Macroarray Detection of Plant RNA Viruses Using Randomly Primed and Amplified Complementary DNAs from Infected Plants
|
|
|
- Lydia Blankenship
- 6 years ago
- Views:
Transcription
1 Virology Macroarray Detection of Plant RNA Viruses Using Randomly Primed and Amplified Complementary DNAs from Infected Plants Bright Agindotan and Keith L. Perry Department of Plant Pathology, 334 Plant Science Building, Cornell University, Ithaca, NY Accepted for publication 4 August ABSTRACT Agindotan, B., and Perry, K. L Macroarray detection of plant RNA viruses using randomly primed and amplified complementary DNAs from infected plants. Phytopathology 97: Membrane-based macroarrays provide a relatively inexpensive technology with the potential to detect hundreds of pathogens in a single assay. For the simultaneous detection of a large number of pathogens, it is necessary to obtain sufficient nucleic acids for labeling, and any amplification reactions need to be performed using unbiased, pathogen-nonspecific primers. A nonradioactive macroarray system is described to test for plant RNA viruses using 70-mer oligonucleotide probes immobilized on nylon membranes. Starting with a total plant RNA extract, complementary DNA (cdna) and second-strand syntheses were carried out using an anchor primer sequence with random pentamers coupled at the 3 end. Subsequent synthesis by polymerase chain reaction using the anchor primer alone resulted in a relatively unbiased amplification of plant and viral RNAs. These cdnas were chemically labeled and the product used as a target in hybridization analyses. The system was validated using RNA extracts from plants infected with Cucumber mosaic virus, Potato virus Y, and Potato leaf roll virus (PLRV). Despite the relative excess of host-derived nonviral sequences, viral RNAs were amplified between 100- and 1,000-fold and were detected in single and mixed infections. The macroarray sensitivity was comparable to that of double-antibody sandwich enzyme-linked immunosorbent assay, with PLRV being detected in sap dilutions of 1:100. The potential for the development of a relatively inexpensive multipathogen detection system is discussed. Additional keywords: anchor primers. The most common methods of plant virus detection are variations on the enzyme-linked immunosorbent assay (ELISA) and the polymerase chain reaction (PCR) (30,52). As a serological technique, ELISA with polyclonal antibodies has the advantage of being robust and detecting most strains of a given virus. The major drawback is that antisera for the detection of each virus of interest must be both available and affordable. PCR-based methods have the advantage of being both extraordinarily sensitive and requiring reagents that are easily accessible and relatively inexpensive. The downside of PCR-based methods is that, in order to be reliable, the 3 ends of the oligonucleotide primers employed must be conserved among all strains of the virus. In most cases, with both types of methods, tests are performed for each pathogen individually. This may not be limiting for regional crop certification programs, where tests screen for only a small number of pathogens (e.g., with potato in North America) (45). In such cases, a collection of specific antisera would allow for ELISA testing, or a few different multiplex PCR assays might suffice. The problem becomes considerably more challenging when monitoring the international movement of seed or vegetative planting stock (e.g., grapevine, citrus, strawberry, or potato). In these cases, all known pathogens must be considered and the amassing of reagents becomes a limiting factor, because of cost and logistics. The most promising technology for the development of multipathogen detection systems has been the advent of microarrays. Thousands of diagnostic nucleic acid probes can be spotted onto a platform (glass slide) and hybridized with labeled targets (i.e., nucleic acids extracted from specimens and chemically modified Corresponding author: K. L. Perry; address: KLP3@cornell.edu DOI: /PHYTO The American Phytopathological Society with a fluorescent or other reporter) (6). A number of microarrays were developed for the detection and typing of animal viruses (9,21 23,42). One of the first and most powerful of these arrays contained 70-mer oligonucleotide probes for the detection of 140 viruses and was employed to detect multiple viruses in human respiratory specimens without the use of sequence-specific primers (49). More recent versions of this pan-viral array include oligonucleotides specific to more than 1,000 viruses, including those from plants (50). Microarrays for the detection of plant viruses have been much more limited in scope and have employed relatively small numbers of probes (1,5,7,8,24). Perhaps greater potential has been realized in the detection of other plant pathogens using membrane-based, reverse dot-blot hybridization assays (20). These assays employed synthetic oligonucleotides and, when spotted at high densities as macroarrays, they approach the power of microarrays. This potential is well illustrated in the work of Levesque and colleagues in the detection of fungal, bacterial, and oomycete plant pathogens (15,25,27). The identification of these pathogens has depended largely on the primer-specific amplification of ribosomal DNA sequences, an approach not applicable to the detection of viral pathogens. For viruses, multiplex PCR has been used to amplify several viral nucleic acids prior to hybridization (26, 28). The constraints to this process are the need to design primers for each virus and the optimization process involved in multiplex PCR. This method is not suitable for the detection of unknown virus nucleic acid sequences. Group- and genera-specific primers also have been used for the amplification of viral sequences (10, 13,16,17,37,38,41); however, there are many groups of viruses for which no effective generic primers are available. Thus, there is a significant need for the application of amplification methods that are relatively sequence independent. This report demonstrates the use of sequence-nonspecific amplified complementary DNAs (cdnas) as substrates for labeling and Vol. 97, No. 1,
2 the detection of plant RNA viruses. Of greater significance is that these approaches can be combined to create multipathogen detection systems of considerably broader application. MATERIALS AND METHODS Hosts, viruses, and RNA extraction. Potato leafroll virus (PLRV) and Potato virus Y (PVY) reference isolates were maintained in potato (Solanum tuberosum) tissue culture plantlets in vitro, as described (46). The PLRV isolates from the collection at Cornell University were PLRV-Sup (in cv. Superior), PLRV-Ken (in cv. Kennebec), PLRV-WI, and PLRV#4. The PVY isolates PVY-Mex and PVY-Oz were maintained in tissue culture in the potato cv. Russet Burbank and in the greenhouse on tobacco (Nicotiana tabacum cv. Burley), respectively. The potato cvs. Cinnabar, Buffalo, and Altura were dually infected with PLRV and PVY and maintained as tissue culture plantlets. Cucumber mosaic virus (CMV) isolates CMV-Mal1 (31) and CMV-Fny (3) were propagated in N. clevelandii. Total RNA was extracted from 200 mg of leaf tissue using an RNeasy Plant mini kit (Qiagen Inc., Valencia, CA). Total RNA (40 µl) was recovered and its concentration estimated by measuring absorbance at 260 nm (39). Reverse transcription and cdna amplification. Total extracted plant RNAs (host and viral) were amplified using a reverse transcription (RT)-PCR procedure based on the strategy of Bohlander et al. (4) for the nonspecific amplification of DNA of unknown sequence (Fig. 1). The RT reactions were prepared with 4 µg of total RNA, 2 µm random anchored primer (either 5 - TGGTAGCTCTTGATCANNNNN-3 or 5 -TGGTAGCTCTTG- ATCANNNNNNNNN-3 ) or virus-specific primer, 2 mm dntp mix, and nuclease-free water in a volume of 10 µl. The mixture was heated for 5 min at 65 C and chilled on ice for 2 min. To the chilled mixture was added 4 µl of 5 first-strand buffer (Invitrogen Corp., Carlsbad, CA), 2 µl of 0.1 M dithiothreitol, 2 µl of RNaseOut ribonuclease inhibitor (Invitrogen) at 40 U/µl, 1.0 µl of M-MLV reverse transcriptase (Invitrogen) at 200 U/µl, and water to a final volume of 20 µl. The mix was incubated at 25 C for 5 min, 35 C for 5 min, and 37 C for 1 h. For real-time or conventional RT-PCR (described below), reverse transcription with the same amount of the total RNA was done with 2 µm Fig. 1. Schematic outline of the strategy used in the macroarray detection of viral sequences. For clarity, the second-strand complementary DNA (cdna) synthesis is shown as a separate step; in the standard protocol, the anchor primer is added immediately after first-strand cdna synthesis and the second strand that will be amplified is synthesized in the first of 35 polymerase chain reaction (PCR) cycles. The outline is abbreviated, with blocking and washing steps not depicted. virus-specific primers such as P392 or P403, but the reaction was carried out at 42 C for 1 h. In the case of the random-primed reactions, the second strand synthesis was mediated by the reverse transcriptase (48) and was carried out by denaturing the cdna for 5 min at 65 C, cooling on ice, doubling the total volume with an addition of all new RT reagents, and repeating the RT reaction conditions with the ramped temperature regime, as described above. Alternatively, the original RT reaction products were used directly in a PCR reaction and the second-strand cdna was synthesized in the first cycle of the PCR, as described below. PCR reactions consisted of 2.5 µl of reverse transcription product, 25 µl of 2 iqsupermix (Bio-Rad Laboratories, Hercules, CA), 20 pmol of anchor primer (5 -AGAGTTGGTAGCTCTTGATC-3 ) for anchor-primed amplification, and water to a total volume of 50 µl. For conventional and real-time RT-PCR, 20 pmol each of the virus-specific reverse and forward primers were added to the PCR mix instead of anchor primer. Additionally, for the real-time PCR, 10 pmol of TaqMan probe (Applied Biosystems, Foster City, CA) also was included in the PCR mix. Anchor-primed amplification was done in a thermocycler (Mycycler; Bio-Rad) with an initial 5-min denaturation step at 94 C; followed by 35 cycles of 94 C for 30 s, 40 C for 30 s, 50 C for 1 min, and 72 C for 2 min; and a final extension step at 72 C for 7 min. For virus-specific amplification, the PCR profile was an initial 5-min denaturation step at 94 C; followed by 35 cycles of 94 C for 30 s, 52 C for 30 s, and 72 C for 1 min; and a final extension step at 72 C for 7 min. For the real-time PCR, the PCR profile was an initial 5 min denaturation step at 94 C, followed by 35 cycles of 94 C for 30 s, 52 C for 30 s, and 72 C for 30 s. Agarose gel electrophoresis, purification, and quantification of PCR products. Unpurified PCR products (5 µl each) and a comparable amount of the reverse transcription products used as input for the PCR reactions were analyzed on a 1.2% agarose gel in 0.5 Tris-acetate EDTA (TAE) buffer (0.02 M Tris-acetate, 0.5 mm EDTA, ph 8.0) with ethidium bromide staining. PCR products were purified using a QIAquick PCR purification kit (Qiagen) and the amount of product estimated by absorbance at 260 nm (39). RT-PCR and real-time quantification of PLRV. For conventional RT-PCR, a 725-bp nucleotide sequence from PLRV (nucleotides 3426 to 4129; reference GenBank accession no. AF453394) was amplified using the forward primer P402 (5 - TCAAGCCTCGTTACATCAACCGGA-3 ) and the reverse primer P403 (5 -TAATACGACTCACTATAGGGAAAGAGTATTTCCC- TTCCACAGTATCCGGCA-3 ); P403 contained a T-7 promoter sequence at its 5 end. The amplified cdna contained the target sequences of the real-time primers, and was transcribed using an mmessage mmachine transcription kit and protocol (Ambion, Inc., Austin, TX). The crna was recovered using the lithium chloride precipitation option in the manufacturer s protocol. The quantity and the copy number of the crna was estimated as described by Olmos et al. (32). The quantity of crna (µg) obtained was quantified by UV absorption at 260 nm (39). The number of bases (N b ) in the crna transcript was 725 and, from its estimated molecular weight (assuming 340 Da per ribonucleotide), the quantity of crna per picomole was calculated as follows: pmol of single-stranded RNA = µg (of single-stranded RNA) (10 6 pg/µg) (1 pmol/340 pg) (1/N b ). Using the Avogadro constant ( molecules/mol), the copy number of crna transcripts was calculated per 1 µl, the volume template used in each of the real-time RT-PCR reactions. Ten-fold serial dilutions of the transcripts were prepared starting from an initial copies (crna at 1.86 ng/µl) to copies per reverse transcription reaction to generate a standard curve. The copy number of PLRV in samples was estimated from the quantity of crna in grams as described by Olmos et. al. (32) and Ginocchio et al. (18). Real-time RT-PCR was done with the various crna amounts and a standard curve (crna copy number versus 120 PHYTOPATHOLOGY
3 threshold cycle [Ct]) was generated. The primer pair and Taq Man probe used were forward primer P393 (5 -CAATCGCCGCT- CAAGAAAGAA-3 ), reverse primer P392 (5 -GGTTGTCCTT- TGTAACACGAATG-3 ), and probe P460 (5-6-FAM-TGAGC- CTCGTCCTCGGGGAACTCC-BHQ-3 ). The copy number of PLRV RNA in the total RNA of infected-plant extract was determined using the standard curve. To estimate the PLRV copy number in 4 µg of total RNA, real-time RT-PCR was done with 4 µg, 0.4 µg, and 40 ng of the total RNA, and the Ct values of these dilutions within the standard curve were used for the estimation. Oligonucleotide probes. Probes of 70 nucleotides were designed for the detection of PLRV, CMV, and PVY. Complete genomic sequences of each of the viruses were retrieved from the National Center for Biotechnology Information (NCBI) database (available online from NCBI website) and aligned using Vector NTI Suite 8 (InforMax, Inc., Frederick, MD). Regions of sequence identity conserved among most or all strains of the virus were identified visually and used for the design of 70-mer oligonucleotides. As positive or reference controls, seven 70-mer probes corresponding to conserved host plant ribosomal RNAs (rrnas) were designed, one for the 5.8S rrna, three for the 18S rrna, and three for the 25S RNA. The oligo Analyzer 3.0 tools (Integrated DNA Technologies, Coralville, IA) were used for analysis of G+C content and temperature (T m ), and to predict hairpin formation and self-annealing properties of each oligonucleotide. Those oligonucleotides selected exhibited T m of 70 C, the least tendency to form homo dimers, and the least stable hairpin structures. The virus specificity of each of these oligonucleotides was evaluated using the Basic Local Alignment Search Tool (BLAST) (2). Oligonucleotides were synthesized commercially (Integrated DNA Technologies, Coralville, IA), and the viral- and rrna-specific oligonucleotides are listed in Table 1. Printing of oligonucleotide probes on membranes. Ribosomal and virus-specific DNA oligonucleotide probes were diluted to 10 and 20 µm, respectively, in 1 spotting buffer (4 µm sodium carbonate buffer, ph 8.0; 3 SSC [1 SSC is 0.15 M TABLE 1. Sequences of DNA oligonucleotide probes used in the macroarray Probe a Sequence Nucleotide position rrna-1 ACT CTC GGC AAC GGA TAT CTC GGC TCT CGC ATC GAT GAA GAA CGT AGC GAA ATG CGA TAC TTG GTG TGA A b rrna-2 TCG GGG GCA TTC GTA TTT CAT AGT CAG AGG TGA AAT TCT TGG ATT TAT GAA AGA CGA ACA ACT GCG AAA G c rrna-3 TCT ATG GGT GGT GGT GCA TGG CCG TTC TTA GTT GGT GGA GCG ATT TGT CTG GTT AAT TCC GTT AAC GAA C c rrna-4 TCA GCT CGC GTT GAC TAC GTC CCT GCC CTT TGT ACA CAC CGC CCG TCG CTC CTA CCG ATT GAA TGG TCC G c rrna-5 AGG CTC GAA GCG ATA CTG ACG TGC AAA TCG TTC GTC TGA CTT GGG TAT AGG GGC GAA AGA CTA ATC GAA C d rrna-6 GTG ACG CGC ATG AAT GGA TTA ACG AGA TTC CCA CTG TCC CTG TCT ACT ATC CAG CGA AAC CAC AGC CAA G d rrna-7 GGA CGG TGG TCA TGG AAG TCG AAA TCC GCT AAG GAG TGT GTA ACA ACT CAC CTG CCG AAT CAA CTA GCC C d PVY-1 AAT TAA AAC AAC TCA ATA CAA CAT AAG AAA AAC AAC GCA AAA ACA CTC ATA AAC GCT TAT TCT CAC TCA A 2 70 e PVY-2 GCA TAC GAC ATA GGA GAA ACT GAG ATG CCA ACT GTG ATG AAT GGG CTT ATG GTT TGG TGC ATT GAA AAT G e PVY-3 ATG CCA ACT GTG ATG AAT GGG CTT ATG GTT TGG TGC ATT GAA AAT GGA ACC TCG CCA AAT GTC AAC GGA G e PVY-4 GGC TTA TGG TTT GGT GCA TTG AAA ATG GAA CCT CGC CAA ATG TCA ACG GAG TTT GGG TTA TGA TGG ATG G e PVY-5 TGA AAC CAA TCG TTG AGA ATG CAA AAC CAA CCC TTA GGC AAA TCA TGG CAC ATT TCT CAG ATG TTG CAG A e PVY-6 ACC CTT AGG CAA ATC ATG GCA CAT TTC TCA GAT GTT GCA GAA GCG TAT ATA GAA ATG CGC AAC AAA AAG G e PVY-7 CTC AGA TGT TGC AGA AGC GTA TAT AGA AAT GCG CAA CAA AAA GGA ACC ATA TAT GCC ACG ATA TGG TTT A e PVY-8 CTC GAC TTT TCG GGT TGG ACG GTG GCA TCA GTA CAC AAG AGG AGA ACA CAG AGA GGC ACA CCA CCG AGG A e PVY-9 CAC AGA GAG GCA CAC CAC CGA GGA TGT CTC TCC AAG TAT GCA TAC TCT ACT TGG AGT CAA GAA CAT GTG A e PVY-10 GTC TCT CCG GAC GAT ATA TAA ATA TTT ACA TAT GCA GTA AGT ATT TTG GCT TTT CCT GTA CTA CTT TTA T e PVY-11 TGC AGT AAG TAT TTT GGC TTT TCC TGT ACT ACT TTT ATC ATA ATT AAT AAT CAG TTT GAA TAT TAC TAA T e PVY-12 TCT GGA TCT ATC TGC TTG GGT GAT GTT GTG ATT TTG TCA TAA CAG TGA CTG TAA ACT TCA ATC AGG AGA C e CMV-1 AAT TCC TAT GGC GAC GTC CTC GTT CAA CAT CAA TGA ATT GGT AGC CTC CCA CGG CGA TAA AGG ACT ACT C f CMV-2 CAG CAG GAG TTT GCT CCC CAC GGC CTA GCT GGT GCC CTC CGC TTG TGT GAA ACT CTC GAT TGT CTA GAC T f CMV-3 TAG ACT CTT TCC CTT CAT CAG GTC TGC GGC AGG ACC TCG TCT TAG ACT TCG GAG GAA GTT GGG TCA CAC A f CMV-4 TTC AAC ATG GAA GCT AAG GTG ATG GAA CCT GCC GTA CCA TAT ATT TGT TCG AAG TTC TTA CTC TCT GAC G g CMV-5 ACT GTC TGA AGT CAC TAA ACA CAT TGT GGT GAA CGG GTT GTC CAT CCA GCT TAC GGC TAA AAT GGT CAG T g CMV-6 ATG GCT TTC CAA GGT ACC AGT AGG ACT TTA ACT CAA CAG TCC TCA GCG GCT ACG TCT GAC GAT CTT CAA A h CMV-7 GAT TCA GTC ACG GAA TAT GAT AAG AAG CTT GTT TCG CGC ATT CAA ATT CGA GTT AAT CCT TTG CCG AAA T h CMV-8 GAT AAG AAG CTT GTT TCG CGC ATT CAA ATT CGA GTT AAT CCT TTG CCG AAA TTT GAT TCT ACC GTG TGG G h CMV-9 GTT GTT GCG CGG GGA ACG GGT TGT CCA TCC AGC TTA CGG CTA AAA TGG TCA GTC GTG TCT TTC ACA CGC C h CMV-10 TTT TTA CGG TGA ACG GGT TGT CCA TCC AGC TTA CGG CTA AAA TGG TCA GTC GTG GAG AAA TCC ACG CCA G h CMV-11 ATG GCT ACT GAG TGT GAC CTA GGC CGG CAT CAT TGG ATG CGC GCT GAT AAT GCT ATT TCA GTC CGG CCC C h CMV-12 CGC GCA TTC AAA TTC GAG TTA ATC CTT TGC CGA AAT TTG ATT CTA CCG TGT GGG TGA CAG TCC GTA AAG T h PLRV-1 TGG ACT TTC AGC ATA GAA GCC TTA TGC TTA ATT TTG CTC GGT TGT ATA ACC AGC TTG ATC TAC AAG GGC i PLRV-2 AGG ATT CGG CTG CCA AAA GCC GGA TCT GAA GCT GAA CTC CAA AGC CTG AAT CTA CAG GCT GCC AGG TGGC i PLRV-3 GCT TTC AAC AAG ATA TCC GTG AAG CAG TCC AGT CCC TTG AGC TAG ACG CTG GTG TAG GCA TTC CCT ATA T i PLRV-4 ATG TCT GTT TCC TTG GTG GAT CAA CTG GTA GCC CGG GTT CTG TTC CAA AAT CAG AAC AAA AGG GAA ATT T i PLRV-5 TCG TGA GCT CGT TGC CAA GCT CCA CCA GTG GTT GGT TCC GAG TGC CAC CAC AAA AGA ACA CTG AAG GAG C i PLRV-6 CGC CGC TCA AGA AGA ACT GGA GTT CCC CGA GGA CGA GGC TCA AGC GAG ACA TTC GTG TTT ACA AAG GAC A i PLRV-7 CCC AAG GAA GTT TCA CCT TCG GGC CGA GTC TAT CAG ACT GTC CGG CAT TCA AGG ATG GAA TAC TCA AGG C i PLRV-8 TAC TCA AGG CCT ACC ATG AGT ATA AGA TCA CAA GCA TCT TAC TTC AGT TCG TCA GCG AGG CCT CTT CCA C i PLRV-9 GAC CCT GGT TAT TCC AAA GAA GAT GTC GCT GCT GCA ACT ATT ATA GCG CAC GGC AGT ATT CAA GAT GGG C i PLRV-10 CCG GAC ATT AAG CGG AAC GAA AGC CGA AAG GTG ATT AGG CTC TCA ACG CCT GCT AGA GAC CGT CGA AAG A i PLRV-11 CTC GAT GAA GGC CGC TAC CTC CTC ATC ATG TCT GTT TCC TTG GTG GAT CAA CTG GTA GCC CGG GTT CTG T i PLRV-12 CCT CGC CGT TCC GGT CAA TAC CAA CAA AAT GCT TTA CAA GTT GAT CCA TGG TTA TAA TCC GGA ATG TGG C i a rrna = ribosomal RNA, PVY = Potato virus Y, CMV = Cucumber mosaic virus, and PLRV = Potato leaf roll virus. b GenBank accession number of 5.8S rrna used to designate probe nucleotide position is U c GenBank accession number of 18S rrna used to designate probe nucleotide position is U d GenBank accession number of 25S rrna used to designate probe nucleotide position is M e GenBank accession number of the PVY reference genome used to designate probe nucleotide position is U f GenBank accession number of the CMV RNA 1 reference used to designate probe nucleotide position is NC_ g GenBank accession number of the CMV RNA 2 reference genome used to designate probe nucleotide position is NC_ h GenBank accession number of the CMV RNA 3 reference genome used to designate probe nucleotide position is D i GenBank accession number of the PLRV reference genome used to designate probe nucleotide position is AY Vol. 97, No. 1,
4 NaCl and 0.15 M sodium citrate]; 50% [vol/vol] dimethy sulfoxide [DMSO]; 0.01% N-lauroylsarcosine; and 1 mg of bromophenol blue). The probes were transferred in 30-µl aliquots into the wells of a polypropylene 384-well microtiter plate (Nalge Nunc International, Rochester, NY). A pin replicator (VP 110; V&P Scientific, Inc., San Diego, CA) for 384-well plates was cleansed as recommended by its manufacturer prior to printing. Hybond-N Plus membranes (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) were cut to the size (12 by 10 mm) of a multiprint device (VP 381; V&P Scientific) and the copier unit (VP 382; V&P Scientific) aligned over the 384-well plate. The oligonucleotides were printed on a membrane on the same spots twice. The pin replicator delivered 0.2 µl per print. Five minutes was allowed for air drying the membrane before the second printing. The printed oligonucleotides were fixed onto the membrane by UV-cross-linking for 2 min at 120 mj/s in a Stratalinker (Strategene Corp., La Jolla, CA). Labeling, hybridization, and detection. The chemical labeling of the purified PCR product was done using an AlkPhos Direct labeling kit (GE Healthcare Bio-Sciences Corp.), following the manufacturer s protocol, except that 200 ng of DNA was labeled with 1 µl of the alkaline phosphatase reagent. Prehybridization and hybridization were done at 55 C using the hybridization protocol provided with the Alkphos Direct kit. Prehybridization and hybridization were done in 50-ml polypropylene tubes (Corning, Inc., Corning, NY). Prior to adding the prehybridization buffer, membranes first were wetted by incubating in 0.5% sodium dodecyl sulfate (SDS) (wt/vol) at 55 C for 1 h followed by a rinse in 0.1 M Tris-HCl, ph 8.4, for 5 min. For every 20 to 25 cm 2, 10 ml of hybridization buffer was used for blocking at 55 C for at least 1 h. After this prehybridization, only 5 ml of the buffer was used for hybridization with 200-ng labeled target DNA. Hybridization was done at 55 C overnight. Membranes were washed two times in a primary wash buffer (2 M urea, 0.1% SDS, 50 mm sodium phosphate, 150 mm NaCl, and 0.2% [wt/vol] blocking agent [supplied with the Alkphos Direct kit]) at 55 C for 15 min each, followed by a washing in a secondary wash buffer (50 mm Tris, 100 mm NaCl, ph 10.0) for 10 min, at room temperature on a shaker. The membranes were incubated with Fig. 2. Representative polymerase chain reaction (PCR) amplification products, using as templates first- and second-strand complementary DNAs (cdnas) synthesized with primers consisting of random pentamers linked to a 5 end anchor sequence. M = DNA ladder of molecular size markers. Lanes 1 to 8: cdnas amplified from eight independent RNA preparations. Lanes 9 to 12: Four reverse-transcriptase (RT) reaction products, which were used as substrates for the PCR reactions depicted in lanes 1 to 4, respectively. The volume of the RT reaction product loaded in each lane of the gel was equivalent to the amount of input cdna added in the lanes for the corresponding PCR products. The RNAs employed were isolated from infected Solanum tuberosum (potato; lanes 1, 2, 5, 6, 7, 8, 9, and 10), Nicotiana clevelandii (lanes 3 and 11), and N. tabacum (tobacco; lanes 4 and 12). CDP-star chemiluminescent (GE Healthcare Bio-Sciences Corp.) reagent for 5 min, drained, and exposed to Chemiluminescence Bio Max film (Kodak Co., Rochester, NY) for 1 h to overnight. Comparing the sensitivity of the macroarray, double-antibody sandwich ELISA, PCR, and real-time PCR. For comparison of double-antibody sandwich (DAS)-ELISA with the PCRbased techniques, uninfected or PLRV-infected potato leaf tissue was sliced into 1-mm-wide pieces and divided into two equal portions of 200 mg each. One half of the sample was extracted for total RNA as described above, while the other half sample was processed for serological analysis by grinding in 2 ml extraction/ conjugate buffer (phosphate buffered saline [39] containing 2% polyvinylpyrrolidone, molecular weight 40,000, and 0.1% ovalbumin). For DAS-ELISA (12), the original PLRV-WI leaf extract was designated 1 and diluted serially into 1 healthy leaf tissue extract to yield dilutions of 1/5, 1/25, 1/125, 1/625 and 1/3,125 or, alternatively, 10 1 through These extracts were tested for the presence of PLRV by using commercial antibodies at recommended dilutions (Agdia, Inc., Elkhart, IN). For RNA analysis to determine the dilution end-point of detection, the RNA concentration was estimated and 4 µg of total RNA extract ( 2 µl) from the infected plant was diluted into an uninfected plant RNA extract to obtain a 10-fold dilution series. For macroarray analyses, 2 µl of each of the total RNA dilutions were amplified by random-primed RT-PCR, as described above, and these products then were labeled and hybridized to membranes. In parallel, conventional RT-PCR and real-time PCR were performed using equivalent volumes of the diluted RNAs. For the purposes of comparing sensitivities, assuming 80 µg of total RNA extracted from 200 mg of infected tissue, the 4 µg of undiluted RNA used for the PCR assays would correspond to a starting 10 mg of leaf tissue. Similarly, for the DAS-ELISA, one well of the microtiter plate with 100 µl of the undiluted ground extract (200 mg of leaf tissues in 2 ml of buffer) would correspond to 10 mg of tissue. RESULTS Amplification of total RNA from virus-infected plants. Total RNAs from virus-infected plants were amplified in a relatively unbiased fashion, as shown schematically in Figure 1. cdna synthesis was primed using random pentamers (or nonamers) linked to the 3 end of an anchor primer of a specific sequence. The same primer was used to prime second-strand cdna synthesis, giving rise to products bound at either end harboring the anchor primer sequence. The cdnas then were added to a reaction mixture with the anchor primer for PCR amplification. The products of three independent amplification experiments initially primed with random pentamers are shown in Figure 2. cdnas from eight independent preparations of total RNAs from leaf tissues of potato, N. clevelandii, and N. tabacum are represented. The amplification products were visualized as a smear of products co-migrating with molecular size markers from 300 to 500 bp up to the well of the gel; faint specific bands also were observed against the background smear. Similar results were obtained if the second-strand cdna was synthesized in a separate reaction with the random primers alone prior to PCR amplification. Comparable results also were obtained using random nonamers instead of pentamers. The faint bands are presumed to represent host sequences recognized and amplified by the anchor primers, but the same bands were not always observed in every reaction. Thus, the total plant RNAs appear to be amplified in a relatively unbiased fashion. The design of virus- and host-specific probes. Twelve oligonucleotides specific to each of the three viruses CMV, PVY, and PLRV were synthesized for spotting onto nylon membranes. Each of the oligonucleotides corresponded and were referenced to the genomic sequences of CMV-Fny (33,35,36), PVY-O (44), or an un-named isolate of PLRV (Table 1). The oligonucleotides 122 PHYTOPATHOLOGY
5 selected were those with >95% sequence identity to the target of interest and with <14% contiguous and 21% noncontiguous sequence identity to unrelated sequences. The oligonucleotides represented different regions of each virus genome, and preference was given to those with G+C content of between 40 and 60%. Based on sequence alignments and BLAST analyses, the oligonucleotides were expected to hybridize to all isolates of the respective virus, but not to host plant-derived nucleic acids. Oligonucleotides specific to plant rrna were synthesized for inclusion on the array in order to serve as internal controls to monitor the relative intensity of hybridization signals from experiment to experiment. Seven rrna-specific 70 mers were designed to hybridize with conserved 5S, 18S, and 25S rrna sequences present in monocotyledonous and dicotyledonous plants and were spotted along the first row and first column of the nylon membrane (Fig. 3A). Labeling and hybridization of target DNA to oligonucleotide probes on the macroarray. The amplified cdnas from virus-infected and uninfected control plants were chemically labeled by conjugation with a heat-stable alkaline phosphatase. Target DNA hybridization to oligonucleotide probes spotted onto nylon membranes was visualized by incubation with a chemiluminescent substrate and exposure to film. The placement of the rrna-specific 70 mers along the top and sides of the membrane facilitated identifying the rows and columns of spotted oligonucleotides and provided a reference for the relative strength of the hybridization signals (Fig. 3B to D). Each spot contained an estimated 8 pmol of viral-specific oligonucleotide or 4 pmol of rrna-specific oligonucleotide. All of the rrna-specific oligonucleotides hybridized to the labeled target, although the probes rrna-1 and rrna-5 yielded weaker hybridization signals and were not visualized in some experiments. The remaining five rdna probes were clearly visualized in all experiments. All three viruses were easily detected in the macroarray, with strong signals observed relative to the background on the membranes (Fig. 3B to D). The labeled target DNA hybridizations were specific to the infecting virus, with no observed cross hybridization to probes for heterologous viruses. Similarly, labeled Fig. 3. Macroarray detection of Cucumber mosaic virus (CMV), Potato virus Y (PVY), and Potato leafroll virus (PLRV) in single and double infections. A, Diagram of the spotting pattern of 70-mer DNA oligonucleotide probes. In each box (e.g., 1a, 1b, 1c, and so on) there are two identical, diagonally spotted oligonucleotides. Ribosomal RNA-specific oligonucleotide reference controls were coded R1, R2, R3, and so on. PLRV-, CMV-, and PVY-specific oligonucleotides were similarly coded L1 to L12, C1 to C12 and P1 to P12, respectively. B to E, Films exposed to hybridized macroarrays for 1 to 3 h, showing the hybridization of polymerase chain reaction-amplified and labeled complementary DNA targets. The RNAs used to prepare the amplified targets were derived from B, Solanum tuberosum infected with PLRV strain WI; C, Nicotiana clevelandii infected with CMV strain Mal-1; D, N. tabacum infected with PVY strain Oz; and E, S. tuberosum doubly infected with PLRV and PVY. Vol. 97, No. 1,
6 cdnas from uninfected plants only hybridized with the reference rdna probes (data not shown). For the detection of PLRV in potato, all of the virus-specific oligonucleotides hybridized with target cdna except for probe PLRV-10 (Fig. 3B). This was true for experiments with target cdna obtained from plants singly infected with four different isolates of PLRV and from plants doubly infected with both PLRV and PVY (Fig. 3E; Table 2). Two strains of CMV representing different genetic subgroups were tested on the array (Fig. 3C; Table 2). Labeled cdnas from N. clevelandii infected with CMV-Mal1 hybridized to all the virus-specific oligonucleotides except probe CMV-1; additionally, hybridization to probe CMV-6 was very weak. In contrast, labeled cdnas from N. clevelandii infected with the genetically distinct CMV-Fny hybridized to all the probes, but the signals from hybridization with probes CMV-9 and CMV-10 were relatively weak (data not shown). Labeled cdnas from N. tabacum and S. tuberosum infected with strains of PVY singly or as mixed infections hybridized to all the virus-specific oligonucleotides except probe PVY-1 (Fig. 3D and E; Table 2). In all cases, probes PVY-9, -10, -11, and -12 yielded signals that were weaker than the others, but clearly distinguishable as positive. In total, PLRV was detected in seven of seven plants tested, CMV in two of two plants, and PVY in six of six plants. Sensitivity of macroarray detection compared with DAS- ELISA, RT-PCR, and real-time RT-PCR. The relative sensitivity of the macroarray was compared with that of three commonly used assays for plant RNA viruses: DAS-ELISA, conventional RT-PCR, and real-time RT-PCR (Fig. 4). In testing for PLRV in S. tuberosum, a 10-fold dilution series was prepared of an infected plant RNA (or sap) extract in an uninfected plant RNA (or sap) extract. The end point of detection in the macroarray was 10 2 (Fig. 4B), comparable with results obtained with the DAS-ELISA (Fig. 4C). Conventional RT-PCR was three orders of magnitude more sensitive, with an end point of detection of 10 2 (Fig. 4A). These levels of detection were observed in two independent experiments using plants infected by either isolate PLRV#4 or PLRV-WI. In real-time RT-PCR analyses of the infected plant RNA extracts, the endpoint of detection was 10 7 in two independent experiments, a further improvement of two orders of magnitude. In order to estimate the viral copy number being detected in these assays, a 10-fold dilution series of a PLRV RNA transcript was employed in real-time RT-PCR experiments and the data were used to prepare a standard curve. These quantitative PCR measurements showed that the minimum copy number of PLRV template detected was for real-time RT-PCR and for the conventional RT-PCR. Furthermore, in the macroarray experiments, PLRV was detected only if there were at least copies of the viral RNA per 4 µg of total RNA (data not shown). The quantitative PCR measurements also were employed to estimate the copy number of viral RNA in sample preparations before and after the standard amplification procedure for the macroarray. In the starting aliquot of 4 µg of total RNA, there were an estimated copies of viral RNA. Following amplification, the copy number increased 100 to 1,000-fold to between and Fig. 4. Comparison of the sensitivity of detection of Potato leafroll virus (PLRV) isolate 4 by reverse-transcriptase polymerase chain reaction (RT- PCR), macroarray, and double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA). A, Agarose gel electrophoresis and ethidium bromide stained RT-PCR products. Lanes 1 to 8 show the products from reactions performed using a dilution series of the RNA template, from 1 (undiluted) to 10 7, respectively. B, Portion of the macroarray showing hybridization to spots of 70-mer oligonucleotides L6 and L7. Columns 1 to 5 represent hybridizations performed using labeled targets prepared using an undiluted virus-infected plant RNA extract or dilutions of 10 1 to 10 4, respectively. C, Histogram showing results from a DAS-ELISA with values for absorption at 405 nm. Bar 1 is the undiluted plant extract, and bars 2 to 6 represent dilutions of the same extract of 10 1 to 10 5, respectively. The horizontal line at an absorbance of 0.07 indicates the threshold level of detection (i.e., three standard deviations above the mean value for uninfected controls). TABLE 2. Macroarray and double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) detection of three plant viruses 124 PHYTOPATHOLOGY Diagnostic assay a PLRV CMV PVY Virus isolate Plant source ELISA Macroarray ELISA Macroarray ELISA Macroarray PLRV-WI Solanum tuberosum + + PLRV#4 S. tuberosum + + PLRV-Sup S. tuberosum + + PLRV-Ken S. tuberosum + + CMV-Mal1 Nicotiana clevelandii + + CMV-Fny N. clevelandii + + PVY-Oz N. tabacum + + PVY-Mex S. tuberosum + + PLRV+PVY-Altura S. tuberosum PLRV+PVY-Buffalo S. tuberosum PLRV+PVY-Cinnabar S. tuberosum a Assays are for the three plant viruses Potato leafroll virus (PLRV), Cucumber mosaic virus (CMV), and Potato virus Y (PVY); + or indicate detection or no detection, respectively, in the specified assay.
7 DAS-ELISA was performed on the same samples in parallel with the macroarray experiments. Results between the two techniques were entirely consistent (Table 2). In the serological assays, the optical density at 405 nm values for infected plant extracts ranged between 3.5 and 11 times the mean value for uninfected leaf sap. DISCUSSION The most significant aspect of the macroarray methodology described in this report is the potential for its use in larger arrays for the detection of all known plant viruses of a given crop group or as part of a multipathogen detection system that also can detect the presence of co-infecting bacteria, fungi, or oomycete pathogens. Although microarrays are unparalleled in their potential for the detection of huge numbers of viruses (49,50) and other pathogens (53), their deployment is limited by the high costs associated with spotting and detection instrumentation, reagents, and dust-free facilities. Membrane-based macroarrays offer a significant advantage with regard to a number of constraints: no specialized instrumentation or facilities are required, materials and reagents are relatively inexpensive, probes can be easily spotted onto small pilot arrays and evaluated for effectiveness, and membranes can be reused multiple times. In these studies, membranes have been used for up to six rounds of hybridization and stripping without any apparent loss in sensitivity. DNA sequences also can be recovered from the membranes, reamplified, and sequenced (11). A remarkable example of such a recovery approach was seen with the use of a microarray in one of the original identifications of the causal agent in an outbreak of severe acute respiratory syndrome (SARS) (50). The methods described here demonstrate a viable strategy for nucleic acid amplification and hybridization that should be effective in detecting virtually any plant RNA virus for which representative sequence information is available. We have adapted a sequence-independent amplification method originally described by Bohlander et al. (4) for the amplification of CMV, PLRV, and PVY from total RNA of infected leaves. CMV, PVY, and PLRV represent plant viruses of relatively high, medium, and low titers, and their amplification from total RNA illustrates the utility of this method to amplify plant viruses present at varying concentrations and in the presence of excess host plant RNA. Particularly encouraging is the detection of a member of the family Luteoviridae (PLRV); as phloem-restricted agents, these collectively are a benchmark for low-titer viruses (51). Because the emphasis of this report is on a description of the methodologies, a broader testing of many viral strains was not undertaken. Even with the limited number of probes (12 oligonucleotides per virus), differences were seen with the hybridization patterns among the PLRV, with each of the four single isolates yielding a unique hybridization pattern (data not shown). Thus, the technique has potential as a means of easily fingerprinting isolates of a virus, although mixed infections will confound interpretation. The amplification reactions were not completely unbiased, because some specific products were amplified preferentially and visualized in stained agarose gels (Fig. 2). These products were not analyzed; however, presumably, they arose from host sequences to which the anchor primer specifically hybridized during PCR. BLAST analysis of plant sequences using the 15 or 13 nucleotides at the 3 end of the anchor primer resulted in multiple hits, but not with any obvious conserved or repetitive sequences; this is consistent with the absence of any specific band that was reproducibly observed. These minor biased products did not interfere with the amplification of viral sequences and, in experiments where copy numbers were quantified, a 100- to 1,000-fold amplification of PLRV cdna was observed. Comparable macroarray results were obtained when amplifications were performed using either random pentamer or nonamer sequences at the 3 end of the anchor primer. During the course of >100 experiments, this macroarray has reproducibly provided results in agreement with those obtained using alternative diagnostic methods. Several factors proved to be critical in obtaining consistent results. Wetting the membrane in the 0.5% SDS and 0.1 M Tris-HCl prior to adding the prehybridization buffer eliminated variable signal and higher background. The optimized labeling and hybridization conditions employed yielded a satisfactory balance of signal and background following a 1- or 3-h exposure of the film; the background usually was excessive with overnight exposures. Accurate measurement of the concentration of the purified amplification products was crucial, because underestimation resulted in low detection signals, whereas overestimation resulted in higher background, presumably due to the higher concentrations of DNA in the hybridization buffer. The labeling reactions were performed using only 25% of the manufacturer s recommended amount of labeling reagent, providing a desired balance between cost and sensitivity. Although the majority of the oligonucleotide probes worked as predicted, their effectiveness varied and had to be determined empirically, not on a sequence basis alone. This was exemplified by the rrna-specific probes rrna-1 and rrna-5 that often yielded weak or no signals. Probe rrna-1 was designed to hybridize to 5.8S sequences and its failure may be due to the small size of the 5.8S RNA. The efficiency of random-primed reverse transcription and amplification would be expected to drop off with the size of the nucleic acid and the concomitant decrease in the number of priming positions. This has implications for the detection of pathogens with very small genomes, such as viroids or viral satellites. The probe PLRV-10 had 100% sequence identity with all database sequences for the virus, but failed to hybridize with any of the seven PLRV isolates tested. The probes PVY-9, -10, -11, and -12 consistently gave rise to relatively weak hybridization signals. These latter results might be related to the secondary structure of the viral RNAs and a resulting poor reverse transcription, amplification, or hybridization. The sensitivity of the described macroarray was comparable with that of DAS-ELISA, with conventional RT-PCR and realtime RT-PCR being 10 3 and 10 5 times more sensitive, respectively. These results are consistent with other reports of RT-PCR providing 10 2 to 10 6 times greater sensitivity than DAS-ELISA (29,34,40) and real-time RT-PCR being 10 2 to 10 4 times more sensitive than conventional RT-PCR (19,32). It is to be expected that reports of sensitivity will vary with sources of antisera and the design and efficacy of primers. The sensitivity of the macroarray could be improved upon with an enrichment step for viral cdnas such as subtractive hybridization. Alternatively, fluorescent labeling of the target could increase the sensitivity of the macroarray, although this would introduce a requirement for more costly instrumentation. The emphasis of this report is methodology for the macroarray detection of RNA plant viruses using a nonradioactive label. Arrays have been developed for the detection of a number of plant pathogens. Some of the most detailed and comprehensive studies have been on the detection of oomycetes (25,47). Other arrays were developed for the detection of pathogens of individual crop plants (15,27,43) or, more specifically, the detection of viral pathogens (5,7,8,24). Oligonucleotide hybridization patterns or fingerprints can be used to differentiate strains of plant viruses (e.g., PVY and Potato virus S [8] or CMV [14]). All of these developments set the stage for the development of remarkably powerful and informative array-based multipathogen detection systems. ACKNOWLEDGMENTS This research was supported by U.S. Department of Agriculture NRI program grant to K. L. Perry. We thank C. Smart, Vol. 97, No. 1,
8 N. Zhang, A. Charkowski, and J. Thompson for assistance in the development of the detection system; A. Lesvesque for protocols and advice; and L. Miller for technical support. LITERATURE CITED 1. Abdullahi, I., Koerbler, M., Stachewicz, H., and Winter, S The 18S rdna sequence of Synchytrium endobioticum and its utility in microarrays for the simultaneous detection of fungal and viral pathogens of potato. Appl. Microbiol. Biotechnol. 68: Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J. H., Zhang, Z., Miller, W., and Lipman, D. J Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25: Banik, M. T., and Zitter, T. A Determination of cucumber mosaic virus titer in muskmelon by enzyme-linked immunosorbent assay and correlation with aphid transmission. Plant Dis. 74: Bohlander, S. K., Espinosa, R., Lebeau, M. M., Rowley, J. D., and Diaz, M. O A method for the rapid sequence-independent amplification of microdissected chromosomal material. Genomics 13: Boonham, N., Walsh, K., Smith, P., Madagan, K., Graham, I., and Barker, I Detection of potato viruses using microarray technology: Towards a generic method for plant viral disease diagnosis. J. Virol. Methods 108: Bowtell, D., and Sambrook, J DNA Microarrays: A Molecular Cloning Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 7. Bystricka, D., Lenz, O., Mraz, I., Dedic, P., and Sip, M DNA microarray: Parallel detection of potato viruses. Acta Virol. 47: Bystricka, D., Lenz, O., Mraz, I., Pilterova, L., Kmoch, S., and Sip, M Oligonucleotide-based microarray: A new improvement in microarray detection of plant viruses. J. Virol. Methods 128: Chizhikov, V., Wagner, M., Ivshina, A., Hoshino, Y., Kapikian, A. Z., and Chumakov, K Detection and genotyping of human group A rotaviruses by oligonucleotide microarray hybridization. J. Clin. Microbiol. 40: Choi, S. K., Choi, J. K., Park, W. M., and Ryu, K. H RT-PCR detection and identification of three species of cucumoviruses with a genus-specific single pair of primers. J. Virol. Methods 83: Chong, K. Y., Chen, C. M., and Choo, K. B Post-hybridization recovery of membrane filter-bound DNA for enzymatic DNA amplification. Biotechniques 14: Clark, A. J., and Perry, K. L Transmissibility of field isolates of soybean viruses by Aphis glycines. Plant Dis. 86: Culley, A. I., Lang, A. S., and Suttle, C. A High diversity of unknown picorna-like viruses in the sea. Nature 424: Deyong, Z., Willingmann, P., Heinze, C., Adam, G., Pfunder, M., Frey, B., and Frey, J. E Differentiation of cucumber mosaic virus isolates by hybridization to oligonucleotides in a microarray format. J. Virol. Methods 123: Fessehaie, A., De Boer, S. H., and Levesque, C. A An oligonucleotide array for the identification and differentiation of bacteria pathogenic on potato. Phytopathology 93: Fitzgibbon, J. E., and Sagripanti, J. L Simultaneous identification of orthopoxviruses and alphaviruses by oligonucleotide macroarray with special emphasis on detection of variola and Venezuelan equine encephalitis viruses. J. Virol. Methods 131: Gibbs, A., and Mackenzie, A A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. J. Virol. Methods 63: Ginocchio, C. C., Zhang, F., Malhotra, A., Manji, R., Sillekens, P., Foolen, H., Overdyk, M., and Peeters, M Development, technical performance, and clinical evaluation of a NucliSens basic kit application for detection of enterovirus RNA in cerebrospinal fluid. J. Clin. Microbiol. 43: Harju, V. A., Skelton, A., Clover, G. R. G., Ratti, C., Boonham, N., Henry, C. M., and Mumford, R. A The use of real-time RT-PCR (TaqMan) and post-elisa virus release for the detection of Beet necrotic yellow vein virus types containing RNA 5 and its comparison with conventional RT-PCR. J. Virol. Methods 123: Kawasaki, E., Saiki, R., and Erlich, H Genetic analysis using polymerase chain reaction-amplified DNA and immobilized oligonucleotide probes: Reverse dot-blot typing. Methods Enzymol. 218: Klaassen, C. H. W., Prinsen, C. F. M., de Valk, H. A., Horrevorts, A. M., Jeunink, M. A. F., and Thunnissen, F DNA microarray format for detection and subtyping of human papillomavirus. J. Clin. Microbiol. 42: Kozal, M. J., Shah, N., Shen, N. P., Yang, R., Fucini, R., Merigan, T. C., Richman, D. D., Morris, D., Hubbell, E. R., Chee, M., and Gingeras, T. R Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat. Med. 2: Lapa, S., Mikheev, M., Shchelkunov, S., Mikhailovich, V., Sobolev, A., Blinov, V., Babkin, I., Guskov, A., Sokunova, E., Zasedatelev, A., Sandakhchiev, L., and Mirzabekov, A Species-level identification of orthopoxviruses with an oligonucleotide microchip. J. Clin. Microbiol. 40: Lee, G. P., Min, B. E., Kim, C. S., Choi, S. H., Harn, C. H., Kim, S. U., and Ryu, K. H Plant virus cdna chip hybridization for detection and differentiation of four cucurbit-infecting Tobamoviruses. J. Virol. Methods 110: Levesque, C. A., Harlton, C. E., and de Cock, A. W. A. M Identification of some oomycetes by reverse dot blot hybridization. Phytopathology 88: Li, J., Chen, S., and Evans, D. H Typing and subtyping influenza virus using DNA microarrays and multiplex reverse transcriptase PCR. J. Clin. Microbiol. 39: Lievens, B., Brouwer, M., Vanachter, A., Levesque, C. A., Cammue, B. P. A., and Thomma, B Design and development of a DNA array for rapid detection and identification of multiple tomato vascular wilt pathogens. FEMS Microbiol. Lett. 223: Lin, B. C., Vora, G. J., Thach, D., Walter, E., Metzgar, D., Tibbetts, C., and Stenger, D. A Use of oligonucleotide microarrays for rapid detection and serotyping of acute respiratory disease-associated adenoviruses. J. Clin. Microbiol. 42: Lunello, P., Mansilla, C., Conci, V., and Ponz, F Ultra-sensitive detection of two garlic potyviruses using a real-time fluorescent (Taqman(R)) RT-PCR assay. J. Virol. Methods 118: Martin, R. R., James, D., and Levesque, C. A Impacts of molecular diagnostic technologies on plant disease management. Annu. Rev. Phytopathol. 38: Ng, J., and Perry, K. L Stability of the aphid transmission phenotype in cucumber mosaic virus. Plant Pathol. 48: Olmos, A., Bertolini, E., Gil, M., and Cambra, M Real-time assay for quantitative detection of non-persistently transmitted Plum pox virus RNA targets in single aphids. J. Virol. Methods 128: Owen, J., Shintaku, M., Aeschleman, P., Tahar, S. B., and Palukaitis, P Nucleotide sequence and evolutionary relationships of cucumber mosaic virus (CMV) strains: CMV RNA 3. J. Gen. Virol. 71: Ratti, C., Budge, G., Ward, L., Clover, G., Rubies-Autonell, C., and Henry, C Detection and relative quantitation of Soil-borne cereal mosaic virus (SBCMV) and Polymyxa graminis in winter wheat using real-time PCR (TaqMan). J. Virol. Methods 122: Rizzo, T. M., and Palukaitis, P Nucleotide-sequence and evolutionary relationships of cucumber mosaic virus (CMV) strains: CMV RNA 2. J. Gen. Virol. 69: Rizzo, T. M., and Palukaitis, P Nucleotide-sequence and evolutionary relationships of Cucumber mosaic virus (CMV) strains: CMV RNA 1. J. Gen. Virol. 70: Robertson, N. L., French, R., and Gray, S. M Use of group-specific primers and the polymerase chain-reaction for the detection and identification of luteoviruses. J. Gen. Virol. 72: Saldarelli, P., Rowhani, A., Routh, G., Minafra, A., and Digiaro, M Use of degenerate primers in a RT-PCR assay for the identification and analysis of some filamentous viruses, with special reference to closteroand vitiviruses of the grapevine. Eur. J. Plant Pathol. 104: Sambrook, J., Fritsch, E. F., and Maniatis, T Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 40. Sanchez-Navarro, J. A., Aparicio, F., Rowhani, A., and Pallas, V Comparative analysis of ELISA, nonradioactive molecular hybridization and PCR for the detection of Prunus necrotic ringspot virus in herbaceous and Prunus hosts. Plant Pathol. 47: Sanchez-Seco, M. P., Echevarria, J. M., Hernandez, L., Estevez, D., Navarro-Mari, J. M., and Tenorio, A Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J. Med. Virol. 71: Shih, S. R., Wang, Y. W., Chen, G. W., Chang, L. Y., Lin, T. Y., Tseng, M. C., Chiang, C., Tsao, K. C., Huang, C. G., Shio, M. R., Tai, J. H., Wang, S. H., Kuo, R. L., and Liu, W. T Serotype-specific detection of enterovirus 71 in clinical specimens by DNA microchip array. J. Virol. Methods 111: Sholberg, P., O Gorman, D., Bedford, K., and Levesque, C. A Development of a DNA macroarray for detection and monitoring of economically important apple diseases. Plant Dis. 89: Singh, M., and Singh, R. P Nucleotide sequence and genome organization of a Canadian isolate of the common strain of potato virus Y (PVYO). Can. J. Plant Pathol. 18: Slack, S. A Seed certification and seed improvement programs. Pages in: Potato Health Management. R. C. Rowe, ed. The 126 PHYTOPATHOLOGY
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH).
 Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC
 Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR
 Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Y-chromosomal haplogroup typing Using SBE reaction
 Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Supplementary. Table 1: Oligonucleotides and Plasmids. complementary to positions from 77 of the SRα '- GCT CTA GAG AAC TTG AAG TAC AGA CTG C
 Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Supplemental Data Supplemental Figure 1.
 Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis
 1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
Hes6. PPARα. PPARγ HNF4 CD36
 SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
Supporting Information
 Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Supporting Information
 Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
Cat. # Product Size DS130 DynaExpress TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1
 Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Arabidopsis actin depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB
 Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Supplementary Figure 1A A404 Cells +/- Retinoic Acid
 Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
Occurrence of viruses in apple and pear orchards in Latvia.
 INTERNATIONAL SCIENTIFIC CONFERENCE Sustainable Fruit Growing: From Plant to Product Occurrence of viruses in apple and pear orchards in Latvia. Neda P pola, Anna K le, Inga Moro ko Bi evska. The economically
INTERNATIONAL SCIENTIFIC CONFERENCE Sustainable Fruit Growing: From Plant to Product Occurrence of viruses in apple and pear orchards in Latvia. Neda P pola, Anna K le, Inga Moro ko Bi evska. The economically
Disease and selection in the human genome 3
 Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular
![strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular](/thumbs/72/66361212.jpg) Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Gene synthesis by circular assembly amplification
 Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
MacBlunt PCR Cloning Kit Manual
 MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Supplemental Data. mir156-regulated SPL Transcription. Factors Define an Endogenous Flowering. Pathway in Arabidopsis thaliana
 Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
PCR analysis was performed to show the presence and the integrity of the var1csa and var-
 Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Multiplexing Genome-scale Engineering
 Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Supporting information for Biochemistry, 1995, 34(34), , DOI: /bi00034a013
 Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
RPA-AB RPA-C Supplemental Figure S1: SDS-PAGE stained with Coomassie Blue after protein purification.
 RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
Supplemental material
 Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
ORFs and genes. Please sit in row K or forward
 ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
ΔPDD1 x ΔPDD1. ΔPDD1 x wild type. 70 kd Pdd1. Pdd3
 Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Legends for supplementary figures 1-3
 High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
Supplemental Information. Human Senataxin Resolves RNA/DNA Hybrids. Formed at Transcriptional Pause Sites. to Promote Xrn2-Dependent Termination
 Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells
 Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Table S1. Bacterial strains (Related to Results and Experimental Procedures)
 Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were
 1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
Supporting Online Information
 Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system
 Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Materials Protein synthesis kit. This kit consists of 24 amino acids, 24 transfer RNAs, four messenger RNAs and one ribosome (see below).
 Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
PCR-based Markers and Cut Flower Longevity in Carnation
 PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
Supplemental Table 1. Primers used for PCR.
 Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
Supplementary Information
 Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR
 Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
hcd1tg/hj1tg/ ApoE-/- hcd1tg/hj1tg/ ApoE+/+
 ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
Sequencing of DNA lesions facilitated by site-specific excision via base. excision repair DNA glycosylases yielding ligatable gaps
 Supporting information Sequencing of DNA lesions facilitated by site-specific excision via base excision repair DNA glycosylases yielding ligatable gaps Jan Riedl, Aaron M. Fleming, and Cynthia J. Burrows*
Supporting information Sequencing of DNA lesions facilitated by site-specific excision via base excision repair DNA glycosylases yielding ligatable gaps Jan Riedl, Aaron M. Fleming, and Cynthia J. Burrows*
SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer
 TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
An engineered tryptophan zipper-type peptide as a molecular recognition scaffold
 SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR
 Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Overexpression Normal expression Overexpression Normal expression. 26 (21.1%) N (%) P-value a N (%)
 SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
Dierks Supplementary Fig. S1
 Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
NAME:... MODEL ANSWER... STUDENT NUMBER:... Maximum marks: 50. Internal Examiner: Hugh Murrell, Computer Science, UKZN
 COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
II 0.95 DM2 (RPP1) DM3 (At3g61540) b
 Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
evaluated with UAS CLB eliciting UAS CIT -N Libraries increase in the
 Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
Supplemental Data. Bennett et al. (2010). Plant Cell /tpc
 BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
Event-specific Method for the Quantification of Soybean SYHT0H2 by Real-time PCR. Validated Method
 EUROPEAN COMMISSION JOINT RESEARCH CENTRE Institute for Health and Consumer Protection Molecular Biology and Genomics Unit Event-specific Method for the Quantification of Soybean SYHT0H2 by Real-time PCR
EUROPEAN COMMISSION JOINT RESEARCH CENTRE Institute for Health and Consumer Protection Molecular Biology and Genomics Unit Event-specific Method for the Quantification of Soybean SYHT0H2 by Real-time PCR
Project 07/111 Final Report October 31, Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines
 Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
SUPPORTING INFORMATION FILE
 Intrinsic and extrinsic connections of Tet3 dioxygenase with CXXC zinc finger modules Nan Liu, Mengxi Wang, Wen Deng, Christine S. Schmidt, Weihua Qin, Heinrich Leonhardt and Fabio Spada Department of
Intrinsic and extrinsic connections of Tet3 dioxygenase with CXXC zinc finger modules Nan Liu, Mengxi Wang, Wen Deng, Christine S. Schmidt, Weihua Qin, Heinrich Leonhardt and Fabio Spada Department of
Inhibition of Hepatitis C Virus Replication by GS-6620, A Potent C-Nucleoside Monophosphate Prodrug
 Inhibition of Hepatitis C Virus Replication by, A Potent C-Nucleoside Monophosphate Prodrug Running Title: In Vitro Pharmacology of Authors: Joy Y. Feng et al. Supplementary Information Table S1. RNA primer
Inhibition of Hepatitis C Virus Replication by, A Potent C-Nucleoside Monophosphate Prodrug Running Title: In Vitro Pharmacology of Authors: Joy Y. Feng et al. Supplementary Information Table S1. RNA primer
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
Supplementary Information. Construction of Lasso Peptide Fusion Proteins
 Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Supporting Information
 Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
11th Meeting of the Science Working Group. Lima, Peru, October 2012 SWG-11-JM-11
 11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
Lecture 11: Gene Prediction
 Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
I. Things to keep in mind before you start this protocol
 Inverse PCR and Sequencing Protocol on 5 Fly Preps For recovery of sequences flanking piggybac elements This protocol is an adaptation of "Inverse PCR and Cycle Sequencing Protocols" by E. Jay Rehm Berkeley
Inverse PCR and Sequencing Protocol on 5 Fly Preps For recovery of sequences flanking piggybac elements This protocol is an adaptation of "Inverse PCR and Cycle Sequencing Protocols" by E. Jay Rehm Berkeley
Event-specific Method for the Quantification of Soybean Event DP Using Real-time PCR. Validated Method
 Event-specific Method for the Quantification of Soybean Event DP-356043-5 Using Real-time PCR Validated Method 29 March 2010 Joint Research Centre Institute for Health and Consumer Protection Molecular
Event-specific Method for the Quantification of Soybean Event DP-356043-5 Using Real-time PCR Validated Method 29 March 2010 Joint Research Centre Institute for Health and Consumer Protection Molecular
Primer Design Workshop. École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria
 Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Supporting Information. Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy
 Supporting Information Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy Agata Olszewska, Radek Pohl and Michal Hocek # * Institute of Organic
Supporting Information Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy Agata Olszewska, Radek Pohl and Michal Hocek # * Institute of Organic
Lecture 19A. DNA computing
 Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ
 Electronic Supplementary Material A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ Xiaoli Zhu 1, Hai Shi 1, Yalan Shen 1,
Electronic Supplementary Material A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ Xiaoli Zhu 1, Hai Shi 1, Yalan Shen 1,
Event-specific Method for the Quantification of Cotton Event MON Using Real-time PCR. Protocol
 Event-specific Method for the Quantification of Cotton Event MON 88913 Using Real-time PCR Protocol 5 May 2009 Joint Research Centre Institute for Health and Consumer Protection Molecular Biology and Genomics
Event-specific Method for the Quantification of Cotton Event MON 88913 Using Real-time PCR Protocol 5 May 2009 Joint Research Centre Institute for Health and Consumer Protection Molecular Biology and Genomics
Wet Lab Tutorial: Genelet Circuits
 Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURB
 Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURBO DNA-free Kit (Ambion). One µg of total RNA was reverse
Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURBO DNA-free Kit (Ambion). One µg of total RNA was reverse
NESTED Sequence-based Typing (SBT) protocol for epidemiological typing of Legionella pneumophila directly from clinical samples
 NESTED Sequence-based Typing (SBT) protocol for epidemiological typing of Legionella pneumophila directly from clinical samples VERSION 2.0 SUMMARY This procedure describes the use of nested Sequence-Based
NESTED Sequence-based Typing (SBT) protocol for epidemiological typing of Legionella pneumophila directly from clinical samples VERSION 2.0 SUMMARY This procedure describes the use of nested Sequence-Based
2
 1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
Supplemental Information. Target-Mediated Protection of Endogenous. MicroRNAs in C. elegans. Inventory of Supplementary Information
 Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
SUPPLEMENTARY MATERIALS
 SUPPLEMENTARY MATERIALS Supplementary Table S1: Water sampling sites in Brisbane River and their characteristics Sampling sites GPS coordinates Site characteristics Suspected source of fecal pollution
SUPPLEMENTARY MATERIALS Supplementary Table S1: Water sampling sites in Brisbane River and their characteristics Sampling sites GPS coordinates Site characteristics Suspected source of fecal pollution
Event-specific Method for the Quantification of maize MON by Real-time PCR. Validated Method
 EUROPEAN COMMISSION JOINT RESEARCH CENTRE Health, Consumers & Reference Materials Food & Feed Compliance Unit Event-specific Method for the Quantification of maize MON 87403 by Real-time PCR Validated
EUROPEAN COMMISSION JOINT RESEARCH CENTRE Health, Consumers & Reference Materials Food & Feed Compliance Unit Event-specific Method for the Quantification of maize MON 87403 by Real-time PCR Validated
G+C content. 1 Introduction. 2 Chromosomes Topology & Counts. 3 Genome size. 4 Replichores and gene orientation. 5 Chirochores.
 1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
SUPPLEMENTARY INFORMATION
 1. RNA/DNA sequences used in this study 2. Height and stiffness measurements on hybridized molecules 3. Stiffness maps at varying concentrations of target DNA 4. Stiffness measurements on RNA/DNA hybrids.
1. RNA/DNA sequences used in this study 2. Height and stiffness measurements on hybridized molecules 3. Stiffness maps at varying concentrations of target DNA 4. Stiffness measurements on RNA/DNA hybrids.
Nongenetic Reprogramming of the Ligand Specificity. of Growth Factor Receptors by Bispecific DNA Aptamers
 Supporting Information For Nongenetic Reprogramming of the Ligand Specificity of Growth Factor Receptors by Bispecific DNA Aptamers Ryosuke Ueki,* Saki Atsuta, Ayaka Ueki and Shinsuke Sando* Department
Supporting Information For Nongenetic Reprogramming of the Ligand Specificity of Growth Factor Receptors by Bispecific DNA Aptamers Ryosuke Ueki,* Saki Atsuta, Ayaka Ueki and Shinsuke Sando* Department
Event-specific Method for the Quantification of Maize Line LY038 Using Real-time PCR. Protocol
 Event-specific Method for the Quantification of Maize Line LY038 Using Real-time PCR Protocol 6 October 2008 Joint Research Centre Institute for Health and Consumer Protection Biotechnology & GMOs Unit
Event-specific Method for the Quantification of Maize Line LY038 Using Real-time PCR Protocol 6 October 2008 Joint Research Centre Institute for Health and Consumer Protection Biotechnology & GMOs Unit
BioInformatics and Computational Molecular Biology. Course Website
 BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
DNA sentences. How are proteins coded for by DNA? Materials. Teacher instructions. Student instructions. Reflection
 DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers
 DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
Genomics and Gene Recognition Genes and Blue Genes
 Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
SUPPLEMENTARY INFORMATION
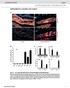 doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
Electronic Supplementary Information. Transcription Monitoring Using Fused RNA with a Dye-Binding Light-Up Aptamer as a Tag: A Blue Fluorescent RNA
 Electronic Supplementary Information for Transcription Monitoring Using Fused RNA with a Dye-Binding Light-Up Aptamer as a Tag: A Blue Fluorescent RNA Shinsuke Sando,* Atsushi Narita, Masayoshi Hayami,
Electronic Supplementary Information for Transcription Monitoring Using Fused RNA with a Dye-Binding Light-Up Aptamer as a Tag: A Blue Fluorescent RNA Shinsuke Sando,* Atsushi Narita, Masayoshi Hayami,
Chapter 13 Chromatin Structure and its Effects on Transcription
 Chapter 13 Chromatin Structure and its Effects on Transcription Students must be positive that they understand standard PCR. There is a resource on the web for this purpose. Warn them before this class.
Chapter 13 Chromatin Structure and its Effects on Transcription Students must be positive that they understand standard PCR. There is a resource on the web for this purpose. Warn them before this class.
Event-specific Method for the Quantification of. Oilseed Rape Line RT73 Using Real-time PCR. Protocol. 07 February 2007
 EUROPEAN COMMISSION DIRECTORATE GENERAL JRC JOINT RESEARCH CENTRE INSTITUTE FOR HEALTH AND CONSUMER PROTECTION COMMUNITY REFERENCE LABORATORY FOR GM FOOD AND FEED Event-specific Method for the Quantification
EUROPEAN COMMISSION DIRECTORATE GENERAL JRC JOINT RESEARCH CENTRE INSTITUTE FOR HEALTH AND CONSUMER PROTECTION COMMUNITY REFERENCE LABORATORY FOR GM FOOD AND FEED Event-specific Method for the Quantification
Annex 6.5 CDC real-time rubella RT-PCR protocol targeting rubella p150 gene (154 nt region)
 Annex 6.5 CDC real-time rubella RT-PCR protocol targeting rubella p150 gene (154 nt region) NOTE: This document is intended to provide basic test method details and is not an SOP. Laboratories need to
Annex 6.5 CDC real-time rubella RT-PCR protocol targeting rubella p150 gene (154 nt region) NOTE: This document is intended to provide basic test method details and is not an SOP. Laboratories need to
Det matematisk-naturvitenskapelige fakultet
 UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
Annex 6.2 CDC real-time measles RT-PCR protocol targeting measles N gene (75 nt region)
 Annex 6.2 CDC real-time measles RT-PCR protocol targeting measles N gene (75 nt region) NOTE: This document is intended to provide basic test method details and is not an SOP. Laboratories need to develop
Annex 6.2 CDC real-time measles RT-PCR protocol targeting measles N gene (75 nt region) NOTE: This document is intended to provide basic test method details and is not an SOP. Laboratories need to develop
A netlike rolling circle nucleic acid amplification technique
 Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2014 Supplementary Information A netlike rolling circle nucleic acid amplification technique Xiaoli Zhu,
Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2014 Supplementary Information A netlike rolling circle nucleic acid amplification technique Xiaoli Zhu,
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Site-Specific Control of Distances between Gold Nanoparticles using Phosphorothioate Anchors on DNA and a Short Bifunctional Molecular Fastener
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Site-Specific Control of Distances between Gold Nanoparticles using Phosphorothioate Anchors on DNA and a Short Bifunctional Molecular Fastener
SUPPLEMENTARY MATERIALS AND METHODS. E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1)
 SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide
 Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide Jochen Hoffmann a,b, Martin Trotter a, Felix von Stetten a,b, Roland Zengerle, a,b,c Günter
Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide Jochen Hoffmann a,b, Martin Trotter a, Felix von Stetten a,b, Roland Zengerle, a,b,c Günter
2ml of 1M stock 10x TBE (1 Litre) Tris Base 107.8g 55g (harmful, wear mask) EDTA 7.4g
 Phytoplasma Detection Protocol Buffers: Hybridisation buffer 100ml hybridisation buffer 2.92g Sodium chloride 4g Blocking reagent (add slowly while stirring) Mix at room temperature for 2 hours Can be
Phytoplasma Detection Protocol Buffers: Hybridisation buffer 100ml hybridisation buffer 2.92g Sodium chloride 4g Blocking reagent (add slowly while stirring) Mix at room temperature for 2 hours Can be
Protocol Rf3 Community Reference Laboratory for GM Food and Feed
 EUROPEAN COMMISSION DIRECTORATE GENERAL JRC JOINT RESEARCH CENTRE INSTITUTE FOR HEALTH AND CONSUMER PROTECTION COMMUNITY REFERENCE LABORATORY FOR GM FOOD AND FEED Event-specific Method for the Quantification
EUROPEAN COMMISSION DIRECTORATE GENERAL JRC JOINT RESEARCH CENTRE INSTITUTE FOR HEALTH AND CONSUMER PROTECTION COMMUNITY REFERENCE LABORATORY FOR GM FOOD AND FEED Event-specific Method for the Quantification
SUPPLEMENTARY INFORMATION
 doi:1.138/nature11496 Cl. 8 Cl. E93 Rag1 -/- 3H9 + BM Rag1 -/- BM CD CD c-kit c-kit c-kit wt Spleen c-kit B22 B22 IgM IgM IgM Supplementary Figure 1. FACS analysis of single-cell-derived pre-b cell clones.
doi:1.138/nature11496 Cl. 8 Cl. E93 Rag1 -/- 3H9 + BM Rag1 -/- BM CD CD c-kit c-kit c-kit wt Spleen c-kit B22 B22 IgM IgM IgM Supplementary Figure 1. FACS analysis of single-cell-derived pre-b cell clones.
SUPPLEMENTAL MATERIAL GENOTYPING WITH MULTIPLEXING TARGETED RESEQUENCING
 SUPPLEMENTAL MATERIAL GENOTYPING WITH MULTIPLEXING TARGETED RESEQUENCING All of the patients and control subjects were sequenced and genotyped in the same way. Shotgun libraries of approximately 250 bp
SUPPLEMENTAL MATERIAL GENOTYPING WITH MULTIPLEXING TARGETED RESEQUENCING All of the patients and control subjects were sequenced and genotyped in the same way. Shotgun libraries of approximately 250 bp
