Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos
|
|
|
- Annabelle Jacobs
- 5 years ago
- Views:
Transcription
1 Development 125, (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos Elaine M. Joseph and Douglas A. Melton* Howard Hughes Medical Institute, Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138, USA *Author for correspondence ( Accepted 30 April; published on WWW 23 June 1998 SUMMARY The Xenopus Vg1 gene, a TGFβ superfamily member, is expressed as a maternal mrna localized to prospective endoderm, and mature Vg1 protein can induce both endodermal and mesodermal markers in embryonic cells. Most previous work on embryonic inducers, including activin, BMPs and Vg1, has relied on ectopic expression to assay for gene function. Here we employ a mutant ligand approach to block Vg1 signaling in developing embryos. The results indicate that Vg1 expression is essential for normal endodermal development and the induction of dorsal mesoderm in vivo. Key words: Xenopus laevis, Vg1, Mutant ligand, Endoderm, Mesoderm, Signaling, Embryonic inducer INTRODUCTION Cells at the vegetal hemisphere of Xenopus blastula have two important roles: to form endoderm and induce mesoderm. The endoderm gives rise to the gastrointestinal tract with associated organs including the lung, liver and pancreas. Induction of mesoderm occurs during the early cleavage stages and leads to formation of the vertebrate body axis with bone, muscle, blood and other mesodermal derivatives. Relatively little is known about the formation of endoderm, but signaling molecules capable of inducing mesoderm have been intensely studied and it is generally agreed that TGFβ proteins play a key role (reviewed in Kessler and Melton, 1994; Jones et al., 1995; Smith et al., 1995), as does signaling by β-catenin (Heasman et al., 1994). There is also evidence that FGF signals are needed for the maintenance of mesoderm that has been induced (Amaya et al., 1994). One TGFβ, Vg1, is uniquely interesting because it is present as a maternal mrna localized to cells at the vegetal hemisphere (Rebagliati et al., 1985; Weeks and Melton, 1987), i.e. the cells that form endoderm and induce mesoderm. When Vg1 mrna is ectopically expressed in Xenopus embryos, large amounts of the inactive prepropolypeptide are synthesized but most of this precursor is not cleaved to give a mature active protein, suggesting that post-translational processing of Vg1 is tightly regulated. This corresponds with the fact that while a large amount of the Vg1 prepropolypeptide is detectable in oocytes and early embryos, mature Vg1 protein is not present at definitively detectable levels (Dale et al., 1989; Tannahill and Melton, 1989). Studies on the zebrafish orthologue, zdvr-1, have similarly shown that unprocessed precursor zdvr-1 protein is distributed throughout the future dorsal-ventral axis and that mature protein has potent inductive activity (Dohrmann et al., 1996). More recently, Cooke and colleagues have characterized a chick orthologue of Vg1, cvg1, and shown that ectopic expression is capable of initiating axis formation in chick embryos (Seleiro et al., 1996). Based on their results and previous work, they suggest that Vg genes may be required for an evolutionarily conserved early step in positioning or inducing the main body axis (Seleiro et al., 1996). In Xenopus, ectopic expression of mature Vg1, using a chimeric construct of the BMP2 pro-region with the Vg1 mature region (BVg1), which directs synthesis of dimers and processing, shows that Vg1 is able to induce both endodermal and mesodermal markers in embryonic explants (Henry et al., 1996; Thomsen and Melton, 1993). The converse experiment, removal of Vg1 activity, has not been accomplished and an endogenous role for Vg1 signaling is open to question. Experiments with truncated TGFβ receptors (specifically, the activin type II receptor) have demonstrated the requirement for TGFβ signals, in general, in mesoderm development, but since this reagent blocks signaling by all TGFβ ligands it has been tested against (i.e. activin, Vg1 and BMP signals), those experiments do not allow for a strong conclusion as to which signals are required (Hemmati-Brivanlou and Melton, 1992; Kessler and Melton, 1995; Schulte-Merker et al., 1994; Hemmati-Brivanlou and Thomsen, 1995). The activinbinding protein follistatin, which blocks signaling by activin and BMP4 but not by Vg1, does not disrupt dorsal mesoderm formation when overexpressed in Xenopus embryos (Fainsod et al., 1997; Kessler and Melton, 1995; Sasai et al., 1995; Schulte-Merker et al., 1994), arguing against activin playing a key role in dorsal mesoderm induction. A dominant negative mutant ligand approach has been used to study medaka development (Wittbrodt and Rosa, 1994); however,
2 2678 E. M. Joseph and D. A. Melton it is unclear whether the activin mutant in this medaka study blocked signaling by other TGFβ members, such as Vg1 and/or BMPs. To address the role of Vg1 in Xenopus and to overcome the lack of specificity observed with truncated receptors, we have used a mutant ligand that appears to selectively block Vg1 signaling. Affected embryos lack dorsal mesoderm and axial structures. These embryos also lack expression of an early endodermal marker, Xlhbox8, a gene coding for a transcription factor expressed in dorsal vegetal pole cells (prospective endoderm) and later in the pancreas (Gamer and Wright, 1995; Henry et al., 1996; Wright et al., 1988). MATERIALS AND METHODS Construction of Vg1 mutants and mrna synthesis The mutants were constructed in the BVg1 chimeric construct using the Altered Sites mutagenesis system (Promega) and were then subcloned into psp64t3 (Thomsen and Melton, 1993). The m109/111 BVg1 construct was provided by G. H. Thomsen (Thomsen and Melton, 1993). Sequencing of the mutant constructs was performed using a Sequenase version 2.0 kit (USB/Amersham) and the sequencing reactions were run through 6% polyacrylamide gels using SequaGel reagents (National Diagnostics). Capped mrna was synthesized in vitro from linearized DNA templates using the Megascript SP6 transcription kit (Ambion). Embryological manipulations and histology Xenopus laevis eggs were fertilized, cultured and injected according to Thomsen and Melton (1993). The staging of embryos was done according to Nieuwkoop and Faber (1967). Histology was done by fixing embryos in MEMFA (0.1 M Mops, ph 7.4, 2 mm EGTA, 1 mm MgSO 4, 3.7% formaldehyde), embedding in paraplast, cutting 8 µm sections, and staining with Giemsa. Reverse transcripion-polymerase chain reactions Reverse transcription-polymerase chain reactions (RT-PCR) were used to study molecular marker expression (according to Wilson and Melton, 1994). The resulting PCR products were run through 5% acrylamide gels. EF1-α was used a control for RNA recovery (Krieg et al., 1989). Primer sequences used for RT-PCR were: EF1-α: U CAGATTGGTGCTGGATATGC/D ACTGCCTTGATGACTCCTAG (20 cycles) (Krieg et al., 1989); Muscle actin: U GCTGACAGAATGCAGAAG/D TTGCTTGGAGGAGTGTGT (20 cycles) (Stutz and Sphor, 1986); αt1-globin: U GCCTACA- ACCTGAGAGTGG/D CAGGCTGGTGAGCTGCCC (25 cycles) (Banville and Williams, 1985); Xbra: U GGATCGTTA- TCACCTCTG/D GTGTAGTCTGTAGCAGCA (25 cycles) (Smith et al., 1991); NCAM: U CACAGTTCCACCAAATGC/D GGAATCAAGCGGTACAGA (25 cycles) (Kinter and Melton, 1987); Xnot: U ATACATGGTTGGCACTGA/D CTCCTACAGT- TCCACATC (25 cycles) (von Dassow et al., 1993); Xlhbox8: U AAGAGAGGAAGAGGCAGTG/D ATAAGAACTAGGCCAGCA (25 cycles) (Wright et al., 1988); Xvent-1: U ACAGCTGAA- ATATTTACAAGAGGAA/D CTGAGAGCTCAGGCAGGAGT (25 cycles) (Gawantka et al., 1995); Xtwi: U AGAAACTG- GAGCTGGATC/D GGCTTCAAAGGCACGACT (25 cycles) (Hopwood et al., 1989). In situ hybridizations Whole-mount in situ hybridizations were performed according to Harland (1991), with modifications to include the use of BM purple Fig. 1. Site-directed mutagenesis used to construct mutations in the mature region of Vg1. (A) Schematic diagram of the mature region of Vg1, based on a figure in Schlunegger and Grutter (1992). The mature protein acts as a dimer connected at the asterisk to the same residue of another subunit. Mutants were designed by presuming that the structure of Vg1 is like of TGFβ2, and are numbered accordingly (Daopin et al., 1992; Schlunegger and Grutter, 1992). The mutant changes were: m23/24/25: Glu to Gly/Phe to Tyr/Lys to Gln; m42/43: Asn to Ser/Asn to Ser; m69: Glu to Gly; m69/71: Glu to Gly/Glu to Gly; m77: Cys to Gly; m78: Cys to Ser; m109: Cys to Gly; m111: Cys to Ser; and m109/111: Cys to Gly/Cys to Ser. (B) Animal cap assay. Fertilized Xenopus laevis eggs were injected with 2-4 ng of capped mrna and animal cap explants were cut from blastula (stage 8) and cultured along with sibling control embryos. (C) RT-PCR analysis of mesodermal markers. Animal caps were injected with 2 ng of various mutant BVg1 mrnas and analyzed for expression of muscle actin and Xbra, two mesodermal markers, at stage 17. The BVg1 loop mutant m69/71 and cysteine mutants m78, m109 and m109/111 lost the ability to induce muscle actin and Xbra. These results were confirmed by animal cap assays using 4 ng of the mutant mrnas (data not shown). EF1-α is used as an RT-PCR control for RNA recovery. Other controls include analysis of uninjected explants, sibling-stage whole embryos, and whole-embryo RNA analyzed without reverse transcriptase (RT).
3 Vg1 signaling in Xenopus 2679 substrate (Boehringer). Digoxigenin mrna probes were made from linear DNA templates using Megascript in vitro RNA synthesis kits (Ambion) and digoxigenin-utp. RESULTS Design of Vg1 mutants Nine mutant versions of Vg1 were designed to possibly act as either dominant negative proteins or competitive antagonists of Vg1 signaling. Site-directed mutagenesis was used to mutate residues in the mature region of Vg1, taking into account the known structures of TGFβ1 and 2 and amino acid conservation among TGFβ members (Daopin et al., 1992; Schlunegger and Grutter, 1992). Mutations were made to alter (1) cysteine residues thought to be involved in a disulfide knot structural motif (m78, m109, m111, m109/111), (2) proposed dimer interface residues (m42/43, m77) and (3) charged residues in loop regions possibly involved in receptor binding (m23/24/25, m69, m69/71) (Fig. 1A). Selection of Vg1 mutants that lack mesoderm inducing activity Before testing whether the mutated Vg1 ligands act in an inhibitory fashion, we first tested whether they lost the ability to induce mesoderm. Induction was assayed by injecting mrna coding for the mutant Vg1 ligands (Thomsen and Melton, 1993) into the animal poles of fertilized Xenopus eggs and testing for mesoderm in animal cap explants (Fig. 1B). Five of the mutant ligands retained the ability to induce mesoderm with varying degrees. These five mutants all induce mesoderm and are not therefore useful for the present study. This includes m23/24/25, which is comparable to the activin mutant ligand used to study medaka development (Wittbrodt and Rosa, 1994) (Fig. 1C). Four of the constructs completely lost the ability to induce the mesodermal markers muscle actin and Xbra (Stutz and Sphor, 1986; Smith et al., 1991), (m69/71, m78, m109 and m109/111), (Fig. 1C) and these were chosen for further study. Selection of Vg1 mutants that block signaling by exogenous Vg1 The ability of four mutant ligands (m69/71, m78, m109 and Fig. 2. Specificity of mutant ligands tested in a coinjection animal cap assay. (A) The ability of mutant Vg1 ligands to block Vg1 signaling was assayed by coinjecting BVg1 mrna with increasing concentrations of mutated BVg1 mrnas. Injection of 10 pg BVg1 mrna induces muscle actin and Xbra in animal caps (assayed by RT-PCR at stage 17). This concentration of BVg1 mrna was coinjected with a 10-fold, 50-fold and 100-fold excess of different mutated BVg1 mrnas. The m109/111 mrna blocked induction of muscle actin and Xbra at a 50-fold and 100-fold excess. Coinjection with a 50-fold excess of the m109/111 BVg1 mrna also blocked induction by AVg1 mrna (data not shown). AVg1 is a construct similiar to BVg1 but with the activin pro-region rather than the BMP2 pro-region (Kessler and Melton, 1995). (B) In a similiar experiment, coinjection with a 100-fold excess of the m78 mrna blocked induction of muscle actin and Xbra. The m69/71 and m109 mutant constructs did not block induction, even at a 100-fold excess (data not shown). (C) The mutants specificity was assayed using a coinjection animal cap assay, in which 2 pg of activin βb mrna (BB) were coinjected with increasing concentrations of mutant BVg1 mrna. At a 100-fold excess, the m109/111 mrna did not block induction by activin (assayed by RT- PCR at stage 17-25). Even at a 500-fold excess of mutant mrna, activin signaling was not blocked. A control for the integrity of the mutant mrna included induction by BVg1 and a block of that induction by a 100-fold excess of the mutant mrna. (D) In a similiar experiment to that shown in C, activin signaling was not blocked by a 100- fold excess of the m78 mrna. These data show that the m78 and m109/111 mutants do not block signaling by activin. (E) The mutants specificity was further analyzed using a coinjection animal cap assay, in which 500 pg Xenopus nodal-related mrna (encoding Xnr1, 2 or 4) was coinjected with 2 ng mutant BVg1 mrna. In this experiment, signaling by the Xenopus nodal-related factors (Xnr1,2,4) was not blocked by the m109/111 mutant mrna (as assayed by RT-PCR at stage 25). (F) In a similiar experiment to that of E, Xnr signaling was not blocked by the m78 mutant mrna.
4 2680 E. M. Joseph and D. A. Melton Fig. 3. Phenotype of whole embryos injected with mutant Vg1 mrna. Mutant Vg1 mrna, m109/111, was injected subequatorially at the one-cell stage, such that half of the sample was injected on one side of the embryo (2 ng/10 nl) and the other half on the opposite side. (A) Severely affected tadpole-stage embryos (at stage 35). (A1a) A severely affected embryo injected with m109/111 BVg1 mrna. (A1b) A severely affected embryo injected with m109/111 AVg1 mrna. The phenotypes of embryos injected with m78 BVg1 mrna were not as extreme as those shown here, consistent with the less efficient block of Vg1 signaling by m78 BVg1 mrna compared to m109/111 BVg1 mrna (see Figs 2A,B and 4). (A1c) A histological section of the embryo shown in A1b. Note the lack of any histological differentiation of mesoderm or endoderm. (A2) A sibling control of the embryo shown in A1a. (B) Analysis of markers by RT-PCR at stage 35. Severely affected mutant Vg1 embryos, such as those shown in A, do not form dorsal mesoderm (lane 1), and muscle actin and the notochord marker Xnot are not expressed; however, expression of the ventral mesodermal marker αt1-globin is slightly increased relative to EF1-α controls. The neural marker NCAM and the endodermal marker Xlhbox8 are not expressed. Results from sibling control tadpoles are shown in lane 2; lane 3 is a control embryo analyzed without reverse transcriptase. (C) Severely affected neurula-stage embryos (at stage 15). (C1) An embryo injected with the m109/111 BVg1 mrna construct. (C2) An embryo injected with tar mrna. (C3) A sibling-stage control embryo. (D) Analysis of markers by RT-PCR at stage 14/15. Embryos were analyzed at the early neurula stage so that expression of muscle actin could be assayed; both the m109/111 BVg1 and tar mrna embryos analyzed here were shown not to express muscle actin (data not shown). Injection of m109/111 BVg1 mrna blocks expression of Xtwi (Hopwood et al., 1989) but does not block expression of Xbra, Xwnt8 or Xvent-1 in whole embryos (lane 1), whereas injection of tar mrna completely blocks expression of all of these mesodermal markers except for Xvent-1, where expression is greatly reduced (lane 2). Lane 3 is an uninjected embryo control and lane 4 is a control embryo treated without reverse transcriptase. m109/111) to block Vg1 signaling was tested by a coinjection animal cap assay. 10 pg of BVg1 mrna (Thomsen and Melton, 1993) were coinjected with increasing concentrations of each of the four mutated mrnas. At a 100-fold excess of mutated mrna, two (m78 and m109/111) block the induction of mesoderm by BVg1 (Fig. 2A,B). Both the m78 and m109/111 mutant ligands act at levels feasible for blocking endogenous Vg1 within a whole embryo. Vg1 is present at an estimated 40 pg mrna/xenopus egg (Rebagliati et al., 1985) therefore 4 ng of either mutant mrna should be needed to block Vg1 signaling in vivo, i.e. in developing embryos. Specificity of Vg1 mutants A truncated activin type II receptor, tar, has little or no specificity for the different TGFβ ligands and can block signaling by both maternally present dorsal mesoderm inducers, activin and Vg1, as well as by BMPs (Hemmati- Brivanlou and Melton, 1992; Kessler and Melton, 1995; Schulte-Merker et al., 1994; Hemmati-Brivanlou and Thomsen, 1995). This broad inhibition limits conclusions that can be drawn using tar to block signaling. The specificity of the mutated Vg1 ligands was tested using a coinjection animal cap assay with activin βb mrna. In this coinjection animal cap assay, neither mutant Vg1 construct m78 nor m109/111, inhibits mesoderm induction by activin (Fig. 2C,D). Likewise, neither mutated Vg1 ligand was shown to inhibit mesoderm induction by any of three Xenopus nodal-related factors (Xnr1, 2 and 4) (Fig. 2E,F). This indicates that the mutants act specifically to block Vg1, rather than nonspecifically inhibiting signaling by all TGFβ ligands. In support of this conclusion, the phenotype of injected embryos and explants is not consistent with the possibility that the mutant Vg1 ligands block BMP signaling (see Results and Discussion below). Whole embryo phenotype To determine the role of Vg1 signaling in whole embryos, mrnas coding for the mutant Vg1 ligands were injected at the one-cell stage, subequatorially at two opposite points, and resulting embryos were characterized by gross phenotype, histology and molecular markers (Fig. 3). Embryos injected with the mutated Vg1 mrnas are phenotypically abnormal. In the extreme cases (see Fig. 3 legend), affected embryos develop without forming dorsal mesoderm or axial structures (Fig. 3A). These embryos are ventralized, as defined by a slight increase in expression of the ventral mesodermal marker αt1-globin (Banville and Williams, 1985) and a lack of expression of the dorsal mesodermal marker muscle actin. Analysis of the notochord marker Xnot (von Dassow et al., 1993) confirms that notochord does not form in these embryos (Fig. 3B). At an earlier stage of development, the extreme severely affected embryos (Fig. 3C) lack expression of muscle actin and Xtwi (Hopwood et al., 1989); however expression of the general mesodermal marker Xbra is not blocked, nor is expression of the ventrolateral mesodermal markers Xwnt8
5 Vg1 signaling in Xenopus 2681 Fig. 4. Examples of the less severe phenotype of tadpole-stage embryos injected with mutant Vg1 mrna. (A1) An embryo injected with the m109/111 AVg1 construct; (A2) an embryo injected with the m109/111 BVg1 construct; (A3) an embryo injected with the m78 BVg1 construct; (A4) a sibling stage control embryo. (B) RT- PCR analysis of the embryos shown in A. Lane 1 is the embryo shown in A1, lane 2 is the embryo shown in A2, lane 3 is the embryo shown in A3, lane 4 is the sibling-stage control embryo shown in A4, and lane 5 is a no-reverse transcriptase control. These less severely affected embryos lack expression of NCAM, Xlhbox8 and Xnot; they differ from the extreme affected embryos shown in Fig. 3 in that they express muscle actin. This level of msucle actin expression is reduced compared to the level of expression in the sibling-stage control embryo and the level of αt1-globin expression is slightly increased relative to this control. (Christian et al., 1991) or Xvent-1 (Gawantka et al., 1995) (Fig. 3D). The expression of Xbra, Xwnt8 and Xvent-1 provides further evidence for the specificity of the mutant Vg1 ligand because expression of these genes is blocked by injection of tar mrna (Hemmati-Brivanlou and Melton, 1992). Injection of the m109/111 mutant mrna led to an extremely severely ventralized phenotype in 10% of the injected embryos (with the BVg1 construct: n=8/76, with the AVg1 construct: n=10/98). Similiar injections with tar mrna led to an extreme phenotype in 15% of the injected embryos (n=10/67), whereas injection of a control mrna, β-globin, resulted in 0% (n=0/93). Within each population of mutant Vg1-injected embryos there was a range of phenotypes. These phenotypes include embryos with a partially disrupted dorsal axis (45% of the embryos injected with the m109/111 BVg1 construct (n=34/76) and 48% of the embryos injected with the AVg1 construct (n=47/98)) and embryos with a normal dorsal axis (n=75/174). Examples of less severely affected embryos are shown in Fig. 4; these embryos have a loss of Xnot expression accompanied by a reduction of muscle actin expression. The mutant Vg1 mrnas were injected to have early expression. At such an early stage of injection, the presumptive dorsal versus ventral sides of the embryo are nearly indistinguishable; therefore, the low number of severely affected embryos may be a reflection of the varied mrna distribution within the population of injected embryos with respect to the prospective dorsal-ventral axis. To further investigate the character of mesoderm present in mutated Vg1-injected embryos, whole-mount in situ hybridizations were performed on batches of injected embryos at the gastrula stage (Fig. 5). This in situ hybridization analysis reveals that Xnr3 (Smith et al., 1995) and chordin (Sasai et al., 1994) are expressed at high levels in the marginal zone of injected embryos, both in a slightly expanded domain compared to sibling contols. Other markers tested, including Xbra (Figs 3D, 5), follistatin (Fig. 5), the ventrolateral mesodermal marker Xwnt8 (Fig. 3D), and the ventral mesodermal markers Xvent1 (Fig. 3D) and Vox-15 (Schmidt et al., 1996) (Fig. 5) do not show an increased level of expression. Given that later, post-gastrula Fig. 5. In situ hybridization analysis of mutated Vg1-injected embryos. Embryos were injected at the one-cell stage with m109/111 BVg1 mrna (as described in the Fig. 3 legend) and whole-mount in situ hybridizations were performed at the early gastrula stage with antisense digoxigenin probes. A representative of each batch of injected embryos is shown, together with a representative of each batch of uninjected control embryos. Injection of mutated Vg1 mrna did not disrupt expression of Xbra, as it did for expression of follistatin (XFS) at this stage, although there was often a lighter area within the domain of Xbra expression, as shown here. Xnr3 and chordin are both highly expressed in the marginal zone regions of injected embryos; Vox-15 expression is not blocked or expanded in comparison to the uninjected controls (see Discussion).
6 2682 E. M. Joseph and D. A. Melton development shows a loss of dorsal mesoderm and a slight increase in ventral mesoderm, as judged by globin expression (Fig. 3), one might have expected that at the gastrula stage the ventral and ventrolateral markers (Xvent1, Vox-15 and Xwnt8) would be increased, but this is not the case. Thus, these data show that the phenotypes resulting from injection of mutated Vg1 mrna are not equivalent to those of a UV-induced ventralization (Darras et al., 1997; Medina et al., 1997; Cooke and Smith, 1987). Effect on vegetal pole explants Gross morphology and histological analysis of affected embryos, injected with the mutated Vg1 mrnas, reveal that organs derived from the endoderm, such as the pancreas and liver, do not develop normally (see Fig. 3). It is difficult to assess the role of Vg1 signaling for endodermal development per se since the mutant Vg1 ligands disrupt dorsal mesoderm formation and earlier work has shown that the development of some endodermal organs requires interactions with embryonic mesoderm (Okada, 1953; Takata, 1960). At the molecular level, expression of the early endodermal marker Xlhbox8 is blocked in embryos injected with the mutated Vg1 mrnas (m78 and m109/111) (Fig. 3B). To determine whether this effect on expression of Xlhbox8 is a direct result of a block to Vg1 signaling, or a secondary effect resulting from a change in the character of mesoderm present, the mutant Vg1 mrnas were injected into the vegetal poles of embryos and mesodermfree vegetal pole explants were cut and cultured (Henry et al., 1996; Gamer and Wright, 1995) (Fig. 6). Autonomous Xlhbox8 expression is blocked by injection of the mutant Vg1 mrnas in these vegetal pole explants, indicating a direct effect of Vg1 signaling on the regionalized expression of this endodermal (pancreatic) marker (Fig. 6). Embryos with a significant loss of dorsal mesoderm, due to the injection of mutated Vg1 mrnas, do not express the neural marker NCAM (Kinter and Melton, 1987) (Figs 3B, 4B). This result contrasts with that obtained by injecting embryos with tar mrna, in which case excess neural structures and NCAM expression accompanies the loss of dorsal and ventral types of mesoderm (Hemmati-Brivanlou and Melton, 1992, 1994; Hemmati-Brivanlou et al., 1994). A further demonstration of the specificity of the mutant Vg1 ligands comes from analyzing NCAM expression in vegetal pole explants. Injection of tar or follistatin mrna induces NCAM in vegetal pole explants (Henry et al., 1996), a neuralization that is thought to occur as a result of inhibiting BMP signals (Hemmati-Brivanlou and Melton, 1994; Hemmati-Brivanlou et al., 1994; Wilson and Hemmati-Brivanlou, 1995). The mutant Vg1 block of Xlhbox8 expression in vegetal pole explants, as in whole embryos, occurs without NCAM expression (Fig. 6). DISCUSSION Mutant Vg1 ligand approach To investigate the role of Vg1, we set out to specifically block Vg1 signaling in vivo. Two options for blocking signaling by secreted proteins in Xenopus are (1) preventing the receptor from responding to the ligand and (2) preventing the ligand from binding to or activating the receptor. Results from previous work involving overexpression of truncated receptors have proved difficult to interpret because some mutant receptors block signaling in a non-specific manner. For example, a truncated activin receptor (tar) blocks signaling by several TGFβs including Vg1, activin, and BMPs (Hemmati-Brivanlou and Melton, 1992; Kessler and Melton, 1995; Schulte-Merker et al., 1994; Hemmati-Brivanlou and Thomsen, 1995). Recently the truncated receptor approach has been refined to allow for a more specific block of activin signaling (Dyson and Gurdon, 1997). However, since the Vg1 receptor has not yet been identified the truncated receptor strategy is not presently feasible as a means to block Vg1 signaling. We have attempted to prevent Vg1 ligand from signaling. Removing the ligand activity of Vg1 is complicated because Vg1 is present both as a maternal mrna and protein. Thus, even if the maternal Vg1 mrna is removed (e.g. by an antisense oligodeoxynucleotide strategy; Heasman et al., 1984) a high level of maternal protein remains available to signal. This stored maternal protein also presents a problem when trying to block signaling using a co-translationally acting mutant ligand approach (Hawley et al., 1995; Wittbrodt and Rosa, 1994). Indeed, antisense strategies have failed to produce a discernable loss-of-function Vg1 phenotype (Rebagliati and Melton, 1987; Woolf, 1991). Therefore, we designed novel Fig. 6. Vegetal pole explant assay for the effects of mutant Vg1 ligand in the absence of mesoderm. The mutant Vg1 effect on the expression of Xlhbox8 was studied by RT-PCR analysis of injected vegetal pole explants (Henry et al., 1996; Gamer and Wright, 1995). 4 ng of mutant Vg1 mrnas were injected into the vegetal poles of embryos at the one-cell stage. At stage 9, vegetal pole explants were cut and cultured until stage These vegetal pole explants lack expression of the mesodermal marker muscle actin. Autonomous expression of Xlhbox8 was shown in uninjected vegetal pole explants (Gamer and Wright, 1995; Henry et al., 1996). Injection of mutant Vg1 mrna inhibited this autonomous expression of Xlhbox8, without induction of NCAM.
7 Vg1 signaling in Xenopus 2683 mature mutant Vg1 ligands which could block Vg1 signaling by preventing the wild-type ligand from binding to receptor sites. The mutant ligands could act directly by dimerizing with wild-type ligand or complexing with receptor subunits. These alternative mechanisms of action are difficult to test since the amount of processed Vg1 is below detectable levels in the embryo and a Vg1 receptor has not yet been cloned. We show that some mutant Vg1 ligands are inactive (i.e. do not induce mesoderm) and can prevent induction by mature Vg1 ligand. The specificity of these reagents was demonstrated by showing that the mutant Vg1 ligands do not affect the inductive activity of activin or Xenopus nodal-related factors. The phenotype of embryos injected with mutant Vg1 ligands in our experiments further suggest that these ligands do not interfere with BMP signaling. Our mutant ligand experiments may also aid our understanding of which amino acid residues are necessary for activity of TGFβ members, by considering which mutant Vg1 ligands lose the ability to induce mesoderm (see Fig. 1). For instance, the m69/71 Vg1 mutant cannot induce mesoderm; this supports the idea that residues on this solvent-exposed loop are involved in the recognition of a ligand by its receptor, as was suggested by the differences in the structure of this loop region of TGFβ1 in nuclear magnetic resonance solution structure compared to the X-ray crystal structure of TGFβ2 (Archer et al., 1983). The m77 Vg1 mutant, which eliminates the cysteine involved in forming a covalent linkage between TGFβ dimer subunits, has only a low level of mesoderm inducing activity. This finding suggests that uncleaved Vg1 protein must be both cleaved and dimerized to have full activity. Finally, the m111 Vg1 mutant (which lacks a conserved cysteine numbered as postion 111 in Fig. 1) retained a high degree of mesoderm inducing activity; Xnr3, a TGFβ superfamily member with signaling activity, lacks this conserved cysteine (Smith et al., 1995). The role of Vg1 signaling BVg1 mrna can induce dorsal mesoderm in an animal cap assay and rescue a complete dorsal axis in a UV-ventralized embryo (Thomsen and Melton, 1993). Blastomeres expressing mature Vg1 are found (by lineage tracing) in the endoderm of ventralized embryos rescued by BVg1 mrna injection, regardless of the site of mrna injection (Kessler and Melton, 1998). The results presented here using mutant Vg1 ligands indicate an in vivo requirement for Vg1 signaling in the normal development of Xenopus embryos. A block of Vg1 signaling prevents formation of dorsal mesoderm and adversely affects endoderm development. Taken together, these results provide evidence that Vg1 is an endogenous Nieuwkoop center activity (Gerhart and Keller, 1986), i.e. the signaling activity of dorsal vegetal cells with an endodermal fate which induce overlying ectodermal cells to form dorsal mesoderm. In addition, there is evidence to support the idea that Vg1 signaling may have a late role in specifying left-right asymmetry (Hyatt et al., 1996; Danos and Yost, 1995). The phenotype of Xenopus embryos injected with the nonspecific truncated activin receptor, tar, is a loss of mesoderm (Hemmati-Brivanlou and Melton, 1992; Schulte-Merker et al., 1994). This result is merely consistent with a role for Vg1, activin and/or BMPs in mesoderm induction. Follistatin, an antagonist of activin (Hemmati-Brivanlou et al., 1994; Nakamura et al., 1990) and BMP (Sasai et al., 1995), but not Vg1 (Kessler and Melton, 1995), has also been injected into embryos. Unlike our mutant Vg1 mrna-injected embryos, these follistatin mrna-injected embryos do not lose dorsal structures; in fact, the embryos are hyperdorsalized, probably due to a block of BMP signals (Kessler and Melton, 1995; Fainsod et al., 1997). Most recently, embryos have been injected with a truncated type II activin receptor (ActRIIBexd) that specifically blocks activin signaling. The injected embryos form mesoderm, apparently in a delayed manner, and lack anterior head structures (Dyson and Gurdon, 1997). Thus, in the most severely affected embryos, blocking signaling by activin does not give the same phenotype as that observed when Vg1 signaling is blocked. The ventralized phenotype of the most severely affected mutant Vg1 mrna embryos lack dorsal mesoderm formation and have defective endoderm formation. Our data are consistent with the proposal that the large amount of Vg1 precursor protein in the vegetal pole of a Xenopus embryo is selectively processed after fertilization in response to a cortical rotation event to give a small amount of the potent mature Vg1 on the presumptive dorsal side (Thomsen and Melton, 1993). This small amount of active protein could then function to initiate dorsal mesoderm formation and axis formation. Like formation of dorsal mesoderm, expression of the endodermal marker Xlhbox8 is dependent on the cortical rotation event (Henry et al., 1996; Gamer and Wright, 1995) and is blocked by the mutant Vg1 ligands. The results presented here extend the conclusions of previously published data and point to a dual role for mature Vg1 signaling in the formation and patterning of dorsal mesoderm and endoderm. Vg1 signaling and the β-catenin pathway The data presented here must be reconciled with the established importance of the β-catenin dorsal patterning pathway. The importance of β-catenin signaling was shown by antisense studies in which β-catenin-depleted embryos had a loss of dorsal structures (Heasman et al., 1984). Interestingly, β-catenin was needed as a signal in both animal and vegetal pole explants for full dorsal induction to occur in recombinant experiments (Heasman et al., 1984). A block of this β-catenin pathway can be rescued by injection of the mesoderm-inducing BVg1 mrna as well as by injection of mrna coding for mesoderm dorsalizing factors downstream of β-catenin (Wylie et al., 1996). The Vg1 signaling pathway could synergize with this β- catenin pathway by activating a downstream component (Cui et al., 1996); in this case, it is possible that Vg1 signaling is initiated prior to or at the same time as the β-catenin pathway. Here we show that affected embyros injected with mutant Vg1 mrnas lose dorsal structures; it is presumed that overexpression of members of the β-catenin signaling pathway could rescue this phenotype by dorsalizing the existing ventral mesoderm. To summarize, a block of either the mature Vg1 signaling pathway (this study) or the β-catenin signaling pathway (Heasman et al., 1984; Wylie et al., 1996) prevents formation of dorsal axial structures in Xenopus; it is noteworthy that a block of either signaling pathway could be compensated for by the overexpression, but not the mere expression, of members of the other pathway. Recently, it has been shown that the spatial expression patterns of genes in the β-catenin signaling pathway, such as Xnr3 and
8 2684 E. M. Joseph and D. A. Melton siamois, are disrupted in UV-ventralized embryos such that the genes are expressed at reduced levels in the vegetal hemisphere rather than at normal levels in the marginal zone (Larabell et al., 1997; Brannon and Kimelman, 1996; Darras et al., 1997; Medina et al., 1997). Chordin, a target of siamois (Carnac et al., 1996), is not expressed in UV-ventralized embryos (Darras et al., 1997). Since our injections of mutated Vg1 mrnas do not prevent cortical rotation from occurring, it is not surprising that members of the β-catenin signaling pathway (i.e. Xnr3 and chordin) are expressed in the marginal zones of the mutant Vg1- injected embryos. Our results are consistant with a possible role for cortical rotation in both the activation of mature Vg1 signaling and the proper localization of members of the β- catenin signaling pathway. An alternative possibility is that the mutant Vg1 injections do not completely prevent mature Vg1 signaling, and that mature Vg1 signaling acts endogenously as a general mesoderm-inducing signal in combination with the β- catenin signaling pathway to form dorsal structures (Watabe et al., 1995); this possible interpretation of the data would leave unanswered the question of how injection of BVg1 mrna can completely rescue a UV- (Thomsen and Melton, 1993) or β- catenin antisense- (Wylie et al., 1996) ventralization and the fact that UV-ventralized embryos lack expression of Xlhbox8 rather than have reduced expression throughout the vegetal hemisphere (Henry et al., 1996; Gamer and Wright, 1995). This work was supported by an NIH training grant to E.M.J. D.A.M. is a Howard Hughes Medical Institute investigator. REFERENCES Amaya, E., Musci, T. J. and Kirschner, M. W. (1994). Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66, Archer, S. J., Bax, A., Roberts, A. B., Sporn, M. B., Ogawa, Y., Piez, K. A. and Weatherbee, J. A. (1993). Transforming growth factor β1: secondary structure as determined by heteronuclear magnetic resonance spectroscopy. Biochemistry 32, Banville, D. and Williams, J. G. (1985). The pattern of expression of Xenopus laevis tadpole alpha-globin genes and the amino acid sequence of the three major alpha-globin polypeptides. Nucl. Acids Res. 13, Brannon, M. and Kimelman, D. (1996). Activation of siamois by the Wnt pathway. Dev. Biol. 180, Carnac, G., Kodjabachian, L., Gurdon, J. B. and Lemaire, P. (1996). The homeobox gene siamois is a target of the Wnt dorsalization pathway and triggers organizer activity in the absence of mesoderm. Development 122, Christian, J. L., McMahon, J. A., McMahon, A. P. and Moon, R. T. (1991). Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm inducing factors may play a role in ventral mesodermal patterning during embryogenesis. Development 111, Cooke, J. and Smith, J. C. (1987). The midblastula cell cycle transition and the character of mesoderm in u.v.-induced nonaxial Xenopus development. Development 99, Cui, Y., Tian, Q. and Christian, J. L. (1996). Synergistic effects of Vg1 and Wnt ligands in the specification of dorsal mesoderm and endoderm. Dev. Biol. 180, Dale, L., Matthews, G., Tabe, L. and Colman, A. (1989). Developmental expression of the protein product of Vg1, a localized maternal mrna in the frog Xenopus laevis. EMBO J. 8, Danos, M. C. and Yost, H. J. (1995). Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development 121, Daopin, S., Piez, K. A., Ogawa, Y. and Davies, D. R. (1992). Crystal structure of transforming growth factor-β2: an unusual fold for the superfamily. Science 257, Darras, S., Marikawa, Y., Elinson, R. P. and Lemaire, P. (1997). Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinants: implications for the patterning of the organiser. Development 124, Dohrmann, C. E., Kessler, D. S. and Melton, D. A. (1996). Induction of axial mesoderm by zdvr-1, the zebrafish orthologue of Xenopus Vg1. Dev. Biol. 175, Dyson, S. and Gurdon, J. B. (1997). Activin signaling has a necessary function in Xenopus early development. Curr. Biol. 7, Fainsod A., Deissler, K., Yelin, R., Marom, K., Epstein, M., Pillemer, G., Steinbeisser, H. and Blum, M. (1997). The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech. Dev. 63, Gamer, L. W. and Wright, C. V. E. (1995). Autonomous endodermal determination in Xenopus: regulation of expression of the pancreatic gene Xlhbox8. Dev. Biol. 171, Gawantka, V., Delius, H., Hirschfeld, K., Blumenstock, C. and Niehrs, C. (1995). Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J. 14, Gerhardt, J. and Keller, R. (1986). Region-specific activities in amphibian gastrulation. Annu. Rev. Cell Biol. 2, Harland, R. M. (1991). In situ hybridization: an improved whole mount method for Xenopus embryos. Methods Cell Biol. 36, Hawley, S. H. B., Wunnenberg-Stapleton, K., Hashimoto, C., Laurent, M. N., Watabe, T., Blumberg, B. W. and Cho, K. W. Y. (1995). Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 9, Heasman, J., Crawford, A., Goldstone, K., Garner-Hamrick, P., Gumbiner, B., Kinter, C., Noro, C. Y. and Wylie, C. (1994). Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79, Hemmati-Brivanlou, A., Kelly, O. G. and Melton, D. A. (1994). Follistatin, an antagonist of activin, is present in the Spemann organizer and displays direct neuralizing activity. Cell 77, Hemmati-Brivanlou, A. and Melton, D. A. (1992). A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature 359, Hemmati-Brivanlou, A. and Melton, D. A. (1994). Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell 77, Hemmati-Brivanlou, A. and Thomsen, G. H. (1995). Ventral mesodermal patterning in Xenopus embryos; expression patterns and activities of BMP2 and BMP4. Dev. Genet. 17, Henry, G. L., Brivanlou, I. H., Kessler, D. S., Hemmati-Brivanlou, A. and Melton, D. A. (1996). TGFβ signals and a pre-pattern in Xenopus laevis endodermal development. Development 122, Hopwood, N. D., Pluck, A. and Gurdon, J. B. (1989). A Xenopus mrna related to Drosophilia twist is expressed in response to induction in the mesoderm and the neural crest. Cell 59, Hyatt, B. A., Lohr, J. L. and Yost, H. J. (1996). Initiation of vertebrate leftright axis formation by maternal Vg1. Nature 384, Jones, C. M., Kuehn, M. R., Hogan, B. L. M., Smith, J. C. and Wright, C. V. E. (1995). Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development 121, Kessler, D. S. and Melton, D. A. (1994). Vertebrate embryonic induction; mesodermal and neural patterning. Science 266, Kessler, D. S. and Melton, D. A. (1995). Induction of dorsal mesoderm by soluble, mature Vg1 protein. Development 121, Kessler, D. S. and Melton, D. A. (1998). Development of endodermal lineages: specification by mature Vg1. Dev. Biol. (in press). Kinter, C. R. and Melton, D. A. (1987). Expression of Xenopus NCAM RNA in ectoderm as an early response to neural induction. Development 99, Krieg, P. A., Varnum, S., Wormington, M. and Melton, D. A. (1989). The mrna encoding elongation factor 1-α (EF1-α) is a major transcript at the mid-blastula transition in Xenopus laevis embryos. Dev. Biol. 133, Larabell, C. A., Torres, M., Rowning, B. A., Yost, C., Miller, J. R., Wu, M., Kimelman, D. and Moon, R. T. (1997). Establishment of the dorsoventral axis in Xenopus embryos is presaged by early asymmetries in β- catenin that are modulated by the Wnt signaling pathway. J. Cell Biol. 136, Medina, A., Wendler, S. R. and Steinbeisser, H. (1997). Cortical rotation is required for the correct spatial expression of nr3, sia and gsc in Xenopus embryos. Int. J. Dev. Biol. 41, Nakamura, T., Takio, K., Eto, Y., Shibai, H., Titani, K. and Sugino, H. (1990). Activin-binding protein from rat ovary is follistatin. Science 247,
9 Vg1 signaling in Xenopus 2685 Nieuwkoop, P. D. and Faber, J. (1967). Normal table of Xenopus laevis. Amsterdam: North Holland. Okada, T. S. (1953). On the role of mesoderm in the differentiation of the presumptive endoderm. Mem. Coll. Sci., Univ. Kyoto 20, Rebagliati, M. R. and Melton, D. A. (1987). Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell 48, Rebagliati, M. R., Weeks, D. L., Harvey, R. P. and Melton, D. A. (1985). Identification and cloning of localized maternal mrnas from Xenopus eggs. Cell 42, Sasai, Y., Lu, B., Steinbeisser, H. and De Robertis, E. M. (1995). Regulation of neural induction by the Chd and BMP-4 antagonistic patterning signals in Xenopus. Nature 376, Sasai, Y., Lu, B., Steinbeisser, H., Geissert, D., Gont, L. K. and De Robertis, E. M. (1994). Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, Schlunegger, M. P. and Grutter, M. G. (1992). An unusual feature revealed by the crystal stucture at 2.2 A resolution of human transforming growth factor-β2. Nature 358, Schulte-Merker, S., Smith, J. C and Dale, L. (1994). Effects of truncated activin and FGF receptors and of follistatin on the inducing activities of BVg1 and activin: does activin play a role in mesoderm induction? EMBO J. 13, Seleiro, E. A. P., Connolly, D. J. and Cooke, J. (1996). Early developmental expression and experimental axis determination by the chicken Vg1 gene. Curr. Biol. 6, Smith, W.C., McKendry, R., Ribisi Jr, S. and Harland, R. M. (1995). A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell 82, Smith, J. C., Price, B. M. J., Green, J. B. A., Weigel, D. and Herrmann, B. G. (1991). Expression of a Xenopus homolog of Brachyury (T) in an immediate-early response to mesoderm induction. Cell 67, Smith, W. C., McKendry, R., Ribisi Jr, S. and Harland, R. M. (1995). A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell 82, Stutz, F. and Sphor, G. (1986). Isolation and characterization of sacromeric actin genes expressed in Xenopus laevis embryos. J. Mol. Biol. 187, Takata, C. (1960). The differentiation in vitro of the isolated endoderm under the influence of the mesoderm in Triturus pyrrhogaster. Embryologia 5, Tannahill, D. and Melton, D. A. (1989). Localized synthesis of the Vg1 protein during early Xenopus development. Development 106, Thomsen, G. H. and Melton, D. A. (1993). Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell 74, von Dassow, G., Schmidt, J. E. and Kimelman, D. (1993). Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeobox gene. Genes Dev. 7, Watabe, T., Kim, S., Candia, A., Rothbacher, U., Hashimoto, C., Inoue, K. and Cho, K. W. Y. (1995). Molecular mechanisms of Spemann s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 9, Weeks, D. L. and Melton, D. A. (1987). A maternal mrna localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGFβ. Cell 51, Wilson, P. A. and Hemmati-Brivanlou, A. (1995). Induction of epidermis and inhibition of neural fate by BMP4. Nature 376, Wilson, P. A. and Melton, D. A. (1994). Mesodermal patterning by an inducer gradient on secondary cell-cell communication. Curr. Biol. 4, Wittbrodt, J. and Rosa, F. M. (1994). Disruption of mesoderm and axis formation in fish by ectopic expression of activin variants; the role of maternal activin. Genes Dev. 8, Woolf, T. M. (1991). PhD Thesis. Harvard University, Cambridge, MA: USA. Wright, C. V. E., Schnegelsberg, P. and De Robertis, E. M. (1988). Xlhbox8: a novel Xenopus homeoprotein restricted to a narrow band of endoderm. Development 105, Wylie, C., Kofron, M., Payne, C., Anderson, R., Hosobuchi, M., Joseph, E. and Heasman, J. (1996). Maternal β-catenin establishes a dorsal signal in early Xenopus embryos. Development 122, Yamashita, H., ten Dijke, P., Huylebroeck, D., Sampath, T. K. andries, M., Smith, J. C., Heldin, C.-H. and Miyazono, K. (1995). Osteogenic protein- 1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 130,
Mesoderm Formation. Fate map of early gastrula. Only two types of mesoderm are induced. Mesoderm induction by the vegetal hemisphere
 Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
RNAs were transcribed from described expression conhibit a similar capacity to synergize with neural-inducing
 DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2
 Developmental Biology 248, 40 51 (2002) doi:10.1006/dbio.2002.0705 The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2 Vered Levy, Karen Marom, Sharon Zins, Natalia
Developmental Biology 248, 40 51 (2002) doi:10.1006/dbio.2002.0705 The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2 Vered Levy, Karen Marom, Sharon Zins, Natalia
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Xenopus GDF6, a new antagonist of noggin and a partner of BMPs
 Development 126, 3347-3357 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV3918 3347 Xenopus GDF6, a new antagonist of noggin and a partner of BMPs Chenbei Chang and Ali Hemmati-Brivanlou*
Development 126, 3347-3357 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV3918 3347 Xenopus GDF6, a new antagonist of noggin and a partner of BMPs Chenbei Chang and Ali Hemmati-Brivanlou*
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm
 Development 122, 3055-3065 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV1088 3055 The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser
Development 122, 3055-3065 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV1088 3055 The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/βcatenin signaling pathway, but not by Vg1, Activin or Noggin
 Development 124, 453-46 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV9489 453 Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/βcatenin signaling pathway,
Development 124, 453-46 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV9489 453 Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/βcatenin signaling pathway,
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural crest induction in Xenopus: evidence for a two-signal model
 Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5
 Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Activin-mediated mesoderm induction requires FGF
 Development 120, 453-462 (1994) Printed in Great Britain The Company of Biologists Limited 1994 453 Activin-mediated mesoderm induction requires FGF Robert A. Cornell and David Kimelman* Department of
Development 120, 453-462 (1994) Printed in Great Britain The Company of Biologists Limited 1994 453 Activin-mediated mesoderm induction requires FGF Robert A. Cornell and David Kimelman* Department of
RAPID COMMUNICATION A Novel TGF-b-like Gene, fugacin, Specifically Expressed in the Spemann Organizer of Xenopus
 DEVELOPMENTAL BIOLOGY 172, 699 703 (1995) RAPID COMMUNICATION A Novel TGF-b-like Gene, fugacin, Specifically Expressed in the Spemann Organizer of Xenopus V. Ecochard,* C. Cayrol,*,1 F. Foulquier,* A.
DEVELOPMENTAL BIOLOGY 172, 699 703 (1995) RAPID COMMUNICATION A Novel TGF-b-like Gene, fugacin, Specifically Expressed in the Spemann Organizer of Xenopus V. Ecochard,* C. Cayrol,*,1 F. Foulquier,* A.
The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways
 Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Activin-Induced Factors Maintain goosecoid Transcription through a Paired Homeodomain Binding Site
 DEVELOPMENTAL BIOLOGY 204, 172 186 (1998) ARTICLE NO. DB989065 Activin-Induced Factors Maintain goosecoid Transcription through a Paired Homeodomain Binding Site Roslyn McKendry, Richard M. Harland, 1
DEVELOPMENTAL BIOLOGY 204, 172 186 (1998) ARTICLE NO. DB989065 Activin-Induced Factors Maintain goosecoid Transcription through a Paired Homeodomain Binding Site Roslyn McKendry, Richard M. Harland, 1
Expression of Siamois and Twin in the blastula Chordin/Noggin signaling center is required for brain formation in Xenopus laevis embryos
 MECHANISMS OF DEVELOPMENT 125 (2008) 58 66 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/modo Expression of Siamois and Twin in the blastula Chordin/Noggin signaling center
MECHANISMS OF DEVELOPMENT 125 (2008) 58 66 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/modo Expression of Siamois and Twin in the blastula Chordin/Noggin signaling center
Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mrnas
 Development 118, 499-507 (1993) Printed in Great Britain The Company of Biologists Limited 1993 499 Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mrnas Herbert Steinbeisser
Development 118, 499-507 (1993) Printed in Great Britain The Company of Biologists Limited 1993 499 Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mrnas Herbert Steinbeisser
Cytochalasin B inhibits morphogenetic movement and muscle differentiation of activin-treated ectoderm in Xenopus
 Develop. Growth Differ. (1999) 41, 41 49 Cytochalasin B inhibits morphogenetic movement and muscle differentiation of activin-treated ectoderm in Xenopus Keiko Tamai, 1 Chika Yokota, 2 Takashi Ariizumi
Develop. Growth Differ. (1999) 41, 41 49 Cytochalasin B inhibits morphogenetic movement and muscle differentiation of activin-treated ectoderm in Xenopus Keiko Tamai, 1 Chika Yokota, 2 Takashi Ariizumi
Patterning of the neural ectoderm of Xenopus laevis by the amino-terminal product of hedgehog autoproteolytic cleavage
 Development 121, 2349-2360 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2349 Patterning of the neural ectoderm of Xenopus laevis by the amino-terminal product of hedgehog autoproteolytic
Development 121, 2349-2360 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2349 Patterning of the neural ectoderm of Xenopus laevis by the amino-terminal product of hedgehog autoproteolytic
Mesendoderm Induction and Reversal of Left Right Pattern by Mouse Gdf1, a Vg1-Related Gene
 Developmental Biology 227, 495 509 (2000) doi:10.1006/dbio.2000.9926, available online at http://www.idealibrary.com on Mesendoderm Induction and Reversal of Left Right Pattern by Mouse Gdf1, a Vg1-Related
Developmental Biology 227, 495 509 (2000) doi:10.1006/dbio.2000.9926, available online at http://www.idealibrary.com on Mesendoderm Induction and Reversal of Left Right Pattern by Mouse Gdf1, a Vg1-Related
Neural Induction. Steven McLoon Department of Neuroscience University of Minnesota
 Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Cardiac looping and the vertebrate left-right axis: antagonism of left-sided Vg1 activity by a right-sided ALK2-dependent BMP pathway
 Development 126, 5195-5205 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV6415 5195 Cardiac looping and the vertebrate left-right axis: antagonism of left-sided Vg1 activity
Development 126, 5195-5205 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV6415 5195 Cardiac looping and the vertebrate left-right axis: antagonism of left-sided Vg1 activity
Single cells can sense their position in a morphogen gradient
 Development 6, 509-57 (999) Printed in Great Britain The Company of Biologists Limited 999 DEV6 509 Single cells can sense their position in a morphogen gradient J. B. Gurdon*, H. Standley, S. Dyson, K.
Development 6, 509-57 (999) Printed in Great Britain The Company of Biologists Limited 999 DEV6 509 Single cells can sense their position in a morphogen gradient J. B. Gurdon*, H. Standley, S. Dyson, K.
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
efgf, Xcad3 and Hox genes form a molecular pathway that establishes the
 Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation
 Development 127, 2227-2238 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV1490 2227 Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled,
Development 127, 2227-2238 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV1490 2227 Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled,
Expression Cloning of Siamois, a Xenopus Homeobox Gene Expressed in Dorsal-Vegetal Cells of Blastulae and Able to Induce a Complete Secondary Axis
 Cell, Vol. 81, 85-94, April 7, 1995, Copyright 1995 by Cell Press Expression Cloning of Siamois, a Xenopus Homeobox Gene Expressed in Dorsal-Vegetal Cells of Blastulae and Able to Induce a Complete Secondary
Cell, Vol. 81, 85-94, April 7, 1995, Copyright 1995 by Cell Press Expression Cloning of Siamois, a Xenopus Homeobox Gene Expressed in Dorsal-Vegetal Cells of Blastulae and Able to Induce a Complete Secondary
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Later Development. Caenorhabditis elegans. Later Processes 10/06/12. Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development
 Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Neural induction. Noggin Chordin Follistatin (Xnr3)
 a bird s eye view Since the discovery of the phenomenon of neural induction by Spemann and Mangold in 94, considerable effort has been invested in identifying the signals produced by the organizer that
a bird s eye view Since the discovery of the phenomenon of neural induction by Spemann and Mangold in 94, considerable effort has been invested in identifying the signals produced by the organizer that
FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula
 RESEARCH ARTICLE 779 Development 134, 779-788 (27) doi:1.1242/dev.2768 FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula Adnan Mir 1,2, Matt Kofron 1, Aaron M. Zorn
RESEARCH ARTICLE 779 Development 134, 779-788 (27) doi:1.1242/dev.2768 FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula Adnan Mir 1,2, Matt Kofron 1, Aaron M. Zorn
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Exam 1 ID#: October 1, 2006
 Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Induction and patterning of the telencephalon in Xenopus laevis
 Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
of MyoD in Xenopus mesoderm
 Proc. Nati. Acad. Sci. USA Vol. 91, pp. 10844-10848, November 1994 Developmental iology Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm CHRISTINE E. HOLT*t,
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 10844-10848, November 1994 Developmental iology Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm CHRISTINE E. HOLT*t,
β-catenin/tcf-regulated transcription prior to the midblastula transition
 Development 129, 5743-5752 2002 The Company of Biologists Ltd doi:10.1242/dev.00150 5743 β-catenin/tcf-regulated transcription prior to the midblastula transition Jing Yang 1, Change Tan 1, Rachel S. Darken
Development 129, 5743-5752 2002 The Company of Biologists Ltd doi:10.1242/dev.00150 5743 β-catenin/tcf-regulated transcription prior to the midblastula transition Jing Yang 1, Change Tan 1, Rachel S. Darken
goosecoid and the organizer
 Development 1992 Supplement, 167-171 (1992) Printed in Great Britain The Company of Biologists Limited 1992 167 goosecoid and the organizer EDDY M. DE ROBERTIS, MARTIN BLUM, CHRISTOF NIEHRS and HERBERT
Development 1992 Supplement, 167-171 (1992) Printed in Great Britain The Company of Biologists Limited 1992 167 goosecoid and the organizer EDDY M. DE ROBERTIS, MARTIN BLUM, CHRISTOF NIEHRS and HERBERT
Regulating Bone Growth and Development with Bone Morphogenetic Proteins
 Regulating Bone Growth and Development with Bone Morphogenetic Proteins PHOEBE S. LEBOY Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6030,
Regulating Bone Growth and Development with Bone Morphogenetic Proteins PHOEBE S. LEBOY Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6030,
Muscle gene activation by induction and the nonrequirement for cell division
 /. Embryol. exp. Morph. 97 Supplement, 75-84 (1986) 75 Printed in Great Britain The Company of Biologists Limited 1986 Muscle gene activation by induction and the nonrequirement for cell division J. B.
/. Embryol. exp. Morph. 97 Supplement, 75-84 (1986) 75 Printed in Great Britain The Company of Biologists Limited 1986 Muscle gene activation by induction and the nonrequirement for cell division J. B.
W Nr genes encode a family of secreted glycoproteins
 Activities of the Wnt-1 Class of Secreted Signaling Factors Are Antagonized by the Wnt-5A Class and by a Dominant Negative Cadherin in Early Xenopus Development Monica A. Tortes, Julia A. Yang-Snyder,
Activities of the Wnt-1 Class of Secreted Signaling Factors Are Antagonized by the Wnt-5A Class and by a Dominant Negative Cadherin in Early Xenopus Development Monica A. Tortes, Julia A. Yang-Snyder,
The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
 Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1
 Development 124, 3177-3184 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV1180 3177 Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer
Development 124, 3177-3184 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV1180 3177 Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer
Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3
 The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
KEY Reproductive cloning Therapeutic cloning
 1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
Conservation and evolutionary divergence in the activity of receptor-regulated smads. Sorrentino et al.
 Conservation and evolutionary divergence in the activity of receptor-regulated smads Sorrentino et al. Sorrentino et al. EvoDevo 2012, 3:22 Sorrentino et al. EvoDevo 2012, 3:22 RESEARCH Open Access Conservation
Conservation and evolutionary divergence in the activity of receptor-regulated smads Sorrentino et al. Sorrentino et al. EvoDevo 2012, 3:22 Sorrentino et al. EvoDevo 2012, 3:22 RESEARCH Open Access Conservation
The Xenopus homologue of Bicaudal-C is a localized maternal mrna that can induce endoderm formation
 Development 127, 2053-2062 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV3185 2053 The Xenopus homologue of Bicaudal-C is a localized maternal mrna that can induce endoderm
Development 127, 2053-2062 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV3185 2053 The Xenopus homologue of Bicaudal-C is a localized maternal mrna that can induce endoderm
Anti-sense RNA injections in fertilized eggs as a test for the function of localized mrnas
 J. Embryol. exp. Morph. 97 Supplement, 211-221 (1986) 211 Printed in Great Britain The Company of Biologists Limited 1986 Anti-sense RNA injections in fertilized eggs as a test for the function of localized
J. Embryol. exp. Morph. 97 Supplement, 211-221 (1986) 211 Printed in Great Britain The Company of Biologists Limited 1986 Anti-sense RNA injections in fertilized eggs as a test for the function of localized
1. 40 points Retinoic acid (structure below) is a small hydrophobic molecule that is derived from vitamin A. Figure by MIT OCW.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 9 7.22 Fall 2005 exam 2 practice
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 9 7.22 Fall 2005 exam 2 practice
Reading. Lecture III. Nervous System Embryology. Biology. Brain Diseases. September 5, Bio 3411 Lecture III. Nervous System Embryology
 Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Applicazioni biotecnologiche
 Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Lecture III. Nervous System Embryology
 Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo
 Mechanisms of Development 97 (2000) 117±131 www.elsevier.com/locate/modo Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo Thomas Kurth, Peter Hausen* Max-Planck-Institut
Mechanisms of Development 97 (2000) 117±131 www.elsevier.com/locate/modo Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo Thomas Kurth, Peter Hausen* Max-Planck-Institut
Patterning of the Brain
 Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Expression Pattern of Xtshz1 and Xtshz3 During Xenopus Early Eye Formation
 SUNY College of Environmental Science and Forestry Digital Commons @ ESF Honors Theses 5-2012 Expression Pattern of Xtshz1 and Xtshz3 During Xenopus Early Eye Formation Katherine L. McKissick Follow this
SUNY College of Environmental Science and Forestry Digital Commons @ ESF Honors Theses 5-2012 Expression Pattern of Xtshz1 and Xtshz3 During Xenopus Early Eye Formation Katherine L. McKissick Follow this
ANAT2341 Embryology Introduction: Steve Palmer
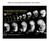 ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
The Role of Pitx Proteins in Early Xenopus Development
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 9-2017 The Role of Pitx Proteins in Early Xenopus Development Ye Jin The Graduate Center,
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 9-2017 The Role of Pitx Proteins in Early Xenopus Development Ye Jin The Graduate Center,
Understanding embryonic head development. ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute
 Understanding embryonic head development ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute Head malformations among the most common category of congenital malformations in
Understanding embryonic head development ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute Head malformations among the most common category of congenital malformations in
Action Range of BMP Is Defined by Its N-Terminal Basic Amino Acid Core
 Current Biology, Vol. 12, 205 209, February 5, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(01)00684-4 Action Range of BMP Is Defined by Its N-Terminal Basic Amino Acid Core Bisei
Current Biology, Vol. 12, 205 209, February 5, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(01)00684-4 Action Range of BMP Is Defined by Its N-Terminal Basic Amino Acid Core Bisei
Neural Induction in the Absence of Mesoderm: -Catenin-Dependent Expression of Secreted BMP Antagonists at the Blastula Stage in Xenopus
 Developmental Biology 234, 161 173 (2001) doi:10.1006/dbio.2001.0258, available online at http://www.idealibrary.com on Neural Induction in the Absence of Mesoderm: -Catenin-Dependent Expression of Secreted
Developmental Biology 234, 161 173 (2001) doi:10.1006/dbio.2001.0258, available online at http://www.idealibrary.com on Neural Induction in the Absence of Mesoderm: -Catenin-Dependent Expression of Secreted
TRANSGENIC ANIMALS. -transient transfection of cells -stable transfection of cells. - Two methods to produce transgenic animals:
 TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
Guided differentiation of ES cell into mesenchymal stem cell
 Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
Hemidesmosome. Focal adhesion
 BIOLOGY 52 - - CELL AND DEVELOPMENTAL BIOLOGY - - FALL 2002 Third Examination - - November, 2002 -------------------------------------------------- Put your name at the top of each page. Please read each
BIOLOGY 52 - - CELL AND DEVELOPMENTAL BIOLOGY - - FALL 2002 Third Examination - - November, 2002 -------------------------------------------------- Put your name at the top of each page. Please read each
Why Study Developmental Neurobiology? Terrific scientific challenge.
 Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms
 Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals
 Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals Hidehiko Inomata, 1,3, * Tatsuo Shibata, 2 Tomoko Haraguchi, 1 and Yoshiki Sasai 1, * 1 Organogenesis
Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals Hidehiko Inomata, 1,3, * Tatsuo Shibata, 2 Tomoko Haraguchi, 1 and Yoshiki Sasai 1, * 1 Organogenesis
Developmental Zoology (ZOO ) Gatrulation
 Developmental Zoology (ZOO 228.1.0) Gatrulation 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis Aging
Developmental Zoology (ZOO 228.1.0) Gatrulation 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis Aging
produces an RNA copy of the coding region of a gene
 1. Transcription Gene Expression The expression of a gene into a protein occurs by: 1) Transcription of a gene into RNA produces an RNA copy of the coding region of a gene the RNA transcript may be the
1. Transcription Gene Expression The expression of a gene into a protein occurs by: 1) Transcription of a gene into RNA produces an RNA copy of the coding region of a gene the RNA transcript may be the
Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have
 Development 125, 579-587 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV6311 579 Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in
Development 125, 579-587 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV6311 579 Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in
ksierzputowska.com Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster
 Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster Research plan: Specific aims: 1. To successfully engineer transgenic Drosophila expressing TALENs
Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster Research plan: Specific aims: 1. To successfully engineer transgenic Drosophila expressing TALENs
Technical tips Session 4
 Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Maternal Dead-End1 is required for vegetal cortical microtubule assembly during Xenopus axis specification
 2334 RESEARCH ARTICLE Development 140, 2334-2344 (2013) doi:10.1242/dev.094748 2013. Published by The Company of Biologists Ltd Maternal Dead-End1 is required for vegetal cortical microtubule assembly
2334 RESEARCH ARTICLE Development 140, 2334-2344 (2013) doi:10.1242/dev.094748 2013. Published by The Company of Biologists Ltd Maternal Dead-End1 is required for vegetal cortical microtubule assembly
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis
 Development 130, 2129-2138 2003 The Company of Biologists Ltd doi:10.1242/dev.00435 2129 Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis Bisei
Development 130, 2129-2138 2003 The Company of Biologists Ltd doi:10.1242/dev.00435 2129 Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis Bisei
Roche Molecular Biochemicals Technical Note No. LC 12/2000
 Roche Molecular Biochemicals Technical Note No. LC 12/2000 LightCycler Absolute Quantification with External Standards and an Internal Control 1. General Introduction Purpose of this Note Overview of Method
Roche Molecular Biochemicals Technical Note No. LC 12/2000 LightCycler Absolute Quantification with External Standards and an Internal Control 1. General Introduction Purpose of this Note Overview of Method
Available online at
 Available online at www.sciencedirect.com R Developmental Biology 257 (2003) 190 204 www.elsevier.com/locate/ydbio Molecular link in the sequential induction of the Spemann organizer: direct activation
Available online at www.sciencedirect.com R Developmental Biology 257 (2003) 190 204 www.elsevier.com/locate/ydbio Molecular link in the sequential induction of the Spemann organizer: direct activation
Neural induction in Xenopus requires inhibition of Wnt-β-catenin signaling
 Developmental Biology 298 (2006) 71 86 www.elsevier.com/locate/ydbio Neural induction in Xenopus requires inhibition of Wnt-β-catenin signaling Elizabeth Heeg-Truesdell a, Carole LaBonne a,b, a Department
Developmental Biology 298 (2006) 71 86 www.elsevier.com/locate/ydbio Neural induction in Xenopus requires inhibition of Wnt-β-catenin signaling Elizabeth Heeg-Truesdell a, Carole LaBonne a,b, a Department
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mot
 Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Chapter 14 Regulation of Transcription
 Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis
 Development 121, 2337-2347 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2337 Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis
Development 121, 2337-2347 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2337 Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/318/5851/794/dc1 Supporting Online Material for A Gene Regulatory Network Subcircuit Drives a Dynamic Pattern of Gene Expression Joel Smith, Christina Theodoris, Eric
www.sciencemag.org/cgi/content/full/318/5851/794/dc1 Supporting Online Material for A Gene Regulatory Network Subcircuit Drives a Dynamic Pattern of Gene Expression Joel Smith, Christina Theodoris, Eric
Localized synthesis of the Vg1 protein during early Xenopus development
 Development 106, 775-785 (1989) Printed in Great Britain The Company of Biologists Limited 1989 775 Localized synthesis of the Vg1 protein during early Xenopus development D. TANNAHILL and D. A. MELTON
Development 106, 775-785 (1989) Printed in Great Britain The Company of Biologists Limited 1989 775 Localized synthesis of the Vg1 protein during early Xenopus development D. TANNAHILL and D. A. MELTON
The secreted EGF-Discoidin factor xdel1 is essential for dorsal development of the Xenopus embryo
 Developmental Biology 306 (2007) 160 169 www.elsevier.com/locate/ydbio The secreted EGF-Discoidin factor xdel1 is essential for dorsal development of the Xenopus embryo Akiko Arakawa a,c,1, Mami Matsuo-Takasaki
Developmental Biology 306 (2007) 160 169 www.elsevier.com/locate/ydbio The secreted EGF-Discoidin factor xdel1 is essential for dorsal development of the Xenopus embryo Akiko Arakawa a,c,1, Mami Matsuo-Takasaki
Sundari Chetty, Felicia Walton Pagliuca, Christian Honore, Anastasie Kweudjeu, Alireza Rezania, and Douglas A. Melton
 A simple tool to improve pluripotent stem cell differentiation Sundari Chetty, Felicia Walton Pagliuca, Christian Honore, Anastasie Kweudjeu, Alireza Rezania, and Douglas A. Melton Supplementary Information
A simple tool to improve pluripotent stem cell differentiation Sundari Chetty, Felicia Walton Pagliuca, Christian Honore, Anastasie Kweudjeu, Alireza Rezania, and Douglas A. Melton Supplementary Information
Practice Exam A. Briefly describe how IL-25 treatment might be able to help this responder subgroup of liver cancer patients.
 Practice Exam 2007 1. A special JAK-STAT signaling system (JAK5-STAT5) was recently identified in which a gene called TS5 becomes selectively transcribed and expressed in the liver upon induction by a
Practice Exam 2007 1. A special JAK-STAT signaling system (JAK5-STAT5) was recently identified in which a gene called TS5 becomes selectively transcribed and expressed in the liver upon induction by a
Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus
 Development 129, 5683-5695 2002 The Company of Biologists Ltd doi:10.1242/dev.00169 5683 Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed
Development 129, 5683-5695 2002 The Company of Biologists Ltd doi:10.1242/dev.00169 5683 Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed
XSIP1 is essential for early neural gene expression and neural differentiation by suppression of BMP signaling
 Developmental Biology 275 (2004) 258 267 www.elsevier.com/locate/ydbio XSIP1 is essential for early neural gene expression and neural differentiation by suppression of BMP signaling Kazuhiro R. Nitta a,
Developmental Biology 275 (2004) 258 267 www.elsevier.com/locate/ydbio XSIP1 is essential for early neural gene expression and neural differentiation by suppression of BMP signaling Kazuhiro R. Nitta a,
The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm
 RESEARCH ARTICLE 451 Development 135, 451-461 (2008) doi:10.1242/dev.008458 The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm Francesca M. Spagnoli and
RESEARCH ARTICLE 451 Development 135, 451-461 (2008) doi:10.1242/dev.008458 The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm Francesca M. Spagnoli and
