Foundations of Materials Science and Engineering Lecture Note 4
|
|
|
- Vivien Terry
- 6 years ago
- Views:
Transcription
1 Foundations of Materials Science and Engineering Lecture Note 4 April 2, 2013 Kwang Kim Yonsei University kbkim@yonsei.ac.kr Y O N Se I
2 Solidification of Metals
3 Solidification of Metals The solidification of metals and their alloys is an important industrial process. Not only do structural alloys start with the casting of ingots for processing into reinforcing bars or structural shapes, but when a metal is welded a small portion of metal near the weld melts and resolidifies. It also serves as a model to represent first order phase transformations in general.
4 Solidification of Metals Metals are melted to produce finished and semi-finished parts. Two steps of solidification Nucleation : Formation of stable nuclei. Growth of nuclei : Formation of grain structure. Thermal gradients define the shape of each grain. (a) Formation of stable nuclei (b) Growth of crystals (c) Grain structure
5 Solidification of Metals Phase transformations involve change in structure and composition rearrangement and redistribution of atoms via diffusion is required. - Nucleation : Formation of stable nuclei. - Growth of nuclei : Formation of grain structure. The process of phase transformation involves: - Nucleation of the new phase(s) - formation of stable small particles (nuclei) of the new phase(s). Nuclei are often formed at grain boundaries and other defects. - Growth of the new phase(s) at the expense of the original phase(s).
6 Ideal Gas Law An ideal gas : all collisions between atoms or molecules are perfectly elastic there are no intermolecular attractive forces a collection of perfectly hard spheres which collide but which otherwise do not interact with each other no potential energy (no force field) all the internal energy is in the form of kinetic energy and any change in internal energy is accompanied by a change in temperature. An ideal gas : characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T).
7 Non- ideal Gas
8 Non ideal Gas Ideal Gas Intermolecular force? : condensation, liquefaction at high P Liquefaction of a real gas No liquefaction of an ideal gas Requirements for liquefaction of a real gas?
9 Phase Equilibria in water
10 Phase Equilibria in water temperature increase pressure increase
11 Phase Equilibria in water Temp. temperature increase V L + V S + L L S pressure increase time
12 Phase Equilibria in water Pressure temperature increase V V +L L pressure increase time
13 Non- ideal Gas D D E D Definition: a physically distinctive form of matter, such as a solid, liquid, gas or At point C plasma. A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet the same state of matter. For example, mixtures can exist in multiple phases, such as an oil phase and an aqueous phase.
14 Pc At Critical Point, Molar volume of liquid = Molar volume of gas Critical Point Critical Temperature Tc Critical Pressure Pc Critical Volume Vc Molar volume of liquid Molar volume of gas Vc
15 Cryophorus : an instrument that illustrates the freezing of water by its own evaporation A cryophorus is a glass container containing liquid water and water vapor. It is used to demonstrate rapid freezing by evaporation. A typical cryophorus has a bulb at one end connected to a tube of the same material. When the liquid water is manipulated into the bulbed end and the other end is connected to a vacuum pump, the gas pressure drops as it is cooled. The liquid water begins to evaporate, producing more water vapor. Evaporation causes the water to cool rapidly to its freezing point and it solidifies suddenly. Finally, a piece of ice disappears as a result of ice sublimation to water vapor/ Vacuum pump Water droplet in a bottle under vacuum
16 Cryophorus 실험 1) 진공상태에위치한 water droplet 의 evaporation 2) 흡열반응인 evaporation 진행 자체의온도가감소 liquid water/vapor 평형상태공존선도달 * 3) 지속적인온도감소에의해 3중점도달 (ice/ liquid water/vapor 평형상태공존점 ) 4) 온도, 압력저하 liquid water/vapor 평형상태공존선에서 solid/vapor 평형공존선으로변화 5) liquid water droplet가 solid ice 로변화 6) solid ice는 sublimation에의해 vapor 상으로승화궁극적으로는 solid ice는모두 sublimation 하여사라짐
17 Nucleation Heterogeneous the new phase appears on the walls of the container, at impurity particles, etc. Homogeneous solid nuclei spontaneously appear within the undercooled phase. solidification of a liquid phase undercooled below the melting temperature as a simple example of a phase transformation.
18 Homogenous Nucleation Homogeneous nucleation occurs when there are no special objects inside a phase which can cause nucleation. For instance when a pure liquid metal is slowly cooled below its equilibrium freezing temperature to a sufficient degree numerous homogeneous nuclei are created by slow-moving atoms bonding together in a crystalline form.
19 Formation of Stable Nuclei Two main mechanisms: Homogenous and heterogeneous. Homogenous Nucleation : First and simplest case. Metal itself will provide atoms to form nuclei. Metal, when significantly undercooled, has several slow moving atoms which bond each other to form nuclei. Cluster of atoms below critical size is called embryo. If the cluster of atoms reach critical size, they grow into crystals. Else get dissolved. Cluster of atoms that are grater than critical size are called nucleus.
20 Solidification of Metals Typical data relevant to the solidification of metals
21 Energies Involved in Homogeneous Nucleation Gibbs Free Energy G = H - TS where H = Enthalpy, T = Absolute Temperature, and S = Entropy Materials Scientists refer to the difference in G between the old and new phases as the driving force for the phase transformation. The release of heat when a metal solidifies indicates that the crystalline phase has a lower Gibbs Free Energy, G, than the liquid. At the equilibrium freezing temperature the Gibbs Free Energy of the liquid and the crystalline phase are equal.
22 Energies Involved in Homogeneous Nucleation Phase transformation of liquid to solid G = G solid -G liquid G liquid G solid G solid G liquid
23 Energies Involved in Homogeneous Nucleation Is the transition from undercooled liquid to a solid spherical particle in the liquid a spontaneous one? That is, is the Gibbs free energy decreases? The formation of a solid nucleus leads to a Gibbs free energy change of ΔG
24 Energies Involved in Homogeneous Nucleation The formation of a solid nucleus leads to a Gibbs free energy change of ΔG
25 Energies Involved in Homogeneous Nucleation (1)Volume free energy (2) Surface energy
26 Energies involved in homogenous nucleation Volume free energy G v (Gibbs Free Energy G = H-TS) Released by liquid to solid transformation. ΔG v is change in free energy per unit volume between liquid and solid. free energy change for a spherical nucleus of radius r is given by r 4 r 3 3 G v Surface energy Gs Required to form new solid surface ΔG s is energy needed to create a surface. γ is specific surface free energy. Then Gs 4 r 2 ΔG s is retarding energy.
27 Energies involved in homogenous nucleation
28 Total Free Energy Total free energy is given by + Since when r=r*, d(δg T )/dr = 0 ΔG s r* 2 G V Nucleus ΔG r* r* ΔG T r Above critical radius r* Below critical radius r* - ΔG v Energy lowered by growing into crystals Energy Lowered by redissolving
29 Critical Radius Versus Undercooling Greater the degree of undercooling, greater the change in volume free energy ΔG v ΔGs does not change significantly. As the amount of undercooling ΔT increases, critical nucleus size decreases. Critical radius is related to undercooling by relation r* 2 T H f m T r* = critical radius of nucleus γ = Surface free energy ΔH f = Latent heat of fusion Δ T = Amount of undercooling. Both r* and G* decrease with increasing undercooling.
30 Critical Radius Versus Undercooling
31 Heterogeneous Nucleation Heterogeneous nucleation occurs much more often than homogeneous nucleation. Heterogeneous nucleation applies to the phase transformation between any two phases of gas, liquid, or solid, typically for example, condensation of gas/vapor, solidification from liquid, bubble formation from liquid, etc. Heterogeneous nucleation forms at preferential sites such as phase boundaries, surfaces (of container, bottles, etc.) or impurities like dust. At such preferential sites, the effective surface energy is lower, thus diminishes the free energy barrier and facilitating nucleation.
32 Heterogeneous Nucleation Heterogeneous nucleation can be considered as a surface catalyzed or assisted nucleation process. The extent of how a surface can catalyze or facilitate the nucleation depends on the contact angle of the nucleus with respect to the substrate. The smaller the angle (or the stronger the wetting of the surface), the lower the free energy change, and the lower the nucleation barrier will be.
33 Heterogeneous Nucleation Critical radius of the nucleus (r*) for a heterogeneous nucleation is the same as that for a homogeneous nucleation, whereas the critical volume of the nucleus (like the droplet for liquid nucleated from gas/vapor phase) is usually smaller for heterogeneous nucleation than for homogeneous nucleation, due to the surface wetting (spreading).
34 Homogeneous nucleation : Total Free Energy Total free energy is given by + Since when r=r*, d(δg T )/dr = 0 ΔG s r* 2 G V Nucleus ΔG r* r* ΔG T r Above critical radius r* Below critical radius r* - ΔG v Energy lowered by growing into crystals Energy Lowered by redissolving
35 Heterogeneous Nucleation
36 Heterogeneous Nucleation γαβ= (gas - liquid), γαδ= (gas - solid), γβδ= (liquid - solid) Consider a droplet of liquid on a flat surface with fixed volume. The total surface energy of the system is a function of the shape of the droplet. The diagram above shows two different shapes of the same volume, but of different surface area leading to different Surface energy: Gs= γαβaαβ+ γαδaαδ+ γβδaβδ where Aαβ,Aαδand Aβδare areas of αβ, αδ and βδ interfaces, respectively
37 Heterogeneous Nucleation Gs= γαβaαβ+ γαδaαδ+ γβδaβδ where Aαβ,Aαδand Aβδare areas of αβ, αδ and βδ interfaces, respectively Assume r be the radius of curvature of the droplet, then: Aβδ= π r2sin2θ Aαδ=A0- π r2sin2θ;a0is the area of αδ interface without β Thus,
38 Heterogeneous Nucleation dgs= {4 π r γαβ (1-cosθ) + 2 π r (γβδ - γαδ) sin2θ} d r + {2 π r2γαβ sinθ + 2 π r2 (γβδ - γαδ) sinθ cosθ}dθ = 0
39 Heterogeneous Nucleation
40 Heterogeneous Nucleation
41 Heterogeneous Nucleation
42 Heterogeneous Nucleation
43 Heterogeneous Nucleation
44 Heterogeneous Nucleation The cloud : Lots of small spherical water droplets too light to fall under gravity The droplets need to freeze to ice which becomes a nucleus capable of attracting water vapor. In cold days it snows and In hot days it rains. The ice : The homogeneous nucleation temperature is 40 C (way too cold) The solution : Heterogeneous nucleation Air Pollution will provide the seeds (OK, not the best solution) Use silver iodide crystals decreases the nucleation T from -40 C to 4 C.
45 Heterogeneous Nucleation Carbonate drinks have carbon dioxide which is dissolved under pressure. When the can is opened the pressure drops and the gas comes out of solution in the form of bubbles. Aluminum and glass are poor catalysts for heterogeneous nucleation.
46 Growth of Crystals and Formation of Grain Structure Nucleus grow into crystals in different orientations. Crystal boundaries are formed when crystals join together at complete solidification. Crystals in solidified metals are called grains. Grains are separated by grain boundaries. More the number of nucleation sites available, more the number of grains formed.
47 Types of Grains Equiaxed Grains: Crystals, smaller in size, grow equally in all directions. Formed at the sites of high concentration of the nuclie. Example:- Cold mold wall Mold Columnar Grains: Long thin and coarse. Grow predominantly in one direction. Formed at the sites of slow cooling and steep temperature gradient. Example:- Grains that are away from the mold wall. Columnar Grains Equiaxed Grains
48 Types of Grains
49 Casting in Industries In industries, molten metal is cast into either semi finished or finished parts. Continuous casting of steel ingots Direct-Chill semicontinuous Casting unit for aluminum
50 Grain Structure in Industrial castings To produce cast ingots with fine grain size, grain refiners are added. Example:- For aluminum alloy, small amount of Titanium, Boron or Zirconium is added. Grain structure of Aluminum cast with (a) and without (b) grain refiners. (a) (b)
51 Solidification of Single Crystal For some applications (Eg: Gas turbine blades-high temperature environment), single crystals are needed. Single crystals have high temperature creep resistance. Latent head of solidification is conducted through solidifying crystal to grow single crystal. Growth rate is kept slow so that temperature at solidliquid interface is slightly below melting point. Growth of single crystal for turbine airfoil.
52 Czochralski Process This method is used to produce single crystal of silicon for electronic wafers. A seed crystal is dipped in molten silicon and rotated. The seed crystal is withdrawn slowly while silicon adheres to seed crystal and grows as a single crystal.
53 Metallic Solid Solutions Alloys are used in most engineering applications. Alloy is an mixture of two or more metals and nonmetals. Example: Cartridge brass is binary alloy of 70% Cu and 30% Zinc. Iconel is a nickel based superalloy with about 10 elements. Solid solution is a simple type of alloy in which elements are dispersed in a single phase.
54 Substitutional Solid Solution Solute atoms substitute for parent solvent atom in a crystal lattice. The structure remains unchanged. Lattice might get slightly distorted due to change in diameter of the atoms. Solute percentage in solvent can vary from fraction of a percentage to 100% Solvent atoms Solute atoms
55 Substitutional Solid Solution The solubility of solids is greater if The diameter of atoms not differ by more than 15% Crystal structures are similar. No much difference in electronegativity (else compounds will be formed). Have some valence. System Atomic radius Difference Examples:- Electronegativity difference Solid Solibility Cu-Zn 3.9% % Cu-Pb 36.7% % Cu-Ni 2.3% 0 100%
56 Interstitial Solid Solution Solute atoms fit in between the voids (interstices) of solvent atoms. Solvent atoms in this case should be much larger than solute atoms. Example:- between 912 and C, interstitial solid solution of carbon in γ iron (FCC) is formed. A maximum of 2.8% of carbon can dissolve interstitially in iron. Iron atoms r00.129nm Carbon atoms r=0.075nm
57 Crystalline Imperfections No crystal is perfect. Imperfections affect mechanical properties, chemical properties and electrical properties. Imperfections can be classified as Zero dimension point deffects. One dimension / line deffects (dislocations). Two dimension deffects. Three dimension deffects (cracks).
58 Point Defects Vacancy Vacancy is formed due to a missing atom. Vacancy is formed (one in atoms) during crystallization or mobility of atoms. Energy of formation is 1 ev. Mobility of vacancy results in cluster of vacancies. Also caused due to plastic defor- -mation, rapid cooling or particle bombardment. Vacancies moving to form vacancy cluster
59 Point Defects - Interstitially Atom in a crystal, sometimes, occupies interstitial site. This does not occur naturally. Can be induced by irradiation. This defects caused structural distortion.
60 Point Defects in Ionic Crystals Complex as electric neutrality has to be maintained. If two appositely charged particles are missing, cationanion divacancy is created. This is scohttky imperfection. Frenkel imperfection is created when cation moves to interstitial site. Impurity atoms are also considered as point defects.
61 Line Defects (Dislocations) Lattice distortions are centered around a line. Formed during Solidification Permanent Deformation Vacancy condensation Different types of line defects are Edge dislocation Screw dislocation Mixed dislocation
62 Edge Dislocation Created by insertion of extra half planes of atoms. Positive edge dislocation Negative edge dislocation Burgers vector Shows displacement of atoms (slip). Burgers vector
63 Screw Dislocation Created due to shear stresses applied to regions of a perfect crystal separated by cutting plane. Distortion of lattice in form of a spiral ramp. Burgers vector is parallel to dislocation line.
64 Mixed Dislocation Most crystal have components of both edge and screw dislocation. Dislocation, since have irregular atomic arrangement will appear as dark lines when observed in electron microscope. Dislocation structure of iron deformed 14% at C
65 Grain Boundaries Grain boundaries, twins, low/high angle boundaries, twists and stacking faults Free surface is also a defect : Bonded to atoms on only one side and hence has higher state of energy Highly reactive Nanomaterials have small clusters of atoms and hence are highly reactive. 65
66 Grain Boundaries Grain boundaries separate grains. Formed due to simultaneously growing crystals meeting each other. Width = 2-5 atomic diameters. Some atoms in grain boundaries have higher energy. Restrict plastic flow and prevent dislocation movement. 3D view of grains 66 Grain Boundaries In 1018 steel
67 Twin Boundaries Twin: A region in which mirror image pf structure exists across a boundary. Formed during plastic deformation and recrystallization. Strengthens the metal. Twin Plane Twin
68 Other Planar Defects Small angle tilt boundary: Array of edge dislocations tilts two regions of a crystal by < 10 o Stacking faults: Piling up faults during recrystallization due to collapsing. Example: ABCABAACBABC FCC fault Volume defects: Cluster of point defects join to form 3-D void.
69 Observing Grain Boundaries - Metallography To observe grain boundaries, the metal sample must be first mounted for easy handling Then the sample should be ground and polished with different grades of abrasive paper and abrasive solution. The surface is then etched chemically. Tiny groves are produced at grain boundaries. Groves do not intensely reflect light. Hence observed by optical microscope.
70 Effect of Etching Unetched Steel 200 X Etched Steel 200 X Unetched Brass 200 X Etched Brass 200 X
71 Grain Size Affects the mechanical properties of the material The smaller the grain size, more are the grain boundaries. More grain boundaries means higher resistance to slip (plastic deformation occurs due to slip). More grains means more uniform the mechanical properties are.
72 Measuring Grain Size ASTM grain size number n is a measure of grain size. N = 2 n-1 N = Number of grains per N < 3 Coarse grained square inch of a polished 4 < n < 6 Medium grained and etched specimen at 100 x. n = ASTM grain size number. 7 < n < 9 Fine grained N > 10 ultrafine grained 200 X 200 X 1018 cold rolled steel, n= cold rolled steel, n=8
73 Average Grain Diameter Average grain diameter more directly represents grain size. Random line of known length is drawn on photomicrograph. Number of grains intersected is counted. Ratio of number of grains intersected to length of line, n L is determined. d = C/n L M C=1.5, and M is magnification 3 inches 5 grains.
74 Transmission Electron Microscope Electron produced by heated tungsten filament. Accelerated by high voltage ( KV) Electron beam passes through very thin specimen. Difference in atomic arrangement change directions of electrons. Beam is enlarged and focused on fluorescent screen. Collagen Fibrils of ligament as seen in TEM
75 Transmission Electron Microscope TEM needs complex sample preparation Very thin specimen needed ( several hundred nanometers) High resolution TEM (HRTEM) allows resolution of 0.1 nm. 2-D projections of a crystal with accompanying defects can be observed. Low angle boundary As seen In HTREM
76 Scanning Electron Microscope Electron source generates electrons. Electrons hit the surface and secondary electrons are produced. The secondary electrons are collected to produce the signal. The signal is used to produce the image. TEM of fractured metal end
CHAPTER 4. Solidification and Crystalline Imperfections in Solids 4-1
 CHAPTER 4 Solidification and Crystalline Imperfections in Solids 4-1 Solidification of Metals Metals are melted to produce finished and semifinished parts. Two steps of solidification Nucleation : Formation
CHAPTER 4 Solidification and Crystalline Imperfections in Solids 4-1 Solidification of Metals Metals are melted to produce finished and semifinished parts. Two steps of solidification Nucleation : Formation
3. Solidification & Crystalline Imperfections
 3. Solidification & Crystalline Imperfections solidification (casting process) of metals divided into two steps (1) nucleation formation of stable nuclei in the melt (2) growth of nuclei into crystals
3. Solidification & Crystalline Imperfections solidification (casting process) of metals divided into two steps (1) nucleation formation of stable nuclei in the melt (2) growth of nuclei into crystals
Learning Objectives. Chapter Outline. Solidification of Metals. Solidification of Metals
 Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Point Defects. Vacancies are the most important form. Vacancies Self-interstitials
 Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
RUNNING HOT. Sub-topics. Fuel cells Casting Solidification
 RUNNING HOT Sub-topics 1 Fuel cells Casting Solidification CONCEPT OF FUEL CELLS International concerns regarding the emission of greenhouse gases and the trend toward distributed power generation are
RUNNING HOT Sub-topics 1 Fuel cells Casting Solidification CONCEPT OF FUEL CELLS International concerns regarding the emission of greenhouse gases and the trend toward distributed power generation are
TOPIC 2. STRUCTURE OF MATERIALS III
 Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Dept.of BME Materials Science Dr.Jenan S.Kashan 1st semester 2nd level. Imperfections in Solids
 Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Engineering materials
 1 Engineering materials Lecture 2 Imperfections and defects Response of materials to stress 2 Crystalline Imperfections (4.4) No crystal is perfect. Imperfections affect mechanical properties, chemical
1 Engineering materials Lecture 2 Imperfections and defects Response of materials to stress 2 Crystalline Imperfections (4.4) No crystal is perfect. Imperfections affect mechanical properties, chemical
12/10/09. Chapter 4: Imperfections in Solids. Imperfections in Solids. Polycrystalline Materials ISSUES TO ADDRESS...
 Chapter 4: ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects be varied and controlled? How do defects affect material
Chapter 4: ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects be varied and controlled? How do defects affect material
Introduction to Engineering Materials ENGR2000 Chapter 4: Imperfections in Solids. Dr. Coates
 Introduction to Engineering Materials ENGR000 Chapter 4: Imperfections in Solids Dr. Coates Learning Objectives 1. Describe both vacancy and self interstitial defects. Calculate the equilibrium number
Introduction to Engineering Materials ENGR000 Chapter 4: Imperfections in Solids Dr. Coates Learning Objectives 1. Describe both vacancy and self interstitial defects. Calculate the equilibrium number
Defect in crystals. Primer in Materials Science Spring
 Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Imperfections, Defects and Diffusion
 Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Chapter 2. Ans: e (<100nm size materials are called nanomaterials)
 Chapter 2 1. Materials science and engineering include (s) the study of: (a) metals (b) polymers (c) ceramics (d) composites (e) nanomaterials (f) all of the above Ans: f 2. Which one of the following
Chapter 2 1. Materials science and engineering include (s) the study of: (a) metals (b) polymers (c) ceramics (d) composites (e) nanomaterials (f) all of the above Ans: f 2. Which one of the following
Strengthening Mechanisms
 ME 254: Materials Engineering Chapter 7: Dislocations and Strengthening Mechanisms 1 st Semester 1435-1436 (Fall 2014) Dr. Hamad F. Alharbi, harbihf@ksu.edu.sa November 18, 2014 Outline DISLOCATIONS AND
ME 254: Materials Engineering Chapter 7: Dislocations and Strengthening Mechanisms 1 st Semester 1435-1436 (Fall 2014) Dr. Hamad F. Alharbi, harbihf@ksu.edu.sa November 18, 2014 Outline DISLOCATIONS AND
From sand to silicon wafer
 From sand to silicon wafer 25% of Earth surface is silicon Metallurgical grade silicon (MGS) Electronic grade silicon (EGS) Polycrystalline silicon (polysilicon) Single crystal Czochralski drawing Single
From sand to silicon wafer 25% of Earth surface is silicon Metallurgical grade silicon (MGS) Electronic grade silicon (EGS) Polycrystalline silicon (polysilicon) Single crystal Czochralski drawing Single
Materials Science. Imperfections in Solids CHAPTER 5: IMPERFECTIONS IN SOLIDS. Types of Imperfections
 In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
Chapter 10, Phase Transformations
 Chapter Outline: Phase Transformations Heat Treatment (time and temperature) Microstructure Kinetics of phase transformations Homogeneous and heterogeneous nucleation Growth, rate of the phase transformation
Chapter Outline: Phase Transformations Heat Treatment (time and temperature) Microstructure Kinetics of phase transformations Homogeneous and heterogeneous nucleation Growth, rate of the phase transformation
Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities)
 Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities) 1 Structural Imperfections A perfect crystal has the lowest internal energy E Above absolute zero
Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities) 1 Structural Imperfections A perfect crystal has the lowest internal energy E Above absolute zero
Chapter 5. Imperfections in Solids
 Chapter 5 Imperfections in Solids Chapter 5 2D Defects and Introduction to Diffusion Imperfections in Solids Issues to Address... What types of defects arise in solids? Can the number and type of defects
Chapter 5 Imperfections in Solids Chapter 5 2D Defects and Introduction to Diffusion Imperfections in Solids Issues to Address... What types of defects arise in solids? Can the number and type of defects
Imperfections in the Atomic and Ionic Arrangements
 Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
(a) Would you expect the element P to be a donor or an acceptor defect in Si?
 MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
IMPERFECTIONSFOR BENEFIT. Sub-topics. Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface
 IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
 Defects in solids http://www.bath.ac.uk/podcast/powerpoint/inaugural_lecture_250407.pdf http://www.materials.ac.uk/elearning/matter/crystallography/indexingdirectionsandplanes/indexing-of-hexagonal-systems.html
Defects in solids http://www.bath.ac.uk/podcast/powerpoint/inaugural_lecture_250407.pdf http://www.materials.ac.uk/elearning/matter/crystallography/indexingdirectionsandplanes/indexing-of-hexagonal-systems.html
Metal working: Deformation processing II. Metal working: Deformation processing II
 Module 28 Metal working: Deformation processing II Lecture 28 Metal working: Deformation processing II 1 Keywords : Difference between cold & hot working, effect of macroscopic variables on deformation
Module 28 Metal working: Deformation processing II Lecture 28 Metal working: Deformation processing II 1 Keywords : Difference between cold & hot working, effect of macroscopic variables on deformation
Crystal Defects. Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K)
 Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
8. Principles of Solidification
 CBE4010 Introduction to Materials Science for Chemical Engineers 8. Principles of Solidification The Driving Force a Phase Change We expect a material to solidify when the liquid cools to just below its
CBE4010 Introduction to Materials Science for Chemical Engineers 8. Principles of Solidification The Driving Force a Phase Change We expect a material to solidify when the liquid cools to just below its
ISSUES TO ADDRESS...
 Chapter 5: IMPERFECTIONS IN SOLIDS School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 4-1 ISSUES TO ADDRESS... What are the solidification mechanisms? What types
Chapter 5: IMPERFECTIONS IN SOLIDS School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 4-1 ISSUES TO ADDRESS... What are the solidification mechanisms? What types
Point coordinates. x z
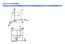 Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Physics of Materials: Defects in Solids. Dr. Anurag Srivastava. Indian Institute of Information Technology and Manegement, Gwalior
 : Defects in Solids Dr. Anurag Srivastava Atal Bihari Vajpayee Indian Institute of Information Technology and Manegement, Gwalior What you have learnt so far? Properties of Materials affected by defects
: Defects in Solids Dr. Anurag Srivastava Atal Bihari Vajpayee Indian Institute of Information Technology and Manegement, Gwalior What you have learnt so far? Properties of Materials affected by defects
Materials and their structures
 Materials and their structures 2.1 Introduction: The ability of materials to undergo forming by different techniques is dependent on their structure and properties. Behavior of materials depends on their
Materials and their structures 2.1 Introduction: The ability of materials to undergo forming by different techniques is dependent on their structure and properties. Behavior of materials depends on their
Defects and Diffusion
 Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Diffusional Transformations in Solids
 Diffusional Transformations in Solids The majority of phase transformations that occur in the solid state take place by thermally activated atomic movements. The transformations that will be dealt with
Diffusional Transformations in Solids The majority of phase transformations that occur in the solid state take place by thermally activated atomic movements. The transformations that will be dealt with
SECTION A. NATURAL SCIENCES TRIPOS Part IA. Friday 4 June to 4.30 MATERIALS AND MINERAL SCIENCES
 NATURAL SCIENCES TRIPOS Part IA Friday 4 June 1999 1.30 to 4.30 MATERIALS AND MINERAL SCIENCES Answer five questions; two from each of sections A and B and one from section C. Begin each answer at the
NATURAL SCIENCES TRIPOS Part IA Friday 4 June 1999 1.30 to 4.30 MATERIALS AND MINERAL SCIENCES Answer five questions; two from each of sections A and B and one from section C. Begin each answer at the
Point coordinates. Point coordinates for unit cell center are. Point coordinates for unit cell corner are 111
 Point coordinates c z 111 Point coordinates for unit cell center are a/2, b/2, c/2 ½ ½ ½ Point coordinates for unit cell corner are 111 x a z 000 b 2c y Translation: integer multiple of lattice constants
Point coordinates c z 111 Point coordinates for unit cell center are a/2, b/2, c/2 ½ ½ ½ Point coordinates for unit cell corner are 111 x a z 000 b 2c y Translation: integer multiple of lattice constants
MT 348 Outline No MECHANICAL PROPERTIES
 MT 348 Outline No. 1 2009 MECHANICAL PROPERTIES I. Introduction A. Stresses and Strains, Normal and Shear Loading B. Elastic Behavior II. Stresses and Metal Failure A. ʺPrincipal Stressʺ Concept B. Plastic
MT 348 Outline No. 1 2009 MECHANICAL PROPERTIES I. Introduction A. Stresses and Strains, Normal and Shear Loading B. Elastic Behavior II. Stresses and Metal Failure A. ʺPrincipal Stressʺ Concept B. Plastic
Tutorial 2 : Crystalline Solid, Solidification, Crystal Defect and Diffusion
 Tutorial 1 : Introduction and Atomic Bonding 1. Explain the difference between ionic and metallic bonding between atoms in engineering materials. 2. Show that the atomic packing factor for Face Centred
Tutorial 1 : Introduction and Atomic Bonding 1. Explain the difference between ionic and metallic bonding between atoms in engineering materials. 2. Show that the atomic packing factor for Face Centred
Metallurgy - Lecture (2) Solidification
 Metallurgy - Lecture (2) Solidification When molten metal enters a mold cavity, its heat is transferred through the mold wall. In the case of pure metals and eutectics, the solidification proceeds layer-bylayer
Metallurgy - Lecture (2) Solidification When molten metal enters a mold cavity, its heat is transferred through the mold wall. In the case of pure metals and eutectics, the solidification proceeds layer-bylayer
Chapter 4: Imperfections (Defects) in Solids
 Chapter 4: Imperfections (Defects) in Solids ISSUES TO ADDRESS... What types of defects exist in solids? How do defects affect material properties? Can the number and type of defects be varied and controlled?
Chapter 4: Imperfections (Defects) in Solids ISSUES TO ADDRESS... What types of defects exist in solids? How do defects affect material properties? Can the number and type of defects be varied and controlled?
Types of manufacturing processes
 Materials processing Metal parts undergo sequence of processes Primary alter the ( raw ) material s basic shape or form. Sand casting Rolling Forging Sheet metalworking Types of manufacturing processes
Materials processing Metal parts undergo sequence of processes Primary alter the ( raw ) material s basic shape or form. Sand casting Rolling Forging Sheet metalworking Types of manufacturing processes
Part II : Interfaces Module 3 : Nucleation of precipitates from a supersaturated matrix
 Part II : Interfaces Module 3 : Nucleation of precipitates from a supersaturated matrix 3.1 Motivation A solid contains many defects: vacancies, dislocations, stacking faults, grain and interphase boundaries,
Part II : Interfaces Module 3 : Nucleation of precipitates from a supersaturated matrix 3.1 Motivation A solid contains many defects: vacancies, dislocations, stacking faults, grain and interphase boundaries,
Dislocations Linear Defects
 Dislocations Linear Defects Dislocations are abrupt changes in the regular ordering of atoms, along a line (dislocation line) in the solid. They occur in high density and are very important in mechanical
Dislocations Linear Defects Dislocations are abrupt changes in the regular ordering of atoms, along a line (dislocation line) in the solid. They occur in high density and are very important in mechanical
Recrystallization Theoretical & Practical Aspects
 Theoretical & Practical Aspects 27-301, Microstructure & Properties I Fall 2006 Supplemental Lecture A.D. Rollett, M. De Graef Materials Science & Engineering Carnegie Mellon University 1 Objectives The
Theoretical & Practical Aspects 27-301, Microstructure & Properties I Fall 2006 Supplemental Lecture A.D. Rollett, M. De Graef Materials Science & Engineering Carnegie Mellon University 1 Objectives The
Chapter 2 Crystal Growth and Wafer Preparation
 Chapter 2 Crystal Growth and Wafer Preparation Professor Paul K. Chu Advantages of Si over Ge Si has a larger bandgap (1.1 ev for Si versus 0.66 ev for Ge) Si devices can operate at a higher temperature
Chapter 2 Crystal Growth and Wafer Preparation Professor Paul K. Chu Advantages of Si over Ge Si has a larger bandgap (1.1 ev for Si versus 0.66 ev for Ge) Si devices can operate at a higher temperature
Chapter 8 Strain Hardening and Annealing
 Chapter 8 Strain Hardening and Annealing This is a further application of our knowledge of plastic deformation and is an introduction to heat treatment. Part of this lecture is covered by Chapter 4 of
Chapter 8 Strain Hardening and Annealing This is a further application of our knowledge of plastic deformation and is an introduction to heat treatment. Part of this lecture is covered by Chapter 4 of
Lecture # 11 References:
 Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC Dr.Haydar Al-Ethari
Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC Dr.Haydar Al-Ethari
Introduction to Materials Science
 EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties
 Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties A perfect crystal, with every atom of the same type in the correct position, does not exist. There always exist crystalline defects,
Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties A perfect crystal, with every atom of the same type in the correct position, does not exist. There always exist crystalline defects,
solvent: component of a solution present in the greatest amount in alloy.
 Phase Equilibrium Diagrams:- Phase equilibrium diagram is a graphic relationship between temperature and weight ratios of elements and alloys contribute to the built of the diagram. Phase diagrams provide
Phase Equilibrium Diagrams:- Phase equilibrium diagram is a graphic relationship between temperature and weight ratios of elements and alloys contribute to the built of the diagram. Phase diagrams provide
SOLIDIFICATION, PHASE DIAGRAM & STEELS
 MODULE TWO SOLIDIFICATION, PHASE DIAGRAM & STEELS 4. SOLIDIFICATION Introduction Mechanism of solidification - crystallization and development of cast structure - nucleation and grain growth - dendritic
MODULE TWO SOLIDIFICATION, PHASE DIAGRAM & STEELS 4. SOLIDIFICATION Introduction Mechanism of solidification - crystallization and development of cast structure - nucleation and grain growth - dendritic
Chapter Outline Dislocations and Strengthening Mechanisms. Introduction
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
Fundamentals of Casting
 Fundamentals of Casting Chapter 11 11.1 Introduction Products go through a series of processes before they are produced Design Material selection Process selection Manufacture Inspection and evaluation
Fundamentals of Casting Chapter 11 11.1 Introduction Products go through a series of processes before they are produced Design Material selection Process selection Manufacture Inspection and evaluation
Materials Engineering. Phase transformation Phase diagrams
 Materials Engineering Phase transformation Phase diagrams Phase Transformation Why is it important for us? o Temperature, chemical composition and pressure can change the properties of materials o Understanding
Materials Engineering Phase transformation Phase diagrams Phase Transformation Why is it important for us? o Temperature, chemical composition and pressure can change the properties of materials o Understanding
Phase change processes for material property manipulation BY PROF.A.CHANDRASHEKHAR
 Phase change processes for material property manipulation BY PROF.A.CHANDRASHEKHAR Introduction The phase of a material is defined as a chemically and structurally homogeneous state of material. Any material
Phase change processes for material property manipulation BY PROF.A.CHANDRASHEKHAR Introduction The phase of a material is defined as a chemically and structurally homogeneous state of material. Any material
Imperfections in atomic arrangements
 MME131: Lecture 9 Imperfections in atomic arrangements Part 2: 1D 3D Defects A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Classifications and characteristics of 1D 3D defects
MME131: Lecture 9 Imperfections in atomic arrangements Part 2: 1D 3D Defects A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Classifications and characteristics of 1D 3D defects
Metals are used by industry for either one or combination of the following properties
 Basic Metallurgy Metals are the backbone of the engineering industry being the most important Engineering Materials. In comparison to other engineering materials such as wood, ceramics, fabric and plastics,
Basic Metallurgy Metals are the backbone of the engineering industry being the most important Engineering Materials. In comparison to other engineering materials such as wood, ceramics, fabric and plastics,
Chapter 9 Phase Diagrams. Dr. Feras Fraige
 Chapter 9 Phase Diagrams Dr. Feras Fraige Chapter Outline Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited
Chapter 9 Phase Diagrams Dr. Feras Fraige Chapter Outline Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited
Solid. Imperfection in solids. Examples of Imperfections #2. Examples of Imperfections #1. Solid
 Solid Imperfection in solids By E-mail: Patama.V@chula.ac.th Solid State of materials Rigid Strong bonding ionic, van der aals, metal bonding normally has crystal structure Examples of Imperfections #
Solid Imperfection in solids By E-mail: Patama.V@chula.ac.th Solid State of materials Rigid Strong bonding ionic, van der aals, metal bonding normally has crystal structure Examples of Imperfections #
Equilibria in Materials
 2009 fall Advanced Physical Metallurgy Phase Equilibria in Materials 09.01.2009 Eun Soo Park Office: 33-316 Telephone: 880-7221 Email: espark@snu.ac.kr Office hours: by an appointment 1 Text: A. PRINCE,
2009 fall Advanced Physical Metallurgy Phase Equilibria in Materials 09.01.2009 Eun Soo Park Office: 33-316 Telephone: 880-7221 Email: espark@snu.ac.kr Office hours: by an appointment 1 Text: A. PRINCE,
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS ev /atom = exp. kt ( =
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
STRENGTHENING MECHANISM IN METALS
 Background Knowledge Yield Strength STRENGTHENING MECHANISM IN METALS Metals yield when dislocations start to move (slip). Yield means permanently change shape. Slip Systems Slip plane: the plane on which
Background Knowledge Yield Strength STRENGTHENING MECHANISM IN METALS Metals yield when dislocations start to move (slip). Yield means permanently change shape. Slip Systems Slip plane: the plane on which
EMA5001 Lecture 15 Other Issues with Metal Solidification by Zhe Cheng
 EMA5001 Lecture 15 Other Issues with Metal Solidification 016 by Zhe Cheng Eutectic Solidification L α + β 67 wt.% Al-wt% Cu eutectic Classification Normal Lamellar or other structures Both solid phase
EMA5001 Lecture 15 Other Issues with Metal Solidification 016 by Zhe Cheng Eutectic Solidification L α + β 67 wt.% Al-wt% Cu eutectic Classification Normal Lamellar or other structures Both solid phase
Module 10. Crystal Defects in Metals I. Lecture 10. Crystal Defects in Metals I
 Module 10 Crystal Defects in Metals I Lecture 10 Crystal Defects in Metals I 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Introduction Keywords:
Module 10 Crystal Defects in Metals I Lecture 10 Crystal Defects in Metals I 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Introduction Keywords:
CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS
 MODULE ONE CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS CRYSTAL STRUCTURE Metallic crystal structures; BCC, FCC and HCP Coordination number and Atomic Packing Factor (APF) Crystal imperfections:
MODULE ONE CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS CRYSTAL STRUCTURE Metallic crystal structures; BCC, FCC and HCP Coordination number and Atomic Packing Factor (APF) Crystal imperfections:
CHAPTER 4 1/1/2016. Mechanical Properties of Metals - I. Processing of Metals - Casting. Hot Rolling of Steel. Casting (Cont..)
 Processing of Metals - Casting CHAPTER 4 Mechanical Properties of Metals - I Most metals are first melted in a furnace. Alloying is done if required. Large ingots are then cast. Sheets and plates are then
Processing of Metals - Casting CHAPTER 4 Mechanical Properties of Metals - I Most metals are first melted in a furnace. Alloying is done if required. Large ingots are then cast. Sheets and plates are then
Dislocations and Plastic Deformation
 Dislocations and Plastic Deformation Edge and screw are the two fundamental dislocation types. In an edge dislocation, localized lattice distortion exists along the end of an extra half-plane of atoms,
Dislocations and Plastic Deformation Edge and screw are the two fundamental dislocation types. In an edge dislocation, localized lattice distortion exists along the end of an extra half-plane of atoms,
Engineering 45 The Structure and Properties of Materials Midterm Examination October 26, 1987
 Engineering 45 The Structure and Properties of Materials Midterm Examination October 26, 1987 Problem 1: (a) The compound CsCl is an ordered arrangement of Cs and Cl over the sites of a BCC lattice. Draw
Engineering 45 The Structure and Properties of Materials Midterm Examination October 26, 1987 Problem 1: (a) The compound CsCl is an ordered arrangement of Cs and Cl over the sites of a BCC lattice. Draw
Kinetics. Rate of change in response to thermodynamic forces
 Kinetics Rate of change in response to thermodynamic forces Deviation from local equilibrium continuous change T heat flow temperature changes µ atom flow composition changes Deviation from global equilibrium
Kinetics Rate of change in response to thermodynamic forces Deviation from local equilibrium continuous change T heat flow temperature changes µ atom flow composition changes Deviation from global equilibrium
Lecture 12: High Temperature Alloys
 Part IB Materials Science & Metallurgy H. K. D. H. Bhadeshia Course A, Metals and Alloys Lecture 12: High Temperature Alloys Metallic materials capable of operating at ever increasing temperatures are
Part IB Materials Science & Metallurgy H. K. D. H. Bhadeshia Course A, Metals and Alloys Lecture 12: High Temperature Alloys Metallic materials capable of operating at ever increasing temperatures are
Module 29. Precipitation from solid solution I. Lecture 29. Precipitation from solid solution I
 Module 29 Precipitation from solid solution I Lecture 29 Precipitation from solid solution I 1 Keywords : Properties of two phase alloys, super saturated solid solutions, historical perspective, solution
Module 29 Precipitation from solid solution I Lecture 29 Precipitation from solid solution I 1 Keywords : Properties of two phase alloys, super saturated solid solutions, historical perspective, solution
Phase Diagrams of Pure Substances Predicts the stable phase as a function of P total and T. Example: water can exist in solid, liquid and vapor
 PHASE DIAGRAMS Phase a chemically and structurally homogenous region of a material. Region of uniform physical and chemical characteristics. Phase boundaries separate two distinct phases. A single phase
PHASE DIAGRAMS Phase a chemically and structurally homogenous region of a material. Region of uniform physical and chemical characteristics. Phase boundaries separate two distinct phases. A single phase
Phase Transformations in Metals Tuesday, December 24, 2013 Dr. Mohammad Suliman Abuhaiba, PE 1
 Ferrite - BCC Martensite - BCT Fe 3 C (cementite)- orthorhombic Austenite - FCC Chapter 10 Phase Transformations in Metals Tuesday, December 24, 2013 Dr. Mohammad Suliman Abuhaiba, PE 1 Why do we study
Ferrite - BCC Martensite - BCT Fe 3 C (cementite)- orthorhombic Austenite - FCC Chapter 10 Phase Transformations in Metals Tuesday, December 24, 2013 Dr. Mohammad Suliman Abuhaiba, PE 1 Why do we study
PHASE EQUILIBRIUM P + F = C + 2
 PHASE EQUILIBRIUM Component: is either pure metal and/or compound of which an alloy is composed. They refer to the independent chemical species that comprise the system. Solid Solution: It consists of
PHASE EQUILIBRIUM Component: is either pure metal and/or compound of which an alloy is composed. They refer to the independent chemical species that comprise the system. Solid Solution: It consists of
Free Electron Model What kind of interactions hold metal atoms together? How does this explain high electrical and thermal conductivity?
 Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Mechanical Lustrous
Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Mechanical Lustrous
Fundamentals of Plastic Deformation of Metals
 We have finished chapters 1 5 of Callister s book. Now we will discuss chapter 10 of Callister s book Fundamentals of Plastic Deformation of Metals Chapter 10 of Callister s book 1 Elastic Deformation
We have finished chapters 1 5 of Callister s book. Now we will discuss chapter 10 of Callister s book Fundamentals of Plastic Deformation of Metals Chapter 10 of Callister s book 1 Elastic Deformation
Introduction to the phase diagram Uses and limitations of phase diagrams Classification of phase diagrams Construction of phase diagrams
 Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Concept of alloying Classification of alloys Introduction to the phase diagram Uses and limitations of phase diagrams Classification of phase diagrams
Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Concept of alloying Classification of alloys Introduction to the phase diagram Uses and limitations of phase diagrams Classification of phase diagrams
the Phase Diagrams Today s Topics
 MME 291: Lecture 03 Introduction to the Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Concept of alloying Classification of alloys Introduction to the phase diagram
MME 291: Lecture 03 Introduction to the Phase Diagrams Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Concept of alloying Classification of alloys Introduction to the phase diagram
CREEP CREEP. Mechanical Metallurgy George E Dieter McGraw-Hill Book Company, London (1988)
 CREEP CREEP Mechanical Metallurgy George E Dieter McGraw-Hill Book Company, London (1988) Review If failure is considered as change in desired performance*- which could involve changes in properties and/or
CREEP CREEP Mechanical Metallurgy George E Dieter McGraw-Hill Book Company, London (1988) Review If failure is considered as change in desired performance*- which could involve changes in properties and/or
Point Defects in Metals
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327 C (600 K). Assume an energy
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327 C (600 K). Assume an energy
CHAPTER INTRODUCTION
 1 CHAPTER-1 1.0 INTRODUCTION Contents 1.0 Introduction 1 1.1 Aluminium alloys 2 1.2 Aluminium alloy classification 2 1.2.1 Aluminium alloys (Wrought) 3 1.2.2 Heat treatable alloys (Wrought). 3 1.2.3 Aluminum
1 CHAPTER-1 1.0 INTRODUCTION Contents 1.0 Introduction 1 1.1 Aluminium alloys 2 1.2 Aluminium alloy classification 2 1.2.1 Aluminium alloys (Wrought) 3 1.2.2 Heat treatable alloys (Wrought). 3 1.2.3 Aluminum
NATURE OF METALS AND ALLOYS
 NATURE OF METALS AND ALLOYS Chapter 4 NATURE OF METALS AND ALLOYS Instructor: Office: MEC 325, Tel.: 973-642-7455 E-mail: samardzi@njit.edu Link to ME 215: http://mechanical.njit.edu/students/merequired.php
NATURE OF METALS AND ALLOYS Chapter 4 NATURE OF METALS AND ALLOYS Instructor: Office: MEC 325, Tel.: 973-642-7455 E-mail: samardzi@njit.edu Link to ME 215: http://mechanical.njit.edu/students/merequired.php
Solid State Transformations
 Solid State Transformations Symmetrical Tilt Boundary The misorientation θ between grains can be described in terms of dislocations (Fig. 1). Inserting an edge dislocation of Burgers vector b is like forcing
Solid State Transformations Symmetrical Tilt Boundary The misorientation θ between grains can be described in terms of dislocations (Fig. 1). Inserting an edge dislocation of Burgers vector b is like forcing
Two marks questions and answers. 1. what is a Crystal? (or) What are crystalline materials? Give examples
 UNIT V CRYSTAL PHYSICS PART-A Two marks questions and answers 1. what is a Crystal? (or) What are crystalline materials? Give examples Crystalline solids (or) Crystals are those in which the constituent
UNIT V CRYSTAL PHYSICS PART-A Two marks questions and answers 1. what is a Crystal? (or) What are crystalline materials? Give examples Crystalline solids (or) Crystals are those in which the constituent
Phase Transformation in Materials
 2015 Fall Phase Transformation in Materials 09. 02. 2015 Eun Soo Park Office: 33-313 Telephone: 880-7221 Email: espark@snu.ac.kr Office hours: by an appointment 1 Introduction Web lecture assistance: http://etl.snu.ac.kr
2015 Fall Phase Transformation in Materials 09. 02. 2015 Eun Soo Park Office: 33-313 Telephone: 880-7221 Email: espark@snu.ac.kr Office hours: by an appointment 1 Introduction Web lecture assistance: http://etl.snu.ac.kr
Department of Mechanical Engineering University of Saskatchewan. ME324.3 Engineering Materials. Mid-Term Examination (Closed Book)
 Student #: Department of Mechanical Engineering University of Saskatchewan ME. Engineering Materials Mid-Term Examination (Closed Book) Instructor: I. Oguocha Time Allowed: h Friday, 9 October 00. Section
Student #: Department of Mechanical Engineering University of Saskatchewan ME. Engineering Materials Mid-Term Examination (Closed Book) Instructor: I. Oguocha Time Allowed: h Friday, 9 October 00. Section
Strengthening Mechanisms. Today s Topics
 MME 131: Lecture 17 Strengthening Mechanisms Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Strengthening strategies: Grain strengthening Solid solution strengthening Work hardening
MME 131: Lecture 17 Strengthening Mechanisms Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Today s Topics Strengthening strategies: Grain strengthening Solid solution strengthening Work hardening
Chapter 7 Dislocations and Strengthening Mechanisms. Dr. Feras Fraige
 Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Metal Casting. Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian Schmid 2008, Pearson Education ISBN No.
 Metal Casting Important factors in casting Solidification of the metal from its molten state and accompanying shrinkage Flow of the molten metal into the mold cavity Heat transfer during solidification
Metal Casting Important factors in casting Solidification of the metal from its molten state and accompanying shrinkage Flow of the molten metal into the mold cavity Heat transfer during solidification
4-Crystal Defects & Strengthening
 4-Crystal Defects & Strengthening A perfect crystal, with every atom of the same type in the correct position, does not exist. The crystalline defects are not always bad! Adding alloying elements to a
4-Crystal Defects & Strengthening A perfect crystal, with every atom of the same type in the correct position, does not exist. The crystalline defects are not always bad! Adding alloying elements to a
Lecture # 11. Line defects (1D) / Dislocations
 Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC References: 1-
Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC References: 1-
Engineering 45 Midterm 02
 UNIVERSITY OF ALIFORNIA ollege of Engineering Department of Materials Science & Engineering Professor R. Gronsky Fall Semester, 5 Engineering 45 Midterm SOLUTIONS INSTRUTIONS LATTIE seating... Please be
UNIVERSITY OF ALIFORNIA ollege of Engineering Department of Materials Science & Engineering Professor R. Gronsky Fall Semester, 5 Engineering 45 Midterm SOLUTIONS INSTRUTIONS LATTIE seating... Please be
Introduction to Engineering Materials ENGR2000 Chapter 7: Dislocations and Strengthening Mechanisms. Dr. Coates
 Introduction to Engineering Materials ENGR2000 Chapter 7: Dislocations and Strengthening Mechanisms Dr. Coates An edge dislocation moves in response to an applied shear stress dislocation motion 7.1 Introduction
Introduction to Engineering Materials ENGR2000 Chapter 7: Dislocations and Strengthening Mechanisms Dr. Coates An edge dislocation moves in response to an applied shear stress dislocation motion 7.1 Introduction
Effect of titanium additions to low carbon, low manganese steels on sulphide precipitation
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2008 Effect of titanium additions to low carbon, low manganese steels on sulphide precipitation
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2008 Effect of titanium additions to low carbon, low manganese steels on sulphide precipitation
University of Pretoria Z Tang (2006) Chapter 8 Studies of acicular ferrite by thin foil TEM
 8.2 Two types of acicular ferrite 8.2.1 Structure with parallel laths There appeared to be two types of acicular ferrite laths that were observed in those alloys cooled with a rapid cooling rate of 47
8.2 Two types of acicular ferrite 8.2.1 Structure with parallel laths There appeared to be two types of acicular ferrite laths that were observed in those alloys cooled with a rapid cooling rate of 47
Introduction to Heat Treatment. Introduction
 MME444 Heat Treatment Sessional Week 01 Introduction to Heat Treatment Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction Can you control the microstructure that formed during cooling of
MME444 Heat Treatment Sessional Week 01 Introduction to Heat Treatment Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction Can you control the microstructure that formed during cooling of
Single vs Polycrystals
 WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
CHAPTER 12. Phase Transformations
 CHAPTER 12 Phase Transformations Introduction Basic concepts The kinetics of phase transformations Metastable versus equilibrium states Isothermal transformation diagrams Continuous cooling transformation
CHAPTER 12 Phase Transformations Introduction Basic concepts The kinetics of phase transformations Metastable versus equilibrium states Isothermal transformation diagrams Continuous cooling transformation
Material Science. Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore India
 Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 3. Imperfections in Solids Learning objectives:
Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 3. Imperfections in Solids Learning objectives:
The Science and Engineering of Materials, 4 th ed Donald R. Askeland Pradeep P. Phulé. Chapter 8 Solid Solutions and Phase Equilibrium
 The Science and Engineering of Materials, 4 th ed Donald R. Askeland Pradeep P. Phulé Chapter 8 Solid Solutions and Phase Equilibrium Objectives of Chapter 8 The goal of this chapter is to describe the
The Science and Engineering of Materials, 4 th ed Donald R. Askeland Pradeep P. Phulé Chapter 8 Solid Solutions and Phase Equilibrium Objectives of Chapter 8 The goal of this chapter is to describe the
INGE Engineering Materials. Chapter 3 (cont.)
 Some techniques used: Chapter 3 (cont.) This section will address the question how do we determine the crystal structure of a solid sample? Electron microscopy (by direct and indirect observations) Scanning
Some techniques used: Chapter 3 (cont.) This section will address the question how do we determine the crystal structure of a solid sample? Electron microscopy (by direct and indirect observations) Scanning
