and δ 13 C DIC δ 13 C DIC
|
|
|
- Charla Horton
- 6 years ago
- Views:
Transcription
1 The effects of agricultural activities and atmospheric acid deposition on carbonate weathering in a small karstic agricultural catchment, Southwest China COBISS: 1.01 Vpliv kmetijske dejavnosti in kislih usedlin iz zraka na preperevanje karbonatov na majhnem kraškem kmetijskem območju na jugozahodu Kitajske Yu Chen 1 1, 2* & Yongjun Jiang Abstract UDC : (513) Yu Chen & Yongjun Jiang : The effects of agricultural activities and atmospheric acid deposition on carbonate weathering in a small karstic agricultural catchment, Southwest China In order to quantify the sources and fluxes of DIC, the effects of the use of N-fertilizers and acid deposition on carbonate weathering have been quantified by hydrochemistry and in Qingmuguan underground river system (QURS) a small karstic agricultural catchment of Southwest China. The results show that: (1) the significant temporal variations for major element concentrations and in different months were observed, especially, of which the groundwater showed significant high concentrations of DIC, Ca 2, Mg 2, NO 3, 2 and in rainy season and fertilizing period in the QURS; (2) the contributions of carbonate dissolution by carbonic acid to total concentrations of (Ca 2 Mg 2 ) and in different months averaged 68.5 % and 81.0 %, respectively. While the contributions of carbonate dissolution by nitric acid originated from the use of N-fertilizers and atmospheric acid deposition to total concentrations of (Ca 2 Mg 2 ) and in different months averaged 11.1 % and 6.7 %, respectively, and the contributions of carbonate dissolution by sulphuric acid originated from the atmospheric acid deposition to total concentrations of (Ca 2 Mg 2 ) and in different months averaged 20.4 % and 12.3 %, respectively; (3) the increased obviously with (Ca 2 Mg 2 )/ of groundwater in the rainy season and fertilizing period indicated that the use of N-fertilizers and atmospheric acid deposition should be responsible for the elevated the and the molar ratio of (Ca 2 Mg 2 )/ in the QURS. Key words: carbonate weathering, karst groundwater, agricultural activities, atmospheric acid deposition, Qingmuguan, Southwest China. Izvleček UDK : (513) Yu Chen & Yongjun Jiang Vpliv kmetijske dejavnosti in kislih usedlin iz zraka na preperevanje karbonatov na majhnem kraškem kmetijskem območju na jugozahodu Kitajske Da bi določili izvor in tok DIC (raztopljen anorganski ogljik) smo vrednotili učinek uporabe dušikovih gnojil in kislih usedlin na preperevanje karbonatov. Pri tem smo se poslužili hidrokemičnih postopkov in analize podzemne vode v kraškem zaledju reke Quinmugan (QURS), ki je del manjšega kmetijskega območja na jugozahodu Kitajske. Rezultati so pokazali naslednje: (1) Izmerjene so bile opazne časovne spremembe v koncentraciji glavnih elementov in v podzemni vodi v različnih mesecih; to je še posebej očitno v deževnem obdobju in času gnojenja, ko so bile zabeležene izrazito povečane koncentracije DIC, Ca 2, Mg 2, NO 3, 2 in ; (2) Delež preperevanja karbonatov zaradi ogljikove kisline je glede na skupne koncentracije (Ca 2 Mg 2 ) in v podzemni vodi v različnih mesecih v povprečju znašal med 68,5 % in 81,0 %. Medtem je delež preperevanja karbonatov zaradi dušikove kisline (posledica uporabe dušikovih gnojil in kislih usedlin iz zraka) glede na skupne koncentracije (Ca 2 Mg 2 ) in v podzemni vodi v različnih mesecih v povprečju znašal med 11,1 % in 6,7 %, delež preperevanja karbonatov zaradi žveplove kisline (posledica kislih usedlin iz zraka) glede na skupne koncentracije (Ca 2 Mg 2 ) in v podzemni vodi v različnih mesecih pa je v povprečju znašal med 20,4 % in 12,3 %; (3) Vsebnost v podzemni vodi se v času deževne sezone občutno poveča skupaj z razmerjem (Ca 2 Mg 2 )/, obdobje gnojenja pa nakazuje, da uporaba dušikovih gnojil in usedanje kislin iz ozračja vplivajo na povišano vsebnost in molarno razmerje (Ca 2 Mg 2 )/ v podzemni vodi v rečnem sistemu QURS. Ključne besede: preperevanje karbonatov, kraška podzemna voda, kmetijske dejavnosti, kisle usedline iz zraka, Qingmuguan, jugozahodna Kitajska. 1 School of Geographical Sciences, Southwest University, Chongqing , China; 2 Karst Environment Laboratory, Southwest University, Chongqing , China. Yu Chen, School of Geographical Sciences, Southwest University, Chongqing, China; chenyu192781@163.com *Corresponding author: Yongjun Jiang, School of Geographical Sciences, Southwest University, Chongqing, China; jiangjyj@swu.edu.cn Received/Prejeto: ACTA CARSOLOGICA 45/1, , POSTOJNA 2016
2 Yu Chen & Yongjun Jiang Introduction The geological-environmental characteristics of karst hydrological systems result in a highly vulnerable system, which is considerably sensitive to external environmental changes. While the increasing environmental pollution, both deliberate and unintentional forms as consequence of human activities, has to a great extent spoiled sensitive karst hydrological systems in Southwest China (Liu et al. 2006, 2008; Jiang et al. 2008, 2009a, 2009b; Jiang 2012; Pu et al. 2011). Concentrations of nitrate and sulphate of karst groundwater in southwest China increase notably as a result of large amount of chemical fertilizers used in agriculture (Liu et al. 2006; Jiang et al. 2008, 2009a, 2009b; Jiang 2012; Pu et al. 2011) and acid deposition (Li et al. 2008, 2010), which could not only influence the quality of karst groundwater, as well as the carbonate weathering process related to the global carbon cycle. Conventionally, the DIC in karst groundwater is dominantly derived from carbonate dissolution by carbonic acid (Eq. 1), which forms from reaction of soil or atmospheric CO 2 with water. NH 4 2O 2 2(Ca 1x Mg x )CO 3 = NO 3 2(1x)Ca 2 2 xmg 2 2 H 2 O (4) Acid deposition, including reactive N and sulfate, has dramatically increased worldwide in the past few decades, of which more than one third of the territory in China (Tang et al. 2010), especially in Southwest China, is suffering from acid deposition. Anthropogenic Nr is released to the atmosphere either as nitrogen oxides (NOx), mainly from combustion, or as ammonia (NH 3 ), mainly from agriculture (Dentener et al. 2006; Paulot et al. 2013). NOx is oxidized in the atmosphere to nitric acid (HNO 3 ) and ammonia can be transformed into NH 4 through chemical reactions and then can be nitrified and produce proton. Sulfate is released to the atmosphere as SOx, predominately from sulfur emissions (fossil fuel combustion) (Dentener et al. 2006), and SOx can be oxidized in the atmosphere to sulfuric acid (H 2 ). Thus, acid deposition can impact carbonate weathering. These processes can be expressed as following equations: (Ca 1x Mg x )CO 3 H 2 O CO 2 (1x)Ca 2 xmg 2 2 (1) (Ca 1x Mg x )CO 3 HNO 3 (1x)Ca 2 xmg 2 NO 3 (5) In this case, half of the DIC (generally, bicarbonate is the dominant DIC species in karst groundwater) in karst groundwater derived from soil/atmospheric CO 2 constitutes an important sink of atmospheric CO 2. However, recently increases in the inorganic carbon flux in karst groundwater have been linked to agricultural activities and acid precipitation (Semhi et al. 2000; Calmels et al. 2007; Li et al. 2008, 2010; Perrin et al. 2008; Barnes & Raymond 2009; Ali & Atekwana 2011; Gandois et al. 2011; Jiang 2013; Yue et al. 2015). The consequence of the use of nitrogen fertilizers (usually in the form of (CO(NH 2 ) 2 ), (NH 4 ) 2, NH 3 and (NH 4 ) 2 PO 4, is the release of protons in the soil during the nitrification process. N fertiliser oxidation produces two protons for every nitrified ammonium ion and then enhances carbonate weathering. These processes can be expressed as following equations: NH 4 2O 2 = NO 3 2H H 2 O (2) 2(Ca 1x Mg x )CO 3 H 2 2(1x)Ca 2 2xMg (6) In these cases, carbonate dissolution by nitric and sulphuric acids are resulting in greater DIC export, which is derived from the carbonate rather than from the CO 2 sequestration. Such enhanced carbonate weathering by anthropogenic acidity inputs could not only influence the element fluxes to riverine systems, as well as the global carbon cycle. Thus, based on these knowledge, this paper presented the results of chemical analysis for the concentrations of major ions, DIC and δ 13 CDIC, and discussed: (1) to quantify the sources and fluxes of DIC in groundwater, and (2) to evaluate the effects of Nfertilizers and acid deposition on carbonate weathering in a small karstic agricultural catchment of Southwest China. CaCO 3 H = Ca 2 (3) 162 ACTA CARSOLOGICA 45/2 2016
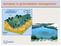
3 The effects of agricultural activities and atmospheric acid deposition on carbonate... Study area The Qingmuguan Underground River System (QURS) is located at the west of Chongqing municipality, Southwest China (Fig. 1). The underground drainage area of the system is approximately 11.4 km 2. The elevation of QURS is between 320~640 m above average sea level. The climate is primarily subtropical monsoonal with a mean annual precipitation of 1100 mm and a mean air temperature of 16.5 C. The monsoonal climate results in a rainy season from April to October and a dry season from November to March. Fig. 1: Location, hydrogeological and land use map in the QURS. ACTA CARSOLOGICA 45/
4 Yu Chen & Yongjun Jiang Geology and hydrogeology The geologic layers of the study area are shown in Fig. 1. The QURS lies in the middle part of the Wentangxia anticline, Chongqing, and the aquifers are mainly underlain by Lower and Middle Triassic strata (Fig. 1). Carbonate rocks (limestone) cover an area of 10 km 2 or about 88 % of the total area. The strata of the anticlinal axis are carbonate rocks of the Lower Triassic Jialingjiang Formation (T 1 j), with limestone being a major lithology, whereas anticlinal wings are carbonate rocks of the Middle Triassic Leikoupo Formation (T 2 l) and sandstones with some coal seams of the Upper Triassic Xujiahe Formation (T 3 xj). Yellow-green calcareous clay rocks of the Lower Leikoupo (T 2 l) overlie the Jialingjiang Group (T 1 j). No sulfate evaporates (gypsum and anhydrite) are exposed in the study area. Due to the banded distribution of carbonate rocks and the presence of relatively impermeable sandstone at two wings combined with a vertical patulous cranny which was well developed in the anticlinal core, basic conditions exist for a formation of a karst trough valley and the development of a karst groundwater system. There are many depressions, caves, dolines and sinkholes in the catchment. In the upper stream, the surface water in Ganjiacao depression, which is the biggest depression, recharges the QURS via Yankou sinkhole. The Qingmuguan Underground River developed in the core of the karst trough valley and flowed along anticlinal axis (Lower Triassic Jialingjiang formation) in a NESW direction with a total length of 7.8 km. In the downstream reach of the QURS, the Jiangjia spring discharges the groundwater of the QURS. High-resolution tracer tests in the catchment indicated a single conduit between Yankou sinkhole and Jiangjia spring (Fig. 1) (He et al. 2010), and the Jiangjia spring is the only outlet of the hydrological system. Flow rates of the spring have large variations around a year from to 3.5 m 3 /s, with an average of 20.5 l/s. The spring water is used by individual households. Land use pattern, vegetation, soil and agricultural practices There are three land use categories (Fig. 1): forested land, paddy land and dry land. The percentages of land use were 64.9 for forested, 11.4 for paddy land and 23.7 for dry land, respectively. Paddy fields are mainly distributed in the Ganjiacao depression, and dry fields are scattered around the bottom of the valley. The crops are rice in paddy field, potato and vegetables in dry field. Vegetation is predominantly subtropical evergreen broadleaved forests. Those plants and crops are C 3 photosynthetic type. The dominant types of soils are mainly limestone soil and yellow soil derived from carbonate rocks and sandstones, respectively. Paddy soil are scattered in the depressions. Farmers principally spread chemical fertilizers on soils in spring and summer seasons, mainly compound fertilizers of the N-P-K type and straight fertilizers such as urea (CO(NH 2 ) 2 ), NH 4 and ammonium sulphate ((NH 4 ) 2 ). Typical amounts of chemical fertilizers used in the catchment are 1200 kg ha 1 yr 1 for rice, 600 kg ha 1 yr 1 for vegetables. Because soil ph and carbonate content are high, buffering with lime is not practiced. Samples and analyses The rainfall was collected from the HOBO weather station (made by OnSET Ltd., USA) installed in the catchment, which the resolution of rainfall was 0.2 mm with a time internal of 15 min. The discharge, water temperature, ph and specific conductivity were monitored at 15 min intervals using a Greenspan CTDP 300 multi-channel data logger. Monthly samples were collected for analysis of major hydrochemical components and isotope in laboratory in Rainwater was collected during 3 events in the catchment from May to July, Temperature, ph, specific conductivity (SC, at 25 C) and dissolved oxygen (DO) and rainwater were measured in the field using a WTW multiparameter probe (Multiline P3 PH/LF-SET) with resolutions of 0.1 C, 0.1, 1 μs cm 1 and 0.1 mg L 1, respectively. NH 4 and rainwater was measured in the field using a D8500 water quality multimeter (HACH, America), with resolution of Ca 2 and were determined by a test kit with a titration pipette (Aquamerck) in the field with resolutions of 2 and 0.1 mmol/l, respectively. Water samples of the spring and rain were collected by injection syringes and were immediately filtered into pre-rinsed plastic containers (1 L) with 0.45 μm filter membranes for ion analyses, one of which was acidified to ph<2 with HNO 3 to preserve cation concentrations. To limit gas exchange, the plastic containers were cooled with ice. Samples for the stable carbon isotope of dissolved inorganic carbon ( ) analysis were filtered 164 ACTA CARSOLOGICA 45/2 2016
5 The effects of agricultural activities and atmospheric acid deposition on carbonate... into 10 ml tubes with 0.2 μm cellulose-acetate filters. Soon after collection, these samples were preserved using three drops of a saturated solution of HgCl 2 to prevent microbial alteration. All samples were kept refrigerated (below 4 C) until analysis. Three representative plant samples (including the root, stem and leaf), rice, bamboo and Cyclobalanopsis, were collected in the catchment. Meanwhile, three soil CO 2 samples were collected, using a capillary tube reaching in m depth and an evacuated glass vessel with Kontes valves, under the corresponding plants. Also, two limestone samples were collected from Lower Trissic Jialingjiang formation and Middle Triassic Leikoupo Formation, respectively. Concentrations of major cations and anions were measured with inductively coupled plasma optical emission spectrometry (ICP-OES) with a resolution of 0.01 and ion chromatography (IC) with a resolution of 0.01, respectively, at the Water Environmental Laboratory of Southwest University. The precision of the IC and ICP analyses was within ±5 % for major elements. For, using the modified method of Atekwana and Krishnamurthy (1998) for stable carbon isotope analysis of dissolved inorganic carbon, a 10 ml water sample was injected by syringe into glass bottles that were pre-filled with 1 ml 85 % phosphoric acid and magnetic stir bars. The CO 2 was extracted and purified after cryogenic removal of H 2 O using a liquid nitrogen ethanol trap. Finally, the CO 2 was transferred cryogenically into a tube for isotope measurement. Plant samples were ultrasonically cleaned for 15 min in deionized water, and dried in an oven at 50 C for 48 h. Then, the plant samples were ground into powder with diameters less than 150 μm to ensure homogeneity, of which 1 to 2 mg was placed into stannum cups for carbon isotope analysis. All analyses for δ 13 C were done at the Geochemistry Laboratory of Southwest University. The carbon isotopic compositions of soil organic matter and plants were determined using an elemental analyzer coupled to an isotope-ratio mass spectrometer (EA-IRMS), and the carbon isotopic compositions of DIC, soil CO 2 and carbonate were determined using Gas Bench-linked with Delta V Plus gas stable isotope-ratio mass spectrometer (Gasbench-IRMS). The results were expressed on a conventional permil scale with respect to the Vienna Pee Dee Belemnite (V-PDB) standard. The overall experimental accuracy for δ 13 C during this study was ±0.2. Results and discussion The δ 13 C of limestone, plants and soil organic matter Isotopic compositions (δ 13 C) of limestone, plants and soil CO 2 are shown in Tab. 1. The δ 13 C of limestone from Jinaglingjiang and Leikoupo information was 0.2 and 0.3, respectively, consistent with typical values of marine limestone. The δ 13 C of plants varied from 26.6 Tab. 1: Isotopic composition (δ 13 C) of limestone, plants and soil CO 2 in QURS. Sample type δ 13 C ( ) Limestone Jialingjiang formation of Lower Trissic limestone (T 1 j) Leikoupo Formation of Middle Triassic limestone (T 2 l) Plant Rice 28.6 Bamboo 28.4 Cyclobalanopsis 26.6 Soil CO 2 Soil CO 2 in a Rice field 24.3 Soil CO 2 in a Bamboo field 23.1 Soil CO 2 in a Cyclobalanopsis field 22.6 to 28.6 with an average value of 27.9, consistent with the major vegetation type using the C 3 carbon fixation pathway in the catchment. The δ 13 C of soil CO 2 ranged from 22.6 to 24.3 with an average value of The in groundwater From the range of ph values it can be deduced that bicarbonate ( ) is the dominant DIC species of the groundwater in the catchment. Therefore, concentrations of total inorganic carbon are expressed as in this article. As shown in Tab. 2, the varied from 12.4 to 10.1 in different months, with a mean value of 11.2 in 2013 in the catchment. Also, as shown in Fig. 2, obvious positive values were observed in the rainy season and fertilizing period, and negative values were observed in the winter, suggesting the was controlled by different geochemical processes in different seasons in the QURS. ACTA CARSOLOGICA 45/
6 Yu Chen & Yongjun Jiang Tab. 2: Major ions concentration and and rainwater in the QURS. Theoretical Contribution of carbonate dissolution by sulphuric acid to total DIC in groundwater % Contribution of carbonate dissolution by nitric acid to total DIC in groundwater % Contribution of carbonate dissolution by carbonic acid to total DIC in groundwater % DO NH 4 NO 3 2 Cl K Na Mg 2 Ca 2 EC μs/cm ph Month Rainfall mm Groundwater Nd Nd Nd Nd Nd Nd Nd Nd Nd Nd Nd Nd Rainwater Chemical characteristics of groundwater and rainwater As shown in Tab. 2, the ph values samples in the QURS ranged from 7.4 to 8.1, averaging 7.7. The Ca 2 concentrations in groundwater varied from 103 to 131, with a mean value of 115. The Mg 2 concentrations varied from 9.6 to 14.5, with a mean value of Ca 2 and Mg 2 dominate the cation concentrations in groundwater, accounted for % of the total cations in groundwater. The NH 4 was not detected in the groundwater samples. was the most abundant anions, and its concentrations ranged from to 382.5, averaging The 2 and NO 3 concentrations varied dramatically, ranging from 23.1 to 59.1 and 12.3 to 38.2 in different months, with a mean concentration of 42.0 and 23.7, respectively. The Cl ranged from 14.3 to 18.5, with a mean concentration of Rainwater showed lower ph values, ranging from 4.9 to 5.1, typically characterized by acid rain. Concentrations of Ca 2, 2 and NO 3 in rainwater ranged from 11 to 12, 11.6 to 12.6 and 6.6 to 7.5, respectively. And other ions concentrations were very low in rainwater. Meanwhile, as shown in Fig. 2, ph showed lower values in rainy season and higher values in the winter, indicating ph was impacted by the dilution effect of rainwater with lower ph in the rainy season. In contrast, the concentrations of Ca 2, Mg 2,, NO 3 and 2 in groundwater tended to be enriched in rainy season, and the dilution effect of rainfall has not been observed in these ions, suggesting these ions were impacted by some geochemical processes which are different from other seasons. Relationship between (Ca 2 Mg 2 ) and in groundwater As shown in Figure 3a, the molar ratio between (Ca 2 Mg 2) and in the QURS varied from 0.56 to 0.63 with a mean value of 0.60 in different months, which higher molar ratio between (Ca 2 Mg 2) and of groundwater were observed in the rainy season and fertilizing period. As indicated by Equation (1), the molar ratio between (Ca 2 Mg 2 ) and released into the groundwater should be 0.5, suggesting that carbonate dissolution is controlled by natural processes involving carbonic 166 ACTA CARSOLOGICA 45/2 2016
7 The effects of agricultural activities and atmospheric acid deposition on carbonate... Fig. 3: Cross plot of (Ca 2 Mg 2 ) vs. (a) and (Ca 2 Mg 2 ) vs. ( NO 3 2 ) (b) in groundwater. Fig. 2: Seasonal variations of rainfall and hydrogeochemistry of groundwater in acid. However, as indicated by Equation (4), (5) and (6), the molar ratio between (Ca 2 Mg 2 ) and released into the groundwater should be 1, suggesting that carbonate dissolution is governed by sulphuric/nitric acid. These suggested that carbonic acid could probably not be a unique weathering agent in the catchment. Mean- while, as stated in section 4.3, higher concentrations of Ca 2, Mg 2,, NO 3 and 2 were observed the rainy season and fertilizing period, suggesting that among the different sources of protons mentioned in the introduction, fertilizers as well as atmospheric acid deposition may play an important role in the carbonate weathering in the catchment. Therefore, the questions arised whether those elements in groundwater originated from carbonate dissolution by carbonic and sulphuric/nitric acids and to which extent of carbonate dissolution were influenced by different acids in different months. Discussion Atmospheric inputs Compared to groundwater, the rainwater showed very low concentrations of Ca 2, Mg 2 and, however, high concentrations of NO 3 and 2 in the catchment. Therefore, it might be concluded that Ca 2, Mg 2 and supplied by atmospheric inputs are low, but nitrate and sulfate inputs are significant. The highest values of nitrate and sulfate were observed during local fertilizer spreading period, suggesting that they partly originate from fertilizer inputs. Meanwhile, a significant amount ACTA CARSOLOGICA 45/
8 Yu Chen & Yongjun Jiang of NH 4 is supplied to the catchment by rainwater, but NH 4 was not detected in groundwater, which could be presumably nitrified in the catchment by soil bacteria and used by plants. [Ca 2 Mg 2 ] carbonate dissolution by sulphuric acid = [ ] carbonate dissolution by sulphuric acid = 2(Ca 2 Mg 2 ) (HCO ) groundwater 3 groundwater (NO 3 ) groundwater (10) Hydrochemistry : carbonate dissolution by carbonic acid versus sulphuric and nitric sulphuric acids As atmospheric inputs of Ca 2, Mg 2 and are very low, and the silicate weathering originated from sandstone with slow dissolution can be neglected, the concentrations of Ca 2, Mg 2 and were mainly originated from carbonate dissolution in the catchment. If the contributions of carbonic, sulphuric and nitric acids in equimolar amounts to the carbonate dissolution were considered, carbonate dissolution becomes (Eq. 7): 4(Ca 1x Mg x )CO 3 H 2 CO 3 HNO 3 H 2 4(1x)Ca 2 4xMg 2 5 NO 3 2 In this case, the molar ratio between (Ca 2 Mg 2 ) and, and (Ca 2 Mg 2 ) and ( NO 3 SO 0 2 ) in groundwater should be 4/5 (0.8) and 4/7 (0.571). However, the molar ratio between (Ca 2 Mg 2 ) and, (Ca 2 Mg 2 ) and ( NO 3 2 ) in different varied from 0.56 to 0.63 with a mean value of 0.60, and from 0.51 to 0.54 with a mean value of 0.52 (Figs. 3a and 3b), which deviate from the expected the molar ratio of 0.8 and 0.57, respectively, indicating carbonate dissolution in those groundwaters controlled by carbonic, sulphuric and nitric acids in different ratios. Meanwhile, it could be assumed that all NO 3 in groundwater could be derived from the nitrification of fertilizer and atmospheric NH 4, and oxidation of atmospheric NOx, due to other NO 3 sources in the catchment. Thus, based on the (1), (5) and (6), the concentrations of [Ca 2 Mg 2 ] and in groundwater resulted from the carbonate dissolution by carbonic, nitric and sulphuric acids can be calculated by following equations: [Ca 2 Mg 2 ] carbonate dissolution by carbonic acid = 1/2[ ] carbonate dissolution by carbonic acid = (8) [( ) groundwater (Ca 2 Mg 2 )] groundwater [Ca 2 Mg 2 ] carbonate dissolution by nitric acid = [ ] carbonate dissolution by nitric acid = [NO 3 ] groundwater (9) 7) The calculated results are presented in Tab. 2. The contributions of carbonate dissolution by carbonic acid to total concentrations of (Ca 2 Mg 2 ) and of groundwater in different months varied from 57.7 % to 80.4 % with mean percentage of 68.5 %, and from 73.2 % to 89.1 % with a mean percentage of 81 %, respectively. While the contributions of carbonate dissolution by nitric and sulphuric acids to total concentrations of (Ca 2 Mg 2 ) and in different months varied from 6.4 % to 16.8 % with a mean percentage of 11.1 %, and 3.7 % to 10.7 % with a mean percentage of 6.7 %, and from 11.3 % to 29.7 % with a mean percentage of 20.4 % and 6.3 % to 18.8 % with a mean percentage of 12.3 %, respectively. Especially, the contributions of carbonate dissolution by carbonic acid to total concentrations of (Ca 2 Mg 2 ) and averaged only 63 % and 77 %, while the contributions of carbonate dissolution by nitric and sulphuric acids to total concentrations of (Ca 2 Mg 2 ) and averaged 37 % and 23 % in rainy season and fertilizing period (from Apr. to Oct.). Therefore, based on the hydrochemical data, it can be concluded that carbonate dissolution was not only controlled by carbonic acid, but also by sulphuric and nitric acids introduced by the use of N-fertilizers and atmospheric acid deposition in the catchment. The in groundwater: carbonate dissolution by carbonic acid versus nitric and sulphuric acids Because the contribution of atmospheric CO 2 to the DIC was minor due to the high partial pressure of CO 2 in groundwater, the DIC in the catchment had two primary sources, soil CO 2 and carbonate bedrock. The δ 13 C of soil CO 2 and limestone averaged 23.3 and 0.25, respectively. Meanwhile, groundwater showed higher DO (Dissolved oxygen) concentrations in different months, indicating it is an open karst system. In general, the fractionation of the carbon isotope composition was around 9 between soil gas CO 2 and (Deines 2004; Zhang et al. 1995). Therefore, DIC in groundwater originated from carbonate dissolving by CO 2 in the catchment was expected to have value of around 14. While the had identical values to the δ 13 C of carbonate (0.25 ) which DIC was derived from carbonate dissolving by sulfur acid or nitric acid. Thus, the in ground- 168 ACTA CARSOLOGICA 45/2 2016
9 The effects of agricultural activities and atmospheric acid deposition on carbonate... water approaching a value of 14 indicated the DIC resulted from carbonate dissolving by soil CO 2, while the in groundwater varying from 0 to 14 suggested the DIC resulted from carbonate dissolving by soil CO 2, HNO 3 and H 2 in the catchment. As shown in Tab. 2, the varied from 12.4 to 10.1 in different months, which deviate from the expected the (14 ) which DIC is derived from carbonate dissolving by natural soil CO 2, suggesting that the DIC not only resulted from carbonate dissolving by natural soil CO 2, but also from carbonate dissolving by sulfur and nitric acids in the catchment in the QURS. Thus, given the δ 13 C soil CO2 (23.3 ), δ 13 C carbonate (0.25 ) and fractionation of isotopic compositions (9 ) in groundwater system, the theoretical value that DIC was derived from different sources (carbonate dissolution and soil CO 2 ) can be calculated by the following equation: and atmospheric acid deposition could be responsible for the elevated DIC concentrations and in the catchment. Meanwhile, an intense positive relationship between the and (Ca 2 Mg 2 )/ was observed (Fig. 4). The increased obviously with (Ca 2 Mg 2 )/ in groundwater collected from the rainy season and fertilizing period, indicating that the use of N-fertilizers and atmospheric acid deposition could be responsible for the elevated the and the molar ratio of (Ca 2 Mg 2 )/. Also, as indicated by Fig. 4, the varying from 10 to 12.5, with a variational molar ratio between (Ca 2 Mg 2 ) and of 0.53 to 0.63 in different months, indicated the carbonate was (11) where is the theoretical value, mc i is the molality of added DIC from the ith source (carbonate dissolution by carbonic acid and sulphuric/nitric acids, respectively), and the δ 13 C i is δ 13 C composition of added DIC from the ith source (soil CO 2 and carbonate). The calculated results of the theoretical values are shown in Tab. 2. The measured value of monthly groundwater sample is much closed to its corresponding calculated value. Thus, the elevated (varying from 11.1 to 10.3 with an average of 10.6 ) in the rainy season and fertilizing period suggested that carbonate dissolving was significantly influenced by nitric and sulphuric acids originated from the use of N-fertilizers and atmospheric acid deposition in the catchment. As indicated in Fig. 2, the increased with DIC in groundwater collected from the rainy season and fertilizing period, indicating that the use of N-fertilizers Fig. 4: Cross plot of (a) vs. (Ca 2 Mg 2 )/ in different seasons. dissolved by soil CO 2 (from C 3 vegetation), HNO 3 and H 2 in the catchment. Thus, the evidences from both of the chemical data and in different months indicated that carbonate dissolution was controlled by natural carbonic acid, and nitric and sulphuric acids originated from the use of N-fertilizers and atmospheric acid deposition in the QURS. Conclusions Although the results of this study are preliminary and the effects of the use of N-fertilizers and acid deposition on carbonate weathering have been quantified in a small karstic agricultural catchment of Southwest China. The significant temporal variations for major element concentrations and in different months were observed in the QURS. The groundwater collected in rainy season and fertilizing period showed significant high concentrations of DIC, Ca 2, ACTA CARSOLOGICA 45/
10 Yu Chen & Yongjun Jiang Mg 2, NO 3, 2 and. These temporal variations could be attributed to carbonate dissolution by natural carbonic acid, and nitric and sulphuric acids introduced by the use of N-fertilizers and atmospheric acid deposition in the QURS. The contributions of carbonate dissolution by carbonic acid to total concentrations of (Ca 2 Mg 2 ) and in different months averaged 68.5 % and 81 %, respectively. While the contributions of carbonate dissolution by nitric and sulphuric acids originated from the use of N-fertilizers and atmospheric acid deposition to total concentrations of (Ca 2 Mg 2 ) and in different months averaged 11.1 % and 6.7 %, and 20.4 % and 12.3 %, respectively. Especially, the contributions of carbonate dissolution by carbonic acid to total concentrations of (Ca 2 Mg 2 ) and averaged only 63 % and 77 %, while the contributions of carbonate dissolution by nitric and sulphuric acids to total concentrations of (Ca 2 Mg 2 ) and averaged 37 % and 23 % in rainy season and fertilizing period (from Apr. to Oct.). The temporal variations of in groundwater (varying from 12.4 to 10.1 ) in different months deviated from the expected the (14 ) of groundwater which DIC is derived from carbonate dissolving by natural soil CO 2, suggesting that the DIC of groundwater not only resulted from carbonate dissolving by natural soil CO 2, but also from carbonate dissolving by sulfur and nitric acids in the QURS. Meanwhile, the increased obviously with (Ca 2 Mg 2 )/ of groundwater in the rainy season and fertilizing period, indicating that the use of N-fertilizers and atmospheric acid deposition should be responsible for the elevated the and the molar ratio of (Ca 2 Mg 2 )/ of groundwater in the QURS. Thus, the evidences from both of the hydrochemical data and in different months indicated that carbonate dissolution was not only controlled by natural carbonic acid, but also by sulphuric and nitric acids introduced by the use of N-fertilizers and atmospheric acid deposition in the QURS. More important is that not only the concentrations of nitrate and sulphate in karst groundwater have been elevated, but also the exports of inorganic carbon and (Ca 2 Mg 2 ) have been enhanced due to carbonate weathering by nitric and sulphuric acids originated from the use of N-fertilizers and atmospheric acid deposition in the catchment. This work is financially supported by National Natural Science Foundation of China (Grant Nos and ), and IGCP598. Acknowledgments References Ali, H.N. & E.A. Atekwana, 2011: The effect of sulfuric acid neutralization on carbonate and stable carbon isotope evolution of shallow groundwater.- Chemical Geology, 284, org/ /j.chemgeo Atekwana, E.A. & R.V. Krishnamurthy, 1998: Seasonal variations of dissolved inorganic carbon and δ 13 C of surface waters: application of a modified gas evolution technique.- Journal of Hydrology, 205, (98) Barnes, R.T. & P.A. Raymond, 2009: The contribution of agricultural and urban activities to inorganic carbon fluxes within temperate watersheds.- Chemical Geology, 266, 34, org/ /j.chemgeo Calmels, D., Gaillardet, J., Brenot, A. & C. France-Lanord, 2007: Sustained sulfide oxidation by physical erosion processes in the Mackenzie River basin: Climatic perspectives.- Geology, 35, 11, Deines, P., 2004: Carbon isotope effects in carbonate systems.- Geochimica et Cosmochimica Acta, 68, gca ACTA CARSOLOGICA 45/2 2016
11 The effects of agricultural activities and atmospheric acid deposition on carbonate... Dentener, F., Drevet, J., Lamarque, J.F., Bey, I., Eickhout, B., Fiore, A.M., Hauglustaine, D., Horowitz, L.W., Krol, M., Kulshrestha, U.C., Lawrence, M., Galy-Lacaux, C., Rast, S., Shindell, D., Stevenson, D., Noije, T. van, Atherton, C., Bell, N., Bergman, D., Butler, T., Cofala, J., Collins, B., Doherty, R., Ellingsen, K., Galloway, J., Gauss, M., Montanaro, V., Müller, J.F., Pitari, G., Rodriguez, J., Sanderson, M., Solmon, F., Strahan, S., Schultz, M., Sudo, K., Szopa S. & O. Wild, 2006: Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation.- Global Biogeochemical Cycles, 20, GB4003, Gandois, L., Perrin, A.-S. & A. Probst, 2011: Impact of nitrogenous fertiliser-induced proton release on cultivated soils with contrasting carbonate contents: a column experiment.- Geochimica et Cosmochimica Acta, 75, gca He, Q., Yang P., Yuan, W., Jiang, Y., Pu, J., Yuan, D. & Y. Kuang, 2010: The use of nitrate, bacteria and fluorescent tracers to characterize groundwater recharge and contamination in a karst catchment, Chongqing, China.- Hydrogeology Journal, 18, /s Jiang, Y., 2012: Sources of sulfur in the Nandong underground river system, southwest China: A chemical and isotopic reconnaissance.- Applied Geochemistry, 27, 8, apgeochem Jiang, Y., 2013: The contribution of human activities to dissolved inorganic carbon fluxes in a karst underground river system: evidence from major elements and in Nandong, Southwest China.- Journal of Contaminant Hydrology, 152, dx.doi.org/ /j.jconhyd Jiang, Y., Wu, Y., Groves, C., Yuan, D. & P. Kambesis, 2009a: Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China.- Journal of Contaminant Hydrology, 109, dx.doi.org/ /j.jconhyd Jiang, Y., Wu, Y. & D. Yuan, 2009b: Human impacts on karst groundwater contamination deduced by coupled nitrogen with strontium isotopes in the Nandong underground river system in Yunan, China.- Environmental Science & Technology, 43, Jiang, Y., Yuan, D., Zhang, C., Zhang, G. & R. He, 2008: Impact of land use change on groundwater quality in a typical karst watershed of southwest China.- Hydrogeology Journal, 16, 4, dx.doi.org/ /s Li, S., Calmel, D., Han, G., Gaillardet, J. & C. Liu, 2008: Sulfuric acid as an agent of carbonate weathering constrained by : Examples from Southwest China.- Earth and Planetary Science Letters, 270, epsl Li, S., Liu, C., Li, J., Lang, Y., Ding, H. & L. Li, 2010: Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints.- Chemical Geology, 277, Liu, C., Li, S., Lang, Y. & H. Xiao, 2006: Using δ 15 N- and δ 18 O-values to identify nitrate sources in karst ground water, Guiyang, southwest China.- Environmental Science & Technology, 40, 22, Liu, C., Lang, Y., Satake, H., Wu, J. & S. Li, 2008: Identification of anthropogenic and natural inputs of sulfate and chloride into the karstic ground water of Guiyang, SW China: Combined δ 37 Cl and δ 34 S approach.- Environmental Science & Technology, 42, 15, es800380w Paulot, F., Jacob, D.J. & D.K. Henze, 2013: Sources and processes contributing to nitrogen deposition: an adjoint model analysis applied to biodiversity hotspots worldwide.- Environmental Science & Technology, 47, 7, org/ /es Perrin, A.-S., Probst, A. & J.-L. Probst, 2008: Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: implications for weathering CO 2 uptake at regional and global scales.- Geochimica et Cosmochimica Acta, 72, gca Pu, J., Yuan, D., He, Q., Wang, Z., Hu, Z. & P. Gou, 2011: High-resolution monitoring of nitrate variations in a typical subterranean karst stream, Chongqing, China.- Environmental Earth Science, 64, 7, Semhi, K., Suchet, P. A., Clauer, N. & J.-L. Probst, 2000: Impact of nitrogen fertilizers on the natural weathering-erosion processes and fluvial transport in the Garonne basin.- Applied Geochemistry, 15, 6, (99) Tang, J., Xu, X., Ba J. & S. Wang, 2010: Trends of the precipitation acidity over China during Chinese Science Bulletin, 55, 17, dx.doi.org/s ACTA CARSOLOGICA 45/
12 Yu Chen & Yongjun Jiang Yue F.-J., Li S.-L., Liu C.-Q., Lang Y.-C. & H. Ding, 2015: Sources and transport of nitrate constrained by the isotopic technique in a karst catchment: an example from Southwest China.- Hydrological Processes, 29, 8, Zhang, J., Quay, P.D. & D.O. Wilbur, 1995: Carbon isotope fractionation during gas-water exchange and dissolution of CO 2.- Geochimica et Cosmochimica Acta, 59, ACTA CARSOLOGICA 45/2 2016
6 SAMPLING OF WATER QUANTITIES, METHODS, POINTS OF ATTENTION
 6 SAMPLING OF WATER QUANTITIES, METHODS, POINTS OF ATTENTION Water sampling intends to provide information on water quality and quantity, and on hydrodynamic properties of systems under observation. To
6 SAMPLING OF WATER QUANTITIES, METHODS, POINTS OF ATTENTION Water sampling intends to provide information on water quality and quantity, and on hydrodynamic properties of systems under observation. To
!"#$%&'()*+,-./0!"#$%&'()*+
 ===== 7 = 3!"#$% 2011 5 ADVANCES IN CLIMATE CHANGE RESEARCH Vol. 7 No. 3 May 2011!"1673-1719 (2011) 03-0157-05!"#$%&'()*+,-./0!"#$%&'()*+ NIO NIOIP NIOIQ R N=!"#$%&!'()L!"#$%&'()*+,-=RQNMMQ O=!"#$% &'()*+,-=RQNMMQP=!"#!
===== 7 = 3!"#$% 2011 5 ADVANCES IN CLIMATE CHANGE RESEARCH Vol. 7 No. 3 May 2011!"1673-1719 (2011) 03-0157-05!"#$%&'()*+,-./0!"#$%&'()*+ NIO NIOIP NIOIQ R N=!"#$%&!'()L!"#$%&'()*+,-=RQNMMQ O=!"#$% &'()*+,-=RQNMMQP=!"#!
Anthropogenic alteration of dissolved inorganic carbon fluxes from watersheds. Pete Raymond Yale School of Forestry and Environmental Studies
 Anthropogenic alteration of dissolved inorganic carbon fluxes from watersheds Pete Raymond Yale School of Forestry and Environmental Studies River Bicarbonate Bicarbonate (HCO 3- ) is the most abundant
Anthropogenic alteration of dissolved inorganic carbon fluxes from watersheds Pete Raymond Yale School of Forestry and Environmental Studies River Bicarbonate Bicarbonate (HCO 3- ) is the most abundant
Chemical Analysis of Ground Waters in Tabriz Area, Northwestern Iran
 Asian Journal of Chemistry Vol. 21, No. 2 (2009), 1217-1224 Chemical Analysis of Ground Waters in Tabriz Area, Northwestern Iran N. MEHRDADI, A. BAGHVAND, H. ETEMADI, NAVID RAZMKHAH* and M. ESMAEILI BIDHENDI
Asian Journal of Chemistry Vol. 21, No. 2 (2009), 1217-1224 Chemical Analysis of Ground Waters in Tabriz Area, Northwestern Iran N. MEHRDADI, A. BAGHVAND, H. ETEMADI, NAVID RAZMKHAH* and M. ESMAEILI BIDHENDI
Nutrient Cycles. Nutrient cycles involve flow of high quality energy from the sun through the environment & of elements.
 Nutrient Cycles Nutrient cycles (= biogeochemical cycles): natural processes that involve the flow of nutrients from the environment (air, water, soil, rock) to living organisms ( ) & back again. Nutrient
Nutrient Cycles Nutrient cycles (= biogeochemical cycles): natural processes that involve the flow of nutrients from the environment (air, water, soil, rock) to living organisms ( ) & back again. Nutrient
Determination of Recharge area using Environmental Isotopes in Karstified Aquifers in Balisan area, Kurdistan Region, Iraq.
 Omed M. Mustafa M.Sc. Environmental Geology Assistant Lecturer Department of Geology College of Science University of Sulaimani Mobile: 009647701540252 Email: omedgeology@gmail.com omed.mustafa@univsul.net
Omed M. Mustafa M.Sc. Environmental Geology Assistant Lecturer Department of Geology College of Science University of Sulaimani Mobile: 009647701540252 Email: omedgeology@gmail.com omed.mustafa@univsul.net
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Sequestration of Atmospheric Carbon Dioxide as Inorganic Carbon under Semi-Arid Forests
 Sequestration of Atmospheric Carbon Dioxide as Inorganic Carbon under Semi-Arid Forests Murray Moinester, Joel Kronfeld, Israel Carmi Tel Aviv University World Conference on Climate Change, October 24-26,
Sequestration of Atmospheric Carbon Dioxide as Inorganic Carbon under Semi-Arid Forests Murray Moinester, Joel Kronfeld, Israel Carmi Tel Aviv University World Conference on Climate Change, October 24-26,
Isotope Hydrology Investigation of the Pawcatuck Watershed. Principle Investigators. Anne I. Veeger
 Isotope Hydrology Investigation of the Pawcatuck Watershed Principle Investigators Anne I. Veeger Introduction There are three new research projects for the FY1999 annual research program for the Water
Isotope Hydrology Investigation of the Pawcatuck Watershed Principle Investigators Anne I. Veeger Introduction There are three new research projects for the FY1999 annual research program for the Water
Nutrient Cycling in an Aquatic Ecosystem
 Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Nutrient Cycling in an Aquatic Ecosystem 2.1 Productivity 2.2 Oxygen 2.3 Salinity 2.4 Carbon 2.5 Nitrogen 2.6 Phosphorous 2.7 Iron 2.8 Sulphur 2.9 Silica 2.3 Salinity of Inland Waters The salinity of freshwaters
Ch. 5 - Nutrient Cycles and Soils
 Ch. 5 - Nutrient Cycles and Soils What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient
Ch. 5 - Nutrient Cycles and Soils What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient
Isotopes in groundwater management IAEA
 Isotopes in groundwater management The arid zone Rainfall is low or intermittent and unreliable The need for water research is greatest because of water scarcity and growth in world population The arid
Isotopes in groundwater management The arid zone Rainfall is low or intermittent and unreliable The need for water research is greatest because of water scarcity and growth in world population The arid
CONTRASTING STREAM WATER NO 3 - AND CA 2+ IN TWO NEARLY ADJACENT CATCHMENTS: THE ROLE OF SOIL CA AND FOREST VEGETATION
 CONTRASTING STREAM WATER NO 3 - AND CA 2+ IN TWO NEARLY ADJACENT CATCHMENTS: THE ROLE OF SOIL CA AND FOREST VEGETATION By: Sheila F Christopher et al. Presented by: Hannah Scholes Outline Introduction
CONTRASTING STREAM WATER NO 3 - AND CA 2+ IN TWO NEARLY ADJACENT CATCHMENTS: THE ROLE OF SOIL CA AND FOREST VEGETATION By: Sheila F Christopher et al. Presented by: Hannah Scholes Outline Introduction
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
ASSESSMENT OF WATER RISE PROBLEM USING ENVIRONMENTAL ISOTOPES AT AL-QURAIN RESIDENTIAL AREA, KUWAIT
 ASSESSMENT OF WATER RISE PROBLEM USING ENVIRONMENTAL ISOTOPES AT AL-QURAIN RESIDENTIAL AREA, KUWAIT H. Bhandary 1, K. Al-Fahad 2, and M. Al-Senafy 3 1 Water Research Center Kuwait Institute for Scientific
ASSESSMENT OF WATER RISE PROBLEM USING ENVIRONMENTAL ISOTOPES AT AL-QURAIN RESIDENTIAL AREA, KUWAIT H. Bhandary 1, K. Al-Fahad 2, and M. Al-Senafy 3 1 Water Research Center Kuwait Institute for Scientific
Impact of Human Activity on the Groundwater Chemical Composition of the South Part of the Poyang Lake Basin
 Available online at www.sciencedirect.com ScienceDirect IERI Procedia 8 (2014 ) 113 118 2014 International Conference on Agricultural and Biosystem Engineering Impact of Human Activity on the Groundwater
Available online at www.sciencedirect.com ScienceDirect IERI Procedia 8 (2014 ) 113 118 2014 International Conference on Agricultural and Biosystem Engineering Impact of Human Activity on the Groundwater
Where does the nitrogen go? Christopher Kelley Washington State University School of the Environment
 Where does the nitrogen go? Christopher Kelley Washington State University School of the Environment Outline My background Research in Eastern Washington o o Background Previous research on nitrate leaching
Where does the nitrogen go? Christopher Kelley Washington State University School of the Environment Outline My background Research in Eastern Washington o o Background Previous research on nitrate leaching
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
CHAPTER 6 GROUNDWATER HYDROCHEMISTRY AND HYDROCHEMICAL PROCESSES
 69 CHAPTER 6 GROUNDWATER HYDROCHEMISTRY AND HYDROCHEMICAL PROCESSES 6.1 GENERAL Groundwater contains a wide range of dissolved solids and contain small amount of dissolved organic matter and gases. Groundwater,
69 CHAPTER 6 GROUNDWATER HYDROCHEMISTRY AND HYDROCHEMICAL PROCESSES 6.1 GENERAL Groundwater contains a wide range of dissolved solids and contain small amount of dissolved organic matter and gases. Groundwater,
NUTRIENT CYCLES REVIEW
 52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
Movement and Storage of Groundwater The Hydrosphere
 Movement and Storage of Groundwater The Hydrosphere The water on and in Earth s crust makes up the hydrosphere. About 97 percent of the hydrosphere is contained in the oceans. The water contained by landmasses
Movement and Storage of Groundwater The Hydrosphere The water on and in Earth s crust makes up the hydrosphere. About 97 percent of the hydrosphere is contained in the oceans. The water contained by landmasses
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 18 September 2012 Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 18 September 2012 Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions
NGUYEN THU THUY (M2) Supervisor: Prof., Maki Tsujimura
 I. Literature review II. Introduction III. Method and Objectives IV. Results and Discussion V. Conclusion NGUYEN THU THUY (M2) Supervisor: Prof., Maki Tsujimura I. Literature review: Water resource issues
I. Literature review II. Introduction III. Method and Objectives IV. Results and Discussion V. Conclusion NGUYEN THU THUY (M2) Supervisor: Prof., Maki Tsujimura I. Literature review: Water resource issues
Analyses for geochemical investigations traditionally report concentrations as weight per volume of the measured ions (mg/l of NO 3 , NO 2
 Nitrate-Nitrogen 55 Nutrients The nutrients nitrogen and phosphorus occur naturally and also may be introduced to groundwater systems from urban and agricultural fertilizer applications, livestock or human
Nitrate-Nitrogen 55 Nutrients The nutrients nitrogen and phosphorus occur naturally and also may be introduced to groundwater systems from urban and agricultural fertilizer applications, livestock or human
Ecosystems and Nutrient Cycles Chapters 3
 Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Journal of Chemical and Pharmaceutical Research, 2013, 5(11): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 13, (11):8388 Research Article ISSN : 97738 CODEN(USA) : JCPRC Hydrochemical characteristics and evolution laws of shallow
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 13, (11):8388 Research Article ISSN : 97738 CODEN(USA) : JCPRC Hydrochemical characteristics and evolution laws of shallow
The influence of urbanization on karst rivers based on nutrient concentration and nitrate dual isotopes: an example from southwestern China
 Acta Geochim (2017) 36(3):446 451 DOI 10.1007/s11631-017-0188-9 ORIGINAL ARTICLE The influence of urbanization on karst rivers based on nutrient concentration and nitrate dual isotopes: an example from
Acta Geochim (2017) 36(3):446 451 DOI 10.1007/s11631-017-0188-9 ORIGINAL ARTICLE The influence of urbanization on karst rivers based on nutrient concentration and nitrate dual isotopes: an example from
Water Resources on PEI: an overview and brief discussion of challenges
 Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
Elements essential for life also cycle through ecosystems.
 13.5 Cycling of Matter KEY CONCEPT Matter cycles in and out of an ecosystem. MAIN IDEAS Water cycles through the environment. Elements essential for life also cycle through ecosystems. VOCABULARY hydrologic
13.5 Cycling of Matter KEY CONCEPT Matter cycles in and out of an ecosystem. MAIN IDEAS Water cycles through the environment. Elements essential for life also cycle through ecosystems. VOCABULARY hydrologic
TRACING SOURCES OF POLLUTION IN GROUNDWATER USING HYDROCHEMICAL AND ISOTOPIC METHODS: BEIRUT AND ITS SUBURBS
 JOURNAL OF ENVIRONMENTAL HYDROLOGY The Electronic Journal of the International Association for Environmental Hydrology On the World Wide Web at http://www.hydroweb.com VOLUME 16 28 TRACING SOURCES OF POLLUTION
JOURNAL OF ENVIRONMENTAL HYDROLOGY The Electronic Journal of the International Association for Environmental Hydrology On the World Wide Web at http://www.hydroweb.com VOLUME 16 28 TRACING SOURCES OF POLLUTION
Hydrogeologic Monitoring at University of Minnesota Outreach, Research and Educational Park (UMore Park), 2011
 Hydrogeologic Monitoring at University of Minnesota Outreach, Research and Educational Park (UMore Park), 2011 Joel T. Groten Dr. E. Calvin Alexander, Jr. January 2012 University of Minnesota Twin Cities
Hydrogeologic Monitoring at University of Minnesota Outreach, Research and Educational Park (UMore Park), 2011 Joel T. Groten Dr. E. Calvin Alexander, Jr. January 2012 University of Minnesota Twin Cities
What is Hydrogeology?
 What is Hydrogeology? It is the comprehensive study of groundwater, its distribution and evolution through time and space, under regional geology. Geohydrology studies water behavior in geological environment,
What is Hydrogeology? It is the comprehensive study of groundwater, its distribution and evolution through time and space, under regional geology. Geohydrology studies water behavior in geological environment,
CHAPTER 6: GEOCHEMICAL CYCLES Daniel J. Jacob, Atmospheric Chemistry, Harvard University, Spring 2017
 CHAPTER 6: GEOCHEMICAL CYCLES Daniel J. Jacob, Atmospheric Chemistry, Harvard University, Spring 2017 THE EARTH: ASSEMBLAGE OF ATOMS OF THE 92 NATURAL ELEMENTS Most abundant elements: oxygen (in solid
CHAPTER 6: GEOCHEMICAL CYCLES Daniel J. Jacob, Atmospheric Chemistry, Harvard University, Spring 2017 THE EARTH: ASSEMBLAGE OF ATOMS OF THE 92 NATURAL ELEMENTS Most abundant elements: oxygen (in solid
Evaluation copy. Total Dissolved Solids. Computer INTRODUCTION
 Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
13.5. Cycling of Matter. Water cycles through the environment.
 13.5 Cycling of Matter VOCABULARY hydrologic cycle biogeochemical cycle nitrogen fixation KEY CONCEPT Matter cycles in and out of an ecosystem. Main Ideas Water cycles through the environment. Elements
13.5 Cycling of Matter VOCABULARY hydrologic cycle biogeochemical cycle nitrogen fixation KEY CONCEPT Matter cycles in and out of an ecosystem. Main Ideas Water cycles through the environment. Elements
Impairment Issues in the Ichetucknee Springs Basin Potential Research Questions and Hypotheses
 Impairment Issues in the Ichetucknee Springs Basin Potential Research Questions and Hypotheses Brian G. Katz U.S. Geological Survey Ichetucknee Springs Research Meeting February 10, 2011 Sources of hydrologic
Impairment Issues in the Ichetucknee Springs Basin Potential Research Questions and Hypotheses Brian G. Katz U.S. Geological Survey Ichetucknee Springs Research Meeting February 10, 2011 Sources of hydrologic
Assessment of Groundwater Quality in and around Neyveli Lignite Mines using GIS and Water Quality Index, Cuddalore District, Tamil Nadu
 Volume 3, Issue 7 December 2014 311 RESEARCH ARTICLE ISSN: 2278-5213 Assessment of Groundwater Quality in and around Neyveli Lignite Mines using GIS and Water Quality Index, Cuddalore District, Tamil Nadu
Volume 3, Issue 7 December 2014 311 RESEARCH ARTICLE ISSN: 2278-5213 Assessment of Groundwater Quality in and around Neyveli Lignite Mines using GIS and Water Quality Index, Cuddalore District, Tamil Nadu
CHEMICAL COMPOSITION OF NATURAL WATERS
 CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
The Comparative Studies on Seasonal Variations of Chemical Fertilizer Residues and Physico-Chemical Characteristics in Different Water Samples
 The Comparative Studies on Seasonal Variations of Chemical Fertilizer Residues and Physico- Department of Studies in Environmental Science, University of Mysore, Mysore-570 006, Karnataka, India, corresponding
The Comparative Studies on Seasonal Variations of Chemical Fertilizer Residues and Physico- Department of Studies in Environmental Science, University of Mysore, Mysore-570 006, Karnataka, India, corresponding
Water Quality Index For Assessment Of Water Quality In South Chennai Coastal Aquifer, Tamil Nadu, India
 International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.4, No.4, pp 1582-1588, Oct-Dec 2012 Water Quality Index For Assessment Of Water Quality In South Chennai Coastal Aquifer,
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.4, No.4, pp 1582-1588, Oct-Dec 2012 Water Quality Index For Assessment Of Water Quality In South Chennai Coastal Aquifer,
Nutrient Cycling & Soils
 Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Lecture 17 Guest Lecturer this week. Prof. Greg Ravizza
 Lecture 17 Guest Lecturer this week. Prof. Greg Ravizza Migrating Reservoirs in the Hydrologic Cycle - Inorganic solubility examples from Rain and River chemistry Migrating Reservoirs in the Hydrologic
Lecture 17 Guest Lecturer this week. Prof. Greg Ravizza Migrating Reservoirs in the Hydrologic Cycle - Inorganic solubility examples from Rain and River chemistry Migrating Reservoirs in the Hydrologic
2.2 Nutrient Cycles in Ecosystems
 2.2 Nutrient Cycles in Ecosystems CARBON CYCLE A. Carbon Facts: Carbon is found in all living matter. Places that carbon is found are called stores or sinks Short-term Stores Long-term Stores - living
2.2 Nutrient Cycles in Ecosystems CARBON CYCLE A. Carbon Facts: Carbon is found in all living matter. Places that carbon is found are called stores or sinks Short-term Stores Long-term Stores - living
Ecosystems: Nutrient Cycles
 Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Natural Ecosystem Change
 Environmental Science Set 3 of 9 Natural Ecosystem Change Presentation MEDIA Version 2 BIOZONE International 2009, 2013 Processes in Carbon Cycling Carbon cycles between the living (biotic) and non-living
Environmental Science Set 3 of 9 Natural Ecosystem Change Presentation MEDIA Version 2 BIOZONE International 2009, 2013 Processes in Carbon Cycling Carbon cycles between the living (biotic) and non-living
Discharge and Geochemical Characteristics of Evey Canyon and Icehouse. Canyon Springs, San Gabriel Mountains, During Extended Drought.
 1 Discharge and Geochemical Characteristics of Evey Canyon and Icehouse Canyon Springs, San Gabriel Mountains, During Extended Drought Paula Soto California State Polytechnic University, Pomona Internship
1 Discharge and Geochemical Characteristics of Evey Canyon and Icehouse Canyon Springs, San Gabriel Mountains, During Extended Drought Paula Soto California State Polytechnic University, Pomona Internship
Evaluating acid rock drainage from road cuts in Tennessee
 Evaluating acid rock drainage from road cuts in Tennessee Michael Bradley, U.S. Geological Survey In cooperation with Tennessee Department of Transportation 14th Annual Technical Forum Geohazards Impacting
Evaluating acid rock drainage from road cuts in Tennessee Michael Bradley, U.S. Geological Survey In cooperation with Tennessee Department of Transportation 14th Annual Technical Forum Geohazards Impacting
The Global Nitrogen Cycle, and Linkages Between C, N, and P Cycles
 OCN 401 The Global Nitrogen Cycle, and Linkages Between C, N, and P Cycles (12.1.11) The Contemporary N Cycle - Basic Facts - Reservoirs and Fluxes Global N and P Budgets - balance between N-fixation and
OCN 401 The Global Nitrogen Cycle, and Linkages Between C, N, and P Cycles (12.1.11) The Contemporary N Cycle - Basic Facts - Reservoirs and Fluxes Global N and P Budgets - balance between N-fixation and
Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p
 OCN 401-Biogeochemical Systems Lecture #12 (10.8.13) Angelos Hannides, hannides@hawaii.edu Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p. 288-308 1. Physical
OCN 401-Biogeochemical Systems Lecture #12 (10.8.13) Angelos Hannides, hannides@hawaii.edu Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p. 288-308 1. Physical
Total Dissolved Solids
 Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
2.2 Nutrient Cycles in Ecosystems. Review How energy flows What is the difference between a food chain, food web, and food pyramid?
 2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
Hydrogeology. Konza LTER Workshop 1 September 2007 Gwen Macpherson
 Hydrogeology Konza LTER Workshop 1 September 2007 Gwen Macpherson What We ve Learned Water flux Aquifer-atmosphere link Stream-aquifer interactions Chemistry Aquifer-Atmosphere Link up to 33% of evapotranspiration
Hydrogeology Konza LTER Workshop 1 September 2007 Gwen Macpherson What We ve Learned Water flux Aquifer-atmosphere link Stream-aquifer interactions Chemistry Aquifer-Atmosphere Link up to 33% of evapotranspiration
5/6/2015. Matter is recycled within and between ecosystems.
 Biogeochemical Cycles/ Nutrient Cycles Biogeochemical Cycle Evaporation Water Cycle Transpiration Condensation Precipitation Runoff Vocabulary Seepage Root Uptake Carbon Cycle Phosphorus Cycle Nitrogen
Biogeochemical Cycles/ Nutrient Cycles Biogeochemical Cycle Evaporation Water Cycle Transpiration Condensation Precipitation Runoff Vocabulary Seepage Root Uptake Carbon Cycle Phosphorus Cycle Nitrogen
7.014 Lecture 20: Biogeochemical Cycles April 1, 2007
 Global Nutrient Cycling - Biogeochemical Cycles 7.14 Lecture 2: Biogeochemical Cycles April 1, 27 Uptake Bioelements in Solution Weathering Precipitation Terrestrial Biomass Decomposition Volatile Elements
Global Nutrient Cycling - Biogeochemical Cycles 7.14 Lecture 2: Biogeochemical Cycles April 1, 27 Uptake Bioelements in Solution Weathering Precipitation Terrestrial Biomass Decomposition Volatile Elements
Nitrogen Pollution and its Impacts
 Nitrogen Pollution and its Impacts OUTLINE: Background forms and cycling Sources Cycling, transport dynamics and loadings from watersheds Landuse and N exports Management Options to reduce N Effects and
Nitrogen Pollution and its Impacts OUTLINE: Background forms and cycling Sources Cycling, transport dynamics and loadings from watersheds Landuse and N exports Management Options to reduce N Effects and
The Global Nitrogen Cycle
 OCN 401 The Global Nitrogen Cycle (11.30.10) Fig. 12.2. Units are 10 12 g N/yr (Tg) Role of N in Biogeochemistry Bioavailability of N (and/or P) can limit NPP on land/oceans; controls size of biomass N
OCN 401 The Global Nitrogen Cycle (11.30.10) Fig. 12.2. Units are 10 12 g N/yr (Tg) Role of N in Biogeochemistry Bioavailability of N (and/or P) can limit NPP on land/oceans; controls size of biomass N
Lakes, Primary Production, Budgets and Cycling
 OCN 401-Biogeochemical Systems Lecture #10 (9.22.11) Lakes, Primary Production, Budgets and Cycling (Schlesinger: Chapter 7) 1. Primary Production and Nutrient Cycling in Lakes Physical aspects and nomenclature
OCN 401-Biogeochemical Systems Lecture #10 (9.22.11) Lakes, Primary Production, Budgets and Cycling (Schlesinger: Chapter 7) 1. Primary Production and Nutrient Cycling in Lakes Physical aspects and nomenclature
Guide 34. Ecosystem Ecology: Energy Flow and Nutrient Cycles. p://www.mordantorange.com/blog/archives/comics_by_mike_bannon/mordant_singles/0511/
 Guide 34 Ecosystem Ecology: Energy Flow and Nutrient Cycles p://www.mordantorange.com/blog/archives/comics_by_mike_bannon/mordant_singles/0511/ Overview: Ecosystems, Energy, and Matter An ecosystem consists
Guide 34 Ecosystem Ecology: Energy Flow and Nutrient Cycles p://www.mordantorange.com/blog/archives/comics_by_mike_bannon/mordant_singles/0511/ Overview: Ecosystems, Energy, and Matter An ecosystem consists
2.2 Nutrient Cycles in Ecosystems
 2.2 Nutrient Cycles in Ecosystems are chemicals required for growth and other life processes. Nutrients move through the biosphere in Nutrients often accumulate in areas called Without interference, generally
2.2 Nutrient Cycles in Ecosystems are chemicals required for growth and other life processes. Nutrients move through the biosphere in Nutrients often accumulate in areas called Without interference, generally
The role of urban forest to reduce rain acid in urban industrial areas
 IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS The role of urban forest to reduce rain acid in urban industrial areas To cite this article: B Slamet et al 2018 IOP Conf. Ser.:
IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS The role of urban forest to reduce rain acid in urban industrial areas To cite this article: B Slamet et al 2018 IOP Conf. Ser.:
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 23 September 2014 Reading: Schlesinger & Bernhardt, Chapter 6 2014 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 23 September 2014 Reading: Schlesinger & Bernhardt, Chapter 6 2014 Frank Sansone 1. Nitrogen cycle Soil nitrogen
ENVE203 Environmental Engineering Ecology (Oct 08, 2012)
 ENVE203 Environmental Engineering Ecology (Oct 08, 2012) Elif Soyer Ecosystem and Physical Environment Cycling of Materials within Ecosystems Energy flows in one direction through an ecosystem Matter moves
ENVE203 Environmental Engineering Ecology (Oct 08, 2012) Elif Soyer Ecosystem and Physical Environment Cycling of Materials within Ecosystems Energy flows in one direction through an ecosystem Matter moves
Irrigation. Branch. Groundwater Quality in the Battersea Drainage Basin
 AGRICULTURE, FOOD AND RURAL DEVELOPMENT Irrigation Branch Groundwater Quality in the Battersea Drainage Basin Groundwater quality in the Battersea drainage basin was monitored between 1995 and 21 to characterize
AGRICULTURE, FOOD AND RURAL DEVELOPMENT Irrigation Branch Groundwater Quality in the Battersea Drainage Basin Groundwater quality in the Battersea drainage basin was monitored between 1995 and 21 to characterize
Geochemical Cycles, Aerosol
 Geochemical Cycles, Aerosol Geochemical cycles: Reservoirs and exchange Carbon Nitrogen Aerosol: What is an aerosol? Sources and sinks of aerosol particles Description of the atmospheric aerosol Literature
Geochemical Cycles, Aerosol Geochemical cycles: Reservoirs and exchange Carbon Nitrogen Aerosol: What is an aerosol? Sources and sinks of aerosol particles Description of the atmospheric aerosol Literature
Ecosystems. Trophic relationships determine the routes of energy flow and chemical cycling in ecosystems.
 AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania
 Hardness Comparisons Hardness Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania 16335. Grade Level: all Duration: 50 minutes Setting:
Hardness Comparisons Hardness Adapted from: An original Creek Connections activity. Creek Connections, Box 10, Allegheny College, Meadville, Pennsylvania 16335. Grade Level: all Duration: 50 minutes Setting:
The Snapshot CONODOGUINET CREEK WATERSHED SNAPSHOT
 CONODOGUINET CREEK WATERSHED SNAPSHOT ABOVE: CONODOGUINET CREEK AT RT 74 BRIDGE FACING DOWNSTREAM The Snapshot The Conodoguinet Watershed Snapshot was a collaborative effort to engage local citizens in
CONODOGUINET CREEK WATERSHED SNAPSHOT ABOVE: CONODOGUINET CREEK AT RT 74 BRIDGE FACING DOWNSTREAM The Snapshot The Conodoguinet Watershed Snapshot was a collaborative effort to engage local citizens in
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 20 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2016 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 20 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2016 Frank Sansone 1. Nitrogen cycle Soil nitrogen
CO 2 (g) + H 2 O = H 2 CO 3 log K H = HCO 3 log K 1 = HCO - 3 = H CO 3 log K 2 = -9.0
 Ocean 400 Chemical Oceanography Winter 2006 Your Name Final Exam Read all questions carefully before you begin to answer. Use the back of the pages if necessary. Points are assigned to each question in
Ocean 400 Chemical Oceanography Winter 2006 Your Name Final Exam Read all questions carefully before you begin to answer. Use the back of the pages if necessary. Points are assigned to each question in
River transport and chemistry. Lecture Outline
 OCN 401 Biogeochemical Systems (10.12.17) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
OCN 401 Biogeochemical Systems (10.12.17) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
Objectives: Define the term biogeochemical cycles. Compare and contrast how carbon, phosphorus, nitrogen, and water cycle through the environment.
 Objectives: Define the term biogeochemical cycles. Compare and contrast how carbon, phosphorus, nitrogen, and water cycle through the environment. Explain how human impact is affecting biogeochemical cycles
Objectives: Define the term biogeochemical cycles. Compare and contrast how carbon, phosphorus, nitrogen, and water cycle through the environment. Explain how human impact is affecting biogeochemical cycles
1. Where are nutrients accumulated or stored for short or long periods?
 Use with textbook pages 68 87. Nutrient cycles Answer the questions below. Comprehension 1. Where are nutrients accumulated or stored for short or long periods? 2. Name a biotic process and an abiotic
Use with textbook pages 68 87. Nutrient cycles Answer the questions below. Comprehension 1. Where are nutrients accumulated or stored for short or long periods? 2. Name a biotic process and an abiotic
Afternoon Lecture Outline. Northern Prairie Hydrology
 Afternoon Lecture Outline 1. Northern Prairies watershed hydrology 2. Solute mass balance in lakes and ponds 3. Simple mass balance simulation using MS Excel 4. Effects of sediment-water exchange on lake
Afternoon Lecture Outline 1. Northern Prairies watershed hydrology 2. Solute mass balance in lakes and ponds 3. Simple mass balance simulation using MS Excel 4. Effects of sediment-water exchange on lake
A study of surface water and groundwater using isotopes in Huaishahe basin in Beijing, China
 106 Sustainability of Groundwater Resources and its Indicators (Proceedings of symposium S3 held during the Seventh IAHS Scientific Assembly at Foz do Iguaçu, Brazil, April 2005). IAHS Publ. 302, 2006.
106 Sustainability of Groundwater Resources and its Indicators (Proceedings of symposium S3 held during the Seventh IAHS Scientific Assembly at Foz do Iguaçu, Brazil, April 2005). IAHS Publ. 302, 2006.
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 19 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2017 Frank Sansone Outline 1. Nitrogen cycle
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 19 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2017 Frank Sansone Outline 1. Nitrogen cycle
Afternoon Lecture Outline. Northern Prairie Hydrology
 Afternoon Lecture Outline 1. Northern Prairies watershed hydrology 2. Solute mass balance in lakes and ponds 3. Simple mass balance simulation using MS Excel 4. Effects of sediment-water exchange on lake
Afternoon Lecture Outline 1. Northern Prairies watershed hydrology 2. Solute mass balance in lakes and ponds 3. Simple mass balance simulation using MS Excel 4. Effects of sediment-water exchange on lake
Radiocarbon Dating, non-bone samples
 Radiocarbon Dating, non-bone samples Standard 15 20 days 7 days University System of Georgia & Emory University Full Processing & $450 $800 $250 Prepared Samples (combustion only) $400 $600 $200 CO2 Gas
Radiocarbon Dating, non-bone samples Standard 15 20 days 7 days University System of Georgia & Emory University Full Processing & $450 $800 $250 Prepared Samples (combustion only) $400 $600 $200 CO2 Gas
Biogeochemical Cycles. {Living World
 Biogeochemical Cycles {Living World What Sustains Life on Earth? Solar energy, the cycling of matter, and gravity sustain the earth s life. Earth's Spheres Atmosphere layer of air that surrounds the Earth
Biogeochemical Cycles {Living World What Sustains Life on Earth? Solar energy, the cycling of matter, and gravity sustain the earth s life. Earth's Spheres Atmosphere layer of air that surrounds the Earth
WATER AND NUTRIENT BALANCES FOR THE TWIN FALLS IRRIGATION TRACT
 WATER AND NUTRIENT BALANCES FOR THE TWIN FALLS IRRIGATION TRACT D. Bjorneberg 1, D. Westermann 1 and N. Nelson 2 1 USDA ARS, Kimberly, ID 2 Kansas State University ABSTRACT Surface water return flow from
WATER AND NUTRIENT BALANCES FOR THE TWIN FALLS IRRIGATION TRACT D. Bjorneberg 1, D. Westermann 1 and N. Nelson 2 1 USDA ARS, Kimberly, ID 2 Kansas State University ABSTRACT Surface water return flow from
The Carbon cycle. Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon
 The Carbon cycle Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon The oceans store much more carbon than the atmosphere and the terrestrial biosphere The oceans essentially
The Carbon cycle Atmosphere, terrestrial biosphere and ocean are constantly exchanging carbon The oceans store much more carbon than the atmosphere and the terrestrial biosphere The oceans essentially
Lecture 18. Soil Acidity, Alkalinity, and Socidity
 Lecture 18 Soil Acidity, Alkalinity, and Socidity 1 Questions ow can acidification occur in soils? ow does p affects availability of N, P, K? ow can acidic soils be managed? Define a saline and sodic soil.
Lecture 18 Soil Acidity, Alkalinity, and Socidity 1 Questions ow can acidification occur in soils? ow does p affects availability of N, P, K? ow can acidic soils be managed? Define a saline and sodic soil.
ScienceDirect. Shallow hydrogeological and hydrochemical characterization of the Aquistore CO 2 sequestration site in Estevan, Saskatchewan, Canada
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 4971 4976 GHGT-12 Shallow hydrogeological and hydrochemical characterization of the Aquistore CO 2 sequestration site
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 4971 4976 GHGT-12 Shallow hydrogeological and hydrochemical characterization of the Aquistore CO 2 sequestration site
WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein!
 Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
USING CERIUM OXIDES AS CATALYSTS FOR THE ABATAMENT OF TRICHLOROETHYLENE BY PLASMA-CATALYSIS ROUTE
 USING CERIUM OXIDES AS CATALYSTS FOR THE ABATAMENT OF TRICHLOROETHYLENE BY PLASMA-CATALYSIS ROUTE Arne M. Vandenbroucke 1, Manuel Mora 2, C. Jimenéz-Sanchidrián 2, Francisco Romero- Salguero 2, Nathalie
USING CERIUM OXIDES AS CATALYSTS FOR THE ABATAMENT OF TRICHLOROETHYLENE BY PLASMA-CATALYSIS ROUTE Arne M. Vandenbroucke 1, Manuel Mora 2, C. Jimenéz-Sanchidrián 2, Francisco Romero- Salguero 2, Nathalie
Fate of Nitrogen in a Ponded Recharge Basin
 Fate of Nitrogen in a Ponded Recharge Basin Michelle E. Pizzulli Gilbert N. Hanson Department of Geosciences SUNY Stony Brook, NY 11794-2100 Introduction In developed areas on Long Island it is estimated
Fate of Nitrogen in a Ponded Recharge Basin Michelle E. Pizzulli Gilbert N. Hanson Department of Geosciences SUNY Stony Brook, NY 11794-2100 Introduction In developed areas on Long Island it is estimated
Chapter 4 Biogeochemical Cycles
 Chapter 4 Biogeochemical Cycles ENERGY FLOW THROUGH ECOSYSTEMS Nature s Building Blocks Matter Energy Laws of Nature Earth s Major Components Ecosystems Ecology and biodiversity Organisms Components and
Chapter 4 Biogeochemical Cycles ENERGY FLOW THROUGH ECOSYSTEMS Nature s Building Blocks Matter Energy Laws of Nature Earth s Major Components Ecosystems Ecology and biodiversity Organisms Components and
Reducing Ammonia Emissions from Agriculture
 Knowledge grows Reducing Ammonia Emissions from Agriculture PURE NUTRIENT Ammonia Emissions Clean Air, Strong Crops Ammonia lost to the air is nitrogen lost for plant growth. At the same time, ammonia
Knowledge grows Reducing Ammonia Emissions from Agriculture PURE NUTRIENT Ammonia Emissions Clean Air, Strong Crops Ammonia lost to the air is nitrogen lost for plant growth. At the same time, ammonia
CHAPTER 13 OUTLINE The Hydrologic Cycle and Groundwater. Hydrologic cycle. Hydrologic cycle cont.
 CHAPTER 13 OUTLINE The Hydrologic Cycle and Groundwater Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Hydrologic cycle The hydrologic cycle is
CHAPTER 13 OUTLINE The Hydrologic Cycle and Groundwater Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. Hydrologic cycle The hydrologic cycle is
The modification of global atmospheric Nitrogen cycling by human activities. David Fowler Centre for Ecology and Hydrology Edinburgh UK
 The modification of global atmospheric Nitrogen cycling by human activities David Fowler Centre for Ecology and Hydrology Edinburgh UK Background The N cycle Global N emissions Oxidised Nitrogen Reduced
The modification of global atmospheric Nitrogen cycling by human activities David Fowler Centre for Ecology and Hydrology Edinburgh UK Background The N cycle Global N emissions Oxidised Nitrogen Reduced
How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package
 How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package Bradley J. Austin, Dirk Philipp, Mike Daniels, and Brian E. Haggard Arkansas Water Resources Center University
How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package Bradley J. Austin, Dirk Philipp, Mike Daniels, and Brian E. Haggard Arkansas Water Resources Center University
Challenges for the sustainable management of urban water supply and sanitation systems Case of the Thiaroye aquifer
 Challenges for the sustainable management of urban water supply and sanitation systems Case of the Thiaroye aquifer Prof. Cheikh B. Gaye G-WADI Global Conference G-WADI more than a decade enhancing water
Challenges for the sustainable management of urban water supply and sanitation systems Case of the Thiaroye aquifer Prof. Cheikh B. Gaye G-WADI Global Conference G-WADI more than a decade enhancing water
Afternoon Lecture Outline
 Afternoon Lecture Outline 1. Northern Prairies watershed hydrology 2. Solute mass balance in lakes and ponds 3. Simple mass balance simulation using MS Excel 4. Effects of sediment-water exchange on lake
Afternoon Lecture Outline 1. Northern Prairies watershed hydrology 2. Solute mass balance in lakes and ponds 3. Simple mass balance simulation using MS Excel 4. Effects of sediment-water exchange on lake
River transport and chemistry
 OCN 401 Biogeochemical Systems (10.15.15) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction - Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
OCN 401 Biogeochemical Systems (10.15.15) (Schlesinger & Bernhardt: Chapter 8) River transport and chemistry Lecture Outline 1. Introduction - Overview 2. Soil Hydraulics & Stream Hydrology 3. Stream Load
Assessment of origin and quality of water by a multi-isotope approach
 Assessment of origin and quality of water by a multi-isotope approach Gerhard Strauch Khalid Al-Mashaikhi, Thomas Müller, Karsten Osenbrück and colleagues from Depts Hydrogeology and Isotope Hydrology
Assessment of origin and quality of water by a multi-isotope approach Gerhard Strauch Khalid Al-Mashaikhi, Thomas Müller, Karsten Osenbrück and colleagues from Depts Hydrogeology and Isotope Hydrology
12 ph. Figure 4. Cumulative plot of ph values from BMU 1. Table 4. Summary of ph values (standard ph units). SMCL: 6.5 to 8.5.
 12 ph Table 4. Summary of ph values (standard ph units). SMCL: 6.5 to 8.5. BMU 1 BMU 2 BMU 5 Values 2,005 778 1,605 Maximum 11.6 8.6 10.4 75th percentile 7.5 7.7 7.4 Median 7.1 7.4 6.9 25th percentile
12 ph Table 4. Summary of ph values (standard ph units). SMCL: 6.5 to 8.5. BMU 1 BMU 2 BMU 5 Values 2,005 778 1,605 Maximum 11.6 8.6 10.4 75th percentile 7.5 7.7 7.4 Median 7.1 7.4 6.9 25th percentile
The Green Revolution
 The Green Revolution Since the 1950s, most increases in global food production have come from increased yields per unit area of cropland. This green revolution has been brought about through the development
The Green Revolution Since the 1950s, most increases in global food production have come from increased yields per unit area of cropland. This green revolution has been brought about through the development
A FIELD DEMONSTRATION OF AN ALTERNATIVE COAL WASTE DISPOSAL TECHNOLOGY GEOCHEMICAL FINDINGS. Paul T. Behum. Liliana Lefticariu. Y.
 A FIELD DEMONSTRATION OF AN ALTERNATIVE COAL WASTE DISPOSAL TECHNOLOGY GEOCHEMICAL FINDINGS Paul T. Behum Office of Surface Mining, Mid-Continent Region, Alton, IL Liliana Lefticariu Department of Geology,
A FIELD DEMONSTRATION OF AN ALTERNATIVE COAL WASTE DISPOSAL TECHNOLOGY GEOCHEMICAL FINDINGS Paul T. Behum Office of Surface Mining, Mid-Continent Region, Alton, IL Liliana Lefticariu Department of Geology,
BC Science Nutrient Cycles in Ecosystems
 BC Science 10 2.2 Nutrient Cycles in Ecosystems Notes Nutrients are chemicals required for growth and other life processes. Nutrients move through the biosphere in nutrient cycles (n.c), or exchanges.
BC Science 10 2.2 Nutrient Cycles in Ecosystems Notes Nutrients are chemicals required for growth and other life processes. Nutrients move through the biosphere in nutrient cycles (n.c), or exchanges.
INDEX FRESH SEMINAR SERIES
 INDEX FRESH SEMINAR SERIES Acidification & ph Control with SO 2 -Sulfurous Acid Generators By Terry R. Gong Harmon Systems International, LLC We provide solutions that benefit the world 1 Presentation
INDEX FRESH SEMINAR SERIES Acidification & ph Control with SO 2 -Sulfurous Acid Generators By Terry R. Gong Harmon Systems International, LLC We provide solutions that benefit the world 1 Presentation
Draft Fact Sheet Butte County Stable Isotope Recharge Study
 Agenda Item #4 Draft Fact Sheet Butte County Stable Isotope Recharge Study Purpose of the Study: To develop a better understanding of how various water sources contribute to recharge of Butte County groundwater.
Agenda Item #4 Draft Fact Sheet Butte County Stable Isotope Recharge Study Purpose of the Study: To develop a better understanding of how various water sources contribute to recharge of Butte County groundwater.
