An ES-Like Pluripotent State in FGF-Dependent Murine ips cells
|
|
|
- Maximilian Emery Elliott
- 6 years ago
- Views:
Transcription
1 An ES-Like Pluripotent State in FGF-Dependent Murine ips cells Bruno Di Stefano 1,9., Christa Buecker 2,3., Federica Ungaro 1, Alessandro Prigione 4, Hsu-Hsin Chen 2,3, Maaike Welling 2,8, Maureen Eijpe 5, Gustavo Mostoslavsky 6, Paul Tesar 7, James Adjaye 4, Niels Geijsen 2,3,8,10 *, Vania Broccoli 1 * 1 Stem Cells and Neurogenesis Unit, Division of Neuroscience, San Raffaele Scientific Institute, Milan, Italy, 2 Center for Regenerative Medicine, Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, United States of America, 3 Harvard Stem Cell Institute, Harvard University, Cambridge, Massachusetts, United States of America, 4 Molecular Embryology and Aging Group, Department of Vertebrate Genomics, Max Planck Institute for Molecular Genetics, Berlin, Germany, 5 Department of Cell Biology, Erasmus Medical Centre, Rotterdam, The Netherlands, 6 Department of Stem Cell Biology and Regenerative Medicine, Boston University School of Medicine, Boston, Massachusetts, United States of America, 7 Department of Genetics, Case Western Reserve University, Cleveland, Ohio, United States of America, 8 Hubrecht Institute for Developmental Biology and Stem Cell Research, Utrecht, The Netherlands, 9 Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, 10 Department of Clinical Sciences of Companion Animals, Utrecht University Veterinary School, Utrecht, The Netherlands Abstract Recent data demonstrates that stem cells can exist in two morphologically, molecularly and functionally distinct pluripotent states; a naïve LIF-dependent pluripotent state which is represented by murine embryonic stem cells (mescs) and an FGFdependent primed pluripotent state represented by murine and rat epiblast stem cells (EpiSCs). We find that derivation of induced pluripotent stem cells (ipscs) under EpiSC culture conditions yields FGF-dependent ipscs from hereon called FGFiPSCs) which, unexpectedly, display naïve ES-like/ICM properties. FGF-iPSCs display X-chromosome activation, multi-lineage differentiation, teratoma competence and chimera contribution in vivo. Our findings suggest that in 129 and Bl6 mouse strains, ipscs can dominantly adopt a naive pluripotent state regardless of culture growth factor conditions. Characterization of the key molecular signalling pathways revealed FGF-iPSCs to depend on the Activin/Nodal and FGF pathways, while signalling through the JAK-STAT pathway is not required for FGF-iPS cell maintenance. Our findings suggest that in 129 and Bl6 mouse strains, ipscs can dominantly adopt a naive pluripotent state regardless of culture growth factor conditions. Citation: Di Stefano B, Buecker C, Ungaro F, Prigione A, Chen H-H, et al. (2010) An ES-Like Pluripotent State in FGF-Dependent Murine ips cells. PLoS ONE 5(12): e doi: /journal.pone Editor: Martin Pera, University of Southern California, United States of America Received July 28, 2010; Accepted December 8, 2010; Published December 30, 2010 Copyright: ß 2010 Di Stefano et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was supported by funding from Telethon (GGP07181) and Italian Ministry of Health (Young Research Award) to V.B., from National Institutes of Health and Dutch Science Organization (NWO) to N.G., and from the Bundesministerium für Bildung und Forschung (01GN0807) and the Max Planck Society to J.A. C.B. was supported by the Gottlieb Daimler- and Karl Benz-Foundation. H.H.C. was supported by the National Science Council (Taiwan, ROC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: The authors have declared that no competing interests exist. * ngeijsen@mgh.harvard.edu (NG); broccoli.vania@hsr.it (VB). These authors contributed equally to this work. Introduction Pluripotent stem cells are characterized by their ability to expand indefinitely in vitro while retaining the capacity to generate derivatives of all three germ layers, both in vitro and in vivo. Sources of pluripotent stem cells include blastocyst embryos, which give rise to embryonic stem cells (ES cells), and the post-implantation epiblast which gives rise to epiblast stem cells (EpiSCs) [1,2]. ES cells and EpiSCs are both pluripotent as they are capable of generating derivatives of the three embryonic germ layers upon in vitro or in vivo differentiation, yet important molecular and functional differences exist between these two pluripotent states. At the molecular level, the ES cell pluripotent state is maintained by a combination of LIF/JAK/STAT3 and BMP4 signaling, while EpiSCs require a combination of bfgf and TGFb/Activin signaling for their continued self-renewal. The different culture conditions that maintain ES cells and EpiSCs are reflected in the morphological, molecular and functional properties of these cells. Murine ES cells form dome-shaped three dimensional colonies and are capable of generating chimeras with functional contribution to all somatic lineages as well as the germline. In contrast, EpiSCs form flatted colonies that are split by mechanical- or collagen-mediated passaging as small clusters of cells, since EpiSCs cannot be passaged as single cells by trypsin digest. EpiSCs are pluripotent and form derivatives of all three germ layers during in vitro differentiation and upon teratoma formation in vivo. Unlike ES cells, EpiSCs can even generate trophoectoderm derivatives in vitro. Yet, fail to integrate with the ICM upon morula aggragation and as a result, chimera forming potential of EpiSCs is very low or even absent. Thus, while EpiSCs are pluripotent, to-date their in vivo developmental potential is limited to teratoma formation. Above results demonstrate that in the mouse, two functionally distinct pluripotent states exist, a naïve LIF-dependent pluripotent state that is compatible with the pre-implantation ICM and a primed FGF-dependent state that is reminiscent of the postimplantation epiblast [3]. PLoS ONE 1 December 2010 Volume 5 Issue 12 e16092
2 The ability to generate ES cell lines is restricted to only a few inbred mouse strains whereas other, so-called non-permissive mouse strains fail to yield ES cells under standard culture conditions, but instead can give rise to to EpiSCs,Pluripotent stem cell lines from other species, including human and rat, share many of the defining characteristics of EpiSCs, suggesting that the EpiSC pluripotent state is the common stable pluripotent state for most strains of mice as well as other species. Interestingly, Hanna and colleagues recently demonstrated that the constitutive ectopic expression of either Klf4 or cmyc allows the derivation of LIFdependent ES-like cells from blastocyst embryos of the nonpermissive NOD mouse strain [4]. In addition, LIF/serumdependent ES-like cell lines can be generated through somatic cell reprogramming of NOD fibroblasts with defined factors (Oct4, Sox2, Klf4, cmyc) that have recently been shown to allow the generation of induced pluripotent stem cells (ips cells) from somatic cells [5,6]. Yet, as with the blastocyst-derived NOD ES cell lines, the stable propagation of NOD ips cells is dependent on the continued ectopic expression of Klf4 or cmyc. Small molecule inhibitors of glycogen synthase kinase beta (GSK3b) and the mitogen-activated protein kinase (MAPK) signaling pathway can replace some of the reprogramming factors during ips cell generation [7], and these inhibitors can similarly stabilize the LIF/serum-dependent pluripotent state in blastocystderived stem cells or ips cells from the the non-permissive NOD mouse strain [4,8,9,10]. Thus, it appears that the LIF-dependent pluripotent state is metastable in NOD mice, meaning it is dependent on either the constitutive expression of ectopic reprogramming factors or the presence of small molecule inhibitors of the GSK3b and/or the MEK/ERK signaling pathway. In the absence of these exogenous factors, NOD ips cells assume a stable EpiSC-like state, even when LIF is present in the culture media. Genetic background appears to play an important role in stabilizing the LIF-dependent pluripotent state, yet its role in defining the FGF-dependent pluripotent stateis less clear. We explored the possibility of generating EpiSCs by ips reprogramming of murine embryonic fibroblasts from the permissive129 and/or BL6 mouse strains in EpiSC culture conditions. Unexpectedly, we found that even in the presence of EpiSC culture conditions, ips cells adopt a naive ICM/ES-like pluripotent state. Thus, it appears that strain-specific genetic elements dictate that in permissive mouse strains, the ES-like pluripotent state is dominant following ips reprogramming Results Generation and molecular analysis of FGF-iPSCs Murine embryonic fibroblasts (MEFs) of E14 Oct4-GFP (BL6/ tgoct4-gfp) were transduced with a cocktail of retroviruses expressing the ips reprogramming factors (Oct4, Sox2, Klf4 and c-myc) as shjown schematically in (Figure 1A). Upon transduction, the cells were passaged with trypsin and then re-plated onto a feeder layer of mitotically inactivated MEFs. From day 7 onwards, infected fibroblasts were maintained in bfgf medium (DMEM, 20% Serum Replacement and 4 ng/ml bfgf). Starting from days 10 12, we observed the emergence of tightly compact colonies, which had reactivated the Oct4-GFP transgene (Figures 1B 1E). On day 17, single colonies were picked, and further propagated in bfgf medium. Unexpectedly, upon subsequent passaging, the cultures uniformly maintained a characteristic murine ES-like morphology, with round and compacted cell clusters expressing Oct4-GFP (Figures 1F 1I) which contrasts sharply with the flattened two-dimensional colony morphology of EpiSCs derived and maintained under the same culture conditions. We name these cells mouse FGF-iPSCs, to distinguish them from conventional LIF-dependent murine ESCs and ipscs. In addition to the ES-like morphology, FGF-iPSCs cultures exhibited homogeneous SSEA-1, but not SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 expression (Fig. 1H, I). In addition, FGF-iPSCs reactivate endogenous Oct4-GFP, Sox2 and Nanog (Fig. 1J Q, Figure S1). Cytogenetic analysis of two independent FGF-iPS cell lines revealed a normal karyotype (2n = 40) even after prolonged culture at high passage number (passage 28, 3 months in culture) (Fig. S1C and data not shown). As expected, bisulfite sequencing demonstrated hypomethylation of the Oct4 promoter region as tested in 12 different CpG islands scattered around 350 bp of the Oct4 minimal promoter (Fig. S1A). Correct establishment of the reprogrammed cell state was confirmed by complete silencing of the exogenous reprogramming factors as revealed by qpcr (Figure S1B). Growth factor culture conditions affect the dynamic of ips reprogramming To examine the effect of the growth factor conditions on the dynamic of the ips reprogramming response, we reprogrammed from 129/BL6 F1 embryonic fibroblasts either in the presence of LIF/serum (standard murine ES/iPS conditions) or in the presence of bfgf (EpiSC conditions). For this purpose, we employed the recently reported STEMCCA inducible lentiviral vector system, which allows the expression of the four reprogramming factors (Oct4, Sox2, Klf4 and cmyc) from a single lentiviral vector in a doxycyclin-inducible manner through the action of the reverse tetracycline transactivator (rtta) at high efficiency (Figure S2A) [11]. Figure 2A schematically displays the experimental setup. Murine embryonic fibroblasts were transduced with the doxycyclin-inducible reprogramming factors and rtta and reprogramming was induced 24 hours after infection (t = 0). At day 1, the sample was split and cells were cultured either in the presence of LIF (mes conditions) or in the presence of bfgf (EpiSC conditions). At set time intervals (between day 5 and day 15), ectopic reprogramming factors were silenced by removal of doxycycline. Colonies were visualized by Crystal Violet staining on day 18. After approximately days, ips colonies appeared under both conditions, and the LIF-derived ips cells displayed a characteristic ES-like colony morphology, whereas we noted ips cells derived in the presence of bfgf displayed the characteristic flattened colony morphology of EpiSCs (Figure S2B). However, the EpiSC-like colonies were unstable, and upon withdrawal of the ectopic reprogramming factors, most of the EpiSC-like ips cells assumed a fibroblast-like morphology, indicating that they were partially reprogrammed and had not activated their endogenous pluripotency program. Indeed, the Oct4-GFP reporter gene present in these cells was not reactivated in the EpiSC-like ips cells, whereas the control ips cells did reactivate Oct4-GFP (not shown). However, in the FGF conditions, few colonies remained after silencing of ectopic reprogramming factors, which could be stably propagated in the presence of bfgf, and yet displayed the characteristic murine ES-like colony morphology. The number of stable FGF-iPS colonies increased with longer reprogramming time, but lagged behind compared to the LIF control ips cells. As shown in figure 2B, in the presence of bfgf, stable ips colonies emerged after 9 days of doxycycline-induced reprogramming, whereas in the presence of LIF, stable colonies were noted 4 days earlier. Thus, it appears that while the culture growth factor conditions affect the dynamic of the ips reprogramming process, with stable colonies emerging late under FGF growth factor conditions, the PLoS ONE 2 December 2010 Volume 5 Issue 12 e16092
3 Generation of Mouse FGF-iPS Cells Figure 1. Generation of FGF-iPS from mouse fibroblasts by retroviral transduction. Schematic diagram illustrating a timetable of the critical steps of the reprogramming protocol utilized. Embryonic mouse fibroblasts were transduced with retroviruses carrying Oct4, Sox2 Klf4 and cmyc and then transferred onto MEF feeder layers in DMEM medium supplemented with 10% FBS. After 4 days the medium was switched to a human ESC culture medium. Colonies activated the Oct4-GFP transgene after twelve days in culture (B C) and are isolated for expansion after days (D E). After picking, single clones give rise to stable cell lines of FGF-iPSCs, which grow as round compacted colonies (F, H) expressing the Oct4-GFP transgene (G, I). Colonies expressing the Oct4-GFP (K, O, S) result positive for alkaline phosphatase activity (AP) (J), and SSEA-1 (M), Sox2 (P) and Nanog (T) immunoistochemistry. Merged figures for Sox2/Oct4-GFP and Nanog/Oct4- immunostainings are showed in Q and U, respectively. DAPI fluorescent staining is labeling cell nuclei (blue) in L, N, R. doi: /journal.pone g001 analysis of the global gene expression profiles of FGF-iPSCs cells, LIF-derived ips cells, murine ESCs and EpiSCs revealed that FGF-iPSCs are highly similar to murine ES- and LIF-derived ips cells, whereas EpiSCs cells form a separate cluster of unrelated cells (Fig. 3B). Starting fibroblasts are absent in this analysis as most of the investigated were not expressed in the cells prior to ipsc reprogramming. Alkaline phosphatase (AP) is a widely used marker distinguishing murine ESCs, which are expressing AP, from EpiSCs, which are negative for this marker. Interestingly, ipscs derived in the presence of bfgf were strongly positive for the AP staining, further confirming their similarity to ESCs (Fig. 4A, B). In addition to the above molecular and morphological characteristics, we examined the epigenetic properties of the FGF-iPSCs. The pluripotency mediator Oct4 is differentially expressed from two distinct enhancer regions; a distal enhancer general outcome of the reprogramming response is not affected by the culture conditions. FGF-iPSCs display molecular and epigenetic features of the ICM/ES cell pluripotent state The emergence of ips cell colonies with typical murine ES-like characteristics under EpiSC culture conditions was unexpected and hence we performed genome-wide expression analysis to further characterize these cells. As shown in Figure 3A, FGFiPSCc display a gene expression pattern characteristic of murine ES cells, including the inner cell mass markers Rex1, Nanog, Oct4, Sox2, Sall4, Gdf3 and Eras. In contrast, typical EpiSC markers, including FGF5, Eomes (also known as Tbr2), FoxA2 and Cer1 were not expressed in FGF-iPSCs (Fig. 3A). Microarray data were confirmed by qpcr expression analysis (the list of used primers is available in table S1) (Fig. 3C). Hierarchical cluster PLoS ONE 3 December 2010 Volume 5 Issue 12 e16092
4 Figure 2. Derivation of murine ips cells in the presence of bfgf by inducible lentiviral transduction. (A) Schematic representation of the strategy used for the reprogramming of murine fibroblasts into ips cells in the presence of LIF or bfgf. (B) Image of an EpiSC-like ips cell colony derived in bfgf. (C) Schematic of the temporal analysis of the emergence of stable ips colonies in the presence of bfgf. At day -1, murine embryonic fibroblasts are infected with the doxycycline-inducible reprogramming factors and rtta. AT day 0, doxycycline is added to the culture media to initiate expression of the ectopic reprogramming factors. On day 1, the sample is split into dishes which are subsequently maintained in the presence of LIF or bfgf. Starting at day 5, doxycycline is withdrawn at set time intervals and emergence of stable, factor-independent colonies is analyzed at day 18. (D) Emergence of stable, factor independent colonies upon ips reprogramming in the presence of bfgf (top panel) or LIF (bottom panel). Indicated on top is the day at which doxycycline was withdrawn from the culture to silence the ectopic reprogramming factors. doi: /journal.pone g002 which drives Oct4 expression in murine ES cells and and a proximal enhancer which mediates Oct4 expression in EpiSCs [12]. Thus, Oct4 enhancer choice is a distinctive feature between ES cells and EpiSCs. As shown in Figure S1D, Oct4 expression is driven by the ES-specific distal enhancer in FGF-derived ips cells, as well as the ES- and LIF-derived ips controls. In contrast, as expected, the proximal enhancer is active in control EpiSCs. In addition, we examined the X-inactivation state of ipsc clones from a female cell line by RNA FISH for Xist. As demonstrated in Figure 4C D the majority of FGF-iPSCs contains two active X chromosomes as demonstrated by the presence of only basal (pinpoint) Xist expression on both X chomosomes as also observed in the mesc control cells (Figure 4C) whereas in some cells an Xist-cloud was observed (Figure 4C, arrow). As expected, FGF-iPSCs robustly display X-inactivation upon differentiation, demonstrating that the cells are capable of X- inactivation (Figure 4C). The percentage of FGF-iPSCs containing a Xist-cloud is quantified in Figure 4D and demonstrated that approximately 90% of the undifferentiated FGF-iPSCs contain two active X-chromosomes, whereas 40% of the FGF-iPSCs display X-inactivation after 4 days of differentiation. The percentage of FGF-iPSCs displaying an Xist-cloud (10%) is higher than X-inactivation observed in control mescs (0.5%) and is perhaps reminiscent of the higher percentage of X-inactivation also observed in human ESCs. Finally, immunofluorescence-based detection of the trimethylated H3 lysine 27 (me3h3k27), a repressive histone modification, revealed the absence of a silent X chromosome in two undifferentiated female FGF-iPS cell lines (Fig. 4E). This is in stark contrast to EpiSCs which exhibit complete X-chromosome inactivation similar to their tissue of origin. Together these data demonstrate that in addition to morphological and molecular similarities, FGF-iPSCs display an epigenetic profile characteristic of mescs as well. Murine FGF-iPSCs are FGF-dependent Despite the common expression of pluripotency genes between LIF or FGF derived ipscs, important differences emerged in the expression levels of genes encoding key factors of the Nodal/ Activin or Jak/Stat3 pathways between the two cell types. In fact, FGF-iPSCs exhibited high expression levels of Nodal and Inhba and, simultaneously, a low expression of genes downstream of the LIF-JAK-STAT3 signalling pathway (Stat3, Jak1, Jak2 and Pim1) in comparison to conventional ESCs and ipscs as detected by microarray profiling and confirmed by qpcr analysis (Fig. 5A, B). To confirm that FGF-iPS are maintained independent of JAK- STAT3 signaling, we cultured FGF-iPSCs in the presence of a JAK inhibitor (JAKi) or a LIF blocking antibody, in order to inhibit Stat3 phosphorylation (Fig. 6A). As shown in Figure 6G, addition of the JAKi inhibitor efficiently eliminates STAT3 phosphorylation under these conditions both in FGF-iPS and conventional mescs, in which STAT3 is robustly activated. FGFiPSCs could be propagated for more than 7 passages in the presence of JAKi inhibitor while maintaining their undifferentiated state and Oct4-GFP endogenous expression (Fig. 6B, C). In contrast, we observed rapid loss of pluripotency gene expression when conventional mouse ESC and/or ipsc were cultured under the same conditions (Fig.6). Furthermore, these cells displayed a strong AP activity and lacked any evident me3h3k27 staining ruling out the induction of Epi-like stem cells in these conditions (Fig. 6D F). Accordingly, FGF-iPSCs maintained for 5 passages in the presence of JAKi inhibitor, retained their characteristic ESClike gene expression profile with expression of ESC-like markers Stra8, Rex1 and Stella (Dppa3) and absence of epiblast marker expression (Cer1, Dkk1 and FGF5) (Fig. 6H). Conversely, inhibition of TGFbeta/Activin signaling using a specific inhibitor of the type I Activin receptor (ALK-I) resulted in rapid FGF-iPSC differentiation, while this inhibitor did not affect mesc selfrenewal (Fig. 6). Control EpiSCs and human ESCs similarly PLoS ONE 4 December 2010 Volume 5 Issue 12 e16092
5 Figure 3. The ES-like pluripotent state is dominant in the 129/BL6 genetic background. (A) Comparative gene expression analysis among conventional murine ESCs, LIF-iPSCs, FGF-iPSCs and EpiSCs for candidate genes of pluripotency. Normalized expression intensity values (scaled median ratio) were obtained from Agilent whole-genome microarrays. Two biological replicates were used for all cell types. (B) Unbiased cluster analysis of global gene expression profiles of two independent FGF-derived ips clones, two independent LIF-derived-iPS clones, two murine ES cell lines and two EpiSC cell lines. (C) qpcr quantification of the pluripotency associated genes Nanog, Sox2, Oct4, Rex1, Fbx15, Eras, Cripto, Cer1, Daz1, Fgf5, Sox17, Dkk1 and Lefty1 in LIF-iPSCs compared to FGF-iPSCs. doi: /journal.pone g003 differentiated upon ALK-1 inhibition (data not shown). In addition, FGF withdrawal or FGF receptor inhibition by the application of SU5402 in FGF-iPSCs for six days resulted in widespread cell death (Figure S3). These findings demonstrate that FGF-iPSCs are maintained independent of the activation of the JAK-Stat3 signalling pathway. Instead, FGF-iPSC self-renewal relies on the continued presence of FGF stimulation and activity of the TGFbeta/Activin signaling cascade. Indeed, downstream mediators of FGF- and TGFbeta/Activin signaling are activated in FGF-iPSCs., We assessed Smad 2/3, Smad 1/5/8 levels in mouse ESCs, LIF-iPSCs and FGF-iPSCs by Western blot analysis. ESCs and LIF-iPSCs showed phosphorylation of Smad 1/5/8, indicating active transduction of Bmp signaling (Fig. 5C). On the contrary, two independent FGF-iPSC lines displayed a strong activation of Smad 2/3 concomitant with undetectable levels of Smad 1/5/8 active forms (Fig. 5C). qpcr analysis confirmed that specific to FGF-iPSCs, Bmp4 expression was significantly down-regulated together with the up-regulation of Gdf3 and Gremlin-1 (Grem1), two well-known Bmp4 antagonists [13,14,15] (Fig. 5B). Taken together, these findings demonstrate that self-renewing FGF-iPSCs exhibit activation of FGF and TGFbeta/Activin downstream signaling pathways and undetectable BMP4 signalling, in contrast to mescs, in which the BMP4 signalling pathway is prominently activated and TGFbeta/ Activin signalling is low (Fig. 5D). Next, to definitively exclude the role of feeder cells in promoting FGF-iPS stem cell properties, we serially cultured FGF-iPSCs on fibronectin-coated plates in the absence of fibroblast feeder cells. At passage 6, corresponding to 5 weeks of culture in these conditions, FGF-iPS colonies revealed strong Oct4-GFP and Nanog endogenous expression as well as evident AP activity (Fig. 7A G). In contrast, FGF-iPSCs did not show inactivation of the X-chromosome as indicated by lack of me3h3k27 staining (Fig. 7H). In line with these findings, FGF-iPSCs expressed Nanog, Rex1 and Stella (Dppa3) at similar levels to those detected when cultured on feeder conditions, and the EpiSC markers Cer1 and FGF5 were not found up-regulated PLoS ONE 5 December 2010 Volume 5 Issue 12 e16092
6 Figure 4. FGF-iPSCs do not share crucial properties of EpiSCs. (A) Colony morphology of FGF ipscs (left), murine ES cells (middle) and EpiSCs (right). Note the dome-shaped, three dimensional morphology of the FGF-iPS cells. (B) Alkaline phosphatase (AP) staining of the cells in (A). FGF-iPSCs are expressing AP at levels comparable with murine ESCs. On the contrary EpiSCs are negative for this markers. (C) FISH analysis show that the majority of FGF-iPSCs contains two active X chromosomes. FGF-iPSCs robustly display X-inactivation upon differentiation. The percentage of FGFiPSCs containing a Xist-cloud is quantified. (D) Approximately 90% of the undifferentiated FGF-iPSCs contain two active X-chromosomes, whereas 40% of the FGF-iPSCs display X-inactivation after 4 days of differentiation. (E) Double immunostaining for me3h3k27 and Oct4-GFP in two female FGF-iPS cell lines. Differentiated cells which lost Oct4 expression show an evident me3h3k27 positive nuclear staining corresponding to the inactivated X-chromosome (arrow). On contrary, undifferentiated Oct4-GFP+ cells do not present any me3h3k27 positive nuclear puncta. White arrow indicates the diagnostic staining for the inactive X chromosome. Inset in D is a higher magnification of a single cell nucleus with a me3h3k27 positive inactivated X-chr. doi: /journal.pone g004 astestedbyqpcr(fig.7i).interestingly,expressionofthestat3- induced gene Socs3 was strongly reduced suggesting that this signaling is generally repressed in these culture conditions (Fig. 6I). Thus, FGF-iPSCs conserved those cardinal molecular and epigenetic features closely associated to pluripotency even when deprived of feeder layers for prolonged time. To test the influence of the growth factor milieu on pluripotency of FGF-iPS cells, we examined the effect of LIF stimulation on PLoS ONE 6 December 2010 Volume 5 Issue 12 e16092
7 Figure 5. FGF-iPSCs show activation of opposite TGFb and FGF intracellular pathways with respect to ESCs and LIF-iPSCs. (A) Heatmap diagram of a small selection of self-renewal related genes that are in common between the three cell types. Shown are genes which are part of the pluripotency machinery, primitive-streak, Jak-Stat pathway and TGFb signaling pathway. (B) qpcr analysis for mrna level quantification of the Nodal, Inhba, Bmp4, Gremlin-1 (Grem1) and Gdf3 genes in FSR-iPSCs and ESCs. (C) Immunoblotting showing the phosphorylation levels of the different SMAD and ERK1/2 proteins and compared among ESCs, LIF-iPSCs and FSR-iPSCs, tested as protein extracts of cell cultures grown in their normal conditions. In some conditions FGF-2 (bfgf) was withdrawn for six days. (D) General outline illustrating the state of activation of the two different molecular branches of the TGFb signaling and their key molecular components in FSR-iPSCs and LIF dependent stem cells (ESCs and LIF-iPSCs). doi: /journal.pone g005 these cells. Upon culture for 10 days in a traditional mouse ESC culture medium (20% Serum, LIF), the vast majority of FGFiPSCs were rapidly induced to differentiate causing the fragmentation of the colonies into numerous polygonal-shaped GFPisolated cells (data not shown). However, few cells tightly adherent in small colonies maintained a strong Oct4-GFP expression. Upon trypsinization into single cells and propagation on MEFs, these cells organized into typical mouse ESCs colonies, a morphology maintained even after extensive expansion (P10, 6 weeks in culture) (Figure S4A D). We termed these cells LIF stimulated FGF-iPSCs to indicate their FGF-iPSC origin. The conversion efficiency was approximately,0.01% comparable process to the recently reported conversion of EpiSCs into mesc-like cells [16]. Furthermore, when culture conditions were switched back to the original FGF culture medium, the cells re-acquired all the FGFiPSC morphological characteristics (Figure S4E, F). These results emphasize once again that FGF-iPS cells do not depend on LIF signals for their continued self-renewal, but instead differentiate when switched to LIF culture conditions. However, similar to the recently reported conversion of EpiSCs into mesc-like cells, a small fraction of FGF-iPSCs can adapt to the LIF culture conditions and convert into a mesc-like state. Murine FGF-iPSCs generate chimeras with germline transmission To determine the developmental potential of FGF-iPSCs, we examined their in vitro and in vivo differentiation. We generated aggregates, termed embryoid bodies (EBs), in which pluripotent stem cells differentiate in a manner closely resembling early embryonic development, with the formation of early derivatives of the three embryonic germ layers and downregulation of pluripotency genes. Indeed, we observed rapid loss of Oct4-GFP expression in FGF-iPSC-derived EBs after 4 days of differentiation (Fig. 8A). EBs plated onto matrigel coated dishes in serum-free medium containing bfgf differentiated into Nestin expressing neuronal cells (ectoderm) (Fig. 8B). When these EBs were incubated on gelatin-coated tissue culture plates in DMEM medium supplemented with 10% FBS for 15 to 20 days, they differentiated into a wide range of cell types including Sox17 positive endoderm progenitors and Sma positive smooth-muscle cells (mesoderm) (Fig. 8C, D). To test pluripotency in vivo, FGF-iPSCs were injected subcutaneously into nude mice. Six weeks after transplantation, teratomas were isolated and histological analysis confirmed the presence of well defined differentiated derivatives of the three embryonic germ PLoS ONE 7 December 2010 Volume 5 Issue 12 e16092
8 Figure 6. Effect of Jaki, ALKi and LIF-antibody on FGF-iPSCs and LIF dependent ES and ips cells. (A) All cell samples were plated onto inactivated MEFs and treated for one week with Jaki, Alki or LIF-blocking antibody to evaluate the dependency from LIF/Stat pathway or TGFb pathway. The percentage of persisting Oct4-GFP positive colonies was evaluated at day 7. Reported values were normalized over the internal control (number of colonies in growth medium without inhibitors, black bars). The number of cell colonies are represented on the Y axis. After treatment with Jaki for 15 days the FGF-iPSCs maintein the morphology (B) and the Oct4-GFP expression (C). (D-E) AP staining reveal the mainteinance of positive colonies in treated cells. (F) After treatment the cells show no X chromosome inactivation. (G) Western blot show the expression of Stat3 and P-Stat3 in FGF-iPSCs and mesc. Stat3 phosphorylation is suppressed by Jaki. (H) Gene expression analysis by qpcr among mescs, FGF-iPSCs, EpiSCs, FGF-iPSCs + Jaki and FGF-iPSCs + alphalif. doi: /journal.pone g006 layers, including neural tissue, adipose tissue, epithelial structures and muscle fibers (Fig. 8E H). The most important functional difference between mescs and EpiSCs is the striking inability of EpiSCs to form chimeras upon morula or blastocyst injection [1,2]. We examined the ability of FGF-iPS cells to integrate into preimplantation stage mouse embryos by aggregating mouse embryos at the 8 cells or morula stage with clumps of FGF-iPSC. GFP-positive FGF-iPSCs readily integrated with the developing inner cell mass and chimeric animals were successfully obtained at a frequency of approximately 24% (11 chimeras out of 46 animals) with a coat-color contribution ranging between 5% to 80% (Fig. 7I,L). Furthermore, germline transmission was obtained from two chimeras, derived from two independent FGF-iPSC lines (#5 and #9), as revealed by coat color and confirmed by the presence of the Oct4- GFP transgene in the offspring (Fig. 8M, N). These results demonstrate that the developmental potential of FGF-iPSCs is comparable to that normally exhibited by conventional LIFdependent ESCs and ipscs and FGF-iPSCs can functionally contribute to chimera formation. We did not test the ability of FGF-iPSCs to generate entirely ipsc-derived mice by tetraploid blastocyst complementation, but analysis of the Dlk1-Dio locus revealed that in at least one FGFiPSC clone the expression of Gtl2 was correct (Figure S5), demonstrating that similar to LIF-dependent ipscs, the imprint status of this locus is not always preserved, but some clones can be identified that show a correct imprint status. Discussion Together, our data demonstrate that derivation of stable ips cells in the presence of bfgf yields two types of colonies. Colonies with morphological characteristics of EpiSCs, which are unstable and remain dependent on the constitutive expression of ectopic reprogramming factors. These are likely partially reprogrammed colonies, since they fail to reactivate endogenous pluripotency genes. In addition, stable, ectopic factor-independent colonies emerge, which display morphological, molecular, epigenetic and functional properties of murine ES cells. These murine FGF-iPSCs are maintained in an FGF-dependent fashion (.40 passages) with a normal karyotype, and display multilineage differentiation in vitro and broad developmental potential in vivo, including the generation of germline competent chimeras. Together our results demonstrate that while the growth factor conditions influence the dynamic of the somatic cell reprogramming response, the ES-like pluripotent state is the dominant PLoS ONE 8 December 2010 Volume 5 Issue 12 e16092
9 Figure 7. Feeder-free culture of FGF-iPSCs. The FGF-iPSCs growth in the absence of feeder layer as colonies expressing Oct4-GFP (A B). (C D) The colonies are positive for alkaline phophatase. (E G) FGF-iPSCs maintain the expression of Nanog in a feeder free condition. (H) Staining for H3K27 on FGF-iPS colonies show the absence of X-chromosome inactivation. doi: /journal.pone g007 endpoint that is achieved independent of the culture growth factor conditions. Several lines of evidence make it highly unlikely that the ES cell pluripotent state is the result of low-level residual LIF activity emerging from the MEF feeders. First, FGF-iPS cells can be maintained under defined culture conditions in the absence of MEF feeders. Second, the FGF-derived ips cells are dependent on bfgf signaling for their continued self-renewal, and are not affected by prolonged inhibition of JAK-STAT signaling. Finally, switching the cells to conventional mes culture conditions with addition of LIF results in FGF-iPSC differentiation, indicating that LIF is in fact incapable of maintaining FGF-iPS cells. FGF-iPSCs and standard ESCs or ipscs do not represent alternative metastable cell states as described for ESCs and EpiSCs, but cells with similar properties sharing an equivalent pluripotency state. Consistent with this, while EpiSCs require the stable exogenous expression of Klf4 or c-myc for their conversion into conventional ESCs or, alternatively, the presence of small molecules that can replace these factors during reprogramming [4,17], FGF-iPSCs can be converted to conventional LIFdependent ipscs by simply switching growth factor culture conditions. A similar approach was shown to efficiently induce the conversion of epiblast cells from the primitive ectoderm into ES-cell-like cells (rescs) [16]. Using an ESC-specific Oct4 distal enhancer reporter cell line, Bao and colleagues demonstrated that approx % of epiblast cells respond to a switch in growth factors from FGF to LIF by upregulating the ESC-specific GFP reporter [16]. This efficiency in resc derivation is much higher than the conversion rate we observed between FGF-iPSCs and LIF-dependent ESCs, indicating a relative more robust epigenetic barrier exists between these two cell types. However, when the same switch was applied to established EpiSC, the efficiency for generating rescs was extremely more reduced and therefore more similar to what described in our experimental conditions. Iinhibition of Erk signalling, either by BMP4-induced upregulation of ID proteins or using small molecule inhibitors, is required in mescs to maintain the ICM-like pluripotent state [18]. It is striking that FGF-dependent ipscs maintain a naive pluripotent state in the presence of strong ERK activity. The morphological and epigenetic similarities between murine EpiSCs and human ESCs suggest that human ESCs, despite their blastocyst origin, exist in a primed pluripotent state. Our data now demonstrate that a naive pluripotent state can be achieved in FGF-dependent murine ipscs. Revealing the molecular mechanisms that install a naive pluripotent state in FGF-iPSCs may provide important cues the identity of human ESCs. Hanna and colleagues recently demonstrated that in the nonpermissive NOD genetic background, the ES-like pluripotent state is metastable, and remains dependent on the ectopic expression of Klf4 or cmyc or the presence of small molecule inhibitors of GSK3b or the MEK-ERK signaling pathway. In this genetic background, the EpiSC pluripotent state appears dominantly stable and is achieved upon withdrawal of ectopic reprogramming factors. Our results demonstrate that the opposite is true for permissive mouse strains such as the 129Sv, C57B/L6 and 129/BL6 F1 genetic backgrounds used in this study. The genetic elements that allow the derivation of ES-cell lines in these strains may be the same that dominantly stabilize the ES-like pluripotent state in ips cells from these strains. The murine ES-like state offers several functional PLoS ONE 9 December 2010 Volume 5 Issue 12 e16092
10 Generation of Mouse FGF-iPS Cells Figure 8. In vitro and in vivo pluripotency of FGF-iPSCs. (A) FGF-iPSCs were detached with collagenase IV from the substrate to induce embryoid body (EB) formation. After 5 days in culture, the majority of EBs downregulate the GFP transgene. (B) EBs plated in N2-medium generate nestin positive neural progenitor cells as highlighted by immunofluorescence with its specific antibody. (C) EBs were plated on gelatin coated dishes containing DMEM medium supplemented with 20% FBS. After 20 days in such condition, islands of beating cardiomyocite became evident (see Movie S1). Cell differentiation was confirmed by immunoistochemistry for the mesodermal marker smooth muscle actin (Sma) (C) and for the endodermal marker Sox17 (D). (E H) Ematoxylin and Eosin histological staining of teratomas, derived by subcutaneously injection of 1 million FGF-iPSCs, showed cartilage (E), adipose tissue (F), gut-like epithelium (G) and muscle (H). (I) Oct4-GFP positive FGF-iPSCs integrate into the inner cell mass of mouse blastocyst after morula aggregation and contribute, thereafter, to viable highly chimaeric animals (on the right) as compared to unmanipulated mice (on the left) (J). (K) Germiline contribution of FGF-iPS #5 and #9 chimera. (L) Genotyping demontrate the presence of the Oct4-GFP transgene in the offspring. doi: /journal.pone g008 advantages over the epiblast-stem cell state, since it allows ready manipulation of the genome combined with the ability to generate chimeras. While gene targeting is commonplace in some mouse strains, other species do not readily allow gene manipulation using similar experimental approaches, since in most species the primed pluripotent state appears to prevail. Primed pluripotent stem cells are refractive to single cell culture, which severely hampers the clonal derivation of mutant cell lines. In addition, the murine ESstate allows the generation of chimeras and may thus facilitate the generation of animal mutants to model human disease in alternative species. Indeed, the recent derivation of rat pluripotent stem cells in a murine ES-like state illustrates this point [9,10]. Understanding the nature of the genetic factors that dominantly stabilize the murine ES-like pluripotent state and uncovering their function in the stabilization of the ES-like pluripotent state is fundamentally important to understanding how to harness the power of this pluripotent state in future research and cell based therapies and with the Institutional Committee for the Good Animal Experimentation of the San Raffaele Scientific Institute (IACUC 423). This study was performed following ethical approval by the institutional review board of San Raffaele Scientific Institute. Culture of murine ES cells, EpiSCs and ipscs Murine ES (Di Stefano et al., 2009) and LIF-iPS cells (Di Stefano et al., 2009) were cultured on feeder layer in ES medium composed by knock-out DMEM medium (Invitrogen) containing 15% fetal bovine serum (Invitrogen), leukemia inhibitor factor (LIF) (Chemicon), 2-b-mercaptoethanol, L-glutamine and nonessential amino acids (Invitrogen) as detailed in Di Stefano et al. (2009). FGF-iPS cells were cultivated on mytomicin-inactivated MEF in human ESC medium containing knock-out DMEM medium (Invitrogen) containing 20% knock-out serum replacement (Invitrogen), 4 ng/ml bfgf (Peprotrech), 2-b-mercaptoethanol, L-glutamine and non-essential amino acids (Invitrogen). FGF-iPSCs were normally passaged in small clumps after treatment with collagenase IV (1 mg/ml) every 3 to 5 days in average. LIF-iPS, EpiSC and FGF-iPScells were cultured on Matrigel-coated dishes for two passages for depletion of feeder cells Materials and Methods Ethics Statement Animal care and experimental procedures were performed in accordance with Decreto Legislativo n. 116 del 27 Gennaio PLoS ONE 10 December 2010 Volume 5 Issue 12 e16092
11 before DNA and RNA isolation. EpiSC [19] were maintained in the same conditions of FGF-iPS. Derivation of ips cells using retroviral vectors Oct4-GFP embryonic or post-natal fibroblasts were obtained from the OG2 transgenic mouse line kindly provided to us by Dr. Michele Boiani (Max Planck Institute, Münster, Germany). Briefly, E15.5 transgenic embryos were isolated, washed repetitively in PBS and then the heads and all the visceral tissues dissected out. The remaining carcasses were washed, minced and dissociated after a trypsin treatment of 309 at 37uC. Single cells and small aggregates were cultured and propagated in DMEM medium (Invitrogen) with 10% FBS (Sigma). For post-natal fibroblasts, clipped tails were rinsed in PBS supplemented with antibiotics and careful minced, before digestion into trypsin for 209 at 37uC. Digested tissue was finely disaggregated with Pasteur pipettes and single cell suspension plated and propagated in DMEM medium (Invitrogen) with 10% FBS (Sigma). For induction of FGF-iPS cells, MEFs were seeded onto gelatin-coated dishes in DMEM medium with 10% serum and transduced by plib-based retroviruses for mouse Oct4, Klf4, Sox2 and c-myc. Two days after, the cells were harvested and replated on mytomicin-inactivated MEF in 100 mm dish and incubated in human ESC medium. After days (for O-S-K-M transduced cells) or days (for O-S-K/O-S-M), the FGF-iPS colonies were picked for expansion on MEF feeder cells in human ESC medium. A schematic diagram of the reprogramming protocol used is shown in Figure 1A. Retroviral production was performed as described before [6]. Briefly, Murine cdnas for Oct4, Klf4, Sox2 and c-myc were amlified by PCR from ES cells cdna and cloned into Moloney-based retroviral vector plib (kindly donated by Dr. Marius Wernig, Stanford University, CA). Plat-E cells were plated at cells per 100 mm dish and incubated overnight. Cells were transfected with 10 mg of vector, according to a conventional CaCl 2 transfection protocol. After 30 h, medium was collected, filtered through 0.44 mm cellulose acetate filters and supplemented with 4 mg/ml Polybrene (Sigma). MEFs were then exposed to viralpolybrene containing supernatants. Derivation of ips cells using STEMCCA vectors Fibroblasts were harvested from tails of Oct4-GFP/R26- M2rtTA transgenic newborn mice and maintained in DMEM with 10% FBS, 2 mm L-Glutamine and Pen/Strep (all Invitrogen). To produce infectious viral particles, 293 T cells cultured on 10 cm dishes were transfected with the STEMCCA vectors together with the packaging plasmids VSV-G and D8.9 using Lipofectamine2000 (Invitrogen). The fibroblasts were transduced at,4000 cells/cm 2 on 10 cm TC dishes with concentrated virus. The transgenes were induced the following day by adding 2 ug/ml Doxycyline (Sigma) to the media. After one more day cells were split onto two new 10 cm dishes and the media was changed to ES or human ESC media. Doxycyline was removed after 15 days and colonies were picked after 3 to 5 more days and maintained as described above. Alkaline phosphatase, immunofluorescence assay and FISH For alkaline phosphatase (AP) staining, cells were fixed with 2% paraformaldehyde for 10 min at RT, washed two times with PBS (Euroclone) and treated with AP solution containing BCIP and NBT (Roche). For immunocytochemistry, cells were fixed with 4% paraformaldehyde for 20 min at RT and washed three times with PBS (Euroclone). Fixed cells were then incubated in blocking buffer containing 5% goat serum (Sigma) and 0,1% Triton X-100 for 30 minutes at RT. The cells were then incubated with primary antibodies diluted in the blocking buffer overnight at 4uC. On the next day, after 3 washes in PBS, the cells were exposed to secondary antibodies at RT for one hour. Primary antibodies included SSEA1 (1:100, Chemicon), Desmin (1:200, kindly donated by G.Cossu), SMA (1:200, kindly donated by L. Naldini), Nanog (1:100, Abcam), Oct4 (1:100, Abcam), Sox2 (1:100, Abcam), me3h3k27 (1:100, kindly donated by G. Testa). Secondary antibodies used were donkey anti-mouse and antirabbit IgG and IgM antibodies conjugated with Alexa 488, Alexa 594 (1:500, Molecular Probes). Nuclear staining was performed with Hoechst (Invitrogen). Pictures were taken using a Nikon Eclipse 3000 fluorescence microscope. For RNA FISH the Xist probe used a 19 kb genomic fragment derived from a lamda clone which covers most of Xist gene. Probes were labeled by nick translation with Spectrum Green-dUTP and hybridized (0.1 ug of probe DNA with 10 ug of salmon sperm DNA per coverslip) in 50% formamide, 2 x SSC, 20% dextran sulfate, 1 mg/ml BSA (Biolabs), and 200 mm VRC, overnight at 37uC. After three washes in 50% formamide, 2 x SSC and three washes in 2 x SSC at 42uC, DNA was counterstained for 3 min in 0.2 mg/ml DAPI, followed by a final wash in 2 x SSC. Samples were mounted in 90% glycerol, 0.1x PBS, 0.1% p- phenylenediamine. Feeder-free culture of FGF-iPSCs FGF-iPS cells were cultivated on fibronectin (Sigma) coated dishes (5 ug/ml) in a knock-out DMEM medium (Invitrogen) containing N2 and B27 and supplemented with 100 ng/ml bfgf (Invitrogen), 50 ng/ml ActivinA (Peprotech), 2-b-mercaptoethanol, L-glutamine and non-essential amino acids (Invitrogen). RT-PCR analysis The RNeasy mini-kit (Qiagen) was used for total RNA isolation and Superscript reverse transcriptase II (Invitrogen) for cdna synthesis starting with 2 mg of total RNA. Amplification of specific genes was carried out using primers shown in Table S1. PCR conditions were as follows: 95uC for 10 minutes; denaturation at 95uC for 30 sec, annealing for 30 sec at a temperature specific for each primer set, and extension at 72uC for 30 sec for 33 cycles and then 72uC for 10 minutes. Luciferase reporter assay Plasmids for the Oct4 enhancer assay were cloned as described [2]. 12 well plates with mes, EpiSCs and FGF-iPS cells were transfected with the 1 ug of the respective Oct4 enhancer plasmid and 0.1 ug of a renilla control plasmid using Lipofectamine2000 (Invitrogen). Luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega) following the manufacturer s protocol. All experiments were carried out in triplicates and normalized to renilla activity to account for differences in transfection efficiencies. Agilent chip hybridization For genome-wide expression analysis, total RNA was extracted using Trizol reagent (Invitrogen) and labeled and hybridized to Agilent Whole Mouse Genome Oligo 4X44K Microarrays (onecolor platform) according to the manufacturer s protocols. The gene expression results were analyzed using GeneSifter microarray analysis software. Illumina bead chip hybridization Biotin-labelled crna was produced using a linear amplification kit (Ambion, Austin, TX, United States). Total RNA was PLoS ONE 11 December 2010 Volume 5 Issue 12 e16092
12 quality-checked by NanoDrop analysis (NanoDrop Technologies, Wilmington, DE, USA) and a quantity of 400 ng was used as input. Chip hybridization, washing, Cy3-streptavidin staining and scanning were performed on the Illumina BeadStation 500 platform (Illumina, San Diego, CA, United States), according to manufacturer s instruction. crna samples were hybridized onto Illumina mouse-8 BeadChips. We hybridized the following samples as biological replicates: ESCs, LIF-iPSCs and FGF-iPSCs. All basic expression data analysis was carried out using the BeadStudio software 3.0. Raw data were background-subtracted and normalized using the rank invariant algorithm and then filtered for significant expression on the basis of negative control beads. Pathway analysis was determined according to Gene Ontology terms or mapped to Kegg pathways using DAVID 2006 ( by using GenBank accession numbers represented by the corresponding chip oligonucleotides as input. Analysis of the transcriptional regulatory circuit was performed using String database 8.0 ( Real-time polymerase chain reaction (qpcr) Real-Time PCR was performed in 384 Well Optical Reaction Plates (Applied Biosystems, Foster City, CA, United States). The PCR mix in each well included 5 ml of SYBRHGreen PCR Master Mix (Applied Biosystems), 1.5 ml each of the forward and reverse primers (5 ng/ml) and 1 ml of single strand cdna (50 ng/ml) in a final reaction volume of 10 ml. qpcr reactions were carried out on the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) using the following program: 50uC for 2 min, 95uC for 10 min, 95uC for 15 s and 60uC for 1 min, 95uC for 15 s, 60uC for 15 s and 95uC for 15 s for a total of 40 cycles. Triplicate amplifications were carried out for each target gene with three wells serving as negative controls. The output data generated by the Sequence Detection System software were transferred to Microsoft Excel for analysis. Quantification was performed through a standard curve-based method and normalized to the expression of the housekeeping gene beta-actin. Primer sequences are provided in Table S1. Western blotting Protein extracts were isolated from ESCs, LIF-iPSCs and FGFiPSCs using a modified RIPA buffer lysis buffer (150 mm NaCl, 10 mm Tris-HCl, 1 mm EDTA, 1% Triton-X100; ph 7.8) supplemented with complete protease inhibitor cocktail (Sigma) added just before use. Protein concentration was determined according to the Bradford method. Equal quantities of proteins were separated by electrophoresis in a 10% SDS-polyacrylamide gel (BioRad, Hercules, CA). Primary antibodies, purchased from Cell Signalling Technology, against SMAD 2/3, Phospho-SMAD 2/3, SMAD 1/5/8, Phospho-SMAD 1/5/8, ERK K, phospho- ERK K, Stat3 and phospho-stat3 were used with the suitable HRP-conjugated secondary antibodies. Bound antibodies on nitrocellulose membrane (Amersham Biosciences) were then detected using the chemiluminescent substrate ECL (Amersham Biosciences). Methylation analysis Methylation analysis was performed as previously described by Blelloch et al. (2006). Genomic DNA extraction was performed using the Blood & Cell Culture DNA Mini Kit (Qiagen). Bisulfite treatment of DNA was achieved using the CpGenome DNA Modification Kit (Chemicon) according to the manufacturer s instructions. The resulting modified DNA was amplified by nested polymerase chain reaction (PCR) using two forward (Met- Oct4 F1 and Met-Oct4 F2) primers and one reverse (Met-Oct4 R) primer listed in Table S1.The first round of PCR was done as follows: 94uC for 4 minutes; five cycles of 94uC for 30 seconds, 56uC for 1 minute ( 1uC per cycle), 72uC for 1 minute; and 30 cycles of 94uC for 30 seconds, 51uC for 45 seconds, and 72uC for 1 minute, 20 seconds. The second round of PCR was 94uC for 4 minutes; 30 cycles of 94uC for 30 seconds, 53.5uC for 1 minute, and 72uC for 1 minute 20 seconds. The resulting amplified products were gel-purified (Promega), subcloned into the TA vector (Invitrogen), and sequenced using the T7 and SP6 primers. Small-molecule compounds The following inhibitors were used at the indicated final concentrations: ALK inhibitor (A8301, Tocris Bioscience; 0.5 mm) and JAK inhibitor I (Calbiochem ; 0.6 mm), SU5402 (Calbiochem , 10 mm). Anti-mouse LIF antibody was purchased by R&D system. Teratoma formation One million of serially passaged FGF-iPS cells were harvested by collagenase IV treatment, diluted in DMEM/F12 and injected subcutaneously into the hind limb muscle of SCID mice. Five to six weeks after the injection all mice developed tumors. Teratomas were removed, fixed overnight in 4% PFA and embedded in Paraffin. 10 um thicken sections were stained with hematoxylin and eosin for conventional morphological assessment. Differentiation of FGF-iPS cells For EB formation, FGF-iPS cells were harvested by treating with 1 mg/ml collagenase IV for one hour. The clumps of cells were then transferred into T25 flasks for 6 days in differentiation medium (knock-out DMEM containing 20% knock-out serum replacement, 2-b-mercaptoethanol, L-glutamine, penicillin-streptomycin and non-essential amino acids). For the generation of cardiomyocytes and Sox17-positive cells, EBs were plated onto gelatin-coated tissue culture dishes at very low density in DMEM medium with 20% animal serum. Medium was replaced every two days. For neural differentiation EBs were cultured for 6 days in differentiation medium and then plated onto matrigel coated dishes in N2-medium. Chimera production Morulas were collected from the oviduct of superovulated CD-1 females mated with CD-1 males 48 hrs earlier by a flushing procedure with BMOC-3 medium. The zona pellucida was removed from the embryos by washing through 3 consecutive drops of Acid Tyrode solution (Sigma). Small clumps of FGF-cells were generated after treatment with collagenase IV. One clump containing between 5 to 15 FGF-iPS cells was added to each embryo and cultured in BMOC-3 medium (Invitrogen) overnight at 37uC 5% in 5% CO(2). The following day, embryos, which had developed into blastocysts, were transferred into the uterus of pseudopregnant recipient CD-1 females mated with vasectomized CD-1 males 2.5 days earlier. Supporting Information Figure S1 Epigenetic, transgene silencing and chromosome stability of FGF-iPSCs. (A) Scheme representing the methylation pattern of the Oct4 promoter in FGF-iPSCs (line 5C) and MEFs. Open circles indicate unmethylated, while filled circles depict methylated CpG dinucleotides. All the CpG dinucleotides tested were unmethylated in FGF-iPSCs. (B) Silencing of retroviral expressed transgenes was assessed by qpcr analysis. (C) A chromosome spread of FGF-iPS cells is presented with a normal PLoS ONE 12 December 2010 Volume 5 Issue 12 e16092
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Xeno-Free Systems for hesc & hipsc. Facilitating the shift from Stem Cell Research to Clinical Applications
 Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Protocol Using the Reprogramming Ecotropic Retrovirus Set: Mouse OSKM to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblast (MEF) cells into induced pluripotent stem (ips) cells in a 6-well format. Transduction
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblast (MEF) cells into induced pluripotent stem (ips) cells in a 6-well format. Transduction
Protocol Reprogramming MEFs using the Dox Inducible Reprogramming Lentivirus Set: Mouse OKSM
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblasts (MEFs) into induced pluripotent stem (ips) cells in a 6-well format. Transduction
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblasts (MEFs) into induced pluripotent stem (ips) cells in a 6-well format. Transduction
Protocol Reprogramming Human Fibriblasts using the Dox Inducible Reprogramming Polycistronic Lentivirus Set: Human 4F2A LoxP
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1.
 Supplementary Figure 1. Quantification of western blot analysis of fibroblasts (related to Figure 1) (A-F) Quantification of western blot analysis for control and IR-Mut fibroblasts. Data are expressed
Supplementary Figure 1. Quantification of western blot analysis of fibroblasts (related to Figure 1) (A-F) Quantification of western blot analysis for control and IR-Mut fibroblasts. Data are expressed
Protocol Using a Dox-Inducible Polycistronic m4f2a Lentivirus to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency.
 Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
APPLICATION NOTE Page 1
 APPLICATION NOTE Page 1 Generation of ips Cells from Oct4-Neo Reporter Embryonic Fibroblasts Dongmei Wu, Yan Gao, Charles Martin, Xun Cheng, Wen Xiong, Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle St.,
APPLICATION NOTE Page 1 Generation of ips Cells from Oct4-Neo Reporter Embryonic Fibroblasts Dongmei Wu, Yan Gao, Charles Martin, Xun Cheng, Wen Xiong, Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle St.,
ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming vector for generating induced pluripotent stem (ips)
 Kit for generating ips cells using ReproRNA -OKSGM, a non-integrating, self-replicating RNA reprogramming vector Product Description ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming
Kit for generating ips cells using ReproRNA -OKSGM, a non-integrating, self-replicating RNA reprogramming vector Product Description ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming
Protocol Reprogramming Human Fibroblasts into ips Cells using the Stemgent Reprogramming Lentivirus Set: Human OKSM
 STEMGENT Page 1 Reprogramming Lentivirus Set: Human OKSM OVERVIEW The following procedure describes the reprogramming of BJ Human Fibroblasts (BJ cells) to induced pluripotent stem (ips) cells using the
STEMGENT Page 1 Reprogramming Lentivirus Set: Human OKSM OVERVIEW The following procedure describes the reprogramming of BJ Human Fibroblasts (BJ cells) to induced pluripotent stem (ips) cells using the
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
A Novel Platform to Enable the High-Throughput Derivation and Characterization of Feeder-Free Human ipscs
 Supplementary Information A Novel Platform to Enable the High-Throughput Derivation and Characterization of Feeder-Free Human ipscs Bahram Valamehr, Ramzey Abujarour, Megan Robinson, Thuy Le, David Robbins,
Supplementary Information A Novel Platform to Enable the High-Throughput Derivation and Characterization of Feeder-Free Human ipscs Bahram Valamehr, Ramzey Abujarour, Megan Robinson, Thuy Le, David Robbins,
Supplementary Figure 1 Characterization of OKSM-iNSCs
 Supplementary Figure 1 Characterization of OKSM-iNSCs (A) Gene expression levels of Sox1 and Sox2 in the indicated cell types by microarray gene expression analysis. (B) Dendrogram cluster analysis of
Supplementary Figure 1 Characterization of OKSM-iNSCs (A) Gene expression levels of Sox1 and Sox2 in the indicated cell types by microarray gene expression analysis. (B) Dendrogram cluster analysis of
SUPPLEMENTARY INFORMATION
 Supplementary Figure S1 Colony-forming efficiencies using transposition of inducible mouse factors. (a) Morphologically distinct growth foci appeared from rtta-mefs 6-8 days following PB-TET-mFx cocktail
Supplementary Figure S1 Colony-forming efficiencies using transposition of inducible mouse factors. (a) Morphologically distinct growth foci appeared from rtta-mefs 6-8 days following PB-TET-mFx cocktail
StemTAG Alkaline Phosphatase Staining Kit
 Product Manual StemTAG Alkaline Phosphatase Staining Kit Catalog Number CBA-300 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES) cells are continuous
Product Manual StemTAG Alkaline Phosphatase Staining Kit Catalog Number CBA-300 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES) cells are continuous
SUPPLEMENTARY INFORMATION
 doi:.38/nature08267 Figure S: Karyotype analysis of ips cell line One hundred individual chromosomal spreads were counted for each of the ips samples. Shown here is an spread at passage 5, predominantly
doi:.38/nature08267 Figure S: Karyotype analysis of ips cell line One hundred individual chromosomal spreads were counted for each of the ips samples. Shown here is an spread at passage 5, predominantly
Assuring Pluripotency of ESC and ipsc Lines
 Assuring Pluripotency of ESC and ipsc Lines Introduction Compared to other cell types, stem cells have the unique ability to self-renew their populations by dividing into identical daughter stem cells
Assuring Pluripotency of ESC and ipsc Lines Introduction Compared to other cell types, stem cells have the unique ability to self-renew their populations by dividing into identical daughter stem cells
hipscs were derived from human skin fibroblasts (CRL-2097) by ectopic
 Generation and characterization of hipscs hipscs were derived from human skin fibroblasts (CRL-2097) by ectopic expression of OCT4, SOX2, KLF4, and C-MYC as previously described 1. hipscs showed typical
Generation and characterization of hipscs hipscs were derived from human skin fibroblasts (CRL-2097) by ectopic expression of OCT4, SOX2, KLF4, and C-MYC as previously described 1. hipscs showed typical
hpsc Growth Medium DXF Dr. Lorna Whyte
 hpsc Growth Medium DXF Dr. Lorna Whyte 27.06.2014 Training from Heidelberg Overview Background: Stem Cells Introduction: Human Pluripotent Stem Cells (hpsc) vs. Adult Stem Cells Promise of PSC Research
hpsc Growth Medium DXF Dr. Lorna Whyte 27.06.2014 Training from Heidelberg Overview Background: Stem Cells Introduction: Human Pluripotent Stem Cells (hpsc) vs. Adult Stem Cells Promise of PSC Research
produced in this study characterized using 3-8 % gradient SDS-PAGE under reducing
 Supplementary Figure 1 Characterisation of newly produced laminins. Human recombinant LN-521 produced in this study characterized using 3-8 % gradient SDS-PAGE under reducing 1 and non-reducing conditions.
Supplementary Figure 1 Characterisation of newly produced laminins. Human recombinant LN-521 produced in this study characterized using 3-8 % gradient SDS-PAGE under reducing 1 and non-reducing conditions.
Chemically defined conditions for human ipsc derivation and culture
 Nature Methods Chemically defined conditions for human ipsc derivation and culture Guokai Chen, Daniel R Gulbranson, Zhonggang Hou, Jennifer M Bolin, Victor Ruotti, Mitchell D Probasco, Kimberly Smuga-Otto,
Nature Methods Chemically defined conditions for human ipsc derivation and culture Guokai Chen, Daniel R Gulbranson, Zhonggang Hou, Jennifer M Bolin, Victor Ruotti, Mitchell D Probasco, Kimberly Smuga-Otto,
Figure S2. Response of mouse ES cells to GSK3 inhibition. Mentioned in discussion
 Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2239 Hepatocytes (Endoderm) Foreskin fibroblasts (Mesoderm) Melanocytes (Ectoderm) +Dox rtta TetO CMV O,S,M,K & N H1 hes H7 hes H9 hes H1 hes-derived EBs H7 hes-derived EBs H9 hes-derived
DOI: 10.1038/ncb2239 Hepatocytes (Endoderm) Foreskin fibroblasts (Mesoderm) Melanocytes (Ectoderm) +Dox rtta TetO CMV O,S,M,K & N H1 hes H7 hes H9 hes H1 hes-derived EBs H7 hes-derived EBs H9 hes-derived
SUPPLEMENTARY FIG. S9. Morphology and DNA staining of cipsc-differentiated trophoblast cell-like cell. Scale bar: 100 mm. SUPPLEMENTARY FIG. S10.
 Supplementary Data SUPPLEMENTARY FIG. S1. Lentivirus-infected canine fibroblasts show YFP expression. (A, B) Uninfected canine skin fibroblasts (CSFs); (C, D) infected CSFs; (E, F) uninfected CTFs; (G,
Supplementary Data SUPPLEMENTARY FIG. S1. Lentivirus-infected canine fibroblasts show YFP expression. (A, B) Uninfected canine skin fibroblasts (CSFs); (C, D) infected CSFs; (E, F) uninfected CTFs; (G,
Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency
 nature methods Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency Akitsu Hotta, Aaron Y L Cheung, Natalie Farra, Kausalia Vijayaragavan, Cheryle A Séguin, Jonathan S Draper,
nature methods Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency Akitsu Hotta, Aaron Y L Cheung, Natalie Farra, Kausalia Vijayaragavan, Cheryle A Séguin, Jonathan S Draper,
Application Note. Laminin/Entactin Complex: A Feeder-Free Surface for Culture of Human Embryonic Stem Cells. Introduction
 Laminin/Entactin Complex: A Feeder-Free Surface for Culture of Human Embryonic Stem Cells Deepa Saxena, Ph.D. and Susan Qian, Ph.D. Corning Incorporated, Tewksbury, MA, USA Application Note Contents 1
Laminin/Entactin Complex: A Feeder-Free Surface for Culture of Human Embryonic Stem Cells Deepa Saxena, Ph.D. and Susan Qian, Ph.D. Corning Incorporated, Tewksbury, MA, USA Application Note Contents 1
PluriQ TM Serum Replacement (PluriQ TM SR)
 PluriQ TM Serum Replacement (PluriQ TM SR) (PluriQ SR) is a defined, serum-free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated
PluriQ TM Serum Replacement (PluriQ TM SR) (PluriQ SR) is a defined, serum-free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated
Components Neural induction basal medium (Cat. # ) 100 ml x 5 Neural induction medium supplement 50x (Cat. # ) 2 ml x 5
 HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
SUPPLEMENTARY INFORMATION
 Full Methods Derivation of resc cell lines Mouse embryos were isolated from E5.5 - E7.5 129/sv or ROSA129/Sv females mated with Oct4-ΔPE-GFP transgenic males with a mixed background of MF1, 129/sv, and
Full Methods Derivation of resc cell lines Mouse embryos were isolated from E5.5 - E7.5 129/sv or ROSA129/Sv females mated with Oct4-ΔPE-GFP transgenic males with a mixed background of MF1, 129/sv, and
gacgacgaggagaccaccgctttg aggcacattgaaggtctcaaacatg
 Supplementary information Supplementary table 1: primers for cloning and sequencing cloning for E- Ras ggg aat tcc ctt gag ctg ctg ggg aat ggc ttt gcc ggt cta gag tat aaa gga agc ttt gaa tcc Tpbp Oct3/4
Supplementary information Supplementary table 1: primers for cloning and sequencing cloning for E- Ras ggg aat tcc ctt gag ctg ctg ggg aat ggc ttt gcc ggt cta gag tat aaa gga agc ttt gaa tcc Tpbp Oct3/4
StemTAG Alkaline Phosphatase Activity Assay Kit (Colorimetric)
 Product Manual StemTAG Alkaline Phosphatase Activity Assay Kit (Colorimetric) Catalog Number CBA-301 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES)
Product Manual StemTAG Alkaline Phosphatase Activity Assay Kit (Colorimetric) Catalog Number CBA-301 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES)
bfgf Supports Human ES Cell Self- Renewal
 APPLICATION NOTE Page 1 bfgf Supports Human ES Cell Self- Renewal Authors: Dongmei Wu, Wen Xiong, Yan Gao, Kristine Guerrero, Yi Chen, Liming Yang, Yang Liu, and Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle
APPLICATION NOTE Page 1 bfgf Supports Human ES Cell Self- Renewal Authors: Dongmei Wu, Wen Xiong, Yan Gao, Kristine Guerrero, Yi Chen, Liming Yang, Yang Liu, and Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle
Electromagnetic Fields Mediate Efficient Cell Reprogramming Into a Pluripotent State
 Electromagnetic Fields Mediate Efficient Cell Reprogramming Into a Pluripotent State Soonbong Baek, 1 Xiaoyuan Quan, 1 Soochan Kim, 2 Christopher Lengner, 3 Jung- Keug Park, 4 and Jongpil Kim 1* 1 Lab
Electromagnetic Fields Mediate Efficient Cell Reprogramming Into a Pluripotent State Soonbong Baek, 1 Xiaoyuan Quan, 1 Soochan Kim, 2 Christopher Lengner, 3 Jung- Keug Park, 4 and Jongpil Kim 1* 1 Lab
StemTAG Alkaline Phosphatase Staining Kit (Purple)
 Product Manual StemTAG Alkaline Phosphatase Staining Kit (Purple) Catalog Number CBA- 306 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES) cells are
Product Manual StemTAG Alkaline Phosphatase Staining Kit (Purple) Catalog Number CBA- 306 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Embryonic stem (ES) cells are
Supplemental Information
 Cell Stem Cell, Volume 15 Supplemental Information Generation of Naive Induced Pluripotent Stem Cells from Rhesus Monkey Fibroblasts Riguo Fang, Kang Liu, Yang Zhao, Haibo Li, Dicong Zhu, Yuanyuan Du,
Cell Stem Cell, Volume 15 Supplemental Information Generation of Naive Induced Pluripotent Stem Cells from Rhesus Monkey Fibroblasts Riguo Fang, Kang Liu, Yang Zhao, Haibo Li, Dicong Zhu, Yuanyuan Du,
Suggested Method Reprogramming MEF Cells into pips Cells using the Stemgent Recombinant Human Protein Set: OSKM-11R
 Page 1 Reprogramming MEF Cells into pips Cells using the Cat. No. 00-0062 OVERVIEW The procedure outlined below describes the reprogramming of mouse embryonic fibroblasts (MEF) by the addition of four
Page 1 Reprogramming MEF Cells into pips Cells using the Cat. No. 00-0062 OVERVIEW The procedure outlined below describes the reprogramming of mouse embryonic fibroblasts (MEF) by the addition of four
T-iPSC. Gra-iPSC. B-iPSC. TTF-iPSC. Supplementary Figure 1. Nature Biotechnology: doi: /nbt.1667
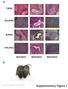 a T-iPSC Gra-iPSC B-iPSC TTF-iPSC Ectoderm Endoderm Mesoderm b Nature Biotechnology: doi:.38/nbt.667 Supplementary Figure Klf4 transgene expression Oct4 transgene expression.7 Fold GAPDH.6.5.4.3 Fold GAPDH
a T-iPSC Gra-iPSC B-iPSC TTF-iPSC Ectoderm Endoderm Mesoderm b Nature Biotechnology: doi:.38/nbt.667 Supplementary Figure Klf4 transgene expression Oct4 transgene expression.7 Fold GAPDH.6.5.4.3 Fold GAPDH
Supplementary data. sienigma. F-Enigma F-EnigmaSM. a-p53
 Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
HUMAN IPSC CULTURE PROTOCOLS
 HUMAN IPSC CULTURE PROTOCOLS FOR TRAINING COURSE IXCELLS BIOTECHNOLOGIES USA, LLC 10340 CAMINO SANTA FE, SUITE C, SAN DIEGO, CA 92121 TABLE OF CONTENTS CHAPTER 1 BEFORE STARTING 2-7 SECTION 1.1 COATING
HUMAN IPSC CULTURE PROTOCOLS FOR TRAINING COURSE IXCELLS BIOTECHNOLOGIES USA, LLC 10340 CAMINO SANTA FE, SUITE C, SAN DIEGO, CA 92121 TABLE OF CONTENTS CHAPTER 1 BEFORE STARTING 2-7 SECTION 1.1 COATING
The Effects of Serum Starvation on Cell Cycle Synchronization
 OSR Journal of Student Research Volume 1 Article 4 2013 The Effects of Serum Starvation on Cell Cycle Synchronization Negin Baghdadchi California State University, San Bernardino Follow this and additional
OSR Journal of Student Research Volume 1 Article 4 2013 The Effects of Serum Starvation on Cell Cycle Synchronization Negin Baghdadchi California State University, San Bernardino Follow this and additional
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
A Murine ESC-like State Facilitates Transgenesis and Homologous Recombination in Human Pluripotent Stem Cells
 Article A Murine ESC-like State Facilitates Transgenesis and Homologous Recombination in Human Pluripotent Stem Cells Christa Buecker, 1,4 Hsu-Hsin Chen, 1,4 Jose Maria Polo, 1,2,4 Laurence Daheron, 1,4
Article A Murine ESC-like State Facilitates Transgenesis and Homologous Recombination in Human Pluripotent Stem Cells Christa Buecker, 1,4 Hsu-Hsin Chen, 1,4 Jose Maria Polo, 1,2,4 Laurence Daheron, 1,4
Selected Techniques Part I
 1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.
 Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
CULTURE ENGINEERING DIFFERENTIATION CHARACTERIZATION. Stem Cell Research Solutions Promotional offers
 CULTURE ENGINEERING DIFFERENTIATION CHARACTERIZATION Stem Cell Research Solutions Promotional offers Stem Cell research products For more than a decade, we have provided you with key tools to address challenges
CULTURE ENGINEERING DIFFERENTIATION CHARACTERIZATION Stem Cell Research Solutions Promotional offers Stem Cell research products For more than a decade, we have provided you with key tools to address challenges
Table S1. Components for knockout serum replacer medium (KSR medium) (500 ml) Component Supplier Catalogue number
 Table S1. Components for knockout serum replacer medium (KSR medium) (500 ml) Component Supplier Catalogue number Stock Concentration Final concentration Volume used Advanced DMEM/F12 Invitrogen 12634010-78%
Table S1. Components for knockout serum replacer medium (KSR medium) (500 ml) Component Supplier Catalogue number Stock Concentration Final concentration Volume used Advanced DMEM/F12 Invitrogen 12634010-78%
Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent
 APPLICATION NOTE Page 1 Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent Authors: Amelia L. Cianci 1, Xun Cheng 1 and Kerry P. Mahon 1,2 1 Stemgent Inc.,
APPLICATION NOTE Page 1 Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent Authors: Amelia L. Cianci 1, Xun Cheng 1 and Kerry P. Mahon 1,2 1 Stemgent Inc.,
Transcriptional regulation of BRCA1 expression by a metabolic switch: Di, Fernandez, De Siervi, Longo, and Gardner. H3K4Me3
 ChIP H3K4Me3 enrichment.25.2.15.1.5 H3K4Me3 H3K4Me3 ctrl H3K4Me3 + E2 NS + E2 1. kb kb +82 kb Figure S1. Estrogen promotes entry of MCF-7 into the cell cycle but does not significantly change activation-associated
ChIP H3K4Me3 enrichment.25.2.15.1.5 H3K4Me3 H3K4Me3 ctrl H3K4Me3 + E2 NS + E2 1. kb kb +82 kb Figure S1. Estrogen promotes entry of MCF-7 into the cell cycle but does not significantly change activation-associated
John Gurdon was testing the hypothesis of genomic equivalence or that when cells divide they retain a full genomic compliment.
 1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Fig. S1 TGF RI inhibitor SB effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of
 Fig. S1 TGF RI inhibitor SB525334 effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of different concentrations of SB525334. Cells were lysed and
Fig. S1 TGF RI inhibitor SB525334 effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of different concentrations of SB525334. Cells were lysed and
Directed differentiation of human embryonic stem cells
 Directed differentiation of human embryonic stem cells TECAN Symposium 2008 Biologics - From Benchtop to Production Wednesday 17 September 2008 Andrew Elefanty Embryonic Stem Cell Differentiation Laboratory,
Directed differentiation of human embryonic stem cells TECAN Symposium 2008 Biologics - From Benchtop to Production Wednesday 17 September 2008 Andrew Elefanty Embryonic Stem Cell Differentiation Laboratory,
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days,
 Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days, respectively, and their mrnas were quantified by real time
Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days, respectively, and their mrnas were quantified by real time
used at a final concentration of 5 ng/ml. Rabbit anti-bim and mouse anti-mkp2 antibodies were
 1 Supplemental Methods Reagents and chemicals: TGFβ was a generous gift from Genzyme Inc. (Cambridge, MA) and was used at a final concentration of 5 ng/ml. Rabbit anti-bim and mouse anti-mkp2 antibodies
1 Supplemental Methods Reagents and chemicals: TGFβ was a generous gift from Genzyme Inc. (Cambridge, MA) and was used at a final concentration of 5 ng/ml. Rabbit anti-bim and mouse anti-mkp2 antibodies
ipsc Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer
 ipsc Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer Wenli Yang 1, Jason A. Mills 2, Spencer Sullivan 3, Ying Liu 1, Deborah L. French 2 and Paul Gadue 2, 1 Institute
ipsc Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer Wenli Yang 1, Jason A. Mills 2, Spencer Sullivan 3, Ying Liu 1, Deborah L. French 2 and Paul Gadue 2, 1 Institute
Supporting Information
 Supporting Information Tal et al. 10.1073/pnas.0807694106 SI Materials and Methods VSV Infection and Quantification. Infection was carried out by seeding 5 10 5 MEF cells per well in a 6-well plate and
Supporting Information Tal et al. 10.1073/pnas.0807694106 SI Materials and Methods VSV Infection and Quantification. Infection was carried out by seeding 5 10 5 MEF cells per well in a 6-well plate and
Jing Liao, Chun Cui, Siye Chen, Jiangtao Ren, Jijun Chen, Yuan Gao, Hui Li, Nannan Jia, Lu Cheng, Huasheng Xiao, and Lei Xiao
 Cell Stem Cell, Volume 4 Supplemental Data Generation of Induced Pluripotent Stem Cell Lines from Adult Rat Cells Jing Liao, Chun Cui, Siye Chen, Jiangtao Ren, Jijun Chen, Yuan Gao, Hui Li, Nannan Jia,
Cell Stem Cell, Volume 4 Supplemental Data Generation of Induced Pluripotent Stem Cell Lines from Adult Rat Cells Jing Liao, Chun Cui, Siye Chen, Jiangtao Ren, Jijun Chen, Yuan Gao, Hui Li, Nannan Jia,
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
Cat# Product Name amounts h NANOG (RFP-Bsd) inducible particles
 Premade Lentiviral Particles for ips Stem Factors For generating induced pluripotent stem (ips) cells or other applications. LVP003 LVP004 LVP005 LVP006 LVP007 LVP008 RESEARCH USE ONLY, not intend for
Premade Lentiviral Particles for ips Stem Factors For generating induced pluripotent stem (ips) cells or other applications. LVP003 LVP004 LVP005 LVP006 LVP007 LVP008 RESEARCH USE ONLY, not intend for
Frequently Asked Questions Stem Cells
 Q: Do you add antibiotics to your media? A: Coriell does not use antibiotics when culturing stem cells. Customers should be aware that inclusion of antibiotics in media may change growth characteristics
Q: Do you add antibiotics to your media? A: Coriell does not use antibiotics when culturing stem cells. Customers should be aware that inclusion of antibiotics in media may change growth characteristics
Long non-coding RNAs and Autophagy in Embryonic stem cells. Lei Han
 Long non-coding RNAs and Autophagy in Embryonic stem cells Lei Han 2011-12-21 Contents Embryonic Stem Cells (ESCs) Long non-coding RNA in ESCs and paper Autophagy in ESCs and paper Embryonic Stem Cells
Long non-coding RNAs and Autophagy in Embryonic stem cells Lei Han 2011-12-21 Contents Embryonic Stem Cells (ESCs) Long non-coding RNA in ESCs and paper Autophagy in ESCs and paper Embryonic Stem Cells
Nanog-Luc. R-Luc. 40 HA-Klf Relative protein level of Klf USP21. Vector USP2 USP21 *** *** *** ***
 :Flag Relative protein level of Relative protein level of : Flag Vec 21LV 21SV 2 : HA HA- HA- HA- HA-Klf4 Relative mrna expression Relative protein level of Relative protein level of Relative protein level
:Flag Relative protein level of Relative protein level of : Flag Vec 21LV 21SV 2 : HA HA- HA- HA- HA-Klf4 Relative mrna expression Relative protein level of Relative protein level of Relative protein level
Data Sheet. SBE Reporter Kit (TGFβ/SMAD signaling pathway) Catalog #: 60654
 Data Sheet SBE Reporter Kit (TGFβ/SMAD signaling pathway) Catalog #: 60654 Background The transforming growth factor beta (TGFβ) signaling pathway is involved in a diverse range of cell processes such
Data Sheet SBE Reporter Kit (TGFβ/SMAD signaling pathway) Catalog #: 60654 Background The transforming growth factor beta (TGFβ) signaling pathway is involved in a diverse range of cell processes such
Biology Open (2015): doi: /bio : Supplementary information
 Fig. S1. Verification of optimal conditions for the generation of LIF-dependent inscs Representative images (from five repeat experiments) of human primary fibroblasts undergoing reprogramming for 21 days
Fig. S1. Verification of optimal conditions for the generation of LIF-dependent inscs Representative images (from five repeat experiments) of human primary fibroblasts undergoing reprogramming for 21 days
ERK and GSK3 Signaling Pathway in cipsc
 ERK and GSK3 Signaling Pathway in cipsc (Experiment Designs & Results) Yangqing Lu, 2/21/2012 1 Background In naïve state ESC, MEK/ERK signaling pathway is dispensable for maintenance of the pluripotence
ERK and GSK3 Signaling Pathway in cipsc (Experiment Designs & Results) Yangqing Lu, 2/21/2012 1 Background In naïve state ESC, MEK/ERK signaling pathway is dispensable for maintenance of the pluripotence
Supplementary Information: Materials and Methods. Immunoblot and immunoprecipitation. Cells were washed in phosphate buffered
 Supplementary Information: Materials and Methods Immunoblot and immunoprecipitation. Cells were washed in phosphate buffered saline (PBS) and lysed in TNN lysis buffer (50mM Tris at ph 8.0, 120mM NaCl
Supplementary Information: Materials and Methods Immunoblot and immunoprecipitation. Cells were washed in phosphate buffered saline (PBS) and lysed in TNN lysis buffer (50mM Tris at ph 8.0, 120mM NaCl
TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1
 AD Award Number: W81XWH-10-1-0181 TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1 PRINCIPAL INVESTIGATOR: Jonathan Chernoff, M.D., Ph.D. CONTRACTING ORGANIZATION: Institute
AD Award Number: W81XWH-10-1-0181 TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1 PRINCIPAL INVESTIGATOR: Jonathan Chernoff, M.D., Ph.D. CONTRACTING ORGANIZATION: Institute
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11070 Supplementary Figure 1 Purification of FLAG-tagged proteins. a, Purification of FLAG-RNF12 by FLAG-affinity from nuclear extracts of wild-type (WT) and two FLAG- RNF12 transgenic
doi:10.1038/nature11070 Supplementary Figure 1 Purification of FLAG-tagged proteins. a, Purification of FLAG-RNF12 by FLAG-affinity from nuclear extracts of wild-type (WT) and two FLAG- RNF12 transgenic
MeCP2. MeCP2/α-tubulin. GFP mir1-1 mir132
 Conservation Figure S1. Schematic showing 3 UTR (top; thick black line), mir132 MRE (arrow) and nucleotide sequence conservation (vertical black lines; http://genome.ucsc.edu). a GFP mir1-1 mir132 b GFP
Conservation Figure S1. Schematic showing 3 UTR (top; thick black line), mir132 MRE (arrow) and nucleotide sequence conservation (vertical black lines; http://genome.ucsc.edu). a GFP mir1-1 mir132 b GFP
The p53-puma axis suppresses ipsc generation
 The p53-puma axis suppresses ipsc generation Yanxin Li, Haizhong Feng, Haihui Gu, Dale W. Lewis, Youzhong Yuan, Lei Zhang, Hui Yu, Peng Zhang, Haizi Cheng, Weimin Miao, Weiping Yuan, Shi-Yuan Cheng, Susanne
The p53-puma axis suppresses ipsc generation Yanxin Li, Haizhong Feng, Haihui Gu, Dale W. Lewis, Youzhong Yuan, Lei Zhang, Hui Yu, Peng Zhang, Haizi Cheng, Weimin Miao, Weiping Yuan, Shi-Yuan Cheng, Susanne
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW)
 Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Mutagenesis and generation of expression vectors Gene expression profiling Lentiviral production and infection of TF1 cells
 Mutagenesis and generation of expression vectors Human c-kit wild-type cdna encoding the short c-kit isoform was excised from vector pbshkitwt (kind gift from Dr. L Gros, France) by Sal I-Acc65 I digestion.
Mutagenesis and generation of expression vectors Human c-kit wild-type cdna encoding the short c-kit isoform was excised from vector pbshkitwt (kind gift from Dr. L Gros, France) by Sal I-Acc65 I digestion.
Percent survival. Supplementary fig. S3 A.
 Supplementary fig. S3 A. B. 100 Percent survival 80 60 40 20 Ml 0 0 100 C. Fig. S3 Comparison of leukaemia incidence rate in the triple targeted chimaeric mice and germline-transmission translocator mice
Supplementary fig. S3 A. B. 100 Percent survival 80 60 40 20 Ml 0 0 100 C. Fig. S3 Comparison of leukaemia incidence rate in the triple targeted chimaeric mice and germline-transmission translocator mice
To isolate single GNS 144 cell clones, cells were plated at a density of 1cell/well
 Supplemental Information: Supplemental Methods: Cell culture To isolate single GNS 144 cell clones, cells were plated at a density of 1cell/well in 96 well Primaria plates in GNS media and incubated at
Supplemental Information: Supplemental Methods: Cell culture To isolate single GNS 144 cell clones, cells were plated at a density of 1cell/well in 96 well Primaria plates in GNS media and incubated at
Premade Lentiviral Particles for ips Stem Factors: Single vector system. For generation of induced pluripotent stem (ips) cells and other applications
 Premade Lentiviral Particles for ips Stem Factors: Single vector system For generation of induced pluripotent stem (ips) cells and other applications RESEARCH USE ONLY, not for diagnostics or therapeutics
Premade Lentiviral Particles for ips Stem Factors: Single vector system For generation of induced pluripotent stem (ips) cells and other applications RESEARCH USE ONLY, not for diagnostics or therapeutics
Authors: Takuro Horii, Masamichi Yamamoto, Sumiyo Morita, Mika Kimura, Yasumitsu Nagao, Izuho Hatada *
 SUPPLEMENTARY INFORMATION Title: p53 Suppresses Tetraploid Development in Mice Authors: Takuro Horii, Masamichi Yamamoto, Sumiyo Morita, Mika Kimura, Yasumitsu Nagao, Izuho Hatada * Supplementary Methods
SUPPLEMENTARY INFORMATION Title: p53 Suppresses Tetraploid Development in Mice Authors: Takuro Horii, Masamichi Yamamoto, Sumiyo Morita, Mika Kimura, Yasumitsu Nagao, Izuho Hatada * Supplementary Methods
The Transfection Collection TCF/LEF Transient Pack Wnt / -catenin Signaling Pathway Catalog #: 79273
 Data Sheet The Transfection Collection TCF/LEF Transient Pack Wnt / -catenin Signaling Pathway Catalog #: 79273 Background The Wnt / -catenin signaling pathway controls a large and diverse set of cell
Data Sheet The Transfection Collection TCF/LEF Transient Pack Wnt / -catenin Signaling Pathway Catalog #: 79273 Background The Wnt / -catenin signaling pathway controls a large and diverse set of cell
Data Sheet. MAPK/ERK Signaling Pathway SRE Reporter HEK293 Cell Line Catalog #: 60406
 Data Sheet MAPK/ERK Signaling Pathway SRE Reporter HEK293 Cell Line Catalog #: 60406 Description The MAPK/ERK signaling pathway is a major participant in the regulation of cell growth and differentiation.
Data Sheet MAPK/ERK Signaling Pathway SRE Reporter HEK293 Cell Line Catalog #: 60406 Description The MAPK/ERK signaling pathway is a major participant in the regulation of cell growth and differentiation.
Supplemental Methods Cell lines and culture
 Supplemental Methods Cell lines and culture AGS, CL5, BT549, and SKBR were propagated in RPMI 64 medium (Mediatech Inc., Manassas, VA) supplemented with % fetal bovine serum (FBS, Atlanta Biologicals,
Supplemental Methods Cell lines and culture AGS, CL5, BT549, and SKBR were propagated in RPMI 64 medium (Mediatech Inc., Manassas, VA) supplemented with % fetal bovine serum (FBS, Atlanta Biologicals,
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture. Catalog Number Guidelines for Use
 Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Anti-HB-EGF (Human) mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Site directed mutagenesis, Insertional and Deletion Mutagenesis. Mitesh Shrestha
 Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
PROTOCOL PluriQ Serum Replacement
 (PluriQ SR) is a serum free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated human embryonic stem cells (hesc) and induced
(PluriQ SR) is a serum free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated human embryonic stem cells (hesc) and induced
NIA Aging Cell Repository
 Certificate of Analysis NIA Aging Cell Repository Human induced Pluripotent Stem Cell (ipsc) Line: AG25370*B Diagnosis Alzheimer Disease, Familial, Type 4 Mutation Reprogramming method Publication(s) describing
Certificate of Analysis NIA Aging Cell Repository Human induced Pluripotent Stem Cell (ipsc) Line: AG25370*B Diagnosis Alzheimer Disease, Familial, Type 4 Mutation Reprogramming method Publication(s) describing
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature06403 Supplementary Information: Nanog safeguards pluripotency and mediates germline development Supplementary Tables SUPPLEMENTARY INFORMATION mrna Forward primer Reverse primer Annealing
doi: 10.1038/nature06403 Supplementary Information: Nanog safeguards pluripotency and mediates germline development Supplementary Tables SUPPLEMENTARY INFORMATION mrna Forward primer Reverse primer Annealing
Intracellular receptors specify complex patterns of gene expression that are cell and gene
 SUPPLEMENTAL RESULTS AND DISCUSSION Some HPr-1AR ARE-containing Genes Are Unresponsive to Androgen Intracellular receptors specify complex patterns of gene expression that are cell and gene specific. For
SUPPLEMENTAL RESULTS AND DISCUSSION Some HPr-1AR ARE-containing Genes Are Unresponsive to Androgen Intracellular receptors specify complex patterns of gene expression that are cell and gene specific. For
Disease Clinical features Genotype
 Disease Clinical features Genotype Alpha 1 Antitrypsin deficiency (A1ATD) Patient 1 65 year old caucasian male, liver transplant recipient, explant biopsy confirmed A1ATD induced liver cirrhosis Patient
Disease Clinical features Genotype Alpha 1 Antitrypsin deficiency (A1ATD) Patient 1 65 year old caucasian male, liver transplant recipient, explant biopsy confirmed A1ATD induced liver cirrhosis Patient
Stem cell transfection guide
 APPLICATION NOTE Stem cell transfection guide Gene delivery solutions Introduction Stem cells continue to show immense promise for the future of regenerative medicine and personalized therapeutic treatments.
APPLICATION NOTE Stem cell transfection guide Gene delivery solutions Introduction Stem cells continue to show immense promise for the future of regenerative medicine and personalized therapeutic treatments.
The Thermo Scientific Nunclon Sphera surface supports formation of embryoid bodies from pluripotent stem cells
 1 The Thermo Scientific surface supports formation of embryoid bodies from pluripotent stem cells Louise Gaarn, Amy Sinor-Anderson, Robert Scott, Cindy Neeley, Joseph Granchelli, and Tina Marwood Application
1 The Thermo Scientific surface supports formation of embryoid bodies from pluripotent stem cells Louise Gaarn, Amy Sinor-Anderson, Robert Scott, Cindy Neeley, Joseph Granchelli, and Tina Marwood Application
LysoTracker Red DND-99 (Invitrogen) was used as a marker of lysosome or acidic
 information MATERIAL AND METHODS Lysosome staining LysoTracker Red DND-99 (Invitrogen) was used as a marker of lysosome or acidic compartments, according to the manufacturer s protocol. Plasmid independent
information MATERIAL AND METHODS Lysosome staining LysoTracker Red DND-99 (Invitrogen) was used as a marker of lysosome or acidic compartments, according to the manufacturer s protocol. Plasmid independent
Supplemental Information. A Mesenchymal-to-Epithelial Transition. Initiates and Is Required for the Nuclear. Reprogramming of Mouse Fibroblasts
 Cell Stem Cell, Volume 7 Supplemental Information A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts Ronghui Li, Jialiang Liang, Su Ni,
Cell Stem Cell, Volume 7 Supplemental Information A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts Ronghui Li, Jialiang Liang, Su Ni,
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
ADVANCED MEDIA TECHNOLOGY
 ADVANCED MEDIA TECHNOLOGY For Human ES and ips Cell Research The right medium makes all the difference in ensuring successful ES and ips cell culture. Stemgent offers high-quality, chemically-defined,
ADVANCED MEDIA TECHNOLOGY For Human ES and ips Cell Research The right medium makes all the difference in ensuring successful ES and ips cell culture. Stemgent offers high-quality, chemically-defined,
supplementary information
 DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
