Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo
|
|
|
- Charlotte Daniel
- 6 years ago
- Views:
Transcription
1 Mechanisms of Development 97 (2000) 117±131 Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo Thomas Kurth, Peter Hausen* Max-Planck-Institut fuèr Entwicklungsbiologie, Abteilung Zellbiologie, Spemannstrasse 35/V, TuÈbingen, Germany Received 16 June 2000; received in revised form 20 July 2000; accepted 21 July 2000 Abstract The appearance of bottle cells at the dorsal vegetal/marginal boundary of Xenopus embryos marks the onset of blastopore formation. The conditions leading to this epithelial activity were investigated by inducing bottle cells ectopically in the animal region with VegT or different members of the transforming growth factor (TGF)-b family. Morphological studies on the ectopic bottle cells indicate their close similarity to the endogenous bottle cells at the dorsal blastopore lip. The subepithelial cells of the induced animal region express mesodermal genes in a pattern reminiscent to that observed on the dorsal lip. Relating this expression pattern to the position of the ectopic bottle cells leads to the conclusion that bottle cells form in regions of high TGF-b signalling. The speci c inhibitory effects of cerberus on ectopically induced bottle cells revealed that nodal related growth factors are the intrinsic signals that elicit bottle cell formation in the normal embryo. In addition, broblast growth factor signalling is an essential precondition for this epithelial response as it is for mesoderm formation. We conclude that bottle cell formation in the epithelial layer of the gastrula is closely linked to mesodermal patterning in the subepithelial tissues. q 2000 Elsevier Science Ireland Ltd. All rights reserved. Keywords: Xenopus laevis; Gastrulation; Bottle cell; Pattern formation; Transforming growth factor b; Activin; Nodal; Fibroblast growth factor; Cerberus 1. Introduction Morphogenetic movements during gastrulation reshape the early embryo into a structure that re ects in many aspects the basic body plan of the phylotype. A special differentiation occurring during gastrulation are the bottle cells that form in the primary epithelium at the periphery of the embryo. Their appearance in gastrulating embryos is widespread throughout the different animal phyla, including arthropods, echinoderms and chordates (Kimberly and Hardin, 1998 and references therein). In Xenopus embryos the formation of bottle cells at the blastopore lip is the rst outwardly discernible sign for the onset of gastrulation. A concentration of pigment at the animal/vegetal border demarcates a eld of epithelial cells with constricted apical surfaces and ask-like cell bodies that stretch inwards. This local distortion of the epithelial layer results in a dent which indicates the incipient blastopore (Hardin and Keller, 1988; Keller, 1981). Bottle cell formation begins dorsally and proceeds later into lateral and ventral regions of the marginal zone. * Corresponding author. Tel.: ; fax: address: peter.hausen@tuebingen.mpg.de (P. Hausen). Despite their widespread occurrence little attention has been paid to the mechanisms that lead to bottle cell formation at the precise position in the embryo and at de ned times during gastrulation. Both positioning and timing of bottle cell formation correlate closely with patterning events in the marginal zone. The question therefore arises whether this correlation is incidental or whether bottle cell formation is causally linked to these events. The patterning processes in the marginal zone are known in some detail (for recent reviews see Harland and Gerhart, 1997; Heasman, 1997; Asashima et al., 1999). Overexpression of dominant negative receptor constructs revealed that both transforming growth factor (TGF)-b and broblast growth factor (FGF) signalling events are required (Amaya et al., 1991, 1993; Hemmati-Brivanlou and Melton, 1992). The spatial restriction of the prospective mesoderm to the marginal zone is thought to be due to an overlap in this zone of two signalling processes with activin-like TGF-b signals contributed by the vegetal hemisphere and FGFsignals contributed by the animal hemisphere (Cornell et al., 1995). Consistent with this model, a variety of activinlike TGF-b signals (e.g. BVg1, activin, Xnr1, -2 and -4) elicit mesodermal properties in animal caps (Asashima et al., 1999), whereas FGF expression in vegetal caps allows for the activation of mesoderm speci c gene expression /00/$ - see front matter q 2000 Elsevier Science Ireland Ltd. All rights reserved. PII: S (00)
2 118 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117±131 there (Cornell et al., 1995). In addition, factors translocated by cortical rotation specify the dorsal region of the marginal zone. This local property becomes more pronounced and spatially con ned when zygotic gene activation leads to elaborate dorsal patterning processes (Kimelman, 1999). To assess the question of how bottle cell formation depends on local patterning in the marginal zone we made use of the fact that animal caps are competent to form ectopic invaginations resembling endogenous lips. This has been demonstrated by Lustig et al. (1996). They discovered a maternally provided vegetal transcription factor that is involved in mesoderm induction and the control of TGF-b signalling molecules. (This factor has been described independently under the names of Xombi, VegT, Antipodian and Brat (Lustig et al., 1996; Zhang and King, 1996; Horb and Thomsen, 1997; Stennard et al., 1996). We decided to use the name VegT here.) VegT-mRNA injected into the animal hemisphere of fertilized eggs induces invaginations of the animal epithelium at the onset of gastrulation, which resembles the process of lip formation in the marginal zone. Using the procedure of mrna-injection we have tried to de ne the signalling conditions which allow for bottle cell formation in the animal cap region of the intact embryo. We show that after mrna injection bona de bottle cells form in regions with high TGF-b signalling activity. The subepithelial cells of the animal cap assume a pattern of gene activity that re ects the graded distribution of the TGF-b signal. Making use of the speci c properties of the growth factor inhibitor cerberus we demonstrate that among the TGF-b signals the nodal related factors are most likely the intrinsic signals that elicit bottle cell formation in vivo. Further, FGF signalling is required to obtain ectopic bottle cells. We conclude that ectopic bottle cell formation occurs under signalling conditions that closely re ect the situation at the dorsal blastopore lip. large, yolky endodermal cells (Fig. 2F). Involution at the endogenous dorsal lip seems to be normal (not shown). At higher concentrations of injected mrna the pigment spot at the injection site is larger (phenotype II, Fig. 2A±D). It represents a eld of bottle cells which, however, show less tendency to invaginate. At most they form a pit-like structure in a multi-layered animal cap. The blastocoel is displaced vegetally (Fig. 2G,H). Dorsal lip formation is initiated but becomes arrested at stage 10.5 (see Fig. 4A). A further rise in the amounts of mrna produces a broad eld of bottle cells (phenotype III, Fig. 2I). Invagination does not occur and the animal material is further contracted towards the pole region. The blastocoel is shifted into the vegetal half and often abuts a single layered vegetal epithelium (Fig. 2K). Endogenous bottle cells do form but show no sign of invagination (arrow in Fig. 2K). At the highest mrna concentrations, all animal pigment is concentrated in a dark spot (phenotype IV). The contraction of animal cap material towards the spot is accompanied by an upward translocation of the peripheral vegetal cell mass and a rupture of the vegetal epithelium in the pole region (Fig. 2L). The types of RNAs used in these experiments induce these phenotypes with different ef ciencies (Fig. 1 and Table 1). In all cases, the mass of induced bottle cells increases with the amount of injected mrna. Any of the TGF-b mrnas can eventually provoke a type IV phenotype and there was no indication of an inhibitory effect on ectopic bottle cell formation exerted by excess amounts of mrna. VegT mrna is less ef cient than the TGF-b mrnas and the response does never exceed phenotype II. The morphological changes in the injected embryos that result from aberrant morphogenetic movements are complex and not easy to explain by current concepts of gastrula morphogenesis. In the following, we concentrate on one aspect of these changes, the formation of ectopic 2. Results 2.1. Bottle cell formation and concomitant morphological changes in embryos overexpressing members of the TGF-b family or VegT in the animal cap TGF-b signalling induces mesodermal properties in the inner animal cap cells (Asashima et al., 1999). To assess the effects of this induction on the morphology of the embryo, mrnas of activin, Xnr-1, BVg1 or VegT were injected into the animal pole region of two to eight cell embryos. The resulting phenotypes of stage 10±11 embryos depend in a systematic manner on the amounts of mrna injected (Fig. 1). Low doses of mrna induce a weak response (phenotype I). Externally, an accumulation of pigment forms around the site of mrna injection (Fig. 2E). Internally, a deep invagination at this site is lined by epithelial cells with bottle cell morphology and surrounded by a compact mass of animal cells. Sometimes the blastocoel roof consists entirely of Fig. 1. Summary of the phenotypic effects achieved by injection of different amounts of mrnas coding for activin-like TGF-b signals (Xnr1, activin, BVg1) and for the transcription factor VegT into the animal hemisphere of Xenopus embryos. Phenotype I: weak accumulation of pigment, invagination of the animal epithelium. Phenotype II: a pigmented spot indicates a bottle cell eld that forms a pit. Phenotype III: large bottle cell eld without any sign of invagination. Phenotype IV: the epithelial cells of the complete animal half transform into bottle cells.
3 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117± Fig. 2. Morphology of the phenotypic effects induced by TGF-b signals or by VegT. (A±E) Gross morphology of ectopic bottle cell elds induced by activin (A, animal view; B, animal (left) and vegetal (right) views of the same embryo), BVg1 (C, animal view), Xnr1 (D, animal view), and VegT (E, animal view). Ectopic bottle cells are indicated by arrows and endogenous bottle cells by arrowheads. (F±L) Histological analysis of embryos injected with TGF-b mrna. (F) The weakest phenotype, characterized by an invagination into the animal cap tissue, is shown here for an embryo injected with 25 pg Xnr1 mrna (phenotype I). (G,H) Ectopic bottle cell eld induced by 100 pg activin mrna (phenotype II), (H) Enlarged view of the bottle cell pit in (G). (I) Immunohistological staining of the cell surface marker b-catenin to visualize ectopically induced bottle cells after injection of 200 pg of BVg1 mrna (phenotype III). (K) Phenotype III after injection of 200 pg activin mrna. The embryo appears as turned upside down with the blastocoel displaced to the vegetal half. The arrowhead indicates endogenous bottle cells. (L) Strong phenotype induced by 200 pg of Xnr1 mrna (phenotype IV). Bars: 200 mm in (G,K,L); 100 mm in (F,H,I). bottle cells. We aim to deduce from experimentally manipulated bottle cell induction the mechanism of how endogenous bottle cell formation is controlled at the blastopore lip of the normal embryo Fine structure of ectopic bottle cells Ectopic bottle cells in the animal region share characteristic structural features with the endogenous bottle cells at the blastopore lip. Their apical surfaces are furnished with small protrusions which distinguish them from the smooth surfaces of adjacent cells (see Fig. 3A,B for endogenous and Fig. 3C±G for induced bottle cells in embryos injected with activin mrna). Transmission electron microscopy (TEM) inspection reveals these microvilli to be rooted in a prominent electron dense submembrane lamentous layer (Fig. 3H,I) that correlates with an accumulation of actin in the apices of the bottle cells (Fig. 3K,M). Further, pigment granules and small vesicles are enriched beneath the apical cortex in both endogenous and induced exogenous bottle cells. In no case could we nd in this analysis any characteristic difference between the two types of bottle cells. In conclusion, high TGF-b signalling leads to the transformation of animal epithelial cells into bottle cells that by morphological means (light microscope, scanning electron microscopy (SEM), TEM) are indistinguishable from those at the blastopore lip indicating that we are dealing with bona de bottle cells. Thus, the pigmentation of the apically constricted cortex provided a useful and reliable indicator of bottle cells in our experiments Timing of ectopic bottle cell formation The notion that ectopic induction by RNA injection yields authentic bottle cells is supported by observations made in timing experiments. Embryos were injected animally with
4 120 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117±131 Table 1 Frequencies and percentages (in parentheses) of phenotypes (I±IV) after injection of Xnr1-, activin-, BVg1-, and VegT-mRNAs into the animal hemisphere mrna Normal phenotype I II III IV No. of embryos ( ˆ 100%) No. of experiments Xnr1 (25 pg) 7 (8) 69 (81) 6 (7) 3 (4) ± 85 3 Xnr1 (50 pg) ± ± 31 (62) 19 (30) ± 50 2 Xnr1 (100 pg) ± 9 (8) 38 (35) 63 (57) ± Xnr1 (200 pg) ± ± 1 (2) 7 (10) 61 (88) 69 3 Activin (25 pg) 26 (39) 41 (61) ± ± ± 67 3 Activin (50 pg) ± 44 (54) 37 (46) ± ± 81 3 Activin (100 pg) 14 (5) 4 (2) 242 (93) ± ± Activin (200 pg) ± ± 30 (26) 85 (74) ± Activin (4 150 pg) ± ± ± ± 29 (100) 29 1 BVg1 (25 pg) 9 (25) 23 (64) 4 (11) ± ± 36 2 BVg1 (50 pg) 4 (7) 22 (38) 32 (55) ± ± 58 2 BVg1 (100 pg) ± 10 (17) 50 (83) ± ± 60 2 BVg1 (200 pg) ± ± 40 (69) 18 (31) ± 58 3 BVg1 (400 pg) ± ± 20 (54) 17 (46) ± 37 2 BVg1 (4 200 pg) ± ± 3 (8) 15 (38) 21 (54) 39 2 VegT (60 pg) 43 (91) 4 (9) ± ± ± 47 2 VegT (125 pg) 32 (71) 13 (29) ± ± ± 45 2 VegT (250 pg) 5 (25) 15 (75) ± ± ± 20 1 VegT (500 pg) 6 (11) 43 (83) 3 (6) ± ± 52 2 VegT (1 ng) 2 (10) 16 (80) 2 (10) ± ± 20 1 activin mrna and the formation of ectopic bottle cells, recognized by pigment concentration in the animal half, was monitored by time lapse videomicroscopy. Some of these embryos were turned upside down to monitor the time of appearance of the endogenous dorsal lip. Animal pigment concentrations appeared at exactly the same time as in the dorsal lip, i.e. at stage 10 of development (Fig. 4A). Later, the endogenous lip becomes arrested in these embryos (compare Fig. 4A,B). Further, the timing of the initiation of endogenous lip formation is not in uenced by the injection of activin mrna (compare Fig. 4A,B). In the normal embryo the formation of bottle cells proceeds over a period of 2 h into the lateral and ventral regions. Thus, if there is a difference at all in the character of dorsal and ventral bottle cells, the induced bottle cells would be, according to this timing experiment, of the dorsal type Spatial pattern of mesodermal gene activity and ectopic bottle cell formation Dorsal bottle cells form in the early gastrula from epithelial cells located at the animal/vegetal border. The presumptive mesoderm there consists of non-epithelial inner cells of the marginal zone, some of them in direct contact with the bottle cells. It may be anticipated that cues from this cellular environment dispose the epithelial cells for their bottle cell fate. Numerous genes are speci cally expressed in this region, of which we chose brachyury (Xbra, Smith et al., 1991) and goosecoid (gsc, Cho et al., 1991) to de ne the cellular environment in which ectopic bottle cell formation occurs. In the normal stage 10 embryo Xbra is expressed in the marginal zone at some distance animalwards off the bottle cell-lined blastopore lip (Fig. 5Ab, see also b 0 ). Transcripts of gsc are present in the dorsal part of the blastopore adjacent to the bottle cells (Fig. 5Aa). Further, gsc is expressed in the deep mesoderm whereas Xbra is expressed more peripherally (Fig. 5Ba). In neither case are the respective transcripts present in the bottle cells themselves (see double in situ hybridization in Fig. 5B). Together with bottle cell formation, gsc- and Xbraexpression are induced in animal caps upon injection of TGF-b mrna. Interestingly, the patterns of expression of the two genes in stage 10 embryos are discrete and resemble the endogenous patterns at the dorsal lip in several aspects. In situ hybridization reveals an area of gsc transcripts around the site of RNA injection. The ectopic bottle cell eld occupies the central part within this domain (Fig. 5Ac,e). By way of contrast, expression of Xbra occurs at some distance from the injection site, leaving a central disc free of transcripts (Fig. 5Ad,f, see also d 0 ). Double in situ hybridization of dissected embryos further showed that the epithelial cells together with the induced bottle cells produce neither of the transcripts. As at the dorsal lip, the gsc domain underlaps the Xbra domain to a certain extent at its proximal border (Fig. 5Bb). A possible explanation of these results is that a gradient of TGF-b signalling elicited by injected mrna is responsible for the concentric shape of the patterns in the animal cap.
5 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117± Fig. 3. Fine structure of endogenous and ectopic bottle cells. (A±E) SEM preparations of the blastopore of an uninjected embryo (stage 10, A,B) and of an ectopic bottle cell eld (arrows in C) in an embryo overexpressing activin in the animal cap (C±E). Both types of bottle cells show characteristic protrusions on their apical surfaces (B,E), which contrasts to the smooth apical surfaces of other epithelial cells. Cell borders are highlighted in (B) and the insert of (E). (F,G) Low-power magni cation TEM pictures which demonstrate the apical microvilli like protrusions on the surface of an ectopic bottle cell (F) as compared to the smooth apical surface of a normal animal cap cell (G). (H) Details of the apical parts of bottle cells from the dorsal blastopore lip (stage 11) at higher magni cation. Microvilli (MV) with electron dense submembraneous material, many small vesicles (V) and pigment granules (PG) are visible. LD, lipid droplet. (I) Apical part of an ectopic bottle cell in the animal cap of an activin-injected embryo. Note the similar organization as compared to the bottle cells in (H). YP, yolk platelet. (K±N) Actin accumulation (arrows in K and M) in the apical cortices of normal (K) and activin-induced (M) bottle cells. Actin was stained with a pan-actin monoclonal antibody. To visualize the cell shapes, the specimens were counterstained for cell surface molecules (cadherins in L and b- catenin in N). Bars: 1 mm in (H,I); 5 mm in (B,F,G) and insert of (E); 20 mm in (A,E); 50 mm in (D); 100 mm in (K±N); 200 mm in (C).
6 122 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117±131 Bottle cell formation and gsc expression in the centre are responses to high doses of TGF-b activity. Xbra is induced at lower and probably repressed at higher levels of TGF-b signals (Latinkic and Smith, 1999; Papin and Smith, 2000). Further experiments were undertaken to substantiate this hypothesis. Different amounts of activin mrna were injected into the animal pole of 4-cell embryos and the embryos were scored at stage 101 for bottle cell formation and Xbra expression. As is illustrated in Fig. 6 the domains of bottle cell formation and of Xbra expression change in a characteristic way. After injection of high doses of activin mrna, a large eld of bottle cells forms and the animal cap remains free of Xbra transcripts (Fig. 6A). Intermediate doses of activin result in a large domain of Xbra expression separated from the injection site by a narrowing zone devoid of Xbra Fig. 4. Time lapse videomicroscopy of early gastrulation. (A) Comparison of the emergence of activin induced ectopic bottle cell pits in the animal hemisphere and the development of the endogenous lip in the marginal/ vegetal region (inverted embryo on the right). (B) Activin induced ectopic bottle cell formation compared to blastopore formation in uninjected embryos. In both cases ectopic and endogenous bottle cells develop roughly at the same time. The endogenous blastopore of the activin injected inverted embryo in (A) is not closed. transcripts. The eld of bottle cells becomes smaller (Fig. 6B). At low concentration of activin a spot of Xbra expression forms at the site of injection but no bottle cells appear (Fig. 6C). Similar concentration dependent ectopic patterns can be found in animal caps of embryos injected with mrnas coding for Xnr1 or BVg1 (not shown). Taken together, these data are consistent with the interpretation that after mrna injection a gradient of TGF-b activity patterns a eld of cells in the animal cap and that ectopic bottle cells form at the apex of the gradient. The similarity of the pattern of gene expression in the ectopic eld and at the dorsal blastopore lip suggests that the same mechanism of bottle cell formation operates in both cases Cerberus inhibits bottle cell formation Cerberus is a secreted protein that binds extracellularly to Xnr1, BMP4 and Xwnt8 and inhibits their signalling activity. The speci city of this inhibitor is illustrated by the fact that it does not bind to or affect signalling by activin or BVg1 (Piccolo et al., 1999). Radial injection of high levels of cerberus mrna into the marginal zone of fertilized eggs completely blocks lip formation and Xbra expression (Bouwmeester et al., 1996; data not shown). To investigate further the effects of cerberus on bottle cell formation, graded amounts of the mrna were injected into two adjacent cells in the marginal zone of 8-cell embryos (Fig. 7Aa±e). Seventy- ve picograms of mrna prevents lip formation on the injected side and reduces the expression of Xbra. A lip forms on the opposite side but no signs of the completion of the blastopore ring were detected. This phenotype is more pronounced after injection of twice the amount of mrna. Injection of the high dose of 600 pg cerberus mrna completely abolishes lip formation but appreciable Xbra expression remains on the non-injected side. Since the injected mrna was restricted to cells on one side of the embryo this observation suggests that secreted cerberus protein can reach distant locations to exert its effect. At a moderate dosis of cerberus mrna a lip may be formed in the vegetal pole region, indicating the potential of the vegetal epithelium to form bottle cells (Fig. 7Af). The injected embryos further display signs of disturbed morphogenesis that are dif cult to interpret. Examples are the blastocoel oor consisting of animal and marginal cells instead of the normal vegetal cells or an apparent decoupling of external and internal gastrulation movements, e.g. involution around an internal lip and translocation of mesendodermal cells to the blastocoel roof in the absence of an external lip (data not shown). In sum, cerberus affects endogenous bottle cell formation, disturbs gastrulation movements and may elicit bottle cell formation ectopically in the vegetal epithelium. Further experiments showed that cerberus inhibits ectopic lip formation induced by Xnr1 but not by activin. Embryos were injected with different amounts of mrnas coding for either activin, Xnr1 or combinations of activin 1 cerberus
7 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117± Fig. 5. (A) Patterns of gene expression in normal embryos (a,b, vegetal view) and in embryos injected with the indicated amounts of mrnas coding for activin (c,d, animal view) or Xnr1 (e,f, animal view). In normal gastrula embryos the dorsal marker goosecoid (gsc) is expressed directly on the lip (a). The transcripts of the panmesodermal marker Xbra form a ring in the marginal zone with a gap between the bottle cells and the Xbra positive region (b). Injection of activin or Xnr1 mrna generates similar ectopic expression patterns of these markers in the animal cap: gsc is expressed near the ectopic bottle cells (c,e), whereas Xbra is induced at some distance to them (d,f). At an intermediate level of injected activin mrna the pattern at the ectopic `lip' closely resembles the wild type situation (compare b 0 with d 0 ). The schematic drawing illustrates the different domains abutting endogenous and ectopic bottle cells. Domain I represents the gap between the Xbra domain (domain II) and the bottle cell region whereas domain III represents a distal Xbra negative domain. Arrows indicate the endogenous bottle cells in a,b and ectopic bottle cells in d. (B) Top: double in situ analysis of gsc (blue) and Xbra (magenta) in control (a) and in embryos injected with activin mrna (b). Whole-mount in situ hybridization was performed on dissected embryos to visualize internal patterns of transcript distribution. Bottom: interpretative drawings of the expression domains in (a) and (b). On the dorsal side of uninjected embryos goosecoid signal is found in deeper mesendodermal regions whereas Xbra-transcripts occur in more peripheral regions (a). (b) Ectopic internal patterns in an embryo injected with activin mrna. The embryo is cut perpendicular to the dorsal±ventral axis. As in the control Xbra and gsc transcripts are separated from each other (arrow). Gsc is expressed in the tissue underneath the bottle cell eld but cannot be found in the bottle cells themselves. Abbreviations: An, animal; bc, blastocoel; D, dorsal; ect, ectopic; end, endogenous; ep, epithelium; Le, left; Ri, right; V, ventral; Veg, vegetal. Asterisks indicate the injection sites in (A,B).
8 124 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117±131 and Xnr1 1 cerberus and scored for ectopic bottle cell elds and Xbra expression (Table 2 and Fig. 7B). Embryos injected with Xnr1-mRNA develop pronounced bottle cell elds and ectopic Xbra expression domains in the animal cap. Coexpression of cerberus together with Xnr1 leads to a complete loss of this phenotype. In contrast, cerberus has little or no effect on the activin induced formation of ectopic bottle cells and ectopic Xbra expression. Thus cerberus speci cally inhibits Xnr1 signalling and does not affect activin signalling as expected (Piccolo et al., 1999). Given the inhibitory effect of cerberus on endogenous lip formation, we conclude from these experiments that TGF-b signals of the nodal class and not of the activin class are essential for the formation of endogenous bottle cells FGF signalling and ectopic bottle cell formation We con rmed previous observations that activin-induced expression of mesodermal markers in animal caps and VegT induced ectopic invaginations depend on FGF signalling (LaBonne and Whitman, 1994; Cornell and Kimelman, 1994; Lustig et al., 1996) by use of a dominant negative form of FGF-receptor (XFD, Amaya et al., 1991, 1993). As shown in Fig. 8A, ectopic bottle cell formation and Xbra expression induced by activin are both inhibited by coinjection of XFD. Likewise, Xnr1-induced ectopic bottle cells are also inhibited by XFD (data not shown). In addition, we monitored the FGF signalling pathway by use of an antibody recognizing speci cally the double phosphorylated form of the extracellular signal regulated protein kinase (dperk; see Christen and Slack, 1999). Noninjected control embryos show a faint staining all over the animal hemisphere, an enhanced signal in the marginal zone, and no staining at all in the vegetal hemisphere. Activin induces an activation of ERK in embryos between stages Fig. 6. Expression of Xbra is regulated by gradients of TGF-b activity. Expansion and retraction of the bottle cell region and/or the adjacent domains I±III can be achieved by injecting different amounts of activin mrna: (A) 200 pg, (B) 100 pg, (C) 15 pg. The schematic drawings illustrate the shifts of the different domains. 8 and 11 (Fig. 8B) that concentrates in the region of ectopic bottle cell formation in the animal hemisphere indicating that the FGF signalling pathway is activated there (Fig. 8C). 3. Discussion The appearance of bottle cells at the vegetal border of the marginal zone is a conspicuous and early sign of blastopore formation at gastrulation. However, it does not indicate the onset of patterning of the marginal region. Mesoderm induction (Ding et al., 1998), expression of Xbra in the marginal zone (Smith et al., 1991) and priming of the nuclei for dorsal gene expression by nuclear translocation of b-catenin (Schneider et al., 1996) occur well in advance of bottle cell formation. Likewise, pregastrula morphogenetic movements, the passing of prospective mesoderm cells around an inner blastopore lip at the tip of Brachet's cleft are initiated before bottle cell formation (Keller, 1986). This suggests that bottle cell formation is embedded in a continuous developmental process that leads to gastrulation. The experiments described in this paper were undertaken to elucidate the speci c developmental conditions that induce the cells of the primary epithelium to form bottle cells by constricting their cell-apices and stretching their cell bodies inwards Ectopic bottle cell formation Inducing TGF-b signalling by injection of activin, Xnr-1, BVg1 or VegT mrnas into the animal region of early embryos results in ectopic bottle cell formation at stage 10 of development. Together with our observation of cerberus induced vegetal invaginations (Fig. 7), we come to the conclusion that the entire peripheral epithelium of the early gastrula embryo is competent to form bottle cells. Translation of the injected TGF-b mrna occurs presumably much earlier. The temporal correlation of ectopic bottle cell formation with the onset of gastrulation indicates therefore that the emergence of competence and not the presence of the signal alone determines the timing of the epithelial response. Ectopic bottle cells and the expression domains of gsc and Xbra are arranged concentrically around the site of mrna injection. This pattern strongly suggests a response to a concentric gradient of TGF-b signalling that forms around the site of mrna injection. It is not known to what extent dispersion of the injected RNA or diffusion of the secreted factors later on contribute to the formation of this gradient. Bottle cells form at the apex of the concentric gradient indicating a requirement for relatively high TGF-b signalling. At low doses the bottle cell eld often forms a lip-like invagination or a pit. Raising the concentration of the injected RNA enlarges the eld of bottle cells until the whole animal epithelium consists of bottle cells. Invaginations are not found in these phenotypes, presumably because the concerted apical constrictions produce a tension in the whole epithelium that hampers invagination and sometimes
9 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117± even causes the vegetal epithelium to rupture. In the animal cap bottle cell formation is apparently not restricted to an optimal TGF-b mrna concentration; it rather occurs wherever a certain threshold of mrna is surpassed. As a result a eld and not a ring of bottle cells forms. Only a limited number of bottle cells occurs upon injection of VegT mrna, even at high concentrations. Presumably, the transcription factor VegT acts by inducing TGF-b gene activity (Clements et al., 1999; Yasuo and Lemaire, 1999; Kofron et al., 1999; Hyde and Old, 2000). Hence the TGF-b mrna level could be in this case transcriptionally regulated, in contrast to the situation where the mrna has been injected. Fig. 9A,B interpret the collected data on the expression of the different markers at the ectopic site of bottle cell formation. Activation of the gsc gene also occurs in the centre of the gradient but the gsc expression domain often appears considerably larger than the eld of bottle cell formation. Fig. 7. Effects of cerberus on bottle cell formation. (A) Injection of increasing amounts of cerberus mrna into one side of the embryo leads to dose dependent effects on bottle cell formation and Xbra expression. Bottle cell appearance is more sensitive than Xbra expression to the overexpression of cerberus (b±e). (a) Uninjected control sibling at stage 12. (f) Histological analysis of an injected embryo. The asterisk indicates the injected side. Note the shift of the invagination site into the vegetal hemisphere (arrow). Bar: 200 mm. (B) Coinjection experiments reveal speci c inhibition of Xnr1 induced ectopic lips by cerberus. Uninjected control embryos (co) or embryos microinjected with the indicated amounts of mrnas coding for Xnr1 (nr), Xnr1 1 cerberus (nr 1 cer), activin (ac) or activin 1 cerberus (ac 1 cer) were scored for ectopic bottle cell formation (a±e) and Xbra expression in the animal cap (a 0 ±e 0 ). Ectopic bottle cells and Xbra expression induced by Xnr1 can be completely inhibited by coinjecting cerberus mrna (c,c 0 ), whereas no effect of cerberus coexpression can be seen in activin injected embryos (e,e 0 ).
10 126 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117±131 Table 2 Cerberus (cer) speci cally inhibits Xnr1-induced ectopic bottle cell elds (percentages in parentheses) mrna Normal phenotype I II III IV No. of embryos ( ˆ 100%) No. of experiments Xnr1 (200 pg) ± ± ± ± 19 (100) 19 1 Xnr1 (200 pg) 1 cer (150 pg) 18 (100) ± ± ± ± 18 1 Xnr1 (100 pg) ± 8 (13) 22 (35) 33 (52) ± 63 2 Xnr1 (100 pg) 1 cer (150 pg) 60 (98) 1 (2) ± ± ± 61 2 Activin (100 pg) 6 (5) 4 (3) 121 (92) ± ± Activin (100 pg) 1 cer (75 pg) ± 1 (4) 22 (96) ± ± 23 1 Activin (100 pg) 1 cer (150 pg) 2 (3) ± 67 (97) ± ± 69 3 Activin (100 pg) 1 cer (300 pg) ± 1 (4) 22 (96) ± ± 23 1 How much the constriction of the bottle cell apices contributes to this effect remains unknown. Xbra behaves differently. The ring like expression domain around the injection site indicates that this gene responds to a de ned range of lower concentrations of TGF-b signals. Similar dose dependent responses of gsc and Xbra to TGF-b signals have been observed previously in animal cap explants or in dissociated cells (Green et al., 1992; Gurdon et al., 1994, 1996, 1999; for review see McDowell and Gurdon, 1999). Unfortunately, bottle cell formation of epithelial cells is not mentioned in these reports. Whether this discrepancy is due to the different experimental conditions, which might in uence the behaviour of epithelial cells, remains elusive. Clearly, bottle cell formation is not a direct effect of the activity of these genes as both gsc and Xbra are expressed in subepithelial cells only. The question therefore remains open, whether at the injection site the epithelium itself produces the TGF-b signal and responds to it in an autocrine fashion or, alternatively, bottle cell formation is a response to signals that arise in the subepithelial cells. The latter case would also allow for the possibility that secondary signals other than the TGF-bs may be involved in bottle cell formation. Studies addressing these questions are in progress. Immunostaining for dperk revealed FGF signalling in the whole animal half of stage 10 embryos, but none in the vegetal half. Consistent with the known stimulation of FGF signalling by Xbra (Isaacs et al., 1994; Schulte-Merker and Smith, 1995), we nd strong dperk staining around ectopic bottle cell elds in a region where also Xbra is expressed. Presumably, FGF signalling is an essential prerequisite for the formation of bottle cells. Consistent with this interpretation, both the formation of ectopic bottle cells in the animal cap and those at the blastopore lip are affected by the dominant negative FGF receptor XFD (Amaya et al., 1991, 1993; Lustig et al., 1996; this study). To summarize, ectopic bottle cells form in animal caps if TGF-b signalling exceeds a certain threshold. The subepithelial cells respond to this condition with gsc expression whereas Xbra is activated at lower levels of TGF-b signalling. Further, the formation of ectopic bottle cells is dependent on functional FGF signalling. Thus, ectopic bottle cells form in the animal cap in an environment reminiscent of the situation at the dorsal blastopore lip. The TGF-b signalling molecules activin, BVg1 and Xnr1 induce bottle cells at ectopic sites, which raises the question which of them is the intrinsic inducer of the bottle cells at the blastopore lip. Nodal-related signals are essentially involved in early patterning of different reference organisms such as mouse, zebra sh and frog (for review see Schier and Shen, 2000). Making use of the speci city of the growth factor inhibitor cerberus, we found that Xnr1 induced formation of ectopic bottle cells is inhibited by cerberus whereas activin induced bottle cell formation remains unaffected. This is in accordance with recently published results of Agius and colleagues (Agius et al., 2000), who showed that a truncated form of cerberus (cer-short), which binds speci cally only to nodal signals (Piccolo et al., 1999), inhibits ectopic `lip' formation induced by Xnr1,2,4 but not that induced by activin, BVg1 or derriere. In our study we used the full-length cerberus, which also impairs BMP and Wnt signalling (Piccolo et al., 1999). Nevertheless, similar to cer-short it does not inhibit activin-induced ectopic bottle cells indicating that BMPs and Wnts are not essential for bottle cell formation A model for bottle cell formation at the blastopore lip Fig. 9C,D present the attempt to assemble the information obtained from our experiments on ectopic bottle cell formation into a model of how bottle cells may arise at the blastopore lip during gastrulation. The position of the bottle cells forming at the boundary between the marginal and the vegetal region indicates, by analogy to ectopic bottle cell formation, a zone of high TGF-b signalling. Among the various TGF-b signals that are able to induce bottle cells in animal caps Xnr1, presumably along with its close relatives Xnr2 and Xnr4, are the intrinsic inducers in the marginal region of the non manipulated embryo. The signal originates in the vegetal region, as a result of the activity of VegT, which is localized there and controls TGF-b transcription (Kofron et al., 1999). The decrement of the signal as it passes into the marginal zone would also explain the
11 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117± Fig. 8. FGF signalling is essential for ectopic bottle cell formation. (A) Coexpression of dominant-negative FGF-receptor (XFD) together with activin inhibits formation of ectopic bottle cell elds (a,c) and Xbra-expression domains (b,d) in the animal cap. (B) Western blot of dperk (double phosphorylated active MAPKinase ERK, arrow) in uninjected control embryos and in embryos injected with activin mrna. Whereas no activin induced effect can be seen at stage 8, an activin dependent induction of dperk signal is observed at stage 11. (C) Whole-mount staining of dperk. (a±c) Control embryos: animal view (a), marginal view (b), vegetal±marginal view (c). (d) Embryos injected with activin mrna, animal view. Note the ectopic dperk signal around ectopic bottle cells in the animal cap. position of the gsc and Xbra expression domains relative to the bottle cells. This arrangement of the different domains occurs on the dorsal side only. Here, nodal signalling is initially enforced by the synergy with the wnt/b-catenin signalling pathway resulting in a dorsal to ventral gradient of nodal activity (Kimelman, 1999; Agius et al., 2000). On the lateral and ventral sides gsc is negatively regulated, e.g. by zygotic BMP4 and Vent1 (Fainsod et al., 1994; Gawantka et al., 1995). Apparently this regulation does not affect lateral and ventral bottle cell formation. Bottle cell formation further requires the FGF signalling pathway. The absence of FGF signalling in the vegetal hemisphere possibly prevents the spreading of bottle cell formation into this region. Accordingly, when TGF-b mrna is applied near to the animal/vegetal border, only the animal cells respond by forming bottle cells and transcribing the marker genes gsc and Xbra (unpublished observations). The notion that FGF signalling does not occur in the vegetal half is consistent with the low vegetal expression levels of the FGF-receptor (Cornell et al., 1995), the FGFresponsive transcription factor ER81 (MuÈnchberg and Steinbeisser, 1999) and the activated ERK (Christen and Slack, 1999; this study). However, on the basis of in vitro assays (LaBonne and Whitman, 1997) it is doubtful that
12 128 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117±131 Fig. 9. Summary of the events generating spatially restricted areas of epithelial cells transforming into bottle cells and of subepithelial cells expressing gsc and Xbra. The concentric patterns in the animal cap of embryos overexpressing TGF-b signals (A) can be interpreted as the result of cellular responses to graded growth factor activities (B). (C) The corresponding patterns in the dorsal lip reveal similar spatial relationships of the bottle cell domain and the expression domains of gsc and Xbra. (D) Model for the control of bottle cell formation in the normal embryo. Due to the combined activities of maternal VegT and Wnt/bcatenin signalling a dorsal/ventral and vegetal/animal gradient of zygotic nodal related growth factors patterns the marginal region in cooperation with FGF signals. The temporal and spatial restriction of the epithelial and subepithelial cellular responses to these signals may be accomplished by the inhibitory actions of e.g. Vent, BMP, or cerberus. The outer epithelial cells (indicated in (B) and in (D) on the dorsal side) express neither of the mesodermal markers and the formation of bottle cells is probably not a direct outcome of their activity. Inhibition of the spreading of bottle cell formation into the vegetal region could be due to a lack of FGF signals there or due to the action of an inhibitory vegetal factor X. As a result of these interactions a narrow eld of bottle cells forms rst on the dorsal side and proceeds thereafter to lateral and ventral regions. For details, see text. Abbreviations: An, animal; D, dorsal; V, ventral; Veg, vegetal. FGF signalling is indeed reduced vegetally. The topic is still under dispute. We have observed that the growth factor inhibitor cerberus can induce ectopic invaginations in the vegetal region. This indicates that a lack of FGF signalling may not be the only barrier that prevents bottle cell formation from spreading vegetally. The function of a vegetal inhibitor for bottle cell formation, which itself depends on nodal or Wnt signalling, would be an obvious, though speculative, explanation. The consecutive spreading of bottle cell formation during gastrulation from the dorsal to the lateral and ventral regions could well be explained by the timing of Xnr1-transcription that progresses from dorsal to ventral within the vegetal/ marginal region (Agius et al., 2000). A wave of Xnr1 signalling in the lower marginal zone could well be envisioned to explain the consecutive formation of the bottle cells. Further, Xnr1 signals are known to induce the transcription of cerberus (Piccolo et al., 1999). Indeed, we have found a strong expression of cerberus around Xnr1 induced ectopic bottle cells (unpublished observation). A negative feedback loop might be generated this way, limiting nodal signalling to a certain time window before it becomes obliterated by the inhibitor. Such a mechanism may be important for controlling the timing of bottle cell formation at the precise location of the marginal to vegetal boundary. The tentative model for bottle cell formation proposed here is in many aspects similar to the models for mesoderm formation in the marginal zone forwarded by Cornell et al. (1995) and recently by Agius et al. (2000). Indeed, our data strongly suggest that the processes of both bottle cell and mesoderm formation are closely interlinked. We were not able to decouple bottle cell formation from the mesodermal properties of the adjacent inner cells. Although the nature of the linkage remains unclear, we propose two alternatives. (1) Bottle cells and subepithelial mesodermal cells are induced independently of each other by a gradient of nodal signalling. In this case the epithelial cells themselves need nodal signals. (2) Inner cells respond to the nodal gradient and produce signals which induce the overlying outer epithelial cells to transform into bottle cells. In this case bottle cell induction is downstream of the initial nodal signalling and depends on properly formed mesoderm. Further studies are required to distinguish between these two alternatives. 4. Experimental procedures 4.1. Embryos and microinjections Adult Xenopus laevis were purchased from the African
13 T. Kurth, P. Hausen / Mechanisms of Development 97 (2000) 117± Xenopus Facility C.C. (South Africa). Embryos were obtained by in vitro fertilization as described (Fey and Hausen, 1990), cultured in 0.1 MBSH (MBSH: 88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO 3, 0.82 mm MgSO 4, 0.41 mm CaCl 2, 0.33 mm Ca(NO 3 ) 2, 10 mm HEPES (ph 7.4), 10 mg/ml streptomycin sulphate and penicillin) and staged according to Nieuwkoop and Faber (1967). mrna injections (5 nl per injection) were performed in 2/3 MBSH containing 4% Ficoll-400 (Sigma, Munich, Germany). After injection the embryos were left in Ficoll solution for 2±3 h and then transferred to 0.1 MBSH for further cultivation Expression constructs and mrna synthesis cdna plasmids containing the full-length sequences of activin (pbsk, restriction with EcoRI, transcription with T3-RNA-Polymerase (Thomsen et al., 1990)), Xnr1 (psp64t, restriction with SmaI, transcription with SP6- RNA-Polymerase (Jones et al., 1995)), and cerberus (pcs, restriction with NotI, transcription with SP6-RNA-Polymerase (Bouwmeester et al., 1996)) were kindly provided by Herbert Steinbeisser. BVg1 cdna (Thomsen and Melton, 1993) was kindly provided by Francois Fagotto and Brat cdna (Horb and Thomsen, 1997) by G. Thomsen. Capped sense mrnas for microinjections were prepared with T3 or SP6 mmessage mmachine kits (Ambion) according to the manufacturer's instructions. The RNA was resuspended in sterile TE buffer (1 mm EDTA, 10 mm Tris±HCl, ph 8.0) and was quanti ed by comparing uorescence intensity to a marker with a known concentration on an ethidium bromide stained agarose gel. Aliquots were stored at 2708C and diluted in sterile injection buffer (88 mm NaCl and 15 mm Tris±HCl, ph 7.5 in sterile water) In situ hybridization Whole-mount in situ hybridization was performed according to the protocol of Harland (1991). The proteinase K step was done 30 min for whole embryos and 20 min for half embryos. Double in situ hybridizations were performed with simultaneous incubation of embryos with DIG- and uorescein-labelled antisense probes followed by sequential antibody and colour reactions as described previously (Epstein et al., 1997; Jowett and Lettice, 1994). DIG- and uorescein-labelled antisense mrnas were synthesized using T7 or SP6-RNA-Polymerases. The following plasmids were used as templates for the transcription of antisense mrna: goosecoid (H7 full-length clone in pbsk, restriction with EcoRI, transcription with T7-RNA-Polymerase (Blumberg et al., 1991; Cho et al., 1991)), Xbra (psp73, restriction with SalI, SP6-RNA-Polymerase (Smith et al., 1991)), and cerberus (pbsk, restriction with EcoRI, transcription with T7-RNA-Polymerase (Bouwmeester et al., 1996)). For subsequent histological analysis specimens stored in methanol were embedded in Technovit 7100 and cut as described in Section Histology and immunostaining Embryos were xed in 20% DMSO in methanol overnight at 2208C (Dent et al., 1989) or pre xed in MEMFA for 2 h at room temperature followed by post- xation in Dent's xative or in ice-cold methanol. Specimens were washed in methanol, in ltrated and embedded in glycolmethacrylate (Technovit 7100, Kulzer, Wehrsheim, Germany) and cut into 5-mm thick serial sections. Sections were stained with 0.5% toluidine blue and 1% borax in water. Single or double immuno uorescent labellings were performed as described previously (Kurth et al., 1999) using the following primary antibodies: P14L (polyclonal rabbit antibody against b-catenin (Schneider et al., 1993)), P35N (polyclonal rabbit antibody against a variety of cadherins (Kurth et al., 1999)) and a mouse monoclonal anti-pan-actin antibody (CL9001, Cedarlane; Hornby, Ontario, Canada (Lessard, 1988)). As secondary antibodies Cy3-coupled goat-anti-rabbit IgGs (Dianova) and Alexa 488-coupled goat-anti-mouse IgGs (Molecular Probes) were applied. For double immunostainings, selective lters for Cy3- and Alexa- uorescent signals were used (AF Analysentechnik, Germany). Sections were analysed with a Zeiss Axioplan microscope equipped with epi uorescence optics. Whole-mount staining of the active double phosphorylated form of the MAPKinase ERK was performed as described by Christen and Slack (1999) using the monoclonal anti-dperk antibody (clone MAPK-YT, Sigma) Time lapse videomicroscopy Embryos in 0.1 MBSH were placed into agar forms with embryo sized pits and xed in position. Using a Sony-CCD camera and the Analysis-program (SIS), pictures were taken every 5 min starting from late blastula stages up to midgastrula stages Western blotting Protein extraction was performed as described previously (MuÈller et al., 1994). Proteins were separated in a 12% acrylamide gel and blotted onto a nitrocellulose membrane. Uniformity of loading was assessed by staining the blot with 0.2% Ponceau S (Sigma). Blotted proteins were stained with anti-dperk antibody followed by a peroxidase coupled secondary goat-anti-mouse antibody (Dianova) and were detected on X-ray lms using the Renaissance chemiluminescence detection system (NEN) Electron microscopy Embryos were xed in a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in 100 mm HEPES (ph 7.4) overnight at 48C and washed several times in 100 mm HEPES. For SEM, embryos were post xed in 1% OsO 4 in
Mesoderm Formation. Fate map of early gastrula. Only two types of mesoderm are induced. Mesoderm induction by the vegetal hemisphere
 Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways
 Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Blastula: An early staged embryo that is made up of sphere shaped cells that surround an inner fluid-filled cavity known as the blastocoel.
 1) Define the following terms: cleavage, blastomere, blastula, blastulation, microlecithal, mesolecithal, megalecithal, centrolecithal, isolecithal, telolecithal, holoblastic cleavage, meroblastic cleavage,
1) Define the following terms: cleavage, blastomere, blastula, blastulation, microlecithal, mesolecithal, megalecithal, centrolecithal, isolecithal, telolecithal, holoblastic cleavage, meroblastic cleavage,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12116 Supplementary Figure 1 Theoretical prediction of temperature profiles. a, sketch of sample, immersed into X-ray beam, its environment (agarose and buffer solution are not distinguished),
doi:10.1038/nature12116 Supplementary Figure 1 Theoretical prediction of temperature profiles. a, sketch of sample, immersed into X-ray beam, its environment (agarose and buffer solution are not distinguished),
-RT RT RT -RT XBMPRII
 Base pairs 2000 1650 1000 850 600 500 400 Marker Primer set 1 Primer set 2 -RT RT RT -RT XBMPRII kda 100 75 50 37 25 20 XAC p-xac A 300 B Supplemental figure 1. PCR analysis of BMPRII expression and western
Base pairs 2000 1650 1000 850 600 500 400 Marker Primer set 1 Primer set 2 -RT RT RT -RT XBMPRII kda 100 75 50 37 25 20 XAC p-xac A 300 B Supplemental figure 1. PCR analysis of BMPRII expression and western
Activin-mediated mesoderm induction requires FGF
 Development 120, 453-462 (1994) Printed in Great Britain The Company of Biologists Limited 1994 453 Activin-mediated mesoderm induction requires FGF Robert A. Cornell and David Kimelman* Department of
Development 120, 453-462 (1994) Printed in Great Britain The Company of Biologists Limited 1994 453 Activin-mediated mesoderm induction requires FGF Robert A. Cornell and David Kimelman* Department of
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
efgf, Xcad3 and Hox genes form a molecular pathway that establishes the
 Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Microinjection Techniques: Injecting through the chorion
 Microinjection Techniques: Injecting through the chorion I. Introduction To investigate the role of a gene during development, overexpression or misexpression of your gene of interest is a fast assay.
Microinjection Techniques: Injecting through the chorion I. Introduction To investigate the role of a gene during development, overexpression or misexpression of your gene of interest is a fast assay.
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex
 Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos
 Development 125, 2677-2685 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV3749 2677 Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos Elaine M. Joseph
Development 125, 2677-2685 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV3749 2677 Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos Elaine M. Joseph
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
 Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm
 Development 122, 3055-3065 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV1088 3055 The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser
Development 122, 3055-3065 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV1088 3055 The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and
 SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
Lecture III. Nervous System Embryology
 Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
MimEX TM GI Human Descending Colon Stem Cells
 MimEX TM GI Human Descending Colon Stem Cells Adult Gastrointestinal Stem Cells for Use With MimEX GI Reagents Catalog Number: MIM006 Size: 1 vial, ~ 100,000 cells PRODUCT DESCRIPTION The MimEX GI Tissue
MimEX TM GI Human Descending Colon Stem Cells Adult Gastrointestinal Stem Cells for Use With MimEX GI Reagents Catalog Number: MIM006 Size: 1 vial, ~ 100,000 cells PRODUCT DESCRIPTION The MimEX GI Tissue
Nestin expression during mouse eye and lens development
 Mechanisms of Development 94 (2000) 287±291 www.elsevier.com/locate/modo Gene expression pattern Nestin expression during mouse eye and lens development Jing Yang 1,2, Wei Bian 1, Xiang Gao, Leping Chen,
Mechanisms of Development 94 (2000) 287±291 www.elsevier.com/locate/modo Gene expression pattern Nestin expression during mouse eye and lens development Jing Yang 1,2, Wei Bian 1, Xiang Gao, Leping Chen,
Reading. Lecture III. Nervous System Embryology. Biology. Brain Diseases. September 5, Bio 3411 Lecture III. Nervous System Embryology
 Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Heparanase Modulates Chondrogenic Factor Signaling And Is Upregulated In Ectopic Cartilage
 Heparanase Modulates Chondrogenic Factor Signaling And Is Upregulated In Ectopic Cartilage Julianne Huegel, Motomi Enomoto-Iwamoto, PhD, Federica Sgariglia, Tricia Bhatti, Eiki Koyama, Maurizio Pacifici.
Heparanase Modulates Chondrogenic Factor Signaling And Is Upregulated In Ectopic Cartilage Julianne Huegel, Motomi Enomoto-Iwamoto, PhD, Federica Sgariglia, Tricia Bhatti, Eiki Koyama, Maurizio Pacifici.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mrnas
 Development 118, 499-507 (1993) Printed in Great Britain The Company of Biologists Limited 1993 499 Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mrnas Herbert Steinbeisser
Development 118, 499-507 (1993) Printed in Great Britain The Company of Biologists Limited 1993 499 Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mrnas Herbert Steinbeisser
The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2
 Developmental Biology 248, 40 51 (2002) doi:10.1006/dbio.2002.0705 The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2 Vered Levy, Karen Marom, Sharon Zins, Natalia
Developmental Biology 248, 40 51 (2002) doi:10.1006/dbio.2002.0705 The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2 Vered Levy, Karen Marom, Sharon Zins, Natalia
Neural crest induction in Xenopus: evidence for a two-signal model
 Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
Gα i Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
SUPPLEMENTARY INFORMATION
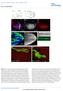 DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
Gα 13 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
For Research Use Only. Not for use in diagnostic procedures. Anti-NRF2 mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. Anti-NRF2 mab CODE No. M200-3 CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 1F2 Mouse IgG1 100 L,
Page 1 For Research Use Only. Not for use in diagnostic procedures. Anti-NRF2 mab CODE No. M200-3 CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 1F2 Mouse IgG1 100 L,
Vegetal rotation, a new gastrulation movement involved in the internalization of the mesoderm and endoderm in Xenopus
 Development 126, 3703-3713 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV6409 3703 Vegetal rotation, a new gastrulation movement involved in the internalization of the mesoderm
Development 126, 3703-3713 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV6409 3703 Vegetal rotation, a new gastrulation movement involved in the internalization of the mesoderm
Why Study Developmental Neurobiology? Terrific scientific challenge.
 Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
supplementary information
 DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
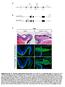 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Solutions for Your Research
 Solutions for Your Research Custom Antibody Services Polyclonal Monoclonal The service we offer is very complete starting from rabbits, mice and rats. The different formats provided by Primm depend on
Solutions for Your Research Custom Antibody Services Polyclonal Monoclonal The service we offer is very complete starting from rabbits, mice and rats. The different formats provided by Primm depend on
Induction and patterning of the telencephalon in Xenopus laevis
 Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Cdc42 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
Supplemental Figure 1 Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical
 Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/βcatenin signaling pathway, but not by Vg1, Activin or Noggin
 Development 124, 453-46 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV9489 453 Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/βcatenin signaling pathway,
Development 124, 453-46 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV9489 453 Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/βcatenin signaling pathway,
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Human/Mouse/Rat Phospho-CREB (S133) Immunoassay. An ELISA-based assay using fluorogenic substrates to measure phosphorylated CREB in whole cells.
 Cell-Based ELISA Human/Mouse/Rat Phospho-CREB (S133) Immunoassay Catalog Number KCB2510 An ELISA-based assay using fluorogenic substrates to measure phosphorylated CREB in whole cells. This package insert
Cell-Based ELISA Human/Mouse/Rat Phospho-CREB (S133) Immunoassay Catalog Number KCB2510 An ELISA-based assay using fluorogenic substrates to measure phosphorylated CREB in whole cells. This package insert
The Dot-Spot Test. a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition. Aurion Newsletter 4
 Aurion Newsletter 4 The Dot-Spot Test a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition Peter F.E.M. van de Plas AURION Costerweg 5 6702 AA Wageningen The
Aurion Newsletter 4 The Dot-Spot Test a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition Peter F.E.M. van de Plas AURION Costerweg 5 6702 AA Wageningen The
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula
 RESEARCH ARTICLE 779 Development 134, 779-788 (27) doi:1.1242/dev.2768 FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula Adnan Mir 1,2, Matt Kofron 1, Aaron M. Zorn
RESEARCH ARTICLE 779 Development 134, 779-788 (27) doi:1.1242/dev.2768 FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula Adnan Mir 1,2, Matt Kofron 1, Aaron M. Zorn
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals
 Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals Hidehiko Inomata, 1,3, * Tatsuo Shibata, 2 Tomoko Haraguchi, 1 and Yoshiki Sasai 1, * 1 Organogenesis
Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals Hidehiko Inomata, 1,3, * Tatsuo Shibata, 2 Tomoko Haraguchi, 1 and Yoshiki Sasai 1, * 1 Organogenesis
Regulation of the Xenopus Xsox17α 1 promoter by co-operating VegT and Sox17 sites
 Developmental Biology 310 (2007) 402 415 www.elsevier.com/developmentalbiology Genomes & Developmental Control Regulation of the Xenopus Xsox17α 1 promoter by co-operating VegT and Sox17 sites Laura Howard,
Developmental Biology 310 (2007) 402 415 www.elsevier.com/developmentalbiology Genomes & Developmental Control Regulation of the Xenopus Xsox17α 1 promoter by co-operating VegT and Sox17 sites Laura Howard,
Nature Medicine doi: /nm.2548
 Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
RNAs were transcribed from described expression conhibit a similar capacity to synergize with neural-inducing
 DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
Supplemental Table 1: Sequences of real time PCR primers. Primers were intronspanning
 Symbol Accession Number Sense-primer (5-3 ) Antisense-primer (5-3 ) T a C ACTB NM_001101.3 CCAGAGGCGTACAGGGATAG CCAACCGCGAGAAGATGA 57 HSD3B2 NM_000198.3 CTTGGACAAGGCCTTCAGAC TCAAGTACAGTCAGCTTGGTCCT 60
Symbol Accession Number Sense-primer (5-3 ) Antisense-primer (5-3 ) T a C ACTB NM_001101.3 CCAGAGGCGTACAGGGATAG CCAACCGCGAGAAGATGA 57 HSD3B2 NM_000198.3 CTTGGACAAGGCCTTCAGAC TCAAGTACAGTCAGCTTGGTCCT 60
Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3
 The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
Supplementary Protocol. sirna transfection methodology and performance
 Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
ab CFSE Fluorescent Cell Labeling Kit
 ab113853 CFSE Fluorescent Cell Labeling Kit Instructions for Use For the durable fluorescent labeling of live cells for fluorescent microscopy and flow cytometry, population growth studies and within sample
ab113853 CFSE Fluorescent Cell Labeling Kit Instructions for Use For the durable fluorescent labeling of live cells for fluorescent microscopy and flow cytometry, population growth studies and within sample
In Situ Hybridization
 In Situ Hybridization Modified from C. Henry, M. Halpern and Thisse labs April 17, 2013 Table of Contents Reagents... 2 AP Buffer... 2 Developing Solution... 2 Hybridization buffer... 2 PBT... 2 PI Buffer
In Situ Hybridization Modified from C. Henry, M. Halpern and Thisse labs April 17, 2013 Table of Contents Reagents... 2 AP Buffer... 2 Developing Solution... 2 Hybridization buffer... 2 PBT... 2 PI Buffer
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
ENDEXT Technology. Instruction manual for protein synthesis. with wheat germ cell-free system
 ENDEXT Technology Instruction manual for protein synthesis with wheat germ cell-free system 1 Protocol Overview Plasmid DNA construction (see Section 3.1) Preparation of plasmid DNA for transcription (see
ENDEXT Technology Instruction manual for protein synthesis with wheat germ cell-free system 1 Protocol Overview Plasmid DNA construction (see Section 3.1) Preparation of plasmid DNA for transcription (see
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Supplementary Figure 1.
 Supplementary Figure 1. Quantification of western blot analysis of fibroblasts (related to Figure 1) (A-F) Quantification of western blot analysis for control and IR-Mut fibroblasts. Data are expressed
Supplementary Figure 1. Quantification of western blot analysis of fibroblasts (related to Figure 1) (A-F) Quantification of western blot analysis for control and IR-Mut fibroblasts. Data are expressed
In-Cell Western Kits I and II
 Odyssey and Aerius Infrared Imaging Systems In-Cell Western Assay Kits I and II Published November, 2006. The most recent version of this protocol is posted at http://biosupport.licor.com/protocols.jsp
Odyssey and Aerius Infrared Imaging Systems In-Cell Western Assay Kits I and II Published November, 2006. The most recent version of this protocol is posted at http://biosupport.licor.com/protocols.jsp
Activin-Induced Factors Maintain goosecoid Transcription through a Paired Homeodomain Binding Site
 DEVELOPMENTAL BIOLOGY 204, 172 186 (1998) ARTICLE NO. DB989065 Activin-Induced Factors Maintain goosecoid Transcription through a Paired Homeodomain Binding Site Roslyn McKendry, Richard M. Harland, 1
DEVELOPMENTAL BIOLOGY 204, 172 186 (1998) ARTICLE NO. DB989065 Activin-Induced Factors Maintain goosecoid Transcription through a Paired Homeodomain Binding Site Roslyn McKendry, Richard M. Harland, 1
Using Sapphire700 Stain and DRAQ5 Stain for Cell Number Normalization
 Using Sapphire700 Stain and DRAQ5 Stain for Cell Number Normalization Developed for: Aerius, Odyssey Classic, Odyssey CLx, and Odyssey Sa Infrared Imaging Systems Please refer to your manual to confirm
Using Sapphire700 Stain and DRAQ5 Stain for Cell Number Normalization Developed for: Aerius, Odyssey Classic, Odyssey CLx, and Odyssey Sa Infrared Imaging Systems Please refer to your manual to confirm
FIGURE S1. Representative images to illustrate RAD51 foci induction in FANCD2 wild-type (wt) and
 Supplementary Figures FIGURE S1. Representative images to illustrate RAD51 foci induction in FANCD2 wild-type (wt) and mutant (mut) cells. Higher magnification is shown in Figure 1A. Arrows indicate cisplatin-induced
Supplementary Figures FIGURE S1. Representative images to illustrate RAD51 foci induction in FANCD2 wild-type (wt) and mutant (mut) cells. Higher magnification is shown in Figure 1A. Arrows indicate cisplatin-induced
Single cells can sense their position in a morphogen gradient
 Development 6, 509-57 (999) Printed in Great Britain The Company of Biologists Limited 999 DEV6 509 Single cells can sense their position in a morphogen gradient J. B. Gurdon*, H. Standley, S. Dyson, K.
Development 6, 509-57 (999) Printed in Great Britain The Company of Biologists Limited 999 DEV6 509 Single cells can sense their position in a morphogen gradient J. B. Gurdon*, H. Standley, S. Dyson, K.
Technical Note Detection of post-immunoprecipitation proteins by Western blot using the Quick Western Kit IRDye 680RD
 Technical Note Detection of post-immunoprecipitation proteins by Western blot using the Quick Western Kit IRDye 680RD Developed for: Aerius, Odyssey Classic, Odyssey CLx and Odyssey Sa Imaging Systems
Technical Note Detection of post-immunoprecipitation proteins by Western blot using the Quick Western Kit IRDye 680RD Developed for: Aerius, Odyssey Classic, Odyssey CLx and Odyssey Sa Imaging Systems
LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS.
 Supplemental Data: LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS. Scott Jepson, Bryan Vought, Christian H.
Supplemental Data: LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS. Scott Jepson, Bryan Vought, Christian H.
Apoptosis assay: Apoptotic cells were identified by Annexin V-Alexa Fluor 488 and Propidium
 Apoptosis assay: Apoptotic cells were identified by Annexin V-Alexa Fluor 488 and Propidium Iodide (Invitrogen, Carlsbad, CA) staining. Briefly, 2x10 5 cells were washed once in cold PBS and resuspended
Apoptosis assay: Apoptotic cells were identified by Annexin V-Alexa Fluor 488 and Propidium Iodide (Invitrogen, Carlsbad, CA) staining. Briefly, 2x10 5 cells were washed once in cold PBS and resuspended
Human IgG ELISpot BASIC
 Human IgG ELISpot BASIC Product Code: 3850-2H CONTENTS: Vial 1 (yellow top) Monoclonal antibodies MT91/145 (1.2 ml) Concentration: 0.5 mg/ml Vial 2 (green top) Biotinylated monoclonal antibodies MT78/145
Human IgG ELISpot BASIC Product Code: 3850-2H CONTENTS: Vial 1 (yellow top) Monoclonal antibodies MT91/145 (1.2 ml) Concentration: 0.5 mg/ml Vial 2 (green top) Biotinylated monoclonal antibodies MT78/145
sides of the aleurone (Al) but it is excluded from the basal endosperm transfer layer
 Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
HPV E6 oncoprotein targets histone methyltransferases for modulating specific. Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu,
 1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
FMF NIRCA PROTOCOL STEP 1.
 FMF NIRCA PROTOCOL STEP 1. After you have isolated patient s DNA and DNA from a healthy donor (wild type), you perform a nested PCR. The primers used to amplify exon 2 and exon 10 of the mefv gene are
FMF NIRCA PROTOCOL STEP 1. After you have isolated patient s DNA and DNA from a healthy donor (wild type), you perform a nested PCR. The primers used to amplify exon 2 and exon 10 of the mefv gene are
Anti-HB-EGF (Human) mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Mouse IgM ELISpot BASIC
 Mouse IgM ELISpot BASIC Product Code: 3885-2H CONTENTS: Vial 1 (green top) Monoclonal antibody MT6A3 (1.2 ml) Concentration: 0.5 mg/ml Vial 2 (yellow top) Biotinylated monoclonal antibody MT9A2 (100 μl)
Mouse IgM ELISpot BASIC Product Code: 3885-2H CONTENTS: Vial 1 (green top) Monoclonal antibody MT6A3 (1.2 ml) Concentration: 0.5 mg/ml Vial 2 (yellow top) Biotinylated monoclonal antibody MT9A2 (100 μl)
IsoFluxTM. System. The next generation of CTC Analysis is here
 IsoFluxTM System The next generation of CTC Analysis is here Product Overview IsoFlux System The next generation of circulating tumor cell analysis is here The IsoFlux System enriches intact rare cells
IsoFluxTM System The next generation of CTC Analysis is here Product Overview IsoFlux System The next generation of circulating tumor cell analysis is here The IsoFlux System enriches intact rare cells
Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna
 Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
Human CEACAM6 ELISA Pair Set
 Human CEACAM6 ELISA Pair Set ( CD66c ) Catalog Number : SEK10823 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human CEACAM6 ELISA Pair Set ( CD66c ) Catalog Number : SEK10823 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Neural Induction. Steven McLoon Department of Neuroscience University of Minnesota
 Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
