1 R01 AI IHD HALFORD, W
|
|
|
- Augustine Hutchinson
- 6 years ago
- Views:
Transcription
1
2 1 R01 AI IHD 1R01AI ILLIAM DESCRIPTION (provided by applicant): More than 20 years have been invested into the testing of herpes simplex virus 2 (HSV-2) subunit vaccines that contain HSV-2's glycoprotein D (gd-2) and a powerful adjuvant. To date, gd-2 subunit vaccines have yielded equivocal protection in human clinical trials. It is imperative that we identify a more effective HSV-2 vaccine. R21 support from the NIH (AI81072) has allowed the P.I. to demonstrate that mutations in the ICP0 gene of HSV-2 yield liveattenuated viruses that are avirulent and yet elicit robust immunity to HSV-2. In side-by-side tests, a live HSV-2 ICP0- virus yields 10 to 100 times better protection against HSV-2 genital herpes relative to a gd-2 subunit vaccine in mice. However, the underlying basis of this superior protection remains unknown. The current research proposal seeks to address this gap in knowledge, and define the mechanisms that allow a novel, live-attenuated HSV-2 vaccine to elicit complete protection against HSV-2 genital herpes. This area of research offers the opportunity to understand, for the first time, the underpinnings of how a highly effective HSV-2 vaccine confers complete protective immunity to HSV-2. Two pivotal questions remain unanswered, and these are: 1. What are the dominant antigens that this live HSV-2 ICP0- virus relies upon to generate robust protection against HSV-2?; and 2. What are the critical effector mechanisms by which a live HSV-2 ICP0- virus-induced immune response rapidly controls HSV-2 challenge virus replication? Preliminary data reveal that immunization with a live HSV-2 ICP0- virus elicits a potent antibody response that targets several novel HSV-2 antigens not previously considered for use in vaccines. Moreover, adoptive transfer of polyclonal anti-hsv-2 antiserum from these immunized animals confers significant protection upon naïve recipients The goal of the studies proposed herein is to identify the dominant antigens and effector mechanisms that underlie effective vaccine-induced protective immunity to HSV-2, and determine if this knowledge may be applied to derive a superior, synthetic HSV-2 vaccine. This central goal will be achieved through completion of the following Specific Aims. Specific Aim 1 To identify the dominant B- and/or T-cell antigens of a liveattenuated HSV-2 ICP0- virus. Specific Aim 2 To determine if the protective immune response elicited by a live HSV-2 ICP0- virus is dependent on complement, antibody, and/or CD8+ T-cells. Specific Aim 3 To determine if immunization with the dominant antigens of a HSV-2 ICP0- virus (identified in Aim 1) elicits protection comparable to that elicited by the actual live HSV-2 ICP0- virus. PUBLIC HEALTH RELEVANCE: Herpes simplex virus-2 (HSV-2) ICP0- viruses are a promising new HSV-2 vaccine candidate. Such live-attenuated viruses may be capable of preventing the spread of genital herpes, which afflicts tens of millions of people. We propose to use such a live HSV-2 ICP0- mutant virus as a tool to determine the underlying mechanisms by which an effective vaccine elicits robust protection against HSV-2 genital herpes in animal models. This results of this analysis will improve our ability to better predict which HSV-2 vaccines are likely to succeed in future human clinical trials. CRITIQUE 1: Significance: 3 Investigator(s): 3 Innovation: 4 Approach: 7 Environment: 4 Overall Impact: HSV-2 is a significant health problem and there is no approved vaccine to prevent HSV disease. This application will utilize an ICP0 deficient HSV-2 strain that elicits exceptionally strong protection against HSV disease in animal models as a probe to determine the protective antigens and immunological components involved in protection. Dr. Halford is a well-trained viral immunologist and has made significant contributions to the HSV field and most recently in the area of HSV vaccines. In addition to the live attenuated vaccine he will use, Dr. Halford has developed novel techniques to
3 1 R01 AI IHD assess spread of challenge virus in immunized recipients. Using an array of strategies, he plans to identify the dominant proteins recognized by B and T cells elicited by the attenuated vaccine, identify the important mechanisms of protection, and test the ability of subunit vaccines composed of the identified dominant proteins to protect mice against HSV-2 disease in comparison to a glycoprotein D recombinant vaccine. Overall, the proposal s strengths lie in the vigorous protection elicited by this vaccine, novel use of a luciferase-expressing HSV-2 strain to assess virus spread as a parameter of protection, and the ability to produce individual HSV-2 proteins to assess immune recognition of virus proteins and for use as subunit vaccine components. There are several weaknesses that diminish enthusiasm for the proposal. The techniques for identifying B cell epitopes are incompletely described. For example, no mention is made of how virion proteins will be assessed for recognition by immune sera, how the results may differ from data from recognition of infected cell lysates, and how these data from the several assays, each potentially analyzing different proteins and perhaps identifying different dominant proteins will be utilized to identify the dominant proteins for inclusion in a vaccine (Aim 3). In a similar light, it is not clear how the B cell frequency data and T cell epitope data will be incorporated into vaccine protein selection, that is, if proteins are recognized by B cells but not T cells, will they be considered for use in a vaccine? Aim 2 contains some technical concerns including immunization of C3-deficient mice and potential difficulties in antigen presentation for CD4+ T cell assays. However, the basic immune reconstitution experiments proposed for this aim are straightforward and should provide the desired information. The enthusiasm for this aim is diminished somewhat by concerns that the PI will find that both HSV-specific T cells and antibody are required for complete protection which will not move the field forward. Aim 3 is interesting conceptually and should test the possibility that the wrong antigens are being tested in HSV vaccines. A strong rationale for the criteria used to select vaccine antigens would strengthen this part of the application. In this aim, Dr. Halford will abandon the live attenuated vaccine approach, which likely is the basis for the strong vaccine-elicited protection observed, and test recombinant proteins in MPL-alum. Given that there will be much effort in determining which proteins are recognized by CD8+ T cells (Aim 1) and in the possibility of CD8+ T cell protection (Aim 2), it is surprising that this approach which will not elicit strong CD8+ T cell responses will be utilized. Overall, the proposal touches on important issues such as which antigens should be included in an HSV vaccine but the weaknesses in some of the approaches and rationale diminish enthusiasm for this interesting and potentially important proposal. 1. Significance: HSV infection is a serious threat to health worldwide. Currently, there is no approved vaccine for HSV. Determining how an effective vaccine works might provide useful information towards development of an effective HSV vaccine. None noted. 2. Investigator(s): Dr. Halford is a well-trained HSV virologist with extensive expertise in both virological and immunological aspects of HSV virology. None noted. 3. Innovation:
4 1 R01 AI IHD Use of a luciferase-expressing HSV-2 to measure virus spread following challenge of immunized mice is a novel approach. The remaining approaches taken are standard and are not novel. 4. Approach: The PI has previously demonstrated that HSV-2 0dNLS provides significant protection against HSV disease in animal models of HSV infection. Availability of luciferase-expressing HSV-2 as a reagent to assess viral spread represents an important reagent and an important assay in assessment of vaccine protection. Utilization of 5 different screening procedures to identify dominant HSV proteins recognized vaccine-elicited serum antibody. Ability to screen individual HSV-2 proteins for detection by antigen-specific T cells is a strength. The reconstitution experiments of aim 2 are relatively straightforward and should provide useful information as to the protective components elicited by the vaccine (with the exception of the C3-/- mice as discussed in weaknesses). The five screening tools available to identify targeted antigens, how they will be utilized, and how the results will be analyzed are not well described. The top 10% of proteins will be identified but the criteria used for determining which will be identified and prioritized for aim 3 studies (most consistently targeted by IgG antibodies vs. most abundant antibody) is not clear. ELISPOT analysis using infected cell lysates may fail to detect B cells specific for non-dominant proteins (minor point). ELISPOT analysis 30 days after the final immunization will likely not represent the peak plasma cell response and lower frequency B cells may not be detected. It is not clear how antibody against non-structural proteins or non-exposed capsid proteins might be protective. A strong rationale for inclusion of such antigens (VP5, ICP10, ICP8) in the screening would be helpful. Many of the CD8 T cell epitopes recognized by C57BL/6 mice have been described and identification of the same proteins in this assay would help validate study results. It is not clear that infection of DC to express individual HSV proteins will feed the exogenous antigen presenting pathway utilized for CD4+ T cell recognition of antigen. Exactly how the B cell and T cell recognition experimental data of Aim 1 will be compiled and used to identify the dominant antigens to be used in Aim 3 studies is not clear. Mice lacking C3 have severely impaired antibody responses. This approach is not useful for Aim 2 studies. Functional resistance to HSV-2 vaginal challenge in Aim 2 studies will not include an analysis of protection against establishment of latency. This is the most clinically relevant measure of protection for a prophylactic vaccine. Immunization with recombinant antigens in MPL-alum will not elicit much of a CD8+ T cell response. It is not clear why the PI has abandoned approaches that might elicit this response.
5 1 R01 AI IHD Experiment shown in Table 5 includes 8 groups with 12 mice/group. This seems as if it might be difficult to show statistical differences among immunization groups. A power analysis to show feasibility of this approach would be helpful. 5. Environment: The environment at Southern Illinois University is excellent for these studies. It is not clear where the immunoprecipitation-mass spec analysis will be performed. Since a number of analyses are proposed, clarification would be helpful. Protections for Human Subjects: Not Applicable (No Human Subjects). Vertebrate Animals: Acceptable All 5 points addressed however inclusion of a power analysis would be helpful. Biohazards: Acceptable Sufficient BSL2 and ABSL2 facilities available. Dr. Halford has extensive experience working with HSV in laboratory and animal models. Resource Sharing Plans: Acceptable Acceptable. Budget and Period of Support: Recommend as Requested. CRITIQUE 2: Significance: 3 Investigator(s): 3 Innovation: 6 Approach: 6 Environment: 4 Overall Impact: The search for an effective HSV vaccine has been elusive. Clinical trials of adjuvanted recombinant glycoproteins have been disappointing and results largely ineffective. This PI proposes to evaluate HSV-2 vaccines in an animal model with a two pronged approach: to explore novel HSV-2 antigens; and to elucidate effector mechanisms that have been highly effective in his hands for an ICP0- attenuated vaccine. The potential value of a vaccine against HSV is compelling from a medical and public health perspective. The lack of current extramural support for the PI and his major collaborator is a concern. There are regulatory concerns about the ultimate application of a disabled but whole-genome derived attenuated vaccine in clinical practice. Indeed, the comment of the PI that a live HSV-2 ICP0- virus may persist at a low level in vaccine recipients, and thus may elicit a protective immune response that endures for months to years, may be correct (although there is no
6 1 R01 AI IHD aim to address this in mice) but may be especially problematic from a human subjects perspective. The extrapolation of findings from IP-mass spectrometry and western blot assay to T cell response with respect to T cell immunity is problematic. Human immunology has already made important advances in identifying novel subunit vaccine candidates and hence the pursuit of these studies in a murine model where the dominant T cell response may or may not be relevant to human health is a concern. These numerous moderate weaknesses limit what is otherwise a satisfactory application from an experienced investigator. 1. Significance: HSV is a medically important pathogen and a high-priority area for vaccine development. Vaccines have proven unsuccessful in the clinic. This effort has expended considerable resources but has been problematic in spite of large clinical trials. The concept that antibody to a single envelope glycoprotein might be sufficient for HSV disease control appears unfortunately to be incorrect based on the outcome of clinical trials. Novel vaccine strategies and immunogens are needed. HSV vaccines based on any sort of attenuated virus derived from the full viral genome are likely to be very problematic from a regulatory perspective. The translation of these findings to human vaccinology is unclear. As has been evidence by many preclinical studies in mice and guinea pigs, approaches that appear protective in an animal model may not be successful in humans. 2. Investigator(s): The PI is a well-established investigator at Southern Illinois University. PI has extensive experience with studying animal models of HSV vaccines. Track record of strong collaborations. Anticipated sabbatical to learn additional immunological techniques is a plus (noted in supplemental materials). Productivity has been good but not outstanding with only 7 papers since 2009 and many are middle author paper. PI does not currently hold extramural funding. 3. Innovation: The magnitude of protection conferred by the ICP0 deletion virus is stunning and very novel. The experimental techniques are in and of themselves fairly standard and not really very innovative. Experiments such as pull-down coupled with mass spect are pretty standard. The
7 1 R01 AI IHD generation of stable cell lines and viral vectors for gene expression is not an innovative technique. Highly innovative approaches using patients with HSV seropositive individuals from the Koelle group (J Clin Invest Feb 1;122(2):654-73) has identified viral ORFs that appear to stand out as important T cell immunogens and possibly subunit vaccine candidates, including UL39 and UL46. Thus, there has been progress in a human immunology model in achieving some of the goals of this grant. It is surprising in this context that this work is not referenced and put into perspective. Preliminary data based on western blot suggests a role for ICP5, 8 and 10 in protective immunity but this is based only on IP-PAGE and there is no data presented to suggest that the T cell response will target these proteins. Hence the hypothesis in C-1.3 that T cell responses to these proteins may be critical does not seem to be supported by any preliminary data. The observation in mice that IgG antibodies elicited in response to HSVserotype-specific and only weakly cross-reacted with linear epitopes in their corresponding HSV-1 proteins would seem to be at odds with observations from clinical trials (Stanberry et al., 2002, N Engl. J Med 347: ) that prior immunity to HSV-1 conferred some protection against acquisition of HSV-2 disease. Some clarification on this point would be valuable. 4. Approach: The application is very well written and reads well. Experimental flow is logical and experiments are well designed and well controlled. The preliminary data don t speak toward the suitability of specific HSV-2 proteins as vaccine targets. Although VP5, ICP8 and ICP10 are pulled dow animals, it does not mean that these proteins, even if they elicit antibody response, are necessarily good vaccine candidates. The concept of pan-hsv antibody as a marker of protective immunity is not supported by clinical observations where antibody titer does not correlate with the risk of disease or protection against recurrence. Aim 2 exploits known effects of gc, ICP47 and ge on immune responsive by employing knockout animals and examining the mechanisms of protection revertants virus (wild type) were evaluated (at sublethal doses) in parallel. animals is effective as stated, it is unclear why a CD8+ knockout mouse would have increased mortality, assuming the antibody response is intact (p. 33). Aim 3 tests the hypothesis that a Synthetic vaccine based on subunit responses to VP5, ICP8, or ICP10 will be protective. It is not clear that small groups of 6 animals will yield sufficient power for discrimation across groups. A rationale for adjuvanted protein subunit vaccine is provided, but it is not clear that this is an appropriate vehicle for T-cell immunogens. 5. Environment: Strong research environment at Southern Illinois University.
8 1 R01 AI IHD Excellent core resources in FACs, sequencing, proteomics, etc. Strong collaboration with Dr. Gershburg who has expertise in these assays. Does not appear to have independent extramural support. Collaborator Dr. Gershburg does not appear to have current extramural support. No institutional letters of support are a concern. No letter of support from Dr. Cohen for provision of gg protein. Protections for Human Subjects: Not Applicable (No Human Subjects) Vertebrate Animals: Acceptable Biohazards: Acceptable Resource Sharing Plans: Unacceptable No resource sharing plan was provided. This would be particularly important for the microarray data as well as development of novel reagents. Budget and Period of Support: Recommend as Requested. CRITIQUE 3: Significance: 5 Investigator(s): 4 Innovation: 5 Approach: 6 Environment: 4 Overall Impact: A vaccine for HSV-2 would be an important advance, not only because of the disease burden of genital herpes in the west but also because there is some evidence that HSV-2 is an important co-factor for HIV acquisition in sub-saharan Africa. The gd vaccine is not very protective, and work from other labs has not provided good support for the idea that adding other major virion glycoproteins to the vaccine formulation adds much to protection. Various live attenuated vaccines have been investigated in various labs for at least two decades; some have been and are in clinical trials. The ICP0 null vaccine described here provides impressive protection, and the goal of elucidating its means of protection and perhaps reproducing it in subunit vaccine formulation is logical. However, the current proposal seems deficient both in background insight into fundamental immune mechanisms and in experimental approach, and seems unlikely to really add much to a field that has in fact been exploring live attenuated HSV-2 vaccines for some time. Dr Halford provides evidence that antibody titers induced by his vaccine are much higher than those induced by the gd vaccine, but he doesn t distinguish neutralizing and other antibody responses. He proposes to use the dominant proteins recognized by antisera from HSV-2 D0 vaccine to induce antibodies, but provides little rationale as to why including antigens that are not targets for
9 1 R01 AI IHD neutralization nor for antibody-mediated cellular cytotoxicity (ADCC) (i.e. expressed on the surface of infected cells), would be useful, other than a rather vague hypothesis that antibodies could induce inflammation and thus attraction of cellular immune components. Assessing immune mechanisms using knockout mice is messy, as these mice develop exaggerated alternative mechanisms during immune development (e.g. MHC I ko mice develop cytotoxic CD4 T cells). Depletion experiments and adoptive transfer, as also proposed, are much more powerful. The means by which the results of SA1 and SA2 would be exploited in SA3 need to be better developed. E.g. if complement is important, what is the implication? Will an attempt to skew the antibody response towards complement fixing isotypes, rather than the Th2-induced isotypes expected from the alum adjuvant, be made? If CD8 T cells are important, a much better means of inducing them needs to be proposed. However, if that s the case there is a real problem in extrapolating from the mouse to the human model, since ICP47 works well in humans but not at all in mice. Budget and Period of Support: Recommend as Requested. Additional Comments to Applicant: Somewhere in the back of my head i have the impression that JAWS II does not express murine MHC class II-- that the endogenous MHC II was deleted and replaced by human MHC II when the line was made. I was struggling to find a reference to this as I was writing this review, so it may be that I'm wrong (and I certainly didn't make a scoring issue of it). But I would urge the applicant to check this out functionally before using JAWS II to characterize CD4 T cell responses in B6 mice. Certainly in the Jiang paper (ref 38) the level (MFI) of MHC II staining on JAWS II was much lower than the BMDCs, even though staining for all other activation markers was comparable. On the other hand, JAWS II DOES express murine MHC I and is a fantastic presenter to CD8 T cells. I hate to make this comment, but the proposal would benefit a lot from a collaboration with a professional immunologist. The analyses and thought processes underlying the grant throughout are rather unsophisticated. NIH has modified its policy regarding the receipt of resubmissions (amended applications). See Guide Notice NOT-OD at html. The impact/priority score is calculated after discussion of an application by averaging the overall scores (1-9) given by all voting reviewers on the committee and multiplying by 10. The criterion scores are submitted prior to the meeting by the individual reviewers assigned to an application, and are not discussed specifically at the review meeting or calculated into the overall impact score. Some applications also receive a percentile ranking. For details on the review process, see
A Live-Attenuated HSV-2 ICP0 2 Virus Elicits 10 to 100 Times Greater Protection against Genital Herpes than a Glycoprotein D Subunit Vaccine
 A Live-Attenuated HSV-2 ICP0 2 Virus Elicits 10 to 100 Times Greater Protection against Genital Herpes than a Glycoprotein D Subunit Vaccine William P. Halford*, Ringo Püschel, Edward Gershburg, Andrew
A Live-Attenuated HSV-2 ICP0 2 Virus Elicits 10 to 100 Times Greater Protection against Genital Herpes than a Glycoprotein D Subunit Vaccine William P. Halford*, Ringo Püschel, Edward Gershburg, Andrew
HSV-2 therapeutic vaccine program. Subunit vaccine candidates
 HSV-2 therapeutic vaccine program Subunit vaccine candidates Our HSV-2 vaccine program Therapeutic subunit vaccine T-cell-based Aim: Best-in-class antigens by engineering Excellent standing (Sept 2017):
HSV-2 therapeutic vaccine program Subunit vaccine candidates Our HSV-2 vaccine program Therapeutic subunit vaccine T-cell-based Aim: Best-in-class antigens by engineering Excellent standing (Sept 2017):
Pan-HSV-2 IgG Antibody in Vaccinated Mice and Guinea Pigs Correlates with Protection against Herpes Simplex Virus 2
 Pan-HSV-2 IgG Antibody in Vaccinated Mice and Guinea Pigs Correlates with Protection against Herpes Simplex Virus 2 William P. Halford*, Joshua Geltz, Edward Gershburg Department of Microbiology and Immunology,
Pan-HSV-2 IgG Antibody in Vaccinated Mice and Guinea Pigs Correlates with Protection against Herpes Simplex Virus 2 William P. Halford*, Joshua Geltz, Edward Gershburg Department of Microbiology and Immunology,
Passive Immunization Trials to Inform Vaccine Design
 Passive Immunization Trials to Inform Vaccine Design Points for Consideration from deliberations held at the August 8, 2014 workshop Contents I. Introduction... 2 II. Types of trials... 2 1. Therapeutic
Passive Immunization Trials to Inform Vaccine Design Points for Consideration from deliberations held at the August 8, 2014 workshop Contents I. Introduction... 2 II. Types of trials... 2 1. Therapeutic
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness
 Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
BIOPHARMACEUTICAL PROCESS EVALUATED FOR VIRAL CLEARANCE
 The purpose of Viral Clearance evaluation is to assess the capability of a manufacturing production process to inactivate and/or remove potential viral contaminants. Experience and knowledge in selecting
The purpose of Viral Clearance evaluation is to assess the capability of a manufacturing production process to inactivate and/or remove potential viral contaminants. Experience and knowledge in selecting
Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion
 Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion Robin Thorpe & Meenu Wadhwa Revised Guideline: Differences from original
Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion Robin Thorpe & Meenu Wadhwa Revised Guideline: Differences from original
Custom Antibodies Services. GeneCust Europe. GeneCust Europe
 GeneCust Europe Laboratoire de Biotechnologie du Luxembourg S.A. 2 route de Remich L-5690 Ellange Luxembourg Tél. : +352 27620411 Fax : +352 27620412 Email : info@genecust.com Web : www.genecust.com Custom
GeneCust Europe Laboratoire de Biotechnologie du Luxembourg S.A. 2 route de Remich L-5690 Ellange Luxembourg Tél. : +352 27620411 Fax : +352 27620412 Email : info@genecust.com Web : www.genecust.com Custom
Herpes Simplex Virus 2 ICP0 2 Mutant Viruses Are Avirulent and Immunogenic: Implications for a Genital Herpes Vaccine
 Herpes Simplex Virus 2 ICP0 2 Mutant Viruses Are Avirulent and Immunogenic: Implications for a Genital Herpes Vaccine William P. Halford*, Ringo Püschel, Brandon Rakowski Department of Microbiology and
Herpes Simplex Virus 2 ICP0 2 Mutant Viruses Are Avirulent and Immunogenic: Implications for a Genital Herpes Vaccine William P. Halford*, Ringo Püschel, Brandon Rakowski Department of Microbiology and
The World Leader in SPR Technology. Jimmy Page, PhD, Biacore, Inc.
 The World Leader in SPR Technology Jimmy Page, PhD, Biacore, Inc. Objectives of Biacore Experiments Yes/No Data» Is there binding?» Ligand Fishing Concentration Analysis: How MUCH? Active Concentration
The World Leader in SPR Technology Jimmy Page, PhD, Biacore, Inc. Objectives of Biacore Experiments Yes/No Data» Is there binding?» Ligand Fishing Concentration Analysis: How MUCH? Active Concentration
Biomarkers, Early Prediction of Vaccine Efficacy and Safety
 Biomarkers, Early Prediction of Vaccine Efficacy and Safety Giuseppe Pantaleo, M.D. Professor of Medicine Head, Division of Immunology and Allergy Executive Director, Swiss Vaccine Research Institute Lausanne
Biomarkers, Early Prediction of Vaccine Efficacy and Safety Giuseppe Pantaleo, M.D. Professor of Medicine Head, Division of Immunology and Allergy Executive Director, Swiss Vaccine Research Institute Lausanne
Biosafety Level Host Range Propagation Comments
 Guidelines BSL for Commonly used Viral Vectors Version 1.0 Office of Animal Care and Institutional Biosafety (OACIB) 1737 West Polk Street (MC 672) 206 Administrative Office Building Chicago, IL 60612
Guidelines BSL for Commonly used Viral Vectors Version 1.0 Office of Animal Care and Institutional Biosafety (OACIB) 1737 West Polk Street (MC 672) 206 Administrative Office Building Chicago, IL 60612
PPTA Regulatory Workshop June 13, 2016
 PPTA Regulatory Workshop June 13, 2016 DOROTY SCOTT Dr. Dorothy Scott is the Branch Chief for the Laboratory of Plasma Derivatives, in the Office of Blood Research and Review, CBER. Her group is responsible
PPTA Regulatory Workshop June 13, 2016 DOROTY SCOTT Dr. Dorothy Scott is the Branch Chief for the Laboratory of Plasma Derivatives, in the Office of Blood Research and Review, CBER. Her group is responsible
The presenter declare no conflict of interest This work was partly supported by Selecta Bioscience
 Modulation of AAV vector dosing and avoidance of capsid immune responses via repeated co-administration of vector with rapamycin tolerogenic nanoparticles Amine Meliani The presenter declare no conflict
Modulation of AAV vector dosing and avoidance of capsid immune responses via repeated co-administration of vector with rapamycin tolerogenic nanoparticles Amine Meliani The presenter declare no conflict
Developing T cell vaccines for HSV-2 Infection
 Developing T cell vaccines for HSV-2 Infection Jessica Baker Flechtner, PhD World Vaccine Congress 24-26 March, 2014 Deep Pipeline of T Cell-Enabled Vaccine Candidates Discovery Pre-clinical Phase 1 Phase
Developing T cell vaccines for HSV-2 Infection Jessica Baker Flechtner, PhD World Vaccine Congress 24-26 March, 2014 Deep Pipeline of T Cell-Enabled Vaccine Candidates Discovery Pre-clinical Phase 1 Phase
Serological Analysis of Herpes Simplex Virus Types 1 and 2
 INFECTION AND IMMUNITY, Jan. 1982, p. 363-367 Vol. 35, No. 1 19-9567/82/1363-5$2./ Serological Analysis of Herpes Simplex Virus Types 1 and 2 with Monoclonal Antibodies LENORE PEREIRA,* DALE V. DONDERO,
INFECTION AND IMMUNITY, Jan. 1982, p. 363-367 Vol. 35, No. 1 19-9567/82/1363-5$2./ Serological Analysis of Herpes Simplex Virus Types 1 and 2 with Monoclonal Antibodies LENORE PEREIRA,* DALE V. DONDERO,
Antibody Structure. Antibodies
 Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibody Structure supports Function
 Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Tutorial for Completing Penn s r s DNA Registration Document
 NIH GUIDELINES FOR RESEARCH INVOLVING RECOMBINANT OR SYNTHETIC NUCLEIC ACID MOLECULES NIH GUIDELINES Tutorial for Completing Penn s r s DNA Registration Document Revision March 7, 2013 Penn s r s DNA Registration
NIH GUIDELINES FOR RESEARCH INVOLVING RECOMBINANT OR SYNTHETIC NUCLEIC ACID MOLECULES NIH GUIDELINES Tutorial for Completing Penn s r s DNA Registration Document Revision March 7, 2013 Penn s r s DNA Registration
Novel T cell antigen discovery technology providing new momentum on a malaria vaccine
 Novel T cell antigen discovery technology providing new momentum on a malaria vaccine World Vaccine Congress 2012 Best Vaccine Startup 2008 Jessica Baker Flechtner, PhD Vice President, Research Genocea
Novel T cell antigen discovery technology providing new momentum on a malaria vaccine World Vaccine Congress 2012 Best Vaccine Startup 2008 Jessica Baker Flechtner, PhD Vice President, Research Genocea
Adeno-Associated Virus titer and aggregation characterization
 Adeno-Associated Virus titer and aggregation characterization Characterization of gold-labeled Adeno-Associated Virus (AAV) and other small viruses by Nanoparticle Tracking Analysis (NTA) PARTICLE CONCENTRATION
Adeno-Associated Virus titer and aggregation characterization Characterization of gold-labeled Adeno-Associated Virus (AAV) and other small viruses by Nanoparticle Tracking Analysis (NTA) PARTICLE CONCENTRATION
IBC protocol Risk Assessment and Determination of NIH Guidelines
 IBC protocol Risk Assessment and Determination of NIH Guidelines The following are points to consider when reviewing all protocols for risk, recommended containment conditions, and determine applicable
IBC protocol Risk Assessment and Determination of NIH Guidelines The following are points to consider when reviewing all protocols for risk, recommended containment conditions, and determine applicable
Issues in production of viral gene transfer vectors. Stefan Kochanek Department of Gene Therapy Ulm University
 Issues in production of viral gene transfer vectors Stefan Kochanek Department of Gene Therapy Ulm University Only few positive results in gene therapy so far - many early phase, few late phase clinical
Issues in production of viral gene transfer vectors Stefan Kochanek Department of Gene Therapy Ulm University Only few positive results in gene therapy so far - many early phase, few late phase clinical
OmniAb. Naturally optimized human antibodies
 OmniAb Naturally optimized human antibodies Transgenic animals for hmab discovery Only company to offer three platforms Patented technology with freedom to operate V L V H C C H 1 hinge C H 2 C H 3 2 28
OmniAb Naturally optimized human antibodies Transgenic animals for hmab discovery Only company to offer three platforms Patented technology with freedom to operate V L V H C C H 1 hinge C H 2 C H 3 2 28
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION
 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS NOTE FOR GUIDANCE 1 : DNA VACCINES NON-AMPLIFIABLE IN EUKARYOTIC CELLS FOR VETERINARY USE
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use CVMP/IWP/07/98-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS NOTE FOR GUIDANCE 1 : DNA VACCINES
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use CVMP/IWP/07/98-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS NOTE FOR GUIDANCE 1 : DNA VACCINES
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging
 QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A
 Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Single and combination HSV-2 glycoprotein vaccines adjuvanted with. CPG or MPL exhibit differential immunity in animal models, but this is
 CVI Accepts, published online ahead of print on 18 August 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05071-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions.
CVI Accepts, published online ahead of print on 18 August 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05071-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions.
OIE Guideline. International Reference Antibody Standards for Antibody Assays. 1. Introduction
 OIE Guideline International Reference Antibody Standards for Antibody Assays 1. Introduction 1.1. Purpose This document provides guidelines for the preparation, validation and distribution of antibodies
OIE Guideline International Reference Antibody Standards for Antibody Assays 1. Introduction 1.1. Purpose This document provides guidelines for the preparation, validation and distribution of antibodies
Global Leader in Viral Vector Technologies
 Global Leader in Viral Vector Technologies Gene Therapy Vaccines - Discovery 1 SIRION BIOTECH ANY GENE TO ANY CELL Expert for viral vectors Competitive proprietary viral vector platforms High-end enabling
Global Leader in Viral Vector Technologies Gene Therapy Vaccines - Discovery 1 SIRION BIOTECH ANY GENE TO ANY CELL Expert for viral vectors Competitive proprietary viral vector platforms High-end enabling
uniqure Announces First Clinical Data From Second Dose Cohort of AMT-060 in Ongoing Phase I/II trial in Patients with Severe Hemophilia B
 uniqure Announces First Clinical Data From Second Dose Cohort of AMT-060 in Ongoing Phase I/II trial in Patients with Severe Hemophilia B -- Second-dose Cohort Demonstrates Dose Response with All Patients
uniqure Announces First Clinical Data From Second Dose Cohort of AMT-060 in Ongoing Phase I/II trial in Patients with Severe Hemophilia B -- Second-dose Cohort Demonstrates Dose Response with All Patients
Microbial Biotechnology agustin krisna wardani
 Microbial Biotechnology agustin krisna wardani 1. The Structure of Microbes Microbes (microorganisms) are tiny organisms that are too small to be seen individually by the naked eye and must be viewed with
Microbial Biotechnology agustin krisna wardani 1. The Structure of Microbes Microbes (microorganisms) are tiny organisms that are too small to be seen individually by the naked eye and must be viewed with
REGISTRATION DOCUMENT FOR RECOMBINANT DNA RESEARCH
 EHRS Date Received: Reg. Doc. No.: REGISTRATION DOCUMENT FOR RECOMBINANT DNA RESEARCH Principal Investigator: Penn ID#: Position Title: School: Department: Mailing Address: Mail Code: Telephone: FAX: E-mail:
EHRS Date Received: Reg. Doc. No.: REGISTRATION DOCUMENT FOR RECOMBINANT DNA RESEARCH Principal Investigator: Penn ID#: Position Title: School: Department: Mailing Address: Mail Code: Telephone: FAX: E-mail:
Gene Expression Analysis Superior Solutions for any Project
 Gene Expression Analysis Superior Solutions for any Project Find Your Perfect Match ArrayXS Global Array-to-Go Focussed Comprehensive: detect the whole transcriptome reliably Certified: discover exceptional
Gene Expression Analysis Superior Solutions for any Project Find Your Perfect Match ArrayXS Global Array-to-Go Focussed Comprehensive: detect the whole transcriptome reliably Certified: discover exceptional
ENCODE RBP Antibody Characterization Guidelines
 ENCODE RBP Antibody Characterization Guidelines Approved on November 18, 2016 Background An integral part of the ENCODE Project is to characterize the antibodies used in the experiments. This document
ENCODE RBP Antibody Characterization Guidelines Approved on November 18, 2016 Background An integral part of the ENCODE Project is to characterize the antibodies used in the experiments. This document
Assessing and Controlling Potency of Vector and Drug Product for Chimeric Antigen Receptor T Cells
 Assessing and Controlling Potency of Vector and Drug Product for Chimeric Antigen Receptor T Cells David M, Hambly, Ph.D. Director, Analytical Development April 4th, 2016 Forward Looking Statements/Safe
Assessing and Controlling Potency of Vector and Drug Product for Chimeric Antigen Receptor T Cells David M, Hambly, Ph.D. Director, Analytical Development April 4th, 2016 Forward Looking Statements/Safe
Biosafety and the NIH Guidelines
 Biosafety and the NIH Guidelines This section will explore: Why the NIH Guidelines are important The definition of recombinant or synthetic nucleic acid research Content of the NIH Guidelines Section III
Biosafety and the NIH Guidelines This section will explore: Why the NIH Guidelines are important The definition of recombinant or synthetic nucleic acid research Content of the NIH Guidelines Section III
Histocompatibility. Revised and effective July 14, Histocompatibility Standard 1 (HC S1)
 Clinical Laboratory s of Practice The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where applicable to the scope of services
Clinical Laboratory s of Practice The following specialty sustaining standards of practices shall be incorporated into the laboratory s quality management system, where applicable to the scope of services
Chicken EpithelialGut CellLines 1
 Chicken EpithelialGut CellLines 1 Content 01 Introduction p 3 02 Characterization p 5 03 Infection and inhibition p 6 04 Protein expression system p 8 05 NutriProof p 10 06 Contact p 12 01...which came
Chicken EpithelialGut CellLines 1 Content 01 Introduction p 3 02 Characterization p 5 03 Infection and inhibition p 6 04 Protein expression system p 8 05 NutriProof p 10 06 Contact p 12 01...which came
Supplementary Figure 1. Utilization of publicly available antibodies in different applications.
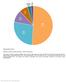 Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Antibodies and Antigens in the Blood Bank 9/7/2015 NAHLA BAKHAMIS 1
 Antibodies and Antigens in the Blood Bank NAHLA BAKHAMIS 9/7/2015 NAHLA BAKHAMIS 1 Outline Antibodies structure, classes and functions Most important Abs in the blood bank effective roles of Abs Zeta potential
Antibodies and Antigens in the Blood Bank NAHLA BAKHAMIS 9/7/2015 NAHLA BAKHAMIS 1 Outline Antibodies structure, classes and functions Most important Abs in the blood bank effective roles of Abs Zeta potential
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development
 Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
ZIKAVAX PARTNERSHIP. Dr Odile LEROY DCVMN Seoul 26 th September 2017
 ZIKAVAX PARTNERSHIP Dr Odile LEROY DCVMN Seoul 26 th September 2017 1 Product Development Partnership THE EUROPEAN VACCINE INITIATIVE IS A PRODUC T DEVELOPMENT PARTNERSHIP WHICH AIMS TO ACCELERATE THE
ZIKAVAX PARTNERSHIP Dr Odile LEROY DCVMN Seoul 26 th September 2017 1 Product Development Partnership THE EUROPEAN VACCINE INITIATIVE IS A PRODUC T DEVELOPMENT PARTNERSHIP WHICH AIMS TO ACCELERATE THE
Antibody Services from GenScript
 Services from GenScript www.genscript.com GenScript USA Inc. 860 Centennial Ave., Piscataway, NJ 08854 USA Toll-Free: 1-877-436-7274 Fax: 1-732-210-0262 1-732-855-5878 Academic Services Pharmaceutical
Services from GenScript www.genscript.com GenScript USA Inc. 860 Centennial Ave., Piscataway, NJ 08854 USA Toll-Free: 1-877-436-7274 Fax: 1-732-210-0262 1-732-855-5878 Academic Services Pharmaceutical
The Biotechnology Education Company. Quantitative ELISA. Storage: See Page 3 for specific storage instructions EXPERIMENT OBJECTIVE:
 The Biotechnology Education Company Revised and Updated Quantitative ELISA Storage: See Page 3 for specific storage instructions EXPERIMENT OBJECTIVE: EDVO-Kit # 278 The objective of this experiment is
The Biotechnology Education Company Revised and Updated Quantitative ELISA Storage: See Page 3 for specific storage instructions EXPERIMENT OBJECTIVE: EDVO-Kit # 278 The objective of this experiment is
Guideline for the Quality, Safety, and Efficacy Assurance of Follow-on Biologics
 Provisional Translation (as of April 19, 2013) PFSB/ELD Notification No. 0304007 March 4, 2009 To: Prefectural Health Department (Bureau) From: Evaluation and Licensing Division, Pharmaceutical and Food
Provisional Translation (as of April 19, 2013) PFSB/ELD Notification No. 0304007 March 4, 2009 To: Prefectural Health Department (Bureau) From: Evaluation and Licensing Division, Pharmaceutical and Food
The current status of vaccine development for control of Salmonella Paratyphi A
 The current status of vaccine development for control of Salmonella Paratyphi A Rodney Carbis (Head Vaccine Development, IVI) 9 th International Conference on Typhoid and Invasive NTS Disease Bali Indonesia
The current status of vaccine development for control of Salmonella Paratyphi A Rodney Carbis (Head Vaccine Development, IVI) 9 th International Conference on Typhoid and Invasive NTS Disease Bali Indonesia
Pharmacology. Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University.
 Pharmacology Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University. 1 PHARMACODYNAMIC STUDIES A. Primary pharmacodynamics primary action in target
Pharmacology Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University. 1 PHARMACODYNAMIC STUDIES A. Primary pharmacodynamics primary action in target
Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines)
 Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines) Jacqueline Corrigan-Curay, J.D. M.D. Acting Director Office of Biotechnology Activities National Institute
Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines) Jacqueline Corrigan-Curay, J.D. M.D. Acting Director Office of Biotechnology Activities National Institute
Applied Protein Services
 Applied Protein Services Applied Protein Services A Window into the Future Development risk and attrition rates remain two of the greatest challenges to a successful biopharmaceutical pipeline. To help
Applied Protein Services Applied Protein Services A Window into the Future Development risk and attrition rates remain two of the greatest challenges to a successful biopharmaceutical pipeline. To help
Transgenic plants: A new biopharmaceutical manufacturing platform
 Transgenic plants: A new biopharmaceutical manufacturing platform Rachel K. Chikwamba 1, Hugh S. Mason 1, Richard Mahoney 2 and Charles J. Arntzen 1 1 - Center for Infectious Disease and 2 - Vaccinology
Transgenic plants: A new biopharmaceutical manufacturing platform Rachel K. Chikwamba 1, Hugh S. Mason 1, Richard Mahoney 2 and Charles J. Arntzen 1 1 - Center for Infectious Disease and 2 - Vaccinology
Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit Protocol
 Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit Protocol (Cat. No.:EK-310-93) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com
Herpes Simplex Virus Type 1 (HSV-1) IgM ELISA Kit Protocol (Cat. No.:EK-310-93) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com
The Role of Mass Spectrometry for Developing Biotherapeutics: Regulatory Perspectives
 The Role of Mass Spectrometry for Developing Biotherapeutics: Regulatory Perspectives Jun Park, Ph.D. Division of Monoclonal Antibodies Office of Biotechnology Products CDER/FDA CASSS, Applications of
The Role of Mass Spectrometry for Developing Biotherapeutics: Regulatory Perspectives Jun Park, Ph.D. Division of Monoclonal Antibodies Office of Biotechnology Products CDER/FDA CASSS, Applications of
FORENSIC SEROLOGY. Chapter PRENTICE HALL 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458
 Chapter 8 FORENSIC SEROLOGY 8-1 Nature of Blood The word blood refers to a highly complex mixture of cells, enzymes, proteins, and inorganic substances. Plasma, which is the fluid portion of blood, is
Chapter 8 FORENSIC SEROLOGY 8-1 Nature of Blood The word blood refers to a highly complex mixture of cells, enzymes, proteins, and inorganic substances. Plasma, which is the fluid portion of blood, is
Modulation of Immune Response in Lambs
 Modulation of Immune Response in Lambs A.S. Leaflet R1473 Jose O. Lopez Virella, graduate research assistant, M. L. Kaeberle, professor, veterinary microbiology Mamadou Niang, graduate research assistant.
Modulation of Immune Response in Lambs A.S. Leaflet R1473 Jose O. Lopez Virella, graduate research assistant, M. L. Kaeberle, professor, veterinary microbiology Mamadou Niang, graduate research assistant.
NIH Guidelines for rdna Research: Tutorial for Completing Penn s rdna Registration Document
 NIH Guidelines for rdna Research: Tutorial for Completing Penn s rdna Registration Document Revision March 3, 2011 Penn s rdna Registration Document Dual Use Research Definition: Research that yields information
NIH Guidelines for rdna Research: Tutorial for Completing Penn s rdna Registration Document Revision March 3, 2011 Penn s rdna Registration Document Dual Use Research Definition: Research that yields information
A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation
 A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation A Risk-based Approach for In Vitro Companion Diagnostics
A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation A Risk-based Approach for In Vitro Companion Diagnostics
RSV vaccine development for Low and Middle Income Countries: Challenges and Progress
 Global Vaccine and Immunization Research Forum RSV vaccine development for Low and Middle Income Countries: Challenges and Progress Claudio F. Lanata, MD, MPH Senior Researcher, Instituto de Investigación
Global Vaccine and Immunization Research Forum RSV vaccine development for Low and Middle Income Countries: Challenges and Progress Claudio F. Lanata, MD, MPH Senior Researcher, Instituto de Investigación
Received 19 September 2008/Returned for modification 4 November 2008/Accepted 13 November 2008
 CLINICAL AND VACCINE IMMUNOLOGY, Jan. 2009, p. 55 60 Vol. 16, No. 1 1556-6811/09/$08.00 0 doi:10.1128/cvi.00351-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Comparison of
CLINICAL AND VACCINE IMMUNOLOGY, Jan. 2009, p. 55 60 Vol. 16, No. 1 1556-6811/09/$08.00 0 doi:10.1128/cvi.00351-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Comparison of
How can innovation in regulatory science inform the regulatory process to facilitate the development of new vaccines?
 How can innovation in regulatory science inform the regulatory process to facilitate the development of new vaccines? Marion F. Gruber, Ph.D. Office of Vaccines Research and Review CBER/FDA Global Vaccine
How can innovation in regulatory science inform the regulatory process to facilitate the development of new vaccines? Marion F. Gruber, Ph.D. Office of Vaccines Research and Review CBER/FDA Global Vaccine
System Biology and Immune Response
 System Biology and Immune Response Fondation Mérieux Conference Centre Les Pensières Veyrier-du-Lac - France June 21-23, 2010 Steering Committee: Nicolas BURDIN Bruno GUY Michael KATZE Jacques LOUIS Ray
System Biology and Immune Response Fondation Mérieux Conference Centre Les Pensières Veyrier-du-Lac - France June 21-23, 2010 Steering Committee: Nicolas BURDIN Bruno GUY Michael KATZE Jacques LOUIS Ray
Developing a MAPS vaccine against Salmonella Typhi and Paratyphi
 Developing a MAPS vaccine against Salmonella Typhi and Paratyphi 10th International Conference on Typhoid and Other Invasive Salmonelloses April 6, 2017 Kampala, Uganda Yingjie Lu, PhD Rick Malley, MD
Developing a MAPS vaccine against Salmonella Typhi and Paratyphi 10th International Conference on Typhoid and Other Invasive Salmonelloses April 6, 2017 Kampala, Uganda Yingjie Lu, PhD Rick Malley, MD
Small-Cap Research. GeoVax Labs Inc. (GOVX-OTC) GOVX: New Ebola vaccine program added, on track to advance its HIV vaccine programs- -Outperform
 Small-Cap Research October 6, 2014 Grant Zeng, CFA 312-265-9466 gzeng@zacks.com scr.zacks.com 10 S. Riverside Plaza, Chicago, IL 60606 GeoVax Labs Inc. (GOVX-OTC) GOVX: New Ebola vaccine program added,
Small-Cap Research October 6, 2014 Grant Zeng, CFA 312-265-9466 gzeng@zacks.com scr.zacks.com 10 S. Riverside Plaza, Chicago, IL 60606 GeoVax Labs Inc. (GOVX-OTC) GOVX: New Ebola vaccine program added,
Peptide libraries: applications, design options and considerations. Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST
 Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
sirna Overview and Technical Tips
 1 sirna Overview and Technical Tips 2 CONTENTS 3 4 5 7 8 10 11 13 14 18 19 20 21 Introduction Applications How Does It Work? Handy Tips Troubleshooting Conclusions Further References Contact Us 3 INTRODUCTION
1 sirna Overview and Technical Tips 2 CONTENTS 3 4 5 7 8 10 11 13 14 18 19 20 21 Introduction Applications How Does It Work? Handy Tips Troubleshooting Conclusions Further References Contact Us 3 INTRODUCTION
OIE Guideline. 1. Introduction
 OIE Guideline INTERNATIONAL REFERENCE STANDARDS FOR ANTIGEN DETECTION ASSAYS 1. Introduction 1.1. Purpose This document provides guidelines for the preparation, validation and distribution of antigens
OIE Guideline INTERNATIONAL REFERENCE STANDARDS FOR ANTIGEN DETECTION ASSAYS 1. Introduction 1.1. Purpose This document provides guidelines for the preparation, validation and distribution of antigens
Beth Hutchins, PhD PhRMA ICH Gene Therapy Discussion Group
 ICH Considerations on General Principles to Address the Risk of Inadvertent Germline Integration of Gene Therapy Vectors and Current Topics on Gene Therapy in USA Beth Hutchins, PhD PhRMA ICH Gene Therapy
ICH Considerations on General Principles to Address the Risk of Inadvertent Germline Integration of Gene Therapy Vectors and Current Topics on Gene Therapy in USA Beth Hutchins, PhD PhRMA ICH Gene Therapy
Development of Multiplex Sensitive Anti-Drug Antibody Assays for CRISPR/Cas9 Gene Therapies
 Development of Multiplex Sensitive Anti-Drug Antibody Assays for CRISPR/Cas9 Gene Therapies September 27, 2017 Junxia Wang editasmedicine.com 1 Overview of the presentation Immunogenicity Introduction
Development of Multiplex Sensitive Anti-Drug Antibody Assays for CRISPR/Cas9 Gene Therapies September 27, 2017 Junxia Wang editasmedicine.com 1 Overview of the presentation Immunogenicity Introduction
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations. Audrey Chang, PhD, Senior Director Development Services
 Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Replacing Analytical Methods for Release and Stability Testing CBER Perspective
 Replacing Analytical Methods for Release and Stability Testing CBER Perspective Presentation at the CMC Strategy Forum January 27, 2014 Lokesh Bhattacharyya Chief, Lab of Analytical Chemistry and Blood
Replacing Analytical Methods for Release and Stability Testing CBER Perspective Presentation at the CMC Strategy Forum January 27, 2014 Lokesh Bhattacharyya Chief, Lab of Analytical Chemistry and Blood
Safe Operating Procedure
 Safe Operating Procedure RECOMBINANT OR SYNTHETIC NUCLEIC ACIDS IBC AND OTHER REVIEW REQUIREMENTS (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) (Revised
Safe Operating Procedure RECOMBINANT OR SYNTHETIC NUCLEIC ACIDS IBC AND OTHER REVIEW REQUIREMENTS (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) (Revised
Title: Combination of a third generation bisphosphonate and replication-competent adenoviruses augments the cytotoxicity on mesothelioma
 Author s response to reviews Title: Combination of a third generation bisphosphonate and replication-competent adenoviruses augments the cytotoxicity on mesothelioma Authors: Masatoshi Tagawa (mtagawa@chiba-cc.jp)
Author s response to reviews Title: Combination of a third generation bisphosphonate and replication-competent adenoviruses augments the cytotoxicity on mesothelioma Authors: Masatoshi Tagawa (mtagawa@chiba-cc.jp)
Learning Objectives. Define RNA interference. Define basic terminology. Describe molecular mechanism. Define VSP and relevance
 Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Antibodies and Antigens In the blood bank
 Antibodies and Antigens In the blood bank 1 Nice game!! http://nobelprize.org/ 2 Karl Landsteiner discovered blood groups in 1901. Awarded Nobel Prize for Physiology or Medicine in 1930 3 Why we study
Antibodies and Antigens In the blood bank 1 Nice game!! http://nobelprize.org/ 2 Karl Landsteiner discovered blood groups in 1901. Awarded Nobel Prize for Physiology or Medicine in 1930 3 Why we study
Received 15 August 2006/Accepted 1 October 2006
 JOURNAL OF VIROLOGY, Dec. 2006, p. 12312 12323 Vol. 80, No. 24 0022-538X/06/$08.00 0 doi:10.1128/jvi.01766-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Linker Insertion Mutations
JOURNAL OF VIROLOGY, Dec. 2006, p. 12312 12323 Vol. 80, No. 24 0022-538X/06/$08.00 0 doi:10.1128/jvi.01766-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Linker Insertion Mutations
Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use.
 1 2 3 18 November 2010 EMA/CHMP/BMWP/86289/2010 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical
1 2 3 18 November 2010 EMA/CHMP/BMWP/86289/2010 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical
Current Best Practices in Commercial Kit Evaluation and Validation for Biomarker Assays
 Current Best Practices in Commercial Kit Evaluation and Validation for Biomarker Assays 10th Workshop on Recent Issues in Bioanalysis, 2016 Paul Rhyne, Ph.D. - Director of Immunoassay Services Copyright
Current Best Practices in Commercial Kit Evaluation and Validation for Biomarker Assays 10th Workshop on Recent Issues in Bioanalysis, 2016 Paul Rhyne, Ph.D. - Director of Immunoassay Services Copyright
Assays and Strategies for Immunogenicity Assessment. Steven J Swanson, Ph.D. Executive Director, Medical Sciences Clinical Immunology, Amgen
 Assays and Strategies for Immunogenicity Assessment Steven J Swanson, Ph.D. Executive Director, Medical Sciences Clinical Immunology, Amgen General Antibody Assay Strategy Correlation of clinical findings
Assays and Strategies for Immunogenicity Assessment Steven J Swanson, Ph.D. Executive Director, Medical Sciences Clinical Immunology, Amgen General Antibody Assay Strategy Correlation of clinical findings
Dr. David D. Lee, Provost and VP for Academic Affairs
 POLICY & PROCEDURE DOCUMENT NUMBER: 2.7000 SECTION: TITLE: Research Institutional Biosafety Committee Policy DATE: November 6, 2017 Authorized by: Dr. David D. Lee, Provost and VP for Academic Affairs
POLICY & PROCEDURE DOCUMENT NUMBER: 2.7000 SECTION: TITLE: Research Institutional Biosafety Committee Policy DATE: November 6, 2017 Authorized by: Dr. David D. Lee, Provost and VP for Academic Affairs
Supplementary Fig. 1 related to Fig. 1 Clinical relevance of lncrna candidate
 Supplementary Figure Legends Supplementary Fig. 1 related to Fig. 1 Clinical relevance of lncrna candidate BC041951 in gastric cancer. (A) The flow chart for selected candidate lncrnas in 660 up-regulated
Supplementary Figure Legends Supplementary Fig. 1 related to Fig. 1 Clinical relevance of lncrna candidate BC041951 in gastric cancer. (A) The flow chart for selected candidate lncrnas in 660 up-regulated
Lichenase fusions improve immunological properties of antigens
 Lichenase fusions improve immunological properties of antigens Konstantin Musiychuk Center for Molecular Biotechnology, Fraunhofer USA 03/27/2012 Lichenase Hydrolysis site of β-glycans Structure of the
Lichenase fusions improve immunological properties of antigens Konstantin Musiychuk Center for Molecular Biotechnology, Fraunhofer USA 03/27/2012 Lichenase Hydrolysis site of β-glycans Structure of the
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
Mouse Factor XII Total ELISA Kit
 Mouse Factor XII Total ELISA Kit Catalog No: IMFXIIKT-TOT Lot No: SAMPLE INTENDED USE This mouse coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor XII
Mouse Factor XII Total ELISA Kit Catalog No: IMFXIIKT-TOT Lot No: SAMPLE INTENDED USE This mouse coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor XII
Do Donors With Non-Deletional Blood Group O Allels Boost Anti-A and Anti-B Titers in Blood Group O Recipients?
 Do Donors With Non-Deletional Blood Group O Allels Boost Anti-A and Anti-B Titers in Blood Group O Recipients? E. A. Scharberg, S. Seyboth, K. Panter, A. Ernst, J. Hagel, E. Richter, G. Rink, K. Janetzko,
Do Donors With Non-Deletional Blood Group O Allels Boost Anti-A and Anti-B Titers in Blood Group O Recipients? E. A. Scharberg, S. Seyboth, K. Panter, A. Ernst, J. Hagel, E. Richter, G. Rink, K. Janetzko,
Chapter 10 Analytical Biotechnology and the Human Genome
 Chapter 10 Analytical Biotechnology and the Human Genome Chapter Outline Enzyme tests and biosensors DNA-based tests DNA analysis technologies Human genome and genome-based analytical methods 1 Enzyme-based
Chapter 10 Analytical Biotechnology and the Human Genome Chapter Outline Enzyme tests and biosensors DNA-based tests DNA analysis technologies Human genome and genome-based analytical methods 1 Enzyme-based
Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings
 Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings from a Randomized Trial Running title: Therapeutic Vaccine for Genital Herpes David I Bernstein 1, Anna Wald 2, Terri Warren 3,
Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings from a Randomized Trial Running title: Therapeutic Vaccine for Genital Herpes David I Bernstein 1, Anna Wald 2, Terri Warren 3,
DNA Microarray Technology
 CHAPTER 1 DNA Microarray Technology All living organisms are composed of cells. As a functional unit, each cell can make copies of itself, and this process depends on a proper replication of the genetic
CHAPTER 1 DNA Microarray Technology All living organisms are composed of cells. As a functional unit, each cell can make copies of itself, and this process depends on a proper replication of the genetic
i) Wild animals: Those animals that do not live under human supervision or control and do not have their phenotype selected by humans.
 The OIE Validation Recommendations provide detailed information and examples in support of the OIE Validation Standard that is published as Chapter 1.1.6 of the Terrestrial Manual, or Chapter 1.1.2 of
The OIE Validation Recommendations provide detailed information and examples in support of the OIE Validation Standard that is published as Chapter 1.1.6 of the Terrestrial Manual, or Chapter 1.1.2 of
Passive vaccination as a global strategy for preventing RSV disease in infants. Filip Dubovsky MD MPH FAAP MedImmune March 2016
 Passive vaccination as a global strategy for preventing RSV disease in infants Filip Dubovsky MD MPH FAAP MedImmune March 2016 Outline for Presentation Rationale for passive immunization for RSV prophylaxis
Passive vaccination as a global strategy for preventing RSV disease in infants Filip Dubovsky MD MPH FAAP MedImmune March 2016 Outline for Presentation Rationale for passive immunization for RSV prophylaxis
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Modulating immune checkpoint pathways like CTLA-4 and PD-1 for cancer immunotherapy has gained much attention in recent years due to their immense
Reviewers' comments: Reviewer #1 (Remarks to the Author): Modulating immune checkpoint pathways like CTLA-4 and PD-1 for cancer immunotherapy has gained much attention in recent years due to their immense
HSV-UL18 DNA vaccine construction and immunodetection.
 Research Article http://www.alliedacademies.org/medical-oncology-therapeutics/ HSV-UL18 DNA vaccine construction and immunodetection. Sinan Fang 1-3#, Qiaoli Wang 1,2#, Lu Wang 1-3, Fan Luo 1-3, Junwei
Research Article http://www.alliedacademies.org/medical-oncology-therapeutics/ HSV-UL18 DNA vaccine construction and immunodetection. Sinan Fang 1-3#, Qiaoli Wang 1,2#, Lu Wang 1-3, Fan Luo 1-3, Junwei
European Regulatory Experiences and Expectations of HCP Analysis and Control
 HCP Strategy Forum, Washington DC, January 26, 2015 www.pei.de European Regulatory Experiences and Expectations of HCP Analysis and Control Blood Products: Dr. Erika Friedl, PEI (DE) Discalimer: The views
HCP Strategy Forum, Washington DC, January 26, 2015 www.pei.de European Regulatory Experiences and Expectations of HCP Analysis and Control Blood Products: Dr. Erika Friedl, PEI (DE) Discalimer: The views
 InCHIP Research Interest Groups Crystal.park@uconn.edu Debbie Cornman @ Deborah.cornman@uconn.edu Debarchana.ghosh@uconn.edu HIV Research Interest Group - Chaired by Seth Kalichman @ Seth.K@uconn.edu Amy.gorin@ucponn.edu
InCHIP Research Interest Groups Crystal.park@uconn.edu Debbie Cornman @ Deborah.cornman@uconn.edu Debarchana.ghosh@uconn.edu HIV Research Interest Group - Chaired by Seth Kalichman @ Seth.K@uconn.edu Amy.gorin@ucponn.edu
Overview of Immunohistochemistry. (with a focus on wax-embedded sections)
 Overview of Immunohistochemistry (with a focus on wax-embedded sections) Overview of Immunohistochemistry (with a focus on wax-embedded sections) Overview of Immunohistochemistry IHC is like cooking. There
Overview of Immunohistochemistry (with a focus on wax-embedded sections) Overview of Immunohistochemistry (with a focus on wax-embedded sections) Overview of Immunohistochemistry IHC is like cooking. There
ANTIBODY IMMUNOGENICITY
 ANTIBODY IMMUNOGENICITY Tolerance Classical Immunity To aggegated Antibody Chiller and Weigle, PNAS, 65:551, 1970; Benjamin and Waldmann, et al, J Exp Med, 163:1539, 1986) THE IMMUNOGENICITY PROBLEM WITH
ANTIBODY IMMUNOGENICITY Tolerance Classical Immunity To aggegated Antibody Chiller and Weigle, PNAS, 65:551, 1970; Benjamin and Waldmann, et al, J Exp Med, 163:1539, 1986) THE IMMUNOGENICITY PROBLEM WITH
Genomic Research: Issues to Consider. IRB Brown Bag August 28, 2014 Sharon Aufox, MS, LGC
 Genomic Research: Issues to Consider IRB Brown Bag August 28, 2014 Sharon Aufox, MS, LGC Outline Key genomic terms and concepts Issues in genomic research Consent models Types of findings Returning results
Genomic Research: Issues to Consider IRB Brown Bag August 28, 2014 Sharon Aufox, MS, LGC Outline Key genomic terms and concepts Issues in genomic research Consent models Types of findings Returning results
Progress in Improving the HIV Envelope Manufacturing Process
 Progress in Improving the HIV Envelope Manufacturing Process Third HIV Env Manufacturing Workshop Division of AIDS/NIAID/NIH and the Global HIV Vaccine Enterprise Phillip Berman Department of Biomolecular
Progress in Improving the HIV Envelope Manufacturing Process Third HIV Env Manufacturing Workshop Division of AIDS/NIAID/NIH and the Global HIV Vaccine Enterprise Phillip Berman Department of Biomolecular
Update from the Center for Biologics Evaluation and Research (CBER): Advancing the Development of Complex Biologic Products
 Update from the Center for Biologics Evaluation and Research (CBER): Advancing the Development of Complex Biologic Products Peter Marks, M.D., Ph.D. FDLI 2017 Outline Products regulated Significance of
Update from the Center for Biologics Evaluation and Research (CBER): Advancing the Development of Complex Biologic Products Peter Marks, M.D., Ph.D. FDLI 2017 Outline Products regulated Significance of
