High-Speed FLIM Data Acquisition by Time-Correlated Single Photon Counting
|
|
|
- Gabriella Hamilton
- 5 years ago
- Views:
Transcription
1 High-Speed FLIM Data Acquisition by Time-Correlated Single Photon Counting Wolfgang Becker 1, Axel Bergmann 1, Giovanni Biscotti 1, Karsten Koenig 2, Iris Riemann 2, Laimonas Kelbauskas 3, Christoph Biskup 3 1 Becker & Hickl GmbH, Nahmitzer Damm 30, Berlin, Germany 2 Fraunhofer Institute for Biomedical Engineering, St. Ingbert, Germany 3 Friedrich Schiller University, Institute of Physiology II, Teichgraben 8, Jena, Germany In this study, we describe a time-correlated single photon counting (TCSPC) technique for multi-wavelength lifetime imaging in laser-scanning microscopes. The technique is based on a four-dimensional histogramming process that records the photon density versus the time in the fluorescence decay, the x-y coordinates of the scanning area and the wavelength. It avoids any time gating or wavelength scanning and, therefore, yields a near-ideal counting efficiency. The decay functions are recorded in a large number of time channels, and the components of a multi-exponential decay can be resolved down to less than 30 ps. A single TCSPC imaging channel works with a high detection efficiency up to a photon count rate of about s -1. A modified version of the TCSPC fluorescence lifetime imaging (FLIM) technique uses several fully parallel detector and TCSPC channels. It operates at a count rate of more than 10 7 photons per second and records double-exponential FLIM data within less than 10 seconds. Keywords: Fluorescence lifetime imaging, FLIM, FRET, autofluorescence, time-correlated single photon counting, TCSPC 1. Introduction Confocal 1 and two-photon laser scanning microscopes 2 have considerably improved the image quality in fluorescence microscopy 3. The optical sectioning capability and the increased contrast make them superior to conventional wide-field fluorescence microscopes. Recent techniques exploit the spectral characteristics of the fluorescence emitted from the sample to better separate different fluorophores 4. However, the fluorescence light emitted by organic molecules is not only characterised by its emission spectrum. It has also a specific lifetime, which can be used to distinguish fluorophores whose spectra are too similar to be separated spectrally. This approach has been particularly useful to distinguish the autofluorescence components in tissues. These components often have poorly defined fluorescence spectra but are clearly distinguished by their fluorescence lifetime 5,6. The ability of FLIM to identify fluorophores has also been used to verify cell transfection 7. The fluorescence lifetime of an excited fluorophore is mostly independent of its concentration, but it can be influenced by its local environment 8. Changes of the fluorescence lifetime can be used as an indicator of the energy transfer rate to the local environment. Thus, FLIM is a direct approach to measure all effects that change the quantum efficiency of a fluorophore. Typical examples are mapping of cell parameters such as ph, ion concentrations or oxygen saturation by fluorescence quenching 9-12, as well as probing protein or DNA structures by lifetime sensitive dyes 13,14. By exploiting fluorescence - or Förster - resonance energy transfer (FRET) fluorescence lifetime measurements can be used to prove the molecular proximity of proteins, which are labelled with appropriate donor and acceptor fluorophores. The rate of energy transfer from the donor to the acceptor depends on the inverse sixth power of the distance between the fluorophores. Because of this steep relationship FRET occurs only within distances of a few nm. Thus, FRET is a direct indicator for the molecular proximity of donor and acceptor fluorophores and an indirect indicator of an association of the protein molecules to which the fluorophores are attached When FRET occurs, both the intensity and the lifetime of the donor fluorescence decrease, whereas the intensity of the acceptor emission is increased. The decrease of the fluorescence lifetime is related to the rate constant of the energy transfer and can be used to estimate the distance between donor and acceptor fluorophores. In contrast to steady-state FRET techniques, FLIM has the advantage of being independent of the local concentrations of donor and acceptor molecules. Therefore, FRET results can be obtained from a single lifetime image of the donor. The fluorescence lifetimes of typical fluorophores used in cell imaging are of the order of a few nanoseconds. However, in the presence of quenchers fluorescence lifetimes can decrease to the sub-nanosecond range. The lifetimes of Proc. SPIE 5323 (2004) 1
2 autofluorescence components can be as short as 100 ps 5,6,15. Even shorter lifetimes are found in dye aggregates 20 and complexes of dyes and metallic nano-particles 21,22. Due to the presence of several fluorophores with different lifetimes or due to non-uniform quenching the fluorescence decay functions observed in cells and tissues are normally multiexponential. Therefore, a lifetime imaging technique should be able to resolve the components of multi-exponential fluorescence decay functions down to less than 100 ps. Moreover, it should be compatible with two-photon and confocal laser scanning microscopes. Rough single-exponential lifetimes can be derived from data containing a few hundred photons per pixel. This is not more than required for a mediocre steady state image. However, multi-exponential lifetime analysis requires much more photons 23. Due to prolonged excitation photobleaching can constitute a problem in precision FLIM experiments. Thus, recording efficiency is an important issue in FLIM experiments. A good FLIM technique should have a high recording efficiency, i.e. it should not discard any photons, neither by gating off photons on the time axis, nor by blocking a part of the emission spectrum by filters. It should record the entire fluorescence decay simultaneously in several detection channels, covering the entire emission spectrum. FLIM techniques are normally classified into frequency domain techniques, and time-domain techniques. Frequency domain techniques measure the phase shift between the high-frequency modulated or pulsed excitation, and the fluorescence signal at the fundamental modulation frequency or its harmonics Time-domain techniques record the fluorescence decay functions directly Although the frequency-domain and the time domain are equivalent, the corresponding signal recording techniques may differ considerably in efficiency, i.e. in the lifetime accuracy obtained for a given number of detected photons 40,41. Both the frequency-domain and time-domain FLIM techniques can use either camera techniques or point-detector scanning techniques. Frequency domain camera techniques are based on modulated image intensifiers, frequency domain point-detector techniques on modulated photomultiplier tubes (PMTs) or on photomultiplier tubes with subsequent electronic mixers. Time domain camera techniques use gated image intensifiers. Time-domain point-detector techniques employ time-correlated single photon counting (TCSPC), or gated photon counting with several parallel time-gates. Camera techniques are used in wide-field microscopes, but they cannot reasonably exploit the benefits of the scanning microscope. Point detector techniques cannot be used for wide field imaging but are compatible with laser scanning microscopes. Therefore, a FLIM technique that can exploit the high spatial resolution of a laser scanning microscope should be a high-efficiency point-detector technique. For the reasons mentioned above it should record images with a timeresolution better than 100 ps, resolve multi-exponential decay functions, and record the fluorescence signal in several wavelength intervals simultaneously. 2. Multi-dimensional TCSPC technique Time-correlated single-photon counting (TCSPC) delivers a near-ideal efficiency and an optimal time-resolution for a given detector. It acquires the signal in a sufficiently high number of time-channels so that multi-exponential decay functions can be resolved 42. For a long time, TCSPC has been considered to be intrinsically slow and one-dimensional and therefore not useful for fluorescence lifetime imaging. Certainly, this reputation came from early flashlamp-based TCSPC lifetime spectrometers which indeed needed extremely long acquisition times. Compared to early TCSPC setups advanced TCSPC devices can be used at count rates which are two orders of magnitude higher and have correspondingly shorter acquisition times. Moreover, advanced TCSPC techniques are able to use a multi-dimensional histogramming process. They can built up a histogram for the photons emitted from the sample not only versus the time axis, but also versus the coordinates of the scanning area, and the wavelength. The principle of multi-dimensional TCSPC is shown in Fig. 1. At the input of the detection system are a number of photomultipliers (PMTs), typically detecting the fluorescence signal in different wavelength intervals. The pulses of the PMTs are combined in the subsequent router into a common timing pulse line. Furthermore, for each photon, the router delivers the number of the PMT in which the photon was detected. The routing technique can be used for several individual PMTs and for multi-anode PMTs. The TCSPC module receives the timing pulse, the PMT channel number, and the scan clock signals (frame sync, line sync and pixel clock) from the scanning unit of the microscope. For each photon, the TCSPC module determines the location within the scanning area (X and Y), the time of the photon with respect to the laser pulse sequence (t), and the 2 Proc. SPIE 5323 (2004)
3 detector channel number (n). These values are used to address the histogram memory in which the detection events are accumulated. Thus, in the memory the distribution of the photon density over X, Y, t, and n is built up. PMTs Channel Router Channel register n Channel / Wavelength Timing Start Time Measurement CFD TAC ADC t Time within decay curve Stop from Laser CFD Frame Sync Line Sync Pixel Clock from Microscope Counter Y Scanning Interface Counter X y x Histogram Memory Detector channel 1 Histogram Memory Detector channel 2 Location within scanning area Histogram Memory Detector channel 3 Histogram Memory Detector channel 4 Figure 1: Principle of multidimensional TCSPC lifetime imaging The data acquisition runs at any scanning speed of the microscope. As many frame scans as necessary to obtain an appropriate signal-to-noise ratio can be accumulated. Due to the synchronisation via the scan clock pulses, the regular zoom and image rotation functions of the microscope act automatically on the TCSPC recording and can be used in the normal way. The recording process does not use any time gating, wavelength scanning, or detector multiplexing. Under ideal conditions all detected photons contribute to the result, and a maximum signal-to-noise ratio for a given fluorescence intensity and acquisition time is obtained. However, the signal processing of a recorded photon causes a dead time during which the TCSPC electronics is unable to process another photon event. As long as the photon detection rate is small compared to the reciprocal dead time of the TCSPC module the counting efficiency is close to 100%. For a detector count rate of the reciprocal dead time the efficiency is 50%, which is still better than for most other signal recording techniques. The recorded count rate at an efficiency of 50% is defined as the maximum useful count rate. For the fastest TCSPC modules the dead time is 100 ns, corresponding to a maximum useful count rate of s -1. Another limitation of the count rate is the pile-up effect. Pile-up results from the fact that a single TCSPC device can record only one photon per excitation pulse. Pile-up is negligible for a count rate of 1% of the laser pulse frequency and remains tolerable up to a count rate of 5% of the laser pulse frequency. For the lasers currently used in lifetime microscopy the pulse frequency is 80 to 90 MHz, corresponding to a pile-up limited count rate of to s -1. It remains questionable whether count rates this high can be obtained from living cells, especially under two-photon excitation. Nevertheless, cells stained with extremely stable fluorophores, or large-area tissue samples under one-photon excitation may yield count rates higher than the limits given above. To record count rates this high in diffuse optical tomography, packages of several TCSPC modules have been operated in a single Pentium PC 43. Here, we applied a similar setup of four TCSPC FLIM cards to microscopic samples. With four detectors, connected to four separate TCSPC channels, the total useful count rate of this system is s Single-Channel TCSPC System The single-channel TCSPC system uses two ultra-fast Hamamatsu R3809U MCP-PMTs connected to the non-descanned detection (NDD) module of a Zeiss LSM 510 laser scanning microscope. The two R3809U MCPs are connected to a single Becker & Hickl SPC-830 TCSPC module via a HRT-41 routing module (Becker & Hickl, Berlin). The instrument response (FWHM) of the system is 28 ps 16. Figure 2 shows an autofluorescence image of aorta tissue recorded in one detector channel. The figure shows the intensity image calculated from the photons in all time channels of the pixels, and a lifetime image. In the bottom panel the fluorescence decay recorded in a selected spot is shown. Obviously, this Proc. SPIE 5323 (2004) 3
4 fluorescence decay could not be approximated by a single exponential function, but had to be approximated by a biexponential function. The parameters of the fit (i.e. lifetime components and intensity coefficients) are shown in the lower right panel. To obtain a lifetime image from the decay data of the pixels, the lifetime components of the double exponential fit are weighted by their intensity coefficients and averaged. In the lifetime image, the mean lifetime obtained for each pixel is encoded by colour. The distribution of the mean lifetime over the entire image is shown in the upper right panel. Figure 2: Fluorescence lifetime image of an aorta tissue sample excited by two-photon absorption. Upper row: Intensity image, lifetime image with mean fluorescence lifetime encoded by colour, distribution of the mean lifetime over the entire image. Lower row: fluorescence decay curve in the selected spot and parameters recovered in the fit. Merging the components of the double exponential decay into a mean lifetime yields a single-exponential approximation of the data but certainly neglects useful information. Fig. 3 shows images of the short and the long lifetime component, and of the ratio of their intensity coefficients, encoded by colour. Figure 3: Images of the individual decay components of the sample shown in Fig. 2. Left: Fast lifetime component (blue to red = 0.1 to 0.5 ns), Centre: Slow lifetime component (blue to red = 1.8 to 3.0 ns), Right: Intensity ratio of fast to slow lifetime component (blue to red = 1.5 to 5.0) The image of the intensity ratio of the lifetime components shows more detail than the lifetime images of the components. It represents directly the relative concentrations of the molecules emitting the fast and slow decay components. 4 Proc. SPIE 5323 (2004)
5 The high time-resolution and the multi-detector capability of the FLIM system makes it excellently suitable for FLIMbased FRET measurements. To get images from the donor (CFP) and the acceptor (YFP) simultaneously, the fluorescence light was split into a 480 ± 15 nm and a 535 ± 13 nm detection channel. Splitting can be achieved either via the Zeiss NDD switch box or via an external dichroic beamsplitter and bandpass filters. The filters were selected to detect the fluorescence of the CFP and the YFP, respectively. Figures 4-6 show measurements of an HEK cell expressing two interacting proteins, which are labeled with CFP and YFP, respectively. Fluorescence was excited by two-photon absorption at 860 nm. The TCSPC images were recorded with 128 x 128 pixels, i.e. with a 4 x 4 binning of the pixels of the regular 512 x 512 LSM 510 scan. The total count rate was reduced to 60,000 s -1 in order to avoid any detectable photobleaching or possible formation of photoisomers. To obtain enough photons for high accuracy data analysis the images were acquired for 20 minutes. Donor and acceptor intensity images of the cell are shown in Fig. 4. The fluorescence decay recorded in the indicated region is shown in Fig. 5. Figure 4: HEK cell expressing two interacting proteins labeled with CFP and YFP. Left: Intensity image recorded in the donor channel. Right: Intensity image recorded in the acceptor channel. The marker indicates the position at which the fluorescence decay curves of Fig 5 are obtained. Figure 5: Fluorescence decay curves in the spots indicated in Fig. 4. Left: Fluorescence decay recorded in the donor channel. Right: Fluorescence decay recorded in the acceptor channel. Donor fluorescence recorded in this channel has been subtracted. The decay functions in the pixels of the donor image are double exponential, with a fast lifetime component representing the quenched and a slow lifetime component emerging from the unquenched donor fraction. A Levenberg-Marquardt fit delivers a fast lifetime component of τ f = 0.68 ns and a slow component of τ s = 2.30 ns. The intensity coefficients are A f = 0.44 and A s = 0.56, respectively. Due to the energy transfer from the CFP to YFP, the YFP fluorescence should be double-exponential as well, with a short lifetime component corresponding to the short lifetime component of the donor. However, a f should be negative, due to gradual transfer of energy from the donor 44. Unfortunately the fluorescence spectrum of the CFP has a long wavelength tail that extends far into the spectrum of the YFP. Therefore the YFP channel detects a considerable amount of CFP fluorescence, so that the true shape of the acceptor decay function is not visible in the raw data. The leakage was determined by imaging cells containing only CFP. It was found to be 60 % for the filter combination used 16. By subtracting the known percentage of CFP leakage from the YFP data, corrected decay curves of the YFP were obtained. The result for the indicated pixel is shown in the right panel of Fig. 5. The decay is double exponential, with a fast lifetime component τ f = 0.66 ns, and a slow component τ s = 2.86 ns. The intensity Proc. SPIE 5323 (2004) 5
6 coefficients are A f = -0.4 and A s = 0.6. Images of the intensity ratios of the lifetime components, A f /A s, for the donor and -A f /A s for the acceptor are shown in Fig. 6. For the donor, A f /A s represents the ratio of the number of quenched and unquenched molecules. Figure 6: Image of the ratio of the intensity coefficients A f / A s. A f / A s is encoded by colour, from 0.2 (blue) to 1.0 (red). Left: Intensity ratios calculated for the donor channel. Right: Intensity ratios calculated for the acceptor channel 4. Parallel Four-Channel TCSPC System To record images at a high count rate a package of four fully parallel TCSPC imaging cards (Becker & Hickl, SPC-144) was used. The fluorescence light was distributed into four detectors (Becker & Hickl PMH-100) without wavelength dispersion. Each detector was connected to an individual TCSPC channel. The image size in all TCSPC channels was 256 x 256 pixels x 64 time channels. Fig. 7 shows a 16 µm section of a mouse kidney (Molecular Probes, F-24630) stained with Alexa Fluor 488, Alexa Fluor 568 phalloidin, and DAPI. The excitation wavelength was tuned to 860 nm. The count rate was about s -1 per TCSPC channel, corresponding to a total count rate of about s -1. Fig 7, left, shows a lifetime image of the combined photon data of all four channels. A double exponential Leveberg-Marquardt fit was applied to the decay data in the individual pixels. The colour of the image represents the amplitude-weighted mean lifetime of the double exponential decay. In Fig. 7, middle and right, the relative amplitudes of the two lifetime components are encoded by colour. The images show that double exponential lifetime analysis of TCSPC data can be used to unmix fluorophores even if they are recorded in the same pixel. In the same way, the relative amounts of associated and free forms of proteins can be retrieved from FRET measurements 15,16,19. Figure 7: Mouse kidney sample stained with Alexa Fluor 488 wheat germ agglutinin, Alexa Fluor 568 phalloidin, and DAPI, recorded with four detectors connected to separate TCSPC channels. Left: Lifetime image, the colour represents amplitude-weighted mean lifetime, blue to red = 0.7 to 1.7 ns. Middle and Right: Images of the intensity coefficients of the fast and slow lifetime components. The relative intensity of the lifetime components is encoded by colour, blue to red = 0.1 to Proc. SPIE 5323 (2004)
7 The good quality of the images shows that TCSPC can be used to obtain double exponential lifetime images in 10 seconds. However, to obtain high count rates high excitation powers have to be used, which can destroy the sample by photodestruction or thermal effects. Fig. 8 shows a sequence of lifetime images obtained from the same specimen shown in Fig. 7. The upper row shows a series of intensity images, which were calculated from subsequent measurements by summing up fluorescence signals of the four detectors and of all time channels in each pixel. The acquisition time of each image was 20 seconds. The initial total count rate was s -1. The lower row shows the distribution of the mean lifetimes over the image. Figure 8: Sequence of images recorded for 20 seconds each. The initial count rate was s -1. Upper row: Intensity images obtained from the recordings in all time channels. Lower row: Distribution of the mean lifetime in the images (from 0.5 to 2.0 ns). Photobleaching and thermal effects cause a progressive loss in intensity and a variation in the mean lifetime. The sequence shows that the high excitation power, to which the sample had to be exposed to achieve high count rates, causes photobleaching and thermal destruction of the sample. Since not all fluorophores are equally susceptible to photobleaching the distribution of the mean lifetime changes during the exposure. The results show that such effects can bias lifetime measurements considerably. 4. Summary TCSPC FLIM in conjunction with laser scanning microscopes delivers multi-exponential fluorescence lifetime images in several detector channels simultaneously. The detectors can either be connected to a single TCSPC card or to separate channels of a package of TCSPC cards operated in a single computer. The single-tcspc system is efficient up to a count rate of a few 10 6 s -1. Via double-exponential lifetime analysis, this system is able to distinguish between different fluorophores in a single pixel or to distinguish between associated and free donor molecules in FRET experiments. The four-channel TCSPC system can be operated at count rates in excess of 10 7 s -1 and delivers double exponential FLIM data in less than 10 s. Imaging at a count rate this high in a two-photon microscope induces noticeable photobleaching and lifetime changes over an acquisition time of a few 10 s. Thus, in practice it is not the TCSPC technique which imposes limits to the maximum count rate. It is rather the sample itself which tolerates only a moderate excitation power. 5. References 1. J.G. White, W.B. Amos, M. Fordham. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J. Cell. Biol. 105, 41-48, W. Denk, J.H. Strickler, W.W. Webb. Two-photon laser scanning fluorescence microscopy. Science 24, 73-76, Proc. SPIE 5323 (2004) 7
8 3. J. Pawley (Editor). Handbook of biological confocal microscopy. Plenum, New York, M.E. Dickinson, C.W. Waters, G. Bearman, R. Wolleschensky, S. Tille, S.E. Fraser, Sensitive imaging of spectrally overlapping fluorochromes using the LSM 510 META. Proc. SPIE, 4620, , K. König, I. Riemann. High resolution optical tomography of human skin with subcellular resolution and picosecond time resolution. J. Biomed. Opt. 8, , D. Schweitzer, A. Kolb, M. Hammer, E. Thamm, Basic investigations for 2-dimensional time-resolved fluorescence measurements at the fundus. Int. Ophthalmol. 23, , U.K. Tirlapur, K. König. Targeted transfection by femtosecond laser. Nature 418, , J.R. Lakowicz. Principles of Fluorescence Spectroscopy, 2nd ed., Plenum Press, New York, W.R.G. Baeyens, D. de Keukeleire, K. Korkidis. Luminescence techniques in chemical and biochemical analysis. Dekker, New York, M.R. Eftink. Fluorescence quenching: Theory and application. In : J.R. Lakowicz, Topics in fluorescence spectrocopy, Vol 2, Plenum Press, New York 1991, pp J.R. Lakowicz, H. Szmacinski. Fluorescence-lifetime based sensing of ph, Ca 2+, and glucose. Sens. Actuator Chem. 11, , H.C. Gerritsen, R. Sanders, A. Draaijer, Y.K. Levine. Fluorescence lifetime imaging of oxygen in cells. J. Fluoresc. 7, 11-16, M.A.M.J. Van Zandvoort, C.J. de Grauw, H.C. Gerritsen, J.L.V. Broers, M.G.A. Egbrink, F.C.S. Ramaekers, D.W. Slaaf, Discrimination of DNA and RNA in cells by a vital fluorescent probe: Lifetime imaging of SYTO13 in healthy and apoptotic cells. Cytometry 47, , J.P. Knemeyer, N. Marmé, M. Sauer. Probes for detection of specific DNA sequences at the single molecule level. Anal. Chem. 72, , W. Becker, K. Benndorf, A. Bergmann, C. Biskup, K. König, U. Tirplapur, Th. Zimmer. FRET Measurements by TCSPC laser scanning microscopy. Proc. SPIE, 4431, 94-98, W. Becker, A. Bergmann, C. Biskup, L. Kelbauskas, T. Zimmer, N. Klöcker, K. Benndorf, High resolution TCSPC lifetime imaging. Proc. SPIE 4963, M. Elangovan, R.N. Day, A. Periasami, Nanosecond fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy to localize the protein interactions in a single cell. J. Microsc. 205, 3-14, M. Tramier, I. Gautier, T. Piolot, S. Ravalet, K. Kemnitz, J. Coppey, C. Durieux, V. Mignotte, M. Coppey-Moisan. Picosecond-hetero-FRET microscopy to probe protein-protein interactions in live cells. Biophys. J. 83, , B.J. Bacskai, J. Skoch, G.A. Hickey, R. Allen, B.T. Hyman. Fluorescence resonance energy transfer determinations using multiphoton fluorescence lifetime imaging microscopy to characterize amyloid-beta plaques. J. Biomed. Opt. 8, , L. Kelbauskas, W. Dietel, Internalization of aggregated photosensitizers by tumor cells: Subcellular time-resolved fluorescence spectroscopy on derivates of pyropheophorbide-a ethers and chlorin e6 under femtosecond one- and two-photon excitation. Photochem. Photobiol. 76, , C.D. Geddes, H. Cao, I. Gryczynski, Z. Gryczynski, J. Fang, J.R. Lakowicz. Metal-enhanced fluorescence (MEF) due to silver colloids on a planar surface: Potential applications of indocyanine green to in vivo imaging. J. Phys. Chem. A 107, , J. Malicka, I. Gryczynski, C.D. Geddes, J.R. Lakowicz. Metal-enhanced emission from indocyanine green: a new approach to in vivo imaging. J. Biomed. Opt , M. Köllner, J. Wolfrum. How many photons are necessary for fluorescence-lifetime measurements? Chem. Phys. Lett. 200, , G.H. Patterson, D.W. Piston. Photobleaching in two-photon exciatation microscopy. Biophys. J. 78, , A.A. Heikal, W.W. Webb. One- and two-photon time-resolved fluorescence spectroscopy of selected fluorescent markers: photobleaching, triple-, and singlet-state dynamics. Biophys. J., 73, , Proc. SPIE 5323 (2004)
9 26. A. Hopf, E. Neher. Highly nonlinear Photodamage in two-photon fluorescence microscopy. Biophys. J., 80, , K. Carlsson, A. Liljeborg. Confocal fluorescence microscopy using spectral and lifetime information to simultaneously record four fluorophores with high channel separation. J. Microsc , J.R. Lakowicz, K. Berndt. Lifetime-selective fluorescence lifetime imaging using an rf phase-sensitive camera. Rev. Sci. Instrum. 62, , P.T.C. So, T. French, E. Gratton. A frequency domain microscope using a fast-scan CCD camera. Proc. SPIE 2137, 83-92, P.T.C. So, T. French, W.M. Yu, K.M. Berland, C.Y. Dong, E. Gratton, Time-resolved fluorescence microscopy using two-photon excitation. Bioimaging 3, 49-63, A. Squire, P.J. Verveer, P.I.H. Bastiens: Multiple frequency fluorescence lifetime imaging microscopy. J. Microsc. 197, , R. Pepperkok, A. Squire, S. Geley, P.I.H. Bastiaens, Simultaneous detection of multiple green fluorescent proteins in live cells by fluorescence lifetime imaging microscopy. Curr. Biol. 9, , M. Straub, S. W. Hell, Fluorescence lifetime three-dimensional microscopy with picosecond precision using a multifocal multiphoton mocroscope. Appl. Phys. Lett. 73, , M.J. Cole, J.Siegel, R. Dowling, M.J. Dayel, D. Parsons-Karavassilis, P.M. French, M.J. Lever, L.O. Sucharov, M.A. Neil, R. Juskaitas, T. Wilson, Time-domain whole-field lifetime imaging with optical sectioning. J. Microsc. 203, , E.P. Buurman, R. Sanders, A. Draaijer, H.C. Gerritsen, J.J.F. van Veen, P.M. Houpt, Y.K. Levine, Fluorescence lifetime imaging using a confocal laser scanning microscope. Scanning 14, , J. Syrtsma, J.M. Vroom, C.J. de Grauw, H.C. Gerritsen. Time-gated fluorescence lifetime imaging and microvolume spectroscopy using two-photon excitation. J. Microsc. 191, 39-51, I. Bugiel, K. König, H. Wabnitz. Investigations of cells by fluorescence laser scanning microscopy with subnanosecond resolution. Lasers in the Life Sciences 3, 47-53, H. Brismar, B. Ulfhake. Fluorescence lifetime measurements in confocal microscopy of neurons labeled with multiple fluorophores. Nat. Biotech. 15, , W. Becker, A. Bergmann, K. Koenig, U. Tirlapur. Picosecond fluorescence lifetime microscopy by TCSPC imaging. Proc. SPIE 4262, , K. Carlsson, J.P. Philip. Theoretical investigation of the signal-to-noise ratio for different fluorescence lifetime imaging techniques. Proc. SPIE 4622, 70-78, J.P. Philip, K. Carlsson, Theoretical investigation of the signal-to-noise ratio in fluorescence lifetime imaging. J. Opt. Soc. Am. A, 20, , D.V. O Connor, D. Phillips. Time Correlated Single Photon Counting. Academic Press, London, W. Becker, A. Bergmann, H. Wabnitz, D. Grosenick, A. Liebert. High count rate multichannel TCSPC for optical tomography. Proc. SPIE 4431, , M.K.Y. Hughes, S. Ameer-Beg, M. Peter, T. Ng. Use of acceptor fluorescence for determining FRET lifetimes. Proc. SPIE 5139, 88-96, 2003 Proc. SPIE 5323 (2004) 9
Advanced time-correlated single photon counting technique for spectroscopy and imaging in biomedical systems
 Advanced time-correlated single photon counting technique for spectroscopy and imaging in biomedical systems Wolfgang Becker 1, Axel Bergmann 1, Giovanni Biscotti 1, Angelika Rück 2 1 Becker & Hickl GmbH,
Advanced time-correlated single photon counting technique for spectroscopy and imaging in biomedical systems Wolfgang Becker 1, Axel Bergmann 1, Giovanni Biscotti 1, Angelika Rück 2 1 Becker & Hickl GmbH,
Fluorescence Lifetime Imaging by Time-Correlated Single-Photon Counting
 MICROSCOPY RESEARCH AND TECHNIQUE 63:58 66 (2004) Fluorescence Lifetime Imaging by Time-Correlated Single-Photon Counting W. BECKER, 1 * A. BERGMANN, 1 M.A. HINK, 2 K. KÖNIG, 3 K. BENNDORF 4, AND C. BISKUP
MICROSCOPY RESEARCH AND TECHNIQUE 63:58 66 (2004) Fluorescence Lifetime Imaging by Time-Correlated Single-Photon Counting W. BECKER, 1 * A. BERGMANN, 1 M.A. HINK, 2 K. KÖNIG, 3 K. BENNDORF 4, AND C. BISKUP
Fluorescence lifetime images and correlation spectra obtained by multi-dimensional TCSPC
 Fluorescence lifetime images and correlation spectra obtained by multi-dimensional TCSPC Wolfgang Becker 1, Axel Bergmann 1, Elke Haustein 2, Zdenek Petrasek 2, Petra Schwille 2, Christoph Biskup 3, Tiemo
Fluorescence lifetime images and correlation spectra obtained by multi-dimensional TCSPC Wolfgang Becker 1, Axel Bergmann 1, Elke Haustein 2, Zdenek Petrasek 2, Petra Schwille 2, Christoph Biskup 3, Tiemo
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Fluorescence Lifetime Imaging Using a Confocal Laser Scanning Microscope
 SCANNING Vol. 14,155-159 (1992) OFAMS. Inc. Received: April 1, 1992 Fluorescence Lifetime Imaging Using a Confocal Laser Scanning Microscope E.P. BUURMAN,*? R. SANDERS,*? A. DRAAIJER," H.C. GERRITSEN,?
SCANNING Vol. 14,155-159 (1992) OFAMS. Inc. Received: April 1, 1992 Fluorescence Lifetime Imaging Using a Confocal Laser Scanning Microscope E.P. BUURMAN,*? R. SANDERS,*? A. DRAAIJER," H.C. GERRITSEN,?
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Special Techniques 1. Mark Scott FILM Facility
 Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope
 High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
Experts in Femtosecond Laser Technology. DermaInspect. Non-invasive multiphoton tomography of human skin
 Experts in Femtosecond Laser Technology DermaInspect Non-invasive multiphoton tomography of human skin In vivo optical biopsies with subcellular spatial resolution based on near infrared femtosecond laser
Experts in Femtosecond Laser Technology DermaInspect Non-invasive multiphoton tomography of human skin In vivo optical biopsies with subcellular spatial resolution based on near infrared femtosecond laser
Analysis of histology specimens using lifetime multiphoton microscopy
 Journal of Biomedical Optics 8(3), 376 380 (July 2003) Analysis of histology specimens using lifetime multiphoton microscopy Kevin W. Eliceiri Ching-Hua Fan* Laboratory for Optical and Computational Instrumentation
Journal of Biomedical Optics 8(3), 376 380 (July 2003) Analysis of histology specimens using lifetime multiphoton microscopy Kevin W. Eliceiri Ching-Hua Fan* Laboratory for Optical and Computational Instrumentation
D e c N o. 2 8
 D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss LSM 710 In Tune with Your Application Enjoy new freedom in selecting fluorescent dyes with In Tune, the new laser system for the LSM 710. Whatever the wavelength, you can match
Microscopy from Carl Zeiss LSM 710 In Tune with Your Application Enjoy new freedom in selecting fluorescent dyes with In Tune, the new laser system for the LSM 710. Whatever the wavelength, you can match
Spectral Separation of Multifluorescence Labels with the LSM 510 META
 Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
The DCS-120 Confocal Scanning FLIM System
 The DCS-120 Confocal Scanning FLIM System An Overview Abstract: The DCS-120 system uses excitation by ps diode lasers or femtosecond titanium-sapphire lasers, fast scanning by galvanometer mirrors, confocal
The DCS-120 Confocal Scanning FLIM System An Overview Abstract: The DCS-120 system uses excitation by ps diode lasers or femtosecond titanium-sapphire lasers, fast scanning by galvanometer mirrors, confocal
Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging
 SciMethods: Applications Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging Kim Doré 1, Jonathan Aow 1, Roberto Malinow 1 & Christian Wilms 2 1 : Department of Neuroscience and
SciMethods: Applications Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging Kim Doré 1, Jonathan Aow 1, Roberto Malinow 1 & Christian Wilms 2 1 : Department of Neuroscience and
Widefield Microscopy Bleed-Through
 In widefield microscopy the excitation wavelengths which illuminate the sample, and the emission wavelengths which reach the CCD camera are selected throughout a filter cube. A filter cube consists of
In widefield microscopy the excitation wavelengths which illuminate the sample, and the emission wavelengths which reach the CCD camera are selected throughout a filter cube. A filter cube consists of
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Global Analysis of Fluorescence Lifetime Imaging Microscopy Data
 Biophysical Journal Volume 78 April 2000 2127 2137 2127 Global Analysis of Fluorescence Lifetime Imaging Microscopy Data Peter J. Verveer, Anthony Squire, and Philippe I. H. Bastiaens Cell Biophysics Laboratory,
Biophysical Journal Volume 78 April 2000 2127 2137 2127 Global Analysis of Fluorescence Lifetime Imaging Microscopy Data Peter J. Verveer, Anthony Squire, and Philippe I. H. Bastiaens Cell Biophysics Laboratory,
Contact Details. Dr Alexander Galkin. Office: MBC Room 186. Tel: (028) Frequency and wavelength.
 Contact Details The electromagnetic spectrum Biological Spectroscopy Dr Alexander Galkin Email: a.galkin@qub.ac.uk Dr Alexander Galkin MSc Biomolecular Function - BBC8045 Office: MBC Room 186 Tel: (028)
Contact Details The electromagnetic spectrum Biological Spectroscopy Dr Alexander Galkin Email: a.galkin@qub.ac.uk Dr Alexander Galkin MSc Biomolecular Function - BBC8045 Office: MBC Room 186 Tel: (028)
Supplementary Table 1. Components of an FCS setup (1PE and 2PE)
 Supplementary Table 1. Components of an FCS setup (1PE and 2PE) Component and function Laser source Excitation of fluorophores Microscope with xy-translation stage mounted on vibration isolated optical
Supplementary Table 1. Components of an FCS setup (1PE and 2PE) Component and function Laser source Excitation of fluorophores Microscope with xy-translation stage mounted on vibration isolated optical
Workshop advanced light microscopy
 Workshop advanced light microscopy Multi-mode confocal laser scanning microscope Jan Willem Borst Laboratory of Biochemistry Biomolecular Networks www.bic.wur.nl MicroSpectroscopy Centre Wageningen Microspectroscopy
Workshop advanced light microscopy Multi-mode confocal laser scanning microscope Jan Willem Borst Laboratory of Biochemistry Biomolecular Networks www.bic.wur.nl MicroSpectroscopy Centre Wageningen Microspectroscopy
The Sweet PIE (Pulsed Interleaved Excitation)
 TECHNICAL NOTE The Sweet PIE (Pulsed Interleaved Excitation) Yuansheng Sun, Ulas Coskun and Shih-Chu Liao ISS, Inc. 1 Introduction to PIE The crosstalk between two fluorescent species causes problems in
TECHNICAL NOTE The Sweet PIE (Pulsed Interleaved Excitation) Yuansheng Sun, Ulas Coskun and Shih-Chu Liao ISS, Inc. 1 Introduction to PIE The crosstalk between two fluorescent species causes problems in
Performance of the Micro Photon Devices PDM 50CT SPAD detector with PicoQuant TCSPC systems
 Technical Note Performance of the Micro Photon Devices PDM 5CT SPAD detector with PicoQuant TCSPC systems Rolf Krahl, Andreas Bülter, Felix Koberling, PicoQuant GmbH These measurements were performed to
Technical Note Performance of the Micro Photon Devices PDM 5CT SPAD detector with PicoQuant TCSPC systems Rolf Krahl, Andreas Bülter, Felix Koberling, PicoQuant GmbH These measurements were performed to
FLIM Fluorescence Lifetime IMaging
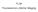 FLIM Fluorescence Lifetime IMaging Fluorescence lifetime t I(t) = F0 exp( ) τ 1 τ = k f + k nr k nr = k IC + k ISC + k bl Batiaens et al, Trends in Cell Biology, 1999 τ τ = fluorescence lifetime (~ns to
FLIM Fluorescence Lifetime IMaging Fluorescence lifetime t I(t) = F0 exp( ) τ 1 τ = k f + k nr k nr = k IC + k ISC + k bl Batiaens et al, Trends in Cell Biology, 1999 τ τ = fluorescence lifetime (~ns to
Welcome! openmicberkeley.wordpress.com. Open Berkeley
 Welcome! openmicberkeley.wordpress.com Agenda Jen Lee: Introduction to FRET Marla Feller: Using FRET sensors to look at time resolved measurements Becky Lamason: Using FRET to determine if a bacterial
Welcome! openmicberkeley.wordpress.com Agenda Jen Lee: Introduction to FRET Marla Feller: Using FRET sensors to look at time resolved measurements Becky Lamason: Using FRET to determine if a bacterial
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
STED microscopy with single light source. TeodoraŞcheul
 STED microscopy with single light source TeodoraŞcheul Dr. Iréne Wang, Dr. Jean-Claude Vial LIPhy, Grenoble, France Summary I. Introduction to STED microscopy II. STED with one laser source 1. Two-photon
STED microscopy with single light source TeodoraŞcheul Dr. Iréne Wang, Dr. Jean-Claude Vial LIPhy, Grenoble, France Summary I. Introduction to STED microscopy II. STED with one laser source 1. Two-photon
Biophotonics II general remarks
 general remarks BIOPHOTONICS I (WS 2017/18) I. Imaging Systems human vision microscopy II. Light Scattering Mie scattering light propagation in tissue BIOPHOTONICS II (SS 2018) III. Biospectroscopy Fluorescence
general remarks BIOPHOTONICS I (WS 2017/18) I. Imaging Systems human vision microscopy II. Light Scattering Mie scattering light propagation in tissue BIOPHOTONICS II (SS 2018) III. Biospectroscopy Fluorescence
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility
 Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Sample region with fluorescent labeled molecules
 FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
Double-pulse fluorescence lifetime measurements
 Journal of Microscopy, Vol. 186, Pt 3, June 1997, pp. 212 220. Received 15 August 1996; accepted 14 January 1997 Double-pulse fluorescence lifetime measurements A. H. BUIST,* M. MÜLLER,* E. J. GIJSBERS,*
Journal of Microscopy, Vol. 186, Pt 3, June 1997, pp. 212 220. Received 15 August 1996; accepted 14 January 1997 Double-pulse fluorescence lifetime measurements A. H. BUIST,* M. MÜLLER,* E. J. GIJSBERS,*
FRET from Multiple Pathways in Fluorophore Labeled DNA
 Supporting Information for FRET from Multiple Pathways in Fluorophore Labeled DNA Joseph S. Melinger 1,*, Ani Khachatrian 1, Mario G. Ancona 1, Susan Buckhout-White 3, Ellen R. Goldman 3, Christopher M.
Supporting Information for FRET from Multiple Pathways in Fluorophore Labeled DNA Joseph S. Melinger 1,*, Ani Khachatrian 1, Mario G. Ancona 1, Susan Buckhout-White 3, Ellen R. Goldman 3, Christopher M.
Imaging Protein-Protein Interactions By Multiphoton FLIM S. M. Ameer-Beg 1, N. Edme 2, M. Peter 2, P. R. Barber 1, T. Ng 2, B. Vojnovic 1.
 Imaging Protein-Protein Interactions By Multiphoton FLIM S. M. Ameer-Beg 1, N. Edme 2, M. Peter 2, P. R. Barber 1, T. Ng 2, B. Vojnovic 1. 1. Advanced Technology Development Group, Gray Cancer Institute,
Imaging Protein-Protein Interactions By Multiphoton FLIM S. M. Ameer-Beg 1, N. Edme 2, M. Peter 2, P. R. Barber 1, T. Ng 2, B. Vojnovic 1. 1. Advanced Technology Development Group, Gray Cancer Institute,
How to Build the World s Fastest Spectrofluorometer. MF 2 Multi-Frequency Fluorometer
 How to Build the World s Fastest Spectrofluorometer MF 2 Multi-Frequency Fluorometer Time is always on your side with a HORIBA Jobin Yvon MF 2 (Multi-Frequency Fluorometer) lifetime spectrofluorometer.
How to Build the World s Fastest Spectrofluorometer MF 2 Multi-Frequency Fluorometer Time is always on your side with a HORIBA Jobin Yvon MF 2 (Multi-Frequency Fluorometer) lifetime spectrofluorometer.
The analysis of fluorescence microscopy images for FRET detection
 The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
Monitoring and Optimizing the Lipopolysaccharides-plasmid DNA interaction by FLIM-FRET
 Transactions on Science and Technology Vol. 4, No. 3-3, 342-347, 2017 Monitoring and Optimizing the Lipopolysaccharides-plasmid DNA interaction by FLIM-FRET Nur Syahadatain Abdul Razak 1#, Clarence M.
Transactions on Science and Technology Vol. 4, No. 3-3, 342-347, 2017 Monitoring and Optimizing the Lipopolysaccharides-plasmid DNA interaction by FLIM-FRET Nur Syahadatain Abdul Razak 1#, Clarence M.
Concept review: Fluorescence
 16 Concept review: Fluorescence Some definitions: Chromophore. The structural feature of a molecule responsible for the absorption of UV or visible light. Fluorophore. A chromophore that remits an absorbed
16 Concept review: Fluorescence Some definitions: Chromophore. The structural feature of a molecule responsible for the absorption of UV or visible light. Fluorophore. A chromophore that remits an absorbed
The new LSM 700 from Carl Zeiss
 The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
The Phasor approach: Application to FRET analysis and Tissue autofluorescence
 The Phasor approach: Application to FRET analysis and Tissue autofluorescence Laboratory for Fluorescence Dynamics University of California at Irvine lfd lfd Outline Background: Lifetime Intro to Fluorescence
The Phasor approach: Application to FRET analysis and Tissue autofluorescence Laboratory for Fluorescence Dynamics University of California at Irvine lfd lfd Outline Background: Lifetime Intro to Fluorescence
Towards Probing Skin Cancer using Endogenous Melanin Fluorescence
 8 Towards Probing Skin Cancer using Endogenous Melanin Fluorescence Andra Colbert, McNair Scholar, Virginia State University Faculty Research Advisor: Dr. Ahmed A. Heikal Associate Professor of Bioengineering
8 Towards Probing Skin Cancer using Endogenous Melanin Fluorescence Andra Colbert, McNair Scholar, Virginia State University Faculty Research Advisor: Dr. Ahmed A. Heikal Associate Professor of Bioengineering
Directional Surface Plasmon Coupled Emission
 Journal of Fluorescence, Vol. 14, No. 1, January 2004 ( 2004) Fluorescence News Directional Surface Plasmon Coupled Emission KEY WORDS: Surface plasmon coupled emission; high sensitivity detection; reduced
Journal of Fluorescence, Vol. 14, No. 1, January 2004 ( 2004) Fluorescence News Directional Surface Plasmon Coupled Emission KEY WORDS: Surface plasmon coupled emission; high sensitivity detection; reduced
Chapter 4. the biological community to assay for protein-protein interactions. FRET describes the
 31 Chapter 4 Determination of nachr stoichiometry using Normalized Försters Resonance Energy Transfer (NFRET) Försters resonance energy transfer (FRET) has become a technique widely used in the biological
31 Chapter 4 Determination of nachr stoichiometry using Normalized Försters Resonance Energy Transfer (NFRET) Försters resonance energy transfer (FRET) has become a technique widely used in the biological
Lab 1: Ensemble Fluorescence Basics
 Lab 1: Ensemble Fluorescence Basics This laboratory module is divided into two sections. The first one is on organic fluorophores, and the second one is on ensemble measurement of FRET (Fluorescence Resonance
Lab 1: Ensemble Fluorescence Basics This laboratory module is divided into two sections. The first one is on organic fluorophores, and the second one is on ensemble measurement of FRET (Fluorescence Resonance
Absorption of an electromagnetic wave
 In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
Fluorescence Nanoscopy 高甫仁 ) Institute of Biophotonics, National Yang Ming University. Outline
 Fluorescence Nanoscopy 高甫仁 ) Fu-Jen Kao ( 高甫仁 Institute of Biophotonics, National Yang Ming University Outline The Abbe s (diffraction) limit and nanoscopy Fundamentals and opportunities of FLIM/FRET Visualizing
Fluorescence Nanoscopy 高甫仁 ) Fu-Jen Kao ( 高甫仁 Institute of Biophotonics, National Yang Ming University Outline The Abbe s (diffraction) limit and nanoscopy Fundamentals and opportunities of FLIM/FRET Visualizing
Presented at the 52nd Annual ASMS Conference on Mass Spectrometry Nashville, 2004
 Electron Autodetachment and Fluorescence Measurements of Trapped ligonucleotides Allison S. Danell and Joel H. Parks RWLAND INSTITUTE AT HARVARD, 1 EDWIN LAND BLVD., CAMBRIDGE, MA 2142 Presented at the
Electron Autodetachment and Fluorescence Measurements of Trapped ligonucleotides Allison S. Danell and Joel H. Parks RWLAND INSTITUTE AT HARVARD, 1 EDWIN LAND BLVD., CAMBRIDGE, MA 2142 Presented at the
Supporting Information for. Electrical control of Förster energy transfer.
 1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
The Green Fluorescent Protein. w.chem.uwec.edu/chem412_s99/ppt/green.ppt
 The Green Fluorescent Protein w.chem.uwec.edu/chem412_s99/ppt/green.ppt www.chem.uwec.edu/chem412_s99/ppt/green.ppt Protein (gene) is from a jellyfish: Aequorea victoria www.chem.uwec.edu/chem412_s99/ppt/green.ppt
The Green Fluorescent Protein w.chem.uwec.edu/chem412_s99/ppt/green.ppt www.chem.uwec.edu/chem412_s99/ppt/green.ppt Protein (gene) is from a jellyfish: Aequorea victoria www.chem.uwec.edu/chem412_s99/ppt/green.ppt
FLUORESCENT PEPTIDES. Outstanding Performance and Wide Application Range
 FLUORESCENT PEPTIDES Peptides and amino acids labeled with and Tide Quencher TM We offer peptides and amino acids tagged with fluorescent dyes. They meet highest demands in fluorescence intensity and photo-stability,
FLUORESCENT PEPTIDES Peptides and amino acids labeled with and Tide Quencher TM We offer peptides and amino acids tagged with fluorescent dyes. They meet highest demands in fluorescence intensity and photo-stability,
Discrimination of DNA and RNA in Cells by a Vital Fluorescent Probe: Lifetime Imaging of SYTO13 in Healthy and Apoptotic Cells
 2002 Wiley-Liss, Inc. Cytometry 47:226 235 (2002) Discrimination of DNA and RNA in Cells by a Vital Fluorescent Probe: Lifetime Imaging of SYTO13 in Healthy and Apoptotic Cells Marc A.M.J. van Zandvoort,
2002 Wiley-Liss, Inc. Cytometry 47:226 235 (2002) Discrimination of DNA and RNA in Cells by a Vital Fluorescent Probe: Lifetime Imaging of SYTO13 in Healthy and Apoptotic Cells Marc A.M.J. van Zandvoort,
Biophotonics?? Biophotonics. technology in biomedical engineering. Advantages of the lightwave
 Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
1. INTRODUCTION 2. EXPERIMENTAL 3. REFERENCES
 1. INTRODUCTION 2. EXPERIMENTAL 3. REFERENCES 1 1. INTRODUCTION Fluorescence spectroscopy is one of the most widely used spectroscopic techniques in the fields of biochemistry and molecular biophysics
1. INTRODUCTION 2. EXPERIMENTAL 3. REFERENCES 1 1. INTRODUCTION Fluorescence spectroscopy is one of the most widely used spectroscopic techniques in the fields of biochemistry and molecular biophysics
A cost-effective fluorescence detection system for pulsed laser analysis
 Susquehanna University Scholarly Commons Chemistry Faculty Publications 2-2015 A cost-effective fluorescence detection system for pulsed laser analysis J. W. Lafferty Susquehanna University N. A. Fox Susquehanna
Susquehanna University Scholarly Commons Chemistry Faculty Publications 2-2015 A cost-effective fluorescence detection system for pulsed laser analysis J. W. Lafferty Susquehanna University N. A. Fox Susquehanna
Basic Principles in Flow Cytometry. Flow Cytometry
 Basic Principles in Flow Cytometry Flow Cytometry» Flow Cytometry is the technological process that allows for the individual measurements of cell fluorescence and light scattering. This process is performed
Basic Principles in Flow Cytometry Flow Cytometry» Flow Cytometry is the technological process that allows for the individual measurements of cell fluorescence and light scattering. This process is performed
Imaging facilities at WUR
 Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Published in: Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications XVII
 Heriot-Watt University Heriot-Watt University Research Gateway Fibre optic time-resolved spectroscopy using CMOS-SPAD arrays Ehrlich, Katjana; Kufcsák, A.; Krstajić, N.; Henderson, R. K.; Thomson, Robert
Heriot-Watt University Heriot-Watt University Research Gateway Fibre optic time-resolved spectroscopy using CMOS-SPAD arrays Ehrlich, Katjana; Kufcsák, A.; Krstajić, N.; Henderson, R. K.; Thomson, Robert
Imaging fluorescence lifetime modulation of a ruthenium-based dye in living cells: the potential for oxygen sensing
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS D: APPLIED PHYSICS J. Phys. D: Appl. Phys. 36 (2003) 1689 1695 PII: S0022-3727(03)57024-3 Imaging fluorescence lifetime modulation of a ruthenium-based
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS D: APPLIED PHYSICS J. Phys. D: Appl. Phys. 36 (2003) 1689 1695 PII: S0022-3727(03)57024-3 Imaging fluorescence lifetime modulation of a ruthenium-based
DeltaPro. DeltaFlex FLUORESCENCE FORENSICS PARTICLE CHARACTERIZATION ELEMENTAL ANALYSIS FLUORESCENCE GRATINGS & OEM SPECTROMETERS OPTICAL COMPONENTS
 DeltaPro DeltaFlex ELEMENTAL ANALYSIS FLUORESCENCE FLUORESCENCE GRATINGS & OEM SPECTROMETERS OPTICAL COMPONENTS FORENSICS PARTICLE CHARACTERIZATION RAMAN SPECTROSCOPIC ELLIPSOMETRY SPR IMAGING Delta Series
DeltaPro DeltaFlex ELEMENTAL ANALYSIS FLUORESCENCE FLUORESCENCE GRATINGS & OEM SPECTROMETERS OPTICAL COMPONENTS FORENSICS PARTICLE CHARACTERIZATION RAMAN SPECTROSCOPIC ELLIPSOMETRY SPR IMAGING Delta Series
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
The Phasor approach: Application to FRET analysis and Tissue autofluorescence
 The Phasor approach: Application to FRET analysis and Tissue autofluorescence Enrico Gratton University of California at Irvine lfd lfd Outline Background: Lifetime Intro to Fluorescence Lifetime Imaging
The Phasor approach: Application to FRET analysis and Tissue autofluorescence Enrico Gratton University of California at Irvine lfd lfd Outline Background: Lifetime Intro to Fluorescence Lifetime Imaging
Fluorescence spectroscopy
 Fluorescence spectroscopy The light: electromagnetic wave Tamás Huber Biophysics seminar Dept. of Biophysics, University of Pécs 05-06. February 2014. 1 Luminescence: light emission of an excited system.
Fluorescence spectroscopy The light: electromagnetic wave Tamás Huber Biophysics seminar Dept. of Biophysics, University of Pécs 05-06. February 2014. 1 Luminescence: light emission of an excited system.
Introduction to Fluorescence Jablonski Diagram
 ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
Multiphoton excitation spectra in biological samples
 Journal of Biomedical Optics 8(3), 329 338 (July 2003) Multiphoton excitation spectra in biological samples Mary E. Dickinson California Institute of Technology Beckman Institute Biological Imaging Center
Journal of Biomedical Optics 8(3), 329 338 (July 2003) Multiphoton excitation spectra in biological samples Mary E. Dickinson California Institute of Technology Beckman Institute Biological Imaging Center
Sep No.20 CONFOCAL APPLICATION LETTER. resolution. Emission. Wavelength LAS AF APPLICATION WIZARD FRET SENSITIZED EMISSION
 CONFOCAL APPLICATION LETTER Sep. 2006 No.20 resolution Green Channel Red Channel Emission Wavelength LAS AF APPLICATION WIZARD FRET SENSITIZED EMISSION FRET with Leica TCS SP5 (LAS AF) S1 Donor S1 Acceptor
CONFOCAL APPLICATION LETTER Sep. 2006 No.20 resolution Green Channel Red Channel Emission Wavelength LAS AF APPLICATION WIZARD FRET SENSITIZED EMISSION FRET with Leica TCS SP5 (LAS AF) S1 Donor S1 Acceptor
Supporting On-line Materials
 Supporting On-line Materials Conformational Analysis of DNA Repair Intermediates by Time-Resolved Fluorescence Spectroscopy Su Lin 1, David P. Horning 2, Jack W. Szostak 2, and John C. Chaput* 1 1 Center
Supporting On-line Materials Conformational Analysis of DNA Repair Intermediates by Time-Resolved Fluorescence Spectroscopy Su Lin 1, David P. Horning 2, Jack W. Szostak 2, and John C. Chaput* 1 1 Center
Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone
 Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone Sven Terclavers LSM780 System overview The Scan Module - Core of the LSM 780 1 V/tunable PTC laser ports (405/440, cw/ps;
Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone Sven Terclavers LSM780 System overview The Scan Module - Core of the LSM 780 1 V/tunable PTC laser ports (405/440, cw/ps;
Exploring metallic nanoparticles in Biophotonics
 Exploring metallic nanoparticles in Biophotonics Renato E. de Araujo Laboratório de Óptica Biomédica e Imagem, Universidade Federal de Pernambuco Recife, PE, Brazil. renato.earaujo@ufpe.br LABORATÓRIO
Exploring metallic nanoparticles in Biophotonics Renato E. de Araujo Laboratório de Óptica Biomédica e Imagem, Universidade Federal de Pernambuco Recife, PE, Brazil. renato.earaujo@ufpe.br LABORATÓRIO
FLUORESCENCE. Matyas Molnar and Dirk Pacholsky
 FLUORESCENCE Matyas Molnar and Dirk Pacholsky 1 Information This lecture contains images and information from the following internet homepages http://micro.magnet.fsu.edu/primer/index.html http://www.microscopyu.com/
FLUORESCENCE Matyas Molnar and Dirk Pacholsky 1 Information This lecture contains images and information from the following internet homepages http://micro.magnet.fsu.edu/primer/index.html http://www.microscopyu.com/
Supplementary Figure 1. The normalized absorption and emission spectra of 605QD
 1..8 65Q Absorbance 65Q Emission Cy5 Absorbance Cy5 Emission 1..8 Extiction Absorption Coefficient.6.4.2. 45 5 55 6 65 7 75 8 Wavelength (nm).6.4.2. Fluorescence Emission Intensity Supplementary Figure
1..8 65Q Absorbance 65Q Emission Cy5 Absorbance Cy5 Emission 1..8 Extiction Absorption Coefficient.6.4.2. 45 5 55 6 65 7 75 8 Wavelength (nm).6.4.2. Fluorescence Emission Intensity Supplementary Figure
Design for Manufacturability (DFM) in the Life Sciences
 T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
DeltaPro. DeltaFlex FORENSICS PARTICLE CHARACTERIZATION ELEMENTAL ANALYSIS FLUORESCENCE GRATINGS & OEM SPECTROMETERS OPTICAL COMPONENTS RAMAN
 DeltaFlex DeltaPro ELEMENTAL ANALYSIS FLUORESCENCE GRATINGS & OEM SPECTROMETERS OPTICAL COMPONENTS FORENSICS PARTICLE CHARACTERIZATION RAMAN SPECTROSCOPIC ELLIPSOMETRY SPR IMAGING Why measure fluorescence
DeltaFlex DeltaPro ELEMENTAL ANALYSIS FLUORESCENCE GRATINGS & OEM SPECTROMETERS OPTICAL COMPONENTS FORENSICS PARTICLE CHARACTERIZATION RAMAN SPECTROSCOPIC ELLIPSOMETRY SPR IMAGING Why measure fluorescence
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Fluorescence Resonance Energy Transfer (FRET) Microscopy
 Applications in Confocal Microscopy Fluorescence Resonance Energy Transfer (FRET) Microscopy Product Info Brochures Confocal Theory Java Tutorials Glossary Applications Image Gallery Resource Links The
Applications in Confocal Microscopy Fluorescence Resonance Energy Transfer (FRET) Microscopy Product Info Brochures Confocal Theory Java Tutorials Glossary Applications Image Gallery Resource Links The
Translational Multimodality Optical Imaging
 Translational Multimodality Optical Imaging Fred S. Azar Xavier Intes Editors 0 ARTECH H O U S E BOSTON LONDON artechhouse.com Contents Foreword Preface xv xvii CHAPTER1 Introduction to Clinical Optical
Translational Multimodality Optical Imaging Fred S. Azar Xavier Intes Editors 0 ARTECH H O U S E BOSTON LONDON artechhouse.com Contents Foreword Preface xv xvii CHAPTER1 Introduction to Clinical Optical
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
Melanin Fluorescence Spectra by Step-wise Three Photon Excitation
 Melanin Fluorescence Spectra by Step-wise Three Photon Excitation Zhenhua Lai a,c, Josef Kerimo a,c, Charles A. DiMario a,b,c a Electrical and Computer Engineering Department,Northeastern University, Boston,
Melanin Fluorescence Spectra by Step-wise Three Photon Excitation Zhenhua Lai a,c, Josef Kerimo a,c, Charles A. DiMario a,b,c a Electrical and Computer Engineering Department,Northeastern University, Boston,
UV MicroTime 200. Crossing the Border towards Deep UV Time-resolved Microscopy of Native Fluorophores
 UV MicroTime 200 Crossing the Border towards Deep UV Time-resolved Microscopy of Native Fluorophores Marcelle König 1, Sebastian Tannert 1, Sandra Orthaus 1, Volker Buschmann 1, Thomas Schönau 1, Kristian
UV MicroTime 200 Crossing the Border towards Deep UV Time-resolved Microscopy of Native Fluorophores Marcelle König 1, Sebastian Tannert 1, Sandra Orthaus 1, Volker Buschmann 1, Thomas Schönau 1, Kristian
Sapphire. Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING
 Sapphire Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING Breakthrough image capture and analysis The Sapphire Biomolecular Imager is a next generation laser scanning system that provides
Sapphire Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING Breakthrough image capture and analysis The Sapphire Biomolecular Imager is a next generation laser scanning system that provides
Two-Photon Microscopy for Deep Tissue Imaging of Living Specimens
 for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
Confocal Microscopy of Electronic Devices. James Saczuk. Consumer Optical Electronics EE594 02/22/2000
 Confocal Microscopy of Electronic Devices James Saczuk Consumer Optical Electronics EE594 02/22/2000 Introduction! Review of confocal principles! Why is CM used to examine electronics?! Several methods
Confocal Microscopy of Electronic Devices James Saczuk Consumer Optical Electronics EE594 02/22/2000 Introduction! Review of confocal principles! Why is CM used to examine electronics?! Several methods
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Fluorescence lifetime imaging in biosciences: technologies and applications
 Front. Phys. China, 2008, 3(1): 88 104 DOI 10.1007/s11467-008-0002-6 REVIEW ARTICLE Raluca NIESNER, Karl-Heinz GERICKE Fluorescence lifetime imaging in biosciences: technologies and applications Higher
Front. Phys. China, 2008, 3(1): 88 104 DOI 10.1007/s11467-008-0002-6 REVIEW ARTICLE Raluca NIESNER, Karl-Heinz GERICKE Fluorescence lifetime imaging in biosciences: technologies and applications Higher
Spectroscopy and Imaging IV
 PROGRESS IN BIOMEDICAL OPTICS AND IMAGING Vol. 16 No. 55 Clinical and Biomedical Spectroscopy and Imaging IV J. Quincy Brown Volker Decked Edifors 22-24 June 2015 Munich, Germany Sponsored by SPIE (United
PROGRESS IN BIOMEDICAL OPTICS AND IMAGING Vol. 16 No. 55 Clinical and Biomedical Spectroscopy and Imaging IV J. Quincy Brown Volker Decked Edifors 22-24 June 2015 Munich, Germany Sponsored by SPIE (United
Structural Analysis of Blended Materials Using Multiphoton. Autofluorescence and Second Harmonic Generation Microscopy
 Structural Analysis of Blended Materials Using Multiphoton Autofluorescence and Second Harmonic Generation Microscopy Hsin-Yuan Tan a,b, Ming-Guo Lin c, Sung-Jan Lin d, Wen-Chu Hsiao a, Liang-Kung Wen
Structural Analysis of Blended Materials Using Multiphoton Autofluorescence and Second Harmonic Generation Microscopy Hsin-Yuan Tan a,b, Ming-Guo Lin c, Sung-Jan Lin d, Wen-Chu Hsiao a, Liang-Kung Wen
single-molecule fluorescence spectroscopy
 single-molecule fluorescence spectroscopy 5 dynamics of a single molecule by FRET michael börsch 18/07/2003 topics theory of fluorescence resonance energy transfer solvent effects and fluorescence quenching
single-molecule fluorescence spectroscopy 5 dynamics of a single molecule by FRET michael börsch 18/07/2003 topics theory of fluorescence resonance energy transfer solvent effects and fluorescence quenching
A simple introduction to multiphoton microscopy
 Journal of Microscopy, Vol. 243, Pt 3 2011, pp. 221 226 Received 29 April 2011; accepted 28 June 2011 doi: 10.1111/j.1365-2818.2011.03532.x A simple introduction to multiphoton microscopy A. USTIONE &
Journal of Microscopy, Vol. 243, Pt 3 2011, pp. 221 226 Received 29 April 2011; accepted 28 June 2011 doi: 10.1111/j.1365-2818.2011.03532.x A simple introduction to multiphoton microscopy A. USTIONE &
MicroTime 200 STED. Super-resolution add-on for the confocal time-resolved microscopy platform
 MicroTime 200 STED Super-resolution add-on for the confocal time-resolved microscopy platform confocal STED 2 Vision The MicroTime 200... The MicroTime 200 is a high-end confocal fluorescence lifetime
MicroTime 200 STED Super-resolution add-on for the confocal time-resolved microscopy platform confocal STED 2 Vision The MicroTime 200... The MicroTime 200 is a high-end confocal fluorescence lifetime
Applications of combined spectral lifetime microscopy for biology
 Applications of combined spectral lifetime microscopy for biology Long Yan, Curtis T. Rueden, John G. White, and Kevin W. Eliceiri Laboratory for Optical and Computational Instrumentation University of
Applications of combined spectral lifetime microscopy for biology Long Yan, Curtis T. Rueden, John G. White, and Kevin W. Eliceiri Laboratory for Optical and Computational Instrumentation University of
Presented at the 51st Annual ASMS Conference on Mass Spectrometry and Allied Topics Montreal, 2003
 FRET Measurements of Trapped Oligonucleotide Anions Allison S. Danell and Joel H. Parks ROWLAND INSTITUTE AT HARVARD, 100 EDWIN LAND BLVD., CAMBRIDGE, MA 02142 Presented at the 51st Annual ASMS Conference
FRET Measurements of Trapped Oligonucleotide Anions Allison S. Danell and Joel H. Parks ROWLAND INSTITUTE AT HARVARD, 100 EDWIN LAND BLVD., CAMBRIDGE, MA 02142 Presented at the 51st Annual ASMS Conference
SUPPLEMENTARY FIGURES
 SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging
 Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging Introduction In this application note, we will discuss the application of quantum dots in fluorescence imaging, both
Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging Introduction In this application note, we will discuss the application of quantum dots in fluorescence imaging, both
Fluorescence resonance energy transfer determinations using multiphoton fluorescence lifetime imaging microscopy to characterize amyloid-beta plaques
 Journal of Biomedical Optics 8(3), 368 375 (July 2003) Fluorescence resonance energy transfer determinations using multiphoton fluorescence lifetime imaging microscopy to characterize amyloid-beta plaques
Journal of Biomedical Optics 8(3), 368 375 (July 2003) Fluorescence resonance energy transfer determinations using multiphoton fluorescence lifetime imaging microscopy to characterize amyloid-beta plaques
Multi-modality imaging of structure and function combining spectral-domain optical coherence and multiphoton microscopy
 Multi-modality imaging of structure and function combining spectral-domain optical coherence and multiphoton microscopy Claudio Vinegoni a, Tyler Ralston a,b, Wei Tan a, Wei Luo a, Daniel L. Marks a,b,
Multi-modality imaging of structure and function combining spectral-domain optical coherence and multiphoton microscopy Claudio Vinegoni a, Tyler Ralston a,b, Wei Tan a, Wei Luo a, Daniel L. Marks a,b,
Effects of Refractive Index and Viscosity on Fluorescence and Anisotropy Decays of Enhanced Cyan and Yellow Fluorescent Proteins
 Journal of Fluorescence, Vol. 15, No. 2, March 2005 ( 2005) DOI: 10.1007/s10895-005-2523-5 Effects of Refractive Index and Viscosity on Fluorescence and Anisotropy Decays of Enhanced Cyan and Yellow Fluorescent
Journal of Fluorescence, Vol. 15, No. 2, March 2005 ( 2005) DOI: 10.1007/s10895-005-2523-5 Effects of Refractive Index and Viscosity on Fluorescence and Anisotropy Decays of Enhanced Cyan and Yellow Fluorescent
Toggling Between Blue and Red Emitting Fluorescent Silver Nanoclusters
 Supporting Information Toggling Between Blue and Red Emitting Fluorescent Silver Nanoclusters Uttam Anand, Subhadip Ghosh and Saptarshi Mukherjee * Department of Chemistry, Indian Institute of Science
Supporting Information Toggling Between Blue and Red Emitting Fluorescent Silver Nanoclusters Uttam Anand, Subhadip Ghosh and Saptarshi Mukherjee * Department of Chemistry, Indian Institute of Science
A quantitative protocol for intensity-based live cell FRET imaging.
 A quantitative protocol for intensity-based live cell FRET imaging. Kaminski CF, Rees EJ, Schierle GS. Methods Mol Biol. 2014; 1076:445-454. Department of Chemical Engineering and Biotechnology, Pembroke
A quantitative protocol for intensity-based live cell FRET imaging. Kaminski CF, Rees EJ, Schierle GS. Methods Mol Biol. 2014; 1076:445-454. Department of Chemical Engineering and Biotechnology, Pembroke
SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION
 APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
