1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
|
|
|
- Vanessa Doyle
- 6 years ago
- Views:
Transcription
1 CHAPTER 3 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from other materials? How are elements classified? National Science Education Standards PS 1a, 1c What Are Elements? Many materials can be broken down into simpler materials. For example, some rocks contain copper. When they are heated in a large furnace, the copper separates from the rest of the rock. Another example is breaking down water by passing electricity through it. The electric current causes hydrogen gas and oxygen gas to form. Some materials cannot be separated or broken down into other materials. An element is a pure substance that cannot be separated into simpler substances by chemical or physical methods. This is how elements are different from all other materials. A pure substance is a material in which all the basic particles are the same. For example, table salt contains particles of sodium chloride. Table salt from anywhere is the same. All pure substances, except for elements, can be broken down into simpler substances. The basic particles of an element are called atoms. Copper is an example of an element. All of the atoms in a piece of pure copper are alike. As shown in the figure below, iron is also an element. STUDY TIP Graphic Organizer In your notebook, make a Concept Map by using the terms element, substance, metal, nonmetal, and metalloid. 1. Compare How does an element differ from other pure substances? The iron atoms in the meteorite from space are the same as the iron atoms in a steel spoon. There are also iron atoms in the cereal, in the boy s braces, and even in his blood. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else. Interactive Textbook 33 Elements, Compounds, and Mixtures
2 SECTION 1 Elements continued STANDARDS CHECK PS 1a A substance has characteristic properties, such as density, a boiling point, and solubility, all of which are independent of the amount of the sample. A mixture of substances often can be separated into the original substances using one or more of the characteristic properties. 3. List What are five physical properties that are characteristics of an element? How Can Elements Be Classified? Elements can be classified based on their properties. There are two types of properties, chemical and physical. Physical properties include hardness, melting point, and density. Chemical properties include reactivity and flammability. We can use properties to tell elements apart. For example, the elements helium and krypton are both colorless, odorless, unreactive gases. However, the elements have different densities (mass per unit volume). Helium is less dense than air, so a helium balloon floats upward. Krypton is denser than air. A krypton-filled balloon, on the other hand, will sink to the floor. The Unique Properties of Elements Critical Thinking 4. Infer Compare the properties of iron with those of cobalt and nickel. How do you think cobalt and nickel are used in manufactured products? Cobalt Melting point: 1,495 C Density: 8.9 g/cm 3 Reactivity: unreactive with oxygen in the air Iron Melting point: 1,535 C Density: 7.9 g/cm 3 Reactivity: reacts by combining with oxygen in the air to form rust. Nickel Melting point: 1,455 C Density: 8.9 g/cm 3 Reactivity: unreactive with oxygen in the air 5. Explain Why can t you use density or reactivity to determine whether a sample is cobalt or nickel? The figure above shows some of the properties of three different elements. The physical properties shown are melting point, electrical and thermal conductivities, and density. Each element has other physical properties, including color, hardness, and texture. The figure also includes a chemical property: the reactivity of the element with oxygen in the air. If you had a piece of metal, you could use these properties to determine which element it is. Iron has different physical and chemical properties than the other two elements. The density of iron is much less than cobalt or nickel, and it reacts with oxygen in the air. We can also use properties to tell nickel and cobalt apart. They have the same density and reactivity, but the melting points of these two elements differ by 40 C. This property can be used to tell them apart. Interactive Textbook 34 Elements, Compounds, and Mixtures
3 SECTION 1 Elements continued How Can Elements Be Sorted? Think about all the different types of dogs that you have seen. Dogs can be classified based on different properties. These include their size, the shape of the ears, or the length of their coat. You can often determine a dog s breed just with a quick glance. The figure below shows three kinds of terriers. They are not exactly alike, but they share certain features. TAKE A LOOK 6. Describe What are some of the physical properties that describe terriers? Even though these dogs are different breeds, they have enough in common to be classified as terriers. The elements can be classified based on properties, just like the dogs in the image. There are three major categories of elements: metals, nonmetals, and metalloids. The elements iron, cobalt, and nickel are all metals. They are not exactly alike, but they have similar properties. Metals tend to be shiny solids (except mercury, which is a shiny liquid). Metals conduct electric current and heat well. Nonmetals do not conduct heat or electric current very well. Many nonmetals are gases. The solid nonmetals have a dull appearance. Metalloids have some of the properties of metals and some of the properties of nonmetals. Metalloids are important in electronics because their electrical conductivity can vary with conditions. Three Major Categories of Elements Property Metals Nonmetals Metalloids Appearance shiny dull some are shiny Conductivity of heat and electricity Malleable can be hammered into sheets Ductile can be made into wires good poor some do yes no some are somewhat malleable yes no some are somewhat ductile Brittle no yes some are 7. Identify What are the three main categories of elements? Say It Explore Applications The properties of metals make them very useful in everyday things. In groups of three or four, make a list of things that you use for cooking that are made of metal. Make another list of things used for cooking that are never made of metal. Discuss why the properties of metal determined which things are in which group. Interactive Textbook 35 Elements, Compounds, and Mixtures
4 Section 1 Review NSES PS 1a, 1c SECTION VOCABULARY element a substance that cannot be separated or broken down into simpler substances by chemical means metal an element that is shiny and conducts heat and electricity well metalloid an element that has properties of both metals and nonmetals nonmetal an element that conducts heat and electricity poorly pure substance a sample of matter, either a single element or a single compound that has definite chemical and physical properties 1. Compare How does the ability to conduct heat differ between metals and nonmetals? 2. Classify Fill in each blank to complete the table. Element Property Classification Copper shiny solid Oxygen gas Silicon electrical conductivity varies depending on conditions 3. Evaluate Assumptions Your friend tells you that all of the electric wires in your home are metals. From what you know about elements, explain whether or not this statement is true. 4. Apply Concepts Several elements are placed between panes of glass in double windows to block heat flow. Should these elements be metals or nonmetals? Why? 5. Calculate Two elements, hydrogen and helium, make up most of the atoms in the universe. 92.7% of atoms are hydrogen and 6.9% of atoms are helium. What percentage of atoms are neither hydrogen nor helium? Show your work. Interactive Textbook 36 Elements, Compounds, and Mixtures
5 K Introduction to Matter Answer Key continued 4. The volume will double. According to Boyle s law, at constant temperature, volume is inversely related to pressure. 5. The volume, temperature, and pressure of a gas are all related. If there is a change in one, it will affect the others as well. SECTION 3 CHANGES OF STATE 1. energy 2. It changes into a gas, or evaporates, or vaporizes. 3. energy 4. the temperature at which it changes from a solid to a liquid C 6. The sweat removes energy from your body as it evaporates. 7. boil 8. beaker of boiling water 9. Water from a lake evaporates, then it condenses to become part of a cloud. 10. evaporation 11. The warmer temperatures cause the water droplets to evaporate. 12. It changes directly from a solid to a gas. 13. The graph is flat, or horizontal. This means that the temperature is constant. Review 1. The particles of a solid only vibrate. The particles of a liquid can move past one another. The particles of a gas are free to move anywhere. 2. Energy is added or removed during a change of state. A change of state does not make a new substance, so changes of state are physical changes. 3. Melting requires energy. Freezing is the removal of energy. Both happen at the same temperature. 4. Both processes change a liquid to a gas. Evaporation is a slower process than boiling. In an open container, you need to heat a liquid in order to boil it. 5. Sublimation requires energy and changes a solid directly to a gas. Condensation gives off energy and changes a gas to a liquid. 6. Property Solid Liquid Gas Attraction between particles Distance between particles Movement of particles strong weaker than in a solid little or no attraction close close far apart They vibrate only. They can move past each other. There is freedom of movement. Chapter 3 Elements, Compounds, and Mixtures SECTION 1 ELEMENTS 1. Elements cannot be separated into simpler substances. 2. the meteorite 3. hardness, melting point, density, boiling point, solubility 4. Because their properties are so similar to iron s, cobalt and nickel can be used where a strong metal is needed, such as in structures, tools, or vehicles. 5. These properties are too similar in the two elements to be used to tell them apart. 6. small size; short, curly hair; shape of face 7. metals, nonmetals, metalloids Review 1. Metals are good heat conductors, and nonmetals are poor heat conductors. 2. Element Property Classification Copper shiny solid metal Oxygen gas nonmetal Silicon electrical conductivity (varies depending on conditions) metalloid 3. Possible answers: Yes, because metals conduct electric current and nonmetals don t. Yes, because electric wires are made of copper or aluminum, which are metals. No, because some metalloids conduct electric current, so it may be possible to use metalloids as wires. 4. The elements are nonmetals because all of the elements that are gases are nonmetals and because metals conduct heat % (92.7% 6.9%) 0.4% Interactive Textbook Answer Key 71 Introduction to Matter
1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
Chapter 11 The Periodic Table
 Chapter 11 The Periodic Table Lesson 1 Using the Periodic Table Textbook pages 391 397 Lesson 2 Metals Textbook pages 401 402 Lesson 3 Nonmetals and Metalloids Textbook pages 409 410 & 413-414 1 Chapter
Chapter 11 The Periodic Table Lesson 1 Using the Periodic Table Textbook pages 391 397 Lesson 2 Metals Textbook pages 401 402 Lesson 3 Nonmetals and Metalloids Textbook pages 409 410 & 413-414 1 Chapter
Metals. Key Concepts. Name Date Class. Chapter 4 Elements and the Periodic Table Section 3 Summary
 Chapter 4 Elements and the Periodic Table Section 3 Summary Metals Key Concepts What are the physical properties of metals? How does the reactivity of metals change across the periodic table? How are synthetic
Chapter 4 Elements and the Periodic Table Section 3 Summary Metals Key Concepts What are the physical properties of metals? How does the reactivity of metals change across the periodic table? How are synthetic
Metals, Nonmetals, and Metalloids
 Metals, Nonmetals, and Metalloids Color and label your periodic table like the one below: Put the colored periodic table to the side, we will glue it in later Find this page, we will begin taking notes:
Metals, Nonmetals, and Metalloids Color and label your periodic table like the one below: Put the colored periodic table to the side, we will glue it in later Find this page, we will begin taking notes:
The City School PAF Chapter
 The City School PAF Chapter Comprehensive Worksheet December 2018 SCIENCE Class 7 Candidate Name: Index Number: Section: Branch/Campus: Date: Maximum Marks: 100 Time Allowed: 1 hour 30 minutes INSTRUCTIONS:
The City School PAF Chapter Comprehensive Worksheet December 2018 SCIENCE Class 7 Candidate Name: Index Number: Section: Branch/Campus: Date: Maximum Marks: 100 Time Allowed: 1 hour 30 minutes INSTRUCTIONS:
Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet
 Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet Name: Sec: Date: Q1.Choose the best answer. 1. Which of the following is an element? a) steam b) sugar c)dry ice d) sulphur 2. Which
Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet Name: Sec: Date: Q1.Choose the best answer. 1. Which of the following is an element? a) steam b) sugar c)dry ice d) sulphur 2. Which
Student Activity: Identifying Matter
 When you have completed this activity, go to Status Check. Physical Science A Unit 1 Student Activity: Identifying Matter Name Date Objectives In this activity, you will: evaluate data on different physical
When you have completed this activity, go to Status Check. Physical Science A Unit 1 Student Activity: Identifying Matter Name Date Objectives In this activity, you will: evaluate data on different physical
Name Class Date. Does it have a crystalline structure? Minerals are crystals. Each mineral has a certain crystal structure that is always the same.
 CHAPTER 1 1 What Is a Mineral? SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: What are minerals? What determines the shape
CHAPTER 1 1 What Is a Mineral? SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: What are minerals? What determines the shape
S1 Building Blocks Summary Notes
 S1 Building Blocks Summary Notes Atoms & Molecules 1 We are developing our understanding of atoms and molecules. Atoms are the simplest building blocks of every substance in the universe. There are just
S1 Building Blocks Summary Notes Atoms & Molecules 1 We are developing our understanding of atoms and molecules. Atoms are the simplest building blocks of every substance in the universe. There are just
History of the Periodic Table
 Periodic Trends History of the Periodic Table Many scientists suspected a pattern in the order of the elements, but were not able to exactly figure it out. Dmitri Mendeleev was the first to create an accurate
Periodic Trends History of the Periodic Table Many scientists suspected a pattern in the order of the elements, but were not able to exactly figure it out. Dmitri Mendeleev was the first to create an accurate
The table shows the students suggestions about the identity of P.
 1 Three students, X, Y and Z, were told that solid P reacts with dilute acids and also conducts electricity. The table shows the students suggestions about the identity of P. Which of the students are
1 Three students, X, Y and Z, were told that solid P reacts with dilute acids and also conducts electricity. The table shows the students suggestions about the identity of P. Which of the students are
The table gives some information about a family of molecules in crude oil. Show information from the table in the most appropriate way on the grid.
 ## The table gives some information about a family of molecules in crude oil. NUMBER OF CARBON ATOMS IN MOLECULE MASS OF MOLECULE (atomic units) 1 16 2 30 4 58 Show information from the table in the most
## The table gives some information about a family of molecules in crude oil. NUMBER OF CARBON ATOMS IN MOLECULE MASS OF MOLECULE (atomic units) 1 16 2 30 4 58 Show information from the table in the most
Science 8. Unit 1. Booklet
 Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
METALS AND NONMETALS
 Suggested time allotment: 5 to 6 hours MODULE 5 METALS AND NONMETALS Elements are the simplest form of substances. This means that whatever you do with an element, it remains to be the same element. Its
Suggested time allotment: 5 to 6 hours MODULE 5 METALS AND NONMETALS Elements are the simplest form of substances. This means that whatever you do with an element, it remains to be the same element. Its
Read pg Answer pg 157 #1-5, & pg159 #2-7
 4.2 Physical Properties HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 Learning Goals I can describe the physical properties of matter
4.2 Physical Properties HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 Learning Goals I can describe the physical properties of matter
21.The smallest part of an element that keeps the same properties of that element. a. Element b. Compound c. Atom d. quark
 21.The smallest part of an element that keeps the same properties of that element a. Element b. Compound c. Atom d. quark 22.A substance made of 2 or more elements chemically combined a. Compound b. Mixture
21.The smallest part of an element that keeps the same properties of that element a. Element b. Compound c. Atom d. quark 22.A substance made of 2 or more elements chemically combined a. Compound b. Mixture
Physical Science Chapter 19. Elements and Their Properties Quick Notes
 Physical Science Chapter 19 Elements and Their Properties Quick Notes 1 19:1 A.Metals conduct heat and electricity, reflect light (luster); are malleable ( ) Can be hammered or rolled into sheets are ductile
Physical Science Chapter 19 Elements and Their Properties Quick Notes 1 19:1 A.Metals conduct heat and electricity, reflect light (luster); are malleable ( ) Can be hammered or rolled into sheets are ductile
CHAPTER 3 Electronic Structure and the Periodic Law
 CHAPTER 3 Electronic Structure and the Periodic Law . Metals, Nonmetals, and Metalloids Metals, Metalloids, and Nonmetals in the Periodic Table Metals, Nonmetals, and Metalloids Metals, Metalloids, and
CHAPTER 3 Electronic Structure and the Periodic Law . Metals, Nonmetals, and Metalloids Metals, Metalloids, and Nonmetals in the Periodic Table Metals, Nonmetals, and Metalloids Metals, Metalloids, and
D =? D = M = 10 g = 5 g/cm 3 M = 10 g V 2 cm 3 V = 2 cm 3 5 g/cm 3
 Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Chemistry Quiz #1 Review
 Name: Chemistry Quiz 1 Review Page 1 of 6 Date: Please check your answers at http://leetz.weebly.com Chemistry Quiz #1 Review Part A: Density Calculations 1. The density of sodium is 0.97 g/cm 3. A sample
Name: Chemistry Quiz 1 Review Page 1 of 6 Date: Please check your answers at http://leetz.weebly.com Chemistry Quiz #1 Review Part A: Density Calculations 1. The density of sodium is 0.97 g/cm 3. A sample
The table below shows some of the properties of the elements cobalt and nickel.
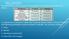 BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.3 Metals
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.3 Metals Metals The physical properties of metals include: shininess- ability to reflect light malleability ability to be hammered or rolled into flat
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.3 Metals Metals The physical properties of metals include: shininess- ability to reflect light malleability ability to be hammered or rolled into flat
1. Which of the following elements has the highest percentage by mass in nature? A. Oxygen B. Aluminium C. Nitrogen D. Silicon
 Class: F.3 ( ) Baptist Lui Ming Choi Secondary School First Term Examination (2013-2014) Date: 6 / 12 / 2013 Name: Form 3 Chemistry Time: 10:20-11:05 a.m. Answer ALL the questions. For Section A, choose
Class: F.3 ( ) Baptist Lui Ming Choi Secondary School First Term Examination (2013-2014) Date: 6 / 12 / 2013 Name: Form 3 Chemistry Time: 10:20-11:05 a.m. Answer ALL the questions. For Section A, choose
Before Statement After
 CHAPTER 10 The Periodic Table LESSON 2 Metals What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the
CHAPTER 10 The Periodic Table LESSON 2 Metals What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the
Organizing the Elements
 Organizing the Elements In a self-service store, the products are grouped according to similar characteristics. With a logical classification system, finding and comparing products is easy. You will learn
Organizing the Elements In a self-service store, the products are grouped according to similar characteristics. With a logical classification system, finding and comparing products is easy. You will learn
Draw a ring around the correct word in the box to complete the sentence.
 Q. Iron is the main structural metal used in the world. (a) The diagram represents the particles in iron, Fe. Draw a ring around the correct word in the box to complete the sentence. Iron is described
Q. Iron is the main structural metal used in the world. (a) The diagram represents the particles in iron, Fe. Draw a ring around the correct word in the box to complete the sentence. Iron is described
Metal and Non Metals
 Metal and Non Metals Malleable Ductile Sonorous Conductor Insulator KEYWORDS Rusting Brass Calcium Chloride Galvanising Reactivity Metals on the Periodic table Metals Non Metals Metals and their properties
Metal and Non Metals Malleable Ductile Sonorous Conductor Insulator KEYWORDS Rusting Brass Calcium Chloride Galvanising Reactivity Metals on the Periodic table Metals Non Metals Metals and their properties
Properties of metals
 For more awesome resources, visit us at www.savemyexams.co.uk/ Properties of metals Question Paper Level IGSE Subject hemistry (060/097) Exam oard ambridge International Examinations (IE) Topic Metals
For more awesome resources, visit us at www.savemyexams.co.uk/ Properties of metals Question Paper Level IGSE Subject hemistry (060/097) Exam oard ambridge International Examinations (IE) Topic Metals
Organizing the Elements
 Organizing the Elements 1 of 28 Organizing the Elements In a self-service store, the products are grouped according to similar characteristics. With a logical classification system, finding and comparing
Organizing the Elements 1 of 28 Organizing the Elements In a self-service store, the products are grouped according to similar characteristics. With a logical classification system, finding and comparing
Properties of Elements
 Properties of Elements Pre-Lab: (Answer questions on your data sheet.) 1. Using a periodic table, determine and write the chemical symbol for each of the eight elements listed on your data sheet. 2. Predict
Properties of Elements Pre-Lab: (Answer questions on your data sheet.) 1. Using a periodic table, determine and write the chemical symbol for each of the eight elements listed on your data sheet. 2. Predict
EXPERIMENT 5. Physical and Chemical Changes Part 1 INTRODUCTION
 EXPERIMENT 5 Physical and Chemical Changes Part 1 INTRODUCTION Matter undergoes many changes. In some cases only the form of the substance (such as physical state, size of particle, or temperature) is
EXPERIMENT 5 Physical and Chemical Changes Part 1 INTRODUCTION Matter undergoes many changes. In some cases only the form of the substance (such as physical state, size of particle, or temperature) is
1a Elements and their combinations account for all the varied
 Physical Sciences 1a Elements and their combinations account for all the varied types of matter in the world. As a basis for understanding this concept: Students know during chemical reactions, the atoms
Physical Sciences 1a Elements and their combinations account for all the varied types of matter in the world. As a basis for understanding this concept: Students know during chemical reactions, the atoms
2 Identifying Minerals
 CHAPTER 1 2 Identifying Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: What seven properties can be used to identify
CHAPTER 1 2 Identifying Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: What seven properties can be used to identify
An Organized Table Worksheet Due Thursday Name: Date: Period:
 An Organized Table Worksheet Due Thursday Name: Date: Period: The Periodic Table of Elements In 1871, the first periodic table was developed by Dmitrii Mendeleev. Mendeleev is known as the father of the
An Organized Table Worksheet Due Thursday Name: Date: Period: The Periodic Table of Elements In 1871, the first periodic table was developed by Dmitrii Mendeleev. Mendeleev is known as the father of the
Key Points. ATOMIC STRUCTURE Atom: the smallest part of an element that still has the characteristics/ properties of that element.
 The Periodic Table Learning Objective Red Yellow Green Date Completed OC3 understand what an element is and recall that all known elements are listed in the Periodic Table; (also know how to use the Table;
The Periodic Table Learning Objective Red Yellow Green Date Completed OC3 understand what an element is and recall that all known elements are listed in the Periodic Table; (also know how to use the Table;
Properties of Elements
 Pre-Lab: (Answer questions on your data sheet.) Properties of Elements 1. Using a periodic table, determine and write the chemical symbol for each of the eight elements listed on your data sheet. 2. Predict
Pre-Lab: (Answer questions on your data sheet.) Properties of Elements 1. Using a periodic table, determine and write the chemical symbol for each of the eight elements listed on your data sheet. 2. Predict
Corresponding Physical or Chemical Property bread rising through the use of baking soda carbonates decompose to form CO 2
 Substance = a chemical (element or mixture) Substance mixture chemical process = chemical reaction (new substances result) physical process = same substances present through out Physical or chemical processes?
Substance = a chemical (element or mixture) Substance mixture chemical process = chemical reaction (new substances result) physical process = same substances present through out Physical or chemical processes?
2.2 Physical Properties
 2.2 Physical Properties A physical property is any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. Examples of Physical
2.2 Physical Properties A physical property is any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. Examples of Physical
REVISION NEW LEARNING
 REVISION I have developed my knowledge of the Periodic Table by considering the properties and uses of a variety of elements relative to their positions. SCN 3-15a S2 Chemistry Metals Lesson 1 NEW LEARNING
REVISION I have developed my knowledge of the Periodic Table by considering the properties and uses of a variety of elements relative to their positions. SCN 3-15a S2 Chemistry Metals Lesson 1 NEW LEARNING
Chapter: The Periodic Table
 Table of Contents Chapter: The Periodic Table Section 1: Introduction to the Periodic Table Section 2: Representative Elements Section 3: Transition Elements 1 Introduction to the Periodic Table Development
Table of Contents Chapter: The Periodic Table Section 1: Introduction to the Periodic Table Section 2: Representative Elements Section 3: Transition Elements 1 Introduction to the Periodic Table Development
6. In this temperature time graph for the heating of H 2O at a constant rate, the segment DE represents the
 1. Which of the following contains particles with the least freedom of motion? A) CO 2( ) B) HCl(aq) C) F 2(g) D) MgBr 2(s) E) C 6H 12O 6(aq) 2. During boiling, the temperature of a pure liquid substance
1. Which of the following contains particles with the least freedom of motion? A) CO 2( ) B) HCl(aq) C) F 2(g) D) MgBr 2(s) E) C 6H 12O 6(aq) 2. During boiling, the temperature of a pure liquid substance
Explain how metalloids are different from metals and nonmetals.
 Explain how metalloids are different from metals and nonmetals. Explain how the periodic table is organized based on physical and chemical properties. Where are the metals, nonmetals, and metalloids located
Explain how metalloids are different from metals and nonmetals. Explain how the periodic table is organized based on physical and chemical properties. Where are the metals, nonmetals, and metalloids located
CONNECTICUT SCIENCE FRAMEWORK. Grade 6
 CONNECTICUT SCIENCE FRAMEWORK Grade 6 Core Themes, Content Standards and Expected Performances Properties of Matter How does the structure of matter affect the properties and uses of materials? 6.1 - Materials
CONNECTICUT SCIENCE FRAMEWORK Grade 6 Core Themes, Content Standards and Expected Performances Properties of Matter How does the structure of matter affect the properties and uses of materials? 6.1 - Materials
Look at the measuring cylinders. What happened to the volume of the water and the wax after freezing? the volume of water... the volume of wax...
 1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
Materials are all substances and include metals, ceramics and plastics as well as natural and new substances.
 National 4 Materials It is hard to imagine life without mobile gadgets such as iphones, ipads and MP3 players. Yet twenty years ago these handy gadgets such as the mobile phone where bigger and cost five
National 4 Materials It is hard to imagine life without mobile gadgets such as iphones, ipads and MP3 players. Yet twenty years ago these handy gadgets such as the mobile phone where bigger and cost five
NOTE: If substances have the SAME VOLUME, they can have DIFFERENT MASSES.
 Grade 8 SCIENCE Chapter 8 questions Page 306 1. When particles are CLOSER TOGETHER, DENSITY is GREATER. 2. Particles in SOLIDS have FEWER SPACES between them. The ATTRACTIVE FORCES between the particles
Grade 8 SCIENCE Chapter 8 questions Page 306 1. When particles are CLOSER TOGETHER, DENSITY is GREATER. 2. Particles in SOLIDS have FEWER SPACES between them. The ATTRACTIVE FORCES between the particles
Naming and Classifying the Elements. nobelium antimony. oxygen arsenic. phosphorus beryllium. platinum bismuth. plutonium boron.
 6.2 Naming and Classifying the Elements To learn more about the origins of the names of the elements, visit www.science.nelson.com GO The names of the elements have varied origins. Some elements are named
6.2 Naming and Classifying the Elements To learn more about the origins of the names of the elements, visit www.science.nelson.com GO The names of the elements have varied origins. Some elements are named
WJEC England GCSE Chemistry. Topic 11: Production, use and disposal of important chemicals and materials. Notes
 WJEC England GCSE Chemistry Topic 11: Production, use and disposal of important chemicals and materials Notes (Content in bold is for Higher Tier only) The Haber process Used to manufacture ammonia, which
WJEC England GCSE Chemistry Topic 11: Production, use and disposal of important chemicals and materials Notes (Content in bold is for Higher Tier only) The Haber process Used to manufacture ammonia, which
3 The Formation, Mining, and Use of Minerals
 CHAPTER 3 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
CHAPTER 3 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
THE PERIODIC TABLE. Chapter 15
 THE PERIODIC TABLE Chapter 15 MENDELEEV 1869 The first version of the periodic table was published by Dmitri Mendeleev, a Russian chemist He arranged the elements in order of increasing atomic mass This
THE PERIODIC TABLE Chapter 15 MENDELEEV 1869 The first version of the periodic table was published by Dmitri Mendeleev, a Russian chemist He arranged the elements in order of increasing atomic mass This
3 The Formation, Mining, and Use of Minerals
 CHAPTER 1 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
CHAPTER 1 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
GCSE BITESIZE Examinations
 GCSE BITESIZE Examinations General Certificate of Secondary Education AQA SCIENCE A CHY1A Unit Chemistry C1a (Products from Rocks) AQA Chemistry Unit Chemistry C1a (Products from Rocks) FOUNDATION TIER
GCSE BITESIZE Examinations General Certificate of Secondary Education AQA SCIENCE A CHY1A Unit Chemistry C1a (Products from Rocks) AQA Chemistry Unit Chemistry C1a (Products from Rocks) FOUNDATION TIER
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Chapters 1&2 Practice Problems
 Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Solids SECTION Critical Thinking
 SECTION 10.3 Solids A gas has neither a definite volume nor a definite shape. A liquid has a definite volume, but not a definite shape. A solid, the third state, has a definite volume and a definite shape.
SECTION 10.3 Solids A gas has neither a definite volume nor a definite shape. A liquid has a definite volume, but not a definite shape. A solid, the third state, has a definite volume and a definite shape.
I. PHYSICAL PROPERTIES PROPERTY METALS NON-METALS
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Name Honors Chemistry / /
 Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Oxygen Formula: O 2 Bonding: covalent Appearance: colourless gas. Oxygen is one of the two main gases in our atmosphere, the other being nitrogen.
 Composition of the air Air is a mixture of gases. The approximate amount if each gas in dry air is shown in the pie chart (right), but you should be aware that air also contains a variable amount of water
Composition of the air Air is a mixture of gases. The approximate amount if each gas in dry air is shown in the pie chart (right), but you should be aware that air also contains a variable amount of water
Creation Settings. can be separated into two or more pure substances by distillation. can be separated into two or more substances by chromatography.
 Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
ALL ABOUT METALS. SKG Tutorials - Class 8
 ALL ABOUT METALS SKG Tutorials - Class 8 Metals 2 Elements can be classified as metals and non-metals. Metals are strong and durable. Thus metals are used so widely for making almost everything. Example:
ALL ABOUT METALS SKG Tutorials - Class 8 Metals 2 Elements can be classified as metals and non-metals. Metals are strong and durable. Thus metals are used so widely for making almost everything. Example:
Iÿtÿÿeÿ/ÿrh Jÿtcket. Tÿlchÿwÿeÿ
 o 0 Iÿtÿÿeÿ/ÿrh Jÿtcket Tÿlchÿwÿeÿ Elements, Compounds, and Mixtures Classify each of the pictures below by placing the correct label in the blanks below: A= Element D-- Mixture of compounds B= Compound
o 0 Iÿtÿÿeÿ/ÿrh Jÿtcket Tÿlchÿwÿeÿ Elements, Compounds, and Mixtures Classify each of the pictures below by placing the correct label in the blanks below: A= Element D-- Mixture of compounds B= Compound
There are a number of steps in the treatment of water to ensure supplies are clean and safe.
 ExamLearn.ie Water Water The water cycle 1. Heat from the sun causes water to evaporate from seas. 2. As water vapour rises it cools. Condensation produces clouds of tiny droplets of water. 3. Cloud rises
ExamLearn.ie Water Water The water cycle 1. Heat from the sun causes water to evaporate from seas. 2. As water vapour rises it cools. Condensation produces clouds of tiny droplets of water. 3. Cloud rises
INTERACTIVE SCIENCE 1B
 INTERACTIVE SCIENCE 1B Workbook Solutions (Enrichment Edition) Chapter 6 MATTER as PARTICLES Part A Sectional Exercise 6.1 Fill in the blanks p.54 1. (a) liquid (b) gas 2. (a) Melting (b) unchanged 3.
INTERACTIVE SCIENCE 1B Workbook Solutions (Enrichment Edition) Chapter 6 MATTER as PARTICLES Part A Sectional Exercise 6.1 Fill in the blanks p.54 1. (a) liquid (b) gas 2. (a) Melting (b) unchanged 3.
Atoms and elements/compounds and mixtures
 Medway LEA Advisory Service Atoms and elements/compounds and mixtures 8E & 8F 26 min 26 marks Q1-L3, Q2-L4, Q3-L4, Q4-L5, Q5-L6, Q6-L7 Medway LEA Advisory Service 1 0 10 C 1. (a) The drawings below show
Medway LEA Advisory Service Atoms and elements/compounds and mixtures 8E & 8F 26 min 26 marks Q1-L3, Q2-L4, Q3-L4, Q4-L5, Q5-L6, Q6-L7 Medway LEA Advisory Service 1 0 10 C 1. (a) The drawings below show
NCERT solutions for Metals and Non Metals
 NCERT solutions for Metals and Non Metals 1 Question 1 Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv)
NCERT solutions for Metals and Non Metals 1 Question 1 Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv)
BASIS Lesson Plan. *Note to teachers: Detailed standards connections can be found at the end of this lesson plan.
 BASIS Lesson Plan Lesson Name: It s Just a Phase! Grade Level Connection(s) NGSS Standards: Grade 2, Physical Science FOSS CA Edition: Grade 3 Physical Science: Matter and Energy Module *Note to teachers:
BASIS Lesson Plan Lesson Name: It s Just a Phase! Grade Level Connection(s) NGSS Standards: Grade 2, Physical Science FOSS CA Edition: Grade 3 Physical Science: Matter and Energy Module *Note to teachers:
Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee
 Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 1 Engineering Materials and Processing Lecture -
Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 1 Engineering Materials and Processing Lecture -
CHAPTER 9. The Uses of Sulphuric Acid
 CHAPTER 9 MANUFACTURED SUBSTANCES IN INDUSTRY SULPHURIC ACID The Uses of Sulphuric Acid 175 POLLUTION of Sulphur DIOXIDE Formation of Acid Rain Burning of Sulphur Burning of Sulphur Dioxide 176 Effect
CHAPTER 9 MANUFACTURED SUBSTANCES IN INDUSTRY SULPHURIC ACID The Uses of Sulphuric Acid 175 POLLUTION of Sulphur DIOXIDE Formation of Acid Rain Burning of Sulphur Burning of Sulphur Dioxide 176 Effect
Triple Beam Balance: add the three together: 700g + 20g + 2.9g = 722.9g Metric base unit for mass is gram.
 6 th Grade 2 nd Nine Week CSA Study Guide 2015-16 SOL 6.1b: Make precise and consistent measurements and estimations. Graduated Cylinder: read from the lowest part of the water line curve. Reading: 56ml
6 th Grade 2 nd Nine Week CSA Study Guide 2015-16 SOL 6.1b: Make precise and consistent measurements and estimations. Graduated Cylinder: read from the lowest part of the water line curve. Reading: 56ml
Strong under tension and compression. Malleable. Low density. Have a dull appearance. Good conductors of electricity and heat
 Revision from Year 10: Properties of Metals and Non-Metals Read CC pp182-183 Use arrows to link the properties with the materials: Strong under tension and compression Malleable Low density Have a dull
Revision from Year 10: Properties of Metals and Non-Metals Read CC pp182-183 Use arrows to link the properties with the materials: Strong under tension and compression Malleable Low density Have a dull
Page 2. Q1.Copper is a transition metal. (a) (i) Where is copper in the periodic table? in the central block. in Group 1. in the noble gas group
 Q1.Copper is a transition metal. (a) (i) Where is copper in the periodic table? Tick ( ) one box. in the central block in Group 1 in the noble gas group (ii) What is a property of copper? Tick ( ) one
Q1.Copper is a transition metal. (a) (i) Where is copper in the periodic table? Tick ( ) one box. in the central block in Group 1 in the noble gas group (ii) What is a property of copper? Tick ( ) one
GRADE 5 SCIENCE. Sampler Streamlined TEKS. STAAR Preparation and Practice
 Sampler GRADE 5 SCIENCE STAAR Preparation and Practice Instruction and practice in all tested TEKS (Grades 3 4) Over 225 authentic STAAR test items 3-step approach for remediation STAAR is a registered
Sampler GRADE 5 SCIENCE STAAR Preparation and Practice Instruction and practice in all tested TEKS (Grades 3 4) Over 225 authentic STAAR test items 3-step approach for remediation STAAR is a registered
DOWNLOAD OR READ : METALS AND METALLOIDS PERIODIC TABLE OF THE ELEMENTS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : METALS AND METALLOIDS PERIODIC TABLE OF THE ELEMENTS PDF EBOOK EPUB MOBI Page 1 Page 2 metals and metalloids periodic table of the elements metals and metalloids periodic pdf metals
DOWNLOAD OR READ : METALS AND METALLOIDS PERIODIC TABLE OF THE ELEMENTS PDF EBOOK EPUB MOBI Page 1 Page 2 metals and metalloids periodic table of the elements metals and metalloids periodic pdf metals
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Topic 9 National 4 Chemistry Summary Notes. Metals and Alloys. Materials
 Topic 9 National 4 Chemistry Summary Notes Metals and Alloys LI 1 Materials Materials are all substances and include: metals ceramics plastics natural substances novel substances. Materials can be used
Topic 9 National 4 Chemistry Summary Notes Metals and Alloys LI 1 Materials Materials are all substances and include: metals ceramics plastics natural substances novel substances. Materials can be used
Classification of matter: Properties *
 OpenStax-CNX module: m38706 1 Classification of matter: Properties * Free High School Science Texts Project This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License
OpenStax-CNX module: m38706 1 Classification of matter: Properties * Free High School Science Texts Project This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License
Physical Science I: Atomic Theory
 Greenwich Public Schools Science Objectives and Grade Level Concepts Grade Six Physical Science I: Atomic Theory CSDE Science Curriculum Standard 6.1: Materials can be classified as pure substances or
Greenwich Public Schools Science Objectives and Grade Level Concepts Grade Six Physical Science I: Atomic Theory CSDE Science Curriculum Standard 6.1: Materials can be classified as pure substances or
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard.
 1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
Aldine ISD Curriculum Guide th Grade Science. Second Nine Weeks Scope and Sequence
 Second Nine Weeks Scope and Sequence The students know the essential elements that make up the Earth: Carbon, Oxygen, Hydrogen, and Nitrogen. Students understand that all living and nonliving elements
Second Nine Weeks Scope and Sequence The students know the essential elements that make up the Earth: Carbon, Oxygen, Hydrogen, and Nitrogen. Students understand that all living and nonliving elements
13. Friction changes mechanical energy into heat energy.
 1. What basic form of energy is present in radioactive substances. A) nuclear B) chemical C) mechanical D) electrical 2. What basic form of energy is present in a blowing wind? A) nuclear B) chemical C)
1. What basic form of energy is present in radioactive substances. A) nuclear B) chemical C) mechanical D) electrical 2. What basic form of energy is present in a blowing wind? A) nuclear B) chemical C)
2.2 Physical Properties
 There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It s easy! Lemonade is yellow and has a tart taste that is hard to miss. A yellow color
There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It s easy! Lemonade is yellow and has a tart taste that is hard to miss. A yellow color
GCSE MARKING SCHEME SUMMER 2016 SCIENCE - CHEMISTRY C1 4462/01/02. WJEC CBAC Ltd.
 GCSE MARKING SCHEME SUMMER 2016 SCIENCE - CHEMISTRY C1 4462/01/02 INTRODUCTION This marking scheme was used by WJEC for the 2016 examination. It was finalised after detailed discussion at examiners' conferences
GCSE MARKING SCHEME SUMMER 2016 SCIENCE - CHEMISTRY C1 4462/01/02 INTRODUCTION This marking scheme was used by WJEC for the 2016 examination. It was finalised after detailed discussion at examiners' conferences
CHEMISTRY WESTMINSTER SCHOOL THE CHALLENGE Thursday 28 April Time allowed: 30 minutes. Please write in black or blue ink.
 WESTMINSTER SCHOOL THE CHLLENGE 2016 CHEMISTRY Thursday 28 pril 2016 Time allowed: 30 minutes Please write in black or blue ink. Write your answers in the spaces provided. For examiner use only Total Mark
WESTMINSTER SCHOOL THE CHLLENGE 2016 CHEMISTRY Thursday 28 pril 2016 Time allowed: 30 minutes Please write in black or blue ink. Write your answers in the spaces provided. For examiner use only Total Mark
Year 8 Reaction and Magnets HW Questions
 Year 8 Reaction and Magnets HW Questions 31 minutes 44 marks Page 1 of 14 Q1. Different elements have a wide variety of properties. The list gives some of them. brittle good electrical conductor good thermal
Year 8 Reaction and Magnets HW Questions 31 minutes 44 marks Page 1 of 14 Q1. Different elements have a wide variety of properties. The list gives some of them. brittle good electrical conductor good thermal
Homework for Unit Vocab for Unit 6; due: 2. Pg 333 (1-5), Pg 335 (1-4), Pg 337 (1-4), Pg 339 (1-6), No sentences; due:
 Unit 6 Heat Homework for Unit 6 1. Vocab for Unit 6; due: 2. Pg 333 (1-5), Pg 335 (1-4), Pg 337 (1-4), Pg 339 (1-6), No sentences; due: 3. Temperature change activity; due: 4. Heat Transfer Homework; due:
Unit 6 Heat Homework for Unit 6 1. Vocab for Unit 6; due: 2. Pg 333 (1-5), Pg 335 (1-4), Pg 337 (1-4), Pg 339 (1-6), No sentences; due: 3. Temperature change activity; due: 4. Heat Transfer Homework; due:
Minerals. This study packet belongs to
 Minerals This study packet belongs to What are minerals? To understand what minerals are, you need to know a bit about what makes up our Earth. Our Earth is made up of about 109 pure substances called.
Minerals This study packet belongs to What are minerals? To understand what minerals are, you need to know a bit about what makes up our Earth. Our Earth is made up of about 109 pure substances called.
4. Where do the names of the elements come from? Some were named as substances before they were known to be elements. sulfur 16
 4. Where do the names of the elements come from? Some were named as substances before they were known to be elements. silver 47 sulfur 16 copper 29 Some are named from the natural substance that they are
4. Where do the names of the elements come from? Some were named as substances before they were known to be elements. silver 47 sulfur 16 copper 29 Some are named from the natural substance that they are
Materials : Metals and Non-Metals
 18 EXEMPLAR PROBLEMS 4 Materials : Metals and Non-Metals MULTIPLE CHOICE QUESTIONS 1. Which of the following is not a metal? (a) copper (c) aluminium (b) sulphur (d) iron 2. The substance that will be
18 EXEMPLAR PROBLEMS 4 Materials : Metals and Non-Metals MULTIPLE CHOICE QUESTIONS 1. Which of the following is not a metal? (a) copper (c) aluminium (b) sulphur (d) iron 2. The substance that will be
3 The Formation, Mining, and Use of Minerals
 CHAPTER 3 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
CHAPTER 3 3 The Formation, Mining, and Use of Minerals SECTION Minerals of the Earth s Crust BEFORE YOU READ After you read this section, you should be able to answer these questions: How do minerals form?
STAAR Vocabulary noticing something about the world around you. using clues to find the answer. everything everywhere. to sort into groups
 Observation noticing something about the world around you Inference using clues to find the answer Matter everything everywhere Classify to sort into groups Physical Property something you observe with
Observation noticing something about the world around you Inference using clues to find the answer Matter everything everywhere Classify to sort into groups Physical Property something you observe with
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example,
 SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
OC30 Conduct a qualitative experiment to detect the presence of dissolved solids in water samples, and test water for hardness (soap test)
 Chemistry: 6. Water Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC14 Use cobalt chloride paper or anhydrous copper sulfate to test
Chemistry: 6. Water Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC14 Use cobalt chloride paper or anhydrous copper sulfate to test
Rusting is an example of corrosion, which is a spontaneous redox reaction of materials with substances in their environment.
 CORROSION WHAT IS CORROSION? Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Rusting is an example of corrosion, which is a spontaneous
CORROSION WHAT IS CORROSION? Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Rusting is an example of corrosion, which is a spontaneous
Contact us:
 Class X Chapter 3 Metals and Non-metals Science Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat.
Class X Chapter 3 Metals and Non-metals Science Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat.
Objective: Classify metals, nonmetals, and metalloids based on their properties.
 Do Now Date: September 2, 2014 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Do Now Date: September 2, 2014 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Properties of Metals and Alloys
 Properties of Metals and Alloys 1 of 19 Boardworks Ltd 2016 Properties of Metals and Alloys 2 of 19 Boardworks Ltd 2016 What is the structure of metals? 3 of 19 Boardworks Ltd 2016 Metal particles are
Properties of Metals and Alloys 1 of 19 Boardworks Ltd 2016 Properties of Metals and Alloys 2 of 19 Boardworks Ltd 2016 What is the structure of metals? 3 of 19 Boardworks Ltd 2016 Metal particles are
Metals. N4 & N5 Homework Questions
 St Peter the Apostle High School Chemistry Department Metals N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
St Peter the Apostle High School Chemistry Department Metals N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
*20GSD2101* Double Award Science: Chemistry. Unit C1 Foundation Tier THURSDAY 14 MAY 2015, MORNING [GSD21] *GSD21* TIME 1 hour.
![*20GSD2101* Double Award Science: Chemistry. Unit C1 Foundation Tier THURSDAY 14 MAY 2015, MORNING [GSD21] *GSD21* TIME 1 hour. *20GSD2101* Double Award Science: Chemistry. Unit C1 Foundation Tier THURSDAY 14 MAY 2015, MORNING [GSD21] *GSD21* TIME 1 hour.](/thumbs/81/82623486.jpg) Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Foundation Tier [GSD21] *GSD21* *G5802* *GSD21* THURSDAY 14 MAY 2015, MORNING
Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Foundation Tier [GSD21] *GSD21* *G5802* *GSD21* THURSDAY 14 MAY 2015, MORNING
PERIODIC TABLE. Group Family Period Valence electrons Energy levels
 PERIODIC TABLE PERIODIC TABLE A visual representation used in Science to organize the elements according to their chemical and physical properties When talking about the Periodic Table, you will hear words
PERIODIC TABLE PERIODIC TABLE A visual representation used in Science to organize the elements according to their chemical and physical properties When talking about the Periodic Table, you will hear words
