Module-6. Dislocations and Strengthening Mechanisms
|
|
|
- Colin Barnett
- 6 years ago
- Views:
Transcription
1 Module-6 Dislocations and Strengthening Mechanisms
2 Contents 1) Dislocations & Plastic deformation and Mechanisms of plastic deformation in metals 2) Strengthening mechanisms in metals 3) Recovery, Recrystallization and Grain growth
3 Plastic deformation Dislocations Permanent plastic deformation is due to shear process atoms change their neighbors. Inter-atomic forces and crystal structure plays an important role during plastic deformation. Cumulative movement of dislocations leads to gross plastic deformation. Edge dislocation move by slip and climb, while screw dislocation move by slip and cross-slip. During their movement, dislocations tend to interact. The interaction is very complex because of number of dislocations moving over many slip systems in different directions.
4 Plastic deformation Dislocations (contd ) Dislocations moving on parallel planes may annihilate each other, resulting in either vacancies or interstitials. Dislocations moving on non-parallel planes hinder each other s movement by producing sharp breaks jog (break out of slip plane), kink (break in slip plane) Other hindrances to dislocation motion interstitial and substitutional atoms, foreign particles, grain boundaries, external grain surface, and change in structure due to phase change. Material strength can be increased by arresting dislocation motion.
5 Plastic deformation mechanisms - Slip Mainly two kinds: slip and twinning. Slip is prominent among the two. It involves sliding of blocks of crystal over other along slip planes. Slip occurs when shear stress applied exceeds a critical value. Slip occurs most readily in specific directions (slip directions) on certain crystallographic planes. Feasible combination of a slip plane together with a slip direction is considered as a slip system. During slip each atom usually moves same integral number of atomic distances along the slip plane.
6 Plastic deformation mechanisms Slip (contd ) Extent of slip depends on many factors - external load and the corresponding value of shear stress produced by it, crystal structure, orientation of active slip planes with the direction of shearing stresses generated. Slip occurs when shear stress applied exceeds a critical value. For single crystal, Schmid defined critical shear stress as R P cos A cos P A cos cos cos cos m cos cos
7 Plastic deformation mechanisms Slip (contd ) In a polycrystalline aggregate, individual grains provide a mutual geometrical constraint on one other, and this precludes plastic deformation at low applied stresses. Slip in polycrystalline material involves generation, movement and (re-)arrangement of dislocations. During deformation, mechanical integrity and coherency are maintained along the grain boundaries. A minimum of five independent slip systems must be operative for a polycrystalline solid to exhibit ductility and maintain grain boundary integrity von Mises. On the other hand, crystal deform by twinning.
8 Slip systems Crystal Occurrence Slip planes Slip directions FCC {111} <110> BCC HCP More common Less common More common Less common {110} {112},{123} Basal plane Prismatic & Pyramidal planes <111> Close packed directions NaCl {110} <110>
9 Plastic deformation mechanisms Twinning It results when a portion of crystal takes up an orientation that is related to the orientation of the rest of the untwined lattice in a definite, symmetrical way. The important role of twinning in plastic deformation is that it causes changes in plane orientation so that further slip can occur. Twinning also occurs in a definite direction on a specific plane for each crystal structure. Crystal Example Twin plane Twin direction FCC Ag, Au, Cu (111) [112] BCC α-fe, Ta (112) [111] HCP Zn, Cd, Mg, Ti (10ˉ12) [ˉ1011]
10 Slip Vs. Twinning Crystal orientation Size (in terms of interatomic distance) Occurs on during/in slip Same above and below the slip plane Multiples Widely spread planes during/in twinning Differ across the twin plane Fractions Every plane of region involved Time required Milli seconds Micro seconds Occurrence On many slip systems simultaneously On a particular plane for each crystal
11 Strengthening mechanisms Material can be increased by hindering dislocation, which is responsible for plastic deformation. Different ways to hinder dislocation motion / Strengthening mechanisms: in single-phase materials - Grain size reduction - Solid solution strengthening - Strain hardening in multi-phase materials - Precipitation strengthening - Dispersion strengthening - Fiber strengthening - Martensite strengthening
12 Strengthening by Grain size reduction It is based on the fact that dislocations will experience hindrances while trying to move from a grain into the next because of abrupt change in orientation of planes. Hindrances can be two types: forcible change of slip direction, and discontinuous slip plane. Smaller the grain size, often a dislocation encounters a hindrance. Yield strength of material will be increased. Yield strength is related to grain size (diameter, d) as Hall- Petch relation: y i kd 1 2 Grain size can be tailored by controlled cooling or by plastic deformation followed by appropriate heat treatment.
13 Strengthening by Grain size reduction (contd ) Grain size reduction improves not only strength, but also the toughness of many alloys. If d is average grain diameter, S v is grain boundary area per unit volume, N L is mean number of intercepts of grain boundaries per unit length of test line, N A is number of grains per unit area on a polished surface: Sv 2N L d 3 3 S v 2N L 6 N Grain size can also be measured by comparing the grains at a fixed magnification with standard grain size charts. Other method: Use of ASTM grain size number (Z). It is related to grain diameter, D (in mm) as follows: D G d A
14 Solid solution strengthening Impure foreign atoms in a single phase material produces lattice strains which can anchor the dislocations. Effectiveness of this strengthening depends on two factors size difference and volume fraction of solute. Solute atoms interact with dislocations in many ways: - elastic interaction - modulus interaction - stacking-fault interaction - electrical interaction - short-range order interaction - long-range order interaction Elastic, modulus, and long-range order interactions are of long-range i.e. they are relatively insensitive to temperature and continue to act about 0.6 Tm.
15 Yield point phenomenon Localized, heterogeneous type of transition from elastic to plastic deformation marked by abrupt elastic-plastic transition Yield point phenomenon. It characterizes that material needs higher stress to initiate plastic flow than to continue it.
16 Yield point phenomenon (contd ) The bands are called Lüders bands / Hartmann lines / stretcher stains, and generally are approximately 45 to the tensile axis. Occurrence of yield point is associated with presence of small amounts of interstitial or substitutional impurities. It s been found that either unlocking of dislocations by a high stress for the case of strong pinning or generation of new dislocations are the reasons for yield-point phenomenon. Magnitude of yield-point effect will depend on energy of interaction between solute atoms and dislocations and on the concentration of solute atoms at the dislocations.
17 Strain hardening Phenomenon where ductile metals become stronger and harder when they are deformed plastically is called strain hardening or work hardening. Increasing temperature lowers the rate of strain hardening. Hence materials are strain hardened at low temperatures, thus also called cold working. During plastic deformation, dislocation density increases. And thus their interaction with each other resulting in increase in yield stress. Dislocation density (ρ) and shear stress (τ) are related as follows: 0 A
18 Strain hardening (contd ) During strain hardening, in addition to mechanical properties physical properties also changes: - a small decrease in density - an appreciable decrease in electrical conductivity - small increase in thermal coefficient of expansion - increased chemical reactivity (decrease in corrosion resistance). Deleterious effects of cold work can be removed by heating the material to suitable temperatures Annealing. It restores the original properties into material. It consists of three stages recovery, recrystallization and grain growth. In industry, alternate cycles of strain hardening and annealing are used to deform most metals to a very great extent.
19 Precipitation & Dispersion hardening Foreign particles can also obstructs movement of dislocations i.e. increases the strength of the material. Foreign particles can be introduced in two ways precipitation and mixing-and-consolidation technique. Precipitation hardening is also called age hardening because strength increases with time. Requisite for precipitation hardening is that second phase must be soluble at an elevated temperature but precipitates upon quenching and aging at a lower temperature. E.g.: Al-alloys, Cu-Be alloys, Mg-Al alloys, Cu-Sn alloys If aging occurs at room temperature Natural aging If material need to be heated during aging Artificial aging.
20 Precipitation & Dispersion hardening (contd ) In dispersion hardening, fine second particles are mixed with matrix powder, consolidated, and pressed in powder metallurgy techniques. For dispersion hardening, second phase need to have very low solubility at all temperatures. E.g.: oxides, carbides, nitrides, borides, etc. Dislocation moving through matrix embedded with foreign particles can either cut through the particles or bend around and bypass them. Cutting of particles is easier for small particles which can be considered as segregated solute atoms. Effective strengthening is achieved in the bending process, when the particles are submicroscopic in size.
21 Precipitation & Dispersion hardening (contd ) Stress (τ) required to bend a dislocation is inversely proportional to the average interspacing (λ) of particles: Gb Interspacing (λ) of spherical particles: where r - particle radius, f - volume fraction 4(1 f 3 f ) r Optimum strengthening occurs during aging once the right interspacing of particles is achieved. - Smaller the particles, dislocations can cut through them at lower stresses - larger the particles they will be distributed at wider distances.
22 Fiber strengthening Second phase can be introduced into matrix in fiber form too. Requisite for fiber strengthening: Fiber material high strength and high modulus Matrix material ductile and non-reactive with fiber material E.g.: fiber material Al 2 O 3, boron, graphite, metal, glass, etc. matrix material metals, polymers Mechanism of strengthening is different from other methods. Higher modulus fibers carry load, ductile matrix distributes load to fibers. Interface between matrix and fibers thus plays an important role. Strengthening analysis involves application of continuum, not dislocation concepts as in other methods of strengthening.
23 Fiber strengthening (contd ) To achieve any benefit from presence of fibers, critical fiber volume which must be exceeded for fiber strengthening to occur: ' mu m f critical ' fu m where σ mu strength of strain hardened matrix, σ m flow stress of matrix at a strain equal to fiber breaking stress, σ fu ultimate tensile strength of the fiber. Minimum volume fraction of fiber which must be exceeded to have real reinforcement: f min fu mu mu ' m ' m
24 Martensite strengthening This strengthening method is based on formation of martensitic phase from the retained high temperature phase at temperatures lower then the equilibrium invariant transformation temperature. Martensite forms as a result of shearing of lattices. Martensite platelets assumes characteristic lenticular shape that minimizes the elastic distortion in the matrix. These platelets divide and subdivide the grains of the parent phase. Always touching but never crossing one another. Martensite platelets grow at very high speeds (1/3rd of sound speed) i.e. activation energy for growth is less. Thus volume fraction of martensite exist is controlled by its nucleation rate.
25 Martensite strengthening (contd ) Martensite platelets attain their shape by two successive shear displacements - first displacement is a homogeneous shear throughout the plate which occurs parallel to a specific plane in the parent phase known as the habit plane, second displacement, the lesser of the two, can take place by one of two mechanisms: slip as in Fe-C Martensite or twinning as in Fe-Ni Martensite. Martensite formation occurs in many systems. E.g.: Fe-C, Fe-Ni, Fe-Ni-C, Cu-Zn, Au-Cd, and even in pure metals like Li, Zr and Co. However, only the alloys based on Fe and C show a pronounced strengthening effect. High strength of Martensite is attributed to its characteristic twin structure and to high dislocation density. In Fe-C system, carbon atoms are also involved in strengthening.
26 Recovery Annealing relieves the stresses from cold working three stages: recovery, recrystallization and grain growth. Recovery involves annihilation of point defects. Driving force for recovery is decrease in stored energy from cold work. During recovery, physical properties of the cold-worked material are restored without any observable change in microstructure. Recovery is first stage of annealing which takes place at low temperatures of annealing. There is some reduction, though not substantial, in dislocation density as well apart from formation of dislocation configurations with low strain energies.
27 Recrystallization This follows recovery during annealing of cold worked material. Driving force is stored energy during cold work. It involves replacement of cold-worked structure by a new set of strain-free, approximately equi-axed grains to replace all the deformed crystals. This is process is characterized by recrystallization temperature which is defined as the temperature at which 50% of material recrystallizes in one hour time. The recrystallization temperature is strongly dependent on the purity of a material. Pure materials may recrystallizes around 0.3 T m, while impure materials may recrystallizes around T m, where T m is absolute melting temperature of the material.
28 Recrystallization laws A minimum amount of deformation is needed to cause recrystallization (Rx). Smaller the degree of deformation, higher will be the Rx temperature. The finer is the initial grain size; lower will be the Rx temperature. The larger the initial grain size, the greater degree of deformation is required to produce an equivalent Rx temperature. Greater the degree of deformation and lower the annealing temperature, the smaller will be the recrystallized grain size. The higher is the temperature of cold working, the less is the strain energy stored and thus Rx temperature is correspondingly higher. The Rx rate increases exponentially with temperature.
29 Grain growth Grain growth follows complete crystallization if the material is left at elevated temperatures. Grain growth does not need to be preceded by recovery and recrystallization; it may occur in all polycrystalline materials. In contrary to recovery and recrystallization, driving force for this process is reduction in grain boundary energy. Tendency for larger grains to grow at the expense of smaller grains is based on physics. In practical applications, grain growth is not desirable. Incorporation of impurity atoms and insoluble second phase particles are effective in retarding grain growth. Grain growth is very strongly dependent on temperature.
Chapter Outline Dislocations and Strengthening Mechanisms. Introduction
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter 7 Dislocations and Strengthening Mechanisms. Dr. Feras Fraige
 Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Strengthening Mechanisms
 Strengthening Mechanisms The ability of a metal/ alloy to plastically deform depends on the ability of dislocations to move. Strengthening techniques rely on restricting dislocation motion to render a
Strengthening Mechanisms The ability of a metal/ alloy to plastically deform depends on the ability of dislocations to move. Strengthening techniques rely on restricting dislocation motion to render a
Strengthening Mechanisms
 ME 254: Materials Engineering Chapter 7: Dislocations and Strengthening Mechanisms 1 st Semester 1435-1436 (Fall 2014) Dr. Hamad F. Alharbi, harbihf@ksu.edu.sa November 18, 2014 Outline DISLOCATIONS AND
ME 254: Materials Engineering Chapter 7: Dislocations and Strengthening Mechanisms 1 st Semester 1435-1436 (Fall 2014) Dr. Hamad F. Alharbi, harbihf@ksu.edu.sa November 18, 2014 Outline DISLOCATIONS AND
Chapter 8 Strain Hardening and Annealing
 Chapter 8 Strain Hardening and Annealing This is a further application of our knowledge of plastic deformation and is an introduction to heat treatment. Part of this lecture is covered by Chapter 4 of
Chapter 8 Strain Hardening and Annealing This is a further application of our knowledge of plastic deformation and is an introduction to heat treatment. Part of this lecture is covered by Chapter 4 of
STRENGTHENING MECHANISM IN METALS
 Background Knowledge Yield Strength STRENGTHENING MECHANISM IN METALS Metals yield when dislocations start to move (slip). Yield means permanently change shape. Slip Systems Slip plane: the plane on which
Background Knowledge Yield Strength STRENGTHENING MECHANISM IN METALS Metals yield when dislocations start to move (slip). Yield means permanently change shape. Slip Systems Slip plane: the plane on which
MT 348 Outline No MECHANICAL PROPERTIES
 MT 348 Outline No. 1 2009 MECHANICAL PROPERTIES I. Introduction A. Stresses and Strains, Normal and Shear Loading B. Elastic Behavior II. Stresses and Metal Failure A. ʺPrincipal Stressʺ Concept B. Plastic
MT 348 Outline No. 1 2009 MECHANICAL PROPERTIES I. Introduction A. Stresses and Strains, Normal and Shear Loading B. Elastic Behavior II. Stresses and Metal Failure A. ʺPrincipal Stressʺ Concept B. Plastic
Dislocations and Plastic Deformation
 Dislocations and Plastic Deformation Edge and screw are the two fundamental dislocation types. In an edge dislocation, localized lattice distortion exists along the end of an extra half-plane of atoms,
Dislocations and Plastic Deformation Edge and screw are the two fundamental dislocation types. In an edge dislocation, localized lattice distortion exists along the end of an extra half-plane of atoms,
14ME406/ME 226. Material science &Metallurgy. Hall Ticket Number: Fourth Semester. II/IV B.Tech (Regular/Supplementary) DEGREE EXAMINATION
 Hall Ticket Number: 14ME406/ME 226 April, 2017 Fourth Semester Time: Three Hours Answer Question No.1 compulsorily. Answer ONE question from each unit. II/IV B.Tech (Regular/Supplementary) DEGREE EXAMINATION
Hall Ticket Number: 14ME406/ME 226 April, 2017 Fourth Semester Time: Three Hours Answer Question No.1 compulsorily. Answer ONE question from each unit. II/IV B.Tech (Regular/Supplementary) DEGREE EXAMINATION
Strengthening Mechanisms. Today s Topics
 MME 131: Lecture 17 Strengthening Mechanisms Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Strengthening strategies: Grain strengthening Solid solution strengthening Work hardening
MME 131: Lecture 17 Strengthening Mechanisms Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Strengthening strategies: Grain strengthening Solid solution strengthening Work hardening
Introduction to Engineering Materials ENGR2000 Chapter 7: Dislocations and Strengthening Mechanisms. Dr. Coates
 Introduction to Engineering Materials ENGR2000 Chapter 7: Dislocations and Strengthening Mechanisms Dr. Coates An edge dislocation moves in response to an applied shear stress dislocation motion 7.1 Introduction
Introduction to Engineering Materials ENGR2000 Chapter 7: Dislocations and Strengthening Mechanisms Dr. Coates An edge dislocation moves in response to an applied shear stress dislocation motion 7.1 Introduction
ME 254 MATERIALS ENGINEERING 1 st Semester 1431/ rd Mid-Term Exam (1 hr)
 1 st Semester 1431/1432 3 rd Mid-Term Exam (1 hr) Question 1 a) Answer the following: 1. Do all metals have the same slip system? Why or why not? 2. For each of edge, screw and mixed dislocations, cite
1 st Semester 1431/1432 3 rd Mid-Term Exam (1 hr) Question 1 a) Answer the following: 1. Do all metals have the same slip system? Why or why not? 2. For each of edge, screw and mixed dislocations, cite
Fundamentals of Plastic Deformation of Metals
 We have finished chapters 1 5 of Callister s book. Now we will discuss chapter 10 of Callister s book Fundamentals of Plastic Deformation of Metals Chapter 10 of Callister s book 1 Elastic Deformation
We have finished chapters 1 5 of Callister s book. Now we will discuss chapter 10 of Callister s book Fundamentals of Plastic Deformation of Metals Chapter 10 of Callister s book 1 Elastic Deformation
Movement of edge and screw dislocations
 Movement of edge and screw dislocations Formation of a step on the surface of a crystal by motion of (a) n edge dislocation: the dislocation line moves in the direction of the applied shear stress τ. (b)
Movement of edge and screw dislocations Formation of a step on the surface of a crystal by motion of (a) n edge dislocation: the dislocation line moves in the direction of the applied shear stress τ. (b)
Materials and their structures
 Materials and their structures 2.1 Introduction: The ability of materials to undergo forming by different techniques is dependent on their structure and properties. Behavior of materials depends on their
Materials and their structures 2.1 Introduction: The ability of materials to undergo forming by different techniques is dependent on their structure and properties. Behavior of materials depends on their
Chapter 7: Dislocations and strengthening mechanisms. Strengthening by grain size reduction
 Chapter 7: Dislocations and strengthening mechanisms Mechanisms of strengthening in metals Strengthening by grain size reduction Solid-solution strengthening Strain hardening Recovery, recrystallization,
Chapter 7: Dislocations and strengthening mechanisms Mechanisms of strengthening in metals Strengthening by grain size reduction Solid-solution strengthening Strain hardening Recovery, recrystallization,
IMPERFECTIONSFOR BENEFIT. Sub-topics. Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface
 IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
Introduction to Materials Science
 EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
CHAPTER 4 1/1/2016. Mechanical Properties of Metals - I. Processing of Metals - Casting. Hot Rolling of Steel. Casting (Cont..)
 Processing of Metals - Casting CHAPTER 4 Mechanical Properties of Metals - I Most metals are first melted in a furnace. Alloying is done if required. Large ingots are then cast. Sheets and plates are then
Processing of Metals - Casting CHAPTER 4 Mechanical Properties of Metals - I Most metals are first melted in a furnace. Alloying is done if required. Large ingots are then cast. Sheets and plates are then
CREEP CREEP. Mechanical Metallurgy George E Dieter McGraw-Hill Book Company, London (1988)
 CREEP CREEP Mechanical Metallurgy George E Dieter McGraw-Hill Book Company, London (1988) Review If failure is considered as change in desired performance*- which could involve changes in properties and/or
CREEP CREEP Mechanical Metallurgy George E Dieter McGraw-Hill Book Company, London (1988) Review If failure is considered as change in desired performance*- which could involve changes in properties and/or
Single vs Polycrystals
 WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
Recrystallization Theoretical & Practical Aspects
 Theoretical & Practical Aspects 27-301, Microstructure & Properties I Fall 2006 Supplemental Lecture A.D. Rollett, M. De Graef Materials Science & Engineering Carnegie Mellon University 1 Objectives The
Theoretical & Practical Aspects 27-301, Microstructure & Properties I Fall 2006 Supplemental Lecture A.D. Rollett, M. De Graef Materials Science & Engineering Carnegie Mellon University 1 Objectives The
Chapter 7: Plastic deformation, Strengthening and Recrystallisation of Metals
 Chapter 7: Plastic deformation, Strengthening and Recrystallisation of Metals What do I need to know? The Mechanism of Plastic formation in single and polycrystalline metals The slip mechanism The slip
Chapter 7: Plastic deformation, Strengthening and Recrystallisation of Metals What do I need to know? The Mechanism of Plastic formation in single and polycrystalline metals The slip mechanism The slip
Metal working: Deformation processing II. Metal working: Deformation processing II
 Module 28 Metal working: Deformation processing II Lecture 28 Metal working: Deformation processing II 1 Keywords : Difference between cold & hot working, effect of macroscopic variables on deformation
Module 28 Metal working: Deformation processing II Lecture 28 Metal working: Deformation processing II 1 Keywords : Difference between cold & hot working, effect of macroscopic variables on deformation
Lecture # 11 References:
 Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC Dr.Haydar Al-Ethari
Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC Dr.Haydar Al-Ethari
Learning Objectives. Chapter Outline. Solidification of Metals. Solidification of Metals
 Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
CHAPTER 3 SELECTION AND PROCESSING OF THE SPECIMEN MATERIAL
 54 CHAPTER 3 SELECTION AND PROCESSING OF THE SPECIMEN MATERIAL 3.1 HIGH STRENGTH ALUMINIUM ALLOY In the proposed work, 7075 Al alloy (high strength) has been identified, as a material for the studies on
54 CHAPTER 3 SELECTION AND PROCESSING OF THE SPECIMEN MATERIAL 3.1 HIGH STRENGTH ALUMINIUM ALLOY In the proposed work, 7075 Al alloy (high strength) has been identified, as a material for the studies on
Chapter 8. Deformation and Strengthening Mechanisms
 Chapter 8 Deformation and Strengthening Mechanisms Chapter 8 Deformation Deformation and Strengthening Issues to Address... Why are dislocations observed primarily in metals and alloys? How are strength
Chapter 8 Deformation and Strengthening Mechanisms Chapter 8 Deformation Deformation and Strengthening Issues to Address... Why are dislocations observed primarily in metals and alloys? How are strength
Dept.of BME Materials Science Dr.Jenan S.Kashan 1st semester 2nd level. Imperfections in Solids
 Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Lecture 12: High Temperature Alloys
 Part IB Materials Science & Metallurgy H. K. D. H. Bhadeshia Course A, Metals and Alloys Lecture 12: High Temperature Alloys Metallic materials capable of operating at ever increasing temperatures are
Part IB Materials Science & Metallurgy H. K. D. H. Bhadeshia Course A, Metals and Alloys Lecture 12: High Temperature Alloys Metallic materials capable of operating at ever increasing temperatures are
NATURE OF METALS AND ALLOYS
 NATURE OF METALS AND ALLOYS Chapter 4 NATURE OF METALS AND ALLOYS Instructor: Office: MEC 325, Tel.: 973-642-7455 E-mail: samardzi@njit.edu Link to ME 215: http://mechanical.njit.edu/students/merequired.php
NATURE OF METALS AND ALLOYS Chapter 4 NATURE OF METALS AND ALLOYS Instructor: Office: MEC 325, Tel.: 973-642-7455 E-mail: samardzi@njit.edu Link to ME 215: http://mechanical.njit.edu/students/merequired.php
Tutorial 2 : Crystalline Solid, Solidification, Crystal Defect and Diffusion
 Tutorial 1 : Introduction and Atomic Bonding 1. Explain the difference between ionic and metallic bonding between atoms in engineering materials. 2. Show that the atomic packing factor for Face Centred
Tutorial 1 : Introduction and Atomic Bonding 1. Explain the difference between ionic and metallic bonding between atoms in engineering materials. 2. Show that the atomic packing factor for Face Centred
TOPIC 2. STRUCTURE OF MATERIALS III
 Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Plastic Deformation and Strengthening Mechanisms in Crystalline Materials
 Plastic Deformation and Strengthening Mechanisms in Crystalline Materials Updated 6 October, 2011 Slip in Polycrystalline Materials and all you ll ever need to know about it in MSE250 and life (unless
Plastic Deformation and Strengthening Mechanisms in Crystalline Materials Updated 6 October, 2011 Slip in Polycrystalline Materials and all you ll ever need to know about it in MSE250 and life (unless
Solid State Transformations
 Solid State Transformations Symmetrical Tilt Boundary The misorientation θ between grains can be described in terms of dislocations (Fig. 1). Inserting an edge dislocation of Burgers vector b is like forcing
Solid State Transformations Symmetrical Tilt Boundary The misorientation θ between grains can be described in terms of dislocations (Fig. 1). Inserting an edge dislocation of Burgers vector b is like forcing
Lecture # 11. Line defects (1D) / Dislocations
 Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC References: 1-
Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC References: 1-
ME254: Materials Engineering Second Midterm Exam 1 st semester December 10, 2015 Time: 2 hrs
 ME254: Materials Engineering Second Midterm Exam 1 st semester 1436-1437 December 10, 2015 Time: 2 hrs Problem 1: (24 points) A o = π/4*d o 2 = π/4*17 2 = 227 mm 2 L o = 32 mm a) Determine the following
ME254: Materials Engineering Second Midterm Exam 1 st semester 1436-1437 December 10, 2015 Time: 2 hrs Problem 1: (24 points) A o = π/4*d o 2 = π/4*17 2 = 227 mm 2 L o = 32 mm a) Determine the following
Materials Science and Engineering: An Introduction
 Materials Science and Engineering: An Introduction Callister, William D. ISBN-13: 9780470419977 Table of Contents List of Symbols. 1 Introduction. 1.1 Historical Perspective. 1.2 Materials Science and
Materials Science and Engineering: An Introduction Callister, William D. ISBN-13: 9780470419977 Table of Contents List of Symbols. 1 Introduction. 1.1 Historical Perspective. 1.2 Materials Science and
Imperfections, Defects and Diffusion
 Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Precipitation Hardening. Outline. Precipitation Hardening. Precipitation Hardening
 Outline Dispersion Strengthening Mechanical Properties of Steel Effect of Pearlite Particles impede dislocations. Things that slow down/hinder/impede dislocation movement will increase, y and TS And also
Outline Dispersion Strengthening Mechanical Properties of Steel Effect of Pearlite Particles impede dislocations. Things that slow down/hinder/impede dislocation movement will increase, y and TS And also
4-Crystal Defects & Strengthening
 4-Crystal Defects & Strengthening A perfect crystal, with every atom of the same type in the correct position, does not exist. The crystalline defects are not always bad! Adding alloying elements to a
4-Crystal Defects & Strengthening A perfect crystal, with every atom of the same type in the correct position, does not exist. The crystalline defects are not always bad! Adding alloying elements to a
Crystal Defects. Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K)
 Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Activation of deformation mechanism
 Activation of deformation mechanism The deformation mechanism activates when a critical amount of mechanical stress imposed to the crystal The dislocation glide through the slip systems when the required
Activation of deformation mechanism The deformation mechanism activates when a critical amount of mechanical stress imposed to the crystal The dislocation glide through the slip systems when the required
Why are dislocations observed primarily in metals CHAPTER 8: DEFORMATION AND STRENGTHENING MECHANISMS
 Why are dislocations observed primarily in metals CHAPTER 8: and alloys? DEFORMATION AND STRENGTHENING MECHANISMS How are strength and dislocation motion related? How do we manipulate properties? Strengthening
Why are dislocations observed primarily in metals CHAPTER 8: and alloys? DEFORMATION AND STRENGTHENING MECHANISMS How are strength and dislocation motion related? How do we manipulate properties? Strengthening
Twins & Dislocations in HCP Textbook & Paper Reviews. Cindy Smith
 Twins & Dislocations in HCP Textbook & Paper Reviews Cindy Smith Motivation Review: Outline Crystal lattices (fcc, bcc, hcp) Fcc vs. hcp stacking sequences Cubic {hkl} naming Hcp {hkil} naming Twinning
Twins & Dislocations in HCP Textbook & Paper Reviews Cindy Smith Motivation Review: Outline Crystal lattices (fcc, bcc, hcp) Fcc vs. hcp stacking sequences Cubic {hkl} naming Hcp {hkil} naming Twinning
Imperfections in the Atomic and Ionic Arrangements
 Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
Engineering Materials
 Engineering Materials Heat Treatments of Ferrous Alloys Annealing Processes The term annealing refers to a heat treatment in which a material is exposed to an elevated temperature for an extended time
Engineering Materials Heat Treatments of Ferrous Alloys Annealing Processes The term annealing refers to a heat treatment in which a material is exposed to an elevated temperature for an extended time
(a) Would you expect the element P to be a donor or an acceptor defect in Si?
 MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
Three stages: Annealing Textures. 1. Recovery 2. Recrystallisation most significant texture changes 3. Grain Growth
 Three stages: Annealing Textures 1. Recovery 2. Recrystallisation most significant texture changes 3. Grain Growth Cold worked 85% Cold worked 85% + stress relieved at 300 C for 1 hr Cold worked 85% +
Three stages: Annealing Textures 1. Recovery 2. Recrystallisation most significant texture changes 3. Grain Growth Cold worked 85% Cold worked 85% + stress relieved at 300 C for 1 hr Cold worked 85% +
Dislocations & Materials Classes. Dislocation Motion. Dislocation Motion. Lectures 9 and 10
 Lectures 9 and 10 Chapter 7: Dislocations & Strengthening Mechanisms Dislocations & Materials Classes Metals: Disl. motion easier. -non-directional bonding -close-packed directions for slip. electron cloud
Lectures 9 and 10 Chapter 7: Dislocations & Strengthening Mechanisms Dislocations & Materials Classes Metals: Disl. motion easier. -non-directional bonding -close-packed directions for slip. electron cloud
CHAPTER INTRODUCTION
 1 CHAPTER-1 1.0 INTRODUCTION Contents 1.0 Introduction 1 1.1 Aluminium alloys 2 1.2 Aluminium alloy classification 2 1.2.1 Aluminium alloys (Wrought) 3 1.2.2 Heat treatable alloys (Wrought). 3 1.2.3 Aluminum
1 CHAPTER-1 1.0 INTRODUCTION Contents 1.0 Introduction 1 1.1 Aluminium alloys 2 1.2 Aluminium alloy classification 2 1.2.1 Aluminium alloys (Wrought) 3 1.2.2 Heat treatable alloys (Wrought). 3 1.2.3 Aluminum
Defects and Diffusion
 Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Point coordinates. Point coordinates for unit cell center are. Point coordinates for unit cell corner are 111
 Point coordinates c z 111 Point coordinates for unit cell center are a/2, b/2, c/2 ½ ½ ½ Point coordinates for unit cell corner are 111 x a z 000 b 2c y Translation: integer multiple of lattice constants
Point coordinates c z 111 Point coordinates for unit cell center are a/2, b/2, c/2 ½ ½ ½ Point coordinates for unit cell corner are 111 x a z 000 b 2c y Translation: integer multiple of lattice constants
Problems to the lecture Physical Metallurgy ( Materialkunde ) Chapter 6: Mechanical Properties
 Institut für Metallkunde und Metallphysik Direktor: Prof. Dr. rer. nat. Günter Gottstein RWTH Aachen, D-52056 Aachen Internet: http://www.imm.rwth-aachen.de E-mail: imm@imm.rwth-aachen.de Tel.: +49 241
Institut für Metallkunde und Metallphysik Direktor: Prof. Dr. rer. nat. Günter Gottstein RWTH Aachen, D-52056 Aachen Internet: http://www.imm.rwth-aachen.de E-mail: imm@imm.rwth-aachen.de Tel.: +49 241
Point Defects. Vacancies are the most important form. Vacancies Self-interstitials
 Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Imperfections in atomic arrangements
 MME131: Lecture 9 Imperfections in atomic arrangements Part 2: 1D 3D Defects A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Classifications and characteristics of 1D 3D defects
MME131: Lecture 9 Imperfections in atomic arrangements Part 2: 1D 3D Defects A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Classifications and characteristics of 1D 3D defects
Introduction to Engineering Materials ENGR2000 Chapter 4: Imperfections in Solids. Dr. Coates
 Introduction to Engineering Materials ENGR000 Chapter 4: Imperfections in Solids Dr. Coates Learning Objectives 1. Describe both vacancy and self interstitial defects. Calculate the equilibrium number
Introduction to Engineering Materials ENGR000 Chapter 4: Imperfections in Solids Dr. Coates Learning Objectives 1. Describe both vacancy and self interstitial defects. Calculate the equilibrium number
Wrought Aluminum I - Metallurgy
 Wrought Aluminum I - Metallurgy Northbrook, IL www.imetllc.com Copyright 2015 Industrial Metallurgists, LLC Course learning objectives Explain the composition and strength differences between the alloy
Wrought Aluminum I - Metallurgy Northbrook, IL www.imetllc.com Copyright 2015 Industrial Metallurgists, LLC Course learning objectives Explain the composition and strength differences between the alloy
Materials Science. Imperfections in Solids CHAPTER 5: IMPERFECTIONS IN SOLIDS. Types of Imperfections
 In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
Defect in crystals. Primer in Materials Science Spring
 Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Physcial Metallurgy and Microstructure of Superalloys
 www.materialstechnology.org Physcial Metallurgy and Microstructure of Superalloys Roger Reed University of Birmingham The definition of superalloys utilized in the classic textbook 'The Superalloys' which
www.materialstechnology.org Physcial Metallurgy and Microstructure of Superalloys Roger Reed University of Birmingham The definition of superalloys utilized in the classic textbook 'The Superalloys' which
Continuous Cooling Diagrams
 Continuous Cooling Diagrams Isothermal transformation (TTT) diagrams are obtained by rapidly quenching to a given temperature and then measuring the volume fraction of the various constituents that form
Continuous Cooling Diagrams Isothermal transformation (TTT) diagrams are obtained by rapidly quenching to a given temperature and then measuring the volume fraction of the various constituents that form
Kinetics - Heat Treatment
 Kinetics - Heat Treatment Nonequilibrium Cooling All of the discussion up till now has been for slow cooling Many times, this is TOO slow, and unnecessary Nonequilibrium effects Phase changes at T other
Kinetics - Heat Treatment Nonequilibrium Cooling All of the discussion up till now has been for slow cooling Many times, this is TOO slow, and unnecessary Nonequilibrium effects Phase changes at T other
Point coordinates. x z
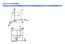 Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Chapter 9 Heat treatment (This chapter covers selective sections in Callister Chap. 9, 10 &11)
 Chapter 9 Heat treatment (This chapter covers selective sections in Callister Chap. 9, 10 &11) Study theme outcomes: After studying this chapter, students should or should be able to: - know and understand
Chapter 9 Heat treatment (This chapter covers selective sections in Callister Chap. 9, 10 &11) Study theme outcomes: After studying this chapter, students should or should be able to: - know and understand
Chapter Outline: Failure
 Chapter Outline: Failure How do Materials Break? Ductile vs. brittle fracture Principles of fracture mechanics Stress concentration Impact fracture testing Fatigue (cyclic stresses) Cyclic stresses, the
Chapter Outline: Failure How do Materials Break? Ductile vs. brittle fracture Principles of fracture mechanics Stress concentration Impact fracture testing Fatigue (cyclic stresses) Cyclic stresses, the
Part VII. Miscellaneous topics
 Part VII. Miscellaneous topics Module 1 : Recovery and recrystallisation Part VII. Miscellaneous topics In this part, we discuss the recovery, recrystallisation and grain growth processes; though these
Part VII. Miscellaneous topics Module 1 : Recovery and recrystallisation Part VII. Miscellaneous topics In this part, we discuss the recovery, recrystallisation and grain growth processes; though these
5. A round rod is subjected to an axial force of 10 kn. The diameter of the rod is 1 inch. The engineering stress is (a) MPa (b) 3.
 The Avogadro's number = 6.02 10 23 1 lb = 4.45 N 1 nm = 10 Å = 10-9 m SE104 Structural Materials Sample Midterm Exam Multiple choice problems (2.5 points each) For each problem, choose one and only one
The Avogadro's number = 6.02 10 23 1 lb = 4.45 N 1 nm = 10 Å = 10-9 m SE104 Structural Materials Sample Midterm Exam Multiple choice problems (2.5 points each) For each problem, choose one and only one
Titanium and titanium alloys. Josef Stráský
 Titanium and titanium alloys Josef Stráský Lecture 2: Fundamentals of Ti alloys Polymorphism Alpha phase Beta phase Pure titanium Titanium alloys alloys alloys alloys Phase transformation β α phase Hardening
Titanium and titanium alloys Josef Stráský Lecture 2: Fundamentals of Ti alloys Polymorphism Alpha phase Beta phase Pure titanium Titanium alloys alloys alloys alloys Phase transformation β α phase Hardening
Chapter 1. The Structure of Metals. Body Centered Cubic (BCC) Structures
 Chapter 1 The Structure of Metals Body Centered Cubic (BCC) Structures Figure 1. The body-centered cubic (bcc) crystal structure: (a) hard-ball model; (b) unit cell; and (c) single crystal with many unit
Chapter 1 The Structure of Metals Body Centered Cubic (BCC) Structures Figure 1. The body-centered cubic (bcc) crystal structure: (a) hard-ball model; (b) unit cell; and (c) single crystal with many unit
CME 300 Properties of Materials. ANSWERS Homework 2 September 28, 2011
 CME 300 Properties of Materials ANSWERS Homework 2 September 28, 2011 1) Explain why metals are ductile and ceramics are brittle. Why are FCC metals ductile, HCP metals brittle and BCC metals tough? Planes
CME 300 Properties of Materials ANSWERS Homework 2 September 28, 2011 1) Explain why metals are ductile and ceramics are brittle. Why are FCC metals ductile, HCP metals brittle and BCC metals tough? Planes
Recrystallization textures in metals and alloys
 Recrystallization textures in metals and alloys Uniaxial deformation Aluminium wire F.C.C. Metals and alloys FCC wires retain deformation texture ([111]+[100]) upon recrystallisation Composition / Purity
Recrystallization textures in metals and alloys Uniaxial deformation Aluminium wire F.C.C. Metals and alloys FCC wires retain deformation texture ([111]+[100]) upon recrystallisation Composition / Purity
Equilibria in Materials
 2009 fall Advanced Physical Metallurgy Phase Equilibria in Materials 09.01.2009 Eun Soo Park Office: 33-316 Telephone: 880-7221 Email: espark@snu.ac.kr Office hours: by an appointment 1 Text: A. PRINCE,
2009 fall Advanced Physical Metallurgy Phase Equilibria in Materials 09.01.2009 Eun Soo Park Office: 33-316 Telephone: 880-7221 Email: espark@snu.ac.kr Office hours: by an appointment 1 Text: A. PRINCE,
1) Fracture, ductile and brittle fracture 2) Fracture mechanics
 Module-08 Failure 1) Fracture, ductile and brittle fracture 2) Fracture mechanics Contents 3) Impact fracture, ductile-to-brittle transition 4) Fatigue, crack initiation and propagation, crack propagation
Module-08 Failure 1) Fracture, ductile and brittle fracture 2) Fracture mechanics Contents 3) Impact fracture, ductile-to-brittle transition 4) Fatigue, crack initiation and propagation, crack propagation
ASE324: Aerospace Materials Laboratory
 ASE324: Aerospace Materials Laboratory Instructor: Rui Huang Dept of Aerospace Engineering and Engineering Mechanics The University of Texas at Austin Fall 2003 Lecture 3 September 4, 2003 Iron and Steels
ASE324: Aerospace Materials Laboratory Instructor: Rui Huang Dept of Aerospace Engineering and Engineering Mechanics The University of Texas at Austin Fall 2003 Lecture 3 September 4, 2003 Iron and Steels
Design of High Strength Wrought Magnesium Alloys!
 Design of High Strength Wrought Magnesium Alloys! Joseph Robson! School of Materials! University of Manchester UK! joseph.robson@manchester.ac.uk! ! Strengthening of Mg! Mg has low density (2/3 Al, 1/4
Design of High Strength Wrought Magnesium Alloys! Joseph Robson! School of Materials! University of Manchester UK! joseph.robson@manchester.ac.uk! ! Strengthening of Mg! Mg has low density (2/3 Al, 1/4
Diffusional Transformations in Solids
 Diffusional Transformations in Solids The majority of phase transformations that occur in the solid state take place by thermally activated atomic movements. The transformations that will be dealt with
Diffusional Transformations in Solids The majority of phase transformations that occur in the solid state take place by thermally activated atomic movements. The transformations that will be dealt with
Part IA Paper 2: Structures and Materials MATERIALS Examples Paper 3 Stiffness-limited Design; Plastic Deformation and Properties
 Engineering Part IA Paper 2: Structures and Materials MATERIALS FIRST YEAR Examples Paper 3 Stiffness-limited Design; Plastic Deformation and Properties Straightforward questions are marked with a Tripos
Engineering Part IA Paper 2: Structures and Materials MATERIALS FIRST YEAR Examples Paper 3 Stiffness-limited Design; Plastic Deformation and Properties Straightforward questions are marked with a Tripos
MSE 170 Midterm review
 MSE 170 Midterm review Exam date: 11/2/2008 Mon, lecture time Place: Here! Close book, notes and no collaborations A sheet of letter-sized paper with double-sided notes is allowed Material on the exam
MSE 170 Midterm review Exam date: 11/2/2008 Mon, lecture time Place: Here! Close book, notes and no collaborations A sheet of letter-sized paper with double-sided notes is allowed Material on the exam
Introduction to Heat Treatment. Introduction
 MME444 Heat Treatment Sessional Week 01 Introduction to Heat Treatment Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction Can you control the microstructure that formed during cooling of
MME444 Heat Treatment Sessional Week 01 Introduction to Heat Treatment Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction Can you control the microstructure that formed during cooling of
Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number:
 Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number: Instructions: Answer all questions and show your work. You will not receive partial credit unless you show your work.
Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number: Instructions: Answer all questions and show your work. You will not receive partial credit unless you show your work.
3, MSE 791 Mechanical Properties of Nanostructured Materials
 3, MSE 791 Mechanical Properties of Nanostructured Materials Module 3: Fundamental Physics and Materials Design Lecture 1 1. What is strain (work) hardening? What is the mechanism for strain hardening?
3, MSE 791 Mechanical Properties of Nanostructured Materials Module 3: Fundamental Physics and Materials Design Lecture 1 1. What is strain (work) hardening? What is the mechanism for strain hardening?
Single crystal defect properties and tensile behavior of high purity Nb from Nb ingot slice
 Single crystal defect properties and tensile behavior of high purity Nb from Nb ingot slice D.C. Baars, P. Darbandi, D. Kang, F. Pourboghrat, T.R. Bieler, C. Compton* Michigan State University, * National
Single crystal defect properties and tensile behavior of high purity Nb from Nb ingot slice D.C. Baars, P. Darbandi, D. Kang, F. Pourboghrat, T.R. Bieler, C. Compton* Michigan State University, * National
Chapter 9: Dislocations & Strengthening Mechanisms. Why are the number of dislocations present greatest in metals?
 Chapter 9: Dislocations & Strengthening Mechanisms ISSUES TO ADDRESS... Why are the number of dislocations present greatest in metals? How are strength and dislocation motion related? Why does heating
Chapter 9: Dislocations & Strengthening Mechanisms ISSUES TO ADDRESS... Why are the number of dislocations present greatest in metals? How are strength and dislocation motion related? Why does heating
Phase Transformations in Metals Tuesday, December 24, 2013 Dr. Mohammad Suliman Abuhaiba, PE 1
 Ferrite - BCC Martensite - BCT Fe 3 C (cementite)- orthorhombic Austenite - FCC Chapter 10 Phase Transformations in Metals Tuesday, December 24, 2013 Dr. Mohammad Suliman Abuhaiba, PE 1 Why do we study
Ferrite - BCC Martensite - BCT Fe 3 C (cementite)- orthorhombic Austenite - FCC Chapter 10 Phase Transformations in Metals Tuesday, December 24, 2013 Dr. Mohammad Suliman Abuhaiba, PE 1 Why do we study
Free Electron Model What kind of interactions hold metal atoms together? How does this explain high electrical and thermal conductivity?
 Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Mechanical Lustrous
Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Mechanical Lustrous
Phase change processes for material property manipulation BY PROF.A.CHANDRASHEKHAR
 Phase change processes for material property manipulation BY PROF.A.CHANDRASHEKHAR Introduction The phase of a material is defined as a chemically and structurally homogeneous state of material. Any material
Phase change processes for material property manipulation BY PROF.A.CHANDRASHEKHAR Introduction The phase of a material is defined as a chemically and structurally homogeneous state of material. Any material
Module 32. Heat treatment of steel II. Lecture 32. Heat treatment of steel II
 Module 32 Heat treatment of steel II Lecture 32 Heat treatment of steel II 1 Keywords : Kinetics of pearlitic transformation, Johnsom Mehl Avrami equation, effect of carbon content on T T T diagram, bainite:
Module 32 Heat treatment of steel II Lecture 32 Heat treatment of steel II 1 Keywords : Kinetics of pearlitic transformation, Johnsom Mehl Avrami equation, effect of carbon content on T T T diagram, bainite:
Recrystallization. Chapter 7
 Chapter 7 Recrystallization 7.1 INTRODUCTION The earlier chapters have described creep as a process where dislocation hardening is accompanied by dynamic recovery. It should be discussed at this point
Chapter 7 Recrystallization 7.1 INTRODUCTION The earlier chapters have described creep as a process where dislocation hardening is accompanied by dynamic recovery. It should be discussed at this point
Dislocations Linear Defects
 Dislocations Linear Defects Dislocations are abrupt changes in the regular ordering of atoms, along a line (dislocation line) in the solid. They occur in high density and are very important in mechanical
Dislocations Linear Defects Dislocations are abrupt changes in the regular ordering of atoms, along a line (dislocation line) in the solid. They occur in high density and are very important in mechanical
INSTITUTE OF AERONAUTICAL ENGINEERING (Autonomous) Dundigal, Hyderabad
 INSTITUTE OF AERONAUTICAL ENGINEERING (Autonomous) Dundigal, Hyderabad -500 043 MECHANICAL ENGINEERING TUTORIAL QUESTION BANK Course Name METALLURGY AND MATERIAL SCIENCE Course Code AME005 Class III Semester
INSTITUTE OF AERONAUTICAL ENGINEERING (Autonomous) Dundigal, Hyderabad -500 043 MECHANICAL ENGINEERING TUTORIAL QUESTION BANK Course Name METALLURGY AND MATERIAL SCIENCE Course Code AME005 Class III Semester
Impurities in Solids. Crystal Electro- Element R% Structure negativity Valence
 4-4 Impurities in Solids 4.4 In this problem we are asked to cite which of the elements listed form with Ni the three possible solid solution types. For complete substitutional solubility the following
4-4 Impurities in Solids 4.4 In this problem we are asked to cite which of the elements listed form with Ni the three possible solid solution types. For complete substitutional solubility the following
Chapter Outline How do atoms arrange themselves to form solids?
 Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Face-centered cubic Body-centered cubic Hexagonal close-packed Close packed
Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures Face-centered cubic Body-centered cubic Hexagonal close-packed Close packed
Objective To study the time and temperature variations in the hardness of Al-4% Cu alloy on isothermal aging.
 EXPERIMENT 8 PRECIPITATION HARDENING IN 2024 ALUMINUM Objective To study the time and temperature variations in the hardness of Al-4% Cu alloy on isothermal aging. Introduction Materials can be hardened
EXPERIMENT 8 PRECIPITATION HARDENING IN 2024 ALUMINUM Objective To study the time and temperature variations in the hardness of Al-4% Cu alloy on isothermal aging. Introduction Materials can be hardened
Ex: NaCl. Ironically Bonded Solid
 Ex: NaCl. Ironically Bonded Solid Lecture 2 THE STRUCTURE OF CRYSTALLINE SOLIDS 3.2 FUNDAMENTAL CONCEPTS SOLIDS AMORPHOUS CRYSTALLINE Atoms in an amorphous Atoms in a crystalline solid solid are arranged
Ex: NaCl. Ironically Bonded Solid Lecture 2 THE STRUCTURE OF CRYSTALLINE SOLIDS 3.2 FUNDAMENTAL CONCEPTS SOLIDS AMORPHOUS CRYSTALLINE Atoms in an amorphous Atoms in a crystalline solid solid are arranged
Chapter 7: Dislocations and strengthening mechanisms
 Chapter 7: Dislocations and strengthening mechanisms Introduction Basic concepts Characteristics of dislocations Slip systems Slip in single crystals Plastic deformation of polycrystalline materials Plastically
Chapter 7: Dislocations and strengthening mechanisms Introduction Basic concepts Characteristics of dislocations Slip systems Slip in single crystals Plastic deformation of polycrystalline materials Plastically
CHAPTER10. Kinetics Heat Treatment
 CHAPTER10 Kinetics Heat Treatment The microstructure of a rapidly cooled eutectic soft solder ( 38 wt%pb 62 wt % Sn) consists of globules of lead-rich solid solution (dark) in a matrix of tin-rich solid
CHAPTER10 Kinetics Heat Treatment The microstructure of a rapidly cooled eutectic soft solder ( 38 wt%pb 62 wt % Sn) consists of globules of lead-rich solid solution (dark) in a matrix of tin-rich solid
Engineering materials
 1 Engineering materials Lecture 2 Imperfections and defects Response of materials to stress 2 Crystalline Imperfections (4.4) No crystal is perfect. Imperfections affect mechanical properties, chemical
1 Engineering materials Lecture 2 Imperfections and defects Response of materials to stress 2 Crystalline Imperfections (4.4) No crystal is perfect. Imperfections affect mechanical properties, chemical
Chapter Outline. How do atoms arrange themselves to form solids?
 Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures! Face-centered cubic! Body-centered cubic! Hexagonal close-packed Close packed
Chapter Outline How do atoms arrange themselves to form solids? Fundamental concepts and language Unit cells Crystal structures! Face-centered cubic! Body-centered cubic! Hexagonal close-packed Close packed
How do you expect the packing factor to vary with coordination number?
 DATA SHEETS: Crystal Structures and Metallography Date: Lab Partners: Body Centered Cubic (BCC) Structure: CN: Atoms touch along: Number of atoms per unit cell: Determination of PF: Face Centered Cubic
DATA SHEETS: Crystal Structures and Metallography Date: Lab Partners: Body Centered Cubic (BCC) Structure: CN: Atoms touch along: Number of atoms per unit cell: Determination of PF: Face Centered Cubic
