RSC Advances.
|
|
|
- Ethan Caldwell
- 6 years ago
- Views:
Transcription
1 This is n Accepted Mnuscript, which hs een through the Royl Society of Chemistry peer review process nd hs een ccepted for puliction. Accepted Mnuscripts re pulished online shortly fter cceptnce, efore technicl editing, formtting nd proof reding. Using this free service, uthors cn mke their results ville to the community, in citle form, efore we pulish the edited rticle. This Accepted Mnuscript will e replced y the edited, formtted nd pginted rticle s soon s this is ville. You cn find more informtion out Accepted Mnuscripts in the Informtion for Authors. Plese note tht technicl editing my introduce minor chnges to the text nd/or grphics, which my lter content. The journl s stndrd Terms & Conditions nd the Ethicl guidelines still pply. In no event shll the Royl Society of Chemistry e held responsile for ny errors or omissions in this Accepted Mnuscript or ny consequences rising from the use of ny informtion it contins.
2 Pge 1 of 13 Corse-grined (CG) Corrosion film CG-1 Nnocrystlline (NC) Corrosion film NC-1 A hydrophoic protective corrosion product film (NC-1) with nno-wires structure is formed on the surfce of nnocrystlline zinc coting.
3 Pge 2 of 13 RSCPulishing Cite this: DOI: /x0xx00000x Received 00th Jnury 2012, Accepted 00th Jnury 2012 DOI: /x0xx00000x Introduction The electrodeposition of zinc coting hs een widely pplied in mechnicl, erospce, utomotive nd construction fields for steel protection ginst corrosion ecuse of its excellent nd comprehensive properties: (i) zinc coting is stle in ir nd it forms lyer of dense lkline zinc cronte film, which plys role in physicl protection of the sustrte in humid environment; (ii) zinc is n nodic coting reltive to cron steel, iron nd low lloy steel, which cn ply role in electrochemicl protection when it forms micro-corrosion ttery with steel nd the dmged re of the coting surfce is not too ig; nd (iii) the cost of electrodeposited zinc coting is low nd the technique is esy ppliction, especilly. The production of electrodeposited zinc is incresing yer y yer Deciphering the formtion mechnism of protective corrosion product lyer from electrochemicl nd nturl corrosion ehviors of nnocrystlline zinc coting Qingyng Li, Zhongo Feng, Lihu Liu, Hong Xu, Wng Ge, Fenghun Li nd Mozhong An, * The corrosion resistnce improvement of zinc coting with the reduction of grin sizes from micro to nno-scles hs long een ttriuted to the formtion of etter protection of corrosion product lyer. However, the formtion mechnism of protective corrosion product lyer hs rrely een studied. Here nnocrystlline zinc cotings re produced y pulse reverse electrodeposition in sulfte th with polycrylmide s the only dditive. The electrochemicl nd nturl corrosion ehviors of electrodeposited nnocrystlline zinc coting in comprison with conventionl corse-grined zinc coting in simulted sewter re investigted. Nnocrystlline zinc coting exhiits distinctly enhnced corrosion resistnce in the simulted sewter compred to corse-grined zinc coting. The enhnced corrosion resistnce of zinc cotings with the reduction of grin sizes from micro (6 µm) to nno-scles (31 nm) is due to the fct tht nnocrystlline zinc coting is chrcterized y high-volume frction of grin oundry, nd the zinc toms t grin oundries possess higher ctivity. It is eneficil for rpidly forming protective corrosion product film with hydrophoic nnowires structure on the surfce of zinc coting during the exposure to simulted sewter, nd therey contriuting to the corrosion resistnce enhncement. Bsed on nlysis results, the possile formtion mechnism of protective corrosion product lyer on the surfce of nnocrystlline zinc coting is discussed in detil. nd it hs ecome one of the indispensle surfce modifiction techniques for the steel nowdys. Despite of this, the electrodeposition of corse-grined zinc cotings just retins the structure nd corrosion property of zinc, nd the success of using zinc s steel coting cn e ttriuted to its scrificil nture, therey the coting must e thick enough to endure the ttck of corrosive environment. However, thick cotings cn led to wste of zinc, s well s poor weldility, nd difficulty in the speculr finish fter pinting. Becuse of these chllenges, the ppliction of electrodeposition of zinc coting is gretly limited in mny fields. It is therefore necessry to develop thinner electrodeposited cotings with excellent corrosion resistnce nd investigte the corrosion ehvior in order to dely the corrosion process. 1-3 This journl is The Royl Society of Chemistry 2013 J. Nme., 2013, 00, 1-3 1
4 Pge 3 of 13 Electrodeposition of nnocrystlline cotings possesses excellent wer resistnce, 4,5 corrosion resistnce, 6,7 ductility, 8,9 hrdness 10,11 nd electrochemicl properties 12 due to its grin size elow 100 nm nd high-volume frction of grin oundry, 13 when compred with conventionl corse-grined cotings. Therefore, it is of gret significnce to improve the corrosion property of conventionl zinc coting through nnoelectrodeposition technology, nd it hs ecome future direction. So fr, severl nnocrystlline zinc cotings hve een otined through direct current or pulse current electrodeposition from vrious glvnizing th (such s lkli, 14 chloride, 15 sulfte, 16 cette 17 or citrte 18 system) with two or more dditives. Menwhile, some relted works hve een done to investigte the corrosion ehvior of nnocrystlline zinc cotings in vrious solutions y different techniques. For exmple, Youssef et l. 3 investigted the corrosion ehvior of nnocrystlline zinc deposit (56 nm grin size) in 0.5 M NOH solution (ph=13.6, 25 ± 1 C) y potentiodynmic polriztion nd impednce mesurements. Rmnusks et l. 14 investigted the corrosion ehvior of nnocrystlline zinc cotings (30~120 nm) in 0.6 M NCl M NHCO 3 solution (ph=6.8, 25 ± 1 C) using polriztion mesurements. G Z Meng et l. 18 studied the pitting corrosion ehvior of nnocrystlline zinc (21.5 nm grin size) in 0.5 M NCl (ph=12, 25 ± 1 C) through sttisticl methods. M C Li et l. 19 lso demonstrted tht the corrosion resistnce of nnocrystlline zinc deposit (43 nm grin size) ws greter thn tht of its corse-grined counterprt in 3.5% NCl solution (open to ir t 25 ± 2 C). There hve een other studies on the corrosion resistnce of nnocrystlline zinc cotings. Almost ll of them suggest tht the etter corrosion resistnce of nnocrystlline zinc cotings minly results from their protective corrosion product lyers However, the formtion mechnism of corrosion product film on the surfce of nnocrystlline zinc coting hs een scrcely studied to dte. In our previous work, the nnocrystlline zinc cotings were produced y pulse reverse electrodeposition nd the triologicl ehvior of nnocrystlline zinc coting ws investigted. 23,24 In this pper, the electrochemicl corrosion nd nturl corrosion ehviors of nnocrystlline zinc coting in simulted sewter re systemticlly investigted, nd these ehviors re compred with those of conventionl corse-grined zinc coting. Specilly, interests re focused on the formtion mechnism of protective corrosion product film on the surfce of nnocrystlline zinc coting. Experimentl Synthesis nd chrcteriztion of zinc cotings According to our previous work, nnocrystlline zinc cotings were produced on cron steel sustrte (4 4 cm 2 ) y pulse Tle 1 Compositions nd operting conditions of electrodeposition th. reverse electrodeposition from sulfte th contining polycrylmide s the only dditive, nd the electrolyte composition, th conditions nd electrodeposition prmeters re listed in Tle 1. 23,24 Besides the nnocrystlline zinc cotings prepred in this study, conventionl corse-grined zinc cotings were lso electrodeposited from sic sulfte th (ZnSO 4 7H 2 O 100 g L -1 nd H 3 BO 3 20 g L -1 ) under the sme experimentl conditions for comprison. All electrodeposition experiments were duplicted nd good reproduciility ws otined. After electrodeposition, zinc cotings were rinsed immeditely in deionized wter, then dried nd sujected directly to chrcteriztion nd property mesurements. Surfce nd cross-sectionl morphologies of zinc cotings were chrcterized y field-emission scnning electron microscope (FESEM, Helios Nnol 600i) with energy dispersive X-ry spectroscopy (EDS) nd tomic force microscope (AFM, Bruker Multimode 8). X-ry diffrction (XRD, Rigku Corportion Dmx-3B) ws crried out using Cu K rdition in order to determine the crystlline texture, crystllogrphic preferred orienttion nd pproximte verge grin size of the cotings. The grin size ws clculted y the Scherrer's formul ccording to eqution (1). 25 = (1) Where D is the grin size (in nm), K is constnt (0.89), λ is the X-ry wvelength (0.154 nm), β is the full width t hlf mxim in 2θ degrees, nd θ is the diffrction ngle. The wettility of zinc cotings ws evluted sed on contct ngles otined y contct ngle goniometer (CA, Dtphysics OCA20) with droplet (3 µl) of wter s n indictor. Typiclly, 3 different points were otined for individul specimens, from which the verge vlues were clculted. All results re reported s the men numer ± stndrd devition. Corrosion ehvior evlution of zinc cotings Chloride-ion-induced corrosion is common sitution for protective zinc cotings such s in mrine environments, 19 hence the sewter is simulted using 3.5 wt.% NCl solution nd pplied s the corrosive medium in this study. Electrochemicl corrosion ehvior: The electrochemicl corrosion ehvior of zinc cotings ws evluted y electrochemicl impednce spectroscopy (EIS) nd potentiodynmic polriztion techniques in erted simulted sewter t room temperture (24 ± 1 C). All electrochemicl mesurements were performed in three-electrode cell using CHI 750D electrochemicl worksttion with pltinum plte, sturted clomel electrode (SCE) nd zinc cotings with geometricl working re of 1 1 cm 2 s the uxiliry, reference Compositions Electrodeposition prmeters Zinc sulfte 100 g L -1 Forwrd pulse current density 3 A dm -2 Boric cid 20 g L -1 Reverse pulse current density 0.3 A dm -2 Polycrylmide 1 g L -1 Forwrd pulse durtion 100 ms ph 1-2 Reverse pulse durtion 10 ms Temperture 23 ± 2 C Duty cycle 20% Mgneticlly stirred mild Time 60 min 2 J. Nme., 2012, 00, 1-3 This journl is The Royl Society of Chemistry 2012
5 Pge 4 of 13 nd working electrode, respectively. The working electrode ws immersed in simulted sewter for 30 min efore pplying the current to estlish stle rest potentil. EIS mesurements were conducted t open-circuit potentil with potentil mplitude of 10 mv, nd frequency rnge from 10-2 Hz to 10 5 Hz. Before conducting EIS experiments, the working electrode ws kept in simulted sewter for 30 min in order to stilize the corrosion potentil. The equivlent circuit simultion progrm ZsimpWin ws used for dt nlysis nd fitting of the impednce dt. Potentiodynmic polriztion curves were otined y chnging the electrode potentil in the rnge of ± 500 mv round the open-circuit potentil (OCP) ginst SCE t scn rte of 1.0 mv S -1. The corrosion potentils (E corr ), nodic nd cthodic Tfel slopes (β nd β c ) were clculted from the polriztion curves using liner extrpoltion method. Then, the corrosion current (i corr ) of zinc cotings ws clculted y mens of the Stern-Gery eqution ccording to eqution (2). 26 = ( ) (2) The liner polriztion resistnce (R p ) ws determined y the slope of current-potentil plot in the rnge of ± 2 mv out the corrosion potentil ccording to eqution (3). 3,27 c =( ) (3) 10 µm After potentiodynmic polriztion test, the surfce morphology nd element composition of zinc cotings ws nlyzed y FESEM nd EDS elementl mpping, respectively. Nturl corrosion ehvior: The nturl corrosion ehvior of zinc cotings ws evluted y n immersion test, nd simulted sewter ws used s the corrosive medium. All experiments were crried out t room temperture (24 ± 1 C) in closed environment with epoxy resin seling tretment, s shown in Fig. S1. In order to ensure zinc cotings in complete contct with corrosive medium, the cotings were immersed in simulted sewter for 30 s efore immersion test. After immersion tests, mesurements y FESEM, EDS, XRD nd contct ngle were crried out for the corroded coting specimens. Moreover, the corrosion resistnce property of corrosion product lyers formed on the coting surfce ws exmined y EIS nd potentiodynmic polriztion techniques. Results nd discussion Electrodeposition of nnocrystlline nd corse-grined zinc cotings The surfce morphologies of corse-grined nd nnocrystlline zinc cotings re shown in Fig. 1. Both cotings re otined t the sme electrodeposition prmeters, s shown in Tle 1. It cn e seen tht the FESEM microgrph of the d 40 nm 10 µm 200 nm Fig. 1 FESEM microgrphs of corse-grined ( nd c) nd nnocrystlline () zinc cotings, nd AFM imge of nnocrystlline (d) zinc coting. This journl is The Royl Society of Chemistry 2012 J. Nme., 2012, 00, 1-3 3
6 Pge 5 of 13 coting otined from sic sulfte th (ZnSO 4 7H 2 O-100 g L - 1 nd H 3 BO 3-20 g L -1 ) shows n irregulr corse-grined size (pproximtely 6 µm), s shown in Fig. 1. In sic sulfte th, the coting displys the hexgonl zinc pltes ligned prllel to the sustrte, indicting tht the hexgonl structure of zinc is preserved in zinc cotings. As clerly illustrted from Fig. 1c, lminted structure with thickness of out 60 nm is detected for corse-grined zinc coting. However, it only chieved nnoscle in the thickness direction not in the three dimensionl grin sizes. By compring with corse-grined zinc coting, the FESEM microgrph (Fig. 1) of nnocrystlline zinc coting electrodeposited from the optimum th shows perfect crystl growth, uniform rrngement of crystls nd refinement in crystl size. We cn lso see tht the corresponding AFM microgrph (Fig. 1d) of nnocrystlline zinc coting shows pproximtely sphericl-shped prticles, nd the grin size is round 40 nm. XRD ptterns (Fig. 2) for the cotings indicte tht the corse-grined zinc coting hs (002) preferred orienttion, while the nnocrystlline zinc coting hs well preferred orienttion long (110) direction nd the verge grin size is 31nm clculted y Scherrer's formul. The clculted result of grin size is consistent with the oservtion y AFM. As discussed in the litertures, 15,28 the formtion of nnocrystlline zinc coting is ttriuted to the presence of dditives (in our optimum th, the only dditive is polycrylmide). Moreover, the cross-sectionl FESEM imges (s shown in Fig. S2) show tht the thickness of corse-grined nd nnocrystlline zinc cotings is 53 nd 41 µm, respectively. It is noteworthy tht, lthough the surfce nnocrystlliztion reduces the thickness of zinc cotings, the nnocrystlline zinc coting exhiits reltively smoother surfce nd more uniform thickness distriution thn tht of corse-grined zinc smple. The thickness decrese of zinc cotings is ttriuted to the fct tht the polycrylmide refines the grin size s well s decreses the current efficiency of zinc electrodeposition. Fig. 2 X-ry diffrction ptterns of corse-grined nd nnocrystlline zinc cotings. Electrochemicl corrosion ehvior of zinc cotings Effective electrochemicl methods of EIS nd potentiodynmic polriztion technique re used to chrcterize the corrosion resistnt properties of zinc cotings in simulted sewter. Typicl Bode nd Nyquist digrms of corse-grined nd nnocrystlline zinc cotings re otined y EIS mesurements in simulted sewter, nd re displyed in Figs. 3 nd 4, respectively. An equivlent circuit shown in Fig. 4 is proposed to simulte the zinc/solution corrosion system ccording to the litertures. 19,29,30 Here, R s is the resistnce corresponding to the ohmic resistnce of the electrolyte, R l nd C l (CPE : Q l ) represent the resistnce nd cpcitnce of porous corrosion product lyer (or constnt phse element of porous corrosion product lyer), R ct nd C dl (CPE : Q dl ) represent the chrge trnsfer resistnce nd doule lyer cpcitnce (or doule lyer constnt phse element), nd Z W is the Wrurg impednce, respectively. The vlues of ll elements otined y fitting re listed in Tle S1. Moreover, the clculted impednces from fit prmeters re lso plotted in Fig. 4, nd show good greement with the experimentl dt for nnocrystlline nd corse-grined zinc cotings, indicting tht the equivlent circuit model provides relile description for the corrosion system. It is ovious tht surfce nnocrystlliztion of zinc cotings results in n increse in the interfce impednce nd in the mximum phse ngle in Fig. 3, indicting n improvement in the corrosion resistnce. 31,32 Moreover, from the Bode phse plots it cn lso e oserved tht the phse ngle do not rech zero t high frequencies for oth cotings, indicting the presence of defects in the coting surfce. 33 As seen from Fig. 4, oth Nyquist plots exhiit single depressed cpcitive semicircles t higher frequencies, which is ttriuted to the chrge trnsfer in comintion with the corrosion product lyers. 19,30 It is noteworthy tht the dimeter of the semicircle increses with the reduction of grin size to nnocrystlline, indicting the much lrger impednce vlues of nnocrystlline zinc coting. This result is lso confirmed y the fitted results (s shown in Tle S1), showing tht the vlues of R ct re much lrger for nnocrystlline zinc coting (R ct(nc) =360.2 Ω cm 2 ) thn for corse-grined zinc coting (R ct(cg) =121.9 Ω cm 2 ). These results ll demonstrte tht the corrosion resistnce of zinc cotings is significntly improved with the reduction of grin sizes. In order to verify the EIS results, the corrosion resistnce properties of nnocrystlline nd corse-grined zinc cotings re lso evluted through potentiodynmic polriztion curves in simulted sewter, s shown in Fig. 5. The corrosion processes of zinc cotings re in ccordnce with those reported y other investigtors, which could e explined y two prtil rections: The cthodic rection is the reduction of oxygen nd leds to n increse in ph. O 2 + 2H 2 O + 4e - 4OH - (4) The nodic rection involves the dissolution of zinc nd leds to decrese in the smple weight. Zn Zn e - (5) A notle difference is tht the curve of nnocrystlline zinc coting shift to the left-hnd side over the entire potentil rnge in comprison with tht of corse-grined zinc coting, which mens tht nnocrystlline zinc coting hs lower oth nodic nd cthodic rection rtes during corrosion process thn corse-grined zinc coting. A summry of electrochemicl prmeters clculted from the potentiodynmic polriztion This journl is The Royl Society of Chemistry 2013 J. Nme., 2013, 00, 1-3 4
7 Pge 6 of 13 Fig. 3 Bode plots of corse-grined nd nnocrystlline zinc cotings in simulte sewter: () Bode mgnitude plots nd () Bode phse plots. R s Fig. 4 Nyquist plots of corse-grined () nd nnocrystlline () zinc cotings in simulte sewter nd equivlent circuit. Symols: mesured dt; lines: simulted dt. curves y Stern-Gery eqution is listed in Tle 2. It is evident tht, lthough the corrosion potentil (E corr ) of corse-grined zinc coting is more positive thn tht of nnocrystlline zinc coting, the nnocrystlline zinc smple exhiits more protective coting thn tht of corse-grined zinc smple. This is evidenced y the lower corrosion current density (i corr ) of nnocrystlline zinc coting. The results firly ccorded with experimentl results reported in Artho Nik's litertures, 16,20,21 nd revel the sme vrition trend in corrosion resistnce with the reduction of grin sizes. The corrosion resistnce of zinc coting is heighted y more thn 1 time with the reduction of grin sizes from micro (6 µm) to nno-scle (31 nm). After potentiodynmic polriztion test, FESEM oservtion nd EDS nlysis re crried out for corse-grined nd nnocrystlline zinc cotings nd re shown in Figs. 6, 7 nd 8, R l C l (CPE : Q l ) C dl (CPE : Q dl ) R ct Z w Fig. 5 Polriztion curves of corse-grined () nd nnocrystlline () zinc cotings in simulted sewter t 25 ± 1 C. Tle 2 Electrochemicl prmeters of corse-grined (CG) nd nnocrystlline (NC) zinc cotings in simulted sewter t 25 ± 1 C Smple E corr mv β mv dec -1 β c mv dec -1 R p Ω cm 2 i corr µa cm -2 CG NC respectively. Significnt differences cn e oserved in the corroded surfces of zinc cotings nd re shown in Fig. 6. There re mny crcks on the surfce of corse-grined zinc coting, nd prts of the coting hve een detched from the coting surfce during the corrosion process (Fig. 6-c), indicting tht the corrosion sites re locl nd corroded dly. The EDS spectrum (Fig. 7) for the mteril detched from the surfce of corse-grined zinc coting confirms it is zinc. As This journl is The Royl Society of Chemistry 2012 J. Nme., 2012, 00, 1-3 5
8 Pge 7 of 13 for nnocrystlline zinc coting, some smll nd shllow etchpits re discretely distriuted over the corroded surfce (Fig. 6d-f), which indictes etter protective surfce film. Menwhile, The EDS elementl mppings for the corroded surfces of zinc cotings re lso detected nd re shown in Fig. 8. After potentiodynmic polriztion test in simulted sewter, oth corrosion products minly consist of Zn, O nd Cl elements. It is noteworthy tht the chemicl distriution on nnocrystlline zinc coting is reltively uniform thn tht on corse-grined zinc coting, lso indicting tht the electrochemicl corrosion of corse-grined zinc coting is locl ehvior. In this cse, the improvement in corrosion resistnce of zinc cotings with the reduction of grin sizes from micro to nno-scles is ttriuted to the fct tht the c d 20 µm 20 µm e 5 µm 5 µm Fig. 6 FESEM imges with different mgnifictions of corse-grined (-c) nd nnocrystlline (d-f) zinc cotings in simulted sewter fter potentiodynmic polriztion test. f 1 µm Fig. 7 FESEM imge nd corresponding EDS pttern for the corrosion products on the surfce of corse-grined zinc coting. 6 J. Nme., 2012, 00, 1-3 This journl is The Royl Society of Chemistry 2012
9 Pge 8 of 13 Imge Zn Imge Zn 20 µm 20 µm O Fig. 8 EDS elementl mppings for Zn, O nd Cl on the surfce of corse-grined () nd nnocrystlline () zinc cotings. nnomterils re chrcterized y high-volume frction of grin oundry, nd the zinc toms tht re t the grin oundries possess higher ctivity. For corse-grined zinc coting, it only chieves the nnoscle in the thickness direction (s shown in Fig. 1c), so the zinc toms tht re in the thickness direction possess higher ctivity. As for nnocrystlline zinc coting, it chieves the nnoscle in three-dimensionl spce, so zinc toms tht re exposed on the surfce of the coting possess higher ctivity. The reduction of grin sizes cn led to the numer increse in ctive toms of the surfce, ccelerting the formtion of corrosion product lyers on the surfce of zinc cotings during the electrochemicl corrosion process. Mny uthors hve reported tht zinc hydroxide chloride lyer forms on the zinc cotings during exposure to NCl solution, nd the corresponding elements cn lso e found in the EDS elementl mpping (s shown in Fig. 8). This lso explins the reson why nnocrystlline zinc coting exhiits more negtive E corr thn corse-grined zinc coting (s shown in Fig. 5). Since there is no ovious pssivtion region in the polriztion curves of zinc cotings, the zinc hydroxide chloride lyer is lwys clssified s pseudopssive lyer. 38 The insolule zinc hydroxide chloride lyers tht cover the surfce of corroded cotings cn inhiit corrosion further, therey decrese the rte of zinc dissolution. 32,34 Moreover, these discrete etch pits on the surfce of nnocrystlline zinc coting re lso n indiction tht corrosion initites from defect sites of the zinc hydroxide chloride film. In order to verify this reltionship etween corrosion resistnce nd corrosion product lyer of zinc cotings, corrosion product lyer is synthesized on the surfce of corse-grined nd nnocrystlline zinc cotings y n immersion test in simulted sewter, furthermore the formtion mechnism nd properties of corrosion product films re systemticlly investigted. The nturl corrosion ehvior of zinc cotings Cl The nturl corrosion ehvior of zinc cotings is evluted y n immersion test in the simulted sewter. XRD nlysis in Fig. 9 revels the corrosion product lyers on oth zinc coting surfces re primrily composed of ZnO fter 100 h of O Cl immersion. According to the literture, 19,39 there my e other corrosion products such s Zn 5 (OH) 8 Cl 2 H 2 O (simonkolleite) nd Zn(OH) 2, which re in minor mount or exist in the form of noncrystl phse, nd therey sometimes they re difficult to e found y XRD. The existence of ZnO should men the pssivtion (or corrosion) of zinc cotings during exposure to simulted sewter, nd the pssivtion ehvior of zinc cotings is minly controlled y the oxygen. Fig. 9 XRD ptterns for corse-grined (CG) nd nnocrystlline (NC) zinc cotings fter 100 h of immersion in simulte sewter. Bsed on nlysis results ove, n isolted system of zinc coting, simulted sewter nd ir re used to simulte the nturl corrosion ehvior of zinc cotings (Fig. S1.). Since the environment is isolted, the oxygen content in the solution re lower thn tht in the ir, indicting tht the corrosion rection of zinc cotings ove the ir/simulted sewter interfce is more complete thn tht of zinc cotings elow the interfce under the sme immersion time. Therefore, the coting nd This journl is The Royl Society of Chemistry 2013 J. Nme., 2013, 00, 1-3 7
10 Pge 9 of 13 simulted sewter system elow the ir/simulted sewter interfce cn e used to simulte the erlier stge of corrosion; the coting nd ir system ove the interfce cn e used to simulte the lter stge of corrosion in the immersion test; nd the coting nd ir system t the interfce corresponds to the middle stge of corrosion. After 100 h of immersion in this isolted system, the FESEM oservtion nd the EDS nlysis re crried out for corsegrined nd nnocrystlline zinc cotings, s shown in Fig. 10. Pnels () nd (d) re the surfce morphologies of corsegrined nd nnocrystlline zinc cotings elow ir/simulted sewter interfce, respectively; pnels () nd (e) re the surfce morphologies of corse-grined nd nnocrystlline zinc cotings t the interfce, respectively; nd pnels (c) nd (f) re the surfce morphologies of corse-grined nd nnocrystlline zinc cotings ove the interfce, respectively. As shown in Fig. 10, oth surfces re covered with corrosion product lyer. In the cse of corse-grined zinc coting, the corrosion ehvior first tkes plce in the thickness direction of the coting (Fig. 10) in the erlier stge of corrosion nd grdully extends to most prt of the coting surfce (Fig. 10), therey forming lyer of complete corrosion product film on the surfce of the coting (Fig. 10c) in the lter stge of corrosion. It is noteworthy tht there re some micro-crcks on the surfces of corrosion product lyer, s shown y the inset in Fig 10c. As for nnocrystlline zinc coting, the surfce hs een completely covered with uniform corrosion product lyer, even in very limited mount of oxygen environment (Fig. 10d). The corrosion product lyer shows n pproximte nno-pillrs structure, s shown y the inset in Fig. 10d, nd experiences the trnsition from nnopillrs to nno-wires with the progress of corrosion, therey ecomes more uniform, finer nd denser in the middle nd lter stge of corrosion (s shown in Fig. 10e nd f ). In order to verify the imges displyed in Figs. 10c nd 10f re true reflection of the surfce morphologies of corrosion product lyers in the lter stge of corrosion nd re not ffected y oxidtion rection, the FESEM nd EDS oservtions re lso crried out for corse-grined nd nnocrystlline zinc cotings fter 100 h of plcement in the sme isolte environment without ny corrosive medium, s shown in Fig. S3. Compred with the surfce morphologies of freshly prepred corse-grined nd nnocrystlline zinc cotings (in Fig. 1), the surfce morphologies of oth cotings (Fig. S3 nd S3c) fter 100 h of plcement in this isolte environment hve not significntly chnged. The EDS results lso indicte tht oth the cotings re lmost no oxidtion (Fig. S3 nd S3d). It implies tht the surfce morphologies displyed in Figs. 10c nd 10f come from zinc cotings corrosion, nd the results of FESEM oservtion re relile. A mesurement of wetting property (Fig. 11) for the corrosion product films on the surfce of corse-grined nd nnocrystlline zinc cotings in the lter stge of corrosion indictes tht, ovementioned morphology differences cuse mrkedly lrger contct ngles for the corrosion product film of nnocrystlline zinc coting (d) thn for the corrosion product film of corse-grined zinc coting (), corse-grined zinc coting () nd nnocrystlline zinc coting (c). According to the studies of Imz et l. 33 nd Teschke et l., 40 mterils with more hydrophoic chrcter re typiclly more resistnt ginst corrosion in queous environments. Hence, ccording to the correltion etween the wettility of given mteril nd its nticorrosion performnce, the corrosion product film of nnocrystlline zinc coting is more resistnt ginst corrosion in queous environments. The difference of formtion mechnisms of corrosion product film etween corse-grined zinc coting nd nnocrystlline zinc coting is ttriuted to the quntity nd position vrition of ctivity sites of zinc cotings with the reduction of grin size (s stted ove). The volume frction of grin oundry increses with the surfce nnocrystlliztion of the coting, thus the numer of the surfce ctivity sites is increses, mking the surfce of nnocrystlline zinc coting rpidly form protective corrosion product film compred to corse-grined zinc coting during exposure to simulted sewter. Moreover, EDS spectrums for corrosion product lyer on the surfce of zinc cotings (in Fig. 10) show tht the films predominntly contin Zn nd O. A summry of tomic oxygen content derived from EDS spectrums is lso given in Fig. S4. It cn e seen tht the tomic oxygen content of corrosion product film on the surfce of nnocrystlline zinc coting is higher thn tht of corrosion product film on the surfce of corsegrined zinc coting elow ir/simulted sewter interfce, indicting tht corrosion rection of nnocrystlline zinc coting is quicker thn tht of corse-grined zinc coting in the erlier stge of corrosion. The surfce of nnocrystlline zinc coting cn rpidly form protective corrosion product film compred to tht of corse-grined zinc coting, nd inhiits the progress of corrosion rection. Therefore the tomic oxygen content of corse-grined zinc coting is higher thn tht of nnocrystlline zinc coting in the middle nd lter stges of corrosion. To explin the nturl corrosion ehvior of corse-grined nd nnocrystlline zinc cotings in simulted sewter, EIS mesurements re crried out t their respective open circuit potentils fter 100 h of immersion. The impednce response of this system is presented in Figs. 12 nd 13, respectively, which exemplify, the Bode (mgnitude nd phse ngle plots) nd Nyquist plots. Experimentl dt is lso compred with the fitted results of equivlent circuit nd shows good coincidence. The EIS spectrum of the corrosion product lyer on nnocrystlline zinc coting is still fitted y the originl equivlent circuit (s shown y the inset in Fig. 4). However, since micro-crcks ppers on the corrosion product lyer of corse-grined zinc coting, the originl equivlent circuit is not pproprite for simulte the zinc/solution corrosion system from the fitting results. Therefore, new equivlent circuit is used to simulte the zinc/solution corrosion system of the corrosion product lyer on corse-grined zinc coting, s shown y the inset in Fig. 13. Here, R s is the resistnce corresponding to the ohmic resistnce of the electrolyte, R l1 (R l2 ) nd C l1 (C l2 ) represent the resistnce nd cpcitnce of porous corrosion product lyer, R ct nd C dl represent the chrge trnsfer resistnce nd doule lyer cpcitnce, nd Z W is the Wrurg impednce, respectively. Tle S2 nd S3 give the fitted results of EIS spectr for the corrosion product lyers of corsegrined nd nnocrystlline zinc cotings in simulted sewter, respectively. Oviously, the impednce of the interfce (Fig. 12), the mximum phse ngle (Fig. 12) nd the semicircle size (Fig. 13) of nnocrystlline zinc coting is igger thn tht of corsegrined zinc coting fter 100 h of immersion in this system. Hence, it cn e concluded tht the corrosion product lyer of nnocrystlline zinc coting exhiits higher resistnce ginst corrosion thn tht of corse-grined zinc coting. Different from preceding oservtion in Fig. 4, the Nyquist plot of 8 J. Nme., 2012, 00, 1-3 This journl is The Royl Society of Chemistry 2012
11 Pge 10 of 13 Air/simulted sewter interfce d e c f 1 µm Aove the interfce Fig. 10 Surfce morphologies nd element compositions for corroded surfces of corse-grined (-c) nd nnocrystlline (d-f) zinc cotings fter the immersion test. The imges (, d), (, e) nd (c, f) correspond to corroded surfces of zinc cotings elow the interfce, t the interfce nd ove the interfce, respectively. This journl is The Royl Society of Chemistry 2013 J. Nme., 2013, 00, Below the interfce
12 Pge 11 of 13 corse-grined zinc coting is composed of two depressed cpcitive semicircles fter 100 h of immersion in simulted sewter, suggesting tht the corrosion mechnism of zinc cotings is chnging. The high frequency semicircle is ttriuted to the chrge trnsfer in comintion with the corrosion products, while low frequency semicircle indictes finite length diffusion process through porous lyer. This chnge is minly scried to the formtion of corrosion product lyer during exposure to simulted sewter. As for nnocrystlline zinc coting, the Nyquist plot just exhiits single depressed cpcitive semicircle t higher frequency, while low frequency semicircle evolves to stright line with the reduction of grin sizes, indicting tht the finite diffusion Fig. 11 The vrition in contct ngle of corse-grined ( nd ) nd nnocrystlline (c nd d) zinc surfces efore nd fter 100 h of immersion in simulted sewter: () nd (d) re the surfce contct ngles of corse-grined () nd nnocrystlline (c) zinc cotings fter 100 h of immersion in simulted sewter (ove ir/simulted sewter interfce prt), respectively. process of corse-grined zinc coting grdully trnsforms into the semi-infinite diffusion process with the reduction of grin sizes. According to the fitted results of equivlent circuit (in Tle S2 nd S3), the R ct of corrosion product lyers on the surfces of corse-grined nd nnocrystlline zinc cotings re nd Ω cm 2, respectively, implying tht the ltter hs higher resistnce ginst corrosion thn the former. Apprently, the corrosion product lyer on the surfce of nnocrystlline zinc coting should e more protective thn tht on the surfce of corse-grined zinc coting. The corrosion inhiition efficiency (η) of corrosion product lyer on the surfce of nnocrystlline zinc coting is clculted y the following eqution: 41 = ( ) ( ) ( ) 100% (6) Where R ct(cg) nd R ct(nc) re the chrge trnsfer resistnce vlues of the corrosion product lyers on corse-grined nd nnocrystlline zinc cotings, respectively. The η of corrosion product lyer on the surfce of nnocrystlline zinc coting is pproximtely %. The corrosion resistnce of corrosion product lyers on the surfces of oth zinc cotings is lso evluted through potentiodynmic polriztion technique in simulted sewter, s shown in Fig.14, with the electrochemicl prmeters (E corr, β, β c, R p nd i corr ) of smples presented in Tle 3. These dt clerly shows tht the corrosion product lyer of nnocrystlline zinc coting possesses higher resistnce ginst corrosion thn tht of corse-grined zinc coting. Compred to the polriztion curves of zinc coting (in Fig. 5), notle difference is tht the corrosion potentil (E corr ) of corrosion product lyer on the surfce of nnocrystlline zinc coting is more positive thn tht on the surfce of corse-grined zinc coting. It implies tht the corrosion product of nnocrystlline zinc coting is more stle thn tht of corse-grined zinc coting in the sme corrosion medium. From the ove results, it is deduced tht the corrosion product lyer of nnocrystlline zinc coting hs more effective corrosion inhiition thn tht of corse-grined zinc in simulted sewter. Fig. 12 Bode plots for the corroded surfces of corse-grined nd nnocrystlline zinc cotings in simulted sewter fter 100 h of immersion: () Bode mgnitude plots nd () Bode phse plots. This journl is The Royl Society of Chemistry 2013 J. Nme., 2013, 00,
13 Pge 12 of 13 Fig. 13 Nyquist plots for the corroded surfces of corsegrined () nd nnocrystlline () zinc cotings in simulted sewter fter 100 h of immersion, nd equivlent circuit of the corrosion product lyer on corse-grined zinc coting. Symols: mesured dt; lines: simulted dt. Fig. 14 Polriztion curves for the corroded surfces of corsegrined () nd nnocrystlline () zinc cotings in simulted sewter fter 100 h of immersion. Tle 3 Electrochemicl prmeters of corse-grined (CG) nd nnocrystlline (NC) zinc in simulted sewter fter 100 h of immersion. Smple E corr mv β mv dec -1 β c mv dec -1 R p Ω cm 2 i corr µa cm -2 CG NC Conclusion R s R l1 The electrochemicl corrosion nd nturl corrosion ehviors of zinc cotings chnging with the reduction of grin sizes re descried in this pper, which is to give insight into the formtion mechnism of protective corrosion product film on C l1 R l2 C l2 C dl R ct the surfce of nnocrystlline zinc coting, nd therey to improve the resistnce ginst corrosion of conventionl corse-grined zinc coting. Bsed on the results from present study, the corrosion ehviors of zinc cotings re simply ssumed s follows: (1) As for the electrochemicl corrosion ehvior of corsegrined nd nnocrystlline zinc cotings in simulted sewter, the etter corrosion resistnce of nnocrystlline zinc coting is ttriuted to the fct tht grin refinement leds to the numer increse in ctive toms of the surfce, ccelerting the formtion of zinc hydroxide chloride lyer on the surfce of nnocrystlline zinc coting. The insolule pseudo-pssive lyers tht cover the surfce of corroded cotings inhiit corrosion further, therey decrese the rte of zinc dissolution. (2) The immersion test proves the corrosion product lyer of nnocrystlline zinc coting hs etter corrosion resistnce thn tht of corse-grined zinc coting. The corrosion ehviors preferentilly tke plce in the grin oundry of zinc cotings during exposure to simulted sewter. For the corse-grined zinc coting, the corrosion ehvior first tkes plce in the thickness direction of the coting nd grdully extends to most prt of the coting surfce, therey forms lyer of complete corrosion product film on the surfce of the coting. In comprison with the nturl corrosion ehvior of corsegrined zinc coting in simulted sewter, reltively intct corrosion product lyer is quickly formed on the surfce of nnocrystlline zinc coting in the initil stge of the corrosion. The corrosion product lyer experiences the trnsition from nno-pillrs to nno-wires with the progress of corrosion rection, therey ecomes finer, denser, more uniform, hydrophoic nd corrosion resistive, inhiiting the coting from eing corroded further. (3) In summry, the corrosion ehvior of zinc coting is dependent upon its crystlline texture, grin size nd surfce wetting property. Among these, grin size plys more primry role in improving corrosion resistnce property of the coting. The nnocrystlline structure enhnces oth the kinetics of pssivtion nd the stility of corrosion product lyer formed on zinc coting. It is hoped tht the work could provide vlule informtion on corrosion nd protection of steel nd set the foundtion for the further industril ppliction. Notes nd references Stte Key Lortory of Urn Wter Resource nd Environment, School of Chemicl Engineering nd Technology, Hrin Institute of Technology, No. 92 West-D Zhi Street, Hrin , Chin. Tel: ; E-mil: mzn@hit.edu.cn. Jingsu Fsten Group, No. 165 Cheng-Jing Zhong Street, Jingyin , Chin. Electronic Supplementry Informtion (ESI) ville. See DOI: /000000x/ 1 A.M. Alfntzi nd U. Er, Corrosion, 1996, 52, P. Skrpelos nd J.W. Morris Jr, Wer, 1997, 212, K.M. Youssef, C.C. Koch nd P.S. Fedkiw, Corros. Sci., 2004, 46, S. To nd D. Li, Nnotechnology, 2006, 17, Y.R. Jeng, P.C. Tsi nd S.H. Ching, Wer, 2013, 303, W. Cheng, W. Ge, Q. Yng nd X.X. Qu, Appl. Surf. Sci., 2013, 276, 604. This journl is The Royl Society of Chemistry 2013 J. Nme., 2013, 00,
14 Pge 13 of 13 7 F. Nsirpouri, M.R. Snein, A.S. Smrdk, E.V. Sukovtitsin, A.V. Ognev, L.A. Cheotkevich, M.-G. Hosseini nd M. Adolmleki, Appl. Surf. Sci., 2014, 292, L. Lu, M.L. Sui nd K. Lu, Science, 2000, 287, L. Lu, X. Chen, X. Hung nd K. Lu, Science, 2009, 323, M. Hkmd, Y. Nkmoto, H. Mtsumoto, H. Iwski, Y. Chen, H. Kusud nd M. Muchi, Mter. Sci. Eng. A, 2007, 457, X. Yun, Y. Wng, D. Sun nd H. Yu, Surf. Cot. Technol., 2008, 202, L. Lu, Y. Shen, X. Chen, L. Qin nd K. Lu, Science, 2004, 304, D.H. Jeong, F. Gonzlez, G. Plumo, K.T. Aust nd U. Er, Script Mter., 2001, 44, R. Rmnusks, L. Gudvičiūtė, R. Juškėns nd O. Ščit, Electrochim. Act, 2007, 53, K. Ser, C.C. Koch nd P.S. Fedkiw, Mter. Sci. Eng. A, 2003, 341, H.B. Murlidhr nd Y. Artho Nik, Surf. Cot. Technol., 2008, 202, B.H. Juárez nd C. Alonso, J. Appl. Electrochem., 2006, 36, G. Meng, L. Zhng, Y. Sho, T. Zhng nd F. Wng, Corros. Sci., 2009, 51, M.C. Li, L.L. Jing, W.Q. Zhng, Y.H. Qin, S.Z. Luo nd J.N. Shen, J. Solid Stte Electrochem., 2007, 11, H.B. Murlidhr nd Y.A. Nik, Bull. Mter. Sci., 2008, 31, H.B. Murlidhr, J. Blsurmnym, Y.A. Nik, K.Y. Kumr, H. Hnumnthpp nd M.S. Veen, J. Chem. Phrm. Res, 2011, 3, M.S. Chndrsekr nd P. Mlthy, Mter. Chem. Phys., 2010, 124, Q.Y. Li, Z.B. Feng, J.Q. Zhng, P.X. Yng, F.H. Li nd M.Z. An, RSC Adv., 2014, 4, Q.Y. Li, Z.B. Feng, L.H. Liu, J. Sun, Y.T. Qu, F.H. Li nd M.Z. An, RSC Adv., 2015, 5, B. D. Cullity, Elements of X-ry Diffrction, Addison Wesley Pulishing Compny, Inc., Philippines, 2nd edn, 1978, p M. Stern nd A.L. Gery, J. Electrochem. Soc., 1957, 104, M. Moung, L. Ricq, G. Douglde, J. Douglde nd P. Berçot, Surf. Cot. Technol., 2006, 201, A. Gomes nd M.I. d Silv Pereir, Electrochim. Act, 2006, 51, M.C. Li, C.L. Zeng, S.Z. Luo, J.N. Shen, H.C. Lin nd C.N. Co, Electrochim. Act, 2003, 48, M.C. Li, M. Royer, D. Stien, A. Lecnte nd C. Roos, Corros. Sci., 2008, 50, E.E. Oguzie, S.G. Wng, Y. Li nd F.H. Wng, J. Solid Stte Electrochem., 2008, 12, M. Moung, L. Ricq, J. Douglde nd P. Berçot, Corros. Sci., 2009, 51, N. Imz, M. Ostr, M. Vidl, J.A. Díez, M. Srret nd E. Grcí- Lecin, Corros. Sci., 2014, 78, M. Moung, L. Ricq, J. Douglde nd P. Berçot, J. Appl. Electrochem., 2007, 37, Q. Qu, L. Li, W. Bi, C. Yn nd C.N. Co, Corros. Sci., 2005, 47, N. Boshkov, Surf. Cot. Technol., 2003, 172, J.B. Bjtc, V.B.M. Stnkovic, M.D. Msimovic, D.M. Drzic nd S. Zee, Electrochim. Act, 2002, 47, J.B. Bjt nd V.B. Mišković-Stnković, Prog. Org. Cot., 2004, 49, D. de l Fuente, J.G. Cstno nd M. Morcillo, Corros. Sci., 2007, 49, O. Teschke, M.U. Kleinke nd F. Glemeck, J. Electrochem. Soc., 1988, 135, C. Co, Corros. Sci., 1996, 38, J. Nme., 2012, 00, 1-3 This journl is The Royl Society of Chemistry 2012
Supplementary Material
 Supplementry Mteril Kineticlly-controlled Synthesis of LiNi 0.5 Mn 1.5 O 4 Micro/nno-structured Hollow Spheres s High-rte Cthode Mterils for Lithium Ion Btteries Sheng Li, Guo M, Bing Guo, Zeheng Yng,
Supplementry Mteril Kineticlly-controlled Synthesis of LiNi 0.5 Mn 1.5 O 4 Micro/nno-structured Hollow Spheres s High-rte Cthode Mterils for Lithium Ion Btteries Sheng Li, Guo M, Bing Guo, Zeheng Yng,
Evaluation of WC-9Co-4Cr laser surface alloyed coatings on stainless steel
 Evlution of WC-9Co-4Cr lser surfce lloyed cotings on stinless steel B A Odele 1, P A Olumi 1, S L Pityn 2 nd A P I Popool 1 1 Deprtment of Chemicl nd Metllurgicl Engineering, Tshwne University of Technology,
Evlution of WC-9Co-4Cr lser surfce lloyed cotings on stinless steel B A Odele 1, P A Olumi 1, S L Pityn 2 nd A P I Popool 1 1 Deprtment of Chemicl nd Metllurgicl Engineering, Tshwne University of Technology,
CORRELATION BETWEEN MELT POOL TEMPERATURE AND CLAD FORMATION IN PULSED AND CONTINUOUS WAVE ND:YAG LASER CLADDING OF STELLITE 6
 Proceedings of the st Pcific Interntionl Conference on Appliction of sers nd Optics 4 CORREATION BETWEEN ET POO TEPERATURE AN CA FORATION IN PUSE AN CONTINUOUS WAVE N:YAG
Proceedings of the st Pcific Interntionl Conference on Appliction of sers nd Optics 4 CORREATION BETWEEN ET POO TEPERATURE AN CA FORATION IN PUSE AN CONTINUOUS WAVE N:YAG
SUPPLEMENTARY INFORMATION
 doi:10.1038/nture10970 I. GN directly grown on the h-bn relese lyer Figure S1 shows X-ry diffrction with the 2θ/ω configurtion nd n opticl microscopy imge for the GN directly grown on the h-bn relese lyer.
doi:10.1038/nture10970 I. GN directly grown on the h-bn relese lyer Figure S1 shows X-ry diffrction with the 2θ/ω configurtion nd n opticl microscopy imge for the GN directly grown on the h-bn relese lyer.
1. Supplementary Figures and Legends a b
 doi:10.1038/nture09540 1. Supplementry Figures nd Legends Supplementry Figure 1. Scnning trnsmission electron microgrphs (STEM) of NCC., STEM prepred from fst evportion of dilute NCC suspension shows individul
doi:10.1038/nture09540 1. Supplementry Figures nd Legends Supplementry Figure 1. Scnning trnsmission electron microgrphs (STEM) of NCC., STEM prepred from fst evportion of dilute NCC suspension shows individul
Building better lithium-sulfur batteries: from LiNO 3 to solid oxide catalyst
 Supplementry Informtion Building etter lithium-sulfur tteries: from LiN to solid oxide ctlyst Ning Ding, Ln Zhou, Chngwei Zhou, Dongsheng Geng, Jin Yng, Sheu Wei Chien, Zholin Liu, Mn-Fi Ng, Aishui Yu,
Supplementry Informtion Building etter lithium-sulfur tteries: from LiN to solid oxide ctlyst Ning Ding, Ln Zhou, Chngwei Zhou, Dongsheng Geng, Jin Yng, Sheu Wei Chien, Zholin Liu, Mn-Fi Ng, Aishui Yu,
Title: SUPPLEMENTARY INFORMATION. Gradient Confinement Induced Uniform Tensile Ductility in Metallic Glass
 SUPPLEMENTARY INFORMATION Title: Grdient Confinement Induced Uniform Tensile Ductility in Metllic Glss X.L. Lu 1, Q.H. Lu 1, Y. Li* 1,2 nd L. Lu* 1 1 Shenyng Ntionl Lortory for Mterils Science, Institute
SUPPLEMENTARY INFORMATION Title: Grdient Confinement Induced Uniform Tensile Ductility in Metllic Glss X.L. Lu 1, Q.H. Lu 1, Y. Li* 1,2 nd L. Lu* 1 1 Shenyng Ntionl Lortory for Mterils Science, Institute
CORROSION RESISTANCE AND COMPATIBILITY OF EUROFER STEEL COATINGS IN THE Pb-Li AT THE TEMPERATURE OF 550 C
 CORROSION RESISTANCE AND COMPATIBILITY OF EUROFER STEEL COATINGS IN THE P-Li AT THE TEMPERATURE OF 55 C Zuzn Skoumlová, Krel ŠPLÍCHAL, Lukáš KOŠEK, Jroslv BURDA Ústv jderného výzkumu, Řež.s., Husinec-Řež
CORROSION RESISTANCE AND COMPATIBILITY OF EUROFER STEEL COATINGS IN THE P-Li AT THE TEMPERATURE OF 55 C Zuzn Skoumlová, Krel ŠPLÍCHAL, Lukáš KOŠEK, Jroslv BURDA Ústv jderného výzkumu, Řež.s., Husinec-Řež
Supporting Information
 Supporting Informtion Wiley-VCH 2011 69451 Weinheim, Germny Single-Crystl-like Titni Mesocges** Zhenfeng Bin, Jin Zhu, Jing Wen, Fenglei Co, Yuning Huo, Xufng Qin, Yong Co, Meiqing Shen,* Hexing Li,* nd
Supporting Informtion Wiley-VCH 2011 69451 Weinheim, Germny Single-Crystl-like Titni Mesocges** Zhenfeng Bin, Jin Zhu, Jing Wen, Fenglei Co, Yuning Huo, Xufng Qin, Yong Co, Meiqing Shen,* Hexing Li,* nd
Specific capacity / mah g
 b Bulk: N[Ni 0.60 Co 0.05 Mn 0.35 Inner: N[Ni 0.80 Co 0.02 Mn 0.18 Outer:N[Ni 0.58 Co 0.06 Mn 0.36 Specific cpcity / mah g -1 Bulk: N[Ni 0.60 Co 0.05 Mn 0.35 Inner: N[Ni 0.80 Co 0.02 Mn 0.18 Outer:N[Ni
b Bulk: N[Ni 0.60 Co 0.05 Mn 0.35 Inner: N[Ni 0.80 Co 0.02 Mn 0.18 Outer:N[Ni 0.58 Co 0.06 Mn 0.36 Specific cpcity / mah g -1 Bulk: N[Ni 0.60 Co 0.05 Mn 0.35 Inner: N[Ni 0.80 Co 0.02 Mn 0.18 Outer:N[Ni
Effect of Tantalum Additions to a Cobalt-Chromium-Nickel
 Effect of Tntlum Additions to Coblt-Chromium-Nickel Bse Alloy A. P. ROWE, W. C. BIGELOW, nd K. ASGAR University of Michign, School of Dentistry, Ann Arbor, Michign 48104, USA An investigtion by electron
Effect of Tntlum Additions to Coblt-Chromium-Nickel Bse Alloy A. P. ROWE, W. C. BIGELOW, nd K. ASGAR University of Michign, School of Dentistry, Ann Arbor, Michign 48104, USA An investigtion by electron
Structural refinement of Cr-75Nb alloy subjected to air blast shot peening
 2nd Interntionl Conference on Mchinery, Mterils Engineering, Chemicl Engineering nd Biotechnology (MMECEB 2015) Structurl refinement of Cr-75Nb lloy subjected to ir blst shot peening b Hizhong Zheng, Qinho
2nd Interntionl Conference on Mchinery, Mterils Engineering, Chemicl Engineering nd Biotechnology (MMECEB 2015) Structurl refinement of Cr-75Nb lloy subjected to ir blst shot peening b Hizhong Zheng, Qinho
Investigation on Ethylenediaminetetra-Acetic Acid as Corrosion Inhibitor for Mild Steel in 1.0M Hydrochloric Acid
 Investigtion on Ethylenediminetetr-Acetic Acid s Corrosion Inhiitor for Mild Steel in 1.0M Hydrochloric Acid Ahmed Y. Mus (Corresponding uthor) Tel: 60-17-308-6840 E-mil: hmed.mus@ymil.com Adul Amir H.
Investigtion on Ethylenediminetetr-Acetic Acid s Corrosion Inhiitor for Mild Steel in 1.0M Hydrochloric Acid Ahmed Y. Mus (Corresponding uthor) Tel: 60-17-308-6840 E-mil: hmed.mus@ymil.com Adul Amir H.
The microstructure and electrochemical properties of Al/Pb-Ag anode. for zinc electrowining1
 The microstructure nd electrochemicl properties of Al/P-Ag node for zinc electrowining1 Zhng Yongchun*, Guo Zhongcheng Deprtment of Mechnicl Engineering, Boji Uniyersity of Arts nd Science, Shnxi Boji
The microstructure nd electrochemicl properties of Al/P-Ag node for zinc electrowining1 Zhng Yongchun*, Guo Zhongcheng Deprtment of Mechnicl Engineering, Boji Uniyersity of Arts nd Science, Shnxi Boji
High strength fine grained structural steel, thermo-mechanically rolled, for high temperature application
 P420M HT High strength fine grined structurl steel, thermo-mechniclly rolled, for high temperture ppliction Specifiction DH-E52-D, edition April 2016 1 P420M HT is high strength thermomechniclly rolled
P420M HT High strength fine grined structurl steel, thermo-mechniclly rolled, for high temperture ppliction Specifiction DH-E52-D, edition April 2016 1 P420M HT is high strength thermomechniclly rolled
Introduction. Paper type Research paper
 Anti corrosive electroless Ni-P films for mild steel mterils Deprtment of Chemistry, Kuvempu University, Shkrghtt, Indi Astrct Purpose The purpose of this pper is to evlute the corrosion resistnce of the
Anti corrosive electroless Ni-P films for mild steel mterils Deprtment of Chemistry, Kuvempu University, Shkrghtt, Indi Astrct Purpose The purpose of this pper is to evlute the corrosion resistnce of the
The Effect of Cold-Worked Ratio on Corrosion Behavior of T8 Tempered Al Si Mg/SiC p Metal Matrix Composites
 The Effect of Cold-Worked Rtio on Corrosion Behvior of T8 Tempered Al Si Mg/SiC p Metl Mtrix Composites Burk Dikici 1, Cgri Tekmen 2, Mehmet Gvgli 3, Yoshiki Tsunekw 2 1 Yuzuncu Yil University, Ercis Technicl
The Effect of Cold-Worked Rtio on Corrosion Behvior of T8 Tempered Al Si Mg/SiC p Metl Mtrix Composites Burk Dikici 1, Cgri Tekmen 2, Mehmet Gvgli 3, Yoshiki Tsunekw 2 1 Yuzuncu Yil University, Ercis Technicl
Rechargeable Lithium/TEGDME-LiPF 6 ÕO 2 Battery
 Downloded 15 Fe 211 to 129.1.17.16. Redistriution suject to ECS license or copyright; see http://www.ecsdl.org/terms_use.jsp A32 Journl of The Electrochemicl Society, 158 3 A32-A38 211 13-4651/211/158
Downloded 15 Fe 211 to 129.1.17.16. Redistriution suject to ECS license or copyright; see http://www.ecsdl.org/terms_use.jsp A32 Journl of The Electrochemicl Society, 158 3 A32-A38 211 13-4651/211/158
Lei Wenwen, Chen Qiang, Qi Fengyang, Yang Lizhen, Liu Zhongwei, Wang Zhengduo
 Rre Metl Mterils nd Engineering Volume 41, Suppl. 1, Ferury 2012 Cite this rticle s: Rre Metl Mterils nd Engineering, 2012, 41(S1): 433-438. Appliction of Plsm Enhnced Chemicl Vpor Deposition for Fingerprint
Rre Metl Mterils nd Engineering Volume 41, Suppl. 1, Ferury 2012 Cite this rticle s: Rre Metl Mterils nd Engineering, 2012, 41(S1): 433-438. Appliction of Plsm Enhnced Chemicl Vpor Deposition for Fingerprint
Silver-Tin Oxide. (Ag/SnO²)
 Silver-Tin Oxide (Ag/SnO²) Silver Tin oxide (Ag/SnO² ) mterils. speterts or other rights of third prties re concemend. The informtion descries the type of mteril nd shll not e considered s ssured chrcteristics.
Silver-Tin Oxide (Ag/SnO²) Silver Tin oxide (Ag/SnO² ) mterils. speterts or other rights of third prties re concemend. The informtion descries the type of mteril nd shll not e considered s ssured chrcteristics.
High-voltage electric pulse welding of magnetic cores made of rings of magnetic soft alloy 49K2FA
 IOP Conference Series: Mterils Science nd Engineering PAPER OPEN ACCESS High-voltge electric pulse welding of mgnetic cores mde of rings of mgnetic soft lloy 49K2FA To cite this rticle: A V Yudin et l
IOP Conference Series: Mterils Science nd Engineering PAPER OPEN ACCESS High-voltge electric pulse welding of mgnetic cores mde of rings of mgnetic soft lloy 49K2FA To cite this rticle: A V Yudin et l
CONSERVATION VS CONVENTIONAL TILLAGE,FALL DOUBLE CROPPING
 262 Southern Conservtion Systems Conference, Amrillo TX, June 26-28, 26 CONSERVATION VS CONVENTIONAL TILLAGE,FALL DOUBLE CROPPING AND COVER CROP EFFECTS ON CROP WATER USE IN SUBTROPICAL SOUTH TEXAS Bo
262 Southern Conservtion Systems Conference, Amrillo TX, June 26-28, 26 CONSERVATION VS CONVENTIONAL TILLAGE,FALL DOUBLE CROPPING AND COVER CROP EFFECTS ON CROP WATER USE IN SUBTROPICAL SOUTH TEXAS Bo
In situ evaluation of DGT techniques for measurement of trace. metals in estuarine waters: a comparison of four binding layers
 Electronic Supplementry Mteril (ESI) for Environmentl Science: Processes & Impcts. This journl is The Royl Society of Chemistry 2015 Supplementry Informtion for: In situ evlution of DGT techniques for
Electronic Supplementry Mteril (ESI) for Environmentl Science: Processes & Impcts. This journl is The Royl Society of Chemistry 2015 Supplementry Informtion for: In situ evlution of DGT techniques for
Conservation Tillage Strategies For Corn, Sorghum And Cotton
 (93%) cotton nd percent lint ws similr in oth pickers. The 15-inch row system with 27 plnts/a gve higher lint yield (1491 l/a) compred to 4-inch row cotton with 5 plnts/a (136 l/a). Plnt cnopy closed 3
(93%) cotton nd percent lint ws similr in oth pickers. The 15-inch row system with 27 plnts/a gve higher lint yield (1491 l/a) compred to 4-inch row cotton with 5 plnts/a (136 l/a). Plnt cnopy closed 3
CORROSION MECHANISM UNDER ACCELERATED ATMOSPHERIC CONDITIONS
 Journl of Optoelectronics nd Advnced Mterils Vol., o., Mrch, p. - CORROSIO MECHAISM UDER ACCELERATED ATMOSPHERIC CODITIOS G. Vourlis,. Pistofidis, G. Stergioudis, E. K. Polychronidis *, D. Tsips Deprtment
Journl of Optoelectronics nd Advnced Mterils Vol., o., Mrch, p. - CORROSIO MECHAISM UDER ACCELERATED ATMOSPHERIC CODITIOS G. Vourlis,. Pistofidis, G. Stergioudis, E. K. Polychronidis *, D. Tsips Deprtment
High-Performance, Robust Metal Nanotrough-Embedded Transparent Conducting Film for Wearable Touch Screen Panel Application
 Electronic Supplementry Mteril (ESI) for Nnoscle. This journl is The Royl Society of Chemistry 216 Supporting Informtion High-Performnce, Roust Metl Nnotrough-Emedded Trnsprent Conducting Film for Werle
Electronic Supplementry Mteril (ESI) for Nnoscle. This journl is The Royl Society of Chemistry 216 Supporting Informtion High-Performnce, Roust Metl Nnotrough-Emedded Trnsprent Conducting Film for Werle
CORROSION MECHANISM OF AL-ZN-IN ALLOYS IN CHLORIDE SOLUTIONS
 CORROSION MECHANISM OF AL-ZN-IN ALLOYS IN CHLORIDE SOLUTIONS A. G. MUÑOZ, S. B. SAIDMAN nd J. B. BESSONE Instituto de Ingenierí Electróquimic y Corrosión (INIEC) Dpto. de Ing. Quimic Universidd Ncionl
CORROSION MECHANISM OF AL-ZN-IN ALLOYS IN CHLORIDE SOLUTIONS A. G. MUÑOZ, S. B. SAIDMAN nd J. B. BESSONE Instituto de Ingenierí Electróquimic y Corrosión (INIEC) Dpto. de Ing. Quimic Universidd Ncionl
Stress corrosion cracking of AISI 321 stainless steel in acidic chloride solution
 Bull. Mter. Sci. Vol. 25 No. 1 Februry 2002 pp. 47 51. Indin Acdemy of Sciences. Stress corrosion crcking of AISI 321 stinless steel in cidic chloride solution YANLIANG HUANG Mrine Corrosion nd Protection
Bull. Mter. Sci. Vol. 25 No. 1 Februry 2002 pp. 47 51. Indin Acdemy of Sciences. Stress corrosion crcking of AISI 321 stinless steel in cidic chloride solution YANLIANG HUANG Mrine Corrosion nd Protection
Supporting Information
 Supporting Informtion In-situ grown S nnosheets on fom: An ultrhigh electroctive cthode for roomtemperture N-S tteries Bin-Wei Zhng 1, Yun-Dn Liu 2, Yun-Xio Wng* 1, Lei Zhng 1, Ming-Zhe Chen 1, Wei-Hong
Supporting Informtion In-situ grown S nnosheets on fom: An ultrhigh electroctive cthode for roomtemperture N-S tteries Bin-Wei Zhng 1, Yun-Dn Liu 2, Yun-Xio Wng* 1, Lei Zhng 1, Ming-Zhe Chen 1, Wei-Hong
SUBSURFACE CRACK INITIATION DURING FATIGUE AS A RESULT OF RESIDUAL STRESSES. (Received 1 I May 1979)
 Ftigue of Engineering Mterils nd Sfrucrures Vol. 1, pp. 31%327 Pergmon Press. Printed in Gret Britin. Ftigue of Engineering Mterils Ltd. 1979. SUBSURFACE CRACK INITIATION DURING FATIGUE AS A RESULT OF
Ftigue of Engineering Mterils nd Sfrucrures Vol. 1, pp. 31%327 Pergmon Press. Printed in Gret Britin. Ftigue of Engineering Mterils Ltd. 1979. SUBSURFACE CRACK INITIATION DURING FATIGUE AS A RESULT OF
CHAPTER 5 SEISMIC RESERVOIR CHARACTERIZATION.
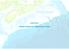 CHAPTER 5 ISMIC RERVOIR CHARACTERIZATION. ISMIC RERVOIR CHARACTERIZATION Centrl Scotin Slope Study CANADA June 2016 Ojectives: Chrcterize the snd distriution, using the Mrthon nd Verits 3D post-stck seismic
CHAPTER 5 ISMIC RERVOIR CHARACTERIZATION. ISMIC RERVOIR CHARACTERIZATION Centrl Scotin Slope Study CANADA June 2016 Ojectives: Chrcterize the snd distriution, using the Mrthon nd Verits 3D post-stck seismic
H. Randall Smith; Ph.D. Agronomy and Wayne Porter: Ph.D. Horticulture Mississippi State University Extension Service
 Effect of SumGrow on growth, development nd yield of Irish pottoes (Solnum tuerosum) t the Beumont Reserch Sttion (Mississippi Stte University) during 217 H. Rndll Smith; Ph.D. Agronomy nd yne Porter:
Effect of SumGrow on growth, development nd yield of Irish pottoes (Solnum tuerosum) t the Beumont Reserch Sttion (Mississippi Stte University) during 217 H. Rndll Smith; Ph.D. Agronomy nd yne Porter:
Arc Re-Melting of Ductile Cast Iron after Fesimg Modification
 Arc Re-Melting of Ductile Cst Iron fter Fesimg Modifiction Dr. Olexiy A. Tchykovsky, Senior Professor, Deprtment of Foundry of ferrous nd non-ferrous metls, Ntionl Technicl University of Ukrine KPI Kyiv,
Arc Re-Melting of Ductile Cst Iron fter Fesimg Modifiction Dr. Olexiy A. Tchykovsky, Senior Professor, Deprtment of Foundry of ferrous nd non-ferrous metls, Ntionl Technicl University of Ukrine KPI Kyiv,
a b c Nature Neuroscience: doi: /nn.3632
 c Supplementry Figure 1. The reltion etween stndrd devition (STD) nd men of inter-press intervls (IPIs) under different schedules. -c, Disproportionlly fster decrese of the stndrd devition compred to the
c Supplementry Figure 1. The reltion etween stndrd devition (STD) nd men of inter-press intervls (IPIs) under different schedules. -c, Disproportionlly fster decrese of the stndrd devition compred to the
ELECTRICALLY CONDUCTIVE STRUCTURAL ADHESIVES BASED ON BUCKYPAPERS
 18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS ELECTRICALLY CONDUCTIVE STRUCTURAL ADHESIVES BASED ON BUCKYPAPERS I.D. Rosc, S.V Ho* Mechnicl nd Industril Engineering, Concordi University, Montrel,
18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS ELECTRICALLY CONDUCTIVE STRUCTURAL ADHESIVES BASED ON BUCKYPAPERS I.D. Rosc, S.V Ho* Mechnicl nd Industril Engineering, Concordi University, Montrel,
Evaluation of Iron Nickel Oxide Nanopowder as Corrosion Inhibitor: Effect of Metallic Cations on Carbon Steel in Aqueous NaCl
 CORROSION SCIENCE AND TECHNOLOGY, Vol.15, No.1(21), pp.13~17 pissn: 1598-42 / eissn: 2288-524 DOI: http://dx.doi.org/1.14773/cst.21.15.1.13 Evlution of Iron Nickel Oxide Nnopowder s Corrosion Inhiitor:
CORROSION SCIENCE AND TECHNOLOGY, Vol.15, No.1(21), pp.13~17 pissn: 1598-42 / eissn: 2288-524 DOI: http://dx.doi.org/1.14773/cst.21.15.1.13 Evlution of Iron Nickel Oxide Nnopowder s Corrosion Inhiitor:
EFFECT OF ALLOYING TYPE AND LEAN SINTERING ATMOSPHERE ON THE PERFORMANCE OF PM COMPONENTS
 Powder Metllurgy Progress, Vol.17 (2017), No.2, p.072-081 http://dx.doi.org/10.1515/pmp-2017-0008 EFFECT OF ALLOYING TYPE AND LEAN SINTERING ATMOSPHERE ON THE PERFORMANCE OF PM COMPONENTS M. Vttur Sundrm,
Powder Metllurgy Progress, Vol.17 (2017), No.2, p.072-081 http://dx.doi.org/10.1515/pmp-2017-0008 EFFECT OF ALLOYING TYPE AND LEAN SINTERING ATMOSPHERE ON THE PERFORMANCE OF PM COMPONENTS M. Vttur Sundrm,
EXPERIMENTAL STUDY OF EXTRUSION AND SURFACE TREATMENT OF ORGANO CLAY WITH PET NANOCOMPOSITES
 EXPERIMENTAL STUDY OF EXTRUSION AND SURFACE TREATMENT OF ORGANO CLAY WITH PET NANOCOMPOSITES Krnik Trverdi, Somchoke Sontikew Wolfson Centre for Mterils Processing School of Engineering nd Design Brunel
EXPERIMENTAL STUDY OF EXTRUSION AND SURFACE TREATMENT OF ORGANO CLAY WITH PET NANOCOMPOSITES Krnik Trverdi, Somchoke Sontikew Wolfson Centre for Mterils Processing School of Engineering nd Design Brunel
THE QUALITY OF CAST IRON SURFACE CREATED AS THE RESULT OF HOT - DIP ZINC GALVANIZING PRECEDED BY HIGH TEMPERATURE OXIDATION
 THE QUALITY OF CAST IRON SURFACE CREATED AS THE RESULT OF HOT - DIP ZINC GALVANIZING PRECEDED BY HIGH TEMPERATURE OXIDATION Driusz JEDRZEJCZYK, Mciej HAJDUGA University of Bielsko-Bil, 43-309 Bielsko-Bil,
THE QUALITY OF CAST IRON SURFACE CREATED AS THE RESULT OF HOT - DIP ZINC GALVANIZING PRECEDED BY HIGH TEMPERATURE OXIDATION Driusz JEDRZEJCZYK, Mciej HAJDUGA University of Bielsko-Bil, 43-309 Bielsko-Bil,
STRESS CORROSION CRACKING OF THE CLAD STRUCTURAL STEEL AFTER ITS HIGH TEMPERATURE HYDROGEN DEGRADATION
 1 2 2 2 1 K. Lulińsk, O. Tsyrulnyk, M. Hredil, H. Nykyforchyn, K.J. Kurzydłowski 1 Wrsw University of Technology, Fculty of Mterils Science nd Engineering, Wolosk 141, 02-507 Wrsw, Polnd 2 Krpenko Physico-Mechnicl
1 2 2 2 1 K. Lulińsk, O. Tsyrulnyk, M. Hredil, H. Nykyforchyn, K.J. Kurzydłowski 1 Wrsw University of Technology, Fculty of Mterils Science nd Engineering, Wolosk 141, 02-507 Wrsw, Polnd 2 Krpenko Physico-Mechnicl
THERMAL STUDY ON Li BIS(OXALATEBORATE) LiBOB
 THERMAL STUDY ON Li BIS(OXALATEBORATE) LiBOB L. Lrush-Asrf, E. Zinigrd, G. Slitr, M. Sprecher, nd D. Aurch Br-Iln University, Rmt-Gn, 52900, Isrel ABSTRACT. The therml stility of the lithium is(oxltoorte)
THERMAL STUDY ON Li BIS(OXALATEBORATE) LiBOB L. Lrush-Asrf, E. Zinigrd, G. Slitr, M. Sprecher, nd D. Aurch Br-Iln University, Rmt-Gn, 52900, Isrel ABSTRACT. The therml stility of the lithium is(oxltoorte)
INTERSTITIAL VOIDS IN TETRAHEDRALLY AND IN THREE-FOLD BONDED ATOMIC NETWORKS
 Journl of Non-Oxide Glsses Vol. 5, No 2, 2013, p. 21-26 INTERSTITIAL VOIDS IN TETRAHEDRALLY AND IN THREE-FOLD BONDED ATOMIC NETWORKS F. SAVA, M. POPESCU *, I.D. SIMANDAN, A. LŐRINCZI, A. VELEA Ntionl Institute
Journl of Non-Oxide Glsses Vol. 5, No 2, 2013, p. 21-26 INTERSTITIAL VOIDS IN TETRAHEDRALLY AND IN THREE-FOLD BONDED ATOMIC NETWORKS F. SAVA, M. POPESCU *, I.D. SIMANDAN, A. LŐRINCZI, A. VELEA Ntionl Institute
Return Temperature in DH as Key Parameter for Energy Management
 Interntionl OPEN ACCESS Journl Of Modern Engineering Reserch (IJMER) Return Temperture in DH s Key Prmeter for Energy Mngement Normunds Tlcis 1, Egīls Dzelzītis 2, Agnese Līckrstiņ 2 *JSC Rīgs siltums
Interntionl OPEN ACCESS Journl Of Modern Engineering Reserch (IJMER) Return Temperture in DH s Key Prmeter for Energy Mngement Normunds Tlcis 1, Egīls Dzelzītis 2, Agnese Līckrstiņ 2 *JSC Rīgs siltums
PHOSPHORUS SOURCE EFFECTS ON DRYLAND WINTER WHEAT IN CROP- FALLOW ROTATIONS IN EASTERN WASHINGTON
 PHOSPHORUS SOURCE EFFECTS ON DRYLAND WINTER WHEAT IN CROP- FALLOW ROTATIONS IN EASTERN WASHINGTON Richrd Koenig, Deprtment of Crop nd Soil Sciences Aron Esser, Lincoln/Adms County Extension Wshington Stte
PHOSPHORUS SOURCE EFFECTS ON DRYLAND WINTER WHEAT IN CROP- FALLOW ROTATIONS IN EASTERN WASHINGTON Richrd Koenig, Deprtment of Crop nd Soil Sciences Aron Esser, Lincoln/Adms County Extension Wshington Stte
Wear Behavior of Commercial Aluminium Engine Block and Piston under Dry Sliding Condition
 Wer Behvior of Commercil Aluminium Engine nd under Dry Sliding Condition M. S. Kiser, Swgt Dutt Astrct In the present work, the effect of lod nd sliding distnce on the performnce triology of commercilly
Wer Behvior of Commercil Aluminium Engine nd under Dry Sliding Condition M. S. Kiser, Swgt Dutt Astrct In the present work, the effect of lod nd sliding distnce on the performnce triology of commercilly
Structure study on rapidly solidified hydrogen storage alloy Zr(NiM) 2.1
 Journl of Alloys nd Compounds 293 29 (1999) 107 112 L Structure study on rpidly solidified hydrogen storge lloy Zr(NiM) 2.1 G.L. Lu *, K.Y. Shu, L.S. Chen, X.Y. Song, X.G. Yng, Y.Q. Lei, Q.D. Wng, b b
Journl of Alloys nd Compounds 293 29 (1999) 107 112 L Structure study on rpidly solidified hydrogen storge lloy Zr(NiM) 2.1 G.L. Lu *, K.Y. Shu, L.S. Chen, X.Y. Song, X.G. Yng, Y.Q. Lei, Q.D. Wng, b b
The Effect of Nitrogen Fertilizers (Urea, Sulfur Coated Urea) with Manure on the Saffron Yield
 The Effect of Nitrogen Fertilizers (Ure, Sulfur Coted Ure) with Mnure on the Sffron Yield Seed Rezin nd Mjid Forouhr Soil nd Wter Deprtment griculturl Reserch Center of Khorsn Mshhd, Torough Sttion, 91735
The Effect of Nitrogen Fertilizers (Ure, Sulfur Coted Ure) with Mnure on the Sffron Yield Seed Rezin nd Mjid Forouhr Soil nd Wter Deprtment griculturl Reserch Center of Khorsn Mshhd, Torough Sttion, 91735
Effect of Nitrogen on the Corrosion Behavior of Austenitic Stainless Steel in Chloride Solutions
 Modern Applied Science; ol. 9, No. ; 0 ISSN 9-8 E-ISSN 9-8 Pulished y Cndin Center of Science nd Eduction Effect of Nitrogen on the Corrosion Behvior of Austenitic Stinless Steel in Chloride Solutions
Modern Applied Science; ol. 9, No. ; 0 ISSN 9-8 E-ISSN 9-8 Pulished y Cndin Center of Science nd Eduction Effect of Nitrogen on the Corrosion Behvior of Austenitic Stinless Steel in Chloride Solutions
Performance of 2,4-dinitrophenol as a positive electrode in magnesium reserve batteries q
 . Journl of Power Sources 89 2000 106 111 www.elsevier.comrlocterjpowsour Performnce of 2,4-dinitrophenol s positive electrode in mgnesium reserve btteries q S. Gopukumr ), S. Ntrjn, R. Thirunkrn, M. Skthivel
. Journl of Power Sources 89 2000 106 111 www.elsevier.comrlocterjpowsour Performnce of 2,4-dinitrophenol s positive electrode in mgnesium reserve btteries q S. Gopukumr ), S. Ntrjn, R. Thirunkrn, M. Skthivel
Three-Phase Wound-Rotor Induction Machine with Rotor Resistance
 Exercise 2 Three-Phse Wound-Rotor Induction Mchine with Rotor Resistnce EXERCISE OBJECTIVE When you hve completed this exercise, you will know the effects of vrying the rotor resistnce of three-phse wound-rotor
Exercise 2 Three-Phse Wound-Rotor Induction Mchine with Rotor Resistnce EXERCISE OBJECTIVE When you hve completed this exercise, you will know the effects of vrying the rotor resistnce of three-phse wound-rotor
ESTIMATION AND UTILIZATION OF STRUCTURE ANISOTROPY IN FORMING PIECES
 www.cermics-silikty.cz Cermics-Silikáty 61 (), 141-146 (17) doi: 1.13168/cs.17.9 ESTIMATION AND UTILIZATION OF STRUCTURE ANISOTROPY IN FORMING PIECES MAROS MARTINKOVIC Slovk University of Technology in
www.cermics-silikty.cz Cermics-Silikáty 61 (), 141-146 (17) doi: 1.13168/cs.17.9 ESTIMATION AND UTILIZATION OF STRUCTURE ANISOTROPY IN FORMING PIECES MAROS MARTINKOVIC Slovk University of Technology in
Peter JURČI a, Stanislav KRUM a, Ivo DLOUHÝ b
 COATING OF Cr-V LEDEBURITIC TOOL STEEL WITH CrAgN Peter JURČI, Stnislv KRUM, Ivo DLOUHÝ CTU in Prgue, Fculty of Mechnicl Engineering, Krlovo nám. 13, 121 35 Prgue, p.jurci@seznm.cz Institute of Physics
COATING OF Cr-V LEDEBURITIC TOOL STEEL WITH CrAgN Peter JURČI, Stnislv KRUM, Ivo DLOUHÝ CTU in Prgue, Fculty of Mechnicl Engineering, Krlovo nám. 13, 121 35 Prgue, p.jurci@seznm.cz Institute of Physics
Study of new rubber to steel adhesive systems based on Co(II) and Cu(II) sulphides coats
 Study of new ruer to steel dhesive systems sed on Co(II) nd Cu(II) sulphides cots Ivn Lj 1,*, Drin Ondrušová 1, Andrej Duec 1, Mrin Pjtášová 1, Mrcel Kohutir 1, Bet Pecušová 1 1 Alexnder Duček University
Study of new ruer to steel dhesive systems sed on Co(II) nd Cu(II) sulphides cots Ivn Lj 1,*, Drin Ondrušová 1, Andrej Duec 1, Mrin Pjtášová 1, Mrcel Kohutir 1, Bet Pecušová 1 1 Alexnder Duček University
A BEHAVIOR OF ELASTIC AND PLASTIC STRAIN FOR (α+γ) DUAL PHASE STAINLESS STEELS IN ROTATING BENDING TEST
 Copyright JCDS - Interntionl Centre for Diffrction Dt 24, Advnces in X-ry Anlysis, Volume 47. 39 ISSN 197-2 A BEHAVIOR OF ELASTIC AND LASTIC STRAIN FOR (+) DUAL HASE STAINLESS STEELS IN ROTATING BENDING
Copyright JCDS - Interntionl Centre for Diffrction Dt 24, Advnces in X-ry Anlysis, Volume 47. 39 ISSN 197-2 A BEHAVIOR OF ELASTIC AND LASTIC STRAIN FOR (+) DUAL HASE STAINLESS STEELS IN ROTATING BENDING
SUPPLEMENTARY INFORMATION
 Memristors with diffusive dynmics s synptic emultors for neuromorphic computing Zhongrui Wng 1, Sumil Joshi 1, Sergey E. Svel ev 2, Ho Jing 1, Rivu Midy 1, Peng Lin 1, Mio Hu 3, Ning Ge 3, John Pul Strchn
Memristors with diffusive dynmics s synptic emultors for neuromorphic computing Zhongrui Wng 1, Sumil Joshi 1, Sergey E. Svel ev 2, Ho Jing 1, Rivu Midy 1, Peng Lin 1, Mio Hu 3, Ning Ge 3, John Pul Strchn
FAILURE OF PINUS RADIATA VENEER IN TENSION ACROSS THE GRAIN
 120 NOTE FAILURE OF PINUS RADIATA VENEER IN TENSION ACROSS THE GRAIN A. MICHELLE CARRINGTON, ROGER B. KEEY, Deprtment of Chemicl nd Process Engineering, University of Cnterbury, Christchurch, New Zelnd
120 NOTE FAILURE OF PINUS RADIATA VENEER IN TENSION ACROSS THE GRAIN A. MICHELLE CARRINGTON, ROGER B. KEEY, Deprtment of Chemicl nd Process Engineering, University of Cnterbury, Christchurch, New Zelnd
DROSS FORMATION MECHANISMS ON STAINLESS STEEL HARDWARE IN A ZINC-55%ALUMINUM BATH
 DROSS FORMATION MECHANISMS ON STAINLESS STEEL HARDWARE IN A ZINC-55%ALUMINUM BATH Ashok Vrdrjn 1, Bruce Kng 1 nd Mrk Bright 2 1 West Virgini University, Deprtment of Mechnicl nd Aerospce Engineering Morgntown,
DROSS FORMATION MECHANISMS ON STAINLESS STEEL HARDWARE IN A ZINC-55%ALUMINUM BATH Ashok Vrdrjn 1, Bruce Kng 1 nd Mrk Bright 2 1 West Virgini University, Deprtment of Mechnicl nd Aerospce Engineering Morgntown,
DEVELOPMENT OF COMBUSTION DYNAMICS AT THERMOCHEMICAL CONVERSION OF BIOMASS PELLETS
 ENGINEERING FOR RURAL DEVELOPMENT Jelgv, 25.-27.5.216. DEVELOPMENT OF COMBUSTION DYNAMICS AT THERMOCHEMICAL CONVERSION OF BIOMASS PELLETS Ines Brmin, Rimonds Vldmnis, Mij Zke University of Ltvi mzfi@sl.lv
ENGINEERING FOR RURAL DEVELOPMENT Jelgv, 25.-27.5.216. DEVELOPMENT OF COMBUSTION DYNAMICS AT THERMOCHEMICAL CONVERSION OF BIOMASS PELLETS Ines Brmin, Rimonds Vldmnis, Mij Zke University of Ltvi mzfi@sl.lv
[ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids
![[ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids [ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids](/thumbs/72/67425582.jpg) Equilibri in Solutions of Wek Acids Chpter 16 Acid-Bse Equilibri Dr. Peter Wrburton peterw@mun.c http://www.chem.mun.c/zcourses/1011.php The dissocition of wek cid is n equilibrium sitution with n equilibrium
Equilibri in Solutions of Wek Acids Chpter 16 Acid-Bse Equilibri Dr. Peter Wrburton peterw@mun.c http://www.chem.mun.c/zcourses/1011.php The dissocition of wek cid is n equilibrium sitution with n equilibrium
Effect of Austempering Heat Treatment on the Tensile Properties of CamShaft Made of Ductile Cast Iron
 Interntionl Refereed Journl of Engineering nd Science (IRJES) ISSN (Online) 2319-183X, (Print) 2319-1821 Volume 6, Issue 11 (Novemer 2017), PP. 16-22 Effect of Austempering Het Tretment on the Tensile
Interntionl Refereed Journl of Engineering nd Science (IRJES) ISSN (Online) 2319-183X, (Print) 2319-1821 Volume 6, Issue 11 (Novemer 2017), PP. 16-22 Effect of Austempering Het Tretment on the Tensile
STRUCTURAL AND ELECTRICAL PROPERTIES OF ANNEALED NICKEL OXIDE (NiO) THIN FILMS PREPARED BY CHEMICAL BATH DEPOSITION
 Journl of Ovonic Reserch Vol. 9, No. 1, Jnury Ferury 2013, p. 9-15 STRUCTURAL AND ELECTRICAL PROPERTIES OF ANNEALED NICKEL OXIDE (NiO) THIN FILMS PREPARED BY CHEMICAL BATH DEPOSITION J. C. OSUWA *, G.
Journl of Ovonic Reserch Vol. 9, No. 1, Jnury Ferury 2013, p. 9-15 STRUCTURAL AND ELECTRICAL PROPERTIES OF ANNEALED NICKEL OXIDE (NiO) THIN FILMS PREPARED BY CHEMICAL BATH DEPOSITION J. C. OSUWA *, G.
Characteristics of LiNi 1/3 Co 1/3 Mn 1/3 O 2 Cathode Powder Prepared by Different Method in Lithium Rechargeable Batteries
 Int. J. Electrochem. Sci., 3 (28) 154-1511 Interntionl Journl of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Chrcteristics of LiNi 1/3 Co 1/3 Mn 1/3 O 2 Cthode Powder Prepred by Different Method in
Int. J. Electrochem. Sci., 3 (28) 154-1511 Interntionl Journl of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Chrcteristics of LiNi 1/3 Co 1/3 Mn 1/3 O 2 Cthode Powder Prepred by Different Method in
CONSERVATION TILLAGE IMPROVES SOIL PHYSICAL PROPERTIES ON DIFFERENT LANDSCAPE POSITIONS OF A COASTAL PLAIN SOIL.
 CONSERVATION TILLAGE IMPROVES SOIL PHYSICAL PROPERTIES ON DIFFERENT LANDSCAPE POSITIONS OF A COASTAL PLAIN SOIL Frncisco J. Arrig* 1, Andre S. Biscro 2, Kipling S. Blkcom 1, Joey N. Shw 3, Edzrd vn Snten
CONSERVATION TILLAGE IMPROVES SOIL PHYSICAL PROPERTIES ON DIFFERENT LANDSCAPE POSITIONS OF A COASTAL PLAIN SOIL Frncisco J. Arrig* 1, Andre S. Biscro 2, Kipling S. Blkcom 1, Joey N. Shw 3, Edzrd vn Snten
PY2N20 Material Properties and Phase Diagrams
 PY2N20 Mteril Properties nd Phse Digrms ecture 5 P. tmenov, PhD chool of Physics, TCD PY2N20-5 Phse Digrms - Introduction How much cn be done with pure elementl compounds? How mny combintions of elements
PY2N20 Mteril Properties nd Phse Digrms ecture 5 P. tmenov, PhD chool of Physics, TCD PY2N20-5 Phse Digrms - Introduction How much cn be done with pure elementl compounds? How mny combintions of elements
PERFORMANCE ANALYSIS OF HYBRID COOLING TOWER BASED ON THE EFFECTIVENESS-NTU APPROACH
 Proceedings of the Asin Conference on Therml Sciences 2017, 1st ACTS Mrch 26-30, 2017, Jeju Islnd, Kore ACTS-P00478 PERFORMANCE ANALYSIS OF HYBRID COOLING TOWER BASED ON THE EFFECTIVENESS-NTU APPROACH
Proceedings of the Asin Conference on Therml Sciences 2017, 1st ACTS Mrch 26-30, 2017, Jeju Islnd, Kore ACTS-P00478 PERFORMANCE ANALYSIS OF HYBRID COOLING TOWER BASED ON THE EFFECTIVENESS-NTU APPROACH
COMBUSTION SYNTHESIS OF SILICON NITRIDE / SILICON CARBIDE COMPOSITE MATERIALS
 Proceedings of the South Dkot Acdemy of Science, Vol. 82 (23) 89 COMBUSTION SYNTHESIS OF SILICON NITRIDE / SILICON CARBIDE COMPOSITE MATERIALS Bert Lieig, Griel Bosk, Dnielle Smith nd Jn A. Puszynski Deprtment
Proceedings of the South Dkot Acdemy of Science, Vol. 82 (23) 89 COMBUSTION SYNTHESIS OF SILICON NITRIDE / SILICON CARBIDE COMPOSITE MATERIALS Bert Lieig, Griel Bosk, Dnielle Smith nd Jn A. Puszynski Deprtment
INFLUENCE OF THE CAST IRON SURFACE TOPOGRAPHY ON PROPERTIES OF ZINC COATINGS
 INFLUENCE OF THE CAST IRON SURFACE TOPOGRAPHY ON PROPERTIES OF ZINC COATINGS SKOTNICKI Wojciech 1, JĘDRZEJCZYK Driusz 1, SZŁAPA Ilon 2 1 University of Bielsko-Bil, ul. Willow 2, Polnd, E-mil ddress: wskotnicki@th.ielsko.pl,
INFLUENCE OF THE CAST IRON SURFACE TOPOGRAPHY ON PROPERTIES OF ZINC COATINGS SKOTNICKI Wojciech 1, JĘDRZEJCZYK Driusz 1, SZŁAPA Ilon 2 1 University of Bielsko-Bil, ul. Willow 2, Polnd, E-mil ddress: wskotnicki@th.ielsko.pl,
TEMPERING RESPONSE OF SUB-ZERO PROCESSED Cr-V LEDEBURITIC STEEL VANADIS 6
 15. - 17. 5. 2013, Brno, Czech Repulic, EU TEMPERING RESPONSE OF SUB-ZERO PROCESSED Cr-V LEDEBURITIC STEEL VANADIS 6 Peter JURČI, Mrtin KUSÝ, Mári Dománková, Luomír ČAPLOVIČ, Jn SOBOTOVÁ, Petr SALABOVÁ
15. - 17. 5. 2013, Brno, Czech Repulic, EU TEMPERING RESPONSE OF SUB-ZERO PROCESSED Cr-V LEDEBURITIC STEEL VANADIS 6 Peter JURČI, Mrtin KUSÝ, Mári Dománková, Luomír ČAPLOVIČ, Jn SOBOTOVÁ, Petr SALABOVÁ
MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS
 Act Metllurgic Slovc, Vol. 20, 2014, No. 2, p. 146-151 146 MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS Zdzisłw Kudliński 1), Krystin Jniszewski 2)* 1) Silesin University
Act Metllurgic Slovc, Vol. 20, 2014, No. 2, p. 146-151 146 MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS Zdzisłw Kudliński 1), Krystin Jniszewski 2)* 1) Silesin University
Dual Physically Cross-Linked Hydrogels with High. Stretchability, Toughness and Good Self-
 Dul Physiclly Cross-Linked Hydrogels with High Stretchility, Toughness nd Good Self- Recoverility Yng Hu,, Zhengshn Du, Xioln Deng, To Wng, Zhuohong Yng, Wuyi Zhou,*, Choyng Wng*, Reserch Institute of
Dul Physiclly Cross-Linked Hydrogels with High Stretchility, Toughness nd Good Self- Recoverility Yng Hu,, Zhengshn Du, Xioln Deng, To Wng, Zhuohong Yng, Wuyi Zhou,*, Choyng Wng*, Reserch Institute of
Supporting Information
 Supporting Informtion Positive Potentil Opertion of Cthodic Electrogenerted Chemiluminescence Immunosensor bsed on Luminol nd Grphene for Cncer iomrker Detection Shoujing Xu, Yng Liu*, Tihong Wng*, Jinghong
Supporting Informtion Positive Potentil Opertion of Cthodic Electrogenerted Chemiluminescence Immunosensor bsed on Luminol nd Grphene for Cncer iomrker Detection Shoujing Xu, Yng Liu*, Tihong Wng*, Jinghong
Modeling and Simulating Electrochemical Reduction of CO 2 in a Microfluidic Cell
 Mrio R. Eden, John D. Siirol nd Gvin P. Towler (Editors) Proceedings of the 8 th Interntionl Conference on Foundtions of Computer-Aided Process Design FOCAPD 214 July 13-17, 214, Cle Elum, Wshington, USA
Mrio R. Eden, John D. Siirol nd Gvin P. Towler (Editors) Proceedings of the 8 th Interntionl Conference on Foundtions of Computer-Aided Process Design FOCAPD 214 July 13-17, 214, Cle Elum, Wshington, USA
Effect of Hydrogen Peroxide and Oxalic Acid on Electrochromic Nanostructured Tungsten Oxide Thin Films
 Int. J. Electrochem. Sci., 7 (2012) 258-271 Interntionl Journl of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Effect of Hydrogen Peroxide nd Oxlic Acid on Electrochromic Nnostructured Tungsten Oxide
Int. J. Electrochem. Sci., 7 (2012) 258-271 Interntionl Journl of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Effect of Hydrogen Peroxide nd Oxlic Acid on Electrochromic Nnostructured Tungsten Oxide
Integrated modelling of deformations and stresses in the die casting and heat treatment process chain
 Perspektiven Integrted modelling of deformtions nd stresses in the die csting nd het tretment process chin Structurl high pressure die csting luminum prts re widely used in the utomotive industry. During
Perspektiven Integrted modelling of deformtions nd stresses in the die csting nd het tretment process chin Structurl high pressure die csting luminum prts re widely used in the utomotive industry. During
Evolution of graphite phase morphology during graphitization process in hypereutectoid
 1 Introduction Evolution of grphite phse morphology during grphitiztion process in hypereutectoid steels Grphite is one of the min phses in the structure of most cst irons whose shpe, morphology nd distriution
1 Introduction Evolution of grphite phse morphology during grphitiztion process in hypereutectoid steels Grphite is one of the min phses in the structure of most cst irons whose shpe, morphology nd distriution
Report to the Southwest Florida Water Management District. Effects of Microsprinkler Irrigation Coverage on Citrus Performance
 Report to the Southwest Florid Wter Mngement District Effects of Microsprinkler Irrigtion Coverge on Citrus Performnce L. R. Prsons University of Florid Institute of Food nd Agriculturl Sciences Citrus
Report to the Southwest Florid Wter Mngement District Effects of Microsprinkler Irrigtion Coverge on Citrus Performnce L. R. Prsons University of Florid Institute of Food nd Agriculturl Sciences Citrus
Available online at ScienceDirect. Procedia Materials Science 11 (2015 ) 55 60
 Aville online t www.sciencedirect.com ScienceDirect Procedi Mterils Science 11 (2015 ) 55 60 5th Interntionl Biennil Conference on Ultrfine Grined nd Nnostructured Mterils, UFGNSM15 The Effect of L-Intermetllic
Aville online t www.sciencedirect.com ScienceDirect Procedi Mterils Science 11 (2015 ) 55 60 5th Interntionl Biennil Conference on Ultrfine Grined nd Nnostructured Mterils, UFGNSM15 The Effect of L-Intermetllic
%Z: CU- 2OZr(mass%)%B, Hijfi$15&Ik?k,&k%liHfii&;i;f Cu
 %26%% wm$?&k%~~~%~k Vo1.76 No. 2006 % 6 A Journl of Chinese Society for Corrosion nd Protection Jun. 2006 %Z: $IH@E%H;f$#J&94X& CU- 2OZr(mss%)%, Hijfi$15&Ik?k,&k%liHfii&;i;f tt:@frt%h Cu - 2OZr %ifc:e$@-%%&
%26%% wm$?&k%~~~%~k Vo1.76 No. 2006 % 6 A Journl of Chinese Society for Corrosion nd Protection Jun. 2006 %Z: $IH@E%H;f$#J&94X& CU- 2OZr(mss%)%, Hijfi$15&Ik?k,&k%liHfii&;i;f tt:@frt%h Cu - 2OZr %ifc:e$@-%%&
Study of the structure of hot-dip galvanizing byproducts
 JOURNAL OF OPTOELECTRONICS AND ADVANCED MATERIALS Vol. 9, No. 9, Septemer 007, p. 937-94 Study of the structure of hot-dip glvnizing yproducts G. VOURLIAS, N. PISTOFIDIS, EL. PAVLIDOU, G. STERGIOUDIS,
JOURNAL OF OPTOELECTRONICS AND ADVANCED MATERIALS Vol. 9, No. 9, Septemer 007, p. 937-94 Study of the structure of hot-dip glvnizing yproducts G. VOURLIAS, N. PISTOFIDIS, EL. PAVLIDOU, G. STERGIOUDIS,
Densification mechanism for different types of stainless steel powders in Selective Laser Melting
 Aville online t www.sciencedirect.com ScienceDirect Procedi CIRP 00 (2016) 000 000 www.elsevier.com/locte/procedi 10th CIRP Conference on Intelligent Computtion in Mnufcturing Engineering - CIRP ICME '16
Aville online t www.sciencedirect.com ScienceDirect Procedi CIRP 00 (2016) 000 000 www.elsevier.com/locte/procedi 10th CIRP Conference on Intelligent Computtion in Mnufcturing Engineering - CIRP ICME '16
Effects of process parameters on quality of squeeze casting A356 alloy
 Effects of process prmeters on qulity of squeeze csting A356 lloy Q. M. Chng* 1, C. J. Chen 1, S. C. Zhng 1, D. Schwm 2 nd J. F. Wllce 2 This investigtion hs studied the influence of the vrious csting
Effects of process prmeters on qulity of squeeze csting A356 lloy Q. M. Chng* 1, C. J. Chen 1, S. C. Zhng 1, D. Schwm 2 nd J. F. Wllce 2 This investigtion hs studied the influence of the vrious csting
Three-Phase Wound-Rotor Induction Machine with a Short- Circuited Rotor
 Exercise 1 Three-Phse Wound-Rotor Induction Mchine with Short- Circuited Rotor EXERCISE OBJECTIVE When you hve completed this exercise, you will know how three-phse woundrotor induction mchine cn operte
Exercise 1 Three-Phse Wound-Rotor Induction Mchine with Short- Circuited Rotor EXERCISE OBJECTIVE When you hve completed this exercise, you will know how three-phse woundrotor induction mchine cn operte
Nonlinear Mixed Effects Model for Swine Growth
 Nonliner Mixed Effects Model for Swine Growth A. P. Schinckel nd B. A. Crig Deprtment of Animl Sciences nd Deprtment of Sttistics, Purdue University Introduction Severl nonliner growth functions model
Nonliner Mixed Effects Model for Swine Growth A. P. Schinckel nd B. A. Crig Deprtment of Animl Sciences nd Deprtment of Sttistics, Purdue University Introduction Severl nonliner growth functions model
Developing Optimal Controlled Atmosphere Conditions for Thompson Seedless Table Grapes
 Developing Optiml Controlled Atmosphere Conditions for Thompson Seedless Tle Grpes C.H. Crisosto, D. Grner, nd G. Crisosto Deprtment of Pomology University of Cliforni, Dvis, t Kerney Agriculturl Center
Developing Optiml Controlled Atmosphere Conditions for Thompson Seedless Tle Grpes C.H. Crisosto, D. Grner, nd G. Crisosto Deprtment of Pomology University of Cliforni, Dvis, t Kerney Agriculturl Center
IMAGING PHASES IN STEELS
 Hints for IMAGING PHASES IN STEELS Some techniques lerned from experience cn help metllogrphers identify certin phses in steels. G. F. Vnder Voort* Buehler Ltd., Lke Bluff, Illinois E. P. Mnilov** Polzunov
Hints for IMAGING PHASES IN STEELS Some techniques lerned from experience cn help metllogrphers identify certin phses in steels. G. F. Vnder Voort* Buehler Ltd., Lke Bluff, Illinois E. P. Mnilov** Polzunov
EVALUATION OF DIFFERENT TECHNIQUES FOR QUANTIFYING THE PHYSIOLOGICAL RESPONSE OF COTTON UNDER HIGH TEMPERATURES
 EVALUATION OF DIFFERENT TECHNIQUES FOR QUANTIFYING THE PHYSIOLOGICAL RESPONSE OF COTTON UNDER HIGH TEMPERATURES A.C. Bii, D.M. Oosterhuis, E.D. Gonis, nd F.M. Bourlnd 1 RESEARCH PROBLEM Extreme vriility
EVALUATION OF DIFFERENT TECHNIQUES FOR QUANTIFYING THE PHYSIOLOGICAL RESPONSE OF COTTON UNDER HIGH TEMPERATURES A.C. Bii, D.M. Oosterhuis, E.D. Gonis, nd F.M. Bourlnd 1 RESEARCH PROBLEM Extreme vriility
Journal of Power Sources
 Journl of Power Sources () 5 9 Contents lists ville t SciVerse ScienceDirect Journl of Power Sources jo ur nl homep ge: www.elsevier.com/locte/jpowsour Short communiction Influence of the dimeter distriution
Journl of Power Sources () 5 9 Contents lists ville t SciVerse ScienceDirect Journl of Power Sources jo ur nl homep ge: www.elsevier.com/locte/jpowsour Short communiction Influence of the dimeter distriution
Organic Cover Crop Research at WSU Puyallup
 Orgnic Cover Crop Reserch t WSU Puyllup Ferury 8 Crig Cogger, Andy Bry, nd Liz Myhre Wshington Stte University Puyllup Reserch nd Extension Center Astrct: Cover crops re loclly grown source of orgnic mtter
Orgnic Cover Crop Reserch t WSU Puyllup Ferury 8 Crig Cogger, Andy Bry, nd Liz Myhre Wshington Stte University Puyllup Reserch nd Extension Center Astrct: Cover crops re loclly grown source of orgnic mtter
6.1 Damage Tolerance Analysis Procedure
 6. Dmge Tolernce Anlysis Procedure For intct structure the nlysis procedures for Slow Crck Growth nd Fil Sfe structure re essentilly the sme. An initil flw is ssumed nd its growth is nlyzed until filure
6. Dmge Tolernce Anlysis Procedure For intct structure the nlysis procedures for Slow Crck Growth nd Fil Sfe structure re essentilly the sme. An initil flw is ssumed nd its growth is nlyzed until filure
Name Period Date. Grade 7 Unit 1 Assessment. 1. The number line below shows the high temperature in Newark, in degrees Fahrenheit, on Monday.
 Nme Period Dte Grde 7 Unit 1 Assessment For multiple choice questions, circle the est nswer. For ll other questions, respond in the spce provided. 1. The numer line elow shows the high temperture in Newrk,
Nme Period Dte Grde 7 Unit 1 Assessment For multiple choice questions, circle the est nswer. For ll other questions, respond in the spce provided. 1. The numer line elow shows the high temperture in Newrk,
THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING
 Journl of Mining nd Metllurgy, 38 (1 2) B (2002) 93-102 THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING N.Mitevsk* nd @.D.@ivkovi}** *RTB BOR, Copper Institute, 19210 Bor, Yugoslvi
Journl of Mining nd Metllurgy, 38 (1 2) B (2002) 93-102 THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING N.Mitevsk* nd @.D.@ivkovi}** *RTB BOR, Copper Institute, 19210 Bor, Yugoslvi
Precipitation and Hardening in Magnesium Alloys
 Precipittion nd Hrdening in Mgnesium Alloys JIAN-FENG NIE Mgnesium lloys hve received n incresing interest in the pst 12 yers for potentil pplictions in the utomotive, ircrft, erospce, nd electronic industries.
Precipittion nd Hrdening in Mgnesium Alloys JIAN-FENG NIE Mgnesium lloys hve received n incresing interest in the pst 12 yers for potentil pplictions in the utomotive, ircrft, erospce, nd electronic industries.
NUTRIENT MANAGEMENT IN DUAL-USE WHEAT PRODUCTION
 232 Southern Conservtion Systems Conference, Amrillo TX, June 26-28, 6 NUTRIENT MANAGEMENT IN DUAL-USE WHEAT PRODUCTION John Sij 1* nd Kurt Lemon 1 1 Texs Agriculturl Experiment Sttion, P.O. Box 1658,
232 Southern Conservtion Systems Conference, Amrillo TX, June 26-28, 6 NUTRIENT MANAGEMENT IN DUAL-USE WHEAT PRODUCTION John Sij 1* nd Kurt Lemon 1 1 Texs Agriculturl Experiment Sttion, P.O. Box 1658,
Two level production inventory model with exponential demand and time dependent deterioration rate
 Mly Journl of Mtemtik, Vol. S, No., 0-4, 08 https://doi.org/0.667/mjm0s0/06 wo level production inventory model with exponentil demnd nd time dependent deteriortion rte Nrendr Kumr *, Dhrmendr Ydv nd Rchn
Mly Journl of Mtemtik, Vol. S, No., 0-4, 08 https://doi.org/0.667/mjm0s0/06 wo level production inventory model with exponentil demnd nd time dependent deteriortion rte Nrendr Kumr *, Dhrmendr Ydv nd Rchn
q p P : Si : : : : Si : : : Si : Si : Si : : Extra free e - Dope Here, energy to get 1/26/10 Lecture Topics: Silicon
 EE 143 Microfbriction Technology Lecture 3 Mterils II 1/26/10 Reding portions throughout Jeger Lecture Topics licon licon Dioxide licon Nitride Aluminum & Other Metls In this course, the focus will be
EE 143 Microfbriction Technology Lecture 3 Mterils II 1/26/10 Reding portions throughout Jeger Lecture Topics licon licon Dioxide licon Nitride Aluminum & Other Metls In this course, the focus will be
Patterson Air Force Base, OH USA
 Low Temperture Superplsticity of Ti-6Al-4V Processed y Wrm Multidirectionl Forging G.A. Slishchev 1,, E.A. Kudrjvtsev 1,, S.V. Zheretsov 1,c, S.L. Semitin 2,d 1 Belgorod Stte University, Poed 85, Belgorod
Low Temperture Superplsticity of Ti-6Al-4V Processed y Wrm Multidirectionl Forging G.A. Slishchev 1,, E.A. Kudrjvtsev 1,, S.V. Zheretsov 1,c, S.L. Semitin 2,d 1 Belgorod Stte University, Poed 85, Belgorod
Study on the inhibition of Mild Steel Corrosion by Cationic Surfactant in HCl Medium
 IOSR Journl of Applied Chemistry (IOSRJAC) ISSN : 2278-5736 Volume 2, Issue 1 (Sep-Oct. 212), PP 45-53 Study on the inhibition of Mild Steel Corrosion by Ctionic Surfctnt in HCl Medium Prthibh B.S 1 P.Kotteeswrn
IOSR Journl of Applied Chemistry (IOSRJAC) ISSN : 2278-5736 Volume 2, Issue 1 (Sep-Oct. 212), PP 45-53 Study on the inhibition of Mild Steel Corrosion by Ctionic Surfctnt in HCl Medium Prthibh B.S 1 P.Kotteeswrn
Supplementary Figure S1 Parylene roughness. Atomic force microscopy image of
 Supplementry Figure S1 Prylene roughness. Atomic force microscopy imge of the thermlly evported prylene lyer on top of the Si/PVA stck. The verge surfce roughness is rms=7nm. 1 c Supplementry Figure S2
Supplementry Figure S1 Prylene roughness. Atomic force microscopy imge of the thermlly evported prylene lyer on top of the Si/PVA stck. The verge surfce roughness is rms=7nm. 1 c Supplementry Figure S2
Evaluation of Winter Canola Grown in 30 inch Rows
 Evlution of Winter Cnol Grown in 3 inch Rows Chd Godsey, Oilseed Cropping Systems Specilist Pst reserch in Oklhom hs indicted tht yield potentil my decrese from to 1% when cnol is grown in 3 inch rows,
Evlution of Winter Cnol Grown in 3 inch Rows Chd Godsey, Oilseed Cropping Systems Specilist Pst reserch in Oklhom hs indicted tht yield potentil my decrese from to 1% when cnol is grown in 3 inch rows,
Population Distribution
 Nme: Period: Why? Popultion Distriution How does popultion distriution ffect the environment? Alsk contins over 127 million cres of untouched forest lnd. It is the lrgest stte in the United Sttes, yet
Nme: Period: Why? Popultion Distriution How does popultion distriution ffect the environment? Alsk contins over 127 million cres of untouched forest lnd. It is the lrgest stte in the United Sttes, yet
