NIH Public Access Author Manuscript Nature. Author manuscript; available in PMC 2013 October 04.
|
|
|
- Morgan Patterson
- 5 years ago
- Views:
Transcription
1 NIH Public Access Author Manuscript Published in final edited form as: Nature April 4; 496(7443): doi: /nature A solution to release twisted DNA during chromosome replication by coupled DNA polymerases Isabel Kurth 1, Roxana E. Georgescu 1, and Mike O Donnell 1,* 1 The Rockefeller University, Howard Hughes Medical Institute, 1230 York Avenue, New York, NY Abstract Chromosomal replication machines contain coupled DNA polymerases that simultaneously replicate the leading and lagging strands 1. However, coupled replication presents a largely unrecognized topological problem. Since DNA polymerase must travel a helical path during synthesis, the physical connection between leading and lagging strand polymerases causes the daughter strands to entwine, or produces extensive buildup of negative supercoils in the newly synthesized DNA 2 4. How DNA polymerases maintain their connection during coupled replication despite these topological challenges is a mystery. Here, we examine the dynamics of the E. coli replisome, by ensemble and single-molecule methods that may solve this topological problem independent of topoisomerases. We find that the lagging strand polymerase frequently releases from an Okazaki fragment before completion, leaving single-strand gaps behind. Dissociation of the polymerase does not result in loss from the replisome due to its contact with the leading-strand polymerase. This behavior, referred to as signal release, had been thought to require a protein, possibly primase, to pry polymerase from incompletely extended DNA fragments 5 7. However, we observe that signal release is independent of primase and does not appear to require a protein trigger at all. Instead, the lagging-strand polymerase is simply less processive in the context of a replisome. Interestingly, when the lagging-strand polymerase is supplied with primed DNA in trans, uncoupling it from the fork, high processivity is restored. Hence, we propose that coupled polymerases introduce topological changes, possibly by accumulation of superhelical tension in the newly synthesized DNA, that cause lower processivity and transient lagging-strand polymerase dissociation from DNA. Coupled leading- and lagging-strand polymerases, as observed in phage and bacterial replisomes 1,8, have dramatic implications for the topology of the daughter DNA products 2 4,9. The topological problem is based on the a priori fact that polymerases generate a helical product, and thus either must travel a helical path or the DNA product must turn behind them. For example, a rotating leading-strand polymerase will take the attached lagging-strand polymerase and wind the DNA duplex 360 around the axis of the leading strand, forming precatenanes in the daughter helices (Figure 1a, left) 2. At the rate of E. coli replication (650 bp/s) rotating polymerases quickly result in an impossible tangle 2. Alternatively, if the DNA products rotate instead of the polymerases, negative supercoils accumulate (Figure 1a, right). Superhelical tension on the lagging strand may be relieved by * Correspondence and requests for materials should be addressed to M.O.D. (odonnel@rockefeller.edu). Supplementary Information is linked to the online version of the paper at Author Contributions I.K. and M.O.D. conceived the project, I.K. and R.G. performed experiments, I.K., R.G. and M.O.D. designed the experiments, analyzed data and wrote the paper. Reprints and permissions information is available at The authors declare no conflict of interest.
2 Kurth et al. Page 2 rotation of single-strand DNA, but SSB forms large superstuctures, which likely constrain swivel motion 10. The energy generated by only 3 4 supercoils 11 is sufficient to disrupt protein-protein and protein-dna interactions 12. Thus topological tension could disrupt the replisome, unless the tension is periodically released. The supercoils produced by coupled polymerases behind the fork are negative supercoils, which can be resolved by Topo I and Topo III 13. However, Topo III is non-essential, and the viability of Topo I mutants has been controversial 14,15. Hence, topoisomerases may participate, but are insufficient to remove negative supercoils produced by coupled replisomes. Interestingly, single-molecule studies demonstrate highly processive DNA synthesis (>100 kb) in the absence of topoisomerases, implying that replisomes possess an intrinsic solution to the problem of coupled replication 16,17. Indeed, Cozzarelli originally proposed that the topological problem could be solved by transient release of one polymerase of a coupled replisome from DNA (Figure 1b), enabling the negative supercoils on both strands to relax 2. If the leading polymerase detaches from DNA, it will rebind the same primer terminus for continued extension. If the lagging polymerase dissociates it will reattach to the same Okazaki fragment and complete it provided a new primed site is not yet formed. During extension of longer Okazaki fragments a new priming event is more likely to occur; thus a dissociated lagging polymerase may rebind the at the new RNA primer, leaving the original Okazaki fragment incomplete (Figure 1b). In fact incomplete Okazaki fragments have been observed in bacterial and phage systems 5 7. Premature polymerase dissociation is referred to as signal release because it has been presumed that the lagging-strand polymerase is signaled by a replisome component to dissociate and alleviate supercoil tension 5 7. To gain insight into the topological challenge of coupled DNA replication, we developed assays to study signal release. The E. coli replisome is assembled on a 5 biotinylated rolling circle substrate and then attached to streptavidin beads (Fig. 2a). The substrate has only three nucleotides on either strand allowing either leading or lagging strand to be labeled depending on which radioactive nucleotide is used. Replication is initiated after wash steps to remove unbound proteins, and ssdna gaps left by signal release are detected by treating the (α- 32 P)-dTTP labeled lagging-strand template with S1 endonuclease to digest the gaps (Supplementary Fig. 1). If all Okazaki fragments are completely extended the product will be S1-resistant. Conversely, 100% signal release will leave gaps between every Okazaki fragment, and S1 digestion will yield products of similar size to Okazaki fragments (Supplementary Fig. 2). An intermediate length of DNA will result if signal release is <100%. The S1 analysis shows an intermediate-sized (α- 32 P)-dTTP labeled DNA (Fig. 2b, lane 2) that migrates between the length of undigested DNA (lane 1) and the size of Okazaki fragments (lane 3). Size analysis of S1-treated products reveals that about one-third of all Okazaki fragments are incomplete (Supplementary Fig. 3, Full Methods). To validate the assay, several controls were performed (Supplementary Fig. 4): 1) S1 digestion eliminates ssdna produced in a reaction without primase (i.e. no lagging-strand synthesis), 2) S1 does not cleave double-strand Okazaki fragments, 3) ssdna gaps produced by signal release can be filled in by addition of Pol II, 4) the presence of complete Okazaki fragments is confirmed using ligase. Earlier studies implied that primase triggers polymerase signal release 5,6. To test this hypothesis, we performed the reaction in the complete absence of primase, using exogenously added DNA primers to initiate Okazaki fragments. However, the result is unchanged (Fig. 2c). Studies in the T4 system suggest that a loaded clamp triggers polymerase release 7. Although clamps cannot be omitted without negating lagging-strand synthesis altogether, titrations of the clamp (+/ clamp loader) do not influence signal
3 Kurth et al. Page 3 release (Supplementary Fig. 5). Importantly, the assays of Fig. 2 remove excess (unbound) polymerase-clamp loader (i.e. Pol III*) and DnaB helicase, and show that primase, soluble polymerase, clamp loader and helicase are not required to trigger polymerase dissociation. We find that increasing Okazaki fragment size by lowering DNA primer concentration results in more frequent signal release (Fig 2d, e and Supplementary Fig. 6). Okazaki fragments of 1 kb give only 20% signal release while 5 kb Okazaki fragments give 80% signal release. Hence, small Okazaki fragments are more likely to be completed than long Okazaki fragments, implying that the lagging-strand polymerase is not very processive. In contrast, studies on model lagging-strand templates show the polymerase has high intrinsic processive and extends a primed 5.4 kb φx174 ssdna without dissociating 18,19. To exclude that this result is an artifact of using DNA primers, we altered Okazaki fragment size by changing the concentration of primase 20, but the results were upheld (Supplementary Fig. 7). That signal release correlates with long Okazaki fragments was confirmed using Pol II to fill in ssdna gaps, demonstrating that ssdna gaps are associated with long Okazaki fragments and yield an average gap size of 800 nt (Supplementary Fig. 8). To further test for a protein circuit that lowers processivity by triggering polymerase release, we designed an assay to directly measure lagging-strand polymerase processivity. Since priming at a replication fork occurs randomly, producing heterogeneous sized products, we attached a 5.4 kb primed φx174 circular ssdna to the lagging-strand polymerase in trans before initiating replication (Fig. 3a). Considering that all replisomal proteins are present, protein-induced signal release should still occur, and the length of extension from the defined primed site will define the processivity of the lagging-strand polymerase. A time-course of replication shows a most surprising result (Fig. 3b). The 5.4 kb φx174 DNA is fully replicated by the lagging polymerase (Fig. 3b, lanes 1 6 and 13 18), in striking contrast to the low processivity during Okazaki fragment synthesis (i.e. 80% dissociation by 5 kb, Fig. 2d, e). We obtained similar results in the presence or absence of primase, confirming that neither primase nor primed sites trigger signal release (Fig. 3b, lanes 7 12 and 19 24). The major difference in reactions with or without the primed φx174 DNA in trans is the connection of the two polymerases through the DNA, indicating that the connection of two polymerases to the same DNA molecule causes a decrease in laggingstrand polymerase processivity. One possible non-protein source is the topological strain produced in DNA due to coupled replication (Fig. 1). Lagging-strand loops are an unlikely source, as bending of large DNA stretches requires very little energy 21. To examine the dynamics of lagging-strand polymerase action we turned to single-molecule studies to determine if signal release is characteristic of all replisomes, and whether it takes place during long replication times. Replication is performed in a flow cell in which the replisome-dna complex is attached to a lipid bilayer. Replicating DNA is held in place at a lipid diffusion barrier (Fig. 4a, and Full Methods). Upon initiating replication, product DNA is observed using TIRF microscopy and a fluorescent dsdna intercalator (see Fig. 4b). We initially performed experiments at 10 μl/min, which translates into an applied force of 0.23 pn on the DNA, far below that needed to disrupt most protein-protein and protein- DNA interactions 12. After replication is complete, we increased the flow to 100 μl/min (1.45 pn) to stretch DNA, enabling examination of the product for ssdna gaps, which appear as regions with decreased fluorescence intensity 16 (Fig. 4b, c). The resolution of the CCD camera is approximately 0.27μm per pixel, the length of about 880 bp of dsdna. Hence ssdna gaps should result in an uneven pixel density over the length of the molecule and enable a quantitative evaluation of the ssdna/dsdna ratio per pixel (Full Methods). All the molecules examined contained pixel intensity differences along their entire length (Fig. 4c, and Supplementary Fig. 9). If the pixel intensity differences are indeed ssdna they
4 Kurth et al. Page 4 FULL METHODS Reagents and proteins should be eliminated upon adding Pol III, which fills gaps, in the flow buffer, and this was the case (Fig. 4d). Summation of the pixel intensities over μm of DNA within nine different DNA molecules indicates 13% ssdna and 87% dsdna (assuming SSB-bound ssdna equals dsdna length 5 (Fig. 4e)). This result is within 2-fold that observed in ensemble studies (i.e. Okazaki fragments average 2.3 kb, and give 65% signal release at 10 nm primase; thus an 800 bp average gap size yields a value of 22.3% ssdna and 77.7% dsdna). Overall, these results demonstrate that signal release occurs over the length of long DNA products and is a characteristic of all replisomes. We also performed replication at a 10-fold higher flow rate (100 μl/min; 1.45 pn) (Fig. 4f, g). Unexpectedly, the amount of ssdna relative to dsdna increased nearly two-fold relative to the 10 μl/min flow rate (Fig. 4h), from 13% at 10 μl/min to 22% at 100 μl/min (Fig. 4e). An explanation for this difference is that when the lagging-strand polymerase undergoes a transient release cycle (signal release), the increased velocity of the flow carries away the old 3 terminus, making the polymerase more likely to associate with the next RNA primer synthesized at the fork (illustration in Fig. 4i), resulting in more ssdna gaps. Notably, previous studies of signal release that suggested a protein trigger are also consistent with polymerase release triggered by torsional constraints due to coupled replication 5 7. For example, in T4, increasing the concentration of clamps may accelerate clamp assembly on new RNA primers and increase the probability that a released polymerase binds the new RNA primed site 7. In the T7 system additional primase may enhance priming frequency providing more opportunity for a dissociated polymerase to bind a new site 5. Negative supercoiling is required for a variety of DNA metabolic actions, including replication, recombination and transcription 22,23. Negative superhelicity is a property of genomic DNA in all organisms and appears constant over the entire genome 24. Hence it is conceivable that coupled leading-/lagging-strand replication provides a method by which negative supercoils can be placed into DNA across the entire genome without topoisomerase action. Our data suggest that signal release is caused by DNA topology and thus may provide a topoisomerase-independent solution to the topological problem incurred by coupled DNA polymerases during replication. The results demonstrate that: (a) signal release does not require primase, (b) the release process is stochastic implying an inherent low processivity of the lagging polymerase, (c) signal release requires connection of both polymerases to the same DNA and (d) addition of DNA in trans eliminates signal release in the presence of all replisomal proteins. Despite the correlation of premature signal release to the topological problem inherent in coupled polymerases, the problem of coupled polymerase action is not definitively solved in this report and will require further studies. Proteins were purified as described:α, ε, γ, τ, δ,δ, χ, ψ, θ, β 26, SSB 27, Pol III* ((αεθ) 3 τ 3 δδ χψ) andγ complex (γ 3 δδ χψ) were reconstituted as described 26,28,29. Solid support bead-based assays were performed using Pol III* containing an ε mutant (D12A and E14A) that eliminates the 3-5 exonuclease activity to prevent removal of the 3 dideoxyterminal nucleotide of the Trap DNA. Replication buffer is 20 mm Tris-HC1 (ph 7.5), 4% glycerol, 0.1 mm EDTA, 40 μg/ml BSA, 5 mm DTT, 10 mm MgOAc 2. S1 buffer is 40 mm NaOAc (ph 4.5), 300 mm NaCl and 2 mm ZnSO 4.
5 Kurth et al. Page 5 Rolling circle replication reactions Twenty μl streptavidin-coupled magnetic beads (Invitrogen) were washed and equilibrated in replication buffer. Replisomes were assembled in 20 μl replication buffer RB (20 mm Tris-HC1 (ph 7.5), 4% glycerol, 0.1 mm EDTA, 40 μg/ml BSA, 5 mm DTT, 10 mm MgOAc 2 ) containing 100 fmol synthetic 5 -biotinylated 100-mer rolling circle DNA (prepared as in 25 ), 60 μm each datp and dgtp, 50 μm ATP-γ-S, 4 pmol DnaB 6, 2.5 pmol β 2, and 0.5 pmol Pol III*. Reactions were incubated 5 min at 37 C prior to immobilization on beads for 10 min at 25 C. Beads were washed three times in 500 μl replication buffer containing 30 μm datp and dgtp, 12 pmol β 2 and 50 μm ATP-γ-S prior to resuspending in 10 μl replication buffer containing 60 μm dctp, 60 μm dgtp and 2.5 pmol β 2. Replication was initiated by addition of 10 μl 0.5 mm ATP, 200 nm primase (unless indicated otherwise), 480 nm SSB 4, 50 μm NTPs and either: 60 μm datp, 10 μm TTP, 3 μci (α 32 P)-TTP to label the lagging-strand template (i.e. leading-strand product is the lagging-strand template in a rolling circle reaction), or 60 μm dttp, 10 μm datp and 3μCi (α 32 P)-dATP to label lagging-strand Okazaki fragments. After 15 sec 5 μl replication buffer containing 20 nm Trap DNA was added to sequester any polymerase that dissociates during replication, thereby preventing it from filling gaps produced by signal release. Indicated concentrations reflect final reaction conditions in 25 μl. Replication was allowed to proceed for a total of 35 sec. The Trap DNA consists of M13mp18 ssdna primed with a 3 dideoxyterminated DNA 30-mer (5 - GTTAAAGGCCGCTTTTGCGGGATCGTCACddC-3 ) onto which the β clamp was preassembled in a 2-min reaction at 37 C containing 4.8 pmol SSB 4, 2.5 pmol β 2, 1 pmol γ- complex. Reactions were quenched by adding 25 μl of 40 mm EDTA and 1% SDS. Where indicated, primase was substituted with 20-mer DNA oligonucleotides (5 - GCAAAAGCAGGCACAGCAAC-3 ). Beads were washed twice in Buffer E (20 mm Tris- HC1 (ph 7.5), 4% glycerol, 0.1 mm EDTA, 40 μg/ml BSA, 5 mm DTT) and once in S1 buffer (200 mm sodium acetate (ph 4.5), 1.5 M NaCl and 10 mm ZnSO 4 ). S1 endonuclease digestion Beads were resuspended in 20 μl S1 buffer and 5 μl of the reaction was withdrawn and incubated with 1 U S1 nuclease (Fermentas) for 7.5 min at 37 C in 15 μl total reaction volume. Reactions were quenched by adding 15 μl of 40 mm HEPES (ph 7.5), 40 mm EDTA and 6% SDS. DNA was released from beads by incubating at 95 C for 3 min and resolved on 0.8 % alkaline agarose gels. Control reactions for S1 assay Where indicated, S1 treatment was performed on either: 0.2 μg supercoiled pik31 plasmid DNA, pik31 linearized with PstI (pik31-psti), pik31 nicked on one strand with Nb.BvCI (pik31-bvci), or 0.4 μg circular M13mp18 ssdna. Rolling circle replication reactions were performed as described above. Reactions that were treated with Pol II were first replicated and quenched as described above, washed three times in replication buffer before resuspending in 25 μl buffer containing 20 nm Pol II, 60 μm dctp and dgtp, 2.5 pmol β 2, 1 pmol γ-complex and 0.5 mm ATP and incubated for 5 min at 37 C. Replication was initiated by adding 60 μm dttp, datp and incubated for 10 min at 37 C. Replication for fill-in reactions with Pol II to label gaps were performed similarly and initiated with datp, dttp and (α 32 P)-dATP, where indicated. Ligation of Okazaki fragments Replication reactions that were treated with T4 DNA ligase (New England Biolabs) were first replicated in the presence of (α 32 P)-dATP and 800 nm 5 -phosphorylated oligonucleotide (5 -P-GCAAAAGCAGGCACAGCAAC-3 ) for lagging-strand synthesis
6 Kurth et al. Page 6 and quenched as described. Beads were washed three times in 20 mm Tris-HC1 (ph 7.5), 4% glycerol, 0.1 mm EDTA, 40 μg/ml BSA, 5 mm DTT. Beads were resuspended in T4 quick ligase buffer (NEB) and 1 μl of T4 DNA quick ligase for 15 min at 25 C. Reactions were quenched by adding 15 μl of 40 mm HEPES (ph 7.5), 40 mm EDTA and 6% SDS. DNA was released from the beads by incubating at 95 C for 3 min and resolved in 0.8 % alkaline agarose gels. Analysis of lagging-strand polymerase processivity on primed φx174 ssdna in trans Data analysis Replisomes were assembled on the biotinylated rolling circle substrate as described above. After the wash steps, beads were resuspended in 20 μl replication buffer containing 8 nm φx174 ssdna (5.4 kb) primed with a DNA oligonucleotide (5 - CAAGCAGTAGTAATTCCTGCTTTATCAAG-3 ), 2.4 pmol SSB 4, 1 pmol β 2, 0.08 pmol γ-complex, 60 μm dctp and 60 μm dgtp for 2 min at 37 C. Beads were immobilized, washed and replication was initiated as described above. Lanes in alkaline gels were scanned using a phosphorimager with ImageQuant Software. The intensity of radioactivity at each pixel was normalized to the corresponding molecular weight in order to correct for the fact that longer products incorporate more radiolabel. Size markers were used as a reference in the gel. Percent signal release was calculated from the size difference of S1 treated leading strand and lagging strand products (fit with a single Gaussian distribution as described in the legend to Supplementary Fig. 3) using the calculation: SR = Signal release; Size (lg) = Size of lagging strand product; Size (ld + S1) = Size of S1 digested leading strand product (i.e. lagging strand template). Single-molecule replication assays Replisomes were assembled onto 530 fmol (10.5 nm) 5 biotinylated 100-mer rolling circle DNA by first incubating with 18 pmol DnaB helicase (365 nm) in 50 μl Buffer A (20 mm Tris- HCl, ph 7.5, 5 mm DTT, 40 μg/ml BSA, 4% glycerol) containing 8 mm MgOAc 2 and 0.25 mm ATP, followed by incubation for 30 sec at 37 C. Then a 25 μl solution of Buffer A containing Pol III* (675 fmol (αεθ)τ 3 δδ ψχ, 27 nm as complex), β 2 (1.85 pmol, 74 nm as dimer), 60 μm each dctp and dgtp and 8 mm MgOAc 2 was added followed by a further 1 min at 37 C. To immobilize the replisome-dna complex in the flow cell, 1 μl of the above reaction was diluted 1000-fold into 1 ml of Buffer B (8 mm MgOAc 2, 60 μm each of dctp and dgtp, and 50 nm Yo-Pro1 in Buffer A), and then the diluted reaction was passed through the flow cell at 500 μl/min for 30 sec, and a further 2 min at 10 μl/min. DNA replication was initiated upon flowing Buffer A containing 60 μm of each dntp, 250 μm each of CTP, TTP, and UTP, 1 mm ATP, 462 nm SSB 4, 100 nm primase, 50 nm β 2, 50 mm potassium glutamate, 50 nm Yo-Pro1, 0.8% glucose, 0.01% β-mercaptoethanol, 0.57U glucose oxidase, and 2.1U catalase. The flow rate was either 10 μl/min or 100 μl/min, as indicated. After 10 min of replication, the flow rate was adjusted to 100 μl/min to analyze replication products.
7 Kurth et al. Page 7 Intensity Analysis of the DNA products In order to correct for flow disturbances in our analysis, we averaged between frames for each DNA strand (over two adjacent pixels); the same operation was performed for an adjacent empty region to serve as background correction that takes into consideration the signal variation of non-uniform intensities displayed by the bilayer. In addition to this background subtraction, we performed a signal normalization. As the DNA strand grows longer and extends further away from the Biotin-Streptavidin anchoring point in the lipid bilayer, there is an additional slow-drift in intensity that is due to higher strand mobility into the flow. Therefore we normalized the slow-drift intensity signal to 100% dsdna by applying a rank-order filter, a method best suited for removing shot noise. This method finds the specified percentile of data points in the data window around each point in the data set (here the corrected line plot intensity) and replaces that point with the percentile. The analysis was performed using Matlab software. Supplementary Material Acknowledgments References Refer to Web version on PubMed Central for supplementary material. We thank Drs. L. Langston, A. Libchaber and B. Michel for helpful suggestions on the manuscript. We are also grateful to NIH (GM 38839) for supporting this work. 1. Kornberg, A.; Baker, TA. DNA replication. 2. W.H. Freeman; Ullsperger, CJ.; Volgodskii, AV.; Cozzarelli, NR. Unlinking of DNA by Topoisomerases During DNA Replication. 9. Springer-Verlag; Hingorani MM, O Donnell M. Sliding clamps: a (tail)ored fit. Curr Biol. 2000; 10:R [PubMed: ] 4. Wyman C, Botchan M. DNA replication. A familiar ring to DNA polymerase processivity. Curr Biol. 1995; 5: [PubMed: ] 5. Hamdan SM, Loparo JJ, Takahashi M, Richardson CC, van Oijen AM. Dynamics of DNA replication loops reveal temporal control of lagging-strand synthesis. Nature. 2009; 457: [PubMed: ] 6. Li X, Marians KJ. Two distinct triggers for cycling of the lagging strand polymerase at the replication fork. J Biol Chem. 2000; 275: [PubMed: ] 7. Yang J, Nelson SW, Benkovic SJ. The control mechanism for lagging strand polymerase recycling during bacteriophage T4 DNA replication. Mol Cell. 2006; 21: [PubMed: ] 8. Johnson A, O Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem. 2005; 74: [PubMed: ] 9. Postow L, Peter BJ, Cozzarelli NR. Knot what we thought before: the twisted story of replication. Bioessays. 1999; 21: [PubMed: ] 10. Chastain PD 2nd, Makhov AM, Nossal NG, Griffith J. Architecture of the replication complex and DNA loops at the fork generated by the bacteriophage t4 proteins. J Biol Chem. 2003; 278: [PubMed: ] 11. Vologodskii AV, Lukashin AV, Anshelevich VV, Frank-Kamenetskii MD. Fluctuations in superhelical DNA. Nucleic Acids Res. 1979; 6: [PubMed: ] 12. Leu FP, Georgescu R, O Donnell M. Mechanism of the E. coli tau processivity switch during lagging-strand synthesis. Mol Cell. 2003; 11: [PubMed: ] 13. Espeli O, Marians KJ. Untangling intracellular DNA topology. Mol Microbiol. 2004; 52: [PubMed: ]
8 Kurth et al. Page Stockum A, Lloyd RG, Rudolph CJ. On the viability of Escherichia coli cells lacking DNA topoisomerase I. BMC Microbiol. 2012; 12:26. [PubMed: ] 15. Stupina VA, Wang JC. Viability of Escherichia coli topa mutants lacking DNA topoisomerase I. J Biol Chem. 2005; 280: [PubMed: ] 16. Yao NY, Georgescu RE, Finkelstein J, O Donnell ME. Single-molecule analysis reveals that the lagging strand increases replisome processivity but slows replication fork progression. Proc Natl Acad Sci U S A. 2009; 106: [PubMed: ] 17. Tanner NA, et al. Real-time single-molecule observation of rolling-circle DNA replication. Nucleic Acids Res. 2009; 37:e27. [PubMed: ] 18. O Donnell ME, Kornberg A. Complete replication of templates by Escherichia coli DNA polymerase III holoenzyme. J Biol Chem. 1985; 260: [PubMed: ] 19. McHenry CS. DNA replicases from a bacterial perspective. Annu Rev Biochem. 2011; 80: [PubMed: ] 20. Wu CA, Zechner EL, Reems JA, McHenry CS, Marians KJ. Coordinated leading- and laggingstrand synthesis at the Escherichia coli DNA replication fork. V. Primase action regulates the cycle of Okazaki fragment synthesis. J Biol Chem. 1992; 267: [PubMed: ] 21. Marko JF, Siggia ED. Stretching DNA. Macromolecules. 1995; 28: Dorman CJ. DNA supercoiling and bacterial gene expression. Sci Prog. 2006; 89: [PubMed: ] 23. Travers A, Muskhelishvili G. A common topology for bacterial and eukaryotic transcription initiation? EMBO Rep. 2007; 8: [PubMed: ] 24. Benyajati C, Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976; 9: [PubMed: ] 25. McInerney P, O Donnell M. Functional uncoupling of twin polymerases: mechanism of polymerase dissociation from a lagging-strand block. J Biol Chem. 2004; 279: [PubMed: ] 26. Onrust R, Finkelstein J, Turner J, Naktinis V, O Donnell M. Assembly of a chromosomal replication machine: two DNA polymerases, a clamp loader, and sliding clamps in one holoenzyme particle. III. Interface between two polymerases and the clamp loader. J Biol Chem. 1995; 270: [PubMed: ] 27. Yao N, Hurwitz J, O Donnell M. Dynamics of beta and proliferating cell nuclear antigen sliding clamps in traversing DNA secondary structure. J Biol Chem. 2000; 275: [PubMed: ] 28. Bailey S, Wing RA, Steitz TA. The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerases. Cell. 2006; 126: [PubMed: ] 29. Georgescu RE, et al. Mechanism of polymerase collision release from sliding clamps on the lagging strand. Embo J. 2009; 28: [PubMed: ]
9 Kurth et al. Page 9 Figure 1. The topological problem caused by coupled leading- and lagging-strand polymerases The figure illustrates the topological problem only for the leading-strand polymerase. a The two Pols (yellow) are coupled through the clamp loader (green). Pols travel a helical path as they synthesize DNA. As the leading Pol spins, the lagging Pol is dragged around this path, twisting the lagging strand around the leading strand (left). Alternatively, the DNA turns instead (right), generating negative supercoils as indicated by the arrow. In either case, the stress can be released by b. transient dissociation of the leading Pol or, c. transient dissociation of the lagging Pol, which could re-bind the incomplete Okazaki fragment or a new RNA primer.
10 Kurth et al. Page 10 Figure 2. Signal release does not require primase and correlates with increasing Okazaki fragment length a, The replisome, consisting of DNA helicase (blue), Pol III* (one clamp loader (green) that binds 3 Pols (yellow); only two Pols are shown for clarity) and β-clamps (red) is assembled on a 5 biotinylated rolling circle DNA, then attached to beads. After unbound proteins are removed, replication is initiated by adding primase, β, SSB, ATP and (α- 32 P)-dNTPs. Reactions are quenched and treated with S1 to cleave gaps left by signal release, prior to analysis on alkaline gels. The newly synthesized leading strand (purple) is the template for lagging-strand synthesis (blue), initiated by RNA primers (red). b, Replication reactions using (α- 32 P)-dTTP either before (lane 1) or after (lane 2) S1 analysis, or using (α- 32 P)- datp (lane 3). c, Reactions in the absence of primase, using 800 nm DNA 20-mers to prime the lagging strand. d, Titration of DNA 20-mers into reactions. e, Plot of Okazaki fragment length versus % signal release.
11 Kurth et al. Page 11 Figure 3. Lagging-strand polymerase processivity is restored using a separate DNA molecule a, The replisome is assembled on DNA as in the legend to Fig. 2a. Unbound proteins are washed away prior to addition of primed φx kb ssdna. b, Reactions are initiated in the presence (lanes 7 12 and 19 24) or absence (lanes 1 6 and 13 18) of primase. Supernatants containing φx174 replication products (lanes 1 12) are separated from beadbound rolling circle products (lanes 13 24).
12 Kurth et al. Page 12 NIH-PA Author Manuscript NIH-PA Author Manuscript Figure 4. Single-molecule TIRF microcopy NIH-PA Author Manuscript a, The replisome is assembled on the rolling circle DNA and attached to a lipid bilayer. Replication is initiated upon flowing replication buffer at 10 μl/min. b, DNA curtain produced at 10 μl/min. c, Normalized line plot intensity of a representative DNA strand. Diagrams at the top illustrate dsdna and ssdna regions. d, Analysis as in c, but with Pol III* in the buffer flow. e. Quantification of line plot analysis at indicated flow rates. n = 9 ± SD for 10 μl/min or n = 13 ± SD for 100 μl/min. f. DNA curtain at 100 μl/min. g. Line plot analysis as in c, at 100 μl/min. h. Histogram relating pixel intensity to dsdna at 10 μl/min (purple) and 100 μl/min (bright blue). i, Scheme at 100 μl/min.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11988 Supplementary Figure 1. Digestion of model DNA substrates. a, Linearized plasmid DNA (pik31- PstI, lanes 1 and 2), supercoiled plasmid (pik31, lanes 3 and 4), singly nicked plasmid
doi:10.1038/nature11988 Supplementary Figure 1. Digestion of model DNA substrates. a, Linearized plasmid DNA (pik31- PstI, lanes 1 and 2), supercoiled plasmid (pik31, lanes 3 and 4), singly nicked plasmid
Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome
 SUPPLEMENTARY ONLINE MATERIAL For manuscript: Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome By Roxana E. Georgescu 1, Isabel Kurth 1 and Mike E. O Donnell 1 1 The Rockefeller
SUPPLEMENTARY ONLINE MATERIAL For manuscript: Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome By Roxana E. Georgescu 1, Isabel Kurth 1 and Mike E. O Donnell 1 1 The Rockefeller
DNA Replication II Biochemistry 302. January 25, 2006
 DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302. Bob Kelm January 28, 2004
 DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
DNA Replication II Biochemistry 302. Bob Kelm January 26, 2005
 DNA Replication II Biochemistry 302 Bob Kelm January 26, 2005 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 Bob Kelm January 26, 2005 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
Chapter 11 DNA Replication and Recombination
 Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
 The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
The replication forks Summarising what we know:
 When does replication occur? MBLG1001 lecture 10 Replication the once in a lifetime event! Full blown replication only occurs once, just before cell division BUT the DNA template is constantly being repaired.
When does replication occur? MBLG1001 lecture 10 Replication the once in a lifetime event! Full blown replication only occurs once, just before cell division BUT the DNA template is constantly being repaired.
Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function. Gary Peter
 Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function Gary Peter Learning Objectives 1. List and explain the mechanisms by which E. coli DNA is replicated 2. Describe
Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function Gary Peter Learning Objectives 1. List and explain the mechanisms by which E. coli DNA is replicated 2. Describe
Enzymes used in DNA Replication
 Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
DNA Replication in Prokaryotes and Eukaryotes
 DNA Replication in Prokaryotes and Eukaryotes 1. Overall mechanism 2. Roles of Polymerases & other proteins 3. More mechanism: Initiation and Termination 4. Mitochondrial DNA replication DNA replication
DNA Replication in Prokaryotes and Eukaryotes 1. Overall mechanism 2. Roles of Polymerases & other proteins 3. More mechanism: Initiation and Termination 4. Mitochondrial DNA replication DNA replication
XactEdit Cas9 Nuclease with NLS User Manual
 XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
The flow of Genetic information
 The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition
 SUPPLEMENTARY INFORMATION Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition Mari-Liis Visnapuu 1 and Eric C. Greene 1 1 Department of Biochemistry &
SUPPLEMENTARY INFORMATION Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition Mari-Liis Visnapuu 1 and Eric C. Greene 1 1 Department of Biochemistry &
Supplemental Materials and Methods
 Supplemental Materials and Methods Proteins and reagents Proteins were purified as described previously: RecA, RecQ, and SSB proteins (Harmon and Kowalczykowski 1998); RecF protein (Morimatsu and Kowalczykowski
Supplemental Materials and Methods Proteins and reagents Proteins were purified as described previously: RecA, RecQ, and SSB proteins (Harmon and Kowalczykowski 1998); RecF protein (Morimatsu and Kowalczykowski
III. Detailed Examination of the Mechanism of Replication A. Initiation B. Priming C. Elongation D. Proofreading and Termination
 Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
DNA Structure Requirements for the Escherichia coli Complex Clamp Loader and DNA Polymerase III Holoenzyme*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 15, Issue of April 14, pp. 11440 11450, 2000 2000 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. DNA Structure
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 15, Issue of April 14, pp. 11440 11450, 2000 2000 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. DNA Structure
The Size and Packaging of Genomes
 DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
DNA Metabolism. I. DNA Replication. A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding
 DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
University of Groningen. Single-molecule studies of DNA replication Geertsema, Hylkje
 University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen. Single-molecule studies of DNA replication Geertsema, Hylkje
 University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
Supplementary Information for. Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment.
 Supplementary Information for Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation Thomas R. Beattie and Stephen D. Bell Sir William Dunn School of Pathology,
Supplementary Information for Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation Thomas R. Beattie and Stephen D. Bell Sir William Dunn School of Pathology,
DNA REPLICATION. Third Stage. Lec. 12 DNA Replication. Lecture No.: 12. A. Watson & Crick (1952) C. Cairns (1963) autoradiographic experiment
 Lec. 12 DNA Replication A. Watson & Crick (1952) Proposed a model where hydrogen bonds break, the two strands separate, and DNA synthesis occurs semi-conservatively in the same net direction. While a straightforward
Lec. 12 DNA Replication A. Watson & Crick (1952) Proposed a model where hydrogen bonds break, the two strands separate, and DNA synthesis occurs semi-conservatively in the same net direction. While a straightforward
Bacterial DNA replication
 Bacterial DNA replication Summary: What problems do these proteins solve? Tyr OH attacks PO4 and forms a covalent intermediate Structural changes in the protein open the gap by 20 Å! 1 Summary: What problems
Bacterial DNA replication Summary: What problems do these proteins solve? Tyr OH attacks PO4 and forms a covalent intermediate Structural changes in the protein open the gap by 20 Å! 1 Summary: What problems
Genetic Information: DNA replication
 Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
Questions from chapters in the textbook that are relevant for the final exam
 Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
University of Groningen
 University of Groningen E. coli DNA replication in the absence of free beta clamps Tanner, Nathan A.; Tolun, Goekhan; Loparo, Joseph J.; Jergic, Slobodan; Griffith, Jack D.; Dixon, Nicholas E.; van Oijen,
University of Groningen E. coli DNA replication in the absence of free beta clamps Tanner, Nathan A.; Tolun, Goekhan; Loparo, Joseph J.; Jergic, Slobodan; Griffith, Jack D.; Dixon, Nicholas E.; van Oijen,
Molecular Biology (2)
 Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Replication. Obaidur Rahman
 Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
DNA Replication I Biochemistry 302. Bob Kelm January 24, 2005
 DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
DNA replication: Enzymes link the aligned nucleotides by phosphodiester bonds to form a continuous strand.
 DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
Requirements for the Genetic Material
 Requirements for the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell-generation to generation. 2. Information Storage Biologically useful information in a stable
Requirements for the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell-generation to generation. 2. Information Storage Biologically useful information in a stable
The highly processive DNA polymerases that replicate cellular
 Colloquium Interaction of the sliding clamp with MutS, ligase, and DNA polymerase I Francisco J. López de Saro* and Mike O Donnell Howard Hughes Medical Institute and The Rockefeller University, 1230 York
Colloquium Interaction of the sliding clamp with MutS, ligase, and DNA polymerase I Francisco J. López de Saro* and Mike O Donnell Howard Hughes Medical Institute and The Rockefeller University, 1230 York
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule.
 1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
Lecture 1 Sunday, 4 March :24 pm
 Lecture 1 Sunday, 4 March 2018 10:24 pm Amino acid side chains can be Hydrophobic, hydrophilic Positive, negatively charged Movement of information OH removed from 2' carbon to make the end more stable
Lecture 1 Sunday, 4 March 2018 10:24 pm Amino acid side chains can be Hydrophobic, hydrophilic Positive, negatively charged Movement of information OH removed from 2' carbon to make the end more stable
Chapter 3: Duplicating the DNA- Replication
 3. Basic Genetics Plant Molecular Biology Chapter 3: Duplicating the DNA- Replication Double helix separation New strand synthesis Plant Biotechnology Lecture 2 1 I've missed more than 9000 shots in my
3. Basic Genetics Plant Molecular Biology Chapter 3: Duplicating the DNA- Replication Double helix separation New strand synthesis Plant Biotechnology Lecture 2 1 I've missed more than 9000 shots in my
Replication of DNA and Chromosomes
 Chapter 9. Replication of DNA and Chromosomes 1. Semiconservative Replication 2. DNA Polymerases and DNA Synthesis In Vitro 3. The Complex Replication Apparatus 4. Unique Aspects of Eukaryotic Chromosome
Chapter 9. Replication of DNA and Chromosomes 1. Semiconservative Replication 2. DNA Polymerases and DNA Synthesis In Vitro 3. The Complex Replication Apparatus 4. Unique Aspects of Eukaryotic Chromosome
Chapter Twelve: DNA Replication and Recombination
 This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
Ren Lab ENCODE in situ HiC Protocol for Tissue
 Ren Lab ENCODE in situ HiC Protocol for Tissue Pulverization, Crosslinking of Tissue Note: Ensure the samples are kept frozen on dry ice throughout pulverization. 1. Pour liquid nitrogen into a mortar
Ren Lab ENCODE in situ HiC Protocol for Tissue Pulverization, Crosslinking of Tissue Note: Ensure the samples are kept frozen on dry ice throughout pulverization. 1. Pour liquid nitrogen into a mortar
University of Groningen. Single-molecule studies of DNA replication Geertsema, Hylkje
 University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
Supporting Information
 Supporting Information Loparo et al. 1.173/pnas.111 SI Methods Coverslip Functionalization. To reduce surface sticking of labeled protein and to tether the DNA construct, glass coverslips were functionalized
Supporting Information Loparo et al. 1.173/pnas.111 SI Methods Coverslip Functionalization. To reduce surface sticking of labeled protein and to tether the DNA construct, glass coverslips were functionalized
Chapter 12. DNA Replication and Recombination
 Chapter 12 DNA Replication and Recombination I. DNA replication Three possible modes of replication A. Conservative entire original molecule maintained B. Semiconservative one strand is template for new
Chapter 12 DNA Replication and Recombination I. DNA replication Three possible modes of replication A. Conservative entire original molecule maintained B. Semiconservative one strand is template for new
Gene Expression- Protein Synthesis
 Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
DNA replication. - proteins for initiation of replication; - proteins for polymerization of nucleotides.
 DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
Role of the Core DNA Polymerase III Subunits at the Replication Fork
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 273, No. 4, Issue of January 23, pp. 2452 2457, 1998 1998 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Role of the Core
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 273, No. 4, Issue of January 23, pp. 2452 2457, 1998 1998 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Role of the Core
Welcome to Class 18! Lecture 18: Outline and Objectives. Replication is semiconservative! Replication: DNA DNA! Introductory Biochemistry!
 Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
University of Groningen
 University of Groningen Proliferating Cell Nuclear Antigen Uses Two Distinct Modes to Move along DNA Kochaniak, Anna B.; Habuchi, Satoshi; Loparo, Joseph J.; Chang, Debbie J.; Cimprich, Karlene A.; Walter,
University of Groningen Proliferating Cell Nuclear Antigen Uses Two Distinct Modes to Move along DNA Kochaniak, Anna B.; Habuchi, Satoshi; Loparo, Joseph J.; Chang, Debbie J.; Cimprich, Karlene A.; Walter,
ARUNAI ACADEMY FOR PG TRB-BOTANY DHARMAPURI REPLICATION - ENZYMES.
 ARUNAI ACADEMY FOR PG TRB-BOTANY DHARMAPURI.9500244679 REPLICATION - ENZYMES DNA HELICASE Sparation of two strands- DNA helicase enzyme functions Unwinds DNA. DNA double helix by breaking the hydrogen
ARUNAI ACADEMY FOR PG TRB-BOTANY DHARMAPURI.9500244679 REPLICATION - ENZYMES DNA HELICASE Sparation of two strands- DNA helicase enzyme functions Unwinds DNA. DNA double helix by breaking the hydrogen
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
You Should Be Able To
 DNA Replica,on You Should Be Able To 1. Describe the func9on of: DNA POL1, DNA POL3, Sliding Clamp, SSBPs, Ligase, Topoisomerase, Helicase, Primase 2. Describe DNA synthesis on the leading and lagging
DNA Replica,on You Should Be Able To 1. Describe the func9on of: DNA POL1, DNA POL3, Sliding Clamp, SSBPs, Ligase, Topoisomerase, Helicase, Primase 2. Describe DNA synthesis on the leading and lagging
Fidelity of DNA polymerase
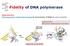 Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Enzymatic assembly of DNA molecules up to several hundred kilobases
 nature methods Enzymatic assembly of DNA molecules up to several hundred kilobases Daniel G Gibson, Lei Young, Ray-Yuan Chuang, J Craig Venter, Clyde A Hutchison III & Hamilton O Smith Supplementary figures
nature methods Enzymatic assembly of DNA molecules up to several hundred kilobases Daniel G Gibson, Lei Young, Ray-Yuan Chuang, J Craig Venter, Clyde A Hutchison III & Hamilton O Smith Supplementary figures
DNA metabolism. DNA Replication DNA Repair DNA Recombination
 DNA metabolism DNA Replication DNA Repair DNA Recombination Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology Central Dogma or Flow of genetic information
DNA metabolism DNA Replication DNA Repair DNA Recombination Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology Central Dogma or Flow of genetic information
Tutorial Week #9 Page 1 of 11
 Tutorial Week #9 Page 1 of 11 Tutorial Week #9 DNA Replication Before the tutorial: Read ECB Chapter 6 p195-207, and review your lecture notes Read this tutorial and create a table of definitions and functions
Tutorial Week #9 Page 1 of 11 Tutorial Week #9 DNA Replication Before the tutorial: Read ECB Chapter 6 p195-207, and review your lecture notes Read this tutorial and create a table of definitions and functions
Fundamental Process. Unit Map. Unit
 Unit 3 Unit Map 3.A DNA replication, repair and recombination 120 3.B RNA synthesis and processing 137 3.C Protein synthesis and processing 151 3.D Control of gene expression at transcription and translation
Unit 3 Unit Map 3.A DNA replication, repair and recombination 120 3.B RNA synthesis and processing 137 3.C Protein synthesis and processing 151 3.D Control of gene expression at transcription and translation
CSIR UGC NET, GATE (ENGINEERING), GATE (Science), IIT-JAM, UGC NET, TIFR, IISc, NIMCET, JEST etc. JNU CEEB SAMPLE THEORY
 JNU CEEB SAMPLE THEORY REPLICATION TYPE OF DNA REPLICATION REPLICATION IN PROKARYOTES REPLICATION IN EUKARYOTES For IIT-JAM, JNU, GATE, NET, NIMCET and Other Entrance Exams 1-C-8, Sheela Chowdhary Road,
JNU CEEB SAMPLE THEORY REPLICATION TYPE OF DNA REPLICATION REPLICATION IN PROKARYOTES REPLICATION IN EUKARYOTES For IIT-JAM, JNU, GATE, NET, NIMCET and Other Entrance Exams 1-C-8, Sheela Chowdhary Road,
Chapter 9. Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination
 Chapter 9 Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination 1 Genetics Genome Chromosome Gene Protein Genotype Phenotype 2 Terms and concepts gene Fundamental unit of heredity
Chapter 9 Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination 1 Genetics Genome Chromosome Gene Protein Genotype Phenotype 2 Terms and concepts gene Fundamental unit of heredity
NEBNext RNase III RNA Fragmentation Module
 SAMPLE PREPARATION NEBNext RNase III RNA Fragmentation Module Instruction Manual NEB #E6146S 100 reactions NEBNext RNase III RNA Fragmentation Module Table of Contents: Description....2 Applications....2
SAMPLE PREPARATION NEBNext RNase III RNA Fragmentation Module Instruction Manual NEB #E6146S 100 reactions NEBNext RNase III RNA Fragmentation Module Table of Contents: Description....2 Applications....2
DNA: Structure & Replication
 DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Protocol for in vitro transcription
 Protocol for in vitro transcription Assemble the reaction at room temperature in the following order: Component 10xTranscription Buffer rntp T7 RNA Polymerase Mix grna PCR DEPC H 2 O volume 2μl 2μl 2μl
Protocol for in vitro transcription Assemble the reaction at room temperature in the following order: Component 10xTranscription Buffer rntp T7 RNA Polymerase Mix grna PCR DEPC H 2 O volume 2μl 2μl 2μl
DNA. Chapter 1. Molecular Diagnostics Fundamentals, Methods and Clinical Applications Second Edition 1/29/2013. Copyright 2012 F.A.
 DNA Chapter 1 1 Deoxyribonucleic acid (DNA) is a genetic information storage system. A T G C T A C G DNA is a polymer of nucleotides. Nucleotides are phosphorylated nucleosides. DNA Nucleosides comprise
DNA Chapter 1 1 Deoxyribonucleic acid (DNA) is a genetic information storage system. A T G C T A C G DNA is a polymer of nucleotides. Nucleotides are phosphorylated nucleosides. DNA Nucleosides comprise
Recitation CHAPTER 9 DNA Technologies
 Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
Supplementary Information
 Supplementary Information Supplementary Figures Figure S1. Study of mgtl translation in vitro. (A) Detection of 5 LR RNA using wild-type and anti-sd (91-95) substituted templates in a transcription-translation
Supplementary Information Supplementary Figures Figure S1. Study of mgtl translation in vitro. (A) Detection of 5 LR RNA using wild-type and anti-sd (91-95) substituted templates in a transcription-translation
Supplementary Information. Synergistic action of RNA polymerases in overcoming the nucleosomal barrier
 Supplementary Information Synergistic action of RNA polymerases in overcoming the nucleosomal barrier Jing Jin, Lu Bai, Daniel S. Johnson, Robert M. Fulbright, Maria L. Kireeva, Mikhail Kashlev, Michelle
Supplementary Information Synergistic action of RNA polymerases in overcoming the nucleosomal barrier Jing Jin, Lu Bai, Daniel S. Johnson, Robert M. Fulbright, Maria L. Kireeva, Mikhail Kashlev, Michelle
Fast-Link DNA Ligation Kits
 Fast-Link DNA Ligation Kits Cat. Nos. LK0750H and LK6201H Available exclusively thru Lucigen. lucigen.com/epibio www.lucigen.com MA086E Fast-Link DNA Ligation Kits 6/2017 1 1. Introduction The Fast-Link
Fast-Link DNA Ligation Kits Cat. Nos. LK0750H and LK6201H Available exclusively thru Lucigen. lucigen.com/epibio www.lucigen.com MA086E Fast-Link DNA Ligation Kits 6/2017 1 1. Introduction The Fast-Link
Your first goal is to determine the order of assembly of the 3 DNA polymerases during initiation.
 MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1 Question 1 You are studying the mechanism of DNA polymerase loading at eukaryotic DNA replication origins. Unlike the situation in bacteria,
MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1 Question 1 You are studying the mechanism of DNA polymerase loading at eukaryotic DNA replication origins. Unlike the situation in bacteria,
CircLigase II ssdna Ligase
 Cat. Nos. CL9021K and CL9025K www.lucigen.com MA298E-CircLigase II ssdna Ligase 2/2018 1 1. Introduction CircLigase II ssdna Ligase is a thermostable ligase that catalyzes iintramolecular ligation (i.e.,
Cat. Nos. CL9021K and CL9025K www.lucigen.com MA298E-CircLigase II ssdna Ligase 2/2018 1 1. Introduction CircLigase II ssdna Ligase is a thermostable ligase that catalyzes iintramolecular ligation (i.e.,
Replication of DNA Virus Genomes. Lecture 7 Virology W3310/4310 Spring 2013
 Replication of DNA Virus Genomes Lecture 7 Virology W3310/4310 Spring 2013 It s all about Initiation Problems faced by DNA replication machinery Viruses must replicate their genomes to make new progeny
Replication of DNA Virus Genomes Lecture 7 Virology W3310/4310 Spring 2013 It s all about Initiation Problems faced by DNA replication machinery Viruses must replicate their genomes to make new progeny
IDTutorial: DNA Replication
 IDTutorial: DNA Replication Introduction In their report announcing the structure of the DNA molecule, Watson and Crick (Nature, 171: 737-738, 1953) observe, It has not escaped our notice that the specific
IDTutorial: DNA Replication Introduction In their report announcing the structure of the DNA molecule, Watson and Crick (Nature, 171: 737-738, 1953) observe, It has not escaped our notice that the specific
Nucleic Acid Structure:
 Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Mechanisms of DNA Synthesis
 Mechanisms of DNA Synthesis Biochemistry 201 Advanced Molecular Biology January 19, 2000 Doug Brutlag Introduction Today's topic concerns the fundamental mechanism of DNA replication as exemplified by
Mechanisms of DNA Synthesis Biochemistry 201 Advanced Molecular Biology January 19, 2000 Doug Brutlag Introduction Today's topic concerns the fundamental mechanism of DNA replication as exemplified by
NEBNext DNA Library Prep Reagent Set for 454
 SAMPLE PREPARATION NEBNext DNA Library Prep Reagent Set for 454 Instruction Manual NEB #E6020S/L 10/50 reactions NEBNext DNA Library Prep Reagent Set for 454 Table of Contents: The Reagent Set Includes...2
SAMPLE PREPARATION NEBNext DNA Library Prep Reagent Set for 454 Instruction Manual NEB #E6020S/L 10/50 reactions NEBNext DNA Library Prep Reagent Set for 454 Table of Contents: The Reagent Set Includes...2
* + * RecA * + + for RecA-dependent strand + + MIT Department of Biology 7.28, Spring Molecular Biology
 MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1) Question 1. 1A. You are studying the function of in vitro along with a lab partner. You run several reactions to examine the requirements
MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1) Question 1. 1A. You are studying the function of in vitro along with a lab partner. You run several reactions to examine the requirements
DNA Replication semiconservative replication conservative replication dispersive replication DNA polymerase
 DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
Clamping down on pathogenic bacteria how to shut down a key DNA polymerase complex
 Clamping down on pathogenic bacteria how to shut down a key DNA polymerase complex Bacterial DNA-replication machinery Pathogenic bacteria that are resistant to the current armoury of antibiotics are an
Clamping down on pathogenic bacteria how to shut down a key DNA polymerase complex Bacterial DNA-replication machinery Pathogenic bacteria that are resistant to the current armoury of antibiotics are an
DNA supercoiling, a critical signal regulating the basal expression of the lac operon in Escherichia coli
 Supplementary Information DNA supercoiling, a critical signal regulating the basal expression of the lac operon in Escherichia coli Geraldine Fulcrand 1,2, Samantha Dages 1,2, Xiaoduo Zhi 1,2, Prem Chapagain
Supplementary Information DNA supercoiling, a critical signal regulating the basal expression of the lac operon in Escherichia coli Geraldine Fulcrand 1,2, Samantha Dages 1,2, Xiaoduo Zhi 1,2, Prem Chapagain
Microarray Protocol for Agilent Inkjet-Deposited Presynthesized Oligo Arrays Aminoallyl Method AfCS Procedure Protocol PP Version 1, 10/20/03
 Microarray Protocol for Agilent Inkjet-Deposited Presynthesized Oligo Arrays AfCS Procedure Protocol PP00000184 Version 1, 10/20/03 The following procedure details the preparation of fluorescently labeled
Microarray Protocol for Agilent Inkjet-Deposited Presynthesized Oligo Arrays AfCS Procedure Protocol PP00000184 Version 1, 10/20/03 The following procedure details the preparation of fluorescently labeled
University of Groningen. Single-molecule studies of DNA replication Geertsema, Hylkje
 University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Single-molecule studies of DNA replication Geertsema, Hylkje IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
NEBNext Quick DNA Library Prep Reagent Set for 454
 LIBRARY PREPARATION NEBNext Quick DNA Library Prep Reagent Set for 454 Instruction Manual NEB #E6080S/L 10/50 reactions AMPURE is a registered trademark of Beckman Coulter, Inc. BIOANALYZER is a registered
LIBRARY PREPARATION NEBNext Quick DNA Library Prep Reagent Set for 454 Instruction Manual NEB #E6080S/L 10/50 reactions AMPURE is a registered trademark of Beckman Coulter, Inc. BIOANALYZER is a registered
Fast-Link DNA Ligation Kits
 Cat. Nos. LK11025, LK0750H, and LK6201H The provide reagents optimized for the construction of recombinant vectors in a short time. Ligation reactions require incubation for as little as 5 minutes at room
Cat. Nos. LK11025, LK0750H, and LK6201H The provide reagents optimized for the construction of recombinant vectors in a short time. Ligation reactions require incubation for as little as 5 minutes at room
Amplification Products for PCR and RT-PCR
 Selection guide Polymerase Hot start Comment UptiTherm DNA pol. no Most economic. Lower error rate than Taq polymerase Available in several formats, master mix including or not dntp, Mg 2+..., in gel format
Selection guide Polymerase Hot start Comment UptiTherm DNA pol. no Most economic. Lower error rate than Taq polymerase Available in several formats, master mix including or not dntp, Mg 2+..., in gel format
Supplementary Information for
 Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
Replisome mechanics: insights into a twin DNA polymerase machine
 Review TRENDS in Microbiology Vol.15 No.4 Molecular Machines of the Cell Replisome mechanics: insights into a twin DNA polymerase machine Richard T. Pomerantz and Mike O Donnell Rockefeller University,
Review TRENDS in Microbiology Vol.15 No.4 Molecular Machines of the Cell Replisome mechanics: insights into a twin DNA polymerase machine Richard T. Pomerantz and Mike O Donnell Rockefeller University,
Mechanism of Transcription Termination by RNA Polymerase III Utilizes a Non-template Strand Sequence-Specific Signal Element
 Molecular Cell Supplemental Information Mechanism of ranscription ermination by RNA Polymerase III Utilizes a Non-template Strand Sequence-Specific Signal Element Aneeshkumar G. Arimbasseri and Richard
Molecular Cell Supplemental Information Mechanism of ranscription ermination by RNA Polymerase III Utilizes a Non-template Strand Sequence-Specific Signal Element Aneeshkumar G. Arimbasseri and Richard
University of Groningen
 University of Groningen Coupling dttp Hydrolysis with DNA Unwinding by the DNA Helicase of Bacteriophage T7 Satapathy, Ajit K.; Kulczyk, Arkadiusz W.; Ghosh, Sharmistha; van Oijen, Antonius; Richardson,
University of Groningen Coupling dttp Hydrolysis with DNA Unwinding by the DNA Helicase of Bacteriophage T7 Satapathy, Ajit K.; Kulczyk, Arkadiusz W.; Ghosh, Sharmistha; van Oijen, Antonius; Richardson,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION A human XRCC4-XLF complex bridges DNA ends. Sara N. Andres 1, Alexandra Vergnes 2, Dejan Ristic 3, Claire Wyman 3, Mauro Modesti 2,4, and Murray Junop 2,4 1 Department of Biochemistry
SUPPLEMENTARY INFORMATION A human XRCC4-XLF complex bridges DNA ends. Sara N. Andres 1, Alexandra Vergnes 2, Dejan Ristic 3, Claire Wyman 3, Mauro Modesti 2,4, and Murray Junop 2,4 1 Department of Biochemistry
Prokaryotic Physiology. March 3, 2017
 1. (10 pts) Explain the replication of both strands of DNA in prokaryotes. At a minimum explain the direction of synthesis, synthesis of the leading and lagging strand, separation of the strands and the
1. (10 pts) Explain the replication of both strands of DNA in prokaryotes. At a minimum explain the direction of synthesis, synthesis of the leading and lagging strand, separation of the strands and the
BIO 311C Spring Lecture 34 Friday 23 Apr.
 BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
Supporting Information
 Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany RNA ligands that distinguish metabolite-induced conformations in the TPP riboswitch Günter Mayer, Marie-Sophie L. Raddatz, Julia D. Grunwald,
Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany RNA ligands that distinguish metabolite-induced conformations in the TPP riboswitch Günter Mayer, Marie-Sophie L. Raddatz, Julia D. Grunwald,
User Manual. Topoisomerase I Assay Kit (plasmid based). Lot Number:
 Copyright TopoGEN, Inc., 2012. All rights reserved. User Manual Topoisomerase I Assay Kit (plasmid based). Catalog Number Catalog Number TG1015-1 100 Reaction Set TG1015-2 250 Reaction Set Lot Number:
Copyright TopoGEN, Inc., 2012. All rights reserved. User Manual Topoisomerase I Assay Kit (plasmid based). Catalog Number Catalog Number TG1015-1 100 Reaction Set TG1015-2 250 Reaction Set Lot Number:
of the Triphosphate of ATP
 A Small Aptamer with Strong and Specific Recognition of the Triphosphate of Peter L. Sazani, Rosa Larralde and Jack W. Szostak Howard Hughes Medical Institute, and Department of Molecular Biology, Massachusetts
A Small Aptamer with Strong and Specific Recognition of the Triphosphate of Peter L. Sazani, Rosa Larralde and Jack W. Szostak Howard Hughes Medical Institute, and Department of Molecular Biology, Massachusetts
NIH Public Access Author Manuscript Cell Cycle. Author manuscript; available in PMC 2010 September 24.
 NIH Public Access Author Manuscript Published in final edited form as: Cell Cycle. 2009 September 1; 8(17): 2686 2691. WHITHER THE REPLISOME: EMERGING PERSPECTIVES ON THE DYNAMIC NATURE OF THE DNA REPLICATION
NIH Public Access Author Manuscript Published in final edited form as: Cell Cycle. 2009 September 1; 8(17): 2686 2691. WHITHER THE REPLISOME: EMERGING PERSPECTIVES ON THE DYNAMIC NATURE OF THE DNA REPLICATION
Answers to Module 1. An obligate aerobe is an organism that has an absolute requirement of oxygen for growth.
 Answers to Module 1 Short Answers 1) What is an obligate aerobe? An obligate aerobe is an organism that has an absolute requirement of oxygen for growth. What about facultative anaerobe? 2) Distinguish
Answers to Module 1 Short Answers 1) What is an obligate aerobe? An obligate aerobe is an organism that has an absolute requirement of oxygen for growth. What about facultative anaerobe? 2) Distinguish
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2013.71 DNA sequencing with electrical conductance measurements of a DNA polymerase Yu-Shiun Chen, Chia-Hui Lee, Meng-Yen Hung, Hsu-An Pan, Jin-Chern Chiou,
SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2013.71 DNA sequencing with electrical conductance measurements of a DNA polymerase Yu-Shiun Chen, Chia-Hui Lee, Meng-Yen Hung, Hsu-An Pan, Jin-Chern Chiou,
