Introduction to Precious Metals
|
|
|
- Silvia York
- 5 years ago
- Views:
Transcription
1 1 / introduction Introduction to Precious Metls Metllurgy for Jewelers & smiths Mrk Grimwde
2 Introduction to precious metls Chpter One Physicl Properties of the Precious Metls The eight individul pure precious metls re chemicl elements, i.e., they cnnot e split up into simpler sustnces. All the toms of n element hve similr structure ut re different to toms of nother element. There re 92 elements nturlly occurring in the erth s crust nd tmosphere rnging from the gs hydrogen (H), with tomic no. 1, to the metl urnium (tomic no. 92). In ddition, there re numer of mn-mde elements produced in tomic rectors hving tomic numers greter thn 92 ut they need not concern us here. The Periodic Tle of Elements In 1869, the Russin chemist Dmitri Mendeleev pulished the Periodic Lw tht sttes the properties of elements re in periodic dependence on their tomic weights. Mny physicl nd chemicl properties of elements oey this lw nd it ws possile for Mendeleev to rrnge the known elements in the form of tle, modern version of which is shown in Figure 1.1. Verticl columns, known s Groups contin elements tht show strong fmily resemlnce with regrds to their physicl nd chemicl chrcteristics. For exmple, Group 1 consists of the elements copper (Cu), silver (Ag), nd gold (Au), nd lthough copper is regrded s se metl, s distinct from the precious metls silver nd gold, its ehvior is similr in mny respects nd, s will e seen in Chpter 5, it only just misses out on eing precious or nole metl. Horizontl rows, known s Periods, lso hve importnt fetures in common. Note, prticulrly tht the six pltinumgroup metls (PGMs) form lock in the center of the tle together with iron (Fe), colt (Co), nd nickel (Ni). This lock is referred to Group VIII. Ech element is numered in scending order from top to ottom nd from left to right. This is the tomic numer, mentioned ove, nd it tells us something out the nture of the toms of ech element. An tom consists of nucleus mde up of electricllypositive chrged prticles clled protons nd electriclly-neutrl prticles clled neutrons, together, with electriclly-negtive chrged prticles clled electrons. For simplicity, the electrons cn e considered to orit the nucleus in series of lyers or shells. Since the tom is electriclly neutrl overll, the numer of protons is equl to the numer of electrons, nd this numer is the tomic numer. Hence, for exmple, gold with tomic numer 79 will hve toms with 79 protons in their nuclei nd 79 electrons in [ 16 ]
3 1 physicl properties of the precious metls Group Period 1 I II III IV V VI VII VIII I II III IV V VI VII B 0 1 H 2 He 2 3 Li 4 Be 5 B 6 C 7 N 8 O 9 F 10 Ne 3 11 N 12 Mg 13 Al 14 Si 15 P 16 S 17 Cl 18 A 4 19 K 20 C 21 Sc 22 Ti 23 V 24 Cr 25 Mn 26 Fe 27 Ni 28 Co 29 Cu 30 Zn 31 G 32 Ge 33 As 34 Se 35 Br 36 Kr 5 37 R 38 Sr 39 Y 40 Zr 41 N 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 S 52 Te 53 I 54 Xe Cs 87 Fr 56 B 88 R *R.E. 89 Ac 72 Hf 90 Th 73 T 91 P 74 W 92 U 75 Re 76 Os 77 Ir 78 Pt 79 Au Trnsurnic Elements 80 Hg 81 Ti 82 P 83 Bi 84 Po 85 At 86 Rn Figure The Periodic Tle of Elements series of shells round ech nucleus. The toms of one element, therefore, differ from those of nother nd, ecuse mny physicl nd chemicl properties such s color, chemicl rectivity, nd reflectivity depend on the tomic structure, these chrcteristics vry from one element to nother. The periodicity discovered y Mendeleev nd others rises from the fct tht similrities in electron orit configurtion periodiclly reoccur with scending tomic numer, hence, the similrities in certin properties in elements within group. The elements re ssigned chemicl symols. Metls tht hve een known efore Romn times hve symols relting to their Ltin nmes. For exmple, the symol for copper is Cu from the Ltin word cuprum, gold is shortened to Au from urum, nd silver is shortened to Ag from rgentum. The sme is true for iron (Fe) nd led (P). Aout ¾ of the 92 elements re metls nd they re chrcterized y their ility to give up their outermost electrons nd the wy their toms ond together. The remining elements re gses nd non-metls. * Rre Erth Metls It is interesting to note tht when Mendeleev constructed his Periodic Tle there were numer of gps due to undiscovered elements. The puliction of the Tle nd Lw enled reserchers to predict nd find mny of these elements, germnium (Ge) eing cse in point. It will e pprent tht there should e n increse in the weight of ech tom (tomic weight) with tomic numer since the numer of protons, electrons, nd incidentlly, neutrons re incresing lmost without exception. The tomic weight of n tom is lmost entirely mde up from the nucleus, i.e., the weights of the protons nd the neutrons. This is ecuse the weight of n electron is only out 1/1800 of tht of the electriclly equivlent proton. A neutron hs out the sme weight s proton. Atomic weight is on comprtive scle using the stndrd of 12 s the weight of cron tom, which hs 6 protons nd 6 neutrons in its nucleus. Tle 1.1 gives the tomic numers nd weights of the precious metls with their tomic sizes ssuming the toms re spheres of certin rdius. The [ 17 ]
4 Introduction to precious metls Element Chemicl Atomic Atomic Atomic Symol Numer Weight Rdius (nm)* Ag Au Ruthenium Ru Rh Plldium Pd Osmium Os Iridium Ir Pltinum Pt Tle 1.1 Atomic chrcteristics of the precious metls. *One nnometer = 10-9 meters. lloying ehvior of metllic elements is prtly determined y the tomic rdii nd the tomic size mismtch of the elements present in the lloy. These fctors lso influence the strength of the lloy. Crystl Structure For our purposes, we cn consider toms to e very smll spheres, the rdii of which depend on the individul element. Oviously, to mke lump of metl tht cn e hndled, very lrge numer of toms must e rought together nd onded in some wy. Before going on to discuss onding nd crystl structure it is importnt to distinguish etween toms nd molecules. Too often, I her jewelers nd metlsmiths refer to the molecules of metl when they should e tlking out toms. Metls do not exist in moleculr form in the wy tht gses such s oxygen nd hydrogen or sustnces like wter nd certin chemicl compounds do. Molecules re generlly specific comintions of toms e.g., oxygen gs consists of molecules ech contining two toms of oxygen; ozone gs hs molecules of three toms of oxygen; the wter molecule is two toms of hydrogen to one tom of oxygen. Wter coming out of tp will consist of very lrge collection of those molecules. A solid chemicl compound cn e considered to e moleculr in the sense tht they exist in certin tom comintions. For exmple, the compound clcium sulphte hemihydrte (CSO₄ ½H₂O), etter known s gypsum (n importnt constituent of lost wx investment powder) hs the comintion of one tom of clcium to one tom of sulfur nd four toms of oxygen with hlf molecule of wter. Even so, it will hve crystl structure s ll true solids do. On the other hnd, pure metls nd most lloys exist s collection of toms nd not s specific comintions of toms. The only exception is in the cse of intermetllic compounds (see lter in Chpter 6) where it is true tht the compound hs fixed, or nerly-fixed, rtio of toms of one metl to nother. Let us now return to the suject of crystl structure of metls nd lloys. At very high tempertures, metl will vporize. Its toms will e fr prt nd will not tke up ny structurl form. As the [ 18 ]
5 1 physicl properties of the precious metls () () (c) Figure 1.2 Atomic rrngement of ) Gses, ) Liquids, nd c) Solids; the three sttes of mtter. temperture is lowered, the vpor condenses to liquid in the sme wy tht wter vpor condenses to stem (minute wter droplets) nd liquid wter. In quntity of liquid, the toms re loosely held together in rndom fshion. There is rudimentry structure of smll clusters of toms tht re continully reking up nd the toms move reltively esily to form new clusters. As the temperture drops still further, the liquid freezes to form solid nd ll the toms tke up positions in regulr geometric rry known s lttice to form crystl structure chrcteristic of tht prticulr sustnce. A two-dimensionl representtion is shown in Figure 1.2. All true solids hve regulr crystl structure whether they re metls or nonmetls. The min fctor tht distinguishes metls from non-metls is the mnner in which their toms re onded in the lttice. Metls hve specil form of onding known s metllic onding in which the toms re not so tightly held s in chemicl compounds (ionic onding) or s in cron in the form of dimonds (covlent onding). With metllic onding, the outermost electrons of the toms flot throughout the lttice s n electron cloud. It is this type of onding tht is responsile for the chrcteristics of ductility (mlleility), toughness nd good electricl nd therml conductivity. Solids tht re ionic- or covlent-onded my e strong nd hrd ut they re not ductile nd will shtter if sujected to sufficiently high force s in hmmer low. There re fourteen wys in which spheres cn e pcked together to form regulr geometric rry. The vst mjority of metls fll into one of three ctegories. The precious metls, with the exceptions of osmium nd ruthenium, hve lttice in which the unit cell or sic uilding lock is known s fce-centered cuic (). This structure hs n tom t ech corner of the cue nd one in the center of ech fce (Figure 1.3). If other similr cells re dded on in ll three directions d infinitum, single metl crystl cn e uilt up tht is sufficiently lrge enough to hndle (Figure 1.4). It will e noticed tht the corner nd fce toms re shred etween djcent cells. Aprt from gold (Au), silver (Ag), pltinum (Pt), plldium (Pd), iridium (Ir) nd rhodium (Rh), other metls include luminium (Al), copper (Cu), nickel (Ni) nd led (P). For resons tht re too complex to discuss in this ook, the metls hve very good mlleility nd ductility s will e recognized from the ove list. Osmium (Os) nd ruthenium (Ru) re exceptions in tht their lttice is close-pcked hexgonl (c.p.h.). The c.p.h. [ 19 ]
6 Introduction to precious metls lttice (Figure 1.5) often displys limited ductility. Indeed, osmium nd ruthenium re notoriously difficult to fricte nd, consequently, their industril uses re somewht limited. Other exmples of c.p.h. metls re zinc (Zn), cdmium (Cd) nd mgnesium (Mg). A third common occurrence ctegory is the ody-centered cuic lttice (.c.c.). Agin, the unit cell is cue with n tom t ech corner ut with n tom lso t the center of the cue insted of in ech fce (Figure 1.6). Exmples of.c.c. metls re chromium (Cr), tungsten (W) nd molydenum (Mo). A few metls cn disply more thn one type of crystl structure. The most notle exmples re iron (Fe) nd titnium (Ti). Iron is.c.c. (ferritic) up to 910 C (1670 F) where it chnges to (ustenitic) simply y the toms moving positions in the solid stte. At pproximtely 1400 C (2552 F) it chnges ck gin to the.c.c. form nd remins so up to the melting point of 1535ºC (2795 F). It is the ferrite to ustenite chnge tht is responsile for the fct tht steels, which re lloys of iron, cn e het treted nd hrdened. Titnium is c.p.h. up to 885 C (1625 F) where it chnges to.c.c.. These metls re sid to undergo n llotropic trnsformtion. However, it is the precious metls nd their crystl structures tht minly concern us. Wht then is the difference etween their lttices? Becuse the sizes of their toms vry from one metl to nother, the dimensions of the unit cell will lso vry. The length of cue edge for gold for instnce, will e different from tht of pltinum or silver. Crystllogrphers cn mesure the cell dimensions y X- Ry diffrction nlysis nd refer to the dimensions s the lttice prmeters for the prticulr metl. It is only necessry to quote the cue edge prmeter for the nd.c.c. metls wheres the c.p.h. metls hve the prmeter for the length of the side of the hexgon nd the c prmeter for the height of the cell (Tle 1.2). Corner Atoms Fce-Centered Atoms Figure 1.3 The fce-centered cuic () cell represented y ) points nd ) spheres where ech fce- centered tom touches its nerest corner toms. Figure 1.4 A lock of eight fce-centered cuic cells illustrting the wy crystl is uilt up. [ 20 ]
7 1 physicl properties of the precious metls Figure 1.5 The close pcked hexgonl (c.p.h.) cell. All toms on the se nd the top plnes re in contct with ech other on tht plne nd the toms on the intermedite plne nest on three of the six niches so formed. Figure 1.6 The Body Centered Cuic (.c.c.) Cell. The center tom touches ech corner tom, ut these do not touch ech other. Density The density of sustnce is defined s its weight per unit volume nd it is usully expressed in grms per cuic centimeter (g cm 3 ) lthough the preferred Interntionl System of Units (SI unit) is kg m 3. Density is function of oth tomic weight nd crystl structure. Therefore, elements with high tomic weights usully hve high densities, prticulrly if their toms re closely pcked together in the lttice. Both the nd the c.p.h. structures represent the closest form of tomic pcking of ll types of lttice. Consequently, it is not surprising to find tht gold, osmium, iridium, nd pltinum re regrded s hevy metls (Tle 1.3). Temperture lso ffects the density vlue ecuse the crystl structure expnds on heting. Density vlues re usully quoted for mesurements tken t 0 C or 2o C (32 F to 68 F) on fully nneled metl. To put the importnce of density into context, consider n item of jewelry mde in either sterling silver or 22 krt gold. Ech contins lmost identicl mounts of silver or gold t ~92.5%. The density of gold is nerly twice tht of silver, hence the item [ 21 ]
8 Introduction to precious metls Metl Structure Lttice prmeter (nm) Pltinum Plldium Iridium Osmium c.p.h. Ruthenium c.p.h. will weigh lmost twice s much in 22k gold s in silver. Similrly, pltinum ring of 950 fineness will e hevier thn n 18k gold ring of the sme design nd dimensions. It is lso n importnt considertion when costing items for mnufcture nd sle s precious metl content, density, nd price per troy ounce hve to e tken into ccount. Metl Density (g cm -³) Pltinum Plldium Iridium Osmium Ruthenium Tle 1.2 Crystl structure nd lttice prmeters * The close pcked hexgonl (c.p.h.) cell hs two chrcteristic dimensions nd hence two prmeters must e stted. The prmeter for the cell is the length of the cue edge c Melting nd Boiling Points The melting (freezing) tempertures of silver, gold, plldium, nd pltinum re of specil interest nd of gret prcticl importnce ecuse they re used s primry nd secondry fixed points on the Interntionl Prcticl Temperture Scle from which ll temperture-mesuring devices re clirted. This scle is periodiclly revised, the ltest eing in 1990, s stndrds of purity nd mesuring techniques re improved. The precious metls re chosen s reference points ecuse they re produced to high degree of purity nd re free from contmintion y oxidtion. Boiling points re more difficult to determine nd wide vrition in vlues hs een reported in the technicl literture over the yers. The vlues quoted in Tle 1.4 re the currently ccepted vlues. However, oiling points re of little interest to metlsmiths. Metl Melting Boiling Point ( C) Point ( C) Pltinum Plldium Iridium Osmium Ruthenium Tle 1.4 Melting nd oiling points of the precious metls. Tle 1.3 Density vlues for the precious metls t 20 C. [ 22 ]
9 1 physicl properties of the precious metls Opticl Properties Much of the fscintion of gold throughout the ges hs een due to its eutiful yellow color nd the high luster or reflectivity tht cn e retined indefinitely ecuse of its resistnce to corrosive ttck y the environment. Copper is the only metl esides gold with chrcteristic color, ut unfortuntely its lcks the corrosion resistnce of gold. Similrly, the esthetic ppel of silver lrgely rises from its rillint white color nd very high reflectivity. It hs the highest reflectivity of ll metls. Unfortuntely, the trnishing ehvior of silver limits its usefulness s reflector. The colors of gold nd copper result from shrp increse in reflectivity within nrrow nd of incresing wvelength of incident light flling on the surfce of the metl. Reflectivity (R) is low t the lue end of the visile light spectrum, (wvelength less thn 500 nnometers (nm)) ut rises drmticlly t the yellow-red-infrred end of the spectrum ( nm). This chnge in reflectivity is ssocited with the nture of the electron configurtions in the tomic structures of gold nd copper ut detiled discussion is eyond the scope of this ook. Figure 1.7 shows the reltionship etween R (on scle 0 to 1, where one equls 100%, nd wvelength for gold, silver, rhodium, nd pltinum. hs high R vlue over the entire visile light spectrum which ccounts for its rillint white luster. Of the PGMs, rhodium hs the highest reflectivity, with men vlue of out 80%. Tht, coupled with lck of trnishing ehvior, mkes it eminently suitle s n electroplted finish for silver nd the soclled white golds. The opticl properties of gold re profoundly ltered y lloying nd this is of considerle importnce to the jeweler Reflectivity, per cent Violet Indigo Blue Green Pltinum Wvelength nm nd goldsmith. The effect of lloying on the color of the krt gold lloys will e discussed more fully in Chpter 9. Much interest hs een shown in stndrdizing colors for gold lloys. The Europen wtch industry dopted coding system prticulrly for electroplted wtchcses lthough it hs lso een pplied for wrought lloys (Tle 1.5). In recent yers, quntittive system of color mesurement hs een developed tht enles lloy mnufcturers nd jewelers to give etter definition of the color of their products. It is the CIELAB system which mesures color in terms of spce coordintes L*, * nd * using color spectrophotometer. L* mesures the lck (0) white (100) coordinte. Metls with high reflectivity or whiteness will hve vlues pproching 100. The * coordinte goes from * vlues representing green Yellow Red Figure 1.7 Reflectivity in precious metls. [ 23 ]
Chapter 9: Phase Diagrams
 Chpter 9: Phse Digrms ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Chpter 9: Phse Digrms ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING
 Journl of Mining nd Metllurgy, 38 (1 2) B (2002) 93-102 THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING N.Mitevsk* nd @.D.@ivkovi}** *RTB BOR, Copper Institute, 19210 Bor, Yugoslvi
Journl of Mining nd Metllurgy, 38 (1 2) B (2002) 93-102 THERMODYNAMICS OF As, Sb AND Bi DISTRIBUTION DURING REVERB FURNACE SMELTING N.Mitevsk* nd @.D.@ivkovi}** *RTB BOR, Copper Institute, 19210 Bor, Yugoslvi
Chapter 3: The Structure of Crystalline Solids
 Chpter 3: The Structure of Crystlline Solids ISSUES TO ADDRESS... How do toms ssemble into solid structures? Exmples of dependence of mteril property on its crystl structure Chpter 3-1 Crystlline mterils...
Chpter 3: The Structure of Crystlline Solids ISSUES TO ADDRESS... How do toms ssemble into solid structures? Exmples of dependence of mteril property on its crystl structure Chpter 3-1 Crystlline mterils...
Types of Solids. Chapter 12. Solids and Modern Materials
 Chpter 12 Solids nd 1 Types of Solids Four generl types of solids. Metllic solids shre network of highly deloclized electrons. Ionic solids re sets of ctions nd nions mutully ttrcted to one nother. 2 Bonding
Chpter 12 Solids nd 1 Types of Solids Four generl types of solids. Metllic solids shre network of highly deloclized electrons. Ionic solids re sets of ctions nd nions mutully ttrcted to one nother. 2 Bonding
Phase Equilibria: Solubility Limit PHASE DIAGRAMS 10 0 Solubility 8 0 Limit ENT 145 Materials Engineering (liquid) atu (liquid solution 4 0
 Temperture (ºC) ter ugr Temperture (ºC) Phse Equilibri: olubility imit PHE DIGM ENT 145 Mterils Engineering Chpter 9 - olution solid, liquid, or gs solutions, single phse Mixture more thn one phse olubility
Temperture (ºC) ter ugr Temperture (ºC) Phse Equilibri: olubility imit PHE DIGM ENT 145 Mterils Engineering Chpter 9 - olution solid, liquid, or gs solutions, single phse Mixture more thn one phse olubility
Chapter 9: PHASE DIAGRAM
 Chpter 9: PHASE DIAGRAM ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Chpter 9: PHASE DIAGRAM ISSUES TO ADDRESS... When we combine two elements... wht is the resulting equilibrium stte? In prticulr, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), nd -- the temperture
Crystal Structure. Dragica Vasileska and Gerhard Klimeck
 Crystl Structure Drgic Vsilesk nd Gerhrd Klimeck Crystl Structure Issues tht re ddressed in this lecture include:. Periodic rry of toms. Fundmentl types of lttices 3. Index system for crystl plnes 4. Simple
Crystl Structure Drgic Vsilesk nd Gerhrd Klimeck Crystl Structure Issues tht re ddressed in this lecture include:. Periodic rry of toms. Fundmentl types of lttices 3. Index system for crystl plnes 4. Simple
SUPPLEMENTARY INFORMATION
 doi:10.1038/nture10970 I. GN directly grown on the h-bn relese lyer Figure S1 shows X-ry diffrction with the 2θ/ω configurtion nd n opticl microscopy imge for the GN directly grown on the h-bn relese lyer.
doi:10.1038/nture10970 I. GN directly grown on the h-bn relese lyer Figure S1 shows X-ry diffrction with the 2θ/ω configurtion nd n opticl microscopy imge for the GN directly grown on the h-bn relese lyer.
Li Mass = 7 amu Melting point C Density 0.53 g/cm 3 Color: silvery
 Blackline Master B02: Mendeleev s Element Cards H Mass = 1 amu Melting point -259 0 C Density 0.0909 g/cm 3 Color: colorless Li Mass = 7 amu Melting point 180.5 0 C Density 0.53 g/cm 3 Be Mass = 9 amu
Blackline Master B02: Mendeleev s Element Cards H Mass = 1 amu Melting point -259 0 C Density 0.0909 g/cm 3 Color: colorless Li Mass = 7 amu Melting point 180.5 0 C Density 0.53 g/cm 3 Be Mass = 9 amu
Chapter 9. Quadratics
 Chpter 9 Qudrtics Artificil Body Prts 9.1 Solving Qudrtic Equtions by Fctoring 9. Completing the Squre 9.3 The Qudrtic Formul 9.4 Eponentil Functions (Growth nd Decy) Chpter Review Chpter Test 147 Section
Chpter 9 Qudrtics Artificil Body Prts 9.1 Solving Qudrtic Equtions by Fctoring 9. Completing the Squre 9.3 The Qudrtic Formul 9.4 Eponentil Functions (Growth nd Decy) Chpter Review Chpter Test 147 Section
Silver-Tin Oxide. (Ag/SnO²)
 Silver-Tin Oxide (Ag/SnO²) Silver Tin oxide (Ag/SnO² ) mterils. speterts or other rights of third prties re concemend. The informtion descries the type of mteril nd shll not e considered s ssured chrcteristics.
Silver-Tin Oxide (Ag/SnO²) Silver Tin oxide (Ag/SnO² ) mterils. speterts or other rights of third prties re concemend. The informtion descries the type of mteril nd shll not e considered s ssured chrcteristics.
PY2N20 Material Properties and Phase Diagrams
 PY2N20 Mteril Properties nd Phse Digrms ecture 5 P. tmenov, PhD chool of Physics, TCD PY2N20-5 Phse Digrms - Introduction How much cn be done with pure elementl compounds? How mny combintions of elements
PY2N20 Mteril Properties nd Phse Digrms ecture 5 P. tmenov, PhD chool of Physics, TCD PY2N20-5 Phse Digrms - Introduction How much cn be done with pure elementl compounds? How mny combintions of elements
Laboratory Plant for Studies Regarding the Behaviour of the Sintered Materials in Oxygen Isotopic Distillation
 6th IASME/WSEAS Interntionl Conference on HEAT TRANSFER, THERMAL ENGINEERING nd ENVIRONMENT (HTE'08) Lortory Plnt for Studies Regrding the Behviour of the Sintered Mterils in Oxygen Isotopic Distilltion
6th IASME/WSEAS Interntionl Conference on HEAT TRANSFER, THERMAL ENGINEERING nd ENVIRONMENT (HTE'08) Lortory Plnt for Studies Regrding the Behviour of the Sintered Mterils in Oxygen Isotopic Distilltion
High strength fine grained structural steel, thermo-mechanically rolled, for high temperature application
 P420M HT High strength fine grined structurl steel, thermo-mechniclly rolled, for high temperture ppliction Specifiction DH-E52-D, edition April 2016 1 P420M HT is high strength thermomechniclly rolled
P420M HT High strength fine grined structurl steel, thermo-mechniclly rolled, for high temperture ppliction Specifiction DH-E52-D, edition April 2016 1 P420M HT is high strength thermomechniclly rolled
Families on the Periodic Table
 Families on the Periodic Table Elements on the periodic table can be grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families
Families on the Periodic Table Elements on the periodic table can be grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families
H. Randall Smith; Ph.D. Agronomy and Wayne Porter: Ph.D. Horticulture Mississippi State University Extension Service
 Effect of SumGrow on growth, development nd yield of Irish pottoes (Solnum tuerosum) t the Beumont Reserch Sttion (Mississippi Stte University) during 217 H. Rndll Smith; Ph.D. Agronomy nd yne Porter:
Effect of SumGrow on growth, development nd yield of Irish pottoes (Solnum tuerosum) t the Beumont Reserch Sttion (Mississippi Stte University) during 217 H. Rndll Smith; Ph.D. Agronomy nd yne Porter:
Chapter 9: Phase Diagrams
 hpter 9: Phse Digrms ISSUES TO ADDRESS... oncepts of Phse, omponent, Equilibrium Phse digrm In prticulr, if we specify... -- composition (e.g., wt% u - wt% Ni), nd -- temperture (T) then... How mny phses
hpter 9: Phse Digrms ISSUES TO ADDRESS... oncepts of Phse, omponent, Equilibrium Phse digrm In prticulr, if we specify... -- composition (e.g., wt% u - wt% Ni), nd -- temperture (T) then... How mny phses
NOTICE CONCERNING COPYRIGHT RESTRICTIONS
 NOTICE CONCERNING COPYRIGHT RESTRICTIONS This document my contin copyrighted mterils. These mterils hve been mde vilble for use in reserch, teching, nd privte study, but my not be used for ny commercil
NOTICE CONCERNING COPYRIGHT RESTRICTIONS This document my contin copyrighted mterils. These mterils hve been mde vilble for use in reserch, teching, nd privte study, but my not be used for ny commercil
Work hard. Be nice. Name: Period: Date: UNIT 2: Atomic Concepts Lesson 1: Give me an element, any element
 1 Name: Period: Date: KIPP NYC College Prep General Chemistry UNIT 2: Atomic Concepts Lesson 1: Give me an element, any element Do Now: By the end of today, you will have an answer to: How do you know
1 Name: Period: Date: KIPP NYC College Prep General Chemistry UNIT 2: Atomic Concepts Lesson 1: Give me an element, any element Do Now: By the end of today, you will have an answer to: How do you know
Course Objectives. MSE 260 Phase Transformations. Prerequisites. Course Outcomes 1/16/2017. Forms of Assessment. Course Outline. Dr.
 ME 26 Phse Trnsformtions Kwme Nkrumh University of cience & Technology, Kumsi, Ghn Dr. Anthony Andrews Deprtment of Mterils Engineering Fculty of Mechnicl nd Chemicl Engineering College of Engineering
ME 26 Phse Trnsformtions Kwme Nkrumh University of cience & Technology, Kumsi, Ghn Dr. Anthony Andrews Deprtment of Mterils Engineering Fculty of Mechnicl nd Chemicl Engineering College of Engineering
PY2N20 Material Properties and Phase Diagrams
 PY2N20 Mteril Properties nd Phse Digrms ecture 6 P. Stmenov, PhD School of Physics, TCD PY2N20-6 Microstructures in Eutectic Systems: I C o < 2 wt% Sn Result: - t extreme ends - polycrystl of grins i.e.,
PY2N20 Mteril Properties nd Phse Digrms ecture 6 P. Stmenov, PhD School of Physics, TCD PY2N20-6 Microstructures in Eutectic Systems: I C o < 2 wt% Sn Result: - t extreme ends - polycrystl of grins i.e.,
MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS
 Act Metllurgic Slovc, Vol. 20, 2014, No. 2, p. 146-151 146 MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS Zdzisłw Kudliński 1), Krystin Jniszewski 2)* 1) Silesin University
Act Metllurgic Slovc, Vol. 20, 2014, No. 2, p. 146-151 146 MACROSCOPIC INVESTIGATIONS OF THE PRIMARY STRUCTURE OF CONTINUOUS CASTING INGOTS Zdzisłw Kudliński 1), Krystin Jniszewski 2)* 1) Silesin University
1. Supplementary Figures and Legends a b
 doi:10.1038/nture09540 1. Supplementry Figures nd Legends Supplementry Figure 1. Scnning trnsmission electron microgrphs (STEM) of NCC., STEM prepred from fst evportion of dilute NCC suspension shows individul
doi:10.1038/nture09540 1. Supplementry Figures nd Legends Supplementry Figure 1. Scnning trnsmission electron microgrphs (STEM) of NCC., STEM prepred from fst evportion of dilute NCC suspension shows individul
q p P : Si : : : : Si : : : Si : Si : Si : : Extra free e - Dope Here, energy to get 1/26/10 Lecture Topics: Silicon
 EE 143 Microfbriction Technology Lecture 3 Mterils II 1/26/10 Reding portions throughout Jeger Lecture Topics licon licon Dioxide licon Nitride Aluminum & Other Metls In this course, the focus will be
EE 143 Microfbriction Technology Lecture 3 Mterils II 1/26/10 Reding portions throughout Jeger Lecture Topics licon licon Dioxide licon Nitride Aluminum & Other Metls In this course, the focus will be
CORRELATION BETWEEN MELT POOL TEMPERATURE AND CLAD FORMATION IN PULSED AND CONTINUOUS WAVE ND:YAG LASER CLADDING OF STELLITE 6
 Proceedings of the st Pcific Interntionl Conference on Appliction of sers nd Optics 4 CORREATION BETWEEN ET POO TEPERATURE AN CA FORATION IN PUSE AN CONTINUOUS WAVE N:YAG
Proceedings of the st Pcific Interntionl Conference on Appliction of sers nd Optics 4 CORREATION BETWEEN ET POO TEPERATURE AN CA FORATION IN PUSE AN CONTINUOUS WAVE N:YAG
INTERSTITIAL VOIDS IN TETRAHEDRALLY AND IN THREE-FOLD BONDED ATOMIC NETWORKS
 Journl of Non-Oxide Glsses Vol. 5, No 2, 2013, p. 21-26 INTERSTITIAL VOIDS IN TETRAHEDRALLY AND IN THREE-FOLD BONDED ATOMIC NETWORKS F. SAVA, M. POPESCU *, I.D. SIMANDAN, A. LŐRINCZI, A. VELEA Ntionl Institute
Journl of Non-Oxide Glsses Vol. 5, No 2, 2013, p. 21-26 INTERSTITIAL VOIDS IN TETRAHEDRALLY AND IN THREE-FOLD BONDED ATOMIC NETWORKS F. SAVA, M. POPESCU *, I.D. SIMANDAN, A. LŐRINCZI, A. VELEA Ntionl Institute
Where do we start? ocreate the Universe oform the Earth and elements omove the elements into their correct positions obuild the atmosphere and oceans
 Where do we start? ocreate the Universe oform the Earth and elements omove the elements into their correct positions obuild the atmosphere and oceans 1 The BIG BANG The Universe was created 13.8 billion
Where do we start? ocreate the Universe oform the Earth and elements omove the elements into their correct positions obuild the atmosphere and oceans 1 The BIG BANG The Universe was created 13.8 billion
Groups of Elements 3B 5B 6B 7B 2 C. 10 Na. 36 Rb. 54 Cs. 86 Fr. 57 Ac. 71 Th. Nitrogen group. Alkali metals. Alkaline earth metals.
 Groups of Elements * * Li He C N O 8 F 9 Ne 0 B Be H Al Si P S Cl Ar 8 K 9 Ca 0 Sc Ti V Cr Mn Fe Co Ni 8 Cu 9 Zn 0 Ga Ge As Se Br Kr Rb Sr 8 Y 9 Zr 0 Nb Mo Tc Ru Rh Pd Ag Cd 8 In 9 Sn 0 Sb Te I Xe Cs Ba
Groups of Elements * * Li He C N O 8 F 9 Ne 0 B Be H Al Si P S Cl Ar 8 K 9 Ca 0 Sc Ti V Cr Mn Fe Co Ni 8 Cu 9 Zn 0 Ga Ge As Se Br Kr Rb Sr 8 Y 9 Zr 0 Nb Mo Tc Ru Rh Pd Ag Cd 8 In 9 Sn 0 Sb Te I Xe Cs Ba
Effect of Tantalum Additions to a Cobalt-Chromium-Nickel
 Effect of Tntlum Additions to Coblt-Chromium-Nickel Bse Alloy A. P. ROWE, W. C. BIGELOW, nd K. ASGAR University of Michign, School of Dentistry, Ann Arbor, Michign 48104, USA An investigtion by electron
Effect of Tntlum Additions to Coblt-Chromium-Nickel Bse Alloy A. P. ROWE, W. C. BIGELOW, nd K. ASGAR University of Michign, School of Dentistry, Ann Arbor, Michign 48104, USA An investigtion by electron
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 7: Chemical systems
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 7: Chemical systems MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 7: Chemical systems MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
ENVIRONMENTAL AUDIT OF THE SITES IMPACTED BY THE PROBO KOALA TOXIC WASTE DUMPING IN ABIDJAN, CÔTE D IVOIRE
 ENVIRONMENTAL AUDIT OF THE SITES IMPACTED BY THE PROBO KOALA TOXIC WASTE DUMPING IN ABIDJAN, CÔTE D IVOIRE This series of fct sheets ws prepred s prt of UN Environment s environmentl udit of the sites
ENVIRONMENTAL AUDIT OF THE SITES IMPACTED BY THE PROBO KOALA TOXIC WASTE DUMPING IN ABIDJAN, CÔTE D IVOIRE This series of fct sheets ws prepred s prt of UN Environment s environmentl udit of the sites
6.1 Damage Tolerance Analysis Procedure
 6. Dmge Tolernce Anlysis Procedure For intct structure the nlysis procedures for Slow Crck Growth nd Fil Sfe structure re essentilly the sme. An initil flw is ssumed nd its growth is nlyzed until filure
6. Dmge Tolernce Anlysis Procedure For intct structure the nlysis procedures for Slow Crck Growth nd Fil Sfe structure re essentilly the sme. An initil flw is ssumed nd its growth is nlyzed until filure
Part 1. Preparation and Color of Solutions. Experiment 1 (2 session lab) Electrons and Solution Color. Pre-lab Report, page 29
 Experiment 1 (2 session lab) Electrons and Solution Color Pre-lab Report, page 29 Session 1 One hour discussion (E2) Two hour lab (E1) Aim to complete Parts 1, 2, and 3 of E1. Part 1. Preparation and Color
Experiment 1 (2 session lab) Electrons and Solution Color Pre-lab Report, page 29 Session 1 One hour discussion (E2) Two hour lab (E1) Aim to complete Parts 1, 2, and 3 of E1. Part 1. Preparation and Color
Chemistry*120* * Name:! Rio*Hondo*College* * * EXAMPLE*EXAM*1*!
 Chemistry*120* * Name: EXAMPLE*EXAM*1* Exam)#1 100)points) Directions:)Answereachquestionbelowtothebestofyourability.Showallworkwherecalculations arerequired.aninformationsheetwithaperiodictableisattachedtothebackoftheexam;youmay
Chemistry*120* * Name: EXAMPLE*EXAM*1* Exam)#1 100)points) Directions:)Answereachquestionbelowtothebestofyourability.Showallworkwherecalculations arerequired.aninformationsheetwithaperiodictableisattachedtothebackoftheexam;youmay
P.M. THURSDAY, 15 January minutes
 Candidate Name Centre Number Candidate Number 0 GCSE 236/01 SCIENCE FOUNDATION TIER CHEMISTRY 1 P.M. THURSDAY, 15 January 2009 45 minutes For Examiner s use Total Mark ADDITIONAL MATERIALS In addition
Candidate Name Centre Number Candidate Number 0 GCSE 236/01 SCIENCE FOUNDATION TIER CHEMISTRY 1 P.M. THURSDAY, 15 January 2009 45 minutes For Examiner s use Total Mark ADDITIONAL MATERIALS In addition
1 Information, Persuasion, and Signalling
 ECON 312: Advertising 1 We will now exmine nother strtegic vrible vilble to firms, tht of dvertising. Industril Orgniztion Advertising 1 Informtion, Persusion, nd Signlling 1.1 Persusion versus Informtion
ECON 312: Advertising 1 We will now exmine nother strtegic vrible vilble to firms, tht of dvertising. Industril Orgniztion Advertising 1 Informtion, Persusion, nd Signlling 1.1 Persusion versus Informtion
A BEHAVIOR OF ELASTIC AND PLASTIC STRAIN FOR (α+γ) DUAL PHASE STAINLESS STEELS IN ROTATING BENDING TEST
 Copyright JCDS - Interntionl Centre for Diffrction Dt 24, Advnces in X-ry Anlysis, Volume 47. 39 ISSN 197-2 A BEHAVIOR OF ELASTIC AND LASTIC STRAIN FOR (+) DUAL HASE STAINLESS STEELS IN ROTATING BENDING
Copyright JCDS - Interntionl Centre for Diffrction Dt 24, Advnces in X-ry Anlysis, Volume 47. 39 ISSN 197-2 A BEHAVIOR OF ELASTIC AND LASTIC STRAIN FOR (+) DUAL HASE STAINLESS STEELS IN ROTATING BENDING
Building initial configuration. Ideal crystals (I)
 Building initil configurtion. Idel crstls (I) An crstlline solid cn be defined in terms of Brvis lttice which specifies the periodic rr in which the repeted units of the crstl re rrnged. Brvis lttice is
Building initil configurtion. Idel crstls (I) An crstlline solid cn be defined in terms of Brvis lttice which specifies the periodic rr in which the repeted units of the crstl re rrnged. Brvis lttice is
Name Period Date. Grade 7 Unit 1 Assessment. 1. The number line below shows the high temperature in Newark, in degrees Fahrenheit, on Monday.
 Nme Period Dte Grde 7 Unit 1 Assessment For multiple choice questions, circle the est nswer. For ll other questions, respond in the spce provided. 1. The numer line elow shows the high temperture in Newrk,
Nme Period Dte Grde 7 Unit 1 Assessment For multiple choice questions, circle the est nswer. For ll other questions, respond in the spce provided. 1. The numer line elow shows the high temperture in Newrk,
IMAGING PHASES IN STEELS
 Hints for IMAGING PHASES IN STEELS Some techniques lerned from experience cn help metllogrphers identify certin phses in steels. G. F. Vnder Voort* Buehler Ltd., Lke Bluff, Illinois E. P. Mnilov** Polzunov
Hints for IMAGING PHASES IN STEELS Some techniques lerned from experience cn help metllogrphers identify certin phses in steels. G. F. Vnder Voort* Buehler Ltd., Lke Bluff, Illinois E. P. Mnilov** Polzunov
CHM101 SECOND EXAM 162. Chemistry 101 SECOND Exam (162) Name: Date: 30/4/2017
 Chemistry 101 SECOND Exam (162) Name: Date: 30/4/2017 Student no. Section: Useful Information:Gas Constant R= 0.08206 L.atm/K.mol, Specific heat of H 2 O=4.18 J/g. C 1 atm. = 760 mmhg. H 1 He 2 1.000 4
Chemistry 101 SECOND Exam (162) Name: Date: 30/4/2017 Student no. Section: Useful Information:Gas Constant R= 0.08206 L.atm/K.mol, Specific heat of H 2 O=4.18 J/g. C 1 atm. = 760 mmhg. H 1 He 2 1.000 4
Practice General Chemistry Speaking Test (I. Gould) (Questions from Chapter 1 of the textbook) hydrogen 1 H
 Practice General hemistry Speaking Test (I. Gould) (Questions from hapter 1 of the textbook) hydrogen 1 1.0079 helium 2 e 4.0026 lithium 3 Li 6.941 beryllium 4 Be 9.012 boron 5 B 10.811 carbon 6 12.0107
Practice General hemistry Speaking Test (I. Gould) (Questions from hapter 1 of the textbook) hydrogen 1 1.0079 helium 2 e 4.0026 lithium 3 Li 6.941 beryllium 4 Be 9.012 boron 5 B 10.811 carbon 6 12.0107
2016 Prelim Essay Question 2
 216 Prelim Essy Question 2 In recent yers, the price of nturl fertilisers for orgnic brown rice production hs risen nd helthy living cmpigns re seeing more consumers switching from nonorgnic white rice
216 Prelim Essy Question 2 In recent yers, the price of nturl fertilisers for orgnic brown rice production hs risen nd helthy living cmpigns re seeing more consumers switching from nonorgnic white rice
ELECTRICALLY CONDUCTIVE STRUCTURAL ADHESIVES BASED ON BUCKYPAPERS
 18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS ELECTRICALLY CONDUCTIVE STRUCTURAL ADHESIVES BASED ON BUCKYPAPERS I.D. Rosc, S.V Ho* Mechnicl nd Industril Engineering, Concordi University, Montrel,
18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS ELECTRICALLY CONDUCTIVE STRUCTURAL ADHESIVES BASED ON BUCKYPAPERS I.D. Rosc, S.V Ho* Mechnicl nd Industril Engineering, Concordi University, Montrel,
Effect of Austempering Heat Treatment on the Tensile Properties of CamShaft Made of Ductile Cast Iron
 Interntionl Refereed Journl of Engineering nd Science (IRJES) ISSN (Online) 2319-183X, (Print) 2319-1821 Volume 6, Issue 11 (Novemer 2017), PP. 16-22 Effect of Austempering Het Tretment on the Tensile
Interntionl Refereed Journl of Engineering nd Science (IRJES) ISSN (Online) 2319-183X, (Print) 2319-1821 Volume 6, Issue 11 (Novemer 2017), PP. 16-22 Effect of Austempering Het Tretment on the Tensile
Three-Phase Wound-Rotor Induction Machine with Rotor Resistance
 Exercise 2 Three-Phse Wound-Rotor Induction Mchine with Rotor Resistnce EXERCISE OBJECTIVE When you hve completed this exercise, you will know the effects of vrying the rotor resistnce of three-phse wound-rotor
Exercise 2 Three-Phse Wound-Rotor Induction Mchine with Rotor Resistnce EXERCISE OBJECTIVE When you hve completed this exercise, you will know the effects of vrying the rotor resistnce of three-phse wound-rotor
2nd International Conference on Electronic & Mechanical Engineering and Information Technology (EMEIT-2012)
 2nd Interntionl Conference on Electronic & Mechnicl Engineering nd Informtion Technology (EMEIT-2012) Comprehensive Evlution of Air Conditioning Cold/Het Source System Bsed on Fuzzy Mthemtics Theory Gng
2nd Interntionl Conference on Electronic & Mechnicl Engineering nd Informtion Technology (EMEIT-2012) Comprehensive Evlution of Air Conditioning Cold/Het Source System Bsed on Fuzzy Mthemtics Theory Gng
The Structure of Metals
 Chpter 1 The Structure of Metls QUALITATIVE PROBLEMS 1.21 Explin your understnding of why the study of the crystl structure of metls is importnt. The study of crystl structure is importnt for number of
Chpter 1 The Structure of Metls QUALITATIVE PROBLEMS 1.21 Explin your understnding of why the study of the crystl structure of metls is importnt. The study of crystl structure is importnt for number of
ADDITIONAL SCIENCE/CHEMISTRY. A.M. TUESDAY, 14 January hour
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
ADDITIONAL SCIENCE/CHEMISTRY. A.M. TUESDAY, 14 January hour
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
Densification mechanism for different types of stainless steel powders in Selective Laser Melting
 Aville online t www.sciencedirect.com ScienceDirect Procedi CIRP 00 (2016) 000 000 www.elsevier.com/locte/procedi 10th CIRP Conference on Intelligent Computtion in Mnufcturing Engineering - CIRP ICME '16
Aville online t www.sciencedirect.com ScienceDirect Procedi CIRP 00 (2016) 000 000 www.elsevier.com/locte/procedi 10th CIRP Conference on Intelligent Computtion in Mnufcturing Engineering - CIRP ICME '16
ADDITIONAL SCIENCE/CHEMISTRY
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 S15-4472-02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER A.M. THURSDAY, 14 May 2015 1 hour For s use Question Maximum Mark Mark
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 S15-4472-02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER A.M. THURSDAY, 14 May 2015 1 hour For s use Question Maximum Mark Mark
Return Temperature in DH as Key Parameter for Energy Management
 Interntionl OPEN ACCESS Journl Of Modern Engineering Reserch (IJMER) Return Temperture in DH s Key Prmeter for Energy Mngement Normunds Tlcis 1, Egīls Dzelzītis 2, Agnese Līckrstiņ 2 *JSC Rīgs siltums
Interntionl OPEN ACCESS Journl Of Modern Engineering Reserch (IJMER) Return Temperture in DH s Key Prmeter for Energy Mngement Normunds Tlcis 1, Egīls Dzelzītis 2, Agnese Līckrstiņ 2 *JSC Rīgs siltums
Materials Science. Energy and Packing. Materials and Packing. Chapter 3: Structures of Metals & Ceramics ISSUES TO ADDRESS...
 In the Nme of God Chpter : Structures of Metls & Cermics ISSUES TO ADDRESS... Mterils Science Dr Mohmmd Heidri-Rrni How do toms ssemble into solid structures? How does the density of mteril depend on its
In the Nme of God Chpter : Structures of Metls & Cermics ISSUES TO ADDRESS... Mterils Science Dr Mohmmd Heidri-Rrni How do toms ssemble into solid structures? How does the density of mteril depend on its
The basic model for inventory analysis
 The bsic model for inventory nlysis Lecture Notes for ME515 Prepred by Joyce Smith Cooper Professor of Mechnicl Engineering University of Wshington cooperjs@uw.edu See Chpter 2 of Heijungs nd Suh (22)
The bsic model for inventory nlysis Lecture Notes for ME515 Prepred by Joyce Smith Cooper Professor of Mechnicl Engineering University of Wshington cooperjs@uw.edu See Chpter 2 of Heijungs nd Suh (22)
SCH 3U1 Solutions Practice Test...visit class website for answers
 Nme: Clss: Dte: SCH 3U1 Solutions Prctice Test...visit clss website for nswers True/Flse Indicte whether the sttement is true or flse. 1. Wter is not very good for dissolving ionic compounds. 2. Adding
Nme: Clss: Dte: SCH 3U1 Solutions Prctice Test...visit clss website for nswers True/Flse Indicte whether the sttement is true or flse. 1. Wter is not very good for dissolving ionic compounds. 2. Adding
Paper Reference (complete below) Edexcel GCSE Additional Science (5018H) Chemistry (5038H) C2 Topics 5 to 8
 Centre No. Paper Reference (complete below) Surname Initial(s) Candidate No. H 1 H Signature Paper Reference(s) 5018H/1H 5038H/1H Edexcel GCSE Additional Science (5018H) Chemistry (5038H) C2 Topics 5 to
Centre No. Paper Reference (complete below) Surname Initial(s) Candidate No. H 1 H Signature Paper Reference(s) 5018H/1H 5038H/1H Edexcel GCSE Additional Science (5018H) Chemistry (5038H) C2 Topics 5 to
STRUCTURAL TRANSFORMATIONS IN ELECTRON BEAM WELDED JOINTS OF CONTINUOUS SAW BLADES. Dumitru OCHIAN, Ion-Dragoş UŢU, Ion MITELEA
 STRUCTURAL TRANSFORMATIONS IN ELECTRON BEAM WELDED JOINTS OF CONTINUOUS SAW BLADES Dumitru OCHIAN, Ion-Drgoş UŢU, Ion MITELEA Politehnic University of Timisor, Pt Victoriei, No. 1, Timisor, Romni, dnochin@yhoo.com,
STRUCTURAL TRANSFORMATIONS IN ELECTRON BEAM WELDED JOINTS OF CONTINUOUS SAW BLADES Dumitru OCHIAN, Ion-Drgoş UŢU, Ion MITELEA Politehnic University of Timisor, Pt Victoriei, No. 1, Timisor, Romni, dnochin@yhoo.com,
PAPER CHEMISTRY, APPLETON, WISCONSIN IPC TECHNICAL PAPER SERIES NUMBER 163 W. J. WHITSITT OCTOBER, 1985
 163 THE INSTITUTE OF PAPER CHEMISTRY, APPLETON, WISCONSIN O E G? o IPC TECHNICAL PAPER SERIES NUMBER 163 O4 o= o COMPRESSIVE STRENGTH RELATIONSHIPS AND FACTORS, C) t C= c. Md Qy W. J. WHITSITT OCTOBER,
163 THE INSTITUTE OF PAPER CHEMISTRY, APPLETON, WISCONSIN O E G? o IPC TECHNICAL PAPER SERIES NUMBER 163 O4 o= o COMPRESSIVE STRENGTH RELATIONSHIPS AND FACTORS, C) t C= c. Md Qy W. J. WHITSITT OCTOBER,
SUPPLEMENTARY INFORMATION
 SI Fig. PrpS is single copy gene k 3. 9... EcoRV EcoRV k 5 BmH Pst c well k HindIII HindIII HindIII.3.5 3.. Southern lots of Ppver genomic DNA from plnts with SS8 hplotypes, hyridized with PrpS proe..
SI Fig. PrpS is single copy gene k 3. 9... EcoRV EcoRV k 5 BmH Pst c well k HindIII HindIII HindIII.3.5 3.. Southern lots of Ppver genomic DNA from plnts with SS8 hplotypes, hyridized with PrpS proe..
This resource contains three different versions of the periodic table, including a blank one for colouring!
 Teaching notes This resource contains three different versions of the periodic table, including a blank one for colouring! It also contains tables of the Group 0, 1 and 7 elements with a few columns for
Teaching notes This resource contains three different versions of the periodic table, including a blank one for colouring! It also contains tables of the Group 0, 1 and 7 elements with a few columns for
[ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids
![[ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids [ HOCl] Chapter 16. Problem. Equilibria in Solutions of Weak Acids. Equilibria in Solutions of Weak Acids](/thumbs/72/67425582.jpg) Equilibri in Solutions of Wek Acids Chpter 16 Acid-Bse Equilibri Dr. Peter Wrburton peterw@mun.c http://www.chem.mun.c/zcourses/1011.php The dissocition of wek cid is n equilibrium sitution with n equilibrium
Equilibri in Solutions of Wek Acids Chpter 16 Acid-Bse Equilibri Dr. Peter Wrburton peterw@mun.c http://www.chem.mun.c/zcourses/1011.php The dissocition of wek cid is n equilibrium sitution with n equilibrium
STRESS CORROSION CRACKING OF THE CLAD STRUCTURAL STEEL AFTER ITS HIGH TEMPERATURE HYDROGEN DEGRADATION
 1 2 2 2 1 K. Lulińsk, O. Tsyrulnyk, M. Hredil, H. Nykyforchyn, K.J. Kurzydłowski 1 Wrsw University of Technology, Fculty of Mterils Science nd Engineering, Wolosk 141, 02-507 Wrsw, Polnd 2 Krpenko Physico-Mechnicl
1 2 2 2 1 K. Lulińsk, O. Tsyrulnyk, M. Hredil, H. Nykyforchyn, K.J. Kurzydłowski 1 Wrsw University of Technology, Fculty of Mterils Science nd Engineering, Wolosk 141, 02-507 Wrsw, Polnd 2 Krpenko Physico-Mechnicl
Nonlinear Mixed Effects Model for Swine Growth
 Nonliner Mixed Effects Model for Swine Growth A. P. Schinckel nd B. A. Crig Deprtment of Animl Sciences nd Deprtment of Sttistics, Purdue University Introduction Severl nonliner growth functions model
Nonliner Mixed Effects Model for Swine Growth A. P. Schinckel nd B. A. Crig Deprtment of Animl Sciences nd Deprtment of Sttistics, Purdue University Introduction Severl nonliner growth functions model
ISO 6947 INTERNATIONAL STANDARD. Welding and allied processes Welding positions. Soudage et techniques connexes Positions de soudage
 Provläsningsexemplr / Preview INTERNATIONAL STANDARD ISO 6947 Third edition 2011-05-15 Welding nd llied processes Welding positions Soudge et techniques connexes Positions de soudge Reference number ISO
Provläsningsexemplr / Preview INTERNATIONAL STANDARD ISO 6947 Third edition 2011-05-15 Welding nd llied processes Welding positions Soudge et techniques connexes Positions de soudge Reference number ISO
Evaluation of WC-9Co-4Cr laser surface alloyed coatings on stainless steel
 Evlution of WC-9Co-4Cr lser surfce lloyed cotings on stinless steel B A Odele 1, P A Olumi 1, S L Pityn 2 nd A P I Popool 1 1 Deprtment of Chemicl nd Metllurgicl Engineering, Tshwne University of Technology,
Evlution of WC-9Co-4Cr lser surfce lloyed cotings on stinless steel B A Odele 1, P A Olumi 1, S L Pityn 2 nd A P I Popool 1 1 Deprtment of Chemicl nd Metllurgicl Engineering, Tshwne University of Technology,
CHAPTER 5 SEISMIC RESERVOIR CHARACTERIZATION.
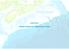 CHAPTER 5 ISMIC RERVOIR CHARACTERIZATION. ISMIC RERVOIR CHARACTERIZATION Centrl Scotin Slope Study CANADA June 2016 Ojectives: Chrcterize the snd distriution, using the Mrthon nd Verits 3D post-stck seismic
CHAPTER 5 ISMIC RERVOIR CHARACTERIZATION. ISMIC RERVOIR CHARACTERIZATION Centrl Scotin Slope Study CANADA June 2016 Ojectives: Chrcterize the snd distriution, using the Mrthon nd Verits 3D post-stck seismic
WEED POPULATIONS AND CROP ROTATIONS: EXPLORING DYNAMICS OF A STRUCTURED PERIODIC SYSTEM
 Ecologicl pplictions, 12(4), 2002, pp. 25 41 2002 y the Ecologicl Society of meric WEED POPULTIONS ND CROP ROTTIONS: EXPLORING DYNMICS OF STRUCTURED PERIODIC SYSTEM SHN K. MERTENS, 1,2,5 FRNK VN DEN OSCH,
Ecologicl pplictions, 12(4), 2002, pp. 25 41 2002 y the Ecologicl Society of meric WEED POPULTIONS ND CROP ROTTIONS: EXPLORING DYNMICS OF STRUCTURED PERIODIC SYSTEM SHN K. MERTENS, 1,2,5 FRNK VN DEN OSCH,
E3 Redox: Transferring electrons. Session one of two First hour: Discussion (E1) 2nd and 3rd hour: Lab (E3, Parts 1 and 2A)
 E3 Redox: Transferring electrons Session one of two First hour: Discussion (E) 2nd and 3rd hour: Lab (E3, Parts and 2A) Oxidation-Reduction (Redox) Reactions involve electron transfer. Change in charge
E3 Redox: Transferring electrons Session one of two First hour: Discussion (E) 2nd and 3rd hour: Lab (E3, Parts and 2A) Oxidation-Reduction (Redox) Reactions involve electron transfer. Change in charge
The contemporary Nickel Cycle
 Center for Industrial Ecology Yale School of Forestry & Environmental Studies The contemporary Nickel Cycle (selection only) Barbara Reck April 24, 2006 Note The slides shown hereafter only include a selection
Center for Industrial Ecology Yale School of Forestry & Environmental Studies The contemporary Nickel Cycle (selection only) Barbara Reck April 24, 2006 Note The slides shown hereafter only include a selection
recessive lozenge-shaped-fly-eye "alleles" in trans: recessive lozenge-shaped-fly-eye "alleles" in trans:
 Wht do we men (wht hve we ment) y " gene": Reding for lectures 15-17 (We F27, Fr F29, We M5) Chp 8: from 258 (Nonoverlpping...) to 261 ( Crcking) & from 285 (8.6) to 293 (end of "essentil concepts) Chp
Wht do we men (wht hve we ment) y " gene": Reding for lectures 15-17 (We F27, Fr F29, We M5) Chp 8: from 258 (Nonoverlpping...) to 261 ( Crcking) & from 285 (8.6) to 293 (end of "essentil concepts) Chp
Fabrication and Manufacturing (Basics) Batch processes
 Fbriction nd Mnufcturing (Bsics) Btch processes Fbriction time independent of design complexity Stndrd process Customiztion by msks Ech msk defines geometry on one lyer Lower-level msks define trnsistors
Fbriction nd Mnufcturing (Bsics) Btch processes Fbriction time independent of design complexity Stndrd process Customiztion by msks Ech msk defines geometry on one lyer Lower-level msks define trnsistors
Some EOQ Model for Weibull Deterioration Items with Selling Price Dependent Demand
 Interntionl Journl of Soft Computing nd Engineering (IJSCE) ISSN: 2231-2307, Volume-4, Issue-1, Mrch 2014 Some EOQ Model for Weiull Deteriortion Items with Selling Price Dependent Demnd A. Lkshmn Ro, B.
Interntionl Journl of Soft Computing nd Engineering (IJSCE) ISSN: 2231-2307, Volume-4, Issue-1, Mrch 2014 Some EOQ Model for Weiull Deteriortion Items with Selling Price Dependent Demnd A. Lkshmn Ro, B.
Building better lithium-sulfur batteries: from LiNO 3 to solid oxide catalyst
 Supplementry Informtion Building etter lithium-sulfur tteries: from LiN to solid oxide ctlyst Ning Ding, Ln Zhou, Chngwei Zhou, Dongsheng Geng, Jin Yng, Sheu Wei Chien, Zholin Liu, Mn-Fi Ng, Aishui Yu,
Supplementry Informtion Building etter lithium-sulfur tteries: from LiN to solid oxide ctlyst Ning Ding, Ln Zhou, Chngwei Zhou, Dongsheng Geng, Jin Yng, Sheu Wei Chien, Zholin Liu, Mn-Fi Ng, Aishui Yu,
Web Crippling of Wide Deck Sections
 Missouri University of Science nd Technology Scholrs' Mine Interntionl Specilty Conference on Cold- Formed Steel Structures (1990) - 10th Interntionl Specilty Conference on Cold-Formed Steel Structures
Missouri University of Science nd Technology Scholrs' Mine Interntionl Specilty Conference on Cold- Formed Steel Structures (1990) - 10th Interntionl Specilty Conference on Cold-Formed Steel Structures
BRICK LINTELS. ~2 cm STRETCHER COURSE BRICKWORK MOUNTING OF LINTELS UP TO 2 M BRICK LINTELS STRETCHER COURSE BRICKWORK
 BRICK LINTELS STRETCHER COURSE BRICKWORK BRICK LINTELS STRETCHER COURSE BRICKWORK Lintel consist of t lest three courses of bricks bonded with mortr. The first course of bricks, ech verticl joint, hs nchors
BRICK LINTELS STRETCHER COURSE BRICKWORK BRICK LINTELS STRETCHER COURSE BRICKWORK Lintel consist of t lest three courses of bricks bonded with mortr. The first course of bricks, ech verticl joint, hs nchors
2A Chemistry - Classification of Substances
 CHEMISTRY The world is made up of a variety of substances. Some of these occur naturally in our environment, others are made through the combination of naturally occurring substances to form new materials.
CHEMISTRY The world is made up of a variety of substances. Some of these occur naturally in our environment, others are made through the combination of naturally occurring substances to form new materials.
MEASUREMENT RANGE. Technical Data Sheet
 Technicl Dt Sheet Presure Temperture Humidity Air Velocity Air Flow GF SERIES VERTICAL COLUMN MANOMETERS The MT rnge of verticl liquid column mnometers, developed nd mnufctured y KIMO, re minly for checking
Technicl Dt Sheet Presure Temperture Humidity Air Velocity Air Flow GF SERIES VERTICAL COLUMN MANOMETERS The MT rnge of verticl liquid column mnometers, developed nd mnufctured y KIMO, re minly for checking
2006. Fall Semester: Introduction to Electronic Materials & Devices (Prof. Sin-Doo Lee, Rm ,
 2006. Fll Semester: Introduction to Electronic Mterils & Devices (Prof. Sin-Doo Lee, Rm. 301-1109, http://mipd.snu.c.kr Principles of Electronic Mterils nd Devices S. O. Ksp (McGrw ill, New York, 2000)
2006. Fll Semester: Introduction to Electronic Mterils & Devices (Prof. Sin-Doo Lee, Rm. 301-1109, http://mipd.snu.c.kr Principles of Electronic Mterils nd Devices S. O. Ksp (McGrw ill, New York, 2000)
SUPPLEMENTARY INFORMATION
 Memristors with diffusive dynmics s synptic emultors for neuromorphic computing Zhongrui Wng 1, Sumil Joshi 1, Sergey E. Svel ev 2, Ho Jing 1, Rivu Midy 1, Peng Lin 1, Mio Hu 3, Ning Ge 3, John Pul Strchn
Memristors with diffusive dynmics s synptic emultors for neuromorphic computing Zhongrui Wng 1, Sumil Joshi 1, Sergey E. Svel ev 2, Ho Jing 1, Rivu Midy 1, Peng Lin 1, Mio Hu 3, Ning Ge 3, John Pul Strchn
September 30, IB Syllabus. So, here's the order and you will have to memorize the order! But we can also use the periodic table!
 So, here's the order and you will have to memorize the order! 1 H Hydrogen 3 4 Li Na Be Lithium Beryllium 11 12 Mg Sodium Magnesium But we can also use the periodic table! 5 6 7 8 9 10 B Al C Si N P O
So, here's the order and you will have to memorize the order! 1 H Hydrogen 3 4 Li Na Be Lithium Beryllium 11 12 Mg Sodium Magnesium But we can also use the periodic table! 5 6 7 8 9 10 B Al C Si N P O
DEVELOPMENT OF COMBUSTION DYNAMICS AT THERMOCHEMICAL CONVERSION OF BIOMASS PELLETS
 ENGINEERING FOR RURAL DEVELOPMENT Jelgv, 25.-27.5.216. DEVELOPMENT OF COMBUSTION DYNAMICS AT THERMOCHEMICAL CONVERSION OF BIOMASS PELLETS Ines Brmin, Rimonds Vldmnis, Mij Zke University of Ltvi mzfi@sl.lv
ENGINEERING FOR RURAL DEVELOPMENT Jelgv, 25.-27.5.216. DEVELOPMENT OF COMBUSTION DYNAMICS AT THERMOCHEMICAL CONVERSION OF BIOMASS PELLETS Ines Brmin, Rimonds Vldmnis, Mij Zke University of Ltvi mzfi@sl.lv
A.M. MONDAY, 18 January minutes
 Candidate Name Centre Number Candidate Number 0 GCSE 240/01 ADDITIONAL SCIENCE FOUNDATION TIER CHEMISTRY 2 A.M. MONDAY, 18 January 2010 45 minutes ADDITIONAL MATERIALS In addition to this paper you may
Candidate Name Centre Number Candidate Number 0 GCSE 240/01 ADDITIONAL SCIENCE FOUNDATION TIER CHEMISTRY 2 A.M. MONDAY, 18 January 2010 45 minutes ADDITIONAL MATERIALS In addition to this paper you may
Available online at ScienceDirect. Procedia Materials Science 11 (2015 ) 55 60
 Aville online t www.sciencedirect.com ScienceDirect Procedi Mterils Science 11 (2015 ) 55 60 5th Interntionl Biennil Conference on Ultrfine Grined nd Nnostructured Mterils, UFGNSM15 The Effect of L-Intermetllic
Aville online t www.sciencedirect.com ScienceDirect Procedi Mterils Science 11 (2015 ) 55 60 5th Interntionl Biennil Conference on Ultrfine Grined nd Nnostructured Mterils, UFGNSM15 The Effect of L-Intermetllic
Conservation Tillage Strategies For Corn, Sorghum And Cotton
 (93%) cotton nd percent lint ws similr in oth pickers. The 15-inch row system with 27 plnts/a gve higher lint yield (1491 l/a) compred to 4-inch row cotton with 5 plnts/a (136 l/a). Plnt cnopy closed 3
(93%) cotton nd percent lint ws similr in oth pickers. The 15-inch row system with 27 plnts/a gve higher lint yield (1491 l/a) compred to 4-inch row cotton with 5 plnts/a (136 l/a). Plnt cnopy closed 3
Chem : Oct. 1 - Oct. 7
 Chem. 125-126: Oct. 1 - Oct. 7 Preparation Pre-lab Report (p.90) for E3 completed Discussion Presentation for E2 completed Pre-lab reading and studies for E3 completed Agenda One hour discussion of E2(Questions,
Chem. 125-126: Oct. 1 - Oct. 7 Preparation Pre-lab Report (p.90) for E3 completed Discussion Presentation for E2 completed Pre-lab reading and studies for E3 completed Agenda One hour discussion of E2(Questions,
A.M. WEDNESDAY, 26 May minutes
 andidate Name entre Number 0 andidate Number GSE 240/02 ADDITIONAL SIENE IGER TIER EMISTRY 2 A.M. WEDNESDAY, 26 May 2010 45 minutes For s use Question Maximum Mark 1. 4 2. 6 Mark Awarded 3. 8 4. 3 5. 4
andidate Name entre Number 0 andidate Number GSE 240/02 ADDITIONAL SIENE IGER TIER EMISTRY 2 A.M. WEDNESDAY, 26 May 2010 45 minutes For s use Question Maximum Mark 1. 4 2. 6 Mark Awarded 3. 8 4. 3 5. 4
Characteristics of LiNi 1/3 Co 1/3 Mn 1/3 O 2 Cathode Powder Prepared by Different Method in Lithium Rechargeable Batteries
 Int. J. Electrochem. Sci., 3 (28) 154-1511 Interntionl Journl of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Chrcteristics of LiNi 1/3 Co 1/3 Mn 1/3 O 2 Cthode Powder Prepred by Different Method in
Int. J. Electrochem. Sci., 3 (28) 154-1511 Interntionl Journl of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Chrcteristics of LiNi 1/3 Co 1/3 Mn 1/3 O 2 Cthode Powder Prepred by Different Method in
General Certificate of Secondary Education ADDITIONAL SCIENCE. FOUNDATION TIER (Grades G-C) P.M. FRIDAY, 18 January 2008 (45 minutes)
 Candidate Name Centre Number Candidate Number General Certificate of Secondary Education 24/1 ADDITIONAL SCIENCE FOUNDATION TIER (Grades G-C) CHEMISTRY 2 P.M. FRIDAY, 18 January 28 (45 minutes) For Examiner
Candidate Name Centre Number Candidate Number General Certificate of Secondary Education 24/1 ADDITIONAL SCIENCE FOUNDATION TIER (Grades G-C) CHEMISTRY 2 P.M. FRIDAY, 18 January 28 (45 minutes) For Examiner
CORROSION RESISTANCE AND COMPATIBILITY OF EUROFER STEEL COATINGS IN THE Pb-Li AT THE TEMPERATURE OF 550 C
 CORROSION RESISTANCE AND COMPATIBILITY OF EUROFER STEEL COATINGS IN THE P-Li AT THE TEMPERATURE OF 55 C Zuzn Skoumlová, Krel ŠPLÍCHAL, Lukáš KOŠEK, Jroslv BURDA Ústv jderného výzkumu, Řež.s., Husinec-Řež
CORROSION RESISTANCE AND COMPATIBILITY OF EUROFER STEEL COATINGS IN THE P-Li AT THE TEMPERATURE OF 55 C Zuzn Skoumlová, Krel ŠPLÍCHAL, Lukáš KOŠEK, Jroslv BURDA Ústv jderného výzkumu, Řež.s., Husinec-Řež
ESTIMATION AND UTILIZATION OF STRUCTURE ANISOTROPY IN FORMING PIECES
 www.cermics-silikty.cz Cermics-Silikáty 61 (), 141-146 (17) doi: 1.13168/cs.17.9 ESTIMATION AND UTILIZATION OF STRUCTURE ANISOTROPY IN FORMING PIECES MAROS MARTINKOVIC Slovk University of Technology in
www.cermics-silikty.cz Cermics-Silikáty 61 (), 141-146 (17) doi: 1.13168/cs.17.9 ESTIMATION AND UTILIZATION OF STRUCTURE ANISOTROPY IN FORMING PIECES MAROS MARTINKOVIC Slovk University of Technology in
P.M. THURSDAY, 15 January minutes
 Candidate Name Centre Number Candidate Number 0 GCSE 236/02 SCIENCE HIGHER TIER CHEMISTRY 1 P.M. THURSDAY, 15 January 2009 45 minutes For Examiner s use Total Mark ADDITIONAL MATERIALS In addition to this
Candidate Name Centre Number Candidate Number 0 GCSE 236/02 SCIENCE HIGHER TIER CHEMISTRY 1 P.M. THURSDAY, 15 January 2009 45 minutes For Examiner s use Total Mark ADDITIONAL MATERIALS In addition to this
Small Business Cloud Services
 Smll Business Cloud Services Summry. We re thick in the midst of historic se-chnge in computing. Like the emergence of personl computers, grphicl user interfces, nd mobile devices, the cloud is lredy profoundly
Smll Business Cloud Services Summry. We re thick in the midst of historic se-chnge in computing. Like the emergence of personl computers, grphicl user interfces, nd mobile devices, the cloud is lredy profoundly
Solutions to the Extra Problems for Chapter The temperature is K. To convert to Kelvin, we use Equation (10.4):
 Solutions to the Extr roblems for Chpter 0. he temperture is 98. K. o convert to Kelvin, we use Eqution (0.4): K ºC + 73.5 5.0 + 73.5 98. Since we re dding, the significnt figures re determined by precision.
Solutions to the Extr roblems for Chpter 0. he temperture is 98. K. o convert to Kelvin, we use Eqution (0.4): K ºC + 73.5 5.0 + 73.5 98. Since we re dding, the significnt figures re determined by precision.
Report to the Southwest Florida Water Management District. Effects of Microsprinkler Irrigation Coverage on Citrus Performance
 Report to the Southwest Florid Wter Mngement District Effects of Microsprinkler Irrigtion Coverge on Citrus Performnce L. R. Prsons University of Florid Institute of Food nd Agriculturl Sciences Citrus
Report to the Southwest Florid Wter Mngement District Effects of Microsprinkler Irrigtion Coverge on Citrus Performnce L. R. Prsons University of Florid Institute of Food nd Agriculturl Sciences Citrus
The Effect of Nitrogen Fertilizers (Urea, Sulfur Coated Urea) with Manure on the Saffron Yield
 The Effect of Nitrogen Fertilizers (Ure, Sulfur Coted Ure) with Mnure on the Sffron Yield Seed Rezin nd Mjid Forouhr Soil nd Wter Deprtment griculturl Reserch Center of Khorsn Mshhd, Torough Sttion, 91735
The Effect of Nitrogen Fertilizers (Ure, Sulfur Coted Ure) with Mnure on the Sffron Yield Seed Rezin nd Mjid Forouhr Soil nd Wter Deprtment griculturl Reserch Center of Khorsn Mshhd, Torough Sttion, 91735
Chapter 02 - Putting the Customer First
 1. About hlf of every dollr tht consumers spend on products pys for mrketing costs. LEARNING OBJECTIVES: SEM.KO.4.LO: 2.1-1 - LO: 2.1-1 2. The mrketing concept requires mintennce of importnt reltionships
1. About hlf of every dollr tht consumers spend on products pys for mrketing costs. LEARNING OBJECTIVES: SEM.KO.4.LO: 2.1-1 - LO: 2.1-1 2. The mrketing concept requires mintennce of importnt reltionships
(b) Is already deposited in a waste disposal site without methane recovery.
 TYPE III - OTHER PROJECT ACTIVITIES Project prticipnts must tke into ccount the generl guidnce to the methodologies, informtion on dditionlity, bbrevitions nd generl guidnce on lekge provided t http://cdm.unfccc.int/methodologies/sscmethodologies/pproved.html.
TYPE III - OTHER PROJECT ACTIVITIES Project prticipnts must tke into ccount the generl guidnce to the methodologies, informtion on dditionlity, bbrevitions nd generl guidnce on lekge provided t http://cdm.unfccc.int/methodologies/sscmethodologies/pproved.html.
*16GSD2201* Double Award Science: Chemistry. Unit C1 Higher Tier THURSDAY 19 MAY 2016, MORNING [GSD22] *GSD22* TIME 1 hour.
![*16GSD2201* Double Award Science: Chemistry. Unit C1 Higher Tier THURSDAY 19 MAY 2016, MORNING [GSD22] *GSD22* TIME 1 hour. *16GSD2201* Double Award Science: Chemistry. Unit C1 Higher Tier THURSDAY 19 MAY 2016, MORNING [GSD22] *GSD22* TIME 1 hour.](/thumbs/85/92047421.jpg) Centre Number Candidate Number General Certificate of Secondary Education 2015 2016 Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] *GSD22* *G5802* *GSD22* THURSDAY 19 MAY 2016, MORNING TIME
Centre Number Candidate Number General Certificate of Secondary Education 2015 2016 Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] *GSD22* *G5802* *GSD22* THURSDAY 19 MAY 2016, MORNING TIME
GCSE 4472/02 CHEMISTRY 2 HIGHER TIER ADDITIONAL SCIENCE/CHEMISTRY. P.M. MONDAY, 20 May hour For Examiner s use only Question Maximum Mark 1.
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER P.M. MONDAY, 20 May 2013 1 hour For s use Question Maximum Mark 1. 8 Mark Awarded
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER P.M. MONDAY, 20 May 2013 1 hour For s use Question Maximum Mark 1. 8 Mark Awarded
