ABSTRACT. Since carbon dioxide from petroleum-derived fuels has become an environmental
|
|
|
- Gwenda Lloyd
- 6 years ago
- Views:
Transcription
1 ABSTRACT KIM, SANGWOOK. Stresses at Electrode-Electrolyte Interface in Lithium-ion Batteries via Multiphysics Modeling (Under the direction of Dr. Hsiao-Ying Shadow Huang.) Since carbon dioxide from petroleum-derived fuels has become an environmental issue, the need for alternative energy sources increases dramatically. One of the most promising energy solutions is the use of lithium-ion batteries as an energy storage system. They exhibit a few favorable characteristics, such as their low cost, high thermal stability, high electrochemical performance, and high specific capacity. However, lithium-ion batteries show capacity loss and impedance rise on the surface of cathode particles during charging and discharging in several conditions. It has been suggested that high current-rates (C-rates) during discharging play an important role in the mechanical and structural deterioration of lithium-insertion materials, thereby progressively reducing the capacity of lithium-ion batteries with each charge and discharge cycle (referred to as rate-capacity fade ). Yet our basic knowledge of how electrode material mechanical stress states contribute to the battery performance and stability is currently extremely limited, hindering materials innovation and the ability to use lithium-ion batteries as energy storage systems. The main objective of this research is to investigate the mechanical stresses on the interface between the cathode and the electrolyte (i.e., a half-cell system). LiFePO4 is used as a cathode material and the combination of several different kinds of electrolyte is considered. Multiphysics finite element models incorporating a Fluid Flow module (CFX), a Transient Thermal module, a Static Structural module, and additional Application Customization Toolkits (ACTs) in ANSYS are developed to study mechanical stresses in the half-cell battery system during discharging.
2 Our results provide a better understanding of mechanical stresses on the interface between the electrode and the electrolyte in lithium-ion batteries in several conditions. We explore (i) the impact of the porosity of electrode-electrolyte interface, (ii) the impact of porous electrodes in a lithium-ion half-cell, and (iii) the impact of phase transformation in porous electrodes. Specifically, we study effects of C-rate, volume fraction (porosity), viscosity, and lithiation stage during a half-cell system discharging. Moreover, our simulations demonstrate that both electrode and electrolyte material properties have greater effects when investigating mechanical stresses on the electrode-electrolyte interface. These computational models would aid on mitigating higher stresses in cathode particles to ensure longer battery cycle life.
3 Copyright 2015 by Sangwook Kim All Rights Reserved
4 Stresses at Electrode-Electrolyte Interface in Lithium-ion Batteries via Multiphysics Modeling by Sangwook Kim A thesis submitted to the Graduate Faculty of North Carolina State University in partial fulfillment of the requirements for the degree of Master of Science Mechanical Engineering Raleigh, North Carolina 2015 APPROVED BY: Dr. Hsiao-Ying Shadow Huang Committee Chair Dr. Brendan T. O Conner Dr. Alexei V. Saveliev
5 DEDICATION To my parents, Soon-tae and Il-ran Kim, and My sister, Yeaeun Kim Nothing would be possible without your support. Thank you for always encouraging me. ii
6 BIOGRAPHY Sangwook Kim was born in Busan, South Korea, on January 3rd, He matriculated at Pusan National University (Busan, Korea) in He served two years from 2010 at Korea Army. After that, he received his Bachelor of Science degree in Mechanical Engineering from PNU in He decided to study abroad to expand his academic and develop global leadership. In 2014, he enrolled in graduate school at North Carolina State University (Raleigh, NC) for pursuing his Master of Science degree in Mechanical Engineering. His main research field is stress analysis in lithium-ion batteries using multiphysics modeling. iii
7 ACKNOWLEDGEMENTS First of all, I would like to thank North Carolina State University and Department of Mechanical & Aerospace Engineering for giving me this opportunity to pursue Master of Science degree in the U.S. Secondly, I would like to thank my advisor, Dr. Hsiao-Ying Shadow Huang for continued guidance and encouragement. I also would like to thank my committee members, Dr. Brendan T. O Conner and Dr. Alexei V. Saveliev for being willing to serve in my committee and providing me invaluable advice. Finally, I would like to thank my family for their support during my life in the U.S. iv
8 TABLE OF CONTENTS LIST OF TABLES... vii LIST OF FIGURES... viii CHAPTER 1: INTRODUCTION Lithium-Ion Battery Promising LiFePO4 Electrode Research at National Laboratory Sandia National Laboratory Brookhaven National Laboratory Current State-of-Art and Limitation Objectives of this Study...12 CHAPTER 2: LITERATURE REVIEW LiFePO4 as a Cathode material Electrolyte Solid-Electrolyte Interphase (SEI) Phase Transformation across Electrode Diffusion-Induced Stress...37 CHAPTER 3: MODEL DEVELOPMENT The Impact of the Porosity of Electrode-Electrolyte Interface The Impact of Porous Electrodes in a Lithium Ion Half- Cell The Impact of Phase Transformation in Porous Electrodes...54 CHAPTER 4: SIMULATION RESULTS AND DISCUSSION Stress Analysis of the Porosity of Electrode-Electrolyte Interface Effect of C-rate Effect of Porosity Effect of Viscosity Effect of Lithiation Conclusion Multiphysics Models for Stress Analysis of Porous Electrodes...68 v
9 4.2.1 Effect of C-rate Effect of Volume Fraction Effect of Viscosity Conclusion Phase Transformation Effect in Porous Electrodes via Multiphysics Modeling Lithiation Effect of C-rate Effect of Volume Fraction Conclusion CHAPTER 5: SUMMARY AND CONCLUSIONS FUTURE WORK...96 REFERENCES...97 APPENDIX A1: Electrode Material Property Change during Discharging A2: Material Property of Electrolyte A3: Supplementary Information on Model A3: Supplementary Information on Model A4: Supplementary Information on Model vi
10 LIST OF TABLES Table 1: Thermal stability characteristics and other factors in cost and environmental issues for battery materials [1] Table 2: Lithium ion diffusivity and electronic conductivity of cathode materials [15] Table 3: Summary of simulations to present Table 4: A candidate of organic electrolyte for lithium-ion batteries Table 5: Material properties for cathode and electrolyte vii
11 LIST OF FIGURES Chapter 1 Figure 1: Comparison of the different battery technologies in terms of gravimetric(x-axis), and volumetric(y-axis) energy density [2] Figure 2: Working mechanism of lithium-ion batteries during discharging [3] Figure 3: Comparison of various cathode materials; LiCoO2, LiMn2O4, LiNiO2, and LiFePO4 [1] Figure 4: SOC mapping obtained via scanning transmission X-ray microscopy (a) 26µm, (b) 18µm, and (c) 6µm from the current collector [6] Figure 5: (a) The number of particles in terms of particle size, and (b) the fraction of delithiated particles plotted as a function of percentile of particle size (lithium-rich particles are in color red and lithium-poor particles are in color green) [6] Figure 6: Schematic illustration for multi-particle LiFePO4 system [7] Chapter 2 Figure 7: Comparison of the discharge capacity of various LiFePO4 electrodes at 25 C [17] Figure 8: Simulation snapshots of 50% SOC electrodes discharged at different C-rates [20] Figure 9: The voltage as a function of the capacity at (a) the first cycle, and (b) the second cycle. The phase fraction of FePO4 during charging as a function of the cell position (y) at (c) the first cycle, and (d) the second cycle in C/7 [18] Figure 10: Discharge capacity versus cycle number for various LiFePO4 electrodes in 0.2 C- rate [18] Figure 11: Normalized 7 Li MAS NMR spectra of (a) carbon coated LiFePO4, and (b) pure LiFePO4 at 25 ºC [19] Figure 12: The SEM images of LiFePO4 carbon coated electrode (a) before cycling, (b) after 10 cycles, (c) after 30 cycles, and (d) after 60 cycles [23] Figure 13: (a) Average SOC (γ) from current collector to separator, and (b) intercalation stresses at the surface of the particles across the electrode during discharge [24] Figure 14: (a) Fe-ion dissolution from LiFePO4 olivine powders in different electrolytes at 30 ºC, and (b) unstable behavior of a LiFePO4 olivine electrode in acidic electrolyte [29] Figure 15: (a) Simulated porous electrode microstructure, and (b) resultant stream lines highlight the tortuous path [33] Figure 16: Average lithium content gradient from cathode to anode at cell depths of discharge (DODs, i.e., % overall lithiated = 50%) as a function of (a) porosity, and (b) C-rate for the three different particle-size distributions [21] viii
12 Figure 17: General reactions at an electrode, an electrode surface region, and bulk solution [34] Figure 18: The Nyquist plots of carbon coated LiFePO4 after 5 charge discharge cycles. (a) 100%, (b) 50%, and (c) 0% SOC at 303 K [39] Figure 19: SEM images of (a) pristine cathodes, (b) pristine anodes, (c) cathodes after 15-day storage, and (d) anodes after 15-day storage [38] Figure 20: (a) XRD spectra in terms of SOC from starting material to 95% SOC, and (b) unit cell volumes and the normalized crystalline phase fraction of triphylite and heterosite [39]. 33 Figure 21: Illustration of the shrinking-core model with the juxtaposition of the two phases and the movement of the phase boundary [11] Figure 22. (a) Contour plots for lithium concentration according to the lithium intercalation, and (b) anisotropic misfit between FePO4 and LiFePO4 along 3 directions according to onedimensional lithium diffusion along [010] [42] Figure 23: (a) Dimensionless concentration profile, (b) radial stress, and (c) tangential stress in terms of location in the spherical particle [45] Figure 24: Diffusion-induced stress fields in a particle using (a) the isotropic model, and (b) the anisotropic model [46] Figure 25: Effect of (a) porosity of anode, and (b) partcile size on the total stress along the thickness of the anode [24] Figure 26: Maximum and minimum stress in terms of C-rate (dashed: 5.5 µm, dotted: 55 µm, and solid: 165 µm electrodes) [47] Chapter 3 Figure 27: (a) Geometry, and (b) mesh of the model 1 composed of electrolyte and cathode domains Figure 28: Project schematic simulation in ANSYS including Fluid Flow (CFX), Transient Thermal, and Static Structural modules Figure 29: (a) Geometry, and (b) mesh of the model 2 composed of electrolyte and cathode domains Figure 30: Project schematic simulation in ANSYS including Fluid Flow (CFX), and Static Structural modules Figure 31: (a) Geometry, and (b) mesh of the model 3 composed of electrolyte and cathode domains Figure 32: Project schematic including Fluid Flow, Static Structural modules, and Application Customization Toolkit (ACT) ix
13 Chapter 4 Figure 33: (a) Velocity vector of electrolyte across electrolyte and electrode domains, and (b) velocity profiles at 3 different locations in the half-cell system (red: inlet boundary, blue: middle of the electrolyte domain, and green: middle of the electrode) Figure 34: Influences of varying C-rate on the normalized normal stress and shear stress (porosity of 0.25 and EC is used as an electrolyte) Figure 35: Relationship between porosity and normalized normal stress (EC is used as an electrolyte) Figure 36: The effect of electrolyte viscosity on normal and shear stresses at 1C (porosity of 0.25) Figure 37: Concentration profile calculated by the transient thermal module in ANSYS. Each model represents lithiation of (a) 20%, and (b) 80% Figure 38: Local concentration in terms of diffusion distance. Each concentration distribution shows lithiation of 20% (a) and 80% (b) Figure 39: Relationship between stresses and lithiation stage at 1C (porosity of 0.25 and EC is used as an electrolyte) Figure 40: Molar volume misfits when lithium-poor phase and lithium-rich phase exist Figure 41: Contour plots of stress and locations of position 1, 2, and 3 in (a) electrode domain and (b) a zoom-in top view of the electrode domain. Snapshots are taking from the 1C model with the volume fraction of 15.5% Figure 42: Influences of C-rate on normalized normal and shear stresses at position 1 (volume fraction of 15.5% and EC is used as an electrolyte) Figure 43: Streamline of model 2 in 1C with the volume fraction of (a) 6.5%, (b) 15.5%, and (c) 30% Figure 44: Contour plots of normal stress (y-axis) in 1C with volume fraction of (a) 6.5%, (b) 15.5%, and (c) 30% at the lithiation of 10% Figure 45: Effect of volume fraction on normalized normal stress in 1C at the lithiation of 10% (EC is used as an electrolyte) Figure 46: Effect of the viscosity of electrolyte on normalized normal stress at specific location in the 1C model at the lithiation of 10% (volume fraction of 15.5%) Figure 47: Concentration distribution at the lithiation of (a) 0%, (b) 15.8%, and (c) 83% in cathode in a 1C model (volume fraction of 15.5%) Figure 48: (a) Contour plot of lithiation at 50% lithiation in 1C (volume fraction of 15.5%), and (b) a 10-nodes tetrahedral type element Figure 49: Contour plot at lithiation stage of (a) 0%, (b) 5%, (c) 10%, (d) 20%, (e) 30%, (f) 50%, (g) 75%, and (h) 100% for 1C model with volume fraction of 15.5% Figure 50: Contour plots of normal stress for the model with volume fraction of 15.5% at 50% lithiation in (a) 1C, and (b) 6C x
14 Figure 51: Effect of C-rate on normal stress at the position 1 during lithiation (volume fraction of 15.5% and EC is used as an electrolyte) Figure 52: Relationship between the surface concentration and the dimensionless discharging time for the models with volume fraction of 15.5% and EC is used as an electrolyte Figure 53: Effect of C-rate on the normalized strain energy during lithiation (volume fraction of 15.5% and EC is used as an electrolyte) Figure 54: Contour plots of normal stress for the model with volume fraction of (a) 3.3%, and (b) 26.8% particle at 50% lithiation in 1C (EC is used as an electrolyte) Figure 55: Effect of volume fraction on normal stress in (a) 1C, (b) 6C, and (c) combined results (EC is used as an electrolyte) Figure 56: Effect of volume fraction on normalized strain energy (a) 1C, and (b) 6C (EC is used as an electrolyte) xi
15 CHAPTER 1: INTRODUCTION 1.1 Lithium-Ion Battery Since carbon dioxide from petroleum-derived fuels has become an environmental issue, the need for alternative energy sources and the replacement of the internal combustion engine increases. The use of lithium-ion batteries is one of the most promising power source. Lithiumion batteries have been used for a wide range of applications such as portable electronics, power tools, and transportation [1]. Especially, the lithium-ion batteries show good potential for the application on hybrid electric vehicles (HEVs), plug-in hybrid electric vehicles (PHEVs), and electric vehicles (EVs). The main reasons why lithium-ion batteries are now the prevailing rechargeable battery system are due to their low cost, low toxicity, high thermal stability, high electrochemical performance, and high specific capacity compared to commonly used batteries such as Ni Cd and Ni MH portable batteries [2]. Gravimetric and volumetric energy densities, which are important properties for cathode materials, determine the energy release rate and energy storage capacity per unit weight or volume. Since a lighter battery with the same energy capacity is easier to carry around, the gravimetric energy density is especially important for portable devices. Especially, in case of electric vehicles, the volumetric energy density becomes a determinant factor, because with the same energy capacity, a smaller battery is easier to fit into a car. Figure 1 demonstrates that lithium-ion batteries show higher gravimetric energy density (W h kg -1 ) and volumetric energy density (W h l -1 ), which are strongly related to the ability to deliver energy in the system. 1
16 Figure 1: Comparison of the different battery technologies in terms of gravimetric(x-axis), and volumetric(y-axis) energy density [2]. 2
17 Figure 2 below portrays how lithium-ion batteries work. The lithium-ion battery cell is made up of five essential components; a positive and a negative electrode, electrolyte, a separator, and current collectors. During the charge and discharge process, lithium ions are shuttled between an anode and a cathode through electrolyte. When lithium ions reached the cathode surface, lithium ion insertion (intercalation) occurs, and it causes diffusion-induced stress (DIS). Graphite is commonly used as an anode, whereas various materials such as LiCoO2, LiMn2O4, LiNiO2, and LiFePO4 are used as a cathode. Moreover, organic-carbonate solvents such as ethylene carbonate (EC) and dimethyl carbonate (DMC) with a lithium salt dissolved are commonly used as an electrolyte. Figure 2: Working mechanism of lithium-ion batteries during discharging [3]. 3
18 1.2 Promising LiFePO4 Electrode In 1997, Padhi et al. [4] proposed LiFePO4 as a cathode material, which shows a higher capacity (170 mah/g) as high as LiCoO2. It also exhibits high volumetric energy (970 WhL -1 ), and volumetric power (1236 WL -1 ) compared to the four mainstream cathode materials as shown in Figure 3 [1]. Since LiFePO4 is made from non-toxic materials and the abundant transition metal (160 billion tons in the Earth), it is an environmental-friendly and cheaper material (Table 1). Moreover, one of the prominent problems of lithium-ion batteries is safety, attributed primarily to the material s thermal stability [5]. In 2013, the United States Federal Aviation Administration (FAA) ordered a review into Boeing 787 Dreamliner due to the battery overheating problems. The lithium-ion batteries of Boeing 787 have used LiCoO2 as a cathode material. Table 1 shows that LiFePO4 exhibits the lowest exothermic peak temperature (289 o C) and heat flow (-6 Wg -1 ). Exothermic peak temperature can be used as a description of selfreaction temperature. The lower the peak temperature, the safer a material is. Negative value of heat flow means electrochemical reaction of electrode is endothermic. Therefore, LiFePO4 shows the higher thermal stability during charge and discharge process. 4
19 Figure 3: Comparison of various cathode materials; LiCoO 2, LiMn 2O 4, LiNiO 2, and LiFePO 4 [1]. Table 1: Thermal stability characteristics and other factors in cost and environmental issues for battery materials [1]. 5
20 1.3 Research at National Laboratory Interesting research on lithium-ion batteries have been conducted by several national laboratories and universities. In this section, a few studies conducted by U.S. national laboratories, such as Sandia National Laboratory and Brookhaven National Laboratory, are introduced Sandia National Laboratory For better performance in lithium-ion batteries in electric vehicles, medical equipment, and aircrafts, Sandia National Lab has been conducting research using LiFePO4 as a cathode material in lithium-ion batteries. Chueh et al. [6] studied the particle-by-particle pathway of lithium intercalation, which is the ability of a molecule to be inserted and extracted from two other molecules. In Figure 4, the lithium-poor particles are represented in color green and indicate nearly charged, while lithium-rich particles are represented in color red and indicate nearly discharged. It has been observed that the average lithium content of the particles (i.e., state-of-charge, SOC) shows nearly same across the cathode, suggesting that lithium ion transport through the electrolyte is relatively fast even under a 1 discharging current-rate (C-rate). A nc-rate requires 1/n hour to fully charge and discharge the battery. Furthermore, only a small fraction of the particles has both lithium-rich and lithium-poor regions during discharging (i.e., few particles with mixed phase marked in yellow are observed). Therefore, the time needed to completely 6
21 charge individual LiFePO4 particle is much shorter (about 50 times shorter) than the time needed to charge the entire electrode. Figure 4: SOC mapping obtained via scanning transmission X-ray microscopy (a) 26µm, (b) 18µm, and (c) 6µm from the current collector [6]. 7
22 From Figure 4, their experimental results suggest that most particles are ellipsoidal, therefore the particle size is later expressed as a length along the long axis of the ellipse. Figure 5 demonstrates the particle-size distributions of all the single-phase particles. The two vertical lines (i.e., a red and a green line) in the Figure 5a indicate the average particle size of lithiumrich particles and lithium-poor particles. It has been observed that the average particle size is 256 nm for lithium-rich LiFePO4 particles and 294 nm for lithium-poor LiFePO4 particles. Even though there is a difference of average particles size between lithium-rich and lithium-poor LiFePO4, Figure 5b displays the fraction of delithiated particles (in color green) and lithiated particles (in color red) plotted as a function of percentile of particle size. That is, lithiation state depends weakly on the particle size. It suggest that increases in particle size does not influence the fraction of delithiation. This result is contrary to the common expectation that smaller particles would delithiate before larger ones. Figure 5: (a) The number of particles in terms of particle size, and (b) the fraction of delithiated particles plotted as a function of percentile of particle size (lithium-rich particles are in color red and lithium-poor particles are in color green) [6]. 8
23 1.3.2 Brookhaven National Laboratory At the Brookhaven National Laboratory, Wang et al. [7] have used X-ray microscopy to track the phase transformation process at nano-scale resolution during charging and discharging. They focused on the reasons why charging under high C-rate constrains LiFePO4 battery performance. Figure 6 compares phase transformation mechanism and intercalation pathway at fast and slow charging rates for a multi-particle LiFePO4 system. It suggests that in a multiple LiFePO4 particle system, phase transformation occurs concurrently at slow C-rate, whereas it proceeds inhomogeneously at faster C-rate [7]. Even at the end of discharging, an incomplete phase transformation marked in red is still detected at faster C-rate (i.e., 5C). To improve the ionic and electron conductivity, they tried to relieve inhomogeneity of phase distribution by using low active material loading and liquid electrolyte. However, inhomogeneity of the phase distribution still remains unsolved in spite of their efforts. The observed inhomogeneity at high C-rates has not been solved until now, thus is regarded as prominent issue, which needs to be settled as soon as possible. 9
24 Figure 6: Schematic illustration for multi-particle LiFePO 4 system [7]. 1.4 Current State-of-Art and Limitation Currently, lithium-ion batteries have become commercially available. Due to their high gravimetric energy density, lithium-ion batteries have become the choices of the energy storage systems for laptops, cell phones, and many other portable applications. For example, Samsung SDI continues to develop lithium-ion batteries for portable electronic devices. One of latest batteries, called ICR A, has 3200mAh current capacity and 12Wh energy capacity [8]. It has the highest capacity among the cylindrical-type lithium-ion batteries and it provides increased hours of laptop usage. Moreover, lithium-ion batteries are commonly used in HEV, PHEV, and EV because of their high volumetric energy density. BMW has released a PHEV in 2015, called BMW i8. It has a 7.1 kwh pack, which suggests that BMW i8 can operate 37 km (23 miles) without gas [9]. 10
25 Since BMW i8 have an additional internal combustion engine, the battery pack can be charged during driving with gas. TESLA has developed a high profile EV, which has received a lot of attention. It is reported that the 85 kwh lithium-ion battery in model S (85D) has an ability to deliver 230 miles (370 km) [10]. Even though lithium-ion batteries have been commercially used in industry due to their many advantages, they have some obstacles and limitations. First of all, LiFePO4 has relatively low electronic conductivity and lithium ion diffusivity comparing to other commonly used cathodes such as LiMn2O4 or LiCoO2 (Table 2). Low electronic conductivity and lithium ion diffusivity of LiFePO4 results in considerable ohmic drops within the electrode [11]. Ohmic drops cause decrease in the terminal voltage during discharge and increase in voltage needed to charge. Secondly, it has been noted that the electrode displays capacity loss in several conditions [12]. Even though a fast C-rate is required to provide a better acceleration for EV, LiFePO4 limited high-rate capability, with considerable loss in utilization with a high C-rate [11]. Moreover, capacity loss is very significant with the longer period of charge and discharge cycles or storage [13]. Thirdly, an irreversible charge loss occurs on the solid-electrolyte interphase (SEI) is observed [14]. It is noted that the SEI plays negative roles on the battery performance via irreversible charge losses, and it harms the battery safety by forming an additional layer on the electrode surface. Table 2: Lithium ion diffusivity and electronic conductivity of cathode materials [15]. Lithium Ion Diffusivity [cm 2 s -1 ] Electronic Conductivity [S cm -1 ] LiCoO ~
26 LiMn2O ~ LiFePO ~ Objectives of this Study It has been observed that the capacity fade with a high C-rate or with a long period of cycling is strongly related to the mechanical stresses inside lithium-ion batteries [12]. Moreover, it has been experimentally observed that stresses due to intercalation and phase transformation can cause local fractures in the active materials of lithium-ion batteries [16]. Yet our basic knowledge of how electrode material mechanical stress states contribute to the battery performance and stability is currently extremely limited, hindering materials innovation and the ability to use lithium-ion battery as an energy storage system. The main objective of this research is to investigate the mechanical stresses on the interface between the cathode and the electrolyte (i.e., a half-cell system). Three distinct multiphysics finite element models are developed aiming to understand (i) the impact of the porosity of electrode-electrolyte interface, (ii) the impact of porous electrodes in a lithium ion half-cell, and (iii) the impact of phase transformation in porous electrodes. A Fluid Flow module (CFX), a Static Structural module, a Transient Thermal module, and additional Application Customization Toolkits (ACTs) in ANSYS are utilized and effects of C-rate, volume fraction (porosity), viscosity, and lithiation on internal stresses inside the electrode are investigated. Table 3 below provides a summary of simulations that are carried out within developed models. Parameters used in the models will discuss in Chapter 3. 12
27 Table 3: Summary of simulations to present Lithiation C-rate Volume Fraction (Particle Size) Viscosity Model 1 0% ~ 100% 1C 5C 10C 20C Pa s 749 Pa s 1900 Pa s Model 2 10% 1C 5C 10C 20C 6.5% (75nm) 15.5% (100nm) 23.6% (115nm) 30% (125nm) 585 Pa s 749 Pa s 1900 Pa s Model 3 0% ~ 100% 1C 2C 6C 8C 3.3% (60nm) 8% (80nm) 15.5% (100nm) 26.8% (120nm) 1900 Pa s 13
28 CHAPTER 2: LITERATURE REVIEW 2.1 LiFePO4 as a Cathode material LiFePO4 is considered as one of the promising materials for a cathode as discussed in Chapter 1.2, however, the capacity loss is very critical at higher C-rate. To overcome this problem, Shrinivasan and Newman [11] have suggested to reduce the capacity loss by decreasing the particle sizes and to improve the conductivity of LiFePO4 by coating the particles with a layer of carbon. With this concept, experimentalists have starting fabricating LiFePO4 with nano-scale particles and carbon coatings [17][18][19]. In Figure 7, LPK142, LPK132, and LPK140 indicate the electrode with carbon coated nano-scale particles (1.5 to 2%wt of carbon, size ranges from 50 to 250nm), carbon free nano-scale particles, and carbon free micro-scale LiFePO4 particles (size ranges from 1 to 5 μm), respectively. Guerfi et al. [17] have reported that the carbon free nano-scale cathode (i.e., LPK 132) exhibits 50% capacity improvement at 10C compared to the carbon free micro-scale cathode (i.e., LPK 140). Moreover, positive effect of carbon coating on the battery performance was confirmed by comparing LPK142 with LPK
29 Figure 7: Comparison of the discharge capacity of various LiFePO 4 electrodes at 25 C [17]. In spite of that the electrode with nano-scale particles exhibits high performance, it is observed battery degrades due to inhomogeneous electrochemical reaction across the cathode, however. Using synchrotron-based X-ray microscopy, Li et al. [20] investigated 3,000 individual particles at various C-rates and measured a fraction of actively intercalating particles in the electrode at different state of charge (SOC) (Figure 8). They have reported that more lithiated particles (marked in red) are located near the separator, and more delithiated particles (marked in green) are observed at a high C-rate. This inhomogeneous lithiation across the electrode was also observed by Strobridge et al. [21]. Moreover, Figure 8 displays that the size of delithiated particles (marked in green) is larger than that of lithiated particles (marked in red). They concluded that an inhomogeneous active population of particles in the electrode directly impacts a decreased in the cycle life and electrochemical performance of lithium-ion batteries. 15
30 Figure 8: Simulation snapshots of 50% SOC electrodes discharged at different C-rates [20]. Strobridge et al. [21] have measured a fraction of nano-scale LiFePO4 electrode to investigate inhomogeneous reaction by using X-ray diffraction (XRD). They have reported that the fraction of LiFePO4 decreases along with the diffusion direction (y-direction) at both the first and the second cycle (Figure 9b, d) because of lithium diffusion limited-kinetics in the electrolyte across the cathode. Moreover, it has been observed that the fraction of LiFePO4 decreases more drastically at the second cycle especially between 15µm and 150µm. More specifically, the gradient of lithium content in the particles, which indicates the inhomogeneous reaction causing battery degradation, is 1.6 Li fraction/mm at the first cycle 16
31 (scan #6, green line in Figure 9b) and 2.7 Li fraction/mm at the second cycle (scan #7, green line in Figure 9d). To understand the reduced battery performance due to the inhomogeneous reaction, a simulation have been performed to examine the hypothesis that the decrease in electronic conductivity at the second cycle affects the inhomogeneity across the electrode. With the same geometry and parameters in the simulations, Strobridge et al. [21] varied the electronic conductivity to observe the change of the battery performance. Their result suggests that an expansion and a contraction of particles during cycling have caused a decrease in electronic conductivity of LiFePO4, which negatively affects the battery performance. 17
32 Figure 9: The voltage as a function of the capacity at (a) the first cycle, and (b) the second cycle. The phase fraction of FePO 4 during charging as a function of the cell position (y) at (c) the first cycle, and (d) the second cycle in C/7 [18]. Several studies have suggested that carbon coating could potentially overcome the capacity loss in LiFePO4 [17][18]. For example, Cho et al. [18] have conducted experiments to examine the effect of carbon content in the cathode. They have used different carbon fraction, 1.25wt.% (Product-0), 1.67wt.% (Product-1), 2.28wt.% (Product-2), and 2.54 wt.% (Product-3), as an additional layer on the surface of cathode, respectively, and compared the battery performance. Figure 10 compares the discharge capacity of pure LiFePO4 with various LiFePO4/C samples. It has been observed that an increase in carbon content of samples shows a reduced capacity loss during cycling, which is related to the increase in electronic conductivity. However, the electrode containing too much carbon (i.e., Product-3 18
33 with 2.54 wt.% carbon) shows lower performance comparing to Product-1 (with 1.67wt.% carbon) and Product-2 (with 2.28wt.% carbon) due to the carbon thickness. They have reported that LiFePO4 coated with a thin and uniform carbon film can deliver maximum discharge capacity of 151mAhg -1 at 0.2C and sustain 415 cycles with 80% of capacity retention [18]. Higher performance of the carbon coated LiFePO4 particles are found due to the higher electron conductivity and more nucleation from more surface area, comparing to a carbon free electrode [22]. Figure 10: Discharge capacity versus cycle number for various LiFePO 4 electrodes in 0.2 C-rate [18]. 19
34 Dupré et al. [19] have utilized 7 Li magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy, electrochemical impedance spectroscopy (EIS), and X-ray photoelectron spectroscopy (XPS) to study the influence of carbon coating of LiFePO4 during storage at the room temperature. Through normalized 7 Li MAS NMR spectra, the reactions of the interphase are evaluated after aging (Figure 11). After one month of contact with electrolyte, the detected NMR signal for the carbon-free LiFePO4 has increased significantly compared to the carbon coated LiFePO4. This suggests that the reaction of the carbon-free electrode is aggravated more than that of carbon coated electrode during storage at the room temperature. Figure 11: Normalized 7 Li MAS NMR spectra of (a) carbon coated LiFePO 4, and (b) pure LiFePO 4 at 25 ºC [19]. 20
35 Another important issue regarding the battery degradation is that cracks are observed in LiFePO4 after cycling. Wang et al. [23] have investigated cracks on the LiFePO4 particles using XRD and SEM. Small flaws were observed in some particles after 10 cycles (Figure 12b). Figure 12c demonstrates that the crack becomes more obviously after 30 cycles. They believe that the cracks, which related to the performance degradation, may be caused by high internal strain energy during lithium intercalation. Thus, the importance of investigating mechanical stresses and strain energy in electrode particles during charging and discharging becomes more significant. 21
36 Figure 12: The SEM images of LiFePO 4 carbon coated electrode (a) before cycling, (b) after 10 cycles, (c) after 30 cycles, and (d) after 60 cycles [23]. Reganathan et al. [24] have published a mathematical model to develop a correlation between the mechanical properties and the performance of the porous battery electrodes. In their study, γ is expressed as the ratio of average concentration of lithium in the particle and τ is defined to present normalized SOC: o i o i γ = C 1,i C l,i max, i = p (1) τ = [ γ] /[ γ max ], i = p, m =given rate of discharge (2) m C/33 22
37 As shown in Figure 13a, the ratio of average concentration of the particles (i.e., γ) across the thickness of the cathode could be realized in terms of normalized SOC (i.e., τ). It has been reported that once the concentration on the surface is saturated when τ is 80%, phase transformation initiates near the separator causing a drastic increase in average concentration γ near the separator above 80% (Figure 13a). Because of the phase transformation, the magnitude of the stress across the electrode increases until τ = 80% (single phase) (Figure 13b), and the stress starts to decrease in this point (not shown in figure). Figure 13: (a) Average SOC (γ) from current collector to separator, and (b) intercalation stresses at the surface of the particles across the electrode during discharge [24]. 2.2 Electrolyte The electrolyte plays an important role in the performance of lithium-ion batteries. Generally, battery electrolytes are optimized for maximizing conductivity and electrochemical stability [25], [26]. Table 4 lists candidates for organic carbonate electrolyte for lithium-ion batteries. 23
38 Table 4: A candidate of organic electrolyte for lithium-ion batteries Electrolyte Melting point [ o C] Boiling point [ o C] Dipole moment [10-30 Cm] Viscosity [mpa s] Propylene Carbonate (PC) a a 16.5 a b Ethylene Carbonate (EC) 36.4 a 238 a 4.87 a a Diethyl Carbonate (DEC) a a 3.15 a c Dimethyl Carbonate (DMC) 4 a 90 a 2.95 a c a Nazri and Pistia [26], b Muhuri and Hazra [27], and c Rodríguez et al. [28] For a maximum conductivity of electrolyte, propylene carbonate seems suitable for lithium-ion batteries since it has higher dipole moment, the measure of molecular polarity, which ensures higher dissociation of the lithium salt [29]. However, the viscosity of propylene carbonate is significantly high compared to other electrolyte such as ethylene carbonate, diethyl carbonate, and dimethyl carbonate. Therefore, linear carbonate (i.e., diethyl carbonate and dimethyl carbonate) with low viscosity, related to high fluidity, needs to be added to assure a fast ionic movement [30]. To compensate the low conductivity of the electrolyte with the low viscosity, thin electrode stacks with a thickness around µm is commonly used for lithium-ion batteries [25]. Moreover, doubling the concentration of lithium ion in the electrolyte can increase the capacity up to 15% [31]. Therefore, studying transport of lithium ion in the electrolyte is of importance. Aurbach et al. [32] have studied the effect of acidic and non-acidic electrolyte on carbon coated cathodes during storage. In Figure 14a, sample #1 containing 3% carbon and 24
39 sample #2 containing nearly 1.5 % of carbon are compared. When they stored the carbon coated cathode in non-acidic electrolyte (i.e., EC-DMC/LiCLO4), Fe-ion dissolution is negligible. Fe-ion dissolution means the loss of the active mass from the electrode and indicates that cathode has become deteriorated. Even at a higher temperature (60 o C), Fe-ion dissolution is also negligible. As the solution becomes more acidic, the dissolution of Fe-ion becomes pronounced and it causes an unstable behavior of electrode (Figure 14b). As expected, the higher temperature also leads to more dissolution of Fe-ion. Particularly, dissolution becomes very noteworthy in water contaminated solution (i.e., EC-DMC/LiPF ppm H2O). It is confirmed that effect of commonly used acidic-electrolyte on cathode during storage period is significant. Figure 14: (a) Fe-ion dissolution from LiFePO 4 olivine powders in different electrolytes at 30 ºC, and (b) unstable behavior of a LiFePO 4 olivine electrode in acidic electrolyte [29]. Generally, tortuosity refers to the ratio of the diffusivity in the free space to the diffusivity in the porous medium. Figure 15 displays the simulated porous electrode microstructure and resultant stream line highlight the tortuous path in the electrolyte. In the 25
40 composite matrix, the diffusion path length increases because lithium-ions are forced to flow around or through the particles [33]. The increased diffusion path length due to the porous medium makes the macroscopic resistivity of the cell increase, and it results in a significant decrease in the total delivered power density and the capacity of the power source. (a) (b) Figure 15: (a) Simulated porous electrode microstructure, and (b) resultant stream lines highlight the tortuous path [33]. Using in-situ energy-dispersive X-ray diffraction, Strobridge et al. [21] have analyzed the average lithium content gradient across the electrode with three different particle size distributions, to study the magnitude of the inhomogeneity, the porosity, and C-rate effects. Figure 16a shows that when the porosity increases to 35% (i.e., relatively low volume fraction), the lithium concentration gradient decreases, which can be related to a higher effective diffusivity of the ions in the electrolyte. Figure 16b suggests that the lithium concentration gradients increase with C-rate. It also suggested that the lithium ion diffusion in the electrolyte becomes more limiting at higher C-rate [21]. 26
41 Figure 16: Average lithium content gradient from cathode to anode at cell depths of discharge (DODs, i.e., % overall lithiated = 50%) as a function of (a) porosity, and (b) C-rate for the three different particle-size distributions [21]. 27
42 2.3 Solid-Electrolyte Interphase (SEI) Figure 17 demonstrates overall electrode reactions characterized by a reductionoxidation mechanism including mass transfer, and chemical reactions [34]. Although an electrode region and a bulk solution region were commonly studied in many papers [20][23][35][36], it is now realized that more research about electrode surface region is needed. Especially, among electrode surface regions, solid-electrolyte interphase (SEI) growth at an interface between the negative electrode and the electrolyte is one of the most focused research areas [37]. Furthermore, it has been reported that lithium ions must also travel through an additional layer between the cathode and the electrolyte, which is similar with the SEI observed on an anode [38][39]. Oxidation (Anode) Reduction (Cathode) Figure 17: General reactions at an electrode, an electrode surface region, and bulk solution [34]. 28
43 The experimental results from Ju et al. [39] suggest the charge exchange process should take place at the interface between LiFePO4 and the electrolyte for pure phase LiFePO4 electrode. From the Nyquist plot in Figure 18, after cycling for 5 times, the SEI is formed on the cathode surface. The migration process of lithium ions through the SEI layer leads to the manifestation of the additional semicircle in the Nyquist plots. Strobridge et al. [21] used XRD to map the evolution of the inhomogeneous electrochemical reaction in electrodes. Their results also show that the poorer electronic wiring could result from both the expansion and the contraction of the particles during cycling and the formation of a SEI layer [21]. Figure 18: The Nyquist plots of carbon coated LiFePO 4 after 5 charge discharge cycles. (a) 100%, (b) 50%, and (c) 0% SOC at 303 K [39]. 29
44 Zhong et al. [40] have used an electrochemical method to investigate SEI film on the surface of the both electrode. Figure 19 below portrays SEM images of electrodes. After 15- day storage, the surfaces of both cathode and anode become coarse (Figure 19c and d) caused by the formation of SEI films. Generally, SEI films deteriorate surface structures of the electrodes. The SEI formation was also confirmed by an increasing internal resistance from 13.8 mω to 14.7 mω after 15-day storage. The internal resistance is caused by the SEI formation since the other resistances including lithium ion diffusion resistance in the electrolyte, charge transfer resistance, and Ohm contact resistance are typically the same for each electrode due to the same battery materials used and in the battery assembly technologies adopted [40]. 30
45 Figure 19: SEM images of (a) pristine cathodes, (b) pristine anodes, (c) cathodes after 15-day storage, and (d) anodes after 15-day storage [38]. 31
46 2.4 Phase Transformation across Electrode Investigating phase stability and phase transformation of electrodes is very important to the battery performance. As current is transferred from the anode to the cathode, an electrochemical reaction occurs inside the particle and it can be represented as below equation 3. Li x FePO 4 charging discharging FePO 4 + xli + + xe (0 x 1), (3) where x represents lithium concentration in the particles. Phase transformation in cathode during charging and discharging has been studied using XRD by Meethong et al. [41]. Figure 20a compares a sequence of XRD spectra in terms of SOC. In XRD spectra, H represents heterosite (i.e., lithium-poor phase), T represents triphylite (i.e., lithium-rich phase), and C denotes the graphite. As charging proceeds, the heterosite peak increases and the triphylite peak decreases at the same time in the XRD spectra. As shown in Figure 20b, the fraction of triphylite (Li1-xFePO4, lithium-rich phase) and heterosite (LiyFePO4, lithium-poor phase) phase changes linearly with the SOC, where x and y is close to 0 and 1, respectively. Additionally, it has been also observed that the unit cell volume changes 6.6% due to the phase transformation, and it is mainly related to the volume misfit causing diffusion-induced stress (DIS) during charging and discharging, which will be discussed in Section
47 Figure 20: (a) XRD spectra in terms of SOC from starting material to 95% SOC, and (b) unit cell volumes and the normalized crystalline phase fraction of triphylite and heterosite [39]. 33
48 The shrinking-core model for a single particle of LiFePO4 has been developed to investigate phase transformation (Figure 21). During discharging, an electrochemical reactions initiates when lithium ions and electrons reach the surface of a single phase particles (LiyFePO4). The phase transformation proceeds from Li1-xFePO4 (lithium-rich phase) to LiyFePO4 (lithium-poor phase) due to the electrochemical reactions. As lithium ions continuously traverse from the surface toward the core of particles, lithiation reaction occurs simultaneously. Finally, whole particle is composed of a single phase particles (Li1- xfepo4) [11]. Neglecting any migration effects, and assuming a concentration-independent diffusion coefficient the governing equation for this process can be written in spherical coordinates as follows: c s = D 2 c s t Li + 2D Li c s r 2 r r (4) Equation 4 is solved with the boundary conditions, D Li c s r = 0 at r = 0, (5) c s = c 0 at t = 0, (6) where D Li is diffusivity of lithium ion in the electrode, c 0 is concentration of FePO4, and c s is concentration of LiFePO4. 34
49 Figure 21: Illustration of the shrinking-core model with the juxtaposition of the two phases and the movement of the phase boundary [11]. The simulation results from Tang et al. [42] suggest that LiFePO4 expands along [100] and [010] but contracts in the [001] direction upon phase transformation caused by lithium intercalation. As shown in Figure 22a, phase transition occurs from a corner because particle corners are the most favored nucleation sites. Anisotropic growth is caused by the anisotropic misfit strain between FePO4 and LiFePO4. The linear misfit strain, caused by volume change during phase transition between FePO4 and LiFePO4, is the largest along [100] (5%) and the smallest along [001] (-1.9%) (Figure 22b) [42]. The misfit strain becomes a critical reason of diffusion-induced stresses during phase transformation. 35
50 Figure 22. (a) Contour plots for lithium concentration according to the lithium intercalation, and (b) anisotropic misfit between FePO 4 and LiFePO 4 along 3 directions according to onedimensional lithium diffusion along [010] [42]. 36
51 2.5 Diffusion-Induced Stress Lithium insertion and removal in lithium-ion battery electrodes can result in diffusion-induced stresses (DIS) upon phase transformation. Capacity fade and fracture of electrode, one of the most significant problems in electrode materials, can be associated with DIS [43][44]. Cheng et al. [45] adopted a thermal stress analysis approach to investigate DIS based on the similarity of the law of heat conduction (Fourier s law) and Fick's laws of diffusion. The diffusion equation in the bulk of the insertion electrode is as follows: Θ I t = D I 2 Θ I, (7) where D I is intercalate diffusion coefficient, Θ I is the local concentration. The stress-strain relationships can be expressed in spherical coordinate system as following equation: ε r = 1 E (σ r 2νσ θ ) Ω cθ I, (8) ε θ = 1 E [(1 ν)σ θ νσ r ] Ω cθ I, (9) where E is Young s modulus, ν is Poisson s ratio, Ω is the partial molar volume of the solute, and c is the concentration of sites available for lithium insertion. The solutions for the normal and tangential stresses are expressed as following equation: σ θ (r) = where Θ av I (r) (3/r 3 r ) r 2 Θ I (r)dr 0 of radius r within the particle of radius R. σ r (r) = 2EΩ c 9(1 ν) [Θ I av (R) Θ I av (r)], (10) EΩ c 9(1 ν) [2Θ I av (R) + Θ I av (r) 3Θ I (r)], (11) is the average concentration in the spherical volume 37
52 From the results presented in the Figure 23a below, it can be seen that when the dimensional time (τ) increases, lithium ions move from the surface (r/r=1) towards the center of the particle (r/r=0). When dimensionless time τ = 0.4, dimensionless concentration ( Θ I (r,t) Θ 0 Θ R Θ 0 ) shows nearly consistent across the electrode, suggesting the particles are fully lithiated. Figure 23b and c demonstrate that dimensionless radial stress is always in tension in the particle, while tangential stress show tension and compression. It has been observed that radial stress initially increases until the dimensionless time τ = and followed by a decrease at a center of particle. In case of dimensionless tangential stress, the highest stress is observed at the center of particle when τ = Thus, both tangential and radial stress increase initially followed by a decrease after reaching certain point. 38
53 Figure 23: (a) Dimensionless concentration profile, (b) radial stress, and (c) tangential stress in terms of location in the spherical particle [45]. 39
54 Malavé et al. [46] have investigated mechanical behaviors within LixCoO2 cathode particles via isotropic and anisotropic computational models. Figure 24 compares DIS fields using isotropic and anisotropic models at t = 1820s (nearly 50% discharged) during 1C. In both cases, compressive stresses observed in the particle interior, while the stresses near the particle surfaces are tensile stresses [46]. The simulation results suggest that the anisotropic model predicts higher tensile stresses than the isotropic model. Especially, higher tensile stresses, which are likely to cause crack initiation and fracture, are observed at the concaved area indicated by arrow. These results suggest that DIS is largely related to analysis models and surface shapes of particles. Figure 24: Diffusion-induced stress fields in a particle using (a) the isotropic model, and (b) the anisotropic model [46]. 40
55 Renganathan et al. [24] have developed a mathematical model to simulate the generation of DIS during the discharge process. They have reported that the intercalation process, related to the elastic deformation of the material, must be considered to quantify the stress generated in the electrode. To simulate the intercalation process, they also used the approach similar to that used to account for thermally induced stresses in a material. The effect of porosity and the particle size in the anode on DIS have been analyzed (Figure 25). It has been observed that the stresses increase with a decrease in the porosity and an increase in the particle size of negative electrode. Figure 25: Effect of (a) porosity of anode, and (b) partcile size on the total stress along the thickness of the anode [24]. 41
56 A lithium ion cell-sandwich mathematical model with porous electrodes has been proposed by Christensen [47] to investigate DIS. It is observed that peak tensile stresses occur at the center of particles and peak compressive stresses occur at the surface of the particles. Figure 26 below represents that higher C-rate and larger particles result in a higher DIS. Besides, it could be observed that extensive fracturing of the cathode occurs exclusively in the region closest to the separator [47]. Figure 26: Maximum and minimum stress in terms of C-rate (dashed: 5.5 µm, dotted: 55 µm, and solid: 165 µm electrodes) [47]. 42
57 CHAPTER 3: MODEL DEVELOPMENT Several published studies have focused on the topic of the effect of mechanical stress in lithium-ion batteries [24][46][45][47][48]. However, these studies emphasized only one component of the battery cells (i.e., only cathode, anode or electrolyte). To better understand electrochemical and mechanical relations in lithium-ion batteries, factors such as (dis)charging current rate (C-rate), the volume fraction of the electrode, the viscosity of the electrolyte, and the phase transformation of the electrode materials, ought to be considered as a whole. Three distinct multiphysics finite element models of half-cell systems (i.e., with electrolyte and cathode materials) are developed in ANSYS Multiphysics (ANSYS, Inc., Canonsburg, Pennsylvania, USA). Model 1 sets up a foundation for studying stresses on the interface between the electrolyte and the electrode. The advantage of this model is that we could investigate variations of mechanical stresses in lithium-ion batteries by different combinations of the porosity, C-rate, and electrolyte viscosity. Moreover, due to the simple geometry of the model, it allows us to evaluate resulting mechanical stresses on the interface between the electrode and the electrolyte quickly. Based on the results, a determinant factor contributing to the stress-induced degradation of the battery could be derived. However, this model is oversimplified by considering the electrode as a continuum media. To this end, the second multiphysics model is developed: a porous medium with various spherical particle sizes in cathode is included in the model 2, by adopting Vijayaraghavan s study [33]. Due to the nature of a porous medium, several interfaces 43
58 between the electrode and the electrolyte can be studied. More insights of the effect of the C- rate, the volume fraction, and electrolyte viscosity are deduced. Based on the results of the second model, we have a better understanding of the electrochemistry-induced mechanical stresses in porous electrodes. To further capture phase transformation induced by lithium ion diffusion in electrode materials, a third multiphysics finite element model is developed. In this model, shrinkingcore model developed by Shrinvasan and Newman [11] is extended by considering several particles and electrolyte. Specifically, changes of the materials properties of the electrode (from lithium-poor phase to lithium-rich phase) are coupled with the lithiation stage during discharging. Stresses at electrode-electrolyte interface in lithium-ion batteries are reinvestigated, particularly on the effects of the C-rate and the volume fraction. Table 5 lists the material properties for cathode and electrolyte used in this study. 44
59 Table 5: Material properties for cathode and electrolyte Parameter Symbol Value Reference Ethylene Carbonate (EC) Density ρ g cm-3 Molar Mass M g/mol Viscosity η 1900 Pa s [26] Dimethyl Carbonate (DMC) Density ρ g cm -3 Molar Mass M g/mol Viscosity η 585 Pa s [28] Diethyl Carbonate (DEC) Density ρ g cm -3 Molar Mass M g/mol Viscosity η 749 Pa s Young s Modulus EX EY EZ GPa GPa GPa FePO 4 Shear Modulus GXY GYZ GxZ 45.6 GPa 32.5 GPa 43 GPa [49] Poisson s Ratio XY YZ XZ
60 Elastic Stiffness C11 C22 C33 C44 C55 C66 C12 C13 C GPa GPa GPa 40.8 GPa 38.2 GPa 42.1 GPa 41.0 GPa 29.1 GPa 29.1 GPa Young s Modulus EX EY EZ GPa GPa GPa Shear Modulus GXY GYZ GxZ 42.4 GPa 34.9 GPa 47.8 GPa LiFePO 4 Poisson s Ratio XY YZ XZ [49] Elastic Stiffness C11 C22 C33 C44 C55 C66 C12 C13 C GPa GPa GPa 40.8 GPa 40.5 GPa 43.9 GPa 61.1 GPa 54.9 GPa 67.7 GPa FePO 4 LiFePO 4 Volume Change Coefficients VX VY VZ [50] 46
61 3.1 The Impact of the Porosity of Electrode-Electrolyte Interface Figure 27a demonstrates the geometry of the model 1 used in the simulations. In order to model the interface between the electrolyte and the cathode, two 0.3μm 0.3μm 0.3μm domains are adopted: one of them is for electrolyte and the other one is for the cathode. The domain size is designed to be resembled 300nm thick LiFePO4 films developed by Sauvage et al. [51]. Thus, the long edge of the simulation domain is set to L=0.6μm. The surface marked in green indicates the electrode-electrolyte interface. Particularly, Fluid- Porous Interface was used in this Fluid Flow module (CFX), in which the cathode material is considered as a continuum media with various porous ratios to simulate the solid domain as a porous electrode. Figure 27b portrays the mesh of the finite element model. The element model contains 1024 elements with the 8-nodes hexahedral (H8) element type. Moreover, the simulations are conducted in a steady-state and ionic flux is not considered as it is our first step to understand the impact of the porosity of electrode-electrolyte interface. Figure 27: (a) Geometry, and (b) mesh of the model 1 composed of electrolyte and cathode domains. 47
62 Figure 28 below shows how the simulations based on the model 1 work in ANSYS. At first, the streamline of the electrolyte, where the lithium ions traverse, is calculated using the Fluid Flow module (CFX). Momentum loss of electrolyte occurs when electrolyte meets the surface of the electrode, and it causes mechanical stresses on the surface of the cathode. The C-rate dependent lithiation stage is calculated using the Transient Thermal module. By combining the results from the Fluid Flow and Transient Thermal modules, the mechanical stresses are investigated on the interface between electrolyte and cathode via the Static Structural module. Figure 28: Project schematic simulation in ANSYS including Fluid Flow (CFX), Transient Thermal, and Static Structural modules. In this simulation, the phase transformation from FePO4 to LiFePO4 during discharging is considered and the material properties of the electrode changes based on the following equation: [C(x)] = x[c] LiFePO 4 + (1 x)[c] FePO 4, (12) 48
63 where [C(x)] is the phase transformation dependent anisotropic material property stiffness matrix, [C] LiFePO 4 is the anisotropic material property stiffness matrix of LiFePO4, [C] FePO 4 is anisotropic material property stiffness matrix of FePO4, and x represents lithiation from the lithium-poor phase to the lithium-rich phase (0 x 1). Equation 13 below indicates generalized Butler-Volmer kinetics usually describing the lithium insertion rate: 1 α α Li,yte J = k 0 γ TS α αli,s [exp ( αeη ) exp ( (1 α)eη )], (13) k B k B T where J is the lithium insertion rate, k0 is the rate constant, αli,yte and αli,s are the respective activities of lithium in the electrolyte and in solid particles, e is the elementary charge, η is the reaction over-potential at the LiFePO4/carbon/electrolyte triple-phase boundary, and γ TS is the activity coefficient of the transition state [52]. The 1C model has J 1C = 0.19 A m 2, the 5C model has J 5C = 0.25 A m 2, the 10C model is with J 10C = 0.31 A m 2, and the 20C model has J 20C = 0.59 A m 2 obtained from Li et al. [20]. To fulfill above insertion rate (J), the current density (I) at the inlet boundary was calculated by equation 14 since the current density can be expressed in terms of the velocities of the ions [36]. I F = c (Li + )v (Li + ) c (PF6 ) v (PF6 ), (14) where I is the current density, F is Faraday s constant, c Li +, c PF6, v Li +, and v PF6 indicates concentration of lithium ion and hexafluorophosphate ion, and velocity of lithium ion and hexafluorophosphate ion, respectively. To simplify the simulations, the concentration and the velocity of hexafluorophosphate ions are disregarded and we assumed that concentration of lithium ion are constant across the electrolyte. 49
64 To investigate the C-rate effects in our finite element models, we also assume that the velocity of lithium ions calculated above is used as an inlet velocity of the electrolyte. Furthermore, due to the process that lithium ions are forced to flow around or through the particles of cathode, resistant occurs on the surface of cathode in the half-cell system. That is, it is assumed that an increase in macroscopic resistivity of the simulation domain is expressed as the momentum loss of electrolyte. 50
65 3.2 The Impact of Porous Electrodes in a Lithium Ion Half- Cell The model presented in the previous section does not consider particle size, shape, and molar volume misfit, therefore, the second multiphysics model is developed: a porous medium with various spherical particle sizes in cathode materials. As shown in Figure 29a, model 2 consists of a 0.3μm 0.3μm 0.6μm rectangular domain for the electrolyte and several spherical particles as electrode materials. Moreover, symmetry boundary conditions and fluid-solid interface boundary conditions are adopted in the second model. Figure 29b displays the mesh of the finite element model. The element model contains elements with the 10-nodes tetrahedral (T10) element type. Figure 29: (a) Geometry, and (b) mesh of the model 2 composed of electrolyte and cathode domains. In this simulation, spherical and nano-size particles ranging from 75nm to 125nm are adopted to investigate the effect of electrode volume fraction; the particle size is selected 51
66 from an experimental observation [53]. When the streamline passes around the cathode particles, the resistant occurs on the electrode-electrolyte surface. The similar assumption in the model 1 that momentum loss of electrolyte causes pressure on the surface of cathode is also used. To determine inlet boundary conditions, the lithium ions transversal diffusion distance at a given time is estimated by the equation 15 [54]: δ i (t) = D i t, (15) where D i is the diffusion coefficient of the electrolyte and δ i is the estimated diffusion distance of lithium ion at a given time. Based on the estimated diffusion distance, the velocity of the electrolyte is calculated by dividing the diffusion length by the different discharging time due to different C-rates (i.e., 1C requires 3600s to reach a fully discharging, 5C requires 720s to reach a fully discharging). In this porous electrode half-cell model the diffusion coefficient of electrolyte is assumed to be a constant across the half-cell system, and with D electrolyte = m 2 s 1 [55]. Details on how to calculate lithiation are discussed in the Section Figure 30 below demonstrates the multiphysics mechanism for stress analysis in the second model. To calculate the electrolyte flow around spherical particles, Fluid Flow module is used. Electrolyte flow produces fluid pressure on the interface between spherical particles and the electrolyte (i.e., solid-fluid interactions). As a result, pressured-induced mechanical stresses are generated in the half-cell system, which is then captured in the Static Structural module as the second step. In this step, spherical particles are modeled as anisotropic materials, as shown in Table 5. Since we focus on lithiation of 10% instead of transient lithiation, lithiation stage is calculated in CFX. From lithiation of 10%, lithium-rich 52
67 phase and lithium-poor phase coexist in the electrode. Therefore, Transient Thermal model is not included in this model. Figure 30: Project schematic simulation in ANSYS including Fluid Flow (CFX), and Static Structural modules. 53
68 3.3 The Impact of Phase Transformation in Porous Electrodes Although reasonable results are obtained from the porous model (model 2) developed in the previous section, where the viscosity of the electrolyte and multiple interfaces are included, however, the C-rate dependent molar volume misfits, lithiation, and associated the phase transformation of the particle during discharging are not incorporated. Therefore, a third model is developed to address these factors. The geometry used in the model 3 is the same as the model 2, as shown in Figure 31. Specifically, changes of the material property of the electrode (from lithium-poor phase to lithium-rich phase) are coupled with the C-rate dependent lithiation stage during discharging in model 3. Figure 31: (a) Geometry, and (b) mesh of the model 3 composed of electrolyte and cathode domains. To simulate phase transformation during discharging, the shrinking-core model developed by Srinivasan and Newman [11] is adopted: the phase transformation of cathode materials is considered and the C-rate dependent volume misfits are adopted from the 54
69 experimental observations [56]. Thus, the importance of lithium-ion intercalation is further emphasized in the third model. The stress is calculated by following equation: [σ] = [C]([ε] 3α Φ), (16) where α is a volume expansion coefficient, Φ is the concentration gradients of lithium ion, and [C] is the phase transformation dependent anisotropic material property matrix. The driving force (flux) of the lithium ions movement is modeled by the heat flux. Since heat flux (flux of lithium ions) is an inlet boundary condition, heat energy (lithium-ion chemical potential) transfers through this domain. Lithiation can be simulated based on similarities between the heat equation (equation 17) and Fick s second law of diffusion (equation 18). T t = α T T, (17) C t = D C, (18) where T is the temperature, αt is a thermal diffusivity, C is the concentration of lithium ion, and D is the diffusion coefficient of lithium ion. In this simulation, the concept of heat transfer and temperature is used for simulating the diffusion and concentration of lithium ion. Thus, the limitation of the model is that the real effects due to the temperature and heat on lithium-ion batteries have to be disregarded. In order to represent various C-rates, different mass fluxes (J) are determined to ensure enough time for lithium ion intercalation and diffusion in both electrolyte and cathode, where the 1C model has J 1C = 0.125Wm 2, the 2C model has J 2C = 0.25Wm 2, the 6C model has J 6C = 0.75Wm 2, and the 8C model has J 8C = 1Wm 2. In addition, a constant diffusion coefficients are used in the model, where D electrolyte = 1 55
70 10 10 m 2 s 1 [55], and D LiFePO4 = D FePO4 = m 2 s 1 [57]. Detail on how to calculate lithiation is discussed in the Section In this study, a computational model integrating experimental results [56] and theoretical analysis [11] is developed through a multiphysics analysis. As shown in Figure 32, the Fluid Flow and Static Structural module, and an Application Customization Toolkit (ACT) are used in the model 3. At first, the concentration distribution of the electrolyte, which enables lithium ions to move toward cathode, and the diffusion of lithium ions in each particles are calculated in the Fluid Flow module. The results generated in the Fluid Flow module are imported into the Static Structural module via a Fluid-Solid Interaction (FSI) transient extension, one of the ACT. FSI transient extension enables interpolating transient CFX results from the CFX mesh to the FEM mesh at each step of the structural analysis. Based on the transferred results from two modules, the diffusion-induced stresses on the cathode particles are calculated in the Static Structural module. Another ACT, called FE info extension, is also used to calculate the lithiation stage. FE info extension details nodes and elements related information in the mechanical interface. 56
71 Figure 32: Project schematic including Fluid Flow, Static Structural modules, and Application Customization Toolkit (ACT). 57
72 CHAPTER 4: SIMULATION RESULTS AND DISCUSSION Sections below details the simulation results that were carried out by ANSYS Multiphysics. The results from the current study provide a better understanding of the mechanical stresses on the electrode-electrolyte interface of a lithium-ion half-cell system. Several parameters such as C-rate, volume fraction (porosity), viscosity, and phase transformation are considered in this study. Since it has been reported that tensile or compressive stresses are highly correlated to fractures in cathode particles [16] and highest stress is observed on the surface of electrode particles [46], we focus on normal stress on the surface of the electrode in our study. 4.1 Stress Analysis of the Porosity of Electrode-Electrolyte Interface Model 1 aims to understand the influences of C-rate, porosity, viscosity, and lithiation on the interface between electrolyte and electrode. Figure 33a displays the velocity vector of electrolyte and Figure 33b shows dimensionless velocity profiles at different locations in the half-cell system: at the inlet boundary (marked in red, location 1), at the middle of electrolyte domain (marked in blue, location 2), and at the middle of electrode domain (marked in green, location 3). Since the maximum value at the middle of electrolyte domain is twice higher than that at the inlet boundary and non-slip boundary conditions are adopted, the electrolyte domain size is sufficient for fluid flow (i.e., electrolyte) to be fully developed. It has been 58
73 observed that the maximum velocity increase by 4 times (i.e. 1/porosity) at the middle of electrode domain due to the porosity of 0.25 in the electrode. Figure 33: (a) Velocity vector of electrolyte across electrolyte and electrode domains, and (b) velocity profiles at 3 different locations in the half-cell system (red: inlet boundary, blue: middle of the electrolyte domain, and green: middle of the electrode) Effect of C-rate Figure 34 below provides the simulation results that demonstrate the relationship between C-rates and the maximum normalized normal and shear stresses. Both normal and shear stresses increase with the increasing C-rate. This relationship was also observed in another battery chemistry (i.e., LiCoO2) [46]. Moreover, the results suggest that normal stress is much higher than the shear stress and it is due to that lithium ions mainly transverse along the diffusion direction, which is normal to the electrode surface, thus higher normal stresses are generated. The similar phenomenon is also reported in LiFePO4, where only electrode was simulated in that study [56]. From the results, higher C-rate results in higher stresses 59
74 during discharging, suggesting damages caused by the mechanical stresses occur in the electrode. The results also provide quantitative data to support the hypothesis that batteries show the performance degradation (e.g., capacity loss) at higher C-rate. Figure 34: Influences of varying C-rate on the normalized normal stress and shear stress (porosity of 0.25 and EC is used as an electrolyte) Effect of Porosity In the model 1, the porosity is calculated according to equation 19 below. = V V V T, (19) where V V is the volume of the void-space, and V T is the bulk volume of the material. Various porosities, 0.1, 0.25, 0.5, and 0.75, are considered and the resulting maximum stresses on the 60
75 interface are analyzed. The plot in Figure 35 below represents that the normal stress decreases in a non-linear fashion as the porosity increases. It could be explained by the relationship of the porosity and the diffusion length in the porous media. Since decreasing in porosity causes an increase in the diffusion length, the electrolyte across the lower porosity electrode needs to move faster to reach a fully discharging, and as a result, a larger normal stress is generated. This result is consistent with the theoretical analysis by Renganathan et al. [24]. We also conduct another simulation with a larger domain size (10μm) (not shown in this thesis), and a similar result about the effect of the porosity of the electrode to the stresses is also observed. Figure 35: Relationship between porosity and normalized normal stress (EC is used as an electrolyte). 61
76 4.1.3 Effect of Viscosity Figure 36 demonstrates that normal and shear stresses increase with the increasing viscosity of electrolyte, suggesting that viscous force generated from the electrolyte has great influence. Moreover, the normal stress is affected by the electrolyte viscosity to a greater degree than the shear stress. The electrolyte Ethylene Carbonate has 3.25 times higher viscosity than Dimethyl Carbonate, and our simulation results show that the normal stress increase more than 3 times. It is confirmed that in spite of the higher conductivity properties in Ethylene Carbonate, the electrolyte with a higher viscosity plays a negative role in the battery performance due to the resulting higher mechanical stresses. It is also observed that the other properties of electrolyte, such as the molar mass and density, do not affect both normal and shear stresses that much. 62
77 Figure 36: The effect of electrolyte viscosity on normal and shear stresses at 1C (porosity of 0.25) Effect of Lithiation LiFePO4 electrode undergoes a phase transformation during charging and discharging. Upon lithium intercalation and diffusion in cathode, lithium-poor phase (FePO4) is transformed into lithium-rich phase (LiFePO4) [11]. In this study, effect of lithiation of the electrode is incorporated and the study from ChiuHuang [56] is employed. The contour plots in Figure 37 shows lithium concentration profiles when the cathode is lithiated 20% and 80%. In this model, the property change according to lithiation stage is considered instead of C-rat dependent lithiation stage. Therefore, maximum concentration (marked in red) is imposed on the surface of lithium-poor phase electrode (marked in blue) to make concentration gradients. Concentration gradients is the driving force of lithium ion diffusion. 63
78 Figure 37: Concentration profile calculated by the transient thermal module in ANSYS. Each model represents lithiation of (a) 20%, and (b) 80%. The lithiation of the electrode is calculated by the following equation: l Lithiation stage = ( C 0 i l dy)/( C 0 s dy), (20) where C s is the concentration when the surface of the cathode is fully saturated (i.e., C s = 1) and C i is the concentration at i (0 i l). l is the total length of cathode (i.e., 0.3μm ). In l equation 20, C 0 i l dy indicates gray area whereas C 0 s dy indicates sum of gray area and yellow area in Figure 38. Since lithium ion diffusion occurs proportional to the surface of the electrode, lithium-ion concentration change is completely independent along the x and z directions. Using equation 20, lithiation is calculated based on the local concentration distribution (Figure 38). 64
79 Figure 38: Local concentration in terms of diffusion distance. Each concentration distribution shows lithiation of 20% (a) and 80% (b). As shown in Figure 39, both normal and shear stresses decrease in non-linear fashion as lithium lithiation increases, suggesting phase transformation has a great effect on mechanical stresses in the half-cell system. 80% lithiation means that electrode consists mostly of lithium-rich phase (LiFePO4), while 20% lithiation indicates electrode consists most of lithium-poor phases (FePO4). This trend is not consistent with the results obtained from ChiuHuang [56], and we believe it may be due to that volume misfits during lithiation are not included. Therefore, we reinvestigate effect of phase transformation and associated volume misfits in Section
80 Figure 39: Relationship between stresses and lithiation stage at 1C (porosity of 0.25 and EC is used as an electrolyte) Conclusion The highest stress has been observed on the surface of electrode particles [46] and normal stresses are highly associated with the fracture in the electrode [16], therefore we focus on studying maximum normal stress on the surface between electrolyte and electrode in the model 1. First, the effect of C-rate was investigated in Figure 34. Both normal and shear stresses increase with an increasing C-rate by adjusting inlet boundary conditions in our simulations. In other words, higher stresses at the electrode-electrolyte interface caused by higher C-rate during discharging, suggesting damages strongly related to the mechanical stresses occur in the electrode. Our results provide qualitative and quantitative information 66
81 on mechanical stresses and could be used to support the experimental observation that the reduced performance such as capacity loss is caused by high C-rate discharging. Secondly, Figure 35 portrays the effect of electrode porosity. It indicates that mechanical stresses on the electrode-electrolyte interface decreases exponentially as the porosity of electrode increases. In the low porosity electrode, an increase in diffusion length results in higher stress. Similar trend was also concluded in LiCoO2 battery materials [24]. In the model with a larger domain size (10μm) (not shown in this thesis), similar result about the effect of the porosity of the electrode to the stresses is also observed. Moreover, we also observed that an increase in the electrolyte viscosity causes higher normal and shear stresses (Figure 36). Even though high viscosity electrolyte ensures high ionic conductivity, it negatively affects the battery performance from a stress analysis point of view. Furthermore, the results also suggest that the effects of molar mass and density of the electrolyte is less important than that of viscosity. In case of lithium concentration, there is an inverse relationship between maximum stress and lithiation (Figure 39). That is, maximum stress on the surface decreases in a nonlinear fashion during discharging process. We suspect that it is because volume misfits during lithiation was not considered in the model 1, therefore further reinvestigation is conducted in next few sections. The results of this analysis contend that stress analysis on the surface of cathode is important during discharging process. 67
82 4.2 Multiphysics Models for Stress Analysis of Porous Electrodes Several experiments have observed volume change (expansion and contraction) in LiFePO4 cathode materials during lithiation [50][58], and therefore volume change and the pressure induced by electrolyte are considered in this study. To incorporate volume change in this study, lithiation stages are considered 10%, where 10% lithium-rich phase and 90% lithium-poor phase are included in the computational models by using the material property in Table 5. Since LiFePO4 has relatively larger molar volume than that of FePO4 and two phases are constrained at a coherent interface, volume misfits are observed. As shown in Figure 41, this higher volume misfit could lead to a tensile stress marked in red. On the other hand, electrolyte pressure causes compressive stress is acting on some parts of the model 2, marked in blue (Figure 41). FePO 4 LiFePO 4 Figure 40: Molar volume misfits when lithium-poor phase and lithium-rich phase exist. 68
83 Since maximum stress occurs on different locations in the model 2, we compare stresses at specific points: position 1, 2, and 3 (Figure 41). As expected, the stress at position 1 is much higher than stress at position 3. Figure 41: Contour plots of stress and locations of position 1, 2, and 3 in (a) electrode domain and (b) a zoom-in top view of the electrode domain. Snapshots are taking from the 1C model with the volume fraction of 15.5% Effect of C-rate Figure 42 below shows that shear and normal stresses at the position 1 increase in a non-linear fashion as C-rate increases. A similar trend is also observed at the position 2 and 3. One interesting observation is that the shear stresses are higher than normal stresses. It is inconsistent with the result from model 1, however. We strongly believe that it is related to the porous electrode with multiple spherical particles. There is a need to further investigate the effect of various shapes of particles such as plate-like particles or ellipsoidal particles. 69
84 Figure 42: Influences of C-rate on normalized normal and shear stresses at position 1 (volume fraction of 15.5% and EC is used as an electrolyte) Effect of Volume Fraction In this study, volume fraction is defined as the local ratio of the volume of cathode particles to the total physical volume. Because of total physical domain size is fixed, volume fraction is proportional to particle size. In Figure 43, streamlines of model 2 with various particle sizes are illustrated. It is assumed that lithium ions traverse along the streamline around spherical particles in this model. The streamlines show that the diffusion length 70
85 increases with an increase in particle size, causing higher velocity of electrolyte in the space between particles. Figure 43: Streamline of model 2 in 1C with the volume fraction of (a) 6.5%, (b) 15.5%, and (c) 30%. Figure 44 displays contour plots of normal stress of the particle size of 75nm, 100nm, and 125nm. The models with 75nm, 100nm, 115nm, and 125nm particles have a volume fraction of 6.5%, 15.5%, 23.6%, 30%, respectively. The surface area of particles increases with the volume fraction, and it causes higher compressive stress caused by electrolyte at a higher volume fraction. Tensile stress is observed due to the volume misfits. However, this results suggest that compressive stress induced by electrolyte is more important factor than the volume misfits of particles at the stress analysis. 71
86 Figure 44: Contour plots of normal stress (y-axis) in 1C with volume fraction of (a) 6.5%, (b) 15.5%, and (c) 30% at the lithiation of 10%. The effect of volume fraction on the normalized normal stress at each position is shown in Figure 45 below. As the volume fraction increases from 15.5% to 30% (i.e., more electrode particles), the normal stress increase by 131%. Studies have shown that with high volume fraction of cathode enabled by using nano-scale particles and carbon black [59], the higher battery performance is observed. From the stress analysis point of view, our results demonstrate that higher volume fraction of electrode (bigger particle sizes) produces higher stresses and may deteriorate the battery performance. It is also observed that the normal 72
87 stress at position 1 is always higher than that at position 3. This can be explained by the experimental results from Li et al. [20], suggesting more lithiation occurs near the electrodeelectrolyte interface (i.e., more lithiation occurs at position 1 than that at position 3). Therefore, the stress induced by fluid pressure at position 1 is higher than any other position due to more lithiation at position 1. Figure 45: Effect of volume fraction on normalized normal stress in 1C at the lithiation of 10% (EC is used as an electrolyte). 73
88 4.2.3 Effect of Viscosity It has been reported that the most important properties of the electrolyte for ionic conductance are the viscosity and dipole moment [25]. Figure 46 below shows the effect of viscosity on the stress at each point. For each electrolyte, normal stress decreases in a nonlinear fashion along the diffusion direction from position 1 to position 3. Even though the viscosity of EC is 3.2 times higher than that of DMC, the normal stress is 3.4 and 3.2 times higher when EC is used at the position 1 and the position 2, respectively. The results suggest that as the distance from the interface increases, the effect of the viscosity of the electrolyte becomes less important. Moreover, EC has 1.65 times higher dipole moment (i.e., suggesting higher ionic conductivity due to the dissociation of the lithium salt) than DMC, the stress increases more than 3 times at position 1 and 2. Even though EC has a higher dipole moment and high viscosity of electrolyte to ensure high fluidity (i.e., ionic movement), EC plays a negative role on the electrode from the stress analysis point of view. Therefore, DEC or DMC are recommended as electrolyte for lithium-ion batteries. However, EC should be added for assuring good cycling battery performance because EC shows very good filmforming ability for carbonaceous anodes [60]. 74
89 Figure 46: Effect of the viscosity of electrolyte on normalized normal stress at specific location in the 1C model at the lithiation of 10% (volume fraction of 15.5%) Conclusion In the model 2, molar volume misfits and fluid pressure are considered to investigate mechanical stresses on the interface. Since Model 2 has several interfaces between the electrolyte and the electrode, the stresses at three specific positions are compared rather than the maximum stress on the interface (Figure 41). First, the effect of C-rate was discussed in Figure 42. As expected, the maximum stresses at every position increase with the increasing C-rate. It is interesting to note that 75
90 shear stresses is higher than normal stresses, which seems to be caused by the spherical shapes of particles. Furthermore, the effect of volume fraction on normal stress at each position is investigated (Figure 45). The volume fraction is determined by the size and number of particles because electrolyte domain size is fixed. Due to a larger contact area between the electrolyte and the electrode, the model with volume fraction of 30% has 1.66 times higher normal stress than 15.5% volume fraction model at position 1. It is also observed that stress induced by fluid pressure at position 1 is always higher than any other position in the electrode and it is due to more lithiation occurs at the position 1. Lastly, Figure 46 demonstrates the effect of viscosity on the stress at 3 locations in the electrode. It is observed that as the distance from the inlet boundary increases, the effect of viscosities is less important. Moreover, it is suggested that in spite of high conductivity property of the high viscosity electrolyte (i.e., EC), resulting mechanical stresses are higher than that when other electrolyte are chosen (i.e., DEC or DME). 76
91 4.3 Phase Transformation Effect in Porous Electrodes via Multiphysics Modeling Reasonable results are obtained from the previous models where the viscosity of the electrolyte and multiple interfaces are included, however, during the course of this thesis, several factors are also important to be included. Therefore, a third model is developed to address factors such as, the C-rate dependent volume change due to the molar volume misfits, lithiation and associated the phase transformation of the particle during discharging. Within the model 3, several simulations were carried out to better understand the influences of C-rate, volume fraction during lithiation and to reinvestigate mechanical stresses at the electrode-electrolyte interface Lithiation To adopt the concept of lithiation, we used a thermal stress analysis approach, which has been widely discussed [44] [48] [56]. Specifically, shrinking-core model developed by Shrinvasan and Newman [11] is used and the thermal diffusivity mimics lithium ion diffusivity. In this study, mass flux of lithium ion (J) is expressed as heat flux, and the temperature gradient represents lithium-ion concentration gradient. The concept depends on the similarities between lithium concertation distribution equation (equation 18) and temperature distribution equation (equation 17). The process of diffusion in both electrolyte and electrode is based on Fick s second law (equation 18). Since the concept of heat and temperature is used for diffusion and concentration of lithium ion, effects of temperature and 77
92 heat on material properties are disregarded. Once mass flux of lithium ion induced on the inlet boundary, the lithium concentration changes as diffusion occurs (i.e., lithiation proceeds). Figure 47 represents a cross-sectional view of whole model which demonstrates concentration distribution in the electrolyte and the cathode. The lithiation of particles shows lithiation of 0%, 15.8%, and 83%, respectively. In this study, it is assumed that conductivity of electrolyte and electrode are constant and the diffusion coefficient are D electrolyte = m 2 s 1 [55], and D LiFePO4 = D FePO4 = m 2 s 1 [57], respectively. Thus, the concentration of electrolyte changes more rapidly than that of the cathode. Even though lithium concentration of electrolyte indicates 100% concentration, only a surface of particles shows 100% concentration (i.e., fully saturated) and the lithiation of the electrode is still in progress. Figure 47: Concentration distribution at the lithiation of (a) 0%, (b) 15.8%, and (c) 83% in cathode in a 1C model (volume fraction of 15.5%). 78
93 In Figure 48a demonstrating a full contour plot at lithiation of 50%, high concentration of lithium ion is marked in red and low concentration of lithium ion is marked in blue. Figure 48b portrays a 10-nodes tetrahedral (T10) type element, which is used in this simulations. Based on the data from each element, lithiation of electrode can be calculated by following equation: Lithiation stage = i V i ( j=10 j=1 C j 10 ), (22) V T where i is the number of elements j is the number of nodes (10 nodes in each element), V T and V i indicates volume of particles for each element, and C j is concentration of lithium ion at each nodes. Figure 48: (a) Contour plot of lithiation at 50% lithiation in 1C (volume fraction of 15.5%), and (b) a 10-nodes tetrahedral type element. 79
94 Figure 49 shows contour plots of the electrode at various lithiation stages. When lithium ions insert into lithium-poor phase particles, lithium-rich phase is initially formed on the surface of particles (Figure 49a-c). While the phase boundary moves toward the center of the particle, the thickness of lithium-rich phase increases (Figure 49 d-g). At the last stage of lithiation (Figure 49h), only lithium-rich phase particles are observed. Figure 49: Contour plot at lithiation stage of (a) 0%, (b) 5%, (c) 10%, (d) 20%, (e) 30%, (f) 50%, (g) 75%, and (h) 100% for 1C model with volume fraction of 15.5% Effect of C-rate Figure 50 below displays contour plots of normal stress in the models with volume fraction of 30% in 1C and 6C, respectively. The stress field within the particles shows concentric, which is caused by the lithium concentration field shown in Figure 48a. As C-rate increases, the increases in the tensile and compressive stress are observed. The six times lager C-rate causes 1.62 times larger in tensile stress and 1.18 times larger in compressive 80
95 stress. It is concluded that the tensile stress is strongly affect by C-rate. Moreover, higher tensile stress is observed near the center of particle, called diffusion-induced stress. The magnitude of maximum compressive stress is 4.05 and 2.94 times larger than that of maximum tensile stress in 1C and 6C, respectively. The results suggest that contraction of particles during discharging (due to phase transformation) and fluid pressure caused by the electrolyte is dominant factors comparing to the particle volume expansion during discharging. It is interesting to point out that the area marked in red and blue continuously decrease in stresses as the particles located away from inlet boundary. That is, particles near an inlet boundary show a higher stress than particles near outlet current collector. It is consistent with the experimental results from Strobridge et al. [21] where inhomogeneous reaction is reported. In addition, the higher stresses of particles closer to the electrodeelectrolyte interface suggested there is a need to mitigate stresses at the separator as it is the location might occur mechanical damages in the battery during discharging. 81
96 Figure 50: Contour plots of normal stress for the model with volume fraction of 15.5% at 50% lithiation in (a) 1C, and (b) 6C. 82
97 The plot portraying the effect of C-rate on normal stress during lithiation is shown in Figure 51. The results show that higher C-rate causes higher normal stress. The maximum stress for 1, 2, 6, and 8C occurs when the lithiation is 0.34, 0.38, 0.49, and 0.59, respectively. It is apparent that as C-rate increases, the peak stress occurs at a higher lithiation. Our results are supported by a mathematical study from Lim et al. [61] and they have reported that the SOC (i.e., lithiation stage) is related to the maximum increased diffusion-induced stresses when increasing the particle size and intercalation rate, with a decreased lithium diffusion coefficient in LiCoO2 batteries [61]. Our results confirm that peak stresses at higher C-rate occurs at a high lithiation stage in LiFePO4 batteries. Moreover, the phenomenon of initially increasing stresses followed by a decrease has been observed in LiMn2O4 [16] battery chemistries. We believe that this result may support the idea of that volume expansion and contraction of particles during phase transformation should be included to better capture the stress evolution during discharging, where the maximum stress is observed at relatively high lithiation stage (Figure 51) instead of at the low lithiation in our model 1 (Section 4.1.4). 83
98 Figure 51: Effect of C-rate on normal stress at the position 1 during lithiation (volume fraction of 15.5% and EC is used as an electrolyte). Figure 52 represents a relationship between the surface concentration and the dimensionless discharging time. As C-rate increases, the lithium concentration gradients increase. Since larger lithium concentration gradients contribute to the higher stresses based on the equation 16 (Section 3.3) [61], it is concluded that higher C-rate results in a higher stress on the cathode. 84
99 Figure 52: Relationship between the surface concentration and the dimensionless discharging time for the models with volume fraction of 15.5% and EC is used as an electrolyte. Figure 53 compares strain energy variation at different C-rates. It is observed that higher C-rate leads to a higher strain energy. Our results supports the findings from Zhang et al. [48], suggesting that higher concentration gradients cause a higher strain energy. We also observe that the strain energy increases initially, reaches a peak value, and then decreases. The high elastic energy from the high C-rate models results in the instability of battery system and particle fracture [44]. From the appearance of Figure 53, it is suggested that higher C-rate plays a critical role in the rapid variation of the mechanical strain energy between lithiation of 0.2 and 0.6. We believe that the results are directly related to the capacity loss at the high C-rate in the lithium-ion batteries. 85
100 Figure 53: Effect of C-rate on the normalized strain energy during lithiation (volume fraction of 15.5% and EC is used as an electrolyte) Effect of Volume Fraction Since the electrolyte domain size is fixed, each model with particle sizes of 60nm, 80nm, 100nm, and 120nm indicates volume fraction of 3.3%, 8.0%, 15.5%, and 26.8%, respectively. Figure 54 below demonstrates contour plots of normal stress for the model with volume fraction of 3.3% and 26.8%. As a volume fraction increase, the increases in the tensile and compressive stress are observed. Interestingly, tensile stress at the cathode with volume fraction of 26.8% is 1.17 times larger than that of the 3.3% volume fraction model, while compressive stress increase by 2.13 times. From these results, it is obvious that compressive stress caused by electrolyte and volume change (expansion and contraction) 86
101 during lithiation is a more critical factor. Moreover, the compressive stress in the electrode is strongly affected by volume fraction of electrode. Based on intensive literature search and review, it is the first study reporting diffusion-induced stresses from the coupled effects of electrolyte and electrode. 87
102 Figure 54: Contour plots of normal stress for the model with volume fraction of (a) 3.3%, and (b) 26.8% particle at 50% lithiation in 1C (EC is used as an electrolyte). 88
103 Figure 55 demonstrates the impact of volume fraction on normal stress in 1C and 6C models. The results suggest that normal stress increases with the increasing volume fraction (i.e., particle size). This results match up with that of model 2. As the volume fraction increases, the surface area of cathode particles, where lithium intercalation occurs, increases. Therefore, higher stress is observed in models with higher volume fraction. Moreover, we also observe that the stress increases initially, reaches a peak value, and then decreases as lithiation proceed and the models with various C-rates show peak stress occurs at nearly the same lithiation stage. Figure 55a and b are combined in to show overall results about effect of C-rate and the volume fraction (Figure 55c). Since higher volume fraction and C-rate result in higher stress, battery degradation could be expected in these conditions. 89
104 Figure 55: Effect of volume fraction on normal stress in (a) 1C, (b) 6C, and (c) combined results (EC is used as an electrolyte). Similar trends with the effect of volume fraction are observed in Figure 56. As lithiation proceeds, the strain energy first increases and then decreases in the models with different volume fractions. The highest value of strain energy for the same volume fraction can be found at approximate lithiation of 0.5. Moreover, it can be seen that higher volume fraction shows higher strain energy. This is consistent with the observations of Strobridge et al. [21]. It has been reported that when the porosity increases, the lithium concentration 90
105 gradient, which indicates battery degradation, decreases. Similarly, the lithium concentration gradient increases with a decrease in porosity [21]. That is, when we consider the relationship between volume fraction and porosity (volume fraction + porosity = 100%), higher volume fraction causes increases in lithium concentration gradient, which is strongly related the capacity loss. Figure 56: Effect of volume fraction on normalized strain energy (a) 1C, and (b) 6C (EC is used as an electrolyte) Conclusion Through the model 3, the effect of various parameters on normal stress and strain energy is investigated. We first calculate the lithiation from local concentration during discharging process (Figure 47). Due to the similarity between Fick s 2 nd law and heat equation, temperature gradient is used in the ANSYS simulations to represent the concentration gradient. 91
106 First, the effects of C-rate on mechanical stresses and strain energy are compared in Figure 51 and Figure 53. Normal stress and strain energy increase with the increasing C-rate. This could be explained by the relationship between the surface concentration and dimensionless discharging time (Figure 52). As lithiation proceeds, the stress and strain energy increases initially followed by a decrease after reaching the peak. Moreover, peak stresses at higher C-rate occur at high lithiation stage in LiFePO4 battery. It is supported by the results from Lim et al. [61]. By considering volume misfits during discharging, the maximum stress is observed at a relatively high lithiation stage instead of low lithiation one with lithium-poor phase. Next, the impact of volume fraction of the positive electrode on the magnitude of stress is portrayed in Figure 55. We observed similar trends with effect of volume fraction. The contour plot in Figure 54 suggests that increases in volume fraction increase both compressive and tensile stress in particles due to the pressure from electrolyte and the diffusion-induced stresses. 92
107 CHAPTER 5: SUMMARY AND CONCLUSIONS In this study, the coupled domains of electrolyte and the cathode particle are modeled to investigate the evolution of stress and strain energy during lithium-ion battery discharging. Model 1, model 2, and model 3 have been developed using Fluid Flow (CFX), Transient Thermal, and Static Structural modules in ANSYS Workbench. The main objective of this research is to provide a better understanding the effect of major parameters of cathode and electrolyte on the battery performance from the stress analysis point of view. Since the highest stress is observed on the surface of electrode particles [46] and normal stress is highly associated with the fracture in the electrode [16], stresses on the interface between electrolyte and electrode are investigated in the model 1. Through the model 1, it can be concluded that there is a linear relationship between mechanical stresses on the surface of cathode when C-rate increases (Figure 34). That is, higher C-rate results in higher internal stress inside the electrode, which may accelerate the crack initiation and progression. The impact of porosity of the positive electrode on the magnitude of stress is discussed in Figure 35. It can be seen that stress on the interface decreases exponentially as the porosity of electrode increases. This result is consistent with the results reported by Renganathan et al. [24]. Similar trend is also observed when the simulations are conducted with a smaller cubic domain (0.3μm). Among electrolyte properties, an influence of the electrolyte viscosity is crucial to the stresses on the surface of cathode (Figure 36). 93
108 However, model 1 did not consider the particle shapes, sizes, and molar volume misfits. Thus, in the model 2, we consider several details of cathode particles (Figure 29). Because of many interfaces between the electrolyte and the electrode in the model 2, the stresses at the specific position are compared (Figure 41). As expected, the stress on the forefront of particles is higher than any other particles. The relationship between the maximum normal stress and other parameters such as C-rate, volume fraction, and viscosity is discussed. It is observed that there is a linear relationship between the maximum stress and C-rate (Figure 42). From Figure 45, it is observed that the model with volume fraction of 30% shows higher normal stress than the model with a volume fraction of 23.6% due to intercalating surface in the half-cell system. It is also observed that diffusion-induced stress at position 1 is higher than any other positions due to more lithiation occurs at that location. It has been reported that this inhomogeneous reaction causes the performance degradation [7],[20],[21]. The impact of viscosity is discussed in Figure 46. As the distance from inlet boundary increases, the effect of the viscosity is less significant. It is concluded that in spite of high conductivity property of Ethylene Carbonate (EC), it is not preferred to be use EC due to its higher viscosity might results in higher mechanical stresses. Since the model 2 did not consider phase transformation during discharging, model 3 was developed to investigate the effect of various parameters on normal stress and strain energy. From the model 3, it is observed that the stress increases initially and after that stress decrease as lithiation proceed. This trend also has been reported in LiCoO2 [61] and LiMn2O4 [16] battery chemistries. Moreover, normal stress and strain energy increase with an 94
109 increasing C-rate (Figure 51 and Figure 53). It is due to that the surface concentration gradient is higher with higher C-rate (Figure 52). The impact of volume fraction of the positive electrode on the magnitude of stress is portrayed in Figure 55. The contour plots in Figure 54 suggest that an increase in volume fraction (i.e., particle sizes) increases both compressive and tensile stress in the half-cell system. Moreover, the higher stress on the forefront of particles has been observed in most cases. It suggests there is a need to relief stresses at a specific location on the electrode-electrolyte interface. Simulation results from three models confirm relationships between mechanical stresses and many design parameters for lithium-ion batteries. It is apparent that the impact of phase transformation dependent mechanical properties and lithium diffusion cannot be neglected for the stress prediction. Furthermore, these results can be used to predict fracture in cathode, which plays negative roles in a battery system. Our study provides quantitative information of mechanical effects in lithium-ion batteries during discharging, specifically, on the interface between electrolyte and electrode. 95
110 5.1 FUTURE WORK In this work, the simulation models have enormous potential for a future study. It has been reported that diffusion-induced stresses could possibly lead to the occurrence of Mode I, Mode II, and Mode III fractures [56]. With an initial crack inside particles of multiphysics models, fracture mechanics can be investigated more accurately during discharging. Since both physics (fluid flow and static structural) were already considered in our study, we could further improve both electrolyte and electrode at the same time. It has been observed that lithium ion diffusion coefficient in electrolyte and cathode is highly affected by the temperature [17],[55]. If the temperature effect in both domains is considered, mechanical stresses on the electrode-electrolyte interface could be reinvestigated. Moreover, cathode material is strongly affected by the surface formation and particle shapes. We could also improve our model by adding coating of the particles with a layer of carbon on the surface of the particle or using plate-like particles, which show higher conductivity by decreasing diffusion distance for lithium ions. Based on the developed models, another battery chemistries could be incorporated, such as LiCoO2, LiMn2O4 by simply changing the material properties and phase transformation mechanisms. Ultimately, the model will be improved by considering a separator and anode materials to be a whole cell system. It will allow us better understand whole battery charging and discharging mechanisms during cycling. 96
111 REFERENCES [1] Y. R. Wang and H. S. Huang, Lithium-Ion Battery Materials and Mechanical Stress Fields, Trans. Control Mech. Syst., vol. 1, no. 5, pp , [2] J.-M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature, vol. 414, no. November, pp , [3] J. Molenda and M. Mole, Composite Cathode Material for Li-Ion Batteries Based on LiFePO4 System., in Metal, Ceramic and Polymeric Composites for Various Uses, InTech, 2011, pp [4] J. B. G. A K Padhi, K S Nanjundaswamy, Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries, J. Electrochem. Soc, vol. 144, no. 4, [5] H. Lundgren, Thermal Aspects and Electrolyte Mass Transport in Lithium- ion Batteries, KTH Royal Institute of Technology, [6] W. C. Chueh, F. El Gabaly, J. D. Sugar, N. C. Bartelt, A. H. McDaniel, K. R. Fenton, K. R. Zavadil, T. Tyliszczak, W. Lai, and K. F. McCarty, Intercalation pathway in many-particle LiFePO4 electrode revealed by nanoscale state-of-charge mapping, Nano Lett., vol. 13, no. 3, pp ,
112 [7] J. Wang, Y. K. Chen-Wiegart, and J. Wang, In operando tracking phase transformation evolution of lithium iron phosphate with hard X-ray microscopy, Nat. Commun., vol. 5, pp. 1 10, [8] Samsung SDI, Cylindrical Rechargeable Laptop Li-ion(Lithium ion) Battery cells. [Online]. Available: [Accessed: 08-Sep-2015]. [9] BMW, BMW i8 : Technical data. [Online]. Available: [Accessed: 08-Sep-2015]. [10] Tesla Motors, Model S_Battery, Performance, and Drive options. [Online]. Available: [Accessed: 08-Sep-2015]. [11] V. Srinivasan and J. Newman, Discharge Model for the Lithium Iron-Phosphate Electrode, J. Electrochem. Soc., vol. 151, no. 10, p. A1517, [12] K. A. S. Joongpyo Shim, Azucena Sierra, THE DEVEOPMENT OF LOW COST LiFePO4-BASED HIGH POWER LITHIUM-ION BATTERIES, Berkeley, CA USA. [13] M. Kassem, J. Bernard, R. Revel, S. Pélissier, F. Duclaud, and C. Delacourt, Calendar aging of a graphite/lifepo4 cell, J. Power Sources, vol. 217, p. 574,
113 [14] P. Verma, P. Maire, and P. Novák, A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries, Electrochim. Acta, vol. 55, no. 22, pp , [15] M. Park, X. Zhang, M. Chung, G. B. Less, and A. M. Sastry, A review of conduction phenomena in Li-ion batteries, J. Power Sources, vol. 195, no. 24, pp , [16] M. Zhu, J. Park, and a. M. Sastry, Fracture Analysis of the Cathode in Li-Ion Batteries: A Simulation Study, J. Electrochem. Soc., vol. 159, no. 4, p. A492, [17] A. Guerfi, N. Ravet, P. Charest, M. Dontigny, M. Petitclerc, M. Gauthier, and K. Zaghib, Temperature Effects on LiFePO 4 Cathode Performance, J. Electrochem. Soc, vol. 3, no. 36, pp. 3 17, [18] Y.-D. Cho, G. T.-K. Fey, and H.-M. Kao, The effect of carbon coating thickness on the capacity of LiFePO4/C composite cathodes, J. Power Sources, vol. 189, no. 1, pp , [19] N. Dupré, J. F. Martin, J. Degryse, V. Fernandez, P. Soudan, and D. Guyomard, Aging of the LiFePO4 positive electrode interface in electrolyte, J. Power Sources, vol. 195, no. 21, pp , [20] Y. Li, F. El Gabaly, T. R. Ferguson, R. B. Smith, N. C. Bartelt, J. D. Sugar, K. R. Fenton, D. A. Cogswell, A. L. D. Kilcoyne, T. Tyliszczak, M. Z. Bazant, and W. C. 99
114 Chueh, Current-induced transition from particle-by particle to concurrent intercalation in phase-separating battery electrodes, Nat. Mater., vol. 13, no. September, pp , [21] F. C. Strobridge, B. Orvananos, M. Croft, H.-C. Yu, R. Robert, H. Liu, Z. Zhong, T. Connolley, M. Drakopoulos, K. Thornton, and C. P. Grey, Mapping the Inhomogeneous Electrochemical Reaction Through Porous LiFePO4-Electrodes in a Standard Coin Cell Battery, Chem. Mater., p , [22] G. Chen, X. Song, and T. J. Richardson, Electron Microscopy Study of the LiFePO[sub 4] to FePO[sub 4] Phase Transition, Electrochem. Solid-State Lett., vol. 9, no. 6, p. A295, [23] D. Wang, X. Wu, Z. Wang, and L. Chen, Cracking causing cyclic instability of LiFePO4 cathode material, J. Power Sources, vol. 140, no. 1, pp , [24] S. Renganathan, G. Sikha, S. Santhanagopalan, and R. E. White, Theoretical Analysis of Stresses in a Lithium Ion Cell, J. Electrochem. Soc., vol. 157, no. 2, p. A155, [25] L. O. Valo en and J. N. Reimers, Transport Properties of LiPF6-Based Li-Ion Battery Electrolytes, J. Electrochem. Soc., vol. 152, no. 5, p. A882, [26] G. P. Gholam-Abbas Nazri, Lithium Batteries: Science and Technology. Springer Science & Business Media,
115 [27] P. K. Muhuri and D. K. Hazra, Density and Viscosity for Propylene Carbonate + 1,2- Dimethoxyethane at , , and K, J. Chem. Eng. Data, vol. 39, pp , [28] A. Rodríguez, J. Canosa, A. Domínguez, and J. Tojo, Viscosities of dimethyl carbonate or diethyl carbonate with alkanes at four temperatures. New UNIFAC- VISCO parameters, J. Chem. Eng. Data, vol. 48, no. 1, pp , [29] D. Aurbach, Y. Talyosef, B. Markovsky, E. Markevich, E. Zinigrad, L. Asraf, J. S. Gnanaraj, and H. J. Kim, Design of electrolyte solutions for Li and Li-ion batteries, Electrochim. Acta, vol. 50, no. 2 3 SPEC. ISS., pp , [30] A. Franco, Rechargeable Lithium Batteries: From Fundamentals to Applications. Elsevier Science, [31] K. L. Gering, Prediction of electrolyte viscosity for aqueous and non-aqueous systems: Results from a molecular model based on ion solvation and a chemical physics framework, Electrochim. Acta, vol. 51, no. 15, pp , [32] D. Aurbach, B. Markovsky, G. Salitra, E. Markevich, Y. Talyossef, M. Koltypin, L. Nazar, B. Ellis, and D. Kovacheva, Review on electrode-electrolyte solution interactions, related to cathode materials for Li-ion batteries, J. Power Sources, vol. 165, no. 2, pp ,
116 [33] B. Vijayaraghavan, D. R. Ely, Y.-M. Chiang, R. Garciá-Garciá, and R. E. Garciá, An Analytical Method to Determine Tortuosity in Rechargeable Battery Electrodes, J. Electrochem. Soc., vol. 159, no. 5, p. A548, [34] G. J. Bauer, A Coupled Finite Element Approach for Electrochemical Systems, TECHNISCHE UNIVERSITÄT MÜNCHEN, [35] A. Nyman, M. Behm, and G. Lindbergh, A Theoretical and Experimental Study of the Mass Transport in Gel Electrolytes II. Experimental Characterization of LiPF6- EC-PC-P(VdF-HFP), J. Electrochem. Soc., vol. 158, no. 6, p. A636, [36] A. Nyman, M. Behm, and G. Lindbergh, Electrochemical characterisation and modelling of the mass transport phenomena in LiPF6-EC-EMC electrolyte, Electrochim. Acta, vol. 53, no. 22, pp , [37] E. Prada, D. Di Domenico, Y. Creff, J. Bernard, V. Sauvant-Moynot, and F. Huet, A Simplified Electrochemical and Thermal Aging Model of LiFePO4-Graphite Li-ion Batteries: Power and Capacity Fade Simulations, J. Electrochem. Soc., vol. 160, no. 4, pp. A616 A628, Feb [38] K. Edström, T. Gustafsson, and J. O. Thomas, The cathode electrolyte interface in the Li-ion battery, Electrochim. Acta, vol. 50, no. 2 3, pp , Nov [39] H. JU, J. WU, and Y. XU, Revisiting the electrochemical impedance behaviour of the LiFePO4/C cathode, J. Chem. Sci, vol. 125, no. 3, pp ,
117 [40] K. Zhong, Y. Cui, X. De Xia, J. J. Xue, P. Liu, and Y. X. Tong, Study on the stability of the LiFePO4 Li-ion battery via an electrochemical method, J. Power Sources, vol. 250, pp , [41] N. Meethong, Y.-H. Kao, M. Tang, H.-Y. Huang, W. C. Carter, and Y.-M. Chiang, Electrochemically Induced Phase Transformation in Nanoscale Olivines LiMPO4 (M = Fe, Mn), Chem. Mater., vol. 20, no. 19, pp , [42] M. Tang, J. F. Belak, and M. R. Dorr, Anisotropic phase boundary morphology in nanoscale olivine electrode particles, J. Phys. Chem. C, vol. 115, pp , [43] R. Deshpande, Y.-T. Cheng, M. W. Verbrugge, and A. Timmons, Diffusion Induced Stresses and Strain Energy in a Phase-Transforming Spherical Electrode Particle, J. Electrochem. Soc., vol. 158, no. 6, p. A718, [44] C.-K. ChiuHuang and H.-Y. Shadow Huang, Stress Evolution on the Phase Boundary in LiFePO4 Particles, J. Electrochem. Soc., vol. 160, no. 11, pp. A2184 A2188, [45] Y.-T. Cheng and M. W. Verbrugge, Diffusion-Induced Stress, Interfacial Charge Transfer, and Criteria for Avoiding Crack Initiation of Electrode Particles, J. Electrochem. Soc., vol. 157, no. 4, p. A508,
118 [46] V. Malavé, J. R. Berger, H. Zhu, and R. J. Kee, A computational model of the mechanical behavior within reconstructed LixCoO2 Li-ion battery cathode particles, Electrochim. Acta, vol. 130, pp , [47] J. Christensen, Modeling Diffusion-Induced Stress in Li-Ion Cells with Porous Electrodes, J. Electrochem. Soc., vol. 157, no. 3, p. A366, [48] X. Zhang, W. Shyy, and A. Marie Sastry, Numerical Simulation of Intercalation- Induced Stress in Li-Ion Battery Electrode Particles, J. Electrochem. Soc., vol. 154, p. S21, [49] T. Maxisch and G. Ceder, Elastic properties of olivine LixFePO4 from first principles, Phys. Rev. B, vol. 73, no. 17, pp. 1 4, [50] G. Kobayashi, S. I. Nishimura, M.-S. S. Park, R. Kanno, M. Yashima, T. Ida, and A. Yamada, Isolation of Solid Solution Phases in Size-Controlled LixFePO4 at Room Temperature, Adv. Funct. Mater., vol. 19, no. 3, pp , [51] F. Sauvage, E. Baudrin, M. Morcrette, and J.-M. Tarascon, Pulsed Laser Deposition and Electrochemical Properties of LiFePO4 Thin Films, Electrochem. Solid-State Lett., vol. 7, no. 1, p. A15, [52] M. Z. Bazant, Theory of chemical kinetics and charge transfer based on nonequilibrium thermodynamics, Acc. Chem. Res., vol. 46, no. 5, pp ,
119 [53] G. Brunetti, D. Robert, P. Bayle-Guillemaud, J. L. Ouvi, E. F. Rauch, J. F. Martin, J. F. Colin, F. Bertin, and C. Cayron, Confirmation of the Domino-Cascade Model by LiFePO4 / FePO4 Precession Electron Diffraction, Chem. Mater., vol. 23, pp , [54] M. Abonnenc, J. Josserand, and H. H. Girault, Sandwich mixer-reactor: influence of the diffusion coefficient and flow rate ratios., Lab Chip, vol. 9, no. 3, pp , [55] K. Hayamizu, Temperature dependence of self-diffusion coefficients of ions and solvents in ethylene carbonate, propylene carbonate, and diethyl carbonate single solutions and ethylene carbonate + diethyl carbonate binary solutions of LiPF 6 studied by NMR, J. Chem. Eng. Data, vol. 57, no. 7, pp , [56] C.-K. ChiuHuang and H.-Y. S. Huang, Critical lithiation for C-rate dependent mechanical stresses in LiFePO4, J. Solid State Electrochem., [57] A. V. Churikov, A. V. Ivanishchev, I. a. Ivanishcheva, V. O. Sycheva, N. R. Khasanova, and E. V. Antipov, Determination of lithium diffusion coefficient in LiFePO4 electrode by galvanostatic and potentiostatic intermittent titration techniques, Electrochim. Acta, vol. 55, no. 8, pp , [58] Q. Liu, H. He, Z. Li, Y. Liu, Y. Ren, W. Lu, J. Lu, E. A. Stach, and J. Xie, Rate- Dependent, Li-Ion Insertion / Deinsertion Behavior of LiFePO 4 Cathodes in Commercial LiFePO 4 Cells,
120 [59] Y.-H. Chen, C.-W. Wang, G. Liu, X.-Y. Song, V. S. Battaglia, and a. M. Sastry, Selection of Conductive Additives in Li-Ion Battery Cathodes, J. Electrochem. Soc., vol. 154, no. 10, p. A978, [60] H. V. Venkatasetty, Electrolytes for rechargeable lithium batteries. Elsevier Ltd., [61] C. Lim, B. Yan, L. Yin, and L. Zhu, Simulation of diffusion-induced stress using reconstructed electrodes particle structures generated by micro/nano-ct, Electrochim. Acta, vol. 75, pp ,
121 APPENDIX A1: Electrode Material Property Change during Discharging Figure A1 : Phase transformation equation. Figure A2: According to phase transformation, change of elastic material properties (temperature 0: lithium-poor phase, temperature 1: lithium-rich phase). 107
122 Figure A3: Thermal properties of electrode. 108
123 A2: Material Property of Electrolyte Figure A4: Material properties of Ethylene Carbonate. 109
124 A3: Supplementary Information on Model 1 Figure A5: Mesh details of model 1 in Fluid Flow (CFX) module 110
125 Figure A6: Mesh details of model 1 in Static Structural module 111
126 Figure A7: SOLID185 3D 8-Node Hexahedral Structural Solid element 112
127 Figure A8: Lithiation stage calculation in model
128 Figure A9: Normal stress across electrode. 114
129 A3: Supplementary Information on Model 2 Figure A10: Mesh details of model 2 in Fluid Flow (CFX) module 115
130 Figure A11: Mesh details of model 2 in Static Structural module 116
131 Figure A12: SOLID92 3D 10-Node Tetrahedral Structural Solid element 117
132 Figure A13: Top view of model2 with 75nm, 100nm, and 125nm particles. Figure A14: (a) Pressure distribution caused by electrolyte, (b) normal stress, and (c) shear stress in 1C. 118
133 Figure A15: (a) Pressure distribution caused by electrolyte, (b) normal stress, and (c) shear stress in 5C. Figure A16: (a) Pressure distribution caused by electrolyte, (b) normal stress, and (c) shear stress in 10C. Figure A17: (a) Pressure distribution caused by electrolyte, (b) normal stress, and (c) shear stress in 20C. 119
134 A4: Supplementary Information on Model 3 Figure A18: Thermal properties of LiFePO 4 in model
135 Figure A19: Thermal properties of ethylene carbonate in model
136 Figure A20: Surface nodes and elements to calculate surface concentration. 122
 Energy Storage Need!! Batteries provide the vital storage link between energy generation and consumption around the world.!! Energy demand is increasing, thus new generation facilities are being built
Energy Storage Need!! Batteries provide the vital storage link between energy generation and consumption around the world.!! Energy demand is increasing, thus new generation facilities are being built
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral
 Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Kuang-Che Hsiao. Supervisor: Prof. Tony West
 New Potential Cathode Materials for Lithium-ion ion Battery - Synthesis and characterization of Li 1+x FePO 4-x N x cathode - Kuang-Che Hsiao Supervisor: Prof. Tony West 08/06/2010 E-mail: dtp09kh@sheffield.ac.uk
New Potential Cathode Materials for Lithium-ion ion Battery - Synthesis and characterization of Li 1+x FePO 4-x N x cathode - Kuang-Che Hsiao Supervisor: Prof. Tony West 08/06/2010 E-mail: dtp09kh@sheffield.ac.uk
Supplementary Figure 1:
 b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
Factors Influencing the Thermal Stability of Lithium Ion Batteries - From Active Materials to State-of-Charge and Degradation
 Factors Influencing the Thermal Stability of Lithium Ion Batteries - From Active Materials to State-of-Charge and Degradation JRC Exploratory Research Workshop Safer Li-Ion Batteries by Preventing Thermal
Factors Influencing the Thermal Stability of Lithium Ion Batteries - From Active Materials to State-of-Charge and Degradation JRC Exploratory Research Workshop Safer Li-Ion Batteries by Preventing Thermal
Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries
 PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
A Quantum Leap Forward for Li-Ion Battery Cathodes
 A Quantum Leap Forward for Li-Ion Battery Cathodes Josh Thomas Ångström Advanced Battery Centre, Uppsala University, Sweden. josh.thomas@mkem.uu.se GCEP Research Symposium: Energy Research Five Years and
A Quantum Leap Forward for Li-Ion Battery Cathodes Josh Thomas Ångström Advanced Battery Centre, Uppsala University, Sweden. josh.thomas@mkem.uu.se GCEP Research Symposium: Energy Research Five Years and
Novel Materials for Lithium-Ion Batteries
 Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
THERMOELECTRIC EFFECTS OF SIZE OF MICROCHANNELS ON AN INTERNALLY COOLED LI-ION BATTERY CELL
 Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition IMECE2016 November 11-17, 2016, Phoenix, Arizona, USA IMECE2016-65729 THERMOELECTRIC EFFECTS OF SIZE OF MICROCHANNELS
Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition IMECE2016 November 11-17, 2016, Phoenix, Arizona, USA IMECE2016-65729 THERMOELECTRIC EFFECTS OF SIZE OF MICROCHANNELS
Nanocrystalline LiFePO4 as cathode material for lithium battery applications S.C SIAH
 Nanocrystalline LiFePO as cathode material for lithium battery applications Abstract S.C SIAH Engineering Science Programme, National University of Singapore Kent Ridge, Singapore 119260 LiFePO was prepared
Nanocrystalline LiFePO as cathode material for lithium battery applications Abstract S.C SIAH Engineering Science Programme, National University of Singapore Kent Ridge, Singapore 119260 LiFePO was prepared
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201704947 Bioinspired, Spine-Like, Flexible, Rechargeable Lithium-Ion
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201704947 Bioinspired, Spine-Like, Flexible, Rechargeable Lithium-Ion
Overview of research
 MICDE-TARDEC Faculty Workshop Overview of research Wei Lu Mechanical Engineering University of Michigan, Ann Arbor weilu@umich.edu September 15, 2017 1 Overall of Research Areas Joining of dissimilar materials,
MICDE-TARDEC Faculty Workshop Overview of research Wei Lu Mechanical Engineering University of Michigan, Ann Arbor weilu@umich.edu September 15, 2017 1 Overall of Research Areas Joining of dissimilar materials,
Supplementary Figure 1 The lithium polysulfide distribution on the patterned electrode.
 Supplementary Figure 1.The lithium polysulfide distribution on the patterned electrode. SEM image of the ITO-carbon electrode after dipping into Li 2 S 8 solution and drying, which shows the random distribution
Supplementary Figure 1.The lithium polysulfide distribution on the patterned electrode. SEM image of the ITO-carbon electrode after dipping into Li 2 S 8 solution and drying, which shows the random distribution
Thermal Behavior of Charged Cathode Materials Studied by Synchrotron-Based X-ray Techniques
 Thermal Behavior of Charged Cathode Materials Studied by Synchrotron-Based X-ray Techniques Won-Sub Yoon 1, *, Kyung-Wan Nam 2, Kyung Yoon Chung 3, Mahalingam Balasubramanian 4, Dong-Hyuk Jang 1, Joengbae
Thermal Behavior of Charged Cathode Materials Studied by Synchrotron-Based X-ray Techniques Won-Sub Yoon 1, *, Kyung-Wan Nam 2, Kyung Yoon Chung 3, Mahalingam Balasubramanian 4, Dong-Hyuk Jang 1, Joengbae
Supporting Information for
 Supporting Information for Self-stabilized solid electrolyte interface on host-free Li metal anode towards high areal capacity and rate utilization Zhenglin Hu 1,3, Shu Zhang 1, Shanmu Dong*,1, Quan Li
Supporting Information for Self-stabilized solid electrolyte interface on host-free Li metal anode towards high areal capacity and rate utilization Zhenglin Hu 1,3, Shu Zhang 1, Shanmu Dong*,1, Quan Li
Chapter 7. Evaluation of Electrode Performance by. Electrochemical Impedance
 Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells
 Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Application Note. 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy
 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy Authors: Dr. Claus
4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy 4D Study of Silicon Anode Volumetric Changes in a Coin Cell Battery using X-ray Microscopy Authors: Dr. Claus
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
Electrochemical performance of lithium-rich layered oxides for
 IBA 2013 Electrochemical performance of lithium-rich layered oxides for electric vehicle applications Jay Hyok Song, Andrei Kapylou, Chang Wook Kim, Yong Chan You, and Sun Ho Kang* SAMSUNG SDI Contents
IBA 2013 Electrochemical performance of lithium-rich layered oxides for electric vehicle applications Jay Hyok Song, Andrei Kapylou, Chang Wook Kim, Yong Chan You, and Sun Ho Kang* SAMSUNG SDI Contents
Investigations on Fatigue of Li-ion batteries
 Investigations on Fatigue of Li-ion batteries HELMUT EHRENBERG INSTITUTE FOR APPLIED MATERIALS ENERGY STORAGE SYSTEMS (IAM-ESS) KIT University of the State of Baden-Wuerttemberg and National Research Center
Investigations on Fatigue of Li-ion batteries HELMUT EHRENBERG INSTITUTE FOR APPLIED MATERIALS ENERGY STORAGE SYSTEMS (IAM-ESS) KIT University of the State of Baden-Wuerttemberg and National Research Center
Supplementary Figure 1. SEM images of LiCoO 2 before (a) and after (b) electrochemical tuning. The size and morphology of synthesized LiCoO 2 and
 Supplementary Figure 1. SEM images of LiCoO 2 before (a) and after (b) electrochemical tuning. The size and morphology of synthesized LiCoO 2 and De-LiCoO 2 particles were almost the same, indicating that
Supplementary Figure 1. SEM images of LiCoO 2 before (a) and after (b) electrochemical tuning. The size and morphology of synthesized LiCoO 2 and De-LiCoO 2 particles were almost the same, indicating that
From Surface To Cell: Understanding the Lithium Ion Battery. The world leader in serving science
 From Surface To Cell: Understanding the Lithium Ion Battery 1 The world leader in serving science Content Discharge Detail the Li-ion Battery industry drivers & trends Our position in industry and our
From Surface To Cell: Understanding the Lithium Ion Battery 1 The world leader in serving science Content Discharge Detail the Li-ion Battery industry drivers & trends Our position in industry and our
Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery
 JFE TECHNICAL REPORT No. 22 (Mar. 2017) Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery SIMAUCHI Yutaka *1 OHMORI Shigekazu *2 IKEMOTO Sachi *3 Abstract: analyzed the microstructure
JFE TECHNICAL REPORT No. 22 (Mar. 2017) Microscopic Structural Analysis of Advanced Anode Material for Lithium Battery SIMAUCHI Yutaka *1 OHMORI Shigekazu *2 IKEMOTO Sachi *3 Abstract: analyzed the microstructure
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
Factors Governing Life of High-Energy Lithium-Ion Cells
 Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Cycle life performance of lithium-ion pouch cells
 Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Model Prediction and Experiments for the Electrode Design Optimization of LiFePO 4 /Graphite Electrodes in High Capacity Lithium-ion Batteries
 Model Prediction and Experiments for the Electrode Design Optimization Bull. Korean Chem. Soc. 2013, Vol. 34, No. 1 79 http://dx.doi.org/10.5012/bkcs.2013.34.1.79 Model Prediction and Experiments for the
Model Prediction and Experiments for the Electrode Design Optimization Bull. Korean Chem. Soc. 2013, Vol. 34, No. 1 79 http://dx.doi.org/10.5012/bkcs.2013.34.1.79 Model Prediction and Experiments for the
All-solid-state Li battery using a light-weight solid electrolyte
 All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
All-solid-state Li battery using a light-weight solid electrolyte Hitoshi Takamura Department of Materials Science, Graduate School of Engineering, Tohoku University Europe-Japan Symposium, Electrical
Reliability of Li-ion Batteries
 IEEE Boston Reliability Seminar 9/11/13 Reliability of Li-ion Batteries Martin Z. Bazant Chemical Engineering & Mathematics MIT Matthew Pinson Peng Bai Dan Cogswell Todd Ferguson Alan Millner Prior funding
IEEE Boston Reliability Seminar 9/11/13 Reliability of Li-ion Batteries Martin Z. Bazant Chemical Engineering & Mathematics MIT Matthew Pinson Peng Bai Dan Cogswell Todd Ferguson Alan Millner Prior funding
Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from
 Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from polyimide used to seal Titanium (Ti) current collectors
Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from polyimide used to seal Titanium (Ti) current collectors
Supporting Information
 Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Accumulation (%) Amount (%) Particle Size 0.1
 100 10 Amount (%) 5 50 Accumulation (%) 0 0.1 1 Particle Size (µm) 10 0 Supplementary Figure 1. The particle size distribution of W-15 at% Cr after 20 hours milling. Supplementary Figure 2. a,b, X-ray
100 10 Amount (%) 5 50 Accumulation (%) 0 0.1 1 Particle Size (µm) 10 0 Supplementary Figure 1. The particle size distribution of W-15 at% Cr after 20 hours milling. Supplementary Figure 2. a,b, X-ray
Sn Wears Super Skin: A New Design For Long Cycling Batteries
 Supporting Information Wears Super Skin: A New Design For Long Cycling Batteries Shuai Kang, Xi Chen, Junjie Niu* Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee
Supporting Information Wears Super Skin: A New Design For Long Cycling Batteries Shuai Kang, Xi Chen, Junjie Niu* Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee
BATTERY SOLUTIONS WITH KYNAR PVDF LITHIUM-ION FOCUS
 BATTERY SOLUTIONS WITH KYNAR PVDF LITHIUM-ION FOCUS BY 2025, THE WORLD WILL MANUFACTURE 8 BILLION LI-ION CELLS Continued market growth requires rapid advances in higher energy density, higher performance
BATTERY SOLUTIONS WITH KYNAR PVDF LITHIUM-ION FOCUS BY 2025, THE WORLD WILL MANUFACTURE 8 BILLION LI-ION CELLS Continued market growth requires rapid advances in higher energy density, higher performance
Maximizing Hydrogen Production of A Solid Oxide Electrolyser Cell
 212 International Conference on Clean and Green Energy IPCBEE vol.27 (212) (212) IACSIT Press, Singapore Maximizing Hydrogen Production of A Solid xide Electrolyser Cell Qiong Cai 1+, Claire S. Adjiman
212 International Conference on Clean and Green Energy IPCBEE vol.27 (212) (212) IACSIT Press, Singapore Maximizing Hydrogen Production of A Solid xide Electrolyser Cell Qiong Cai 1+, Claire S. Adjiman
PASSIVE THERMAL MANAGEMENT OF LITHIUM-ION BATTERIES USING LATENT HEAT STORAGE MATERIALS
 PASSIVE THERMAL MANAGEMENT OF LITHIUM-ION BATTERIES USING LATENT HEAT STORAGE MATERIALS WHITE PAPER Joe Kelly - Materials Scientist, March 2015 Rev: 2 INTRODUCTION As demand steadily grows for more powerful
PASSIVE THERMAL MANAGEMENT OF LITHIUM-ION BATTERIES USING LATENT HEAT STORAGE MATERIALS WHITE PAPER Joe Kelly - Materials Scientist, March 2015 Rev: 2 INTRODUCTION As demand steadily grows for more powerful
CHAPTER-VII SUMMARY AND CONCLUSIONS
 CHAPTER-VII SUMMARY AND CONCLUSIONS Chapter-VII Summary and Conclusions Sr. No. Title Page No. 7.1 Summary 167 7.2 Conclusions.. 171 CHAPTER SEVEN Summary and Conclusions 7.1: Summary The technologies
CHAPTER-VII SUMMARY AND CONCLUSIONS Chapter-VII Summary and Conclusions Sr. No. Title Page No. 7.1 Summary 167 7.2 Conclusions.. 171 CHAPTER SEVEN Summary and Conclusions 7.1: Summary The technologies
measurements. The spectra show electrolyte in contact with the carbon fibre based gas diffusion layer
 Supplementary Figure 1. 7 Li NMR spectra of electrolyte in contact with potential substrates for in-situ NMR measurements. The spectra show electrolyte in contact with the carbon fibre based gas diffusion
Supplementary Figure 1. 7 Li NMR spectra of electrolyte in contact with potential substrates for in-situ NMR measurements. The spectra show electrolyte in contact with the carbon fibre based gas diffusion
Supporting Information
 Supporting Information Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries Xuanpeng Wang, Xiaoming Xu, Chaojiang Niu*, Jiashen Meng, Meng Huang, Xiong Liu,
Supporting Information Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries Xuanpeng Wang, Xiaoming Xu, Chaojiang Niu*, Jiashen Meng, Meng Huang, Xiong Liu,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION SUPPLEMENTARY DISCUSSION AND FIGURES 1. Chemical and Structural Characterization (a) Grazing-incidence small-angle X-ray scattering (GISAXS) The structural evolution of the mesoporous
SUPPLEMENTARY INFORMATION SUPPLEMENTARY DISCUSSION AND FIGURES 1. Chemical and Structural Characterization (a) Grazing-incidence small-angle X-ray scattering (GISAXS) The structural evolution of the mesoporous
Investigations on polarization losses in planar Solid Oxide Fuel Cells
 Investigations on polarization losses in planar Solid Oxide Fuel Cells S.Senthil Kumar, Akshay Iyer, B. ShriPrakash, S.T. Aruna CSIR National Aerospace Laboratories Bangalore-560017 Presentation at COMSOL
Investigations on polarization losses in planar Solid Oxide Fuel Cells S.Senthil Kumar, Akshay Iyer, B. ShriPrakash, S.T. Aruna CSIR National Aerospace Laboratories Bangalore-560017 Presentation at COMSOL
De-ionized water. Nickel target. Supplementary Figure S1. A schematic illustration of the experimental setup.
 Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE POROSITY EVOLUTION MODEL
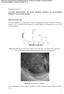 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Supporting Information
 Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
Batteries for Vehicular Applications
 Batteries for Vehicular Applications Venkat Srinivasan * Staff Scientist Lawrence Berkeley National Laboratory March 2, 2008 *vsrinivasan@lbl.gov Range Specific c Energy (W Wh/kg) 1000 100 10 Relative
Batteries for Vehicular Applications Venkat Srinivasan * Staff Scientist Lawrence Berkeley National Laboratory March 2, 2008 *vsrinivasan@lbl.gov Range Specific c Energy (W Wh/kg) 1000 100 10 Relative
Particle Size Effects in Lithium ion batteries
 Particle Size Effects in Lithium ion batteries Marnix Wagemaker1, Swapna Ganapathy1, Deepak P. Singh1, Wouter J.H. Borghols1,2, Ugo Lafont1, Lucas Haverkate1, Vanessa Peterson3, Gordon J. Kearley3, Fokko
Particle Size Effects in Lithium ion batteries Marnix Wagemaker1, Swapna Ganapathy1, Deepak P. Singh1, Wouter J.H. Borghols1,2, Ugo Lafont1, Lucas Haverkate1, Vanessa Peterson3, Gordon J. Kearley3, Fokko
In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries
 Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
Towards High-Safety Potassium-Sulfur Battery Using. Potassium Polysulfide Catholyte and Metal-Free Anode
 Supporting Information Towards High-Safety Potassium-Sulfur Battery Using Potassium Polysulfide Catholyte and Metal-Free Anode Jang-Yeon Hwang, Hee Min Kim, Chong S. Yoon, Yang-Kook Sun* Department of
Supporting Information Towards High-Safety Potassium-Sulfur Battery Using Potassium Polysulfide Catholyte and Metal-Free Anode Jang-Yeon Hwang, Hee Min Kim, Chong S. Yoon, Yang-Kook Sun* Department of
Fundamental Chemistry of Sion Power Li/S Battery. Yuriy Mikhaylik Sion Power Corporation, 9040 South Rita Road, Tucson, Arizona, 85747, USA
 Fundamental Chemistry of Sion Power Li/S Battery Yuriy Mikhaylik Sion Power Corporation, 9040 South Rita Road, Tucson, Arizona, 85747, USA Outline Thermodynamics of Li-S Discharge-charge mechanism in the
Fundamental Chemistry of Sion Power Li/S Battery Yuriy Mikhaylik Sion Power Corporation, 9040 South Rita Road, Tucson, Arizona, 85747, USA Outline Thermodynamics of Li-S Discharge-charge mechanism in the
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016)
 6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
Design and fabrication of all-solid-state rechargeable lithium batteries using ceramic electrolytes
 International Symposium on Electrical Fatigue in Functional Materials September 15, 2014 Sellin, Rügen, Germany Design and fabrication of all-solid-state rechargeable lithium batteries using ceramic electrolytes
International Symposium on Electrical Fatigue in Functional Materials September 15, 2014 Sellin, Rügen, Germany Design and fabrication of all-solid-state rechargeable lithium batteries using ceramic electrolytes
COMSOL Multiphysics Simulation of Flow in a Radial Flow Fixed Bed Reactor (RFBR)
 COMSOL Multiphysics Simulation of Flow in a Radial Flow Fixed Bed Reactor (RFBR) Anthony G. Dixon *,1, Dominic Polcari 1, Anthony Stolo 1 and Mai Tomida 1 1 Department of Chemical Engineering, Worcester
COMSOL Multiphysics Simulation of Flow in a Radial Flow Fixed Bed Reactor (RFBR) Anthony G. Dixon *,1, Dominic Polcari 1, Anthony Stolo 1 and Mai Tomida 1 1 Department of Chemical Engineering, Worcester
Supporting Information. Carbon Welding by Ultrafast Joule Heating
 Supporting Information Carbon Welding by Ultrafast Joule Heating Yonggang Yao, 1,(a) Kun Fu, 1,(a) Shuze Zhu, 2 Jiaqi Dai, 1 Yanbin Wang, 1 Glenn Pastel, 1 Yanan Chen, 1 Tian Li, 1 Chengwei Wang, 1 Teng
Supporting Information Carbon Welding by Ultrafast Joule Heating Yonggang Yao, 1,(a) Kun Fu, 1,(a) Shuze Zhu, 2 Jiaqi Dai, 1 Yanbin Wang, 1 Glenn Pastel, 1 Yanan Chen, 1 Tian Li, 1 Chengwei Wang, 1 Teng
Enhancement of connectivity and flux pinning in MgB2 superconducting bulks and wires
 University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2009 Enhancement of connectivity and flux pinning in MgB2 superconducting
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2009 Enhancement of connectivity and flux pinning in MgB2 superconducting
PVP-Functionalized Nanometer Scale Metal Oxide Coatings for. Cathode Materials: Successful Application to LiMn 2 O 4 Spinel.
 PVP-Functionalized Nanometer Scale Metal Oxide Coatings for Cathode Materials: Successful Application to LiMn 2 O 4 Spinel Nanoparticles Hyesun Lim, Jaephil Cho* Department of Applied Chemistry Hanyang
PVP-Functionalized Nanometer Scale Metal Oxide Coatings for Cathode Materials: Successful Application to LiMn 2 O 4 Spinel Nanoparticles Hyesun Lim, Jaephil Cho* Department of Applied Chemistry Hanyang
Development of LSCF: CGO Composite Cathodes for SOFCs by Suspension Spraying and Sintering
 Development of LSCF: CGO Composite Cathodes for SOFCs by Suspension Spraying and Sintering R. Costa *, R. Spotorno, Z. Ilhan, A. Ansar German Aerospace Center, Institute of Technical Thermodynamics, Pfaffenwaldring
Development of LSCF: CGO Composite Cathodes for SOFCs by Suspension Spraying and Sintering R. Costa *, R. Spotorno, Z. Ilhan, A. Ansar German Aerospace Center, Institute of Technical Thermodynamics, Pfaffenwaldring
Advanced Lithium-ion Battery Manufacturing R&D
 EVS28 KINTEX, Korea, May 3-6, 2015 Advanced Lithium-ion Battery Manufacturing R&D James F. Miller Argonne National Laboratory, Argonne, Illinois, USA 60439 Introduction I. The cost of lithium-ion batteries
EVS28 KINTEX, Korea, May 3-6, 2015 Advanced Lithium-ion Battery Manufacturing R&D James F. Miller Argonne National Laboratory, Argonne, Illinois, USA 60439 Introduction I. The cost of lithium-ion batteries
Next Generation Anodes for Li-Ion Cells: How to Achieve Both High Capacity and Cycle Stability When Using Silicon Metal
 Next Generation Anodes for Li-Ion Cells: How to Achieve Both High Capacity and Cycle Stability When Using Silicon Metal introduction Jeff Norris CEO +1.803.528.0941 JNorris@ParacleteEnergy.com Michigan
Next Generation Anodes for Li-Ion Cells: How to Achieve Both High Capacity and Cycle Stability When Using Silicon Metal introduction Jeff Norris CEO +1.803.528.0941 JNorris@ParacleteEnergy.com Michigan
The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning
 2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
IBM Almaden June 27, Seongmin Ha, Dongho Koo, Kyu Tae Lee * Chemical and Biological Engineering Seoul National University
 IBM Almaden June 27, 2017 Seongmin Ha, Dongho Koo, Kyu Tae Lee * Chemical and Biological Engineering Seoul National University (ktlee@snu.ac.kr) 1) Introduction 2) Failure mechanism of a redox mediator
IBM Almaden June 27, 2017 Seongmin Ha, Dongho Koo, Kyu Tae Lee * Chemical and Biological Engineering Seoul National University (ktlee@snu.ac.kr) 1) Introduction 2) Failure mechanism of a redox mediator
Electrochemical impedance study of initial lithium ion intercalation into graphite powders
 Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Superior Additive of Exfoliated RuO 2 Nanosheet. Oxide over Graphene
 Supporting Information Superior Additive of Exfoliated RuO 2 Nanosheet for Optimizing the Electrode Performance of Metal Oxide over Graphene Seul Lee, a, Xiaoyan Jin a, In Young Kim, a Tae-Ha Gu, a Ji-Won
Supporting Information Superior Additive of Exfoliated RuO 2 Nanosheet for Optimizing the Electrode Performance of Metal Oxide over Graphene Seul Lee, a, Xiaoyan Jin a, In Young Kim, a Tae-Ha Gu, a Ji-Won
Andrew Lamb Magefekt. Batteries
 Andrew Lamb Magefekt Batteries Batteries Notes Where the fuel goes Energy density batteries What is a battery A Battery is a reversible chemical process that requires the movement of Electrons to reduce
Andrew Lamb Magefekt Batteries Batteries Notes Where the fuel goes Energy density batteries What is a battery A Battery is a reversible chemical process that requires the movement of Electrons to reduce
Supporting Information. Hematite photoanode with gradient structure shows an unprecedentedly low onset
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information Hematite photoanode with gradient structure shows an unprecedentedly
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information Hematite photoanode with gradient structure shows an unprecedentedly
Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface
 Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface Xiaobiao Wu a, Sihui Wang a, Xiaochen Lin a, Guiming Zhong a, Zhengliang Gong
Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface Xiaobiao Wu a, Sihui Wang a, Xiaochen Lin a, Guiming Zhong a, Zhengliang Gong
PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES
 PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES A. Concheso, R. Santamaría, R. Menéndez, R. Alcántara #, P. Lavela #, J.L. Tirado # Instituto Nacional del Carbón (CSIC), Apdo.
PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES A. Concheso, R. Santamaría, R. Menéndez, R. Alcántara #, P. Lavela #, J.L. Tirado # Instituto Nacional del Carbón (CSIC), Apdo.
Supporting Information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information High performance All-Solid-State Li-Se Batteries induced
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information High performance All-Solid-State Li-Se Batteries induced
On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage
 On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage Pradeep R. Guduru School of Engineering, Brown University V. Sethuraman, M. Chon, S. Nadimpalli Collaborators:
On the Role of Mechanics in the Design and Performance of Electrode Materials for Energy Storage Pradeep R. Guduru School of Engineering, Brown University V. Sethuraman, M. Chon, S. Nadimpalli Collaborators:
Batteries for Mobile Applications
 Batteries for Mobile Applications Dr A.R. Armstrong and Dr A.D. Robertson, School of Chemistry, University of St. Andrews February 2002 Portable electronic devices are an increasingly vital element of
Batteries for Mobile Applications Dr A.R. Armstrong and Dr A.D. Robertson, School of Chemistry, University of St. Andrews February 2002 Portable electronic devices are an increasingly vital element of
Submitted By Maksim V. Tyufekchiev Somyi Hur. Submitted on April 23, 2013
 PROJECT NUMBER: MQP YW1-YW11 Developing a Low-Cost Methodology for Fabricating All-Solid-State Lithium-Ion Battery A Major Qualifying Project Submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE
PROJECT NUMBER: MQP YW1-YW11 Developing a Low-Cost Methodology for Fabricating All-Solid-State Lithium-Ion Battery A Major Qualifying Project Submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE
Morphology controlled synthesis of monodispersed manganese. sulfide nanocrystals and their primary application for supercapacitor
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Simulation of Atmospheric Air Micro Plasma Jet for Biomedical Applications
 Simulation of Atmospheric Air Micro Plasma Jet for Biomedical Applications Luke T. Gritter 1, Jeffrey S. Crompton *1, and Kyle C. Koppenhoefer 1 1 AltaSim Technologies, LLC 130 E. Wilson Bridge Rd, Suite
Simulation of Atmospheric Air Micro Plasma Jet for Biomedical Applications Luke T. Gritter 1, Jeffrey S. Crompton *1, and Kyle C. Koppenhoefer 1 1 AltaSim Technologies, LLC 130 E. Wilson Bridge Rd, Suite
SUPPLEMENTARY INFORMATION
 High Electrochemical Activity of the Oxide Phase in Model Ceria- and Ceria-Ni Composite Anodes William C. Chueh 1,, Yong Hao, WooChul Jung, Sossina M. Haile Materials Science, California Institute of Technology,
High Electrochemical Activity of the Oxide Phase in Model Ceria- and Ceria-Ni Composite Anodes William C. Chueh 1,, Yong Hao, WooChul Jung, Sossina M. Haile Materials Science, California Institute of Technology,
State of Lithium Ion Battery Research
 State of Lithium Ion Battery Research Professor Vanessa Wood Department of Information Technology and Electrical Engineering ETH Zürich 2/5/2018 1 Lithium ion batteries can be used for many applications
State of Lithium Ion Battery Research Professor Vanessa Wood Department of Information Technology and Electrical Engineering ETH Zürich 2/5/2018 1 Lithium ion batteries can be used for many applications
Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries
 /8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
/8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
Striving for energy density
 Striving for energy density HIGH VOLTAGE MATERIALS FOR LITHIUM ION BATTERIES Public C. Brünig / G. Nuspl BL Energy Storage 08.10.2014 2 Public, Striving for energy density Table of contents Corporate introduction
Striving for energy density HIGH VOLTAGE MATERIALS FOR LITHIUM ION BATTERIES Public C. Brünig / G. Nuspl BL Energy Storage 08.10.2014 2 Public, Striving for energy density Table of contents Corporate introduction
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes
 In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
Modeling of HTPEM Fuel Cell Start-Up Process by Using Comsol Multiphysics
 Modeling of HTPEM Fuel Cell Start-Up Process by Using Comsol Multiphysics Y. Wang *1,2, J. Kowal 1,2 and D. U. Sauer 1,2,3 1 Electrochemical Energy Conversion and Storage Systems Group, Institute for Power
Modeling of HTPEM Fuel Cell Start-Up Process by Using Comsol Multiphysics Y. Wang *1,2, J. Kowal 1,2 and D. U. Sauer 1,2,3 1 Electrochemical Energy Conversion and Storage Systems Group, Institute for Power
Supporting Information. Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries
 Supporting Information Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries Yu Lei,, Lei Qin,, Ruliang Liu,, Kah Chun Lau, Yiying Wu, Dengyun Zhai, *, Baohua Li, and Feiyu Kang *,
Supporting Information Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries Yu Lei,, Lei Qin,, Ruliang Liu,, Kah Chun Lau, Yiying Wu, Dengyun Zhai, *, Baohua Li, and Feiyu Kang *,
High energy all-solid-state lithium batteries with
 Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
PHYSICAL REVIEW LETTERS 107,
 Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
Real-time Measurement of Stress and Damage Evolution During Initial Lithiation of Crystalline Silicon M. J. Chon, 1 V.A. Sethuraman, 1 A. McCormick, 1 V. Srinivasan, 2 P. R. Guduru 1,* 1 School of Engineering,
Main presentation title
 Main presentation title Presentation sub-title Developments in battery chemistries Dr. Marcel Meeus (Umicore): Agenda 1. Umicore materials supplier to the battery industry 2. Generic insight battery technologies,
Main presentation title Presentation sub-title Developments in battery chemistries Dr. Marcel Meeus (Umicore): Agenda 1. Umicore materials supplier to the battery industry 2. Generic insight battery technologies,
Improving cyclic performance of Si anode for lithium-ion batteries by forming an intermetallic skin
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Fabrication and thermal properties of Al 2 TiO 5 /Al 2 O 3 composites
 Materials Science-Poland, Vol. 28, No. 3, 2010 Fabrication and thermal properties of Al 2 TiO 5 /Al 2 O 3 composites M. LI, F. CHEN, Q. SHEN *, L. ZHANG State Key Lab of Advanced Technology for Materials
Materials Science-Poland, Vol. 28, No. 3, 2010 Fabrication and thermal properties of Al 2 TiO 5 /Al 2 O 3 composites M. LI, F. CHEN, Q. SHEN *, L. ZHANG State Key Lab of Advanced Technology for Materials
Advanced Analytical Chemistry Lecture 13. Chem 4631
 Advanced Analytical Chemistry Lecture 13 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Advanced Analytical Chemistry Lecture 13 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Supplementary Information. Capacity fade in high energy silicon-graphite electrodes for lithium-ion batteries
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Information Capacity fade in high energy silicon-graphite electrodes for lithium-ion
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Information Capacity fade in high energy silicon-graphite electrodes for lithium-ion
CAPACITY AND POWER FADE IN LITHIUM-ION BATTERIES
 CAPACITY AND POWER FADE IN LITHIUM-ION BATTERIES A Dissertation Presented to The Academic Faculty by Tapesh Joshi In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School
CAPACITY AND POWER FADE IN LITHIUM-ION BATTERIES A Dissertation Presented to The Academic Faculty by Tapesh Joshi In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School
Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO 4
 836 Bull. Korean Chem. Soc. 2011, Vol. 32, No. 3 ChangKyoo Park et al. DOI 10.5012/bkcs.2011.32.3.836 Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO ChangKyoo
836 Bull. Korean Chem. Soc. 2011, Vol. 32, No. 3 ChangKyoo Park et al. DOI 10.5012/bkcs.2011.32.3.836 Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO ChangKyoo
Lithium Ion Batteries Lecture WS 2016/2017
 Ulm, 12.12.2016 Lithium Ion Batteries Lecture WS 2016/2017 Margret Wohlfahrt-Mehrens Zentrum für Sonnenenergie- und Wasserstoff-Forschung (ZSW) Baden-Württemberg - 1 - Major types of reaction: Insertion
Ulm, 12.12.2016 Lithium Ion Batteries Lecture WS 2016/2017 Margret Wohlfahrt-Mehrens Zentrum für Sonnenenergie- und Wasserstoff-Forschung (ZSW) Baden-Württemberg - 1 - Major types of reaction: Insertion
Electrode and Molecular Architectures for Iron based Multivalent Systems
 Electrode and Molecular Architectures for Iron based Multivalent Systems Jagjit Nanda Materials Science and Technology Division 2 nd MRES, North Eastern University August 20 th 2014 Collaborators S. K.
Electrode and Molecular Architectures for Iron based Multivalent Systems Jagjit Nanda Materials Science and Technology Division 2 nd MRES, North Eastern University August 20 th 2014 Collaborators S. K.
Influence of particles-matrix interphase on stress distribution in particulate composite with polymer matrix
 Applied and Computational Mechanics 1 (2007) 143-148 Influence of particles-matrix interphase on stress distribution in particulate composite with polymer matrix Z. Majer a,b, *, P. Hutař a,, L. Náhlík
Applied and Computational Mechanics 1 (2007) 143-148 Influence of particles-matrix interphase on stress distribution in particulate composite with polymer matrix Z. Majer a,b, *, P. Hutař a,, L. Náhlík
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Deterioration mechanism of LiNi 0.8 Co 0.15 Al 0.05
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Deterioration mechanism of LiNi 0.8 Co 0.15 Al 0.05
AggiE-Challenge: Final Report
 Faculty Advisors: P. P. Mukherjee, H. Liang Graduate Student Mentors: P. Barai, C. Chen, S. Cho, C. Lopez AggiE-Challenge: Final Report Analysis of Thermo-Mechano-Electrochemical Behavior in Lithium-Ion
Faculty Advisors: P. P. Mukherjee, H. Liang Graduate Student Mentors: P. Barai, C. Chen, S. Cho, C. Lopez AggiE-Challenge: Final Report Analysis of Thermo-Mechano-Electrochemical Behavior in Lithium-Ion
School of Materials Science and Engineering, South China University of Technology,
 Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Three-Dimensional Phase Field Based Finite Element Study on Li Intercalation-Induced Stress in Polycrystalline LiCoO2
 Three-Dimensional Phase Field Based Finite Element Study on Li Intercalation-Induced Stress in Polycrystalline LiCoO2 Linmin Wu 1, Yi Zhang 1, Yeon-Gil Jung 2, Jing Zhang 1, * 1 Department of Mechanical
Three-Dimensional Phase Field Based Finite Element Study on Li Intercalation-Induced Stress in Polycrystalline LiCoO2 Linmin Wu 1, Yi Zhang 1, Yeon-Gil Jung 2, Jing Zhang 1, * 1 Department of Mechanical
Volume Change from Materials to Cell Level and Its Influence on Battery Lifetime
 AABC Europe Volume Change from Materials to Cell Level and Its Influence on Battery Lifetime 31.01.2017 Mainz Prof. Dr.-Ing. Andreas Jossen, Bernhard Rieger Institute for Electrical Energy Storage Technology
AABC Europe Volume Change from Materials to Cell Level and Its Influence on Battery Lifetime 31.01.2017 Mainz Prof. Dr.-Ing. Andreas Jossen, Bernhard Rieger Institute for Electrical Energy Storage Technology
Vacuum and Atmospheric Coating and Lamination Techniques Applied to Li-S Battery Fabrication
 Vacuum and Atmospheric Coating and Lamination Techniques Applied to Li-S Battery Fabrication AIMCAL Web Coating Conference Paper AB5, 1:00 PM Wednesday, October 26, 2011 The Rechargeable Battery Company
Vacuum and Atmospheric Coating and Lamination Techniques Applied to Li-S Battery Fabrication AIMCAL Web Coating Conference Paper AB5, 1:00 PM Wednesday, October 26, 2011 The Rechargeable Battery Company
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED
 FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
