Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly
|
|
|
- Tracy McDonald
- 6 years ago
- Views:
Transcription
1 Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly Colleen T. Skau a, Sergey V. Plotnikov b, Andrew. oyle c, and Clare M. Waterman a,1 a Cell Biology and Physiology Center, National Heart Lung and Blood Institute, and c Cell Biology Section, Laboratory of Cell and evelopmental Biology, National Institute of ental and Craniofacial Research, NIH, Bethesda, M 2892; and b epartment of Cell and Systems Biology, University of Toronto, Toronto, ON, Canada M5S 3G5 Edited by Martin A. Schwartz, Yale School of Medicine, New Haven, CT, and accepted by the Editorial Board March 31, 215 (received for review March 18, 215) filaments and integrin-based focal adhesions (FAs) form integrated systems that mediate dynamic cell interactions with their environment or other cells during migration, the immune response, and tissue morphogenesis. How adhesion-associated actin structures obtain their functional specificity is unclear. Here we show that the formin-family actin nucleator, inverted formin 2 (INF2), localizes specifically to FAs and dorsal stress fibers (SFs) in fibroblasts. High-resolution fluorescence microscopy and manipulation of INF2 levels in cells indicate that INF2 plays a critical role at the SF FA junction by promoting actin polymerization via free barbed end generation and centripetal elongation of an FAassociated actin bundle to form dorsal SF. INF2 assembles into FAs during maturation rather than during their initial generation, and once there, acts to promote rapid FA elongation and maturation into tensin-containing fibrillar FAs in the cell center. We show that INF2 is required for fibroblasts to organize fibronectin into matrix fibers and ultimately 3 matrices. Collectively our results indicate an important role for the formin INF2 in specifying the function of fibrillar FAs through its ability to generate dorsal SFs. Thus, dorsal SFs and fibrillar FAs form a specific class of integrated adhesionassociated actin structure in fibroblasts that mediates generation and remodeling of ECM. INF2 integrin actin fluorescence microscopy The dynamic connection between the forces generated in the actomyosin cytoskeleton and integrin-mediated focal adhesions (FAs) to the extracellular matrix (ECM) is essential for many physiological processes including cell migration, vascular formation and function, the immune response, and tissue morphogenesis. These diverse functions are mediated by distinct cellular structures including protruding lamellipodia containing nascent FAs that mediate haptotaxis (1), ventral adhesive actin waves that mediate leukocyte transmigration through endothelia (2, 3), and stress fibers (SFs) and FAs that drive fibrillarization of ECM in developing embryos (4, 5). The coordination and interdependence of actin and integrin-based adhesion in these specialized cellular structures are rooted in their biochemical interdependence. Activation of integrins to their high-affinity ECM binding state requires the actin cytoskeleton (6). In turn, integrin engagement with ECM induces signaling that mediates actin polymerization and contractility downstream of Rho GTPases (6, 7). ECM-engaged integrins also affect cytoskeletal organization by physically linking the contractile actomyosin system to extracellular anchorage points (7). Thus, adhesion-associated actin structures are integrated systems that mediate cellular functions requiring coordination of intracellular cytoskeletal forces with ECM binding. Mesenchymal cells generally possess two main types of adhesion-associated actin structures: protruding lamellipodia containing nascent FAs at the cell edge and linear actin bundles in the cell body connected to FAs. Compared with architecturally invariant lamellipodia, adhesion-associated actin bundle structures, including filopodia, the perinuclear actin cap/transmembrane actin-associated nuclear lines, trailing edge bundles, and dorsal SFs, are more diverse in their morphology and less well understood in their architecture and function (8 1). The most-studied actin bundle structure is perhaps dorsal SFs, noncontractile bundles associated at one end with a ventral FA near the cell edge and that extend radially toward the cell center and join with dorsal actin arcs on their other end. How the functional specificity of dorsal SFs is generated apart from the many other distinct adhesion-associated actin bundle structures is not well understood. The functional specificity of adhesion-associated actin structures could be generated either on the adhesion side by compositional differences in FA proteins or on the actin side by differences in the nucleation mechanism and actin binding proteins. On the adhesion side, it is well known that different integrin family members bind distinct types of ECM (11, 12). However, cells adhered to different ECMs all form common structures including lamellipodia, filopodia, and multiple types of SFs. In addition to different integrins, FA function could be regulated by the process of maturation in which FAs undergo stereotypical dynamic changes in composition and morphology driven by actomyosin-mediated cellular tension (13, 14). Nascent FAs contain integrins, focal adhesion kinase (FAK), a-actinin, Significance The ability of cells to interact with and remodel their extracellular environment is a critical process in developmental morphogenesis, wound healing, and cancer. How the physical and chemical responses of fibroblasts to the extracellular matrix are integrated across the cell remains a major open question. Previous data has shown a major role for the actin cytoskeleton in coordinating deposition and organization of the extracellular matrix by fibroblasts. Our study examines the role of inverted formin 2 (INF2), a protein known to create new actin filaments, in mediating cellular response to extracellular conditions and control of extracellular matrix remodeling. We find that INF2 is responsible for generating specific actin structures and specialized integrin-based fibrillar adhesions that are required for remodeling of the fibronectin extracellular matrix by fibroblasts. Author contributions: C.T.S., A..., and C.M.W. designed research; C.T.S. performed research; A... contributed new reagents/analytic tools; C.T.S. and S.V.P. analyzed data; and C.T.S. and C.M.W. wrote the paper. The authors declare no conflict of interest. This article is a PNAS irect Submission. M.A.S. is a guest editor invited by the Editorial Board. 1 To whom correspondence should be addressed. watermancm@nhlbi.nih.gov. This article contains supporting information online at 173/pnas /-/CSupplemental. CELL BIOLOGY PNAS PLUS PNAS Published online April 27, 215 E2447 E2456
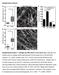
2 and paxillin (13, 15). When tension is applied, nascent FAs grow and recruit hundreds of proteins, including talin, vinculin, and zyxin (16). These mature FAs then either disassemble or further mature into tensin-containing fibrillar FAs that are responsible for fibronectin fibrillogenesis (17). Thus, the changes in FA size and protein content that accompany FA maturation could give rise to functional specialization of adhesion/actin systems. On the other hand, actin filaments in migrating cells are generated by two main classes of nucleators: the Arp2/3 complex and formins (18). ifferent nucleating proteins generate different actin organization and geometries, which could in turn dictate functional specificity of adhesions. Arp2/3 forms the branched network in lamellipodia and is thought to be linked to nascent FAs through interaction with FAK (19 21) or vinculin (22). The formin family of actin nucleators, which generates linear actin bundles (23), is more diverse, although formins share a common actin assembly core domain (24), (25). Recent work has begun to ascribe the generation of particular actin structures to some of the 15 formins in mammalian cells, particularly members of the diaphanous family and FHO1 (26 29). Specifically regarding dorsal SFs, evidence points strongly to polymerization by a formin family member (23, 3 32) but no formin has ever been localized to these SFs or their associated FAs in motile cells. Thus, although formins are clearly critical for forming distinct actin structures, whether they cooperate with FA proteins to specify the function of adhesion-associated actin structures in the cell is unclear. We hypothesized that inverted formin 2 (INF2), found in our recent FA proteome (33), may play a critical role in the formation and functional specificity of adhesion-associated actin structures. INF2 is expressed in cells in two isoforms, one containing a membrane-targeting CAAX-motif that plays a role in mitochondrial fission (34) and a non-caax isoform whose function is not well characterized. INF2 is an unusual formin insofar as it contains, in addition to the FH1 FH2 domains that polymerize actin, a WH2-like domain at the C terminus (35) that binds actin monomers to regulate autoinhibition, and also mediates filament severing (35, 36). INF2 also interacts with and inhibits members of the diaphanous family of formin proteins (37). INF2 therefore could have multiple possible roles at FAs in local modulation of actin. Here we explore the role of INF2 in mouse embryonic fibroblasts (MEFs). We find for the first time to our knowledge strong localization of an endogenous formin to FAs at the distal tips of dorsal SFs where it is required for actin polymerization at FAs to form dorsal SFs. We show that INF2 plays a role in controlling morphological, but not compositional maturation of FAs. Strikingly, INF2 is responsible for the formation of one specific class of FAs, the fibrillar FAs that organize the ECM; disruption of INF2 leads to defects in ECM fibrillogenesis. Thus, our study demonstrates that INF2 mediates the formation of dorsal SFs and fibrillar FAs, which together comprise a specific integrated adhesion-associated actin structure responsible for the fibrillogenesis of ECM by fibroblasts. Results The INF2 Formin Localizes to Lamellipodia, orsal SFs, and FAs. To understand the role of the formin INF2 in organization of the actin cytoskeleton in fibroblast function, we first sought to determine the actin structures with which it associates. Primary MEFs plated on fibronectin (FN)-coated coverslips were rapidly fixed with acetone and costained for endogenous INF2, F-actin, and canonical markers of SFs (Fig. 1A) and subjected to spinning disk confocal microscopy. As reported previously (34), INF2 localized to narrow fibril structures in the center of the cell presumed to be mitochondria (Fig. S1, blue arrow). Costaining of INF2 and F-actin showed that INF2 was restricted to a subset A α-in INF2 INF2/Atn Tpm INF2 INF2/Tpm MIIA INF2 INF2/MIIA INF2 Vinculin INF2 of actin bundles in the lamella and to the lamellipodial actin meshwork at the cell edge (Fig. 1 A C). To determine the identity of the INF2-containing actin bundles, we performed colocalization analysis with specific SF markers. Immunofluorescence analysis showed that INF2 localized along radial actin bundles that also contained α-actinin and tropomyosins (Fig. 1 A and E). These SFs extended perpendicularly to the leading edge and INF2 was concentrated at their distal tips where it colocalized with α-actinin. We then costained for myosin IIA, which is known to associate with transverse arcs B Bottom C E Top INF2 INF2 Stress Fiber Proteins α-in Tpm Focal Adhesion Proteins N.S P-Tyr Vinculin Fig. 1. Formin INF2 localizes to dorsal SFs at the junction with FAs. (A) Representative confocal micrographs of INF2 immunofluorescence (colorcoded green) with Alexa-488 phalloidin staining of actin and immunofluorescence of the actin binding proteins α-actinin (Top, color-coded red), pantropomyosin (Middle, red) and myosin IIA (Bottom, red) in acetone-fixed MEFs. White boxes indicate area zoomed below. White arrowheads indicate colocalization at actin structures. Red arrowheads indicate lack of colocalization. [Scale bar, (Top) 1μm; (Bottom) 5μm.] (B) Maximal projection of confocal Z series of Alexa-488 phalloidin staining of actin (Left) and immunofluorescence of INF2 (Right). Color scale of z position is shown at the Lower Left. White arrowheads indicate colocalization along dorsal SFs. Arrows indicate dorsal actin structures (white) that lack INF2 (red). (Scale bar, 1 μm.) (C) Representative confocal micrographs of Alexa-488 phalloidin staining of actin (green) with INF2 (red) immunofluorescence with overlay shown. White boxes indicate area zoomed below. White arrowhead indicates colocalization of actin and INF2 at cell edge. [Scale bar, (Top) 1μm; (Bottom) 5μm.] () Representative confocal micrographs of INF2 immunofluorescence (green) and immunofluorescence of the focal FA proteins paxillin (Top, red) and vinculin (Bottom, red). White boxes indicate area zoomed below. White arrowheads indicate colocalization at FAs. [Scale bar, (Top) 1μm; (Bottom) 5μm.] (E) Bar graph of Pearson s coefficient of colocalization between INF2 and SF proteins (Top) orfaproteins(bottom). n = 6 cells per condition. Error bars: S. P < 5;, not significant, Student s t test. E Skau et al.
3 but is largely absent from dorsal SFs (23, 38). Myosin IIA localized to arcs around the cell sides and actin bundles in the cell body, but had reduced levels on INF2-decorated bundles extending perpendicular to the edge (Fig. 1A, Bottom). To confirm that the SFs decorated with INF2 were in fact dorsal SFs, we generated 3 projections from confocal stacks and tracked these actin bundles in the Z direction from the ventral cell surface and found that they extended radially toward the cell center and up toward the dorsal cortex (Fig. 1B). Endogenous INF2 is therefore localized specifically to dorsal actin SFs in the lamella. Because INF2 localization appeared to extend distally beyond the termini of dorsal SFs, we hypothesized that INF2 was associated with FAs. To test our hypothesis, we examined colocalization of INF2 and FA proteins. INF2 partially colocalized with paxillin at FAs in the lamellum, but extended more proximally than the bulk of paxillin (Fig. 1 and E). In contrast, INF2 colocalized more strongly with the FA adaptor protein vinculin (Fig. 1 and E), which localizes to the actin FA interface (39). Together, these results show that in addition to mitochondria (34), endogenous INF2 localizes specifically to dorsal SFs and the SF FA junction in MEFs, as well as lamellipodia. INF2 Is Required for orsal SFs in Lamellae. We next sought to determine the role of INF2 in organization of the actin cytoskeleton. We used sirna to decrease INF2 protein levels by 66% (Fig. 2A), then acetone-fixed and stained mock-transfected control and INF2 knockdown MEFs (INF2 K, Fig. 2) with antibodies to INF2 and paxillin, and examined them by spinning disk confocal microscopy. These experiments showed that sirna treatment resulted in loss of focal adhesion staining with the anti-inf2 antibody in individual cells. Loss of INF2 consistently reduced cell area compared with controls (Fig. 2 B and C), possibly due to metabolic deficiencies induced by reduced INF2 activity at mitochondria (34). Reexpression of the human INF2 (and thus refractory to sirna targeting the mouse protein) isoform lacking the mitochondrial targeting sequence fused at its C terminus to EGFP (henceforth referred to as INF2-GFP) at 121% of endogenous level showed that this isoform did not localize to mitochondria in the cell center and failed to rescue the defect in cell size, but localized to the cytoskeleton and FA similar to the endogenous protein (Fig. 2C and see Fig. 4), and rescued cytoskeletal and FA phenotypes induced by INF2 K (see below). We therefore used this construct for the remainder of our studies. espite the small size of INF2 K cells, formaldehyde fixation and staining with fluorescent phalloidin showed that they had a well-developed actin cytoskeleton, albeit with marked differences in organization compared with mock-transfected controls. cells displayed a lamellipodium typified by a bright band of dense actin at the cell edge and possessed dorsal and ventral SFs and transverse arcs in the lamellum (Fig. 2 B and ). Cells lacking INF2 possessed similar transverse arcs (Fig. 2B), but displayed a dramatic loss of actin bundles perpendicular to the leading edge in the lamella and cell center (Fig. 2 B ), as well as a slightly wider lamellipodium (Fig. S2). We focused our study on understanding the defect in SFs in the absence of INF2. Compared with controls, INF2 K cells lacked the SFs that normally extend radially through the lamella from the cell edge toward the top of the cell as seen in 3 projections, and also exhibited increased cell height (Fig. 2 B and C). Quantitative analysis showed that compared with control, INF2 K significantly reduced the number and length of actin bundles (Fig. 2C). Reexpression of INF2-GFP in INF2 K MEFs rescued SF number, but not their length, likely because of their smaller cell area (Fig. 2C). Remaining bundles in INF2 K cells were localized along the sides and rear of the cell, although weak radial bundles were sometimes present in the lamella. INF2 Protein, Frac A 1.6 INF2 sirna Con K Resc Con 15 h 24 h 48 h B C Avg Size, μm 2 x 1 MEFs Bottom Cell Area MEF INF2K Rescue INF2K Rescue INF2K Rescue MEFs INF2 K MEFs # SF per cell1 INF2 Top α-in α-in Tm (pan) # of Stress Fibers Bottom Immunostaining with the SF markers pan-tropomyosin, α-actinin, myosin II A, myosin II B, and phosphorylated myosin II regulatory light chain showed that remaining actin bundles along the sides and rear of INF2 K cells contained the appropriate contractile machinery; however, the myosin II marker proteins were largely absent from weak radial bundles, suggesting that they were remnants of dorsal SFs (Fig. 2 and Fig. S3). Together, these data indicate that INF2 is specifically required for formation of dorsal SFs to reduce cell height (4). Avg Length, μm INF2 K MEF INF2 Top Stress Fiber Length Tm (pan) Fig. 2. INF2 controls SF morphology and lamellipodial width. (A, Left Top) Bar graph of the average level of depletion and reexpression of INF2 in four independent experiments. Error bar: S. (Bottom) Representative image of Western blot showing levels of INF2 in mock-transfected control (Con) and MEF transfected with sirna targeting INF2 (INF2 K) for 15, 24, and 48 h. (Right) Representative confocal micrographs of Alexa-488 phalloidin staining of actin with immunofluorescence of INF2 in control and INF2 K MEF showing loss of INF2 at FA in INF2 K. White boxes indicate area zoomed below. [Scale bar, (Top) 1μm; (Bottom) 5μm.] Note the difference in size of the scale bar in control and INF2 K cells. (B) Black and white images: Representative confocal micrographs of Alexa-488 phalloidin staining of actin in control and MEFs transfected with sirnas targeting INF2 (INF2 K). Color images: Maximal projection of confocal Z series of Alexa-488 phalloidin staining of actin in control and INF2 K MEFs. Color scale of z position is shown at Upper Right. (Scalebar,1μm.) (C) Bar graphs of the averagecellarea(left),thenumberofsfspercell(center), and length of SFs (Right) in control MEFs, INF2 K MEFs, or INF2 K MEFs reexpressing the human INF2 isoform lacking the mitochondrial targeting sequence fused to GFP (rescue) n = 2 cells per condition. Error bar: S. P < 1,, not significant, Student s t test. () Representative confocal micrographs of Alexa-488 phalloidin (green) staining of actin with immunofluorescence of the actin-binding proteins α-actinin (Top, red) and pan-tropomyosin (Bottom, red) in control and INF2 K MEFs. White boxes indicate area zoomed below. White arrowheads indicate linear actin bundles. [Scale bar, (Top) 1μm; (Bottom) 5μm.] CELL BIOLOGY PNAS PLUS Skau et al. PNAS Published online April 27, 215 E2449
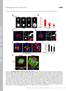
4 A MEFs B Barbed Ends MEFs MEFs s GFP-Pxn mapple- GFP-Pxn Barbed Ends mapple- GFP-Pxn mapple- C (-GFP) Bleach Time INF2K (-GFP) Bleach s GFP-Pxn Normalized Fluorescence mapple- Time Rescue (mapple-) Bleach Time.3.1 Elongation Rate, μm/min G- Incorporation at FA Stress Fiber Elongation Rate.8 INF2K INF2 K Rescue Fig. 3. INF2 mediates barbed end formation and actin assembly at FAs. (A, Left) Representative confocal micrographs of permeabilized mock-transfected control and MEFs transfected with sirnas targeting INF2 (INF2 K) showing Alexa-488 phalloidin (green) staining of F-actin together with X-rhodamine labeled G-actin (red) incorporation (barbed ends) and immunofluorescence of paxillin (blue) with color overlay shown. White boxes indicate area zoomed below. White arrowheads indicate presence of G-actin incorporation at FAs. Red arrowhead indicates reduced incorporation. [Scale bar, (Left) 1μm; (Right) 5μm.] Note the difference in size of the scale bar in control and INF2 K cells. (Right) Bar graph of G-actin incorporation at FAs as measured by the ratio of X-rhodamine G-actin fluorescence to Alexa-488 phalloidin fluorescence at FAs. n = 5 cells, at least 25 FAs per condition. Error bar: S. P < 5;, not significant, Student s t test. (B, Left) Representative TIRF micrographs of control and INF2 K MEF expressing EGFP-paxillin (Top, green) and mapple-actin (Center, red). Color overlay is shown at Bottom. White boxes indicated area zoomed at Right. (Scale bar, 1 μm.) Right: TIRF time lapse image sequences of an actin bundle (Center, red) elongating out of a paxillin-marked FA (Top, green) with color overlay shown (Bottom). Time in seconds is shown. White arrowheads indicate a single FA and associated actin. (Scale bar, 2 μm.) (C, Left) Representative confocal time lapse image sequence of EGFP-actin SF in mock-transfected or INF2 K MEF, or mapple-actin SF in INF2 K cells reexpressing the human INF2-GFP isoform lacking the mitochondrial targeting sequence (rescue). Red bar indicates area photobleached at time. Time in seconds is shown. (Scale bar, 1 μm.) (Center) Kymograph projection of rearward movement/recovery of bleached region. Arrowhead indicates time of bleach. (Right) Bar graph of average SF elongation rate in mock transfected, INF2 K, or INF2-GFP rescue. n = 1 SFs per condition. Error bar: S. P < 1, Student s t test. INF2 Promotes Barbed End Formation and Assembly at FA. Our observation that INF2 contributes to dorsal SF formation suggests that INF2 may regulate actin assembly specifically at FAs. To test this prediction, we first determined the role of INF2 in generation of assembly-competent free barbed ends of actin filaments in control and INF2 K cells (Fig. 3A). By gently permeabilizing cells in the presence of rhodamine-labeled G-actin, fixing and costaining with fluorescent phalloidin and an antibody against paxillin, we were able to visualize sites of incorporation of new actin monomers onto free barbed ends, total F-actin, and FAs (Fig. 3A) (41). Spinning disk confocal images revealed incorporation of rhodamine-actin indicating the location of free barbed ends along the leading edge of the lamellipodium and at FAs at the termini of dorsal SFs in control cells (Fig. 3A). Quantification of the ratio of rhodamine-actin to phalloidin fluorescence showed that compared with control, INF2 K cells had a slight but insignificant reduction in rhodamine-actin fluorescence in lamellipodia (Fig. S2E), but a strongly significant decrease in rhodamine-actin incorporation at FAs (Fig. 3A). Therefore, INF2 promotes formation of polymerization-competent free barbed ends of actin filaments at FAs, whereas other mechanisms contribute to barbed end formation in lamellipodia. We then analyzed the role of INF2 in actin dynamics in dorsal SFs at FAs in living cells. We coexpressed mapple-actin and EGFP-paxillin in MEFs in the presence and absence of sirnas targeting INF2 and imaged cells by time-lapse TIRF microscopy (Fig. 3B). Examination of movies of control cells showed paxillin-containing nascent FAs formed within the lamellipodium concomitant with leading edge protrusion, as previously reported (Fig. 3B and Movies S1 and S2) (42). Nascent adhesions forming in the lamellipodium initially lacked actin bundles and the majority underwent rapid disassembly within about a minute as the trailing edge of the lamellipodium moved beyond them. For the nascent FAs that remained after the lamellipodium advanced, a fine actin bundle appeared at the proximal end of the FAs and the FAs began to elongate (Fig. 3B, Right and Movies S1 and S2). These linear bundles continued to extend from the proximal side of the FAs as the FAs grew (Fig. 3B, Right). INF2 K MEFs also nucleated nascent FAs in protruding lamellipodia (Fig. 3B). In contrast with control cells, however, these FAs failed to sprout actin bundles (Fig. 3B, Right and Movies S3 and S4). Rather than linear filaments, a dense actin meshwork associated with these FAs (Fig. 3B). These structures remained associated with FAs for many minutes after the edge advanced, but displayed little-tono directional elongation (Fig. 3B, Right and Movies S3 and S4). Some actin aggregates in INF2 K MEFs appeared to span or connect multiple small FAs (Fig. 3B, Right), a phenomenon not typical of dorsal SFs. The few weak radial actin structures we observed were associated with the sides of a protrusion (Fig. 3B) or coalesced as multiple globules merged (Fig. 3B, Right). These results show that INF2 is required for forming actin bundles at the proximal edge of maturing FAs in the lamellum. E245 Skau et al.
5 A C INF2-GFP mcherry-pxn INF2-GFP mapple-pxn T INF2-GFP Phospho paxillin Fluorescence, a.u. To determine the role of INF2 in elongation of actin filaments in dorsal SFs at FAs, we photobleached a narrow stripe across an EGFP-actin labeled SFs just proximal to its terminus at a FA, and monitored the bleached zone by time-lapse spinning disk confocal microscopy (23). In control cells, the bleached area did not recover fluorescence within 3 s, but during that time moved away from the SF terminus at the FA at a consistent rate (Fig. 3C and Movie S5), indicating elongation of the actin bundle distal to the bleach mark at its site of attachment to the FA, as previously reported (23). Although INF2 K induced loss of most dorsal SFs, we were able to bleach a stripe across remnant weak radial bundles attached to FAs in the lamella. This experiment showed that, like controls, the bleached mark in INF2 K cells did not recover fluorescence. However, in contrast to control MEFs, in INF2 K cells bleached marks on actin bundles remained almost stationary relative to the FA, moving away from the FA at a significantly lower rate than control (Fig. 3C and Movie S4). In INF2 K cells reexpressing INF2-GFP, movement of the bleached SF stripe away from the FA was recovered (Fig. 3C). Together, these data show that INF2 is required for promoting actin polymerization and forming free barbed ends at FAs to mediate formation of actin bundles at maturing FAs and dorsal SFs. INF2 Is Recruited to FAs uring Myosin II-ependent Maturation. Our data show that INF2 localizes at the FA SF interface and promotes dorsal SF formation (Figs. 1 3). Because the SF interface with the FA forms as nascent FAs mature (42), we hypothesized that INF2 may assemble into FAs during maturation. To test this hypothesis, we determined the dynamic localization of INF2 to FAs during assembly and maturation. We cotransfected MEFs with INF2-GFP together with mapple- or mcherry-tagged paxillin as a marker of FAs and performed time-lapse TIRF microscopy (Fig. 4 A C). In control cells, mapple-paxillin appeared as diffraction-limited nascent FA punctae in protruding lamellipodia, most of which disassembled, but a fraction B Fluorescence, a.u ist. along edge 5 INF2-GFP s mapple-pxn Phosphopaxillin Nascent FA INF2 Mature FA INF2 INF2-GFP istance along edge, μm 15 Fig. 4. INF2 localizes to FAs during maturation. (A) Representative TIRF micrographs of MEFs cotransfected with human INF2 isoform lacking the mitochondrial targeting sequence fused to EGFP (INF2-GFP, Left, green) and mcherry-paxillin (Center, red) with color overlay shown (Right). White boxes indicate area zoomed below. Arrowheads indicate a paxillinmarked FA (white) that lacks INF2-GFP (red). Blue and purple lines in the overlay indicate the position of line scans used for quantification of fluorescence intensities in nascent (Upper graph in B) and mature (Lower graph in B) FAs, respectively. [Scale bar, (Top) 3μm; (Bottom) 2μm.] (B) Fluorescence intensities of mcherry-paxillin (red) and INF2-GFP (green) along the lines in A. (C, Left) Representative TIRF micrographs of MEFs cotransfected with INF2-GFP (Left, green) and mapplepaxillin (Center, red) with color overlay shown. White box indicates the area shown in the kymograph in the Inset as well as in the zoomed time-lapse series at Right. (Inset) Kymographs perpendicular to the leading edge across the FA are shown in the white box., distance; T, time. White arrowheads indicate earliest appearance of INF2-GFP. (Scale bar, 1 μm.) (Right) Time-lapse TIRF time lapse images of the cell edge and FA marked by INF2-GFP (green, Top) and paxillin (red, Center). Time in seconds is indicated. (Scale bar, 1 μm.) White arrowhead indicates an FA born at the cell edge. () Representative confocal micrographs of MEF transfected with INF2-GFP (Left, green) treated for 2 h with 5 μm blebbistatin then fixed and stained for paxillin phosphorylated on tyrosine 31 (phosphopaxillin, red, Center) with color overlay shown. White box indicates area zoomed below. White arrowhead indicates a paxillin-marked FA that lacks INF2-GFP (red arrowhead). Blue line in Left indicates the position of the line scan used for quantification of fluorescence intensities of phospho-paxillin and INF2-GFP in nascent FA on the graph at Right. [Scalebar,(Top) 1μm; (Bottom) 2μm.] of which exited the advancing lamellipodium and elongated centripetally as they matured (Fig. 4A and Movies S6 and S7). INF2-GFP localized to lamellipodia, making it difficult to directly determine whether INF2 was at nascent FAs (Fig. 4A). However, fluorescence intensity line scans along the protruding edge of cells revealed no specific coconcentration of INF2-GFP above the lamellipodial localization in punctate mcherrypaxillin labeled nascent FAs, and in regions just proximal to lamellipodia, young, slightly elongated FAs in the earliest stage of maturation contained little-to-no INF2-GFP (Fig. 4A, red arrowhead). Larger FAs located deeper in the lamella exhibited high concentrations of INF2-GFP (Fig. 4 A and B). Two-color kymograph analysis of individual FAs confirmed that nascent FAs exiting the advancing, INF2-rich lamellipodium lacked INF2-GFP (Fig. 4C, Inset and Movie S7), followed by strong INF2-GFP localization throughout the length of growing FAs, and finally specific concentration of INF2-GFP in the proximal end of fully mature FAs, presumably at the junction with SFs (Fig. 4C). Therefore, INF2 assembles into FAs as they mature. Proteins such as vinculin or zyxin that assemble into FAs during maturation are recruited in a myosin II contractilitydependent manner (13, 43, 44). To determine if INF2 recruitment to FAs was contractility dependent, we examined the response of INF2 localization to inhibition of myosin II ATPase activity. We treated cells transfected with INF2-GFP with blebbistatin and stained them with antibodies to paxillin phosphorylated on Y31 (phosphopaxillin) as a marker of immature FAs (45) and imaged them by confocal microscopy. Blebbistatin treatment resulted in the formation of lamellipodia containing diffraction-limited phosphopaxillin punctae and loss of mature FAs in the lamella and cell center (Fig. 4). Colocalization line scans showed that in the absence of myosin II activity, INF2 was localized in a relatively uniform band along the cell edge but did not appear specifically enriched in phosphopaxillin FA punctae (Fig. 4). Together, these results show that INF2 is absent from nascent CELL BIOLOGY PNAS PLUS Skau et al. PNAS Published online April 27, 215 E2451
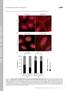
6 A MEFs B C Adhesion Size, um 2 Avg Adhesion Size Con INF2KRescue E FAK Frac of Adhesions.8 MEFs VASP Pxn Vinc Pxn Tensin F Adhesion istribution Avg Aspect Ratio 5 INF2 K >2. um 2 Con INF2K Rescue <.3 um um 2 Aspect Ratio FAK VASP Pxn Vinc Pxn Tensin INF2-GFP Rescue MEFs INF2-GFP Tensin INF/Tens Fig. 5. INF2 is critical for morphological maturation of FAs. (A) Representative confocal micrographs of mock-transfected control MEF and MEF transfected with sirnas targeting INF2 (INF2 K) showing Alexa-488 phalloidin staining of actin (green) with immunofluorescence of paxillin (red). White boxes indicate area zoomed below. White arrowheads show termination of SF at FA. [Scale bar, (Top) 1 μm; (Bottom) 5μm.] Note the difference in size of the scale bar in control and INF2 K cells. (B ) Quantification of FA morphometry from analysis of fluorescence images of paxillin in control, INF2 K, and INF2 K cells reexpressing the human INF2-GFP isoform lacking the mitochondrial targeting sequence (rescue). n = 15 cells, at least 9 FAs per condition. (B and ) Bar graphs of FA area (B) and aspect ratio (). Error bars: S. P < 1; P < 5;, not significant, Student s t test. (C) Box and whisker plot of FA size distribution, means are indicated by red bars. (E) Representativeconfocalmicrographs ofimmunofluorescenceofpaxillin (Pxn) or VASP (both green) with FA proteins found at nascent (FAK, Top, red), maturing [vinculin (Vinc), Middle, red] and fibrillar (tensin, Bottom, red) FAsincontrol(Left) andinf2k (Right) MEFs. Whiteboxesindicateareas zoomedbelow; whitedashed boxes indicate area zoomed on bottom row. White arrows indicate nascent FA, arrowheads denote mature FA, red arrow indicates lack of colocalization of tensin with paxillin in nascent FA. [Scale bar, (Top) 1μm; (Bottom Left) 5μm.] (F) Immunofluorescence of tensin and INF2-GFP in INF2-GFP re-expressing MEF. White boxes indicate areas zoomed below. Arrowheads denote colocalization of tensin and INF2 at central adhesion. [Scale bar, (Top)1μm; (Bottom)5μm.] FAs, but is recruited to FAs during myosin II-dependent FA growth and maturation. INF2 Promotes Formation of Large and Fibrillar FAs. Our observation that INF2 is recruited during FA growth suggests that it may be required for FA maturation. To test this hypothesis, we examined the role of INF2 in FA morphology, composition, and dynamics (Figs. 5 and 6). First, to characterize FA morphology, we stained cells in the presence (INF2 K) or absence (control) of sirnas targeting INF2 for actin and paxillin. Compared with control, INF2 K cells lacked extended FAs in the cell center, and their peripheral FAs were smaller (Fig. 5 A ). Rather than existing as a very narrow band at the cell edge, these small FAs were present further into the lamellum than in control cells (Fig. 5 A and E). Analysis of FA area confirmed this finding, showing that compared with control, INF2 K cells had significantly smaller FAs (Fig. 5 A and B). Furthermore, in controls, approximately one-third of the total FAs were nascent FAs (<.3 μm 2 )and two-thirds were medium (.3 2. μm 2 ) or large mature FAs (>2. μm 2, Fig. 5C), whereas INF2 K shifted the distribution such that over 8% of the FAs were nascent and greatly decreased the fraction of medium and large FAs. In control cells, FAs were oblong in shape (aspect ratio > 3), whereas FAs in INF2 K were significantly rounder and less extended (Fig. 5). FA size was rescued by expression of INF2-GFP in INF2 K cells, although reexpression did not fully rescue FA aspect ratio, likely because the cells remained smaller than average (Figs. 2 A and E,5B and, and 6G). Thus, INF2 reduces nascent FAs and promotes large, oblong FAs in the cell center. Because we found that INF2 increases FA size, we hypothesized that INF2 may also promote the protein compositional changes that accompany FA growth during maturation. We costained control and INF2 K cells for paxillin and markers of nascent (FAK, refs. 13, 15), mature (vinculin and zyxin, refs. 13, 15), and fibrillar (tensin, refs. 15, 17) FAs and imaged them by confocal microscopy (Fig. 5E and Fig. S4A). cells possessed diffraction-limited nascent FAs at their edges marked by paxillin, vasodilator-stimulated phosphoprotein (VASP), and FAK, whereas small oblong FAs at the lamellipodium lamellum border as well as extended FAs in the lamellum also contained vinculin and zyxin (Fig. 5E and Fig. S4A). Staining for tensin showed strong staining of long FAs in the cell center and dim or variable staining of small FAs in the cell periphery (Fig. 5E). In INF2 K cells, the small round FAs in the cell periphery exhibited the same complement of proteins seen in nascent and small FAs of control cells, including paxillin, FAK, vinculin, zyxin, and variable levels of tensin (Fig. 5E and Fig. S4A). However, INF2 K cells completely lacked tensin-containing extended FAs in the cell center (Fig. 5E). Localization of tensin to oblong FAs formed in INF2 K cells reexpressing INF2-GFP was recovered, although due to small cell size, fewer FAs were observed in the cell center (Fig. 5F). Thus, INF2 is not required for compositional maturation of FAs, but is critical for forming large, central, tensin-containing FAs. To determine if the signaling properties of FAs are modulated by INF2, we examined tyrosine phosphorylation of FA proteins that occurs in response to integrin engagement to ECM. We coimmunolocalized paxillin together with either total phosphotyrosine or with antibodies specific to FAK phosphorylated on Y397 (phospho-fak) or phosphopaxillin in control and INF2 K cells (Fig. S4B). Confocal imaging showed that FAs in both control and INF2 K cells contained high levels of tyrosine phosphorylation, phospho-fak, and phosphopaxillin (Fig. S4B). Together, these results show that INF2 reduces the fraction of nascent FAs and is also required for formation of fibrillar FAs in the cell center, but not recruitment of FA proteins or basic aspects of integrin signaling. E Skau et al.
7 A INF2 K INF2 K NA nucleated/ 1 sec/μm 2 B s/12 s INF2K+INF2-GFP Fig. 6. INF2 promotes centripetal elongation and turnover of nascent FAs. (A) TIRF micrographs from s 12 s mcherry- time-lapse image series of the cell edge in mocktransfected control and MEF transfected with sir- s 15 s s/15s NAs targeting INF2 (INF2 K) expressing EGFPpaxillin. The color overlay shows the two time s 12 s s/12 s INF2-GFP points 12 s apart; purple FAs were assembled in the s 15 s s/15s time gap, green FAs disassembled during the time gap, and white FAs remained present throughout the 12-time gap. (Scale bar, 2 μm.) (B, Left) Representative TIRF micrograph of INF2 K cells reex- C INF2-GFP; mcherry-pxn pressing the human INF2-GFP isoform lacking the GFP-Pxn s T mitochondrial targeting sequence (INF2-GFP, green) and mcherry-paxillin (red). (Right) TIRF micrographs from time-lapse image series of the cell edge in control and INF2 K MEFs expressing INF2-GFP. The overlay shows the two time points differentially color encoded [t = s(green)andt = 15 s (purple)]. (Scale bar, 5 μm.) (C) Representative GFP-Pxn s T TIRF micrographs of control and INF2 K MEF transfected with EGFP-paxillin. White rectangle highlights the region shown in time-lapse sequence in. ashed white box highlights the region shown in overlay in A. (Scale bar, 1 μm.) () TIRF time-lapse micrographs of FA dynamics in E F G control and INF2 K MEF; FA marker EGFP-paxillin; Nucleation Rate Maturation Fraction 6 Adhesion Lifetime time in seconds is shown. ashed red line indicates lines along FA used for kymograph analyses (Right) 5 4 of FA growth., distance; T, time. (Scale bar, 1 μm.).15 3 ashed white line highlights the slope, indicative.1 2 oftherateoffagrowth.(e G) Bar graphs of nascent FA formation rate per micrometer of cell edge 5 1 INF2 K INF2 K INF2 K (nucleation rate, E), the fraction of nascent FAs that undergo maturation (maturation fraction, F), and average FA lifetime (G), in control and INF2 K MEF. FA marker, paxillin. n = 5 cells, at least 2 adhesions per condition. Error bar: S. P < 1;, not significant, Student s t test. Frac that Elongate INF2 Promotes Turnover of Nascent FAs and Is Critical to FA Growth and Elongation. To determine how INF2 inhibits nascent FAs and promotes fibrillar FAs, we performed time-lapse TIRF microscopy of FA dynamics and quantitative image analysis (Fig. 6 A F). First, we tracked the overall dynamic behavior of FAs by creating color-encoded time overlays that compared GFP-paxillin marked FAs near the cell edge before and after a 12-s time interval (Fig. 6A). In this analysis, purple represents FAs newly assembled during the 12-s interval, green shows FAs that disassembled during the time interval, and white FAs were constant throughout (Fig. 6A and Movie S8). This showed that in 12 s in control MEFs, many FAs formed near the leading edge and many FAs disassembled in the lamella, but few remained constant, suggesting a rapid FA assembly and turnover cycle. In INF2 K MEFs, although many FAs formed near the leading edge in 12 s, most of them remained constant and very few turned over in the lamella, indicating a reduction in FA turnover compared with control. Coexpression of INF2-GFP and mcherry paxillin in INF2 K cells rescued rapid FA assembly and turnover dynamics (Fig. 6B and Movie S8). Time-lapse image series at 5-s intervals and kymograph analysis showed that in control MEFs, most nascent FAs turned over rapidly as the leading edge and lamellipodium advanced, whereas a subset underwent rapid centripetal elongation as they matured in the lamellum (Fig. 6 C and and Movie S9) (42). In contrast, most of the nascent FAs in INF2 K MEFs remained round puncta even as the leading edge advanced and new FAs were nucleated in front of them. The few FAs that did grow in INF2 K cells either expanded radially as two neighboring punctate FAs merged or grew centripetally much more slowly than control cells (Fig. 6 and Movie S9) and never extended into the cell center. To determine the precise defect in FA dynamics, we performed quantitative analysis of FA nucleation rate (number of nascent FAs formed/unit time/unit lamellipodial area), maturation fraction (the fraction of nascent FAs that do not disassemble in the leading edge but go on to elongate), and lifetime (time from appearance to disappearance of individual FAs) on image series acquired at 5-s intervals. This analysis showed that, as suspected from examination of time overlays, there was no difference in FA nucleation rate in control and INF2 K MEFs (Fig. 6E). Once nascent FAs nucleated, nearly 25% of them matured in the lamellum of control cells, whereas in INF2 K cells only 5% of nascent FAs matured in this fashion (Fig. 6F). Furthermore, the lack of nascent FA turnover in INF2 K cells resulted in a significant threefold increase in FA lifetime compared with control (Fig. 6G). Together these results suggest that INF2 reduces nascent FAs not by inhibiting their formation, but by promoting both their turnover and transition to rapid elongation and likely their eventual transition to central fibrillar FAs. INF2 Regulates Traction Force and ECM Fibrillogenesis. Our results show that INF2 is critical to regulation of the actin cytoskeleton and FAs. To determine the physiological significance of these functions of INF2, we examined the requirement for INF2 in traction force and generation of ECM by fibroblasts. First, we used high-resolution traction-force microscopy (TFM) (46) to determine the force exerted by control and INF2 K cells expressing GFP-paxillin by plating on compliant, 8.6-kPa FN-coupled polyacrylamide gels embedded with fluorescent beads to serve as fiducial marks of cell-generated substrate displacement (Fig. 7A). FAs in control and INF2 K cells were plated on compliant substrates resembled FAs in cells on glass; FAs in INF2 K cells were smaller than those in control cells (Fig. 7A). TFM analysis showed that cells lacking INF2 generated significantly higher stress on the substrate than controls (Fig. 7A). This demonstrates that INF2 attenuates traction force on the ECM, in agreement with the notion that large FAs exert little force on the substrate compared with nascent FAs (47). LIfetime, min CELL BIOLOGY PNAS PLUS Skau et al. PNAS Published online April 27, 215 E2453
8 A Extracellular Matrix Production B CM INF2K CM C MEFs INF2 K Rescue GFP- INF2K FN FN FN Traction Force Microscopy Fibronectin Fibronectin Fluorescence a.u. Normalized Traction Force, nn/μm Stress Exerted by Cells INF2 K Rescue INF2 K Fig. 7. INF2 regulates traction force and ECM fibrillogenesis. (A, Left) Representative confocal images of EGFP-paxillin in mock-transfected control and MEF transfected with sirnas targeting INF2 (INF2 K) plated on fiducial-marked polyacrylamide substrates of stiffness, 8.6 kpa. (Scale bar, 1 μm.) Note the difference in size of the scale bar in control and INF2 K cells. (Right) Bar graph of normalized traction force generated on substrates by control and INF2 K cells as determined by Fourier transform traction microscopy (46). P < 5, Student s t test. n = 5 cells per condition. (B) Representative phase-contrast images of cell-derived matrix produced by mock-transfected control human foreskin fibroblasts (HFFs) or HFFs transfected with shrna targeting INF2. White boxes indicate area zoomed below. [Scale bar, (Top) 4 μm; (Bottom) 1 μm.] (C) Representative confocal micrographs of Alexa-488 phalloidin staining of actin (green) with immunofluorescence of paxillin (red) and FN (blue) in control and INF2 K MEF. White boxes indicate area zoomed below. White arrowheads indicate fibrils of FN near FA, red arrowhead indicates diffuse FN. [Scale bar, (Top) 2μm; (Bottom) 5μm.] Note the difference in size of the scale bar in control and INF2 K cells. (, Left) Representative confocal micrographs of control MEF, INF2 K MEF, or INF2-K MEF reexpressing INF2- GFP (rescue) transfected with mapple-actin (green) and plated on FN-coated coverslips. Images were acquired 6 h after Alexa- 647 labeled FN (red) was added to the media and allowed to be incorporated into fibrils. White boxes indicate area zoomed below. White arrowheads indicate accumulation of labeled FN at the termini of stress fibers; red arrowhead indicates lack of FN accumulation. [Scale bar, (Left) 1μm; (Right) 5μm.] (Right) Graph of average labeled FN fluorescence under each cell normalized to cell area. n = 1 cells per condition, error bar = S. P < 5;, not significant, Student s t test. The major physiological function of fibroblasts, deposition and organization of FN-containing ECM, is mediated by tensinpositive fibrillar FAs (48). The reduced FA size and unusual distribution of tensin in cells lacking INF2 suggests INF2 may be critical for ECM fibrillogenesis. We first tested the requirement for INF2 in the ability of human foreskin fibroblasts (HFFs) to generate cell-derived matrices (CMs). We used HFFs as opposed to MEFs because shrna plasmids to human INF2 continually inhibited INF2 expression for the long time periods required for CM generation, compared with the transient knockdown obtained for sirna in MEFs. Cells expressing either the GFP-tagged shrna or GFP alone as a control were plated at confluency for 7 d and supplemented with ascorbic acid to promote deposition of ECM, after which cells were lysed and washed out, leaving behind CMs (Fig. 7B). Phase-contrast imaging showed that the CM deposited by control cells was homogeneous and dense, with thick, phase-dense bundles of aligned and interconnected fibrils (Fig. 7B). Conversely, compared with control, the CM produced by cells lacking INF2 was more heterogeneous in density and isotropic in organization, with regions completely lacking thick fibrils and much less interconnected mesh (Fig. 7B). We then determined the requirement for INF2 in FN fibrillogenesis. We plated control and INF2 K cells on glass, allowed them to deposit and reorganize FN for 24 h, then fixed and costained for FN and paxillin to determine the organization of FN relative to FAs (Fig. 7C). cells deposited FN and organized it into fibrils associated with paxillin-containing FAs toward the center of the cell (Fig. 7C). Cells lacking INF2, however, were unable to form FN fibrils. Some FN accumulated around INF2 K cells, but it remained diffuse in the cell center or formed irregular blobs in the periphery that were not strongly associated with FAs (Fig. 7C). To determine if the defect of INF2 K cells in fibrillogenesis was due to lack of FN secretion or an inability to fibrillarize plasma FN, we assayed the ability of living cells to incorporate fluorescently labeled FN added to the media into ECM fibrils. This showed that control cells efficiently assembled fluorescent FN into fibrillar structures close to large central FAs at the termini of SFs (Fig. 7, Top row). In contrast, INF2 knockdown cells exhibited a lack of fluorescent FN fibrils under the cell, although hazy fluorescence was present (Fig. 7, Middle row). Reexpression of INF2-GFP in INF2 K cells rescued the defect, with these cells regaining the ability to form FN fibrils at the termini of SFs in the center of the cell (Fig. 7, Bottom row). Together, these data show that INF2 is required for the ability of fibroblasts to organize ECM and fibrillarize FN on both long and short timescales. iscussion Our results show for the first time to our knowledge that the formin INF2 localizes specifically to FAs, dorsal SFs, and lamellipodia in fibroblasts. Although it is well accepted that formin proteins act at FAs to mediate SF formation (23, 3 32), the ability to localize a formin to FAs has eluded the field. This localization is quite distinct from the CAAX membrane-targeted isoform of INF2, which localizes to mitochondria and the endoplasmic reticulum (34, 49). We find that FA-associated INF2 plays a critical role in promoting formation of polymerizationcompetent free actin barbed ends that mediate the centripetal elongation of an FA-associated actin bundle to form a dorsal SF. INF2 assembles into FAs in a contractility-dependent manner during FA maturation rather than during initial FA generation, E Skau et al.
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Changes in E-cadherin rigidity sensing regulate cell adhesion
 Changes in E-cadherin rigidity sensing regulate cell adhesion Caitlin Collins a, Aleksandra K. Denisin b,c, Beth L. Pruitt b,c, and W. James Nelson a,1 a Department of Biology, Stanford University, Stanford,
Changes in E-cadherin rigidity sensing regulate cell adhesion Caitlin Collins a, Aleksandra K. Denisin b,c, Beth L. Pruitt b,c, and W. James Nelson a,1 a Department of Biology, Stanford University, Stanford,
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
Journal of Cell Science Supplementary Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Supporting Information
 Supporting Information Shao et al. 10.1073/pnas.1504837112 SI Materials and Methods Immunofluorescence and Immunoblotting. For immunofluorescence, cells were fixed with 4% paraformaldehyde and permeabilized
Supporting Information Shao et al. 10.1073/pnas.1504837112 SI Materials and Methods Immunofluorescence and Immunoblotting. For immunofluorescence, cells were fixed with 4% paraformaldehyde and permeabilized
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
Supplementary Figures
 Supplementary Figures Supplementary Figure 1: Collagen gel fibril sizes in vitro and in vivo. (a-b) Box and whisker plots for collagen fibril/bundle diameter (A) and length (B) for HR and FB16 matrices.*
Supplementary Figures Supplementary Figure 1: Collagen gel fibril sizes in vitro and in vivo. (a-b) Box and whisker plots for collagen fibril/bundle diameter (A) and length (B) for HR and FB16 matrices.*
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
Functions of Nonmuscle Myosin II in Assembly of the Cellular Contractile System
 Functions of Nonmuscle Myosin II in Assembly of the Cellular Contractile System Maria Shutova 1,2, Changsong Yang 1, Jury M. Vasiliev 2, Tatyana Svitkina 1 * 1 Department of Biology, University of Pennsylvania,
Functions of Nonmuscle Myosin II in Assembly of the Cellular Contractile System Maria Shutova 1,2, Changsong Yang 1, Jury M. Vasiliev 2, Tatyana Svitkina 1 * 1 Department of Biology, University of Pennsylvania,
supplementary information
 DOI: 10.1038/ncb1992 Figure S1 (a-b) Verification of actin flow patter using photobleaching of actin:gfp and Lifeact:RFP in migrating dendritic cells (DCs). (a) Actin:GFP and Lifeact:RFP double-trafected
DOI: 10.1038/ncb1992 Figure S1 (a-b) Verification of actin flow patter using photobleaching of actin:gfp and Lifeact:RFP in migrating dendritic cells (DCs). (a) Actin:GFP and Lifeact:RFP double-trafected
Description: Nuclear morphology and dynamics in nontargeting sirna transfected cells. HeLa Kyoto
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Journal of Cell Science Supplementary Material
 Figure S4 A His-(RBCPS)T15 His-(_BCPS) T15 His-PXN His-PXN+T15 His-PXN+ (RB_PS)T15 Myc-Ub IP:Ni-NTA 6M GuHCL WB: anti-myc WB: anti-his (Ub)n His-PXN His-(RBCPS)T15 His-(_BCPS)T15 + + + + + + + + + -100
Figure S4 A His-(RBCPS)T15 His-(_BCPS) T15 His-PXN His-PXN+T15 His-PXN+ (RB_PS)T15 Myc-Ub IP:Ni-NTA 6M GuHCL WB: anti-myc WB: anti-his (Ub)n His-PXN His-(RBCPS)T15 His-(_BCPS)T15 + + + + + + + + + -100
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends.
 SUPPLEMENTAL DATA Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends. Supplemental Experimental Procedures Dominant negative nesprin KASH (DN KASH) plasmid construction-
SUPPLEMENTAL DATA Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends. Supplemental Experimental Procedures Dominant negative nesprin KASH (DN KASH) plasmid construction-
No wash 2 Washes 2 Days ** ** IgG-bead phagocytosis (%)
 Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity
 Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Article. Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices
 Current Biology 23, 1607 1619, September 9, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.06.053 Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices
Current Biology 23, 1607 1619, September 9, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.06.053 Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices
ANAT Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales. UNSW Copyright Notice
 ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Stargazin regulates AMPA receptor trafficking through adaptor protein. complexes during long term depression
 Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Prospéri et al.,
 Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
(B) Comparable expression of major integrin subunits and glycoproteins on the surface of resting WT and Lnk -/- platelets.
 Supplemental Figure S1. Characteristics of Lnk -/- platelets. (A) Electron micrographs of resting platelets showing the normal intracellular structure of Lnk -/ platelets. Samples were fixed with 4% PFA
Supplemental Figure S1. Characteristics of Lnk -/- platelets. (A) Electron micrographs of resting platelets showing the normal intracellular structure of Lnk -/ platelets. Samples were fixed with 4% PFA
supplementary information
 DOI: 10.1038/ncb2156 Figure S1 Depletion of p114rhogef with different sirnas. Caco-2 (a) and HCE (b) cells were transfected with individual sirnas, pools of the two sirnas or the On Target (OnT) sirna
DOI: 10.1038/ncb2156 Figure S1 Depletion of p114rhogef with different sirnas. Caco-2 (a) and HCE (b) cells were transfected with individual sirnas, pools of the two sirnas or the On Target (OnT) sirna
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
This is an open access publisher version of an article that appears in:
 This is an open access publisher version of an article that appears in: PLOS ONE The internet address for this paper is: https://publications.icr.ac.uk/11345/ Published text: JE Sero, CK Thodeti, A Mammoto,
This is an open access publisher version of an article that appears in: PLOS ONE The internet address for this paper is: https://publications.icr.ac.uk/11345/ Published text: JE Sero, CK Thodeti, A Mammoto,
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in
 Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
Plutoni et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Plutoni et al., http ://www.jcb.org /cgi /content /full /jcb.201505105 /DC1 THE JOU RNAL OF CELL BIO LOGY Figure S1. Cell morphology during migration. (a) Protein extracts (20
Supplemental material JCB Plutoni et al., http ://www.jcb.org /cgi /content /full /jcb.201505105 /DC1 THE JOU RNAL OF CELL BIO LOGY Figure S1. Cell morphology during migration. (a) Protein extracts (20
Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering.
 Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering. The journal welcomes manuscripts on the following topics: synthetic
Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering. The journal welcomes manuscripts on the following topics: synthetic
Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated
 Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Cell motility requires the spatial and temporal coordination
 JCB: Article Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration Violaine D. Delorme-Walker, 1,2 Jeffrey R. Peterson, 3 Jonathan Chernoff, 3 Clare
JCB: Article Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration Violaine D. Delorme-Walker, 1,2 Jeffrey R. Peterson, 3 Jonathan Chernoff, 3 Clare
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
 DOI: 0.038/ncb337 a RFP-Zyxin Actin b RFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling, positive tilting of radial fibers c GFP-VASP Actin d GFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling,
DOI: 0.038/ncb337 a RFP-Zyxin Actin b RFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling, positive tilting of radial fibers c GFP-VASP Actin d GFP Fluorescence 0 0 0 6 5 4 non-swirling CCW swirling,
Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis. 3/9/17 ChE 575
 Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis 3/9/17 ChE 575 When, Where, Why do cells migrate? 1. Neutrophil Migration to Battle Infection 2. Development 3. Wound Healing 4. Disease 2 Wound
Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis 3/9/17 ChE 575 When, Where, Why do cells migrate? 1. Neutrophil Migration to Battle Infection 2. Development 3. Wound Healing 4. Disease 2 Wound
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various
 Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
In vitro EGFP-CALI comprehensive analysis. Liang CAI. David Humphrey. Jessica Wilson. Ken Jacobson. Dept. of Cell and Developmental Biology
 In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
TRACTION and STRESS MICROSCOPY for CELLS:
 TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
Quantitative analysis of Bidirectional Signaling (qbids)
 Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
T H E J O U R N A L O F C E L L B I O L O G Y
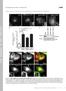 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Flag-Rac Vector V12 V12 N17 C40. Vector C40 pakt (T308) Akt1. Myc-DN-PAK1 (N-SP)
 a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
Cell-Extracellular Matrix Interactions
 Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
Focal adhesions (FAs) regulate cell migration. Vinculin,
 JCB: ARTICLE Vinculin controls focal adhesion formation by direct interactions with talin and actin Jonathan D. Humphries, 1 Pengbo Wang, 1 Charles Streuli, 1 Benny Geiger, 2 Martin J. Humphries, 1 and
JCB: ARTICLE Vinculin controls focal adhesion formation by direct interactions with talin and actin Jonathan D. Humphries, 1 Pengbo Wang, 1 Charles Streuli, 1 Benny Geiger, 2 Martin J. Humphries, 1 and
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Biophysics of contractile ring assembly
 Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides
 Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides targeting RCP (SMARTPool (RCP) or two individual oligos (RCP#1
Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides targeting RCP (SMARTPool (RCP) or two individual oligos (RCP#1
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Modeling cytoskeleton self-assembly
 Modeling cytoskeleton self-assembly Dimitrios Vavylonis Department of Physics, Lehigh University BIOS 10 Lehigh University Oct 30, 2009 Cell organization How does the cell achieve internal organization?
Modeling cytoskeleton self-assembly Dimitrios Vavylonis Department of Physics, Lehigh University BIOS 10 Lehigh University Oct 30, 2009 Cell organization How does the cell achieve internal organization?
Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways
 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways Grégory Giannone 1,2 and Michael. Sheetz 1 1 Department of
Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways Grégory Giannone 1,2 and Michael. Sheetz 1 1 Department of
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Elizabeth F Noratel, Chere L Petty, Jessica S Kelsey, Hoa N Cost, Nisha Basappa and Daphne D Blumberg *
 Noratel et al. BMC Cell Biology 2012, 13:29 RESEARCH ARTICLE Open Access The adhesion modulation protein, AmpA localizes to an endocytic compartment and influences substrate adhesion, actin polymerization
Noratel et al. BMC Cell Biology 2012, 13:29 RESEARCH ARTICLE Open Access The adhesion modulation protein, AmpA localizes to an endocytic compartment and influences substrate adhesion, actin polymerization
Supporting Information
 Supporting Information Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Vincent Fitzpatrick, Laure Fourel, Olivier Destaing,
Supporting Information Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Vincent Fitzpatrick, Laure Fourel, Olivier Destaing,
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental Movie Legend.
 Supplemental Movie Legend. Transfected T cells were dropped onto SEE superantigen-pulsed Raji B cells (approximate location indicated by circle). Maximum-intensity projections from Z-stacks (17 slices,
Supplemental Movie Legend. Transfected T cells were dropped onto SEE superantigen-pulsed Raji B cells (approximate location indicated by circle). Maximum-intensity projections from Z-stacks (17 slices,
ABSTRACT. constantly interact with and adapt to their environment by exerting forces to mechanically
 ABSTRACT Title of dissertation: THE ROLE OF ACTIN NETWORKS IN CELLULAR MECHANOSENSING Mikheil Azatov, Doctor of Philosophy, 2015 Dissertation directed by: Professor Arpita Upadhyaya Department of Physics
ABSTRACT Title of dissertation: THE ROLE OF ACTIN NETWORKS IN CELLULAR MECHANOSENSING Mikheil Azatov, Doctor of Philosophy, 2015 Dissertation directed by: Professor Arpita Upadhyaya Department of Physics
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines
 Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines Further information can be found at: http://stke.sciencemag.org/sites/default/files/researcharticlerevmsinstructions_0.pdf.
Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines Further information can be found at: http://stke.sciencemag.org/sites/default/files/researcharticlerevmsinstructions_0.pdf.
5 HARNESSING THE PURSE STRING FOR
 107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
Supplemental Information. Pacer Mediates the Function of Class III PI3K. and HOPS Complexes in Autophagosome. Maturation by Engaging Stx17
 Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
Supplementary Figure 1. Espn-1 knockout characterization. (a) The predicted recombinant Espn-1 -/- allele was detected by PCR of the left (5 )
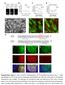 Supplementary Figure 1. Espn-1 knockout characterization. (a) The predicted recombinant Espn-1 -/- allele was detected by PCR of the left (5 ) homologous recombination arm (left) and of the right (3 )
Supplementary Figure 1. Espn-1 knockout characterization. (a) The predicted recombinant Espn-1 -/- allele was detected by PCR of the left (5 ) homologous recombination arm (left) and of the right (3 )
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Post-translational modification
 Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
T H E J O U R N A L O F C E L L B I O L O G Y
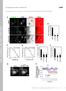 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Yamaguchi et al., http://www.jcb.org/cgi/content/full/jcb.201009126/dc1 S1 Figure S2. The expression levels of GFP-Akt1-PH and localization
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Yamaguchi et al., http://www.jcb.org/cgi/content/full/jcb.201009126/dc1 S1 Figure S2. The expression levels of GFP-Akt1-PH and localization
Asier Jayo 1, Maddy Parsons 1 and Josephine C Adams 2* Abstract
 RESEARCH ARTICLE Open Access A novel Rho-dependent pathway that drives interaction of fascin-1 with p-lin-11/isl-1/mec-3 kinase (LIMK) 1/2 to promote fascin-1/actin binding and filopodia stability Asier
RESEARCH ARTICLE Open Access A novel Rho-dependent pathway that drives interaction of fascin-1 with p-lin-11/isl-1/mec-3 kinase (LIMK) 1/2 to promote fascin-1/actin binding and filopodia stability Asier
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Craft et al., http://www.jcb.org/cgi/content/full/jcb.201409036/dc1 Figure S1. GFP -tubulin interacts with endogenous -tubulin. Ciliary
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Craft et al., http://www.jcb.org/cgi/content/full/jcb.201409036/dc1 Figure S1. GFP -tubulin interacts with endogenous -tubulin. Ciliary
Supplementary Figure 1. Immunoprecipitation of synthetic SUMOm-remnant peptides using UMO monoclonal antibody. (a) LC-MS analyses of tryptic
 Supplementary Figure 1. Immunoprecipitation of synthetic SUMOm-remnant peptides using UMO 1-7-7 monoclonal antibody. (a) LC-MS analyses of tryptic digest from HEK293 cells spiked with 6 SUMOmremnant peptides
Supplementary Figure 1. Immunoprecipitation of synthetic SUMOm-remnant peptides using UMO 1-7-7 monoclonal antibody. (a) LC-MS analyses of tryptic digest from HEK293 cells spiked with 6 SUMOmremnant peptides
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Nature Publishing Group
 s u p p l e m e n ta r y i n f o r m at i o n Figure S1 Ang1 induces translocation of Tie1 and Tie2 to TritonX-100 insoluble cell junctions connected to actin microfilaments. (a) Tie2 is present in cell-cell
s u p p l e m e n ta r y i n f o r m at i o n Figure S1 Ang1 induces translocation of Tie1 and Tie2 to TritonX-100 insoluble cell junctions connected to actin microfilaments. (a) Tie2 is present in cell-cell
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 CELL BIOLOGY BIO-2B06 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 CELL BIOLOGY BIO-2B06 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in
