NIH Public Access Author Manuscript Cancer Cell. Author manuscript; available in PMC 2010 December 8.
|
|
|
- Holly Maria Martin
- 6 years ago
- Views:
Transcription
1 NIH Public Access Author Manuscript Published in final edited form as: Cancer Cell December 8; 16(6): doi: /j.ccr Tissue-penetrating delivery of compounds and nanoparticles into tumors Kazuki N. Sugahara 1,5, Tambet Teesalu 1,5, Priya Prakash Karmali 2, Venkata Ramana Kotamraju 1, Lilach Agemy 1, Olivier M. Girard 3, Douglas Hanahan 4, Robert F. Mattrey 3, and Erkki Ruoslahti 1,2,* 1 Vascular Mapping Center, Burnham Institute for Medical Research at UCSB, Biology II Bldg., University of California, Santa Barbara, CA Cancer Research Center, Burnham Institute for Medical Research, N. Torrey Pines Rd., La Jolla, CA Department of Radiology, University of California, San Diego, 408 Dickinson Street, San Diego, CA Department of Biochemistry and Biophysics, Diabetes and Comprehensive Cancer Centers, University of California, San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, U.S.A. SUMMARY Poor penetration of drugs into tumors is a major obstacle in tumor treatment. We describe a strategy for peptide-mediated delivery of compounds deep into the tumor parenchyma that employs a tumor homing peptide, irgd (CRGDK/RGPD/EC). Intravenously injected compounds coupled to irgd bound to tumor vessels and spread into the extravascular tumor parenchyma, whereas conventional RGD peptides only delivered the cargo to the blood vessels. irgd homes to tumors through a 3-step process: The RGD motif mediates binding to αv integrins on tumor endothelium, a proteolytic cleavage then exposes a binding motif for neuropilin-1, which mediates penetration into tissue and cells. Conjugation to irgd significantly improved the sensitivity of tumor imaging agents and enhanced the activity of an anti-tumor drug. Keywords tissue-penetrating peptide; tumor targeting; tumor blood vessels; integrins; neuropilin-1; MRI; tumor treatment SIGNIFICANCE Targeted delivery of compounds to tumor vessels and tumor cells can enhance tumor detection and therapy. Docking-based (synaphic) targeting strategies use peptides, antibodies and other molecules that bind to tumor vessels and tumor cells to deliver more drug to tumors than to normal tissues. A major obstacle to applying this principle has been the limited transport of the targeted payload into tumor parenchyma. The irgd peptide we describe here overcomes this limitation and establishes a capability for tissue-penetrating drug delivery. *Correspondence: ruoslahti@burnham.org, (phone), (fax). 5 These authors contributed equally to this work.
2 Sugahara et al. Page 2 INTRODUCTION RESULTS Identification of irgd The vasculature in different tissues expresses distinct biochemical signatures, the vascular zip codes (Ruoslahti, 2002; Ruoslahti and Rajotte, 2000). Vascular zip codes can serve as targets for docking-based (synaphic) delivery of diagnostics and therapeutics. αv-integrins are highly expressed in tumor vasculature, where they can be accessed with peptides containing the RGD integrin recognition motif (Eliceiri and Cheresh, 2001; Pierschbacher and Ruoslahti, 1984; Ruoslahti, 2002; Ruoslahti, 2003). RGD-based synaphic targeting has been successfully used to deliver drugs, biologicals (Arap et al., 1998; Curnis et al., 2004), imaging agents (Sipkins et al., 1998), viruses (Pasqualini et al., 1997; Wickham, 2000), and nanoparticles (Murphy et al., 2008) to tumor vasculature. However, crossing the vascular wall and penetrating into the tumor parenchyma against the elevated interstitial pressure in tumors remains a major challenge in tumor therapy (Heldin et al., 2004; Jain, 1990). We have recently identified a consensus R/KXXR/K motif as a mediator of cell and tissue penetration (Teesalu et al., 2009). The receptor for the R/KXXR/K motif was shown to be neuropilin-1. This motif is not active unless it occupies a C-terminal position in the peptide; we refer to this position effect as the C-end Rule (CendR). The interaction between the CendR motif and neuropilin-1 appears to be a key determinant for penetration of biological barriers. For example, vascular endothelial growth factor (VEGF)-165 and certain semaphorins bind to neuropilin-1 through C-terminal CendR motifs and thereby increase vascular permeability (Acevedo et al., 2008; Jia et al., 2006; Soker et al., 1998; Teesalu et al., 2009). In addition, many viruses possess CendR motifs within their capsid proteins, and often require proteolytic cleavage to expose the CendR motif to be infective, a process that requires penetration of biological barriers (Steinhauer, 1999; Teesalu et al., 2009). One such virus, HTLV-1, has been shown to utilize its CendR motif (KPXR) to bind to and internalize into immune cells in a neuropilin-1-dependent fashion to infect the cells (Lambert et al., 2009). We hypothesized that the tissue-penetrating properties of the CendR system could be used to deliver drugs and nanoparticles into tumor parenchyma, beyond the vascular barrier. Here we report results obtained with a peptide that combines tumor-homing and CendR-dependent tissue-penetrating properties. We also evaluate the potential of the technology for clinical applications by performing magnetic resonance imaging (MRI) and tumor treatment studies. We used a cyclic CX 7 C (C = cysteine; X = any amino acid) peptide library displayed on T7 phage (diversity approximately 10 9 ; Hoffman et al., 2003) to identify peptides that recognize tumor blood vessels in experimental metastasis mouse models of human prostate cancer (Figure S1). Three rounds of ex vivo phage display selection with cell suspensions from bone tumors were followed by one in vivo selection for homing to the bone tumors (Hoffman et al., 2003). The resulting phage pools bound to tumor-derived cell suspensions times more than the original library, and the binding to the tumor cell suspensions was 5 times higher than to cell suspensions from normal bone (Figure 1A). Individual phage clones were randomly picked from the phage pools and sequenced. Phage that contained the RGD motif (Pierschbacher and Ruoslahti, 1984; Ruoslahti, 2003) within three related sequences, CRGDKGPDC, CRGDRGPDC, and CRGDKGPEC, dominated in the selected pools (Figure 1B). CRGDKGPDC, which was most frequent, bound to cultured PPC1 human prostate cancer cells at 4 C, and appeared to internalize into them at 37 C (Figure 1C), and was named irgd (internalizing-rgd).
3 Sugahara et al. Page 3 Homing of irgd to tumors We synthesized a fluorescein-labeled irgd (FAM-iRGD) and intravenously injected the peptide into tumor-bearing mice. FAM-iRGD accumulated in tumor tissue in every model we tested; these include orthotopic xenografts of prostate, pancreatic ductal, and breast cancer, bone and brain xenografts of prostate carcinoma, and genetically engineered models of de novo pancreatic neuroendocrine (islet), pancreatic ductal, and cervical cancer (summarized in Table S1). The tumors, but not normal tissues, were strongly fluorescent under UV light (Figure 2A and Figure S2). Confocal microscopy revealed accumulation of FAM-iRGD peptide in and around tumor vessels and in tumor parenchyma (Figure 2B), but not in normal tissues (Figure S3). Remarkably, irgd phage (diameter about 65 nm; Sokoloff et al., 2000) and a synthetic irgd-coated nanoparticle, self-assembling micelles (diameter nm; Arleth et al., 2005;Karmali et al., 2009), also reached extravascular tumor parenchyma (Figures 2B). Two conventional RGD peptides and their corresponding peptide-displaying phage, CRGDC and RGD-4C, which both have strong affinities for αv integrins (Koivunen et al., 1993;Koivunen et al., 1995), also homed to tumors, but accumulated only in and around tumor blood vessels, and did not disperse throughout the interstitium like irgd (Figure 2B). Quantification of the area of peptide homing further demonstrated that irgd homes to and spreads within the tumor tissue far more efficiently than CRGDC (Figure 2C). The difference in the distribution of payloads linked to irgd and other RGD peptides was even more pronounced when a genetically engineered model of de novo pancreatic ductal adenocarcinoma (PDAC; Hezel et al., 2006) with an extensive amount of interstitium was used as the target (Figure S4). Whole body imaging of PDAC mice injected with FAM-iRGD micelles labeled with the near-infrared dye, Cy7, produced a strong and specific signal in the tumors, illustrating the potential of irgd for tumor targeting (Figure S5). Integrin-dependent binding of irgd to tumor cells The in vivo homing of irgd to tumors is dependent on the RGD motif. Control peptides including a non-integrin binding variant (Pierschbacher and Ruoslahti, 1984; Ruoslahti, 2003), CRGEKGPDC (irge), produced minimal tumor fluorescence (Figure 3A). Coinjecting an excess of unlabeled irgd peptide reduced the accumulation of FAM-iRGD in tumors, whereas irge peptide did not have such effect (Figure 3B). The in vitro binding of irgd to cancer cells also required the RGD motif because the binding of irgd phage to PPC1 cells at 4 C was inhibited dose dependently by free irgd peptide, but not by irge (Figure 3C). The RGD-directed integrins αvβ3, αvβ5, and α5β1, are upregulated in angiogenic endothelial cells and certain tumor cells (Eliceiri and Cheresh, 2001; Ruoslahti, 2002). PPC1 cells express αvβ5 and α5β1, but not αvβ3 (Figure S6A). An anti-αvβ5 antibody almost completely inhibited irgd phage binding to PPC1 cells, whereas inhibitory antibodies against α5β1 and other integrins had no effect (Figure S6B). M21 human melanoma cells (Cheresh and Spiro, 1987; Figure S7) expressing both αvβ3 and αvβ5 bound irgd phage, whereas variants lacking expression of these integrins did not, confirming the αv integrin dependency of irgd binding (Figure 3D). The binding of the irgd phage was reduced by both anti-αvβ3 or anti-αvβ5 (Figure 3D), indicating that irgd recognizes both of these integrins. The binding affinity of synthetic irgd peptide to purified αvβ3 integrin was K d = 17.8 ± 8.6 nm, and K d = 61.7 ± 13.3 nm to αvβ5 integrin (Table S2). The affinities of two previously identified cyclic RGD peptides, RGD-4C and CRGDC (Koivunen et al., 1993;Koivunen et al., 1995), for αvβ3 and αvβ5 were also in the same range, as shown by measuring the ability of each peptide to block irgd binding (Table S2). Thus, differences in integrin-binding specificity or affinity do not explain the distinct ability of irgd from the other two RGD
4 Sugahara et al. Page 4 peptides to penetrate tumor cells and tissue. Rather, the evidence implicates a second motif in irgd, dubbed the CendR motif, in this capability. irgd as a CendR peptide irgd contains a cryptic CendR motif, RGDK/R, and possesses CendR-like tissue and cell penetrating activities (Teesalu et al., 2009). However, the CendR motif in irgd is not C- terminal, which is a prerequisite for CendR activity (Teesalu et al., 2009). Therefore, we hypothesized that irgd, having been recruited to cell surfaces through the RGD-integrin interactions, is proteolytically processed and that the processing generates a C-terminal RGDK/ R sequence capable of binding to neuropilin-1 (schematized in Figure 4). To investigate this proteolysis hypothesis, we first examined whether irgd binds to PPC1 cells in a CendR-dependent manner after being treated by a protease to expose a C-terminal arginine or lysine; PPC1 cells have substantial levels of neuropilin-1 expression (Teesalu et al., 2009). Since the protease(s) that we postulate to cleave irgd is unknown, we chose to use trypsin for this purpose, as its specificity predicts that it would produce a derivative irgd peptide with the requisite C-terminal arginine or lysine. As shown in Figure 5A, trypsin treatment of irgd phage enhanced the binding of irgd phage to PPC1 cells to a level comparable to a prototypic CendR peptide, RPARPAR (Teesalu et al., 2009). Trypsin had no effect on phage expressing CRGDC (Figure 5A) or RGD-4C (not shown). The binding at 4 C of the trypsin-treated irgd phage was blocked by UV-treated non-infectious phage expressing RPARPAR, but not by phage displaying a peptide in which the CendR motif was disrupted by addition of an alanine residue to the C-terminus (RPARPARA; Teesalu et al., 2009). The binding of intact irgd phage was not affected by RPARPAR (not shown), supporting our hypothesis that irgd does not exhibit CendR features unless its CendR motif is activated by exposure at the C-terminus. To determine whether the CendR motif in irgd is indeed activated by cellular proteases, we incubated FAM-iRGD that carries the fluorophore at its N-terminus with PPC1 prostate cancer cells, and isolated intracellular products by affinity chromatography on anti-fitc (FAM) antibodies. To prevent cytoplasmic proteolysis, while allowing proteolysis at the cell surface, the incubation was done in the presence of a proteasome inhibitor. We detected no intracellular full-length FAM-iRGD, but recovered the FAM-CRGDK fragment (Figure S8). When irgd with FAM on its C-terminus was used, no intracellular irgd fragment was recovered (not shown). These results show that CRGDK, the N-terminal half of irgd with a C-terminal CendR motif, is the cell-penetrating fragment of irgd, as postulated in Figure 4. Prompted by the results indicating that proteolytically released CRGDK is the active cell penetrating component of irgd, we engineered phage expressing CRGDK, and found that it bound to and penetrated into PPC1 cells. The binding of the CRGDK phage was inhibited by CRGDK peptide indicating a specific binding (Figure 5B). The binding process was also inhibited by the RPARPAR peptide, which has a C-terminal arginine, but not by RPARPARA (Figure 5B). Antibodies against various integrins, including αv integrins, had no effect on the CRGDK binding, indicating that the binding is CendR-dependent and does not involve integrins (not shown). Moreover, an antibody against neuropilin-1, the receptor for CendR peptides (Teesalu et al., 2009), reduced the binding (Figure 5C). CRGDK phage did not substantially bind to or penetrate into M21 cells, which express only minimal amounts of neuropilin-1 (Figure S9). However, forced expression of neuropilin-1 in these cells (Teesalu et al., 2009) increased the binding (Figure 5D) and penetration (not shown) approximately 3.5 fold. Affinity measurements revealed that the CRGDK peptide binds to neuropilin-1 with a K d (1.4 ± 0.6 µm; Table S2) similar to that of RPARPAR (K d = 1.7 ± 0.2 µm; Teesalu et al., 2009). These results indicate that CRGDK binds to cells and penetrates into them using the CendR pathway.
5 Sugahara et al. Page 5 In keeping with the possibility that the RGDK CendR element is the cell-penetrating sequence in irgd, both CRGDK and RPARPAR UV-treated phage inhibited irgd phage penetration into PPC1 cells at 37 C (Figure 5E). Anti-neuropilin-1 also inhibited the penetration of irgd phage (Figure 5F), but had little effect on binding of irgd phage to PPC1 cells at 4 C (not shown). We further tested the relative roles of the RGD and RXXK motifs in irgd by using the irge phage, which does not bind to integrins due to the disrupted RGD motif (Pierschbacher and Ruoslahti, 1984; Ruoslahti, 2003), but still contains a CendR motif, RXXK. The irge phage did penetrate PPC1 cells at 37 C, and both RPARPAR and CRGDK inhibited the penetration (Figure 5E), implicating the CendR pathway. The penetration was less effective than that of irgd, presumably because irge lacks integrin binding that would concentrate the phage at the cell surface. These results indicate that irgd penetrates cells through the CendR pathway utilizing the RXXK sequence, and that the penetration is likely facilitated by initial binding to integrins through RGD. Confocal microscopy showed that irgd phage and neuropilin-1 co-localized in cultured cells (Figure S10), supporting the involvement of the neuropilin-1-dependent CendR pathway in the tumor cell penetration by irgd. Phage displaying an irgd variant that lacks the CendR motif (CRGDGGPDC) or the two other conventional RGD peptides, CRGDC and RGD-4C, did not co-localize with neuropilin-1, nor did they penetrate the cells efficiently. Neuropilin-1-dependent penetration of irgd within tumor tissue We next investigated the neuropilin-1 dependence of in vivo tissue penetration by irgd into PDAC. Pre-injection of tumor-bearing mice with a function-blocking anti-neuropilin-1 antibody inhibited the penetration of irgd phage in tumor tissue. The phage were trapped in the tumor blood vessels or stayed in close association with the vessels (Figure 6A, right panel). Pre-injection of control IgG did not affect the spreading of irgd (Figure 6A, left panel). Phage expressing the irgd variant that lacks the CendR motif but retains RGD (CRGDGGPDC) targeted tumor blood vessels, but did not spread into tumor tissue (not shown). Studies on the time dependence of irgd homing and penetration further supported the importance of αv integrin and neuropilin-1 expression in this process. FAM-iRGD peptide injected intravenously into mice bearing PDAC tumors initially co-localized with tumor blood vessels, which were positive for both αv integrins and neuropilin-1 (Figure 6B, upper panels, arrows). The peptide subsequently extravasated, presumably because it induces increased permeability in tumor vessels (Teesalu et al., 2009), and gradually appeared within tumor cells in ductal structures (Figure 6B, middle panels). Most of the tumor cells were positive for αv integrins (Figure 6B, left panels). Importantly, tumor cells strongly positive for neuropilin-1 were particularly effective at accumulating and retaining FAM-iRGD (Figure 6B, right panels). Similar results were obtained in an orthotopic prostate cancer xenograft model; the irgd peptide also homed to areas that over-express both αv integrins and neuropilin-1 in these tumors. The human 22Rv1 prostate cancer cells used in the model express αv-integrins and neuropilin-1 on the cell surface (Figures S11A and S11B). irgd in tumor imaging and treatment To investigate the potential of irgd for clinical applications, we performed MRI and therapeutic targeting experiments. For MRI, mice bearing 22Rv1 orthotopic xenografts were injected intravenously with irgd peptide-linked superparamagnetic iron oxide nanoworms (about 80 nm long and 30 nm thick; Park et al., 2009; Simberg et al., 2007). Iron oxide nanoparticles are evidenced as hypointensities in T2-weighted MR images (McAteer et al., 2007). In addition to hypointense vascular signals, the irgd-nanoworms gave low intensity regions that spread throughout the tumor, while CRGDC-nanoworms only decreased the intensity of the tumor vasculature (Figure 7, T2-weighted MR images). Untargeted-nanoworms
6 Sugahara et al. Page 6 DISCUSSION produced no detectable signal under identical imaging conditions. Confocal microscopy of the tumors confirmed the enhanced tissue penetration of the irgd-nanoworms (Figure 7, right most panels). Both the MRI results and optical imaging (Figure S5) indicate that irgd is capable of delivering diagnostics to tumors, and that tumors are more efficiently visualized with this peptide than with conventional RGD peptides. The ability of irgd to deliver anti-cancer drugs was investigated by treating mice bearing orthotopic 22Rv1 tumors with irgd-coated abraxane, a 130 nm nanoparticle consisting of albumin-embedded paclitaxel (Haley and Frenkel, 2008; Karmali et al., 2009). In vitro, irgdabraxane inhibited the proliferation of 22Rv1 cells more efficiently than abraxane conjugated with a cyclic RGD peptide without a CendR motif (CRGDC; Koivunen et al., 1993) or abraxane alone (Figure S12A). Intravenously injected irgd-abraxane spread more within tumor tissue than the other abraxane formulations (Figures S12B). Quantification of the drug showed that 8 fold more abraxane accumulated in the tumors from injections of irgd-abraxane than of non-targeted-abraxane (Figure 8A). CRGDC-abraxane concentration was only about 2 fold higher than that of non-targeted-abraxane in the tumors. In line with these results, irgdabraxane treatment resulted in significant inhibition of tumor growth at a dose at which untargeted abraxane showed no significant effect (Figure 8B); the slight reduction in tumor growth in the CRGDC group was not statistically significant. Treatment with the irgd peptide alone at a dose equivalent to its molar amount in irgd-abraxane did not affect tumor growth, indicating that the effect of irgd-abraxane was not due to the disruption of the integrin signaling by the irgd peptide. An additional treatment study in a subcutaneous 22Rv1 tumor model with time-dependent tumor volume measurements confirmed the treatment data obtained with the orthotopic tumors (Figure S13B). A similar biodistribution of the abraxane formulations was also observed in the subcutaneous and orthotopic tumors (Figure S13A). We next tested the efficacy of irgd-abraxane in a tumor model unrelated to 22Rv1. We chose orthotopic tumors generated with the BT474 human breast cancer cell line, which expresses both αv integrins and neuropilin-1 at the cell surface (Figure S14). In addition, the BT474 cells are more resistant to abraxane (paclitaxel) than 22Rv1 as shown in cytotoxicity assays (Figure S15). When injected intravenously into the tumor mice, the irgd-abraxane accumulated in the tumor 11 fold more than non-targeted-abraxane and about 4 fold more than the CRGDCabraxane (Figure 8C). Despite the resistance of the BT474 cells to paclitaxel, the irgdabraxane significantly inhibited the tumor growth in vivo (Figure 8D). The other abraxane formulations or irgd peptide alone did not show significant effects at the same dose. Together, these results clearly demonstrate the efficacy of irgd in drug delivery. Our results delineate a technology to deliver diagnostics and therapeutics into the extravascular tumor parenchyma using a unique tumor-specific homing peptide, irgd. The irgd peptide follows a multi-step tumor targeting mechanism; the intact peptide binds to the surface of cells expressing αv integrins, where it is proteolytically cleaved to produce the CRGDK fragment. This fragment then binds to neuropilin-1 and penetrates tumor cells and tissues (schematized in Figure 4). Several pieces of data support the model. First, the affinity of irgd for αv integrins is in the mid to low nanomolar range, similar to that of RGD peptides previously used in tumor targeting (Koivunen et al., 1993;Koivunen et al., 1995). Significantly, the proteolytically processed CRGDK fragment we identified within the targeted cells has lost most of its affinity to the integrins (about 50- to 150-fold reduction in affinity), which is in agreement with the observation that RGDK peptides lack cell attachment activity (Pierschbacher and Ruoslahti, 1984). Instead, the CRGDK fragment acquires an affinity for neuropilin-1 that is stronger than its residual affinity for αv integrins. These changes likely facilitate the transfer of CRGDK from integrins to neuropilin-1, and the resulting penetration activities. Each step in this multi-
7 Sugahara et al. Page 7 step process evidently adds to the tumor specificity of irgd. The expression of αv integrins is largely restricted to tumors (and other sites of angiogenesis or tissue repair), and neuropilin-1 is elevated in multiple tumor types (Eliceiri and Cheresh, 2001;Pellet-Many et al., 2008;Ruoslahti, 2002). The same may be true of the yet unknown processing protease(s). For example, matriptase, a membrane-bound protease, which preferentially cleaves proteins after a sequence similar to the R/KXXR/K CendR motif, is over-expressed in a number of tumor types (Uhland, 2006). The initial recruitment of irgd to cell surfaces appears to be crucial for its pronounced tumor targeting ability, since the related but non-integrin-binding peptide irge showed only modest uptake into cultured cells and was inefficient in targeting tumors in vivo. The presence of the tumor-specific recruitment element, RGD, distinguishes irgd from some previously described tumor-targeting peptides. Jiang et al. (Jiang et al., 2004) have described a design for tumor-homing peptides, in which a cationic cell-penetrating peptide is tethered to a negatively charged sequence that blocks the cell penetrating activity. The tether contains a recognition sequence for a protease known to be elevated in tumors. These authors achieved a 3-fold increase in tumor homing. The greater homing we achieved with irgd (12-fold over a control peptide) is likely due to the RGD-directed specific homing of the intact peptide. In addition, the recruitment of irgd to the cell surface through the RGD-integrin interaction is probably needed for the proteolytic cleavage that triggers the subsequent tumor penetration, as protease inhibitors are generally inactive on cell surfaces, but block proteolysis elsewhere (Hall et al., 1991). The unbiased screening we performed in identifying irgd may also have selected for a protease that is more readily available to cleave an incoming peptide than the proteases with known expression but unknown availability in tumors. Integrins shuttle between the cell surface and intracellular compartments (Pellinen and Ivaska, 2006). Certain viral pathogens take advantage of this mechanism in entering cells (Pellinen and Ivaska, 2006). However, as shown by our results, the cell penetrating activity of irgd is far greater than that of conventional RGD peptides. It far exceeds what can be accomplished with conventional RGD peptides and their mimics, which only take payloads to tumor vessels (this study; Murphy et al., 2008; Pasqualini et al., 1997). The strong tumor MRI signals provided by irgd-coated iron oxide nanoworms and the enhanced tumor growth suppression by irgd-linked abraxane demonstrate the potential of this peptide in tumor targeting. The molecular mechanism of the rapid tumor tissue penetration of irgd remains to be elucidated. However, several lines of evidence suggest that it may involve the so-called vascular permeabilization in the tumor induced by the CendR property of irgd. Molecules such as VEGF-165 and some semaphorins that have exposed CendR motifs increase vascular permeability (Jia et al., 2006; Acevedo et al., 2008). In addition, we have recently demonstrated that a prototypic CendR peptide RPARPAR induces vascular permeability (Teesalu et al., 2009). Some previously described tumor-specific cell-penetrating peptides that contain cryptic CendR sequences may share the same CendR-dependent tissue penetration mechanism (Hoffman et al., 2003; Joyce et al., 2003; Laakkonen et al., 2002; Porkka et al., 2002). For example, LyP-1 (CGNKRTRGC; Laakkonen et al., 2002), a cyclic peptide with a binding site for a specific receptor (Fogal et al., 2008) contains a cryptic CendR motif, KRTR. Like irgd nanoparticles, LyP-1-coated nanoparticles extravasate into tumor tissue within minutes after an intravenous injection (Karmali et al., 2009; Laakkonen et al., 2002; von Maltzahn et al., 2008). CendR involvement in the activities of LyP-1 and other tumor-penetrating homing peptides remains to be studied, but seems likely. irgd and the CendR system may help bring the magic bullet treatment of cancer closer to reality.
8 Sugahara et al. Page 8 EXPERIMENTAL PROCEDURES Tumor models Animal experimentation was performed according to procedures approved by the Animal Research Committees at the University of California, Santa Barbara, San Diego, and San Francisco, and the Burnham Institute for Medical Research. Xenografts were created by injecting nude mice with 10 6 human cancer cells orthotopically, subcutaneously, or into the tibia and the brain: prostate cancers PC-3 (Yang et al., 1999), PPC1 (Zhang et al., 2006), and 22Rv1 (Drake et al., 2005), pancreatic cancer MIA PaCa-2 (Sugahara et al., 2008), and breast cancer BT474 (Rusnak et al., 2001). Disseminated prostate tumors were generated by injecting 10 6 GFP-PC-3 cells (Yang et al., 1999) into the left ventricle of the heart of nude mice. Tumors were monitored with the X-ray system of the Image Station In Vivo FX (Eastman Kodak Company, Rochster, NY) or the Illumatool Bright Light System LT-9900 (Lightools Research, Encinitas, CA). Transgenic mice were maintained as described (Arbeit et al., 1994; Hanahan, 1985; Hezel et al., 2006). In vivo peptide and phage homing Preparation of micelles Approximately 200 µg of FAM-labeled synthetic peptides (Karmali et al., 2009) were intravenously injected into tumor-bearing mice and allowed to circulate for 15 min to 2 hrs. Tissues were collected and observed under UV light (Illumatool Bright Light System LT-9900), and processed for immunofluorescence (Karmali et al., 2009) or immunohistochemistry (Sugahara et al., 2008). To quantify the homing area of peptides within tumors, cryo-sections immunohistochemically stained with an anti-fitc antibody were scanned with the Scanscope CM-1 scanner and analyzed with the ImageScope software (Aperio Technologies, Vista, CA; Fogal et al., 2008). To assess phage homing (Zhang et al., 2006), 10 9 plaque-forming units (pfu) of T7 phage were intravenously injected into tumor-bearing mice, and allowed to circulate for 15 min. The mice were perfused through the heart with PBS containing 1% BSA and tissues were harvested for immunofluorescence. In some experiments, 50 µg of function-blocking anti-neuropilin-1 antibody (R&D Systems, Minneapolis, MN) or goat IgG (Abcam, Cambridge, MA) was intravenously injected into the tumor mice 15 min prior to the phage injections. The phage were allowed to circulate for 10 min prior to perfusion and collection of the tumors and other tissues. Lipids were purchased from Avanti Polar Lipids (Alabaster, AL). DSPE-PEG 2,000 -irgd(fam) was prepared by coupling FAM-iRGD peptide bearing a cysteine on its N-terminus to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-maleimide (polyethylene glycol) 2,000 (DSPE-PEG 2,000 -maleimide) at 1:1 molar ratio at room temperature for 4 hrs. DSPE-PEG 2,000 -FAM was prepared by coupling 1,2-distearoyl-sn-glycero-3- phosphoethanolamine-n-amino(polyethylene glycol) 2,000 (DSPE-PEG 2,000 -amine) with NHS-Fluorescein (Pierce Biotechnology, Rockford, IL) at a 1:1 molar ratio for 1 hr at room temperature. DSPE-PEG 2,000 -Cy7 was prepared similarly using 1,2-distearoyl-sn-glycero-3- phosphoethanolamine-n-amino(polyethylene glycol) 2,000 and Cy7-NHS ester (GE Healthcare, UK). DSPE-PEG 2,000 -irgd(fam), DSPE-PEG 2,000 -amine, and DSPE-PEG 2,000 -Cy7 in 3:6.7:0.3 molar ratios were dissolved in chloroform/methanol (3:1, v/v). The solvent was evaporated, and the dried lipid film was kept under vacuum for 8 hrs and allowed to swell in PBS for 2 hrs at 60 C. The vial was vortexed and sonicated to produce micelles. The micelles were
9 Sugahara et al. Page 9 sequentially filtered through 0.2 µm and 0.1 µm filters, and washed with sterile PBS to remove unreacted peptides. Control Cy7 micelles were prepared using DSPE-PEG 2,000 -FAM in place of DSPE-PEG 2,000 -irgd(fam). The micelles were nm in diameter as measured in deionized water by dynamic laser light scattering (refractive index, 1.59; viscosity, 0.89) on a Malvern Zetasizer Nano (Malvern, UK). Optical in vivo imaging of micelle-peptide conjugates Immunofluorescence PDAC mice were injected with 100 µl of 1 mm micelles in PBS. After 3 hrs, the mice were anesthetized, shaved, and subjected to whole body imaging with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Tissue sections were processed as described (Karmali et al., 2009). Cells were grown on collagen I-coated coverslips (BD Biosciences, San Jose, CA) overnight, and incubated with 10 8 pfu/ml of T7 phage for 30 min. The cells were fixed in 4% paraformaldehyde, and stained with antibodies and DAPI (Molecular Probes, Eugene, OR). The primary antibodies were rat anti-mouse CD31 monoclonal antibody (BD Biosciences), and rabbit anti-human αv integrin (Chemicon, Temecula, CA), rabbit anti-human neuropilin-1 (Chemicon), mouse anti-human neuropilin-1 (Miltenyi Biotec, Auburn, CA), and rabbit anti-t7 phage (Teesalu et al., 2009) polyclonal antibodies. The secondary antibodies, Alexa594 goat antibodies to mouse, rat, and rabbit IgG and Alexa488 donkey anti-rabbit antibody were from Molecular Probes. Cells and tissue sections were examined by a Fluoview 500 confocal microscope (Olympus America, Center Valley, PA). In vitro phage binding and penetration assays Flow cytometry Suspended cells (10 6 cells in DMEM containing 1% BSA) were incubated with 10 8 pfu/ml of T7 phage for 1 hr at 4 C. The cells were washed 4 times with the binding buffer, lysed with lysogeny broth containing 1% NP-40, and titrated. Phage penetration assays used the same procedure, except that the cells were incubated with phage at 37 C, and that an acidic buffer (500 mm sodium chloride, 0.1 M glycine, 1% BSA, ph 2.5) was substituted for the binding buffer in the second wash to remove the phage that bound to the cell surface. Inhibitors of binding and penetration were added 20 min prior to incubation with phage. Non-infectious phage were prepared by treating phage with UV for 8 min in DMEM containing 1% BSA. The UV-inactivated phage particles expressing about 200 peptides per particle were used as multivalent inhibitors. Free synthetic peptides, mouse antibodies against human α1, α2, αvβ3, αvβ5, α5β1, α4, or αv integrins and integrin subunits (Chemicon), goat anti-rat neuropilin-1 (R&D Systems), with mouse and goat IgG isotype controls (Abcam) were also tested. In some cases, 10 9 pfu phage were treated with 50 μg/ml of crystalline trypsin for 5 min at 37 C before use. The proteolytic reaction was terminated with 5 mg/ml of soy-bean inhibitor. The experiments were performed as described (Sugahara et al., 2003) except that 1 mm of MgSO 4, CaCl 2, and MnCl 2 were added to the buffer containing the integrin antibodies. The antibodies were the same as in the cell binding assays, and were detected with an Alexa488 goat anti-mouse or goat anti-rabbit antibody (Molecular Probes). The cells were analyzed with an EasyCyte Plus System (Guava Technologies, Hayward, CA). FAM-iRGD fragment isolation PPC1 cells (10 7 cells in DMEM) were treated with 10 µm carbobenzoxyl-leucinyl-leucinylleucinal (MG132; EMD Chemicals, Gibbstown, NJ) for 30 min at 37 C to inhibit proteasomes, and incubated with 20 µm of irgd peptide labeled with FAM at the N-terminus or C-terminus.
10 Sugahara et al. Page 10 Affinity measurements The cells were washed once with acidic buffer and lysed in MPER (Pierce Biotechnology) containing protease inhibitors (Complete Mini EDTA-free; Roche Applied Science, Indianapolis, IN) on ice for 30 min. The sample was centrifuged for 30 min at 12,000 rpm. The supernatant was applied onto an anti-fitc affinity column, and after washing, bound peptides were eluted with glycine-hcl, ph 2.8. The eluate was subjected to mass spectrometry. Binding affinities of irgd and CRGDK to αvβ3 and αvβ5 integrins (US Biological, Swampscott, MA) and to neuropilin-1 (R&D Systems) were quantified by an ELISA by measuring IC 50. First, saturation binding assays were performed. Microtiter wells coated with 5 µg/ml of the purified proteins were incubated for 1 hr at room temperature with various concentrations of biotinylated irgd or CRGDK peptide in a HEPES-based buffer containing 1 mm of MgSO 4 and CaCl 2 for integrin binding and PBS for neuropilin-1 binding. After washing with the same buffer added with 0.01% Tween 20, streptavidin-conjugated horseradish peroxidase (Vector laboratories, Burlingame, CA) was added to the wells and incubated for 30 min at room temperature. Peptide binding was quantified with 2,2-azino-bis (3-etylbenzthiazoline-6-sulfonic acid; Sigma-Aldrich, St. Louis, MO) as a substrate. In subsequent competition studies, microtiter wells coated with the proteins were incubated with various concentrations of non-labeled test peptides and a biotinylated reporter peptide at a concentration that gave half maximal binding in the saturation binding assay. After 1 hr incubation at room temperature, the binding of the biotinylated peptide was quantified as above. Affinities were determined from the inhibition data as described (Müller, 1980). Magnetic Resonance Imaging Nude mice bearing 22Rv1 orthotopic human prostate tumors were injected intravenously with superparamagnetic iron oxide nanoworms (Park et al., 2009) coated with irgd or CRGDC peptides, or untargeted-nanoworms at a dose of 5 mg/kg of iron. Each animal received Niliposomes (0.2 µmol of Ni) intravenously 1 hr prior to the nanoworms to increase the half-life of the nanoworms (Simberg et al., 2007). The mice were repeatedly imaged before and 3 and 7 hrs after injection of the nanoworms. For each scan, the mice were anesthetized with isoflurane and repositioned into a 30 mm diameter mouse coil. The axial plains were carefully matched to previous scans by measuring the height of the sections and comparing the vascular patterns in the images. Iron sensitive MRI scans consisting of T2-weighted fast spin-echo were acquired using a 3-Tesla MR imager (GE Healthcare, Milwaukee, WI). The conditions used were; repetition time/echo time = 6.4 s/70 ms, echo train length = 32, readout bandwidth = ± 15.6 khz, in-plane spatial resolution = 220 µm, field of view = (3.5 cm) 2, slice thickness = 1 mm, number of excitation = 3. After imaging, tissues of interest were harvested without perfusion and processed for immunofluorescence. Tumor treatment studies Peptide-conjugated abraxanes were prepared and characterized as described (Karmali et al., 2009). For in vitro cytotoxicity studies, 22Rv1 or BT474 cells were seeded in 96-well culture plates ( cells per well) and incubated overnight. The cells were incubated with various concentrations of the conjugates for 30 min at room temperature, and washed with fresh culture media. MTT assays (Invitrogen) to assess cell viability were performed on the cells 48 hrs later. For in vivo tumor treatment studies, nude mice bearing 2 week-old 22Rv1 orthotopic xenografts (typically about 250 mm 3 in tumor volume) were intravenously injected with the abraxane conjugates. The conjugates were given every other day for 14 days at a paclitaxel equivalent of 3 mg/kg/injection. The irgd peptide control was administered in an equivalent amount of irgd in each irgd-abraxane dose. Mice bearing subcutaneous 22Rv1 tumors were treated similarly for 12 days, and orthotopic BT474 tumors for 20 days. The experiments were
11 Sugahara et al. Page 11 Abraxane quantification Statistical analysis terminated according to the guidelines by the Animal Research Committee at the University of California, Santa Barbara. To study the homing pattern of the abraxane conjugates in the 22Rv1 orthotopic tumors, the conjugates were intravenously injected to tumor bearing mice at a dose of 3 mg/kg, and allowed to circulate for 3 hrs. The mice were perfused through the heart, and tissues of interest were harvested and processed for immunofluorescence. Mice bearing 22Rv1 or BT474 tumors were intravenously injected with the abraxane conjugates at a paclitaxel equivalent of 9 mg/kg/injection. After 3 hours, the mice were perfused through the heart, and tissues of interest were harvested. The tissues were homogenized in cold RIPA buffer (Pierce Biotechnology) containing protease inhibitors (Complete Mini EDTAfree) and kept on ice for 30 min. The samples were then centrifuged for 30 min at 14,000 rpm. The abraxane concentration in the supernatant was quantified with an ELISA: abraxane was captured with a taxol antibody (Novus Biologicals, Litleton, CO) coated onto a 96-well plate and detected with a human albumin antibody labeled with biotin (US Biological). Data were analyzed by two-tailed Student s t-test and one-way analysis of variance (ANOVA) followed by suitable post-hoc test. The details are given in Table S3. Supplementary Material Acknowledgments REFERENCES Refer to Web version on PubMed Central for supplementary material. We thank Drs. Michael J. Sailor and Ji-Ho Park for advice in the synthesis of iron oxide nanoworms, Dr. David A. Cheresh for the M21 and Dr. Robert M. Hoffman for the GFP-PC-3 cell lines, Dr. Eva Engvall for comments on the manuscript, and Drs. Lianglin Zhang and Tero Järvinen for advice on the phage display screens. We also thank Dr. Elizabeth Allen and Ehud Drori for support with transgenic mice, Jacqueline Corbeil for help with MRI, and Roslind Varghese for editing. This work was supported by NCI grants CA104898, CA115410, CA119414, and CA119335, and grant W81XWH from the Department of Defense. Acevedo LM, Barillas S, Weis SM, Göthert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 2008;111: [PubMed: ] Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998;279: [PubMed: ] Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J. Virol 1994;68: [PubMed: ] Arleth L, Ashok B, Onyuksel H, Thiyagarajan P, Jacob J, Hjelm RP. Detailed structure of hairy mixed micelles formed by phosphatidylcholine and PEGylated phospholipids in aqueous media. Langmuir 2005;21: [PubMed: ] Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem 1987;262: [PubMed: ] Curnis F, Gasparri A, Sacchi A, Longhi R, Corti A. Coupling tumor necrosis factor-alpha with alphav integrin ligands improves its antineoplastic activity. Cancer Res 2004;64: [PubMed: ] Drake JM, Gabriel CL, Henry MD. Assessing tumor growth and distribution in a model of prostate cancer metastasis using bioluminescence imaging. Clin. Exp. Metastasis 2005;22: [PubMed: ]
12 Sugahara et al. Page 12 Eliceiri BP, Cheresh DA. Adhesion events in angiogenesis. Curr. Opin. Cell Biol 2001;13: [PubMed: ] Fogal V, Zhang L, Krajewski S, Ruoslahti E. Mitochondrial/cell-surface protein p32/gc1qr as a molecular target in tumor cells and tumor stroma. Cancer Res 2008;68: [PubMed: ] Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol 2008;26: [PubMed: ] Hall SW, Humphries JE, Gonias SL. Inhibition of cell surface receptor-bound plasmin by α 2 -antiplasmin and α 2 -macroglobulin. J. Biol. Chem 1991;266: [PubMed: ] Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 1985;315: [PubMed: ] Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat. Rev. Cancer 2004;4: [PubMed: ] Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006;20: [PubMed: ] Hoffman JA, Giraudo E, Singh M, Zhang L, Inoue M, Porkka K, Hanahan D, Ruoslahti E. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell 2003;4: [PubMed: ] Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev 1990;9: [PubMed: ] Jia H, Bagherzadeh A, Hartzoulakis B, Jarvis A, Löhr M, Shaikh S, Aqil R, Cheng L, Tickner M, Esposito D, et al. Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in KDR signaling. J. Biol. Chem 2006;281: [PubMed: ] Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2004;101: [PubMed: ] Joyce JA, Laakkonen P, Bernasconi M, Bergers G, Ruoslahti E, Hanahan D. Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell 2003;4: [PubMed: ] Karmali PP, Kotamraju VR, Kastantin M, Black M, Missirlis D, Tirrell M, Ruoslahti E. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine 2009;5: [PubMed: ] Koivunen E, Gay DA, Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J. Biol. Chem 1993;268: [PubMed: ] Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N.Y.) 1995;13: [PubMed: ] Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med 2002;8: [PubMed: ] Lambert S, Bouttier M, Vassy R, Seigneuret M, Petrow-Sadowski C, Janvier S, Heveker N, Ruscetti FW, Perret G, Jones KS, Pique C. HTLV-1 uses HSPG and neuropilin 1 for entry by molecular mimicry of VEGF165. Blood. 2009in press McAteer MA, Sibson NR, von Zur Muhlen C, Schneider JE, Lowe AS, Warrick N, Channon KM, Anthony DC, Choudhury RP. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat. Med 2007;13: [PubMed: ] Müller R. Calculation of average antibody affinity in anti-hapten sera from data obtained by competitive radioimmunoassay. J. Immunol. Methods 1980;34: [PubMed: ] Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc. Natl. Acad. Sci. USA 2008;105: [PubMed: ] Park JH, von Maltzahn G, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ. Systematic Surface Engineering of Magnetic Nanoworms for In vivo Tumor Targeting. Small 2009;5: [PubMed: ]
13 Sugahara et al. Page 13 Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat. Biotechnol 1997;15: [PubMed: ] Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem. J 2008;411: [PubMed: ] Pellinen T, Ivaska J. Integrin traffic. J. Cell Sci 2006;119: [PubMed: ] Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984;309: [PubMed: ] Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Natl. Acad. Sci. USA 2002;99: [PubMed: ] Ruoslahti E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002;2: [PubMed: ] Ruoslahti E. The RGD story: a personal account. Matrix Biol 2003;22: [PubMed: ] Ruoslahti E, Rajotte D. An address system in the vasculature of normal tissues and tumors. Annu. Rev. Immunol 2000;18: [PubMed: ] Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM. The effects of the novel, reversible epidermal growth factor receptor/ ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer. Ther 2001;1: [PubMed: ] Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, Derfus AM, Yang M, Hoffman RM, Bhatia S, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl. Acad. Sci. USA 2007;104: [PubMed: ] Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphavbeta3-targeted magnetic resonance imaging. Nat. Med 1998;4: [PubMed: ] Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998;92: [PubMed: ] Sokoloff AV, Bock I, Zhang G, Sebestyén MG, Wolff JA. The interactions of peptides with the innate immune system studied with use of T7 phage peptide display. Mol. Ther 2000;2: [PubMed: ] Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 1999;258:1 20. [PubMed: ] Sugahara KN, Hirata T, Tanaka T, Ogino S, Takeda M, Terasawa H, Shimada I, Tamura J, ten Dam GB, van Kuppevelt TH, Miyasaka M. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44- dependent motility in tumor cells. Cancer Res 2008;68: [PubMed: ] Sugahara KN, Murai T, Nishinakamura H, Kawashima H, Saya H, Miyasaka M. Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J. Biol. Chem 2003;278: [PubMed: ] Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end Rule: peptides with C-terminal arginine cause neuropilin-1 dependent internalization, vascular leakage and tissue penetration. Proc. Natl. Acad. Sci. USA. 2009in press Uhland K. Matriptase and its putative role in cancer. Cell. Mol. Life Sci 2006;63: [PubMed: ] von Maltzahn G, Ren Y, Park JH, Min DH, Kotamraju VR, Jayakumar J, Fogal V, Sailor MJ, Ruoslahti E, Bhatia SN. In vivo tumor cell targeting with "click" nanoparticles. Bioconjug. Chem 2008;19: [PubMed: ] Wickham TJ. Targeting adenovirus. Gene Ther 2000;7: [PubMed: ] Yang M, Jiang P, Sun FX, Hasegawa S, Baranov E, Chishima T, Shimada H, Moossa AR, Hoffman RM. A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res 1999;59: [PubMed: ] Zhang L, Giraudo E, Hoffman JA, Hanahan D, Ruoslahti E. Lymphatic zip codes in premalignant lesions and tumors. Cancer Res 2006;66: [PubMed: ]
14 Sugahara et al. Page 14 Figure 1. Identification of irgd peptide (A) A representative example of the enrichment obtained in phage library screens on prostate cancer bone metastases. Three screens with similar results were performed. (B) RGD peptides selected in the screens. Approximately 50 individual clones were randomly picked for sequencing from phage pools recovered in the final round of ex vivo phage display. Clones that gave unsuccessful sequencing results were omitted during the analysis. The proportion of each RGD peptide is shown. (C) Binding and internalization to PPC1 human prostate cancer cells of phage expressing the CRGDKGPDC peptide. PPC1 cells were incubated with phage displaying CRGDKGPDC or a polyglycine control peptide CG 7 C for 1 hr at 4 C or 37 C. To assess internalization, phage bound at the cell surface was removed by washing the cells with an acid buffer. Note that the internalization of CRGDKGPDC phage occurs at 37 C but not at 4 C. Statistical analysis was performed with Student s t-test. n = 3, error bars, s.e.m.; triple asterisk, p <
15 Sugahara et al. Page 15 Figure 2. In vivo tumor homing of irgd peptide (A) Approximately 200 µg of FAM-iRGD or control peptide in PBS was intravenously injected into Kras G12D, p48-cre, Ink4a +/ mice bearing de novo pancreatic ductal adenocarcinoma. The peptides were allowed to circulate for 2 hrs and organs were collected and viewed under UV light. Arrowheads point to the tumors. Dotted lines show where the organs were placed. Representative images from four experiments are shown. (B) Confocal images of orthotopic 22Rv1 human prostate cancer xenografts from mice injected with the indicated peptides, phage, and micelles. irgd was compared to a similar integrinbinding but non-penetrating peptide, CRDGC. The circulation time was 2 hrs for the free peptides, 15 min for the phage, and 3 hrs for the micelles. Red: CD31; green: peptides, phage,
16 Sugahara et al. Page 16 or micelles; blue: nuclei. Arrows point to CRGDC compounds in or just outside the vessel walls, illustrating its homing to the tumor vasculature. Representative fields from multiple sections of five tumors are shown. Scale bars = 50 µm. (C) Quantification of tumor homing area of irgd and CRGDC peptides. Cryo-sections of 22Rv1 orthotopic tumors from mice injected with FAM-iRGD or FAM-CRGDC peptide were immunohistochemically stained with an anti-fitc antibody. The samples were subjected to image analysis with Scanscope CM-1 scanner for quantification of the FAM-positive areas. Statistical analysis was performed with Student s t-test. n = 3; error bars, s.e.m.; triple asterisk, p <
17 Sugahara et al. Page 17 Figure 3. irgd binds to αv integrins (A) Quantification of the in vivo distribution of irgd and control peptides. FAM-iRGD; a non-integrin-binding irgd mutant, FAM-CRGEKGPDC (FAM-iRGE); and a FAM-labeled cyclic polyglycine control peptide, FAM-CG 7 C were injected into PDAC mice as described in the legend of Figure 2A. Fluorescence in each tissue was quantified with Image J. (B) Inhibition of the tumor homing of FAM-iRGD with non-labeled irgd. A 10-fold excess of unlabeled irgd peptide or irge peptide was injected 30 min before the injection of FAMiRGD in PDAC mice. Fluorescence was quantified as above. FAM-iRGD homing without injection of unlabeled peptide was considered as 100%.
18 Sugahara et al. Page 18 (C) Dose-dependent inhibition of irgd phage binding to PPC1 prostate cancer cells by synthetic irgd peptide and irge. irgd phage binding without inhibitors was considered as 100%. (D) Inhibition of irgd phage binding to αv integrin expressing M21 cells by antibodies against integrins or control mouse IgG (left panel). Lack of irgd phage binding to M21 cells selected for low level of αv integrin expression (M21L; right panel). Statistical analyses were performed with Student s t-test in panels (A) and (B), and ANOVA in panels (C) and (D). n = 3; error bars, s.e.m.; single asterisk, p < 0.05; double asterisk, p < 0.01; triple asterisk, p <
19 Sugahara et al. Page 19 Figure 4. Multistep binding and penetration mechanism of irgd The irgd peptide accumulates at the surface of αv integrin-expressing endothelial and other cells in tumors. The RGD motif mediates the integrin binding. The peptide is cleaved by a cell surface-associated protease(s) to expose the cryptic CendR element, RXXK/R, at the C- terminus (red dotted line). The CendR element then mediates binding to neuropilin-1, with resulting penetration of cells and tissues. The peptide can penetrate into tumor cells and tissues with a cargo, such as a simple chemical or a nanoparticle, provided that the cargo is attached to the N-terminus of the irgd peptide because the disulfide bond apparently breaks before the peptide is internalized (black line).
20 Sugahara et al. Page 20 Figure 5. CendR motif in irgd penetration of tumor cells (A) The penetration of trypsin-treated irgd phage within PPC1 cells pre-treated or not with non-infectious RPARPAR or RPARPARA phage. (B) Inhibition of CRGDK phage binding to PPC1 by synthetic CRGDK, RPARPAR, and RPARPARA peptides. CRGDK phage binding without inhibitors was considered as 100%. (C) CRGDK phage binding to PPC1 cells treated with anti-neuropilin-1 blocking antibodies (anti-nrp-1) or control goat-igg. (D) CRGDK phage binding to M21 cells transfected with neuropilin-1 cdna to induce forced expression of neuropilin-1 (NRP-1), vector alone, or without transfection.
21 Sugahara et al. Page 21 (E) Inhibition of irgd and irge phage penetration into PPC1 by non-infectious phage displaying the CendR peptides RPARPAR and CRGDK. (F) Dose-dependent inhibition of irgd phage penetration of PPC1 cells by anti-neuropilin-1 antibodies (anti-nrp-1) to block neuropilin-1 function. Statistical analyses were performed with ANOVA in panels (A), (B), and (E), and Student s t-test in panels (C), (D), and (F). n = 3; error bars, s.e.m.; single asterisk, p < 0.05; double asterisk, p < 0.01; triple asterisk, p <
22 Sugahara et al. Page 22 NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript Figure 6. Penetration of irgd within tumor tissue involves neuropilin-1 (A) Neuropilin-1-dependent spreading of irgd phage within tumor tissue. Confocal images of PDAC tumors from transgenic mice pre-injected with a function-blocking anti-neuropilin-1 antibody (anti-nrp-1) or an IgG-control, followed by injection with the irgd phage. Green, phage; red, CD31; blue, DAPI. Arrows and asterisks represent blood vessels and tumor ducts, respectively. Representative images from three independent sets of studies are shown. Scale bars = 50 µm. (B) Time-dependent homing of FAM-iRGD peptide (green) in relation to the expression of αv integrins (red, left panels) and neuropilin-1 (red, right panels) in PDACs. The blood vessels targeted by FAM-iRGD were positive for both αv integrins and neuropilin-1 (arrows). The
23 Sugahara et al. Page 23 inset shows CD31 staining (magenta) of a blood vessel targeted by FAM-iRGD. Nearly all tumor ducts examined were positive for αv integrins. Tumor cells (arrowheads) and tumor ducts (asterisks) also strongly positive for neuropilin-1 were particularly effective in internalizing and retaining FAM-iRGD. Representative images from three independently studied tumors at each time point are shown. Scale bars = 50 µm.
24 Sugahara et al. Page 24 NIH-PA Author Manuscript NIH-PA Author Manuscript Figure 7. Tumor imaging with irgd-coated iron oxide nanoworms NIH-PA Author Manuscript T2-weighted MR images of mice bearing orthotopic 22Rv1 human prostate tumors. The mice were injected intravenously with iron oxide nanoworms coated with irgd, CRGDC, or no peptide (5 mg/kg of iron). Shown are axial images through the tumors acquired by repeated imaging before the nanoworm injection (Pre-injection) and 3 hrs or 7 hrs after the injection. The orientation of the tumors is slightly different between the time points because the mice were anesthetized for each scan and reintroduced in the MRI instrument. Gadolinium (Gd) was used as a reference for T1 and Feridex (Fe) for T2 imaging agents. Note the wide hypointensity areas indicating the spreading of irgd-nanoworms in the tumor interstitium. CRGDCnanoworms only decreased the intensity of tumor vessels, and the untargeted-nanoworms gave no signal in the tumor. Arrows point to the vasculature. T, tumor; B, urinary bladder. The rightmost panels represent the nanoworm distribution in tumor tissue examined by confocal microscopy. Green, nanoworms; red, CD31; blue, DAPI. Scale bars = 100 µm. The images are representative of multiple tumor mice; irgd-nanoworms, n = 5; CRGDC-nanoworms, n = 3; untargeted-nanoworms, n = 3.
Kazuki N. Sugahara, Tambet Teesalu, Priya Prakash Karmali, Venkata Ramana Kotamraju, Lilach
 Cancer Cell, Volume 16 Supplemental Data Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors Kazuki N. Sugahara, Tambet Teesalu, Priya Prakash Karmali, Venkata Ramana Kotamraju, Lilach
Cancer Cell, Volume 16 Supplemental Data Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors Kazuki N. Sugahara, Tambet Teesalu, Priya Prakash Karmali, Venkata Ramana Kotamraju, Lilach
Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors
 Article Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors Kazuki N. Sugahara, 1,5 Tambet Teesalu, 1,5 Priya Prakash Karmali, 2 Venkata Ramana Kotamraju, 1 Lilach Agemy, 1 Olivier M.
Article Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors Kazuki N. Sugahara, 1,5 Tambet Teesalu, 1,5 Priya Prakash Karmali, 2 Venkata Ramana Kotamraju, 1 Lilach Agemy, 1 Olivier M.
TITLE: Probing Tumor Microenvironment with In Vivo Phage Display
 AD Award Number: W81XWH-12-1-0174 TITLE: Probing Tumor Microenvironment with In Vivo Phage Display PRINCIPAL INVESTIGATOR: Kazuki N. Sugahara, M.D., Ph.D. CONTRACTING ORGANIZATION: Sanford Burnham Medical
AD Award Number: W81XWH-12-1-0174 TITLE: Probing Tumor Microenvironment with In Vivo Phage Display PRINCIPAL INVESTIGATOR: Kazuki N. Sugahara, M.D., Ph.D. CONTRACTING ORGANIZATION: Sanford Burnham Medical
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/science.1183057/dc1 Supporting Online Material for Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs Kazuki N. Sugahara, Tambet Teesalu,
www.sciencemag.org/cgi/content/full/science.1183057/dc1 Supporting Online Material for Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs Kazuki N. Sugahara, Tambet Teesalu,
Tumor tissues or cells were homogenized and proteins were extracted using
 SUPPLEMENTAL MATERIALS AND METHODS Western Blotting Tumor tissues or cells were homogenized and proteins were extracted using T-PER tissue protein extraction buffer. Protein concentrations were determined
SUPPLEMENTAL MATERIALS AND METHODS Western Blotting Tumor tissues or cells were homogenized and proteins were extracted using T-PER tissue protein extraction buffer. Protein concentrations were determined
Transtumoral targeting enabled by a novel neuropilin-binding peptide
 (2012) 31, 3754 3763 & 2012 Macmillan Publishers Limited All rights reserved 0950-9232/12 www.nature.com/onc ORIGINAL ARTICLE Transtumoral targeting enabled by a novel neuropilin-binding peptide L Roth
(2012) 31, 3754 3763 & 2012 Macmillan Publishers Limited All rights reserved 0950-9232/12 www.nature.com/onc ORIGINAL ARTICLE Transtumoral targeting enabled by a novel neuropilin-binding peptide L Roth
TITLE: Highly specific targeting of the TMPRSS2/ERG fusion gene in prostate cancer using liposomal nanotechnology
 AD Award Number: W81XWH-09-1-0385 TITLE: Highly specific targeting of the TMPRSS2/ERG fusion gene in prostate cancer using liposomal nanotechnology PRINCIPAL INVESTIGATOR: Bulent Ozpolat, M.D., Ph.D. Michael
AD Award Number: W81XWH-09-1-0385 TITLE: Highly specific targeting of the TMPRSS2/ERG fusion gene in prostate cancer using liposomal nanotechnology PRINCIPAL INVESTIGATOR: Bulent Ozpolat, M.D., Ph.D. Michael
Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor
 SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
Table S1. List of antibodies used including isotype controls, biotinylated. secondaries, and fluorophore conjugated tertiary antibodies.
 Table S1. List of antibodies used including isotype controls, biotinylated secondaries, and fluorophore conjugated tertiary antibodies. Antibody Description Distributor Catalogue number Working Concentration
Table S1. List of antibodies used including isotype controls, biotinylated secondaries, and fluorophore conjugated tertiary antibodies. Antibody Description Distributor Catalogue number Working Concentration
CONTRACTING ORGANIZATION: Sanford Burnham Medical Research Institute La Jolla, CA 92037
 AD Award Number: W81XWH-12-1-0173 TITLE: Probing Tumor Microenvironment with In Vivo Phage Display PRINCIPAL INVESTIGATOR: Dr. Erkki Ruoslahti CONTRACTING ORGANIZATION: Sanford Burnham Medical Research
AD Award Number: W81XWH-12-1-0173 TITLE: Probing Tumor Microenvironment with In Vivo Phage Display PRINCIPAL INVESTIGATOR: Dr. Erkki Ruoslahti CONTRACTING ORGANIZATION: Sanford Burnham Medical Research
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
phab Amine and Thiol Reactive Dyes
 TECHNICAL MANUAL phab Amine and Thiol Reactive Dyes Instructions for Use of Products G9831, G9835, G9841 and G9845 Revised 12/17 TM451 phab Amine and Thiol Reactive Dyes All technical literature is available
TECHNICAL MANUAL phab Amine and Thiol Reactive Dyes Instructions for Use of Products G9831, G9835, G9841 and G9845 Revised 12/17 TM451 phab Amine and Thiol Reactive Dyes All technical literature is available
SUPPLEMENTARY FIGURES and LEGENDS
 DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency Nikolas Stefan 1, 3, Patricia Martin-Killias 1, 3, Sascha Wyss-Stoeckle 1,
DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency Nikolas Stefan 1, 3, Patricia Martin-Killias 1, 3, Sascha Wyss-Stoeckle 1,
BEH.462/3.962J Molecular Principles of Biomaterials Spring 2003
 Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Supporting information. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo
 Supporting information Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo Greg Thurber 1, Katy Yang 1, Thomas Reiner 1, Rainer Kohler 1, Peter Sorger 2, Tim Mitchison
Supporting information Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo Greg Thurber 1, Katy Yang 1, Thomas Reiner 1, Rainer Kohler 1, Peter Sorger 2, Tim Mitchison
Ultrasound Contrast Agents for Molecular Imaging
 Ultrasound Contrast Agents for Molecular Imaging Design and Development Joshua Rychak, PhD Vice President for Research Targeson, Inc Adj. Assistant Professor Dept of Bioengineering University of California,
Ultrasound Contrast Agents for Molecular Imaging Design and Development Joshua Rychak, PhD Vice President for Research Targeson, Inc Adj. Assistant Professor Dept of Bioengineering University of California,
phab Amine and Thiol Reactive Dyes
 TECHNICAL MANUAL phab Amine and Thiol Reactive Dyes Instructions for Use of Products G9831, G9835, G9841 and G9845 4/15 TM451 phab Amine and Thiol Reactive Dyes All technical literature is available at:
TECHNICAL MANUAL phab Amine and Thiol Reactive Dyes Instructions for Use of Products G9831, G9835, G9841 and G9845 4/15 TM451 phab Amine and Thiol Reactive Dyes All technical literature is available at:
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1
 Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
A histone H1-binding-aptide based apoptosis-imaging probe for monitoring
 Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information A histone H1-binding-aptide based apoptosis-imaging probe for monitoring
Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information A histone H1-binding-aptide based apoptosis-imaging probe for monitoring
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
Supplementary Figure 1 - Characterization of rbag3 binding on macrophages cell surface.
 Supplementary Figure 1 - Characterization of rbag3 binding on macrophages cell surface. (a) Human PDAC cell lines were treated as indicated in Figure 1 panel F. Cells were analyzed for FITC-rBAG3 binding
Supplementary Figure 1 - Characterization of rbag3 binding on macrophages cell surface. (a) Human PDAC cell lines were treated as indicated in Figure 1 panel F. Cells were analyzed for FITC-rBAG3 binding
What is an Aptamer? smallest unit of repeating structure
 What is an Aptamer? apto: mer: to fit smallest unit of repeating structure Aptamers are single stranded folded oligonucleotides that bind to molecular (protein) targets with high affinity and specificity
What is an Aptamer? apto: mer: to fit smallest unit of repeating structure Aptamers are single stranded folded oligonucleotides that bind to molecular (protein) targets with high affinity and specificity
APPLICATION NOTE Rev. 7/2017, v4.0 Fluorescent Nanodiamonds: Bio-applications. Physical and Fluorescence Properties
 APPLICATION NOTE Rev. 7/2017, v4.0 Fluorescent Nanodiamonds: Bio-applications Fluorescent nanodiamonds (FNDs) offer a unique alternative to currently existing fluorescent biomarkers. With exceptional photo
APPLICATION NOTE Rev. 7/2017, v4.0 Fluorescent Nanodiamonds: Bio-applications Fluorescent nanodiamonds (FNDs) offer a unique alternative to currently existing fluorescent biomarkers. With exceptional photo
Supporting Information
 Supporting Information Agemy et al. 10.1073/pnas.1114518108 SI Methods Cell Lines and Tumors. HUVEC (Lonza) were cultured using EBM- 2 medium with endothelial cell growth supplement (Lonza). Human astrocytoma
Supporting Information Agemy et al. 10.1073/pnas.1114518108 SI Methods Cell Lines and Tumors. HUVEC (Lonza) were cultured using EBM- 2 medium with endothelial cell growth supplement (Lonza). Human astrocytoma
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells
 Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Supplementary Information for
 Supplementary Information for Siglec-7 Engagement by GBS -protein Suppresses Pyroptotic Cell Death of Natural Killer Cells Jerry J. Fong a,b, Chih-Ming Tsai a,b, Sudeshna Saha a,b, Victor Nizet a,c,d Ajit
Supplementary Information for Siglec-7 Engagement by GBS -protein Suppresses Pyroptotic Cell Death of Natural Killer Cells Jerry J. Fong a,b, Chih-Ming Tsai a,b, Sudeshna Saha a,b, Victor Nizet a,c,d Ajit
Supplementary Table 1. PCR amplification conditions for each primer pair. Primer sequence
 - 1 - Supplementary Tables Supplementary Table 1. PCR amplification conditions for each primer pair Primer sequence FN1 S - CAAAGCAAGCCCGGTTGT AS - CGCTCCCACTGTTGATTTATCTG ITGα2 S - TTAGGTTACTCTGTGGCTGCAATT
- 1 - Supplementary Tables Supplementary Table 1. PCR amplification conditions for each primer pair Primer sequence FN1 S - CAAAGCAAGCCCGGTTGT AS - CGCTCCCACTGTTGATTTATCTG ITGα2 S - TTAGGTTACTCTGTGGCTGCAATT
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit
 ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
Adenovirus Titration Kit
 Adenovirus Titration Kit Catalog # LF-RK0001(1 kit) Immunostaining method for Quantitative Detection of Adenovirus For research use only Not for diagnostic or therapeutic procedures AbFrontier Science
Adenovirus Titration Kit Catalog # LF-RK0001(1 kit) Immunostaining method for Quantitative Detection of Adenovirus For research use only Not for diagnostic or therapeutic procedures AbFrontier Science
Study Small Molecule-Membrane Protein Binding Kinetics with. Nanodisc and Charge Sensitive Optical Detection
 Support Information Study Small Molecule-Membrane Protein Binding Kinetics with Nanodisc and Charge Sensitive Optical Detection Guangzhong Ma 1,2, Yan Guan 1,3, Shaopeng Wang 1*, Han Xu 4*, Nongjian Tao
Support Information Study Small Molecule-Membrane Protein Binding Kinetics with Nanodisc and Charge Sensitive Optical Detection Guangzhong Ma 1,2, Yan Guan 1,3, Shaopeng Wang 1*, Han Xu 4*, Nongjian Tao
York University School of Medicine, NY. Bhagavathi A. Narayanan, PhD 1, Caroline Cunnigham 1 and Amitabha Mazumder, MD 2. Title
 Title Suppression of Human Multiple Myeloma Cell Growth by TBL-12 in Combination with low doses of Velcade: Insight in to the modulation of IL-6/STAT-3 mechanisms Bhagavathi A. Narayanan, PhD 1, Caroline
Title Suppression of Human Multiple Myeloma Cell Growth by TBL-12 in Combination with low doses of Velcade: Insight in to the modulation of IL-6/STAT-3 mechanisms Bhagavathi A. Narayanan, PhD 1, Caroline
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
In Vitro Angiogenesis Assay Kit
 In Vitro Angiogenesis Assay Kit Catalog Number KA1323 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
In Vitro Angiogenesis Assay Kit Catalog Number KA1323 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
CytoSelect Tumor-Endothelium Adhesion Assay
 Product Manual CytoSelect Tumor-Endothelium Adhesion Assay Catalog Number CBA-215 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cancer metastasis comprises several
Product Manual CytoSelect Tumor-Endothelium Adhesion Assay Catalog Number CBA-215 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cancer metastasis comprises several
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
SUPPLEMENTARY INFORMATION
 VOLUME: 1 ARTICLE NUMBER: 0011 In the format provided by the authors and unedited. In situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy Chao Wang 1, 2, Wujin
VOLUME: 1 ARTICLE NUMBER: 0011 In the format provided by the authors and unedited. In situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy Chao Wang 1, 2, Wujin
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
Description of supplementary material file
 Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
BD IMag. Streptavidin Particles Plus - DM. Technical Data Sheet. Product Information
 Technical Data Sheet Streptavidin Particles Plus - DM Product Information Material Number: Size: Storage Buffer: 557812 5 ml Aqueous buffered solution containing BSA and 0.09% sodium azide. Description
Technical Data Sheet Streptavidin Particles Plus - DM Product Information Material Number: Size: Storage Buffer: 557812 5 ml Aqueous buffered solution containing BSA and 0.09% sodium azide. Description
0.5% Triton X-100 for 5 min at room temperature. Fixed and permeabilized cells were
 1 Supplementary Methods Immunohistochemistry EBC-1 cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by 0.5% Triton X-100 for 5 min at room temperature. Fixed and permeabilized
1 Supplementary Methods Immunohistochemistry EBC-1 cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by 0.5% Triton X-100 for 5 min at room temperature. Fixed and permeabilized
Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information
 Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information 1. Supplementary Figure S1-S10: Pages 2-11 2. Supplementary References:
Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information 1. Supplementary Figure S1-S10: Pages 2-11 2. Supplementary References:
Supplementary Figure 1 (A), (B), and (C) Docking of a physiologic ligand of integrin αvβ3, the tenth type III RGD domain of wild-type fibronectin
 Supplementary Figure 1 (A), (B), and (C) Docking of a physiologic ligand of integrin αvβ3, the tenth type III RGD domain of wild-type fibronectin (A), D1-CD2 (B), and the D1-CD2 variant 3 (C) to Adomain
Supplementary Figure 1 (A), (B), and (C) Docking of a physiologic ligand of integrin αvβ3, the tenth type III RGD domain of wild-type fibronectin (A), D1-CD2 (B), and the D1-CD2 variant 3 (C) to Adomain
1. Cross-linking and cell harvesting
 ChIP is a powerful tool that allows the specific matching of proteins or histone modifications to regions of the genome. Chromatin is isolated and antibodies to the antigen of interest are used to determine
ChIP is a powerful tool that allows the specific matching of proteins or histone modifications to regions of the genome. Chromatin is isolated and antibodies to the antigen of interest are used to determine
*Corresponding author. Tel: ;
 1 SUPPLEMENTARY DATA 2 3 4 5 6 7 8 9 10 11 Integrin 2 1 in nonactivated conformation can induce focal adhesion kinase signaling Maria Salmela 1, Johanna Jokinen 1,2, Silja Tiitta 1, Pekka Rappu 1, Holland
1 SUPPLEMENTARY DATA 2 3 4 5 6 7 8 9 10 11 Integrin 2 1 in nonactivated conformation can induce focal adhesion kinase signaling Maria Salmela 1, Johanna Jokinen 1,2, Silja Tiitta 1, Pekka Rappu 1, Holland
nanoprecipitation mpeg-pla 2 nd Emulsion mpeg-pla in DCM
 THERAPEUTIC NANOTECHNOLOGY LAB MODULE Location: BioNano Lab, 3119 Micro and Nanotechnology Laboratory (MNTL) Instructor: Jianjun Cheng, Assistant Professor of Materials Science and Engineering Lab Assistants:
THERAPEUTIC NANOTECHNOLOGY LAB MODULE Location: BioNano Lab, 3119 Micro and Nanotechnology Laboratory (MNTL) Instructor: Jianjun Cheng, Assistant Professor of Materials Science and Engineering Lab Assistants:
Page 1 of 5. Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR Cy µg (~7.5 nmol) MIR 7901
 Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Supplementary methods
 Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
FIVEphoton Biochemicals
 Apo E ELISA Kit Biotin Detection Antibody Format 96T. General Protocol. FIVEphoton Biochemicals For research use only. Not for diagnostics. Part No. mapoe-biotin Use this protocol as a general guide. For
Apo E ELISA Kit Biotin Detection Antibody Format 96T. General Protocol. FIVEphoton Biochemicals For research use only. Not for diagnostics. Part No. mapoe-biotin Use this protocol as a general guide. For
Atomic Force Microscope (AFM) analysis of GO and RGD-pyrene/GO complex
 Supporting information Materials and apparatus 1-Pyrenebutyric acid (PBA), N,N-Diisopropylethylamine (DIEA), Dipyrrolidino(Nsuccinimidyloxy)carbenium hexafluorophosphate (HSPyU), N,N-dimethylformamide
Supporting information Materials and apparatus 1-Pyrenebutyric acid (PBA), N,N-Diisopropylethylamine (DIEA), Dipyrrolidino(Nsuccinimidyloxy)carbenium hexafluorophosphate (HSPyU), N,N-dimethylformamide
A sensitive direct human telomerase activity assay Scott B Cohen & Roger R Reddel
 A sensitive direct human telomerase activity assay Scott B Cohen & Roger R Reddel Supplementary figure and text: Supplementary Figure 1 Titration of the sheep polyclonal htert antibody. Supplementary Methods
A sensitive direct human telomerase activity assay Scott B Cohen & Roger R Reddel Supplementary figure and text: Supplementary Figure 1 Titration of the sheep polyclonal htert antibody. Supplementary Methods
A New Peptide Drug Modality; Helix-Loop-Helix Technology. Interprotein Corporation
 A New Peptide Drug Modality; Helix-Loop-Helix Technology Interprotein Corporation 1 Interprotein Corporation Location: Osaka, Japan Year Established: 2001 CEO & President: Masato Hosoda www.interprotein.com
A New Peptide Drug Modality; Helix-Loop-Helix Technology Interprotein Corporation 1 Interprotein Corporation Location: Osaka, Japan Year Established: 2001 CEO & President: Masato Hosoda www.interprotein.com
with pentaglycine (GGGGG) peptide motifs. Reaction conditions: (1) hexamethylene
 Supplementary Figure 1. Sequential surface reaction scheme to modify polyurethane catheters with pentaglycine (GGGGG) peptide motifs. Reaction conditions: (1) hexamethylene diisocyanate/triethylamine;
Supplementary Figure 1. Sequential surface reaction scheme to modify polyurethane catheters with pentaglycine (GGGGG) peptide motifs. Reaction conditions: (1) hexamethylene diisocyanate/triethylamine;
Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP.
 Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP. Supplementary Figure 2. Synthesis of CP-PTX conjugate. Supplementary
Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP. Supplementary Figure 2. Synthesis of CP-PTX conjugate. Supplementary
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
More choices, better detection.
 3 Protein Detection More choices, better detection. Perform Western blotting experiments faster and easier with our new Thermo Scientific Pierce G2 Fast Blotter and accessories, and push your microscopy
3 Protein Detection More choices, better detection. Perform Western blotting experiments faster and easier with our new Thermo Scientific Pierce G2 Fast Blotter and accessories, and push your microscopy
Supplementary Figure 1 A green: cytokeratin 8
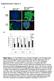 Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
PRODUCT DATA SHEET. Carboxylated Fluorescent Gold Nanoparticles. Description. Characteristics
 PRODUCT DATA SHEET Carboxylated Fluorescent Gold Nanoparticles Description Cytodiagnostics carboxylated fluorescent gold nanoparticles is a unique product that combines our Cyto fluorescent dyes and gold
PRODUCT DATA SHEET Carboxylated Fluorescent Gold Nanoparticles Description Cytodiagnostics carboxylated fluorescent gold nanoparticles is a unique product that combines our Cyto fluorescent dyes and gold
Zenon Goat IgG Labeling Kits
 Product Information Revised: 19 June 2007 Quick Facts Storage upon receipt: 2 6 C Protect from light Abs/Em: See Table 1 Unlabeled IgG antibody Zenon labeling reagent (labeled Fab fragment) Introduction
Product Information Revised: 19 June 2007 Quick Facts Storage upon receipt: 2 6 C Protect from light Abs/Em: See Table 1 Unlabeled IgG antibody Zenon labeling reagent (labeled Fab fragment) Introduction
InnoZyme TACE Activity Kit Cat. No. CBA042
 User Protocol CBA042 Rev. 26 January 2006 JSW Page 1 of 5 InnoZyme TACE Activity Kit Cat. No. CBA042 Note that this user protocol is not lot-specific and is representative of the current specifications
User Protocol CBA042 Rev. 26 January 2006 JSW Page 1 of 5 InnoZyme TACE Activity Kit Cat. No. CBA042 Note that this user protocol is not lot-specific and is representative of the current specifications
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry of cytochrome c and mitochondria.
 PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
Supplementary Information
 Supplementary Information Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors Wang et al. Supplementary Figure 1. The effect of Pt/Co ratio
Supplementary Information Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors Wang et al. Supplementary Figure 1. The effect of Pt/Co ratio
Supporting information
 Supporting information Bioengineered Boronic Esters Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment Wei Lv 1, 3#, Jianpei Xu 1#, Xiaoqi
Supporting information Bioengineered Boronic Esters Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment Wei Lv 1, 3#, Jianpei Xu 1#, Xiaoqi
Human IFN-α (Interferon-Alpha) Pre-Coated ELISA Kit
 Human IFN-α (Interferon-Alpha) Pre-Coated ELISA Kit Catalog No: 90-2235 1 96 well Format (96 tests) Detection Range: 15.6 1000 pg/ml Sensitivity:
Human IFN-α (Interferon-Alpha) Pre-Coated ELISA Kit Catalog No: 90-2235 1 96 well Format (96 tests) Detection Range: 15.6 1000 pg/ml Sensitivity:
Sulfenylated Protein Cell-Based Detection Kit
 Sulfenylated Protein Cell-Based Detection Kit Item No. 600320 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL
Sulfenylated Protein Cell-Based Detection Kit Item No. 600320 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL
tel: IRON OXIDE-BASED SUPERPARAMAGNETIC CONTRAST AGENTS
 tel:4006551678 email: fige007@163.com IRON OXIDE-BASED SUPERPARAMAGNETIC CONTRAST AGENTS Molday ION TM product line is a family of iron oxide-based superparamagnetic contrast reagents designed to label
tel:4006551678 email: fige007@163.com IRON OXIDE-BASED SUPERPARAMAGNETIC CONTRAST AGENTS Molday ION TM product line is a family of iron oxide-based superparamagnetic contrast reagents designed to label
Molecular Imaging. by Magnetic Resonance. Silvio Aime. University of Torino (Italy) Department of Chemistry IFM & Molecular Imaging Center
 Molecular Imaging by Magnetic Resonance Silvio Aime University of Torino (Italy) Department of Chemistry IFM & Molecular Imaging Center Molecular Imaging In vivo characterization and quantitative measurement
Molecular Imaging by Magnetic Resonance Silvio Aime University of Torino (Italy) Department of Chemistry IFM & Molecular Imaging Center Molecular Imaging In vivo characterization and quantitative measurement
Real-Time Monitoring of Arsenic Trioxide Release and Delivery
 Real-Time Monitoring of Arsenic Trioxide Release and Delivery by Activatable T 1 Imaging Zhenghuan Zhao, Xiaomin Wang, Zongjun Zhang, Hui Zhang, Hanyu Liu, Xianglong Zhu, Hui Li, Xiaoqin Chi, Zhenyu Yin,
Real-Time Monitoring of Arsenic Trioxide Release and Delivery by Activatable T 1 Imaging Zhenghuan Zhao, Xiaomin Wang, Zongjun Zhang, Hui Zhang, Hanyu Liu, Xianglong Zhu, Hui Li, Xiaoqin Chi, Zhenyu Yin,
A Role for Streptococcal Collagen-Like Protein 1 (Scl-1) in M1T1 Group A
 Supplemental Information for: A Role for Streptococcal Collagen-Like Protein 1 (Scl-1) in M1T1 Group A Streptococcus Resistance to Neutrophil Extracellular Traps Simon Döhrmann 1, Sabina Anik 1, Joshua
Supplemental Information for: A Role for Streptococcal Collagen-Like Protein 1 (Scl-1) in M1T1 Group A Streptococcus Resistance to Neutrophil Extracellular Traps Simon Döhrmann 1, Sabina Anik 1, Joshua
Application Note AN001
 Testing hybridoma supernatants with the Spots On Dots Antibody Screening Kit Application Note AN1 Table of Contents Overview... 2 Figure 1. Screening of hybridomas raised against peptide antigens... 3
Testing hybridoma supernatants with the Spots On Dots Antibody Screening Kit Application Note AN1 Table of Contents Overview... 2 Figure 1. Screening of hybridomas raised against peptide antigens... 3
64 CuCl 2 in 50 µl 0.1N NaOAc buffer, and 20 µg of each DOTA-antibody conjugate in 40 µl
 Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-16-1-0595 TITLE: Prostate-Specific Membrane Antigen (PSMA) Targeted Bio-orthogonal Therapy for Metastatic Prostate Cancer PRINCIPAL INVESTIGATOR: Dmitri Artemov., Ph.D. CONTRACTING
AWARD NUMBER: W81XWH-16-1-0595 TITLE: Prostate-Specific Membrane Antigen (PSMA) Targeted Bio-orthogonal Therapy for Metastatic Prostate Cancer PRINCIPAL INVESTIGATOR: Dmitri Artemov., Ph.D. CONTRACTING
Human TIMP-2. Pre-Coated ELISA Kit
 Human TIMP-2 (Metalloproteinase Inhibitor-2) Pre-Coated ELISA Kit Catalog No: 90-2125 1 96 well Format (96 tests) Detection Range: 0.156 10 ng/ml Sensitivity: < 0.094 ng/ml This immunoassay kit allows
Human TIMP-2 (Metalloproteinase Inhibitor-2) Pre-Coated ELISA Kit Catalog No: 90-2125 1 96 well Format (96 tests) Detection Range: 0.156 10 ng/ml Sensitivity: < 0.094 ng/ml This immunoassay kit allows
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay. Josephine MY Ko and Maria Li Lung *
 In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
Flow Cytometry - The Essentials
 Flow Cytometry - The Essentials Pocket Guide to Flow Cytometry: 1. Know your Cytometer 2. Understanding Fluorescence and Fluorophores 3. Gating Process 4. Controls 5. Optimization 6. Panel Building 7.
Flow Cytometry - The Essentials Pocket Guide to Flow Cytometry: 1. Know your Cytometer 2. Understanding Fluorescence and Fluorophores 3. Gating Process 4. Controls 5. Optimization 6. Panel Building 7.
The Development of an Indirect Competitive. Immunomagnetic-Proximity Ligation Assay for Small-Molecule. Detection
 The Development of an Indirect Competitive Immunomagnetic-Proximity Ligation Assay for Small-Molecule Detection Xuecheng Jiang, a Zhenhong Zhu, ab Zhihao Sun, a Luming Wang, a Lixiao Zhou, a Hanqiang Miao,
The Development of an Indirect Competitive Immunomagnetic-Proximity Ligation Assay for Small-Molecule Detection Xuecheng Jiang, a Zhenhong Zhu, ab Zhihao Sun, a Luming Wang, a Lixiao Zhou, a Hanqiang Miao,
Supporting Information: Core-Shell Nanoparticle-Based Peptide Therapeutics and Combined. Hyperthermia for Enhanced Cancer Cell Apoptosis
 Supporting Information: Core-Shell Nanoparticle-Based Peptide Therapeutics and Combined Hyperthermia for Enhanced Cancer Cell Apoptosis Birju P. Shah a, Nicholas Pasquale a, Gejing De b, Tao Tan b, Jianjie
Supporting Information: Core-Shell Nanoparticle-Based Peptide Therapeutics and Combined Hyperthermia for Enhanced Cancer Cell Apoptosis Birju P. Shah a, Nicholas Pasquale a, Gejing De b, Tao Tan b, Jianjie
Dynamic light scattering (DLS)-based immunoassay for. ultrasensitive detection of tumor marker protein
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2016 Dynamic light scattering (DLS)-based immunoassay for ultrasensitive detection of
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2016 Dynamic light scattering (DLS)-based immunoassay for ultrasensitive detection of
Supplementary Information Temperature-responsive Gene Silencing by a Smart Polymer
 Supplementary Information Temperature-responsive Gene Silencing by a Smart Polymer Mingming Wang, Yiyun Cheng * Shanghai Key Laboratory of Regulatory Biology, School of Life Sciences, East China Normal
Supplementary Information Temperature-responsive Gene Silencing by a Smart Polymer Mingming Wang, Yiyun Cheng * Shanghai Key Laboratory of Regulatory Biology, School of Life Sciences, East China Normal
SensoLyte Plus 520 MMP-1 Assay Kit *Fluorimetric and Enhanced Selectivity*
 SensoLyte Plus 520 MMP-1 Assay Kit *Fluorimetric and Enhanced Selectivity* Catalog # 72012 Kit Size 96 Assays in 96-well plate Optimized Performance: Optimal conditions for detecting MMP-1 activity in
SensoLyte Plus 520 MMP-1 Assay Kit *Fluorimetric and Enhanced Selectivity* Catalog # 72012 Kit Size 96 Assays in 96-well plate Optimized Performance: Optimal conditions for detecting MMP-1 activity in
Data Sheet CD137/NF-κB Reporter - HEK293 Recombinant Cell Line Catalog # 79289
 Data Sheet CD137/NF-κB Reporter - HEK293 Recombinant Cell Line Catalog # 79289 Background Human CD137 (4-1BB; TNFRS9) is an inducible co-stimulatory molecule that activates T cells. CD137:CD137L-mediated
Data Sheet CD137/NF-κB Reporter - HEK293 Recombinant Cell Line Catalog # 79289 Background Human CD137 (4-1BB; TNFRS9) is an inducible co-stimulatory molecule that activates T cells. CD137:CD137L-mediated
This Document Contains:
 This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
ApoTrack Cytochrome c Apoptosis ICC Antibody
 ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and nonapoptotic
ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and nonapoptotic
AnaTag HiLyte Fluor 488 Microscale Protein Labeling Kit
 AnaTag HiLyte Fluor 488 Microscale Protein Labeling Kit Revision number: 1.3 Last updated: April 2018 Catalog # AS-72048 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor
AnaTag HiLyte Fluor 488 Microscale Protein Labeling Kit Revision number: 1.3 Last updated: April 2018 Catalog # AS-72048 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor
AnaTag HiLyte Fluor 750 Microscale Protein Labeling Kit
 AnaTag HiLyte Fluor 750 Microscale Protein Labeling Kit Catalog # 72044 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 750 SE to proteins (e.g., IgG). It provides ample
AnaTag HiLyte Fluor 750 Microscale Protein Labeling Kit Catalog # 72044 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 750 SE to proteins (e.g., IgG). It provides ample
Supporting Information
 Supporting Information Supplementary Figure-1 Results of Phage Display Screening Against TNT The sequences seen in Supplementary Figure-1 represent the phages sequenced after the third and fourth rounds
Supporting Information Supplementary Figure-1 Results of Phage Display Screening Against TNT The sequences seen in Supplementary Figure-1 represent the phages sequenced after the third and fourth rounds
Supplementary Materials for. Combinatory screening of DNA aptamers for molecular imaging of HER2 in cancer
 Supplementary Materials for Combinatory screening of DNA aptamers for molecular imaging of HER2 in cancer Guizhi Zhu, Huimin Zhang,, Orit Jacobson, Zhantong Wang, Haojun Chen,, Xiangyu Yang, ǁ, Gang Niu,
Supplementary Materials for Combinatory screening of DNA aptamers for molecular imaging of HER2 in cancer Guizhi Zhu, Huimin Zhang,, Orit Jacobson, Zhantong Wang, Haojun Chen,, Xiangyu Yang, ǁ, Gang Niu,
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Award Number: W81XWH TITLE: Direct inhibition of Skp2 for the Treatment of Advanced Prostate Cancer. PRINCIPAL INVESTIGATOR: Hyun-Suk Lim
 AD Award Number: W81XWH-11-1-0286 TITLE: Direct inhibition of Skp2 for the Treatment of Advanced Prostate Cancer PRINCIPAL INVESTIGATOR: Hyun-Suk Lim CONTRACTING ORGANIZATION: Indiana University Indianapolis,
AD Award Number: W81XWH-11-1-0286 TITLE: Direct inhibition of Skp2 for the Treatment of Advanced Prostate Cancer PRINCIPAL INVESTIGATOR: Hyun-Suk Lim CONTRACTING ORGANIZATION: Indiana University Indianapolis,
The SMARTag TM ADC Technology Platform
 The SMARTag TM ADC Technology Platform 2 3 4 World Class Protein Production Capability New State of the Art Facility Madison, WI Expanded GPEx Cell Line Engineering capacity Flexible Non cgmp production
The SMARTag TM ADC Technology Platform 2 3 4 World Class Protein Production Capability New State of the Art Facility Madison, WI Expanded GPEx Cell Line Engineering capacity Flexible Non cgmp production
Supporting information. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and MRI contrast agent
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Supporting information Magnetic nanoscale metal organic frameworks for potential targeted
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Supporting information Magnetic nanoscale metal organic frameworks for potential targeted
Engineering tumors with 3D scaffolds
 Engineering tumors with 3D scaffolds Claudia Fischbach, Ruth Chen, Takuya Matsumoto, Tobias Schmelzle, Joan S Brugge, Peter J Polverini & David J Mooney Supplementary figures and text: Supplementary Figure
Engineering tumors with 3D scaffolds Claudia Fischbach, Ruth Chen, Takuya Matsumoto, Tobias Schmelzle, Joan S Brugge, Peter J Polverini & David J Mooney Supplementary figures and text: Supplementary Figure
Tissue Acetyl-Histone H4 ChIP Kit
 Tissue Acetyl-Histone H4 ChIP Kit Catalog Number KA0672 48 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Tissue Acetyl-Histone H4 ChIP Kit Catalog Number KA0672 48 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
AnaTag 5-FAM Protein Labeling Kit
 AnaTag 5-FAM Protein Labeling Kit Catalog # 72053 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate 5-FAM SE (5-carboxyfluorescein) to proteins (e.g., IgG). It provides ample materials
AnaTag 5-FAM Protein Labeling Kit Catalog # 72053 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate 5-FAM SE (5-carboxyfluorescein) to proteins (e.g., IgG). It provides ample materials
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
Human CEACAM6 ELISA Pair Set
 Human CEACAM6 ELISA Pair Set ( CD66c ) Catalog Number : SEK10823 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human CEACAM6 ELISA Pair Set ( CD66c ) Catalog Number : SEK10823 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Supplementary Information. Trojan Horse Nanotheranostics with Dual Transformability and Multifunctionality. for Highly Effective Cancer Treatment
 Supplementary Information Trojan Horse Nanotheranostics with Dual Transformability and Multifunctionality for Highly Effective Cancer Treatment Xue et. al. Supplementary Figure 1. Synthetic route of PhD
Supplementary Information Trojan Horse Nanotheranostics with Dual Transformability and Multifunctionality for Highly Effective Cancer Treatment Xue et. al. Supplementary Figure 1. Synthetic route of PhD
Supplementary Figure 1. CryoTEM images of the barcoded nanoparticles (a) and
 a b Supplementary Figure 1. CryoTEM images of the barcoded nanoparticles (a) and size measurements of the particles (b). Liposomes were loaded with DNA barcodes and were imaged using cryo-tem and measured
a b Supplementary Figure 1. CryoTEM images of the barcoded nanoparticles (a) and size measurements of the particles (b). Liposomes were loaded with DNA barcodes and were imaged using cryo-tem and measured
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81 XWH-06-1-0482 TITLE: Targeting Therapy Resistant Tumor Vessels PRINCIPAL INVESTIGATOR: Erkki Ruoslahti, M.D., Ph.D. CONTRACTING ORGANIZATION: Burnham Institute La Jolla, CA 92037 REPORT
AD Award Number: W81 XWH-06-1-0482 TITLE: Targeting Therapy Resistant Tumor Vessels PRINCIPAL INVESTIGATOR: Erkki Ruoslahti, M.D., Ph.D. CONTRACTING ORGANIZATION: Burnham Institute La Jolla, CA 92037 REPORT
