CELL ORGANELLES AT UNCOATED CRYOFRACTURED SURFACES AS VIEWED WITH THE SCANNING ELECTRON MICROSCOPE
|
|
|
- Brian Jennings
- 5 years ago
- Views:
Transcription
1 J. Cell Sci. 21, (1976) 47 Printed in Great Britain CELL ORGANELLES AT UNCOATED CRYOFRACTURED SURFACES AS VIEWED WITH THE SCANNING ELECTRON MICROSCOPE P. S. WOODS AND MYRON C. LEDBETTER Biology Department, Queens College, Flushing, New York 11367, U.S.A. and Biology Department, Brookhaven National Laboratory, Upton, New York 11973, U.S.A. SUMMARY A method of direct visualization of cell organelles by scanning electron microscopy (SEM) is described. Plant and animal tissues fixed in glutaraldehyde and osmium tetroxide are treated with the ligand thiocarbohydrazide and a second osmium tetroxide solution, to increase their osmium content. Tissues are then dehydrated, infiltrated with an epoxy monomer, and together solidified with dry ice and fractured. The pieces are transferred to pure acetone, critical-point dried, attached to stubs with silver paint and viewed by SEM. The ligating procedure increases the osmium concentration at its original bonding site sufficiently to render the tissue electrically conductive, thus obviating the need for metallic coating. The organelles at the fractured surface are revealed in relation to their osmium incorporation rather than by surface irregularities as with coating methods. The image derived from the uncoated surface approaches in resolution that of transmission electron micrographs of thin sections. A portion of the image arising from a small distance below the surface, while at progressively lower resolution, provides some 3-dimensional information about cell fine structure. INTRODUCTION Observation of plant or animal cell organelles by scanning electron microscopy (SEM) based on surface irregularities has been demonstrated by others using various methods (Guttman & Styskal, 1971; Lim, 1971; Panessa & Gennaro, 1972, 1973; Humphreys & Wodzicki, 1972; Tanaka & lino, 1972; Humphreys, Spurlock & Johnson, 1974). In one method, Tanaka & lino (1972) exposed the contents of cells by cracking frozen, osmium-fixed tissues infiltrated with monomeric epoxy resin. These workers identified some of the organelles by small surface irregularities in the otherwise smooth break after the pieces were dried and coated with a heavy metal. We repeated this method and found by careful stereoscopic observation of pairs of micrographs that some of the secondary electron signal was derived from below the coated surface, presumably from the osmium absorbed by particular structures during fixation. We sought ways to enhance that part of the image which comes from the osmium-rich organelles, while diminishing that part which shows surface irregularities. After several attempts we found a way to view the fractured surface directly without coating, thus permitting us to observe the organelles at and just beneath the
2 48 P. S. Woods and M. C. Ledbctter surface at relatively high resolution. This was accomplished by using the thiocarbohydrazide (TCH)-osmium ligating method developed by Seligman, Wasserkrug & Hanker (1966) to stain tissue sections for transmission electron microscopy (TEM), and later modified by Kelley, Dekker & Bluemink (1973) to render bulk tissues electrically conductive for surface viewing with the SEM. Using this procedure, it became apparent that osmium ligation in combination with resin cracking provides a new way to study cell fine structure with the SEM. We present here a more extensive account of our preliminary findings reported earlier (Woods & Ledbetter, 1974). MATERIALS AND METHODS Preparation of tissues prior to fixation We explored the usefulness of the method for plants and animals by using corn root tips and portions of tadpole tail. Corn (Zea mays L.) seeds were germinated in the dark at 18 C between layers of thick filter paper moistened with distilled water and grown for 8 or 9 days prior to fixation. Frog (Rana catesbeiana Shaw) tadpoles approximately 6 cm long were collected in the wild and maintained in the laboratory in 15-I. tanks under conditions similar to those in nature. A relaxed condition of the tail muscle was achieved prior to fixation by forcing the tadpole to succumb to exhaustion. Samples were taken from root tips and small pieces of tadpole tail (2 mm') containing epidermis and muscle. Buffers and washes The buffers for the fixing solutions and washes were 005 M throughout, with Sorenson's phosphate at ph 6-8 being used for the corn roots and cacodylate at ph 7-4 being used for the tadpole tails. Washes in buffer were done 3 times at i-h intervals, and washes in water were done 4 times at 05-h intervals. Washes after OsO 4 and TCH treatment were at 45 C C to ensure removal of free reagent and prevent the deposition of TCH crystals in the tissue. Preparation of the TCH The often pinkish TCH crystals (05 g in a small beaker) were washed by repeated rinsing with distilled water until colourless. The washed crystals in 25 ml of water were heated to 60 C C for a few minutes, and then cooled to room temperature for 1 h to attain saturation at that temperature. During preparation the solution was shielded from strong light and before use was filtered by syringe through a Millipore filter of o^y-fim pore size. Dehydration and infiltration of tissue Dehydration of samples was done in a graded series of acetone-water mixtures at concentrations of 10, 30, 50, 70, 90% and pure acetone, all carried out in an ice bath to minimize osmotic effects, with changes at 05-h intervals. The specimens in pure acetone were warmed to room temperature and 2 more changes of acetone were made to assure dryness. Infiltration with epoxy resin monomer (Epon 812) was through a series of 25, 50, and 75% monomer in acetone at 2-h intervals, followed by pure monomer overnight and daily changes of pure monomer for 3 more days using a tissue rotator set at 1 rev/min. Fracturing, drying and mounting of tissue on stubs Tissues to be cracked in epoxy monomer were positioned in a shallow well drilled in a 6-mm aluminium plate with about half of the tissue projecting from the well and with a minimum of resin left in the well. The plate with the tissue was chilled with powdered dry ice to solidify
3 Cell organelles viewed with SEM 49 the resin with the tissue. To do this it was found convenient to use an ice bucket with a tightly fitted lid to prevent ice crystals from forming on the tissue. Cracking was accomplished by a single thrust of a knife precooled with liquid nitrogen. Dry ice may be used to chill the knife if care is taken to keep the knife thoroughly cooled prior to making the fracture. The plate and all of the cracked pieces of tissue were plunged into acetone at room temperature. The cracked tissues were collected in vials, washed a few times with pure acetone to remove the resin and dried by the critical point method of Anderson (1951) using liquid CO 2. Acetone was the transition fluid (Porter, Kelley & Andrews, 1972). The dried pieces were attached to aluminium stubs with silver paint and examined uncoated by SEM at from 10 to 30 kev with the fracture face of the specimen oriented nearly normal to the electron beam. Outline of the procedure The excised tissues to be prepared for SEM were: (1) fixed in buffered glutaraldehyde (3 %) f r J h an d 6 % for 2 h, the latter finally heated to 45 C for 15 min for more complete fixation, cooled to room temperature and washed in buffer; (2) treated in 2% buffered OsO 4 overnight and washed in water at 45 C; (3) treated with saturated TCH at room temperature for 3 min, heated to 45 C for 15 min, washed with water at 45 C and cooled to room temperature; (4) treated with 1 % aqueous OsO, for 1 h and rinsed with water; (5) dehydrated in cold acetone; (6) infiltrated with epoxy resin monomer; (7) chilled, cracked, and washed in acetone; (8) dried by the critical point method; and (9) mounted on stubs and observed by SEM. For some specimens steps 3 and 4 were repeated (osmium ligated twice) to increase the osmium content even further. Procedure for TEM Samples for TEM were processed through steps 1 through 5 (above), infiltrated in an epoxy mixture based on Luft (1961), polymerized at 60 C C for 3 days, sectioned with a diamond knife, and observed without further staining at 80 kev. RESULTS Figs. 1 and 2 compare the results achieved by the two methods of rendering tissue electrically conductive. In Fig. 1 a xylem initial cell in interphase from a corn root tip was fractured and coated with gold according to the procedures of Tanaka & lino (1972). The limits of the cell are outlined by the wall, which appears bright due to the roughness of the fracture through the fibrous wall. Conspicuous vacuoles appear within the cytoplasm especially in the upper portion of the figure. A large nucleus is located centrally, with its lighter nucleolus and presumed heterochromatin bodies against the darker nucleoplasm. Presumably most of the secondary electrons detected here arise from the gold coat applied to reveal the surface and provide a conductive path for electrons; however, stereoscopic study of pairs of such images lead us to the conclusion that a portion of the signal, notably that from parts of the nucleolus and chromatin, originates from below the coat, probably from the denser concentrations of osmium present in these regions. The remainder of the cytoplasm shows some irregularities in the fracture plane induced by various organelles associated with such cells, but interpretation is difficult at best. In contrast, Fig. 2 shows a cell probably in interphase and is from a corn root tip rendered electrically conductive by osmium ligation. The root was fractured in the same manner as that used for the cell in Fig. 1, but in this case is viewed uncoated. In this method sufficient
4 P. S. Woods and M. C. Ledbetter
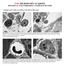
5 Cell organelles viewed with SEM 51 reduced osmium has accumulated in the specimen to provide a conductive path by which excess electrons may pass from the specimen to the grounded stub, thus avoiding charging effects. The image in Fig. 2 is derived almost exclusively from secondary electrons with little contribution from primary backscattered electrons as determined by the almost undetectable image displayed upon reversal of the V from the collector screen to 100 V to visualize backscattered electrons. The signal of secondary electrons will vary with the concentration of osmium within the tissue, rather than with surface irregularities, as for a metal-coated specimen. This difference in the way images are formed is illustrated by the appearance of the cell wall which has a relatively low affinity for osmium and as a consequence appears dark in Fig. 2 though bright in Fig. 1. The contents of the vacuoles appear similar with the 2 methods because of their particulate nature and osmium affinity; however, the limiting vacuolar membrane, or tonoplast, stands out in bright contrast in Fig. 2 because of the high osmium affinity relative to the surrounding cytoplasm, whereas such contrast is lacking in the metal-coated sample (Fig. 1), which shows the roughsurfaced cytoplasm simply terminating abruptly at the tonoplast. Membranes form a prominent feature of the cytoplasm in the uncoated fractured cell treated by the ligation method (Fig. 2), making it possible to identify such structures as the endoplasmic reticulum, nuclear envelope and mitochondria with cristae. These, as well as dictyosomes, and small vesicles making up the cell plate of cells in telophase have already been demonstrated (Woods & Ledbetter, 1974) in similarly treated corn roots. This is what one would expect from the distribution of osmium in cells as seen in thin section by TEM. Besides the highly visible membranes, the nucleolus and condensed heterochromatin bodies (barely visible in coated specimens) stand out in bold contrast against the darker nucleoplasm. The more diffuse euchromatin is also visible. In the coated specimen (Fig. 1) the image formed from secondary electrons reveals chiefly surface characteristics and shows little of the underlying osmium concentrations; however, in the uncoated, ligated sample, the secondary electron picture indicates osmium concentrations not only at the fracture surface, but also deeper within the specimen. This enhanced depth of imaging is illustrated in Figs. 3 and 4. Fig. 1. A scanning electron micrograph of a gold-coated xylem initial cell in interphase from a corn root tip fixed in buffered glutaraldehyde then OsOj and fractured in frozen epoxy resin monomer. Identifiable structures are: nucleus (n), nucleolus {mi), vacuoles (i>) and cell wall (cw). The structure marked he is presumed to be heterochromatin. Other organelles of the cytoplasm though detectable are of uncertain identity, x Fig. 2. A scanning electron micrograph of an uncoated meristematic cell probably in interphase from a corn root tip processed by the osmium ligation method but otherwise fixed and fractured as in Fig. 1. The richness of structure revealed in contrast to Fig. 1 is striking. The limits of the nucleus are clearly defined by its envelope (ne). Within the nucleoplasm the nucleolus (nu), condensed heterochromatin (lie) and diffuse euchromatin (ec) are seen in high contrast. Cytoplasmic organelles include: vacuoles (v), endoplasmic reticulum (er), and mitochondria (m) with cristae. x 8500.
6 P. S. Woods and M. C. Ledbetter
7 Cell organelles viewed with SEM 53 In Fig. 3 the fracture plane passes through a large filamentous mitochondrion in 3 places (arrows). That portions of the mitochondrion between breaks lie below the fractured surface is obvious from the micrograph of this uncoated and ligated specimen. From the predominant absence of condensed chromatin and general appearance of the nucleus the cell in Fig. 3 is judged to be in interphase. In Fig. 4 there are portions of 2 cells, the upper of which is in metaphase and shows various organelles distributed around the central zone of condensed chromosomes appearing as bright objects lying in a darker field. The nuclear envelope is lacking, as expected. Stereo views of pairs of micrographs of this cell give the impression that the chromosomes are rounded, with the less well defined and darker portions lying below the fractured surface. As is apparent in the micrograph, the microtubules of the spindle are not distinguishable. The web-like material that is visible in the spindle region (Fig. 4) is inconsistent with the image of microtubules usually obtained by TEM. In the lower cell of this illustration the nuclear envelope is clearly intact and, from the distribution of condensed chromatin, we estimate the cell to be in mid-prophase. Figs. 5 and 6 are of similarly treated cells from the epidermis of a frog tadpole tail as seen respectively in thin section by TEM and in fractured surface by SEM. Fixation and ligation of the 2 samples was identical and both are viewed without further metallic staining or coating. The identifiable structures in Fig. 5 include the nucleus with its double membrane, plasma membrane, endoplasmic reticulum, mitochondria, dense pigment granules, keratin filaments, desmosomes, basal lamina, orthogonal arrays of collagen and intercellular canaliculi. Most of the structures identified in Fig. 5 are also seen in Fig. 6, though with reversed contrast. The general location of the basal lamina with its associated filaments is visible as a bright zone adjacent to the collagen fibres. It is impossible to distinguish individual keratin filaments or ribosomes in this micrograph (Fig. 6); however, the outer and inner membranes of the nuclear envelope including pores (arrows of Fig. 6A) are easily visualized. Figs. 7 and 8 illustrate, respectively, a transmission micrograph and a scanning micrograph of striated muscle from frog tadpole tail. All of the bands and filaments typical of relaxed myofibrils seen in the transmission micrograph of Fig. 7 are also visible in the scanning micrograph of Fig. 8. These structures include the Z lines marking the limits of the sarcomere of each myofibril, the I bands with actin filaments Fig. 3. A scanning electron micrograph of portion of an uncoated cell in interphase from a corn root tip osmium ligated and processed as in Fig. 2. A large filamentous mitochondrion (m) is seen to be fractured in 3 places (arrows). Other cytoplasmic organelles include: vacuoles (v) and endoplasmic reticulum (er). Part of the nucleus with its nuclear envelope (ne), nucleolus (mi) and heterochromatin (he) are also visible. The euchromatin is highly dispersed in this nucleus, x Fig. 4. A scanning electron micrograph of portions of two uncoated cells in mitosis from a corn root tip osmium ligated and fractured as in Figs. 2 and 3. The upper cell is in metaphase and shows the condensed chromosomes (c) surrounded with less-dense material. The lower cell is in mid-prophase and shows condensed chromosomes (c), nucleolus (mi) and nuclear envelope (ne). x 8500.
8 ii!mt^:^.^^t :: P. S. Woods and M. C. Ledbetter
9 Cell organdies viewed with SEM 55 extending parallel with the myofibrils and spanning part way into the A bands, the myosin filaments also extending parallel with the myofibrils and forming the A bands, the H bands bisecting the A bands and the M lines in turn bisecting the H bands. The membranes of the sarcoplasmic reticulum are visible in both micrographs. While the triads, clearly seen in Fig. 7, are not as obvious in Fig. 8, others of our scanning micrographs show this structure more clearly. DISCUSSION It is worthwhile to consider the possibility that osmium depositions during the ligating step do not take place exclusively at the initial sites of osmium bonding. In all of our work we fix and ligate relatively large (2 mm 3 ) pieces of tissue. The transmission micrographs of Figs. 5 and 7 are from thin sections taken deep within the tissue block. It is clear that during ligation most of the added osmium is deposited at the site where osmium was first bound to cellular components during fixation. For the most part these micrographs are typical of transmission micrographs of thin sections stained by conventional means; however, our transmission micrographs of tadpole tails consistently show fine, randomly distributed grains within the tissue. These are especially clear at high magnification as in Fig. 7. The composition and cause of their appearance is not known. It is possible that they are composed of osmium and that they arise because of inability to remove completely either the osmic acid of the fixative, or the TCH during the ligating step; however, their presence in the animal but not the plant material may reflect differences in the ph or buffer systems used (cacodylate for the animal and phosphate for the plant material). Quite obviously their presence in fractured tissue would tend to degrade the image at the cryofractured surface. It is disappointing that ribosomes and microtubules of the spindle are not visualized by this method. It is reasonable to expect structures of this size (24-27 nm) to be resolved if they are preserved by the procedure with sufficient metal attached. There is evidence that ligation may be the limiting step, in that examination of the ligated material in thin section by TEM revealed that the spindle microtubules of the corn roots are difficult to visualize and they and ribosomes in both the corn and tadpole are poorly defined in comparison to similar material lacking the TCH treatment. Fig. 5. A transmission electron micrograph of portions of cells from the epidermis of a tadpole tail fixed and processed in bulk by the osmium ligation method. The thin section received no additional metallic treatment. Structures visible include: nucleus (n), nuclear envelope (ne), plasma membrane (pm), endoplasmic reticulum (er), mitochondria (m), pigment granules (pg), keratin filaments (kf), desmosomes (d), basal lamina (bl), collagen (co) and intercellular canaliculi (ic). x Fig. 6. A scanning electron micrograph of portions of uncoated cells from the epidermis of a tadpole tail osmium ligated and processed similarly to the plant roots of Figs Labelling as in Fig. 5. x Fig. 6A. An enlarged view of the nucleus of Fig. 6 showing the z unit membranes of the nuclear envelope and nuclear pores (arrows), x
10 P. S. Woods and M. C. Ledbetter
11 Cell organelles viewed with SEM 57 In regard to the best resolutions attained in our scanning micrographs taken at 20 or 30 kev the thinnest regions of the unit membranes that make up the nuclear envelope of the basal cell nucleus shown in Fig. 6 A were measured at approximately 17 nm. Also, in Fig. 8 the smallest filaments in the I bands of this scanning micrograph were measured at approximately 15 nm. In both of these cases the values approach 1 o nm which is the present limit of our SEM and possibly is a practical limit for this method. Although the material of the I-band regions is known to be composed of actin filaments of approximately 5 nm diameter, it is assumed that the structures visualized in Fig. 8 represent aggregates of the smaller actin filaments rather than individuals. It should be pointed out that osmium ligation in combination with resin cracking does not preclude getting information about the surface. Though not illustrated here, we found that SEM images of the more usual sort, restricted to surface irregularities such as seen in Fig. 1, may be obtained from our uncoated, ligated samples by either tilting the mounted specimen to steep angles, by using accelerating voltages of 5 kev or less, or by coating with metal after viewing of the uncoated sample is completed. We chose the method derived from Tanaka & lino (1972) to fracture our tissues, but there is good reason to believe that equivalent results could be had by using the method of Humphreys et al. (1974) in which ethanol-infiltrated tissue is fractured after cooling in liquid nitrogen. Our results show that low-temperature fracturing of osmium-ligated tissue is a useful preparative method for biological material to be examined uncoated by SEM. Plant and animal material so prepared can be viewed at low magnification to study the relationships of tissues and cells or to locate cells of particular interest. Selected areas then can be viewed at higher magnification to study details of cell contents at a resolution approaching that of thin-sectioned material viewed by TEM. In addition some 3-dimensional information from below the fractured surface is made available and this can be especially useful as an aid in interpreting complex structure. This research was carried out at Brookhaven National Laboratory under the auspices of the U.S. Energy Research and Development Administration. We are grateful to Mr R. N. Ruffing and Mr Walter J. Geisbusch for technical assistance rendered. Fig. 7. A transmission electron micrograph of relaxed striated muscle from a tadpole tail fixed and processed in bulk by the osmium ligation method. The thin section received no additional metallic treatment. Structures visible include: Z lines (Z) marking the limits of the sarcomere of each myofibril, I bands (/) with actin filaments extending parallel with the myofibrils and spanning into the A bands (^4), the myosin filaments also extending parallel with the myofibrils and forming the A bands, the H bands (H) bisecting the A bands and the M lines (M) in turn bisecting the H bands. The sarcoplasmic reticulum (sr) and triads or tubular system (ts) are also visible, x Fig. 8. A scanning electron micrograph of uncoated relaxed striated muscle from a tadpole tail osmium ligated twice but otherwise processed similarly to the tissues illustrated in Figs. 2-4 and 6. x
12 58 P. S. Woods and M. C. Ledbetter REFERENCES ANDERSON, T. F. (195I). Techniques for the preservation of three dimensional structures in specimens for the electron microscope. Trans. N.Y. Acad. Set. 13, GUTTMAN, H. N. & STYSKAL, R. C. (1971). Preparation of suspended cells for SEM examination of internal cellular structures. In Scanning Electron Microscopy/i97i, 4th A. Proc. IIT Res. Inst., Chicago, 111., pp HUMPHREYS, W. J., SPURLOCK, B. O. & JOHNSON, J. S. (1974). Critical point drying of ethanolinfiltrated, cryofractured biological specimens for scanning electron microscopy. In Scanning Electron Microscopy/1974, 7th A. Proc. IIT Res. Inst., Chicago, 111., pp HUMPHREYS, W. J. & WODZICKI, T. J. (1972). Methods for viewing by scanning electron microscopy the interior organization of protoplasts of plant cells. In 30th A. Proc. Electron Microsc. Soc. Am. (ed. C. J. Arceneaux), pp , Los Angeles, California. KELLEY, R. O., DEKKER, R. A. & BLUEMINK, J. G. (1973). Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J. Ultrastruct. Res. 45, LIM, D. J. (1971). Scanning electron microscopic observation on non-mechanically cryofractured biological tissue. In Scanning Electron Microscopy/1971, 4th A. Proc. IIT Res. Inst., Chicago, 111., pp LUFT, H. J. (1961). Improvements in epoxy resin embedding methods. J. biophys. biochem. Cytol. 9, PANESSA, B. J. & GENNARO, J. F. (1972). A method for direct observation of botanical tissue and intracellular contents by SEM. In 30th A. Proc. Electron Microsc. Soc. Am. (ed. C. J. Areceneaux), pp Los Angeles, California. PANESSA, B. J. & GENNARO, J. F. (1973). Use of potassium iodide/lead acetate for examining uncoated specimens. In Scanning Electron Microscopy/1973, 6th A. Proc. IIT Res. Inst., Chicago, 111., PORTER, K. R., KELLEY, D. & ANDREWS, P. M. (1972). The preparation of cultured cells and soft tissues for scanning electron microscopy. In 5th A. Stereoscan Colloquium, Kent Cambridge Scientific Co., Morton Grove, 111., pp SELIGMAN, A. M., WASSERKRUG, H. L. & HANKER, J. S. (1966). A new staining method (OTO) for enhancing contrast of lipid-containing membranes and droplets in osmium tetroxidefixed tissue with osmiophilic thiocarbohydrazide (TCH). J. Cell Biol. 30, TANAKA, K. & IINO, A. (1972). Frozen resin cracking method for scanning electron microscopy and its application to cytology. In 30^ A. Proc. Electron Microsc. Soc. Am. (ed. C. J. Arceneaux), pp Los Angeles, California. WOODS, P. S. & LEDBETTER, M. C. (1974). A method of direct visualization of plant cell organelles for scanning electron microscopy. In 32nd A. Proc. Electron Microsc. Soc. Am. (ed. C. J. Arceneaux), pp St Louis, Missouri. (Received 13 September 1975)
CHAPTER 1. Aspects of the Three-Dimensional Intracellular Organization of Mesocarp Cells as Revealed by Scanning Electron Microscopy
 CHAPTER 1 Aspects of the Three-Dimensional Intracellular Organization of Mesocarp Cells as Revealed by Scanning Electron Microscopy Reprinted from Protoplasma by permission of Springer-Verlag Publishers,
CHAPTER 1 Aspects of the Three-Dimensional Intracellular Organization of Mesocarp Cells as Revealed by Scanning Electron Microscopy Reprinted from Protoplasma by permission of Springer-Verlag Publishers,
Chapter. Methods for and. Analysis of Plant Cell Tissue Ultrastructure. Contents. 1.1 Introduction. John E. Mayfield and William V.
 In: Dashek, William V., ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press: pp. 3-11. Chapter 1. 1997. Chapter Methods for and Analysis of Plant Cell Tissue Ultrastructure
In: Dashek, William V., ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press: pp. 3-11. Chapter 1. 1997. Chapter Methods for and Analysis of Plant Cell Tissue Ultrastructure
COPYRIGHTED MATERIAL. Tissue Preparation and Microscopy. General Concepts. Chemical Fixation CHAPTER 1
 CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
Improved Fixation of Cellulose-Acetate Reverse-Osmosis Membrane for Scanning Electron Microscopy
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Feb. 1985, p. 446450 0099-2240/85/020446-05$02.00/0 Copyright 1985, American Society for Microbiology Vol. 49, No. 2 Improved Fixation of Cellulose-Acetate Reverse-Osmosis
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Feb. 1985, p. 446450 0099-2240/85/020446-05$02.00/0 Copyright 1985, American Society for Microbiology Vol. 49, No. 2 Improved Fixation of Cellulose-Acetate Reverse-Osmosis
2. Know the parts of a light microscope and general rules for using and focusing a microscope, such as:
 SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions: Biology Mounting medium Telophase Organelle Cell Theory Cell cycle Cytokinesis
SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions: Biology Mounting medium Telophase Organelle Cell Theory Cell cycle Cytokinesis
Published Online: 1 February, 1960 Supp Info: on October 30, 2018 jcb.rupress.org Downloaded from
 Published Online: 1 February, 1960 Supp Info: http://doi.org/10.1083/jcb.7.1.197 Downloaded from jcb.rupress.org on October 30, 2018 BRIEF NOTES 197 The Use of Potassium Permanganate as an Electron-Dense
Published Online: 1 February, 1960 Supp Info: http://doi.org/10.1083/jcb.7.1.197 Downloaded from jcb.rupress.org on October 30, 2018 BRIEF NOTES 197 The Use of Potassium Permanganate as an Electron-Dense
2. Know the parts of a light microscope and general rules for using and focusing a microscope, such as:
 SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions: Biology Mounting medium Telophase Organelle Cell Theory Cell cycle Cytokinesis
SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions: Biology Mounting medium Telophase Organelle Cell Theory Cell cycle Cytokinesis
BIOLOGICAL SAMPLE PREPARATION FOR TEM OBSERVATION. TEM Seminar Nov 16, 2017 Astari Dwiranti, Ph.D
 BIOLOGICAL SAMPLE PREPARATION FOR TEM OBSERVATION TEM Seminar Nov 16, 2017 Astari Dwiranti, Ph.D Why do we need EM for biological samples? (O'Connor and Adams, 2010) Why do we need EM for biological samples?
BIOLOGICAL SAMPLE PREPARATION FOR TEM OBSERVATION TEM Seminar Nov 16, 2017 Astari Dwiranti, Ph.D Why do we need EM for biological samples? (O'Connor and Adams, 2010) Why do we need EM for biological samples?
Introduction to Botany. Lecture 11
 Introduction to Botany. Lecture 11 Alexey Shipunov Minot State University September 26, 2014 Shipunov (MSU) Introduction to Botany. Lecture 11 September 26, 2014 1 / 21 Outline 1 Questions and answers
Introduction to Botany. Lecture 11 Alexey Shipunov Minot State University September 26, 2014 Shipunov (MSU) Introduction to Botany. Lecture 11 September 26, 2014 1 / 21 Outline 1 Questions and answers
THE MAGNITUDE OF THE EXTRACELLULAR SPACE IN ELECTRON MICROGRAPHS OF SUPERFICIAL AND DEEP REGIONS OF THE CEREBRAL CORTEX
 J. Cell Sci. 6, 793-805 (1970) 793 Printed in Great Britain THE MAGNITUDE OF THE EXTRACELLULAR SPACE IN ELECTRON MICROGRAPHS OF SUPERFICIAL AND DEEP REGIONS OF THE CEREBRAL CORTEX A. VAN HARREVELD AND
J. Cell Sci. 6, 793-805 (1970) 793 Printed in Great Britain THE MAGNITUDE OF THE EXTRACELLULAR SPACE IN ELECTRON MICROGRAPHS OF SUPERFICIAL AND DEEP REGIONS OF THE CEREBRAL CORTEX A. VAN HARREVELD AND
1. Carry the microscope in an upright position with both hands and place the base of the microscope 5cm from the edge of the bench
 The Microscope Operating the compound light microscope 1. Carry the microscope in an upright position with both hands and place the base of the microscope 5cm from the edge of the bench 2. Check that lenses
The Microscope Operating the compound light microscope 1. Carry the microscope in an upright position with both hands and place the base of the microscope 5cm from the edge of the bench 2. Check that lenses
EXPERIMENT 2 Cell Fractionation and DNA Isolation
 EXPERIMENT 2 Cell Fractionation and DNA Isolation Background Information A. The Eukaryotic Cell Cells are frequently classified into two basic types: prokaryotic and eukar yotic. The prokaryotic cell,
EXPERIMENT 2 Cell Fractionation and DNA Isolation Background Information A. The Eukaryotic Cell Cells are frequently classified into two basic types: prokaryotic and eukar yotic. The prokaryotic cell,
(From the Department of Pathology, Albert Einstein College of Medicine, Yeshiva University, New York)
 PRESERVATION OF THE FINE STRUCTURE OF ISOLATED LIVER CELL PARTICULATES WITH POLYVINYLPYRROLLIDONE- SUCROSE* BY ALEX B. NOVIKOFF, 1M.D. (From the Department of Pathology, Albert Einstein College of Medicine,
PRESERVATION OF THE FINE STRUCTURE OF ISOLATED LIVER CELL PARTICULATES WITH POLYVINYLPYRROLLIDONE- SUCROSE* BY ALEX B. NOVIKOFF, 1M.D. (From the Department of Pathology, Albert Einstein College of Medicine,
Monday: Y42 G53 Tuesday: Y42 G53 Wednesday: Y42 J11
 Locations: Irchel building 42, Level H and F Locations: Irchel building 42, Level H and F Self-study sessions: Monday: Y42 G53 Tuesday: Y42 G53 Wednesday: Y42 J11 1 Center for Microscopy and Image Analysis
Locations: Irchel building 42, Level H and F Locations: Irchel building 42, Level H and F Self-study sessions: Monday: Y42 G53 Tuesday: Y42 G53 Wednesday: Y42 J11 1 Center for Microscopy and Image Analysis
Introduction to Histology
 Introduction to Histology The name "Histology" is derived from the Greek word for a tissue "Histos", and "-logos" = the study of It is tightly bounded to molecular biology, genetics, immunology and other
Introduction to Histology The name "Histology" is derived from the Greek word for a tissue "Histos", and "-logos" = the study of It is tightly bounded to molecular biology, genetics, immunology and other
Introduction to Electron Microscopy Andres Kaech
 Center for Microscopy and Image Analysis Introduction to Electron Microscopy Andres Kaech The types of electron microscopes Transmission electron microscope (TEM) Scanning electron microscope (SEM) 1 The
Center for Microscopy and Image Analysis Introduction to Electron Microscopy Andres Kaech The types of electron microscopes Transmission electron microscope (TEM) Scanning electron microscope (SEM) 1 The
Introduction to Histology
 Introduction to Histology Histology The term "Histology" is derived from the Greek word for a tissue "Histos", and "-logos" = the study of Histology : Is the study of tissues and how they are arranged
Introduction to Histology Histology The term "Histology" is derived from the Greek word for a tissue "Histos", and "-logos" = the study of Histology : Is the study of tissues and how they are arranged
(From the Division of Biological and Medical Research, Argonne National Laboratory, Lemont, Illinois) Methods and Materials
 OBSERVATIONS ON FIBRILLOGENESIS IN THE CONNECTIVE TISSUE OF THE CHICK EMBRYO WITH THE AID OF SILVER IMPREGNATION* BY F. WASSERMANN, M.D., AND L. KUBOTA (From the Division of Biological and Medical Research,
OBSERVATIONS ON FIBRILLOGENESIS IN THE CONNECTIVE TISSUE OF THE CHICK EMBRYO WITH THE AID OF SILVER IMPREGNATION* BY F. WASSERMANN, M.D., AND L. KUBOTA (From the Division of Biological and Medical Research,
NUCLEUS. Fig. 2. Various stages in the condensation of chromatin
 NUCLEUS Animal cells contain DNA in nucleus (contains ~ 98% of cell DNA) and mitochondrion. Both compartments are surrounded by an envelope (double membrane). Nuclear DNA represents some linear molecules
NUCLEUS Animal cells contain DNA in nucleus (contains ~ 98% of cell DNA) and mitochondrion. Both compartments are surrounded by an envelope (double membrane). Nuclear DNA represents some linear molecules
A COMPARATIVE STUDY OF NUCLEOCYTOPLASMIC INTERACTIONS
 A COMPARATIVE STUDY OF NUCLEOCYTOPLASMIC INTERACTIONS CARL M. FELDHERR. From the Department of Anatomical Sciences, University of Florida, Gainesville, Florida 32601 It is evident that the pores of the
A COMPARATIVE STUDY OF NUCLEOCYTOPLASMIC INTERACTIONS CARL M. FELDHERR. From the Department of Anatomical Sciences, University of Florida, Gainesville, Florida 32601 It is evident that the pores of the
Introduction to histology and its methods of study
 Introduction to histology and its methods of study Li shulei lishulei@tom.com Department of Histology & Embryology 1 What is histology Definition Cell: smallest units functions in the human body Tissue
Introduction to histology and its methods of study Li shulei lishulei@tom.com Department of Histology & Embryology 1 What is histology Definition Cell: smallest units functions in the human body Tissue
Improved Method of Embedding with Epoxy Resin 'Quetol
 Okajimas Folia Anat. Jpn., 58(4-6): 661-674, March 1982 Improved Method of Embedding with Epoxy Resin 'Quetol 651' for Both Light and Electron Microscopic Observation of Identical Sites in Semi-thin Sections
Okajimas Folia Anat. Jpn., 58(4-6): 661-674, March 1982 Improved Method of Embedding with Epoxy Resin 'Quetol 651' for Both Light and Electron Microscopic Observation of Identical Sites in Semi-thin Sections
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
by JAKOB R~LI and PER R. FLOOD, Institute of Anatomy, University of Bergen, 5000 Bergen, Norway Paper accepted 4 November 1977
 CJ Journal of Microscopy, Vol. 112, Pt 3, April 1978, pp. 359-364. Paper accepted 4 November 1977 A simple method for the determination of thickness and grain size of deposited films as used on non-conductive
CJ Journal of Microscopy, Vol. 112, Pt 3, April 1978, pp. 359-364. Paper accepted 4 November 1977 A simple method for the determination of thickness and grain size of deposited films as used on non-conductive
Investigation of cellular uptake mechanisms by correlative TEM and SIM
 Collaborative Research Center (SFB 1278) Investigation of cellular uptake mechanisms by correlative TEM and SIM Rainer Heintzmann, Fengjiao Ma, Institute of Physical Chemistry Stephanie Höppener, Martin
Collaborative Research Center (SFB 1278) Investigation of cellular uptake mechanisms by correlative TEM and SIM Rainer Heintzmann, Fengjiao Ma, Institute of Physical Chemistry Stephanie Höppener, Martin
Cells and Tissues. Overview CELLS
 Cells and Tissues WIll The basic unit of structure and function in the human body is the cell. Each of a cell's parts, or organelles, as well as the entire cell, is organized to perform a specific function.
Cells and Tissues WIll The basic unit of structure and function in the human body is the cell. Each of a cell's parts, or organelles, as well as the entire cell, is organized to perform a specific function.
Scanning electron microscopy of corneal wound healing in the rabbit. Barrett G. Haik and Marilyn L. Zimny
 Scanning electron microscopy of corneal wound healing in the rabbit Barrett G. Haik and Marilyn L. Zimny Corneal lesions 7.5 mm. in diameter were made with an ocidar trephine in rabbits. The time periods
Scanning electron microscopy of corneal wound healing in the rabbit Barrett G. Haik and Marilyn L. Zimny Corneal lesions 7.5 mm. in diameter were made with an ocidar trephine in rabbits. The time periods
COMPLEX OF EUGLENA GRACILIS
 Published Online: 1 February, 1965 Supp Info: http://doi.org/10.1083/jcb.24.2.253 Downloaded from jcb.rupress.org on May 12, 2018 THE ULTRASTRUCTURE OF THE PELLICLE COMPLEX OF EUGLENA GRACILIS JOACHIM
Published Online: 1 February, 1965 Supp Info: http://doi.org/10.1083/jcb.24.2.253 Downloaded from jcb.rupress.org on May 12, 2018 THE ULTRASTRUCTURE OF THE PELLICLE COMPLEX OF EUGLENA GRACILIS JOACHIM
Vampirococcus sucking on Chromatium
 July 1993 MBL Microbial Diversity (n ni-i-i-i showed that not only hroniatiunz but also Thiocapsa served as a host. The goal of this study was to enrich and if possible characterize further Vampirococcus
July 1993 MBL Microbial Diversity (n ni-i-i-i showed that not only hroniatiunz but also Thiocapsa served as a host. The goal of this study was to enrich and if possible characterize further Vampirococcus
THE USE OF LEAD CITRATE AT HIGH ph AS AN ELECTRON-OPAQUE STAIN IN ELECTRON MICROSCOPY
 THE USE OF LEAD CITRATE AT HIGH ph AS AN ELECTRON-OPAQUE STAIN IN ELECTRON MICROSCOPY EDWARD S. REYNOLDS. From the Department of Anatomy, Harvard Medical School, Boston Aqueous solutions of lead salts
THE USE OF LEAD CITRATE AT HIGH ph AS AN ELECTRON-OPAQUE STAIN IN ELECTRON MICROSCOPY EDWARD S. REYNOLDS. From the Department of Anatomy, Harvard Medical School, Boston Aqueous solutions of lead salts
INVESTIGATIVE OPHTHALMOLOGY. Critical point drying of soft biological material for the scanning electron microscope
 March 1972 Volume 11, Number 3 INVESTIGATIVE OPHTHALMOLOGY Critical point drying of soft biological material for the scanning electron microscope Morton E. Smith and Edward H. Finke An apparatus for the
March 1972 Volume 11, Number 3 INVESTIGATIVE OPHTHALMOLOGY Critical point drying of soft biological material for the scanning electron microscope Morton E. Smith and Edward H. Finke An apparatus for the
plasma membrane Golgi apparatus cytoplasm lysosome endoplasmic reticulum nucleus nucleolus ribosome mitochondrion
 Demonstrate understanding of life processes at the cellular level 1 Achievement Standard 91156 Demonstrate understanding of life processes at the cellular level BIOLOGY 2.4 Externally assessed 4 credits
Demonstrate understanding of life processes at the cellular level 1 Achievement Standard 91156 Demonstrate understanding of life processes at the cellular level BIOLOGY 2.4 Externally assessed 4 credits
Biochemistry and Cell Biology
 Biochemistry and Cell Biology Monomersare simple molecules that can be linked into chains. (Nucleotides, Amino acids, monosaccharides) Polymersare the long chains of monomers. (DNA, RNA, Cellulose, Protein,
Biochemistry and Cell Biology Monomersare simple molecules that can be linked into chains. (Nucleotides, Amino acids, monosaccharides) Polymersare the long chains of monomers. (DNA, RNA, Cellulose, Protein,
Preparation of tissues for study
 Preparation of tissues for study HISTOLOGY : It is the branch of science which deals with the microscopic study of normal tissue HISTOPATHOLOGY : It is the branch of science which deals with the microscopic
Preparation of tissues for study HISTOLOGY : It is the branch of science which deals with the microscopic study of normal tissue HISTOPATHOLOGY : It is the branch of science which deals with the microscopic
A rapid method for electron microscopic examination of blood cells
 Journal of Clinical Pathology, 1979, 32, 162-1 67 A rapid method for electron microscopic examination of blood cells ANTONIO COPPOLA From the Department ofpathology, College of Medicine, Downstate Medical
Journal of Clinical Pathology, 1979, 32, 162-1 67 A rapid method for electron microscopic examination of blood cells ANTONIO COPPOLA From the Department ofpathology, College of Medicine, Downstate Medical
Chapter 13. The Nucleus. The nucleus is the hallmark of eukaryotic cells; the very term eukaryotic means having a "true nucleus".
 Chapter 13 The Nucleus The nucleus is the hallmark of eukaryotic cells; the very term eukaryotic means having a "true nucleus". Fig.13.1. The EM of the Nucleus of a Eukaryotic Cell 13.1. The Nuclear Envelope
Chapter 13 The Nucleus The nucleus is the hallmark of eukaryotic cells; the very term eukaryotic means having a "true nucleus". Fig.13.1. The EM of the Nucleus of a Eukaryotic Cell 13.1. The Nuclear Envelope
AP 5301/8301 LABORATORY MANUAL
 AP 5301/8301 LABORATORY MANUAL Department of Physics & Materials Science City University of Hong Kong Contents Table of Contents. 1 Project 1: Scanning Electron Microscopy (SEM). 2 Project 2: Microscopic
AP 5301/8301 LABORATORY MANUAL Department of Physics & Materials Science City University of Hong Kong Contents Table of Contents. 1 Project 1: Scanning Electron Microscopy (SEM). 2 Project 2: Microscopic
Reinforcement. Cells and Life CHAPTER 1 LESSON 1
 Reinforcement Cells and Life LESSON 1 Directions: In numbers 1 through 4 below, a code letter has been substituted for each letter of the alphabet. To find out what the sentence says, use the following
Reinforcement Cells and Life LESSON 1 Directions: In numbers 1 through 4 below, a code letter has been substituted for each letter of the alphabet. To find out what the sentence says, use the following
Supporting Protocols
 Supporting Protocols This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec ECM Hydrogels. Introduction Cells and organoids may form complex
Supporting Protocols This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec ECM Hydrogels. Introduction Cells and organoids may form complex
Chapter 5 DNA and Chromosomes
 Chapter 5 DNA and Chromosomes DNA as the genetic material Heat-killed bacteria can transform living cells S Smooth R Rough Fred Griffith, 1920 DNA is the genetic material Oswald Avery Colin MacLeod Maclyn
Chapter 5 DNA and Chromosomes DNA as the genetic material Heat-killed bacteria can transform living cells S Smooth R Rough Fred Griffith, 1920 DNA is the genetic material Oswald Avery Colin MacLeod Maclyn
On colouring epon-embedded tissue sections with Sudan black B or Nile blue A for light microscopy
 109 On colouring epon-embedded tissue sections with Sudan black B or Nile blue A for light microscopy By S. M. McGEE-RUSSELL and N. B. SMALE (From the Electron Microscopy Laboratory, Virus Research Unit,
109 On colouring epon-embedded tissue sections with Sudan black B or Nile blue A for light microscopy By S. M. McGEE-RUSSELL and N. B. SMALE (From the Electron Microscopy Laboratory, Virus Research Unit,
Dark-Field Electron Microscopy of Thin Sections of
 JOURNAL OF BACTERIOLOGY, Oct. 1974, p. 527-531 Copyright 0 1974 American Society for Microbiology Vol. 120, No. 1 Printed in U.S.A. Dark-Field Electron Microscopy of Thin Sections of Trichosporon cutaneum
JOURNAL OF BACTERIOLOGY, Oct. 1974, p. 527-531 Copyright 0 1974 American Society for Microbiology Vol. 120, No. 1 Printed in U.S.A. Dark-Field Electron Microscopy of Thin Sections of Trichosporon cutaneum
Biology unit. Big Idea. Cells are derived from cells Part 1 Review, what s in a cell!?
 Biology unit Big Idea Cells are derived from cells Part 1 Review, what s in a cell!? (cell nucleus and DNA-Gr 10 material for next year) http://www.popsci.com/science/article/2013-03/watch-absolutely-beautiful-animatedexplainer-dna
Biology unit Big Idea Cells are derived from cells Part 1 Review, what s in a cell!? (cell nucleus and DNA-Gr 10 material for next year) http://www.popsci.com/science/article/2013-03/watch-absolutely-beautiful-animatedexplainer-dna
Which hydrogel preparation for immunostaining protocol should I use?
 Protocol: Preparation of TissueSpec hydrogels for immunostaining This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec matrix hydrogels.
Protocol: Preparation of TissueSpec hydrogels for immunostaining This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec matrix hydrogels.
Cell cycle: Cell growth and division (when the replication and segregation of chromosomes occurs). Interphase: Interphase Chromosomes
 Chromosomes exist in different states throughout the life of a cell Cell cycle: Cell growth and division (when the replication and segregation of chromosomes occurs). Interphase: Interphase Chromosomes
Chromosomes exist in different states throughout the life of a cell Cell cycle: Cell growth and division (when the replication and segregation of chromosomes occurs). Interphase: Interphase Chromosomes
Conditions Critical for Optimal Visualization of Bacteriophage
 JOURNAL OF VIROLOGY, June 1975, p. 1498-1503 Copyright 0 1975 American Society for Microbiology Vol. 15, No. 6 Printed in U.S.A. Conditions Critical for Optimal Visualization of Bacteriophage Adsorbed
JOURNAL OF VIROLOGY, June 1975, p. 1498-1503 Copyright 0 1975 American Society for Microbiology Vol. 15, No. 6 Printed in U.S.A. Conditions Critical for Optimal Visualization of Bacteriophage Adsorbed
SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions:
 SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions: Apoptosis Cancer Cell membrane Cell specialization Cell wall Centriole Chloroplast
SNC 2DI Exam Review: Biology Unit 1. Understand the meaning of the following terms. Be able to recognize their definitions: Apoptosis Cancer Cell membrane Cell specialization Cell wall Centriole Chloroplast
Methods BRIEF NOTES 125
 Published Online: 25 January, 1958 Supp Info: http://doi.org/10.1083/jcb.4.1.125 Downloaded from jcb.rupress.org on October 13, 2018 BRIEF NOTES 125 The Fine Structure of Kappa in Killer Stock 51 of Paramecium
Published Online: 25 January, 1958 Supp Info: http://doi.org/10.1083/jcb.4.1.125 Downloaded from jcb.rupress.org on October 13, 2018 BRIEF NOTES 125 The Fine Structure of Kappa in Killer Stock 51 of Paramecium
1 The role of scanning electron microscopy in cell and molecular biology: SEM basics, past accomplishments, and new frontiers
 1 The role of scanning electron microscopy in cell and molecular biology: SEM basics, past accomplishments, and new frontiers 1.1 Introduction New developments in scanning electron microscopy (SEM) have
1 The role of scanning electron microscopy in cell and molecular biology: SEM basics, past accomplishments, and new frontiers 1.1 Introduction New developments in scanning electron microscopy (SEM) have
. Viability of colonies was then assessed using the WST-1 reagent as described above, and normalized relative to untreated controls.
 Cell viability analysis in the absence of disaggregation To assess cell viability in the absence of disaggregation, quintuplicate samples of cells at 5 x 1 5 /ml were treated with mab (1 µg/ml) for 24
Cell viability analysis in the absence of disaggregation To assess cell viability in the absence of disaggregation, quintuplicate samples of cells at 5 x 1 5 /ml were treated with mab (1 µg/ml) for 24
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
The principles and practice of electron microscopy
 The principles and practice of electron microscopy Second Edition Ian M. Watt CAMBRIDGE UNIVERSITY PRESS Contents Preface tofirstedition page ix Preface to second edition xi 1 Microscopy with light and
The principles and practice of electron microscopy Second Edition Ian M. Watt CAMBRIDGE UNIVERSITY PRESS Contents Preface tofirstedition page ix Preface to second edition xi 1 Microscopy with light and
Resolution of Microscopes Visible light is nm Dry lens(0.5na), green(530nm light)=0.65µm=650nm for oil lens (1.4NA) UV light (300nm) = 0.13µm f
 Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
1/24/2012. Cell. Plasma Membrane
 Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division Cell Basic unit of structure and function in body
Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division Cell Basic unit of structure and function in body
Staining and Embedding the Whole Mouse Brain for Electron Microscopy
 Staining and Embedding the Whole Mouse Brain for Electron Microscopy Shawn Mikula, Jonas Binding, Winfried Denk Supplementary Item Supplementary Figure 1 Supplementary Table 1 Supplementary Table 2 Supplementary
Staining and Embedding the Whole Mouse Brain for Electron Microscopy Shawn Mikula, Jonas Binding, Winfried Denk Supplementary Item Supplementary Figure 1 Supplementary Table 1 Supplementary Table 2 Supplementary
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Transmission Electron Microscopic Study of Antibiotic Action on Klebsiella pneumoniae Biofilm
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2002, p. 2679 2683 Vol. 46, No. 8 0066-4804/02/$04.00 0 DOI: 10.1128/AAC.46.8.2679 2683.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2002, p. 2679 2683 Vol. 46, No. 8 0066-4804/02/$04.00 0 DOI: 10.1128/AAC.46.8.2679 2683.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
Practical 2P8 Transmission Electron Microscopy
 Practical 2P8 Transmission Electron Microscopy Originators: Dr. M. L. Jenkins and Prof. J. M. Titchmarsh What you should learn from this practical Science This practical ties-in with the lecture course
Practical 2P8 Transmission Electron Microscopy Originators: Dr. M. L. Jenkins and Prof. J. M. Titchmarsh What you should learn from this practical Science This practical ties-in with the lecture course
Practical 2P8 Transmission Electron Microscopy
 Practical 2P8 Transmission Electron Microscopy Originators: Dr. N.P. Young and Prof. J. M. Titchmarsh What you should learn from this practical Science This practical ties-in with the lecture course on
Practical 2P8 Transmission Electron Microscopy Originators: Dr. N.P. Young and Prof. J. M. Titchmarsh What you should learn from this practical Science This practical ties-in with the lecture course on
Silver Diffusion Bonding and Layer Transfer of Lithium Niobate to Silicon
 Chapter 5 Silver Diffusion Bonding and Layer Transfer of Lithium Niobate to Silicon 5.1 Introduction In this chapter, we discuss a method of metallic bonding between two deposited silver layers. A diffusion
Chapter 5 Silver Diffusion Bonding and Layer Transfer of Lithium Niobate to Silicon 5.1 Introduction In this chapter, we discuss a method of metallic bonding between two deposited silver layers. A diffusion
EMS MICROSCOPY ACADEMY BIOLOGICAL TEM WORKSHOP: A COMPLETE PICTURE
 Examples of the endless possibilities in the field of Microscopy Bone Marrow: Transmission electron microscope image of a thin section cut through an area of bone marrow area near the cartilage/bone interface
Examples of the endless possibilities in the field of Microscopy Bone Marrow: Transmission electron microscope image of a thin section cut through an area of bone marrow area near the cartilage/bone interface
A combined method for correlative 3D imaging of biological samples from macro to nano scale
 7 8 9 0 7 8 9 A combined method for correlative D imaging of biological samples from macro to nano scale Manuela Kellner,, *, Marko Heidrich, *, Raoul-Amadeus Lorbeer, Georgios C. Antonopoulos, Lars Knudsen,,
7 8 9 0 7 8 9 A combined method for correlative D imaging of biological samples from macro to nano scale Manuela Kellner,, *, Marko Heidrich, *, Raoul-Amadeus Lorbeer, Georgios C. Antonopoulos, Lars Knudsen,,
Foundations in Microbiology Seventh Edition
 Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 3 Tools of the Laboratory: The Methods for Studying Microorganisms Copyright The McGraw-Hill Companies, Inc. Permission
Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 3 Tools of the Laboratory: The Methods for Studying Microorganisms Copyright The McGraw-Hill Companies, Inc. Permission
A Comparison of Techniques Useful for Preparing Nematodes for Scanning Electron Microscopy 1
 Journal of Nematology 18(4):479-487. 1986. The Society of Nematologists 1986. A Comparison of Techniques Useful for Preparing Nematodes for Scanning Electron Microscopy 1 J. D. EISENBACK ~ Abstract: Second-stage
Journal of Nematology 18(4):479-487. 1986. The Society of Nematologists 1986. A Comparison of Techniques Useful for Preparing Nematodes for Scanning Electron Microscopy 1 J. D. EISENBACK ~ Abstract: Second-stage
Supplementary Information
 Supplementary Information Atmospheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine Au nanodots for SERS sensing Samuel Yick, Zhao Jun Han and Kostya (Ken)
Supplementary Information Atmospheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine Au nanodots for SERS sensing Samuel Yick, Zhao Jun Han and Kostya (Ken)
Cell Structure and Function
 Cell Structure and Function Dead White Men Who Discovered (and were made of) Cells: Anton Van Leeuwenhoek Robert Hooke Where the Magic Happened Schleiden Cell Theory All plants are made of cells Schwann
Cell Structure and Function Dead White Men Who Discovered (and were made of) Cells: Anton Van Leeuwenhoek Robert Hooke Where the Magic Happened Schleiden Cell Theory All plants are made of cells Schwann
The Nucleus, DNA and Chromatin Structure
 The Nucleus, DNA and Chromatin Structure Size of the human genom: The diploid human genome contains approximately 6 billion base pairs of DNA packaged into 23 chromosome pairs. Each diploid cell therefore
The Nucleus, DNA and Chromatin Structure Size of the human genom: The diploid human genome contains approximately 6 billion base pairs of DNA packaged into 23 chromosome pairs. Each diploid cell therefore
HIGH RESOLUTION SCANNING ELECTRON MICROSCOPY OF ISOLATED AND IN SITU CYTOSKELETAL ELEMENTS
 Published Online: 1 October, 1979 Supp Info: http://doiorg/101083/jcb831249 Downloaded from jcbrupressorg on September 30, 2018 HIGH RESOLUTION SCANNING ELECTRON MICROSCOPY OF ISOLATED AND IN SITU CYTOSKELETAL
Published Online: 1 October, 1979 Supp Info: http://doiorg/101083/jcb831249 Downloaded from jcbrupressorg on September 30, 2018 HIGH RESOLUTION SCANNING ELECTRON MICROSCOPY OF ISOLATED AND IN SITU CYTOSKELETAL
Wednesday 11 January 2012 Morning
 Wednesday 11 January 2012 Morning AS GCE BIOLOGY F211 Cells, Exchange and Transport *F210010111* Candidates answer on the Question Paper. OCR supplied materials: Insert (inserted) Other materials required:
Wednesday 11 January 2012 Morning AS GCE BIOLOGY F211 Cells, Exchange and Transport *F210010111* Candidates answer on the Question Paper. OCR supplied materials: Insert (inserted) Other materials required:
1.B Fabrication of Plastic Shells
 LLE REVIEW, Volume 42 1.B Fabrication of Plastic Shells Low-atomic-number ablators are a requirement in future direct-drive highgain capsule designs to reduce preheat; hence, they produce a more efficient
LLE REVIEW, Volume 42 1.B Fabrication of Plastic Shells Low-atomic-number ablators are a requirement in future direct-drive highgain capsule designs to reduce preheat; hence, they produce a more efficient
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called.
 Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Permanganate Fixation of Plant Cells
 Permanganate Fiation of Plant Cells By HILTON H. MOLLENHAUER, Ph.D. (From The University of Teas Southwestern Medical School, Dallas) PLATES 222 ~O 224 (Received for publication, June 22, 1959) ABSTRACT
Permanganate Fiation of Plant Cells By HILTON H. MOLLENHAUER, Ph.D. (From The University of Teas Southwestern Medical School, Dallas) PLATES 222 ~O 224 (Received for publication, June 22, 1959) ABSTRACT
Histological preparation of embryonic and adult zebrafish eyes
 Histological preparation of embryonic and adult zebrafish eyes Richard J. Nuckels 1 and Jeffrey M. Gross 1,2,3 1 Section of Molecular Cell and Developmental Biology 2 Institute of Cell and Molecular Biology
Histological preparation of embryonic and adult zebrafish eyes Richard J. Nuckels 1 and Jeffrey M. Gross 1,2,3 1 Section of Molecular Cell and Developmental Biology 2 Institute of Cell and Molecular Biology
LIPID STAINING FOR THE ELECTRON MICROSCOPE: A NEW METHOD
 y. Cell Sci. 19, 425-437 (i97s) 425 Printed in Great Britain LIPID STAINING FOR THE ELECTRON MICROSCOPE: A NEW METHOD V.B. WIGGLES WORTH Department of Zoology, University of Cambridge, Downing Street,
y. Cell Sci. 19, 425-437 (i97s) 425 Printed in Great Britain LIPID STAINING FOR THE ELECTRON MICROSCOPE: A NEW METHOD V.B. WIGGLES WORTH Department of Zoology, University of Cambridge, Downing Street,
A Study of Potassium Permanganate 'Fixation' for Electron Microscopy
 241 A Study of Potassium Permanganate 'Fixation' for Electron Microscopy By S. BRADBURY AND G. A. MEEK (From the Department of Human Anatomy, South Parks Road, Oxford) With five plates (figs, i, 2, 4,
241 A Study of Potassium Permanganate 'Fixation' for Electron Microscopy By S. BRADBURY AND G. A. MEEK (From the Department of Human Anatomy, South Parks Road, Oxford) With five plates (figs, i, 2, 4,
Vocab Word 1: Interphase
 Vocab Word 1: Interphase Interphase is the phase of the cell cycle in which a typical cell spends most of its life. During this phase, the cell copies its DNA in preparation for mitosis. Interphase is
Vocab Word 1: Interphase Interphase is the phase of the cell cycle in which a typical cell spends most of its life. During this phase, the cell copies its DNA in preparation for mitosis. Interphase is
A Study of the Argentaffin (Kultschitzky) Cells in frozen-dried Tissue by Phase-Contrast Microscopy and Ultra-Violet Light By A. C.
 289 A Study of the Argentaffin (Kultschitzky) Cells in frozen-dried Tissue by Phase-Contrast Microscopy and Ultra-Violet Light By A. C. CHRISTIE (From the Royal Cancer Hospital, London. Present address,
289 A Study of the Argentaffin (Kultschitzky) Cells in frozen-dried Tissue by Phase-Contrast Microscopy and Ultra-Violet Light By A. C. CHRISTIE (From the Royal Cancer Hospital, London. Present address,
Practical Of Genetics
 Practical Of Genetics 1. Students will be able to demonstrate a microtechnique for reliable chromosomal analysis of leucocytes obtained from peripheral blood. 2. Students will be able to prepare a karyotype
Practical Of Genetics 1. Students will be able to demonstrate a microtechnique for reliable chromosomal analysis of leucocytes obtained from peripheral blood. 2. Students will be able to prepare a karyotype
A comparative study on electrorheological properties of various silica/conducting polymer core/shell nanospheres
 Supplementary Information for: A comparative study on electrorheological properties of various silica/conducting polymer core/shell nanospheres Jin-Yong Hong and Jyongsik Jang* World Class University (WCU)
Supplementary Information for: A comparative study on electrorheological properties of various silica/conducting polymer core/shell nanospheres Jin-Yong Hong and Jyongsik Jang* World Class University (WCU)
Specimen configuration
 APPLICATIONNOTE Model 1040 NanoMill TEM specimen preparation system Specimen configuration Preparing focused ion beam (FIB) milled specimens for submission to Fischione Instruments. The Model 1040 NanoMill
APPLICATIONNOTE Model 1040 NanoMill TEM specimen preparation system Specimen configuration Preparing focused ion beam (FIB) milled specimens for submission to Fischione Instruments. The Model 1040 NanoMill
PYROTECHNIC RESIDUES ANALYSIS DETECTION AND ANALYSIS OF CHARACTERISTIC PARTICLES BY SEM-EDS
 PYROTECHNIC RESIDUES ANALYSIS DETECTION AND ANALYSIS OF CHARACTERISTIC PARTICLES BY SEM-EDS Susan A. PHILLIPS Forensic Explosives Laboratory, Defence Evaluation and Research Agency, Fort Halstead, Sevenoaks,
PYROTECHNIC RESIDUES ANALYSIS DETECTION AND ANALYSIS OF CHARACTERISTIC PARTICLES BY SEM-EDS Susan A. PHILLIPS Forensic Explosives Laboratory, Defence Evaluation and Research Agency, Fort Halstead, Sevenoaks,
Chromosomes. M.Sc. Biotechnology. Hawler Medical University, Iraq
 Chromosomes Bashdar Mahmud Hussen M.Sc. Biotechnology Hawler Medical University, Iraq bashdar@res.hmu.edu.iq bmhscience@yahoo.com History of Chromosome Karl Nagali (1842) E. Russow (1872) first description
Chromosomes Bashdar Mahmud Hussen M.Sc. Biotechnology Hawler Medical University, Iraq bashdar@res.hmu.edu.iq bmhscience@yahoo.com History of Chromosome Karl Nagali (1842) E. Russow (1872) first description
Immuno-Labelling Cryosections
 Thin sections of biological material, mounted on nickel or gold grids, can be labelled by floating them, section-side down, on small, 10 µl, droplets of antibody. This process is conveniently carried out
Thin sections of biological material, mounted on nickel or gold grids, can be labelled by floating them, section-side down, on small, 10 µl, droplets of antibody. This process is conveniently carried out
Technical Note. Tissue Section Imaging. Published August The most recent version of this Technical Note is posted at licor.com/bio/support.
 Technical Note Tissue Section Imaging Published August 2017. The most recent version of this Technical Note is posted at licor.com/bio/support. Page 2 - Tissue Section Imaging Table of Contents Page I.
Technical Note Tissue Section Imaging Published August 2017. The most recent version of this Technical Note is posted at licor.com/bio/support. Page 2 - Tissue Section Imaging Table of Contents Page I.
Carnegie Mellon MRSEC
 Carnegie Mellon MRSEC Texture, Microstructure & Anisotropy, Fall 2009 A.D. Rollett, P. Kalu 1 ELECTRONS SEM-based TEM-based Koseel ECP EBSD SADP Kikuchi Different types of microtexture techniques for obtaining
Carnegie Mellon MRSEC Texture, Microstructure & Anisotropy, Fall 2009 A.D. Rollett, P. Kalu 1 ELECTRONS SEM-based TEM-based Koseel ECP EBSD SADP Kikuchi Different types of microtexture techniques for obtaining
Cell Division. Use Target Reading Skills. This section explains how cells grow and divide.
 Name Date Class Cell Processes Guided Reading and Study Cell Division This section explains how cells grow and divide. Use Target Reading Skills As you read, make a cycle diagram that shows the events
Name Date Class Cell Processes Guided Reading and Study Cell Division This section explains how cells grow and divide. Use Target Reading Skills As you read, make a cycle diagram that shows the events
Cell Nucleus. Chen Li. Department of Cellular and Genetic Medicine
 Cell Nucleus Chen Li Department of Cellular and Genetic Medicine 13 223 chenli2008@fudan.edu.cn Outline A. Historical background B. Structure of the nucleus: nuclear pore complex (NPC), lamina, nucleolus,
Cell Nucleus Chen Li Department of Cellular and Genetic Medicine 13 223 chenli2008@fudan.edu.cn Outline A. Historical background B. Structure of the nucleus: nuclear pore complex (NPC), lamina, nucleolus,
Crystal Growth and Buoyancy- Driven Convection Currents
 Crystal Growth and Buoyancy- Driven Convection Currents Objective: To observe buoyancy-driven covection currents that are created as crystals grow in a crystal growing solution. Science Standards: Science
Crystal Growth and Buoyancy- Driven Convection Currents Objective: To observe buoyancy-driven covection currents that are created as crystals grow in a crystal growing solution. Science Standards: Science
scatter electrons and can easily he distinguished
 THE JOURNAL OF INVESTIOATIVE DERMATOLOGY Copyright 1968 by The Williams & Wilkins Co. Vol. 50, No. 4 Printed in U.S.A. PERCUTANEOUS ABSORPTION OF MERCURY IN MAN 1. A STUDY BY ELECTRON MICROSCOPY OF THE
THE JOURNAL OF INVESTIOATIVE DERMATOLOGY Copyright 1968 by The Williams & Wilkins Co. Vol. 50, No. 4 Printed in U.S.A. PERCUTANEOUS ABSORPTION OF MERCURY IN MAN 1. A STUDY BY ELECTRON MICROSCOPY OF THE
ULTRASTRUCTURAL STUDIES ON THE SURFACE MEMBRANE OF THE MOUSE EGG
 J. Cell Sci. 22, 345-353 (1976) 345 Printed in Great Britain ULTRASTRUCTURAL STUDIES ON THE SURFACE MEMBRANE OF THE MOUSE EGG DEBORAH D. EAGER, M. H. JOHNSON AND K. W. THURLEY Department of Anatomy, Downing
J. Cell Sci. 22, 345-353 (1976) 345 Printed in Great Britain ULTRASTRUCTURAL STUDIES ON THE SURFACE MEMBRANE OF THE MOUSE EGG DEBORAH D. EAGER, M. H. JOHNSON AND K. W. THURLEY Department of Anatomy, Downing
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
CELL BIOLOGY - CLUTCH CH CYTOSKELETON AND CELL MOVEMENT.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
!! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
Structural study of Caenorhabditis elegans
 aterial Life Science Application Note Structural study of Caenorhabditis elegans related instruments Leica E ICE, Leica E AFS2 edical Industrial anufacturing Natural Resources 2 Structural study of Caenorhabditis
aterial Life Science Application Note Structural study of Caenorhabditis elegans related instruments Leica E ICE, Leica E AFS2 edical Industrial anufacturing Natural Resources 2 Structural study of Caenorhabditis
Chapter 3. DNA Replication & The Cell Cycle
 Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology
 Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
PROTOCOL. Collagen I Thin Gel Coating of Alvetex Scaffold. Introduction. Method. Page 1
 Page 1 Introduction Extra-cellular matrix (ECM) coating of in vitro culture surfaces is commonly used to enhance cellsubstrate adhesion, encourage cell-matrix signalling and to protect shear-sensitive
Page 1 Introduction Extra-cellular matrix (ECM) coating of in vitro culture surfaces is commonly used to enhance cellsubstrate adhesion, encourage cell-matrix signalling and to protect shear-sensitive
ARTHUR J. HALE. From the Division of Pathology, Imperial Cancer Research Fund, I,incoln's Inn Fields, London, England
 HISTORADIOGRAPHIC IDENTIFICATION OF ALKALINE PHOSPItATASE ARTHUR J. HALE. From the Division of Pathology, Imperial Cancer Research Fund, I,incoln's Inn Fields, London, England The technique of historadiography
HISTORADIOGRAPHIC IDENTIFICATION OF ALKALINE PHOSPItATASE ARTHUR J. HALE. From the Division of Pathology, Imperial Cancer Research Fund, I,incoln's Inn Fields, London, England The technique of historadiography
