Specification of the Retinal Fate of Mouse Embryonic Stem Cells by Ectopic Expression of Rx/rax, a Homeobox Gene
|
|
|
- Tyrone Wheeler
- 6 years ago
- Views:
Transcription
1 MOLECULAR AND CELLULAR BIOLOGY, May 2004, p Vol. 24, No /04/$ DOI: /MCB Copyright 2004, American Society for Microbiology. All Rights Reserved. Specification of the Retinal Fate of Mouse Embryonic Stem Cells by Ectopic Expression of Rx/rax, a Homeobox Gene Yoko Tabata, 1 Yasuo Ouchi, 1 Haruyuki Kamiya, 2 Toshiya Manabe, 2,3 Ken-ichi Arai, 1 and Sumiko Watanabe 1 * Division of Molecular and Developmental Biology 1 and Division of Neuronal Network, 3 Department of Basic Medical Sciences, Institute of Medical Science, University of Tokyo, Minato-ku, Tokyo , and Division of Cell Biology and Neurophysiology, Department of Neuroscience, Faculty of Medicine, Kobe University, Chuo-ku, Kobe , 2 Japan Received 26 November 2003/Returned for modification 1 February 2004/Accepted 11 February 2004 With the goal of generating retinal cells from mouse embryonic stem (ES) cells by exogenous gene transfer, we introduced the Rx/rax transcription factor, which is expressed in immature retinal cells, into feeder-free mouse ES cells (CCE). CCE cells expressing Rx/rax as well as enhanced green fluorescent protein (CCE-RX/E cells) proliferated and remained in the undifferentiated state in the presence of leukemia inhibitory factor, as did parental ES cells. We made use of mouse embryo retinal explant cultures to address the differentiation ability of grafted ES cells. Dissociated embryoid bodies were treated with retinoic acid for use as donor cells and cocultured with retina explants for 2 weeks. In contrast to the parental CCE cells, which could not migrate into host retinal cultures, CCE-RX/E cells migrated into the host retina and extended their process-like structures between the host retinal cells. Most of the grafted CCE-RX/E cells became located in the ganglion cell and inner plexiform layers and expressed ganglion and horizontal cell markers. Furthermore, these grafted cells had the electrophysiological properties expected of ganglion cells. Our data thus suggest that subpopulations of retinal neurons can be generated in retinal explant cultures from grafted mouse ES cells ectopically expressing the transcription factor Rx/rax. The neural retina is a part of the central nervous system (CNS), and regeneration of the retina from retinal stem cells or other sources by transplantation is a critical issue from both clinical and neurobiological points of view. Although a report of successful regeneration of the CNS has appeared in the literature (33), such has not been the case for the vertebrate neural retina. Transplantation of neural stem cells into the retina has been assumed to be particularly difficult in terms of the cells and their ability to survive, migrate, and establish morphological and functional connectivity with their hosts (24). Even though some success has been achieved by transplanting stem cells, less than 1% of them repopulate and become integrated into the normal adult retina (36, 42). A recent report indicated an essential role for reactive astroglial cells in preventing neural graft integration after transplantation into the adult retina (24). The neural retina consists of seven principal cell types, and these cells are derived from multipotent retinal progenitor cells (26). Several lines of evidence indicated that retinal cell diversification is achieved by the sequential production of cell types in a defined histogenetic order (26). A set of transcription factors such as Pax6, Rx/rax, Six3, Six6, and Lhx2 are known to play a role in initiating vertebrate eye development (18). But the exact role of these factors in regulating the development of a complex population from uncommitted retinal progenitor cells has not been clarified. * Corresponding author. Mailing address: Department of Molecular and Developmental Biology, Institute of Medical Science, University of Tokyo, Shirokanedai, Minato-ku, Tokyo , Japan. Phone: Fax: sumiko@ims.u -tokyo.ac.jp. The gene encoding the Rx/rax transcription factor (3, 9) belongs to a subfamily of the paired-like homeobox genes (12), and the homeodomain region of Rx/rax is remarkably conserved among vertebrates (27). Rx/rax was first isolated by two independent groups, one using a cdna library made from Xenopus animal cap ectoderm induced by treatment with ammonium chloride (27) and the other using degenerate PCR to amplify specific classes of genes expressed in the rat retina at E19 and P4 (9). Rx/rax is expressed in the anterior neural fold, including areas that will give rise to the ventral forebrain and optic vesicles in the early mouse embryo; and then, once the optic vesicles have formed, Rx/rax expression becomes restricted to the ventral diencephalon and the optic vesicles (27). This expression pattern is also remarkably conserved among vertebrates (27). Targeted knockouts of Rx/rax in mice eliminates eye formation (27), and an eyeless inbred mouse strain was shown to have a mutation in its Rx/rax gene (38), indicating the essential role of Rx/rax in vertebrate eye development. In keeping with these observations, gain-of-function experiments indicated the ability of Rx/rax to promote retina formation. Injection of Xenopus rx1 (Xrx1) synthetic RNA into 4 to 8 cell stage Xenopus embryos resulted in the development of ectopic retinal pigmented epithelium between the eyes and the neural tube (27). Another report showed that Xrx1 was able to define the retina-diencephalon territory in the anterior neural plate (1). Although Rx/rax has the structure of a typical transcription factor, the targets of Rx/rax are not well defined. The involvement of Rx/rax in photoreceptor-specific gene expression was reported previously (23), but the nature of the targets of early eye development is not known. Embryonic stem (ES) cells, being an unlimited source for 4513
2 4514 TABATA ET AL. MOL. CELL. BIOL. cell therapy, have been discussed in terms of their ability to generate specific cell lineages in vitro. ES cells possess the capacity to generate neurons and glial cells that express markers characteristic of specific classes of these cells (19, 39). Furthermore, successful enrichment of a specific type of neuron was achieved by expression of an exogenous gene (22). There are several reports describing attempts to differentiate ES cells into cells of the retinal cell lineage. Coculture of ES cell-derived neural progenitors with postnatal day 1 retinal cells resulted in expression of photoreceptor lineage markers in a subset of ES cells (43). However, cells expressing photoreceptor-specific markers did not display typical photoreceptor morphology. A subset of cells also expressed bipolar markers, but it was not conclusive whether or not the cells expressing those markers were retinal cells, as these markers are expressed elsewhere in the CNS. Others reported that subretinally transplanted ES cells could rescue photoreceptor cells from degeneration in a mouse model of progressive retinal degeneration, but no direct differentiation from ES cells to retinal cells was evidenced (34). Recently, generation of pigmented epithelium and lentoids from primate ES cells by stromal cell-derived inducing activity (SDIA) was reported (20, 31). In the views of the current state of the literature, the successful generation of neural retina cells from ES cells is an important goal that remains to be achieved. Here we show that subpopulations of retinal neurons can be generated in retinal explant cultures from grafted mouse ES cells ectopically expressing Rx/rax, a transcription factor known to be expressed in immature retinal cells. The retinal neurons generated by these ES cells expressed ganglion and horizontal cell markers and showed the electrophysiological properties expected of ganglion cells. MATERIALS AND METHODS Generation of Rx/rax-expressing mouse ES cells and differentiation. A fulllength cdna fragment for mouse Rx/rax was cloned into CAG-KS, which contained the CAG promoter followed by multicloning sites derived from Blue Script. Stable clones of feeder-free ES cells (CCE) expressing enhanced green fluorescent protein (EGFP) (CCE-E), Rx/rax (CCE-RX), Chx10 (CCE-CH), Rx/rax as well as EGFP (CCE-RX/E), or Chx10 as well as EGFP (CCE-CH/E) were established by transfection of CCE ( cells) with CAG-Rx/rax and/or CAG-EGFP in 400 l of opti MEM (Gibco) by electroporation (0.3 kv, 250 F using Bio-Rad Gene Pulser Xcell). Clones were selected by use of G418 (500 g/ml) and/or hygromycin (500 g/ml). CCE clones expressing only the neomycin gene were also established for control experiments. Selected clones were screened by PCR or immunoblot analysis of the gene product of the introduced gene. For differentiation of ES cells, embryoid bodies (EBs) were formed by culturing trypsinized ES cells in leukemia inhibitory factor (LIF)-free medium in 10-cm bacterial plates. Half of the medium was changed every 2 days, and retinoic acid (RA) (Sigma) at a final concentration of 0.1 mg/ml was added at day 4. CCE cells and their stable clones were cultured in gelatin-coated plates (Samitomo) with high-glucose Dulbecco s modified Eagle s medium (DMEM) (Nikken, Kyoto, Japan) containing 10 3 U of human LIF (ESGRO; Chemicon, Temecula, Calif.) per ml, 20% fetal calf serum (Gibco), 10 mm MEM nonessential amino acids solution (Gibco), 200 mm L-glutamine (Cosmobio, Tokyo, Japan), a nucleoside mixture (Sigma), 2-mercaptoethanol (Sigma), and penicillin and streptomycin (Gibco). The SDIA method was done as described previously (19). Mouse retina explant cultures and transplantation of ES cells. Retina explant cultures were prepared as described previously (14). Briefly, eyes were isolated from E17.5 mice (Japan SLC) and placed on Millicell chamber filters (Millipore; diameter of 30 mm and pore size of 0.4 m). Then, the neural retina was isolated on the filter and placed with the ganglion cell layer facing upwards. The filters were inserted into six-well plates and cultured in 1 ml of medium comprising 50% MEM-HEPES (Gibco), 25% Hanks balanced salt solution (Gibco), and 25% heat-inactivated horse serum (JRH Biosciences) supplemented with 200 M L-glutamine, and 5.75-mg/ml glucose, 100-U/ml penicillin, and 100- g/ml streptomycin (Gibco). ES cells (undifferentiated, those that had formed EBs, or EBs treated with RA for 3 days) were trypsinized (0.25%) and washed with high-glucose DMEM containing 20% fetal calf serum. Then, 10 5 cells were placed on the surface of explanted retina at day 1 of the culture period. Immunostaining of explant culture. Immunostaining of sectioned explant cultures was done as described previously (14). Stained samples were sealed with Vecta Shield (Vector Laboratories, Inc.) containing DAPI (4,6 -diamidino-2- phenylindole) for nuclear staining and examined under a Zeiss Axioplan microscope. Images were processed by using Adobe Photoshop (Adobe Systems). The primary antibodies used were polyclonal anti-gfp (Clontech Laboratories), monoclonal anti-huc/hud neuronal protein (Molecular Probes), monoclonal anti-islet-1 (Developmental Studies Hybridoma Bank), monoclonal anti-protein kinase C (PKC) (Ab-2; Oncogene Research Products), monoclonal anti-glutamine synthetase (Chemicon), monoclonal anti-neurofilament (NF) 160 (Sigma), and polyclonal anti-calbindin-d-28k (Chemicon). Secondary antibodies anti-rabbit immunoglobulin G-Alexa Fluor 488 and anti-mouse immunoglobulin G-Alexa Fluor 546 (Molecular Probes) were used for visualizing the binding sites of the primary antibodies. RT-PCR. For all the reverse transcription (RT)-PCR analyses, mrna was purified from ES cells treated variously by using a Fast Track 2.9 kit (Invitrogen), and cdna was synthesized from the mrna by Superscript II (Gibco BRL). Sequences of primers for Hnf1, Hnf4, Brachyury, Gata1, Bmp4, and Hprt were as previously reported (6). All primer sets were tested for several different cycling numbers (15 to 40 cycles) by using ExTaq (Takara), and the semiquantitative cycle number was determined for each primer set. Bands were visualized with ethidium bromide. For RT-PCR of SDIA samples, mixtures of PA6 and ES cells were harvested, and cdnas were prepared as described above. As a control, PA6 cells alone and ES cells isolated by cell sorter were also used. Retrovirus construction and production. Full-length Rx/rax was inserted into retrovirus vector pmx-ires-egfp, and the resultant plasmid was used to transfect ecotropic packaging cells PLAT-E (29). Cell supernatants containing retrovirus were harvested after 2 days and concentrated by using a centrifugal filter device (Millipore). Two days from the initiation of cultures, the retinal explants were exposed to the virus solution, and then the cells were washed with medium. Explants were harvested at day 14, and frozen sections were prepared and immunostained with appropriate antibodies to examine the expression of marker proteins. Electrophysiology. After 2 weeks of culture, the retinal explant with or without ES-derived cells was placed on the stage of the microscope with the ganglion cell layer upward and continuously superfused with a solution (119 mm NaCl, 2.5 mm KCl, 1.3 mm MgSO 4, 2.5 mm CaCl 2, 1.0 mm NaH 2 PO 4, 26.2 mm NaHCO 3, 11 mm glucose, 100 M picrotoxin) that had been equilibrated with 95% O 2 and 5% CO 2. Whole-cell voltage-clamped recordings were made at 80 mv with patch pipettes filled with an internal solution containing 135 mm potassium methanesulfonate, 8 mm NaCl, 10 mm HEPES, 2 mm Mg 2 ATP, and 0.3 mm Na 3 GTP (ph 7.3 adjusted with KOH; osmolarity, 300 mosm). All recordings were made at room temperature. Five CCE-RX/E-derived cells, five CCE-Ederived cells, and six nonfluorescent cells were examined. RESULTS Expression of Rx/rax or Chx10 in mouse feeder-free ES cells (CCE). To modify ES cells for preferential differentiation into retinal cells by forced expression of exogenous genes, we first examined whether or not some genes known to be expressed in retinal progenitor cells were expressed in CCE cells (feederfree ES cells). Undifferentiated CCE cells, the EBs formed by them, and EBs treated with RA for 4 days to induce neural lineage differentiation were examined by using the semiquantitative PCR with RT-PCR (Fig. 1A). Pax6 was weakly expressed in the EBs and slightly up-regulated in the RA-treated ones, which is in accordance with previous results obtained by using a different type of ES cell (11). In contrast, among Rx/rax, Six3, Chx10, Lhx2, and Six6 (the latter two are not shown in the figure), all of which are known to be expressed in retinal
3 VOL. 24, 2004 RETINAL FATE SPECIFICATION OF MOUSE ES CELLS 4515 FIG. 1. Characterization of CCE-ES cells and their transformants expressing Rx/rax or Chx10. (A) Semiquantitative RT-PCR of genes expressed in retinal progenitor cells in CCE (feeder-free mouse) ES cells. cdna from EBs formed by CCE, EBs treated with RA, and eyes from E15 mouse embryos were used. (B) Morphology of CCE, CCE-CH, and CCE-RX cells. (C) Proliferation of CCE, CCE-RX, and CCE-CH cells. Cells were seeded in 6-cm plates, and cell numbers were counted every 2 days. Values are the average of two independent samples. Experiments were done twice, and essentially the same results were obtained. (D) Differentiation molecular markers were examined by semiquantitative RT-PCR analysis of EB cultures taken at different time points. progenitor cells (26), none of them were expressed in either EBs or RA-treated EBs formed from CCE-ES cells. Undifferentiated CCE cells did not express any of these genes (data not shown). Among these genes, we chose Rx/rax and Chx10 for expression in ES cells. We introduced full-length cdna of mouse Rx/rax or mouse Chx10 under the control of the CAG promoter into CCE-ES cells (CCE-RX and CCE-CH, respectively). The morphology of these ES clones cultured in the presence of LIF was indistinguishable from that of the parental CCE (Fig. 1B). The expression level of SSEA-1, which is a marker of mouse immature ES cells (35), showed no difference between these cells and the parental CCE (data not shown). Furthermore, their proliferation, which was determined by counting the cell number, was almost the same, at least up to the sixth day (Fig. 1C). Taken together, these findings indicate that Rx/rax or Chx10 did not prevent ES cells from sustaining their undifferentiated state. To examine whether or not the introduced gene, Rx/rax or Chx10, altered the differentiation ability of CCE, we examined by semiquantitative RT-PCR the expression of molecular markers of endoderm and mesoderm differentiation in ES cells prepared from EB cultures at different time points (Fig. 1D). The expression of Hnf1 and Hnf4, which are endoderm markers (6), was induced at day 8 of EB formation from parental CCE cells, and these genes were induced in EBs formed by CCE-RX cells with almost the same time course. Similarly, the expression patterns of Brachyury, Gata1, and Bmp4, all of which are mesodermal differentiation markers (7, 21, 40), in CCE-RX cells were comparable to those in parental CCE cells. It thus appears that the expression of Rx/rax did not affect the normal differentiation of ES cells induced by EB formation. Neural differentiation of CCE-RX or CCE-CH by the SDIA method. We next examined the nature of these ES cells once they had differentiated into cells of the neural lineage. The SDIA method is an excellent protocol to cause such differentiation (19). According to the original protocol, we cultured CCE, CCE-RX, or CCE-CH cells on PA6 stromal cells in serum-free medium. After several different culture periods, the cells were immunostained with -tubulin type III antibody (TuJ1). Figure 2 shows 8-day samples stained with TuJ1. More than 95% of the colonies were TuJ1 reactive, irrespective of the cell type cultured; and 80 to 90% of the colonies reacted with anti-map2 antibody, again irrespective of the cell type (data not shown). When we examined the morphology of the cells, the shape of their processes was strikingly different for parental CCE and CCE-RX cells (Fig. 2). Most processes of
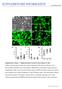
4 4516 TABATA ET AL. MOL. CELL. BIOL. FIG. 2. Differentiation of CCE, CCE-RX, and CCE-CH cells into the neural lineage by the SDIA method. Anti- -tubulin type III antibody (TuJ1) staining patterns of day 8 SDIA cultures of CCE, CCE- RX, and CCE-CH cells. CCE-RX cells were bundled, giving a thick morphology. The length of processes in CCE and CCE-RX cells was not significantly different, though CCE-RX colonies of relatively small size tended to have longer bundled processes. In contrast, CCE-CH cells were morphologically indistinguishable from CCE cells. Since the observation of bundled processes suggested a change in the expression pattern of cell surface proteins, we examined the expression of several membrane proteins by semiquantitative RT-PCR; however, no apparent difference was observed for CCE and CCE-RX cells (data not shown). We then examined several other transcription factors reported to play roles in anterior neural plate or retina development by semiquantitative RT-PCR (data not shown). We first confirmed that none of the genes were expressed in PA6. The weak expression was observed only with Msx2 in undifferentiated ES cells. The expression of Lhx2, Msx2, Pax6, Tbx5, BF-1, Vax, and Otx2 was induced at day 8 in CCE, CCE-CH, and CCE-RX cells cocultured with PA6. We did the same set of experiments three times using independently prepared cdna, and none of these genes showed a significant difference in the expression levels among CCE, CCE-CH, and CCE-RX cells. Use of mouse embryonic retinal explant culture for transplantation experiments. As the mouse embryonic retinal explant culture is an excellent in vitro differentiation system (14), we chose it for use in our study. Retinas were isolated from mouse embryos at day 17.5 and placed on a filter with the ganglion cell layer on the top. At this developmental stage, ganglion cells and some other early differentiating cells started to differentiate (26), and the ganglion cell layer and ventricular zone could be distinguished morphologically when cross sections of the retina were examined (data not shown). After 2 weeks in culture, all of the retinal subpopulations differentiated properly. To test whether this culture can be used as a host of engrafted ES cells for examining their differentiation ability in the retinal environment, we first used mouse embryonic retinal cells obtained from transgenic mice expressing EGFP (16) as donor cells. The retinas of E16.5 transgenic mice strongly expressing EGFP were dispersed with trypsin, and then 10 5 cells were piled onto the surface of the retina explant and cocultured for 2 weeks. Transverse sections of frozen samples revealed that the transplanted retinal cells migrated into the host retina and codifferentiated into a subpopulation of retinal cells, as judged from morphology and marker expression (data not shown). We thus used this culture system to examine the ability of ES cells to differentiate into retinal cells. Transplantation of ES cells into the mouse embryonic retinal explant culture. For transplantation analysis, CCE stable clones expressing either Rx or Chx10 together with EGFP were made and designated as CCE-RX/E and CCE-CH/E, respectively. As a control, CCE clones expressing EGFP alone were also established (CCE-E). We examined the characters of newly established cells by exactly the same set of experiments whose data were given in Fig. 1 and 2, and we found all of the properties of the cells were indistinguishable from those of CCE, CCE-RX, and CCE-CH (data not shown). We first tried to transplant ES cells induced into the neural lineage by the SDIA method. We isolated ES cells from PA6 feeder cells by treatment of cultures with trypsin followed by cell sorting using a FACS-Vantage (Becton Dickinson). But the results were erratic, possibly due to low viability of the isolated ES cells. So we changed the protocol to that involving EB formation followed by RA treatment. We made EBs from CCE-E cells and treated them with RA for 3 days, beginning from the fourth day after starting the EB cultures. Then, the colonies were fully dissociated with trypsin, and 10 5 cells were seeded onto the surface of each of several retinal explant cultures and cocultured for 2 weeks. Frozen transverse sections FIG. 3. Transplantation of ES cells into mouse retina explant cultures. (A) EBs were formed from CCE-E, CCE-RX/E, or CCE-CH/E cells, treated with RA, and cocultured on the surface of mouse retina explant cultures. Views of paraformaldehyde-fixed transverse frozen sections immunostained with anti-egfp antibody (left and middle panels) are shown. Nuclei are visualized by DAPI staining (left and right panels). (B) Appearance of CCE-RX/E cells in the retina explant at 1 and 3 weeks. Views of paraformaldehyde-fixed transverse frozen sections immunostained with anti-egfp antibody and incubated with DAPI. (C) Immunostaining of markers of various retinal subpopulations in frozen sections of mouse retinal explant cultures containing CCE-RX/E cells. The following antibodies were used: anti-glutamine synthetase (GS) (Müller glia cells), anti-nf160 (horizontal cells), anti-calbindin-d-28k (horizontal cells), anti-pkc (bipolar cells), anti-hu (ganglion and amacrine cells), and anti-islet-1 (ganglion, amacrine, and bipolar cells). All samples except for the calbindin-d-28k sample were double stained with anti-egfp antibody. (D) Views of the surface ganglion layer containing CCE-E or CCE-RX/E cells are shown.
5 VOL. 24, 2004 RETINAL FATE SPECIFICATION OF MOUSE ES CELLS 4517
6 4518 TABATA ET AL. MOL. CELL. BIOL. FIG. 4. Electrophysiological recordings from retinal explant cultures. (A) Differential interference contrast and fluorescent images of the CCE-RX/E-derived cells in the retinal explant culture under the whole-cell voltage-clamped condition. Representative recordings of whole-cell currents from CCE-RX/E-derived cells (B), CCE-E-derived cells (E), and nonfluorescent host retinal cells (F) in the presence or absence of the ionotropic glutamate receptor antagonists CNQX (10 M) and D-AP5 (25 M). (C) Ten consecutive spontaneous responses recorded in CCE-RX/E-derived cells are superimposed (superimpose) and averaged (average). (D) A voltage step from 80 to 30 mv evoked an inward current (asterisk) in CCE-RX/E-derived cells, and the current was completely blocked by the sodium channel blocker TTX (1 M). For the electrophysiological study, five CCE-RX/E derived cells, five CCE-E-derived cells, and six nonfluorescent cells were examined. showed that these cells did not migrate into the retina but remained stacked on the surface of the host retina as a large cluster (Fig. 3A, upper panels). With some samples, we observed that the CCE-E-derived cells occupied most of the culture, with only fragments reminiscent of the host retina being found (data not shown). In both cases, antibodies against retinal markers such as NF160, PKC, calbindin-d-28k, and glutamine synthetase did not bind to the ES cell-derived cells (data not shown). Similar results were observed with 1- or 3-week cultures (data not shown), suggesting that the CCE-E cells could not migrate into the retinal explants or differentiate, unlike the case for embryonic retinal cell transplantation. We then examined the fate of CCE-CH/E and CCE-RX/E cells in the retinal explant cultures. Dissociated CCE-CH/E and CCE-RX/E cells, as donor cells, were prepared from EBs treated with RA for 3 days. Cells (10 5 ) were seeded on the top of a retina explant culture, and their fate was traced by examining EGFP expression. After 2 weeks into the culture period, the observation of transverse sections revealed that CCE- CH/E cells had accumulated on the surface of the host retina, as the parental CCE-E cells had done (Fig. 3A, middle panels). In contrast, CCE-RX cells migrated into the host retina and extended their process-like structures between the host retinal cells (Fig. 3A, lower panels). Most of these ES-derived cells were located in the ganglion cell layer as well as in the inner nuclear layer (INL). We also examined the transplanted CCE- RX/E cells on the retinal explant culture at earlier and later periods of culture (Fig. 3B). After 1 week in culture, CCE- RX/E cells had already migrated into the host retina; and after 3 weeks, most of the INL and outer plexiform layer were filled with EGFP-positive cells, and long processes had formed. We also transplanted undifferentiated CCE-RX/E cells or EBs formed from them, but in neither case did the cells migrate into the host retina, suggesting that in vitro priming of cells to the neuronal lineage by RA treatment is essential for the integration of ES-derived cells into the retina. Expression of retinal cell-type markers in transplanted ES cells. Next we analyzed the expression of retinal cell-type markers in CCE-RX/E-derived cells in the retinal explant cultures by immunostaining transverse frozen sections prepared from 2-week cultures. Immunostaining patterns for markers of cells in the INL such as Müller glia, amacrine, bipolar, and horizontal cells are shown in Fig. 3C. Anti-glutamine synthetase, which recognizes Müller glia cells, mostly did not recognize CCE-RX/E-derived cells. We particularly examined the cells with Müller glia-like morphology, which had their cell body in the INL and extended processes vertically, but these ES-derived cells rarely expressed glutamine synthetase. Another Müller glia cell marker, anti-cyclin D3 (10), did not recognize CCE-RX/E-derived cells (data not shown). On the other hand, an antibody specific for NF160 in the axon terminals of horizontal cells (15) reacted with CCE-RX/E-derived cells located along the outer plexiform layer. The possibility that the CCE-RX/E-derived cells had differentiated into horizontal cells was further supported by their reactivity with anticalbindin-d-28k antibody and by the shared morphology characteristic of this specific cell type. Anti-PKC, a marker of bipolar cells, did not recognize CCE-RX/E-derived cells. CCE- RX/E-derived cells located in the INL were also not immunoreactive with anti-hu, which recognizes ganglion and amacrine cells, or with anti-islet-1 (37), which binds to ganglion, amacrine, and bipolar cells, suggesting that CCE-RX/E-derived cells did not differentiate into amacrine or bipolar cells. On the other hand, CCE-RX/E-derived cells in the ganglion cell layer expressed Islet-1. Taken together, our data suggest that by forced expression of Rx/rax, we succeeded in causing ES cells to differentiate into ganglion as well as horizontal cells by coculturing them with retinal explants. When cells were observed from the surface of the culture, CCE-E cells were polygonal in shape, and CCE-RX/E cells on the ganglion cell layer side of the host retina showed a neuronlike morphology and had long processes (Fig. 3D). Electrophysiological properties of transplanted ES cells. To examine the functionality of ES-derived cells in the retina, we next analyzed the electrophysiological properties of grafted CCE-RX/E-derived cells in the ganglion cell layer (Fig. 4A). Whole-cell recordings from CCE-RX/E-derived cells showed spontaneous bursting activity, which was completely suppressed by the combination of CNQX (a non-n-methyl-d-aspartate receptor antagonist) and D-AP5 (an N-methyl-D-aspartate receptor antagonist) (Fig. 4B). Each spontaneous inward current observed in the CCE-RX/E-derived cells looked like
7 VOL. 24, 2004 RETINAL FATE SPECIFICATION OF MOUSE ES CELLS 4519 TABLE 1. Marker expression of retrovirus-transduced retinal explant cultures a Antibodies against: Population (%) of reactive cells from virus-encoded: EGFP RX-IRES- EGFP b GS Cyclin D NF160 Not detected PKC Not detected a Retrovirus encoding EGFP or Rx/rax-IRES-EGFP was used for transduction of retina explant cultures, and after 2 weeks, frozen transverse sections were analyzed for marker expression by immunostaining. Populations (%) of antibody-reactive cells among the EGFP-positive cells were determined by counting independent samples. b IRES, internal ribosome entry site. the synaptic response of an intact central synapse, suggesting that these responses were spontaneous excitatory postsynaptic currents (Fig. 4C). Depolarizing voltage steps caused an inward current followed by a slower outward current in the CCE-RX/E-derived cells (Fig. 4D). Tetrodotoxin, a blocker of voltage-gated sodium channels, eliminated the inward current, leaving the slow outward current, presumably mediated by voltage-gated potassium channels. This finding indicates the presence of sodium channels in these cells, which is one of the most critical criteria for distinguishing neurons from other types of cells (Fig. 4D). In contrast, CCE-E-derived cells never showed such bursting activity (Fig. 4E) or inward sodium currents (data not shown). Current recordings from the cells without fluorescence (most likely host retinal ganglion cells) exhibited spontaneous bursting activity characteristic of the developing retina (28, 41), which was also inhibited by CNQX or by D-AP5 (Fig. 4F). Since grafted CCE-RX/E cells displayed electrophysiological characteristics similar to those of the host ganglion cells, we conclude that the CCE-RX/E-derived cells had became functionally integrated into the neural circuit of the host retina. Transduction of retinal explant cultures with retrovirus containing Rx/rax. Previous studies on the in vivo retroviral transduction of rat pup retinas at P1 with Rx/rax showed that more than 90% of the Rx/rax-infected cells had Müller glia-like morphology and expressed Müller glia cell markers (10). We introduced Rx/rax by retrovirus into mouse E17.5 retinal explant cultures, but the populations of cyclin-d3 or glutamine synthetase-positive cells were not significantly different from those of the control retina under the conditions used (Table 1). Thus, we speculate that Rx/rax has different functions at different stages of retinal development. DISCUSSION Although the use of ES cells for regenerative medicine as an unlimited source of various types of cells has been widely discussed, their successful use for neural retina regeneration has not been reported. We took advantage of the suitability of ES cells for genetic engineering to accomplish efficient differentiation of ES cells into retinal cells. We showed that the expression of Rx/rax, a homeobox gene, permitted ES cells to migrate into the host retina, where at least a subset of the FIG. 5. Expression of various forebrain- and retina-expressed genes in CCE-CH/E and CCE-RX/E cells. Various forebrain- and retina-expressed genes were examined by semiquantitative RT-PCR analysis using mrna prepared from EBs and from EBs treated with RA of CCE, CCE-CH/E, and CCE-RX/E cells. genetically manipulated ES cells differentiated into ganglion and horizontal cells, as judged from the morphological, immunohistochemical, and electrophysiological data. Although the character of the undifferentiated ES cells did not seem to be changed by the expression of Rx/rax, a drastic morphological change in Rx/rax-expressing ES cells was observed when the cells were caused to differentiate into neural lineage, suggesting nervous system-specific effects of Rx/rax. It is possible that Rx/rax cooperates with neuron-specific (transcription) factors to activate target genes; however, biochemical characterization of such a partner(s) of Rx/rax has not been reported. A Rx/rax target sequence was found in photoreceptor-specific genes (23). To examine target genes of Rx/rax, we conducted semiquantitative RT-PCR of various forebrain- and retina-expressed genes using mrna of EBs and EBs treated with RA prepared from CCE, CCE-CH/E, and CCE-RX/E cells, but no clear specific modification of the gene expression pattern in Rx/rax-expressing ES cells was observed (Fig. 5). So we have changed our strategy to DNA-chip analysis to reveal possible target genes in a comprehensive way by using samples
8 4520 TABATA ET AL. MOL. CELL. BIOL. of EBs treated with RA prepared from CCE-E and CCE- RX/E cells, and a panel of genes that were suppressed or enhanced by Rx/rax expression in ES cells was revealed (unpublished observations). Experiments to clarify the physiological roles of the genes are now under way. Chx10 is a homeodomain transcription factor that is expressed in immature retinal progenitor cells (25). We could not observe any change in CCE properties in terms of morphology or differentiation activity due to the expression of Chx10. Although Chx10 is expressed in retinal progenitor cells, there is no argument suggesting Chx10 to be a master regulator of retinal development. As retinal progenitor cells become postmitotic and differentiate, Chx10 expression is terminated in all cell types except bipolar cells. The elimination of Chx10 products results in congenital microphathalmia in humans (8) and mice (2), which is characterized by small eyes, cataracts, iris coloboma, and blindness. The absence of bipolar cells and the low number of retinal cells in this disorder suggest that Chx10 acts to promote the bipolar cell fate (14) and to regulate cell number (13), respectively. Since the proliferation activity of undifferentiated CCE-CH cells did not show augmentation over that of the parental CCE cells, the proliferation-promoting activity of Chx10 is assumed to be cell-type specific. Transplanted CCE-RX/E cells were observed only in the ganglion cell layer and INL. Ganglion and horizontal cells become differentiated relatively early (4). It thus appears that the transplanted CCE-RX/E cells mimicked the endogenous order of cell fate determination. In normal retinal development, the influence of epigenetic cues supplied from the microenvironment to determine the differentiation fate of the retinal progenitor has been suggested. From experiments using cocultures of different stages of progenitor and host cells, it was suggested that the responsiveness of progenitors to stagespecific epigenetic cues was dictated by intrinsic factors (30). On the other hand, a recent study showed that the competence of progenitor cells was not irreversibly fated (17). The role of the retinal environment for differentiation of ES cells in the present study needs to be examined by a carefully designed set of control experiments. One of the striking results obtained from the transplantation experiments was the ability of CCE cells expressing Rx/rax to migrate into the host retina. The results from SDIA cultures, showing bundled processes of CCE-RX/E cells, also suggested a change in the expression pattern of surface proteins in CCE- RX/E cells. In the case of neural transplantation into the adult CNS, it is suggested that retinal glial cells constitute a barrier preventing the mature host retina from being infiltrated by the transplanted cells (24). Only limited information is available regarding reactive glia formation in explant cultures of mouse embryonic retina, and so we need to examine the role of reactive glia cells in our system by using glial fibrillary acidic protein (GFAP) and vimentin knockout mice (32). We have preliminarily examined several known surface proteins by semiquantitative RT-PCR but found no significant change. We are continuing to examine the change in cell surface protein expression by conducting proteomics experiments. A previous study on misexpression of Rx/rax in the neonatal rat retina by in vivo retroviral transduction showed increased formation of Müller glia cells and suggested the role of Hes1 transcription factor downstream of Rx/rax (10). Preliminary examination of Hes1 expression by semiquantitative RT-PCR indicated no augmentation of the Hes1 gene in CCE-RX/E cells of undifferentiated or RA-treated EBs (data not shown), suggesting that enhancement of Hes1 by Rx/rax requires a certain cell type(s) or certain conditions provided from the cellular environment. We also failed to cause differentiation of E17 retinal cells into Müller glia cells by transduction with retrovirus-encoded Rx/ras, which indicates that the differentiation stage of retinal cells may be important in defining the biological functions of Rx/rax in retinal cells. Accordingly, it is possible that CCE-ES cells are not competent to receive signals to promote Müller glia cell formation by Rx/rax expression. The zebra fish orthologue of Rx/rax helps to define the region of the forebrain fated to give rise to retinal tissue and may be involved in the cellular migrations that lead to splitting of the retinal field and formation of the optic primordia (5). Furthermore, Xenopus embryos injected with Rx/rax developed ectopic retinal tissue, suggesting the importance of Rx/rax for retinal establishment (1, 27). We speculate that forced expression of Rx/rax in ES cells gives specification for neuronal differentiation into retinal cells, thus changing the properties of ES cells and resulting in their migration into the host retinal cells and expression of retinal subpopulation markers. Since the retinal environment is required to specify the differentiation of CCE-RX/E cells into retinal cells, further examination of interacting signaling mechanisms between retina and ES cells may give us better insight into the mechanism underlying regeneration of retinal cells. Furthermore, our findings open the prospect of successful therapeutic application of ES cells for the treatment of degenerative retinal diseases and the injured retina. ACKNOWLEDGMENTS We thank M. Watanabe and Y. Aoki for technical assistance, and we are also grateful to S. Nishikawa for CCE-ES cells, M. Okabe for EGFP transgenic mice, and T. Kitamura for retrovirus vector and packaging cells. We thank R. Kageyama for advice on mouse embryo retinal cultures and for critical reading of the manuscript, as well as M. Takeichi, M. Kuno, and A. Yoshimura for discussion and critical reading of the manuscript. This work was supported by RIKEN Center of Developmental Biology, Kobe, Japan, and by special coordination funds for promoting science and technology from the Ministry of Education, Science, Sport, Culture, and Technology of Japan. REFERENCES 1. Andreazzoli, M., G. Gestri, D. Angeloni, E. Menna, and G. Barsacchi Role of Xrx1 in Xenopus eye and anterior brain development. Development 126: Burmeister, M., J. Novak, M.-Y. Liang, S. Basu, L. Ploder, N. L. Hawes, D. Vidgen, F. Hoover, D. Godman, V. I. Kalnins, T. H. Roderick, B. A. Taylor, M. H. Hankin, and R. R. McInnes Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nature 12: Casarosa, S., M. Andreazzoli, A. Simeone, and G. Barsacchi Xrx1, a novel Xenopus homeobox gene expressed during eye and pineal gland development. Mech. Dev. 61: Cepko, C. L., C. P. Austin, X. Yang, M. Alexiades, and D. Ezzedine Cell fate determination in the retina. Proc. Natl. Acad. Sci. USA 93: Chuang, J. C., and P. A. Raymond Zebrafish genes rx1 and rx2 help define the region of forebrain that gives rise to retina. Dev. Biol. 231: Cristofano, A. D., B. Pesce, C. Cordon-Cardo, and P. P. Pandolfi Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19: Elefanty, A. G., L. Robb, R. Birner, and C. G. Begley Hematopoieticspecific genes are not induced during in vitro differentiation of scl-null embryonic stem cells. Blood 90:
9 VOL. 24, 2004 RETINAL FATE SPECIFICATION OF MOUSE ES CELLS Ferda Percin, E., L. A. Ploder, J. J. Yu, K. Arici, D. J. Horsford, A. Rutherford, B. Bapat, D. W. Cox, A. M. Duncan, V. I. Kalnins, A. Kocak-Altintas, J. C. Sowden, E. Traboulsi, M. Sarfarazi, and R. R. McInnes Human microphthalmia associated with mutations in the retinal homeobox gene Chx10. Nat. Genet. 25: Furukawa, T., C. A. Kozak, and C. L. Cepko rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc. Natl. Acad. Sci. USA 94: Furukawa, T., S. Mukherjee, Z.-Z. Bao, E. M. Morrow, and C. L. Cepko rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron 26: Gajovic, S., L. St.-Onge, Y. Yokota, and P. Gruss Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation 62: Galliot, B., C. de Vargas, and D. Miller Evolution of homeobox genes: Q50 Paired-like genes founded the Paired class. Dev. Genes Evol. 209: Green, E. S., J. L. Stubbs, and E. M. Levine Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development 130: Hatakeyama, J., K. Tomita, T. Inoue, and R. Kageyama Roles of homeobox and bhlh genes in specification of a retinal cell type. Development 128: Haverkamp, S., and H. Wassle Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 424: Ikawa, M., S. Yamada, T. Nakanishi, and M. Okabe Green fluorescent protein (GFP) as a vital marker in mammals. Curr. Top. Dev. Biol. 44: James, J., A. V. Das, S. Bhattacharya, D. M. Chacko, X. Zhao, and I. Ahmad In vitro generation of early-born neurons from late retinal progenitors. J. Neurosci. 23: Jean, D., K. Ewan, and P. Gruss Molecular regulators involved in vertebrate eye development. Mech. Dev. 76: Kawasaki, H., K. Mizuseki, S. Nishikawa, S. Kaneko, Y. Kuwana, S. Nakanishi, S. I. Nishikawa, and Y. Sasai Induction of midbrain dopaminergic neurons from ES cells by stromal cell derived inducing activity. Neuron 28: Kawasaki, H., H. Suemori, K. Mizuseki, K. Watanabe, F. Urano, H. Ichinose, M. Haruta, M. Takahashi, K. Yoshikawa, S. Nishikawa, N. Nakatsuji, and Y. Sasai Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. USA 99: Keller, G., M. Kennedy, T. Papayannopoulou, and M. V. Wiles Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. Biol. 13: Kim, J.-H., J. M. Auerbach, J. A. Rodriguez-Gomez, I. Velasco, D. Gavin, N. Lumelsky, S.-H. Lee, J. Nguyen, R. Sanchez-Pernaute, K. Bankiewicz, and R. McKay Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson s disease. Nature 418: Kimura, A., D. Singh, E. F. Wawrousek, M. Kikuchi, M. Nakamura, and T. Shinohara Both PCE-1/RX and PTX/CRX interactions are necessary for photoreceptor-specific gene expression. J. Biol. Chem. 275: Kinouchi, R., M. Takeda, L. Yang, U. Wilhelmsson, A. Lundkvist, M. Pekny, and D.-F. Chen Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 6: Liu, I. S. C., J.-D. Chen, L. Ploder, D. Vidgen, D. van der Kooy, V. I. Kalnins, and R. R. McInnes Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron 13: Marquardt, T., and P. Gruss Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 25: Mathers, P. H., A. Grinberg, K. A. Mahon, and M. Jamrich The Rx homeobox gene is essential for vertebrate eye development. Nature 387: Meister, M., R. O. L. Wong, D. A. Baylor, and C. J. Shatz Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252: Morita, S., T. Kojima, and T. Kitamura Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7: Morrow, E. M., M. J. Belliveau, and C. L. Cepko Two phases of rod photoreceptor differentiation during rat retinal development. J. Neurosci. 18: Ooto, S., M. Haruta, Y. Honda, H. Kawasaki, Y. Sasai, and M. Takahashi Induction of the differentiation of lentoids from primate embryonic stem cells. Investig. Ophthalmol. Vis. Sci. 44: Pekny, M., C. B. Johansson, C. Eliasson, J. Stakeberg, A. Wallen, T. Perlmann, U. Lendahl, C. Betsholtz, C.-H. Berthold, and J. Frisen Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 145: Rossi, F., and E. Cattaneo Neural stem cell therapy for neurological diseases: dreams and reality. Nat. Rev. Neurosci. 3: Schraermeyer, U., G. Thumann, T. Luther, N. Kociok, S. Armhold, K. Kruttwig, C. Andressen, K. Addicks, and K. U. Bartz-Schmidt Subretinally transplanted embryonic stem cells rescue photoreceptor cells from degeneration in the RCS rats. Cell Transplant. 10: Solter, D., and B. B. Knowles Monoclonal antibody defining a stagespecific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 75: Takahashi, M., T. D. Palmer, J. Takahashi, and F. H. Gage Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol. Cell. Neurosci. 12: Thor, S., J. Ericson, T. Brannstrom, and T. Edlund The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron 7: Tucker, P., L. Laemle, A. Munson, S. Kanekar, E. R. Oliver, N. Brown, H. Schlecht, M. Vetter, and T. Glaser The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis 31: Wichterle, H., I. Lieberam, J. A. Porter, and T. M. Jessell Directed differentiation of embryonic stem cells into motor neurons. Cell 110: Winnier, G., M. Blessing, P. A. Labosky, and B. L. Hogan Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9: Wong, R. O. L Retinal waves and visual system development. Annu. Rev. Neurosci. 22: Young, M. J., J. Ray, S. J. Whiteley, H. Klassen, and F. H. Gage Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol. Cell. Neurosci. 16: Zhao, X., J. Liu, and I. Ahmad Differentiation of embryonic stem cells into retinal neurons. Biochem. Biophys. Res. Commun. 297:
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Protocol Using a Dox-Inducible Polycistronic m4f2a Lentivirus to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent
 APPLICATION NOTE Page 1 Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent Authors: Amelia L. Cianci 1, Xun Cheng 1 and Kerry P. Mahon 1,2 1 Stemgent Inc.,
APPLICATION NOTE Page 1 Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent Authors: Amelia L. Cianci 1, Xun Cheng 1 and Kerry P. Mahon 1,2 1 Stemgent Inc.,
Protocol Using the Reprogramming Ecotropic Retrovirus Set: Mouse OSKM to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblast (MEF) cells into induced pluripotent stem (ips) cells in a 6-well format. Transduction
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblast (MEF) cells into induced pluripotent stem (ips) cells in a 6-well format. Transduction
Mesenchymal Progenitor Cells (MPCs) Involvement In Ectopic Fat Formation In Muscular Dystrophy
 Mesenchymal Progenitor Cells (MPCs) Involvement In Ectopic Fat Formation In Muscular Dystrophy Jihee Sohn, MS 1, Ying Tang 2, Bing Wang, MD,PhD 3, Aiping Lu 4, Johnny Huard, Ph.D 5. 1 university of pittsburgh,
Mesenchymal Progenitor Cells (MPCs) Involvement In Ectopic Fat Formation In Muscular Dystrophy Jihee Sohn, MS 1, Ying Tang 2, Bing Wang, MD,PhD 3, Aiping Lu 4, Johnny Huard, Ph.D 5. 1 university of pittsburgh,
Culture and differentiation of embryonic stem cells. Hong-Lin Su Department of Life Sciences, National Chung-Hsing University
 Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Xeno-Free Systems for hesc & hipsc. Facilitating the shift from Stem Cell Research to Clinical Applications
 Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE.
 NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
Protocol Reprogramming MEFs using the Dox Inducible Reprogramming Lentivirus Set: Mouse OKSM
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblasts (MEFs) into induced pluripotent stem (ips) cells in a 6-well format. Transduction
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblasts (MEFs) into induced pluripotent stem (ips) cells in a 6-well format. Transduction
MicroRNAs Modulate Hematopoietic Lineage Differentiation
 Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
Honours and PostDoctorate Research Projects. Lions Eye Institute, Perth
 Honours and PostDoctorate Research Projects Available from 1 st Semester, 2018 Lions Eye Institute, Perth 1 About the Lions Eye Institute. The Lions Eye Institute is a not-for-profit centre of excellence
Honours and PostDoctorate Research Projects Available from 1 st Semester, 2018 Lions Eye Institute, Perth 1 About the Lions Eye Institute. The Lions Eye Institute is a not-for-profit centre of excellence
Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia
 Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia labeled with the specific marker 4C4 (magenta) are
Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia labeled with the specific marker 4C4 (magenta) are
Therapeutic Cell Replacement. Steven McLoon Department of Neuroscience University of Minnesota
 Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Neuronal Death Neurons are lost due to four main causes: Trauma Toxin Hypoxia (typically loss of air or blood
Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Neuronal Death Neurons are lost due to four main causes: Trauma Toxin Hypoxia (typically loss of air or blood
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
APPLICATION NOTE Page 1
 APPLICATION NOTE Page 1 Generation of ips Cells from Oct4-Neo Reporter Embryonic Fibroblasts Dongmei Wu, Yan Gao, Charles Martin, Xun Cheng, Wen Xiong, Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle St.,
APPLICATION NOTE Page 1 Generation of ips Cells from Oct4-Neo Reporter Embryonic Fibroblasts Dongmei Wu, Yan Gao, Charles Martin, Xun Cheng, Wen Xiong, Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle St.,
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Notch1 (forward: 5 -TGCCTGTGCACACCATTCTGC-3, reverse: Notch2 (forward: 5 -ATGCACCATGACATCGTTCG-3, reverse:
 Supplementary Information Supplementary Methods. RT-PCR. For mouse ES cells, the primers used were: Notch1 (forward: 5 -TGCCTGTGCACACCATTCTGC-3, reverse: 5 -CAATCAGAGATGTTGGAATGC-3 ) 1, Notch2 (forward:
Supplementary Information Supplementary Methods. RT-PCR. For mouse ES cells, the primers used were: Notch1 (forward: 5 -TGCCTGTGCACACCATTCTGC-3, reverse: 5 -CAATCAGAGATGTTGGAATGC-3 ) 1, Notch2 (forward:
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10202 Supplementary Figure 1: Rapid generation of neurons from human ES cells. a, Phase contrast image of feeder-free culture of human ES cells infected with Brn2, Ascl1, Myt1l (BAM)
doi:10.1038/nature10202 Supplementary Figure 1: Rapid generation of neurons from human ES cells. a, Phase contrast image of feeder-free culture of human ES cells infected with Brn2, Ascl1, Myt1l (BAM)
Code No Retrovirus Packaging Kit Ampho
 Code No. 6161 Retrovirus Packaging Kit Ampho Precautions for the use of this product Please follow the guideline for experiments using recombinant DNA issued by the relevant authorities and the safety
Code No. 6161 Retrovirus Packaging Kit Ampho Precautions for the use of this product Please follow the guideline for experiments using recombinant DNA issued by the relevant authorities and the safety
TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1
 AD Award Number: W81XWH-10-1-0181 TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1 PRINCIPAL INVESTIGATOR: Jonathan Chernoff, M.D., Ph.D. CONTRACTING ORGANIZATION: Institute
AD Award Number: W81XWH-10-1-0181 TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1 PRINCIPAL INVESTIGATOR: Jonathan Chernoff, M.D., Ph.D. CONTRACTING ORGANIZATION: Institute
Supplementary Protocol. sirna transfection methodology and performance
 Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Suggested Method Reprogramming MEF Cells into pips Cells using the Stemgent Recombinant Human Protein Set: OSKM-11R
 Page 1 Reprogramming MEF Cells into pips Cells using the Cat. No. 00-0062 OVERVIEW The procedure outlined below describes the reprogramming of mouse embryonic fibroblasts (MEF) by the addition of four
Page 1 Reprogramming MEF Cells into pips Cells using the Cat. No. 00-0062 OVERVIEW The procedure outlined below describes the reprogramming of mouse embryonic fibroblasts (MEF) by the addition of four
Protocol Reprogramming Human Fibriblasts using the Dox Inducible Reprogramming Polycistronic Lentivirus Set: Human 4F2A LoxP
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
Flow cytometry Stained cells were analyzed and sorted by SORP FACS Aria (BD Biosciences).
 Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency
 nature methods Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency Akitsu Hotta, Aaron Y L Cheung, Natalie Farra, Kausalia Vijayaragavan, Cheryle A Séguin, Jonathan S Draper,
nature methods Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency Akitsu Hotta, Aaron Y L Cheung, Natalie Farra, Kausalia Vijayaragavan, Cheryle A Séguin, Jonathan S Draper,
hpsc Growth Medium DXF Dr. Lorna Whyte
 hpsc Growth Medium DXF Dr. Lorna Whyte 27.06.2014 Training from Heidelberg Overview Background: Stem Cells Introduction: Human Pluripotent Stem Cells (hpsc) vs. Adult Stem Cells Promise of PSC Research
hpsc Growth Medium DXF Dr. Lorna Whyte 27.06.2014 Training from Heidelberg Overview Background: Stem Cells Introduction: Human Pluripotent Stem Cells (hpsc) vs. Adult Stem Cells Promise of PSC Research
Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of
 Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Therapeutic Cell Replacement. Steven McLoon Department of Neuroscience University of Minnesota
 Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Dec 13) Thursday (Dec 14) Friday (Dec 15) 9:00-10:00am Surdyk s Café in
Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Dec 13) Thursday (Dec 14) Friday (Dec 15) 9:00-10:00am Surdyk s Café in
Stem Cells & Neurological Disorders. Said Ismail Faculty of Medicine University of Jordan
 Stem Cells & Neurological Disorders Said Ismail Faculty of Medicine University of Jordan Outline: - Introduction - Types & Potency of Stem Cells - Embryonic Stem Cells - Adult Stem Cells - ipscs -Tissue
Stem Cells & Neurological Disorders Said Ismail Faculty of Medicine University of Jordan Outline: - Introduction - Types & Potency of Stem Cells - Embryonic Stem Cells - Adult Stem Cells - ipscs -Tissue
Guided differentiation of ES cell into mesenchymal stem cell
 Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 7: Modern Genetics Notes Using Gene Sequencing Genome = all of an organism s DNA, including mitochondrial/chloroplast DNA. Polymerase chain reaction (PCR) is used to amplify
Edexcel (B) Biology A-level Topic 7: Modern Genetics Notes Using Gene Sequencing Genome = all of an organism s DNA, including mitochondrial/chloroplast DNA. Polymerase chain reaction (PCR) is used to amplify
Supplementary Methods
 Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Developmental Biology 3230 Exam 1 (Feb. 6) NAME
 DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
Supplementary Methods
 Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Stem Cells: Introduction and Prospects in Regenerative Medicine.
 Stem Cells: Introduction and Prospects in Regenerative Medicine www.gothamgazette.com/.../stemcell/stem_cell.jpg Ode to a Stem Cell, Part II by VCW There once was stem cell stuck in the hood Dividing endlessly,
Stem Cells: Introduction and Prospects in Regenerative Medicine www.gothamgazette.com/.../stemcell/stem_cell.jpg Ode to a Stem Cell, Part II by VCW There once was stem cell stuck in the hood Dividing endlessly,
Components Neural induction basal medium (Cat. # ) 100 ml x 5 Neural induction medium supplement 50x (Cat. # ) 2 ml x 5
 HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
Schematic representation of the endogenous PALB2 locus and gene-disruption constructs
 Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Piscataway, NJ February 2007
 Final Progress Report for the New Jersey Commission on Spinal Cord Research Submitted by David P. Crockett, PhD for the late Ira B. Black, MD Department of Neuroscience and Cell Biology UMDNJ-Robert Wood
Final Progress Report for the New Jersey Commission on Spinal Cord Research Submitted by David P. Crockett, PhD for the late Ira B. Black, MD Department of Neuroscience and Cell Biology UMDNJ-Robert Wood
Chapter 20 Recombinant DNA Technology. Copyright 2009 Pearson Education, Inc.
 Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Department of Clinical Application, Cebter for ips cell Research and Application, 53 Kawahara-cho Shogoin Sakyo-ku, Kyoto,
 Department of Clinical Application, Cebter for ips cell Research and Application, Kyoto University 53 Kawahara-cho Shogoin Sakyo-ku, Kyoto, 606-8507 JAPAN Phone: 81-75-366-7066, Fax: 81-75-366-7071 Daisuke
Department of Clinical Application, Cebter for ips cell Research and Application, Kyoto University 53 Kawahara-cho Shogoin Sakyo-ku, Kyoto, 606-8507 JAPAN Phone: 81-75-366-7066, Fax: 81-75-366-7071 Daisuke
GENESDEV/2007/ Supplementary Figure 1 Elkabetz et al.,
 GENESDEV/2007/089581 Supplementary Figure 1 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 2 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 3 Elkabetz et al., GENESDEV/2007/089581
GENESDEV/2007/089581 Supplementary Figure 1 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 2 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 3 Elkabetz et al., GENESDEV/2007/089581
The retina is a component of the central nervous system
 Potential for neural regeneration after neurotoxic injury in the adult mammalian retina Sotaro Ooto*, Tadamichi Akagi*, Ryoichiro Kageyama, Joe Akita*, Michiko Mandai*, Yoshihito Honda*, and Masayo Takahashi*
Potential for neural regeneration after neurotoxic injury in the adult mammalian retina Sotaro Ooto*, Tadamichi Akagi*, Ryoichiro Kageyama, Joe Akita*, Michiko Mandai*, Yoshihito Honda*, and Masayo Takahashi*
Input. Pulldown GST. GST-Ahi1
 A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
John Gurdon was testing the hypothesis of genomic equivalence or that when cells divide they retain a full genomic compliment.
 1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Human ESC-derived Neural Progenitor Expansion Starter Panel. Reagents for the expansion of human neural progenitors.
 Human ESC-derived Neural Progenitor Expansion Starter Panel Catalog Number SC016 Reagents for the expansion of human neural progenitors. This package insert must be read in its entirety before using this
Human ESC-derived Neural Progenitor Expansion Starter Panel Catalog Number SC016 Reagents for the expansion of human neural progenitors. This package insert must be read in its entirety before using this
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Reprogramming of Murine Somatic Cells Using mir302/367 lentivirus Modification by: Frederick Anokye-Danso, Ph.D
 Reprogramming of Murine Somatic Cells Using mir302/367 lentivirus Modification by: Frederick Anokye-Danso, Ph.D MATERIALS Media Sodium Pyruvate (Mediatech; Cat# MT25-000-CI) Leukemia Inhibitory Factor
Reprogramming of Murine Somatic Cells Using mir302/367 lentivirus Modification by: Frederick Anokye-Danso, Ph.D MATERIALS Media Sodium Pyruvate (Mediatech; Cat# MT25-000-CI) Leukemia Inhibitory Factor
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Generation of NSCs from hpscs in SDC medium.
 Supplementary Figure 1 Generation of NSCs from hpscs in SDC medium. (a) Q-PCR of pluripotent markers (OCT4, NANOG), neural markers (SOX1, PAX6, N-Cadherin), markers for the other germ layers (T, EOMES,
Supplementary Figure 1 Generation of NSCs from hpscs in SDC medium. (a) Q-PCR of pluripotent markers (OCT4, NANOG), neural markers (SOX1, PAX6, N-Cadherin), markers for the other germ layers (T, EOMES,
OmicsLink shrna Clones guaranteed knockdown even in difficult-to-transfect cells
 OmicsLink shrna Clones guaranteed knockdown even in difficult-to-transfect cells OmicsLink shrna clone collections consist of lentiviral, and other mammalian expression vector based small hairpin RNA (shrna)
OmicsLink shrna Clones guaranteed knockdown even in difficult-to-transfect cells OmicsLink shrna clone collections consist of lentiviral, and other mammalian expression vector based small hairpin RNA (shrna)
Nature Medicine doi: /nm.2548
 Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A
 Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES
 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.
 LEGENDS TO SUPPLEMENTARY FIGURES Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.2) were stained with the antibodies oct3/4
LEGENDS TO SUPPLEMENTARY FIGURES Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.2) were stained with the antibodies oct3/4
OriGene GFC-Arrays for High-throughput Overexpression Screening of Human Gene Phenotypes
 OriGene GFC-Arrays for High-throughput Overexpression Screening of Human Gene Phenotypes High-throughput Gene Function Validation Tool Introduction sirna screening libraries enable scientists to identify
OriGene GFC-Arrays for High-throughput Overexpression Screening of Human Gene Phenotypes High-throughput Gene Function Validation Tool Introduction sirna screening libraries enable scientists to identify
p27 Kip1 and p57 Kip2 Regulate Proliferation in Distinct Retinal Progenitor Cell Populations
 The Journal of Neuroscience, June 15, 2001, 21(12):4259 4271 p27 Kip1 and p57 Kip2 Regulate Proliferation in Distinct Retinal Progenitor Cell Populations Michael A. Dyer and Constance L. Cepko Department
The Journal of Neuroscience, June 15, 2001, 21(12):4259 4271 p27 Kip1 and p57 Kip2 Regulate Proliferation in Distinct Retinal Progenitor Cell Populations Michael A. Dyer and Constance L. Cepko Department
Supporting Information
 Supporting Information Patel et al. 10.1073/pnas. SI Materials and Methods Cells and Reagents. Hepatocyte-derived H35 cells were grown in high glucose DMEM (Gibco) media, supplemented with 10% FBS, 2%
Supporting Information Patel et al. 10.1073/pnas. SI Materials and Methods Cells and Reagents. Hepatocyte-derived H35 cells were grown in high glucose DMEM (Gibco) media, supplemented with 10% FBS, 2%
Differentiation. Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU)
 Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays
 Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays Elizabeth J. Abraham, Ph.D. BD Biosciences Outline Overview of surfaces and culture
Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays Elizabeth J. Abraham, Ph.D. BD Biosciences Outline Overview of surfaces and culture
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP)
 Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP) in the cortex (area 1 as defined in Fig. 2a), and corpus
Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP) in the cortex (area 1 as defined in Fig. 2a), and corpus
PluriQ TM Serum Replacement (PluriQ TM SR)
 PluriQ TM Serum Replacement (PluriQ TM SR) (PluriQ SR) is a defined, serum-free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated
PluriQ TM Serum Replacement (PluriQ TM SR) (PluriQ SR) is a defined, serum-free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated
Genetics and Genomics in Medicine Chapter 9 Questions
 Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
Lecture 24 Differentiation and stem cells
 Lecture 24 Differentiation and stem cells *Stem cells and differentiation in plants Totipotency Stem cells in animals Therapeutic use Cloning Therapeutic Reproductive Therapeutic cloning in humans Stem
Lecture 24 Differentiation and stem cells *Stem cells and differentiation in plants Totipotency Stem cells in animals Therapeutic use Cloning Therapeutic Reproductive Therapeutic cloning in humans Stem
A Novel Platform to Enable the High-Throughput Derivation and Characterization of Feeder-Free Human ipscs
 Supplementary Information A Novel Platform to Enable the High-Throughput Derivation and Characterization of Feeder-Free Human ipscs Bahram Valamehr, Ramzey Abujarour, Megan Robinson, Thuy Le, David Robbins,
Supplementary Information A Novel Platform to Enable the High-Throughput Derivation and Characterization of Feeder-Free Human ipscs Bahram Valamehr, Ramzey Abujarour, Megan Robinson, Thuy Le, David Robbins,
Directed differentiation of human embryonic stem cells
 Directed differentiation of human embryonic stem cells TECAN Symposium 2008 Biologics - From Benchtop to Production Wednesday 17 September 2008 Andrew Elefanty Embryonic Stem Cell Differentiation Laboratory,
Directed differentiation of human embryonic stem cells TECAN Symposium 2008 Biologics - From Benchtop to Production Wednesday 17 September 2008 Andrew Elefanty Embryonic Stem Cell Differentiation Laboratory,
Mice TRAMP mice were maintained in a C57BL/6J background. Syngeneic UBI-GFP/BL6 mice were used for bone marrow engraftment. 2
 Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Supplemental Figure 1 (Figure S1), related to Figure 1 Figure S1 provides evidence to demonstrate Nfatc1Cre is a mouse line that directed gene
 Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supporting Information
 Supporting Information Wang et al. 10.1073/pnas.1008382107 SI Methods Tissue Fixation. Adult rats, mice, and Oprd1 exon 1-deleted mice (Jackson Lab) were anesthetized and perfused with 20 ml of warm saline,
Supporting Information Wang et al. 10.1073/pnas.1008382107 SI Methods Tissue Fixation. Adult rats, mice, and Oprd1 exon 1-deleted mice (Jackson Lab) were anesthetized and perfused with 20 ml of warm saline,
The poly(a) tail blocks RDR6 from converting self mrnas into substrates for gene silencing
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17036 The poly(a) tail blocks RDR6 from converting self mrnas into substrates for gene silencing
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17036 The poly(a) tail blocks RDR6 from converting self mrnas into substrates for gene silencing
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming vector for generating induced pluripotent stem (ips)
 Kit for generating ips cells using ReproRNA -OKSGM, a non-integrating, self-replicating RNA reprogramming vector Product Description ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming
Kit for generating ips cells using ReproRNA -OKSGM, a non-integrating, self-replicating RNA reprogramming vector Product Description ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming
Spécification cellulaire: développement de la rétine. Michel Cayouette Neurobiologie Cellulaire IRCM
 Spécification cellulaire: développement de la rétine Michel Cayouette (michel.cayouette@ircm.qc.ca) Neurobiologie Cellulaire IRCM How is cell diversity generated during CNS development?? Intrinsic vs Environmental
Spécification cellulaire: développement de la rétine Michel Cayouette (michel.cayouette@ircm.qc.ca) Neurobiologie Cellulaire IRCM How is cell diversity generated during CNS development?? Intrinsic vs Environmental
RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by. 5 AGACACAAACACCAUUGUCACACUCCACAGC; Rand-2 OMe,
 Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Figure S2. Response of mouse ES cells to GSK3 inhibition. Mentioned in discussion
 Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
Supporting Information
 Supporting Information Tal et al. 10.1073/pnas.0807694106 SI Materials and Methods VSV Infection and Quantification. Infection was carried out by seeding 5 10 5 MEF cells per well in a 6-well plate and
Supporting Information Tal et al. 10.1073/pnas.0807694106 SI Materials and Methods VSV Infection and Quantification. Infection was carried out by seeding 5 10 5 MEF cells per well in a 6-well plate and
International Journal of Pharma and Bio Sciences
 Research Article Cell Biology International Journal of Pharma and Bio Sciences ISSN 0975-6299 EFFECT OF ANTIBIOTICS ON MESENCHYMAL STROMAL CELLS UNDER XENO-FREE CULTURE CONDITIONS FOR CLINICAL USE JAIANAND
Research Article Cell Biology International Journal of Pharma and Bio Sciences ISSN 0975-6299 EFFECT OF ANTIBIOTICS ON MESENCHYMAL STROMAL CELLS UNDER XENO-FREE CULTURE CONDITIONS FOR CLINICAL USE JAIANAND
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
2014 Pearson Education, Inc. CH 8: Recombinant DNA Technology
 CH 8: Recombinant DNA Technology Biotechnology the use of microorganisms to make practical products Recombinant DNA = DNA from 2 different sources What is Recombinant DNA Technology? modifying genomes
CH 8: Recombinant DNA Technology Biotechnology the use of microorganisms to make practical products Recombinant DNA = DNA from 2 different sources What is Recombinant DNA Technology? modifying genomes
Self-organization of neural patterns and structures in 3D culture of stem cells
 Invited Paper Self-organization of neural patterns and structures in 3D culture of stem cells Yoshiki Sasai Organogenesis and Neurogenesis Group, RIKEN Center for Developmental Biology, Kobe 650-0047,
Invited Paper Self-organization of neural patterns and structures in 3D culture of stem cells Yoshiki Sasai Organogenesis and Neurogenesis Group, RIKEN Center for Developmental Biology, Kobe 650-0047,
Nr2e1 regulates retinal lamination and the development of Müller glia, S-cones, and glycineric amacrine cells during retinogenesis
 Corso-Díaz and Simpson Molecular Brain (2015) 8:37 DOI 10.1186/s13041-015-0126-x RESEARCH Nr2e1 regulates retinal lamination and the development of Müller glia, S-cones, and glycineric amacrine cells during
Corso-Díaz and Simpson Molecular Brain (2015) 8:37 DOI 10.1186/s13041-015-0126-x RESEARCH Nr2e1 regulates retinal lamination and the development of Müller glia, S-cones, and glycineric amacrine cells during
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplementary Data. Generation and Characterization of Mixl1- Inducible Embryonic Stem Cells Under the Control of Oct3/4 Promoter or CAG Promoter
 Supplementary Data Generation and Characterization of Mixl1- Inducible Embryonic Stem Cells Under the Control of Oct3/4 Promoter or CAG Promoter We first introduced an expression unit composed of a tetresponse
Supplementary Data Generation and Characterization of Mixl1- Inducible Embryonic Stem Cells Under the Control of Oct3/4 Promoter or CAG Promoter We first introduced an expression unit composed of a tetresponse
Induced Pluripotent Stem Cell
 Induced Pluripotent Stem Cell When eventually mastered, the processes involved in the obtaining, cultivating and differencing ESC into cells of clinical interest, another practical limitation should be
Induced Pluripotent Stem Cell When eventually mastered, the processes involved in the obtaining, cultivating and differencing ESC into cells of clinical interest, another practical limitation should be
KEY Reproductive cloning Therapeutic cloning
 1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
Peter Dy, Weihuan Wang, Pallavi Bhattaram, Qiuqing Wang, Lai Wang, R. Tracy Ballock, and Véronique Lefebvre
 Developmental Cell, Volume 22 Supplemental Information Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes Peter Dy, Weihuan Wang, Pallavi Bhattaram,
Developmental Cell, Volume 22 Supplemental Information Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes Peter Dy, Weihuan Wang, Pallavi Bhattaram,
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/310/5753/1487/dc1 Supporting Online Material for Stem Cell Self-Renewal Controlled by Chromatin Remodeling Factors Rongwen Xi and Ting Xie* *To whom correspondence should
www.sciencemag.org/cgi/content/full/310/5753/1487/dc1 Supporting Online Material for Stem Cell Self-Renewal Controlled by Chromatin Remodeling Factors Rongwen Xi and Ting Xie* *To whom correspondence should
gacgacgaggagaccaccgctttg aggcacattgaaggtctcaaacatg
 Supplementary information Supplementary table 1: primers for cloning and sequencing cloning for E- Ras ggg aat tcc ctt gag ctg ctg ggg aat ggc ttt gcc ggt cta gag tat aaa gga agc ttt gaa tcc Tpbp Oct3/4
Supplementary information Supplementary table 1: primers for cloning and sequencing cloning for E- Ras ggg aat tcc ctt gag ctg ctg ggg aat ggc ttt gcc ggt cta gag tat aaa gga agc ttt gaa tcc Tpbp Oct3/4
CH 8: Recombinant DNA Technology
 CH 8: Recombinant DNA Technology Biotechnology the use of microorganisms to make practical products Recombinant DNA = DNA from 2 different sources What is Recombinant DNA Technology? modifying genomes
CH 8: Recombinant DNA Technology Biotechnology the use of microorganisms to make practical products Recombinant DNA = DNA from 2 different sources What is Recombinant DNA Technology? modifying genomes
