JBC Papers in Press. Published on September 20, 2004 as Manuscript M
|
|
|
- Everett Hopkins
- 6 years ago
- Views:
Transcription
1 JBC Papers in Press. Published on September 0, 00 as Manuscript M000 Expression and secretion of Salmonella Pathogenicity Island- virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL Brian K. Coombes 1, Nat F. Brown 1, Yanet Valdez 1, John H. Brumell 1, B. Brett Finlay 1,,* Michael Smith Laboratories 1 and Departments of Biochemistry and Molecular Biology, Microbiology and Immunology, University of British Columbia, Vancouver, B.C. VT 1Z Canada * Correspondence to: Dr. B. Brett Finlay, Ph.D. Biotechnology Laboratory -1 University Boulevard University of British Columbia Vancouver, British Columbia, Canada VT 1Z Phone: (0)-- Fax: (0)--0 bfinlay@interchange.ubc.ca Running title: SPI- secretion activity is required for translocon expression Current address: Infection, Immunity, Injury and Repair Program, Hospital for Sick Children, Toronto, Ontario, MG 1X, Canada and Department of Molecular and Medical Genetics, University of Toronto 1 Copyright 00 by The American Society for Biochemistry and Molecular Biology, Inc.
2 Summary Salmonella pathogenicity island (SPI) encodes a type III secretion system pivotal to the intracellular survival of Salmonella and consequently for virulence in mammals. SPI- encodes virulence factors (called effectors) that are secreted and translocated into the host cell, the type III secretion apparatus responsible for the delivery of these effectors and a two-component regulatory system that regulates intracellular expression of SPI-. Salmonella SPI- secretion activity appears to be induced in response to acidification of the vacuole in which it resides and replicates. Here we show that the expression of the SPI- proteins, SseB and SseD (filament and pore forming components of the secretion apparatus, respectively) in response to acidification requires an intact secretion system and SsaL, a Salmonella homologue of SepL, a regulator required for type III-dependent secretion of translocators but not effectors in attaching and effacing gastrointestinal pathogens. In addition, we show that the expression of SPI-- encoded effectors is acid-regulated but can be uncoupled from the expression of filament and translocon components, thus showing a differential requirement of SsaL for expression. The secretion and translocation of SPI--encoded effectors requires SsaL, but SsaL is dispensable for the secretion of SPI- effectors encoded in other pathogenicity loci, suggesting a secretion regulation function for SsaL. Further, we demonstrate that the differential expression of adjacent genes within the ssea operon (ssed, ssee) occurs at the transcriptional level. These data indicate that a Salmonella SPI- activation state is achieved by an acid-regulated response that requires a functional SPI- secretion system that includes SsaL. These data also suggest the existence of a previously unrecognized regulatory element within SPI- for the effector operon region downstream of ssed that might demarcate the expression of translocators and effectors.
3 Introduction Intracellular survival of S. enterica serovar Typhimurium (S. Typhimurium) requires a set of virulence proteins encoded in the chromosomal pathogenicity island designated Salmonella Pathogenicity Island (SPI) - (1,). SPI- is a kb virulence locus that encodes a type III secretion system (TTSS) and related proteins, called effectors which are substrates of this secretion apparatus. Infection of macrophages by Salmonella activates the SPI- virulence locus, which allow Salmonella to establish a replicative vacuole called the Salmonella-containing vacuole (SCV) inside host cells. SPI-- dependent activities are responsible for SCV maturation along the endosomal pathway to prevent bacterial degradation in phagolysosomes, for interfering with trafficking of NADPH oxidase-containing vesicles to the SCV (), remodeling of host cell microfilaments (,) and microtubule networks (,), both of which play a role in maintaining the integrity of the SCV membrane and directing SCV traffic. As such, the regulation of SPI- is integral to Salmonella pathogenesis. SPI- TTSS substrates share a similar temporal pattern of expression within host cells and are activated in response to environmental cues presumably sensed by the bacteria in the SCV lumen. Within SPI- these substrates include effectors, and SseB (a component of the oligomeric filament structure of the type III apparatus), and SseC and SseD (the pore-forming translocation complex, or translocon) (). To date, only three SPI--encoded effectors, SseF, SseG (,) and probably SsaB (SpiC) (), are documented to be translocated into host cells in an SPI--dependent fashion although SPI- substrates are also encoded outside SPI- in other pathogenicity islands (1) and in lysogenic prophages (1) that are not genetically linked to SPI-. The coordinated expression of SPI- secretion substrates is controlled by the SPI--encoded two-component regulatory system SsrA/SsrB (1,1) and the upstream regulators OmpR/EnvZ which modulate the expression of the ssrab operon (1). Once activated, SsrB acts on multiple promoters in SPI- and in various regions of the genome (1,1) to induce synthesis of SPI- translocator and effector substrates. Several open reading frames (ORFs) encoded within SPI- remain uncharacterized including ssal, encoding a protein with homology to the locus of enterocyte effacement
4 (LEE)-encoded sepl of the attaching and effacing pathogens enterohemorrhagic E. coli (EHEC) (1), enteropathogenic E. coli (EPEC) and Citrobacter rodentium (1). We recently identified sepl in a genetic screen and showed it is required for type III secretion in C. rodentium of the translocon complex, EspA, B, and D, but not for the secretion of the LEE-encoded effector, Tir (0). Interestingly, ssal and sepl contain no other homologues in any other pathogenic bacteria with type III secretion systems indicating that they probably are not part of the TTSS core complex, which is generally well-conserved among type III-containing pathogens (1). Previous work on the environmental cues within the host cell that activate SPI- gene expression and type III secretion has implicated Mg + and PO - ion limitation and ph (1,1,,). Since the ph of the SCV falls below. within 0 minutes after its formation inside infected host cells (), it is possible that this environmental cue plays a prominent regulatory role in SPI- induction. In vitro conditions resembling the intracellular environment of the SCV such as low ph of minimal medium activate the secretion activity of SPI- (,), but less is known about how acidic ph regulates SPI- gene expression. In two previous studies, the ph of minimal medium did not have an effect on the expression of SPI- genes () or on a SPI- substrate encoded within a prophage outside of SPI- (1). However, it was also reported that acidic ph of minimal medium activated the ssra promoter in wild-type Salmonella (1), highlighting an apparent discrepancy given that SsrA and SsrB are required for SPI- gene expression. To examine the way in which Salmonella responds to acidification of the vacuole in which it resides, we analysed the expression and secretion of SPI- effectors and translocators under ph conditions that mimic those of the SCV. We demonstrate that expression and secretion of both translocators and effectors occurs only in acidic minimal medium and not in minimal medium at neutral ph. A Salmonella strain with a mutation in ssar (a conserved type III apparatus component) is deficient in not only the secretion of effectors and translocators but also in the expression of translocators at acidic ph. A Salmonella strain deficient in ssal, an uncharacterized SPI- ORF with homology to the secretion regulator sepl of attaching and effacing pathogens, displays a phenotype distinct to the ssar mutant in
5 that it restricts the expression and secretion of translocators encoded within SPI-, yet is dispensable for secretion of SPI- effectors encoded in other pathogenicity loci. We also demonstrate that after acidification, a functional type III secretion system and SsaL are required for optimal activation of the ssea promoter which controls the expression of the SPI- filament and pore-forming components, but show that expression of the downstream effectors is differentially regulated with respect to type III secretion and SsaL.
6 Experimental Procedures Bacterial strains and mutant construction S. Typhimurium strain SL1 was used as the wild-type strain throughout this study and all mutants used in these experiments are isogenic derivatives of SL1. Strains used in this work are listed in Table 1. S. Typhimurium and Escherichia coli strains were routinely cultured in LB broth supplemented with the appropriate antibiotics to maintain plasmids as required. A non-polar, unmarked, in-frame deletion of ssal was generated by allelic exchange from a counter-selectable suicide vector expressing SacB (). First, the ssal open reading frame and approximately 1 kb of flanking genomic DNA upstream and downstream of ssal, were amplified by PCR using the oligonucleotide primers BKC ( CCA GAG TAT CGG CAA TTG CTT ) and BKC ( AAC AGC CTC ACT CAT CGA CAT ) to generate a 0 bp DNA fragment. This fragment was cloned into pcr.1 (Invitrogen) to generate a plasmid template that was amplified by inverse PCR using BKC ( ACG CGT CGA CCA TCG CTA CCT CTT TTA TCT TCA C ) and BKC ( ACG CGT CGA CAA GTC GGT TTT ATT CTG ATA CCT GGC, SalI sites underlined). This PCR product was digested with SalI and then ligated to generate an internal, in-frame deletion allele of ssal that eliminated the coding region for amino acids -, which was confirmed by DNA sequencing. The ssal deletion allele was cloned into the unique XbaI and SacI sites of pre () and transformed into E. coli SM λpir () to generate a donor strain for conjugation. pre-δssal was conjugated into S. Typhimurium SL1 and merodiploid colonies were isolated, grown for h in LB broth without antibiotic selection, diluted and then plated onto agar containing 1% (w/v) tryptone, 0. % (w/v) yeast extract, % (w/v) sucrose and incubated at 0 C overnight. Sucrose-resistant colonies were selected and the presence of the ssal deletion was confirmed by PCR, restriction enzyme analysis, and DNA sequencing. Plasmid construction
7 To generate a wild-type allele of ssal under the control of its native promoter, SL1 chromosomal DNA was amplified by PCR using primers BKC ( CCG CTC GAG ACA TCT CGG GGA GAA CCA TGA A ; XhoI site underlined) and BKC0 ( AGG AAG CTT ATG ATG AGC CAG AAA GCC AA ; HindIII site underlined), which included the ssal stop codon. The resulting fragment was digested with XhoI and HindIII and cloned into the unique XhoI/HindIII sites of pwsk. To generate wild-type alleles of ssea-d under the control of a constitutive promoter, chromosomal DNA was amplified by PCR using primers ssea-f ( ATG GGA TCC TGT ATA TGG AGG GGA ATG ATG ; BamHI site underlined) and ssed-r ( ATG GTC GAC TTA CCT CGT TAA TGC CCG GAG ; SalI site underlined). The resulting -bp fragment was cloned into pwsk1 as a unique BamHI/SalI fragment under the control of the laczα promoter. An epitope-tagged version of SsaL was created by fusing a double hemagglutinin tag (HA) to the carboxyl terminus of SsaL. SsaL and its native promoter were amplified with the primers BKC1 ( ACG CGT CGA CAC ATC TCG GGG AGA ACC ATG AA ) and BKC ( CGG GAT CCG AAT AAA ACC TGA TTT ATC TTT ACT TCA CG ), which eliminated the ssal stop codon. The resulting PCR product was digested with SalI/BamHI and cloned into the SalI/BglII sites of pbkc-ha, to generate the ssal-ha fusion, which was then moved into pwsk to generate an ampicillin-resistant clone for expression in Salmonella. A transcriptional fusion of the ssea promoter to an artificial operon consisting of tnpr and lacz was constructed by PCR amplifying the promoter region of ssea using oligos psseaf ( ATA CTC GAG CGT ATT CTT GAT TTT CAT CGG TG ; XhoI site underlined) and pssear ( ATA CAA TTG CCC TTT CAG CAA GCT GTT GAC ; MfeI site underlined) and cloning the product into pivetn cut with XhoI and MfeI. The resulting plasmid was integrated into the Salmonella chromosome by homologous recombination. Similar transcriptional fusions to lacz were created but fused at ssed (oligonucleotides ATG CAA TTG CTG GTA ATA CCA GTG CTA CGT and ATG CTC GAG ACC GGC ATA TTT GAA ACC GTG ) and ssee (oligonucleotides ATG GAA TTC ACC ATT GCT CTA TTT CTT GCA C and ATG CTC GAG ACC GGC ATA TTT GAA ACC GTG ). All constructs were confirmed by DNA
8 sequencing and transformed into the appropriate Salmonella strains by either electroporation or conjugation. Plasmid pssrab was kindly provided by Dr. M. Hensel, Erlangen, Germany. Plasmids encoding the sepl gene and upstream regulatory region from enteropathogenic and enterohemorrhagic E. coli were kindly provided by Dr. W. Deng, University of British Columbia. A list of plasmids used in this work is outlined in Table Recombinant SPI- proteins and generation of antibodies Polyclonal antibodies to SseB, SseD, SseE and SseG were generated by repeated immunization of New Zealand white rabbits with 1 mg recombinant glutathione S-transferase (GST) fusions of each protein. Each GST fusion was constructed by PCR amplification of the effector gene from genomic DNA of S. Typhimurium SL1 using the following primers: SseB, forward -GCA GGA TCC ATG TCT TCA GGA AAC ATC TTA TGG-, reverse -CGT GTC GAC TCA TGA GTA CGT TTT CTG CGC TAT- ; SseD, forward -GCA GGA TCC ATG GAA GCG AGT AAC GTA GCA CTG-, reverse -CGT GTC GAC TTA CCT CGT TAA TGC CCG GAG TAT- ; SseE, forward -GCA GGA TCC ATG GTG CAA GAA ATA GAG CAA TGG-, reverse -CGT GTC GAC TTA AAA ACG TCG CTG GAT AAG ATG- ; SseG, forward - GCA GGA TCC ATG AAA CCT GTT AGC CCA AAT GCT-, reverse -CGT GTC GAC TTA CTC CGG CGC ACG TTG TTC TGG-. After digestion with BamHI and SalI (sites underlined above), the PCR product was cloned into pgexp-1 (Pharmacia Biotech). This plasmid was transformed into the BL1 strain of E. coli and overexpression of recombinant fusion proteins was accomplished with 1 mm IPTG for h (1h for expression of SseG). SseB was purified using glutathione-coupled beads (Sigma) according to standard protocols (). Purification of SseD, SseE and SseG was performed using the sarkosyl lysis procedure described by Frangioni and Neel (0). Raw antisera were affinity-purified using Sepharose beads (Pharmacia) with covalently coupled antigen. Nonspecific cross-reactivity of the antibodies was minimized by incubation with acetone powders of the BL1 strain that was used to overexpress the recombinant fusion proteins.
9 Cell culture HeLa cells were maintained in Dulbecco s Modified Eagle medium (DMEM; Hyclone, Logan, Utah) supplemented with % fetal calf serum (FCS). For immunofluorescence studies, HeLa cells were seeded onto 1 cm sterile glass coverslips in -well tissue culture dishes and incubated for approximately 1 h prior to infection. For gentamicin protection assays, RAW. murine macrophages were used between passages -1 and maintained in DMEM supplemented with % FCS. RAW. cells were seeded in -well culture dishes 1 h prior to infection. All cell lines were cultured at C in % CO Analysis of Salmonella mutants in mice Female BALB/c mice (- weeks old) were purchased from Harlan Laboratories (Indianapolis, IN). Mice were housed in sterilized, filter-top cages under specific pathogen-free conditions at the University of British Columbia Animal Facility. The protocols used here were in direct accordance with animal care guidelines as outlined by the University of British Columbia s Animal Care Committee and the Canadian Council on the Use of Laboratory Animals. Salmonella cultures were grown overnight in LB broth and 1 then diluted in phosphate-buffered saline to give approximately 1. cfu/ml. Groups of mice (n=) 1 1 were infected by intraperitoneal injection with 0. ml of diluted bacterial suspension, followed by daily monitoring throughout the study period. Mice that showed signs of extreme distress were euthanized Gentamicin protection assays RAW. cells were infected with opsonized stationary phase bacteria as described previously (1). At and 1 h after infection, gentamicin-treated cells were washed with PBS and then lysed in 0. ml of 1% Triton X-0, 0.1% SDS in PBS. Lysates were diluted in PBS and plated onto LB agar followed by incubation at C. Colonies were enumerated and expressed as colony forming units (cfu) /ml. The fold increase in the number of intracellular bacteria was determined by dividing the cfu values at 1 h by the cfu values at h post infection for each condition.
10 In vitro secretion assays In vitro culture conditions were developed based on minimal medium used to induce expression and secretion of SPI- genes (,). Salmonella strains were grown overnight in LB broth, washed twice in low phosphate, low magnesium-containing medium (LPM) and then inoculated at a 1:0 dilution in ml of LPM medium at either ph.0 or ph.. The composition of LPM medium was: mm KCl,. mm (NH ) SO, 0. mm K SO, mm glycerol (0.% v/v), 0.1% casamino acids, µm MgCl, µm PO -, 0 mm Tris-HCl (for titration to ph.0), or 0 mm -[N-morpholino]ethanesulfonic acid (for titration to ph.). Cultures were grown at C with shaking for - h after which the optical density at 00 nm was measured. Bacteria were collected by centrifugation for minutes at 1,000 rpm ( C). The supernatant was passed through a 0. µm filter and then precipitated with trichloroacetic acid (% final concentration, v/v) at C for -1 h Analysis of secreted proteins The TCA-insoluble fraction from above was collected by centrifugation, washed with ice-cold acetone and solubilized with a volume of SDS-sample buffer (0 mm Tris-HCl, ph., 0% glycerol, % SDS, 0.00% bromophenol blue and 00 mm dithiothreitol) adjusted according to the OD 00 of the original culture. When necessary, solubilized secreted proteins were neutralized with an appropriate volume of non-titrated Tris. The bacterial pellet fraction from above was also dissolved in a volume of SDS-sample buffer adjusted according to the OD 00 of the original culture. Proteins from equivalent numbers of bacterial cells, as determined by OD 00 readings, were separated on % or 1% SDSpolyacrylamide gels, transferred to nitrocellulose membranes and then blocked in Tris-buffered saline containing 0.1% (v/v) Tween 0 (TBST) and % (w/v) powdered non-fat milk for 1 h at room temperature. Blots were incubated with the following primary antibodies in TBST + % non-fat milk: rabbit affinity-purified antibodies raised against recombinant SseB, SseD, SseG and SseE (1:0), mouse anti-ha monoclonal antibody (1:000; Covance), mouse anti-dnak monoclonal antibody (1:00;
11 Stressgen). Secondary antibodies conjugated to horseradish peroxidase were used at a 1:000 dilution in TBST for 1 h at room temperature. Antibody complexes were detected using enhanced chemiluminescence (Amersham Biosciences) Chemiluminescent β-galactosidase assays ssea promoter activity was examined using transcriptional fusions to lacz and a chemiluminescence assay described by Kehres et al. (). Approximately 1 kb of chromosomal DNA upstream of the ssea start codon was transcriptionally fused to the lacz gene and was used to generate a single copy chromosomal fusion, P ssea ::lacz, in wild-type Salmonella and in various SPI- mutants by homologous recombination as described above. Similar lacz reporter strains were constructed but terminated at different regions of the ssea operon, including ssed and ssee to generate P ssed ::lacz and P ssee ::lacz. LacZ reporter strains were cultured overnight in LB medium, then washed twice in LPM medium and inoculated into fresh LPM medium at either ph.0 or ph. to give an optical density reading of approximately 0.0 at 00 nm. Cultures were incubated with shaking at C for - h and samples were removed every hour for enumeration of colony forming units and for β-galactosidase activity assays. For β-galactosidase assays, 0. ml of the culture was removed and the bacteria were pelleted by centrifugation. The supernatant was discarded and the bacterial pellet was stored at 0 C until the end of the experiment. Each pellet fraction was resuspended in 0. ml of PBS and then 0 µl of chloroform was added to lyse the bacteria. µl of the lysate was transferred to a well of a black microtitre plate containing 0 µl of Galacto-Star substrate mix (Applied Biosystems, Bedford, MA). Reactions were incubated for 0 min at room temperature and then plates were read by using the luminescence detection function of the Spectrafluor Plus (TECAN, Austria). Light emission was expressed as relative light units (RLU) per bacteria based on cfu determinations derived from matched bacterial cultures. Each condition was performed in quadruplicate and averaged.
12 Results Salmonella requires low ph and an intact SPI- type III secretion apparatus for secretion of filament and translocon components. To examine the molecular basis of SPI- gene expression we performed in vitro secretion assays to study the SPI- translocon. We first established and optimised in vitro conditions for SPI- secretion assays using minimal medium previously reported to induce the expression of SPI- genes () and affinity purified antibodies against SseB and SseD. Using this optimised procedure we were able to reproducibly isolate SPI--secreted protein from 1. ml of non-stationary culture without the need to extract the bacterial surface with hexadecane () or mechanical shearing (), which had previously been required to detect sufficient levels of SPI- translocon components. Wild-type Salmonella and a Salmonella inva::kan mutant that has a generalized defect in SPI-1-mediated type III secretion were then tested for the ability to secrete the filament component, SseB and the translocon component, SseD, when the bacteria were grown in low phosphate, low magnesium-containing medium (LPM) at ph.0 or ph.. In secreted protein fractions, SseB and SseD were detected from wild-type and SPI-1 mutant bacterial cultures grown only in LPM medium at ph. and not in LPM medium at ph.0 (Figure 1A). SseB and SseD secretion required SsaR, a conserved component of type III secretion systems since an ssar mutant did not secrete these molecules under these same conditions. To control for the presence of cytosolic proteins in secreted protein fractions due to bacterial lysis, we probed each fraction with an antibody against the cytoplasmic molecule, DnaK, which was negative in all cases. 0 1 Expression of filament and translocon components requires acidic ph and an intact SPI- type III secretion apparatus. Because the ssar mutant did not secrete SseB and SseD into the SPI- secreted fraction, we tested whole bacterial cell lysates for the expression of these molecules in LPM medium at ph.. Interestingly, the ΔssaR strain, was deficient in cellular pools of both SseB and SseD in LPM medium at acidic ph (Figure 1
13 1B). Based on these data, we hypothesized that a functional SPI- type III secretion system was necessary for full expression of SseB and SseD. Since minimal medium at neutral ph does not support SPI- type III secretion (), Salmonella cells grown in LPM medium at ph.0 should show reduced expression of translocators. To test this, we cultured Salmonella to mid log phase in LPM medium at ph.0 and then probed whole bacterial cell lysates for SseB and SseD. As shown in Figure B, Salmonella grown in LPM medium at ph.0 showed little to no SseB or SseD protein in whole cell lysates, indicating a phdependent control of translocon expression. The growth kinetics of Salmonella in LPM medium at ph. or.0 was similar over a -hour growth period (Figure 1D) and all protein gel lanes were loaded with protein from equivalent numbers of bacteria Salmonella cultures grown to stationary phase show altered SPI- gene expression Some reports examining SseB expression from wild-type stationary phase cultures demonstrated that SseB accumulation was ph-independent in minimal medium (,). Because we found very limited SseB and SseD expression from Salmonella grown in LPM at ph.0, we sought to confirm that wild-type Salmonella grown to stationary phase no longer exhibit ph-regulated SPI- gene expression. Wild-type Salmonella and ΔssaL and ΔssaR mutants were grown to stationary phase by overnight growth in LPM medium at either neutral or acidic ph, following previously published methods (,). Whole bacterial cell fractions were then probed for the presence of SseB. Consistent with previous reports, wild-type Salmonella grown under these conditions expressed SseB when grown in both neutral and acidic minimal medium (Figure 1C). The SPI- mutants tested in these experiments did not express SseB in neutral LPM medium but did express a small amount of SseB when grown in LPM medium at acidic ph. Based on these data, we hypothesize that Salmonella grown to stationary phase demonstrate decreased fidelity of SPI- regulation, possibly due to an independent regulatory mechanism not encoded within SPI-, or because of acidification by the bacteria of the medium during prolonged culture to stationary phase. 1
14 The SepL homologue, SsaL, is required for translocator secretion and expression. Our laboratory has shown that the LEE-encoded secretion regulator SepL is required for secretion of the translocon components EspA, EspB and EspD, but not for secretion of the effector molecule, Tir in attaching and effacing pathogens (0). sepl has a single homologue in sequence databases, called ssal, which is an uncharacterised ORF within SPI-. To test whether SsaL is required for secretion of SPI- translocators, we constructed an in-frame, unmarked deletion in the ssal gene of SPI- and performed expression and secretion assays for SseB and SseD. SsaL was required for secretion (see Figure ) and full expression (Fig 1B) of both SseB and SseD. Together, these results demonstrate that a functional SPI- TTSS, including SsaL, is required for the expression of the SPI- translocon Complementation studies in the ΔssaL strain To rule out a possible inability of the ΔssaL strain to respond to environmental stimuli, such as low magnesium, that might limit SPI- expression and subsequent SseB and SseD accumulation, we overexpressed in a ΔssaL mutant background the SPI- two-component regulator, SsrA/SsrB, from a lowcopy plasmid, which was previously shown to decrease the environmental constraints imposed by various media on SPI- gene expression in Salmonella (). Transformation of ΔssaL with pssrab did not restore cytoplasmic pools of SseB and SseD in the ΔssaL strain (Figure A), suggesting that environmental effects unique to the ΔssaL strain are unlikely. As a control for the function of this plasmid, we expressed pssrab in wild-type Salmonella and confirmed SseB accumulation in both low and high Mg + -containing medium as reported in the original publication () (data not shown). Further, the presence of pssrab and high magnesium did not compensate for the lack of SsaL in terms of SseB expression (data not shown). We then tested the ability of the ΔssaL strain to express and secrete SseB when complemented with a plasmid that expresses SsaL from its native promoter. In this series of experiments, complementation of the ssal mutant with a wild-type ssal allele restored both expression and secretion of SseB to a level comparable to the wild-type strain harbouring the same complementation plasmid (Figure B). As a 1
15 1 1 control in this series of experiments, we tested SseB expression and secretion in a Salmonella mutant with an inactive allele of ssrb, the transcriptional activator component of the SsrA/SsrB SPI- two-component system. As expected, the ssrb mutant strain neither expressed nor secreted SseB under conditions that induced SseB expression and secretion from wild-type cells and from the complemented ssal mutant (Figure B). To test the possibility that SepL from enterohemorrhagic E. coli might complement the ΔssaL strain, we expressed sepl from EHEC in both wild-type Salmonella and the ΔssaL strain and measured the expression and secretion of SseB. Expression of SepL in wild-type Salmonella lead to an increase in the amount of secreted SseB, which is consistent with its role as a secretion regulator in EHEC and EPEC (0) (Figure C). Interestingly, expression of SepL in the ΔssaL strain lead to an increase in the amount of SseB in whole bacteria lysates, but could not restore secretion of SseB (Figure C). These data suggest that while SepL can complement some SsaL activity involved in expression of SseB, SsaL performs an additional function in controlling subsequent secretion that cannot be compensated for by the presence of SepL Cytoplasmic SseB is not labile in the absence of SPI--dependent type III secretion. One possible explanation for the lack of SseB and SseD in Salmonella cells grown in LPM medium at ph.0, and in ΔssaL and ΔssaR mutants at ph. is that these type III secretion substrates could be degraded in the absence of secretion. To test this possibility, we constructed Salmonella strains that expressed SseB along with its chaperone, SseA (,), from a constitutive promoter and then tested these strains for SseB using expression and secretion assays. As shown in Figure A, Salmonella strains containing either an empty plasmid or the sseb expression construct did not secrete SseB in LPM at ph.0 as expected from the above experiments. However, when these same strains expressed SseB from the constitutive lac promoter, SseB was detected in the secreted protein fraction from wild-type Salmonella grown in LPM medium at ph. but not from the ssar or ssal mutants (Fig A), thereby confirming that both SsaR and SsaL are required for translocator secretion. We also tested whole bacterial lysates from 1
16 1 1 the same strains for the presence of SseB. As shown in Figure B, SseB accumulated in bacteria grown in LPM medium at ph.0 when SseB was expressed from the constitutive lac promoter, indicating that in the absence of SPI--dependent type III secretion, intracellular SseB is not degraded in the bacterial cytosol. In LPM medium at ph., both the ΔssaL and ΔssaR strains expressing constitutive SseB also accumulated non-secreted, intracellular pools of SseB with no visible breakdown products (Figure B). These data demonstrate that intracellular SseB is not inherently labile in the absence of SPI--dependent type III secretion and is stably maintained in the cytoplasm of type III secretion mutants and when Salmonella is subjected to environmental conditions that restrict SPI- secretion activity. It was also noted that in wild-type Salmonella which over-expressed SseA B, -C, and D, the levels of SseB secreted into the culture supernatant was lower than for non-transformed wild-type cells (Figure A). It is possible that this resulted from the increased competition for the SPI- apparatus by three over-expressed type III secretion substrates (SseBCD), or possibly due to titration by overexpressed SseC or SseD of an essential endogenous chaperone required for SseB secretion The SPI- regulator SsaL is required for Salmonella virulence in vitro and in vivo. To validate the significance of SsaL for Salmonella disease, we tested the ability of the ssal mutant strain to replicate in macrophages and for virulence in vivo. The ΔssaL strain did not replicate in the murine macrophage cell line RAW. (Figure A). The numbers of intracellular wild-type Salmonella increased approximately.-fold over 1 h whereas the ssal deletion strain showed a net decrease of intracellular bacteria over the same time period. The numbers of intracellular bacteria at h was similar for both wild-type and the ssal mutant indicating that invasion was not affected (data not shown). The level of attenuation was similar to that of a Salmonella strain deleted for an essential SPI- chaperone (ssea) or a conserved type III apparatus component (ssar). The ΔssaL strain was also attenuated for intracellular replication in HeLa cells, which was evident at time points longer than h post-infection (data not shown). The intracellular replication defect in the ΔssaL strain was due to the loss of ssal since 1
17 complementation of the allele on a plasmid under the control of its native promoter restored intracellular growth (Figure A). Since the ability to cause a lethal systemic infection in mice is predicated on the ability of Salmonella to replicate within macrophages, we tested whether the ΔssaL strain was attenuated for virulence in this model. As shown in Figure B, wild-type Salmonella caused a lethal infection in mice whereas the ΔssaL strain did not. The ΔssaR strain was also attenuated in the mouse model of systemic infection, as expected. Complementation of the ssal mutant with a wild-type ssal allele restored virulence in vivo (Figure B) SsaL requires an acidic minimal medium and the SPI- regulator, SsrB for expression. To further characterize the expression requirements for SsaL, we constructed a plasmid that expressed SsaL with a C-terminal HA epitope and expressed this construct in wild-type Salmonella and in ΔssaR and ΔssaL mutants. Expression of SsaL did not occur in minimal medium at neutral ph, consistent with the expression patterns observed for other SPI--encoded molecules (Figure A). However, upon acidification of the medium, expression of SsaL was induced in wild-type Salmonella and in ΔssaR and ΔssaL mutants (Figure A), indicating that SPI- type III secretion activity is not required for SsaL production. SsaL-HA was also not a substrate for SPI--dependent type III secretion (Figure A). To test whether expression of the ssal gene is regulated by the SsrA/SsrB system in response to low ph, we expressed SsaL-HA in a Salmonella mutant with an inactive allele of ssrb. Expression of SsaL was completely abolished in the absence of SsrB (Figure B), indicating that the role for SsaL in expression/secretion of the filament and pore-forming components of SPI- is downstream from the activation of SsrA/SsrB. SsaL is required for secretion and translocation of SPI--encoded effectors but not for effectors encoded outside of SPI-. 1
18 Because the ssal homologue, sepl, found in LEE-containing enteropathogens is dispensable for type III secretion of effectors but not translocon components (0), we tested whether a ΔssaL strain showed a similar phenotype. We performed secretion assays as described in the Materials and Methods and probed bacterial cell fractions and secreted protein fractions for the SPI--encoded molecules, SseG and SseE. SseG is a bona fide SPI--dependent type III secreted effector (,), whereas SseE has not been previously characterized as an effector molecule. As demonstrated in Figure A, none of the Salmonella strains expressed SseG in LPM medium at ph.0, which is consistent with the regulatory pattern observed for SseB and D. SseE was observed in very limited amounts in LPM medium at ph.0. However, growth of Salmonella in LPM medium at ph. induced the expression of both SseG and SseE to similar levels in wild-type cells and in the SPI- mutants (ΔssaR and ΔssaL), and a SPI-1 mutant (inva::kan) (Fig A). Wild-type bacteria and SPI-1 mutant bacteria secreted SseG into the culture supernatant whereas the ΔssaR and ΔssaL strains did not secrete SseG under acidic conditions shown previously to induce SPI- secretion (Fig B). SseE was expressed under SPI--inducing conditions in all the strain backgrounds tested, but was not recovered in detectable amounts from the concentrated secreted fraction of either wild-type bacteria or SPI-1 mutant bacteria (Fig B). To further examine other SPI- effectors that are encoded in other pathogenicity loci outside of SPI-, we tested the secretion of PipB, a SPI- effector that localizes to the membrane portion of Salmonella-induced filaments (Sif) and vacuoles containing bacteria (1), and SopD, a SPI- effector that localizes to late endosomes following translocation into host cells (). Neither PipB (Figure C) or SopD (data not shown) was secreted by the ΔssaR strain, yet these proteins were detected in secreted protein fractions from the ΔssaL strain, indicating a phenotypic difference between a general SPI- secretion mutant (ΔssaR) and a mutant lacking SsaL. To verify that expression of PipB in these strains did not affect expression of SseB or SseD, we tested whole cell lysates from strains expressing PipB. As expected, expression of PipB did not restore the expression of SseB or SseD in any of the strains tested (Figure D). To examine the effect of ssal deletion on the translocation of PipB into host cells, we used immunofluorescence to examine 1
19 whether this strain could translocate epitope-tagged PipB into epithelial cells. As expected, PipB was not translocated into host cells by the ΔssaL strain (data not shown), thus confirming its defect for assembling a translocation filament and translocon pore The ssea promoter demonstrates ph-dependent activation in LPM medium. The above experiments examining SseB expressed from a constitutive lac promoter (Figure ) demonstrated that SseB was not degraded in the absence of SPI--dependent type III secretion. To test whether this pattern of expression was reflected at the transcriptional level, we expressed from the bacterial chromosome a single copy transcriptional fusion of the ssea promoter fused to lacz (P ssea ::lacz) and measured β-galactosidase activity in various strains grown in LPM medium that was titrated to neutral or acidic ph. ssea lies immediately upstream of sseb and is part of the same transcriptional unit. As shown in Figure A, β-galactosidase activity was significantly lower in wild-type Salmonella grown in LPM medium at ph.0, compared to the same reporter strain grown in LPM medium at ph. at all time points tested. Together with the SseB stability data from Figure, these results suggest that the lack of SseB and SseD in Salmonella grown in LPM medium at neutral ph is due to decreased activity of the ssea promoter Mutations in ssar and ssal have a negative effect on the transcriptional activation of the ssea promoter. To test further whether the decreased amounts of SseB and SseD in the ΔssaL and ΔssaR mutant backgrounds was reflected at the transcriptional level, we constructed the chromosomal integration of the P ssea ::lacz reporter in ΔssaL and ΔssaR mutant backgrounds and performed time course experiments using the P ssea ::lacz reporter strains grown in LPM medium at ph. and ph.0. Viable counts were also performed on the same bacterial cultures such that β-galactosidase activity was normalized to bacterial counts at each time point. Consistent with our protein expression data, ssea induction was reduced 1
20 significantly in ssal and ssar mutant backgrounds at low ph compared with wild-type cells (Figure B). Also consistent with the protein data, β-galactosidase activity from cultures grown at neutral ph were significantly lower than those at ph. and did not differ between wild-type Salmonella and the ΔssaR and ΔssaL mutants (Figure C). Taken together, these data verify the requirement for (i) acidification and (ii) SPI- secretion activity including SsaL for activation of the ssea promoter Transcriptional activity of genes downstream of the pore-forming translocon genes can be uncoupled from the requirement of type III secretion and SsaL. The expression experiments shown in Figure demonstrated that while the expression of effectors downstream of the filament and pore-forming genes (SseB and SseD) was also ph-dependent, the ΔssaR and ΔssaL strains expressed similar amounts of SseE and SseG compared to wild-type Salmonella, which contrasts with the expression pattern for the upstream genes. To examine this, we constructed singlecopy chromosomal transcriptional fusions of lacz to the ssed gene and the immediately downstream gene, ssee, and measured β-galactosidase activity from wild-type, ΔssaR and ΔssaL reporter strains. SseD is the second component of the pore-forming translocon (). As shown in Figure, β-galactosidase activity from Salmonella strains with the P ssed ::lacz reporter was comparable to the activity for the P ssea ::lacz fusion in terms of magnitude and demonstrated lower activity in the ΔssaR and ΔssaL mutants compared to wild-type. β-galactosidase activity from the P ssee ::lacz reporter were not significantly different between wild-type Salmonella or the ΔssaR and ΔssaL mutants, thus confirming the expression patterns observed previously for SseE. β-galactosidase activity from the P ssee ::lacz reporter strains was approximately -fold higher than either the P ssed ::lacz or P ssea ::lacz reporters in all strains at all time points tested (Figure ). Together with the protein expression data, these data suggest that expression of the genes downstream of the filament (sseb) and pore-forming component of the SPI- translocon (ssed) can be uncoupled from the expression of the upstream genes with respect to the requirement of type III secretion and the presence of SsaL. 0
21 Discussion We have identified a regulatory mechanism for the SPI- translocon components SseB and SseD that is responsive to acidic ph and requires a functional SPI- TTSS. A Salmonella strain with a mutation in the SPI--encoded molecule, SsaL, and a second Salmonella strain with a mutation in a conserved SPI- type III secretion apparatus component displayed a similar phenotype for translocator expression and secretion, but differed in their competency for secretion of SPI- effectors encoded outside of SPI-. This pattern of expression was reflected at the transcriptional level and was reproducible using both environmental and genetic approaches to block SPI--dependent type III secretion. It is known that the two-component regulatory system comprised of SsrA/SsrB positively regulates SPI--encoded genes. SsrA is the putative sensor kinase component and SsrB is the transcriptional activator that acts on promoters in SPI- and in other regions of the genome. Using transcriptional fusions to gfp, Lee and colleagues demonstrated that the promoter controlling ssrab was activated in acidic minimal medium but not in minimal medium at neutral ph (1). We therefore reasoned that genes controlled by SsrA/SsrB (such as the ssea operon) should also demonstrate acidinduced activation. We found that components of the SPI- filament (SseB), translocon (SseD), and effectors (SseG, SseE) required acidified minimal medium for expression and accumulation within wildtype bacteria. It was of interest to find that SseE was localized to bacterial cells but was not found in the secreted fraction from any of the Salmonella strains tested. Although there are currently no published data on SseE, based on its localization within Salmonella cells we hypothesize that it could play a chaperone role or other accessory role for SPI--dependent type III secretion. The lack of an ssee homologue in any pathogenic bacterium suggests it might play a role unique to the function of SPI-. As mentioned, a previous report demonstrated that activation of the ssra promoter required minimal medium at acidic ph (1). However, two other studies investigating SPI- gene expression (,) reported that the expression of SPI- genes was ph-independent. These data are difficult to reconcile with each other given that SsrA/SsrB is a requisite activator of SPI- gene expression. Our results are in agreement with the former study (1) and show that activation of the ssea promoter and 1
22 expression of both translocon components (SseB, SseD) and effectors (SseG, SseE) requires acidic ph. This ph-inducible activation was SsrB-dependent, since no SPI- protein accumulation or secretion was seen in an ssrb mutant. Given that the SCV acidifies to below ph. within 0 minutes after uptake into bone marrow-derived macrophages (), its likely that an acidic ph is a major environmental cue determining not only activation of SPI- secretion, but also inducing the expression of SPI--encoded genes. We suspect that the bacterial growth stage represents an important contribution to the regulation of SPI-, since we found that growth of Salmonella to stationary phase in neutral minimal medium conditions which were used in previous studies that did not find ph-dependent SPI- gene expression caused accumulation of SseB in wild-type Salmonella but not in ssal or ssar mutants. Whether this represents a stress response by the bacteria will require additional experimentation. It should be noted that in our expression and secretion experiments, all bacterial cultures were tested during exponentialphase growth, after to h of incubation in minimal medium. The sensitivity of our expression and secretion assays allowed us to perform these experiments before the bacterial cultures reached saturation in stationary phase and allowed us to detect SPI- secreted proteins from culture volumes of less that two ml without the need to extract the bacterial surface by chemical or mechanical means conditions which were previously reported as being necessary to detect SPI- secreted proteins in vitro (,). The data presented here are consistent with the hypothesis that the ph-dependent secretion of a putative transcriptional repressor may play a role in controlling SPI- gene expression and that SsaL might be involved in this process. In such a model, repression of the translocon promoter upstream of ssea would occur in the absence of SPI- type III secretion, which is inhibited during bacterial growth in neutral minimal medium or disabled in SPI- apparatus mutants. Following activation of SPI-- dependent type III secretion, as when bacteria encounter an acidified intracellular environment, the active secretion of a putative repressor would lower its cytoplasmic concentration and de-repress the translocon promoter, facilitating SsrB-mediated expression of SseB, C, and D. Given that type III secretion systems have shown a hierarchal secretion of regulators, translocators and effectors (), it is possible that a SPI- TTSS intermediate exists in which the apparatus is permissive for secretion of regulatory
23 controllers prior to engaging translocators and effector substrates. This transitional structure would represent a secretion-competent but not translocation-competent intermediate in which substrates could be secreted into the SCV lumen prior to formation of the filament and translocon pore. Given that ssal mutants constrain the expression of the SPI- translocon it is possible that SsaL is involved in such a transitional secretion complex. This hypothesis is supported by data from Cirillo and colleagues (), who showed that a Salmonella sseb mutant (that can still secrete, but not translocate, SPI- substrates) could still activate promoters controlling the SPI- molecules, SpiA (SsaC), SscB and SsrA. This group also reported that SPI- gene expression did not require an intact secretion apparatus. However, this conclusion may be limited insofar as the expression studies were performed with a limited number of SPI- mutants containing plasmid-based transcriptional fusions to GFP, whereas it has been observed that SPI- gene expression can be improperly regulated when SPI- genes are expressed from medium copy plasmids from their native promoters [B. Coombes, B. Finlay, unpublished data and ()]. It is possible that an increased copy number of SPI- promoters in these instances could titrate out a putative repressor molecule leading to the observed gene expression patterns from plasmid-based studies. While it is possible that SsaL is part of the core type III apparatus, genetic and phenotypic evidence might suggest a more complex role. First, SsaL has only one homologue (SepL) in type III secretion systems from other pathogens, whereas it has been recognized that the core structure of the type III apparatus is generally well conserved (1,). Second, SepL from attaching and effacing gastrointestinal pathogens has been shown to regulate the transition from translocon to effector secretion (0) and is dispensable for secretion of type III effectors. Third, SepL from EHEC can moderately complement an ssal mutation in Salmonella for expression of SseB, but not for secretion, suggesting a bifunctional role for SsaL in SPI-, and lastly, an ssal mutant retains the ability to secrete non-spi--encoded effectors, while a generalized secretion mutant (ΔssaR) does not. Interestingly, Day and Lee (0) recently hypothesized that a SPI-1-encoded protein called OrgC functions as a SPI-1-secreted repressor of SPI-1 virulence genes. This hypothesis was based on
24 phenotypic studies using an orgc deletion mutant and by comparing the gene synteny of orgc to that of virulence genes in other pathogens. In particular, the position of orgc in the SPI-1 operon, prghijkorgabc corresponds to the position of a transcriptional repressor, lcrq in the Yersinia virulence plasmid (0). While the hypothesis that OrgC is a transcriptional repressor of SPI-1 was not formally tested by Day and Lee, it remains plausible that a corresponding mechanism exists to repress SPI- gene expression until the appropriate intracellular environmental cue(s) is sensed. It was of interest that SPI--encoded effectors and translocators were repressed in a similar fashion in minimal medium at neutral ph, but there was no difference in SseE or SseG expression between wild-type, ssar and ssal mutants in acidic minimal medium. It is possible that SPI--encoded effectors downstream of ssed are subject to different regulatory control compared to the translocators, despite being present in the same putative operon. Transcription fusions of lacz to various regions of this operon, including ssed and the immediately downstream gene, ssee confirmed that the expression of upstream translocators and downstream effectors has different requirements with respect to type III secretion and the presence of SsaL. These data also suggest the presence of a previously unrecognised regulatory element that might act specifically on genes downstream of the translocon, since the activity of the P ssee ::lacz reporter was significantly greater than two upstream reporters. Indeed, there is already evidence that SPI- genes in the same transcriptional unit contain distinct promoters that are differentially activated. For example, Feng and colleagues (1) recently reported that unlike many two-component regulatory systems, regulation of the sensor kinase SsrA is partially uncoupled from regulation of the response regulator SsrB in SPI-, owing to their distinct promoters. Emerging evidence implies that combinatorial pair-wise interactions of different response regulators can interact at the same promoter, suggesting an increase in the complexity of prokaryotic transcriptional regulation akin to that seen in eukaryotic systems (-). Since SPI- gene regulation is integral to Salmonella pathogenesis, it is reasonable to speculate that virulence gene regulation in Salmonella is under complex regulatory control and insight into this process will lead to gains in understanding how intracellular Salmonella interact with host cells to cause disease.
25 Acknowledgements We thank M. Wickham, J. Puente, P. Hardwidge and G. Grassl for helpful discussions and insightful comments. We also acknowledge W. Deng for sharing unpublished data on Citrobacter rodentium sepl, G. Gotto for assistance with antibody production and M. Hensel for providing pssrab. This work was supported by grants to B.B.F. from the Canadian Institutes of Health Research and the Howard Hughes Medical Institute. B.K.C. is the recipient of a CIHR postdoctoral fellowship and a Michael Smith Foundation for Health Research fellowship. J.H.B. is the recipient of a CIHR New Investigator Award. B.B.F. is a CIHR Distinguished Investigator, an HHMI International Research Scholar and the University of British Columbia Peter Wall Distinguished Professor.
26 References Ochman, H., Soncini, F. C., Solomon, F., and Groisman, E. A. (1) Proc. Natl. Acad. Sci. U.S.A., Shea, J. E., Hensel, M., Gleeson, C., and Holden, D. W. (1) Proc. Natl. Acad. Sci. U. S. A., -. Vazquez-Torres, A., Xu, Y., Jones-Carson, J., Holden, D. W., Lucia, S. M., Dinauer, M. C., Mastroeni, P., and Fang, F. C. (000) Science, 1-1. Miao, E. A., Brittnacher, M., Haraga, A., Jeng, R. L., Welch, M. D., and Miller, S. I. (00) Mol. Microbiol., Meresse, S., Unsworth, K. E., Habermann, A., Griffiths, G., Fang, F., Martinez-Lorenzo, M. J., Waterman, S. R., Gorvel, J.-P., and Holden, D. W. (001) Cell. Microbiol., -. Brumell, J. H., Goosney, D. L., and Finlay, B. B. (00) Traffic, 0-1. Guignot, J., Caron, E., Beuzon, C., Bucci, C., Kagan, J., Roy, C., and Holden, D. W. (00) J. Cell. Sci., -. Nikolaus, T., Deiwick, J., Rappl, C., Freeman, J. A., Schroder, W., Miller, S. I., and Hensel, M. (001) J. Bacteriol. 1, 0-0. Hansen-Wester, I., Stecher, B., and Hensel, M. (00) Infect. Immun. 0, -. Kuhle, V., and Hensel, M. (00) Cell. Microbiol., 1-. Uchiya, K.-i., Barbieri, M. A., Funato, K., Shar, A. H., Stahl, P. D., and Groisman, E. A. (1) EMBO J. 1, - 1. Knodler, L. A., Celli, J., Hardt, W. D., Vallance, B. A., Yip, C., and Finlay, B. B. (00) Mol. Microbiol., Ho, T. D., Figueroa-Bossi, N., Wang, M., Uzzau, S., Bossi, L., and Slauch, J. M. (00) J. Bacteriol. 1, - 1. Worley, M. J., Ching, K. H., and Heffron, F. (000) Mol. Microbiol., Lee, A. K., Detweiler, C. S., and Falkow, S. (000) J. Bacteriol. 1, Garmendia, J., Beuzon, C. R., Ruiz-Albert, J., and Holden, D. W. (00) Microbiology 1, - 1. Miao, E. A., Freeman, J. A., and Miller, S. I. (00) J. Bacteriol. 1, Kresse, A. U., Beltrametti, F., Muller, A., Ebel, F., and Guzman, C. A. (000) J. Bacteriol. 1, 0-1. Deng, W., Li, Y., Vallance, B. A., and Finlay, B. B. (001) Infect. Immun., - 0. Deng, W., Puente, J. L., Gruenheid, S., Li, Y., Vallance, B. A., Vazquez, A., Barba, J., Ibarra, J. A., O'Donnell, P., Metalnikov, P., Ashman, K., Lee, S., Goode, D., Pawson, T., and Finlay, B. B. (00) Proc. Natl. Acad. Sci. U. S. A. 1, Hueck, C. J. (1) Microbiol. Mol. Biol. Rev., -. Beuzon, C. R., Banks, G., Deiwick, J., Hensel, M., and Holden, D. W. (1) Mol. Microbiol., 0-1. Deiwick, J., Nikolaus, T., Erdogan, S., and Hensel, M. (1) Mol. Microbiol. 1, 1-1. Rathman, M., Sjaastad, M. D., and Falkow, S. (1) Infect. Immun., -. Rappl, C., Deiwick, J., and Hensel, M. (00) FEMS Microbiol. Lett., -. Pelicic, V., Reyrat, J. M., and Gicquel, B. (1) Mol. Microbiol. 0, 1-
27 Edwards, R. A., Keller, L. H., and Schifferli, D. M. (1) Gene 0, 1-1. Miller, V. L., and Mekalanos, J. J. (1) J. Bacteriol., -. Ausubel, F. W., Brent, R., Kingston, R. E., Moore, D. D., and Smith, J. A. (1) Current protocols in molecular biology, Wiley Interscience, New York 0. Frangioni, J. V., and Neel, B. G. (1) Anal. Biochem., Coombes, B. K., Brown, N. F., Kujat-Choy, S., Vallance, B. A., and Finlay, B. B. (00) Microbes Infect., 1-0. Kehres, D. G., Zaharik, M. L., Finlay, B. B., and Maguire, M. E. (000) Mol. Microbiol., -00. Ruiz-Albert, J., Mundy, R., Yu, X. J., Beuzon, C. R., and Holden, D. W. (00) Microbiology 1, 0-. Zurawski, D. V., and Stein, M. A. (00) Mol. Microbiol., -. Salcedo, S. P., and Holden, D. W. (00) EMBO J., Brumell, J. H., Kujat-Choy, S., Brown, N. F., Vallance, B. A., Knodler, L. A., and Finlay, B. B. (00) Traffic, -. Wulff-Strobel, C. R., Williams, A. W., and Straley, S. C. (00) Mol. Microbiol., -. Cirillo, D. M., Valdivia, R. H., Monack, D. M., and Falkow, S. (1) Mol. Microbiol. 0, 1-1. Blocker, A., Komoriya, K., and Aizawa, S. (00) Proc Natl Acad Sci U S A 0, Day, J. B., and Lee, C. A. (00) Infect. Immun. 1, 0-1. Feng, X., Oropeza, R., and Kenney, L. J. (00) Mol. Microbiol., 1-. Senear, D. F., Perini, L. T., and Gavigan, S. A. (1) Methods Enzymol., 0-. Gavigan, S. A., Nguyen, T., Nguyen, N., and Senear, D. F. (1) J. Biol. Chem., -1. Jennings, M. P., and Beacham, I. R. (1) Mol. Microbiol., 1-1. Brumell, J. H., Rosenberger, C. M., Gotto, G. T., Marcus, S. L., and Finlay, B. B. (001) Cell. Microbiol., -. Galan, J. E., and Curtiss, R., rd. () Infect. Immun., Stein, M. A., Leung, K. Y., Zwick, M., Garcia-del Portillo, F., and Finlay, B. B. (1) Mol. Microbiol. 0, -1. Wang, R. F., and Kushner, S. R. () Gene 0, 1-1
28 1 1 1 Figure Legends Figure 1. Expression and SPI- secretion of translocators is acid-inducible and requires SsaL and a functional SPI- type III secretion system. (A). Analysis of SPI- secreted proteins. Bacterial cultures were grown in low-phosphate, low-magnesium (LPM) medium at neutral or acidic ph and SseB (top panel) and SseD (middle panel) were detected in the secreted protein fraction by Western blot analysis. Secreted protein fractions were also probed for an intracellular marker, DnaK (bottom panel). (B) Detection of SseB and SseD in Salmonella lysates. Whole bacterial cell lysates from the cultures from (A) were subjected to Western blot analysis for SseB and SseD. Lysates were also probed for DnaK (bottom panel). (C). Detection of SseB from stationary phase Salmonella cultures. Bacterial cultures were grown for 1 h to stationary phase in the indicated media and subjected to Western blot analysis for SseB in whole cell lysates. Expression and secretion assays were repeated five times with similar results. All proteins fractions were normalized to OD 00 readings for each culture, and (D) differences between the growth of cultures in acidic or neutral minimal medium were not detected Figure. Complementation studies of ΔssaL. (A) Effect of overexpression of SsrA/B. ΔssaL Salmonella containing the plasmid, pssrab were grown in LPM medium (ph.) and protein from equal numbers of cfu were subjected to Western analysis for SseB (top panel), SseD (middle panel) and DnaK (bottom panel). (B) Complementation of SseB expression by re-introduction of a wild-type ssal allele. Wildtype Salmonella and a ΔssaL strain each containing pssal were grown in LPM medium (ph.) and protein from equal numbers of bacteria were subjected to Western analysis for SseB in the whole bacterial fraction (pellet) and the secreted protein fraction. As a control, protein samples were probed for the bacterial cytoplasmic molecule, DnaK. (C) The SsaL homologue, SepL from EHEC, moderately restores expression of SseB in whole bacterial lysates (pellet) but does not restore secretion of SseB. The sepl gene from EHEC was expressed in wild-type Salmonella and the ΔssaL mutant by growing cultures in
29 LPM medium at ph.0 or.. SseB in whole bacterial lysates and in secreted proteins fractions was detected by Western blot. Figure. SseB is stable in the cytoplasm in the absence of SPI- type III secretion. Wild-type Salmonella and the ΔssaL and ΔssaR strains expressing SseA, -B, -C, and D from the constitutive lac promoter (pssea-d c ) were grown in LPM medium at neutral and acidic ph. Each strain containing an empty plasmid (pwsk1) was also cultured under identical conditions. SseB was detected in the secreted protein fraction (A) and the whole bacterial lysate (B) from each of the strains by Western blotting. Lanes contain proteins from equal numbers of bacteria as determined by OD 00 readings Figure. ssal is required for Salmonella virulence in vitro and in vivo. (A). Gentamicin protection assays. RAW. murine macrophages were infected with wild-type Salmonella and the SPI- mutants shown and the numbers of intracellular bacteria were enumerated at h and 0 h after infection. Data are presented as the mean fold increase in intracellular bacteria with standard deviation from three separate experiments. (B). Survival plots for Balb/c mice (n=) infected with wild-type S. Typhimurium (black squares), ΔssaR S. Typhimurium (black diamonds), ΔssaL S. Typhimurium (black triangles), and the complemented ΔssaL strain (black circles). Percentage of the mice surviving on each day after infection is shown. Replication and virulence experiments were repeated three times Figure. Expression of ssal requires acidic ph and the SPI- regulator, SsrB. (A) Salmonella strains that expressed SsaL-HA or that were transformed with empty plasmid were grown in LPM medium at ph.0 and. and cell fractions were probed for the presence of SsaL-HA. SsaL-HA was not detected in LPM (ph.0) medium in any of the strains tested, but was induced upon acidification of the minimal medium (upper panel). SsaL-HA was not detected in the secreted fraction from any of the strains tested (lower panel). (B) Expression of ssal is SsrB-dependent. A Salmonella mutant with an inactive allele of
30 ssrb was tested for expression of epitope-tagged ssal. Whereas wild-type Salmonella expressed SsaL- HA under acidic conditions, SsaL-HA expression was abolished in the absence of SsrB. Figure. SPI--encoded effectors are expressed in acidic minimal medium and require SsaL for secretion but secretion of non-spi--encoded effectors is retained. Wild-type Salmonella and ssal, ssar, and inva mutants were grown in LPM medium at neutral and acidic ph and tested for presence of SseG and SseE in whole bacterial lysates (A), and in secreted protein fractions (B) As a control, each fraction was also probed for DnaK. SPI- effectors encoded outside of the SPI- pathogenicity island including PipB (C) were also examined for their expression and secretion from various Salmonella strains. (D) Expression of PipB does not restore the expression of SseB or SseD in whole bacterial lysates Figure. Activation of the ssea promoter requires acidic minimal medium and a functional SPI- type III secretion system. (A). Wild-type Salmonella with a single chromosomal integration of a P ssea ::lacz reporter fusion was grown in LPM medium at ph.0 and ph. for the times indicated in the Figure. β- galactosidase activity and bacterial counts were measured at each time point and data are expressed as the relative light units of β-galactosidase activity per 1 cfu. Data are the means with standard errors from triplicate determinations. (B). SL1 P ssea ::lacz (closed squares), ΔssaR P ssea ::lacz (open circles) and ΔssaL P ssea ::lacz (closed diamonds) strains were grown in LPM medium at ph. and β- galactosidase activity was measured at the indicated time points. Data are the means with standard errors from quadruplicate determinations. *, p<0.01 compared to wild-type strain. (C). Salmonella reporter strains were cultured in LPM medium at neutral ph and β-galactosidase activity was measured as described above. Data are the means with standard errors from quadruplicate determinations and the experiments were repeated at least three times. 0
31 Figure. Differential regulation and activity of ssed and ssee::lacz reporters. Single-copy chromosomal integrations of the reporters, P ssed ::lacz (solid lines) and P ssee ::lacz (dashed lines) were constructed in wild-type Salmonella (SL1) and in the ΔssaR and ΔssaL mutants. β-galactosidase activity was measured at the indicated time points from whole bacterial lysates and expressed as relative light units of β-galactosidase activity per 1 cfu. Data are the means with standard errors from quadruplicate determinations. p<0.0 for wt P ssed ::lacz compared to ΔssaR P ssed ::lacz or ΔssaL P ssed ::lacz; p<0.01 for wt P ssed ::lacz compared to wt P ssee ::lacz. 1
32 TABLE 1. Bacterial strains and plasmids used in this study Strain or plasmid Genotype or Description Source or Reference Strains S. enterica sv. Typhimurium SL1 wild-type, Sm R ATCC SL1 ΔssaR SL1 ssar -, unmarked deletion, Sm R () SL1 ΔssaL SL1 ssal -, unmarked in-frame deletion, Sm r this work SL1 ΔsseA SL1 ssea -, unmarked in-frame deletion, Sm R (1) SB SL1 inva::kan, Sm R () SL1 ΔpipB SL1 pipb -, unmarked in-frame deletion, Sm R (1) SL1 ΔsifA SL1 sifa -, unmarked in-frame deletion, Sm R () SL1 ssrb::kan SL1 ssrb::kan, Sm R, transduced by P from s (1) NB SL1 PsseA::lacZ, Sm R, Amp R this work NB SL1 ssar -, PsseA::lacZ, Sm R, Amp R this work NB SL1 ssal -, PsseA::lacZ, Sm R, Amp R this work NB1 SL1 PsseD::lacZ, Sm R, Amp R this work NB1 SL1 ssar -, PsseD::lacZ, Sm R, Amp R this work NB1 SL1 ssal -, PsseD::lacZ, Sm R, Amp R this work NB1 SL1 PsseE::lacZ, Sm R, Amp R this work NB0 SL1 ssar -, PsseE::lacZ, Sm R, Amp R this work NB SL1 ssal -, PsseE::lacZ, Sm R, Amp R this work Escherichia coli DHα general cloning strain our collection DHα λpir for propagation of π-dependent plasmid our collection SM λpir thi thr leu tona lacy supe reca::rp--tc::mu Km λpir () Plasmids PCR.1 Amp R, Kan R, general cloning plasmid Invitrogen pwsk psc1 ori f1 ori laczα Amp R, low copy vector () pwsk1 psc1 orif1 ori laczα, Km R, low copy vector () pbkc-ha pwsk1-based vector, contains HA (BglII/HindIII) our collection pssrab ssrab cloned into pwsk () pssal produces SsaL from native promoter in pwsk, Amp R this work pssal-ha produces SsaL with C-terminal tandem HA fusion, Amp R this work pre orit oriv sacb1, Cm R, counterselectable suicide vector () pivet-ssea ssea promoter fused to lacz, cloned into pivetn, Amp R this work preδssal pre carrying deletion allele of ssal corresponding this work to amino acids through, Cm R pssea-d C -bp fragment of ssea through ssed cloned into this work pwsk1 under lac promoter ppipb-ha produces PipB with C-terminal tandem HA fusion (1) from pipb promoter, Cm R psopd-ha produces SopD with C-terminal tandem HA fusion () from sopd promoter, Cm R Sm, streptomycin; Amp, ampicillin; Cm, chloramphenicol; Km, kanamycin
33 A Secreted wt ssar ssal inva::kan wt ssar ssal inva::kan SseB B Whole cell lysate wt wt ssar ssal inva::kan ssar ssal inva::kan SseB SseD SseD DnaK DnaK LPM (ph.0) LPM (ph.) LPM (ph.0) LPM (ph.) D C Whole cell lysate (stationary phase) wt ssar ssal wt ssar ssal LPM (ph.0) LPM (ph.) SseB Optical density O.D LPM (ph.) 0 1 Time (h) LPM (ph.0) wt wt (pssal) ssar ssar (pssal) ssal ssal (pssal) Time (h) Coombes et al. Figure 1
34 A Whole cell lysate wt ssar ssal inva::kan ssal (pssrab) SseB B pellet wt wt (pssal) ssal ssal (pssal) ssrb::kan ssar SseB SseD secreted SseB DnaK pellet DnaK secreted DnaK C pellet wt wt (pehec-sepl) ssal ssal (pehec-sepl) wt wt (pehec-sepl) ssal ssal (pehec-sepl) SseB secreted SseB ph.0 ph. pellet DnaK secreted DnaK Coombes et al. Figure
35 A kda - Secreted wt wt (pwsk1) wt (pssea-d c ) ssar (pwsk1) ssar (pssea-d c ) ssal (pwsk1) ssal (pssea-d c ) wt wt (pwsk1) wt (pssea-d c ) ssar (pwsk1) ssar (pssea-d c ) ssal (pwsk1) ssal (pssea-d c ) - SseB LPM (ph.0) LPM (ph.) DnaK B kda.- - Whole cell lysate wt wt (pwsk1) wt (pssea-d c ) ssar (pwsk1) ssar (pssea-d c ) ssal (pwsk1) ssal (pssea-d c ) wt wt (pwsk1) wt (pssea-d c ) ssar (pwsk1) ssar (pssea-d c ) ssal (pwsk1) ssal (pssea-d c ) SseB LPM (ph.0) LPM (ph.) DnaK Coombes et al. Figure
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Supplemental Data Supplemental Figure 1.
 Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Supplemental material
 Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Supplemental material Diversity of O-antigen repeat-unit structures can account for the substantial sequence variation of Wzx translocases Yaoqin Hong and Peter R. Reeves School of Molecular Bioscience,
Supplementary. Table 1: Oligonucleotides and Plasmids. complementary to positions from 77 of the SRα '- GCT CTA GAG AAC TTG AAG TAC AGA CTG C
 Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Supplementary Table 1: Oligonucleotides and Plasmids 913954 5'- GCT CTA GAG AAC TTG AAG TAC AGA CTG C 913955 5'- CCC AAG CTT ACA GTG TGG CCA TTC TGC TG 223396 5'- CGA CGC GTA CAG TGT GGC CAT TCT GCT G
Cat. # Product Size DS130 DynaExpress TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1
 Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Product Name: Kit Component TA PCR Cloning Kit (ptakn-2) Cat. # Product Size DS130 TA PCR Cloning Kit (ptakn-2) 20 reactions Box 1 (-20 ) ptakn-2 Vector, linearized 20 µl (50 ng/µl) 1 2 Ligation Buffer
Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were
 1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
1 Supplemental methods 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 Quantitative reverse-transcription PCR. Transcript levels of flgs, flgr, flia and flha were monitored by quantitative reverse-transcription
Arabidopsis actin depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB
 Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Arabidopsis actin depolymerizing factor mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB Files in this Data Supplement: Supplemental Table S1 Supplemental Table
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR
 Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Lecture 10, 20/2/2002: The process of solution development - The CODEHOP strategy for automatic design of consensus-degenerate primers for PCR 1 The problem We wish to clone a yet unknown gene from a known
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
PCR analysis was performed to show the presence and the integrity of the var1csa and var-
 Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Supplementary information: Methods: Table S1: Primer Name Nucleotide sequence (5-3 ) DBL3-F tcc ccg cgg agt gaa aca tca tgt gac tg DBL3-R gac tag ttt ctt tca ata aat cac tcg c DBL5-F cgc cct agg tgc ttc
Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC
 Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Disease and selection in the human genome 3
 Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Supporting Information
 Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
Supporting Information Transfection of DNA Cages into Mammalian Cells Email: a.turberfield@physics.ox.ac.uk Table of Contents Supporting Figure 1 DNA tetrahedra used in transfection experiments 2 Supporting
MCB421 FALL2005 EXAM#3 ANSWERS Page 1 of 12. ANSWER: Both transposon types form small duplications of adjacent host DNA sequences.
 Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Dierks Supplementary Fig. S1
 Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
Dierks Supplementary Fig. S1 ITK SYK PH TH K42R wt K42R (kinase deficient) R29C E42K Y323F R29C E42K Y323F (reduced phospholipid binding) (enhanced phospholipid binding) (reduced Cbl binding) E42K Y323F
ΔPDD1 x ΔPDD1. ΔPDD1 x wild type. 70 kd Pdd1. Pdd3
 Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Supplemental Fig. S1 ΔPDD1 x wild type ΔPDD1 x ΔPDD1 70 kd Pdd1 50 kd 37 kd Pdd3 Supplemental Fig. S1. ΔPDD1 strains express no detectable Pdd1 protein. Western blot analysis of whole-protein extracts
Supplementary Figure 1A A404 Cells +/- Retinoic Acid
 Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
Supplementary Figure 1A A44 Cells +/- Retinoic Acid 1 1 H3 Lys4 di-methylation SM-actin VEC cfos (-) RA (+) RA 14 1 1 8 6 4 H3 Lys79 di-methylation SM-actin VEC cfos (-) RA (+) RA Supplementary Figure
MacBlunt PCR Cloning Kit Manual
 MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
MacBlunt PCR Cloning Kit Manual Shipping and Storage MacBlunt PCR Cloning Kits are shipped on dry ice. Each kit contains a box with cloning reagents and an attached bag with Eco-Blue Competent Cells (optional).
Supplemental Data. mir156-regulated SPL Transcription. Factors Define an Endogenous Flowering. Pathway in Arabidopsis thaliana
 Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
SUPPLEMENTARY MATERIALS AND METHODS. E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1)
 SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
SUPPLEMENTARY MATERIALS AND METHODS E. coli strains, plasmids, and growth conditions. Escherichia coli strain P90C (1) dinb::kan (lab stock) derivative was used as wild-type. MG1655 alka tag dinb (2) is
Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR
 Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis
 1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
ORFs and genes. Please sit in row K or forward
 ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
ORFs and genes Please sit in row K or forward https://www.flickr.com/photos/teseum/3231682806/in/photostream/ Question: why do some strains of Vibrio cause cholera and others don t? Methods Mechanisms
PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells
 Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
Supplementary Information for: PGRP negatively regulates NOD-mediated cytokine production in rainbow trout liver cells Ju Hye Jang 1, Hyun Kim 2, Mi Jung Jang 2, Ju Hyun Cho 1,2,* 1 Research Institute
strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular
![strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular](/thumbs/72/66361212.jpg) Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Hes6. PPARα. PPARγ HNF4 CD36
 SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
SUPPLEMENTARY INFORMATION Supplementary Table Positions and Sequences of ChIP primers -63 AGGTCACTGCCA -79 AGGTCTGCTGTG Hes6-0067 GGGCAaAGTTCA ACOT -395 GGGGCAgAGTTCA PPARα -309 GGCTCAaAGTTCAaGTTCA CPTa
Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR
 Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
Supplementary Methods Antibodies Anti-Pim-1 (Cat#3247), anti-met (Cat#3127), anti-ron (Cat#2654), Anti-EGFR (Cat#2646), anti-igf1r (Cat#3018), anti-insr (Cat#3020), anti-akt (pan, Cat#4691), anti-phospho-akt
Table S1. Bacterial strains (Related to Results and Experimental Procedures)
 Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
An engineered tryptophan zipper-type peptide as a molecular recognition scaffold
 SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
SUPPLEMENTARY MATERIAL An engineered tryptophan zipper-type peptide as a molecular recognition scaffold Zihao Cheng and Robert E. Campbell* Supplementary Methods Library construction for FRET-based screening
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Multiplexing Genome-scale Engineering
 Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Multiplexing Genome-scale Engineering Harris Wang, Ph.D. Department of Systems Biology Department of Pathology & Cell Biology http://wanglab.c2b2.columbia.edu Rise of Genomics An Expanding Toolbox Esvelt
Supplemental Information. Human Senataxin Resolves RNA/DNA Hybrids. Formed at Transcriptional Pause Sites. to Promote Xrn2-Dependent Termination
 Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
Supplemental Information Molecular Cell, Volume 42 Human Senataxin Resolves RNA/DNA Hybrids Formed at Transcriptional Pause Sites to Promote Xrn2-Dependent Termination Konstantina Skourti-Stathaki, Nicholas
Y-chromosomal haplogroup typing Using SBE reaction
 Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Schematic of multiplex PCR followed by SBE reaction Multiplex PCR Exo SAP purification SBE reaction 5 A 3 ddatp ddgtp 3 T 5 A G 3 T 5 3 5 G C 5 3 3 C 5 ddttp ddctp 5 T 3 T C 3 A 5 3 A 5 5 C 3 3 G 5 3 G
Materials Protein synthesis kit. This kit consists of 24 amino acids, 24 transfer RNAs, four messenger RNAs and one ribosome (see below).
 Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Protein Synthesis Instructions The purpose of today s lab is to: Understand how a cell manufactures proteins from amino acids, using information stored in the genetic code. Assemble models of four very
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Information
 Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supporting Online Information
 Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH).
 Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Supporting information for Biochemistry, 1995, 34(34), , DOI: /bi00034a013
 Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Supporting information for Biochemistry, 1995, 34(34), 10807 10815, DOI: 10.1021/bi00034a013 LESNIK 10807-1081 Terms & Conditions Electronic Supporting Information files are available without a subscription
Gene synthesis by circular assembly amplification
 Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
Gene synthesis by circular assembly amplification Duhee Bang & George M Church Supplementary figures and text: Supplementary Figure 1. Dpo4 gene (1.05kb) construction by various methods. Supplementary
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system
 Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Converting rabbit hybridoma into recombinant antibodies with effective transient production in an optimized human expression system Dr. Tim Welsink Molecular Biology Transient Gene Expression OUTLINE Short
Expression of Recombinant Proteins
 Expression of Recombinant Proteins Uses of Cloned Genes sequencing reagents (eg, probes) protein production insufficient natural quantities modify/mutagenesis library screening Expression Vector Features
Expression of Recombinant Proteins Uses of Cloned Genes sequencing reagents (eg, probes) protein production insufficient natural quantities modify/mutagenesis library screening Expression Vector Features
RPA-AB RPA-C Supplemental Figure S1: SDS-PAGE stained with Coomassie Blue after protein purification.
 RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
RPA-AB RPA-C (a) (b) (c) (d) (e) (f) Supplemental Figure S: SDS-PAGE stained with Coomassie Blue after protein purification. (a) RPA; (b) RPA-AB; (c) RPA-CDE; (d) RPA-CDE core; (e) RPA-DE; and (f) RPA-C
Overexpression Normal expression Overexpression Normal expression. 26 (21.1%) N (%) P-value a N (%)
 SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
SUPPLEMENTARY TABLES Table S1. Alteration of ZNF322A protein expression levels in relation to clinicopathological parameters in 123 Asian and 74 Caucasian lung cancer patients. Asian patients Caucasian
Supplementary Information. Construction of Lasso Peptide Fusion Proteins
 Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide
 SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide former/ working Description a designation Plasmids pes213a b pes213-tn5
SUPPLEMENTAL TABLE S1. Additional descriptions of plasmid constructions and the oligonucleotides used Plasmid or Oligonucleotide former/ working Description a designation Plasmids pes213a b pes213-tn5
Project 07/111 Final Report October 31, Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines
 Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
Project 07/111 Final Report October 31, 2007. Project Title: Cloning and expression of porcine complement C3d for enhanced vaccines Project Leader: Dr Douglas C. Hodgins (519-824-4120 Ex 54758, fax 519-824-5930)
Genomics and Gene Recognition Genes and Blue Genes
 Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Supporting Information
 Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Supporting Information Barderas et al. 10.1073/pnas.0801221105 SI Text: Docking of gastrin to Constructed scfv Models Interactive predocking of the 4-WL-5 motif into the central pocket observed in the
Legends for supplementary figures 1-3
 High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
High throughput resistance profiling of Plasmodium falciparum infections based on custom dual indexing and Illumina next generation sequencing-technology Sidsel Nag 1,2 *, Marlene D. Dalgaard 3, Poul-Erik
S4B fluorescence (AU)
 A S4B fluorescence (AU) S4B fluorescence (AU) dsbb csgba csgd dsbb csgba bcsa 5000 * NS NS 4000 * 3000 2000 1000 0 ΔcsgBAΔbcsA ΔcsgDΔdsbBΔbcsA ΔcsgBA ΔdsbBΔcsgBA ΔcsgDΔdsbB B -1000 4000 * * NS 3500 * 3000
A S4B fluorescence (AU) S4B fluorescence (AU) dsbb csgba csgd dsbb csgba bcsa 5000 * NS NS 4000 * 3000 2000 1000 0 ΔcsgBAΔbcsA ΔcsgDΔdsbBΔbcsA ΔcsgBA ΔdsbBΔcsgBA ΔcsgDΔdsbB B -1000 4000 * * NS 3500 * 3000
Lecture 11: Gene Prediction
 Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
II 0.95 DM2 (RPP1) DM3 (At3g61540) b
 Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
NAME:... MODEL ANSWER... STUDENT NUMBER:... Maximum marks: 50. Internal Examiner: Hugh Murrell, Computer Science, UKZN
 COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
COMP710, Bioinformatics with Julia, Test One, Thursday the 20 th of April, 2017, 09h30-11h30 1 NAME:...... MODEL ANSWER... STUDENT NUMBER:...... Maximum marks: 50 Internal Examiner: Hugh Murrell, Computer
2
 1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
1 2 3 4 5 6 7 Supplemental Table 1. Magnaporthe oryzae strains generated in this study. Strain background Genotype Strain name Description Guy-11 H1:RFP H1:RFP Strain expressing Histone H1- encoding gene
Supporting Information
 upporting Information hiota et al..73/pnas.159218 I Materials and Methods Yeast trains. Yeast strains used in this study are described in Table 1. TOM22FLAG, a yeast haploid strain for expression of C-terminally
upporting Information hiota et al..73/pnas.159218 I Materials and Methods Yeast trains. Yeast strains used in this study are described in Table 1. TOM22FLAG, a yeast haploid strain for expression of C-terminally
Glutathione (GSH)-Decorated Magnetic Nanoparticles for Binding Glutathione-S-transferase (GST) Fusion Protein and Manipulating Live Cells
 Glutathione (GSH)-Decorated Magnetic Nanoparticles for Binding Glutathione-S-transferase (GST) Fusion Protein and Manipulating Live Cells Yue Pan, Marcus J. C. Long, Xinming Li, Junfeng Shi, Lizbeth Hedstrom,
Glutathione (GSH)-Decorated Magnetic Nanoparticles for Binding Glutathione-S-transferase (GST) Fusion Protein and Manipulating Live Cells Yue Pan, Marcus J. C. Long, Xinming Li, Junfeng Shi, Lizbeth Hedstrom,
evaluated with UAS CLB eliciting UAS CIT -N Libraries increase in the
 Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
Supplementary Figures Supplementary Figure 1: Promoter scaffold library assemblies. Many ensembless of libraries were evaluated in this work. As a legend, the box outline color in top half of the figure
SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer
 TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
SUPPORTING INFORMATION FILE
 Intrinsic and extrinsic connections of Tet3 dioxygenase with CXXC zinc finger modules Nan Liu, Mengxi Wang, Wen Deng, Christine S. Schmidt, Weihua Qin, Heinrich Leonhardt and Fabio Spada Department of
Intrinsic and extrinsic connections of Tet3 dioxygenase with CXXC zinc finger modules Nan Liu, Mengxi Wang, Wen Deng, Christine S. Schmidt, Weihua Qin, Heinrich Leonhardt and Fabio Spada Department of
Supplemental Table 1. Primers used for PCR.
 Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
Supplemental Table 1. Primers used for PCR. Gene Type Primer Sequence Genotyping and semi-quantitative RT-PCR F 5 -TTG CCC GAT CAC CAT CTG TA-3 rwa1-1 R 5 -TGT AGC GAT CAA GGC CTG ATC TAA-3 LB 5 -TAG CAT
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
SUPPORTING INFORMATION Investigation of the Biosynthesis of the Lasso Peptide Chaxapeptin Using an E. coli-based Production System Helena Martin-Gómez, Uwe Linne, Fernando Albericio, Judit Tulla-Puche,*
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
Supplementary Figures Supplementary Fig. 1 Characterization of GSCs. a. Immunostaining of primary GSC spheres from GSC lines. Nestin (neural progenitor marker, red), TLX (green). Merged images of nestin,
Nongenetic Reprogramming of the Ligand Specificity. of Growth Factor Receptors by Bispecific DNA Aptamers
 Supporting Information For Nongenetic Reprogramming of the Ligand Specificity of Growth Factor Receptors by Bispecific DNA Aptamers Ryosuke Ueki,* Saki Atsuta, Ayaka Ueki and Shinsuke Sando* Department
Supporting Information For Nongenetic Reprogramming of the Ligand Specificity of Growth Factor Receptors by Bispecific DNA Aptamers Ryosuke Ueki,* Saki Atsuta, Ayaka Ueki and Shinsuke Sando* Department
Supporting Information
 Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Supporting Information Table S1. Oligonucleotide sequences used in this work Oligo DNA A B C D CpG-A CpG-B CpG-C CpG-D Sequence 5 ACA TTC CTA AGT CTG AAA CAT TAC AGC TTG CTA CAC GAG AAG AGC CGC CAT AGT
Engineering D66N mutant using quick change site directed mutagenesis. Harkewal Singh 09/01/2010
 Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
G+C content. 1 Introduction. 2 Chromosomes Topology & Counts. 3 Genome size. 4 Replichores and gene orientation. 5 Chirochores.
 1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
Supplemental Data. Bennett et al. (2010). Plant Cell /tpc
 BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
BRN1 ---------MSSSNGGVPPGFRFHPTDEELLHYYLKKKISYEKFEMEVIKEVDLNKIEPWDLQDRCKIGSTPQNEWYFFSHKDRKYPTGS 81 BRN2 --------MGSSSNGGVPPGFRFHPTDEELLHYYLKKKISYQKFEMEVIREVDLNKLEPWDLQERCKIGSTPQNEWYFFSHKDRKYPTGS 82 SMB
hcd1tg/hj1tg/ ApoE-/- hcd1tg/hj1tg/ ApoE+/+
 ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
ApoE+/+ ApoE-/- ApoE-/- H&E (1x) Supplementary Figure 1. No obvious pathology is observed in the colon of diseased ApoE-/me. Colon samples were fixed in 1% formalin and laid out in Swiss rolls for paraffin
7.03 Problem Set 3 Due before 5 PM on Wednesday, October 18 Hand in answers in recitation section or in the box outside of
 7.03 Problem Set 3 Due before 5 PM on Wednesday, October 18 Hand in answers in recitation section or in the box outside of 68-120 1. The following DNA sequence fragment comes from the middle of a bacterial
7.03 Problem Set 3 Due before 5 PM on Wednesday, October 18 Hand in answers in recitation section or in the box outside of 68-120 1. The following DNA sequence fragment comes from the middle of a bacterial
Det matematisk-naturvitenskapelige fakultet
 UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: MBV4010 Arbeidsmetoder i molekylærbiologi og biokjemi I MBV4010 Methods in molecular biology and biochemistry I Day of exam: Friday
Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURB
 Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURBO DNA-free Kit (Ambion). One µg of total RNA was reverse
Supplementary Methods Quantitative RT-PCR. For mrna, total RNA was prepared using TRIzol reagent (Invitrogen) and genomic DNA was eliminated with TURBO DNA-free Kit (Ambion). One µg of total RNA was reverse
Lecture 19A. DNA computing
 Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
Lecture 19A. DNA computing What exactly is DNA (deoxyribonucleic acid)? DNA is the material that contains codes for the many physical characteristics of every living creature. Your cells use different
Chapter 13 Chromatin Structure and its Effects on Transcription
 Chapter 13 Chromatin Structure and its Effects on Transcription Students must be positive that they understand standard PCR. There is a resource on the web for this purpose. Warn them before this class.
Chapter 13 Chromatin Structure and its Effects on Transcription Students must be positive that they understand standard PCR. There is a resource on the web for this purpose. Warn them before this class.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3240 Supplementary Figure 1 GBM cell lines display similar levels of p100 to p52 processing but respond differentially to TWEAK-induced TERT expression according to TERT promoter mutation
DOI: 10.1038/ncb3240 Supplementary Figure 1 GBM cell lines display similar levels of p100 to p52 processing but respond differentially to TWEAK-induced TERT expression according to TERT promoter mutation
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Multiplexed Detection of Lung Cancer Cells at the Single-Molecule
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Multiplexed Detection of Lung Cancer Cells at the Single-Molecule
Supplementary Figure 1 A) MMC provokes a rapid replication arrest. We used a strain with a dnaats allele to
 Supplementary Figure 1 A) MMC provokes a rapid replication arrest. We used a strain with a dnaats allele to block the initiation of replication when placed at 40 C. The ori/ter ratio was monitored by qpcr.
Supplementary Figure 1 A) MMC provokes a rapid replication arrest. We used a strain with a dnaats allele to block the initiation of replication when placed at 40 C. The ori/ter ratio was monitored by qpcr.
Primer Design Workshop. École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria
 Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Primer Design Workshop École d'été en géné-que des champignons 2012 Dr. Will Hintz University of Victoria Scenario You have discovered the presence of a novel endophy5c organism living inside the cells
Cloning and Expression of a Haloacid Dehalogenase Enzyme. By: Skyler Van Senior Research Advisor: Dr. Anne Roberts
 Cloning and Expression of a Haloacid Dehalogenase Enzyme By: Skyler Van Senior Research Advisor: Dr. Anne Roberts utline The gene being cloned is JHP1130 from Helicobacter pylori (H. pylori) JHP1130 is
Cloning and Expression of a Haloacid Dehalogenase Enzyme By: Skyler Van Senior Research Advisor: Dr. Anne Roberts utline The gene being cloned is JHP1130 from Helicobacter pylori (H. pylori) JHP1130 is
Yeast BioBrick Assembly (YBA)
 Yeast BioBrick Assembly (YBA) Standardized method for vector assembly of BioBrick devices via homologous recombination in Saccharomyces cerevisiae Martin Schneider, Leonard Fresenborg, Virginia Schadeweg
Yeast BioBrick Assembly (YBA) Standardized method for vector assembly of BioBrick devices via homologous recombination in Saccharomyces cerevisiae Martin Schneider, Leonard Fresenborg, Virginia Schadeweg
CHE-3H84: Protein Engineering Past Exam Papers
 CHE-3H84: Protein Engineering Past Exam Papers Sorted by Topic then Year Knowledge-Based Engineering of Proteins, Large Scale Production of Recombinant Proteins, and Protein Purification Dr. Hemmings 2006/7
CHE-3H84: Protein Engineering Past Exam Papers Sorted by Topic then Year Knowledge-Based Engineering of Proteins, Large Scale Production of Recombinant Proteins, and Protein Purification Dr. Hemmings 2006/7
Supplementary Fig. 1. Isolation and in vitro expansion of EpCAM + cholangiocytes. For collagenase perfusion, enzyme solution was injected from the
 Supplementary Fig. 1. Isolation and in vitro expansion of EpCAM + cholangiocytes. For collagenase perfusion, enzyme solution was injected from the portal vein for digesting adult livers, whereas it was
Supplementary Fig. 1. Isolation and in vitro expansion of EpCAM + cholangiocytes. For collagenase perfusion, enzyme solution was injected from the portal vein for digesting adult livers, whereas it was
Luo et al. Supplemental Figures and Materials and Methods
 Luo et al. Supplemental Figures and Materials and Methods The supplemental figures demonstrate that nuclear NFAT is situated at PODs, overexpressed PML does not increase NFAT nuclear localization, and
Luo et al. Supplemental Figures and Materials and Methods The supplemental figures demonstrate that nuclear NFAT is situated at PODs, overexpressed PML does not increase NFAT nuclear localization, and
Table S2. Oligonucleotide primers used to amplify pile and tcpa genes. Restriction endonuclease sites are underlined. Oligonucleotide Gene/ Sequence
 The metal binding site Even though metals were not added to the crystallization mixture, a strong positive peak (56 sigma) in the fo fc electron density map clearly shows the tetrahedral coordination of
The metal binding site Even though metals were not added to the crystallization mixture, a strong positive peak (56 sigma) in the fo fc electron density map clearly shows the tetrahedral coordination of
The B1 Protein Guides the Biosynthesis of a Lasso Peptide
 The B1 Protein Guides the Biosynthesis of a Lasso Peptide Shaozhou Zhu 1,2, Christopher D. Fage 1, Julian D. Hegemann 1, Andreas Mielcarek 1, Dushan Yan 1, Uwe Linne 1 & Mohamed A. Marahiel*,1 1 Department
The B1 Protein Guides the Biosynthesis of a Lasso Peptide Shaozhou Zhu 1,2, Christopher D. Fage 1, Julian D. Hegemann 1, Andreas Mielcarek 1, Dushan Yan 1, Uwe Linne 1 & Mohamed A. Marahiel*,1 1 Department
SUPPLEMENTAL DATA SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL DATA SUPPLEMENTAL FIGURE LEGENDS SUPPLEMENTAL FIGURE S1. Identification of BmCREC. (A) Amino acid sequences of BmCREC show the peptides identified in LC-MS/MS analysis (marked by red letters
SUPPLEMENTAL DATA SUPPLEMENTAL FIGURE LEGENDS SUPPLEMENTAL FIGURE S1. Identification of BmCREC. (A) Amino acid sequences of BmCREC show the peptides identified in LC-MS/MS analysis (marked by red letters
Supplementary Figure 1 Tmod3 expression and phosphorylation. (a) Expression of Tmod3 in 3T3-L1 preadipocytes and differentiated adipocytes.
 1 2 3 1 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Supplementary Figure 1 Tmod3 expression and phosphorylation. (a) Expression of Tmod3 in 3T3-L1 preadipocytes and differentiated
1 2 3 1 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Supplementary Figure 1 Tmod3 expression and phosphorylation. (a) Expression of Tmod3 in 3T3-L1 preadipocytes and differentiated
SUPPLEMENTARY INFORMATION
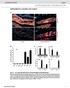 doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
doi: 10.1038/nature07182 SUPPLEMENTAL FIGURES AND TABLES Fig. S1. myf5-expressing cells give rise to brown fat depots and skeletal muscle (a) Perirenal BAT from control (cre negative) and myf5-cre:r26r3-yfp
Supplemental Information. Target-Mediated Protection of Endogenous. MicroRNAs in C. elegans. Inventory of Supplementary Information
 Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
Supporting Information
 Supporting Information Figure S1. (A) Schematic representation of PCP GrsA fused N-terminal to MBP with and without linker (yellow) in between PCP and MBP. The numbers denote the site of the fusion and
Supporting Information Figure S1. (A) Schematic representation of PCP GrsA fused N-terminal to MBP with and without linker (yellow) in between PCP and MBP. The numbers denote the site of the fusion and
Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers
 DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
DNA sentences. How are proteins coded for by DNA? Materials. Teacher instructions. Student instructions. Reflection
 DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
DNA sentences How are proteins coded for by DNA? Deoxyribonucleic acid (DNA) is the molecule of life. DNA is one of the most recognizable nucleic acids, a double-stranded helix. The process by which DNA
Electronic Supplementary Information Sensitive detection of polynucleotide kinase using rolling circle amplification-induced chemiluminescence
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sensitive detection of polynucleotide kinase
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sensitive detection of polynucleotide kinase
11th Meeting of the Science Working Group. Lima, Peru, October 2012 SWG-11-JM-11
 11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
11th Meeting of the Science Working Group Lima, Peru, 15-19 October 2012 Russian population genetics studies of jack mackerel in the South Pacific P.K.Afanasiev M.A.Rabchun A.I.Glubokov Introduction. In
PCR-based Markers and Cut Flower Longevity in Carnation
 PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
PCRbased Markers and Cut Flower Longevity in Carnation Laura De Benedetti, Luca Braglia, Simona Bruna, Gianluca Burchi *, Antonio Mercuri and Tito Schiva Istituto Sperimentale per la Floricoltura, Corso
A Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion,
 TITLE A Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion, Survival, and Cancer Cell Behaviors AUTHORS Carolyn R. Shurer *, Marshall J. Colville *, Vivek K. Gupta,
TITLE A Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion, Survival, and Cancer Cell Behaviors AUTHORS Carolyn R. Shurer *, Marshall J. Colville *, Vivek K. Gupta,
Wet Lab Tutorial: Genelet Circuits
 Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Supplementary Information
 Supplementary Information Load-dependent modulation of non-muscle myosin-2a function by tropomyosin 4.2 Nikolas Hundt 1, Walter Steffen 2, Salma Pathan-Chhatbar 1, Manuel H. Taft 1, Dietmar J. Manstein
Supplementary Information Load-dependent modulation of non-muscle myosin-2a function by tropomyosin 4.2 Nikolas Hundt 1, Walter Steffen 2, Salma Pathan-Chhatbar 1, Manuel H. Taft 1, Dietmar J. Manstein
BioInformatics and Computational Molecular Biology. Course Website
 BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
BioInformatics and Computational Molecular Biology Course Website http://bioinformatics.uchc.edu What is Bioinformatics Bioinformatics upgrades the information content of biological measurements. Discovery
Table S1. Sequences of mutagenesis primers used to create altered rdpa- and sdpa genes
 Supplementary Table and Figures for Structural Basis for the Enantiospecificities of R- and S-Specific Phenoxypropionate/α-Ketoglutarate Dioxygenases by Tina A. Müller, Maria I. Zavodszky, Michael Feig,
Supplementary Table and Figures for Structural Basis for the Enantiospecificities of R- and S-Specific Phenoxypropionate/α-Ketoglutarate Dioxygenases by Tina A. Müller, Maria I. Zavodszky, Michael Feig,
