1.1 Definition of Environmental Fluid Dynamics
|
|
|
- Melina Cross
- 5 years ago
- Views:
Transcription
1 Chapter 1 Introduction 1.1 Definition of Environmental Fluid Dynamics Environmental fluid dynamics concerns the flow of fluid in the environment and its use and management. Human activity is integrated into the environment in which we live. Our health and comfort is determined by the flow and quality of the air we breathe and the water we drink. We harness air and water flows for power and discharge waste into the atmosphere, oceans and rivers. In order to continue sustainable economic activity it is necessary to manage these flows to maintain their quality. Much of our environment is fluid; obviously the atmosphere, oceans, lakes and rivers. Indeed on longer time scales even the solid earth can be considered as fluid, responsible for the motion of the continental plates (figure 1.1) and the formation of mountains. 1 Our concerns also span wide ranges of spatial and temporal scales. From short term, local concerns like the proximity of passive cigarette smoke, to long term, wide ranging flows that influence climate change. And, since we live on the earth, environmental fluid dynamics has much in common and significant overlap with geophysical fluid dynamics, which is concerned with the dynamics of the oceans and atmosphere. For the purposes of this course, we restrict our definition of environmental fluid dynamics to those flows where humans provide some kind of engineering role. This could be the management of a lake as a supply of drinking water, or the harnessing of the wind for wind power or to ventilate a building. The distinction from geophysical fluid dynamics is not precise, and the edges are 1 Alfred Wegener ( ), a German meteorologist and geologist, was the first person to propose the theory of continental drift. In his book, Origin of Continents and Oceans, he calculated that 200 million years ago the continents were originally joined together, forming a large supercontinent. He named this supercontinent Pangaea, meaning All-earth. He was also known for his papers on lunar craters, which he correctly believed were the result of impacts rather than vulcanism. 5
2 6 CHAPTER 1. INTRODUCTION Figure 1.1: Schematic of the outer layers of the earth. From blurred. But since we can not, as yet, change the weather or the flow of the Gulf Stream, we consider the dynamics of these and similar flows to be a topic in geophysical fluid dynamics rather than environmental fluid dynamics. Engineered flows, such as for example, the flow inside an engine or a gas pipeline, are primarily determined by the geometry - the boundary conditions. Geophysical flows are broadly independent of boundary conditions; instead they are governed by the internal dynamics. Environmental fluid dynamics is concerned with flows that span both aspects - they are natural flows but where the effects if boundaries is usually important (figure 1.5). For example we will study the sea breeze. Figure 1.6 shows the winds measured at La Jolla during the summer. This figure shows that the wind has a strong diurnal (daily) cycle, with on-shore winds in the afternoon. This sea breeze is driven by the land-sea temperature contrast, and so depends on the local boundary conditions at the coast. It plays a large role in pollutant transport in coastal regions, such as the San Diego - Los Angeles corridor. However, the sea breeze, which is a form of gravity current, could equally well be studied as a geophysical flow, since the flow is really set by the internal dynamics associated with the density changes in the air.
3 1.1. DEFINITION OF ENVIRONMENTAL FLUID DYNAMICS 7 Figure 1.2: Tectonic plates.
4 8 CHAPTER 1. INTRODUCTION Figure 1.3: The continental plates. From Figure 1.4: Schematic of processes involved in plate tectonics. Note the presence of convective plumes and stable stratification. From
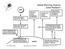
5 1.1. DEFINITION OF ENVIRONMENTAL FLUID DYNAMICS 9 Engineering Fluid Dynamics boundary controlled Environmental Fluid Dynamics internal dynamics and boundaries Geophysical Fluid Dynamics internal dynamics Figure 1.5: Schematic of Environmental Fluid Dynamics.
6 10 CHAPTER 1. INTRODUCTION Figure 1.6: Wind speed and direction measured at the Scripps Institution of Oceanography pier in July Note the clear diurnal cycle with the maximum onshore wind corresponding to the sea breeze. Environmental engineering is essentially an interdisciplinary subject, since environmental effects include chemical and biological changes. Sustainable engineering also needs to work within the economic and policy framework, possibly changing these to be more environmentally responsible. Similarly, environmental fluid dynamics, particularly the study of the physics of environmental flows, is only one part of the environmental picture. It is, however, the central part, since without an understanding of the physics it is very difficult to remediate or prevent biogeochemical problems in the environment. As we will see the physics is complex and the equations that govern these flows and their consequences are tough to solve. It is almost always necessary to make approximations both to the real environmental problem and to the equations that describe them in order to get solutions. This process is modeling, whereby a problem is analyzed and broken down to its essential parts. Often models, the answers from which are increasingly the basis of decisions costing millions of dollars, are used as black boxes. It is essential to realize that these models are approximate and have limitations in their applicability and accuracy. The job of the environmental engineer is to be aware of these limitations and to interpret the results in defensible terms. The purpose of this course is to provide some of the background required for this difficult, challenging and important task. Our environment consists of the atmosphere and the oceans. We begin with
7 1.2. THE ATMOSPHERE 11 Figure 1.7: Smoke and SO 2 concentrations compared to deaths per day in London during December a discussion of the atmosphere, partly because we are all exposed to it and also since it has most of the features that concerns environmental fluid dynamics. 1.2 The atmosphere We all rely on the atmosphere to provide us with oxygen for life. We have become increasingly aware since the start of the industrial revolution that industry can pollute this air, with deleterious effects on our health, on flora and fauna and even on buildings. Many cities in Europe have been cleaning the grime from their medieval buildings which has built up since the 18 th century (figure 1.11). Recent increases in childhood occurrences of asthma in many countries have been attributed to emissions from vehicles. The infamous smog in London in 1952, in which 4000 people died, was the spur for the first cleanair act. This is the first piece of environmental legislation, which has been a model for environmental regulations in an increasing numbers of areas. Figure 1.7 shows the fatalities during the smog, showing that they are correlated in time with smoke and SO 2 concentration. A similar correlation was also noted in New York during a pollution episode in 1966 (figure 1.8). Since that time legislation has caused a significant reduction in the emissions and smoke concentration. Figure 1.9 shows the reduction from the period This reduction in emissions is mainly due to the introduction of smokeless coal for domestic use. Further reductions since then have mainly been due to reduction in vehicle emissions. It is now possible to predict pollu-
8 12 CHAPTER 1. INTRODUCTION Figure 1.8: Number of deaths in London and New York air pollution episodes as a function of the product of SO 2 and suspended particulate concentrations. From Larsen (1970).
9 1.2. THE ATMOSPHERE 13 Figure 1.9: Smoke concentration in London from 1952 to From Hov, Isaksen & Hesstvedt (1976). tion from emissions with spatial resolution of 10m (figure 1.10). The atmosphere consists of 3 layers: the surface layer, the troposphere and the stratosphere (figure 1.12). Figure 1.13 shows the Standard atmosphere, with the average temperature plotted against height. The vertical scales are height and pressure and density. The density of the air decreases rapidly with height, so that most of the mass of the atmosphere is contained in the lower 10km. Since momentum is proportional to the mass, this implies that the dynamical processes are most significant near the surface and get weaker with height. Exercise was the 50 th anniversary of the great London fog. Look on the web to see what you can find about the fog and what has happened since to mitigate the problem Atmospheric surface layer This is the lower most part of the atmosphere and it is the most affected by diurnal variations. It extends from the ground to about 1-2 km during the day when the convective heating by the sun is the strongest. At night it is generally
10 14 CHAPTER 1. INTRODUCTION Figure 1.10: Air pollution map of San Diego, obtained using ADMS Urban. much shallower. This region of the atmosphere is where we live and to a large extent is where we release pollutants and extract energy. The wind is turbulent and changes direction with height, the latter due to the rotation of the earth. The temperature is fairly constant with height. As the day progresses the temperature near the ground increases as a result of the solar heating. This heating causes the air to convect, and mix with the air above. This convection increases the depth of the surface mixed layer during the day. A schematic of this process is shown in figure At night (figure 1.14 (a)) the ground cools by long wave radiation and so the temperature increases with height. This stratification is stable (see 3.2) and, since it is the opposite of the usual situation where the temperature decreases with height, this is called an inversion. If the lower part of the atmosphere is stirred by the air flow, the temperature will be uniform up to some height h, and the ground temperature will increase by 1 2hT z, where T z is the initial temperature gradient. Convection adds extra heat into this lower layer as shown in figure 1.14 (c). If it is assumed that the convection extends up to the height where the ground temperature equals the temperature at z = h, then the additional heat that is added is again 1 2hT z. We will discuss whether this is realistic in 3.3. We will see later on that these changes in the temperature profiles near the ground have a profound effect on the flow in the lower atmosphere, with important implications for the spread of pollutants released from ground sources such as vehicles, industrial plants etc.
11 THE ATMOSPHERE Figure 1.11: The Senate House (left) and Caius College, Cambridge in June 2003, showing the effects of air pollution Troposphere This is the region above the ASL to about 10 km. The temperature decreases with height, largely as a result of the decrease in pressure. Most of the mass of the atmosphere is contained below the top of the troposphere, called the tropopause. In order to determine how the temperature of the air decreases with height in a stationary atmosphere, we need to do a little thermodynamics. Dry air is well represented by the perfect gas equation p = ρrt, (1.1) where p is the pressure, ρ is the density, T is the temperature (in Kelvin) and R is the gas constant. We consider what happens when a small volume of air (a parcel) is moved vertically in a stationary atmosphere. The first law of thermodynamics states that the change in heat content dq is given by dq = cv dt + pdv, (1.2)
12 16 CHAPTER 1. INTRODUCTION Figure 1.12: The atmosphere. From
13 1.2. THE ATMOSPHERE 17 Figure 1.13: Temperature variation with geopotential height of the US Standard Atmosphere (solid line) This consists of straight line segments with breaks at 11, 20, 32, 47, 51 and 71 km. The surface temperature is 15 o C and the gradients, starting from the surface, are -6.5, 0, 1.0, 2.8, 0, -2.8 and -2.0 K km 1, respectively. The dashed line shows the lowest and highest monthly mean temperatures obtained for any location between the equator and the pole, while the dotted lines shows estimates of the 1% maximum and minimum temperatures that occur during the warmest and coldest months, respectively, in the most extreme locations. The scale at the right gives the pressures and densities at 10km intervals for the standard profile. From NOAA/NASA/USAF, 1976.
14 18 CHAPTER 1. INTRODUCTION z z z h h (a) T (b) T (c) T Figure 1.14: Schematic of development of the temperature during the day. (a) Night time conditions, where the temperature is coolest near the ground due to longwave radiation. This is known as an inversion. (b) Redistribution of the temperature caused simply by mixing fluid from below without any heat input. (c) The development when there is heat input and mixing.
15 1.2. THE ATMOSPHERE 19 z z z h h ρ ρ ρ (a) (b) (c) Figure 1.15: Schematic of development of the density profile during the day corresponding to the temperature variation shown in figure where c V is the specific heat at constant volume and dv is the volume change of the parcel. For an adiabatic change, in which no heat is exchanged between the parcel and its surroundings, dq = 0, and writing V = 1 ρ, and using the equation of state (3.10) we have dp = (c V + R)dT = c p dt, (1.3) where c p = c V + R is the specific heat at constant pressure. For a fluid at rest, the pressure (force per unit area) is the weight (per unit area) of the fluid above, i.e. p = ρgdz, (1.4) where the integral is from the point in question to the top of the fluid. This equation is usually expressed as a differential equation dp = gρ(z), (1.5) dz where the negative sign results from the fact that z is measured upwards and the pressure increases downwards. The equation (1.5) is known as the hydrostatic equation.
16 20 CHAPTER 1. INTRODUCTION From (1.3) we have Then (1.5) gives dp dt = c p. (1.6) dt dz = g c p, (1.7) and the right hand side of (1.7) is known as the adiabatic lapse rate. This means that if a parcel of air is raised adiabatically its temperature will decrease at the rate given by (1.7). For air this rate is about 10 K km 1. Figure 1.13 shows that the observed temperature decrease is very close this value. Consequently, this implies that the troposphere is close to thermodynamic equilibrium, in that the temperature distribution is close to that achieved simply by having the air at rest. We will return to this point later Stratosphere The stratosphere is the region above the tropopause, i.e above 10 km, and it is generally marked by an increase in temperature with height. As a result this region of the atmosphere is stably stratified (see 3.2) and there is little vertical mixing. This is one reason why the ozone hole (figure??) that occurs in the stratospheric winter hemisphere and the anthropogenic CFCs that contribute to its formation persist for a long time. Exercise 1.2 Find out the scale and persistence of the ozone hole. Why does it vary from year to year? What is a Dobson unit? 1.3 Climate change The begin of industrialization was the industrial revolution which began in England in the 18 th century. It was largely driven by technological innovations such as the use of steam 2, iron and the organization of labor. Since then industrial activity has spread around the globe and increases continually. This activity uses natural resources and produces waste products. Such activity is only sustainable if it does not deplete resources or create waste disposal problems to the extent that the activity can no longer continue Radiation balance The energy for all life and motion on the earth is received as radiation from the sun. Figure 1.19 shows the radiation balance of the earth. The average energy flux from the sun at the mean radius of the earth is called the solar constant S 2 James Watt ( ) invented the steam engine.
17 1.3. CLIMATE CHANGE 21 Figure 1.16: Monthly average of ozone concentration in October over Antarctica. From
18 22 CHAPTER 1. INTRODUCTION Figure 1.17: CO 2 concentration measured in the Pacific. S = 1.376kWm 2 (1.8) Thus a 1 m diameter dish in space would collect about 1 kw; enough to run a small electric heater. The total energy received from the sun per unit time is E = πr 2 S (1.9) where R is the radius of the earth. Since the surface area of the earth is 4πR 2, the average amount of energy received per unit area per unit time at the earth s surface is 1 4 S = 344Wm 2 (1.10) If the earth s axis were not tilted the average flux would vary from π 1 S at the equator to zero at the poles. However, since the axis is tilted (23.5 o ) the average flux is as shown in figure Not all the incoming energy is absorbed. A fraction α is reflected or scattered, and so the average flux absorbed is 1 4 (1 α) S = 240Wm 2 (1.11) The amount reflected or scattered is about 100 Wm 2, and is fairly independent
19 1.3. CLIMATE CHANGE 23 Figure 1.18: CO 2 concentration and temperature change from the present over the past 160,000 years.
20 24 CHAPTER 1. INTRODUCTION Figure 1.19: Incoming radiation as a function of latitude. The upper solid curve is the average incoming radiation and the lower solid curve is the average amount absorbed. The dashed curve is the amount of outgoing radiation. of latitude. The number α is called the albedo 3 of the earth and has a value of about α = Radiative equilibrium models The absorption of energy causes the surface to warm up until it radiates the same amount of energy to space. When the surface reaches temperature T, the amount of energy E radiated is given by Stefan s law 4 3 Albedo is the fraction of light that is reflected by a body or surface. It is commonly used in astronomy to describe the reflective properties of planets, satellites, and asteroids. Albedo is usually differentiated into two general types: normal albedo and bond albedo. The former, also called normal reflectance, is a measure of a surface s relative brightness when illuminated and observed vertically. The normal albedo of snow, for example, is nearly 1.0, whereas that of charcoal is about Investigators frequently rely on observations of normal albedo to determine the surface compositions of satellites and asteroids. The albedo, diameter, and distance of such objects together determine their brightness. Bond albedo, defined as the fraction of the total incident solar radiation reflected by a planet back to space, is a measure of the planet s energy balance. (It is so named for the American astronomer George P. Bond, who in 1861 published a comparison of the brightness of the Sun, the Moon, and Jupiter.) The value of bond albedo is dependent on the spectrum of the incident radiation because such albedo is defined over the entire range of wavelengths. Earth-orbiting satellites have been used to measure the Earth s bond albedo. The most recent values obtained are approximately The Moon, which has a very tenuous atmosphere and no clouds, has an albedo of By contrast, that of Venus, which is covered by dense clouds, is (from a google search leading to a web site at the University of Oregon). Albedo features of Mars may be viewed at 4 The law (and constant) were discovered in 1879 by the Austrian physicist Josef Stefan (183593)
21 1.3. CLIMATE CHANGE 25 Figure 1.20: Schematic of the greenhouse effect. From E = σt 4, (1.12) where σ = Wm 2 K 4. Such an equilibrium would give a temperature at the equator of 270 K, and 150 K at the South Pole and 170 K at the North Pole. The difference between these temperatures and those on the earth are a result of the atmosphere and oceans: (a) some radiation can be absorbed within the atmosphere itself (b) the atmosphere and oceans can transport heat from one location to another The greenhouse effect A schematic of the radiation into and from the earth s atmosphere is shown in figure Infrared radiation is trapped in the atmosphere by greenhouse gases, mainly CO 2, methane and water vapour. Consider a greenhouse modelled as a sheet of glass above the ground as shown in figure The glass is
22 26 CHAPTER 1. INTRODUCTION Short waves Long waves I (1-e)U B Glass I U B Ground Figure 1.21: Schematic of the radiation balance caused by greenhouse gases. transparent to wavelengths less than 4mm, and partially absorbs radiation of longer wavelengths. Initially the glass is cold, and a downward flux I of solar radiation is switched on. The ground will warm up to a temperature T g, and emit long wave radiation with an upward flux U given by Stefan s law 5 U = σt g 4. (1.13) Almost all of the radiation has wavelengths above 4 mm (the range is mm at typical temperatures), and a fraction e of this will be absorbed by the glass. The glass will also warm up and emit radiation B. Equilibrium is reached when the upward and downward fluxes match Solving (1.13) and (1.14) gives I = (1 e)u + B = U B. (1.14) 5 Joseph Stefan (Slovene Joef Stefan) ( ) was a Slovene mathematician, physicist and poet. There always something will remain, that we shall not know, why?
23 1.3. CLIMATE CHANGE 27 Figure 1.22: Radiation balance of the atmosphere. σt g 4 = U = I(1 e 2 ) 1. (1.15) Thus T g is higher than it would be in the absence of the glass (e = 0). Consider the case where the glass absorbs all the long wave radiation (e = 1). Then I = B, and the glass would reach the same temperature as the ground in the absence of glass. Since the underside of the glass is at the same temperature, it radiates a downward flux B of long wave radiation, so the ground receives a total flux of I + B = 2I. Thus by Stefan s law the temperature is higher by 2 1/4 = In the atmosphere the absorption occurs over a range of heights but the principle is the same (Chamberlain (1978)). An estimate of the radiation balance is given in figure Problem 1.1 Figure 1.15 is a schematic of the development of the density profile near the ground corresponding (approximately) to the temperature profiles shown in figure (a) is the initial profile. Calculate the change in potential energy per unit area of the stratification in cases (b) and (c) in terms of the initial density gradient ρ z and the height h. Assuming that the difference between (b) and (c) is due to solar radiation, explain why the change in potential energy is positive in one case and negative in the other. Problem 1.2 Find the value of c p for air and for water. Hence calculate the adiabatic lapse rate for both the atmosphere and the ocean. Given that the temperature gradient in the lowest 11km of the atmosphere is -6.5 K km 1, find the effective temperature gradient called the potential temperature gradient once the effects of compressibility have been removed. Problem 1.3 Stefan s law (1.12) is for a black body (i.e. a perfect emitter). A more
24 28 CHAPTER 1. INTRODUCTION general form is E = e m σt 4, where 0 e m 1 is the emissivity of the emitting surface. Calculate the greenhouse enhancement of the surface temperature for (a) e m = 0.5, e = 1; (b) e m = 1, e = 0.5, where e is the emissivity of the atmosphere. Comment on the implications for the earth s climate of changing the characteristics of the earth surface and the emissivity of the atmosphere.
Chapter 4: The Global Energy System
 Discovering Physical Geography Third Edition by Alan Arbogast Chapter 4: The Global Energy System The Electromagnetic Spectrum and Solar Energy Solar Energy as Radiation Electromagnetic energy transmitted
Discovering Physical Geography Third Edition by Alan Arbogast Chapter 4: The Global Energy System The Electromagnetic Spectrum and Solar Energy Solar Energy as Radiation Electromagnetic energy transmitted
Planetary Energy Balance
 Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
The Earth s Global Energy Balance
 The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
High School Climate Science Curriculum Course learning goals. October 2011
 1 High School Climate Science Curriculum Course learning goals October 2011 Current Climate 1. Earth climate is determined by a balance between absorbed sunlight and emitted infrared radiation. Because
1 High School Climate Science Curriculum Course learning goals October 2011 Current Climate 1. Earth climate is determined by a balance between absorbed sunlight and emitted infrared radiation. Because
- geographic patterns of energy balance
 (1 of 10) Further Reading: Chapter 04 of the text book Outline - geographic patterns of energy balance - net radiation - meridional transport (2 of 10) Introduction Previously, we discussed the energy
(1 of 10) Further Reading: Chapter 04 of the text book Outline - geographic patterns of energy balance - net radiation - meridional transport (2 of 10) Introduction Previously, we discussed the energy
Lecture 22: Atmospheric Chemistry and Climate
 Lecture 22: Atmospheric Chemistry and Climate Required Reading: FP Chapter 14 (only sections that I cover) Suggested Introductory Reading: Jacob Chapter 7 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring
Lecture 22: Atmospheric Chemistry and Climate Required Reading: FP Chapter 14 (only sections that I cover) Suggested Introductory Reading: Jacob Chapter 7 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring
How is the atmosphere different from outer space? a mixture of gases that surrounds the Earth
 Chapter 15 Atmosphere Section 1 Objectives Describe the composition of Earth's atmosphere. Explain why air pressure changes with altitude. Explain how air temperature changes with atmospheric composition.
Chapter 15 Atmosphere Section 1 Objectives Describe the composition of Earth's atmosphere. Explain why air pressure changes with altitude. Explain how air temperature changes with atmospheric composition.
Greenhouse gases. A snow-covered surface refl ects massive amounts of sunlight and therefore has a cooling effect on the climate.
 A k t u e l N a t u r v i d e n s k a b 2 0 0 9 G R E E N H O U S E G A S E S 13 Greenhouse gases - and their impact on the climate The greenhouse effect is the best understood and well mapped of the mechanisms
A k t u e l N a t u r v i d e n s k a b 2 0 0 9 G R E E N H O U S E G A S E S 13 Greenhouse gases - and their impact on the climate The greenhouse effect is the best understood and well mapped of the mechanisms
Earth s Climate from Space. Richard Allan Department of Meteorology University of Reading
 Earth s Climate from Space Richard Allan Department of Meteorology University of Reading Earth s energy balance in space S πr 2 4πr 2 Outgoing Thermal Radiative Energy Absorbed Solar Radiative Energy
Earth s Climate from Space Richard Allan Department of Meteorology University of Reading Earth s energy balance in space S πr 2 4πr 2 Outgoing Thermal Radiative Energy Absorbed Solar Radiative Energy
Radiative forcing of climate change
 Radiative forcing of climate change Joanna D. Haigh Imperial College of Science, Technology and Medicine, London Radiative forcing concept, definition and applications On a global and annual average, and
Radiative forcing of climate change Joanna D. Haigh Imperial College of Science, Technology and Medicine, London Radiative forcing concept, definition and applications On a global and annual average, and
Environmental Engineering Atmosphere & pollution 2
 Environmental Engineering Atmosphere & pollution 2 Global radiation Greenhouse effect Kyoto protocol David Zumr Dpt. of Drainage, Irrigation and Landscape Eng. 1/ insolation from the Sun Electromagnetic
Environmental Engineering Atmosphere & pollution 2 Global radiation Greenhouse effect Kyoto protocol David Zumr Dpt. of Drainage, Irrigation and Landscape Eng. 1/ insolation from the Sun Electromagnetic
Lecture 7 Global Warming/Climate Change (Observations and Attribution of Cause) METR/ENVS 113 Spring Semester 2011 May 3, 2011
 Lecture 7 Global Warming/Climate Change (Observations and Attribution of Cause) METR/ENVS 113 Spring Semester 2011 May 3, 2011 Reading Henson Rough Guide Chapter 1 Pages 75 127; 215; 227-244 Other pages
Lecture 7 Global Warming/Climate Change (Observations and Attribution of Cause) METR/ENVS 113 Spring Semester 2011 May 3, 2011 Reading Henson Rough Guide Chapter 1 Pages 75 127; 215; 227-244 Other pages
Welcome to ATMS 111 Global Warming.
 Welcome to ATMS 111 Global Warming http://www.atmos.washington.edu/2010q1/111 Censored Spotted by Jennifer Le Today Review and Finish up The Greenhouse Effect - RG p 21-30 A rogues gallery of greenhouse
Welcome to ATMS 111 Global Warming http://www.atmos.washington.edu/2010q1/111 Censored Spotted by Jennifer Le Today Review and Finish up The Greenhouse Effect - RG p 21-30 A rogues gallery of greenhouse
Energy and the Earth. Key words: Incoming Solar Radiation, Electromagnetic wave, Greenhouse effect, conduction, convection, radiation.
 S c i e n c e Energy and the Earth Key words: Incoming Solar Radiation, Electromagnetic wave, Greenhouse effect, conduction, convection, radiation. Energy transfer Heat is energy in transit from warmer
S c i e n c e Energy and the Earth Key words: Incoming Solar Radiation, Electromagnetic wave, Greenhouse effect, conduction, convection, radiation. Energy transfer Heat is energy in transit from warmer
The Chemistry of Climate Change. Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy
 The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
T8-1 [166 marks] Which energy resource is renewable? A. Natural gas B. Uranium C. Biogas D. Coal
![T8-1 [166 marks] Which energy resource is renewable? A. Natural gas B. Uranium C. Biogas D. Coal T8-1 [166 marks] Which energy resource is renewable? A. Natural gas B. Uranium C. Biogas D. Coal](/thumbs/78/78599564.jpg) T8-1 [166 marks] 1. Which energy resource is renewable? A. Natural gas B. Uranium C. Biogas D. Coal 2. For a black-body at absolute temperature T the power emitted per unit area is P. What is the power
T8-1 [166 marks] 1. Which energy resource is renewable? A. Natural gas B. Uranium C. Biogas D. Coal 2. For a black-body at absolute temperature T the power emitted per unit area is P. What is the power
Radiative Forcing Components
 Radiative Forcing Components Content Definition of Radiative Forcing Radiation Balance Climate sensitivity Solar forcing Forcing due to atmospheric gas Definition of Radiative Forcing In climate science,
Radiative Forcing Components Content Definition of Radiative Forcing Radiation Balance Climate sensitivity Solar forcing Forcing due to atmospheric gas Definition of Radiative Forcing In climate science,
Some resources (more websites later)
 Some resources (more websites later) Intergovernmental Panel Climate Change 2001: The Scientific Basis at http://www.ipcc.ch/pub/reports.htm John Houghton Global Warming - the complete briefing Cambridge
Some resources (more websites later) Intergovernmental Panel Climate Change 2001: The Scientific Basis at http://www.ipcc.ch/pub/reports.htm John Houghton Global Warming - the complete briefing Cambridge
Air Pollution Chapter 21. Atmosphere as a Resource
 Air Pollution Chapter 21 Atmosphere as a Resource Atmospheric Composition Nitrogen 78.08% Oxygen 20.95% Argon 0.93% Carbon dioxide 0.04% Ecosystem services Blocks UV radiation Moderates the climate Redistributes
Air Pollution Chapter 21 Atmosphere as a Resource Atmospheric Composition Nitrogen 78.08% Oxygen 20.95% Argon 0.93% Carbon dioxide 0.04% Ecosystem services Blocks UV radiation Moderates the climate Redistributes
Climate: Earth s Dynamic Equilibrium
 Climate: Earth s Dynamic Equilibrium review session CCIU April 30, 2016 High-school standard HS-ESS2-4 focuses on the role energy flows play in Earth s climate HS-ESS2-4 Use a model to describe how variations
Climate: Earth s Dynamic Equilibrium review session CCIU April 30, 2016 High-school standard HS-ESS2-4 focuses on the role energy flows play in Earth s climate HS-ESS2-4 Use a model to describe how variations
Lecture 29 Air Pollution. Air Pollution. Clean Boundary Layer. Clean Boundary Layer
 Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
How things work college course/cumulative global warming exam/testbank
 How things work college course/cumulative global warming exam/testbank From Wikiversity Contents 1 GlobalWarmingCumulative 1.1 GlobalWarmingCumulative v1s1 1.1.1 Key to GlobalWarmingCumulative v1s1 1.2
How things work college course/cumulative global warming exam/testbank From Wikiversity Contents 1 GlobalWarmingCumulative 1.1 GlobalWarmingCumulative v1s1 1.1.1 Key to GlobalWarmingCumulative v1s1 1.2
1 Characteristics of the Atmosphere
 CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
How Can Thermal Effects Be Explained?
 How Can Thermal Effects Be Explained? Lesson 6, Part 3: Climate Science The Enhanced Greenhouse Effect The Earth will maintain equilibrium (constant stable temperature level) if the energy coming in is.
How Can Thermal Effects Be Explained? Lesson 6, Part 3: Climate Science The Enhanced Greenhouse Effect The Earth will maintain equilibrium (constant stable temperature level) if the energy coming in is.
Chapter 11: Atmosphere
 To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
Lecture 8: Anthropogenic Climate Change. Instructor: Prof. Johnny Luo. Acknowledge: IPCC
 Lecture 8: Anthropogenic Climate Change Instructor: Prof. Johnny Luo Acknowledge: IPCC Energy Budget Fluid (atmosphere/ ocean) motions to redistribute energy Energy imbalance (forcing) and climate change
Lecture 8: Anthropogenic Climate Change Instructor: Prof. Johnny Luo Acknowledge: IPCC Energy Budget Fluid (atmosphere/ ocean) motions to redistribute energy Energy imbalance (forcing) and climate change
FOLLOW: Green House on Twitter
 Jan 31, 2012 Recommend 773 208 By Wendy Koch, USA TODAY Updated 1d 13h ago CAPTION By William Fernando Martinez, AP A new NASA study tries to lay to rest the skepticism about climate change, especially
Jan 31, 2012 Recommend 773 208 By Wendy Koch, USA TODAY Updated 1d 13h ago CAPTION By William Fernando Martinez, AP A new NASA study tries to lay to rest the skepticism about climate change, especially
Scientific Foundation of Climate Change. Human Responsibility for Climate Change
 Scientific Foundation of Climate Change EOH 468 CSU Northridge Spring 2010 Peter Bellin, CIH, Ph.D. 1 Human Responsibility for Climate Change The IPCC finds that it is very likely that emissions of heat-trapping
Scientific Foundation of Climate Change EOH 468 CSU Northridge Spring 2010 Peter Bellin, CIH, Ph.D. 1 Human Responsibility for Climate Change The IPCC finds that it is very likely that emissions of heat-trapping
Final Exam (2011) Name EVST201a/G&G 140a The Atmosphere, Ocean and Environmental Change
 Name EVST201a/G&G 140a The Atmosphere, Ocean and Environmental Change Final Exam (2011) Useful physical and mathematical constants: R = 8314 J / kmole Kelvin ; σ = 5.735 10 8 Wm 2K 4 ; π = 3.14159 = 6.674
Name EVST201a/G&G 140a The Atmosphere, Ocean and Environmental Change Final Exam (2011) Useful physical and mathematical constants: R = 8314 J / kmole Kelvin ; σ = 5.735 10 8 Wm 2K 4 ; π = 3.14159 = 6.674
Composition and Energy AOSC 200 Tim Canty
 Composition and Energy AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Atmospheric composition cont. Energy transfer Lecture 03 Sept 5 2017 1 Today s Weather
Composition and Energy AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Atmospheric composition cont. Energy transfer Lecture 03 Sept 5 2017 1 Today s Weather
Energy, power and climate change
 Energy, power and climate change Energy degradation and power generation Thermal energy may be completely converted to work in a single process, but continuous conversion of this energy into work requires
Energy, power and climate change Energy degradation and power generation Thermal energy may be completely converted to work in a single process, but continuous conversion of this energy into work requires
08 Energy, Power and climate change review answers
 08 Energy, Power and climate change review answers Power generation 1. Copy and complete: Thermal energy may be completely converted into work in a single process such as the adiabatic expansion of a gas
08 Energy, Power and climate change review answers Power generation 1. Copy and complete: Thermal energy may be completely converted into work in a single process such as the adiabatic expansion of a gas
2 Atmospheric Heating
 CHAPTER 15 2 Atmospheric Heating SECTION The Atmosphere BEFORE YOU READ After you read this section, you should be able to answer these questions: How does energy travel from the sun to Earth? What are
CHAPTER 15 2 Atmospheric Heating SECTION The Atmosphere BEFORE YOU READ After you read this section, you should be able to answer these questions: How does energy travel from the sun to Earth? What are
The IPCC Working Group I Assessment of Physical Climate Change
 The IPCC Working Group I Assessment of Physical Climate Change Martin Manning Director, IPCC Working Group I Support Unit 1. Observed climate change 2. Drivers of climate change 3. Attribution of cause
The IPCC Working Group I Assessment of Physical Climate Change Martin Manning Director, IPCC Working Group I Support Unit 1. Observed climate change 2. Drivers of climate change 3. Attribution of cause
Winter 2009: ATMS/OCN/ESS 588 The Global Carbon Cycle and Greenhouse Gases. Course Goals
 PCC 588 - January 6 and 8 2009 Winter 2009: ATMS/OCN/ESS 588 The Global Carbon Cycle and Greenhouse Gases T,Th 12:00-1:20 pm OSB 25 Course Goals The course focuses on factors controlling the global cycle
PCC 588 - January 6 and 8 2009 Winter 2009: ATMS/OCN/ESS 588 The Global Carbon Cycle and Greenhouse Gases T,Th 12:00-1:20 pm OSB 25 Course Goals The course focuses on factors controlling the global cycle
Radiation and Climate Change
 Radiation and Climate Change Earth s Energy Balance Forcing and Feedback Implications for climate change including the global water cycle Introduction / Motivations Past societies e.g. Jared Diamond: Collapse
Radiation and Climate Change Earth s Energy Balance Forcing and Feedback Implications for climate change including the global water cycle Introduction / Motivations Past societies e.g. Jared Diamond: Collapse
ATM S 211 Final Examination June 4, 2007
 ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
The Effects of Volcano-Induced Ozone Depletion on Short-Lived Climate Forcing in the Arctic
 C53C-0852 The Effects of Volcano-Induced Ozone Depletion on Short-Lived Climate Forcing in the Arctic Peter L. Ward US Geological Survey Retired Teton Tectonics Jackson, WY 307-733-3664 cell 307-413-4055
C53C-0852 The Effects of Volcano-Induced Ozone Depletion on Short-Lived Climate Forcing in the Arctic Peter L. Ward US Geological Survey Retired Teton Tectonics Jackson, WY 307-733-3664 cell 307-413-4055
Name: Class: Date: 6. Most air pollution is produced by a. thermal inversions. c. ozone layer depletion. b. fuel burning. d. volcanic eruptions.
 Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
The greenhouse effect
 Sources of CO 2 It is an undisputable fact that the carbon dioxide concentration in the atmosphere is increasing, see next page for the evidence. There are of course many sources of the CO 2 in the atmosphere,
Sources of CO 2 It is an undisputable fact that the carbon dioxide concentration in the atmosphere is increasing, see next page for the evidence. There are of course many sources of the CO 2 in the atmosphere,
Global Warming and the Hydrological Cycle
 Global Warming and the Hydrological Cycle Climate Change Projections Wet regions will become wetter Dry regions will become drier Precipitation will occur less frequently Precipitation will be more intense
Global Warming and the Hydrological Cycle Climate Change Projections Wet regions will become wetter Dry regions will become drier Precipitation will occur less frequently Precipitation will be more intense
Sea ice field at time of annual minimum extent. NASA
 Sea ice field at time of annual minimum extent. NASA Climate Models & Climate Sensitivity: A Review Sea ice field at time of annual minimum extent. NASA Paul Kushner Department of Physics, University of
Sea ice field at time of annual minimum extent. NASA Climate Models & Climate Sensitivity: A Review Sea ice field at time of annual minimum extent. NASA Paul Kushner Department of Physics, University of
Chapter 17: Atmospheric Science and Air Pollution I. Central Case: The 1952 Killer Smog of London
 Chapter 17: Atmospheric Science and Air Pollution I. Central Case: The 1952 Killer Smog of London A. Thick smog first settled over the city on December 5, 1952, when many residents stoked: B. A wind finally
Chapter 17: Atmospheric Science and Air Pollution I. Central Case: The 1952 Killer Smog of London A. Thick smog first settled over the city on December 5, 1952, when many residents stoked: B. A wind finally
Three Connected Interactives
 Three Connected Interactives Carbon Dioxide and the Carbon Cycle Earth s Energy Flows and Climate Impacts of Climate Change in the Pacific Region Climate Change: Causes and Impacts Human Activities More
Three Connected Interactives Carbon Dioxide and the Carbon Cycle Earth s Energy Flows and Climate Impacts of Climate Change in the Pacific Region Climate Change: Causes and Impacts Human Activities More
Radiative forcing of gases, aerosols and, clouds.
 Lecture 23. Radiative forcing of gases, aerosols and, clouds. 1. Concepts of radiative forcing, climate sensitivity, and radiation feedbacks. 2. Radiative forcing of anthropogenic greenhouse gases. 3.
Lecture 23. Radiative forcing of gases, aerosols and, clouds. 1. Concepts of radiative forcing, climate sensitivity, and radiation feedbacks. 2. Radiative forcing of anthropogenic greenhouse gases. 3.
Heating and Warming: Sensitivity of Earth s Climate to Atmospheric CO 2
 LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Heating and Warming: Sensitivity of Earth s Climate to Atmospheric CO 2 Presented by: Scott Denning and Randy Russell September 24, 2012 6:30 p.m. 8:00 p.m. Eastern
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Heating and Warming: Sensitivity of Earth s Climate to Atmospheric CO 2 Presented by: Scott Denning and Randy Russell September 24, 2012 6:30 p.m. 8:00 p.m. Eastern
Global Climatic Change. GEOG/ENST 2331 Lecture 22 Ahrens: Chapter 16
 Global Climatic Change GEOG/ENST 2331 Lecture 22 Ahrens: Chapter 16 Global Climatic Change! Review: Radiation balance! Enhanced greenhouse effect! human-induced change! Climate feedbacks Climatic change!
Global Climatic Change GEOG/ENST 2331 Lecture 22 Ahrens: Chapter 16 Global Climatic Change! Review: Radiation balance! Enhanced greenhouse effect! human-induced change! Climate feedbacks Climatic change!
Ground-Source Heat Pumps Whence the Energy? Last Update 15/6/10
 1. he Question Ground-Source Heat Pumps Whence the Energy? Last Update 15// Domestic ground source heat pump installations are increasingly common as a means of both saving money on fuel bills and reducing
1. he Question Ground-Source Heat Pumps Whence the Energy? Last Update 15// Domestic ground source heat pump installations are increasingly common as a means of both saving money on fuel bills and reducing
TOPIC # 15 GLOBAL WARMING & ANTHROPOGENIC FORCING (cont.)
 TOPIC # 15 GLOBAL WARMING & ANTHROPOGENIC FORCING (cont.) Part B RADIATIVE FORCING Class Notes pp 89 THE KEY TO IT ALL: p 89 RADIATIVE FORCING (linked to the Energy Balance!) expressed in Watts per square
TOPIC # 15 GLOBAL WARMING & ANTHROPOGENIC FORCING (cont.) Part B RADIATIVE FORCING Class Notes pp 89 THE KEY TO IT ALL: p 89 RADIATIVE FORCING (linked to the Energy Balance!) expressed in Watts per square
The Global Water Cycle
 The Global Water Cycle Water Unusual properties Central role in biogeochemistry Agent in global weathering cycles Water Outline Most abundant molecule on earths surface (1) Water: Properties and importance
The Global Water Cycle Water Unusual properties Central role in biogeochemistry Agent in global weathering cycles Water Outline Most abundant molecule on earths surface (1) Water: Properties and importance
GLOBAL WARMING COMPUTER LAB
 GLOBAL WARMING COMPUTER LAB A COMPUTER SIMULATION PROGRAM ON TEMPERATURE CHANGE AND SEA LEVEL RISING After performing this computer simulation lab you will be able to: 1) understand the greenhouse effect
GLOBAL WARMING COMPUTER LAB A COMPUTER SIMULATION PROGRAM ON TEMPERATURE CHANGE AND SEA LEVEL RISING After performing this computer simulation lab you will be able to: 1) understand the greenhouse effect
PHY392S Physics of Climate. Lecture 1. Introduction
 PHY392S Physics of Climate Lecture 1 Introduction Slides based on material from Prof. K. Strong PHY392S - Physics of Climate Lecture 1, Page 1 Some Definitions Weather the fluctuating state of the atmosphere
PHY392S Physics of Climate Lecture 1 Introduction Slides based on material from Prof. K. Strong PHY392S - Physics of Climate Lecture 1, Page 1 Some Definitions Weather the fluctuating state of the atmosphere
Chapter 19 Global Change. Wednesday, April 18, 18
 Chapter 19 Global Change Module 62 Global Climate Change and the Greenhouse Effect After reading this module you should be able to distinguish among global change, global climate change, and global warming.
Chapter 19 Global Change Module 62 Global Climate Change and the Greenhouse Effect After reading this module you should be able to distinguish among global change, global climate change, and global warming.
Measurement of Carbon Dioxide Concentration in the Outdoor Environment
 R32 Project: Measurement of Carbon Dioxide Concentration in the Outdoor Environment Woo Ka Ming Physics Department, from Chinese University of Hong Kong, CONTENTS: Introduction 1. Data Analysis: 1.1 Measurements
R32 Project: Measurement of Carbon Dioxide Concentration in the Outdoor Environment Woo Ka Ming Physics Department, from Chinese University of Hong Kong, CONTENTS: Introduction 1. Data Analysis: 1.1 Measurements
Earth s Atmosphere Lecture 14 2/28/2013
 Earth s Atmosphere Lecture 14 2/28/2013 MRS 1 Due Tuesday The Earth s atmosphere has changed substantially over our planet s history First gases surrounding Earth were originally hydrogen and helium (during
Earth s Atmosphere Lecture 14 2/28/2013 MRS 1 Due Tuesday The Earth s atmosphere has changed substantially over our planet s history First gases surrounding Earth were originally hydrogen and helium (during
Earth energy budget and balance
 Earth energy budget and balance 31% total reflection (3% clouds. 8% surface) 69% absorption( 0% clouds, 49% surface) Reflection is frequency dependent but will be treated as average value for visible light
Earth energy budget and balance 31% total reflection (3% clouds. 8% surface) 69% absorption( 0% clouds, 49% surface) Reflection is frequency dependent but will be treated as average value for visible light
Air Pollution Meteorology
 Air Pollution Meteorology 1 Air Pollution Meteorology Air Pollution Transport by Convection Adiabatic Lapse Rate Atmospheric Stability Air Parcel Buoyancy Chimney Plumes Plume Shape vs. Atmospheric Stabilty
Air Pollution Meteorology 1 Air Pollution Meteorology Air Pollution Transport by Convection Adiabatic Lapse Rate Atmospheric Stability Air Parcel Buoyancy Chimney Plumes Plume Shape vs. Atmospheric Stabilty
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32)
 Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Global warming. Models for global warming Sand analogy
 8.10 Global warming Assessment statements 8.6.1 Describe some possible models of global warming. 8.6. State what is meant by the enhanced greenhouse effect. 8.6.3 Identify the increased combustion of fossil
8.10 Global warming Assessment statements 8.6.1 Describe some possible models of global warming. 8.6. State what is meant by the enhanced greenhouse effect. 8.6.3 Identify the increased combustion of fossil
Grade 10 Academic Science Climate Change Unit Test
 Grade 10 Academic Science Climate Change Unit Test Part A - Multiple Choice: Circle the most correct answer. 1. What is the difference between weather and climate? a. Weather deals with wind and precipitation;
Grade 10 Academic Science Climate Change Unit Test Part A - Multiple Choice: Circle the most correct answer. 1. What is the difference between weather and climate? a. Weather deals with wind and precipitation;
Digging Deeper SOLAR ENERGY. Forms of Solar Energy
 a) Is the wind speed the same in the morning; the afternoon; the evening? b) Move your anemometer to another location. Is it windier in other places? c) Do trees or buildings block the wind? 7. Back in
a) Is the wind speed the same in the morning; the afternoon; the evening? b) Move your anemometer to another location. Is it windier in other places? c) Do trees or buildings block the wind? 7. Back in
Climate Change U C Irvine OLLI Class, Winter, 2013: SC 206 by Gary Oberts and Dennis Silverman Using Talks by NOAA for OLLI
 Climate Change U C Irvine OLLI Class, Winter, 2013: SC 206 by Gary Oberts and Dennis Silverman Using Talks by NOAA for OLLI Radiative Forcing Dennis Silverman Department of Physics and Astronomy, U C Irvine
Climate Change U C Irvine OLLI Class, Winter, 2013: SC 206 by Gary Oberts and Dennis Silverman Using Talks by NOAA for OLLI Radiative Forcing Dennis Silverman Department of Physics and Astronomy, U C Irvine
Global Warming Science Solar Radiation
 SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
Chapter 5. The Earth s Atmosphere
 Chapter 5 The Earth s Atmosphere Layers of the Earth Earth largest of the inner planets Gravity strong enough to hold gases. Lots of spheres Equator divided the Earth into two hemispheres Lithosphere-
Chapter 5 The Earth s Atmosphere Layers of the Earth Earth largest of the inner planets Gravity strong enough to hold gases. Lots of spheres Equator divided the Earth into two hemispheres Lithosphere-
The Greenhouse Effect
 Name: Date: The Greenhouse Effect This document provides an overview of the earth's atmospheric "greenhouse effect" by briefly exploring the atmospheres of nearby planets and discussing our atmosphere's
Name: Date: The Greenhouse Effect This document provides an overview of the earth's atmospheric "greenhouse effect" by briefly exploring the atmospheres of nearby planets and discussing our atmosphere's
SCI-6 SOL Practice Questions_6th Grade Exam not valid for Paper Pencil Test Sessions
 SCI-6 SOL Practice Questions_6th Grade Exam not valid for Paper Pencil Test Sessions [Exam ID:392MWT 1 The quality of pond water can be determined by identifying the number and types of organisms found
SCI-6 SOL Practice Questions_6th Grade Exam not valid for Paper Pencil Test Sessions [Exam ID:392MWT 1 The quality of pond water can be determined by identifying the number and types of organisms found
GREENHOUSE GASES 3/14/2016. Water Vapor, CO 2, CFCs, Methane and NO x all absorb radiation Water vapor and CO 2 are the primary greenhouse gases
 GREENHOUSE EFFECT The earth is like a greenhouse The atmosphere acts like the glass which lets the sun s rays pass through. The earth absorbs this as heat energy and keeps it in, only letting a little
GREENHOUSE EFFECT The earth is like a greenhouse The atmosphere acts like the glass which lets the sun s rays pass through. The earth absorbs this as heat energy and keeps it in, only letting a little
Earth s Atmosphere Lecture 14 3/6/2014
 Earth s Atmosphere Lecture 14 3/6/2014 MRS 1 Due Tuesday Second exam will be postponed until after spring break The sun drives the climate of Earth http://www.spaceweather.com/images2002/18mar02/cme_c3_big.gif
Earth s Atmosphere Lecture 14 3/6/2014 MRS 1 Due Tuesday Second exam will be postponed until after spring break The sun drives the climate of Earth http://www.spaceweather.com/images2002/18mar02/cme_c3_big.gif
Concentrations of several of these greenhouse gases (CO 2, CH 4, N 2 O and CFCs) have increased dramatically in the last hundred years due to human
 Global Warming 1.1 The facts: With no atmosphere surrounding the earth the surface temperature would be 17 o C. However, due to the greenhouse gases in the atmosphere that absorb infrared radiation emitted
Global Warming 1.1 The facts: With no atmosphere surrounding the earth the surface temperature would be 17 o C. However, due to the greenhouse gases in the atmosphere that absorb infrared radiation emitted
Session 14 Unit VI CLIMATIC CHANGE AND GLOBAL WARMING
 Session 14 Unit VI CLIMATIC CHANGE AND GLOBAL WARMING Dr. H.S. Ramesh Professor of Environmental Engineering S.J. College of Engineering, Mysore 570 006 Carbon di-oxide is a natural constituent of atmosphere,
Session 14 Unit VI CLIMATIC CHANGE AND GLOBAL WARMING Dr. H.S. Ramesh Professor of Environmental Engineering S.J. College of Engineering, Mysore 570 006 Carbon di-oxide is a natural constituent of atmosphere,
History of significant air pollution events
 Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Earth s Dynamic Climate
 UNIT 3 Earth s Dynamic Climate Topic 3.1: What is climate, and how has it changed during Earth s history? Topic 3.2 : Where are the effects of climate change felt, and what is their impact? Topic 3.5:
UNIT 3 Earth s Dynamic Climate Topic 3.1: What is climate, and how has it changed during Earth s history? Topic 3.2 : Where are the effects of climate change felt, and what is their impact? Topic 3.5:
Ecology. What is the role of the Sun s Energy in Earth s spheres?
 Ecology What is the role of the Sun s Energy in Earth s spheres? http://video.nationalgeographic.com/video/news/101- videos/151201-climate-change-bill-nye-news https://www.youtube.com/watch?v=hs0so6loe-8
Ecology What is the role of the Sun s Energy in Earth s spheres? http://video.nationalgeographic.com/video/news/101- videos/151201-climate-change-bill-nye-news https://www.youtube.com/watch?v=hs0so6loe-8
Introduction to Environmental Physics
 Introduction to Environmental Physics Planet Earth, Life and Climate Nigel Mason Department of Physics and Astronomy University College, London, UK. Peter Hughes Kingsway College, London, UK. with Randall
Introduction to Environmental Physics Planet Earth, Life and Climate Nigel Mason Department of Physics and Astronomy University College, London, UK. Peter Hughes Kingsway College, London, UK. with Randall
Introduction. August 27, 2014
 Introduction August 27, 2014 Atmospheric sciences focuses on understanding the atmosphere of the earth and other planets. The motivations for studying atmospheric sciences are largely: weather forecasting,
Introduction August 27, 2014 Atmospheric sciences focuses on understanding the atmosphere of the earth and other planets. The motivations for studying atmospheric sciences are largely: weather forecasting,
Greenhouse Effect & Climate Change
 Greenhouse Effect & Climate Change Greenhouse Effect Light energy from the sun (solar radiation) is either reflected or absorbed by the Earth. Greenhouse Effect When it is absorbed by the Earth (or something
Greenhouse Effect & Climate Change Greenhouse Effect Light energy from the sun (solar radiation) is either reflected or absorbed by the Earth. Greenhouse Effect When it is absorbed by the Earth (or something
Global Perspective on Climate Change
 Global Perspective on Climate Change Dr. Barrie Pittock, PSM Former Leader of Climate Impact Group, CSIRO Lead Author IPCC Reports, 1995, 1998, 2001 Editor, AGO Climate Change Guide 2003 Author, Climate
Global Perspective on Climate Change Dr. Barrie Pittock, PSM Former Leader of Climate Impact Group, CSIRO Lead Author IPCC Reports, 1995, 1998, 2001 Editor, AGO Climate Change Guide 2003 Author, Climate
TODAY: TOPIC #6 WRAP UP!! Atmospheric Structure & Composition
 TODAY: TOPIC #6 WRAP UP!! Atmospheric Structure & Composition There s one more thing to correct in our the depiction of incoming Solar....... the atmosphere is NOT totally TRANSPARENT to INCOMING Solar
TODAY: TOPIC #6 WRAP UP!! Atmospheric Structure & Composition There s one more thing to correct in our the depiction of incoming Solar....... the atmosphere is NOT totally TRANSPARENT to INCOMING Solar
Earth and Space Science (Earth's Atmosphere) Grade 7 Science Grade 7 Science Start Date: December 02, 2013 End Date : December 20, 2013
 Unit Overview Atmospheric properties Content Elaborations The atmosphere has different properties at different elevations and contains a mixture of gases that cycle through the lithosphere, biosphere,
Unit Overview Atmospheric properties Content Elaborations The atmosphere has different properties at different elevations and contains a mixture of gases that cycle through the lithosphere, biosphere,
ANSWER KEY TO THE PRACTICE QUESTIONS ON THE FINAL EXAM STUDY GUIDE
 ANSWER KEY TO THE PRACTICE QUESTIONS ON THE FINAL EXAM STUDY GUIDE -- 2013 1. Y (visible) 2. Z (infrared) 3. X (ultraviolet) 4. d (the absorption is occurring almost entirely in the infrared (LW) part
ANSWER KEY TO THE PRACTICE QUESTIONS ON THE FINAL EXAM STUDY GUIDE -- 2013 1. Y (visible) 2. Z (infrared) 3. X (ultraviolet) 4. d (the absorption is occurring almost entirely in the infrared (LW) part
Atmosphere, the Water Cycle and Climate Change
 Atmosphere, the Water Cycle and Climate Change OCN 623 Chemical Oceanography 16 April 2013 (Based on previous lectures by Barry Huebert) 2013 F.J. Sansone 1. The water cycle Outline 2. Climate and climate-change
Atmosphere, the Water Cycle and Climate Change OCN 623 Chemical Oceanography 16 April 2013 (Based on previous lectures by Barry Huebert) 2013 F.J. Sansone 1. The water cycle Outline 2. Climate and climate-change
Greenhouse Effect. How we stay warm
 Greenhouse Effect How we stay warm The Sun s energy reaches Earth through Radiation (heat traveling through Space) How much solar radiation reaches Earth? The Earth s surface only absorbs 51% of incoming
Greenhouse Effect How we stay warm The Sun s energy reaches Earth through Radiation (heat traveling through Space) How much solar radiation reaches Earth? The Earth s surface only absorbs 51% of incoming
FINAL EXAM STUDYING JUMP START REVIEW. Some review from earlier in the semester and some Q s on more recent topics...
 FINAL EXAM STUDYING JUMP START REVIEW Some review from earlier in the semester and some Q s on more recent topics.... The wavelength range of infrared 1. < 0.4 micrometers radiation. 2. > 0.7 micrometers
FINAL EXAM STUDYING JUMP START REVIEW Some review from earlier in the semester and some Q s on more recent topics.... The wavelength range of infrared 1. < 0.4 micrometers radiation. 2. > 0.7 micrometers
Lecture 28: Radiative Forcing of Climate Change
 Lecture 28: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
Lecture 28: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
ASTRONOMY 161. Introduction to Solar System Astronomy. Class 16
 ASTRONOMY 161 Introduction to Solar System Astronomy Class 16 Earth s Atmosphere Monday, February 19 Earth s Atmosphere: Key Concepts (1) The Earth s atmosphere consists mainly of nitrogen (N 2 ) and
ASTRONOMY 161 Introduction to Solar System Astronomy Class 16 Earth s Atmosphere Monday, February 19 Earth s Atmosphere: Key Concepts (1) The Earth s atmosphere consists mainly of nitrogen (N 2 ) and
Earth's Atmosphere. Atmospheric Layers. Atmospheric Layers
 Earth's Atmosphere Today we will talk about the part of Earth that is most important to our survival - the atmosphere Earth's atmosphere is unique in the Solar System and has changed greatly over time
Earth's Atmosphere Today we will talk about the part of Earth that is most important to our survival - the atmosphere Earth's atmosphere is unique in the Solar System and has changed greatly over time
Introduction. Introduction. Introduction. Outline Last IPCC report : 2001 Last IPCC report :
 Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Radiative Forcing and
 Radiative Forcing and Feedbacks in Climate Change Júlio C. S. Chagas Entrenamiento en Modelado Numérico de Escenarios de Cambios Climáticos Cachoeira Paulista, 30 de Agosto 4 de Septiembre de 2009. Definitions
Radiative Forcing and Feedbacks in Climate Change Júlio C. S. Chagas Entrenamiento en Modelado Numérico de Escenarios de Cambios Climáticos Cachoeira Paulista, 30 de Agosto 4 de Septiembre de 2009. Definitions
K39: The Key Evidence That Current Global Warming is Human-Caused
 K39: The Key Evidence That Current Global Warming is Human-Caused Here are 13 lines of Evidence GW = AGW (Global Warming = Anthropogenic Global Warming) Evidence #1: A Warming Troposphere with a Cooling
K39: The Key Evidence That Current Global Warming is Human-Caused Here are 13 lines of Evidence GW = AGW (Global Warming = Anthropogenic Global Warming) Evidence #1: A Warming Troposphere with a Cooling
Carbon Dioxide and Global Warming Case Study
 Carbon Dioxide and Global Warming Case Study Key Concepts: Greenhouse Gas Carbon dioxide El Niño Global warming Greenhouse effect Greenhouse gas La Niña Land use Methane Nitrous oxide Radiative forcing
Carbon Dioxide and Global Warming Case Study Key Concepts: Greenhouse Gas Carbon dioxide El Niño Global warming Greenhouse effect Greenhouse gas La Niña Land use Methane Nitrous oxide Radiative forcing
CONTENTS. Introduction x
 CONTENTS Introduction x Chapter 1: Climate 1 Solar Radiation and Temperature 2 The Distribution of Radiant Energy from the Sun 2 The Effects of the Atmosphere 3 Average Radiation Budgets 6 Surface-Energy
CONTENTS Introduction x Chapter 1: Climate 1 Solar Radiation and Temperature 2 The Distribution of Radiant Energy from the Sun 2 The Effects of the Atmosphere 3 Average Radiation Budgets 6 Surface-Energy
Climate Change and Air Quality
 Climate Change and Air Quality SW PA Air Quality Action June 6, 2007 Peter J. Adams Associate Professor Civil and Environmental Engineering Engineering and Public Policy Outline Climate Change Primer What
Climate Change and Air Quality SW PA Air Quality Action June 6, 2007 Peter J. Adams Associate Professor Civil and Environmental Engineering Engineering and Public Policy Outline Climate Change Primer What
Climate Change. Black-Body Radiation. Factors that affect how an object absorbs, emits (radiates), and reflects EM radiation incident on them:
 Climate Change Black-Body Radiation Factors that affect how an object absorbs, emits (radiates), and reflects EM radiation incident on them: 1) Nature of the surface: material, shape, texture, etc. 2)
Climate Change Black-Body Radiation Factors that affect how an object absorbs, emits (radiates), and reflects EM radiation incident on them: 1) Nature of the surface: material, shape, texture, etc. 2)
Global Warming and Climate Change
 Global Warming and Climate Change 1800s: Scientists knew that: If the earth were a bare, airless rock, the surface would be much colder than it actually is. Why? Tens of thousands of years ago, thick layers
Global Warming and Climate Change 1800s: Scientists knew that: If the earth were a bare, airless rock, the surface would be much colder than it actually is. Why? Tens of thousands of years ago, thick layers
Chapter 13 The Earths Atmosphere
 Chapter 3 The Earths Atmosphere Name: Class: Date: Time: 79 minutes Marks: 79 marks Comments: Page of 28 The bar chart shows some of the gases in the atmospheres of Earth today and Mars today. (b) Complete
Chapter 3 The Earths Atmosphere Name: Class: Date: Time: 79 minutes Marks: 79 marks Comments: Page of 28 The bar chart shows some of the gases in the atmospheres of Earth today and Mars today. (b) Complete
Convection Conduction
 L 18 Thermodynamics [3] Review Heat transfer processes convection conduction Greenhouse effect Climate change Ozone layer Review The temperature of a system is a measure of the average kinetic energy of
L 18 Thermodynamics [3] Review Heat transfer processes convection conduction Greenhouse effect Climate change Ozone layer Review The temperature of a system is a measure of the average kinetic energy of
Weather, Climate and Wetlands: Understanding the Terms and Definitions
 Weather, Climate and Wetlands: Understanding the Terms and Definitions Jan Pokorný and Hanna Huryna Contents Introduction... 1 Solar Energy Flux Between Sun and Earth... 4 Main Fluxes of Solar Energy in
Weather, Climate and Wetlands: Understanding the Terms and Definitions Jan Pokorný and Hanna Huryna Contents Introduction... 1 Solar Energy Flux Between Sun and Earth... 4 Main Fluxes of Solar Energy in
Analyze the causes and effects of air pollution. Acid precipitation 1.
 Lesson 4 Air Quality HE.6.C.1.3, LA.6.2.2.3, MA.6.A.3.6, SC.6.E.7.5, SC.6.E.7.9, SC.6.N.1.1, SC.6.N.1.4 Skim or scan the heading, boldfaced words, and pictures in the lesson. Identify or predict three
Lesson 4 Air Quality HE.6.C.1.3, LA.6.2.2.3, MA.6.A.3.6, SC.6.E.7.5, SC.6.E.7.9, SC.6.N.1.1, SC.6.N.1.4 Skim or scan the heading, boldfaced words, and pictures in the lesson. Identify or predict three
Climate Change: Demystifying the Application of Earth Systems Models for Climate Science
 Climate Change: Demystifying the Application of Earth Systems Models for Climate Science Richard B. Rood Cell: 301-526-8572 2525 Space Research Building (North Campus) rbrood@umich.edu http://clasp.engin.umich.edu/people/rbrood
Climate Change: Demystifying the Application of Earth Systems Models for Climate Science Richard B. Rood Cell: 301-526-8572 2525 Space Research Building (North Campus) rbrood@umich.edu http://clasp.engin.umich.edu/people/rbrood
Global Warming: What is the role of aerosol?
 Global Warming: What is the role of aerosol? Barbara Wyslouzil, Sept. 10 2007 Outline Aerosols 101 The greenhouse effect Global temperature records The global warming problem How do aerosols play a role
Global Warming: What is the role of aerosol? Barbara Wyslouzil, Sept. 10 2007 Outline Aerosols 101 The greenhouse effect Global temperature records The global warming problem How do aerosols play a role
