Quan Zhao, Helen Cumming, Loretta Cerruti, John M. Cunningham, and Stephen M. Jane
|
|
|
- Ginger Atkinson
- 6 years ago
- Views:
Transcription
1 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 40, Issue of October 1, pp , by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Site-specific Acetylation of the Fetal Globin Activator NF-E4 Prevents Its Ubiquitination and Regulates Its Interaction with the Histone Deacetylase, HDAC1* Received for publication, May 10, 2004, and in revised form, July 21, 2004 Published, JBC Papers in Press, July 24, 2004, DOI /jbc.M Quan Zhao, Helen Cumming, Loretta Cerruti, John M. Cunningham, and Stephen M. Jane From the Rotary Bone Marrow Research Laboratory, Royal Melbourne Hospital, Parkville, Victoria 3050 Australia and the Division of Experimental Hematology, St. Jude Children s Research Hospital, Memphis, Tennessee Acetylation provides one mechanism by which the functional diversity of individual transcription factors can be expanded. This is valuable in the setting of complex multigene loci that are regulated by a limited number of proteins, such as the human -globin locus. We have studied the role of acetylation in the regulation of the transcription factor NF-E4, a component of a protein complex that facilitates the preferential expression of the human -globin genes in fetal erythroid cells. We have shown that NF-E4 interacts directly with, and serves as a substrate for, the acetyltransferase co-activator PCAF. Acetylation of NF-E4 is restricted to a single residue (Lys 43 ) in the amino-terminal domain of the protein and results in two important functional consequences. Acetylation of NF-E4 prolongs the protein halflife by preventing ubiquitin-mediated degradation. This stabilization is PCAF-dependent, since enforced expression in fetal/erythroid cells of a mutant form of PCAF lacking the histone acetyltransferase domain (PCAF HAT) decreases NF-E4 stability. Acetylation of Lys 43 also reduces the interaction between NF-E4 and HDAC1, potentially maximizing the activating ability of the factor at the -promoter. These results provide further demonstration that co-activators, such as PCAF, can influence individual transcription factor properties at multiple levels to alter their effects on gene expression. Proteins with intrinsic histone acetyltransferase (HAT) 1 activity function as co-activators of transcription through direct acetylation of specific lysine residues within the N-terminal tails of core histones (1). This modification leads to destabilization of local nucleosomal structure with induction of an open chromatin configuration, allowing access of the transcriptional * This work was supported by National Health and Medical Research Council of Australia, NIH Grants PO1 HL and RO1 HL (to S. M. J.), the Wellcome Trust (to S. M. J.), Cancer Centre Support CORE Grant P30 CA 21765, the American Lebanese Syrian Associated Charities, and the Assisi Foundation of Memphis. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. To whom correspondence should be addressed: Rotary Bone Marrow Research Laboratory, c/o Royal Melbourne Hospital Post Office, Grattan Street, Parkville, Victoria 3050, Australia. Tel.: ; Fax: ; jane@wehi.edu.au. 1 The abbreviations used are: HAT, histone acetyltransferase; EKLF, erythroid Kruppel-like factor; SSP, stage selector protein complex; SSE, stage selector element; GFP, green fluorescent protein; GST, glutathione S-transferase; TSA, trichostatin A; HA, hemagglutinin; Ub, ubiquitin; CBP, CREB-binding protein; CREB, camp-response elementbinding protein. This paper is available on line at machinery to core promoters (reviewed in Refs. 2 4). The covalent modification of histones by acetylation also provides recognition sites for factors involved in gene activation or repression (5). Two of the most widely studied protein families with acetylase activity are the GCN5/PCAF (1, 6) and p300/ CBP proteins (7, 8). The substrates for these factors are far more diverse than the histone proteins alone and include transcription factors such as p53 (9), GATA-1 (10, 11), erythroid Kruppel-like factor (EKLF) (12), SCL (13), E2F1 (14), transcriptional co-regulators (15), DNA-binding proteins (16), retroviral proteins (17), and nonnuclear proteins (18). The sequelae of acetylation of these proteins range from altered DNA binding or cellular localization to changes in protein stability or protein-protein interactions (reviewed in Ref. 19). These significant changes in function occur in the context of modification of a small number of lysine residues within an individual protein and thus provide mechanisms to expand the roles for single factors in the regulation of complex multigenic loci. One such locus is the -like globin cluster in which a linear array of five genes is expressed in a highly regulated tissuespecific and developmentally specific manner (20). To date, very few transcription factors that specifically influence the temporal profile of globin gene expression have been identified. One of these, EKLF is a red cell-specific activator that is critical for switching on high level adult -globin expression (21 23). EKLF binds to the CACCC element in the proximal -promoter, leading to chromatin remodeling and transcriptional activation (24 28). The transcriptional activity of EKLF is regulated by site-specific acetylation by p300 and CBP that results in an enhanced activation potential of the protein and increased binding to the SWI/SNF chromatin remodeling complex (12, 29). NF-E4 is another globin-specific transcription factor (30). This protein forms the stage selector protein complex (SSP) with the ubiquitous transcription factor CP2 (31). The SSP facilitates the interaction of the -globin genes with the powerful enhancer elements contained in the locus control region in fetal erythroid cells through binding to the stage selector element (SSE) in the proximal -promoter (32). Enforced expression of NF-E4 in the human fetal/erythroid K562 cell line and human cord blood progenitors leads to increased fetal globin gene expression (30). In cord blood progenitors, where active competition between fetal and adult globin genes occurs, enforced expression of NF-E4 also leads to a reduction in -globin gene expression (30). In this study, we demonstrate that NF-E4 is a direct target of the co-activator PCAF. The resultant acetylation of Lys-43 in NF-E4 results in both an increase in the stability of the protein due to diminished targeting by ubiquitination and an alteration in protein-protein interactions that favor transcriptional 转载
2 41478 Acetylation Controls Stability and Interactions of NF-E4 activation. Thus, a single amino acid modification results in complementary functional changes that enhance the ability of NF-E4 to positively regulate fetal globin gene expression. EXPERIMENTAL PROCEDURES Cell Culture K562 cells were grown in RPMI medium 1640 supplemented with 10% fetal bovine serum. 293T cells were cultured in Dulbecco s modified Eagle s medium containing 10% fetal bovine serum. All cells were grown at 37 C and in 5% CO 2 supplemented with 50 units of penicillin/ml and 50 g of streptomycin/ml. Reagents and Antibodies [ 14 C]Acetyl-CoA (59 mci/mmol) and sodium [ 3 H]acetate (5 Ci/mmol) were purchased from Amersham Biosciences and PerkinElmer Life Sciences, respectively. Tran 35 S-label was obtained from ICN (Costa Mesa, CA). Trichostatin A (TSA), sodium butyrate, acetyl-coa, ubiquitin, MG132, and cycloheximide were from Sigma. Peroxidase-conjugated goat anti-mouse and monoclonal antirabbit immunoglobulin G, monoclonal anti-flag (M2), and anti-ubiquitin antibodies were from Sigma. Monoclonal anti-ha (12CA5) antibody was from Roche Applied Science. Monoclonal anti-acetyl lysine and anti-hdac1 antibodies were from Upstate (Waltham, MA). Anti- PCAF and anti-tubulin antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-NF-E4-specific antibody was generated by immunizing rabbits with a C-terminal synthetic peptide with the amino acid sequence LKTDSALEQTPQQLPSLHLS coupled to keyhole limpet hemocyanin. Plasmids, Transfections, Luciferase, and -Galactosidase Assays The expression vectors GST-NF-E4 and GST-CP2 in pgex were described previously (30, 31). For the GST-NF-E4 truncation constructs, PCR fragments corresponding to the shortened coding sequences were generated with BamHI and XhoI ends and were cloned into pgex vectors. The plasmids pcx-pcaf, pcx-pcaf HAT, MSCV-IRES- PCAF-GFP, and MSCV-IRES-PCAF HAT-GFP were kindly provided by Dr. Steven Brandt (13). Plasmids expressing GST-PCAF-( ) and GST-CBP-( ) were kindly provided by Dr. Tony Kouzarides (14). The plasmid pcdna3.1-flag-ubiquitin was kindly provided by Dr. Ivan Dikic. The in vitro transcription/translation plasmid for PCAF-(1 832) was cloned by inserting an EcoRI/XhoI fragment from MSCV-IRES-PCAF-GFP into vector psp72. Mutation of GST-NF-E4 to GST-K43R was accomplished with the QuikChange mutagenesis system (Stratagene, La Jolla, CA). Retroviral vectors expressing HA- NF-E4 and HA-K43R were constructed by cloning fragments obtained by PCR amplification of coding sequences of pgex-nf-e4 and pgex- K43R into MSCV-IRES-GFP. The integrity of all constructs that generated fusion proteins was confirmed by DNA sequencing. Stable K562 cell lines overexpressing HA-NF-E4, HA-K43R, PCAF, and PCAF HAT were generated according to the protocols described previously, except that only the top 10% of GFP-positive cells were collected (30). For the co-immunoprecipitation experiment, 293T cells were transiently transfected with various plasmids by a calcium phosphate method (Invitrogen). For the reporter gene assays, native K562 cells or stable K562 cell lines overexpressing HA-NF-E4 and HA-K43R were co-transfected with HS2 53 -Luciferase, containing hypersensitivity site 2 of the -globin locus control region linked to the 53 -promoter relative to the CAP site and the firefly luciferase reporter gene (32), and pch110, containing the -galactosidase gene driven by SV40 promoter as an internal transfection efficiency control by electroporation. For the TSA induction experiments, the transfected cells were divided into two aliquots, one of which was cultured in various concentrations of TSA for 20 h and the other in standard medium alone. For all other transfection experiments, cells were harvested after 36 h and lysed, and luciferase activity was determined on a Monolight 2001 luminometer using the luciferase assay kit (Promega). -Galactosidase activity was measured in a spectrophotometer using the enzyme assay system (Promega), and the relative luciferase activities were calculated by dividing the luciferase activity by the -galactosidase activity to correct for transfection efficiency. Three independent transfections were performed in duplicate. Metabolic Labeling For 35 S labeling, K562 cells were grown in RPMI supplemented with 5% dialyzed fetal bovine serum containing Tran 35 S-label (20 Ci/ml; a mixture of cysteine and methionine) for 4 h. For 3 H labeling, K562 cells were cultured in RPMI containing sodium [ 3 H]acetate (1 mci/ml) and 2 M TSA for 2 h. Extracts were prepared and boiled after adding 2 sample buffer (125 mm Tris-HCl, ph 6.8, 4% SDS, 20% glycerol, 2% -mercaptoethanol) to disrupt interactions between proteins before being subjected to immunoprecipitation with specific anti-nf-e4 antibody. The immunoprecipitated samples on beads were extensively washed with stringent washing buffer (500 mm NaCl, 50 mm Tris-HCl, ph 8.0, 5 mm EDTA, 1% Triton X-100, 0.1% SDS), resolved on SDS-PAGE, and analyzed by autoradiography. Immunoprecipitation and Immunoblotting Cells were lysed in icecold lysis buffer (150 mm NaCl, 50 mm Tris-HCl, ph 8.0, 1 mm EDTA, 1% Nonidet P-40, 10 mm sodium butyrate) containing a protease inhibitor mixture (Roche Applied Science) and cleared by centrifugation. Immunoprecipitations were carried out by adding appropriate antibodies plus protein G-Sepharose beads, followed by incubation at 4 C. The immunoprecipitates were washed extensively, subjected to SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were incubated with various specific antibodies and then washed extensively prior to incubation with peroxidase-conjugated anti-rabbit or antimouse immunoglobulin G. After further extensive washes, the blots were visualized by using ECL reagents (Amersham Biosciences). All immunoprecipitations were performed in duplicate. Recombinant Protein Expression and GST Pull-down Assay GSTfusion proteins were produced in BL21 Escherichia coli as described previously (30). 35 S-Labeled NF-E4 and PCAF synthesized using the T7 TNT kit (Promega) and Tran 35 S-label (ICN) were incubated with GST fusion proteins prebound to glutathione beads at 4 C overnight. The samples were washed extensively and subjected to SDS-PAGE. The gels were dried and analyzed by autoradiography. Determination of Protein Half-life K562 cells stably expressing HA- NF-E4, HA-K43R, PCAF, and PCAF HAT were treated with cycloheximide (100 M) to stop protein synthesis and incubated for the indicated times. Cells were lysed, and lysates were subjected to SDS-PAGE and Western blotting probed with the indicated specific antibodies. The protein signals were scanned and quantitated by PhosphorImager analysis software (Amersham Biosciences). In Vitro Protein Acetylation Assay 2 3 g of the indicated GST fusion protein or SCL and 200 ng of acetyltransferase protein were incubated in a reaction containing 50 mm Tris-HCl, ph 8.0, 10% glycerol, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 10 mm sodium butyrate, and 30 M acetyl-coenzyme A or 1 l of[ 14 C]acetyl- CoA for 1 h at 30 C. The reaction mixture was subjected to SDS-PAGE and analyzed by autoradiography of dried gels or by Western blotting with anti-acetyl lysine antibody. In Vitro Ubiquitination Assay Recombinant protein was used as the substrate in a ubiquitin reaction containing 20 mm Hepes, ph 7.5, 5 mm MgCl 2,2mM dithiothreitol, 2 mm ATP, 5 g of ubiquitin, 20 M MG132, and 5 l of crude rabbit reticulocyte (Promega) for 1 h at 30 C. In some cases, GST fusion proteins were acetylated prior to the ubiquitination reaction by PCAF in the presence or absence of unlabeled acetyl-coa. The samples were subjected to SDS-PAGE and transferred to nitrocellulose membranes followed by determination of ubiquitination by Western blotting analysis. In Vivo Ubiquitination Assay 293T cells were transfected with pcdna3.1-flag-ubiquitin, and cell lysate was prepared 36 h later. For immunoprecipitation, 1 mg of protein was incubated with -HA antibody at 4 C overnight before protein G beads were added for 2 h. The beads were washed twice with NaCl (1 M) in Tris-buffered saline supplemented with Nonidet P-40 (1%), -mercaptoethanol (0.05%), and EDTA (1 mm). Proteins were loaded onto SDS-PAGE followed by immunoblot analysis with the indicated antibodies and enhanced chemiluminescence detection. Electrophoretic Mobility Shift Assay The electrophoretic mobility shift assay was performed as previously described (31) using recombinant proteins. In some cases, recombinant NF-E4 was acetylated in vitro prior to binding DNA. Specific antibodies against NF-E4 or CP2 or specific or nonspecific cold oligonucleotide competitors in molar excess were added to the reaction mixture for 30 min on ice before the addition of the labeled SSE oligonucleotides. RESULTS Acetylation of NF-E4 in Vitro and in Vivo Distinct functional parallels exist between the globin regulatory transcription factors EKLF and NF-E4. Both factors are expressed throughout erythroid ontogeny and yet exert their predominant effects at distinct developmental time points. Acetylation of EKLF is critical for its role as an adult globin gene activator, and we postulated that a similar post- translational modification might also regulate NF-E4 function. We therefore examined the ability of NF-E4 to serve as a substrate for two acetyltransferases, PCAF and CBP. A purified GST-NF-E4 fusion protein was incubated with recombinant CBP or PCAF in the presence of [ 14 C]acetyl-CoA and the incorporation of [ 14 C]ac-
3 Acetylation Controls Stability and Interactions of NF-E FIG. 1. Acetylation of NF-E4 by PCAF in vitro and in vivo. A, purified GST and GST-NF-E4 and SCL cleaved from GST were incubated with [ 14 C]acetyl-CoA and bacterially expressed PCAF or CBP as indicated. Reaction products were fractionated by SDS-PAGE, and the dried gel was exposed to x-ray film. B, human K562 cells were pulselabeled with either [ 35 S]methionine/cysteine or [ 3 H]acetate in the presence of 50 nm TSA and lysed. Lysates were subjected to immunoprecipitation (IP) with NF-E4 antibody or preimmune sera (PI), and the resulting immunoprecipitates were fractionated by SDS-PAGE and analyzed for incorporation of [ 35 S]methionine/cysteine or [ 3 H]acetate by autoradiography. C, 293T cells were transfected with expression vectors for HA-tagged NF-E4 and FLAG-tagged PCAF or PCAF that lacked the histone acetyltransferase domain (PCAF HAT). Cellular extracts from the transfected cells were immunoprecipitated with preimmune sera or anti-ha antibody and immunoblotted (IB) with either anti-ha antibody ( -HA) or antibody specific for acetylated lysine ( -AcK). Extracts were also directly immunoblotted with anti-flag antibody, which detects both PCAF species ( -Flag). etate determined by SDS-PAGE and autoradiography. Recombinant SCL (TAL1), which is acetylated in vitro by both acetyltransferases (13), served as the positive control, and GST alone served as the negative control. As shown in Fig. 1A, acetylation of GST-NF-E4 was observed in the presence of PCAF (lane 3) but not CBP (lane 2). Incorporation of [ 14 C]acetate was dependent on the presence of NF-E4, since GST alone remained unlabeled (lane 1). SCL was acetylated in the presence of both co-activators (lanes 4 and 5). To determine whether NF-E4 was acetylated in vivo in a fetal/erythroid environment, human K562 cells were pulse-labeled with either [ 35 S]methionine/cysteine or sodium [ 3 H]acetate in the presence of TSA. Cellular extract was subjected to immunoprecipitation with anti-nf-e4 antibody, and precipitates were washed under stringent conditions. As a control, extracts were also immunoprecipitated with preimmune sera. As shown in Fig. 1B, NF-E4-specific antisera detected both the 35 S-labeled NF-E4 (left panel) and acetylated NF-E4 (right panel). Neither species was detected with preimmune sera. To directly examine the role of PCAF in the acetylation of NF-E4 in a cellular context, we co-transfected the human cell line 293T with mammalian expression vectors containing a hemagglutinin (HA) epitope-tagged NF-E4 (HA-NF- E4), and either the wild-type PCAF cdna (PCAF) tagged with a FLAG epitope or a mutant PCAF that lacked the histone acetyltransferase domain (PCAF HAT) tagged with a FLAG epitope, or vector alone (13, 14). Cellular extracts from the transfected cells were immunoprecipitated with preimmune sera (lane 1) or anti-ha antibody (lanes 2 4) and immunoblotted with either anti-ha antibody ( -HA) or antibody specific for acetylated lysine ( -AcK) (Fig. 1C). An increase in the level of acetylated NF-E4 was observed in the presence of PCAF compared with vector alone (compare lanes 2 and 3). The
4 41480 Acetylation Controls Stability and Interactions of NF-E4 FIG. 2. NF-E4 and PCAF interact in vitro and in cellular extracts. A, purified GST and GST fusion proteins containing amino acids of PCAF (GST-PCAF-( )) or full-length CP2 (GST-CP2) preadsorbed to glutathione-sepharose beads were incubated with 35 S-labeled in vitro transcribed/translated (IVT) NF-E4. Specifically bound protein was eluted from washed beads and visualized by autoradiography after SDS-PAGE. Input represents 20% of the in vitro translated NF-E4 used in the assay. Each experiment was performed in duplicate. B, purified GST and GST fusion proteins containing amino acids 1 25, 1 48, , and (top panel) of NF-E4 preadsorbed to glutathione-sepharose beads were incubated with 35 S-labeled PCAF. Specifically bound protein was eluted from washed beads and visualized by autoradiography (bottom panel) after SDS-PAGE. Input represents 5% of the in vitro translated PCAF used in the assay. C, 293T cells were transfected with expression vectors for HA-tagged NF-E4 and FLAG-tagged PCAF. Cellular extracts were immunoprecipitated (IP) with antibody to the FLAG epitope ( -Flag) or an unrelated antibody (mock) and analyzed by immunoblotting (IB) with anti-ha and anti-pcaf antibodies. NF-E4 protein was identified only in precipitates using anti-flag antisera. D, K562 cell extract was immunoprecipitated with either anti-nf-e4 or anti-pcaf antibodies. Preimmune sera served as the control. Immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with anti-pcaf antibody. PCAF protein was detected in precipitates from both anti-nf-e4 and anti-pcaf antibodies but not preimmune sera. level of acetylated NF-E4 was decreased in cells transfected with PCAF HAT, consistent with its dominant negative role (lane 4). The expression of HA-NF-E4 and FLAG-PCAF/ PCAF HAT were similar in the transfected lines (lower panels). These findings indicate that NF-E4 serves as a substrate for the acetyltransferase, PCAF. NF-E4 Interacts with PCAF in Vitro and in Vivo Acetylation of transcription factors is usually mediated by a direct interaction between the factor and the specific acetyltransferase. To determine whether NF-E4 interacted directly with PCAF, we performed glutathione S-transferase (GST)-chromatography assays (Fig. 2A). In vitro transcribed/translated [ 35 S]methionine-labeled NF-E4 (lane 1) was applied to glutathione-sepharose beads adsorbed with GST alone (lane 2); GST-PCAF-( ) (lane 3), which contains key regulatory domains including the HAT domain; and a positive control, GST-CP2 (lane 4). The labeled protein was retained on both the GST-PCAF and GST-CP2 matrices but not on GST alone. To further localize the region of NF-E4 that interacted with PCAF, we generated a series of truncation deletions of NF-E4 as GST fusion proteins and examined their ability to retain [ 35 S]methionine-labeled PCAF (Fig. 2B). Only NF-E4 fusion proteins containing the first 25 amino acids were capable of interacting with PCAF (lanes 3 5). In contrast, fusion proteins that lacked only the N-terminal 17 amino acids did not interact (lane 6), and no PCAF was retained on the GST alone matrix (lane 2). To confirm the interaction in a cellular context, we transfected 293T cells with mammalian expression vectors containing the NF-E4 cdna tagged with a hemagglutinin epitope (HA-NF-E4) and the PCAF cdna tagged with a FLAG epitope (FLAG- PCAF) (Fig. 2C). Cellular extract was immunoprecipitated with an unrelated antibody (mock) or anti-flag antibody and immunoblotted with either anti-ha antibody (top panel) or anti-flag antibody (bottom panel). Both PCAF and NF-E4
5 Acetylation Controls Stability and Interactions of NF-E FIG.3.NF-E4 is acetylated on lysine 43. A, purified GST and GST fusion proteins containing amino acids 1 25, 1 48, , and of NF-E4 preadsorbed to glutathione-sepharose beads were incubated with acetyl-coa and bacterially expressed PCAF. Reaction products were fractionated by SDS-PAGE and immunoblotted (IB) with anti-acetyl lysine antibody. The integrity of the fusion proteins used (denoted by asterisks) is shown in the accompanying immunoblot probed with anti-gst antisera. B, the predicted amino acid sequence of wild-type NF-E4 from residue 26 to 48 is shown with Lys-43 in boldface type. The sequence of the K43R mutant is shown below. GST fusion proteins containing the full-length wild-type NF-E4 sequences or the K43R mutant sequences were incubated with acetyl-coa and bacterially expressed PCAF. Reaction products were fractionated by SDS-PAGE and immunoblotted with anti-acetyl lysine antibody ( -AcK) or anti-nf-e4 antibody ( - NF-E4). C, 293T cells were transfected with expression vectors for HA-tagged NF-E4 and HA-tagged K43R NF-E4. Cellular extracts were immunoprecipitated (IP) with antibody to the HA epitope ( - HA) and analyzed by immunoblotting with anti-ha and anti-ack antibodies. were immunoprecipitated with the anti-flag antibody, but not the unrelated antibody, indicating that the two proteins interact in a cellular context. Immunoprecipitation of lysate from nontransfected cells with the anti-flag antibody failed to bring down NF-E4 (data not shown). We then examined whether this interaction occurred in the absence of enforced expression of the proteins. K562 cell extract was immunoprecipitated with either preimmune sera or antibody to NF-E4 or PCAF, and the precipitates were electrophoresed and immunoblotted with anti-pcaf antibody. As shown in Fig. 2D, PCAF co-immunoprecipitated with NF-E4 (lane 2), indicating that these two factors form a complex in native cells. No PCAF was observed with preimmune sera (lane 1). Identifying Acetylation Sites in NF-E4 To identify the residue(s) in NF-E4 that are acetylated by PCAF, we utilized our series of GST-NF-E4 truncation mutants (Fig. 3A, left panel). The proteins were subjected to a PCAF-dependent in vitro acetylation assay using unlabeled acetyl-coa, and protein acetylation was detected using an antibody specific for acetylated lysine ( -AcK) (Fig. 3A, top right panel). Full-length NF-E4 (amino acids 1 179) (top panel, lane 5) and NF-E4 (amino acids 1 48) (lane 3) were both acetylated in vitro. In contrast, NF-E4 lacking the first 48 amino acids (lane 4), NF-E4 (amino acids 1 25) (lane 2), and GST alone (lane 1) remained unacetylated, despite significant levels of GST fusion protein expression (Fig. 3A, bottom right panel). This acetylation pattern was duplicated when acetylated lysine was detected by incorporation of [ 14 C]acetyl-CoA in the in vitro assay (data not shown). These results localized the site of acetylation of NF-E4 to the residues between 26 and 48 and indicated that the acetylation status of the NF-E4 protein was accurately reflected by Western analysis with the antibody specific for acetylated lysine. Acetylation of proteins involves the attachment of an acetyl group to the side chain of lysine residues. Examination of the predicted amino acid sequence of NF-E4 in this region revealed a single potential site for NF-E4 acetylation at lysine 43. To determine whether this residue was acetylated by PCAF in vitro, we generated a full-length NF-E4 protein in which the lysine at position 43 was altered to arginine (K43R) (Fig. 3B, top panel). Cleaved GST fusion proteins of the full-length wildtype NF-E4 and K43R mutant were incubated with PCAF in
6 41482 Acetylation Controls Stability and Interactions of NF-E4 FIG. 4. Acetylation of NF-E4 does not affect DNA binding. Recombinant NF-E4 and CP2 were used in electrophoretic mobility shift assays as indicated with a 32 P-labeled probe containing the SSE sequence from the proximal -promoter. For lanes 2 7, increasing amounts of NF-E4 were incubated with PCAF in the presence or absence of acetyl-coa prior to the addition of rcp2 and the electrophoretic mobility shift assays. Acetylation of the recombinant protein was confirmed by immunoblotting with anti-ack antibody (data not shown). The CP2 NF-E4 complexes were disrupted with anti-nf-e4 and anti-cp2 antibodies (lanes 8 and 9) and excess unlabeled SSE probe (lanes 11 13). No effect was observed with an unrelated unlabeled probe (lane 14). The SSP complex is shown, and the band marked with the asterisk is a higher order CP2 NF-E4 complex that is altered by both antibodies and specific unlabeled probe. the presence of acetyl-coa and sodium butyrate, separated by SDS-PAGE, and immunoblotted with anti-acetyl lysine antibody or anti-nf-e4 antibody. As shown in Fig. 3B (bottom panel), despite producing abundant mutant and wild-type proteins as detected with anti-nf-e4 antibody, no acetylation was detected in the setting of the K43R mutation. This mutation did not affect the ability of PCAF to bind to NF-E4 or the nuclear localization of the protein (data not shown). To examine the effect of the K43R mutation on NF-E4 acetylation in vivo, we transfected 293T cells with mammalian expression vectors containing the HA-tagged NF-E4 cdna (HA-NF-E4) or a tagged NF-E4 cdna containing the K43R mutation (HA- K43R). Extract from both lines was immunoprecipitated with -HA antisera and immunoblotted with either -AcK or -HA. As shown in Fig. 3C, despite abundant expression of the mutant and wild type NF-E4 proteins as demonstrated with anti-ha antibody ( -HA), only a faint band was detected in the setting of the K43R mutation ( -AcK). In contrast, the wildtype protein displayed prominent acetylation consistent with Lys-43 acting as the sole site of in vitro acetylation and the major site of in vivo PCAF-dependent acetylation. NF-E4 DNA Binding Is Not Affected by Acetylation in Vitro The DNA-binding properties of many transcription factors are altered by acetylation (19). To examine the effects of this modification on NF-E4 DNA binding, we performed an electrophoretic mobility shift assay with recombinant CP2 and NF-E4 and an SSE probe from the human -promoter (Fig. 4). Under the conditions used, recombinant CP2 failed to bind the SSE probe in isolation (lane 1). Recombinant NF-E4 was preincubated with PCAF in the presence and absence of acetyl-coa using the conditions defined in Fig. 1A. Both forms of NF-E4 failed to bind the SSE in isolation (data not shown). When increasing amounts of unacetylated NF-E4 (lanes 2 4) or acetylated NF-E4 (lanes 5 7) were combined with rcp2 and probe, a robust fast migrating complex was observed. The formation of this complex was unaffected by the acetylation status of the rnf-e4 but was altered by specific antibodies to both CP2 and NF-E4 (lanes 8 and 9). A weaker, slower migrating complex that was similarly unaffected by the acetylation status of NF-E4 was also noted. We attributed this to a higher order NF-E4 CP2 complex (30), since it was dependent on the amount of NF-E4 added and was supershifted with both antibodies. To further confirm the specificity of the protein-dna complexes, we examined the effects of specific and nonspecific unlabeled oligonucleotides. As shown in lanes 11 13, the presence of a fold molar excess of unlabeled SSE probe resulted in diminution/loss of the SSP complexes. In contrast, a 100-fold excess of an unrelated unlabeled oligonucleotide did not alter the SSP SSE complex (lane 14). These findings indicate that acetylation of NF-E4 does not alter its DNA binding properties. Acetylation Stabilizes NF-E4 Protein by Inhibiting Its Ubiquitination Acetylation has recently been shown to increase the stability of a number of diverse transcription factors (14, 33, 34). We examined the influence of acetylation on NF-E4 protein stability by determining the half-life of endogenous NF-E4 in K562 cells stably transfected with retroviral vectors carrying wild-type PCAF or PCAF HAT or the empty vector as a control (Fig. 5A). Cellular extracts were prepared at various time points after cycloheximide treatment and subjected to SDS-PAGE and immunoblotted with antibodies to NF-E4 or tubulin as a control. The expression of PCAF and PCAF HAT was shown to be comparable in the two cell lines by immunoblotting extracts with -PCAF (top right panel). As shown in data from a representative experiment (left panel) and graphically from all experiments (bottom right panel), an increase in endogenous NF-E4 stability was observed in the cells transfected with wild-type PCAF compared with vector alone. In contrast, the half-life of NF-E4 in K562 cells transfected with
7 Acetylation Controls Stability and Interactions of NF-E FIG. 5. Acetylation of NF-E4 increases its stability and inhibits its ubiquitination. A, K562 cells were stably transfected with an empty vector or vectors containing wild type PCAF or PCAF HAT. Thirty-six hours after transfection, cells were treated with cycloheximide (CHX) for the indicated times, and the levels of NF-E4 in the cell lysates were determined as described under Experimental Procedures. The results of a representative experiment are shown (left panel). The levels of PCAF and PCAF HAT in the transfected cells were measured by Western blotting using antisera to PCAF (right panel, top). To ensure that equal amounts of protein were loaded in each well, the levels of tubulin in the samples were estimated by Western blotting using anti-tubulin antibodies ( -Tub). The amount of NF-E4 at each time point is plotted as a percentage of the amount at the start of the chase normalized to the tubulin levels and represents the average S.E. of the mean of three independent experiments (right panel). B, K562 cells were stably transfected with vectors containing HA-tagged wild type or K43R acetylation-deficient NF-E4. Cells were processed as in A, and a representative experiment is shown (left panel). The amount of HA-NF-E4 or HA-K43R at each time point is plotted as a percentage of the amount at the start of the chase normalized to tubulin and represents the average the S.E. of the mean of three independent experiments (right panel). C, GST-HA-NF-E4 was used as the substrate in in vitro ubiquitination reactions in the presence or absence of rabbit reticulocyte lysate (RRL). Where indicated, GST-HA-NF-E4 was acetylated prior to the ubiquitination reaction (lane 2). The samples were resolved by SDS-PAGE, and the ubiquitination of NF-E4 was determined with anti-ubiquitin antibodies ( -Ub). The polyubiquitinated NF-E4 is marked as NF-E4-Ub n. The amount of HA-tagged NF-E4 and its acetylation were determined with anti-ha ( -HA) and anti-acetyl lysine ( -AcK) antibodies, respectively. D, 293T cells were stably transfected with an empty vector or vectors containing HA-tagged wild type or K43R acetylation-deficient NF-E4. These lines were subsequently transfected with a mammalian expression vector containing FLAG-tagged ubiquitin or the vector alone, as a control. Thirty-six hours after transfection, cell lysates were immunoprecipitated (IP) with anti-ha antibodies ( -HA) and resolved by SDS-PAGE. The ubiquitination of the wild type and mutant NF-E4 was determined by Western blotting (IB) using anti-ubiquitin antibodies ( -Ub) or anti-flag antibodies ( -Flag). In the top panel, the asterisk denotes a nonspecific band, and no ubiquitinated species are seen in this panel. Inthesecond panel, the polyubiquitinated NF-E4 species are marked as NF-E4-Ub n, and the immunoglobulin heavy chain is also shown. The amounts of HA-tagged wild type and mutant protein were determined in the cell lysates by Western blotting with anti-ha antibodies (third panel), and the expression of FLAG-tagged ubiquitin is shown in the bottom panel. PCAF HAT was significantly reduced, suggesting that the stability of NF-E4 is dependent on PCAF-mediated acetylation of Lys-43. We therefore compared the stability of wild type and acetylation-deficient (K43R) NF-E4. K562 cells were stably transfected with retroviral vectors carrying HA-tagged wild-type (HA-NF-E4) or K43R mutant NF-E4 (HA-K43R), and extracts were processed as described above (Fig. 5B, left panel). The half-life of the transfected, tagged HA-NF-E4 was less than 1 h (right panel), considerably shorter than the endogenous NF-E4 (Fig. 5A). We attributed this to levels of endogenous PCAF expression that were limiting in the context of a marked increase in NF-E4 expression in the transfected cells. Surprisingly, the half-life of the acetylation-deficient HA-K43R was almost 3 h(right panel), 3 times longer than the wild type protein. The levels and stability of the control protein, tubulin, were comparable in extracts from both transduced lines. These results suggested that acetylation of lysine 43 plays an important role in the stabilization of the NF-E4 protein in cells. However, they also suggest that lysine 43 may also play a role in the targeted degradation of NF-E4. The enhanced stability of both acetylated NF-E4 and the acetylation-deficient K43R mutant was reminiscent of the findings reported with the transcription factors Smad7 and the SREBP family (33, 34). In these proteins, the sites of acetylation that enhance protein stabilization are also the lysine residues targeted by ubiquitination. Consequently, either acetylation or mutation of these lysine residues increases protein stability. To determine whether acetylation of NF-E4 altered the susceptibility of the protein to ubiquitination, we utilized recombinant NF-E4 in both acetylated and nonacetylated forms as substrates in reconstituted in vitro ubiquitination assays (Fig. 5C, top panel). Nonacetylated NF-E4 was ubiquitinated in the presence of rabbit reticulocyte lysate (RRL) (lane 3) and migrated as a high molecular weight polyubiquitinated species (NF-E4-Ub n ). Acetylation of NF-E4 markedly reduced its ubiquitination (compare lanes 2 and 3). No ubiquitinated protein was observed in the absence of the rabbit reticulocyte lysate (lane 1). The amount of HA-NF-E4 used in each experiment was equivalent as demonstrated by immunoblotting with anti-ha antisera (lower panel), and acetylation was confirmed in the presence of PCAF and Ac-CoA by immunoblotting with anti-ack antisera (middle panel). This finding suggests that acetylation of Lys-43 blocks the ubiquitination of NF-E4. To
8 41484 Acetylation Controls Stability and Interactions of NF-E4 examine the effect of mutation of Lys-43 on the ubiquitination of NF-E4 in a cellular context, we utilized the 293T cells stably transfected with retroviral vectors containing the HA-tagged wild-type (HA-NF-E4) or K43R mutant NF-E4 (HA-K43R) or the empty vector control (Fig. 5D). The levels of expression of the tagged NF-E4 forms were similar in both lines, with a small increase in the K43R protein level reflecting its increased stability (shown in -HA, third panel). These lines were then transfected with a mammalian expression vector containing FLAG-tagged ubiquitin ( Flag-Ub, second panel) or the empty vector (w/o Flag-Ub, top panel). The levels of expression of the FLAG-Ub were equivalent in all three lines (bottom panel). Cellular extracts were prepared and immunoprecipitated with anti-ha antibody prior to immunoblotting with either antiubiquitin or anti-flag antibody. In the absence of FLAG-Ub expression (w/o Flag-Ub, top panel), the -HA-immunoprecipitate from all three lines contained no ubiquitinated NF-E4 species, with only a nonspecific band (asterisk) visualized in all three lanes. We attributed this to low levels of endogenous ubiquitin expression in these lines. When FLAG-Ub was expressed ( Flag-Ub, second panel), high molecular weight polyubiquitinated NF-E4 species (NF-E4-Ub n ) were seen in the immunoprecipitates from the wild type NF-E4-expressing cells. These polyubiquitinated proteins were not seen in cells containing the empty vector, and more significantly, they were not seen in the K43R-expressing cells. These results suggest that lysine 43 is also targeted by ubiquitination in vivo. Effect of K43R Mutation on NF-E4 Transcriptional Activity We predicted that functional effects of stabilization of NF-E4 would be reflected by an increase in the activity of the -promoter. To test this, we utilized the stable K562 cells in which expression of wild type or K43R mutant NF-E4 had been enforced and a vector-transduced line as a control. These lines were then transiently transfected with a plasmid containing the -promoter truncated to 53 relative to the CAP site linked to a luciferase reporter gene (32). This construct contains the SSE and the TATA box but none of the other known upstream regulatory elements and is therefore specifically responsive to NF-E4. A -galactosidase-expressing plasmid was also used to monitor transfection efficiency. As shown in Fig. 6A, a greater than 2-fold increase in -promoter activity was observed in cells expressing the wild-type NF-E4. Surprisingly, the increase in -promoter activity observed in the K43R-expressing cells was significantly less than in the wild-type cells despite the higher level of expression of the mutant protein (Fig. 6B). This finding suggested that stabilization of the NF-E4 protein was insufficient in isolation to enhance the protein s activation potential and that acetylation of the protein must play an additional role in NF-E4-mediated -gene activation. To examine this further, we investigated the effects of the histone deacetylase inhibitor TSA on -promoter activity in a transient transfection assay. K562 cells were co-transfected with the -promoter-luciferase reporter and the -galactosidase-expressing plasmid and divided into three flasks, two of which were induced with TSA in varying concentrations. As shown in Fig. 6C, a dose-dependent increase in -promoter activity was seen with TSA induction. This finding was consistent with the enhancement of endogenous -gene expression seen with this compound (35, 36). Diminished Binding of Acetylated NF-E4 to HDAC1 Altered protein-protein interactions are a well established consequence of transcription factor acetylation (19). The affinity of EKLF for the SWI/SNF chromatin-remodeling complex is enhanced by this modification (29), prompting us to consider whether acetylation of NF-E4 could influence its protein interactions to enhance its activation potential. We had previously FIG. 6. Acetylated NF-E4 activates a minimal -globin promoter. A, K562 cells were co-transfected with an empty vector or vectors containing HA-tagged wild type or the acetylation-deficient K43R NF-E4 mutant and a human -globin promoter reporter construct containing the 53 -promoter (relative to the CAP site) linked to a firefly luciferase reporter gene. A -galactosidase-expressing vector was used to monitor transfection efficiency. After 36 h, cell lysates were prepared and assayed for luciferase and -galactosidase activity as described under Experimental Procedures. The results represent the average S.E. of the mean of three independent experiments performed in duplicate. B, the levels of HA-NF-E4 and HA-K43R were determined in the cell lysates by Western blotting (IB) using anti-ha antibodies ( -HA). The levels of tubulin in the samples were estimated by Western blotting using anti-tubulin antibodies ( -Tub). C, K562 cells were transfected with the human -globin promoter fragment linked to a firefly luciferase reporter gene. A -galactosidase-expressing vector was used to monitor transfection efficiency. After the transfection, the cells were divided into three and grown in the presence and absence of the stated concentrations of TSA for 20 h. Cell lysates were then prepared and assayed for luciferase and -galactosidase activity as described under Experimental Procedures. The results represent the average S.E. of three independent experiments performed in duplicate. demonstrated that NF-E4 and the histone deacetylase HDAC1 were co-immunoprecipitated from extract derived from K562 cells using anti-nf-e4 antibody (Fig. 7A). HDAC1 is a member
9 Acetylation Controls Stability and Interactions of NF-E FIG. 7. Reduced binding of HDAC1 to acetylated NF-E4. A, K562 cell extract was immunoprecipitated with anti-nf-e4 antibodies ( -NF-E4). Preimmune sera served as the control (PI). Immunoprecipitates (IP) were fractionated by SDS-PAGE and immunoblotted (IB) with anti-hdac1 antibody ( -HDAC1). HDAC1 protein was detected in precipitates from anti-nf-e4 antibody but not preimmune sera. B, GST-HA-NF-E4 coupled to glutathione-sepharose beads was preincubated with PCAF in the presence and absence of acetyl-coa (Ac-CoA) prior to the addition of K562 cell extract. GST alone served as the control. Specifically bound proteins were eluted from washed beads and resolved by SDS-PAGE. The presence of CP2 and HDAC1 in the eluates was detected by Western blotting with their respective antisera ( -CP2 and -HDAC1). The level of HA-NF-E4 on the beads and the acetylation status of bound NF-E4 in each experiment were determined by Western blotting using anti-ha ( -HA) and anti-acetyl lysine ( -AcK) antibodies, respectively. The input of CP2 and HDAC1 is shown on the left. Each experiment was performed in triplicate. of a family of deacetylases involved in transcriptional repression and gene silencing (reviewed in Ref. 37). To determine whether acetylation of NF-E4 altered its affinity for HDAC1, we utilized recombinant GST-HA-NF-E4 in both acetylated and nonacetylated forms and a GST alone control in pull-down experiments with K562 cell extract (Fig. 7B). Acetylation of the recombinant protein was confirmed by immunoblotting with anti-ack antibody (bottom panel). Analysis of the eluate from the three GST matrices with anti-hdac1 antibody ( -HDAC1) demonstrated that nonacetylated NF-E4 bound significantly greater amounts of HDAC1 derived from K562 extract than acetylated NF-E4. In contrast, CP2 from K562 cell extract was retained equally on both acetylated and nonacetylated NF-E4 as detected by CP2 antibody ( -CP2). GST alone bound neither protein. These findings suggest that acetylation of NF-E4 plays a dual role in enhancing the activation potential of the factor via increased protein stability and diminished association with the transcriptional repressor, HDAC1. DISCUSSION Our previous studies have suggested that NF-E4 plays a role in regulation of the human -globin locus through activation of the -genes and competitive silencing of -gene expression (30). The mechanisms underlying these activities are gradually emerging, and appear to hinge on both critical protein/protein interactions and post-translational modification of the factor. In the fetal erythroid environment, NF-E4 is a component of a multiprotein complex that contains the transcriptional activator CP2 (30, 31). We now provide evidence that the acetyltransferase PCAF also complexes with NF-E4 in this environment and covalently modifies the protein. This modification has a number of sequelae, including increased stability of the protein through resistance to ubiquitination and alteration of protein partners to favor interactions that promote transcriptional activation. The PCAF-interacting domain of NF-E4 is contained within the amino-terminal region of the NF-E4 protein. Although the full-length NF-E4 protein contains a number of lysine residues, it appears that in the context of PCAF, only the single residue at position 43 is acetylated. We found no evidence that CBP directly modifies the NF-E4 protein. In this regard, NF-E4 is unlike several of the other erythroid transcription factors that bind to the globin locus, including GATA-1, EKLF, and p45 NF-E2, all of which are acetylated by CBP (10 12, 38). Interestingly, these factors have all been directly implicated in recruitment of acetyltransferases to the adult -globin promoter (29, 39, 40). In contrast, the fetal globin regulatory factors, NF-E4 and FKLF-2, are both acetylated and functionally modified by PCAF, raising the possibility that stage-specific recruitment of acetyltransferases could occur at the globin locus (41). This could provide a potential explanation for the paradox of the highly stage-specific effects of factors such as EKLF and NF-E4 that are observed despite expression of these factors at all stages of erythroid development. The functional consequences of acetylation of several of the globin locus binding factors have been examined previously. Modification of the MafG component of NF-E2 augments the DNA binding activity of this complex (38). Enhanced DNA binding has also been reported for acetylated GATA-1 (10), although there is some conjecture about this conclusion (11). We observed no effect of acetylation on the DNA-binding properties of NF-E4, a finding that parallels the lack of effect of acetylation on EKLF binding to its cognate site (29). The effects of acetylation on EKLF involve enhanced interaction between the factor and the SWI/SNF chromatin-remodeling complex (29). This results in maximization of the activation potential of EKLF at the -promoter. The acetylation of NF-E4 also appears to maximize its activation potential through increased levels of the protein and a diminished association with the transcriptional repressor HDAC1. HDAC1 is a member of a family of proteins involved in mammalian gene silencing through deacetylation of core histones (37). Several HDAC-dependent co-repressor complexes have been identified in mammalian cells including msin3a (42 47) and NuRD/Mi2 (48, 49). Although the nature and role of such complexes are yet to be established at the -promoter, our identification of HDAC1 and more recently another NuRD component, SHARP (50) 2 as protein partners of NF-E4 suggest the potential for an NF-E4-dependent repressor complex to assemble on the promoter. As such, it appears that NF-E4 may play a dual role in fetal globin gene regulation depending on the developmental context. Modification of a number of transcription factors has been shown to influence their interaction with HDAC1. The phosphorylation status of NF- B determines whether it associates with CBP/p300 or HDAC1 (51). The acetylation status of MyoD and YY1 also influences the ability of these factors to bind HDAC1 (52, 53). In both cases, acetylation increases the interaction with the deacetylase, in contrast to the reduced association of acetylated NF-E4 with HDAC1 that we observed. An- 2 S. M. Jane and J. M. Cunningham, unpublished observations.
10 41486 Acetylation Controls Stability and Interactions of NF-E4 other hematopoietic transcription factor, SCL, has also been shown to have an impaired interaction with the msin3a repressor complex including HDAC1 when acetylated (13). This may provide a novel mechanism to expand the potential roles of a factor at a single complex locus. The enhanced stability of NF-E4 we observed in the context of acetylation has been reported for a number of transcription factors, including E2F1, members of the SREBP family, and Smad7 (14, 33, 34). In the latter two examples, it was demonstrated that specific lysine residues were targeted by ubiquitination and that acetylation of these residues prevented the subsequent ubiquitination of the protein. Our data indicate that a similar mechanism governs NF-E4 stability. The potential link between acetylation status of proteins and their ubiquitination has been strengthened by the identification of ubiquitin pathway components in the HDAC6 complex (54). NF-E4 degradation may be dependent on its deacetylation and ubiquitination, and it is tempting to speculate that this may be mediated by NF-E4 serving as a substrate for HDAC1. Our future studies will explore this possibility. Acknowledgments We are grateful to T. Kouzarides and S. Brandt and I. Dikic for providing plasmids. REFERENCES 1. Brownell, J. E., Zhou, J., Ranalli, T., Kobayashi, R., Edmondson, D. G., Roth, S. Y., and Allis, C. D. (1996) Cell 84, Roth, S. Y., Denu, J. M., and Allis, C. D. (2001) Annu. Rev. Biochem. 70, Struhl, K. (1998) Genes Dev. 12, Workman, J. L., and Kingston, R. E. (1998) Annu. Rev. Biochem. 67, Strahl, B. D., and Allis C. D. (2000) Nature 403, Yang, X. J., Ogryzko, V. V., Nishikawa, J., Howard, B. H., and Nakatani, Y. (1996) Nature 382, Bannister, A. J., and Kouzarides, T. (1996) Nature 384, Ogryzko, V. V., Schiltz, R. L., Russanova, V., Howard, B. H., and Nakatini, Y. (1996) Cell 87, Gu, W., and Roeder, R. G. (1997) Cell 90, Boyes, J., Byfield, P., Nakatani, Y., and Ogryzko, V. (1998) Nature 396, Hung, H. L., Lau, J., Kim, A. Y., Weiss, M. J., and Blobel, G. A. (1999) Mol. Cell. Biol. 19, Zhang, W., and Bieker, J. J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95, Huang, S., Qiu, Y., Shi, Y., Xu, Z., and Brandt, S. J. (2000) EMBO J. 19, Martinez-Balbas, M. A., Bauer, U. M., Nielsen, S. J., Brehm, A., and Kouzarides, T. (2000) EMBO J. 19, Chen, H., Lin, R. J., Xie, W., Wilpitz, D., and Evans, R. M. (1999) Cell 98, Munshi, N., Merika, M., Yie, J., Senger, K., Chen, G., and Thanos, D. (1998) Mol. Cell 2, Kiernan, R. E., Vanhulle, C., Schiltz, L., Adam, E., Xiao, H., Maudoux, F., Calomme, C., Burny, A., Nakatani, Y., Jeang, K. T., Benkirane, M., and Van Lint, C. (1999) EMBO J. 18, L Hernault, S. W., and Rosenbaum, J. L. (1985) Biochemistry 24, Kouzarides, T. (2000) EMBO J. 19, Stamatoyannopoulos, G., Nienhuis, A. W., Majerus, P. W., and Varmus, H. (1994) The Molecular Basis of Blood Diseases, 2nd Ed., W.B. Saunders, Philadelphia, PA 21. Miller, I. J., and Bieker, J. J. (1993) Mol. Cell. Biol. 13, Nuez, B., Michalovich, D., Bygrave, A., Ploemacher, R., and Grosveld, F. (1995) Nature 375, Perkins, A. C., Sharpe, A. H., and Orkin, S. H. (1995) Nature 375, Armstrong, J. A., Bieker, J. J., and Emerson, B. M. (1998) Cell 95, Brown, R. C., Pattison, S., van Ree, J., Coghill, E., Perkins, A., Jane, S. M., and Cunningham, J. M. (2002) Mol. Cell. Biol. 22, Donze, D., Townes, T. M., and Bieker, J. J. (1995) J. Biol. Chem. 270, Feng, W. C., Southwood, C. M., and Bieker, J. J. (1994) J. Biol. Chem. 269, Wijgerde, M., Gribnau, J. Trimborn, T., Nuez, B., Philipsen, S., Grosveld, F., and Fraser, P. (1996) Genes Dev. 10, Zhang, W., Kadam, S., Emerson, B. M., and Bieker, J. J. (2001) Mol. Cell. Biol. 21, Zhou, W.-L., Clouston, D. R., Wang, X., Cerruti, L., Cunningham, J. M., and Jane, S. M. (2000) Mol. Cell. Biol. 20, Jane, S. M., Nienhuis, A. W., and Cunningham, J. M. (1995) EMBO J. 14, Jane, S. M., Ney, P. A., Vanin, E. F., Gumucio, D. L., and Nienhuis, A. W. (1992) EMBO J. 11, Giandomenico, V., Simonsson, M., Gronroos, E., and Ericsson, J. (2003) Mol. Cell. Biol. 23, Gronroos, E., Hellman, U., Heldin, C. H., and Ericsson, J. (2002) Mol. Cell. 10, McCaffrey, P. G., Newsome, D. A., Fibach, E., Yoshida, M., and Su, M. S.-S. (1997) Blood 90, Cao, H., Stamatoyannopoulos, G., and Jung, M. (2004) Blood 103, de Ruijter, A. J., van Gennip, A. H., Caron, H. N., Kemp, S., and van Kuilenburg, A. B. (2003) Biochem. J. 370, Hung, H. L., Kim, A. Y., Hong, W., Rakowski, C., and Blobel, G. A. (2001) J. Biol. Chem. 276, Kiekhaefer, C. M., Grass, J. A., Johnson, K. D., Boyer, M. E., and Bresnick, E. H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99, Letting, D. L., Rakowski, C., Weiss, M. J., and Blobel, G. A. (2003) Mol. Cell. Biol. 23, Song, C. Z., Keller, K., Murata, K., Asano, H., and Stamatoyannopoulos, G. (2002) J. Biol. Chem. 277, Alland, L., Muhle, R., Hou, H., Jr., Potes, J., Chin, L., Schreiber-Agus, N., and DePinho, R. A. (1997) Nature 387, Hassig, C. A., Fleischer, T. C., Billin, A. N., Schreiber, S. L., and Ayer, D. E. (1997) Cell 89, Heinzel, T., Lavinsky, R. M., Mullen, T. M., Soderstrom, M., Laherty, C. D., Torchia, J., Yang, W. M., Brard, G., Ngo, S. D., Davie, J. R., Seto, E., Eisenman, R. N., Rose, D. W., Glass, C. K., and Rosenfeld, M. G. (1997) Nature 387, Laherty, C. D., Yang, W., M., Sun, J. M., Davie, J. R., Seto, E., and Eisenman, R. N. (1997) Cell 89, Nagy, L., Kao, H. Y., Chakravarti, D., Lin, R. J., Hassig, C. A., Ayer, D. E., Schreiber, S. L., and Evans, R. M. (1997) Cell 89, Tong, J. K., Hassig, C. A., Schnitzler, G. R., Kingston, R. E., and Schreiber, S. L. (1998) Nature 395, Xue, Y., Wong, J., Moreno, G. T., Young, M. K., Cote, J., and Wang, W. (1998) Mol. Cell 2, Zhang, Y., LeRoy, G., Seelig, H. P., Lane, W. S., and Reinberg, D. (1998) Cell 95, Shi, Y., Downes, M., Xie, X., Kao, H. Y., Ordentlich, P., Tsai, C. C., Hon, M., and Evans, R. M. (2001) Genes Dev. 15, Zhong, H., May, M. J., Jimi, E., and Ghosh, S. (2002) Mol. Cell 9, Mal, A., Sturniolo, M., Schiltz, R. L., Ghosh, M. K., and Harter, M. L. (2001) EMBO J. 20, Yao, Y. L., Yang, W. M., and Seto, E. (2001) Mol. Cell. Biol. 21, Seigneurin-Berny, D., Verdel, A., Curtet, S., Lemercier, C., Garin, J., Rousseaux, S., and Khochbin, S. (2001) Mol. Cell. Biol. 21,
SUPPLEMENTARY INFORMATION
 The Supplementary Information (SI) Methods Cell culture and transfections H1299, U2OS, 293, HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. H1299 and 293 cells were
The Supplementary Information (SI) Methods Cell culture and transfections H1299, U2OS, 293, HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. H1299 and 293 cells were
Supplementary data. sienigma. F-Enigma F-EnigmaSM. a-p53
 Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplemental Data. LMO4 Controls the Balance between Excitatory. and Inhibitory Spinal V2 Interneurons
 Neuron, Volume 61 Supplemental Data LMO4 Controls the Balance between Excitatory and Inhibitory Spinal V2 Interneurons Kaumudi Joshi, Seunghee Lee, Bora Lee, Jae W. Lee, and Soo-Kyung Lee Supplemental
Neuron, Volume 61 Supplemental Data LMO4 Controls the Balance between Excitatory and Inhibitory Spinal V2 Interneurons Kaumudi Joshi, Seunghee Lee, Bora Lee, Jae W. Lee, and Soo-Kyung Lee Supplemental
Supplementary information
 Supplementary information The E3 ligase RNF8 regulates KU80 removal and NHEJ repair Lin Feng 1, Junjie Chen 1 1 Department of Experimental Radiation Oncology, The University of Texas M. D. Anderson Cancer
Supplementary information The E3 ligase RNF8 regulates KU80 removal and NHEJ repair Lin Feng 1, Junjie Chen 1 1 Department of Experimental Radiation Oncology, The University of Texas M. D. Anderson Cancer
Supplementary Information: Materials and Methods. Immunoblot and immunoprecipitation. Cells were washed in phosphate buffered
 Supplementary Information: Materials and Methods Immunoblot and immunoprecipitation. Cells were washed in phosphate buffered saline (PBS) and lysed in TNN lysis buffer (50mM Tris at ph 8.0, 120mM NaCl
Supplementary Information: Materials and Methods Immunoblot and immunoprecipitation. Cells were washed in phosphate buffered saline (PBS) and lysed in TNN lysis buffer (50mM Tris at ph 8.0, 120mM NaCl
Analysing protein protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate Hong Wang and Xin Zeng
 Analysing protein protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate Hong Wang and Xin Zeng Department of Molecular Genetics, Biochemistry and Microbiology,
Analysing protein protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate Hong Wang and Xin Zeng Department of Molecular Genetics, Biochemistry and Microbiology,
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Supplemental Online Material. The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/-
 #1074683s 1 Supplemental Online Material Materials and Methods Cell lines and tissue culture The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/- knock-out animals
#1074683s 1 Supplemental Online Material Materials and Methods Cell lines and tissue culture The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/- knock-out animals
Nature Structural & Molecular Biology: doi: /nsmb.1583
 Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Functional Interaction between Coactivators CBP/p300, PCAF, and Transcription Factor FKLF2*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 9, Issue of March 1, pp. 7029 7036, 2002 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Functional Interaction
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 9, Issue of March 1, pp. 7029 7036, 2002 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Functional Interaction
HPV E6 oncoprotein targets histone methyltransferases for modulating specific. Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu,
 1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
The microtubule-associated tau protein has intrinsic acetyltransferase activity. Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and
 SUPPLEMENTARY INFORMATION: The microtubule-associated tau protein has intrinsic acetyltransferase activity Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and Virginia M.Y. Lee Cohen
SUPPLEMENTARY INFORMATION: The microtubule-associated tau protein has intrinsic acetyltransferase activity Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and Virginia M.Y. Lee Cohen
Supporting Information
 Supporting Information Su et al. 10.1073/pnas.1211604110 SI Materials and Methods Cell Culture and Plasmids. Tera-1 and Tera-2 cells (ATCC: HTB- 105/106) were maintained in McCoy s 5A medium with 15% FBS
Supporting Information Su et al. 10.1073/pnas.1211604110 SI Materials and Methods Cell Culture and Plasmids. Tera-1 and Tera-2 cells (ATCC: HTB- 105/106) were maintained in McCoy s 5A medium with 15% FBS
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
Fisher (Fairlawn, NJ) and Sigma-Aldrich (St. Louis, MO) and were used without further. (Promega) and DpnI (New England Biolabs, Beverly, MA).
 175 Appendix III Chapter 4 Methods General. Unless otherwise noted, reagents were purchased from the commercial suppliers Fisher (Fairlawn, NJ) and Sigma-Aldrich (St. Louis, MO) and were used without further
175 Appendix III Chapter 4 Methods General. Unless otherwise noted, reagents were purchased from the commercial suppliers Fisher (Fairlawn, NJ) and Sigma-Aldrich (St. Louis, MO) and were used without further
Cell culture and drug treatment. HEK 293 cells were cultured in DMEM (Gibco-BRL)
 Supplementary materials Detailed methods Cell culture and drug treatment. HEK 293 cells were cultured in DMEM (Gibco-BRL) supplemented with 10% fetal bovine serum. To inhibit glucosidase Ι and ΙΙ, castanospermine
Supplementary materials Detailed methods Cell culture and drug treatment. HEK 293 cells were cultured in DMEM (Gibco-BRL) supplemented with 10% fetal bovine serum. To inhibit glucosidase Ι and ΙΙ, castanospermine
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.
 Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Supplementary information
 Supplementary information Table of Content: Supplementary Results... 2 Supplementary Figure S1: Experimental validation of AP-MS results by coimmunprecipitation Western blot analysis.... 3 Supplementary
Supplementary information Table of Content: Supplementary Results... 2 Supplementary Figure S1: Experimental validation of AP-MS results by coimmunprecipitation Western blot analysis.... 3 Supplementary
Supplementary Figure 1. The Hsp70 acetylation level is related to the co-chaperone binding of Hsp70 under various stress conditions.
 Supplementary Figure 1. The Hsp70 acetylation level is related to the co-chaperone binding of Hsp70 under various stress conditions. 1 (a) Etoposide treatment gradually changes acetylation level and co-chaperone
Supplementary Figure 1. The Hsp70 acetylation level is related to the co-chaperone binding of Hsp70 under various stress conditions. 1 (a) Etoposide treatment gradually changes acetylation level and co-chaperone
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Information
 Supplementary Information Supplementary Figures Figure S1. Study of mgtl translation in vitro. (A) Detection of 5 LR RNA using wild-type and anti-sd (91-95) substituted templates in a transcription-translation
Supplementary Information Supplementary Figures Figure S1. Study of mgtl translation in vitro. (A) Detection of 5 LR RNA using wild-type and anti-sd (91-95) substituted templates in a transcription-translation
Transcriptional Regulation in Eukaryotes
 Transcriptional Regulation in Eukaryotes Concepts, Strategies, and Techniques Michael Carey Stephen T. Smale COLD SPRING HARBOR LABORATORY PRESS NEW YORK 2000 Cold Spring Harbor Laboratory Press, 0-87969-537-4
Transcriptional Regulation in Eukaryotes Concepts, Strategies, and Techniques Michael Carey Stephen T. Smale COLD SPRING HARBOR LABORATORY PRESS NEW YORK 2000 Cold Spring Harbor Laboratory Press, 0-87969-537-4
Mechanism of Induction and Suppression of Antiviral Immunity Directed by Virus-Derived Small RNAs in Drosophila
 Cell Host & Microbe, Volume 4 Supplemental Data Mechanism of Induction and Suppression of Antiviral Immunity Directed by Virus-Derived Small RNAs in Drosophila Roghiyh Aliyari, Qingfa Wu, Hong-Wei Li,
Cell Host & Microbe, Volume 4 Supplemental Data Mechanism of Induction and Suppression of Antiviral Immunity Directed by Virus-Derived Small RNAs in Drosophila Roghiyh Aliyari, Qingfa Wu, Hong-Wei Li,
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Transcriptional regulation of BRCA1 expression by a metabolic switch: Di, Fernandez, De Siervi, Longo, and Gardner. H3K4Me3
 ChIP H3K4Me3 enrichment.25.2.15.1.5 H3K4Me3 H3K4Me3 ctrl H3K4Me3 + E2 NS + E2 1. kb kb +82 kb Figure S1. Estrogen promotes entry of MCF-7 into the cell cycle but does not significantly change activation-associated
ChIP H3K4Me3 enrichment.25.2.15.1.5 H3K4Me3 H3K4Me3 ctrl H3K4Me3 + E2 NS + E2 1. kb kb +82 kb Figure S1. Estrogen promotes entry of MCF-7 into the cell cycle but does not significantly change activation-associated
Supplementary methods Shoc2 In Vitro Ubiquitination Assay
 Supplementary methods Shoc2 In Vitro Ubiquitination Assay 35 S-labelled Shoc2 was prepared using a TNT quick Coupled transcription/ translation System (Promega) as recommended by manufacturer. For the
Supplementary methods Shoc2 In Vitro Ubiquitination Assay 35 S-labelled Shoc2 was prepared using a TNT quick Coupled transcription/ translation System (Promega) as recommended by manufacturer. For the
transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected with the wwp-luc reporter, and FLAG-tagged FHL1,
 Supplementary Data Supplementary Figure Legends Supplementary Figure 1 FHL-mediated TGFβ-responsive reporter transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected
Supplementary Data Supplementary Figure Legends Supplementary Figure 1 FHL-mediated TGFβ-responsive reporter transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected
Supplemental Information
 Supplemental Information ATP-dependent unwinding of U4/U6 snrnas by the Brr2 helicase requires the C-terminus of Prp8 Corina Maeder 1,3, Alan K. Kutach 1,2,3, and Christine Guthrie 1 1 Department of Biochemistry
Supplemental Information ATP-dependent unwinding of U4/U6 snrnas by the Brr2 helicase requires the C-terminus of Prp8 Corina Maeder 1,3, Alan K. Kutach 1,2,3, and Christine Guthrie 1 1 Department of Biochemistry
Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity.
 Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity. (A) Amino acid alignment of HDA5, HDA15 and HDA18. The blue line
Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity. (A) Amino acid alignment of HDA5, HDA15 and HDA18. The blue line
Histone H3 Acetylation Antibody Panel Pack II Base Catalog # C K23A Histone H3K23ac (Acetyl H3K23) Polyclonal Antibody 25 µg 4 C 20 C
 Histone H3 Acetylation Antibody Panel Pack II Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K23A Histone H3K23ac (Acetyl H3K23) Polyclonal Antibody 25 µg 4 C
Histone H3 Acetylation Antibody Panel Pack II Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K23A Histone H3K23ac (Acetyl H3K23) Polyclonal Antibody 25 µg 4 C
Supplementary Materials and Methods
 Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
The poly(a) tail blocks RDR6 from converting self mrnas into substrates for gene silencing
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17036 The poly(a) tail blocks RDR6 from converting self mrnas into substrates for gene silencing
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17036 The poly(a) tail blocks RDR6 from converting self mrnas into substrates for gene silencing
TITLE: Restoration of Wild-Type Activity to Mutant p53 in Prostate Cancer: A Novel Therapeutic Approach
 AD Award Number: W81XWH-05-1-0109 TITLE: Restoration of Wild-Type Activity to Mutant p53 in Prostate Cancer: A Novel Therapeutic Approach PRINCIPAL INVESTIGATOR: James Manfredi, Ph.D. CONTRACTING ORGANIZATION:
AD Award Number: W81XWH-05-1-0109 TITLE: Restoration of Wild-Type Activity to Mutant p53 in Prostate Cancer: A Novel Therapeutic Approach PRINCIPAL INVESTIGATOR: James Manfredi, Ph.D. CONTRACTING ORGANIZATION:
Fig. S1 TGF RI inhibitor SB effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of
 Fig. S1 TGF RI inhibitor SB525334 effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of different concentrations of SB525334. Cells were lysed and
Fig. S1 TGF RI inhibitor SB525334 effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of different concentrations of SB525334. Cells were lysed and
Cdc42 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
Supplemental Materials and Methods
 Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Post-translational modification
 Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
For Research Use Only. Not for use in diagnostic procedures.
 Printed December 13, 2011 Version 1.0 For Research Use Only. Not for use in diagnostic procedures. DDDDK-tagged Protein PURIFICATION GEL with Elution Peptide (MoAb. clone FLA-1) CODE No. 3326 / 3327 PURIFICATION
Printed December 13, 2011 Version 1.0 For Research Use Only. Not for use in diagnostic procedures. DDDDK-tagged Protein PURIFICATION GEL with Elution Peptide (MoAb. clone FLA-1) CODE No. 3326 / 3327 PURIFICATION
supplementary information
 DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
Supplementary Information Supplementary Figure 1
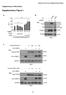 Bararia, Kwok et al, Supplementary Page 1 Supplementary Information Supplementary Figure 1 S1 Bararia, Kwok et al, Supplementary Page 2 Supplementary Figure 1 (cont.) S2 Bararia, Kwok et al, Supplementary
Bararia, Kwok et al, Supplementary Page 1 Supplementary Information Supplementary Figure 1 S1 Bararia, Kwok et al, Supplementary Page 2 Supplementary Figure 1 (cont.) S2 Bararia, Kwok et al, Supplementary
Gα i Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
RheB Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by
 Supplementary Methods and Figures Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer s disease treatment Wenchao Sun 1, Seongsoo Lee 1,2, Xiaoran
Supplementary Methods and Figures Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer s disease treatment Wenchao Sun 1, Seongsoo Lee 1,2, Xiaoran
3.1 The Role of the Enzymatic Activity of Sir2 for Efficient. The Sir proteins are recruited to DNA by site-specific DNA-binding proteins and
 35 3 RESULTS 3.1 The Role of the Enzymatic Activity of Sir2 for Efficient Association of the SIR Complex with DNA The Sir proteins are recruited to DNA by site-specific DNA-binding proteins and subsequently
35 3 RESULTS 3.1 The Role of the Enzymatic Activity of Sir2 for Efficient Association of the SIR Complex with DNA The Sir proteins are recruited to DNA by site-specific DNA-binding proteins and subsequently
Supporting Online Material
 Supporting Online Material 1. Materials and Methods 2. Supporting online figures 3. Supporting online references 1. Material and Methods Plasmid constructs All AvrPphB and protease inactive AvrPphB() constructs
Supporting Online Material 1. Materials and Methods 2. Supporting online figures 3. Supporting online references 1. Material and Methods Plasmid constructs All AvrPphB and protease inactive AvrPphB() constructs
Rab5 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
SUPPLEMENTARY INFORMATION
 (Supplementary Methods and Materials) GST pull-down assay GST-fusion proteins Fe65 365-533, and Fe65 538-700 were expressed in BL21 bacterial cells and purified with glutathione-agarose beads (Sigma).
(Supplementary Methods and Materials) GST pull-down assay GST-fusion proteins Fe65 365-533, and Fe65 538-700 were expressed in BL21 bacterial cells and purified with glutathione-agarose beads (Sigma).
b alternative classical none
 Supplementary Figure. 1: Related to Figure.1 a d e b alternative classical none NIK P-IkBa Total IkBa Tubulin P52 (Lighter) P52 (Darker) RelB (Lighter) RelB (Darker) HDAC1 Control-Sh RelB-Sh NF-kB2-Sh
Supplementary Figure. 1: Related to Figure.1 a d e b alternative classical none NIK P-IkBa Total IkBa Tubulin P52 (Lighter) P52 (Darker) RelB (Lighter) RelB (Darker) HDAC1 Control-Sh RelB-Sh NF-kB2-Sh
Ubiquitin (76 aa) UB genes encode linear fusions of UB either to itself (poly-ub genes) or to other proteins these fusions are cleaved by
 Ubiquitin (76 aa) UB genes encode linear fusions of UB either to itself (poly-ub genes) or to other proteins these fusions are cleaved by deubiquitylases (DUBs) yielding mature Ub deubiquitylase FLAG 3
Ubiquitin (76 aa) UB genes encode linear fusions of UB either to itself (poly-ub genes) or to other proteins these fusions are cleaved by deubiquitylases (DUBs) yielding mature Ub deubiquitylase FLAG 3
EXPERIMENTAL PROCEDURES
 EXPERIMENTAL PROCEDURES Cell culture and antibodies-human colorectal cancer HCT-116 cells, human embryonic kidney 293 cells and human normal breast epithelial cells MCF-10A cells were cultured as recommended
EXPERIMENTAL PROCEDURES Cell culture and antibodies-human colorectal cancer HCT-116 cells, human embryonic kidney 293 cells and human normal breast epithelial cells MCF-10A cells were cultured as recommended
Comparison of different methods for purification analysis of a green fluorescent Strep-tag fusion protein. Application
 Comparison of different methods for purification analysis of a green fluorescent Strep-tag fusion protein Application Petra Sebastian Meike Kuschel Stefan Schmidt Abstract This Application Note describes
Comparison of different methods for purification analysis of a green fluorescent Strep-tag fusion protein Application Petra Sebastian Meike Kuschel Stefan Schmidt Abstract This Application Note describes
1. Cross-linking and cell harvesting
 ChIP is a powerful tool that allows the specific matching of proteins or histone modifications to regions of the genome. Chromatin is isolated and antibodies to the antigen of interest are used to determine
ChIP is a powerful tool that allows the specific matching of proteins or histone modifications to regions of the genome. Chromatin is isolated and antibodies to the antigen of interest are used to determine
StressXpress Ubiquitin Detection Kit. Catalog# SKT-131 (20 Assay Kit) Discovery through partnership Excellence through quality
 Discovery through partnership Excellence through quality StressXpress Ubiquitin Detection Kit Catalog# SKT-131 (20 Assay Kit) Capture and detection of ubiquitinated proteins TABLE OF CONTENTS GENERAL INFORMATION
Discovery through partnership Excellence through quality StressXpress Ubiquitin Detection Kit Catalog# SKT-131 (20 Assay Kit) Capture and detection of ubiquitinated proteins TABLE OF CONTENTS GENERAL INFORMATION
Supplemental Data. Lee et al. Plant Cell. (2010) /tpc Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2.
 Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2. (A) Protein structures of DWA1 and DWA2. WD40 region was determined based on the NCBI conserved domain databases (B, C) Schematic representation
Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2. (A) Protein structures of DWA1 and DWA2. WD40 region was determined based on the NCBI conserved domain databases (B, C) Schematic representation
RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by. 5 AGACACAAACACCAUUGUCACACUCCACAGC; Rand-2 OMe,
 Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Chromatin and Transcription
 Chromatin and Transcription Chromatin Structure Chromatin Represses Transcription Nucleosome Positioning Histone Acetylation Chromatin Remodeling Histone Methylation CHIP Analysis Chromatin and Elongation
Chromatin and Transcription Chromatin Structure Chromatin Represses Transcription Nucleosome Positioning Histone Acetylation Chromatin Remodeling Histone Methylation CHIP Analysis Chromatin and Elongation
Protein Translation Study Label Protein with S35 Methionine in Cells Salma Hasan and Isabelle Plo *
 Protein Translation Study Label Protein with S35 Methionine in Cells Salma Hasan and Isabelle Plo * INSERM U1009, Gustave Roussy, Villejuif, France *For correspondence: isabelle.plo@gustaveroussy.fr [Abstract]
Protein Translation Study Label Protein with S35 Methionine in Cells Salma Hasan and Isabelle Plo * INSERM U1009, Gustave Roussy, Villejuif, France *For correspondence: isabelle.plo@gustaveroussy.fr [Abstract]
supplementary information
 DOI: 10.1038/ncb2172 Figure S1 p53 regulates cellular NADPH and lipid levels via inhibition of G6PD. (a) U2OS cells stably expressing p53 shrna or a control shrna were transfected with control sirna or
DOI: 10.1038/ncb2172 Figure S1 p53 regulates cellular NADPH and lipid levels via inhibition of G6PD. (a) U2OS cells stably expressing p53 shrna or a control shrna were transfected with control sirna or
The role of erna in chromatin looping
 The role of erna in chromatin looping Introduction Enhancers are cis-acting DNA regulatory elements that activate transcription of target genes 1,2. It is estimated that 400 000 to >1 million alleged enhancers
The role of erna in chromatin looping Introduction Enhancers are cis-acting DNA regulatory elements that activate transcription of target genes 1,2. It is estimated that 400 000 to >1 million alleged enhancers
Supplementary Fig.1 Luton
 Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supplementary Figure 1. BES1 specifically inhibits ABA responses in early seedling
 Supplementary Figure 1. BES1 specifically inhibits ABA responses in early seedling development. a. Exogenous BR application overcomes the hypersensitivity of bzr1-1d seedlings to ABA. Seed germination
Supplementary Figure 1. BES1 specifically inhibits ABA responses in early seedling development. a. Exogenous BR application overcomes the hypersensitivity of bzr1-1d seedlings to ABA. Seed germination
Supplementary material for: Materials and Methods:
 Supplementary material for: Iron-responsive degradation of iron regulatory protein 1 does not require the Fe-S cluster: S.L. Clarke, et al. Materials and Methods: Fe-S Cluster Reconstitution: Cells treated
Supplementary material for: Iron-responsive degradation of iron regulatory protein 1 does not require the Fe-S cluster: S.L. Clarke, et al. Materials and Methods: Fe-S Cluster Reconstitution: Cells treated
SUPPLEMENTAL MATERIAL. Supplemental material contains Supplemental Figure Legends and Supplemental Figures 1 to
 SUPPLEMENTAL MATERIAL Supplemental material contains Supplemental Figure Legends and Supplemental Figures 1 to 6. SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Overview of the mechanisms by which
SUPPLEMENTAL MATERIAL Supplemental material contains Supplemental Figure Legends and Supplemental Figures 1 to 6. SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Overview of the mechanisms by which
SUPPLEMENTAL MATERIALS SIRTUIN 1 PROMOTES HYPEROXIA-INDUCED LUNG EPITHELIAL DEATH INDEPENDENT OF NRF2 ACTIVATION
 SUPPLEMENTAL MATERIALS SIRTUIN PROMOTES HYPEROXIA-INDUCED LUNG EPITHELIAL DEATH INDEPENDENT OF NRF ACTIVATION Haranatha R. Potteti*, Subbiah Rajasekaran*, Senthilkumar B. Rajamohan*, Chandramohan R. Tamatam,
SUPPLEMENTAL MATERIALS SIRTUIN PROMOTES HYPEROXIA-INDUCED LUNG EPITHELIAL DEATH INDEPENDENT OF NRF ACTIVATION Haranatha R. Potteti*, Subbiah Rajasekaran*, Senthilkumar B. Rajamohan*, Chandramohan R. Tamatam,
Supporting Information
 Supporting Information Powell and Xu 10.1073/pnas.0807274105 SI Methods Design of Estrogen Receptor (ER) Fusion Constructs for Use in Bioluminescence Resonance Energy Transfer (BRET) Assay. Because the
Supporting Information Powell and Xu 10.1073/pnas.0807274105 SI Methods Design of Estrogen Receptor (ER) Fusion Constructs for Use in Bioluminescence Resonance Energy Transfer (BRET) Assay. Because the
The retroviral vectors encoding WT human SIRT1 or a mutant of SIRT in which a
 Supporting online material Constructs The retroviral vectors encoding WT human SIRT1 or a mutant of SIRT in which a critical histidine in the deacetylase domain of SIRT1 has been replaced by a tyrosine
Supporting online material Constructs The retroviral vectors encoding WT human SIRT1 or a mutant of SIRT in which a critical histidine in the deacetylase domain of SIRT1 has been replaced by a tyrosine
The Transfection Collection TCF/LEF Transient Pack Wnt / -catenin Signaling Pathway Catalog #: 79273
 Data Sheet The Transfection Collection TCF/LEF Transient Pack Wnt / -catenin Signaling Pathway Catalog #: 79273 Background The Wnt / -catenin signaling pathway controls a large and diverse set of cell
Data Sheet The Transfection Collection TCF/LEF Transient Pack Wnt / -catenin Signaling Pathway Catalog #: 79273 Background The Wnt / -catenin signaling pathway controls a large and diverse set of cell
Site-Directed Mutagenesis. Mutations in four Smad4 sites of mouse Gat1 promoter
 Supplement Supporting Materials and Methods Site-Directed Mutagenesis. Mutations in four Smad4 sites of mouse Gat1 promoter were independently generated using a two-step PCR method. The Smad4 binding site
Supplement Supporting Materials and Methods Site-Directed Mutagenesis. Mutations in four Smad4 sites of mouse Gat1 promoter were independently generated using a two-step PCR method. The Smad4 binding site
Supporting Information
 Supporting Information Deng et al. 10.1073/pnas.1515692112 SI Materials and Methods FPLC. All fusion proteins were expressed and purified through a three-step FPLC purification protocol, as described (20),
Supporting Information Deng et al. 10.1073/pnas.1515692112 SI Materials and Methods FPLC. All fusion proteins were expressed and purified through a three-step FPLC purification protocol, as described (20),
Arf6 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Arf6 Activation Assay Kit Catalog Number: 82401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Arf6 Activation Assay Kit Catalog Number: 82401 20 assays NewEast Biosciences 1 Table of Content Product
Supplementary Information
 Supplementary Information Peroxiredoxin-2 and STAT3 form a redox relay for H 2 O 2 signaling Mirko C. Sobotta 1, Willy Liou 1, Sarah Stöcker 1, Deepti Talwar 1, Michael Oehler 1, Thomas Ruppert 2, Annette
Supplementary Information Peroxiredoxin-2 and STAT3 form a redox relay for H 2 O 2 signaling Mirko C. Sobotta 1, Willy Liou 1, Sarah Stöcker 1, Deepti Talwar 1, Michael Oehler 1, Thomas Ruppert 2, Annette
Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg
![Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg](/thumbs/84/90687567.jpg) Supplementary information Supplementary methods PCNA antibody and immunodepletion Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg extracts, one volume of protein
Supplementary information Supplementary methods PCNA antibody and immunodepletion Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg extracts, one volume of protein
Supporting Information
 Supporting Information Drugs Modulate Interactions Between the First Nucleotide-Binding Domain and the Fourth Cytoplasmic Loop of Human P-glycoprotein Tip W. Loo and David M. Clarke Department of Medicine
Supporting Information Drugs Modulate Interactions Between the First Nucleotide-Binding Domain and the Fourth Cytoplasmic Loop of Human P-glycoprotein Tip W. Loo and David M. Clarke Department of Medicine
B Western blot of the p150caf-1 complex
 Loyola et al., Supplementary Figure 1 A MS data of the HP1α complex RIF1 SPT6 SMARCA4 POGZ CAF-1 p150 KAP-1 HP1α 200 116 97.1 CAF-1 p150 tnasp Importin4 B Western blot of the p150caf-1 complex Input mock
Loyola et al., Supplementary Figure 1 A MS data of the HP1α complex RIF1 SPT6 SMARCA4 POGZ CAF-1 p150 KAP-1 HP1α 200 116 97.1 CAF-1 p150 tnasp Importin4 B Western blot of the p150caf-1 complex Input mock
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature10928 Materials and Methods 1. Plant material and growth conditions. All plant lines used were in Col-0 background unless otherwise specified. pif4-101 mutant
SUPPLEMENTARY INFORMATION doi:10.1038/nature10928 Materials and Methods 1. Plant material and growth conditions. All plant lines used were in Col-0 background unless otherwise specified. pif4-101 mutant
Supplementary Information: Materials and Methods. GST and GST-p53 were purified according to standard protocol after
 Supplementary Information: Materials and Methods Recombinant protein expression and in vitro kinase assay. GST and GST-p53 were purified according to standard protocol after induction with.5mm IPTG for
Supplementary Information: Materials and Methods Recombinant protein expression and in vitro kinase assay. GST and GST-p53 were purified according to standard protocol after induction with.5mm IPTG for
5.2 Protein purification
 Purification of a His 6 -tagged Green Fluorescent Protein (GFP). Protein purification.. Purification of a His 6 -tagged Green Fluorescent Protein (GFP) Principle You can add either a N- or C-terminal His
Purification of a His 6 -tagged Green Fluorescent Protein (GFP). Protein purification.. Purification of a His 6 -tagged Green Fluorescent Protein (GFP) Principle You can add either a N- or C-terminal His
Supplementary methods
 Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
GST Fusion Protein Purification Kit
 Glutathione Resin GST Fusion Protein Purification Kit Cat. No. L00206 Cat. No. L00207 Technical Manual No. TM0185 Version 01042012 Index 1. Product Description 2. Related Products 3. Purification Procedure
Glutathione Resin GST Fusion Protein Purification Kit Cat. No. L00206 Cat. No. L00207 Technical Manual No. TM0185 Version 01042012 Index 1. Product Description 2. Related Products 3. Purification Procedure
AP Biology Gene Expression/Biotechnology REVIEW
 AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
Figure S1. Sequence alignments of ATRIP and ATR TopBP1 interacting regions.
 A H. sapiens 204 TKLQTS--ERANKLAAPSVSH VSPRKNPSVVIKPEACS-PQFGKTSFPTKESFSANMS LP 259 B. taurus 201 TKLQSS--ERANKLAVPTVSH VSPRKSPSVVIKPEACS-PQFGKPSFPTKESFSANKS LP 257 M. musculus 204 TKSQSN--GRTNKPAAPSVSH
A H. sapiens 204 TKLQTS--ERANKLAAPSVSH VSPRKNPSVVIKPEACS-PQFGKTSFPTKESFSANMS LP 259 B. taurus 201 TKLQSS--ERANKLAVPTVSH VSPRKSPSVVIKPEACS-PQFGKPSFPTKESFSANKS LP 257 M. musculus 204 TKSQSN--GRTNKPAAPSVSH
Supplementary Information for. Regulation of Rev1 by the Fanconi Anemia Core Complex
 Supplementary Information for Regulation of Rev1 by the Fanconi Anemia Core Complex Hyungjin Kim, Kailin Yang, Donniphat Dejsuphong, Alan D. D Andrea* *Corresponding Author: Alan D. D Andrea, M.D. Alan_dandrea@dfci.harvard.edu
Supplementary Information for Regulation of Rev1 by the Fanconi Anemia Core Complex Hyungjin Kim, Kailin Yang, Donniphat Dejsuphong, Alan D. D Andrea* *Corresponding Author: Alan D. D Andrea, M.D. Alan_dandrea@dfci.harvard.edu
SUPPLEMENTAL FIGURES AND TABLES
 SUPPLEMENTAL FIGURES AND TABLES A B Flag-ALDH1A1 IP: α-ac HEK293T WT 91R 128R 252Q 367R 41/ 419R 435R 495R 412R C Flag-ALDH1A1 NAM IP: HEK293T + + - + D NAM - + + E Relative ALDH1A1 activity 1..8.6.4.2
SUPPLEMENTAL FIGURES AND TABLES A B Flag-ALDH1A1 IP: α-ac HEK293T WT 91R 128R 252Q 367R 41/ 419R 435R 495R 412R C Flag-ALDH1A1 NAM IP: HEK293T + + - + D NAM - + + E Relative ALDH1A1 activity 1..8.6.4.2
IgG TrueBlot Protocol for Mouse, Rabbit or Goatderived Antibodies - For Research Use Only
 IgG TrueBlot Protocol for Mouse, Rabbit or Goatderived Antibodies - For Research Use Only Introduction The IgG TrueBlot for mouse, rabbit, or goat-derived antibodies represents unique series of respective
IgG TrueBlot Protocol for Mouse, Rabbit or Goatderived Antibodies - For Research Use Only Introduction The IgG TrueBlot for mouse, rabbit, or goat-derived antibodies represents unique series of respective
Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit
 Application Note 13 RNA Sample Preparation Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit B. Lam, PhD 1, P. Roberts, MSc 1 Y. Haj-Ahmad, M.Sc., Ph.D 1,2 1 Norgen
Application Note 13 RNA Sample Preparation Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit B. Lam, PhD 1, P. Roberts, MSc 1 Y. Haj-Ahmad, M.Sc., Ph.D 1,2 1 Norgen
Supporting Information
 Supporting Information Üstün and Börnke, 2015 Figure S1: XopJ does not display acetyltransferase activity. Autoacetylation activity in vitro. Acetylation reactions using MBP, MBP-XopJ, GST and GST-HopZ1a
Supporting Information Üstün and Börnke, 2015 Figure S1: XopJ does not display acetyltransferase activity. Autoacetylation activity in vitro. Acetylation reactions using MBP, MBP-XopJ, GST and GST-HopZ1a
Polyclonal ARHGAP25 antibody was prepared from rabbit serum after intracutaneous
 Preparation and purification of polyclonal antibodies Polyclonal ARHGAP25 antibody was prepared from rabbit serum after intracutaneous injections of glutathione S-transferase-ARHGAP25-(509-619) (GST-coiled
Preparation and purification of polyclonal antibodies Polyclonal ARHGAP25 antibody was prepared from rabbit serum after intracutaneous injections of glutathione S-transferase-ARHGAP25-(509-619) (GST-coiled
Figure S1. USP-46 is expressed in several tissues including the nervous system
 Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1154040/dc1 Supporting Online Material for Selective Blockade of MicroRNA Processing by Lin-28 Srinivas R. Viswanathan, George Q. Daley,* Richard I. Gregory* *To whom
www.sciencemag.org/cgi/content/full/1154040/dc1 Supporting Online Material for Selective Blockade of MicroRNA Processing by Lin-28 Srinivas R. Viswanathan, George Q. Daley,* Richard I. Gregory* *To whom
ReliaBLOT TM. IP/Western Blot Reagents Cat. No. WB120, rabbit
 ReliaBLOT TM Introduction: IP/Western Blot Reagents Cat. No. WB120, rabbit ReliaBLOT TM IP/Western Blot Reagents and Procedures (patent pending) provide an improved method for the detection of immunoprecipitated
ReliaBLOT TM Introduction: IP/Western Blot Reagents Cat. No. WB120, rabbit ReliaBLOT TM IP/Western Blot Reagents and Procedures (patent pending) provide an improved method for the detection of immunoprecipitated
His-Spin Protein Miniprep
 INSTRUCTIONS His-Spin Protein Miniprep Catalog No. P2001 (10 purifications) and P2002 (50 purifications). Highlights Fast 5 minute protocol to purify His-tagged proteins from cell-free extracts Screen
INSTRUCTIONS His-Spin Protein Miniprep Catalog No. P2001 (10 purifications) and P2002 (50 purifications). Highlights Fast 5 minute protocol to purify His-tagged proteins from cell-free extracts Screen
Western blot troubleshooting guide
 Specializing in Secondary Antibodies and Conjugates Western blot troubleshooting guide Optimize your Western blotting with Jackson ImmunoResearch Secondary antibodies Troubleshooting for better blots Western
Specializing in Secondary Antibodies and Conjugates Western blot troubleshooting guide Optimize your Western blotting with Jackson ImmunoResearch Secondary antibodies Troubleshooting for better blots Western
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Fig. 1 Kinetics of appearence of the faster migrating form of Bcl-10.
 α-cd3 + α-cd28: Time (min): + + + + + + + + + 0 5 15 30 60 120 180 240 300 360 360 n.s. Supplementary Fig. 1 Kinetics of appearence of the faster migrating form of. Immunoblot of lysates from Jurkat cells
α-cd3 + α-cd28: Time (min): + + + + + + + + + 0 5 15 30 60 120 180 240 300 360 360 n.s. Supplementary Fig. 1 Kinetics of appearence of the faster migrating form of. Immunoblot of lysates from Jurkat cells
Molecular Cloning. Joseph Sambrook. David W. Russell A LABORATORY MANUAL COLD SPRING HARBOR LABORATORY PRESS VOLUME.
 VOLUME Molecular Cloning A LABORATORY MANUAL THIRD EDITION www.molecularcloning.com Joseph Sambrook PETER MACCALLUM CANCER INSTITUTE AND THE UNIVERSITY OF MELBOURNE, AUSTRALIA David W. Russell UNIVERSITY
VOLUME Molecular Cloning A LABORATORY MANUAL THIRD EDITION www.molecularcloning.com Joseph Sambrook PETER MACCALLUM CANCER INSTITUTE AND THE UNIVERSITY OF MELBOURNE, AUSTRALIA David W. Russell UNIVERSITY
Cell extracts and western blotting RNA isolation and real-time PCR Chromatin immunoprecipitation (ChIP)
 Cell extracts and western blotting Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed with lysis buffer. 1 Total cell extracts were separated by SDS-PAGE and transferred to nitrocellulose
Cell extracts and western blotting Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed with lysis buffer. 1 Total cell extracts were separated by SDS-PAGE and transferred to nitrocellulose
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
