ATTEMPT TO IDENTIFY NOVEL IFT MUTANT THROUGH PCR SEQUENCING AND ANALYSIS OF CHLAMYDOMONAS REINHARDTII FLAGELLAR MUTANTS
|
|
|
- Willis Summers
- 6 years ago
- Views:
Transcription
1 ATTEMPT TO IDENTIFY NOVEL IFT MUTANT THROUGH PCR SEQUENCING AND ANALYSIS OF CHLAMYDOMONAS REINHARDTII FLAGELLAR MUTANTS An Undergraduate Research Scholars Thesis by CATHERINE MARIE HERNANDEZ Submitted to Honors and Undergraduate Research Texas A&M University in partial fulfillment of the requirements for the designation as UNDERGRADUATE RESEARCH SCHOLAR Approved by Research Advisor: Dr. Hongmin Qin May 2013 Major: Biology
2 TABLE OF CONTENTS Page TABLE OF CONTENTS. 1 ABSTRACT. 2 DEDICATION. 4 ACKNOWLEDGMENTS...5 NOMENCLATURE 6 CHAPTER I INTRODUCTION... 7 II MATERIALS AND METHODS Culturing mutant strains.. 12 DNA extraction PCR reaction and sequencing..13 Back-cross III RESULTS.. 22 IV DISCUSSION 28 REFERENCES.. 30 APPENDIX A 32 APPENDIX B 33 1
3 ABSTRACT Attempt to Identify Novel IFT Mutant through PCR Sequencing and Analysis of Chlamydomonas reinhardtii Flagellar Mutants. (May 2013) Catherine Marie Hernandez Department of Biology Texas A&M University Research Advisor: Professor Hongmin Qin Department of Biology The flagella organelle is crucial to many different cells and organisms because it allows for sensation and movement throughout a matrix. The Intraflagellar Transport system (IFT) is of particular interest since it helps build and maintain the flagella through anterograde and retrograde transport. Chlamydomonas reinhardtii is a commonly used model to study the mechanisms of how the flagella organelle functions because its flagella organelle is highly conserved and similar to human cilia and flagella. One route in which to study the flagella organelle is through the creation and analysis of flagellar mutants. Six possible C. reinhardtii IFT mutants (created from a previous transformation process and confirmed through a phototaxis test) were chosen to perform PCR on. These regions of disrupted DNA used for PCR were hypothesized to be related to IFT. The DNA was sent to be sequenced in order to determine what sections of the C. reinhardtii genome were disrupted by the transformation process. Conclusive results are currently being determined. The consequences of improper functioning flagella can be devastating to a cell. Through this research, the flagella organelle and its IFT processes can be 2
4 better understood, and the findings may even aid in the discovery of cures for flagellar diseases (such as polycystic disease, retinitis pigmentosa, etc.). 3
5 DEDICATION I would like to dedicate this thesis to my grandfather, who has taught me that education and the pursuit of knowledge is the most important task to accomplish in life because no one can ever take that away from you. I would also like to dedicate this thesis to my parents, who have given me all that I need and much more, and have provided me with the means to pursue a great education. 4
6 ACKNOWLEDGEMENTS I would like to thank Professor Qin for believing in my abilities to work in the lab, for letting me use the materials in the lab, and for allowing me to have this wonderful undergraduate research experience. I would also like to thank the Technician, Graduate, and Undergraduate students in the lab, who were very helpful and welcoming to me. I would also like to thank The Louis Stokes Alliance for Minority Participation; without this organization, I probably would not have been involved with research in Professor Qin s lab in the first place. Finally, I want to thank the Undergraduate Research Scholars Program for allowing me to participate in their program, for giving me funding for my project, and for guiding me as I write my Thesis. 5
7 NOMENCLATURE Wt Wild type Chlamydomonas reinhardtii cells, cc125 (mt +) mt Hygr TAP HEPA ddh 2 O PCR TAP-N IFT bp DMSO Mating type Hygromycin B antibiotic Tris-acetate-phosphate media High-efficiency particulate air filter Double distilled water Polymerase chain reaction Nitrogen-deficient TAP media Intraflagellar transport Base pair Dimethyl sulfoxide 6
8 CHAPTER I INTRODUCTION There are many instances where the flagella organelle has proven to be crucial to the longevity of cells (Seravin, 1992). One example is seen in the ability of different cells and organisms to move towards food or away from toxins through opposing rotations of the flagella (Meadows, 2011). IFT is a critical component of flagellar function since its role is to build and maintain the flagella. This is achieved through the anterograde and retrograde transport of motor-like proteins along the microtubules of the flagella. If IFT or other important components of flagellar machinery are mutated, basic cellular functions would be compromised. These flagellar mutations may lead to human diseases such as Kartagener syndrome (Afzelius et al., 2001), polycystic kidney disease (Pazour et al., 2000), retinitis pigmentosa (Pazour et al., 2002), and male infertility due to immobile sperm (Ibanez-Tallon et al., 2003). Further study and analysis of the genetic makeup of the flagella will bring the scientific community one step closer to discovering the genetic culprit of these diseases and, quite possibly, their cures. Chlamydomonas reinhardtii is a photosynthetic, unicellular, green alga that depends on the flagella organelle for movement and sensation throughout a matrix. Considering this organelle s functions, the flagellum is very important and crucial for C. reinhardtii s wellbeing. This is especially true since this organism is photosynthetic. Without a proper functioning flagellum, the cells would have trouble moving towards a light source that is important for obtaining energy for the cell (as with vascular plants) and even aids to the growth of the cell (Rochaix, 2001). C. 7
9 reinhardtii is not only is an excellent model that demonstrates why the flagellum is important, but also provides a means for in depth study of this organelle. The flagella organelle can be studied further by the creation and analysis of C. reinhardtii flagellar mutants. Mutants are created through a transformation process which is illustrated in Figure 1. Figure 1. Transformation Process of Chlamydomonas reinhardtii. Figure 1. The phyg3 plasmid was linearized at ScaI and inserted into the membranes of Wt Chlamydomonas reinhardtii cells that were punched with holes. The linearized plasmid entered the genome of the cells, disrupting various sites in the DNA. These disruptions led to the creation of different C. reinhardtii mutants. The first step in this process was the linearization of the phyg3 plasmid at the ScaI restriction enzyme site. This plasmid is of known sequence and contains a Hygromycin B resistance gene called Streptomyces hygroscopicus aminoglycoside phosphotransferase. The linearized plasmid was then inserted into the C. reinhardtii cells through holes that were punched into the membrane of the cells. Once in the cell, the plasmid entered the genome of the C. reinhardtii Wt cells 8
10 (Manshouri et al., 2012). These plasmids, when in the genome of C. reinhardtii cells, caused various mutations by disrupting important genes in C. reinhardtii (Qin, ). A phototaxis test was then performed on the newly synthesized mutants in order to differentiate C. reinhardtii flagellar mutants from other C. reinhardtii mutants (Manshouri et al., 2012). An illustration of this test can be seen in Figure 2. Figure 2. Phototaxis Test. A. B. C. Figure 2. Possible C. reinhardtii flagellar mutants were isolated from other mutants using a phototaxis test. Figure 2A. Phototaxis box. This test consisted of placing tubes of inoculated mutants in a black box that had a slit. The box was then placed near a light source. Figure 2B. Non-flagellar mutants. If the cells were able to move towards the light, they were assumed to be non-flagellar mutants. Figure 2C. Flagellar mutants. If the cells could not move towards the light, then they were assumed to be possible flagellar mutants. 9
11 This phototaxis screening test is similar to what Wallace and his lab presented in their paper The Mother Centriole Plays an Instructive Role in Defining Cell Geometry. (Wallace et al., 2007). Its first step consisted of placing test tubes inoculated with mutant strains into a black box. A slit that runs across the box allowed the only light that is exposed to the cultures to shine through. The tubes were left in the box for approximately ten minutes. After the inoculated tubes are removed, if the cells have migrated towards the light (which will be known if they are condensed into a line where the slit exposed the test tubes to the light), this indicated that the test was positive and they were probably not flagellar mutants. If the inoculated tubes were removed with the cells still evenly dispersed throughout the tube, then the test was negative and the strains can be assumed to be flagellar mutants (Manshouri et al., 2012). After the flagellar mutants were distinguished from the other C. reinhardtii mutants, each strain was inoculated into TAP media, cultured, and observed under the microscope. Its phenotypic characteristics were identified and recorded, and the strains were streaked onto media. Single colony isolation was also performed in order to assure that the cells are only of one particular strain. These strains were then plated on slants and stored for later use (Manshouri et al., 2012). The research that will be performed on these mutants will help identify what regions of the C. reinhardtii genome are vital to flagellar function through PCR sequencing. Procedures such as backcross and rescue can then be performed to verify that only one gene is associated with the corresponding mutant phenotype, and therefore necessary for proper flagella function. After, further study can be conducted to determine why the particular gene is vital to flagellar function 10
12 and methods to restore the flagellar function of the mutant can be determined. This research is important because it may help in understanding IFT better and may even provide clues as to what regions of DNA are damaged in flagellar diseases (polycystic kidney disease, retinitis pigmentosa, etc.). 11
13 CHAPTER II MATERIALS AND METHODS Culturing mutant strains The strains utilized include six flagellar mutants from a previous transformation using a plasmid cut at the ScaI site (Manshouri et al., 2012): 2P40, 6P1, 6P7, 6P9, 6P10, and 6P11. The strains were streaked from slants onto 1.2% (of agar) TAP plates containing Hygr (10 µg/ml). This was done under sterile conditions (under a HEPA system using a sterile inoculating loop). The instructions for making TAP media can be found in Appendix A. The plates were then placed under light for several days (to allow for growth) and stored for later use. DNA extraction Fresh cells (about a week old) were transferred from the Hygr TAP plates and inoculated in a 250 ml Erlenmeyer flask containing approximately 200 ml of liquid TAP. This was done under sterile conditions. The cells were cultured using a Kimble serological pipette attached to an aeration system under light for two days. The cells should be a light, deep green color throughout the media. The cells were then spun down using a 5810 R Eppendorf centrifuge. The supernatant was discarded and the cells were resuspended in about 20 µl of ddh 2 O. This solution was transferred to a 1.5 ml microcentrifuge tube. The DNA was then extracted using a Genomic DNA Purification Kit from Fermentas Life Sciences. The DNA was stored in -20 C for later use. 12
14 PCR reaction and sequencing Figure 3 illustrates the principle of how PCR was used to amplify specific regions of C. reinhardtii DNA. Figure 3. Principle of PCR. A. 13
15 B. C. 14
16 D. Figure 3. The route in which PCR is able to amplify specific regions of the C. reinhardtii genome. Figure 3A. Restriction Enzyme Digestion. DNA is cut by a restriction enzyme at a specific cut site creating restriction fragments of DNA. Figure 3B. Ligation. Cassettes are ligated to the ends of the restriction fragments, but two nicks are left in the DNA. Figure 3C. First PCR. The genomic DNA containing the plasmid is amplified. The nicks allow for this specific amplification since the C1 primer will stop at the nick and S1/L1 is needed to complete amplification. Figure 3D. Second PCR. The amplified DNA is further specified by using C2 and S2/L2 primers which are closer to the genomic DNA. Further runs are similar to the Second PCR concept. This figure was adapted from Takara s LA PCR in vitro Cloning Kit. Restriction enzyme digestion of DNA Four reactions for each mutant were prepared in 1.5 ml microcentrifuge tubes containing 5 µl of Fermentas Restriction enzyme (either HindIII, PstI, SacII, or ApaI, 10 u/ µl), 5 µl of their 15
17 corresponding Fermentas Restriction enzyme buffer (10X), 40 µl maximum amount of extracted DNA, and enough ddh 2 O to bring the final volume up to 50 µl. This mixture was incubated at 37 C for three to five hours. Ethanol precipitation was then used to produce a DNA pellet that was dissolved in 5 L of ddh 2 O. This was stored at -20 C until further use. Ligation Cassettes were prepared by adding 10 µl of the first Cassette and 10 µl of the second Cassette (0.1 µmol/ml) for each particular restriction enzyme in a PCR tube. These tubes were then heated at 94 C for ten minutes using a PCR machine. Reactions were then setup in PCR tubes using 5 µl of the DNA solution from the Restriction Enzyme Digestion, 3 µl of T4 ligase (3 u/ µl), 3 µl of T4 ligation buffer (10X), 2.5 µl of each reaction s corresponding prepared cassette, and enough ddh 2 O to bring the final reaction volume to 30 µl. The cassettes were synthesized by Integrated DNA Technologies and their sequences can be seen in Table 1 in Appendix B. These reactions were then run on the PCR machine for approximately eight hours at a constant temperature of 16 C. The DNA was collected using ethanol precipitation and the DNA pellet was dissolved in 5 µl of ddh 2 O. The tubes were stored at -20 C until further use. First run 1 µl of the ligation product was added to 13.5 L of ddh 2 O in a PCR tube and heated at 94 C for ten minutes using the PCR machine. Reactions were then setup using the 14.5 µl of the ligation solution, 0.5 µl of C1 primer (1 x 10-5 mmol/ml), 0.5 µl of S1or L1 primer (1 x 10-5 mmol/ml), 5 µl buffer (5X), 5 µl GC melt buffer (5X), 0.25 µl of Polymerase mix (5X), and 16
18 0.5 µl dntp (10X). The final reaction volume was µl. All primers were synthesized by Integrated DNA Technologies and their sequences can be seen in Table 2 in Appendix B. The PCR reactions were then run in a PCR machine using the program seen in Figure 4. After the reactions were run in the PCR program, the PCR products were stored in -20 C until further use. Figure 4. First Run PCR Program. Figure 4. Program utilized for first run PCR products. Time is displayed in minutes:seconds. For the second run, annealing time is reduced to 1:30 and extension time is reduced to 2:30. Annealing time/temperature, extension time/temperature, number of cycles, and the template dilution vary for the third run and further runs based on the assessment of the quality of bands in the agarose gel. Second run PCR products from the first run were diluted 200X with ddh 2 O. Reactions were prepared by adding 1µL of the diluted first run template, 1 µl of C2 primer (1 x 10-5 mmol/ml), 1 µl of S2 or L2 primer (1 x 10-5 mmol/ml), 5 µl GoTaq buffer (5X), 0.25 µl of GoTaq enzyme (5 u/ 17
19 µl), 0.4 µl dntp (10X), 0.75 µl of DMSO, 1 µl of MgCl 2, and 14.5 µl of ddh 2 O. The annealing time of the PCR program was shortened to 1.5 minutes and the extension time was shortened to 2.5 minutes. After the program was completed, the PCR tubes were stored in -20 C until further use. Gel electrophoresis 5 µl of the PCR products were loaded and ran on a 1 % (of agarose) agarose gel with a 1 kb DNA ladder using electrophoresis at a rate of 96 volts. If the bands were bright and sharp (indicating a good concentration and specificity of the DNA), the second run reactions were prepared again using a 50 µl final volume and run in the second run PCR program. When the program finished, the total volume of the reactions were loaded onto a 1% agarose gel and ran at a slower rate of 80 volts. Those bands could then be used for the next step of the procedure. If the bands needed to be brighter or less smeared after the initial second run, further runs were performed. Further runs The procedure for the runs following the first two runs was basically the same as the procedure for the second run. The only differences were that the right and left primers (S and L) were changed to S3 and L3 for the third run and S4 and L4 for a fourth run if it was needed. The annealing time/temperature, extension time/temperature, number of cycles, and template dilution could also differ from the first two runs depending on the quality of the bands. 18
20 DNA gel extraction Once the bands on the agarose gel were bright and sharp, the bands were cut precisely from the gel using a sterile knife and UV light. The DNA was then extracted from the gel using the Fermentas Life Sciences GeneJET Gel Extraction Kit. The DNA was stored in -20 C until further use. DNA sequencing and analysis The DNA was added to a 1.5 ml microcentrifuge tube containing a reaction mixture adhering to the guidelines that the sequencing company stipulated. The company that sequenced the target DNA was Eurofins MWG Operon (located in Huntsville, Alabama). The results were then analyzed using an online database called the National Center for Biotechnology Information s Blast Server and the Sequencher 4.8 computer program. Back-cross Mutant strain preparation The mutant strains were streaked onto large 1.2 % TAP plates containing Hygr in a concentrated area of approximately 1 cm in width. The cc124 (mt -) strain was streaked so that cells were covering the entire TAP plate. Both of these steps were done under sterile conditions. The plates were placed under light until a thick layer of cells were present. The cells were then transferred to TAP-N plates (instructions for preparation in Appendix A) and kept under light for three days (Jiang et al., 2009). 19
21 Mating The strains were transferred from the TAP-N plates to a 50 ml Erlenmeyer flask containing 2.5 ml of sterile water. Each strain and its corresponding Wt mating culture had about the same cell concentration. These steps were performed under sterile conditions. The cells were then placed under bright light and agitated (placed on a shaker) for two hours. After two hours, the cells become extremely motile (motility was checked under the microscope using 20 µl of cells and 40X objective). After motility was checked, cc124 strain and mutant strains were combined together into a 50 ml Erlenmeyer flask. This step was also performed under sterile conditions. The combination of cells were left under bright light without agitation. The amount of time that it took for the cells to mate varied from thirty minutes to four hours depending on the mutant strain. 300 µl of the mating mixture was then plated on 4% TAP plates in four separate spots. The mating mixture was not agitated during this process. The plates were then dried under the HEPA system until the liquid on the plates was absorbed. The plates were left in light for eighteen hours and after, wrapped in foil and stored for five to seven days (Jiang et al., 2009). Random spore evaluation The vegetative cells were gently scraped off with a blade under sterile conditions using a dissection microscope. Zygotes were present in the yellow-orange edges of the drops. A small piece of TAP media was cut out that contained approximately 30 zygotes. This piece was then dragged over a 1.5% TAP plate to spread out the zygotes. The newly streaked plate was held 5 cm over chloroform for 1.5 minutes in order to the kill the remaining vegetative cells. The plate was wrapped in paper towels and grown under light for 16 to 20 hours. 300 µl of sterile water was added to the plate. The cells were able to swim at this point (cells were checked by using the 20
22 dissection microscope) and the water and cells were spread over the plate in order to make sure the spores were separated. The plates were grown under light until single colonies formed. 96 colonies were picked and inoculated into a 96 well plate containing liquid TAP media. The phenotypes were checked using the dissection microscope in order to screen only flagellar mutants. Backcross was repeated on selected colonies four more times (mated to both + and strains to determine mating type) (Jiang et al., 2009). 21
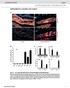
23 CHAPTER III RESULTS DNA extraction did not work on mutants 6P9, 6P1, or 6P11. The inoculated mutants may have been cultured for too long. Rounds of PCR were performed on 2P40, 6P7, and 6P10 mutants. The results for the second round of PCR can be seen in Figure 5. Figure 5. Second round of PCR on 2P40, 6P7, and 6P10. Figure 5. Agarose gel (1%) after second round of PCR, running at about 96 volts. 12 µl was loaded into each well. 100 bp ladder was used on either side of the lanes. Left side of PCR did not get any results for any of the three mutants. The right side got results for only 6P7 and 6P10 mutants. The HindIII bands, about 1000 bp and 500 bp, were chosen for a third round of PCR for the 6P7 mutant. The reason for this is because the SacII and ApaI bands are too short, having about bp. Shortened bands are not favorable, since their sequences may only contain the insertional plasmid itself, or may not have enough genomic DNA to specify the exact location of the insert in the genomic DNA of Chlamydomonas reinhardtii. 6P10 only had a band for ApaI; this band 22
24 was also long enough for sequencing, having around 650 bp. This band was also chosen for a third round of PCR. The results for the third round of PCR can be seen in Figure 6. Figure 6. Third round of PCR on 6P7 and 6P10. Figure 6. Agarose gel (1%) after second round of PCR, running at about 83 volts. 6 µl was loaded into each well and a 500 bp ladder was used. Bands were obtained for each sample. 1 µl of the template for the third round of PCR was diluted in 200 µl of ddh 2 O. The program used for the third round of PCR was the same as the second round of PCR except that the annealing temperature was increased to 56.5 C. Both mutants got the same bands for the third round of PCR as they did in the second round of PCR. This is a good result since this means that these bands are specific. However, the concentrations do not look as good for either sample as they did in the second round of PCR. Therefore, the second round of PCR was performed again in the exact same manner as before, except that a 50 µl reaction was prepared for each mutant instead of a 25 µl reaction. A greater 23
25 volume of DNA is preferred when sending a sample for sequencing. The results from the second attempt of second PCR can be seen in Figure 7. Figure 7. Second round of PCR on 6P7 and 6P10 (50 µl). Figure 7. Agarose gel (1%) after second attempt of second round of PCR, running at about 83 volts. 6 µl of 500 bp ladder was loaded into first well. 50 µl of 6P7 DNA sample was loaded into second well and 50 µl of 6P10 DNA sample was loaded into third well. Bands were obtained for each sample. The bands from this second attempt at a second run were of good concentration. Therefore, the two darker bands from 6P7 were cut out and sent for sequencing and the band from 6P10 was cut out and sent for sequencing. The sequencing results for each sample can be seen in Figure 8, Figure 9, and Figure
26 Figure 8. Sequencing Results for 6P7, 500 bp. Figure 8. The sequencing results sent by Operon for the 500 bp band from the 6P7 mutant. There were 410 total bases. The sequencing for each bp was specific, but not well amplified. This means that the DNA was probably not of good quality. 25
27 Figure 9. Sequencing Results for 6P7, 1000 bp. Figure 9. The sequencing results sent by Operon for the 1000 bp band from the 6P7 mutant. There were 652 total bases. The sequencing for the bp between was specific and well amplified. However, this only gives 200 bp sequenced well. This band was supposed to be 1000 bp long. The fact that the sequencing did not sequence 800 of the bp well means that the DNA was not of good quality. 26
28 Figure 10. Sequencing Results for 6P10, 650 bp. Figure 10. The sequencing results sent by Operon on the 650 bp band from 6P10 mutant. There were 278 total bases. The sequencing for each bp was specific, but not very amplified. This means that the quality of the DNA was not very good. After analyzing the sequences of the mutant DNA using the Sequencher program 4.8, it was found that the sequences contained only the sequence of the insertional plasmid. This gives us no information about the location of the plasmid insert in the genomes of the two different mutants. 27
29 CHAPTER IV DISUCUSSION As stated before, if the bands that are sent for sequencing have too few bp, then the sequence may contain only the insertional plasmid. During this project, that is exactly what happened. Even though it was determined that the 1000 bp, 650 bp, and 500 bp bands would be long enough, this was not the case. The sequences of all the bands were only the sequence of the insertional plasmid. Unfortunately, these results do not give the information about where in the mutants genomes the plasmid inserted. Therefore, the genes responsible for the mutants phenotypes are still unknown. One reason why this may have occurred is that the extracted mutant DNA was not of good quality. If the DNA was not of good quality, then the primers during the PCR process may have had trouble adhering to the genomic DNA. The primers instead may have adhered only to the insertional plasmid. Therefore, only the plasmid DNA was amplified and sequenced. One of the major steps in obtaining good DNA is that the cells which you extract the DNA from must be of good quality. The cells that were cultured before extracting the DNA from them must have been bad quality cells. This means that during the next trial, the cells should be cultured for two days at most. Although conclusive results have not been achieved yet, this method does work. This is known from previous research conducted last summer (the region of the genome where the plasmid 28
30 inserted was successfully determined in one mutant). There are also promising aspects of the research conducted this year. The band quality that has been obtained following consecutive rounds of PCR during this project has been better than achieved last summer. This means that this PCR technique is being performed better than before. If this research is continued, while paying close attention to the quality of cells from which DNA will be extracted, conclusive results will be achieved. Hopefully, one of these six particular mutants is a novel IFT mutant. If there is a novel IFT mutant, then further research on that mutant can be conducted and the mechanisms of IFT can be better understood. This may lead to the better understanding of the cause of flagellar diseases, and may someday even aid in the discovery of their cures. 29
31 REFERENCES Afzelius, B. A., Mossberg, B., and Bergstrom, S. E. (2001). Immotile cilia syndrome (primary ciliary dyskinesia), including Kartagener syndrome. in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D. New York: McGraw-Hill Publishers, p Feldman, J. L., Geimer, S., and Wallace, M. F. (2007). The Mother Centriole Plays and Instructive Role in Defining Cell Geometry. PLoS Biol. 5(149). Retrieved September 15, 2012, from Ibanez-Tallon, I., Heintz, N., and Omran, H. (2003). To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet. 12. Retrieved September 15, 2012, from Jiang, X. and Stern, D. (2009). Mating and tetrad separation of Chlamydomonas reinhardtii for genetic analysis. J. Vis. Exp. (30), e /1274, DOI : /1274. Manshouri, R., Hernandez, C., Richey, E., Mathis, G., Lyuksyutova, E., and Qin, H. (2012). Generating and sequencing Chlamydomonas reinhardtii flagella mutants via random insertional mutagenesis and RESDA-PCR. Unpublished manuscript, Texas A&M University, College Station, TX. Meadows, R. (2011). How Bacteria Shift Gears. PLoS Biol. 9(5). Retrieved September 15, 2012, from 30
32 Pazour, G. J., Baker, S. A., Deane, J. A., Cole, D. G., Dickert, B. L., Rosenbaum, J. L., Witman, G. B., and Besharse, J. C. (2002). The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 157, Pazour, G. J., Dickert, B. L., Vucica, Y., Seeley, E. S., Rosenbaum, J.L., Witman, G. B., and Cole, D. G. (2000). Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 151, Qin, H. ( ). Creating Chlamydomonas reinhardtii insertional mutants. Research. Lecture conducted from Texas A&M University, College Station, TX. Rochaix, J-D. (2001). Assembly, function, and dynamics of the photosynthetic machinery in Chlamydomonas reinhardtii. Plant Physiology. 127, Seravin, L. N. (1992). Eukaryotes devoid of the most important cellular organelles (flagella, Golgi apparatus, mitochondria) and the main task of organellology. Tsitologyia. 34,
33 APPENDIX A TAP Media Preparation (500 ml) 1. Add 7.5 g of Agar and about approximately 200 ml of purified water to a 1L flask. 2. Wash the Agar three times with purified water. After washed, add purified water so the resulting volume is about 300 ml. 3. Add the following solutions in order to the 1L flask: m m m m m 5 ml Tris (100X, 4 C) 5 ml TAP salts (100X, 4 C) 500 µl Phosphate (4 C) 500 µl Trace metals (Hunter trace element, 4 C) 500 µl Glacial acetic acid 4. Use purified water to bring the final volume to 500 ml. 5. Autoclave the media for 30 minutes and cool in a 50 C water bath for 30 minutes. 6. If wanted, add antibiotic (10 µg/ml) and pour the media into plates. Allow to media to harden and collect plates in a bag. This step should be done under sterile conditions. 7. Store plates at 4 C. TAP-N Media Preparation (500 ml) All the steps from TAP Media Preparation should be followed except TAP-N (nitrogen deficient) salts are used in place of TAP salts. 32
34 APPENDIX B Table 1. Cassettes synthesized for PCR reaction. Cassette Name Sequence Length (bp) HindIII Cassette-1 HindIII Cassette-2 PstI Cassette-1 PstI Cassette-2 SacII Cassette-1 SacII Cassette-2 ApaI Cassette-1 ApaI Cassette-2 5 -GTA CAT ATT GTC GTT AGA ACG CGT AAT ACG ACT CAC TAT AGG GAG A-3 5 -AGC TTC TCC CTA TAG TGA GTC GTA TTA CGC GTT CTA ACG ACA ATA TGT AC-3 5 -GTA CAT ATT GTC GTT AGA ACG CGT AAT ACG ACT CAC TAT AGG GAG ACT GCA-3 5 -GTC TCC CTA TAG TGA GTC GTA TTA CGC GTT CTA ACG ACA ATA TGT AC-3 5 -GTA CAT ATT GTC GTT AGA ACG CGT AAT ACG ACT CAC TAT AGG GAG ACC GC-3 5 -GGT CTC CCT ATA GTG AGT CGT ATT ACG CGT TCT AAC GAC AAT ATG TAC-3 5 -GTA CAT ATT GTC GTT AGA ACG CGT AAT ACG ACT CAC TAT AGG GAG AGG GCC-3 5 -CTC TCC CTA TAG TGA GTC GTA TTA CGC GTT CTA ACG ACA ATA TGT AC Table 2. Primers synthesized for PCR reaction. Primer Name Sequence Length (bp) Cassette Primer C1 5 -GTA CAT ATT GTC GTT AGA ACG CGT AAT ACG ACT CA-3 35 Cassette Primer C2 5 -CGT TAG AAC GCG TAA TAC GAC TCA CTA TAG GGA GA-3 35 Right Primer S1 5 -AAG AGT ATG AGT ATT CAA CAT TTC CGT GTC GCC-3 33 Right Primer S2 5 -AGA AAC GCT GGT GAA AGT AAA AGA TGC TGA AG-3 32 Right Primer S3 5 -AAG ATG CTG AAG ATC AGT TGG GTG C-3 25 Right Primer S4 5 -CGT TTT CCA ATG ATG AGC ACT-3 21 Left Primer L1 5 -GCA ACT TTA TCC GCC TCC ATC CAG TCT AT-3 29 Left Primer L2 5 -CGC TCG TCG TTT GGT ATG GCT TCA-3 24 Left Primer L3 5 -GGC GAG TTA CAT GAT CCC CCA TGT T-3 25 Left Primer L4 5 -GAT CGT TGT CAG AAG TAA GTT GGC-3 24 Left Primer L5 5 -TTC TCT TAC TGT CAT GCC ATC
Cat. # Product Size DS130 DynaExpress TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1
 Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
MacBlunt PCR Cloning Kit Manual
 MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR
 Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular
![strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular](/thumbs/72/66361212.jpg) Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Arabidopsis actin depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB
 Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC
 Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplemental Data Supplemental Figure 1.
 Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were
 1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
Y-chromosomal haplogroup typing Using SBE reaction
 Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Supplementary. Table 1: Oligonucleotides and Plasmids. complementary to positions from 77 of the SRα '- GCT CTA GAG AAC TTG AAG TAC AGA CTG C
 Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis
 1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
Supplemental material
 Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Engineering D66N mutant using quick change site directed mutagenesis. Harkewal Singh 09/01/2010
 Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH).
 Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Gene synthesis by circular assembly amplification
 Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
PCR analysis was performed to show the presence and the integrity of the var1csa and var-
 Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Hes6. PPARα. PPARγ HNF4 CD36
 SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
Supplementary Figure 1A A404 Cells +/- Retinoic Acid
 Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
Supporting information for Biochemistry, 1995, 34(34), , DOI: /bi00034a013
 Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Materials Protein synthesis kit. This kit consists of 24 amino acids, 24 transfer RNAs, four messenger RNAs and one ribosome (see below).
 Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Supplemental Data. mir156-regulated SPL Transcription. Factors Define an Endogenous Flowering. Pathway in Arabidopsis thaliana
 Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
ORFs and genes. Please sit in row K or forward
 ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
An engineered tryptophan zipper-type peptide as a molecular recognition scaffold
 SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
ΔPDD1 x ΔPDD1. ΔPDD1 x wild type. 70 kd Pdd1. Pdd3
 Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Supporting Information
 Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells
 Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Disease and selection in the human genome 3
 Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Supporting Information
 Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
SUPPLEMENTARY MATERIALS AND METHODS. E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1)
 SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
Supplementary Information
 Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Legends for supplementary figures 1-3
 High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
Supplementary Information. Construction of Lasso Peptide Fusion Proteins
 Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
Supporting Online Information
 Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
I. Things to keep in mind before you start this protocol
 Inverse PCR and Sequencing Protocol on 5 Fly Preps For recovery of sequences flanking piggybac elements This protocol is an adaptation of "Inverse PCR and Cycle Sequencing Protocols" by E. Jay Rehm Berkeley
Inverse PCR and Sequencing Protocol on 5 Fly Preps For recovery of sequences flanking piggybac elements This protocol is an adaptation of "Inverse PCR and Cycle Sequencing Protocols" by E. Jay Rehm Berkeley
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
Multiplexing Genome-scale Engineering
 Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Table S1. Bacterial strains (Related to Results and Experimental Procedures)
 Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Overexpression Normal expression Overexpression Normal expression. 26 (21.1%) N (%) P-value a N (%)
 SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
2
 1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
Project 07/111 Final Report October 31, Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines
 Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
A netlike rolling circle nucleic acid amplification technique
 Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2014 Supplementary Information A netlike rolling circle nucleic acid amplification technique Xiaoli Zhu,
Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2014 Supplementary Information A netlike rolling circle nucleic acid amplification technique Xiaoli Zhu,
Dierks Supplementary Fig. S1
 Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system
 Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
RPA-AB RPA-C Supplemental Figure S1: SDS-PAGE stained with Coomassie Blue after protein purification.
 RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer
 TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
Lecture 11: Gene Prediction
 Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
NAME:... MODEL ANSWER... STUDENT NUMBER:... Maximum marks: 50. Internal Examiner: Hugh Murrell, Computer Science, UKZN
 COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
Supplemental Table 1. Primers used for PCR.
 Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
Yeast BioBrick Assembly (YBA)
 Yeast BioBrick Assembly (YBA) Standardized method for vector assembly of BioBrick devices via homologous recombination in Saccharomyces cerevisiae Martin Schneider, Leonard Fresenborg, Virginia Schadeweg
Yeast BioBrick Assembly (YBA) Standardized method for vector assembly of BioBrick devices via homologous recombination in Saccharomyces cerevisiae Martin Schneider, Leonard Fresenborg, Virginia Schadeweg
PCR-based Markers and Cut Flower Longevity in Carnation
 PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR
 Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
S4B fluorescence (AU)
 A S4B fluorescence (AU) S4B fluorescence (AU) dsbb csgba csgd dsbb csgba bcsa 5000 * NS NS 4000 * 3000 2000 1000 0 ΔcsgBAΔbcsA ΔcsgDΔdsbBΔbcsA ΔcsgBA ΔdsbBΔcsgBA ΔcsgDΔdsbB B -1000 4000 * * NS 3500 * 3000
A S4B fluorescence (AU) S4B fluorescence (AU) dsbb csgba csgd dsbb csgba bcsa 5000 * NS NS 4000 * 3000 2000 1000 0 ΔcsgBAΔbcsA ΔcsgDΔdsbBΔbcsA ΔcsgBA ΔdsbBΔcsgBA ΔcsgDΔdsbB B -1000 4000 * * NS 3500 * 3000
Genomics and Gene Recognition Genes and Blue Genes
 Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Sequencing of DNA lesions facilitated by site-specific excision via base. excision repair DNA glycosylases yielding ligatable gaps
 Supporting information Sequencing of DNA lesions facilitated by site-specific excision via base excision repair DNA glycosylases yielding ligatable gaps Jan Riedl, Aaron M. Fleming, and Cynthia J. Burrows*
Supporting information Sequencing of DNA lesions facilitated by site-specific excision via base excision repair DNA glycosylases yielding ligatable gaps Jan Riedl, Aaron M. Fleming, and Cynthia J. Burrows*
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide
 SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide former/ working Description a designation Plasmids pes213a b pes213-tn5
SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide former/ working Description a designation Plasmids pes213a b pes213-tn5
Lecture 19A. DNA computing
 Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
evaluated with UAS CLB eliciting UAS CIT -N Libraries increase in the
 Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
hcd1tg/hj1tg/ ApoE-/- hcd1tg/hj1tg/ ApoE+/+
 ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
Supporting Information
 Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Det matematisk-naturvitenskapelige fakultet
 UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
Supplemental Information. Human Senataxin Resolves RNA/DNA Hybrids. Formed at Transcriptional Pause Sites. to Promote Xrn2-Dependent Termination
 Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
Supporting Information. Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy
 Supporting Information Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy Agata Olszewska, Radek Pohl and Michal Hocek # * Institute of Organic
Supporting Information Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy Agata Olszewska, Radek Pohl and Michal Hocek # * Institute of Organic
Supplemental Data. Bennett et al. (2010). Plant Cell /tpc
 BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
II 0.95 DM2 (RPP1) DM3 (At3g61540) b
 Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
G+C content. 1 Introduction. 2 Chromosomes Topology & Counts. 3 Genome size. 4 Replichores and gene orientation. 5 Chirochores.
 1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
BioInformatics and Computational Molecular Biology. Course Website
 BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
11th Meeting of the Science Working Group. Lima, Peru, October 2012 SWG-11-JM-11
 11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
Complexity of the Ruminococcus flavefaciens FD-1 cellulosome reflects an expansion of family-related protein-protein interactions
 Complexity of the Ruminococcus flavefaciens FD-1 cellulosome reflects an expansion of family-related protein-protein interactions Vered Israeli-Ruimy 1,*, Pedro Bule 2,*, Sadanari Jindou 3, Bareket Dassa
Complexity of the Ruminococcus flavefaciens FD-1 cellulosome reflects an expansion of family-related protein-protein interactions Vered Israeli-Ruimy 1,*, Pedro Bule 2,*, Sadanari Jindou 3, Bareket Dassa
Primer Design Workshop. École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria
 Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR
 Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
NESTED Sequence-based Typing (SBT) protocol for epidemiological typing of Legionella pneumophila directly from clinical samples
 NESTED Sequence-based Typing (SBT) protocol for epidemiological typing of Legionella pneumophila directly from clinical samples VERSION 2.0 SUMMARY This procedure describes the use of nested Sequence-Based
NESTED Sequence-based Typing (SBT) protocol for epidemiological typing of Legionella pneumophila directly from clinical samples VERSION 2.0 SUMMARY This procedure describes the use of nested Sequence-Based
Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers
 DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
Table S1. Sequences of mutagenesis primers used to create altered rdpa- and sdpa genes
 Supplementary Table and Figures for Structural Basis for the Enantiospecificities of R- and S-Specific Phenoxypropionate/α-Ketoglutarate Dioxygenases by Tina A. Müller, Maria I. Zavodszky, Michael Feig,
Supplementary Table and Figures for Structural Basis for the Enantiospecificities of R- and S-Specific Phenoxypropionate/α-Ketoglutarate Dioxygenases by Tina A. Müller, Maria I. Zavodszky, Michael Feig,
MCB421 FALL2005 EXAM#3 ANSWERS Page 1 of 12. ANSWER: Both transposon types form small duplications of adjacent host DNA sequences.
 Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Molecular Techniques Third-year Biology
 PLANNING Genetics Lab practices Molecular Techniques. Genetics Lab practices protocol. 2015-16 PCR-DIRECTED MUTAGENESIS, MOLECULAR CLONING AND RESTRICTION ANALYSIS Sessions 1 & 2 (2x3 hours): PCR-directed
PLANNING Genetics Lab practices Molecular Techniques. Genetics Lab practices protocol. 2015-16 PCR-DIRECTED MUTAGENESIS, MOLECULAR CLONING AND RESTRICTION ANALYSIS Sessions 1 & 2 (2x3 hours): PCR-directed
Homework. A bit about the nature of the atoms of interest. Project. The role of electronega<vity
 Homework Why cited articles are especially useful. citeulike science citation index When cutting and pasting less is more. Project Your protein: I will mail these out this weekend If you haven t gotten
Homework Why cited articles are especially useful. citeulike science citation index When cutting and pasting less is more. Project Your protein: I will mail these out this weekend If you haven t gotten
Wet Lab Tutorial: Genelet Circuits
 Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
SUPPLEMENTAL MATERIAL GENOTYPING WITH MULTIPLEXING TARGETED RESEQUENCING
 SUPPLEMENTAL MATERIAL GENOTYPING WITH MULTIPLEXING TARGETED RESEQUENCING All of the patients and control subjects were sequenced and genotyped in the same way. Shotgun libraries of approximately 250 bp
SUPPLEMENTAL MATERIAL GENOTYPING WITH MULTIPLEXING TARGETED RESEQUENCING All of the patients and control subjects were sequenced and genotyped in the same way. Shotgun libraries of approximately 250 bp
DNA sentences. How are proteins coded for by DNA? Materials. Teacher instructions. Student instructions. Reflection
 DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
Sexing Bovine Preimplantation Embryos by PCR
 Sexing Bovine Preimplantation Embryos by PCR Katherine E.M. Hendricks 1, Leydson F. Martins 2, Justin M. Fear 1 and Peter J. Hansen 1 1 Dept. of Animal Sciences, University of Florida and Department of
Sexing Bovine Preimplantation Embryos by PCR Katherine E.M. Hendricks 1, Leydson F. Martins 2, Justin M. Fear 1 and Peter J. Hansen 1 1 Dept. of Animal Sciences, University of Florida and Department of
Supporting Information
 upporting Information hiota et al..73/pnas.159218 I Materials and Methods Yeast trains. Yeast strains used in this study are described in Table 1. TOM22FLAG, a yeast haploid strain for expression of C-terminally
upporting Information hiota et al..73/pnas.159218 I Materials and Methods Yeast trains. Yeast strains used in this study are described in Table 1. TOM22FLAG, a yeast haploid strain for expression of C-terminally
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Site-Specific Control of Distances between Gold Nanoparticles using Phosphorothioate Anchors on DNA and a Short Bifunctional Molecular Fastener
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Site-Specific Control of Distances between Gold Nanoparticles using Phosphorothioate Anchors on DNA and a Short Bifunctional Molecular Fastener
Microsatellite Library Protocol
 Last Update 11/26/03 D Drown Modified from T. Garner Protocol Microsatellite Library Protocol Outline of Protocol DNA Extraction (1 overnight [optional], 3 hrs setup) Digestion and Size Fractionation (8
Last Update 11/26/03 D Drown Modified from T. Garner Protocol Microsatellite Library Protocol Outline of Protocol DNA Extraction (1 overnight [optional], 3 hrs setup) Digestion and Size Fractionation (8
Supplemental Information. Target-Mediated Protection of Endogenous. MicroRNAs in C. elegans. Inventory of Supplementary Information
 Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
A Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion,
 TITLE A Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion, Survival, and Cancer Cell Behaviors AUTHORS Carolyn R. Shurer *, Marshall J. Colville *, Vivek K. Gupta,
TITLE A Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion, Survival, and Cancer Cell Behaviors AUTHORS Carolyn R. Shurer *, Marshall J. Colville *, Vivek K. Gupta,
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURB
 Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURBO DNA-free Kit (Ambion). One µg of total RNA was reverse
Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURBO DNA-free Kit (Ambion). One µg of total RNA was reverse
Amplified Analysis of DNA by the Autonomous Assembly of Polymers Consisting of DNAzyme Wires
 Supporting information for the paper: Amplified Analysis of DNA by the Autonomous Assembly of Polymers Consisting of DNAzyme Wires Fuan Wang, Johann Elbaz, Ron Orbach, Nimrod Magen and Itamar Willner*
Supporting information for the paper: Amplified Analysis of DNA by the Autonomous Assembly of Polymers Consisting of DNAzyme Wires Fuan Wang, Johann Elbaz, Ron Orbach, Nimrod Magen and Itamar Willner*
High-throughput cloning and expression in recalcitrant bacteria
 High-throughput cloning and expression in recalcitrant bacteria Eric R Geertsma & Bert Poolman Supplementary text and figures: Supplementary Figure 1 Frequency of SfiI sites yielding identical 3 extensions
High-throughput cloning and expression in recalcitrant bacteria Eric R Geertsma & Bert Poolman Supplementary text and figures: Supplementary Figure 1 Frequency of SfiI sites yielding identical 3 extensions
ITS Sequencing in Millepora. 10/09 Subcloning DNA Fragments into pbluescript Preparation of pbluescript Vector
 Page 1 of 5 10/09 Subcloning DNA Fragments into pbluescript Preparation of pbluescript Vector 1. Digest 1 µg of pbluescript with Eco RI 2. Following digestion, add 0.1 volumes of 3M sodium acetate (ph
Page 1 of 5 10/09 Subcloning DNA Fragments into pbluescript Preparation of pbluescript Vector 1. Digest 1 µg of pbluescript with Eco RI 2. Following digestion, add 0.1 volumes of 3M sodium acetate (ph
APPENDIX S1. The SSR-patchwork protocol for SSR libraries.
 Di Maio and De Castro Applications in Plant Sciences 2013 1(1): 1200158. Appendix S1 Page 1 APPENDIX S1. The SSR-patchwork protocol for SSR libraries. LEGEND * HINT ATTENTION REST I. DNA extraction and
Di Maio and De Castro Applications in Plant Sciences 2013 1(1): 1200158. Appendix S1 Page 1 APPENDIX S1. The SSR-patchwork protocol for SSR libraries. LEGEND * HINT ATTENTION REST I. DNA extraction and
Supplemental Data. The Survival Advantage of Olfaction. in a Competitive Environment. Supplemental Experimental Procedures
 Supplemental Data The Survival Advantage of Olfaction in a Competitive Environment Kenta Asahina, Viktoryia Pavlenkovich, and Leslie B. Vosshall Supplemental Experimental Procedures Experimental animals
Supplemental Data The Survival Advantage of Olfaction in a Competitive Environment Kenta Asahina, Viktoryia Pavlenkovich, and Leslie B. Vosshall Supplemental Experimental Procedures Experimental animals
Appendix 1a. Microsatellite analysis of P1-hyg, P2-neo and their. Amplified cdna (base pairs) P1-hyg P2-neo All progeny A A
 Supplementary information Appendix 1a. Microsatellite analysis of P1-hyg, P2-neo and their double drug resistant progeny (ABI 377). Primer set a,b Amplified cdna (base pairs) P1-hyg P2-neo All progeny
Supplementary information Appendix 1a. Microsatellite analysis of P1-hyg, P2-neo and their double drug resistant progeny (ABI 377). Primer set a,b Amplified cdna (base pairs) P1-hyg P2-neo All progeny
Protein Structure Analysis
 BINF 731 Protein Structure Analysis http://binf.gmu.edu/vaisman/binf731/ Iosif Vaisman COMPUTATIONAL BIOLOGY COMPUTATIONAL STRUCTURAL BIOLOGY COMPUTATIONAL MOLECULAR BIOLOGY BIOINFORMATICS STRUCTURAL BIOINFORMATICS
BINF 731 Protein Structure Analysis http://binf.gmu.edu/vaisman/binf731/ Iosif Vaisman COMPUTATIONAL BIOLOGY COMPUTATIONAL STRUCTURAL BIOLOGY COMPUTATIONAL MOLECULAR BIOLOGY BIOINFORMATICS STRUCTURAL BIOINFORMATICS
Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide
 Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide Jochen Hoffmann a,b, Martin Trotter a, Felix von Stetten a,b, Roland Zengerle, a,b,c Günter
Solid-phase PCR in a picowell array for immobilizing and arraying 100,000 PCR products to a microscope slide Jochen Hoffmann a,b, Martin Trotter a, Felix von Stetten a,b, Roland Zengerle, a,b,c Günter
