Chromosome arrangement within a bacterium Aurelio A. Teleman*, Peter L. Graumann*, Daniel Chi-Hong Lin, Alan D. Grossman and Richard Losick*
|
|
|
- Ashlynn Carpenter
- 6 years ago
- Views:
Transcription
1 1102 Research Paper Chromosome arrangement within a bacterium Aurelio A. Teleman*, Peter L. Graumann*, Daniel Chi-Hong Lin, Alan D. Grossman and Richard Losick* Background: The contour length of the circular chromosome of bacteria is greater than a millimeter but must be accommodated within a cell that is only a few micrometers in length. Bacteria do not have nucleosomes and little is known about the arrangement of the chromosome inside a prokaryotic cell. Results: We have investigated the arrangement of chromosomal DNA within the bacterium Bacillus subtilis by using fluorescence microscopy to visualize two sites on the chromosome simultaneously in the same cell. Indirect immunofluorescence with antibodies against the chromosome partition protein Spo0J were used to visualize the replication origin region of the chromosome. Green fluorescent protein fused to the lactose operon repressor LacI was used to decorate tandem copies of the lactose operon operator laco. A cassette of tandem operators was separately inserted into the chromosome near the origin (359 ), near the replication terminus (181 ), or at two points in between (90 and 270 ). The results show that the layout of the chromosome is dynamic but is principally arranged with the origin and terminus maximally apart and the quarter points of the chromosome in between. Addresses: *Department of Molecular and Cellular Biology, The Biological Laboratories, Harvard University, Cambridge, Massachusetts 02138, USA. Department of Biology, Building , Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA. Correspondence: Richard Losick losick@biosun.harvard.edu Received: 22 July 1998 Revised: 24 August 1998 Accepted: 24 August 1998 Published: 21 September , 8: Ltd ISSN Conclusions: The use of cytological methods to visualize two chromosomal sites in the same cell has provided a glimpse of the arrangement of a bacterial chromosome. We conclude that, to a first approximation, the folding of the bacterial chromosome is consistent with, and may preserve, the linear order of genes on the DNA. Background All cells face the challenge of packing their genome into a small space. Genomes are DNA molecules of extraordinary length, often hundreds of times longer than the cells in which they are contained, and must be highly compacted to form the chromosomes of higher cells and the nucleoids of bacteria. At the same time, the genome must remain functional and accessible to the DNA replication, chromosome segregation, and gene transcription machinery of the cell. In eukaryotes, this problem is solved, in part, by wrapping DNA around nucleosomes and then wrapping the nucleosomes into higher-order structures to create fibers of approximately 30 nm in diameter. Despite the high degree of compaction, the nucleosome structure remains dynamic. Chromatin is, for example, subject to reversible covalent and non-covalent modifications that render specific genes accessible to the transcription machinery in response to specific regulatory signals. Bacteria, on the other hand, do not have nucleosomes, and how the circular chromosome of many prokaryotes is folded in the nucleoid is largely unknown [1]. Some compaction is achieved by maintenance of the DNA in a supercoiled state as a result of underwinding [2,3]. Abundant proteins that bind to DNA and cause it to bend are likely to achieve most compaction; these proteins include HU, HNS, FIS and IHF [4 6]. Of special significance is the discovery in bacteria of homologs of a family of eukaryotic structural maintenance of chromosomes proteins (SMCs), which are involved in chromosome folding [7]. Mutants of smc in Bacillus subtilis exhibit decondensed chromosomes and defects in chromosome segregation [8,9]. Mutants of Escherichia coli mukb, which is a functional analog of SMC, exhibit similar defects in chromosome structure and segregation [10]. We are concerned here with the architecture of the chromosome in the bacterial cell. How is the circular genome organized within the nucleoid and what is the location of specific regions on the chromosome within the cell? This problem has been difficult to investigate because of the small size of bacteria (~2 µm in length) and the absence of methods for visualizing specific regions of the chromosome within cells. Recently, however, the application of cytological methods in conjunction with fluorescence microscopy has made it possible to localize specific chromosomal segments within the intact cell. These methods include the use of fluorescence in situ hybridization (FISH) [11,12], the insertion into the chromosome of tandem copies of the lactose operon operator, which can
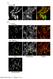
2 Research Paper Arrangement of a bacterial chromosome Teleman et al be visualized by the use of green fluorescent protein (GFP) fused to the lactose operon repressor LacI (GFP LacI) [13 17], and the use of GFP fused to, or antibodies raised against, chromosome partition proteins that bind to the replication origin region of the chromosome in certain bacteria [18 20]. In the case of B. subtilis, the partition protein known as Spo0J binds to a cluster of at least eight sites spread out over a distance of about 0 kb around the origin of replication [21] and is believed to cause the origin region to fold into a higher-order structure [8,21]. The use of these methods has led to the conclusion that during chromosome segregation the replication origin region of the chromosome tends to be located towards the cell pole at the outer edge of the nucleoid and that the terminus tends to be located towards the cell center. In newborn cells with a single origin, the replication origin region is generally near one pole of the cell and the terminus near the other. To investigate the organization of the chromosome within the cell, we visualized two sites simultaneously on the chromosome of B. subtilis. To visualize the replication origin region, we used antibodies against the Spo0J protein [18,19,21,22] and immunofluorescence microscopy. The second site was visualized by insertion into the chromosome of a laco cassette, which was decorated with GFP LacI [15,16]. Because the laco cassette could be inserted at various sites, we reasoned that we would be able to compare simultaneously the position of the replication origin region with that of any other site on the chromosome at which the cassette had been inserted. Results and discussion Co-localization of Spo0J and the replication origin region First, we investigated whether Spo0J would co-localize with a laco cassette that had been inserted into the chromosome (at 359 ) near the origin of replication in a strain (AT63) that had been engineered to produce GFP LacI. A complication was that the fixation procedure employed to detect Spo0J by immunofluorescence lowered the level of fluorescence from GFP LacI (compared with the level in unfixed living cells). Nonetheless, sufficient signal was retained for both Spo0J (red) and GFP LacI (green) to be detected in the same cell. Cells were grown at 25 C in defined minimal medium with glucose as the carbon source. The low temperature increased the intensity of the GFP fluorescence and the relatively slow growth rate (doubling time of ~100 min) reduced the number of initiation events per cell. The field of cells displayed in Figure 1a shows that fluorescence from Spo0J partially or substantially coincided with that from the GFP LacI-decorated laco cassette (the yellow color in the overlay panel indicates coincidence of GFP fluorescence and immunofluorescence). The position of the fluorescent foci relative to the boundaries of the cells can be seen in the Nomarski image, although, because of the lysozyme treatment in the immunofluorescence procedure, the images are less clear than for untreated living cells. Figure 1 Simultaneous localization of Spo0J and a laco cassette that has been inserted at one of four different sites on the chromosome. (a) Cells harboring a laco cassette at 359 (origin; strain AT63). (b) Cells harboring a laco cassette at 90 (strain AT64). (c) Cells harboring a laco cassette at 270 (strain AT61). (d) Cells harboring a laco cassette at 181 (terminus; strain AT62). Each row contains four images of the same field; for (b d), the two rows show two separate sample fields. The first panel in each row is an immunofluorescence image of Spo0J, the second panel is an image of fluorescence from GFP LacI bound to the operator cassette, the third panel is a Nomarski differential interference contrast (DIC) image, and the last panel is a color-coded overlay of the GFP fluorescence (green) and Spo0J immunofluorescence (red). Spo0J immunodetection micrographs were deconvoluted by Exhaustive Photon Reassignment software (Scanalytics) to remove out-of-focus background light. laco position (a) 359º (b) 90º (c) 270º (d) 181º Spo0J GFP Nomarski Overlay
3 1104, Vol 8 No 20 Figure 2 Percentage of Spo0J foci 359º 90º 270º 181º during the cell cycle. Using time-lapse fluorescence microscopy and the laco cassette decorated with GFP LacI, we had previously reported that, after duplication, replication origin regions move towards opposite poles of the cell, often separating abruptly during a brief period of the cell cycle [16]. We found that Spo0J behaves in a similar fashion. We recorded the movement of Spo0J in living cells using a GFP fusion and time-lapse microscopy. Starting with a cell having a single Spo0J focus at the mid-cell position, we observed duplication of the focus and movement of the resulting foci towards opposite poles of the cell (Figure 3). The speed of separation from the time (12 16 min) when two Spo0J foci were first resolvable to the time (60 min) when they stopped moving apart was µm/min. From 11 such time-course experiments, we calculated an average speed of separation of about 0.08 µm/min (s.d. = 0.06 µm/min) and an average distance of separation of 1.1 µm (s.d. = 0.4 µm) when measured between the centers of the two foci. 0 Coincident Adjacent Separate Distribution of distances between Spo0J foci and laco cassette foci. Immunofluorescence microscopy using Spo0J-specific antibodies was used to detect Spo0J localization in strains producing GFP LacI and containing a laco cassette that had been inserted into the chromosome at 359, 90, 270, or 181. All Spo0J foci were divided into three classes: those with a spatially coincident GFP signal (referred to in the figure as Coincident ), those with an overlapping but not quite coincident GFP signal ( Adjacent ), and those that could be resolved and separated from any GFP signal ( Separate ). The total number of Spo0J foci counted for 359, 90, 270 and 181 were 123, 175, 270 and 164, respectively. Tabulating the results for 123 Spo0J foci, we found that 87% of the foci were coincident with a laco cassette. A further 9% overlapped with, but were not substantially superimposable with, the laco cassette (Figure 2); in such cases, the laco cassette seemed to lie adjacent to the Spo0J focus. In the remaining 4% of the cells there was no visible signal from the GFP LacI, and so we could not determine whether Spo0J was closely associated with the laco cassette. In summary, the cytological evidence indicates that, within the resolution limits of light microscopy, Spo0J molecules principally localized at or near the replication origin region of the chromosome, a finding that is fully consistent with the results of previous investigations of the subcellular distribution of Spo0J [18,19,21,22]. Spo0J movement during the cell cycle To investigate further the association of Spo0J with the replication origin, we examined the behavior of Spo0J These measurements underestimate the maximum velocity because a substantial portion of the total separation of foci was often observed to occur over a short period of the cell cycle (although this was not the case in the example of Figure 3). Figure 4 plots the separation of Spo0J foci as a function of time for three time-course experiments. Figure 4a corresponds to the time-course of Figure 3, whereas Figure 4b documents a case in which most separation occurred over a short time. Measuring the rate of movement during the period of most rapid separation of Spo0J foci for 11 time-course experiments, we calculated an average peak velocity of separation of 0.17 µm/min (s.d. = 0.07 µm/min) and a maximal velocity of 0.31 µm/min. In toto, these results are similar to those obtained previously using the laco cassette to monitor movement of the origin region. We conclude that Spo0J co-localizes with the replication origin region of the chromosome and that it remains associated with, and behaves in a manner similar to, the origin region during the entire course of the cell cycle. The velocities that we observe for Spo0J GFP and GFP LacI are approximately an order of magnitude more rapid than those recently reported by Sharpe and Errington [23] for Spo0J GFP. One possible explanation for the discrepancy is that our measurements were collected for cells that were undergoing a duplication of a single origin focus whereas those of Sharpe and Errington [23] were collected from cells undergoing a duplication of two foci. A second explanation could be that our measurements were collected from individual living cells over the course of the cell cycle whereas those of Sharpe and Errington [23] were collected from separate fixed cells of a DNA replication mutant at various times after a temperature shift.
4 Research Paper Arrangement of a bacterial chromosome Teleman et al Figure 3 Figure 4 Time (min) (a) (b) (a) Distance (µm) Distance (µm) Distance (µm) (b) (c) Time (min) Time (min) Time (min) Graphical representation of the time-course of separation of Spo0J GFP foci during the cell cycle. (a) Plot corresponding to the time-course of Figure 3 showing gradual separation of Spo0J GFP foci. (b) Plot showing abrupt separation of Spo0J GFP foci. (c) A second example of more gradual Spo0J GFP separation, as in (a) Time-lapse images of Spo0J GFP movement during the cell cycle. Cells producing a Spo0J GFP fusion (strain RL1746) were grown to mid-exponential phase in defined minimal glucose medium and then placed on a slide coated with minimal medium that had been solidified with 1% agarose. Time-lapse photography was used to follow (a) cell growth and (b) Spo0J GFP movement in a single cell. Pictures of (a) Nomarski DIC and (b) GFP fluorescence were taken at 4 min intervals. Time elapsed from the start of the sequence is indicated on the left. Cell boundaries are indicated for reference by white ticks on the fluorescence image. Although the use of Spo0J GFP and of the laco cassette decorated with GFP LacI yielded similar results, some differences were noted between the two procedures. For example, the percentage of cells with a single fluorescent focus from the origin region was higher with the laco cassette method (29%) than with Spo0J GFP (10%; A.A.T., Undergraduate Honors Thesis, Harvard University, 1998). Conversely, the percentage of cells with two replication origin foci was lower with the laco cassette method (67%) than with Spo0J GFP (77%). We suspect that insertion of the laco cassette in the origin region and its decoration with GFP LacI causes a slight perturbation of the normal processes of chromosome replication and segregation. If, as postulated previously, Spo0J causes the origin region of the chromosome to fold up into a higherorder structure [8,21], then perhaps the insertion of tandem copies of laco decorated with GFP LacI interferes with the proper assembly of this structure. In addition, the presence of the laco cassette bound by GFP LacI might also delay separation of newly replicated origin regions. Finally, we note that Spo0J foci visualized either by indirect immunofluorescence (Figure 1) or by use of a GFP fusion (data not shown) appeared to be somewhat larger than the foci from laco cassettes decorated with GFP LacI. Conceivably, the larger size of Spo0J foci reflects the fact that the partition protein binds to multiple sites dispersed over a wide region around the replication origin [21].
5 1106, Vol 8 No 20 Visualizing the origin and the terminus regions of the chromosome in the same cell Knowing that Spo0J could be reliably used to visualize the replication origin region of the chromosome, we visualized the replication origin and terminus regions of the chromosome simultaneously in the same cell. For this purpose we used antibodies against Spo0J to visualize the origin region and GFP LacI to visualize the terminus region in a strain (AT62) in which a cassette of tandem laco repeats had been inserted into the chromosome at 181. Examples of cells that had been fluorescently labeled in this way at the origin and the terminus regions are shown in Figure 1d. In confirmation and extension of previous results with cells that were singly labeled at the origin or the terminus [15,16], the images show that Spo0J foci were generally localized near the cell poles and that fluorescence from GFP LacI was principally visible near the mid-cell position. Most often, only a single focus of GFP LacI was present, which presumably represented either one terminus region which had not yet replicated or two newly replicated terminus regions which had not separated sufficiently to be resolved. Sometimes, however, two distinct GFP LacI foci could be seen near the midcell, indicating that the terminus region had replicated and the two sister chromosomes had moved apart sufficiently to be resolved by light microscopy. These results are entirely consistent with those of Niki and Hiraga [12], who recently used FISH to visualize the origin and terminus regions of the E. coli chromosome. Tabulating the separation of origin and terminus regions for 164 images (Figure 2), we found that in most cases (68%) Spo0J foci were clearly resolved and separated from the GFP LacI foci. In a minority (25%) of the images the terminus signal partially overlapped with, but was not coincident with, the origin signal; that is, the Spo0J and GFP LacI foci seemed to be adjacent. Finally, in a small number of cases (7%) the Spo0J focus was coincident with the GFP LacI signal. Thus, in sharp contrast to the distribution pattern observed with cells harboring the operator cassette in the origin region, these results indicate that the origin and terminus regions generally occupy distinct regions in the cell. Resolving the replication origin region from 90 and from 270 on the chromosome Knowing that the replication origin and the terminus are generally well separated from each other in the cell, we investigated the disposition of two sites lying at intermediate positions on the chromosome. For these experiments we created GFP LacI-producing strains harboring the laco cassette at positions corresponding to 90 (strain AT64) and 270 (strain AT61) and used Spo0J as a marker for the origin region of the chromosome. We observed three principal patterns of localization (Figure 2). In the first pattern, representing the predominant class (60% and 52%, respectively, for the 90 and 270 cassettes; Figure 2), GFP LacI foci were seen at positions immediately adjacent to the Spo0J foci. Examples of this pattern are shown in Figure 1b,c. In the second pattern (representing 29% and 28%, respectively, for the 90 and 270 cassettes; Figure 2), Spo0J foci were clearly separated from GFP LacI foci. Often, in these cases, the Spo0J foci were located near the cell poles and one or two GFP LacI dots were observed at or near the mid-cell position. In the third pattern (representing 11% and 20%, respectively, for the 90 and 270 cassettes), the GFP LacI foci were coincident with the Spo0J foci. We conclude that the 90 and 270 sites on the chromosome are generally situated at distinct positions in the cell from the replication origin region. Also, because GFP LacI foci at the 90 and 270 loci were predominantly located adjacent to Spo0J foci whereas GFP LacI foci at the terminus region were predominantly separated from Spo0J foci, we infer that 90 and 270 in the chromosome are generally located at intermediate positions in the cell between the origin and terminus regions (Figure 5). Another way to analyze the images is to count the number of GFP LacI dots in cells exhibiting two polar Spo0J foci. When the laco cassette is located near the terminus, cells are frequently observed that have two Spo0J foci near opposite poles and only one GFP LacI focus. As discussed above, this pattern corresponded to cells in which the terminus region of the chromosome had not yet undergone replication or cells in which the chromosome had been completely duplicated but the termini had not separated sufficiently to be resolved by microscopy. Tabulating the results for cells with bipolar foci of Spo0J, we found that 77% of cells harboring a laco cassette at 181 had one GFP LacI dot, whereas only 28% and 20% of cells harboring a laco cassette at 90 and 270, respectively, had this localization pattern. Most cells (that is, the Figure 5 0º 90º 270º 1º A model of chromosome arrangement in the cell. The cartoon shows the nucleoid as a dense core with loops extruding towards the cell membrane. In general, the DNA is locally compacted (that is, it doesn t string back and forth between the poles of the cell), and the chromosome is usually arranged such that origin regions are towards the cell poles, termini are towards the mid-cell position and the 90 and 270 regions are in between.
6 Research Paper Arrangement of a bacterial chromosome Teleman et al remaining 72% and %, respectively) containing bipolar Spo0J foci and harboring a laco cassette at either 90 or 270 had two GFP LacI dots. Our interpretation of these results is that the 90 and 270 regions of the chromosome separate earlier than does the terminus region, which indicates that segregation propagates from the origin through the 90 and 270 regions towards the terminus. Time-lapse microscopy of strains in which the laco cassette was integrated into the 181 region of the chromosome showed dynamics of separation similar to the origin regions, though not as fast and as far apart [16]. In this study, the 90 and 270 regions were found to separate in a rapid fashion similar to the origin and terminus regions of the chromosome (data not shown), which suggests that the force that separates the B. subtilis chromosomes is applied not only at the origin but also, at least, at the most distant and the intermediate regions. The arrangement of the chromosome is not fixed Our findings are consistent with the view that the chromosome is often arranged in the nucleoid with the origin at one end, the terminus at the other end, and 90 and 270 sites in between (Figure 5). It is important to emphasize, however, that this is a preferred arrangement, and that the chromosome can, and frequently does, exhibit other patterns. For example, when the laco cassette was near the terminus, which is usually near the mid-cell position, 12% (n = 315) of the cells exhibited a localization pattern in which the GFP LacI signal was closer to the cell pole than was the Spo0J focus. Similarly, when the laco cassette was at 90 or 270, fluorescent dots from GFP LacI were found at a more polar position than Spo0J in 23% and 21% of the cases respectively (n = 92 and n = 147 respectively). These observations reinforce the findings from time-lapse studies indicating that the chromosome is dynamic and undergoes substantial changes in location and organization during the course of the cell cycle. Conclusions We have used fluorescence microscopy to visualize two sites on the chromosome simultaneously in the same cell. We used indirect immunofluorescence with antibodies against the partition protein Spo0J to visualize the replication origin region of the chromosome and GFP LacI to visualize tandem copies of laco that had been separately inserted into the chromosome at four quarter points (359, 90, 181 and 270 ). We draw two principal sets of conclusions, one concerning the location of Spo0J on the chromosome and the other concerning the overall architecture of the chromosome. First, in confirmation and extension of previous work [18,19,21,22], we conclude that Spo0J binds near the origin of DNA replication and that it remains associated with the origin region throughout the cell cycle. This conclusion is based on the high proportion of Spo0J foci that were closely associated with foci from a laco cassette that had been inserted at the origin and on the similarity in the behavior of Spo0J (as visualized by use of a GFP fusion) in time-lapse images of living cells to that previously observed for laco. We also note that Spo0J foci (as visualized either by indirect immunofluorescence or by use of a fusion to GFP) were somewhat larger than laco foci. This observation is consistent with, and could be a reflection of, the fact that Spo0J binds to multiple sites spread out over a large distance around the origin of DNA replication [21]. With regard to the architecture of the chromosome, we conclude that the arrangement of the chromosome is dynamic and that individual sites can be found at a variety of locations in the cell. Nonetheless, in the main, the origin and the terminus regions of the chromosome tend to be maximally apart whereas the 90 and 270 positions on the chromosome tend to exhibit an intermediate level of separation from the origin (Figure 5). Thus, the use of cytological methods to visualize two chromosomal regions simultaneously has given us a glimpse of the arrangement of the chromosome in a bacterial cell. To a first approximation, the folding of the chromosome is consistent with, and may preserve, the linear order of genes on the DNA. Materials and methods Strain constructions RL1746, which corresponds to DCL233 [18], produces Spo0J GFP and contains a spo0j null mutation. The Spo0J GFP fusion protein is functional and the strain is phenotypically Spo0J + [18]. An integration plasmid (pat19) for directing the synthesis of GFP LacI under the control of the promoter for the veg gene in the chromosome was described previously [16]. In the present work, a derivative of pat19 was created by site-directed mutagenesis using the primers ATO19 (5 -CCAACACTTGTCACTACTCTCACTTATGGTGTTCAA- TGC-3 ) and ATO20 (5 -GCATTGAACACCATAAGTGAGAGTAGT- GACAAGTGTTGG-3 ). This created pat27, which contained a Phe to Leu change at codon 64 of gfp laci in addition to the Ser to Thr change already present at codon 65. The resulting F64L, S65T double-mutant GFP was several-fold brighter in our experiments than the S65T singlemutant protein. The pat27 plasmid was introduced into the chromosome of B. subtilis strain PY79 by transformation followed by selection for resistance to erythromycin (Erm R ; 5 µg/ml) to yield strain AT60. Four plasmids were created to insert a cassette of laco tandem repeats at either 359, 90, 181 or 270 on the B. subtilis chromosome. Each plasmid was derived from pat12 [15], which contains a chloramphenicol-resistance gene and 256 copies of laco, by insertion of a copy of a segment of DNA from each of the four chromosomal positions. Plasmids pat14 and pat15 contain sequences from the 181 (cged) and 359 (spo0j) regions of the chromosome, respectively, as described previously [15]. pat26 (90 ) was created by insertion of a 0.7 kb segment of DNA from yheh between the AatII and BamHI sites of pat12. The 0.7 kb DNA fragment was amplified by PCR from the chromosome of strain PY79 using primers ATO17 (5 - TCTAGACGTCAAGGCGAAACCGTAG-3 ) and ATO18 (5 -TCTAG- GATCCTGGGTTTTTGAGCGT-3 ) and was then digested with AatII and BamHI. Similarly, pat25 (270 ) was created by introducing into the HindIII site of pat12 a 1 kb DNA from cots that was amplified from
7 1108, Vol 8 No 20 chromosomal DNA by PCR using as primers ATO13 (5 -TCTAG- GATCCATCGACCATGTGGCG-3 ) and ATO14 (5 -TCTAGGATC- CGTCAATTCGCCTCCC-3 ) and then treated with HindIII. To generate strains harboring laco cassettes at 270, 90, 181 and 359, plasmids pat25, pat26, pat14 or pat15 were used to transform wild-type PY79 followed by selection for chloramphenicol resistance (5 µg/ml) to create AT52, AT53, AT54 and AT55. These were then transformed with chromosomal DNA from AT60, selecting for Erm R, to generate strains AT61, AT64, AT62 and AT63, respectively, which contain a laco cassette and produce GFP LacI. Finally, to increase the number of lac operators in these strains, AT61, AT62, AT63 and AT64 were grown at 30 C on Luria Bertani (LB) plates containing an elevated concentration of chloramphenicol (25 µg/ml), a procedure that selected for tandem duplications of the integrated laco-cassette-containing plasmids. Growth conditions and fluorescence microscopy To obtain reasonably bright GFP LacI signals, strains were grown in the following manner before fixation for immunofluorescence microscopy. Cell cultures (2 ml) were prepared by inoculating L broth with single colonies from each strain and allowed to grow for 6 h at 37 C. This culture was then diluted at various concentrations into defined minimal glucose medium [24] and allowed to grow overnight (~12 hr) at 25 C. During growth, fluorescence from GFP LacI was dim. As cells reached stationary phase, the GFP LacI signal became brighter, probably as a result of more and more GFP molecules reaching maturation, which is a slow process. Later, as the cells became older, the intensity of GFP LacI signal decreased. The culture with the brightest signal was diluted 1:10 into minimal medium (2 ml) and allowed to grow at 25 C for 110 min. Time-lapse photomicroscopy showed that a subpopulation of cells began to grow immediately after dilution and that approximately half of the cells were growing by 75 min after dilution [16]. The GFP LacI signal become less bright 110 min after dilution, perhaps due to the dilution of mature GFP LacI among progeny cells. Cells were fixed for immunofluorescence using methanol (MeOH) as modified from a previously described procedure [25]. This was found to preserve GFP fluorescence better than fixation with glutaraldehyde and paraformaldehyde [15]. Culture (1 ml) growing in minimal medium was collected by centrifugation and resuspended in µl of its own medium. This volume of cells was then injected into 1 ml 99% MeOH previously equilibrated at 20 C, gently rocked back and forth, and then rapidly placed back at 20 C for at least 10 min. These cells were then placed on a poly-lysine coated slide and allowed to dry fully (~5 min) before lysis and incubation with antibodies. Cells were blocked using 2% BSA/PBS overnight at 4 C. Polyclonal rabbit anti-spo0j antibodies [18] were used at a 1:16,000 dilution for 1 h at room temperature. Antirabbit Cy3 secondary antibodies (Molecular Probes) were used at a 1:300 dilution and also incubated for 1 h at room temperature. Nomarski and GFP images were acquired and processed as previously described [16]. Spo0J images were deconvoluted to remove out-offocus light as follows. A piezoelectric device was installed on the objective lens of the microscope to move the plane of focus by 0.1 µm increments in the direction perpendicular to the microscope stage. An electric shutter was also installed to electrically control emission of the excitation light from the ultraviolet bulb of the fluorescence microscope. Image acquisition, shutter control and voltage on the piezoelectric device were all controlled by Metamorph image acquisition software. A stack of images was acquired for each field of interest with a vertical spacing of 0.1 µm. The stack was then deconvoluted by the Exhaustive Photon Reassignment software package (Scanalytics) to a convergence of with a smoothing factor of 10. Time-lapse images of Spo0J GFP movement Time-lapse images of Spo0J GFP movement were obtained using strain RL1746 as described previously [16], except that the images were collected during the mid-exponential phase of cell growth. Acknowledgements P.L.G. is a postdoctoral fellow of the Deutsche Forschungsgemeinschaft and D.C-H.L. was supported in part by a predoctoral training grant from the NSF. We are grateful to C. Webb for guidance in fluorescence microscopy techniques. This work was supported by NIH grants GM18568 and GM41934 to R.L. and A.D.G., respectively. References 1. Trun NJ, Marko JF: Architecture of a bacterial chromosome. ASM News 1998, 64: Pettijohn DE, Pfenninger O: Supercoils in prokaryotic DNA restrained in vivo. Proc Natl Acad Sci USA 19, 77: Sinden RR, Pettijohn DE: Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci USA 1981, 78: Nash HA. The HU and IHF proteins: accessory factors for complex protein DNA assemblies. In Regulation of Gene Expression in Escherichia coli. Edited by Lin ECC, Lynch AS. Austin, Texas: R.G. Landes Company; 1996: Norris V, Turnock G, Sigee D: The Escherichia coli enzoskeleton. Mol Microbiol 1996, 19: Köhler P, Marahiel MA: Association of the histone-like protein HBsu with the nucleoid of Bacillus subtilis. J Bacteriol 1997, 179: Koshland D, Strunnikov A: Mitotic chromosome condensation. Annu Rev Cell Dev Biol 1996, 12: Britton RA, Lin DC-H, Grossman AD: Characterization of a procaryotic SMC protein involved in chromosome partitioning. Genes Dev 1998, 12: Moriya S, Tsujikawa E, Hassan AKM, Asai K, Kodama T, Osagawara N: A Bacillus subtilis gene encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol Microbiol 1998, 29: Niki H, Imamura R, Kitaoka M, Yamanaka K, Ogura T, Hiraga S: E. coli MukB protein involved in chromosome partition forms a homodimer with a rod-and-hinge structure having DNA binding and ATP/GTP binding activities. EMBO J 1992, 11: Niki H, Hiraga S: Subcellular distribution of actively partitioning F plasmid during the cell division cycle in E. coli. Cell 1997, 90: Niki H, Hiraga S: Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev 1998, 12: Straight AF, Belmont AS, Robinett CC, Murray AW: GFP tagging of budding yeast chromosomes reveals that protein protein interactions can mediate sister chromatid cohesion. Curr Biol 1996, 6: Robinett CC, Straight AF, Li G, Willhelm C, Sudlow G, Murray AW, Belmont AS: In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J Cell Biol 1996, 135: Webb CD, Teleman A, Gordon S, Straight A, Belmont A, Lin DC-H, Grossman AD, Wright A, Losick R: Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cels of B. subtilis. Cell 1997, 88: Webb CD, Graumann PL, Kahana J, Teleman AA, Silver P, Losick R: Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol Microbiol 1998, 28: Gordon S, Sitnikov D, Webb CD, Teleman A, Losick R, Murray AW, Wright A: Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell 1997, 90: Lin DC-H, Levin PA, Grossman AD: Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc Natl Acad Sci USA 1997, 94: Glaser P, Sharpe ME, Raether B, Perego M, Ohlsen K, Errington J: Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev 1997, 11: Mohl DA, Gober JW: Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell 1997, 88: Lin DC-H, Grossman AD: Identification and characterization of a bacterial chromosome partitioning site. Cell 1998, 92:
8 Research Paper Arrangement of a bacterial chromosome Teleman et al Lewis PJ, Errington J: Direct evidence for active segregation of oric regions of the Bacillus subtilis chromosome and co-localization with the Spo0J partitioning protein. Mol Microbiol 1997, 25: Sharpe ME, Errington J: A fixed distance for separation of newly duplicated copies of oric in Bacillus subtilis: implications for coordination of chromosome segregation and cell division. Mol Microbiol 1998, 28: Jaacks KJ, Healy J, Losick R, Grossman AD: Identification and characterization of genes controlled by the sporulation regulatory gene spo0h in Bacillus subtilis. J Bacteriol 1989, 171: Hiraga S, Ichinose C, Niki H, Yamazoe M: Cell cycle-dependent duplication and bidirectional migration of SeqA-associated DNA protein complexes in E. coli. Mol Cell 1998, 1: Because operates a Continuous Publication System for Research Papers, this paper has been published on the internet before being printed. The paper can be accessed from for further information, see the explanation on the contents page.
PETER L. GRAUMANN* Department of Molecular and Cellular Biology, The Biological Laboratories, Harvard University, Cambridge, Massachusetts 02138
 JOURNAL OF BACTERIOLOGY, Nov. 2000, p. 6463 6471 Vol. 182, No. 22 0021-9193/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Bacillus subtilis SMC Is Required for Proper
JOURNAL OF BACTERIOLOGY, Nov. 2000, p. 6463 6471 Vol. 182, No. 22 0021-9193/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Bacillus subtilis SMC Is Required for Proper
The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 10661 10666, September 1999 Biochemistry The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation RASMUS B. JENSEN AND
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 10661 10666, September 1999 Biochemistry The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation RASMUS B. JENSEN AND
Materials and methods. by University of Washington Yeast Resource Center) from several promoters, including
 Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
Lac Operon contains three structural genes and is controlled by the lac repressor: (1) LacY protein transports lactose into the cell.
 Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
-7 ::(teto) ::(teto) 120. GFP membranes merge. overexposed. Supplemental Figure 1 (Marquis et al.)
 P spoiie -gfp -7 ::(teto) 120-91 ::(teto) 120 GFP membranes merge overexposed Supplemental Figure 1 (Marquis et al.) Figure S1 TetR-GFP is stripped off the teto array in sporulating cells that contain
P spoiie -gfp -7 ::(teto) 120-91 ::(teto) 120 GFP membranes merge overexposed Supplemental Figure 1 (Marquis et al.) Figure S1 TetR-GFP is stripped off the teto array in sporulating cells that contain
Biology 201 (Genetics) Exam #3 120 points 20 November Read the question carefully before answering. Think before you write.
 Name KEY Section Biology 201 (Genetics) Exam #3 120 points 20 November 2006 Read the question carefully before answering. Think before you write. You will have up to 50 minutes to take this exam. After
Name KEY Section Biology 201 (Genetics) Exam #3 120 points 20 November 2006 Read the question carefully before answering. Think before you write. You will have up to 50 minutes to take this exam. After
Molecular Cell Biology - Problem Drill 06: Genes and Chromosomes
 Molecular Cell Biology - Problem Drill 06: Genes and Chromosomes Question No. 1 of 10 1. Which of the following statements about genes is correct? Question #1 (A) Genes carry the information for protein
Molecular Cell Biology - Problem Drill 06: Genes and Chromosomes Question No. 1 of 10 1. Which of the following statements about genes is correct? Question #1 (A) Genes carry the information for protein
DNA AND CHROMOSOMES. Genetica per Scienze Naturali a.a prof S. Presciuttini
 DNA AND CHROMOSOMES This document is licensed under the Attribution-NonCommercial-ShareAlike 2.5 Italy license, available at http://creativecommons.org/licenses/by-nc-sa/2.5/it/ 1. The Building Blocks
DNA AND CHROMOSOMES This document is licensed under the Attribution-NonCommercial-ShareAlike 2.5 Italy license, available at http://creativecommons.org/licenses/by-nc-sa/2.5/it/ 1. The Building Blocks
Enzyme that uses RNA as a template to synthesize a complementary DNA
 Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
A) The constituent monomer of DNA and RNA. C) The basic structural unit of chromatin with "bead-on-a-string" morphology
 MATCHING. Choose the item in column 2 that best matches each item in column 1. Please select the best match for each term. 1) Centromere 2) Nucleoid 3) Nucleotide 4) Chromosome 5) Nucleosome A) The constituent
MATCHING. Choose the item in column 2 that best matches each item in column 1. Please select the best match for each term. 1) Centromere 2) Nucleoid 3) Nucleotide 4) Chromosome 5) Nucleosome A) The constituent
Bi 1x Spring 2016: LacI Titration
 Bi 1x Spring 2016: LacI Titration 1 Overview In this experiment, you will measure the effect of various mutated LacI repressor ribosome binding sites in an E. coli cell by measuring the expression of a
Bi 1x Spring 2016: LacI Titration 1 Overview In this experiment, you will measure the effect of various mutated LacI repressor ribosome binding sites in an E. coli cell by measuring the expression of a
Name_BS50 Exam 3 Key (Fall 2005) Page 2 of 5
 Name_BS50 Exam 3 Key (Fall 2005) Page 2 of 5 Question 1. (14 points) Several Hfr strains derived from the same F + strain were crossed separately to an F - strain, giving the results indicated in the table
Name_BS50 Exam 3 Key (Fall 2005) Page 2 of 5 Question 1. (14 points) Several Hfr strains derived from the same F + strain were crossed separately to an F - strain, giving the results indicated in the table
DNA: the thread of life
 DNA: the thread of life Lectured by Chompunuch Virunanon This presentation Partial Fulfillment of the Requirements for the 2303107 General Biology teaching, Department of Biology Chulalongkorn University
DNA: the thread of life Lectured by Chompunuch Virunanon This presentation Partial Fulfillment of the Requirements for the 2303107 General Biology teaching, Department of Biology Chulalongkorn University
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Supporting information
 Supporting information Construction of strains and plasmids To create ptc67, a PCR product obtained with primers cc2570-162f (gcatgggcaagcttgaggacggcgtcatgt) and cc2570+512f (gaggccgtggtaccatagaggcgggcg),
Supporting information Construction of strains and plasmids To create ptc67, a PCR product obtained with primers cc2570-162f (gcatgggcaagcttgaggacggcgtcatgt) and cc2570+512f (gaggccgtggtaccatagaggcgggcg),
Department of Molecular Biology, Princeton University, Washington Rd, Princeton, NJ 08544
 JB Accepts, published online ahead of print on 21 November 2008 J. Bacteriol. doi:10.1128/jb.00862-08 Copyright 2008, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
JB Accepts, published online ahead of print on 21 November 2008 J. Bacteriol. doi:10.1128/jb.00862-08 Copyright 2008, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Genes - DNA - Chromosome. Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology
 Genes - DNA - Chromosome Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology DNA Cellular DNA contains genes and intragenic regions both of which may
Genes - DNA - Chromosome Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology DNA Cellular DNA contains genes and intragenic regions both of which may
Tala Saleh. Tamer Barakat ... Anas Abu. Humaidan
 7 Tala Saleh Tamer Barakat... Anas Abu. Humaidan Some Information in this lecture may not be mentioned by the Dr. as thoroughly as this sheet. But they cannot be overlooked for a better understanding,
7 Tala Saleh Tamer Barakat... Anas Abu. Humaidan Some Information in this lecture may not be mentioned by the Dr. as thoroughly as this sheet. But they cannot be overlooked for a better understanding,
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene. Andrew ElBardissi, The Pennsylvania State University
 Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Module Code: BIO00007C
 Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Genetics Time Allowed: 1 Hour 30 Minutes Marking Scheme: Total marks available for
Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Genetics Time Allowed: 1 Hour 30 Minutes Marking Scheme: Total marks available for
CHAPTER 9 DNA Technologies
 CHAPTER 9 DNA Technologies Recombinant DNA Artificially created DNA that combines sequences that do not occur together in the nature Basis of much of the modern molecular biology Molecular cloning of genes
CHAPTER 9 DNA Technologies Recombinant DNA Artificially created DNA that combines sequences that do not occur together in the nature Basis of much of the modern molecular biology Molecular cloning of genes
Mcbio 316: Exam 3. (10) 2. Compare and contrast operon vs gene fusions.
 Mcbio 316: Exam 3 Name (15) 1. Transposons provide useful tools for genetic analysis. List 5 different uses of transposon insertions. ANSWER: Many answers are possible, however, if multiple items on the
Mcbio 316: Exam 3 Name (15) 1. Transposons provide useful tools for genetic analysis. List 5 different uses of transposon insertions. ANSWER: Many answers are possible, however, if multiple items on the
Gene expression. What is gene expression?
 Gene expression What is gene expression? Methods for measuring a single gene. Northern Blots Reporter genes Quantitative RT-PCR Operons, regulons, and stimulons. DNA microarrays. Expression profiling Identifying
Gene expression What is gene expression? Methods for measuring a single gene. Northern Blots Reporter genes Quantitative RT-PCR Operons, regulons, and stimulons. DNA microarrays. Expression profiling Identifying
Design. Construction. Characterization
 Design Construction Characterization DNA mrna (messenger) A C C transcription translation C A C protein His A T G C T A C G Plasmids replicon copy number incompatibility selection marker origin of replication
Design Construction Characterization DNA mrna (messenger) A C C transcription translation C A C protein His A T G C T A C G Plasmids replicon copy number incompatibility selection marker origin of replication
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Genetics Time Allowed: 1 hour and 30 minutes Marking Scheme: Total marks available
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Genetics Time Allowed: 1 hour and 30 minutes Marking Scheme: Total marks available
2/6/18. file:///users/jlc/ Downloads/ DNAi_replicatio n_vo2-lg.mov. 3x10 13 cells (Bianconi et al. Ann Hum Biol, 2013) DNA Replication in vivo
 file:///users/jlc/ Downloads/ DNAi_replicatio n_vo2-lg.mov 3x10 13 cells (Bianconi et al. Ann Hum Biol, 2013) 10 12 replicating cells (Cairns: Genetics (2006)174,1069). Lose about 1/3 of these per day
file:///users/jlc/ Downloads/ DNAi_replicatio n_vo2-lg.mov 3x10 13 cells (Bianconi et al. Ann Hum Biol, 2013) 10 12 replicating cells (Cairns: Genetics (2006)174,1069). Lose about 1/3 of these per day
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr.
 MIT Department of Biology 7.01: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel iv) Would Xba I be useful for cloning? Why or why not?
MIT Department of Biology 7.01: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel iv) Would Xba I be useful for cloning? Why or why not?
Chromosome segregation is fundamental to the transmission
 Does RNA polymerase help drive chromosome segregation in bacteria? Jonathan Dworkin and Richard Losick* Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138 Contributed
Does RNA polymerase help drive chromosome segregation in bacteria? Jonathan Dworkin and Richard Losick* Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138 Contributed
3.1 The Role of the Enzymatic Activity of Sir2 for Efficient. The Sir proteins are recruited to DNA by site-specific DNA-binding proteins and
 35 3 RESULTS 3.1 The Role of the Enzymatic Activity of Sir2 for Efficient Association of the SIR Complex with DNA The Sir proteins are recruited to DNA by site-specific DNA-binding proteins and subsequently
35 3 RESULTS 3.1 The Role of the Enzymatic Activity of Sir2 for Efficient Association of the SIR Complex with DNA The Sir proteins are recruited to DNA by site-specific DNA-binding proteins and subsequently
Molecular Genetics Techniques. BIT 220 Chapter 20
 Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Chromosomes. M.Sc. Biotechnology. Hawler Medical University, Iraq
 Chromosomes Bashdar Mahmud Hussen M.Sc. Biotechnology Hawler Medical University, Iraq bashdar@res.hmu.edu.iq bmhscience@yahoo.com History of Chromosome Karl Nagali (1842) E. Russow (1872) first description
Chromosomes Bashdar Mahmud Hussen M.Sc. Biotechnology Hawler Medical University, Iraq bashdar@res.hmu.edu.iq bmhscience@yahoo.com History of Chromosome Karl Nagali (1842) E. Russow (1872) first description
Chapter 20 Recombinant DNA Technology. Copyright 2009 Pearson Education, Inc.
 Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Chapter 20 Recombinant DNA Technology Copyright 2009 Pearson Education, Inc. 20.1 Recombinant DNA Technology Began with Two Key Tools: Restriction Enzymes and DNA Cloning Vectors Recombinant DNA refers
Chapter 10: Classification of Microorganisms
 Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
Selected Techniques Part I
 1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
AP Biology Gene Expression/Biotechnology REVIEW
 AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
Delve AP Biology Lecture 7: 10/30/11 Melissa Ko and Anne Huang
 Today s Agenda: I. DNA Structure II. DNA Replication III. DNA Proofreading and Repair IV. The Central Dogma V. Transcription VI. Post-transcriptional Modifications Delve AP Biology Lecture 7: 10/30/11
Today s Agenda: I. DNA Structure II. DNA Replication III. DNA Proofreading and Repair IV. The Central Dogma V. Transcription VI. Post-transcriptional Modifications Delve AP Biology Lecture 7: 10/30/11
ONTARIO SCIENCE CENTRE. Teacher Guide. Way to Glow Program
 ONTARIO SCIENCE CENTRE Teacher Guide Way to Glow Program Table of Contents Bacterial transformation background information 3 Experimental procedure 5 Expected results 7 Post-program activity sheet 8 Post-program
ONTARIO SCIENCE CENTRE Teacher Guide Way to Glow Program Table of Contents Bacterial transformation background information 3 Experimental procedure 5 Expected results 7 Post-program activity sheet 8 Post-program
Biological consequences of site specific recombination: integration, excision, deletion
 Biological consequences of site specific recombination: integration, excision, deletion The types of DNA rearrangements promoted by a large number of site specific recombination systems and their physiological
Biological consequences of site specific recombination: integration, excision, deletion The types of DNA rearrangements promoted by a large number of site specific recombination systems and their physiological
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
SCREENING AND PRESERVATION OF DNA LIBRARIES
 MODULE 4 LECTURE 5 SCREENING AND PRESERVATION OF DNA LIBRARIES 4-5.1. Introduction Library screening is the process of identification of the clones carrying the gene of interest. Screening relies on a
MODULE 4 LECTURE 5 SCREENING AND PRESERVATION OF DNA LIBRARIES 4-5.1. Introduction Library screening is the process of identification of the clones carrying the gene of interest. Screening relies on a
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM Part I: Definitions. Homology: Reverse transcriptase. Allostery: cdna library
 Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Reverse transcriptase Allostery: cdna library Transformation Part II Short Answer 1. Describe the reasons for
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Reverse transcriptase Allostery: cdna library Transformation Part II Short Answer 1. Describe the reasons for
DNA replication: Enzymes link the aligned nucleotides by phosphodiester bonds to form a continuous strand.
 DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
Student Manual. pglo Transformation
 Student Manual pglo Transformation Lesson 1 Introduction to Transformation In this lab you will perform a procedure known as genetic transformation. Remember that a gene is a piece of DNA which provides
Student Manual pglo Transformation Lesson 1 Introduction to Transformation In this lab you will perform a procedure known as genetic transformation. Remember that a gene is a piece of DNA which provides
GENE REGULATION slide shows by Kim Foglia modified Slides with blue edges are Kim s
 GENE REGULATION slide shows by Kim Foglia modified Slides with blue edges are Kim s 2007-2008 Bacterial metabolism Bacteria need to respond quickly to changes in their environment STOP GO if they have
GENE REGULATION slide shows by Kim Foglia modified Slides with blue edges are Kim s 2007-2008 Bacterial metabolism Bacteria need to respond quickly to changes in their environment STOP GO if they have
The fusion used in most of these studies (MinC4-GFP) contains GFP and
 Gregory, Becker and Pogliano, Supplemental Materials page 1 Supplemental Methods Description of the MinC and MinD GFP insertions The fusion used in most of these studies (MinC4-GFP) contains GFP and flanking
Gregory, Becker and Pogliano, Supplemental Materials page 1 Supplemental Methods Description of the MinC and MinD GFP insertions The fusion used in most of these studies (MinC4-GFP) contains GFP and flanking
Problem Set 8. Answer Key
 MCB 102 University of California, Berkeley August 11, 2009 Isabelle Philipp Online Document Problem Set 8 Answer Key 1. The Genetic Code (a) Are all amino acids encoded by the same number of codons? no
MCB 102 University of California, Berkeley August 11, 2009 Isabelle Philipp Online Document Problem Set 8 Answer Key 1. The Genetic Code (a) Are all amino acids encoded by the same number of codons? no
However, only a fraction of these genes are transcribed in an individual cell at any given time.
 All cells in an organism contain the same set of genes. However, only a fraction of these genes are transcribed in an individual cell at any given time. It is the pattern of gene expression that determines
All cells in an organism contain the same set of genes. However, only a fraction of these genes are transcribed in an individual cell at any given time. It is the pattern of gene expression that determines
7 Gene Isolation and Analysis of Multiple
 Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 7 Gene Isolation and Analysis of Multiple
Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 7 Gene Isolation and Analysis of Multiple
Supplementary Information
 Supplementary Information Supplementary Figures Figure S1. Study of mgtl translation in vitro. (A) Detection of 5 LR RNA using wild-type and anti-sd (91-95) substituted templates in a transcription-translation
Supplementary Information Supplementary Figures Figure S1. Study of mgtl translation in vitro. (A) Detection of 5 LR RNA using wild-type and anti-sd (91-95) substituted templates in a transcription-translation
Fig. S1. CrgA intracellular levels in M. tuberculosis. Ten and twenty micrograms of
 Supplementary data Fig. S1. CrgA intracellular levels in M. tuberculosis. Ten and twenty micrograms of cell free protein lysates from WT M. tuberculosis (Rv) together with various known concentrations
Supplementary data Fig. S1. CrgA intracellular levels in M. tuberculosis. Ten and twenty micrograms of cell free protein lysates from WT M. tuberculosis (Rv) together with various known concentrations
Chapter Twelve: DNA Replication and Recombination
 This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
(i) A trp1 mutant cell took up a plasmid containing the wild type TRP1 gene, which allowed that cell to multiply and form a colony
 1. S. pombe is a distant relative of baker s yeast (which you used in quiz section). Wild type S. pombe can grow on plates lacking tryptophan (-trp plates). A mutant has been isolated that cannot grow
1. S. pombe is a distant relative of baker s yeast (which you used in quiz section). Wild type S. pombe can grow on plates lacking tryptophan (-trp plates). A mutant has been isolated that cannot grow
Molecular Biology: Gene cloning
 Molecular Biology: Gene cloning Author: Prof Marinda Oosthuizen Licensed under a Creative Commons Attribution license. CLONING VECTORS The central component of a gene cloning experiment is the vector or
Molecular Biology: Gene cloning Author: Prof Marinda Oosthuizen Licensed under a Creative Commons Attribution license. CLONING VECTORS The central component of a gene cloning experiment is the vector or
Supplemental Data. Farmer et al. (2010) Plant Cell /tpc
 Supplemental Figure 1. Amino acid sequence comparison of RAD23 proteins. Identical and similar residues are shown in the black and gray boxes, respectively. Dots denote gaps. The sequence of plant Ub is
Supplemental Figure 1. Amino acid sequence comparison of RAD23 proteins. Identical and similar residues are shown in the black and gray boxes, respectively. Dots denote gaps. The sequence of plant Ub is
Chapter 14 Regulation of Transcription
 Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Packaging of P22 DNA requires a pac site while packaging of lambda DNA requires a cos site. Briefly describe:
 1). (12 Points) Packaging of P22 DNA requires a pac site while packaging of lambda DNA requires a cos site. Briefly describe: 1. The mechanisms used by P22 and lambda to package DNA. P22 uses a headfull
1). (12 Points) Packaging of P22 DNA requires a pac site while packaging of lambda DNA requires a cos site. Briefly describe: 1. The mechanisms used by P22 and lambda to package DNA. P22 uses a headfull
5. Which of the following enzymes catalyze the attachment of an amino acid to trna in the formation of aminoacyl trna?
 Sample Examination Questions for Exam 3 Material Biology 3300 / Dr. Jerald Hendrix Warning! These questions are posted solely to provide examples of past test questions. There is no guarantee that any
Sample Examination Questions for Exam 3 Material Biology 3300 / Dr. Jerald Hendrix Warning! These questions are posted solely to provide examples of past test questions. There is no guarantee that any
Chapter 6 - Molecular Genetic Techniques
 Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Compartmentalization of prokaryotic DNA replication
 FEMS Microbiology Reviews 29 (2005) 25 47 www.fems-microbiology.org Compartmentalization of prokaryotic DNA replication Alicia Bravo *, Gemma Serrano-Heras, Margarita Salas Instituto de Biología Molecular
FEMS Microbiology Reviews 29 (2005) 25 47 www.fems-microbiology.org Compartmentalization of prokaryotic DNA replication Alicia Bravo *, Gemma Serrano-Heras, Margarita Salas Instituto de Biología Molecular
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION
 M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
The partitioning protein ParB of SLP2 binds palindromic repeats in its own coding sequence
 The partitioning protein ParB of SLP2 binds palindromic repeats in its own coding sequence Chin-Chen Hsu 1, Carton W Chen 1 1 Faculty of Life Sciences and Institute of Genome Sciences, National Yang-Ming
The partitioning protein ParB of SLP2 binds palindromic repeats in its own coding sequence Chin-Chen Hsu 1, Carton W Chen 1 1 Faculty of Life Sciences and Institute of Genome Sciences, National Yang-Ming
Chapter 15 Recombinant DNA and Genetic Engineering. Restriction Enzymes Function as Nature s Pinking Shears
 Chapter 15 Recombinant DNA and Genetic Engineering In this chapter you will learn How restriction enzyme work and why they are essential to DNA technology. About various procedures such as cloning and
Chapter 15 Recombinant DNA and Genetic Engineering In this chapter you will learn How restriction enzyme work and why they are essential to DNA technology. About various procedures such as cloning and
Types of nucleic acid
 RNA STRUCTURE 1 Types of nucleic acid DNA Deoxyribonucleic acid RNA ribonucleic acid HOCH 2 O OH HOCH 2 O OH OH OH OH (no O) ribose deoxyribose 2 Nucleic acids consist of repeating nucleotide that have
RNA STRUCTURE 1 Types of nucleic acid DNA Deoxyribonucleic acid RNA ribonucleic acid HOCH 2 O OH HOCH 2 O OH OH OH OH (no O) ribose deoxyribose 2 Nucleic acids consist of repeating nucleotide that have
Q2 (1 point). How many carbon atoms does a glucose molecule contain?
 Q1 (1 point). Name three amino acids that are typically found at the surface of integrated membrane proteins.. Q2 (1 point). How many carbon atoms does a glucose molecule contain? Q3 (1 point). What do
Q1 (1 point). Name three amino acids that are typically found at the surface of integrated membrane proteins.. Q2 (1 point). How many carbon atoms does a glucose molecule contain? Q3 (1 point). What do
An estimate of the physical distance between two linked markers in Haemophilus influenzae
 J. Biosci., Vol. 13, No. 3, September 1988, pp. 223 228. Printed in India. An estimate of the physical distance between two linked markers in Haemophilus influenzae Ε. Β. SAMIWALA, VASUDHA P. JOSHI and
J. Biosci., Vol. 13, No. 3, September 1988, pp. 223 228. Printed in India. An estimate of the physical distance between two linked markers in Haemophilus influenzae Ε. Β. SAMIWALA, VASUDHA P. JOSHI and
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/315/5819/1709/dc1 Supporting Online Material for RISPR Provides Acquired Resistance Against Viruses in Prokaryotes Rodolphe Barrangou, Christophe Fremaux, Hélène Deveau,
www.sciencemag.org/cgi/content/full/315/5819/1709/dc1 Supporting Online Material for RISPR Provides Acquired Resistance Against Viruses in Prokaryotes Rodolphe Barrangou, Christophe Fremaux, Hélène Deveau,
Bi 8 Lecture 10. Ellen Rothenberg 4 February 2016
 Bi 8 Lecture 10 Bacterial regulation, II Ellen Rothenberg 4 February 2016 Not all bacterial promoters use the same σ factors, and this provides added regulation capability Most sigma factors are related
Bi 8 Lecture 10 Bacterial regulation, II Ellen Rothenberg 4 February 2016 Not all bacterial promoters use the same σ factors, and this provides added regulation capability Most sigma factors are related
CHAPTER 20 DNA TECHNOLOGY AND GENOMICS. Section A: DNA Cloning
 Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Genetic Background Page 1 PHAGE P22
 Genetic Background Page 1 PHAGE P22 Growth of P22. P22 is a temperate phage that infects Salmonella by binding to the O-antigen, part of the lipopolysaccharide on the outer membrane. After infection, P22
Genetic Background Page 1 PHAGE P22 Growth of P22. P22 is a temperate phage that infects Salmonella by binding to the O-antigen, part of the lipopolysaccharide on the outer membrane. After infection, P22
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 MOLECULAR BIOLOGY BIO-2B02 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 MOLECULAR BIOLOGY BIO-2B02 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question
Chapter 2. An Introduction to Genes and Genomes
 PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 2 An Introduction to Genes and Genomes Lectures by Lara Dowland Chapter Contents
PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 2 An Introduction to Genes and Genomes Lectures by Lara Dowland Chapter Contents
7.1 Techniques for Producing and Analyzing DNA. SBI4U Ms. Ho-Lau
 7.1 Techniques for Producing and Analyzing DNA SBI4U Ms. Ho-Lau What is Biotechnology? From Merriam-Webster: the manipulation of living organisms or their components to produce useful usually commercial
7.1 Techniques for Producing and Analyzing DNA SBI4U Ms. Ho-Lau What is Biotechnology? From Merriam-Webster: the manipulation of living organisms or their components to produce useful usually commercial
Identification and Characterization of a Bacterial Chromosome Partitioning Site
 Identification and Characterization of a Bacterial Chromosome Partitioning Site The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Identification and Characterization of a Bacterial Chromosome Partitioning Site The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation
(A) Extrachromosomal DNA (B) RNA found in bacterial cells (C) Is part of the bacterial chromosome (D) Is part of the eukaryote chromosome
 Microbiology - Problem Drill 07: Microbial Genetics and Biotechnology No. 1 of 10 1. A plasmid is? (A) Extrachromosomal DNA (B) RNA found in bacterial cells (C) Is part of the bacterial chromosome (D)
Microbiology - Problem Drill 07: Microbial Genetics and Biotechnology No. 1 of 10 1. A plasmid is? (A) Extrachromosomal DNA (B) RNA found in bacterial cells (C) Is part of the bacterial chromosome (D)
d. reading a DNA strand and making a complementary messenger RNA
 Biol/ MBios 301 (General Genetics) Spring 2003 Second Midterm Examination A (100 points possible) Key April 1, 2003 10 Multiple Choice Questions-4 pts. each (Choose the best answer) 1. Transcription involves:
Biol/ MBios 301 (General Genetics) Spring 2003 Second Midterm Examination A (100 points possible) Key April 1, 2003 10 Multiple Choice Questions-4 pts. each (Choose the best answer) 1. Transcription involves:
Chromatin. Structure and modification of chromatin. Chromatin domains
 Chromatin Structure and modification of chromatin Chromatin domains 2 DNA consensus 5 3 3 DNA DNA 4 RNA 5 ss RNA forms secondary structures with ds hairpins ds forms 6 of nucleic acids Form coiling bp/turn
Chromatin Structure and modification of chromatin Chromatin domains 2 DNA consensus 5 3 3 DNA DNA 4 RNA 5 ss RNA forms secondary structures with ds hairpins ds forms 6 of nucleic acids Form coiling bp/turn
BIOLOGY 205 Midterm II - 19 February Each of the following statements are correct regarding Eukaryotic genes and genomes EXCEPT?
 BIOLOGY 205 Midterm II - 19 February 1999 Name Multiple choice questions 4 points each (Best 12 out of 13). 1. Each of the following statements are correct regarding Eukaryotic genes and genomes EXCEPT?
BIOLOGY 205 Midterm II - 19 February 1999 Name Multiple choice questions 4 points each (Best 12 out of 13). 1. Each of the following statements are correct regarding Eukaryotic genes and genomes EXCEPT?
The plasmid shown to the right has an oriv and orit at the positions indicated, and is known to replicate bidirectionally.
 Name Microbial Genetics, BIO 410/510 2008 Exam II The plasmid shown to the right has an oriv and orit at the positions indicated, and is known to replicate bidirectionally. 1.) Indicate where replication
Name Microbial Genetics, BIO 410/510 2008 Exam II The plasmid shown to the right has an oriv and orit at the positions indicated, and is known to replicate bidirectionally. 1.) Indicate where replication
Zool 3200: Cell Biology Exam 2 2/20/15
 Name: TRASK Zool 3200: Cell Biology Exam 2 2/20/15 Answer each of the following short and longer answer questions in the space provided; circle the BEST answer or answers for each multiple choice question
Name: TRASK Zool 3200: Cell Biology Exam 2 2/20/15 Answer each of the following short and longer answer questions in the space provided; circle the BEST answer or answers for each multiple choice question
Plant Molecular and Cellular Biology Lecture 9: Nuclear Genome Organization: Chromosome Structure, Chromatin, DNA Packaging, Mitosis Gary Peter
 Plant Molecular and Cellular Biology Lecture 9: Nuclear Genome Organization: Chromosome Structure, Chromatin, DNA Packaging, Mitosis Gary Peter 9/16/2008 1 Learning Objectives 1. List and explain how DNA
Plant Molecular and Cellular Biology Lecture 9: Nuclear Genome Organization: Chromosome Structure, Chromatin, DNA Packaging, Mitosis Gary Peter 9/16/2008 1 Learning Objectives 1. List and explain how DNA
Schematic representation of the endogenous PALB2 locus and gene-disruption constructs
 Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Mechanism of DNA segregation in prokaryotes: Replicon pairing by parc of plasmid R1 (partitioning centromere)
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8550 8555, July 1998 Biochemistry Mechanism of DNA segregation in prokaryotes: Replicon pairing by parc of plasmid R1 (partitioning centromere) RASMUS BUGGE JENSEN*,
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8550 8555, July 1998 Biochemistry Mechanism of DNA segregation in prokaryotes: Replicon pairing by parc of plasmid R1 (partitioning centromere) RASMUS BUGGE JENSEN*,
Supplementary Information
 Supplementary Information Fluorescent protein choice The dual fluorescence channel ratiometric promoter characterization plasmid we designed for this study required a pair of fluorescent proteins with
Supplementary Information Fluorescent protein choice The dual fluorescence channel ratiometric promoter characterization plasmid we designed for this study required a pair of fluorescent proteins with
3 Designing Primers for Site-Directed Mutagenesis
 3 Designing Primers for Site-Directed Mutagenesis 3.1 Learning Objectives During the next two labs you will learn the basics of site-directed mutagenesis: you will design primers for the mutants you designed
3 Designing Primers for Site-Directed Mutagenesis 3.1 Learning Objectives During the next two labs you will learn the basics of site-directed mutagenesis: you will design primers for the mutants you designed
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
32 Gene regulation in Eukaryotes Lecture Outline 11/28/05. Gene Regulation in Prokaryotes and Eukarykotes
 3 Gene regulation in Eukaryotes Lecture Outline /8/05 Gene regulation in eukaryotes Chromatin remodeling More kinds of control elements Promoters, Enhancers, and Silencers Combinatorial control Cell-specific
3 Gene regulation in Eukaryotes Lecture Outline /8/05 Gene regulation in eukaryotes Chromatin remodeling More kinds of control elements Promoters, Enhancers, and Silencers Combinatorial control Cell-specific
Chapter 5 DNA and Chromosomes
 Chapter 5 DNA and Chromosomes DNA as the genetic material Heat-killed bacteria can transform living cells S Smooth R Rough Fred Griffith, 1920 DNA is the genetic material Oswald Avery Colin MacLeod Maclyn
Chapter 5 DNA and Chromosomes DNA as the genetic material Heat-killed bacteria can transform living cells S Smooth R Rough Fred Griffith, 1920 DNA is the genetic material Oswald Avery Colin MacLeod Maclyn
Characteristics of bacterial Plasmid : Size : Conformation : Replication origin of replication : Replication Protein :
 Characteristics of bacterial Plasmid : Size : Conformation : Replication origin of replication : Replication Protein : Definition of Plasmid Plasmids are extrachromosomal circular, double stranded DNA
Characteristics of bacterial Plasmid : Size : Conformation : Replication origin of replication : Replication Protein : Definition of Plasmid Plasmids are extrachromosomal circular, double stranded DNA
Chapter 13A: Viral Basics
 Chapter 13A: Viral Basics 1. Viral Structure 2. The Viral Life Cycle 3. Bacteriophages 1. Viral Structure What exactly is a Virus? Viruses are extremely small entities that are obligate intracellular parasites
Chapter 13A: Viral Basics 1. Viral Structure 2. The Viral Life Cycle 3. Bacteriophages 1. Viral Structure What exactly is a Virus? Viruses are extremely small entities that are obligate intracellular parasites
MCB 102 University of California, Berkeley August 11 13, Problem Set 8
 MCB 102 University of California, Berkeley August 11 13, 2009 Isabelle Philipp Handout Problem Set 8 The answer key will be posted by Tuesday August 11. Try to solve the problem sets always first without
MCB 102 University of California, Berkeley August 11 13, 2009 Isabelle Philipp Handout Problem Set 8 The answer key will be posted by Tuesday August 11. Try to solve the problem sets always first without
Lecture 22: Molecular techniques DNA cloning and DNA libraries
 Lecture 22: Molecular techniques DNA cloning and DNA libraries DNA cloning: general strategy -> to prepare large quantities of identical DNA Vector + DNA fragment Recombinant DNA (any piece of DNA derived
Lecture 22: Molecular techniques DNA cloning and DNA libraries DNA cloning: general strategy -> to prepare large quantities of identical DNA Vector + DNA fragment Recombinant DNA (any piece of DNA derived
Chapter 2 The Structure of Genes and Genomes. Electron micrograph of a metaphase chromosome
 Chapter 2 The Structure of Genes and Genomes Electron micrograph of a metaphase chromosome Genetic information is stored in double stranded DNA DNA structure, building blocks: (A) Bases DNA structure,
Chapter 2 The Structure of Genes and Genomes Electron micrograph of a metaphase chromosome Genetic information is stored in double stranded DNA DNA structure, building blocks: (A) Bases DNA structure,
Designing and creating your gene knockout Background The rada gene was identified as a gene, that when mutated, caused cells to become hypersensitive
 Designing and creating your gene knockout Background The rada gene was identified as a gene, that when mutated, caused cells to become hypersensitive to ionizing radiation. However, why these mutants are
Designing and creating your gene knockout Background The rada gene was identified as a gene, that when mutated, caused cells to become hypersensitive to ionizing radiation. However, why these mutants are
Chapter 18. Regulation of Gene Expression
 Chapter 18 Regulation of Gene Expression 2007-2008 Control of Prokaryotic (Bacterial) Genes 2007- Bacterial metabolism Bacteria need to respond quickly to changes in their environment STOP GO if they have
Chapter 18 Regulation of Gene Expression 2007-2008 Control of Prokaryotic (Bacterial) Genes 2007- Bacterial metabolism Bacteria need to respond quickly to changes in their environment STOP GO if they have
Name Per AP: CHAPTER 27: PROKARYOTES (Bacteria) p559,
 AP: CHAPTER 27: PROKARYOTES (Bacteria) p559, 561-564 1. How does the bacterial chromosome compare to a eukaryotic chromosome? 2. What is a plasmid? 3. How fast can bacteria reproduce? 4. What is a bacterial
AP: CHAPTER 27: PROKARYOTES (Bacteria) p559, 561-564 1. How does the bacterial chromosome compare to a eukaryotic chromosome? 2. What is a plasmid? 3. How fast can bacteria reproduce? 4. What is a bacterial
Split-Operon Control of a Prophage Gene*
 Proceeding8 of the National Academy of Sciences Vol. 65, No. 2, pp. 331-336, February 1970 Split-Operon Control of a Prophage Gene* L. Elizabeth Bertani DEPARTMENT OF MICROBIAL GENETICS, KAROLINSKA INSTITUTET,
Proceeding8 of the National Academy of Sciences Vol. 65, No. 2, pp. 331-336, February 1970 Split-Operon Control of a Prophage Gene* L. Elizabeth Bertani DEPARTMENT OF MICROBIAL GENETICS, KAROLINSKA INSTITUTET,
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Genome manipulation by homologous recombination in Drosophila Xiaolin Bi and Yikang S. Rong Date received (in revised form): 9th May 2003
 Xiaolin Bi is a post doctoral research fellow at the Laboratory of Molecular Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA. Yikang S. Rong is the principal
Xiaolin Bi is a post doctoral research fellow at the Laboratory of Molecular Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA. Yikang S. Rong is the principal
NUCLEUS. Fig. 2. Various stages in the condensation of chromatin
 NUCLEUS Animal cells contain DNA in nucleus (contains ~ 98% of cell DNA) and mitochondrion. Both compartments are surrounded by an envelope (double membrane). Nuclear DNA represents some linear molecules
NUCLEUS Animal cells contain DNA in nucleus (contains ~ 98% of cell DNA) and mitochondrion. Both compartments are surrounded by an envelope (double membrane). Nuclear DNA represents some linear molecules
GENETIC ENGINEERING worksheet
 Section A: Genetic Engineering Overview 1. What is genetic engineering? 2. Put the steps of genetic engineering in order. Recombinant product is isolated, purified and analyzed before marketing. The DNA
Section A: Genetic Engineering Overview 1. What is genetic engineering? 2. Put the steps of genetic engineering in order. Recombinant product is isolated, purified and analyzed before marketing. The DNA
