Supporting Information Lin et al.
|
|
|
- Bruno Simmons
- 6 years ago
- Views:
Transcription
1 Supporting Information Lin et al. SI Materials and Methods. Isolation and culture of human MSCs and ECFCs Human watmscs were isolated from normal discarded subcutaneous white adipose tissue obtained during clinically indicated procedures in accordance with an Institutional Review Board-approved protocol, as previously described (1). Human bmmscs were isolated from the mononuclear cell fraction of bone marrow aspirates as previously described (2). Both, watmscs and bmmscs were cultured on uncoated plates using MSC-medium: MSCGM (Lonza) supplemented with 10% MSC-qualified FBS (Hyclone), 1x glutamine-penicillin-streptomycin (GPS; Invitrogen). All experiments were carried out with MSCs between passages 4-6. Human ECFCs were isolated from umbilical cord blood samples in accordance with an Institutional Review Board-approved protocol as previously described (3). ECFCs were cultured on 1% gelatin-coated plates using ECFC-medium: EGM-2 (except for hydrocortisone; Lonza) supplemented with 20% FBS, 1x GPS. All experiments were carried out with ECFCs between passages 5-8. In vivo cell engraftment Animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee at Children s Hospital Boston in an AAALAC-approved facility. Human MSCs (1.2x10 6 cells), resuspended in 200 µl of ice-cold Phenol Red-free Matrigel (BD Bioscience) and in the presence or absence of ECFCs (8x10 5 cells), were subcutaneously injected on the back of 6-week-old male athymic nu/nu mice (Massachusetts General Hospital, Boston, MA) or GFP transgenic severe combined immunodeficiency (SCID) mice. GFP transgenic SCID mice were generated by crossing GFP-expressing UBI-GFP/BL6 mice with C57BL/6J-SCID mice (Jackson Laboratories). Alternatively, on indicated experiments, bovine collagen type 1 (Trevigen) based gels were used instead of Matrigel, as previously described (4). On indicated experiments, supplements were added to the cell-matrigel mixture prior to implantation: BMP-2 (R&D Systems; 2 µg/implant); PDGF-BB (R&D Systems; 100 ng/implant); PDGFR inhibitor Tyrphostin AG1296 (Sigma; 1.33 µg/implant); or concentrated ECFCconditioned medium (100 µl/implants). Conditioned media were generated for 24 h by 8x10 5 ECFCs in EBM-2, 5% FBS, and then concentrated 10-fold. Mice were euthanized and grafts were explanted after either 2, 7, or 28 days. On indicated experiments, rhodamine-conjugated Ulex europaeus agglutinin I lectin (UEA-1; Vector Laboratories) (100 µl; 1 mg/ml in saline) was intravenously injected into the tail vein of implant-bearing mice 10 min before harvesting the implants. Cell retrieval and sorting Grafts were removed from euthanized mice and cells were retrieved by enzymatic (1 mg/ml collagenase and 2.5 U/mL dispase) digestion for 1 h at 37 C. Retrieved cells were then prepared into single-cell suspensions and sorted into hcd90 + and hcd90 - cells by magneticactivated cell sorting (MACS) using magnetic beads (Miltenyi Biotec) coated with anti-human CD90 antibodies. Alternatively, retrieved cells were sorted into hcd90 + /PDGFR-β + and hcd90 + / 1
2 PDGFR-β - cells by fluorescence-activated cell sorting (FACS) using a FACSAria II 5-LASER sorter system (BD Bioscience). Sorted cells were analyzed either immediately or after an indicated period of culture. Analyses included (1) expression of cell markers, (2) CFU-F activity, (3) in vitro differentiation potential, and (4) in vivo engraftment potential in secondary mice. Histological analyses Explanted grafts were fixed overnight in 10% buffered formalin, embedded in paraffin and sectioned (7 µm-thick). Hematoxylin and eosin (H&E)-stained sections were examined for the presence of adipocytes, adipocyte density (adipocytes/mm 2 ), and adipocyte size (µm 2 ) using ImageJ 1.47v software (National Institutes of Health). Von kossa-stained sections were used to measure tissue mineralization (% of section area mineralized) using ImageJ. For immunostaining, sections were deparaffinized and antigen retrieval was carried out with tris EDTA buffer (10 mm Tris-Base, 2 mm EDTA, 0.05 % Tween-20, ph 9.0), as previously described (2). Sections were then blocked for 30 min in 5 10 % blocking serum and incubated with primary antibodies for 1 h at room temperature. Primary antibodies used are detailed in Supplemental Experimental Procedures. Horseradish peroxidase-conjugated mouse secondary antibody (1:200; Vector Laboratories) and 3,3 -diaminobenzidine (DAB) were used for detection of hcd31, followed by hematoxylin counterstaining and Permount mounting. Fluorescent staining was performed using either Texas Red- or FITC-conjugated secondary antibodies (1:200) followed by DAPI counterstaining (Vector Laboratories). On indicated experiments, additional fluorescent staining was performed using rhodamine-conjugated UEA-1 (1:200). Sections taken from indicated murine tissues and from normal discarded human dermis and adipose tissues served as control. Ex vivo multilineage differentiation Adipogenesis: confluent MSCs were cultured for 10 days in low-glucose DMEM with 10% FBS, 1x GPS, and adipogenic supplements (5 µg/ml insulin, 1 µm dexamethasone, 0.5 mm isobutylmethylxanthine, 60 µm indomethacin, 1 µm rosiglitazone). Differentiation into adipocytes was assessed by Oil Red O staining. Cells cultured in medium lacking adipogenic supplements served as a negative control. Osteogenesis: confluent MSCs were cultured for 21 days in low-glucose DMEM with 10% FBS, 1x GPS, and osteogenic supplements (1 µm dexamethasone, 10 mm β- glycerophosphate, 60 µm ascorbic acid-2-phosphate). Osteogenic differentiation was assessed by alkaline phosphatase and von Kossa staining. Cells cultured in medium lacking osteogenic supplements served as a negative control. Chondrogenesis: suspensions of MSCs were gently centrifuged in 15 ml polypropylene centrifuge tubes (500,000 cells/tube). Pellets were cultured in high-glucose DMEM medium with 1x GPS, and chondrogenic supplements (1X insulin-transferrin-selenium, 1 µm dexamethasone, 100 µm ascorbic acid-2-phosphate, and 10 ng/ml TGF-β3). After 21 days, pellets were fixed in 10% buffered formalin, cryoprotected in 30% (w/v) sucrose solution, embedded in O.C.T. medium, and sectioned (8 µm-thick) using a cryostat microtome. Chondrogenic differentiation was assessed by the presence of glycosaminoglycans with Alcian Blue staining. MSCs cultured in the absence of TGF-β3 served as negative controls and failed to form compact spheroids. Smooth muscle myogenesis: MSCs were co-cultured for 7 days with ECFCs (1:1 ratio) on fibronectin-coated, 2-well Permanox chamber slides at a density of 1x10 5 cell/well using ECFC- 2
3 medium. Expression of smooth muscle myosin heavy chain (MYH11) was evaluated by immunofluorescence using a rabbit monoclonal antibody followed by anti-rabbit FITCconjugated secondary antibody. ECFCs were stained with rhodamine-conjugated UEA-1 (1:200). Monocultures of MSCs served as negative control. Protein array Samples of conditioned media were collected from monocultures of MSCs and ECFCs and from co-cultures of MSCs+ECFCs (1:1 ratio). Cells were cultured in EBM-2, 5% FBS for 24 h and collected media were filtered (0.2 µm) and then concentrated 10-fold (Amicon Ultra centrifugal filters; 3 kda cut off; Millipore). The presence of angiogenic cytokines and growth factors was evaluated with a human angiogenesis protein array (R&D Systems Inc.), according to the manufacturer s instructions. Antigen-antibody complexes were visualized using LumiGLO substrate (Kirkegaard & Perry Laboratories, Inc.) and chemiluminescent sensitive film (Kodak). Densitometry was performed by image analysis (ImageJ) to estimate the amount of protein present in each sample. sirna silencing Three sirna constructs targeting human PDGF-B, as well as the negative control sirna construct, were obtained from Origene (Trilencer-27 sirna; SR303420). Each sirna construct (100 pmole) was transfected into 2x10 6 ECFCs by electroporation using Amaxa HUVEC Nuclefector kit (Lonza). After 48 h, mrna were isolated from transfected cells and qrt-pcr was carried out to validate the efficiency of sirna silencing. For in vivo cell implantation, ECFCs 24 h after sirna transfection were used. Colony-forming unit-fibroblast (CFU-F) activity MSCs were plated at clonal density (500 cell/100 cm 2 ) onto 1% gelatin-coated plates using MSC-medium. Medium was refreshed every 4 days. At day 10, colonies containing 200 cells were scored under a fluorescence microscope following nuclei staining with DAPI. On indicated experiments, colonies were also examined for expression of green fluorescence and binding of rhodamine-conjugated UEA-1 identify the presence of murine GFP + colonies and human ECFCs, respectively. Quantitative RT-PCR Quantitative RT-PCR (qrt-pcr) was carried out in RNA lysates prepared from either cells in culture or explanted grafts. Total RNA was isolated with a RNeasy kit (QIAGEN), and cdna was prepared using reverse transcriptase III (Invitrogen), according to the manufacturer s instructions. Multigene transcriptional profiling, a form of qrt-pcr, was used to determine the number of mrna copies per cell normalized to ribosomal 18S rrna abundance, as previously described (4). On indicated analyses, gene expression was normalized to human β-actin (ACTB) expression. Real-time PCR primer sequences are displayed in Table S1. Heat maps were generated using the Gene-E software package ( software/gene-e/). Lysates from human adult peripheral blood mononuclear cells (PBMNCs), aortic smooth muscle cells (SMCs; Lonza), and dermal fibroblasts (NHDFs; Lonza) served as control. Flow cytometry 3
4 Flow cytometric analyses were performed using a Guava easy Cyte HT FACS flow cytometer (Millipore). Antibody labeling was carried out for 20 min on ice followed by 3 washes with PBS supplemented with 1% BSA and 0.2 mm EDTA. Cell staining was followed by fixation with 1% paraformaldehyde. All fluorophore-conjugated antibodies used are displayed in Table S2. Cell apoptosis was evaluated following APC-conjugated Annexin V and propidium iodide (PI) staining (Annexin V Apoptosis Detection Kit; ebioscience), according to the manufacturer s instructions. Immunofluorescence Immunofluorescence was carried out using primary antibodies described in Table S2, followed by fluorescently-conjugated secondary antibodies (1:200; Vector Laboratories) and Vectashield mounting medium with DAPI (Vector Laboratories). Murine dermal ECs (m-decs) and adipose tissue-derived MSCs (m-watmscs) served as control. Microscopy Images were taken using an Axio Observer Z1 inverted microscope (Carl Zeiss) and AxioVision Rel. 4.8 software. Phase microscopy images were taken with an AxioCam MRm camera and 5x/ 0.16 or 10x/0.3 objective lens. Fluorescent images were taken with an ApoTome.2 Optical sectioning system (Carl Zeiss) and 20x/0.8 or 40x/1.4 oil objective lens. Non-fluorescent images were taken with an AxioCam MRc5 camera using a 40x/1.4 objective oil lens. Statistical analyses Data were expressed as mean ± standard error of the mean (SEM). Means were compared using unpaired Student s t tests. Comparisons between multiple groups were performed by ANOVA followed by bonferroni post-test analysis. All statistical analyses were performed using GraphPad Prism v.5 software (GraphPad Software Inc). P<0.05 was considered statistically significant. SI References 1. Lin RZ, et al. (2013) Human white adipose tissue vasculature contains endothelial colonyforming cells with robust in vivo vasculogenic potential. Angiogenesis 16(4): Melero-Martin JM, et al. (2008) Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 103(2): Melero-Martin JM, et al. (2007) In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109(11): Lin RZ, et al. (2011) Induction of erythropoiesis using human vascular networks genetically engineered for controlled erythropoietin release. Blood 118(20):
5 Table S1 - Primer sequences used in the study * Gene Forward Reverse β-actin (ACTB) CTGGAACGGTGAAGGTGACA AGTCCTCGGCCACATTGTG 18S rrna TGTCTCAAAGATTAAGCCATGCA GCGACCAAAGGAACCATAACTG CD90 GCCTAACGGCCTGCCTAGT GGGTGAACTGCTGGTATTCTCAT CD73 TATCCGGTCGCCCATTGAT GGCAATACAGCAGCCAGGTT PDGF-A CACAGCATCCGGGACCTC AGGCTGGTGTCCAAAGAATCC PDGF-B CTCGATCCGCTCCTTTGATG GGGTCATGTTCAGGTCCAACTC PDGFR-β GGTGGGCACACTACAATTTGC GGTGGGTAGGCCTCGAACA CD31 CACCTGGCCCAGGAGTTTC AGTACACAGCCTTGTTGCCATGT vwf GTCGAGCTGCACAGTGACATG GCACCATAAACGTTGACTTCCA VE-cadherin GAACCCAAGATGTGGCCTTTAG GATGTGACAACAGCGAGGTGTAA VEGFR-2 GGCAAATGTGTCAGCTTTGTACA GGTCACGTGGAAGGAGATCAC enos AGATCTCCGCCTCGCTCAT GTCTCGGAGCCATACAGGATTG CD45 GTGGAGAAAGGACGCATGCT TGCCAAGAGTTTAAGCCACAAA CD11b TCGAGTACGTGCCACACCAA TTCCGAAGCTCAGCCAGAA CD14 GAACTGACGCTCGAGGACCTA GCAAGTCCTGTGGCTTCCA PPARγ GCCAAGCTGCTCCAGAAAAT GAGCGGGTGAAGACTCATGTC FABP4 TGGTGGTGGAATGCGTCAT CAACGTCCCTTGGCTTATGC C/EBPα GCCTTCAGCATTGCCTAGGA GGGCACAGAGGCCAGATACA Leptin GTATGCCTTCCAGAAACGTGATC GGCCAGCACGTGAAGAAGA Adiponectin CCACTATGATGGCTCCACTGGTA AGGCTGACCTTCACATCCTTCA Adiposin TGGTTGGTCTTTATTGAGCACCTA GAGATGGAGTCGCGCTACGT LPL TGTGGTGGACTGGCTGTCA TCCTGTCCCACCAGTTTGGT RUNX2 CCTCACTGAGAGCCGCTTCT AGGGACATGCCTGAGGTGACT SOX9 CCCCAACAGATCGCCTACAG TCTGGTGGTCGGTGTAGTCGTA Osterix TGAGCTGGAGCGTCATGTG GGTGGTCGCTTCGGGTAAA Osteopontin CGAGGAGTTGAATGGTGCATAC CATCCAGCTGACTCGTTTCATAA Osteocalcin AAGAGACCCAGGCGCTACCT AACTCGTCACAGTCCGGATTG SM22 CTGCAGGAGGGAAAGCATGT AGGTCGTCCGTAGCCTGTCA α-sma GGAAGGACCTGTATGCCAACA AGCGCGGTGATCTCTTTCTG MYH11 CAGCCCAGGATGATGAGATGT TCCTCGCTGAAACCCATGA PDGFR-α CCTGGCTAAGAATCTCCTTGGA CAGCAGCCACCGTGAGTTC Desmin TCCAGTCCTACACCTGCGAGAT GGCAAATCGGTCCTCCAAT Ang1 CGCTGCCATTCTGACTCACATA CCGGTTATATCTTCTCCCACTGTTT * Primers were designed to specifically recognize human, but not murine, genes. Primers for 18S rrna were valid for both human and mouse. 5
6 Table S2 - Antibodies used in the study Antibody Vendor Clone Dilution * PE-conjugated mouse anti-human CD90 BD Pharmingen 5E10 5 µl/10 6 cells (MACS) 1:100 (FC) APC-conjugated mouse anti-human CD90 ebioscience 5E10 1:100 (FC) PE-conjugated mouse anti-human CD44 BD Pharmingen 515 1:100 (FC) FITC-conjugated mouse anti-human CD105 R&D Systems :100 (FC) PE-conjugated mouse anti-human CD29 BD Pharmingen MAR4 1:100 (FC) PE-conjugated mouse anti-human CD73 BD Pharmingen AD2 1:100 (FC) APC-conjugated mouse anti-human PDGFR-β BioLegend 18A2 1:10 (FC) FITC-conjugated mouse anti-human CD31 BD Pharmingen WM59 1:10 (FC) PE-conjugated mouse anti-human CD31 Ancell 158-2B3 1:100 (FC) FITC-conjugated mouse anti-human CD45 BD Pharmingen 2D1 1:100 (FC) FITC-conjugated rat anti-mouse CD45 BD Pharmingen 30-F11 1 µl/10 6 cells (MACS) 1:100 (FC) PE-Cy5-conjugated rat anti-mouse CD45 BD Pharmingen 30-F11 1:100 (FC) PE -conjugated rat anti-mouse CD29 ebioscience HMb1-1 1:100 (FC) APC-conjugated rat anti-mouse CD31 ebioscience 390 1:50 (FC) Mouse anti-human Vimentin Abcam V9 1:200 (IF) 1:200 (IHF) Rabbit anti-myh11 Biomedical BT-562 1:200 (IF) Technology, Inc. Mouse anti-human CD31 DakoCytomation JC70A 1:50 (IHC) Goat anti-perilipin-a Abcam ab :200 (IF) Rabbit anti-osterix Abcam ab :200 (IF) Rabbit anti-pdgfr-β Cell Signaling 28E1 1:200 (IF) Rabbit anti-αsma Abcam ab5694 1:200 (IHF) Rabbit anti-gfp Abcam ab290 1:200 (IHF) TexasRed -conjugated horse anti-mouse IgG Vector Laboratories TI :200 (IF) 1:200 (IHF) FITC-conjugated horse anti-mouse IgG Vector Laboratories FI :200 (IHF) Peroxidase-conjugated horse anti-mouse IgG Vector Laboratories PI :200 (IHC) Texas Red-conjugated goat anti-rabbit IgG Vector Laboratories TI :200 (IF) Texas Red -conjugated goat anti-rabbit IgG Vector Laboratories TI :200 (IHF) FITC-conjugated goat anti-rabbit IgG Vector Laboratories FI :200 (IHF) * MACS: Magnetic-activated cell sorting; FC: flow cytometry; IF: immunofluorescence cell staining; IHC: immunohistochemistry staining; IHF: immuno-histofluorescence staining. 6
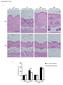
7 Fig. S1. Isolation and phenotypical characterization of human MSCs. (A) Characteristic spindleshape morphology of MSC colonies derived from normal white adipose tissue (watmscs) and bone marrow aspirates (bmmscs) (n = 3 separate subjects per tissue). (B) Cytometric analysis of cultured MSCs revealed uniform expression of mesenchymal cell markers (CD90, CD44, CD105, CD29, CD73 and PDGFR-β) and lack of CD31 and CD45 expression. Red and bluelined histograms represent cells stained with fluorescent antibodies. Isotype-matched controls are overlaid in solid grey histograms. (C) Heat map generated from qrt-pcr analysis of cultured MSCs revealed high expression of mesenchymal markers (top) and low expression of endothelial (middle) and hematopoietic (bottom) markers. mrna data are normalized to ribosomal 18S rrna abundance. Human blood-derived ECFCs and PBMNCs served as controls. 7
8 Fig. S2. Ex vivo multilineage differentiation of MSCs. (A) Induction of adipogenic differentiation (10 days) assessed by intracellular accumulation of oil droplets (oil red O staining). Inset depicts watmscs under non-inducing conditions. (B) Upregulation of PPAR-γ following adipogenic induction. Analyses were performed by qrt-pcr and data normalized to ribosomal 18S rrna abundance. (C) Ex vivo induction of osteogenic differentiation (21 days) assessed by calcium deposition (von Kossa staining). Inset depicts watmscs under non-inducing conditions. (D) Upregulation of RUNX2 following osteogenic induction. Data normalized to 18S rrna abundance. (E) Induction of chondrogenic differentiation (21 days) assessed in pellet culture by glycosaminoglycan deposition (Alcian blue staining). (F) Induction of smooth-muscle-myogenic differentiation (7 days) assessed by expression of smooth muscle myosin heavy chain (MYH11) upon direct contact with ECFCs. ECFCs were identified by binding of rhodamine-conjugated UEA-1 (red). Cell nuclei were counterstained with DAPI (blue). Scale bars: 100 µm (A, C, and F) and 200 µm (E). Bars represent mean ± SEM (n = 3). * P < ** P <
9 Fig. S3. In vivo pro-vasculogenic properties of MSCs. watmscs and bmmscs with and without ECFCs were combined in Matrigel and the mixture subcutaneously injected into nude mice for 7 days. Microvessel density was determined as the number of total and human (ECFC-lined) blood vessels per unit of area in explants. Bars represent mean ± SEM (n = 4). 9
10 10
11 Fig. S4. Identification of transplanted human MSCs and ECFCs by flow cytometry. (A) Human MSCs were subcutaneously transplanted with ECFCs into nude mice. Grafts were explanted at day 7 and the retrieved cells analyzed by flow cytometry. Cells were identified as follows: nonhematopoietic cells (mcd45 - cells; R1 gate); human MSCs (mcd45 - /hcd90 + /hcd31 - cells; R2 gate); human ECFCs (mcd45 - /hcd90 - /hcd31 + cells; R3 gate); murine non-hematopoietic cells (mcd45 - /hcd90 - /hcd31 - cells; R4 gate); murine ECs (mcd45 - /mcd29 + /mcd31 + cells; R5 gate); and other murine stromal cells (mcd45 - /mcd29 + /mcd31 - cells; R6 gate). (B) Flow cytometry analysis of cells retrieved from grafts that were seeded with MSCs but without ECFCs. (C) Flow cytometry analysis of cells retrieved from grafts that were seeded with murine cells (m-watmscs + m-decs). (D) Flow cytometry analysis of cells retrieved from native murine subcutaneous white adipose tissue. (E-H) Cytometric quantification of cells retrieved from grafts at day 7: (E) human ECFCs; (F) human MSCs; (G) murine ECs; and (H) other murine stromal cells. Bars represent mean ± SEM (n = 4 mice per group). * P < 0.05, ** P < 0.01, and *** P <
12 Fig. S5. Magnetic-activated sorting of MSCs retrieved from subcutaneous grafts. (A) Human MSCs were subcutaneously transplanted with ECFCs into GFP-SCID mice. Grafts were explanted at day 7 and the retrieved cells sorted into hcd90 + and hcd90 - cells by magneticactivated cell sorting (MACS) using magnetic beads coated with anti-human CD90 antibodies. (B-D) Flow cytometry analysis of retrieved cells. Cells were identified as either murine (R1 gate) or human (R2) by virtue of GFP expression. Cytometric analyses were performed on: (B) nonsorted retrieved cells, (C) sorted hcd90 + cells, and (D) sorted hcd90 - cells. 12
13 Fig. S6. Identification of colonies formed by human MSCs retrieved from subcutaneous grafts. MSCs were subcutaneously transplanted with ECFCs into GFP-SCID mice. Engrafted MSCs were retrieved from explants (day 7) and selected as hcd90 + cells by MACS (see Fig. S4.). CFU-F activity of retrieved MSCs was tested at clonal density (500 cell/100 cm 2 ). Phase contrast microscopy (top) and immunofluorescence (bottom) was used to reveal the identity of the colonies formed. (A) Colonies formed by murine cells were identified as GFP + /UEA-1 - cells. (B) Colonies formed by human ECFCs were identified as GFP - /UEA-1 + cells. (C) Colonies formed by human MSCs were identified as GFP - /UEA-1 - cells and categorized into small, intermediate and large colonies. Scale bars: 50 µm. 13
14 Fig. S7. Engraftment and adipogenic differentiation of human MSCs in collagen-fibrin gels. (A) Human watmscs (1.2x10 6 cells) were subcutaneously transplanted into nude mice with or without ECFCs (8x10 5 cells) using collagen-fibrin gels. H&E staining revealed differential adipocyte abundance (28 days). Insets depict representative macroscopic views of the explants. (B) Histological quantification of adipocyte density in explants (28 days). Sections from native murine subcutaneous white adipose tissue (WAT) served as control. Scale bars: 200 µm (top panels), 50 µm (bottom panels), and 5 mm (insets). Bars represent mean ± SEM (n = 4 mice per group). ** P < 0.01 compared to grafts with MSCs alone. 14
15 Fig. S8. Expression of vimentin by human MSCs. (A) Immunofluorescent staining of human bmmscs and watmscs using an antibody against human vimentin (h-vimentin; red) and FITCphalloidin for F-actin (green). Murine dermal ECs (m-decs) and adipose tissue-derived MSCs (m-watmscs) served as negative control and did not express h-vimentin. (B) Fluorescent staining of sections taken from indicated murine tissues using an antibody against human vimentin (h-vimentin; green) and rhodamine-phalloidin for F-actin (red). (C) Fluorescent staining of tissue sections from human dermis using antibodies against human vimentin (h-vimentin; green) and UEA-1 (red). (D) Fluorescent staining of tissue sections from human subcutaneous adipose tissue using antibodies against human vimentin (h-vimentin; green) and perilipin-a (red). Cell nuclei were counterstained with DAPI (blue; A-D). Scale bars: 20 µm (A), 100 µm (B, C right panel, and D), and 200 µm (C left panel). 15
16 Fig. S9. Adipogenic differentiation of human MSCs in GFP-SCID mice. (A) Human MSCs (both bmmscs and watmscs) were subcutaneously transplanted into GFP-SCID mice with ECFCs. Grafts were explanted at day 28. (B) Immunohistochemical analysis of adipocytes in explanted grafts. Adipocytes were identified by expression of perilipin-a (red). Murine cells were identified by expression of GFP (green). Cell nuclei were counterstained with DAPI (blue). Dashed white lines mark the boundary between implants (right) and host tissue (left). White asterisks indicate human adipocytes. Yellow asterisks indicate host murine adipocytes. Scale bars: 200 µm (top panels) and 50 µm (bottom panels). 16
17 Fig. S10. PDGF-B mrna expression in ECFCs. ECFCs were transplanted into nude mice with watmscs, retrieved at day 7, and immediately sorted into hcd31 + and hcd31 - cells. (A) mrna expression of CD31 in both hcd31 + and hcd31 - cell fractions. (B) mrna expression of PDGF- B in both hcd31 + and hcd31 - cell fractions. mrna data are normalized to ribosomal 18S rrna abundance. (C) Comparison of PDGF-B expression level between ECFCs prior to implantation (in vitro) and ECFCs retrieved from the graft at day 7 (in vivo). mrna data are normalized to human β-actin (ACTB) expression. Bars represent mean ± SEM (n = 3). * P <
18 Fig. S11. In vivo inhibition of PDGFR signaling compromises MSC clonogenic and differentiation potential. MSC were implanted into GFP-SCID mice with ECFCs and with or without PDGFR inhibitor (AG1296) and then retrieved and selected as hcd90 + cells at day 7. (A) CFU-F activity of retrieved hcd90 + MSCs. (B) Quantitative CFU-F activity of retrieved MSCs from replicate grafts. (C) Ex vivo adipogenic and osteogenic differentiation potential of retrieved MSCs at clonal density (n = 16 clones per group). (D) Percentage of MSC clones with double differentiation potential. Bars represent mean ± SEM (n=3 mice per group). 18
19 Fig. S12. Effect of PDGFR inhibitor AG1296 on vascular network formation. (A) watmscs and (B) bmmscs were subcutaneously transplanted into nude mice with ECFCs and PDGFR inhibitor AG1296. BMP-2 was added to grafts seeded with bmmscs. Grafts were explanted at day 7 and analyzed histologically. H&E staining (left panels) revealed the presence of numerous blood vessels containing murine erythrocytes. Immunohistochemistry using antibodies against human CD31 (hcd31) revealed that most lumens were lined by human cells (right panels). Scale bars: 100 µm. 19
20 Fig. S13. Effect of AG1296 on MSC function. (A) Dose-dependent effect of AG1296 on watmsc proliferation assessed as fold increase in cell number after 48 h. (B) Proliferation of watmscs in basal medium upon stimulation with PDGF-BB (50 ng/ml) in the presence or absence of AG1296 (10 µm). (C) Non-treated and H2O2-treated (500 µm) watmscs were assessed for apoptosis by flow cytometry (annexin-v+) in the presence or absence of PDGF-BB (50 ng/ml), AG1296 (10 µm), and ECFC-CM. (D) Dose-dependent effect of AG1296 on adipogenic differentiation (10 days) assessed by intracellular accumulation of oil droplets (oil red O staining; top panels). Induction of osteogenic differentiation (21 days) assessed by calcium deposition (von Kossa staining; bottom panels). Insets depict watmscs under non-inducing conditions. Scale bars: 100 µm. Bars represent mean ± SEM (n = 3). * P < 0.05; ** P < 0.01; *** P <
21 Fig. S14. Effect of silencing PDGF-B expression in ECFCs. (A) Downregulation of PDGF-B mrna expression in ECFCs treated with three separate sirna constructs targeting PDGF-B. (B) Unaltered PDGF-A mrna expression in ECFCs treated with PDGF-B sirna. mrna values were measured by qrt-pcr and values were normalized to a control sirna. (C) watmscs were transplanted into nude mice with either ECFCs, ECFCs transfected with control sirna, or ECFCs transfected with PDGF-B sirna (construct #2; red bar). All sirna transfections were carried out on ECFCs 24 h prior to implantation. The number of viable MSCs was quantified at day 2 by flow cytometry in explanted grafts. Bars represent mean ± SEM (n = 2). 21
22 Fig. S15. Effect of PDGF-BB on long term differentiation of MSC. (A) watmscs were subcutaneously transplanted into nude mice with either ECFCs or 100 ng PDGF-BB. H&E staining revealed the presence of adipocyte at day 28. Immunohistochemical analysis of adipocytes in explanted grafts by expression of perilipin-a (red). Human MSC-derived cells were identified by expression of h-vimentin (green). Cell nuclei were counterstained with DAPI (blue). Yellow asterisks indicate human adipocytes. (B) bmmscs were subcutaneously transplanted into nude mice with either ECFCs or 100 ng PDGF-BB for 28 days. All grafts were stimulated with BMP-2 at day 7. Von kossa staining revealed calcium deposition at day 28. Immunohistochemical analysis of osteoblasts in explanted grafts by expression of osterix (red). Human MSC-derived cells were identified by expression of h-vimentin (green). Yellow arrowheads indicate human osteoblasts. White arrowheads indicate murine osteoblasts. Scale bars: 200 µm (top) and 50 µm (bottom). 22
23 Fig. S16. Dual perivascular and interstitial localization of engrafted MSCs. (A) watmscs were subcutaneously transplanted into nude mice in the presence or absence of ECFCs. Grafts were explanted at day 7 and analyzed histologically. Immunofluorescent staining of explanted grafts revealed dual perivascular and interstitial localization of donor MSCs. Human MSCs were identified by expression of h-vimentin (green). Perivascular cells were identified by expression of α-sma (red). Cell nuclei were counterstained with DAPI (blue). Yellow arrowheads indicate perivascular MSCs. White arrowheads indicate interstitial MSCs. Scale bars: 50 µm. (B) Quantification of perivascular/interstitial MSCs (ratio) in grafts explanted at day 7. Bars represent mean ± SEM (n = 3 mice per group). 23
24 Fig. S17. Ex vivo and in vivo expression of PDGFR-β by human MSCs. (A) Cytometric analysis revealed uniform ex vivo expression of PDGFR-β on cultured MSCs derived from both adipose tissue (watmscs) and bone marrow (bmmscs). Red and blue-lined histograms represent MSCs stained with fluorescent antibody against PDGFR-β. Isotype-matched controls are overlaid in solid grey histograms. (B) MSCs were subcutaneously transplanted with ECFCs into GFP-SCID mice. Grafts were explanted at day 7 and the retrieved cells analyzed by flow cytometry. watmscs were identified as GFP-/hCD31-/hCD90+ cells (left panel) and displayed a dichotomized expression of PDGFR-β (right panel). (C) MSCs were subcutaneously transplanted with ECFCs into GFP-SCID mice. Grafts were explanted at day 7 and the retrieved MSCs sorted into GFP-/hCD31-/hCD90+/PDGFR-β+ and GFP-/hCD31-/hCD90+/PDGFR-β- cells by FACS. (D) Phase microscopy (left panels) and immunofluorescent staining (right panels) of FACS-sorted MSCs revealed that PDGFR-β- MSCs acquired expression of PDGFR-β (green) in culture. Ex vivo culture time corresponded to either 3 h or 5 days post sorting. Cell nuclei were counterstained with DAPI (blue). Scale bars: 100 µm (phase) and 20 µm (immunofluorescence). 24
Figure S1. Phenotypic characterization of transfected ECFC. (a) ECFC were transfected using a lentivirus with a vector encoding for either human EPO
 Figure S1. Phenotypic characterization of transfected ECFC. (a) ECFC were transfected using a lentivirus with a vector encoding for either human EPO (epoecfc) or LacZ (laczecfc) under control of a cytomegalovirus
Figure S1. Phenotypic characterization of transfected ECFC. (a) ECFC were transfected using a lentivirus with a vector encoding for either human EPO (epoecfc) or LacZ (laczecfc) under control of a cytomegalovirus
Mice TRAMP mice were maintained in a C57BL/6J background. Syngeneic UBI-GFP/BL6 mice were used for bone marrow engraftment. 2
 Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
TF-1a lymphoblastic leukemia cell line: marking with GFP, phenotyping and sorting
 Supplemental Material Supplemental Methods TF-1a lymphoblastic leukemia cell line: marking with GFP, phenotyping and sorting In order to determine if the multi-parameter FACS approach would be successful
Supplemental Material Supplemental Methods TF-1a lymphoblastic leukemia cell line: marking with GFP, phenotyping and sorting In order to determine if the multi-parameter FACS approach would be successful
Supplementary Information. Conversion of vascular endothelial cells into multipotent stem-like cells
 Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
used for all the experiments.
 Expanded Methods Isolation and culture of endothelial progenitor cells Human umbilical cord blood was obtained from the Brigham and Women s Hospital in accordance with an Institutional Review Board approved
Expanded Methods Isolation and culture of endothelial progenitor cells Human umbilical cord blood was obtained from the Brigham and Women s Hospital in accordance with an Institutional Review Board approved
Supplemental Information. Materials and methods.
 Supplemental Information Materials and methods. Cell culture. hmscs were isolated from bone marrow of 3 male donors, undergoing orthopedic surgery (mean age 69.7). Cells were cultured in high glucose DMEM
Supplemental Information Materials and methods. Cell culture. hmscs were isolated from bone marrow of 3 male donors, undergoing orthopedic surgery (mean age 69.7). Cells were cultured in high glucose DMEM
F4/80, CD11b, Gr-1, NK1.1, CD3, CD4, CD8 and CD19. A-antigen was detected with FITCconjugated
 SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
Isolation, culture, and transfection of primary mammary epithelial organoids
 Supplementary Experimental Procedures Isolation, culture, and transfection of primary mammary epithelial organoids Primary mammary epithelial organoids were prepared from 8-week-old CD1 mice (Charles River)
Supplementary Experimental Procedures Isolation, culture, and transfection of primary mammary epithelial organoids Primary mammary epithelial organoids were prepared from 8-week-old CD1 mice (Charles River)
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1
 Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
Supplementary Figures
 Supplementary Figures Fig. S1. Specificity of perilipin antibody in SAT compared to various organs. (A) Images of SAT at E18.5 stained with perilipin and CD31 antibody. Scale bars: 100 μm. SAT stained
Supplementary Figures Fig. S1. Specificity of perilipin antibody in SAT compared to various organs. (A) Images of SAT at E18.5 stained with perilipin and CD31 antibody. Scale bars: 100 μm. SAT stained
Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer
 SUPPLEMENTAL METHODS Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer For Celigo experiments, 0.1 ml containing 5 x 10 4 cells was seeded into 96 well plates for 30 min
SUPPLEMENTAL METHODS Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer For Celigo experiments, 0.1 ml containing 5 x 10 4 cells was seeded into 96 well plates for 30 min
Supplemental Information Inventory
 Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.
 LEGENDS TO SUPPLEMENTARY FIGURES Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.2) were stained with the antibodies oct3/4
LEGENDS TO SUPPLEMENTARY FIGURES Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.2) were stained with the antibodies oct3/4
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Department of Cardiac Surgery, Boston Children's Hospital, Harvard Medical School, Boston, MA.
 Title: Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential Short title: Endothelial colony-forming cells in adipose tissue Authors
Title: Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential Short title: Endothelial colony-forming cells in adipose tissue Authors
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Reprogramming of Dermal Fibroblasts into Osteo-Chondrogenic Cells
 Stem Cell Reports, Volume 8 Supplemental Information Reprogramming of Dermal Fibroblasts into Osteo-Chondrogenic Cells with Elevated Osteogenic Potency by Defined Transcription Factors Yinxiang Wang, Ming-Hoi
Stem Cell Reports, Volume 8 Supplemental Information Reprogramming of Dermal Fibroblasts into Osteo-Chondrogenic Cells with Elevated Osteogenic Potency by Defined Transcription Factors Yinxiang Wang, Ming-Hoi
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang *
 In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
Supplemental methods Supplemental figure and legend...7. Supplemental table.. 8
 Supplemental Digital Content (SDC) Contents Supplemental methods..2-6 Supplemental figure and legend...7 Supplemental table.. 8 1 SDC, Supplemental Methods Flow cytometric analysis of intracellular phosphorylated
Supplemental Digital Content (SDC) Contents Supplemental methods..2-6 Supplemental figure and legend...7 Supplemental table.. 8 1 SDC, Supplemental Methods Flow cytometric analysis of intracellular phosphorylated
supplementary information
 DOI: 10.1038/ncb2015 Figure S1 Confirmation of Sca-1 CD34 stain specificity using isotypematched control antibodies. Skeletal muscle preparations were stained and gated as described in the text (Hoechst
DOI: 10.1038/ncb2015 Figure S1 Confirmation of Sca-1 CD34 stain specificity using isotypematched control antibodies. Skeletal muscle preparations were stained and gated as described in the text (Hoechst
Nature Medicine: doi: /nm.4169
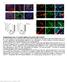 Supplementary Fig.1. EC-specific deletion of Ccm3 by Cdh5-CreERT2. a. mt/mg reporter mice were bred with Cdh5CreERT2 deleter mice followed by tamoxifen feeding from P1 to P3. mg expression was specifically
Supplementary Fig.1. EC-specific deletion of Ccm3 by Cdh5-CreERT2. a. mt/mg reporter mice were bred with Cdh5CreERT2 deleter mice followed by tamoxifen feeding from P1 to P3. mg expression was specifically
Discarded thymus tissue from infant heart operations were mechanically minced into < 3mm fragments
 Supplementary Information Materials and Methods Cell Isolation and Culture Discarded thymus tissue from infant heart operations were mechanically minced into < 3mm fragments under sterile conditions. Tissue
Supplementary Information Materials and Methods Cell Isolation and Culture Discarded thymus tissue from infant heart operations were mechanically minced into < 3mm fragments under sterile conditions. Tissue
Therapeutic angiogenesis via solar cell facilitated electrical
 Supporting information Therapeutic angiogenesis via solar cell facilitated electrical stimulation Gun-Jae Jeong 1,, Jin Young Oh 2,, Yeon-Ju Kim 3,, Suk Ho Bhang 4, Hyeon-Ki Jang 5, Jin Han 1, Jeong-Kee
Supporting information Therapeutic angiogenesis via solar cell facilitated electrical stimulation Gun-Jae Jeong 1,, Jin Young Oh 2,, Yeon-Ju Kim 3,, Suk Ho Bhang 4, Hyeon-Ki Jang 5, Jin Han 1, Jeong-Kee
Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis
 Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis Diptiman Chanda 1*, Ashish Kurundkar 1, Sunad Rangarajan 1, Morgan Locy 1, Karen Bernard 1,
Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis Diptiman Chanda 1*, Ashish Kurundkar 1, Sunad Rangarajan 1, Morgan Locy 1, Karen Bernard 1,
Supplementary Methods
 Supplementary Methods Antibodies Rabbit polyclonal VEGFR-1, VEGFR-2, Angiopoietin-1, Angiopoietin- 2 and GAPDH specific antibodies were from Santa Cruz Biotechnology. Rabbit polyclonal Akt1, Akt2, ps473
Supplementary Methods Antibodies Rabbit polyclonal VEGFR-1, VEGFR-2, Angiopoietin-1, Angiopoietin- 2 and GAPDH specific antibodies were from Santa Cruz Biotechnology. Rabbit polyclonal Akt1, Akt2, ps473
Engineering tumors with 3D scaffolds
 Engineering tumors with 3D scaffolds Claudia Fischbach, Ruth Chen, Takuya Matsumoto, Tobias Schmelzle, Joan S Brugge, Peter J Polverini & David J Mooney Supplementary figures and text: Supplementary Figure
Engineering tumors with 3D scaffolds Claudia Fischbach, Ruth Chen, Takuya Matsumoto, Tobias Schmelzle, Joan S Brugge, Peter J Polverini & David J Mooney Supplementary figures and text: Supplementary Figure
Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells
 Angiogenesis (2012) 15:443 455 DOI 10.1007/s10456-012-9272-2 ORIGINAL PAPER Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells Ruei-Zeng Lin Rafael Moreno-Luna
Angiogenesis (2012) 15:443 455 DOI 10.1007/s10456-012-9272-2 ORIGINAL PAPER Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells Ruei-Zeng Lin Rafael Moreno-Luna
Mouse Mesenchymal Stem Cell Functional Identification Kit
 Mouse Mesenchymal Stem Cell Functional Identification Kit Catalog Number SC010 Reagents for the identification of mouse bone marrow-derived stem cells (BMSC)/mesenchymal stem cells (MSC) by in vitro functional
Mouse Mesenchymal Stem Cell Functional Identification Kit Catalog Number SC010 Reagents for the identification of mouse bone marrow-derived stem cells (BMSC)/mesenchymal stem cells (MSC) by in vitro functional
Title: Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells
 Title: Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells Running title: Tissue-specific MSCs facilitate vasculogenesis Authors and Affiliations: Ruei-Zeng
Title: Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells Running title: Tissue-specific MSCs facilitate vasculogenesis Authors and Affiliations: Ruei-Zeng
Supplementary Figure 1 A green: cytokeratin 8
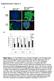 Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Human Bone Marrow Derived Mesenchymal Stem Cell (MSC) Care Manual
 Human Bone Marrow Derived Mesenchymal Stem Cell (MSC) Care Manual INSTRUCTION MANUAL ZBM0101.001 SHIPPING CONDITIONS Human Bone Marrow Derived MSC (HBMMSC-F) Orders are delivered via Federal Express courier.
Human Bone Marrow Derived Mesenchymal Stem Cell (MSC) Care Manual INSTRUCTION MANUAL ZBM0101.001 SHIPPING CONDITIONS Human Bone Marrow Derived MSC (HBMMSC-F) Orders are delivered via Federal Express courier.
Methods Western blot analysis of plg Quantification of plasminogen accumulation by ELISA Immunohistochemical analysis
 Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and
 Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Supplementary Table-1: List of genes that were identically matched between the ST2 and
 Supplementary data Supplementary Table-1: List of genes that were identically matched between the ST2 and ST3. Supplementary Table-2: List of genes that were differentially expressed in GD2 + cells compared
Supplementary data Supplementary Table-1: List of genes that were identically matched between the ST2 and ST3. Supplementary Table-2: List of genes that were differentially expressed in GD2 + cells compared
Mayumi Egawa, Kaori Mukai, Soichiro Yoshikawa, Misako Iki, Naofumi Mukaida, Yohei Kawano, Yoshiyuki Minegishi, and Hajime Karasuyama
 Immunity, Volume 38 Supplemental Information Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4 Mayumi Egawa, Kaori Mukai, Soichiro
Immunity, Volume 38 Supplemental Information Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4 Mayumi Egawa, Kaori Mukai, Soichiro
Flow cytometry Stained cells were analyzed and sorted by SORP FACS Aria (BD Biosciences).
 Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor
 SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
Application Note. Introduction. Kerry Thompson, Jeff Partridge, Paula Flaherty, Susan Qian, and Deepa Saxena
 Page 1 BD PureCoat ECM Mimetic Cultureware Fibronectin Peptide: Novel Synthetic, Animalfree Surface for Culture of Human Bone Marrow-derived Mesenchymal Stem Cells Kerry Thompson, Jeff Partridge, Paula
Page 1 BD PureCoat ECM Mimetic Cultureware Fibronectin Peptide: Novel Synthetic, Animalfree Surface for Culture of Human Bone Marrow-derived Mesenchymal Stem Cells Kerry Thompson, Jeff Partridge, Paula
g: relative centrifugal force R: radius of the rotor S: speed of the centrifuge in revolutions per minute
 Propagation of Mesenchymal Stem Cells The density of the colonies is the primary consideration in determining when the cells should be passaged. Cells grow densely should be passaged, even though the cells
Propagation of Mesenchymal Stem Cells The density of the colonies is the primary consideration in determining when the cells should be passaged. Cells grow densely should be passaged, even though the cells
7-amino actinomycin D (7ADD) was added to all samples 10 minutes prior to analysis on the flow cytometer in order to gate 7AAD viable cells.
 Antibody staining for Ho uptake analyses For HSC staining, 10 7 BM cells from Ho perfused mice were stained with biotinylated lineage antibodies (CD3, CD5, B220, CD11b, Gr-1, CD41, Ter119), anti Sca-1-PECY7,
Antibody staining for Ho uptake analyses For HSC staining, 10 7 BM cells from Ho perfused mice were stained with biotinylated lineage antibodies (CD3, CD5, B220, CD11b, Gr-1, CD41, Ter119), anti Sca-1-PECY7,
Antibody used for FC Figure S1. Multimodal characterization of NIR dyes in vitro Figure S2. Ex vivo analysis of HL60 cells homing
 Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Establishing sirna assays in primary human peripheral blood lymphocytes
 page 1 of 7 Establishing sirna assays in primary human peripheral blood lymphocytes This protocol is designed to establish sirna assays in primary human peripheral blood lymphocytes using the Nucleofector
page 1 of 7 Establishing sirna assays in primary human peripheral blood lymphocytes This protocol is designed to establish sirna assays in primary human peripheral blood lymphocytes using the Nucleofector
bronchial epithelial cells (I). Bronchi are outlined with dashed line. Scale bars = 25 µm, if not
 Supplemental Figure S1: ronchial epithelial cell polarity and integrity is maintained in bronchi. (A-E) Staining for selected markers of bronchial cell differentiation and intracellular compartments is
Supplemental Figure S1: ronchial epithelial cell polarity and integrity is maintained in bronchi. (A-E) Staining for selected markers of bronchial cell differentiation and intracellular compartments is
Cell were phenotyped using FITC-conjugated anti-human CD3 (Pharmingen, UK)
 SUPPLEMENTAL MATERIAL Supplemental Methods Flow cytometry Cell were phenotyped using FITC-conjugated anti-human CD3 (Pharmingen, UK) and anti-human CD68 (Dako, Denmark), anti-human smooth muscle cell α-actin
SUPPLEMENTAL MATERIAL Supplemental Methods Flow cytometry Cell were phenotyped using FITC-conjugated anti-human CD3 (Pharmingen, UK) and anti-human CD68 (Dako, Denmark), anti-human smooth muscle cell α-actin
Confocal immunofluorescence microscopy
 Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Vandetanib was kindly provided by AstraZeneca (Macclesfield, United Kingdom).
 Supplemental Experimental Procedures Reagents Vandetanib was kindly provided by AstraZeneca (Macclesfield, United Kingdom). Erlotinib and BV were both obtained from the institutional pharmacy. For in vivo
Supplemental Experimental Procedures Reagents Vandetanib was kindly provided by AstraZeneca (Macclesfield, United Kingdom). Erlotinib and BV were both obtained from the institutional pharmacy. For in vivo
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM
 Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
Supplementary Figure 1. Characterization of the POP2 transcriptional and post-transcriptional regulatory elements. (A) POP2 nucleotide sequence
 1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW
 Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW Lyset, the human platelet derived supplement for cell culture Among the different alternatives to animal serum, platelet derived preparations
Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW Lyset, the human platelet derived supplement for cell culture Among the different alternatives to animal serum, platelet derived preparations
a KYSE270-CON KYSE270-Id1
 a KYSE27-CON KYSE27- shcon shcon sh b Human Mouse CD31 Relative MVD 3.5 3 2.5 2 1.5 1.5 *** *** c KYSE15 KYSE27 sirna (nm) 5 1 Id2 Id2 sirna 5 1 sirna (nm) 5 1 Id2 sirna 5 1 Id2 [h] (pg per ml) d 3 2 1
a KYSE27-CON KYSE27- shcon shcon sh b Human Mouse CD31 Relative MVD 3.5 3 2.5 2 1.5 1.5 *** *** c KYSE15 KYSE27 sirna (nm) 5 1 Id2 Id2 sirna 5 1 sirna (nm) 5 1 Id2 sirna 5 1 Id2 [h] (pg per ml) d 3 2 1
Modeling Cardiomyocyte Differentiation:
 icell Cardiac Progenitor Cells Prototype Application Protocol Wnt- and Activin/TGFβ-inhibitor Induction with Flow Cytometry Analysis Introduction The ability of cardiac progenitor cells to proliferate
icell Cardiac Progenitor Cells Prototype Application Protocol Wnt- and Activin/TGFβ-inhibitor Induction with Flow Cytometry Analysis Introduction The ability of cardiac progenitor cells to proliferate
1. Paraffin section slides can be stored at room temperature for a long time.
 Immunohistochemistry (IHC) Protocols Immunohistochemistry (IHC) Protocol of Paraffin Section 1. Fix dissected tissues with 10% formalin for no less than 48 hours at room temperature. Inadequately fixation
Immunohistochemistry (IHC) Protocols Immunohistochemistry (IHC) Protocol of Paraffin Section 1. Fix dissected tissues with 10% formalin for no less than 48 hours at room temperature. Inadequately fixation
Single-cell suspensions of C57BL/6J splenocytes were incubated with CD5 beads
 S S Single-cell suspensions of C57BL/6J splenocytes were incubated with CD5 beads (Miltenyi Biotech) and T cells were positively selected by magnetically activated cell sorting (MACS). 50x10 6 or greater
S S Single-cell suspensions of C57BL/6J splenocytes were incubated with CD5 beads (Miltenyi Biotech) and T cells were positively selected by magnetically activated cell sorting (MACS). 50x10 6 or greater
3D Endothelial Pericyte Coculturing Kit Product Description Kit Components
 3D Endothelial Pericyte Coculturing Kit (3D-EPC) Cat #8728 Product Description Blood vessels are responsible for transporting blood throughout the body as part of the circulatory system. Their main components
3D Endothelial Pericyte Coculturing Kit (3D-EPC) Cat #8728 Product Description Blood vessels are responsible for transporting blood throughout the body as part of the circulatory system. Their main components
Supplementary Data. In Vivo Bone Formation by impcs Materials and methods. Results. Conclusions
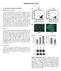 Supplementary Data In Vivo Bone Formation by impcs Materials and methods Induced pluripotent stem cell (ipsc)-derived mesenchymal stem cell (MSC)-like progenitor cells (impcs) (1 10 6 M-iMPC-GMs in 10
Supplementary Data In Vivo Bone Formation by impcs Materials and methods Induced pluripotent stem cell (ipsc)-derived mesenchymal stem cell (MSC)-like progenitor cells (impcs) (1 10 6 M-iMPC-GMs in 10
5.4 PERIODONTAL LIGAMENT STEM CELL (PDLSC) ISOLATION. Having validated that the isolated PDL stromal cells possessed the major phenotypic
 5.4 PERIODONTAL LIGAMENT STEM CELL (PDLSC) ISOLATION Having validated that the isolated PDL stromal cells possessed the major phenotypic characteristics of their tissue of origin, we next focused on identifying
5.4 PERIODONTAL LIGAMENT STEM CELL (PDLSC) ISOLATION Having validated that the isolated PDL stromal cells possessed the major phenotypic characteristics of their tissue of origin, we next focused on identifying
For in vitro killing assays with lysed cells, neutrophils were sonicated using a 550 Sonic
 Supplemental Information Cell Host & Microbe, Volume 8 Statins Enhance Formation of Phagocyte Extracellular Traps Ohn A. Chow, Maren von Köckritz-Blickwede, A. Taylor Bright, Mary E. Hensler, Annelies
Supplemental Information Cell Host & Microbe, Volume 8 Statins Enhance Formation of Phagocyte Extracellular Traps Ohn A. Chow, Maren von Köckritz-Blickwede, A. Taylor Bright, Mary E. Hensler, Annelies
Supplementary Information: ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells
 Supplementary Information: ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells E. M. Morandi, R. Verstappen, M. E. Zwierzina, S. Geley, G.
Supplementary Information: ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells E. M. Morandi, R. Verstappen, M. E. Zwierzina, S. Geley, G.
Isolation of mouse monocytes: Mouse monocytes were isolated using a modified
 Supplemental Material Extended Methods Isolation of mouse monocytes: Mouse monocytes were isolated using a modified method described (1). Citrated mouse whole blood was mixed with equal volume of PBS,
Supplemental Material Extended Methods Isolation of mouse monocytes: Mouse monocytes were isolated using a modified method described (1). Citrated mouse whole blood was mixed with equal volume of PBS,
Mammosphere formation assay. Mammosphere culture was performed as previously described (13,
 Supplemental Text Materials and Methods Mammosphere formation assay. Mammosphere culture was performed as previously described (13, 17). For co-culture with fibroblasts and treatment with CM or CCL2, fibroblasts
Supplemental Text Materials and Methods Mammosphere formation assay. Mammosphere culture was performed as previously described (13, 17). For co-culture with fibroblasts and treatment with CM or CCL2, fibroblasts
Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days,
 Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days, respectively, and their mrnas were quantified by real time
Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days, respectively, and their mrnas were quantified by real time
Cell viability. Cell viability was examined by MTT assay (Sigma-Aldrich).
 Supplementary Materials Supplementary materials and methods Cell culture. Primary human dermal fibroblasts (DFs) were isolated from full-thickness skin samples. Tissue samples were dissected into small
Supplementary Materials Supplementary materials and methods Cell culture. Primary human dermal fibroblasts (DFs) were isolated from full-thickness skin samples. Tissue samples were dissected into small
a, b-immnunostainings of lcmn and SSM tissue for HMB45 (A, left panel) and CD44 (B, left panel) using
 Figure S1 a HMB45 HMB45 + cells (% of total cells) 15 1 5 CD44 CD44 + cells (% of total cells) 25 2 15 1 5 In vivo characterization of. a, -Immnunostainings of and tissue for HMB45 (A, left panel) and
Figure S1 a HMB45 HMB45 + cells (% of total cells) 15 1 5 CD44 CD44 + cells (% of total cells) 25 2 15 1 5 In vivo characterization of. a, -Immnunostainings of and tissue for HMB45 (A, left panel) and
PCCS Growth Media, Cell Tagging, Cell Separation Final Assignment. Igneris Rosado-Erazo. Panama College of Cell Science
 Running Head: Growth Media, Cell Tagging, Cell Separation PCCS Growth Media, Cell Tagging, Cell Separation Final Assignment Igneris Rosado-Erazo Panama College of Cell Science In partial fulfillment of
Running Head: Growth Media, Cell Tagging, Cell Separation PCCS Growth Media, Cell Tagging, Cell Separation Final Assignment Igneris Rosado-Erazo Panama College of Cell Science In partial fulfillment of
Nature Immunology: doi: /ni.1744
 Macrophage colony stimulating factor induces macrophage proliferation and survival through a pathway involving DAP12 and β-catenin Karel Otero, Isaiah R Turnbull, Pietro Luigi Poliani *, William Vermi
Macrophage colony stimulating factor induces macrophage proliferation and survival through a pathway involving DAP12 and β-catenin Karel Otero, Isaiah R Turnbull, Pietro Luigi Poliani *, William Vermi
STEMdiff Mesenchymal Progenitor Kit
 Defined culture kit for derivation and expansion of mesenchymal progenitor cells Catalog #05240 1 Kit Product Description STEMdiff Mesenchymal Progenitor Kit is a defined culture kit consisting of animal
Defined culture kit for derivation and expansion of mesenchymal progenitor cells Catalog #05240 1 Kit Product Description STEMdiff Mesenchymal Progenitor Kit is a defined culture kit consisting of animal
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation
 Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
VDL602.2 RAPID ASSAY FOR DETERMINING ADENOVIRAL VECTOR TITER
 1. Purpose 1.1. The purpose of this protocol is to determine the number of infectious adenoviral particles. 1.2. The starting material is purified adenoviral vectors. 1.3. This protocol is based upon the
1. Purpose 1.1. The purpose of this protocol is to determine the number of infectious adenoviral particles. 1.2. The starting material is purified adenoviral vectors. 1.3. This protocol is based upon the
CRISPR-based gene disruption in murine HSPCs. Ayumi Kitano and Daisuke Nakada
 CRISPR-based gene disruption in murine HSPCs Ayumi Kitano and Daisuke Nakada Nakada Lab Protocol INTRODUCTION We recently described fast, efficient, and cost-effective methods to directly modify the genomes
CRISPR-based gene disruption in murine HSPCs Ayumi Kitano and Daisuke Nakada Nakada Lab Protocol INTRODUCTION We recently described fast, efficient, and cost-effective methods to directly modify the genomes
Immunofluorescent staining and flow cytometric analysis of cells
 UCAN-U 0003 Version 1 November 2010 Page 1 of 7 CMCI (Center for molecular and Cellular Intervention) University Medical Center Utrecht Written By Name Function Date Signature Mark Klein Lab manager Conformation
UCAN-U 0003 Version 1 November 2010 Page 1 of 7 CMCI (Center for molecular and Cellular Intervention) University Medical Center Utrecht Written By Name Function Date Signature Mark Klein Lab manager Conformation
Supporting Information
 Supporting Information Chakrabarty et al. 10.1073/pnas.1018001108 SI Materials and Methods Cell Lines. All cell lines were purchased from the American Type Culture Collection. Media and FBS were purchased
Supporting Information Chakrabarty et al. 10.1073/pnas.1018001108 SI Materials and Methods Cell Lines. All cell lines were purchased from the American Type Culture Collection. Media and FBS were purchased
Cell culture and drug treatment. Lineage - Sca-1+ CD31+ EPCs were cultured on
 Supplemental Material Detailed Methods Cell culture and drug treatment. Lineage - Sca-1+ CD31+ EPCs were cultured on 5µg/mL human fibronectin coated plates in DMEM supplemented with 10% FBS and penicillin/streptomycin
Supplemental Material Detailed Methods Cell culture and drug treatment. Lineage - Sca-1+ CD31+ EPCs were cultured on 5µg/mL human fibronectin coated plates in DMEM supplemented with 10% FBS and penicillin/streptomycin
Mesenchymal Stem/Stromal Cells
 Mesenchymal Stem/Stromal Cells ABOUT US Irvine Scientific, a member of JX Holdings group, is a worldwide leader in the design, manufacture and distribution of medical devices, including Cell Therapy, Industrial
Mesenchymal Stem/Stromal Cells ABOUT US Irvine Scientific, a member of JX Holdings group, is a worldwide leader in the design, manufacture and distribution of medical devices, including Cell Therapy, Industrial
Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential
 DOI 10.1007/s10456-013-9350-0 ORIGINAL PAPER Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential Ruei-Zeng Lin Rafael Moreno-Luna
DOI 10.1007/s10456-013-9350-0 ORIGINAL PAPER Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential Ruei-Zeng Lin Rafael Moreno-Luna
For Research Use Only. Not for use in diagnostic procedures. Anti-NRF2 mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. Anti-NRF2 mab CODE No. M200-3 CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 1F2 Mouse IgG1 100 L,
Page 1 For Research Use Only. Not for use in diagnostic procedures. Anti-NRF2 mab CODE No. M200-3 CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 1F2 Mouse IgG1 100 L,
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured
 Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Poietics Rat Mesenchymal Stem Cells Instructions for Use
 Poietics Rat Mesenchymal Stem Cells Instructions for Use Lonza Walkersville, Inc. www.lonza.com scientific.support@lonza.com Scientific Support: 800-521-0390 Document # IS-PT-2505 08/09 Walkersville, MD
Poietics Rat Mesenchymal Stem Cells Instructions for Use Lonza Walkersville, Inc. www.lonza.com scientific.support@lonza.com Scientific Support: 800-521-0390 Document # IS-PT-2505 08/09 Walkersville, MD
Phagocytosis Assay Kit (IgG PE)
 Phagocytosis Assay Kit (IgG PE) Item No. 600540 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Phagocytosis Assay Kit (IgG PE) Item No. 600540 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information
 Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information 1. Supplementary Figure S1-S10: Pages 2-11 2. Supplementary References:
Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information 1. Supplementary Figure S1-S10: Pages 2-11 2. Supplementary References:
Gene Forward (5 to 3 ) Reverse (5 to 3 ) Accession # PKA C- TCTGAGGAAATGGGAGAACC CGAGGGTTTTCTTCCTCTCAA NM_011100
 Supplementary Methods: Materials. BRL37344, insulin, 3-isobutyl-1-methylxanthine, dibutyryl camp (Bt2-cAMP) and 8-Bromoadenosine 3,5 -cyclic monophosphate sodium (8-br-cAMP), cilostamide, adenosine deaminase,
Supplementary Methods: Materials. BRL37344, insulin, 3-isobutyl-1-methylxanthine, dibutyryl camp (Bt2-cAMP) and 8-Bromoadenosine 3,5 -cyclic monophosphate sodium (8-br-cAMP), cilostamide, adenosine deaminase,
A collection of 31 splenic specimens from patients (42.4±17.7 years old) with ITP
 Supplemental Materials & Methods Patients and patient samples. A collection of 31 splenic specimens from patients (42.4±17.7 years old) with ITP requiring splenectomy and 36 splenic specimens obtained
Supplemental Materials & Methods Patients and patient samples. A collection of 31 splenic specimens from patients (42.4±17.7 years old) with ITP requiring splenectomy and 36 splenic specimens obtained
Protocol Using a Dox-Inducible Polycistronic m4f2a Lentivirus to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
WT Day 90 after injections
 Supplementary Figure 1 a Day 1 after injections Day 9 after injections Klf5 +/- Day 1 after injections Klf5 +/- Day 9 after injections BLM PBS b Day 1 after injections Dermal thickness (μm) 3 1 Day 9 after
Supplementary Figure 1 a Day 1 after injections Day 9 after injections Klf5 +/- Day 1 after injections Klf5 +/- Day 9 after injections BLM PBS b Day 1 after injections Dermal thickness (μm) 3 1 Day 9 after
Yalin Emre, Magali Irla, Isabelle Dunand- Sauthier, Romain Ballet, Mehdi Meguenani, Stephane Jemelin, Christian Vesin, Walter Reith and Beat A.
 Supplementary information Thymic epithelial cell expansion through matricellular protein CYR61 boosts progenitor homing and T- cell output Yalin Emre, Magali Irla, Isabelle Dunand- Sauthier, Romain Ballet,
Supplementary information Thymic epithelial cell expansion through matricellular protein CYR61 boosts progenitor homing and T- cell output Yalin Emre, Magali Irla, Isabelle Dunand- Sauthier, Romain Ballet,
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Supplemental Materials and Methods
 Supplemental Materials and Methods 125 I-CXCL12 binding assay KG1 cells (2 10 6 ) were preincubated on ice with cold CXCL12 (1.6µg/mL corresponding to 200nM), CXCL11 (1.66µg/mL corresponding to 200nM),
Supplemental Materials and Methods 125 I-CXCL12 binding assay KG1 cells (2 10 6 ) were preincubated on ice with cold CXCL12 (1.6µg/mL corresponding to 200nM), CXCL11 (1.66µg/mL corresponding to 200nM),
Protocol Reprogramming Human Fibriblasts using the Dox Inducible Reprogramming Polycistronic Lentivirus Set: Human 4F2A LoxP
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
Marilyn G. Rimando, Hao-Hsiang Wu, Yu-An Liu, Chien-Wei Lee, Shu-Wen Kuo, Yin-
 Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Supplementary Figure 1. Reconstitution of human-acquired lymphoid system in
 Supplementary Figure 1. Reconstitution of human-acquired lymphoid system in mouse NOD/SCID/Jak3 null mice were transplanted with human CD34 + hematopoietic stem cells. (Top) Four weeks after the transplantation
Supplementary Figure 1. Reconstitution of human-acquired lymphoid system in mouse NOD/SCID/Jak3 null mice were transplanted with human CD34 + hematopoietic stem cells. (Top) Four weeks after the transplantation
Initial genotyping of all new litters was performed by Transnetyx (Memphis,
 SUPPLEMENTAL INFORMATION MURILLO ET AL. SUPPLEMENTAL EXPERIMENTAL PROCEDURES Mouse Genotyping Initial genotyping of all new litters was performed by Transnetyx (Memphis, USA). Assessment of LoxPpα recombination
SUPPLEMENTAL INFORMATION MURILLO ET AL. SUPPLEMENTAL EXPERIMENTAL PROCEDURES Mouse Genotyping Initial genotyping of all new litters was performed by Transnetyx (Memphis, USA). Assessment of LoxPpα recombination
