Student Activity: Identifying Matter
|
|
|
- Hilary Curtis
- 5 years ago
- Views:
Transcription
1 When you have completed this activity, go to Status Check. Physical Science A Unit 1 Student Activity: Identifying Matter Name Date Objectives In this activity, you will: evaluate data on different physical and chemical properties of matter to determine the identity of unknown substances explain why various substances behave differently under specific conditions Activities Read the following information and, using your knowledge of different physical and chemical properties of matter, answer the questions that follow. Physical Properties. Imagine you are a sculptor and, for your next project, need a that is shiny and easy to bend. Suppose the should also have a very high melting point so that it can withstand a lot of heat. You should realize from the lesson on physical and chemical properties of matter that things such as melting point and boiling point, whether a bends easily, and its appearance are all different physical properties of s. Physical properties are characteristics that can be observed without changing the material. Some physical properties that you should be familiar with are the state of matter, ph, density, melting point, boiling point, brittleness, and hardness. You should recall that brittleness describes how easily different substances break or shatter. For example, you saw in the lesson that glass, which is very brittle, shatters when it is hit with a hammer. In contrast, aluminum, which is not very brittle, does not break, and only changes shape. Hardness describes how hard or soft a is. There are different scales used to measure and compare the hardness of different elements, which you can learn about later. In general, you should know that softer s are easier to bend and shape than harder s are. When you studied the lesson on the periodic table, you learned that s have certain physical properties that can be used to differentiate them from other elements. Two of these properties are malleability and ductility. Malleability is a measure of how easily a can be hammered into different shapes. Gold is the most malleable of all the s. In fact it is so malleable that as little as one ounce of gold can be hammered into a thin sheet about 100 square feet in size! Ductility describes how a can be shaped into wires. Gold is also extremely ductile. One ounce of gold can be stretched into a wire that extends about 8 kilometers in length! You also learned that s are usually good conductors of things such as heat and electricity. Thermal conductivity is a measure of how well a substance can transfer, or conduct, heat. In contrast, electrical conductivity is a measure of how well a substance can transfer electricity. Copper for example, has a high thermal conductivity, which is why it is often found on the bottoms of pots and pans. Copper also has a high electrical conductivity, which is why many electrical wires are made out of copper. In fact, copper has the highest electrical conductivity of any, except for silver. 1
2 Chemical Properties. In contrast to physical properties, chemical properties of matter describe how a substance behaves in a chemical reaction. Chemical properties include things such as reactivity, solubility, and corrosion. As you know, reactivity is a measure of how a substance reacts when placed with another substance. A chemical reaction occurs when one or more substances changes to form a new substance. For example, table salt is the result of a chemical reaction between two different elements, sodium and chloride. Some substances, such as chlorine, are highly reactive. Mixing chlorine with ammonia can produce a very toxic gas, which is why you should be careful not to mix household cleaning products. However, other substances, such as noble gases, are known as inert. Inert gases are gases that very rarely react with other substances. If you have ever stirred sugar in iced tea, you are familiar with solubility. Solubility is a measure of how much solute will dissolve in a solvent at a certain temperature. If you have studied the lesson on mixtures and solutions, you know that increasing the temperature will usually increase the rate at which solid and liquid solutes dissolve, and decrease the rate at which gases dissolve. You should also recall that a solute is a substance that is dissolved in another substance. A solvent is a substance that can dissolve other substances. For example, salt is highly soluble in water. When you mix the two, salt is considered the solute, and water is the solvent. Other substances, such as gold, have a low solubility in water and do not dissolve as easily. If you have ever seen pipes that look as though they have been eaten away, you are familiar with what is known as corrosion. Corrosion occurs when s break down after repeated exposure to air, water, or even other chemicals. For example, when some s are exposed to oxygen from water or air, they rust, turning a reddish-brown color. Rust forms when the reacts with oxygen, in a process known as oxidation. Corrosion commonly occurs on s such as iron and steel. However, adding things to some s can help prevent corrosion. For example, stainless steel is resistant to corrosion because it is a mix of iron, nickel, and chrome. In contrast, iron by itself is much more likely to corrode. Interestingly, corrosion occurs more quickly in moist air or when the s are exposed to salt. This is why cars near the ocean tend to experience more corrosion than cars farther inland. 2
3 1. Imagine you are a lab assistant helping Dr. Rusty Mettle identify different types of matter for various experiments that he is performing. Help Dr. Mettle complete his experiments by using the chart and answering the questions that follow. Physical Properties of Various Chemical Elements Element Appearanc e State of 25 C Density (kg/m 3 25 C Hardness (how easily it bends or breaks) Melting Point ( C) Boiling Point ( C) Electrical Conductivity Thermal Conductivity Argon colorless, odorless, tasteless gas N/A ( ) ( )185.7 low low Barium silver solid 3,510 soft 729 1,898 low low Boron Bromine Calcium Chlorine Copper Fluorine Gold yellowbrown crystal red-brown with bad odor silvery yellowish green reddishorange greenish yellow brightyellow solid 2,340 hard 2,300 4,002 low low liquid 3,119 N/A N/A low solid 1,550 soft 839 1,484 medium medium gas N/A ( ) ( )33.9 N/A low solid 8,920 soft 1, ,567 high high gas 1.69 N/A ( ) ( ) N/A low solid 19,300 soft 1, ,807 high high Iron silver-white solid 7,874 medium 1,811 3,134 low low Krypton colorless, odorless, tasteless gas 3.71 N/A ( ) ( )153.2 N/A low Mercury silver liquid 13,570 soft low low Rubidium silver-white solid 1,532 soft low low Silver silver solid 10,490 soft 961 2,163 high high Sulfur pale-yellow non solid 1,960 soft low low A. Suppose Dr. Rusty Mettle is looking for a that he can bend and shape very easily, which also conducts electricity well. He would like a that has between 15,000 and 20,000 kg per m 3. Which substance or substance should he choose? 3
4 B. Now, Dr. Mettle is looking for a that he can bend and shape easily. He wants to make wires out of the that will transfer electrical current very well. He can melt some s, but his melting equipment can only reach 1,000 C. Which substance or substances should he choose? C. Dr. Mettle needs a substance that does not conduct electricity well to take up some space in an experiment that requires pressure in the container. He needs a material that will remain in the gas phase unless it is at very cold temperatures. At cold temperatures, the material should change from a liquid to a gas very quickly, as long as Dr. Mettle adds heat. It should also change from a solid to a liquid to a gas within a temperature range of less than 10 Celsius. Which substance or substances could he use? D. Dr. Mettle is trying to identify a that he can pour into thin cylindrical tubes. The material needs to remain in the liquid phase over a wide range of temperatures. It should melt above 30 C but not boil below 350 C. The material needs to be very heavy with a mass of at least 10,000 kg/m 3. Which substance or substances should he choose? E. Dr. Mettle knows that if he tried to mix the element sodium (which is not shown in the chart) with water, it will explode. He needs to identify a different that is not as reactive, does not conduct heat very well, and changes from a solid to a liquid at a temperature between 1,775 C and 2,030 C. Using the information from the table, and this list, which substance or substances should he choose? Most Reactive sodium calcium iron copper silver gold Least Reactive 2. Suppose Dr. Mettle mixed 3 different substances in water labeled substance X, substance Y, and substance Z. Two grams of substance X dissolved at room temperature. When heat was added to the reaction, 3 grams of substance X dissolved completely. Only 1.5 grams of substance Y dissolved at room temperature and when heat was added to the reaction. Substance Z did not dissolve at all and sank to the bottom of the flask at many different temperatures. What does this tell you about the solubility of each of the 3 substances in water? 4
5 3. Suppose Dr. Mettle found some old pipes in his laboratory that had corroded away. He says that they are made of steel. You compare those with some other pipes that do not look nearly as worn down. He says that those pipes are made out of galvanized steel, which is coated with zinc. What does that tell you about zinc and steel in reference to corrosion? Explain. 4. Suppose Dr. Mettle has 2 flasks that he has filled with a liquid. He added a different gas to each flask but forgot to label them. Unfortunately, both of the gases are colorless, so he can not easily tell them apart. He knows that 1 gas is inert and that the other reacts with many different things. How can Dr. Mettle tell which gas he put into each flask? Explain. 5
The table shows the students suggestions about the identity of P.
 1 Three students, X, Y and Z, were told that solid P reacts with dilute acids and also conducts electricity. The table shows the students suggestions about the identity of P. Which of the students are
1 Three students, X, Y and Z, were told that solid P reacts with dilute acids and also conducts electricity. The table shows the students suggestions about the identity of P. Which of the students are
1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
Metal and Non Metals
 Metal and Non Metals Malleable Ductile Sonorous Conductor Insulator KEYWORDS Rusting Brass Calcium Chloride Galvanising Reactivity Metals on the Periodic table Metals Non Metals Metals and their properties
Metal and Non Metals Malleable Ductile Sonorous Conductor Insulator KEYWORDS Rusting Brass Calcium Chloride Galvanising Reactivity Metals on the Periodic table Metals Non Metals Metals and their properties
Look at the measuring cylinders. What happened to the volume of the water and the wax after freezing? the volume of water... the volume of wax...
 1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
Properties of metals
 For more awesome resources, visit us at www.savemyexams.co.uk/ Properties of metals Question Paper Level IGSE Subject hemistry (060/097) Exam oard ambridge International Examinations (IE) Topic Metals
For more awesome resources, visit us at www.savemyexams.co.uk/ Properties of metals Question Paper Level IGSE Subject hemistry (060/097) Exam oard ambridge International Examinations (IE) Topic Metals
1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 3 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 3 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
Key Points. ATOMIC STRUCTURE Atom: the smallest part of an element that still has the characteristics/ properties of that element.
 The Periodic Table Learning Objective Red Yellow Green Date Completed OC3 understand what an element is and recall that all known elements are listed in the Periodic Table; (also know how to use the Table;
The Periodic Table Learning Objective Red Yellow Green Date Completed OC3 understand what an element is and recall that all known elements are listed in the Periodic Table; (also know how to use the Table;
Oman College of Management & Technology COURSE NAME: MATERIALS SCIENCE PROPOSED BY: DR.MOHAMED ALNEJEM SEMESTER: SECOND 2015/2016 CHAPTER (7): METALS
 Oman College of Management & Technology COURSE NAME: MATERIALS SCIENCE PROPOSED BY: DR.MOHAMED ALNEJEM SEMESTER: SECOND 2015/2016 CHAPTER (7): METALS 1 CHAPTER 7 : 2 METALS 3 Which of these things are
Oman College of Management & Technology COURSE NAME: MATERIALS SCIENCE PROPOSED BY: DR.MOHAMED ALNEJEM SEMESTER: SECOND 2015/2016 CHAPTER (7): METALS 1 CHAPTER 7 : 2 METALS 3 Which of these things are
Metals, Nonmetals, and Metalloids
 Metals, Nonmetals, and Metalloids Color and label your periodic table like the one below: Put the colored periodic table to the side, we will glue it in later Find this page, we will begin taking notes:
Metals, Nonmetals, and Metalloids Color and label your periodic table like the one below: Put the colored periodic table to the side, we will glue it in later Find this page, we will begin taking notes:
Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet
 Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet Name: Sec: Date: Q1.Choose the best answer. 1. Which of the following is an element? a) steam b) sugar c)dry ice d) sulphur 2. Which
Science Class 8 Topic: Elements And Compounds Reinforcement Worksheet Name: Sec: Date: Q1.Choose the best answer. 1. Which of the following is an element? a) steam b) sugar c)dry ice d) sulphur 2. Which
I. PHYSICAL PROPERTIES PROPERTY METALS NON-METALS
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Rusting is an example of corrosion, which is a spontaneous redox reaction of materials with substances in their environment.
 CORROSION WHAT IS CORROSION? Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Rusting is an example of corrosion, which is a spontaneous
CORROSION WHAT IS CORROSION? Corrosion is the deterioration of a metal as a result of chemical reactions between it and the surrounding environment. Rusting is an example of corrosion, which is a spontaneous
I. PHYSICAL PROPERTIES. PROPERTY METALS NON-METALS 1.Lustre Metals have shining surface. They do not have shining surface.
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
An Organized Table Worksheet Due Thursday Name: Date: Period:
 An Organized Table Worksheet Due Thursday Name: Date: Period: The Periodic Table of Elements In 1871, the first periodic table was developed by Dmitrii Mendeleev. Mendeleev is known as the father of the
An Organized Table Worksheet Due Thursday Name: Date: Period: The Periodic Table of Elements In 1871, the first periodic table was developed by Dmitrii Mendeleev. Mendeleev is known as the father of the
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE. 4.3 Metals
 NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.3 Metals Metals The physical properties of metals include: shininess- ability to reflect light malleability ability to be hammered or rolled into flat
NOTES ON CHAPTER 4: ELEMENTS AND THE PERIODIC TABLE 4.3 Metals Metals The physical properties of metals include: shininess- ability to reflect light malleability ability to be hammered or rolled into flat
History of the Periodic Table
 Periodic Trends History of the Periodic Table Many scientists suspected a pattern in the order of the elements, but were not able to exactly figure it out. Dmitri Mendeleev was the first to create an accurate
Periodic Trends History of the Periodic Table Many scientists suspected a pattern in the order of the elements, but were not able to exactly figure it out. Dmitri Mendeleev was the first to create an accurate
2.3 Chemical Changes corrosion Kinds of Corrosion
 2.3 Chemical Changes Have you ever wondered why metal car bodies rust but plastic bumpers do not? As you know, different substances have different physical properties, such as colour and hardness, and
2.3 Chemical Changes Have you ever wondered why metal car bodies rust but plastic bumpers do not? As you know, different substances have different physical properties, such as colour and hardness, and
CO forms CO 2. forms. (a) The coke reacts with the oxygen in the air to form carbon dioxide. C + O 2
 1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
Before Statement After
 CHAPTER 10 The Periodic Table LESSON 2 Metals What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the
CHAPTER 10 The Periodic Table LESSON 2 Metals What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the
2.2 Physical Properties
 There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It s easy! Lemonade is yellow and has a tart taste that is hard to miss. A yellow color
There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It s easy! Lemonade is yellow and has a tart taste that is hard to miss. A yellow color
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Metals And Their Properties- Physical and Chemical
 Metals And Their Properties- Physical and Chemical All the things around us are made of 100 or so elements. These elements were classified by Lavoisier in to metals and non-metals by studying their properties.
Metals And Their Properties- Physical and Chemical All the things around us are made of 100 or so elements. These elements were classified by Lavoisier in to metals and non-metals by studying their properties.
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard.
 1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
*20GSD2101* Double Award Science: Chemistry. Unit C1 Foundation Tier THURSDAY 14 MAY 2015, MORNING [GSD21] *GSD21* TIME 1 hour.
![*20GSD2101* Double Award Science: Chemistry. Unit C1 Foundation Tier THURSDAY 14 MAY 2015, MORNING [GSD21] *GSD21* TIME 1 hour. *20GSD2101* Double Award Science: Chemistry. Unit C1 Foundation Tier THURSDAY 14 MAY 2015, MORNING [GSD21] *GSD21* TIME 1 hour.](/thumbs/81/82623486.jpg) Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Foundation Tier [GSD21] *GSD21* *G5802* *GSD21* THURSDAY 14 MAY 2015, MORNING
Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Foundation Tier [GSD21] *GSD21* *G5802* *GSD21* THURSDAY 14 MAY 2015, MORNING
ALL ABOUT METALS. SKG Tutorials - Class 8
 ALL ABOUT METALS SKG Tutorials - Class 8 Metals 2 Elements can be classified as metals and non-metals. Metals are strong and durable. Thus metals are used so widely for making almost everything. Example:
ALL ABOUT METALS SKG Tutorials - Class 8 Metals 2 Elements can be classified as metals and non-metals. Metals are strong and durable. Thus metals are used so widely for making almost everything. Example:
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Chemistry Quiz #1 Review
 Name: Chemistry Quiz 1 Review Page 1 of 6 Date: Please check your answers at http://leetz.weebly.com Chemistry Quiz #1 Review Part A: Density Calculations 1. The density of sodium is 0.97 g/cm 3. A sample
Name: Chemistry Quiz 1 Review Page 1 of 6 Date: Please check your answers at http://leetz.weebly.com Chemistry Quiz #1 Review Part A: Density Calculations 1. The density of sodium is 0.97 g/cm 3. A sample
1. Which of the following elements has the highest percentage by mass in nature? A. Oxygen B. Aluminium C. Nitrogen D. Silicon
 Class: F.3 ( ) Baptist Lui Ming Choi Secondary School First Term Examination (2013-2014) Date: 6 / 12 / 2013 Name: Form 3 Chemistry Time: 10:20-11:05 a.m. Answer ALL the questions. For Section A, choose
Class: F.3 ( ) Baptist Lui Ming Choi Secondary School First Term Examination (2013-2014) Date: 6 / 12 / 2013 Name: Form 3 Chemistry Time: 10:20-11:05 a.m. Answer ALL the questions. For Section A, choose
(A) Copper. (B) Zinc. (C) Iron. (D) Wood. (A) Zinc. (B) Wood. (C) Rubber. (D) Plastic.
 Downloaded from MATERIALS : METALS AND NON-METALS 1.Give one example of each: metals and non-metals. 2.Name the metal, which is the best conductor of heat and electricity. 3.Name the property by which
Downloaded from MATERIALS : METALS AND NON-METALS 1.Give one example of each: metals and non-metals. 2.Name the metal, which is the best conductor of heat and electricity. 3.Name the property by which
NCERT solutions for Metals and Non Metals
 NCERT solutions for Metals and Non Metals 1 Question 1 Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv)
NCERT solutions for Metals and Non Metals 1 Question 1 Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv)
2.2 Physical Properties
 2.2 Physical Properties A physical property is any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. Examples of Physical
2.2 Physical Properties A physical property is any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. Examples of Physical
The table gives some information about a family of molecules in crude oil. Show information from the table in the most appropriate way on the grid.
 ## The table gives some information about a family of molecules in crude oil. NUMBER OF CARBON ATOMS IN MOLECULE MASS OF MOLECULE (atomic units) 1 16 2 30 4 58 Show information from the table in the most
## The table gives some information about a family of molecules in crude oil. NUMBER OF CARBON ATOMS IN MOLECULE MASS OF MOLECULE (atomic units) 1 16 2 30 4 58 Show information from the table in the most
85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO 3
 Metal and metal reactivity / Section 2 / Sect2pp.doc / S. W. Tse / P.1 85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO
Metal and metal reactivity / Section 2 / Sect2pp.doc / S. W. Tse / P.1 85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO
Reactivity Series. Question Paper. Save My Exams! The Home of Revision. Module Double Award (Paper 1C) Chemistry of the Elements.
 Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk Reactivity Series Question Paper Level GCSE Subject Chemistry Exam Board Edexcel IGCSE
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at www.savemyexams.co.uk Reactivity Series Question Paper Level GCSE Subject Chemistry Exam Board Edexcel IGCSE
Page 1 of 15. Website: Mobile:
 Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor conductor of heat. (i) Metal that
Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor conductor of heat. (i) Metal that
Contact us:
 Class X Chapter 3 Metals and Non-metals Science Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat.
Class X Chapter 3 Metals and Non-metals Science Question 1: Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat.
Project: Corrosion of Iron
 Project: Corrosion of Iron Potential Credits: /15 Name: Goal: The goal of this lab is to help you acquaint you with the process of corrosion and to investigate how it can be slowed. You will be provided
Project: Corrosion of Iron Potential Credits: /15 Name: Goal: The goal of this lab is to help you acquaint you with the process of corrosion and to investigate how it can be slowed. You will be provided
Covered with a thin layer of oxide at ordinary temperatures.
 1 More about Metals Physical properties of metals In general metals have luster, are malleable and ductile, good conductors of heat and electricity and have high boiling and melting points and nonmetals
1 More about Metals Physical properties of metals In general metals have luster, are malleable and ductile, good conductors of heat and electricity and have high boiling and melting points and nonmetals
UNIT 2 - MATTER & CHANGE
 ELEMENTS & THEIR SYMBOLS NOTES H hydrogen Ni nickel He helium Cu copper Li lithium Zn zinc Be beryllium As arsenic B boron Se selenium C carbon Br bromine N nitrogen Kr krypton O oxygen Rb rubidium F fluorine
ELEMENTS & THEIR SYMBOLS NOTES H hydrogen Ni nickel He helium Cu copper Li lithium Zn zinc Be beryllium As arsenic B boron Se selenium C carbon Br bromine N nitrogen Kr krypton O oxygen Rb rubidium F fluorine
Physical Science Chapter 19. Elements and Their Properties Quick Notes
 Physical Science Chapter 19 Elements and Their Properties Quick Notes 1 19:1 A.Metals conduct heat and electricity, reflect light (luster); are malleable ( ) Can be hammered or rolled into sheets are ductile
Physical Science Chapter 19 Elements and Their Properties Quick Notes 1 19:1 A.Metals conduct heat and electricity, reflect light (luster); are malleable ( ) Can be hammered or rolled into sheets are ductile
Read pg Answer pg 157 #1-5, & pg159 #2-7
 4.2 Physical Properties HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 Learning Goals I can describe the physical properties of matter
4.2 Physical Properties HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 HOMEWORK Read pg 149-159 Answer pg 157 #1-5, & pg159 #2-7 Learning Goals I can describe the physical properties of matter
Chapter 3: Metals and Non-metals Question 1: Define amphoteric oxides. Give two examples. Answer: Oxides that react with both acids and bases to form
 Chapter 3: Metals and Non-metals Question 1: Define amphoteric oxides. Give two examples. Oxides that react with both acids and bases to form salt and water are known as amphoteric oxides. Examples: PbO
Chapter 3: Metals and Non-metals Question 1: Define amphoteric oxides. Give two examples. Oxides that react with both acids and bases to form salt and water are known as amphoteric oxides. Examples: PbO
4. Where do the names of the elements come from? Some were named as substances before they were known to be elements. sulfur 16
 4. Where do the names of the elements come from? Some were named as substances before they were known to be elements. silver 47 sulfur 16 copper 29 Some are named from the natural substance that they are
4. Where do the names of the elements come from? Some were named as substances before they were known to be elements. silver 47 sulfur 16 copper 29 Some are named from the natural substance that they are
Chapter 3 Metals and Non-metals
 Chapter 3 Metals and Non-metals Intext Questions On Page 40 Question 1: Give an example of a metal which (i) (ii) (iii) (iv) Is a liquid at room temperature Can be easily cut with a knife. Is the best
Chapter 3 Metals and Non-metals Intext Questions On Page 40 Question 1: Give an example of a metal which (i) (ii) (iii) (iv) Is a liquid at room temperature Can be easily cut with a knife. Is the best
TEST I REVIEW. 1. All of the following are properties of antimony. Which one is not a
 TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
Metals and Non-metals
 Metals and Non-metals Q.1. Define amphoteric oxides. Give two examples. Oxides that react with both acids and bases to form salt and water are known as amphoteric oxides. Examples: PbO and Al2O3. Q.2.
Metals and Non-metals Q.1. Define amphoteric oxides. Give two examples. Oxides that react with both acids and bases to form salt and water are known as amphoteric oxides. Examples: PbO and Al2O3. Q.2.
Class 8 Chapter 04 Metals and Non - metals Study Notes
 Class 8 Chapter 04 Metals and Non - metals Study Notes FOR FORMATIVE AND SUMMATIVE ASSESSMENT A. MULTIPLE-CHOICE QUESTIONS: 1. Which of the following is true for all metals? a. They are hard solids. b.
Class 8 Chapter 04 Metals and Non - metals Study Notes FOR FORMATIVE AND SUMMATIVE ASSESSMENT A. MULTIPLE-CHOICE QUESTIONS: 1. Which of the following is true for all metals? a. They are hard solids. b.
THE PERIODIC TABLE. Chapter 15
 THE PERIODIC TABLE Chapter 15 MENDELEEV 1869 The first version of the periodic table was published by Dmitri Mendeleev, a Russian chemist He arranged the elements in order of increasing atomic mass This
THE PERIODIC TABLE Chapter 15 MENDELEEV 1869 The first version of the periodic table was published by Dmitri Mendeleev, a Russian chemist He arranged the elements in order of increasing atomic mass This
Unit 9F Patterns of reactivity. About the unit. Expectations. Science Year 9. Where the unit fits in
 Science Year 9 Unit 9F Patterns of reactivity About the unit In this unit pupils: learn that although metals react in a similar way with oxygen, water and acids, some react more readily than others establish
Science Year 9 Unit 9F Patterns of reactivity About the unit In this unit pupils: learn that although metals react in a similar way with oxygen, water and acids, some react more readily than others establish
Unit I PART I. MATH TOOLS FOR CHEMISTRY I. The Metric System The metric system is the scientific system of units of measurement
 CHEMISTRY 100 LECTURE Unit I PART I. MATH TOOLS FOR CHEMISTRY I. The Metric System The metric system is the scientific system of units of measurement Length Volume Mass METRIC BASIC UNITS LENGTH MASS VOLUME
CHEMISTRY 100 LECTURE Unit I PART I. MATH TOOLS FOR CHEMISTRY I. The Metric System The metric system is the scientific system of units of measurement Length Volume Mass METRIC BASIC UNITS LENGTH MASS VOLUME
Topic 9 National 4 Chemistry Summary Notes. Metals and Alloys. Materials
 Topic 9 National 4 Chemistry Summary Notes Metals and Alloys LI 1 Materials Materials are all substances and include: metals ceramics plastics natural substances novel substances. Materials can be used
Topic 9 National 4 Chemistry Summary Notes Metals and Alloys LI 1 Materials Materials are all substances and include: metals ceramics plastics natural substances novel substances. Materials can be used
Oxidation and Reduction
 Oxidation and Reduction An oxidation reaction is one in which oxygen is added to a substance. Example: Methane is oxidised when it burns in air. Oxygen is added to the carbon in methane, forming carbon
Oxidation and Reduction An oxidation reaction is one in which oxygen is added to a substance. Example: Methane is oxidised when it burns in air. Oxygen is added to the carbon in methane, forming carbon
Chapter 11 The Periodic Table
 Chapter 11 The Periodic Table Lesson 1 Using the Periodic Table Textbook pages 391 397 Lesson 2 Metals Textbook pages 401 402 Lesson 3 Nonmetals and Metalloids Textbook pages 409 410 & 413-414 1 Chapter
Chapter 11 The Periodic Table Lesson 1 Using the Periodic Table Textbook pages 391 397 Lesson 2 Metals Textbook pages 401 402 Lesson 3 Nonmetals and Metalloids Textbook pages 409 410 & 413-414 1 Chapter
REVISION NEW LEARNING
 REVISION I have developed my knowledge of the Periodic Table by considering the properties and uses of a variety of elements relative to their positions. SCN 3-15a S2 Chemistry Metals Lesson 1 NEW LEARNING
REVISION I have developed my knowledge of the Periodic Table by considering the properties and uses of a variety of elements relative to their positions. SCN 3-15a S2 Chemistry Metals Lesson 1 NEW LEARNING
1. METALS 2. FERRIC METALS 3. NON-FERRIC METALS 4. WORKING WITH METALS 5. METAL FORMING TECHNIQUES 6. ENVIRONMENTAL IMPACT OF METAL EXTRACTION
 METALS: 1. METALS 2. FERRIC METALS 3. NON-FERRIC METALS 4. WORKING WITH METALS 5. METAL FORMING TECHNIQUES 6. ENVIRONMENTAL IMPACT OF METAL EXTRACTION 1. METALS: Metals are chemical elements found in nature
METALS: 1. METALS 2. FERRIC METALS 3. NON-FERRIC METALS 4. WORKING WITH METALS 5. METAL FORMING TECHNIQUES 6. ENVIRONMENTAL IMPACT OF METAL EXTRACTION 1. METALS: Metals are chemical elements found in nature
2.2 Physical Properties. Physical Properties. Notes due EOC
 Physical Properties Notes due EOC There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It s easy! Lemonade is yellow and has a tart taste that
Physical Properties Notes due EOC There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It s easy! Lemonade is yellow and has a tart taste that
Level 1 Chemistry, 2013
 90933 909330 1SUPERVISOR S Level 1 Chemistry, 2013 90933 Demonstrate understanding of aspects of selected elements 9.30 am Thursday 21 November 2013 Credits: Four Achievement Achievement with Merit Achievement
90933 909330 1SUPERVISOR S Level 1 Chemistry, 2013 90933 Demonstrate understanding of aspects of selected elements 9.30 am Thursday 21 November 2013 Credits: Four Achievement Achievement with Merit Achievement
WJEC England GCSE Chemistry. Topic 11: Production, use and disposal of important chemicals and materials. Notes
 WJEC England GCSE Chemistry Topic 11: Production, use and disposal of important chemicals and materials Notes (Content in bold is for Higher Tier only) The Haber process Used to manufacture ammonia, which
WJEC England GCSE Chemistry Topic 11: Production, use and disposal of important chemicals and materials Notes (Content in bold is for Higher Tier only) The Haber process Used to manufacture ammonia, which
Corresponding Physical or Chemical Property bread rising through the use of baking soda carbonates decompose to form CO 2
 Substance = a chemical (element or mixture) Substance mixture chemical process = chemical reaction (new substances result) physical process = same substances present through out Physical or chemical processes?
Substance = a chemical (element or mixture) Substance mixture chemical process = chemical reaction (new substances result) physical process = same substances present through out Physical or chemical processes?
Materials : Metals and Non-Metals
 18 EXEMPLAR PROBLEMS 4 Materials : Metals and Non-Metals MULTIPLE CHOICE QUESTIONS 1. Which of the following is not a metal? (a) copper (c) aluminium (b) sulphur (d) iron 2. The substance that will be
18 EXEMPLAR PROBLEMS 4 Materials : Metals and Non-Metals MULTIPLE CHOICE QUESTIONS 1. Which of the following is not a metal? (a) copper (c) aluminium (b) sulphur (d) iron 2. The substance that will be
Creation Settings. can be separated into two or more pure substances by distillation. can be separated into two or more substances by chromatography.
 Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
2A Chemistry - Classification of Substances
 CHEMISTRY The world is made up of a variety of substances. Some of these occur naturally in our environment, others are made through the combination of naturally occurring substances to form new materials.
CHEMISTRY The world is made up of a variety of substances. Some of these occur naturally in our environment, others are made through the combination of naturally occurring substances to form new materials.
S1 Building Blocks Summary Notes
 S1 Building Blocks Summary Notes Atoms & Molecules 1 We are developing our understanding of atoms and molecules. Atoms are the simplest building blocks of every substance in the universe. There are just
S1 Building Blocks Summary Notes Atoms & Molecules 1 We are developing our understanding of atoms and molecules. Atoms are the simplest building blocks of every substance in the universe. There are just
Part 1 Pre-16 The platinum story
 Materials 1. Food 3 3 Part 1 Pre-16 The platinum story Teacher s notes This section includes information on platinum (which can be used along with the video), questions to test comprehension, a word search
Materials 1. Food 3 3 Part 1 Pre-16 The platinum story Teacher s notes This section includes information on platinum (which can be used along with the video), questions to test comprehension, a word search
Metals. N4 & N5 Homework Questions
 St Peter the Apostle High School Chemistry Department Metals N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
St Peter the Apostle High School Chemistry Department Metals N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
Nickel Electroplating
 Nickel Electroplating In a galvanic or voltaic electrochemical cell, the spontaneous reaction occurs and electrons flow from the anode (oxidation) to the cathode (reduction). In an electrolytic cell, a
Nickel Electroplating In a galvanic or voltaic electrochemical cell, the spontaneous reaction occurs and electrons flow from the anode (oxidation) to the cathode (reduction). In an electrolytic cell, a
1. What are amphoteric oxides? Give two examples of amphoteric oxides.
 1. What are amphoteric oxides? Give two examples of amphoteric oxides. Amphoteric oxides are the oxides, which react with both acids and bases to form salt and water. E.g. ZnO and Al 2 O 3. 2. Name two
1. What are amphoteric oxides? Give two examples of amphoteric oxides. Amphoteric oxides are the oxides, which react with both acids and bases to form salt and water. E.g. ZnO and Al 2 O 3. 2. Name two
ST EDWARD S OXFORD 13+ Entrance Assessment SCIENCE 1 Hour Candidate Name
 ST EDWARD S OXFORD 13+ Entrance Assessment 2013-14 SCIENCE 1 Hour Candidate Name Question 1: (a) The diagram below shows two different cells. Complete the table below with the names of the numbered structures.
ST EDWARD S OXFORD 13+ Entrance Assessment 2013-14 SCIENCE 1 Hour Candidate Name Question 1: (a) The diagram below shows two different cells. Complete the table below with the names of the numbered structures.
Elements. The periodic table organizes elements by their chemical properties. Main Idea. Key Terms group period nonmetal family metal metalloid
 Section 3 5B, 5C s The periodic table organizes elements by their chemical properties. Some elements are metals. Some elements are nonmetals or metalloids. Elements Key Terms group period nonmetal family
Section 3 5B, 5C s The periodic table organizes elements by their chemical properties. Some elements are metals. Some elements are nonmetals or metalloids. Elements Key Terms group period nonmetal family
Our country, our future S2 CHEMISTRY DURATION: 2 HOUR
 Our country, our future S2 CHEMISTRY Exam 1 DURATION: 2 HOUR INSTRUCTIONS: This paper consists of two sections A and B, Attempt all questions in section A and B For section A, circle the most correct alternative
Our country, our future S2 CHEMISTRY Exam 1 DURATION: 2 HOUR INSTRUCTIONS: This paper consists of two sections A and B, Attempt all questions in section A and B For section A, circle the most correct alternative
METALS AND THEIR COMPOUNDS
 METALS AND THEIR COMPOUNDS Metals are elements whose atoms ionize by electron loss, while non-metals are elements whose atoms ionize by electron gain. Metals are in groups 1, 2 and 3 of the periodic table.
METALS AND THEIR COMPOUNDS Metals are elements whose atoms ionize by electron loss, while non-metals are elements whose atoms ionize by electron gain. Metals are in groups 1, 2 and 3 of the periodic table.
Electricity and Chemistry
 Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Reactivity Series. Question Paper. Cambridge International Examinations. Score: /39. Percentage: /100
 Reactivity Series Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International Examinations Metals Reactivity Series Question Paper Time llowed: 47 minutes Score:
Reactivity Series Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International Examinations Metals Reactivity Series Question Paper Time llowed: 47 minutes Score:
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example,
 SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SAMPLE PAGES PAGES. Extraction of metals from metal oxides. mixture of iron sand and coal are heated as they move down kiln, by force of gravity
 Unit 11.5 Metals and Non-metals Topic 3: Extraction of metals and corrosion In the previous two Topics we looked at the physical and chemical properties of metals. In Topic 3 we now examine how metals
Unit 11.5 Metals and Non-metals Topic 3: Extraction of metals and corrosion In the previous two Topics we looked at the physical and chemical properties of metals. In Topic 3 we now examine how metals
Double Award Science: Chemistry
 New Specification Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Higher Tier ML [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING
New Specification Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Higher Tier ML [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING
Suggest one reason why spoons are electroplated. ... Why is hydrogen produced at the negative electrode and not sodium?
 Q1.This question is about electrolysis. (a) Metal spoons can be coated with silver. This is called electroplating. Suggest one reason why spoons are electroplated. (b) When sodium chloride solution is
Q1.This question is about electrolysis. (a) Metal spoons can be coated with silver. This is called electroplating. Suggest one reason why spoons are electroplated. (b) When sodium chloride solution is
Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee
 Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 1 Engineering Materials and Processing Lecture -
Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 1 Engineering Materials and Processing Lecture -
Naming and Classifying the Elements. nobelium antimony. oxygen arsenic. phosphorus beryllium. platinum bismuth. plutonium boron.
 6.2 Naming and Classifying the Elements To learn more about the origins of the names of the elements, visit www.science.nelson.com GO The names of the elements have varied origins. Some elements are named
6.2 Naming and Classifying the Elements To learn more about the origins of the names of the elements, visit www.science.nelson.com GO The names of the elements have varied origins. Some elements are named
INDEX METALS FERRIC METALS NON-FERRIC METALS WORKING WITH METALS METAL FORMING TECHNIQUES ENVIRONMENTAL IMPACT OF METAL EXTRACTION
 METALS INDEX METALS FERRIC METALS NON-FERRIC METALS WORKING WITH METALS METAL FORMING TECHNIQUES ENVIRONMENTAL IMPACT OF METAL EXTRACTION METALS CHEMICAL ELEMENTS FOUND IN NATURE IN SOLID STATE AT ROOM
METALS INDEX METALS FERRIC METALS NON-FERRIC METALS WORKING WITH METALS METAL FORMING TECHNIQUES ENVIRONMENTAL IMPACT OF METAL EXTRACTION METALS CHEMICAL ELEMENTS FOUND IN NATURE IN SOLID STATE AT ROOM
Iron filings (Fe) 56g IRON + SULPHUR IRON SULPHIDE
 W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
Draw a ring around the correct word in the box to complete the sentence.
 Q. Iron is the main structural metal used in the world. (a) The diagram represents the particles in iron, Fe. Draw a ring around the correct word in the box to complete the sentence. Iron is described
Q. Iron is the main structural metal used in the world. (a) The diagram represents the particles in iron, Fe. Draw a ring around the correct word in the box to complete the sentence. Iron is described
Properties A Metal B Non- metal Electronic configuration?? Nature of oxides?? Oxidizing or reducing action?? Conduction of heat and electricity??
 CLASS: X NCERT (CBSE) SCIENCE: Chemistry Page: 1 Question 1: Compare the properties of a typical metal and a non-metal on the basis of the following. Fill in Column A, B. Properties A Metal B Non- metal
CLASS: X NCERT (CBSE) SCIENCE: Chemistry Page: 1 Question 1: Compare the properties of a typical metal and a non-metal on the basis of the following. Fill in Column A, B. Properties A Metal B Non- metal
 CHAPTER 3 METALS AND NON-METALS About 118 elements are known today. There are more than 90 metals, 22 non metals and a few metalloids. Sodium (Na), potassium (K), magnesium(mg), aluminium(al), calcium(ca),
CHAPTER 3 METALS AND NON-METALS About 118 elements are known today. There are more than 90 metals, 22 non metals and a few metalloids. Sodium (Na), potassium (K), magnesium(mg), aluminium(al), calcium(ca),
Materials are all substances and include metals, ceramics and plastics as well as natural and new substances.
 National 4 Materials It is hard to imagine life without mobile gadgets such as iphones, ipads and MP3 players. Yet twenty years ago these handy gadgets such as the mobile phone where bigger and cost five
National 4 Materials It is hard to imagine life without mobile gadgets such as iphones, ipads and MP3 players. Yet twenty years ago these handy gadgets such as the mobile phone where bigger and cost five
Metals. Key Concepts. Name Date Class. Chapter 4 Elements and the Periodic Table Section 3 Summary
 Chapter 4 Elements and the Periodic Table Section 3 Summary Metals Key Concepts What are the physical properties of metals? How does the reactivity of metals change across the periodic table? How are synthetic
Chapter 4 Elements and the Periodic Table Section 3 Summary Metals Key Concepts What are the physical properties of metals? How does the reactivity of metals change across the periodic table? How are synthetic
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS SUB-TOPIC 9.3 TO 5 EXTRACTION OF METALS; RECYLING OF METALS; IRON LEARNING OUTCOMES
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 9: METALS 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 9: METALS SUB-TOPIC 9.3 TO 5 EXTRACTION OF METALS; RECYLING OF METALS; IRON LEARNING OUTCOMES
3 Metals and Non-Metals
 3 Intext Questions On Page 40 Question 1. Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor
3 Intext Questions On Page 40 Question 1. Give an example of a metal which (i) is a liquid at room temperature. (ii) can be easily cut with a knife. (iii) is the best conductor of heat. (iv) is a poor
Page 2. Q1.Copper is a transition metal. (a) (i) Where is copper in the periodic table? in the central block. in Group 1. in the noble gas group
 Q1.Copper is a transition metal. (a) (i) Where is copper in the periodic table? Tick ( ) one box. in the central block in Group 1 in the noble gas group (ii) What is a property of copper? Tick ( ) one
Q1.Copper is a transition metal. (a) (i) Where is copper in the periodic table? Tick ( ) one box. in the central block in Group 1 in the noble gas group (ii) What is a property of copper? Tick ( ) one
How Do You Choose Cookware?
 Cookin' Chem Activity 6 How Do You Choose Cookware? GOALS In this activity you will: Explore the concept of specific heat capacity. Experimentally determine the specific heat capacity of various substances.
Cookin' Chem Activity 6 How Do You Choose Cookware? GOALS In this activity you will: Explore the concept of specific heat capacity. Experimentally determine the specific heat capacity of various substances.
Chemistry IGCSE Paper 6 revision guide
 Chemistry IGCSE Paper 6 revision guide You can change the temperature and concentration used (not both at the same time though) You need to keep the diameter of the conical flask the same, if it is
Chemistry IGCSE Paper 6 revision guide You can change the temperature and concentration used (not both at the same time though) You need to keep the diameter of the conical flask the same, if it is
METALS AND NONMETALS
 Suggested time allotment: 5 to 6 hours MODULE 5 METALS AND NONMETALS Elements are the simplest form of substances. This means that whatever you do with an element, it remains to be the same element. Its
Suggested time allotment: 5 to 6 hours MODULE 5 METALS AND NONMETALS Elements are the simplest form of substances. This means that whatever you do with an element, it remains to be the same element. Its
The table below shows some of the properties of the elements cobalt and nickel.
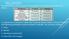 BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
Chapters 1&2 Practice Problems
 Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Chapter No. 3 Metals and Non Metals (I) (Multiple choice questions)
 Chapter No. 3 Metals and Non Metals (I) (Multiple choice questions) Q1. Which of the following metal forms amphoteric oxides? 1) Copper 2) Silver 3) Aluminium 4) Iron Q2. Aqua regia is a mixture of 1)
Chapter No. 3 Metals and Non Metals (I) (Multiple choice questions) Q1. Which of the following metal forms amphoteric oxides? 1) Copper 2) Silver 3) Aluminium 4) Iron Q2. Aqua regia is a mixture of 1)
SAMPLE. MEM05051A Select welding processes. MEM05 Metal and Engineering Training Package. Learner guide Version 1
 MEM05 Metal and Engineering Training Package MEM05051A Select welding processes Learner guide Version 1 Training and Education Support Industry Skills Unit Meadowbank Product Code: 5721 Acknowledgments
MEM05 Metal and Engineering Training Package MEM05051A Select welding processes Learner guide Version 1 Training and Education Support Industry Skills Unit Meadowbank Product Code: 5721 Acknowledgments
Elements. Students must include the following information in their superhero element profile:
 TEACHER MATERIALS Directions: Students will choose an element from the list below and create a superhero profile. On the first page, students will design a superhero and create a tagline that embodies
TEACHER MATERIALS Directions: Students will choose an element from the list below and create a superhero profile. On the first page, students will design a superhero and create a tagline that embodies
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET
 INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
N Goalby chemrevise.org
 4.10 Using Resources Humans use the Earth s resources to provide warmth, shelter, food and transport. Natural resources, supplemented by agriculture, provide food, timber, clothing and fuels. Finite resources
4.10 Using Resources Humans use the Earth s resources to provide warmth, shelter, food and transport. Natural resources, supplemented by agriculture, provide food, timber, clothing and fuels. Finite resources
Metals. General information
 Metals. General information Read and copy the following text: Metals are divided into two groups: Metals that contain iron are called Ferrous Metals (Alloys). Metals that do not contain iron are called
Metals. General information Read and copy the following text: Metals are divided into two groups: Metals that contain iron are called Ferrous Metals (Alloys). Metals that do not contain iron are called
