BIOC 312. Gehring Lecture 1 Jan 12
|
|
|
- Aubrey Dina West
- 6 years ago
- Views:
Transcription
1 Gehring Lecture 1 Jan 12 BIOC 312 Lecture Plan for Gehring (all lectures): 1) nomenclature, purine biosynthesis 2) pyrimidine biosynth, formation of tri-diphosphates, ribonucleotide reductase 3) Thymidylate synth, salvage pathway, folate metabolism Readings: Will be talking about how purines and pyrimidines are made de novo, also how they are degraded from other compounds in food ect. and something called the salvage pathway. De Novo Path -synth from starting materials -conserved in eukaryotes Salvage Path -recovery of bases -more varied than de novo What do you need to know for this section? - Nomenclature - Origin of atoms - Key products and Intermediates - Sites of regulation - Structure and numbering of bases and nucleotides Don t need to know - names of all intermediates - Reaction mechanisms Purine/pyrimidine cube: black is de novo, blue is salvage and degradation Key to the lectures is knowing the terminology! 1
2 Gehring Lecture 1 Jan 12 Nomenclature Nucleotides are made up of 3 parts - base, nucleoside, nucleotide base is the aromatic part, note Nitrogens -side means no phosphate -tide means there is a phosphate Purines are double rings Pyrimidines are single rings Know these images Two types of sugar: - ribose/deoxyribose Some properties - ß-glycosidic bonds - D sugars, furanose conformation - 2 OH difference between DNA and RNA Need to learn numbering scheme of sugars/purines/pyrimidines - Sugar: Primed numbers, start at O, clockwise - Pyrimidines: Nitrogens at 1/3, sugar attaches to N1 - Purines: Nitrogens at 1,3,7,9, sugar attaches at N9 Neither have nitrogens at N5! Just base Adenine (Ade) Guanine (Gua) Cytosine (Cyt) Uracil (Ura) Thymine (Thy) Nucleoside (X-ribose) Adenosine (Ado) Guanosine (Guo) Cytidine (Cyd) Uridine (Urd) Deoxythymidine (dthd) Nucleotide (X-ribose-phosphate) Adenylic Acid Adenylate AMP Guanylic Acid Cyanylate GMP Cytidylic Acid Cytidylate CMP Uridylic Acid Uridylate UMP Deoxythymidylic Acid Thymidylate/Deoxythymidylate dtmp 2
3 Gehring Lecture 1 Jan 12 Metabolites (intermediates) - Hypoxanthine / Inosine / Inosinate (IMP) - Xanthine / Xantholine / Xanthylate (XMP) - Orotate (orotic acid) / Orotidylate (OMP) - Uric Acid Modified bases found in DNA (not going to learn about, but understand why) - Bacteria will modify DNA in order to detect foreign DNA - Do so through restriction modification - small amounts Modified bases found in trna - trna are heavily modified (sometimes up to 25%) - rrnas contain methylated bases Cofactors, vitamins, signalling molecules - i.e camp, NAD, FAD, SAM, UDP-glucose, Coenzyme A Purine Biosynthesis PRPP, glutamine, aspartate, CO2, glucine, formate > IMP Origin of Purines 11 STEPS Gehring s Cheat sheet - Purine ring is assembled on ribose phosphate - #1 5-phosphate -> PRPP - #2 -> 5-phosphoribosylamine regulation at steps 1/2 pathway committed at step 2-11 STEPS to make IMP (inosine monophosphate) - 7 high energy phosphate bonds Remember this! *Note* Some textbooks start AFTER synth of PRPP PRPP Recall Nomencalture: when just the ribose, no need for primes, when it is nucloside/tide need the primes on numbering When oxygen is below = alpha, above = beta 3
4 Gehring Lecture 1 Jan 12 1) Synthesis PRPP - key intermediate in BOTH nucleic acid and amino acid synth because in both, need complex regulation (in step 1/2) - Start with alpha sugar anomer (inversion next step) STEP 1 2) Phosphoribosylamine - Committing step Site of purine specific regulation Cell prevents this step from running backwards by getting rid of pyrophosphate (orange in step 2) - Anomeric Inversion to form ß anomer - Release of diphosphate (PPi) irreversible happens because concentration in cell of pyrophosphate is low in the cell (enzyme that degrades it) - Glutamine is the N donor STEP 2 Nitrogen Donation Recall Bioc 311: Nitrogen donors Nitrogen from glutamine comes from the side chain (circled) Nitrogen from aspartate comes from backbone (circled) Nitrogen donation occurs through a conserved mechanism: - Phosphoester intermediate - Aldehyde or ketone > amine - Phosphate from ATP or GTP - N from glutamine side-chain OR aspartic acid backbone 4
5 Gehring Lecture 1 Jan ) Synthesis of 1st cycle - Step 3 is the addition of glycine 3 atoms of cycle complex regulation - Then step 4 is addition of C from N 10 formyl THF - Step 5 another N from glutamine - Step 6 is Cyclization nucleophilic attack by enamine N9 on C=O 7-10) Prep for second ring synthesis - Step 7 is the addition of HCO2 - Then Another N from aspartate this time in 2 steps N-succinyl adduct release of fumarate - Step 10 is Then C from N 10 formyl THF again THF (Tetrahydrofolate) (p1062 voet) - Important cofactor, always used to add carbon (similar to how SAM only transfers Me groups) - C units are attached either at N5, N10 or to both Carries a carbon unit in three different oxidation states Carries 1 carbon unit in five different chemical forms - Comes from folic acid in leaves (vitamin B9 or Bc) - Active in reduced form, THF Nitrogens in red carry the carbons Oxidation levels of C groups carried by THF Oxidation Level Group Carried THF Derivatives Methanol Methyl ( CH3) N5-methyl-THF Formaldehyde Methylene ( CH2 ) N5,N10-Methylene-THF Formate Formyl ( CH=O) Formimino ( CH=NH) Methenyl ( CH=) N5-formyl-THF or N10-formyl-THF N5-formimino-THF N5,N10-methenyl-THF 5
6 Gehring Lecture 1 Jan 12 Examples of THF derivatives: - (left) formyl group attached to N10 - (right) methylene group connecting N10 and N5 We only need to know these two THF derivatives, but know about how the other forms are named, be able to identify the group carried, oxidation levels, where the N5, N10 is, and how many carbon units it will donate as a result. 11) Synthesis of IMP - Inosine monophosphate purine base intermediate branch point for AMP and GMP biosynthesis - Second ring closure via dehydration, no ATP required Synth of AMP & GMP from IMP Cell needs to make a decision, therefore there needs to be regulation! - General rule: When you put extra nitrogens, you put on oxygen first, then nitrogen. When you degrade (i.e take off) nitrogens, you take nitrogen off then oxygen Therefore always through hypoxanthine/ xanthine Components AMP/GMP synth Make AMP Aspartate + GTP Make GMP (via XMP) Glutamine + ATP Make AMP: - Aspartate + GTP to add on the aspartate - Fumarate off, leaving the N Make GMP: - Add oxygen (makes XMP) - get N from glutamine 6
7 Gehring Lecture 1 Jan 12 Regulation of Purine Synthesis - Inhibition: Presence of AMP and GMP inhibit the conversion from IMP Making these uses ATP which is used to make PRPP, therefore inhibition ATP also used in steps 3-7 of cycle, inhibition - Feed forward By PRPP, activates path to 5-Phosphoribosylamine Purine Cube: - Three levels: Bases (bottom), nucleosides, nucleotides (top) - Recall: Black is de novo synthesis (starts at PPRP) 7
8 Gehring Lecture 2 Jan 14 BIOC 312 Lecture 2 Plan: - Pyrimidine Biosynthesis - Formation of Tri-/Diphosphates - Ribonucleotide Reductase (RNA >DNA) Additions to last lecture: Intermediate in generation of IMP, AICAR (product of step 9, purine synth) is a banned substance, used as a performance enhancing drug. Pyrimidine Pathway CO2, glutamine, Aspartate, PRPP > UMP Complications in pyrimidine pathway (and pyrimidine cube) due to uracil being in RNA and not DNA, where Thymine is in DNA and not RNA Origin of Atoms - Most molecules comes from Aspartate - N from glutamine - Carbon from HCO3 Importance of Charged Intermediates during synthesis: - Charged intermediates of IMP/UMP synthesis will NOT be able to cross membranes - Purines are built up from PRPP, meaning there is always a charged phosphate - Pyrimidines are assembled separately, PRPP at end carboxylic acid from aspartate is kept in order to give the charge, and prevent leakage through membranes 1-3) Carbamoyl Phosphate Synthesis & Cyclization Recall: BIOC 311 Urea Cycle, in mitochondria - Carbamoyl phosphate synthetase - Two enzymes in animals. Only one in bacteria mitochondrial (urea cycle) cytosolic (pyrimidine synthesis) - Regulation between animals and bacteria is different step 1 regulated animals, step 2 in bacteria PALA inhibits 8
9 Gehring Lecture 2 Jan 14 - First step uses glutamine and carbamoyl phosphate synthesis II (cytosolic) - Second step uses aspartate and aspartate transcarbamoylase (ATCase) - Third step is cyclization by condensation Studies of ATCase in bacteria These studies were essential in figuring out allosteric regulation. - Testing reaction rate against aspartate concentration ATP added, rate up, Km down (activated) CTP added, rate down, Km up (inhibited) This is because C (a pyrimidine) will slightly inhibit the reaction rate allosterically - ACTase is inhibited by PALA via competitive inhibition. PALA binds ACTase at its active sites to block its function Concept of Channelling - Idea that often in a pathway, enzymes are located in such a way that the product is fed directly into the second similar to an assembly line. - Enzymes tend to form complexes to see these channels through. - Pyrimidine pathway is split up into 3 sections based on this concept, with the enzymes in a complex: Step 1/2/3 has CPS, ATCase, and dehydrogenase in a complex Step 4 occurs at mitochondria Step 5/6 has OPRT and OMP decarboxylase in a complex Point of Channelling: 1) Decreases amount of intermediates 2) Increases throughput 4) Oxidation at mitochondrial membranes - at mitochondria - uses quinone - produces Orotate Orotic Aciduria High levels of orotate in the blood which is then secreted in urine - Caused by loss of steps 5/6 in this pathway 5-6) Addition of PRPP and decarboxylation - OPRT and OMP decarboxylase form a complex - UMP final Product 9
10 Gehring Lecture 2 Jan 14 Pyrimidine Regulation Regulation is at step #1 in animals, and step #2 in bacteria - Inhibition by pyrimidine nucleoside triphosphates (i.e CTP and UTP) this is because CTP and UTP are used to make RNA, the final product - Activation by ATP and PRPP Formation of Di/Tri Phosphates Overview Diphosphates are made via base specific kinases (non-specific for ribose/deoxyribose) for example: - Adenylate Kinase (d)amp + ATP <==> (d)adp + ADP - Uridylate Kinase (d)ump + ATP <==> (d)udp + ADP Remember that udridylate and uridine are different due to the former having phosphates on it. Triphosphates are made via ubiquitous non-specific kinase: Nucleoside diphosphate kinase (d)ndp + (d)n TP <==> (d)ntp + (d)n DP Both run close to thermodynamic equilibrium, therefore G ~ 0 - Enzymes are catalysts so the process is reversible, and depends on Gº, which depends on the concentration of reactants - Concentration of Products/Reactants = 1 CTP Synthesis Function: CTP is a coenzyme in many metabolic reactions i.e synthesis of glycerophospholipids and the glycosylation of proteins. It also allosterically inhibits ACTase Recall: steps 1-3 in pyrimidine synthesis involving ACTase - Made at the level of triphosphate - From UTP - N from glutamine Study of Intracellular nucleotides and PRPP in E. coli Rule of seven dictates the ratios of nucleotides and RNA to DNA in the cell, know the table below. Therefore, you have inhibition by the NTPs due to their higher concentration of it in the cell. Relative concentrations of nucleotides in the cell Concentration Ratio RNA : DNA NTP : dntp NTP : NDP NDP : NMP Value 7 : 1 7 : 1 7 : 1 7 : 1 10
11 Gehring Lecture 2 Jan 14 Know these concentrations: Specific concentrations of nucleotides and PRPP in the cell Substrate ATP GTP IMP PRPP Concentration 3 mm 1 mm 0.15 mm 0.5 mm Main Ideas: - Moving between mono, di and triphosphates are reversible reactions and are in thermodynamic equilibrium - Relative concentrations are determined by energy charge of the cell i.e [ATP] / [AMP] - Base-specific nucleotide kinases determine which nucleotides become triphosphates Synthesis of Deoxyribose (dntp) (p.1119 voet&voet) Why is DNA the genetic material? - Removing the 2 OH makes the DNA phosphodiester backbone more stable. - The base thymine allows spontaneous deamidation of cytosine to be detected therefore when cell sees U in DNA, it knows it was from deamidation Reduction is by NADPH DNA is more reduced than RNA (OIL RIG, gain electrons, OH is more electron withdrawing) The removal of OH (reduction of RNA to DNA) is carried out at level of DNPs (diphosphates). - Reducing equivalents can be NADH or NADPH: [NADPH] / [NADP + ] = 100 (reducing power, usually catabolic paths) [NAD + ] / [NADH] = 1000 (oxidizing power, usually anabolic paths) - Origin of reducing power of NTP to dntp is from NADPH - As a general rule, when you want to reduce something, you use NADPH Redox Review REDOX in terms of Oxygen transfer REDOX in terms of Hydrogen transfer REDOX in terms of electron transfer Oxidation is gain of O Oxidation is loss of H Oxidation is loss of e- Reduction is loss of O Reduction is gain of H Reduction is gain of e- Use OIL RIG for hydrogen and electrons, oxygen is the opposite! 11
12 Gehring Lecture 2 Jan 14 Ribonucleotide Reductase The actual reduction of NTP to dntp is carried out by ribonucleotide reductase (RR) There are a few intermediates to this process (that form a complex?) - Thioredoxin reductase; have cysteine residues that can be reduced (to thiols) - Thioredoxin; also has cysteine residues that are reduced to thiols Many varieties of this enzyme, with many different cofactors. i.e viruses bring their own RR in order to make lots of DNA, turning this off will stop the virus! Some properties of RR: - Two allosteric sites: Activity site - regulated by ATP/dATP levels, controls whether active or inactive Specificity site - regulated by datp/dgtp/dttp, controls which bases are reduced - The substrate binding site is a Tyr free radical Inhibited by hydroxyurea, which binds Tyr directly to inhibit Also inhibited by 8-hydroquinoline, which chelates the Fe3+ RR is a theraputic target for many viruses and cancers which want to make lots of DNA, since RR creates the building blocks that make up DNA Regulation of RiboReductase Two main types of regulation for this enzyme: 1) Feedback inhibition -by the products 2) Feedforward -also by the products, but to other bases 12
13 Gehring Lecture 2 Jan 14 Note that the cube has many arrows, but often they represent the same enzyme - i.e Ribonucleotide reductase, UDP to dudp, CDP to dcdp Additions to Pyrimidine Pathway Problem: Need a method to keep uridine on only ribose, and thymine on only deoxyribose. - A specific enzyme UTPase hydrolyzes dump to decrease its concentration and prevent incorperation into DNA dutp diphosphatase If dutp somehow gets incorporated into DNA, there is an enzyme that will remove it called Uracil-DNA Glycosylase. - Works by hydrolyzing the bond between the 1 C and the N on uracil. - Afterward, endonucleases, ollowed by polymerases and ligases will repair the DNA - Easier to prevent rather than repair 13
14 Gehring Lecture 3 Jan 19 BIOC 312 Lecture 3 Plan: - Thymidylate Synthase - Salvage Pathway (degradation) - Folate metabolism Thymidylate Synthase Synthesis of dtmp from dump by thymidylate synthase, with N 5,N 10 -methylene-thf as the methyl donor. - Required for DNA synthesis but not RNA synthesis. - Synthesis at level of monophosphate - Majority of dump come from action of dutpase on dutp Thymidylate synthase puts a methyl group on the 5 position of the uracil base. The methyl group comes from N 5,N 10 -methylene-thf (indirectly from tetrahydrofolate) - Carbon that is transferred, at the time of transfer, is a different oxidation state than the carbon on the product methylene donated has oxidation state of formaldehyde reduced methyl group has oxidation state of methanol - The reduction comes at the expense of tetrahydroflate > dihydrofolate TS Cycle 1) THF is oxidized to DHF THF in the form of N 5,N 10 -methylene-thf, is reduced by thymidylate synthase to DHF converts dump to dtmp 2) DHF is reduced to THF DHF is reduced by dihydrofolate reductase (DHFR) using NADPH + H + as the reducing agent produces THF 3) Regeneration of N 5,N 10 -methylene-thf THF has a methyl group from a serine molecule added to it this is facilitated by serine hydroxymethyl transferase serine > glycine 14
15 Gehring Lecture 3 Jan 19 Inhibitors to TS Cycle FdUMP is an inhibitor of TS - TS and THF will both bind to this enzyme as it is very similar to dump - Since the F on the 5 position is very electronegative, it cannot be removed - Complex is irreversibly locket as enzyme-fdump-thf this specific inhibition has been used in cancer therapy to stop cell from replicating DNA and therefore inhibits proliferation Inhibitors that undergo all or part of the catalytic reaction before inactivating the enzyme are called mechanism-based inhibitors, aka suicide inhibitors Methotrexate, Aminopterin, Trimethoprim - all inhibit DHFR - These inhibitors are DHF analogs, they have similar compositions - They competitively bind to DHFR, and are essentially irreversibly bound - They are used as anti-cancer and antibacterial agents i.e treat cancer patient with methotrexate, then 5 hours later rescue with 5-formyl-THF i.e trimethoprim has higher affinity for bacterial DHFR, therefore used as antibacterial i.e methotrexate will suppress immune system, can use to reduce arthritis inflammation Sulfonamides (Sulfa Drugs) Are antibiotics, such as sulfanilamide, that are structural analogs of the p-aminobenzoic acid constituent of THF. - Act by competitive inhibition of the p-aminobenzoic constituent acid in THF synthesis - Bacteria synthesize DHF de novo from p-aminobenzoic acid, humans get it from diet therefore sulfonamides will affect bacteria but not humans Of the many oxidizes form of THF, we only need to know two: Used in de novo purine synthesis Used in TS Cycle 15
16 Gehring Lecture 3 Jan 19 Salvage Pathway Salvage Pathway Enzymes Overview Pathway Reaction Enzymes Conversion of base to ribonucleotide Interconversion of bases and nucleosides Conversion of nucleoside to nucleotide Base exchange into deoxynucleosides Interconversion by base alterations Reutilization of nucleotides PRPP + base X > rnmp + PPi ribose 1-P + base N < > nucleoside N + Pi deoxyribose 1-P + base N < > deoxynucleoside N + Pi nucleoside N + ATP > NMP + ADP base X + deoxynucleoside Y < > base Y + deoxynucleoside X Adenine > hypoxanthine cytosine > uracil rnmp > rndp; dnmp > dndp rndp < > rntp; dndp < > dntp phosphoribosyl* transferase nucleoside phosphorylase nucleoside kinase nucleoside transglycosylases deaminases nucleoside** monophosphate kinase nucleoside diphosphate kinase Recall: *Have already seen phosphoribosyl transferase, part of the pyrimidine de novo pathway (step 5 addition of PRPP to orotic acid to make OMP) **Seen nucleoside monophosphate/diphosphate kinase as part of the de novo pathway. Phosphoribosyl Transferase In mammals, purines are salvaged, mostly, from two different enzymes: - Adenine Phosphoribosyltransferase (APRT) - Hypoxanthine-Guanine phosphoribosyltransferase (HGPRT) Has general formula: Base X + PRPP > rnmp + PPi Lesch-Nyhan disease results from genetic loss of HGPRT activity - symptoms: gout and mental retardation 16
17 Gehring Lecture 3 Jan 19 Nucleoside Phosphorylase & Nucleoside Transglycosylase Nuclosides may be degraded into the free base and ribose, or ribose-1-p through nucleosidases and nucleoside phosphrylase - nucleosidases break down the nucleosides into free base and ribose - nucleoside phosphorylase breaks down the nucleoside into free base and r-1-p Note that the breakdown of a nucleoside, as well as the formation of one will change the sugar from the alpha to beta anomer and vice versa - the nucleoside is in the beta anomer R-1-P or dr-1-p Ribonucleoside/ Deoxyribonucleoside The molecule may use the above mechanism, which is reversible, to remove and then reattach a new base, however this action is more efficiently performed by nucleoside transglycosylases. - this exchange of bases is only for deoxynucleosides - there is some specificity to avoid putting a thymine, for example, onto a ribose Other Salvage Pathway Enzymes Nucleoside kinase will convert nucleosides to nucleotides - uses ATP Deaminase interconvert bases by alterations - i.e Adenine > Hypoxanthine, or Cytosine > Uracil Mutases used to move around constituents on the same molecule 17
18 Gehring Lecture 3 Jan 19 Catabolism of Purines Overview The diagram below outlines the major pathways in purine catabolism, in which all paths lead to uric acid. Many of the intermediates are reused in salvage pathways. - Specifically R-1-P, which is a product of PNP-catalyzed reaction, can be isomerized by phosphoribomutase, to the PRPP precursor R-5-P The first step of purine degradation is the removal of extra-cyclic nitrogens with deaminases. SCID (Sever Combined Immunodeficiencies) are a set of genetic defects that can be caused by several sources - Mutations in adenosine deaminase (ADA) causing deficiency In the absence of active ADA, deoxyadenosine is phosphorylated to yield levels of datp that are 50-fold greater than normal. This high concentration of datp inhibits ribonucleotide reductase, thereby preventing the synthesis of the other dntps, choking off DNA synthesis and thus cell proliferation. Gout is the buildup of uric acid in joints. By blocking the degradation of xanthine to uric acid (via xanthine oxidase) one can then treat this disease. - High PRPP levels may indicate gout, due to it being feed-forward on the purine pathway 18
19 Gehring Lecture 3 Jan 19 Uric Acid This is the final degradation product of purines - birds use uric acid to eliminate nitrogen in their systems - uric acid is less soluble at low ph, which conserves water at the same time - fish release urea directly into the water Ammonia is the easiest way to get rid of nitrogen, however it is toxic, and therefore must be converted into other forms to be safely released. Catabolism of Pyrimidines Overview First step is deamidation, then reducing the ring. 19
20 Gehring Lecture 3 Jan 19 Kalle Gehring s Favourite Inhibitors Name Metabolite Antagonized Enzyme(s) Inhibited Notes Purine Synthesis & Degradation Azaserine, 6-diazo-5-oxo-Lnorleucine (DON) Glutamine Aminotransferase Uses in cancer therapy, however is cytotoxic 6-mercaptopurine, thioimp IMP purine nucleotide synthesis and interconversions Represses de novo purine biosynth, specifically IMP to GMP/AMP Converted to thioimp in cells Allopurinol hypoxanthine xanthine oxidase Used to treat gout Pyrimidine Synthesis Phosphoacetylasparta te (PALA) Glutamine Aminotransferase Uses in cancer therapy, however is cytotoxic 6-azauridine, azaump IMP purine nucleotide synthesis and interconversions Represses de novo purine biosynth, specifically IMP to GMP/AMP Leads to orotic aciduria by inhibiting decarboxylation of OMP Folate Synthesis Sulfonamides, dapsone p-aminobenzoate Folate Biosynthesis Amethopterin (methotraxate), Aminopterin, Trimethoprim Folate, dihydrofolate folate (dihydrofolate) reductase DNA Synthesis Hydroxyurea, RR, peptidomimetics enzyme free radical ribonucleotide reductase Hydroxyurea: quenches the RR tyrosine radical, has anticancer treatment uses and inhibits DNA synthesis 5-fluorouracin (FU), FU deoxynucleoside, dfump dump thymidylate synthetase AZT, dideoxycytidine dntp DNA polymerase, reverse transcriptase Blocks chain elongation by DNA pol 20
number Done by Corrected by Doctor
 number 34 Done by Abdulrahman Alhanbali Corrected by Mohammad Mahmoud Tarabeih Doctor Diala Nucleotide metabolism 1 P age In this lecture we will talk about nucleotides; their structures, the synthesis
number 34 Done by Abdulrahman Alhanbali Corrected by Mohammad Mahmoud Tarabeih Doctor Diala Nucleotide metabolism 1 P age In this lecture we will talk about nucleotides; their structures, the synthesis
number Done by Corrected by Doctor Diala
 number 34 Done by Abdulrahman Alhanbali Corrected by Mohammad Mahmoud Tarabeih Doctor Diala 1 P a g e Nucleotide metabolism In this lecture we will talk about nucleotides; their structures, the synthesis
number 34 Done by Abdulrahman Alhanbali Corrected by Mohammad Mahmoud Tarabeih Doctor Diala 1 P a g e Nucleotide metabolism In this lecture we will talk about nucleotides; their structures, the synthesis
Lipid Metabolism. Lipid Metabolism. Lipid Metabolism
 Chem 352 - Lecture 10 Lipid, Amino Acid, and Nucleotide Metabolism Part III: Nucleotide Metabolism 1 Lipid Metabolism 2-1 Chem 352, Lecture 10, Part I: Lipid Metabolism 2 Lipid Metabolism 2-2 Question:
Chem 352 - Lecture 10 Lipid, Amino Acid, and Nucleotide Metabolism Part III: Nucleotide Metabolism 1 Lipid Metabolism 2-1 Chem 352, Lecture 10, Part I: Lipid Metabolism 2 Lipid Metabolism 2-2 Question:
Biosynthesis of nucleotides
 Biosynthesis of nucleotides De novo pathways De novo purine nucleotide synthesis Regulation of purine biosynthesis De novo pyrimidine nucleotides biosynthesis Salvage pathways Lesch-Nyhan syndrome Why
Biosynthesis of nucleotides De novo pathways De novo purine nucleotide synthesis Regulation of purine biosynthesis De novo pyrimidine nucleotides biosynthesis Salvage pathways Lesch-Nyhan syndrome Why
Nucleotide Metabolism Biochemistry by Lippincott pp
 Nucleotide Metabolism Biochemistry by Lippincott pp 291-306 Metabolism: CONCEPT Ø Metabolism is the totality of an organism s chemical reactions. Ø A metabolic pathway begins with a specific molecule and
Nucleotide Metabolism Biochemistry by Lippincott pp 291-306 Metabolism: CONCEPT Ø Metabolism is the totality of an organism s chemical reactions. Ø A metabolic pathway begins with a specific molecule and
I. NUCLEOTIDES. Nitrogenous bases are frequently represented by single letter abbreviations. The abbreviations for several bases are shown below:
 Biochemistry II Nucleotide Metabolism I. NUCLEOTIDES Nitrogenous bases are frequently represented by single letter abbreviations. The abbreviations for several bases are shown below: Common Nitrogenous
Biochemistry II Nucleotide Metabolism I. NUCLEOTIDES Nitrogenous bases are frequently represented by single letter abbreviations. The abbreviations for several bases are shown below: Common Nitrogenous
Nucleotide Metabolism
 Nucleotide Metabolism Nucleotide Synthesis De Novo Pathway Synthesize purine and pyrimidine nucleotides from low M.W. precursors Salvage Pathway synthesize nucleotides from nucleosides of nucleobases NB:
Nucleotide Metabolism Nucleotide Synthesis De Novo Pathway Synthesize purine and pyrimidine nucleotides from low M.W. precursors Salvage Pathway synthesize nucleotides from nucleosides of nucleobases NB:
Biochem for the Boards 2nd Edition
 Biochem for the Boards! Biochem for the Boards 2nd Edition Active Learning Workbook for the BDE 1, DDS, MA Bonus Chapter ucleotide Metabolism Socrates Publishing Co. ew York, Y Page 1 ucleotide Metabolism
Biochem for the Boards! Biochem for the Boards 2nd Edition Active Learning Workbook for the BDE 1, DDS, MA Bonus Chapter ucleotide Metabolism Socrates Publishing Co. ew York, Y Page 1 ucleotide Metabolism
GENERAL NUCLEIC ACID BIOCHEMISTRY 461, FALL A two (2) unit course for non-biochemistry majors only.
 GENERAL NUCLEIC ACID BIOCHEMISTRY 461, FALL 2010 A two (2) unit course for non-biochemistry majors only. PREREQUISITES: Biology 181 Organic Chemistry (Chem 241a,b) Concurrent/previous registration in Biochemistry
GENERAL NUCLEIC ACID BIOCHEMISTRY 461, FALL 2010 A two (2) unit course for non-biochemistry majors only. PREREQUISITES: Biology 181 Organic Chemistry (Chem 241a,b) Concurrent/previous registration in Biochemistry
Student Name Biochem. 461 Exam 1 Key, September 23, 2010
 Student Name Biochem. 461 Exam 1 Key, September 23, 2010 (100 POINTS TOTAL) ANSWER ALL QUESTIONS IN THE SPACES PROVIDED [10 pts] We will learn in Chapter 28 that ribonuclosides and deoxyribonucleosides
Student Name Biochem. 461 Exam 1 Key, September 23, 2010 (100 POINTS TOTAL) ANSWER ALL QUESTIONS IN THE SPACES PROVIDED [10 pts] We will learn in Chapter 28 that ribonuclosides and deoxyribonucleosides
Nucleotide Metabolism
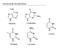 Nucleotide Metabolism Adenine Guanidine Uracil Thymine Cytosine Last Week Nucleotide Metabolism - Mostly, we think of nucleotides as being part of RNA/DNA, but now that we ve studied metabolism, we know
Nucleotide Metabolism Adenine Guanidine Uracil Thymine Cytosine Last Week Nucleotide Metabolism - Mostly, we think of nucleotides as being part of RNA/DNA, but now that we ve studied metabolism, we know
Password: LECTURE 7: NUCLEOTIDE METABOLISM
 http://smtom.lecture.ub.ac.id/ Password: LECTURE 7: NUCLEOTIDE METABOLISM 51 All humans are 99,7% identical at DNA sequence level, and yet all of us carry a significant number of glitches in our genomes.
http://smtom.lecture.ub.ac.id/ Password: LECTURE 7: NUCLEOTIDE METABOLISM 51 All humans are 99,7% identical at DNA sequence level, and yet all of us carry a significant number of glitches in our genomes.
Nucleotide Metabolism December 12-17, 2008 Dr. Robert Lyons. See: for supplementary course materials.
 1 2 ucleotide Metabolism December 12-17, 2008 Dr. Robert Lyons See: http://seqcore.brcf.med.umich.edu/mcb500 for supplementary course materials. Medical relevance of nucleotide pathways Critical in cancer
1 2 ucleotide Metabolism December 12-17, 2008 Dr. Robert Lyons See: http://seqcore.brcf.med.umich.edu/mcb500 for supplementary course materials. Medical relevance of nucleotide pathways Critical in cancer
Biomolecules: nucleotides
 Biomolecules: nucleotides Nucleotides and nucleic acids Nucleic acids are linear hetero-polymers adapted to maintain and transmit the genetic information Depending on their sugar moiety they can be classified
Biomolecules: nucleotides Nucleotides and nucleic acids Nucleic acids are linear hetero-polymers adapted to maintain and transmit the genetic information Depending on their sugar moiety they can be classified
36. The double bonds in naturally-occuring fatty acids are usually isomers. A. cis B. trans C. both cis and trans D. D- E. L-
 36. The double bonds in naturally-occuring fatty acids are usually isomers. A. cis B. trans C. both cis and trans D. D- E. L- 37. The essential fatty acids are A. palmitic acid B. linoleic acid C. linolenic
36. The double bonds in naturally-occuring fatty acids are usually isomers. A. cis B. trans C. both cis and trans D. D- E. L- 37. The essential fatty acids are A. palmitic acid B. linoleic acid C. linolenic
Unless otherwise noted, the content of this course material is licensed under a Creative Commons Attribution Share Alike 3.0 License.
 Unless otherwise noted, the content of this course material is licensed under a Creative Commons Attribution Share Alike 3.0 License. Copyright 2007, Robert Lyons. The following information is intended
Unless otherwise noted, the content of this course material is licensed under a Creative Commons Attribution Share Alike 3.0 License. Copyright 2007, Robert Lyons. The following information is intended
Nucleotide Metabolism
 Nucleotide Metabolism Dr. Chalermchai Mitrpant Outline Content Page Function of nucleotide 2 Nucleotide synthesis 3-6 Purine synthesis and its regulation 3-4 Pyrimidine synthesis and its regulation 5-6
Nucleotide Metabolism Dr. Chalermchai Mitrpant Outline Content Page Function of nucleotide 2 Nucleotide synthesis 3-6 Purine synthesis and its regulation 3-4 Pyrimidine synthesis and its regulation 5-6
Nucleic Acids. Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology 1
 Nucleic Acids Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology 1 Topics : 2 hrs - Nucleic acid ----------------------------- Nucleic acid structure
Nucleic Acids Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology 1 Topics : 2 hrs - Nucleic acid ----------------------------- Nucleic acid structure
Paper 4: Biomolecules and Their Interactions Module 12: Bases, Sugars, Nucleosides and Nucleotides Introduction Nucleic acids are involved in storage
 Paper 4: Biomolecules and Their Interactions Module 12: Bases, Sugars, Nucleosides and Nucleotides Introduction Nucleic acids are involved in storage and transfer of genetic information. The double helical
Paper 4: Biomolecules and Their Interactions Module 12: Bases, Sugars, Nucleosides and Nucleotides Introduction Nucleic acids are involved in storage and transfer of genetic information. The double helical
Structure and Function of Nucleic Acids
 Structure and Function of Nucleic Acids E T Nyahangare Class Assignment 1. Write notes and outline the role of the following in protein biosynthesis a. DNA replication b. Transcription c. Genetic code
Structure and Function of Nucleic Acids E T Nyahangare Class Assignment 1. Write notes and outline the role of the following in protein biosynthesis a. DNA replication b. Transcription c. Genetic code
Enzymes, ATP and Bioenergetics
 Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College Enzymes, ATP and Bioenergetics Bioenergetics Bioenergetics can be defined as energy transfer mechanisms occurring within living organisms.
Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College Enzymes, ATP and Bioenergetics Bioenergetics Bioenergetics can be defined as energy transfer mechanisms occurring within living organisms.
Biosynthesis. Amino acids, N-containing molecules Nucleotides
 Biosynthesis Amino acids, N-containing molecules Nucleotides 1 Modified Fig 22-9 p. 827 Biosynthesis of A.A. Glucose All C derived from intermediates in Glycolysis 3-Phosphoglycerate (3PG) Phosphoenolpyruvate
Biosynthesis Amino acids, N-containing molecules Nucleotides 1 Modified Fig 22-9 p. 827 Biosynthesis of A.A. Glucose All C derived from intermediates in Glycolysis 3-Phosphoglycerate (3PG) Phosphoenolpyruvate
For more information about how to cite these materials visit
 Author(s): Dr. Robert Lyons, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http:// creativecommons.org/licenses/by-sa/3.0/
Author(s): Dr. Robert Lyons, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http:// creativecommons.org/licenses/by-sa/3.0/
Chemistry of Heterocyclic Compounds
 Chemistry of Heterocyclic Compounds Porphyrins Purines and pyrimidines Nucleosides and nucleotides Introduction to Heterocyclic Compounds Cyclic compounds with one or more other elements along with carbon
Chemistry of Heterocyclic Compounds Porphyrins Purines and pyrimidines Nucleosides and nucleotides Introduction to Heterocyclic Compounds Cyclic compounds with one or more other elements along with carbon
Biochemistry 694:301 Final Exam, Deis
 Biochemistry 694:301 Final Exam, Deis Mon., May 8, 2006 Name Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth two points. Answer the
Biochemistry 694:301 Final Exam, Deis Mon., May 8, 2006 Name Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth two points. Answer the
Biochemistry Prof. S. Dasgupta Department of Chemistry. Indian Institute of Technology Kharagpur. Lecture - 16 Nucleic Acids - I
 Biochemistry Prof. S. Dasgupta Department of Chemistry. Indian Institute of Technology Kharagpur Lecture - 16 Nucleic Acids - I We start our discussion on Nucleic Acids and their components. Before we
Biochemistry Prof. S. Dasgupta Department of Chemistry. Indian Institute of Technology Kharagpur Lecture - 16 Nucleic Acids - I We start our discussion on Nucleic Acids and their components. Before we
Paper : 03 Structure and Function of Biomolecules II Module: 02 Nucleosides, Nucleotides and type of Nucleic Acids
 Paper : 03 Structure and Function of Biomolecules II Module: 02 Nucleosides, Nucleotides and type of Nucleic Acids Principal Investigator Paper Coordinators Prof. Sunil Kumar Khare, Professor, Department
Paper : 03 Structure and Function of Biomolecules II Module: 02 Nucleosides, Nucleotides and type of Nucleic Acids Principal Investigator Paper Coordinators Prof. Sunil Kumar Khare, Professor, Department
* What are the nucleic acids?
 Nucleic Acids This lecture is meant to be a refreshment, it s a brief introduction to nucleic acids since we ll be given a full course about it next year in molecular biology. One last lecture and you
Nucleic Acids This lecture is meant to be a refreshment, it s a brief introduction to nucleic acids since we ll be given a full course about it next year in molecular biology. One last lecture and you
A. Incorrect! A sugar residue is only part of a nucleotide. Go back and review the structure of nucleotides.
 Organic Chemistry - Problem Drill 24: ucleic Acids o. 1 of 10 1. What are the components of a nucleotide? (A) A sugar residue (B) A sugar residue + a nitrogenous base (C) A sugar residue + a nitrogenous
Organic Chemistry - Problem Drill 24: ucleic Acids o. 1 of 10 1. What are the components of a nucleotide? (A) A sugar residue (B) A sugar residue + a nitrogenous base (C) A sugar residue + a nitrogenous
CHAPTER 8 Nucleotides and Nucleic Acids
 CHAPTER 8 Nucleotides and Nucleic Acids Key topics Biological function of nucleotides and nucleic acids Structures of common nucleotides Structure of double-stranded DNA Structures of ribonucleic acids
CHAPTER 8 Nucleotides and Nucleic Acids Key topics Biological function of nucleotides and nucleic acids Structures of common nucleotides Structure of double-stranded DNA Structures of ribonucleic acids
Part IV => DNA and RNA. 4.1 Nucleotide Properties 4.1a Nucleotide Nomenclature 4.1b DNA Sequencing
 Part IV => DNA and RNA 4.1 Nucleotide Properties 4.1a Nucleotide Nomenclature 4.1b DNA Sequencing The Molecular Dogma of Life DNA carries genetic information in the form of its sequence of nucleotides
Part IV => DNA and RNA 4.1 Nucleotide Properties 4.1a Nucleotide Nomenclature 4.1b DNA Sequencing The Molecular Dogma of Life DNA carries genetic information in the form of its sequence of nucleotides
Chemistry of Heterocyclic Compounds. Porphyrins Purines and pyrimidines Nucleosides and nucleotides
 Chemistry of Heterocyclic Compounds Porphyrins Purines and pyrimidines Nucleosides and nucleotides Introduction to Heterocyclic Compounds Cyclic compounds with one or more other elements along with carbon
Chemistry of Heterocyclic Compounds Porphyrins Purines and pyrimidines Nucleosides and nucleotides Introduction to Heterocyclic Compounds Cyclic compounds with one or more other elements along with carbon
Chapter 5: Microbial Metabolism (Part I)
 Chapter 5: Microbial Metabolism (Part I) Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism (Part I) Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living organism. These chemical reactions are generally of two types: Catabolic:
BIO 311C Spring Lecture 16 Monday 1 March
 BIO 311C Spring 2010 Lecture 16 Monday 1 March Review Primary Structure of a portion of a polypeptide chain backbone of Polypeptide chain R-groups of amino acids Native conformation of a dimeric protein,
BIO 311C Spring 2010 Lecture 16 Monday 1 March Review Primary Structure of a portion of a polypeptide chain backbone of Polypeptide chain R-groups of amino acids Native conformation of a dimeric protein,
NUCLEIC ACID METABOLISM. Omidiwura, B.R.O
 NUCLEIC ACID METABOLISM Omidiwura, B.R.O Nucleic Acids Nucleic acids are molecules that store information for cellular growth and reproduction There are two types of nucleic acids: - deoxyribonucleic acid
NUCLEIC ACID METABOLISM Omidiwura, B.R.O Nucleic Acids Nucleic acids are molecules that store information for cellular growth and reproduction There are two types of nucleic acids: - deoxyribonucleic acid
Chapter 24: Carbohydrates and Nucleic Acids
 Chem 225 otes Page 138 I. Introduction Chapter 24: Carbohydrates and ucleic Acids A. Definitions and aming Conventions Carbohydrate: a sugar. A polyhydroxy aldehyde or ketone with the molecular formula
Chem 225 otes Page 138 I. Introduction Chapter 24: Carbohydrates and ucleic Acids A. Definitions and aming Conventions Carbohydrate: a sugar. A polyhydroxy aldehyde or ketone with the molecular formula
Nucleic acids deoxyribonucleic acid (DNA) ribonucleic acid (RNA) nucleotide
 Nucleic Acids Nucleic acids are molecules that store information for cellular growth and reproduction There are two types of nucleic acids: - deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) These
Nucleic Acids Nucleic acids are molecules that store information for cellular growth and reproduction There are two types of nucleic acids: - deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) These
Enzymes Part III: regulation I. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation I Dr. Mamoun Ahram Summer, 2017 Mechanisms of regulation Expression of isoenzymes Regulation of enzymatic activity Inhibitors Conformational changes Allostery Modulators Reversible
Enzymes Part III: regulation I Dr. Mamoun Ahram Summer, 2017 Mechanisms of regulation Expression of isoenzymes Regulation of enzymatic activity Inhibitors Conformational changes Allostery Modulators Reversible
The nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
 Nucleic acids are macromolecules composed of chains of mononucleotides joined by phosphodiester bonds. The nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are universal
Nucleic acids are macromolecules composed of chains of mononucleotides joined by phosphodiester bonds. The nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are universal
Nucleic Acids. By Sarah, Zach, Joanne, and Dean
 Nucleic Acids By Sarah, Zach, Joanne, and Dean Basic Functions Carry genetic information (DNA storing it) Protein synthesis Helps in cell division (DNA replicates itself) RNA- numerous functions during
Nucleic Acids By Sarah, Zach, Joanne, and Dean Basic Functions Carry genetic information (DNA storing it) Protein synthesis Helps in cell division (DNA replicates itself) RNA- numerous functions during
Lecture 8. Chromosome. The Nuclei. Two Types of Nucleic Acids. Genes. Information Contained Within Each Cell
 Information Contained Within Each Cell Lecture 8 Nucleic Acids and Protein Synthesis Chapter 23: Section 1-5 Most higher organisms reproduce sexually! Sperm cell + Egg cell! Fertilized egg The wondrous
Information Contained Within Each Cell Lecture 8 Nucleic Acids and Protein Synthesis Chapter 23: Section 1-5 Most higher organisms reproduce sexually! Sperm cell + Egg cell! Fertilized egg The wondrous
Syllabus for GUTS Lecture on DNA and Nucleotides
 Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
DNA and RNA are both made of nucleotides. Proteins are made of amino acids. Transcription can be reversed but translation cannot.
 INFORMATION TRANSFER Information in cells Properties of information Information must be able to be stored, accessed, retrieved, transferred, read and used. Information is about order, it is basically the
INFORMATION TRANSFER Information in cells Properties of information Information must be able to be stored, accessed, retrieved, transferred, read and used. Information is about order, it is basically the
Super Models. Nucleic Acid Bases (nucleobases) Molecular Model Kit
 Super Models ucleic Acid Bases (nucleobases) Molecular Model Kit Copyright 2015 Ryler Enterprises, Inc. Recommended for ages 10 - adult! Caution: Atom centers and vinyl tubing are a choking hazard. Do
Super Models ucleic Acid Bases (nucleobases) Molecular Model Kit Copyright 2015 Ryler Enterprises, Inc. Recommended for ages 10 - adult! Caution: Atom centers and vinyl tubing are a choking hazard. Do
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA
 RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
Chapter 3 Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Nucleic acids -1 Purines
 Nucleic acids -1 Purines Purine metabolism Purine Biosynthesis: 5-Aminoimidazole ribonucleotide is carboxylated and this is followed by the addition of an aspartic acid to form 5-Aminoimidazole-
Nucleic acids -1 Purines Purine metabolism Purine Biosynthesis: 5-Aminoimidazole ribonucleotide is carboxylated and this is followed by the addition of an aspartic acid to form 5-Aminoimidazole-
8 Nucleotides and Nucleic Acids W. H. Freeman and Company
 8 Nucleotides and Nucleic Acids 2013 W. H. Freeman and Company 1 Week 8 Nucleotides and Nucleic Acids 8.1 Some Basics 8.2 Nucleic Acid Structure 8.3 Nucleic Acid Chemistry 8.4 Other Functions of Nucleotides
8 Nucleotides and Nucleic Acids 2013 W. H. Freeman and Company 1 Week 8 Nucleotides and Nucleic Acids 8.1 Some Basics 8.2 Nucleic Acid Structure 8.3 Nucleic Acid Chemistry 8.4 Other Functions of Nucleotides
PETER PAZMANY CATHOLIC UNIVERSITY Consortium members SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
 SEMMELWEIS UIVERSITY PETER PAZMAY ATLI UIVERSITY Development of omplex urricula for Molecular Bionics and Infobionics Programs within a consortial* framework** onsortium leader PETER PAZMAY ATLI UIVERSITY
SEMMELWEIS UIVERSITY PETER PAZMAY ATLI UIVERSITY Development of omplex urricula for Molecular Bionics and Infobionics Programs within a consortial* framework** onsortium leader PETER PAZMAY ATLI UIVERSITY
Metabolism BIOL 3702: Chapter 10
 Metabolism BIOL 3702: Chapter 10 Introduction to Metabolism u Metabolism is the sum total of all the chemical reactions occurring in a cell u Two major parts of metabolism: v Catabolism Ø Large, more complex
Metabolism BIOL 3702: Chapter 10 Introduction to Metabolism u Metabolism is the sum total of all the chemical reactions occurring in a cell u Two major parts of metabolism: v Catabolism Ø Large, more complex
CHAPTER 22: Nucleic Acids & Protein Synthesis. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 22: Nucleic Acids & Protein Synthesis General, rganic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 22: Nucleic Acids & Protein Synthesis Learning bjectives: q Nucleosides & Nucleo@des:
CHAPTER 22: Nucleic Acids & Protein Synthesis General, rganic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 22: Nucleic Acids & Protein Synthesis Learning bjectives: q Nucleosides & Nucleo@des:
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA
 AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
1.5 Nucleic Acids and Their Functions Page 1 S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.5 Nucleic Acids and their functions Page 1 From the syllabus: 1.5 Nucleic Acids and Their Functions Page 1 S. Preston 1 l. Nucleic
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.5 Nucleic Acids and their functions Page 1 From the syllabus: 1.5 Nucleic Acids and Their Functions Page 1 S. Preston 1 l. Nucleic
Chemistry of Heterocyclic Compounds. Porphyrins Purines and pyrimidines Nucleosides and nucleotides
 Chemistry of Heterocyclic Compounds Porphyrins Purines and pyrimidines Nucleosides and nucleotides Introduction to Heterocyclic Compounds. Cyclic compounds with one or more other elements along with carbon
Chemistry of Heterocyclic Compounds Porphyrins Purines and pyrimidines Nucleosides and nucleotides Introduction to Heterocyclic Compounds. Cyclic compounds with one or more other elements along with carbon
Feedback D. Incorrect! No, although this is a correct characteristic of RNA, this is not the best response to the questions.
 Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
DNA and RNA Structure Guided Notes
 Nucleic acids, especially DNA, are considered as the key biomolecules that guarantee the continuity of life. DNA is the prime genetic molecule which carry all the hereditary information that's passed from
Nucleic acids, especially DNA, are considered as the key biomolecules that guarantee the continuity of life. DNA is the prime genetic molecule which carry all the hereditary information that's passed from
Nucleic Acid Structure:
 Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
DNA and RNA Structure. Unit 7 Lesson 1
 Unit 7 Lesson 1 Students will be able to: Explain the structure and function of the DNA and RNA. Illustrate the structure of nucleotide. Summarize the differences between DNA and RNA. Identify the different
Unit 7 Lesson 1 Students will be able to: Explain the structure and function of the DNA and RNA. Illustrate the structure of nucleotide. Summarize the differences between DNA and RNA. Identify the different
The discovery of the role of RNA RNA structure, synthesis and function
 Central Dogma The discovery of the role of RNA RNA structure, synthesis and function! Fundamental observations in genetics!! Genes are located in nuclei (in eukaryotes)!! Polypeptides are synthesised in
Central Dogma The discovery of the role of RNA RNA structure, synthesis and function! Fundamental observations in genetics!! Genes are located in nuclei (in eukaryotes)!! Polypeptides are synthesised in
Chapter 17 Nucleic Acids and Protein Synthesis
 Chapter 17 Nucleic Acids and Protein Synthesis Nucleic Acids Nucleic acids are the components that make up the genetic material DNA (deoxyribonucleic acid). DNA is a macromolecule which contains all the
Chapter 17 Nucleic Acids and Protein Synthesis Nucleic Acids Nucleic acids are the components that make up the genetic material DNA (deoxyribonucleic acid). DNA is a macromolecule which contains all the
Biochemistry study of the molecular basis of life
 Biochemistry : An Introduction Biochemistry study of the molecular basis of life n Study of the chemistry of living organisms Studies organic molecules & organic reactions in living organisms n Living
Biochemistry : An Introduction Biochemistry study of the molecular basis of life n Study of the chemistry of living organisms Studies organic molecules & organic reactions in living organisms n Living
Lecture Overview. Overview of the Genetic Information. Marieb s Human Anatomy and Physiology. Chapter 3 DNA & RNA Protein Synthesis Lecture 6
 Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 3 DNA & RNA Protein Synthesis Lecture 6 Lecture Overview The Genetic Information Structure of DNA/RNA DNA Replication Overview of protein synthesis
Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 3 DNA & RNA Protein Synthesis Lecture 6 Lecture Overview The Genetic Information Structure of DNA/RNA DNA Replication Overview of protein synthesis
Take-Home Quiz II. Spring 2005 Semester
 Take-Home Quiz II General Instructions and Information: Obtain an answer sheet from the instructor and legibly write your name in the appropriate space. After placing your name and quiz version on the
Take-Home Quiz II General Instructions and Information: Obtain an answer sheet from the instructor and legibly write your name in the appropriate space. After placing your name and quiz version on the
BIOLOGY 311C - Brand Spring 2008
 BIOLOGY 311C - Brand Spring 2008 NAME (printed very legibly) Key UT-EID EXAMINATION 3 Before beginning, check to be sure that this exam contains 7 pages (including front and back) numbered consecutively,
BIOLOGY 311C - Brand Spring 2008 NAME (printed very legibly) Key UT-EID EXAMINATION 3 Before beginning, check to be sure that this exam contains 7 pages (including front and back) numbered consecutively,
Transcription. The sugar molecule found in RNA is ribose, rather than the deoxyribose found in DNA.
 Transcription RNA (ribonucleic acid) is a key intermediary between a DNA sequence and a polypeptide. RNA is an informational polynucleotide similar to DNA, but it differs from DNA in three ways: RNA generally
Transcription RNA (ribonucleic acid) is a key intermediary between a DNA sequence and a polypeptide. RNA is an informational polynucleotide similar to DNA, but it differs from DNA in three ways: RNA generally
}Nucleosides NUCLEIC ACIDS. Nucleic acids are polymers Monomer---nucleotides Nitrogenous bases Purines Pyrimidines Sugar Ribose Deoxyribose
 DNA STRUCTURE NUCLEIC ACIDS Nucleic acids are polymers Monomer---nucleotides Nitrogenous bases Purines Pyrimidines Sugar Ribose Deoxyribose Phosphates +nucleoside=nucleotide }Nucleosides The Sugars The
DNA STRUCTURE NUCLEIC ACIDS Nucleic acids are polymers Monomer---nucleotides Nitrogenous bases Purines Pyrimidines Sugar Ribose Deoxyribose Phosphates +nucleoside=nucleotide }Nucleosides The Sugars The
Bio 366: Biological Chemistry II Test #3, 100 points
 Bio 366: Biological Chemistry II Test #3, 100 points READ THIS: Take a numbered test and sit in the seat with that number on it. Remove the numbered sticker from the desk, and stick it on the back of the
Bio 366: Biological Chemistry II Test #3, 100 points READ THIS: Take a numbered test and sit in the seat with that number on it. Remove the numbered sticker from the desk, and stick it on the back of the
Chapter 4: How Cells Work
 Chapter 4: How Cells Work David Shonnard Department of Chemical Engineering 1 Presentation Outline: l l l l l Introduction : Central Dogma DNA Replication: Preserving and Propagating DNA Transcription:
Chapter 4: How Cells Work David Shonnard Department of Chemical Engineering 1 Presentation Outline: l l l l l Introduction : Central Dogma DNA Replication: Preserving and Propagating DNA Transcription:
Chapter 4: How Cells Work
 Chapter 4: How Cells Work David Shonnard Department of Chemical Engineering 1 Presentation Outline: Introduction : Central Dogma DNA Replication: Preserving and Propagating DNA Transcription: Sending the
Chapter 4: How Cells Work David Shonnard Department of Chemical Engineering 1 Presentation Outline: Introduction : Central Dogma DNA Replication: Preserving and Propagating DNA Transcription: Sending the
DNA, RNA, Replication and Transcription
 Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College DNA, RNA, Replication and Transcription The metabolic processes described earlier (glycolysis, cellular respiration, photophosphorylation,
Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College DNA, RNA, Replication and Transcription The metabolic processes described earlier (glycolysis, cellular respiration, photophosphorylation,
CHAPTER 31 NUCLEIC ACIDS AND HEREDITY SOLUTIONS TO REVIEW QUESTIONS. ƒ H. Guanine H O. Uracil
 CAPTER 31 UCLEIC ACIDS AD EREDITY SLUTIS T REVIEW QUESTIS 1. The five nitrogen bases found in nucleotides: 3 C 2 Adenine Thymine Guanine Uracil 2 2 Cytosine 2. A nucleoside is a purine or pyrimidine base
CAPTER 31 UCLEIC ACIDS AD EREDITY SLUTIS T REVIEW QUESTIS 1. The five nitrogen bases found in nucleotides: 3 C 2 Adenine Thymine Guanine Uracil 2 2 Cytosine 2. A nucleoside is a purine or pyrimidine base
Dina Al-Tamimi. Faisal Nimri. Ma amoun Ahram. 1 P a g e
 1 Dina Al-Tamimi Faisal Nimri Ma amoun Ahram 1 P a g e **Difference between Molecular Biology and Genetics: Molecular Biology: is a fancy term of biochemistry. It is the science that deals with DNA, RNA
1 Dina Al-Tamimi Faisal Nimri Ma amoun Ahram 1 P a g e **Difference between Molecular Biology and Genetics: Molecular Biology: is a fancy term of biochemistry. It is the science that deals with DNA, RNA
Unit II Problem 3 Genetics: Summary of Basic Concepts in Molecular Biology
 Unit II Problem 3 Genetics: Summary of Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated
Unit II Problem 3 Genetics: Summary of Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated
DNA Replication AP Biology
 DNA Replication 2007-2008 Watson and Crick 1953 1953 article in Nature Directionality of DNA You need to number the carbons! u it matters! u 3 refers to the 3 carbon on the sugar u 5 refers to the 5 carbon
DNA Replication 2007-2008 Watson and Crick 1953 1953 article in Nature Directionality of DNA You need to number the carbons! u it matters! u 3 refers to the 3 carbon on the sugar u 5 refers to the 5 carbon
DNA Replication AP Biology
 DNA Replication 2007-2008 Double helix structure of DNA It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material.
DNA Replication 2007-2008 Double helix structure of DNA It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material.
Proofreading, post-replication modification of DNA. Mitesh Shrestha
 Proofreading, post-replication modification of DNA Mitesh Shrestha Proofreading During DNA replication (copying), most DNA polymerases can check their work with each base that they add. This process is
Proofreading, post-replication modification of DNA Mitesh Shrestha Proofreading During DNA replication (copying), most DNA polymerases can check their work with each base that they add. This process is
Microbial Metabolism. PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 5 Microbial Metabolism Big Picture: Metabolism Metabolism is the buildup and breakdown of nutrients
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 5 Microbial Metabolism Big Picture: Metabolism Metabolism is the buildup and breakdown of nutrients
Metabolism. BIOL 3702: Chapter 10. Introduction to Metabolism. Energy and Work. BIOL 3702: Chapter 10 AY Dr. Cooper 1. Metabolism (cont.
 Metabolism BIOL 3702: Chapter 10 Introduction to Metabolism u Metabolism is the sum total of all the chemical reactions occurring in a cell u Two major parts of metabolism: v Catabolism Ø Large, more complex
Metabolism BIOL 3702: Chapter 10 Introduction to Metabolism u Metabolism is the sum total of all the chemical reactions occurring in a cell u Two major parts of metabolism: v Catabolism Ø Large, more complex
Rapid Learning Center Presents. Teach Yourself High School Biology in 24 Hours. and Functions
 Rapid Learning Center Chemistry :: Biology :: Physics :: Math Rapid Learning Center Presents Teach Yourself High School Biology in 24 Hours Gene e Structures and Functions High School Biology Rapid Learning
Rapid Learning Center Chemistry :: Biology :: Physics :: Math Rapid Learning Center Presents Teach Yourself High School Biology in 24 Hours Gene e Structures and Functions High School Biology Rapid Learning
DNA Replication AP Biology
 DNA Replication 2007-2008 Watson and Crick 1953 article in Nature Double helix structure of DNA It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible
DNA Replication 2007-2008 Watson and Crick 1953 article in Nature Double helix structure of DNA It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible
Please contact us if any parts are missing or damaged. DNA Backbone
 DNA Starter Kit Contents 1-Group Set Contents 12 Red Foam Adenine Bases (DNASK-R-A) 12 Blue Foam Cytosine Bases (DNASK-R-C) 12 Green Foam Guanine Bases (DNASK-R-G) 12 Yellow Foam Thymine Bases (DNASK-R-T)
DNA Starter Kit Contents 1-Group Set Contents 12 Red Foam Adenine Bases (DNASK-R-A) 12 Blue Foam Cytosine Bases (DNASK-R-C) 12 Green Foam Guanine Bases (DNASK-R-G) 12 Yellow Foam Thymine Bases (DNASK-R-T)
Nucleic Acids. The Ribose Sugar
 Nucleic cids adenosine triphosphate (TP) a mononucleotide Nucleic acids consist of: a ribose (aldopentose) sugar; a heteroaromatic, glycosidic base; a phosphoester. ribonucleic acid (RN) a polynucleotide
Nucleic cids adenosine triphosphate (TP) a mononucleotide Nucleic acids consist of: a ribose (aldopentose) sugar; a heteroaromatic, glycosidic base; a phosphoester. ribonucleic acid (RN) a polynucleotide
Control of Metabolic Processes
 Control of Metabolic Processes Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College As described earlier, the metabolic processes occurring within living organisms (glycolysis, respiration,
Control of Metabolic Processes Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College As described earlier, the metabolic processes occurring within living organisms (glycolysis, respiration,
Chapter 11. Nucleic Acids. Nucleotides. BCH 4053 Spring 2001 Chapter 11 Lecture Notes. Slide 1. Slide 2. Slide 3. Chapter 11, page 1
 BC 4053 Spring 2001 Chapter 11 Lecture otes 1 Chapter 11 ucleotides and ucleic Acids 2 ucleic Acids Two classes DA (Deoxyribonucleic Acid) RA (Ribonucleic Acid) Polymers of nucleotides DA carries genetic
BC 4053 Spring 2001 Chapter 11 Lecture otes 1 Chapter 11 ucleotides and ucleic Acids 2 ucleic Acids Two classes DA (Deoxyribonucleic Acid) RA (Ribonucleic Acid) Polymers of nucleotides DA carries genetic
Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Nucleic Acids. OpenStax College. 1 DNA and RNA
 OpenStax-CNX module: m44403 1 Nucleic Acids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be
OpenStax-CNX module: m44403 1 Nucleic Acids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be
Unit IX Problem 3 Genetics: Basic Concepts in Molecular Biology
 Unit IX Problem 3 Genetics: Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated to synthesize
Unit IX Problem 3 Genetics: Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated to synthesize
Double helix structure of DNA
 Replication Double helix structure of It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material. Watson & Crick
Replication Double helix structure of It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material. Watson & Crick
What is necessary for life?
 Life What is necessary for life? Most life familiar to us: Eukaryotes FREE LIVING Or Parasites First appeared ~ 1.5-2 10 9 years ago Requirements: DNA, proteins, lipids, carbohydrates, complex structure,
Life What is necessary for life? Most life familiar to us: Eukaryotes FREE LIVING Or Parasites First appeared ~ 1.5-2 10 9 years ago Requirements: DNA, proteins, lipids, carbohydrates, complex structure,
Chapter 10. Chapter 10 Nucleotides and Nucleic Acids. Nobel Prize Reginald H. Garrett Charles M. Grisham
 Reginald H. Garrett Charles M. Grisham Chapter 10 Nucleotides and Nucleic Acids Chapter 10 We have discovered the secret of life. Francis Crick, to patrons of The Eagle, a pub in Cambridge, England (1953)
Reginald H. Garrett Charles M. Grisham Chapter 10 Nucleotides and Nucleic Acids Chapter 10 We have discovered the secret of life. Francis Crick, to patrons of The Eagle, a pub in Cambridge, England (1953)
translation The building blocks of proteins are? amino acids nitrogen containing bases like A, G, T, C, and U Complementary base pairing links
 The actual process of assembling the proteins on the ribosome is called? translation The building blocks of proteins are? Complementary base pairing links Define and name the Purines amino acids nitrogen
The actual process of assembling the proteins on the ribosome is called? translation The building blocks of proteins are? Complementary base pairing links Define and name the Purines amino acids nitrogen
DNA and RNA: Structure and Function. 阮雪芬 May 14, 2004
 DNA and RNA: Structure and Function 阮雪芬 May 14, 2004 Two Fundamental types of nucleic acids participate as genetic molecules DNA: deoxyribonucleic acid Found in the chromosome form in the cell s nucleus
DNA and RNA: Structure and Function 阮雪芬 May 14, 2004 Two Fundamental types of nucleic acids participate as genetic molecules DNA: deoxyribonucleic acid Found in the chromosome form in the cell s nucleus
Resources. How to Use This Presentation. Chapter 10. Objectives. Table of Contents. Griffith s Discovery of Transformation. Griffith s Experiments
 How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
Hole s Essentials of Human Anatomy & Physiology
 Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Amended by
Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Amended by
Case 16 Allosteric Regulation of ATCase
 Case 16 Allosteric Regulation of ATCase Focus concept An enzyme involved in nucleotide synthesis is subject to regulation by a variety of combinations of nucleotides. rerequisites roperties of allosteric
Case 16 Allosteric Regulation of ATCase Focus concept An enzyme involved in nucleotide synthesis is subject to regulation by a variety of combinations of nucleotides. rerequisites roperties of allosteric
Enzymes. Most known enzymes are large globular proteins.
 Chapter 10 Enzymes Enzymes Most known enzymes are large globular proteins. There are 27,383 proteins in the human genome. 12,000 have unknown functions and 6,000 behave as enzymes. Enzymes are necessary
Chapter 10 Enzymes Enzymes Most known enzymes are large globular proteins. There are 27,383 proteins in the human genome. 12,000 have unknown functions and 6,000 behave as enzymes. Enzymes are necessary
What is necessary for life?
 Life What is necessary for life? Most life familiar to us: Eukaryotes FREE LIVING Or Parasites First appeared ~ 1.5-2 10 9 years ago Requirements: DNA, proteins, lipids, carbohydrates, complex structure,
Life What is necessary for life? Most life familiar to us: Eukaryotes FREE LIVING Or Parasites First appeared ~ 1.5-2 10 9 years ago Requirements: DNA, proteins, lipids, carbohydrates, complex structure,
Nucleic Acids and Proteins
 DNA Structure 7.1 Nucleic Acids and Proteins Topic 7 DNA is composed of subunits called nucleotides The four types of bases on nucleotides are: Adenine, Guanine, Cytosine, and Thyamine Purines: two ring
DNA Structure 7.1 Nucleic Acids and Proteins Topic 7 DNA is composed of subunits called nucleotides The four types of bases on nucleotides are: Adenine, Guanine, Cytosine, and Thyamine Purines: two ring
