Molecular Architecture of Synaptic Actin Cytoskeleton in Hippocampal Neurons Reveals a Mechanism of Dendritic Spine Morphogenesis
|
|
|
- June Greer
- 6 years ago
- Views:
Transcription
1 Molecular Biology of the Cell Vol. 21, , January 1, 2010 Molecular Architecture of Synaptic Actin Cytoskeleton in Hippocampal Neurons Reveals a Mechanism of Dendritic Spine Morphogenesis Farida Korobova and Tatyana Svitkina Department of Biology, The University of Pennsylvania, Philadelphia, PA Submitted July 22, 2009; Revised September 28, 2009; Accepted October 23, 2009 Monitoring Editor: Paul Forscher Excitatory synapses in the brain play key roles in learning and memory. The formation and functions of postsynaptic mushroom-shaped structures, dendritic spines, and possibly of presynaptic terminals, rely on actin cytoskeleton remodeling. However, the cytoskeletal architecture of synapses remains unknown hindering the understanding of synapse morphogenesis. Using platinum replica electron microscopy, we characterized the cytoskeletal organization and molecular composition of dendritic spines, their precursors, dendritic filopodia, and presynaptic boutons. A branched actin filament network containing Arp2/3 complex and capping protein was a dominant feature of spine heads and presynaptic boutons. Surprisingly, the spine necks and bases, as well as dendritic filopodia, also contained a network, rather than a bundle, of branched and linear actin filaments that was immunopositive for Arp2/3 complex, capping protein, and myosin II, but not fascin. Thus, a tight actin filament bundle is not necessary for structural support of elongated filopodia-like protrusions. Dynamically, dendritic filopodia emerged from densities in the dendritic shaft, which by electron microscopy contained branched actin network associated with dendritic microtubules. We propose that dendritic spine morphogenesis begins from an actin patch elongating into a dendritic filopodium, which tip subsequently expands via Arp2/3 complex-dependent nucleation and which length is modulated by myosin II-dependent contractility. INTRODUCTION Dendritic spines are small protrusions on the surface of neuronal dendrites that form the postsynaptic component of the excitatory synapse and play important roles in learning and memory. Alterations in dendritic spines are found in many types of mental retardation and other neurological disorders (Calabrese et al., 2006). By morphology, dendritic spines are usually classified as mushroom shaped, thin (or elongated), and stubby, and their shape is thought to correlate with the strength and activity of the synapse (Bourne and Harris, 2008). A mushroom spine has a bulbous head connected to the dendrite by a constricted neck (or stalk); thin spines have a smaller head, and stubby spines lack a neck. However, these categories are not separated by clearcut boundaries but rather describe a continuum of shapes (Arellano et al., 2007). A distinctive feature of dendritic spines is the postsynaptic density (PSD), a large assembly of receptors and signaling proteins associated with the membrane of the spine head at the junction with a presynaptic bouton of the axon (Fifkova and Delay, 1982; Sheng and Hoogenraad, 2007). Dendritic spines are highly dynamic (Matus, 2005) and their formation, maturation, and plasticity heavily depend on the actin cytoskeleton remodeling (Ethell and Pasquale, This article was published online ahead of print in MBC in Press ( ) on November 4, Address correspondence to: Tatyana M. Svitkina (svitkina@sas. upenn.edu). Abbreviations used: DIV, days in vitro; EM, electron microscopy; PSD, postsynaptic density. 2005; Cingolani and Goda, 2008). However, the underlying mechanisms and even the structural organization of actin filaments in spines are poorly understood (Halpain, 2000; Rao and Craig, 2000; Ethell and Pasquale, 2005; Tada and Sheng, 2006), apparently because of their small size combined with complex organization. These features critically require electron microscopy (EM) to fully understand the biology of dendritic spines. The major types of actin filament organization across cell types and subcellular organelles include protrusive structures, such as branched networks in lamellipodia (Svitkina et al., 1997; Svitkina and Borisy, 1999) and parallel bundles in filopodia (Svitkina et al., 2003), and contractile structures, such as bundles and networks of linear filaments with mixed polarity (Verkhovsky et al., 1995). Early EM studies of dendritic spines by using thin-section (Fifkova and Delay, 1982; Markham and Fifkova, 1986) or freeze-fracture (Landis and Reese, 1983; Hirokawa, 1989) techniques detected long filaments, as well as a meshwork of short, potentially branched, actin filaments in spines, but they failed to provide a cohesive picture of their entire cytoskeleton. More recent light microscopic and functional approaches revealed that proteins normally involved in generation of protrusive branched networks, such as the Arp2/3 complex (Racz and Weinberg, 2008), WAVE1 (Kim et al., 2006; Hotulainen et al., 2009), cortactin (Hering and Sheng, 2003), N-WASP (Wegner et al., 2008), profilin (Ackermann and Matus, 2003), and cofilin (Racz and Weinberg, 2006; Hotulainen et al., 2009), were present in spines. However, myosin II (Cheng et al., 2000; Ryu et al., 2006) and -actinin (Wyszynski et al., 1997) were also found there suggesting formation of contractile bundles or networks. Kinetically, dendritic spines contain dynamic and stable subpopulations of actin (Honkura et al., 2008), which may correspond to distinct types of actin fila by The American Society for Cell Biology 165
2 F. Korobova and T. Svitkina ment arrays within spines. In general, a common belief is that the head of a spine should be similar to lamellipodia and contain an Arp2/3 complex-dependent branched network, whereas the spine neck is probably maintained by an axial actin filament bundle (Halpain, 2000; Rao and Craig, 2000; Tada and Sheng, 2006; Hotulainen et al., 2009). However, these ideas have not been directly proven and the actin filament organization in spines remains uncertain. Even less is known about the structure of presynaptic actin, which is believed to have a dual function of restraining synaptic vesicles and of directing them toward the synapse (Cingolani and Goda, 2008). Dendritic spines are thought to derive from dendritic filopodia that establish the initial contact with an axon, although other models also exist (Papa et al., 1995; Ethell and Pasquale, 2005; Yoshihara et al., 2009). The cytoskeletal organization and molecular composition of dendritic filopodia remain totally unknown. They may be similar to conventional filopodia found in neuronal growth cones and at the leading edge of other migrating cells that contain a tight bundle of long uniformly oriented actin filaments in their interior (Small et al., 2002; Svitkina et al., 2003; Korobova and Svitkina, 2008). Alternatively, dendritic filopodia may be structurally similar to dendritic spines (Papa et al., 1995), which structure as mentioned earlier is also unknown. These conflicting considerations require an experimental approach to investigate the actual structure of dendritic filopodia. Here, we used platinum replica EM to characterize the cytoskeletal organization and molecular composition of dendritic spines and dendritic filopodia in dissociated cultures of hippocampal neurons and revealed novel features of both structures essential for understanding of their morphogenesis and dynamics. MATERIALS AND METHODS Cell Culture and Transfection For high density cultures, hippocampal neurons were isolated as described previously (Wilcox et al., 1994) and were kindly provided by Dr. M. Dichter (The University of Pennsylvania, Philadelphia, PA). Every 5 d, one third of culture medium was replaced by fresh medium. For transfection with enhanced yellow fluorescent protein (EYFP)-actin (Clontech, Mountain View, CA) or mcherry-actin (Yang et al., 2009), hippocampal neurons were plated on 35-mm glass-bottomed dishes at 100,000 cells/dish concentration and transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) after 5 6 days in vitro (DIV). Transfected cells were analyzed on 14 DIV. Antibodies The following rabbit polyclonal antibodies were used: p16-arc (Vignjevic et al., 2003), Arp3 (Santa Cruz Biotechnology, Santa Cruz, CA), and p34-arc subunits of Arp2/3 complex (Millipore, Billerica, MA), nonmuscle myosin II from bovine spleen (Verkhovsky et al., 1987), and capping protein (from D. Schafer, University of Virginia). The following mouse monoclonal antibodies were used: fascin (Millipore), microtubule-associated protein (MAP2) (Sigma- Aldrich, St. Louis, MO), N-cadherin (Santa Cruz Biotechnology; gift from W. J. Nelson, Stanford University, Stanford, CA), -tubulin (Sigma-Aldrich), and PSD-95 (Abcam, Cambridge, MA). Secondary fluorescently labeled antibodies and AlexaFluor 488-, 594-, and 647-labeled phalloidins were from Invitrogen, secondary rabbit or mouse antibodies conjugated to 12- or 18-nm colloidal gold were from Jackson ImmunoResearch Laboratories (West Grove, PA). Immunofluorescence and Light Microscopy Immunofluorescence staining of cells growing on glass coverslips was performed after cell extraction for 5 min with 1% Triton X-100 in PEM buffer [100 mm piperazine-n,n -bis(2-ethanesulfonic acid)-koh, ph 6.9, 1 mm MgCl 2, and 1 mm EGTA] containing 2% polyethelene glycol (mol. wt. 35,000) and 5 M phalloidin and fixation with 4% paraformaldehyde in phosphate-buffered saline (PBS) or 0.2% glutaraldehyde in 0.1 M Na-cacodylate, ph 7.3. Glutaraldehyde-fixed samples were quenched with 2 mg/ml NaBH 4. For F-actin staining, fluorescently labeled phalloidin (0.033 M) was added to the secondary antibody solution. For fascin immunostaining, extracted cells were fixed with 50% methanol in PBS and washed with PBS. For time-lapse imaging, cells were cultured in glass-bottomed dishes and kept on the microscope stage in the TC-MIS miniature incubator (Bioscience Tools, Santa Clara, CA) at 36.5 C in the atmosphere containing 5% CO 2 and 20% O 2. For correlative immunostaining, marked coverslips were used to help with cell identification (Svitkina, 2007). Immediately after acquisition of 10- to 12-min-long movies with 2- to 3-s time intervals, the medium in the dish was changed to 4% paraformaldehyde in PBS, and cells were immunostained as described above. Light microscopy was performed using an inverted microscope (Eclipse TE2000; Nikon, Tokyo, Japan) equipped with Planapo numerical aperture or objectives and Cascade 512B chargecoupled device (CCD) camera (Roper Scientific, Trenton, NJ) driven by Meta- Morph imaging software (Molecular Devices, Sunnyvale, CA). Electron Microscopy Samples for platinum replica EM, correlative light and electron microscopy, immuno-em, and S1 decoration were processed as described previously (Svitkina, 2007; Korobova and Svitkina, 2008). For nonextracted samples, neuronal cultures were fixed sequentially with 2% glutaraldehyde in Nacacodylate, and with aqueous solutions of 0.2% uranyl acetate, 0.1% tannic acid, and 1% OsO 4. For Arp2/3 complex immunogold staining, cells were extracted as for immunofluorescence, fixed with 0.2% glutaraldehyde, quenched with NaBH 4, and incubated in a mixture of p16-arc, Arp3, and p34-arc antibodies. For N-cadherin, myosin II, and capping protein immunostaining, unfixed cells extracted with 1% Triton X-100 and 4% polyethelene glycol in PEM buffer were incubated for 15 min with a primary antibody in the PEM buffer containing 5 M unlabeled phalloidin, washed with the PEM buffer, fixed with 0.2% glutaraldehyde, stained with secondary antibody, and postfixed with 2% glutaraldehyde. For PSD-95 staining, cells were extracted/ fixed for 5 min with a mixture of 0.5% Triton X-100 and 1% paraformaldehyde in PEM buffer with 5 M phalloidin, washed in the same buffer, incubated with primary antibodies in PEM buffer for 15 min, postfixed with 2% glutaraldehyde, and stained with secondary antibody. Myosin S1 (gift from Dr. L. Tilney) was centrifuged at 100,000 g for 30 min before use. For correlative light and electron microscopy, EYFP- or mcherry-actin expressing cells were located on marked glass-bottomed dishes and phase-contrast and fluorescent images were acquired immediately before extraction and within 2 min after addition of the extraction solution. Then cells were fixed and processed for EM, as described previously (Svitkina, 2007; Korobova and Svitkina, 2008). Samples were analyzed using JEM 1011 transmission EM (JEOL USA, Peabody, MA) operated at 100 kv. Images were captured by ORIUS W CCD camera (Gatan, Warrendale, PA) and presented in inverted contrast. Identification of gold particles in replica EM samples was performed at high magnification after contrast enhancement to distinguish them from other bright objects in the samples. Thus, gold particles showed up as solid white circles in contrast to filament ends, for example, which usually have a donutlike appearance. Image Analysis and Statistics All morphometric measurements were done using MetaMorph or Photoshop (Adobe Systems, Mountain View, CA) software packages and repeated for at least two independent experiments. Data were analyzed using Excel software (Microsoft, Redmond, CA). Significance was determined using a two-tailed t test. For quantification of F-actin and Arp2/3 complex contents in filopodia, filopodia were manually selected on phalloidin-stained images using the MetaMorph selection tool and selection was transferred to corresponding Arp2/3 complex-stained images. Average fluorescence intensities of selected regions from both sets of images were recorded after background subtraction. To quantify the distribution of PSD-95 and N-cadherin immunogold labeling in dendritic spines relative to the adjacent axon, we measured the shortest distances between individual gold particles and the nearest microtubule or intermediate filament in the axonal shaft in EM samples. Gold particles occurring on the other side of the first axonal fiber were assigned negative numbers. Only gold particles located in the distal half of the spine, were included into quantification to exclude irrelevant labeling in the dendritic shafts where both PSD-95 and N-cadherin are also present. If the axon and the dendrite were not parallel, the distal half of the spine was determined by drawing a bisector for the angle between them until it intersects the spine. RESULTS Identification of Spines Platinum replica EM is advantageous for investigation of the cytoskeletal organization of whole-mount samples at high resolution (Svitkina, 2007). However, detergent extraction, which is required for this technique, dissolves membranes making cell boundaries undetectable and has a potential to perturb the cytoskeletal organization. Therefore, we performed control experiments to identify dendritic spines, de- 166 Molecular Biology of the Cell
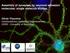
3 Actin Cytoskeleton in Synapses termine their polarity, and test their preservation in EM samples. First, we performed replica EM of nonextracted hippocampal neurons in which cell boundaries remain preserved (Figure 1). Neurites in these cultures usually formed complex networks or aligned bundles (Figure 1A). Dendritic spines were abundant in mature cultures fixed after DIV and could be recognized by their mushroom shape and/or ability to make contacts (presumably, synapses) with adjacent neurites (Figure 1, A D). An axon interacting with the spine head was usually thinner than the dendrite, from which the spine emerged. The spine necks frequently expanded not only at the tip forming a bulbous head, but also at the root, although to a lesser extent, forming a deltashaped base (Figure 1C). At 7 11 DIV, spines were rare, but dendritic filopodia abundant (Figure 1, E H). Compared with straight and uniform growth cone filopodia in the same samples (data not shown), dendritic filopodia were very polymorphic. Occasionally, they made contacts with other neurites (Figure 1H). At this stage of development, dendrites also formed small lamellipodia-like protrusions (data not shown). We also stained hippocampal cultures with a dendritic marker MAP2 (Caceres et al., 1984) and phalloidin to distinguish dendrites and axons (Supplemental Figure S1). A strong MAP2 signal in dendrites correlated with prominent F-actin staining and a greater thickness of neurites; in contrast, MAP2-depleted axons contained much less F-actin and were on average thinner. Finally, we used correlative EM of EYFP-actin expressing neurons to visualize the same spines sequentially by light and EM (Supplemental Figure S2). These results showed that detergent extraction and EM processing did not alter the shape or actin distribution in spines. They also revealed characteristic features of the spine cytoskeleton, such as a greater density of the actin filaments in spine heads, compared with spine bases. Based on these experiments, we applied following criteria to identify the spine polarity in EM samples (Supplemental Figure S3): a greater thickness of dendrites and abundance of actin filaments there, compared with axons; a greater size and higher actin filaments density in spine heads, as compared with bases; and a more distinct delta-like shape of bases, as compared with more polymorphic heads. Actin Cytoskeleton Organization in Dendritic Spines Mature DIV hippocampal neurons were used for EM analysis of dendritic spines. Axons and dendrites could be recognized by axial bundles of microtubules and intermediate filaments in their interior, and dendrites also contained significant amount of actin filaments (Supplemental Figure S3). A typical mushroom spine contained three compartments: a bulbous head contacting the axon, a constricted neck in the middle, and a delta-shaped base at the junction with the dendrite (Figures 2 and 3 and Supplemental Figure S3). The spine head typically contained a dense network of short cross-linked actin filaments, in which branched filaments were clearly visible in relatively sparse regions (Figure 2A). Long filaments were also sometimes observed in the spine head. Surprisingly, the actin network in the dendritic spine head seemed to directly interact with axonal microtubules and intermediate filaments (Figure 2). Proximity of actin-rich spine heads to microtubules in the axon was also seen by fluorescence microscopy (Supplemental Figure S4). However, part of this network likely belongs to the presynaptic bouton, rather than to the dendritic spine (see Figure 1. Morphology of dendritic spines (A D) and dendritic filopodia (E H). Replica EM of nonextracted hippocampal neurons after 14 (A D) or 10 (E H) DIV. (A) Network of neurites with dendritic spines (arrows); two spines contact other neurites (axons). The cell body producing the major dendrite is located 20 m away from the left margin of the panel. (B D) Examples of mushroom (B), thin (C), and stubby (D) spines. (E) Distal region of a dendrite with polymorphic dendritic filopodia. (F H) Examples of dendritic filopodia. Bars, 2 m (A and E), 1 m (B D, F, and G), or 0.5 m (H). below). Accordingly, occasional chunks of nonfibrillar material in the spine head, which might represent nonextracted PSD fragments, were found either next to the axonal micro- Vol. 21, January 1,
4 F. Korobova and T. Svitkina Figure 2. Cytoskeletal organization of dendritic spines: head and neck. EM of extracted 14 DIV neurons. Mushroom (A) and thin (B) spines associate with dendrites at the base (bottom) and with axons by the head (top). Unlabeled insets show the smaller versions of the corresponding main panels with axons colorcoded in purple, dendrites in yellow, and spines in cyan. See Supplemental Figure S3 for determination of the orientation of the spine shown in A. Thick fibers in both neurites represent microtubules. Red asterisks indicate putative PSD fragments. Yellow boxes are enlarged in panels with corresponding numbers. Box 1, interaction of putative PSD (arrow) from the spine head with axonal intermediate filaments (green); actin filaments are shown in cyan and a microtubule in red. Boxes 2, 3, and 5, branched actin filaments (cyan) in the head (2), at the neck head junction (3), and in the neck (5) of respective spines. Insets in panels 2 and 3 show nonpseudocolored regions outlined by yellow boxes. Dashed arrows in panel 3 indicate potential filament breakage. Box 4, interaction of actin filaments (cyan) with an axonal microtubule (red). Bars, 0.2 m (A and B). tubules and intermediate filaments, or slightly away from them (Figure 2A). In the spine neck, actin filaments formed an anisometric network, which consisted of loosely arranged actin filaments that might be longitudinally oriented, but only infrequently formed tight bundles. The number of filaments varied significantly along the length of the neck and many filaments did not span the entire neck. Filaments began or ended with unbound ends but also branched off of the side of another filament (Figure 2B). At the neck head junction, the number of actin filaments abruptly increased toward the head, which usually occurred through extensive branching of neck filaments entering the head (Figure 2A). The spine base is a previously unrecognized structural compartment of the spine. It usually contained relatively long actin filaments converging from a broad area in the dendrite toward the neck in a delta-shaped configuration (Figures 2 and 3). These filaments either continued into the neck, or terminated within the base (Figure 3C). In many spines, the base also contained branched filaments (Figure 168 3A). Surprisingly, the actin filaments of the spine base frequently seemed to begin directly from the microtubule network in the dendrite and subsequently amplified through branching (Figures 2 and 3), whereas others originated from other actin structures in the dendrite. Some spines did not have a well-formed base (Figure 3B). Other morphological types of spines displayed the same structural plan. However, thin spines (Figure 2B) had longer necks and narrower heads, whereas stubby spines seemed to lack a neck and consisted of a patch of a densely branched actin network that likely represented the spine head or a merge of the base and the head (Figure 3B). The dimensions of spine compartments varied broadly even within one morphological class of spines blurring the distinction between the classes. Some mushroom-shaped dendritic protrusions not making contacts with axons had nonetheless a similar structural organization as the contact-forming spines (data not shown). Decoration of actin filaments with the myosin II subfragment 1 (S1) confirmed that actin filaments were the major cytoskeletal component of spines (Figure 3C). HowMolecular Biology of the Cell
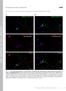
5 Actin Cytoskeleton in Synapses Figure 3. Cytoskeletal organization of dendritic spines: base. EM of extracted neurons after 11 (A) or 14 (B and C) DIV shows undecorated (A and B) or S1-decorated (C) mushroom (A and C) or stubby (B) spines. The spine in A also may be considered stubby. Unlabeled insets show the smaller versions of the corresponding main panels with axons color-coded in purple, dendrites in yellow, and spines in cyan. Yellow boxes are enlarged in panels with corresponding numbers. Box 1, interaction of actin filaments (cyan) with axonal microtubules (red). Boxes 2 and 3, branched actin filaments (cyan) in the spine bases associate with dendritic microtubules (red). Box 4, linear actin filaments in the spine base have unbound ends (arrows) and are arranged in a delta-shaped configuration. S1-decorated actin filaments have a rough bumpy outline that distinguishes them from undecorated thin filaments (arrowheads). Bars, 0.2 m (A C). ever, nondecorated filaments of 6 nm in thickness (including an 4-nm platinum layer) were also detected in different spine compartments (Figure 3C). Thus, the cytoskeletons of the head, neck, and base of a mature spine are not dramatically different from each other and consist of a mixture of branched and linear filaments, but in different combinations. Branched filaments dominate in the head, whereas linear filaments are more prominent in the base, and the neck may contain different ratios of both. The mutual alignment of actin filaments also varies between compartments being most prominent in the neck, but not to an extent of forming a tight bundle. This organization is conserved among all spine classes, mushroom, thin and stubby, but the dimensions of individual compartments Vol. 21, January 1, 2010 vary broadly up to complete absence of a neck and/or a base. Molecular Markers in Dendritic Spines The presence of branched actin networks in different spine compartments suggested involvement of the Arp2/3 complex. Indeed, immunofluorescence staining detected Arp2/3 complex in the majority of heads and in 30 40% of necks and bases of mushroom or thin spines (Figure 4, A and D). In stubby spines, the Arp2/3 complex was found throughout the spine. Small actin patches that were usually present in dendrites also contained the Arp2/3 complex. Immunolocalization of another common marker of branched networks, heterodimeric capping protein (Svitkina et al., 2003), 169
6 F. Korobova and T. Svitkina Figure 4. Immunofluorescence staining of cytoskeletal proteins in spines. (A C) Low-magnification (top) and high-magnification (bottom) images of dendritic spines in 14 DIV hippocampal neurons stained with phalloidin (green) and indicated antibodies (red). Dendritic spines are marked by arrowheads. (D F) Percentage of spine compartments immunopositive for indicated proteins in the population of spines. Data are collected from three experiments and expressed as mean SD (137 spines for the Arp2/3 complex; 90 spines for capping protein, and 121 spines for myosin II). Bars, 2 m (A C). gave similar results (Figure 4E). An actin filament crosslinker, fascin, which is characteristic for conventional filopodia (Svitkina et al., 2003), was virtually absent from dendritic spines (Supplemental Figure S5). Apparently seamless transition between dendritic and axonal cytoskeletons at the synaptic junction in the detergentextracted samples prompted us to attempt to elucidate the position of a boundary between two cells using immuno-em of N-cadherin, a transmembrane junctional molecule involved in synapse formation (Bourne and Harris, 2008), and PSD-95, a scaffolding protein in the PSD that has perijunctional localization (Tada and Sheng, 2006). As expected for the membrane-associated molecules, a significant fraction of both proteins was dissolved by the detergent treatment during immuno-em processing, but a fraction of them remained. Although labeling was not dense, it was specific, as gold particles were not detected in inappropriate locations and they were absent when primary antibodies were omitted. Furthermore, the staining pattern was consistent between spines and experiments. Gold particles in both cases tended to localize at some distance from axonal microtubules and intermediate filaments within the actin network in 170 spine heads (Figure 5A and Supplemental S6). Because the intensity of labeling was limited to approximately five to six gold particles per spine, it was not possible to precisely demarcate the interface between the axon and the spine in each individual case. Therefore, to quantitatively evaluate the label distribution and thus an average position of the boundary, we measured the distance between gold particles labeling N-cadherin or PSD-95 and the closest definite cytoskeletal component of an axon, usually a microtubule or an intermediate filament. The quantification produced similar results for both markers. Thus, gold particles labeling N-cadherin and PSD-95 were localized at distances of nm (mean SD; 10 spines, 59 gold particles) and nm (12 spines, 60 gold particles) from axons, respectively, supporting an idea that a fraction of the actin network at the spine tips, in the order of several hundreds nanometers, probably belongs to the axon rather than to the dendrite. The structure of the network was similar on the both sides of the putative boundary revealed by the N-cadherin or PSD-95 labeling. Additional gold particles of N-cadherin staining were also found in dendrites consistent with frequent bundling of dendrites in these cultures (Figure 1A). Molecular Biology of the Cell
7 Actin Cytoskeleton in Synapses Figure 5. Immunogold EM of cytoskeleton-associated proteins in spines. Unlabeled insets show the smaller versions of the corresponding main panels with axons color-coded in purple, dendrites in yellow, and spines in cyan. (A) N-cadherin. (B) Arp2/3 complex. (C) Capping protein. (D) Myosin II. Gold particles showing protein localization are highlighted in orange. Red boxes are enlarged in panels with corresponding numbers to show noncolorized gold particles (arrowheads). Box 5 shows a linear cluster of gold particles at the bottom. Bar, 0.2 m. Immunogold EM of Arp2/3 complex and capping protein gave similar results (Figure 5, B and C) and showed that all analyzed mushroom or thin spines (N 23 and 22 spines for Arp2/3 complex and capping protein, respectively) contained gold particles in the head, and approximately half of spines was labeled in the neck (11 and 14 spines, respectively) and the base (13 spines for each protein). The density of gold particles in necks and bases was usually lower, than in heads. Stubby spines had even distribution of gold particles throughout the body. Next, we evaluated the precise distribution of myosin II in spines, which presence in spines was reported previously (Morales and Fifkova, 1989; Ryu et al., 2006). Immunofluorescence staining revealed a punctate, or sometimes diffuse, distribution Vol. 21, January 1, 2010 of myosin II in dendritic spines, dendrites, and the cell body (Figure 4, C and F). Myosin puncta likely corresponded to myosin II bipolar filaments (Verkhovsky et al., 1995), whereas diffuse staining might correspond to individual cytoskeletonassociated myosin II molecules. In spines, myosin II puncta predominantly localized to the neck and a lower part of the head, and to a lesser extent to the base. Immunogold EM revealed similar distribution of myosin II between spine compartments (Figure 5D). Thus, of 11 mushroom or thin spines analyzed, the neck (10 spines) and the lower part of the head (9 spines) were most frequently labeled, whereas the base labeling was less frequent (5 spines). However, gold particles in the neck frequently formed linear clusters, possibly reflecting formation of my171
8 F. Korobova and T. Svitkina Figure 6. Cytoskeletal organization of dendritic filopodia and patches. EM of extracted 10 DIV hippocampal neurons. (A) A typical filopodium containing actin network in the base and the shaft. (B) A rare type of filopodia with a relatively well-shaped actin filament bundle, which however contains branched filaments and linear filaments of variable lengths. (C) S1 decorated filopodium. Red boxes 1 and 2 are enlarged in numbered panels at right to show actin filaments of mixed polarity. Adjacent asymmetric units of S1 decoration are highlighted in alternating red and cyan colors. The compound orange arrowheads point toward the pointed ends of filaments. (D) Filopodium stained with myosin II antibody contains gold particles (orange) distributed along the length as linear clusters (boxes) or single particles. Red boxes 3 and 4 are enlarged in panels with corresponding numbers at left to show noncolorized gold particles (arrowheads). (E) Filopodium containing prominent patches of branched actin network at the tip and in the lower shaft (arrowheads). (F and G) Dendritic patches of branched actin network with three-dimensional (F) or flattened (G) morphology. Bars, 0.5 m. osin II filaments, whereas in the head they were usually scattered as single particles (Figure 5D). Actin Cytoskeleton Organization and Cytoskeletal Components in Dendritic Filopodia The dendritic filopodia were investigated by replica EM by using 10 DIV hippocampal neurons (Figure 6). Their struc172 ture was strikingly different from that of conventional filopodia (Svitkina et al., 2003; Korobova and Svitkina, 2008), but similar to that of spine necks. It consisted of loosely aligned actin filaments of varying length with some branched filaments and occasional bundles (Figure 6, A and B). In addition, patches of a branched network were associated with filopodial shafts and even more frequently with filopodial Molecular Biology of the Cell
9 Actin Cytoskeleton in Synapses Figure 7. Molecular markers and dynamics of dendritic filopodia. (A D) Fluorescence staining of cytoskeletal components in filopodia of 7 11 DIV hippocampal neurons by phalloidin (green) and indicated antibodies (red) shown at low magnification (top, A C, left, D) or high magnification (bottom, A C, right, D). Dendritic filopodia in A C are indicated by arrowheads. Boxes 1 and 2 from D are enlarged in side panels with corresponding numbers. Unlabeled top panel shows a dendritic filopodium from another dendrite. Box 2 shows fascin-positive growth cone filopodia in contrast to dendritic filopodia not containing significant amounts of fascin. (E and F) Time-lapse phasecontrast sequences showing initiation of dendritic filopodia (arrows) from phase-dense spots (E) or small lamellipodia (F) in 10 DIV neurons. Bottom panel in E shows a colorcoded image from the top panel to emphasize the phase-dense patches (purple). Time intervals are 6 s (E) or 3 s (F). Bars, 2 m (A C), 5 m (F), and 5 m (E and F). tips producing prominent bulges there (Figure 6, A and E). The base of dendritic filopodia was similar to that of spines, both in the shape and cytoskeletal organization (Figure 6). Decoration of actin filaments with myosin S1 revealed a small fraction of actin filaments with barbed ends facing away from the tip (Figure 6C), which is unusual for conventional filopodia. Besides filopodia, patches of highly branched actin network, either relatively flat like in lamellipodia (Figure 6G), or more three-dimensional (Figure 6F), were associated with dendrites in these cultures. By immunofluorescence staining, the Arp2/3 complex and capping protein were present in 65% (N 54) and 69% (N 74), respectively, of dendritic filopodia (Figure 7, A and B) unlike the conventional filopodia, which lack these proteins (Svitkina and Borisy, 1999; Svitkina et al., 2003). Conversely, fascin was absent in dendritic filopodia, but abundant in conventional growth cone filopodia (Figure 7D). A majority of dendritic filopodia (88%; N 77) contained myosin II, especially in the proximal regions (80% of the myosin II-positive filopodia) (Figure 7C). Immunogold EM with the myosin II antibody revealed both single gold particles and their linear clusters in dendritic filopodia (Figure 6D). To understand how dendritic filopodia may be formed, we followed their dynamics in 10 DIV neuronal cultures by time-lapse phase-contrast microscopy. Filopodia usually originated from phase-dense dynamic patches or small lamellipodia associated with the dendritic shafts (Figure 7, E F, and Supplemental Videos S1 and S2). These precursors of filopodia likely correspond to the patches of branched actin filaments we noted in EM samples (Figure 6F). Newly formed, as well as some preexisting filopodia, were highly dynamic, whereas other preexisting filopodia were stable and expressed relatively little dynamics. To address a possibility that stable and dynamic filopodia may differ in the contents of key cytoskeletal components, we evaluated the amounts of F-actin and the Arp2/3 complex by fluorescence microscopy in filopodia with pre-recorded behavior. Quantification of fluorescence intensities of filopodia stained with fluorescent phalloidin and Arp2/3 complex antibodies revealed no statistically significant differences in either F-actin or Arp2/3 contents between stable and dynamic filopodia Vol. 21, January 1,
10 F. Korobova and T. Svitkina Figure 8. Model for actin cytoskeleton organization in dendritic protrusions and for spine morphogenesis. The actin cytoskeleton in dendritic patches (left), dendritic filopodia (middle), and dendritic spines (right) has similar organization consisting of a mixed network of linear and branched actin filaments (blue) anchored to microtubules (red) or actin filaments in the dendritic shaft. The process of spine formation probably begins with the formation of a dendritic patch, which then elongates into a dendritic filopodium. On receiving of appropriate signals, the filopodium undergoes maturation into a spine; this process probably involves activation of the Arp2/3 complex followed by formation of a dense branched actin network, which drives the expansion of the filopodial tip into a spine head. Presynaptic bouton forming a synapse with the spine head spine contains a similar network of actin filaments. Membrane is shown in gray. (Supplemental Figure S7), consistent with our EM data showing comparable organization of the actin cytoskeleton in the entire population of filopodia. DISCUSSION Although the important role of dendritic spines in synaptic transmission is well established, the full understanding of their biology is critically delayed by the absence of their high-resolution structure. Diverse diagrams of the actin cytoskeleton organization in spines existing in the literature rather reflect a range of speculations than actual knowledge (Halpain, 2000; Rao and Craig, 2000; Ethell and Pasquale, 2005; Tada and Sheng, 2006), but they reveal how acutely this information is needed. In this study, we have characterized the cytoskeletal organization of dendritic spines and dendritic filopodia in their entirety and at high resolution and proposed a model of spine morphogenesis driven by actin cytoskeleton remodeling (Figure 8). Dendritic Spines Out of three structural domains of a mushroom spine, the base is a previously unrecognized compartment whose structure ranges from an elaborate mixture of branched and linear filaments to a few converging linear filaments. This variability resembles a situation with basal regions of conventional filopodia, in which fraction of linear filaments increases with filopodia maturation (Svitkina et al., 2003). This similarity suggests that the spine base may be a remainder of a precursor structure, which gradually changes its organization with its age. The spine neck, unexpectedly, is supported not by an axial bundle of actin filaments, but by a longitudinally stretched network of branched and linear filaments of different lengths, which are only roughly aligned with each other. Although we cannot completely exclude a possibility that this structure is an artifact of the technique, there are several arguments against such possibility. First, by correlative EM we do not reveal significant reorganization and/or loss of 174 actin in spines. Second, the presence of Arp2/3 complex and capping protein in spine necks is also consistent with their network-like organization. Third, replica technique successfully reveals long bundled filaments in other cases (Svitkina et al., 2003; Yang et al., 2007; Korobova and Svitkina, 2008). Finally, our results do not actually conflict with previous EM data obtained by other techniques showing occasional episodes of bundling by thin section EM (Fifkova and Delay, 1982; Markham and Fifkova, 1986) or roughly aligned filaments by freeze-fracture EM (Landis and Reese, 1983; Hirokawa, 1989). Because detection of branched filaments and filament ends in sectioned or fractured samples is difficult, replica EM of whole-mount samples used here provides more interpretable images. The spine head undergoes constant actin-dependent shape changes (morphing), probably regulated by synaptic stimulation (Tada and Sheng, 2006; Bourne and Harris, 2008). Dynamic actin-dependent processes are frequently associated with branched actin networks nucleated by the Arp2/3 complex (Pollard and Borisy, 2003; Goley and Welch, 2006). Such networks were also expected to function in spine heads (see Introduction). Our data support these expectations by showing extensively branched actin network in the distal regions of spines. Although replica EM is very beneficial for revealing the cytoskeletal architecture, it is limited by necessity to remove the plasma membrane to expose the cytoskeleton. Consequently, it is not possible to determine precisely where the spine head ends. To overcome this problem, at least partially, we used immunogold labeling of N-cadherin and PSD-95 in conditions that partly preserve these plasma membrane-associated components. These data allowed us to suggest that an apparently single piece of actin network connecting a spine to the axon, in fact, consists of pre- and postsynaptic subsets. This idea is more consistent with the available data, compared with an alternative possibility of actin filaments from the spine head directly interacting with axonal microtubules. Indeed, transmembrane adhesion receptors mediating cell cell junctions usually interact through additional proteins with the actin cytoskeleton on both sides of the junction, rather than with microtubules. Furthermore, there is convincing evidence that presynaptic actin exists and is important for synaptic vesicle trafficking (Cingolani and Goda, 2008). Our data suggest that the presynaptic actin cytoskeleton consists of a branched actin network similar to that in the spine head and is also associated with microtubules. In adaptive dynamic systems, like dendritic spines, protrusion and retraction cooperate in generating a proper shape, which may explain the presence of myosin II in spines (Morales and Fifkova, 1989; Ryu et al., 2006). We found that myosin II is biased toward the neck and proximal regions of the head, suggesting that contraction mainly occurs there. Filamentous myosin II in spines suggests the presence actin filaments of mixed polarity. However, we could not obtain enough filaments with interpretable polarity to test this prediction. Because the quality of decoration was good elsewhere, such as in growth cones, we assume that actin filaments in spines are overloaded with other actin-binding proteins preventing filament saturation with S1, as required for polarity determination. Dendritic Filopodia and Spine Morphogenesis Dendritic filopodia can transform into morphologically mature spines (Yoshihara et al., 2009). Their structure remained totally unknown but was assumed to resemble that of conventional filopodia. Strikingly, we found that dendritic Molecular Biology of the Cell
11 Actin Cytoskeleton in Synapses filopodia had network-like cytoskeletal organization, which is unusual for highly elongated membrane protrusions, where a tight actin filament bundle is considered to be obligatory (Chhabra and Higgs, 2007). However, we have shown recently that bundled actin filaments are dispensable for maintaining filopodia induced by the membrane-deforming I-BAR domain of IRSp53, although polymerized actin is required (Yang et al., 2009). Dendritic filopodia provide a naturally occurring example of this kind. The network-like organization of dendritic filopodia probably makes them more plastic allowing for frequent changes of direction (Portera-Cailliau et al., 2003). The structural organization of dendritic filopodia suggests potential mechanisms of their differentiation into spines. By live imaging, the filopodium-to-spine transformation occurs as swelling of the filopodial tip and shortening (Marrs et al., 2001). The swelling may occur through Arp2/3 complexdependent actin filament branching at the filopodial tip, which would drive the head expansion. Filopodia with sizable patches of branched actin network at the tip may represent transitional states in this process. The filopodia shortening during spine maturation was proposed to involve myosin II (Ryu et al., 2006). We indeed detected myosin II in the shafts of dendritic filopodia and also observed actin filaments of mixed polarity there. Both features are quite unusual for conventional filopodia, which are devoid of myosin II and have uniformly oriented filaments, but are consistent with contractile properties of dendritic filopodia. Another important question is the origin of dendritic filopodia. By light microscopy, they seem to grow directly from a dendrite (Dailey and Smith, 1996), usually from pre-existing actin patches (Andersen et al., 2005) or, in our experiments, from phase-dense spots or small lamellipodia. These sites of filopodia initiation likely correspond to patches of a branched actin network in dendrites, which may subsequently become the filopodial base, thus explaining the presence of branched filaments there. These branched filaments may be responsible for incorporation of labeled actin into roots of dendritic filopodia (Hotulainen et al., 2009). The mechanism of transformation of a relatively isometric patch into an elongated protrusion remains unclear. One possibility is contribution of membrane-deforming proteins, such as IRSp53 (Choi et al., 2005; Mattila and Lappalainen, 2008), which can induce and support tubular membrane protrusions (Saarikangas et al., 2009; Yang et al., 2009). Enhanced polymerization of actin filaments assisted by formin mdia2 may also contribute to this process (Hotulainen et al., 2009). Surprisingly, the actin structures in neurons frequently reside directly on the microtubule array. Moreover, some actin filaments seem to branch off of a microtubule in the neurite suggesting involvement of a microtubule-associated actin filament nucleator(s) or actin microtubule cross-linkers, which remain to be identified. In conclusion, the cytoskeletal organization and molecular composition of dendritic spines and dendritic filopodia suggest a likely sequence of cytoskeletal reorganizations underlying the spine morphogenesis (Figure 8). We propose that the process begins with the formation of a small patch of branched actin network, which might be nucleated in association with dendritic microtubules. The patch subsequently elongates into a dendritic filopodium, which due to plastic networklike organization of its cytoskeleton is able to perform a wide range of movements searching for an axon. When an appropriate signal is received, it triggers the head formation by inducing extensive branching of actin filaments. Myosin II-dependent contractility within the head and the neck then modulates the shape of the spine to fit the requirements of synaptic transmission. This model provides a conceptual framework for future studies which would uncover structural reorganizations of the cytoskeleton occurring at different stages of synapse morphogenesis and specific roles of individual proteins in these processes. ACKNOWLEDGMENTS We thank Drs. M. A. Dichter, D. A. Schafer, W. J. Nelson, and L. G. Tilney for generous gifts of reagents and cells and D. Zinshteyn for technical assistance. This work is supported by National Institutes of Health grants GM and RR (to T. S.). REFERENCES Ackermann, M., and Matus, A. (2003). Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat. Neurosci. 6, Andersen, R., Li, Y., Resseguie, M., and Brenman, J. E. (2005). Calcium/ calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J. Neurosci. 25, Arellano, J. I., Benavides-Piccione, R., Defelipe, J., and Yuste, R. (2007). Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front. Neurosci. 1, Bourne, J. N., and Harris, K. M. (2008). Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 31, Caceres, A., Banker, G., Steward, O., Binder, L., and Payne, M. (1984). MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 315, Calabrese, B., Wilson, M. S., and Halpain, S. (2006). Development and regulation of dendritic spine synapses. Physiology 21, Cheng, X. T., Hayashi, K., and Shirao, T. (2000). Non-muscle myosin IIB-like immunoreactivity is present at the drebrin-binding cytoskeleton in neurons. Neurosci. Res. 36, Chhabra, E. S., and Higgs, H. N. (2007). The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, Choi, J., Ko, J., Racz, B., Burette, A., Lee, J. R., Kim, S., Na, M., Lee, H. W., Kim, K., Weinberg, R. J., and Kim, E. (2005). Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J. Neurosci. 25, Cingolani, L. A., and Goda, Y. (2008). Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, Dailey, M. E., and Smith, S. J. (1996). The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, Ethell, I. M., and Pasquale, E. B. (2005). Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 75, Fifkova, E., and Delay, R. J. (1982). Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J. Cell Biol. 95, Goley, E. D., and Welch, M. D. (2006). The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, Halpain, S. (2000). Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci. 23, Hering, H., and Sheng, M. (2003). Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 23, Hirokawa, N. (1989). The arrangement of actin filaments in the postsynaptic cytoplasm of the cerebellar cortex revealed by quick-freeze deep-etch electron microscopy. Neurosci. Res. 6, Honkura, N., Matsuzaki, M., Noguchi, J., Ellis-Davies, G. C., and Kasai, H. (2008). The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 57, Hotulainen, P., Llano, O., Smirnov, S., Tanhuanpaa, K., Faix, J., Rivera, C., and Lappalainen, P. (2009). Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 185, Kim, Y., et al. (2006). Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442, Vol. 21, January 1,
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity
 Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
Assembly of synapses by neuronal adhesion molecules: single molecule studies
 Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
Supporting Online Material Material and Methods
 Supporting Online Material Material and Methods Live-cell DIC recordings PtK 1 cells (ATCC, Manassas, VA) were filmed on a Nikon (Nikon Instruments, Melville, NY) inverted microscope equipped with 60x
Supporting Online Material Material and Methods Live-cell DIC recordings PtK 1 cells (ATCC, Manassas, VA) were filmed on a Nikon (Nikon Instruments, Melville, NY) inverted microscope equipped with 60x
Functions of Nonmuscle Myosin II in Assembly of the Cellular Contractile System
 Functions of Nonmuscle Myosin II in Assembly of the Cellular Contractile System Maria Shutova 1,2, Changsong Yang 1, Jury M. Vasiliev 2, Tatyana Svitkina 1 * 1 Department of Biology, University of Pennsylvania,
Functions of Nonmuscle Myosin II in Assembly of the Cellular Contractile System Maria Shutova 1,2, Changsong Yang 1, Jury M. Vasiliev 2, Tatyana Svitkina 1 * 1 Department of Biology, University of Pennsylvania,
ANAT Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales. UNSW Copyright Notice
 ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Actin Capping Protein Is Required for Dendritic Spine Development and Synapse Formation
 10228 The Journal of Neuroscience, July 13, 2011 31(28):10228 10233 Brief Communications Actin Capping Protein Is Required for Dendritic Spine Development and Synapse Formation Yanjie Fan 1, Xin Tang 2,
10228 The Journal of Neuroscience, July 13, 2011 31(28):10228 10233 Brief Communications Actin Capping Protein Is Required for Dendritic Spine Development and Synapse Formation Yanjie Fan 1, Xin Tang 2,
DCLK-immunopositive. Bars, 100 µm for B, 50 µm for C.
 Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplementary Information
 Supplementary Information Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction Hideto Takahashi 1, Kei-ichi Katayama 2, Kazuhiro Sohya 3,4, Hiroyuki Miyamoto 4,5,
Supplementary Information Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction Hideto Takahashi 1, Kei-ichi Katayama 2, Kazuhiro Sohya 3,4, Hiroyuki Miyamoto 4,5,
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Chapter 15. Cytoskeletal Systems. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Moutin et al., http://www.jcb.org/cgi/content/full/jcb.201110101/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Tagged Homer1a and Homer are functional and display different
Supplemental material Moutin et al., http://www.jcb.org/cgi/content/full/jcb.201110101/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Tagged Homer1a and Homer are functional and display different
Practice Exam 3 MCBII
 1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Filopodial Initiation and a Novel Filament-organizing Center, the Focal Ring
 Molecular Biology of the Cell Vol. 12, 2378 2395, August 2001 Filopodial Initiation and a Novel Filament-organizing Center, the Focal Ring Michael Steketee, Kenneth Balazovich, and Kathryn W. Tosney* Department
Molecular Biology of the Cell Vol. 12, 2378 2395, August 2001 Filopodial Initiation and a Novel Filament-organizing Center, the Focal Ring Michael Steketee, Kenneth Balazovich, and Kathryn W. Tosney* Department
COPYRIGHTED MATERIAL. Tissue Preparation and Microscopy. General Concepts. Chemical Fixation CHAPTER 1
 CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
Polarization of actin cytoskeleton is reduced in dendritic protrusions during early spine development in hippocampal neuron
 MBoC ARTICLE Polarization of actin cytoskeleton is reduced in dendritic protrusions during early spine development in hippocampal neuron Vedakumar Tatavarty*, Sulagna Das*, and Ji Yu Center for Cell Analysis
MBoC ARTICLE Polarization of actin cytoskeleton is reduced in dendritic protrusions during early spine development in hippocampal neuron Vedakumar Tatavarty*, Sulagna Das*, and Ji Yu Center for Cell Analysis
Immunofluorescence and phalloidin labeling of mammalian cells
 Immunofluorescence and phalloidin labeling of mammalian cells 2 Contents Materials for immunofluorescence and phalloidin labeling of mammalian cells...1 Immunofluorescence-labelling on cultivated adherent
Immunofluorescence and phalloidin labeling of mammalian cells 2 Contents Materials for immunofluorescence and phalloidin labeling of mammalian cells...1 Immunofluorescence-labelling on cultivated adherent
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
by Neurobasal medium (supplemented with B27, 0.5mM glutamine, and 100 U/mL
 Supplementary Materials and methods Neuronal cultures and transfection The hippocampus was dissected from E8 rat embryos, dissociated, and neurons plated onto glass coverslips coated with poly-ornithine
Supplementary Materials and methods Neuronal cultures and transfection The hippocampus was dissected from E8 rat embryos, dissociated, and neurons plated onto glass coverslips coated with poly-ornithine
2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non
 Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Immuno-Labelling Cryosections
 Thin sections of biological material, mounted on nickel or gold grids, can be labelled by floating them, section-side down, on small, 10 µl, droplets of antibody. This process is conveniently carried out
Thin sections of biological material, mounted on nickel or gold grids, can be labelled by floating them, section-side down, on small, 10 µl, droplets of antibody. This process is conveniently carried out
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Serine phosphorylation of ephrinb2 regulates trafficking of synaptic
 Serine phosphorylation of ephrinb2 regulates trafficking of synaptic AMPA receptors Clara L. Essmann, Elsa Martinez, Julia C. Geiger, Manuel Zimmer, Matthias H. Traut, Valentin Stein, Rüdiger Klein and
Serine phosphorylation of ephrinb2 regulates trafficking of synaptic AMPA receptors Clara L. Essmann, Elsa Martinez, Julia C. Geiger, Manuel Zimmer, Matthias H. Traut, Valentin Stein, Rüdiger Klein and
Supplementary Information:
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2015 Supplementary Information: 3D Printed Nervous System on a Chip Blake N. Johnson, a,b Karen
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2015 Supplementary Information: 3D Printed Nervous System on a Chip Blake N. Johnson, a,b Karen
Supplementary Figure 1. Pathologically phosphorylated Tau is localized to the presynaptic terminals of Alzheimer s disease (AD) patient brains.
 Supplementary Figure 1. Pathologically phosphorylated Tau is localized to the presynaptic terminals of Alzheimer s disease (AD) patient brains. Ultrathin (70 nm) sections of healthy control or AD patient
Supplementary Figure 1. Pathologically phosphorylated Tau is localized to the presynaptic terminals of Alzheimer s disease (AD) patient brains. Ultrathin (70 nm) sections of healthy control or AD patient
Nature Structural & Molecular Biology: doi: /nsmb.2996
 Supplementary Figure 1 Negative-stain EM of native cytoplasmic dynein. (a) A representative micrograph of negatively stained dynein, with the flexible dimeric molecules circled in white. (b) Reference-free
Supplementary Figure 1 Negative-stain EM of native cytoplasmic dynein. (a) A representative micrograph of negatively stained dynein, with the flexible dimeric molecules circled in white. (b) Reference-free
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
THE MAGNITUDE OF THE EXTRACELLULAR SPACE IN ELECTRON MICROGRAPHS OF SUPERFICIAL AND DEEP REGIONS OF THE CEREBRAL CORTEX
 J. Cell Sci. 6, 793-805 (1970) 793 Printed in Great Britain THE MAGNITUDE OF THE EXTRACELLULAR SPACE IN ELECTRON MICROGRAPHS OF SUPERFICIAL AND DEEP REGIONS OF THE CEREBRAL CORTEX A. VAN HARREVELD AND
J. Cell Sci. 6, 793-805 (1970) 793 Printed in Great Britain THE MAGNITUDE OF THE EXTRACELLULAR SPACE IN ELECTRON MICROGRAPHS OF SUPERFICIAL AND DEEP REGIONS OF THE CEREBRAL CORTEX A. VAN HARREVELD AND
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
-RT RT RT -RT XBMPRII
 Base pairs 2000 1650 1000 850 600 500 400 Marker Primer set 1 Primer set 2 -RT RT RT -RT XBMPRII kda 100 75 50 37 25 20 XAC p-xac A 300 B Supplemental figure 1. PCR analysis of BMPRII expression and western
Base pairs 2000 1650 1000 850 600 500 400 Marker Primer set 1 Primer set 2 -RT RT RT -RT XBMPRII kda 100 75 50 37 25 20 XAC p-xac A 300 B Supplemental figure 1. PCR analysis of BMPRII expression and western
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Superresolution Pattern Recognition Reveals the Architectural Map of the
 Supplementary Information Superresolution Pattern Recognition Reveals the Architectural Map of the Ciliary Transition Zone T. Tony Yang a, Jimmy Su b, Won-Jing Wang c, Branch Craige d, George B. Witman
Supplementary Information Superresolution Pattern Recognition Reveals the Architectural Map of the Ciliary Transition Zone T. Tony Yang a, Jimmy Su b, Won-Jing Wang c, Branch Craige d, George B. Witman
Supplemental Figure 1 Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical
 Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced
 Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Kanaani et al., http://www.jcb.org/cgi/content/full/jcb.200912101/dc1 Figure S1. The K2 rabbit polyclonal antibody is specific for GAD67,
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Kanaani et al., http://www.jcb.org/cgi/content/full/jcb.200912101/dc1 Figure S1. The K2 rabbit polyclonal antibody is specific for GAD67,
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier
 Supporting Information In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier Yang Song, Dan Du, Lei Li, Jun Xu, Prashanta Dutta *,, and Yuehe Lin *, School of Mechanical
Supporting Information In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier Yang Song, Dan Du, Lei Li, Jun Xu, Prashanta Dutta *,, and Yuehe Lin *, School of Mechanical
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and
 Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Modeling cytoskeleton self-assembly
 Modeling cytoskeleton self-assembly Dimitrios Vavylonis Department of Physics, Lehigh University BIOS 10 Lehigh University Oct 30, 2009 Cell organization How does the cell achieve internal organization?
Modeling cytoskeleton self-assembly Dimitrios Vavylonis Department of Physics, Lehigh University BIOS 10 Lehigh University Oct 30, 2009 Cell organization How does the cell achieve internal organization?
Supplementary Figure 1. Espn-1 knockout characterization. (a) The predicted recombinant Espn-1 -/- allele was detected by PCR of the left (5 )
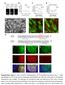 Supplementary Figure 1. Espn-1 knockout characterization. (a) The predicted recombinant Espn-1 -/- allele was detected by PCR of the left (5 ) homologous recombination arm (left) and of the right (3 )
Supplementary Figure 1. Espn-1 knockout characterization. (a) The predicted recombinant Espn-1 -/- allele was detected by PCR of the left (5 ) homologous recombination arm (left) and of the right (3 )
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Prospéri et al.,
 Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Ultra small Immunogold labeling. Optimizing Signal-noise ratios.
 Ultra small Immunogold labeling & Optimizing Signal-noise ratios. Jan L.M. Leunissen and Hong Yi * Aurion Immuno Gold Reagents, Costerweg 5, 6702 AA Wageningen, The Netherlands Dept Anatomy & Structural
Ultra small Immunogold labeling & Optimizing Signal-noise ratios. Jan L.M. Leunissen and Hong Yi * Aurion Immuno Gold Reagents, Costerweg 5, 6702 AA Wageningen, The Netherlands Dept Anatomy & Structural
T H E J O U R N A L O F C E L L B I O L O G Y
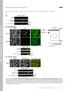 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Supplementary Information. Tau co-organizes dynamic microtubule and actin networks. and Isabelle Arnal 1,2,*
 Supplementary Information Tau co-organizes dynamic microtubule and actin networks Auréliane Elie 1,2, Elea Prezel 1,2, Christophe Guérin 3, Eric Denarier 1,2,4, Sacnicte Ramirez- Rios 1,2, Laurence Serre
Supplementary Information Tau co-organizes dynamic microtubule and actin networks Auréliane Elie 1,2, Elea Prezel 1,2, Christophe Guérin 3, Eric Denarier 1,2,4, Sacnicte Ramirez- Rios 1,2, Laurence Serre
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 PCR-genotyping of the three mouse models used in this study and controls for behavioral experiments after semi-chronic Pten inhibition. a-c. DNA from App/Psen1 (a), Pten tg (b) and
Supplementary Figure 1 PCR-genotyping of the three mouse models used in this study and controls for behavioral experiments after semi-chronic Pten inhibition. a-c. DNA from App/Psen1 (a), Pten tg (b) and
The definitive version is available at www3.intersci. Supporting_Information.pdf (Supporting Information) Instructions for use
 Title Nonmuscle myosin II folds into a 10S form via two po Kiboku, Takayuki; Katoh, Tsuyoshi; Nakamura, Akio; K Author(s) Masayuki CitationGenes to Cells, 18(2): 90-109 Issue Date 2013-02 Doc URL http://hdl.handle.net/2115/53054
Title Nonmuscle myosin II folds into a 10S form via two po Kiboku, Takayuki; Katoh, Tsuyoshi; Nakamura, Akio; K Author(s) Masayuki CitationGenes to Cells, 18(2): 90-109 Issue Date 2013-02 Doc URL http://hdl.handle.net/2115/53054
ANAT Cell Biology Lecture 12 School of Medical Sciences The University of New South Wales
 ANAT3231 - Cell Biology Lecture 12 School of Medical Sciences The University of New South Wales Prof Peter Gunning Microfilaments, 2010 Sllide 1 UNSW Copyright Notice COMMONWEALTH OF AUSTRALIA Copyright
ANAT3231 - Cell Biology Lecture 12 School of Medical Sciences The University of New South Wales Prof Peter Gunning Microfilaments, 2010 Sllide 1 UNSW Copyright Notice COMMONWEALTH OF AUSTRALIA Copyright
The cytoskeletal system
 The cytoskeletal system Types, polymerization, characteristics and mechanical properties of cytoskeletal filaments. Associated protein. 24.09.2013. Cytoskeleton The cellular "scaffold" or "skeleton" within
The cytoskeletal system Types, polymerization, characteristics and mechanical properties of cytoskeletal filaments. Associated protein. 24.09.2013. Cytoskeleton The cellular "scaffold" or "skeleton" within
F- actin Dynamics in Dendritic Spines
 F- actin Dynamics in Dendritic Spines Mikko Koskinen Dissertationes Scholae Doctoralis Ad Sanitatem Investigandam Universitatis Helsinkiensis Neuroscience Center And Division of Physiology and Neuroscience
F- actin Dynamics in Dendritic Spines Mikko Koskinen Dissertationes Scholae Doctoralis Ad Sanitatem Investigandam Universitatis Helsinkiensis Neuroscience Center And Division of Physiology and Neuroscience
Nature Methods: doi: /nmeth Supplementary Figure 1. Retention of RNA with LabelX.
 Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0
 Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0 Table of Contents General ICC Protocol 3 Synaptic Marker ICC Protocol 5 Recommended Markers 6 Technical Support 7 2 General
Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0 Table of Contents General ICC Protocol 3 Synaptic Marker ICC Protocol 5 Recommended Markers 6 Technical Support 7 2 General
T H E J O U R N A L O F C E L L B I O L O G Y
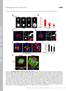 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true?
 Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true? A. COPII and COPI vesicles are transported with dynein. B. COPII and COPI vesicles
Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true? A. COPII and COPI vesicles are transported with dynein. B. COPII and COPI vesicles
The Dot-Spot Test. a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition. Aurion Newsletter 4
 Aurion Newsletter 4 The Dot-Spot Test a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition Peter F.E.M. van de Plas AURION Costerweg 5 6702 AA Wageningen The
Aurion Newsletter 4 The Dot-Spot Test a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition Peter F.E.M. van de Plas AURION Costerweg 5 6702 AA Wageningen The
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
SUPPLEMENTARY INFORMATION
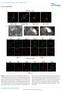 DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Supporting Information
 Supporting Information Shao et al. 10.1073/pnas.1504837112 SI Materials and Methods Immunofluorescence and Immunoblotting. For immunofluorescence, cells were fixed with 4% paraformaldehyde and permeabilized
Supporting Information Shao et al. 10.1073/pnas.1504837112 SI Materials and Methods Immunofluorescence and Immunoblotting. For immunofluorescence, cells were fixed with 4% paraformaldehyde and permeabilized
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
CENTER FOR BRAIN EXPERIMENT
 CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Guidance of microtubule growth and organization by F-actin
 Chapter 4 Guidance of microtubule growth and organization by F-actin In this chapter we study interactions between dynamic microtubules and actin filament bundles. We find that microtubule growth can be
Chapter 4 Guidance of microtubule growth and organization by F-actin In this chapter we study interactions between dynamic microtubules and actin filament bundles. We find that microtubule growth can be
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
ab CFSE Fluorescent Cell Labeling Kit
 ab113853 CFSE Fluorescent Cell Labeling Kit Instructions for Use For the durable fluorescent labeling of live cells for fluorescent microscopy and flow cytometry, population growth studies and within sample
ab113853 CFSE Fluorescent Cell Labeling Kit Instructions for Use For the durable fluorescent labeling of live cells for fluorescent microscopy and flow cytometry, population growth studies and within sample
1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called.
 Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Biophysics of Molecules
 Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
CELL BIOLOGY - CLUTCH CH CYTOSKELETON AND CELL MOVEMENT.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
!! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA. Figure Legends
 Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA Figure Legends Suppl. Fig. S1. Antigenic determinants of the Panx2 antibodies employed in this study. (A) Schematic of mouse Panx2
Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA Figure Legends Suppl. Fig. S1. Antigenic determinants of the Panx2 antibodies employed in this study. (A) Schematic of mouse Panx2
Supplemental Information Control of apico-basal epithelial polarity by the microtubule minus-end binding protein CAMSAP3 and spectraplakin ACF7
 Supplemental Information Control of apico-basal epithelial polarity by the microtubule minus-end binding protein CAMSAP3 and spectraplakin ACF7 Ivar Noordstra, Qingyang Liu, Wilco Nijenhuis, Shasha Hua,
Supplemental Information Control of apico-basal epithelial polarity by the microtubule minus-end binding protein CAMSAP3 and spectraplakin ACF7 Ivar Noordstra, Qingyang Liu, Wilco Nijenhuis, Shasha Hua,
Development Team. Molecular Cell Biology Cytoskeleton ZOOLOGY. Head, Department of Zoology, University of Delhi
 Paper No. : 15 Module : 11 Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Paper Coordinator: Prof. S. Basu Modak Department of Zoology, University
Paper No. : 15 Module : 11 Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Paper Coordinator: Prof. S. Basu Modak Department of Zoology, University
ab CytoPainter ER Staining Kit Red Fluorescence
 ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in
 Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
