Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm
|
|
|
- Dwight Goodman
- 6 years ago
- Views:
Transcription
1 Development 127, (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm Masashi Kishi 1,2,3, Kenji Mizuseki 1, Noriaki Sasai 1,3, Hiroshi Yamazaki 1, Kohei Shiota 2, Shigetada Nakanishi 1 and Yoshiki Sasai 1,3,4, * 1 Department of Biological Sciences, and 2 Department of Anatomy and Developmental Biology, Faculty of Medicine; 3 Department of Medical Embryology and Neurobiology, Institute for Frontier Medical Sciences, Kyoto University, Sakyo, Kyoto , Japan 4 PRESTO, JST, Japan *Author for correspondence ( sasai@phy.med.kyoto-u.ac.jp) Accepted 22 November 1999; published on WWW 26 January 2000 SUMMARY From early stages of development, Sox2-class transcription factors (Sox1, Sox2 and Sox3) are expressed in neural tissues and sensory epithelia. In this report, we show that Sox2 function is required for neural differentiation of early Xenopus ectoderm. Microinjection of dominantnegative forms of Sox2 (dnsox2) mrna inhibits neural differentiation of animal caps caused by attenuation of BMP signals. Expression of dnsox2 in developing embryos suppresses expression of N-CAM and regional neural markers. We have analyzed temporal requirement of Sox2- mediated signaling by using an inducible dnsox2 construct fused to the ligand-binding domain of the glucocorticoid receptor. Attenuation of Sox2 function both from the late blastula stage and from the late gastrula stage onwards causes an inhibition of neural differentiation in animal caps and in whole embryos. Additionally, dnsox2-injected cells that fail to differentiate into neural tissues are not able to adopt epidermal cell fate. These data suggest that Sox2- class genes are essential for early neuroectoderm cells to consolidate their neural identity during secondary steps of neural differentiation. Key words: Dominant-negative, Neural differentiation, Neuroectoderm, Sox, Xenopus INTRODUCTION Recent molecular studies on neural induction have identified key molecules that regulate neural differentiation of embryonic ectoderm. In Xenopus, neural inducers such as Noggin, Chordin and Follistatin are secreted by the Spemann organizer and can promote neuralization of animal cap ectoderm (Lamb et al., 1993; Hemmati-Brivanlou et al., 1994; Sasai et al., 1995). These factors inactivate BMP4 by binding to it in the extracellular space. The role of BMP4 is to inhibit neural differentiation and to promote epidermal differentiation of ectoderm (Wilson and Hemmati-Brivanlou, 1995; Sasai et al., 1995). BMP4 signaling through BMP receptors is mediated by the Smad system as well as possibly by the TAB/TAK system (Massague, 1998; Shibuya et al., 1998). As a consequence, downstream genes such as GATA1 and Msx1 are induced as primary response genes. Overexpression of GATA1 and Msx1 can also suppress neuralization of ectoderm caused by neural inducers (Xu et al., 1997; Suzuki et al., 1997). Several genes that promote neural differentiation have also been isolated. Xenopus homologues of Drosophila odd-paired (Zic-related factors) are expressed in the neuroectoderm of early gastrulae and can initiate neural differentiation of animal cap ectoderm when overexpressed (Nakata et al., 1997; Mizuseki et al., 1998a). A Zic1-related zinc finger factor, called opl, is reported to promote neuronal differentiation when combined with Noggin (Kuo et al., 1998). SoxD is a distant member of the Sox gene family and its transcription is regulated by Chd/BMP signals (Mizuseki et al., 1998b). Regulation of SoxD is positively controlled by Zicr1 and negatively by GATA1 and Msx1. SoxD transcripts first appear during late blastula stages in a pan-ectodermal fashion. SoxD expression becomes limited to the neuroectoderm around the time of mid-gastrulation and remains pan-neural during the rest of embryogenesis. Microinjection of SoxD mrna causes neural differentiation of ectoderm both in vivo and in the animal cap (Mizuseki et al., 1998b). A POU-class homeobox gene, Xlpou2, which is expressed in the neuroectoderm of frog gastrulae, is also capable of inducing neuralization in a similar manner to Zic and SoxD (Witta et al., 1995). Another group of genes acting in early neurogenesis is vertebrate homologues of Drosophila proneural and neurogenic genes (Ma et al., 1996; Lee, 1997). In Xenopus, Xngnr1 (a neurogenin-related bhlh factor) is expressed in the primary neuron precursors and can induce neuronal differentiation in vivo and in the animal cap. Other bhlh factors (e.g., Xash3, NeuroD, Xath3) are also expressed in early neural plate and seem to play positive roles in parallel with or downstream of Xngnr1. The Sox gene family encode Sry-related transcription factors containing an HMG DNA-binding domain (Pevny and Lovell- Badge, 1997). The Sox family are subdivided into several subfamily groups on the basis of structural similarity (Wegner,
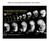
2 792 M. Kishi and others 1999). Sox2 and its closely related genes (Sox1 and Sox3) are classified as subfamily group B genes, and share more than 90% amino acid residue identities in their DNA-binding domains. These values are significantly higher than those found among DNA-binding domains of different subfamily groups. For instance, Sox9, which belongs to group E, shares about 60% amino acid identity to Sox2 in the DNA-binding domain. Sox1, Sox2 and Sox3 are expressed in early neuroectoderm of many vertebrate embryos and are also expressed in the forming lens (Wegner, 1999 and references therein). Sox2-class factors bind to the promotor region of a crystallin gene and transactivate this gene when working with lens-specific cofactors (Kamachi et al., 1995). Although the Sox1 gene knockout has been shown to cause defects in lens maturation (Nishiguchi et al., 1998), little is yet known about the role of Sox2-class genes in early neural differentiation. Two recent studies have reported possible involvement of Sox2-class factors in early steps of neural differentiation. In Xenopus, a combination of Sox2 and bfgf can promote posterior neural differentiation in the animal cap. Neither exposure to FGF nor overexpression of Sox2 alone can initiate neural differentiation, suggesting that Sox2 can change the responsiveness of the ectoderm to FGF signaling (Mizuseki et al., 1998a). Mammalian cell culture studies also suggested a possible role of a Sox2 subfamily member. Neural differentiation of P19 embryonic carcinoma cells requires two distinct signals; exposure to retinoic acid and cell aggregation. Overexpression of Sox1 can substitute for the requirement of retinoic acid (Pevny et al., 1998). To further understand the roles of Sox2-class genes in early neural development, we attempted to perform loss-of-function studies by using dominant-negative forms of Sox2 (dnsox2). Generally, the dominant-negative approach, as compared to the gene disruption strategy, is expected to have an advantage in a system where multiple closely related genes are present. In the case of Sox2, at least three subfamily members (Sox1, Sox2, Sox3), are expressed in mouse and chick neuroectoderm (Uwanogho et al., 1995; Collignon et al., 1996; Kamachi et al., 1995). In Xenopus, Sox3 transcripts are detected in developing nervous systems as well as in the maternal RNA pool (Penzel et al., 1997), although isolation of Xenopus Sox1 has not yet been reported. By using dnsox2, we provide data showing that signaling mediated by Sox2-class factors is required for proper differentiation of Xenopus neuroectodermal cells into neural tissues. MATERIALS AND METHODS Plasmid construction and in vitro mrna synthesis To generate Sox2BD( ) construct, the Sox2-coding region lacking the most of the HMG domain (corresponding to residues ) was amplified by PCR and inserted into the EcoRI-XhoI site of pcs2-nls vector (Rupp et al., 1994). For FLAG-tagged Sox2BD( ), the FLAG epitope sequence was added to Sox2BD( ) at the carboxyl terminus by PCR and the product was subcloned as above. For Sox2-EnR and Sox9-EnR, the cdna fragments corresponding to the HMG domain (residues and , respectively) obtained by PCR were first subcloned into pcs2-nls vector. Then, Drosophila engrailed fragment containing the repressor region (amino acid residues 2-298; Conlon et al., 1996) was added to the carboxyl termini of the Sox2 and Sox9 HMG domains (pcs-nls-sox2enr and pcs-nls- Sox9EnR; the resultant constructs have a EcoRI and a XbaI sites at 5 and 3 ends, respectively). For Sox2-GR, Sox3-GR, Sox9-GR and Sox2BD( )-GR plasmids, the Xbra cdna fragment was removed Fig. 1. Dominant-negative effects of Sox2BD( ) and Sox2-EnR on wild-type Sox2. (A) Structures of two dnsox2 constructs. (Top) Wild type; (middle) Sox2BD( ), lacking most of the DNA-binding HMG domain (black box). (Bottom) Sox2-EnR, the Sox2 HMG domain was fused to the Drosophila engrailed repressor region (grey box) at the carboxyl terminus. (B-L) Neural differentiation of the animal caps was analyzed with the pan-neural N-CAM marker at stage 19. Injection of the FGF4 expression plasmid (B) or control plasmid (inset) did not induce N-CAM expression in the explants. Coinjection of wild-type Sox2 mrna with the FGF4 plasmid caused neural differentiation (C). This neural differentiation was suppressed by coinjecting Sox2BD( ) mrna (D) or Sox2-EnR mrna (G) in the caps injected with Sox2 mrna and the FGF4 plasmid. This suppression was reversed by expressing Sox2-GR in the presence of 10 µm Dex (E,H) but not in its absence (insets). Sox9-GR did not rescue N-CAM expression even with Dex (F,I). SoxD mrna injection caused neural differentiation of animal caps (J). This neuralization was not inhibited by coinjection of Sox2BD( ) or Sox2-EnR mrna (K,L). In each injection, the total amounts of RNA and DNA were made constant by adding an adequate amount of control lacz mrna and plasmid DNA. The injected amounts were 10 pg for FGF4 plasmid, 100 pg for Sox2 and SoxD, 400 pg for Sox2BD( ), 50 pg for Sox2-EnR, 200 pg for Sox2-GR and 200 pg for Sox9-GR mrna.
3 Sox2 requirement for neural differentiation 793 Fig. 2. Injection of dnsox2 mrnas suppresses neural differentiation of animal cap explants caused by dnbmpr. (A) Expression of N-CAM in normal embryos at stage 19, when the animal caps below were harvested. (B-I) Effects of dnsox2 were examined with the N-CAM marker in the animal cap assay. Injection of dnbmpr (C) but not of control lacz (B) mrna induced N-CAM expression. The neural differentiation caused by dnbmpr was inhibited by coinjecting Sox2BD( ) mrna (D) or Sox2-EnR mrna (G). This inhibition was reversed by Sox2-GR coinjection in the presence of Dex (E,H) but not in its absence (not shown). The inhibitory effect of Sox2BD( ) or Sox2-EnR was not counteracted by Sox9-GR even with Dex treatment (F,I). In each injection, the total amounts of RNA were made constant by adding an adequate amount of control mrna. The injected amounts of mrna were 50 pg for dnbmpr, 400 pg for Sox2BD( ), 50 pg for Sox2-EnR, 200 pg for Sox2-GR, 200 pg for Sox9-GR. from psp64t-xbra-gr-ha (Tada et al., 1997) and replaced with the corresponding Sox cdna inserts. The FGF4-expressing vector was generated by inserting the coding region of Xenopus FGF4 (Isaacs et al., 1992) obtained by PCR into the HindIII-PstI site of pcska (Condie et al., 1990) which contains the Xenopus actin promotor. The cdna fragments obtained by PCR were verified by sequencing. To synthesize sense mrnas, the plasmids above were linearized with NotI (Sox2BD( ), FLAG-tagged Sox2BD( )), BssHII(Sox2-EnR, Sox9-EnR), or SalI (Sox2-GR, Sox3-GR, Sox9-GR, Sox2BD( )-GR) and subjected to in vitro transcription with SP6 polymerase (mmessage mmachine, Ambion). Embryonic manipulations, RNA and DNA injection and dexamethasone treatment Xenopus laevis eggs were inseminated in vitro with testis homogenate. Embryos were kept in 0.1 Barth solution until indicated stages. Animal cap explants were prepared at stage 9 and cultured in 1 LCMR + 0.2% BSA unless mentioned. In experiments for neuralizing activity of Sox2 with FGF4, animal caps were excised at stage Synthetic mrna and expression plasmids were injected into animal blastomeres of 8-cell embryos to overexpress the products in the ectoderm. Dexamethasone was purchased from Sigma and the stock solution was made at 10 mm in ethanol. A final 10 µm concentration of Dex was added to the culture medium at the stage mentioned and was not removed until fixation. All the injection experiments were carried out at least twice and gave reproducible results. The statistical values given in the text were from one representative experiment. In each series of experiments, total amount of injected RNA and DNA was adjusted by adding neutral lacz mrna or control lacz plasmid. Whole-mount in situ hybridization Whole-mount in situ hybridization analysis was performed as described (Chitnis et al., 1995) with minor modifications. For doublecolor staining, fixed embryos were incubated with one fluoresceinlabeled and one DIG-labeled antisense RNA probes. The samples were then incubated with anti-fluorescein-pod and anti-dig-ap antibodies (Boehringer) and stained sequentially with DAB (first) and with BM Purple (second). Signals in the animal caps were Fig. 3. Injection of Sox2BD( ) inhibits neural differentiation in vivo. Whole-mount in situ hybridization analyses at the open neural plate stage (dorsal view; upside, anterior). (A-C) Both embryos were injected with synthetic mrnas into each animal blastomere at the 8-cell stage: control lacz mrna (600 pg; A), Sox2BD( ) mrna (400 pg pg lacz; B) or Sox2BD( ) + Sox2-GR (400 pg pg; C). N-CAM expression in the neural plate was severely suppressed in Sox2BD( )-injected embryos while Sox2-GR coinjection rescued N-CAM expression in the presence of Dex. (D-I) 400 pg of Sox2BD( ) mrna (right) or control mrna (left) were injected into each animal blastomere of the 8-cell embryo. Injection of Sox2BD( ) had little effect on the expression of the dorsal mesodermal markers Chd or MyoD (D,E) while the proneural gene Xngnr1 (F), the neuronal marker N- tubulin (G), the neural crest markers Slug (H) and fkh6 (I) were significantly suppressed. Note that the fkh6 expression was inhibited in presumptive head crest regions (arrow) but not in posterior mesoderm (arrowhead).
4 794 M. Kishi and others photographed after counterstained with Bouin s fixative (yellow) and cleared in Murray s solution (Mizuseki et al., 1998a). Antisense RNA probes were prepared as described previously (Mizuseki et al., 1998b). The MyoD (Hopwood et al., 1989) and HoxB9 (Cho et al., 1991) probes were synthesized from pm3 (kind gift of J. Gurdon; linearized with BamHI) and px4 (kind gift of C. Wright; linearized with XbaI; Wright et al., 1990) with SP6 and T3 RNA polymerases, respectively. The plasmids containing Xenopus Zic2 (pbs-sk-zic2) and fkh6 (pbs-sk-fkh6) were isolated in our previous differential screen (Mizuseki et al., 1998a; GenBank accession number AB and AB014661, respectively). The Zic2 cdna that we isolated contains a DNA sequence 95% identical to the Zic2 sequence previously reported (Brewster et al., 1998) and is likely a different allele due to pseudotetraploidity of Xenopus laevis. The Sox2 probe was prepared from the plasmid that contains Sox2 5 -UTR sequence which does not overlap with that of Sox2BD( ) (pcs2-sox25utr). The Bluescript plasmids above and pcs2-sox25utr were linearized with EcoRI and transcribed with T7 RNA polymerase. Western blot and RT-PCR analysis Western blot analysis was performed by using anti-flag M2 antibody and PVDF membrane filters (Immobilon, Millipore). Signals were visualized by using chemiluminescence reagents (Renaissance, NEN). Total RNA was prepared from stage 19 animal caps by the acid-phenol-gtc method (Trizol, Gibco-BRL) and was subjected to DNase I treatment to get rid of trace of genomic DNA (Message Clean Kit, GenHunter). RT-PCR analysis was performed as described previously (Mizuseki et al., 1998a). Confocal microscopy Embryos were injected with RNAs at the 8-cell stage and cultured until neurula stages. Embryos were fixed with MEMFA + 1% glutaraldehyde, embedded in 2% agarose gel and sectioned transversely with vibratome. Confocal images of injected tracer GFP and nuclear DAPI staining were collected on an Olympus BX-50 confocal microscope using appropriate filters. RESULTS Inhibition of Sox2 activity by dominant-negative Sox2 constructs Two different candidate constructs for Sox2 dominantnegative mutants were generated (Fig. 1A). The first construct (middle) lacks most of the DNA-binding HMG domain (Sox2BD( )). In our previous study, a similar construct for another Sox gene, SoxD, worked efficiently as a dominantnegative mutant (SoxDBD( ); Mizuseki et al., 1998b). In the second construct (bottom), the HMG domain of Sox2 is fused with the engrailed repressor domain (Sox2-EnR). In Xenopus studies, a number of reports have shown that a chimeric construct of a DNA-binding and the engrailed repressor domains successfully functions as a dominant-inhibitory mutant (Conlon et al., 1996). Effects of both mutants on wild-type Sox2 activity were determined in animal cap assays. As previously reported (Mizuseki et al., 1998a), when wild-type Sox2 and FGF4 were overexpressed, the neural marker N-CAM was induced in animal cap explants (84%, n=32) (Fig. 1B,C). When Sox2BD( ) mrna was coinjected with the wild-type Sox2 mrna, this induction was strongly suppressed (84%, n=49) (Fig. 1D). We next asked if this suppression could be reversed by increasing wild-type Sox2 mrna. However, it proved that higher doses of Sox2 mrna showed significant toxic effects. To avoid toxicity, we took advantage of a glucocorticoid receptor ligand-binding domain (GR) fusion strategy. It has been shown that activities of transcription factors fused with GR can be controlled by administration of dexamethasone (Dex; Kolm and Sive, 1995). In this study, the GR-fusion strategy was adopted to reduce the non-specific toxic effects by holding Sox2-GR inactive until required. In this manner, timing of activation of exogenous Sox2 could be closer to that of endogenous Sox2 (on and after stage 9) and the toxicity was avoided. Sox2, Sox2BD( ) and Sox2-GR mrna were injected into 8- cell-stage embryos. Animal caps were prepared at stage and cultured with or without Dex. Coinjection of Sox2-GR clearly rescued expression of the neural differentiation marker N-CAM in animal caps cultured with Dex (80%, n=44; Fig. 1E) whereas in the absence of Dex it did not reverse suppression by Sox2BD( ) (n=31; Fig. 1E inset). Administration of Dex alone did not affect N-CAM expression (n=23; not shown). Injection of Sox3-GR rescued the neural differentiation suppressed by Sox2BD( ) in a similar manner to that observed for Sox2-GR (89%, n=27; not shown). In contrast, Sox9-GR failed to rescue the phenotype (n=25), showing that the effects are specific to Sox2-class members but not to Sox genes in general (Fig. 1F). These data demonstrated that Sox2BD( ) can function in the Xenopus ectoderm as a dominant-negative construct specific to Sox2-class factors. Effects of Sox2-EnR were examined by the same approach as described above for Sox2BD( ). Injection of Sox2-EnR mrna suppressed induction of N-CAM expression caused by Sox2 and FGF in animal caps (82%, n=22; Fig. 1G) while injection of Sox9-EnR did not (n=37; not shown). The suppression effect of Sox2-EnR was reversed by coinjecting with Sox2-GR in the presence of Dex (89%, n=44; Fig. 1H) but not in its absence (n=40; inset). Sox9-GR did not rescue the differentiation suppressed by Sox2-EnR (n=40; Fig. 1I). These results show that Sox2-EnR can also be used as a dominantnegative mutant. A previous study has shown that another Sox family factor, SoxD, is involved in early neural differentiation (Mizuseki et al., 1998b). Therefore, we tested the effect of dnsox2 constructs on the neuralizing activity of SoxD (Fig. 1J). Injection of Sox2BD( ) and Sox2-EnR mrna (at the same amounts used above) did not significantly suppressed N-CAM induction caused by SoxD (Fig. 1K,L; n=30 each). This suggests that Sox2 signaling does not function downstream of SoxD in the regulation of neural differentiation. Sox2-mediated signaling is required for neural differentiation of animal caps We next asked if Sox2-mediated signaling is required for neural differentiation of animal caps caused by attenuated BMP signals. Overexpression of a dominant-negative BMP receptor (dnbmpr) in the animal cap shuts off endogenous BMP signals that are antineuralizing and initiates neural differentiation (Hemmati-Brivanlou and Melton, 1997). Fig. 2C shows the induction of N-CAM expression by dnbmpr injection in animal caps (100%, n=32). When Sox2BD( ) or Sox2-EnR was coinjected with dnbmpr, the animal caps failed to express the pan-neural marker N-CAM (Fig. 2D, 93%, n=59; Fig. 2G, 92%, n=39). This suppression of neural differentiation
5 Sox2 requirement for neural differentiation 795 was reversed by coinjecting Sox2-GR in the presence of Dex (Fig. 2E, 85%, n=34; Fig. 2H, 88%, n=34) but not in its absence (n=32 and 29, respectively; not shown). Sox9-GR did not reverse the suppression by dnsox2 (Fig. 2F and I; n=36 and 41, respectively). These data demonstrate that signaling mediated by Sox2 is required for animal cap ectoderm to differentiate into neural tissues. Sox2 signaling is essential for neural development in vivo The effects of dnsox2 expression on neural development were tested in vivo. In the following in vivo studies, only Sox2BD( ) mrna was utilized since Sox2-EnR mrna injection, which worked well in animal cap assays, frequently caused exogastrulation when used in studies in vivo. It is difficult to evaluate neural formation in an embryo with exogastrulation because the interaction between ectoderm and mesoderm is impaired. When 400 pg of Sox2BD( ) mrna was injected into all the animal blastomeres of 8-cell embryos, N-CAM expression was inhibited in mid-neurula embryos (97%, n=71; Fig. 3A,B). N- CAM expression could be rescued by Sox2-GR injection with Dex (87%, n=23; Fig. 3C) but not without Dex (n=21; not shown), indicating that this suppression was not due to nonspecific toxic effects of Sox2BD( ). Dorsal mesodermal markers such as Chordin (Chd; Fig. 3D; Sasai et al., 1994) and MyoD (Fig. 3E; Hopwood et al., 1989) were intact (n=28, each), suggesting that the primary effect of dnsox2 occurred in the ectoderm rather than in the mesoderm. Formation of primary neurons as depicted by neurogenin (Xngnr1) and N- tubulin (N-tub) was severely inhibited (Fig. 3F, 90%, n=20; Fig. 3G, 91%, n=21). Early neural crest markers such as Slug and forkhead 6 (fkh6) were clearly suppressed (Fig. 3H, 90%, n=29; Fig. 3I, 100%, n=23). In the latter case, fkh6 expression was suppressed only in the neural crest regions (arrow) but not in the posterior mesoderm (arrowhead). Collectively, these results show that overexpression of dnsox2 inhibits early neural development in general, including formation of the CNS, primary neurons and neural crest cells. Sox2 signaling is required for expression of both anterior and posterior neural markers We next asked if signaling mediated by Sox2 is required for regional neural markers. This question is important since our previous studies have shown that SoxD is required for neural formation only in the anterior regions (Mizuseki et al., 1998b). Overexpression of SoxDBD( ) caused suppression of the anterior neural marker Otx2 (Fig. 4A, arrow; 87%, n=31), with the posterior spinocaudal marker HoxB9 unaffected (arrowhead). In contrast, overexpression of Sox2BD( ) significantly decreased both anterior and posterior neural markers: Otx2 (forebrain; Fig. 4B, 92%, n=36), Xanf1 (anterior neural ridge and pituitary gland; Fig. 4C, 100%, n=35), En2 (Midbrain-hindbrain boundary; Fig. 4D, 86%, n=22), Krox20 (hindbrain; Fig. 4E, 88%, n=24) and HoxB9 (spinal cord and posterior mesoderm; Fig. 4F, 91%, n=21). In Fig. 4F, Sox2BD( ) suppressed HoxB9 expression only in the neural tissues (arrowhead) but not in the mesoderm (arrow). This observation is in good agreement with the ectoderm-specific expression of Sox2. These data show that Sox2-mediated signaling is essential for general neural differentiation as well as for regional specification. Effects of dominant-negative Sox2 on expression of early neural markers The data above examined the effects of dnsox2 on neural markers in the neurula embryo or in the animal cap of the equivalent stage. Next, expression of earlier neural marker genes was determined in embryos injected with dnsox2. Effects of dnsox2 were assayed in early neuroectoderm by using two early neural markers, Xenopus Zic2 (Fig. 5A,C,E) and Sox2 (Fig. 5B,D,F). In the latter case, the 5 UTR sequence not overlapping with injected Sox2BD( ) mrna was used as a probe. At stage 11 (early gastrula), expression of Zic2 and Sox2 in embryos injected with Sox2BD( ) were not significantly suppressed as compared to that in the control embryos (n=38 and 30, respectively), showing that early neural markers were intact in the early neuroectoderm (Fig. 5A,B). At stage 12 (late gastrula) and stage 14 (early neurula), expression of the two neural markers was reduced in the Sox2BD( )-injected embryo significantly (Fig. 5C, 83%, n=30; Fig. 5D, 84%, n=31; Fig. 5E, 97%, n=39; Fig. 5F, 92%, n=37). To test if Sox2BD( ) protein accumulates at the gastrula stages, FLAG-tagged Sox2BD( ) mrna, which has a similar dominant-negative activity to Sox2BD( ) mrna, was injected into each animal blastomere of 8-cell embryos. Western blot analysis showed that translated FLAG-tagged dnsox2 products were accumulated at high levels during early gastrula stages (Fig. 5G; stages 10 and 11), suggesting that the lack of dnsox2 effects on early markers at stage 11 was not due to shortage of the gene product. These results show two phases of effects of dnsox2. First, injection of dnsox2 mrna does not block formation of early neuroectoderm in the light of neural-specific gene markers (Fig. 5A,B). Second, initially formed neuroectoderm expressing Zic2 and Sox2 fails to maintain the expression of neural-specific genes when Sox2-mediated signaling is inhibited (Fig. 5C-F). This suggests that Sox2 plays an essential role in maintenance or consolidation of neural fate of the cells in which initiation of neural differentiation has already taken place. Sox2 signaling is necessary for late gastrula neuroectoderm to adopt neural fate To determine the timing of Sox2 requirement in early neural development, we made a chimeric construct of Sox2BD( ) with GR (Sox2BD( )-GR; Fig. 6A), which was predicted to move into the nucleus under the control of exogenous Dex (Tada et al., 1997). In the absence of Dex, injection of Sox2BD( )-GR mrna per se did not affect N-CAM expression of the animal cap induced by dnbmpr (n=29; Fig. 6B,E). However, when Dex was applied to the culture saline from stage 9 (late blastula stage) onwards or from stage 12 (late gastrula stage) onwards, significant inhibition of neural differentiation was observed in each case (Fig. 6F, 100%, n=23; Fig. 6G, 82%, n=51). These data show that Sox2-mediated signaling is required for neural differentiation to proceed beyond late gastrula stages. Similar results were obtained when Sox2BD( )-GR was applied in vivo. Overexpression of Sox2BD( )-GR mrna per se did not affect expression of N-CAM (n=33; Fig. 6I). When Sox2BD( )-GR-injected embryos were treated with Dex from stage 9 or stage 12 onwards, clear decrease of N-CAM expression was observed (Fig. 6J, 88%, n=25; Fig. 6K, 85%,
6 796 M. Kishi and others Fig. 4. Injection of Sox2BD( ) inhibits expression of both anterior and posterior neural markers. Whole-mount in situ hybridization analyses were performed with regional neural markers in neurula embryos. (A) SoxDBD( ) mrna (right) or control lacz mrna (left) was injected into each animal blastomere of the 8-cell embryo (400 pg of mrna/cell). DIG-labeled Otx2 and HoxB9 probes were used simultaneously for double-label in situ hybridization. In the embryo injected with SoxDBD( ), the expression of the forebrain marker Otx2 (arrow) was suppressed whereas that of the spinocaudal marker HoxB9 (arrowhead) was not reduced. (B-F) Embryos were injected with Sox2BD( ) mrna (right) or control mrna (left) in a manner comparable to that for SoxDBD( ) mrna injections. Injection of Sox2BD( ) inhibited expression of Otx2 (B), Xanf1 (C), En2 (D), Krox20 (E), and HoxB9 (F). Note that inhibition of HoxB9 expression was detected only in the posterior neural tube (arrowhead) but not in the posterior mesoderm (arrow). n=27). Application of Dex alone did not cause any change in neural differentiation of either the animal caps or the neural plate in vivo (Fig. 6C,D, n=23 and 20, respectively). The data above demonstrate that Sox2-mediated signaling is required in the ectoderm at or following late gastrula stages, when the neuroectoderm has already expressed early neural genes such as Zic genes (see Fig. 5). We next tested whether Sox2- mediated signaling is required at later stages. N-CAM expression starts at the end of gastrulation (stage 13 by in situ hybridization). When Dex was applied from stage 14 onwards, N-CAM expression decreased in the animal caps (75%, n= 24; Fig. 6H) and embryos injected with Sox2BD( )-GR (74%, n=34; Fig. 6L), suggesting that Sox2-class factors play an essential role in the maintenance of late neural markers such as N-CAM. Neural precursors with reduced Sox2 signaling are not able to become either neural or epidermal cells Finally, we analyzed the fate of the cells that failed to differentiate into neural tissues due to lack of Sox2 function. Fig. 7A shows RT-PCR analysis of injected animal caps. Overexpression of dnbmpr suppressed the epidermal marker Keratin and induced the neural marker N-CAM (lane 2, 3). Sox2BD( ) significantly suppressed this N-CAM induction by dnbmpr whereas Keratin expression remained undetectable (lane 4). This is not due to inhibitory effects of Sox2BD( ) directly on epidermogenesis since Keratin expression was intact in the caps that were injected with Sox2BD( ) but without dnbmpr (lane 5). In addition, Sox2BD( ) did not induce the cement gland marker XAG-1 at the cost of N-CAM (lane 4). These data demonstrate that the cells in which neural differentiation has been initiated can neither proceed toward neural fate nor adopt an epidermal fate when Sox2 function is inhibited. This is consistent with the idea that Sox2-class factors are required for a secondary stage of differentiation of Fig. 5. Effects of Sox2BD( ) on early neural marker genes. (A-F) Embryos were injected with Sox2BD( ) mrna (right) or control mrna (left) as described for Fig. 4. Whole-mount in situ hybridization was performed with Zic2 (A,C,E) and Sox2-5 UTR (B,D,F) probes. No significant inhibition of Zic2 or Sox2 expression was detected at stage 11 (A,B). In embryos at stage 12 and 14, injection of Sox2BD( ) reduced expression of both early neural markers (C-F). (G) Accumulation of Sox2BD( ) protein (FLAGtagged) shown by western blot analysis. Embryos injected with FLAG-tagged Sox2BD( ) mrna were harvested at stages indicated above each lane. Western blotting with anti-flag antibody (upper panel) gave a single band of the expected size (25 kda) in each lane. This 25 kda band was not detected when control lacz-injected embryos were used (not shown). CBB staining of SDS-PAGE (lower panel) is presented as loading controls.
7 Sox2 requirement for neural differentiation 797 Fig. 6. Temporal requirement of Sox2-mediated signaling during neural differentiation. Sox2BD( )-GR was generated by adding the GR sequence to the carboxyl terminus of Sox2BD( ) (A, bottom). (B) N-CAM expression in animal caps neuralized with dnbmpr. Application of Dex did not affect N-CAM mrna expression either in the animal caps injected with dnbmpr (C) or in the embryo injected with control LacZ mrna (D). In animal cap assays (E-H), neural differentiation triggered by dnbmpr was not inhibited by coinjecting Sox2BD( )-GR mrna in the absence of Dex (E) while the induction of N-CAM in the injected caps was significantly suppressed when Dex was added to the culture medium from stage 9 onwards (F), stage 12 (G) or stage 14 onwards (H). (I-L) 200 pg of Sox2BD( )-GR mrna was injected into each animal blastomere of the 8-cell embryo. Without Dex treatment, injection of Sox2BD( )-GR had little effect on N-CAM expression (I). Significant suppression of N-CAM was observed when the injected embryos were treated with Dex from stage 9 onwards (J), stage 12 (K) or stage 14 (L) onwards. The extent of suppression of (H) and (L) tended to be less complete than that found in Dex treatment from earlier stages (F,G,J,K). neuroectoderm rather than for the initial binary decision (neural or epidermal). This idea was also tested in vivo. At the 8-cell stage, 400 pg of Sox2BD( ) mrna was injected into one animal blastomere and the embryo was analyzed with double in situ hybridization at stage 15 (mid-neurula). In the control embryo (Fig. 7B), N- CAM (purple) and Keratin (brown) signals were detected in neural and epidermal tissues, respectively, leaving no gap between the two markers. In contrast, the Sox2BD( )-injected embryo (Fig. 7C) had a reduced N-CAM-expressing area, and showed the appearance of a N-CAM-negative, Keratinnegative region instead (arrow; 93%, n=40). The area expressing XAG-1 did not expand in the embryos injected with Sox2BD( ) (n=25; not shown). These results indicate that Fig. 7. Sox2BD( ) does not promote epidermogenesis at the cost of neural tissues. (A) RT-PCR analysis of stage 19 animal caps injected with control (lane 2), dnbmpr (lane 3), dnbmpr + Sox2BD( ) (lane 4) or Sox2BD( ) (lane 5) mrna. Primers specific for N-CAM, epidermal Keratin, XAG-1, muscle-actin (M-actin) and the control EF-1α were used. (B,C) Double-labeled in situ hybridization with N-CAM (purple) and Keratin (brown) probes. The embryos were injected with control mrna (B) or Sox2BD( ) mrna (C) at one animal blastomere of the 8-cell embryo. The neural plate region that failed to express N-CAM (arrow) did not give Keratin signals, either. (D,E) Confocal microscopy images of the neural plate region of neurula embryos. Green fluorescence shows the progeny of the blastomere that was injected with control lacz and EGFP mrna (D) or Sox2BD( ) and EGFP mrna (E). The insets are low magnification images showing the nuclear DAPI staining. The brackets indicate the areas shown in high magnification GFP images. n, notochord; arrows, cuboidal cells in the superficial layer; arrowheads, cells in the sensorial layer.
8 798 M. Kishi and others Sox2BD( ) inhibits differentiation of neuroectoderm cells without re-directing the cells into epidermal fate. Finally, we examined morphological changes of the ectodermal cells injected with dnsox2 mrna. One animal blastomere of the 8-cell embryo was injected with tracer GFP mrna together with either Sox2BD( ) or control mrna. The embryos were fixed at the mid-neurula stage, sectioned and analyzed with confocal microscopy. The cells derived from the blastomere injected with control and GFP mrna formed twolayered neuroectoderm characteristic of Xenopus embryos (Fig. 7D; inset, nuclear staining at a lower magnification). The superficial layer was composed of short cuboidal cells (arrow) and the deep (sensorial) layer contained tall columnar neuroepitheial cells (arrowheads). In contrast, the Sox2BD( )- injected cells in the neural plate region did not show either columnar or squamous apprearance in the sensorial layer (arrows in Fig. 7E; n=10). This is in good agreement with the data above shown with N-CAM and Keratin markers. Later during the tailbud stages, Sox2BD( )-injected ectoderm started to peel off from the embryo (100%, n=35; not shown). We tested by TUNEL assay (Hensey and Gautier, 1997) whether the injected cells went through massive apoptosis. Both at late neurula stages and at tailbud stages, only a small number of embryos showed elevated TUNEL signals in the injected regions (2.6%, n=75; not shown), suggesting that improper differentiation changed adhesion characteristics of the cells during tailbud stages without causing programmed cell death. DISCUSSION Inhibition of Sox2-mediated function by dominantnegative Sox2 constructs The Sox family factors including Sox2 are known to require cofactors (or partners) for their transactivation activities (Kamachi et al., 1995). The HMG domain is responsible for DNA-binding and the rest of the protein is required for interaction with cofactors and for transactivation (Kamachi et al., 1999). Using distinct regions of Sox2, we generated two different mutant Sox2 constructs that have dominant-inhibitory effects over wild-type Sox2. One contained the Sox2 DNAbinding domain and was fused with the en repressor region. This is now a frequently used method of making a dominantnegative factor in Xenopus studies, and it is believed that the dominant-negative chimera works by binding to target genes (Conlon et al., 1996). The other construct that we used was a Sox2 cdna lacking most of the DNA-binding domain. A similar construct was successfully used in the case of SoxD (Mizuseki et al., 1998b). Since it cannot bind to the target DNA, it is likely to act by competing with endogenous Sox2 and Sox2-like factors for the essential cofactors. Both constructs showed an efficient dominant-inhibitory effect on neuralizing activity of Sox2 in the FGF-treated animal caps (Fig. 1). Rescue experiments demonstrated that the inhibition was not due to secondary toxic effects. This allowed us to test if Sox2 is required for neural differentiation, which is known to involve the blockade of BMP signaling. Requirement of Sox2-mediated signaling in neural differentiation of Xenopus ectoderm The dominant-negative experiments showed that signaling mediated by Sox2 is required for Xenopus ectoderm to express neural markers such as N-CAM. In animal caps injected with dnbmpr, neural differentiation was inhibited by coinjection of dnsox2 mrnas. The conclusion was strengthened by the observation that two constructs containing distinct Sox2 regions (Sox2BD( ) and Sox2-EnR) gave similar results. In vivo studies showed that Sox2-mediated signaling is essential for all the neural markers studied at neurula stages. Sox2BD( ) prevented the dorsal ectoderm from expressing the pan-neural marker N-CAM, the neuronal markers N-tubulin and neurogenin, and the neural crest markers Slug and fkh6 (Fig. 3). Thus, both CNS and neural crest formations require Sox2 signaling by the time of neurula stages. This effect was unlikely to be mediated secondarily by suppressing development of dorsal mesoderm (which can induce neural tissues) since the Chd and MyoD markers were intact (Fig. 3D,E). Differential requirement for Sox2 and SoxD signals in early neural development In our previous reports, we identified two distinct classes of Sox genes expressed in the frog gastrula ectoderm. Sox2 is expressed in the presumptive neural ectoderm from late blastula stages onwards, while SoxD expression is first panectodermal and becomes neural-specific during mid-gastrula stages. In structure, SoxD is distantly related to other Sox factors including Sox2. Beside the conserved HMG DNAbinding domain, SoxD shows no homology to any Sox genes. Overexpression of SoxD induces neural differentiation of ectoderm while that of Sox2 per se is not sufficient to initiate neural differentiation in animal cap ectoderm. In animal cap assays, injection of dnsoxd and that of dnsox2 have similar effects. Both inhibit expression of the pan-neural marker N-CAM induced by dnbmpr. However, in vivo studies show different outcomes. Injection of dnsoxd suppresses development of the anterior neural tissues, mostly forebrain, whereas overexpression of Sox2BD( ) prevents differentiation of neural tissues in general, both in the anterior and posterior regions. This demonstrates that Sox2 and SoxD have distinct roles in neural development. Possible timing of requirement of Sox2 in Xenopus neuroectoderm By using an inducible Sox2BD( ) construct fused to GR, we attempted to analyze temporal requirement of Sox2 signaling for neural differentiation. This construct showed inhibitory effects on neural differentiation of ectoderm in vivo and in the animal cap, in a Dex-dependent manner. The common understanding concerning the conditional regulation of GRfused nuclear factors is that the GR domain is tethered to the cytoplasmic protein HSP90 and is released upon binding to a GR ligand (reviewed in Smith and Toft, 1993). As discussed above, Sox2BD( ) is likely to act by competing for cofactors with endogenous Sox2 and its related proteins. Therefore, nuclear localization of Sox2BD( ) protein may be essential for its dominant-negative effects. This explains how Sox2BD( )- GR activity is dependent on Dex. A similar usage of GR to make a conditional dominant-negative construct was recently reported (Horb and Thomsen, 1999). Both the animal cap experiments and in vivo studies showed that inhibition of neural differentiation was seen by activation
9 Sox2 requirement for neural differentiation 799 of dnsox2 not only from the late blastula stage but also from the late gastrula stage onwards. In amphibia (newt), classical experiments have shown that neural fate of dorsal ectoderm is determined by the time of late gastrula stages (Spemann, 1918). Although detailed studies have not yet been reported in Xenopus as to when the neuroectoderm becomes irreversibly neural, columnarization of neuroectoderm cells is seen by histology within the sensorial layer during late gastrulation (stage 12-13; Hausen and Ribesell, 1991). These observations strongly suggest that the dorsal ectoderm of late gastrula has received and responded to inductive neuralizing signals. In our study (Fig. 6), late gastrula neuroectoderm could not further differentiate into neural tissues when Sox2 signaling was inhibited, suggesting that Sox2-class factors are essential for presumptive neural ectoderm to further differentiate beyond gastrula stages. In this study, we demonstrated that Sox2 signaling is required during secondary stages of neural differentiation starting from late gastrula. However, it remains unclear whether Sox2 function is necessary for the initial step of neural induction which occurs around stage 10. One possible experiment is to analyze effects of inducible Sox2BD( ) by adding Dex at stage 9 and removing it by stage 12. However, this experiment proves difficult since hydrophobic Dex can be deposited efficiently in the yolk lipid, making the Dex removal incomplete. Instead, we examined effects of Sox2BD( ) mrna injection on expression of Zic2 and Sox2 transcripts at the early gastrula stage (Fig. 5). In this case, Sox2BD( ) did not suppress expression of these two neural markers at stage 11, suggesting that inhibition of Sox2 signaling has little effects on initial steps of neural induction. Permissive role of Sox2 in neural development As described previously, overexpression of Sox2 mrna per se has little effects on animal cap ectoderm. When combined with FGF, Sox2 can modify the responsiveness of animal cap cells to FGF neuralizing signals (Mizuseki et al., 1998a; Fig. 1). Sox2 alone is not sufficient to direct cells to neural fate but rather plays a role in changing the competence of the ectoderm. When 100 pg of Sox2 mrna was injected into all the animal cells of 8-cell embryos, overexpression of Sox2 had very weak effects, if any, on the expression of neural markers at neurula stages (our unpublished observations). Higher doses of Sox2 mrna caused non-specific effects such as exogastrulation. Therefore, as of yet, we have no particular evidence for instructive roles of Sox2 in early neural development. In this study, we reported data that support permissive roles of Sox2 in developing nervous systems. Sox2 and/or its close relatives are necessary for the ectoderm to develop into neural tissues during secondary steps of neural differentiation. However, it remains to be known exactly which members of the Sox2-subfamily are responsible for this role. Being so closely related in structure and in expression pattern, it is likely that Sox2-subfamily genes have largely redundant functions. Additionally, it also remains to be clarified precisely which signaling steps in the downstream cascade of neural induction require the presence of Sox2-class factors. To study detailed gene interaction between early regulators of neural differentiation, analysis of the promoters of early neural marker genes by using the transgenic frog technique may prove useful (Kroll and Amaya, 1998). This new technique should provide us with information about the mode of action of Sox2 on neural-specific transcription in future. In closing, our present results have provided the first solid evidence for an essential role of Sox2-class genes in the forming neuroectoderm. Further studies on the role of Sox2 should help us dissect the regulatory cascade of neural development into individual steps. We thank Drs Jim Smith, Masazumi Tada, Chris Wright, Ralph Rupp, Richard Harland and John Gurdon for kind gifts of plasmids. We are grateful to Drs Sylvia Evans, Kumlesh Dev, Kimiko Fukuda and members of the Sasai lab for valuable comments on this manuscript and Hisato Kondoh for discussion on dnsox2 construction. This work was supported by grants from the Ministry of Education, PREST program, the Organization of Pharmaceutical Safety and Research, Takeda Foundation, Naito Foundation, Mochida Foundation, Yamanouchi Foundation and Kowa Foundation. REFERENCES Brewster, R., Lee, J. and Ruiz i Altaba, A. (1998). Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature 393, Chitnis, A., Henrique, D., Lewis, J., Ish-Horowicz, D. and Kintner, C. (1995). Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 375, Cho, K. W., Morita, E. A., Wright, C. V. and De Robertis, E. M. (1991). Overexpression of a homeodomain protein confers axis-forming activity to uncommitted Xenopus embryonic cells. Cell 65, Collignon, J., Sockanathan, S., Hacker, A., Cohen-Tannoudji, M., Norris, D., Rastan, S., Stevanovic, M., Goodfellow, P. N. and Lovell-Badge, R. (1996). A comparison of the properties of Sox3 with Sry and two related genes, Sox1 and Sox2. Development 122, Condie, B. G., Brivanlou, A. H. and Harland, R. M. (1990). Most of the homeobox-containing Xhox 3 transcripts in early Xenopus embryos cannot encode a homeodomain protein. Mol. Cell Biol. 10, Conlon, F. L., Sedgwick, S. G., Weston, K. M. and Smith, J. C. (1996). Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122, Hausen, P. and Riebesell, M. (1991). The Histology (Plate 20). In The Early Development of Xenopus Laevis, pp Berlin: Springer-Verlag. Hemmati-Brivanlou, A., Kelly, O. G. and Melton, D. A. (1994). Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77, Hemmati-Brivanlou, A. and Melton, D. (1997). Vertebrate neural induction. Annu Rev Neurosci. 20, Hensey, C. and Gautier, J. (1997) A developmental timer that regulates apotosis at the onset of gastrulation. Mech. Dev. 69, Hopwood, N. D., Pluck, A. and Gurdon, J. B. (1989). MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 8, Horb, M. E. and Thomsen, G. H. (1999). Tbx5 is essential for heart development. Development 126, Isaacs, H. V., Tannahill, D. and Slack, J. M. (1992). Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development 114, Kamachi, Y., Sockanathan, S., Liu, Q., Breitman, M., Lovell-Badge, R. and Kondoh, H. (1995). Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 14, Kamachi, Y., Cheah, K. E. and Kondoh, H. (1999). Mechamism of regulatory target selection by Sox high-mobility-group domain proteins as revealed by comparison of Sox1/2/3 and Sox9. Mol. Cell. Biol. 19, Kolm, P. J. and Sive, H. L. (1995). Efficient hormone-inducible protein function in Xenopus laevis. Dev. Biol. 171, Kroll, K. L. and Amaya, E. (1996). Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, Kuo, J. S., Patel, M., Gamse, J., Merzdorf, C., Liu, X., Apekin, V. and Sive,
10 800 M. Kishi and others H. (1998). Opl: a zinc finger protein that regulates neural determination and patterning in Xenopus. Development 125, Lamb, T. M., Knecht, A. K., Smith, W. C., Stachel, S. E., Economides, A. N., Stahl, N., Yancopolous, G. D. and Harland, R. M. (1993). Neural induction by the secreted polypeptide noggin. Science 262, Lee, J. E. (1997). Basic helix-loop-helix genes in neural development. Curr. Opin. Neurobiol. 7, Ma, Q., Kintner, C. and Anderson, D. J. (1996). Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87, Massague, J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem. 67, Mizuseki, K., Kishi, M., Matsui, M., Nakanishi, S. and Sasai, Y. (1998a). Xenopus Zic-related-1 and Sox2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125, Mizuseki, K., Kishi, M., Shiota, K., Nakanishi, S. and Sasai, Y. (1998b). SoxD: an essential mediator of induction of anterior neural tissues in Xenopus embryos. Neuron 21, Nakata, K., Nagai, T., Aruga, J. and Mikoshiba, K. (1997). Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc. Natl Acad. Sci. USA 94, Nishiguchi, S., Wood, H., Kondoh, H., Lovell-Badge, R. and Episkopou, V. (1998). Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12, Penzel, R., Oschwald, R., Chen, Y., Tacke, L. and Grunz, H. (1997). Characterization and early embryonic expression of a neural specific transcription factor xsox3 in Xenopus laevis. Int. J. Dev. Biol. 41, Pevny, L. H. and Lovell-Badge, R. (1997). Sox genes find their feet. Curr. Opin. Genet. Dev. 7, Pevny, L. H., Sockanathan, S., Placzek, M. and Lovell-Badge, R. (1998). A role for SOX1 in neural determination. Development 125, Rupp, R. A., Snider, L. and Weintraub, H. (1994). Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 8, Sasai, Y., Lu, B., Steinbeisser, H., Geissert, D., Gont, L. K. and De Robertis, E. M. (1994). Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, Sasai, Y., Lu, B., Steinbeisser, H. and De Robertis, E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, Shibuya, H., Iwata, H., Masuyama, N., Gotoh, Y., Yamaguchi, K., Irie, K., Matsumoto, K., Nishida, E. and Ueno, N. (1998). Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 17, Smith, D. F. and Toft, D. O. (1993). Steroid receptors and their associated proteins. Mol. Endocrinol. 7, Spemann, H. (1918). Über die Determination der ersten Organanlagen des Amphibienembryo. Wilhelm Roux Arch. EntwMech. Org. 43, Suzuki, A., Ueno, N. and Hemmati-Brivanlou, A. (1997). Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 124, Tada, M., O Reilly, M. A. and Smith, J. C. (1997). Analysis of competence and of Brachyury autoinduction by use of hormone- inducible Xbra. Development 124, Uwanogho, D., Rex, M., Cartwright, E. J., Pearl, G., Healy, C., Scotting, P. J. and Sharpe, P. T. (1995). Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech. Dev. 49, Wegner, M. (1999). From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 27, Wilson, P. A. and Hemmati-Brivanlou, A. (1995). Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, Witta, S. E., Agarwal, V. R. and Sato, S. M. (1995). XlPOU 2, a noggininducible gene, has direct neuralizing activity. Development 121, Wright, C. V., Morita, E. A., Wilkin, D. J. and De Robertis, E. M. (1990). The Xenopus XlHbox 6 homeo protein, a marker of posterior neural induction, is expressed in proliferating neurons. Development 109, Xu, R. H., Kim, J., Taira, M., Lin, J. J., Zhang, C. H., Sredni, D., Evans, T. and Kung, H. F. (1997). Differential regulation of neurogenesis by the two Xenopus GATA-1 genes. Mol. Cell Biol. 17,
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Neural Induction. Steven McLoon Department of Neuroscience University of Minnesota
 Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
efgf, Xcad3 and Hox genes form a molecular pathway that establishes the
 Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Neural induction. Noggin Chordin Follistatin (Xnr3)
 a bird s eye view Since the discovery of the phenomenon of neural induction by Spemann and Mangold in 94, considerable effort has been invested in identifying the signals produced by the organizer that
a bird s eye view Since the discovery of the phenomenon of neural induction by Spemann and Mangold in 94, considerable effort has been invested in identifying the signals produced by the organizer that
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
ANAT2341 Embryology Introduction: Steve Palmer
 ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5
 Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Figure S1. Figure S2. Figure S3 HB Anti-FSP27 (COOH-terminal peptide) Ab. Anti-GST-FSP27(45-127) Ab.
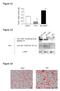 / 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
/ 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Culture and differentiation of embryonic stem cells. Hong-Lin Su Department of Life Sciences, National Chung-Hsing University
 Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
SUPPLEMENTARY INFORMATION
 doi:.38/nature899 Supplementary Figure Suzuki et al. a c p7 -/- / WT ratio (+)/(-) p7 -/- / WT ratio Log X 3. Fold change by treatment ( (+)/(-)) Log X.5 3-3. -. b Fold change by treatment ( (+)/(-)) 8
doi:.38/nature899 Supplementary Figure Suzuki et al. a c p7 -/- / WT ratio (+)/(-) p7 -/- / WT ratio Log X 3. Fold change by treatment ( (+)/(-)) Log X.5 3-3. -. b Fold change by treatment ( (+)/(-)) 8
7.013 Practice Quiz
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
CHAPTER 20 DNA TECHNOLOGY AND GENOMICS. Section A: DNA Cloning
 Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Fatchiyah
 Fatchiyah Email: fatchiya@yahoo.co.id RNAs: mrna trna rrna RNAi DNAs: Protein: genome DNA cdna mikro-makro mono-poly single-multi Analysis: Identification human and animal disease Finger printing Sexing
Fatchiyah Email: fatchiya@yahoo.co.id RNAs: mrna trna rrna RNAi DNAs: Protein: genome DNA cdna mikro-makro mono-poly single-multi Analysis: Identification human and animal disease Finger printing Sexing
Reading. Lecture III. Nervous System Embryology. Biology. Brain Diseases. September 5, Bio 3411 Lecture III. Nervous System Embryology
 Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms
 Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
AP Biology Gene Expression/Biotechnology REVIEW
 AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
AP Biology Gene Expression/Biotechnology REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Gene expression can be a. regulated before transcription.
Lecture III. Nervous System Embryology
 Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Charles Shuler, Ph.D. University of Southern California Los Angeles, California Approved for Public Release; Distribution Unlimited
 AD Award Number: DAMD17-01-1-0100 TITLE: Smad-Mediated Signaling During Prostate Growth and Development PRINCIPAL INVESTIGATOR: Charles Shuler, Ph.D. CONTRACTING ORGANIZATION: University of Southern California
AD Award Number: DAMD17-01-1-0100 TITLE: Smad-Mediated Signaling During Prostate Growth and Development PRINCIPAL INVESTIGATOR: Charles Shuler, Ph.D. CONTRACTING ORGANIZATION: University of Southern California
Recombinant DNA Technology. The Role of Recombinant DNA Technology in Biotechnology. yeast. Biotechnology. Recombinant DNA technology.
 PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University C H A P T E R 8 Recombinant DNA Technology The Role of Recombinant DNA Technology in Biotechnology Biotechnology?
PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University C H A P T E R 8 Recombinant DNA Technology The Role of Recombinant DNA Technology in Biotechnology Biotechnology?
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Trasposable elements: Uses of P elements Problem set B at the end
 Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Guided differentiation of ES cell into mesenchymal stem cell
 Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
Guided differentiation of ES cell into mesenchymal stem cell Takumi Era Division of Molecular Neurobiology Institute of Molecular Embryology and Genetics, Kumamoto University Differentiation pathways of
RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by. 5 AGACACAAACACCAUUGUCACACUCCACAGC; Rand-2 OMe,
 Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression: Transcription
 Gene Expression: Transcription The majority of genes are expressed as the proteins they encode. The process occurs in two steps: Transcription = DNA RNA Translation = RNA protein Taken together, they make
Gene Expression: Transcription The majority of genes are expressed as the proteins they encode. The process occurs in two steps: Transcription = DNA RNA Translation = RNA protein Taken together, they make
Chapter 25: Regulating Eukaryotic Transcription The Ligand Responsive Activators
 Chapter 25: Regulating Eukaryotic Transcription The Ligand Responsive Activators At least 5 potential gene expression control points Superfamily of Gene Regulators Activation of gene structure Initiation
Chapter 25: Regulating Eukaryotic Transcription The Ligand Responsive Activators At least 5 potential gene expression control points Superfamily of Gene Regulators Activation of gene structure Initiation
32 Gene regulation in Eukaryotes Lecture Outline 11/28/05. Gene Regulation in Prokaryotes and Eukarykotes
 3 Gene regulation in Eukaryotes Lecture Outline /8/05 Gene regulation in eukaryotes Chromatin remodeling More kinds of control elements Promoters, Enhancers, and Silencers Combinatorial control Cell-specific
3 Gene regulation in Eukaryotes Lecture Outline /8/05 Gene regulation in eukaryotes Chromatin remodeling More kinds of control elements Promoters, Enhancers, and Silencers Combinatorial control Cell-specific
Conservation and evolutionary divergence in the activity of receptor-regulated smads. Sorrentino et al.
 Conservation and evolutionary divergence in the activity of receptor-regulated smads Sorrentino et al. Sorrentino et al. EvoDevo 2012, 3:22 Sorrentino et al. EvoDevo 2012, 3:22 RESEARCH Open Access Conservation
Conservation and evolutionary divergence in the activity of receptor-regulated smads Sorrentino et al. Sorrentino et al. EvoDevo 2012, 3:22 Sorrentino et al. EvoDevo 2012, 3:22 RESEARCH Open Access Conservation
Construction of plant complementation vector and generation of transgenic plants
 MATERIAL S AND METHODS Plant materials and growth conditions Arabidopsis ecotype Columbia (Col0) was used for this study. SALK_072009, SALK_076309, and SALK_027645 were obtained from the Arabidopsis Biological
MATERIAL S AND METHODS Plant materials and growth conditions Arabidopsis ecotype Columbia (Col0) was used for this study. SALK_072009, SALK_076309, and SALK_027645 were obtained from the Arabidopsis Biological
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Learning Objectives. Define RNA interference. Define basic terminology. Describe molecular mechanism. Define VSP and relevance
 Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
DNA Binding Domains: Structural Motifs. Effector Domain. Zinc Fingers. Zinc Fingers, continued. Zif268
 DNA Binding Domains: Structural Motifs Studies of known transcription factors have found several motifs of protein design to allow sequence-specific binding of DNA. We will cover only three of these motifs:
DNA Binding Domains: Structural Motifs Studies of known transcription factors have found several motifs of protein design to allow sequence-specific binding of DNA. We will cover only three of these motifs:
Biology 201 (Genetics) Exam #3 120 points 20 November Read the question carefully before answering. Think before you write.
 Name KEY Section Biology 201 (Genetics) Exam #3 120 points 20 November 2006 Read the question carefully before answering. Think before you write. You will have up to 50 minutes to take this exam. After
Name KEY Section Biology 201 (Genetics) Exam #3 120 points 20 November 2006 Read the question carefully before answering. Think before you write. You will have up to 50 minutes to take this exam. After
Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product.
 Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Microinjection Techniques: Injecting through the chorion
 Microinjection Techniques: Injecting through the chorion I. Introduction To investigate the role of a gene during development, overexpression or misexpression of your gene of interest is a fast assay.
Microinjection Techniques: Injecting through the chorion I. Introduction To investigate the role of a gene during development, overexpression or misexpression of your gene of interest is a fast assay.
John Gurdon was testing the hypothesis of genomic equivalence or that when cells divide they retain a full genomic compliment.
 1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Supplementary Methods
 Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Developmental Biology 3230 Exam 1 (Feb. 6) NAME
 DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
Chemical hijacking of auxin signaling with an engineered auxin-tir1
 1 SUPPLEMENTARY INFORMATION Chemical hijacking of auxin signaling with an engineered auxin-tir1 pair Naoyuki Uchida 1,2*, Koji Takahashi 2*, Rie Iwasaki 1, Ryotaro Yamada 2, Masahiko Yoshimura 2, Takaho
1 SUPPLEMENTARY INFORMATION Chemical hijacking of auxin signaling with an engineered auxin-tir1 pair Naoyuki Uchida 1,2*, Koji Takahashi 2*, Rie Iwasaki 1, Ryotaro Yamada 2, Masahiko Yoshimura 2, Takaho
SureSilencing sirna Array Technology Overview
 SureSilencing sirna Array Technology Overview Pathway-Focused sirna-based RNA Interference Topics to be Covered Who is SuperArray? Brief Introduction to RNA Interference Challenges Facing RNA Interference
SureSilencing sirna Array Technology Overview Pathway-Focused sirna-based RNA Interference Topics to be Covered Who is SuperArray? Brief Introduction to RNA Interference Challenges Facing RNA Interference
Chapter 47. Development
 Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
 Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
NAME TA SEC Problem Set 4 FRIDAY October 15, Answers to this problem set must be inserted into the box outside
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 4 FRIDAY October 15,
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 4 FRIDAY October 15,
Introducing new DNA into the genome requires cloning the donor sequence, delivery of the cloned DNA into the cell, and integration into the genome.
 Key Terms Chapter 32: Genetic Engineering Cloning describes propagation of a DNA sequence by incorporating it into a hybrid construct that can be replicated in a host cell. A cloning vector is a plasmid
Key Terms Chapter 32: Genetic Engineering Cloning describes propagation of a DNA sequence by incorporating it into a hybrid construct that can be replicated in a host cell. A cloning vector is a plasmid
Regulation of cellular differentiation by tissue specific transcription factor
 Regulation of cellular differentiation by tissue specific transcription factor T. Endoh, M. Mie, Y. Yanagida, M. Aizawa, E. Kobatake Department of Biological Information, Graduate School of Bioscience
Regulation of cellular differentiation by tissue specific transcription factor T. Endoh, M. Mie, Y. Yanagida, M. Aizawa, E. Kobatake Department of Biological Information, Graduate School of Bioscience
Jung-Nam Cho, Jee-Youn Ryu, Young-Min Jeong, Jihye Park, Ji-Joon Song, Richard M. Amasino, Bosl Noh, and Yoo-Sun Noh
 Developmental Cell, Volume 22 Supplemental Information Control of Seed Germination by Light-Induced Histone Arginine Demethylation Activity Jung-Nam Cho, Jee-Youn Ryu, Young-Min Jeong, Jihye Park, Ji-Joon
Developmental Cell, Volume 22 Supplemental Information Control of Seed Germination by Light-Induced Histone Arginine Demethylation Activity Jung-Nam Cho, Jee-Youn Ryu, Young-Min Jeong, Jihye Park, Ji-Joon
Supplemental Data. LMO4 Controls the Balance between Excitatory. and Inhibitory Spinal V2 Interneurons
 Neuron, Volume 61 Supplemental Data LMO4 Controls the Balance between Excitatory and Inhibitory Spinal V2 Interneurons Kaumudi Joshi, Seunghee Lee, Bora Lee, Jae W. Lee, and Soo-Kyung Lee Supplemental
Neuron, Volume 61 Supplemental Data LMO4 Controls the Balance between Excitatory and Inhibitory Spinal V2 Interneurons Kaumudi Joshi, Seunghee Lee, Bora Lee, Jae W. Lee, and Soo-Kyung Lee Supplemental
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr.
 MIT Department of Biology 7.01: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel iv) Would Xba I be useful for cloning? Why or why not?
MIT Department of Biology 7.01: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel iv) Would Xba I be useful for cloning? Why or why not?
SUPPLEMENTARY INFORMATION
 b Supplementary Figure 1. Analysis of Vkg-Dpp interactions. a, In vitro pulldown showing binding of Dpp-HA to the GST-VkgC fusion protein. No interaction is detected between denatured Dpp-HA and GST-Vkg.
b Supplementary Figure 1. Analysis of Vkg-Dpp interactions. a, In vitro pulldown showing binding of Dpp-HA to the GST-VkgC fusion protein. No interaction is detected between denatured Dpp-HA and GST-Vkg.
What s the most complex problem in biology?
 Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Genetic Engineering & Recombinant DNA
 Genetic Engineering & Recombinant DNA Chapter 10 Copyright The McGraw-Hill Companies, Inc) Permission required for reproduction or display. Applications of Genetic Engineering Basic science vs. Applied
Genetic Engineering & Recombinant DNA Chapter 10 Copyright The McGraw-Hill Companies, Inc) Permission required for reproduction or display. Applications of Genetic Engineering Basic science vs. Applied
Lecture 20: Drosophila melanogaster
 Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Recent technology allow production of microarrays composed of 70-mers (essentially a hybrid of the two techniques)
 Microarrays and Transcript Profiling Gene expression patterns are traditionally studied using Northern blots (DNA-RNA hybridization assays). This approach involves separation of total or polya + RNA on
Microarrays and Transcript Profiling Gene expression patterns are traditionally studied using Northern blots (DNA-RNA hybridization assays). This approach involves separation of total or polya + RNA on
Problem Set 8. Answer Key
 MCB 102 University of California, Berkeley August 11, 2009 Isabelle Philipp Online Document Problem Set 8 Answer Key 1. The Genetic Code (a) Are all amino acids encoded by the same number of codons? no
MCB 102 University of California, Berkeley August 11, 2009 Isabelle Philipp Online Document Problem Set 8 Answer Key 1. The Genetic Code (a) Are all amino acids encoded by the same number of codons? no
A subclass of HSP70s regulate development and abiotic stress responses in Arabidopsis thaliana
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Journal of Plant Research A subclass of HSP70s regulate development and abiotic stress responses in Arabidopsis thaliana Linna Leng 1 Qianqian Liang
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Journal of Plant Research A subclass of HSP70s regulate development and abiotic stress responses in Arabidopsis thaliana Linna Leng 1 Qianqian Liang
Melton, D.W. (1994) Gene targeting in the mouse. Bioessays 16:633-8
 Reverse genetics - Knockouts Paper to read for this section : Melton, D.W. (1994) Gene targeting in the mouse. Bioessays 16:633-8 Up until now, we ve concentrated on ways to get a cloned gene. We ve seen
Reverse genetics - Knockouts Paper to read for this section : Melton, D.W. (1994) Gene targeting in the mouse. Bioessays 16:633-8 Up until now, we ve concentrated on ways to get a cloned gene. We ve seen
Comparison of Commercial Transfection Reagents: Cell line optimized transfection kits for in vitro cancer research.
 Comparison of Commercial Transfection Reagents: Cell line optimized transfection kits for in vitro cancer research. by Altogen Labs, 11200 Manchaca Road, Suite 203 Austin TX 78748 USA Tel. (512) 433-6177
Comparison of Commercial Transfection Reagents: Cell line optimized transfection kits for in vitro cancer research. by Altogen Labs, 11200 Manchaca Road, Suite 203 Austin TX 78748 USA Tel. (512) 433-6177
Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines
 Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines Further information can be found at: http://stke.sciencemag.org/sites/default/files/researcharticlerevmsinstructions_0.pdf.
Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines Further information can be found at: http://stke.sciencemag.org/sites/default/files/researcharticlerevmsinstructions_0.pdf.
SOX2 antibody - Embryonic Stem Cell Marker (ab15830) datasheet
 -Embryonic Stem Cell Marker (ab15830) 1/8 ページ d Products: Stem Cells >> Germline Stem Cells >> Embryonic Germ Cells SOX2 antibody - Embryonic Stem Cell Marker (ab15830) datasheet Product Name Product type
-Embryonic Stem Cell Marker (ab15830) 1/8 ページ d Products: Stem Cells >> Germline Stem Cells >> Embryonic Germ Cells SOX2 antibody - Embryonic Stem Cell Marker (ab15830) datasheet Product Name Product type
2054, Chap. 14, page 1
 2054, Chap. 14, page 1 I. Recombinant DNA technology (Chapter 14) A. recombinant DNA technology = collection of methods used to perform genetic engineering 1. genetic engineering = deliberate modification
2054, Chap. 14, page 1 I. Recombinant DNA technology (Chapter 14) A. recombinant DNA technology = collection of methods used to perform genetic engineering 1. genetic engineering = deliberate modification
Molecular Cell Biology - Problem Drill 11: Recombinant DNA
 Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
2. Outline the levels of DNA packing in the eukaryotic nucleus below next to the diagram provided.
 AP Biology Reading Packet 6- Molecular Genetics Part 2 Name Chapter 19: Eukaryotic Genomes 1. Define the following terms: a. Euchromatin b. Heterochromatin c. Nucleosome 2. Outline the levels of DNA packing
AP Biology Reading Packet 6- Molecular Genetics Part 2 Name Chapter 19: Eukaryotic Genomes 1. Define the following terms: a. Euchromatin b. Heterochromatin c. Nucleosome 2. Outline the levels of DNA packing
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10163 Supplementary Table 1 Efficiency of vector construction. Process wells recovered efficiency (%) Recombineering* 480 461 96 Intermediate plasmids 461 381 83 Recombineering efficiency
doi:10.1038/nature10163 Supplementary Table 1 Efficiency of vector construction. Process wells recovered efficiency (%) Recombineering* 480 461 96 Intermediate plasmids 461 381 83 Recombineering efficiency
Biotech Applications Nucleic acid therapeutics, Antibiotics, Transgenics. BIT 220 End of Chapter 22 (Snustad/Simmons)
 Biotech Applications Nucleic acid therapeutics, Antibiotics, Transgenics BIT 220 End of Chapter 22 (Snustad/Simmons) Nucleic Acids as Therapeutic Agents Many diseases (cancer, inflammatory diseases) from
Biotech Applications Nucleic acid therapeutics, Antibiotics, Transgenics BIT 220 End of Chapter 22 (Snustad/Simmons) Nucleic Acids as Therapeutic Agents Many diseases (cancer, inflammatory diseases) from
Role of Bmi-1 in Epigenetic Regulation. During Early Neural Crest Development
 Role of Bmi-1 in Epigenetic Regulation During Early Neural Crest Development Thesis by Jane I. Khudyakov In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute
Role of Bmi-1 in Epigenetic Regulation During Early Neural Crest Development Thesis by Jane I. Khudyakov In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute
7 Gene Isolation and Analysis of Multiple
 Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 7 Gene Isolation and Analysis of Multiple
Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 7 Gene Isolation and Analysis of Multiple
Protocol for Genome Editing via the RNA-guided Cas9 Nuclease in. Zebrafish Embryos 1
 Protocol for Genome Editing via the RNA-guided Cas9 Nuclease in Zebrafish Embryos 1 1. In vitro synthesis of capped Cas9 mrna The full length of humanized Cas9 cdnas with double NLS were cloned into pxt7
Protocol for Genome Editing via the RNA-guided Cas9 Nuclease in Zebrafish Embryos 1 1. In vitro synthesis of capped Cas9 mrna The full length of humanized Cas9 cdnas with double NLS were cloned into pxt7
The Genetic Basis of Development
 Chapter 21 The Genetic Basis of Development Overview: From Single Cell to Multicellular Organism The application of genetic analysis and DNA technology Has revolutionized the study of development PowerPoint
Chapter 21 The Genetic Basis of Development Overview: From Single Cell to Multicellular Organism The application of genetic analysis and DNA technology Has revolutionized the study of development PowerPoint
Read the question carefully before answering. Think before you write. If I can not read your handwriting, I will count the question wrong.
 Name KEY Note Total points added up to only 98 points so everyone received 2 free points to make total points 100. Biology 201 (Genetics) Exam #3 23 November 2004 Read the question carefully before answering.
Name KEY Note Total points added up to only 98 points so everyone received 2 free points to make total points 100. Biology 201 (Genetics) Exam #3 23 November 2004 Read the question carefully before answering.
Developmental Biology BY1101 P. Murphy
 Developmental Biology BY1101 P. Murphy Lecture 7 Cellular differentiation and the regulation of gene expression. In this lecture we looked at two main questions: How is gene expression regulated? (revision
Developmental Biology BY1101 P. Murphy Lecture 7 Cellular differentiation and the regulation of gene expression. In this lecture we looked at two main questions: How is gene expression regulated? (revision
Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression
 Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression LVP336 LVP336-PBS LVP339 LVP339-PBS LVP297 LVP297-PBS LVP013 LVP013-PBS LVP338 LVP338-PBS LVP027 LVP027-PBS LVP337 LVP337-PBS
Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression LVP336 LVP336-PBS LVP339 LVP339-PBS LVP297 LVP297-PBS LVP013 LVP013-PBS LVP338 LVP338-PBS LVP027 LVP027-PBS LVP337 LVP337-PBS
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
PrimePCR Assay Validation Report
 Gene Information Gene Name SRY (sex determining region Y)-box 6 Gene Symbol Organism Gene Summary Gene Aliases RefSeq Accession No. UniGene ID Ensembl Gene ID SOX6 Human This gene encodes a member of the
Gene Information Gene Name SRY (sex determining region Y)-box 6 Gene Symbol Organism Gene Summary Gene Aliases RefSeq Accession No. UniGene ID Ensembl Gene ID SOX6 Human This gene encodes a member of the
of MyoD in Xenopus mesoderm
 Proc. Nati. Acad. Sci. USA Vol. 91, pp. 10844-10848, November 1994 Developmental iology Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm CHRISTINE E. HOLT*t,
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 10844-10848, November 1994 Developmental iology Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm CHRISTINE E. HOLT*t,
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Multiple choice questions (numbers in brackets indicate the number of correct answers)
 1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
Supplemental Data. Lee et al. Plant Cell. (2010) /tpc Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2.
 Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2. (A) Protein structures of DWA1 and DWA2. WD40 region was determined based on the NCBI conserved domain databases (B, C) Schematic representation
Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2. (A) Protein structures of DWA1 and DWA2. WD40 region was determined based on the NCBI conserved domain databases (B, C) Schematic representation
Roche Molecular Biochemicals Technical Note No. LC 10/2000
 Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Supplemental Information. Role of phosphatase of regenerating liver 1 (PRL1) in spermatogenesis
 Supplemental Information Role of phosphatase of regenerating liver 1 (PRL1) in spermatogenesis Yunpeng Bai ;, Lujuan Zhang #, Hongming Zhou #, Yuanshu Dong #, Qi Zeng, Weinian Shou, and Zhong-Yin Zhang
Supplemental Information Role of phosphatase of regenerating liver 1 (PRL1) in spermatogenesis Yunpeng Bai ;, Lujuan Zhang #, Hongming Zhou #, Yuanshu Dong #, Qi Zeng, Weinian Shou, and Zhong-Yin Zhang
Lecture 25 (11/15/17)
 Lecture 25 (11/15/17) Reading: Ch9; 328-332 Ch25; 990-995, 1005-1012 Problems: Ch9 (study-guide: applying); 1,2 Ch9 (study-guide: facts); 7,8 Ch25 (text); 1-3,5-7,9,10,13-15 Ch25 (study-guide: applying);
Lecture 25 (11/15/17) Reading: Ch9; 328-332 Ch25; 990-995, 1005-1012 Problems: Ch9 (study-guide: applying); 1,2 Ch9 (study-guide: facts); 7,8 Ch25 (text); 1-3,5-7,9,10,13-15 Ch25 (study-guide: applying);
Problem Set 4
 7.016- Problem Set 4 Question 1 Arginine biosynthesis is an example of multi-step biochemical pathway where each step is catalyzed by a specific enzyme (E1, E2 and E3) as is outlined below. E1 E2 E3 A
7.016- Problem Set 4 Question 1 Arginine biosynthesis is an example of multi-step biochemical pathway where each step is catalyzed by a specific enzyme (E1, E2 and E3) as is outlined below. E1 E2 E3 A
Chapter 6 - Molecular Genetic Techniques
 Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
HiPer RT-PCR Teaching Kit
 HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
Methods of Biomaterials Testing Lesson 3-5. Biochemical Methods - Molecular Biology -
 Methods of Biomaterials Testing Lesson 3-5 Biochemical Methods - Molecular Biology - Chromosomes in the Cell Nucleus DNA in the Chromosome Deoxyribonucleic Acid (DNA) DNA has double-helix structure The
Methods of Biomaterials Testing Lesson 3-5 Biochemical Methods - Molecular Biology - Chromosomes in the Cell Nucleus DNA in the Chromosome Deoxyribonucleic Acid (DNA) DNA has double-helix structure The
7.17: Writing Up Results and Creating Illustrations
 7.17: Writing Up Results and Creating Illustrations A Results Exercise: Kansas and Pancakes Write a 5-sentence paragraph describing the results illustrated in this figure: - Describe the figure: highlights?
7.17: Writing Up Results and Creating Illustrations A Results Exercise: Kansas and Pancakes Write a 5-sentence paragraph describing the results illustrated in this figure: - Describe the figure: highlights?
