Supporting Information
|
|
|
- Arlene Sutton
- 6 years ago
- Views:
Transcription
1 Supporting Information Shao et al /pnas SI Materials and Methods Immunofluorescence and Immunoblotting. For immunofluorescence, cells were fixed with 4% paraformaldehyde and permeabilized using either 0.2% Triton X-100 at room temperature or 0.003% digitinon on ice (1). Cells were then blocked with 3% BSA in PBS for 1 h followed by primary and secondary antibody staining for 1 h each. F-actin was labeled by TRITC or Alexa-488 conjugated phalloidin (Life Technologies) for min after permeabilization. Antibodies for immunofluorescence were rabbit polyclonal anti-filamin A (SAB ; Sigma), mouse antinesprin 2 (gift from Brian Burke), rabbit polyclonal anti-inf2 ( AP; Proteintech), rabbit polyclonal anticofilin (ab11062; Abcam), mouse anti- Nup153 (gift from Brian Burke), nonmuscle myosin heavy-chain II-A polyclonal antibody (PRB-440P; Covance), nonmuscle myosin heavy-chain II-B polyclonal antibody (PRB-445P; Covance), and secondary antibody (Alexa Fluor-405, -488, -568, and -647; Life Technologies). For the G-actin incorporation assay, 0.4 μm Alexa-568 labeled G-actin (Life Technologies) was added into cytoskeletal stabilizing buffer (containing either CaCl 2 or EGTA) supplemented with 0.003% digitonin (2). NIH 3T3 cells were permeabilized using this buffer for 7 min at room temperature. Cells were then washed with cytoskeletal stabilizing buffer and fixed in 4% paraformaldehyde. For immunoblotting, transfected NIH 3T3 cells were lysed in 1 SDS loading buffer. Samples were then subjected to sonication and run in SDS/PAGE gels. Proteins were transferred to PVDF membranes at 100 V for 1.5 h and blocked for 30 min with 5% low-fat milk before the addition of primary antibodies. Primary antibodies were incubated for either 1 h at room temperature or overnight at 4 C. After washing, the membrane was incubated with HRP-conjugated secondary antibodies for 20 min. Bound antibodies were detected by HRP Chemiluminescent Reagent (Thermo Scientific). Antibodies for immunoblotting were mouse monoclonal anti α-tubulin (T6199; Sigma), rabbit polyclonal anti-inf2 ( AP; Proteintech), and rabbit polyclonal anticofilin (ab11062; Abcam). Microscopy and Data Analysis. Confocal image acquisition and fluorescence recovery after photobleaching (FRAP) were done on a Nikon A1R Confocal System using 60 or 100 oil-immersion objectives (N.A. 1.4). In FRAP, cells transfected with EGFPβ-actin were plated on fibronectin-coated rectangular micropatterns. Three rectangular regions of interest (ROIs) were selected within the perinuclear region, cell periphery, and their midpoint. Photobleaching with maximum laser power (488 nm) was applied to all ROIs for 10 s simultaneously. Time-lapse images before and after photobleaching were acquired every 2 s. For perinuclear actin analysis, images of a single confocal section at or near the maximum nuclear projected area were chosen. The confocal section is 500 nm in thickness. A rectangular ROI (10 30 μm 2 ) was drawn at the perinuclear area, which covers part of the nuclear rim and part of the perinuclear cytoplasmic region. In experiments stimulated by force, the ROI was put distal from the site of force application. This way of measuring perinuclear actin has been calibrated by three other methods as shown in Fig. S2 A and B. The analysis was done using the MATLAB Image Processing Toolbox by thresholding ER and nuclear intensity. To measure the peripheral actin intensity during force application, EGFP-Lifeact transfected cells were plated on fibronectin-coated rectangular micropatterns (1,800 μm 2 ; aspect ratio of 1:5). Peripheral actin is measured within a 15 5-μm ROI at the cell periphery, where ER is undetectable based on the sum of all Z sections. Fluorescence images, except for perinuclear actin calibration, were analyzed using the Fiji Image Processing Package ImageJ. The color-coded images in Figs. 2 and 3 are shown by fire scale to indicate the level of fluorescence intensity. All data were normalized by dividing the value before force application or A23187 treatment. Analyses of significant differences were carried out using two-tailed unpaired Student s t test. Data with error bars present means ± SEM. Mathematical Modeling. We use the following coarse-grained model to describe the bulk of actin in the cell. We simulate three actin densities: the G-actin density, GðtÞ, and two F-actin densities, F N ðtþ and F P ðtþ. The first density, F N ðtþ, describes actin filaments associated with INF2 near the nucleus, and the second one, F P ðtþ, describes other peripheral filaments associated with other formins and branching/nucleating agents, like Arp2/3 complexes. We track the densities as functions of time t without describing the spatial distributions and transport of actin. The justification for this is that characteristic diffusion time is the distance between the edge of the nucleus and edge of the cell squared divided by the actin monomer diffusion coefficient: ð20 μmþ 2 =ð20 μm 2 =sþ 20 s. This time is shorter than the characteristic timescale on the order of 100 s for the transient change of actin in response to the calcium peak. Furthermore, we do not model tens of reactions of actin species with actin binding proteins and nucleotide exchange, clumping all of these reactions into simple first-order reactions of transitioning between the three states of actin. Equations describing three actin densities have the form df N = k N d dt ðtþf N + k N a ðgþg df P = k P d dt ðtþf P + k P a G dg dt = kn d ðtþf N + k P d ðtþf P k N a ðgþg. [S1] The meanings of the terms on the right sides can be gleaned from the scheme above. The rates of F-actin assembly are proportional to the G-actin density; we assume that the polymerizable fraction of actin monomers is simply proportional to the total G-actin density; k P a is the constant proportionality coefficient in the expression for the peripheral actin rate of assembly. Similarly, k N a ðgþ is the proportionality coefficient in the expression for the perinuclear actin rate of assembly, which depends on the monomer density; k N d ðtþ and kp dðtþ are the rates of disassembly of the perinuclear and peripheral F-actin, respectively, which are functions 1of16
2 of time because of the transient calcium peak. If the steady-state levels of the three actin densities before the perturbation are F N, F P, and G, then we can introduce the normalized densities f N = F N =F N, f P = F P =F N, and g = G=F N, respectively. Furthermore, based on the FRAP data (Fig. S8 A and B), we use the characteristic turnover time for the peripheral actin network T 50 s as the timescale and introduce the nondimensionalized time variable τ = t=t. (Specifically, we used the FRAP data reported in Table S3, fitted the recovery data to exponential functions, and found that the fit is good if the time constant T 50 s.) In the nondimensional form, the dynamic equations are df N dτ = r Nð cðτþf N + αðgþgþ df P dτ = r Pð cðτþf P + sgþ dg dτ = r NðcðτÞf N αðgþgþ + r P ðcðτþf P sgþ. [S2] Here, factors r P and r N account for acceleration of the actin network assembly when calcium transient effectively increases disassembly caused by the increase of the nascent barbed ends, which is one of the consequences of breaking actin filaments into smaller filaments. In the simulations, we use r P = 2, and considering that the FRAP data show that the perinuclear actin turns over 1.5 times faster than the peripheral actin, r N = 3. Parameter s accounts for how much of the total amount of actin not associated with INF2 in the cell is greater than that associated with INF2. Nonrigorous analysis of the micrographs suggests that this ratio is close to one order of magnitude; in the simulations, we use s = 5. From the FRAP data reported in Table S3, the G-actin concentration is about 20% of the total F-actin concentration. Therefore, assuming s = 5, the total amounts of the perinuclear F- and G-actin are similar in the steady state, and in the calculations, we use equal amounts of the total perinuclear F- and G-actin. Function cðtþ describes the increase of the disassembly rate over the baseline caused by the calcium transient. We assume that this function is proportional to the calcium concentration. We fitted the calcium concentration as a function of time observed in this study with the analytical function: p cðτþ = ffiffi τ exp τ 2. [S3] Finally, function αðgþ describes the dependence of the INF2- mediated actin assembly rate on the G-actin concentration. We use the following step-like function: αðgþ = 1 + exp½hðg g 0ÞŠ ð1 + exp½hðg g 0 ÞŠÞ. [S4] Effectively, we assume that, when the G-actin density increases 1.5-fold relative to the baseline (g 0 = 1.5), the respective assembly rate goes up two times. Coefficient h = 10 describes the steepness of the threshold effect. We solve Eqs. S2 S4 numerically and obtain the solutions reported in Fig. S8C. To test another model that INF2 formins are activated by calcium more directly and not through the effect on the F-actin disassembly and the consequent activation of the formins by G-actin we implement the following changes in the model: we keep the F-actin disassembly rate constants k N d ðtþ = const and kp dðtþ = const, and we make the perinuclear actin assembly rate a function of not G-actin but time, assuming that the perinuclear actin assembly rate is proportional to the calcium concentration. This assumption makes the rate k N d cðτþ = p ffiffi τ expð τ 2 Þ.Themodelhasthe dimensional form and the nondimensional form df N = k N d dt F N + k N a ðtþg df P = k P d dt F P + k P a G dg dt = kn d F N + k P d F P k N a ðtþg df N dτ = r Nð f N + cðτþgþ df P dτ = r Pð f P + scðτþgþ dg dτ = r Nðf N cðτþgþ + r P ðf P cðτþgþ. [S5] [S6] We solve Eqs. S3, S5, and S6 numerically and obtain the solutions reported in Fig. S8D. 1. Crisp M, et al. (2006) Coupling of the nucleus and cytoplasm: Role of the LINC complex. J Cell Biol 172(1): Hirata H, Tatsumi H, Sokabe M (2008) Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci 121(Pt 17): of16
3 A B C Nomalized Perinuclear Intensity Fig. S1. (A) F-actin imaging at the focal plane corresponding to (Upper) the maximal nuclear projection area and (Lower) z-section views of a cell labeled by EGFP-Lifeact (Left) before and (Right) after force application. Red and white arrows indicate perinuclear actin and peripheral actin, respectively. (B) EGFP- Lifeact and phase-contrast images of the cell (Top) before and (Middle and Bottom) after force application. In Bottom Left, a phalloidin-labeled perinuclear F-actin structure (red) in the same cell is shown after being subjected to immediate fixation on force application. (Bottom Right) This structure colocalizes with EGFP-Lifeact as seen in the merged image. (C, Left) EGFP-α-actinin and (C, Center) phase-contrast images of a cell (Upper) before and (Lower) after force application and (C, Right) a plot of normalized perinuclear α-actinin intensity over time. (Scale bars: 10 μm.) 3of16
4 A B C Nomalized Perinuclear Actin Intensity D E ROI rim ER ER-rim Nomalized F-actin Intensity Perinuclear Peripheral Fig. S2. (A) Example of perinuclear actin measurement by four different methods. Single-section images of a cell labeled by EGFP-Lifeact, DsRed-ER, and Hoechst as well as the merged image are shown in Upper. Images were analyzed using MATLAB to create four masks: total ER, nuclear rim, ER exclusive of the nuclear rim (ER-rim), and a manually drawn perinuclear ROI. The white-colored regions in Lower indicate the masks of each method, under which the mean actin intensity was measured over time. (B) Plots of perinuclear actin intensity on force application obtained from 20 cells and the four methods of measurement as described in A. Data are given as means ± SEM. (C) z-projection images of a cell transfected with EGFP-Lifeact and DsRed-ER and the merged image. Actin at the narrow periphery of cell where ER is undetectable, as cropped by the rectangular boxes, is referred to as peripheral actin. (D, Left) Z-projection and (D, Center) single-section images of EGFP-Lifeact labeled cell plated on fibronectin-coated rectangular substrate (1,800 μm 2 ); regions that indicate peripheral ( 15 5 μm) and perinuclear ( μm) F-actin subjected to the intensity measurement are shown by rectangular boxes, respectively. (D, Right) Micrographs of peripheral actin on force application are shown at 10-s time intervals. (E) Perinuclear and peripheral F-actin intensity change on force application. Peripheral F-actin intensity is measured based on all Z stacks of the Lifeact images, whereas perinuclear F-actin intensity is measured on a single section with the maximal nuclear projection area. Data are given as means ± SEM of 20 cells. (Scale bar: 10 μm.) 4of16
5 Fig. S3. (A, Left) EGFP-Lifeact and (A, Right) phase-contrast images of a cell growing on poly-l-lysine coated substrate (Upper) before and (Lower) after force application. The white arrow indicates the actin rim around the nucleus (N). (B) Cells labeled by EGFP-Lifeact and pretreated with inhibitors of focal adhesion kinase (FAK; PF-562,271; 10 μm; Upper) before and (Lower) after force application. (C) Digitonin-permeabilized cells labeled by Alexa-568 actin in (Left) Ca 2+ - containing and (Right) Ca 2+ -free buffers. (D) Intensity of Alexa-568 actin at the perinuclear rim in cells incubated with Ca 2+ -containing and Ca 2+ -free buffers. Data are normalized by the total Alexa-568 actin intensity of each image. More than 10 cells were used for the measurements in each group. Data are presented as means ± SEM. *P < (Scale bars: 10 μm.) 5of16
6 A B Fig. S4. (A) Fluorescence staining of Nup153 (blue), a nuclear pore complex component localized at the inner nuclear membrane, and F-actin (phalloidin; red) in EGFP-Lifeact (green) transfected cells permeabilized by either (Upper) digitonin or (Lower) Triton X on immediate fixation after A23187 treatment. Note that digitonin does not permeabilize the nuclear membranes. (B) Fluorescence staining of (Upper) myosin IIA and (Lower) myosin IIB together with F-actin (phalloidin) in the cells treated with A (Scale bars: 10 μm.) 6of16
7 A B Nomalized Perinuclear Actin Intensity nm Nomalized Perinuclear Actin Intensity nm C D Nomalized Perinuclear Actin Intensity nm Nomalized Perinuclear Actin Intensity nm E Nomalized Perinuclear Actin Intensity Fig. S5. Plots of normalized perinuclear actin intensity in EGFP-Lifeact transfected cells on treatment with (A) 20, (B) 50,(C) 100, or (D) 200 nm Latrunculin A. Latrunculin A was added at 0 s. Zero of four situations show significant perinuclear actin assembly. Data are given as means ± SEM in more than five cells for each situation. (E) EGFP-Lifeact fluorescence images of cells pretreated with 200 nm Latrunculin A (Left) before and (Center) after the addition of A23187 and (Right) corresponding plots of normalized perinuclear actin intensity over time in seven cells. (Scale bar: 10 μm.) 7of16
8 A B C E D Fig. S6. (A) Cells labeled by EGFP-Lifeact and pretreated with inhibitors of Arp2/3 (CK-666; 100 μm), Rho (C3 transferase; 1 μg/ml), or Rho kinase (Y-27632; 10 μm; Upper) before and (Lower) after force application. (B) Cells labeled by red fluorescent protein (RFP)-Lifeact and pretreated with 25 μm Blebbistatin (Left) before and (Right) after the addition of A Images at the focal plane corresponding to the maximal nuclear projection area are shown. (C) Immunoblots showing knockdown of cofilin-1 in NIH 3T3 cells. Tubulin content is shown as an internal control. (D) Perinuclear actin intensity in nontreated (NT) and A treated control and cofilin-1 knockdown (KD) cells. Data are given as means ± SEM from more than 40 cells. NS, P > *P < (E) Fluorescence staining of actin (phalloidin; green) and cofilin (red) in cells transfected with cofilin-1 sirnas. Images show that, in cofilin-1 knockdown cells (indicated by arrows), the perinuclear actin rim still forms on A23187 treatment like in control cells. (Scale bars: 10 μm.) 8of16
9 A B C Fig. S7. (A) Effect of calcium ionophore A23187 on cells (Upper) not expressing and (Lower) expressing GFP-KASH. Nesprin2 (purple) and F-actin (phalloidin; red) were stained. Note that, in cells expressing GFP-KASH, the actin rim appeared, despite the absence of nesprin2 at the nuclear envelope. (B) Z projection of filamin A immunostaining in NIH 3T3 cells. (C) Effect of A23187 on WT and filamin A KO mouse embryonic fibroblast cells. Note that the actin rim emerged, despite filamin A being absent. (Scale bars: 10 μm.) 9of16
10 A B Normalized Mean Intensity perinuclear middle peripheral C Normalized Intensity Ca 2+ Perinuclear F-actin Peripheral F-actin G-actin D Normalized Intensity Ca 2+ Perinuclear F-actin Peripheral F-actin G-actin Fig. S8. (A) Time-lapse images of FRAP experiments on cells transfected with EGFP-actin. N indicates the nucleus. (Scale bar: 10 μm.) (B) Normalized actin intensity during FRAP in the regions at the cell periphery, perinuclear area, and midpoint as shown by the boxes in A. Cumulative data of five independent experiments are shown. Data are given as means ± SEM. (C and D) Numerical simulations of the actin dynamics in response to the Ca 2+ burst that is used as the input variable. (C) The model assumes that the Ca 2+ burst causes F-actin disassembly and that G-actin activates INF2 formin. (D) In this model, Ca 2+ -induced actin depolymerization is not considered. The model assumes that the Ca 2+ burst activates INF2-driven perinuclear actin polymerization independently of the actin disassembly process. 10 of 16
11 Table S1. Maximal signal and half-time (t 1/2 )ofca 2+ and actin increase on force application and A23187 treatment Stimulation_increase of signal Maximal signal (fold change) t 1/2 (s) No. of cells Force_Ca ± ± Force_Actin 1.43 ± ± A23187_Ca ± ± A23187_Actin 1.63 ± ± Data are presented as means ± SEM. Table S2. Half-time (t 1/2 ) and recovery rate of FRAP on EGFPactin at cell periphery, middle, and perinuclear regions Region t 1/2 (s) Recovery rate (indicating mobile fraction) Perinuclear ± ± 0.02 Middle ± ± 0.04 Peripheral ± ± 0.03 Numbers are presented as means ± SEM from five experiments. 11 of 16
12 Table S3. Data obtained from FRAP presented in Fig. S8B were used for mathematical model fitting Perinuclear Middle Peripheral t (s) Photobleaching at this point of 16
13 Table S3. Cont. Perinuclear Middle Peripheral t (s) of 16
14 Movie S1. Movie showing reversible perinuclear actin assembly on three mechanical stimulations. (Upper) A 3T3 NIH fibroblast is transfected with EGFP- Lifeact to label actin. The movie is 34 times faster than real time. This movie is related to Fig. 1 B and C. Movie S1 14 of 16
15 Movie S2. Movie showing fluorescence intensity of (Upper) the Ca 2+ indicator G-CaMP (a fusion of green fluorescent protein, calmodulin, and M13, a peptide sequence from myosin light chain kinase) and (Lower) red fluorescent protein (RFP)-Lifeact on force application. The color code is in fire scale and was prepared using ImageJ. The movie is 17 times faster than real time. This movie is related to Fig. 2A. Movie S2 15 of 16
16 Movie S3. Movie showing fluorescence intensity of (Upper) the Ca 2+ indicator G-CaMP (a fusion of green fluorescent protein, calmodulin, and M13, a peptide sequence from myosin light chain kinase) and (Lower) red fluorescent protein (RFP)-Lifeact on addition of calcium ionophore A The color code is in fire scale and was prepared using ImageJ. The movie is 30 times faster than real time. This movie is related to Fig. 3A. Movie S3 16 of 16
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Supplemental data. Supplemental Materials and Methods
 Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends.
 SUPPLEMENTAL DATA Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends. Supplemental Experimental Procedures Dominant negative nesprin KASH (DN KASH) plasmid construction-
SUPPLEMENTAL DATA Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends. Supplemental Experimental Procedures Dominant negative nesprin KASH (DN KASH) plasmid construction-
Journal of Cell Science Supplementary Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1
 Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Prospéri et al.,
 Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
Supplementary Fig.1 Luton
 Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
0.5% Triton X-100 for 5 min at room temperature. Fixed and permeabilized cells were
 1 Supplementary Methods Immunohistochemistry EBC-1 cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by 0.5% Triton X-100 for 5 min at room temperature. Fixed and permeabilized
1 Supplementary Methods Immunohistochemistry EBC-1 cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by 0.5% Triton X-100 for 5 min at room temperature. Fixed and permeabilized
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured
 Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Farnesoid X Receptor and its ligands inhibit the function of platelets
 Farnesoid X Receptor and its ligands inhibit the function of platelets Article Accepted Version Figures and Legends Moraes, L. A., Unsworth, A. J., Vaiyapuri, S., Ali, M. S., Sasikumar, P., Sage, T., Flora,
Farnesoid X Receptor and its ligands inhibit the function of platelets Article Accepted Version Figures and Legends Moraes, L. A., Unsworth, A. J., Vaiyapuri, S., Ali, M. S., Sasikumar, P., Sage, T., Flora,
Supplementary Figure Legends
 Supplementary Figure Legends Figure S1 gene targeting strategy for disruption of chicken gene, related to Figure 1 (f)-(i). (a) The locus and the targeting constructs showing HpaI restriction sites. The
Supplementary Figure Legends Figure S1 gene targeting strategy for disruption of chicken gene, related to Figure 1 (f)-(i). (a) The locus and the targeting constructs showing HpaI restriction sites. The
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of
 Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information
 Supplementary Information Supplementary Figure 1: Over-expression of CD300f in NIH3T3 cells enhances their capacity to phagocytize AC. (a) NIH3T3 cells were stably transduced by EV, CD300f WT or CD300f
Supplementary Information Supplementary Figure 1: Over-expression of CD300f in NIH3T3 cells enhances their capacity to phagocytize AC. (a) NIH3T3 cells were stably transduced by EV, CD300f WT or CD300f
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity
 Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in
 Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Confocal immunofluorescence microscopy
 Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
Supplementary Information
 Supplementary Information stability is regulated by CK2-dependent interaction with R2TP complex Patrick von Morgen 1,2, Kamila Burdova 1, Thomas G. Flower 3, Nicola J. O'Reilly 4, Simon J. Boulton 5, Stephen
Supplementary Information stability is regulated by CK2-dependent interaction with R2TP complex Patrick von Morgen 1,2, Kamila Burdova 1, Thomas G. Flower 3, Nicola J. O'Reilly 4, Simon J. Boulton 5, Stephen
 DOI: 0.038/ncb337 a RFP-Zyxin Actin b RFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling, positive tilting of radial fibers c GFP-VASP Actin d GFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling,
DOI: 0.038/ncb337 a RFP-Zyxin Actin b RFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling, positive tilting of radial fibers c GFP-VASP Actin d GFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling,
Description of supplementary material file
 Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Description: Nuclear morphology and dynamics in nontargeting sirna transfected cells. HeLa Kyoto
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
FIGURE S1. Representative images to illustrate RAD51 foci induction in FANCD2 wild-type (wt) and
 Supplementary Figures FIGURE S1. Representative images to illustrate RAD51 foci induction in FANCD2 wild-type (wt) and mutant (mut) cells. Higher magnification is shown in Figure 1A. Arrows indicate cisplatin-induced
Supplementary Figures FIGURE S1. Representative images to illustrate RAD51 foci induction in FANCD2 wild-type (wt) and mutant (mut) cells. Higher magnification is shown in Figure 1A. Arrows indicate cisplatin-induced
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Cdc42 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
No wash 2 Washes 2 Days ** ** IgG-bead phagocytosis (%)
 Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplemental figures Supplemental Figure 1: Fluorescence recovery for FRAP experiments depicted in Figure 1.
 Supplemental figures Supplemental Figure 1: Fluorescence recovery for FRAP experiments depicted in Figure 1. Percent of original fluorescence was plotted as a function of time following photobleaching
Supplemental figures Supplemental Figure 1: Fluorescence recovery for FRAP experiments depicted in Figure 1. Percent of original fluorescence was plotted as a function of time following photobleaching
Gα 13 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
Gα i Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
Supplemental Data Supplementary Figure Legends and Scheme Figure S1.
 Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplement Figure 1. Plin5 Plin2 Plin1. KDEL-DSRed. Plin-YFP. Merge
 Supplement Figure 1 Plin5 Plin2 Plin1 KDEL-DSRed Plin-YFP Merge Supplement Figure 2 A. Plin5-Ab MitoTracker Merge AML12 B. Plin5-YFP Cytochrome c-cfp merge Supplement Figure 3 Ad.GFP Ad.Plin5 Supplement
Supplement Figure 1 Plin5 Plin2 Plin1 KDEL-DSRed Plin-YFP Merge Supplement Figure 2 A. Plin5-Ab MitoTracker Merge AML12 B. Plin5-YFP Cytochrome c-cfp merge Supplement Figure 3 Ad.GFP Ad.Plin5 Supplement
Supplementary Figures
 Supplementary Figures Supplementary Figure S1. Inhibition kinetics of p53/hdm2 binding induced by Nutlin 3 on live cells. Reproducibility of the p53/hdm2 binding disruption kinetics induced by Nutlin 3
Supplementary Figures Supplementary Figure S1. Inhibition kinetics of p53/hdm2 binding induced by Nutlin 3 on live cells. Reproducibility of the p53/hdm2 binding disruption kinetics induced by Nutlin 3
over time using live cell microscopy. The time post infection is indicated in the lower left corner.
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Supplementary Movie 1 Description: Fusion of NBs. BSR cells were infected
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Supplementary Movie 1 Description: Fusion of NBs. BSR cells were infected
Flag-Rac Vector V12 V12 N17 C40. Vector C40 pakt (T308) Akt1. Myc-DN-PAK1 (N-SP)
 a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
Organelle Atlas APPENDIX TO CHAPTER 4 4A.1
 APPENDIX TO CHAPTER 4 This atlas contains images of cellular organelles visualized using a wide variety of reagents and techniques and demonstrates the diversity of methods for exploring the cell. The
APPENDIX TO CHAPTER 4 This atlas contains images of cellular organelles visualized using a wide variety of reagents and techniques and demonstrates the diversity of methods for exploring the cell. The
Supplementary Figure 1
 Supplementary Figure a.5 fold of increase.5 plaa plaa with LY plaa /piaa plaa /piaa with LY b 5 5 5 plaa plaa /piaa No LY with LY No LY with LY c 6X fluorescence plaa plaa with LY plaa /piaa plaa /piaa
Supplementary Figure a.5 fold of increase.5 plaa plaa with LY plaa /piaa plaa /piaa with LY b 5 5 5 plaa plaa /piaa No LY with LY No LY with LY c 6X fluorescence plaa plaa with LY plaa /piaa plaa /piaa
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Supporting Information
 Supporting Information Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers Weibo Guo, 1,2
Supporting Information Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers Weibo Guo, 1,2
Supplementary information
 Supplementary information Table of Content: Supplementary Results... 2 Supplementary Figure S1: Experimental validation of AP-MS results by coimmunprecipitation Western blot analysis.... 3 Supplementary
Supplementary information Table of Content: Supplementary Results... 2 Supplementary Figure S1: Experimental validation of AP-MS results by coimmunprecipitation Western blot analysis.... 3 Supplementary
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Post-expansion antibody delivery, after epitope-preserving homogenization.
 Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Supporting Information
 Supporting Information Deng et al. 10.1073/pnas.1515692112 SI Materials and Methods FPLC. All fusion proteins were expressed and purified through a three-step FPLC purification protocol, as described (20),
Supporting Information Deng et al. 10.1073/pnas.1515692112 SI Materials and Methods FPLC. All fusion proteins were expressed and purified through a three-step FPLC purification protocol, as described (20),
Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and
 SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
Visualizing mechanical tension across membrane receptors with a fluorescent sensor
 Nature Methods Visualizing mechanical tension across membrane receptors with a fluorescent sensor Daniel R. Stabley, Carol Jurchenko, Stephen S. Marshall, Khalid S. Salaita Supplementary Figure 1 Fabrication
Nature Methods Visualizing mechanical tension across membrane receptors with a fluorescent sensor Daniel R. Stabley, Carol Jurchenko, Stephen S. Marshall, Khalid S. Salaita Supplementary Figure 1 Fabrication
Immunofluorescence and phalloidin labeling of mammalian cells
 Immunofluorescence and phalloidin labeling of mammalian cells 2 Contents Materials for immunofluorescence and phalloidin labeling of mammalian cells...1 Immunofluorescence-labelling on cultivated adherent
Immunofluorescence and phalloidin labeling of mammalian cells 2 Contents Materials for immunofluorescence and phalloidin labeling of mammalian cells...1 Immunofluorescence-labelling on cultivated adherent
Supplementary Methods
 Supplementary Methods Antibodies For immunocytochemistry, the following antibodies were used: mouse anti-γ-h2ax (Upstate), rabbit anti-γ-h2ax (Abcam), rabbit anti-53bp1 (Novus), mouse anti-atm-phosphoserine1981
Supplementary Methods Antibodies For immunocytochemistry, the following antibodies were used: mouse anti-γ-h2ax (Upstate), rabbit anti-γ-h2ax (Abcam), rabbit anti-53bp1 (Novus), mouse anti-atm-phosphoserine1981
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
Supplementary Figure S1
 Supplementary Figure S1 Figure S1. CENP- FP constructs target to centromeres irrespective of site of tagging. FP- CENP and CENP- FP constructs were transfected into U2OS cells and counterstained with anti-
Supplementary Figure S1 Figure S1. CENP- FP constructs target to centromeres irrespective of site of tagging. FP- CENP and CENP- FP constructs were transfected into U2OS cells and counterstained with anti-
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
14_integrins_EGFR
 α1 Integrin α1 -/- fibroblasts from integrin α1 knockout animals Figure S1. Serum-starved fibroblasts from α -/- 1 and +/+ mice were stimulated with 10% FBS for 30 min. (FBS) or plated on collagen I (CI)
α1 Integrin α1 -/- fibroblasts from integrin α1 knockout animals Figure S1. Serum-starved fibroblasts from α -/- 1 and +/+ mice were stimulated with 10% FBS for 30 min. (FBS) or plated on collagen I (CI)
vector company modification/annotation insert
 Supplemental information Plasmids Table 1. List of constructs used in the study. vector company modification/annotation insert pegfp-c1 Mikhaylova et al. 2009 Caln1 (NM_001077201.1) pegfp-c1 Caln1 ΔC (aa
Supplemental information Plasmids Table 1. List of constructs used in the study. vector company modification/annotation insert pegfp-c1 Mikhaylova et al. 2009 Caln1 (NM_001077201.1) pegfp-c1 Caln1 ΔC (aa
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
Supplemental Movie Legend.
 Supplemental Movie Legend. Transfected T cells were dropped onto SEE superantigen-pulsed Raji B cells (approximate location indicated by circle). Maximum-intensity projections from Z-stacks (17 slices,
Supplemental Movie Legend. Transfected T cells were dropped onto SEE superantigen-pulsed Raji B cells (approximate location indicated by circle). Maximum-intensity projections from Z-stacks (17 slices,
Supporting Information for. Bull s Eye Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation
 Supporting Information for Bull s Eye Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation Bo Chen, Yilong Jia, Yuan Gao, Lucero Sanchez, Stephen M. Anthony, and Yan Yu * Department
Supporting Information for Bull s Eye Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation Bo Chen, Yilong Jia, Yuan Gao, Lucero Sanchez, Stephen M. Anthony, and Yan Yu * Department
SUPPLEMENTARY INFORMATION
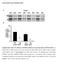 SUPPLEMENTARY INFORMATION Supplementary Figure S1. Efficacy of STIM1 knockdown by electroporation of STIM1 sirnas. (a) Western blot of FDB muscles from 4 mice treated with either STIM1-specific sirna (KD)
SUPPLEMENTARY INFORMATION Supplementary Figure S1. Efficacy of STIM1 knockdown by electroporation of STIM1 sirnas. (a) Western blot of FDB muscles from 4 mice treated with either STIM1-specific sirna (KD)
2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non
 Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated
 Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Materials and Methods
 Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Input. Pulldown GST. GST-Ahi1
 A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
by Neurobasal medium (supplemented with B27, 0.5mM glutamine, and 100 U/mL
 Supplementary Materials and methods Neuronal cultures and transfection The hippocampus was dissected from E8 rat embryos, dissociated, and neurons plated onto glass coverslips coated with poly-ornithine
Supplementary Materials and methods Neuronal cultures and transfection The hippocampus was dissected from E8 rat embryos, dissociated, and neurons plated onto glass coverslips coated with poly-ornithine
Khaled_Fig. S MITF TYROSINASE R²= MITF PDE4D R²= Variance from mean mrna expression
 Khaled_Fig. S Variance from mean mrna expression.8 2 MITF.6 TYROSINASE.4 R²=.73.2.8.6.4.2.8.6.4.2.8.6.4.2 MALME3M SKMEL28 UACC257 MITF PDE4D R²=.63 4.5 5 MITF 3.5 4 PDE4B 2.5 3 R²=.2.5 2.5.8.6.4.2.8.6.4.2
Khaled_Fig. S Variance from mean mrna expression.8 2 MITF.6 TYROSINASE.4 R²=.73.2.8.6.4.2.8.6.4.2.8.6.4.2 MALME3M SKMEL28 UACC257 MITF PDE4D R²=.63 4.5 5 MITF 3.5 4 PDE4B 2.5 3 R²=.2.5 2.5.8.6.4.2.8.6.4.2
PCNA appears in two populations of slow and fast diffusion with a constant ratio throughout S-phase in replicating mammalian cells
 PCNA appears in two populations of slow and fast diffusion with a constant ratio throughout S-phase in replicating mammalian cells Patrick J. M. Zessin 1, Anje Sporbert 2, *, Mike Heilemann 1, * 1 Institute
PCNA appears in two populations of slow and fast diffusion with a constant ratio throughout S-phase in replicating mammalian cells Patrick J. M. Zessin 1, Anje Sporbert 2, *, Mike Heilemann 1, * 1 Institute
Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides
 Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides targeting RCP (SMARTPool (RCP) or two individual oligos (RCP#1
Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides targeting RCP (SMARTPool (RCP) or two individual oligos (RCP#1
DCLK-immunopositive. Bars, 100 µm for B, 50 µm for C.
 Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
The definitive version is available at www3.intersci. Supporting_Information.pdf (Supporting Information) Instructions for use
 Title Nonmuscle myosin II folds into a 10S form via two po Kiboku, Takayuki; Katoh, Tsuyoshi; Nakamura, Akio; K Author(s) Masayuki CitationGenes to Cells, 18(2): 90-109 Issue Date 2013-02 Doc URL http://hdl.handle.net/2115/53054
Title Nonmuscle myosin II folds into a 10S form via two po Kiboku, Takayuki; Katoh, Tsuyoshi; Nakamura, Akio; K Author(s) Masayuki CitationGenes to Cells, 18(2): 90-109 Issue Date 2013-02 Doc URL http://hdl.handle.net/2115/53054
Rab5 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
SUPPLEMENTARY INFORMATION FILE
 SUPPLEMENTARY INFORMATION FILE Existence of a microrna pathway in anucleate platelets Patricia Landry, Isabelle Plante, Dominique L. Ouellet, Marjorie P. Perron, Guy Rousseau & Patrick Provost 1. SUPPLEMENTARY
SUPPLEMENTARY INFORMATION FILE Existence of a microrna pathway in anucleate platelets Patricia Landry, Isabelle Plante, Dominique L. Ouellet, Marjorie P. Perron, Guy Rousseau & Patrick Provost 1. SUPPLEMENTARY
T H E J O U R N A L O F C E L L B I O L O G Y
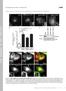 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
