The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains
|
|
|
- Leo Dixon
- 6 years ago
- Views:
Transcription
1 The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains Daniel P. Szeto 1 and David Kimelman 2 Department of Biochemistry, University of Washington, Seattle, Washington 98195, USA The vertebrate musculature is produced from a visually uniform population of mesodermal progenitor cells (MPCs) that progressively bud off somites populating the trunk and tail. How the MPCs are regulated to continuously release cells into the presomitic mesoderm throughout somitogenesis is not understood. Using a genetic approach to study the MPCs, we show that a subset of MPCs are set aside very early in zebrafish development, and programmed to cell-autonomously enter the tail domain beginning with the 16th somite. Moreover, we show that the trunk is subdivided into two domains, and that entry into the anterior trunk, posterior trunk, and tail is regulated by interactions between the Nodal and bone morphogenetic protein (Bmp) pathways. Finally, we show that the tail MPCs are held in a state we previously called the Maturation Zone as they wait for the signal to begin entering somitogenesis. [Keywords: Bmp signaling; Nodal; T-box genes; MZoep; somite] Supplemental material is available at Received March 29, 2006; revised version accepted May 12, The mesodermal progenitor cells (MPCs) are a population of undifferentiated progenitor cells that originate in the early gastrula embryo (for review, see Schier et al. 1997; Kimelman 2006). By the end of gastrulation, they have moved to the very posterior end of the embryo in a region called the tailbud. Throughout gastrulation and somitogenesis, the MPCs continuously contribute cells to the presomitic mesoderm, producing a species-specific number of segments using a complex clock and wavefront mechanism to segment the presomitic mesoderm (for review, see Tam et al. 2000; Holley and Takeda 2002; Dubrulle and Pourquie 2004). The release of cells from the MPC population into the presomitic mesoderm needs to be controlled such that a sufficient number of cells continuously enter the presomitic mesoderm to form somites, yet enough precursors remain behind to form the complete set of somites. We previously provided evidence that cells from the MPC population first enter a transitional Maturation Zone at the very posterior end of the embryo, marked by the overlapping expression of the T-box transcription factors no tail, spadetail, and tbx6, as they make the commitment to enter 1 Present address: Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA. 2 Corresponding author. kimelman@u.washington.edu; FAX (206) Article is online at the presomitic mesoderm (Griffin and Kimelman 2002) (this zone is not the same as the maturation front at the anterior of the presomitic mesoderm, where the somite border forms). These results suggest that the decision to enter the presomitic mesoderm involves a multistep process that is under tight regulatory control. Several lines of evidence indicate that the zebrafish tail somites are genetically different from the trunk somites even though the mechanism of forming the trunk and tail somites is indistinguishable (Ho and Kane 1990; Halpern et al. 1993; Mullins et al. 1996; Ober and Schulte-Merker 1999; Agathon et al. 2003). One key regulator of tail formation in zebrafish as well as in Xenopus is the bone morphogenetic protein (Bmp) pathway (Ober and Schulte-Merker 1999; Gonzalez et al. 2000; Beck et al. 2001; Hammerschmidt and Mullins 2002; Myers et al. 2002; Agathon et al. 2003; De Robertis and Kuroda 2004; Munoz-Sanjuan and Hemmati-Brivanlou 2004). Recent studies have shown that Bmp signaling is required during early gastrulation for the specification of the tail (Pyati et al. 2005; Connors et al. 2006), suggesting that the tail MPCs might be uniquely specified at early stages of development and held in an undifferentiated state until the tail somites are ready to form. Unfortunately, it is very difficult to study this process because the trunk and tail MPCs are intermixed at the gastrula stage and within the early tailbud (Kanki and Ho GENES & DEVELOPMENT 20: by Cold Spring Harbor Laboratory Press ISSN /06;
2 Szeto and Kimelman 1997; Warga and Nüsslein-Volhard 1999), and thus it is not possible to uniquely identify the tail MPCs within an embryo until they are in the process of contributing to the tail somites. To circumvent this problem, we investigated the use of a genetic approach. Embryos lacking maternal and zygotic One-eyed pinhead (MZoep), an essential coreceptor for Nodal signaling (Gritsman et al. 1999), form only a small tail-like structure and do not form any trunk somites (Gritsman et al. 1999; Carmany- Rampey and Schier 2001). To determine if this structure is a true tail, we carefully compared the development of wild-type and MZoep embryos. In every case, MZoep embryos did not start forming somites until the wildtype embryos formed their first tail somite (the 16th somite), and the MZoep embryos continued to form somites at the wild-type rate until the wild-type embryos formed their final somite (Supplementary Fig. S1). This result indicated that MZoep embryos could provide a unique source of tail MPCs for analysis. We show here that MZoep provides a valuable system for studying the subdivision of the zebrafish body into trunk and tail domains. We show that the trunk is subdivided into an anterior and posterior domain, and that the decision of cells to enter the anterior trunk, posterior trunk, and tail is regulated by an interplay between Nodal and Bmp signaling, as well as by a trunk-promoting signal. Analyzing the expression of marker genes, we show that MZoep cells are held in a state equivalent to the Maturation Zone before they are ready to contribute to the tail. We propose that Nodal and Bmp signals control a regulator, which determines when the MPCs can first leave the Maturation Zone and commit to entering the presomitic mesoderm. In this way, some cells enter somitogenesis early and others are held in reserve, ensuring that a continuous supply of cells enter the presomitic mesoderm throughout somitogenesis. Results Somite fate of MZoep MPCs Within the context of the MZoep embryo, MZoep MPCs are only able to contribute to the tail (Carmany-Rampey and Schier 2001). To determine if MZoep MPCs cellautonomously recognize when to enter the tail, or if their fate can be changed when surrounded with MPCs entering the trunk and tail, we examined the ability of MZoep cells to contribute to trunk and tail somites in a wild-type background using cell transplantation. Small groups of fluorescently labeled cells were removed from the prospective mesodermal region of MZoep embryos at 5 h post-fertilization (hpf; 5 hpf = 40% epiboly) and transplanted into the prospective mesodermal region of wild-type embryos at the same stage (Fig. 1A). It is important to note that the transplanted cells will randomly end up in different positions across the dorsoanterior ventroposterior axis, and thus individual cells are potentially exposed to different intercellular signaling factors in the wild-type embryos. We generated 90 chimeric embryos and observed fluorescent MZoep cells in several regions including the neural tube, brain, and somites at 24 hpf when the muscle cells are easily distinguished from other cell types morphologically (Fig. 1B; data not shown). These results are generally consistent with the previous fate map of MZoep embryos (Carmany-Rampey and Schier 2001). To Figure 1. Homochronic cell transplantation. (A) Schematic depiction of cell transplant experiment showing 5-hpf MZoep cells transplanted into a 5-hpf wild-type host embryo. (B) Representative picture of a chimeric embryo. White arrows denote the boundary between trunk and tail regions. (C) Graph showing the most anterior somite populated by transplanted MZoep cells. The bars in the graph are differently colored in the trunk and tail regions for clarity. (D F) Transplants of 4-hpf MZoep cells into 4-hpf wild-type host embryos. Note that MZoep cells only populate the tail when transplanted at 5 hpf, whereas they also populate the posterior trunk when transplanted at 4 hpf GENES & DEVELOPMENT
3 Mesodermal progenitor cell specification determine the contribution of MZoep cells specifically to wild-type somites, we scored chimeric embryos for the most anterior somite containing a fluorescent cell, and represented this in a graphical format. Importantly, MZoep cells were never observed to contribute to the trunk somites (somites 1 15) (Fig. 1C). In order to ensure that the restriction of the MZoep muscle cells to the tail was not an artifact of the transplantation procedure, we transplanted rhodamine (red)- labeled wild-type cells and fluorescein (green)-labeled MZoep cells together into wild-type host embryos at 5 hpf (Fig. 2A). Whereas red fluorescent wild-type cells were found throughout the trunk and tail somites, green fluorescent MZoep cells were only found in the tail somites (Fig. 2B,C). Our results demonstrate that 5-hpf MZoep MPCs only contribute to the tail somites, and that the MZoep MPCs exclusively populate the tail domain despite the presence of wild-type cells contributing to the trunk and tail somites. Timing of commitment to the tail fate Our cell transplantation data indicated that the MZoep MPCs are committed to a tail fate by 5 hpf. To determine if MZoep MPCs can only ever contribute to the tail somites or if this is a property they acquire during early development, we transplanted cells from MZoep embryos at 3.3 and 4 hpf (high and sphere stages, respectively) into wild-type host embryos of the same stages (Fig. 1D). When MZoep cells were transplanted at 4 hpf and earlier, they populated both the trunk and tail (Fig. 1E,F; data not shown). These results suggest that the fates of the MZoep MPCs are not fixed, and that they acquire their identity as tail MPCs between 4 and 5 hpf. What changes between 4 and 5 hpf that causes the MZoep MPCs to become committed to a tail fate by 5 hpf? One possibility is that the fate of the MPCs becomes fixed between 4 and 5 hpf such that the 5-hpf cells do not respond to a new environment (Fig. 3A, Model 1). A second possibility is that the prospective tail cells need to be exposed to a tail-promoting signal during the 4 5-hpf interval (Fig. 3A, Model 2). When MZoep cells are transplanted at 4 hpf into 4-hpf wild-type embryos, some of the MPCs would no longer be exposed to the tail signal in this model because they randomly ended up in a region of the wild-type embryo that does express the tail signal, and these cells therefore would adopt a trunk fate. A third possibility is that a trunk-promoting signal is present in wild-type embryos only prior to 5 hpf (Fig. 3A, Model 3). In this scenario, transplantation of MZoep cells randomly into wild-type embryos at 4 hpf would Figure 2. Two-color cell transplantation. (A) Schematic depiction of the experiment. Fivehour-post-fertilization wild-type cells containing rhodamine dextran (red) and 5-hpf MZoep cells containing fluorescein dextran (green) were cotransplanted into 5-hpf wildtype host embryos. (B) A representative embryo. White arrows denote the boundary between the trunk and tail regions. (C) Graph showing the most anterior somite populated by transplanted wild-type cells (labeled in red) and MZoep cells (labeled in green). The anterior somite limit for MZoep cells in this experiment was somite 16, whereas wild-type cells were found as far anterior as somite 1. (D,E) Same as in A and B except that the donor cells and host embryo were all at 4 hpf. (F) Graph showing the most anterior somite populated by transplanted wild-type cells (labeled in red) and MZoep cells (labeled in green). The anterior somite limit for MZoep cells in this experiment was somite 9, whereas wild-type cells were found as far anterior as somite 1. GENES & DEVELOPMENT 1925
4 Szeto and Kimelman Figure 3. Heterochronic cell transplantation. (A) The three models are shown. At the top of each model is the hypothesis; in the middle is the experimental test, with a cartoon of a fish showing the predicted result based on the hypothesis; and at bottom is the result, either yes or no. Only Model 3 fits the data. (B) Schematic depiction of the experiment showing 5-hpf MZoep cells transplanted into 4-hpf wild-type host embryos. (C) Representative picture of a chimeric embryo. White arrows denote the boundary between trunk and tail regions. (D) Graph showing the most anterior somite populated by transplanted MZoep cells. (E G) Transplants of 4-hpf MZoep cells into 5-hpf wild-type host embryos. Note that 5-hpf MZoep cells populate the posterior trunk and tail when transplanted into 4-hpf hosts, whereas 4-hpf MZoep cells only populate the tail when transplanted into 5-hpf hosts. expose some of them to the trunk-promoting signal, whereas transplanting MZoep cells into wild-type embryos at 5 hpf would not expose them to the trunk promoting signal for enough time to change the fate of the MZoep cells. To distinguish between these possibilities, we used heterochronic cell transplantation by transplanting 5-hpf MZoep cells into 4-hpf wild-type hosts (Fig. 3B) and transplanting 4-hpf MZoep cells into 5-hpf wild-type hosts (Fig. 3E). If cells lost competence to change their trunk-tail fate by 5 hpf, then transplantation of 5-hpf MZoep cells into 4-hpf wild-type embryos would result in MZoep cells only populating the tail (Fig. 3A, Model 1). Instead, we found that transplanting 5-hpf MZoep cells to earlier wild-type embryos caused some of them to populate the trunk (Fig. 3C,D). If a tail-promoting signal was disrupted by transplantation at 4 hpf, we predicted that transplantation of 4-hpf MZoep cells into 5-hpf wild-type hosts would cause the MZoep cells to populate the trunk and tail, whereas transplantation of 5-hpf MZoep cells into 4-hpf wild-type hosts would result in MZoep cells only populating the tail (Fig. 3A, Model 2). This is not what we observed (Fig. 3C,D,F,G). Conversely, if a trunk-promoting signal was present in wild-type embryos up until 5 hpf, we predicted that transplantation of 5-hpf MZoep cells into 4-hpf wild-type hosts would cause the MZoep cells to populate the trunk and tail, whereas transplantation of 4-hpf MZoep cells into 5-hpf wild-type hosts would result in MZoep cells only populating the tail (Fig. 3A, Model 3). This exactly matches the experimental results (Fig. 3C,D,F,G). We therefore conclude that wild-type embryos express a trunk-promoting signal that MZoep cells must first encounter prior to 5 hpf to adopt a trunk fate. Moreover, since MZoep embryos lack Nodal signaling and do not form a trunk, we conclude that the trunk-promoting signal is Nodal dependent GENES & DEVELOPMENT
5 Mesodermal progenitor cell specification Nodal signaling specifies the anterior trunk Although MZoep cells can contribute to trunk somites when transplanted into wild-type hosts at 4 hpf, we noticed that they never contributed to somites anterior to somite 9 in either homochronic or heterochronic transplants (Figs. 1F, 3D). This was not related to the age of the MZoep cells since transplantation of 3.3-hpf MZoep cells produced exactly the same result as a 4-hpf transplantation (data not shown). To rule out the possibility that this result was an artifact of our transplantation technique, we cotransplanted 4-hpf red fluorescent wildtype cells and 4-hpf green fluorescent MZoep cells into 4-hpf wild-type hosts (Fig. 2D). Whereas wild-type cells populated all regions of the embryo, the cotransplanted MZoep cells were not found anterior to somite 9 (Fig. 2E,F). Our results suggest that MZoep cells lack an essential property that allows them to populate the anterior trunk even when exposed to the trunk-promoting signal. We reasoned that the failure of the MZoep cells to receive a direct Nodal signal might be the cause of this deficit, which we tested by expressing a cell-autonomous activator of the Nodal pathway (a constitutively active Alk4 receptor, CA-Alk4) (Chang et al. 1997) in MZoep embryos, and then transplanting cells from these embryos at 5 hpf into 5-hpf wild-type hosts (Fig. 4A). As shown in Figure 4, MZoep cells expressing the CA-Alk4 populate the most anterior somites (cf. Figs. 4B,C and 1B,C). Thus, Nodal signaling is cell-autonomously required in cells to form the anterior, but not the posterior, trunk somites. Bmp signaling is required to establish the trunk tail boundary What instructs the MZoep cells transplanted at 5 hpf to only enter the tail domain? Studies in Xenopus and zebrafish have shown that Bmp signaling is important for forming a tail, and thus it was a strong candidate factor (Mullins et al. 1996; Kishimoto et al. 1997; Ober and Schulte-Merker 1999; Dick et al. 2000; Gonzalez et al. 2000; Beck et al. 2001; Hammerschmidt and Mullins 2002; Myers et al. 2002; Agathon et al. 2003; De Robertis and Kuroda 2004; Munoz-Sanjuan and Hemmati-Brivanlou 2004; Pyati et al. 2005). We investigated the role of Bmp signaling in the development of the MPCs by examining the ability of 5-hpf MZoep MPCs expressing a dominant-negative Bmp receptor (dnbmpr) (Pyati et al. 2005) to contribute to the somites in wild-type hosts using cell transplantation (Fig. 4D). Whereas uninjected MZoep cells contribute only to the tail (Fig. 1B,C), MZoep cells injected with dnbmpr RNA were also found in the posterior trunk (Fig. 4E,F). Conversely, whereas uninjected 4-hpf MZoep cells transplanted into 4-hpf wild-type hosts produced many embryos with cells in the posterior trunk (Fig. 1F), 4-hpf MZoep cells injected with 138 pg of bmp2b RNA (Kishimoto et al. 1997) transplanted into wild-type host embryos at 4 hpf had a reduced number of trunk cells (Supplementary Fig. S2A C). We confirmed this finding by showing that the proportion of 4-hpf MZoep cells contributing to the trunk decreased with increasing amounts of injected bmp2b RNA (Supplementary Fig. S2D). We note that even at the highest doses of injected bmp2b RNA we Figure 4. Regulation of MZoep somite commitment by Nodal and Bmp. (A) Schematic depiction of the experiment showing 5-hpf MZoep cells expressing a constitutive activator of the Nodal pathway (CA-Alk4) transplanted into 5-hpf wild-type host embryos. (B) Representative picture of a chimeric embryo. (C) Graph showing the most anterior somite populated by transplanted MZoep cells. Note that activation of the Nodal pathway causes the 5-hpf MZoep cells to enter the anterior trunk (cf. Fig. 1A C). (D F) Same as A C except that the MZoep cells express an inhibitor of Bmp signaling (dnbmpr) and the donor cells and host embryos are at 5 hpf. Note that inhibiting the Bmp pathway causes 5-hpf MZoep cells to populate the trunk (cf. Fig. 1A C). GENES & DEVELOPMENT 1927
6 Szeto and Kimelman were not able to convert all of the MPCs to a tail fate. This might indicate that Bmp signaling alone is not able to promote the tail fate in all MPCs, or it might be more simply due to the fact that RNA injected at the one-cell stage frequently is not incorporated into all of the embryonic blastomeres (our unpublished results). These results demonstrate that Bmp signaling plays an essential role in establishing the boundary between the posterior trunk and the tail. Since MZoep cells can be converted to a trunk fate by inhibiting Bmp signaling, we suggest that Bmp inhibitory factors such as Chordin, Noggin, and Follistatin are candidates to be the trunk signal. A second means of regulating Bmp signaling in zebrafish involves Fibroblast growth factor (Fgf) signaling (Furthauer et al. 1997, 2004). Three fgfs are expressed in a dorsal ventral gradient in the late blastula zebrafish embryo (Furthauer et al. 1997, 2004; Draper et al. 2003), and they have been shown to inhibit the transcription of bmp2b, bmp4, and bmp7 during the late blastula stages, which is important for restricting bmp transcripts to the ventral side of the embryo (Furthauer et al. 1997, 2004). Since the fgfs were also candidates to be components of the trunk-promoting signal, we examined whether fgf overexpression in MZoep cells would cause them to enter the posterior trunk. As shown in Figure 5, MZoep cells overexpressing Fgf entered the posterior trunk when transplanted at 5 hpf (cf. Figs. 5A C and 1A C). Tail progenitors are held in the Maturation Zone state during early somitogenesis The formation of the somites from the MPCs involves progressive changes in T-box gene expression starting with an initial expression of no tail (ntl) (Schulte-Merker et al. 1992), progressing to the activation of spadetail (spt) (Griffin et al. 1998) and tbx6 (Hug et al. 1997) in the Maturation Zone, and finally to the down-regulation of ntl and the activation of tbx24 (Nikaido et al. 2002) in the early presomitic mesoderm (Griffin and Kimelman 2002; Nikaido et al. 2002). Since we found that the MZoep embryos formed only tail MPCs, we recognized that we could use the MZoep embryos to determine whether the tail MPCs are normally held in the Maturation Zone state before they are ready to contribute to the somites. We examined the expression of each of the four T-box genes during the early stages of development. ntl is expressed in MZoep embryos during the gastrula stages, although at decreased levels compared with wild-type embryos, as previously noted (Gritsman et al. 1999), and it continues to be expressed at wild-type levels in the tailbud throughout somitogenesis, although not in the notochord, which is absent in MZoep (Fig. 6, cf. E H and A D). Similarly, spt and tbx6 are expressed throughout gastrulation and somitogenesis in MZoep embryos (Supplementary Fig. S3). In contrast, tbx24, the marker of the early presomitic mesoderm (Nikaido et al. 2002), is not expressed in MZoep embryos until after the 12- somite stage, whereas it is expressed in wild-type embryos throughout the gastrula and somitogenesis stages (Fig. 6, cf. I L and M P). Since there is a delay between when cells first enter the presomitic mesoderm and when they are incorporated into a morphologically apparent somite, we suggest that the cells first expressing tbx24 at the 12-somite stage will be the cells that end up in the first tail somite (somite 16). To confirm that the MZoep tail MPCs fail to enter the early presomitic mesoderm prior to the 12-somite stage, we also examined the expression of Wnt Inhibitory Factor-1 (WIF-1) (Hsieh et al. 1999). Like tbx24, WIF-1 is expressed in the early presomitic mesoderm but not in the tailbud. Whereas WIF-1 is expressed from the end of gastrulation through somitogenesis in wild-type embryos, it is not expressed in MZoep embryos until the 12-somite stage, paralleling the expression of tbx24 (Supplementary Fig. S4). We conclude that the tail MPCs are held in the Maturation Zone state, expressing ntl, spt, and tbx6, but prevented from expressing markers of the early presomitic mesoderm such as tbx24 and WIF-1. Discussion Regional development of zebrafish body Our studies using cell transplants have demonstrated that the zebrafish body is divided into three domains, which the MPCs recognize: the anterior trunk, the posterior trunk, and the tail (Fig. 7A). When transplanted among wild-type cells that contribute to all three domains, the MZoep cells transplanted at 5 hpf contribute only to the tail, whereas MZoep cells transplanted at 4 Figure 5. Fgf overexpression causes MZoep cells to enter the posterior trunk. (A) Schematic depiction of the experiment showing 5-hpf MZoep cells overexpressing Fgf4 transplanted into 5-hpf wild-type host embryos. (B) Representative picture of a chimeric embryo. (C) Graph showing the most anterior somite populated by transplanted MZoep cells. Note that Fgf overexpression causes 5-hpf MZoep cells to enter the posterior trunk (cf. Fig. 1A C) GENES & DEVELOPMENT
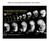
7 Mesodermal progenitor cell specification Figure 6. Tail MPCs express ntl but not tbx24 prior to the 12-somite stage. (A D) Expression of ntl in wild-type embryos. (E H) Expression of ntl in MZoep embryos. (I L) Expression of tbx24 in wild-type embryos. (M P) Expression of tbx24 in MZoep embryos. A, E, I, and M are animal pole views with dorsal to the top. The other panels are posterior views with dorsal to the top. hpf contribute to the posterior trunk and tail. Intriguingly, this subdivision of the zebrafish body is also observed with several zebrafish mutants; mutants in integrin 5 fail to properly form just the anterior trunk somites (Julich et al. 2005), whereas MZoep, ntl, spt, and strong bozozok;chordino mutants (Ho and Kane 1990; Halpern et al. 1993; Griffin et al. 1998; Gritsman et al. 1999; Gonzalez et al. 2000), as well as embryos with perturbed dorsal organizer formation (Ober and Schulte- Merker 1999), have phenotypes that specifically affect either the trunk or tail somites. Thus, the timing of MPC commitment to the somites is likely to reflect a basic subdivision of the zebrafish body into three genetically distinct regions. At the posterior end of the embryo, we show that Bmp is necessary to establish the trunk tail boundary since MZoep cells expressing a dominant-negative Bmp receptor no longer recognize this boundary (Fig. 4F). Whereas previous studies in zebrafish have shown that Bmp signaling is necessary for tail formation (Mullins et al. 1996; Ober and Schulte-Merker 1999; Gonzalez et al. 2000; Hammerschmidt and Mullins 2002; Agathon et al. 2003; Pyati et al. 2005; Connors et al. 2006), we show that Bmp signaling plays a critical role in precisely setting the boundary between the trunk and tail domains. This is in general agreement with previous studies demonstrating that overexpression of Bmps promotes posteriorization of the embryo, whereas inhibition of Bmp signaling results in anteriorization (for review, see Hammerschmidt and Mullins 2002). A second important role of Bmp signaling is to regulate the morphogenesis of cells on the ventral side of the zebrafish embryo. Whereas dorsal and lateral cells converge toward the dorsal side and extend along the future anterior posterior axis, the ventral cells, which will contribute many of the tail MPCs (Warga and Nüsslein-Volhard 1999; Carmany-Rampey and Schier 2001), exhibit a zone of no convergence no extension behavior, and instead migrate to the vegetal pole of the embryo where the tailbud forms at the end of gastrulation (Myers et al. 2002). The specific morphogenetic properties of the ventral cells depends on high-level Bmp signaling (Myers et al. 2002). Thus, Bmp signaling not only determines exactly when the tail cells enter the somitogenesis program, but it also controls their morphogenesis so that they will end up in the tailbud, ready to contribute to the formation of the tail somites (Kanki and Ho 1997). Regulation of the zebrafish body by Bmps and Nodals We propose that the different domains within the somites are established through Nodal and Bmp signaling (Fig. 7A). Nodal signaling within the MPCs is necessary for cells to ingress during gastrulation (Carmany- Rampey and Schier 2001), and we show here that expression of a constitutively activate Nodal receptor causes MZoep cells to contribute to the anterior trunk (Fig. 4C). This result shows that the only defect in MZoep embryos with regards to forming trunk MPCs is due to a defect in the Nodal signaling pathway and not due to other possible roles of Oep (Warga and Kane 2003). Figure 7. Model for the mesodermal progenitor cell commitment regulator. (A) Schematic diagram showing the three domains within the somites. The anterior trunk region (somites 1 8) requires a direct Nodal signaling input. The posterior trunk region (somites 9 15) requires a trunk signal that is activated by Nodal signaling. The tail region (somite 16 and beyond) requires a Bmp signal. (B) The MPC commitment regulator. In the presence of low Nodal and Bmp signaling, MPCs begin to enter at somite 9 (S9). High levels of Nodal signaling set the regulator for entry at somite 1 (S1), whereas high levels of Bmp signaling set the regulator for entry at somite 16 (S16). GENES & DEVELOPMENT 1929
8 Szeto and Kimelman Regulation of posterior trunk formation Our data suggest that the posterior trunk is regulated by a trunk signal, which is activated by Nodal signaling since the trunk fails to form in MZoep embryos (Fig. 7A). Since MZoep cells can contribute to the trunk when Bmp signaling is inhibited, we suggest that the trunk signal includes several Bmp inhibitory molecules, which are known to work in an overlapping fashion (Khokha et al. 2005). In support of this hypothesis, MZoep mutants fail to express noggin1 and follistatin (Ragland and Raible 2004) and have reduced expression of chordin (Gritsman et al. 1999; Ragland and Raible 2004), supporting a role for Nodal signaling in regulating these Bmp inhibitors. An additional set of candidates to regulate Bmp signaling are the Fgfs, which inhibit bmp transcription (Furthauer et al. 1997, 1999). fgf8 is not expressed in MZoep embryos until the mid-gastrula stages, whereas fgf3 expression is initiated but not maintained in early MZoep, embryos and it is not expressed again until the mid-gastrula stages (Mathieu et al. 2004) (the expression of fgf24 in MZoep has not been reported). Together with reduced or absent expression of the Bmp inhibitors, this results in a reduction of dorsal organizer gene expression and an expanded expression of bmp2b and Bmp-regulated genes in MZoep embryos (Gritsman et al. 1999; Ragland and Raible 2004). We therefore suggest that the Nodal-dependent trunk-promoting signal present in the late blastula wild-type embryo is composed of the Bmp inhibitors (Chordin, Noggin1, and Follistatin), which bind the Bmps and prevent them from interacting with their receptors, and the Fgfs, which regulate the transcription of the bmps. Our heterochronic experiments indicate that the trunk signal is only present in wild-type embryos prior to 5 hpf. Thus if 4-hpf or 5-hpf MZoep cells are transplanted into 4-hpf wild-type embryos, they can contribute to the trunk, whereas if the MZoep cells are transplanted into 5-hpf wild-type embryos, they only contribute to the tail. It is important to note that when the MZoep cells are transplanted into wild-type embryos, they randomly enter into different positions along the dorsoanterior ventroposterior axis. Thus, some MZoep cells will end up in the ventroposterior region where they are exposed to high levels of Bmp signaling, whereas other MZoep cells will end up in lateral and dorsoanterior regions where they will be exposed to much lower levels of Bmp signaling. This is likely to explain why only some of the MZoep cells transplanted into 4-hpf wild-type embryos contribute to the trunk. We do not know why MZoep cells transplanted into 5-hpf wild-type embryos are not converted to a trunk fate since the Bmp inhibitors continue to be expressed in embryos after 5 hpf. One possibility is that the length of time that MPCs are exposed to the inhibitors is critical, and that they first have to see the trunk signal prior to 5 hpf. However, it is also becoming increasingly clear that the regulation of Bmp signaling in the early vertebrate embryo is quite complex (for review, see Kimelman and Szeto 2006). An intriguing possibility is that while the inhibitors are expressed after 5 hpf, their activity is diminished, and thus they are no longer able to convert the transplanted cells to a trunk fate. Understanding whether or not the Bmp inhibitors change in potency during development will be essential to distinguish between these ideas. Regulation of MPC commitment to somitogenesis Our data are consistent with a model in which the MPCs contain a regulator that instructs them when to first begin entering the somites (Fig. 7B). We suggest that highlevel Nodal signaling drives the cells to enter at somite 1 (the anterior trunk), whereas high-level Bmp signaling instructs cells to begin entering at somite 16 (the tail). Since cells lacking both Nodal and Bmp signaling (MZoep cells expressing the dominant-negative Bmp receptor) contribute to the posterior trunk, we suggest that cells that receive low levels of Nodal and Bmp signals are programmed to enter at somite 9. There is good evidence for a gastrula-stage gradient of Bmp signaling across the dorsoanterior ventroposterior axis and a gradient of Nodal signaling across the animal vegetal axis (for review, see Schier and Talbot 2005; Kimelman 2006), and we suggest that these overlapping gradients determine when each of the MPCs enters into the somitogenesis program. Before the regulator tells cells to begin entering the somites, cells wait in the Maturation Zone state until they are ready to enter the presomitic mesoderm, suggesting that the regulator acts by not only preventing cells from leaving the Maturation Zone, but also by blocking the expression of a subset of genes including tbx24 and WIF-1. We note that the regulator does not tell cells exactly when to enter the presomitic mesoderm, but instead specifies when they can first begin entering. We suggest that the regulator plays an important role by preventing subsets of the MPCs from entering the somite-forming program early to ensure that there is a continuous supply of MPCs throughout somitogenesis to allow all of the somites to form. In the absence of Bmp signaling, for example, the tail muscles are absent since they have been converted to a trunk fate as shown in Xenopus studies (Lane et al. 2004), resulting in an expansion of the trunk muscles and severely deleterious development of the embryo. Thus, an interplay between Nodal and Bmp signals provides critical information to the MPCs, resulting in the formation of distinct domains within the zebrafish body musculature. Materials and methods Embryos and RNA injections Zebrafish embryos were obtained by natural spawning of adult AB/WIK wild-type and MZoep mutant zebrafish. RNAs were synthesized from Asp718 linearized CS2-dnBmpR (Pyati et al. 2005), CS2-zBmp2b (Kishimoto et al. 1997), and CS2-fgf4 templates using the mmessage Machine Kit (Ambion) and dissolved in RNase-free sterile water. RNA (at concentrations indicated in 1930 GENES & DEVELOPMENT
9 Mesodermal progenitor cell specification the text) was injected into one- to two-cell stage zebrafish embryos. Injected embryos were collected at the appropriate stages for in situ hybridization analysis. Cell transplantation Single and double cell transplantations were performed at the various developmental stages indicated in text. Briefly, donor embryos were labeled at the one-cell stage with 2% (10,000 MW) fluorescein dextran or rhodamine dextran (Molecular Probes) and allowed to develop to the desired stage. Cells from a donor embryo were removed using a forged micropipette and transferred to the margin of an unlabeled wild-type host embryo. The resulting chimeric embryo was allowed to develop to 24 h. The location of the labeled donor cells within the trunk and tail regions was monitored under an epi-fluorescent microscope. Images were captured using a digital camera and stored as Adobe Photoshop files for manipulation and analysis. Scoring chimeric embryos After cell transplantation, chimeric embryos were allowed to develop for 24 h. Under an epi-fluorescent microscope, embryos with fluorescein-labeled MZoep donor cells in the developing somites were identified and taken out for further evaluation. Each of these selected chimeric embryos was scored for the most anterior somite containing a fluorescein-labeled MZoep donor cell. The somite number was plotted along the X-axis, and the number of chimeric embryos with a fluorescent cell at that position was plotted on the Y-axis. In situ hybridization Whole-mount in situ hybridization was performed using digoxigenin-labeled antisense RNA probes and visualized using antidigoxigenin Fab fragment conjugated to alkaline phosphatase (Roche Molecular Biochemicals) as described (Szeto and Kimelman 2004). Riboprobes were made from DNA templates, which were linearized and transcribed with either SP6 or T7 RNA polymerases. Embryos were processed and hybridized as described (Szeto and Kimelman 2004). Acknowledgments We thank Ujwal J. Pyati, Douglas C. Weiser, and Ashley E. Webb for critical comments on this manuscript; Ali Hemmati- Brivanlou for providing CA-Alk4; Bruce W. Draper for fgf4; and Jen C. Hsieh for providing WIF-1. We are very grateful to David Raible for allowing us to frequently use his transplant apparatus. This work was supported by an NSF grant to D.K. References Agathon, A., Thisse, C., and Thisse, B The molecular nature of the zebrafish tail organizer. Nature 424: Beck, C.W., Whitman, M., and Slack, J.M The role of BMP signaling in outgrowth and patterning of the Xenopus tail bud. Dev. Biol. 238: Carmany-Rampey, A. and Schier, A.F Single-cell internalization during zebrafish gastrulation. Curr. Biol. 11: Chang, C., Wilson, P.A., Mathews, L.S., and Hemmati-Brivanlou, A A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development 124: Connors, S.A., Tucker, J.A., and Mullins, M.C Temporal and spatial action of Tolloid (Mini fin) and Chordin to pattern tail tissues. Dev. Biol. 293: De Robertis, E.M. and Kuroda, H Dorsal ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20: Dick, A., Hild, M., Bauer, H., Imai, Y., Maifeld, H., Schier, A.F., Talbot, W.S., Bouwmeester, T., and Hammerschmidt, M Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127: Draper, B.W., Stock, D.W., and Kimmel, C.B Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development 130: Dubrulle, J. and Pourquie, O Coupling segmentation to axis formation. Development 131: Furthauer, M., Thisse, C., and Thisse, B A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development 124: Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev. Biol. 214: Furthauer, M., Van Celst, J., Thisse, C., and Thisse, B Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development 131: Gonzalez, E.M., Fekany-Lee, K., Carmany-Rampey, A., Erter, C., Topczewski, J., Wright, C.V., and Solnica-Krezel, L Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes & Dev. 14: Griffin, K.J. and Kimelman, D One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nat. Cell Biol. 4: Griffin, K.J.P., Amacher, S.L., Kimmel, C.B., and Kimelman, D Molecular identification of spadetail: Regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development 125: Gritsman, K., Zhang, J., Cheng, S., Heckscher, E., Talbot, W.S., and Schier, A.F The EGF-CFC protein One-eyed pinhead is essential for Nodal signaling. Cell 97: Halpern, M.E., Ho, R.K., Walker, C., and Kimmel, C.B Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75: Hammerschmidt, M. and Mullins, M Dorsoventral patterning in the zebrafish: Bone morphogenetic proteins and beyond. In Results and problems in cell differentiation (ed. L. Solnica-Krezel), pp Springer-Verlag, Berlin. Ho, R.K. and Kane, D.A Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature 348: Holley, S.A. and Takeda, H Catching a wave: The oscillator and wavefront that create the zebrafish somite. Semin. Cell Dev. Biol. 13: Hsieh, J.C., Kodjabachian, L., Rebbert, M.L., Rattner, A., Smallwood, P.M., Samos, C.H., Nusse, R., Dawid, I.B., and Nathans, J A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398: Hug, B., Walter, V., and Grunwald, D.J tbx6, a Brachyuryrelated gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev. Biol. 183: Julich, D., Geisler, R., and Holley, S.A Integrin 5 and Delta/Notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev. Cell 8: Kanki, J.P. and Ho, R.K The development of the posterior body in zebrafish. Development 124: GENES & DEVELOPMENT 1931
10 Szeto and Kimelman Khokha, M.K., Yeh, J., Grammer, T.C., and Harland, R.M Depletion of three BMP antagonists from Spemann s organizer leads to a catastrophic loss of dorsal structures. Dev. Cell 8: Kimelman, D Mesoderm induction: From caps to chips. Nat. Rev. Genet. 7: Kimelman, D. and Szeto, D.P Chordin cleavage is sizzling. Nat. Cell Biol. 8: Kishimoto, Y., Lee, K.H., Zon, L., Hammerschmidt, M., and Schulte-Merker, S The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124: Lane, M.C., Davidson, L., and Sheets, M.D BMP antagonism by Spemann s organizer regulates rostral-caudal fate of mesoderm. Dev. Biol. 275: Mathieu, J., Griffin, K., Herbomel, P., Dickmeis, T., Strahle, U., Kimelman, D., Rosa, F.M., and Peyrieras, N Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development 131: Mullins, M.C., Hammerschmidt, M., Kane, D.A., Odenthal, J., Brand, M., van Eeden, F.J., Furutani-Seiki, M., Granato, M., Haffter, P., Heisenberg, C.P., et al Genes establishing dorsoventral pattern formation in the zebrafish embryo: The ventral specifying genes. Development 123: Munoz-Sanjuan, I. and Hemmati-Brivanlou, A.H Modulation of Bmp signaling during vertebrate gastrulation. In Gastrulation (ed. C.D. Stern), pp Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. Myers, D.C., Sepich, D.S., and Solnica-Krezel, L Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev. Biol. 243: Nikaido, M., Kawakami, A., Sawada, A., Furutani-Seiki, M., Takeda, H., and Araki, K Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nat. Genet. 31: Ober, E.A. and Schulte-Merker, S Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev. Biol. 215: Pyati, U.J., Webb, A.E., and Kimelman, D Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development 132: Ragland, J.W. and Raible, D.W Signals derived from the underlying mesoderm are dispensable for zebrafish neural crest induction. Dev. Biol. 276: Schier, A.F. and Talbot, W.S Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39: Schier, A.F., Neuhauss, S.C., Helde, K.A., Talbot, W.S., and Driever, W The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development 124: Schulte-Merker, S., Ho, R.K., Herrmann, B.G., and Nüsslein- Vollhard, C The protein product of the zebrafish homologue of the mouse T gene is expressed in the nuclei of the germ ring and the notochord of the early embryo. Development 116: Szeto, D.P. and Kimelman, D Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development 131: Tam, P.P., Goldman, D., Camus, A., and Schoenwolf, G.C Early events of somitogenesis in higher vertebrates: Allocation of precursor cells during gastrulation and the organization of a meristic pattern in the paraxial mesoderm. Curr. Top. Dev. Biol. 47: Warga, R.M. and Kane, D.A One-eyed pinhead regulates cell motility independent of Squint/Cyclops signaling. Dev. Biol. 261: Warga, R.M. and Nüsslein-Volhard, C Origin and development of the zebrafish endoderm. Development 126: GENES & DEVELOPMENT
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Mesoderm Formation. Fate map of early gastrula. Only two types of mesoderm are induced. Mesoderm induction by the vegetal hemisphere
 Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation
 Corrigendum Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation Daniel P. Szeto and David Kimelman Development 131, 3751-3760. The labelling in Fig. 6 of this article
Corrigendum Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation Daniel P. Szeto and David Kimelman Development 131, 3751-3760. The labelling in Fig. 6 of this article
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
The role of the yolk syncytial layer in germ layer patterning in zebrafish
 Development 127, 4681-4689 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV3237 4681 The role of the yolk syncytial layer in germ layer patterning in zebrafish Shaw-Ree Chen and
Development 127, 4681-4689 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV3237 4681 The role of the yolk syncytial layer in germ layer patterning in zebrafish Shaw-Ree Chen and
Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement
 Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement Hwei-Jan Hsu 1,2, Ming-Ren Liang 3, Chao-Tsen Chen 3, and Bon-Chu Chung 1 * Abstract Embryonic cell movement is essential
Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement Hwei-Jan Hsu 1,2, Ming-Ren Liang 3, Chao-Tsen Chen 3, and Bon-Chu Chung 1 * Abstract Embryonic cell movement is essential
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Neural Induction. Steven McLoon Department of Neuroscience University of Minnesota
 Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
BMP signaling restricts hemato-vascular development from lateral mesoderm during somitogenesis
 RESEARCH ARTICLE 2177 Development 133, 2177-2187 (2006) doi:10.1242/dev.02386 BMP signaling restricts hemato-vascular development from lateral mesoderm during somitogenesis Sunny Gupta 1, Hao Zhu 2, Leonard
RESEARCH ARTICLE 2177 Development 133, 2177-2187 (2006) doi:10.1242/dev.02386 BMP signaling restricts hemato-vascular development from lateral mesoderm during somitogenesis Sunny Gupta 1, Hao Zhu 2, Leonard
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5
 Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Patterning of the Brain
 Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Reading. Lecture III. Nervous System Embryology. Biology. Brain Diseases. September 5, Bio 3411 Lecture III. Nervous System Embryology
 Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Lecture III. Nervous System Embryology
 Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Lecture 20: Drosophila embryogenesis
 Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
1. 40 points Retinoic acid (structure below) is a small hydrophobic molecule that is derived from vitamin A. Figure by MIT OCW.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 9 7.22 Fall 2005 exam 2 practice
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 9 7.22 Fall 2005 exam 2 practice
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3
 The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
Later Development. Caenorhabditis elegans. Later Processes 10/06/12. Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development
 Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Neural Induction in the Absence of Mesoderm: -Catenin-Dependent Expression of Secreted BMP Antagonists at the Blastula Stage in Xenopus
 Developmental Biology 234, 161 173 (2001) doi:10.1006/dbio.2001.0258, available online at http://www.idealibrary.com on Neural Induction in the Absence of Mesoderm: -Catenin-Dependent Expression of Secreted
Developmental Biology 234, 161 173 (2001) doi:10.1006/dbio.2001.0258, available online at http://www.idealibrary.com on Neural Induction in the Absence of Mesoderm: -Catenin-Dependent Expression of Secreted
Differentiation. Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU)
 Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Extraembryonic Signals under the Control of MGA, Max, and Smad4 Are Required for Dorsoventral Patterning
 Article Extraembryonic Signals under the Control of MGA, Max, and Smad4 Are Required for Dorsoventral Patterning Yuhua Sun, 1 Wei-Chia Tseng, 1 Xiang Fan, 1 Rebecca Ball, 1 and Scott T. Dougan 1, * 1 Department
Article Extraembryonic Signals under the Control of MGA, Max, and Smad4 Are Required for Dorsoventral Patterning Yuhua Sun, 1 Wei-Chia Tseng, 1 Xiang Fan, 1 Rebecca Ball, 1 and Scott T. Dougan 1, * 1 Department
Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms
 Development 127, 1081-1093 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV6431 1081 Mesendoderm and left-right brain, heart and gut development are differentially regulated by
Development 127, 1081-1093 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV6431 1081 Mesendoderm and left-right brain, heart and gut development are differentially regulated by
The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
 Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
Developmental Biology 229, 287 306 (2001) doi:10.1006/dbio.2000.9950, available online at http://www.idealibrary.com on The Pitx2 Homeobox Protein Is Required Early for Endoderm Formation and Nodal Signaling
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Regulating Bone Growth and Development with Bone Morphogenetic Proteins
 Regulating Bone Growth and Development with Bone Morphogenetic Proteins PHOEBE S. LEBOY Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6030,
Regulating Bone Growth and Development with Bone Morphogenetic Proteins PHOEBE S. LEBOY Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6030,
RNAs were transcribed from described expression conhibit a similar capacity to synergize with neural-inducing
 DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways
 Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Biphasic wnt8a Expression Is Achieved Through Interactions of Multiple Regulatory Inputs
 a DEVELOPMENTAL DYNAMICS 241:1062 1075, 2012 RESEARCH ARTICLE Biphasic wnt8a Expression Is Achieved Through Interactions of Multiple Regulatory Inputs Anand Narayanan and Arne C. Lekven* Background: Vertebrate
a DEVELOPMENTAL DYNAMICS 241:1062 1075, 2012 RESEARCH ARTICLE Biphasic wnt8a Expression Is Achieved Through Interactions of Multiple Regulatory Inputs Anand Narayanan and Arne C. Lekven* Background: Vertebrate
Cell Lineage in the Development of the Leech Nervous System
 Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
KEY Reproductive cloning Therapeutic cloning
 1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Exam 1 ID#: October 1, 2006
 Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Lecture 20: Drosophila melanogaster
 Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Bmp Suppression in Mangrove Killifish Embryos Causes a Split in the Body Axis
 Bmp Suppression in Mangrove Killifish Embryos Causes a Split in the Body Axis Sulayman Mourabit, Michael W. Moles, Emma Smith, Ronny van Aerle, Tetsuhiro Kudoh* Biosciences, College of Life & Environmental
Bmp Suppression in Mangrove Killifish Embryos Causes a Split in the Body Axis Sulayman Mourabit, Michael W. Moles, Emma Smith, Ronny van Aerle, Tetsuhiro Kudoh* Biosciences, College of Life & Environmental
Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals
 Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals Hidehiko Inomata, 1,3, * Tatsuo Shibata, 2 Tomoko Haraguchi, 1 and Yoshiki Sasai 1, * 1 Organogenesis
Scaling of Dorsal-Ventral Patterning by Embryo Size-Dependent Degradation of Spemann s Organizer Signals Hidehiko Inomata, 1,3, * Tatsuo Shibata, 2 Tomoko Haraguchi, 1 and Yoshiki Sasai 1, * 1 Organogenesis
Why Study Developmental Neurobiology? Terrific scientific challenge.
 Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
cyclops encodes a nodal-related factor involved in midline signaling
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9932 9937, August 1998 Developmental Biology cyclops encodes a nodal-related factor involved in midline signaling MICHAEL R. REBAGLIATI*, REIKO TOYAMA*, PASCAL HAFFTER,
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9932 9937, August 1998 Developmental Biology cyclops encodes a nodal-related factor involved in midline signaling MICHAEL R. REBAGLIATI*, REIKO TOYAMA*, PASCAL HAFFTER,
Conserved Patterns of Cell Movements during Vertebrate Gastrulation. Review. Lilianna Solnica-Krezel
 Current Biology, Vol. 15, R213 R228, March 29, 2005, 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2005.03.016 Conserved Patterns of Cell Movements during Vertebrate Gastrulation Review Lilianna
Current Biology, Vol. 15, R213 R228, March 29, 2005, 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2005.03.016 Conserved Patterns of Cell Movements during Vertebrate Gastrulation Review Lilianna
NIH Public Access Author Manuscript Nat Chem Biol. Author manuscript; available in PMC 2012 September 01.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Chem Biol. ; 8(3): 270 276. doi:10.1038/nchembio.772. Spatiotemporal resolution of the Ntla transcriptome in axial mesoderm development
NIH Public Access Author Manuscript Published in final edited form as: Nat Chem Biol. ; 8(3): 270 276. doi:10.1038/nchembio.772. Spatiotemporal resolution of the Ntla transcriptome in axial mesoderm development
Understanding embryonic head development. ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute
 Understanding embryonic head development ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute Head malformations among the most common category of congenital malformations in
Understanding embryonic head development ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute Head malformations among the most common category of congenital malformations in
Developmental Zoology (ZOO ) Gatrulation
 Developmental Zoology (ZOO 228.1.0) Gatrulation 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis Aging
Developmental Zoology (ZOO 228.1.0) Gatrulation 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis Aging
What s the most complex problem in biology?
 Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
ANAT2341 Embryology Introduction: Steve Palmer
 ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
HOW DOES Wnt SIGNALING POSTERIORIZE THE NEURAL PLATE?
 HOW DOES Wnt SIGNALING POSTERIORIZE THE NEURAL PLATE? An Undergraduate Research Scholars Thesis By JEFFREY JOON TAEK LEE Submitted to Honors and Undergraduate Research Texas A&M University In partial fulfillment
HOW DOES Wnt SIGNALING POSTERIORIZE THE NEURAL PLATE? An Undergraduate Research Scholars Thesis By JEFFREY JOON TAEK LEE Submitted to Honors and Undergraduate Research Texas A&M University In partial fulfillment
Neural crest induction in Xenopus: evidence for a two-signal model
 Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
Assignment 11. Which of the following is true of the gray crescent region of frog eggs?
 Assignment 11 1. Multiple-choice (1 point) Which of the following is true of the gray crescent region of frog eggs? It contains mainly yolk. It may be seen in an unfertilized egg. Its content is usually
Assignment 11 1. Multiple-choice (1 point) Which of the following is true of the gray crescent region of frog eggs? It contains mainly yolk. It may be seen in an unfertilized egg. Its content is usually
7.012 Problem Set 5. Question 1
 Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Induction and patterning of the telencephalon in Xenopus laevis
 Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Culture and differentiation of embryonic stem cells. Hong-Lin Su Department of Life Sciences, National Chung-Hsing University
 Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
Chapter 47. Development
 Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Neural induction. Noggin Chordin Follistatin (Xnr3)
 a bird s eye view Since the discovery of the phenomenon of neural induction by Spemann and Mangold in 94, considerable effort has been invested in identifying the signals produced by the organizer that
a bird s eye view Since the discovery of the phenomenon of neural induction by Spemann and Mangold in 94, considerable effort has been invested in identifying the signals produced by the organizer that
Supplemental Information. Antagonistic Activities of Sox2. and Brachyury Control the Fate Choice. of Neuro-Mesodermal Progenitors
 Developmental Cell, Volume 42 Supplemental Information Antagonistic Activities of Sox2 and Brachyury Control the Fate Choice of Neuro-Mesodermal Progenitors Frederic Koch, Manuela Scholze, Lars Wittler,
Developmental Cell, Volume 42 Supplemental Information Antagonistic Activities of Sox2 and Brachyury Control the Fate Choice of Neuro-Mesodermal Progenitors Frederic Koch, Manuela Scholze, Lars Wittler,
The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm
 Development 122, 3055-3065 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV1088 3055 The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser
Development 122, 3055-3065 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV1088 3055 The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms
 Research article 383 Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms Jing Xie and Shannon Fisher* The McKusick-Nathans Institute of Genetic Medicine, The
Research article 383 Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms Jing Xie and Shannon Fisher* The McKusick-Nathans Institute of Genetic Medicine, The
Nature Immunology: doi: /ni.3694
 Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
efgf, Xcad3 and Hox genes form a molecular pathway that establishes the
 Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Development 122, 3881-3892 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3573 3881 efgf, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency.
 Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation
 801 Development 133, 801-811 doi:10.1242/dev.02250 Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation Fabian Rentzsch 1, *,, Jinli Zhang 2, *, Carina
801 Development 133, 801-811 doi:10.1242/dev.02250 Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation Fabian Rentzsch 1, *,, Jinli Zhang 2, *, Carina
Deciphering Complexity in Biology: Induction of Embryonic Cell Differentiation by Morphogen Gradients
 Complexity and Analogy in Science Pontifical Academy of Sciences, Acta 22, Vatican City 2014 www.pas.va/content/dam/accademia/pdf/acta22/acta22-derobertis.pdf Deciphering Complexity in Biology: Induction
Complexity and Analogy in Science Pontifical Academy of Sciences, Acta 22, Vatican City 2014 www.pas.va/content/dam/accademia/pdf/acta22/acta22-derobertis.pdf Deciphering Complexity in Biology: Induction
Hemidesmosome. Focal adhesion
 BIOLOGY 52 - - CELL AND DEVELOPMENTAL BIOLOGY - - FALL 2002 Third Examination - - November, 2002 -------------------------------------------------- Put your name at the top of each page. Please read each
BIOLOGY 52 - - CELL AND DEVELOPMENTAL BIOLOGY - - FALL 2002 Third Examination - - November, 2002 -------------------------------------------------- Put your name at the top of each page. Please read each
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15
 Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mot
 Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Essential role for Csk upstream of Fyn and Yes in zebrafish gastrulation
 Mechanisms of Development 124 (2007) 129 136 www.elsevier.com/locate/modo Essential role for Csk upstream of Fyn and Yes in zebrafish gastrulation Chris Jopling, Jeroen den Hertog * Hubrecht Laboratory,
Mechanisms of Development 124 (2007) 129 136 www.elsevier.com/locate/modo Essential role for Csk upstream of Fyn and Yes in zebrafish gastrulation Chris Jopling, Jeroen den Hertog * Hubrecht Laboratory,
HD1 - Introduction to Human Development
 Human Development Ed Laufer, PhD Course Director elaufer@columbia.edu Ping Feng Course Administrator pf2013@columbia.edu Michael Shen, PhD Patricia Ducy, PhD Michael Gershon, MD Cathy Mendelsohn, PhD Richard
Human Development Ed Laufer, PhD Course Director elaufer@columbia.edu Ping Feng Course Administrator pf2013@columbia.edu Michael Shen, PhD Patricia Ducy, PhD Michael Gershon, MD Cathy Mendelsohn, PhD Richard
Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm
 Development 129, 4335-4346 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV2859 4335 Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm Tetsuhiro
Development 129, 4335-4346 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV2859 4335 Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm Tetsuhiro
A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish
 Developmental Cell Supplemental Information A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish Julien Ablain, Ellen M. Durand, Song Yang, Yi Zhou, and Leonard I. Zon % larvae
Developmental Cell Supplemental Information A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish Julien Ablain, Ellen M. Durand, Song Yang, Yi Zhou, and Leonard I. Zon % larvae
BMP-2 and BMP-4 signalling in the developing spinal cord of human and rat embryos
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 74, No. 3, pp. 359 364 DOI: 10.5603/FM.2015.0054 Copyright 2015 Via Medica ISSN 0015 5659 www.fm.viamedica.pl BMP-2 and BMP-4 signalling in the developing
O R I G I N A L A R T I C L E Folia Morphol. Vol. 74, No. 3, pp. 359 364 DOI: 10.5603/FM.2015.0054 Copyright 2015 Via Medica ISSN 0015 5659 www.fm.viamedica.pl BMP-2 and BMP-4 signalling in the developing
Solution Key Problem Set
 Solution Key- 7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as
Solution Key- 7.013 Problem Set 5-2013 Question 1 During a summer hike you suddenly spot a huge grizzly bear. This emergency situation triggers a fight or flight response through a signaling pathway as
fga phenotypes were also analyzed in the tracheal system, the organ responsible for
 SUPPLEMENTARY TEXT Tracheal defects fga phenotypes were also analyzed in the tracheal system, the organ responsible for oxygen delivery in the fly. In fga homozygous mutant alleles, we observed defects
SUPPLEMENTARY TEXT Tracheal defects fga phenotypes were also analyzed in the tracheal system, the organ responsible for oxygen delivery in the fly. In fga homozygous mutant alleles, we observed defects
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
Blastula: An early staged embryo that is made up of sphere shaped cells that surround an inner fluid-filled cavity known as the blastocoel.
 1) Define the following terms: cleavage, blastomere, blastula, blastulation, microlecithal, mesolecithal, megalecithal, centrolecithal, isolecithal, telolecithal, holoblastic cleavage, meroblastic cleavage,
1) Define the following terms: cleavage, blastomere, blastula, blastulation, microlecithal, mesolecithal, megalecithal, centrolecithal, isolecithal, telolecithal, holoblastic cleavage, meroblastic cleavage,
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Muscle gene activation by induction and the nonrequirement for cell division
 /. Embryol. exp. Morph. 97 Supplement, 75-84 (1986) 75 Printed in Great Britain The Company of Biologists Limited 1986 Muscle gene activation by induction and the nonrequirement for cell division J. B.
/. Embryol. exp. Morph. 97 Supplement, 75-84 (1986) 75 Printed in Great Britain The Company of Biologists Limited 1986 Muscle gene activation by induction and the nonrequirement for cell division J. B.
Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity
 Development 127, 2143-2154 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV9708 2143 Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity Kweon Yu
Development 127, 2143-2154 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV9708 2143 Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity Kweon Yu
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and
 Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Aquatic Vertebrates Platform Services (See services description below, page 3)
 Aquatic Vertebrates Platform Services (See services description below, page 3) Services 1. Micro-injection of DNA, morpholino or mrna in zebrafish and medaka embryos. 2. Generation of stable zebrafish
Aquatic Vertebrates Platform Services (See services description below, page 3) Services 1. Micro-injection of DNA, morpholino or mrna in zebrafish and medaka embryos. 2. Generation of stable zebrafish
The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2
 Developmental Biology 248, 40 51 (2002) doi:10.1006/dbio.2002.0705 The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2 Vered Levy, Karen Marom, Sharon Zins, Natalia
Developmental Biology 248, 40 51 (2002) doi:10.1006/dbio.2002.0705 The Competence of Marginal Zone Cells to Become Spemann s Organizer Is Controlled by Xcad2 Vered Levy, Karen Marom, Sharon Zins, Natalia
and rag1 as Markers for the Development of the Zebrafish Immune System
 bug journal vol. 2, 1999 early development of the immune system. For these purposes, the lab previously cloned the recombination activating genes, rag1 and rag2 (Willett et al., 1997 a). These genes are
bug journal vol. 2, 1999 early development of the immune system. For these purposes, the lab previously cloned the recombination activating genes, rag1 and rag2 (Willett et al., 1997 a). These genes are
The Genetic Basis of Development
 Chapter 21 The Genetic Basis of Development Overview: From Single Cell to Multicellular Organism The application of genetic analysis and DNA technology Has revolutionized the study of development PowerPoint
Chapter 21 The Genetic Basis of Development Overview: From Single Cell to Multicellular Organism The application of genetic analysis and DNA technology Has revolutionized the study of development PowerPoint
7.013 Practice Quiz
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/318/5851/794/dc1 Supporting Online Material for A Gene Regulatory Network Subcircuit Drives a Dynamic Pattern of Gene Expression Joel Smith, Christina Theodoris, Eric
www.sciencemag.org/cgi/content/full/318/5851/794/dc1 Supporting Online Material for A Gene Regulatory Network Subcircuit Drives a Dynamic Pattern of Gene Expression Joel Smith, Christina Theodoris, Eric
Supplemental Information. Boundary Formation through a Direct. Threshold-Based Readout. of Mobile Small RNA Gradients
 Developmental Cell, Volume 43 Supplemental Information Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients Damianos S. Skopelitis, Anna H. Benkovics, Aman Y. Husbands,
Developmental Cell, Volume 43 Supplemental Information Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients Damianos S. Skopelitis, Anna H. Benkovics, Aman Y. Husbands,
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene. Andrew ElBardissi, The Pennsylvania State University
 Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Conservation and evolutionary divergence in the activity of receptor-regulated smads. Sorrentino et al.
 Conservation and evolutionary divergence in the activity of receptor-regulated smads Sorrentino et al. Sorrentino et al. EvoDevo 2012, 3:22 Sorrentino et al. EvoDevo 2012, 3:22 RESEARCH Open Access Conservation
Conservation and evolutionary divergence in the activity of receptor-regulated smads Sorrentino et al. Sorrentino et al. EvoDevo 2012, 3:22 Sorrentino et al. EvoDevo 2012, 3:22 RESEARCH Open Access Conservation
