Patterning Surface-bound Microtubules through Reversible DNA Hybridization
|
|
|
- Cynthia Brooks
- 5 years ago
- Views:
Transcription
1 Patterning Surface-bound Microtubules through Reversible DNA Hybridization NANO LETTERS 2004 Vol. 4, No Gayatri Muthukrishnan, Caitlin A. Roberts, Yi-Chun Chen, Jeffrey D. Zahn, and William O. Hancock* Department of Bioengineering, PennsylVania State UniVersity, UniVersity Park, PennsylVania Received July 24, 2004; Revised Manuscript Received August 31, 2004 ABSTRACT Biomolecular motors have great potential as transporters and actuators in microscale devices. Existing efforts toward harnessing kinesin motors have involved microtubule movements over immobilized motors. The reverse geometry has distinct advantages, but progress has been hindered by the difficulty of immobilizing patterned and aligned microtubules on surfaces. Here we show that microtubules can be reversibly patterned with microscale resolution through DNA hybridization, and that these DNA-functionalized microtubules support the movement of kinesin-coated beads. Because of the unique transport and force generating capabilities of biological molecular machines, there is considerable interest in incorporating them into nano- and microscale hybrid devices. Kinesins are transport motors that use the chemical energy of ATP hydrolysis to carry intracellular cargo in eukaryotic cells. Their tracks, microtubules, are protein polymers, 25 nm diameter and tens of microns long. By isolating these proteins from cells and reconstituting their activity in engineered microscale systems, these biological machines provide a powerful system for transporting material at microscale dimensions, for microactuation of MEMS devices, and for manipulating and assembling nanoparticles into useful materials. Until now, most applications of the kinesin-microtubule system have utilized the upside-down geometry, in which motors are immobilized on surfaces and microtubules are transported over them. By fabricating microscale channels and functionalizing them with kinesin motors, a number of groups have shown that the direction of microtubule motion can be controlled, a prerequisite for useful transport. 1-5 Because the microtubules can be functionalized with biotin or antibodies, this geometry works well for microscale transport. However, to fully realize the potential of these motors, methods must be developed to immobilize microtubules with control over their position and orientation. This orientation is key: in cells microtubules are oriented with their slow growing minus-ends at the center of the cell and their fast growing plus-ends at the periphery, and motors move unidirectionally along these tracks. The first advantage of replicating this cellular geometry is that it provides greater * Corresponding author. wohbio@engr.psu.edu. Phone: Fax: control over cargo transport-recombinant kinesins can be engineered with an almost limitless range of cargo attachment domains, and the arrangement of microtubules determines their transport direction. A second motivation is that to generate the nanonewton forces required to power MEMS and NEMS devices, it is necessary to team the activity of many motors working along an array of parallel microtubules. The goal of this study is to develop methods to reversibly pattern microtubules on engineered surfaces, with the eventual goal of controlling the orientation of surfaceimmobilized microtubules and 3D microtubule assemblies. It is well established that microtubules can be noncovalently attached to surfaces while retaining their functional properties as substrates for kinesin movement. Microtubules, which have a net negative charge, 6 bind well to amino-silane functionalized surfaces; 7 biotinylated microtubules bind well to streptavidin-functionalized surfaces; 8 and, in the presence of the non-hydrolyzable nucleotide analog AMP-PNP, microtubules bind tightly to kinesin-functionalized surfaces. 9 Using these methods, it has been shown that kinesin can carry beads, 9 gold nanowires, 4 or micron-scale silicon microchips 10 along surface-immobilized microtubules, but in these studies there was no control over the transport direction. There has been some success to date in aligning microtubules on surfaces. Both fluid flow 11 and the application of electric fields 12 have been shown to orient gliding microtubules. In another study, Limberis et al. used antibodies complementary to alpha-tubulin to bind the minus-ends of microtubules to surfaces and then used fluid flow to align the filaments on the surface. 13 To make an aligned microtubule array, Brown and Hancock immobilized short microtubule seeds on a patterned surface, polymerized long microtubules selectively /nl048816b CCC: $27.50 Published on Web 10/01/ American Chemical Society
2 Table 1. oligonucleotide name Oligo A Oligo A Oligo B FITCOligoA List of Oligonucleotides a oligonucleotide sequence 5 -(biotin) taacatt CGCATTCAGGAT-3 5 -(biotin) taacatt ATCCTGAATGCG-3 5 -CCGGAATTGGCC taacatt (biotin)-3 5 -(fluorescein) taacatt ATCCTGAATGCG-3 a T M of A-A overlap ) 36 C. from these plus-ends, and then aligned the filaments using fluid flow and cross-linked them to the surface. 14 However, while these techniques achieved a degree of microtubule alignment, they provide little control over positioning, they do not result in a large number of immobilized microtubules, and they are not optimal approaches for incorporating microtubules into microscale devices. Our approach is to use the specificity and reversibility of DNA hybridization to immobilize oriented microtubules on surfaces. In other systems, DNA oligonucleotides have been successfully used to create nanostructural assemblies. For example, to exploit the optical properties of colloidal gold nanoparticles for sensor applications, particles functionalized with different oligonucleotides were reversibly aggregated by a bridging strand of DNA, causing a shift in the optical absorbance spectrum. 15 DNA-directed assembly of Au nanowires on surfaces has also been achieved by coating nanowires with oligonucleotides through Au-thiol linkages. 16 Here, we use DNA hybridization to pattern immobilized microtubules on surfaces. The eventual goal is to functionalize the two ends of microtubules with different DNA oligonucleotides, pattern complementary oligonucleotides at defined sites on a surface, and use the specificity of DNA hybridization to properly orient the microtubules. In this study we develop a method to attach single-stranded oligonucleotides to microtubules, we use two approaches to pattern complementary oligonucleotides on glass surfaces, we establish proper buffer conditions to enable reversible hybridization while maintaining microtubule stability, and we demonstrate that these DNA-functionalized microtubules remain functional tracks for kinesin motors. To make microtubules, tubulin protein was isolated from calf brains using established procedures, 17 polymerized at 37 C, and stabilized with 10 µm paclitaxel in BRB80 buffer (80 mm PIPES, 1 mm EGTA, 1 mm MgCl 2, ph 6.9) as previously described. 18 Microtubules were fluorescently labeled with rhodamine, and a subset of tubulin was covalently labeled with biotin as previously described. 18 Microtubules were polymerized with different proportions of unlabeled, rhodamine-labeled, and biotinylated tubulin. Single-stranded oligonucleotides (Proligo, Inc.) were attached to microtubules via biotin-avidin chemistry using two different methods. Both take advantage of the fact that avidin has four biotin binding sites and can be used as a bridge. In the first approach, microtubules (684 nm final tubulin concentration with a ratio of 4:4:1 unlabeled/ rhodamine/biotinylated tubulin) were polymerized and stabilized with 10 µm paclitaxel, 76 nm neutravidin was added, Figure 1. Diagrammatic representation of microtubule immobilization through DNA hybridization, showing the eventual goal of functionalizing the microtubule with different oligonucleotides on each end. the solution vortexed for 20 s, and then incubated with 760 nm of OligoA (Table 1) for 20 min to make OligoA-functionalized microtubules (Mt-OligoA). To determine whether the microtubule-bound oligonucleotides retained their function, the microtubules were incubated with 760 nm of a fluorescently labeled complementary strand, FITCOligoA (Table 1), and visualized by fluorescence microscopy using a Nikon E600 microscope (1.3 NA, 100 objective) coupled to a CCD camera (Genwac GW902H, gamma factor ) 0.45) with G-2E/C filter for TRITC (rhodamine) and B2E/C for FITC (fluorescein). Since the FITC fluorescence signal was very low, the concentration of biotinylated tubulin was increased (820 nm final tubulin concentration with 0.8:0.8:1 ratio of unlabeled/ rhodamine/biotinylated tubulin), and the neutravidin (300 nm) and both oligonucleotide concentrations (3 µm) increased proportionally. To remove background fluorescence, this solution was centrifuged for 5 min at 30 psi in a Beckman Airfuge, resuspended in BRB80 buffer plus 10 µm paclitaxel, and visualized. Oligo-functionalized microtubules were clearly seen both under TRITC and FITC filters, while the nonfunctionalized microtubules were seen only under TRITC (Figure 2). In the above method, removing free neutravidin by centrifugation and resuspension before adding the oligonucleotides caused the microtubules to aggregate and depolymerize, especially at higher biotinylated tubulin concentrations. To remove the unbound neutravidin, another method using magnetic beads was devised. In this method, 1.9 µm OligoA was incubated with a 5-fold excess of neutravidin for 10 min to bind e 1 oligonucleotide per neutravidin. Simultaneously, 3.84 µm streptavidin-coated magnetic beads (0.83 µm diameter, Bangs Laboratories, Inc.) were incubated for 10 min with an identical concentration of complementary oligonucleotide (OligoA, Table 1). These two solutions were then mixed and the oligonucleotides allowed to hybridize for 20 min. A magnetic separator (Bangs Laboratories, Inc.) was used to separate the magnetic beads and OligoAneutravidin complexes from free neutravidin. The pellet was then resuspended in TE buffer (10 mm Tris, 1 mm EDTA, ph 8.0) and heated at 42 C for 2 min to denature the DNA, and the magnetic beads were pulled out of solution, leaving a supernatant of OligoA-labeled neutravidin. Finally, 50 µl of this solution was incubated with 100 µl of biotinylated microtubules (684 nm final tubulin concentration with ratio of 4:4:1 unlabeled/rhodamine/biotinylated tubulin) to obtain oligonucleotide-functionalized microtubules (Mt-OligoA) Nano Lett., Vol. 4, No. 11, 2004
3 Figure 2. Oligonucleotide-functionalized microtubules. (a) View under TRITC filter showing rhodamine labeled microtubules, (b) same view under FITC filter showing FITCOligoA hybridized to OligoA functionalized microtubules. (c) Digitally overlapped image of (a) and (b). Nonfunctionalized microtubules are not detectable under the FITC filter. To attach these oligo-functionalized microtubules to surfaces and determine the sequence specificity of microtubule binding, glass coverslip (Corning, no. 1 1 / 2,18mm squares) surfaces were functionalized with different oligonucleotides. To quantify microtubule binding, the number of microtubules bound to the surface after 20 min was compared to a surface of kinesin in adenosine 5 -(β, γ- imido)triphosphate (AMP-PNP). 19,20 Flow cells were constructed from coverslips, glass slides (FisherFinest Premium), and two-sided tape. Neutravidin (10 µm) was flowed in, followed by 10 µm biotinylated oligonucleotide and the Mt- OligoA solution (456 nm tubulin). The sample was incubated for 20 min. Finally, unbound microtubules were washed out with motility buffer (0.2 mg/ml casein, 10 µm paclitaxel, 20 mm D-glucose, 0.02 mg/ml glucose oxidase, mg/ ml catalase, 0.5% β-mercaptoethanol in BRB80), and the surface visualized. The results obtained by the two methods described to make Mt-OligoA gave results within 20% of each other, so the data were pooled. Microtubules bound with very high specificity. There was 95% specific binding on the complementary OligoA surface, and 3.53% and 2.77% nonspecific binding on OligoA and OligoB surfaces, respectively (Figure 3b). The relative binding to a neutravidin surface devoid of oligonucleotides was 1.02%. In these experiments, the buffer (BRB80: 80 mm PIPES, 1 mm EGTA, 1 mm MgCl 2, ph 6.9) was optimized for microtubule stability and is of much higher ionic strength than standard DNA buffers. We hypothesized that the elevated ionic strength may decrease the hybridization specificity somewhat, so in an attempt to reduce the nonspecific binding even further, we repeated the experiment in a lower salt buffer BRB12 (12 mm PIPES, 1 mm EGTA, 2 mm MgCl 2, ph 6.9). This more dilute buffer reduced the absolute amount of nonspecific binding, but it also reduced the degree of specific binding to an equal degree (Figure 3c). Finally, since casein was used as a blocking agent while patterning in further experiments, the relative amount of Mt- OligoA binding to a surface treated with 0.5 mg/ml casein was tested and found to be < 0.5%. To test the reversibility of hybridization, we heated the flow cells to denature the double-stranded DNA hybrid. Increasing the temperature by placing the flow cell at 52 C for 10 min on a heat block and washing in warm buffer (60 µl BRB µm paclitaxel at 52 C) effectively detached the microtubules from the surface. Before heating, the number of microtubules on the surface was 179 ( 11 (N ) 10 screens from 2 flow cells); after heating, only 3 ( 1.7 microtubules remained. To confirm that microtubules were not simply depolymerizing from this procedure, a solution of microtubules in a flow cell was heated in a similar manner for 10 min at 52 C. No measurable depolymerization was detected. Importantly, when oligo-functionalized microtubules were introduced back into the flow cell, they bound to the surface, demonstrating that detachment was due to melting of the DNA duplex and not due to the DNA coming off of the surface. After establishing that DNA hybridization can be used to attach microtubules to homogeneous surfaces, we investigated whether this approach can be used to pattern microtubules at defined locations on surfaces. As a first approach to patterning, we used microcontact printing, a soft lithography technique that uses a PDMS (silicone) stamp to deposit molecules on surfaces. 21 The importance of binding biological ligands to surfaces for bioassays and bioelectronic devices has expanded the use of this technique to biological molecules such as enzymes, antibodies, other proteins, and DNA. 22,23 After lithographically etching a pattern into a silicon wafer, the stamp was made by pouring PDMS over the wafer, curing it overnight and peeling it off. The stamp was first inked with a solution of 10 µm neutravidin that adsorbed onto the PDMS. The stamp was then dried and pressed onto a coverslip, transferring the neutravidin (Figure 4a). A flow cell was prepared using this coverslip, 0.5 mg/ ml casein was introduced for 5 min to block the nonpatterned surface, and then 1 µm OligoA was flowed in and allowed to bind for 20 min. Finally, 10 µm biotin was flowed in to block any free biotin-binding sites. To confirm that the oligonucleotide was properly patterned, 1 µm fluores- Nano Lett., Vol. 4, No. 11,
4 Figure 4. Microcontact printing of neutravidin on a glass coverslip. (a) View under TRITC filter, showing rhodamine-labeled neutravidin. (b) View under FITC filter, showing FITCOligoA hybridized to OligoA. Reduced pattern contrast is a result of the higher camera gain needed for the faint fluorescence signal. When the slide was observed through the FITC filter before introducing FITCOligoA, no fluorescence from the rhodamine was observed. Figure 3. Relative microtubule binding to oligo-functionalized surfaces. (a) Sketch of oligonucleotide functionalization and hybridization strategy. (b) Results of Mt-OligoA binding to surfaces in 80 mm PIPES buffer, normalized to binding to a kinesin surface in AMP-PNP. (c) Results in 12 mm PIPES buffer. Oligonucleotide sequences are listed in Table 1. cently labeled complementary oligonucleotide (FITCOligoA ) was introduced into the flow cell, incubated for 20 min, and imaged. High specificity was observed (Figure 4b). Having patterned the oligonucleotides, we proceeded to pattern microtubules by incubating oligo-functionalized microtubules together with complementary oligonucleotide patterned surfaces. After 20 min of incubation, unbound microtubules were removed by washing thoroughly with motility buffer (described above). We observed clear patterns of microtubules with limited nonspecific binding (Figure 5a). Figure 5b shows limited bridging of microtubules across two patterned areas (around two per screen). In future work, smaller islands of immobilized DNA will be used to create high-density polarized bridges for force generation or biomolecular separations. As an alternative to microcontact printing, we also used parylene dry lift-off, a technique developed by the Craighead group to pattern biomolecules on surfaces. 24 This process, which involves lithographically patterning a weakly bound polymer on a surface, adsorbing biomolecules to the patterned surface, and then peeling off the polymer, overcomes both the elastomer sagging and protein transfer problems of PDMS stamps. The technique has been used to immobilize E. coli and rat basophilic leukemia cells through patterned antibodies and poly-l-lysine. 24 Our approach followed published procedures. 24 Parylene was vapor deposited on glass coverslips and coated first with the adhesion promoter hexamethyldisilizane, and then 1.8 µm of Shipley 1818 positive photoresist. The sample was soft baked at 95 C for 90 s followed by exposure with UV light through a photomask for 6 s, and developed with Shipley CD27. The exposed parylene and remaining photoresist were then removed by an oxygen plasma etch (300 millitorr, 300 W for 15 min). After patterning the parylene, 10 µl of10µm neutravidin was allowed to adsorb to the glass surface for 30 min, and the parylene was peeled off using a razor blade and tweezers. A flow cell was then 2130 Nano Lett., Vol. 4, No. 11, 2004
5 Figure 5. Microtubules immobilized on patterned surface. Clear microtubule patterns can be observed with limited nonspecific binding. In (b) bridging between to patterns 10 µm apart can be seen. Bands on figure edges are estimated positions of the oligonucleotide patterns. constructed using this coverslip, and the oligonucleotide of interest was flowed in. Figure 6a shows a sharp pattern of a fluorescently labeled oligonucleotide (FITCOligoA ) hybridized to patterned complementary oligonucleotides (OligoA) on the surface. Figure 6b shows oligonucleotide-functionalized microtubules (Mt-OligoA) binding to surfaces patterned with complementary oligonucleotides (OligoA ). Like the microcontact printing approach, high specificity of microtubule binding was observed. Microtubules could be removed from the pattern by heating the sample at 52 C for 10 min. Although oligonucleotide functionalization works well to immobilize microtubules on surfaces, it is important that this microtubule modification does not disrupt the ability of kinesins to walk along the filaments. To confirm that oligonucleotide-functionalized microtubules remain good substrates for motors, we first performed a microtubule gliding assay. His-tagged Drosophila melanogaster kinesin heavy chain motors were adsorbed to a glass surface and oligonucleotide-functionalized microtubules were flowed in along with ATP and antifade following standard procedures. 25 DNA-functionalized microtubules moved at 592 ( 67 nm/s Figure 6. Parylene dry lift-off patterning. (a) Image of fluorescently labeled oligonucleotides hybridized to complementary oligonucleotides on the neutravidin surface. (b) Oligo-functionalized microtubules (Mt-OligoA) bound to complementary oligonucleotide (OligoA ) patterned surfaces. (mean ( SD, N ) 10), compared to 583 ( 55 nm/s (N ) 10) for standard rhodamine-labeled microtubules. We next tested whether surface-bound microtubules can support the movement of kinesin-coated beads. Kinesin motors were adsorbed to 200-nm silica beads as previously described 26 at a stoichiometry of approximately 50 motors/ bead, and microtubules were attached to the surface of a coverslip by DNA hybridization as described above. The beads were then introduced into the flow cell in the presence of 1 mm MgATP, and the sample was visualized by differential interference contrast microscopy. Beads moved along DNA-functionalized microtubules at a speed of 658 ( 79 nm/s (mean ( SD, N ) 3) (Figure 7), while beads moved along unlabeled microtubules adsorbed to a standard amino-silane coated surface at 760 ( 432 nm/s (N ) 3). Hence, DNA functionalization and surface attachment did not affect the ability of the microtubules to support kinesinbased motility. For long-distance transport, both the length and density of surface-bound microtubules will need to be increased significantly. Nano Lett., Vol. 4, No. 11,
6 Figure 7. Kinesin-functionalized beads moving along microtubules immobilized via DNA hybridization. The pictures are 1 s apart. In (d), the bead has reached the end of the microtubule and is diffusing away. Images were obtained with a Nikon TE2000 inverted microscope, NA oil objective, and Sony XC-ST-50 CCD camera with background subtraction by an Argus-20 (Hamamatsu, Inc.). The approach described here shows specific immobilization of microtubules, no loss in microtubule functionality, and reversibility of the attachment by denaturing the doublestranded DNA. The ability to pattern oligonucleotides on the surface using microcontact printing or parylene lift-off as shown, or by other potentially better methods such as dippen lithography or microarray spotting enables microtubule patterning with high resolution. With the present technique, the surface and the microtubules use the same biotin-avidin binding chemistry, creating the potential for cross-linking. This can be improved in the future by, for example, using thiolated oligos on Au surfaces, or covalently cross-linking oligonucleotides to aminoterminated glass surfaces. Also, covalently cross-linking oligonucleotides to microtubules may increase microtubule stability and the potential for long-term storage. The next step toward aligning microtubules on surfaces is defining their polarity and, in turn, the path taken by motors associated with them. To achieve this, we envision making segmented microtubules with different oligonucleotides on each end and immobilizing them on surfaces patterned with different complementary oligonucleotides. In this way, the patterns of the surface-bound oligonucleotides define the position and orientation of the microtubules, enabling the assembly of a range of 2D and 3D microtubule structures. Using this geometry, recombinant kinesins can be engineered with a range of different cargo binding domains to transport biomolecules, nanoparticles, cells, or other objects along these tracks. Finally, for actuating microdevices using the kinesin-microtubule system, this microtubule immobilization approach allows parallel arrays of microtubules to be created, enabling the activity of many kinesins to be summed. Acknowledgment. This work was funded by NSF NER award ( ) to W.O.H and J.D.Z, and by the PSU NSF MRSEC Center for Nanoscale Science (DMR ). We thank Yangrong Zhang for help in purifying kinesin motors, Kihong Ahn for assistance with the bead assay, and Zachary Donhauser for image processing expertise. References (1) Dennis, J. R.; Howard, J.; Vogel, V. Nanotechnology 1999, 10, (2) Hess, H.; Clemmens, J.; Qin, D.; Howard, J.; Vogel, V. Nano Lett. 2001, 1, (3) Moorjani, S. G.; Jia, L.; Jackson, T. N.; Hancock, W. O. Nano Lett. 2003, 3, (4) Jia, L.; Moorjani, S. G.; Jackson, T. N.; Hancock, W. O. Biomed. MicrodeV. 2004, 6, (5) Hiratsuka, Y.; Tada, T.; Oiwa, K.; Kanayama, T.; Uyeda, T. Q. Biophys. J. 2001, 81, (6) Nogales, E.; Whittaker, M.; Milligan, R. A.; Downing, K. H. Cell 1999, 96, (7) Svoboda, K.; Schmidt, C. F.; Schnapp, B. J.; Block, S. M. Nature 1993, 365, (8) Gittes, F.; Meyhofer, E.; Baek, S.; Howard, J. Biophys. J. 1996, 70, (9) Vale, R. D.; Schnapp, B. J.; Reese, T. S.; Sheetz, M. P. Cell 1985, 40, (10) Limberis, L.; Stewart, R. J. Nanotechnology 2000, 11, (11) Stracke, R.; Bohm, K. J.; Burgold, J.; Schacht, H.-J.; Unger, E. Nanotechnology 2000, 11, (12) Stracke, R.; Bohm, K. J.; Wollweber, L.; Tuszynski, J. A.; Unger, E. Biochem. Biophys. Res. Commun. 2002, 293, (13) Limberis, L.; Magda, J. J.; Stewart, R. J. Nano Lett. 2001, 1, (14) Brown, T. B.; Hancock, W. O. Nano Lett. 2002, 2, (15) Mirkin, C. A.; Letsinger, R. L.; Mucic, R. C.; Storhoff, J. J. Nature 1996, 382, (16) Mbindyo, J. K. N.; Reiss, B. D.; Martin, B. R.; Keating, C. D.; Natan, M. J.; Mallouk, T. E. AdV. Mater. 2001, 13, (17) Williams, R. C., Jr.; Lee, J. C. Methods Enzymol. 1982, 85 Pt B, (18) Hyman, A.; Drechsel, D.; Kellogg, D.; Salser, S.; Sawin, K.; Steffen, P.; Wordeman, L.; Mitchison, T. Methods Enzymol. 1991, 196, (19) Kinesin binds very tightly to microtubules in the presence of AMP- PNP, a non-hydrolyzable analog of ATP. 20 Here, we pretreat the surface with 0.5 mg/ml casein for 5 min, and subsequently incubate it with His-tagged Drosophila melangoster kinesin heavy chain for 5 min, followed by microtubules in motility solution containing 1 mm MgAMP-PNP instead of MgATP. (20) Lasek, R. J.; Brady, S. T. Nature 1985, 316, (21) Whitesides, G. M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D. E. Annu. ReV. Biomed. Eng. 2001, 3, (22) Chen, C. S.; Mrksich, M.; Huang, S.; Whitesides, G. M.; Ingber, E. D. Science 1997, (23) Xu, C. T., P.; Ersoz, M.; Fletcher, P. D. I.; Paunov, V. N. J. Mater. Chem. 2003, 13, (24) Ilic, B. C.; Craighead, H. G. Biomed. MicrodeV. 2000, 2, (25) Hancock, W. O.; Howard, J. J. Cell Biol. 1998, 140, (26) Coy, D. L.; Hancock, W. O.; Wagenbach, M.; Howard, J. Nat. Cell Biol. 1999, 1, NL048816B 2132 Nano Lett., Vol. 4, No. 11, 2004
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation
 A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements
 Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements NANO LETTERS 2003 Vol. 3, No. 5 633-637 Samira G. Moorjani, Lili Jia, Thomas N.
Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements NANO LETTERS 2003 Vol. 3, No. 5 633-637 Samira G. Moorjani, Lili Jia, Thomas N.
Self assembly and organization of nanofibers using biological molecular motors
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Motility of CoFe 2 O 4 nanoparticle-labelled microtubules in magnetic fields
 Motility of CoFe 2 O 4 nanoparticle-labelled microtubules in magnetic fields B.M. Hutchins, M. Platt, W.O. Hancock and M.E. Williams Abstract: Kinesin motor proteins transport intracellular cargo unidirectionally
Motility of CoFe 2 O 4 nanoparticle-labelled microtubules in magnetic fields B.M. Hutchins, M. Platt, W.O. Hancock and M.E. Williams Abstract: Kinesin motor proteins transport intracellular cargo unidirectionally
584 IEEE TRANSACTIONS ON ADVANCED PACKAGING, VOL. 28, NO. 4, NOVEMBER Vivek Verma, William O. Hancock, and Jeffrey M.
 584 IEEE TRANSACTIONS ON ADVANCED PACKAGING, VOL. 28, NO. 4, NOVEMBER 2005 Micro- and Nanofabrication Processes for Hybrid Synthetic and Biological System Fabrication Vivek Verma, William O. Hancock, and
584 IEEE TRANSACTIONS ON ADVANCED PACKAGING, VOL. 28, NO. 4, NOVEMBER 2005 Micro- and Nanofabrication Processes for Hybrid Synthetic and Biological System Fabrication Vivek Verma, William O. Hancock, and
Nano-Scale Engineering III Bio-Molecular Motors for Engineering
 Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
A smart dust biosensor powered by kinesin motors
 Supplementary Information (SI) to accompany A smart dust biosensor powered by kinesin motors Thorsten Fischer, Ashutosh Agarwal and Henry Hess* *Henry Hess University of Florida Department of Materials
Supplementary Information (SI) to accompany A smart dust biosensor powered by kinesin motors Thorsten Fischer, Ashutosh Agarwal and Henry Hess* *Henry Hess University of Florida Department of Materials
Aligning Bacterial Cellulose
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
Nanotechnological Applications of Biomolecular Motor Systems. Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden
 Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
communications Directing Transport of CoFe 2 O 4 -Functionalized Microtubules with Magnetic Fields** Microtubule transport
 communications DOI: 10.1002/smll.200600410 Microtubule transport Directing Transport of CoFe 2 O 4 -Functionalized Microtubules with Magnetic Fields** Benjamin M. Hutchins, Mark Platt, William O. Hancock,*
communications DOI: 10.1002/smll.200600410 Microtubule transport Directing Transport of CoFe 2 O 4 -Functionalized Microtubules with Magnetic Fields** Benjamin M. Hutchins, Mark Platt, William O. Hancock,*
Supplementary Information. Arrays of Individual DNA Molecules on Nanopatterned Substrates
 Supplementary Information Arrays of Individual DNA Molecules on Nanopatterned Substrates Roland Hager, Alma Halilovic, Jonathan R. Burns, Friedrich Schäffler, Stefan Howorka S1 Figure S-1. Characterization
Supplementary Information Arrays of Individual DNA Molecules on Nanopatterned Substrates Roland Hager, Alma Halilovic, Jonathan R. Burns, Friedrich Schäffler, Stefan Howorka S1 Figure S-1. Characterization
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
SCIENTIFIC REPORT. regarding the implementation of the project between December December 2014
 SCIENTIFIC REPORT regarding the implementation of the project between December 013 - December 014 Title of the project: DETECTION AND IDENTIFICATION OF BIOMOLECULES OF MEDICAL INTEREST BY USING MAGNETIC
SCIENTIFIC REPORT regarding the implementation of the project between December 013 - December 014 Title of the project: DETECTION AND IDENTIFICATION OF BIOMOLECULES OF MEDICAL INTEREST BY USING MAGNETIC
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules. Dr. Carissa M. Soto
 Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
NanoFabrication Systems DPN. Nanofabrication Systems. A complete line of instruments and tools for micro and nanopatterning applications
 DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning applications DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning
DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning applications DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning
Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive Detection of Proteins. Jwa-Min Nam, C. Shad Thaxton, and Chad A. Mirkin
 1 Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive Detection of Proteins Jwa-Min Nam, C. Shad Thaxton, and Chad A. Mirkin Department of Chemistry and Institute for Nanotechnology, Northwestern University,
1 Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive Detection of Proteins Jwa-Min Nam, C. Shad Thaxton, and Chad A. Mirkin Department of Chemistry and Institute for Nanotechnology, Northwestern University,
High-throughput single-dna molecule microscopy using automated micropatterning
 Laurence Salomé High-throughput single-dna molecule microscopy using automated micropatterning enjamin Berteloite BIOSOFT October 2016 B I O S O F T Innopsys/CNRS joint lab: BIOSOFT Biosoft is: An open
Laurence Salomé High-throughput single-dna molecule microscopy using automated micropatterning enjamin Berteloite BIOSOFT October 2016 B I O S O F T Innopsys/CNRS joint lab: BIOSOFT Biosoft is: An open
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology
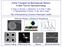 Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NPLANTS.2015.111 The non-processive rice kinesin-14 OsKCH1 transports actin filaments along microtubules with two distinct velocities Figure S1 The OsKCH1 constructs used in this study Schematic
DOI: 10.1038/NPLANTS.2015.111 The non-processive rice kinesin-14 OsKCH1 transports actin filaments along microtubules with two distinct velocities Figure S1 The OsKCH1 constructs used in this study Schematic
Biosensors. DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses)
 Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5837/513/dc1 Supporting Online Material for Spring-Loaded Mechanism of DNA Unwinding by Hepatitis C Virus NS3 Helicase Sua Myong,* Michael M. Bruno, Anna M. Pyle,
www.sciencemag.org/cgi/content/full/317/5837/513/dc1 Supporting Online Material for Spring-Loaded Mechanism of DNA Unwinding by Hepatitis C Virus NS3 Helicase Sua Myong,* Michael M. Bruno, Anna M. Pyle,
Light-Controlled Molecular Shuttles Made from Motor Proteins Carrying Cargo on Engineered Surfaces
 Light-Controlled Molecular Shuttles Made from Motor Proteins Carrying Cargo on Engineered Surfaces NANO LETTERS 2001 Vol. 1, No. 5 235-239 Henry Hess, John Clemmens, Dong Qin, Jonathon Howard, and Viola
Light-Controlled Molecular Shuttles Made from Motor Proteins Carrying Cargo on Engineered Surfaces NANO LETTERS 2001 Vol. 1, No. 5 235-239 Henry Hess, John Clemmens, Dong Qin, Jonathon Howard, and Viola
mrnadembeads Purification Mini Kit (cat #06011) Instruction manual for mrna purification
 mrnadembeads Purification Mini Kit (cat #06011) Instruction manual for mrna purification Ademtech * Bioparc BioGalien 27 Allée Charles Darwin * 33600 Pessac France Tel : + 33 (0) 57 02 02 01, Fax : + 33
mrnadembeads Purification Mini Kit (cat #06011) Instruction manual for mrna purification Ademtech * Bioparc BioGalien 27 Allée Charles Darwin * 33600 Pessac France Tel : + 33 (0) 57 02 02 01, Fax : + 33
Microarray Industry Products
 Via Nicaragua, 12-14 00040 Pomezia (Roma) Phone: +39 06 91601628 Fax: +39 06 91612477 info@lifelinelab.com www.lifelinelab.com Microarray Industry Products Page 10 NBT / BCPIP Chromogenic phosphatase
Via Nicaragua, 12-14 00040 Pomezia (Roma) Phone: +39 06 91601628 Fax: +39 06 91612477 info@lifelinelab.com www.lifelinelab.com Microarray Industry Products Page 10 NBT / BCPIP Chromogenic phosphatase
Controlling the Direction of Kinesin-Driven Microtubule Movements along Microlithographic Tracks
 Biophysical Journal Volume 81 September 2001 1555 1561 1555 Controlling the Direction of Kinesin-Driven Microtubule Movements along Microlithographic Tracks Yuichi Hiratsuka,* Tetsuya Tada, Kazuhiro Oiwa,
Biophysical Journal Volume 81 September 2001 1555 1561 1555 Controlling the Direction of Kinesin-Driven Microtubule Movements along Microlithographic Tracks Yuichi Hiratsuka,* Tetsuya Tada, Kazuhiro Oiwa,
DNA Microarray Technology
 2 DNA Microarray Technology 2.1 Overview DNA microarrays are assays for quantifying the types and amounts of mrna transcripts present in a collection of cells. The number of mrna molecules derived from
2 DNA Microarray Technology 2.1 Overview DNA microarrays are assays for quantifying the types and amounts of mrna transcripts present in a collection of cells. The number of mrna molecules derived from
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition
 SUPPLEMENTARY INFORMATION Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition Mari-Liis Visnapuu 1 and Eric C. Greene 1 1 Department of Biochemistry &
SUPPLEMENTARY INFORMATION Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition Mari-Liis Visnapuu 1 and Eric C. Greene 1 1 Department of Biochemistry &
Antibody Array User s Guide
 Antibody Microarray User s Guide Rev. 9.1 Page 1 TABLE OF CONTENTS Introduction... 2 How it works... 3 Experimental considerations... 4 Components... 4 Additional Material Required... 6 Reagent Preparation...
Antibody Microarray User s Guide Rev. 9.1 Page 1 TABLE OF CONTENTS Introduction... 2 How it works... 3 Experimental considerations... 4 Components... 4 Additional Material Required... 6 Reagent Preparation...
7/24/2012. DNA Probes. Hybridization and Probes. CLS 420 Immunology & Molecular Diagnostics. Target Sequences. Target Sequences. Nucleic Acid Probes
 Hybridization and Probes CLS 420 Immunology & Molecular Diagnostics Molecular Diagnostics Techniques: Hybridization and Probes Nucleic acid probes: A short, known sequence of DNA or RNA Used to detect
Hybridization and Probes CLS 420 Immunology & Molecular Diagnostics Molecular Diagnostics Techniques: Hybridization and Probes Nucleic acid probes: A short, known sequence of DNA or RNA Used to detect
Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome
 SUPPLEMENTARY ONLINE MATERIAL For manuscript: Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome By Roxana E. Georgescu 1, Isabel Kurth 1 and Mike E. O Donnell 1 1 The Rockefeller
SUPPLEMENTARY ONLINE MATERIAL For manuscript: Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome By Roxana E. Georgescu 1, Isabel Kurth 1 and Mike E. O Donnell 1 1 The Rockefeller
Nanoimprinting in Polymers and Applications in Cell Studies. Albert F. YEE Chemical Engineering & Materials Science UC Irvine
 Nanoimprinting in Polymers and Applications in Cell Studies Albert F. YEE Chemical Engineering & Materials Science UC Irvine Presentation outline Motivation Reversal imprinting Soft inkpad imprinting on
Nanoimprinting in Polymers and Applications in Cell Studies Albert F. YEE Chemical Engineering & Materials Science UC Irvine Presentation outline Motivation Reversal imprinting Soft inkpad imprinting on
Protocol. Epoxy Slide For Protein Array Applications. Version 2.1. February 2014
 Protocol Epoxy Slide For Protein Array Applications Version 2.1 February 2014 For Technical Assistance, Please contact Z Biotech, LLC Fitzsimons Bioscience Park Center 12635 E. Montview Blvd., Suite 214
Protocol Epoxy Slide For Protein Array Applications Version 2.1 February 2014 For Technical Assistance, Please contact Z Biotech, LLC Fitzsimons Bioscience Park Center 12635 E. Montview Blvd., Suite 214
Streptavidin Particles Technical Information
 Streptavidin Particles Technical Information Streptavidin Streptavidin is a protein (MW of approx. 66,000) made up of four identical subunits, each containing a high affinity binding site for biotin (KD
Streptavidin Particles Technical Information Streptavidin Streptavidin is a protein (MW of approx. 66,000) made up of four identical subunits, each containing a high affinity binding site for biotin (KD
RayBio Apoptosis Detection Kit, MitoCapture
 RayBio Apoptosis Detection Kit, MitoCapture User Manual Version 1.0 Mar 17, 2015 RayBio Apoptosis Detection Kit, MitroCapture Protocol (Cat#: 68MC-Apo-S100) RayBiotech, Inc. We Provide You With Excellent
RayBio Apoptosis Detection Kit, MitoCapture User Manual Version 1.0 Mar 17, 2015 RayBio Apoptosis Detection Kit, MitroCapture Protocol (Cat#: 68MC-Apo-S100) RayBiotech, Inc. We Provide You With Excellent
Biotin 3' End DNA Labeling Kit
 INSTRUCTIONS Biotin 3' End DNA Labeling Kit 3747 N. Meridian Road P.O. Box 117 Rockford, IL 61105 89818 1290.4 Number Description 89818 Biotin 3' End DNA Labeling Kit, sufficient reagents to perform 20
INSTRUCTIONS Biotin 3' End DNA Labeling Kit 3747 N. Meridian Road P.O. Box 117 Rockford, IL 61105 89818 1290.4 Number Description 89818 Biotin 3' End DNA Labeling Kit, sufficient reagents to perform 20
DPN 5000 System. Figure 1: The DPN 5000 System. Page 1 of 5. Created on 9/9/2011 Revision
 Introduction NanoInk s is a dedicated, versatile instrument capable of nanopatterning a variety of materials with nanoscale accuracy and precision. With NanoInk s proprietary MEMs devices and deposition
Introduction NanoInk s is a dedicated, versatile instrument capable of nanopatterning a variety of materials with nanoscale accuracy and precision. With NanoInk s proprietary MEMs devices and deposition
Surface Enhanced Raman Scattering Tags for Rapid and Homogeneous Detection of Circulating Tumor Cells in Presence of Human Whole Blood
 Supporting Information Surface Enhanced Raman Scattering Tags for Rapid and Homogeneous Detection of Circulating Tumor Cells in Presence of Human Whole Blood Michael Y. Sha*, Hongxia Xu, Michael J. Natan
Supporting Information Surface Enhanced Raman Scattering Tags for Rapid and Homogeneous Detection of Circulating Tumor Cells in Presence of Human Whole Blood Michael Y. Sha*, Hongxia Xu, Michael J. Natan
Self-Contained, Biomolecular Motor-Driven Protein Sorting and Concentrating in an Ultrasensitive Microfluidic Chip
 Self-Contained, Biomolecular Motor-Driven Protein Sorting and Concentrating in an Ultrasensitive Microfluidic Chip NANO LETTERS 2008 Vol. 8, No. 4 1041-1046 Chih-Ting Lin, Ming-Tse Kao, Katsuo Kurabayashi,*,
Self-Contained, Biomolecular Motor-Driven Protein Sorting and Concentrating in an Ultrasensitive Microfluidic Chip NANO LETTERS 2008 Vol. 8, No. 4 1041-1046 Chih-Ting Lin, Ming-Tse Kao, Katsuo Kurabayashi,*,
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Modulating the microtubule assembly and dynamics by altering the chemical environment
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2016 Modulating the microtubule assembly and dynamics by altering the chemical environment
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2016 Modulating the microtubule assembly and dynamics by altering the chemical environment
+ M III. IMAP Screening Express Kit Product #8073 Quantity: 8000, 20 µl reactions (80 µl final volumes) Low FP. M III High FP.
 Product Insert IMAP Screening Express Kit Product #8073 Quantity: 8000, 20 µl reactions (80 µl final volumes) Introduction About the IMAP Screening Express Kit The IMAP Screening Express Kit is used to
Product Insert IMAP Screening Express Kit Product #8073 Quantity: 8000, 20 µl reactions (80 µl final volumes) Introduction About the IMAP Screening Express Kit The IMAP Screening Express Kit is used to
Oligonucleotide Loading Determines Cellular Uptake of DNA- Modified Gold Nanoparticles
 Supporting Information for: Oligonucleotide Loading Determines Cellular Uptake of DNA- Modified Gold Nanoparticles David A. Giljohann, Dwight S. Seferos, Pinal C. Patel, Jill E. Millstone, Nathaniel L.
Supporting Information for: Oligonucleotide Loading Determines Cellular Uptake of DNA- Modified Gold Nanoparticles David A. Giljohann, Dwight S. Seferos, Pinal C. Patel, Jill E. Millstone, Nathaniel L.
(a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The
 1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
Mater. Res. Soc. Symp. Proc. Vol Materials Research Society
 Mater. Res. Soc. Symp. Proc. Vol. 940 2006 Materials Research Society 0940-P13-12 A Novel Fabrication Technique for Developing Metal Nanodroplet Arrays Christopher Edgar, Chad Johns, and M. Saif Islam
Mater. Res. Soc. Symp. Proc. Vol. 940 2006 Materials Research Society 0940-P13-12 A Novel Fabrication Technique for Developing Metal Nanodroplet Arrays Christopher Edgar, Chad Johns, and M. Saif Islam
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
BioMag SelectaPure mrna Purification System
 Corporate Headquarters 400 Valley Road Warrington, PA 18976 1-800-523-2575 FAX 1-800-343-3291 Email: info@polysciences.com www.polysciences.com Europe - Germany Polysciences Europe GmbH Handelsstr. 3 D-69214
Corporate Headquarters 400 Valley Road Warrington, PA 18976 1-800-523-2575 FAX 1-800-343-3291 Email: info@polysciences.com www.polysciences.com Europe - Germany Polysciences Europe GmbH Handelsstr. 3 D-69214
Using Magnetic Nanoparticles to Enhance Gene Transfection. on Magneto-electroporation Microchips
 Materials Science Forum Online: 2006-01-15 ISSN: 1662-9752, Vols. 505-507, pp 661-666 doi:10.4028/www.scientific.net/msf.505-507.661 2006 Trans Tech Publications, Switzerland Using Magnetic Nanoparticles
Materials Science Forum Online: 2006-01-15 ISSN: 1662-9752, Vols. 505-507, pp 661-666 doi:10.4028/www.scientific.net/msf.505-507.661 2006 Trans Tech Publications, Switzerland Using Magnetic Nanoparticles
Introduction to Nanofabrication: Top Down to Bottom Up
 Welcome to NACK s Webinar Introduction to Nanofabrication: Top Down to Bottom Up NACK is an NSF-funded ATE Resource Center supporting faculty in Nanotechnology Education Hosted by MATEC Networks www.matecnetworks.org
Welcome to NACK s Webinar Introduction to Nanofabrication: Top Down to Bottom Up NACK is an NSF-funded ATE Resource Center supporting faculty in Nanotechnology Education Hosted by MATEC Networks www.matecnetworks.org
Nanobiotechnology. Place: IOP 1 st Meeting Room Time: 9:30-12:00. Reference: Review Papers. Grade: 50% midterm, 50% final.
 Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Dynamic Re-organization of Individual Adhesion Nanoclusters in Living Cells by Ligand Patterned Surfaces**
 Supplementary information: SMALL Dynamic Re-organization of Individual Adhesion Nanoclusters in Living Cells by Ligand Patterned Surfaces** Ruth Diez-Ahedo, Davide Normanno, Olga Esteban, GertJan Bakker,
Supplementary information: SMALL Dynamic Re-organization of Individual Adhesion Nanoclusters in Living Cells by Ligand Patterned Surfaces** Ruth Diez-Ahedo, Davide Normanno, Olga Esteban, GertJan Bakker,
What is Nano-Bio? Non-Covalent Interactions
 - - What is Nano-Bio? Physicist: Biotech: Biologists: -study of molecular interactions -application of nano-tools to study biological systems. -application of nano-tools to detect, treat, and prevent disease
- - What is Nano-Bio? Physicist: Biotech: Biologists: -study of molecular interactions -application of nano-tools to study biological systems. -application of nano-tools to detect, treat, and prevent disease
Micromotors Powered by Enzyme Catalysis
 Supporting Information Micromotors Powered by Enzyme Catalysis Krishna K. Dey, 1 Xi Zhao, 1, Benjamin M. Tansi, 1, Wilfredo J. Méndez-Ortiz, 2 Ubaldo M. Córdova-Figueroa 2, * Ramin Golestanian, 3, * and
Supporting Information Micromotors Powered by Enzyme Catalysis Krishna K. Dey, 1 Xi Zhao, 1, Benjamin M. Tansi, 1, Wilfredo J. Méndez-Ortiz, 2 Ubaldo M. Córdova-Figueroa 2, * Ramin Golestanian, 3, * and
DNA Hybridization and Detection
 Chapter 6 DNA Hybridization and Detection Fluorescence Polarization Detection of DNA Hybridization........................................................ 6-2 Introduction.............................................................................................................
Chapter 6 DNA Hybridization and Detection Fluorescence Polarization Detection of DNA Hybridization........................................................ 6-2 Introduction.............................................................................................................
The Pennsylvania State University. The Graduate School APPROACHES TO HYBRID SYNTHETIC DEVICES. A Dissertation in. Engineering Science and Mechanics
 The Pennsylvania State University The Graduate School Department of Engineering Science and Mechanics APPROACHES TO HYBRID SYNTHETIC DEVICES A Dissertation in Engineering Science and Mechanics by Vivek
The Pennsylvania State University The Graduate School Department of Engineering Science and Mechanics APPROACHES TO HYBRID SYNTHETIC DEVICES A Dissertation in Engineering Science and Mechanics by Vivek
Supplementary Figures 1-6
 1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
DYNAMICBIOSENSORS. Chip functionalization DNA-encoded addressing and chip configuration. Technology Information #120
 Technology Information #120 Chip functionalization DNA-encoded addressing and chip configuration The switchsense biochip features 24 detection spots, which are arranged in 2 or 4 seperate flow channels.
Technology Information #120 Chip functionalization DNA-encoded addressing and chip configuration The switchsense biochip features 24 detection spots, which are arranged in 2 or 4 seperate flow channels.
Fluorescence Imaging with One Nanometer Accuracy Lab
 I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
The Pennsylvania State University. The Graduate School. Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF
 The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering
The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering
2. Pyrosequencing Assay Design
 2. Pyrosequencing Assay Design 2.1 Guidelines for PCR set-up and primer design 2.1.1 PCR primer design Design of PCR primers follows standard rules, i.e. calculated Tm of 62-65 C, primer length of about
2. Pyrosequencing Assay Design 2.1 Guidelines for PCR set-up and primer design 2.1.1 PCR primer design Design of PCR primers follows standard rules, i.e. calculated Tm of 62-65 C, primer length of about
Rapid Prototyping of Purification Platforms
 Rapid Prototyping of Purification Platforms Tom Huang, Nathan Mosier, Michael Ladisch Department of Agricultural and Biological Engineering Weldon School of Biomedical Engineering Laboratory of Renewable
Rapid Prototyping of Purification Platforms Tom Huang, Nathan Mosier, Michael Ladisch Department of Agricultural and Biological Engineering Weldon School of Biomedical Engineering Laboratory of Renewable
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/333/6041/456/dc1 Supporting Online Material for Cilia-Like Beating of Active Microtubule Bundles Timothy Sanchez, David Welch, Daniela Nicastro, Zvonimir Dogic* *To
www.sciencemag.org/cgi/content/full/333/6041/456/dc1 Supporting Online Material for Cilia-Like Beating of Active Microtubule Bundles Timothy Sanchez, David Welch, Daniela Nicastro, Zvonimir Dogic* *To
Propidium Iodide. Catalog Number: Structure: Molecular Formula: C 27H 34I 2N 4. Molecular Weight: CAS #
 Catalog Number: 195458 Propidium Iodide Structure: Molecular Formula: C 27H 34I 2N 4 Molecular Weight: 668.45 CAS # 25535-16-4 Physical Description: Dark red crystals Description: Reagent used for the
Catalog Number: 195458 Propidium Iodide Structure: Molecular Formula: C 27H 34I 2N 4 Molecular Weight: 668.45 CAS # 25535-16-4 Physical Description: Dark red crystals Description: Reagent used for the
Surface Plasmon Resonance Analyzer
 Surface Plasmon Resonance Analyzer 5 6 SPR System Based on Microfluidics Wide Dynamic Range Kinetic Analysis by Detection of Association /Dissociation of Bio-Molecules Measuring of Mass Change below
Surface Plasmon Resonance Analyzer 5 6 SPR System Based on Microfluidics Wide Dynamic Range Kinetic Analysis by Detection of Association /Dissociation of Bio-Molecules Measuring of Mass Change below
PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%)
 1 AFFINITY HIS-TAG PURIFICATION PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%) DESCRIPTION Nickel NTA Magnetic Agarose Beads are products that allow rapid and easy small-scale purification of
1 AFFINITY HIS-TAG PURIFICATION PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%) DESCRIPTION Nickel NTA Magnetic Agarose Beads are products that allow rapid and easy small-scale purification of
Nonspecific binding of 10 nm Cy5-labeled DinB on nine different surfaces, measured by the number of DinB spots over an imaging area of 2,500 µm 2.
 Supplementary Figure 1 Nonspecific binding of 10 nm Cy5-labeled DinB on nine different surfaces, measured by the number of DinB spots over an imaging area of 2,500 µm 2. Free DinB was washed out using
Supplementary Figure 1 Nonspecific binding of 10 nm Cy5-labeled DinB on nine different surfaces, measured by the number of DinB spots over an imaging area of 2,500 µm 2. Free DinB was washed out using
The Development of an Indirect Competitive. Immunomagnetic-Proximity Ligation Assay for Small-Molecule. Detection
 The Development of an Indirect Competitive Immunomagnetic-Proximity Ligation Assay for Small-Molecule Detection Xuecheng Jiang, a Zhenhong Zhu, ab Zhihao Sun, a Luming Wang, a Lixiao Zhou, a Hanqiang Miao,
The Development of an Indirect Competitive Immunomagnetic-Proximity Ligation Assay for Small-Molecule Detection Xuecheng Jiang, a Zhenhong Zhu, ab Zhihao Sun, a Luming Wang, a Lixiao Zhou, a Hanqiang Miao,
Elecrtonic Supplementary Information. Application of quantum dot barcodes prepared using biological self-assembly to multiplexed immunoassays
 Elecrtonic Supplementary Information Application of quantum dot barcodes prepared using biological self-assembly to multiplexed immunoassays Sakandar Rauf, Andrew Glidle and Jonathan M Cooper Department
Elecrtonic Supplementary Information Application of quantum dot barcodes prepared using biological self-assembly to multiplexed immunoassays Sakandar Rauf, Andrew Glidle and Jonathan M Cooper Department
Technical tips Session 4
 Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Polymer-based Microfabrication
 Polymer-based Microfabrication PDMS SU-8 PMMA Hydrogel 1 Soft Lithography Developed by Whitesides, et. al A set of techniques for microfabrication based on the use of lithography, soft substrate materials
Polymer-based Microfabrication PDMS SU-8 PMMA Hydrogel 1 Soft Lithography Developed by Whitesides, et. al A set of techniques for microfabrication based on the use of lithography, soft substrate materials
Comparison of different methods for purification analysis of a green fluorescent Strep-tag fusion protein. Application
 Comparison of different methods for purification analysis of a green fluorescent Strep-tag fusion protein Application Petra Sebastian Meike Kuschel Stefan Schmidt Abstract This Application Note describes
Comparison of different methods for purification analysis of a green fluorescent Strep-tag fusion protein Application Petra Sebastian Meike Kuschel Stefan Schmidt Abstract This Application Note describes
Department of Mechanical Engineering, University of Washington, Seattle, WA, 98195
 Supplementary information Contact Angle Changes Induced by Immunocomplex Formation Jong-Hoon Kim, a Amy Q. Shen, a Kyong-Hoon Lee, b Gerard A. Cangelosi c and Jae-Hyun Chung* a a Department of Mechanical
Supplementary information Contact Angle Changes Induced by Immunocomplex Formation Jong-Hoon Kim, a Amy Q. Shen, a Kyong-Hoon Lee, b Gerard A. Cangelosi c and Jae-Hyun Chung* a a Department of Mechanical
Electronic supplementary information (ESI) Kinetic Study of DNA Hybridization on DNA-modified Gold Nanoparticles. with Engineered Nano-Interfaces
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic supplementary information (ESI) Kinetic Study of DNA Hybridization on DNA-modified
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic supplementary information (ESI) Kinetic Study of DNA Hybridization on DNA-modified
The preparation of native chromatin from cultured human cells.
 Native chromatin immunoprecipitation protocol The preparation of native chromatin from cultured human cells. All solutions need to be ice cold. Sucrose containing solutions must be made up fresh on the
Native chromatin immunoprecipitation protocol The preparation of native chromatin from cultured human cells. All solutions need to be ice cold. Sucrose containing solutions must be made up fresh on the
PRODUCT DATA SHEET. Carboxylated Gold Nanoparticles. Description. Features. Storage. Applications. Handling. Characteristics
 PRODUCT DATA SHEET Carboxylated Gold Nanoparticles Description Cytodiagnostics carboxylated gold nanoparticles are available with two different lengths of PEG surface spacers, i.e. 3000Da and 5000Da offering
PRODUCT DATA SHEET Carboxylated Gold Nanoparticles Description Cytodiagnostics carboxylated gold nanoparticles are available with two different lengths of PEG surface spacers, i.e. 3000Da and 5000Da offering
Study Small Molecule-Membrane Protein Binding Kinetics with. Nanodisc and Charge Sensitive Optical Detection
 Support Information Study Small Molecule-Membrane Protein Binding Kinetics with Nanodisc and Charge Sensitive Optical Detection Guangzhong Ma 1,2, Yan Guan 1,3, Shaopeng Wang 1*, Han Xu 4*, Nongjian Tao
Support Information Study Small Molecule-Membrane Protein Binding Kinetics with Nanodisc and Charge Sensitive Optical Detection Guangzhong Ma 1,2, Yan Guan 1,3, Shaopeng Wang 1*, Han Xu 4*, Nongjian Tao
Typical probes. Slides per pack Aminosilane. Long oligo- Slide AStar None D surface. nucleotides
 Aminosilane coating Nexterion Slide A+ and Slide AStar Overview Type of coating Immobilization method Typical probes Ordering information Nexterion product Barcode option Item number Slides per pack Aminosilane
Aminosilane coating Nexterion Slide A+ and Slide AStar Overview Type of coating Immobilization method Typical probes Ordering information Nexterion product Barcode option Item number Slides per pack Aminosilane
Solid Phase cdna Synthesis Kit
 #6123 v.02.09 Table of Contents I. Description... 2 II. Kit components... 2 III. Storage... 2 IV. Principle... 3 V. Protocol V-1. Preparation of immobilized mrna... 4 Protocol A: Starting from Tissue or
#6123 v.02.09 Table of Contents I. Description... 2 II. Kit components... 2 III. Storage... 2 IV. Principle... 3 V. Protocol V-1. Preparation of immobilized mrna... 4 Protocol A: Starting from Tissue or
Sensitive Detection of Small Molecules by Competitive Immunomagnetic-Proximity Ligation Assay
 Sensitive Detection of Small Molecules by Competitive Immunomagnetic-Proximity Ligation Assay Shuyan Cheng a, Feng Shi a, Xuecheng Jiang a, Luming Wang a, Weiqing Chen b, Chenggang Zhu a * a College of
Sensitive Detection of Small Molecules by Competitive Immunomagnetic-Proximity Ligation Assay Shuyan Cheng a, Feng Shi a, Xuecheng Jiang a, Luming Wang a, Weiqing Chen b, Chenggang Zhu a * a College of
ab Oligonucleotide Conjugation Kit
 Version 2 Last updated 6 April 2017 ab218260 Oligonucleotide Conjugation Kit For the covalent conjugation of antibodies to oligonucleotides. This product is for research use only and is not intended for
Version 2 Last updated 6 April 2017 ab218260 Oligonucleotide Conjugation Kit For the covalent conjugation of antibodies to oligonucleotides. This product is for research use only and is not intended for
ab Oligonucleotide Conjugation Kit
 Version 2 Last updated 6 April 2017 ab218260 Oligonucleotide Conjugation Kit For the covalent conjugation of antibodies to oligonucleotides. This product is for research use only and is not intended for
Version 2 Last updated 6 April 2017 ab218260 Oligonucleotide Conjugation Kit For the covalent conjugation of antibodies to oligonucleotides. This product is for research use only and is not intended for
Figure SI-1. Fabrication process of the microchip. (A) Heater and sensor. 1) Au/Cr
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplemental Information 1. Microfluidic Device Fabrication Figure SI-1. Fabrication process
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplemental Information 1. Microfluidic Device Fabrication Figure SI-1. Fabrication process
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Multiplexed Detection of Lung Cancer Cells at the Single-Molecule
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Multiplexed Detection of Lung Cancer Cells at the Single-Molecule
SUPPLEMENTARY FIGURES and LEGENDS
 DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency Nikolas Stefan 1, 3, Patricia Martin-Killias 1, 3, Sascha Wyss-Stoeckle 1,
DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency Nikolas Stefan 1, 3, Patricia Martin-Killias 1, 3, Sascha Wyss-Stoeckle 1,
Kinetics Review. Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets)
 Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension
 Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
MagSi Beads. Magnetic Silica Beads for Life Science and Biotechnology study
 MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
Controlled Microtubules Transport on Patterned Non-fouling Surfaces
 Controlled Microtubules Transport on Patterned Non-fouling Surfaces R. C. Lipscomb 1, J. Clemmens 2, Y. Hanein 1, M. R. Holl 1, V. Vogel 2, B. D. Ratner 2, 3, D. D. Denton 1 and K. F. Böhringer 1 1 Electrical
Controlled Microtubules Transport on Patterned Non-fouling Surfaces R. C. Lipscomb 1, J. Clemmens 2, Y. Hanein 1, M. R. Holl 1, V. Vogel 2, B. D. Ratner 2, 3, D. D. Denton 1 and K. F. Böhringer 1 1 Electrical
Technical Seminar 22th Jan 2013 DNA Origami
 Technical Seminar 22th Jan 2013 DNA Origami Hitoshi Takizawa, PhD Agenda 1) Basis of structural DNA nanotechnology 2) DNA origami technique (2D, 3D, complex shape) 3) Programmable nanofactory 4) Application
Technical Seminar 22th Jan 2013 DNA Origami Hitoshi Takizawa, PhD Agenda 1) Basis of structural DNA nanotechnology 2) DNA origami technique (2D, 3D, complex shape) 3) Programmable nanofactory 4) Application
Epoxy Microarray Slides
 Introduction Epoxy Slides provide a uniform substrate for the creation of high-quality nucleic acid microarrays leveraging the advantage of a high throughput, parallel, and multiplex format. The substrate
Introduction Epoxy Slides provide a uniform substrate for the creation of high-quality nucleic acid microarrays leveraging the advantage of a high throughput, parallel, and multiplex format. The substrate
Educational Note # 1. Applications of Magnetic Bead Technology with Versa Workstation in Genomics & Proteomics
 Educational Note # 1 Applications of ic Bead Technology with Versa Workstation in Genomics & Proteomics I. Introduction ic separation technology, using magnetic particles, is a quick and easy method for
Educational Note # 1 Applications of ic Bead Technology with Versa Workstation in Genomics & Proteomics I. Introduction ic separation technology, using magnetic particles, is a quick and easy method for
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 215 Supporting Information Quantitative Description of Thermodynamic and Kinetic Properties of the
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 215 Supporting Information Quantitative Description of Thermodynamic and Kinetic Properties of the
A Straw-Housed Paper-based Colorimetric Antibody-antigen Sensor
 Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2016 Supporting Information A Straw-Housed Paper-based Colorimetric Antibody-antigen Sensor
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2016 Supporting Information A Straw-Housed Paper-based Colorimetric Antibody-antigen Sensor
Self-assembly of oligonucleotides
 Self-assembly of oligonucleotides Dr. K. Uma Maheswari Professor, School of Chemical & Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 9 Table of Contents 1 APPLICATIONS
Self-assembly of oligonucleotides Dr. K. Uma Maheswari Professor, School of Chemical & Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 9 Table of Contents 1 APPLICATIONS
Synthetic Biology for
 Synthetic Biology for Plasmids and DNA Digestion Plasmids Plasmids are small DNA molecules that are separate from chromosomal DNA They are most commonly found as double stranded, circular DNA Typical plasmids
Synthetic Biology for Plasmids and DNA Digestion Plasmids Plasmids are small DNA molecules that are separate from chromosomal DNA They are most commonly found as double stranded, circular DNA Typical plasmids
Alternative MicroFabrication and Applications in Medicine and Biology
 Alternative MicroFabrication and Applications in Medicine and Biology Massachusetts Institute of Technology 6.152 - Lecture 15 Fall 2003 These slides prepared by Dr. Hang Lu Outline of Today s Materials
Alternative MicroFabrication and Applications in Medicine and Biology Massachusetts Institute of Technology 6.152 - Lecture 15 Fall 2003 These slides prepared by Dr. Hang Lu Outline of Today s Materials
Supplemental Information
 Supplemental Information Solid-phase Extraction and Purification of Membrane Proteins Using a UV-modified PMMA Microfluidic Bioaffinity µspe Device Katrina N. Battle, 1 Joshua M. Jackson, 2 Małgorzata
Supplemental Information Solid-phase Extraction and Purification of Membrane Proteins Using a UV-modified PMMA Microfluidic Bioaffinity µspe Device Katrina N. Battle, 1 Joshua M. Jackson, 2 Małgorzata
