The Pennsylvania State University. The Graduate School. Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF
|
|
|
- Ursula Wilson
- 5 years ago
- Views:
Transcription
1 The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering by Maruti Uppalapati 2008 Maruti Uppalapati Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2008
2 The dissertation of Maruti Uppalapati was reviewed and approved* by the following: ii William O. Hancock Associate Professor of Bioengineering Dissertation Advisor Co-Chair of Committee Thomas N. Jackson Robert E. Kirby Chair Professor of Electrical Engineering Co-Chair of Committee Ahmed A. Heikal Associate Professor of Bioengineering Richard J. Cyr Professor of Biology Ryan S. Clement Assistant Professor of Bioengineering Herbert H. Lipowsky Professor and Head of the Department of Bioengineering *Signatures are on file in the Graduate School
3 ABSTRACT iii Kinesins are microtubule based motor proteins that play important roles in intracellular transport and cell division in eukaryotic cells. Because this motion can be recapitulated in synthetic environments using purified components, the kinesinmicrotubule system is an excellent model for biologically derived nanoscale motion. The first objective of this thesis was to develop microscale transport systems that use kinesin-driven microtubules as carriers to transport and sort cargo in lab-on-a-chip devices. The second objective was to develop in vitro models that mimic the microtubule organization found in cells, to study the role of kinesin motors in cellular processes such as cell division. By optimizing materials, surfaces and geometries, functional kinesins motors and microtubules were successfully integrated into enclosed microchannels. A threetier hierarchical system of microfluidic channels that links the microscale transport channels to macroscopic fluid connections was used. Shallow microchannels (5 m wide and 1 m deep) were etched in a glass substrate and bonded to a cover glass using PMMA as an adhesive. Intermediate channels (~100 μm wide) serve as reservoirs and connect to 250 μm deep microchannels that hold fine gauge tubing for fluid injection. High surface-to-volume ratio in microchannels results in gradients of motor adsorption. To solve the problems of high surface-to-volume ratio in these systems, we developed an approach, using a headless kinesin construct, to eliminate gradients in motor adsorption and microtubule binding in the enclosed channels. This enables precise control of kinesin density in the microchannels and maximizes microtubule movement. The confinement geometry (channel cross-section 5 μm x 1 μm) enables long-distance movement (~5 mm) and directional control, which are prerequisites for developing hybrid
4 iv lab-on-chip devices using biomotor-driven transport. We further showed that constructing a circular ring using these microchannels generates a high density ensemble of isopolar microtubules driven by kinesin motors. These aligned microtubules can be used for microscale transport applications or as a model in vitro system for studying kinesin-driven microtubule organization in cells. The stability and limited shelf life of these motor proteins and their associated protein filaments is a barrier to implementing of kinesin-driven transport in devices. We demonstrated that freeze-drying or critical point drying kinesins adsorbed to glass surfaces extends their lifetime from days to more than four months. Further, photoresist deposition and removal can be carried out on these motor-adsorbed surfaces without loss of motor function. The methods developed here are an important step towards realizing the integration of biological motors into practical devices, and these approaches can be extended to patterning and preserving other proteins immobilized on surfaces. A number of tools have been developed to manipulate microtubules in solution, including optical tweezers, fluid flow, magnetic fields, and DC electrophoresis, however each approach has its limitations. AC electrokinetics provides a novel tool for manipulating and organizing microtubules in solution, enabling new experimental geometries for investigating and controlling the interactions of microtubules and microtubule motors in vitro. By fabricating microelectrodes on glass substrates and generating AC electric fields across solutions of microtubules in low ionic strength buffers, we were able to collect and align bundles of microtubules and to measure the electrical properties of microtubules in solution. We found that AC electric fields result in electroosmotic flow, electrothermal flow and dielectrophoresis of microtubules, which can be controlled by varying the solution conductivity, AC frequency, and electrode
5 v geometry. These experiments demonstrate that AC electrokinetics provides a powerful new tool for kinesin-driven transport applications. Furthermore, by maximizing dielectrophoretic forces and minimizing electroosmotic and electrothermal flows, microtubules could be assembled into opposed asters, reminiscent of the mitotic spindle in dividing cells. However the microtubules in these asters were not sorted for polarity. We developed kinesin motor patterning and neutravidin patterning techniques and used them to organize microtubules in the asters an isopolar manner, similar to microtubule organization in a mitotic spindle. These assembled structures serve as an in vitro model for investigating the role of microtubule motors in development and maintenance of the mitotic spindle.
6 TABLE OF CONTENTS vi List of Figures..viii Acknowledgements...xvii Foreword.xviii Chapter 1 Literature review: Nanotechnology of kinesins and microtubules Introduction Kinesins and Microtubules In vitro assays of kinesin-driven movement Harnessing kinesin driven motion Kinesins as cargo carriers : bead assay geometry Microtubules as cargo carriers: gliding assay geometry Long term stability and preservation of motility assays Nanotechnological tools to study kinesins and microtubules Thesis outline and objectives.19 Chapter 2 Microtubule transport, concentration and alignment in enclosed microfluidic channels Introduction Materials and Methods Results and Discussion Conclusion Chapter 3 Enhancing the Stability of Kinesin Motors for Microscale Transport Applications Introduction Materials and Methods Results and Discussion Conclusion... 45
7 vii Chapter 4 Microtubule Alignment and Manipulation Using AC Electrokinetics Introduction Materials and Methods Results and Discussion AC Electroosmotic Flow Electrothermal flow Microtubule accumulation due to electrohydrodynamic flows Microtubule dielectrophoresis Estimating dielectrophoretic forces on microtubules Kinesin motility in low ionic strength buffers Conclusion Chapter 5 Organizing bipolar arrays of microtubules to create in vitro models of mitotic spindles Introduction Materials and Methods Results and Discussion Kinesin patterning approach Neutravidin patterning approach Conclusion 87 Chapter 6 Conclusions and Suggestions for Future Work Harnessing kinesin-driven motion for lab-on-a-chip devices In vitro models of microtubule organization mimicking mitotic spindle 77 References..98
8 LIST OF FIGURES viii Figure 1.1: Microtubules are non-covalent polymers of tubulin heterodimers which form a hollow cylindrical filament with 25nm diameter.2 Figure 1.2: Conventional kinesin is a tetrameric protein with identical heavy chains and light chains.3 Figure 0.3: Microtubule motility assays. (a) In the microtubule gliding assay, microtubules are visualized moving over a lawn of motor proteins absorbed to the microscope coverglass. (b) Single, fluorescently labelled motor proteins can be visualized moving along microtubules by total internal reflection microscopy in the single molecule motility assay(rogers and Scholey, 2004)..4 Figure 1.4: Schematic for biosensor/bioseparation device based kinesin-driven transport (Jia et al., 2004)... 6 Figure 1.5: Fluid flow aligns free floating plus-ends of microtubules in the direction of flow (Limberis et al., 2001)..9 Figure 1.6: Tight arrays of isopolar microtubules (Scale bar 5 µm)(brown and Hancock, 2002)...10
9 ix Figure 1.7: Microtubule movement confined within narrow SU8 photoresist channels (Moorjani et al., 2003) Figure 1.8: Unidirectional motion of microtubules can be obtained using rectifier patterns in photoresist channels. The rectifier allows microtubules moving from left to right (A) while microtubules moving from right to left are redirected (Hiratsuka et al., 2001) Figure 1.9: Demonstration of molecular sorting. (A) Color image of a mixture of red- and greenlabeled microtubules approaching a Y junction. Electrical force is used to steer microtubules carrying green and red fluorophores into the right and left reservoirs, respectively. (B) Example of successful sorting events for a green- and a red-labeled microtubule. As a function of time, first a green microtubule is steered into the right reservoir (t e 10 s), and subsequently a red microtubule is sent into the left reservoir (van den Heuvel et al., 2006b)..15 Figure 1.10: Localized activation of microtubule motility by UV light spot. Left: Two images (pseudocolored in green and red and separated by 200 s in time) are overlaid, showing the illumination zone and the movement of microtubules with radius-dependent velocity, due to the ATP sequestration by hexokinase in solution. Right: Velocity profile plotted as a function of distance from UV spot and hexokinase concentration (Tucker et al., 2008)... 17
10 x Figure 1.11: Demonstration of centering of bead by microtubule polymerization forces in a microchamber, an example of an in vitro model to study the role of microtubule polymerization in centering the centrosome with cell. (Scale bar 10 µm, Images 3 min apart) (Holy et al., 1997)...20 Figure 1.12: Self organization of microtubules into asters and vortices (Nedelec et al., 1997)...20 Figure 2.1: Hierarchical microchannel design. Macrochannels (250 μm deep) enable sample introduction. Intermediate channels (100 μm wide and 1 µm deep) connect to microchannels (5 μm wide and 1 μm deep) where microtubule motility is observed. Bottom panel shows a completed sample including the coverglass bonded using PMMA adhesive, and tubing for sample injection...25 Figure 2.2: Eliminating kinesin motor gradients using a headless kinesin construct. Left: Image of an intermediate channels when only full-length motors are used. The microtubules bind avidly to the macro channel (to the left of the intermediate channel) and the proximal portion of the intermediate channel (left side of the image). The number of microtubules entering microchannels (located to the right of the intermediate channel) is therefore very small. Right: When full-length kinesin motors are combined with headless kinesin, the motor density is reduced and the gradient in the intermediate channel is eliminated. The edge of the macro channel can be seen at left..25
11 xi Figure 2.3: Directional rectifier constructed using enclosed microfabricated channels. (a) Expected paths of microtubules in the rectifier pattern (gray traces follow microtubules moving in desired direction, black traces follow microtubules moving in opposite direction. (b) Microtubules entering from reservoir at left (not shown) travel down either arm and continue through to the other arm, reversing their direction. Microtubules entering from the right collide with the base of the structure and are directed into one of the two arms.28 Figure 2.4: SEM image of the microtubule storage ring. Microtubules originate in the intermediate channels and are transported by immobilized kinesins into the microchannels. The microchannels are designed so that filaments traveling counterclockwise in the ring are retained, while any traveling clockwise should exit the ring, be reversed by the rectifiers and return in a counterclockwise orientation...30 Figure 2.5: Microtubule movement and concentration in a microfabricated ring. A: Microtubule movement shortly after injecting microtubules into the device. Microtubules are moving in both directions in this case. B: Image of concentration ring after 90 min of accumulation; almost all microtubules are moving counterclockwise in this case. C and D: Fluorescence intensity profiles taken along lines in panel B, for estimating the number of microtubules in the ring. Single microtubule peaks in panel C were obtained in a sparsely populated area, and together with the integrated fluorescence cross-section in panel D used to calculate the number of microtubules in the ring.. 31 Figure 3.1: Microtubule motility in critical point-dried samples. A) The fraction of microtubules moving following different durations of storage at 4 o C. At each time point,
12 45 video screens [54 m x 70 m] were analyzed from a total of three flow cells. B) The xii number of microtubules bound per video screen. C) Screen captures showing microtubule movement following 5 weeks of storage. Frames are 4 sec apart, scale bar is 15 µm 41 Figure 3.2: Microtubule motility in freeze-dried samples. A) The fraction of microtubules moving following different durations of storage at 4 o C. At each time point, 45 video screens [54 m x 70 m] were analyzed from a total of three flow cells. B) The number of microtubules bound per video screen. C) Screen captures showing microtubule movement following 5 weeks of storage. Frames are 4 sec apart, scale bar is 15 µm..43 Figure 3.3: Using photolithography to pattern critical point dried motors on glass substrates. A) The patterning process. Photoresist is deposited on top of the motorfunctionalized surface, patterened using contact lithography, developed to pattern motors in specific regions, and then stripped to expose functional motors. B) Microtubule motility on a region of the surface protected by the photoresist (top) and a region of the surface where motors were exposed (bottom) Figure 4.1: Electrode design and AC field-driven microtubule accumulation. A: Optical micrograph of Cr electrodes 15 µm wide and 12 mm long, separated by a gap of 20 µm. B: Accumulation of microtubules resulting from application of 40 V p-p at 500 khz across the electrodes. C: Schematic of electrohydrodynamic flows leading to microtubule
13 xiii accumulation. Green arrows denote electroosmotic flows and red arrows denote electrothermal flows 56 Figure 4.2: Mechanism of AC electroosmotic flow. A) During each half cycle, counter ions accumulate on each electrode. Tangential electric field components (E x ) lead to coulombic forces (F c ) that are directed away from the gap, irrespective of the electrode polarity. B) The resulting ion fluxes cause electroosmotic fluid flow away from the electrode gap. C) Using the same electrode geometry as in the experiments, a simulation of the tangential components of the electric field in the x-y plane just above the surface. The resulting ion flows result in inward fluid flow toward the center of the electrodes from all edges.. 57 Figure 4.3: Simulation and experimental observation of electrothermal flows resulting from microscope illumination. A) Simulation of electrothermal flow, showing the flow vectors above two 100 µm long electrodes separated by a 20 µm gap (side view). Note that near the electrode surface, flow is observed towards the gap. B) Experimental flow profiles (top view) observed by tracking fluorescent beads at a height of ~7 µm above the electrode surface. The traces show the track taken by each particle in the 5 sec preceding the image. For clarity, only one electrode is shown, and every bead is observed to move toward the gap separating the electrodes. C) Particle velocities along a line perpendicular to the electrode at a distance of 50 µm from the gap. Velocities are maximal directly above the electrode and fall of with increasing lateral distances away from the electrode edge..62
14 Figure 4.4: Frequency dependence of microtubule accumulation. Electrodes are 10 µm xiv wide and separated by a 20 µm gap. BRB12 buffer was used with 40 V p-p field stimulation. Insets show the start points and end point of the accumulation zone (Scale bar 20 µm). Distances are measured from the edge each electrode and are displayed as mean +/- standard deviation from 4 to 6 determinations for each point...64 Figure 4.5: Positive dielectrophoresis of microtubules using castellated electrodes at 5 MHz frequency and 35 V p-p amplitude in BRB3 buffer..67 Figure 4.6: The dependence of microtubule accumulation on AC frequency using opposed electrodes. At high frequencies, dielectrophoresis dominates, while at lower frequencies, electroosmotic flows push the microtubules away from the electrode gap. Experiments were performed in BRB12 buffer with 30V p-p stimulation. Frequencies were: A) 100 khz, B) 250 khz, C) 500 khz, D) 1 MHz, E) 2.5 MHz 67 Figure 4.7: Dielectrophoresis of microtubules. A: Simulation of the electric field and electric field gradients using opposed electrodes. Simulations used 35 V p-p amplitude in BRB6 buffer. Red arrows denote the electric field vectors, and colors denote E 2. To better visualize the data, values are scaled logarithmically and gradients less than V 2 /m 3 are not shown. B: Dielectrophoretic accumulation of microtubules at electrode edges agree with the simulations (AC voltage of 35 V p-p, 5 MHz in BRB6 buffer) 71
15 xv Figure 5.1: Left: Features of the bipolar mitotic spindle(gadde and Heald, 2004) Right: Immunofluorescence image of mitotic spindle in tissue cell. Green microtubules, blue chromosomes and red, TPX2 spindle pole component.(wittmann et al., 2001).. 75 Figure 5.2: Pathways for mitotic spindle assembly. Left :Centrosome mediated Right: Chromosome mediated.. 76 Figure 5.3: Patterning of neutravidin using deep UV illumination. (a)-(c) Top side exposure approach (d) Effective patterning demonstrated by binding rhodamine labeled biotinylated BSA. (e)-(g) Back side exposure technique. (h) effective patterning demonstrated by microtubule binding..80 Figure 5.4: Spindle-like accumulation of microtubules. A: Opposed microtubule asters generated by dielectrophoretic accumulation of microtubules at electrode edges. AC voltage of 35 V p-p, 5 MHz was applied across the electrodes, and microtubules were suspended in BRB12 buffer.. 82 Figure 5.5: A: Schematic for sorting microtubules by polarity. Microtubules accumulate at the center of the electrode with their minus-ends aligned along the long axis of electrode. Microtubules of opposite orientations move in different directions resulting in sorting of microtubules for polarity. B: Dynamic asters formed as microtubules walk off the edges of electrodes. (Arrows mark the end of electrodes). 84
16 xvi Figure 5.6: Polarized dynamic asters formed at electrode edges. Microtubules accumulate at the edges of the electrode. Conventional kinesin motors patterned on electrodes capture and pull microtubules, with their minus-ends facing the edge, inwards while leaving the oppositely oriented microtubules alone Figure 5.7: Assembling spindle-like microtubule structures A: Schematic of approach B: Assembled microtubules with their minus-ends (red) bound to electrodes and plusends overlapping in the center (green) 86 Figure 6.1: A: Electroosmotic accumulations of microtubules in microchannels (locations marked by arrows) at 100 khz 150V P-P AC field in BRB12 buffer B: Complex flow patterns resulting in the formation of vortices (pointed by arrows) at 1 MHz 150 V P-P AC field. Scale bar 20 µm.. 92
17 ACKNOWLEDGEMENTS xvii I would like to thank my advisor William Hancock and co-advisor Thomas Jackson and my committee members for the support and guidance throughout the course of graduate studies. I would also like to thank members of Hancock Lab and Jackson Lab for assistance with experiments. Finally I would like to thank my family members for their patience and support.
18 FOREWORD xviii Most of the work presented in this thesis was done in close collaboration with Ying-Ming Huang, graduate student in Electrical Engineering advised by Thomas Jackson. The results and the text of Chapters 2, 3 and 4 were previously published in these following papers: Uppalapati, M., Y.M. Huang, T.N. Jackson, W.O. Hancock. Microtubule alignment and manipulation using AC electrokinetics, Small (Accepted) Uppalapati, M., Y.M. Huang, T.N Jackson, W.O. Hancock Enhancing the Stability of Biomolecular Motors for Microscale Transport Applications, Lab-on-a-chip, 8: Huang. Y.M., M. Uppalapati, T.N. Jackson, W.O. Hancock Microtubule transport, concentration and alignment in enclosed microfluidic channels, Biomedical Microdevices 9(2): ( shared first author)
19 1 Chapter 1 Introduction: Nanotechnology of kinesins and microtubules 1.1 Introduction One of the outstanding problems in nanotechnology is the difficulty of manipulating and transporting materials at the micro- and nano-scales. Motor proteins, such as kinesins, are ideal for active transport of materials at this size range. Kinesins are microtubule based motor proteins that play a major role in intracellular transport of cargo and cell division in eukaryotic cells. These motor proteins utilize the chemical energy of ATP hydrolysis to walk on protein filaments, called microtubules. Kinesins and microtubules can be purified and the motor-driven motion can be reconstituted in vitro. By incorporating kinesins and microtubules in synthetic environments, the transport abilities of these proteins can be utilized for applications in biological sensing, microactuation and nano-scale manipulation of materials. In addition, nanotechnology can be used to develop tools to study the cellular function of kinesins and microtubules. This chapter reviews these promising applications and the progress towards realizing these goals. 1.2 Kinesins and Microtubules Eukaryotic cells use motor proteins for intracellular trafficking of organelles and macromolecules. These proteins also play an important role in organizing the mitotic spindle and generating critical movements necessary for segregating chromosomes
20 2 during cell division. Motor proteins move along the cytoskeletal filament rails namely, actin filaments and microtubules. Microtubules are hollow cylindrical protein polymers, 25 nm in diameter (See Figure 1.1). The subunits of microtubules are heterodimers of α- and β-tubulin. These subunits associate in a head-to-tail fashion to form linear protofilaments that associate laterally to form hollow cylindrical polymers (Desai and Mitchison, 1997). The head-to-tail attachment of the subunits makes the microtubules polar structures. This polarity is evident from the polymerization rates at the ends: the faster growing end is called the plus end (β-tubulin exposed) and the slower growing end is called the minus end (α-tubulin exposed) (Desai and Mitchison, 1997). This polarity is key for directionality of microtubule based motor proteins namely, kinesins and dyneins. - end + end Figure 1.1: Microtubules are non-covalent polymers of tubulin heterodimers which form a hollow cylindrical filament with 25nm diameter (Picture source: Conventional kinesin is the first discovered and most studied member of the kinesin superfamily (Hirokawa and Takemura, 2004). It is a heterotetramer with two identical heavy chains and two light chains. (See Figure 1.2) The heavy chain has a motor domain followed by a neck-linker region and α-helical coiled coil region. The
21 3 heavy chains dimerize to form the functional motor protein with two motor domains followed by rod-like coiled coil structure (Hirokawa et al., 1989). The motor domains are on the N-terminus and the light chains bind to the C-terminus of the heavy chains. The C-terminal tail and the light chains bind to cargo and different isoforms of light chains are present to allow variation in cargo binding (Hirokawa, 1998). The motor domain contains the microtubule binding site and the ATP binding site. The two motor domains bind alternately to adjacent tubulin subunits and use the energy of ATP hydrolysis to walk along a single protofilament towards the end of the microtubule (Hackney, 1994a). Kinesin takes hundreds of steps before detaching from a microtubule (Block et al., 1990; Howard et al., 1989) and can sustain a load of upto 6pN (Svoboda and Block, 1994). The motors proteins have high torsional flexibility and can swivel freely to bind microtubules (Hunt and Howard, 1993). This property allows several motors attached to the same cargo to work together, irrespective of their orientation, thereby generating sufficient forces to move large cargo. Figure 1.2: Conventional kinesin is a tetrameric protein with identical heavy chains and light chains (Lodish et al., 2007).
22 1.3 In vitro assays of kinesin-driven movement 4 The kinesin-microtubule transport system can be reconstituted in vitro using purified components. Drosophila melanogaster kinesin heavy chain can be expressed and purified from bacteria (Yang et al., 1989) and tubulin can be purified from bovine brains (Williams and Lee, 1982) and labeled with fluorescent dyes (Hyman et al., 1991). The motion generated by these proteins can be visualized using the bead assay (Block et al., 1990) or the microtubule gliding assay (Hancock and Howard, 1998). In the microtubule gliding assay, the cellular geometry is inverted, here the motors are adsorbed on the surface and microtubules bind and move on the kinesin coated surface. The microtubule movement can be observed using fluorescence microscopy or DIC (Differential interference contrast) microscopy with background subtraction. In the bead assay geometry, motor coated beads or fluorescently labeled motors walk on microtubule rails immobilized on surface (see Fig.1.3). These assays have generated a large body of biophysical understanding of motor proteins. Additionally, they serve as starting points for harnessing the kinesin-microtubule system for in vitro microscale transport applications Figure 1.3: Microtubule motility assays. Left: In the microtubule gliding assay, microtubules are visualized moving over a lawn of motor proteins absorbed to the microscope coverglass. Right: Single, fluorescently labeled motor proteins can be visualized moving along microtubules by total internal reflection microscopy in the single molecule motility assay (Rogers and Scholey, 2004).
23 1.4 Harnessing kinesin driven motion 5 Kinesin-microtubule transport can be reconstituted in vitro using the microtubule gliding assay or the bead assay as discussed in the above section. The realization of active transport, using the bead assay geometry, has potential applications in manipulation of materials at the nanoscale. By laying microtubule tracks, cargo can be transported to specific locations. In addition, several motors bound to the same cargo can cooperate to generate sufficient forces to move large cargo. This property can potentially be used for microscale actuation where motors can move parts, such as valves in microfluidic devices In the gliding assay geometry, motors are immobilized on the surface and microtubules bind to motors and are pushed along the surface. Depending on the density of motors used several motors interact with the same microtubule providing forces proportional to the length of the microtubule. Since multiple motors are involved, microtubules move a long distance before detaching. Compared the bead assay where diffusion plays more of a role, the motility in the gliding assay is more robust and therefore a better alternative for harnessing kinesin driven motion for transport applications. There has been considerable interest in utilizing kinesin driven microtubule motion for transport applications in lab-on-a-chip devices (Hancock, 2006; Hess, 2006). Lab-on-a-chip devices are diagnostic/sensing devices based on microfluidics. There are several advantages of incorporating motors in hybrid microfluidic devices. First, motors can transport cargo against concentration gradients. Second, no bulk fluid flow or pumping mechanisms are required, easing the fabrication of such devices. Third, microtubules/motors can be modified to bind specific cargo, thereby can be used for biosensing and separation. The potential for such devices is illustrated in
24 6 Figure 1.4, which shows a design plan for a biosensor/ separation device for detecting viral RNA. Here microtubules act as mobile probes that can bind, transport, concentrate and separate cargo (in this case RNA) from bulk fluid. Figure 1.4: Schematic for biosensor/bioseparation device based kinesin-driven transport (Jia et al., 2004) Kinesins as cargo carriers: bead assay geometry Kinesin motors can be functionalized with synthetic cargo and can potentially be used for microscale manipulation of materials, provided we can define organized microtubule tracks. In eukaryotic cells, microtubules are organized in an isopolar manner where all the minus-ends are organized into centrosome and plus-ends pointing towards the cell wall. This organization allows the transport of intracellular cargo from the center of the cell to periphery by plus-end directed motors and vice-versa for the minus-end directed motors. In the bead assay geometry, microtubules are immobilized on the
25 7 surface and kinesin motors move on those immobilized tracks, similar to the geometry in the cell. However to obtain directed transport in vitro microtubules need to be immobilized with a proper orientation. In addition, to immobilizing microtubules in an isopolar manner a sufficient density of microtubules is required. The average run length of conventional kinesin motor is ~1 µm (Block et al., 1990; Howard et al., 1989), after which the motor detaches, diffuses and rebinds to microtubules. Low density of microtubules increases the diffusion component and therefore undermines active transport by kinesin motors. A key advantage of getting high densities of isopolar microtubules is that multiple motors on the same cargo can bind microtubules and forces can be summed up to move large objects. This has applications in micro-actuation for moving micro/nanofabricated structures in miniaturized devices. Progress towards realizing these goals are discussed below. Functionalization of cargo Kinesin motors can be adsorbed nonspecifically on a variety of materials including glass and metal surfaces. However the surfaces should be passivated with casein to prevent binding of kinesin head domain to the surface. Specific binding of cargo can be achieved by replacing the kinesin tail with biotin (Berliner et al., 1994) and bind cargo using the avidin-biotin chemistry. Muthukrishnan et.al(muthukrishnan et al., 2006) functionalized motors with quantum dots derivatized with biotinylated PEG and bound them to biotinylated kinesin using neutravidin. A wide variety of proteins as well as DNA oligomers can also be functionalized with biotin and therefore can be attached as cargo to the kinesin motor.
26 8 Microtubule Immobilization Surface properties are important for immobilizing microtubules, while preserving their function. Microtubules bind to hydrophobic surfaces, however strongly hydrophobic surfaces do not support kinesin motility (Turner et al., 1995). Microtubules can be immobilized on positively charged surfaces such as polylysine and aminosilanes, since tubulin is negatively charges at neutral ph, with good kinesin motility (Turner et al., 1995). Another method for immobilizing microtubules is to use surface bound kinesins to bind them and cross-link the motors and microtubules using glutaraldehyde (Prots et al., 2003). Alternatively rigor mutant motors which bind microtubules irreversibly (Nakata and Hirokawa, 1995) can also be used to attach microtubules to surfaces. Isopolar organization of immobilized microtubules In flow conditions where hydrodynamic shear flows dominate Brownian motion, rigid filaments like microtubules align with the flow direction (Limberis et al., 2001). Limberis et. al (Limberis et al., 2001) used an antibody specific to the end of the microtubule to bind them to the surface while the use of a pluronic surfactant prevented non-specific binding of microtubules the surface. Flow was used to align the free floating plus-ends in the same direction and 0.2% methyl cellulose was used to increase the viscosity of the solution to prevent the microtubule from diffusing back once the flow was stopped (See Figure 1.5). This resulted in 90% of +ends of microtubules pointing towards the flow direction.
27 9 Figure 1.5: Fluid flow aligns free floating plus-ends of microtubules in the direction of flow (Limberis et al., 2001) Brown et. al (Brown and Hancock, 2002) patterned APTES (3- aminopropyltriethoxy silane) surfaces to bind microtubules in the patterned region. These microtubules were extended exclusively at their plus-ends by polymerizing free tubulin under conditions where no new microtubules can be formed. NEM (N-ethyl maleimide) modified tubulin was used to block growth from the minus-ends of seed microtubules. After sufficient growth, the plus-ends of the microtubule extended into unpatterned area where they were free in solution. Flow was used to align these free plus-ends. After the alignment, kinesin motors were introduced to tack down these microtubules to the surface, as they bind both to the surface and microtubules, and kinesins and microtubules were crosslinked using glutaraldehyde. This process resulted in a tight array of aligned microtubules organized in an isopolar manner (See Figure 1.6) In another approach, Prots et al. (Prots et al., 2003) demonstrated isopolar organization of microtubules using the gliding assay geometry and fluid flow to reorient the direction of their movement. In the gliding assay, surface immobilized kinesins bind microtubules and push them along the surface with their ends leading. By reorienting
28 10 Figure 1.6: Tight arrays of isopolar microtubules (Scale bar 5 µm)(brown and Hancock, 2002) the direction of microtubule movement with fluid flow, all of the microtubule minus-ends face downstream of the flow direction. After alignment, the microtubules were held in this orientation by crosslinking them to the motors using glutaraldehyde. Drawbacks and Scope for Improvement Since all the techniques described for obtaining isopolar organization involve fluid flow, the geometric shape of microtubule tracks are limited to those that enable bulk fluid flow. Strategies for getting isopolar organization independent of fluid flow would allow the development of more complex patterns. While long distance transport of large cargo by multiple motors for nearly ~5mm (Limberis et al., 2001) has been demonstrated, single motors detach frequently. The density of microtubules achieved by the above techniques still needs improvement to achieve long distance transport by single motors.
29 Microtubules as cargo carriers: gliding assay geometry In the gliding assay, because the motors are flexible(hunt and Howard, 1993) they can pivot and bind to microtubules. Thereby the direction of microtubule motion is determined by the orientation of the leading end. Since the ends of microtubules are normally randomly oriented, microtubules move in all possible directions. For harnessing microtubule motion for device applications, it is important to achieve long distance directed transport of microtubules. In order to obtain net directional motion it is important to reorient the microtubules in an isopolar manner, such that all the microtubules move in the same direction. The progress towards achieving control over microtubule motion and transport of cargo is described below. Functionalizing microtubules with cargo Biotinylated tubulin (Hyman et al., 1991) can be prepared from established protocols. Using avidin-biotin chemistry, a wide variety of cargo, including nanoparticles (Bachand et al., 2004) and DNA oligomers (Muthukrishnan et al., 2004) can be functionalized on biotinylated microtubules prepared from biotinylated tubulin. While heavily loading microtubules with cargo can impede the motility, moderate loading of cargo does not affect motility. (Bachand et al., 2005) Raab et. al (Raab and Hancock, 2008) demonstrated the functionalization of microtubules with molecular beacons which can sense the binding of DNA/RNA molecules. Molecular beacons are made of hairpin DNA oligomers containing a fluorescent donor and acceptor on the two ends of the oligomer. In the normal state because of the hairpin structure the close proximity of donor and acceptor results in quenching of donor fluorescence. The binding of DNA/RNA results in opening of the
30 12 hairpin thereby increasing the donor fluorescence. In another study Hirabayashi et al. (Hirabayashi et al., 2006) functionalized microtubules with malachite-green which shows enhanced fluorescence when it binds to RNA containing the malachite green binding aptamer sequence. These techniques for detecting RNA are useful for biosensor devices for detection of presence of viruses. However, for bioseparation applications the ability to release cargo is important. The previous approaches that use biotin-avidin chemistry to bind cargo, permanently attaches the cargo to the microtubule. Taira et al. (Taira et al., 2008) demonstrated the ability to release cargo by functionalization of microtubules with DNA oligomers and nanoparticles with the complimentary DNA sequence, so that the cargo can bind by hybridization of DNA oligomers. The cargo was then unloaded by cleaving the DNA with a restriction enzyme. Redirecting microtubule motion using physical barriers It has been shown that microfabricated surface features such as photoresist walls can redirect microtubule motion (Cheng et al., 2005; Clemmens et al., 2003; Hess et al., 2003; Moorjani et al., 2003; van den Heuvel et al., 2005b). Using narrow channels, with motors functionalized only on the surface between channel walls, the microtubule movement can be confined effectively to two directions (see Figure 1.7). By designing special patterns in these channels, the bidirectional motion can be made unidirectional (van den Heuvel et al., 2005b) (See Figure 1.8). Selective functionalization of motors only in the region between the channel walls was achieved by using ampiphilic surfactant molecules that selectively bind to the hydrophobic photoresist walls while enabling motor functionalization in the hydrophilic region between the walls.
31 13 Figure 1.7: Microtubule movement confined within narrow SU8 photoresist channels (Moorjani et al., 2003) Figure 1.8: Unidirectional motion of microtubules can be obtained using rectifier patterns in photoresist channels. The rectifier allows microtubules moving from left to right (A) while microtubules moving from right to left (B) are redirected (Hiratsuka et al., 2001) However, microtubules can detach from the kinesin coated surface and diffuse away after colliding with physical barriers (Moorjani et al., 2003; van den Heuvel et al., 2005b). The result is that microtubules are continually lost from the surface, making it difficult to obtain high densities of oriented microtubules or to achieve long distance transport. Even microfabrication approaches that achieve excellent directional uniformity have been reported to lose up to half of the microtubules over time. (van den Heuvel et al., 2005b). Three-dimensional confinement of kinesin-driven microtubule transport is
32 14 necessary to prevent microtubule loss. In addition, enclosed microchannels are essential for developing hybrid lab-on-a chip devices using motor-based transport of molecular or cellular cargo in microfluidic channels. Partial three-dimensional confinement, achieved using undercut channels, was shown to reduce microtubule loss, (Clemmens et al., 2004) but this approach is incompatible with microfluidics applications because the channels are open to bulk solution. Redirecting microtubules using electric/magnetic fields Microtubules are negatively charged at neutral ph. In DC electric fields microtubules undergo electrophoresis and move towards the +ve electrodes(jia et al., 2004). DC electric fields have been used to redirect microtubule gliding towards the +ve electrodes (Kim et al., 2007). van den Heuvel et al. (van den Heuvel et al., 2006) demonstrated the utility of this technique by using DC fields to sort microtubules moving in three-dimensionally confined microchannels based on their fluorescence, (see Figure 1.9) which is an important step towards realizing bioseparation devices discussed in Figure 1.6. In AC electric fields, dielectrophoretic forces act on particles depending on the polarizability of the particles compared to the surrounding medium. Microtubules experience dielectrophoretic forces in non-uniform fields and torque in uniform electric fields (Böhm et al., 2005; Jia et al., 2004; Minoura and Muto, 2006). Therefore dielectrophoretic forces, in principle can be used for controlling microtubule motion but this has not yet been demonstrated. Microtubules can be functionalized with magnetic nanoparticles using biotinavidin chemistry(platt et al., 2005). Hutchins et al.(hutchins et al., 2007) functionalized microtubules at their leading ends with magnetic nanoparticles using segmented
33 15 microtubules with biotinylated tubulin at the minus end and normal tubulin at the plusend. Segmented microtubules were obtained by extending biotinylated microtubule seeds exclusively from their plus-ends using NEM tubulin. The heavy loading of the biotinylated segement with nanoparticles prevented the segment from binding while the normal tubulin segment bound to kinesins. Magnetic fields were used to redirect the free leading end of the microtubule to redirect microtubule motion. Figure 1.9: Demonstration of molecular sorting. (A) Color image of a mixture of red- and green labeled microtubules approaching a Y junction. Electrical force is used to steer microtubules carrying green and red fluorophores into the right and left reservoirs, respectively. (B) Example of successful sorting events for a green- and a red-labeled microtubule. As a function of time, first a green microtubule is steered into the right reservoir (t e 10 s), and subsequently a red microtubule is sent into the left reservoir. (van den Heuvel et al., 2006) Spatial and temporal control of microtubule motility The ability to turn the motility on and off both spatially and temporally has added utility in devices based microtubule transport. Hess et al. (Hess et al., 2001) demonstrated that caged ATP can be used as fuel and can be activated by UV light. Therefore motion can be initiated by treating the sample to UV light to turn on the device. Once the UV light is turned off, the motion continues until the generated ATP is consumed. Hexokinase can be used to convert ATP to ADP to turn off the motility more
34 16 rapidly. When the sample was blanket exposed to UV though, the turn on time is fast, the turn off time is in order of hours without hexokinase and order minutes with hexokinase. In a recent report Tucker et al. (Tucker et al., 2008) improved on the temporal resolution of switching motility by localizing the UV illumination to 15 µm or 25 µm. This results in activation locally around the illumination spot (See Figure 1.10). When the UV light is turned off, the motility stops in 10 s without hexokinase and 1s with hexokinase. This improved temporal resolution is due to the rapid diffusion of ATP, leading to its dilution in the surrounding solution. However, for device applications larger activation areas are required thereby reducing the temporal resolution. The spatial resolution of activation depends on the time of exposure and diffusion of activated ATP from the exposed area. The use of hexokinase improves the spatial resolution as it acts as a sink for diffusing ATP. Yokokawa et al. (Yokokawa et al., 2004), used a microfluidic approach to flush ATP and hexokinase sequentially to turn on and turn off motility. However, the temporal resolution in this case is dependent on the flow rate and the size of the channel. In another approach Nomura et al. (Nomura et al., 2006) used caged peptides derived from kinesin C-terminus sequence. The C-terminus of kinesin is known to interact with the head domains to inhibit motility. Therefore, by manufacturing caged peptides that can be activated by UV light, the motility can be turned off by UV exposure. However in this case the motility was not completely turned off, it only resulted in a reduction of velocity. Motility can also be turned on and off by controlling the surface properties. Ionov et al. (Ionov et al., 2006) demonstrated reversible switching of microtubule motility using a thermoresponsive polymer. In this study kinesin molecules were adsorbed onto PNIPAM (poly(n-isopropylacrylamide)) grafted silicon substrates, resulting in kinesin
35 17 Figure 1.10: Localized activation of microtubule motility by UV light spot. Left: Two images (pseudocolored in green and red and separated by 200 s in time) are overlaid, showing the illumination zone and the movement of microtubules with radius-dependent velocity, due to the ATP sequestration by hexokinase in solution. Right: Velocity profile plotted as a function of distance from UV spot and hexokinase concentration (Tucker et al., 2008) binding in the interstices between PNIPAM. PNIPAM chains are in an extended configuration below 32 O C and in a compact folded state above this critical temperature. The extended configuration sterically hinders microtubule binding, while the compact state allows microtubule binding, resulting in motility. Therefore by switching between 27 O C and 35 O C, the microtubules bind and unbind, thereby enabling switching of motility. In another study Martin et al. (Martin et al., 2007) used the doping state of a conductive polymer (poly(ch2oh-edot)) to modulate kinesin motility. When the polymer was electrochemically switched from a dedoped state to a conducting doped state, the
36 velocity of microtubule gliding on this surface decreased by 35% and this process was reversible Long term stability and preservation of motility assays The progress towards developing devices powered by kinesin motors is promising, but a key limitation of such devices is their stability over time. Proteins degrade, denature and lose function over time when stored at room temperature. Yokokawa et. al(yokokawa et al., 2005) demonstrated that motility could be cryopreserved at -85 o C for 30 days when 20% sucrose was used as a cryoprotectant. However, for device applications storing at -85 o C requires the end user to have access to deep freezing equipment. Seetharam et al. (Seetharam et al., 2006) demonstrated that lyophilizing the samples can help preserve motility for 24 days when stored at room temperature. However, only 50% of the microtubules were motile in these storage conditions. Clearly better methods of preservation are required for realization of biomotor powered devices. 1.6 Nanotechnological tools to study kinesins and microtubule cell biology While the kinesin-microtubule system has applications in nanotechnology, several tools developed through nanoscience can be used to obtain fundamental insights into the cell biology of kinesins and microtubules. Using an artificial in vitro system, Holy et al. (Holy et al., 1997) studied the effect of microtubule polymerization forces against the cell cortex on centering of the centrosome within the cell, (See Figure 1.11). They demonstrated the polymerization forces are sufficient to center an artificial
37 19 microtubule aster using a microfabricated enclosed chamber to mimic the confined enivironment of a cell. Shek et al. (Schek et al., 2007; Schek and Hunt, 2005) polymerized microtubules against microfabricated photoresist barriers, whiie trapping the beads attached to the opposing end of microtubule. This enabled observation of microtubule polymerization forces and steps in tubulin assembly at molecular resolution using optical tweezers. In dividing cells, self organization of microtubules and motors leads to the formation of mitotic spindle during cell division. To better understand this complex process, Nedelec et al. (Nedelec et al., 1997) studied the self organization of purified microtubules and bifunctional kinesin motors in confined microfabricated chambers. A large variety of assembled structures including asters and vortices were demonstrated (See Figure 1.12). This is an important step in understanding the complex phenomenon mitotic spindle assembly. 1.7 Thesis outline and objectives The underlying theme of harnessing biomotor driven transport is the ability to organize microtubules in an isopolar manner and to assemble a sufficient number of microtubules to enable transport of significant amounts of cargo. Chapter 2 demonstrates the use of enclosed microchannels to orient microtubules in an isopolar manner and to assemble high densities of microtubules. Chapter 3 addresses the issue of long term preservation of microtubule motility, which is a key limitation of hybrid devices incorporating proteins.
38 20 Figure 1.11: Demonstration of centering of bead by microtubule polymerization forces in a microchamber, an example of an in vitro model to study the role of microtubule polymerization in centering the centrosome with cell. (Scale bar 10 µm, Images 3 min apart) (Holy et al., 1997) Figure 1.12: Self organization of microtubules into asters and vortices( Nedelec et al., 1997) While we and others have shown that DC electric fields can be successfully used to guide microtubule motion, there are several drawbacks including buffer depletion and electrolysis, which can affect motility. These problems can be avoided by using AC electric fields. Chapter 4 investigates the use AC electric fields to manipulate and organize microtubules. In Chapter 5, protein patterning and AC electric fields are used to organize microtubules into bipolar structures similar to their organization in the mitotic spindle.
39 Chapter 2 21 Microtubule transport, concentration and alignment in enclosed microfluidic channels 2.1 Introduction In eukaryotic cells, kinesin motor proteins transport intracellular cargo and provide the mechanical forces underlying mitotic spindle morphogenesis and chromosome separation (Hirokawa, 1998; Sharp et al., 2000). In the microtubule gliding assay,(hancock and Howard, 1998) an in vitro assay for kinesin generated movement, microtubules are propelled along the surface by surface-immobilized kinesin motors. There has been considerable interest in utilizing kinesin-driven microtubule motion for nano/microscale transport applications in lab-on-a-chip devices with microtubules as carrier proteins (Jia et al., 2004; Stracke et al., 2000; Yokokawa et al., 2004). However, to harness this system for useful applications, it is necessary to both control the direction of microtubule motion and to assemble sufficient densities of microtubules. In the gliding assay geometry, the microtubules move randomly in various directions. Since the motors are flexible, they can pivot and bind to microtubules. Therefore the direction of microtubule motion is determined by the orientation of the microtubule minus-ends. The minus-ends are oriented randomly in a typical gliding assay leading to microtubule motion in various directions. A number of groups have shown that microfabricated surface features such as walls and channels can be used to confine, redirect and control the trajectory of kinesin-driven microtubules (Clemmens et al., 2003; Hess et al., 2002; Hiratsuka et al., 2001; Moorjani et al., 2003). However, in most of these demonstrations the top of the channels are open to the bulk solution and because filaments can detach from the kinesin coated surface and diffuse away,
40 22 microtubules are lost from the surface over time (half of the observed population in some designs (Clemmens et al., 2004; van den Heuvel et al., 2005b)). This makes it difficult to obtain controlled motion over long distances or generate high densities of oriented microtubules, which are required for transporting significant amounts of cargo over distances relevant for device applications. Hence it is important to confine microtubule motion three-dimensionally to prevent microtubule loss to bulk solution. Here we demonstrate successful three-dimensional confinement of microtubules in microfluidic channels. This confinement geometry (channel cross-section ~5 μm x ~1 μm) enables long-distance movement (~5 mm) and directional control, prerequisites for developing biomotor-driven transport for hybrid lab-on-chip devices. We further show that constructing a circular ring using these microchannels generates a high density ensemble of isopolar microtubules driven by kinesin motors. These aligned microtubules can be used for microscale transport applications or as a model in vitro system for studying kinesin-driven microtubule organization in cells. 2.2 Materials and Methods Kinesin and microtubules Full-length hexahis-tagged Drosophila conventional kinesin was used for all motility experiments. In some experiments, headless kinesin was used, which is a modified kinesin construct that contains the rod and tail domains and a hexahis tag, but lacks its motor domain.(hancock and Howard, 1998) All motors were expressed in bacteria and purified by Ni column chromatography as previously described.(coy et al., 1999; Hancock and Howard, 1998) Tubulin was purified from bovine brains and labeled
41 23 with rhodamine as previously described.(hyman et al., 1991; Williams and Lee, 1982) Microtubules were polymerized by mixing 32µM rhodamine-labeled tubulin, 4 mm MgCl 2, 1 mm GTP and 5% DMSO in BRB80 buffer (80 mm PIPES, 1 mm EGTA, 1 mm MgCl 2, ph 6.9 with KOH), incubating at 37 o C for 20 min, and then diluting into a solution containing 10 μm paclitaxel. To immobilize motors inside the microchannels, a solution containing 4 mg/ml casein, 7.5 µg/ml conventional kinesin and 32 µg/ml headless kinesin in BRB80 buffer was introduced. This solution was flushed with a second solution containing 0.64µM rhodamine-labeled microtubules, 10mM ATP, 50µM paclitaxel and antifade reagents (0.1M D-glucose, 0.1 mg/ml glucose oxidase, 0.04 mg/ml catalase and 0.35M β- mercaptoethanol) in BRB80 buffer. These concentrations are five to twenty times higher than our standard levels (Moorjani et al., 2003), to compensate for the low volume to surface area of the microchannels. Microscopy and image analysis Movement of the confined microtubules was observed by epifluorescence microscopy (Nikon E600, 100x 1.3 N.A. objective). Images recorded on videotape were digitized using Scion Image (Scion Corporation). The intensity values were then corrected for the gamma function of the camera (Genwac GW-902H) and the background noise was subtracted using Image J. To estimate the microtubule density in the microchannels, the integrated fluorescence intensity across the channel was measured and compared to the intensity of single isolated microtubules.
42 2.3 Results and Discussion 24 Fabrication of microchannels In order to confine microtubules, we developed an approach for fabricating enclosed microchannels that are compatible with kinesin motors and microtubules, enable fluorescence imaging of microtubule movement, and provide fluidic connections for sample introduction. The design consists of a three tier hierarchical structure (Figure 2.1) that links microscale transport channels to macroscopic fluid connections. Shallow microchannels (~5 m wide and ~1 m deep) for microtubule motility experiments connect to intermediate channels (~100 μm wide) that serve as reservoirs and also connect to 250 μm deep macrochannels that hold fine gauge tubing for simple external fluid connections. The micro, intermediate, and macro scale channels are etched in a Schott D263 glass substrate and bonded to a cover glass using polymethyl methacrylate (PMMA) as an adhesive. Controlling motor density in enclosed channels In the microtubule gliding assay the characteristics of microtubule transport are influenced by the density of kinesin motors immobilized on the surface. While the transport speed is virtually unchanged over three orders of magnitude of motor density, the rate that microtubules land on the motor functionalized surface and the distance they move before detaching and diffusing away both vary strongly with the motor density (Hancock and Howard, 1998; Howard et al., 1989). Furthermore, there is an optimal
43 25 Macrochannel Intermediate channel Microchannel 2.5in X2.5in 600µm 30µm Figure 2.1: Hierarchical microchannel design. Macrochannels (250 μm deep) enable sample introduction. Intermediate channels (100 μm wide and 1 µm deep) connect to microchannels (5 μm wide and 1 μm deep) where microtubule motility is observed. Bottom panel shows a completed sample including the coverglass bonded using PMMA adhesive, and tubing for sample injection. 20 µm 20 µm Figure 2.2: Eliminating kinesin motor gradients using a headless kinesin construct. Left: Image of an intermediate channels when only full-length motors are used. The microtubules bind avidly to the macro channel (to the left of the intermediate channel) and the proximal portion of the intermediate channel (left side of the image). The number of microtubules entering microchannels (located to the right of the intermediate channel) is therefore very small. Right: When full-length kinesin motors are combined with headless kinesin, the motor density is reduced and the gradient in the intermediate channel is eliminated. The edge of the macro channel can be seen at left.
44 26 motor surface density for redirecting kinesin-driven microtubules using microfabricated walls. At low motor densities collisions with walls tend to cause microtubules to detach from the surface and diffuse away, while at high motor densities microtubules stall when encountering sharp features and vertical walls, which are needed for proper redirection (Moorjani et al., 2003). Compared to open top channels, enclosed microchannels reduce the impact of both of these factors. At low motor densities, microtubules that detach can rapidly rebind. Further, because motors are present on all internal surfaces and there are few sharp edges, microtubules rarely stall and are faithfully redirected even at very high motor densities. However, while motor adsorption is homogeneous with open channels, the high surface to volume ratio of enclosed channels results in motors preferentially adsorbing to the upstream end, creating a gradient in motor adsorption along the channels. High uniform motor densities can be achieved throughout the device by flowing large volumes of motor solution through the channels. However, high motor densities in the intermediate channels result in rapid attachment of microtubules at the junction between the macro channel and the intermediate channel. The junction thus acts as a filter, preventing microtubules from reaching the distal end of the intermediate channel and the microchannel. Figure 2.2 A shows the steep gradient of microtubule binding in the intermediate channel adjacent to the macro channel junction. Because this filtering effect is more severe for long microtubules, only short microtubules are seen in the intermediate channels (Figure 2.2 A). Over time, the long microtubules form a dense network that prevents any microtubules from entering the intermediate channel. We solved this motor gradient problem using a modified kinesin that includes the rod and tail domains that mediate motor interactions with surfaces, but lacks the motor domain and hence does not bind microtubules (Hancock and Howard, 1998). When this
45 27 headless kinesin protein is introduced into the enclosed channels along with the fulllength functional motors, it competes for surface binding with the functional motors, and hence limits the number of functional motors that can adsorb onto the surface. When 32 μg/ml headless motors were combined with 7.5 μg/ml functional kinesin motors and flushed into the channels, the functional motor density was optimal for long distance transport and no motor gradient was apparent (Figure 2.2 B). Hence, despite the high surface area inherent in these small enclosed microchannels, by varying the ratio of headless motors to functional motors, the functional motor density can be specified over a wide range of densities and optimal motility can be achieved throughout all regions of the device. Confinement and redirection in enclosed microchannels The confinement geometry in the microscale channels effectively confines microtubules to move in two directions (towards entry and exit points). To transform bidirectional motion into unidirectional motion, we designed a rectification pattern (Figure 2.3) that permits microtubules entering from one end to pass through, while redirecting microtubules moving from opposite end. Figure 2.3 (a) shows representative paths of two microtubules in the rectifier pattern. Filaments entering from the right (grey traces) buckle at the base of the rectifier, travel leftward in one or the other arms and exit from the left, while microtubules entering from the left (black traces) continue through the entire circuit and exit from the left. To show the rectification ability of the structure, microtubules were introduced from left entrance and observed traveling through the pattern; 96% (N=50) of microtubules entering from the left were successfully redirected and exited to the left. Importantly, even though microtubules occasionally detach from
46 28 the motor-coated surface, they remain in the channel and reattach within a few seconds. Also the small confinement geometry (5µm x 1 µm) prevents the dissociated microtubules from reorienting in the opposite direction. Movement is observed on not only the bottom and sidewalls of the channel, which are glass, but also the top, which is coated with a layer of PMMA. 20 µm (a) (b) Figure 2.3: Directional rectifier constructed using enclosed microfabricated channels. (a) Expected paths of microtubules in the rectifier pattern (gray traces follow microtubules moving in desired direction, black traces follow microtubules moving in opposite direction. (b) Microtubules entering from reservoir at left (not shown) travel down either arm and continue through to the other arm, reversing their direction. Microtubules entering from the right collide with the base of the structure and are directed into one of the two arms. Long term confinement and concentration of microtubules in a circular ring In harnessing microtubule based transport and in developing experimental models of complex cellular processes, one recurring challenge has been concentrating significant numbers of microtubules that are parallel and uniformly oriented. A number of approaches have been investigated including immobilizing short microtubule seeds
47 29 and growing filaments preferentially off of one end (Brown and Hancock, 2002), binding microtubules to functionalized surfaces through one end and using flow to push them onto the surface (Limberis et al., 2001), and reorienting kinesin-driven microtubules using fluid flow or electric fields (Prots et al., 2003; Stracke et al., 2002; van den Heuvel et al., 2006a). Furthermore, microfabricated open top structures have been shown to assemble moderate numbers of parallel and uniformly oriented microtubules (Clemmens et al., 2004; Hess et al., 2002; Lin et al., 2006), and the rectifier described above acts to collect uniformly oriented filaments. However, we sought to create a channel geometry that sorts and concentrates dense bundles of uniformly oriented microtubules as a tool for transport applications and to mimic cellular microtubule architectures. We created a microtubule storage ring designed to collect and concentrate uniformly aligned microtubules driven by kinesin motors (Figure 2.4). As with the directional rectifier, motors were adsorbed and observed to be functional on all interior surfaces. Blocking motor activity on the side walls, a prerequisite for open channels (Cheng et al., 2005; Hiratsuka et al., 2001; Moorjani et al., 2003), is not required. The design consists of a circular ring with entry and exit channels that connect to reservoirs (intermediate channels) 100μm wide where microtubules are introduced. Microtubules that are transported into the 60 μm diameter circular track from the adjoining reservoir are guided in a counterclockwise direction by the curved walls, while those moving clockwise typically exit the structure within one half revolution (Figure 2.4). Microtubules that detach from the surface rebind and continue movement. Rectifiers are included on each end of the ring to return any microtubules that escape the ring (Figure 2.5). Because microtubules continuously enter from the reservoirs at either end, the number of oriented microtubules builds over time, resulting in a dense bundle of aligned
48 30 microtubules after 30 minutes, much higher density than has been demonstrated for patterns in non-enclosed undercut channels (Clemmens et al., 2004; Lin et al., 2006). The structure can be continually monitored under the microscope, and we found that microtubules are retained and continue to move for more than 90 minutes. Intermediate channels Concentration ring Rectifiers 200 µm Figure 2.4: SEM image of the microtubule storage ring. Microtubules originate in the intermediate channels and are transported by immobilized kinesins into the microchannels. The microchannels are designed so that filaments traveling counterclockwise in the ring are retained, while any traveling clockwise should exit the ring, be reversed by the rectifiers and return in a counterclockwise orientation Early after introducing microtubules, individual filaments can be observed being guided around the ring, but at later times the microtubules are sufficiently dense to prevent simple counting. To determine the number of microtubules present after 90 minutes, the fluorescence intensity was integrated across the channel, and the value divided by the integrated intensity of an individual microtubule (see Methods for details). This image analysis indicated that the ring cross section contains 162 ± 40 microtubules (mean SD, N = 6 cross-sections). Assuming an average microtubule length of 10 µm, the total number of microtubules in the ring is approximately Based on their
49 Intensity Intensity typical speed of ~0.8 μm/s, microtubules present in the ring for the entire 90 minutes move a distance of 5 mm µm 20 µm A B Single microtubule peak Pixel Pixel C D Figure 2.5: Microtubule movement and concentration in a microfabricated ring. A: Microtubule movement shortly after injecting microtubules into the device. Microtubules are moving in both directions in this case. B: Image of concentration ring after 90 min of accumulation; almost all microtubules are moving counterclockwise in this case. C and D: Fluorescence intensity profiles taken along lines in panel B, for estimating the number of microtubules in the ring. Single microtubule peaks in panel C were obtained in a sparsely populated area, and together with the integrated fluorescence cross-section in panel D used to calculate the number of microtubules in the ring.
50 32 A second intriguing observation was that despite the large surface to volume ratio in the channel, the kinesin-driven transport continued for over 90 minutes, meaning the ATP fuel is not being used up. The microtubule gliding velocity in the ring shown in Figure 7, was nm/s (mean SD, N = 25) shortly after introducing the microtubules and nm/s after 90 minutes. Data from Schief et al (Schief et al., 2004), who measured the gliding velocity of conventional kinesin at a range of ATP and ADP concentrations, indicate that with an initial concentration of 10 mm ATP, conversion of ATP to ADP will cause a proportional decrease in the gliding velocity. This small reduction after 90 minutes of operation implies there is a ~5% decrease in ATP from 10 mm to 9.5 mm. Because of the small volumes and continuous consumption of ATP by the motors, ATP depletion (and the accompanying ADP buildup) would be expected to be a limiting factor in the lifetime of this device. However, we show here that this is not the case because diffusion of ATP from the reservoirs formed by the intermediate channels is sufficient to replenish ATP consumed in the microchannel ring. The first question is: at the 90 minute time point when ~3000 microtubules are in the ring, what is the ATP consumption rate? By comparison to standard microtubule gliding assays in flow cells (Hancock and Howard, 1998), we estimate that microtubules are being propelled by roughly 10 motors each. Biochemical assays have shown that conventional kinesin hydrolyzes approximately 100 ATP per second when walking along microtubules and roughly 0.01 ATP per second when not interacting with microtubules (Hackney, 1994b). This low fuel consumption when motors are not engaged with microtubules is one important factor slowing the ATP depletion. The volume of the ring (a 60 μm diameter annulus of width ~5 μm and depth ~1 μm) is approximately 1 pl, and the initial ATP concentration is 10 mm, meaning the ring contains 10 fmol or 6 x 10 9 molecules of ATP.
51 For 3000 microtubules with ten motors moving each microtubule, the motors would be 33 predicted to burn 3 x 10 6 molecules of ATP per second, which means that without replenishment all of the ATP would be hydrolyzed in roughly 30 minutes. In our design, the circular ring is connected to intermediate channel reservoirs (100 μm wide and 2 mm long) by microchannels that are 5 μm wide, 1 μm deep and 200 μm long. Given the ATP consumption rate calculated above, we can calculate that diffusion of ATP through this 200 μm connecting channel is sufficient to maintain high concentrations in the ring. Using a diffusion constant D = 3 x m 2 /s for ATP (Rostovtseva and Bezrukov, 1998) and taking a maximal concentration gradient of 10 mm in the intermediate channel and zero in the ring, the maximum flux from the reservoirs into the ring is 9 x 10 7 ATP/s, which is 30-times the estimated consumption rate calculated above. Therefore, a concentration gradient of 0.3 mm (leaving a steadystate concentration of 9.7 mm ATP in the ring) should be sufficient to balance the consumption of ATP in the ring with diffusional flux of ATP from the reservoir. Because the reservoir volume is 200 times the ring volume, and the macrochannel volume is 2500 times the reservoir volume, ATP depletion should not be a problem in operation of this device. In addition to diffusion of ATP, small pressure changes in the tubing and macrochannels can cause flow from the reservoirs into the small bore microchannels, replenishing the ATP supply. Based on the 3 x 10 6 ATP/s consumption rate calculated above, a flow of 10 fl/s, corresponding to a linear velocity of 2 μm/s in the 5 μm 2 microchannels, would be sufficient to maintain a concentration of 9.5 mm ATP in the ring. However, because microtubules that transiently detach from the surface move slower than the ~0.8 μm/s motor-driven rate, we don t believe that this degree of bulk flow is present during our measurements, although we can t rule out that slower flows
52 34 are playing a role. In either case the heirarchical design, consisting of microchannels with a small active volume connected to intermediate and macrochannels ensures that active motors in the enclosed microchannels are provided with sufficient fuel for longterm operation. 2.4 Conclusion The kinesin-microtubule system is an intriguing model for biologically driven transport in engineered microenvironments. In cells these motors provide long distance transport of intracellular cargo, and by immobilizing them on surfaces, long distance microtubule transport can be achieved in vitro under a range of experimental conditions. To harness this transport system for lab-on-a-chip analytical devices, it is necessary to encapsulate the motors and microtubules in enclosed microchannels. This work provides a simple and robust strategy for fabricating these microdevices, and demonstrates that kinesin function, microtubule stability, and sufficient ATP levels are maintained in these systems. Furthermore, novel geometries such as the concentration ring enable control of microtubule transport that cannot be achieved using the open top channels employed in most previous studies. Finally, by gaining improved control over the movement, orientation, and number of microtubules, these systems provide novel tools that can be used to probe fundamental issues in subcellular organization. For example, an aligned bundle of microtubules mimics the axon of neurons, which are filled with aligned microtubules to enable intracellular transport to and from the synapse. The mitotic spindle is another structure consisting of a pair of opposed bundles of uniformly oriented microtubules. By combining microtubule binding proteins and other molecular
53 motors with these aligned microtubules, it may be possible to build refined analogs of these cellular structures test minimal models of their assembly and maintenance. 35
54 Chapter 3 36 Enhancing the Stability of Kinesin Motors for Microscale Transport Applications 3.1 Introduction In eukaryotic cells kinesin motor proteins use the energy from ATP hydrolysis to transport intracellular cargo along protein filaments called microtubules. In vitro, kinesin-driven motion can be observed with purified components using the microtubule gliding assay, in which motors are adsorbed on a surface and microtubules are pushed along the surface by these motors (Hancock and Howard, 1998; Rogers and Scholey, 2004). These transport dynamics can be utilized for active transport in devices, with microtubules as carrier proteins, and there has been considerable interest in developing hybrid devices harnessing the mechanical motion provided by these motors. Recent progress towards developing kinesin-driven devices includes functionalization of microtubules with a variety of cargo and control of microtubule motion using microfabricated channels and electric fields (Cheng et al., 2005; Hess, 2006; Hess et al., 2004; Hirabayashi et al., 2006; Hiratsuka et al., 2001; Huang et al., 2007; Jia et al., 2004; Kim et al., 2007; Moorjani et al., 2003; Muthukrishnan et al., 2006; Muthukrishnan et al., 2004; van den Heuvel et al., 2006). However a major issue in integrating these biological components into functional hybrid devices is the limited shelf-life of kinesin motors and microtubules. Motor proteins degrade rapidly (hours to days) (Brunner et al., 2004) when the solution is stored at room temperature, which limits their utility from the standpoint of manufacturing devices. Hence, methods for improving the stability of conventional
55 37 kinesin motor proteins for long term storage are needed. One straightforward approach for storing such hybrid biological devices would be to freeze-dry (lyophilize) them to preserve protein function, and then reconstitute them just before use. By removing the water, which is the medium for most chemical degradation pathways, freeze drying stabilizes proteins. However, protein structure and function can be compromised during both the freezing and the drying transitions, and several chemical degradation reactions such as deamidation, peptide bond cleavage and oxidation can occur in the solid state after freeze drying (Lai and Topp, 1999). In a recent report, Seetharam et al. (Seetharam et al., 2006) established that flow cells containing immobilized kinesins and microtubules can be lyophilized and reconstituted, but after four weeks of storage less than half of the microtubules were still motile, a significant degradation of activity. Critical point drying is another method that is commonly used for drying biological specimens (Burstyn and Bartlett, 1975). The critical point is the state of continuity between the liquid and vapor states where the surface tension is zero. By displacing the aqueous buffer sequentially with acetone and liquid CO 2 and then raising the temperature and pressure of the CO 2 to the critical point where the liquid state ceases to exist, the damaging effects of surface tension that occur in air drying are eliminated. There have been no reports to date using critical point drying for preserving motor proteins immobilized on surfaces. Here, we show that critical point drying is an effective and relatively simple method of stabilizing proteins immobilized on glass surfaces that doesn t require formulations of cryo- and lyoprotectants. Furthermore, this approach can be extended to patterning functional motors on the surface using traditional photolithography approaches of photoresist deposition, patterning, and removal, while preserving motor functionality on the surface.
56 3.2 Materials and Methods 38 Microtubules and Kinesin Drosophila melanogaster conventional kinesin heavy chain was bacterially expressed and purified as previously described(hancock and Howard, 1998). Bovine brain tubulin was purified and rhodamine labeled as previously described(williams and Lee, 1982). Microtubules were polymerized by mixing 32 µm rhodamine-labeled tubulin, 4 mm MgCl 2, 1 mm GTP and 5% DMSO in BRB80 buffer (80 mm PIPES, 1 mm EGTA, 1 mm MgCl 2, ph 6.9 with KOH) and incubating at 37 o C for 20 min. Polymerized microtubules were stabilized with 10µM paclitaxel. Freeze-Drying Flow cells were constructed by attaching a coverslip to a glass slide with double-stick tape and were sequentially incubated with casein solution (0.5 mg/ml casein in BRB80 buffer for 5 min) and kinesin solution (~5 µg/ml kinesin, 1 mm ATP, 0.2 mg/ml casein, 10% glycerol and 5% w/v sucrose in BRB80 buffer) for 5 min each. These flow cells were dried in a lyophilizing container surrounded by dry-ice, which was connected to a vacuum of µm Hg and a trap temperature of -84 o C. The dry ice was allowed to sublime and vacuum was held for 8 hrs. As the dry-ice sublimed, the sample temperature increased, allowing mass transfer of water from sample to trap. These flow cells were stored at 4 o C in a box containing Drierite. Critical Point Drying Glass coverslips were treated with casein (0.5 mg/ml casein in BRB80) and kinesin (~5 µg/ml kinesin, 1 mm ATP, 0.2 mg/ml casein in BRB80 buffer) solutions for 5 min each. The coverslips were dehydrated by sequentially incubating the sample in 50%,
57 39 75% and 100% acetone/ethanol. The samples were then loaded in a closed drying chamber where the acetone/ethanol was displaced by liquid CO 2. The temperature of the chamber was increased from 10 o C to the critical point of CO 2 (31.1 o C, 73 atm pressure) where the liquid vapor interface vanishes. These coverslips were stored at 4 o C in a box containing Drierite. Patterning dried motors Shipley 1811 photoresist (Shipley Corporation) was spin-coated on critical pointdried coverslips and the samples were dried overnight at room temperature. This method contrasts with the standard process where the photoresist is baked at 90 C for 90 seconds to drive off excessive solvent, as these temperatures denature motor proteins. The photoresist was then patterned by UV exposure through a photo mask using a Karl Suss contact aligner. Exposed photoresist was removed by soaking the sample for 60 s in Microposit MF 351 developer diluted 1:4 in DI water, which created regions with no motor activity. Finally, the remaining photoresist was removed by flowing acetone over the sample to expose regions containing functional kinesin motors. Microtubule motility Freeze dried flow cells and flow cells constructed with critical point-dried coverslips were reconstituted with motility solution (~32 nm microtubules, 1 mm ATP, 10 µm paclitaxel, 0.2 mg/ml casein, 20 mm D-glucose, 0.02 mg/ml glucose oxidase, mg/ml catalase, 0.5% β-mercaptoethanol in BRB80 buffer). Microtubule movements were observed by fluorescence microscopy and videotaped for further analysis (Huang et al., 2007). Microtubules shorter than 1 m were excluded from analysis.
58 3.3 Results and Discussion 40 Functionally, kinesin motor proteins have two modes of failure: they can denature completely and lose their ability to bind microtubules, or they can inactivate such that they bind microtubules irreversibly. While complete denaturation can sometimes be compensated for by starting with very high motor concentrations, inactivated motors stall microtubule movement and thereby reduce the functionality of microtubule-based devices. In order to compare the effectiveness of different drying methods in preserving the motor functionality, both the number microtubules per given area and the fraction of microtubules moving were used as metrics for assessing motor function. To assess motor survival after storage, critical point-dried coverslips were used to construct flow cells, motility solution was introduced, and the resulting microtubule movements analyzed. In critical point drying, the aqueous solution must first be replaced by a miscible solvent, which is then replaced by liquid CO 2. When ethanol was used as the dehydrant, very few microtubules were observed, consistent with ethanol denaturing or desorbing the surface-adsorbed kinesin motors. Similar results were obtained when the freezing step was omitted and the aqueous buffer was replaced by ethanol followed by the introduction of microtubule-containing motility solution, indicating that it is most likely exposure to the ethanol that denatures the motors. In contrast, when acetone was used as a dehydrant, numerous microtubules bound to the surface upon reconstitution with motility solution, and a large fraction of them moved. To test the suitability of these critical point-dried samples for long term use, the samples were stored at 4 o C and tested at 3, 12 and 20 weeks. As seen in Figure 3.1, the number of microtubules bound to the
59 41 motor-functionalized surface was roughly constant over the 20 week period, and the fraction of moving microtubules remained near 100%. These data suggest that after critical point drying, storage for 20 weeks results in very little inactivation or denaturation of the surface-adsorbed kinesin motors. Figure 3.1: Microtubule motility in critical point-dried samples. A) The fraction of microtubules moving following different durations of storage at 4 o C. At each time point, 45 video screens [54 m x 70 m] were analyzed from a total of three flow cells. B) The number of microtubules bound per video screen. C) Screen captures showing microtubule movement following 5 weeks of storage. Frames are 4 sec apart, scale bar is 15 µm. As a comparison, kinesin-functionalized coverslips were freeze dried and stored for up to 20 weeks at 4 o C and then reconstituted with motility solution and
60 42 analyzed. The movement of microtubules after storage for 20 weeks is shown in Figure 3.2. As seen in Figure 3.2, the fraction of moving microtubules was high, indicating that few motors were inactivated. In both critical point-dried and freezedried samples, a subset of microtubules moved in a spiral pattern, presumably pivoting around one irreversibly bound motor at their leading end. However the stalled and spiraling microtubules comprised only a small fraction of the bound filaments. The number of microtubules on the surface was somewhat lower for freeze dried than for critical point-dried samples, indicating that a significant fraction of the motors were denatured by freeze drying. With no storage time, the freezedried samples had roughly one-third fewer bound microtubules than the critical pointdried samples (this difference was statistically significant, unpaired t-test P=0.003) and the number fell following storage. These freeze- drying results can be compared to the results from Seetharam et al. (Seetharam et al., 2006) who found that when flow cells containing both kinesin motors and microtubules were freeze-dried, stored at either room temperature or -80 o C and reconstituted, less half of the microtubules moved after 24 days of storage. The high percentage of motile filaments observed here could be a result of introducing fresh microtubules into the flow cells, and because lyophilized microtubules are commercially available *, one strategy would be to reconstitute the microtubules and the motor-functionalized surfaces separately and then combine them. Notably, in both the present experiments and those of Seetharam et al, there was significant heterogeneity observed across different regions of the surface, which may indicate that by optimizing specific experimental variables, these freeze drying results could be improved. * Cytoskeleton, Inc, Denver, CO
61 43 For designing hybrid microscale devices that use the kinesin-microtubule system for transport or actuation, it is important to be able to pattern kinesin motors in specific regions on a surface, for instance to guide microtubules in one direction. To date, it has been shown that by patterning photoresist (Hess and Vogel, 2001; Hiratsuka et al., 2001; Moorjani et al., 2003) or by nanoimprinting a fluorinated polymer on a protein adsorbent surface such as glass (Cheng et al., 2005), Figure 3.2: Microtubule motility in freeze-dried samples. A) The fraction of microtubules moving following different durations of storage at 4 o C. At each time point, 45 video screens [54 m x 70 m] were analyzed from a total of three flow cells. B) The number of microtubules bound per video screen. C) Screen captures showing microtubule movement following 5 weeks of storage. Frames are 4 sec apart, scale bar is 15 µm.
62 44 functional kinesin proteins can be patterned on surfaces. However, all of these approaches lead to three dimensional surfaces, and it would be advantageous both to be able to pattern motors on flat two-dimensional surfaces and to use traditional photolithography processes on kinesin-functionalized surfaces. One advantage of critical point drying is that because the aqueous buffer is removed prior to drying, the salt residues that remain following freeze drying are eliminated, resulting in a smooth surface that is amenable to photoresist deposition. Using critical point-dried samples, we investigated whether it is possible to deposit photoresist on top of the preserved motors and pattern this photoresist to create patterns of functional surface-adsorbed kinesins. In initial studies the motors did not survive treatment with Shipley 1811 developing solution, but we found that the motors were stable in acetone (Verma et al., 2005), which is used to strip the photoresist. Hence, when a UV-exposed region of photoresist is developed, the underlying motors come into contact with the developing solution and either denature or are removed, while motors in the unexposed region are protected by the photoresist. After the remaining photoresist is removed using acetone, motility solution is introduced and the microtubules observed under the microscope. As seen in Figure 3.3, in the region where the photoresist was not exposed, microtubules were observed binding to the surface and moving across it, while no activity was observed in the exposed regions. The edges of the patterns were somewhat diffuse because the normal photoresist baking step was not used. However the differences in the patterned and unpatterned regions were very clear. The number of microtubules bound per screen was 18.2 ± 4.1 (mean ± SD; n=6), which is roughly 3- fold lower than unpatterned critical point-dried samples (Figure 1), possibly due to incomplete stripping of the photoresist. Nevertheless, this result shows that a
63 45 population of surface-adsorbed kinesin motors can be dried, covered with photoresist, and then exposed to acetone, and a significant fraction of the motors still retain their activity. This result expands the range of microfabrication techniques that can be used with kinesin motors, and suggests that similar approaches could be used to pattern antibodies or other functional proteins on surfaces. Figure 3.3: Using photolithography to pattern critical point dried motors on glass substrates. A) The patterning process. Photoresist is deposited on top of the motorfunctionalized surface, patterened using contact lithography, developed to pattern motors in specific regions, and then stripped to expose functional motors. B) Microtubule motility on a region of the surface protected by the photoresist (top) and a region of the surface where motors were exposed (bottom). (Scale bar 15 µm). 3.4 Conclusion Future microdevices that interface biological components with engineered materials have great promise, but the fragility of the protein components often outweighs the improved functionality they provide. For these devices to become realistic, it is important to develop approaches for preserving protein function over
64 46 time in novel device geometries. Because hybrid microdevices that incorporate the kinesin-microtubule system are at the forefront of this research area, methods for storing kinesin-functionalized samples long-term are needed. Here, we show that both critical point drying and freeze drying kinesin-functionalized glass coverslips extend the lifetime of the motors to months. Furthermore, following critical point drying, motors can be patterned using traditional photolithography approaches, which results in two-dimensional surfaces that contain both motor-functionalized and motorfree regions. These approaches extend the range of fabrication processes that can be used with kinesin motors, and they can be extended to other functional proteins immobilized on surfaces for sensors and other devices.
65 47 Chapter 4 Microtubule Alignment and Manipulation Using AC Electrokinetics 4.1 Introduction Microtubules are structurally polar cytoskeletal filaments that serve as tracks for kinesin and dynein motor proteins and provide mechanical integrity to the cell. In eukarytotic cells, kinesins use the energy of ATP hydrolysis to move unidirectionally along the microtubule surface, transporting intracellular cargo and driving the segregation of replicated chromosomes during cell division (Hirokawa et al., 1998), among other tasks. The components of the microtubule-kinesin motor system can be purified and motility reconstituted in vitro using the microtubule gliding assay (Howard et al., 1989), or bead assay (Block et al., 1990). Building on these in vitro assays, there has been considerable interest in exploiting this biologically derived nanoscale motion for manipulation, assembly and active transport of materials at the nanoscale (Hess, 2006; Hess and Vogel, 2001; Jia et al., 2004; Limberis et al., 2001). However, to harness this biologically derived motion for such applications, it is necessary to develop tools to control microtubule-based motility. In addition, the development of in vitro techniques to harness these motors can also be used to manipulate microtubules into structures resembling the intracellular organization of microtubules, thereby providing tools for studying fundamental cellular processes such as axonal transport and mitosis. Existing techniques for controlling microtubule motion on kinesin-coated surfaces include physical barriers and DC electric fields. Physical barriers such as
66 48 microfabricated channels can guide the direction of microtubules driven by immobilized kinesin motors by buckling and redirecting the front end of moving microtubules (Cheng et al., 2005; Clemmens et al., 2004; Hiratsuka et al., 2001; Moorjani et al., 2003; van den Heuvel et al., 2006). We recently built on this work by creating fully enclosed microchannels functionalized with kinesin motors and generating high density ensembles of uniformly oriented microtubules (Huang et al., 2007). Electric fields can also be used to manipulate microtubules in vitro. In DC electric fields, microtubules undergo electrophoresis and move towards positive electrodes (Böhm et al., 2005; Jia et al., 2004; van den Heuvel et al., 2005a). Precise electrophoretic control of microtubule transport has been demonstrated using DC electric fields (Kim et al., 2007; van den Heuvel et al., 2006). However, using DC electric fields in microchannels often results in electrophoresis of buffer ions, which poisons microtubule motility, and electrolysis that leads to bubble generation and electrode damage. Here, we characterize the behavior of microtubules in AC electric fields and show that AC electrokinetic phenomena can be used to organize microtubules into novel and useful assemblies in vitro. In non-uniform AC electric fields, dielectrophoretic forces act on particles whose polarizability differs from that of the surrounding medium. If the particle is more polarizable than the surrounding medium, positive dielectrophoresis is observed, causing the particle to move towards regions of high field gradient (Pohl, 1978). Negative dielectrophoresis is observed when the particle is less polarizable than the surrounding medium, resulting in particle movement towards low field gradient regions. Dielectrophoresis of relatively large particles such as latex beads, cells, viruses, and bacteria has been reported (for review see (Gonzalez and Remcho, 2005)). However, significant electric field gradients are required to manipulate particles as small as individual microtubules. Using lithography to fabricate microelectrodes on surfaces
67 enables the generation of such field gradients, but these high fields also generate electrohydrodynamic flows, which can potentially overpower the dielectrophoretic forces 49 (Castellanos et al., 2003). Dielectrophoresis has been used to manipulate macromolecules such as DNA (Asbury et al., 2002), actin filaments (Asokan et al., 2003) and microtubules (Jia et al., 2004), but the effect of electrohydrodynamic flows was not addressed in these studies. Moreover, we found that the dielectrophoresis of microtubules reported previously (Jia et al., 2004) depends strongly on small changes in experimental variables such as electrode geometry and buffer conditions. While electrorotation experiments on microtubules in uniform AC electric fields have been used to estimate electrical properties of microtubules(böhm et al., 2005; Minoura and Muto, 2006), there are no comprehensive studies using dielectrophoretic forces generated by non-uniform AC fields to manipulate, accumulate, and characterize the electrical properties of microtubules. There have been reports of accumulations of latex particles, bacteria, yeast, and large DNA molecules in non-uniform AC electric fields that cannot be explained by dielectrophoretic forces alone (Bown and Meinhart, 2006; Green et al., 2000b; Hoettges et al., 2003; Wu et al., 2005). Such accumulations have primarily been attributed to AC electroosmotic flows (Bhatt et al., 2005; Hoettges et al., 2003; Wu et al., 2005). At sufficiently low AC frequencies, an induced double layer of ions builds up on the electrode surfaces during each half cycle. AC electroosmotic flow results from the movement of these ions due to non-uniform electric fields tangential the surface (Ramos et al., 1999). The other type of electrohydrodynamic flow generated by AC fields in conductive buffers is electrothermal flow. Electrothermal flows arise due to temperature gradients in the solution, which give rise to gradients in conductivity and permittivity. In the presence of an AC electric field, the conductivity and permittivity gradients produce
68 50 body forces on the fluid that result in convective flows (Ramos et al., 1998). If dielectrophoretic forces are to be used to manipulate particles, then AC electroosmotic and electrothermal flows must be minimized. However, when coupled with dielectrophoretic forces, these electrohydrodynamic flows can also be used to accumulate and concentrate particles on electrodes, and this phenomenon can in principle be used to accumulate microtubules. Here we investigate the effects of buffer concentration, field strength and frequency, and electrode geometry on the behavior of microtubules in non-uniform AC fields. We identify optimal experimental conditions for minimizing electrohydrodynamic flows such that significant dielectrophoretic forces can be achieved and kinesin-driven microtubule motility is preserved. Furthermore, by characterizing the frequency and conductivity dependence of the positive dielectrophoretic forces, we estimate the conductivity of bovine brain microtubules. Finally, we demonstrate that experimental conditions can be chosen to maximize AC electroosmotic accumulation of high densities of microtubules. By choosing appropriate electrode geometries, this accumulation results in dense bundles of aligned (parallel and antiparallel) microtubules that can be used to investigate the role that motor proteins play in organizing microtubules into the mitotic spindle during cell division. 4.2 Experimental Section Microtubules and kinesin Tubulin was purified from bovine brains (Williams and Lee, 1982) and labeled with rhodamine (Hyman et al., 1991) as previously described. Microtubules were
69 51 polymerized by mixing 32 μm rhodamine-labeled tubulin, 4 mm MgCl 2, 1 mm GTP and 5% DMSO in BRB80 buffer (80 mm PIPES, 1 mm EGTA, 1 mm MgCl 2, ph 6.9 with KOH), incubating at 37 C for 20 min, and then diluting into a solution containing 10 μm paclitaxel. The microtubules were pelleted using a Beckman Airfuge at 30 psi and were resuspended in BRB3, BRB6, BRB12, BRB16 and BRB20 buffers (3 mm, 6 mm, 12 mm, 16 mm and 20 mm PIPES, respectively, with 1 mm EGTA and 1 mm MgCl 2 ). Buffer conductivities were measured using an AKTA FPLC system (Amersham Biosciences) and found to vary linearly with increasing PIPES concentration. The linear fit for the conductivity as a function of PIPES concentration was σ buffer (ms/m) = 11.4 x [PIPES (mm)] The nominal ph of these buffers was 7.0, but for some experiments the ph of the low molarity buffers was raised, as noted in the text. Antifade reagents (20 mm D- glucose, 0.02 mg/ml glucose oxidase, mg/ml catalase and 70 mm β- mercaptoethanol) were added to the buffer and the microtubules were observed using epifluorescence microscopy (Nikon E600, N.A. water immersion objective). Fluorescent microtubules were visualized using a Genwac GW-902H CCD camera and recorded to videotape. Frames were digitized using Scion Image (Scion Corporation), and image analysis carried out using Image J. In some experiments, 1 µm diameter polystyrene beads (Bang Laboratories) labeled with rhodamine were added to the microtubule solution as tracer particles to determine flow velocities. Full-length Drosophila conventional kinesin was bacterially expressed and purified using previously published protocols (Hancock and Howard, 1998).
70 52 Electrode fabrication and application of AC electric fields To generate AC electric fields in our microchambers, Cr microelectrodes were patterned on glass and these surfaces assembled into flow chambers. Masks used for photolithography were fabricated using a Mann 3600 pattern generator. Before electrode fabrication, glass substrates were cleaned by immersion in acetone and isopropyl alcohol in an ultrasonic bath for 10 minutes each, followed by immersion in piranha solution (H 2 SO 4 : H 2 O 2 = 4:1) for 20 minutes. The piranha treatment both cleans the glass and results in a high-surface-energy OH-saturated surface that improves the adhesion of deposited layers. To pattern electrodes, a 100 nm Cr layer was deposited onto the cleaned glass surface using an Edward E306A sputtering system. Shipley 1811 photoresist was then spun on top of the Cr layer and this photoresist patterned through the photomask using a Karl Suss MA-55 aligner. The exposed photoresist and underlying Cr were removed by wet etching, and the remaining unexposed photoresist dissolved by acetone to expose the patterned electrodes. Flow chambers were assembled on the electrode-patterned glass substrates using a coverslip and 3M double-sided tape as spacers. The surfaces were blocked with casein (0.5 mg/ml casein in BRB80) and then flushed with 64 nm microtubules and 0.2 mg/ml casein in appropriate buffer containing antifade reagents. The leads of the electrodes were connected to a function generator (BK precision, Yorba Linda, CA, USA) and AC electric fields with frequencies varying from 10 khz to 10 MHz were applied with a maximum peak-peak voltage of 40V. At low frequencies, Faradaic charging of the electrodes occurs due electrochemical reactions at the electrode surface, which can affect the electroosmotic flow (Wu et al., 2005). Since such reactions often damage the electrodes, in the present work we avoided frequencies below 10 khz.
71 53 Motility in low molarity buffers To study kinesin function in low molarity buffers, flow cells were prepared with Fisher s Finest glass slides and Corning 18 mm coverglass, using 3M double-sided tape as spacers. The flow cells were incubated with 0.5 mg/ml casein for 5 min, flushed with a motor solution containing 5 µg/ml kinesin, 0.2 mg/ml casein and 1 mm ATP, and incubated for 5 min. Motility solution (64 nm microtubules, 10 µm paclitaxel, 20 mm D- glucose, 0.02 mg/ml glucose oxidase, mg/ml catalase and 0.07 M β- mercaptoethanol) was then introduced into the flow cell and microtubule movement was visualized. Initial experiments used BRB3, BRB6 and BRB12 all at ph 7.0. For additional experiments the ph values of BRB12, BRB6 and BRB3 were increased to 7.5, 8.0 and 9.0, respectively, and the buffers were degassed. Finite element model simulations Because AC electrokinetics is a complex phenomenon involving a combination of dielectrophoretic forces and electroosmotic and electrothermal fluid flows, we used finite element modeling using the COMSOL Multiphysics package to predict and understand the observed phenomena. For predicting the direction of electroosmotic flows, the tangential electric fields surrounding each electrode were simulated using COMSOL Multiphysics in the 3D conductivity mode. The model consisted of two electrodes 0.1 µm high, 10 µm wide and 400 µm long, separated by a gap of 20 µm. The left electrode was set to +5 V and the right electrode set to -5V, the solution conductivity was set to 170 ms/m, and the solution permittivity set to The flow chamber was 100 µm high. To model the predicted electrothermal flow, the Electrostatics, Convection and Conduction, and Incompressible Navier-Stokes application modes were used in a two dimensional simulation. The flow chamber in the simulation was 400 µm wide and 200
72 54 µm high, and the electrodes were placed on the bottom surface with zero thickness and an electrode gap of 20 µm. Equation 2 (see Results and Discussion) was used calculate body forces due to Joule heating, and electrode heating from illumination was modeled by setting the temperature of the electrodes to 10 C above the surrounding environment. The simulation used a 40 V p-p 1.5 MHz AC potential across the electrodes, a fluid conductivity of S/m (BRB6 buffer), a dynamic viscosity of kg/m/s for the fluid, and an initial fluid velocity of zero. For calculating dielectrophoretic forces, the Electric Currents mode was used to calculate the spatial distribution of both the electric field E and the square of the electric field gradient, E 2 in a simulated chamber 100 µm by 150 µm with height of 10 µm. The simulation modeled a pair of 15 µm wide electrodes separated by a 20 µm gap, with 35 V p-p applied across the electrodes in BRB6 buffer. 4.3 Results and Discussion The goal of this work is to develop AC electrokinetics as a tool for manipulating microtubules in solution and organizing microtubules into aligned bundles on surfaces. The accumulation of microtubules on electrodes is described below, followed by an analysis of the roles played by AC electroosmotic flow, electrothermal flow, and dielectrophoresis. The first step of this work was to fabricate a pair of electrodes on a glass surface and observe the behavior of microtubules in solution when an AC voltage was applied. Chrome microelectrodes 15 µm wide and 12 mm long, separated by a gap of 20 µm were patterned on glass substrates using conventional lithography techniques (Figure
73 55 4.1A). The substrates were then assembled into experimental chambers, and rhodamine-labeled microtubules suspended in BRB16 buffer (16 mm PIPES, 1 mm MgCl 2, 1 mm EGTA, ph 6.9) were introduced. An AC voltage of 40 V p-p was applied across the electrodes, and the resulting microtubule behavior was observed by fluorescence microscopy. At frequencies above 500 khz, bulk solution flow was observed along the electrodes, with the dominant flow being directed inward towards the gap separating the electrode pair. At 500 khz and below, dense bundles of microtubules accumulated along the centerline of the electrodes as far as 400 µm away from the gap separating the electrodes (see Figure 4.1B). Upon closer inspection, there were two distinct flow patterns (as shown in Figure 4.1C). The first flow pattern occurred in the solution, roughly 7 µm above the electrodes and was directed towards the electrode gap, while the second flow pattern occurred at the electrode surface and formed vortices perpendicular to the long axis of the electrodes. These electrohydrodynamic flows continued indefinitely as long as the AC voltage across the electrodes was maintained, and they stopped instantly when the field was switched off. We next set out to understand the origin of these flow patterns AC Electroosmotic Flow AC electroosmotic flows occur in non-uniform fields at low frequencies where tangential electric fields act on the induced double layer of ions on the electrode surface (Ramos et al., 1999). The ion movement due to coulomb forces creates a drag on the bulk fluid that results in electroosmotic flow. The mechanism of AC electroosmosis is illustrated in Figure 4.2. In the half cycle shown in Figure 4.2A, a positive potential is applied to the right electrode and a negative potential to the left electrode. This results in the accumulation of negatively charged ions on the right electrode and positive ions
74 56 on the left electrode. Due to the action of tangential electric field components, the coulomb forces on the double layer are always pointed inward, irrespective of the polarity of the electrodes, and have maximal amplitude closest to the gap separating the two electrodes. This results in symmetric flow on both electrodes with ions moving inwards away from the gap (Figure 4.2B). A 200 µm B C Figure 4.1: Electrode design and AC field-driven microtubule accumulation. A: Optical micrograph of Cr electrodes 15 µm wide and 12 mm long, separated by a gap of 20 µm. B: Accumulation of microtubules resulting from application of 40 V p-p at 500 khz across the electrodes. C: Schematic of electrohydrodynamic flows leading to microtubule accumulation. Green arrows denote electroosmotic flows and red arrows denote electrothermal flows.
75 57 A B C Figure 4.2: Mechanism of AC electroosmotic flow. A) During each half cycle, counter ions accumulate on each electrode. Tangential electric field components (E x ) lead to coulombic forces (F c ) that are directed away from the gap, irrespective of the electrode polarity. B) The resulting ion fluxes cause electroosmotic fluid flow away from the electrode gap. C) Using the same electrode geometry as in the experiments, a simulation of the tangential components of the electric field in the x-y plane just above the surface. The resulting ion flows result in inward fluid flow toward the center of the electrodes from all edges.
76 58 The velocity of fluid flow is given by (Ramos et al., 1999): E t q v (1) where E t is the tangential component of electric field, q is the surface charge density in the diffuse double layer, is the reciprocal Debye length and is the solution viscosity. In AC fields, both the induced charge as well as the potential drop across the solution are frequency dependent. At low frequencies, there is capacitive charging of the double layer, and the potential drop is mainly across the double layer, resulting in E t tending to zero. At high frequencies, the ions can not move fast enough to form a double layer during each half cycle, therefore q tends to zero. Hence, the strongest electroosmotic flows are achieved at intermediate frequencies (Ramos et al., 1999). A flow pattern was observed, consisting of vortices near the electrode edges oriented perpendicular to the long axis of the electrodes (Figure 4.1C). This flow disappeared at high frequencies, consistent with electroosmotic flow (Ramos et al., 1999). Because the direction of electroosmotic flow is determined by the direction of the tangential electric field at the electrode surface, we used finite element modeling to simulate the tangential electric fields surrounding each electrode. The simulated tangential field lines at a plane 0.5 µm above the electrode surface are shown in Figure 4.2C. Except for regions of the electrodes closest to the gap, the field direction along most of the length of each electrode is perpendicular to the long edge of the electrode. Hence, during each half cycle, counterions moving to the electrode surface to form the double layer will create inward flows from the edge that will meet at the centerline of the electrode and rise upward, setting up vortices. As discussed below, the microtubule
77 accumulations along the electrodes (i.e. Figure 4.1B) primarily result from these inward AC electroosmotic flows Electrothermal flow Electrothermal flows result from temperature gradients in the solution, which give rise to gradients in the solution conductivity and permittivity. Temperature gradients can be generated from external sources or through Joule heating (Green et al., 2001), but a major mechanism for generating the temperature gradients that drive electrothermal flow has been found to be illumination from the light source of the microscope, which leads to absorptive heating of the electrodes (Green et al., 2000a; Green et al., 2001). To understand and quantify electrothermal flow in our system, we carried out simulations and performed experiments using tracer particles to map the electrothermal flows. The body force on a fluid in terms of permittivity and conductivity gradients is given by (Ramos et al., 1998): 1 ( ) 1 Re ( T. E) E E 2 i 2 f e 2 T.(2) 1 T 1 T where is the conductivity and is the permittivity of the solution, T is the temperature, E is the applied electric field, and Re denotes the real component of the complex number. The first term on the right hand side, which dominates at low frequencies, represents Coulomb forces due to the space charge density generated by the conductivity and permittivity gradients. The second term on the right hand side, which dominates at high frequencies, represents dielectric forces. For the electrode geometries used here, the temperature gradient and therefore the permittivity and
78 60 conductivity gradients are expected to be uniform along the length of the electrodes. However, the field strength is highest near the gap separating the electrodes. Previous work using similar electrode geometries showed that temperature gradients lead to inward bulk fluid flow along each electrode that converges at the electrode gap to create vertical flow normal to the surface at the electrode gap (Green et al., 2000a; Green et al., 2001). To model the expected electrothermal flow profile for our electrode geometry, we carried out finite element simulations using a pair of electrodes separated by a 20 µm gap. The electrode temperatures were set to 10 o C above ambient to simulate electrode heating from the microscope illumination, and a 35 V p-p, 1.5 MHz AC voltage was applied across them. As seen from the streamlines in Figure 4.3A, the simulation predicted inward flow along the electrode surfaces that converged to a vertical flow in the electrode gap, with a return path in solution. To characterize the speed and direction of electrothermal flow in our experimental chambers, the movement of 1 µm fluorescent polystyrene beads in solution was quantified at different illumination intensities. One test of whether observed flows are electrothermal in nature is to reduce the microscope illumination (reducing the associated heating of the electrodes) and test whether flow velocities are diminished. In the 100 µm thick experimental chamber, the bottom surface contained a pair of 15 µm wide electrodes separated by a 20 µm gap, similar to Figure 1A. A 30 V p-p, 500 khz AC voltage was applied across the electrodes and the bead movements observed by fluorescence microscopy. As seen in Figure 4.3 B and C, at a height of ~7 µm above the surface, the beads moved parallel to electrodes toward the gap and flow velocities fell with increasing distance away from the electrodes. These flow trajectories are similar to electrothermal flow observed by others (i.e. Figure 3 in (Green et al., 2001)) and qualitatively agree with our simulations. As expected for
79 61 electrothermal flow, when neutral density filters were used to reduce the illumination of the 100 W mercury arc lamp on the microscope by a factor of 64, there was an 85% decrease in the flow velocity of tracer particles. When the electric field was switched off while maintaining constant illumination, the flow stopped immediately, demonstrating that the flows are not due to simple convection. Hence, we conclude that the inwarddirected bulk flows observed along the electrodes were electrothermal in origin and that the temperature gradients driving this flow were primarily due to heating of the electrodes by the microscope illumination Microtubule accumulation due to electrohydrodynamic flows One behavior that we consistently observed at frequencies below 500 khz was the accumulation of microtubules along the centerline of the two electrodes up to hundreds of microns away from the electrode gap. At these frequencies, microtubules did not accumulate near the electrode gap (Figure 4.1B), and interestingly, microtubules could be observed moving away from the electrode gap. This behavior has not been documented using this electrode geometry or with particles having aspect ratios approaching microtubules, and for biological experiments this phenomenon could provide a model for the microtubule organization in neurons or a geometry to test the sorting of mixed orientation filaments by molecular motors. As described below, we believe that this accumulation is driven by electroosmotic flow that sweeps the microtubules into the center of the electrode, and that dielectrophoretic forces that attract microtubules to the electrode edges may also be playing a role. As seen in Figure 4.2C, away from the electrode gaps the electric field vector parallel to the surface is directed perpendicular to the electrode axis and is strongest at the edges of the electrodes. This field distribution sets up electroosmotic flows that, at
80 Velocity ( m/s) 62 A B C On electrode Electrode Electrode 20 µm 0 to to to to 40 Figure 4.3: Simulation and experimental observation of electrothermal flows resulting from microscope illumination. A) Simulation of electrothermal flow, showing the flow vectors above two 100 µm long electrodes separated by a 20 µm gap (side view). Note that near the electrode surface, flow is observed towards the gap. B) Experimental flow profiles (top view) observed by tracking fluorescent beads at a height of ~7 µm above the electrode surface. The traces show the track taken by each particle in the 5 sec preceding the image. For clarity, only one electrode is shown, and every bead is observed to move toward the gap separating the electrodes. C) Particle velocities along a line perpendicular to the electrode at a distance of 50 µm from the gap. Velocities are maximal directly above the electrode and fall of with increasing lateral distances away from the electrode edge. Distance from edge of electrode ( m)
81 63 the surface, are directed toward the center of the electrode, and which converge to a stagnation point at the centerline and flow upwards into solution from there. The result of this flow is a pair of vorticies (see Figure 4.1C) with an energy minimum at the centerline, where the flow velocity is zero and shear forces are balanced. Microtubules oriented parallel to the electrodes accumulate at the centerline of the electrodes, and any filaments not precisely aligned are swept away by the vortex flow. One argument for electroosmotic flow being the driver for microtubule accumulation is that the degree of accumulation correlates with the predicted and observed magnitude and direction of electroosmotic flow. At the electrode surface, the calculated tangential electric field (Figure 4.2C) falls with increasing distances away from the electrode gap, and the rate of electroosmotic flow, as observed by following fluorescent beads or microtubules in solution, similarly fell at distances away from the electrode gap. The fact that microtubules accumulated at these positions of maximal electroosmotic flow suggests a mechanism in which a minimum organizing flow rate is necessary to overcome Brownian motion of the microtubules in solution. As seen in Figure 4.4, the region over which microtubules accumulate depends strongly on the AC excitation frequency. At frequencies above 500 khz there was no accumulation, but at lower frequencies the accumulation zone grew and the accumulation start point moved farther away from the gap. At 50 khz, the microtubule accumulation zone began 15 µm from the electrode gap and continued for over 400 µm. Because at lower frequencies there is more time during each half cycle to accumulate the ion double layer (see Equation 1), electroosmotic flows are expected to be larger at lower frequencies, and qualitative observations supported this. Because the tangential electric field is strongest directly adjacent to the electrode gap (Figure 4.2C), and because the electroosmotic flow is predicted and observed to be directed away from the electrode gap in this region, the
82 64 fact that the microtubule accumulation begins farther from the gap and extends for a longer distance at lower frequencies is consistent with electroosmotic flow driving the microtubule accumulation. Figure 4.4: Frequency dependence of microtubule accumulation. Electrodes are 10 µm wide and separated by a 20 µm gap. BRB12 buffer was used with 40 V p-p field stimulation. Insets show the start points and end point of the accumulation zone (Scale bar 20 µm). Distances are measured from the edge each electrode and are displayed as mean +/- standard deviation from 4 to 6 determinations for each point. In previous AC electrokinetics experiments, the accumulation of latex particles observed by others has been attributed to a combination of AC electroosmotic flows and positive dielectrophoretic forces. For instance, using large electrodes so that only a single vortex is formed on each electrode (in contrast to the pairs of vortices in our experiments), Green and Morgan (Green and Morgan, 1998) and Hoettges et al. (Hoettges et al., 2003) found that positive dielectrophoretic forces attract particles towards the electrode edges, while electroosmotic flows push the particles away from
83 65 the edges. These opposing forces lead to particles accumulating at a stagnation point whose position is frequency dependent. A recent study that used AC electrokinetics to accumulate viruses on surface electrodes also concluded that, while positive dielectrophoresis plays a role, the drag forces generated by electrohydrodynamic flows are the main driving force for accumulation (Docoslis et al., 2007). While AC electroosmotic flow appears to be the dominant mechanism for microtubule accumulation in our experiments, there is good evidence that dielectrophoresis is also playing an important role. First, the densities of microtubules observed on the electrodes are difficult to explain without attractive dielectrophoretic forces playing a role. Second, in buffers above 16 mm PIPES where electroosmotic flows are evident but no dielectrophoresis is seen, microtubules do not accumulate on the electrodes. Third, under some conditions two bands of microtubules were observed on the electrodes (data not shown), consistent with a balance between dielectrophoretic forces directed toward the electrode edges and electroosmotic flows directed toward the electrode midline. However, because dielectrophoresis is expected to be negligible at distances far from the electrode gap, accumulations of microtubules observed hundreds of microns away from the electrode gaps (Figure 4.4) must be predominantly driven by electroosmotic forces. It is possible that these microtubules are being collected on the electrodes closer to the gap and are migrating long distances along the electrodes by outward electroosmotic flow Microtubule dielectrophoresis To develop dielectrophoresis as a tool for manipulating microtubules and to characterize the electrical properties of microtubules, we identified experimental
84 66 conditions where dielectrophoresis dominates and quantified the dielectrophoretic forces on microtubules. When a 30 V p-p, 5 MHz AC potential difference was applied across a pair of castellated electrodes, which are routinely used in dielectrophoresis studies because they have clearly defined regions at their tips where electric field gradients are maximized, microtubules accumulated at the high field gradient regions, indicating positive dielectrophoresis (Figure 4.5). Others have observed similar accumulations of actin filaments in AC fields (Arsenault et al., 2007). One difficulty in characterizing dielectrophoresis is that electrohydrodynamic flows can overwhelm the dielectrophoretic forces (Castellanos et al., 2003). Using the paired electrodes shown in Figure 1, we systematically analyzed the dependence of microtubule dielectrophoresis on the AC frequency and buffer conductivity. As discussed above, at low frequencies AC electroosmotic flow away from the electrode gap plays a dominant role, while at high frequencies electrothermal flow toward the gap dominates. We characterized this in more detail by observing the microtubule accumulation at the tips of the opposed electrodes (the region of highest electric field gradient) as function of frequency in BRB12 buffer (Figure 4.6). At frequencies from 100 khz to 1 MHz (Figure 4.6A-D), microtubules are attracted to the electrode tips by dielectrophoresis, but are pushed away from the gap by AC electroosmotic flow. In contrast, at 2.5 MHz, electroosmotic flows are balanced by electrothermal flows directed toward the gap, and dielectrophoresis dominates (Figure 4.6E). Above roughly 7 MHz, electrothermal flows toward the electrode gap dominate. Hence, there is a range of frequencies centered around 5 MHz, where electroosmotic and electrothermal flows are balanced, providing a window where dielectrophoretic forces on microtubules can be analyzed.
85 67 20 µm Figure 4.5: Positive dielectrophoresis of microtubules using castellated electrodes at 5 MHz frequency and 35 V p-p amplitude in BRB3 buffer. A B C D E 30µm Figure 4.6: The dependence of microtubule accumulation on AC frequency using opposed electrodes. At high frequencies, dielectrophoresis dominates, while at lower frequencies, electroosmotic flows push the microtubules away from the electrode gap. Experiments were performed in BRB12 buffer with 30V p-p stimulation. Frequencies were: A) 100 khz, B) 250 khz, C) 500 khz, D) 1 MHz, E) 2.5 MHz.
86 68 In a conducting medium, particles that have a different polarizability than the medium are attracted to regions of high field gradient. The dielectrophoretic force on a particle is given by (Morgan and Green, 2003): 1 ~ 2 F DEP Re E..(3) 4 where Re[ ~ ] is real part of the effective polarizability of the particle (also called the Claussius-Mossotti factor), E is the electric field, and is the volume of the particle. Microtubules can be considered as prolate ellipsoids with their major axis >> minor axes. The effective polarizability of a prolate ellipsoid along the major axis when the major axis aligns with the electric field is given by Morgan and Green (Morgan and Green, 2003) as: ~ ~ m ~ p m m..(4) ~ ~ where and p ~ m are the complex permittivities of the particle and the medium, respectively. These complex permittivities are given by: ~ m i m m ~ p i p p..(5) where p, p, m and m are the permittivity and conductivity of the particle and the medium, respectively. Equations 3-5 can be combined to obtain a relationship for the dielectrophoretic force as a function of the conductivities and permittivities of the particle and the medium: F DEP m p m m p m m E (6) 4 m m
87 69 In the 5 MHz range where electrohydrodynamic flows are minimal, the dielectrophoretic forces from Equation 6 are dominated by conductivity differences between the microtubule and the buffer; only at much higher frequencies, permittivity differences are expected to play a role. Consistent with this, greater accumulations of microtubules were seen in lower ionic strength buffers than in higher ionic strength buffers (data not shown). One implication of this simplification is that the effective conductivity of microtubules can be estimated by finding the solution conductivity at which dielectrophoretic forces disappear (i.e. ). This zero force approach was p m used by Hughes et al to obtain an estimate of 100 ms/m for the internal conductivity of Herpes Simplex Virus type I particles (Hughes et al., 2002). Using identical electrodes to Figure 6 and a frequency of 2.5 MHz, we measured the accumulation of microtubules as a function of buffer conductivity. Robust microtubule accumulation was clearly observed in BRB6 and BRB12 buffers, very few microtubules accumulated at the electrode tips in BRB16 buffer, and no microtubule accumulation was observed in BRB20 buffer. Assuming that microtubule accumulation is due to dielectrophoretic forces, this puts the apparent conductivity of microtubules very close to the conductivity of our BRB16 buffer, which we measured to be 250 ms/m (see Methods). Because the apparent microtubule conductivity is dominated by the ion double layer surrounding this negatively charged polymer, this value holds for the buffers used in these experiments and is expected to vary with buffer composition. This microtubule conductivity value is roughly two-fold higher than the 120 ms/m measured by Minoura and Muto using microtubule electrorotation experiments (Minoura and Muto, 2006). In that work, the buffer consisted of 1 mm MES plus 10 µm MgCl 2.
88 Estimating dielectrophoretic forces on microtubules By using estimates for the microtubule and buffer permittivities and conductivities, and by calculating the square of the electric field gradient ( E 2 ), it is possible to use Equation 6 to calculate the magnitude of the dielectrophoretic forces on microtubules in our experiments. In Figure 4.7A, finite element modeling was used to calculate the spatial distribution of both the electric field E and the square of the electric field gradient, 2 E. The simulations used an electrode geometry identical to Figure 6 (15 µm wide electrodes separated by a 20 µm gap), with 35 V applied across the electrodes in BRB6 buffer. The maximum 2 E occurs near the edges of the electrodes and falls steeply with distance away from the edges, which qualitatively agrees with the microtubule accumulations observed in Figure 4.6A. Using the microtubule and buffer conductivities (250 ms/m and 116 ms/m, respectively), the known dimensions of microtubules (25 nm diameter), the calculated maximal 2 E (~10-20 V 2 /m 3, Figure 4.7) and reported relative permittivity of microtubules (1 to 100 [22]), we estimated the dielectrophoretic forces on microtubules in our experiments. At a frequency of 5 MHz in BRB6 buffer, the maximal dielectrophoretic force per unit length of microtubule was estimated to be ~10 pn/µm. Varying the microtubule permittivity from 1 to 100 changed this value by less than 5% at 5 MHz, demonstrating that dielectrophoresis of microtubules is dominated by conductivity differences and not permittivity differences between microtubules and the bufffer in which they are suspended. It should also be noted that calculating the overall force on a microtubule requires integrating over the
89 71 A B 15 µm Figure 4.7: Dielectrophoresis of microtubules. A: Simulation of the electric field and electric field gradients using opposed electrodes. Simulations used 35 V p-p amplitude in BRB6 buffer. Red arrows denote the electric field vectors, and colors denote E 2. To better visualize the data, values are scaled logarithmically and gradients less than V 2 /m 3 are not shown. B: Dielectrophoretic accumulation of microtubules at electrode edges agree with the simulations (AC voltage of 35 V p-p, 5 MHz in BRB6 buffer). entire length of the microtubule, and that these maximum forces are localized to the electrode edges. Nonetheless, as shown in Figures 4.5 and 4.6, these forces are sufficient to attract and align microtubules between the electrodes. Furthermore,
90 72 previous work using DC electric fields and magnetic fields showed that forces on the order of 1-5 pn/µm are sufficient to redirect microtubules being transported along kinesin functionalized surfaces (Hutchins et al., 2007; van den Heuvel et al., 2006) Kinesin motility in low ionic strength buffers Having identified buffer ionic strengths and AC frequencies over which positive dielectrophoresis can be used to manipulate microtubules, it is important for future work on kinesin-driven microtubule transport to ensure that kinesin motors retain their function under these same conditions. To test whether kinesin functionality is retained in these low ionic strength buffers, microtubule gliding experiments were carried out in BRB12, BRB6 and BRB3 at ph 7.0. We found that in BRB12 and BRB6, microtubules moved for 30 and 6 minutes, respectively before depolymerizing, but in BRB3 microtubules depolymerized within one minute. An antifade system is used in these experiments to scavenge dissolved oxygen from solution and thus prevent the formation of fluorophoreinduced oxygen free radicals that bleach fluorophores and damage proteins. The end product of this reaction is gluconic acid, which leads to a decrease in the solution ph over time. Therefore, as the molarity of the buffer decreases, the buffering capacity is diminished, resulting in earlier loss of motility. In order to circumvent this problem, solutions were degassed prior to use, and the flow chambers were sealed with vacuum grease to block atmospheric oxygen from dissolving in the buffers. Additionally, in order to allow some working room before sealing the flow cell, the initial ph of the low molarity buffers was increased (see Methods) to compensate for the initial production of gluconic acid. By following this protocol, we were able to extend the lifetime of motility in all three low ionic strength buffers to greater than 3 hours. Hence, in future experiments it should
91 be possible to use dielectrophoresis to manipulate microtubules driven by immobilized kinesin motors Conclusion Because microelectrodes can be fabricated on glass surfaces with virtually any geometry, AC electrokinetics has the potential to be a powerful tool for manipulating microtubules in experimental work or for applications integrating biomotors and microtubules into microengineered devices. We found that when solutions of taxolstabilized microtubules are subjected to AC electric fields, three AC electrokinetic phenomena become apparent: electroosmotic flow, electrothermal flow, and dielectrophoresis. Interestingly, along long (12 mm) narrow (10-20 µm) electrodes, the electroosmotic and electrothermal flows result in dense bundles microtubules aligning along the long axis of the electrodes. This result has implications in transport applications where microtubules can be concentrated from solution or for studying kinesin motility along bundles of microtubules similar to the axon, provided that subpopulations of parallel and antiparallel oriented microtubules can be sorted out. These aligned bundles of mixed orientation microtubules are also a convenient model to test the organizing activities of tetrameric Eg5 and other mitotic motors. Finally, by minimizing the electrohydrodynamic flows, we identified conditions in which pn-scale dielectrophoretic forces on microtubules can be achieved. These experiments lay the foundation for applying AC electrokinetics as a tool to for studying the role of molecular motors in mitotic spindle function, and for guiding microtubules in motor-driven transport applications.
92 Chapter 5 74 Organizing bipolar arrays of microtubules to create in vitro models of mitotic spindles 5.1 Introduction Mitosis is the process in cell division by which the duplicated chromosomes are segregated into the two emerging daughter cells. The segregation of chromosomes is achieved using the complex protein machinery of the mitotic spindle. The mitotic spindle consists of microtubules organized in a bipolar manner with the ends of microtubules focused at the two poles. The +ends of the microtubule either bind to the kinetochores or interact with chromosome arms and microtubules from the other pole (spindle microtubules) (see Figure 5.1). It is hypothesized that the bipolar spindle is formed by either centrosome-mediated assembly, chromosome-mediated assembly or a combination of these pathways. (see Figure 5.2). The centrosome-mediated assembly usually occurs in somatic animal cells, while chromosome-mediated assembly is known to occur in cells lacking centrosomes (Compton, 2000). In the centrosome-mediated assembly, microtubules nucleate at the centrosome. The centrosome is duplicated to form two asters, which are pushed apart during prophase. The highly dynamic microtubules +ends search and bind kinetechores of chromosomes. The search and capture leads binding of microtubules from each pole to the sister kinetochores of a chromosome. After microtubule binding, the chromosomes congress towards the center and align at midpoint of spindle at metaphase. In the chromosome-mediated assembly, the microtubules are nucleated near chromosomes and bind to chromosomes. The ends of these microtubules are pushed away from the chromosomes and are crosslinked and then focused into poles. In both pathways, the organization of the
93 75 microtubules into the mitotic spindle is mediated by populations of motors acting on the spindle and is controlled by various signaling factors. (Scholey et al., 2003) The roles of these motors vary depending on the assembly pathway. In some cells, these mechanisms may not be exclusive and the bipolar spindle can be established by a combination of both these pathways. Therefore it is important to generate tools to study this complex phenomenon of spindle assembly and the role of kinesin motor proteins. Figure 5.1: Left: Features of the bipolar mitotic spindle(gadde and Heald, 2004) Right: Immunofluorescence image of mitotic spindle in tissue cell. Green microtubules, blue chromosomes and red, TPX2 spindle pole component.(wittmann et al., 2001) The assembly of the spindle and chromosome segregation is a complex process involving many motor proteins and signaling factors. Several synergistic and antagonist mechanisms exist to ensure fidelity in segregating chromosomes. One approach to understanding the roles of different motor proteins, has been to knockout individual or combination of motors proteins and examine the resulting phenotype (Goshima and Vale, 2003; Zhu et al., 2005). The complimentary approach is to purify the motors and perform in vitro experiments to characterize these motors. By building an in vitro model of the bipolar mitotic spindle in a bottom-up approach, the effects of individual and combinations of motors can be studied without the interference from other force
94 generating mechanisms. This information can supplement the information obtained from knockout experiments to obtain a better understanding of mitosis. 76 Figure 5.2: Pathways for mitotic spindle assembly. Left :Centrosome-mediated Right: Chromosome-mediated While in vitro experiments have been conducted to study the biophysics of individual motors such as Ncd and Eg5, very few experiments have examined microtubule organization by ensembles of motors. The organization of microtubules into asters and vortices was demonstrated by mixing microtubules with Ncd and streptavidin conjugated multimer kinesins (Nedelec et al., 1997). Spindle-like shapes with multiple poles were reported when combinations of microtubules, Ncd and oligomeric motors were used (Surrey et al., 2001). However these structures are in solution and are not isolated, thereby making it difficult to manipulate and study the function of different proteins. Here we demonstrate the spindle-like assembly of bipolar microtubule structures using protein patterning and AC Electrokinetics. As described in Chapter 4, AC
95 77 electrokinetics was used to accumulate high densities of microtubules at center of the electrode or at the edges, depending on the frequency of electric field. Conventional kinesin motors were patterned on these electrodes to sort the accumulated microtubules to generate polarized asters. In order to immobilize these microtubules in a bipolar manner, neutravidin was patterned on electrodes using UV light. Segmented microtubules with biotin segments at ends were prepared and accumulated at the edges of electrodes using dielectrophoretic forces. Only microtubules with biotin at the ends bind to the electrode resulting in a spindle-like structure with the ends focused at the edge of the electrodes. This is the first demonstration of an in vitro model for microtubule organization mimicking the mitotic spindle. 5.2 Materials and Methods Microtubules and Kinesin Tubulin was purified from bovine brains (Williams and Lee, 1982) and labeled with rhodamine (Hyman et al., 1991) as previously described. Microtubules were polymerized by mixing 32 μm rhodamine-labeled tubulin, 4 mm MgCl 2, 1 mm GTP and 5% DMSO in BRB80 buffer (80 mm PIPES, 1 mm EGTA, 1 mm MgCl 2, ph 6.9 with KOH), incubating at 37 C for 20 min, and then diluting into a solution containing 10 μm paclitaxel. The microtubules were pelleted using a Beckman Airfuge at 30 psi and were resuspended in BRB 6 and BRB12 buffer (6 mm and12 mm PIPES, respectively, with 1 mm EGTA and 1 mm MgCl 2 ) before applying AC electric fields. The ph of BRB6 and BRB12 were 8.5 and 7.5 respectively. For some experiments segmented microtubules were required. Biotinylated tubulin and NEM tubulin was prepared as described previously. Biotinylated tubulin was
96 78 mixed with rhodamine tubulin (2dyes/subunit) in the ratio 5:1 and polymerized as described above. The microtubules were diluted 100 times into BRB80 buffer containing 10 μm paclitaxel. 200 μl of this solution was pelleted using airfuge and resuspended in 100 μl of polymerization mix (1.6 μm Alexa647 labeled tubulin, 6 μm NEM tubulin, 4 mm MgCl 2, 1 mm GTP and 5% DMSO in BRB80 buffer). The solution was sheared and incubated at 37 C for 25 min. 10 μm paclitaxel was added to stabilize the polymerized microtubules. Full-length Drosophila conventional kinesin was bacterially expressed and purified using previously published protocols (Hancock and Howard, 1998). Electrode Fabrication Microelectrodes were fabricated on quartz and glass substrates. The fabrication process is the same as described in previous chapter. Typical electrodes were 100 µm long and 5-15 µm wide with opposing electrodes facing each other with a 20 µm gap. In another experiment the electrodes were offset by 40 µm. Kinesin and Neutravidin Patterning Octadecyltrichlorosilane (OTS) was patterned on electrode samples such that OTS is present only on the glass surface making it hydrophobic while the metal surface is hydrophilic. The surface was blocked with 0.5 mg/ml casein in BRB12 and then flushed with a solution containing 0.2 mg/ml casein, 1% Triton X-100, 1mM ATP and 5 µg/ml of kinesin in BRB12. The ampiphilic molecule Triton X-100 binds to hydrophobic surface and sterically prevents microtubule binding. Thereby kinesins are functional only on the electrode surface.
97 79 We developed a novel approach for directly patterning neutravidin protein by exposure to deep UV irradiation. For these experiments, neutravidin was physically adsorbed onto electrode samples by incubating them with 1mg/ml neutravidin in BRB80 buffer for 5 min. The samples were then flushed with BRB12 and then dehydrated using acetone and air dried. BRB12 was used to minimize salt residues on the sample. Unlike kinesin motors which require critical point drying or freeze-drying as discussed in Chapter 3, neutravidin was stable with air drying after dehydration in acetone. In preliminary experiments we found that 254nm wavelength UV did not affect proteins even after exposure for 1 hr, however deep UV light (~185nm wavelength) can denature within a seconds to minutes. To pattern surface-adsorbed neutravidin, quartz masks were used to maximize transmission in the DUV, and a UV-blocking coating was used to mask the light from selected regions of the surface. In another approach quartz substrates were used and the samples were exposed from the back side, with the electrodes masking the UV light. Effective neutravidin patterning was observed with these techniques (See Figure 5.3). AC Electrokinetics Flow chambers were assembled on the electrode-patterned glass substrates using a coverslip and 3M double-sided tape as spacers. The surfaces were blocked with casein (0.5 mg/ml casein in BRB12) and then flushed with motility solution (0.64 nm microtubules, 10 µm paclitaxel, 20 mm D-glucose, 0.1 mg/ml glucose oxidase, 0.04 mg/ml catalase, 0.2 mg/ml casein, 1mM ATP and 0.07 M β-mercaptoethanol) in BRB12. The leads of the electrodes were connected to a function generator (BK precision, Yorba
98 Figure 5.3: Patterning of neutravidin using deep UV illumination. (a)-(c) Top side exposure approach (d) Effective patterning demonstrated by binding rhodamine labeled biotinylated BSA. (e)-(g) Back side exposure technique. (h) effective patterning demonstrated by microtubule binding 80
99 81 Linda, CA, USA) and AC electric fields with frequencies varying from 100 khz to 5 MHz were applied with a maximum peak-peak voltage of 40V. Microtubules were observed using epifluorescence microscopy (Nikon E600, N.A. water immersion objective) and electrodes were observed using an overlay of transillumination. The samples were imaged using a Genwac GW-902H CCD camera and recorded to videotape. Frames were digitized using Scion Image (Scion Corporation). 5.3 Results and Discussion In vitro approaches for studying spindle assembly require isolated structures of microtubules organized in a manner similar to their organization in the spindle. In the mitotic spindle microtubules are organized in bipolar manner with the minus ends of the microtubules focused at the two poles and the plus-ends overlapping at the center and interacting with chromosomes. In order to develop in vitro models of the mitotic spindle, high densities (similar to the cellular concentrations) of organized microtubules are required. We demonstrated accumulations of high densities of microtubules using AC electric fields in Chapter 4. We found that AC electric fields result in electroosmotic flow, electrothermal flow and dielectrophoresis of microtubules, which can be controlled by varying the solution conductivity, AC frequency, and electrode geometry. At low frequencies (<500 khz, 40V P-P; See Figure 4.1B) microtubules accumulated at the center of electrodes and are aligned parallel to the long axis of the electrode due to electroosmotic flows. Under optimal conditions where flows were minimized (BRB6 buffer, 40V P-P, 5MHz), spindle-like accumulations of microtubules were observed at the edges of the electrode (see Figure 5.4)
100 82 15 µm Figure 5.4: Spindle-like accumulation of microtubules. A: Opposed microtubule asters generated by dielectrophoretic accumulation of microtubules at electrode edges. AC voltage of 35 V p-p, 5 MHz was applied across the electrodes, and microtubules were suspended in BRB12 buffer. However, in these accumulations microtubules are of mixed orientation and they must be sorted for polarity. Also since the microtubules diffuse away after the field is turned off, strategies to bind microtubules in the right orientation are required. Here we report two approaches to obtain polarized organization of high-densities of microtubules. In the first approach, kinesin motors are patterned on the electrodes. These motors sort the microtubules that accumulate on these electrodes resulting in polarized asters. In the second approach neutravidin was patterned on the electrodes and biotin was functionalized on the ends of microtubules to bind microtubules in the right orientation resulting in spindle-like structures. These approaches are discussed below Kinesin patterning approach Kinesin motors were patterned on the electrodes and microtubules were accumulated at the center of the electrode using 100 khz 40 V P-P AC field in BRB12 buffer. As the microtubules accumulate they bind to motors on the electrode and start
101 83 moving. Since the minus-ends are aligned along the long axis of he electrode either towards or away from the electrode gap, the microtubules move either towards the gap or away from the gap between electrodes (See Figure 5.5A). Once the microtubules walk off the edge of the electrode, they diffuse and rebind. As more microtubules walk off the edge a dynamic polarized microtubule aster (see Figure 5.5B) is created with their plus-ends on the edge of the electrodes (as the microtubules move with minus-end leading forward). When 5 MHz 40V P-P AC field was used microtubules accumulate at the edges of electrodes. The motors on the edge capture the microtubules with their minus-ends facing the edge, and pull them inwards while microtubules with their plus-ends facing the edge remain at the edge and thereby forming a polarized aster at edges of each electrode (see Figure 5.6). In both cases of low frequency accumulation at center and high frequency accumulation at the edge, the orientation of microtubules in the asters is opposite to the microtubule orientation in the mitotic spindle with the minus-ends facing the center. Nevertheless these polarized asters can be used to study the minus end focusing and bundling activities of ended motors such as Ncd. By patterning minus-ended motors on the electrode structures of right orientation can be obtained Neutravidin patterning approach In the previous approach, the asters were dynamic and microtubules diffuse away once the field is turned off. Moreover the AC electric field accumulation of microtubules requires low molarity buffers. While low molarity buffers do not affect conventional kinesin, the buffer may not be optimal for other motors. Therefore we need
102 A 84 B 20 µm Figure 5.5: A: Schematic for sorting microtubules by polarity. Microtubules accumulate at the center of the electrode with their minus-ends aligned along the long axis of electrode. Microtubules of opposite orientations move in different directions resulting in sorting of microtubules for polarity. B: Dynamic asters formed as microtubules walk off the edges of electrodes. (Arrows mark the end of electrodes)
103 85 10 µm Figure 5.6: Polarized dynamic asters formed at electrode edges. Microtubules accumulate at the edges of the electrode. Conventional kinesin motors patterned on electrodes capture and pull microtubules, with their minus-ends facing the edge, inwards while leaving the oppositely oriented microtubules alone. to immobilize microtubules, so that electric field can be turned off enabling buffer exchange. Figure 5.7A shows the schematic of the strategy to immobilize microtubules into spindle-like structures. Here segmented microtubules with biotin-tubulin at the minus-end and normal tubulin at the plus-end are used and neutravidin is patterned on the electrode surface. When microtubules are accumulated at the edge of the electrode only the microtubules with their minus ends facing the edge will bind to patterned neutravidin. When the field is turned off the unbound microtubules diffuse away generating polarized asters with plus-ends of the microtubule at the center and minusends bound to the electrodes. Segmented microtubules were diluted 100 fold in BRB6 with antifade components and flushed onto the neutravidin patterned surface. AC Electric field (40V P-P 5 MHz) was applied to accumulate microtubules on the edges of the electrode. Microtubules with the ends facing the edge, bind to the neutravidin patterned electrode, while the other microtubules do not bind and diffuse away. Therefore after turning off the field microtubules are immobilized in the right orientation. This
104 86 A Pattern neutravidin on electrodes Biotin tubulin Make segmented Mts with biotin at -end Acummulate MTs on edge so that ends can bind to neutravidin and other microtubules diffuse away B 5 µm Figure 5.7: Assembling spindle-like microtubule structures A: Schematic of approach B: Assembled microtubules with their minus-ends (red) bound to electrodes and plusends overlapping in the center (green)
105 process is repeated twice to get more microtubules immobilized on the electrode. Figure 5.7B shows the spindle-like structures on the electrode after this process Conclusion In vitro models for studying mitotic motors have been developed. Using patterned kinesins and AC electric fields, polarized asters microtubules with their plus-ends focused on the electrode have been generated. This can used as model to study the end focusing activity of Ncd and other minus-end bundling proteins. By patterning neutravidin and using segmented microtubules with biotin at minus-end, bipolar asters were formed on opposing electrodes similar to the microtubule organization in mitotic spindles with the electrodes as poles. This is the first demonstration of an in vitro model of the mitotic spindle. The techniques developed here enable the study of the effect of ensembles of motors on spindle structure and thereby can provide insight into the cellular process of spindle assembly and chromosome segregation.
106 Chapter 6 88 Conclusions and Suggestion for Future Work Kinesin are microtubule based motor proteins which are involved intracellular transport of cargo and cell division. There has been considerable interest in harnessing the kinesin-microtubule transport system for applications in manipulation of materials at the nanoscale, microactuation and cargo transport in lab-on-a-chip devices. While there has been progress in various aspects of integrating the kinesin-microtubule transport system for these in vitro applications, several technological issues need to be solved for practical integration of kinesins and microtubules. The first aim of the thesis was to develop technologies to harness kinesin-driven transport for applications in lab-on-a-chip devices. In addition, nanotechnology tools can be used to study the cell biology of kinesins and microtubules. The second aim of was to develop in vitro models of microtubules mimicking their cellular organization. 6.1 Harnessing kinesin-driven motion for lab-on-a-chip devices Lab-on-a-chip devices integrate one or several laboratory functions on a chip. Typical advantages of using a lab-on-chip device include, smaller sample volume, faster sample analysis and high throughput due to parallelization. While the development of such devices is primarily based on microfluidics technology, there has been significant interest in using kinesin-driven microtubule motion in such devices. Integration of kinesin-driven motion in microfluidic devices has several advantages including the ability to transport analytes against bulk flow and concentration gradients and the ability to bind
107 89 and transport analytes for separation and sensing. The use of kinesin-driven motion can also simplify the fabrication of such devices by eliminating the need for elaborate pumping mechanisms when compared to traditional microfluidics technology. Practical integration of kinesin-driven microtubule motion requires the development of the following core technologies: i) The ability to achieve functional kinesin-driven motion in enclosed microfluidic channels ii) The ability to control microtubule motion and generate long-distance motion at the scale required for lab-on-a-chip devices iii) The ability to load and release cargo on microtubules iv) The ability to detect the presence of analyte for diagnostic applications v) The ability to sort microtubules based on the presence of cargo for separation applications While many research groups are addressing these issues, my research focused on developing technologies for generating controlled long-distance motion of kinesindriven microtubules in microfluidic channels and developing technologies for sorting microtubules using AC electric fields. In addition techniques were developed for longterm preservation of motors which addresses the key issue of shelf life of devices incorporating kinesin motor proteins. Integration of kinesins and microtubules in microfluidic channels and long-distance transport As discussed in Chapter 2, we developed techniques of functionalizing kinesins and microtubules in enclosed microchannels and optimized channel geometries to demonstrate controlled long distance transport (~5mm) of high densities of microtubules.
108 90 This is a significant improvement over previous approaches to control microtubules using open microchannels, (Cheng et al., 2005; Clemmens et al., 2003; Hess et al., 2003; Hiratsuka et al., 2001; Moorjani et al., 2003; van den Heuvel et al., 2005b) which are not compatible with microfluidic channels. While this study demonstrates that kinesin-microtubule system can be successfully integrated in microfluidic channels for transport applications, there is room for improving the functionalization process. The high surface-to-volume ratio in these channels results in a gradient of motor density across the length of the channel. While these gradients can be eliminated by saturating the surface with motors, the high motor density results in clogging of the channels. Therefore an optimal motor density is required for robust microtubule motion. The problem of sharp motor gradients was alleviated by competitive binding with a headless kinesin (KRT). While it is expected that kinesin and KRT should occupy the binding sites according to the ratio of concentrations, the higher diffusion coefficient of KRT still leads to a gradient in functional motor concentration. Therefore the optimal ratio of concentrations between kinesin and KRT varies with the length of the microchannel. A systematic study should be performed to quantify the motor density across the length of the channel for different kinesin-krt ratios. The results of this study will be useful for generalization of this approach for different channel geometries. In another approach, a kinesin mutant that cannot bind microtubules can be used instead of KRT, which will eliminate the gradients arising due to difference in diffusion coefficients. Manipulating Microtubules using AC Electric Fields Several techniques have been developed to control and manipulate microtubules including physical barriers, surface modification, DC electric fields and magnetic fields (Ionov et al., 2006; Jia et al., 2004; Platt et al., 2005; van den Heuvel et al., 2005b).
109 91 However, each of these techniques has its limitations. We explored the use of AC Electrokinetics as a tool to manipulate and sort microtubules. In Chapter 3, dielectrophoretic forces and electrohydrodynamic flows were studied as function of applied voltage, frequency and buffer ionic strengths and optimal conditions were identified to assemble high densities of microtubules at precise locations. At frequencies below 1 MHz, dense bundles of microtubules accumulated at the center of electrodes with AC electroosmotic flow as the main driver of this accumulation. At 5 MHz electrohydrodynamic flows were minimized and microtubules accumulated at the edges of electrodes due to dielectrophoresis. These experiments demonstrate that AC electrokinetics provides a powerful new tool for kinesin-driven transport applications. However the observed phenomena of AC Electrokinetics in flow cells (as in Chapter 3) cannot be easily translated to the geometry of the microfluidic channels. The phenomena of AC Electrokinetics is highly dependent on the distribution of electric field lines and this distribution is expected to vary with flow cell height and the presence of insulating surfaces, as in the case of microfluidic channel walls. Moreover, since AC electroosmotic flow is a surface phenomenon, the high surface area of the microfluidic channel is expected to affect the observed flow patterns. Figure 6.1A shows the accumulation of microtubules in a microfluidic channel (cross section 5 µm wide x 1 µm high) using BRB 12 and 100 khz 150V p-p electric field. The microtubule accumulation does not occur along the center of electrodes as observed in flow cells (Figure 3.1), and the accumulation points are defined by the geometry of the microchannel and the location of electrodes. The accumulation at specific locations suggests local minima of tangential electric field component at these positions. Figure 6.1B shows the development of vortices in microchannels when a 1 MHz electric field is applied. A complete understanding of these observations requires a 3D simulation of
110 92 electrothermal, electrosmotic and dielectrophoretic forces in the microchannnel. While accumulation of high densities and vortices can be used to enable mixing of solutions and binding cargo to microtubules, a better understanding of these AC electrokinetic phenomena will be useful for guiding the design of electrodes and their locations in the channel geometry. A B Figure 6.1: A: Electroosmotic accumulations of microtubules in microchannels (locations marked by arrows) at 100 khz 150V p-p AC field in BRB12 buffer B: Complex flow patterns resulting in the formation of vortices (pointed by arrows) at 1 MHz 150 V p-p AC field. Scale bar 20 µm At 5 MHz, electrohydrodynamic flows are also minimized in microchannels, enabling the use of dielectrophoresis to guide and sort microtubules. However the DEP
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Self assembly and organization of nanofibers using biological molecular motors
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
The Pennsylvania State University. The Graduate School. College of Engineering MICRO-SCALE HYBRID BIOLOGICAL-ENGINEERED DEVICES
 The Pennsylvania State University The Graduate School College of Engineering MICRO-SCALE HYBRID BIOLOGICAL-ENGINEERED DEVICES POWERED BY BIOMOLECULAR MOTORS A Thesis in Electrical Engineering by Ying-Ming
The Pennsylvania State University The Graduate School College of Engineering MICRO-SCALE HYBRID BIOLOGICAL-ENGINEERED DEVICES POWERED BY BIOMOLECULAR MOTORS A Thesis in Electrical Engineering by Ying-Ming
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology
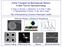 Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
The Pennsylvania State University. The Graduate School APPROACHES TO HYBRID SYNTHETIC DEVICES. A Dissertation in. Engineering Science and Mechanics
 The Pennsylvania State University The Graduate School Department of Engineering Science and Mechanics APPROACHES TO HYBRID SYNTHETIC DEVICES A Dissertation in Engineering Science and Mechanics by Vivek
The Pennsylvania State University The Graduate School Department of Engineering Science and Mechanics APPROACHES TO HYBRID SYNTHETIC DEVICES A Dissertation in Engineering Science and Mechanics by Vivek
The Pennsylvania State University. The Graduate School. The College of Engineering INTEGRATED ELECTRONICS AND FLUIDIC MEMS FOR BIOENGINEERING
 The Pennsylvania State University The Graduate School The College of Engineering INTEGRATED ELECTRONICS AND FLUIDIC MEMS FOR BIOENGINEERING A Dissertation in Electrical Engineering by Ho Him Raymond Fok
The Pennsylvania State University The Graduate School The College of Engineering INTEGRATED ELECTRONICS AND FLUIDIC MEMS FOR BIOENGINEERING A Dissertation in Electrical Engineering by Ho Him Raymond Fok
Aligning Bacterial Cellulose
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
Nanotechnological Applications of Biomolecular Motor Systems. Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden
 Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Nano-Scale Engineering III Bio-Molecular Motors for Engineering
 Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
Supplementary Figures 1-6
 1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation
 A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules. Dr. Carissa M. Soto
 Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
NanoFabrication Systems DPN. Nanofabrication Systems. A complete line of instruments and tools for micro and nanopatterning applications
 DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning applications DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning
DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning applications DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler
 Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler 23.10. Zelle 30.10. Biologische Makromoleküle I 06.11. Biologische Makromoleküle II 13.11. Nukleinsäuren-Origami (DNA, RNA) 20.11.
Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler 23.10. Zelle 30.10. Biologische Makromoleküle I 06.11. Biologische Makromoleküle II 13.11. Nukleinsäuren-Origami (DNA, RNA) 20.11.
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Biomolecular motors at the intersection of. nanotechnology and polymer science
 Biomolecular motors at the intersection of nanotechnology and polymer science Ashutosh Agarwal 1 and Henry Hess 2 * 1 Department of Materials Science and Engineering, University of Florida, Gainesville,
Biomolecular motors at the intersection of nanotechnology and polymer science Ashutosh Agarwal 1 and Henry Hess 2 * 1 Department of Materials Science and Engineering, University of Florida, Gainesville,
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
(a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The
 1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Progress in Polymer Science
 Progress in Polymer Science 35 (2010) 252 277 Contents lists available at ScienceDirect Progress in Polymer Science journal homepage: www.elsevier.com/locate/ppolysci Biomolecular motors at the intersection
Progress in Polymer Science 35 (2010) 252 277 Contents lists available at ScienceDirect Progress in Polymer Science journal homepage: www.elsevier.com/locate/ppolysci Biomolecular motors at the intersection
Contents Preface xiii Introduction Fabrication and manufacturing technology for optical MEMS
 Contents Preface xiii 1 Introduction 1 1.1 Optical MEMS and optofluidics 1 1.2 History 1 1.2.1 Processes and materials 1 1.2.2 Early devices and systems 2 1.3 Progress in optical MEMS and optofluidics
Contents Preface xiii 1 Introduction 1 1.1 Optical MEMS and optofluidics 1 1.2 History 1 1.2.1 Processes and materials 1 1.2.2 Early devices and systems 2 1.3 Progress in optical MEMS and optofluidics
Biosensors. DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses)
 Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
Rapid Prototyping of Purification Platforms
 Rapid Prototyping of Purification Platforms Tom Huang, Nathan Mosier, Michael Ladisch Department of Agricultural and Biological Engineering Weldon School of Biomedical Engineering Laboratory of Renewable
Rapid Prototyping of Purification Platforms Tom Huang, Nathan Mosier, Michael Ladisch Department of Agricultural and Biological Engineering Weldon School of Biomedical Engineering Laboratory of Renewable
CYTOSKELETON, MOTORPROTEINS.
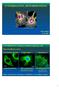 CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
Activity Subject Area(s) Associated Unit Associated Lesson Activity Title Header Image 1 ADA Description: Caption: Image file: Source/Rights:
 Activity Subject Area(s) Biology Associated Unit Associated Lesson Activity Title Breaking News: Molecular Trucks Riding Inside Cells! Header Image 1 ADA Description: An ant carries a 1 millimeter square
Activity Subject Area(s) Biology Associated Unit Associated Lesson Activity Title Breaking News: Molecular Trucks Riding Inside Cells! Header Image 1 ADA Description: An ant carries a 1 millimeter square
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures. Xin Zhang Associate Professor.
 Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
me239 mechanics of the cell homework biopolymers - motivation 3.1 biopolymers - motivation 3. biopolymers biopolymers biopolymers
 3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
Microtubule Assembly Dynamics at the Nanoscale
 Microtubule Assembly Dynamics at the Nanoscale Melissa K. Gardner, Henry T. Schek III*, Alan J. Hunt, and David J. Odde Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455
Microtubule Assembly Dynamics at the Nanoscale Melissa K. Gardner, Henry T. Schek III*, Alan J. Hunt, and David J. Odde Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension
 Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Supporting Information: Model Based Design of a Microfluidic. Mixer Driven by Induced Charge Electroosmosis
 Supporting Information: Model Based Design of a Microfluidic Mixer Driven by Induced Charge Electroosmosis Cindy K. Harnett, Yehya M. Senousy, Katherine A. Dunphy-Guzman #, Jeremy Templeton * and Michael
Supporting Information: Model Based Design of a Microfluidic Mixer Driven by Induced Charge Electroosmosis Cindy K. Harnett, Yehya M. Senousy, Katherine A. Dunphy-Guzman #, Jeremy Templeton * and Michael
BIOC 463A Expt. 4: Column Chromatographic Methods Column Chromatography
 Column Chromatography Chromatography is the process use to separate molecules based on SOME physical property of the molecule: Mass (i.e. size) Charge Affinity for ligands or substrates Hydrophobic interactions
Column Chromatography Chromatography is the process use to separate molecules based on SOME physical property of the molecule: Mass (i.e. size) Charge Affinity for ligands or substrates Hydrophobic interactions
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature08627 Supplementary Figure 1. DNA sequences used to construct nucleosomes in this work. a, DNA sequences containing the 601 positioning sequence (blue)24 with a PstI restriction site
doi: 10.1038/nature08627 Supplementary Figure 1. DNA sequences used to construct nucleosomes in this work. a, DNA sequences containing the 601 positioning sequence (blue)24 with a PstI restriction site
Biophysics of Molecules
 Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Cell cycle oscillations
 Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
FABRICATION OF MICROFLUIDIC CHANNELS USING MICROFIBERS FOR APPLICATIONS IN BIOTECHNOLOGY
 FABRICATION OF MICROFLUIDIC CHANNELS USING MICROFIBERS FOR APPLICATIONS IN BIOTECHNOLOGY Tom Huang, 1,5 Woo-Jin Chang, 2 Demir Akin, 2 Rafael Gomez, 2 Rashid Bashir, 2,3 Nathan Mosier, 4 and Michael R.
FABRICATION OF MICROFLUIDIC CHANNELS USING MICROFIBERS FOR APPLICATIONS IN BIOTECHNOLOGY Tom Huang, 1,5 Woo-Jin Chang, 2 Demir Akin, 2 Rafael Gomez, 2 Rashid Bashir, 2,3 Nathan Mosier, 4 and Michael R.
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Cell position. Edge. Core Mean Std. Deviation
 Diameter (um) 20 *** 15 10 5 0 Edge Cell position Core Mean Std. Deviation Edge 10.69 2.664 Core 12.61 2.632 Supplementary Figure S1: Measuring cell diameter. Cell diameters were obtained by ImageJ using
Diameter (um) 20 *** 15 10 5 0 Edge Cell position Core Mean Std. Deviation Edge 10.69 2.664 Core 12.61 2.632 Supplementary Figure S1: Measuring cell diameter. Cell diameters were obtained by ImageJ using
Micro-fluidic Chip for Flow Cytometry Jeff Wang
 Micro-fluidic Chip for Flow Cytometry Jeff Wang September, 2015 ABSTRACT This lab course is intended to give students hands-on experience with microfabrication. The project is to make a micro-fluidic chip
Micro-fluidic Chip for Flow Cytometry Jeff Wang September, 2015 ABSTRACT This lab course is intended to give students hands-on experience with microfabrication. The project is to make a micro-fluidic chip
Biophysics of contractile ring assembly
 Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements
 Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements NANO LETTERS 2003 Vol. 3, No. 5 633-637 Samira G. Moorjani, Lili Jia, Thomas N.
Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements NANO LETTERS 2003 Vol. 3, No. 5 633-637 Samira G. Moorjani, Lili Jia, Thomas N.
Three major types of cytoskeleton
 The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
Self-assembly of oligonucleotides
 Self-assembly of oligonucleotides Dr. K. Uma Maheswari Professor, School of Chemical & Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 9 Table of Contents 1 APPLICATIONS
Self-assembly of oligonucleotides Dr. K. Uma Maheswari Professor, School of Chemical & Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 9 Table of Contents 1 APPLICATIONS
Nanobiotechnology. Place: IOP 1 st Meeting Room Time: 9:30-12:00. Reference: Review Papers. Grade: 50% midterm, 50% final.
 Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Dark- Field Total Internal Reflection Microscopy for the Study of Kinesin Motor Proteins
 Dark- Field Total Internal Reflection Microscopy for the Study of Kinesin Motor Proteins Christopher Pfeiffer- Kelly Penn State College of Engineering Summer REU Program (CERI) Abstract: Kinesin motor
Dark- Field Total Internal Reflection Microscopy for the Study of Kinesin Motor Proteins Christopher Pfeiffer- Kelly Penn State College of Engineering Summer REU Program (CERI) Abstract: Kinesin motor
Comsol Multiphysics Simulations of Microfluidic Systems for Biomedical Applications
 Excerpt from the Proceedings of the COMSOL Conference 008 Hannover Comsol Multiphysics Simulations of Microfluidic Systems for Biomedical Applications Maria Dimaki *,1, Jacob Moresco Lange 1, Patricia
Excerpt from the Proceedings of the COMSOL Conference 008 Hannover Comsol Multiphysics Simulations of Microfluidic Systems for Biomedical Applications Maria Dimaki *,1, Jacob Moresco Lange 1, Patricia
Fluorescence Imaging with One Nanometer Accuracy Lab
 I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEERING NANOPARTICLES IN BIOMEDICINE
 UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEERING NANOPARTICLES IN BIOMEDICINE Challenges The challenges are: in-situ analysis and in vivo at micro level recognize biomolecules functionality
UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEERING NANOPARTICLES IN BIOMEDICINE Challenges The challenges are: in-situ analysis and in vivo at micro level recognize biomolecules functionality
BE.430/2.795//6.561/10.539/HST.544. Final Exam. Handed out: Monday, Dec. 6, 2004 Due: Thursday, Dec. 9 by 5pm
 BE.430/2.795//6.561/10.539/HST.544 Final Exam Handed out: Monday, Dec. 6, 2004 Due: Thursday, Dec. 9 by 5pm Problem 1 (30 %) ALG Notes Problem 2.6.3 Parts a f (not g) (Page 199) Problem 2 (35 %) When patients
BE.430/2.795//6.561/10.539/HST.544 Final Exam Handed out: Monday, Dec. 6, 2004 Due: Thursday, Dec. 9 by 5pm Problem 1 (30 %) ALG Notes Problem 2.6.3 Parts a f (not g) (Page 199) Problem 2 (35 %) When patients
Development Team. Molecular Cell Biology Cytoskeleton ZOOLOGY. Head, Department of Zoology, University of Delhi
 Paper No. : 15 Module : 11 Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Paper Coordinator: Prof. S. Basu Modak Department of Zoology, University
Paper No. : 15 Module : 11 Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Paper Coordinator: Prof. S. Basu Modak Department of Zoology, University
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
SUPPLEMENTARY INFORMATION
 A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane Jonathan R. Burns, Astrid Seifert, Niels Fertig, Stefan Howorka NATURE NANOTECHNOLOGY
A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane Jonathan R. Burns, Astrid Seifert, Niels Fertig, Stefan Howorka NATURE NANOTECHNOLOGY
Bioengineering: Where Biology Meets Bioengineering
 Bioengineering: Where Biology Meets Bioengineering What is Bioengineering? The application of engineering principles to biology. Thermodynamics Fluid mechanics Heat & mass transfer Materials Reaction engineering
Bioengineering: Where Biology Meets Bioengineering What is Bioengineering? The application of engineering principles to biology. Thermodynamics Fluid mechanics Heat & mass transfer Materials Reaction engineering
A rapid and practical technique for real-time monitoring of biomolecular interactions using mechanical responses of macromolecules
 A rapid and practical technique for real-time monitoring of biomolecular interactions using mechanical responses of macromolecules Mehmet C. Tarhan,,*, Nicolas Lafitte, Yannick Tauran,, Laurent Jalabert,
A rapid and practical technique for real-time monitoring of biomolecular interactions using mechanical responses of macromolecules Mehmet C. Tarhan,,*, Nicolas Lafitte, Yannick Tauran,, Laurent Jalabert,
Module 16: Gel filtration: Principle, Methodology & applications. Dr. Savita Yadav Professor Department of Biophysics AIIMS, New Delhi
 PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module 16: Gel filtration: Principle, Methodology & applications Dr. Savita Yadav Professor Department of Biophysics AIIMS, New Delhi Module 16 Gel filtration:
PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module 16: Gel filtration: Principle, Methodology & applications Dr. Savita Yadav Professor Department of Biophysics AIIMS, New Delhi Module 16 Gel filtration:
TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS. Kathryn P. Trogden
 TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS Kathryn P. Trogden A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill
TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS Kathryn P. Trogden A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill
Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules
 Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
DEPArray Technology. Sorting and Recovery of Rare Cells
 DEPArray Technology Sorting and Recovery of Rare Cells Delivering pure, single, viable cells The DEPArray system from Silicon Biosystems is the only automated instrument that can identify, quantify, and
DEPArray Technology Sorting and Recovery of Rare Cells Delivering pure, single, viable cells The DEPArray system from Silicon Biosystems is the only automated instrument that can identify, quantify, and
Particle Motion Analysis Reveals Nanoscale Bond Characteristics and Enhances Dynamic Range for Biosensing
 Particle Motion Analysis Reveals Nanoscale Bond Characteristics and Enhances Dynamic Range for Biosensing Emiel W. A. Visser *,,, Leo J. van IJzendoorn,, Menno W. J. Prins,, Department of Applied Physics,
Particle Motion Analysis Reveals Nanoscale Bond Characteristics and Enhances Dynamic Range for Biosensing Emiel W. A. Visser *,,, Leo J. van IJzendoorn,, Menno W. J. Prins,, Department of Applied Physics,
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA
 AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
DNA Biosensors. Anand Jagota 16 November 2015
 DNA Biosensors Anand Jagota 16 November 2015 1 Market, Unmet Needs Worldwide In-vitro diagnostics ~$ 50 Billion and growing Nucleic Acid diagnostics ~$9 Billion Health, Security, Pathogen Detection, etc.
DNA Biosensors Anand Jagota 16 November 2015 1 Market, Unmet Needs Worldwide In-vitro diagnostics ~$ 50 Billion and growing Nucleic Acid diagnostics ~$9 Billion Health, Security, Pathogen Detection, etc.
Nanoimprinting in Polymers and Applications in Cell Studies. Albert F. YEE Chemical Engineering & Materials Science UC Irvine
 Nanoimprinting in Polymers and Applications in Cell Studies Albert F. YEE Chemical Engineering & Materials Science UC Irvine Presentation outline Motivation Reversal imprinting Soft inkpad imprinting on
Nanoimprinting in Polymers and Applications in Cell Studies Albert F. YEE Chemical Engineering & Materials Science UC Irvine Presentation outline Motivation Reversal imprinting Soft inkpad imprinting on
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology
 Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Nature Structural & Molecular Biology: doi: /nsmb.2996
 Supplementary Figure 1 Negative-stain EM of native cytoplasmic dynein. (a) A representative micrograph of negatively stained dynein, with the flexible dimeric molecules circled in white. (b) Reference-free
Supplementary Figure 1 Negative-stain EM of native cytoplasmic dynein. (a) A representative micrograph of negatively stained dynein, with the flexible dimeric molecules circled in white. (b) Reference-free
Enhancement of electrochemical biosensor performances using redox cycling at 3D sub-micrometer scale electrode architectures.
 Enhancement of electrochemical biosensor performances using redox cycling at 3D sub-micrometer scale electrode architectures Heungjoo Shin School of Mechanical and Nuclear Engineering Contents 1 Introduction
Enhancement of electrochemical biosensor performances using redox cycling at 3D sub-micrometer scale electrode architectures Heungjoo Shin School of Mechanical and Nuclear Engineering Contents 1 Introduction
Final exam. Please write your name on the exam and keep an ID card ready.
 Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Visualizing mechanical tension across membrane receptors with a fluorescent sensor
 Nature Methods Visualizing mechanical tension across membrane receptors with a fluorescent sensor Daniel R. Stabley, Carol Jurchenko, Stephen S. Marshall, Khalid S. Salaita Supplementary Figure 1 Fabrication
Nature Methods Visualizing mechanical tension across membrane receptors with a fluorescent sensor Daniel R. Stabley, Carol Jurchenko, Stephen S. Marshall, Khalid S. Salaita Supplementary Figure 1 Fabrication
Packaging Commercial CMOS Chips for Lab on a Chip Integration
 Supporting Information for Packaging Commercial CMOS Chips for Lab on a Chip Integration by Timir Datta-Chaudhuri, Pamela Abshire, and Elisabeth Smela Biocompatibility Although the supplier s instructions
Supporting Information for Packaging Commercial CMOS Chips for Lab on a Chip Integration by Timir Datta-Chaudhuri, Pamela Abshire, and Elisabeth Smela Biocompatibility Although the supplier s instructions
Patterning Surface-bound Microtubules through Reversible DNA Hybridization
 Patterning Surface-bound Microtubules through Reversible DNA Hybridization NANO LETTERS 2004 Vol. 4, No. 11 2127-2132 Gayatri Muthukrishnan, Caitlin A. Roberts, Yi-Chun Chen, Jeffrey D. Zahn, and William
Patterning Surface-bound Microtubules through Reversible DNA Hybridization NANO LETTERS 2004 Vol. 4, No. 11 2127-2132 Gayatri Muthukrishnan, Caitlin A. Roberts, Yi-Chun Chen, Jeffrey D. Zahn, and William
MSD Immuno-Dot-Blot Assays. A division of Meso Scale Diagnostics, LLC.
 MSD Immuno-Dot-Blot Assays Example: High Throughput Western Blots Replacements Traditional Western Blots High content Molecular weight and immunoreactivity Labor and protein intensive Inherently low throughput
MSD Immuno-Dot-Blot Assays Example: High Throughput Western Blots Replacements Traditional Western Blots High content Molecular weight and immunoreactivity Labor and protein intensive Inherently low throughput
Application Note #124 VITA: Quantitative Nanoscale Characterization and Unambiguous Material Identification for Polymers
 Local thermal analysis identifies polymer AFM image of polymer blend Application Note #124 VITA: Quantitative Nanoscale Characterization and Unambiguous Material Identification for Polymers VITA module
Local thermal analysis identifies polymer AFM image of polymer blend Application Note #124 VITA: Quantitative Nanoscale Characterization and Unambiguous Material Identification for Polymers VITA module
UV15: For Fabrication of Polymer Optical Waveguides
 CASE STUDY UV15: For Fabrication of Polymer Optical Waveguides Master Bond Inc. 154 Hobart Street, Hackensack, NJ 07601 USA Phone +1.201.343.8983 Fax +1.201.343.2132 main@masterbond.com CASE STUDY UV15:
CASE STUDY UV15: For Fabrication of Polymer Optical Waveguides Master Bond Inc. 154 Hobart Street, Hackensack, NJ 07601 USA Phone +1.201.343.8983 Fax +1.201.343.2132 main@masterbond.com CASE STUDY UV15:
MagSi Beads. Magnetic Silica Beads for Life Science and Biotechnology study
 MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
Current ( pa) Current (pa) Voltage (mv) Voltage ( mv)
 Current ( pa) 3000 2000 1000 0-1000 -2000-3000 a -400-200 0 200 400 Voltage (mv) P1 P2 P3 P4 P5 P6 P7 P8 P9 P10 P11 P12 P13 P14 P15 P16 P17 P18 Average Current (pa) 4500 3000 1500 0-1500 -3000-4500 b -400-200
Current ( pa) 3000 2000 1000 0-1000 -2000-3000 a -400-200 0 200 400 Voltage (mv) P1 P2 P3 P4 P5 P6 P7 P8 P9 P10 P11 P12 P13 P14 P15 P16 P17 P18 Average Current (pa) 4500 3000 1500 0-1500 -3000-4500 b -400-200
ULTRAPRECISION MICROMACHINING OF MICROFLUIDIC DEVICES BY USE OF A HIGH-SPEED AIRBEARING SPINDLE
 ULTRAPRECISION MICROMACHINING OF MICROFLUIDIC DEVICES BY USE OF A HIGH-SPEED AIRBEARING SPINDLE Chunhe Zhang 1, Allen Y. Yi 1, Lei Li 1, L. James Lee 1, R. Ryan Vallance 2, Eric Marsh 3 1 The Ohio State
ULTRAPRECISION MICROMACHINING OF MICROFLUIDIC DEVICES BY USE OF A HIGH-SPEED AIRBEARING SPINDLE Chunhe Zhang 1, Allen Y. Yi 1, Lei Li 1, L. James Lee 1, R. Ryan Vallance 2, Eric Marsh 3 1 The Ohio State
Chemotaxis assay using µ-slide Chemotaxis
 Chemotaxis assay using µ-slide Chemotaxis 1. General information The µ-slide Chemotaxis is a tool for observing chemotactical responses of adherent migrating cells over extended periods of time. The linear
Chemotaxis assay using µ-slide Chemotaxis 1. General information The µ-slide Chemotaxis is a tool for observing chemotactical responses of adherent migrating cells over extended periods of time. The linear
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
acgh studies using GeneFix collected and purified saliva DNA
 Application note: GFX-4 acgh studies using GeneFix collected and purified saliva DNA 1. Background 1.1 Basic Principles Array comparative genomic hybridization (acgh) is a novel technique for the detection
Application note: GFX-4 acgh studies using GeneFix collected and purified saliva DNA 1. Background 1.1 Basic Principles Array comparative genomic hybridization (acgh) is a novel technique for the detection
UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change
 A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
Molecular Communication: Simulation of a Molecular Motor Communication System
 Molecular Communication: Simulation of a Molecular Motor Communication System Michael Moore, Akihiro Enomoto, Tadashi Nakano, Tatsuya Suda Department of Computer Science Donald Bren School of Information
Molecular Communication: Simulation of a Molecular Motor Communication System Michael Moore, Akihiro Enomoto, Tadashi Nakano, Tatsuya Suda Department of Computer Science Donald Bren School of Information
Chem Lecture 5 Catalytic Strategies
 Chem 452 - Lecture 5 Catalytic Strategies 111026 Enzymes have evolved an array of different strategies or enhancing the power and specificity of the reactions they catalyze. For numerous enzymes the details
Chem 452 - Lecture 5 Catalytic Strategies 111026 Enzymes have evolved an array of different strategies or enhancing the power and specificity of the reactions they catalyze. For numerous enzymes the details
MST Starting Guide Monolith NT.115
 MST Starting Guide Monolith NT.115 Contents 1. How to design an experiment 2. Before you start 3. Assay Setup Pretests 4. Assay Setup 5. MST-experiment using temperature control 6. Data Interpretation
MST Starting Guide Monolith NT.115 Contents 1. How to design an experiment 2. Before you start 3. Assay Setup Pretests 4. Assay Setup 5. MST-experiment using temperature control 6. Data Interpretation
Practice Exam 3 MCBII
 1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
Isolation of Protein
 Isolation of Protein Ultra-centrifugation http://irfanchemist.wordpress.com/2009/04/19/isolation-of-protein / Protein solutions of various masses or densities may separated based on the time it takes to
Isolation of Protein Ultra-centrifugation http://irfanchemist.wordpress.com/2009/04/19/isolation-of-protein / Protein solutions of various masses or densities may separated based on the time it takes to
BASIC MOLECULAR GENETIC MECHANISMS Introduction:
 BASIC MOLECULAR GENETIC MECHANISMS Introduction: nucleic acids. (1) contain the information for determining the amino acid sequence & the structure and function of proteins (1) part of the cellular structures:
BASIC MOLECULAR GENETIC MECHANISMS Introduction: nucleic acids. (1) contain the information for determining the amino acid sequence & the structure and function of proteins (1) part of the cellular structures:
Solutions to 7.02 Quiz II 10/27/05
 Solutions to 7.02 Quiz II 10/27/05 Class Average = 83 Standard Deviation = 9 Range Grade % 87-100 A 43 74-86 B 39 55-73 C 17 > 54 D 1 Question 1 (56 points) While studying deep sea bacteria, you discover
Solutions to 7.02 Quiz II 10/27/05 Class Average = 83 Standard Deviation = 9 Range Grade % 87-100 A 43 74-86 B 39 55-73 C 17 > 54 D 1 Question 1 (56 points) While studying deep sea bacteria, you discover
Lab-on-a-Chip (LOC) Miniaturization on micro- and nanoscale.
 Lab-on-a-Chip (LOC) Miniaturization on micro- and nanoscale http://nanob2a.cin2.es/publication/articles/integrated-optical-devices-for-lab-on-a-chip-biosensing-applications, downloaded 14.04.16 www.kit.edu
Lab-on-a-Chip (LOC) Miniaturization on micro- and nanoscale http://nanob2a.cin2.es/publication/articles/integrated-optical-devices-for-lab-on-a-chip-biosensing-applications, downloaded 14.04.16 www.kit.edu
Supplementary Figure 1. Binding capacity of the nanoparticles. Protein A-coated magnetic nanoparticles were mixed with FITC-antibody (antibody
 Supplementary Figure 1. Binding capacity of the nanoparticles. Protein A-coated magnetic nanoparticles were mixed with FITC-antibody (antibody conjugated with FITC) and PE-antibody (antibody conjugated
Supplementary Figure 1. Binding capacity of the nanoparticles. Protein A-coated magnetic nanoparticles were mixed with FITC-antibody (antibody conjugated with FITC) and PE-antibody (antibody conjugated
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
DNA Microarray Technology
 2 DNA Microarray Technology 2.1 Overview DNA microarrays are assays for quantifying the types and amounts of mrna transcripts present in a collection of cells. The number of mrna molecules derived from
2 DNA Microarray Technology 2.1 Overview DNA microarrays are assays for quantifying the types and amounts of mrna transcripts present in a collection of cells. The number of mrna molecules derived from
Movement at the Molecular Level
 Movement at the Molecular Level Diffusion: = 6 D t (D 6 π µ a) Typical numbers: 10 nm protein in water D= 10-10 m 2 /s.in cells D= 10-12 m 2 /s (D= 10-14 m 2 /s lipids) [] 1/2 =1 µm, t ~0.2
Movement at the Molecular Level Diffusion: = 6 D t (D 6 π µ a) Typical numbers: 10 nm protein in water D= 10-10 m 2 /s.in cells D= 10-12 m 2 /s (D= 10-14 m 2 /s lipids) [] 1/2 =1 µm, t ~0.2
Chapter 15. Cytoskeletal Systems. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
CELL BIOLOGY - CLUTCH CH CYTOSKELETON AND CELL MOVEMENT.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
!! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
