A thesis presented to the faculty of the Voinovich School of Leadership & Public Affairs
|
|
|
- Raymond Cain
- 5 years ago
- Views:
Transcription
1 The Recovery of an AMD-impacted Stream Treated by Steel Slag Leach Beds: a Case Study in the East Branch of Raccoon Creek, Ohio A thesis presented to the faculty of the Voinovich School of Leadership & Public Affairs In partial fulfillment of the requirements for the degree Master of Science Caleb M. Hawkins May Caleb M. Hawkins. All Rights Reserved.
2 2 This thesis titled The Recovery of an AMD-impacted Stream Treated by Steel Slag Leach Beds: a Case Study in the East Branch of Raccoon Creek, Ohio by CALEB M. HAWKINS has been approved for the Program of Environmental Studies and the Voinovich School of Leadership & Public Affairs by Natalie A. Kruse-Daniels Professor of Environmental Studies Mark Weinberg Director, Voinovich School of Leadership & Public Affairs
3 3 ABSTRACT HAWKINS, CALEB M., M.S., May 215, Environmental Studies The Recovery of an AMD-impacted Stream Treated by Steel Slag Leach Beds: A Case Study in the East Branch of Raccoon Creek, Ohio Director of Thesis: Natalie A. Kruse Coal mining is disruptive to ecosystems and causes both chemical and physical changes in the local environment that alter water chemistry, habitat quality, and aquatic biota. The East Branch of Raccoon Creek, Ohio is highly impacted by preregulation coal mining and contains 1 steel slag leach beds that passively treat low ph, Fe and Al-rich waters. Water chemistry, sediment chemistry, aquatic macroinvertebrate richness and diversity, habitat quality, and stream gradient were examined along the mainstem and a headwater tributary. Gradient showed no significant relationship with sediment quality. Higher flows increased instream concentrations of Fe and increased the portion of suspended Fe in the water column. Macroinvertebrate richness and diversity varies from good to very poor and is negatively correlated with sediment As, Cu, Fe and Mn, and multiple water quality parameters such as specific conductivity (µs/cm), ph, and TDS (mg/l).
4 4 DEDICATION This thesis is dedicated to my grandfather, who nurtured my appreciation for rivers and the places they lead.
5 5 ACKNOWLEDGMENTS This thesis and field research would not have been possible without a generous gift from the American Electric Power Watershed Restoration Program. I am indebted to my advisor, Dr. Natalie Kruse-Daniels, who was instrumental in bringing me to Ohio University and crucial to my development as a scientist. I would also like to thank Drs. Dina Lopez and Kelly Johnson for their service on my committee, and their dedication to better understand aquatic ecosystems and environmental restoration. Many staff from the Voinovich School, the Appalachian Watershed Research Group, and the Raccoon Creek Partnership were helpful and essential to my success. Specifically, I would like to thank Jen Bowman, Steve Porter, Amy Mackey, Sarah Landers, and volunteers who assisted me with field work. Lastly, I would like to thank my family and friends for supporting me in this endeavor.
6 TABLE OF CONTENTS Abstract... 3 Dedication... 4 Acknowledgments... 5 List of Tables... 7 List of Figures... 8 Chapter 1: Introduction... 9 Chapter 2: Methods and Study Area Page 2.1 Site Description Climate, Landuse, Soils, Geology, and Streamflow Acid Mine Drainage Impacts Chapter 3: Results and Discussion Field Parameters Aqueous Geochemistry Varying Flow, Instream Fe Profiles and Chemical Alkalinity Loads Macroinvertebrate Assessments MAIS Scores and Water and Sediment Chemistry Interactions Identifying Zones of Recovery Chapter 4: Conclusions Changes in Water Chemistry Depending on Flow Chemical and Physical Parameters that Influence Recovery Gradient, Velocity, and Grain Size Limitations Recovery in East Branch References... 6 Appendix 1 Field Parameters Appendix 2 Water Quality... 7 Appendix 3: June Loadings Along Mainstem Appendix 4: August Chemical Loadings Along MAinstem in mg/l and TDS/TSS Loads for June, August, and January Appendix 5: Excerpt from ODNR Document Stating the Maxiumum Limit for As in Leachate from Steel Slag Appendix 6: List of Macroinvertebrate Taxa found in East Branch in 213 and
7 7 LIST OF TABLES Page Table 1: Samples Sites and Description... 2 Table 2: Historic Biological Survey Sites and Scores Table 3: Summary Statistic from East Branch Sites Prior to Study Table 4: Summary Statistics from Water Quality Samples: Aug 213 to Feb Table 5: Summary Statistics of Sediment Chemistry Analytes Table 6: Sediment Metal Concentrations Table 7: Spearman Rank Correlation Matrix of Sediment Metals Table 8: Sediment Quality Guidelines Table 9: Gradient Measurements and Grain Size Table 1: MAIS and QHEI Scores from 213 and Table 11: Grain size, MAIS, and Sediment Chemistry Correlations Table 12: June Water Quality and MAIS Correlation Significance Table 13: August Water Quality and MAIS Correlation Significance Table 14: January Water Quality and MAIS Correlation Significance... 57
8 8 LIST OF FIGURES Page Figure 1: Location Map of East Branch, Raccoon Creek Figure 2: Study Area Site Map Figure 3: Schematic of East Branch Figure 4: Historic Acid Loads in East Branch Figure 5: Author with Pygmy Meter at EB Figure 6: Stream Bottom Precipitate at EB199.1 and EB Figure 7: Al, Fe, and Mn Concentrations in Mainstem during Three Sampling Events... 3 Figure 8: Al, Fe, and Mn Concentration in Laurel Run during Three Sampling Events Figure 9: Ca and SO 4 in Mainstem during Three Sampling Events Figure 1: Ca and SO 4 in Laurel Run during Three Sampling Events Figure 11: Mg, Na, and Total Alkalinity in Mainstem Figure 12: Mg, Na, and Total Alkalinity in Laurel Run Figure 13: Instream Fe Concentrations in June and August Figure 14: June Alkalinity, Fe, and Al Loadings and Delta Loadings Figure 15: August Alkalinity, Fe, and Al Loadings and Delta Loadings... 4 Figure 16: Fe. Ni, and Zn Compared to Grain Size by River Kilometer Figure 17: Grain size and Gradient by River Kilometer Figure 18: MAIS Scores by Year... 5
9 9 CHAPTER 1: INTRODUCTION Coal mining holds an important socioeconomic and environmental legacy in Central Appalachia and many other parts of the world. Coal continues to provide about 4% of global electricity production and will continue to be an important resource for years to come. The process of coal mining is disruptive to ecosystems and incurs both chemical and physical changes in the local environment that alter water and habitat quality in receiving water bodies. Many deleterious environmental effects can be attributed to physical and chemical changes at mining sites. Mine waters may contain elevated levels of TSS (total suspended solids), TDS (total dissolved solids), hardness, sulfate, nitrates, and metals (Tiwary, 21). The environmental impacts of mining vary depending on the scale of mining operations and the geochemistry of the overburden or source rock. This thesis focuses on the reclamation and restoration of the East Branch of Raccoon Creek, Ohio. East Branch was highly impacted by preregulation coal mining and is undergoing restoration by steel slag leach beds. This study looks at the water chemistry, sediment chemistry, gradient, and macroinvertebrate richness and diversity within East Branch. 1.1 Generation of Acid Mine Drainage Acidic and sulfur-rich waters are sometimes produced by industrial effluent, but they are predominantly created by operations in the mining industry (Johnson & Hallberg, 25). Both underground and surface mining increase the surface area of rock strata and sulfide minerals, which then come into contact with air and water. In coal-producing regions, as in southeastern Ohio, the dominant sulfide mineral is typically pyrite (Skousen et al., 2). However, sulfide minerals and mineral complexes can vary. Lead, copper, and zinc mines contain sulfide mineral complexes such as sphaelerite, chalcopyrite, and galena that contribute to the generation of AMD (Skousen et al., 2). Acid mine drainage (AMD) is produced when air and water come in to contact with sulfide minerals, creating a series of oxidizing reactions that produce acidity. These reactions, which are catalyzed by the acidophilic, sulfide-oxidizing bacteria, Thiobacillus ferrooxidans (Younger et al., 22), may be described by the following equations: 2 FeS O H 2O 2 Fe SO H+ 4 Fe 2+ + O H+ 4 Fe H 2O 4 Fe H 2O 4 Fe(OH) H+ FeS Fe H 2O 15 Fe SO H+ The acidity produced by these reactions lowers the ph in receiving watersheds and affects aquatic biota and ecological processes. AMD waters may have low ph, high specific conductivity, high
10 1 sulfate, and elevated dissolved metal (Al, Cu, Zn, Mn, Pb) and metalloid (As, Se) concentrations (Lottermoser, 21). These metals impair the biological functions of benthic macroinvertebrates and fish (Jennings et al., 28; MacCausland & McTammany, 27). Many watersheds lack enough natural alkalinity to buffer AMD, thus, acidity persists in the water column. 1.2 Acid Mine Drainage Treatment Many technologies have been developed to treat mine water and watersheds impacted by AMD. Treatments incorporate chemicals and processes that restore water to net alkaline conditions and low metal concentrations. AMD treatments generally fall into two classifications: active and passive. In active treatments, a chemical agent is physically added to acid mine drainage (Fripp et al., 2). Some of the chemical compounds involved in active AMD treatments include limestone, hydrated lime, soda ash, caustic soda, pebble quicklime, and ammonia (Skousen et al., 2). Passive treatments rely on naturally occurring chemical and physical processes to treat AMD (Skousen et al., 2). Passive AMD treatments may utilize aerobic wetlands, anaerobic wetlands, open limestone channels, anoxic limestone drains, successive alkalinity producing systems, sulfate-reducing bioreactors, permeable reactive barriers, and passive alkaline addition (Doshi, 26). In the Appalachian region of Ohio, passive alkalinity addition treatment systems consist of hydro-powered lime dosers, successive alkalinity producing systems (SAPS), and steel slag leach beds (SSLBs). These systems use calcium carbonate, calcium oxides, or calcium hydroxides to neutralize acidity, or organic matter to remove sulfate from AMD. The basic chemical reaction for neutralization using calcium oxide is shown in the following equations: CaO + H 2O Ca(OH) 2 2Fe H+ + 4Ca(OH) 2 2Fe(OH)3 + 4Ca H 2O Although these techniques increase the lotic system s buffering capacity, they are expensive and sometimes susceptible to failure. 1.3 Ecological Recovery: Influence of Chemical and Physical Stressors Research on AMD and its effects on biota and ecological processes has been conducted extensively in the United States and throughout the world. Research in the Appalachian region of the of the United States has been conducted in Pennsylvania (MacCausland & McTammany, 27), Virginia (Cherry et al., 21), Ohio (Dsa et al., 28; Johnson et al., 214; Kruse et al., 213; Verb & Vis, 2), and West Virginia (McClurg et al., 27). Physical stressors (the
11 11 deposition of metal oxides, sedimentation) and chemical stressors (low ph, high concentrations of dissolved metals) affect aquatic ecology at multiple trophic and functional levels. Numerous studies indicate that increased ph and alkalinity do not necessarily create an environment that is suitable for full biological recovery (Cravotta & Bilger, 21; Hogsden & Harding, 211; McClurg et al., 27; Yoder & Rankin, 1996). Functional measures of ecosystem processes such as leaf litter breakdown, microbial respiration, and nutrient spiraling are impaired and do not necessarily match gradients of restored water chemistry (Hogsden & Harding, 211;Bott et al., 212; Johnson et al., 214). Furthermore, acid mine drainage impacted ecosystems respond differently to chemical and physical stresses (Niyogi et al., 22). Benthic macroinvertebrates are good indicators of stream health because they respond to anthropogenic changes in water quality and habitat (Kenney et al., 29), and benthic macroinvertebrates are relatively sedentary and depict local conditions (Barbour et al.,1999). Though it is agreed that benthic marcoinvertebrates are good indicators of general stream health, there is some debate about the exact mechanisms of toxicology, and the role that toxicology plays with habitat quality and abundance. Numerous studies have found that pore-water and water column metal concentrations are more indicative of benthic macroinvertebrate populations than sediment metal concentrations (Beltman et al., 1999; Burton, 1991; Chapman et al., 1998; Kemble et al., 1994). 1.4 Sediment Deposition and Topography: The Fate of Precipitated Metals Even at circumneutral ph, stream geomorphology may influence biological recovery as sediments and precipitates settle, erode, and interact with water chemistry. The addition of alkalinity restores water chemistry to circumneutral ph, but it also leads to large amounts of precipitates in the form of minerals and colloids (Kruse et al., 213). The precipitation of metal and unreacted alkaline material adds to the suspended solid load of the stream and increases turbidity. Although the precipitation of metal sulfides, Mn-oxides, and Fe-hydroxides can cause toxic metals such as Cu, Cd, Pb, and Zn to sorb to bonding sites, trace metals can remain in solution even at circumneutral ph if an insufficient amount of sorbent is formed (Lee et al., 22; Lynch et al., 214). Furthermore, the locations of minerals within the stream channel may influence the concentration of metals in the water column. For example, minerals within a stream riffle are exposed to more dissolved oxygen. An increase in dissolved oxygen can oxidize minerals and create low-ph conditions that remobilize trace metals (Hudson-Edwards, 23).
12 12 This suggests that interactions between sediment and aqueous chemistry change as the physical position of the sediment changes. Recovery within impacted watersheds relies on constant interactions between chemical and physical factors that are punctuated by episodic flushes of acidity and high energy events. Biota are affected by periodic and chronic stressors. With this in mind, it is important to consider both physical and chemical parameters of streams undergoing AMD-remediation. A better understanding of the interplay between chemical and physical factors that influence biological recovery will maximize the efficacy of restoration. This study examines the relationships between aqueous chemistry, sediment chemistry, topography, stream velocity, habitat, and macroinvertebrates. Metals can be remobilized by changes in physical and chemical conditions, therefore sources of alkalinity and the gradient must be considered in remediation (Kruse et al., 213). Considering that treatment in East Branch is taking place within the stream channel, the physical and chemical factors, such as gradient, topography, and ph may influence sediment and metal deposition. 1.5 Identification of Impaired Zones Alkalinity additions from both treatment and natural sources, as well as stream velocity, determine the length of the impaired zones in streams undergoing treatment with lime dosers (Kruse et al., 213). This is important to consider for treatment options, particularly in economically depressed regions and areas with limited space, where expensive sediment ponds cannot be constructed and substrate removal cannot be performed (Kruse et al., 213). Biological recovery may be inhibited further if a stream reach is not long enough to create an impaired or sacrifice zone. 1.6 Objectives The objectives of this study are: To characterize the linear geochemical data downstream of steel slag leach beds in lowflow and high-flow conditions To examine the influence that gradient and velocity have on sediment deposition, sediment metal concentration and partitioning, and aqueous chemical parameters To compare chemical data with macroinvertebrate scores (MAIS) in order to see relationships between chemical parameters and biological recovery
13 13 To identify if zones of recovery exist within the East Branch subwatershed
14 14 CHAPTER 2: METHODS AND STUDY AREA 2.1 Site Description The East Branch subwatershed of Raccoon Creek has a drainage area of km 2. East Branch is located in Vinton and Hocking counties (Figures 1-3). Prior to the Surface Mining Control and Reclamation Act (SMCRA) of 1977, a total of 62 surface mining permits were issued within the subwatershed (Laverty, 25). Local geology in the most northern portion of Raccoon Creek Watershed consist of sandstones, shales, and carbonates from the Pottsville and Allegheny formations (Hindall, 1984). Within East Branch, the most frequently mined coals were the Lower Kittaning (No. 5) and Middle Kittaning (No. 6) coal seams (Hindall, 1984). Mining activities left highwalls, mine spoils, last cut lakes, and coal fragments exposed throughout the subwatershed (Laverty, 25). Figure 1: Location of East Branch Subwatershed within Raccoon Creek Watershed
15 15 Figure 2: Water quality sites, MAIS sites, and SSLB discharges. Note three main tributaries to the East Branch Mainstem: Laurel Run, EB12 (Coonville), and EB16 (Starr). Steel slag leach bed construction occurred in three phases from Treatment sites are denoted by phase and have a narrative name. 2.2 Climate, Landuse, Soils, Geology, and Streamflow The East Branch Subwatershed in approximately 7% forested and <1% of the area is covered by water and wetlands. East Branch is 12.4 km long and mean annual precipitations at the basin centroid is 11 cm (USGS, 212). The soils within the study area are well-drained, belong to the Shelocta-Brownsville-Latham-Steinsburg series (United States Department of Agriculture, n.d.), and are derived from sandstone and shale (Hindall, 1984). Mean annual streamflow at the mouth of East Branch is 611 L/s (USGS, 212). The bedrock of Raccoon Creek consists of alternating beds of sandstone, shale, clay, coal, and limestone that are Pennsylvanian in age (Hindall, 1984). The Allegheny formation, which consists of sandstone and shale and minor amount of limestone, is the predominate formation in the central headwaters of Raccoon
16 16 Creek (Rice et al., 22). The Allegheny formation contains thirteen coal layers. The middle Kittanning (No.6) coal seam was the seam that was most often mined in East Branch headwaters (Rice et al., 22) Figure 3: Schematic of East Branch showing tributaries and treatment systems. 2.3 Acid Mine Drainage Impacts AMD impacts in East Branch come predominantly from three main tributaries and extensive surface mining in the upper reaches of the mainstem. EB12 (Coonville Tributary), EB16 (Starr Tributary), EB19 (Laurel Run Road Tributary), and the area above EB21 were severely impaired (Figure 2). AMD is generated from partially reclaimed mines on the hilltops (1-2 ft. above the stream valley) along the Laurel Run tributary and mainstem along Sanner Road (Laverty, 25). There are 5 AMD-impacted streams that discharge into the Laurel Run mainstem and 7-AMD impacted streams that discharge into the mainstem above EB21 Sanner road (Laverty, 25). The resulting AMD from these tributaries made East Branch the largest acid contributor to Raccoon Creek prior to reclamation (The Voinovich School, 212). Subsequent projects, however, have reduced metal loads and restored the mouth of East Branch to circumneutral ph. Reclamation projects in East Branch headwaters were funded by a United States Environmental Protection Agency EPA 319 grant and completed by the Raccoon Creek Partnership (RCP), the Ohio Environmental Protection Agency (OEPA), and the Ohio
17 17 Department of Natural Resources Division of Mineral Resources Management (ODNR-DMRM). Reclamation includes eleven steel slag leach beds that were constructed from 27 to 29 at a cost of more than $2,, (Kruse et al., 212). The principal goal of these was to reduce impacts on the mainstem of Raccoon creek. Projects were implemented on two tributaries and in the headwaters of the mainstem in East Branch. East Branch Phase I Reclamation Project was completed in December 28 and included gob pile reclamation, the construction of two settling ponds, and 6 steel slag leach beds (Starr, Poling, Cabin, Stallion, and Yost) (Figure 2). One steel slag leach bed at the Yost site is not functioning. Phase II and III reclamation were completed in 21 and 211, and included the construction of 5 steel slag leach beds (Northwoods, Forest, Kern Hollow, and Winnifred) (Figure 2). Chemical and physical data have been collected at lower river miles in East Branch since 1979 by the United States Geological Survey (USGS), ODNR, and RCP. The acid loads at the mouth, shown in Figure 4, have decreased markedly since The majority of chemical sampling events occurred for post-reclamation construction monitoring after 28. Biological (fish and macroinvertebrate) data have been collected in East Branch since 25, with the addition of one Ohio Environmental Protection Agency sampling event in 1995 (Table 2). Samples sites in this study are referenced by site name and river kilometer (Figure 2 and Table 1). 2.4 Water Quality Water samples from mainstem and tributary locations were taken downstream and between steel slag bed discharges in base-flow and high-flow conditions. Samples were taken in August 213, June 214, August 214, and January 215. Filtered and non-filtered samples were collected in 25 ml plastic bottles and preserved with 5 ml of 2% nitric acid for cation analysis. Another set of non-filtered samples were collected in 1 liter flexible cubitainers and evacuated of air. Non-preserved samples were kept at <4 degrees Celsius and analyzed for acidity, alkalinity, ph, total dissolved solids, and anions. Water samples were taken in collaboration with the Raccoon Creek Partnership and then sent to the Ohio Department of Natural Resources Division of Mineral Resources Management Cambridge Analytical Laboratory using Perken Elmer Optima 2 Inductively Coupled Plasma, a Dionex ICS-2 Ion Chromatography system, and a Brinkmann Automated Titration system. Field parameters such as ORP (mv), specific conductivity (µs/cm), TDS (ppm), ph, and dissolved oxygen (mg/l) were taken with a Myron Ultrameter 6p or YSI 6 XLM multiprobe datasonde that were calibrated each sampling day.
18 Jan-78 Oct-8 Jul-83 Mar-86 Dec-88 Sep-91 Acidity Loading (kg/day) Jun-94 Mar-97 Dec-99 Sep-2 May-5 Feb-8 Nov-1 Aug-13 Figure 4: Acid loads (kg/day) at the mouth of East Branch (EB1, RM.1) from 1979 to 213. Laverty (25) suggests that reductions in acid load from 1979 to 25 may indicate a decrease in the reactivity of mining wastes. Sampling for alkalinity and acidity loads took place in August 213, May 214, and September 214. Water quality sites and alkalinity load sites are listed in Table 1. Samples that were not analyzed in the field were preserved at 4 degrees Celsius and examined within 24 hours of sampling. Acidity titrations were conducted using Hach Method 822, and alkalinity titrations were conducted using Hach Method 823 (Hach Company, 23). Alkalinity loads were calculated using the concentrations of alkalinity and acidity, and the discharge: 1 1 Adapted from (Korenowsky, 212)
19 Net alkalinity ( mg s ) = alkalinity (mg) acidity (mg L L ) Q L s = Q (cfs) x Net Alkalinity Load ( mg ) = Net alkalinity (mg) x Q l/s s L Net Alkalinity Load ( kg ) = Net Alkalinity Load (mg) x.864 day s 2.5 Discharge Discharge was measured in cubic feet per second at the same sites as water chemistry and alkalinity using a Pygmy meter or Baski Cutthroat Flume (Buchanan & Somers, 1969) (Figure 5). When needed, absent discharge measurements were calculated using regressions of measured flow vs drainage area. In larger streams channels, the Pygmy meter was used at a minimum of 16 locations within the stream profile with the meter at 6% below the surface of the water. Where discharge was low or the stream channel was narrow, a Baski Cutthroat flume (4 or 8 ) was used to measure discharge. Flumes were allowed to stabilize for approximately five minutes and readings were taken. Velocity was calculated from June 214 discharge measurements using a weighted mean approach. 19
20 2 Table 1: Sample sites and descriptions Site ID Description River Kilometer Drainage Area MSBC1 EB and WB Confluence 178 (Raccoon Creek) km 2 WB1 Mouth of West Branch km 2 EB1 Mouth of East Branch km 2 EB47 Tick Ridge Rd km 2 EB8 Dexter km 2 EB13 EB Mainstem at Coonville km 2 EB15 East of SR56 Mainstem km 2 EB17 West of SR56 Mainstem km 2 EB21 Mainstem EB km 2 EB211 Downstream of Poling SSLB km 2 EB212 Downstream of Yost SSLB km 2 EB25 Downstream of Cabin SSLB km 2 EB27 Upstream of Treatment km 2 EB16 Starr Tributary km 2 EB196 Laurel Run Mainstem Downstream km 2 EB199.1 Laurel Run Mainstem Downstream km 2 EB194 Laurel Run Mainstem Downstream km 2 EB197 Laurel Run Mainstem Downstream km 2 EB199 Laurel Run Mainstem Upstream of Treatments km 2
21 21 Figure 5: Author measures discharge with Pygmy meter at EB13 during the January chemical sampling event. Ambient air temperature was well below freezing and runoff from snowmelt was not entering the creek. 2.6 Sediment Chemistry and Grain Size Sediments were sampled in August 214 with a scoop from soft sediment at each water quality sample site. Samples were taken from the top 3 cm of material on the stream bottom in pools. Sediment sampling was conducted in accordance with the Ohio EPA sediment sampling guide and USEPA SOP #216 (USEPA, 1994 and OEPA, 21). Three locations within the stream profile were taken and mixed to create the sample. Samples were placed into plastic, sealable bags and transported below 4 degrees Celsius. The trowel used for sampling was
22 decontaminated using non-phosphate soap, acetone, and deionized water between sampling sites. Sediments were stored in a refrigerator below 4 degrees Celsius and were then dried in an oven at approximately 95 degrees C. A subset of sediment samples were sieved for grain size analysis using USGS No. 4, 1, 18, 35, 6, and 14 sieves. Percent mass was calculated for each grain size using these formulas: Fraction i = Measured mass (g) Total Mass (g) 22 Fraction Finer = 1 Fraction i (Finer) Percent Finer by Mass = Fraction Finer X 1 Average grain size was determined using the following formula 2 : X i = (Grain size SizeA + Grain size SizeB )/2 X = X i f i Sediments reserved for digestions were homogenized and sieved to a diameter of < 2 mm. Samples were digested in the Geological Sciences Geochemistry Laboratory using repeated additions of HNO 3 and HCl as suggested by EPA method 35B (USEPA, 1995). Sediments were analyzed at the Ohio University Institute for Sustainable Energy and the Environment (ISEE) using ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy). Analytes include Al, As, Ca, Fe, Mg, Na, K, Mn, Zn, Cd, Ni, Co, Cu, Cr, and Pb. Concentration in leachate was converted to concentration in sediment using the formula below: mg solute l x.5 l.5 g x 13 g 1 kg = mg solute kg sediment 2.7 Biological Sampling The Macroinvertebrate Aggregated Index for Streams (MAIS) was used to evaluate the richness and diversity of insect taxa at the family-level (Smith & Voshell, 1997). Three kick net samples and 2 dip-net samples were conducted within riffles in a 15 m stream reach at 5 sites: EB1, EB47, EB8, EB15, and EB17. Samples were taken between June 15 and September 15 in both 213 and 214. Identification of insects was conducted by Raccoon Creek Watershed 2 Adapted from (Kruse et al., 213)
23 23 Coordinator, Amy Mackey. MAIS samples were processed by ODNR in accordance with the adapted metrics for the Western Allegheny Plateau. Historic bioassessments are shown in Table Habitat The Qualitative Habitat Evaluation Index (QHEI) is a method used to evaluate physical characteristics and in-stream morphology in streams with drainage areas of >1 mi 2 (Ohio Environmental Protection Agency, 26). QHEI is typically used with the Index of Biotic Integrity (IBI) as a method to evaluate habitat in stream reaches where fish shocking has been performed. The QHEI is performed by a crew leader who thoroughly analyzes stream characteristics over a 2m reach. There are six indicators for this assessment: substrate type and quality (2pts), instream cover (2pts), channel morphology (2pts), bank erosion and riparian zone (1pts), pool and riffle quality (2pts), and gradient (1pts). The QHEI has a maximum score of 1 points. The target QHEI score for Warmwater Habitat is 6 points (Rankin, 1989). The author conducted QHEI assessments at all MAIS sites in 213 and the OEPA conducted QHEI assessments at EB1 and EB47 in Slope A leaf-off survey was used to determine slope between thalwegs in riffles at water quality sites. An optical total station (surveying equipment used for building construction) was used to identify the elevations of riffle thalwegs during the winter in order to utilize increased visibility while leaves were down. Longitudinal distance exceeded a minimum of 6 times the channel width in order to accurately measure a representative gradient (Pers. Comm. Dr. Gregory Springer). At least one gradient measurement was taken at each water quality site. Average gradient was determined for sites with multiple measurements. Detailed methods for measuring stream slope with survey equipment have been published by the USGS (Fitzpatrick et al., 1998). 2.1 Statistical Analysis All statistical analysis was performed using Excel (Microsoft, 21), SPSS Version 21 (IBM SPSS Statistics for Windows, 212), or RStudio (RStudio, 215). Descriptive statistics were generated for water quality parameters at every site. Sediment metal concentrations and aqueous metal concentrations were compared to MAIS values using Spearman rank correlations. RStudio was used to run Kruskal Wallis tests.
24 24 Table 2: Historic biological survey sites and scores since Indices included in this tables include Invertebrate Community Index, the Index for Biotic Integrity, and the Macroinvertebrate Aggregated Index for Streams. Site Year Metric Score Narrative Evaluation HABITAT EB ICI 1 Poor * EB1 25 ICI 1 Poor * EB1 26 MAIS 8 Poor * EB1 27 MAIS 12 Good * EB1 28 MAIS 6 Very Poor * EB1 29 MAIS 12 Good * EB1 21 MAIS 11 Poor * EB1 21 IBI 28 Scale: EB1 211 MAIS 9 Poor * EB1 212 MAIS 13 Good * EB1 213 MAIS 1 Poor * Site Year Metric Score Narrative Score HABITAT EB47 21 MAIS 5 Very Poor * EB47 22 MAIS 3 Very Poor * EB47 22 MAIS Very Poor * EB47 26 IBI 24 Scale: EB47 29 MAIS 11 Poor * EB47 21 IBI 28 Scale: EB47 21 MAIS 13 Good * EB IBI 28 Scale: EB MAIS 12 Good * EB MAIS 15 Good * * Habitat evaluation was not performed
25 25 CHAPTER 3: RESULTS AND DISCUSSION 3.1 Field Parameters Field parameters were observed eight distinct times from August 213 to February 215 and include oxidation-reduction potential (ORP), temperature (Celsius), specific conductivity (µs/cm), ph, discharge (L/s), and dissolved oxygen (mg/l). Complete field parameter and water chemistry data are shown in Appendix 1. Water temperature varies throughout the sample days as ambient air temperature typically rises through the day. Table 3 shows the summary statistics for 31 parameters for historical data that was recorded in East Branch prior to this project. Laboratory ph values range from 2.58 to 13.3, as sample locations vary from diffuse AMD sources to alkaline treatment sources. Table 4 contains summary statistics of parameters measured during the study period. Note that the ph range is less than historic data summary statistics. Field ph values range from 4.34 to All field parameters from water quality sampling events are shown in Appendix 1. Field ph at the two most downstream sites (EB1 and EB47) is circumneutral in all events. Specific conductivity is very high (typically higher than 1 µs/cm) in the Laurel Run tributary (EB19, EB196, EB194, EB199.1, EB197 and EB199). In the Laurel Run tributary conductivity is high and drops slightly while going downstream, until it reaches its lowest value at EB19, just before the confluence with the mainstem. Specific conductivity in the mainstem behaves differently, in that conductivity values in the headwaters (EB27, EB25, EB212, EB211, and EB21) rise from 7 µs/cm to over 1 µs/cm and do not drop until RKM 5.15 at EB8.
26 26 Table 3: Summary Statistics of All East Branch Sample Sites from 1996 to 213; prior to study Unit N min max median mean SD Temperature Celsius Q L/s Dissolved O 2 mg/l Dissolved O 2 percentage ORP mv ph lab SI ph field SI SC lab µs/cm SC field µs/cm Acidity lab mg/l Acidity field mg/l Alkalinity lab mg/l TDS mg/l TSS mg/l Sulfate mg/l Cl Total mg/l Ca Total mg/l Dissolved Ca mg/l Mg Total mg/l Dissolved Mg mg/l Na Total mg/l Dissolved Na mg/l K Total mg/l Dissolved K mg/l Fe Total mg/l Dissovled Fe mg/l Mn Total mg/l Dissolved Mn mg/l Al Total mg/l Dissolved Al mg/l Hardness mg/l
27 27 Table 4: Summary Statistics of Water Quality Samples Taken from Aug 213 to Feb 215 N min max median mean SD Temperature Celsius Q L/s Dissolved O 2 mg/l Dissolved O 2 percentage ORP mv ph lab SI ph field SI SC lab µs/cm SC field µs/cm acidity lab mg/l alkalinity lab mg/l TDS mg/l TSS mg/l Sulfate mg/l Cl Total mg/l Ca Total mg/l Dissolved Ca mg/l Mg Total mg/l Dissolved Mg mg/l Na Total mg/l Dissolved Na mg/l K Total mg/l Dissolved K mg/l Fe Total mg/l Dissovled Fe mg/l Mn Total mg/l Dissolved Mn mg/l Al Total mg/l Aqueous Geochemistry Full water chemistry parameters for three sampling events are shown in Appendix 2. Overall, aqueous chemistry samples showed that higher concentrations of metal occur near treatment systems and the concentration decreases towards the mouth at EB1. The highest suspended metal concentrations occur near treatment systems.
28 28 Figures 7 shows dissolved and suspended Fe, Mn, and Al concentrations along the mainstem of East Branch. The highest observed aluminum concentration (8.5 mg/l) along the mainstem occurred in June 214 at EB25, which is downstream of the site 7 slag bed discharge. Al may be derived from Al precipitates and detrital clays. In June and August 214, the highest Al concentrations were observed at EB25, below the site 7 discharge. In January 215, however, the highest Al concentrations are observed at EB 211, below the Poling treatment tributary. Mn concentrations are highest at EB211 in all three sampling events. Fe concentrations are highest at EB25 in all 3 sampling events. Overall, Fe, Mn, and Al concentrations were lower during the August 214 sampling event. Increases in ph occur along river kilometer from headwaters to mouth. In the June sampling event, the concentration of Fe is reduced from > 4 mg/l to less than 1 mg/l after the first steel slag-treated tributary enters the mainstem. Most of the Fe at EB25 is in suspended form and the ph is around 5. This suggests that the addition of treatment water incurs the precipitation of Fe-hydroxide (Figures 6a and 6b), or that high flow conditions entrain Fe colloids and increase the suspended solid load in the mainstem. The concentration of Fe at the next sampling site is < 1 mg/l. This suggests that Fe-hydroxide has precipitated or deposited before reaching EB212, or that dilution has occurred. Studies at Iron Mountain Mine in California have shown that precipitates formed at ph 6.5 or higher produce ferrihydrite or goethite and water that has ph values of 2. to 4.5 mostly precipitate schwertmannite with small amounts of goethite (Bigham et al., 1996). A full mineral analysis such as the Hewett Fork investigation conducted by Schleich (214) would be needed to determine the exact nature of precipitates in East Branch. Al concentrations behave similarly to Fe at EB25 and EB212, however, the concentration rises sharply.5 km downstream at EB211. At EB21 most of the Al is in suspended form and Al concentration is reduced to < 1 mg/l at EB 17. Al precipitates between ph 4. and 9.5 (Andersson & Hansson, 21). Mn precipitated in large amounts at EB25 in the August 214 sampling event. Low flow conditions allowed for steel slag treatment to raise the ph of the mainstem to Mn precipitates completely at ph of 1 but can be partially precipitated at a ph of 8 if allowed enough residence time (Lovett, 1997). June and January sampling events revealed that Mn concentrations peaked at EB211 and then steadily declined toward the mouth at EB1. However, in the August sampling event, EB47 had the lowest Mn concentration. Mn is predominately in dissolved form. Physical riparian and stream features may influence aqueous
29 29 Mn concentrations, as Mn kinetics are influenced by exposure to sunlight and the growth of O 2 generating bacteria and algae in warmer weather (Rose et al., 23). Many factors determine the rate of Mn oxidation including ph, O 2, light, surfaces of Mn-oxides and Fe-oxides, and other cations and anions in solution (Rose et al., 23). Figure 8 shows Fe, Al, and Mn concentration in Laurel Run during three sampling events. In contrast to the East Branch mainstem, Al concentrations are less than 1 mg/l and ph decreases from upstream to downstream in all Laurel Run sampling events. In June 214 and January 215, the highest observed concentration on Fe and Mn occur at EB197, just below the Phase III treated tributary. In both June and January EB197 has high suspended concentrations of Fe and Mn, thus suggesting that a high ph causes precipitation of Fe and Mn-oxides. In low-flow conditions of August, the highest concentrations of Fe and Mn occur at EB194, which is below the Kern Hollow treatment system. Figures 6a and 6b (left to right): Fe-hydroxide and other precipitates come out of solution and rest on the stream bottom. Colloidal precipitates in the oxygenated portion of a riffle at EB199.1 (left) are white in color. Precipitates downstream of EB197 (right) are more indicative of typical yellow boy.
30 3 mg/l and ph a: June 214 Site ID and RKM Fe_dissolved_mgl Fe Suspended Al_dissolved_mgl Al Suspended Mn_dissolved_mgl Mn Suspended ph mg/l and ph Cabin Yost Poling Starr / EB16 7b: August 214 Site ID and RKM 1 Cabin Yost Poling Starr / EB16 7c: January mg/l and ph Site ID and RKM Figures 7a-7c: Concentration of Al, Fe, Mn, and ph in the mainstem during three sampling events. Full chemical analysis upstream of EB25 only occurred in August, however, field parameter were taken. Field ph is also shown for MSBC1 in August. The highest and lowest values of total metal vary depending on the sampling event. Vertical lines represent the river kilometer where treated tributaries enter the mainstem. Legend at bottom of 7a.
31 31 mg/l and ph Winnifred Kern Forest Northwoods 8a: June 214 Site / RKM Fe_dissolved_mgl Fe Suspended Mn_dissolved_mgl Mn Suspended Al_dissolved_mgl Al Suspended ph mg/l and ph b: August 214 Site ID/RKM Fe_Dissolved Fe_Suspended Al_Dissolved Al_Suspended Mn_Dissolved Mn_Suspended ph mg/l and ph c: January 215 Fe_dissolved_mgl Fe Suspended Mn_dissolved_mgl Mn Suspended Site ID/RKM Al_dissolved_mgl Al Suspended ph Figures 8a-8c: Total Al, Fe, and Mn at Laurel Run during three sampling events. EB199 was not sampled in January 215. Note high proportions of suspended metal at EB197 and lower overall concentrations at EB19. Vertical lines represent the river kilometer where treated tributaries enter the mainstem.
32 32 Figures 9-12 show the behavior of Ca, SO 4, Mg, Na, and alkalinity along RKM. Sulfate behaves differently in all three sampling events. In June 214, the highest sulfate concentration is observed in the headwaters of the mainstem just below the site 7 SSLB discharge. In August 214, the highest sulfate concentration is observed at EB8 below RKM 4.. The maximum concentration of sulfate in January 215, however, occurs below the confluence at EB17. A sharp increase in sulfate concentration is observed at EB16 in June and August 214. Trends in Ca concentrations in all three events are similar. Ca concentrations rise consistently until the EB16 tributary enters the mainstem, then concentrations slowly decline towards the mouth of East Branch. Figure 1 shows the Ca and SO 4 concentrations in the Laurel Run tributary during three sampling events. In August 214 and January 215 sulfate concentrations rise in the headwaters systems and then sharply decrease following the input of the tributary that is treated by the Forest SSLB. Concentrations continue to decrease steadily towards the mouth of Laurel Run. In June 214, sulfate concentrations decrease sharply between EB197 and EB194, after the Kern Hollow SSLB. Figure 11 shows the Mg, Na, and alkalinity concentrations along the mainstem. In all sampling events, there are variations in alkalinity around the treatment tributaries. In August 214, there was a sharp increase in alkalinity downstream of the site 7 discharge. That alkalinity is consumed rapidly by acid addition in less than one RKM and then continues to fluctuate in the vicinity of treatment systems. A similar pattern is observed in June 214, except with no spike in alkalinity after the site 7 system. After EB15, just below the EB16 tributary, alkalinity consistently increase up to the mouth at EB1. In January 215, however, alkalinity continues to decrease until after EB13 at RKM Figure 12 shows Mg, Na, and alkalinity concentrations along Laurel Run. Alkalinity appears to persist in the water column longer during low flow conditions.
33 33 mg/l Cabin Yost Poling 12 Stallion 1 Starr / EB16 8 RKM 6 9a: June Sulfate Calcium 9b: August 214 mg/l RKM c: January 215 mg/l RKM Figure 9a-9c: Ca and SO 4 concentration along the mainstem. SO 4 concentrations are highest at EB47 in the August sampling event (~9 mg/l). EB47 had the highest MAIS score in both years, but the site may be subject to episodic pulses of acidity, SO 4, and dissolved toxic metals. Ca concentrations have similar patterns in all sampling events but are slightly higher in the June sampling event. This is most likely due to higher discharge from steel slag leach beds. Vertical lines represent the river kilometer at which treated tributaries enter the mainstem.
34 34 1 Figure 1a: June mg/l Calcium Sulfate RKM Figure 1b: August mg/l 6 4 Calciu m RKM Figure 1c: January mg/l Calcium Sulfate RKM Figure 1a-1c: Ca and SO 4 in Laurel Run Tributary during three sampling events. In June, SO 4 and Ca peak at EB197 below the Winnifred treatment and then steadily decline towards the mouth of EB19. Vertical lines represent the river kilometer at which treated tributaries enter the mainstem.
35 35 mg/l Cabin Yost 14 Poling 12 Stallion 1 Starr / EB a: June 214 Alkalinity Mg Na RKM b: August 214 Alkalinity Mg Na 5 mg/l RKM c: January mg/l RKM Figure 11a-11c: Alkalinity, Mg, and Na along mainstem in three sampling events. In June, alkalinity concentration spikes at EB25 and is rapidly consumed before the next treatment tributary. Alkalinity concentration are consistently lowest below the Poling treatment tributary. Vertical lines represent the river kilometer at which treated tributaries enter the mainstem.
36 Winnifred Kern Forest Northwoods 12a: June 214 mg/l RKM b: August 214 mg/l Alkalinity Mg Na RKM c: January mg/l RKM Figure 12a-12c: Alkalinity, Mg, and Na along Laurel Run tributary. Vertical lines indicate RKM where treated tributary enter Laurel Run. August reveals a higher retention of alkalinity in the two uppermost sites.
37 Varying Flow, Instream Fe Profiles and Chemical Alkalinity Loads Instream Fe concentrations for the June and August sampling events are presented in Figures 13a and 13b. The highest concentrations of Fe occur at headwater sites and the flow regime alters the location and proportions of dissolved and particulate Fe. Flow at the mouth at EB1 in the June sampling event was about 95 L/s and well below the mean annual flow of 611 L/s. However, higher flow conditions increased suspended Fe loads and concentrations. Particulate Fe was higher at most sites in the June sampling event. Resuspension of Fe sediments in high flow has been found to be the major source of higher Fe concentrations and Fe loads in other watersheds (Mayes et al., 28). Instream Fe:SO 4 ratio is lower in the higher flow event, suggesting that entrained Fe is more important to Fe concentrations in the water column than direct mine inputs (Mayes et al., 28). Mean Fe:SO 4 ratios for all sampling sites in June and August are 1:581 and 1:112, respectively. This is to be expected, as East Branch is only minimally affected by underground mine inputs coming from the EB16 tributary. Total Fe concentrations are slightly higher downstream of EB16 in August. This may be due to underground mine discharge that is not as variable as surface flow. Al, Fe, and Total Alkalinity loads and delta loads for the mainstem are presented in Figures 14a, 14b, 14c and 15a, 15b, 15c. Figures 13a and 14a show the difference in alkalinity loads and alkalinity inputs based on two sampling events. The largest influx of alkalinity occurred at EB8 in the June sampling event, however, in August EB8 is where there is a decline in delta alkalinity loads. This may be due to an error in discharge measurement for August. Discharge at EB8 was 4 L/s higher than discharge at the mouth for this data. This is unlikely, as East Branch is a gaining watershed in all other sampling events. Peaks in Fe and Al loads occur a different river miles in the two sampling events. All flows for load calculation are measured flows and not interpolated.
38 38 June 214 August 214 Figures 13a (left) and 13b (right) show total Fe concentration and partitioning of particulate and dissolved forms in two sampling events. Figure 13a features concentrations in June and Figure 13b features concentrations in August. The June event features higher total concentrations in the headwaters near treatment systems but a decrease to <1mg/L rapidly site towards the mouth at EB1. High amounts of particulate Fe in the headwaters are a result of alkaline addition. The August sampling event feature lower portions of particulate Fe in the headwaters and a slightly higher total concentration at the mouth. The highest concentrations of Fe is found in the mainstem in June (4.51 mg/l) and the highest concentration in August is found just below the Kern Hollow treatment tributary in August (3.57 mg/l)
39 39 a kg/day RKM Alk loading kg/day 6 4 Delta Alk Loading kg/day 2-5 b RKM kg/day Fe loading kg/day Fe Delta Laoding kg/day c kg/day RKM Al Loading kg/day Delta Al Loading -5 Figures 14a-14c: June Total Alkalinity, Fe, and Al loading and delta loadings along the mainstem. Alkalinity loads increase consistently along the mainstem.
40 4 15a mg/l Alkalintiy Loading Delta Alk 15b mg/l Fe Loading Delta Fe 15c mg/l Al Loading Delta Al Figures 15a, 15b, and 15c: August Total Alkalinity, Fe, and Al loadings. There are spikes in Al and Fe at EB13.
41 Sediment Geochemistry, Size, and Spatial Distribution Sediment samples were taken in June 214. Maximum sediment concentrations of Al, Fe, and Mn are 1874 mg/kg, 928 mg/kg, and 145 mg/kg, respectively (Table 5). Table 6 shows the concentration (mg/kg) of metal per site. EB21 sediment samples contained the highest concentrations of Fe (928 mg/kg) and Pb (42 mg/kg). The mouth of Laurel Run, EB19, featured the highest concentrations of Al (1874 mg/kg), As (18 mg/kg), Co (67 mg/kg), and Cu (319 mg/kg). The highest instance of Ca occurred almost 4.5 RKM downstream of the Laurel Run confluence at EB17. EB197, which is located directly downstream of the Phase III Winnifred SSLB, contained the highest occurrences of Ba (17 mg/kg), Mn (145 mg/kg), Ni (75 mg/kg), and Zn (143 mg/kg). The highest concentration of Sr was found upstream all SSLB discharges EB199. Cd was below detection limits at all sites. Table 7 shows a Spearman correlation matrix of sediment metals, average grain size (mm), and velocity (m/s). Gradient (m/km) and Na (mg/kg) did not produce any significant correlation and are not displayed in the matrix. Grain size produced significant correlations with As, Ba, Ca, Co, Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Si. Only correlations at with 95% confidence level or better are shown. Cu and Fe have the highest correlation coefficient at.99 and significance at the.1 level. As and Fe had a significant positive correlation (Spearman s Rho =.86). Feoxyhydroxides can remove arsenite and arsenate from the water column through sorption (Bednar et al., 25) and the precipitation of schwertmannite, jarosite, and goethite can control the natural attenuation of As (Asta et al., 29). As in East Branch may come from natural (lithogenic) sources or be derived from treatment systems. As occurs naturally with pyrite and the metalloid may constitute up to 1% of the mineral in arsenian pyrites (Abraitis et al., 24). As is also present in steel slag leach bed effluent at low-levels. Steel slag in treatment systems must conform to standards that require As leachate in a TCLP leaching procedure to be less than <34 µg/l (Appendix 5). The high Spearman s Rho suggests that As may be adsorbed or coprecipitated with Fe, however, some literature suggests that coprecipitation of As and Fe is not as effective at alkaline, high-ph conditions (Brix, 1993). More study needs to be conducted in order to identify the exact sources and mechanisms of As attenuation in watersheds undergoing treatment by steel slag leach beds. Trace metals typically sorb more readily to small sediments (Ewais et al., 2; Singh et al., 1999), however, in this study many metals had a high positive correlation (R 2 >.5) with grain size. This may suggest that chemical conditions control the deposition of metals more than
42 42 physical conditions in the upper reaches of East Branch. Metals are adsorbed into sediments by cation exchange or chemisorption (Sheoran & Sheoran, 26). Sedimentation is not only dependent on physical processes, but precipitation and coprecipitation must aggregate metals into particles that are large enough to settle (Sheoran & Sheoran, 26). Therefore, the mechanism for sediment metal concentrations may be sorption or coprecipitation. Grain size (mm) and metal concentration (mg/kg dw) appear to have a positive relationship until RKM 9 below the EB16 tributary (Figure 16). Grain size and sediment metal concentrations behave similarly above RKM 9 for Al, As, Ba, Co, Cr, Fe, Pb, Ni, Mg, Sr, and Zn. Velocity is negatively correlated with grain size (Spearman s Rho = -.64, P <.5). This may be a function of sampling error. Stream velocity was calculated using pygmy measurements from the June sampling event. This data set may not have provided enough measurements to provide accurate correlations. Average velocity at each site may provide positive correlations with grain size.
43 43 Table 5: Summary statistics for sediment chemistry analytes (N =17). Cd is below detection at all sites. All values in mg/kg dw. Metal Al As Ba Ca Cd Co Cr Cu Fe K Mg Mn Na Ni Pb Si Sr Zn min 5612 Bd bd max bd mean bd Table 6: Sediment metal concentration (mg/kg dw) at samples sites along mainstem and Laurel Run sampled in June Al concentrations do not produce a linear decline in concentrations towards the mouth at EB1. The highest Ca concentration occurred downstream of the Winnifred treatment on Laurel Run. Site RKM Al As Ba Ca Cd Co Cr Cu Fe K Mg Mn Na Ni Pb Si Sr Zn EB BD BD EB BD BD EB BD BD EB BD EB BD EB BD EB BD EB BD BD EB BD BD EB BD EB BD BD EB BD EB BD EB BD EB BD EB BD EB BD
44 Table 7: Spearmen rank correlation matrix for sediment metals and grain size. Topographical gradient (m/km) and Na produced no significant correlations and are not included in the table. Velocity had a significant, negative correlation with grain size. P values *<.5, <.1, < Grain Size Velocity (m/s) Al As Ba Ca Co Cr Cu Fe K Mg Mn Ni Pb Si Sr Zn Grain Size * *.6* *.52*.59* Velocity 1 (m/s) * -.68* -.64* * -.59* -.6* Al * As Ba *.56* * Ca *.5* Co Cr Cu Fe * K Mg Mn Ni Pb 1 Si 1 Sr 1.88 Zn 1
45 45 Fe Concentration mg/kg dw RKM 16a Grain Size mm Fe Average Grain Size Ni Concentration mg/kg dw Ni RKM Average Grain Size 16b Grain Size mm 16c Zn Concentration mg/kg dw RKM Zn Average Grain Size Grain Size mm Figure 16a-16c: Sediment Fe, Ni, and Zn and grain size along river mile. The nature of the relationship between each metal and grain size changes around RKM 9. In the headwaters, grain size and sediment metal concentrations appear to be loosely positively correlated, although Zn did not produce a significant correlation.
46 Exceedances of Sediment Quality Guidelines Table 8 shows the threshold effect concentration and probable effect concentrations of metals. Threshold effect concentration is the level under which toxic effects occur rarely. Probable effect concentration is the level above which toxic effects are expected to occur frequently (Smith et al., 1996). Sediment metal concentrations exceed threshold effect levels for As, Pb, Ni, and Zn and exceed probable effect concentration limits for Cu, Pb, and Ni (MacDonald et al., 2). Fe, Al, and Mn concentrations are notably high and exceed sediment quality guidelines. Fe concentrations exceeded probable effect concentrations at 11 sites. Probable effect concentrations for Fe were exceeded at MAIS sites EB8, EB15, and EB17. Probable effect concentrations for Mn were exceeded at 11 sites and one MAIS site. The MAIS site that exceeded Mn PEC limits was EB15, which also received the lowest MAIS score in the two years of this study ( ). Table 8: Sediment Quality Guidelines (concentration in mg/kg dw). * indicates exceedance in this study (MacDonald et al., 2; Persaud et al., 1993) Metals (mg/kg DW) Consensus-based TEC Consensus-based PEC Arsenic 9.79* 33 Cadmium Chromium Copper 31.6* 149* Iron 2* 4* Lead 35* 128* Manganese 46* 11* Nickel 22.7* 48.6* Zinc 121* 459
47 Gradient The geomorphology of watersheds has an impact on the sediment transport and the mobility of sediment-bound contaminants. One of the principle goals of AMD treatment is to reduce the TSS and TDS through precipitation and sedimentation (Younger, 22). In Appalachian Ohio, the stream channels in treated watersheds become a zone for sediment deposition (Kruse et al., 213). Low gradient zones are needed for metal deposition downstream of all acid sources in an AMD-impacted catchment (Kruse et al., 213).The lowest gradient observed occurred at EB13, which is below the majority of acid sources within East Branch. EB15 is the last major acid source coming into the mainstem. Gradient had almost no monotonic relationship with average gain size (Spearman s Rho =.7, P =.2). Gradient was measured at all water quality and sediment chemistry sites. Gradient measurements are shown in Table 9. The highest gradient was observed at EB21, and the lowest gradient was observed at EB13. Gradient did not significantly correlate with TDS, TSS, Ca, Mg, Al, Fe, Mn concentrations in June 214, TDS, TSS, Ca, Mg, Al, Fe, Mn concentrations in August 214, or TSS, Ca, Mg, Al, Fe, Mn concentrations (suspended, total, and dissolved) for January 215. Figure 17 shows the grain size and gradient along RKM.
48 48 Table 9: Gradient Measurements and Grain Size Average Site Average m/km Average Deviation Grain Size N EB EB EB EB EB EB EB EB EB EB EB EB EB EB EB EB EB Gradient m/km Figure RKM Grain Size mm Gradient Grain Size Figure 17: Grain size and gradient. Spearman correlations produced no significant relationships between gradient and grain size or sediment metals.
49 Macroinvertebrate Assessments The Macroinvertebrate Aggregated Index for Streams (MAIS) scores for five East Branch sites are shown in Table 1. The lowest score in both years occurred at EB15. The highest score was observed at EB47 in 213. In 214, EB1 and EB47 received scores of 14. Improved status of MAIS is determined by regression. R 2 values greater than.4 and P values <.1 indicate improved status. MAIS scores from 214 produced a gradient of recovery. Target MAIS scores for Warmwater Habitat are 12 (Kinney, 26; The Voinovich School, 212). It is difficult to ascertain the effects that habitat is having on MAIS scores, as EB17 appears to have high habitat quality and low MAIS scores: 8 in 213 and 7 in 214. Fluctuations in habitat quality may explain changes in MAIS score, but more research needs to be conducted in East Branch to identify statistically significant trends. However, the MAIS score at EB1 was the highest ever recorded at that site, and QHEI was also the highest (Figure 18a). EB47 saw a reduction in habitat quality since a 211 peak at 81. QHEI and MAIS score at EB47 dropped 1 point from 213 to 214, but with 214 data EB47 has recovered status (R 2 =.6171, P =.6) (Figure 18b). Table 1: MAIS and QHEI scores for 213 and 214 Site 213 Narrative QHEI 214 Narrative QHEI MAIS Category 213 MAIS Category 214 EB1 1 Poor Good 68 EB47 15 Good Good 72 EB8 13 Good Poor * EB15 5 Very poor 67 6 Very poor * EB17 8 Poor 7 7 Very poor *
50 5 MAIS Score R² = Year MAIS Score R² = Year 18b Figure 18a show MAIS scores at EB1 over 9 years; R 2 =.2681, P=.15. Figure 18b shows MAIS scores at EB47 over 6 years; R 2 =.6171, P = Integrated Data Assessment MAIS Scores and Sediment Interactions Data for grain size analysis, MAIS scores, and sediment chemistry are presented in Table 11. As concentrations that were below detection limits were input into the Spearman correlations as. mg/kg or half of the detection limit which was.25 mg/kg. Both of these concentrations produced significant negative correlations for both years of MAIS data. Table 11 shows As p- value with below detection limit concentrations run at. mg/kg. Negative correlations between 213 MAIS scores and sediment chemistry from 214 were significant for As, Cu, Fe, and Mn. Negative correlations between 214 MAIS scores and sediment chemistry from 214 were significant only for As. However, As did not exceed TEC concentration at any MAIS site. Sediment Cu and Fe concentrations exceeded PEC limits at the two poorest sites: EB15 and
51 51 EB17. Sediment Mn exceeded PEC limits at one site: EB15. As was the only sediment metal that was significant for both years of MAIS data, however, this should be interpreted with caution. First, sediments were collected in the summer of 214 but correlated better with MAIS scores from 213. Second, below detection limits were run in the correlation as. mg/kg and did not meet the required assumption that the relationship is monotonic and the data set is limited. EB47 exceeded PEC limits for Ni but received the best MAIS score in both years. Grain size and MAIS scores did not produce any significant correlations MAIS Scores and Water and Sediment Chemistry Interactions Tables 12, 13, and 14 depict water chemistry variables from three sampling events and correlation significance with MAIS scores from 214. MAIS correlated significantly with 16 of out 27 parameters for the June 214 sampling event: specific conductivity, alkalinity, TDS, sulfate, Ca, Mg, K, Fe, Mn, Al, and hardness. For the August sampling event, ORP, ph, alkalinity, Cl, Total Ca, Mn, and Al correlated significantly with MAIS score. Al, Mn, and ph correlated significantly in all sampling events. Similar to Kruse et al. (213) and Dsa et al. (28), aqueous chemical parameters appear to have more of an effect on macroinvertebrate populations than sediment contaminants. However, Spearman correlations between MAIS and sediment metals did produce some significant correlations. The two most upstream MAIS sites, EB15 and EB17, received the lowest MAIS scores in both years. These sites were net acidic in June 214 and August 214, and EB15 had a ph of < 5. in August 214. EB15 at RKM 8.8 received the lowest MAIS in both years. Dissolved aluminum concentrations at EB15 are > 1 mg/l in June and August, and ph is < 5. in August. This would suggest that Al is in the more toxic monomeric form at this site on a seasonal basis (Gensemer & Playle, 1999). Although EB47 contained the highest level of sediment aluminum of any of the MAIS sites, it received the highest MAIS score in both years. Similar to findings in Kruse et al. 213, this would suggest that sediment Al has less of effect on the benthic macroinvertebrate community than other metals. MAIS scores from 213 correlated significantly with sediment As, Fe, Cu, and Mn, and MAIS scores in 214 only correlated significantly with sediment As. Arsenic contamination has been shown to have a strong impact on benthic macroinvertebrate communities (Mori, 1999). Sediment arsenic concentration correlated with MAIS score in both
52 52 years. However, sediment As concentrations at MAIS sites did not exceed the Threshold Effect Concentration Limit, which is 9.79 mg/kg dw (MacDonald et al., 2). The chemical speciation of arsenic was not investigated in sediment or the water column. Arsenic toxicity and bioaccumulation are very dependent on the chemical form (Moore & Ramamoorthy, 1984) and As (III) is more toxic than As (V) (Ferguson & Gavis, 1972). More detailed investigations are needed to identify the exact mechanism of arsenic uptake in southeast Ohio. A study in Corsica, however, (Culioli et al., 29) found that shredders and scrapers contained elevated concentrations of metalloids (As and Sb), although sediment As concentrations in that study were much higher than in East Branch (mean µg/g dw). A study in Spain revealed that As concentrations in Hydropsyche larvae were not correlated with As concentrations in the sediment or water (Solà et al., 24), but another study found in Italy showed that gatherers, predators and filterers take up increasing amounts of As as the sediment concentration increases (Santoro, 29). Sediment concentrations of Fe and Cu correlated significantly with 213 MAIS values. Fe concentrations in the sediment may have an effect on MAIS score at the two uppermost sites, as Fe sediment concentrations are above the Probable Effect Concentration (PEC) threshold of 4, mg/kg dw established in Canada (Persaud et al., 1993). Aqueous concentrations of Fe were less than < 1 mg/l at all MAIS site in all sampling events. Sediment Fe concentrations exceeded Threshold Effect Concentrations (2, mg/kg dw) at all MAIS sites. Mn is toxic to benthic organisms, specifically in reducing conditions, and the kinetics of precipitation are slow (Lasier, 2). Sediment Mn exceeded Probable Effect Concentration limits (11 mg/kg dw) at EB15, which received very poor designations in both years. Dissolved and total manganese concentrations correlated significantly with MAIS 214 scores in both the June 214 and August 214 sampling event. 3.8 Identifying Zones of Recovery More benthic macroinvertebrate bioassessments need to be conducted in order to confirm zones of recovery, however, data from 213 and 214 resemble a gradient of recovery. Sites EB1 and EB47 both obtained good scores in 214, and EB47 was the only site to obtain a good rating in both years. Based on MAIS, EB1 and EB47 are in the recovered zone of East Branch. Moreover, EB1 received an IBI score of 4 in 214. EB8 has only two years of data which produced a score of 13 and a score 9. This may suggest that EB8 is in the recovered zone or in the transition zone. EB15 and EB17 had score of poor and very poor in both years,
53 53 suggesting that the sites are the impaired zone. Although no bioassessments have been conducted upstream of EB17, sediment and water chemistry may indicate significant differences in recovery status. In order to test this hypothesis, all water quality sites were assigned narrative descriptions of recovered, transition, or impaired status and a Kruskal-Wallis test was performed. EB1, EB47, and EB8 were assigned recovered status because they received good scores in at least one year. EB15 and EB17 were assigned transition statues because they had MAIS scores of both poor and very poor. All other sites were assigned impaired status. The Kruskal-Wallis (Kruskal & Wallis, 1952) is a nonparametric test that evaluates differences among three or more sample groups based on non-normally distributed continuous variables. Tests of sediment metal concentration revealed no differences between recovered, transition, and impaired sites. The only June water quality variables that differed across the three groups were SO 4 concentration (mg/l) (Chi-squared = , DF = 2, P-value =.25), total Al (Chi-squared = , DF = 2, P-value =.344), and dissolved Al (Chi-squared = , DF = 2, P-Value =.2454). SO 4 (mg/l) did not identify differences between zones of recovery groups in August water quality data, but both Total Al (mg/l) and dissolved Al (mg/l) identified differences between assigned zones of recovery with P-values of.1184 and.1993, respectively. These results suggest that chemical variables may be used to identify biological zones of recovery. This also reinforces the idea that chemical stressors vary seasonally. SO 4 concentrations may be more influential in high-flow conditions and Al concentrations may be influential in various flow regimes in East Branch. Further study is needed is need to confirm the seasonal effects of aqueous chemical variables on biota in AMD-impacted watersheds.
54 54 Table 11: Grain size, MAIS Scores, and Sediment Chemistry and Spearman Significance. BD detection limits for As were run at. mg/kg and.25 mg/kg. Both concentrations produced significant negative correlations in both years. Confidence at 95% level was considered significant. Mn in 214 had a P-value of.89. RKM Gradient Grain m/km Size Al As Ba Ca Co Cr Cu Fe K Mg Mn Na Ni Pb Si Sr Zn BD BD BD P value 213 P value * *.374* * *
55 55 Table 12: June water quality parameters and MAIS Spearman correlation significance RKM temp_c Q (L/s) DO_mgl PH_lab SC acidity alkalinity TDS TSS sulfate Cl P value MAIS > *.21.*.*.32.*.17 RKM Cl total Ca total Ca dissolved Mg total Mg dissolved Na total Na dissolved K total K dissolved Fe Total Fe dissolved Mn total_mgl P value MAIS *.*.*.*.8.55.*.*.*.23.* RKM Mn dissolved Al Total Al Dissolved hardness P value MAIS 214.*.*.3*.*
56 56 Table 13: August 214 Water Quality Parameters and MAIS correlation significance (chemicals, sulfate, alkalinity, and acidity in mg/l) RKM temp_c LPS ORP ph_lab SC Acidity lab Alkalinity lab TDS TSS Sulfate MAIS 214 P-value.8*.17.*.* * RKM Cl total Ca total Ca dissolved Mg_total Mg_dissolved Na_total Na_dissolved K total K dissolved Fe total MAIS 214 P-value.8.13 > RKM Fe_dissolved Mn_total Mn_dissolved Al_total Al_dissolved hardness MAIS 214 P-value *.*.55
57 Table 14: January WQ Parameters vs MAIS 214 significance *estimated discharge based on regression (chemicals, sulfate, alkalinity, and acidity in mg/l) RKM Discharge LPS ORP PH_lab SC Acidity alkalinity TDS TSS sulfate Cl total * P-Value MAIS * >.5.*.* >.5.* >.5 >.5 Ca Ca Mg Mg Na Na K K Fe Fe RKM Total dissolved Total Dissolved Total Dissolved Total Dissolved Total Dissolved P-Value MAIS 214 >.5 >.5 >.5.* >.5.66 RKM Al Total Al Dissolved Mn Total Mn Dissolved Hardness P-Value MAIS 214.*.* >.5 >.5 >.5 57
58 58 CHAPTER 4: CONCLUSIONS 4.1 Changes in Water Chemistry Depending on Flow Metal and acidity concentrations and loadings are influenced by changes in flow regime. These changes are further influenced by steel slag bed treatment systems (alkaline additions) and how they interact with instream chemistry acid rock drainage. Overall, treatment systems effectively buffer acidity in the headwaters and Laurel Run tributary and raise the ph to circumneutral values by RKM 9.1. However, evidence from this study suggest that acidity concentrations are higher at MAIS sites during low flow conditions. This may be due to two factors: 1) Treatment systems in the headwaters are rainfall dependent and do not treat the stream in extremely dry conditions; 2) the EB16 tributary may have more influence on lower-river kilometer stream chemistry in low-flow months because it is fed by underground mines. Furthermore, aqueous contaminant concentrations may spike in flushing events that occur after long dry periods as is recognized in multiple publications (Kennedy, 1971; Nordstrom, 29). These spikes may expose macroinvertebrate populations to episodic stress (MacCausland & McTammany, 27). 4.2 Chemical and Physical Parameters that Influence Recovery The state of contaminants in various flow conditions along the stream may influence biological recovery. MAIS scores from 213 had significant inverse correlations with As, Mn, Fe, and Cu. MAIS scores from 214 had significant inverse correlations with As. More research needs to be conducted to determine the level at which As, Mn, and Fe sediment metal concentrations influence benthic macroinvertebrates, and whether yearly changes in sediment chemistry affect biota. Water-borne contaminants may also influence MAIS scores in East Branch as specific conductivity, alkalinity, TDS, sulfate, Fe, Mn, and Al were all inversely correlated with benthic macroinvertebrate abundance and diversity. The mobilization of metalrich sediment may smother and negatively influence macroinvertebrate populations are well Gradient, Velocity, and Grain Size Limitations Gradient measurements did not produce significant correlations with velocity, grain size, or any sediment metals; this may be a result of sampling methodology bias. In some areas gradient measurement were only taken once because of visibility or a lack of straight stream segments. Therefore, gradient values may not be an accurate representation of stream topography.
59 59 Furthermore, stream bed morphology is highly variable, and drop is not consistent. This is also a factor that may influence velocity measurements, as well. Velocity calculations are based off of one segment in the stream profile and not the entire stream reach where gradient was measured. More velocity measurements may need to be taken in order to determine mean velocity for sites. Dye-tracer methods may reveal more accurate stream velocity measurements if dye-tracer was used to see velocity within a reach where gradient had been measured. Velocity and average grain size were negatively correlated. 4.4 Recovery in East Branch Treatment by steel slag leach bed is effective at restoring the mouth of East Branch to cicumneutral ph. This also creates a gradient of improving aqueous and sediment conditions that may influence MAIS scores. The Kruskal-Wallis method was used to test differences between recovered, transition, and impaired sites. Tests showed the SO 4 and Al may be used to separate sample groups into recovery status. More study needs to be performed in order to further identify the factors that influence macroinvertebrate abundance and diversity and fish populations in East Branch and other AMD-remediated watersheds.
60 6 REFERENCES Abraitis, P., Pattrick, R., & Vaughan, D. (24). Variations in the compositional, textural and electrical properties of natural pyrite: a review. International Journal of Mineral Processing, 74(1), Andersson, E., & Hansson, H. (21). Precipitation of reactive aluminium hydroxide from an acidic aluminium sulphate solution by addition of sodium hydroxide. Asta, M. P., Cama, J., Martínez, M., & Giménez, J. (29). Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. Journal of Hazardous Materials, 171(1), Barbour, M. T., Gerritsen, J., Snyder, B., & Stribling, J. (1999). Rapid bioassessment protocols for use in streams and wadeable rivers. USEPA, Washington. Bednar, A., Garbarino, J., Ranville, J., & Wildeman, T. (25). Effects of iron on arsenic speciation and redox chemistry in acid mine water. Journal of Geochemical Exploration, 85(2), Beltman, D. J., Clements, W. H., Lipton, J., & Cacela, D. (1999). Benthic invertebrate metals exposure, accumulation, and community level effects downstream from a hard rock mine site. Environmental Toxicology and Chemistry, 18(2), Bigham, J., Schwertmann, U., Traina, S., Winland, R., & Wolf, M. (1996). Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochimica et Cosmochimica Acta, 6(12), Bott, T. L., Jackson, J. K., McTammany, M. E., Newbold, J. D., Rier, S. T., Sweeney, B. W., & Battle, J. M. (212). Abandoned coal mine drainage and its remediation: impacts on stream ecosystem structure and function. Ecological Applications, 22(8), Brix, H. (1993). Macrophyte-mediated oxygen transfer in wetlands: transport mechanisms and rates. Constructed Wetlands for Water Quality Improvement, Buchanan, T. J., & Somers, W. P. (1969). Discharge measurements at gaging stations: US Geological Survey Techniques of Water-Resources Investigations, book 3, chap. Burton, G. A. (1991). Assessing the toxicity of freshwater sediments. Environmental Toxicology and Chemistry, 1(12), Chapman, P. M., Wang, F., Janssen, C., Persoone, G., & Allen, H. E. (1998). Ecotoxicology of metals in aquatic sediments: binding and release, bioavailability, risk assessment, and remediation. Canadian Journal of Fisheries and Aquatic Sciences, 55(1),
61 61 Cherry, D., Currie, R., Soucek, D., Latimer, H., & Trent, G. (21). An integrative assessment of a watershed impacted by abandoned mined land discharges. Environmental Pollution, 111(3), Cravotta Iii, C., & Bilger, M. (21). Water-quality trends for a stream draining the Southern Anthracite Field, Pennsylvania. Geochemistry: Exploration, Environment, Analysis, 1(1), Culioli, J.-L., Fouquoire, A., Calendini, S., Mori, C., & Orsini, A. (29). Trophic transfer of arsenic and antimony in a freshwater ecosystem: A field study. Aquatic Toxicology, 94(4), Doshi, S. M. (26). Bioremediation of acid mine drainage using sulfate-reducing bacteria. US Environmental Protection Agency, Office of Solid Waste and Emergency Response and Office of Superfund Remediation and Technology Innovation, 65. Dsa, J. V., Johnson, K. S., Lopez, D., Kanuckel, C., & Tumlinson, J. (28). Residual toxicity of acid mine drainage-contaminated sediment to stream macroinvertebrates: relative contribution of acidity vs. metals. Water, Air, and Soil Pollution, 194(1-4), Ewais, T. A., Grant, A., & Fattah, A. A. (2). The role of surface coatings on sediments in sediment: water partitioning of trace elements and radionuclides. Journal of Environmental Radioactivity, 49(1), Ferguson, J. F., & Gavis, J. (1972). A review of the arsenic cycle in natural waters. Water Research, 6(11), Fitzpatrick, F. A., Waite, I. R., D Arconte, P. J., Meador, M. R., Maupin, M. A., & Gurtz, M. E. (1998). Revised methods for characterizing stream habitat in the National Water-Quality Assessment Program. US Department of the Interior, US Geological Survey. Fripp, J., Ziemkiewicz, P. F., & Charkavorki, H. (2). Acid mine drainage treatment. DTIC Document. Gensemer, R. W., & Playle, R. C. (1999). The bioavailability and toxicity of aluminum in aquatic environments. Critical Reviews in Environmental Science and Technology, 29(4), Hindall, S. (1984). Effects of surface coal-mine reclamation on stream quality in a small watershed near Nelsonville, southeastern Ohio. Water-Resources Investigations Report (USA).
62 62 Hogsden, K. L., & Harding, J. S. (211). Consequences of acid mine drainage for the structure and function of benthic stream communities: a review. Freshwater Science, 31(1), Hudson-Edwards, K. (23). Sources, mineralogy, chemistry and fate of heavy metal-bearing particles in mining-affected river systems. Mineralogical Magazine, 67(2), IBM SPSS Statistics for Windows. (212). (Version 21.). Armonk, NY: IBM Corp. Jennings, S. R., Blicker, P. S., Neuman, D. R., & Reclamation Research Group. (28). Acid mine drainage and effects on fish health and ecology: a review. Reclamation Research Group. Johnson, D. B., & Hallberg, K. B. (25). Acid mine drainage remediation options: a review. Science of the Total Environment, 338(1), Johnson, K. S., Thompson, P. C., Gromen, L., & Bowman, J. (214). Use of leaf litter breakdown and macroinvertebrates to evaluate gradient of recovery in an acid mine impacted stream remediated with an active alkaline doser. Environmental Monitoring and Assessment, 186(7), Kemble, N. E., Brumbaugh, W. G., Brunson, E. L., Dwyer, F. J., Ingersoll, C. G., Monda, D. P., & Woodward, D. F. (1994). Toxicity of metal contaminated sediments from the upper clark fork river, montana, to aquatic invertebrates and fish in laboratory exposures. Environmental Toxicology and Chemistry, 13(12), Kennedy, V. C. (1971). Silica variation in stream water with time and discharge. Nonequilibrium Systems in Natural Water Chemistry, 16, Kenney, M. A., Sutton-Grier, A. E., Smith, R. F., & Gresens, S. E. (29). Benthic macroinvertebrates as indicators of water quality: the intersection of science and policy. Terrestrial Arthropod Reviews, 2(2), Kinney, C. J. (26). A comparison of two methods of bioassessment in streams. Korenowsky, R. (212, June). Hydrogeochmeical Analysis of Alkalinity and Heavy Metal Inputs on Water and Sediment Quality of an Acid Mine Drainage Remediated Stream: Hewett Fork, Southeastern Ohio. Ohio University. Kruse, N. A., DeRose, L., Korenowsky, R., Bowman, J. R., Lopez, D., Johnson, K., & Rankin, E. (213). The role of remediation, natural alkalinity sources and physical stream parameters in stream recovery. Journal of Environmental Management, 128,
63 63 Kruse, N. A., Mackey, A. L., Bowman, J. R., Brewster, K., & Riefler, R. G. (212). Alkalinity production as an indicator of failure in steel slag leach beds treating acid mine drainage. Environmental Earth Sciences, 67(5), Kruskal, W. H., & Wallis, W. A. (1952). Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association, 47(26), Lafferty, B. (25, February 14). Re: East Branch/Yost Track (EB24) Water Quality Update. Lasier, P., Winger, P., & Bogenrieder, K. (2). Toxicity of manganese to Ceriodaphnia dubia and Hyalella azteca. Archives of Environmental Contamination and Toxicology, 38(3), Lee, G., Bigham, J. M., & Faure, G. (22). Removal of trace metals by coprecipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown Mining District, Tennessee. Applied Geochemistry, 17(5), Lottermoser, B. (21). Mine wastes: characterization, treatment and environmental impacts. Springer. Lovett, R. J. (1997). Removal of manganese from acid mine drainage. Journal of Environmental Quality, 26(4), Lynch, S. F., Batty, L. C., & Byrne, P. (214). Environmental risk of metal mining contaminated river bank sediment at redox-transitional zones. Minerals, 4(1), MacCausland, A., & McTammany, M. (27). The impact of episodic coal mine drainage pollution on benthic macroinvertebrates in streams in the Anthracite region of Pennsylvania. Environmental Pollution, 149(2), MacDonald, D. D., Ingersoll, C. G., & Berger, T. (2a). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39(1), MacDonald, D. D., Ingersoll, C. G., & Berger, T. (2b). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39(1), Mayes, W., Gozzard, E., Potter, H., & Jarvis, A. (28). Quantifying the importance of diffuse minewater pollution in a historically heavily coal mined catchment. Environmental Pollution, 151(1), McClurg, S. E., Petty, J. T., Mazik, P. M., & Clayton, J. L. (27). Stream ecosystem response to limestone treatment in acid impacted watersheds of the Allegheny Plateau. Ecological Applications, 17(4),
64 64 Microsoft. (21). Microsoft Excel. Redmond, Washington. Moore, J. W., & Ramamoorthy, S. (1984). Heavy metals in natural waters: applied monitoring and impact assessment. Mori, C., Orsini, A., & Migon, C. (1999). Impact of arsenic and antimony contamination on benthic invertebrates in a minor Corsican river. Hydrobiologia, 392(1), Nordstrom, D. K. (29). Acid rock drainage and climate change. Journal of Geochemical Exploration, 1(2), Ohio Environmental Protection Agency. (26, June). Methods for Assessing Habitat in Flowing Waters: Using the Qualitative Habitat Evaluation Index (QHEI). Persaud, D., Jaagumagi, R., & Hayton, A. (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario: Report. Water Resources Branch, Ontario Ministry of the Environment. Rankin, E. T., & Ohio, E. (1989). The qualitative habitat evaluation index [QHEI]: Rationale, methods, and application. State of Ohio Environmental Protection Agency. Rice, C., Hoy, J., Hoy, R., Last, J., Farley, M., Grow, J., Simon, K. (22). Acid mine drainage abatement and treatment (AMDAT) plan for the headwaters of the Raccoon Creek watershed. Raccoon Creek Partnership, Athens. Rose, A. W., Means, B., & Shah, P. (23). Methods for passive removal of manganese from acid mine drainage (Vol. 12). Presented at the Proceedings 23 West Virginia Surface Mine Drainage Task Force Symposium. RStudio. (215). (Version 3.1.). Boston, MA: RStudio. Retrieved from Santoro, A., Blo, G., Mastrolitti, S., & Fagioli, F. (29). Bioaccumulation of heavy metals by aquatic macroinvertebrates along the Basento River in the South of Italy. Water, Air, and Soil Pollution, 21(1-4), Schleich, K. L. (214). Geochemical Modeling of Processes Affecting Water and Sediment Chemistry and their Relationship to Biological Recovery in an Acid Mine Drainage Remediated Stream. Sheoran, A., & Sheoran, V. (26). Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Minerals Engineering, 19(2), Singh, A. K., Hasnain, S., & Banerjee, D. (1999). Grain size and geochemical partitioning of heavy metals in sediments of the Damodar River a tributary of the lower Ganga, India. Environmental Geology, 39(1), 9 98.
65 65 Skousen, J. G., Sexstone, A., & Ziemkiewicz, P. F. (2). Acid mine drainage control and treatment. Agronomy, 41, Smith, E. P., & Voshell, J. R. (1997). Studies of benthic macroinvertebrates and fish in streams within EPA Region 3 for development of biological indicators of ecological condition. Virginia Polytechnic Institute and State University. Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Field, L. J. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22(3), Solà, C., Burgos, M., Plazuelo, Á., Toja, J., Plans, M., & Prat, N. (24). Heavy metal bioaccumulation and macroinvertebrate community changes in a Mediterranean stream affected by acid mine drainage and an accidental spill (Guadiamar River, SW Spain). Science of the Total Environment, 333(1), The Voinovich School. (212). East Branch Phase I. Retrieved from Tiwary, R. (21). Environmental impact of coal mining on water regime and its management. Water, Air, and Soil Pollution, 132(1-2), United States Department of Agriculture. (n.d.). Web Soil Survey. USGS. (212). The Streamstats Program. U.S. Geologicial Survey. Retrieved from Verb, R. G., & Vis, M. L. (2). Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and nonimpacted sites. Journal of the North American Benthological Society, 19(2), Yoder, C. O., & Rankin, E. T. (1996). Assessing the condition and status of aquatic life designated uses in urban and suburban watersheds. Effects of Watershed Development and Management on Aquatic Ecosystems. American Society of Civil Engineers, New York, Younger, P. L., Banwart, S. A., & Hedin, R. S. (22). Mine Water Hydrology. Springer.
66 66 APPENDIX 1 FIELD PARAMETERS Appendix 1.1 Field Parameters August 4-5, 213 Site ID Temperature Discharge (l/s) DO DO percent ph field SC Field EB EB EB NOT TAKEN EB EB EB EB EB EB EB EB EB NOT TAKEN EB EB EB
67 67 Appendix 1.2 Field Parameters June 3-4, 214 Site ID RKM temp Discharge (LPS) DO (mg/l) Field ph SC (µs/cm) EB EB EB EB EB EB EB EB EB EB EB EB EB EB EB EB
68 68 Appendix 1.3 Field Parameters September 15, 214 Discharge site_id RKM temp_c (LPS) ORP PH_field conductivity_field_uscm EB EB EB EB EB EB EB EB EB EB EB EB EB EB EB
69 69 Appendix 1.4 Field Parameters January 27, 215 site_id RKM temp_c Q (lps) ORP PH_field conductivity_field_uscm EB EB NOT TAKEN EB EB EB EB EB EB EB NOT TAKEN EB EB NOT TAKEN EB EB EB EB EB EB NOT TAKEN
70 7 APPENDIX 2 WATER QUALITY Appendix 2.1 June Water Quality
71 71 Appendix 2.2 August Water Quality Appendix 2.3 January Water Quality
72 Cl concentration at EB197 is 5.86 mg/l 72
73 APPENDIX 3: JUNE LOADINGS ALONG MAINSTEM 73
74 74 APPENDIX 4: AUGUST CHEMICAL LOADINGS ALONG MAINSTEM IN MG/L AND TDS/TSS LOADS FOR JUNE, AUGUST, AND JANUARY RKM June TSS Load June TDS Load Aug TSS Load Aug TDS Load January TSS Load January TDS Load
75 APPENDIX 5: EXCERPT FROM ODNR DOCUMENT STATING THE MAXIUMUM LIMIT FOR AS IN LEACHATE FROM STEEL SLAG 75
76 76 APPENDIX 6: LIST OF MACROINVERTEBRATE TAXA FOUND IN EAST BRANCH IN 213 AND MAIS Taxon in East Branch EB1 EB47 EB8 EB15 EB17 Taxon Taxon Taxon Taxon Taxon Simuliidae 191 Hydropsychidae 65 Hydropsychidae 113 Hydropsychidae 61 Hydropsychidae 69 Chironomidae 82 Elmidae 46 Elmidae 74 Cambaridae 3 Cambaridae 32 Cambaridae 24 Chironomidae 33 Simuliidae 31 Chironomidae 6 Chironomidae 6 Aeshnidae 18 Baetidae 23 Baetidae 25 Libellulidae 5 Libellulidae 5 Hydropsychidae 18 Corydalidae 17 Chironomidae 16 Sialidae 3 Aeshnidae 4 Caenidae 16 Leuctridae 15 Tipulidae 14 Aeshnidae 2 Sialidae 4 Dryopidae 16 Dryopidae 12 Dryopidae 12 Calopterygidae 2 Tipulidae 4 Tipulidae 16 Tipulidae 12 Cambaridae 9 Corduligastridae 2 Corduligastridae 3 Baetidae 13 Cambaridae 1 Sialidae 9 Dryopidae 1 Saldidae 3 Elmidae 9 Aeshnidae 8 Ceratopogonidae 7 Elmidae 1 Dryopidae 2 Gomphidae 9 Caenidae 8 Corydalidae 7 Limnephilidae 1 Elmidae 2 Gammaridae 7 Oligochaeta 5 Caenidae 6 Tipulidae 1 Hydrophilidae 2 Ceratopogonidae 6 Simuliidae 4 Dixidae 5 Caenidae 1 Coenagrionidae 4 Gomphidae 3 Physidae 4 Calopterygidae 1 Gyrinidae 4 Isotomidae 3 Aeshnidae 3 Coenagrionidae 1 Sialidae 4 Perlidae 3 Gomphidae 3 Isotomidae 1 Corduligastridae 3 Sialidae 3 Libellulidae 3 Polycentropodidae 1 Calopterygidae 2 Calopterygidae 2 Dytiscidae 2 Leptoceridae 2 Leptophlebiidae 2 Limnephilidae 2 Limnephilidae 2 Libellulidae 2 Calopterygidae 1 Asellidae 1 Polycentropodidae 2 Coenagrionidae 1 Corydalidae 1 Saldidae 2 Corduligastridae 1 Dixidae 1 Ceratopogonidae 1 Empididae 1 Dytiscidae 1 Dixidae 1 Heptageniidae 1 Empididae 1 Dytiscidae 1 Hydrophilidae 1 Leuctridae 1 Empididae 1 Isotomidae 1 Libellulidae 1 Gammaridae 1 Leuctridae 1 Oligochaeta 1 Heptageniidae 1 Staphylinidae 1 Physidae 1 Philopotamidae 1 Saldidae 1 Pyralidae 1
77 MAIS Taxon in East Branch 7/7/14-7/9/14 EB1 EB47 EB8 EB15 EB17 Taxon Taxon Taxon Taxon Taxon Hydropsychidae 72 Hydropsychidae 147 Hydropsychidae 33 Hydropsychidae 2 Hydropsychidae 74 Elmidae 67 Chironomidae 81 Chironomidae 8 Cambaridae 6 Cambaridae 25 Aeshnidae 22 Elmidae 45 Baetidae 64 Tipulidae 4 Dryopidae 14 Baetidae 16 Cambaridae 2 Elmidae 27 Limnephilidae 2 Tipulidae 5 Chironomidae 15 Simuliidae 17 Sialidae 13 Ceratopogonidae 1 Limnephilidae 3 Cambaridae 14 Dryopidae 15 Cambaridae 7 Chironomidae 1 Coenagrionidae 2 Dryopidae 13 Tipulidae 15 Corydalidae 7 Coenagrionidae 1 Sialidae 2 Simuliidae 11 Baetidae 14 Philopotamidae 7 Corduligastridae 1 Aeshnidae 1 Gyrinidae 8 Corydalidae 13 Dryopidae 6 Dytiscidae 1 Ceratopogonidae 1 Coenagrionidae 6 Empididae 9 Dixidae 5 Haliplidae 1 Corydalidae 1 Corydalidae 5 Calopterygidae 8 Empididae 5 Isotomidae 1 Gerridae 1 Limnephilidae 5 Leuctridae 8 Aeshnidae 4 Staphylinidae 1 Gomphidae 1 Perlidae 5 Sialidae 8 Ceratopogonidae 4 Libellulidae 1 Philopotamidae 4 Libellulidae 7 Culicidae 4 Polycentropodidae 1 Tipulidae 4 Aeshnidae 6 Staphylinidae 4 Saldidae 1 Caenidae 3 Culicidae 4 Gomphidae 3 Calopterygidae 3 Limnephilidae 4 Saldidae 3 Sialidae 3 Coenagrionidae 3 Tipulidae 3 Ceratopogonidae 2 Leptoceridae 3 Gerridae 2 Leptoceridae 2 Isotomidae 2 Gyrinidae 2 Leuctridae 2 Saldidae 2 Heptageniidae 2 Corduligastridae 1 Ceratopogonidae 1 Simuliidae 2 Corixidae 1 Corduligastridae 1 Caenidae 1 Dixidae 1 Gyrinidae 1 Calopterygidae 1 Gomphidae 1 Hydrophilidae 1 Dytiscidae 1 Heptageniidae 1 Perlidae 1 Gammaridae 1 Isotomidae 1 Philopotamidae 1 Haliplidae 1 Libellulidae 1 Polycentropodidae 1 Limnephilidae 1 Staphylinidae 1 Perlidae 1
78 Thesis and Dissertation Services
The recovery of an AMDimpacted stream treated by steel slag leach beds: a case study in the East Branch of Raccoon Creek
 The recovery of an AMDimpacted stream treated by steel slag leach beds: a case study in the East Branch of Raccoon Creek NAAMLP Conference, Columbus, OH September 21-24, 214 Caleb Hawkins Master of Science
The recovery of an AMDimpacted stream treated by steel slag leach beds: a case study in the East Branch of Raccoon Creek NAAMLP Conference, Columbus, OH September 21-24, 214 Caleb Hawkins Master of Science
Effects of Precipitation on the Acid Mine Drainage Impacted Hewett Fork Watershed Understanding Storm Response
 Effects of Precipitation on the Acid Mine Drainage Impacted Hewett Fork Watershed Understanding Storm Response Zeb Martin Ohio University Contents Project Overview Objectives of the Research Project Area
Effects of Precipitation on the Acid Mine Drainage Impacted Hewett Fork Watershed Understanding Storm Response Zeb Martin Ohio University Contents Project Overview Objectives of the Research Project Area
The effects of a plug of alkaline water in an acid stressed watershed
 The effects of a plug of alkaline water in an acid stressed watershed Natalie Kruse, Amy Mackey Ohio University, Athens, Ohio, USA, krusen@ohio.edu Extended Abstract Alkaline addition is a common strategy
The effects of a plug of alkaline water in an acid stressed watershed Natalie Kruse, Amy Mackey Ohio University, Athens, Ohio, USA, krusen@ohio.edu Extended Abstract Alkaline addition is a common strategy
Steel Slag Leach Bed Longevity
 Steel Slag Leach Bed Longevity September 24 th 2014 National Association of Abandoned Mine Land Programs Conference Sarah Landers Raccoon Creek Water Quality Specialist Ohio Valley RC&D and Ohio University
Steel Slag Leach Bed Longevity September 24 th 2014 National Association of Abandoned Mine Land Programs Conference Sarah Landers Raccoon Creek Water Quality Specialist Ohio Valley RC&D and Ohio University
November 15 th, 2012 WMAO 2012 Fall Conference 100 Years of Watershed Events. Amy Mackey Raccoon Creek Watershed Coordinator
 November 15 th, 2012 WMAO 2012 Fall Conference 100 Years of Watershed Events Amy Mackey Raccoon Creek Watershed Coordinator Surface Mining Control & Reclamation Act of 1977 (SMCRA) watershed event most
November 15 th, 2012 WMAO 2012 Fall Conference 100 Years of Watershed Events Amy Mackey Raccoon Creek Watershed Coordinator Surface Mining Control & Reclamation Act of 1977 (SMCRA) watershed event most
Otter Creek Watershed TMDL Project. Stakeholder Meeting June 6, 2013
 Otter Creek Watershed TMDL Project Stakeholder Meeting June 6, 2013 1 Meeting Purpose Meet with watershed & technical advisory group members and watershed landowners to provide basic Otter Creek TMDL project
Otter Creek Watershed TMDL Project Stakeholder Meeting June 6, 2013 1 Meeting Purpose Meet with watershed & technical advisory group members and watershed landowners to provide basic Otter Creek TMDL project
2011 NPS Report - Raccoon Creek Watershed
 Kingston Laurelville The Raccoon Creek Partnership is a local partnership working towards conservation, stewardship, and restoration of the watershed for a healthier stream and community. The partnership
Kingston Laurelville The Raccoon Creek Partnership is a local partnership working towards conservation, stewardship, and restoration of the watershed for a healthier stream and community. The partnership
Pebble Project Surface Water Quality Program Streams, Seeps, and Ponds
 Day1_1445_Surface Water Quality_PLP_McCay.mp3 Pebble Project Surface Water Quality Program Streams, Seeps, and Ponds Mark Stelljes SLR International February 1, 2012 Outline Stream Program Overview Results
Day1_1445_Surface Water Quality_PLP_McCay.mp3 Pebble Project Surface Water Quality Program Streams, Seeps, and Ponds Mark Stelljes SLR International February 1, 2012 Outline Stream Program Overview Results
Design and Early Field Performance of AMD Treatment Systems Developed Under the Appalachian Clean Streams Initiative
 Design and Early Field Performance of AMD Treatment Systems Developed Under the Appalachian Clean Streams Initiative Paul Ziemkiewicz and Jennifer Simmons National Mine Land Reclamation Center West Virginia
Design and Early Field Performance of AMD Treatment Systems Developed Under the Appalachian Clean Streams Initiative Paul Ziemkiewicz and Jennifer Simmons National Mine Land Reclamation Center West Virginia
Black Branch Acid Mine Drainage Remediation. ADEM Nonpoint Source Conference January 15,2015
 Black Branch Acid Mine Drainage Remediation Project ADEM Nonpoint Source Conference January 15,2015 Black Branch Small Watershed Drains approximately 3.3 square Miles Mulberry Fork Watershed Lost Creek
Black Branch Acid Mine Drainage Remediation Project ADEM Nonpoint Source Conference January 15,2015 Black Branch Small Watershed Drains approximately 3.3 square Miles Mulberry Fork Watershed Lost Creek
Impact of Coal Mine Reclamation Using Coal Combustion By-products (CCBs) on Groundwater Quality: Two Case Studies
 Impact of Coal Mine Reclamation Using Coal Combustion By-products (CCBs) on Groundwater Quality: Two Case Studies C.-M. Cheng 1,, T. Butalia 1, W. Wolfe 1, R. Baker 1 N. Yencho 1, N. Mauger 1, J. Massey-Norton
Impact of Coal Mine Reclamation Using Coal Combustion By-products (CCBs) on Groundwater Quality: Two Case Studies C.-M. Cheng 1,, T. Butalia 1, W. Wolfe 1, R. Baker 1 N. Yencho 1, N. Mauger 1, J. Massey-Norton
ACID MINE DRAINAGE ABATEMENT AND TREATMENT (AMDAT) PLAN FOR THE HEADWATERS OF THE RACCOON CREEK WATERSHED
 ACID MINE DRAINAGE ABATEMENT AND TREATMENT (AMDAT) PLAN FOR THE HEADWATERS OF THE RACCOON CREEK WATERSHED by Chip Rice J.B. Hoy Rachael Hoy Ohio University, Institute for Local Government Administration
ACID MINE DRAINAGE ABATEMENT AND TREATMENT (AMDAT) PLAN FOR THE HEADWATERS OF THE RACCOON CREEK WATERSHED by Chip Rice J.B. Hoy Rachael Hoy Ohio University, Institute for Local Government Administration
Summary of Preliminary Results of EPA Remedial Investigation Molycorp molybdenum mine Questa, New Mexico
 Summary of Preliminary Results of EPA Remedial Investigation Molycorp molybdenum mine Questa, New Mexico Prepared by Steve Blodgett, Technical Advisor Rio Colorado Reclamation Committee Introduction On
Summary of Preliminary Results of EPA Remedial Investigation Molycorp molybdenum mine Questa, New Mexico Prepared by Steve Blodgett, Technical Advisor Rio Colorado Reclamation Committee Introduction On
ABSTRACT: Clean sampling and analysis procedures were used to quantify more than 70 inorganic chemical constituents (including 36 priority
 ABSTRACT: Clean sampling and analysis procedures were used to quantify more than 70 inorganic chemical constituents (including 36 priority pollutants), organic carbon and phenols, and other characteristics
ABSTRACT: Clean sampling and analysis procedures were used to quantify more than 70 inorganic chemical constituents (including 36 priority pollutants), organic carbon and phenols, and other characteristics
2015 STREAM HEALTH REPORT
 2015 STREAM HEALTH REPORT AN EVALUATION OF WATER QUALITY, BIOLOGY, AND ACID MINE DRAINAGE RECLAMATION IN FIVE WATERSHEDS: RACCOON CREEK, MONDAY CREEK, SUNDAY CREEK, HUFF RUN, AND LEADING CREEK. CREATED
2015 STREAM HEALTH REPORT AN EVALUATION OF WATER QUALITY, BIOLOGY, AND ACID MINE DRAINAGE RECLAMATION IN FIVE WATERSHEDS: RACCOON CREEK, MONDAY CREEK, SUNDAY CREEK, HUFF RUN, AND LEADING CREEK. CREATED
A FIELD DEMONSTRATION OF AN ALTERNATIVE COAL WASTE DISPOSAL TECHNOLOGY GEOCHEMICAL FINDINGS. Paul T. Behum. Liliana Lefticariu. Y.
 A FIELD DEMONSTRATION OF AN ALTERNATIVE COAL WASTE DISPOSAL TECHNOLOGY GEOCHEMICAL FINDINGS Paul T. Behum Office of Surface Mining, Mid-Continent Region, Alton, IL Liliana Lefticariu Department of Geology,
A FIELD DEMONSTRATION OF AN ALTERNATIVE COAL WASTE DISPOSAL TECHNOLOGY GEOCHEMICAL FINDINGS Paul T. Behum Office of Surface Mining, Mid-Continent Region, Alton, IL Liliana Lefticariu Department of Geology,
The Snapshot CONODOGUINET CREEK WATERSHED SNAPSHOT
 CONODOGUINET CREEK WATERSHED SNAPSHOT ABOVE: CONODOGUINET CREEK AT RT 74 BRIDGE FACING DOWNSTREAM The Snapshot The Conodoguinet Watershed Snapshot was a collaborative effort to engage local citizens in
CONODOGUINET CREEK WATERSHED SNAPSHOT ABOVE: CONODOGUINET CREEK AT RT 74 BRIDGE FACING DOWNSTREAM The Snapshot The Conodoguinet Watershed Snapshot was a collaborative effort to engage local citizens in
ACID MINE DRAINAGE TREATMENT VIA ALKALINE INJECTION TECHNOLOGY 1
 ACID MINE DRAINAGE TREATMENT VIA ALKALINE INJECTION TECHNOLOGY 1 G.A. Canty and J.W. Everett 2 Abstract. The Oklahoma Conservation Commission conducted a demonstration project to investigate the feasibility
ACID MINE DRAINAGE TREATMENT VIA ALKALINE INJECTION TECHNOLOGY 1 G.A. Canty and J.W. Everett 2 Abstract. The Oklahoma Conservation Commission conducted a demonstration project to investigate the feasibility
Today s Webinar: Types of Monitoring and Assessment Data and What They Mean
 Welcome to the 1 st Webinar in the Series Monitoring & Assessment for Watershed Plans: Identifying, Accessing, and Using Data to Protect and Restore Indiana s Waters Each Monday in May at noon Today s
Welcome to the 1 st Webinar in the Series Monitoring & Assessment for Watershed Plans: Identifying, Accessing, and Using Data to Protect and Restore Indiana s Waters Each Monday in May at noon Today s
ATTENUATION OF METAL CONCENTRATIONS IN TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, MAY 1994 THROUGH MAY 1995
 ATTENUATION OF METAL CONCENTRATIONS IN TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, MAY 1994 THROUGH MAY 1995 By Robert W. Stogner, Sr. U.S. Geological Survey, WRD 201 W. 8th Street, Suite 200 Pueblo,
ATTENUATION OF METAL CONCENTRATIONS IN TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, MAY 1994 THROUGH MAY 1995 By Robert W. Stogner, Sr. U.S. Geological Survey, WRD 201 W. 8th Street, Suite 200 Pueblo,
Carbon Dioxide Pretreatment of AMD for Limestone Diversion Wells
 Carbon Dioxide Pretreatment of AMD for Limestone Diversion Wells ABSTRACT Barnaby J. Watten NBS Research and Development Laboratory Wellsboro, Pennsylvania Michael F. Schwartz Freshwater Institute Sheperdstown,
Carbon Dioxide Pretreatment of AMD for Limestone Diversion Wells ABSTRACT Barnaby J. Watten NBS Research and Development Laboratory Wellsboro, Pennsylvania Michael F. Schwartz Freshwater Institute Sheperdstown,
Introduction. Methods
 Introduction Brush Creek is located in the south central part of the Roaring Fork Watershed. It is between the drainages of Maroon Creek and Snowmass Creek and is the major creek flowing below the Snowmass
Introduction Brush Creek is located in the south central part of the Roaring Fork Watershed. It is between the drainages of Maroon Creek and Snowmass Creek and is the major creek flowing below the Snowmass
ALKALINE FOUNDATION DRAINS AND ALKALINE AMENDMENTS FOR AMD CONTROL IN COAL REFUSE PILES 1
 ALKALINE FOUNDATION DRAINS AND ALKALINE AMENDMENTS FOR AMD CONTROL IN COAL REFUSE PILES 1 David L. Brant and Paul F. Ziemkiewicz 2 Abstract: Coal refuse, a by product of cleaning coal is normally disposed
ALKALINE FOUNDATION DRAINS AND ALKALINE AMENDMENTS FOR AMD CONTROL IN COAL REFUSE PILES 1 David L. Brant and Paul F. Ziemkiewicz 2 Abstract: Coal refuse, a by product of cleaning coal is normally disposed
1556 by Georgius Agricola. In the United States 10,000 miles of streams and surface
 USE OF SULFATE REDUCING BACTERIA IN ACID MINE DRAINAGE TREATMENT Thomas J. Powers U. S. Environmental Protection Agency Risk Reduction Engineering Laboratory 26 West Martin Luther King Drive Cincinnati,
USE OF SULFATE REDUCING BACTERIA IN ACID MINE DRAINAGE TREATMENT Thomas J. Powers U. S. Environmental Protection Agency Risk Reduction Engineering Laboratory 26 West Martin Luther King Drive Cincinnati,
9. Cadmium, Cd (atomic no. 48)
 9. Cadmium, Cd (atomic no. 48) - Cd is comparatively rare in the environment - Median concentrations of Cd in soils and sediments range from about 0.04 to 1.8 mg/kg. - Cadmium concentrations are elevated
9. Cadmium, Cd (atomic no. 48) - Cd is comparatively rare in the environment - Median concentrations of Cd in soils and sediments range from about 0.04 to 1.8 mg/kg. - Cadmium concentrations are elevated
Golden Sunlight Mine Bio-Treatment of Acid Producing Waste. By Rory Tibbals Operations Superintendent
 Golden Sunlight Mine Bio-Treatment of Acid Producing Waste By Rory Tibbals Operations Superintendent Golden Sunlight Mine Gold Producing Mine 2.5 Million Ounces Produced 20 Year Operation Ore and All
Golden Sunlight Mine Bio-Treatment of Acid Producing Waste By Rory Tibbals Operations Superintendent Golden Sunlight Mine Gold Producing Mine 2.5 Million Ounces Produced 20 Year Operation Ore and All
Re-plumbing Roadside Ditch Networks
 Re-plumbing Roadside Ditch Networks Ditches Improving management to reduce flooding, water pollution, and in-stream erosion and habitat degradation Rebecca Schneider Dept. Natural Resources Cornell University,
Re-plumbing Roadside Ditch Networks Ditches Improving management to reduce flooding, water pollution, and in-stream erosion and habitat degradation Rebecca Schneider Dept. Natural Resources Cornell University,
Water Chemistry. Water 101
 Water Chemistry Water 101 I. Introduction A. Water is not pure Many different kinds of chemicals dissolved in it Ions, organic chemicals, organic matter, particulate matter, and gases can all be in water
Water Chemistry Water 101 I. Introduction A. Water is not pure Many different kinds of chemicals dissolved in it Ions, organic chemicals, organic matter, particulate matter, and gases can all be in water
THE NORTH BRANCH OF THE POTOMAC RIVER: RESULTS OF TWO YEARS OF LIME DOSING
 THE NORTH BRANCH OF THE POTOMAC RIVER: RESULTS OF TWO YEARS OF LIME DOSING INTRODUCTION Joseph E. Mills Maryland Department of the Environment Bureau of Mines 160 S. Water Street Frostburg, Maryland 21532
THE NORTH BRANCH OF THE POTOMAC RIVER: RESULTS OF TWO YEARS OF LIME DOSING INTRODUCTION Joseph E. Mills Maryland Department of the Environment Bureau of Mines 160 S. Water Street Frostburg, Maryland 21532
Advanced Planning Tools for Optimization of AMD Treatment
 Advanced Planning Tools for Optimization of AMD Treatment Jon Fripp, P.E., Hydraulic Engineer US Army Corps of Engineers, Baltimore District Baltimore, MD James M. Stiles, Ph.D., P.E., Environmental Engineer
Advanced Planning Tools for Optimization of AMD Treatment Jon Fripp, P.E., Hydraulic Engineer US Army Corps of Engineers, Baltimore District Baltimore, MD James M. Stiles, Ph.D., P.E., Environmental Engineer
An Alternative Alkaline Addition for Direct Treatment of Acid Mine Drainage
 An Alternative Alkaline Addition for Direct Treatment of Acid Mine Drainage Jennifer S. Simmons * and Paul Ziemkiewicz National Mine Land Reclamation Center Abstract The primary objective of the National
An Alternative Alkaline Addition for Direct Treatment of Acid Mine Drainage Jennifer S. Simmons * and Paul Ziemkiewicz National Mine Land Reclamation Center Abstract The primary objective of the National
El Dorado Hydroelectric Project FERC Project No Water Quality Monitoring Report
 El Dorado Hydroelectric Project FERC Project No. 184 21 Water Quality Monitoring Report EL DORADO IRRIGATION DISTRICT 289 Mosquito Road Placerville, CA 95667 March 211 1. Introduction The El Dorado Irrigation
El Dorado Hydroelectric Project FERC Project No. 184 21 Water Quality Monitoring Report EL DORADO IRRIGATION DISTRICT 289 Mosquito Road Placerville, CA 95667 March 211 1. Introduction The El Dorado Irrigation
Metals in the Benthic Macroinvertebrates in Coal Creek, Crested Butte, CO
 Metals in the Benthic Macroinvertebrates in Coal Creek, Crested Butte, CO Scarlett E. Graham July 1, 26 University of Colorado at Boulder Environmental Engineering REU Summer Student Overview Motivation
Metals in the Benthic Macroinvertebrates in Coal Creek, Crested Butte, CO Scarlett E. Graham July 1, 26 University of Colorado at Boulder Environmental Engineering REU Summer Student Overview Motivation
Evaluating acid rock drainage from road cuts in Tennessee
 Evaluating acid rock drainage from road cuts in Tennessee Michael Bradley, U.S. Geological Survey In cooperation with Tennessee Department of Transportation 14th Annual Technical Forum Geohazards Impacting
Evaluating acid rock drainage from road cuts in Tennessee Michael Bradley, U.S. Geological Survey In cooperation with Tennessee Department of Transportation 14th Annual Technical Forum Geohazards Impacting
Impact of Acid Mine Drainage on Streams in Southeastern Ohio: Importance of Biological Assessments
 Impact of Acid Mine Drainage on Streams in Southeastern Ohio: Importance of Biological Assessments Abstract Dr. Ben J. Stuart, P.E., Assistant Professor Ohio University Department of Civil Engineering
Impact of Acid Mine Drainage on Streams in Southeastern Ohio: Importance of Biological Assessments Abstract Dr. Ben J. Stuart, P.E., Assistant Professor Ohio University Department of Civil Engineering
MINING POLLUTION OF STREAM SEDIMENTS OF THE SMOLNIK STREAM. O. Lintnerová, P. Šottník, S. Šoltés
 MINING POLLUTION OF STREAM SEDIMENTS OF THE SMOLNIK STREAM O. Lintnerová, P. Šottník, S. Šoltés Comenius University Bratislava, Faculty of Natural Sciences, Mlynská dolina G, 842 15 Bratislava, Slovakia,
MINING POLLUTION OF STREAM SEDIMENTS OF THE SMOLNIK STREAM O. Lintnerová, P. Šottník, S. Šoltés Comenius University Bratislava, Faculty of Natural Sciences, Mlynská dolina G, 842 15 Bratislava, Slovakia,
Historical Water Quality Data Analysis, Pearson Creek, Springfield, Missouri
 The Ozarks Environmental and Water Resources Institute (OEWRI) Missouri State University (MSU) Historical Water Quality Data Analysis, Pearson Creek, Springfield, Missouri Prepared by: Marc R. Owen, M.S.,
The Ozarks Environmental and Water Resources Institute (OEWRI) Missouri State University (MSU) Historical Water Quality Data Analysis, Pearson Creek, Springfield, Missouri Prepared by: Marc R. Owen, M.S.,
Monitoring Stormwater Best Management Practices: Why Is It Important and What To Monitor
 Monitoring Stormwater Best Management Practices: Why Is It Important and What To Monitor Scott D. Struck, Ph.D. US EPA, Urban Watershed Management Branch New Jersey Water Monitoring Workshop 4/20/2006
Monitoring Stormwater Best Management Practices: Why Is It Important and What To Monitor Scott D. Struck, Ph.D. US EPA, Urban Watershed Management Branch New Jersey Water Monitoring Workshop 4/20/2006
Acidity and Alkalinity:
 Evaluation of Pollution Sources to Lake Glenville Quarterly Report December 2018 Kimberlee K Hall, PhD Environmental Health Program, Western Carolina University Summary Chemical and microbial analysis
Evaluation of Pollution Sources to Lake Glenville Quarterly Report December 2018 Kimberlee K Hall, PhD Environmental Health Program, Western Carolina University Summary Chemical and microbial analysis
An Evaluation of Antes Creek. Antes Creek has a drainage area of square miles and is a tributary of the
 An Evaluation of Antes Creek Introduction Antes Creek has a drainage area of 55.75 square miles and is a tributary of the West Branch of the Susquehanna near Jersey Shore, PA. The purpose of this study
An Evaluation of Antes Creek Introduction Antes Creek has a drainage area of 55.75 square miles and is a tributary of the West Branch of the Susquehanna near Jersey Shore, PA. The purpose of this study
Selected Abandoned Mined Land Reclamation Projects and Passive Treatment in Ohio
 Selected Abandoned Mined Land Reclamation Projects and Passive Treatment in Ohio By Mitchell E. Farley 1 and Paul Ziemkiewicz 2 1 Environmental Scientist, ODNR Div. of Mineral Resources Management, 34
Selected Abandoned Mined Land Reclamation Projects and Passive Treatment in Ohio By Mitchell E. Farley 1 and Paul Ziemkiewicz 2 1 Environmental Scientist, ODNR Div. of Mineral Resources Management, 34
The Mahoning Valley Mushroom Farm North Lima, Ohio AMD Investigation
 The Mahoning Valley Mushroom Farm North Lima, Ohio AMD Investigation Department of Geology and Environmental Science The University of Akron Theresa McQuade Ohio Department of Natural Resources Division
The Mahoning Valley Mushroom Farm North Lima, Ohio AMD Investigation Department of Geology and Environmental Science The University of Akron Theresa McQuade Ohio Department of Natural Resources Division
Carp Creek 2013 Summary Report
 Monitoring Activity in the Carp River Watershed In 2012, Mississippi Valley Conservation Authority (MVCA) worked with Friends of the Carp River (FCR) on a preliminary assessment of the Carp River. This
Monitoring Activity in the Carp River Watershed In 2012, Mississippi Valley Conservation Authority (MVCA) worked with Friends of the Carp River (FCR) on a preliminary assessment of the Carp River. This
Nutrients. Water Quality Planning Branch Water Division Arkansas Department of Environmental Quality
 Nutrients Water Quality Planning Branch Water Division Arkansas Department of Environmental Quality Water Quality & Biological Data Water Quality Standards & Criteria Assessment Permit Limitations Impairment
Nutrients Water Quality Planning Branch Water Division Arkansas Department of Environmental Quality Water Quality & Biological Data Water Quality Standards & Criteria Assessment Permit Limitations Impairment
Analysis of Metals in Water, Stream Sediments and Floodplain Soils Collected March 21-23, 2005 from the Bayou Creek System. David J.
 Analysis of Metals in Water, Stream Sediments and Floodplain Soils Collected March 21-23, 2005 from the Bayou Creek System David J. Price DRAFT REPORT December 7, 2006 Submitted to Nicole Burpo and Jon
Analysis of Metals in Water, Stream Sediments and Floodplain Soils Collected March 21-23, 2005 from the Bayou Creek System David J. Price DRAFT REPORT December 7, 2006 Submitted to Nicole Burpo and Jon
Low Maintenance Passive Treatment Systems for Mining Site Contaminants. Robert C. Thomas, Ph.D
 Low Maintenance Passive Treatment Systems for Mining Site Contaminants Robert C. Thomas, Ph.D Acid rock drainage (ARD) forms when sulfide minerals are exposed to oxygen and water during large-scale land
Low Maintenance Passive Treatment Systems for Mining Site Contaminants Robert C. Thomas, Ph.D Acid rock drainage (ARD) forms when sulfide minerals are exposed to oxygen and water during large-scale land
Mount Polley Mining Corporation an Imperial Metals company Box 12 Likely, BC V0L 1N0 T F
 Mount Polley Mining Corporation an Imperial Metals company Box 12 Likely, BC V0L 1N0 T 250.790.2215 F 250.790.2613 November 24, 2016 Ministry of Environment Mining Operations Environmental Protection 2080
Mount Polley Mining Corporation an Imperial Metals company Box 12 Likely, BC V0L 1N0 T 250.790.2215 F 250.790.2613 November 24, 2016 Ministry of Environment Mining Operations Environmental Protection 2080
PHYSICAL-CHEMICAL TREATMENT OF METALS AND RADIONUCLIDES IN THE SATURATED ZONE USING COLLOIDAL BUFFERS EOS Remediation, LLC.
 PHYSICAL-CHEMICAL TREATMENT OF METALS AND RADIONUCLIDES IN THE SATURATED ZONE USING COLLOIDAL BUFFERS - 12515 Yenjung Lai 1, Robert C. Borden 1, Ed Alperin 2 1 North Carolina State University, Raleigh,
PHYSICAL-CHEMICAL TREATMENT OF METALS AND RADIONUCLIDES IN THE SATURATED ZONE USING COLLOIDAL BUFFERS - 12515 Yenjung Lai 1, Robert C. Borden 1, Ed Alperin 2 1 North Carolina State University, Raleigh,
2012 Mill Creek Watershed Habitat Assessment Level 3 Project Study Plan Results
 2012 Mill Creek Watershed Habitat Assessment Level 3 Project Study Plan Results Cranberry Run This project study plan was financed through a Sub-grant from the Ohio EPA with funds from the State of Ohio.
2012 Mill Creek Watershed Habitat Assessment Level 3 Project Study Plan Results Cranberry Run This project study plan was financed through a Sub-grant from the Ohio EPA with funds from the State of Ohio.
Ecotoxicology studies with sediment, pore water, and surface water from the Palmerton Zinc site
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2009 Ecotoxicology studies with sediment, pore water, and surface
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2009 Ecotoxicology studies with sediment, pore water, and surface
Impact of an Agricultural Point Source on Macroinvertebrate Communities and Water Quality on the Lamoille River
 Impact of an Agricultural Point Source on Macroinvertebrate Communities and Water Quality on the Lamoille River Fundamentals of Ecology 12/2/05 Introduction The purpose of this study was to determine if
Impact of an Agricultural Point Source on Macroinvertebrate Communities and Water Quality on the Lamoille River Fundamentals of Ecology 12/2/05 Introduction The purpose of this study was to determine if
GEOCHEMICAL MODULE FOR AMDTreat
 GEOCHEMICAL MODULE FOR AMDTreat Charles A. Cravotta III and David L. Parkhurst, U.S. Geological Survey Brent P. Means, Robert M. McKenzie, and Bill Arthur, U.S. Office of Surface Mining Reclamation and
GEOCHEMICAL MODULE FOR AMDTreat Charles A. Cravotta III and David L. Parkhurst, U.S. Geological Survey Brent P. Means, Robert M. McKenzie, and Bill Arthur, U.S. Office of Surface Mining Reclamation and
SOUTH BRANCH SUBWATERSHED
 SOUTH BRANCH SOUTH BRANCH SUBWATERSHED The South Branch Subwatershed includes the South Branch drainage area plus all the drainage area along the main stream to the south from the mouth of Surrena Run
SOUTH BRANCH SOUTH BRANCH SUBWATERSHED The South Branch Subwatershed includes the South Branch drainage area plus all the drainage area along the main stream to the south from the mouth of Surrena Run
Recovery of Deckers Creek from Acid Mine Drainage. Martin Christ Water Remediation Director Friends of Deckers Creek
 Recovery of Deckers Creek from Acid Mine Drainage Martin Christ Water Remediation Director Friends of Deckers Creek Table of Contents Location of the Deckers Creek watershed Geological setting Mining Flood
Recovery of Deckers Creek from Acid Mine Drainage Martin Christ Water Remediation Director Friends of Deckers Creek Table of Contents Location of the Deckers Creek watershed Geological setting Mining Flood
Little Cypress Bayou Special Study - Subwatershed 1.10
 - Subwatershed 1.1 Sabine River Authority of Texas August 31, 21 Prepared in Cooperation with the Texas Natural Resource Conservation Commission Under the Authorization of the Texas Clean Rivers Act Table
- Subwatershed 1.1 Sabine River Authority of Texas August 31, 21 Prepared in Cooperation with the Texas Natural Resource Conservation Commission Under the Authorization of the Texas Clean Rivers Act Table
Treatability of Organic and Radioactive Emerging Contaminants in Stormwater Runoff
 Treatability of Organic and Radioactive Emerging Contaminants in Stormwater Runoff Robert Pitt, Ph.D., P.E., D.WRE, BCEE, University of Alabama Shirley Clark, Ph.D., P.E., D.WRE, Penn State - Harrisburg
Treatability of Organic and Radioactive Emerging Contaminants in Stormwater Runoff Robert Pitt, Ph.D., P.E., D.WRE, BCEE, University of Alabama Shirley Clark, Ph.D., P.E., D.WRE, Penn State - Harrisburg
Impact of ph on Soil and Groundwater Remediation RE3 Conference November 14, 2012 Brad Elkins Technical Sales Representative
 Impact of ph on Soil and Groundwater Remediation RE3 Conference November 14, 2012 Brad Elkins Technical Sales Representative 919.873.2204 Importance of ph Impacts conditions of silts and clays Controls
Impact of ph on Soil and Groundwater Remediation RE3 Conference November 14, 2012 Brad Elkins Technical Sales Representative 919.873.2204 Importance of ph Impacts conditions of silts and clays Controls
UPPER GALLATIN RIVER WATERSHED
 216 UPPER GALLATIN RIVER WATERSHED WATER QUALITY REPORT Kristin Gardner, PhD Executive Director, Gallatin River Task Force Stephanie Lynn Education and Communications Coordinator, Gallatin River Task Force
216 UPPER GALLATIN RIVER WATERSHED WATER QUALITY REPORT Kristin Gardner, PhD Executive Director, Gallatin River Task Force Stephanie Lynn Education and Communications Coordinator, Gallatin River Task Force
Nutrient distributions and the interaction between coastal wetlands and the nearshore of Lake Ontario
 Nutrient distributions and the interaction between coastal wetlands and the nearshore of Ontario Krista Chomicki and Gary Bowen International Association of Great s Research, 25 Type Durham Region Coastal
Nutrient distributions and the interaction between coastal wetlands and the nearshore of Ontario Krista Chomicki and Gary Bowen International Association of Great s Research, 25 Type Durham Region Coastal
Hydrology and Water Quality. Water. Water 9/13/2016. Molecular Water a great solvent. Molecular Water
 Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Gumboot Run Biological Assessment McKean County, Pennsylvania
 Gumboot Run Biological Assessment McKean County, Pennsylvania By: Elias J Heferle Water Pollution Biologist II Pennsylvania Department of Environmental Protection Knox District Mining Office January 2005
Gumboot Run Biological Assessment McKean County, Pennsylvania By: Elias J Heferle Water Pollution Biologist II Pennsylvania Department of Environmental Protection Knox District Mining Office January 2005
CANADA BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT
 CANADA BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT WATER QUALITY ASSESSMENT OF KETTLE RIVER AT MIDWAY (1972 2) Prepared by: BWP Consulting Kamloops, B.C. January, 23 Environment Canada Environnement
CANADA BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT WATER QUALITY ASSESSMENT OF KETTLE RIVER AT MIDWAY (1972 2) Prepared by: BWP Consulting Kamloops, B.C. January, 23 Environment Canada Environnement
Application of New Leaching Protocols for Assessing Beneficial Use of Solid Wastes in Florida. Technical Awareness Group Meeting June 30 th, 2015
 Application of New Leaching Protocols for Assessing Beneficial Use of Solid Wastes in Florida Technical Awareness Group Meeting June 30 th, 2015 Background Historically a number of different tests have
Application of New Leaching Protocols for Assessing Beneficial Use of Solid Wastes in Florida Technical Awareness Group Meeting June 30 th, 2015 Background Historically a number of different tests have
Hydrology and Water Quality. Water. Water 9/11/2018. Molecular Water a great solvent. Molecular Water
 Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Earth Science Department, University of Arkansas at Little Rock, 2801 S University Ave Little Rock, AR 72204
 Determining the Extent of Contamination and Potential Health Effects from Water Quality of the Oklahoma Section of the Historic Tri-State Mining District William Ketcheside 1 (wdketcheside@ualr.edu), Laura
Determining the Extent of Contamination and Potential Health Effects from Water Quality of the Oklahoma Section of the Historic Tri-State Mining District William Ketcheside 1 (wdketcheside@ualr.edu), Laura
TAPPAN LAKE 9/30/2014 RAPID WATERSHED INVENTORY INTRODUCTION
 TAPPAN LAKE 9/30/2014 RAPID WATERSHED INVENTORY Profile Contents Introduction Physical Description Land Use Map Resource Concerns Census and Social Data Progress/Status References INTRODUCTION Tappan Lake
TAPPAN LAKE 9/30/2014 RAPID WATERSHED INVENTORY Profile Contents Introduction Physical Description Land Use Map Resource Concerns Census and Social Data Progress/Status References INTRODUCTION Tappan Lake
CHEMICAL AND MICROBIOLOGICAL MODIFICATION OF ACID MINE DRAINAGE USING CONSTRUCTED TYPHA WETLANDS
 CHEMICAL AND MICROBIOLOGICAL MODIFICATION OF ACID MINE DRAINAGE USING CONSTRUCTED TYPHA WETLANDS ABSTRACT J.P. Calabrese A.J. Sexstone D.K. Bhumbla J.C. Sencindiver G.K. Bissonnette J.G. Skousen Division
CHEMICAL AND MICROBIOLOGICAL MODIFICATION OF ACID MINE DRAINAGE USING CONSTRUCTED TYPHA WETLANDS ABSTRACT J.P. Calabrese A.J. Sexstone D.K. Bhumbla J.C. Sencindiver G.K. Bissonnette J.G. Skousen Division
Study Justification. Coordinated Resource Management Project Little Snake River Conservation District
 Trends in Surface-Water Quality of the Muddy Creek asin: Carbon County, Wyoming C.. Ellison, Q. D. Skinner and L. S. Hicks Department of Renewable Resources University of Wyoming Funded by: Little Snake
Trends in Surface-Water Quality of the Muddy Creek asin: Carbon County, Wyoming C.. Ellison, Q. D. Skinner and L. S. Hicks Department of Renewable Resources University of Wyoming Funded by: Little Snake
ACID MINE DRAINAGE TREATMENT IN GREENS RUN BY AN ANOXIC LIMESTONE DRAIN
 ACID MINE DRAINAGE TREATMENT IN GREENS RUN BY AN ANOXIC LIMESTONE DRAIN Introduction Troy Titchenell and Jeff Skousen Anker Energy and West Virginia University From its headwaters in Pocahontas, Randolph
ACID MINE DRAINAGE TREATMENT IN GREENS RUN BY AN ANOXIC LIMESTONE DRAIN Introduction Troy Titchenell and Jeff Skousen Anker Energy and West Virginia University From its headwaters in Pocahontas, Randolph
U.S. Department of the Interior U.S. Geological Survey
 Evaluation of stream quality and relations between stream biological conditions and environmental variables in urbanizing watersheds of northeastern Kansas Teresa Rasmussen, U.S. Geological Survey, Lawrence,
Evaluation of stream quality and relations between stream biological conditions and environmental variables in urbanizing watersheds of northeastern Kansas Teresa Rasmussen, U.S. Geological Survey, Lawrence,
Figure 1. Platte River Sub-Watersheds and Monitoring Locations.
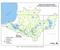 Figure 1. Platte River Sub-Watersheds and Monitoring Locations. 10 2 1 9 7 BL Pond 8 Hatchery 6 3 5 4 1 Platte River at Fewins Rd 6 B. Creek to Hatchery Spring 2 Platte River at Stone Bridge 7 Platte River
Figure 1. Platte River Sub-Watersheds and Monitoring Locations. 10 2 1 9 7 BL Pond 8 Hatchery 6 3 5 4 1 Platte River at Fewins Rd 6 B. Creek to Hatchery Spring 2 Platte River at Stone Bridge 7 Platte River
Benthic Invertebrate Biomonitoring Program for the Obed Mountain Mine
 Submitted to: November, 2011 Prepared by: Pisces Environmental Consulting Services Ltd. 25J 37337 Burnt Lake Trail, Red Deer County, Alberta T4S 2K5 --this page left blank for printing purposes-- Table
Submitted to: November, 2011 Prepared by: Pisces Environmental Consulting Services Ltd. 25J 37337 Burnt Lake Trail, Red Deer County, Alberta T4S 2K5 --this page left blank for printing purposes-- Table
Flambeau Mine On-Site Surface Water Monitoring Program (See Appendix I for maps with site locations)
 Flambeau Mine On-Site Surface Water Monitoring Program (See Appendix I for maps with site locations) Controlling Document and Specifications COC Stipulation Monitoring Plan (Dec 2007) Frequency: Spring/Fall,
Flambeau Mine On-Site Surface Water Monitoring Program (See Appendix I for maps with site locations) Controlling Document and Specifications COC Stipulation Monitoring Plan (Dec 2007) Frequency: Spring/Fall,
RACCOON CREEK WATERSHED
 RACCOON CREEK WATERSHED The Raccoon Creek Watershed Project is a local partnership working towards conservation, stewardship, and restoration of the watershed for a healthier stream and community. The
RACCOON CREEK WATERSHED The Raccoon Creek Watershed Project is a local partnership working towards conservation, stewardship, and restoration of the watershed for a healthier stream and community. The
ASSESSMENT OF MACROINVERTEBRATE COMMUNITIES IN AN ACID MINE DRAINAGE IMPACTED STREAM IN THE GEORGE S CREEK WATERSHED, ALLEGANY COUNTY, MARYLAND
 ASSESSMENT OF MACROINVERTEBRATE COMMUNITIES IN AN ACID MINE DRAINAGE IMPACTED STREAM IN THE GEORGE S CREEK WATERSHED, ALLEGANY COUNTY, MARYLAND JULY 23, 2014 ABSTRACT Macroinvertebrates are a diverse group
ASSESSMENT OF MACROINVERTEBRATE COMMUNITIES IN AN ACID MINE DRAINAGE IMPACTED STREAM IN THE GEORGE S CREEK WATERSHED, ALLEGANY COUNTY, MARYLAND JULY 23, 2014 ABSTRACT Macroinvertebrates are a diverse group
IMPLEMENTATION PLAN FOR JOHNSON CREEK
 IMPLEMENTATION PLAN FOR JOHNSON CREEK TIOGA COUNTY, PENNSYLVANIA Prepared for: The Arnot Sportsmen Association, Inc. P.O. Box 142 Arnot, PA 16911 Prepared by: Alder Run Engineering, LLC Civil & Environmental
IMPLEMENTATION PLAN FOR JOHNSON CREEK TIOGA COUNTY, PENNSYLVANIA Prepared for: The Arnot Sportsmen Association, Inc. P.O. Box 142 Arnot, PA 16911 Prepared by: Alder Run Engineering, LLC Civil & Environmental
TOTAL MAXIMUM DAILY LOAD (TMDL)
 TOTAL MAXIMUM DAILY LOAD (TMDL) For ph In Crab Orchard Creek Located In The Cumberland & Morgan County, Tennessee Prepared by: Tennessee Department of Environment and Conservation Division of Water Pollution
TOTAL MAXIMUM DAILY LOAD (TMDL) For ph In Crab Orchard Creek Located In The Cumberland & Morgan County, Tennessee Prepared by: Tennessee Department of Environment and Conservation Division of Water Pollution
Dissolved Organic Carbon Augmentation:
 National Conference on Mining Influenced Waters Dissolved Organic Carbon Augmentation: An Innovative Tool for Managing Operational and Closure-Phase Impacts from Mining on Surface Water Resources Charles
National Conference on Mining Influenced Waters Dissolved Organic Carbon Augmentation: An Innovative Tool for Managing Operational and Closure-Phase Impacts from Mining on Surface Water Resources Charles
Is There AMD In This Stream?
 Is There AMD In This Stream? Adapted from: Is There Mine Drainage Impacting This Stream? in AMD Biology Module. St. Vincent College Environmental Education Center, 2002. Abandoned Mine Drainage Grade Level:
Is There AMD In This Stream? Adapted from: Is There Mine Drainage Impacting This Stream? in AMD Biology Module. St. Vincent College Environmental Education Center, 2002. Abandoned Mine Drainage Grade Level:
Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania
 Hardness Comparisons Hardness Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania 16335. Grade Level: all Duration: 50 minutes Setting:
Hardness Comparisons Hardness Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania 16335. Grade Level: all Duration: 50 minutes Setting:
IMPLEMENTATION PLAN FOR HUBLER RUN
 IMPLEMENTATION PLAN FOR HUBLER RUN CLEARFIELD COUNTY, PENNSYLVANIA Prepared for: The West Branch Sportsman s Association, Inc. 2657 Schoonover Road Kylertown, PA 16847 Prepared by: Alder Run Engineering
IMPLEMENTATION PLAN FOR HUBLER RUN CLEARFIELD COUNTY, PENNSYLVANIA Prepared for: The West Branch Sportsman s Association, Inc. 2657 Schoonover Road Kylertown, PA 16847 Prepared by: Alder Run Engineering
Semi-Passive Bioreactors and RCTS Lime Treatment of Acid Mine Drainage
 Semi-Passive Bioreactors and RCTS Lime Treatment of Acid Mine Drainage Timothy K. Tsukamoto, Ph.D. TKT Consulting, LLC TKTtim@gmail.com Bridging the Gap Between Passive and Active PASSIVE ACTIVE Limestone
Semi-Passive Bioreactors and RCTS Lime Treatment of Acid Mine Drainage Timothy K. Tsukamoto, Ph.D. TKT Consulting, LLC TKTtim@gmail.com Bridging the Gap Between Passive and Active PASSIVE ACTIVE Limestone
DEVELOPING A WATERSHED IMPROVEMENT PLAN TO MEET MULTIPLE COMMUNITY OBJECTIVES IN GAINESVILLE AND HALL COUNTY, GEORGIA
 DEVELOPING A WATERSHED IMPROVEMENT PLAN TO MEET MULTIPLE COMMUNITY OBJECTIVES IN GAINESVILLE AND HALL COUNTY, GEORGIA Chrissy, Thom 1, David Dockery 2, Kevin McInturff 3, Betsy Massie 1, and Lauren Murphy
DEVELOPING A WATERSHED IMPROVEMENT PLAN TO MEET MULTIPLE COMMUNITY OBJECTIVES IN GAINESVILLE AND HALL COUNTY, GEORGIA Chrissy, Thom 1, David Dockery 2, Kevin McInturff 3, Betsy Massie 1, and Lauren Murphy
CREATES: Cheat River Ephemeral Access Treatment and Enhancement Strategy
 CREATES: Cheat River Ephemeral Access Treatment and Enhancement Strategy Mission Statement: The CREATES team plans to revitalize and recover the streams entering the southern 20 miles of the Cheat River
CREATES: Cheat River Ephemeral Access Treatment and Enhancement Strategy Mission Statement: The CREATES team plans to revitalize and recover the streams entering the southern 20 miles of the Cheat River
Passive treatment of mine drainage: Options, challenges, and possible future developments
 Passive treatment of mine drainage: Options, challenges, and possible future developments Adam P Jarvis Newcastle University, UK Definitions Active Treatment is the improvement of water quality by methods
Passive treatment of mine drainage: Options, challenges, and possible future developments Adam P Jarvis Newcastle University, UK Definitions Active Treatment is the improvement of water quality by methods
Temporal Dynamics of Benthic Macroinvertebrate Communities and Their Response to Elevated TDS in Appalachian Coalfield Streams
 Temporal Dynamics of Benthic Macroinvertebrate Communities and Their Response to Elevated TDS in Appalachian Coalfield Streams Powell River Project Annual Report 2011 2012 Elizabeth Boehme Virginia Water
Temporal Dynamics of Benthic Macroinvertebrate Communities and Their Response to Elevated TDS in Appalachian Coalfield Streams Powell River Project Annual Report 2011 2012 Elizabeth Boehme Virginia Water
LIMNOLOGY SPRING 2010 BIOL 362 / WMAN 446. Week 8: Water Chemistry, Pollution Loads, and Organic Matter Dynamics
 LIMNOLOGY SPRING 2010 BIOL 362 / WMAN 446 Week 8: Water Chemistry, Pollution Loads, and Organic Matter Dynamics INTRODUCTION On your last field lab, you took several water samples (i.e. filtered, unfiltered,
LIMNOLOGY SPRING 2010 BIOL 362 / WMAN 446 Week 8: Water Chemistry, Pollution Loads, and Organic Matter Dynamics INTRODUCTION On your last field lab, you took several water samples (i.e. filtered, unfiltered,
THE SUGAR CREEK COAL REFUSE PILE AND MINE DRAINAGE DISCHARGE RECLAMATION PROJECT
 THE SUGAR CREEK COAL REFUSE PILE AND MINE DRAINAGE DISCHARGE RECLAMATION PROJECT Eric E. Cavazza, P.E., Thomas C. Malesky, P.E., and Pamela J. Milavec Pennsylvania Department of Environmental Protection
THE SUGAR CREEK COAL REFUSE PILE AND MINE DRAINAGE DISCHARGE RECLAMATION PROJECT Eric E. Cavazza, P.E., Thomas C. Malesky, P.E., and Pamela J. Milavec Pennsylvania Department of Environmental Protection
Stimulation of Natural Attenuation of Metals in Acid Mine Drainage through Water and Sediment Management at Abandoned Copper Mines
 Stimulation of Natural Attenuation of Metals in Acid Mine Drainage through Water and Sediment Management at Abandoned Copper Mines Gijsbert D. Breedveld 1,2, Franziska Klimpel 2, Marianne Kvennås 1, Gudny
Stimulation of Natural Attenuation of Metals in Acid Mine Drainage through Water and Sediment Management at Abandoned Copper Mines Gijsbert D. Breedveld 1,2, Franziska Klimpel 2, Marianne Kvennås 1, Gudny
Comparative Study on Total Suspended Solids and Nutrients of the Las Vegas Wash Between the Demonstration Weir and its Terminus at the Las Vegas Bay
 Comparative Study on Total Suspended Solids and Nutrients of the Las Vegas Wash Between the Demonstration Weir and its Terminus at the Las Vegas Bay Delta September 2006 Comparative Study on Total Suspended
Comparative Study on Total Suspended Solids and Nutrients of the Las Vegas Wash Between the Demonstration Weir and its Terminus at the Las Vegas Bay Delta September 2006 Comparative Study on Total Suspended
FINAL. DEPARTMENT OF THE ENVIRONMENT 1800 Washington Boulevard, Suite 540 Baltimore, Maryland Submitted to:
 Watershed Report for Biological Impairment of the Watershed, Garrett County, Maryland Biological Stressor Identification Analysis Results and Interpretation DEPARTMENT OF THE ENVIRONMENT 1800 Washington
Watershed Report for Biological Impairment of the Watershed, Garrett County, Maryland Biological Stressor Identification Analysis Results and Interpretation DEPARTMENT OF THE ENVIRONMENT 1800 Washington
Know What You re Monitoring! Since not all of us are water chemists, here is a brief understanding of what your different tools measure
 1 Know What You re Monitoring! Since not all of us are water chemists, here is a brief understanding of what your different tools measure Extech EC400 meters Salinity (SAL) Total Dissolved Solids (TDS)
1 Know What You re Monitoring! Since not all of us are water chemists, here is a brief understanding of what your different tools measure Extech EC400 meters Salinity (SAL) Total Dissolved Solids (TDS)
Nick Shepherd University of Oklahoma
 Nick Shepherd University of Oklahoma Introduction Hypotheses & Objectives Methods Results Conclusions 2 Introduction 3 4 Introduction: Castor canadensis Life cycle 10 year life expectancy in wild Sexual
Nick Shepherd University of Oklahoma Introduction Hypotheses & Objectives Methods Results Conclusions 2 Introduction 3 4 Introduction: Castor canadensis Life cycle 10 year life expectancy in wild Sexual
REPORT ON RABBIT CREEK SPECIAL STUDY SUBWATERSHED 5.19
 REPORT ON RABBIT CREEK SPECIAL STUDY SUBWATERSHED 5.19 Sabine River Authority of Texas August 31, 23 Prepared in Cooperation with the Texas Commission on Environmental Quality Under the Authorization of
REPORT ON RABBIT CREEK SPECIAL STUDY SUBWATERSHED 5.19 Sabine River Authority of Texas August 31, 23 Prepared in Cooperation with the Texas Commission on Environmental Quality Under the Authorization of
RIMINI ROAD IMPROVEMENT PROJECT RESTORATION OF ABANDONED MINE SITES PROJECT DRAFT REPORT
 RIMINI ROAD IMPROVEMENT PROJECT RESTORATION OF ABANDONED MINE SITES PROJECT DRAFT REPORT Prepared by U.S. Army Corps of Engineers Omaha District Omaha, Nebraska August 2004 Table of Contents 1 Introduction...
RIMINI ROAD IMPROVEMENT PROJECT RESTORATION OF ABANDONED MINE SITES PROJECT DRAFT REPORT Prepared by U.S. Army Corps of Engineers Omaha District Omaha, Nebraska August 2004 Table of Contents 1 Introduction...
2012 Nutrient Regulations Update
 2012 Nutrient Regulations Update OWEA Government Affairs Workshop March 1, 2012 Guy Jamesson, PE, BCEE Malcolm Pirnie, The Water Division of ARCADIS Imagine the result Agenda Nutrient impacts Nutrient
2012 Nutrient Regulations Update OWEA Government Affairs Workshop March 1, 2012 Guy Jamesson, PE, BCEE Malcolm Pirnie, The Water Division of ARCADIS Imagine the result Agenda Nutrient impacts Nutrient
Riffle Beetles to Riparian Buffers
 We care about WateR. it S What We do. Riffle Beetles to Riparian Buffers Exploring Methods of Assessing Surface Water Quality Grade level: 7-12 objective: Students will evaluate the quality of two water
We care about WateR. it S What We do. Riffle Beetles to Riparian Buffers Exploring Methods of Assessing Surface Water Quality Grade level: 7-12 objective: Students will evaluate the quality of two water
Nicholas A. Reckinger. June 11, 2007
 Comparison of Phosphorus Forms at Different Spatial Scales and Assessment of an Area-Weighted P-Index to Multi-Field Watersheds Nicholas A. Reckinger June 11, 2007 Watershed Activities Affect Water Quality
Comparison of Phosphorus Forms at Different Spatial Scales and Assessment of an Area-Weighted P-Index to Multi-Field Watersheds Nicholas A. Reckinger June 11, 2007 Watershed Activities Affect Water Quality
Limiting growth factors. Water Temperature Nutrients
 Limiting growth factors Water Temperature Nutrients What are they? Nutrient Dynamics Why do we care? Stream chemistry study Why do nutrients matter? Limit primary production Affect decomposition (+/ )
Limiting growth factors Water Temperature Nutrients What are they? Nutrient Dynamics Why do we care? Stream chemistry study Why do nutrients matter? Limit primary production Affect decomposition (+/ )
Chemical Reduction processes for In Situ Soluble Metals Remediation and Immobilization in Groundwater
 Chemical Reduction processes for In Situ Soluble Metals Remediation and Immobilization in Groundwater 2014 RPIC Federal Contaminated Sites National Workshop Ottawa 2014 Prepared by Jean Paré, P. Eng. Chemco
Chemical Reduction processes for In Situ Soluble Metals Remediation and Immobilization in Groundwater 2014 RPIC Federal Contaminated Sites National Workshop Ottawa 2014 Prepared by Jean Paré, P. Eng. Chemco
