A Single Intravenous AAV9 Injection Mediates Bilateral Gene Transfer to the Adult Mouse Retina
|
|
|
- Ashley Johnson
- 6 years ago
- Views:
Transcription
1 A Single Intravenous AAV9 Injection Mediates Bilateral Gene Transfer to the Adult Mouse Retina Alexis-Pierre Bemelmans 1,2,3,4., Sandra Duqué 5., Christel Rivière 6, Stéphanie Astord 5, Mélissa Desrosiers 1,2,3, Thibault Marais 5, José-Alain Sahel 1,2,3,7, Thomas Voit 5, Martine Barkats 5 * 1 INSERM, U968, Paris, France, 2 Institut de la Vision, Université Pierre et Marie Curie Paris 6 - UM80, Paris, France, 3 UMR_7210, CNRS, Paris, France, 4 Molecular Imaging Research Center (MIRCen) and CNRS URA2210, Commissariat à l Energie Atomique et aux Energies Alternatives (CEA), Département des Sciences du Vivant (DSV), Institut d Imagerie Biomédicale (I2BM), Fontenay-aux-Roses, France, 5 UM76 UPMC-AIM UMR S974, INSERM U 974, CNRS UMR 7215, Institut de Myologie, Université Pierre et Marie Curie Paris 6, Paris, France, 6 Généthon, Evry, France, 7 INSERM-DHOS CIC 503, Centre Hospitalier National d Ophtalmologie des Quinze-Vingts, Paris, France Abstract Widespread gene delivery to the retina is an important challenge for the treatment of retinal diseases, such as retinal dystrophies. We and others have recently shown that the intravenous injection of a self-complementary (sc) AAV9 vector can direct efficient cell transduction in the central nervous system, in both neonatal and adult animals. We show here that the intravenous injection of scaav9 encoding green fluorescent protein (GFP) resulted in gene transfer to all layers of the retina in adult mice, despite the presence of a mature blood-eye barrier. Cell morphology studies and double-labeling with retinal cell-specific markers showed that GFP was expressed in retinal pigment epithelium cells, photoreceptors, bipolar cells, Müller cells and retinal ganglion cells. The cells on the inner side of the retina, including retinal ganglion cells in particular, were transduced with the highest efficiency. Quantification of the cell population co-expressing GFP and Brn-3a showed that 45% of the retinal ganglion cells were efficiently transduced after intravenous scaav9-gfp injection in adult mice. This study provides the first demonstration that a single intravenous scaav9 injection can deliver transgenes to the retinas of both eyes in adult mice, suggesting that this vector serotype is able to cross mature blood-eye barriers. This intravascular gene transfer approach, by eliminating the potential invasiveness of ocular surgery, could constitute an alternative when fragility of the retina precludes subretinal or intravitreal injections of viral vectors, opening up new possibilities for gene therapy for retinal diseases. Citation: Bemelmans A-P, Duqué S, Rivière C, Astord S, Desrosiers M, et al. (2013) A Single Intravenous AAV9 Injection Mediates Bilateral Gene Transfer to the Adult Mouse Retina. PLoS ONE 8(4): e doi: /journal.pone Editor: Alfred Lewin, University of Florida, United States of America Received March 19, 2012; Accepted March 15, 2013; Published April 15, 2013 Copyright: ß 2013 Bemelmans et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was supported by the Université Pierre et Marie Curie (UPMC), the Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS), and the Association Française contre les Myopathies (AFM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: The authors have declared that no competing interests exist. * m.barkats@institut-myologie.org. These authors contributed equally to this work. Introduction A number of inherited human blindness disorders, some monogenic and others with multi-factorial inheritance, are particularly amenable to gene therapy. It would therefore be theoretically possible to treat retinal diseases, such as retinitis pigmentosa (RP), which is caused by gene mutations in photoreceptors or retinal pigment epithelium (RPE) [1], and glaucoma, which is caused by gene mutations in retinal ganglion cells (RGC) [2], by transferring specific genes into the appropriate retinal cell population. The delivery of transgenes to retinal cells in animal models generally requires subretinal or intravitreal injections of viral vectors, such as adeno-associated virus (AAV) or lentiviral vectors. Genes can generally be delivered to the inner side of the retina through the intravitreal injection of AAV vectors but, due to vector accumulation at the vitreoretinal junction, only localized transgene expression can be obtained in RGC, and deep retinal layers have never been successfully transduced in healthy retina [3 5]. By contrast, the subretinal route of delivery can be used to target the RPE and/or the photoreceptors with lentiviral or AAV vectors [6 10]. AAV is a particularly promising vector for gene therapy for retinal diseases, due to its weak immunogenicity, its ability to infect all retinal cell types and its potential for the long-term expression of transgenes [3,11]. Several clinical trials have recently shown that the subretinal injection of RPE65- encoding AAV yields significant visual improvement in patients with Leber s congenital amaurosis (LCA), a severe form of retinal degeneration affecting children [12 14]. The subretinal injection of gene vectors is generally considered to be safe [6,15], but this surgical procedure induces detachment of the retina at the site of injection, leading to localized trauma and possible retinal thinning and cell destruction [16 18]. If prolonged, retinal detachment can induce the apoptotic cell death of photoreceptor cells, leading to a loss of vision [19]. In the first clinical trials of gene therapy for LCA [12 14], the subretinal injection of RPE65-expressing AAV caused temporary retinal detachment, which resolved spontaneously in most cases. However, the development of a macular hole in one case highlights the risks of such surgery [20,21]. Subretinal gene vector delivery is also of questionable value in gene therapy for retinal disorders increasing the likelihood of retinal detachment, such as X-linked PLOS ONE 1 April 2013 Volume 8 Issue 4 e61618
2 juvenile retinoschisis [5], or diseases affecting the subretinal space, such as the wet-form of age-related macular degeneration, in which the development of subretinal choroidal neovascular membranes or subretinal hemorrhage is responsible for most of the vision loss [22]. Finally, subretinal injection usually limits transgene delivery to the area surrounding the injection site, and this is not ideal for the treatment of diseases requiring the transduction of cells throughout the retina [5]. The translation of retinal gene transfer into clinical practice might therefore require alternative delivery routes to subretinal injection. The transfer of genes to cells throughout the retinas of both eyes via a single systemic injection of viral gene vectors would constitute an attractive non invasive strategy for the treatment of retinal diseases. However, systemic gene transfer to the retina is hampered by the tight junctions of the blood-eye barrier, which prevents the passage of viral vectors from the bloodstream into the subretinal space, particularly in adults [23]. We and others have shown that the self-complementary AAV9 vector (scaav9) has a remarkable ability to mediate widespread transgene expression in the brain and spinal cord following its intravenous injection into both neonatal and adult animals [24 26], suggesting that this vector can cross the blood-brain barrier (BBB). The therapeutic potential of this systemic approach was recently demonstrated in a mouse model of spinal muscular atrophy (SMA), a devastating neuromuscular disorder caused by mutations or deletions of the Survival of Motor Neuron (SMN) gene [27 30]. In these pioneering studies, mice intravenously injected with SMN-encoding scaav9 during the perinatal period displayed an impressive rescue of SMA. The basis of this robust transduction of central nervous system (CNS) cells after the systemic delivery of scaav9 remains unclear. It is thought to involve differential BBB transport, but it remains unclear how AAV9 crosses the BBB and whether this mechanism is different from that of other serotypes in vivo. The superiority of scaav9 for the systemic transduction of nerve cells may be due to various factors, including capsid-interacting blood factors, strong neural cell tropism or intracellular trafficking, the rapid uncoating of virion shells in cells and delayed blood clearance [31,32]. In this study, we investigated whether the systemic injection of scaav9 could mediate transduction of the retina in adult mice despite the presence of functional blood-eye barriers. We found that the intravenous injection of scaav9 into adult mice resulted in gene transfer to all cell layers of the retina, with the predominant transduction of RGC and ciliary bodies. This study suggests that this vector could cross mature blood-eye barriers, and constitutes the basis for future development of a non invasive alternative to the current methods of viral gene delivery to the retina. Materials and Methods Animals This study was performed on adult (6 to 8 weeks old, female) C57Bl6 mice purchased from Charles River Laboratories (Les Oncins, France). All animal experiments were carried out in accordance with European guidelines for the care and use of experimental animals. AAV Vector Production AAV vectors express GFP or mouse secreted alkaline phosphatase (mseap) under the control of the cytomegalovirus immediate early (CMV) promoter. Self-complementary genome-containing plasmids were constructed by deleting the D sequence and the terminal resolution site from one of the inverted terminal repeats. The production of serotype 9 AAV has been described elsewhere [24]. Briefly, pseudotyped AAV9 and AAV2 vectors were generated by packaging AAV2-based recombinant single-stranded (ss) or self-complementary (sc) genomes into the AAV9 or AAV2 capsids. Virions were produced by transfecting HEK293 cells with (i) the adenovirus helper plasmid (pxx6-80), (ii) the AAV packaging plasmid encoding the rep2 and the cap2 or the cap9 genes, and (iii) the AAV2 shuttle plasmid containing the gene encoding GFP or mseap in a ss or sc genome. Recombinant vectors (raav) were purified by double-cscl ultracentrifugation followed by dialysis against the formulation buffer of the vector stocks, namely phosphate-buffered saline containing 0.5 mm MgCl2 and 1.25 mm KCl (PBS-MK; five buffer changes, 3 hours per round of dialysis). Physical particles were quantified by realtime PCR. Vector titers are expressed as viral genomes per milliliter (vg/ml). Peripheral Administration of AAV Vectors In adults, AAV vectors were administered peripherally by injection into the tail vein at 8 weeks of age. The animals were restrained in a tube, facilitating manipulation of the tail. A 30- gauge needle attached to a 1 ml syringe was inserted into the tail vein and 500 ml of the viral solution was injected over a period of about 30 seconds. Real-time PCR Quantification of Vector Genome Copy Number in the Retina Enucleated whole eyes were snap-frozen in liquid nitrogen and stored at 280uC until further processing. Frozen tissues were lysed in 700 ml of nuclear lysis buffer (Wizard genomic DNA extraction kit, Promega, Charbonnières-les-Bains, France). Tissues were homogenized by four 30-second pulses with an Ultra-Turrax homogenizer, to ensure complete lysis. Cell membranes and debris were pelleted by centrifugation for 2 minutes at 10,0006g and 4uC. A sample of the supernatant was collected for the mseap quantification assay, and genomic DNA containing the AAV vector genome was purified according to the manufacturer s instructions. For each sample, we used 72 ng of genomic DNA as the template. Vector genome copy number was determined by a real-time PCR assay with primers and a probe corresponding to the inverted terminal repeat region (ITR) of the AAV vector genome common to ss and scaav vectors. The sequences of the primer pair and the Taqman probe were 59-CTCCATCAC- TAGGGGTTCCTTG-39 (AAV-Fw), 59-GTAGATAAGTAG- CATGGC-39 (AAV-Rev) and 59-TAGTTAATGATTAACC- CAA-39 (AAV-MGBprobe). Amplification of the Titin gene was used to normalize the results with respect to the number of AAV vector genome copies per cell. The sequences of the primer pair and the Taqman probe were 59-AAAACGAGCAGTGACGT- GAGC-39 (Titin-Fw), 59-TTCAGTCATGCTGCTAGCGC-39 (Titin-Rev) and 59-TGCACGGAAGCGTCTCGTCTCAGCT- 39 (Titin-VIC/TAMRAprobe). Serial dilutions of the raav vector plasmid were used to generate a standard curve for the determination of vector genome copy numbers. Real-time PCR was carried out and results were analyzed with the ABI Prism 7700 sequence Detection System (Applied Biosystems, Foster City CA, USA). mseap Quantification Assay mseap activity in the eye lysate supernatant was quantified in a chemiluminescence assay. Endogenous alkaline phosphatase was inactivated by heating at 65uC for 5 minutes and the heat-resistant mseap activity was measured by adding reaction buffer and the PLOS ONE 2 April 2013 Volume 8 Issue 4 e61618
3 PLOS ONE 3 April 2013 Volume 8 Issue 4 e61618
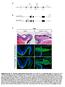
4 Figure 1. GFP expression in the retina after the intravenous delivery of scaav9-gfp in adult mice. Retinal cross sections were treated for GFP immunofluorescence (green) and counterstained with DAPI (blue), four weeks after the injection of vg of scaav9-gfp into the tail veins of eight-week-old mice. GFP was detected in all retina layers (A D) and in the ciliary bodies (CB in A). Transduction efficiency was particularly high in the RGC layer (B C) but GFP was also expressed in the various cell types of the inner nuclear layer (INL), including cells with the morphology of bipolar cells (arrowheads in C) and of Müller cells (arrows in D). Rare GFP-positive photoreceptors (asterisks in B and D) and RPE cells (arrowheads in D and F) were also detected. (E G) High magnification of GFP-positive (E) Müller cells, (F) RPE cells and (G) photoreceptors. RPE: retinal pigment epithelium; ONL: outer nuclear layer; INL: inner nuclear layer; RGC: retinal ganglion cell layer. Scale bar: 200 mm in A and B; 70 mm inc;50mm ind; 20 mm in E G. A, B and E G are epifluorescence images; C and D are confocal images. doi: /journal.pone g001 CPSD chemiluminescence substrate of the Tropix system, according to the manufacturer s instructions (Applied Biosystems, Foster City CA, USA). Chemiluminescence was then determined with a luminometer (Perkin Elmer). Activity levels are expressed as ng of mseap and were determined by comparison with a standard curve for purified human placental alkaline phosphatase. Results were normalized on the basis of protein concentration, determined with the Nano-orange protein quantification assay (Invitrogen, Cergy-Pontoise, France). Histological Processing Mice were deeply anesthetized with 10 mg.kg 21 xylazine and 100 mg.kg 21 ketamine and transcardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by ice-cold 4% paraformaldehyde in PBS. The eyes were enucleated and post-fixed by overnight incubation in the same fixative, cryoprotected by overnight incubation in 15% sucrose in PBS, frozen in cold isopentane (250uC) and cut on a cryostat into 14 mm sections, which were stored at 280uC until further processing. For immunofluorescence labeling, sections were rinsed twice in PBS, blocked by incubation for one hour in PBS supplemented with 10% normal goat serum (NGS) and 0.2% Triton X-100 and incubated overnight at 4uC with primary antibodies in PBS supplemented with 1% NGS and 0.2% Triton X-100. Anti-GFP antibody (1:800; Abcam, Cambridge, UK), anti-brn-3a antibody (1:19000; Millipore, Billericay MA, USA) and anti-chx10 antibody (1:300; Santa Cruz Biotechnology, Santa Cruz CA, USA) were used. Sections were then rinsed in PBS, incubated with fluorescently labeled secondary antibodies (Alexa-Fluor conjugates, 1:1,000; Invitrogen, Cergy-Pontoise, France), labeled with 49,69-diamidino-2-phenylindol (DAPI) and mounted in Mowiol 4 88 reagent (Sigma-Aldrich, Lyon, France) and covered with a coverslip for examination under a DM6000-B epifluorescence microscope (Leica Microsystèmes, Nanterre, France). Brn-3a- and GFP-positive cells were counted by eye at 206 magnification. Results We investigated the efficiency of retina cell transduction following the intravenous delivery of scaav9 vectors in adult mice, by injecting a scaav9 vector expressing the murine secreted alkaline phosphatase (mseap) under the transcriptional control of the cytomegalovirus (CMV) promoter (10 12 vector genomes [vg] per mouse) into the tail vein of eight-week-old mice. Four weeks after injection, quantitative PCR analysis on genomic DNA from the whole eyes showed that the vector was present in eye tissue (0.320+/20.184, n = 3). Furthermore, significant mseap activity was detected in eye protein extracts from the scaav9-treated mice (10.15+/25.86 pg of mseap per mg of protein, n = 3). These results strongly suggest that scaav9 was able to reach the ocular tissues after intravenous injection in adult mice, probably by crossing mature blood-eye barriers. We tested this hypothesis by analyzing the pattern of retinal cell transduction after the intravenous injection of a scaav9 vector expressing the green fluorescent protein (GFP) under transcriptional control of the CMV promoter into adult mice. We injected scaav9-cmv-gfp ( vg per mouse) into the tail vein of eight-week-old mice (n = 6) and assessed transgene expression in immunofluorescence analyses carried out four weeks later. Multiple layers of the retina expressed GFP to various extents in all the animals. GFP was detected in the choroid, the RPE, the photoreceptor and outer nuclear layers (ONL) (rod/cones), the outer plexiform and inner nuclear layers (INL) (bipolar and horizontal cells), the inner plexiform and RGC layers (amacrine and ganglion cells), for example (Figure 1). Strong GFP expression was also detected along the ganglion cell axons forming the optic nerve and within the ciliary bodies (Figure 2). No specific immunostaining was detected in control mice that received no injection (data not shown). To demonstrate the specificity of scaav9 to transduce retinal neurons after peripheral administration in adult mice, another serotype was administered according to the same experimental design. We selected AAV serotype 2, a vector which does not cross the BRB and which is particularly efficient for ganglion cell transduction following intravitreal injection in mice [33]. New batches of AAV2 and AAV9 vectors were produced and injected into the tail vein of adult mice ( vg/mice, n = 3 mice for each serotype). One month after injection, numerous GFP expressing retinal cells were clearly detected in the 6 eyes of the scaav9-injected mice (Figure 3). In accordance to the previous experiment, the retinal cell layer most efficiently transduced by scaav9 was the RGC layer (Figure 3). In contrast, no GFP expression was detected in the retina of any of the 6 eyes from the scaav2-injected mice, which confirmed that the BRB was not disrupted by the gene transfer procedure. We then investigated the nature of the GFP-positive cells in the RGC layer, by double-labeling retina sections with antibodies directed against GFP and Brn-3a, a POU Domain transcription factor specifically expressed in the nuclei of the retinal ganglion cells within the retina [34]. A large proportion of the GFP-positive cells located in the RGC layer were found to be retinal ganglion cells (Figure 4 A C). We also investigated the colocalization of GFP and Chx10, a transcription factor specifically expressed in the nucleus of bipolar cells [35]. This analysis identified the GFPpositive cells located in the INL as bipolar cells (Figure 4 D F). It is noteworthy that only a negligible number of ganglion cell were visible on the latter sections taken from the central part of the retina (as denoted by the thickness of the retinal nerve fiber layer [RNFL]). At this level of the retina the number of transduced- RGC is low and not representative of the whole retina. We quantified the efficiency of gene transfer to the retinal ganglion cells of the RGC layer (the most efficiently transduced retina layer), by determining the number of GFP-positive cells, the number of Brn-3a-positive cells, and the number of cells expressing both markers, on transverse sections of the retina at the level of the optic nerve (n = 6, one eye per mouse). We found that cells per retinal section expressed GFP in the RGC layer, and that of these cells also expressed Brn-3a. The Brn-3a-positive cell population was estimated at cells per retinal section. Thus, almost 45% of the Brn-3a-positive retinal PLOS ONE 4 April 2013 Volume 8 Issue 4 e61618
5 Systemic scaav9 Gene Transfer to the Retina Figure 2. GFP expression in the optic nerve and the ciliary body of intravenous scaav9-gfp injected adult mice. Representative cross sections of the optic nerve (A) and the ciliary body (B) treated for GFP immunofluorescence (green) and stained with DAPI (blue), four weeks after the injection of vg of scaav9-gfp into the tail veins of eight-week-old mice. In (A), the boundaries of the retinal nerve fiber layer (originating from the RGC) are clearly demarcated by their pattern of GFP expression (arrowheads) (arrows: GFP-positive axons in the optic nerve). (B) High magnification of the ciliary body, showing strong GFP expression in the epithelial cells. ON: optic nerve; RET: retina; TM: trabecular meshwork. Scale bar: 100 mm. doi: /journal.pone g002 body (the blood-aqueous barrier), and the other preventing outward movement from the retina into the blood (the bloodretinal barrier) [23]. We found that retinal ganglion cells were the principal cells transduced in the retina after the intravenous injection of scaav9 in adult mice. These findings suggest that scaav9 may be delivered to the neural retina either directly from the retinal circulation, by crossing the blood-retinal barrier, or indirectly, entering the aqueous and vitreous humors via the ciliary bodies the structural equivalent of the blood-aqueous barrier to reach its final destination, the retinal cells. The ciliary processes and the adjacent retinal cells appeared to be strongly transduced after intravenous scaav9 injection, suggesting that at least some of the vector passed across the tight junctions between the non pigmented cells of the ciliary epithelium. These findings are of particular importance because systemic AAV9-mediated transduction of the retina has previously been reported to be dependent on the age of the animal, with efficient transduction observed only in neonatal or fetal animals [39 42]. Such discrepancies between our data and previous work from several groups may be due to the use in our study of a selfcomplementary genome-based AAV9, or to species- differences in the vector tropism. For example, Bostick et al. showed that the systemic injection of single-stranded (ss) AAV9 mediated gene transfer to the inner layer of the retina in neonatal mice, but that systemic ssaav9 gene transfer was inefficient in adults [39], suggesting the superiority of the scaav9 versus its single-stranded ganglion cells in the RGC layer were efficiently transduced after the intravenous delivery of scaav9-gfp in adult mice. Discussion Recombinant scaav9 is currently considered to be one of the most promising vectors for human gene therapy because this serotype transduces the cells of various organs, including the heart, liver, skeletal muscle, brain and spinal cord, highly efficiently after its systemic administration [24,25,36 38]. The demonstration of broad gene delivery to neurons after systemic scaav9 injection [24,25] and the therapeutic proof-of-principle of this method in a mouse model of SMA [27 29] have paved the way for the clinical development of intravenous scaav9 gene therapy for SMA in Europe and the USA. This study provides the first demonstration that scaav9 can transduce ocular tissues following its intravenous injection in adult mice. One month after the injection of a scaav9 encoding a reporter gene in eight-week-old mice, transgene expression was detected in multiple layers of the retina, in the optic nerve and in the ciliary bodies. These findings suggest that scaav9 may cross the mature blood-eye barrier, which, in adult mammalian eyes, consists of tissue layers separating the neural retina and the transparent refractive media from the circulating blood. Like the BBB, there are two main barrier systems in the eye: one essentially regulating inward movements from the blood into the eye at the level of the ciliary PLOS ONE 5 April 2013 Volume 8 Issue 4 e61618
6 PLOS ONE 6 April 2013 Volume 8 Issue 4 e61618
7 Figure 3. Systemic injection of AAV serotype 2 does not lead to transduction of the neural retina. GFP expression in representative cross-sections of the retina of adult mice one month after systemic administration of vg scaav-gfp of serotype 9 (A F) or serotype 2 (G L) in adult mice (n = 3 per condition). GFP expression was detected in the neural retina in all mice from the serotype 9 treated-group (panel A to F are from three different animals). As expected, the highest transduction efficiency was observed at the level of the RGC layer. In contrast, no GFP expression was detected in the retina after AAV serotype 2 injection (panels G to L are from three different animals). Left panels: GFP immunofluorescence; right panels: merged view of GFP (green) and dapi (blue). ONL: outer nuclear layer; INL: inner nuclear layer; RGC: retinal ganglion cell layer. Scale bar: 100 mm. doi: /journal.pone g003 counterpart. Also, RPE cells but not other retinal cells were found to be targeted when scaav9 vectors were delivered to other species such as the cat or the dog [40]. The use of different promoters could also explain the disparity between the studies. In the present study, we used a CMV promoter, which is known to drive high and constitutive expression of foreign genes in a variety of neurons and in retinal ganglion cells in particular [43], while for instance Bostick et al. used a RSV promoter in adults and neonates [39], and Dalkara et al. used a CAG promoter in neonates [42]. The method of AAV preparation (CsCl or Iodixanol purification) could also impact both the transduction pattern and efficiency by inducing changes in the vector tropism [44]. Finally, the use of different doses of vector could also be the cause of differences between studies, but this remains particularly difficult to establish because of the various titration methods used in different laboratories. This first report of efficient retinal gene delivery by systemic AAV9 treatment in adult animals might have important implications for clinical applications. Indeed, the possibility of targeting retina cells in adult patients soon after the onset of symptoms is an essential prerequisite of gene therapy for most retinal diseases. In this context, systemic AAV9 gene delivery may be indicated, in particular, for the treatment of Leber s hereditary optic neuropathy (LHON), a genetic disease of young adults leading to the preferential degeneration of RGCs and their axons (optic nerve). Another advantage of systemic gene therapy is its potential application to retinal diseases for which intravitreal or subretinal injections would be too risky. For example, high intraocular pressure (IOP) in glaucoma is a major risk factor thought to play a major role in RGC degeneration. Local gene vector injection, which would further increase IOP and induce an inflammatory reaction, could thus be replaced by the systemic delivery of scaav9 vectors. This route of administration would also be particularly advantageous for the two forms of age-related macular degeneration (AMD), in which abnormal neovascularization of the choroid is associated with a risk of bleeding and irreversible damage to the retina ( wet AMD) or the accumulation of cellular debris (drusen) between the retina and the choroid ( dry AMD). Both forms of AMD lead to retinal detachment and could benefit advantageously from a systemic gene therapy. In the present study, the use of a ubiquitous promoter resulted in a non-specific expression of the transgene in the whole retina and in a number of nervous and non-nervous organs [24] following scaav9 systemic delivery. The use of this method for specific delivery of transgenes to the retina might therefore involve utilization of cell-specific promoters, such as the Thy1.1 promoter Figure 4. GFP expression in RGC and bipolar cells. Representative retina sections from adult mice treated for double-immunofluorescence analysis four weeks after the injection of vg of scaav9-gfp vectors into the tail vein. (A C) Double-labeling of transduced RGCs for GFP (green) and Brn-3a (red) (arrowheads indicate double-labeled RGCs and arrows indicate cells expressing GFP only). A transduced photoreceptor is highlighted with an asterisk. (D F) Double-labeling of bipolar cells for GFP (green) and Chx10 (red). The arrow indicates a Chx10-positive bipolar cell with high levels of GFP. The retinal nerve fiber layer (RNFL), labeled for GFP due to the transduction of upstream RGC, is indicated by an asterisk. No RGC can be seen on this panel which illustrates the central part of the retina (as demonstrated by the thickness of the RNFL). ONL: outer nuclear layer; INL: inner nuclear layer; RGC: retinal ganglion cell layer. Scale bar: 50 mm. doi: /journal.pone g004 PLOS ONE 7 April 2013 Volume 8 Issue 4 e61618
8 for RGC specific expression [45], or the myocilin promoter, which may allow preferential expression in the ciliary body [46]. Although tissue specific promoters generally drive lower expression than ubiquitous promoters in many tissues, in some cases, tissue-specific promoters were found to be superior to ubiquitous promoters in vitro and in vivo [47,48]. In the retina, Miyoshi et al. (1997) found the rhodopsin promoter to be significantly superior to CMV in vivo to drive transgene expression in the photoreceptors (x2.5 higher levels of expression in the photoreceptors than the CMV promoter) [49]. However, in addition to its possible use to treat diseases limited to the eye, systemic injection of scaav9 vectors might also be useful for treating pathological conditions affecting both the eye and other organs. For example, systemic gene therapy may be a particularly suitable approach to the treatment of syndromic retinitis pigmentosa (Usher syndrome), a disease in which both the retina and the sensory neurons of the ear are impaired [50], and for the treatment of many lysosomal storage diseases, such as Gaucher s disease, which affects peripheral organs (the lung, liver and spleen in particular) and in which patients also present neurological symptoms and eye problems, such as glaucoma. The Bardet-Biedl syndrome, a genetic multisystem disorder, would also benefit in an advantageous way from systemic gene therapy using AAV9 vectors [51]. Another important finding of this study was the highly efficient transduction of the ciliary body, an epithelium within the eye that is responsible for producing the aqueous humor. Following systemic scaav9 transduction, ciliary bodies could thus be used References 1. den Hollander AI, Black A, Bennett J, Cremers FP (2010) Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J Clin Invest 120: Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, et al. (1997) Identification of a gene that causes primary open angle glaucoma. Science 275: Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, et al. (2009) Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 17: Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, et al. (2010) Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther 21: Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, et al. (2009) Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther 16: Bemelmans AP, Bonnel S, Houhou L, Dufour N, Nandrot E, et al. (2005) Retinal cell type expression specificity of HIV-1-derived gene transfer vectors upon subretinal injection in the adult rat: influence of pseudotyping and promoter. J Gene Med 7: Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, et al. (2006) Effective gene therapy with nonintegrating lentiviral vectors. Nat Med 12: Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, et al. (2001) Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 28: Boye SE, Boye SL, Pang J, Ryals R, Everhart D, et al. (2010) Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS One 5: e Sun X, Pawlyk B, Xu X, Liu X, Bulgakov OV, et al. (2010) Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther 17: Buch PK, Bainbridge JW, Ali RR (2008) AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene Ther 15: Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, et al. (2008) Effect of gene therapy on visual function in Leber s congenital amaurosis. N Engl J Med 358: Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, et al. (2008) Safety and efficacy of gene transfer for Leber s congenital amaurosis. N Engl J Med 358: Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, et al. (2008) Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 19: as biological pumps, to ensure the long-term secretion of therapeutic proteins (such as anti-vegf antibodies [52]) into the anterior and posterior chambers of the eye or into the vitreous humor [53]. In summary, we report here the capacity of intravenous scaav9 to target multiple retinal layers in adult animals in a non invasive manner, with a predominant transduction of the retinal ganglion cells, the optic nerve and the ciliary bodies. Improving the efficiency of transduction in all retinal layers (particularly the photoreceptors), for example by the use of tyrosine to phenylalanine mutant AAV9 [41], and inducing specificity of expression to the retina using cell-specific promoters would present this intravascular approach as a basis for alternative developments to current subretinal or intravitreal injection. Attempts to translate this strategy into clinical practice for humans should also take into account possible species differences in the retinal tropism of AAV9 vectors. Acknowledgments We thank Peggy Barbe and Gwenaëlle Auregan for their excellent technical support. Author Contributions Critically read the manuscript: TV JAS. Conceived and designed the experiments: APB SD MB. Performed the experiments: APB SD CR SA MD TM. Analyzed the data: APB SD MB. Wrote the paper: APB MB. 15. Bennett J, Wilson J, Sun D, Forbes B, Maguire A (1994) Adenovirus vectormediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci 35: Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, et al. (2007) Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther 14: Cheng L, Toyoguchi M, Looney DJ, Lee J, Davidson MC, et al. (2005) Efficient gene transfer to retinal pigment epithelium cells with long-term expression. Retina 25: Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, et al. (2008) AAVmediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci 49: Abouzeid H, Wolfensberger TJ (2006) Macular recovery after retinal detachment. Acta Ophthalmol Scand 84: Bainbridge JW, Ali RR (2008) Success in sight: The eyes have it! Ocular gene therapy trials for LCA look promising. Gene Ther 15: Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, et al. (2010) Gene therapy for Leber s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18: Bird AC (2010) Therapeutic targets in age-related macular disease. J Clin Invest 120: Cunha-Vaz JG (1997) The blood-ocular barriers: past, present, and future. Doc Ophthalmol 93: Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, et al. (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 17: Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, et al. (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27: Barkats M (2007) Widespread Gene Delivery to Motor Neurons Using Peripheral Injection of AAV Vectors. 27. Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, et al. (2011) Intravenous scaav9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet 20: Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, et al. (2010) Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 28: Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, et al. (2010) Systemic delivery of scaav9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci Transl Med 2: 35ra Passini MA, Cheng SH (2011) Prospects for the gene therapy of spinal muscular atrophy. Trends Mol Med 17: PLOS ONE 8 April 2013 Volume 8 Issue 4 e61618
9 31. Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, et al. (2011) Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19: Kotchey NM, Adachi K, Zahid M, Inagaki K, Charan R, et al. (2011) A potential role of distinctively delayed blood clearance of recombinant adenoassociated virus serotype 9 in robust cardiac transduction. Mol Ther 19: Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, et al. (2009) Highefficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 17: Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, Nieto-Lopez L, Canovas-Martinez I, et al. (2009) Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci 50: Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, et al. (1994) Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron 13: Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, et al. (2006) Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 14: Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, et al. (2006) Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 99: e Zincarelli C, Soltys S, Rengo G, Rabinowitz JE (2008) Analysis of AAV serotypes 1 9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16: Bostick B, Ghosh A, Yue Y, Long C, Duan D (2007) Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther 14: Joussemet B, Belbellaa B, Mendes-Madeira A, Bucher T, Briot-Nivard D, et al. (2011) Neonatal systemic delivery of scaav9 in rodents and large animals results in gene transfer to RPE cells in the retina. Exp Eye Res 93: Rahim AA, Wong AM, Hoefer K, Buckley SM, Mattar CN, et al. (2011) Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. FASEB J 25: Dalkara D, Byrne LC, Lee T, Hoffmann NV, Schaffer DV, et al. (2011) Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther. 43. Aartsen WM, van Cleef KW, Pellissier LP, Hoek RM, Vos RM, et al. (2010) GFAP-driven GFP expression in activated mouse Muller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS One 5: e Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP (2008) AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 16: Hayworth CR, Rojas JC, Gonzalez-Lima F (2008) Transgenic mice expressing cyan fluorescent protein as a reporter strain to detect the effects of rotenone toxicity on retinal ganglion cells. J Toxicol Environ Health A 71: Tamm ER (2002) Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res 21: Pacak CA, Sakai Y, Thattaliyath BD, Mah CS, Byrne BJ (2008) Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther 6: Xu R, Janson CG, Mastakov M, Lawlor P, Young D, et al. (2001) Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther 8: Miyoshi H, Takahashi M, Gage FH, Verma IM (1997) Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci U S A 94: Williams DS (2008) Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res 48: Simons DL, Boye SL, Hauswirth WW, Wu SM (2011) Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci U S A 108: Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, et al. (2007) Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci 48: Bishop PN, Takanosu M, Le Goff M, Mayne R (2002) The role of the posterior ciliary body in the biosynthesis of vitreous humour. Eye (Lond) 16: PLOS ONE 9 April 2013 Volume 8 Issue 4 e61618
vision is our mission NASDAQ: OPHT October 2018
 vision is our mission NASDAQ: OPHT October 2018 Forward-looking Statements Any statements in this presentation about Ophthotech s future expectations, plans and prospects constitute forwardlooking statements
vision is our mission NASDAQ: OPHT October 2018 Forward-looking Statements Any statements in this presentation about Ophthotech s future expectations, plans and prospects constitute forwardlooking statements
Chapter 86 Advances in AAV Vector Development for Gene Therapy in the Retina
 Chapter 86 Advances in AAV Vector Development for Gene Therapy in the Retina Timothy P. Day, Leah C. Byrne, David V. Schaffer and John G. Flannery Abstract Adeno-associated virus (AAV) is a small, non-pathogenic
Chapter 86 Advances in AAV Vector Development for Gene Therapy in the Retina Timothy P. Day, Leah C. Byrne, David V. Schaffer and John G. Flannery Abstract Adeno-associated virus (AAV) is a small, non-pathogenic
Quality Control Assays
 QUALITY CONTROL An integral part of the Penn Vector Core is its robust quality control program which is carried out by a separate quality control group. Quality control assays have been developed and optimized
QUALITY CONTROL An integral part of the Penn Vector Core is its robust quality control program which is carried out by a separate quality control group. Quality control assays have been developed and optimized
VECTOR SYSTEMS. Vector Systems
 VECTOR SYSTEMS Vector Systems The vector systems currently available through Penn Vector Core are those derived from adenoassociated virus (AAV), adenovirus and lentivirus. The Core offers AAV-based vectors
VECTOR SYSTEMS Vector Systems The vector systems currently available through Penn Vector Core are those derived from adenoassociated virus (AAV), adenovirus and lentivirus. The Core offers AAV-based vectors
Lecture 7 Adeno-associated viral vectors
 Lecture 7 Adeno-associated viral vectors Viral vectors, continued 19th November 2012 AAV adenovirus 2 Infectious cycle of AAV 3 Adeno-associated viruses AAV Small, non-pathogenic single stranded DNA viruses.
Lecture 7 Adeno-associated viral vectors Viral vectors, continued 19th November 2012 AAV adenovirus 2 Infectious cycle of AAV 3 Adeno-associated viruses AAV Small, non-pathogenic single stranded DNA viruses.
Innovative Non-Viral Gene Therapies for the Treatment of Ocular Diseases
 Innovative Non-Viral Gene Therapies for the Treatment of Ocular Diseases Innovative Non-Viral Gene Therapies for the Treatment of Ocular Diseases Company Clinical stage company developing innovative non-viral
Innovative Non-Viral Gene Therapies for the Treatment of Ocular Diseases Innovative Non-Viral Gene Therapies for the Treatment of Ocular Diseases Company Clinical stage company developing innovative non-viral
CRN 18-mo progress update
 CRN 18-mo progress update Gene transfer studies for cystinosis Principal investigator: Vasiliki Kalatzis, Ph.D. Co-investigator: Eric J. Kremer, Ph.D. Affiliation: Institut Génétique Moléculaire de Montpellier
CRN 18-mo progress update Gene transfer studies for cystinosis Principal investigator: Vasiliki Kalatzis, Ph.D. Co-investigator: Eric J. Kremer, Ph.D. Affiliation: Institut Génétique Moléculaire de Montpellier
A Novel Approach to Ocular Gene Therapy: Evaluation of Suprachoroidally Administered Non-Viral DNA Nanoparticles in Rabbits
 A Novel Approach to Ocular Gene Therapy: Evaluation of Suprachoroidally Administered Non-Viral DNA Nanoparticles in Rabbits Szilárd Kiss, MD Division Chief, Retina Service Director of Clinical Research
A Novel Approach to Ocular Gene Therapy: Evaluation of Suprachoroidally Administered Non-Viral DNA Nanoparticles in Rabbits Szilárd Kiss, MD Division Chief, Retina Service Director of Clinical Research
AAVanced Concentration Reagent
 AAVanced Concentration Reagent Catalog # AAV100A-1, AAV110A-1 A limited-use label license covers this product. By use of this product, you accept the terms and conditions outlined in the Licensing and
AAVanced Concentration Reagent Catalog # AAV100A-1, AAV110A-1 A limited-use label license covers this product. By use of this product, you accept the terms and conditions outlined in the Licensing and
HH67.2, A PROMISING NOVEL ADENO-ASSOCIATED VIRUS (AAV) VECTOR, SHOWS IMPROVED MUSCLE SPECIFICITY AND INFECTIVITY COMPARED TO AAV9. Carrie B.
 HH67.2, A PROMISING NOVEL ADENO-ASSOCIATED VIRUS (AAV) VECTOR, SHOWS IMPROVED MUSCLE SPECIFICITY AND INFECTIVITY COMPARED TO AAV9 Carrie B. Martin A thesis submitted to the faculty at the University of
HH67.2, A PROMISING NOVEL ADENO-ASSOCIATED VIRUS (AAV) VECTOR, SHOWS IMPROVED MUSCLE SPECIFICITY AND INFECTIVITY COMPARED TO AAV9 Carrie B. Martin A thesis submitted to the faculty at the University of
Mutagenesis and Expression of Mammalian Clotting Factor IX. Mark McCleland The Children s Hospital of Philadelphia and Lycoming College
 Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Gene Therapy for MYO7A USH1B. Shannon Boye, Ph.D. Assistant Professor Department of Ophthalmology University of Florida
 Gene Therapy for MYO7A USH1B Shannon Boye, Ph.D. Assistant Professor Department of Ophthalmology University of Florida Hearing Vision Vestibular Function (balance) Type 1 Type 2 Type 3 Profound deafness
Gene Therapy for MYO7A USH1B Shannon Boye, Ph.D. Assistant Professor Department of Ophthalmology University of Florida Hearing Vision Vestibular Function (balance) Type 1 Type 2 Type 3 Profound deafness
Gaining Momentum in Gene Therapy
 Gaining Momentum in Gene Therapy Corporate Presentation November 2018 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results
Gaining Momentum in Gene Therapy Corporate Presentation November 2018 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results
Manufacturing Viral Gene Therapy Vectors: General Approaches and Challenges John T. Gray
 Manufacturing Viral Gene Therapy s: 1 Dr. Vice President, Research & Development Audentes Therapeutics, Inc. San Francisco, USA Natural virus life cycle Late Early Viral Virally infected cell Naïve cell
Manufacturing Viral Gene Therapy s: 1 Dr. Vice President, Research & Development Audentes Therapeutics, Inc. San Francisco, USA Natural virus life cycle Late Early Viral Virally infected cell Naïve cell
AAVpro Titration Kit (for Real Time PCR) Ver.2
 Cat. # 6233 For Research Use AAVpro Titration Kit (for Real Time PCR) Ver.2 Product Manual Table of Contents I. Description... 4 II. Components... 6 III. Storage... 6 IV. Materials Required but not Provided...
Cat. # 6233 For Research Use AAVpro Titration Kit (for Real Time PCR) Ver.2 Product Manual Table of Contents I. Description... 4 II. Components... 6 III. Storage... 6 IV. Materials Required but not Provided...
Phase 1 SMA Type 2 Trial Initiation and Study Design. December 2017
 Phase 1 SMA Type 2 Trial Initiation and Study Design December 2017 Disclaimers This presentation contains forward-looking statements, including statements about: the timing, progress and results of preclinical
Phase 1 SMA Type 2 Trial Initiation and Study Design December 2017 Disclaimers This presentation contains forward-looking statements, including statements about: the timing, progress and results of preclinical
Visionary Science for Life Changing Cures OIS 2017
 Visionary Science for Life Changing Cures OIS 2017 1 Forward Looking Statements Today s presentation includes forward-looking statements intended to qualify for the Safe Harbor from liability established
Visionary Science for Life Changing Cures OIS 2017 1 Forward Looking Statements Today s presentation includes forward-looking statements intended to qualify for the Safe Harbor from liability established
Honours and PostDoctorate Research Projects. Lions Eye Institute, Perth
 Honours and PostDoctorate Research Projects Available from 1 st Semester, 2018 Lions Eye Institute, Perth 1 About the Lions Eye Institute. The Lions Eye Institute is a not-for-profit centre of excellence
Honours and PostDoctorate Research Projects Available from 1 st Semester, 2018 Lions Eye Institute, Perth 1 About the Lions Eye Institute. The Lions Eye Institute is a not-for-profit centre of excellence
Utility of a Recombinant Adeno-Associated Viral Vector Reference Standard BY Terence R. Flotte, Parris Burd, and Richard O. Snyder
 Utility of a Recombinant Adeno-Associated Viral Vector Reference Standard BY Terence R. Flotte, Parris Burd, and Richard O. Snyder Recombinant Adeno-associated viral (raav) vectors are known to be efficient
Utility of a Recombinant Adeno-Associated Viral Vector Reference Standard BY Terence R. Flotte, Parris Burd, and Richard O. Snyder Recombinant Adeno-associated viral (raav) vectors are known to be efficient
Gene Therapy: Fellow s Conference Fred Hutchinson Cancer Research Center. Zandra K. Klippel
 Gene Therapy: Did we finally get it right? Fellow s Conference Fred Hutchinson Cancer Research Center University it of Washington Zandra K. Klippel March 2012 Introduction >6000 human diseases are monogenic
Gene Therapy: Did we finally get it right? Fellow s Conference Fred Hutchinson Cancer Research Center University it of Washington Zandra K. Klippel March 2012 Introduction >6000 human diseases are monogenic
VECTOR SAFETY INFORMATION. AAV Vectors: Material Information
 VECTOR SAFETY INFORMATION AAV Vectors: Material Information AAV vectors contain recombinant transgene sequences (e.g. encoding reporter or therapeutic genes) flanked by the AAV inverted terminal repeats
VECTOR SAFETY INFORMATION AAV Vectors: Material Information AAV vectors contain recombinant transgene sequences (e.g. encoding reporter or therapeutic genes) flanked by the AAV inverted terminal repeats
Inherited Retinal Diseases research at Moorfields
 Recruiting Research Studies Inherited Retinal Diseases research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the
Recruiting Research Studies Inherited Retinal Diseases research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the
Retinal. alfred t. kamajian
 Retinal alfred t. kamajian 52 november 2012 From inherited retinal dystrophies to AMD, the pace of gene therapy is picking up, spurred on by recent success with Leber congenital amaurosis. An update on
Retinal alfred t. kamajian 52 november 2012 From inherited retinal dystrophies to AMD, the pace of gene therapy is picking up, spurred on by recent success with Leber congenital amaurosis. An update on
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
AAVpro Helper Free System
 For Research Use Cat. # 6230, 6650-6657, 6668, 6669, 6673 AAVpro Helper Free System Product Manual For China/Korea/India/Europe Table of Contents I. Description... 4 II. Components... 7 III. IV. Storage...13
For Research Use Cat. # 6230, 6650-6657, 6668, 6669, 6673 AAVpro Helper Free System Product Manual For China/Korea/India/Europe Table of Contents I. Description... 4 II. Components... 7 III. IV. Storage...13
ARTICLE DISCUSSION: MARCH 2018 IRB MEMBER TRAINING
 ARTICLE DISCUSSION: SEVERE TOXICITY IN NONHUMAN PRIMATES AND PIGLETS FOLLOWING HIGH- DOSE INTRAVENOUS ADMINISTRATION OF AN ADENO-ASSOCIATED VIRUS VECTOR EXPRESSING HUMAN SMN MARCH 2018 IRB MEMBER TRAINING
ARTICLE DISCUSSION: SEVERE TOXICITY IN NONHUMAN PRIMATES AND PIGLETS FOLLOWING HIGH- DOSE INTRAVENOUS ADMINISTRATION OF AN ADENO-ASSOCIATED VIRUS VECTOR EXPRESSING HUMAN SMN MARCH 2018 IRB MEMBER TRAINING
J. Fraser Wright, Ph.D.
 Towards Better Characterization of Recombinant AAV Gene Transfer Vectors: Case Studies Illustrating Challenges with Dose Determining Vector Concentration methods J. Fraser Wright, Ph.D. Well Characterized
Towards Better Characterization of Recombinant AAV Gene Transfer Vectors: Case Studies Illustrating Challenges with Dose Determining Vector Concentration methods J. Fraser Wright, Ph.D. Well Characterized
Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of
 Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
Issues in production of viral gene transfer vectors. Stefan Kochanek Department of Gene Therapy Ulm University
 Issues in production of viral gene transfer vectors Stefan Kochanek Department of Gene Therapy Ulm University Only few positive results in gene therapy so far - many early phase, few late phase clinical
Issues in production of viral gene transfer vectors Stefan Kochanek Department of Gene Therapy Ulm University Only few positive results in gene therapy so far - many early phase, few late phase clinical
Pre-existing anti-viral vector antibodies in gene therapy
 Pre-existing anti-viral vector antibodies in gene therapy Impact on assays, study conduct and data interpretation Mark N Milton, Executive Director DMPK-Bx, Novartis AAPS NBC, May 2016 Gene Therapy Treatment
Pre-existing anti-viral vector antibodies in gene therapy Impact on assays, study conduct and data interpretation Mark N Milton, Executive Director DMPK-Bx, Novartis AAPS NBC, May 2016 Gene Therapy Treatment
The Effects of Scaffold Rigidity on Retinal Pigment Epithelial Cells. Corina White Symposium on Biomaterials Science 24 October 2016
 The Effects of Scaffold Rigidity on Retinal Pigment Epithelial Cells Corina White Symposium on Biomaterials Science 24 October 2016 Background Physiology The retina is the light-responsive tissue layer
The Effects of Scaffold Rigidity on Retinal Pigment Epithelial Cells Corina White Symposium on Biomaterials Science 24 October 2016 Background Physiology The retina is the light-responsive tissue layer
vision is our mission
 vision is our mission NASDAQ: OPHT March 2019 Forward-looking Statements Any statements in this presentation about Ophthotech s future expectations, plans and prospects constitute forward-looking statements
vision is our mission NASDAQ: OPHT March 2019 Forward-looking Statements Any statements in this presentation about Ophthotech s future expectations, plans and prospects constitute forward-looking statements
ViraBind Lentivirus Concentration and Purification Kit
 Product Manual ViraBind Lentivirus Concentration and Purification Kit Catalog Number VPK-091 VPK-091-5 5 preps 25 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus
Product Manual ViraBind Lentivirus Concentration and Purification Kit Catalog Number VPK-091 VPK-091-5 5 preps 25 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus
More information. Page 2
 GenSight Biologics, Pixium Vision and Fondation Voir et Entendre join forces and benefit from a total 18.5 million funding for SIGHT AGAIN as part of the Investment for the Future This public - private
GenSight Biologics, Pixium Vision and Fondation Voir et Entendre join forces and benefit from a total 18.5 million funding for SIGHT AGAIN as part of the Investment for the Future This public - private
ViraBind Lentivirus Concentration and Purification Kit
 Product Manual ViraBind Lentivirus Concentration and Purification Kit Catalog Number VPK-090 2 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus vectors based on
Product Manual ViraBind Lentivirus Concentration and Purification Kit Catalog Number VPK-090 2 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus vectors based on
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Gene therapy for retinitis pigmentosa
 Gene therapy for retinitis pigmentosa Robert E MacLaren Professor of Ophthalmology University of Oxford & Honorary Consultant Vitreoretinal Surgeon Oxford University Hospitals, Moorfields Eye Hospital
Gene therapy for retinitis pigmentosa Robert E MacLaren Professor of Ophthalmology University of Oxford & Honorary Consultant Vitreoretinal Surgeon Oxford University Hospitals, Moorfields Eye Hospital
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Gaining Momentum in Gene Therapy
 Gaining Momentum in Gene Therapy Corporate Deck March 6, 2019 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results that
Gaining Momentum in Gene Therapy Corporate Deck March 6, 2019 Forward-looking Statements Statements contained in this document regarding matters, events, statistics, or clinical or financial results that
Article: "Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilit 1 / 16. Ultrasound" Malova Anna
 Article: "Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound" 06.04.2013 Article: "Noninvasive and Targeted Gene Delivery into the Brain Using 06.04.2013
Article: "Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound" 06.04.2013 Article: "Noninvasive and Targeted Gene Delivery into the Brain Using 06.04.2013
Adenovirus Titration Kit
 Adenovirus Titration Kit Catalog # LF-RK0001(1 kit) Immunostaining method for Quantitative Detection of Adenovirus For research use only Not for diagnostic or therapeutic procedures AbFrontier Science
Adenovirus Titration Kit Catalog # LF-RK0001(1 kit) Immunostaining method for Quantitative Detection of Adenovirus For research use only Not for diagnostic or therapeutic procedures AbFrontier Science
Recombinant Adeno- Associated Virus (raav) Delivered shrna. - A Technical Guide
 Efficient Gene Knockdown via Recombinant Adeno- Associated Virus (raav) Delivered shrna - A Technical Guide Revision 201602.01 ViGene Biosciences 2016 RESEARCH USE ONLY. Not for use in diagnostic procedures
Efficient Gene Knockdown via Recombinant Adeno- Associated Virus (raav) Delivered shrna - A Technical Guide Revision 201602.01 ViGene Biosciences 2016 RESEARCH USE ONLY. Not for use in diagnostic procedures
ViraBind PLUS Lentivirus Concentration and Purification Kit
 Product Manual ViraBind PLUS Lentivirus Concentration and Purification Kit Catalog Number VPK-095 2 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus vectors based
Product Manual ViraBind PLUS Lentivirus Concentration and Purification Kit Catalog Number VPK-095 2 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus vectors based
AAVpro Helper Free System
 For Research Use Cat. # 6230, 6652, 6655 AAVpro Helper Free System Product Manual For USA Table of Contents I. Description... 4 II. Components... 6 III. Storage... 9 IV. Materials Required but not Provided...
For Research Use Cat. # 6230, 6652, 6655 AAVpro Helper Free System Product Manual For USA Table of Contents I. Description... 4 II. Components... 6 III. Storage... 9 IV. Materials Required but not Provided...
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days
 Animal injections and tissue processing In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days before they received any injections. Mice were injected with sesame seed oil
Animal injections and tissue processing In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days before they received any injections. Mice were injected with sesame seed oil
The Molecular Virology Support Core: Adenoviral Vectors and Beyond
 The : Adenoviral Vectors and Beyond Christoph A. Kahl, Ph.D. Viral Vector Workshop, May 5th, 2011 Outline 1) Overview of the MVSC 2) Adenoviral Vectors 3) Expertise and Services Overview What does the
The : Adenoviral Vectors and Beyond Christoph A. Kahl, Ph.D. Viral Vector Workshop, May 5th, 2011 Outline 1) Overview of the MVSC 2) Adenoviral Vectors 3) Expertise and Services Overview What does the
Hemophilia and Gene Therapy
 Hemophilia and Gene Therapy Jackie Chu June 4, 2008 Overview Hemophilia, the disease Gene therapy Hemophilia as a target for gene therapy Gene delivery systems Clinical trials New methods Future of gene
Hemophilia and Gene Therapy Jackie Chu June 4, 2008 Overview Hemophilia, the disease Gene therapy Hemophilia as a target for gene therapy Gene delivery systems Clinical trials New methods Future of gene
Adenoviral Expression Systems. Lentivirus is not the only choice for gene delivery. Adeno-X
 Adenoviral Expression Systems Lentivirus is not the only choice for gene delivery 3 Adeno-X Why choose adenoviral gene delivery? Table I: Adenoviral vs. Lentiviral Gene Delivery Lentivirus Adenovirus Infects
Adenoviral Expression Systems Lentivirus is not the only choice for gene delivery 3 Adeno-X Why choose adenoviral gene delivery? Table I: Adenoviral vs. Lentiviral Gene Delivery Lentivirus Adenovirus Infects
Supplementary Methods
 Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
ViraBind Lentivirus Purification Kit
 Product Manual ViraBind Lentivirus Purification Kit Catalog Number VPK-104 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus vectors based on the human immunodeficiency
Product Manual ViraBind Lentivirus Purification Kit Catalog Number VPK-104 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lentivirus vectors based on the human immunodeficiency
Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products
 Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products Approved by the IPRP Management Committee on 3 June 2018 12 April 2018 Table of Contents 1. Position Statement... 3 2. Executive
Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products Approved by the IPRP Management Committee on 3 June 2018 12 April 2018 Table of Contents 1. Position Statement... 3 2. Executive
AAVpro Purification Kit (AAV2)
 Cat. # 6232 For Research Use AAVpro Purification Kit (AAV2) Product Manual Table of Contents I. Description... 4 II. Components... 6 III. Storage... 6 IV. Materials Required but not Provided... 6 V. Protocol...
Cat. # 6232 For Research Use AAVpro Purification Kit (AAV2) Product Manual Table of Contents I. Description... 4 II. Components... 6 III. Storage... 6 IV. Materials Required but not Provided... 6 V. Protocol...
Intravitreal and sub-retinal injections of plasmid DNA and electroporation in P0 pups
 Intravitreal and sub-retinal injections of plasmid DNA and electroporation in P0 pups Protocol modified from: Retinal Gene Delivery by raav and DNA Electroporation, Aditya Venkatesh et all, Current Protocols
Intravitreal and sub-retinal injections of plasmid DNA and electroporation in P0 pups Protocol modified from: Retinal Gene Delivery by raav and DNA Electroporation, Aditya Venkatesh et all, Current Protocols
GC GC - AAV2/8-4E10
 SUPPLEMENTARY INFORMATION doi:.38/nature660 a Luciferase Expression (Photons/Sec) c f 12 11 11 GC - AAV2/8-Luciferase 9 GC - AAV2/8-Luciferase 0 8 16 24 32 40 48 56 64 Weeks Post-AAV Injection Human IgG
SUPPLEMENTARY INFORMATION doi:.38/nature660 a Luciferase Expression (Photons/Sec) c f 12 11 11 GC - AAV2/8-Luciferase 9 GC - AAV2/8-Luciferase 0 8 16 24 32 40 48 56 64 Weeks Post-AAV Injection Human IgG
Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides
 Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides Laboratory Sciences, MPI Research, A Charles River Company Amy Smith, BA, Senior Director, Bioanalytical/Analytical
Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides Laboratory Sciences, MPI Research, A Charles River Company Amy Smith, BA, Senior Director, Bioanalytical/Analytical
Input. Pulldown GST. GST-Ahi1
 A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
AAV vectors for gene therapy. Any Gene to Any Cell
 AAV vectors for gene therapy Any Gene to Any Cell % Population WHY WORK WITH SIRION? OVERCOME MAJOR ROAD BLOCKS IN AAV GENE THERAPY Invent improved AAV vectors with optimized transduction and expression
AAV vectors for gene therapy Any Gene to Any Cell % Population WHY WORK WITH SIRION? OVERCOME MAJOR ROAD BLOCKS IN AAV GENE THERAPY Invent improved AAV vectors with optimized transduction and expression
Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia
 Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia labeled with the specific marker 4C4 (magenta) are
Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia labeled with the specific marker 4C4 (magenta) are
Page 1 of 5. Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR Cy µg (~7.5 nmol) MIR 7901
 Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Rod Outer Segment Development Influences AAV-Mediated Photoreceptor Transduction After Subretinal Injection
 University of Massachusetts Medical School escholarship@umms UMass Metabolic Network Publications UMass Metabolic Network 6-1-2017 Rod Outer Segment Development Influences AAV-Mediated Photoreceptor Transduction
University of Massachusetts Medical School escholarship@umms UMass Metabolic Network Publications UMass Metabolic Network 6-1-2017 Rod Outer Segment Development Influences AAV-Mediated Photoreceptor Transduction
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Figure S1 is related to Figure 1B, showing more details of outer segment of
 Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Somatic gene therapy for hypertension
 Brazilian Journal of Medical and Biological Research (2000) 33: 715-721 Gene therapy ISSN 0100-879X 715 Somatic gene therapy for hypertension Department of Physiology, College of Medicine, University of
Brazilian Journal of Medical and Biological Research (2000) 33: 715-721 Gene therapy ISSN 0100-879X 715 Somatic gene therapy for hypertension Department of Physiology, College of Medicine, University of
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
Visionary Science for Life Changing Cures 2018
 Visionary Science for Life Changing Cures 2018 1 Forward Looking Statements Today s presentation includes forward-looking statements intended to qualify for the Safe Harbor from liability established by
Visionary Science for Life Changing Cures 2018 1 Forward Looking Statements Today s presentation includes forward-looking statements intended to qualify for the Safe Harbor from liability established by
Investigating Critical Challenges in the Gene Therapy Field
 Investigating Critical Challenges in the Gene Therapy Field Mapping out the Landscape & Discussing Future Strategies Sander van Deventer Managing Partner Forbion Capital Partners CSO and General Manager
Investigating Critical Challenges in the Gene Therapy Field Mapping out the Landscape & Discussing Future Strategies Sander van Deventer Managing Partner Forbion Capital Partners CSO and General Manager
sirna Overview and Technical Tips
 1 sirna Overview and Technical Tips 2 CONTENTS 3 4 5 7 8 10 11 13 14 18 19 20 21 Introduction Applications How Does It Work? Handy Tips Troubleshooting Conclusions Further References Contact Us 3 INTRODUCTION
1 sirna Overview and Technical Tips 2 CONTENTS 3 4 5 7 8 10 11 13 14 18 19 20 21 Introduction Applications How Does It Work? Handy Tips Troubleshooting Conclusions Further References Contact Us 3 INTRODUCTION
10/07/2018. Liver directed gene therapy. - An overview. DNA/RNA therapies for Wilson s disease. The liver, organ of choice. Highly vascularised
 DNA/RNA therapies for Wilson s disease Julien Baruteau - Great Ormond Street Institute of Child Health, University College London, UK Metabolic Medicine, Great Ormond Street Hospital, London, UK PLAN Landscape
DNA/RNA therapies for Wilson s disease Julien Baruteau - Great Ormond Street Institute of Child Health, University College London, UK Metabolic Medicine, Great Ormond Street Hospital, London, UK PLAN Landscape
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
Target Selection in the area of Gene Therapy Harald Petry, PhD
 Target Selection in the area of Gene Therapy Harald Petry, PhD Chief Scientific Officer Forward-looking statements This presentation contains forward-looking statements that involve substantial risks and
Target Selection in the area of Gene Therapy Harald Petry, PhD Chief Scientific Officer Forward-looking statements This presentation contains forward-looking statements that involve substantial risks and
Supporting Information
 Supporting Information Fujii et al. 10.1073/pnas.1217563110 A Human Cen chromosome 4q ART3 NUP54 SCARB2 FAM47E CCDC8 Tel B Bac clones RP11-54D17 RP11-628A4 Exon 1 2 34 5 6 7 8 9 10 11 12 5 3 PCR region
Supporting Information Fujii et al. 10.1073/pnas.1217563110 A Human Cen chromosome 4q ART3 NUP54 SCARB2 FAM47E CCDC8 Tel B Bac clones RP11-54D17 RP11-628A4 Exon 1 2 34 5 6 7 8 9 10 11 12 5 3 PCR region
Retinal guanylate cyclase-1 (GC1) encoded by GUCY2D
 Retina Long-term Preservation of Cone Photoreceptors and Restoration of Cone Function by Gene Therapy in the Guanylate Cyclase-1 Knockout (GC1KO) Mouse Sanford L. Boye, 1 Thomas Conlon, 2,3 Kirsten Erger,
Retina Long-term Preservation of Cone Photoreceptors and Restoration of Cone Function by Gene Therapy in the Guanylate Cyclase-1 Knockout (GC1KO) Mouse Sanford L. Boye, 1 Thomas Conlon, 2,3 Kirsten Erger,
ViraBind PLUS Retrovirus Concentration and Purification Kit
 Product Manual ViraBind PLUS Retrovirus Concentration and Purification Kit Catalog Number VPK-135 2 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Retroviral gene transfer
Product Manual ViraBind PLUS Retrovirus Concentration and Purification Kit Catalog Number VPK-135 2 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Retroviral gene transfer
Retinal Degenerations
 Treatments and Clinical Trials for Retinal Degenerations Jacque Duncan, M.D. UCSF Kearn Family Center for the Study of Retinal Degeneration December 4, 2015 Financial Disclosures Grant Support Consultant/Scientific
Treatments and Clinical Trials for Retinal Degenerations Jacque Duncan, M.D. UCSF Kearn Family Center for the Study of Retinal Degeneration December 4, 2015 Financial Disclosures Grant Support Consultant/Scientific
AAVpro Extraction Solution
 Cat. # 6235 For Research Use AAVpro Extraction Solution Product Manual Table of Contents I. Description... 4 II. Components... 4 III. Storage... 4 IV. Materials Required but not Provided... 4 V. Protocol...
Cat. # 6235 For Research Use AAVpro Extraction Solution Product Manual Table of Contents I. Description... 4 II. Components... 4 III. Storage... 4 IV. Materials Required but not Provided... 4 V. Protocol...
ViraBind PLUS Retrovirus Concentration and Purification Mega Kit
 Product Manual ViraBind PLUS Retrovirus Concentration and Purification Mega Kit Catalog Number VPK-136 VPK-136-5 2 preps 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual ViraBind PLUS Retrovirus Concentration and Purification Mega Kit Catalog Number VPK-136 VPK-136-5 2 preps 10 preps FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Supplementary Materials and Methods
 Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Supplemental Information. A Rationally Engineered Capsid Variant of AAV9. for Systemic CNS-Directed and Peripheral
 OMTM, Volume 9 Supplemental Information A Rationally Engineered Capsid Variant of AAV9 for Systemic CNS-Directed and Peripheral Tissue-Detargeted Gene Delivery in Neonates Dan Wang, Shaoyong Li, Dominic
OMTM, Volume 9 Supplemental Information A Rationally Engineered Capsid Variant of AAV9 for Systemic CNS-Directed and Peripheral Tissue-Detargeted Gene Delivery in Neonates Dan Wang, Shaoyong Li, Dominic
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Single and Double Stranded Transgene Expression. of the Green Fluorescent Protein (GFP) within AAV9. with a focus on the myocardium
 Single and Double Stranded Transgene Expression of the Green Fluorescent Protein (GFP) within AAV9 with a focus on the myocardium Daniel Greenberg Briarcliff High School 1 Abstract Recent studies and trials
Single and Double Stranded Transgene Expression of the Green Fluorescent Protein (GFP) within AAV9 with a focus on the myocardium Daniel Greenberg Briarcliff High School 1 Abstract Recent studies and trials
Supplemental Materials and Methods
 Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Datasheet for HEK293/EGFP- AAVS1- Puro Stable Cell Line
 Datasheet for HEK293/EGFP- AAVS1- Puro Stable Cell Line Catalog number: Product: Description: SL573 HEK293 cell line stably expressing EGFP from AAVS1 locus. This product is a cell line stably expressing
Datasheet for HEK293/EGFP- AAVS1- Puro Stable Cell Line Catalog number: Product: Description: SL573 HEK293 cell line stably expressing EGFP from AAVS1 locus. This product is a cell line stably expressing
Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa)
 Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa) Cat. No. EK0474 96 Tests in 8 x 12 divisible strips Background Neurotrophin-3 (NT-3) is a new member of the nerve growth factor gene family which plays an important
Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa) Cat. No. EK0474 96 Tests in 8 x 12 divisible strips Background Neurotrophin-3 (NT-3) is a new member of the nerve growth factor gene family which plays an important
Report from the CAT expert meeting
 16 March 2018 EMA/CAT/60852/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Report from the CAT expert meeting Scientific and regulatory considerations for adeno-associated
16 March 2018 EMA/CAT/60852/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Report from the CAT expert meeting Scientific and regulatory considerations for adeno-associated
Retinal Degeneration Progression Changes Lentiviral Vector Cell Targeting in the Retina
 Retinal Degeneration Progression Changes Lentiviral Vector Cell Targeting in the Retina Maritza Calame 1, Maité Cachafeiro 1, Stéphanie Philippe 1, Karine Schouwey 1, Meriem Tekaya 1, Dana Wanner 1, Chamsy
Retinal Degeneration Progression Changes Lentiviral Vector Cell Targeting in the Retina Maritza Calame 1, Maité Cachafeiro 1, Stéphanie Philippe 1, Karine Schouwey 1, Meriem Tekaya 1, Dana Wanner 1, Chamsy
*To whom correspondence and reprint requests should be addressed. Fax: (33)
 ARTICLE doi:10.1016/s1525-0016(03)00098-4 Recombinant Adeno-associated Virus Serotype 4 Mediates Unique and Exclusive Long-Term Transduction of Retinal Pigmented Epithelium in Rat, Dog, and Nonhuman Primate
ARTICLE doi:10.1016/s1525-0016(03)00098-4 Recombinant Adeno-associated Virus Serotype 4 Mediates Unique and Exclusive Long-Term Transduction of Retinal Pigmented Epithelium in Rat, Dog, and Nonhuman Primate
A roadmap for bringing FKRP gene therapies into clinical use
 A roadmap for bringing FKRP gene therapies into clinical use Jeffrey S. Chamberlain, Ph.D. McCaw Chair in Muscular Dystrophy Wellstone Muscular Dystrophy Research Center - Seattle Depts. of Neurology,
A roadmap for bringing FKRP gene therapies into clinical use Jeffrey S. Chamberlain, Ph.D. McCaw Chair in Muscular Dystrophy Wellstone Muscular Dystrophy Research Center - Seattle Depts. of Neurology,
Supporting information. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo
 Supporting information Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo Greg Thurber 1, Katy Yang 1, Thomas Reiner 1, Rainer Kohler 1, Peter Sorger 2, Tim Mitchison
Supporting information Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo Greg Thurber 1, Katy Yang 1, Thomas Reiner 1, Rainer Kohler 1, Peter Sorger 2, Tim Mitchison
ICH CONSIDERATIONS Oncolytic Viruses
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS.
 Supplemental Data: LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS. Scott Jepson, Bryan Vought, Christian H.
Supplemental Data: LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS. Scott Jepson, Bryan Vought, Christian H.
Revised: RG-RV2 by Fukuhara et al.
 Supplemental Figure 1 The generation of Spns2 conditional knockout mice. (A) Schematic representation of the wild type Spns2 locus (Spns2 + ), the targeted allele, the floxed allele (Spns2 f ) and the
Supplemental Figure 1 The generation of Spns2 conditional knockout mice. (A) Schematic representation of the wild type Spns2 locus (Spns2 + ), the targeted allele, the floxed allele (Spns2 f ) and the
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5837/477/dc1 Supporting Online Material for AAV Vector Integration Sites in Mouse Hepatocellular Carcinoma Anthony Donsante, Daniel G. Miller, Yi Li, Carole Vogler,
www.sciencemag.org/cgi/content/full/317/5837/477/dc1 Supporting Online Material for AAV Vector Integration Sites in Mouse Hepatocellular Carcinoma Anthony Donsante, Daniel G. Miller, Yi Li, Carole Vogler,
StemTAG Alkaline Phosphatase Activity Assay Kit (Colorimetric)
 Product Manual StemTAG Alkaline Phosphatase Activity Assay Kit (Colorimetric) Catalog Number CBA-301 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES)
Product Manual StemTAG Alkaline Phosphatase Activity Assay Kit (Colorimetric) Catalog Number CBA-301 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES)
Impact of Retinoic acid induced-1 (Rai1) on Regulators of Metabolism and Adipogenesis
 Impact of Retinoic acid induced-1 (Rai1) on Regulators of Metabolism and Adipogenesis The mammalian system undergoes ~24 hour cycles known as circadian rhythms that temporally orchestrate metabolism, behavior,
Impact of Retinoic acid induced-1 (Rai1) on Regulators of Metabolism and Adipogenesis The mammalian system undergoes ~24 hour cycles known as circadian rhythms that temporally orchestrate metabolism, behavior,
Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and
 Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
We are committed to translating ground-breaking science into genomic therapies that transform patients lives
 We are committed to translating ground-breaking science into genomic therapies that transform patients lives Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model
We are committed to translating ground-breaking science into genomic therapies that transform patients lives Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model
