gingivalis prtt Gene, Coding for Protease Activity
|
|
|
- Opal Andrews
- 6 years ago
- Views:
Transcription
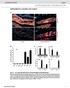
1 INFECrION AND IMMUNITY, Jan. 1993, p /93/ $02.00/0 Copyright 1993, American Society for Microbiology Vol. 61, No. 1 Isolation and Characterization of the Porphyromonas gingivalis prtt Gene, Coding for Protease Activity JUN-ICHI OTOGOTO AND HOWARD K. KURAMITSU* Department of Pediatric Dentistry, University of Texas Health Science Center at San Antonio, San Antonio, Texas Received 8 June 1992/Accepted 3 November 1992 The prtt gene, coding for trypsinlike proteolytic activity, has been isolated from Porphyromonas gingivalis ATCC This gene is present immediately downstream from the sod gene on a 5.9-kb DNA fragment from the organism isolated in Escherichia coli. The complete nucleotide sequence of the gene was determined, and the deduced amino acid sequence of the enzyme corresponds to a 53.9-kDa protein with an estimated pl of Gelatin-sodium dodecyl sulfate-polyacrylamide gel electrophoresis zymography also indicated a similar molecular size for the protease. The enzyme was purified to near homogeneity following anion-exchange and gel-filtration chromatography. The purified enzyme also exhibited a single protein species with a size of approximately 53 kda. Enzyme activity was strongly dependent upon the presence of reducing agents (dithiothreitol, cysteine, and 2-mercaptoethanol) and was also stimulated in the presence of calcium ions. A comparison of the properties of the prtt gene product with comparable parameters of proteases previously purified from different strains of P. gingivalis suggested that the cloned protease represents a previously uncharacterized enzyme. Although human periodontal diseases appear to result from complex interactions between the host and a variety of anaerobic microorganisms, a variety of approaches have strongly suggested an important role for Porphyromonas gingivalis in periodontal tissue destruction (28). Several potential virulence factors, including the elaboration of protease activity, have been identified for these organisms (17). These enzymes may play a role in periodontal disease by degrading protective host immunogloblins (10, 15), hydrolyzing host proteins to provide required amino acids for growth (4), and aiding in the destruction of host connective tissue (30). One prominent enzymatic activity expressed by these organisms (6, 7, 31, 32, 36) is that related to the hydrolysis of the synthetic trypsin substrate BAPNA (N-abenzoyl-DL-arginine-p-nitroanilide). However, several laboratories have demonstrated that enzymes with distinct properties present in both the culture fluids (7, 8, 21, 31) and associated with the cellular membranes of P. gingivalis (7, 8, 19, 22, 26, 29) express BAPNA-hydrolyzing activity. In view of the possibility of autodegradation of the proteases, it is not clear how many distinct proteases are produced by each strain of these organisms. One approach to answering this question is to utilize molecular genetic techniques to isolate the genes for the individual proteases. Several recent communications (23, 32) have reported on the cloning of protease genes from P. gingivalis. In the latter investigation, we identified a protease activity expressed from the Escherichia coli plasmid psl containing a 5.9-kb DNA fragment from P. gingivalis ATCC The present study demonstrates that this activity is not mediated by the collagenase expressed by theprtc gene present on the insert fragment but is due to a distinct gene, prtt, located downstream from the sod gene recently characterized on this same DNA fragment (5). The properties of this protease are compared with those of other proteases previously characterized from different strains of P. gingivalis. * Corresponding author. 117 MATERIALS AND METHODS Bacteria and plasmids. E. coli HB101 and MV1184 (34) were maintained and grown in Luria-Bertani (LB) broth as previously described (1). Plasmid vectors puc18 and puc19 (35) were used for subcloning and expression studies while pbluescript SK+ and KS+ (Stratagene, La Jolla, Calif.) were utilized for nucleotide sequencing. DNA manipulations. Restriction endonuclease digestion and ligation of DNA fragments were carried out according to the directions of the suppliers. The isolation of DNA and transformation of E. coli cells were carried out as previously described (1). Enzyme assays. For routine enzyme assays, E. coli strains harboring recombinant plasmids were grown in 10 ml of LB medium containing ampicillin (50,ug/ml) and 0.14 mm isopropyl-p-d-thiogalactopyranoside (IPTG) at 37 C for 18 h. The cultures were harvested by centrifugation at 5,000 x g for 10 min, and the cells were resuspended in 1 ml of 50 mm Tris-HCl buffer (TB) (ph 8.0) and sonicated for a total time of 10 min (with intermittent cooling) in a Microson Ultrasonic Disruptor (Heat Systems, Farmingdale, N.Y.). The supernatant fluids obtained following centrifugation at 5,000 x g for 5 min served as the crude extracts. Subsequently, this crude extract was centrifuged at 160,000 x g for 1 h, and the supernatant fluid was used as the membrane-free fraction. The resulting pellet was mixed with TB containing 1% Triton X-100 and centrifuged at 160,000 x g for 1 h, and the supernatant fluid was used as the membrane-bound fraction. Trypsinlike protease activity was determined following hydrolysis of the synthetic chromogenic substrate BAPNA (Sigma Chemical Co., St. Louis, Mo.). The enzyme samples (0.1 ml) were mixed with 0.7 ml of reaction mixture (5 mm CaCl2, 2 mm mercaptoethanol, 0.2 mm BAPNA in TB) and incubated at 37 C for 30 min. The reaction was terminated following the addition of 0.2 ml of 50% acetic acid, and the absorbance of the solutions was measured at 410 nm. One unit of enzyme was defined as the amount of enzyme required to release 1.0,umol ofp-nitroanilide per min at 37 C in the standard assay.
2 118 OTOGOTO AND KURAMITSU Two assays were utilized to determine gelatinase activity. Gelatinase activity was routinely determined by visual detection of the dissolution of the gelatin matrix of X-ray film as previously described (20). Samples (20,ul) were spotted onto the film, incubated for 18 h at 37 C, and flushed with water; the film was observed for zones of clearing, indicating gelatinase activity. In addition, gelatinase activity was also determined following gelatin-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) zymography (9). Following electrophoresis of enzyme samples in 0.1% gelatin-conjugated gels, the gels were washed gently with 2.5% Triton X-100 (twice for 30 min) to remove SDS and finally with detergent containing TB. The washed gel was then incubated for 18 h at 37 C in 0.1 M glycine-naoh (ph 8.3) and stained with Coomassie brilliant blue R to visualize gelatinase bands. The degradation of potential protein substrates by the cloned enzyme was examined following SDS-PAGE analysis of the reaction mixtures (12). Utilizing a 10% acrylamide separating gel and a 4.5% stacking gel, reaction mixtures (containing 15,ul of enzyme and 5 pg of protein substrate incubated for 24 h at 37 C) were heated at 100 C for 3 mn and subjected to electrophoresis (16). The gels were then stained with Coomassie brilliant blue R to visualize protein degradation. Enzyme purification procedure. E. coli subclone KS1 (see Fig. 2) was grown in 4 liters of LB broth at 37 C for 18 h, and the washed cells were resuspended in 20 ml of TB buffer. The bacterial cells were sonicated, and the suspensions were centrifuged at 5,000 x g for 15 min to remove the cellular debris and produce a crude extract. The supernatant fluids were then filtered through a Millipore filter (0.22-p,m-poresize), and the filtrate was injected into a Mono-Q HR5/5 anion-exchange chromatography column (Pharmacia-LKB Biotechnology, Inc., Piscataway, N.J.). The column was eluted with a gradient of 0 to 1 M NaCl in TB, and the fractions exhibiting BAPNA hydrolysis and gelatinase activities were pooled and concentrated through a Centricon-10 ultrafilter (Amicon Corp., Danvers, Mass.). The partially purified protease present in the flow-through fractions was next applied to a TSK-G3000SW gel-filtration column (Pharmacia-LKB) and eluted with TB. The active fractions were again pooled, concentrated through a Centricon ultrafilter, and stored at -20 C. Protein concentrations were routinely determined by the modified Bradford method (3), with bovine serum albumin as a standard. Cystatin was kindly provided by M. J. Levine (State University of New York, Buffalo, N.Y.) and histatin-5 was provided by Y. Kuboki (Hokkaido University, Sapporo, Japan). DNA sequence analysis. The complete nucleotide sequence of the prtt gene was determined from both DNA strands by utilizing the dideoxy nucleotide sequencing strategy (24). Overlapping DNA fragments were subcloned into pbluescript SK+ or KS+ and deleted with exonuclease III and mung bean nuclease (11). Sequencing was carried out with cx-35s-datp, Sequenase T7 DNA polymerase (United States Biochemical Corp., Cleveland, Ohio), and universal sequencing primers. Sequence analysis was carried out with the Pustell Sequence Analysis Programs (International Biotech, Inc., New Haven, Conn.). Nucleotide sequence accession number. The P. gingivalis prtt gene sequence has been deposited in the GenBank data base under accession number M KDa FIG. 1. Gelatin-SDS zymography of crude extracts containing proteins expressed from recombinant plasmids. Zymography was carried out as described in the text. Lanes: 1, HB101 (ppl-lambda); 2, JM109 (puc19); 3, KS1, JM109 (puc19); 4, HB101 (psl). See Fig. 2 for orientation of DNA fragments. RESULTS Isolation of the prtt gene. The 5.9-kb DNA fragment containing the prtc (32) and sod (5) genes of P. gingivalis was originally isolated following screening of plasmid clone banks for protease activity on skim-milk-agar plates. However, the collagenase activity expressed from the prtc gene did not degrade the major milk protein casein or gelatin (32). By contrast, an enzyme expressed from plasmid psl from the original clone displayed gelatinase activity (Fig. 1). Furthermore, deletion of the region downstream from the sod gene resulted in the loss of gelatinase activity (32). These results suggested that a gene coding for protease activity was present in the deleted region. In order to confirm this possibility, various subcloned fragments from the psl insert were isolated and inserted into puc18 and puc19 (Fig. 2). These results indicated that insertion of the 1.1-kb KpnI-PstI or 3.7-kb Kjpnl-SalI fragment into puc19 resulted in subclones expressing BAPNA hydrolysis activity, but only the latter fragment expressed gelatinase activity (Table 1). Subsequent nucleotide sequencing (see below) revealed that the former fragment expressed a truncated, but enzymatically active, proteinase. However, insertion of both DNA fragments into puc18 did not result in similar activities. This suggested that transcription of the gene responsible for the protease activity was initiated from the lac promoter of the plasmid and not from a P. gingivalis promoter. In addition, sod KP= PS prtt I_I INFECT. IMMUN. KSII ---I KS2 = 0 1Kb FIG. 2. Subcloning analysis of the prtt gene. Fragments from the 5.9-kb P. gingivalis DNA insert of plasmid ps1 were subcloned into either puc18 or puc19. prtc, collagenase gene; sod, superoxide dismutase gene.
3 VOL. 61, 1993 TABLE 1. Activities of subcloned fragmentsa Subclone Vet Trypsinlike pro- Gelatinase Hemagglutination fragment ector tease activityb activity' activityd KP puc puc PS puc puc KS1 puc puc KS2' puc puc puc puc19 pslf ppl lambda ppl lambda a The DNA fragments were subcloned into the vectors and the resulting plasmid-containing strains were examined for indicated activities. E. coli cells containing the recombinant plasmids were grown in 100 ml of LB medium containing ampicillin (50 p.g/ml) and 0.14 mm IPTG at 37'C for 18 h. The cultures were harvested, suspended in 1 ml of 50 mm TB (ph 8.0), and sonicated. b Trypsinlike protease activity was detected following hydrolysis of the chromogenic substrate BAPNA. c Each sample was assayed for enzymatic activity by the X-ray film method. d Hemagglutinating activity was assayed using sheep erythrocytes (19). e Plasmid containing deletion of the KpnI-SalI fragment. f Reference 32. most of the activity in crude extracts was located in the membrane-bound fraction (data not shown). Plasmid psl also expressed hemagglutinin activity, which was expressed from subclones containing the PstI-SalI fragment in puc18 and the KpnI-SalI fragment in puc18. Therefore, these results suggested that the putative protease was not responsible for hemagglutinin activity. Likewise, deletion of the KpnI-SalI fragment indicated that both activities were encoded by genes located downstream from the sod gene. Nucleotide sequencing of the prtt gene. Both DNA strands downstream from the sod gene were sequenced, and one long open reading frame corresponding to a 53.9-kDa protein was detected (Fig. 3). This molecular size was approximately equal to that estimated for the gelatinase for which activity was expressed from psl following gelatin-sds- PAGE analysis (Fig. 1). The protein would be initiated at base position 239 (Fig. 3), which was 8 bp downstream from an AGA sequence found in E. coli ribosome binding sites (27). However, sequences corresponding to -10 and -35 promoter consensus sequences could not be detected upstream from the gene. In addition, no sequence typical of bacterial signal sequences could be detected in the amino terminus of the deduced protein. The unprocessed protein appears to be highly basic, with an estimated pi of This gene has been named prtt (protease with trypsinlike activity). The calculated G+C ratio for the prtt gene was 45.4%, which corresponds well to the ratio of 46 to 48% previously determined for chromosomal DNA from strains of P. gingivalis (25) and the value of 48% determined from the sod gene of strain (5). Purification of the trypsinlike protease. In order to charac- prtt PROTEASE 119 terize the protein product of the prtt gene, the protease was purified from the E. coli subclone containing plasmid pks1. Since the deduced amino acid sequence indicated that the enzyme was highly basic, crude extracts of the subclone were initially chromatographed on a Mono-Q anion-exchange column. As predicted from the pi of the cloned enzyme, the protease was not retained by the column at ph 8.5. The flow-through fractions were pooled, concentrated, and then subjected to chromatography on a TSK G3000 gel-filtration column. Several peaks of BAPNA hydrolytic activity were detected, but only one of these displayed gelatinase activity. This latter fraction was concentrated and represented an approximately 64-fold purification of protease activity, with an overall yield of 11% (Table 2). Analysis of the concentrated enzyme following SDS-PAGE yielded a single protein band with an estimated molecular size of 53 kda (Fig. 4). This value is similar to that estimated from the deduced amino acid sequence of theprtt gene (Fig. 3) and that determined following gelatin zymography of crude extracts of the original psl clone (Fig.1). Characterization of the purified protease. Maximum protease activity of the purified enzyme was observed near ph 8.5 (data not shown) in the presence of Ca2+ (Table 3). Mg2+ ions were somewhat inhibitory, while Mn2' had only a slight stimulatory effect on enzyme activity. The enzyme did not appear to be a metalloenzyme since EDTA did not inhibit activity. The synthetic protease inhibitors TLCK (N-a-tosyl- L-lySyl chloromethyl ketone) and PMSF (phenylmethylsulfonyl fluoride) produced little or only marginal inhibitory activity. Essential sulfhydryl groups did not appear to be important for maximum activity since PCMB (p-chloromercuribenzoic acid) produced only a small inhibitory effect. However, like several previously characterized P. gingivalis proteases (31), the prtt gene product was strongly stimulated in the presence of the reducing agents dithiothreitol, cysteine, and 2-mercaptoethanol. Two salivary peptides, histatin and cystatin (18), which have been demonstrated to influence protease activity, had little effect on the trypsinlike protease. Despite the strong hydrolytic activity on the trypsin substrate BAPNA by the enzyme (Table 1), little activity against the homologous lysine-containing substrate BLPNA (Nbenzoyl-DL-lysine-p-nitroanilide) could be detected (data not shown). Therefore, unlike classical trypsin, the prtt gene product appears to be specific for arginine-containing peptide bonds. Since the protease could hydrolyze gelatin, it was of interest to determine whether the enzyme was active on native collagens. Analysis of proteolysis following SDS- PAGE revealed that the purified enzyme did not degrade native type I or type IV collagens (data not shown) following incubation at either 30 or 37 C for 18 h. In addition, the enzyme did not hydrolyze laminin, fibronectin, lysozyme, bovine serum albumin, or human immunoglobulin G or M. However, the enzyme could be shown to degrade casein in addition to gelatin (Fig. 5 and 6). Two fractions from the gel-filtration column (fractions 1 and 12) exhibited BAPNA hydrolytic activity but no gelatinase activity (Fig. 5). These fractions may represent altered forms of the P. gingivalis proteinase or E. coli endogenous proteinases. Furthermore, only a low degree of apparent self-digestion of the enzyme was detected following incubation for 15 h at 4 or 37 C. DISCUSSION The present communication describes the isolation of the P. gingivalis prtt gene, coding for a protease with activity
4 120 OTOGOTO AND KURAMITSU INFEcr. IMMUN. A BqlII A GAT CTT TGG AGT ATT GTT GAC TGG GAT ATTl GTA GAA TCT CGG TAT TAA GTA ACC CCA TTG TGC ACT TTG CAC AAT ACA TAA GGT ATA TGC CTG TGC CAA GAA CCG ATC GGG TGT CTC GGC AGG GCT TCT TCT TTT TCT CTT TTC GTT GTT CAC TAA CAG CCG AAT CAA AGC AAA AGA AAA AAG AAA CGG T'! TTC CCT CAA TCC TAT CAA GCC T'm TCA GAA AAG ATC AGG AAC ATG TAC CAT GTA TAT CCG AAT TGG TTT AGG CTT CAA AAT TT! CCC GTT MNt Tyr His Val Tyr Pro Asn Trp Phe Arg Leu Gln Asn Phe Pro Val TGC TAC TCC TCA AAA CGC GGT TCG GAA ACT TTT TTA TTT TGG CGT GGG AAG ACC AAA Cys Tyr Ser Ser Lys Ar; Gly Ser Glu Thr Ph. Lou Ph. Trp Arg Gly Lys Thr Lys AAA TTC TCA CGC CAC AAC GAA AAA AAT CTC GCG CCA CTT TTT CAG GGA ATA CGC GCC Lys Ph. S.r Arg His Asn Glu Lys Asn Lou Ala Pro L.u Phe Gln Gly Ile Arg Ala ACA ATC GGA GCT TTT CCG GTT CGT ATT T'! TTG AAT AGC TCC TTT TGC AAA CAA GCC Thr Ile Gly Ala Phe Pro Val Arg Ile Ph. Lou Asn Ser Ser Ph. Cys Lys Gln Ala * * 0 * * 0 GAG GCA ATC GAC AAA AAT CTG CCG AGG ATT CAC CTT CCG GGA AAA GAC TI! CCT GAG Glu Ala Ile Asp Lys Asn Lou Pro Arg Ile His Lou Pro Gly Lys Asp Pho Pro Glu *0 * * 0 * GAA AAG ATC GAA AAG ATT CGT TCA CAC ATA CAT TTA CGC AAA AAT TGT CCG ACA AAA Glu Lys Ile Glu Lys Ile Arg Ser His I1e His Lou Arg Lys Asn Cys Pro Thr Lys A"A TTA CAT TTG CAC CCG AAT AAA GAT GGA CAG GTA CCT CAG CTG GAT ACA GCA ATA Arg Lou His Lou His Pro Asn Lys Asp Gly Gln Val Ala Gln Lou Asp Thr Ala Ile GCC TTC GA GCT ATC GGC CTG GGG TCG AAT CCC AGC CTG ATC ACC TCA AAA AGG GCA Ala Phe Glu Ala Ile Gly Lou Gly Ser Asn Pro Ser Lou I1- Thr S-r Lys Arg Ala TGC GCA AGT ATC CTT TTN TAT CTC TGT AAC TI! AAT AAT CGT CCT TI! TTA TCA TTA Cys Ala Ser Ile Lou Lou Tyr Lou Cys Asn Ph. Asn Asn Arg Pro Pho Lou Sor L.u OTC GAC GTT AAA GAG aat GAC GAT TAT CCT TCT TCT CCC ATC TCC TGC TTC GAA CAA Val Asp Val Lys Glu Asp Clu Asp Tyr Pro Ser S-r Ala I1 Sor Cys Pho Glu Gln CAA AAC CAA aat GCA ATA ATC AAA COT ATC TTC TAC ACc TTA GcG CTA TTA TTA CTG Gln Asn Gln Asn Ala Il Hot Lys Ar; I1 Pho Tyr Thr Lou Gly Lou Lou Lou Lou a TCT CTC CCT ATN cc CAa WCA GGA cco GTG ACA CGA TCA AAG CCG AAC A"A CN cta Cys Lou Pro Not Lou Gln Ala Gly Pro Val Thr Ar Sor Lys Pro Asn Ar; Lu Lou FIG. 3. Nucleotide sequence and deduced amino acid sequence of the P. gingivalis prtt gene. A potential ribosome binding site (SD) as well as the locations of the restriction endonuclease sites is indicated. against the synthetic trypsin substrate BAPNA. Several ally inhibited by leupeptin (Table 3). Furthermore, all of proteases with similar specificity isolated from different P. these enzymes are serine proteases. By contrast, the prtt gingivalis strains have also been described previously (2, 6, protease is insensitive to inhibitors of this class of enzymes, 7, 31, 33, 36). These enzymes differ in terms of cellular such as PMSF (Table 3). location and sensitivity to inhibitors, as well as in estimated The molecular size of the cloned prtt protease estimated molecular size. A comparison of the properties of these following SDS-PAGE analysis of the purified enzyme (Fig. enzymes with those of the prtt protease suggests that the 4) and gelatin-sds-page zymography (Fig. 1) and from the latter enzyme may represent a unique enzyme which has not deduced amino acid sequence is consistent with a value of 53 been previously identified. For example, all the P. gingivalis kda. This size is most similar to that estimated for the trypsinlike proteases examined have been reported to be trypsinlike protease purified from strain 381 of P. gingivalis strongly inhibited by the synthetic protease inhibitor leupep- (33). However, because of the multiple proteases produced tin (13, 19, 33). However, the prtt protease is only margin- by these organisms (9), caution should be exercised in
5 VOL. 61, 1993 prtt PROTEASE 121 B AGA ACT m TTG CCA AAC GAC AAC CCA CGT TGT CTT CAT CGA CTG CGA GTC TCC GGA Arg Thr Phe Lou Pro Asn Asp Asn Pro Arg Cys Lou His Arg Leu Arg Val Ser Gly * * Pstl * * * TGG ATT TCG m ACA AAG CTG CAG AAA GAG AGG AGG CAC TAT TCT TCG TTT TCA ATC Trp Ile Ser Phe Thr Lys Leu Gln Lys Glu Arg Arg His Tyr Ser Ser Phe Sr Ile GAG GAG AGA AAG ACG GAT TTC TCC TCG TCG CAG CGG ATT ATC GGT TCC CGG AGT GAT Glu Glu Arg Lys Thr Asp Phe Ser Ser Ser Gln Arg Ile Ile Gly Ser Arg Ser Asp CGG ATA TGC TTT CkA GGA AAA CTT CGT ATG GGC GTA TGC CGG ACA ATC TCA CGG GGT Arg Ile Cys Ph- Gln Gly Lys Lou Arg Not Gly Val Cys Arg Thr Ile Sr Arg Gly * * * * * TGT CAA AGG CTA GAa CGT GAA ATG CTT GCT GTA ATG GAC GGC AAG GCA GAG CCG ATA Cys Gln Arg Lou Glu Arg Glu Not Lou Ala Val Hot Asp Gly Lys Ala Glu Pro Ile GAT CCT ATC CGT GAA GAC MAG CCT ACA CGG ACC TGC CAT CAT CCA TTG CCC CTA TTT Asp Pro Ile Arg Glu Asp Lys Pro Thr Arg Thr Cys His His Pro Lou Pro Lou Phc * * * BamI * * * TGG AAA CGG GCG AAC ATG CAT CGG ATC COT ACG ATA CCG GGT GCT TTA TTG CTG GCC Trp Lys Arg Aio Asn Met His Arg Iie Arg Thr Ile Pro Gly Ala Lou Lou Lou Ala * * * * * GGC TCT ATG CCT ATG ACA ACC TCT TOG TCA GTA GAG TAT CCT CAT CGC CTG ACT CCT Gly Sr Met Pro Mot Thr Thr Scr Trp Sr Val Glu Tyr Pro His Arg Lou Thr Pro GTG CCA AGC GAa CCC GGA TTG AGT AGA TTG AGA TAC MAA TTA CCG TTA CTC ATG CCA Val Pro Ser Glu Pro Gly Lou Sr Arg Lou Arg Tyr Lys Lou Pro Lou Liu Not Pro * * * * * CCC CAT CCC CAG TTG AAG TGG aac GTT CCO TCT GGT TCO TAT CCA TCO CAA ACG AAA Pro His Pro Gln Lou Lys Trp Asn Val Pro Sr Gly Sr Tyr Pro Sr Gln Thr Lys S GCA TGT CCA TCG ACC GTC TGC ACC GGC ATA ATA CAC AGO TCT GTT TTC TGC CAG TTC Ala Cys Pro Sr Thr Val Cys Thr Gly I1- I1 His Arg Sr Val Ph- Cys Gln Phe * * 0 CTT ACG AAT CAT GTC TTC ATT CTA GCC GGT TCO CTG GTG ACO GTC TTA GCG GTG CAA Lou Thr Asn His Val Phe Ile Lou Ala Gly Ser Lou Val Thr Val Lou Ala Val Gln * * * 0 0 * CGO ATT CGC TAC ATG ACA GCG CAT GAC AGT GGT CTA CTA ACA GAT CGG AAA GCA CGA Arg Ile Arg Tyr Net Thr Ala His Asp Sr Gly Lou Lou Thr Asp Arq Lys Ala Arg * * * 0 * * CAT GTA TTC CTC MAT GTA TGA TCA aac GTC AAT ATT GAA TCA GGA GCT TGT AAA aat His Val Phe Lou Asn Val determining the precise molecular size for proteins purified from these organisms. Despite the differences detected between the clonedprtt protease and the trypsinlike proteases previously character- TABLE GAC AM GCO CAA GC CCC GTC CTG GAA TO T FIG. 3-Continued. Purification of the trypsinlike protease Total Total Sp act Yield Purification Purification step protein activity (U/mg) (%) (fold) (mg) (U) Crude extract Mono-Q HR5/ TSK G3000SW ized from P. gingivalis, all of these enzymes are strongly stimulated by reducing agents. However, by contrast to most of the trypsinlike proteases from these organisms, the cloned enzyme and the enzyme purified from strain 381 (33) are not strongly inhibited by sulfhydryl reagents. Therefore, it appears that the prtt protease is not a classical serine protease or cysteine protease. In addition, this enzyme is not a classical trypsin since it does not degrade the synthetic substrate BLPNA (containing a lysine residue instead of arginine). Therefore, the protease appears to hydrolyze peptide bonds containing arginine at the carboxyl side of the hydrolyzed peptide bond. Recently, two proteases purified from P. gingivalis have been reported to display similar substrate specificity (4, 13). However, it is not clear whether
6 122 OTOGOTO AND KURAMITSU INFEcr. IMMUN KDa FIG. 4. SDS-PAGE analysis of the purified enzyme. Purified enzyme (2.Lg) from the TSK-G3000SW column was concentrated by a Centricon-10 ultrafilter. Molecular size standards (in kilodaltons) are indicated on the right. the prtt gene product is identical to either of these two enzymes since distinct differences (substrate specificity and preliminary amino acid sequence comparisons) between the enzymes have been detected. Several of the trypsinlike proteases isolated from P. gingivalis were isolated from culture supernatant fluids (7, 13, 21, 31) while others were isolated from cellular fractions (7, 8, 19, 22, 26, 29). However, the demonstrations that these organisms release considerable amounts of membrane vesicles containing membrane-associated proteins into the culture fluids (30) make it difficult to assess the cellular location of some of these enzymes. Therefore, the cellular location of the prtt protease in strain has not been determined. The majority of the protease activity expressed from the cloned prtt gene was associated with the E. coli membrane fraction, although a significant amount was also present in the membrane-free fraction (data not shown). Therefore, the protease could be primarily membrane associated in E. coli but released following the sonication treatment. TABLE Effects of enzyme inhibitorsa Effector Concn (mm) Relative activity None 100 TLCK Leupeptin Cystatin Histatin EDTA PCMB PMSF Dithiothreitol L-Cysteine Mercaptoethanol CaCl MgCl MnCl a Purified protease was assayed for BAPNA hydrolyzing activity in the presence of the indicated effectors in the absence of Ca and 2-mercaptoethanol. In addition, the inhibitors were preincubated with the enzyme before the addition of the substrate. 200K1 X) 97~ ' *-* A4 0 ' - Gelatin FIG. 5. The effects of the trypsinlike protease on gelatin. Fractions from the TSK-G3000SW gel-filtration column were assayed for gelatinase activity following SDS-PAGE. Lanes: 1, fraction 1 plus gelatin; 2, fraction 12 plus gelatin; 3, fraction 20 (prtt gene product) plus gelatin; 4, gelatin alone. Preliminary characterization of a protease gene isolated from P. gingivalis W83 has been recently reported (23). However, a comparison of the properties of this enzyme with those of theprtt protease revealed that their molecular sizes following SDS-PAGE analysis are distinct and that both enzymes exhibit different sensitivities to EDTA and 2-mercaptoethanol. Recent Southern blot analysis utilizing plasmid psl as a probe has suggested that the prtc, sod, and prtt genes are present in the same relative orientation on the chromosome of representative strains from the three major serotypes of P. gingivalis (14). In addition, the results of that investigation using Northern (RNA) blot analysis indicated that the mrna coding for the prtc collagenase may be large enough to include the prtt protease. Therefore, possible coexpression of two enzymes involved in collagen degradation (gelatin is denatured type I collagen) would suggest that the major role of the prtt protease may be the digestion of collagen fragments generated from the action of the prtc collagenase. However, it is of interest that no apparent signal sequence could be detected in the deduced amino acid sequence of the proteinase. Therefore, it is not clear how this protein would be compartmentalized (outer membrane vesicles) to function in exogenous collagen breakdown. Further characterization of the mrna corresponding to these genes as well as the specificities of these two enzymes will be required to confirm this hypothesis. In addition, the recent development of a gene transfer system for generating specific mutants in P. gingivalis (5a) will allow direct testing of this hypothesis with the prtc and prtt genes. Recently, it has been reported that the hemagglutinin of P. gingivalis 381 is identical to a trypsinlike protease produced t4' 21KflaKl) _ (53KDa)n3a - - *w 46 r* 21.5 FIG. 6. Effects of trypsinlike protease on casein and self-digestion. Protein degradation was analyzed by SDS-PAGE. Lanes: 1, 2 p.g of purified protease stored at 4 C; 2, 2,ug of enzyme incubated at 37 C for 18 h; 3 and 4, 2,ug of enzyme incubated with 1,ug of casein at 37'C for 18 h; 5, 1,ug of casein.
7 VOL. 61, 1993 by these organisms (19). Therefore, it was of great interest that the P. gingivalis 5.9-kb DNA fragment on plasmid psl expressed both BAPNA hydrolysis as well as hemagglutinating activities (Table 1). However, subcloning of this fragment revealed that the prtt protease was not responsible for hemagglutinin activity and that this latter activity was expressed from a gene downstream from the prtt gene. Preliminary sequencing of this region has revealed the presence of an open reading frame corresponding to a truncated protein, and further characterization of this putative gene is now in progress. ACKNOWLEDGMENTS We express our appreciation to T. Kato for advice in several aspects of the current study. This investigation was supported in part by National Institutes of Health grant DE REFERENCES 1. Aoki, H., T. Shiroza, M. Hayakawa, S. Sato, and H. K. Kuramitsu Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect. Immun. 53: Birkedal-Hansen, H., R E. Taylor, J. J. Zambon, P. K. Barua, and M. E. Neiders Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J. Periodontal. Res. 23: Bradford, M. H A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: Chen, Z., J. Potempa, A. Polanowski, S. Renvert, M. Wikstrom, and J. Travis Stimulation of proteinase and amidase activities in Porphyromonas (Bacteroides) gingivalis by amino acids and peptides. Infect. Immun. 59: Choi, J., N. Takahashi, T. Kato, and H. K. Kuramitsu Isolation, expression, and nucleotide sequence of the sod gene from Porphyromonas gingivalis. Infect. Immun. 59: a.Dyer, D. Personal communication. 6. Endo, J., M. Otsuka, M. Sato, and R. Nakamura Cleavage action of a trypsin-like protease from Bacteroidesgingivalis 381 on reduced egg-white lysozyme. Arch. Oral Biol. 34: Fujimura, S., and T. Nakamura Isolation and characterization of a protease from Bacteroides gingivalis. Infect. Immun. 55: Fujimura, S., and T. Nakamura Purification and characterization of a 43 kda protease of Porphyromonas gingivalis. Oral Microbiol. Immunol. 5: Grenier, D., G. Chao, and B. M. McBride Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect. Immun. 57: Grenier, D., D. Mayrand, and B. C. McBride Further studies on the degradation of immunoglobulins by black-pigmented Bacteroides. Oral Microbiol. Immunol. 4: Heinkoff, S Unidirectional digestion with exonuclease II creates targeted breakpoints for DNA sequencing. Gene 28: Heussen, C., and E. B. Dowdle Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 102: Hinode, D., H. Hayashi, and R. Nakamura Purification and characterization of three types of proteases from culture supernatants of Porphyromonas gingivalis. Infect. Immun. 59: Kato, T., N. Takahashi, and H. K. Kuramitsu Sequence analysis and characterization of the Porphyromonas gingivalis prtc gene expressing a novel collagenase activity. J. Bacteriol. 174: Kilian, M Degradation of immunoglobulins Al, A2, and G by suspected principal periodontal pathogens. Infect. Immun. 34: prtt PROTEASE Laemmli, U. K Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227: Mayrand, D., and S. C. Holt Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol. Rev. 52: Nishikata, M., T. Kanehira, H. Oh, H. Tani, M. Tazaki, and Y. Kuboki Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem. Biophys. Res. Commun. 174: Nishikata, M., and F. Yoshimura Characterization of Porphyromonas (Bacteroides) gingivalis hemagglutinin as a protease. Biochem. Biophys. Res. Commun. 178: Norqvist, A., B. Norrman, and H. Wolf-Walz Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect. Immun. 58: Ono, M., K. Okuda, and I. Takazoe Purification and characterization of a thiol-protease from Bacteroides gingivalis strain 381. Oral Microbiol. Immunol. 2: Otsuka, M., J. Endo, D. Hinode, A. Nagata, R. Maehara, M. Sato, and R. Nakamura Isolation and characterization of protease from culture supematant of Bacteroides gingivalis. J. Periodontal. Res. 22: Park, Y., and B. C. McBride Cloning of a Porphyromonas (Bacteroides) gingivalis protease gene and characterization of its product. FEMS Microbiol. Lett. 92: Sanger, F., S. Nicklen, and A. R Coulson DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74: Shah, H. N., and M. D. Collins Proposal for reclassification of Bacteroides asaccharolyticus, Bacteroides gingivalis, and Bacteroides endodontalis in a new genus, Porphyromonas. Int. J. Syst. Bacteriol. 38: Shah, H. N., and S. E. Gharbia Lysis of erythrocytes by the secreted cysteine proteinase of Porphyromonas gingivalis W83. FEMS Microbiol. Lett. 61: Shine, J., and L. Dalgarno The 3' terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71: Slots, J., and R. J. Genco Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J. Dent. Res. 63: Smalley, J. W., and A. J. Birss Trypsin-like enzyme activity of the extracellular membrane vesicles of Bacteroides gingivalis W50. J. Gen. Microbiol. 133: Smalley, J. W., A. J. Birss, and C. A. Shuttleworth The degradation of type I collagen and human plasma fibronectin by the trypsinlike enzyme and extracellular membrane vesicles of Bacteroides gingivalis W50. Arch. Oral Biol. 33: Sorsa, T., V. J. Uitto, K. Suomalainen, H. Turto, and S. Lindy A trypsinlike protease from Bacteroides gingivalis: partial purification and characterization. J. Periodontal Res. 22: Takahashi, N., T. Kato, and H. K. Kuramitsu Isolation and preliminary characterization of the Porphyromonas gingivalis prtc gene expressing collagenase activity. FEMS Microbiol. Lett. 84: Tsutsui, H., T. Kinouchi, Y. Wakano, and Y. Ohnishi Purification and characterization of a protease from Bacteroides gingivalis 381. Infect. Immun. 55: Vieira, J., and J. Messing The puc plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19: Vieira, J., and J. Messing Production of single-stranded plasmid DNA. Methods Enzymol. 153: Yoshimura, F., M. Nishikata, T. Suzuki, C. I. Hoover, and E. Newbrun Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch. Oral Biol. 29:
Materials Protein synthesis kit. This kit consists of 24 amino acids, 24 transfer RNAs, four messenger RNAs and one ribosome (see below).
 Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
ORFs and genes. Please sit in row K or forward
 ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Project 07/111 Final Report October 31, Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines
 Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
Disease and selection in the human genome 3
 Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Lecture 19A. DNA computing
 Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR
 Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
NAME:... MODEL ANSWER... STUDENT NUMBER:... Maximum marks: 50. Internal Examiner: Hugh Murrell, Computer Science, UKZN
 COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
G+C content. 1 Introduction. 2 Chromosomes Topology & Counts. 3 Genome size. 4 Replichores and gene orientation. 5 Chirochores.
 1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
Supplemental Data Supplemental Figure 1.
 Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Cat. # Product Size DS130 DynaExpress TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1
 Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Homework. A bit about the nature of the atoms of interest. Project. The role of electronega<vity
 Homework Why cited articles are especially useful. citeulike science citation index When cutting and pasting less is more. Project Your protein: I will mail these out this weekend If you haven t gotten
Homework Why cited articles are especially useful. citeulike science citation index When cutting and pasting less is more. Project Your protein: I will mail these out this weekend If you haven t gotten
Supplementary. Table 1: Oligonucleotides and Plasmids. complementary to positions from 77 of the SRα '- GCT CTA GAG AAC TTG AAG TAC AGA CTG C
 Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Lecture 11: Gene Prediction
 Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Arabidopsis actin depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB
 Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
RPA-AB RPA-C Supplemental Figure S1: SDS-PAGE stained with Coomassie Blue after protein purification.
 RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
Det matematisk-naturvitenskapelige fakultet
 UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
Expression of Recombinant Proteins
 Expression of Recombinant Proteins Uses of Cloned Genes sequencing reagents (eg, probes) protein production insufficient natural quantities modify/mutagenesis library screening Expression Vector Features
Expression of Recombinant Proteins Uses of Cloned Genes sequencing reagents (eg, probes) protein production insufficient natural quantities modify/mutagenesis library screening Expression Vector Features
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
PCR analysis was performed to show the presence and the integrity of the var1csa and var-
 Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Supplemental material
 Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Primer Design Workshop. École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria
 Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Supporting Information
 Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
Supplementary Figure 1A A404 Cells +/- Retinoic Acid
 Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
MacBlunt PCR Cloning Kit Manual
 MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis
 1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
Codon Bias with PRISM. 2IM24/25, Fall 2007
 Codon Bias with PRISM 2IM24/25, Fall 2007 from RNA to protein mrna vs. trna aminoacid trna anticodon mrna codon codon-anticodon matching Watson-Crick base pairing A U and C G binding first two nucleotide
Codon Bias with PRISM 2IM24/25, Fall 2007 from RNA to protein mrna vs. trna aminoacid trna anticodon mrna codon codon-anticodon matching Watson-Crick base pairing A U and C G binding first two nucleotide
Gene synthesis by circular assembly amplification
 Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
Supporting information for Biochemistry, 1995, 34(34), , DOI: /bi00034a013
 Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC
 Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Hes6. PPARα. PPARγ HNF4 CD36
 SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were
 1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
Table S1. Bacterial strains (Related to Results and Experimental Procedures)
 Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Protein Structure Analysis
 BINF 731 Protein Structure Analysis http://binf.gmu.edu/vaisman/binf731/ Iosif Vaisman COMPUTATIONAL BIOLOGY COMPUTATIONAL STRUCTURAL BIOLOGY COMPUTATIONAL MOLECULAR BIOLOGY BIOINFORMATICS STRUCTURAL BIOINFORMATICS
BINF 731 Protein Structure Analysis http://binf.gmu.edu/vaisman/binf731/ Iosif Vaisman COMPUTATIONAL BIOLOGY COMPUTATIONAL STRUCTURAL BIOLOGY COMPUTATIONAL MOLECULAR BIOLOGY BIOINFORMATICS STRUCTURAL BIOINFORMATICS
Y-chromosomal haplogroup typing Using SBE reaction
 Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Multiplexing Genome-scale Engineering
 Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
ΔPDD1 x ΔPDD1. ΔPDD1 x wild type. 70 kd Pdd1. Pdd3
 Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
National PHL TB DST Reference Center PSQ Reporting Language Table of Contents
 PSQ Reporting Language Table of Contents Document Page Number PSQ for Rifampin 2-6 Comparison table for rpob Codon Numbering 2 rpob mutation list (new numbering system) 3-5 rpob interpretations 6 PSQ for
PSQ Reporting Language Table of Contents Document Page Number PSQ for Rifampin 2-6 Comparison table for rpob Codon Numbering 2 rpob mutation list (new numbering system) 3-5 rpob interpretations 6 PSQ for
Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR
 Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Dierks Supplementary Fig. S1
 Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular
![strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular](/thumbs/72/66361212.jpg) Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Supplementary Information
 Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells
 Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Supplementary Information. Construction of Lasso Peptide Fusion Proteins
 Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Lezione 10. Bioinformatica. Mauro Ceccanti e Alberto Paoluzzi
 Lezione 10 Bioinformatica Mauro Ceccanti e Alberto Paoluzzi Dip. Informatica e Automazione Università Roma Tre Dip. Medicina Clinica Università La Sapienza Lezione 10: Sintesi proteica Synthesis of proteins
Lezione 10 Bioinformatica Mauro Ceccanti e Alberto Paoluzzi Dip. Informatica e Automazione Università Roma Tre Dip. Medicina Clinica Università La Sapienza Lezione 10: Sintesi proteica Synthesis of proteins
Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH).
 Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
 www.lessonplansinc.com Topic: Gene Mutations WS Summary: Students will learn about frame shift mutations and base substitution mutations. Goals & Objectives: Students will be able to demonstrate how mutations
www.lessonplansinc.com Topic: Gene Mutations WS Summary: Students will learn about frame shift mutations and base substitution mutations. Goals & Objectives: Students will be able to demonstrate how mutations
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system
 Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Supporting Online Information
 Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
for Programmed Chemo-enzymatic Synthesis of Antigenic Oligosaccharides
 Supporting Information Design of α-transglucosidases of Controlled Specificity for Programmed Chemo-enzymatic Synthesis of Antigenic Oligosaccharides Elise Champion ±,,,, Isabelle André ±,,, Claire Moulis
Supporting Information Design of α-transglucosidases of Controlled Specificity for Programmed Chemo-enzymatic Synthesis of Antigenic Oligosaccharides Elise Champion ±,,,, Isabelle André ±,,, Claire Moulis
Supporting Information
 Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Supporting Information
 Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Overexpression Normal expression Overexpression Normal expression. 26 (21.1%) N (%) P-value a N (%)
 SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR
 Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
Creation of A Caspese-3 Sensing System Using A Combination of Split- GFP and Split-Intein
 Supplementary Information Creation of A Caspese-3 Sensing System Using A Combination of Split- GFP and Split-Intein Seiji Sakamoto,* Mika Terauchi, Anna Hugo, Tanner Kim, Yasuyuki Araki and Takehiko Wada*
Supplementary Information Creation of A Caspese-3 Sensing System Using A Combination of Split- GFP and Split-Intein Seiji Sakamoto,* Mika Terauchi, Anna Hugo, Tanner Kim, Yasuyuki Araki and Takehiko Wada*
Supplemental Data. mir156-regulated SPL Transcription. Factors Define an Endogenous Flowering. Pathway in Arabidopsis thaliana
 Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
Supplemental Information. Human Senataxin Resolves RNA/DNA Hybrids. Formed at Transcriptional Pause Sites. to Promote Xrn2-Dependent Termination
 Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
SUPPLEMENTARY MATERIALS AND METHODS. E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1)
 SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer
 TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
PROTEIN SYNTHESIS Study Guide
 PART A. Read the following: PROTEIN SYNTHESIS Study Guide Protein synthesis is the process used by the body to make proteins. The first step of protein synthesis is called Transcription. It occurs in the
PART A. Read the following: PROTEIN SYNTHESIS Study Guide Protein synthesis is the process used by the body to make proteins. The first step of protein synthesis is called Transcription. It occurs in the
FROM DNA TO GENETIC GENEALOGY Stephen P. Morse
 1. GENES, CHROMOSOMES, AND DNA Chromosomes FROM DNA TO GENETIC GENEALOGY Stephen P. Morse (steve@stevemorse.org) Every human cell = 46 chromosomes (1 to 22 in pairs, 2 sex chromosomes) Male: sex chromosomes
1. GENES, CHROMOSOMES, AND DNA Chromosomes FROM DNA TO GENETIC GENEALOGY Stephen P. Morse (steve@stevemorse.org) Every human cell = 46 chromosomes (1 to 22 in pairs, 2 sex chromosomes) Male: sex chromosomes
evaluated with UAS CLB eliciting UAS CIT -N Libraries increase in the
 Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
S4B fluorescence (AU)
 A S4B fluorescence (AU) S4B fluorescence (AU) dsbb csgba csgd dsbb csgba bcsa 5000 * NS NS 4000 * 3000 2000 1000 0 ΔcsgBAΔbcsA ΔcsgDΔdsbBΔbcsA ΔcsgBA ΔdsbBΔcsgBA ΔcsgDΔdsbB B -1000 4000 * * NS 3500 * 3000
A S4B fluorescence (AU) S4B fluorescence (AU) dsbb csgba csgd dsbb csgba bcsa 5000 * NS NS 4000 * 3000 2000 1000 0 ΔcsgBAΔbcsA ΔcsgDΔdsbBΔbcsA ΔcsgBA ΔdsbBΔcsgBA ΔcsgDΔdsbB B -1000 4000 * * NS 3500 * 3000
INTRODUCTION TO THE MOLECULAR GENETICS OF THE COLOR MUTATIONS IN ROCK POCKET MICE
 The Making of the The Fittest: Making of the Fittest Natural Selection Natural and Adaptation Selection and Adaptation Educator Materials TEACHER MATERIALS INTRODUCTION TO THE MOLECULAR GENETICS OF THE
The Making of the The Fittest: Making of the Fittest Natural Selection Natural and Adaptation Selection and Adaptation Educator Materials TEACHER MATERIALS INTRODUCTION TO THE MOLECULAR GENETICS OF THE
Genomic Sequence Analysis using Electron-Ion Interaction
 University of Aizu, Graduation Thesis. March, 25 s1985 1 Genomic Sequence Analysis using Electron-Ion Interaction Potential Masumi Kobayashi s1985 Supervised by Hiroshi Toyoizumi Abstract This paper proposes
University of Aizu, Graduation Thesis. March, 25 s1985 1 Genomic Sequence Analysis using Electron-Ion Interaction Potential Masumi Kobayashi s1985 Supervised by Hiroshi Toyoizumi Abstract This paper proposes
Genomics and Gene Recognition Genes and Blue Genes
 Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Assignment 13. In the Griffith experiment, why did mice die when injected with live R bacteria plus heatkilled
 Assignment 13 1. Multiple-choice (1 point) In the Griffith experiment, why did mice die when injected with live R bacteria plus heatkilled S bacteria? Some of the S bacteria were still alive. The R bacteria
Assignment 13 1. Multiple-choice (1 point) In the Griffith experiment, why did mice die when injected with live R bacteria plus heatkilled S bacteria? Some of the S bacteria were still alive. The R bacteria
Supplemental Data. Bennett et al. (2010). Plant Cell /tpc
 BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
An engineered tryptophan zipper-type peptide as a molecular recognition scaffold
 SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
2
 1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
CHE-3H84: Protein Engineering Past Exam Papers
 CHE-3H84: Protein Engineering Past Exam Papers Sorted by Topic then Year Knowledge-Based Engineering of Proteins, Large Scale Production of Recombinant Proteins, and Protein Purification Dr. Hemmings 2006/7
CHE-3H84: Protein Engineering Past Exam Papers Sorted by Topic then Year Knowledge-Based Engineering of Proteins, Large Scale Production of Recombinant Proteins, and Protein Purification Dr. Hemmings 2006/7
Thr Gly Tyr. Gly Lys Asn
 Your unique body characteristics (traits), such as hair color or blood type, are determined by the proteins your body produces. Proteins are the building blocks of life - in fact, about 45% of the human
Your unique body characteristics (traits), such as hair color or blood type, are determined by the proteins your body produces. Proteins are the building blocks of life - in fact, about 45% of the human
High-throughput cloning and expression in recalcitrant bacteria
 High-throughput cloning and expression in recalcitrant bacteria Eric R Geertsma & Bert Poolman Supplementary text and figures: Supplementary Figure 1 Frequency of SfiI sites yielding identical 3 extensions
High-throughput cloning and expression in recalcitrant bacteria Eric R Geertsma & Bert Poolman Supplementary text and figures: Supplementary Figure 1 Frequency of SfiI sites yielding identical 3 extensions
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide
 SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide former/ working Description a designation Plasmids pes213a b pes213-tn5
SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide former/ working Description a designation Plasmids pes213a b pes213-tn5
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
hcd1tg/hj1tg/ ApoE-/- hcd1tg/hj1tg/ ApoE+/+
 ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
Table S1. Sequences of mutagenesis primers used to create altered rdpa- and sdpa genes
 Supplementary Table and Figures for Structural Basis for the Enantiospecificities of R- and S-Specific Phenoxypropionate/α-Ketoglutarate Dioxygenases by Tina A. Müller, Maria I. Zavodszky, Michael Feig,
Supplementary Table and Figures for Structural Basis for the Enantiospecificities of R- and S-Specific Phenoxypropionate/α-Ketoglutarate Dioxygenases by Tina A. Müller, Maria I. Zavodszky, Michael Feig,
Supplemental Table 1. Primers used for PCR.
 Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
MCB421 FALL2005 EXAM#3 ANSWERS Page 1 of 12. ANSWER: Both transposon types form small duplications of adjacent host DNA sequences.
 Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
II 0.95 DM2 (RPP1) DM3 (At3g61540) b
 Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Legends for supplementary figures 1-3
 High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
Long ssdna Preparation Kit (LsODN Preparation Kit)
 Product Name: Long ssdna Preparation Kit (LsODN Preparation Kit) Cat. # Product Size DS610 Long ssdna Preparation Kit for 1.5 kb (LsODN Preparation Kit) plsodn-1 10 μg (0.5 μg/μl) plsodn-2d 10 μg (0.5
Product Name: Long ssdna Preparation Kit (LsODN Preparation Kit) Cat. # Product Size DS610 Long ssdna Preparation Kit for 1.5 kb (LsODN Preparation Kit) plsodn-1 10 μg (0.5 μg/μl) plsodn-2d 10 μg (0.5
Long ssdna Preparation Kit (LsODN Preparation Kit)
 Product Name: Long ssdna Preparation Kit (LsODN Preparation Kit) Cat. # Product Size DS610 Long ssdna Preparation Kit for 1.5 kb (LsODN Preparation Kit) plsodn-1 10 μg (0.5 μg/μl) plsodn-2d 10 μg (0.5
Product Name: Long ssdna Preparation Kit (LsODN Preparation Kit) Cat. # Product Size DS610 Long ssdna Preparation Kit for 1.5 kb (LsODN Preparation Kit) plsodn-1 10 μg (0.5 μg/μl) plsodn-2d 10 μg (0.5
Engineering D66N mutant using quick change site directed mutagenesis. Harkewal Singh 09/01/2010
 Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
BioDynamics Laboratory Inc.
 Product Name: Long ssdna Preparation Kit (LsODN Preparation Kit) Cat. # Product Size DS610 Long ssdna Preparation Kit for 1.5 kb (LsODN Preparation Kit) plsodn-1 10 µg (0.5 µg/µl) plsodn-2d 10 µg (0.5
Product Name: Long ssdna Preparation Kit (LsODN Preparation Kit) Cat. # Product Size DS610 Long ssdna Preparation Kit for 1.5 kb (LsODN Preparation Kit) plsodn-1 10 µg (0.5 µg/µl) plsodn-2d 10 µg (0.5
Glutathione (GSH)-Decorated Magnetic Nanoparticles for Binding Glutathione-S-transferase (GST) Fusion Protein and Manipulating Live Cells
 Glutathione (GSH)-Decorated Magnetic Nanoparticles for Binding Glutathione-S-transferase (GST) Fusion Protein and Manipulating Live Cells Yue Pan, Marcus J. C. Long, Xinming Li, Junfeng Shi, Lizbeth Hedstrom,
Glutathione (GSH)-Decorated Magnetic Nanoparticles for Binding Glutathione-S-transferase (GST) Fusion Protein and Manipulating Live Cells Yue Pan, Marcus J. C. Long, Xinming Li, Junfeng Shi, Lizbeth Hedstrom,
BioInformatics and Computational Molecular Biology. Course Website
 BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
DNA sentences. How are proteins coded for by DNA? Materials. Teacher instructions. Student instructions. Reflection
 DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
Supporting Information
 Supporting Information CLOSTRIDIOLYSIN S: A POST-TRANSLATIONALLY MODIFIED BIOTOXIN FROM CLOSTRIDIUM BOTULINUM David J. Gonzalez 1, Shaun W. Lee 9, Mary E. Hensler 6, Andrew L. Markley 1, Samira Dahesh
Supporting Information CLOSTRIDIOLYSIN S: A POST-TRANSLATIONALLY MODIFIED BIOTOXIN FROM CLOSTRIDIUM BOTULINUM David J. Gonzalez 1, Shaun W. Lee 9, Mary E. Hensler 6, Andrew L. Markley 1, Samira Dahesh
Introduction to Bioinformatics Dr. Robert Moss
 Introduction to Bioinformatics Dr. Robert Moss Bioinformatics is about searching biological databases, comparing sequences, looking at protein structures, and more generally, asking biological questions
Introduction to Bioinformatics Dr. Robert Moss Bioinformatics is about searching biological databases, comparing sequences, looking at protein structures, and more generally, asking biological questions
BIOSTAT516 Statistical Methods in Genetic Epidemiology Autumn 2005 Handout1, prepared by Kathleen Kerr and Stephanie Monks
 Rationale of Genetic Studies Some goals of genetic studies include: to identify the genetic causes of phenotypic variation develop genetic tests o benefits to individuals and to society are still uncertain
Rationale of Genetic Studies Some goals of genetic studies include: to identify the genetic causes of phenotypic variation develop genetic tests o benefits to individuals and to society are still uncertain
Sequencing of DNA lesions facilitated by site-specific excision via base. excision repair DNA glycosylases yielding ligatable gaps
 Supporting information Sequencing of DNA lesions facilitated by site-specific excision via base excision repair DNA glycosylases yielding ligatable gaps Jan Riedl, Aaron M. Fleming, and Cynthia J. Burrows*
Supporting information Sequencing of DNA lesions facilitated by site-specific excision via base excision repair DNA glycosylases yielding ligatable gaps Jan Riedl, Aaron M. Fleming, and Cynthia J. Burrows*
Cloning and Expression of a Haloacid Dehalogenase Enzyme. By: Skyler Van Senior Research Advisor: Dr. Anne Roberts
 Cloning and Expression of a Haloacid Dehalogenase Enzyme By: Skyler Van Senior Research Advisor: Dr. Anne Roberts utline The gene being cloned is JHP1130 from Helicobacter pylori (H. pylori) JHP1130 is
Cloning and Expression of a Haloacid Dehalogenase Enzyme By: Skyler Van Senior Research Advisor: Dr. Anne Roberts utline The gene being cloned is JHP1130 from Helicobacter pylori (H. pylori) JHP1130 is
PCR-based Markers and Cut Flower Longevity in Carnation
 PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
11th Meeting of the Science Working Group. Lima, Peru, October 2012 SWG-11-JM-11
 11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
