DEPRESSION is linked to a reduction in serotonin
|
|
|
- Chester Peters
- 6 years ago
- Views:
Transcription
1 Copyright Ó 2008 by the Genetics Society of America DOI: /genetics Regulation of Serotonin Biosynthesis by the G Proteins Ga o and Ga q Controls Serotonin Signaling in Caenorhabditis elegans Jessica E. Tanis,* James J. Moresco,,1 Robert A. Lindquist,2 and Michael R. Koelle,3 *Department of Molecular, Cellular, and Developmental Biology, Department of Genetics and Department of Molecular Biophysics and Biochemistry, Yale University School of Medicine, New Haven, Connecticut Manuscript received August 3, 2007 Accepted for publication October 26, 2007 ABSTRACT To analyze mechanisms that modulate serotonin signaling, we investigated how Caenorhabditis elegans regulates the function of serotonergic motor neurons that stimulate egg-laying behavior. Egg laying is inhibited by the G protein Ga o and activated by the G protein Ga q.wefoundthatga o and Ga q act directly in the serotonergic HSN motor neurons to control egg laying. There, the G proteins had opposing effects on transcription of the tryptophan hydroxylase gene tph-1, which encodes the rate-limiting enzyme for serotonin biosynthesis. Antiserotonin staining confirmed that Ga o and Ga q antagonistically affect serotonin levels. Altering tph-1 gene dosage showed that small changes in tph-1 expression were sufficient to affect egglaying behavior. Epistasis experiments showed that signaling through the G proteins has additional tph-1- independent effects. Our results indicate that (1) serotonin signaling is regulated by modulating serotonin biosynthesis and (2) Ga o and Ga q act in the same neurons to have opposing effects on behavior, in part, by antagonistically regulating transcription of specific genes. Ga o and Ga q have opposing effects on many behaviors in addition to egg laying and may generally act, as they do in the egg-laying system, to integrate multiple signals and consequently set levels of transcription of genes that affect neurotransmitter release. 1 Present address: Department of Cell Biology, The Scripps Research Institute, La Jolla, CA Present address: Whitehead Institute for Biomedical Research, Cambridge, MA Corresponding author: Department of Molecular Biophysics and Biochemistry, Yale University School of Medicine, SHM CE30, New Haven, CT michael.koelle@yale.edu DEPRESSION is linked to a reduction in serotonin signaling since it is alleviated by selective serotonin reuptake inhibitors (SSRIs), which increase serotonin signaling at synapses (Lucki 1998). A better understanding of depression requires knowledge of the basic mechanisms that regulate serotonin signaling. The best-characterized example of serotonin signaling in a genetically tractable model organism occurs in the Caenorhabditis elegans egglaying system (Schafer 2005). Egg-laying results from contraction of egg-laying muscles (ELMs), which are stimulated by serotonin released from the hermaphrodite-specific motor neurons (HSNs) (Trent et al. 1983). Egg-laying rate is strongly regulated (Schafer 2005) and thus provides a model for analyzing mechanisms that modulate serotonin signaling. Mutants for the neural G proteins Ga o and Ga q lay eggs too frequently and too rarely, respectively (Mendel et al. 1995; Ségalat et al. 1995; Brundage et al. 1996), and thus may be defective in mechanisms that adjust serotonin signaling in the egg-laying system. Ga o is the most abundant G protein in the human brain and mediates signaling by many neurotransmitters (Sternweis and Robishaw 1984; Jiang et al. 2001), but the mechanism of Ga o signaling remains unclear. Ga o and Ga q have opposing effects on many C. elegans behaviors, apparently through opposing effects of these G proteins on neurotransmitter release (Lackner et al. 1999; Miller et al. 1999; Nurrish et al. 1999). Ga o activity decreases the abundance of the synaptic vesicle priming protein UNC-13S at presynaptic termini of C. elegans ventral cord neurons (Nurrish et al. 1999) and negatively regulates synaptic active zone size in C. elegans GABAergic neurons (Yeh et al. 2005). Although these results potentially reveal mechanisms by which Ga o acts to inhibit neurotransmitter release, it is unknown to what extent either of the aforementioned changes is actually responsible for the effects of Ga o on behavior. It is also unknown whether Ga o and Ga q antagonize each other by functioning in the same cells or by acting in different cells. Each behavior affected by the G proteins is controlled by multiple neurons. Ga o and Ga q are expressed in every C. elegans neuron as well as in some muscles, including all cells of the egg-laying system (Mendel et al. 1995; Ségalat et al. 1995; Bastiani et al. 2003). Although some data suggest that the G proteins may act in the HSNs and/or ELMs (Ségalat et al. 1995; Bastiani et al. 2003; Shyn et al. 2003; Moresco and Koelle 2004), their site(s) of action in the egg-laying system remain unclear because this issue has not been systematically analyzed. Genetics 178: ( January 2008)
2 158 J. E. Tanis et al. Here we have manipulated G-protein signaling in specific cells of the egg-laying system to determine where Ga o and Ga q act to regulate egg laying and have also visualized functional consequences of G-protein signaling using fluorescent reporters. Our results show that Ga o and Ga q function in the HSNs to have antagonistic effects on transcription of the tryptophan hydroxylase gene tph-1. This alters serotonin level in the HSNs and, ultimately, the rate of egg-laying behavior, apparently by setting the level of serotonin release from the HSNs. MATERIALS AND METHODS Nematode culture: C. elegans strains were maintained at 20 under standard conditions, and double- and triple-mutant strains were generated using standard genetic techniques (Brenner 1974). The wild-type strain was Bristol N2; a list of all other strains used in this study can be found in the supplemental Materials and Methods at org/supplemental/. Mutations used were as follows: egl- 30(n686), goa-1(n1134), goa-1(sa734), lin-15(n765ts), ser-4(ok512), tph-1(mg280), unc-4(e120), and ardp2. Transgenes for cell-specific expression: Cell-specific expression transgenes were based on a set of vectors that each contained a cell-specific promoter, a polylinker into which a cdna of interest can be inserted, and the unc untranslated region. To determine the specificity of the cellspecific promoters, we inserted cdnas for DsRed2, GFP, and cyan fluorescent protein (CFP) into the ventral cord type C neuron (HSN, VC) and ELM expression vectors, respectively, and generated a separate chromosomally integrated transgene for each construct. Strains carrying these transgenes were outcrossed and analyzed independently to show that each construct used drove expression in only one cell type of the egg-laying system. The transgenes were crossed together to obtain the triple-labeled strain LX975. In separate experiments, when we generated transgenic strains carrying the same plasmids as in LX975, co-injected and integrated as a single transgene, we observed a significant loss of specificity (data not shown). It is possible that enhancers from one promoter drive expression of fluorescent genes in other plasmids incorporated in the same transgenic array. Thus, for all experiments involving expression in more than one cell type, we generated separate integrated transgenes and crossed them together to avoid this problem. cdnas for signaling proteins were inserted into the vectors at exactly the same sites as the cdnas for the fluorescent reporter proteins described above to minimize factors that might alter expression specificity. Each cell-specific promoter was used to drive cdnas encoding GOA-1, GOA-1 Q205L, the S1 subunit of PTX, EGL-30, and EGL-30 Q205L. The HSN promoter was also used to drive expression of SNB-1TGFP or UNC- 13STGFP, cotransforming with a construct to express DsRed2 in the HSNs. Our UNC-13STGFP cassette was modified from that of Nurrish et al. (1999) to remove sequences 59 of the transcription start site and the 5th through 10th introns (to remove the promoter at the 59-end of the gene and an internal promoter). All integrated transgenes used in this article were generated by co-injection with a lin-15 rescuing marker plasmid into lin-15(n765ts) animals and integrated using psoralen/uv mutagenesis. Integrated strains were outcrossed at least four times to lin-15(n765ts). Supplemental Tables S1 and S2 ( contain information on plasmids and integrated transgenes. Fluorescence microscopy: Triple-labeled C. elegans were immobilized with 10 mm levamisole, and a Zeiss LSM 510 confocal microscope was used to obtain a Z-stack image. Channel unmixing (LSM 510 software) was used to eliminate bleed-through observed between the CFP/GFP channels. The three-dimensional reconstruction in Figure 1 and supplemental Movie S1 ( was created using Volocity software (Improvision). Quantitative fluorescence microscopy was performed on a Zeiss LSM 510 confocal microscope (363 objective with 34 zoom), and image analysis was carried out using Volocity software. For imaging, late fourth larval stage (L4) animals were isolated, cultured 40 hr at 20 to produce staged adults, and immobilized with 3 mm levamisole. The left HSN (HSNL) cell-body images were single optical sections through the center of the cell body. Mean DsRed2 intensity in the HSNL cell body minus the nuclear area was measured using Volocity. All significant differences described were also significant without subtraction of the nuclear region. GOA-1 and EGL-30 effects on tph-1 reporter transgene expression levels were reproduced with five independent integrated transgenes (P, for each; Student s t-test was used for all statistical comparisons in this work), although the fold changes seen varied between transgenes used, as GOA-1 increased expression 1.5- to 2.1-fold and EGL-30 decreased expression 1.8- to 6.3-fold. Since the transgene vsis108 integrated on chromosome III, which allowed us to easily cross it with goa-1, egl-30, and all other integrated transgenes, vsis108 was used for experiments in Figure 5 and in supplemental Figure S4 ( org/supplemental/). Antiserotonin immunofluorescence staining was carried out on staged adult worms (see supplemental Materials and Methods) and imaging of the stained HSNLs was performed as described above for the fluorescent reporters. Quantitative analysis of the antiserotonin staining was performed by taking the mean fluorescence intensity from the entire cell body. Further details about image acquisition and SNB-1TGFP and UNC-13STGFP analysis can be found in the supplemental Materials and Methods. Egg-laying assays: Unlaid eggs and early-stage laid eggs were quantitated as described (Chase and Koelle 2004). Staged adults were obtained by picking late L4 animals and culturing them for 40 hr at 20. tph-1 gene dosage: Animals carrying different numbers of functional copies of the tph-1 gene were isolated by picking non-unc cross progeny from the following genetic crosses: (i) tph-1(mg280) males 3 tph-1(mg280); unc-4(e120) hermaphrodites (zero functional copies); (ii) tph-1(mg280) males 3 unc- 4(e120) hermaphrodites (one functional copy); (iii) N2 males 3 unc-4(e120) hermaphrodites (two functional copies); (iv) ardp2 males 3 tph-1(mg280); unc-4(e120) hermaphrodites (two functional copies); and (v) ardp2 males 3 unc-4(e120) hermaphrodites (three functional copies). This experiment was carried out twice (n ¼ 60/genotype each time). Similar significant results were observed each time; Figure 7 contains data from one experiment. RESULTS Cell-specific promoters for the egg-laying system: We set out to genetically manipulate G-protein signaling in the three individual cell types of the egg-laying system to determine in which cells Ga o and Ga q act to control egg laying. The egg-laying system consists of two HSN and six VC motor neurons, which synapse onto the ELM cells (White et al. 1986). We and others have developed a set of three cell-specific promoters that can be used to
3 G Proteins Control Serotonin Synthesis 159 Figure 1. Visualization of neurons and muscles in the egg-laying system of living animals. (A) Confocal image of the egg-laying system with each cell type expressing a different fluorescent protein. (B D) Images from the same animal as in A showing the different fluorescence channels individually to demonstrate promoter specificity. (B) Red fluorescent protein (DsRed2) expression driven by the HSN-specific promoter. (C) GFP expression driven by the VC-specific promoter. (D) CFP expression driven by the ELM-specific promoter. (E) Bright-field image of the same animal. In B and C, arrowheads indicate cell bodies and boxes surround regions that contain synaptic varicosities; in B E, asterisks specify the position of the vulva; and in E, the bracket identifies an egg. Bars, 10 mm. Some of the cells that make up the egg-laying system cannot be seen in these images. The HSNs are a pair of bilaterally symmetric neurons, and only the left cell (HSNL) is seen in A and B. The two VC cell bodies visible in A and C are VC4 and VC5. Three additional VC cell bodies lie anteriorly, and one posteriorly to the field of view shown. Of the 16 total ELM cells, those visible in A and D are the two left vm1 and the two left vm2 cells (each vm2 lies over a vm1 and each vm1/vm2 pair appears as a single unit in the two-dimensional images shown). vm1 and vm2 cells on the right side of the animal are outside of the focal planes imaged. The uterine muscle cells are very thin ELM cells that show weak CFP fluorescence and are also out of view in A and D. express proteins in the HSNs, VCs, or ELMs (Harfe and Fire 1998; Bany et al. 2003; Moresco and Koelle 2004). To analyze promoter specificity, we used the three promoters to transgenically express three different fluorescent proteins (Figure 1A). Confocal images of the transgenic animals demonstrated that each fluorescent protein was expressed in one cell type and was not detectable in the other two cell types of the egg-laying system (Figure 1, B D). Although each promoter also directed expression in one or two additional cell types not related to egg laying (see supplemental Materials and Methods at their specificity within the egg-laying system allowed us to express signaling proteins in individual cell types of this system and to examine the consequences on behavior. In the course of this experiment, we were able for the first time to simultaneously image the cells of the egg-laying system in living animals and visualize spatial relationships among these cells. Figure 1A is a twodimensional representation of a three-dimensional confocal image of the egg-laying system shown in a rotation in supplemental Movie S1 ( org/supplemental/). Our fluorescence imaging showed that the HSN and VC processes formed varicosities that contacted each other and the ELMs (boxed regions in Figure 1, B and C). The HSN processes were otherwise,1.0 mm in diameter, and the varicosities ranged from 3.6 to 6.4 mm across their largest dimension. These varicosities appeared to be the sites of synapses, as confirmed below. A number of features of the VCs and HSNs were variable between animals, including the precise paths of the processes and the number, size, and shape of the varicosities. However, the presence of the varicosities and their spatial relationships to each other and to the ELMs were consistent. Ga o functions in the HSNs to inhibit egg laying: In which cell(s) of the egg-laying system is Ga o signaling sufficient to have inhibitory effects on behavior? To determine this, we generated chromosomally integrated transgenes to express a constitutively active mutant form of GOA-1, the C. elegans Ga o ortholog, in the HSNs, VCs, or ELMs. The mutant protein used, GOA-1 Q205L, is analogous to mutant forms of mammalian Ga s and Ga i, which cannot hydrolyze GTP and thus are locked in the active GTP-bound state (Mendel et al. 1995). We determined where GOA-1 signaling was sufficient to rescue the hyperactive egg-laying behavior of a goa-1 mutant by crossing the chromosomally integrated GOA- 1 Q205L transgenes into the mutant and counting the number of eggs retained in utero. For these and subsequent experiments, we used the goa-1(n1134) mutant (Ségalat et al. 1995), which lacks the N-terminal myristoylation site in GOA-1, preventing membrane localization and thus blocking most or all neurotransmitter signaling by GOA-1. While the wild type retained eggs in utero, the goa-1 mutant retained only unlaid eggs because it laid eggs too often (Figure 2, A C). Expression of GOA-1 Q205L in the HSNs of the goa-1 mutant rescued the hyperactive egg-laying defect and actually caused the retention of even more eggs ( ) than in the wild type, presumably due to excess GOA-1 signaling (Figure 2, A and D). An even greater gain-of-function effect was observed when the same transgene was used to express GOA-1 Q205L in the HSNs of wild-type animals, resulting in accumulation of eggs/animal (Figure 2A). These results are consistent with those observed when GOA-1 Q205L was expressed from extrachromosmal transgenes in the HSNs of wildtype animals (Moresco and Koelle 2004). By coexpressing a fluorescent protein with GOA-1 Q205L in the HSNs and examining the cells, we determined that the accumulation of unlaid eggs in these animals was not
4 160 J. E. Tanis et al. Figure 2. GOA-1 functions in the HSNs to inhibit egg laying. (A) Average number of eggs retained by wild-type animals, goa-1 mutants, and transgenic animals in which GOA-1 Q205L was expressed in the individual cell types of the egg-laying system. Expression of GOA-1 Q205L in the HSNs rescued the hyperactive egg-laying phenotype of the goa-1 mutant and reduced egg laying in the wild-type background, causing retention of many unlaid eggs. n ¼ 30 for each measurement. Error bars show 95% confidence intervals and asterisks indicate P, The goa-1(n1134) partial loss-offunction mutant was used here and throughout the rest of this work, except where otherwise specified. (B D) Representative animals corresponding to the genotypes measured by bars labeled B D in A. In B E, arrows point to unlaid eggs; asterisks indicate the vulva. (E) Wild-type animal with the catalytic subunit of PTX expressed specifically in the HSNs to inactivate GOA-1. This phenocopies the hyperactive egg-laying behavior seen in the goa-1 loss-of-function mutant (compare to C). the result of detectable defects in HSN development or morphology (data not shown). Neither rescue of the goa-1 mutant egg-laying behavior nor a gain-of-function effect in wild-type animals was detected when chromosomally integrated transgenes were used to express GOA-1 Q205L in the VCs or ELMs (Figure 2A). Additional rescue results and controls can be found in supplemental Figure S1A ( including experiments expressing wild-type GOA-1 rather than the activated GOA-1 Q205L. Our results suggest that GOA-1 signaling in the HSNs, but not in the VCs or ELMs, is sufficient to inhibit egg laying. To determine in which cell(s) of the egg-laying system GOA-1 signaling is necessary to inhibit egg laying, we used the cell-specific promoters to express the catalytic subunit of pertussis toxin (PTX), which inactivates Ga o proteins by ADP ribosylation. Ubiquitous expression of an S1 subunit of the PTX transgene in C. elegans results in a phenotype indistinguishable from that of goa-1 null mutants and suppresses the effects of GOA-1 Q205L, indicating that GOA-1 is inactivated by PTX (Darby and Falkow 2001). Four other C. elegans Ga subunits (GPA-1, GPA-3, GPA-4, and GPA-16) are distantly related to Ga o and contain the conserved cysteine that is covalently modified by PTX activity. However, none of these proteins are expressed in the HSNs or the VCs, and only GPA-16 is expressed in the ELMs ( Jansen et al. 1999). Thus, PTX can be used to specifically inactivate GOA-1 in the HSNs and VCs, but likely acts on both GOA-1 and GPA-16 in the ELMs. Expression of PTX in the HSNs of wild-type animals led to the retention of only eggs, compared to in the wild-type control, and thus phenocopied the hyperactive egg laying of the goa-1 mutant (Figure 2E). Expression of PTX in the VCs or ELMs did not cause hyperactive egg-laying behavior (supplemental Figure S1B at supplemental/). These results demonstrate that GOA-1 signaling in the HSN neurons is necessary for GOA-1 to inhibit egg-laying behavior. Our results show that the HSNs are the principal sites where GOA-1 acts to inhibit egg laying. Since the HSN neurons release the neurotransmitter serotonin to stimulate egg laying (Trent et al. 1983; Waggoner et al. 1998), it is possible that GOA-1 inhibits egg-laying behavior by preventing serotonin release from the HSNs. Although we did not observe any evidence of GOA-1 function in the VCs or ELMs in our rescue experiments, we cannot exclude the possibility that GOA-1 may have some minor function in these cells, as has been previously suggested (Ségalat et al. 1995; Shyn et al. 2003). Shyn et al. (2003) saw effects of GOA-1 on the ELMs in animals lacking HSNs and VCs and thus suggested that GOA-1 functions in ELMs. However, these effects could also be the result of GOA-1 signaling in a cell outside the three cell types (HSN, VC, and ELM) that have been thought to compose the egg-laying system. Recently, GOA-1 was shown to act in the uv1/utse neuroendocrine cells to inhibit egg laying ( Jose et al. 2007), and the observations of Shyn et al. (2003) could be explained by GOA-1 function in the uv1/utse rather than in the ELMs. Ga q functions in the HSNs and ELMs to stimulate egg laying: Analogous experiments were used to determine in which cell(s) signaling by the C. elegans Ga q ortholog EGL-30 is sufficient to stimulate egg laying. egl-30 mutants are defective in egg-laying behavior and,
5 G Proteins Control Serotonin Synthesis 161 Figure 3. EGL-30 functions in the HSNs and ELMs to stimulate egg laying. (A) Average number of eggs retained by wild-type animals, egl-30 mutants, and transgenic animals in which EGL-30 Q205L was expressed individually in the cells of the egg-laying system. Expression of EGL-30 Q205L in the HSNs led to partial rescue of the egl-30 mutant phenotype, while expression in the ELMs resulted in full rescue. Expression of EGL-30 Q205L in the HSNs or ELMs in a wild-type background resulted in strong stimulation of egg laying. n ¼ 30 for each measurement. Asterisks over brackets indicate values significantly different from the egl-30 control, while asterisks over individual bars indicate values that are significantly different from the wild-type control (P, ). Error bars show 95% confidence intervals. The egl-30(n686) partial loss-of-function mutant was used here and throughout the rest of this work. (B D) Representative animals corresponding to the genotypes measured by bars labeled B D in A. Arrows point to unlaid eggs; asterisks indicate the vulva. as a result, eggs accumulated in each animal, compared to eggs in the wild-type animals (Figure 3, A and B). We crossed chromosomally integrated transgenes that expressed constitutively active EGL-30 Q205L in the HSNs, VCs, or ELMs in an egl-30 mutant strain and determined where such expression was sufficient to rescue the egl-30 egg-laying defective phenotype. The egl-30(n686) partial loss-of-function mutant was used for these experiments because complete loss of EGL-30 results in lethality (Brundage et al. 1996). Expression of EGL-30 Q205L in the HSNs led to partial rescue ( eggs), while expression in the ELMs was sufficient for full rescue ( eggs; Figure 3, A, C, and D). Rescue effects were also observed when wild-type EGL-30 was expressed in the HSNs or ELMs of the egl-30 mutant (supplemental Figure S1C at It had earlier been suggested that EGL-30 acts in the ELMs to transduce signaling by serotonin released from the HSNs (Bastiani et al. 2003). Our results are consistent with this idea and also indicate that EGL-30 functions in the HSNs to allow these neurons to stimulate the ELMs. The effects of EGL-30 signaling in the HSNs cannot be fully assessed only by expressing EGL-30 Q205L in the HSNs of the egl-30 partial loss-of-function mutant, since the reduced EGL-30 function in the ELMs of these mutants prevents the animals from responding fully to HSN function. Thus, we crossed the transgene expressing EGL-30 Q205L in the HSNs into a wild-type background, and this resulted in strong hyperactive egg laying, as these animals accumulated only eggs compared to the eggs retained by the nontransgenic control (Figure 3A). This gain-of-function effect demonstrates that, when the ELMs are fully functional, EGL-30 signaling in the HSNs is sufficient to strongly stimulate egg laying. Expression of EGL-30 Q205L in the ELMs, but not in the VCs, of wild-type animals was also sufficient to induce a gain-of-function hyperactive egg-laying phenotype (Figure 3A). These results suggest that EGL-30 acts in the HSNs to stimulate release of serotonin and/or other neurotransmitters onto the ELMs, and also in the ELMs, to transduce signaling by neurotransmitters released from the HSNs. Furthermore, these data demonstrate that GOA-1 and EGL-30 antagonize each other by acting in the same neurons, the HSNs. This establishes the HSNs as model neurons for studying the mechanism by which GOA-1 and EGL- 30 have antagonistic effects on serotonin release. No detectable changes in HSN synapse morphology or presynaptic markers in Ga o mutants: We used the HSN-specific promoter to express fluorescently tagged proteins to visualize potential changes at the HSN synapses that might reveal the mechanism by which GOA-1 affects neural function. We generated an integrated transgene to coexpress the red fluorescent protein DsRed2, to fill out the HSNs, with GFP-tagged synaptobrevin (SNB-1), which localizes to synaptic vesicles and labels presynaptic termini (Nonet 1999). HSN function was not significantly altered by expression of these fluorescent proteins since animals carrying the transgene did not exhibit egg-laying defects (supplemental Figure S2 at The transgene was crossed into wild-type and goa-1 mutant backgrounds, and we obtained three-dimensional confocal images of the synaptic region of the HSNL (Figure 4, A I). We quantitatively analyzed SNB-1TGFP fluorescence to look for effects of GOA-1 on presynaptic termini and DsRed2 fluorescence to look for effects on HSNL morphology (see supplemental Materials and Methods).
6 162 J. E. Tanis et al. Figure 4. Morphology and synaptobrevintgfp labeling of presynaptic varicosities in the HSNs of wild-type and goa-1 mutant animals. (A) DsRed2 and (B) synaptobrevintgfp (SNB- 1:::GFP) expressed in a wild-type HSNL. (C) Merge, with nonsynaptic regions indicated by brackets, and three synaptic varicosities indicated by arrowheads. This image corresponds with the boxed region in Figure 1B. The green line between the two small arrows in B, also seen in C I, is an artifact caused by the vulval slit, and not GFP fluorescence. (D I) Representative images of SNB-1TGFP in the HSNL process. (D F) Wild-type and (G I) goa-1 mutant synapses. The three examples for each genotype demonstrate the variability in the morphology, location, and size of the synapses. (A I) Bar, 5 mm. ( J) The average number of SNB-1TGFP varicosities is not significantly different in goa-1 mutants compared to the wild type (P ¼ 0.66). (K) Total synaptic volume (mm 3 ) per HSN is not significantly different in goa-1 mutants compared to the wild type (P ¼ 0.37). In J and K, error bars indicate standard error; n $ 8 for all measurements. The SNB-1TGFP fluorescence perfectly coincided with the varicosities in the HSNL process, indicating that the HSN synapses are located in the varicosities (Figure 4, A C). Considerable variability was observed in the shape of the synaptic varicosities in wild-type HSNLs; however, there were consistently between two and four varicosities per process within 10 mm of the vulval slit (Figure 4, D F). Analysis of SNB-1TGFP indicated that the overall number of synaptic varicosities as well as total synaptic volume was not altered in the HSNs of the goa-1 mutant compared to the wild type (Figure 4, J and K). Analysis of DsRed2 fluorescence showed no difference between wild-type and goa-1 mutant animals in any parameter of HSNL morphology examined, including average width of the HSN process in nonsynaptic regions, mean distance across the largest dimension of the synaptic varicosities, or occurrence of branches emanating from a subset of varicosities (data not shown). A previous study in ventral cord motor neurons showed that a GFP-tagged version of the synaptic vesicle priming protein UNC-13S accumulated at presynaptic termini at higher levels in goa-1 mutants than in wildtype animals (Nurrish et al. 1999). To determine if GOA-1 regulates egg laying by having this effect in the HSNL, we generated a chromosomally integrated transgene to coexpress both DsRed2 and the same UNC- 13STGFP protein used by Nurrish et al. (1999) in the HSNs. Using three-dimensional confocal images, we quantitated UNC-13STGFP fluorescence in the HSNL synaptic region. UNC-13STGFP was concentrated 1.7- fold in the synaptic vs. nonsynaptic regions of the HSNL in both wild-type and goa-1 mutant animals (supplemental Figure S3 at The 1.7-fold synaptic enrichment of this diffuse UNC-13 isoform at wild-type HSN synapses is likely similar to the modest UNC-13STGFP enrichment at ventral nerve cord synapses, although UNC-13STGFP enrichment at ventral cord synapses could not be quantitated since it was visualized within a bundle of many neural processes (Nurrish et al. 1999). Our inability to detect an effect of GOA-1 on UNC-13STGFP in the HSNL similar to the effect seen in ventral cord neurons by Nurrish et al. (1999) could be due to differences between the cell types or imaging methodologies used. The lack of any
7 G Proteins Control Serotonin Synthesis 163 detectable change in synaptic morphology or in presynaptic markers in the HSNL of goa-1 mutants led us to look for other mechanisms by which GOA-1 might affect serotonin release from the HSN neurons. Ga o and Ga q regulate expression of the tryptophan hydroxylase gene: In the course of the synaptic protein localization experiments, we came across an unanticipated and striking effect of GOA-1 and EGL-30 signaling on the HSN neurons. As detailed below, mutations in goa-1 and egl-30 had opposite effects on the levels of fluorescent proteins expressed using the HSN-specific promoter, suggesting that expression from this promoter is regulated by GOA-1 and EGL-30 signaling. This phenomenon is not likely to have affected the cell-specific rescue experiments using this promoter presented in Figures 1 and 2, as expression from the promoter remains specific to the HSNs (plus one other cell type outside the egg-laying system) in all genotypes examined. However, the different expression levels observed in the goa-1 and egl-30 mutants are of great interest because the promoter used in these experiments is that of tph-1, the sole C. elegans gene encoding the tryptophan hydroxylase enzyme that acts at the rate-limiting step in serotonin biosynthesis (Sze et al. 2000). Mutations in goa-1 caused a twofold increase in expression of fluorescent transgenes driven by the tph-1 promoter in the HSNL (Figure 5, A C), suggesting that GOA-1 signaling reduces expression from the tph-1 promoter. A sixfold decrease in fluorescence intensity in the HSNL was observed in egl-30 mutants, indicating that EGL-30 signaling stimulates expression from the tph-1 promoter (Figure 5, A, B, D, and E). The opposing effects of GOA-1 and EGL-30 on tph-1 expression were reproduced using five independent chromosomally integrated transgenes (data not shown). Each consisted of the tph-1 promoter and 23 bp of the tph-1 59 untranslated region, followed by coding sequences for a variety of DsRed2 or GFP proteins and the unc untranslated region. An analogous GFP transgene in which the tph-1 promoter was replaced by the unc-86 promoter was also expressed in the HSNs (Adler et al. 2006) but no changes in expression levels were observed in goa-1 and egl-30 mutant animals compared to the wild type (Figure 5A). These results show that GOA-1 and EGL-30 antagonistically regulate expression from the tph-1 promoter, presumably by regulating transcription. We tested whether GOA-1 and EGL-30 act cell autonomously in the HSNs to have their opposing effects on tph-1 expression. We crossed the chromosomally integrated transgenes that had previously been used to manipulate GOA-1 and EGL-30 function in the HSNs into one of the tph-1 promotertdsred2 transcriptional reporter strains. Expression of PTX specifically in the HSNs to inactivate GOA-1 caused a threefold increase in fluorescence intensity (Figure 5F). This effect was greater than that observed using the goa-1 mutation (Figure 5A), likely because PTX causes more complete Figure 5. GOA-1 and EGL-30 regulate expression of a tph-1 reporter transgene. (A) Average intensity of DsRed2 driven by the tph-1 promoter and GFP driven by the unc-86 promoter in wild-type, goa-1, and egl-30 animals. The intensity of DsRed2 expressed from the tph-1 promoter was greater in goa-1 mutants compared to the wild type, while the intensity was much lower in egl-30 mutants. The intensity of the GFP expressed from the unc-86 promoter was not significantly different in goa-1 or egl-30 mutants compared to the wild type. For A and F H, n ¼ 30 for each genotype, error bars show 95% confidence intervals, and asterisks indicate values significantly different from the respective control (P, ). The fluorescence unit scales shown in different panels cannot be directly compared as different microscope settings were used. (B E) Representative images of DsRed2 expression in HSNL cell bodies from (B) the wild type, (C) goa-1, and (D) egl-30. (E) The same egl-30 HSNL cell body as seen in D imaged with increased gain to show the presence of the HSNL cell body. (B E) Bar, 3 mm. (F) Inactivating GOA-1 in the HSN neurons by expressing the catalytic subunit of PTX from the HSNspecific promoter results in a significant increase in expression of DsRed2 from the tph-1 promoter. (G) Expression of GOA-1 Q205L in the HSNs of the goa-1 mutant decreases expression of DsRed2 from the tph-1 promoter. (H) Expression of EGL-30 Q205L in the HSNs of the egl-30 mutant increases expression of DsRed2 from the tph-1 promoter. inactivation of GOA-1 than the partial loss-of-function goa-1 mutation used. Expression of GOA-1 Q205L in the HSNs of the goa-1 mutant caused a 2.5-fold reduction in expression levels of the tph-1 reporter transgene (Figure 5G). EGL-30 Q205L expression in the HSNs of the egl-30 mutant caused a 2.3-fold increase in expression
8 164 J. E. Tanis et al. levels of the tph-1 reporter transgene (Figure 5H). A 1.5- fold increase in expression levels of the tph-1 reporter transgene was also observed when wild-type EGL-30 was expressed in the HSNs of the egl-30 mutant ( fluorescence intensity units compared to fluorescence intensity units in the egl-30 mutant control). These results show that GOA-1 and EGL-30 act cell autonomously to regulate tph-1 expression in the HSNs. We considered the possibility that serotonin released from the HSNs signals through GOA-1-coupled autoreceptors, as suggested by Shyn et al. (2003), to feedback inhibit tph-1 expression and serotonin production in the HSNs. However, we saw no effects on tph-1 reporter expression in tph-1 mutants (supplemental Figure S4 at which lack serotonin (Sze et al. 2000), or in mutants for the SER-4 G protein-coupled serotonin receptor (supplemental Figure S4), which inhibits egg laying by an unknown mechanism (Dempsey et al. 2005). We also saw no decrease in tph-1 reporter expression in the HSNs when we chronically exposed animals to 7.5 mm serotonin (data not shown). Thus, we were unable to detect evidence for serotonin signaling causing feedback inhibition of tph-1 expression. Ga o and Ga q regulate serotonin levels in the HSNs: Since tryptophan hydroxylase functions at the ratelimiting step in serotonin biosynthesis (Jéquier et al. 1967), changes in tph-1 transcription could affect serotonin levels. To analyze serotonin levels in the HSNs, animals were fixed and stained with an antiserotonin primary antibody, followed by a fluorescent secondary antibody. The HSN cell body, process, and synaptic varicosities stain visibly using this method (Figure 6A). This staining is specific to serotonin since it was absent in the tph-1 null mutant (data not shown). We quantitated antiserotonin stain intensity to determine relative serotonin levels in the HSNs of wild-type, goa-1, and egl-30 animals. The goa-1 mutant showed a 1.3-fold increase in antiserotonin staining in the HSNs (Figure 6, B, C, and E), suggesting that the increase in tph-1 transcription seen in the goa-1 mutant caused an increase in serotonin synthesis. A 1.3-fold increase in antiserotonin staining was also observed in animals in which GOA-1 was inactivated by PTX expression in the HSNs (data not shown). A 1.3-fold decrease in antiserotonin staining was observed in the HSNs of the egl-30 mutant (Figure 6, D and E), indicating that the decrease in tph-1 transcription seen in the egl-30 mutant resulted in a decrease in serotonin synthesis. The intensity of the antiserotonin staining is not likely a linear reflection of serotonin levels, and the 30% changes in staining in the G-protein mutants may not indicate the actual magnitude of changes in serotonin levels. However, our results do show that these changes are significant. GOA-1 and EGL-30 signaling may affect serotonin levels in all serotonergic neurons, or this phenomenon could be HSN specific. Thus, we analyzed the effects of Figure 6. GOA-1 and EGL-30 regulate serotonin levels in the HSN and ADF neurons. (A) Antiserotonin staining in a wild-type HSNL. Arrowhead indicates the HSN cell body, a box surrounds the region that contains synaptic varicosities, and the asterisk specifies the position of the vulva. (B D) Representative images of serotonin staining in HSN cell bodies from (B) the wild type, (C) the goa-1(sa734) null mutant, and (D) the egl-30 mutant. (A D) Bar, 5 mm. (E) Mean intensity of serotonin staining in the HSNs of wild-type, goa-1(sa734), and egl-30 animals. The serotonin level in the HSNs of the goa-1 mutant was significantly higher than in the wild type, while the serotonin level was significantly lower in the egl-30 mutant. n. 24 for all genotypes. In E H, the fluorescence unit scales cannot be directly compared as different microscope settings were used; error bars show 95% confidence intervals and asterisks indicate values that are significantly differentfrom therespective controls (P, 0.01). (F) Mean intensity of serotonin staining in the ADFs of wild-type, goa-1(sa734), and egl-30 animals. The serotonin level in the ADFs of the goa-1 mutant was significantly higher than in the wild type, while the serotonin level was significantly lower in the egl-30 mutant. n ¼ 30 for all genotypes. (G) Expression of GOA-1 Q205L in the HSNs of the goa-1(n1134) mutant significantly decreases serotonin levels in the HSNs. (H) Expression of EGL- 30 Q205L in the HSNs of the egl-30 mutant significantly increases serotoninlevelsinthehsns.ingandh,n¼30forallgenotypes.
9 G Proteins Control Serotonin Synthesis 165 G-protein mutations on serotonin levels in two other pairs of C. elegans serotonergic neurons, the ADFs and NSMs. In the ADFs, the G proteins affected serotonin levels and tph-1 transcription as observed in the HSNs. Antiserotonin staining increased 1.3-fold in the goa-1 mutant and decreased 1.6-fold in the egl-30 mutant (Figure 6F), and analogous changes in tph-1 reporter transgene expression were also seen in the ADFs (data not shown). However, the G proteins caused different effects in the NSM neurons compared to those seen in the HSNs. Antiserotonin staining decreased 1.4- to 1.6- fold in the NSMs of both the goa-1 and the egl-30 mutants (data not shown). These results indicate that there is cell-type specificity in G-protein regulation of tph-1 expression, demonstrating that this phenomenon must be studied in specific identified neurons. We determined whether GOA-1 and EGL-30 act cell autonomously to have their opposing effects on serotonin levels in the HSNs. The chromosomally integrated transgenes used to manipulate GOA-1 and EGL-30 function in the HSNs were crossed into the goa-1 and egl-30 mutants, respectively, and we quantitated antiserotonin stain intensity in these strains. Compared to the goa-1 mutant with no transgene, expression of GOA-1 Q205L in the HSNs of the goa-1 mutant caused a 1.6-fold reduction in HSN serotonin staining (Figure 6G). EGL-30 Q205L expression in the HSNs of the egl-30 mutant caused a 1.5-fold increase in HSN serotonin staining compared to the nontransgenic egl-30 mutant (Figure 6H). These results show that GOA-1 and EGL-30 act cell autonomously to regulate serotonin levels in the HSNs. Small changes in tph-1 expression levels significantly alter egg-laying behavior: Thus far we have established that GOA-1 and EGL-30 function antagonistically in the HSNs to regulate transcription from the tph-1 promoter, which affects serotonin biosynthesis in the HSNs. Our results suggest a model in which changes in serotonin levels in the HSNs lead to changes in the amount of serotonin released from the HSNs, which could account for effects of GOA-1 and EGL-30 signaling on egg-laying behavior (Figure 7A). To determine if modest changes in tph-1 expression actually affect egg laying, we analyzed the egg-laying behavior of animals carrying zero, one, two, or three functional copies of the tph-1 gene. Each wild-type chromosome II carries one functional copy of tph-1, while a chromosome II with a null mutation in tph-1 carries zero functional copies. A free duplication of a portion of chromosome II containing tph-1 was used to add an extra functional copy of the gene. We performed genetic crosses to isolate animals carrying different combinations of these chromosomes. Animals assayed for the dosage experiments were precisely genotypically matched to the controls to which they are compared, such that the only difference was in the number of functional copies of tph-1. Altering the dosage of tph-1 in this way allowed us to generate animals that should have 50% changes in tph-1 expression. We found that these small changes in tph-1 cause significant changes in egg-laying behavior. Animals carrying one functional copy of tph-1 retained significantly more eggs in utero ( eggs) than control animals that carried two functional copies of tph-1 ( eggs), while complete loss of tph-1 (zero functional copies) led to the retention of many unlaid eggs ( eggs; Figure 7B). Increasing the number of functional copies of tph-1 to three led to the retention of significantly fewer eggs ( eggs) than the respective control with only two functional copies ( eggs; Figure 7C). Since even small changes in tph-1 expression are sufficient to cause significant changes in egg laying, the changes in tph-1 expression caused by GOA-1 and EGL-30 signaling appear to be a physiologically significant mechanism for altering egg-laying behavior. Since tph-1 is expressed in multiple neurons, we tested whether TPH-1 acts in the HSNs to regulate egg-laying behavior. We determined in which cells TPH-1 signaling was sufficient to rescue the egg-laying-deficient behavior of the tph-1 mutant by generating extrachromosomal transgenes to express the tph-1 cdna in different serotonergic neurons. Expression of the tph-1 cdna plus GFP from the HSN promoter caused the tph-1 null mutant to retain significantly fewer eggs ( eggs) than the tph-1 null mutant expressing only GFP from the HSN promoter ( eggs; Figure 7D). It is likely that only partial rescue of the tph-1 egg-laying defect was observed because of the mosaic expression associated with extrachromosomal transgenes. Since the HSN promoter also results in expression in the serotonergic NSM neurons, we expressed the tph-1 cdna from a control NSM-specific promoter (Moresco and Koelle 2004) and did not see rescue of the tph-1 null mutant ( eggs; Figure 7D). This indicates that the rescue effects observed with the HSN promoter are the result of tph-1 expression in the HSNs, not in the NSMs. To determine the extent to which regulation of tph-1 transcription in the HSNs accounts for G-protein regulation of egg laying, we crossed together the tph-1 null mutation with the chromosomally integrated transgenes that express PTX or EGL-30 Q205L in the HSNs. Transgenic expression of PTX in the HSNs of the tph-1 null mutant produced animals that retained significantly fewer eggs ( eggs) than the tph-1 null mutant with no transgene ( eggs; Figure 7E). Expression of EGL-30 Q205L in the HSNs of the tph-1 mutant also led to the retention of significantly fewer unlaid eggs ( eggs) compared to the nontransgenic tph-1 control (Figure 7E). Since inactivation of GOA-1 and increased EGL-30 signaling still have effects in the absence of tph-1 function, GOA-1 and EGL-30 signaling must have additional consequences beyond regulation of tph-1 transcription. The HSNs apparently release other neurotransmitters in addition to serotonin to stimulate egg laying, since animals lacking HSNs (Trent et al. 1983) have a more severe egg-laying defect than
10 166 J. E. Tanis et al. do tph-1 mutants (Sze et al. 2000). Thus, the G proteins may regulate synthesis and/or release of these other neurotransmitters to have their additional affects on egg laying. DISCUSSION We used C. elegans egg-laying behavior to investigate the basic mechanisms that modulate serotonin signaling. Our results show that antagonistic signaling through Ga o and Ga q in the HSN motor neurons alters transcription of the tryptophan hydroxylase gene tph-1, altering serotonin biosynthesis to change serotonin content in the HSNs. We showed that even small changes in tph-1 expression levels significantly alter egg-laying behavior, demonstrating that regulation of tph-1 is a significant (albeit not the only) mechanism by which Ga o and Ga q control behavior. Serotonin signaling is regulated at the level of serotonin biosynthesis: How does altering serotonin content alter the ability of the HSNs to stimulate the postsynaptic egg-laying muscles? We suggest that increased cytosolic serotonin leads to increased loading into synaptic vesicles and thus to greater release of serotonin and greater postsynaptic response. The amount of neurotransmitter released by a single depolarization event of a presynaptic neuron dictates the magnitude of postsynaptic response, as postsynaptic and extrasynaptic receptors are not saturated by such events (Bunin and Wightman 1998; Liu et al. 1999; Ishikawa et al. 2002). Regulating the content of synaptic vesicles is a potential mechanism for regulating neurotransmitter release, as Figure 7. Changes in tph-1 expression account, in part, for regulation of egg laying. (A) Model in which GOA-1 and EGL-30 function antagonistically in the HSNs to regulate expression from the tph-1 promoter and serotonin levels to control the amount of serotonin release and egg-laying behavior. (B) Average number of unlaid eggs in animals carrying various doses of the tph-1 gene (n ¼ 60 for each genotype). (1) indicates a wild-type copy of tph-1; (Ø) stands for tph- 1(mg280), the canonical null allele of tph-1. All animals in this experiment were also heterozygous for the recessive marker mutation unc-4(e120), which was necessary to verify some genotypes and was included in the others for consistency. Error bars indicate 95% confidence intervals and asterisks indicate significant differences (P, ). (C) The average number of unlaid eggs per animal (n ¼ 60 for each genotype); error bars and significance were determined as in B. Dp, the chromosomal duplication ardp2, which duplicates part of chromosome II, including tph-1. The duplication causes the animals used in C to not be genotypically matched to those used in B, accounting for the differences between the control animals (two copies of tph-1) in the two separate experiments. (D) Average number of eggs retained by wild-type animals and transgenic tph-1 null mutants in which the tph-1 cdna plus GFP or GFP alone was expressed from either the HSN or the NSM cell-specific promoter. Expression of the tph-1 cdna from the HSN promoter led to partial rescue of the tph-1 mutant phenotype, while expression from the NSM promoter did not result in rescue. n ¼ 50 for all genotypes (10 animals from five independent transgenic lines). The asterisk over the bracket indicates a significant difference from the respective tph-1 null control (P, ). Error bars show 95% confidence intervals. (E) Average number of eggs retained by wild-type animals, tph-1 mutants, and transgenic animals expressing PTX or EGL-30 Q205L in the HSNs in either a wild-type or a tph-1 background. Expression of either PTX or EGL- 30 Q205L in the HSNs led to the retention of fewer unlaid eggs compared to the nontransgenic controls, even in the tph-1 null mutant. n ¼ 30 for all genotypes. Error bars show 95% confidence intervals and asterisks indicate values significantly different from the nontransgenic controls (P, ).
mod-1::mcherry unc-47::gfp RME ser-4::gfp vm2 vm2 vm2 vm2 VNC
 A mod-1::mcherry unc-47::gfp RME B ser-4::gfp vm2 vm2 VNC vm2 vm2 VN Figure S1 Further details of mod- 1 and ser- 4 reporter expression patterns. (A) Adult head region showing mod- 1::mCherry and unc-
A mod-1::mcherry unc-47::gfp RME B ser-4::gfp vm2 vm2 VNC vm2 vm2 VN Figure S1 Further details of mod- 1 and ser- 4 reporter expression patterns. (A) Adult head region showing mod- 1::mCherry and unc-
(A) Schematic depicting morphology of cholinergic DA and DB motor neurons in an L1
 SUPPLEMENTAL MATERIALS Supplemental Figure 1 Figure S1. Expression of the non-alpha nachr subunit ACR-2. (A) Schematic depicting morphology of cholinergic DA and DB motor neurons in an L1 animal. Wide-field
SUPPLEMENTAL MATERIALS Supplemental Figure 1 Figure S1. Expression of the non-alpha nachr subunit ACR-2. (A) Schematic depicting morphology of cholinergic DA and DB motor neurons in an L1 animal. Wide-field
Receptors and Other Signaling Proteins Required for Serotonin Control of Locomotion in Caenorhabditis elegans
 Receptors and Other Signaling Proteins Required for Serotonin Control of Locomotion in Caenorhabditis elegans The MIT Faculty has made this article openly available. Please share how this access benefits
Receptors and Other Signaling Proteins Required for Serotonin Control of Locomotion in Caenorhabditis elegans The MIT Faculty has made this article openly available. Please share how this access benefits
Two classes of silencing RNAs move between Caenorhabditis elegans tissues.
 Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Antony M Jose, Giancarlo A Garcia, and Craig P Hunter. Supplementary Figures, Figure Legends, and Tables. Supplementary Figure
Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Antony M Jose, Giancarlo A Garcia, and Craig P Hunter. Supplementary Figures, Figure Legends, and Tables. Supplementary Figure
An N-terminal Region of Caenorhabditis elegans RGS Proteins EGL-10 and EAT-16 Directs Inhibition of G o Versus G q Signaling*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 49, Issue of December 6, pp. 47004 47013, 2002 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. An N-terminal
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 49, Issue of December 6, pp. 47004 47013, 2002 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. An N-terminal
Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs
 Neuron Supplemental Information Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs Frédéric J. Hoerndli, Rui Wang, Jerry E. Mellem, Angy Kallarackal, Penelope J. Brockie,
Neuron Supplemental Information Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs Frédéric J. Hoerndli, Rui Wang, Jerry E. Mellem, Angy Kallarackal, Penelope J. Brockie,
Two classes of genetic pathways I) Developmental/synthetic pathways II) Regulatory/binary switch pathways
 Two classes of genetic pathways I) Developmental/synthetic pathways II) Regulatory/binary switch pathways I) Developmental pathways Synthesis and/or assembly of molecule(s), subcellular, cellular or multicellular
Two classes of genetic pathways I) Developmental/synthetic pathways II) Regulatory/binary switch pathways I) Developmental pathways Synthesis and/or assembly of molecule(s), subcellular, cellular or multicellular
SUPPLEMENTARY INFORMATION
 Figure S1. lev-9 mutants are resistant to levamisole. The levamisole dose-response curves indicate that lev-9 mutants are partially resistant to levamisole similar to lev-10(kr26) mutants. unc-29(x29)
Figure S1. lev-9 mutants are resistant to levamisole. The levamisole dose-response curves indicate that lev-9 mutants are partially resistant to levamisole similar to lev-10(kr26) mutants. unc-29(x29)
Trasposable elements: Uses of P elements Problem set B at the end
 Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Figure S1. USP-46 is expressed in several tissues including the nervous system
 Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Enhancer genetics Problem set E
 Enhancer genetics Problem set E How are specific cell types generated during development? There are thought to be 10,000 different types of neurons in the human nervous system. How are all of these neural
Enhancer genetics Problem set E How are specific cell types generated during development? There are thought to be 10,000 different types of neurons in the human nervous system. How are all of these neural
b number of motif occurrences
 supplementary information DOI: 1.138/ncb194 a b number of motif occurrences number of motif occurrences number of motif occurrences I II III IV V X TTGGTCAGTGCA 3 8 23 4 13 239 TTGGTCAGTGCT 7 5 8 1 3 83
supplementary information DOI: 1.138/ncb194 a b number of motif occurrences number of motif occurrences number of motif occurrences I II III IV V X TTGGTCAGTGCA 3 8 23 4 13 239 TTGGTCAGTGCT 7 5 8 1 3 83
TOUCH, hearing, taste, vision, smell, and temperature
 Copyright Ó 2007 by the Genetics Society of America DOI: 10.1534/genetics.106.065516 A Specific Subset of Transient Receptor Potential Vanilloid-Type Channel Subunits in Caenorhabditis elegans Endocrine
Copyright Ó 2007 by the Genetics Society of America DOI: 10.1534/genetics.106.065516 A Specific Subset of Transient Receptor Potential Vanilloid-Type Channel Subunits in Caenorhabditis elegans Endocrine
Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by hemeresponsive
 Supplemental Data Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by hemeresponsive gene-2 Caiyong Chen 1, Tamika K. Samuel 1, Michael Krause 2, Harry A. Dailey 3, and Iqbal
Supplemental Data Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by hemeresponsive gene-2 Caiyong Chen 1, Tamika K. Samuel 1, Michael Krause 2, Harry A. Dailey 3, and Iqbal
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
4 Mutant Hunts - To Select or to Screen (Perhaps Even by Brute Force)
 Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 4 Mutant Hunts - To Select or to Screen
Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 4 Mutant Hunts - To Select or to Screen
Advanced Genetics. Why Study Genetics?
 Advanced Genetics Advanced Genetics Why Study Genetics? Why Study Genetics? 1. Historical and aesthetic appreciation Why Study Genetics? 1. Historical and aesthetic appreciation 2. Practical applications
Advanced Genetics Advanced Genetics Why Study Genetics? Why Study Genetics? 1. Historical and aesthetic appreciation Why Study Genetics? 1. Historical and aesthetic appreciation 2. Practical applications
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level *4441518365* BIOLOGY 9700/42 Paper 4 A2 Structured Questions October/November 2012 2 hours Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Level *4441518365* BIOLOGY 9700/42 Paper 4 A2 Structured Questions October/November 2012 2 hours Candidates
Lecture 8: Transgenic Model Systems and RNAi
 Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Sydney Brenner: Present
 Sydney Brenner: 1927 - Present Born in the small town of Germiston, South Africa Won a scholarship to the University of Witwatersrand at the age of 15 Received his PhD from Exeter College, Oxford Made
Sydney Brenner: 1927 - Present Born in the small town of Germiston, South Africa Won a scholarship to the University of Witwatersrand at the age of 15 Received his PhD from Exeter College, Oxford Made
Nature Structural & Molecular Biology: doi: /nsmb.3308
 Supplementary Figure 1 Analysis of CED-3 autoactivation and CED-4-induced CED-3 activation. (a) Repeat experiments of Fig. 1a. (b) Repeat experiments of Fig. 1b. (c) Quantitative analysis of three independent
Supplementary Figure 1 Analysis of CED-3 autoactivation and CED-4-induced CED-3 activation. (a) Repeat experiments of Fig. 1a. (b) Repeat experiments of Fig. 1b. (c) Quantitative analysis of three independent
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
a) Stock 4: 252 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.
![a) Stock 4: 252 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males. a) Stock 4: 252 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.](/thumbs/91/106088469.jpg) Drosophila Problem Set 2018 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
Drosophila Problem Set 2018 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
a) Stock 4: 126 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.
![a) Stock 4: 126 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males. a) Stock 4: 126 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.](/thumbs/88/116538069.jpg) Drosophila Problem Set 2012 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
Drosophila Problem Set 2012 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
Problem set D answers
 Problem set D answers 1. You find a mouse with no tail. In order to determine whether this mouse carries a new mutation, you cross it to a normal mouse. All the F1 progeny of this cross are wild type.
Problem set D answers 1. You find a mouse with no tail. In order to determine whether this mouse carries a new mutation, you cross it to a normal mouse. All the F1 progeny of this cross are wild type.
Caenorhabditis elegans: The Heavyweight Champ of Gene Knockout Technology
 Caenorhabditis elegans: The Heavyweight Champ of Gene Knockout Technology Rachel Pan The nematode (worm) Caenorhabditis elegans was the first multicellular organism to have its genome, or complete DNA
Caenorhabditis elegans: The Heavyweight Champ of Gene Knockout Technology Rachel Pan The nematode (worm) Caenorhabditis elegans was the first multicellular organism to have its genome, or complete DNA
An antibiotic selection marker for nematode transgenesis
 nature methods An antibiotic selection marker for nematode transgenesis Rosina Giordano-Santini, Stuart Milstein, Nenad Svrzikapa, Domena Tu, Robert Johnsen, David Baillie, Marc Vidal & Denis Dupuy Supplementary
nature methods An antibiotic selection marker for nematode transgenesis Rosina Giordano-Santini, Stuart Milstein, Nenad Svrzikapa, Domena Tu, Robert Johnsen, David Baillie, Marc Vidal & Denis Dupuy Supplementary
Biol 432L Midterm Oct 6, 2008 Name: 1. Midterm 1, Answer Key Oct. 26, 2009
 Biol 432L Midterm Oct 6, 2008 Name: 1 Midterm 1, Answer Key Oct. 26, 2009 Honor Pledge: I have neither given nor received any unauthorized help on this exam: Name Printed: Signature: 1a. (2 pts) Imagine
Biol 432L Midterm Oct 6, 2008 Name: 1 Midterm 1, Answer Key Oct. 26, 2009 Honor Pledge: I have neither given nor received any unauthorized help on this exam: Name Printed: Signature: 1a. (2 pts) Imagine
Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product.
 Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Liu et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Liu et al., http ://www.jcb.org /cgi /content /full /jcb.201506081 /DC1 THE JOU RNAL OF CELL BIO LOGY Figure S1. Identification of sorf-1 and sorf-2. (A) RNAi of ZK563.5 increases
Supplemental material JCB Liu et al., http ://www.jcb.org /cgi /content /full /jcb.201506081 /DC1 THE JOU RNAL OF CELL BIO LOGY Figure S1. Identification of sorf-1 and sorf-2. (A) RNAi of ZK563.5 increases
Genetics C Examination #3 - December 7, 1999
 Genetics C3032 - Examination #3 - December 7, 1999 General instructions: Don't Panic. Be sure your name is on every page and that you write your exam in ink. Answer the questions in the space provided.
Genetics C3032 - Examination #3 - December 7, 1999 General instructions: Don't Panic. Be sure your name is on every page and that you write your exam in ink. Answer the questions in the space provided.
MUTANT: A mutant is a strain that has suffered a mutation and exhibits a different phenotype from the parental strain.
 OUTLINE OF GENETICS LECTURE #1 A. TERMS PHENOTYPE: Phenotype refers to the observable properties of an organism, such as morphology, growth rate, ability to grow under different conditions or media. For
OUTLINE OF GENETICS LECTURE #1 A. TERMS PHENOTYPE: Phenotype refers to the observable properties of an organism, such as morphology, growth rate, ability to grow under different conditions or media. For
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15
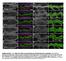 Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Fig. S1. Phosphorylation of eif2 by PEK-1 is increased in ire-1 mutants. Fig. S2. ire-1
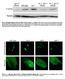 Fig. S1. Phosphorylation of eif2a by PEK-1 is increased in ire-1 mutants. Representative western blot of phosphorylated eif2a and tubulin of day-0 wild-type animals and ire-1 mutants treated with control,
Fig. S1. Phosphorylation of eif2a by PEK-1 is increased in ire-1 mutants. Representative western blot of phosphorylated eif2a and tubulin of day-0 wild-type animals and ire-1 mutants treated with control,
unc-33/crmp and ankyrin organize microtubules and localize kinesin to polarize
 Supplementary Information unc-33/crmp and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting Tapan A. Maniar 1, Miriam Kaplan 1, George J. Wang 2, Kang Shen 2, Li Wei
Supplementary Information unc-33/crmp and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting Tapan A. Maniar 1, Miriam Kaplan 1, George J. Wang 2, Kang Shen 2, Li Wei
TWENTY FIRST CENTURY SCIENCE BIOLOGY A TUESDAY 17 JUNE 2008 GENERAL CERTIFICATE OF SECONDARY EDUCATION A222/02. UNIT 2 Modules B4 B5 B6 (Higher Tier)
 H GENERAL CERTIFICATE OF SECONDARY EDUCATION A222/02 TWENTY FIRST CENTURY SCIENCE BIOLOGY A UNIT 2 Modules B4 B5 B6 (Higher Tier) TUESDAY 17 JUNE 2008 Morning Time: 40 minutes *CUP/T44358* Candidates answer
H GENERAL CERTIFICATE OF SECONDARY EDUCATION A222/02 TWENTY FIRST CENTURY SCIENCE BIOLOGY A UNIT 2 Modules B4 B5 B6 (Higher Tier) TUESDAY 17 JUNE 2008 Morning Time: 40 minutes *CUP/T44358* Candidates answer
[Presented by: Andrew Howlett, Cruise Slater, Mahmud Hasan, Greg Dale]
![[Presented by: Andrew Howlett, Cruise Slater, Mahmud Hasan, Greg Dale] [Presented by: Andrew Howlett, Cruise Slater, Mahmud Hasan, Greg Dale]](/thumbs/96/128035026.jpg) Mutational Dissection [Presented by: Andrew Howlett, Cruise Slater, Mahmud Hasan, Greg Dale] Introduction What is the point of Mutational Dissection? It allows understanding of normal biological functions
Mutational Dissection [Presented by: Andrew Howlett, Cruise Slater, Mahmud Hasan, Greg Dale] Introduction What is the point of Mutational Dissection? It allows understanding of normal biological functions
Sperm cells are passive cargo of the pollen tube in plant fertilization
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
Applicazioni biotecnologiche
 Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Supplementary Methods
 Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and
 Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
GENETICS - CLUTCH CH.15 GENOMES AND GENOMICS.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF GENOMICS Genomics is the study of genomes in their entirety Bioinformatics is the analysis of the information content of genomes - Genes, regulatory sequences,
!! www.clutchprep.com CONCEPT: OVERVIEW OF GENOMICS Genomics is the study of genomes in their entirety Bioinformatics is the analysis of the information content of genomes - Genes, regulatory sequences,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
7.012 Problem Set 5. Question 1
 Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Supporting Information
 Supporting Information Lee et al. 10.1073/pnas.1000866107 SI Experimental Procedures Plasmid and Strain Construction. (p)odr-3::gfp::egl-4 was constructed by excising the coding region of GFP and the first
Supporting Information Lee et al. 10.1073/pnas.1000866107 SI Experimental Procedures Plasmid and Strain Construction. (p)odr-3::gfp::egl-4 was constructed by excising the coding region of GFP and the first
Genetics Lab Biology 322 Fall 2013
 Genetics Lab Biology 322 Fall 2013 CAENORHABDITIS ELEGANS AND MENDEL'S SECOND LAW REVISITED: Independent assortment versus linkage of gene pairs during gamete formation Allele and genotype symbolism: application
Genetics Lab Biology 322 Fall 2013 CAENORHABDITIS ELEGANS AND MENDEL'S SECOND LAW REVISITED: Independent assortment versus linkage of gene pairs during gamete formation Allele and genotype symbolism: application
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Enhancers mutations that make the original mutant phenotype more extreme. Suppressors mutations that make the original mutant phenotype less extreme
 Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
12 Interaction of Genes
 12 Interaction of Genes Yeast genetics has been particularly amenable for identifying and characterizing gene products that directly or indirectly interact with each other, especially when two mutations
12 Interaction of Genes Yeast genetics has been particularly amenable for identifying and characterizing gene products that directly or indirectly interact with each other, especially when two mutations
Automated analysis of embryonic gene expression with cellular resolution in C. elegans
 Automated analysis of embryonic gene expression with cellular resolution in C. elegans John Isaac Murray, Zhirong Bao, Thomas J Boyle, Max E Boeck, Barbara L Mericle, Thomas J Nicholas, Zhongying Zhao,
Automated analysis of embryonic gene expression with cellular resolution in C. elegans John Isaac Murray, Zhirong Bao, Thomas J Boyle, Max E Boeck, Barbara L Mericle, Thomas J Nicholas, Zhongying Zhao,
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Kanaani et al., http://www.jcb.org/cgi/content/full/jcb.200912101/dc1 Figure S1. The K2 rabbit polyclonal antibody is specific for GAD67,
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Kanaani et al., http://www.jcb.org/cgi/content/full/jcb.200912101/dc1 Figure S1. The K2 rabbit polyclonal antibody is specific for GAD67,
Cell autonomous vs. cell non-autonomous gene function
 Cell autonomous vs. cell non-autonomous gene function In multicellular organisms, it is important to know in what cell(s) the activity of a gene is required. - while RNA expression can be highly informative,
Cell autonomous vs. cell non-autonomous gene function In multicellular organisms, it is important to know in what cell(s) the activity of a gene is required. - while RNA expression can be highly informative,
fga phenotypes were also analyzed in the tracheal system, the organ responsible for
 SUPPLEMENTARY TEXT Tracheal defects fga phenotypes were also analyzed in the tracheal system, the organ responsible for oxygen delivery in the fly. In fga homozygous mutant alleles, we observed defects
SUPPLEMENTARY TEXT Tracheal defects fga phenotypes were also analyzed in the tracheal system, the organ responsible for oxygen delivery in the fly. In fga homozygous mutant alleles, we observed defects
Mos1 insertion. MosTIC protocol-11/2006. repair template - Mos1 transposase expression - Mos1 excision - DSB formation. homolog arm.
 MosTIC (Mos1 excision induced Transgene Instructed gene Conversion) Valérie Robert (vrobert@biologie.ens.fr) and Jean-Louis Bessereau (jlbesse@biologie.ens.fr) (November 2006) Introduction: MosTIC (Robert
MosTIC (Mos1 excision induced Transgene Instructed gene Conversion) Valérie Robert (vrobert@biologie.ens.fr) and Jean-Louis Bessereau (jlbesse@biologie.ens.fr) (November 2006) Introduction: MosTIC (Robert
Integrins are the receptors than mediate adhesion between the dorsal and ventral surfaces of the wing.
 Problem set 8 answers 1. Integrins are alpha/beta heterodimeric receptors that bind to molecules in the extracellular matrix. A primary role of integrins is in adhesion, ensuring that cells attach to the
Problem set 8 answers 1. Integrins are alpha/beta heterodimeric receptors that bind to molecules in the extracellular matrix. A primary role of integrins is in adhesion, ensuring that cells attach to the
ONE proposed mechanism for establishing and omi and Kidokoro 2000; Waters and Smith 2000).
 Copyright 2005 by the Genetics Society of America DOI: 10.1534/genetics.104.031286 Convergent, RIC-8-Dependent G Signaling Pathways in the Caenorhabditis elegans Synaptic Signaling Network Nicole K. Reynolds,
Copyright 2005 by the Genetics Society of America DOI: 10.1534/genetics.104.031286 Convergent, RIC-8-Dependent G Signaling Pathways in the Caenorhabditis elegans Synaptic Signaling Network Nicole K. Reynolds,
7.013 Practice Quiz
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
Construct Design and Cloning Guide for Cas9-triggered homologous recombination
 Construct Design and Cloning Guide for Cas9-triggered homologous recombination Written by Dan Dickinson (ddickins@live.unc.edu) and last updated December 2013. Reference: Dickinson DJ, Ward JD, Reiner
Construct Design and Cloning Guide for Cas9-triggered homologous recombination Written by Dan Dickinson (ddickins@live.unc.edu) and last updated December 2013. Reference: Dickinson DJ, Ward JD, Reiner
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature7866 1.8 Fraction Bordering Fraction Aggregating.6.4.2 1.8.6.4.2 Promoter Head neurons expressing npr-1 cdna Genotype WT - - URX AQR ALN AVM gcy-35 sax-7 gcy-32+ gpa-3 RMG ILs URX AQR
doi: 1.138/nature7866 1.8 Fraction Bordering Fraction Aggregating.6.4.2 1.8.6.4.2 Promoter Head neurons expressing npr-1 cdna Genotype WT - - URX AQR ALN AVM gcy-35 sax-7 gcy-32+ gpa-3 RMG ILs URX AQR
Nature Methods: doi: /nmeth Supplementary Figure 1. Neither driver nor effector alone displays expression of GFP
 Supplementary Figure 1 Neither driver nor effector alone displays expression of GFP Comparison of GFP fluorescence in a driver- only strain (a) or an effector-only strain (b) to their driver + effector
Supplementary Figure 1 Neither driver nor effector alone displays expression of GFP Comparison of GFP fluorescence in a driver- only strain (a) or an effector-only strain (b) to their driver + effector
Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. A. Fire et al. (1998) Nature Vol 391:
 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans A. Fire et al. (1998) Nature Vol 391: 806-810 1 Outline 1. Introduction 2. Objective 3. Results 4. Mechanism 5.
Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans A. Fire et al. (1998) Nature Vol 391: 806-810 1 Outline 1. Introduction 2. Objective 3. Results 4. Mechanism 5.
Name AP Biology Mrs. Laux Take home test #11 on Chapters 14, 15, and 17 DUE: MONDAY, DECEMBER 21, 2009
 MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
Supplemental Data. Farmer et al. (2010) Plant Cell /tpc
 Supplemental Figure 1. Amino acid sequence comparison of RAD23 proteins. Identical and similar residues are shown in the black and gray boxes, respectively. Dots denote gaps. The sequence of plant Ub is
Supplemental Figure 1. Amino acid sequence comparison of RAD23 proteins. Identical and similar residues are shown in the black and gray boxes, respectively. Dots denote gaps. The sequence of plant Ub is
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Analysis of unique sequences isolated in Sort 14. Unique sequences are listed next to their frequency of occurrence, in parenthesis. Dithiol resistance is
Supplementary Figure 1 Supplementary Figure 1. Analysis of unique sequences isolated in Sort 14. Unique sequences are listed next to their frequency of occurrence, in parenthesis. Dithiol resistance is
Intragenic suppressors: true revertants
 Suppressor Genetics: intragenic and informational suppressors We have covered one approach used in genetics to study gene function: classical forward genetics where an investigator selects or screens for
Suppressor Genetics: intragenic and informational suppressors We have covered one approach used in genetics to study gene function: classical forward genetics where an investigator selects or screens for
Explain why the scientists used the same restriction endonuclease enzymes on each DNA sample
 Q1.Some populations of flies are becoming resistant to insecticides intended to kill them. Scientists developed a method for finding out whether a fly was carrying a recessive allele, r, that gives resistance
Q1.Some populations of flies are becoming resistant to insecticides intended to kill them. Scientists developed a method for finding out whether a fly was carrying a recessive allele, r, that gives resistance
Lecture 20: Drosophila embryogenesis
 Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Test Bank for Molecular Cell Biology 7th Edition by Lodish
 Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Mcbio 316: Exam 3. (10) 2. Compare and contrast operon vs gene fusions.
 Mcbio 316: Exam 3 Name (15) 1. Transposons provide useful tools for genetic analysis. List 5 different uses of transposon insertions. ANSWER: Many answers are possible, however, if multiple items on the
Mcbio 316: Exam 3 Name (15) 1. Transposons provide useful tools for genetic analysis. List 5 different uses of transposon insertions. ANSWER: Many answers are possible, however, if multiple items on the
Nature Biotechnology: doi: /nbt.4166
 Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1119481/dc1 Supporting Online Material for LIN-12/Notch Activation Leads to MicroRNA-Mediated Down-Regulation of Vav in C. elegans Andrew S. Yoo and Iva Greenwald* *To
www.sciencemag.org/cgi/content/full/1119481/dc1 Supporting Online Material for LIN-12/Notch Activation Leads to MicroRNA-Mediated Down-Regulation of Vav in C. elegans Andrew S. Yoo and Iva Greenwald* *To
TRANSGENIC ANIMALS. transient. stable. - Two methods to produce transgenic animals:
 Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
Supporting information
 Supporting information Construction of strains and plasmids To create ptc67, a PCR product obtained with primers cc2570-162f (gcatgggcaagcttgaggacggcgtcatgt) and cc2570+512f (gaggccgtggtaccatagaggcgggcg),
Supporting information Construction of strains and plasmids To create ptc67, a PCR product obtained with primers cc2570-162f (gcatgggcaagcttgaggacggcgtcatgt) and cc2570+512f (gaggccgtggtaccatagaggcgggcg),
Lecture 2-3: Using Mutants to study Biological processes
 Lecture 2-3: Using Mutants to study Biological processes Objectives: 1. Why use mutants? 2. How are mutants isolated? 3. What important genetic analyses must be done immediately after a genetic screen
Lecture 2-3: Using Mutants to study Biological processes Objectives: 1. Why use mutants? 2. How are mutants isolated? 3. What important genetic analyses must be done immediately after a genetic screen
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat
 Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Chapter 3. Clonal selection
 Chapter 3. Clonal selection I have called this principle, by which each slight variation, if useful, is preserved, by the term of Natural Selection -Charles Darwin, On the Origin of Species, 1859 4 The
Chapter 3. Clonal selection I have called this principle, by which each slight variation, if useful, is preserved, by the term of Natural Selection -Charles Darwin, On the Origin of Species, 1859 4 The
species- Mus musculus Engineering the mouse genome David Ornitz
 species- Mus musculus Engineering the mouse genome David Ornitz How do we analyze gene function in mice? Gene addition (transgenic approach) Permits GOF, DN and knockdown experiments Ectopic (spatial or
species- Mus musculus Engineering the mouse genome David Ornitz How do we analyze gene function in mice? Gene addition (transgenic approach) Permits GOF, DN and knockdown experiments Ectopic (spatial or
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
2007 lecture 4 sterile mutations at the MAT locus C. elegans screens and genetic analysis null phenotypes. Sternberg 2007 Bi190 Caltech
 2007 lecture 4 sterile mutations at the MAT locus C. elegans screens and genetic analysis null phenotypes Saccharomyces cerevisiae (budding yeast) Life Cycle a α mating a/α zygote sporulation -C -N germination
2007 lecture 4 sterile mutations at the MAT locus C. elegans screens and genetic analysis null phenotypes Saccharomyces cerevisiae (budding yeast) Life Cycle a α mating a/α zygote sporulation -C -N germination
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/323/5920/1500/dc1 Supporting Online Material for RSY-1 Is a Local Inhibitor of Presynaptic Assembly in C. elegans Maulik R. Patel and Kang Shen To whom correspondence
www.sciencemag.org/cgi/content/full/323/5920/1500/dc1 Supporting Online Material for RSY-1 Is a Local Inhibitor of Presynaptic Assembly in C. elegans Maulik R. Patel and Kang Shen To whom correspondence
9. What proteins will be affected by mutations in the trans-acting elements? Cis-acting elements?
 6. What regulates the expression of a gene? 7. What are the cis- and trans-acting elements? 8. Can a deficiency in a trans-acting element be overcome by the addition of another copy of the gene to a cell?
6. What regulates the expression of a gene? 7. What are the cis- and trans-acting elements? 8. Can a deficiency in a trans-acting element be overcome by the addition of another copy of the gene to a cell?
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Negative selection analysis of sgrnas targeting all Brd4 exons comparing day 2 to day 10 time points. Systematic evaluation of 64 Brd4 sgrnas in negative selection experiments, targeting
Supplementary Figure 1 Negative selection analysis of sgrnas targeting all Brd4 exons comparing day 2 to day 10 time points. Systematic evaluation of 64 Brd4 sgrnas in negative selection experiments, targeting
elegans controls the time of expression of
 The h'n-14 locus of Caenorhabditis. elegans controls the time of expression of specific postembryonic developmental events Victor Ambros 1 and H. Robert Horvitz Department of Biology, Massachusetts nstitute
The h'n-14 locus of Caenorhabditis. elegans controls the time of expression of specific postembryonic developmental events Victor Ambros 1 and H. Robert Horvitz Department of Biology, Massachusetts nstitute
Section 3. Synthetic combinations of various. termination defective rho, nusg and. nusa mutants: Spectrum of efficiency of
 Section 3 Synthetic combinations of various termination defective rho, nusg and nusa mutants: Spectrum of efficiency of Rho-dependent transcription termination Introduction As described in Sections 1 and
Section 3 Synthetic combinations of various termination defective rho, nusg and nusa mutants: Spectrum of efficiency of Rho-dependent transcription termination Introduction As described in Sections 1 and
Supplemental Data. Benstein et al. (2013). Plant Cell /tpc
 Supplemental Figure 1. Purification of the heterologously expressed PGDH1, PGDH2 and PGDH3 enzymes by Ni-NTA affinity chromatography. Protein extracts (2 µl) of different fractions (lane 1 = total extract,
Supplemental Figure 1. Purification of the heterologously expressed PGDH1, PGDH2 and PGDH3 enzymes by Ni-NTA affinity chromatography. Protein extracts (2 µl) of different fractions (lane 1 = total extract,
Supplemental Information. Glutamylation Regulates Transport, Specializes. Function, and Sculpts the Structure of Cilia
 Current Biology, Volume 27 Supplemental Information Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia Robert O'Hagan, Malan Silva, Ken C.Q. Nguyen, Winnie Zhang,
Current Biology, Volume 27 Supplemental Information Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia Robert O'Hagan, Malan Silva, Ken C.Q. Nguyen, Winnie Zhang,
(a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for
 Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Information
 Supplementary Information COP1 E3 ligase protects HYL1 to retain microrna biogenesis Seok Keun Cho 1, Samir Ben Chaabane 1, Pratik Shah 1, Christian Peter Poulsen 1, and Seong Wook Yang 1 * 1 Laboratory
Supplementary Information COP1 E3 ligase protects HYL1 to retain microrna biogenesis Seok Keun Cho 1, Samir Ben Chaabane 1, Pratik Shah 1, Christian Peter Poulsen 1, and Seong Wook Yang 1 * 1 Laboratory
Supplemental Data. Na Xu et al. (2016). Plant Cell /tpc
 Supplemental Figure 1. The weak fluorescence phenotype is not caused by the mutation in At3g60240. (A) A mutation mapped to the gene At3g60240. Map-based cloning strategy was used to map the mutated site
Supplemental Figure 1. The weak fluorescence phenotype is not caused by the mutation in At3g60240. (A) A mutation mapped to the gene At3g60240. Map-based cloning strategy was used to map the mutated site
Chapter 17. From Gene to Protein
 Chapter 17 From Gene to Protein Overview: The Flow of Genetic Information The information content of DNA is in the form of specific sequences of nucleotides The DNA inherited by an organism leads to specific
Chapter 17 From Gene to Protein Overview: The Flow of Genetic Information The information content of DNA is in the form of specific sequences of nucleotides The DNA inherited by an organism leads to specific
7.22 ANSWERS to Problem set #1
 7.22 ANSWERS to Problem set #1 1. List whether the pairs marked by the arrows are orthologous or paralogous. α tubulin (mouse) α tubulin (fly) α tubulin (worm) α tubulin (yeast) paralogous Tubulin β tubulin
7.22 ANSWERS to Problem set #1 1. List whether the pairs marked by the arrows are orthologous or paralogous. α tubulin (mouse) α tubulin (fly) α tubulin (worm) α tubulin (yeast) paralogous Tubulin β tubulin
Supplementary information
 Supplementary information Supplementary figures Figure S1 Level of mycdet1 protein in DET1 OE-1, OE-2 and OE-3 transgenic lines. Total protein extract from wild type Col0, det1-1 mutant and DET1 OE lines
Supplementary information Supplementary figures Figure S1 Level of mycdet1 protein in DET1 OE-1, OE-2 and OE-3 transgenic lines. Total protein extract from wild type Col0, det1-1 mutant and DET1 OE lines
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
SUPPLEMENTARY INFORMATION
 SULEMENTARY INFORMATION doi:10.1038/nature12117 Supplementary Discussion Although loss of leads to initiation of postembryonic development even under starvation conditions, ectopic overexpression of under
SULEMENTARY INFORMATION doi:10.1038/nature12117 Supplementary Discussion Although loss of leads to initiation of postembryonic development even under starvation conditions, ectopic overexpression of under
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
