Saccharomyces cerevisiae
|
|
|
- Holly Bruce
- 6 years ago
- Views:
Transcription
1 Review For reprint orders, please contact Proteomic analysis of Saccharomyces cerevisiae Trong Khoa Pham and Phillip C Wright CNTENTS Importance of proteomics in Saccharomyces cerevisiae Proteomics as a tool for the identification & quantitation of S. cerevisiae proteins Identification & quantitation of S. cerevisiae proteins in proteomics experiments Proteomics in the study of S. cerevisiae networks Proteomic analysis of S. cerevisiae protein modifications Expert commentary Five-year view Information resources Financial & competing interests disclosure Key issues References Affiliations Author for correspondence The University of Sheffield, Biological & Environmental Systems Group, Department of Chemical & Process Engineering, Mappin Street, Sheffield, S1 3JD, UK Tel.: Fax: p.c.wright@sheffield.ac.uk KEYWRDS: 2DE, ICAT, itraq, protein interactome, proteomic analysis, identification and quantitation, S. cerevisiae, SILAC Nowadays, proteomics is recognized as one of the fastest growing tools in many areas of research. This is especially true for the study of Saccharomyces cerevisiae, as it is considered to be a model organism for eukaryotic cells. Proteomic analysis provides an insight into global protein expressions from identification to quantitation, from localization to function, and from individual to network systems. Moreover, many methods for identification and quantitation of proteins based on tandem mass spectrometry workflows have recently been developed and widely applied in S. cerevisiae. The current methods and issues in the proteomic analysis of S. cerevisiae are reviewed here. Expert Rev. Proteomics 4(6), (2007) Importance of proteomics in Saccharomyces cerevisiae Proteomic analysis, which can be defined as the analysis of the protein complement expressed by a genome [1], has become increasingly attractive due to its potential to elucidate properties of biological systems that cannot be discovered using the DNA sequence or mrna expression alone [2]. In this respect, Saccharomyces cerevisiae is an important model organism for that purpose, because it has a well-understood physiology, a large number of mutant strains and the availability of a genome sequence with relatively good annotation [3]. The proteomic analysis of S. cerevisiae is the next step in the analysis of biological systems of this domain, after genomics and transcriptomics. The global analysis of proteins is now receiving significant attention over that of genes, since the expression of proteins provides a direct understanding of the function and regulation of cells in response to their environments. In comparison with gene expression analysis at the mrna level, proteomic expression analysis provides in-depth information on biological systems, and pathways, since the measurements concentrate on the actual biological effector molecules [4]. Moreover, genomics/ transcriptomics has some disadvantages, such as: Gene transcription levels only provide a rough estimate of their expression level in terms of proteins, as the mrna molecules may be degraded rapidly, or translated inefficiently, resulting in discordance with the protein abundance Some transcripts generate more than one protein via alternative post-translational modifications (PTMs) and alternative splicing Some proteins only reveal their functions after PTM Some proteins interact with other proteins and only demonstrate functionality in the presence of these additional molecules The relationship between DNA, RNA and protein levels are shown in FIGURE 1: DNA is known as an information storage level that instructs the later construction of RNA and proteins RNA plays a key role in translating the genetic information from DNA to proteins Proteins play important roles in cell activities from constructing cells to metabolic pathways and other networks Briefly, the main aims of proteomics in studying S. cerevisiae include (FIGURE 1): Identification of proteins and the comparison of protein expression changes in response to different states or environmental changes Characterization theof cellular functions of each protein(s) / Future Drugs Ltd ISSN
2 Pham & Wright Investigation of the interaction of proteins in regulatory networks to reconstruct some metabolic pathways or to establish the protein protein interaction networks This tool can be also used in the study of PTMs such as glycosylation, phosphorylation and lipid modification In terms of S. cerevisiae protein analysis, the most important initial issue is the identification and quantitation of proteins in different states or conditions. Therefore, these issues have been discussed in detail in this review. Proteomics as a tool for the identification & quantitation of S. cerevisiae proteins Since protein identification is also a key part of the quantitation procedure (particularly for shotgun proteomics), this review aims to focus on quantitative methods more than identification methods. To date, traditional quantitative proteomics has been predominantly performed by 2D electrophoresis (2DE). Recently, various techniques for protein quantitation by mass spectrometry (MS) have been developed, ranging from labelfree quantitation [5,6] to label-based quantitation [7 10]. Proteomic quantitation can be performed either in vivo (metabolic labeling) or in vitro (protein and peptide labeling). Basically, the label-based proteomic quantitation can be performed at three levels: cell, protein and peptide levels. The cell-labeling approach is usually thought of as metabolic labeling and includes metabolic labeling ( 15 N or 13 C) [7] and stable-isotope labeling of amino acids in culture (SILAC) [8]. The labeling approaches at the protein and peptide levels are mostly based on employing stable isotopic tags, such as trypsin digestion of Genetics Genomics Proteomics Interactomics Transcription Translation Identification and quantitation Proteins metabolites Proteins proteins Regulatory mechanisms DNA mrna Protein Function of each protein Figure 1. Relationship of DNA, RNA and protein in terms of omics. The main functions of proteomics in the study of Saccharomyces cerevisiae are also illustrated. protein in [ 18 ]-water [9], isotope-coded affinity tags (ICAT) [4] or itraq (FIGURE 2) [10]. Recently, the development of an isotope-coded protein-labeling (ICPL) technique by Schmidt et al. has contributed a new method for quantitative proteomic analysis by applying a label to free amino acid groups of proteins (lysine and N-terminus) [11]. However, this technique has not yet been applied in S. cerevisiae, although it has been applied for quantitative proteomic analysis of the membrane proteome in Halobacterium salinarum (a halophilic archaeon) [12]. The authors believe that this technique will be applied in S. cerevisiae soon, since compared with ICAT, an increased number of peptides contributing to protein quantitation have been observed, the protein samples were easy to label and nearly all downstream protein or peptide separation techniques are usable [12]. The majority of the proteomic quantitation work performed to date has been relative quantitation. However, absolute quantitation (AQUA) is clearly highly desirable for a deeper understanding and several emerging techniques are meeting with success in this regard (e.g., AQUA [13,14] and QConCat [15,16] workflows) (FIGURE 2). The advantages of the labeling techniques are the ability to simultaneously (and hopefully automatically) identify and quantify targets from complex protein mixtures [8]. With metabolic labeling, the labeling step is performed by incorporation of the isotopes during cell growth. Proteins in S. cerevisiae cells are labeled with a stable-isotope-labeled carbon or N source (e.g., 13 C-glucose, 15 N-ammonium sulfate) or via the SILAC method. In practice, cells are grown under different conditions containing either compounds with natural isotope abundance, or those containing the heavy isotope. The cells from different conditions are then mixed, and protein extraction performed (it is possible to do an extraction first, with the proteins being mixed after extraction, but this is more likely to create errors) (FIGURE 3). A traditional 1D- or 2D-gelbased workflow or shotgun proteomics workflow can then be followed. Peptides (usually) are then identified using MS. The relative abundance of a peptide is calculated based on the areas under the heavy and the light versions of the same peptide fragmented by MS. For in vivo labeling with 14 N and 15 N, for example, there is a mass shift in the resultant peptides from the two different (labeled and unlabeled) media observed Post-translational modification during MS analysis. The N-label can be found in both the backbone and sidechain N atoms, and thus the mass shift cannot (easily) be used to predict peptides from unknown sequences [17]. As a result, this method is not advantageous for highly complex samples such as cell lysates, because using heavy-isotope-containing compounds essentially doubles the 794 Expert Rev. Proteomics 4(6), (2007)
3 Saccharomyces cerevisiae proteomics 2D gels Shotgun Identification Proteomic analysis of S. cerevisiae Quantitation Based on gels Labeling technique Free label Absolute Gels comparision DIGE Cells Proteins Peptides Peak intensity of peptides Number of MS/MS spectra AQUA QConCAT 13 C/ 12 C 15 N/ 14 N SILAC ICAT itraq [ 18 ]-water Figure 2. Identification and quantitation methods that have been applied to the proteomic analysis of Saccharomyces cerevisiae. AQUA: Absolute quantitation; DIGE: Differential in-gel electrophoresis; ICAT: Isotope-coded affinity tags; SILAC: Stable-isotope labeling of amino acids in culture. complexity of the sample submitted to the mass spectrometer. Furthermore, the problem of increased complexity is also found for most chemical labels (except itraq and ExacTag ). A favorable alternative technique to metabolic labeling is the use of a post-growth labeling strategy. In this case, all cells are grown in normal media with no heavy isotope enrichment. In this case, prior to mixing, the cell/proteins or peptides are labeled with tags. A typical method for protein labeling is ICAT (TABLE 1) [4]. For peptide labeling, the labeling step is performed after the digestion step via a number of potential methods (e.g., itraq [10]) or during the digestion step using 18 -labeled water. Then, these peptides are fractionated and analyzed by MS/MS. ne issue that can be addressed here is the use of label-free proteomic methods for quantitation. For shotgun proteomic approaches, the analysis of complex samples can face two crucial issues: the level of randomness observed in data-dependent acquisitions of peptide ions; and the probability that peptide ions from more abundant proteins would be selected more frequently [6]. Moreover, the peptide hits and spectral counts are related to protein abundance [18,19], and proteins with higher abundance levels have a higher probability of being identified [6]. The application of stable-isotope labeling methods offers a good measurement of small changes between proteins. By accessing the analysis of the reproducibility and variability of spectra sampling for 600 proteins identified in each of nine experiments, Liu et al. suggested that the reproducibility of spectra sampling across experiments is good and the linear dynamic range exceeds that of stable-isotope labeling. However, there is a note that spectral sampling accurately reflects relatively abundant proteins, but is less accurate at measuring small differences between different samples [6]. The completed genome sequence of S. cerevisiae provides excellent information for performing biological assays on each protein encoded by this genome, allowing the analysis of hundreds or thousands of proteins using single protein chips or protein arrays [20]. These techniques have been applied for studies of functional proteomics [20 22], PTMs, protein protein interactions [23] and metabolite protein interactions [24] in S. cerevisiae. Among these applications, the investigations of protein protein interactions using protein chips or protein arrays have been widely used and are discussed briefly later in the section entitled Proteomics in the study of S. cerevisiae networks. Comparison of methods The 2DE approach is the classical technique for the identification and quantitation of proteins for proteomics applications. 2DE works by comparing both the positions and intensities of spots on a series of gels. Since the workflow is based on gel-to-gel comparisons, it may be affected by spot position and lead to problems in detecting the differences between gels [25]. To meet the requirements of data validity, numerous repeated gel experiments should be performed to ensure the statistical significance of results [26]. Furthermore, another problem of 2D polyacrylamide gel electrophoresis (PAGE) approaches is the limited dynamic range; however, this problem can be overcome by the application of fluorescent dyes. To improve the power of this technique, both technical solutions and analysis software platforms have been developed. ne of the solutions for the technical aspects is the application of 795
4 Pham & Wright Metabolic labeling Protein labeling Peptide labeling Cells in sample 1 Cells in sample 2 Cells in sample 1 Cells in sample 2 Cells in sample 1 Cells in sample 2 In vivo labeling In vivo labeling Protein extraction Protein extraction Protein extraction Protein extraction Protein extraction Protein extraction Labeling Labeling Digestion Digestion Mixed proteins Mixed proteins Labeling Labeling Digestion Digestion Mixed peptides SCX SCX SCX MS/MS analysis Figure 3. verview of the workflows involved in the various labeling methods. Note that the pooling of the two samples in the metabolic labeling approach can be performed before protein extraction. SCX: Strong cation exchange. differential in-gel electrophoresis (DIGE), an approach that enables the comparison of two samples in the same gel [27]. Briefly, proteins in two samples are labeled with one of two fluorescent dyes and then mixed with a third labeled mixture of two samples as an internal calibration [27]. After separation on 2DE gels, proteins are detected by different lasers and then images are overlapped to reveal changes in protein abundance. The most significant advantage of this method is increased ease of comparison, since the samples are run together, resulting in the elimination of gel-to-gel variation [25]. To overcome problems inherent in the 2DE workflow, gel-free (or shotgun) proteomics methods based on orthogonal liquid chromatography (LC) have been developed (for example see [28]), thereby enabling the potential for quantitation on the mass spectrometer. The ICAT shotgun proteomics quantitation technique sought to address the problem inherent in MS, in which the detection is based on ionization of peptides [4]. Since ionization efficiency is affected by a number of factors, the peak intensities of the same peptides may be difficult to compare (and thus use for quantitation) across samples. To overcome this, in ICAT, a covalent modification of cysteine residues in the peptide pools derived from the samples of interest with light- and heavy-isotope labels means that these peptides can then be mixed and injected into the mass spectrometer as a single sample. Quantitation can be achieved for this duplex sample set using the peak intensities that correlate with peptide abundance. Since this technique is based on the labeling of cysteine residues, problems occur since most proteins contain few cysteine residues. Moreover, ICAT is not suitable for PTM analysis because of this low abundance of cysteine. The development of itraq provided a powerful tool for measuring differential protein expression changes, because the labels attach to amine groups, removing the limitation inherent in ICAT of labeling cysteine groups only [10]. In the itraq workflow, PTM information contained in proteins is 796 Expert Rev. Proteomics 4(6), (2007)
5 Saccharomyces cerevisiae proteomics conserved. The itraq reagents also enhance MS/MS fragmentation, therefore yielding more confident results [29]. Compared with ICAT, itraq requires fewer chromatographic steps, thus minimizing sample loss and saving time. While other stable isotopic labeling methods use MS spectra for quantitation, itraq uses the relative abundance of reporter ions obtained from MS/MS spectra. A key advantage of this technique is that it allows labeling of up to four (and soon eight) samples in a single experiment. Therefore, this method is very useful for the quantification of proteins from multiplexed samples because it saves experimental time and enables enhanced opportunities for adding replicates to generate more statistically confident data [30 32]. Since each method has inherent advantages and disadvantages (TABLE 1), the question that arises is which method is useful for studying S. cerevisiae proteomes? If we are concerned about the protein abundance, itraq and ICAT offer great advantages since these techniques do not have the restriction of 2DE resolution for difficult proteins [25]. If we are concerned about PTM analysis, 2DE may be a good choice, since itraq and ICAT rarely identify more than a few peptides per protein, which may impact on the ability to detect PTMs. Identification & quantitation of S. cerevisiae proteins in proteomics experiments Number of publications Using the search engine Scrius, which links to BioMed Central, Science Direct, MEDLINE/PubMed, PubMed Central and others, with the keywords (with journal source option) S. cerevisiae and alternatively with itraq, ICAT, SILAC, DIGE, 18, 15 N and 13 C, the numbers of publications as a function of year (from 2000 to the present) are shown in FIGURE 4 [201]. In 2006, there was a boom in the publication of proteomic quantitation methods, due to the appearance of seven main methods found in that year. The highest numbers of publications mentioning itraq, SILAC and 18 were also found in that year. ICAT reached a maximum in However, it is necessary to point out that these publications are not only research articles, but review articles are also included, since we wished to gauge the relative numbers of review and research articles. For example, 20 articles were found by searching with keywords S. cerevisiae and itraq, but amongst these results, only six original research articles applied this method for S. cerevisiae. Therefore, the application of itraq to the study of S. cerevisiae proteomes is still relatively rare, although this method has many advantages, as summarized in TABLE 1. Identification of the S. cerevisiae proteome S. cerevisiae has been widely used as a model organism in proteomic studies. 2DE is known as a powerful tool to detect hundreds of proteins by combining this technique with MS/MS for identification. At the dawn of 2DE applications for S. cerevisiae, the number of proteins identified in a single study based on 2DE was more than 400, leading to the construction of yeast gel reference maps [33 37], as well as gaining an overview of global proteins changed in response to stress conditions, such as cadmium [38], lithium [39], H 2 2 [40] and sorbic acid [41]. However, this technique is time-consuming because of the process of spot-by-spot analysis, and it is biased against low-abundance proteins, integral membrane proteins and proteins with extremes in isoelectric point or molecular weight, leading to the development new methods, such as shotgun proteomics, to address these issues [28]. The application of shotgun proteomics has been used successfully to study S. cerevisiae proteomes. The complete genome of S. cerevisiae contains approximately 6300 genes (from the NCBI database). Recently, 1504 [42] and 1484 [28] proteins were found in offline (combining strong cation exchange and reversed-phase) or online (LC-MS) systems, respectively. Moreover, 3019 proteins were also found using a 3D-LC-MS/MS system [43]. For the metabolic labeling technique, the use of 2DE gels coupled with MS/MS is still the preferred choice. Application of [ 2 H]-leucine is a widely used metabolic labeling technique, since this amino acid is abundant in S. cerevisiae, with theoretically 65% of the trypsin-digested peptides containing at least one leucine residue [44]. Moreover, the use of this labeled amino acid leads to a reduction in the search space for protein identification [44]. To overcome the problems of classical proteomics, in that the comparison is only based on comparing the abundance of proteins in cells in two different states, which does not provide information about the dynamic mechanisms when the system changes from one state to another, Bratt et al. applied [ 2 H]-leucine metabolic labeling to determine the dynamics of protein turnover [45]. Metabolic labeling can also be performed with [ 13 C 6 ]-lysine. The combination of this labeled amino acid and data-dependent multiplexed MS/MS for identification and characterization in S. cerevisiae was reported by Berger et al. [46]. Additional mixtures of the labeled amino acids [ 2 H 3 ]-methionine, [ 2 H 3 ]-serine and [ 2 H 2 ]-tyrosine have also been demonstrated as having utility for S. cerevisiae proteomics [47]. However, the application of these deuterated amino acids might lead to problems in subsequent nano-reversed-phase high-performance LC separation because of slight changes in hydrophobicity compared with the undeuterated species. Therefore, when both of these species are separated by LC, false quantification results might occur if this is not accounted for. Localization of proteins As aforementioned, one of the substantial aims of proteomics is the identification of proteins, followed by the determination of their location in the cell. Protein location in the cellular microenvironment is very important for the understanding of protein functions and their interactions. The investigation of protein localization is growing to large-scale analysis that is mostly performed by the application of robust MS based on proteomics methods to obtain an inventory of biochemically isolated organelles containing hundreds of proteins [48]. After purification, organelles are then analyzed by MS. ne of the most interesting foci for this type of investigation is the study of mitochondria in S. cerevisiae, 797
6 Pham & Wright Table 1. Introduction and comparison of methods for quantitation. Names Reagents and functions Methods 2DE/DIGE Coomassie blue stain or silver stain used as traditional stains Fluorescent protein stains that are used to increase quantitative accuracy Recently, stains specific for post-translational modifications have been developed Mixture of proteins is separated first by isoelectric point on a gel strip, and then by molecular weight on SDS-PAGE gels Comparison is based on the position and intensities of spots on 2DE gels SILAC Heavy or light form of an essential amino acid as a medium, such as [ 13 C 6 ]-arginine, [ 13 C 6 ]-lysine, [D 3 ]-leucine Isotopic label added into peptides via metabolic labeling in the culture ICAT Two isotopic reagents that are a heavy isotope (e.g., contains eight deuteriums) and a light isotope (e.g., contains no deuterium) Each reagent includes three elements: (FIGURE 6 B) - An affinity tag (biotin) that is used to isolate ICAT-labeled peptides - A linker that is incorporated stable isotopes (e.g., 1 H or 2 H) - An iodoacetamide-reactive group which is reacted with thiol groups (cysteines; original: [ 1 H]- and [ 2 H]-isotopic ICAT tags; or [ 12 C]- and [ 13 C]- isotope pairing) Two samples are needed for one ICAT experiment Each sample is denatured, reduced, and labeled with isotopic reagent Protein samples are then mixed and trypsin digested Cysteine-tagged peptides are enriched by affinity chromatography of the biotin tag using an avidin column Enriched-peptides are then fractionated by RP-HPLC alone or in combination with SCX Peptides fractions are submitted to the mass spectrometer for identification and quantitation of peptides The comparison is based on isotopic tagging of cysteine residues that can be seen on MS/MS itraq Four reagents: 114, 115, 116 and 117 (but coming soon with eight regents: 113, 114, 115, 116, 117, 118, 119 and 121) Each reagent include three elements: (FIGURE 6A) - Reporter group (mass for 4-plex, or and 121 for 8-plex) that is used to quantify their respective samples - Balance group (mass for 4-plex, or and 84 for 8-plex) that is used to maintain each isotopic tag at exactly the same mass - Amine-specific peptide-reactive group that is used to react with all primary amines, including N-terminus and the ε-amino group of lysine side-chain, to label all peptides Four samples are needed for one itraq experiment (soon eight samples) Each sample is denatured, reduced, alkylated and trypsin digested Subsequently, peptides in each sample are labeled with itraq reagents and then combined The labeled-peptides mixture is then fractionated using SCX chromatography Fractions are introduced to MS/MS for identification and quantitation of peptides and then proteins Radioactive labeling method Heavy form of an essential amino acid, such as [ 14 C]-leucine or [ 35 S]-methionine The same as for the SILAC method 16 / 18 Water with two forms: H 2 18 and H 2 16 Two samples are digested with trypsin in two forms of water H 2 18, and H 2 16 The atoms of 18 and 16 are attached to the C-termini of trypsin-digested peptides Two samples are then combined and analyzed by MS/MS The quantitation is performed based on the relative intensity of paired peptides with a 4-Da mass difference detected by MS/MS Label-free quantitation No reagent is used, the quantitation is based on MS/MS data only Quantitation can be approached based on: - The peak intensity measurement of peptides - The number of MS/MS spectra per protein detected 2DE: 2D electrophoresis; AQUA: Absolute quantitation; CID: Collision-induced dissociation; DIGE: Differential in-gel electrophoresis; ESI: Electrospray ionization; HPLC: High-performance liquid chromatography; ICAT: Isotope-coded affinity tags; MS: Mass spectrometry; MS/MS: Tandem MS; PAGE: Polyacrylamide gel electrophoresis; PTM: Post-translational modification; RP: Reversed phase; SCX: Strong cation exchange; SDS: Sodium dodecyl sulfate; SILAC: Stable-isotope labeling of amino acids in culture. 798 Expert Rev. Proteomics 4(6), (2007)
7 Saccharomyces cerevisiae proteomics Table 1. Introduction and comparison of methods for quantitation (cont.). Advantages Disadvantages Ref. Accuracy in comparison due to at least triplicate for each phenotype Ease of performance Sequential Labor intensive Difficult to automate Not sensitive for basic, hydrophobic and large molecular mass proteins At least triplicate gels for each sample and, thus, cost, time and laborious to analyze and perform Does not require multiple chemical processing steps as well as purification of protein samples Not always suitable for all experimental samples (requires growth on isotope-enriched substrate) [8] Good for complex samples because only a few peptides per proteins are used for analysis nly two samples can be used for one experiment The excess of biotin in the sample matrix (e.g., serum) may reduce the effectiveness of the affinity column because of binding-site competition Sometimes, the isobaric between ICAT peptides and nontagged peptides eluting from the affinity chromatography results in false-positive and false-negative identification The loss of collision energy because of the ICAT tag during MS fragmentation results in sequencing difficulties A loss of peptide redundancy of a given protein can be found because of peptide-selective strategies Possible difficulties for PTM analysis in the case of only one or a few peptides containing low-abundance cysteine, which is used as a representative for protein identification [4] The label is cleaved in the MS before quantification Quantitative comparison can be performed between up to four samples (and soon eight samples) within a single experiment High validation and good for quantitative comparison of PTM and subproteome The signal intensity is increased because the isotopically labeled peptides are isobaric and all contribute to one ion species that is used for CID and observed in the MS Not so good for the identification and quantitation of low-abundance proteins, as well as samples with high complexity Time consuming The noise of untagged isobaric chemicals may confuse MS sequencing of the labeled peptides Problem of protein variants [10] Superior for differential detection and furthermore for particular quantitation Excellent for studying rapid phosphorylation changes Labor cost [62,63] Simple procedure High efficiency Lower cost for experiments compared with other labeling methods The 4-Da mass difference is not big enough to detect by ESI ion trap MS/MS Loss of peptide redundancy of a given protein can be found because of peptide-selective strategies Interchange between the label and the solvent may occur under acidic conditions, for example in RP-LC operation [9] Lower cost for experiments compared with other methods Quantitation is affected by ionization efficiency and chromatography conditions [5,6] 2DE: 2D electrophoresis; AQUA: Absolute quantitation; CID: Collision-induced dissociation; DIGE: Differential in-gel electrophoresis; ESI: Electrospray ionization; HPLC: High-performance liquid chromatography; ICAT: Isotope-coded affinity tags; MS: Mass spectrometry; MS/MS: Tandem MS; PAGE: Polyacrylamide gel electrophoresis; PTM: Post-translational modification; RP: Reversed phase; SCX: Strong cation exchange; SDS: Sodium dodecyl sulfate; SILAC: Stable-isotope labeling of amino acids in culture
8 Pham & Wright Table 1. Introduction and comparison of methods for quantitation (cont.). Names Reagents and functions Methods Protein AQUA Selected isotope labeled ( 13 C, 15 N) peptides are used as an internal standard Each AQUA peptide is a synthetic tryptic peptide incorporating one stable-isotope-labeled amino acid, of 6 10 Da in molecular weight An optimal tryptic peptide corresponding to a protein of interest can be ordered from the Sigma AQUA Peptide Library An optimal tryptic peptide corresponding to a protein of interest is selected and then synthesized incorporating stable-isotope ( 13 C, 15 N)-labeled amino acid The known quantity of AQUA peptide is subsequently added to protein sample extracted from biological sample The sample is then proteolysed with an enzyme Peptide mixture is analyzed by MS/MS The quantitation is performed by comparing the signal intensities of native peptide and AQUA peptide QconCAT Artificial genes are designed de novo to direct the biosynthesis of novel proteins that are assemblies of signature Qpeptides Qpeptides have arginine or lysine at the C-terminus and are used as internal standards for peptides derived from the digestion of protein samples Performed via the design, synthesis and expression of artificial genes that encode concatenated proteolytic peptides used as surrogates for the protein of interest Artificial genes are transformed into and expressed in a heterologous organism such as a bacteria (Escherichia coli) to create an expression strain The expression strain is then grown in either media containing isotope labeled source (e.g., 15 N or 13 C) or containing specific stable-isotopelabeled amino acids so that the artificial proteins are fully labeled The artificial proteins (QconCAT) are then purified and quantified before being mixed with a complex mixture of proteins The sample is then proteolysed to peptides, and these peptides are analyzed by MS/MS The quantitation is performed by comparing the signal intensities of native peptides (proteins) and Qpeptides (QconCAT) 2DE: 2D electrophoresis; AQUA: Absolute quantitation; CID: Collision-induced dissociation; DIGE: Differential in-gel electrophoresis; ESI: Electrospray ionization; HPLC: High-performance liquid chromatography; ICAT: Isotope-coded affinity tags; MS: Mass spectrometry; MS/MS: Tandem MS; PAGE: Polyacrylamide gel electrophoresis; PTM: Post-translational modification; RP: Reversed phase; SCX: Strong cation exchange; SDS: Sodium dodecyl sulfate; SILAC: Stable-isotope labeling of amino acids in culture. with various membrane-associated proteins also being of great interest. More details on progress in these areas are detailed in the following subsections. Proteomics of mitochondria Mitochondria play a central role in bioenergetics, apoptosis and the metabolism of lipids, amino acids and iron [49]. Many studies have been based on genome and functional systematics to investigate S. cerevisiae mitochondria, but the identification and characterization of mitochondrial proteins has not been completed yet [50]. How many distinct proteins can be found in a mitochondrion? To answer this question, many studies were carried out to localize the yeast proteome [51], where the large-scale analyses of S. cerevisiae subcellular proteomes using general [52] or global methods [53] were used. To date, large-scale analyses of protein localization in S. cerevisiae have been performed based on transposon-mediated random epitope tagging [54] and plasmid-based overexpression of epitope-tagged proteins [52]. However, these techniques have some disadvantages; for example, important localization signals can be interrupted because of the epitope tagging of a partial open reading frame, or the overexpression of proteins might oversupply intracellular transport mechanisms [53]. Therefore, Hu et al. tagged each open reading frame in its chromosomal location (with green fluorescent protein [GFP]) through oligonucleotide-directed homologous recombination to create a yeast strain. The GFP was then monitored in living cells using fluorescence microscopy [53]. Using large-scale analysis of protein localization by immunolocalizing 2744 epitope-tagged yeast proteins (covering 45% of the yeast theoretical proteome), Kumar et al. determined that 47% of yeast proteins are cytoplasmic, 13% are mitochondrial, 13% are exocytic and 27% are nuclear/nuclear associated [52]. From the 13% of mitochondrial-located proteins (332 proteins), it can be estimated that the yeast mitochondria contains approximately 800 different proteins [52]. Studies of yeast mitochondria were also performed using protein protein interactions [22], MS (based on 2DE) of mitochondria [55] and computational predictions of mitochondrial proteins [56]. Recently, studies were performed to reduce the proportion of missing mitochondrial proteins in S. cerevisiae. Reduction of the proportion to 10% with the identification of 749 proteins was reported, of which 436 (58.1%) proteins are mitochondrial proteins, 208 (27.7%) proteins have not been localized so far and 106 (14.1%) are localized in other cellular compartments [49]. The identification of 527 mitochondrial proteins by GFP tagging was also carried out [53]. The prefractionation of cellular components has been a very important step for the study of organelles [57], because this helps reduce sample complexity, granting access to 800 Expert Rev. Proteomics 4(6), (2007)
9 Saccharomyces cerevisiae proteomics Table 1. Introduction and comparison of methods for quantitation (cont.). Advantages Disadvantages Ref. High sensitivity, and more accurate compared with quantitative PCR or northern blotting Suitable for the study of gene silencing in low-abundance proteins Can be used for any silent gene since an AQUA peptide can be formed using only an amino acid sequence Study of quantitative proteomics can be performed on specific proteins, as well as specific amino acid modification Suitable for absolute quantitation of large numbers of multiplexed proteins Each protein to be quantified requires at least one stable-isotope-labeled peptide synthesized independently, resulting in high cost Each peptide must be purified and quantified Not suitable for the study of global protein expression since a huge amount of synthesized peptides is required The selection of peptides to use as surrogates is restricted Protocol is moderately complex [14] [15] 2DE: 2D electrophoresis; AQUA: Absolute quantitation; CID: Collision-induced dissociation; DIGE: Differential in-gel electrophoresis; ESI: Electrospray ionization; HPLC: High-performance liquid chromatography; ICAT: Isotope-coded affinity tags; MS: Mass spectrometry; MS/MS: Tandem MS; PAGE: Polyacrylamide gel electrophoresis; PTM: Post-translational modification; RP: Reversed phase; SCX: Strong cation exchange; SDS: Sodium dodecyl sulfate; SILAC: Stable-isotope labeling of amino acids in culture. low-abundance proteins and gaining additional information concerning protein localization [58]. Many techniques have been used to isolate distinct cell compartments; for example, free-low-electrophoresis, gradient centrifugation and immunoprecipitation. However, proteomes of entire organelles are too complex for sufficient separation using 1DE methods. Therefore, many different approaches have been used as an inventory of mitochondrial proteomes to facilitate the analysis on a molecular level [49,50]. Recently, a total of 851 proteins were identified using multidimensional LC-MS/MS, 1DE sodium dodecyl sulfate (SDS) PAGE combined with nano-lc-ms/ms and 2DE-PAGE with MALDI mass fingerprinting [58]. Furthermore, a comparison of these methods was also made, while 2DE-PAGE has an advantage in the separation of protein isoforms and quantitative profiling, 1DE-PAGE with nano-lc-ms/ms and multidimensional LC-MS/MS are suitable for efficient protein identification because they are less biased against distinct classes of proteins [58]. Proteomics of nuclear & plasma membranes Since the nucleus is separated from the cytosol by a double membrane, the exchange of molecules between the cytosol and the inner nucleus happens via nuclear pores. Moreover, the nuclear membrane has a complex structure as well as a high dynamic behavior. Therefore, an understanding of nuclear membrane structure is very important. The first genome-wide screen to identify inner nuclear membrane proteins was performed by Murthi and Hopper, by transforming 4850 proteins of a S. cerevisiae deletion strain collection of nonessential genes in a 96-well format [59]. Due to the relatively poor solubility of proteins during the isoelectric focusing process, most intermembrane proteins are not detected on 2DE-gel maps. With the aim of analyzing yeast plasma membrane proteins, various kinds of procedures have been used, including optimization of the purification protocol to reduce contaminating membranes and cytosolic proteins, as well as the improvement of 2DE gels using the cationic detergent cetyl-trimethyl ammonium bromide, coupled with SDS for the first and second dimensions [60]. As a result, proteins in 50 spots were identified, in which both known and unknown plasma proteins were discovered [60]. Following that, based on 2DE-gels, Delom et al. used ion-exchange chromatography/lithium dodecyl sulfate-page to investigate plasma membrane proteins in response to the antifungal agent calcofluor, where approximately 90 proteins were identified and clustered [61]. Therefore, the combination of subcellular fractionation coupled with 2DE-gels was successfully applied in identifying the plasma membrane proteins
10 Pham & Wright 25 itraq 18 Number of publications ICAT SILAC 15 N 13 C DIGE Year Figure 4. Number of publications related to the proteomic analysis of Saccharomyces cerevisiae. DIGE: Differential in-gel electrophoresis; ICAT: Isotope-coded affinity tags; SILAC: Stable-isotope labeling of amino acids in culture. Quantitative proteome of S. cerevisiae As mentioned previously, there are currently three crucial methods of quantitation applied to the proteomic analysis of S. cerevisiae, including metabolic labeling with stable isotopes, radioactive isotopes (e.g., [ 14 C]-leucine [62] or [ 35 S]-methionine [63]) and stableisotope tagging methods. Recently, other approaches termed label-free methods have been applied to quantitative proteomics [64 66], but their application in S. cerevisiae is still rare [67]. Recently, many studies using metabolic labeling as well as stable-isotopic tagging have been deployed to determine quantitative protein expression changes in S. cerevisiae. [ 2 H 10 ]-leucine was used as a labeled amino acid in synthesis-complete media, and the relative comparison between [ 1 H 10 ] and [ 2 H 10 ] cultures provided reliable data for the identification and quantitation of the S. cerevisiae proteome from wild-type to nonessential gene-null mutant strains [68]. In terms of the quantitative proteome, the comparison between wild-type and mutant strains was necessary to characterize the protein functions of S. cerevisiae under different states or conditions. In that context, ICAT was also used to characterize proteomic changes in S. cerevisiae between the wildtype HFY1200 strain and a mutant HFY871 strain where 1029 distinct proteins were detected [69]. Moreover, the application of ammonium- 15 N sulfate and ammonium- 14 N sulfate combined with the multidimensional protein identification technology (MudPIT) technique was also used for a quantitative proteomic investigation of S. cerevisiae, where 638 unique proteins from N-peptides and 664 unique proteins from 1642 unique 15 N-peptides, leading to a total of 842 proteins were identified using this technique. This study offers a useful tool for quantitative proteomic analysis of a variety of biological systems [70]. In another study, ammonium- 15 N sulfate and ammonium- 14 N sulfate were also used to study the nonspecific protease, protein K, in a quantitative shogun proteomic investigation using normalized spectral abundance factors for relative quantitation. As a result, 719 unique proteins were found for each of the three independent biological replicates, and 84 proteins demonstrated significant differential expression between two growth conditions ( 15 N and 14 N). Moreover, this study offers a nonspecific protease dataset can be used to generate high confidence with a low false-discovery rate, resulting in high-confidence spectral counts that can be used for quantitative proteomics [71]. The most significant application of S. cerevisiae in industry is based on fermentation, especially for the generation of ethanol. To date, most S. cerevisiae proteomic analyses are based on 2DE, with many studies investigating protein expressions under glucose-exhausted conditions. ne of the pioneering studies was performed by Bouncherie, where six new proteins were found in glucose-limited conditions and the synthesis of approximately 95% of the proteins synthesized in the log phase was turned off [72]. Similar studies were applied to investigate the response of S. cerevisiae under stress conditions, such as cadmium [38], oxidative [40] and hyperosmolarity [73] stress. In the case of cadmium stress, there were 54 up- and 43 downregulated proteins detected, with the expression of these proteins being related to the biosynthesis of sulfur amino acids, and the data reported that glutathione and thioredoxin played 802 Expert Rev. Proteomics 4(6), (2007)
11 Saccharomyces cerevisiae proteomics important roles in the thiol redox system in response to this cadmium stress [38]. In the study of H 2 2 -mediated oxidative stress, the synthesis of at least 115 proteins was stimulated, while the expression of 52 proteins was repressed. There was a decrease in protein synthesis and an increase in protein degradation pathways [40]. In the study of hyperosmolarity stress ( M NaCl), 73 proteins were differentially expressed by more than threefold in 1.4 M NaCl, 40% of these proteins were downregulated, and the expression of these proteins was related to the dissimilation of dihydroxyacetone [73]. In the first study where ICAT (developed by Gygi [4]) was used for quantitation of S. cerevisiae, proteins in steady-state on galactose as well as in two different states responding to changes in carbon source to galactose or ethanol were made [4]. The data presented suggested that this technique had high accuracy, as well as demonstrating that this ICAT workflow showed potential for being a good tool for automated, quantitative and global proteome analysis of S. cerevisiae [4]. After the release of the ICAT technique, another study, using solid-phase isotope labeling (for details, see [74]) was used for global quantitative proteomics in comparison with the ICAT method. The solid-phase isotope labeling technique demonstrated some advantages when compared with the ICAT method, such as being less time-consuming, eliminating the effect of proteolytic enzymes (trypsin) as well as strong denaturants and detergents (urea, SDS), and enabling the analysis of more than two samples. This technique was applied to investigate the S. cerevisiae proteome changes in response to induction with galactose of cells originally grown in raffinose, in which more proteins were identified and quantified than in the Gygi ICAT study (the implication being that this technique is more sensitive than ICAT) [4]. For example, after induction with galactose, multiple peptides from Gal1p, Gal2p, Gal7p and Galxp were identified and quantified by the solid-phase workflow, while only one peptide per protein was detected by ICAT for the equivalent proteins [74]. Later, a modification of ICAT reagents (cicat) was applied to the large-scale quantitative proteomic investigation of S. cerevisiae responding to salinity stress (NaCl). In this study, a total of 560 proteins were identified and quantified, 51 proteins changed their expressions more than twofold, in which four RNA-binding proteins were upregulated under this stress condition. Furthermore, proteins relating to amino acid metabolism were also upregulated, suggesting that salt stress and amino acid starvation generate overlapping cellular responses [75]. As mentioned previously, although the newer labeling method of itraq has many advantages, its application for the study of the S. cerevisiae proteome is still rare. The first application of itraq in S. cerevisiae was published by Ross et al. in 2004 [10]. A proteomic comparison of three strains (wild-type, upf1δ and xrn1δ) was performed in this study. A total of 1217 proteins were identified, suggesting that the regulation of the strains upf1δ and xrn1δ was similar. Most of the upregulated proteins were related to amino acid metabolism, pantothenate and CoA biosynthesis, starch and sucrose metabolism, and purine and pyrimidine metabolism, whilst most of the downregulated proteins in the xrn1δ strain were structural components of the ribosome [10]. The next application of itraq in S. cerevisiae was carried out by Husnik et al. (2006) in an attempt to improve the quality of wine fermentation [76]. Two yeast strains (ML01 and S92) were used, and itraq technical replicates were also applied. f the 559 proteins identified, 119 proteins were related to carbohydrate and amino acid metabolism, and pyrimidine, purine, fatty acid, ergosterol and formate biosynthesis. No recorded difference in protein expression was found between ML01 and S92 strains. Recently, the application of itraq in S. cerevisiae was carried out by Pham and coworkers to understand how S. cerevisiae responds to very high gravity conditions aimed at improving ethanol fermentation efficiency [30 32]. In this work, technical replicates, as well as multiple MS/MS injections were also applied. A total of 413 proteins were identified from three MS/MS injections. Most proteins associated with glycolysis increased their expression, whilst amino acid and heat-shock proteins decreased their expression, leading to a decrease in cell viability and proliferation. Moreover, due to the redox balance in the cells, glycerol was also generated. Therefore, to optimize ethanol fermentation under very high glucose conditions, the deletion of some key genes in generating glycerol is necessary [30]. AQUA is very important for providing an insight into the response of yeast to its environments. The first study of a quantitative comparison of mrna abundance and protein expression levels was carried out by Gygi et al., where the relative amounts of protein and mrna with the respective codon bias values for the corresponding genes was made [2]. Later, a S. cerevisiae fusion library where each open reading frame was tagged with a high-affinity epitope and then expressed from its natural chromosomal location was established to facilitate global protein analyses [77]. Approximately 80% of the proteome was expressed during normal growth conditions; moreover, many proteins including essential proteins and transcription factors, which were not detected by other proteomic techniques, were detected using this technique [77]. Therefore, the monitoring of protein expression cannot be replaced by mrna microarray technology since the changes in protein abundance and mrna abundance only moderately correlate with each other [78]. Proteomics in the study of S. cerevisiae networks The interactions of proteins in a network can be divided into two main systems. These are the interaction of proteins with metabolites (protein metabolite), and proteins with proteins (protein protein). Protein metabolite interactions are performed via metabolic pathways, in which each protein catalyzes the reaction(s) of one (or more) specific substrate(s). A significant number of metabolic pathways in S. cerevisiae have been discovered, with constant updating underway. The sources of these metabolic pathways, the functions of each gene/protein, the protein localization databases and the interaction networks can be accessed free of charge at the databases (as listed in the Information resources section). Moreover, systems biology studies/groups with a focus on S. cerevisiae are growing (see Information resources section for details)
12 Pham & Wright The study of protein metabolite interactions networks led to the establishment of metabolic engineering in S. cerevisiae. The aim of metabolic engineering is to increase cell production (e.g., ethanol [79] and glycerol [80]) via optimization of genetic and regulatory processes. Under certain conditions, expression of metabolic pathways will predominate, and therefore, the proper reconstruction of these metabolic pathways is necessary to aid a deeper understanding and then metabolic engineering. Proteomics can aid in this. For example, FIGURE 5 illustrates the reconstruction of glycolysis/gluconeogenesis in the relationship with sucrose metabolism and the biosynthesis of amino acids. Protein expression changes are depicted in relationship with metabolites. With the aim of investigating interactions between protein and metabolite networks, proteome chips were also used, where 5800 yeast open reading frames ( 80% of the predicted yeast proteins) were cloned, overexpressed and the resulting corresponding proteins were purified [21]. This method offered an advantage that a comprehensive set of individual proteins can be directly assayed in vitro for many kinds of activities, such as protein lipid interactions and protein drug interactions [21]. Moreover, by probing a high-density microarray of small molecules formed by diversity-oriented synthesis with fluorescently labeled Ure2p, this technique was used to investigate several small compounds that bind Ure2p, such as uretupamine that Hxk1p(+1.5; +1.7) Glk1p (+1.4; +1.3) Pgi1p (+1.5; +1.5) Pfk1p (+1.1; -3.8) Pfk2p(1.0; -4.6) Tps2p(-2.5; -7.0) Nth2p Glucose Trehalose UDP-glucose Pgm2p(+2.4; +2.6) UDPG phosphorylase Glucose 6-phosphate Glucose-1P Fructose 6-phosphate Fructose 1,6 biphosphate Fba1p Fbp1p Gph1p(-1.2; -3.7) Trehalose-6P Tps1p (-2.5; -4.3) Starch; Glycogen Gsy1p Glycogen; Aminolse Glc3p(-1.3; -3.6) Starch and sucrose metabolism Dihydroxyacetone phosphate Tpl1p Gpd1p (+1.9; +1.8) Gut2p Glycerol-3-phosphate Gpp1p Hor2p Glycerol Gut1p Glyceraldehyde 3-phosphate Tdh1p (+1.7; +1.9) 1,3-Diphosphoglycerate Pgk1p 3-Phosphoglycerate Gpm1p L-Aspartate Dps1p (-1.3; -3.3) Asn2p Asp1p L-Asparagine Ded81p(-1.7; -2.5) Alanine and aspartate metabolism Arg1p Aat2p (+1.1; +1.6) 2-xoglutarate Kgd1p gd1p S-Succinyldihydrolipoamide Gdh1p(-1.6; -2.1) L-AsparaginyltRNA(Asn) L-AspartyltRNA(Asn) L-AspartyltRNA(Asp) L-Argininosuccinate Mdh1p (-1.3; -2.0) Fumarate Arg4p (-1.6; -5.9) Sdh4p Malate Cit2p Mls1p Fum1p(-1.7; -2.8) Glycoxylate Succinate Lsc2p Succinyl-CoA Citrate cycle (TCA cycle) xaloacetate Kgd2p Ldp1p Pck1p, Ppc1p, Jpm2p Icl2p Icl1p Citrate Aco1p Isocitrate Idh1p(-1.6; -2.6) Lysine biosynthesis Cit2p L-Glutamate 2-Phosphoglycerate Eno1p Aro4p (-1.1; -3.1) Phosphoenolpyruvate 7P-2-Dehydro-3-deoxy- D-arabinoheptonate Pyk1p Pyk2p Aro1p Pyruvate Pdc1p(+1.2; +1.5) 3-Dehydroquinate Pdc5p (+1.9; +1.9) Pdc6p (+1.5; +1.7) Eno1p 3-Dehydroshikimate Acs1p Acetaldehyde Acs2p Aro1p Ald6p (+1.8; +2.1) Adh1p (+1.5; +1.6) 5-0-(1-Carboxyvinyl)- Acetate Adh2p 3-phosphoshikimate Ethanol Aro7p Aro2p Trp2p Chorismate Acetyl-CoA Pha2p Phenylpyruvate Prephenate Tyr1p Aat1p His5p His5p Aat1p Phenylalanine Tyrosine Frs2p Tys1p Anthranilate Trp2p, Trp4p Trp1p, Trp3p (3-Indolyl)-glycerolphosphate Trp5p(-1.8; -2.1) L-Tryptophan Glutamate metabolism Phe-tRNA Phenylalanine metabolism Tyr-tRNA Tyrosine metabolism Tryptophan metabolism Phenylalanine, tyrosine and tryptophan biosynthesis Figure 5. Reconstruction of central carbon metabolism in relationship to the TCA cycle, starch and sucrose metabolism, and the biosynthesis of amino acids. TCA: Tricarboxylic acid. Reproduced with permission from J. Proteome Res. 5(12), (2006). Copyright 2006 American Chemical Society [30]. 804 Expert Rev. Proteomics 4(6), (2007)
13 Saccharomyces cerevisiae proteomics A Balance group N N N Reporter group Reactive group B HN S Biotin tag NH X X N H X X Labeled linker X X X X N H Reactive group Figure 6. Structure of regents used for itraq (A) or ICAT (B) techniques [4,10]. X: Heavy or light isotope. activates a glucose-sensitive transcriptional pathway downstream of Ure2p. This technique is a valuable method to identify small molecules that bind to a protein of interest [24]. As mentioned previously, one of the most important roles of proteomics in S. cerevisiae is to characterize both proteins expressions and their functions on a global scale. The interaction of a protein not only provides the functions of proteins, but also plays a key role in the building of complex systems. In the visual representations of complex networks, each protein is presented as a point (or node), and a line is representative of the physical relationships between proteins. To establish a network of protein protein interactions, much genetic and proteomic information (e.g., gene and protein expression, transcription factor binding, and PTM locations and types) is needed on the global scale [81]. Most proteins rarely work by themselves, and they always interact with other biomolecules to express their functions, with these interactions being the basis of life. Therefore, by studying protein interaction networks (protein interactome), we can understand the fundamentals of the life of a molecular system. Although the genome sequence of S. cerevisiae was completed in 1996 [82], a complete comprehension of the protein protein interaction networks in S. cerevisiae (or any organism for that matter) is still a big challenge [83]. The first network model established at the proteome level is known as the two-hybrid system. This system, developed by Fields in 1989, is based on the modular domain structure of the transcription factor GAL4, consisting of a DNA-binding domain and transcription activation [84]. In the two-hybrid system approach, a protein of interest (X) is expressed as a hybrid protein with the GAL4 DNA-binding domain, and another protein of interest (Y) is expressed by the GAL4 activation domain. If X interacts with Y (two hybrid proteins), transcription of a gene regulated by UAS G occurs [84]. This system has high sensitivity in detecting protein protein interactions in vivo without information of protein molecules [83]. But this model cannot be applied for the interactions of three or more proteins and those depending on PTMs [83]. This model is also poor at determining the interactions of membrane proteins; moreover, in some cases the inference of the interaction withdrawn from this model might be irrelevant to the physiology of cells [83]. However, this model is still used as a standard technique in biology [83]. Two large-scale yeast two-hybrid screens were used to characterize the protein protein interactions, whereby 957 putative interactions related to 1004 proteins were detected [23]. A network of 2358 interactions established among 1548 proteins was also characterized, and this network provided connections of global protein protein interaction patterns based on functional groups or localization assignments, and cross-connections [85]. Moreover, pioneering work on protein protein interaction networks using large-scale analyses of protein complexes in S. cerevisiae as performed by Ho et al. [86] and Gavin et al. [22]. There were 3617 associated proteins detected using highthroughput MS protein complexes identification, when 10% of predicted yeast proteins were used as baits [86]. As a result, large numbers of protein complexes were identified and many new interactions were discovered, such as signaling pathways and the DNA response [86]. Moreover, using tandem affinity purification (TAP) and MS to characterize multiprotein complexes in S. cerevisiae, 232 distinct multiprotein complexes were detected and 344 proteins were proposed for new cellular roles, including 231 proteins with no previous functional annotation [22]
MBios 478: Mass Spectrometry Applications [Dr. Wyrick] Slide #1. Lecture 25: Mass Spectrometry Applications
![MBios 478: Mass Spectrometry Applications [Dr. Wyrick] Slide #1. Lecture 25: Mass Spectrometry Applications MBios 478: Mass Spectrometry Applications [Dr. Wyrick] Slide #1. Lecture 25: Mass Spectrometry Applications](/thumbs/74/71368870.jpg) MBios 478: Mass Spectrometry Applications [Dr. Wyrick] Slide #1 Lecture 25: Mass Spectrometry Applications Measuring Protein Abundance o ICAT o DIGE Identifying Post-Translational Modifications Protein-protein
MBios 478: Mass Spectrometry Applications [Dr. Wyrick] Slide #1 Lecture 25: Mass Spectrometry Applications Measuring Protein Abundance o ICAT o DIGE Identifying Post-Translational Modifications Protein-protein
Quantitative mass spec based proteomics
 Quantitative mass spec based proteomics Tuula Nyman Institute of Biotechnology tuula.nyman@helsinki.fi THE PROTEOME The complete protein complement expressed by a genome or by a cell or a tissue type (M.
Quantitative mass spec based proteomics Tuula Nyman Institute of Biotechnology tuula.nyman@helsinki.fi THE PROTEOME The complete protein complement expressed by a genome or by a cell or a tissue type (M.
Proteomics. Proteomics is the study of all proteins within organism. Challenges
 Proteomics Proteomics is the study of all proteins within organism. Challenges 1. The proteome is larger than the genome due to alternative splicing and protein modification. As we have said before we
Proteomics Proteomics is the study of all proteins within organism. Challenges 1. The proteome is larger than the genome due to alternative splicing and protein modification. As we have said before we
Proteomics. Manickam Sugumaran. Department of Biology University of Massachusetts Boston, MA 02125
 Proteomics Manickam Sugumaran Department of Biology University of Massachusetts Boston, MA 02125 Genomic studies produced more than 75,000 potential gene sequence targets. (The number may be even higher
Proteomics Manickam Sugumaran Department of Biology University of Massachusetts Boston, MA 02125 Genomic studies produced more than 75,000 potential gene sequence targets. (The number may be even higher
Enhancers mutations that make the original mutant phenotype more extreme. Suppressors mutations that make the original mutant phenotype less extreme
 Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Proteomics: A Challenge for Technology and Information Science. What is proteomics?
 Proteomics: A Challenge for Technology and Information Science CBCB Seminar, November 21, 2005 Tim Griffin Dept. Biochemistry, Molecular Biology and Biophysics tgriffin@umn.edu What is proteomics? Proteomics
Proteomics: A Challenge for Technology and Information Science CBCB Seminar, November 21, 2005 Tim Griffin Dept. Biochemistry, Molecular Biology and Biophysics tgriffin@umn.edu What is proteomics? Proteomics
Outline and learning objectives. From Proteomics to Systems Biology. Integration of omics - information
 From to Systems Biology Outline and learning objectives Omics science provides global analysis tools to study entire systems How to obtain omics - What can we learn Limitations Integration of omics - In-class
From to Systems Biology Outline and learning objectives Omics science provides global analysis tools to study entire systems How to obtain omics - What can we learn Limitations Integration of omics - In-class
NPTEL
 NPTEL Syllabus Proteomics: Principles and Techniques - Video course COURSE OUTLINE An introduction to proteomics: Basics of protein structure and function, An overview of systems biology, Evolution from
NPTEL Syllabus Proteomics: Principles and Techniques - Video course COURSE OUTLINE An introduction to proteomics: Basics of protein structure and function, An overview of systems biology, Evolution from
Kinetics Review. Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets)
 Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Proteomics And Cancer Biomarker Discovery. Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar. Overview. Cancer.
 Proteomics And Cancer Biomarker Discovery Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar Overview Proteomics Cancer Aims Tools Data Base search Challenges Summary 1 Overview
Proteomics And Cancer Biomarker Discovery Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar Overview Proteomics Cancer Aims Tools Data Base search Challenges Summary 1 Overview
Advances in analytical biochemistry and systems biology: Proteomics
 Advances in analytical biochemistry and systems biology: Proteomics Brett Boghigian Department of Chemical & Biological Engineering Tufts University July 29, 2005 Proteomics The basics History Current
Advances in analytical biochemistry and systems biology: Proteomics Brett Boghigian Department of Chemical & Biological Engineering Tufts University July 29, 2005 Proteomics The basics History Current
Exam 2 BIO200, Winter 2012
 Exam 2 BIO200, Winter 2012 Name: Multiple Choice Questions: Circle the one best answer for each question. (2 points each) 1. The 5 cap structure is often described as a backwards G. What makes this nucleotide
Exam 2 BIO200, Winter 2012 Name: Multiple Choice Questions: Circle the one best answer for each question. (2 points each) 1. The 5 cap structure is often described as a backwards G. What makes this nucleotide
AP Biology Gene Expression/Biotechnology REVIEW
 AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
Lecture 8: Affinity Chromatography-III
 Lecture 8: Affinity Chromatography-III Key words: Chromatography; Affinity chromatography; Protein Purification During this lecture, we shall be studying few more examples of affinity chromatography. The
Lecture 8: Affinity Chromatography-III Key words: Chromatography; Affinity chromatography; Protein Purification During this lecture, we shall be studying few more examples of affinity chromatography. The
Laboratory Water Quality Affects Protein Separation by 2D Gel Electrophoresis
 Laboratory Water Quality Affects Protein Separation by 2D Gel Electrophoresis 2D gel electrophoresis remains a dominant technique in proteomics. Obtaining high quality gels requires careful and tedious
Laboratory Water Quality Affects Protein Separation by 2D Gel Electrophoresis 2D gel electrophoresis remains a dominant technique in proteomics. Obtaining high quality gels requires careful and tedious
Purification: Step 1. Lecture 11 Protein and Peptide Chemistry. Cells: Break them open! Crude Extract
 Purification: Step 1 Lecture 11 Protein and Peptide Chemistry Cells: Break them open! Crude Extract Total contents of cell Margaret A. Daugherty Fall 2003 Big Problem: Crude extract is not the natural
Purification: Step 1 Lecture 11 Protein and Peptide Chemistry Cells: Break them open! Crude Extract Total contents of cell Margaret A. Daugherty Fall 2003 Big Problem: Crude extract is not the natural
Purification: Step 1. Protein and Peptide Chemistry. Lecture 11. Big Problem: Crude extract is not the natural environment. Cells: Break them open!
 Lecture 11 Protein and Peptide Chemistry Margaret A. Daugherty Fall 2003 Purification: Step 1 Cells: Break them open! Crude Extract Total contents of cell Big Problem: Crude extract is not the natural
Lecture 11 Protein and Peptide Chemistry Margaret A. Daugherty Fall 2003 Purification: Step 1 Cells: Break them open! Crude Extract Total contents of cell Big Problem: Crude extract is not the natural
Proteomics Technology Note
 Targeted Proteomics for the Identification of Nucleic Acid Binding Proteins in E. coli: Finding more low abundant proteins using the QSTAR LC/MS/MS System, Multi- Dimensional Liquid Chromatography, Pro
Targeted Proteomics for the Identification of Nucleic Acid Binding Proteins in E. coli: Finding more low abundant proteins using the QSTAR LC/MS/MS System, Multi- Dimensional Liquid Chromatography, Pro
Genome Biology and Biotechnology
 Genome Biology and Biotechnology 10. The proteome Prof. M. Zabeau Department of Plant Systems Biology Flanders Interuniversity Institute for Biotechnology (VIB) University of Gent International course
Genome Biology and Biotechnology 10. The proteome Prof. M. Zabeau Department of Plant Systems Biology Flanders Interuniversity Institute for Biotechnology (VIB) University of Gent International course
The Two-Hybrid System
 Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine The Two-Hybrid System Carolina Vollert & Peter Uetz Institut für Genetik Forschungszentrum Karlsruhe PO Box 3640 D-76021 Karlsruhe
Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine The Two-Hybrid System Carolina Vollert & Peter Uetz Institut für Genetik Forschungszentrum Karlsruhe PO Box 3640 D-76021 Karlsruhe
Metabolomics in Systems Biology
 Metabolomics in Systems Biology Basil J. Nikolau W.M. Keck Metabolomics Research Laboratory Iowa State University February 7, 2008 Outline What is metabolomics? Why is metabolomics important? What are
Metabolomics in Systems Biology Basil J. Nikolau W.M. Keck Metabolomics Research Laboratory Iowa State University February 7, 2008 Outline What is metabolomics? Why is metabolomics important? What are
Faster, easier, flexible proteomics solutions
 Agilent HPLC-Chip LC/MS Faster, easier, flexible proteomics solutions Our measure is your success. products applications soft ware services Phospho- Anaysis Intact Glycan & Glycoprotein Agilent s HPLC-Chip
Agilent HPLC-Chip LC/MS Faster, easier, flexible proteomics solutions Our measure is your success. products applications soft ware services Phospho- Anaysis Intact Glycan & Glycoprotein Agilent s HPLC-Chip
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Peptide libraries: applications, design options and considerations. Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST
 Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Understanding life WITH NEXT GENERATION PROTEOMICS SOLUTIONS
 Innovative services and products for highly multiplexed protein discovery and quantification to help you understand the biological processes that shape life. Understanding life WITH NEXT GENERATION PROTEOMICS
Innovative services and products for highly multiplexed protein discovery and quantification to help you understand the biological processes that shape life. Understanding life WITH NEXT GENERATION PROTEOMICS
Multiple choice questions (numbers in brackets indicate the number of correct answers)
 1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
Application Note # ET-20 BioPharma Compass: A fully Automated Solution for Characterization and QC of Intact and Digested Proteins
 Application Note # ET-20 BioPharma Compass: A fully Automated Solution for Characterization and QC of Intact and Digested Proteins BioPharma Compass TM is a fully automated solution for the rapid characterization
Application Note # ET-20 BioPharma Compass: A fully Automated Solution for Characterization and QC of Intact and Digested Proteins BioPharma Compass TM is a fully automated solution for the rapid characterization
NPTEL VIDEO COURSE PROTEOMICS PROF. SANJEEVA SRIVASTAVA
 LECTURE-06 PROTEIN PURIFICATION AND PEPTIDE ISOLATION USING CHROMATOGRAPHY TRANSCRIPT Welcome to the proteomics course. Today, we will talk about protein purification and peptide isolation using chromatography
LECTURE-06 PROTEIN PURIFICATION AND PEPTIDE ISOLATION USING CHROMATOGRAPHY TRANSCRIPT Welcome to the proteomics course. Today, we will talk about protein purification and peptide isolation using chromatography
Molecular characterization, detection & quantitation of biological products Purin Charoensuksai, PhD
 Molecular characterization, detection & quantitation of biological products Purin Charoensuksai, PhD Department of Biopharmacy, Faculty of Pharmacy, Silpakorn University Example of critical checkpoints
Molecular characterization, detection & quantitation of biological products Purin Charoensuksai, PhD Department of Biopharmacy, Faculty of Pharmacy, Silpakorn University Example of critical checkpoints
Algorithm for Matching Additional Spectra
 Improved Methods for Comprehensive Sample Analysis Using Protein Prospector Peter R. Baker 1, Katalin F. Medzihradszky 1 and Alma L. Burlingame 1 1 Mass Spectrometry Facility, Dept. of Pharmaceutical Chemistry,
Improved Methods for Comprehensive Sample Analysis Using Protein Prospector Peter R. Baker 1, Katalin F. Medzihradszky 1 and Alma L. Burlingame 1 1 Mass Spectrometry Facility, Dept. of Pharmaceutical Chemistry,
CHAPTER 20 DNA TECHNOLOGY AND GENOMICS. Section A: DNA Cloning
 Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Exam MOL3007 Functional Genomics
 Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit
 Application Note 13 RNA Sample Preparation Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit B. Lam, PhD 1, P. Roberts, MSc 1 Y. Haj-Ahmad, M.Sc., Ph.D 1,2 1 Norgen
Application Note 13 RNA Sample Preparation Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit B. Lam, PhD 1, P. Roberts, MSc 1 Y. Haj-Ahmad, M.Sc., Ph.D 1,2 1 Norgen
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Tony Mire-Sluis Vice President, Corporate, Product and Device Quality Amgen Inc
 The Regulatory Implications of the ever increasing power of Mass Spectrometry and its role in the Analysis of Biotechnology Products Where do we draw the line? Tony Mire-Sluis Vice President, Corporate,
The Regulatory Implications of the ever increasing power of Mass Spectrometry and its role in the Analysis of Biotechnology Products Where do we draw the line? Tony Mire-Sluis Vice President, Corporate,
Proteomics. Areas of Application for Proteomics. Most Commonly Used Proteomics Techniques: Limitations: Examples
 Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Molecular Cell Biology - Problem Drill 11: Recombinant DNA
 Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
MagSi Beads. Magnetic Silica Beads for Life Science and Biotechnology study
 MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
Roche Molecular Biochemicals Technical Note No. LC 10/2000
 Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
CHAPTER 9 DNA Technologies
 CHAPTER 9 DNA Technologies Recombinant DNA Artificially created DNA that combines sequences that do not occur together in the nature Basis of much of the modern molecular biology Molecular cloning of genes
CHAPTER 9 DNA Technologies Recombinant DNA Artificially created DNA that combines sequences that do not occur together in the nature Basis of much of the modern molecular biology Molecular cloning of genes
Protein Microarrays?
 Protein Microarrays? Protein Chemistry/Proteomics Unit and the Neuroscience Program,Biomedicum Helsinki E-Mail: marc.baumann@helsinki.fi (http://research.med.helsinki.fi/corefacilities/proteinchem) What
Protein Microarrays? Protein Chemistry/Proteomics Unit and the Neuroscience Program,Biomedicum Helsinki E-Mail: marc.baumann@helsinki.fi (http://research.med.helsinki.fi/corefacilities/proteinchem) What
Liver Mitochondria Proteomics Employing High-Resolution MS Technology
 Liver Mitochondria Proteomics Employing High-Resolution MS Technology Jenny Ho, 1 Loïc Dayon, 2 John Corthésy, 2 Umberto De Marchi, 2 Antonio Núñez, 2 Andreas Wiederkehr, 2 Rosa Viner, 3 Michael Blank,
Liver Mitochondria Proteomics Employing High-Resolution MS Technology Jenny Ho, 1 Loïc Dayon, 2 John Corthésy, 2 Umberto De Marchi, 2 Antonio Núñez, 2 Andreas Wiederkehr, 2 Rosa Viner, 3 Michael Blank,
SUMOstar Gene Fusion Technology
 Gene Fusion Technology NEW METHODS FOR ENHANCING FUNCTIONAL PROTEIN EXPRESSION AND PURIFICATION IN INSECT CELLS White Paper June 2007 LifeSensors Inc. 271 Great Valley Parkway Malvern, PA 19355 www.lifesensors.com
Gene Fusion Technology NEW METHODS FOR ENHANCING FUNCTIONAL PROTEIN EXPRESSION AND PURIFICATION IN INSECT CELLS White Paper June 2007 LifeSensors Inc. 271 Great Valley Parkway Malvern, PA 19355 www.lifesensors.com
BIOLOGY LTF DIAGNOSTIC TEST DNA to PROTEIN & BIOTECHNOLOGY
 Biology Multiple Choice 016074 BIOLOGY LTF DIAGNOSTIC TEST DNA to PROTEIN & BIOTECHNOLOGY Test Code: 016074 Directions: Each of the questions or incomplete statements below is followed by five suggested
Biology Multiple Choice 016074 BIOLOGY LTF DIAGNOSTIC TEST DNA to PROTEIN & BIOTECHNOLOGY Test Code: 016074 Directions: Each of the questions or incomplete statements below is followed by five suggested
Protein preparation and digestion. P. aeruginosa strains were grown in in AB minimal medium
 Supplementary data itraq-based Proteomics Analyses Protein preparation and digestion. P. aeruginosa strains were grown in in AB minimal medium supplemented with 5g L -1 glucose for 48 hours at 37 C with
Supplementary data itraq-based Proteomics Analyses Protein preparation and digestion. P. aeruginosa strains were grown in in AB minimal medium supplemented with 5g L -1 glucose for 48 hours at 37 C with
Genetics Lecture 21 Recombinant DNA
 Genetics Lecture 21 Recombinant DNA Recombinant DNA In 1971, a paper published by Kathleen Danna and Daniel Nathans marked the beginning of the recombinant DNA era. The paper described the isolation of
Genetics Lecture 21 Recombinant DNA Recombinant DNA In 1971, a paper published by Kathleen Danna and Daniel Nathans marked the beginning of the recombinant DNA era. The paper described the isolation of
Please purchase PDFcamp Printer on to remove this watermark. DNA microarray
 DNA microarray Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. A DNA microarray is a multiplex technology used in molecular biology. It consists of
DNA microarray Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. A DNA microarray is a multiplex technology used in molecular biology. It consists of
Synthetic Biology. Sustainable Energy. Therapeutics Industrial Enzymes. Agriculture. Accelerating Discoveries, Expanding Possibilities. Design.
 Synthetic Biology Accelerating Discoveries, Expanding Possibilities Sustainable Energy Therapeutics Industrial Enzymes Agriculture Design Build Generate Solutions to Advance Synthetic Biology Research
Synthetic Biology Accelerating Discoveries, Expanding Possibilities Sustainable Energy Therapeutics Industrial Enzymes Agriculture Design Build Generate Solutions to Advance Synthetic Biology Research
Analysis of two mitochondrial functions in Saccharomyces cerevisiae
 PhD thesis Analysis of two mitochondrial functions in Saccharomyces cerevisiae Zsuzsanna Fekete Supervisors: Gyula Kispál, MD, PhD, Katalin Sipos, MD, PhD University of Pécs Medical School Pécs, 2010 Introduction
PhD thesis Analysis of two mitochondrial functions in Saccharomyces cerevisiae Zsuzsanna Fekete Supervisors: Gyula Kispál, MD, PhD, Katalin Sipos, MD, PhD University of Pécs Medical School Pécs, 2010 Introduction
ARBRE-P4EU Consensus Protein Quality Guidelines for Biophysical and Biochemical Studies Minimal information to provide
 ARBRE-P4EU Consensus Protein Quality Guidelines for Biophysical and Biochemical Studies Minimal information to provide Protein name and full primary structure, by providing a NCBI (or UniProt) accession
ARBRE-P4EU Consensus Protein Quality Guidelines for Biophysical and Biochemical Studies Minimal information to provide Protein name and full primary structure, by providing a NCBI (or UniProt) accession
Small-Molecule Drug Target Identification/Deconvolution Technologies
 Small-Molecule Drug Target Identification/Deconvolution Technologies Case-Studies Shantani Target ID Technology Tool Box Target Deconvolution is not Trivial = A single Tool / Technology May Not necessarily
Small-Molecule Drug Target Identification/Deconvolution Technologies Case-Studies Shantani Target ID Technology Tool Box Target Deconvolution is not Trivial = A single Tool / Technology May Not necessarily
The Five Key Elements of a Successful Metabolomics Study
 The Five Key Elements of a Successful Metabolomics Study Metabolomics: Completing the Biological Picture Metabolomics is offering new insights into systems biology, empowering biomarker discovery, and
The Five Key Elements of a Successful Metabolomics Study Metabolomics: Completing the Biological Picture Metabolomics is offering new insights into systems biology, empowering biomarker discovery, and
Strep-Tactin XT Spin Column
 Strep-Tactin XT Spin Column Purification Protocol Last date of revision Last June date 2017 of revision June 2017 Version PR90-0001 Version PR90-0001 For research use only Important licensing information
Strep-Tactin XT Spin Column Purification Protocol Last date of revision Last June date 2017 of revision June 2017 Version PR90-0001 Version PR90-0001 For research use only Important licensing information
Supplementary Figure 1. Utilization of publicly available antibodies in different applications.
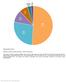 Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Expand Your Research with Metabolomics and Proteomics. Christine Miller Omics Market Manager ASMS 2017
 Expand Your Research with Metabolomics and Proteomics Christine Miller Omics Market Manager ASMS 2017 New Additions to Agilent Omics Workflows Acquisition Ion Mobility Q-TOF IM All Ions MS/MS Find Features
Expand Your Research with Metabolomics and Proteomics Christine Miller Omics Market Manager ASMS 2017 New Additions to Agilent Omics Workflows Acquisition Ion Mobility Q-TOF IM All Ions MS/MS Find Features
Total Test Questions: 66 Levels: Grades Units of Credit: 1.0 STANDARD 2. Demonstrate appropriate use of personal protective devices.
 DESCRIPTION Biotechnology is designed to create an awareness of career possibilities in the field of biotechnology. Students are introduced to diagnostic and therapeutic laboratory procedures that support
DESCRIPTION Biotechnology is designed to create an awareness of career possibilities in the field of biotechnology. Students are introduced to diagnostic and therapeutic laboratory procedures that support
Towards unbiased biomarker discovery
 Towards unbiased biomarker discovery High-throughput molecular profiling technologies are routinely applied for biomarker discovery to make the drug discovery process more efficient and enable personalised
Towards unbiased biomarker discovery High-throughput molecular profiling technologies are routinely applied for biomarker discovery to make the drug discovery process more efficient and enable personalised
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology
 Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Contents... vii. List of Figures... xii. List of Tables... xiv. Abbreviatons... xv. Summary... xvii. 1. Introduction In vitro evolution...
 vii Contents Contents... vii List of Figures... xii List of Tables... xiv Abbreviatons... xv Summary... xvii 1. Introduction...1 1.1 In vitro evolution... 1 1.2 Phage Display Technology... 3 1.3 Cell surface
vii Contents Contents... vii List of Figures... xii List of Tables... xiv Abbreviatons... xv Summary... xvii 1. Introduction...1 1.1 In vitro evolution... 1 1.2 Phage Display Technology... 3 1.3 Cell surface
Lecture 25 (11/15/17)
 Lecture 25 (11/15/17) Reading: Ch9; 328-332 Ch25; 990-995, 1005-1012 Problems: Ch9 (study-guide: applying); 1,2 Ch9 (study-guide: facts); 7,8 Ch25 (text); 1-3,5-7,9,10,13-15 Ch25 (study-guide: applying);
Lecture 25 (11/15/17) Reading: Ch9; 328-332 Ch25; 990-995, 1005-1012 Problems: Ch9 (study-guide: applying); 1,2 Ch9 (study-guide: facts); 7,8 Ch25 (text); 1-3,5-7,9,10,13-15 Ch25 (study-guide: applying);
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION
 M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
Solutions to Quiz II
 MIT Department of Biology 7.014 Introductory Biology, Spring 2005 Solutions to 7.014 Quiz II Class Average = 79 Median = 82 Grade Range % A 90-100 27 B 75-89 37 C 59 74 25 D 41 58 7 F 0 40 2 Question 1
MIT Department of Biology 7.014 Introductory Biology, Spring 2005 Solutions to 7.014 Quiz II Class Average = 79 Median = 82 Grade Range % A 90-100 27 B 75-89 37 C 59 74 25 D 41 58 7 F 0 40 2 Question 1
Total Test Questions: 71 Levels: Grades Units of Credit: 1.0 STANDARD 1 STUDENTS WILL INVESTIGATE THE PAST, PRESENT, AND FUTURE APPLICATIONS OF
 DESCRIPTION Biotechnology is designed to create an awareness of career possibilities in the field of biotechnology. Students are introduced to diagnostic and therapeutic laboratory procedures that support
DESCRIPTION Biotechnology is designed to create an awareness of career possibilities in the field of biotechnology. Students are introduced to diagnostic and therapeutic laboratory procedures that support
Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge
 Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge G. Scott*, H. Gaus #, B. Rivera*, and M. McGinley* *Phenomenex,
Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge G. Scott*, H. Gaus #, B. Rivera*, and M. McGinley* *Phenomenex,
Enabling Systems Biology Driven Proteome Wide Quantitation of Mycobacterium Tuberculosis
 Enabling Systems Biology Driven Proteome Wide Quantitation of Mycobacterium Tuberculosis SWATH Acquisition on the TripleTOF 5600+ System Samuel L. Bader, Robert L. Moritz Institute of Systems Biology,
Enabling Systems Biology Driven Proteome Wide Quantitation of Mycobacterium Tuberculosis SWATH Acquisition on the TripleTOF 5600+ System Samuel L. Bader, Robert L. Moritz Institute of Systems Biology,
MOLECULAR BIOLOGY OF EUKARYOTES 2016 SYLLABUS
 03-442 Lectures: MWF 9:30-10:20 a.m. Doherty Hall 2105 03-742 Advanced Discussion Section: Time and place to be announced Probably Mon 4-6 p.m. or 6-8p.m.? Once we establish who is taking the advanced
03-442 Lectures: MWF 9:30-10:20 a.m. Doherty Hall 2105 03-742 Advanced Discussion Section: Time and place to be announced Probably Mon 4-6 p.m. or 6-8p.m.? Once we establish who is taking the advanced
LC/MS/MS Solutions for Biomarker Discovery QSTAR. Elite Hybrid LC/MS/MS System. More performance, more reliability, more answers
 LC/MS/MS Solutions for Biomarker Discovery QSTAR Elite Hybrid LC/MS/MS System More performance, more reliability, more answers More is better and the QSTAR Elite LC/MS/MS system has more to offer. More
LC/MS/MS Solutions for Biomarker Discovery QSTAR Elite Hybrid LC/MS/MS System More performance, more reliability, more answers More is better and the QSTAR Elite LC/MS/MS system has more to offer. More
Proteomics. Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Limitations: Examples
 Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Self-test Quiz for Chapter 12 (From DNA to Protein: Genotype to Phenotype)
 Self-test Quiz for Chapter 12 (From DNA to Protein: Genotype to Phenotype) Question#1: One-Gene, One-Polypeptide The figure below shows the results of feeding trials with one auxotroph strain of Neurospora
Self-test Quiz for Chapter 12 (From DNA to Protein: Genotype to Phenotype) Question#1: One-Gene, One-Polypeptide The figure below shows the results of feeding trials with one auxotroph strain of Neurospora
Chapter 10 Analytical Biotechnology and the Human Genome
 Chapter 10 Analytical Biotechnology and the Human Genome Chapter Outline Enzyme tests and biosensors DNA-based tests DNA analysis technologies Human genome and genome-based analytical methods 1 Enzyme-based
Chapter 10 Analytical Biotechnology and the Human Genome Chapter Outline Enzyme tests and biosensors DNA-based tests DNA analysis technologies Human genome and genome-based analytical methods 1 Enzyme-based
Spectrum Mill MS Proteomics Workbench. Comprehensive tools for MS proteomics
 Spectrum Mill MS Proteomics Workbench Comprehensive tools for MS proteomics Meeting the challenge of proteomics data analysis Mass spectrometry is a core technology for proteomics research, but large-scale
Spectrum Mill MS Proteomics Workbench Comprehensive tools for MS proteomics Meeting the challenge of proteomics data analysis Mass spectrometry is a core technology for proteomics research, but large-scale
Introduction to Assay Development
 Introduction to Assay Development A poorly designed assay can derail a drug discovery program before it gets off the ground. While traditional bench-top assays are suitable for basic research and target
Introduction to Assay Development A poorly designed assay can derail a drug discovery program before it gets off the ground. While traditional bench-top assays are suitable for basic research and target
Purification of (recombinant) proteins. Pekka Lappalainen, Institute of Biotechnology, University of Helsinki
 Purification of (recombinant) proteins Pekka Lappalainen, Institute of Biotechnology, University of Helsinki Physical properties of proteins that can be applied for purification -size -charge (isoelectric
Purification of (recombinant) proteins Pekka Lappalainen, Institute of Biotechnology, University of Helsinki Physical properties of proteins that can be applied for purification -size -charge (isoelectric
Learning Objectives. Define RNA interference. Define basic terminology. Describe molecular mechanism. Define VSP and relevance
 Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Lecture 23: Metabolomics Technology
 MBioS 478/578 Bioinformatics Mark Lange Lecture 23: Metabolomics Technology Definitions and Background Technologies Nuclear Magnetic Resonance Mass Spectrometry General Introduction Fourier-Transform Mass
MBioS 478/578 Bioinformatics Mark Lange Lecture 23: Metabolomics Technology Definitions and Background Technologies Nuclear Magnetic Resonance Mass Spectrometry General Introduction Fourier-Transform Mass
Application Note. Authors. Abstract
 Automated, High Precision Tryptic Digestion and SISCAPA-MS Quantification of Human Plasma Proteins Using the Agilent Bravo Automated Liquid Handling Platform Application Note Authors Morteza Razavi, N.
Automated, High Precision Tryptic Digestion and SISCAPA-MS Quantification of Human Plasma Proteins Using the Agilent Bravo Automated Liquid Handling Platform Application Note Authors Morteza Razavi, N.
Proteomics software at MSI. Pratik Jagtap Minnesota Supercomputing institute
 Proteomics software at MSI. Pratik Jagtap Minnesota Supercomputing institute http://www.mass.msi.umn.edu/ Proteomics software at MSI. proteomics : emerging technology proteomics workflow search algorithms
Proteomics software at MSI. Pratik Jagtap Minnesota Supercomputing institute http://www.mass.msi.umn.edu/ Proteomics software at MSI. proteomics : emerging technology proteomics workflow search algorithms
Proteomics and Cancer
 Proteomics and Cancer Japan Society for the Promotion of Science (JSPS) Science Dialogue Program at Niitsu Senior High School Niitsu, Niigata September 4th 2006 Vladimir Valera, M.D, PhD JSPS Postdoctoral
Proteomics and Cancer Japan Society for the Promotion of Science (JSPS) Science Dialogue Program at Niitsu Senior High School Niitsu, Niigata September 4th 2006 Vladimir Valera, M.D, PhD JSPS Postdoctoral
Zool 3200: Cell Biology Exam 3 3/6/15
 Name: Trask Zool 3200: Cell Biology Exam 3 3/6/15 Answer each of the following questions in the space provided; circle the correct answer or answers for each multiple choice question and circle either
Name: Trask Zool 3200: Cell Biology Exam 3 3/6/15 Answer each of the following questions in the space provided; circle the correct answer or answers for each multiple choice question and circle either
2012 GENERAL [5 points]
![2012 GENERAL [5 points] 2012 GENERAL [5 points]](/thumbs/75/72452386.jpg) GENERAL [5 points] 2012 Mark all processes that are part of the 'standard dogma of molecular' [ ] DNA replication [ ] transcription [ ] translation [ ] reverse transposition [ ] DNA restriction [ ] DNA
GENERAL [5 points] 2012 Mark all processes that are part of the 'standard dogma of molecular' [ ] DNA replication [ ] transcription [ ] translation [ ] reverse transposition [ ] DNA restriction [ ] DNA
SUMOylated protein capture kit
 SUMOylated protein capture kit Cat no. A010-100 Capture and detect SUMOylated proteins High capacity, high specificity SUMO binding matrix Fast, convenient protein isolation using purification system provided
SUMOylated protein capture kit Cat no. A010-100 Capture and detect SUMOylated proteins High capacity, high specificity SUMO binding matrix Fast, convenient protein isolation using purification system provided
Využití cílené proteomiky pro kontrolu falšování potravin: identifikace peptidových markerů v mase pomocí LC- Q Exactive MS/MS
 Využití cílené proteomiky pro kontrolu falšování potravin: identifikace peptidových markerů v mase pomocí LC- Q Exactive MS/MS Michal Godula Ph.D. Thermo Fisher Scientific The world leader in serving science
Využití cílené proteomiky pro kontrolu falšování potravin: identifikace peptidových markerů v mase pomocí LC- Q Exactive MS/MS Michal Godula Ph.D. Thermo Fisher Scientific The world leader in serving science
DNA Microarray Technology
 CHAPTER 1 DNA Microarray Technology All living organisms are composed of cells. As a functional unit, each cell can make copies of itself, and this process depends on a proper replication of the genetic
CHAPTER 1 DNA Microarray Technology All living organisms are composed of cells. As a functional unit, each cell can make copies of itself, and this process depends on a proper replication of the genetic
Year III Pharm.D Dr. V. Chitra
 Year III Pharm.D Dr. V. Chitra 1 Genome entire genetic material of an individual Transcriptome set of transcribed sequences Proteome set of proteins encoded by the genome 2 Only one strand of DNA serves
Year III Pharm.D Dr. V. Chitra 1 Genome entire genetic material of an individual Transcriptome set of transcribed sequences Proteome set of proteins encoded by the genome 2 Only one strand of DNA serves
Module I: Introduction
 Module I: Introduction 20.109 Lecture 1 3 February, 2011 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology Appreciating nucleic acids (RNA in particular) as more
Module I: Introduction 20.109 Lecture 1 3 February, 2011 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology Appreciating nucleic acids (RNA in particular) as more
2054, Chap. 14, page 1
 2054, Chap. 14, page 1 I. Recombinant DNA technology (Chapter 14) A. recombinant DNA technology = collection of methods used to perform genetic engineering 1. genetic engineering = deliberate modification
2054, Chap. 14, page 1 I. Recombinant DNA technology (Chapter 14) A. recombinant DNA technology = collection of methods used to perform genetic engineering 1. genetic engineering = deliberate modification
XactEdit Cas9 Nuclease with NLS User Manual
 XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
Detecting Challenging Post Translational Modifications (PTMs) using CESI-MS
 Detecting Challenging Post Translational Modifications (PTMs) using CESI-MS Sensitive workflow for the detection of PTMs in biological samples from small sample volumes Klaus Faserl, 1 Bettina Sarg, 1
Detecting Challenging Post Translational Modifications (PTMs) using CESI-MS Sensitive workflow for the detection of PTMs in biological samples from small sample volumes Klaus Faserl, 1 Bettina Sarg, 1
Chapter 13 - Concept Mapping
 Chapter 13 - Concept Mapping Using the terms and phrases provided below, complete the concept map showing the discovery of DNA structure. amount of base pairs five-carbon sugar purine DNA polymerases Franklin
Chapter 13 - Concept Mapping Using the terms and phrases provided below, complete the concept map showing the discovery of DNA structure. amount of base pairs five-carbon sugar purine DNA polymerases Franklin
BIBC 103 Learning Goals with Supporting Learning Outcomes
 BIBC 103 Learning Goals with Supporting Learning Outcomes 1) Basic Lab Skills A. Conceptual understanding and moderate level of hands-on proficiency in making laboratory solutions, including understanding
BIBC 103 Learning Goals with Supporting Learning Outcomes 1) Basic Lab Skills A. Conceptual understanding and moderate level of hands-on proficiency in making laboratory solutions, including understanding
Newsletter Issue 7 One-STrEP Analysis of Protein:Protein-Interactions
 www.iba-biotagnology.com Newsletter Issue 7 One-STrEP Analysis of Protein:Protein-Interactions Strep-tag and One-STrEP-tag PPI Analysis with the co-precipitation/ mass spectrometry approach 3 Background
www.iba-biotagnology.com Newsletter Issue 7 One-STrEP Analysis of Protein:Protein-Interactions Strep-tag and One-STrEP-tag PPI Analysis with the co-precipitation/ mass spectrometry approach 3 Background
2. Outline the levels of DNA packing in the eukaryotic nucleus below next to the diagram provided.
 AP Biology Reading Packet 6- Molecular Genetics Part 2 Name Chapter 19: Eukaryotic Genomes 1. Define the following terms: a. Euchromatin b. Heterochromatin c. Nucleosome 2. Outline the levels of DNA packing
AP Biology Reading Packet 6- Molecular Genetics Part 2 Name Chapter 19: Eukaryotic Genomes 1. Define the following terms: a. Euchromatin b. Heterochromatin c. Nucleosome 2. Outline the levels of DNA packing
Chapter 11: Regulation of Gene Expression
 Chapter Review 1. It has long been known that there is probably a genetic link for alcoholism. Researchers studying rats have begun to elucidate this link. Briefly describe the genetic mechanism found
Chapter Review 1. It has long been known that there is probably a genetic link for alcoholism. Researchers studying rats have begun to elucidate this link. Briefly describe the genetic mechanism found
Heterologous protein expression systems
 IMBB-FORTH Heterologous protein expression systems What are they? Protein expression systems producing desired polypeptides from recombinant genes. Plasmids carry and express the desired recombinant genes
IMBB-FORTH Heterologous protein expression systems What are they? Protein expression systems producing desired polypeptides from recombinant genes. Plasmids carry and express the desired recombinant genes
RNA Isolation and Technology Applications. Nadine Nassif Senior Research Scientist Promega Corporation
 RNA Isolation and Technology Applications Nadine Nassif Senior Research Scientist Promega Corporation verview Brief overview of basic RNA/DNA chemistry. verview of total and poly(a+) RNA isolation. Discuss
RNA Isolation and Technology Applications Nadine Nassif Senior Research Scientist Promega Corporation verview Brief overview of basic RNA/DNA chemistry. verview of total and poly(a+) RNA isolation. Discuss
number Done by Corrected by Doctor Hamed Al Zoubi
 number 3 Done by Neda a Baniata Corrected by Waseem Abu Obeida Doctor Hamed Al Zoubi Note: it is important to refer to slides. Bacterial genetics *The main concepts we will talk about in this lecture:
number 3 Done by Neda a Baniata Corrected by Waseem Abu Obeida Doctor Hamed Al Zoubi Note: it is important to refer to slides. Bacterial genetics *The main concepts we will talk about in this lecture:
BIOTECHNOLOGY. Course Syllabus. Section A: Engineering Mathematics. Subject Code: BT. Course Structure. Engineering Mathematics. General Biotechnology
 BIOTECHNOLOGY Subject Code: BT Course Structure Sections/Units Section A Section B Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit 7 Section C Section D Section E Topics Engineering Mathematics General
BIOTECHNOLOGY Subject Code: BT Course Structure Sections/Units Section A Section B Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit 7 Section C Section D Section E Topics Engineering Mathematics General
2D gels and other high-resolution separations and analysis of intact proteins in biological i l samples
 February 2, 2010 2D gels and other high-resolution separations and analysis of intact proteins in biological i l samples Helen Kim 934-3880 helenkim@uab.edu Dept of Pharmacology and Toxicology McCallum
February 2, 2010 2D gels and other high-resolution separations and analysis of intact proteins in biological i l samples Helen Kim 934-3880 helenkim@uab.edu Dept of Pharmacology and Toxicology McCallum
STUDY GUIDE SECTION 10-1 Discovery of DNA
 STUDY GUIDE SECTION 10-1 Discovery of DNA Name Period Date Multiple Choice-Write the correct letter in the blank. 1. The virulent strain of the bacterium S. pneumoniae causes disease because it a. has
STUDY GUIDE SECTION 10-1 Discovery of DNA Name Period Date Multiple Choice-Write the correct letter in the blank. 1. The virulent strain of the bacterium S. pneumoniae causes disease because it a. has
