Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary
|
|
|
- Blanche Foster
- 6 years ago
- Views:
Transcription
1 Research Article 3527 Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary Yulia A. Komarova 1,2, *, Ivan A. Vorobjev 2 and Gary G. Borisy 1 1 Department of Cell and Molecular Biology, Northwestern University Medical School, Chicago, IL 60611, USA 2 Laboratory of Cell Motility, A. N. Belozersky Institute, Moscow State University, Moscow, Russia *Author for correspondence ( y-komarova@northwestern.edu) Accepted 20 June 2002 Journal of Cell Science 115, The Company of Biologists Ltd Summary Microtubule dynamics were investigated in CHO and NRK cells by novel experimental approaches designed to evaluate the microtubule behavior in the cell interior. These approaches were: (1) laser photobleaching of a path through the centrosome; (2) direct observation of microtubules in centrosome-containing cytoplasts; (3) GFP-CLIP-170 expression as a marker for microtubule plus end growth; and (iv) sequential subtraction analysis. The combination of these approaches allowed us to obtain data where the density of microtubules had previously prevented conventional methods to be applicable. In the steady state, nascent microtubules grew persistently from the centrosome towards the cell margin. Frequently, they arrived at the cell margin without undergoing any transition to the shortening phase. In contrast to the growth of microtubules, shortening of the plus ends from the periphery was non-persistent; that is, rescue was frequent and the extent of shortening showed a distribution of lengths reflecting a stochastic process. The combination of persistent growth and a cell boundary led to a difference in apparent microtubule behavior in the cell interior compared with that near the cell margin. Whereas microtubules in the cell interior showed asymmetric transition frequencies, their behavior near the cell margin showed frequent fluctuations between phases of shortening and growth. Complete microtubule turnover was accomplished by the relatively rare episodes of shortening back to the centrosome. Release from the centrosome with subsequent minus end shortening also occurred but was a minor mechanism for microtubule turnover compared with the plus end pathway. We propose a life cycle for a microtubule which consists of rapid growth from the centrosome to the cell margin followed by an indefinite period of fluctuations of phases of shortening and growth. We suggest that persistent growth and asymmetric transition frequencies serve the biological function of providing a mechanism by which microtubules may rapidly accommodate to the changing shape and advancing edge of motile cells. Key words: Microtubules, Dynamics, Mammalian cell culture line Introduction Rapid remodeling of the microtubule (MT) array is important in large motile cells because such cells are constantly in the process of changing their shape. In fibroblast-like cells, MTs form a radial array emerging from the centrosome with minus ends tethered at the centrosome and plus ends extending towards the cell periphery (reviewed by Desai and Mitchison, 1997; Keating and Borisy, 1999). Although many studies have dealt with aspects of MT dynamics, a full understanding of the MT life cycle has yet to be achieved. In particular, the first phases, namely formation at the centrosome and growth in the cell interior have not been analyzed directly because of technical limitations imposed by the high density of MTs in the centrosomal region. In previous studies, the dynamics and turnover of MTs in cultured cell lines have been analyzed primarily using fluorescently labeled tubulin. The picture that has emerged from these studies is that MTs in vivo undergo frequent transitions between phases of growth and shortening (Sammak et al., 1987; Cassimeris et al., 1988; Shelden and Wadsworth, 1993; Waterman-Storer and Salmon, 1997; Yvon and Wadsworth, 1997). However, several considerations suggest that this picture is incomplete. One basic point is that almost all observations of individual MT dynamics in vivo have been made near the cell margin. This is because the cell is thinner and the MT density is lower near the edge of the cell, thus permitting better visualization of individual MTs. Consequently, MT dynamics in the cell interior have remained essentially unexplored and the formal possibility exists that they are different from that at the cell margin. A second point is that MT dynamics in vivo show complexity not seen in vitro. In vitro, MT dynamics are characterized by transitions between well defined phases of growth and shortening (Mitchison and Kirschner, 1984a; Mitchison and Kirschner, 1984b; Horio and Hotani, 1986; Walker et al., 1988). In contrast, MTs in many cell types do not show well defined phases. In addition, MTs in vivo frequently are quiescent, neither growing nor shortening, a behavior termed pause. Growth and shortening are highly variable in terms of velocity, duration and extent but, in general, tend to be brief. For example, the mean growth length
2 3528 Journal of Cell Science 115 (17) has been reported as 1.3 µm in PtK 1 cells and 3.2 µm in CHO cells (Shelden and Wadsworth, 1993). The difficulty in clearly defining phases of growth and shortening in vivo led us (Vorobjev et al., 1999; Vorobjev et al., 1997) to introduce an alternative description of MT dynamics. MT dynamics were considered as a 1-dimensional random walk of their plus ends along the cell radius and their overall properties were characterized by two parameters a diffusion coefficient and a drift coefficient. The diffusion coefficient is a measure of the amplitude (squared) of growth and shortening excursions per unit time, while the drift parameter represents the imbalance of growth and shortening excursions over time. This framework provided an analysis of dynamics not dependent upon detailed assumptions of growth or shortening behavior. The diffusion and drift parameters permitted prediction of the steady state length distribution of MTs and the time for turnover of the MT population. However, turnover times predicted from reported dynamic instability parameters were substantially greater than experimental determinations (Vorobjev et al., 1997). A similar conclusion was drawn from Monte Carlo analysis using the dynamic instability model (Gliksman et al., 1993). Proceeding from the assumption that MT plus ends undergo stochastic excursions such as the random walk observed in the lamellar region of PtK 1 cells (Vorobjev et al., 1997) or in fish melanophores (Vorobjev et al., 1999), the time for a nascent MT plus end starting from the centrosome to grow to the cell margin (or to shorten from the cell margin back to the centrosome) will be a few hours for a typical cultured cell of radius 25 µm. Such a long time seems incompatible with the requirements for rapid cytoskeletal remodeling in cell motility behavior. This disparity between theoretical and experimental analyses of MT turnover is a third point suggesting the incompleteness of our understanding of MT dynamics in vivo. Thus, we considered that the dynamics of MTs in the cell interior may somehow be different from that near the cell margin. Supporting this view, a few studies have reported the capacity of MTs in vivo to show behavior other than rapid fluctuations between shortening and growth. MTs have been observed to persistently grow into the lamellipodia of newt lung cells (Waterman-Storer and Salmon, 1997) and to continuously elongate into newly formed protrusions of HGF-stimulated PtK 1 cells (Wadsworth, 1999). These considerations taken together motivated us to reinvestigate the life cycle of MTs by procedures designed to evaluate their behavior in the cell interior and, in particular, in the vicinity of the centrosome. By employing a combination of novel approaches, we found that MT behavior in the cell interior indeed differed from that near the cell margin. Remarkably, nascent MTs freshly nucleated at the centrosome grew persistently until they approached the cell margin. Only then did they display the frequent fluctuations between growth and shortening that are considered to be the hallmark of dynamic instability. We suggest a revised view of MT dynamics in vivo in which the default condition of a nascent MT is persistent growth. In this view, dynamic instability at the cell margin results from the behavior of the MT system operating under the constraint of a boundary condition. Materials and Methods Cell culture and digital fluorescence imaging CHO-K1 and NRK cells were grown in F-10 medium supplemented with 10% fetal bovine serum and antibiotics on coverslips with photoetched locator grids (Bellco Glass, Vineland, NJ). For observation of MT dynamics in vivo, cells were cultured for 2 days after plating and were microinjected with Cy3-tagged tubulin at a needle concentration of 10 mg/ml. Cells were kept on the microscope stage at C during observation and temperature was measured before and after each experiment. Cells injected with Cy3-tubulin were treated with the oxygen-depleting preparation, Oxyrase (Oxyrase, Ashland, OH), to reduce photodamage and photobleaching (Mikhailov and Gundersen, 1995). Injected cells were observed on a Nikon Diaphot 300 inverted microscope equipped with a Plan 100, 1.25 NA objective using a Cy3 filter set for observations of Cy3-labeled MTs and a GFP filter set for observation of GFP-CLIP-170 in transiently transfected cells. Images of 16-bit depth were collected with a CH350 slow scan, cooled CCD camera (Photometrics, Tucson, AZ) driven by Metamorph imaging software (Universal Imaging, Westchester, PA). The image was projected onto the CCD chip at a magnification of 250, which corresponded to a resolution of 1 pixel=0.09 µm (11.1 pixels per µm). Time-lapse series of images were collected at 3-5 second intervals. 16-bit images were processed and rescaled with Metamorph software and 8-bit images were prepared for presentation with Adobe PhotoShop (Adobe Systems, Mountain View, CA). To highlight MTs of interest in some figures, color overlays were painted with opacity of 15% within the color mode layer in Adobe PhotoShop. Path photobleaching Photobleaching was performed on a Zeiss IM-35 inverted microscope using a 3 W argon ion laser as described elsewhere (Keating et al., 1997), except that the cells were incubated at 37 C. The laser beam was shaped into an approximately 20 3 µm bar using a cylindrical lens and a Neofluar 100, 1.3 NA objective. The zone was placed to photobleach a path across a cell with the centrosome at its center. Preparation of cytoplasts Cytoplasts were prepared by a modification of a described method (Karsenti et al., 1984). Briefly, 2 days after plating onto coverslips cells were treated with nocodazole (1 µg/ml) and cytochalasin D (1.5 µg/ml) for 90 minutes. Coverslips were then placed cells-down into centrifuge tubes containing culture medium with drugs and were centrifuged at 10,000 g for 25 minutes to enucleate the cells. Enucleation resulted in about equal numbers of cytoplasts containing or lacking the centrosome. Coverslips were washed with fresh medium to remove drugs and incubated for 2 hours for complete recovery of MTs in the cytoplasts. For observation of MTs, cells were microinjected with Cy3-tagged tubulin before enucleation. For simultaneous observation of MTs and CLIP-170 (see next section), cells were first transfected with and allowed to express GFP-CLIP-170, GFP-CLIP-170-positive cells were microinjected with Cy3-tubulin and then cytoplasts were prepared. Transfection of cells by microinjection Cells were transfected by glass capillary microinjection of DNA into the nuclei following a previously described procedure (Perez et al., 1999) with slight modification and were observed after hours. Briefly, pcb6-myc-gfp-clip-170 was used at a needle concentration of 20 µg/ml, which gave sufficiently low expression levels of recombinant protein without addition of cycloheximide. For visualization of the centrosome in some experiments, HsCen-2-GFP DNA (human centrin) was mixed with pcb6-myc-gfp-clip-170 DNA that had the same promoter. The final needle concentration of each vector was the same, 20 µg/ml. Subtraction analysis The position of active ends of MTs and the length of growth or
3 Life cycle of MTs 3529 shortening episodes were analyzed by subtraction of sequential images (I n I n+1) in time-lapse series as described previously (Vorobjev et al., 1999). The resultant difference images identified the extent of growth or shortening as black or white domains, respectively, at the ends of individual MTs. The threshold (minimal length) for determination of shortening or growth in subtraction analysis was set to 0.45 µm that is, 5 pixels in the digital image. Lateral displacements of MTs were distinguished from growth and shortening episodes because they gave a parallel arrangement of long black and white segments, whereas growth or shortening gave single short segments that were either black or white but not both. Analysis of microtubule dynamics The following parameters of MT dynamics were determined: instantaneous rates of growth and shortening; drift and diffusion coefficients; and mean velocity of growth. The lengths of individual MTs were measured from the centrosome (0,0 position of a centrosome) and life histories of MTs were plotted as length (µm) vs time (seconds). Instantaneous velocities were measured as displacement of the plus end divided by the time between successive images (3-5 seconds) in a time-lapse series. The minimum displacement that was measurable was two pixels in the digital image, corresponding to 0.18 µm in the cell. Histograms of instantaneous velocities were generated for the MT population and the mean values and s.d. were calculated. The histogram of MT growth rate included all displacements (growth episodes, rare pauses or shortening episodes) that occurred during MT growth from the centrosome to the cell margin, defined here as a zone 3 µm from the cell boundary. MT dynamics were treated as a 1D random walk characterized by diffusion and drift coefficients. Data handling was performed using SigmaPlot software (Jandel Scientific, San Rafael, CA) as described elsewhere (Vorobjev et al., 1999). The drift coefficient, v d, is a measure of the imbalance of growth and shortening, whereas the diffusion coefficient D is a measure of the absolute magnitude (squared) of the growth and shortening excursions at MT ends per unit time. For calculation of these coefficients for MT plus ends, we used direct imaging of MTs as well as subtraction analysis of those images. Originally, life histories of MTs over a second interval were used to construct plots of displacement and variance vs time (Vorobjev et al., 1999). Here we simplified the data collection and used instantaneous displacements to evaluate the coefficients. Positions of MT plus ends were determined in sequential images and a histogram of displacements was generated. The mean displacement calculated from this histogram represents the drift coefficient and the variance represents an estimate of the diffusion coefficient: v d =, Σ(s i) Σ(t i) where s i is displacement of a MT end between two sequential frames, and Σ(t i) is total time of observation of displacements included in the histogram, and Σ((s i s d) 2 ) D=, Σ(t i) where s i is displacement of a MT end between two sequential frames, s d is mean displacement, and Σ(t i) is total time of observation. Analysis of instantaneous displacement histograms is simple and gives an advantage that any MT whose plus end is visible in two consecutive frames is taken into account. Mean velocity of MT growth was determined using direct observation of Cy3-labeled MTs and the CLIP-170 approach. For the direct observation approach, MTs were monitored until they reached 90% of cell radius. The velocity of apparent CLIP-170 movement was determined by tracking the head of the individual CLIP-170-positive structure from the centrosome until it disappeared within the cytoplasm or near the plasma membrane. The extent of persistent growth was expressed both as an absolute distance and as a percentage of the local cell radius. For the latter calculation, the local cell radius was determined as the straight line distance from the centrosome to the cell margin in the direction of MT plus end or CLIP displacement. Results Direct analysis of MT dynamics in the cell interior Two problems have previously hindered the direct observation of MT nucleation at the centrosome. First, MT density at the centrosome is high, precluding visualization of individual MTs. Second, the cell is generally thick in the region of the centrosome, which results in substantial out-of-focus fluorescence that degrades imaging of individual MTs. To overcome these obstacles, we have employed two approaches. In the first approach, we photobleached a path across the centrosomal region to diminish the fluorescence density of preexisting MTs. This permitted observation of nascent MTs growing from the centrosome in the direction of the path. In the second approach, we generated cytoplasts that, because they lack the nucleus, are much thinner than whole cells, thus facilitating direct observation of individual MTs while still close to the centrosome. MT behavior analyzed after path photobleaching Cy3-tubulin was microinjected into cells and allowed to incorporate into MTs. The MTs in CHO cells are arranged in a predominantly radial pattern with the centrosome at its focus. An argon ion laser and an optical track with a cylindrical lens were used to photobleach a path across the cell with the centrosome in its center. MTs that were nucleated at the centrosome after the photobleaching and which grew in the direction of the path could then be visualized continuously beginning within seconds of their birth (Fig. 1A). The nascent MTs displayed remarkable behavior not previously reported in animal cells. They showed negligible, if any, dynamic instability. Typically, their leading (presumably plus) ends grew persistently until they were at the cell periphery (Fig. 1B). As an operational definition, we consider the cell periphery to be a zone of ~15% of the cell radius (3 µm). On average, 68% of the MTs reached the cell margin without transition to a shortening phase and continuous MT growth phases sometimes exceeded 20 µm. The growth rate derived from the frequency histogram of instantaneous rates (Fig. 1C) was 17.8±13.8 µm/minute (n=33 MTs; 10 cells; total time of observation=1228 seconds). The histogram confirmed that episodes of shortening (8.4%) or pause (0.6%) comprised a minor fraction of nascent MT behavior. At the same time that nascent MTs were growing persistently in the interior, life history plots of MTs at the periphery of the cell showed that they displayed characteristic dynamic instability, both before and after photobleaching (data not shown). Therefore, the experimental protocol did not detectably affect MT behavior. Thus, observations after path photobleaching suggest that nascent MTs in the cell interior have different behavior than those at the periphery. However, a limitation of this approach was that only those MTs that were oriented along the path
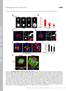
4 3530 Journal of Cell Science 115 (17) (usually 2-4 per cell) could be followed, limiting the size of the data set. To obtain a more comprehensive picture we examined cytoplasts where multiple MTs could be tracked starting close to the centrosome. MT behavior in centrosome-containing cytoplasts Enucleation of cells results in cytoplasts that are smaller and flatter than whole cells. The cytoplasts generally contained a reduced number of MTs (~50%) compared with those in intact cells, suggesting that some nucleating material was lost or damaged during enucleation. Nevertheless, centrosome-containing cytoplasts retained a strong radial array of MTs with the centrosome at its center. The combination of thinness and reduced number of MTs allowed the centrosome to be clearly visible and MTs growing from it to be readily traceable (Fig. 2A). Thus, it was possible to identify an individual MT shortly after it was nucleated and to monitor its elongation in cytoplasts without the need for path photobleaching. MTs grew with rare pauses or shortening episodes (Fig. 2B) at a rate of 15.8±5.9 µm/minute (50 MTs; 8 cytoplasts), which was similar to that in whole cells after path photobleaching. Thus, the property of persistent MT growth was retained after enucleation. As a measure of MT persistence, we estimated the frequency of transition from growth to shortening. Times were recorded for the growth of nascent MTs from the moment they could be detected near the centrosome until they had a catastrophe (shortening episode >0.5 µm) or arrived at the cell periphery, whichever came first. For MTs that arrived at the cell periphery without a detectable shortening episode, we logged their times but did not log a catastrophe. Since MTs in whole cells and centrosome-containing cytoplasts behaved similarly, we combined data from both protocols. For 52 MTs analyzed, only 12 transitions to shortening were logged in 2337 seconds of observation time for a catastrophe frequency of second 1. As MTs approached the cell margin, their behavior changed. Continued observation of MTs for at least 3 minutes, starting from the centrosome (Fig. 2B) or selecting them near the plasma membrane (Fig. 2C) showed that the majority of plus ends displayed typical dynamic instability near the cell margin. MT behavior within the 3 µm zone from the cell margin was characterized by an apparent catastrophe frequency of 0.08 second 1 (207 events for 2710 seconds, 61 MTs analyzed in 17 cells and cytoplasts), that is, 16-fold higher than that in the cell interior. Thus, as in the path photobleaching experiments, dynamic instability behavior was observed only at the cell periphery. Selective visualization of growing MT ends with CLIP-170 The apparent difference in MT behavior in the cell interior as opposed to the cell periphery represents a significant departure from our understanding of MT dynamics. Therefore, it seemed advisable to seek confirmation of these conclusions by an independent approach. Ideally, one would want to visualize MT growing ends without the background contributed by the rest of the MTs. Such selective visualization seemed possible through the use of CLIP-170 as a marker for MT elongation (Perez et al., 1999). Although Perez et al. showed that CLIP-170 colocalized with the ends of some growing MTs and was not targeted to stationary or shortening MTs, they were not able to determine whether CLIP-170 was targeted to all growing MTs or only to a certain subset. Thus, we first needed to evaluate Fig. 1. Path photobleaching reveals persistent MT growth in the interior of a CHO cell. (A) Upper panels: low magnification images of Cy3-labeled MTs before and after bleaching a zone approximately 20 3 µm passing through the centrosomal region. The path of reduced fluorescence allows visualization of nascent centrosomal MTs. Lower panels: three MTs oriented along the direction of the path (colored yellow, red and green) can be seen to elongate persistently. Numbers in top-left corners indicate time in seconds. Images were acquired every 4 seconds. Bar, 5 µm. (B) Life history plots (length vs time) of colored MTs. Zero length represents position of the centrosome; zero time is time of bleaching. MT plus ends arrived at the plasma membrane in ~60 seconds. (C) Instantaneous velocities of nascent MTs were measured as the displacement of MT ends between successive frames. The histogram shows that episodes of shortening and pause were infrequent.
5 Life cycle of MTs 3531 Fig. 2. Life cycle of MTs visualized in centrosomecontaining CHO cytoplasts. (A) Time-lapse sequence shows selected panels from life histories of four MTs. (B) Two MTs (colored blue and red) were nucleated at the centrosome (at 3 seconds and 39 seconds, respectively) and persistently grew towards the cell margin. These MTs underwent transition to shortening only after they arrived at the cell margin (arrowheads: red MT, at 102 seconds; blue MT at 120 seconds). The length of the blue MT is greater than the red MT because it grew in a curved path and along the cell margin before beginning to shorten. (C) Two MTs (colored yellow and green) were initially tracked while near the cell margin. These MTs showed repeated episodes of growth and shortening near the membrane and the green MT eventually underwent a shortening excursion back to the centrosome. Numbers in the top-left corners indicate time in seconds. Bar, 5 µm. Life history plots constructed as indicated in Fig. 1 legend. this point. To do so, we compared MT growth with GFP-CLIP- 170 behavior using a double labeling protocol for cytoplasts. Intact cells were transfected by microinjection of GFP-tagged CLIP-170 DNA into the nuclei. To avoid potential problems with overexpression, we selected cells at 10 hours after transfection expressing low levels of fluorescent GFP-CLIP These GFP-positive cells were then injected with Cy3- labeled tubulin and incubated for minutes prior to cytoplast preparation. Colocalization of GFP-CLIP-170 (red) and ends of MTs (green) in a centrosome-containing cytoplast is shown in Fig. 3A. Displacement of the CLIP-170 label in successive frames was identical to the growth of MT plus ends (Fig. 3B). CLIP-170 labeling disappeared within 5 seconds (time resolution of our double channel time-lapse series, 2.5 seconds per channel) when a MT paused or underwent a transition from growth to shortening (Fig. 3C). Several typical CLIP-170 and MT histories are plotted in Fig. 3D. Invariably (n>100), CLIP-170 tracks were coincident with MT tip displacement, thus indicating that CLIP-170 was targeted to all growing ends and validating their use as a tool for selectively visualizing MT growth. Persistent MT growth confirmed by long CLIP-170 tracks A prediction of persistent growth of MTs starting from the centrosome is that CLIP-170 will be associated with a MT tip almost continuously until the MT plus end approaches the cell margin. Therefore, we used the movement of CLIP-170 labeled zones as a tool to evaluate MT growth in the cell interior. For visualization of the centrosome in some experiments, GFP-Hscen2 DNA (human centrin expressing construct) was mixed with GFP-CLIP-170 DNA and they were used together for transfection. We evaluated MT growth both in intact cells and in cytoplasts containing a centrosome. As predicted, GFP-CLIP- 170-labeled zones displayed long excursions over time (continuous duration frequently >1 minute) through the cell interior (Fig. 4A). Track diagrams were built from successive positions of CLIP-170-labeled zones on individual MTs. The tracks showed that CLIP-170 zones moved radially outward from the centrosome. The mean unbroken length of GFP- CLIP-170 tracks was 17.3±4.8 µm (n=78) in whole cells (Fig. 4B) and 15.8±5.8 µm/minute (n=40) in cytoplasts containing a centrosome. The mean velocity of CLIP-170 zones was 16.5±6.0 µm/minute (n=110) for whole cells and 18.3±4.8 µm/minute (n=39) for the cytoplasts. The velocity of CLIP-170 movement was similar to that determined for MT growth by direct observation, both in whole cells and cytoplasts. We may conclude from these measurements both that CLIP-170 movement is a good proxy for MT growth and that cytoplasts are a good proxy for intact cells. Normalizing the length distribution of CLIP tracks against the distance between the centrosome and the cell margin (Fig. 4C) shows that the majority of MTs nucleated at the centrosome reached ~85%
6 3532 Journal of Cell Science 115 (17) Fig. 3. Selective visualization of growing MT ends with CLIP CHO cells were transfected by nuclear microinjection of GFP-CLIP- 170 DNA, allowed to express GFP-CLIP protein, then microinjected with Cy3-tubulin and, finally, enucleated to generate cytoplasts. Time-lapse sequences were obtained to show dynamics of MTs and CLIP-170. (A) Low magnification of MTs, CLIP- 170 and merged image (green, Cy3-MTs; red, CLIP-170). (B) Time-lapse sequence of region boxed in panel A. Two dynamic MTs are indicated by arrowheads. CLIP-170 is present at their plus ends during growth phases but disappears within 5 seconds after transition from growth to pause or shortening phase. Numbers in top-left corners indicate time in seconds. Interval between acquisitions of images in alternating channels was 2.5 seconds, which gave an interval between successive images in either the GFP or Cy3 channel of 5 seconds. Scale bar, 5 µm. (C) Life history plots of MTs (green) and CLIP-170 tracks (red) indicated by arrowheads in panel B. CLIP-170 data points were time-shifted by 2.5 seconds to compensate for the delay between acquisition of CLIP and MT images. Absence of red data points from segments of the plots indicates when CLIP-170 disappeared from the MT end. (D) Example plots of nascent MTs (green) growing off the centrosome and corresponding CLIP-170 tracks (red) after time-shifting of 2.5 s. Plots were arbitrarily staggered along the time axis for clarity. Scales of axes indicated in upper right corner of graph. For some MTs either near the centrosome or in areas of high MT density, the MT end could not be clearly visualized. Nevertheless, CLIP-170 movement could be seen. This is represented in the plots as red points without corresponding green ones. In all cases, clear growing MTs had CLIP-170 at their ends. (84±16%) of the cell radius without catastrophes or long pauses. This result signifies that most MTs persistently grow from their time of birth at the centrosome until their plus end nears the cell margin. Because new CLIP-170 labeled zones continuously appeared near the centrosome, it was possible to use their appearance to estimate the frequency of MT formation at the centrosome, defined operationally as a circle of 2 µm radius drawn about the focus of the MT array. With this criterion, we estimated the rate of nucleation of MTs by the centrosome in CHO cells to be 5.6±2.3 per minute (178 CLIP-170 zones; 7 cells; 1997 seconds). Comparison of persistent growth and treadmilling The only previously reported instance of persistent plus end growth in mammalian cells is the MT treadmilling observed in vivo in the absence of a centrosome (Rodionov and Borisy, 1997; Rodionov et al., 1999). In our previous study (Rodionov et al., 1999) we suggested that treadmilling in cytoplasts lacking a centrosome resulted from exposed MT minus ends whose depolymerization caused an elevated tubulin pool that suppressed transitions from growth to shortening phase at the MT plus end. Contrary to that suggestion, this study has uncovered persistent growth of MT plus ends in the absence of substantial numbers of free minus ends. Therefore, we reevaluated the question of whether the presence of the centrosome had any significant effect on the rate of plus end growth. With our improved procedures, we compared cytoplasts containing and lacking the centrosome. In centrosome-free cytoplasts, MTs spontaneously appeared in the cytoplasm, then translocated by means of treadmilling and eventually arrived at a cell margin (Fig. 5A). After reaching the cell margin, plus ends often became paused and MTs disassembled because of minus end depolymerization. The rate of growth of the plus end during treadmilling was measured in the same way as for MTs growing from the centrosome in intact cells or centrosome-containing cytoplasts. From time-lapse observations of Cy3-labeled MTs, the rate of plus end growth during treadmilling was 20.3±8.3 µm/minute (n=66; 11 cytoplasts). In steady-state treadmilling, growth of the plus ends must be balanced by minus end shortening. Consistent with this expectation, the rate of minus end shortening in the cytoplasts lacking a centrosome was 18.2±5.8 µm/minute (n=66; 11 cytoplasts). Our measurement by direct observation of mean plus end
7 Life cycle of MTs 3533 Fig. 4. Persistent MT growth confirmed by long CLIP-170 tracks. GFP-CLIP-170 movement was analyzed by timelapse microscopy. Images were acquired every 3 seconds. (A) Three next-nearest frames are shown, displaying radial organization and movement of CLIP-170 patches. Tips of four CLIP-170 patches are indicated over time by arrowheads. Numbers in the upper left corner indicate time in seconds; bar, 5 µm. Diagram shows 25 CLIP-170 tracks over a 90 second period of which four tracks (1-4) correspond to CLIP patches indicated by arrowheads on the images. Many tracks are long indicating continuous MT growth; radial pattern of tracks permits identification of the centrosome region (dashed circle) as the point from which the tracks originate. The centrosome is indicated as two dots in the diagram. (B) Histogram of the length distribution of CLIP-170 tracks. Mean length, 17.3±4.8 µm (n=78). (C) Histogram of length distribution of CLIP tracks normalized against the distance between the centrosome and plasma membrane. The histogram shows that the majority of MTs born at the centrosome grow persistently to ~85% of the cell radius. growth rate includes pauses and shortening episodes. To compare the real growth potential of the plus ends these episodes have to be excluded. The CLIP-170 approach provides a natural realization of this algorithm since CLIP disappears from the plus ends when they stop. The tracks of CLIP-170 in cytoplasts lacking a centrosome (Fig. 5B) gave the MT plus end growth rate as 22.0±10.1 µm/minute (n=75; 7 cytoplasts), while in centrosome-containing cytoplasts, the measured rate was 18.3±4.8 µm/minute (n=39; 5 cytoplasts), a difference of 20%. Thus, by both measures, MT growth was slightly faster in cytoplasts lacking a centrosome, consistent with an elevated tubulin pool. However, since persistent growth is a regular feature of MTs in intact cells and centrosomecontaining cytoplasts, the creation of free minus ends is clearly not a prerequisite for this phenomenon. Treadmilling MTs permitted an additional test of the localization of CLIP-170 exclusively at growing ends. Since one end of a treadmilling MT is shortening while the other is growing, CLIP-170 is expected to be present at the leading (plus) end but absent from the trailing (minus) end. This prediction was confirmed by direct dual label observation (Fig. 5C). Thus, CLIP-170 can be used as a polarity marker for the growing MT plus end. Shortening of MTs Polymer balance of treadmilling MTs comes at the level of individual MTs with shortening from the minus end being equal, on average, to growth at the plus end. However, in intact cells or centrosome-containing cytoplasts, minus ends are tethered at the centrosome. Since release from the centrosome and minus end shortening is infrequent (Keating and Borisy, 1999; Waterman-Storer and Salmon, 1997; Vorobjev et al., 1999), most of the balance must come from the dynamics of the plus end. Therefore, the persistent growth of MT plus ends in the cell interior must be balanced primarily by shortening of other MT plus ends. The steady state requirement of balance of growth and shortening holds true for every position in the cell, including the centrosome. Since nascent MTs are born at the centrosome at the rate of 5.6±2.3 per minute, we predicted that an equivalent rate of MT shortening back to the centrosome should be observed. To evaluate this issue, we randomly selected MTs having their plus ends close to the margin and monitored their fate (Fig. 6A). In contrast to growth, shortening was not persistent. Shortening occurred at a velocity of 28.8±14.1 µm/minute (n=474; 10 cells) (Fig. 6B,C). Frequency histograms of the distances shortened showed an approximately exponential decay (Fig. 6D) with the mean distance being 2.9±3.4 µm. These properties are consistent with a first-order process of transition back to the growing state (rescue). The rescue frequency was determined to be 0.12 second 1 (91 transitions, 100 MTs, 735 seconds of observation) by tracking MTs shortening back from the margin until they began to grow. Thus, rescue as opposed to catastrophe was frequent leading to small shortening excursions being common and long ones rare. Nevertheless, shortening back to the centrosome could be directly observed, albeit at low frequency. Since MTs that shortened long distances were generally lost as their plus ends neared the congested area around the centrosome, we scored, as a more reliable estimate, the number of MTs that shortened by two-thirds of the cell radius. Out of all episodes of shortening, we obtained 6.4±2.7 per minute that shortened more than two-thirds of the distance to the centrosome. Besides shortening from the plus end, infrequent releases of MTs from the centrosome also occurred. Released MTs had a short lifespan and rapidly depolymerized from the minus end
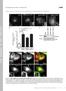
8 3534 Journal of Cell Science 115 (17) Fig. 5. MT treadmilling and GFP-CLIP-170. MT dynamics and GFP-CLIP-170 movement were analysed in cytoplasts lacking the centrosome. (A) MTs and CLIP-170 images (two left pictures) and time lapse sequence of merged images with GFP- CLIP-170 set to the red channel and MTs to the green channel, respectively. Plus and minus ends of treadmilling MTs are indicated by arrowheads. CLIP-170 labels the plus ends of treadmilling MTs while minus ends are always CLIP-170- negative. Numbers in the top-left corner indicate time in seconds; bar, 5 µm. (B) Example histories of CLIP-170 tracks in cytoplasts lacking a centrosome. Individual plots are arbitrarily staggered along the time axis for clarity. Scale of axes is indicated in the top-right corner of the graph. (C) Life history plot of a treadmilling MT (plus and minus ends are green) and CLIP-170 (red). Time shift between plus end history and CLIP- 170 history is 2.5 seconds. CLIP-170 persists at the growing plus end of the treadmilling MT. (1.0/minute) was approximately equal to plus end birth (5.6 MTs/minute). This rough equality confirms that the MT system in CHO cells is indeed in steady state and it indicates that balance at the centrosome in CHO cells comes primarily (~85%) from the plus end pathway with the minus end pathway making a minor (~15%) but significant contribution. (Fig. 6E). In cytoplasts, the frequency of releases was 1.0±0.5 MT per centrosome per minute (58 releases, 11 cytoplasts). Thus, plus end death (6.4/minute) + minus end release Length distribution of MTs The persistent growth of nascent MTs is an apparently paradoxical result because it is not clear how this behavior can be reconciled with the dynamic instability behavior observed near the cell margin. To address this question, we employed the conceptual framework of diffusion plus drift and the procedure of sequential subtraction analysis to obtain the necessary data. Sequential subtraction images (Fig. 7A) permit identification of growing and shortening MT ends as black or white line segments, respectively, even in regions of high MT Fig. 6. Analysis of MT shortening. (A) Time-lapse sequence shows a rare event of plus end shortening (arrowheads) from the plasma membrane back to the centrosome within 12 seconds. (B) Histogram of instantaneous rates of MT shortening from the plasma membrane. (C) Length distribution of MT shortening episodes. MT ends adjacent to the cell margin were followed and the length of a continuous shortening episode was determined. The histogram shows small shortening episodes were frequent and long ones rare (n=319). (D) Time-lapse sequence shows release of a MT from the centrosome followed by shortening from the minus end (arrowheads). Time in seconds; bars, 5 µm.
9 Life cycle of MTs 3535 Fig. 7. Population analysis of MTs. (A) Sequential subtraction analysis for identification of growth and shortening. Image of Cy3-labeled MTs from a time-lapse series and corresponding differential image (I n I n+1) obtained by subtracting from an image (I n) the next image (I n+1) in the series. Black and white segments represent MT growth and shortening, respectively, during the time interval. Five trapezoidal zones, each onefifth of the cell radius, indicate the regions where growth and shortening events were scored. In the two right-hand panels, the fifth trapezoid is enlarged and growth and shortening excursions are shown in colors, green and red, respectively. Parallel black and white segments arise from lateral shifts of MTs and were not scored. (B) Frequency distribution of growth and shortening velocities in region of of cell radius fraction obtained by subtraction analysis. (C) Drift coefficient as calculated from histogram of growth and shortening velocities. Drift is positive and fairly uniform within the cell interior, dropping to negative values only near the cell boundary. (D) Distribution of MT ends. The position of active MT ends (growing or shortening) was scored by subtraction analysis and assigned to one of five trapezoidal zones shown in panel A (n=7421 episodes; 4244 growing; 3177 shortening; 4 cells). Data were fit to a single exponential function. The exponential functions taken separately for growth or shortening episodes were essentially identical. density, which otherwise preclude direct observation of individual MTs. MTs that do not undergo a displacement (i.e. are stable or paused) between successive frames become canceled out and do not appear in the subtraction image. The velocities of growing and shortening were determined from the length of the line segments in the differential images divided by the time between acquisitions of the images. Frequency histograms of the velocities were bimodal with growth events slower than shortening but more frequent. An example histogram is shown in Fig. 7B for the region between of the cell radius. For the same region, the fraction of growing MTs, f g, was 0.70 and their velocity, v g, was 17.0±5.9 µm/minute, n=372, while the fraction of shortening MTs, f s, was 0.30 and their velocity, v s, was 29.7±13.4 µm/minute, n=156. The drift coefficient, v d, reflects the imbalance of growth over shortening and is calculated either as v d =v g f g v s f s or as the mean of the velocity histogram. Based on this histogram analysis, calculated values of the drift coefficient were ~5 µm/minute in the cell interior, declining somewhat towards the cell margin (Fig. 7C), indicating that throughout the cell interior, growth was strongly favored over shortening. Close to the cell margin (<3 µm) the calculated drift coefficient was negative ( 2.7±0.4 µm/minute; n=235 growth events; 197 shortening events), reflecting the increase in proportion of shortening events because of the influence of the cell boundary. It should be noted that since paused MTs do not appear in the subtraction images, they do not contribute to the calculation of drift. As a check on the result, we also evaluated displacement histograms obtained from direct fluorescence imaging in the cell interior. Such histograms were also bimodal although now a population of paused MTs was detected. In the cell interior, paused MTs were infrequent (15%) while near the cell margin they were abundant (70%). In the cell interior, velocities and drift coefficients (data not shown) were similar to those obtained from subtraction analysis. However, near the cell margin, the larger fraction of pauses resulted in a proportionate decrease in the absolute value of the drift coefficient. The diffusion coefficient represents fluctuations around the behavior predicted from drift and is calculated as the variance of the velocity frequency histogram. Calculation gave the apparent diffusion coefficient as 13.1±1.3 µm 2 /minute for the inner, 15.1±1.1 µm 2 /minute for the middle and 13.1±1.3 µm 2 /minute for the outer thirds of the cell, respectively. The ratio of coefficients, diffusion/drift was ~3 µm and did not
10 3536 Journal of Cell Science 115 (17) change significantly along the cell radius. As shown previously (Vorobjev et al., 1999), positive drift predicts a non-random distribution of MT ends. To determine the distribution of MT ends along the cell radius, the whole cell was divided into five radial zones. Counts of black or white line segments showed that MT ends, both growing and shortening, were rare near the centrosome and increased exponentially towards the cell margin (Fig. 7D). Thus, in CHO cells, the length distribution of dynamic MT ends is an ascending one and most MTs are long. From an exponential curve fit to the data in Fig. 7D, the ratio of the coefficients was independently estimated as 5.8 µm, which is within a factor of 2 of the measured value. Thus, the diffusion plus drift framework is sufficient to account for the observed distribution of MT ends. Persistent growth is a characteristic feature of cultured cells Since persistent growth in CHO cells seemed a remarkable property, it was important to test its generality. Consequently, we examined the MT life cycle in NRK cells and cytoplasts obtained from them. For these cells, we observed persistent growth of MTs towards the plasma membrane at a velocity of 17.4±5.7 µm/minute (data not shown). Sequential subtraction analysis of NRK cytoplasts gave a bimodal distribution similar to that for CHO cells (data not shown) with a drift coefficient in the cell interior of 4.0±1.1 µm/minute (n=488, 5 cytoplasts). Additional support for this conclusion was obtained by calculation of the drift coefficient from data available in the literature. Using the expression presented earlier, v d =v g f g v s f s, we calculate drift coefficients of 4.4 µm/minute for the lamellae of NRK fibroblasts (Mikhailov and Gundersen, 1998), 3.4 µm/minute for the periphery of CHO cells (Shelden and Wadsworth, 1993), and 3.8 µm/minute for MTs growing parallel to the cell edge in newt lung cells (Waterman-Storer and Salmon, 1997). Thus, an apparent imbalance of growth over shortening is a characteristic feature of many cultured cells. We conclude that persistent growth and positive drift are widespread features of MT dynamics. Discussion In this study, we employed a combination of novel approaches to examine MT behavior in the cell interior. The results provide an updated picture of the MT life cycle in cells with a radial array originating from the centrosome (Fig. 8A). A MT is born by nucleation on a centrosomal template, it grows through persistent elongation by addition of subunits at the plus end; in the vicinity of the cell margin, the MT undergoes fluctuations in length through episodes of plus end shortening and growing; and, finally, it dies when one of the stochastic episodes of shortening is of sufficient length to depolymerize it all the way back to the centrosome. An alternative path of death is release from the centrosome followed by persistent shortening from the minus end. In CHO and other fibroblasts, these properties combine to generate an apparent imbalance of growth over shortening (drift) for individual MTs in the cell interior that leads in the steady-state to a distribution of MT plus ends that steeply climbs as a function of position along the cell radius. This view of the MT life cycle and steady state differs from Fig. 8. MT life cycle and steady state length distribution (A). Life history of a typical MT reconstructed from data from two individual MTs. The first part (up to time axis break) traces persistent growth towards the cell boundary followed by catastrophes induced by the boundary that are rescued at high frequency. The second part (after time axis break) shows the rare event of shortening back to the centrosome. (B) Diagram of radial array of MTs showing distribution of lengths in accordance with exponential function fitted to data from subtraction analysis. Long MTs dominate over short ones. previous work in three ways: (1) that the default behavior of MTs in the cell interior is to grow persistently; (2) that MTs at the cell periphery show dynamic instability type behavior because of a boundary effect; and (3) that positive drift generates an ascending distribution of MT lengths. Persistent growth of nascent microtubules The frequency of transition from growth to shortening of a MT in the cell interior was determined to be very low, second 1, which equates to a half-time for the growing phase of 140 seconds. Assuming a radius of 20 µm for a typical CHO cell and a growth velocity for the MT plus end of 17 µm/minute, a MT would require only ~70 seconds to grow the distance of the cell radius, which means that a nascent MT typically arrives at the cell margin before it has had a chance to shorten. Assuming a first-order process of transition to shortening, we calculate that an average of 72% of nascent MTs starting at the centrosome would still be in the growth phase by the time they reached the cell margin. This result is essentially the same as the experimentally observed value of 68% for cells. Cytoplasts are smaller than cells and therefore they have a higher percentage of MTs that arrive at the margin without shortening. Thus, persistent growth of a nascent MT can be understood as resulting from a combination of high elongation velocity and low catastrophe frequency. This remarkable feature of the MT life cycle was not previously recognized. The major explanation for this oversight is most likely that observations of MT dynamics in vivo have generally been made where it is technically easier to obtain the data namely, at the cell periphery, where the cytoplasm is thin and MT density is relatively low, thus facilitating visualization of individual MTs. An implicit and untested assumption has been that MT dynamics parameters evaluated at the cell periphery are valid throughout the cell. Our results demonstrate that, at least in CHO and NRK cells, this is not the case.
Contribution of plus and minus end pathways to microtubule turnover
 Journal of Cell Science 112, 2277-2289 (1999) Printed in Great Britain The Company of Biologists Limited 1999 JCS0417 2277 Contribution of plus and minus end pathways to microtubule turnover I. A. Vorobjev
Journal of Cell Science 112, 2277-2289 (1999) Printed in Great Britain The Company of Biologists Limited 1999 JCS0417 2277 Contribution of plus and minus end pathways to microtubule turnover I. A. Vorobjev
SUPPLEMENTARY INFORMATION
 DOI: 1.18/ncb46 a Damage Fracture s s 7 5 16 1 9 26 5 µm 5 µm b Position of photo-damage site in cells 15 Counts 1 5-14 -12-1 -8-6 -4-2 Distance from damage site to tip (µm) Supplementary Figure 1 Effect
DOI: 1.18/ncb46 a Damage Fracture s s 7 5 16 1 9 26 5 µm 5 µm b Position of photo-damage site in cells 15 Counts 1 5-14 -12-1 -8-6 -4-2 Distance from damage site to tip (µm) Supplementary Figure 1 Effect
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
Cytoplasmic assembly of microtubules in cultured cells
 Journal of Cell Science 110, 2635-2645 (1997) Printed in Great Britain The Company of Biologists Limited 1997 JCS4467 2635 Cytoplasmic assembly of microtubules in cultured cells Ivan A. Vorobjev 1, Tatyana
Journal of Cell Science 110, 2635-2645 (1997) Printed in Great Britain The Company of Biologists Limited 1997 JCS4467 2635 Cytoplasmic assembly of microtubules in cultured cells Ivan A. Vorobjev 1, Tatyana
Long-term dynamics of CA1 hippocampal place codes
 Long-term dynamics of CA1 hippocampal place codes Yaniv Ziv, Laurie D. Burns, Eric D. Cocker, Elizabeth O. Hamel, Kunal K. Ghosh, Lacey J. Kitch, Abbas El Gamal, and Mark J. Schnitzer Supplementary Fig.
Long-term dynamics of CA1 hippocampal place codes Yaniv Ziv, Laurie D. Burns, Eric D. Cocker, Elizabeth O. Hamel, Kunal K. Ghosh, Lacey J. Kitch, Abbas El Gamal, and Mark J. Schnitzer Supplementary Fig.
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supplementary Figures 1-6
 1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Nature Methods: doi: /nmeth Supplementary Figure 1. Real-time deformability cytometry: contour detection and theoretical modeling.
 Supplementary Figure 1 Real-time deformability cytometry: contour detection and theoretical modeling. (a) Image of cell deformed in constriction; contour (red) according to image analysis algorithm. Scale
Supplementary Figure 1 Real-time deformability cytometry: contour detection and theoretical modeling. (a) Image of cell deformed in constriction; contour (red) according to image analysis algorithm. Scale
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Aoki et al.,
 JCB: SUPPLEMENTAL MATERIAL Aoki et al., http://www.jcb.org/cgi/content/full/jcb.200609017/dc1 Supplemental materials and methods Calculation of the raw number of translocated proteins First, the average
JCB: SUPPLEMENTAL MATERIAL Aoki et al., http://www.jcb.org/cgi/content/full/jcb.200609017/dc1 Supplemental materials and methods Calculation of the raw number of translocated proteins First, the average
In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating. melanoma cells
 In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells Yun He 1,, Lidai Wang 1,,, Junhui Shi 1,, Junjie Yao 1, Lei Li 1, Ruiying Zhang 1, Chih-Hsien
In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells Yun He 1,, Lidai Wang 1,,, Junhui Shi 1,, Junjie Yao 1, Lei Li 1, Ruiying Zhang 1, Chih-Hsien
Figures and figure supplements
 RESEARCH ARTICLE Figures and figure supplements Dendritic mitochondria reach stable positions during circuit development Michelle C Faits et al Faits et al. elife 2015;5:e11583. DOI: 10.7554/eLife.11583
RESEARCH ARTICLE Figures and figure supplements Dendritic mitochondria reach stable positions during circuit development Michelle C Faits et al Faits et al. elife 2015;5:e11583. DOI: 10.7554/eLife.11583
Supporting Online Material
 Supporting Online Material Two Distinct Actin Networks Drive the Protrusion of Migrating Cells A. Ponti, M. Machacek, S. L. Gupton, C. M. Waterman-Storer, G. Danuser This document contains: Experimental
Supporting Online Material Two Distinct Actin Networks Drive the Protrusion of Migrating Cells A. Ponti, M. Machacek, S. L. Gupton, C. M. Waterman-Storer, G. Danuser This document contains: Experimental
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Die casting Design Simulation
 Die casting Design Simulation Introduction In the process of zinc alloy die casting, feeding the die is of crucial importance both for the quality, the strength and the finish of the product and for the
Die casting Design Simulation Introduction In the process of zinc alloy die casting, feeding the die is of crucial importance both for the quality, the strength and the finish of the product and for the
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
MT minus end catastrophe
 DOI: 1.138/ncb3241 MT minus end catastrophe frequency [s -1 ].2.15.1.5 control mgfp- TPX2 * * mgfp- TPX2 mini Supplementary Figure 1. TPX2 reduces catastrophes at the microtubule minus ends. Modified box-and-whiskers
DOI: 1.138/ncb3241 MT minus end catastrophe frequency [s -1 ].2.15.1.5 control mgfp- TPX2 * * mgfp- TPX2 mini Supplementary Figure 1. TPX2 reduces catastrophes at the microtubule minus ends. Modified box-and-whiskers
Section 10. Junaid Malek, M.D.
 Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
ATTACHMENT 12: CDISCO Description and Sensitivity to Input Parameters
 ATTACHMENT 12: CDISCO Description and Sensitivity to Input Parameters INTRODUCTION The A11. ISCO Spreadsheet Design Tool (a.k.a. Conceptual Design for ISCO or CDISCO) was developed with support from ESTCP
ATTACHMENT 12: CDISCO Description and Sensitivity to Input Parameters INTRODUCTION The A11. ISCO Spreadsheet Design Tool (a.k.a. Conceptual Design for ISCO or CDISCO) was developed with support from ESTCP
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Comparative Genomic Hybridization
 Comparative Genomic Hybridization Srikesh G. Arunajadai Division of Biostatistics University of California Berkeley PH 296 Presentation Fall 2002 December 9 th 2002 OUTLINE CGH Introduction Methodology,
Comparative Genomic Hybridization Srikesh G. Arunajadai Division of Biostatistics University of California Berkeley PH 296 Presentation Fall 2002 December 9 th 2002 OUTLINE CGH Introduction Methodology,
U 'NDERSTANDING the behavior of kinetochore microtubules. Kinetochore Microtubule Dynamics and the Metaphase-Anaphase Transition
 Kinetochore Microtubule Dynamics and the Metaphase-Anaphase Transition Ye Zhai, Paul J. Kronebusch, and Gary G. Borisy Laboratory of Molecular Biology, University of Wisconsin-Madison, Madison, Wisconsin
Kinetochore Microtubule Dynamics and the Metaphase-Anaphase Transition Ye Zhai, Paul J. Kronebusch, and Gary G. Borisy Laboratory of Molecular Biology, University of Wisconsin-Madison, Madison, Wisconsin
Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules
 Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
CMTC depend on the distance from the nucleation site. (Sammak et al., 1987) but are independent of distance
 Molecular Biology of the ell Vol. 4, 135-15, October 1993 How the Transition Frequencies of Microtubule Dynamic Instability (Nucleation, atastrophe, and Rescue) Regulate Microtubule Dynamics in Interphase
Molecular Biology of the ell Vol. 4, 135-15, October 1993 How the Transition Frequencies of Microtubule Dynamic Instability (Nucleation, atastrophe, and Rescue) Regulate Microtubule Dynamics in Interphase
Supplementary Figure 1. FRET probe labeling locations in the Cas9-RNA-DNA complex.
 Supplementary Figure 1. FRET probe labeling locations in the Cas9-RNA-DNA complex. (a) Cy3 and Cy5 labeling locations shown in the crystal structure of Cas9-RNA bound to a cognate DNA target (PDB ID: 4UN3)
Supplementary Figure 1. FRET probe labeling locations in the Cas9-RNA-DNA complex. (a) Cy3 and Cy5 labeling locations shown in the crystal structure of Cas9-RNA bound to a cognate DNA target (PDB ID: 4UN3)
Supplementary Figure 1. Diagram showing software and hardware used for head fixation and water delivery, as well as picam-based imaging.
 Supplementary Figure 1. Diagram showing software and hardware used for head fixation and water delivery, as well as picam-based imaging. 1 Supplementary Figure 2. Layout of training cage for self-head-fixation.
Supplementary Figure 1. Diagram showing software and hardware used for head fixation and water delivery, as well as picam-based imaging. 1 Supplementary Figure 2. Layout of training cage for self-head-fixation.
Supplementary Figures
 Supplementary Figures Supplementary Figure S1. Inhibition kinetics of p53/hdm2 binding induced by Nutlin 3 on live cells. Reproducibility of the p53/hdm2 binding disruption kinetics induced by Nutlin 3
Supplementary Figures Supplementary Figure S1. Inhibition kinetics of p53/hdm2 binding induced by Nutlin 3 on live cells. Reproducibility of the p53/hdm2 binding disruption kinetics induced by Nutlin 3
(B) Comparable expression of major integrin subunits and glycoproteins on the surface of resting WT and Lnk -/- platelets.
 Supplemental Figure S1. Characteristics of Lnk -/- platelets. (A) Electron micrographs of resting platelets showing the normal intracellular structure of Lnk -/ platelets. Samples were fixed with 4% PFA
Supplemental Figure S1. Characteristics of Lnk -/- platelets. (A) Electron micrographs of resting platelets showing the normal intracellular structure of Lnk -/ platelets. Samples were fixed with 4% PFA
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
Supplementary Information
 Supplementary Information Supplementary Figures S1-S9 Supplementary Tables S1 - S2 Supplementary Methods Supplementary References 1-8 1 Supplementary Figure 1. GFP-TipAct domain architecture and localisation
Supplementary Information Supplementary Figures S1-S9 Supplementary Tables S1 - S2 Supplementary Methods Supplementary References 1-8 1 Supplementary Figure 1. GFP-TipAct domain architecture and localisation
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Supplemental Information. Dynamic Organization of Chromatin Domains. Revealed by Super-Resolution Live-Cell Imaging
 Molecular Cell, Volume 67 Supplemental Information Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging Tadasu Nozaki, Ryosuke Imai, Mai Tanbo, Ryosuke Nagashima, Sachiko
Molecular Cell, Volume 67 Supplemental Information Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging Tadasu Nozaki, Ryosuke Imai, Mai Tanbo, Ryosuke Nagashima, Sachiko
Amnis ImageStream : Technical Reports & Applications
 Amnis ImageStream : Technical Reports & Applications ImageStream : Flow Cytometry and Microscopy in a Single Platform The ImageStream achieves true multispectral Imaging in Flow by combining microscopy
Amnis ImageStream : Technical Reports & Applications ImageStream : Flow Cytometry and Microscopy in a Single Platform The ImageStream achieves true multispectral Imaging in Flow by combining microscopy
Imaging of BacMam Transfected U-2 OS Cells
 A p p l i c a t i o n N o t e Imaging of BacMam Transfected U-2 OS Cells Optimization of Transfection Conditions Using the Cytation 3 Multi- Mode Reader and Gen5 Data Analysis Software Paul Held Ph. D.
A p p l i c a t i o n N o t e Imaging of BacMam Transfected U-2 OS Cells Optimization of Transfection Conditions Using the Cytation 3 Multi- Mode Reader and Gen5 Data Analysis Software Paul Held Ph. D.
Fibrin Clots Are Equilibrium Polymers That Can Be Remodeled Without Proteolytic Digestion
 Supplementary Information Fibrin Clots Are Equilibrium Polymers That Can Be Remodeled Without Proteolytic Digestion Irina N. Chernysh 1, Chandrasekaran Nagaswami 1, Prashant K. Purohit 2 and John W. Weisel
Supplementary Information Fibrin Clots Are Equilibrium Polymers That Can Be Remodeled Without Proteolytic Digestion Irina N. Chernysh 1, Chandrasekaran Nagaswami 1, Prashant K. Purohit 2 and John W. Weisel
Transient and Succession-of-Steady-States Pipeline Flow Models
 Transient and Succession-of-Steady-States Pipeline Flow Models Jerry L. Modisette, PhD, Consultant Jason P. Modisette, PhD, Energy Solutions International This paper is copyrighted to the Pipeline Simulation
Transient and Succession-of-Steady-States Pipeline Flow Models Jerry L. Modisette, PhD, Consultant Jason P. Modisette, PhD, Energy Solutions International This paper is copyrighted to the Pipeline Simulation
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Mathematical Modeling of Dynamic Instability in Microtubules
 Mathematical Modeling of Dynamic Instability in Microtubules Christine Lucille Kuryla 2013 NSF/REU Program Department of Physics, University of Notre Dame Advisors: Mark Alber, Dept. of Applied and Computational
Mathematical Modeling of Dynamic Instability in Microtubules Christine Lucille Kuryla 2013 NSF/REU Program Department of Physics, University of Notre Dame Advisors: Mark Alber, Dept. of Applied and Computational
Add Live, Dead and Total Dyes. Overnight Variable 30 min. 10 min 5 min. 30 min
 Cell Viability Analysis using Calcein AM, Propidium Iodide and Hoechst Application Description Celigo Application Plate Type Major Steps Cell viability analysis using Calcein AM, Propidium Iodide and Hoechst
Cell Viability Analysis using Calcein AM, Propidium Iodide and Hoechst Application Description Celigo Application Plate Type Major Steps Cell viability analysis using Calcein AM, Propidium Iodide and Hoechst
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
Supplementary Information
 Supplementary Information Trapping and Detection of Nanoparticles and Cells Using a Parallel Photonic Nanojet Array Yuchao Li, Hongbao Xin, Xiaoshuai Liu, Yao Zhang, Hongxiang Lei*, and Baojun Li* State
Supplementary Information Trapping and Detection of Nanoparticles and Cells Using a Parallel Photonic Nanojet Array Yuchao Li, Hongbao Xin, Xiaoshuai Liu, Yao Zhang, Hongxiang Lei*, and Baojun Li* State
4 Image Analysis of plastic deformation in the fracture of paper
 4 Image Analysis of plastic deformation in the fracture of paper 4.1 Introduction As detailed in Chapter 2, one of the fundamental problems that arises in the estimation of the fracture toughness of an
4 Image Analysis of plastic deformation in the fracture of paper 4.1 Introduction As detailed in Chapter 2, one of the fundamental problems that arises in the estimation of the fracture toughness of an
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
Chapter 15. Cytoskeletal Systems. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
Supplementary material to Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease
 Supplementary material to Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease Gyula Batta, Lilla Soltész, Tamás Kovács, Tamás Bozó, Zoltán
Supplementary material to Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease Gyula Batta, Lilla Soltész, Tamás Kovács, Tamás Bozó, Zoltán
Interphase microtubule dynamics are cell typespecific
 Iowa State University From the SelectedWorks of Maura McGrail 1990 Interphase microtubule dynamics are cell typespecific P. Wadsworth, University of Massachusetts - Amherst M. McGrail, University of Massachusetts
Iowa State University From the SelectedWorks of Maura McGrail 1990 Interphase microtubule dynamics are cell typespecific P. Wadsworth, University of Massachusetts - Amherst M. McGrail, University of Massachusetts
Effect of Cytochalasin on Average Pseudopodia Length in Amoeba Proteus
 Effect of Cytochalasin on Average Pseudopodia Length in Amoeba Proteus Alex Waciega Independent Research Project Report Cell Biology 219 December 3, 2008 Introduction In our experiment we studied the effects
Effect of Cytochalasin on Average Pseudopodia Length in Amoeba Proteus Alex Waciega Independent Research Project Report Cell Biology 219 December 3, 2008 Introduction In our experiment we studied the effects
Brightfield and Fluorescence Imaging using 3D PrimeSurface Ultra-Low Attachment Microplates
 A p p l i c a t i o n N o t e Brightfield and Fluorescence Imaging using 3D PrimeSurface Ultra-Low Attachment Microplates Brad Larson, BioTek Instruments, Inc., Winooski, VT USA Anju Dang, S-BIO, Hudson,
A p p l i c a t i o n N o t e Brightfield and Fluorescence Imaging using 3D PrimeSurface Ultra-Low Attachment Microplates Brad Larson, BioTek Instruments, Inc., Winooski, VT USA Anju Dang, S-BIO, Hudson,
Quantifying biological forces involved in the process of cell-mediated cytolysis
 Quantifying biological forces involved in the process of cell-mediated cytolysis Experimentation based on responses of living cells (Cell-based assays) represents an important stratum in biological and
Quantifying biological forces involved in the process of cell-mediated cytolysis Experimentation based on responses of living cells (Cell-based assays) represents an important stratum in biological and
DEPArray Technology. Sorting and Recovery of Rare Cells
 DEPArray Technology Sorting and Recovery of Rare Cells Delivering pure, single, viable cells The DEPArray system from Silicon Biosystems is the only automated instrument that can identify, quantify, and
DEPArray Technology Sorting and Recovery of Rare Cells Delivering pure, single, viable cells The DEPArray system from Silicon Biosystems is the only automated instrument that can identify, quantify, and
An Advanced Reliability Improvement and Failure Analysis Approach to Thermal Stress Issues in IC Packages
 An Advanced Reliability Improvement and Failure Analysis Approach to Thermal Stress Issues in IC Packages Michael Hertl 1, Diane Weidmann 1, and Alex Ngai 2 1 Insidix, 24 rue du Drac, F-38180 Grenoble/Seyssins,
An Advanced Reliability Improvement and Failure Analysis Approach to Thermal Stress Issues in IC Packages Michael Hertl 1, Diane Weidmann 1, and Alex Ngai 2 1 Insidix, 24 rue du Drac, F-38180 Grenoble/Seyssins,
Three major types of cytoskeleton
 The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
SI Appendix. Supporting Materials (references cited here can be found in the reference list in the main text):
 SI Appendix Supporting Materials (references cited here can be found in the reference list in the main text): SupplementaryText Figs. S1 to S13 Table S1 Captions for Supporting Movies S1 to S5 Other Supporting
SI Appendix Supporting Materials (references cited here can be found in the reference list in the main text): SupplementaryText Figs. S1 to S13 Table S1 Captions for Supporting Movies S1 to S5 Other Supporting
Fluorescence Imaging with One Nanometer Accuracy Lab
 I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
Supporting Information for. Electrical control of Förster energy transfer.
 1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
7/14/2015. Single-cell-level measurements of transcription heterogeneity of highly mobile identical genes. Enrico Gratton and Paolo Annibale
 7//5 Single-cell-level measurements of transcription heterogeneity of highly mobile identical genes Enrico Gratton and Paolo Annibale Laboratory for Florescence Dynamics University of California, Irvine
7//5 Single-cell-level measurements of transcription heterogeneity of highly mobile identical genes Enrico Gratton and Paolo Annibale Laboratory for Florescence Dynamics University of California, Irvine
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Craft et al., http://www.jcb.org/cgi/content/full/jcb.201409036/dc1 Figure S1. GFP -tubulin interacts with endogenous -tubulin. Ciliary
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Craft et al., http://www.jcb.org/cgi/content/full/jcb.201409036/dc1 Figure S1. GFP -tubulin interacts with endogenous -tubulin. Ciliary
Proceedings 1 st International Conference on New Forming Technology, Sept , Harbin, China, pp
 Proceedings 1 st International Conference on New Forming Technology, Sept -9, Harbin, China, pp. 5-9 Water jet forming of steel beverage cans Dr. Wilko C. Emmens Corus Research, Development & Technology,
Proceedings 1 st International Conference on New Forming Technology, Sept -9, Harbin, China, pp. 5-9 Water jet forming of steel beverage cans Dr. Wilko C. Emmens Corus Research, Development & Technology,
Microtubule Assembly Dynamics at the Nanoscale
 Microtubule Assembly Dynamics at the Nanoscale Melissa K. Gardner, Henry T. Schek III*, Alan J. Hunt, and David J. Odde Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455
Microtubule Assembly Dynamics at the Nanoscale Melissa K. Gardner, Henry T. Schek III*, Alan J. Hunt, and David J. Odde Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN 55455
Membrane Potential Assays Using the ValiScreen Human Kv1.3 Voltage-Gated K + Channel Cell Line on the EnVision Multilabel Plate Reader
 TECHNICAL BRIEF ValiScreen Stable Recombinant Ion Channel Cell Line and the EnVision Multilabel Plate Reader Membrane Potential Assays Using the ValiScreen Human Kv1.3 Voltage-Gated K + Channel Cell Line
TECHNICAL BRIEF ValiScreen Stable Recombinant Ion Channel Cell Line and the EnVision Multilabel Plate Reader Membrane Potential Assays Using the ValiScreen Human Kv1.3 Voltage-Gated K + Channel Cell Line
NovoCyte Flow Cytometer
 NovoCyte Flow Cytometer The Flow Cytometer for Everyone 2 Experience the NovoCyte Advantage Focus on advancing your research. Let the flow cytometer do the rest. NovoCyte Flow Cytometer High Performance
NovoCyte Flow Cytometer The Flow Cytometer for Everyone 2 Experience the NovoCyte Advantage Focus on advancing your research. Let the flow cytometer do the rest. NovoCyte Flow Cytometer High Performance
3D Transfection System
 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
PROTOCOL. Live Cell Imaging of 3D Cultures Grown on Alvetex Scaffold Using Confocal Microscopy. Introduction
 Page 1 Introduction Live cell imaging allows real time monitoring of cell shape and form during cell differentiation, proliferation and migration. Alvetex Scaffold offers a unique ability to study cell
Page 1 Introduction Live cell imaging allows real time monitoring of cell shape and form during cell differentiation, proliferation and migration. Alvetex Scaffold offers a unique ability to study cell
Supplementary Figure 1: Two modes of low concentration of BsSMC on a DNA (a) Protein staining (left) and fluorescent imaging of Cy3 (right) confirm
 Supplementary Figure 1: Two modes of low concentration of BsSMC on a DNA (a) Protein staining (left) and fluorescent imaging of Cy3 (right) confirm that BsSMC was labeled with Cy3 NHS-Ester. In each panel,
Supplementary Figure 1: Two modes of low concentration of BsSMC on a DNA (a) Protein staining (left) and fluorescent imaging of Cy3 (right) confirm that BsSMC was labeled with Cy3 NHS-Ester. In each panel,
Supplementary Figure S1 ǀ Power-time-effect for the recrystallization of melt-quenched amorphous bits (erase process with substrate at room
 Supplementary Figure S1 ǀ Power-time-effect for the recrystallization of melt-quenched amorphous bits (erase process with substrate at room temperature). Three different zones can be distinguished. In
Supplementary Figure S1 ǀ Power-time-effect for the recrystallization of melt-quenched amorphous bits (erase process with substrate at room temperature). Three different zones can be distinguished. In
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12116 Supplementary Figure 1 Theoretical prediction of temperature profiles. a, sketch of sample, immersed into X-ray beam, its environment (agarose and buffer solution are not distinguished),
doi:10.1038/nature12116 Supplementary Figure 1 Theoretical prediction of temperature profiles. a, sketch of sample, immersed into X-ray beam, its environment (agarose and buffer solution are not distinguished),
United States Environmental Protection Agency Research and Development Project Summary. K.L. Revzan, W.J. Fisk, and R.G. Sextro
 6EPA United States Environmental Protection Agency Research and Development Project Summary Air and Energy Engineering Research Laboratory Research Triangle Park, NC 27711 EPA/600/SR-92/119 December 1992
6EPA United States Environmental Protection Agency Research and Development Project Summary Air and Energy Engineering Research Laboratory Research Triangle Park, NC 27711 EPA/600/SR-92/119 December 1992
Division of Chemistry and Chemical Engineering, California Institute of Technology 2
 Supporting Information Real-time, Digital LAMP with Commercial Microfluidic Chips Reveals the Interplay of Efficiency, Speed, and Background Amplification as a Function of Reaction Temperature and Time
Supporting Information Real-time, Digital LAMP with Commercial Microfluidic Chips Reveals the Interplay of Efficiency, Speed, and Background Amplification as a Function of Reaction Temperature and Time
Design Principles of Length Control of Cytoskeletal Structures
 Design Principles of Length Control of Cytoskeletal Structures Lishibanya Mohapatra 1, Bruce L. Goode 2, Predrag Jelenkovic 3, Rob Phillips 4, Jane Kondev 5,* 1 Department of Physics, Brandeis University,
Design Principles of Length Control of Cytoskeletal Structures Lishibanya Mohapatra 1, Bruce L. Goode 2, Predrag Jelenkovic 3, Rob Phillips 4, Jane Kondev 5,* 1 Department of Physics, Brandeis University,
Transmission Electron Microscopic Study of Antibiotic Action on Klebsiella pneumoniae Biofilm
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2002, p. 2679 2683 Vol. 46, No. 8 0066-4804/02/$04.00 0 DOI: 10.1128/AAC.46.8.2679 2683.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2002, p. 2679 2683 Vol. 46, No. 8 0066-4804/02/$04.00 0 DOI: 10.1128/AAC.46.8.2679 2683.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
CHO herg-duo Cell Line
 B SYS GmbH CHO herg-duo Cell Line Specification Sheet B SYS GmbH B SYS GmbH CHO herg DUO Cells Page 2 TABLE OF CONTENTS 1. BACKGROUND...3 1.1. DRUG-INDUCED QT PROLONGATION...3 1.2. REGULATORY ISSUES...3
B SYS GmbH CHO herg-duo Cell Line Specification Sheet B SYS GmbH B SYS GmbH CHO herg DUO Cells Page 2 TABLE OF CONTENTS 1. BACKGROUND...3 1.1. DRUG-INDUCED QT PROLONGATION...3 1.2. REGULATORY ISSUES...3
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures. Xin Zhang Associate Professor.
 Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Intercalation-based single-molecule fluorescence assay to study DNA supercoil
 Intercalation-based single-molecule fluorescence assay to study DNA supercoil dynamics Mahipal Ganji, Sung Hyun Kim, Jaco van der Torre, Elio Abbondanzieri*, Cees Dekker* Supplementary information Supplementary
Intercalation-based single-molecule fluorescence assay to study DNA supercoil dynamics Mahipal Ganji, Sung Hyun Kim, Jaco van der Torre, Elio Abbondanzieri*, Cees Dekker* Supplementary information Supplementary
Loose, M., Fischer-Friedrich, E., Ries, J., Kruse, K., Schwille, P. Science, Vol. 320,
 Spatial Regulators for Bacterial Cell Division Self-Organize into Surface Waves in Vitro Loose, M., Fischer-Friedrich, E., Ries, J., Kruse, K., Schwille, P. Science, Vol. 320, 9.5.2008 Maximilian Hörner
Spatial Regulators for Bacterial Cell Division Self-Organize into Surface Waves in Vitro Loose, M., Fischer-Friedrich, E., Ries, J., Kruse, K., Schwille, P. Science, Vol. 320, 9.5.2008 Maximilian Hörner
Super Resolution Imaging Solution Provider. Imaging Future
 Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
REGULATION REQUIREMENTS FOR WIND GENERATION FACILITIES
 REGULATION REQUIREMENTS FOR WIND GENERATION FACILITIES Randy Hudson and Brendan Kirby Oak Ridge National Laboratory Oak Ridge, Tennessee Yih-Huei Wan National Renewable Energy Laboratory Golden, Colorado
REGULATION REQUIREMENTS FOR WIND GENERATION FACILITIES Randy Hudson and Brendan Kirby Oak Ridge National Laboratory Oak Ridge, Tennessee Yih-Huei Wan National Renewable Energy Laboratory Golden, Colorado
A Probabilistic Approach to Defining Freeway Capacity and Breakdown
 A Probabilistic Approach to Defining Freeway Capacity and Breakdown MATT LORENZ LILY ELEFTERIADOU The Pennsylvania Transportation Institute The Pennsylvania State University 201 Transportation Research
A Probabilistic Approach to Defining Freeway Capacity and Breakdown MATT LORENZ LILY ELEFTERIADOU The Pennsylvania Transportation Institute The Pennsylvania State University 201 Transportation Research
Cell Cycle-Dependent Changes in Microtubule Dynamics in Living Cells Expressing Green Fluorescent Protein- Tubulin V
 Molecular Biology of the Cell Vol. 12, 971 980, April 2001 Cell Cycle-Dependent Changes in Microtubule Dynamics in Living Cells Expressing Green Fluorescent Protein- Tubulin V Nasser M. Rusan, Carey J.
Molecular Biology of the Cell Vol. 12, 971 980, April 2001 Cell Cycle-Dependent Changes in Microtubule Dynamics in Living Cells Expressing Green Fluorescent Protein- Tubulin V Nasser M. Rusan, Carey J.
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Dynamics of DCL cell division and its relation to cell dispersion during epiboly of A. nigripinnis. (a) Temporal changes in the cumulative number of DCL cell
1 Supplementary Figures Supplementary Figure 1 Dynamics of DCL cell division and its relation to cell dispersion during epiboly of A. nigripinnis. (a) Temporal changes in the cumulative number of DCL cell
Stable chromosomal units determine the spatial and temporal organization of DNA replication
 Research Article 5353 Stable chromosomal units determine the spatial and temporal organization of DNA replication Nicolas Sadoni 1, M. Cristina Cardoso 2, Ernst H. K. Stelzer 3, Heinrich Leonhardt 1,2
Research Article 5353 Stable chromosomal units determine the spatial and temporal organization of DNA replication Nicolas Sadoni 1, M. Cristina Cardoso 2, Ernst H. K. Stelzer 3, Heinrich Leonhardt 1,2
Kirti Kanaujiya, Yugesh Mani Tiwari
 International Journal of Scientific & Engineering Research, Volume 6, Issue 9, September-2015 1336 Experimental Investigation on Solidification Rate and Grain Size for Centrifugal Casting Kirti Kanaujiya,
International Journal of Scientific & Engineering Research, Volume 6, Issue 9, September-2015 1336 Experimental Investigation on Solidification Rate and Grain Size for Centrifugal Casting Kirti Kanaujiya,
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Localization Microscopy
 Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
