The State of the Atmosphere is affected by the Biosphere, and vice versa. This lecture will focus on the chemical composition of the atmosphere and
|
|
|
- Moris Jenkins
- 6 years ago
- Views:
Transcription
1 The State of the Atmosphere is affected by the Biosphere, and vice versa. This lecture will focus on the chemical composition of the atmosphere and study how the biosphere has changed it and continues to change it. The thrust of this lecture will be on the Breathing of the Biophsere 1
2 There is humor in our science, e.g. New Yorker cartoon 2
3 3
4 The state of the climate/weather affects the rate processes by which trace gases are produced and consumed by plants and microbes. Conversely, the amount of biogenic material, produced and consumed by plants and microbes, affects the state of the atmosphere. Here is yet another feedback! 4
5 It is big questions like this that drive scientists and their curiosity to learn more. Can you think of some additional questions? 5
6 6
7 The atmosphere is a thin layer across the top of the planet. 99.9% of it is within 50 km, compared to the 6300 km radius of the Earth. On clear days we view the sky as blue. 7
8 Constituents in the atmosphere and their relative fractions.. The atmosphere is dominated by N 2 and inert gas. Oxygen levels remain high (21%) and are out of equilibrium, because biological production meets biological and chemical consumption, consequently O2 levels remain in steady state. 8
9 James Lovelock is the originator of the Gaia Hypothesis Recommended Reading: Gaia, a new look at life on earth / J. E. Lovelock. Lovelock, James, 1919 Oxford ; New York : Oxford University Press, LocationCall No.Status Main (Gardner) Stacks QH313.L68 c.2 AVAILABLE I got to meet Lovelock circa 1982 when I was a postdoc in Oak Ridge, TN and hear him describe his recent book and new and evolving ideas. It started to change my thinking, as I had come from an agricultural and atmospheric science background. The field of Earth System Science was brand new and evolving quickly. His idea that life affected the state and evolution of the chemical composition of the atmosphere resonated with me and made sense. 9
10 A key feature of the presence of life is that the concentrations of reactive gases like Oxygen and Methane remain higher than they would be if equilibrium chemistry was able to proceed. The emission of these gases by life sustains their elevated values. 10
11 A fundamental question we all should know the answer to. But for those who are non science majors this is a great starting point 11
12 12
13 content/uploads/2010/12/blue sky night sky.jpg 13
14 The composition of the atmosphere has close connections with the biosphere, and vice versa, as well with poetry and literature. These connections will be explored in this lecture 14
15 In Beijing and other Megacities, humans are injecting so much pollution and pollution forming chemicals into the sky that the sky is not blue and is opaque with smog. Unless we understand the biosphere and try and rectify emissions of pollution into the sky this can be the fate for many of us and already is in much of China, India, Mexico City, etc 15
16 Most of the Atmosphere is dinitrogen and it is opaque to sunlight and longwave, terrestrial energy. Greenhouse gases are important because they absorb longwave radiation, reradiate it back to the surface and warm the surface to hospitable temperatures; longwave energy interacts with molecules like H2O, CH4, CO2, O3, causing them to resonate in the forms of vibration, rotation, stretching 16
17 There are 4 major zones: 1) the Troposphere, where we live and where there is Weather; 2) the Stratosphere, where there is the ozone layer, where jets fly and is stable, hence the narrow contrails we see. The uppermost layers are the 3) Mesosphere and the 4) Thermosphere where there is less than 0.1% of the atmosphere, based on pressure. Most important: is that 99.9% of the atmosphere is below 50 km. Temperature decreases with height in the Troposphere and it Increases with height in the Stratosphere. Zones where temperature decreases with height allows convection and mixing to occur. Zones where temperature increases with height suppress convection and mixing and are more stable. The stratosphere is stably stratified and explains why jet contrails don t dissipate fast. Why is the Temperate warm in the stratosphere? This is where the stratospheric ozone layer resides. The absorption of shortwave, high energy ultraviolet light causes the temperature of this region to be elevated. The temperature of the Thermosphere may be high, but there is so little atmosphere with enough heat capacity, I suspect you would not be able to go there shirtless. 17
18 The pressure decreases exponentially with height. One is at about one half of the atmosphere at about 5.5 km, about 18,000 feet, like many mountains in the Andes. If you climbed Denali (> 20,000 ft) in Alaska you would be above one half of the atmosphere 18
19 If we are to understand the composition of different gases in the atmosphere we must first be aware of the Law of Partial Pressures 19
20 What are the Units?? 20
21 How can we weigh the atmosphere? We can use the laws of physics to compute the mass of the atmosphere by knowing the surface area of the planet, pressure at the surface and the acceleration of gravity. Remember Pressure is Force per area and Force is Mass times acceleration. So Pressure is Mass of the Atmosphere times the acceleration of gravity (9.8 m s 2 ) divided by the Surface area of the planet. So we can solve for Mass 21
22 Here is the math kpa/9.80 *4 * pi * ( ^3)^2 = ^18 kg 22
23 So if we know the mixing ratio of a gas, like CO2, we can also compute its mass in the atmosphere. Why do these simple computations? In this lecture and those to follow I want you to be able to interconvert information on the atmospheric burden of gases like CO2 in terms of mass vs their mixing ratio. This will help you better understand if we release x Pg C in the next year or decade what that will do to the mixing ratio. With this equation, if we know the net flux of CO2 into the atmosphere we can compute future concentrations 23
24 Revisit Principle of Conservation of Mass as it defines how Concentrations in the atmosphere change with time. It is due to a Flux Convergence/Divergence. In other words how Fluxes differ across the top and bottom of the control volume. 24
25 Definition of Turnover Time 25
26 Definition of Flux and Flux Density. A visual example is the number of cars passing some reference point per day. 26
27 Here is a conceptual overview diagram of the various gases that have either biospheric sinks or sources. Also shown are the general flux density magnitudes. Different gases are associated with vegetation, with aerobic and oxic soils, wetlands and anaerobic or anoxic soils. 27
28 A resistance network, with an analogy to an electronic circuit, is used to quantify and describe the biophysical controls of trace gas exchange Fluxes tend to be a balance between demand and supply To estimate Flux rates we can define the biosphere as a networks of resistors acting across a current defined by the difference in the trace gas mixing ratio in the atmosphere and at the surface. Resistors in the network include those associated with turbulence and mixing in the atmosphere, with the development of a boundary layer at the interface between plants/soil/atmosphere. Then there are biological barriers through which gases pass. These are the pores on leaves, called stomata, which are active and open and close, depending on environmental and biological conditions 28
29 The CO2 Molecule. It is 44 g per mole. 29
30 CO2 absorbs longwave, infrared radiation. This property helps explain why and how CO2 is an effective greenhouse gas. Its major IR absorption wave numbers (2350 and 667 cm 1) or wavelengths (4.25 and um) correspond with the spectrum of terrestrial longwave energy that is emitted from the surface (~ 298 K) according to Planck s law. CO2 molecules will absorb this outgoing energy, then re radiate it back to the surface (and outward). It is this re radiation of longwave energy that affects the surface radiation balance, positively, causing the temperature of the surface to be warmer than the radiative temperature of the planet, allowing water to be liquid and life to flourish. Too much CO 2, like on Venus, can cause the surface to be so hot that it can melt lead. 30
31 The change in [CO2] is due to the imbalance between sources and sinks. Here are the major sources and sinks in action. We will cover the actual fluxes in the future lecture on the global carbon balance. 31
32 The is the Mauna Loa Station in Hawaii where Dave Keeling started the long record of CO2 observations. It is a remote location in the Pacific Ocean, where CO2 can be well mixed and produce a good representative measure of the world s CO2. Visit the web site and take a virtual tour of the observatory and look at some of the records 32
33 This is the iconic figure of how the burden of CO 2 has changed in our atmosphere over the past 50+ years. It is a direct measure of the breathing of the biosphere and provide evidence how carbon dioxide from fossil fuel emissions is accumulating in our atmosphere. Go to the NOAA web site, down load the data and plot it yourself
34 Most land is in the northern hemisphere, so during the northern hemisphere summer the sink due to photosynthesis out paces sources, so there is a seasonal draw down in CO2 34
35 Look at amplitude in the north and southern hemisphere..look at phase difference in peaks and troughs. The greatest seasonal amplitudes of CO2 are towards the North pole. Amplitudes are much smaller in the southern hemisphere because it is dominated by oceans, which experience less dramatic swings in carbon uptake and release with the change in the growing season. 35
36 Politicians are always saying there is no proof we are changing the climate through changing the composition of the CO2 in the atmosphere ; Ben Carson, a 2016 presidential candidate said it a couple of days ago. Here is the Proof. Fossil fuels are plant based so their emission reflects the isotopic signature of C 3 photosynthesis. These plants prefer molecules of 12 CO 2 over heavier isotopes consisting of 13 CO 2. Over time the plant retain an isotopic signal of about 25 parts per mill. In the preindustrial age the atmosphere maintained an isotopic signal of about 6.4 per mill. So the combustion of fossil fuel with a 25 parts per mill signal has diluted the signal of the atmosphere since the industrial age. The isotopic record is a smoky gun evidence that fossil fuel combustion is changing the CO2 burden of the atmosphere. δ13c = Rsample Rstandard x 1000 Rstandard where R = 13C/12C 36
37 PeeDee Belimnite. Example of how the stable isotope ratio changes with the delta notation, that is commonly used.. Bottom line, as del 13C becomes more negative, there is less 13 C relative to 12 C in the material, making it isotopically lighter In general there is about 1% 13 C in the air compared to 12 C. 37
38 This figure bookends 3 Eras of CO 2 change over the last 800k years. Part A is shows how CO 2 rose and fell during periods of inter glaciation and glaciation. Part B sees the rise in CO 2 as societies form after the Dark Age and transition into the Industrial Era. Part C shows the more rapid rise as our energy and economy became vibrant and carbonized We will look at each of these in more detail. 38
39 Is CO2 greater day or night?; Winter or Summer 39
40 Properties. 16 g/mole, atmospheric lifetime is about 12 years 40
41 Methane also absorbed outgoing terrestrial radiation in spectral lines associated with outgoing, longwave radiation cm 1; 3321 nm 1300 cm 1; 7692 nm A kilogram of methane is 25 times more effective than a kilogram of CO2 in absorbing IR radiation over a 100 time scale. The greenhouse warming potential considers the spectral bands of IR absorption and the lifetime of the chemical compound in the atmosphere. 41
42 Methane concentrations have increased rapidly over the past 200 years, due to human associated activities. Today, methane levels exceed 1750 ppb 42
43 ATMOSPHERIC SCIENCEMethane on the Rise AgainEuan G. Nisbet, Edward J. Dlugokencky, and Philippe Bousquet Science 31 January 2014: 343 (6170), [DOI: /science ] 43
44 Like CO2 there is a greater amplitude and more production of methane in the northern hemisphere than the southern hemisphere. The north is dominated by land, with sources like rice paddies, cows, termites and gas wells..sinks of methane are from the hydroxyl radical, OH, called the detergent of the atmosphere The amplitudes of the north and south hemisphere are out of phase due to the timing of the warmer summers that stimulate enzymatic activity and life and OH activity. 44
45 Like CO 2 there has been an ebb and flow in the methane concentration between ice ages and inter glacial periods. These reflect feedbacks among warmth and biological activity and changes in the composition of the atmosphere that reinforce or inhibit greenhouse warming of the surface by trace gases. Here Methane peaks at about 700 ppb during the interglacial periods and reaches a nadir at about 350 ppb during the depth of the ice age. In other words, methane concentrations repeatedly varied by a factor of two between glacial and interglacial periods. 45
46 Methane has various routes of transport and destruction after it is produced in anoxic sediments. Methane can be transported through slow diffusion through the water column. At night if the surface is cooler than the deeper water layers, convection will occur and this can increase transport rates and efficiencies. As methane moves through the water column the water will become oxygenated and this will lead to reactions that will destroy methane. A more effective means of transport is ebullition, a fancy word for bubbles. If you look at a methane producing pond under still conditions you will see bubbles. Plant mediated xylem transport is another effective means of methane transport. Wetland plants have large vessels, called aerenchyma. These evolved to enhance oxygen diffusion to the roots for respiration in low oxygen sediments. But doors and routes evolved to facility the uptake of one gas will also yield opportunities for losses of another (like stomata, CO2 and water vapor). Here, methane can take a preferred route of transport to the atmosphere. Finally, there is a link between plants, exudation of recent photosynthesis by the roots and the stimulation of methane by archaea. 46
47 Example of aerenchyma cross section of wetland plants 47
48 Shows alternative routes of electrons from organic matter before methane can be produced. Methane production will be inhibited if other electron acceptors, like O2, iron, nitrate and sulfate, are present in the sediments 48
49 Methane can be produced by two routes after the fermentation of organic matter. One route involves the reaction between CO2 and H2. The other involves the breakdown of acetate, CH 3 COO + H + > CO 2 + CH 4 49
50 Cows, sheep and termite have archaea in their stomachs to help them to break down and digest organic matter, releasing methane in the meantime 50
51 Cow methane emissions will increase with diet, variety and season. How to estimate cow methane emissions? One clever method is the dual tracer technique. Scientists put a sulfur dioxide permeation tube in the belly of cows that emits SO2 at a known rate at a known temperature. By measuring the ratio of SO2 and CH4 measured at the cows mouth, and knowing the SO2 emission rate, the methane emission rate can be computed. 51
52 What is a Terragram?? Methane is produced directly and indirectly from human activities. What are the direct routes?...energy production and biomass burning What are the indirect sources? Enteric fermentation from cattle production and rice cultivation Enteric fermentation occurs in the guts of cows 52
53 53
54 We have seen in earlier lectures the co evolution of life, life forms, oxygen and evolution 54
55 Oxygen is form by the splitting of water during the first step of photosynthesis 55
56 One of the early assumptions of the homeostatic, self regulatory, power of Gaia was the near constancy of oxygen over millions of years. Watson and Lovelock argued about a feedback between oxygen and fire. They did some lab experiments which asserted that dry paper would ignite if O2 levels exceeded 25% 56
57 57
58 Geochemical modelers infer that O2 levels weren t so constant back to the start of the Phanerozoic. Data of Berner counter Lovelock and Watson that O2 was constant Phanerozoic, period of revealed life; Maximum of O2 corresponds with evolution of vascular plants, whose lignin was difficult to decompose and resulted in significant carbon burial in swamps and sediments. We talk about multiple constraints and interconnected consistency. The elevated O2 of the carboniferous was associated with gigantism in insects. They can only breathe effectively through their surface, so they needed higher O2 to overcome the surface to volume ratio that came with large size. 58
59 The Build up of CO2 by fossil fuel combustion consumes oxygen. Hence, we are seeing a downward trend in O2. But the background is so large we don t expect to run out of air. Figure from Ralph Keeling 59
60 60
61 61
62 There is good ozone in the stratosphere and bad ozone in the troposphere 62
63 Shows the location and implications of good and bad ozone. Good ozone is in the Stratosphere; it absorbs high energy uv light and protects life from its effects, like mutations. Bad ozone is in the troposphere, affecting the air we breathe, human and plant health 63
64 64
65 g Ozone is not well mixed like other long lived greenhouse gases. While there are regional differences in absolute ozone, in general there is a world wide increase in its concentration. Earliest data are at the turn of the 20 th Century in Paris, where concentrations were below 20 ppm. The density of monitoring networks have improved, as so the instruments, so the modern record is viewed as more reliable. 65
66 Observed trends in ozone 66
67 67
68 Simple set of reactions that lead to the photochemical production of ozone 68
69 The fresh smell in the woods are volatile organic hydrocarbons, like monoterpenes and isoprene. They play pivotal roles in the production of photochemcial ozone 69
70 Whether ozone is produced depends on the combination of VOX and Nox concentrations 70
71 VOCs play many roles in regards to producing aerosols that can reflect light or act as cloud condensation nucleii. Chemically, they play a central role in ozone production and for biology they help plants defend against pathogens and attract pollenators. 71
72 72
73 High energy, and very short wave ultra violet light splits O 2 molecules and the single O molecules can combine with other O 2 molecules and form ozone, O 3 The production of ozone is not unchecked due to its destruction by CFCs and nitrogen compounds, which are leading to the ozone hole over the Antarcrtic 73
74 Uv light breaks up CFCs, releasing a chlorine atom, Cl. This cleaves O3, ozone, to form ClO. Other O molecules split ClO to enable free Cl atoms to repeat the set of reactions. 74
75 Ozone in the stratosphere is depleted from Cl from CFC and NO from N 2 O. Single O atoms cause N2O to form 2 NO molecules and the NO leads to the destruction of O3. N 2 O + O(1D) > 2NO NO + O3 > NO2 + O2 NO2 + O > NO + O2 Net: O + O3 > 2 O2 75
76 Map showing the Antarctic ozone hole in 2003 Susan Solomon, a UCB graduate, was instrumental in discovering the mechanism for ozone destruction in the Statosphere. She conducted many studies in Antarctica. 76
77 An overview of the ozone hole is produced by NASA Hole Poster_hiRes_508.pdf 77
78 78
79 We will cover nitrogen gases later in this course. 79
80 f1.jpg 80
81 81
82 82
83 Here are some Key points.. Help identify others gleaned from this lecture. 83
84 84
TODAY: TOPIC #6 WRAP UP!! Atmospheric Structure & Composition
 TODAY: TOPIC #6 WRAP UP!! Atmospheric Structure & Composition There s one more thing to correct in our the depiction of incoming Solar....... the atmosphere is NOT totally TRANSPARENT to INCOMING Solar
TODAY: TOPIC #6 WRAP UP!! Atmospheric Structure & Composition There s one more thing to correct in our the depiction of incoming Solar....... the atmosphere is NOT totally TRANSPARENT to INCOMING Solar
Topic # 7 Part II ATMOSPHERIC STRUCTURE & CHEMICAL COMPOSITION
 Topic # 7 Part II ATMOSPHERIC STRUCTURE & CHEMICAL COMPOSITION All about the GASES IN THE ATMOSPHERE, esp. GREENHOUSE GASES! Class Notes pp 37-41 REVIEW: ATMOSPHERIC STRUCTURE The changes in temperature
Topic # 7 Part II ATMOSPHERIC STRUCTURE & CHEMICAL COMPOSITION All about the GASES IN THE ATMOSPHERE, esp. GREENHOUSE GASES! Class Notes pp 37-41 REVIEW: ATMOSPHERIC STRUCTURE The changes in temperature
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32)
 Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Energy and the Earth. Key words: Incoming Solar Radiation, Electromagnetic wave, Greenhouse effect, conduction, convection, radiation.
 S c i e n c e Energy and the Earth Key words: Incoming Solar Radiation, Electromagnetic wave, Greenhouse effect, conduction, convection, radiation. Energy transfer Heat is energy in transit from warmer
S c i e n c e Energy and the Earth Key words: Incoming Solar Radiation, Electromagnetic wave, Greenhouse effect, conduction, convection, radiation. Energy transfer Heat is energy in transit from warmer
The September Equinox is today: Sep 23rd! It s considered the traditional end of Summer and the beginning of Fall
 More coming up in Topic #11 (class notes p 61) The September Equinox is today: Sep 23rd! It s considered the traditional end of Summer and the beginning of Fall The Sun s rays have greatest intensity right
More coming up in Topic #11 (class notes p 61) The September Equinox is today: Sep 23rd! It s considered the traditional end of Summer and the beginning of Fall The Sun s rays have greatest intensity right
Topic # 7 ATMOSPHERIC STRUCTURE & CHEMICAL COMPOSITION
 Topic # 7 ATMOSPHERIC STRUCTURE & CHEMICAL COMPOSITION All about the GASES IN THE ATMOSPHERE, esp. GREENHOUSE GASES! Class Notes pp 37-41 OBJECTIVES: To understand: -- the VERTICALSTRUCTURE of the atmosphere
Topic # 7 ATMOSPHERIC STRUCTURE & CHEMICAL COMPOSITION All about the GASES IN THE ATMOSPHERE, esp. GREENHOUSE GASES! Class Notes pp 37-41 OBJECTIVES: To understand: -- the VERTICALSTRUCTURE of the atmosphere
Module 7: Combustion and Environment Lecture 36: Atmosphere. The Lecture Contains: Atmosphere. Chemical Emission From Combustion
 The Lecture Contains: Atmosphere Chemical Emission From Combustion Chemicals From Combustion (Contd..) file:///d /Web%20Course/Dr.%20D.P.%20Mishra/Local%20Server/FOC/lecture36/36_1.htm[10/5/2012 4:32:17
The Lecture Contains: Atmosphere Chemical Emission From Combustion Chemicals From Combustion (Contd..) file:///d /Web%20Course/Dr.%20D.P.%20Mishra/Local%20Server/FOC/lecture36/36_1.htm[10/5/2012 4:32:17
1 Characteristics of the Atmosphere
 CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
Greenhouse Effect. The Greenhouse Effect
 Greenhouse Effect The Greenhouse Effect Greenhouse gases let short-wavelength radiation come into the Earth s atmosphere from the sun. However, they absorb and re-radiate Earth s long-wavelength radiation
Greenhouse Effect The Greenhouse Effect Greenhouse gases let short-wavelength radiation come into the Earth s atmosphere from the sun. However, they absorb and re-radiate Earth s long-wavelength radiation
Global Warming Science Solar Radiation
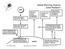 SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
Earth's Atmosphere. Atmospheric Layers. Atmospheric Layers
 Earth's Atmosphere Today we will talk about the part of Earth that is most important to our survival - the atmosphere Earth's atmosphere is unique in the Solar System and has changed greatly over time
Earth's Atmosphere Today we will talk about the part of Earth that is most important to our survival - the atmosphere Earth's atmosphere is unique in the Solar System and has changed greatly over time
Energy, Greenhouse Gases and the Carbon Cycle
 Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Physics 100 Lecture 17. The Greenhouse Effect and Global Warming April 2, 2018
 1 Physics 100 Lecture 17 The Greenhouse Effect and Global Warming April 2, 2018 2 Class Quiz Ch. 7: Suppose your car burned bituminous coal instead of gasoline. How much coal would provide the same energy
1 Physics 100 Lecture 17 The Greenhouse Effect and Global Warming April 2, 2018 2 Class Quiz Ch. 7: Suppose your car burned bituminous coal instead of gasoline. How much coal would provide the same energy
The next 2 weeks. Reading: IPCC (2007), Chap 7 (sections 7.4 and 7.5)
 PCC 588 Jan 15 The next 2 weeks Th. Jan 15: non-co 2 greenhouse gases CH 4 and N 2 O Tu. Jan 20: non-co 2 greenhouse gases: ozone, halocarbons Th. Jan 22: Aerosols and Climate Tu. Jan 27: Paper discussion
PCC 588 Jan 15 The next 2 weeks Th. Jan 15: non-co 2 greenhouse gases CH 4 and N 2 O Tu. Jan 20: non-co 2 greenhouse gases: ozone, halocarbons Th. Jan 22: Aerosols and Climate Tu. Jan 27: Paper discussion
Lecture 28: Radiative Forcing of Climate Change
 Lecture 28: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
Lecture 28: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
The Earth s Global Energy Balance
 The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
Introduction. Introduction. Introduction. Outline Last IPCC report : 2001 Last IPCC report :
 Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Thursday Sep 25th SIT WITH YOUR GROUP TODAY! Topic # 6 Atmospheric Structure & Chemical Composition
 Thursday Sep 25th SIT WITH YOUR GROUP TODAY! Topic # 6 Atmospheric Structure & Chemical Composition Self Test 4 & RQ-4 on The Laws of Thermodynamics are now posted. The readings that will prepare you for
Thursday Sep 25th SIT WITH YOUR GROUP TODAY! Topic # 6 Atmospheric Structure & Chemical Composition Self Test 4 & RQ-4 on The Laws of Thermodynamics are now posted. The readings that will prepare you for
Chapter 19 Global Change. Wednesday, April 18, 18
 Chapter 19 Global Change Module 62 Global Climate Change and the Greenhouse Effect After reading this module you should be able to distinguish among global change, global climate change, and global warming.
Chapter 19 Global Change Module 62 Global Climate Change and the Greenhouse Effect After reading this module you should be able to distinguish among global change, global climate change, and global warming.
Lecture 27: Radiative Forcing of Climate Change
 Lecture 27: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
Lecture 27: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
ANSWER KEY TO THE PRACTICE QUESTIONS ON THE FINAL EXAM STUDY GUIDE
 ANSWER KEY TO THE PRACTICE QUESTIONS ON THE FINAL EXAM STUDY GUIDE -- 2013 1. Y (visible) 2. Z (infrared) 3. X (ultraviolet) 4. d (the absorption is occurring almost entirely in the infrared (LW) part
ANSWER KEY TO THE PRACTICE QUESTIONS ON THE FINAL EXAM STUDY GUIDE -- 2013 1. Y (visible) 2. Z (infrared) 3. X (ultraviolet) 4. d (the absorption is occurring almost entirely in the infrared (LW) part
GLOBAL WARMING COMPUTER LAB
 GLOBAL WARMING COMPUTER LAB A COMPUTER SIMULATION PROGRAM ON TEMPERATURE CHANGE AND SEA LEVEL RISING After performing this computer simulation lab you will be able to: 1) understand the greenhouse effect
GLOBAL WARMING COMPUTER LAB A COMPUTER SIMULATION PROGRAM ON TEMPERATURE CHANGE AND SEA LEVEL RISING After performing this computer simulation lab you will be able to: 1) understand the greenhouse effect
Gases and the atmosphere
 Gases and the atmosphere Chemical reactions in the atmosphere Ozone cycle Ozone depletion Greenhouse effect Global Warming Pollution in the troposphere 1 Gases and the atmosphere Review Composition of
Gases and the atmosphere Chemical reactions in the atmosphere Ozone cycle Ozone depletion Greenhouse effect Global Warming Pollution in the troposphere 1 Gases and the atmosphere Review Composition of
G-5 ACTIVITY ON VOLCANISM & CLIMATE THE ANSWERS!
 G-5 ACTIVITY ON VOLCANISM & CLIMATE THE ANSWERS! #1. List 4 reasons why Tambora in 1815 resulted in the largest GLOBAL cooling: #1 Low latitude eruption both hemispheres #2 Large amount of eruptive material
G-5 ACTIVITY ON VOLCANISM & CLIMATE THE ANSWERS! #1. List 4 reasons why Tambora in 1815 resulted in the largest GLOBAL cooling: #1 Low latitude eruption both hemispheres #2 Large amount of eruptive material
The Earth s Atmosphere-I. GEOL 1350: Introduction To Meteorology
 The Earth s Atmosphere-I GEOL 1350: Introduction To Meteorology 1 Overview What is the composition of Atmosphere? How did the atmosphere arrive at its current state? 2 Earth s Atmosphere Earth s atmosphere
The Earth s Atmosphere-I GEOL 1350: Introduction To Meteorology 1 Overview What is the composition of Atmosphere? How did the atmosphere arrive at its current state? 2 Earth s Atmosphere Earth s atmosphere
Other GHGs. IPCC Climate Change 2007: The Physical Science Basis
 Other GHGs IPCC Climate Change 2007: The Physical Science Basis 1 Atmospheric Chemistry and other long-lived GHG during the industrial period 1750-2000 The radiative forcing of climate during the period
Other GHGs IPCC Climate Change 2007: The Physical Science Basis 1 Atmospheric Chemistry and other long-lived GHG during the industrial period 1750-2000 The radiative forcing of climate during the period
Atmospheric Chemistry (Option 1B)
 Atmospheric Chemistry (Option 1B) Introduction Option 1B consists of the following subsections: 1B.1 Oxygen 1B.2 Nitrogen 1B.3 Carbon Dioxide 1B.4 Atmospheric Pollution 1B.5 The Ozone Layer Some chemistry
Atmospheric Chemistry (Option 1B) Introduction Option 1B consists of the following subsections: 1B.1 Oxygen 1B.2 Nitrogen 1B.3 Carbon Dioxide 1B.4 Atmospheric Pollution 1B.5 The Ozone Layer Some chemistry
Chapter 4: The Global Energy System
 Discovering Physical Geography Third Edition by Alan Arbogast Chapter 4: The Global Energy System The Electromagnetic Spectrum and Solar Energy Solar Energy as Radiation Electromagnetic energy transmitted
Discovering Physical Geography Third Edition by Alan Arbogast Chapter 4: The Global Energy System The Electromagnetic Spectrum and Solar Energy Solar Energy as Radiation Electromagnetic energy transmitted
Lecture 11: Global Warming. Human Acticities. Natural Climate Changes. Global Warming: Natural or Man-Made CO 2 CH 4
 Lecture 11: Global Warming Human Acticities CO 2 CH 4 The initial appearance of human species: last 100,000 to 200,000 years Development of the first civilization: the last 10,000 years What is the sensitivity
Lecture 11: Global Warming Human Acticities CO 2 CH 4 The initial appearance of human species: last 100,000 to 200,000 years Development of the first civilization: the last 10,000 years What is the sensitivity
Gases and the atmosphere
 Gases and the atmosphere Chemical reactions in the atmosphere Ozone cycle Ozone depletion Greenhouse effect Global Warming Pollution in the troposphere 1 Gases and the atmosphere Review Composition of
Gases and the atmosphere Chemical reactions in the atmosphere Ozone cycle Ozone depletion Greenhouse effect Global Warming Pollution in the troposphere 1 Gases and the atmosphere Review Composition of
Winter 2009: ATMS/OCN/ESS 588 The Global Carbon Cycle and Greenhouse Gases. Course Goals
 PCC 588 - January 6 and 8 2009 Winter 2009: ATMS/OCN/ESS 588 The Global Carbon Cycle and Greenhouse Gases T,Th 12:00-1:20 pm OSB 25 Course Goals The course focuses on factors controlling the global cycle
PCC 588 - January 6 and 8 2009 Winter 2009: ATMS/OCN/ESS 588 The Global Carbon Cycle and Greenhouse Gases T,Th 12:00-1:20 pm OSB 25 Course Goals The course focuses on factors controlling the global cycle
A MINI FINAL EXAM REVIEW: SOME PRACTICE QUESTIONS
 A MINI FINAL EXAM REVIEW: SOME PRACTICE QUESTIONS FIRST -- The answers to the G-5 GROUP ACTIVITY on VOLCANISM & CLIMATE G-5 VOLCANISM & CLIMATE ACTIVITY #1. List 4 reasons why Tambora in 1815 resulted
A MINI FINAL EXAM REVIEW: SOME PRACTICE QUESTIONS FIRST -- The answers to the G-5 GROUP ACTIVITY on VOLCANISM & CLIMATE G-5 VOLCANISM & CLIMATE ACTIVITY #1. List 4 reasons why Tambora in 1815 resulted
I. Pollutants A. Harmful substances the enter the environment
 I. Pollutants A. Harmful substances the enter the environment II. Two Classifications A. Particulates 1. Tiny substances (liquid or solid) suspended in the atmosphere 2. Examples: Dust, Ash, and Soot 3.
I. Pollutants A. Harmful substances the enter the environment II. Two Classifications A. Particulates 1. Tiny substances (liquid or solid) suspended in the atmosphere 2. Examples: Dust, Ash, and Soot 3.
Chapter 11: Atmosphere
 To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
Some resources (more websites later)
 Some resources (more websites later) Intergovernmental Panel Climate Change 2001: The Scientific Basis at http://www.ipcc.ch/pub/reports.htm John Houghton Global Warming - the complete briefing Cambridge
Some resources (more websites later) Intergovernmental Panel Climate Change 2001: The Scientific Basis at http://www.ipcc.ch/pub/reports.htm John Houghton Global Warming - the complete briefing Cambridge
History of significant air pollution events
 Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
CALIFORNIA EDUCATION AND THE ENVIRONMENT INITIATIVE
 Water Vapor: A GHG Lesson 3 page 1 of 2 Water Vapor: A GHG Water vapor in our atmosphere is an important greenhouse gas (GHG). On a cloudy day we can see evidence of the amount of water vapor in our atmosphere.
Water Vapor: A GHG Lesson 3 page 1 of 2 Water Vapor: A GHG Water vapor in our atmosphere is an important greenhouse gas (GHG). On a cloudy day we can see evidence of the amount of water vapor in our atmosphere.
Chapter 15 Air Pollution and Stratospheric Ozone Depletion
 Chapter 15 Air Pollution and Stratospheric Ozone Depletion Air Pollution Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough
Chapter 15 Air Pollution and Stratospheric Ozone Depletion Air Pollution Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough
The Atmospheric System 6.1
 The Atmospheric System 6.1 What is the atmosphere? Layer of gas that surrounds our planet. The atmosphere is a dynamic system with inputs, outputs, storages and flows. Heat and pollutants are carried
The Atmospheric System 6.1 What is the atmosphere? Layer of gas that surrounds our planet. The atmosphere is a dynamic system with inputs, outputs, storages and flows. Heat and pollutants are carried
Greenhouse Effect Student Activity Book
 I. Introduction Greenhouse Effect Student Activity Book The greenhouse effect is an increase in the average temperature of the Earth. It happens because certain gases absorb infrared heat that would normally
I. Introduction Greenhouse Effect Student Activity Book The greenhouse effect is an increase in the average temperature of the Earth. It happens because certain gases absorb infrared heat that would normally
Greenhouse Effect Activity Key
 I. Introduction Greenhouse Effect Activity Key The greenhouse effect is an increase in the average temperature of the Earth. It happens because certain gases absorb infrared heat that would normally be
I. Introduction Greenhouse Effect Activity Key The greenhouse effect is an increase in the average temperature of the Earth. It happens because certain gases absorb infrared heat that would normally be
Today. Terrestrial Planets. Atmospheres Climate. Factors affecting atmospheres. Earth, Venus, Mars. Greenhouse effect from planetary perspective
 Today Terrestrial Planets Earth, Venus, Mars Atmospheres Climate Greenhouse effect from planetary perspective Factors affecting atmospheres EXAM NEXT TIME Why the sky is blue Atmosphere scatters blue light
Today Terrestrial Planets Earth, Venus, Mars Atmospheres Climate Greenhouse effect from planetary perspective Factors affecting atmospheres EXAM NEXT TIME Why the sky is blue Atmosphere scatters blue light
Climate Dynamics (PCC 587): Climate Forcings
 Climate Dynamics (PCC 587): Climate Forcings DARGAN M. W. FRIERSON UNIVERSITY OF WASHINGTON, DEPARTMENT OF ATMOSPHERIC SCIENCES DAY 7: 10-16-13 Outline of This Topic Climate forcings Things that directly
Climate Dynamics (PCC 587): Climate Forcings DARGAN M. W. FRIERSON UNIVERSITY OF WASHINGTON, DEPARTMENT OF ATMOSPHERIC SCIENCES DAY 7: 10-16-13 Outline of This Topic Climate forcings Things that directly
FINAL EXAM STUDYING JUMP START REVIEW. Some review from earlier in the semester and some Q s on more recent topics...
 FINAL EXAM STUDYING JUMP START REVIEW Some review from earlier in the semester and some Q s on more recent topics.... The wavelength range of infrared 1. < 0.4 micrometers radiation. 2. > 0.7 micrometers
FINAL EXAM STUDYING JUMP START REVIEW Some review from earlier in the semester and some Q s on more recent topics.... The wavelength range of infrared 1. < 0.4 micrometers radiation. 2. > 0.7 micrometers
Name: Class: Date: 6. Most air pollution is produced by a. thermal inversions. c. ozone layer depletion. b. fuel burning. d. volcanic eruptions.
 Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
3/4/2014. Air Pollution. Chapter 15 Air Pollution and Stratospheric Ozone Depletion. Major Air Pollutants. Primary Pollutants
 Air Pollution Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings,
Air Pollution Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings,
High School Climate Science Curriculum Course learning goals. October 2011
 1 High School Climate Science Curriculum Course learning goals October 2011 Current Climate 1. Earth climate is determined by a balance between absorbed sunlight and emitted infrared radiation. Because
1 High School Climate Science Curriculum Course learning goals October 2011 Current Climate 1. Earth climate is determined by a balance between absorbed sunlight and emitted infrared radiation. Because
Chapter 5. The Earth s Atmosphere
 Chapter 5 The Earth s Atmosphere Layers of the Earth Earth largest of the inner planets Gravity strong enough to hold gases. Lots of spheres Equator divided the Earth into two hemispheres Lithosphere-
Chapter 5 The Earth s Atmosphere Layers of the Earth Earth largest of the inner planets Gravity strong enough to hold gases. Lots of spheres Equator divided the Earth into two hemispheres Lithosphere-
How is the atmosphere different from outer space? a mixture of gases that surrounds the Earth
 Chapter 15 Atmosphere Section 1 Objectives Describe the composition of Earth's atmosphere. Explain why air pressure changes with altitude. Explain how air temperature changes with atmospheric composition.
Chapter 15 Atmosphere Section 1 Objectives Describe the composition of Earth's atmosphere. Explain why air pressure changes with altitude. Explain how air temperature changes with atmospheric composition.
Leif Backman HENVI Seminar February 19, 2009
 Methane Sources and Sinks Leif Backman HENVI Seminar February 19, 2009 Background Atmospheric methane Sources & Sinks Concentration variations & trends Objective & methods Objective & Goals Research plan
Methane Sources and Sinks Leif Backman HENVI Seminar February 19, 2009 Background Atmospheric methane Sources & Sinks Concentration variations & trends Objective & methods Objective & Goals Research plan
9th Period Environmental Science Chapter 15: The Atmosphere
 Section 15.1: Earth s Atmosphere 9th Period Environmental Science Chapter 15: The Atmosphere Properties of the Atmosphere: nitrogen, oxygen, water vapor and 1% other gases. air pressure is higher at the
Section 15.1: Earth s Atmosphere 9th Period Environmental Science Chapter 15: The Atmosphere Properties of the Atmosphere: nitrogen, oxygen, water vapor and 1% other gases. air pressure is higher at the
Carbon Dioxide and Global Warming Case Study
 Carbon Dioxide and Global Warming Case Study Key Concepts: Greenhouse Gas Carbon dioxide El Niño Global warming Greenhouse effect Greenhouse gas La Niña Land use Methane Nitrous oxide Radiative forcing
Carbon Dioxide and Global Warming Case Study Key Concepts: Greenhouse Gas Carbon dioxide El Niño Global warming Greenhouse effect Greenhouse gas La Niña Land use Methane Nitrous oxide Radiative forcing
Effect of Aviation on Atmospheric Composition
 Effect of Aviation on Atmospheric Composition Cynthia Whaley, Kimberly Strong, Zen Mariani, and Steven Barrett (MIT) UTIAS Colloquium on Sustainable Aviation 15-16 May 2013 Outline Introduction Composition
Effect of Aviation on Atmospheric Composition Cynthia Whaley, Kimberly Strong, Zen Mariani, and Steven Barrett (MIT) UTIAS Colloquium on Sustainable Aviation 15-16 May 2013 Outline Introduction Composition
Climate Change and Ozone Loss
 Climate Change and Ozone Loss During the past 900,000 years, the earth has undergone a series of cold glacial periods followed by warmer interglacial periods. The past 10,000 years has been an interglacial
Climate Change and Ozone Loss During the past 900,000 years, the earth has undergone a series of cold glacial periods followed by warmer interglacial periods. The past 10,000 years has been an interglacial
Major Volcanic Eruptions in the past. Major Volcanic Eruptions in the past. Volcanic Eruptions and Global Temperature
 Mechanism of Volcanic Perturbation Amount of sunlight scattered depends greatly on size and amount of aerosol particles The global monitoring of aerosols began in ~1980 Hence, the history of the amplitude
Mechanism of Volcanic Perturbation Amount of sunlight scattered depends greatly on size and amount of aerosol particles The global monitoring of aerosols began in ~1980 Hence, the history of the amplitude
Figure 1 - Global Temperatures - A plot from the EarthScience Centre at
 GLOBAL WARMING Global warming is evidenced by a steady rise in average global temperatures, changing climate, the fact that snow cover has decreased 10% over the past half-century and that glaciers have
GLOBAL WARMING Global warming is evidenced by a steady rise in average global temperatures, changing climate, the fact that snow cover has decreased 10% over the past half-century and that glaciers have
Chemistry in the Environment
 Chemistry in the Environment Section 261 Earth s Atmosphere In your textbook, read about the terms used to describe the physical and chemical properties of Earth s atmosphere Complete each statement 1
Chemistry in the Environment Section 261 Earth s Atmosphere In your textbook, read about the terms used to describe the physical and chemical properties of Earth s atmosphere Complete each statement 1
Global Climate Change. The sky is falling! The sky is falling!
 Global Climate Change The sky is falling! The sky is falling! 1 Global Climate Change Radiative Equilibrium, Solar and Earth Radiation Atmospheric Greenhouse Effect Greenhouse Gases Global Climate Change
Global Climate Change The sky is falling! The sky is falling! 1 Global Climate Change Radiative Equilibrium, Solar and Earth Radiation Atmospheric Greenhouse Effect Greenhouse Gases Global Climate Change
1) The Changing Carbon Cycle
 1) The Changing Carbon Cycle WG1 Chapter 6, figure 1 The numbers represent carbon reservoirs in Petagrams of Carbon (PgC; 10 15 gc) and the annual exchanges in PgC/year. The black numbers and arrows show
1) The Changing Carbon Cycle WG1 Chapter 6, figure 1 The numbers represent carbon reservoirs in Petagrams of Carbon (PgC; 10 15 gc) and the annual exchanges in PgC/year. The black numbers and arrows show
B1 Biogeochemical Systems
 B1 Biogeochemical Systems Carbon Cycle What elements makes life possible? Carbon based life Key component of all known naturally occurring life on Earth Unique properties make it ideal for construction
B1 Biogeochemical Systems Carbon Cycle What elements makes life possible? Carbon based life Key component of all known naturally occurring life on Earth Unique properties make it ideal for construction
Climate Change U C Irvine OLLI Class, Winter, 2013: SC 206 by Gary Oberts and Dennis Silverman Using Talks by NOAA for OLLI
 Climate Change U C Irvine OLLI Class, Winter, 2013: SC 206 by Gary Oberts and Dennis Silverman Using Talks by NOAA for OLLI Radiative Forcing Dennis Silverman Department of Physics and Astronomy, U C Irvine
Climate Change U C Irvine OLLI Class, Winter, 2013: SC 206 by Gary Oberts and Dennis Silverman Using Talks by NOAA for OLLI Radiative Forcing Dennis Silverman Department of Physics and Astronomy, U C Irvine
The Chemistry of Climate Change. Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy
 The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
Atmosphere. Earth s Atmosphere
 chapter 15 3 Atmosphere section 1 Earth s Atmosphere Before You Read Imagine you are on a spaceship looking down at Earth. Would the view be perfectly clear? What do you think you might see surrounding
chapter 15 3 Atmosphere section 1 Earth s Atmosphere Before You Read Imagine you are on a spaceship looking down at Earth. Would the view be perfectly clear? What do you think you might see surrounding
OVERVIEW AND INTRO TO CLIMATE SCIENCE MIT SUMMER HSSP, 2016 WEEK 1
 OVERVIEW AND INTRO TO CLIMATE SCIENCE MIT SUMMER HSSP, 2016 WEEK 1 COURSE OVERVIEW THIS IS GOING TO BE FUN (I HOPE ) JOSH S BACKGROUND MIT: 2 nd Year Ph.D. Student Researching Atmospheric Chemistry U.C.
OVERVIEW AND INTRO TO CLIMATE SCIENCE MIT SUMMER HSSP, 2016 WEEK 1 COURSE OVERVIEW THIS IS GOING TO BE FUN (I HOPE ) JOSH S BACKGROUND MIT: 2 nd Year Ph.D. Student Researching Atmospheric Chemistry U.C.
The Effects of Volcano-Induced Ozone Depletion on Short-Lived Climate Forcing in the Arctic
 C53C-0852 The Effects of Volcano-Induced Ozone Depletion on Short-Lived Climate Forcing in the Arctic Peter L. Ward US Geological Survey Retired Teton Tectonics Jackson, WY 307-733-3664 cell 307-413-4055
C53C-0852 The Effects of Volcano-Induced Ozone Depletion on Short-Lived Climate Forcing in the Arctic Peter L. Ward US Geological Survey Retired Teton Tectonics Jackson, WY 307-733-3664 cell 307-413-4055
EARTH S ATMOSPHERE, PAST AND PRESENT
 EARTH S ATMOSPHERE, PAST AND PRESENT 1. Introduction 2. Evolution of Earth s atmosphere 3. Present-day composition 4. Atmospheric density and pressure 5. Atmospheric structure 6. Air pollution Earth s
EARTH S ATMOSPHERE, PAST AND PRESENT 1. Introduction 2. Evolution of Earth s atmosphere 3. Present-day composition 4. Atmospheric density and pressure 5. Atmospheric structure 6. Air pollution Earth s
Planetary Energy Balance
 Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Earth s Atmosphere Lecture 14 3/6/2014
 Earth s Atmosphere Lecture 14 3/6/2014 MRS 1 Due Tuesday Second exam will be postponed until after spring break The sun drives the climate of Earth http://www.spaceweather.com/images2002/18mar02/cme_c3_big.gif
Earth s Atmosphere Lecture 14 3/6/2014 MRS 1 Due Tuesday Second exam will be postponed until after spring break The sun drives the climate of Earth http://www.spaceweather.com/images2002/18mar02/cme_c3_big.gif
Three Connected Interactives
 Three Connected Interactives Carbon Dioxide and the Carbon Cycle Earth s Energy Flows and Climate Impacts of Climate Change in the Pacific Region Climate Change: Causes and Impacts Human Activities More
Three Connected Interactives Carbon Dioxide and the Carbon Cycle Earth s Energy Flows and Climate Impacts of Climate Change in the Pacific Region Climate Change: Causes and Impacts Human Activities More
ASTRONOMY 161. Introduction to Solar System Astronomy. Class 16
 ASTRONOMY 161 Introduction to Solar System Astronomy Class 16 Earth s Atmosphere Monday, February 19 Earth s Atmosphere: Key Concepts (1) The Earth s atmosphere consists mainly of nitrogen (N 2 ) and
ASTRONOMY 161 Introduction to Solar System Astronomy Class 16 Earth s Atmosphere Monday, February 19 Earth s Atmosphere: Key Concepts (1) The Earth s atmosphere consists mainly of nitrogen (N 2 ) and
Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica?
 Ozone Depletion and Climate Change Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica? In a recent (last August 2016) BBC documentary on the Antarctic weather changes, it has
Ozone Depletion and Climate Change Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica? In a recent (last August 2016) BBC documentary on the Antarctic weather changes, it has
2 Atmospheric Heating
 CHAPTER 15 2 Atmospheric Heating SECTION The Atmosphere BEFORE YOU READ After you read this section, you should be able to answer these questions: How does energy travel from the sun to Earth? What are
CHAPTER 15 2 Atmospheric Heating SECTION The Atmosphere BEFORE YOU READ After you read this section, you should be able to answer these questions: How does energy travel from the sun to Earth? What are
Climate Change Frequently Asked Questions Scrambled Information Source: EPA Climate Change FAQ
 Climate Change Frequently Asked Questions Scrambled Information Source: EPA Climate Change FAQ Instructions: The questions and answers below have been scrambled. Cut the answers and questions apart. Separate
Climate Change Frequently Asked Questions Scrambled Information Source: EPA Climate Change FAQ Instructions: The questions and answers below have been scrambled. Cut the answers and questions apart. Separate
Main Natural Sources of Greenhouse Gases
 Main Natural Sources of Greenhouse Gases Content Atmospheric Composition Composition of the Earth s Atmosphere Greenhouse Gases The Radiative Forcing bar chart: AR5 version Natural Greenhouse Gases Water
Main Natural Sources of Greenhouse Gases Content Atmospheric Composition Composition of the Earth s Atmosphere Greenhouse Gases The Radiative Forcing bar chart: AR5 version Natural Greenhouse Gases Water
Environmental Science Std.-9 Chp.7 Atmosphere and Climate
 Environmental Science Std.-9 Chp.7 Atmosphere and Climate 2018-19 Q.1. Name the layers of the atmosphere. Troposphere Stratosphere Mesosphere Thermosphere Exosphere GREENHOUSE EARTH: Q.2. (a) What is the
Environmental Science Std.-9 Chp.7 Atmosphere and Climate 2018-19 Q.1. Name the layers of the atmosphere. Troposphere Stratosphere Mesosphere Thermosphere Exosphere GREENHOUSE EARTH: Q.2. (a) What is the
Atmospheric Chemistry
 Atmospheric Chemistry Research Dr. Husam T. Majeed Department of Atmospheric Sciences 2nd Year Class First Semester 2018-2019 Course Syllabus 1. Introduction & Review Atmospheric Chemistry Box Models and
Atmospheric Chemistry Research Dr. Husam T. Majeed Department of Atmospheric Sciences 2nd Year Class First Semester 2018-2019 Course Syllabus 1. Introduction & Review Atmospheric Chemistry Box Models and
Analyze the causes and effects of air pollution. Acid precipitation 1.
 Lesson 4 Air Quality HE.6.C.1.3, LA.6.2.2.3, MA.6.A.3.6, SC.6.E.7.5, SC.6.E.7.9, SC.6.N.1.1, SC.6.N.1.4 Skim or scan the heading, boldfaced words, and pictures in the lesson. Identify or predict three
Lesson 4 Air Quality HE.6.C.1.3, LA.6.2.2.3, MA.6.A.3.6, SC.6.E.7.5, SC.6.E.7.9, SC.6.N.1.1, SC.6.N.1.4 Skim or scan the heading, boldfaced words, and pictures in the lesson. Identify or predict three
Climate Change, Greenhouse Gases and Aerosols
 Climate Change, Greenhouse Gases and Aerosols J Srinivasan J Srinivasan is a Professor at the Centre for Atmospheric and Oceanic Sciences at Indian Institute of Science, Bangalore. He was a lead author
Climate Change, Greenhouse Gases and Aerosols J Srinivasan J Srinivasan is a Professor at the Centre for Atmospheric and Oceanic Sciences at Indian Institute of Science, Bangalore. He was a lead author
Atmospheric chemistry Summary
 Atmospheric chemistry Summary Pontus Roldin Div. Nuclear Physics Department of Physics Lund University Summary 1 Chapman mechanism (1930) The Chapman mechanism for stratospheric ozone (1) O 2 + hn O +
Atmospheric chemistry Summary Pontus Roldin Div. Nuclear Physics Department of Physics Lund University Summary 1 Chapman mechanism (1930) The Chapman mechanism for stratospheric ozone (1) O 2 + hn O +
The Greenhouse Effect
 Name: Date: The Greenhouse Effect This document provides an overview of the earth's atmospheric "greenhouse effect" by briefly exploring the atmospheres of nearby planets and discussing our atmosphere's
Name: Date: The Greenhouse Effect This document provides an overview of the earth's atmospheric "greenhouse effect" by briefly exploring the atmospheres of nearby planets and discussing our atmosphere's
Lecture Presentation. Chapter 18. Chemistry of the Environment. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 18 James F. Kirby Quinnipiac University Hamden, CT Atmosphere The atmosphere consists of the troposphere, stratosphere (combined 99.9 mass %), mesosphere, and thermosphere.
Lecture Presentation Chapter 18 James F. Kirby Quinnipiac University Hamden, CT Atmosphere The atmosphere consists of the troposphere, stratosphere (combined 99.9 mass %), mesosphere, and thermosphere.
Climate Change. Some solar radiation is reflected by Earth and the atmosphere. Earth s Surface
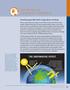 Q& A n The Basics of Greenhouse gases affect Earth s energy balance and climate The Sun serves as the primary energy source for Earth s climate. Some of the incoming sunlight is reflected directly back
Q& A n The Basics of Greenhouse gases affect Earth s energy balance and climate The Sun serves as the primary energy source for Earth s climate. Some of the incoming sunlight is reflected directly back
Radiative forcing of climate change
 Radiative forcing of climate change Joanna D. Haigh Imperial College of Science, Technology and Medicine, London Radiative forcing concept, definition and applications On a global and annual average, and
Radiative forcing of climate change Joanna D. Haigh Imperial College of Science, Technology and Medicine, London Radiative forcing concept, definition and applications On a global and annual average, and
Chapter 15. Atmosphere Notes
 Chapter 15 Atmosphere Notes The Air Around You Weather: The condition of the Earth s atmosphere at a particular time and place The Air Around You Atmosphere: the envelope of gases that surround the planet
Chapter 15 Atmosphere Notes The Air Around You Weather: The condition of the Earth s atmosphere at a particular time and place The Air Around You Atmosphere: the envelope of gases that surround the planet
Chapter 18 Chemistry of the Environment
 Chapter 18 Chemistry of the Environment Earth s Atmosphere Outer Regions of the Atmosphere Ozone in the Upper Atmosphere Chemistry of the Troposphere You will need to read the Course Pack to compliment
Chapter 18 Chemistry of the Environment Earth s Atmosphere Outer Regions of the Atmosphere Ozone in the Upper Atmosphere Chemistry of the Troposphere You will need to read the Course Pack to compliment
AOSC 433/633 & CHEM 433/633 Atmospheric Chemistry and Climate
 Revision 1: 20 Feb 2013 Page 1 of 6 AOSC 433/633 & CHEM 433/633 Atmospheric Chemistry and Climate Problem Set #2 (150 points total) Due: Thurs, 21 Feb 2013 (start of class) Late penalty: 10 points per
Revision 1: 20 Feb 2013 Page 1 of 6 AOSC 433/633 & CHEM 433/633 Atmospheric Chemistry and Climate Problem Set #2 (150 points total) Due: Thurs, 21 Feb 2013 (start of class) Late penalty: 10 points per
Climate Change Vocabulary Global Challenges for the 21 st Century Tony Del Vecchio, M.Ed. Atmosphere
 Atmosphere The mixture of gases surrounding the Earth. The Earth's atmosphere consists of about 79.1% nitrogen (by volume), 20.9% oxygen, 0.036% carbon dioxide and trace amounts of other gases. The atmosphere
Atmosphere The mixture of gases surrounding the Earth. The Earth's atmosphere consists of about 79.1% nitrogen (by volume), 20.9% oxygen, 0.036% carbon dioxide and trace amounts of other gases. The atmosphere
Greenhouse Effect. How we stay warm
 Greenhouse Effect How we stay warm The Sun s energy reaches Earth through Radiation (heat traveling through Space) How much solar radiation reaches Earth? The Earth s surface only absorbs 51% of incoming
Greenhouse Effect How we stay warm The Sun s energy reaches Earth through Radiation (heat traveling through Space) How much solar radiation reaches Earth? The Earth s surface only absorbs 51% of incoming
Answers to the Questions Posed by Judge Alsup
 Answers to the Questions Posed by Judge Alsup Don Wuebbles Department of Atmospheric Sciences University of Illinois SF March 21, 2018 Date Name of Meeting 1 Addressing Some Questions for the Tutorial
Answers to the Questions Posed by Judge Alsup Don Wuebbles Department of Atmospheric Sciences University of Illinois SF March 21, 2018 Date Name of Meeting 1 Addressing Some Questions for the Tutorial
Environmental Engineering Atmosphere & pollution 2
 Environmental Engineering Atmosphere & pollution 2 Global radiation Greenhouse effect Kyoto protocol David Zumr Dpt. of Drainage, Irrigation and Landscape Eng. 1/ insolation from the Sun Electromagnetic
Environmental Engineering Atmosphere & pollution 2 Global radiation Greenhouse effect Kyoto protocol David Zumr Dpt. of Drainage, Irrigation and Landscape Eng. 1/ insolation from the Sun Electromagnetic
GLOBAL BIOGEOCHEMICAL CYCLES. GEOG/ENST 3331 Lecture 10 Turco: Chapter 10; Dearden and Mitchell: Chapter 4
 GLOBAL BIOGEOCHEMICAL CYCLES GEOG/ENST 3331 Lecture 10 Turco: Chapter 10; Dearden and Mitchell: Chapter 4 Assignment 4 1. Suppose that a layer of air 1000 m thick has conditional stability. A rising parcel
GLOBAL BIOGEOCHEMICAL CYCLES GEOG/ENST 3331 Lecture 10 Turco: Chapter 10; Dearden and Mitchell: Chapter 4 Assignment 4 1. Suppose that a layer of air 1000 m thick has conditional stability. A rising parcel
Radiative Forcing Components
 Radiative Forcing Components Content Definition of Radiative Forcing Radiation Balance Climate sensitivity Solar forcing Forcing due to atmospheric gas Definition of Radiative Forcing In climate science,
Radiative Forcing Components Content Definition of Radiative Forcing Radiation Balance Climate sensitivity Solar forcing Forcing due to atmospheric gas Definition of Radiative Forcing In climate science,
Hudson River Estuary Climate Change Lesson Project. Grades 5-8 Teacher s Packet. Lesson 8. Carbon Through the Seasons
 Grades 5-8 Teacher s Packet Lesson 8 Carbon Through the Seasons Teacher s Packet 2 Carbon Through the Seasons NYS Intermediate Level Science Standard 1: Analysis, Inquiry and Design/Scientific Inquiry
Grades 5-8 Teacher s Packet Lesson 8 Carbon Through the Seasons Teacher s Packet 2 Carbon Through the Seasons NYS Intermediate Level Science Standard 1: Analysis, Inquiry and Design/Scientific Inquiry
TOPIC # 16 GLOBAL WARMING & ANTHROPOGENIC FORCING
 TOPIC # 16 GLOBAL WARMING & ANTHROPOGENIC FORCING TODAY s 3 KEY CONCEPTS: Carbon / Forests / Deforestation Computer Model Evidence for Anthropogenic GW Forcing Tying it all together w/ RADIATIVE FORCING
TOPIC # 16 GLOBAL WARMING & ANTHROPOGENIC FORCING TODAY s 3 KEY CONCEPTS: Carbon / Forests / Deforestation Computer Model Evidence for Anthropogenic GW Forcing Tying it all together w/ RADIATIVE FORCING
ATM S 211 Final Examination June 4, 2007
 ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
Case 3:17-cv WHA Document Filed 03/23/18 Page 1 of 14 EXHIBIT 4
 Case 3:17-cv-06011-WHA Document 183-4 Filed 03/23/18 Page 1 of 14 EXHIBIT 4 Case 3:17-cv-06011-WHA Document 183-4 Filed 03/23/18 Page 2 of 14 Answers to the Questions Posed by Judge Alsup Don Wuebbles
Case 3:17-cv-06011-WHA Document 183-4 Filed 03/23/18 Page 1 of 14 EXHIBIT 4 Case 3:17-cv-06011-WHA Document 183-4 Filed 03/23/18 Page 2 of 14 Answers to the Questions Posed by Judge Alsup Don Wuebbles
Chapter Two: Cycles of Matter (pages 32-65)
 Biology 20 Chapter 2.1_keyed Chapter Two: Cycles of Matter (pages 32-65) 2.1 The Role of Water in the Cycles of Matter (pages 34 40) Due to its ability to form hydrogen bonds, water has several unique
Biology 20 Chapter 2.1_keyed Chapter Two: Cycles of Matter (pages 32-65) 2.1 The Role of Water in the Cycles of Matter (pages 34 40) Due to its ability to form hydrogen bonds, water has several unique
Vocabulary for Section 1.1: Pressure. Atmosphere. Troposphere. Ionosphere O zone Layer. Ultraviolet radiation. Chlorofluorocarbon (CFC) Stratosphere
 Name: Date: Module I: Section 1.1 Vocabulary and Study Guide for: Atmosphere Objectives: You will learn to identify the gases in Earth s atmosphere. You will learn to describe the structure of Earth s
Name: Date: Module I: Section 1.1 Vocabulary and Study Guide for: Atmosphere Objectives: You will learn to identify the gases in Earth s atmosphere. You will learn to describe the structure of Earth s
Human Impact on the Environment: Part I
 Human Impact on the Environment: Part I The late Alan Gregg pointed out that human population growth within the ecosystem was closely analogous to the growth of malignant tumor cells, that man was acting
Human Impact on the Environment: Part I The late Alan Gregg pointed out that human population growth within the ecosystem was closely analogous to the growth of malignant tumor cells, that man was acting
1. The diagram below shows a greenhouse.
 1. The diagram below shows a greenhouse. 5. A gradual increase in atmospheric carbon dioxide would warm Earth s because carbon dioxide is a A) poor reflector of ultraviolet radiation B) good reflector
1. The diagram below shows a greenhouse. 5. A gradual increase in atmospheric carbon dioxide would warm Earth s because carbon dioxide is a A) poor reflector of ultraviolet radiation B) good reflector
