Potential Environmental Impacts of Vapours
|
|
|
- Ruby Jennings
- 6 years ago
- Views:
Transcription
1 Potential Environmental Impacts of Vapours Learning Outcome When you complete this module you will be able to: Explain the impact of gases and vapours on the environment. Learning Objectives Here is what you will be able to do when you complete each objective: 1. List the common domestic, industrial, and naturally occurring gases and vapours that have environmental impact. 2. List the sources of the common gases and vapours. 3. Describe the current methods of gas and vapour conditions and disposal. 4. Describe alternative methods of reducing gas and vapour pollution. 1
2 GASEOUS POLLUTANTS The gases and vapours responsible for environmental pollution are numerous and the full impact of individual gases in combination with other gases and elements is not thoroughly understood. This module will emphasize gases that are responsible for acid rain and the so called greenhouse gases. Pollutant Sulphur oxides Nitrous oxides Carbon dioxide Carbon Monoxide Methane Chlorofluorocarbons Ozone Problem produced Responsible for about 70% of acid rain. Responsible for about 30% of acid rain, produce smog and trap heat that increase greenhouse effect The main heat trapping gas responsible for the greenhouse effect A very poisonous gas. A green house gas. Destroy ozone after rising to the stratosphere Hazardous to health and corrosive to many materials in the lower atmosphere Table 1 Common Gaseous Pollutants HOW POLLUTANT GASES ARE PRODUCED Sulphur Oxides Sulphur oxides [sulphur dioxide (SO 2 ) and sulphur trioxide (SO 3 )] are produced whenever a fuel containing sulphur is burned. Sulphur is contained in most coals and fuel oils used by industry. Low sulphur fuels are much more expensive than high sulphur fuels hence the use of high sulphur fuel has a great advantage from an economic standpoint. Some mining and smelting operations discharge great quantities of sulphur oxides to the atmosphere. Pulp mills are also a source of sulphur oxides. Sulphur oxides also enter the atmosphere from natural sources. Some hot springs emit sulphur oxides, and during a volcanic eruption millions of tons of sulphur oxides may be introduced into the atmosphere. 2
3 Nitrogen Oxides (NO x ) Oxygen and nitrogen can react to form several different compounds; nitric oxide (NO), nitrous oxide (N 2 O), nitrogen dioxide (NO 2 ), nitrogen trioxide (N 2 O 3 ), and nitrogen pentoxide (N 2 O 5 ) are some of the possibilities. These gases have varying degrees of stability and are referred to collectively as NO x. In many cases more than one of these gases is given off by a single source. Nitric oxide (NO) and nitrogen dioxide (NO 2 ) are the major NO x gases formed in the combustion process (e.g. in boilers and internal combustion engines. NO 2 formation is favoured at temperatures below 1000 K, while NO formation is favoured above that temperature. The total NO x formed during a combustion process increases with an increase in temperature and an increase in excess O 2 in the burning zone. Usually more than 90% of the NO x formed in the combustion zone is NO. Some of this NO then combines with the excess O 2 to form NO 2. This latter reaction occurs as the temperature of the exhaust gases drops below 1000 K. The rate of reaction slows until it ceases as temperatures approach ambient, and some of the NO will decompose back to O 2 and N 2 before being discharged from the exhaust system. Some decomposition of NO does occur slowly in the atmosphere. NO and NO 2 convert back and forth as components in the photochemical smog problem. When water is added, NO 2 is a contributor to the formation of nitric acid (HNO 3 ), which accounts for roughly 30% of the acidity in most acid rain. Carbon Dioxide (CO 2 ) Any time a fossil fuel is burned CO 2 is produced. It is one of the most important gases on our planet because all plant life depends on CO 2. All animal life produces CO 2 and forest fires are also large contributors of this gas. CO 2 cannot be eliminated from the combustion process as long as the fuel contains carbon. Carbon Monoxide (CO) Carbon monoxide, an extremely poisonous gas, is produced in any combustion process that does not have sufficient air. One of the greatest producers of CO is the internal combustion engine because there is insufficient time for complete combustion to take place. Overloaded boilers, forest fires, and burning refuse can also produce CO. Methane (CH 4 ) Methane is the main component of natural gas. It is a product of decomposed organic matter such as in swamps and sewage lagoons and is formed in the digestion processes of animals, especially ruminants. 3
4 Chlorofluorocarbons (CFCs) These are man-made products that are used as refrigerants, as a foaming agent for some plastics, as propellants in aerosol sprays, as cleaning agents and in Halon fire extinguishers. Ozone (O 3 ) Ozone is a form of oxygen which contains three oxygen atoms or O 3. In the upper atmosphere, O 3 is formed by the action of high intensity sunlight on O 2. Only a small percentage of the resulting ozone works down to the lower atmosphere and ground level. The upper level ozone is of critical importance as it shields the earth s surface from hazardous ultraviolet and other radiation. CFCs not only produce a greenhouse effect, but they also destroy this upper level ozone layer. Ozone at the lower levels is produced by electrical arcs such as from brushes in electric motors, arc welding, and lightning. The action of sunlight on a mixture of NO x and volatile organic compounds (frequently referred to as photochemical smog) also produces ozone in this zone. ACID RAIN Acid rain is formed when NO x and SO 2 in the atmosphere undergo complex and little understood changes in a medium of water vapour and sunlight. The resulting interaction produces sulphuric acid and nitric acid which precipitate with rain, snow, hail, sleet, fog, and in some cases deposit on the ground in a dry form. Since the acid can come down in either a wet or dry form, the condition is better referred to as acid deposition. The contaminants which form acid rain can be carried great distances from their source. The smogs of California deposit their pollution in the snows of Colorado. The snows melt and the acid runoff leaches hazardous minerals from the mountains. Some of the meltwater returns in the rivers as a water source for California. Effects of Acid Rain on Lakes and Rivers When the acid level increases in a lake, microscopic life dies and interference with the reproductive cycle of aquatic animals becomes evident. Metals leached from the soil, in higher concentrations than normal, restrict the ability of some animals to breathe properly, for example, fish gills become fouled and the fish suffocate. 4
5 The first melt of spring releases a surge of acid from the snow. In this toxic meltwater many eggs and hatchlings cannot survive. In lakes and streams, clams, snails and crayfish are first to die, then insects such as the mayfly, dragonfly, damsel fly and other larvae. Then by acid attack or starvation, the amphibians and fish die off. In Ontario, Quebec, and the Northeastern United States about 2500 lakes per year are dying. In other parts of the world acid rain is also a problem; for instance in Sweden acid rain is blamed for as many as dead lakes. Effects of Acid Rain on Plant Life Forests are being killed by acid rain. Some problems are directly created by acid deposition on the leaves, however the greater problem appears to be the effects of acid deposition on the soil. Nutrients normally dissolved and used by the tree roots have either been leached away and lost, or toxic quantities of other materials are taken into solution by the acidic groundwater. These materials interfere with the plants ability to acquire proper nourishment. The result is that plants are weakened and die. We must realize that these effects impact on all plant life, not just the forests. Effects of Acid Rain on People Acid rain causes both economic and health impacts. The effect on maple trees in Eastern Canada and the Northeastern United States is resulting in a diminishing maple sugar industry. Logging operations are being curtailed, and reforestation efforts are futile in heavily impacted areas; fruit trees produce substandard fruit, or die off; vegetables become discoloured and unhealthy, making them difficult to market; fish from certain waters may be banned for human consumption due to the impact of acid rain on the watercourse. The full extent of the economic impact of acid rain is difficult to assess, but the above examples provide some indicator of how widespread the impact may be. The effects of acid rain on human health are varied. Acid water can leach toxic heavy metals from the soil, as well as copper and lead from plumbing systems causing a toxic hazard to humans. Studies suggest that sulphur dioxide in the air causes bronchitis, emphysema, and a strain on the heart and circulatory system. Some recent reports suggest a possible relationship between acid haze and an increase in some types of cancer; probably due to the blocking of sunlight at the wavelength needed by humans to produce Vitamin D in the body. 5
6 THE GREENHOUSE EFFECT The earth has existed for a considerable period of time in an equilibrium state, where the amount of energy received from the sun is balanced with an equal amount of energy radiated from the earth back into space. Some high energy radiation incoming from the sun (such as X-rays and high energy ultraviolet light) encounters upper atmosphere ozone and is absorbed or reflected back into space. Slightly lower energy radiation (such as visible light and infrared radiation) passes through the atmosphere to ground level. At ground level, this radiation is largely absorbed, and eventually radiated back towards space at a lower energy level. Some gases known as the greenhouse gases will allow high energy radiation to pass through but restrict the transmission of the lower energy radiation that would normally move towards space. The relatively stable concentration of greenhouse gases accounts for the earth s overall average temperature being reasonably stable for many centuries. With an increase in greenhouse gases, more outgoing radiation is trapped by the atmosphere causing an increase in average temperatures. The increasing average temperatures can cause drought conditions in presently fertile growing regions; and polar ice masses may be reduced, causing rising ocean levels which would impact severely on man s activities. AI5_fig1.gif Figure 1 Greenhouse Effect 6
7 GREENHOUSE GASES Water Vapour The water vapour content of the air depends on its temperature. The warmer the air the more moisture it can hold and water vapour can hold a tremendous amount of heat. By itself it cannot produce a greenhouse effect but water vapour amplifies the effect of other gases. Carbon Dioxide Carbon dioxide is the principal green house gas, accounting for about 50% of the total problem. The amount of CO 2 in the atmosphere is estimated to be increasing by about 1/2% per year. That may not sound like much but it means an addition of 5 x 10 9 tons of carbon/year. About one-half of that carbon is used by plants or absorbed by the oceans, and the rest stays in the atmosphere. Methane Methane is believed to comprise about 20% of the greenhouse gas problem. The carbon in methane makes it a heat trapping gas like carbon dioxide, and in fact it has about thirty times the activity of CO 2. Although the greenhouse effect of methane is much higher than that of CO 2, methane has an estimated half-life of about 7-10 years compared to 500 years for CO 2. Some scientists are concerned that methane is responsible for preventing the atmosphere from ridding itself of CFCs. Chlorofluorocarbons CFCs contribute about 15% of the greenhouse gas problem. CFCs are very stable man-made compounds that help destroy ozone after they reach the stratosphere. Ozone shields the planet from harmful cosmic radiation that can cause cancer. In the lower atmosphere CFCs are able to absorb infrared rays about 10,000 times as effectively as carbon dioxide making them powerful greenhouse gases. Nitrous Oxide Nitrous oxide (N 2 O) (laughing gas) is very stable, lasting an estimated 150 years or longer in the atmosphere. This gas is one of the gases contributing to the green house effect, accounting for about 10% of the problem. When it rises to the stratosphere it is responsible for destroying ozone. Nitrous oxide levels in the atmosphere are estimated to be rising at a rate of 0.25% per year. 7
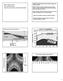
8 Ozone Ozone in the lower atmosphere is a pollutant that is responsible for about 5% of the greenhouse effect. This gas is an irritant to the eyes and respiratory system. In the stratosphere, ozone is very beneficial because it blocks out cancer causing ultra violet radiation. In the lower atmosphere it is a hazard in that it is dangerous to health and corrosive to many products including rubber. WHAT IS BEING DONE TO REDUCE HARMFUL GASES? Sulphur Dioxide Removal At this point the student may wish to refer to Learning Module ENVS 6011 ( ), Gaseous and Noise Pollutants, for additional information on the removal of sulphur dioxide from stack gases. Many plants have met environmental regulations by utilizing naturally occurring low sulphur, western source coal. Others have achieved the required emission levels by installing flue gas desulphurization (FGD) systems. The FGD systems are categorized as nonregenerable or regenerable. Nonregenerable systems are the best developed and most widely applied. Most of the processes involve wet scrubbing of the combustion flue gas in a gasliquid contractor using lime or limestone, alkaline fly ash with supplemental lime or limestone, sodium carbonate and dilute sulphuric acid as the scrubbing medium. A typical limestone system is shown in Fig. 2. Efficiency for these systems can be as high as 90 to 95% with combustion gases containing up to 5,000 ppm SO 2. A regenerable FGD system is shown in Fig. 3 its advantage being that the SO 2 recovered is converted into a marketable by-product such as sulphur, sulphuric acid, or liquid SO 2. These units have a higher initial cost, some of which may be recovered by the sale of the by-product. Some of these processes have been installed in systems over 100 MW capacity. Wet scrubbing units use a solution of magnesium oxide to remove the SO 2. 8
9 AI5_fig2.gif Figure 2 Limestone Wet Scrubbing System (Courtesy of Babcock & Wilcox) AI5_fig3.gif Figure 3 Regenerable Wet Scrubbing System (Courtesy of Babcock & Wilcox) 9
10 Oxides of Nitrogen Unlike sulphur oxides, which are formed only from the sulphur contained in the fuel, nitrogen oxides (NO x ) are formed from both fuel-bound nitrogen and nitrogen contained in the combustion air introduced into the furnace. The NO x produced from nitrogen in the fuel is dependent on the: (i) Percent of nitrogen in the fuel. (ii) Oxygen availability in the combustion zone. (iii) Reactivity of nitrogen compounds contained in the fuel. The conversion of nitrogen in the air to NO x is highly dependent on temperature. The formation of NO x proceeds rapidly at combustion zone temperatures in excess of 1650 C. By maintaining the combustion temperature below 1650 C, the NO x emission can be reduced. There are several methods of reducing the formation of NO x as discussed in the following sections. 1. Two Stage Combustion This is the most effective method of reducing the formation of NO x. Initial combustion takes place in a fuel rich environment. The remaining air for combustion, and excess air, is introduced in an area away from the initial combustion zone to complete the combustion process. By reducing the availability of oxygen in the combustion zone the fuel-bound nitrogen is less likely to be converted to NO x. This process also prolongs combustion thus reducing the flame temperature which reduces the conversion of atmospheric nitrogen to NO x. 2. Low Excess Air Operation By firing with the least amount of excess air as possible, while still maintaining carbon losses to a minimum, the O 2 available is reduced. This means that the amount of fuel-bound nitrogen converted to NO x is reduced. The amount of NO x formed due to nitrogen in the air is also reduced due to the lower availability of O 2 and reduced quantities of nitrogen entering the combustion zone. 3. Gas Recirculation On gas- and oil-fired units, gas recirculation into the combustion zone provides dilution of the combustion air thus prolonging combustion and reducing the flame temperature. Since all the NO x from gas-firing and 50% of the NO x from oilfiring are produced by thermally converted nitrogen, gas recirculation to the burners appears to be effective on overall NO x reduction. With coal-firing the effect of this method of NO x reduction is minimal. 10
11 4. Dual Register Burner Fig. 4 shows a dual register burner developed by Babcock and Wilcox. This burner limits turbulence thus reducing the peak flame temperature at the burner. The reduced turbulence also delays combustion producing a slower burning flame in the combustion zone. While maintaining stable efficient operation the burner has demonstrated a 50% reduction in NO x over previous high turbulence burner designs. Utilizing this type of burner in conjunction with low excess air operation has produced an acceptable reduction of NO x. Figure 4 Dual Register Burner (Courtesy of Babcock & Wilcox) Diluting Pollution To reduce pollution in the immediate area of stack emissions, super stacks have been built. The local pollution has been reduced because the emissions are discharged higher into the atmosphere. This solution of the problem has only spread the problem over a greater area and has not reduced the amount of gases that have adverse environmental impacts. 11
12 ALTERNATIVES Solar Energy Solar energy is a nonpolluting source of energy. The energy is free but harnessing it is expensive. It was reported in 1989 in the New York Times that the conversion of solar energy to electrical power could become comparable in efficiency to conventional power generation. Tremendous gains in the development of solar cells has taken place in the last few years and if fossil fuel prices dictate, more research will take place. It is possible to reduce SO 2 and NO x emissions to an acceptable level, if laws are made strict enough, but as long as we burn fossil fuels the amount of CO 2 in the atmosphere will continue to increase. Hydrogen Fuel Using hydrogen as a fuel is not a new idea, but the methods of production, storage, and handling must be refined before it receives wide acceptance. Hydrogen is an ideal fuel in that the only product of combustion is water vapour. A kilogram of hydrogen delivers about 4.25 times more energy than a kilogram of carbon when burned completely. Hydrogen is often stored in cryogenic containers to keep the storage pressures from being unreasonably high. Even a small hydrogen leak poses an explosion hazard due to the wide range in the explosive limits. Hydrogen rich fuels such as methane and propane contribute less CO 2 pollution to the air than regular gasoline or diesel fuels. A greater usage of these fuels would reduce equipment costs, but the higher demand would likely result in a price increase. Carbon Dioxide Removal From The Atmosphere Sometimes we cannot see the forest for the trees. Many scientists are urging mass reforestation of the earth. Trees require CO 2 and it is estimated that 10,000,000 acres of new forest would use up all the CO 2 that would be emitted by power plants in the next 10 years. There are solvents that can remove CO 2 from power plant emissions but the process is cost prohibitive at the present. The CO 2 removed using this method could be injected into oil fields to maintain formation pressure. CFCs It appears that these man made substances will be phased out and alternate refrigerants will be used. Possibly refrigeration systems like the ammonia and lithium bromide absorption systems will be given more research and development. These systems produce no pollution and can be energy savers by making use of low level waste heat that is otherwise dumped into the atmosphere. 12
Section 4 The Air We Breathe
 Section 4 The Air We Breathe Key Concept Air is an important natural resource that is affected by human activities. What You Will Learn Air pollution is caused by human activities, such as burning fossil
Section 4 The Air We Breathe Key Concept Air is an important natural resource that is affected by human activities. What You Will Learn Air pollution is caused by human activities, such as burning fossil
Acid deposition accumulation of potential acid-forming particles on a surface acids can result from natural causes
 1 Air Quality Issues: Part 2 - Acid Deposition, Greenhouse Gases EVPP 111 Lecture Dr. Largen 2 Air Quality Issues Air Pollution Indoor Air Pollution Acid Deposition Greenhouse Gases & Global Warming 3
1 Air Quality Issues: Part 2 - Acid Deposition, Greenhouse Gases EVPP 111 Lecture Dr. Largen 2 Air Quality Issues Air Pollution Indoor Air Pollution Acid Deposition Greenhouse Gases & Global Warming 3
CLIMATE CHANGE AND ACID RAIN. Mr. Banks 7 th Grade Science
 CLIMATE CHANGE AND ACID RAIN Mr. Banks 7 th Grade Science COMPOSITION OF AIR? COMPOSITION OF AIR? 78% Nitrogen 21% Oxygen 0.93% Argon and other noble gases 0.04% carbon dioxide Variable amounts of water
CLIMATE CHANGE AND ACID RAIN Mr. Banks 7 th Grade Science COMPOSITION OF AIR? COMPOSITION OF AIR? 78% Nitrogen 21% Oxygen 0.93% Argon and other noble gases 0.04% carbon dioxide Variable amounts of water
Name: Class: Date: 6. Most air pollution is produced by a. thermal inversions. c. ozone layer depletion. b. fuel burning. d. volcanic eruptions.
 Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Atmosphere Web quest
 Atmosphere Web quest 1. What are the four main layers of the atmosphere? Troposphere Stratosphere Mesosphere Thermosphere Ionosphere Exsosphere 2. Which layer is closest to space? Exosphere (upper layer
Atmosphere Web quest 1. What are the four main layers of the atmosphere? Troposphere Stratosphere Mesosphere Thermosphere Ionosphere Exsosphere 2. Which layer is closest to space? Exosphere (upper layer
Criteria Pollutants. Sulfur Dioxide (SO 2 ) Nitrogen Oxides (NOx)
 1) Sulfur dioxide 2) Nitrogen oxides 3) Carbon monoxide 4) Ozone 5) Particulates 6) Lead Criteria Pollutants Sulfur Dioxide (SO 2 ) SO 2 is a colorless gas that is formed from the combustion of sulfur-containing
1) Sulfur dioxide 2) Nitrogen oxides 3) Carbon monoxide 4) Ozone 5) Particulates 6) Lead Criteria Pollutants Sulfur Dioxide (SO 2 ) SO 2 is a colorless gas that is formed from the combustion of sulfur-containing
Just what is Acid Rain?
 Acid Rain Just what is Acid Rain? Acid Rain is the term used to describe the ways in which acid precipitates out of the atmosphere. Acid Rain is more accurately termed acid deposition. There are two types
Acid Rain Just what is Acid Rain? Acid Rain is the term used to describe the ways in which acid precipitates out of the atmosphere. Acid Rain is more accurately termed acid deposition. There are two types
Air Pollution. Asian Brown Cloud. Developed Countries have reduced emissions recently
 Study Questions 1. Compare and contrast primary vs. secondary pollutants, giving examples of each. 2. Compare and contrast indoor vs. outdoor pollution, listing specific examples and sources of each. 3.
Study Questions 1. Compare and contrast primary vs. secondary pollutants, giving examples of each. 2. Compare and contrast indoor vs. outdoor pollution, listing specific examples and sources of each. 3.
History of significant air pollution events
 Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
ANSWERS: Combustion. 2C3H8(g) + 7O2(g) 6CO(g) + 8H2O(g)
 ANSWERS: Combustion organic molecule methane equation for complete combustion CH4(g) + 2O2(g) CO2(g) + 2H2O(g) equation for incomplete combustion 4CH4(g) + 5O2(g) 2CO(g) + 2C(s) + 8H2O(g) methanol 2CH3OH(l)
ANSWERS: Combustion organic molecule methane equation for complete combustion CH4(g) + 2O2(g) CO2(g) + 2H2O(g) equation for incomplete combustion 4CH4(g) + 5O2(g) 2CO(g) + 2C(s) + 8H2O(g) methanol 2CH3OH(l)
Air Pollution. GEOL 1350: Introduction To Meteorology
 Air Pollution GEOL 1350: Introduction To Meteorology 1 Overview Types and Sources of Air Pollutants Factors That Affect Air Pollution Air Pollution and the Urban Environment 2 Air pollutants are airborne
Air Pollution GEOL 1350: Introduction To Meteorology 1 Overview Types and Sources of Air Pollutants Factors That Affect Air Pollution Air Pollution and the Urban Environment 2 Air pollutants are airborne
Earth as a System. Chapter 2. Table of Contents. Section 1 Earth: A Unique Planet. Section 2 Energy in the Earth System.
 Earth as a System Table of Contents Section 1 Earth: A Unique Planet Section 2 Energy in the Earth System Section 3 Ecology Section 1 Earth: A Unique Planet Objectives Describe the size and shape of Earth.
Earth as a System Table of Contents Section 1 Earth: A Unique Planet Section 2 Energy in the Earth System Section 3 Ecology Section 1 Earth: A Unique Planet Objectives Describe the size and shape of Earth.
HUMAN IMPACT on the BIOSPHERE part 4
 HUMAN IMPACT on the BIOSPHERE part 4 Charting a course for the Future http://www.claybennett.com/pages2/mistletoe.html ENVIRONMENTAL PROBLEMS DEAD ZONES OZONE DEPLETION ACID RAIN GLOBAL WARMING WASTE http://www.acmecompany.com/stock_thumbnails/13808.greenhouse_effect_2.jpg
HUMAN IMPACT on the BIOSPHERE part 4 Charting a course for the Future http://www.claybennett.com/pages2/mistletoe.html ENVIRONMENTAL PROBLEMS DEAD ZONES OZONE DEPLETION ACID RAIN GLOBAL WARMING WASTE http://www.acmecompany.com/stock_thumbnails/13808.greenhouse_effect_2.jpg
Foundation Course. Semester 3 THREATS TO THE ENVIRONMENT
 Foundation Course Semester 3 THREATS TO THE ENVIRONMENT INTRODUCTION Atmosphere, water and soil are the most important components of environment in which we live. Atmospheric factors like rainfall, humidity,
Foundation Course Semester 3 THREATS TO THE ENVIRONMENT INTRODUCTION Atmosphere, water and soil are the most important components of environment in which we live. Atmospheric factors like rainfall, humidity,
GLOBAL ENVIRONMENTAL PROBLEMS
 GLOBAL ENVIRONMENTAL PROBLEMS DR. SIREEN ALKHALDI, BDS, DRPH EPIDEMIOLOGY AND BIOSTATISTICS, 2 ND YEAR, 2017/ 2018 MEDICAL SCHOOL, THE UNIVERSITY OF JORDAN DEFINITION: ENVIRONMENT Environment is: The
GLOBAL ENVIRONMENTAL PROBLEMS DR. SIREEN ALKHALDI, BDS, DRPH EPIDEMIOLOGY AND BIOSTATISTICS, 2 ND YEAR, 2017/ 2018 MEDICAL SCHOOL, THE UNIVERSITY OF JORDAN DEFINITION: ENVIRONMENT Environment is: The
Major Air Pollutants
 Major Air Pollutants 1 Particulate Matter Particulate refers to all substances that are not gases. It can be suspended droplets / solid particles / mixture of two. Size: 100 µm to 0.1 µm and less. Particulates
Major Air Pollutants 1 Particulate Matter Particulate refers to all substances that are not gases. It can be suspended droplets / solid particles / mixture of two. Size: 100 µm to 0.1 µm and less. Particulates
BIOGEOCHEMICAL CYCLES INTRODUCTION THE CYCLING PROCESS TWO CYCLES: CARBON CYCLE NITROGEN CYCLE HUMAN IMPACTS GLOBAL WARMING AQUATIC EUTROPHICATION
 BIOGEOCHEMICAL CYCLES INTRODUCTION THE CYCLING PROCESS TWO CYCLES: CARBON CYCLE NITROGEN CYCLE HUMAN IMPACTS GLOBAL WARMING AQUATIC EUTROPHICATION BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through
BIOGEOCHEMICAL CYCLES INTRODUCTION THE CYCLING PROCESS TWO CYCLES: CARBON CYCLE NITROGEN CYCLE HUMAN IMPACTS GLOBAL WARMING AQUATIC EUTROPHICATION BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through
Cycles of Ma,er. Lesson Overview. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
POLLUTION. Water Pollution Air Pollution
 POLLUTION Water Pollution Air Pollution Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The presence of a substance in the environment that prevents
POLLUTION Water Pollution Air Pollution Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The presence of a substance in the environment that prevents
Ch. 5 - Nutrient Cycles and Soils
 Ch. 5 - Nutrient Cycles and Soils What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient
Ch. 5 - Nutrient Cycles and Soils What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient
POLLUTION. Water Pollution Air Pollution
 POLLUTION Water Pollution Air Pollution Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The presence of a substance in the environment that prevents
POLLUTION Water Pollution Air Pollution Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The presence of a substance in the environment that prevents
CALIFORNIA EDUCATION AND THE ENVIRONMENT INITIATIVE
 Water Vapor: A GHG Lesson 3 page 1 of 2 Water Vapor: A GHG Water vapor in our atmosphere is an important greenhouse gas (GHG). On a cloudy day we can see evidence of the amount of water vapor in our atmosphere.
Water Vapor: A GHG Lesson 3 page 1 of 2 Water Vapor: A GHG Water vapor in our atmosphere is an important greenhouse gas (GHG). On a cloudy day we can see evidence of the amount of water vapor in our atmosphere.
Water cycles through ecosystems.
 Water cycles through ecosystems. Water is stored on Earth s surface in lakes, rivers, and oceans. Water is found underground, filling the spaces between soil particles and cracks in rocks. Large amounts
Water cycles through ecosystems. Water is stored on Earth s surface in lakes, rivers, and oceans. Water is found underground, filling the spaces between soil particles and cracks in rocks. Large amounts
Directed Reading. Section: Global Change. than in the rest of the United States. b. In the United States and Canada, many lakes are dying as their ph
 Section: Global Change In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Scientists have discovered that acid rain is caused
Section: Global Change In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Scientists have discovered that acid rain is caused
ACID RAIN. CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007
 ACID RAIN CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007 More accurate term may be acid deposition Occurs in two forms wet deposition (acidic rain, fog, and snow) dry deposition
ACID RAIN CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007 More accurate term may be acid deposition Occurs in two forms wet deposition (acidic rain, fog, and snow) dry deposition
Chapter 20 Air Pollution
 Chapter 20 Air Pollution Overview of Chapter 20 Atmosphere as a Resource Types and Sources of Air Pollution Effects of Air Pollution Controlling Air Pollution in the US Ozone Depletion in the Stratosphere
Chapter 20 Air Pollution Overview of Chapter 20 Atmosphere as a Resource Types and Sources of Air Pollution Effects of Air Pollution Controlling Air Pollution in the US Ozone Depletion in the Stratosphere
Overview of Chapter 19
 19 Air Pollution Overview of Chapter 19 Atmosphere as a Resource Types and Sources of Air Pollution Effects of Air Pollution Controlling Air Pollution in the US Ozone Depletion in the Stratosphere Acid
19 Air Pollution Overview of Chapter 19 Atmosphere as a Resource Types and Sources of Air Pollution Effects of Air Pollution Controlling Air Pollution in the US Ozone Depletion in the Stratosphere Acid
Global Warming. By William K. Tong. Adjunct Faculty, Earth Science Oakton Community College
 Global Warming By William K. Tong Adjunct Faculty, Earth Science Oakton Community College What Is Global Warming? According to the National Academy of Sciences, the Earth's surface temperature has risen
Global Warming By William K. Tong Adjunct Faculty, Earth Science Oakton Community College What Is Global Warming? According to the National Academy of Sciences, the Earth's surface temperature has risen
Chapter 2 9/15/2015. Chapter 2. Penny Boat. 2.1 The Role of Water in Cycles of Matter
 Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Announcements. Pollution week continues. Thinking about pollution. Why are polar bears so contaminated?
 Announcements Grades for exam 2 have been posted March 7 th - Last day to submit LEAD summary to TA, extra credit videos due next Tuesday (no late videos will be accepted) Next Thursday, Environmental
Announcements Grades for exam 2 have been posted March 7 th - Last day to submit LEAD summary to TA, extra credit videos due next Tuesday (no late videos will be accepted) Next Thursday, Environmental
Non-Renewable Energy Resources: How do dead things power our lives?
 Non-Renewable Energy Resources: How do dead things power our lives? Life requires energy it is stored, transferred, and converted Ultimate source of energy for life the Sun For humans, our source of energy
Non-Renewable Energy Resources: How do dead things power our lives? Life requires energy it is stored, transferred, and converted Ultimate source of energy for life the Sun For humans, our source of energy
LESSON 3 OTHER LAND RESOURCES C H A P T E R 6, C O N S E R V I N G O U R R E S O U R C E S
 LESSON 3 OTHER LAND RESOURCES C H A P T E R 6, C O N S E R V I N G O U R R E S O U R C E S OBJECTIVES Compare renewable and nonrenewable resources. Describe how human activities affect the environment.
LESSON 3 OTHER LAND RESOURCES C H A P T E R 6, C O N S E R V I N G O U R R E S O U R C E S OBJECTIVES Compare renewable and nonrenewable resources. Describe how human activities affect the environment.
MODULE I. Learning Objectives
 MODULE I Learning Objectives To make the students aware of history of air pollution; definition of air pollution and various types of sources and classification of air pollutants. Lecture 1 Lecture 2 Lecture
MODULE I Learning Objectives To make the students aware of history of air pollution; definition of air pollution and various types of sources and classification of air pollutants. Lecture 1 Lecture 2 Lecture
THE INTRODUCTION THE GREENHOUSE EFFECT
 THE INTRODUCTION The earth is surrounded by atmosphere composed of many gases. The sun s rays penetrate through the atmosphere to the earth s surface. Gases in the atmosphere trap heat that would otherwise
THE INTRODUCTION The earth is surrounded by atmosphere composed of many gases. The sun s rays penetrate through the atmosphere to the earth s surface. Gases in the atmosphere trap heat that would otherwise
1 Characteristics of the Atmosphere
 CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
The atmosphere. The atmosphere is layered. Inversions affect air quality 3/2/2015. The sun influences weather and climate
 The atmosphere Chapter 13 Atmosphere Absorbs radiation and moderates climate Transports and recycles water and nutrients Human activity is now changing the amount of some gases CO 2, methane (CH 4 ), ozone
The atmosphere Chapter 13 Atmosphere Absorbs radiation and moderates climate Transports and recycles water and nutrients Human activity is now changing the amount of some gases CO 2, methane (CH 4 ), ozone
TOPIC-NATURAL RESOURCES NATURAL RESOURCES
 TOPIC-NATURAL RESOURCES ENVIRONMENT: The physical, biological and social aspects of our surroundings in which we live is known as environment.the natural environment consists of things which have been
TOPIC-NATURAL RESOURCES ENVIRONMENT: The physical, biological and social aspects of our surroundings in which we live is known as environment.the natural environment consists of things which have been
Form 4 Chapter 9: Endangered Ecosystem
 Form 4 Chapter 9: Endangered Ecosystem 1. Pollution: Any undesirable change in the physical, chemical or biological characteristics of the natural environment, brought about by human activities. 2. When
Form 4 Chapter 9: Endangered Ecosystem 1. Pollution: Any undesirable change in the physical, chemical or biological characteristics of the natural environment, brought about by human activities. 2. When
2.2 Nutrient Cycles in Ecosystems. Review How energy flows What is the difference between a food chain, food web, and food pyramid?
 2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
Lecture 29 Air Pollution. Air Pollution. Clean Boundary Layer. Clean Boundary Layer
 Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
Pollution of Air and Water
 104 18 EXEMPLAR PROBLEMS Pollution of Air and Water MULTIPLE CHOICE QUESTIONS 1. Air is a mixture of various gases. One of the gases is 21% part of the air and is essential for the survival of human beings.
104 18 EXEMPLAR PROBLEMS Pollution of Air and Water MULTIPLE CHOICE QUESTIONS 1. Air is a mixture of various gases. One of the gases is 21% part of the air and is essential for the survival of human beings.
5. Local winds result from pressure differences between high and low pressure systems. They can be very intense.
 Unit 5: Air Pollution Objectives: 1. Recognize that pollution affects the air, land, freshwater and the oceans. 2. Discuss the composition of the Earth's atmosphere and the distribution of its gasses.
Unit 5: Air Pollution Objectives: 1. Recognize that pollution affects the air, land, freshwater and the oceans. 2. Discuss the composition of the Earth's atmosphere and the distribution of its gasses.
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32)
 Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
How Ecosystems Work Section 1. Chapter 5 How Ecosystems Work Section 1: Energy Flow in Ecosystems DAY 1
 Chapter 5 How Ecosystems Work Section 1: Energy Flow in Ecosystems DAY 1 Life Depends on the Sun Energy from the sun enters an ecosystem when plants use sunlight to make sugar molecules. This happens through
Chapter 5 How Ecosystems Work Section 1: Energy Flow in Ecosystems DAY 1 Life Depends on the Sun Energy from the sun enters an ecosystem when plants use sunlight to make sugar molecules. This happens through
TROPICS: insolation high year round, high sun angle and ~ constant duration
 GE 101, February 6, 14 Finish insolation variation Global environmental issues associated with insolation TRPICS: insolation high year round, high sun angle and ~ constant duration MID-LATITUDES: insolation
GE 101, February 6, 14 Finish insolation variation Global environmental issues associated with insolation TRPICS: insolation high year round, high sun angle and ~ constant duration MID-LATITUDES: insolation
Threats to Our Atmosphere
 Threats to Our Atmosphere A Reading A Z Level W Leveled Reader Word Count: 1,831 LEVELED READER W Written by Shaun Taylor Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Threats to Our Atmosphere A Reading A Z Level W Leveled Reader Word Count: 1,831 LEVELED READER W Written by Shaun Taylor Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Chapter 19: Global Change
 1 Summary Of the Case Study Polar Bear population in the Antarctic going down because temperatures are going up and melting the caps. Polar bears are losing their habitat, they also can t get their food
1 Summary Of the Case Study Polar Bear population in the Antarctic going down because temperatures are going up and melting the caps. Polar bears are losing their habitat, they also can t get their food
Brain Wrinkles. Acid Rain in Germany, Air Pollution in the United Kingdom, & the Nuclear Disaster in Chernobyl, Ukraine
 Acid Rain in Germany, Air Pollution in the United Kingdom, & the Nuclear Disaster in Chernobyl, Ukraine STANDARDS: SS6G8 Explain environmental issues in Europe. a. Explain the causes and effects of acid
Acid Rain in Germany, Air Pollution in the United Kingdom, & the Nuclear Disaster in Chernobyl, Ukraine STANDARDS: SS6G8 Explain environmental issues in Europe. a. Explain the causes and effects of acid
Chapter Introduction. Matter. Ecosystems. Chapter Wrap-Up
 Chapter Introduction Lesson 1 Lesson 2 Lesson 3 Abiotic Factors Cycles of Matter Chapter Wrap-Up Energy in Ecosystems How do living things and the nonliving parts of the environment interact? What do you
Chapter Introduction Lesson 1 Lesson 2 Lesson 3 Abiotic Factors Cycles of Matter Chapter Wrap-Up Energy in Ecosystems How do living things and the nonliving parts of the environment interact? What do you
Population Biology. Biology 2201 Unit IV
 Population Biology Biology 2201 Unit IV Population Biology The study of populations is referred to as demography. The characteristics of populations usually studied are size, density and growth rate. Important
Population Biology Biology 2201 Unit IV Population Biology The study of populations is referred to as demography. The characteristics of populations usually studied are size, density and growth rate. Important
THE COMBUSTION OF HYDROCARBONS. I love the smell of napalm in the morning smells like victory!
 THE COMBUSTION OF HYDROCARBONS I love the smell of napalm in the morning smells like victory! Carbon monoxide is a toxic gas that can be produced during the combustion of a carbon-based fuel such as propane.
THE COMBUSTION OF HYDROCARBONS I love the smell of napalm in the morning smells like victory! Carbon monoxide is a toxic gas that can be produced during the combustion of a carbon-based fuel such as propane.
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore
 Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore GREENHO U SE EFFECT For the complete encyclopedic entry with media
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore GREENHO U SE EFFECT For the complete encyclopedic entry with media
Environmental Impacts of. Energy Production
 CH2356 Energy Engineering Environmental Impacts of Energy Production Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH2356 Energy Engineering Environmental Impacts of Energy Production Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
greenhouse effect 1 of 5
 This website would like to remind you: Your browser (Apple Safari 4) is out of date. Update your browser for more security, comfort and the best experience on this site. Encyclopedic Entry greenhouse effect
This website would like to remind you: Your browser (Apple Safari 4) is out of date. Update your browser for more security, comfort and the best experience on this site. Encyclopedic Entry greenhouse effect
Environmental Impact: Nuclear Energy in Comparison with other Alternatives. Eric D. Graham
 Environmental Impact: Nuclear Energy in Comparison with other Alternatives Eric D. Graham Contents Introduction Greenhouse Gases Solid Waste Wildlife Effects Land Resource Use Other Effects Conclusion
Environmental Impact: Nuclear Energy in Comparison with other Alternatives Eric D. Graham Contents Introduction Greenhouse Gases Solid Waste Wildlife Effects Land Resource Use Other Effects Conclusion
BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through living organisms and the physical environment.
 BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through living organisms and the physical environment. BIOCHEMIST: Scientists who study how LIFE WORKS at a CHEMICAL level. The work of biochemists has
BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through living organisms and the physical environment. BIOCHEMIST: Scientists who study how LIFE WORKS at a CHEMICAL level. The work of biochemists has
2. All of the following are primary air pollutants except a. carbon monoxide. c. sulfur oxides. b. nitric acid. d. VOCs. ANS: B DIF: 1 REF: 1 OBJ: 1
 Chapter 12 Air MULTIPLE CHOICE 1. What pollutant forms when automobile emissions react with oxygen gas and ultraviolet rays? a. ozone c. radon b. carbon dioxide d. sulfur dioxide A DIF: 1 REF: 1 OBJ: 1
Chapter 12 Air MULTIPLE CHOICE 1. What pollutant forms when automobile emissions react with oxygen gas and ultraviolet rays? a. ozone c. radon b. carbon dioxide d. sulfur dioxide A DIF: 1 REF: 1 OBJ: 1
What Exactly is a Greenhouse Gas?
 1 What Exactly is a Greenhouse Gas? You may have stood in a greenhouse and felt the heat, but what do greenhouse gases have to do with greenhouses? A greenhouse gas is any gas that absorbs and re-emits
1 What Exactly is a Greenhouse Gas? You may have stood in a greenhouse and felt the heat, but what do greenhouse gases have to do with greenhouses? A greenhouse gas is any gas that absorbs and re-emits
What is the carbon cycle?
 What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,454 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,454 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
Chapter 5: How Ecosystems Work Section 1, Energy Flow in Ecosystems
 Life Depends on the Sun Chapter 5: How Ecosystems Work Section 1, Energy Flow in Ecosystems Energy from the sun enters an ecosystem when plants use sunlight to make sugar molecules. This happens through
Life Depends on the Sun Chapter 5: How Ecosystems Work Section 1, Energy Flow in Ecosystems Energy from the sun enters an ecosystem when plants use sunlight to make sugar molecules. This happens through
MLA Header: coal oil natural gas burning of fossil fuels volcanoes photosynthesis respiration ocean sugar greenhouse decayed
 MLA Header: s worksheet Please answer the following using the words in the text box. Carbon coal oil natural gas burning of fossil fuels volcanoes photosynthesis respiration ocean sugar greenhouse decayed
MLA Header: s worksheet Please answer the following using the words in the text box. Carbon coal oil natural gas burning of fossil fuels volcanoes photosynthesis respiration ocean sugar greenhouse decayed
Ecosystems: Nutrient Cycles
 Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Conserving Land and Soil (continued)
 Name Date Class Land, Water, and Air Resources Guided Reading and Study Conserving Land and Soil (continued) Types of Land Use 1. Complete the concept map. Uses of land that change the land include 2.
Name Date Class Land, Water, and Air Resources Guided Reading and Study Conserving Land and Soil (continued) Types of Land Use 1. Complete the concept map. Uses of land that change the land include 2.
Downloaded from
 Question 14.1: Define environmental chemistry. Environmental chemistry is the study of chemical and biochemical processes occurring in nature. It deals with the study of origin, transport, reaction, effects,
Question 14.1: Define environmental chemistry. Environmental chemistry is the study of chemical and biochemical processes occurring in nature. It deals with the study of origin, transport, reaction, effects,
Biosphere & Biogeochemical Cycles
 Biosphere & Biogeochemical Cycles Biosphere Sphere of living organisms All the regions of the earth and its atmosphere in which living organisms are found or can live. Interacts with all the other spheres
Biosphere & Biogeochemical Cycles Biosphere Sphere of living organisms All the regions of the earth and its atmosphere in which living organisms are found or can live. Interacts with all the other spheres
Closed Systems A closed system is a system in which energy, but not matter is exchanged with the surroundings.
 2.2 Notes Objectives Compare an open system with a closed system. List the characteristics of Earth s four major spheres. Identify the two main sources of energy in the Earth system. Identify four processes
2.2 Notes Objectives Compare an open system with a closed system. List the characteristics of Earth s four major spheres. Identify the two main sources of energy in the Earth system. Identify four processes
Ecology Part 2: How Ecosystems Work
 Ecology Part 2: How Ecosystems Work Name: Unit 2 1 In this second part of Unit 2, our big idea questions are: SECTION 1 How is energy transferred from the Sun to producers and then to consumers? Why do
Ecology Part 2: How Ecosystems Work Name: Unit 2 1 In this second part of Unit 2, our big idea questions are: SECTION 1 How is energy transferred from the Sun to producers and then to consumers? Why do
High-energy Hydrogen II Teacher Page
 High-energy Hydrogen II Teacher Page Video: Hydrogen - The Pollution Solution Student Objectives will be able to explain how fossil fuels have caused our pollution problem will be able to explain how hydrogen
High-energy Hydrogen II Teacher Page Video: Hydrogen - The Pollution Solution Student Objectives will be able to explain how fossil fuels have caused our pollution problem will be able to explain how hydrogen
Name Class Date. In the space provided, write the letter of the term or phrase that best matches the description.
 Skills Worksheet Concept Review MATCHING In the space provided, write the letter of the term or phrase that best matches the description. 1. ground-level ozone 2. scrubber 3. radon gas 4. nitrogen oxides
Skills Worksheet Concept Review MATCHING In the space provided, write the letter of the term or phrase that best matches the description. 1. ground-level ozone 2. scrubber 3. radon gas 4. nitrogen oxides
The Cycling of Matter
 Section 2 Objectives Describe the short-term and long-term process of the carbon cycle. Identify one way that humans are affecting the carbon cycle. List the three stages of the nitrogen cycle. Describe
Section 2 Objectives Describe the short-term and long-term process of the carbon cycle. Identify one way that humans are affecting the carbon cycle. List the three stages of the nitrogen cycle. Describe
Energy, Greenhouse Gases and the Carbon Cycle
 Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Biology 2201 Populations. Unit 4
 Biology 2201 Populations Unit 4 Population Growth The study of populations is referred to as demography. The characteristics of populations usually studied are size, density and growth rate. A population
Biology 2201 Populations Unit 4 Population Growth The study of populations is referred to as demography. The characteristics of populations usually studied are size, density and growth rate. A population
How Ecosystems Work Section 1. Chapter 5 How Ecosystems Work Section 1: Energy Flow in Ecosystems DAY 1
 Chapter 5 How Ecosystems Work Section 1: Energy Flow in Ecosystems DAY 1 Life Depends on the Sun Energy from the sun enters an ecosystem when plants use sunlight to make sugar molecules. This happens through
Chapter 5 How Ecosystems Work Section 1: Energy Flow in Ecosystems DAY 1 Life Depends on the Sun Energy from the sun enters an ecosystem when plants use sunlight to make sugar molecules. This happens through
Human Activity and Climate Change
 Human Activity and Climate Change Textbook pages 482 501 Section 11.1 11.2 Summary Before You Read How might climate change affect the region where you live? Record your thoughts in the lines below. What
Human Activity and Climate Change Textbook pages 482 501 Section 11.1 11.2 Summary Before You Read How might climate change affect the region where you live? Record your thoughts in the lines below. What
Climate Change and Ozone Depletion Notes. Chapter 20
 Climate Change and Ozone Depletion Notes Chapter 20 PAST CLIMATE AND THE GREENHOUSE EFFECT Over the past 900,000 years, the troposphere has experienced prolonged periods of global cooling and global warming.
Climate Change and Ozone Depletion Notes Chapter 20 PAST CLIMATE AND THE GREENHOUSE EFFECT Over the past 900,000 years, the troposphere has experienced prolonged periods of global cooling and global warming.
POLLUTION. Water Pollution Atmospheric Pollution The Atmosphere: Climate Change and Ozone Depletion
 POLLUTION Water Pollution Atmospheric Pollution The Atmosphere: Climate Change and Ozone Depletion Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The
POLLUTION Water Pollution Atmospheric Pollution The Atmosphere: Climate Change and Ozone Depletion Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The
Directions 1. Activate students' prior knowledge about secondary pollutants. 1 of 10. Activitydevelop
 Activitydevelop Pollutants Making More Pollutants How do pollutants interact with the environment to create more pollution, and what effects do secondary pollutants have on the environment and human health?
Activitydevelop Pollutants Making More Pollutants How do pollutants interact with the environment to create more pollution, and what effects do secondary pollutants have on the environment and human health?
Gaseous and Noise Pollutants
 Gaseous and Noise Pollutants Learning Outcome When you complete this module you will be able to: Describe the nature, environmental impacts and control methods for gaseous and noise pollutants in a power
Gaseous and Noise Pollutants Learning Outcome When you complete this module you will be able to: Describe the nature, environmental impacts and control methods for gaseous and noise pollutants in a power
Pollution of the Atmosphere
 Pollution of the Atmosphere LESSON 2 Guiding Question: What are the sources of air pollution? Explain how both natural processes and human activities can cause air pollution. Describe how air pollutants
Pollution of the Atmosphere LESSON 2 Guiding Question: What are the sources of air pollution? Explain how both natural processes and human activities can cause air pollution. Describe how air pollutants
1 An Interconnected Planet
 CHAPTER 6 1 An Interconnected Planet SECTION The Environment KEY IDEAS As you read this section, keep these questions in mind: How are humans and the environment connected? What is the difference between
CHAPTER 6 1 An Interconnected Planet SECTION The Environment KEY IDEAS As you read this section, keep these questions in mind: How are humans and the environment connected? What is the difference between
Class IX Chapter 14 Natural Resources Science
 Question 1: How is our atmosphere different from the atmospheres on Venus and Mars? Earth s atmosphere is different from those of Venus and Mars. This difference lies essentially in their compositions.
Question 1: How is our atmosphere different from the atmospheres on Venus and Mars? Earth s atmosphere is different from those of Venus and Mars. This difference lies essentially in their compositions.
5/12/15. We depend on environment for. Food Water Air Shelter Fuel, etc. Environmental science the study of the impact of humans on the environment
 List examples of chemical pollution from industry, agriculture, or everyday use. What are some possible effects that these pollutants can have on the environment? Doerfler Biology I How are humans and
List examples of chemical pollution from industry, agriculture, or everyday use. What are some possible effects that these pollutants can have on the environment? Doerfler Biology I How are humans and
Chapter 11 Fossil Fuels
 Chapter 11 Fossil Fuels I. Energy Sources and Consumption A. Energy sources that were used were obtained locally and now they are worldwide Fossil fuels Nuclear energy Electricity B. Energy consumption
Chapter 11 Fossil Fuels I. Energy Sources and Consumption A. Energy sources that were used were obtained locally and now they are worldwide Fossil fuels Nuclear energy Electricity B. Energy consumption
Chapter Two: Cycles of Matter (pages 32-65)
 Biology 20 Chapter 2.1_keyed Chapter Two: Cycles of Matter (pages 32-65) 2.1 The Role of Water in the Cycles of Matter (pages 34 40) Due to its ability to form hydrogen bonds, water has several unique
Biology 20 Chapter 2.1_keyed Chapter Two: Cycles of Matter (pages 32-65) 2.1 The Role of Water in the Cycles of Matter (pages 34 40) Due to its ability to form hydrogen bonds, water has several unique
LABEL AND EXPLAIN THE PROCESSES AT EACH NUMBER IN THE DIAGRAM ABOVE
 HYDROLOGIC CYCLE 3 4 5 2 5 1B 6B 1A 6A 7 6C LABEL AND EXPLAIN THE PROCESSES AT EACH NUMBER IN THE DIAGRAM ABOVE 1A. Evaporation of water from oceans 1B. Evaporation of water from land sources (water and
HYDROLOGIC CYCLE 3 4 5 2 5 1B 6B 1A 6A 7 6C LABEL AND EXPLAIN THE PROCESSES AT EACH NUMBER IN THE DIAGRAM ABOVE 1A. Evaporation of water from oceans 1B. Evaporation of water from land sources (water and
What is the carbon cycle?
 What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,160 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,160 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
Master 5.1, Newspaper Articles. Special Edition December 14. Special Edition March 17
 Master 5.1, Newspaper Articles THE DAILY HERALD Special Edition December 14 Study Forecasts Future Food Shortage A new study published in the Journal of World Agriculture raises concerns that in the future
Master 5.1, Newspaper Articles THE DAILY HERALD Special Edition December 14 Study Forecasts Future Food Shortage A new study published in the Journal of World Agriculture raises concerns that in the future
Chapter 11 Industry and Manufacturing
 AP Human Geography Chapter 11 Industry and Manufacturing Key Issues Where is industry distributed? Why are situation and site factors important? Why does industry cause pollution? Why are situation and
AP Human Geography Chapter 11 Industry and Manufacturing Key Issues Where is industry distributed? Why are situation and site factors important? Why does industry cause pollution? Why are situation and
Section 6.1: A Changing Landscape. Name: Block: Date:
 Section 6.1: A Changing Landscape Name: Block: Date: 1. Our daily activities impact the quality of Earth s natural resources:,, a. These activities are:,, 2. The Effect of Human Activity :Agriculture a.
Section 6.1: A Changing Landscape Name: Block: Date: 1. Our daily activities impact the quality of Earth s natural resources:,, a. These activities are:,, 2. The Effect of Human Activity :Agriculture a.
CHAPTER 4: CHARACTERISTICS IN ECOSYSTEMS
 1 CHAPTER 4: CHARACTERISTICS IN ECOSYSTEMS 4.3. FACTORS AFFECTING ECOSYSTEMS Pages 101-107 Nelson 1. ABIOTIC FACTORS IN TERRESTRIAL ECOSYSTEMS 2 abiotic factors are the non-living components of an ecosystem
1 CHAPTER 4: CHARACTERISTICS IN ECOSYSTEMS 4.3. FACTORS AFFECTING ECOSYSTEMS Pages 101-107 Nelson 1. ABIOTIC FACTORS IN TERRESTRIAL ECOSYSTEMS 2 abiotic factors are the non-living components of an ecosystem
Chapter 11: Atmosphere
 To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
Section Objectives: Explain biodiversity and its importance. Relate various threats to the loss of biodiversity.
 Section Objectives: Explain biodiversity and its importance. Relate various threats to the loss of biodiversity. Biological Diversity Biodiversity refers to the variety of species in a specific area. The
Section Objectives: Explain biodiversity and its importance. Relate various threats to the loss of biodiversity. Biological Diversity Biodiversity refers to the variety of species in a specific area. The
The Global Reaction to Water and Air Pollution
 The Global Reaction to Water and Air Pollution By History.com, adapted by Newsela staff on 05.30.17 Word Count 900 Level 1160L White plume smoke containing many pollutants is emitted from a quenching tower
The Global Reaction to Water and Air Pollution By History.com, adapted by Newsela staff on 05.30.17 Word Count 900 Level 1160L White plume smoke containing many pollutants is emitted from a quenching tower
Overview of Chapter 11
 11 Fossil Fuels Overview of Chapter 11 Fossil Fuels Coal Coal Reserves Coal mining Environmental Effects of Burning Coal Oil and Natural Gas Exploration for Oil and Natural Gas Oil and Natural Gas reserves
11 Fossil Fuels Overview of Chapter 11 Fossil Fuels Coal Coal Reserves Coal mining Environmental Effects of Burning Coal Oil and Natural Gas Exploration for Oil and Natural Gas Oil and Natural Gas reserves
Carbon is an element. It is part of oceans, air, rocks, soil and all living things. Carbon doesn t stay in one place. It is always on the move!
 The Carbon Cycle Carbon is an element. It is part of oceans, air, rocks, soil and all living things. Carbon doesn t stay in one place. It is always on the move! Carbon moves from the atmosphere to plants.
The Carbon Cycle Carbon is an element. It is part of oceans, air, rocks, soil and all living things. Carbon doesn t stay in one place. It is always on the move! Carbon moves from the atmosphere to plants.
What is air pollution?
 Air Pollution 1 What is air pollution? Air pollution is a mixture of natural and man-made substances in the air we breathe. It is typically separated into two categories: outdoor air pollution and indoor
Air Pollution 1 What is air pollution? Air pollution is a mixture of natural and man-made substances in the air we breathe. It is typically separated into two categories: outdoor air pollution and indoor
1st English Speech on Speaker: David REPOLUSK Topic: Acid rain Class: 5.HBa. Speech: ACID RAIN. I am presenting my speech about ACID RAIN.
 Speech: ACID RAIN I am presenting my speech about ACID RAIN. 1. INTRODUCTION Acid rain is caused by burning fossil fuels like coal and oil and allowing them to pollute the atmosphere. This form of pollution
Speech: ACID RAIN I am presenting my speech about ACID RAIN. 1. INTRODUCTION Acid rain is caused by burning fossil fuels like coal and oil and allowing them to pollute the atmosphere. This form of pollution
Introduction to Water Quality Parameters
 Introduction to Water Quality Parameters Directions: Your group will be assigned one or two Water Quality (WQ) parameters to read about and present to the class. 1. Read the explanations for your assigned
Introduction to Water Quality Parameters Directions: Your group will be assigned one or two Water Quality (WQ) parameters to read about and present to the class. 1. Read the explanations for your assigned
