Controls for Immunohistochemistry: The Histochemical Society s Standards of Practice for Validation of Immunohistochemical Assays
|
|
|
- April Bell
- 6 years ago
- Views:
Transcription
1 545224JHCXXX / Hewitt et al.immunohistochemistry Controls research-article2014 Perspective Journal of Histochemistry & Cytochemistry 2014, Vol. 62(10) The Author(s) 2014 Reprints and permissions: sagepub.com/journalspermissions.nav DOI: / jhc.sagepub.com Controls for Immunohistochemistry: The Histochemical Society s Standards of Practice for Validation of Immunohistochemical Assays Stephen M. Hewitt, Denis G. Baskin, Charles W. Frevert, William L. Stahl, and Eduardo Rosa-Molinar Center for Cancer Research, National Cancer Institute, Bethesda, Maryland (SMH); Department of Veterans Affairs Puget Sound Health Care System and Department of Medicine (DGB, WLS); Department of Comparative Medicine (CWF); Departments of Neurology and Physiology and Biophysics (WLS) University of Washington, Seattle, Washington; The Histochemical Society, Seattle, Washington (WLS); Biological Imaging Group, University of Puerto Rico-Rio Piedras (ERM); and Institute of Neurobiology, University of Puerto Rico-Medical Sciences (ERM) Summary Immunohistochemistry is widely used in biomedical research to localize specific epitopes of molecules in cells and tissues. The validity of interpretations based on immunohistochemistry requires appropriate positive and negative controls that are often not reported in publications. This omission may lead to incorrect interpretations and irreproducible results in the literature and contribute to wasted time, effort, and resources as well as erosion of confidence in scientific investigation by the general public, legislative bodies and funding agencies. The present article summarizes essential controls required for validation of immunohistochemical findings and represents a standard of practice for the use of immunohistochemistry in research and diagnostic investigations. Adherence to the guidelines described in the present article can be cited by authors as support for the validity of interpretations of the immunohistochemistry reported in their publications. (J Histochem Cytochem 62: , 2014) Keywords Immunohistochemistry, immunocytochemistry, antibodies, controls, validation, assay, standards Précis Immunohistochemistry is no different from any other technique: An experimental approach for which the quality of the results relies entirely on the analyte, reagents, precise experimental technique, correct application of controls and, ultimately, an accurate interpretation of the data based on all of the aforementioned factors. In this article, the essential nature of appropriate controls in immunohistochemical assay is discussed. Introduction The sine qua non of validating research findings is the use of appropriate controls in the design and performance of experiments and assays. Unfortunately, in the case of immunohistochemistry, although the need for controls is well established (Baskin 2009; Burry 2000, 2010; 2011; Frevert et al. 2013), it is the experience of the authors that critical controls are often not performed or reported in ensuing publications. This neglect has resulted in the publication of unverified and irreproducible findings in the literature (Couchman 2014; Collins and Tabak 2014). Contributing to this problem is the fact that many high-impact journals that publish immunohistochemical data do not require authors to report controls for immunohistochemistry and, what is perhaps more regrettable, the controls that are published often Received for publication April 16, 2014; accepted July 4, Corresponding Author: Stephen M. Hewitt, MD, PhD, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, MSC 4605, Bethesda, MD 20892, USA. genejock@helix.nih.gov
2 694 Hewitt et al. are inappropriate or misinterpreted. Realizing that controls are an essential component of experimental design in all scientific investigations, we insist that valid interpretations of immunohistochemical assays cannot be made in the absence of minimally appropriate controls. Furthermore, controls must be described and included in the ensuing publications. Simply stated, an immunohistochemical assay that lacks controls cannot be validly interpreted. Period. It s equally important not to conclude that a molecule is present when in fact it is not, as it is to conclude that it is absent when in fact it is present. Both can lead to erroneous scientific conclusions and clinical misdiagnoses. The possibility of both errors must be considered when designing an immunohistochemical assay. It is important to understand that controls either positive or negative can never prove the identity or the presence or absence of a molecule in a tissue sample using immunohistochemical techniques. With proper controls, however, the investigator can build a convincing case for the presence or absence of a probed molecule. This is the best that can be done, but it must be done correctly to be credible and reproducible. To get a snapshot of the attitude of many journals for the importance of controls in papers that incorporate immunohistochemical methods, we examined a sample of 29 articles that had immunohistochemical data in the Journal of Histochemistry & Cytochemistry and 71 additional publications in eight other high-impact cell biology and pathologyoriented journals that routinely publish data and images based on immunohistochemistry. Specifically, we were interested to know the extent to which control results are readily identifiable in articles that included immunohistochemistry in these journals. We also reviewed guidelines for authors of all of the selected journals. Of the 100 articles in nine journals, up to 80% of papers do not mention controls and 89% of guidelines for authors for these journals do not require or even mention controls. This informal survey sheds light on one reason that controls are often omitted from publications: journals (and, by inference, their reviewers and editors) either do not understand the basic principles of immunohistochemical controls or they do not consider them sufficiently important to require inclusion in their articles. This sadly influences investigators to have an uncritical attitude towards using valid controls in their studies. Accordingly, we recommend that the guidelines for authors for all journals should include a requirement that controls must be described and included in manuscripts that report results of immunohistochemical studies. To avoid inaccurate conclusions on a false-positive or a false-negative result, a minimum of a positive and a negative control should be run for all immunohistochemical assays. Moreover, journals and their editors should require reviewers to comment on whether appropriate minimal positive and negative controls were used. Here, we briefly describe these controls and how they should be correctly interpreted. Antibody Specificity Essential to valid interpretation of immunohistochemical staining is the selection of antibodies that have been validated to detect specific epitopes. This issue is perhaps the most critical control and is discussed extensively elsewhere (Gore 2013; Fervert et al 2013; Saper 2009; Saper and Sawchenko 2003). The case for specificity is often established independent of binding to epitopes in tissues or cells. This usually involves the separation of proteins that differ in size, charge, or conformation using gel electrophoresis. The proteins are then transferred from the gel to a membrane (i.e., western blot), where they are stained with an antibody and/or antibodies specific to the target protein to resolve the issue of the cross-reactivity of antibodies (Marchalant et al. 2014). Although this specificity is usually provided by the manufacturer of the antibody, ideally the proteins separated in the western blot should be obtained from the same tissue or cells as the antibody will be used for immunohistochemistry. The latter is often not feasible for an investigator but, nevertheless, the investigator must realize that this meaning of specificity applies only to the property of antibodies (either polyclonal or monoclonal) to recognize specific epitopes but does not prove the identity for binding of a stained targeted molecule in a tissue sample. Unfortunately, the so-called absorption control is often incorrectly offered to as a negative control as support for the specificity of immunostaining of a targeted molecule. This procedure is carried out by mixing the antibody with the target protein before applying the antibody to the tissue slice. If immunohistochemical staining in the tissue is blocked by this procedure, the staining observed with the unabsorbed antibody is often considered to be specific. However, this result only demonstrates that the antibody is specific for the molecule to which it was generated a fact already demonstrated by western blots but does not demonstrate that the antibody is binding to the same target molecule in the tissue. Unfortunately, in the spirit of considering the need for controls, reviewers often ask for absorption controls but this result (i.e., blocking of immunostaining) is a weak control for making conclusions about the identity of targeted molecules in the tissue (Holmseth et al. 2012). Positive Controls Positive controls in immunohistochemistry protocols are specimens containing the target molecule in its known location (e.g., specific cell type, intracellular compartment, among others) and whose histomorphology and cytomorphology can be visualized by a stain (i.e., the fluorochrome of a chromogenic molecule). Generally, these characteristics are initially deduced from other experimental evidence, including fractional western blots, overexpression, knock-down, experiments on cells, or studies carried out on transgenic animals known to express
3 Immunohistochemistry Controls 695 the target molecule. Conversely, the antibody should not produce staining in genetically engineered mice where the target molecule has been confirmed to be deleted. By and large, the use of cell lines, especially those transfected to over-express a target, are not recommended as appropriate positive controls, as many assays lack the appropriate dynamic range and calibration to ensure appropriate comparison between a cell line and tissue. The most rigorous positive control is the positive anatomical control; i.e., where the presence of the antigen in the specimen is known a priori and is not the target of the experimental treatment. This can be a known site of expression of the target molecule in the specimen (i.e., internal positive control ) or a separate specimen (e.g., a slide) that is known to contain the molecule targeted by the antibody (i.e., external positive control ). For example, an assay that uses an antibody that is claimed to be specific for insulin should include sections of pancreas that have islets of Langerhans; the antibody should target and visualize only the insulin-producing islet beta cells. The antibody should have been demonstrated not to cross-react with closely related molecules of the insulin-like peptide family. Interpretation of positive controls can be problematic in the case of antibodies that are directed against molecules that are expressed ubiquitously or are expressed at very low levels, and this criterion for specificity may be impossible in clinical settings. Nevertheless, in the absence of such controls, the investigator is obligated to (and journals should demand) that authors be circumspect about interpretations derived from such assays. Negative Controls The goal of a negative control is to demonstrate that the reaction visualized is due to the interaction of the epitope of the target molecule and the paratope of the antibody/affinity reagent. It is absolutely critical for investigators to be wary of claims made about the specificity of antibodies used for immunohistochemistry, especially for antibodies obtained from commercial sources. Although a manufacturer may demonstrate specificity of an antibody by western blot analysis, one cannot conclude that immunohistochemistry with the same antibody reveals the same target molecule in a tissue sample. Thus, in addition to the molecule targeted by the antibody, the results of an immunohistochemical assay (e.g., stain ) can reveal the presence of structurally related molecules as well as the binding of the antibodies used in the assay (both primary and secondary) nonspecifically to other cellular and tissue components. Accordingly, journals should require of authors to demonstrate (or cite evidence) that the antibody recognizes a protein within the tissue of interest that migrates appropriately in the gel electrophoresis and stains with antibodies specific to the target protein, as demonstrated by a western blot. Unfortunately, a common negative control seen in many articles in the literature is to perform the immunohistochemical assay with a primary antibody in tandem with a specimen that is not exposed to the primary antibody; that is to say, with the primary antibody omitted. Investigators claim and, regrettably, journal reviewers and editors accept this as control for the specificity of the staining for the antigen targeted by the antibody. This is a profound error. The absence of staining when the primary antibody is omitted is a control for nonspecific binding of the secondary antibody; this result is not evidence for the specificity of staining with the primary antibody. However, in some instances, it is an important additional control in assay development. It does not distinguish between staining that results from immunoglobulins binding at antigen binding sites in the F(ab) 2 variable regions (e.g., implicit for specific binding and immunoglobulins interacting with cell and tissue components in the Fc region) or due to nonspecific mechanisms. The proper negative control in this instance is substitution of serum or isotype-specific immunoglobulins at the same protein concentration as the primary antibody. Regrettably, the use of isotype-specific immunoglobulins (or normal or preimmune serum) as a substitute for the primary antibody has fallen out of favor as a negative control for staining specificity. In the instance of a noncommercial polyclonal antibody, pre-immune sera should be used, and when pre-immune sera is not available (commercial poly-clonal antibodies and all monoclonal antibodies) an isotype-specific immunoglobulin should be appropriately substituted. This control should be an absolute minimum requirement by journals for publishing studies that incorporate immunohistochemistry. It should be understood, however, that so-called specific antibodies, both polyclonal and monoclonal, can also bind nonspecifically due to Fc binding and other mechanisms, leading to the possibility that both specific and non-specific staining observed with such an antibody in a tissue section could not be validly distinguished without further controls (i.e., some of the staining is specific and some is non-specific). In this situation (where the primary antibody appears to show nonspecific binding), an absorption control can be useful in distinguishing specific from non-specific staining; the staining that is absent when the absorbed antibody is used (against a background of persistent, non-specific staining) is likely to indicate specific antibody binding sites. Standards of Practice for Immunohistochemistry Recognizing that the omission or improper use of controls is a serious problem limiting the validity as well as the reproducibility of much published data derived from the use of immunohistochemistry, we recommend that biomedical
4 696 Hewitt et al. Table 1. Essential Elements Pertaining to Controls in Immunohistochemical Assays that should be Included in Peer-reviewed Manuscripts*. 1. Documentation or reference to a western blot confirming an antibody:antigen binding to detect the target biomolecule of appropriate molecular size in a cellular lysate (not in vitro synthesized or immunogen-only). 2. A clear statement as to the nature of the immunohistochemical positive control that was performed. 3. A clear statement as to the nature of the immunohistochemical negative control that was performed. A. Negative Controls should be performed with pre-immune or isotype-specific sera. B. Negative Controls that omit the primary antibody only are inadequate in and of themselves. 4. The use of genetically engineered controls, as well as sirna, with PCR confirmation can serve as positive and negative controls. 5. The use of an alternative means of controls, including absorption controls, are inadequate to demonstrate specificity. *Additional recommendations concerning immunohistochemical assays will be defined in additional articles in this series. journals include specific requirements regarding the inclusion of controls for immunohistochemistry in their instructions for authors, as an element of describing immunohistochemical assays. The guidelines outlined in this article are the first step in defining a standard of practice for immunohistochemistry. Additional articles will outline additional points that should be addressed to ensure adequate validation and description of immunohistochemical assays. The end product of this larger effort will be a unified manuscript outlining the specification of immunohistochemical assays in the peer-reviewed literature, and the individual articles will serve as the in-depth information on each of those points. Although reproducibility is a concern, the validity of reported results is the more serious problem. The findings with a poor antibody can generate reproducible, but invalid, conclusions if proper controls are absent. Reproducibility can become a problem when different antibodies to the same antigen produce different results and controls are lacking to sort out the validity of different findings. In Table 1, we outline the elements with reference to controls that journal editors should require to be included in submitted manuscripts that report immunohistochemical data. The results of a positive control and a negative control as well as data supporting the claimed specificity of the primary antibodies for the target molecules should be reported. Characterization of antigen-antibody specificity should rely on confirmation of a one-protein:one-antibody relationship, or determination of the presence of shared epitopes. When shared epitopes are present, additional controls are essential. Approaches such as absorption control and the omission of a primary antibody should never be accepted as sole negative controls for specificity of staining in the absence of clear statements by the authors that these controls do not unequivocally demonstrate specificity of staining. The points outlined in Table 1 can be cited by authors as support for the validity of interpretations of the immunohistochemistry reported in their publications. Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Funding The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Partial funding for SMH s research was provided by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. DGB acknowledges the Research and Development Service of the Department of Veterans Health Administration, Department of Veterans Affairs, and the Cellular and Molecular Imaging Core of the Diabetes Research Center at the University of Washington (NIH grant P30DK017047), for providing partial funding for this research.. DGB is the recipient of a Department of Veterans Affairs Senior Research Career Scientist award. ERM acknowledges NIH (GM108470), NSF (NSF and NSF ), and the Puerto Rico Science Technology and Research Trust (Agreement No: ) for providing partial funding for this research. CWF acknowledges NIH (P30 DK089507). References Baskin DG (2009). The rationale and application of controls for immunocytochemistry in Baskin DB, Stahl WL (eds.) The Histochemical Society Annual Short Course: Principles & Applications of Immunocytochemistry, Seattle: The Histochemical Society, pp Burry RW (2000). Specificity controls for immunocytochemical methods. J Histochem Cytochem 48: Burry RW (2010). Immunocytochemistry: a practical guide for bio-medical research. New York: Springer. Burry RW (2011). Controls for Immunocytochemistry: An Update. J Histochem Cytochem 59:6-12. Collins FS, Tabak LA (2014). Policy: NIH plans to enhance reproducibility. Nature 505: Couchman JR (2014). Peer Review and Reproducibility. Crisis or Time for Course Correction? J Histochem Cytochem 62: Frevert CW, Johnson B, Stahl WL (2013). Immunohistochemistry: Antibody Specificity in Mitchell RN, McManus LM (eds.)
5 Immunohistochemistry Controls 697 Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms. Oxford, United Kingdom: Elsevier Ltd. Gore A (2013). Editorial: antibody validation requirements for articles published in endocrinology. Endocrinology 154: Holmseth S, Zhou Y, Follin-Arbelet VV, Lehre KP, Bergles DE, Danbolt NC (2012). Specificity controls for immunocytochemistry: The antigen preadsorption test can lead to inaccurate assessment of antibody specificity. J Histochem Cytochem 60: Marchalant Y, Brownjohn PW, Bonnet A, Kleffmann T, Ashton JC (2014). Validating antibodies to the cannabinoid CB2 receptor: Antibody sensitivity is not evidence of antibody specificity. J Histochem Cytochem 62: Saper CB (2009). A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem 57:1-5. Saper CB, Sawchenko PE (2003). Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol 465:
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging
 QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
Product Datasheet and Instructions for Use
 Product Datasheet and Instructions for Use Product Code: MP-138-CM01 (0.1ml conc) MP-138-CM05 (0.5ml conc) MP-138-CM1 (1ml conc) Product Description: Biotinylated Bromodeoxyuridine (BrdU) Concentrated
Product Datasheet and Instructions for Use Product Code: MP-138-CM01 (0.1ml conc) MP-138-CM05 (0.5ml conc) MP-138-CM1 (1ml conc) Product Description: Biotinylated Bromodeoxyuridine (BrdU) Concentrated
A Guide to the Perplexed on the Specificity of Antibodies
 Volume 57(1): 1 5, 2009 Journal of Histochemistry & Cytochemistry http://www.jhc.org PERSPECTIVE A Guide to the Perplexed on the Specificity of Antibodies Clifford B. Saper Department of Neurology and
Volume 57(1): 1 5, 2009 Journal of Histochemistry & Cytochemistry http://www.jhc.org PERSPECTIVE A Guide to the Perplexed on the Specificity of Antibodies Clifford B. Saper Department of Neurology and
INVESTIGATION OF THE BINDING SPECIFICITY OF IGF-IR USING MONOCLONAL ANTIBODIES
 INVESTIGATION OF THE BINDING SPECIFICITY OF IGF-IR USING MONOCLONAL ANTIBODIES By Mehrnaz Keyhanfar, Pharm.D. A thesis submitted to the University of Adelaide, South Australia in fulfilment of the requirements
INVESTIGATION OF THE BINDING SPECIFICITY OF IGF-IR USING MONOCLONAL ANTIBODIES By Mehrnaz Keyhanfar, Pharm.D. A thesis submitted to the University of Adelaide, South Australia in fulfilment of the requirements
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam will have 40 multiple choice questions.
 MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
a. Hypoxanthine was present in the media. MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No.
 MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
BCH 462. Single Radial Immunodiffusion and Immuno-electrophoresis
 BCH 462 Single Radial Immunodiffusion and Immuno-electrophoresis Immunoassays tests include: 1. Precipitation. 2. Agglutination. 3. Immunofluorescence. 4. Radioimmunoassay (RIA). 5. Enzyme-Linked Immuno
BCH 462 Single Radial Immunodiffusion and Immuno-electrophoresis Immunoassays tests include: 1. Precipitation. 2. Agglutination. 3. Immunofluorescence. 4. Radioimmunoassay (RIA). 5. Enzyme-Linked Immuno
Supplementary Figure 1. Utilization of publicly available antibodies in different applications.
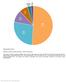 Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Uniform Labeling of Blocks and Slides in Surgical Pathology
 Uniform Labeling of Blocks and Slides in Surgical Pathology Guideline from the College of American Pathologists and the National Society for Histotechnology Published : Archives of Pathology and Laboratory
Uniform Labeling of Blocks and Slides in Surgical Pathology Guideline from the College of American Pathologists and the National Society for Histotechnology Published : Archives of Pathology and Laboratory
September 8, General Comments:
 September 8, 2009 AMP Comments on the draft report from the Agency for Healthcare Research and Quality (AHRQ): AHRQ Draft Report on Quality, Regulation and Clinical Utility of Laboratorydeveloped Tests.
September 8, 2009 AMP Comments on the draft report from the Agency for Healthcare Research and Quality (AHRQ): AHRQ Draft Report on Quality, Regulation and Clinical Utility of Laboratorydeveloped Tests.
ELONA: the evolution of ELISA Banushan Balansethupathy, Andrew Brentnall Aptamer Group Ltd
 ELONA: the evolution of ELISA Banushan Balansethupathy, Andrew Brentnall Aptamer Group Ltd Enzyme-linked immunosorbent assay (ELISA) was developed for the detection and quantification of proteins, peptides
ELONA: the evolution of ELISA Banushan Balansethupathy, Andrew Brentnall Aptamer Group Ltd Enzyme-linked immunosorbent assay (ELISA) was developed for the detection and quantification of proteins, peptides
Module IB. Histochemistry. Martin Špaček, MD. (
 Module IB Histochemistry Martin Špaček, MD (E-mail: m.spacek@centrum.cz) http://www.lf3.cuni.cz/histologie What is histochemistry? It is a histological technique used for studying chemistry of tissues
Module IB Histochemistry Martin Špaček, MD (E-mail: m.spacek@centrum.cz) http://www.lf3.cuni.cz/histologie What is histochemistry? It is a histological technique used for studying chemistry of tissues
Efforts to Improve Data Transparency at the JBC
 Efforts to Improve Data Transparency at the JBC Roger J. Colbran Associate Editor Credit: Amanda Fosang, Associate Editor 2 Why Improve Transparency? Irreproducibility of pre-clinical studies Negative
Efforts to Improve Data Transparency at the JBC Roger J. Colbran Associate Editor Credit: Amanda Fosang, Associate Editor 2 Why Improve Transparency? Irreproducibility of pre-clinical studies Negative
Practical Applications of Immunology (Chapter 18) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus
 Practical Applications of Immunology (Chapter 18) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Source for figures and content: Tortora, G.J. Microbiology
Practical Applications of Immunology (Chapter 18) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Source for figures and content: Tortora, G.J. Microbiology
Product Datasheet and Instructions for Use
 Product Datasheet and Instructions for Use Product Code: MP-323-CM01 (0.1ml conc) MP-323-CM05 (0.5ml conc) Product Description: CD24 Concentrated Monoclonal Antibody Control Number: 901-323-052510 ISO
Product Datasheet and Instructions for Use Product Code: MP-323-CM01 (0.1ml conc) MP-323-CM05 (0.5ml conc) Product Description: CD24 Concentrated Monoclonal Antibody Control Number: 901-323-052510 ISO
Product Datasheet and Instructions for Use
 Product Code: MP-109-CM01 (0.1ml conc) MP-109-CM05 (0.5ml conc) MP-109-CM1 (1ml conc) MP-109-PM6 (6ml RTU) Product Description: Androgen Receptor Concentrated and Prediluted Monoclonal Antibody Control
Product Code: MP-109-CM01 (0.1ml conc) MP-109-CM05 (0.5ml conc) MP-109-CM1 (1ml conc) MP-109-PM6 (6ml RTU) Product Description: Androgen Receptor Concentrated and Prediluted Monoclonal Antibody Control
CURRICULUM VITAE. Educational Qualification: DEGREE INSTITUTION YEAR PROGRESS. Bharathidasan University Tamil Nadu
 CURRICULUM VITAE M.BHARATH VIJAYARAGAVAN Masters in Biomedical sciences Nationality: Indian E-mail: bharathragavan@gmail.com Mobile: 91-9911738539 Sex: Male; Age: 23 Objective: Cell Signaling is an important
CURRICULUM VITAE M.BHARATH VIJAYARAGAVAN Masters in Biomedical sciences Nationality: Indian E-mail: bharathragavan@gmail.com Mobile: 91-9911738539 Sex: Male; Age: 23 Objective: Cell Signaling is an important
Recent Studies of Follow-On Biologics Are Based on Seriously Flawed Assumptions:
 Recent Studies of Follow-On Biologics Are Based on Seriously Flawed Assumptions: PCMA Study Potential Savings That Might Be Realized By the Medicare Program From Enactment Of Legislation Such As The Access
Recent Studies of Follow-On Biologics Are Based on Seriously Flawed Assumptions: PCMA Study Potential Savings That Might Be Realized By the Medicare Program From Enactment Of Legislation Such As The Access
GUIDELINES ON MEDICAL DEVICES. IVD GUIDANCE : Research Use Only products A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES
 Ref. Ares(2015)2031363-13/05/2015 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology
Ref. Ares(2015)2031363-13/05/2015 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology
CHAPTER 24. Immunology
 CHAPTER 24 Diagnostic i Microbiology and Immunology Growth-Dependent Diagnostic Methods Isolation of Pathogens from Clinical Specimens Proper sampling and culture of a suspected pathogen is the most reliable
CHAPTER 24 Diagnostic i Microbiology and Immunology Growth-Dependent Diagnostic Methods Isolation of Pathogens from Clinical Specimens Proper sampling and culture of a suspected pathogen is the most reliable
Position Description November 2018
 Position Description November 2018 MISSION The mission of St Vincent s Institute (SVI) is to carry out the highest quality laboratory based biomedical research, in order to make discoveries that will improve
Position Description November 2018 MISSION The mission of St Vincent s Institute (SVI) is to carry out the highest quality laboratory based biomedical research, in order to make discoveries that will improve
Immunological Applications. Chapter 8: Background
 Immunological Applications Chapter 8: Background The Immune System Types of Immunity Innate The natural immunity present at birth Acquired A specific response to foreign substances. Some cells remember
Immunological Applications Chapter 8: Background The Immune System Types of Immunity Innate The natural immunity present at birth Acquired A specific response to foreign substances. Some cells remember
Your Antibody Source. prosci-inc.com. Extensive Antibody Services Broad Antibody Catalog
 Your Source prosci-inc.com Extensive Services Broad Catalog Company Overview Established in 1998, ProSci Incorporated is a leading provider of high performance antibodies and custom antibody services.
Your Source prosci-inc.com Extensive Services Broad Catalog Company Overview Established in 1998, ProSci Incorporated is a leading provider of high performance antibodies and custom antibody services.
Comparing MSD Electrochemiluminescent Detection Technology to Traditional ELISA for Clinical Applications
 Enzyme- linked immunosorbent assays (s) are widely used in clinical settings for detecting substances such as antibodies, peptides, or hormones. However, assays have been gaining in popularity due to advantages
Enzyme- linked immunosorbent assays (s) are widely used in clinical settings for detecting substances such as antibodies, peptides, or hormones. However, assays have been gaining in popularity due to advantages
Assays for Immunogenicity: Are We There Yet?
 Assays for Immunogenicity: Are We There Yet? Mark Wener, MD Department of Laboratory Medicine & Rheumatology Division Department of Medicine University of Washington Seattle, WA 98195 wener@uw.edu Goals:
Assays for Immunogenicity: Are We There Yet? Mark Wener, MD Department of Laboratory Medicine & Rheumatology Division Department of Medicine University of Washington Seattle, WA 98195 wener@uw.edu Goals:
Novocastra Liquid Mouse Monoclonal Antibody Insulin
 Novocastra Liquid Mouse Monoclonal Antibody Insulin Product Code: NCL-L-INSULIN Leica Biosystems Newcastle Ltd Balliol Business Park West Benton Lane Newcastle Upon Tyne NE12 8EW United Kingdom ( +44 191
Novocastra Liquid Mouse Monoclonal Antibody Insulin Product Code: NCL-L-INSULIN Leica Biosystems Newcastle Ltd Balliol Business Park West Benton Lane Newcastle Upon Tyne NE12 8EW United Kingdom ( +44 191
Custom Antibody Services. Antibodies Designed Just for You. HuCAL Recombinant Monoclonal Antibody Generation Service
 Custom Antibody Services Antibodies Designed Just for You HuCAL Recombinant Monoclonal Antibody Generation Service Antibodies Designed Just for You What if there was a technology that would allow you to
Custom Antibody Services Antibodies Designed Just for You HuCAL Recombinant Monoclonal Antibody Generation Service Antibodies Designed Just for You What if there was a technology that would allow you to
ENCODE RBP Antibody Characterization Guidelines
 ENCODE RBP Antibody Characterization Guidelines Approved on November 18, 2016 Background An integral part of the ENCODE Project is to characterize the antibodies used in the experiments. This document
ENCODE RBP Antibody Characterization Guidelines Approved on November 18, 2016 Background An integral part of the ENCODE Project is to characterize the antibodies used in the experiments. This document
Common Issues in Qualification and Validation of Analytical Procedures
 Common Issues in Qualification and Validation of Analytical Procedures Alexey Khrenov, PhD OTAT/CBER/FDA CMC Strategy Forum January 29, 2018 - Washington, DC Disclaimer These comments are an informal communication
Common Issues in Qualification and Validation of Analytical Procedures Alexey Khrenov, PhD OTAT/CBER/FDA CMC Strategy Forum January 29, 2018 - Washington, DC Disclaimer These comments are an informal communication
Application of Biacore Technology
 Principles and typical results Application of Biacore Technology Common types of Biacore analyses Specificity analysis Is my molecule of interest specific for its target? Multiple binding analysis In which
Principles and typical results Application of Biacore Technology Common types of Biacore analyses Specificity analysis Is my molecule of interest specific for its target? Multiple binding analysis In which
Interplay of Cells involved in Therapeutic Agent Immunogenicity. Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology
 Interplay of Cells involved in Therapeutic Agent Immunogenicity Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology Disclosure The author works with Amicus on an immunogenicity project
Interplay of Cells involved in Therapeutic Agent Immunogenicity Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology Disclosure The author works with Amicus on an immunogenicity project
INOS. Colorimetric Cell-Based ELISA Kit. Catalog #: OKAG00807
 INOS Colorimetric Cell-Based ELISA Kit Catalog #: OKAG00807 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research Purposes Only. Not Intended
INOS Colorimetric Cell-Based ELISA Kit Catalog #: OKAG00807 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research Purposes Only. Not Intended
CONTRACTING ORGANIZATION: Albert Einstein College of Medicine Bronx, NY 10461
 AD Award Number: W81XWH-08-1-0011 TITLE: Defining B. Anthracis Protective Antigen Antigenic Domains PRINCIPAL INVESTIGATOR: Arturo Casadevall, M.D., Ph.D. CONTRACTING ORGANIZATION: Albert Einstein College
AD Award Number: W81XWH-08-1-0011 TITLE: Defining B. Anthracis Protective Antigen Antigenic Domains PRINCIPAL INVESTIGATOR: Arturo Casadevall, M.D., Ph.D. CONTRACTING ORGANIZATION: Albert Einstein College
Advanced Therapeutic Antibody Discovery with Multiplexed Screening
 Advanced Therapeutic Antibody Discovery with Multiplexed Screening White Paper Scientists need powerful tools that can deliver results to fully understand the ability of candidate antibodies to interrupt
Advanced Therapeutic Antibody Discovery with Multiplexed Screening White Paper Scientists need powerful tools that can deliver results to fully understand the ability of candidate antibodies to interrupt
General Comments. Misinformation in Immunohistochemistry. Common Misinformation s in Immunohistochemistry 4/13/2017
 Common Misinformation s in Immunohistochemistry Tri State Meeting May 3, 2017 Steven Westra Reagent Product Specialist Leica Biosystems Misinformation in Immunohistochemistry Flood of New Markers Diagnostic
Common Misinformation s in Immunohistochemistry Tri State Meeting May 3, 2017 Steven Westra Reagent Product Specialist Leica Biosystems Misinformation in Immunohistochemistry Flood of New Markers Diagnostic
Product Datasheet. PIEZO1 Antibody NBP Unit Size: 0.1 ml
 Product Datasheet PIEZO1 Antibody NBP1-78537 Unit Size: 0.1 ml Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles. Publications: 3 Protocols, Publications, Related Products,
Product Datasheet PIEZO1 Antibody NBP1-78537 Unit Size: 0.1 ml Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles. Publications: 3 Protocols, Publications, Related Products,
Androgen Receptor (Phospho-Tyr363) Colorimetric Cell-Based ELISA Kit. Catalog #: OKAG02138
 Androgen Receptor (Phospho-Tyr363) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02138 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research
Androgen Receptor (Phospho-Tyr363) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02138 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology
 Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
College of American Pathologists Laboratory Accreditation Program. AP for Histotechs and Secretaries. Learning Objectives
 College of American Pathologists AP for Histotechs and Secretaries Francis E. Sharkey, M.D., FCAP University of Texas Health Science Center - San Antonio February 20th, 2008 Copyright 2008 College of American
College of American Pathologists AP for Histotechs and Secretaries Francis E. Sharkey, M.D., FCAP University of Texas Health Science Center - San Antonio February 20th, 2008 Copyright 2008 College of American
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Microarray Industry Products
 Via Nicaragua, 12-14 00040 Pomezia (Roma) Phone: +39 06 91601628 Fax: +39 06 91612477 info@lifelinelab.com www.lifelinelab.com Microarray Industry Products Page 10 NBT / BCPIP Chromogenic phosphatase
Via Nicaragua, 12-14 00040 Pomezia (Roma) Phone: +39 06 91601628 Fax: +39 06 91612477 info@lifelinelab.com www.lifelinelab.com Microarray Industry Products Page 10 NBT / BCPIP Chromogenic phosphatase
Product Datasheet and Instructions for Use
 Product Code: MP-066-CM01 (0.1ml conc) MP-066-CM05 (0.5ml conc) MP-066-CM1 (1ml conc) MP-066-PM6 (6ml RTU) Product Description: Neurofilament Concentrated and Prediluted Monoclonal Antibody Control Number:
Product Code: MP-066-CM01 (0.1ml conc) MP-066-CM05 (0.5ml conc) MP-066-CM1 (1ml conc) MP-066-PM6 (6ml RTU) Product Description: Neurofilament Concentrated and Prediluted Monoclonal Antibody Control Number:
WESTERN BLOT. BCH462- Practical
 WESTERN BLOT BCH462- Practical What is Antigen [Ag]? What is Antibody [Ab]? Immunoassay: is a test that uses the highly specific and selective antigen-antibody reactions forming antibody and antigen complexes
WESTERN BLOT BCH462- Practical What is Antigen [Ag]? What is Antibody [Ab]? Immunoassay: is a test that uses the highly specific and selective antigen-antibody reactions forming antibody and antigen complexes
HOST CELL PROTEIN & BIOPROCESSING REAGENT DEVELOPMENT
 HOST CELL PROTEIN & BIOPROCESSING REAGENT DEVELOPMENT INTRODUCTION Biopharmaceuticals require products to be free of residual host cell protein (HCP) contaminants from the bioprocessing workflow. To evaluate
HOST CELL PROTEIN & BIOPROCESSING REAGENT DEVELOPMENT INTRODUCTION Biopharmaceuticals require products to be free of residual host cell protein (HCP) contaminants from the bioprocessing workflow. To evaluate
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 19001 Second edition 2013-03-15 In vitro diagnostic medical devices Information supplied by the manufacturer with in vitro diagnostic reagents for staining in biology Dispositifs
INTERNATIONAL STANDARD ISO 19001 Second edition 2013-03-15 In vitro diagnostic medical devices Information supplied by the manufacturer with in vitro diagnostic reagents for staining in biology Dispositifs
Antigens & Antibodies II. Polyclonal antibodies vs Monoclonal antibodies
 A Brief Review of Antibody Structure A Brief Review of Antibody Structure The basic antibody is a dimer of dimer (2 heavy chain-light chain pairs) composed of repeats of a single structural unit known
A Brief Review of Antibody Structure A Brief Review of Antibody Structure The basic antibody is a dimer of dimer (2 heavy chain-light chain pairs) composed of repeats of a single structural unit known
Anti-HB-EGF (Human) mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
ELISA. An introduction to the basic principles and assay formats. Innova Biosciences ltd All rights reserved
 ELISA An introduction to the basic principles and assay formats Agenda Agenda: Introduction to ELISA The different assay types Assay development tips Data analysis tips Troubleshooting tips Indirect vs.
ELISA An introduction to the basic principles and assay formats Agenda Agenda: Introduction to ELISA The different assay types Assay development tips Data analysis tips Troubleshooting tips Indirect vs.
Site directed mutagenesis, Insertional and Deletion Mutagenesis. Mitesh Shrestha
 Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
CytoGLOW. IKK-α/β. Colorimetric Cell-Based ELISA Kit. Catalog #: CB5358
 CytoGLOW IKK-α/β Colorimetric Cell-Based ELISA Kit Catalog #: CB5358 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research Purposes Only.
CytoGLOW IKK-α/β Colorimetric Cell-Based ELISA Kit Catalog #: CB5358 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research Purposes Only.
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION
 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
METHODS IN CELL BIOLOGY EXAM II, MARCH 26, 2008
 NAME KEY METHODS IN CELL BIOLOGY EXAM II, MARCH 26, 2008 1. DEFINITIONS (30 points). Briefly (1-3 sentences, phrases, word, etc.) define the following terms or answer question. A. depot effect refers to
NAME KEY METHODS IN CELL BIOLOGY EXAM II, MARCH 26, 2008 1. DEFINITIONS (30 points). Briefly (1-3 sentences, phrases, word, etc.) define the following terms or answer question. A. depot effect refers to
Practical What & Where of Rigor and Reproducibility in the NIH Application. SIU School of Medicine Office of Grants & Contracts with Sophia Ran, PhD
 Practical What & Where of Rigor and Reproducibility in the NIH Application SIU School of Medicine Office of Grants & Contracts with Sophia Ran, PhD Recent Training Sessions NIH Regional Seminar Rigor and
Practical What & Where of Rigor and Reproducibility in the NIH Application SIU School of Medicine Office of Grants & Contracts with Sophia Ran, PhD Recent Training Sessions NIH Regional Seminar Rigor and
Cytomics in Action: Cytokine Network Cytometry
 Cytomics in Action: Cytokine Network Cytometry Jonni S. Moore, Ph.D. Director, Clinical and Research Flow Cytometry and PathBioResource Associate Professor of Pathology & Laboratory Medicine University
Cytomics in Action: Cytokine Network Cytometry Jonni S. Moore, Ph.D. Director, Clinical and Research Flow Cytometry and PathBioResource Associate Professor of Pathology & Laboratory Medicine University
DECLARATION OF ANDREW SAXON, M.D. knowledge, and if called to testify, I could competently do so.
 0 WILSHIRE BOULEVARD, TH FLOOR LOS ANGELES, CALIFORNIA 00-0 TELEPHONE --00 FAX --0 DECLARATION OF ANDREW SAXON, M.D. I, Andrew Saxon, M.D., declare as follows:. I am over years of age. I know the following
0 WILSHIRE BOULEVARD, TH FLOOR LOS ANGELES, CALIFORNIA 00-0 TELEPHONE --00 FAX --0 DECLARATION OF ANDREW SAXON, M.D. I, Andrew Saxon, M.D., declare as follows:. I am over years of age. I know the following
Risk Management in IVD Producer Relation between manufacturer and user. S.M.Boutorabi DCLS, PhD
 Risk Management in IVD Producer Relation between manufacturer and user S.M.Boutorabi DCLS, PhD IVD Manufacturer Requirements International Standard ISO 13485-2016 Medical Devices Quality Management Systems
Risk Management in IVD Producer Relation between manufacturer and user S.M.Boutorabi DCLS, PhD IVD Manufacturer Requirements International Standard ISO 13485-2016 Medical Devices Quality Management Systems
VisUCyte TM HRP Polymer-DAB Cell & Tissue Staining Kit
 VisUCyte TM HRP Polymer-DAB Cell & Tissue Staining Kit For the detection of goat, mouse, rabbit, rat, or sheep primary IgG Antibodies with a biotin-free detection system. Size: 50 Tests Secondary Antibody-HRP
VisUCyte TM HRP Polymer-DAB Cell & Tissue Staining Kit For the detection of goat, mouse, rabbit, rat, or sheep primary IgG Antibodies with a biotin-free detection system. Size: 50 Tests Secondary Antibody-HRP
WVU FLOW CYTOMETRY & SINGLE CELL CORE FACILITY
 WVU FLOW CYTOMETRY & SINGLE CELL CORE FACILITY Newsletter Volume 5, issue 1 July 2018 Finding and Validating Antibodies Part 1 Inside this Issue 1-2 Finding and Validating Antibodies Part 1 3 4 Flow Cytometers
WVU FLOW CYTOMETRY & SINGLE CELL CORE FACILITY Newsletter Volume 5, issue 1 July 2018 Finding and Validating Antibodies Part 1 Inside this Issue 1-2 Finding and Validating Antibodies Part 1 3 4 Flow Cytometers
T-cell response. Taken from NIAID: s.aspx
 T-cell receptor T-cell response 1. Macrophage or dendritic cell digest antigen bacteria, virus 2. Fragments of Ag bind to major histo-compatiblity (MHC) proteins in macrophage. 3. MHC I-Ag fragment expressed
T-cell receptor T-cell response 1. Macrophage or dendritic cell digest antigen bacteria, virus 2. Fragments of Ag bind to major histo-compatiblity (MHC) proteins in macrophage. 3. MHC I-Ag fragment expressed
Evaluation of Tissue Adhesion and Staining Performance of BOND Adhesive Microscope Slides: A Comparative Study on the BOND-III and BOND-MAX Platforms
 Evaluation of Tissue Adhesion and Staining Performance of BOND Adhesive Microscope Slides: A Comparative Study on the BOND-III and BOND-MAX Platforms By Brandon Pohl, Sue Kapoor, Stanley E. Hansen, Travis
Evaluation of Tissue Adhesion and Staining Performance of BOND Adhesive Microscope Slides: A Comparative Study on the BOND-III and BOND-MAX Platforms By Brandon Pohl, Sue Kapoor, Stanley E. Hansen, Travis
Molecular Techniques. 3 Goals in Molecular Biology. Nucleic Acids: DNA and RNA. Disclaimer Nucleic Acids Proteins
 Molecular Techniques Disclaimer Nucleic Acids Proteins Houpt, CMN, 9-30-11 3 Goals in Molecular Biology Identify All nucleic acids (and proteins) are chemically identical in aggregate - need to identify
Molecular Techniques Disclaimer Nucleic Acids Proteins Houpt, CMN, 9-30-11 3 Goals in Molecular Biology Identify All nucleic acids (and proteins) are chemically identical in aggregate - need to identify
Manufactured by. Zyagen Barnes Canyon Road San Diego, CA 92121, USA
 Alkaline Phosphatase Immunohistochemistry Detection kits For detection of mouse, rabbit, goat, rat, sheep, chicken, guinea pig, and human primary antibodies Size: 500 Tests Catalog #: AK-011, Mouse Kit
Alkaline Phosphatase Immunohistochemistry Detection kits For detection of mouse, rabbit, goat, rat, sheep, chicken, guinea pig, and human primary antibodies Size: 500 Tests Catalog #: AK-011, Mouse Kit
Post-translational modification
 Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Lecture 5: 8/31. CHAPTER 5 Techniques in Protein Biochemistry
 Lecture 5: 8/31 CHAPTER 5 Techniques in Protein Biochemistry Chapter 5 Outline The proteome is the entire set of proteins expressed and modified by a cell under a particular set of biochemical conditions.
Lecture 5: 8/31 CHAPTER 5 Techniques in Protein Biochemistry Chapter 5 Outline The proteome is the entire set of proteins expressed and modified by a cell under a particular set of biochemical conditions.
Comments on Use of Databases for Establishing the Clinical Relevance of Human Genetic Variants
 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 The American Society of Human Genetics 9650 Rockville Pike Bethesda, MD 20814 24 December
Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 The American Society of Human Genetics 9650 Rockville Pike Bethesda, MD 20814 24 December
DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE
 DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE EXECUTIVE SUMMARY Oncologists reviewing blood and tissue test results face the same problems: Is the
DIAGNOSE AND TREAT WITH ANTIBODIES THAT RECOGNIZE NATIVE HUMAN PROTEIN EPITOPES IN BLOOD AND TISSUE EXECUTIVE SUMMARY Oncologists reviewing blood and tissue test results face the same problems: Is the
The World Leader in SPR Technology. Jimmy Page, PhD, Biacore, Inc.
 The World Leader in SPR Technology Jimmy Page, PhD, Biacore, Inc. Objectives of Biacore Experiments Yes/No Data» Is there binding?» Ligand Fishing Concentration Analysis: How MUCH? Active Concentration
The World Leader in SPR Technology Jimmy Page, PhD, Biacore, Inc. Objectives of Biacore Experiments Yes/No Data» Is there binding?» Ligand Fishing Concentration Analysis: How MUCH? Active Concentration
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion
 Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion Robin Thorpe & Meenu Wadhwa Revised Guideline: Differences from original
Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion Robin Thorpe & Meenu Wadhwa Revised Guideline: Differences from original
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A
 Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Solutions for Your Research
 Solutions for Your Research Custom Antibody Services Polyclonal Monoclonal The service we offer is very complete starting from rabbits, mice and rats. The different formats provided by Primm depend on
Solutions for Your Research Custom Antibody Services Polyclonal Monoclonal The service we offer is very complete starting from rabbits, mice and rats. The different formats provided by Primm depend on
Immunohistochemistry guide
 Immunohistochemistry guide overview immunohistochemistry Overview Immunohistochemistry is a laboratory technique utilized for the visual detection of antigens in tissue. When working with cells this technique
Immunohistochemistry guide overview immunohistochemistry Overview Immunohistochemistry is a laboratory technique utilized for the visual detection of antigens in tissue. When working with cells this technique
EGFR (Phospho-Ser695)
 Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 EGFR (Phospho-Ser695) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02090 Please read the provided manual entirely
Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 EGFR (Phospho-Ser695) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02090 Please read the provided manual entirely
Key Aspects of Non-Clinical Pharmacology and Pharmacokinetics in the Evaluation of Safety
 Safeguarding public health Key Aspects of Non-Clinical Pharmacology and Pharmacokinetics in the Evaluation of Safety David R Jones (david.jones@mhra.gsi.gov.uk) Expert Pharmacotoxicologist, Medicines and
Safeguarding public health Key Aspects of Non-Clinical Pharmacology and Pharmacokinetics in the Evaluation of Safety David R Jones (david.jones@mhra.gsi.gov.uk) Expert Pharmacotoxicologist, Medicines and
Immunoglobulins. (1 of 2)
 Immunoglobulins (1 of 2) Immunoglobulins (Igs) = antibodies Each B cell synthesizes Igs of single specificity for a specific epitope B cell receptors (BCRs) are the Igs on B cell surface Humoral immunity
Immunoglobulins (1 of 2) Immunoglobulins (Igs) = antibodies Each B cell synthesizes Igs of single specificity for a specific epitope B cell receptors (BCRs) are the Igs on B cell surface Humoral immunity
IMMUNOPRECIPITATION TROUBLESHOOTING TIPS
 IMMUNOPRECIPITATION TROUBLESHOOTING TIPS Creative Diagnostics Abstract Immunoprecipitation (IP) is the technique of precipitating a protein antigen out of solution using an antibody that specifically binds
IMMUNOPRECIPITATION TROUBLESHOOTING TIPS Creative Diagnostics Abstract Immunoprecipitation (IP) is the technique of precipitating a protein antigen out of solution using an antibody that specifically binds
College of American Pathologists. Statement to the National Institutes of Health on the proposed Genetic Testing Registry.
 College of American Pathologists Statement to the National Institutes of Health on the proposed Genetic Testing Registry July 12, 2010 College of American Pathologists 1350 I Street, NW, Suite 590 Washington,
College of American Pathologists Statement to the National Institutes of Health on the proposed Genetic Testing Registry July 12, 2010 College of American Pathologists 1350 I Street, NW, Suite 590 Washington,
Applicable To Employees of the Gundersen Palmer Lutheran Hospitals and Clinics laboratories.
 Subject ABO Forward Grouping - D Antigen Typing - Palmer Index Number Lab-8791 Section Laboratory Subsection Regional/Affiliates Category Departmental Contact Betty Tilleraas Last Revised 10/31/2017 References
Subject ABO Forward Grouping - D Antigen Typing - Palmer Index Number Lab-8791 Section Laboratory Subsection Regional/Affiliates Category Departmental Contact Betty Tilleraas Last Revised 10/31/2017 References
Cyfra 21-1 IRMA. Product information Information about other products is available at: Userś Manual DE52100
 Product information Information about other products is available at: www.demeditec.com Userś Manual Cyfra 21-1 IRMA The CYFRA 21.1 IRMA system provides a direct in vitro quantitative determination of
Product information Information about other products is available at: www.demeditec.com Userś Manual Cyfra 21-1 IRMA The CYFRA 21.1 IRMA system provides a direct in vitro quantitative determination of
Custom Antibodies & Recombinant Proteins
 Custom Antibodies & Recombinant Proteins INTRODUCTION Custom services to meet your research and development requirements Improvements in health, medicine and diagnostics over the past century can be largely
Custom Antibodies & Recombinant Proteins INTRODUCTION Custom services to meet your research and development requirements Improvements in health, medicine and diagnostics over the past century can be largely
91 005oo II lllt!!lll
 AD-A238 967 0 12, 1991OAJIk July 12, 1991 Grant No: N00014-89-J-3073 Trimester Report 3/15/91-7/12/91 P.I. H. Shaw Warren, M.D. Massachusetts General Hospital FLEC1 TE Boston, MA 02114 JUL 301991j i. Work
AD-A238 967 0 12, 1991OAJIk July 12, 1991 Grant No: N00014-89-J-3073 Trimester Report 3/15/91-7/12/91 P.I. H. Shaw Warren, M.D. Massachusetts General Hospital FLEC1 TE Boston, MA 02114 JUL 301991j i. Work
ICANtibodies TM. Has discovered more than 200 different antibodies for less than 2 years.
 An innovative antibody development technology using breakthrough nanotaxis leading to the antigen expression by the chosen animal species. Has discovered more than 200 different antibodies for less than
An innovative antibody development technology using breakthrough nanotaxis leading to the antigen expression by the chosen animal species. Has discovered more than 200 different antibodies for less than
ASSESSMENT OF CLINICAL FLOW CYTOMETRY LABORATORY PRACTICE. First Edition Australasian Cytometry Society Laboratory Assessment Document
 ASSESSMENT OF CLINICAL FLOW CYTOMETRY LABORATORY PRACTICE First Edition 2018 Australasian Cytometry Society Laboratory Assessment Document Paper-based publications This work is copyright. You may reproduce
ASSESSMENT OF CLINICAL FLOW CYTOMETRY LABORATORY PRACTICE First Edition 2018 Australasian Cytometry Society Laboratory Assessment Document Paper-based publications This work is copyright. You may reproduce
ONE-HOUR Western TM Multiplex Kit II
 ONE-HOUR Western TM Multiplex Kit II Technical Manual No. 0256 Version 06192009 I Description... 1 II Kit Contents.. 2 III Related Products 2 IV Key Features. 2 V Storage... 2 VI ONE-HOUR Multiplex Western
ONE-HOUR Western TM Multiplex Kit II Technical Manual No. 0256 Version 06192009 I Description... 1 II Kit Contents.. 2 III Related Products 2 IV Key Features. 2 V Storage... 2 VI ONE-HOUR Multiplex Western
Easy-WESTERN-II Quick
 Easy-WESTERN-II Quick Primary Antibody Detection Reagent for Western Blots User Manual for Quick Antigen Detection or Multi Antigen Detection Immediately after receiving the kit, read the section titled
Easy-WESTERN-II Quick Primary Antibody Detection Reagent for Western Blots User Manual for Quick Antigen Detection or Multi Antigen Detection Immediately after receiving the kit, read the section titled
Kathy O Kane Kreutzer, M.Ed., Office of Faculty Affairs, School of Medicine March, 2017
 SOM Authorship Guidelines, Recent Updates to the ICMJE Uniform Requirements for Scholarship, and the Emerging Role of Social Media in Monitoring Scholarship Kathy O Kane Kreutzer, M.Ed., Office of Faculty
SOM Authorship Guidelines, Recent Updates to the ICMJE Uniform Requirements for Scholarship, and the Emerging Role of Social Media in Monitoring Scholarship Kathy O Kane Kreutzer, M.Ed., Office of Faculty
Antibodies and Antigens In the blood bank
 Antibodies and Antigens In the blood bank 1 Nice game!! http://nobelprize.org/ 2 Karl Landsteiner discovered blood groups in 1901. Awarded Nobel Prize for Physiology or Medicine in 1930 3 Why we study
Antibodies and Antigens In the blood bank 1 Nice game!! http://nobelprize.org/ 2 Karl Landsteiner discovered blood groups in 1901. Awarded Nobel Prize for Physiology or Medicine in 1930 3 Why we study
Regulatory Issues and Drug Product Approval for Biopharmaceuticals
 Regulatory Issues and Drug Product Approval for Biopharmaceuticals Vinod P. Shah, Ph. D. FIP Scientific Secretary Biotech 2007 Southern African Regional and International Regulatory Biotechnology Workshop
Regulatory Issues and Drug Product Approval for Biopharmaceuticals Vinod P. Shah, Ph. D. FIP Scientific Secretary Biotech 2007 Southern African Regional and International Regulatory Biotechnology Workshop
Contents. 11 The Use of Epitope Tags in Histochemistry References... 98
 Contents 1 Antibodies for Immunohistochemistry... 1 1.1 Structure of Antibodies... 2 1.2 Polyclonal Antibodies... 4 1.3 Mouse Monoclonal Antibodies... 4 1.4 Rabbit Monoclonal Antibodies... 5 1.5 Protein
Contents 1 Antibodies for Immunohistochemistry... 1 1.1 Structure of Antibodies... 2 1.2 Polyclonal Antibodies... 4 1.3 Mouse Monoclonal Antibodies... 4 1.4 Rabbit Monoclonal Antibodies... 5 1.5 Protein
Title: Production and characterisation of monoclonal antibodies against RAI3 and its expression in human breast cancer
 Author's response to reviews Title: Production and characterisation of monoclonal antibodies against RAI3 and its expression in human breast cancer Authors: Hannah Jörißen (hannah.joerissen@molbiotech.rwth-aachen.de)
Author's response to reviews Title: Production and characterisation of monoclonal antibodies against RAI3 and its expression in human breast cancer Authors: Hannah Jörißen (hannah.joerissen@molbiotech.rwth-aachen.de)
Biotechnology Pathway
 Biotechnology Pathway The Biotechnology Systems (BS) Career Pathway encompasses the study of using data and scientific techniques to solve problems concerning living organisms with an emphasis on applications
Biotechnology Pathway The Biotechnology Systems (BS) Career Pathway encompasses the study of using data and scientific techniques to solve problems concerning living organisms with an emphasis on applications
Nature Structural & Molecular Biology: doi: /nsmb.1583
 Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the linearity range by following this protocol:
 Exam Mol 3008 May 2009 Subject 1 (15p) ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the linearity range by following this protocol: Reaction solutions: 50
Exam Mol 3008 May 2009 Subject 1 (15p) ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the linearity range by following this protocol: Reaction solutions: 50
Formalization of the MESF Unit of Fluorescence Intensity
 Cytometry Part B (Clinical Cytometry) 57B:1 6 (2004) Report Formalization of the MESF Unit of Fluorescence Intensity Abe Schwartz, 1 Adolfas K. Gaigalas, 2 Lili Wang, 2 Gerald E. Marti, 3 Robert F. Vogt,
Cytometry Part B (Clinical Cytometry) 57B:1 6 (2004) Report Formalization of the MESF Unit of Fluorescence Intensity Abe Schwartz, 1 Adolfas K. Gaigalas, 2 Lili Wang, 2 Gerald E. Marti, 3 Robert F. Vogt,
Jeffrey W. Elias, PhD UCD SOM Office of Research Grants Facilitation.
 NIH IS CHANGING/ENHANCING THE REVIEW CRITERIA AND FOCUSING ON MISSING OPPORTUNITIES TO EXAMINE SEX DIFFERENCES JOURNALS ARE CHANGING/ENHANCING THE REVIEW CRITERIA Jeffrey W. Elias, PhD UCD SOM Office of
NIH IS CHANGING/ENHANCING THE REVIEW CRITERIA AND FOCUSING ON MISSING OPPORTUNITIES TO EXAMINE SEX DIFFERENCES JOURNALS ARE CHANGING/ENHANCING THE REVIEW CRITERIA Jeffrey W. Elias, PhD UCD SOM Office of
Disruption of antigenic variation proves crucial for effective parasite vaccine. Supplementary Results
 Disruption of antigenic variation proves crucial for effective parasite vaccine Fernando D. Rivero, Alicia Saura, Cesar G. Prucca, Pedro G. Carranza, Alessandro Torri, and Hugo D. Lujan Supplementary Results
Disruption of antigenic variation proves crucial for effective parasite vaccine Fernando D. Rivero, Alicia Saura, Cesar G. Prucca, Pedro G. Carranza, Alessandro Torri, and Hugo D. Lujan Supplementary Results
