Novel Tissue-Engineered Biodegradable Material for Reconstruction of Vascular Wall
|
|
|
- Erick Holmes
- 5 years ago
- Views:
Transcription
1 Novel Tissue-Engineered Biodegradable Material for Reconstruction of Vascular Wall Shigemitsu Iwai, MD, Yoshiki Sawa, MD, Satoshi Taketani, MD, Kei Torikai, MD, Koichiro Hirakawa, MS, and Hikaru Matsuda, MD Division of Cardiovascular Surgery, Department of Surgery, Osaka University Graduate School of Medicine, Osaka, and Senko Medical Instrument Manufacturing Co, Ltd, Tokyo, Japan Background. To solve several problems with artificial grafts, we sought to develop a novel bioengineered material that can promote tissue regeneration without ex vivo cell seeding and that has sufficient durability to be used for artery reconstruction. Here, we tested whether this biodegradable material could accelerate the in situ regeneration of autologous cardiovascular tissue, especially of the arterial wall, in various models of cardiovascular surgeries. Methods. The tissue-engineered patch was fabricated by compounding a collagen-microsponge with a biodegradable polymeric scaffold composed of polyglycolic acid knitted mesh, reinforced on the outside with woven polylactic acid. Tissue-engineered patches without precellularization were grafted into the porcine descending aorta (n 5), the porcine pulmonary arterial trunk (n 8), or the canine right ventricular outflow tract (as the large graft model; n 4). Histologic and biochemical assessments were performed 1, 2, and 6 months after the implantation. Results. There was no thrombus formation in any animal. Two months after grafting, all the grafts showed good in situ cellularization by hematoxylin/eosin and immunostaining. The quantification of the cell population by polymerase chain reaction showed a large number of endothelial and smooth muscle cells 2 months after implantation. In the large graft model, the architecture of the patch was similar to that of native tissue 6 months after implantation. Conclusions. A tissue-engineered patch made of our biodegradable polymer and collagen-microsponge provided good in situ regeneration at both the venous and arterial wall, suggesting that this patch can be used as a novel surgical material for the repair of the cardiovascular system. (Ann Thorac Surg 2005;80:1821 8) 2005 by The Society of Thoracic Surgeons Tissue engineering represents a promising approach to the in vitro creation of living, autologous tissue replacements with the potential to grow or be repaired or remodeled [1]. Particularly for the surgical treatment of congenital heart disease, there is a substantial need for such implantation materials. Recently, a biodegradable material for cardiovascular surgery was developed and is being used clinically [2 5]. However, its usefulness for reconstructing the arterial wall has not yet been demonstrated. Moreover, the ex vivo cell-seeding pretreatment ( precellularization ) that was reported previously, is complicated, invasive, and can lead to infection [2 4]. Therefore, the development of a biodegradable material that allows good in situ cellularization without precellularization with sufficient durability for arterial reconstruction is an important strategy. Recently, a novel tissue-engineered material made of a biodegradable polymer and collagen-microsponge was reported that had good cell-attachment properties because of its cellular affinity and porous three-dimensional structure [6]. We recently reported that this tissueengineered prosthesis showed good in situ cellularization and a sufficient durability for vascular reconstruction, and its engraftment led to the regeneration of autologous vessel tissue in a dog model [7]. In the present study, we evaluated whether this newly developed tissue-engineered material could, even without precellularization, accelerate in situ cellularization by inducing the proliferation and differentiation of endothelial and smooth muscle progenitor cells after implantation in a large animal model of arterial wall reconstruction that could be used to simulate various cardiovascular surgeries. Material and Methods Scaffold Design The tissue-engineered patch (TEP) was fabricated by compounding a collagen-microsponge with a biodegradable polymeric scaffold composed of a polyglycolic acid (PGA) knitted mesh that was reinforced on the outside with woven polylactic acid (PLA) (Fig 1A). The PGA/PLA mesh was provided by Senko Medical Instrument Mfg Co, Ltd (Tokyo, Japan). The techniques for preparing the TEP with the collagen-microsponge were described in Accepted for publication March 28, Address correspondence to Dr Sawa, 2-2 Yamada-oka, Suita, Osaka, , Japan; sawa@surg1.med.osaka-u.ac.jp. Dr Hirakawa discloses a financial relationship with Senko Medical Instrument Manufacturing Co, Ltd by The Society of Thoracic Surgeons /05/$30.00 Published by Elsevier Inc doi: /j.athoracsur
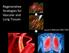
2 1822 IWAI ET AL Ann Thorac Surg TISSUE-ENGINEERED GRAFT 2005;80: Fig 1. Formation of a biodegradable scaffold reinforced with woven polylactic acid mesh (arrow) cross-linked with collagen-microsponge (A). Scanning electron microscopy image of the tissue-engineered patch shows the uniformly distributed and interconnected pore structure (pore size m) of the collagen-microsponge (B) (magnification 40 ). detail by Chen and colleagues [6]. Briefly, the PGA/PLA mesh was immersed in a bovine type I collagen 0.5% solution (Koken Co, Ltd, Tokyo, Japan). It was then frozen at 80 C and lyophilized under vacuum to allow the collagen-microsponges to form. The collagenmicrosponges were further cross-linked by treatment with glutaraldehyde vapor. The completed patch was 800- m thick. A scanning electron microscopy (SEM) image of the patch is shown in Figure 1B. In Vitro Study To verify the cellular affinity and proliferation of the collagen-microsponge, three sets of PGA mesh (10 10 mm), including collagen-microsponge (n 5), a simple collagen-coat, or no collagen (n 5), were cultured dynamically with seeded endothelial cells ( cells) from the canine vena saphena magna. The numbers of attached cells were determined by the 3-[4.5 dimethylthiazol 2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (DO JINDO Laboratories, Japan) on experimental days 1 and 3. In Vivo Study To examine the TEP in various situations, three experimental groups were used for TEP implantation. One was a porcine model (body weight, 15 kg) in which the TEP was implanted into the descending aorta for histologic evaluation and to test the patch s durability in the systemic circulation. Anesthesia was induced with an intramuscular bolus infusion of 3 mg/kg ketamine and 3 mg/kg barbiturate, and maintained by intravenous continuous infusion of propofol (5 mg/kg/hour). The heart was approached by a left anterolateral thoracotomy, and the chest was entered through the fourth intercostal space. The descending aorta was partially clamped and a TEP (20 15 mm) without precellularization was implanted using 5-0 monofilament running sutures. The TEP was explanted 2 months after implantation (n 5) and evaluated by further experiments. Another model was implantation of the TEP into the porcine (body weight, 15 kg) pulmonary arterial trunk, which we used to evaluate early endothelialization and histologic changes. Using the above surgical approach, the pulmonary arterial trunk was partially clamped. A TEP (20 15 mm) without precellularization was implanted using 5-0 monofilament running sutures. The TEP was explanted either one (n 3) or two (n 5) months after implantation. The explanted TEP was evaluated by further experiments. The final model was implantation into the canine right ventricular outflow tract to evaluate a larger size graft and midterm follow-up. Mongrel dogs (body weight, 20 kg) (n 4) were used. Anesthesia was induced with an intravenous bolus infusion of 3 mg/kg ketamine and 3 mg/kg sodium barbiturate, and maintained by inhalation of sevofluorane. Normothermic cardiopulmonary bypass was performed by means of the femoral artery and right atrial venous cannulation. With the heart beating, the pulmonary trunk to the right ventricular outflow tract was incised longitudinally. A large TEP (50 25 mm) without precellularization was implanted using 5-0 monofilament running sutures. Morphologic changes in the large patch model with right ventricular outflow tract reconstruction were evaluated by right ventricular angiography 6 months after the implantation. After the termination, the TEP was explanted and evaluated by further experiments. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Histologic and Immunohistologic Examination Explanted tissue specimens were studied as hematoxylin/eosin, elastica van Gieson, and von Kossa stained paraffin or immunostained frozen sections. The antibodies for immunohistochemistry included monoclonal antibodies against CD31 (clone JC/70A, DAKO, Carpinteria, CA), -smooth muscle actin (clone HHF35, DAKO), and
3 Ann Thorac Surg IWAI ET AL 2005;80: TISSUE-ENGINEERED GRAFT Table 1. The Primers and a for Quantitative Reverse Transcription-Polymerase Chain Reaction GAPDH Forward primer 5=-CTGCACCACCAACTGCTTAGC-3= 5=-GCCATGCCAGTGAGCTTCC-3= 5=-CCTGGCCAAGGTCATCCATGACCACTTC-3= vwf Forward primer 5=-ATGGAGTACACGGCTTTGCTG-3= 5=-CAGGCACATGCTGTGACACAT-3= 5=-ATGAATGTCCACCTCCTCTTCAGACCGG-3= VEGF Forward primer 5=-GACGTCTACCAGCGCAGCTACT-3= 5=-TTTGATCCGCATAATCTGCATG-3= 5=-TTCCAGGAGTACCCCGATGAGATCGA-3= SM22 Forward primer 5=-GCTCCATTTGCTTGAAGACCAT-3= 5=-GTAATGCAGTGTGGCCCTGA-3= 5=-CTCAAAATCACGCCGTTCTTCAGCCA-3= ACTA2 Forward primer 5=-TATGTGGCTATTCAGGCGGTG-3= 5=-AGGATCTTCATGAGGTAGTCGGTG-3= 5=-ATGGTGTCACCCACAACGTGCCCATT-3= Vimentin Forward primer 5=-AGGTGGCAATCTCAATGTCGA-3= 5=-AAATGAGTACCGGAGACAGGTGC-3= 5=-CTCTTCCATTTCCCGCATCTGGCGTT-3= 1823 ACTA2 vascular smooth muscle -actin 2; GAPDH glyceraldehyde-3-phosphate dehydrogenase; SM22 smooth muscle 22 ; VEGF vascular endothelial growth factor; vwf von Willebrand factor. vimentin (clone V9, DAKO), and a polyclonal antibody against von Willebrand factor (Factor VIII related antigen, rabbit, N1505, DAKO). Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) To quantify the cellular population in the explanted TEPs 2 months after implantation, the expression of von Willebrand factor (vwf), vascular endothelial growth factor (VEGF), vascular smooth muscle -actin 2 (ACTA2), smooth muscle 22 (SM22 ), and vimentin were determined with quantitative real-time RT-PCR, as described previously [8]. The primers and a fluorogenic probe are shown in Table 1. The quantitative real-time RT-PCRs were carried out in quadruplicate using the TaqMan RT-PCR kit (ABI) in the Applied Biosystems 7700 sequence detector system (Applied Biosystems, Foster City, CA), according to the manufacturer s instructions. We first determined the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) messenger RNA (mrna) levels in each sample by RT-PCR using differentially diluted, heat-treated, single-stranded complementary DNA (cdna) mixtures under different PCR cycle conditions, and then amplified the target cdnas using defined amounts of heat-denatured, single-stranded cdna mixtures containing an equal amount of GAPDH cdna. Standard curves covering the range from 10 2 to 10 7 copies were constructed using the in vitro synthesized transcripts. For an assay to be considered successful, the correlation coefficient of the standard curve had to be greater than The levels of these mrnas were compared with those of the native pulmonary artery and descending aorta from the same animal and expressed as the percentage of the native content. Biochemical Examination A 4-hydroxyproline assay was used to measure the collagen content in the explanted TEPs 2 months after implantation. The elastin content was quantified by determining the dry weight of the insoluble material after delipidation in acetone-diethyl ether and solubilization in 0.1 N NaOH. The levels of these extracellular components of the explanted TEPs were compared with those of the native vascular tissue from the same animal and expressed as the percentage of the native content. Statistical Analysis All results were expressed as the mean SD and analyzed using a statistical analysis software package (Stat-View version 5.0; Abacus Concept, Inc, Berkeley, CA). One-way analysis of variance, Bonferroni s test, and paired t test were used to analyze the data. A pvalue of less than 0.05 was considered statistically significant. In quantitative RT-PCR and biochemical examination, statistical analyses were performed to compare explanted TEP tissue with native vascular tissue. Results In Vitro Study On experimental day 1, the number of seeded cells attached to the PGA mesh with the collagenmicrosponge was significantly higher than to the PGA mesh alone, by the MTT assay (PGA mesh with collagenmicrosponge, ; PGA mesh with collagen-coat, ; PGA mesh alone, ) (p 0.05). On day 3, the proliferation of cells seeded onto the PGA mesh with collagen-microsponge was significantly higher than the proliferation on the PGA mesh with a
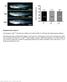
4 1824 IWAI ET AL Ann Thorac Surg TISSUE-ENGINEERED GRAFT 2005;80: ) (Fig 2). These results proved that the collagenmicrosponge had a higher cellular affinity than did the collagen-coated PGA mesh or the polymeric scaffold alone. Fig 2. The attachment and proliferation of seeded cells cultured on the polyglycolic acid (PGA) mesh with collagen-microsponge were significantly higher than when the culture was done on PGA mesh with a simple collagen-coat or on PGA mesh alone. * p 0.05 versus PGA mesh only; ** p 0.05 versus PGA mesh with collagen-coat and PGA mesh only; PGA mesh only; PGA mesh with collagencoat; ΠPGA mesh with collagen-microsponge. (MTT 3-[4.5 dimethylthiazol 2-yl]-2,5-diphenyltetrazolium bromide.) collagen-coat or the PGA mesh alone (PGA mesh with collagen-microsponge, ; PGA mesh with collagen-coat, ; PGA mesh alone, ) (p In Vivo Study All the animals survived the operative procedure for the in vivo experiments. Macroscopic inspection of the explanted TEP showed no intimal thickening in TEP and no aneurism formation, even in the aortic implantation model (Figs 3A and 3B). No patch showed thrombus formation on the internal surface of the TEP, even though we did not provide anticoagulant therapy (Figs 3A and 4A). Right ventricular angiography of the large patch model performed 6 months after implantation showed no evidence of stenosis or aneurismal change (Fig 5). Histologic Findings In the descending aortic model 2 months after implantation, the histologic examination showed almost complete absorption of the PGA polymer and good recellularization, as assessed by hematoxylin eosin staining. The CD31 and -smooth muscle actin staining showed uniform cellularization of endothelial cells and SMCs (Figs 3C and 3D). In the pulmonary arterial model, hematoxylin-eosin staining showed excellent in situ cellularization of the TEP 1 month after implantation (Fig 4C [1 month]), and Fig 3. Macroscopic and histologic findings of the descending aorta model, 2 months after implantation. Macroscopically, there was no aneurysm formation in any animal (A) and (B). The CD31 (C) and -smooth muscle actin staining (D) showed uniform cellularization of the endothelial and smooth muscle cells (magnification 100 ).
5 Ann Thorac Surg IWAI ET AL 2005;80: TISSUE-ENGINEERED GRAFT 1825 Fig 4. Macroscopic (A and B) and histologic (C E) findings of the pulmonary trunk model 1 month and 2 months after implantation. Macroscopically, there was no thrombus formation or neointimal thickness in any sample (A and B). Hematoxylin-eosin staining (C) (magnification 100 ) showed in situ cellularization by 1 month (1 month [C]). The CD31 staining (D) (100 ) showed a monolayer of endothelial cells by 1 month (1 month [D]). Alpha-smooth muscle actin staining (E) (100 ) revealed the parallel alignment of smooth muscle cells at 2 months (2 months [E]). CD31 staining showed a monolayer of endothelial cells covering the surface of the TEP (Fig 4D [1 month]). Alpha-smooth muscle actin staining revealed a parallel alignment of smooth muscle cells (SMCs) 2 months after implantation (Fig 4E [2 months]). In the large patch model 6 months after implantation, no calcification was detected by von Kossa staining, and the architecture of the implanted TEP was similar to that Fig 5. Right ventricular angiography of the large patch model. Arrow indicates tissue engineered patch. There was no stenosis or aneurysmal change from the right ventricular outflow tract to the pulmonary artery. of the native pulmonary artery wall (Fig 6A). Scanning electron microscope observations showed that the luminal surface of the TEP was uniformly covered by a confluent endothelium (Fig 6B). Elastica van Gieson staining revealed the expansion of native tissue into the TEP (Fig 6C). Factor VIII staining showed a number of microvessels in the regenerated tissue (Fig 6D). Quantitative RT-PCR The expression of vwf mrna in the TEPs was comparable with that in the native vascular tissue in both the porcine descending aortic and pulmonary arterial implantation models, even 2 months after implantation (descending aortic model, % of native tissue [p 0.26]; pulmonary arterial model, % of native tissue [p 0.39]). The VEGF mrna expression in both groups was higher than that of native vascular tissue (descending aortic model, % of native tissue [p 0.13]; pulmonary arterial model, % of native tissue [p 0.12]). This may have been the result of angiogenesis in the TEP. The ACTA2 and SM22 expression were also at a level similar to that of the native tissue 2 months after implantation (descending aortic model, ACTA2, 84 15% of native tissue [p 0.16]; pulmonary arterial model, SM22, 72 24% of native tissue [p 0.17]). Vimentin expression revealed less excessive fibroblast migration in the TEP tissue (descending aortic model, 93 59% of native tissue [p 0.78]; pulmonary arterial model, 56 36% of native tissue [p 0.31]).
6 1826 IWAI ET AL Ann Thorac Surg TISSUE-ENGINEERED GRAFT 2005;80: Fig 6. Histologic and scanning electron microscopy findings of large tissue engineered patch ( TEP) model 6 months after implantation. Hematoxylin-eosin staining (magnification 100 ) showed that the architecture of the implanted TEP was similar to that of the native pulmonary artery wall (A). The scanning electron microscopy observations (500 ) showed that the luminal surface of the TEP was uniformly covered by a confluent endothelium (B). Elastica van Gieson staining (100 ) revealed the expansion of native tissue into the TEP (C). Factor VIII staining (100 ) showed a number of microvessels in the regenerated tissue (D). Biochemical Examination The 4-hydroxyproline assay demonstrated that 2 months after implantation, the patch had almost the same collagen content as the native vascular tissue (descending aortic model, % of native tissue [p 0.26]; pulmonary arterial model, 92 6% of native tissue [p 0.83]). The elastin content increased to half that of native tissue 2 months after implantation (descending aortic model, 63 15% of native tissue [p 0.64]; pulmonary arterial model, 47 25% of native tissue [p 0.24]). Comment In this study, we demonstrated the feasibility of using a bioengineered material made of collagen-microsponge and a biodegradable polymer scaffold to promote autologous vascular tissue regeneration without precellularization in various situations, especially arterial reconstruction. Among the experimental animals, whether they underwent arterial or venous reconstruction, there was no case of neointimal hyperplasia, thrombus or aneurysm formation, or rupture, even in the animals whose implant was left in place for 6 months. These findings suggested the TEP patch had sufficient durability after implantation to sustain vascular reconstruction. In our previous study, the PGA patch showed good mechanical strength. However, reinforcement of the patch seemed to be necessary in some cases: for example, in pulmonary hypertension and systemic circulation. Except for this caveat, PGA appeared to be a useful biodegradable polymer with a longer degradation time than 6 months, and with the potential to reinforce the wall of the graft [9]. Therefore, we applied a woven PLA mesh to reinforce the outside of the patch to avoid rupture and aneurysm formation in the present study. Our histologic findings showed the architecture of the implanted TEP was similar to the native vascular wall. No calcification was detected 6 months after implantation. The cellular population, which was determined by RT- PCR, demonstrated excellent in situ cellularization of the TEP. The increase in the collagen content would be reflected by quantifying both the bovine collagen of the initial graft and the newly generated porcine collagen, which could not be discriminated in this experiment. However, the increase in the elastin content to half that of native tissue would only be due to newly generated porcine elastin. In our study, we documented that the percentage of cellular and extracellular components in the graft had increased to levels similar to those in native tissue at six months [7]. The present results suggested that the collagenmicrosponge facilitated the in vivo attachment and spreading of autologous cells throughout the patch, and did so without precellularization. Previously, the redifferentiation of dedifferentiated cells was shown in vitro using a biodegradable polymer with collagenmicrosponge [10]. We also showed that the biodegradable scaffold without the collagen-microsponge resulted
7 Ann Thorac Surg IWAI ET AL 2005;80: TISSUE-ENGINEERED GRAFT in immature regeneration and thrombus formation. The cellular affinity for collagen and the interconnected pore structure of microsponge accelerate the in situ regeneration of autologous tissue without precellularization or the addition of growth factors. Regarding the precellularization, conventional tissue engineering techniques have required autologous cell harvesting and ex vivo culture before implantation. To avoid these pretreatments, a bone marrow cell-seeding technique was recently reported by Matsumura and colleagues [5]. The idea of using bone marrow cells has attracted much interest. The results with bone marrow cells inspired us to test whether we could promote the regeneration of vascular tissue from vascular progenitor cells. Our concept of inducing vascular progenitor cell growth is based on the idea of supplying an in situ bioreactor and incubator for circulating progenitor cells. Therefore, our findings on the efficacy of this material without precellularization may not be reproducible if the graft is used in an organ without direct contact with circulating blood. In our previous study, we proved that there was no remarkable difference in the histologic and biochemical findings after implantation of this material into the dog pulmonary artery between groups with and without precellularization, from 2 to 6 months. A significant difference may exist earlier than 2 months after implantation. However, we found endothelialization of the TEP even by 1 month and observed histologic changes associated with regeneration. In the present study, we obtained good in situ regeneration with many favorable histologic characteristics. Although the precise mechanisms behind the in situ regeneration have not been elucidated, we can speculate the following: (1) circulating vascular progenitor cells attach to the TEP and differentiate, as previously demonstrated [11 16]; (2) cellular and extracellular components from the native tissue expand into the TEP; (3) angiogenesis occurs in the newly formed tissue; and (4) fibroblasts that migrated from the adventitia side of the vessel proliferated in the TEP. Nonetheless, even though our findings seem to reveal site-specific regeneration of vascular tissue, further studies are needed for proof. Such experiments will be important in creating ideal conditions for autologous vascular cells to migrate and to proliferate in situ in artificial grafts. Thus our TEP, made of biodegradable polymer and collagen-microsponge, is a candidate for a novel surgical material; it should be useful for reconstructing autologous tissue in cardiovascular surgery, without the use of foreign material, to avoid later complications. The limitations of this study are the relatively small number of animals used and the short durations of the observations. Moreover, for the clinical use of this graft in the repair of congenital heart disease to be viable, its durability and potential for growth should be evaluated over a long-term follow-up period. The use of a xenogenous substance such as bovine collagen may also be a limitation for clinical application, although prosthetic vascular grafts coated with collagen are already widely used in clinical situations. As the next step, we should consider the use of atelocollagen, which carries lower immunologic and infectious risks. Nevertheless, we believe that our strategy of using this material without precellularization may be a useful option for a novel tissue engineering technique, especially for neonatal and pediatric cardiovascular surgery, which cannot wait for cell culture. Further investigation will be directed towards evaluating its potential and safety for clinical applications. In the present study, a bioengineered patch made of biodegradable polymer and collagen-microsponge used without precellularization, led to excellent histologic findings and was durable. This bioengineered patch is a promising material for cardiovascular surgery that could promote the in situ cellularization that leads to the regeneration of autologous vessel tissue, in both the venous and arterial wall. References Langer R, Vacanti JP. Tissue engineering. Science 1993;260: Shinoka T, Shum-Tim D, Ma PX, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg 1998;115: Hoerstrup SP, Kadner A, Melnitchouk S, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation 2002;106:I Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shinoka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials 2003;24: Matsumura G, Tomita SM, Shinoka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation 2003;108: Chen G, Ushida T, Tateishi T. A biodegradable hybrid sponge nested with collagen-microsponges. J Biomed Mater Res 2000;51: Iwai S, Sawa Y, Ichikawa H, et al. Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis. J Thorac Cardiovasc Surg 2004;128: Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res 1996;6: Ozawa T, Mickle DA, Weisel RD, et al. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg 2002;124: Chen G, Sato T, Ushida T, Hirochika R, Tateishi T. Redifferentiation of dedifferentiated bovine chondrocytes when cultured in vitro in a PLGA-collagen hybrid mesh. FEBS Lett 542;2003: Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological neovascularization. Circ Res 1999;85: Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998;92: Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000;408: Han CL, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res 2001;38: Shimizu K, Sugiyama S, Aikawa M, et al. Host bone marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med 2001;7: Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 2002;8:403 9.
In the history of cardiovascular surgery, glutaraldehyde-fixed xenograft,
 Surgery for Congenital Heart Disease Iwai et al Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis Shigemitsu Iwai, MD a Yoshiki Sawa, MD a Hajime Ichikawa,
Surgery for Congenital Heart Disease Iwai et al Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis Shigemitsu Iwai, MD a Yoshiki Sawa, MD a Hajime Ichikawa,
Tissue Engineering for Blood Vessels
 Regenerative Medicine Tissue Engineering for Blood Vessels JMAJ 47(6): 288 293, 2004 Narutoshi HIBINO and Toshiharu SHIN OKA Department of Cardiovascular Surgery, Heart Institute of Japan, Tokyo Women
Regenerative Medicine Tissue Engineering for Blood Vessels JMAJ 47(6): 288 293, 2004 Narutoshi HIBINO and Toshiharu SHIN OKA Department of Cardiovascular Surgery, Heart Institute of Japan, Tokyo Women
Tissue Engineered Vascular Grafts
 Tissue Engineered Vascular Grafts Jeffrey H. Lawson, M.D., Ph.D. Chief Medical Officer Humacyte Incorporated Professor of Surgery and Pathology Duke University Medical Center Disclosure Information Financial
Tissue Engineered Vascular Grafts Jeffrey H. Lawson, M.D., Ph.D. Chief Medical Officer Humacyte Incorporated Professor of Surgery and Pathology Duke University Medical Center Disclosure Information Financial
1) Determining the best cell sources and scaffold materials for TEHV development.
 Broadly speaking, my primary research interests focus on the development and application of methodologies that can be employed in the basic understanding and optimization of tissue engineered heart valves
Broadly speaking, my primary research interests focus on the development and application of methodologies that can be employed in the basic understanding and optimization of tissue engineered heart valves
New Drugs in the Sky: Coatings & Drug Elution for Improving Stents and Vascular Prostheses Beat H. Walpoth, MD
 New Drugs in the Sky: Coatings & Drug Elution for Improving Stents and Vascular Prostheses Beat H. Walpoth, MD Department of Cardiovascular Surgery, University Hospital of Geneva, Switzerland Coatings
New Drugs in the Sky: Coatings & Drug Elution for Improving Stents and Vascular Prostheses Beat H. Walpoth, MD Department of Cardiovascular Surgery, University Hospital of Geneva, Switzerland Coatings
We demonstrated the feasibility of using tissueengineered
 TISSUE ENGINEERING: Part C Volume 21, Number 1, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/ten.tec.2014.0160 Comparison of a Closed System to a Standard Open Technique for Preparing Tissue-Engineered Vascular
TISSUE ENGINEERING: Part C Volume 21, Number 1, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/ten.tec.2014.0160 Comparison of a Closed System to a Standard Open Technique for Preparing Tissue-Engineered Vascular
Department of Geriatric Medicine, Graduate School of Medicine
 (ONLINE DATA SUPPLEMENT) RENIN ANGIOTENSIN SYSTEM MODULATES OXIDATIVE STRESS- INDUCED ENDOTHELIAL CELL APOPTOSIS IN RATS Masahiro Akishita, MD, PhD 1 ; Kumiko Nagai, MS 1 ; Hang Xi, MD, PhD 1 ; Wei Yu,
(ONLINE DATA SUPPLEMENT) RENIN ANGIOTENSIN SYSTEM MODULATES OXIDATIVE STRESS- INDUCED ENDOTHELIAL CELL APOPTOSIS IN RATS Masahiro Akishita, MD, PhD 1 ; Kumiko Nagai, MS 1 ; Hang Xi, MD, PhD 1 ; Wei Yu,
Tissue Engineering of the Mitral Valve Leaflets and Abdominal Aorta
 Medizinische Hochschhule Hannover Dr Morticelli L Supervisor: Dr Korossis S Niedersächsischen Zentrum für Biomedizintechnik und Implantatforschung The Tissue Engineering (TE) Concept Tissue Engineering
Medizinische Hochschhule Hannover Dr Morticelli L Supervisor: Dr Korossis S Niedersächsischen Zentrum für Biomedizintechnik und Implantatforschung The Tissue Engineering (TE) Concept Tissue Engineering
Valvular heart disease is an important source of morbidity. Tissue Engineering of Heart Valves: In Vitro Experiences
 Tissue Engineering of Heart Valves: In Vitro Experiences Ralf Sodian, MD, Simon P. Hoerstrup, MD, Jason S. Sperling, MD, Sabine H. Daebritz, MD, David P. Martin, PhD, Frederick J. Schoen, MD, PhD, Joseph
Tissue Engineering of Heart Valves: In Vitro Experiences Ralf Sodian, MD, Simon P. Hoerstrup, MD, Jason S. Sperling, MD, Sabine H. Daebritz, MD, David P. Martin, PhD, Frederick J. Schoen, MD, PhD, Joseph
Thoughts and Progress
 aor_1007 69..91 Artificial Organs 35(1):69 91, Wiley Periodicals, Inc. 2010, Copyright the Authors Artificial Organs 2010, International Center for Artificial Organs and Transplantation and Wiley Periodicals,
aor_1007 69..91 Artificial Organs 35(1):69 91, Wiley Periodicals, Inc. 2010, Copyright the Authors Artificial Organs 2010, International Center for Artificial Organs and Transplantation and Wiley Periodicals,
Bioengineered AV Grafts: They Are Finally Happening! Synthetic Vascular Dialysis Grafts. Vascular Grafts and Failure. Disclosure Information
 Bioengineered AV Grafts: They Are Finally Happening! Jeffrey H. Lawson, M.D., Ph.D. Departments of Surgery and Pathology Duke University Medical Center Durham, North Carolina Lawson JH, FINANCIAL DISCLOSURE:
Bioengineered AV Grafts: They Are Finally Happening! Jeffrey H. Lawson, M.D., Ph.D. Departments of Surgery and Pathology Duke University Medical Center Durham, North Carolina Lawson JH, FINANCIAL DISCLOSURE:
Introduction to Cell/ Biomaterial Engineering
 Introduction to Cell/ Biomaterial Engineering Module 3, Lecture 1! 20.109 Spring 2011! Topics for Lecture 1 Introduction to tissue engineering! motivation! basic principles + examples! Introduction to
Introduction to Cell/ Biomaterial Engineering Module 3, Lecture 1! 20.109 Spring 2011! Topics for Lecture 1 Introduction to tissue engineering! motivation! basic principles + examples! Introduction to
Stem Cell Derived, Tissue-Engineered Pulmonary Artery Augmentation Patches In Vivo
 THORACIC SURGERY DIRECTORS ASSOCIATION AWARD SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you
THORACIC SURGERY DIRECTORS ASSOCIATION AWARD SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you
White Paper: Textile Engineered Tissue Scaffolds Offer Advances in Hollow Organ Regenerations. Peter D. Gabriele Director, Emerging Technology
 White Paper: Textile Engineered Tissue Scaffolds Offer Advances in Hollow Organ Regenerations Peter D. Gabriele Director, Emerging Technology Regenerative medicine (RM) holds the potential to address some
White Paper: Textile Engineered Tissue Scaffolds Offer Advances in Hollow Organ Regenerations Peter D. Gabriele Director, Emerging Technology Regenerative medicine (RM) holds the potential to address some
Mechano-dependent biosynthetic response of microintegrated cells in elastomeric scaffolds.
 Mechano-dependent biosynthetic response of microintegrated cells in elastomeric scaffolds. Lauren Anderson, Department of Bioengineering, The Pennsylvania State University Dr. Michael Sacks, Mentor, Department
Mechano-dependent biosynthetic response of microintegrated cells in elastomeric scaffolds. Lauren Anderson, Department of Bioengineering, The Pennsylvania State University Dr. Michael Sacks, Mentor, Department
Scaffolds Covalently Immobilized with VEGF and Angiopoietin-1 to Promote Angiogenesis In Engineered Cardiac Tissues
 Scaffolds Covalently Immobilized with VEGF and Angiopoietin-1 to Promote Angiogenesis In Engineered Cardiac Tissues Loraine L.Y. Chiu 1 and Milica Radisic 1,2,3 1 Department of Chemical Engineering and
Scaffolds Covalently Immobilized with VEGF and Angiopoietin-1 to Promote Angiogenesis In Engineered Cardiac Tissues Loraine L.Y. Chiu 1 and Milica Radisic 1,2,3 1 Department of Chemical Engineering and
BEH.462/3.962J Molecular Principles of Biomaterials Spring 2003
 Lecture 6: Biodegradable Polymers for Tissue Engineering Last time: Today: enzymatic degradation of solid polymers Engineering biological recognition of polymers Designing polymers for tissue engineering
Lecture 6: Biodegradable Polymers for Tissue Engineering Last time: Today: enzymatic degradation of solid polymers Engineering biological recognition of polymers Designing polymers for tissue engineering
Cellular repair of damaged organs. Repopulating scaffoldings in kidney and liver
 Cellular repair of damaged organs Repopulating scaffoldings in kidney and liver Mireia Caralt, MD PhD Servei Cirurgia HBP i Trasplantaments March 29, 2017 Introduction Strategies to increase the number
Cellular repair of damaged organs Repopulating scaffoldings in kidney and liver Mireia Caralt, MD PhD Servei Cirurgia HBP i Trasplantaments March 29, 2017 Introduction Strategies to increase the number
Life Valve Project (FP7)
 Life Valve Project (FP7) Prof. Dr. Dr. Simon P. Hoerstrup University and University Hospital Zurich; Switzerland Cardiovascular Surgery Research / Regenerative Medicine Center 1 LifeValve - Living autologous
Life Valve Project (FP7) Prof. Dr. Dr. Simon P. Hoerstrup University and University Hospital Zurich; Switzerland Cardiovascular Surgery Research / Regenerative Medicine Center 1 LifeValve - Living autologous
Functional Assessment and Clinical Outcome
 Tissue Engineering Functional Assessment and Clinical Outcome STEVEN A. GOLDSTEIN Orthopaedic Research Laboratories, Department of Orthopaedic Surgery, University of Michigan, Ann Arbor, Michigan 48109,
Tissue Engineering Functional Assessment and Clinical Outcome STEVEN A. GOLDSTEIN Orthopaedic Research Laboratories, Department of Orthopaedic Surgery, University of Michigan, Ann Arbor, Michigan 48109,
A NANOFIBROUS HYDROGEL FOR BONE TISSUE ENGINEERING
 A NANOFIBROUS HYDROGEL FOR BONE TISSUE ENGINEERING Umadevi Kandalam, PhD Assistant Professor Department of Pediatric Dentistry College of Dental Medicine Nova Southeastern University Fort Lauderdale, Florida
A NANOFIBROUS HYDROGEL FOR BONE TISSUE ENGINEERING Umadevi Kandalam, PhD Assistant Professor Department of Pediatric Dentistry College of Dental Medicine Nova Southeastern University Fort Lauderdale, Florida
Mechano-dependent Biosynthetic Response of Micro-integrated Cells in Elastomeric Scaffolds
 Mechano-dependent Biosynthetic Response of Micro-integrated Cells in Elastomeric Scaffolds Lauren Anderson 1,2, John Stella 3, and Dr. Michael Sacks 3 1 Bioengineering and Bioinformatics Summer Institute,
Mechano-dependent Biosynthetic Response of Micro-integrated Cells in Elastomeric Scaffolds Lauren Anderson 1,2, John Stella 3, and Dr. Michael Sacks 3 1 Bioengineering and Bioinformatics Summer Institute,
Artificial blood vessels
 Artificial blood vessels S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of
Artificial blood vessels S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of
ISTINYE UNIVERSITY INSTITUTE OF HEALTH SCIENCES DEPARTMENT OF STEM CELL AND TISSUE ENGINEERING (THESIS) COURSE DESCRIPTIONS
 ISTINYE UNIVERSITY INSTITUTE OF HEALTH SCIENCES DEPARTMENT OF STEM CELL AND TISSUE ENGINEERING (THESIS) COURSE DESCRIPTIONS 1 st SEMESTER Adult Stem Cell Biology 5 ECTS In this course, the characteristics
ISTINYE UNIVERSITY INSTITUTE OF HEALTH SCIENCES DEPARTMENT OF STEM CELL AND TISSUE ENGINEERING (THESIS) COURSE DESCRIPTIONS 1 st SEMESTER Adult Stem Cell Biology 5 ECTS In this course, the characteristics
Living, Autologous Pulmonary Artery Conduits Tissue Engineered From Human Umbilical Cord Cells
 Living, Autologous Pulmonary Artery Conduits Tissue Engineered From Human Umbilical Cord Cells Simon P. Hoerstrup, MD, Alexander Kadner, MD, Christian Breymann, MD, Christine F. Maurus, MD, Christina I.
Living, Autologous Pulmonary Artery Conduits Tissue Engineered From Human Umbilical Cord Cells Simon P. Hoerstrup, MD, Alexander Kadner, MD, Christian Breymann, MD, Christine F. Maurus, MD, Christina I.
UNIT CELL PROCESSES UNDERLYING TISSUE ENGINEERING AND REGENERATIVE MEDICINE
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/20.441/HST522J UNIT CELL PROCESSES UNDERLYING TISSUE ENGINEERING AND REGENERATIVE
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/20.441/HST522J UNIT CELL PROCESSES UNDERLYING TISSUE ENGINEERING AND REGENERATIVE
Present and future of regenerative medicine. Liver Transplantation
 Present and future of regenerative medicine Liver Transplantation Mireia Caralt, MD PhD Servei Cirurgia HBP i Trasplantaments March 19, 2015 Introduction Strategies to increase the number of organs EXPAND
Present and future of regenerative medicine Liver Transplantation Mireia Caralt, MD PhD Servei Cirurgia HBP i Trasplantaments March 19, 2015 Introduction Strategies to increase the number of organs EXPAND
ACCEPTED MANUSCRIPT. In situ heart valve tissue engineering: Employing the innate immune response to do the
 Accepted Manuscript In situ heart valve tissue engineering: Employing the innate immune response to do the hard work F. Zafar, MD, D.L.S. Morales, MD PII: S0022-5223(18)30839-0 DOI: 10.1016/j.jtcvs.2018.03.059
Accepted Manuscript In situ heart valve tissue engineering: Employing the innate immune response to do the hard work F. Zafar, MD, D.L.S. Morales, MD PII: S0022-5223(18)30839-0 DOI: 10.1016/j.jtcvs.2018.03.059
Corneal Reconstruction Using Tissue- Engineered Epithelial Cell Sheets Fabricated ex vivo From Autologous Oral Mucosal Epithelium
 Corneal Reconstruction Using Tissue- Engineered Epithelial Cell Sheets Fabricated ex vivo From Autologous Oral Mucosal Epithelium Kohji Nishida, M.D., Ph.D. Department of Ophthalmology Tohoku University
Corneal Reconstruction Using Tissue- Engineered Epithelial Cell Sheets Fabricated ex vivo From Autologous Oral Mucosal Epithelium Kohji Nishida, M.D., Ph.D. Department of Ophthalmology Tohoku University
Transplant & Preparation Center
 Transplant & Preparation Center Raymond L. Benza M.D. FACC Allegheny General Hospital At AGH, we have established a team to facilitate the rapid collection of lungs removed at transplant from patients
Transplant & Preparation Center Raymond L. Benza M.D. FACC Allegheny General Hospital At AGH, we have established a team to facilitate the rapid collection of lungs removed at transplant from patients
Original Article Biodegradation and biocompatibility of a degradable chitosan vascular prosthesis
 Int J Clin Exp Med 2015;8(3):3498-3505 www.ijcem.com /ISSN:1940-5901/IJCEM0004997 Original Article Biodegradation and biocompatibility of a degradable chitosan vascular prosthesis Xiaoying Kong 1, Wenhua
Int J Clin Exp Med 2015;8(3):3498-3505 www.ijcem.com /ISSN:1940-5901/IJCEM0004997 Original Article Biodegradation and biocompatibility of a degradable chitosan vascular prosthesis Xiaoying Kong 1, Wenhua
DEVELOPMENT OF FUNCTIONAL BIOENGINEERED MUSCLE MODELS AND A NOVEL MICRO-PERFUSION SYSTEM. Louise Hecker
 DEVELOPMENT OF FUNCTIONAL BIOENGINEERED MUSCLE MODELS AND A NOVEL MICRO-PERFUSION SYSTEM by Louise Hecker A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of
DEVELOPMENT OF FUNCTIONAL BIOENGINEERED MUSCLE MODELS AND A NOVEL MICRO-PERFUSION SYSTEM by Louise Hecker A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of
White Paper: Designing Functional Fabrics for Today s Heart Valves and Beyond
 White Paper: Designing Functional Fabrics for Today s Heart Valves and Beyond Ryan Heniford Business Development Director Peter Gabriele Director, Emerging Technology How fabrics can be used in heart valves,
White Paper: Designing Functional Fabrics for Today s Heart Valves and Beyond Ryan Heniford Business Development Director Peter Gabriele Director, Emerging Technology How fabrics can be used in heart valves,
Supplementary information Activation of AMP-activated protein kinase
 Supplementary information Activation of AMP-activated protein kinase 2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo Shuangxi Wang 1,2,5, Cheng Zhang 1,2,5, Miao Zhang
Supplementary information Activation of AMP-activated protein kinase 2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo Shuangxi Wang 1,2,5, Cheng Zhang 1,2,5, Miao Zhang
Stem cells and tissue engineering
 Stem cells and tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Stem cells and tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Future implications of regenerative medicine on assisted reproductive technology. Regenerative medicine. History of Regenerative medicine
 Gyndolomiti: 2nd Congress on Gynaecology and Obstetrics February 1 6, 2015 St. Kassian / South Tyrol Regenerative medicine Future implications of regenerative medicine on assisted reproductive technology
Gyndolomiti: 2nd Congress on Gynaecology and Obstetrics February 1 6, 2015 St. Kassian / South Tyrol Regenerative medicine Future implications of regenerative medicine on assisted reproductive technology
Introduction to Cell and Biomaterial Engineering! Module 3, Lecture 1!! Spring 2014!
 Introduction to Cell and Biomaterial Engineering! Module 3, Lecture 1!! 20.109 Spring 2014! Topics for Lecture 1! Introduction to tissue engineering! motivation! basic principles! examples! Introduction
Introduction to Cell and Biomaterial Engineering! Module 3, Lecture 1!! 20.109 Spring 2014! Topics for Lecture 1! Introduction to tissue engineering! motivation! basic principles! examples! Introduction
Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis
 Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis Yan Liu 1,*, Zhipeng Han 1,*, Yingying Jing 1,*, Xue Yang 1, Shanshan Zhang 1, Chen
Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis Yan Liu 1,*, Zhipeng Han 1,*, Yingying Jing 1,*, Xue Yang 1, Shanshan Zhang 1, Chen
Figure S Relative MUC4 transcript level* CD18/HPAF CD18/HPAF-Scr CD18/HPAF-siMUC4
 Figure S1 Relative MUC4 transcript level* 1.4 1.2 1 0.8 0.6 0.4 0.2 0 CD18/HPAF CD18/HPAF-Scr CD18/HPAF-siMUC4 Figure S2 * * CD18/HPAF-Scr CD18/HPAF-siMUC4 CD18/HPAF-Scr CD18/HPAF-siMUC4 Figure S3 CD18/HPAF-Scr
Figure S1 Relative MUC4 transcript level* 1.4 1.2 1 0.8 0.6 0.4 0.2 0 CD18/HPAF CD18/HPAF-Scr CD18/HPAF-siMUC4 Figure S2 * * CD18/HPAF-Scr CD18/HPAF-siMUC4 CD18/HPAF-Scr CD18/HPAF-siMUC4 Figure S3 CD18/HPAF-Scr
Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW
 Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW Lyset, the human platelet derived supplement for cell culture Among the different alternatives to animal serum, platelet derived preparations
Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW Lyset, the human platelet derived supplement for cell culture Among the different alternatives to animal serum, platelet derived preparations
Regenerative Strategies for Vascular and Lung Tissues. Laura E Niklason MD, PhD
 Regenerative Strategies for Vascular and Lung Tissues Laura E Niklason MD, PhD Disclosure: Some of this work in this presentation is from Humacyte Inc. Niklason is a founder of Humacyte, and holds stock
Regenerative Strategies for Vascular and Lung Tissues Laura E Niklason MD, PhD Disclosure: Some of this work in this presentation is from Humacyte Inc. Niklason is a founder of Humacyte, and holds stock
Fibronectin-Hepatocyte Growth Factor Enhances Reendothelialization in Tissue-Engineered Heart Valve
 CARDIOVASCULAR Fibronectin-Hepatocyte Growth Factor Enhances Reendothelialization in Tissue-Engineered Heart Valve Takeyoshi Ota, MD, Yoshiki Sawa, MD, PhD, Shigemitsu Iwai, MD, Takashi Kitajima, PhD,
CARDIOVASCULAR Fibronectin-Hepatocyte Growth Factor Enhances Reendothelialization in Tissue-Engineered Heart Valve Takeyoshi Ota, MD, Yoshiki Sawa, MD, PhD, Shigemitsu Iwai, MD, Takashi Kitajima, PhD,
cardiovascular cell Solutions
 The Essentials of Life Science Research Globally Delivered cardiovascular cell Solutions We love what we do. And now we do it even better! As part of your portfolio for success, ATCC now provides a range
The Essentials of Life Science Research Globally Delivered cardiovascular cell Solutions We love what we do. And now we do it even better! As part of your portfolio for success, ATCC now provides a range
Promising Future for Ex vivo Tissue Fabrication. Eiji Kobayashi, MD, PhD Department of Organ Fabrication, Keio University School of Medicine, Japan
 Promising Future for Ex vivo Tissue Fabrication Eiji Kobayashi, MD, PhD Department of Organ Fabrication, Keio University School of Medicine, Japan The research for fabricating tissue/organs through cultivation
Promising Future for Ex vivo Tissue Fabrication Eiji Kobayashi, MD, PhD Department of Organ Fabrication, Keio University School of Medicine, Japan The research for fabricating tissue/organs through cultivation
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation
 Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Introduction to Cell- Biomaterial Engineering!
 Introduction to Cell- Biomaterial Engineering! Module 3, Lecture 1! 20.109 Spring 2010! Topics for Lecture 1!! Introduction to tissue engineerin! motivation"! basic principles + examples"! Introduction
Introduction to Cell- Biomaterial Engineering! Module 3, Lecture 1! 20.109 Spring 2010! Topics for Lecture 1!! Introduction to tissue engineerin! motivation"! basic principles + examples"! Introduction
Bioreactors in tissue engineering
 Bioreactors in tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Bioreactors in tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Supplemental Information
 Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
1 Publishable summary
 1 Publishable summary 1.1 Summary of project objectives Valve replacement with mechanical or bioprosthetic prostheses is the most common intervention for valvular disease, with almost 300.000 annual replacements
1 Publishable summary 1.1 Summary of project objectives Valve replacement with mechanical or bioprosthetic prostheses is the most common intervention for valvular disease, with almost 300.000 annual replacements
Subject Index. Antithrombotic drugs See Drugs, antithrombotic
 STP898-EB/Sep. 1986 Subject Index A Absorbable vascular grafts, 197-217 Acellular vascular matrix, 219-235 Aneurysm, 58, 98 See also Failure, vascular grafts Animal experimental models baboon, endothelial
STP898-EB/Sep. 1986 Subject Index A Absorbable vascular grafts, 197-217 Acellular vascular matrix, 219-235 Aneurysm, 58, 98 See also Failure, vascular grafts Animal experimental models baboon, endothelial
InVivo Therapeutics. Developing Innovative Products for Spinal Cord Injury
 1 Developing Innovative Products for Spinal Cord Injury 2 Forward-Looking Statements Before we begin, we would like to remind everyone that during our presentation, we will be making forward-looking statements
1 Developing Innovative Products for Spinal Cord Injury 2 Forward-Looking Statements Before we begin, we would like to remind everyone that during our presentation, we will be making forward-looking statements
CHALLENGES OF 3D BIOPRINTING IN CLINICAL PRACTICE
 CENTRE DE THÉRAPIE TISSULAIRE & CELLULAIRE CHALLENGES OF 3D BIOPRINTING IN CLINICAL PRACTICE Pr. D. Dufrane MD, PhD 3D-BIOPRINTING: MYTH OR REALITY? 2 REGENERATIVE MEDICINE FOR ORGAN AND TISSUE A LARGE
CENTRE DE THÉRAPIE TISSULAIRE & CELLULAIRE CHALLENGES OF 3D BIOPRINTING IN CLINICAL PRACTICE Pr. D. Dufrane MD, PhD 3D-BIOPRINTING: MYTH OR REALITY? 2 REGENERATIVE MEDICINE FOR ORGAN AND TISSUE A LARGE
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells
 Cell research (2003); 13(5):335-341 Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells GAN SHEN *, HSIAO CHIEN TSUNG *, CHUN FANG WU, XIAO YIN LIU,
Cell research (2003); 13(5):335-341 Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells GAN SHEN *, HSIAO CHIEN TSUNG *, CHUN FANG WU, XIAO YIN LIU,
Technical Review. Real time PCR
 Technical Review Real time PCR Normal PCR: Analyze with agarose gel Normal PCR vs Real time PCR Real-time PCR, also known as quantitative PCR (qpcr) or kinetic PCR Key feature: Used to amplify and simultaneously
Technical Review Real time PCR Normal PCR: Analyze with agarose gel Normal PCR vs Real time PCR Real-time PCR, also known as quantitative PCR (qpcr) or kinetic PCR Key feature: Used to amplify and simultaneously
Des cellules-souches dans le poumon : pourquoi faire?
 Des cellules-souches dans le poumon : pourquoi faire? Karl-Heinz Krause Dept. of Pathology and Immunology, Medical Faculty Dept. of Genetic and Laboratory Medicine, University Hospitals Geneva, Switzerland
Des cellules-souches dans le poumon : pourquoi faire? Karl-Heinz Krause Dept. of Pathology and Immunology, Medical Faculty Dept. of Genetic and Laboratory Medicine, University Hospitals Geneva, Switzerland
Lecture 6: Programmed/Pulsed Drug Delivery and Drug Delivery in Tissue Engineering
 Lecture 6: Programmed/Pulsed Drug Delivery and Drug Delivery in Tissue Engineering Last time: principles of controlled release from solid polymers Today: Reading: Pulsatile/regulated/multifactor controlled
Lecture 6: Programmed/Pulsed Drug Delivery and Drug Delivery in Tissue Engineering Last time: principles of controlled release from solid polymers Today: Reading: Pulsatile/regulated/multifactor controlled
Importance of inflammation reaction of scaffold for the application of regenerative medicine
 Review ArticleInflammation reaction of scaffold for regenerative medicine 178 Review Article Importance of inflammation reaction of scaffold for the application of regenerative medicine Gilson Khang Department
Review ArticleInflammation reaction of scaffold for regenerative medicine 178 Review Article Importance of inflammation reaction of scaffold for the application of regenerative medicine Gilson Khang Department
Flow cytometry Stained cells were analyzed and sorted by SORP FACS Aria (BD Biosciences).
 Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges
 Annals of Biomedical Engineering, Vol. 34, No. 12, December 2006 ( C 2006) pp. 1799 1819 DOI: 10.1007/s10439-006-9163-z Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges KAREN
Annals of Biomedical Engineering, Vol. 34, No. 12, December 2006 ( C 2006) pp. 1799 1819 DOI: 10.1007/s10439-006-9163-z Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges KAREN
Supplemental Table S1. RT-PCR primers used in this study
 Supplemental Table S1. RT-PCR primers used in this study -----------------------------------------------------------------------------------------------------------------------------------------------
Supplemental Table S1. RT-PCR primers used in this study -----------------------------------------------------------------------------------------------------------------------------------------------
Tissue Engineering of Autologous Human Heart Valves Using Cryopreserved Vascular Umbilical Cord Cells
 Tissue Engineering of Autologous Human Heart Valves Using Cryopreserved Vascular Umbilical Cord Cells Ralf Sodian, MD, Cora Lueders, PhD, Liv Kraemer, MD, Wolfgang Kuebler, MD, Mehdi Shakibaei, MD, Bruno
Tissue Engineering of Autologous Human Heart Valves Using Cryopreserved Vascular Umbilical Cord Cells Ralf Sodian, MD, Cora Lueders, PhD, Liv Kraemer, MD, Wolfgang Kuebler, MD, Mehdi Shakibaei, MD, Bruno
Development 142: doi: /dev : Supplementary Material
 Development 142: doi:1.42/dev.11687: Supplementary Material Figure S1 - Relative expression of lineage markers in single ES cell and cardiomyocyte by real-time quantitative PCR analysis. Single ES cell
Development 142: doi:1.42/dev.11687: Supplementary Material Figure S1 - Relative expression of lineage markers in single ES cell and cardiomyocyte by real-time quantitative PCR analysis. Single ES cell
Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges
 Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges
 Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters.
Heart Valve Tissue Engineering: Concepts, Approaches, Progress, and Challenges The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters.
Supplementary Methods
 Supplementary Methods Antibodies Rabbit polyclonal VEGFR-1, VEGFR-2, Angiopoietin-1, Angiopoietin- 2 and GAPDH specific antibodies were from Santa Cruz Biotechnology. Rabbit polyclonal Akt1, Akt2, ps473
Supplementary Methods Antibodies Rabbit polyclonal VEGFR-1, VEGFR-2, Angiopoietin-1, Angiopoietin- 2 and GAPDH specific antibodies were from Santa Cruz Biotechnology. Rabbit polyclonal Akt1, Akt2, ps473
Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells
 Cell Research (2003); 13(5):335-341 Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells GAN SHEN*, HSIAO CHIEN TSUNG*, CHUN FANG WU, XIAO YIN LIU,
Cell Research (2003); 13(5):335-341 Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells GAN SHEN*, HSIAO CHIEN TSUNG*, CHUN FANG WU, XIAO YIN LIU,
Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells. in the injured sites
 Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites Yunyuan Li, Reza Baradar Jalili, Aziz Ghahary Department of Surgery, University of British Columbia,
Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites Yunyuan Li, Reza Baradar Jalili, Aziz Ghahary Department of Surgery, University of British Columbia,
Optimal Biomaterial for Creation of Autologous Cardiac Grafts
 Optimal Biomaterial for Creation of Autologous Cardiac Grafts Tsukasa Ozawa, MD, PhD; Donald A. G. Mickle, MD, MSc; Richard D. Weisel, MD; Nobuya Koyama, MD, PhD; Sumiko Ozawa, MD; Ren-Ke Li, MD, PhD Background
Optimal Biomaterial for Creation of Autologous Cardiac Grafts Tsukasa Ozawa, MD, PhD; Donald A. G. Mickle, MD, MSc; Richard D. Weisel, MD; Nobuya Koyama, MD, PhD; Sumiko Ozawa, MD; Ren-Ke Li, MD, PhD Background
Course Handbook MSc in Bioengineering Tissue Engineering Specialisation
 Course Handbook 2013-2014 MSc in Bioengineering Tissue Engineering Specialisation 1 Course Objectives & Learning Outcomes This programme aims to give a sound and broad basis in tissue engineering. In particular,
Course Handbook 2013-2014 MSc in Bioengineering Tissue Engineering Specialisation 1 Course Objectives & Learning Outcomes This programme aims to give a sound and broad basis in tissue engineering. In particular,
PAPER. Functional Small-Diameter Human Tissue Engineered Arterial Grafts in an Immunodeficient Mouse Model
 PAPER Functional Small-Diameter Human Tissue Engineered Arterial Grafts in an Immunodeficient Mouse Model Preliminary Findings Gregory N. Nelson, BS; Tamar Mirensky, MD; Matthew P. Brennan, MD; Jason D.
PAPER Functional Small-Diameter Human Tissue Engineered Arterial Grafts in an Immunodeficient Mouse Model Preliminary Findings Gregory N. Nelson, BS; Tamar Mirensky, MD; Matthew P. Brennan, MD; Jason D.
Affinity. A Paradigm Shift in Skeletal Reconstruction
 Affinity A Paradigm Shift in Skeletal Reconstruction INTRODUCING TRS AFFINITY SKELETAL RECONSTRUCTION BREAKTHROUGH Tissue Regeneration Systems (TRS ) is a start-up medical device company commercializing
Affinity A Paradigm Shift in Skeletal Reconstruction INTRODUCING TRS AFFINITY SKELETAL RECONSTRUCTION BREAKTHROUGH Tissue Regeneration Systems (TRS ) is a start-up medical device company commercializing
Bone Marrow as a Cell Source for Tissue Engineering Heart Valves
 THORACIC SURGERY DIRECTORS ASSOCIATION AWARD The Thoracic Surgery Directors Association (TSDA) Resident Research Award, sponsored by Medtronic, Inc, was established in 1990 to encourage resident research
THORACIC SURGERY DIRECTORS ASSOCIATION AWARD The Thoracic Surgery Directors Association (TSDA) Resident Research Award, sponsored by Medtronic, Inc, was established in 1990 to encourage resident research
Supplementary Information. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro
 Supplementary Information Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro Yu-suke Torisawa 1, Catherine S. Spina 1,2, Tadanori Mammoto 3, Akiko Mammoto 3, James C. Weaver 1, Tracy
Supplementary Information Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro Yu-suke Torisawa 1, Catherine S. Spina 1,2, Tadanori Mammoto 3, Akiko Mammoto 3, James C. Weaver 1, Tracy
Journal of Biomedical Materials Research, Vol. 20, (1986) John Wiley & Sons, Inc.
 Journal of Biomedical Materials Research, Vol. 20, 861-865 (1986) 0 1986 John Wiley & Sons, Inc. 862 Characterization of morphologic and mechanical properties of surgical mesh fabrics C. C. Chu, B. Pourdeyhimi,*
Journal of Biomedical Materials Research, Vol. 20, 861-865 (1986) 0 1986 John Wiley & Sons, Inc. 862 Characterization of morphologic and mechanical properties of surgical mesh fabrics C. C. Chu, B. Pourdeyhimi,*
Mice TRAMP mice were maintained in a C57BL/6J background. Syngeneic UBI-GFP/BL6 mice were used for bone marrow engraftment. 2
 Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
York University School of Medicine, NY. Bhagavathi A. Narayanan, PhD 1, Caroline Cunnigham 1 and Amitabha Mazumder, MD 2. Title
 Title Suppression of Human Multiple Myeloma Cell Growth by TBL-12 in Combination with low doses of Velcade: Insight in to the modulation of IL-6/STAT-3 mechanisms Bhagavathi A. Narayanan, PhD 1, Caroline
Title Suppression of Human Multiple Myeloma Cell Growth by TBL-12 in Combination with low doses of Velcade: Insight in to the modulation of IL-6/STAT-3 mechanisms Bhagavathi A. Narayanan, PhD 1, Caroline
Supplementary Data. Plasmid -2486CAT containing the 2.5 kb fragment of VE-cadherin promoter region (-2486
 Supplementary Data Materials and Methods Generation and Genotyping of Transgenic Mice Plasmid -2486CAT containing the 2.5 kb fragment of VE-cadherin promoter region (-2486 to +24) was a gift from P. Huber.
Supplementary Data Materials and Methods Generation and Genotyping of Transgenic Mice Plasmid -2486CAT containing the 2.5 kb fragment of VE-cadherin promoter region (-2486 to +24) was a gift from P. Huber.
Supplementary Materials and Methods:
 Supplementary Materials and Methods: Preclinical chemoprevention experimental design Mice were weighed and each mammary tumor was manually palpated and measured with digital calipers once a week from 4
Supplementary Materials and Methods: Preclinical chemoprevention experimental design Mice were weighed and each mammary tumor was manually palpated and measured with digital calipers once a week from 4
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
Supplementary Figure 1. TSA (10 nmol/l), non-class-selective HDAC inhibitor, potentiates
 Supplementary Figure 1. TSA (10 nmol/l), non-class-selective HDAC inhibitor, potentiates vascular calcification (VC). (a) Von Kossa staining shows that TSA potentiated the Pi-induced VC. Scale bar, 100
Supplementary Figure 1. TSA (10 nmol/l), non-class-selective HDAC inhibitor, potentiates vascular calcification (VC). (a) Von Kossa staining shows that TSA potentiated the Pi-induced VC. Scale bar, 100
Reagents and cell culture Mcl-1 gene expression: real-time quantitative RT-PCR In vitro PP2A phosphatase assay Detection of Mcl-1 in vivo
 Reagents and cell culture Antibodies specific for caspase 3, PARP and GAPDH were purchased from Cell Signaling Technology Inc. (Beverly, MA). Caspase inhibitor z-vad-fmk and ROS scavenger N-acetyl-Lcysteine
Reagents and cell culture Antibodies specific for caspase 3, PARP and GAPDH were purchased from Cell Signaling Technology Inc. (Beverly, MA). Caspase inhibitor z-vad-fmk and ROS scavenger N-acetyl-Lcysteine
Molecular Cell Biology - Problem Drill 11: Recombinant DNA
 Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
A PVA_PCL_Bioglass Composite with Potential Implications for Osteochondral Tissue Engineering.
 A PVA_PCL_Bioglass Composite with Potential Implications for Osteochondral Tissue Engineering. Journal: 2009 MRS Fall Meeting Manuscript ID: Draft Symposium: Symposium RR Date Submitted by the Author:
A PVA_PCL_Bioglass Composite with Potential Implications for Osteochondral Tissue Engineering. Journal: 2009 MRS Fall Meeting Manuscript ID: Draft Symposium: Symposium RR Date Submitted by the Author:
EMA s role & responsibility for the development of modern/advanced therapies
 EMA s role & responsibility for the development of modern/advanced therapies Agenda Current regulatory picture Overall Regulatory framework New Committee at EMA Current Activities & Challenges Egbert Flory
EMA s role & responsibility for the development of modern/advanced therapies Agenda Current regulatory picture Overall Regulatory framework New Committee at EMA Current Activities & Challenges Egbert Flory
Detection of Histone Modifications at Specific Gene Loci in Single Cells in Histological Sections
 Detection of Histone Modifications at Specific Gene Loci in Single Cells in Histological Sections Delphine Gomez; Laura L Shankman; Anh T Nguyen; Gary K Owens. Supplementary Figures 1-13 Supplementary
Detection of Histone Modifications at Specific Gene Loci in Single Cells in Histological Sections Delphine Gomez; Laura L Shankman; Anh T Nguyen; Gary K Owens. Supplementary Figures 1-13 Supplementary
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Methods in Bioengineering: 3D Tissue Engineering
 Methods in Bioengineering: 3D Tissue Engineering Berthiaume, Francois ISBN-13: 9781596934580 Table of Contents Preface Chapter 1. Chemical Modification of Porous Scaffolds Using Plasma Polymers 1.1. Introduction
Methods in Bioengineering: 3D Tissue Engineering Berthiaume, Francois ISBN-13: 9781596934580 Table of Contents Preface Chapter 1. Chemical Modification of Porous Scaffolds Using Plasma Polymers 1.1. Introduction
Index 311. overview, 84, 85 pretreatment, 85 probe preparation, 85 washing, 85, 91. G GeneChip, see DNA microarray
 Index 309 Index A AIDS, see Human immunodeficiency virus Allelotyping, microdissection advantages, 69, 70 ovarian cancer, data acquisition and analysis, 74 DNA extraction, 72, 73, 76 materials, 70, 71
Index 309 Index A AIDS, see Human immunodeficiency virus Allelotyping, microdissection advantages, 69, 70 ovarian cancer, data acquisition and analysis, 74 DNA extraction, 72, 73, 76 materials, 70, 71
Alex A. Aimetti, PhD Sr. Director, Medical Education October 29, InVivo Therapeutics
 Translation of Biomaterial-based Therapies for the Treatment of Acute and Chronic Spinal Cord Injury: The Neuro-Spinal Scaffold and Bioengineered Neural Trails Alex A. Aimetti, PhD Sr. Director, Medical
Translation of Biomaterial-based Therapies for the Treatment of Acute and Chronic Spinal Cord Injury: The Neuro-Spinal Scaffold and Bioengineered Neural Trails Alex A. Aimetti, PhD Sr. Director, Medical
10:10-10:22. YIA-1 A study of newly established human peripheral blood monocyte-derived ips cell line used in allergy research 10:22-10:32
 10:10-10:22 YIA-1 A study of newly established human peripheral blood monocyte-derived ips cell line used in allergy research 10:22-10:32 EPA-1 Integration of conventional cell viability assays- recruiting
10:10-10:22 YIA-1 A study of newly established human peripheral blood monocyte-derived ips cell line used in allergy research 10:22-10:32 EPA-1 Integration of conventional cell viability assays- recruiting
Enhancement of bone formation by genetically-engineered bone marrow stromal cells expressing BMP-2, VEGF and angiopoietin-1
 Biotechnol Lett (2009) 31:1183 1189 DOI 10.1007/s10529-009-0007-4 ORIGINAL RESEARCH PAPER Enhancement of bone formation by genetically-engineered bone marrow stromal cells expressing BMP-2, VEGF and angiopoietin-1
Biotechnol Lett (2009) 31:1183 1189 DOI 10.1007/s10529-009-0007-4 ORIGINAL RESEARCH PAPER Enhancement of bone formation by genetically-engineered bone marrow stromal cells expressing BMP-2, VEGF and angiopoietin-1
BUILT TO CONCENTRATE. Magellan is an autologous concentration system that delivers concentrated platelets and cells at the point of care.
 BUILT TO CONCENTRATE Magellan is an autologous concentration system that delivers concentrated platelets and cells at the point of care. DELIVER PERSONALIZED MEDICINE Every patient has a unique biology
BUILT TO CONCENTRATE Magellan is an autologous concentration system that delivers concentrated platelets and cells at the point of care. DELIVER PERSONALIZED MEDICINE Every patient has a unique biology
Department of Metallurgy and Materials Engineering, Katholieke Universiteit Leuven, B Leuven, Belgium; 2
 Micro-CT: a powerful tool for the characterization and evaluation of the bone formatting capacity of calcium phosphate stem cells tissue engineering constructs G. Kerckhofs 1,2, SJ. Roberts 2,3, M. Wevers
Micro-CT: a powerful tool for the characterization and evaluation of the bone formatting capacity of calcium phosphate stem cells tissue engineering constructs G. Kerckhofs 1,2, SJ. Roberts 2,3, M. Wevers
TISSUE ENGINEERING AND REGENERATION: TECHNOLOGIES AND GLOBAL MARKETS
 TISSUE ENGINEERING AND REGENERATION: TECHNOLOGIES AND GLOBAL MARKETS HLC101B August 2014 Yojana Jeevane Project Analyst ISBN: 1-56965-894-3 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
TISSUE ENGINEERING AND REGENERATION: TECHNOLOGIES AND GLOBAL MARKETS HLC101B August 2014 Yojana Jeevane Project Analyst ISBN: 1-56965-894-3 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
Nanosystems in regenerative medicine. Jöns Hilborn Materials Chemistry The Ångström Laboratory Uppsala University Sweden
 Nanosystems in regenerative medicine Jöns Hilborn Materials Chemistry The Ångström Laboratory Uppsala University Sweden Outline Motivation for tissue regeneration Cell based approaches Material based
Nanosystems in regenerative medicine Jöns Hilborn Materials Chemistry The Ångström Laboratory Uppsala University Sweden Outline Motivation for tissue regeneration Cell based approaches Material based
REMEDI. Regenerative Medicine Institute (REMEDI) NUI Galway, Ireland GENERAL PRESENTATION. Director: Prof. Frank Barry
 Regenerative Medicine Institute (REMEDI) NUI Galway, Ireland Director: Prof. Frank Barry GENERAL PRESENTATION Contact person in NEWGEN: Dr. Jessica Hayes Working Group Involvement: Member of Working Group
Regenerative Medicine Institute (REMEDI) NUI Galway, Ireland Director: Prof. Frank Barry GENERAL PRESENTATION Contact person in NEWGEN: Dr. Jessica Hayes Working Group Involvement: Member of Working Group
Ceramics, Cracow, Poland
 Chemically and physically functionalized carbon composites a prospective material for tissue treatment S.Blazewicz 1*, E.Stodolak 1, E. Staszkow 2, T.Mikolajczyk 3 1 AGH University of Science and Technology,
Chemically and physically functionalized carbon composites a prospective material for tissue treatment S.Blazewicz 1*, E.Stodolak 1, E. Staszkow 2, T.Mikolajczyk 3 1 AGH University of Science and Technology,
Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats
 Surgery for Congenital Heart Disease Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats Tsukasa Ozawa, MD, PhD, Donald A. G. Mickle,
Surgery for Congenital Heart Disease Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats Tsukasa Ozawa, MD, PhD, Donald A. G. Mickle,
-- Laki J. Rousou, MS, MD et. al. -- Boston, Massachusetts. -- Ann Thorac Surg 2009;87:62 70.
 -- Laki J. Rousou, MS, MD et. al -- Boston, Massachusetts. -- Ann Thorac Surg 2009;87:62 70. Presented by: Int. 黃柏翔 Date: 2009 /02 / 25 Patient outcome after CABG surgery greatly influenced by patency
-- Laki J. Rousou, MS, MD et. al -- Boston, Massachusetts. -- Ann Thorac Surg 2009;87:62 70. Presented by: Int. 黃柏翔 Date: 2009 /02 / 25 Patient outcome after CABG surgery greatly influenced by patency
