SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells
|
|
|
- Gilbert Beasley
- 6 years ago
- Views:
Transcription
1 Article SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells Jeffrey Biernaskie, 1,6, * Maryline Paris, 1 Olena Morozova, 4 B. Matthew Fagan, 5 Marco Marra, 4 Larysa Pevny, 5 and Freda D. Miller 1,2,3, * 1 Developmental and Stem Cell Biology Group, Hospital for Sick Children 2 Department of Molecular Genetics 3 Department of Physiology University of Toronto, Toronto, ON M5G 1X5, Canada 4 Genome Sciences Centre, BC Cancer Agency, Vancouver, BC V5Z 4S6, Canada 5 Department of Genetics, University of North Carolina, Chapel Hill, NC 27599, USA 6 Present address: Department of Comparative Biology and Experimental Medicine, Faculty of Veterinary Medicine, University of Calgary, 3300 Hospital Drive, Calgary, AB T2N 4N1, Canada *Correspondence: jeff.biernaskie@ucalgary.ca (J.B.), fredam@sickkids.ca (F.D.M.) DOI /j.stem SUMMARY Despite the remarkable regenerative capacity of mammalian skin, an adult dermal stem cell has not yet been identified. Here, we investigated whether skin-derived precursors (SKPs) might fulfill such a role. We show that SKPs derive from Sox2 + hair follicle dermal cells and that these two cell populations are similar with regard to their transcriptome and functional properties. Both clonal SKPs and endogenous Sox2 + cells induce hair morphogenesis, differentiate into dermal cell types, and home to a hair follicle niche upon transplantation. Moreover, hair follicle-derived SKPs self-renew, maintain their multipotency, and serially reconstitute hair follicles. Finally, grafting experiments show that follicle-associated dermal cells move out of their niche to contribute cells for dermal maintenance and wound-healing. Thus, SKPs derive from Sox2 + follicle-associated dermal precursors and display functional properties predicted of a dermal stem cell, contributing to dermal maintenance, wound-healing, and hair follicle morphogenesis. INTRODUCTION The skin is a unique organ that undergoes continuous cell turnover and harbors significant regenerative capacity in order to repair environmentally mediated insults. At least some of this regenerative capacity is due to somatic tissue stem cells, including basal layer and hair follicle dermal stem cells (Fuchs, 2009) and melanocyte stem cells (Nishimura et al., 2005). However, a dermal stem cell, responsible for maintaining and repairing the dermis, has not yet been described. The dermis is a complex tissue comprised of many cell types, including dermal fibroblasts, myofibroblasts, adipocytes, blood vessels, nerves, and sensory receptors such as Merkel cells. The dermis also contributes the inductive mesenchymal cells necessary for regulating hair follicle morphogenesis, a cyclic process that occurs continuously throughout the life of many mammals. During embryogenesis, the dermis develops from mesenchymal precursors that generate dermal fibroblasts and adipocytes and produce the inductive hair follicle cells. Thus, by analogy to other somatic tissue stem cells, one possibility is that a multipotent embryonic mesenchymal precursor persists into adulthood, thereby providing the dermal stem cell activity necessary for homeostatic maintenance and regeneration of this tissue. Support for the presence of such a multipotent dermal precursor comes from work showing that, in some animals, dermal cells play a key role in limb regeneration (Muneoka et al., 1986; Kragl et al., 2009) and can directly differentiate into the skeletal cells necessary to form an exoskeleton (Vickaryous and Hall, 2008). In this regard, we previously isolated a multipotent precursor cell from rodent and human dermis that differentiated into mesodermal and peripheral neural progeny including adipocytes, skeletogenic cell types, and Schwann cells (McKenzie et al., 2006). These cells, termed SKPs for skin-derived precursors, displayed properties similar to embryonic neural crest precursors, and within facial dermis were derived from the neural crest (Fernandes et al., 2004). Interestingly, the dermal papillae (DP) of hair follicles appear to comprise one niche for SKPs, based upon coincident patterns of gene expression and upon the finding that cells with properties of SKPs can be cultured from adult whisker follicle papillae (Fernandes et al., 2004; Hunt et al., 2007; Joannides et al., 2004). Because DP mesenchymal cells are essential for hair follicle induction (Jahoda et al., 1984; Oliver, 1967) and because it has been suggested that DP cells might be dermal precursors (Gharzi et al., 2003), we asked whether SKPs might represent a previously unrecognized dermal stem cell. Here, we provide evidence in support of this idea, showing that SKPs derive from Sox2 + follicle-associated precursors and that they can contribute dermal cells for tissue maintenance, wound-healing, and hair follicle morphogenesis. RESULTS Endogenous Hair Follicle DS and DP Cells Express the Stem Cell Gene Sox2 and Generate SKPs when Cultured To investigate whether SKPs originate from endogenous hair follicle dermal cells, we took advantage of our finding that 610 Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
2 Sox2 is expressed by SKPs, as detected by RT-PCR and immunostaining of neonatal murine SKP spheres (Figures 1A and 1B). Immunostaining for Sox2 in neonatal murine back skin showed its expression in follicle DP and lower dermal sheath (DS) cells (Figure 1C). We confirmed this localization in a mouse with EGFP knocked in to the Sox2 locus (Ellis et al., 2004). Within skin, Sox2:EGFP-expressing cells first appeared in the embryonic dermal condensates that precede hair and whisker follicle formation (Figure 1D; Figures S1A and S1B available online), as recently published (Driskell et al., 2009). At birth, when hair follicles were in the anagen growth phase, Sox2:EGFP was expressed in all awl, auchene, and guard hair follicle DS and DP (Figures 1E and 1F). In adulthood, Sox2:EGFP was expressed in DP and DS cells of anagen, but not catagen/telogen follicles (Figures 1G 1I), suggesting that expression was dynamically regulated. Sox2:EGFP was also expressed in dermal cells of adult whisker follicles (Figure S1C) and in a small number of cells in close proximity to the hair follicle bulge (Figure S1D). These latter cells did not express K17, K15, nestin, PDGFRa, the melanocyte marker DCT, or the Schwann cell marker P0 (data not shown). However, a few expressed the dermal precursor marker K5 (Figure S1D). To determine whether Sox2 + follicle cells gave rise to SKPs, we prospectively isolated them by using flow cytometry. Approximately 1% 3% and 0.1% 1% of neonatal and adult back skin cells, respectively, were Sox2:EGFP positive (Figures S2A and S2B). Similar results were obtained with facial skin (Figures S2C and S2D). Most Sox2:EGFP + cells expressed PDGFRa, but not CD34, a marker for bulge dermal cells and dermal fibroblasts (Figure 1J). Sorted Sox2:EGFP + cells from neonatal and adult back and facial skin gave rise to EGFP + SKP spheres when cultured in SKPs conditions, and they were enriched in this ability relative to Sox2:EGFP cells (Figures 1K and 1N). Quantification showed that adult Sox2:EGFP + back skin cells were enriched 4-fold and 10-fold relative to unsorted and EGFP cells (Figures 1L and 1M). SKP spheres generated from Sox2: EGFP + cells expressed the SKPs markers fibronectin, nestin, versican, and a-sma (Figure 1O), and when differentiated for 14 days, generated biii-tubulin + neurons and a-sma + myofibroblasts (Figures 1P and 1Q) that had lost their EGFP expression. Thus, anagen hair follicles are a primary niche for SKP-forming dermal precursors. Prospectively Isolated Sox2:EGFP + Dermal Cells Home to Hair Follicles, Induce Hair Follicle Morphogenesis, and Differentiate into Neural and Dermal Cell Types Because SKPs generate peripheral neural cells, we differentiated sorted Sox2:EGFP + cells versus Sox2:EGFP cells under neural conditions. Only Sox2:EGFP + cells generated nestin + precursors (data not shown) or biii-tubulin + neurons (Figure 2A). We then asked whether the sorted cells could induce hair follicle morphogenesis, as predicted by their anatomical localization, by using an ex vivo hair reconstitution patch assay (Zheng et al., 2005). These experiments showed that Sox2:EGFP +, CD34 cells induced hair morphogenesis when mixed with neonatal C57/Bl6 dermal cells and transplanted beneath the dermis of adult nude mice for 12 days (Figure 2B). In addition, Sox2: EGFP cells were depleted in this inductive ability relative to total dermal cells (Figure 2C). To ask whether Sox2:EGFP + cells could also generate differentiated dermal progeny, we transduced sorted Sox2:EGFP +, PDGFRa +, CD34 back skin cells with an RFP-expressing retrovirus (to allow tracing if Sox2:EGFP expression was lost) and transplanted them into NOD/SCID mouse back skin for 3 weeks. For comparison, we used Sox2:EGFP cells. Remarkably, the Sox2:EGFP-positive, but not -negative, cells homed back to their DP and DS follicle niche, where they appropriately expressed the DP markers NCAM, CD133, and Sox2:EGFP itself (Figures 2D 2G; Figures S2E S2G). Quantification confirmed this selective homing (Figures 2H and 2I). In contrast, Sox2:EGFP + cells that integrated into the interfollicular dermis expressed the dermal fibroblast markers PDGFRa, PDGFRb, fibronectin, and CD34 (Figures 2J and 2K; Figure S2I) but no longer expressed EGFP or CD133 (Figure S2H). Thus, Sox2: EGFP + follicle dermal cells home to a hair follicle niche, induce hair follicles, generate interfollicular dermal cell types, and differentiate into neural cells that are never normally found within skin. Global Gene Expression Analysis of SKPs versus Sox2:EGFP + Dermal Cells To ask whether SKPs maintain properties of their Sox2 + dermal precursor parents, we performed global gene expression analysis, comparing four primary passage neonatal back skin SKP preparations with five sorted neonatal Sox2:EGFP + CD34 back skin cell isolates. RNA samples were analyzed on Affymetrix GeneChip Mouse Gene 1.0 ST Array. Spearman rank correlation analysis demonstrated that the two groups were very similar, with intersample correlations ranging from 0.86 to 0.94 (Figure 3B). For comparison, intrasample analysis demonstrated correlations of and for Sox2:EGFP + and SKP samples, respectively (Figure 3B). We also analyzed a subset of 36 genes previously associated with follicle dermal cells including versican, BMP4, CD133, alkaline phosphatase, and corin. Sox2:EGFP + cells and SKPs expressed these genes at similar levels (Figure 3A). Some genes were, however, expressed at significantly different levels based on the F-statistic with Benjamini-Hochberg multiple testing correction implemented in the LIMMA Bioconductor package (Smyth, 2004). Of 22,102 nonredundant protein-coding genes assayed by the array, 281 and 310 were significantly upregulated at least 2-fold (Figure 3C). Ingenuity pathway analysis demonstrated that most of these were associated with metabolic pathways or growth factor signaling (Figure S3A). SKPs were relatively enriched in genes associated with O-glycan, phenylalanine, tyrosine and tryptophan biosynthesis, and the citrate cycle, and Sox2:EGFP + cells were in genes associated with ERK and PI3- kinase-akt signaling, and glycosphingolipid biosynthesis. These differences were probably due to differences in environment rather than cell identity, because of those transcription factors differentially expressed 2-fold or more (the range was 2- to 4.3- fold), none were cell type specific or associated with dermal or stem cell identity (Figure S3B). Thus, SKPs and Sox2:EGFP + cells are highly similar at the transcriptional level, arguing that culturing does not fundamentally change the identity of the endogenous dermal precursors. Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc. 611
3 Figure 1. Sox2 Is Dynamically Expressed in the Hair Follicle DP and DS (A) Neonatal murine SKP sphere immunostained for Sox2 (red). (B) RT-PCR showing Sox2 expression in two neonatal murine SKP cultures. Embryonic stem cells (mescs) were a positive control. (C) Longitudinal section of a neonatal mouse follicle showing Sox2 + DP cells (red, arrowheads). Right panel shows Sox2-positive (arrows) and -negative (arrowhead) DP cells at higher magnification. (D) Sox2:EGFP + cells (green) in an E15.5 whisker follicle. (E and F) Dorsal skin sections from P2 Sox2:EGFP mice immunostained for EGFP (green), the dermal markers keratin 5 (E) or keratin 15 (F; both red), and the DP marker versican (blue). In (E), arrows denote anagen hair follicles. In (F), arrowheads indicate EGFP + DS cells and arrows EGFP + DP cells. (G I) Dorsal skin sections of adult Sox2:EGFP mice immunostained for EGFP (green) and NCAM (H, red) or keratin 17 (I, red). (G) and (H) show EGFP + cells in anagen hair follicles, with arrows denoting the DP and arrowheads the lower DS. (I) shows a telogen hair follicle where the DP (indicated by hatched lines) is negative for Sox2:EGFP expression. (J Q) Sox2:EGFP + hair follicle cells proliferate in FGF2 and EGF to generate multipotent SKP spheres. (J) Flow cytometric analysis of neonatal Sox2:EGFP backskin cells sorted for EGFP (x axis) and PDGFRa or CD34 (y axis). Red boxes indicate gates for prospective isolation experiments. 612 Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
4 SKPs Differentiate into Dermal Cell Types within Intact Skin To ask whether SKPs were also functionally similar to Sox2: EGFP + dermal precursors, we transplanted neonatal YFPexpressing murine or adult GFP-expressing rat SKPs into adult NOD/SCID mouse back skin. At 2 3 weeks posttransplant, both populations of genetically tagged cells were located throughout the dermis (Figure 4A). These cells were morphologically similar to the endogenous fibroblasts and expressed the dermal fibroblast markers PDGFRa, collagen type I, vimentin, fibronectin (Figures 4B 4D), and fibroblast-specific antigen (data not shown) (>90% expressed fibroblast-specific antigen or collagen type I, 73% PDGFRa). Some also coexpressed the hyaluronic acid receptor, CD44 or the myofibroblast protein a-sma (Figure 4E) (80% and 7% expressed a-sma in the upper and lower dermis, respectively). Some transplanted cells were present in the adipocyte-rich hypodermis, where they expressed fatty acid binding protein and displayed adipocyte morphology (Figure 4F). Genetically tagged transplanted cells were never observed within the dermis. Thus, SKPs, like Sox2 + dermal precursors, differentiate into dermal cell types. SKPs Actively Integrate into a Hair Follicle Niche Intriguingly, some transplanted SKPs homed back to the follicle DP and DS (Figures 4A and 4G), where they appropriately expressed the DP markers versican (Figure 4H; Figure S4A), p75ntr, and NCAM (data not shown), or the DS marker a-sma (Figure 4I). Some transplanted cells in the DS also expressed the proliferation marker Ki67 (Figure 4J), consistent with the fact that DS but not DP cells proliferate. Transplanted cells did not differentiate into Pax3 + or tyrosinase + melanocytes (Figure 4H; Figure S4B). The specificity of this integration was shown by transplanting neonatal murine YFP + forebrain neurospheres (NSCs), adult rat GFP + bone marrow MSCs, and dermal fibroblasts from nonhairy plantar skin. The NSCs survived poorly and never associated with follicles, whereas MSCs and plantar skin dermal fibroblasts survived but were never found within the DP (Figures 4K and 4L; Figures S4C S4E). To determine whether follicle cycling alters this homing behavior, we dlated or shaved back skin of NOD/SCID mice (dlation induces follicles to enter anagen) prior to transplantation. Dlation caused adult rat SKPs to integrate into 2- to 3- fold more hair follicles than did shaving (28.5 ± 4.9 versus 8.0 ± 1.6 follicles), with the DP of each positive follicle containing 6- fold more transplanted cells (10.16 ± 1.0 versus 1.52 ± 0.14) (Figures 4N and 4O). Similar results were obtained with neonatal murine SKPs (Figure 4M). Finally, we asked whether SKPs hair follicle integration was modified by skin wounding. Neonatal murine YFP + SKPs were transplanted adjacent to punch wounds on NOD/SCID mouse back skin. 3 4 weeks later, many transplanted cells were present within the scar, where they expressed fibroblastspecific antigen, collagen type 1, fibronectin, and the myofibroblast protein a-sma (Figures 4P 4R; Figures S4F and S4G) (16% expressed high a-sma). Interestingly, transplanted cells were also present in DP and DS of immature-appearing hair follicles (Figures 4S and 4T), with the DP cells appropriately expressing versican (Figure 4T) and p75ntr (data not shown), suggesting that SKPs contribute to new follicle formation in wounded skin. SKPs Regulate Hair Follicle Morphogenesis To ask whether SKPs induce hair follicle morphogenesis, as do Sox2:EGFP + cells, we performed patch assays. As positive controls, we used neonatal dermal cells, which generated many hair follicles (Figures 5A and 5B), and as negative controls MSCs or NSCs, which generated no or very few follicles (Figures S5A and S5B). Interestingly, adult rat GFP + SKPs were highly enriched for hair follicle-inductive ability (Figures 5E and 5F), generating follicles where the entire DS and DP was comprised of genetically tagged cells (Figures 5C and 5D). Neonatal YFPexpressing murine SKPs also induced follicle formation (data not shown). We next performed in vivo experiments, taking advantage of the finding that the size of the DP determines follicle size (Ibrahim and Wright, 1982). GFP + adult rat SKPs were injected into dlated adult NOD/SCID mouse back skin and analyzed after 8 weeks. Many transplanted cells differentiated into interfollicular dermal fibroblasts (Figure 5G), and many comprised the entire DP and DS of correctly oriented follicles (Figures 5G and 5H). Remarkably, relative to endogenous murine hairs, hairs induced by rat SKPs were longer (10.41 ± 0.23 mm versus 7.96 ± 0.11 mm; p < ) and had increased follicle bulb diameter ( ± 4.99 mm versus ± 2.51 mm; p < 0.01) and hair fiber width (49.26 ± 0.87 mm versus ± 0.83 mm; p < 0.001) (n = 8, with 3 animals quantified) (Figures 5I and 5J). Although many of these follicles were in anagen (Figure 5H), some were in catagen/telogen phase (Figure 5K), indicating that follicle-associated SKPs cycle with their newly induced hairs. To confirm that rat SKPs induced larger follicles, we performed patch assays. Adult rat SKPs instructed mouse dermal cells to generate larger follicles than did mouse dermal cells, with an almost 2-fold increase in bulb diameter (Figures 5L 5N). Confocal microscopy demonstrated that the increased rat SKP follicle size correlated with almost four times more NCAM + DP cells (Figures 5O 5Q) (88% of these cells were GFP + ). (K) Sox2:EGFP-positive (top) and -negative (bottom) neonatal backskin cells cultured for 7 days in SKPs conditions. Arrows denote SKP spheres. (L and M) Quantification (M) of SKP spheres generated from sorted adult Sox2:EGFP + (L) versus Sox2:EGFP (M) back skin cells in experiments similar to that in (K). Total back skin cells from the same mice were used for comparison (*p < 0.05, **p < 0.001; n = 4). Error bars represent SEM. (N) Sox2:EGFP-positive (top) and -negative (bottom) cells sorted from adult facial skin and cultured in SKPs conditions for 7 days. (O) SKP spheres generated from Sox2:EGFP + adult back skin cells immunostained for EGFP (green), and the SKP markers fibronectin (blue) and nestin (red; merge is on right) or versican (blue) and a-sma (red; merge is on the right). (P and Q) Sorted adult Sox2:EGFP + back skin (P) or facial skin (Q) cells were cultured as SKPs, differentiated under neural conditions for days, and immunostained for the neuronal marker biii-tubulin (red in both) and a-sma (green in Q). In some panels, cells were counterstained with Hoechst (blue), as indicated. Scale bars represent 25 mm in (A) and (C, left), 10 mm in (C, right), 50 mm in (E) (H), and 100 mm in (D), (I), (K), (L), and (N) (Q). See also Figure S1. Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc. 613
5 Figure 2. Sox2:EGFP Prospectively Identifies Endogenous Dermal Precursors that Induce Hair Follicles, Home to a Hair Follicle Niche, and Differentiate into Neural and Dermal Cell Types (A) Sorted Sox2:EGFP-positive and -negative neonatal back skin cells differentiated for 12 days under neural conditions and immunostained for biii-tubulin (red). Total cells were sorted but gated only for live cells. (B) Patch assays with sorted Sox2:EGFP +, CD34 neonatal back skin dermal cells. The left panel shows brightfield and the right fluorescence illumination. Arrowheads denote EGFP + DP. (C) Number of hair follicles generated in patch assays with total or Sox2:EGFP neonatal dermal cells (n = 3). Error bars represent mean ± SD. (D K) Sorted, uncultured Sox2:EGFP +, CD34, PDGFRa + (D, F K), or Sox2:EGFP (E) neonatal back skin cells were transplanted into adult NOD/SCID mouse back skin and analyzed after 2 weeks. All sorted cells were infected with an RFP-expressing retrovirus. (D) Immunostaining for RFP (red) and keratin 5 (green). Arrows denote follicle DP containing transplanted cells, with the right panel at higher magnification. (E) Immunostaining for RFP (red) on skin transplanted with Sox2:EGFP cells. (F and G) High-magnification confocal images of hair follicles immunostained for RFP (F, G, red) and Sox2:EGFP (F, green) plus the DP marker CD133 (F, blue) or keratin-5 (G, green) plus PDGFRb (G, blue). In (F) the arrowhead and arrow denote Sox2:EGFP + and CD133 + cells, respectively. In (G) the arrow denotes RFP +, PDGFRb + cells in the DS. ORS, outer root sheath. 614 Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
6 Figure 3. Microarray Analysis Demonstrates that SKPs and Sox2:EGFP + Cells Are Transcriptionally Similar Microarray gene expression profiling of sorted Sox2:EGFP +, CD34 neonatal dermal cells versus primary passage neonatal murine SKPs. (A) Heat map showing the relative expression levels of 36 hair follicle dermal genes in 5 independent Sox2:EGFP cell isolates (red bar on the top left) and 4 independent SKP cultures (blue bar on the top right). Relative expression levels are color-coded as per the color key. The cluster analysis on top shows that some Sox2:EGFP + cell samples are more related to SKPs than they are to each other. (B) Spearman rank correlations were computed for every pair of microarray experiments. The resulting color-coded correlation matrix reveals the similarity of the transcriptomes of SKPs and Sox2:EGFP + cells. (C) Venn diagram illustrating that 281/22,102 and 310/22,102 genes are upregulated by at least 2-fold in SKPs and Sox2:EGFP cells, respectively, while an additional 2418 and 2291 genes are significantly upregulated by less than 2-fold. See also Figure S3. SKPs Clonally Differentiate into Dermal Cell Types and Induce Hair Follicle Morphogenesis To ask whether single SKPs could both induce follicle morphogenesis and differentiate into dermal cell types, as expected for a dermal stem cell, we generated clones of adult rat SKPs. Patch assays showed that, of seven clonally derived rat SKP lines passaged at least six times (approximately 8 12 weeks in culture), five induced hair follicle formation (Figures 6A and 6B). Indeed, when 50 spheres from one clone were mixed with total neonatal murine skin cells, 30 ± 2 hair follicles had DP entirely comprised of GFP + SKPs. This activity was persistent; one clone induced follicle formation after 11 months in culture, albeit inefficiently (Figure 6C). Consistent with these results, three of three clones transplanted into adult NOD/SCID mouse skin reconstituted hair follicle DP and DS in vivo (Figure 6D). Transplanted clone cells also differentiated into fibronectin and vimentin + interfollicular dermal fibroblasts and a-sma + myofibroblasts (Figures 6D and 6E). Thus, single SKPs were multipotent with regard to these dermal activities. SKPs Maintain Their Multipotency and Capacity to Self-Renew within the Hair Follicle Niche and Serially Reconstitute Hair Follicles One of the most striking assays of in vivo stem cell functionality is serial repopulation by hematopoietic stem cells. We therefore asked whether follicle-derived DS and DP cells could self-renew as SKPs and sequentially induce and repopulate hair follicles (Figure 6F). Hair follicles were generated in patch assays with adult rat GFP + SKPs, and cells from these follicles (Figure 6G) were cultured in SKPs conditions; days later, genetically tagged spheres were observed (Figure 6H). When these spheres were passaged and put back into patch assays, they again induced follicle formation (Figure 6I). With this approach, we (H and I) Number of hair follicles (H) or follicle DP (I) containing RFP + transplanted cells as shown in (D) (G). **p < Error bars represent SEM. (J and K) Immunostaining of interfollicular dermis for RFP (red) and CD34 (J, blue) plus keratin 5 (J, green) or fibronectin (K, pseudocolored green, center panel) plus PDGFRa (pseudocolored green, right panel). Arrows show cells positive for RFP and CD34 (J), or for RFP, fibronectin, and PDGFRa (K). hf, hair follicle. Nuclei were stained with Hoechst (blue), as indicated. Scale bars represent 100 mm in (A), (D), and (E); 25 mm in (F) and (J); and 10 mm in (G) and (K). See also Figure S2. Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc. 615
7 Figure 4. SKPs Regenerate the Dermis and Integrate into a Hair Follicle Niche upon Transplantation into Adult Skin (A and B) Back skin transplanted with YFP-tagged neonatal mouse SKPs 2 weeks earlier and immunostained for YFP (green) and PDGFRa (B, red). Arrows and arrowheads in (A) show transplanted cells in the interfollicular dermis and the DP and DS of follicles, respectively. Arrows in (B) denote double-labeled cells. (C E) GFP-expressing adult rat SKPs transplanted into dlated adult NOD/SCID mouse dermis 21 days earlier, and immunostained for GFP (green) and collagen type 1 (C, red), vimentin and fibronectin (D, red and blue, respectively), or a-sma (E, red). Arrows denote double-labeled (C, E) or triple-labeled (D) cells. (F) GFP-tagged cells (green) within the hypodermis expressing the adipocyte marker fatty acid binding protein (red, arrow). (G J) Hair follicles containing neonatal murine YFP + SKPs 2 4 weeks posttransplantation as in (A). (G) Hair follicle with YFP-labeled cells (green) in the DP (arrow) and DS (arrowhead). (H) Follicle triple-labeled for YFP (green), the DP marker versican (blue), and the melanoblast marker pax3 (red). The arrow and arrowhead indicate the DP and DS. (I and J) Cross-sections of follicles showing transplanted cells (green) in the DS (arrow in I, arrowhead in J) expressing a -sma (I, red) or Ki67 (J, red), but not e-cadherin (I, blue, an dermal marker). In (J), DP cells (arrow) are positive for versican (blue). (K and L) Telogen follicles in skin transplanted 4 weeks earlier with GFP-tagged rat plantar dermal fibroblasts (K) or SKPs (L), immunostained for GFP (green) and keratin 15 (red). Only SKPs are in the DP (arrows, denoted by hatched lines). (M) Representative hair follicles containing neonatal murine YFP + SKPs (green) 3 weeks after skin was shaved or dlated. Hatched lines denote the DP. 616 Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
8 serially generated SKP spheres and reinduced hair follicles up to three times (Figure 6J). To ask whether these follicle-derived SKPs also maintained their multipotentiality, we took four different approaches. First, we transplanted them into adult NOD/SCID mouse back skin for 4 weeks. Many transplanted cells differentiated into PDGFRa and collagen type I + interfollicular dermal fibroblasts (Figures 6K 6M), and some integrated into the DS and DP of endogenous follicles (Figure 6K), where they expressed versican and NCAM in the DP and fibronectin in the DS (data not shown). Second, we differentiated cells in culture conditions that support neural and mesodermal differentiation of neonatal SKPs (Fernandes et al., 2004). Follicle-derived SKPs generated adipocytes, a-sma + myofibroblasts or smooth muscle cells, nestin + precursors, and biiitubulin + neurons (Figures 6N 6Q). Third, we transplanted follicle-derived SKPs distal to a sciatic nerve crush in NOD/ SCID mice, an environment that instructs naive SKPs to differentiate into Schwann cells (McKenzie et al., 2006). Immunocytochemistry of the distal nerve 6 weeks posttransplant identified GFP + cells closely aligned with axons expressing the Schwann cell markers P0 and p75ntr (Figures 6R and 6S). Finally, we transplanted follicle-derived SKPs into the embryonic chick neural crest migratory stream at HH stage 18, as we have done for total neonatal SKPs (Fernandes et al., 2004). Analysis 3 days later showed that follicle-derived SKPs migrated out of the neural tube into neural crest targets such as the spinal nerve and DRGs, similar to murine SKPs and adult rat clonal SKPs (Figure 6T). Intriguingly, some total and follicle-derived SKPs migrated to the presumptive dermis, and at late time points, some of these expressed the DP marker versican (Figure 6U). Thus, follicleassociated SKPs display properties of dermal stem cells. Lineage Tracing of Transplanted Follicles Demonstrates that Follicular DP and DS Cells Contribute to Dermal Maintenance and Wound Healing To ask whether the endogenous Sox2 + dermal precursors are recruited out of their niche to contribute differentiated dermal cells, we performed punch wounds on Sox2:EGFP mice. At 36 hr, Sox2:EGFP + cells were limited to hair follicles. However, by 72 hr, EGFP + cells were seen exiting hair follicles and streaming into the interfollicular dermis toward the wound site (Figures 7A 7H). Some of these emigrating cells coexpressed the DP/DS marker NCAM, but many were negative (Figures 7C and 7E), indicating that they were losing their follicle-associated phenotype. To further define this phenomenon, we used a transplantationbased lineage tracing approach. Genetically tagged hair follicles were made in patch assays with either rat GFP + SKPs or neonatal dermal cells (Figure 7I) and transplanted under adult nude mouse back skin. Over the ensuing 4 weeks, tagged follicles integrated and grew to generate small patches of hair (Figure 7J). Analysis at this time point showed that many GFPtagged cells were retained within engrafted hair follicles, but that many had also migrated into the adjacent interfollicular dermis where they expressed fibroblast-specific antigen, CD34, and collagen type I (Figures 7K 7N; Figure S6). Similar results were obtained with both follicle populations. In some animals, we also injured the skin adjacent to transplanted SKP-derived hairs with a punch wound (Figure 7O). Analysis 4 weeks later showed that GFP + cells had migrated into the regenerated, newly healed dermis where they expressed CD44 and collagen type I (Figures 7P 7R). Thus, DP/DS cells can contribute differentiated cells to the dermis. DISCUSSION These studies support a number of major conclusions. First, our studies identify a Sox2 + endogenous dermal precursor within the follicle DP and DS that, when prospectively isolated, homes back to a hair follicle niche, induces hair follicle morphogenesis, and displays neural and dermal potential. Second, we show that these endogenous precursors generate SKPs when cultured, and that these two cell populations are very similar with regard to their transcriptome and their functional properties when transplanted into the dermis. Third, we demonstrate that SKPs can clonally reconstitute the dermis and induce hair follicle morphogenesis, properties predicted of a dermal stem cell. Fourth, the serial reconstitution experiments show that SKPs maintain their multipotentiality and their ability to self-renew within their hair follicle niche and that they can serially induce hair follicle formation. Finally, our follicle graft/lineage tracing studies argue that Sox2 + dermal precursors can be recruited out of their niche to contribute differentiated dermal cells. Together, these experiments provide evidence for a hair follicle-associated dermal precursor that functions to regulate hair morphogenesis and to maintain the intact or injured dermis and that, when cultured, generate SKPs that display all the predicted properties of multipotent dermal stem cells. On the basis of these findings, we propose that these adult dermal precursors derive from a subset of embryonic mesenchymal precursors within the presumptive dermis that interact with dermal precursors and undergo a transition to a Sox2 + precursor state, as recently suggested (Driskell et al., 2009). We also propose that hair follicles provide a niche for maintenance of the mesenchymal precursor phenotype, and that in adult life, these Sox2 + follicle cells both regulate follicle morphogenesis and serve as a reservoir of dermal precursor activity, contributing dermal cells for the normal and injured dermis. The broader mesenchymal potential displayed by SKPs (Lavoie (N and O) Number of follicles containing GFP + cells (N) or GFP + cells within the DP of individual follicles (O) after transplantation of adult rat GFP + SKPs into dlated (n = 4) versus shaved (n = 4) skin. **p < 0.01, ***p < Error bars represent SEM. (P T) Skin 3 (P R) or 4 (S and T) weeks after transplantation of neonatal YFP-tagged murine SKPs (green in all panels) adjacent to a back skin punch wound. In (P), transplanted cells are present at the site of injection (arrows) and within the regenerated tissue (denoted by dashed lines). (Q and R) Transplanted cells immunostained for fibroblast-specific antigen (Q, red, arrows) or for a-sma and fibronectin (R, arrowhead, red and blue, respectively). (S and T) Transplanted cells are also present in peg-like hair follicles at the boundary of the wound (S, arrowheads) within the DP and DS (T, arrow and arrowheads). Cells in the DP express versican (T, red). Some sections were counterstained with Hoechst (blue) or fluorescent Nissl (red), as indicated. Epi, dermis. Scale bars represent 200 mm in (A), (P), and (S); 16 mm in (B) (E); 25 mm in (G) (M) and (Q); and 50 mm in (F), (R), and (T). See also Figure S4. Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc. 617
9 Figure 5. SKPs, but Not Other Adult Stem Cells, Reconstitute a Hair Follicle Niche and Instruct Epidermal Cells to Generate Hair Follicles (A and B) Patch assays at 12 days, combining neonatal C57/Bl6 murine dermal cells with (A) and without (B) 10 6 neonatal rat dermal cells, visualized by phase illumination (arrowheads denote hair follicles, which are black). (C and D) Combined phase and fluorescence illumination of similar patch assays with 10 6 GFP-expressing adult rat SKPs (green) (arrow and arrowheads in D denote the DP and DS, respectively). (E) Number of hair follicles with GFP + or YFP + DP as shown in (C) and (D), with 10 6 adult rat GFP + SKPs or MSCs, or neonatal murine YFP + dermal cells or NSCs. **p < relative to dermis only, ***p < relative to dermis only and to dermis (n = 6 for adult rat SKPs, and 3 for other groups). Error bars represent SEM. (F) Quantification of hair follicle numbers as in (E), where adult rat SKPs were compared to neonatal rat dermal cells. ***p < relative to dermal cells (n = 3 and 4 each for 10 5 and 10 6 cells, respectively). Error bars represent SEM. (G K) Adult GFP-expressing rat SKPs (green) were injected into the back skin of adult NOD/SCID mice for 8 weeks. (G and H) Immunostaining for GFP (green). Arrows indicate transplanted cells in hair follicle anagen DP. (I and J) Chimeric rat/mouse hairs were longer (I) and thicker (J) than endogenous pelage hairs. (K) Some transplanted GFP + cells comprised the DP of telogen hair follicles (arrows). (L and M) Hair follicles from patch assays where 10 6 mouse neonatal dermal cells (L) or dissociated adult rat GFP-expressing SKPs (M, green) were used. 618 Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
10 et al., 2009) and whisker follicle papillae (Jahoda et al., 2003; Wojciechowicz et al., 2008) suggests that they may also serve as precursors for adipocytes and/or blood vessel smooth muscle cells. That DP mesenchymal cells induce hair follicle morphogenesis is well established (Jahoda et al., 1984; Oliver, 1967). A precursor function for DP cells has also been previously suggested based upon their ability to contribute cells to healing wounds upon transplantation and to generate nondermal cell types when cultured (Waters et al., 2007). The data we present here provide strong evidence that this is indeed the case. Moreover, our finding that both DP and DS cells express Sox2 suggests that these anatomically distinct cells may in fact represent a common precursor population. In support of this idea, previous studies showed that cells move between the DS and DP (Tobin et al., 2003) and that lower DS cells can regenerate the DP (McElwee et al., 2003). We suggest that because DP cells rarely divide, they may represent a quiescent stem cell population, with DS cells functioning as a transit-amplifying population that ultimately provides differentiated cell types. Alternatively, DS cells may represent dermal stem cells and provide cells both for dermal differentiation and for the DP, where the cells acquire their inductive properties and function to regulate follicle morphogenesis. One of the surprising findings reported here is the ability of transplanted Sox2:EGFP + cells and the SKPs they generate to home back to their hair follicle niche. Interestingly, a hint of this activity was previously obtained in experiments transplanting GFP + DP/DS cells into ear skin (McElwee et al., 2003). These findings may reflect a previously undescribed mechanism for recruiting DS cells into the DP and/or for allowing re-establishment of the DS/DP when hair follicles re-enter the hair growth cycle. Clearly, SKPs are attracted to cells that are similar, as seen in patch assays where single dissociated SKP cells aggregated to form structures that nucleated hair follicle formation. Although the relevant attractive signal is unknown, evidence that it is cell intrinsic comes from our data showing that more rat than mouse cells aggregate to form the DP, even within the same tissue environment. A second surprising finding is that Sox2:EGFP expression was regulated during the hair cycle; DP and DS cells did not express Sox2 during catagen or telogen. Although some cells exit the DP during hair follicle regression (Tobin et al., 2003), many are retained, implying that at least some precursors regulate Sox2 expression in response to their external microenvironment. Sox2 is associated with maintenance of a stem cell state (Avilion et al., 2003), so this may suggest that the niche regulates the stemness of these dermal precursors. However, a recent study also showed Sox2:EGFP expression in the DP of developing hair follicles and provided evidence that the level of expression was associated with follicle type, with small zig-zag hairs expressing low or no Sox2:EGFP (Driskell et al., 2009). Thus, Sox2 expression may be associated with multiple functional properties, and it will be important to determine whether Sox2 is a readout for different functional states and/or whether it actually regulates the properties of these dermal precursors. One key question in the stem cell field has been whether multipotent precursors like SKPs actually reflect endogenous precursor cells, or whether they are cells that dedifferentiate in culture. Data presented here showing that prospectively isolated Sox2 + follicle DP and DS cells give rise to SKPs, and that these Sox2 + cells are very similar to SKPs with regard to their transcriptome and functional properties, argue that SKPs accurately reflect this endogenous precursor population. This finding, together with our data showing that SKPs display all of the properties predicted of dermal stem cells, provide strong support for the idea that the Sox2 + precursors we have identified here are endogenous dermal stem cells that serve to induce hair morphogenesis and maintain the dermis. EXPERIMENTAL PROCEDURES Tissue Culture SKPs were generated from dorsal back skin of neonatal (P1 7) YFP-expressing mice (Hadjantonakis et al., 1998) (Jackson Laboratory) or P0 P3 and adult (5 10 week) GFP-expressing Sprague Dawley rats (SLC, Japan) and cultured as described (Fernandes et al., 2004) at 1,000 20,000 cells/ml. Spheres were passaged at least twice for transplants. For clones, secondary SKPs were grown at 1,000 cells/ml, and single clonal spheres were isolated and expanded at least 5 weeks. MSCs were isolated from adult GFP-expressing rat bone marrow (generous gift of Fabio Rossi, U.B.C.) and cultured at 50,000 cells/ml in Mesencult human MSC medium plus 10% FBS (Stem Cell Technologies). Neurospheres were made from P1 lateral ventricles as described (Reynolds and Weiss, 1992). SKPs were differentiated in vitro under previously defined conditions for neurons, Schwann cells, and SMA + cells (Biernaskie et al., 2006; McKenzie et al., 2006). Adipocytes were differentiated in DMEM-F12 containing 1% penicillin streptomycin, 10% FBS, dexamethasone (1 mm, Sigma), isobutylmethylxanthine (1 mm, Sigma), and insulin (20 mg/ml, GIBCO/Invitrogen). In Vivo Experiments For transplants, to 10 6 passaged, dissociated murine (n = 8) or rat (n = 12) SKPs were injected into dlated (n = 11) or shaved (n = 10) back skin of adult NOD/SCID mice (Charles River Laboratories). Alternatively, a full thickness 4 mm wide biopsy punch was made, and SKPs were injected adjacent to the wound (n = 10). Controls were performed with MSCs (n = 4) or NSCs (n = 4). For in vivo hair follicle morphogenesis experiments, adult rat SKPs were transplanted (n = 8), and quantification was performed with hairs from each of three experiments. Awl hairs were used for width measurements. Punch wounds were performed on Sox2:EGFP mice (n = 4) as for transplants. Nerve transplants were performed as described (McKenzie et al., 2006). In ovo transplants were performed as described (Fernandes et al., 2004) at Hamilton/Hamburger stage 18 with analysis at stage All procedures were approved by the Hospital for Sick Children Animal Care (N) Hair bulb diameter in follicles similar to those in (L) and (M) (n = 2 independent experiments). **p = (O Q) Hair follicles were generated in patch assays with either YFP + mouse or GFP + rat SKPs. (O and P) A single hair follicle derived from rat SKPs at low (O) and high (P) magnification, immunostained for GFP (green) and NCAM (red), which marks DP and DS cells, and Hoechst to delineate cell nuclei. (Q) Number of cells in the DP of follicles generated from mouse versus rat SKPs, as shown in (P). Nuclei were stained with propidium iodide (red), fluorescent Nissl (red), or Hoechst (blue), as indicated. Epi, dermis; hypo, hypodermis. Scale bars represent 500 mm in (A) and (C); 1 mm in (B); 50 mm in (D) and (H); 100 mm in (G), (K) (M), and (O); and 20 mm in (P). See also Figure S5. Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc. 619
11 A B C D E dp dp SKP fibronectin SKP vimentin F G H 14d I J SKP hoechst SKP pdgfra hoechst SKP collag I hoechst K L M hypo N oil red O α sma hoechst O P nestin hoechst β-tub hoechst Q SKP p75 hoechst R S T SKP P0 U SKP hoechst versican Figure 6. SKPs Clonally Reconstitute the Dermis and Induce Hair Follicle Formation and Can Serially Reconstitute the Hair Follicle Niche (A E) Analysis of one adult rat GFP + SKP clone in patch assays (A C) or by transplantation into adult mouse dermis (D, E). After 2 4 (A, B) or 11 (C) months of culturing, clonal SKPs (green) induced the formation of hair follicles, where they comprised the DS and DP (arrowheads in A, arrow in C). (D, E) After 12 weeks in culture, cells from the same clone were transplanted and skin immunostained for GFP (green) and fibronectin (D, red, arrowheads) or vimentin (E, red, arrows). Arrow in (D) denotes the DP. (F U) SKPs isolated from their hair follicle niche self-renew, serially reconstitute hair follicles, and remain multipotent. (F) Schematic showing the serial reconstitution assay of hair morphogenesis. (G and H) A follicle isolated from a patch assay with adult rat GFP-labeled cells. Tagged cells isolated from the boxed area generated GFP + SKP spheres (H, arrows) after 12 days in culture as seen by phase (top) and fluorescence (bottom) illumination. (I) Cells from these follicle-derived spheres induced formation of secondary hair follicles in the patch assay (arrows). (J) In tertiary follicle reconstitutions, follicle-derived SKPs generated hair follicles, but many also aggregated into DP-like structures surrounded by black melanocytes (arrow). 620 Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
12 Committee and were within the guidelines of the Canadian Council of Animal Care. Hair Follicle Induction Assay 10 6 SKPs (n = 6 adult rat, 4 neonatal murine), neonatal (n = 2), or adult (n = 3) rat dermal cells, neonatal murine NSCs (n = 3), or adult rat MSCs (n = 3) were mixed with 10,000 neonatal dermal aggregates ( to single cells) and injected into adult nude mouse (nu/nu; Charles River) back skin for days as described (Zheng et al., 2005). SKPs, MSCs, and NSCs were passaged 1 5 times. For sorted cells, cells were mixed with 10,000 dermal cells. Individual bulb diameters (50 follicles/graft; n = 2 grafts each) were measured with Volocity acquisition software (Improvision) and a Leica MZ16F stereomicroscope. To quantify DP cell number, confocal image stacks spanning the entire DP were collected and all NCAM + and NCAM +, GFP + cells were counted. For serial reconstitutions, patches were digested in collagenase (Type XI) at 37 C for 1 hr or follicles with GFP + DP (n = hairs/experiment) were individually dissected and digested with 0.25% trypsin-edta; similar results were obtained with both approaches (n = 3 adult, 2 neonate rat SKPs isolations). Single cells were liberated by gentle trituration and cultured in SKPs medium at 2,000 to 20,000 cells/ml for 14 days. Follicle Grafting Experiments Follicles generated in patch assays were dissected as above and transplanted into a back skin incision on adult Nude or NOD/SCID mice, and those that grew were analyzed at 3 4 weeks (n = 4 adult rat SKPs, 2 neonatal rat dermal cells). Alternatively, punch wounds were made adjacent to the graft at 3 weeks, and skin analyzed 4 weeks later (n = 3). data were analyzed for significant differential expression via the LIMMA Bioconductor package, with the F-statistic with Benjamini-Hochberg (BH) multiple testing correction implemented in the ebayes function. Genes with BH-corrected p value < 0.01 were considered statistically significant (Smyth, 2004). Differentially expressed genes were analyzed with Ingenuity Pathway Analysis software ( for functional enrichment. Immunocytochemistry and Histology Antibodies are listed in Supplemental Experimental Procedures. Immunocytochemistry was performed as described (Fernandes et al., 2004, 2006; McKenzie et al., 2006), and acquisition and colocalization was confirmed by collecting z-stacks comprised of 0.2 mm to 1 mm optical slices with a Hamamatsu spinning disk confocal microscope fitted to a Zeiss Axiovert 200 inverted microscope (Quorum Technologies). Statistics All data are represented as mean ± SEM. Data (with the exception of the microarray analyses) were analyzed with two-tailed t tests or one-way ANOVA where appropriate. All experiments were done at least in triplicate, unless otherwise noted. ACCESSION NUMBERS Microrray data are deposited in the NIH GEO repository (accession number GSE18690): Cell Sorting Back and facial skin from neonatal (n = 15) or adult (n = 9) Sox2:EGFP mice (Ellis et al., 2004) were dissociated to single cells and sorted for EGFP expression on a MoFlo (Dako) or FACsAria (Becton Dickinson) with viable cells identified by propidium iodide exclusion. In some experiments, cells were stained with APC-conjugated anti-pdgfra (1:100; ebioscience) and/or biotin-conjugated anti-cd34 (1:100; ebioscience) followed by a PECy7-conjugated streptavidin secondary antibody (1:1000; BD Biosciences). Gates were set according to single stained positive controls and negative (secondary only/ isotype) controls. For transplants, sorted cells were incubated in medium containing RFP retrovirus and 2 mg/ml polybrene for 18 hr (a gift from Drs. Akitsu Hotta and James Ellis, HSC) and washed extensively, and 300,000 cells were injected (n = 4 Sox2:EGFP + PDGFRa + CD34 cells and 5 Sox2:EGFP cells). Microarray and Bioinformatics P4 Sox2:EGFP +, CD34 cells were sorted directly into RNAlater (Ambion). RNA was extracted and quality assessed from sorted cells and primary passage P4 SKPs with Trizol (Invitrogen) and an Agilent BioAnalyzer. RNA samples were analyzed on Affymetrix GeneChip Mouse Gene 1.0 ST Arrays, and background was corrected and normalized with standard RMA procedure implemented in the Affymetrix Expression Console software. Preprocessed SUPPLEMENTAL DATA Supplemental Data include Supplemental Experimental Procedures and six figures and can be found with this article online at cell-stem-cell/supplemental/s (09) ACKNOWLEDGMENTS This work was funded by grants from CIHR, the Canadian Stem Cell Network, and HHMI. F.D.M. is an HHMI International Research Scholar and CRC chairholder, and J.B. was funded by a CIHR fellowship during the course of this work. We thank Fabio Rossi (U.B.C.) for supplying MSCs, Ben Alman and Sophia Cheon (H.S.C.) for help with skin wounding assays, Akitsu Hotta and James Ellis for supplying RFP retrovirus, and Richard LeBaron (U.T.S.A.) and Pierre Coulombe (Johns Hopkins) for providing antibodies. We also thank Dennis Aquino and Sherry Zhao for valuable technical assistance. Received: February 15, 2009 Revised: August 6, 2009 Accepted: October 27, 2009 Published: December 3, 2009 (K M) Skin transplanted with GFP + follicle-derived SKPs for 4 weeks. Transplanted cells (green) integrated into follicle DS and DP (K, arrows) and contributed to the dermis (K, arrowheads), where they expressed PDGFRa (L, red) and collagen type 1 (M, red). Arrows indicate double-labeled cells. (N and O) Follicle-derived SKPs generated adipocytes, as indicated by the lipophilic dye oil red O (N, arrows), and a-sma + cells, potentially myofibroblasts (O, arrow) in mesodermal differentiations. (P and Q) When differentiated under neurogenic conditions, they generated nestin + cells after 5 days (P, red, arrows) and biii-tubulin + cells after 14 days (Q, red, arrow). (R and S) Sciatic nerve sections 6 weeks after transplant of follicle-derived SKPs, showing that some transplanted cells expressed the Schwann cell markers p75ntr (R, arrow) and P0 (S, arrowheads). (T and U) Transplantation of follicle-derived SKPs into the chick neural crest migratory stream (H.H. stage 18). (T) Quantification after 3 days in ovo showed follicle-derived (n = 6) and clonal (n = 8) SKPs behaved like total SKPs (n = 9), migrating to the ventral nerve or DRG and skin, with some remaining close to the neural tube. (U) After 8 days in ovo, some of the transplanted cells that were in the dermis (green) were versican + (red, arrows). Nuclei were stained with Hoechst (blue), as indicated. Epi, dermis. Scale bars represent 100 mm in (A) (D), (H), and (P); 50 mm in (E), (G), (N), (O), (Q), and (U); 25 mm in (L), (M), (R), and (S); 200 mm in (I) and (K); and 250 mm in (J). Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc. 621
13 A B C D Sox2:EGFP ncam hoechst Sox2:EGFP E F G wound Sox2:EGFP hoechst wound Sox2:EGFP ncam hoechst H Sox2:EGFP hoechst Sox2:EGFP hoechst I J K GFP hoechst L GFP hoechst DP GFP cd34 hoechst M N GFP fibroblast hoechst O 28d post P GFP hoechst Q GFP cd44 hoechst R GFP collagen I hoechst Figure 7. Lineage Tracing of Hair Follicle Dermal Cells (A H) Immunostaining for EGFP (green) and NCAM (C, E, red) in Sox2:EGFP skin 3 days after a punch wound. (B) (E) show hair follicles in the vicinity of the injury, with (B) and (C) being higher magnification images of the boxed area in (A), while (D) and (E) show the same field. (F) (H) show the region directly adjacent to the wound, with (H) being a higher magnification image. Arrows show Sox2:EGFP + cells outside of hair follicles. (I R) Lineage tracing demonstrates that follicular DP and DS cells contribute to dermal maintenance and wound healing. (I) Schematic dcting lineage tracing procedure. Hair follicles were generated in patch assays with GFP-tagged rat SKPs or uncultured neonatal rat dermal cells, and newly formed genetically tagged follicles were carefully dissected and grafted on to the back skin of a recipient mouse. Skin was analyzed 4 8 weeks later. (J) Photograph of an adult nude mouse that had received a transplant of chimeric hair follicles as in (I) 3 weeks earlier. (K N) Back skin of a mouse as in (I) 4 weeks posttransplant immunostained for GFP (green) and CD34 (M, red, arrows) or fibroblast-specific antigen (N, red, arrows). Arrows and arrowheads in (K) denote GFP-tagged cells in follicle DP and DS and interfollicular dermis, respectively. Arrows in (L) denote GFP + cells leaving the hair follicle. (O) Photograph of a transplant similar to (J), except that two adjacent punch wounds (hatched lines) were made 3 weeks after the initial hair follicle graft, and skin was analyzed 4 weeks later. (P R) Skin sectioned as shown by the red hatched line in (O), and immunostained for GFP (green) and CD44 (red, Q) or collagen type I (red, R). Arrows denote transplanted cells in (P) and double-labeled cells in (Q) and (R). Nuclei were stained with Hoechst (blue). Scale bars represent 200 mm in (K) and (P) and 25 mm in (B), (C), (E), (L) (N), (Q), and (R). See also Figure S Cell Stem Cell 5, , December 4, 2009 ª2009 Elsevier Inc.
Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type
 Article Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type Waleed Rahmani, 1 Sepideh Abbasi, 1 Andrew Hagner, 1 Eko Raharjo, 1 Ranjan Kumar,
Article Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type Waleed Rahmani, 1 Sepideh Abbasi, 1 Andrew Hagner, 1 Eko Raharjo, 1 Ranjan Kumar,
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS Convergent Genesis of an Adult Neural Crest-Like Dermal Stem Cell from Distinct Developmental Origins HIROYUKI JINNO, a,b OLENA MOROZOVA, c KAREN L. JONES, a JEFFREY A. BIERNASKIE,
TISSUE-SPECIFIC STEM CELLS Convergent Genesis of an Adult Neural Crest-Like Dermal Stem Cell from Distinct Developmental Origins HIROYUKI JINNO, a,b OLENA MOROZOVA, c KAREN L. JONES, a JEFFREY A. BIERNASKIE,
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
Supplementary Table-1: List of genes that were identically matched between the ST2 and
 Supplementary data Supplementary Table-1: List of genes that were identically matched between the ST2 and ST3. Supplementary Table-2: List of genes that were differentially expressed in GD2 + cells compared
Supplementary data Supplementary Table-1: List of genes that were identically matched between the ST2 and ST3. Supplementary Table-2: List of genes that were differentially expressed in GD2 + cells compared
Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells. in the injured sites
 Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites Yunyuan Li, Reza Baradar Jalili, Aziz Ghahary Department of Surgery, University of British Columbia,
Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites Yunyuan Li, Reza Baradar Jalili, Aziz Ghahary Department of Surgery, University of British Columbia,
Stem Cell Reports Ar ticle
 Ar ticle Direct Genesis of Functional Rodent and Human Schwann Cells from Skin Mesenchymal Precursors Matthew P. Krause, 1,2,9 Shaalee Dworski, 1,3,9 Konstantin Feinberg, 1,2,9 Karen Jones, 1,2,9 Adam
Ar ticle Direct Genesis of Functional Rodent and Human Schwann Cells from Skin Mesenchymal Precursors Matthew P. Krause, 1,2,9 Shaalee Dworski, 1,3,9 Konstantin Feinberg, 1,2,9 Karen Jones, 1,2,9 Adam
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05574 SUPPLEMENTARY INFORMATION Supplementary Results Additional Materials and Methods Analysis of epidermal wholemounts and sections: Tail epidermis. The patterned organisation of tail
doi: 10.1038/nature05574 SUPPLEMENTARY INFORMATION Supplementary Results Additional Materials and Methods Analysis of epidermal wholemounts and sections: Tail epidermis. The patterned organisation of tail
Supplemental Information Inventory
 Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Supplemental Figure 1 (Figure S1), related to Figure 1 Figure S1 provides evidence to demonstrate Nfatc1Cre is a mouse line that directed gene
 Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
ANNOUNCEMENTS. HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134)
 ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
GENESDEV/2007/ Supplementary Figure 1 Elkabetz et al.,
 GENESDEV/2007/089581 Supplementary Figure 1 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 2 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 3 Elkabetz et al., GENESDEV/2007/089581
GENESDEV/2007/089581 Supplementary Figure 1 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 2 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 3 Elkabetz et al., GENESDEV/2007/089581
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Supplementary Information. Conversion of vascular endothelial cells into multipotent stem-like cells
 Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Supplementary Information
 Supplementary Information Supplementary Figures Supplementary Figure 1. qrt-pcr analysis of the expression of candidate factors including SOX2, MITF, PAX3, DLX5, FOXD3, LEF1, MSX1, PAX6, SOX2 and SOX9
Supplementary Information Supplementary Figures Supplementary Figure 1. qrt-pcr analysis of the expression of candidate factors including SOX2, MITF, PAX3, DLX5, FOXD3, LEF1, MSX1, PAX6, SOX2 and SOX9
Induction of Hair Growth by Fibroblast-Secreted WNTs. Gail Naughton and Frank Zeigler Histogen, Inc.
 Induction of Hair Growth by Fibroblast-Secreted WNTs Gail Naughton and Frank Zeigler Histogen, Inc. Fibroblast Secreted Factors Formed during tissue-engineering manufacturing process by primary human foreskin
Induction of Hair Growth by Fibroblast-Secreted WNTs Gail Naughton and Frank Zeigler Histogen, Inc. Fibroblast Secreted Factors Formed during tissue-engineering manufacturing process by primary human foreskin
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/9/e1500973/dc1 Supplementary Materials for Pharmacologic inhibition of JAK-STAT signaling promotes hair growth Sivan Harel, Claire A. Higgins, Jane E. Cerise,
advances.sciencemag.org/cgi/content/full/1/9/e1500973/dc1 Supplementary Materials for Pharmacologic inhibition of JAK-STAT signaling promotes hair growth Sivan Harel, Claire A. Higgins, Jane E. Cerise,
Flow cytometry Stained cells were analyzed and sorted by SORP FACS Aria (BD Biosciences).
 Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
A population of Nestin expressing progenitors in the cerebellum exhibits increased tumorigenicity
 A population of Nestin expressing progenitors in the cerebellum exhibits increased tumorigenicity Peng Li 1,2, Fang Du 1, Larra W. Yuelling 1, Tiffany Lin 3, Renata E. Muradimova 1, Rossella Tricarico
A population of Nestin expressing progenitors in the cerebellum exhibits increased tumorigenicity Peng Li 1,2, Fang Du 1, Larra W. Yuelling 1, Tiffany Lin 3, Renata E. Muradimova 1, Rossella Tricarico
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12783 Bone marrow transplantation experiments While we confirmed that CD45+ bone marrow derived cells contributed to the dermis during normal homeostasis and wound healing (Fig. E9d,
doi:10.1038/nature12783 Bone marrow transplantation experiments While we confirmed that CD45+ bone marrow derived cells contributed to the dermis during normal homeostasis and wound healing (Fig. E9d,
Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis
 Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis Yan Liu 1,*, Zhipeng Han 1,*, Yingying Jing 1,*, Xue Yang 1, Shanshan Zhang 1, Chen
Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis Yan Liu 1,*, Zhipeng Han 1,*, Yingying Jing 1,*, Xue Yang 1, Shanshan Zhang 1, Chen
Supplementary Figures
 Supplementary Figures Supplementary Figure 1: Phenotypically defined hematopoietic stem and progenitor cell populations show distinct mitochondrial activity and mass. (A) Isolation by FACS of commonly
Supplementary Figures Supplementary Figure 1: Phenotypically defined hematopoietic stem and progenitor cell populations show distinct mitochondrial activity and mass. (A) Isolation by FACS of commonly
(A) Schematic illustration of sciatic nerve ligation. P, proximal; D, distal to the ligation site.
 SUPPLEMENTRY INFORMTION SUPPLEMENTL FIGURES Figure S1. () Schematic illustration of sciatic nerve ligation. P, proximal; D, distal to the ligation site. () Western blot of ligated and unligated sciatic
SUPPLEMENTRY INFORMTION SUPPLEMENTL FIGURES Figure S1. () Schematic illustration of sciatic nerve ligation. P, proximal; D, distal to the ligation site. () Western blot of ligated and unligated sciatic
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and
 Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Guided differentiation of ES cell into mesenchymal stem cell
 Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
NPTEL Biotechnology Tissue Engineering. Stem cells
 Stem cells S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of IITs and IISc
Stem cells S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of IITs and IISc
initial single-cell analysis, with a pragmatic focus on surface markers with the highest potential for
 Supplementary Figure 1: Summary of the exclusionary approach to surface marker selection for initial single-cell analysis, with a pragmatic focus on surface markers with the highest potential for protein
Supplementary Figure 1: Summary of the exclusionary approach to surface marker selection for initial single-cell analysis, with a pragmatic focus on surface markers with the highest potential for protein
Durham E-Theses. The in vitro plasticity of hair follicle dermal and mesenchymal stem cells. Emmerson, Robert
 Durham E-Theses The in vitro plasticity of hair follicle dermal and mesenchymal stem cells Emmerson, Robert How to cite: Emmerson, Robert (2008) The in vitro plasticity of hair follicle dermal and mesenchymal
Durham E-Theses The in vitro plasticity of hair follicle dermal and mesenchymal stem cells Emmerson, Robert How to cite: Emmerson, Robert (2008) The in vitro plasticity of hair follicle dermal and mesenchymal
Tissue Engineering. Mesenchymal Stem Cells for. Tissue Engineering
 Tissue Engineering Mesenchymal Stem Cells for Tissue Engineering Reference: Culture of Cells for Tissue Engineering (Culture of Specialized Cells), Chapter 2 Shu-Ping Lin, Ph.D. Date: 03.20.2012 Institute
Tissue Engineering Mesenchymal Stem Cells for Tissue Engineering Reference: Culture of Cells for Tissue Engineering (Culture of Specialized Cells), Chapter 2 Shu-Ping Lin, Ph.D. Date: 03.20.2012 Institute
supplementary information
 DOI: 10.1038/ncb2015 Figure S1 Confirmation of Sca-1 CD34 stain specificity using isotypematched control antibodies. Skeletal muscle preparations were stained and gated as described in the text (Hoechst
DOI: 10.1038/ncb2015 Figure S1 Confirmation of Sca-1 CD34 stain specificity using isotypematched control antibodies. Skeletal muscle preparations were stained and gated as described in the text (Hoechst
5.4 PERIODONTAL LIGAMENT STEM CELL (PDLSC) ISOLATION. Having validated that the isolated PDL stromal cells possessed the major phenotypic
 5.4 PERIODONTAL LIGAMENT STEM CELL (PDLSC) ISOLATION Having validated that the isolated PDL stromal cells possessed the major phenotypic characteristics of their tissue of origin, we next focused on identifying
5.4 PERIODONTAL LIGAMENT STEM CELL (PDLSC) ISOLATION Having validated that the isolated PDL stromal cells possessed the major phenotypic characteristics of their tissue of origin, we next focused on identifying
a KYSE270-CON KYSE270-Id1
 a KYSE27-CON KYSE27- shcon shcon sh b Human Mouse CD31 Relative MVD 3.5 3 2.5 2 1.5 1.5 *** *** c KYSE15 KYSE27 sirna (nm) 5 1 Id2 Id2 sirna 5 1 sirna (nm) 5 1 Id2 sirna 5 1 Id2 [h] (pg per ml) d 3 2 1
a KYSE27-CON KYSE27- shcon shcon sh b Human Mouse CD31 Relative MVD 3.5 3 2.5 2 1.5 1.5 *** *** c KYSE15 KYSE27 sirna (nm) 5 1 Id2 Id2 sirna 5 1 sirna (nm) 5 1 Id2 sirna 5 1 Id2 [h] (pg per ml) d 3 2 1
Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP +
 Supplementary Figure 1 Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP + haematopoietic cells in the intestine. GFP + cells from E15.5 intestines were obtained after collagenase
Supplementary Figure 1 Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP + haematopoietic cells in the intestine. GFP + cells from E15.5 intestines were obtained after collagenase
SUPPLEMENTARY INFORMATION
 VOLUME: 1 ARTICLE NUMBER: 0011 In the format provided by the authors and unedited. In situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy Chao Wang 1, 2, Wujin
VOLUME: 1 ARTICLE NUMBER: 0011 In the format provided by the authors and unedited. In situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy Chao Wang 1, 2, Wujin
Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.
 LEGENDS TO SUPPLEMENTARY FIGURES Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.2) were stained with the antibodies oct3/4
LEGENDS TO SUPPLEMENTARY FIGURES Figure S1. Phenotypic characterization of AND-1_WASKO cell lines. AND- 1_WASKO_C1.1 (WASKO_C1.1) and AND-1_WASKO_C1.2 (WASKO_C1.2) were stained with the antibodies oct3/4
Antibody used for FC Figure S1. Multimodal characterization of NIR dyes in vitro Figure S2. Ex vivo analysis of HL60 cells homing
 Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Figure S1. Schematic of myeloid lineage reporter mouse model used in this study.
 Supplementary Figure Legends Figure S1. Schematic of myeloid lineage reporter mouse model used in this study. (A) The Lyzs-Cre mouse was crossed with Gt(ROSA)26Sor tm1(eyfp)cos/ J mice to label cells expressing
Supplementary Figure Legends Figure S1. Schematic of myeloid lineage reporter mouse model used in this study. (A) The Lyzs-Cre mouse was crossed with Gt(ROSA)26Sor tm1(eyfp)cos/ J mice to label cells expressing
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Table of Contents. 2.1 NeuroCult NCFC Assay Kit (Rat) Components Additional Required Reagents Required Equipment...
 i Table of Contents 1.0 Overview of the NeuroCult NCFC Assay 2.0 Materials 2.1 NeuroCult NCFC Assay Kit (Rat) Components... 4 2.2 Additional Required Reagents... 4 2.3 Required Equipment... 4 3.0 Preparation
i Table of Contents 1.0 Overview of the NeuroCult NCFC Assay 2.0 Materials 2.1 NeuroCult NCFC Assay Kit (Rat) Components... 4 2.2 Additional Required Reagents... 4 2.3 Required Equipment... 4 3.0 Preparation
Nature Immunology: doi: /ni Supplementary Figure 1
 Supplementary Figure 1 BALB/c LYVE1-deficient mice exhibited reduced lymphatic trafficking of all DC subsets after oxazolone-induced sensitization. (a) Schematic overview of the mouse skin oxazolone contact
Supplementary Figure 1 BALB/c LYVE1-deficient mice exhibited reduced lymphatic trafficking of all DC subsets after oxazolone-induced sensitization. (a) Schematic overview of the mouse skin oxazolone contact
Fundamental properties of Stem Cells
 Stem cells Learning Goals: Define what a stem cell is and describe its general properties, using hematopoietic stem cells as an example. Describe to a non-scientist the current progress of human stem cell
Stem cells Learning Goals: Define what a stem cell is and describe its general properties, using hematopoietic stem cells as an example. Describe to a non-scientist the current progress of human stem cell
Wound Repair and Regeneration
 Wound Repair and Regeneration Mark A. Carlson, MD Department of Surgery University of Nebraska Medical Center Omaha VA Medical Center Omaha, Nebraska USA UNMC Dept Surgery Research Conference, 3/24/10
Wound Repair and Regeneration Mark A. Carlson, MD Department of Surgery University of Nebraska Medical Center Omaha VA Medical Center Omaha, Nebraska USA UNMC Dept Surgery Research Conference, 3/24/10
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and
 Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
UNIVERSITY OF CALGARY. Investigating the regulatory roles of Platelet-derived. Growth Factor in the dermal stem cell niche. Garrett Evan Moffatt
 UNIVERSITY OF CALGARY Investigating the regulatory roles of Platelet-derived Growth Factor in the dermal stem cell niche by Garrett Evan Moffatt A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN
UNIVERSITY OF CALGARY Investigating the regulatory roles of Platelet-derived Growth Factor in the dermal stem cell niche by Garrett Evan Moffatt A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN
Xeno-Free Systems for hesc & hipsc. Facilitating the shift from Stem Cell Research to Clinical Applications
 Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang *
 In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency
 nature methods Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency Akitsu Hotta, Aaron Y L Cheung, Natalie Farra, Kausalia Vijayaragavan, Cheryle A Séguin, Jonathan S Draper,
nature methods Isolation of human ips cells using EOS lentiviral vectors to select for pluripotency Akitsu Hotta, Aaron Y L Cheung, Natalie Farra, Kausalia Vijayaragavan, Cheryle A Séguin, Jonathan S Draper,
Mice TRAMP mice were maintained in a C57BL/6J background. Syngeneic UBI-GFP/BL6 mice were used for bone marrow engraftment. 2
 Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Supplementary Figures
 Supplementary Figures Fig. S1. Specificity of perilipin antibody in SAT compared to various organs. (A) Images of SAT at E18.5 stained with perilipin and CD31 antibody. Scale bars: 100 μm. SAT stained
Supplementary Figures Fig. S1. Specificity of perilipin antibody in SAT compared to various organs. (A) Images of SAT at E18.5 stained with perilipin and CD31 antibody. Scale bars: 100 μm. SAT stained
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
7-amino actinomycin D (7ADD) was added to all samples 10 minutes prior to analysis on the flow cytometer in order to gate 7AAD viable cells.
 Antibody staining for Ho uptake analyses For HSC staining, 10 7 BM cells from Ho perfused mice were stained with biotinylated lineage antibodies (CD3, CD5, B220, CD11b, Gr-1, CD41, Ter119), anti Sca-1-PECY7,
Antibody staining for Ho uptake analyses For HSC staining, 10 7 BM cells from Ho perfused mice were stained with biotinylated lineage antibodies (CD3, CD5, B220, CD11b, Gr-1, CD41, Ter119), anti Sca-1-PECY7,
Isolation, culture, and transfection of primary mammary epithelial organoids
 Supplementary Experimental Procedures Isolation, culture, and transfection of primary mammary epithelial organoids Primary mammary epithelial organoids were prepared from 8-week-old CD1 mice (Charles River)
Supplementary Experimental Procedures Isolation, culture, and transfection of primary mammary epithelial organoids Primary mammary epithelial organoids were prepared from 8-week-old CD1 mice (Charles River)
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Piscataway, NJ February 2007
 Final Progress Report for the New Jersey Commission on Spinal Cord Research Submitted by David P. Crockett, PhD for the late Ira B. Black, MD Department of Neuroscience and Cell Biology UMDNJ-Robert Wood
Final Progress Report for the New Jersey Commission on Spinal Cord Research Submitted by David P. Crockett, PhD for the late Ira B. Black, MD Department of Neuroscience and Cell Biology UMDNJ-Robert Wood
Nature Biotechnology: doi: /nbt.4166
 Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Developing Targeted Stem Cell Therapeutics for Cancer. Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013
 Developing Targeted Stem Cell Therapeutics for Cancer Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013 The Challenge of Drug Delivery for Brain Cancer Stem Cells
Developing Targeted Stem Cell Therapeutics for Cancer Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013 The Challenge of Drug Delivery for Brain Cancer Stem Cells
Stem cells in Development
 ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA. Figure Legends
 Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA Figure Legends Suppl. Fig. S1. Antigenic determinants of the Panx2 antibodies employed in this study. (A) Schematic of mouse Panx2
Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA Figure Legends Suppl. Fig. S1. Antigenic determinants of the Panx2 antibodies employed in this study. (A) Schematic of mouse Panx2
MeCP2. MeCP2/α-tubulin. GFP mir1-1 mir132
 Conservation Figure S1. Schematic showing 3 UTR (top; thick black line), mir132 MRE (arrow) and nucleotide sequence conservation (vertical black lines; http://genome.ucsc.edu). a GFP mir1-1 mir132 b GFP
Conservation Figure S1. Schematic showing 3 UTR (top; thick black line), mir132 MRE (arrow) and nucleotide sequence conservation (vertical black lines; http://genome.ucsc.edu). a GFP mir1-1 mir132 b GFP
Stem cells in Development
 ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
1. Introduction. 1 Page 1
 1. INTRODUCTION Osteoblast differentiation is the key component of the bone formation, growth and remodeling. During this incompletely understood process a subpopulation of multipotential mesenchymal stem
1. INTRODUCTION Osteoblast differentiation is the key component of the bone formation, growth and remodeling. During this incompletely understood process a subpopulation of multipotential mesenchymal stem
Stem Cell Reports Repor t
 Repor t Sox2-Mediated Regulation of Adult Neural Crest Precursors and Skin Repair Adam P.W. Johnston, 1,5 Sibel Naska, 1,2,5 Karen Jones, 1 Hiroyuki Jinno, 1,4,6 David R. Kaplan, 2,3 and Freda D. Miller
Repor t Sox2-Mediated Regulation of Adult Neural Crest Precursors and Skin Repair Adam P.W. Johnston, 1,5 Sibel Naska, 1,2,5 Karen Jones, 1 Hiroyuki Jinno, 1,4,6 David R. Kaplan, 2,3 and Freda D. Miller
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 1.138/NMAT3777 Biophysical regulation of epigenetic state and cell reprogramming Authors: Timothy L. Downing 1,2, Jennifer Soto 1,2, Constant Morez 2,3,, Timothee Houssin
SUPPLEMENTARY INFORMATION DOI: 1.138/NMAT3777 Biophysical regulation of epigenetic state and cell reprogramming Authors: Timothy L. Downing 1,2, Jennifer Soto 1,2, Constant Morez 2,3,, Timothee Houssin
John Gurdon was testing the hypothesis of genomic equivalence or that when cells divide they retain a full genomic compliment.
 1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1: Vector maps of TRMPV and TRMPVIR variants. Many derivatives of TRMPV have been generated and tested. Unless otherwise noted, experiments in this paper use
Supplementary Figure 1 Supplementary Figure 1: Vector maps of TRMPV and TRMPVIR variants. Many derivatives of TRMPV have been generated and tested. Unless otherwise noted, experiments in this paper use
Development 142: doi: /dev : Supplementary Material
 Development 142: doi:1.42/dev.11687: Supplementary Material Figure S1 - Relative expression of lineage markers in single ES cell and cardiomyocyte by real-time quantitative PCR analysis. Single ES cell
Development 142: doi:1.42/dev.11687: Supplementary Material Figure S1 - Relative expression of lineage markers in single ES cell and cardiomyocyte by real-time quantitative PCR analysis. Single ES cell
Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning.
 Supplemental Information Inventory of Supplemental Information Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning. Figure S2. Related to Figure 4, to validate Npn-1 signaling
Supplemental Information Inventory of Supplemental Information Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning. Figure S2. Related to Figure 4, to validate Npn-1 signaling
Lgr5 marks cycling, yet long-lived, hair follicle stem cells.
 Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Viljar Jaks 1,2,*, Nick Barker 3,*, Maria Kasper 1,*, Johan H. van Es 3, Hugo J. Snippert 3, Hans Clevers 3 and Rune Toftgård 1 1 Karolinska
Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Viljar Jaks 1,2,*, Nick Barker 3,*, Maria Kasper 1,*, Johan H. van Es 3, Hugo J. Snippert 3, Hans Clevers 3 and Rune Toftgård 1 1 Karolinska
Supplemental Material
 Supplemental Material Figure S1.(A) Immuno-staining of freshly isolated Myf5-Cre:ROSA-YFP fiber (B) Satellite cell enumeration in WT and cko mice from resting hind limb muscles. Bulk hind limb muscles
Supplemental Material Figure S1.(A) Immuno-staining of freshly isolated Myf5-Cre:ROSA-YFP fiber (B) Satellite cell enumeration in WT and cko mice from resting hind limb muscles. Bulk hind limb muscles
Supporting Information
 Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Supplementary Figure 1 Characterization of OKSM-iNSCs
 Supplementary Figure 1 Characterization of OKSM-iNSCs (A) Gene expression levels of Sox1 and Sox2 in the indicated cell types by microarray gene expression analysis. (B) Dendrogram cluster analysis of
Supplementary Figure 1 Characterization of OKSM-iNSCs (A) Gene expression levels of Sox1 and Sox2 in the indicated cell types by microarray gene expression analysis. (B) Dendrogram cluster analysis of
Supplementary Figure 1.
 Supplementary Figure 1. Quantification of western blot analysis of fibroblasts (related to Figure 1) (A-F) Quantification of western blot analysis for control and IR-Mut fibroblasts. Data are expressed
Supplementary Figure 1. Quantification of western blot analysis of fibroblasts (related to Figure 1) (A-F) Quantification of western blot analysis for control and IR-Mut fibroblasts. Data are expressed
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation
 Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Galactosidase staining of the skin of heterozygous (ColI-Cre x RBP-J loxp/wt ) (+/-) (A) and
 Suppl. Figure 1. ColI-Cre transgene activity in RBP-Jk loxp x R26R mice. (A and B) - Galactosidase staining of the skin of heterozygous (ColI-Cre x RBP-J loxp/wt ) (+/-) (A) and homozygous (ColI-Cre x
Suppl. Figure 1. ColI-Cre transgene activity in RBP-Jk loxp x R26R mice. (A and B) - Galactosidase staining of the skin of heterozygous (ColI-Cre x RBP-J loxp/wt ) (+/-) (A) and homozygous (ColI-Cre x
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM
 Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
A Comparative Study of Upconverting Nanoparticles Versus Lentiviral GFP Transduction for Labeling Mesenchymal Stem Cells
 A Comparative Study of Upconverting Nanoparticles Versus Lentiviral GFP Transduction for Labeling Mesenchymal Stem Cells Artem Kutikov, B.S., Liang Zhao, Gang Han, Ph.D., Jie Song, Ph.D.. University of
A Comparative Study of Upconverting Nanoparticles Versus Lentiviral GFP Transduction for Labeling Mesenchymal Stem Cells Artem Kutikov, B.S., Liang Zhao, Gang Han, Ph.D., Jie Song, Ph.D.. University of
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
A NANOFIBROUS HYDROGEL FOR BONE TISSUE ENGINEERING
 A NANOFIBROUS HYDROGEL FOR BONE TISSUE ENGINEERING Umadevi Kandalam, PhD Assistant Professor Department of Pediatric Dentistry College of Dental Medicine Nova Southeastern University Fort Lauderdale, Florida
A NANOFIBROUS HYDROGEL FOR BONE TISSUE ENGINEERING Umadevi Kandalam, PhD Assistant Professor Department of Pediatric Dentistry College of Dental Medicine Nova Southeastern University Fort Lauderdale, Florida
Nature Immunology: doi: /ni.3694
 Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Simplest synthetic pathways (Ch. 7)
 Simplest synthetic pathways (Ch. 7) A. Symbolism of organ synthesis. B. The central question of organ synthesis. C. What is required to synthesize an organ? D. Trans-organ rules of synthesis. A. Symbolism
Simplest synthetic pathways (Ch. 7) A. Symbolism of organ synthesis. B. The central question of organ synthesis. C. What is required to synthesize an organ? D. Trans-organ rules of synthesis. A. Symbolism
Application Note. Introduction. Kerry Thompson, Jeff Partridge, Paula Flaherty, Susan Qian, and Deepa Saxena
 Page 1 BD PureCoat ECM Mimetic Cultureware Fibronectin Peptide: Novel Synthetic, Animalfree Surface for Culture of Human Bone Marrow-derived Mesenchymal Stem Cells Kerry Thompson, Jeff Partridge, Paula
Page 1 BD PureCoat ECM Mimetic Cultureware Fibronectin Peptide: Novel Synthetic, Animalfree Surface for Culture of Human Bone Marrow-derived Mesenchymal Stem Cells Kerry Thompson, Jeff Partridge, Paula
Supplementary Figure 1. Antigens generated for mab development (a) K9M1P1-mIgG and hgh-k9m1p1 antigen (~37 kda) expression verified by western blot
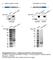 Supplementary Figure 1. Antigens generated for mab development (a) K9M1P1-mIgG and hgh-k9m1p1 antigen (~37 kda) expression verified by western blot (vector: ~25 kda). (b) Silver staining was used to assess
Supplementary Figure 1. Antigens generated for mab development (a) K9M1P1-mIgG and hgh-k9m1p1 antigen (~37 kda) expression verified by western blot (vector: ~25 kda). (b) Silver staining was used to assess
Supporting Information
 Supporting Information Chakrabarty et al. 10.1073/pnas.1018001108 SI Materials and Methods Cell Lines. All cell lines were purchased from the American Type Culture Collection. Media and FBS were purchased
Supporting Information Chakrabarty et al. 10.1073/pnas.1018001108 SI Materials and Methods Cell Lines. All cell lines were purchased from the American Type Culture Collection. Media and FBS were purchased
Supplementary Figure 1. Effect of FRC-specific ablation of Myd88 on PP and mln organization.
 Supplementary Figure 1 Effect of FRC-specific ablation of Myd88 on PP and mln organization. (a) PP numbers in 8 10 week old Cre-negative littermate (Ctrl) and Myd88-cKO mice (n = 11 mice; each dot represents
Supplementary Figure 1 Effect of FRC-specific ablation of Myd88 on PP and mln organization. (a) PP numbers in 8 10 week old Cre-negative littermate (Ctrl) and Myd88-cKO mice (n = 11 mice; each dot represents
Figure S1. Phenotypic characterization of transfected ECFC. (a) ECFC were transfected using a lentivirus with a vector encoding for either human EPO
 Figure S1. Phenotypic characterization of transfected ECFC. (a) ECFC were transfected using a lentivirus with a vector encoding for either human EPO (epoecfc) or LacZ (laczecfc) under control of a cytomegalovirus
Figure S1. Phenotypic characterization of transfected ECFC. (a) ECFC were transfected using a lentivirus with a vector encoding for either human EPO (epoecfc) or LacZ (laczecfc) under control of a cytomegalovirus
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplementary Figure 1 A green: cytokeratin 8
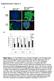 Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis
 Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis Diptiman Chanda 1*, Ashish Kurundkar 1, Sunad Rangarajan 1, Morgan Locy 1, Karen Bernard 1,
Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis Diptiman Chanda 1*, Ashish Kurundkar 1, Sunad Rangarajan 1, Morgan Locy 1, Karen Bernard 1,
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
Ex Vivo Imaging and Genetic Manipulation of Mouse Hair Follicle Bulge Stem Cells
 Methods in Molecular Biology DOI 10.1007/7651_2018_136 Springer Science+Business Media New York 2018 Ex Vivo Imaging and Genetic Manipulation of Mouse Hair Follicle Bulge Stem Cells Daniel Haensel, Melissa
Methods in Molecular Biology DOI 10.1007/7651_2018_136 Springer Science+Business Media New York 2018 Ex Vivo Imaging and Genetic Manipulation of Mouse Hair Follicle Bulge Stem Cells Daniel Haensel, Melissa
supplementary information
 DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
Nature Biotechnology: doi: /nbt.4086
 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Stem cell: a cell capable of 1) tissue plasticity - make different cell types 2) infinite self renewal through asymmetric division
 Stem cell: a cell capable of 1) tissue plasticity - make different cell types 2) infinite self renewal through asymmetric division stem cell stem cell skin muscle nerve Properties of STEM cells Plasticity
Stem cell: a cell capable of 1) tissue plasticity - make different cell types 2) infinite self renewal through asymmetric division stem cell stem cell skin muscle nerve Properties of STEM cells Plasticity
Supplementary Data. In Vivo Bone Formation by impcs Materials and methods. Results. Conclusions
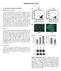 Supplementary Data In Vivo Bone Formation by impcs Materials and methods Induced pluripotent stem cell (ipsc)-derived mesenchymal stem cell (MSC)-like progenitor cells (impcs) (1 10 6 M-iMPC-GMs in 10
Supplementary Data In Vivo Bone Formation by impcs Materials and methods Induced pluripotent stem cell (ipsc)-derived mesenchymal stem cell (MSC)-like progenitor cells (impcs) (1 10 6 M-iMPC-GMs in 10
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Dec 04, 2013
 Stem Cells Junfeng Ji ( 纪俊峰 ), PhD Professor Research Center of Stem Cell and Developmental Biology School of Medicine Zhejiang University Email: jijunfeng@zju.edu.cn Dec 04, 2013 Totipotent Pluripotent
Stem Cells Junfeng Ji ( 纪俊峰 ), PhD Professor Research Center of Stem Cell and Developmental Biology School of Medicine Zhejiang University Email: jijunfeng@zju.edu.cn Dec 04, 2013 Totipotent Pluripotent
At the conclusion of this lesson you should be able to:
 Learning Objectives At the conclusion of this lesson you should be able to: Understand the key terms and definitions regarding stem cells Differentiate between the adult and embryonic stem cells Differentiate
Learning Objectives At the conclusion of this lesson you should be able to: Understand the key terms and definitions regarding stem cells Differentiate between the adult and embryonic stem cells Differentiate
