NANOTECHNOLOGICAL APPLICATIONS OF BIOMOLECULAR MOTOR SYSTEMS
|
|
|
- Aron Blankenship
- 6 years ago
- Views:
Transcription
1 ARTICLE DE FOND NANOTECHNOLOGICAL APPLICATIONS OF BIOMOLECULAR MOTOR SYSTEMS BY STEFAN DIEZ AND JONATHON HOWARD Biomolecular motors are the active workhorses of cells [1]. They are complexes of two or more proteins that convert chemical energy, usually in the form of the high-energy phosphate bond of ATP, into directed motion. The most familiar motor is the protein myosin: it drives the contraction of a muscle by moving along a filament formed from the protein actin. However, it turns out that all cells, not just specialized muscle cells, contain motors that move cellular components such as proteins, mitochondria and chromosomes from one part of the cell to another. Fig. 1 These motors include relatives of muscle myosin (that also move along actin filaments), as well as members of the kinesin and dynein families of proteins. The latter motors Kinesin-microtubule assays. a) In a cellular environment, kinesin motors transport cargo, such as membrane-bounded vesicles, along microtubule tracks (Image courtesy of Graham Johnson of and The Scripps Research Institute). The motility of molecular motors can be reconstituted in vitro in b) gliding assays (where the filaments are propelled by surface-bound motor molecules) and c) stepping assays (where the filaments are immobilized on the surface and motors walk on them). d) Sequence of fluorescent micrographs showing the movement of a kinesin motor (labeled with the green fluorescent protein) along a microtubule (red) in a singlemolecule stepping assay. Images were acquired at the indicated times using total-internal-reflection fluorescence microscopy. SUMMARY Biological cells contain molecular machines that perform complex mechanical tasks such as intracellular transport, chromosome separation and muscle contraction. These processes are driven by biomolecular motors, proteins that convert the chemical energy of ATP directly into mechanical work. Recent advances in understanding how such motor proteins work have raised the possibility that they might find applications as nanomachines. For example, they could be used as molecule-sized robots that work in molecular factories where small, but intricate structures are made on tiny assembly lines, that construct networks of molecular conductors and transistors for use as electrical circuits, or that continually patrol inside adaptive materials and repair them when necessary. Thus biomolecular motors could form the basis of bottom-up approaches for constructing, active structuring and maintenance at the nanometer scale. move along another type of filament called the microtubule (see Figure 1a). The reason that motors are necessary in cells is that diffusion is too slow to efficiently transport molecules from where they are made, typically near the nucleus, to where they are used, often at the periphery of the cell. For example, the passive diffusion of a small protein to the end of a 1-meter-long neuron would take approximately 1000 years, yet kinesin moves it in a week. This corresponds to a speed of 1 2 μm/s, which is typical of biomolecular motors [2]. Actin filaments and microtubules form a network of highways within cells, and localized cues are used to target specific cargoes to specific sites in the cell [3]. Using filaments and motors, cells build highly complex and active structures on the molecular (nanometer) scale. Little imagination is needed to envisage employing biomolecular motors to build molecular robots. Biomolecular motors are unusual machines that do what no man-made machines do: they convert chemical energy to mechanical energy directly rather than via an interme- Stefan Diez <diez@mpi-cbg.de> and Jonathon Howard <howard@mpicbg.de>, Max Planck Institute of Molecular Cell Biology and Genetics, Pfotenhauerstrass 108, Dresden, Germany LA PHYSIQUE AU CANADA / Vol. 65, No. 1 ( janvier à mars 2009 ) C 7
2 diate such as heat or electrical energy. This is essential because the confinement of heat, for example, on the nanometer scale is not possible because of its high diffusivity in aqueous solutions [2]. As energy converters, biomolecular machines are highly efficient. The chemical energy available from the hydrolysis of ATP is 100x10-21 J = 100 pn nm (under physiological conditions where the ATP concentration is 1 mm and the concentrations of the products ADP and phosphate are 0.01 mm and 1 mm, respectively). With this energy, a kinesin molecule is able to perform an 8 nm step against a load of 6 pn [2]. The energy efficiency is therefore nearly 50 %. For the rotary motor F 1 F 0 - ATPase synthase which uses the electrochemical gradient across mitochondrial and bacterial membranes to generate ATP, the efficiency is reported to lie between 80 % and 100 % [4]. The high efficiency demonstrates that, like other biological systems, the operation of biological motors has been optimized through evolution. A B High efficiency is but one feature that makes biomolecular motors attractive for nanotechnological applications. Other features are: 1) they are small and can therefore operate in a highly parallel manner, 2) they are easy to produce and can be modified through genetic engineering, 3) they are extremely cheap. For example, 20 x 10 9 kinesin motors can be acquired for one US cent from commercial suppliers (1 mg = 3.3 x motors cost $1500, Cytoskeleton, Inc., Colorado) and the price could be significantly decreased if production were scaled up, and 4) a wide array of biochemical tools has been developed to manipulate these proteins outside the cell. This report focuses on linear motors that generate force as they move along intracellular filaments. In addition to myosin and kinesin mentioned above, linear motors also include enzymes that move along DNA and RNA. Another class of motors are rotary motors that generate torque via the rotations of a central core within a larger protein complex. They include ATP synthase, mentioned above, as well as the motor that drives bacterial motility. Representatives of both categories have been used to manipulate molecules and nanoparticles. RECONSTRUCTION OF MOTILITY SYS- TEMS IN VITRO Fig. 2 The general setups for studying cytoskeletal motor proteins outside cells the so-called motility assays are depicted in Figure 1. In the gliding assay, the motors are immobilized on a surface and the filaments glide over the assembly (Figure 1b). In the stepping assay, the filaments are laid out on the surface where they form tracks for Examples of a kinesin-microtubule transport system operating in a synthetic environment. a) Microtubules are propelled over the surface by immobilized kinesin motors in the presence of ATP. Cargo (e. g. quantum dots) is to be picked up at point A and moved to point B. To reliably guide the movement of gliding filaments, a combination of topographical and chemical surface modifications has proven most efficient. b) Example of a lithographically structured Si-chip where the bottoms of 300-nm deep channels are coated by gold (Au) in order to achieve a selective binding of motor proteins. The surrounding regions are blocked by polyethylene glycol which prevents protein adhesion (from Ref. [12]). c) Fluorescent image showing the kinesin-driven, unidirectional movement (red dotted line) of a rhodamine-labeled microtubule (red) along a chemically and topographically structured channel. d) Biotemplated stamping of kinesin nanotracks without topographical surface modifications. Kinesin molecules, which are bound in an oriented manner to the lattice of a template microtubule, are transferred onto the surface by a stamping process. After adsorption the template microtubule is released when the deposited motor molecules propel the microtubule off the generated track in the presence of ATP. The same molecules will move and guide the transport microtubules (from Ref. [13]). the motors to move along (Figure 1c, d). Both assays are performed in aqueous solution, where the environmental conditions are chosen similar to those present in cells. Movement is 8 C PHYSICS IN CANADA / VOL. 65, NO. 1 ( January-March 2009 )
3 observed under the light microscope using fluorescence markers or high-contrast techniques. At the Max Planck Institute for Molecular Cell Biology and Genetics Dresden (MPI-CBG) the depicted motility assays are currently applied in combination with novel optical imaging techniques to further the biophysical understanding of biomolecular motors [5 8]. Variations on these assays have been used to reconstitute linear motility on the four types of filaments actin filaments, microtubules, DNA and RNA. The gliding motility assay is the most promising setup for nanotechnological usage. For example, a simple application would be to employ a moving filament to pickup cargo at point A, move it along a user-defined path to point B, and then release it (see Figure 2a). However, for use in nanotechnological applications, the movement of gliding filaments has to be controllable in space and time. A number of methods to control the path along which filaments glide, a process that we call 'guiding', have been developed. Specifically, static spatial control has been achieved using topographical features [9], chemical surface modifications [10], and a combination of both [11,12] (see Figure 2b-d). While it is possible to use chemical and topographical patterning to guide filaments, it is more difficult to control the direction of movement along the path. This difficulty arises because the orientation in which motors bind to a uniform surface is not controlled. However, because the motors bind stereospecifically to the filament, they will exert force in only one direction. Consequently, only the orientation of the filament determines its direction of motion and directionality has so far only been achieved by the design of sophisticated guiding geometries. For example, unidirectional movement of filaments can be achieved if arrow-and ratchet-based guiding structures are employed [10 12] (Figure 2c). However, the generation of topographical surface structures is costly and labor-intensive. It is therefore desirable to produce non-topographical tracks of motor proteins, leading to reliable microtubule guiding when the structural widths are small (below 1 μm, see Figure 2d) [13]. Regarding temporal control mechanisms, motors can be reversibly switched off and on by regulating the concentration of fuel, or by adding and removing inhibitors. The ATP concentration can be rapidly altered by flowing in a new solution. In such a setup, the kinesin-dependent movement of microtubules can be stopped within 1 s and restarted within 10 s [20]. An alternative method to control energy supply is to use photoactivatable ATP. In this method, a flash of UV light is used to release ATP from a derivatized, non-functional precursor; an ATP-consuming enzyme is also present to return the ATP concentration to low levels following release. Using such a system, microtubule movement has been repeatedly started and stopped [9], though the start-up and slow-down times were slow, in the order of minutes. In order to develop novel mechanisms for controlling microtubule motility by external signals, we measured the height at which microtubules glide over a kinesin-coated surface in the CONTROL OVER MOTOR ACTIVITY BY EXTERNAL SIGNALS Spatial control in a dynamic manner can be achieved by the application of external forces. For example, actin filaments and microtubules both possess negative net charges, and consequently, in the presence of a uniform electric field, will experience a force directed towards the positive electrode. It is possible to apply high enough electric fields to steer motor-driven filaments in a specified direction [14-16]. Because the refractive index of protein differs from that of water, filaments become electrically polarized in the presence of an electric field, and consequently in a non-uniform field they move in the direction of highest field strength. This so-called dielectrophoretic force has been used to direct the gliding of actin filaments on a myosin-coated substrate [17]. Directional control of microtubule gliding has also been achieved using hydrodynamic flow fields [18-20]. Fig. 3 Control of microtubule motility on switchable polymer surfaces. a) Kinesin motors are embedded amongst thermoresponsive PNIPAM molecules on a substrate surface. Repeated changes in the temperature result in the reversible switching of PNIPAM chains between the expanded conformation (where microtubules are repelled from the surface and cannot bind to the kinesin heads) and the collapsed conformation (where microtubules can glide unhindered on the kinesin molecules). The number of microtubules gliding on the surface thus varies as a function of temperature (LCST: lower critical solution temparature, PGMA: poly glycidyl methacrylate). b) Proposed incorporation of switchable polymers into guiding channels. Upon external signal, incoming microtubule transporters can be derailed (when the PNIPAM gate is closed) or they can continue to travel along the channel when the PNIPAM gate is open (Image courtesy of Leonid Ionov, Max Planck Institute for Molecular Cell Biology and Genetics Dresden). LA PHYSIQUE AU CANADA / Vol. 65, No. 1 ( janvier à mars 2009 ) C 9
4 presence of ATP. To perform height measurements with nanometer precision, we used fluorescence-interference contrast (FLIC) microscopy, which is based on the self-interference of fluorescent light from objects near a reflecting surface (similar to the setup shown in Figure 2a). We determined that kinesin-1 molecules elevate gliding microtubules about 20 nm above the surface [7]. While this value is significantly lower than the contour length of the motor molecule (in the order of 70 nm), it is consistent with the segmented structure of the molecule. Given the low height of filament movement above the substrate material, another possibility to influence motility is provided by dynamic switching of the surface properties. Towards this end, we embedded in collaboration with the Leibniz Institute of Polymer Research Dresden kinesin molecules within a thin layer of the thermoresponsive polymer poly(n-isopropyl acrylamide) (PNIPAM) (see Figure 3a). PNI- PAM chains adopt a compact or extended conformation above or below the lower critical solution temperature (32 C), respectively. We observed that microtubules can land and glide on a PNIPAM-kinesin surface at high temperature (when the polymer chains are compacted and the surface-attached kinesin molecules are accessible for the microtubules) or are released from the surface at low temperature [21]. This process can be repeated multiple times demonstrating the reversibility of the effect. While we were so far only able to regulate the motility on a global scale, we are currently working towards implementing smart polymer systems into more sophisticated environments (Figure 3b). Fortuitously, many proteins possess natural regulatory mechanisms and, once understood, these might offer additional means to regulate the motors in vitro. Examples include the regulation of myosins by phosphorylation and calcium/calmodulin [22] and the inhibition of kinesin by its cargo-binding tail domain [23]. Because such natural controls might not always be applicable in a synthetic environment, there is strong interest in the development of artificial control mechanisms for motor proteins. Towards this end, metal-ion binding sites have been genetically engineered into the F1- ATPase motor. The binding of ions at the engineered site immobilizes the moving parts of the motor thus inhibiting its rotation [24]. ATP-driven rotation can be restored by the addition of metal ion chelators. Along these lines we are currently working on the genetic engineering of motors that potentially provide temporal control mechanisms switched by temperature and light. FIRST TECHNOLOGICAL APPLICATIONS In addition to these basic techniques for controlling motion, some simple applications of the gliding assay have been demonstrated. These include the transport of streptavidin-coated beads [9] and virus particles [25], the sorting of macromolecular assemblies [26], and the measurement of forces in the pn range [27]. In a collaboration with the BioNanoStructuring group at the Max Bergmann Center of Biomaterials Dresden we used chemically modified microtubules to transport and stretch individual Fig. 4 First nanotechnological applications of kinesin-microtubule transport systems. a) Setup of DNA-nanocircuits: Biotinylated microtubules are used to transport and stretch individual λ-phage DNA molecules that are biotinylated at their ends and attached to the microtubule via a streptavidin linkage (from Ref. [28]). b) Fluorescence micrograph of a moving microtubule (red) transporting (white arrows) and stretching individual DNA molecules (green) (scale bar 5 μm). This technique may offer an efficient mechanism for the generation of multidimensional, DNA-based networks, which can be metallized nano electronic applications. c) Surface imaging by motile microtubules: Schematic diagram of kinesin-driven microtubule nanoprobes crossing shallow or deep pits of a transparent silicon oxide layer above a reflective silicon mirror. d) Due to interference effects, a maximum projection of the microtubule trajectories provides a topographical image of the surface with nanometer height resolution (unpublished work). λ-phage DNA molecules across a surface [28] (see Figure 4a and b) and extended this approach to the manipulation of bifunctional DNA molecules, where individual DNA molecules were simultaneously bound to a structured gold surface (via thiolization of one DNA end) and to biotinylated microtubules (via biotinylation of the other DNA end and streptavidin) [29]. This technique, in contrast to existing nanotechnology tools, enables the parallel yet individual manipulation of many molecules and may offer an efficient mechanism for the generation of multidimensional DNA-based networks which, 10 C PHYSICS IN CANADA / VOL. 65, NO. 1 ( January-March 2009 )
5 after metallization, can be used for nano-electronic applications. Secondly, we employed gliding microtubules as self-propelled probes to measure local height information using FLICmicroscopy. At high motor densities, the trajectories of the flexible microtubules reproduce the topography of the surface (Figure 4c). Utilizing a patterned silicon oxide layer, kinesindriven microtubules moved over the edges and changed their distance to the underlying silicon substrate accordingly. By projecting the maximum intensities of all microtubule trajectories onto a single image, the topography of the various patches is reproduced (Figure 4d). In this application, the microtubules can be regarded as massively parallel, extremely sensitive height probes. In combination with the above mentioned advancements in the directional control of microtubules on surfaces, this nano-probing could bridge the gap between global probing techniques such as imaging ellipsometry, and local techniques such as atomic force microscopy. Moreover, deep pits and cavities could be imaged that are inaccessible by larger probes. OUTLOOK The first steps have been made towards the operation of biomolecular motors in engineered environments. However, many advances are necessary before these motors can be used in nanotechnological applications such as molecular factories and building circuits. An immediate task is to improve the spatial and temporal control over the motors. By combining improved surface techniques with the application of external electric, magnetic and/or optical fields it should be possible, in the near future, to stretch and collide single molecules, to control cargo loading and unloading, and to sort and pool molecules. A crucial longer-term goal is to control the position and orientation of motors with molecular precision. This means placing motors with an accuracy of ~10 nm on a surface and controlling their orientation within a few degrees. In this way both the location and the direction of motion of filaments can be controlled. Additionally, the robustness of motors must be increased. Motors operate only in aqueous solutions and under a restricted range of solute concentrations and temperatures. While it is inconceivable that protein-based motors could operate in a non-aqueous environment, two approaches to increase their robustness can be envisaged. First, motors could be purified from thermophilic or halophilic bacteria some of which grow at high temperatures and high salt concentrations. Second, a genetic screening approach might reveal mutations that allow motors to operate in less restrictive or different conditions. A longer-term goal is to use the design principles learnt from the study of biomolecular motors to build purely artificial nanomotors that can operate in air or vacuum. This is a daunting prospect and it is not even clear what fuel(s) might be used. We finish up by pointing out that the high order and nanometer-scale periodicity of DNA, actin filaments and microtubules make them ideal scaffolds on which to erect three-dimensional nanostructures. While these features have been exploited to produce DNA-based structures [30], the use of DNA motors to address specific sites (based on nucleotide sequence) has not, to our knowledge, been realized. Some years ago it was proposed that the regular lattice of microtubules might serve as substrates for molecular computing and information storage [31,32]. While these ideas seem impracticable in the context of the living organism, they may be realizable for biomolecular motors operating in engineered environments. Already now, medical applications can benefit tremendously from biomolecular motors in artificial environments. Recent studies have revealed that kinesin, dynein and myosin play significant roles in the pathogenesis of a variety of diseases. For example, kinesin deficiencies have been identified as cause for Charcot-Marie-Tooth disease and some kidney diseases. Dynein deficiencies can lead to chronic infections of the respiratory tract as cilia fail to function without dynein. Defects in muscular myosin predictably cause myopathies, whereas defects in unconventional myosin are the cause for Usher syndrome and deafness [33]. Understanding the basic principles of motor operation will thus allow to design specific drugs to enhance (or inhibit) the (mal)function of these motors [34]. Here, the in vitro reconstitution of particular subcellular transport systems will provide the testbeds for a high-throughput drug screening [35]. Moreover, when operated as molecular sorting devices, motor-driven filaments can be used to collect and concentrate specific reagents that are present in a test sample at extremely low concentration. When combined with micro-chip technologies, such systems might form the base for a new class of point-of-care molecular detection and diagnosis devices. ACKNOWLEDGEMENTS The authors are grateful to their group members for being excellent research partners. The groups of Michael Mertig (Max Bergmann Center of Biomaterials Dresden) and Manfred Stamm (Leibniz Institute of Polymer Research Dresden) are acknowledged for very fruitful collaborations on the DNA stretching and the switchable polymer surfaces, respectively. Particular thanks to Jonne Helenius (BioTec Dresden) for his contribution to an earlier version of this article as well as to Franziska Friedrich (MPI-CBG) for her continued support with scientific illustrations. The presented work has been mainly funded by the BMBF (junior group grant 03 N 8712), the VolkswagenStiftung and the Max-Planck-Society. REFERENCES 1. B. Alberts, The cell as a collection of protein machines: preparing the next generation of molecular biologists, Cell, 92, (1998). 2. J. Howard, Mechanics of Motor Proteins and the Cytoskeleton, Sunderland, MA: Sinauer Associates (2001). 3. B. Alberts, Molecular biology of the cell, 4th edition, New York: Garland Science (2002). 4. K. Kinosita, Jr., R. Yasuda, H. Noji, S. Ishiwata, M. Yoshida, F1-ATPase: a rotary motor made of a single molecule, Cell, 93, p (1998). LA PHYSIQUE AU CANADA / Vol. 65, No. 1 ( janvier à mars 2009 ) C 11
6 5. J. Helenius, G. Brouhard, Y. Kalaidzidis, S. Diez, J. Howard, The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends, Nature, 441, (2006). 6, V. Varga, J. Helenius, K. Tanaka, A.A. Hyman, T.U. Tanaka, J. Howard, Yeast kinesin-8 depolymerizes microtubules in a lengthdependent manner, Nature Cell Biology, 8, 957 U960 (2006). 7. J. Kerssemakers, J. Howard, H. Hess, S. Diez, The distance that kinesin-1 holds its cargo from the microtubule surface measured by fluorescence interference contrast microscopy, Proceedings of the National Academy of Sciences of the United States of America, 103, (2006). 8. C. Leduc, F. Ruhnow, J. Howard, S. Diez, Detection of fractional steps in cargo movement by the collective operation of kinesin-1 motors, Proceedings of the National Academy of Sciences of the United States of America, 104, (2007). 9. H. Hess, J. Clemmens, D. Qin, J. Howard, V. Vogel, Light-controlled molecular shuttles made from motor proteins carrying cargo on engineered surfaces, Nano Letters, 1, (2001). 10. H. Hess, J. Clemmens, C.M. Matzke, G.D. Bachand, B.C. Bunker, V. Vogel, Ratchet patterns sort molecular shuttles, Applied Physics a (Materials Science Processing), A75, (2002). 11. Y. Hiratsuka, T. Tada, K. Oiwa, T. Kanayama, T.Q.P. Uyeda, Controlling the direction of kinesin-driven microtubule movements along microlithographic tracks, Biophysical Journal, 81, (2001). 12. M.G.L. van den Heuvel, C.T. Butcher, R.M.M. Smeets, S. Diez, C. Dekker, High rectifying efficiencies of microtubule motility on kinesin-coated gold nanostructures, Nano Letters, 5, , (2005). 13. C. Reuther, L. Hajdo, R. Tucker, A.A. Kasprzak, S. Diez, Biotemplated nanopatterning of planar surfaces with molecular motors, Nano Letters, 6, , (2006). 14. D. Riveline, A. Ott, F. Julicher, D.A. Winkelmann, O. Cardoso, J.J. Lacapere, S. Magnusdottir, J.L. Viovy, L. Gorre-Talini, J. Prost, Acting on actin: the electric motility assay, European Biophysics Journal with Biophysics Letters, 27, , (1998). 15. R. Stracke, K.J. Bohm, L. Wollweber, J.A. Tuszynski, E. Unger, Analysis of the migration behaviour of single microtubules in electric fields, Biochemical and Biophysical Research Communications, 293, , (2002). 16. M.G.L. van den Heuvel, M.P. De Graaff, C. Dekker, Molecular sorting by electrical steering of microtubules in kinesin-coated channels, Science, 312, , (2006). 17. S.B. Asokan, L. Jawerth, R.L. Carroll, R.E. Cheney, S. Washburn, R. Superfine, Two-Dimensional Manipulation and Orientation of Actin-Myosin Systems with Dielectrophoresis, Nano Letters, 3, , (2003). 18. K.J. Bohm, R. Stracke, P. Muhlig, E. Unger, Motor protein-driven unidirectional transport of micrometer-sized cargoes across isopolar microtubule arrays, Nanotechnology, 12, , (2001). 19. I. Prots, R. Stracke, E. Unger, K.J. Bohm, Isopolar microtubule arrays as a tool to determine motor protein directionality Cell Biology International, 27, , (2003). 20. F.U. Gast, P.S. Dittrich, P. Schwille, M. Weigel, M. Mertig, J. Opitz, U. Queitsch, S. Diez, B. Lincoln, F. Wottawah, S. Schinkinger, J. Guck, J. Kas, J. Smolinski, K. Salchert, C. Werner, C. Duschl, M.S. Jager, K. Uhlig, K.; Geggier, P.; Howitz, S., The microscopy cell (MicCell), a versatile modular flowthrough system for cell biology, biomaterial research, and nanotechnology. In: Microfluidics and Nanofluidics, 2, 21 36, (2006). 21. L. Ionov, M. Stamm, S. Diez, Reversible switching of microtubule motility using thermoresponsive polymer surfaces, Nano Letters, 6, , (2006). 22. J.R. Sellers, H.V. Goodson, Motor proteins 2: myosin, Protein Profile, 2, , (2006). 23. D.L. Coy, W.O. Hancock, M. Wagenbach, J. Howard, Kinesin s tail domain is an inhibitory regulator of the motor domain, Nat Cell Biol, 1, , (1999). 24. H. Liu, J.J. Schmidt, G.D. Bachand, S.S. Rizk, L.L. Looger, H.W. Hellinga, C.D. Montemagno, Control of a biomolecular motorpowered nanodevice with an engineered chemical switch, Nat Mater, 1, , (2002). 25. G.D. Bachand, S.B. Rivera, A. Carroll-Portillo, H. Hess, M. Bachand, Active capture and transport of virus particles using a biomolecular motor-driven, nanoscale antibody sandwich assay, Small, 2, , (2006). 26. L. Ionov, M. Stamm, S. Diez, Size sorting of protein assemblies using polymeric gradient surfaces, Nano Letters, 5, , (2005). 27. H. Hess, J. Howard, V. Vogel, A piconewton forcemeter assembled from microtubules and kinesins, Nano Letters, 2, , (2002). 28. S. Diez, C. Reuther, C. Dinu, R. Seidel, M. Mertig, W. Pompe, J. Howard, Stretching and Transporting DNA Molecules Using Motor Proteins, Nano Letters, 3, , (2003). 29. C.Z. Dinu, J. Opitz, W. Pompe, J. Howard, M. Mertig, S. Diez, Parallel manipulation of bifunctional DNA molecules on structured surfaces using kinesin-driven microtubules, Small, 2, , (2006). 30. N.C. Seeman, DNA in a material world, Nature, 421, , (2003). 31. S.R. Hameroff, R.C. Watt, Information processing in microtubules, J Theor Biol., 98, , (1982). 32. R. Penrose, Consciousness, the brain, and spacetime geometry: an addendum. Some new developments on the Orch OR model for consciousness, Ann N Y Acad Sci., 929, , (2001). 33. N. Hirokawa, R. Tekamura, Biochemical and molecular characterization of diseases linked to motor proteins, Trends in Biochemical Sciences, 28, , (2003). 34. R. Sakowicz, M.S. Berdelis, K. Ray, C.L. Blackburn, C. Hopmann, D.J. Faulkner, L.S.B. Goldstein, A marine natural product inhibitor of kinesin motors, Science, 280, , (1998). 35. See, for example: 12 C PHYSICS IN CANADA / VOL. 65, NO. 1 ( January-March 2009 )
Nanotechnological Applications of Biomolecular Motor Systems. Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden
 Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Nano-Scale Engineering III Bio-Molecular Motors for Engineering
 Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler
 Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler 23.10. Zelle 30.10. Biologische Makromoleküle I 06.11. Biologische Makromoleküle II 13.11. Nukleinsäuren-Origami (DNA, RNA) 20.11.
Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler 23.10. Zelle 30.10. Biologische Makromoleküle I 06.11. Biologische Makromoleküle II 13.11. Nukleinsäuren-Origami (DNA, RNA) 20.11.
Biosensors. DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses)
 Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology
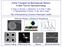 Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
NanoFabrication Systems DPN. Nanofabrication Systems. A complete line of instruments and tools for micro and nanopatterning applications
 DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning applications DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning
DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning applications DPN Nanofabrication Systems A complete line of instruments and tools for micro and nanopatterning
Nanoimprinting in Polymers and Applications in Cell Studies. Albert F. YEE Chemical Engineering & Materials Science UC Irvine
 Nanoimprinting in Polymers and Applications in Cell Studies Albert F. YEE Chemical Engineering & Materials Science UC Irvine Presentation outline Motivation Reversal imprinting Soft inkpad imprinting on
Nanoimprinting in Polymers and Applications in Cell Studies Albert F. YEE Chemical Engineering & Materials Science UC Irvine Presentation outline Motivation Reversal imprinting Soft inkpad imprinting on
Rotary DNA Motors INTRODUCTION THE FLASHING FIELD MODEL
 Biophysical Journal Volume 69 December 1995 2256-2267 Rotary DNA Motors Charles Doering,* Bard Ermentrout,* and George 0ster "Center for Nonlinear Studies, Los Alamos National Laboratory, Los Alamos, New
Biophysical Journal Volume 69 December 1995 2256-2267 Rotary DNA Motors Charles Doering,* Bard Ermentrout,* and George 0ster "Center for Nonlinear Studies, Los Alamos National Laboratory, Los Alamos, New
Biomolecular motors at the intersection of. nanotechnology and polymer science
 Biomolecular motors at the intersection of nanotechnology and polymer science Ashutosh Agarwal 1 and Henry Hess 2 * 1 Department of Materials Science and Engineering, University of Florida, Gainesville,
Biomolecular motors at the intersection of nanotechnology and polymer science Ashutosh Agarwal 1 and Henry Hess 2 * 1 Department of Materials Science and Engineering, University of Florida, Gainesville,
Controlled Microassembly and Transport of Nano- and Micro-components using Bacteria. Sylvain Martel
 Controlled Microassembly and Transport of Nano- and Micro-components using Bacteria Sylvain Martel NanoRobotics Laboratory Department of Computer and Software Engineering, and Institute of Biomedical Engineering
Controlled Microassembly and Transport of Nano- and Micro-components using Bacteria Sylvain Martel NanoRobotics Laboratory Department of Computer and Software Engineering, and Institute of Biomedical Engineering
Movement at the Molecular Level
 Movement at the Molecular Level Diffusion: = 6 D t (D 6 π µ a) Typical numbers: 10 nm protein in water D= 10-10 m 2 /s.in cells D= 10-12 m 2 /s (D= 10-14 m 2 /s lipids) [] 1/2 =1 µm, t ~0.2
Movement at the Molecular Level Diffusion: = 6 D t (D 6 π µ a) Typical numbers: 10 nm protein in water D= 10-10 m 2 /s.in cells D= 10-12 m 2 /s (D= 10-14 m 2 /s lipids) [] 1/2 =1 µm, t ~0.2
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension
 Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Progress in Polymer Science
 Progress in Polymer Science 35 (2010) 252 277 Contents lists available at ScienceDirect Progress in Polymer Science journal homepage: www.elsevier.com/locate/ppolysci Biomolecular motors at the intersection
Progress in Polymer Science 35 (2010) 252 277 Contents lists available at ScienceDirect Progress in Polymer Science journal homepage: www.elsevier.com/locate/ppolysci Biomolecular motors at the intersection
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
Activity Subject Area(s) Associated Unit Associated Lesson Activity Title Header Image 1 ADA Description: Caption: Image file: Source/Rights:
 Activity Subject Area(s) Biology Associated Unit Associated Lesson Activity Title Breaking News: Molecular Trucks Riding Inside Cells! Header Image 1 ADA Description: An ant carries a 1 millimeter square
Activity Subject Area(s) Biology Associated Unit Associated Lesson Activity Title Breaking News: Molecular Trucks Riding Inside Cells! Header Image 1 ADA Description: An ant carries a 1 millimeter square
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules. Dr. Carissa M. Soto
 Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
Self assembly and organization of nanofibers using biological molecular motors
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology
 Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Final exam. Please write your name on the exam and keep an ID card ready.
 Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Biophysics of Molecules
 Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Dark- Field Total Internal Reflection Microscopy for the Study of Kinesin Motor Proteins
 Dark- Field Total Internal Reflection Microscopy for the Study of Kinesin Motor Proteins Christopher Pfeiffer- Kelly Penn State College of Engineering Summer REU Program (CERI) Abstract: Kinesin motor
Dark- Field Total Internal Reflection Microscopy for the Study of Kinesin Motor Proteins Christopher Pfeiffer- Kelly Penn State College of Engineering Summer REU Program (CERI) Abstract: Kinesin motor
Bone-mimetic materials Molecular Devices
 Bone-mimetic materials Molecular Devices Last Time: Today: Reading: organic-templated inorganics structure and assembly of native bone mimicking bone structure/assembly bio/synthetic hybrid molecular devices
Bone-mimetic materials Molecular Devices Last Time: Today: Reading: organic-templated inorganics structure and assembly of native bone mimicking bone structure/assembly bio/synthetic hybrid molecular devices
Aligning Bacterial Cellulose
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures. Xin Zhang Associate Professor.
 Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Technical Seminar 22th Jan 2013 DNA Origami
 Technical Seminar 22th Jan 2013 DNA Origami Hitoshi Takizawa, PhD Agenda 1) Basis of structural DNA nanotechnology 2) DNA origami technique (2D, 3D, complex shape) 3) Programmable nanofactory 4) Application
Technical Seminar 22th Jan 2013 DNA Origami Hitoshi Takizawa, PhD Agenda 1) Basis of structural DNA nanotechnology 2) DNA origami technique (2D, 3D, complex shape) 3) Programmable nanofactory 4) Application
CYTOSKELETON, MOTORPROTEINS.
 CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
(a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The
 1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
OpenPlex. Multiplex Label-Free Interaction Analysis. Open Research Platform
 OpenPlex Multiplex Label-Free Interaction Analysis Open Research Platform OpenPlex Get information about: Kinetics Affinity Specificity Concentration Relative binding OpenPlex is the ideal solution for
OpenPlex Multiplex Label-Free Interaction Analysis Open Research Platform OpenPlex Get information about: Kinetics Affinity Specificity Concentration Relative binding OpenPlex is the ideal solution for
During solution evaporation, there are two major competing evaporation-driven effects, coffee ring effect and Marangoni flow.
 Abstract Evaporation driven particle packing has been investigated to reveal interesting patterns at micrometer to millimeter scale. While the microscopic structures of these patterns are well characterized,
Abstract Evaporation driven particle packing has been investigated to reveal interesting patterns at micrometer to millimeter scale. While the microscopic structures of these patterns are well characterized,
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
DPN 5000 System. Figure 1: The DPN 5000 System. Page 1 of 5. Created on 9/9/2011 Revision
 Introduction NanoInk s is a dedicated, versatile instrument capable of nanopatterning a variety of materials with nanoscale accuracy and precision. With NanoInk s proprietary MEMs devices and deposition
Introduction NanoInk s is a dedicated, versatile instrument capable of nanopatterning a variety of materials with nanoscale accuracy and precision. With NanoInk s proprietary MEMs devices and deposition
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation
 A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
Practice Exam 3 MCBII
 1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
Molecular Communication: Simulation of a Molecular Motor Communication System
 Molecular Communication: Simulation of a Molecular Motor Communication System Michael Moore, Akihiro Enomoto, Tadashi Nakano, Tatsuya Suda Department of Computer Science Donald Bren School of Information
Molecular Communication: Simulation of a Molecular Motor Communication System Michael Moore, Akihiro Enomoto, Tadashi Nakano, Tatsuya Suda Department of Computer Science Donald Bren School of Information
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Nanobiotechnology. Place: IOP 1 st Meeting Room Time: 9:30-12:00. Reference: Review Papers. Grade: 50% midterm, 50% final.
 Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Biological Nanomachines
 Biological Nanomachines Yann R Chemla Dept. of Physics, University of Illinois at Urbana Champaign Saturday Physics for Everyone, Sept. 14, 2013 Biophysics at Illinois Part I: WHAT IS BIOPHYSICS? Physicists
Biological Nanomachines Yann R Chemla Dept. of Physics, University of Illinois at Urbana Champaign Saturday Physics for Everyone, Sept. 14, 2013 Biophysics at Illinois Part I: WHAT IS BIOPHYSICS? Physicists
The Pennsylvania State University. The Graduate School. Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF
 The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering
The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering
DNA Biosensors. Anand Jagota 16 November 2015
 DNA Biosensors Anand Jagota 16 November 2015 1 Market, Unmet Needs Worldwide In-vitro diagnostics ~$ 50 Billion and growing Nucleic Acid diagnostics ~$9 Billion Health, Security, Pathogen Detection, etc.
DNA Biosensors Anand Jagota 16 November 2015 1 Market, Unmet Needs Worldwide In-vitro diagnostics ~$ 50 Billion and growing Nucleic Acid diagnostics ~$9 Billion Health, Security, Pathogen Detection, etc.
Nanosensors. Rachel Heil 12/7/07 Wentworth Institute of Technology Department of Electronics and Mechanical Professor Khabari Ph.D.
 Nanosensors Rachel Heil 12/7/07 Wentworth Institute of Technology Department of Electronics and Mechanical Professor Khabari Ph.D. There are many advances in nanotechnology that if perfected could help
Nanosensors Rachel Heil 12/7/07 Wentworth Institute of Technology Department of Electronics and Mechanical Professor Khabari Ph.D. There are many advances in nanotechnology that if perfected could help
What is Nano-Bio? Non-Covalent Interactions
 - - What is Nano-Bio? Physicist: Biotech: Biologists: -study of molecular interactions -application of nano-tools to study biological systems. -application of nano-tools to detect, treat, and prevent disease
- - What is Nano-Bio? Physicist: Biotech: Biologists: -study of molecular interactions -application of nano-tools to study biological systems. -application of nano-tools to detect, treat, and prevent disease
UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEERING NANOPARTICLES IN BIOMEDICINE
 UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEERING NANOPARTICLES IN BIOMEDICINE Challenges The challenges are: in-situ analysis and in vivo at micro level recognize biomolecules functionality
UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEERING NANOPARTICLES IN BIOMEDICINE Challenges The challenges are: in-situ analysis and in vivo at micro level recognize biomolecules functionality
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Metrology at the Nanoscale What are the Grand Challenges?
 Metrology at the Nanoscale What are the Grand Challenges? Research Challenges for Nanomanufacturing Systems February 11-12, 2008 National Science Foundation Arlington, VA Kevin W. Lyons Manufacturing Engineering
Metrology at the Nanoscale What are the Grand Challenges? Research Challenges for Nanomanufacturing Systems February 11-12, 2008 National Science Foundation Arlington, VA Kevin W. Lyons Manufacturing Engineering
Supplementary Figures 1-6
 1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
NANO-COMMUNICATIONS: AN OVERVIEW
 NANO-COMMUNICATIONS: AN OVERVIEW I. F. AKYILDIZ Georgia Institute of Technology BWN (Broadband Wireless Networking) Lab & Universitat Politecnica de Catalunya EntriCAT (Center for NaNoNetworking in Catalunya)
NANO-COMMUNICATIONS: AN OVERVIEW I. F. AKYILDIZ Georgia Institute of Technology BWN (Broadband Wireless Networking) Lab & Universitat Politecnica de Catalunya EntriCAT (Center for NaNoNetworking in Catalunya)
Detection of local protein structures along DNA using solid-state nanopores
 Detection of local protein structures along DNA using solid-state nanopores nanopore Stefan Kowalczyk Adam Hall Cees Dekker RecA-DNA filament (Nano Letters cover September 2009) Bremen 29-06-2009 Main
Detection of local protein structures along DNA using solid-state nanopores nanopore Stefan Kowalczyk Adam Hall Cees Dekker RecA-DNA filament (Nano Letters cover September 2009) Bremen 29-06-2009 Main
Cell cycle oscillations
 Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Single Molecules Trapped for Study
 Single Molecules Trapped for Study Michelle D. Wang PHYSICS To help us understand some of life s mysteries, we have developed precision optical instruments and techniques to look at important molecules
Single Molecules Trapped for Study Michelle D. Wang PHYSICS To help us understand some of life s mysteries, we have developed precision optical instruments and techniques to look at important molecules
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Reading for lecture 11
 Reading for lecture 11 1. Optical Tweezers, Myosin 2. Atomic Force Microscopy (AFM) 3. Single-Molecule Fluorescence Microscopy 4. Patch-Clamp 5. Genetic Techniques Key references are included in italics
Reading for lecture 11 1. Optical Tweezers, Myosin 2. Atomic Force Microscopy (AFM) 3. Single-Molecule Fluorescence Microscopy 4. Patch-Clamp 5. Genetic Techniques Key references are included in italics
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Kinetics Review. Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets)
 Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Lecture 5. Biomolecular Self-assembly (and Detection)
 10.524 Lecture 5. Biomolecular Self-assembly (and Detection) Instructor: Prof. Zhiyong Gu (Chemical Engineering & UML CHN/NCOE Nanomanufacturing Center) Lecture 6: Biomolecular Self-assembly Table of Contents
10.524 Lecture 5. Biomolecular Self-assembly (and Detection) Instructor: Prof. Zhiyong Gu (Chemical Engineering & UML CHN/NCOE Nanomanufacturing Center) Lecture 6: Biomolecular Self-assembly Table of Contents
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
Nanotechnology for Molecular and Cellular Manipulation
 Nanotechnology for Molecular and Cellular Manipulation Logan Liu Micro and Nano Technology Lab Department of Electrical & Computer Engineering University of Illinois Physical Systems Nano vs. Bio Micro
Nanotechnology for Molecular and Cellular Manipulation Logan Liu Micro and Nano Technology Lab Department of Electrical & Computer Engineering University of Illinois Physical Systems Nano vs. Bio Micro
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Your Name: MID TERM ANSWER SHEET SIN: ( )
 MIDTERM EXAMINATION (October 23, 2008) BIOE150. Introduction to Bio-Nanoscience & Bio-Nanotechnology Professor Seung-Wuk Lee Fall Semester, 2008 0. Write down your name and the last digit of your SIN in
MIDTERM EXAMINATION (October 23, 2008) BIOE150. Introduction to Bio-Nanoscience & Bio-Nanotechnology Professor Seung-Wuk Lee Fall Semester, 2008 0. Write down your name and the last digit of your SIN in
Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules
 Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Module I: Introduction Lecture 1 4 February, 2010
 Module I: Introduction 20.109 Lecture 1 4 February, 2010 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology A powerful and accessible strategy (SELEX) for identifying
Module I: Introduction 20.109 Lecture 1 4 February, 2010 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology A powerful and accessible strategy (SELEX) for identifying
Module I: Introduction
 Module I: Introduction 20.109 Lecture 1 3 February, 2011 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology Appreciating nucleic acids (RNA in particular) as more
Module I: Introduction 20.109 Lecture 1 3 February, 2011 Introduction to: Module Overview Fundamental concepts and techniques in molecular biology Appreciating nucleic acids (RNA in particular) as more
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
EE 45X Biomedical Nanotechnology. Course Proposal
 EE 45X Biomedical Nanotechnology 1 Introduction Jie Chen ECERF W6-019 492-9820 jchen@ece.ualberta.ca Oct. 15, 2008 The purpose of this document is to propose a new course in the area of Biomedical Nanotechnology
EE 45X Biomedical Nanotechnology 1 Introduction Jie Chen ECERF W6-019 492-9820 jchen@ece.ualberta.ca Oct. 15, 2008 The purpose of this document is to propose a new course in the area of Biomedical Nanotechnology
Self-Contained, Biomolecular Motor-Driven Protein Sorting and Concentrating in an Ultrasensitive Microfluidic Chip
 Self-Contained, Biomolecular Motor-Driven Protein Sorting and Concentrating in an Ultrasensitive Microfluidic Chip NANO LETTERS 2008 Vol. 8, No. 4 1041-1046 Chih-Ting Lin, Ming-Tse Kao, Katsuo Kurabayashi,*,
Self-Contained, Biomolecular Motor-Driven Protein Sorting and Concentrating in an Ultrasensitive Microfluidic Chip NANO LETTERS 2008 Vol. 8, No. 4 1041-1046 Chih-Ting Lin, Ming-Tse Kao, Katsuo Kurabayashi,*,
Sept 25 Biochemical Networks. Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks
 Sept 25 Biochemical Networks Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks Background Chemotaxis- signal transduction network Bacterial Chemotaxis Flagellated bacteria
Sept 25 Biochemical Networks Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks Background Chemotaxis- signal transduction network Bacterial Chemotaxis Flagellated bacteria
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements
 Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements NANO LETTERS 2003 Vol. 3, No. 5 633-637 Samira G. Moorjani, Lili Jia, Thomas N.
Lithographically Patterned Channels Spatially Segregate Kinesin Motor Activity and Effectively Guide Microtubule Movements NANO LETTERS 2003 Vol. 3, No. 5 633-637 Samira G. Moorjani, Lili Jia, Thomas N.
Directed Assembly of Nanoparticles for Biosensing Applications
 NSF Nanoscale Science and Engineering Center for High-rate Nanomanufacturing (CHN) www.nano.neu.edu Directed Assembly of Nanoparticles for Biosensing Applications Ahmed Busnaina, Director, NSF Nanoscale
NSF Nanoscale Science and Engineering Center for High-rate Nanomanufacturing (CHN) www.nano.neu.edu Directed Assembly of Nanoparticles for Biosensing Applications Ahmed Busnaina, Director, NSF Nanoscale
SCREENING AND PRESERVATION OF DNA LIBRARIES
 MODULE 4 LECTURE 5 SCREENING AND PRESERVATION OF DNA LIBRARIES 4-5.1. Introduction Library screening is the process of identification of the clones carrying the gene of interest. Screening relies on a
MODULE 4 LECTURE 5 SCREENING AND PRESERVATION OF DNA LIBRARIES 4-5.1. Introduction Library screening is the process of identification of the clones carrying the gene of interest. Screening relies on a
BIOSENOSRS BIO 580. Nanobiosensors WEEK-13 Fall Semester. Faculty: Dr. Javed H. Niazi KM Faculty of Engineering & Natural Sciences Sabanci University
 BIOSENOSRS BIO 580 Nanobiosensors WEEK-13 Fall Semester Faculty: Dr. Javed H. Niazi KM Faculty of Engineering & Natural Sciences Sabanci University Topics that will be covered in the course History of
BIOSENOSRS BIO 580 Nanobiosensors WEEK-13 Fall Semester Faculty: Dr. Javed H. Niazi KM Faculty of Engineering & Natural Sciences Sabanci University Topics that will be covered in the course History of
Leen hajeer. Odai Bani-Monia. Diala abu-hassan
 17 Leen hajeer Odai Bani-Monia Diala abu-hassan We talked last time about actin filaments as a component of the cytoskeleton. In this sheet we are going to talk about second component which is: Microtubules
17 Leen hajeer Odai Bani-Monia Diala abu-hassan We talked last time about actin filaments as a component of the cytoskeleton. In this sheet we are going to talk about second component which is: Microtubules
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Carbohydrate Clustering to Bio-sensing Application
 Faifan Tantakitti (Student ID 0632263) Application for Mary Gates Research Scholarship 1 November 6, 2009 Carbohydrate Clustering to Bio-sensing Application My motivations for getting involved: Growing
Faifan Tantakitti (Student ID 0632263) Application for Mary Gates Research Scholarship 1 November 6, 2009 Carbohydrate Clustering to Bio-sensing Application My motivations for getting involved: Growing
What is bionanotechnology?
 In today s lecture, we will cover: What is bionanotechnology? What is bionanotechnology? Bionanotechnology is nanotechnology that uses biological starting materials, biological design principles or has
In today s lecture, we will cover: What is bionanotechnology? What is bionanotechnology? Bionanotechnology is nanotechnology that uses biological starting materials, biological design principles or has
MICROFABRICATION OF OPTICALLY ACTIVE InO X MICROSTRUCTURES BY ULTRASHORT LASER PULSES
 Journal of Optoelectronics and Advanced Materials Vol. 4, No. 3, September 2002, p. 809-812 MICROFABRICATION OF OPTICALLY ACTIVE InO X MICROSTRUCTURES BY ULTRASHORT LASER PULSES Foundation for Research
Journal of Optoelectronics and Advanced Materials Vol. 4, No. 3, September 2002, p. 809-812 MICROFABRICATION OF OPTICALLY ACTIVE InO X MICROSTRUCTURES BY ULTRASHORT LASER PULSES Foundation for Research
me239 mechanics of the cell homework biopolymers - motivation 3.1 biopolymers - motivation 3. biopolymers biopolymers biopolymers
 3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NPLANTS.2015.111 The non-processive rice kinesin-14 OsKCH1 transports actin filaments along microtubules with two distinct velocities Figure S1 The OsKCH1 constructs used in this study Schematic
DOI: 10.1038/NPLANTS.2015.111 The non-processive rice kinesin-14 OsKCH1 transports actin filaments along microtubules with two distinct velocities Figure S1 The OsKCH1 constructs used in this study Schematic
University of York. BA, BSc, and MSc Degree Examinations Department : BIOLOGY. Title of Exam: Molecular machines. Time Allowed: 2 hours
 Examination Candidate Number: Desk Number: University of York BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular machines Time Allowed: 2 hours Marking Scheme: Total
Examination Candidate Number: Desk Number: University of York BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular machines Time Allowed: 2 hours Marking Scheme: Total
Chapter 17. Molecular Motors. to accompany Biochemistry, 2/e by Reginald Garrett and Charles Grisham. Biochemistry 2/e - Garrett & Grisham
 Chapter 17 Molecular Motors to accompany Biochemistry, 2/e by Reginald Garrett and Charles Grisham All rights reserved. Requests for permission to make copies of any part of the work should be mailed to:
Chapter 17 Molecular Motors to accompany Biochemistry, 2/e by Reginald Garrett and Charles Grisham All rights reserved. Requests for permission to make copies of any part of the work should be mailed to:
Harnessing biological motors to engineer systems for nanoscale transport and assembly
 Harnessing biological motors to engineer systems for nanoscale transport and assembly Living systems use biological nanomotors to build life s essential molecules such as DNA and proteins as well as to
Harnessing biological motors to engineer systems for nanoscale transport and assembly Living systems use biological nanomotors to build life s essential molecules such as DNA and proteins as well as to
Selected Techniques Part I
 1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
The Pennsylvania State University. The Graduate School APPROACHES TO HYBRID SYNTHETIC DEVICES. A Dissertation in. Engineering Science and Mechanics
 The Pennsylvania State University The Graduate School Department of Engineering Science and Mechanics APPROACHES TO HYBRID SYNTHETIC DEVICES A Dissertation in Engineering Science and Mechanics by Vivek
The Pennsylvania State University The Graduate School Department of Engineering Science and Mechanics APPROACHES TO HYBRID SYNTHETIC DEVICES A Dissertation in Engineering Science and Mechanics by Vivek
Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc.
 15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
Ionscope SICM. About Ionscope. Scanning Ion Conductance Microscopy. Ionscope A brand of OpenIOLabs Limited
 SICM About is a brand of OpenIOLabs Ltd, headquartered in Cambridge UK, is the worldleader in (SICM), a rapidly emerging Scanning Probe Microscopy (SPM) technique which allows nanoscale topographical mapping
SICM About is a brand of OpenIOLabs Ltd, headquartered in Cambridge UK, is the worldleader in (SICM), a rapidly emerging Scanning Probe Microscopy (SPM) technique which allows nanoscale topographical mapping
Centre for Synthetic Biology
 Centre for Synthetic Biology Preface Introduction The Groningen Centre for Synthetic Biology (CSB) was established to foster research expertise in the emerging field of synthetic biology. It incorporates
Centre for Synthetic Biology Preface Introduction The Groningen Centre for Synthetic Biology (CSB) was established to foster research expertise in the emerging field of synthetic biology. It incorporates
MagSi Beads. Magnetic Silica Beads for Life Science and Biotechnology study
 MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
Introduction and Techniques
 Single-Molecule Manipulation Experiments of Biological Molecules I: Introduction and Techniques Department of Physics & Astronomy Rice University chkiang@rice.edu www.chkiang.rice.edu Outline Single-molecule
Single-Molecule Manipulation Experiments of Biological Molecules I: Introduction and Techniques Department of Physics & Astronomy Rice University chkiang@rice.edu www.chkiang.rice.edu Outline Single-molecule
Unit 1 Human cells. 1. Division and differentiation in human cells
 Unit 1 Human cells 1. Division and differentiation in human cells Stem cells Describe the process of differentiation. Explain how differentiation is brought about with reference to genes. Name the two
Unit 1 Human cells 1. Division and differentiation in human cells Stem cells Describe the process of differentiation. Explain how differentiation is brought about with reference to genes. Name the two
Motility of CoFe 2 O 4 nanoparticle-labelled microtubules in magnetic fields
 Motility of CoFe 2 O 4 nanoparticle-labelled microtubules in magnetic fields B.M. Hutchins, M. Platt, W.O. Hancock and M.E. Williams Abstract: Kinesin motor proteins transport intracellular cargo unidirectionally
Motility of CoFe 2 O 4 nanoparticle-labelled microtubules in magnetic fields B.M. Hutchins, M. Platt, W.O. Hancock and M.E. Williams Abstract: Kinesin motor proteins transport intracellular cargo unidirectionally
Cell Structure and Function
 Cell Structure and Function Dead White Men Who Discovered (and were made of) Cells: Anton Van Leeuwenhoek Robert Hooke Where the Magic Happened Schleiden Cell Theory All plants are made of cells Schwann
Cell Structure and Function Dead White Men Who Discovered (and were made of) Cells: Anton Van Leeuwenhoek Robert Hooke Where the Magic Happened Schleiden Cell Theory All plants are made of cells Schwann
Chapter 10: Classification of Microorganisms
 Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
2.3 Quantum Dots (QDs)
 2.3 Quantum Dots (QDs) QDs are inorganic nanocrystals, approximately 1 10 nm in size, with unique optical properties of broad excitation, narrow size-tunable emission spectra, high photochemical stability,
2.3 Quantum Dots (QDs) QDs are inorganic nanocrystals, approximately 1 10 nm in size, with unique optical properties of broad excitation, narrow size-tunable emission spectra, high photochemical stability,
Micro-fluidic Chip for Flow Cytometry Jeff Wang
 Micro-fluidic Chip for Flow Cytometry Jeff Wang September, 2015 ABSTRACT This lab course is intended to give students hands-on experience with microfabrication. The project is to make a micro-fluidic chip
Micro-fluidic Chip for Flow Cytometry Jeff Wang September, 2015 ABSTRACT This lab course is intended to give students hands-on experience with microfabrication. The project is to make a micro-fluidic chip
