Copyright. Yun-Sheng Chen
|
|
|
- Thomas Tate
- 6 years ago
- Views:
Transcription
1 Copyright by Yun-Sheng Chen 2012
2 The Dissertation Committee for Yun-Sheng Chen Certifies that this is the approved version of the following dissertation: Contrast and Sensitivity Enhanced Molecular Imaging Using Photoacoustic Nanoamplifiers Committee: Stanislav Emelianov, Supervisor Ahmed Tewfik Sanjay Banerjee Andrew Dunn Konstantin Sokolov
3 Contrast and Sensitivity Enhanced Molecular Imaging Using Photoacoustic Nanoamplifiers by Yun-Sheng Chen, B.S.; M.S.; M.S. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin August 2012
4 Dedication To my family.
5 Acknowledgements I sincerely thank my advisor, Prof. Stanislav Emelianov, for his guidance and great support during the course of my Ph.D. study. I feel very lucky that I came to meet Stas when I was looking for supervisors four years ago. And I am extremely grateful that he provided me with such a great opportunity to work on science that is not only fun, deep, but also can be useful and beneficial to human health. I have also learned from Stas these years beyond science that how a great leader should be. Everything I learned here in this group is invaluable and will benefit me for the many years to come. Thank you Stas! I am also grateful to Dr. Wolfgang Frey, I am one of the luckiest who has two mentors to guide me in my study. Dr. Frey, you are my mentor, and those tremendous discussions with you have brought me with enormous brilliant ideas and have stimulated me for my future research. I also own thanks to my colleagues, Dr. Andrei Karpiouk, Dr. Salavat Aglyamov, Soon-Joon Yoon, Jason Cook, Doug Yeager, Geoff Luke, Dr. Kimberly Homan, Seung- Yun Nam, Seung-Soo Kim, Pratixa Joshi; and special thanks to Justina Tam from Dr. Sokolov's lab for her help and useful discussions. At last, I would like to thank my family. I want to thank my parents for their never-ending love and support during these years and their great help when our baby daughter was born. To my wife, without your help and love, I would never come this far. To my sweet baby, your come is my happiest moment and your happiness means everything to me. v
6 Contrast and Sensitivity Enhanced Molecular Imaging Using Photoacoustic Nanoamplifiers Yun-Sheng Chen, Ph.D. The University of Texas at Austin, 2012 Supervisor: Stanislav Emelianov Molecular imaging is an emerging imaging principle which can visually represent the biological processes both spatially and temporally down to the sub-cellular level in vivo. The outcome of this research is expected to have a profound impact on facilitating the early diagnosis of diseases, accelerating the development of new drugs, and improving the efficacy of therapy. In general, molecular imaging highly relies on probes to sense the occurrence of molecular biological events, and to generate signals which could be picked up by diagnostic imaging modalities. The advances in the design of molecular probes not only have equipped traditional anatomical medical imaging with new capabilities but also, in some cases, stimulated developments of new imaging modalities and renaissance of existing medical imaging modalities. One of these is photoacoustic imaging, which as an emerging medical imaging modality, unites the merits from both optical imaging and ultrasound imaging. It shares with optical imaging, that it uses non-ionizing radiation and provides higher contrast and higher sensitivity than ultrasound imaging. Unlike optical imaging, which requires ballistic photons for imaging, photoacoustic imaging requires only diffusive photons to excite the ultrasound signal from the imaging target; therefore, it is capable of imaging much deeper into the tissue. In combination with molecular probes, photoacoustic molecular imaging has been vi
7 demonstrated by several research groups using various photoacoustic molecular probes. However, the molecular probes used for most of these studies were contrast agents simply adopted from other optical imaging modalities. Our research on photoacoustic contrast agents indicated that the mechanism of photoacoustic signal generation from nanometer-sized contrast agents is distinct from that of optically homogeneous materials, such as tissue. We have discovered that, the amplitude of the photoacoustic signal generated from nano-contrast agents depends not only on the optical absorption of the particles, but more importantly, on the dynamic process of the heat conduction from the nanoparticles to the ambient, and the thermal properties of the surrounding materials. Based on our finding, we explored and further improved the photoacoustic response of the nanoparticles by exploiting the heat conduction process between the nanoparticle and its surrounding materials and by manipulating the excitations. This research allows to create optimized molecular specific contrast enhanced photothermal stable probes which can aid photoacoustic imaging and image guided photothermal cancer therapy. vii
8 Table of Contents List of Tables... xi List of Figures... xii Chapter 1 Introduction The Demand of Molecular Imaging The Challenge of Molecular Imaging Photoacoustic Imaging PA Contrast Agents Overall Research Goals References...14 Chapter 2 Design, Synthesis, and Characterization of Gold Nanorods Optical Properties of Gold Nanorods Surface Plasmons and Localized Surface Plasmon Resonance Mie Theory Rayleigh-Gans Calculation Numerical Calculation on Gold Nanorods...25 Discrete Dipole Approximation...27 Spectral Calculation Using Finite Difference Time Domain Method Optical Properties Inside Human Body Optical Properties of Tissue Optical Properties of Blood Synthesis of Gold Nanorods Characterization of Gold Nanorods Results and Discussions Referencess...39 Chapter 3 Design and Preparation of Photostable Photoacoustic Nanocontrast Agents Synthesis of Silica Coated Gold Nanorods...44 viii
9 3.2 Characterization of Silica Coated Gold Nanorods Results and Discussions References...62 Chapter 4 Characterization of Photoacoustic Nanoamplifier Preparation and Characterization of Nanoparticles Ultrasound and Photoacoustic Imaging of Phantoms Photoacoustic Signal Measurement of Gold Nanoparticles Results and Discussions References...91 Chapter 5 The Mechanism of Photoacoustic Generation in Nanoparticle Solutions Synthsis of Hydrophobic Silica Coated Gold Nanorods Photoacoustic Measurements and Analysis Estimation of the Photoacoustic Signal for a Nanosphere in a Fluid Sphere with no Shell Sphere with a thin shell Characterization of Gold Nanorods Results and Discussions References Chapter 6 Development of Dye Doped Molecular Specific Photoacoustic Nanoamplifier Molecular Probes Specific Targeting of Nanoparticle Active Targeting Using Antibody as Biomarker Directional Conjugation of Antibody to Silica Surface Multi-Imaging Molecular Probe Based on PA Nanoamplifier Methods Preparation of Dye Doped Silica Coated Gold Nanorods Fluorescent Labeling of Antibody Determing Degree of Labeling Surface Functionalization of Silica Surface Maleimide-PEG 5K Functionalized Nanoagents ix
10 Thiol Functionalized Nanoagents Epoxide Functionalized Nanoagents Conjugation of Antibodies on Silica Surface Directional Conjugation Random Directional Conjugation Cytotoxicity Test Optical Microscopy Imaging Results And Discussions Preparation of Dye Doped Silica Coated Gold Nanorods Antibody Conjugation of Silica Coated Gold Nanorods Cytotoxicity Test Of Antibody Conjugation Of Silica Coated Gold Nanorods References Chapter 7 Design of Multipulse Imaging System The Design Principles of Multipulse System for Dynamic Photoacoustic Imaging Optimization of the Excitation Source to Enhance the Contrast of Photoacoustic Imaging Multiple Beam Interferometer Phantom Imaging Results and Discussions References Chapter 8 Future Directions Future Direction for Photoacoustic Molecular Imaging References Bibliography x
11 List of Tables Table 6.1: Monoclonal antibodies approved for human therapy by FDA and/or EMEA xi
12 List of Figures Figure 1.1: Cartoon illustration on the general idea of molecular imaging and its role in cancer therapy. (a) molecular probe specifically targets to cancer cell; (b) pulsed laser induces photoacoustic signal to generate photoacoustic images to image cancer at the cell level; (c) when cw laser irradiation is on, the nanocontrast agents are heated up and locally annihilate cancer cells without damaging the surrounding tissue....8 Figure 2.1: (a) The absorption coefficient of in-vitro measured biological tissue between spectral range of 400 nm to 2000 nm; (b) The reduced scattering coefficients of the averaged experimental data Figure 2.2: The optical spectra of oxygenated and deoxygenated blood...33 Figure 2.3: (a) Longitudinal plasmon spectra with different aspect ratios of the coreshell structured nanorods; (b) Linear relationship of the aspect ratio of the rod versus the peak wavelength Figure 2.4: Slow growth seed mediated method for nanorod synthesis. The TEM images show the nanorod dimension has been successfully reduced from an average length of 125 nm to 65 nm Figure 3.1: Measured UV-Vis extinction spectra of (a) gold nanorods before 300 laser pulse irradiation, and (b) same gold nanorods after 300 laser pulse irradiation. The insets display corresponding TEM images Figure 3.2: Experimental set up for examining of the thermal stability of uncoated and coated gold nanorods. (a) The 96 well set up; (b) photoacoustic signal calibration set up xii
13 Figure 3.3: (a) Normalized extinction efficiency spectra reveal the change of resonance wavelength with respect to a change in the thickness of the silica shell. (b) The shift of peaks as a function of silica thickness show increasing wavelength with increasing thickness and saturation of the trend when the thickness is around 3 times of the effective radius of the gold nanorod core Figure 3.4: (a) The comparison of simulation and experimental results of longitudinal surface plasmon spectra of PEG-coated gold nanorod and silica-coated gold nanorod, the experimental data (in solid black and red curves) well matches the experimental data (in red and black dots) for both kinds of nanorods. The nanorod has an aspect ratio of 3.127, with thickness of the PEG layer around 8nm, and silica coating around 24nm. The effective radius of the gold nanorod in the core is 8.145nm. (b) Shows left to right: SEM image of bare gold nanorod with around 40nm length and 10 nanometer diameter end-caps; SEM image of the silica coated gold nanorod which gives spectra in red curve of Figure 3 (a); Element mapping of a silica coated gold nanorod showing different composite of the core-shell nanorod (Hitachi S-5500 Scanning Electron Microscope/Scanning Transmission Electron Microscopy) xiii
14 Figure 3.5: (a) Experimental and simulated extinction spectra of the PEG-coated gold nanorods, and silica-coated gold nanorods, where solid blue ( ) and solid red ( ) curves correspond to experimental data of PEGcoated and silica-coated gold nanorods. The dotted blue (----), dotted red (----), and dotted green (----) curves correspond to FDTD calculated data of PEG-coated, porous silica-coated, and fused silica-coated gold nanorods, respectively. (b) TEM image illustrates one example of the silica-coated gold nanorods produced Figure 3.6: Measured UV-Vis extinction spectra of (a) CTAB-coated gold nanorods, (b) PEGylated (PEG-coated) gold nanorods, (c) 6 nm silica-coated gold nanorods, and (d) 20 nm silica-coated gold nanorods before and after laser irradiation with various fluences Figure 3.7: TEM images show the morphology evolutions of various gold nanorods before and after 300 pulses of 20mJ/cm 2 laser irradiation. (a) and (b): PEG coated gold nanorods; (c) and (d): 6 nm silica-coated gold nanorods; (e) and (f): 20 nm silica-coated gold nanorods Figure 3.8: FDTD simulated absorption cross section spectra for unpolarized light for various gold nanoparticles. Ellipsoids have the same volume as the original nanorod and are labeled as the fraction of the long axis relative to the original nanorod length. Shortened silica-coated nanorods are labeled for their reduction at each end xiv
15 Figure 3.9: (a) The TEM image illustrates the 6 nm silica-coated gold nanorods after irradiated by 300 pulses of 20 mj/cm 2 laser light; the yellow circles indicate the nanorods which have hollow gaps between gold and silica. (b) The zoom-in view of the hollow gaps of a silica-coated gold nanorod. The image shows the gaps were formed in the tips and about 2 nm Figure 3.10: Photoacoustic signal intensity of PEG-coated gold nanorods (red symbols) and silica-coated gold nanorods (blue symbols) versus number of pulses with fluence (a) 4 mj/cm 2 and (b) 18 mj/cm Figure 4.1: (a) Photograph of the phantom prepared for ultrasound and photoacoustic imaging. (b) Schematic illustration of the experimental setup. (c) A block diagram of the experimental setup for the combined photoacoustic and ultrasound imaging...68 Figure 4.2: (a) Photograph of the phantom prepared for ultrasound and photoacoustic imaging. (b) Schematic illustration of the experimental setup. (c) A block diagram of the experimental setup for the combined photoacoustic and ultrasound imaging...69 Figure 4.3: A block diagram of the experimental setup for the characterization of the photoacoustic response of gold nanoparticles Figure 4.4: Schematic illustration of the experimental setup for measuring the photoacoustic response from (a) aqueous solution of nanorods and (b) nanorods in oil...73 xv
16 Figure 4.5: (a) TEM image of PEGylated gold nanorods; TEM images of gold-silica core-shell nanorods with (b) 6 ± 0.5 nm (N = 100) and (c) 20 ± 3.6 nm (N = 100) thickness of silica coating; (d) SEM image of gold-silica coreshell nanorods with 75 ± 5.0 nm (N = 100) thickness of silica coating (the silica shell is too thick for TEM) Figure 4.6: (a) Ultrasound, (b) photoacoustic, and (c) combined ultrasound and photoacoustic images (top to bottom) of inclusions containing (I) PEGylated gold nanorods and gold-silica core-shell nanorods with (II) 6 nm silica coating, (III) 20 nm silica coating, and (IV) 75 nm silica coating (left to right). Each image covers 6 mm by 6 mm field of view Figure 4.7: (a) Amplitude of the photoacoustic signal from the background (green dots) and inclusions (blue dots) containing gold nanorods with varying thickness of the silica coating. The error bars show plus/minus one standard error. (b) Contrast-to-noise ratios (CNRs) of the photoacoustic images of the inclusions containing gold nanorods with varying thickness of the silica coating Figure 4.8: (a) Extinction spectra of a gold nanorod solution before and after 6 nm, 20 nm, and 75 nm silica coating. (b) FDTD simulated absorption (solid curve) and scattering (dashed curve) cross-section spectra of gold nanorods before (black line) and after coating with 10 nm (red line), 20 nm (blue line), and 60 nm (orange line) silica. The simulations were performed using the refractive index of fused silica. Note that scattering is nearly 2 orders of magnitude lower than the absorption xvi
17 Figure 4.9: Average amplitudes of the photoacoustic signal from three types of gold nanorods before and after silica coating. The error bar represents plus/minus one standard deviation of the photoacoustic signal measured using 60 laser pulses of low (4 mj/cm 2 ) fluence...85 Figure 4.10: Photoacoustic signal amplitude of gold nanorods with varying thickness of the silica coating in (a) silica growth solution (IPA containing 26 vol% water). (b) water, and (c) silicon oil. In polar environment the signal is amplified, and shows a maximum at ~20 nm shell thickness, while in nonpolar environment the signal monotonically decreases. The vertical error bar represents plus/minus one standard deviation of the photoacoustic signal measured using 60 laser pulses of low (4 mj/cm 2 ) fluence. The horizontal error bars represent plus/minus one standard deviation of the silica thickness measured by TEM xvii
18 Figure 4.11: Schematic summary of the proposed thermal transport processes from the nanoparticle to the environment and the resulting temporal profiles of the temperature (T) near the surface and the amplitude of the photoacoustic signal (P) far from the surface of the nanoparticle. (a) A bare nanoparticle with high interfacial resistance leads to a broadened temperature profile and a smaller amplitude of the photoacoustic pressure signal; (b): introducing a silica shell leads to a minimal interfacial resistance between gold (Au) and SiO 2 and SiO 2 and water. The resulting sharper temperature profile, and because the temperature profile is at a larger distance, the photoacoustic signal is increased; (c) a thick shell leads to a broadened temperature peak and again a decrease in the photoacoustic signal, although it may still be higher than that for the bare nanoparticle Figure 5.1: Schematic illustration the combined photoacoustic/ultrasound system used in this study, where M1-3 represent mirror 1 to 3; L1-2 are lens 1-2; ND represent neutral density filters; BS represent beam splitter; PM represent power meter, and UT represent ultrasound transducer. In the dashed square, it shows the close-up schematic illustration of the sample irradiated by a pulsed laser beam while photoacoustic transients were measured using the ultrasound transducer Figure 5.2: (a) Schematic representation of the photoacoustic response generated from a sphere and the surrounding liquid. (b) Generalized temperature profile without interfacial resistance as a function of time and distance, summarizing the major assumptions made for the analytical pressure amplitude estimation xviii
19 Figure 5.3: TEM images of silica coated nanospheres with silica shell of (a) 6nm, (b) 18nm, and (c) 38nm, respectively Figure 5.4: Photoacoustic signal amplitude of gold nanospheres with varying silica shell thickness as a function of temperature in different environments: (a) water (b) silicon oil (c) toluene. The silica thickness is: 0 nm (circle), 6 nm (triangle), 18 nm (square), and 38 nm (rhombus) Figure 5.5: Temperature dependence of the Gruneisen parameter (left axis) for water (open circle) and toluene (open triangle), and of the measured photoacoustic signal (right axis) of water (solid circle) and silicon oil (solid square). The error bar represents the standard error of 16 signals of eight pulses each Figure 5.6: (a) Measured photoacoustic signal of water as a function of temperature compared to the calculated Grüneisen parameter (line). (b) Fits of the photoacoustic signal of the nanoparticles in water at varying shell thickness with the thermal expansion coefficient of water. (c) Results of the fits in (b) showing the relative and the absolute nanoparticle contribution pnp, and the proportionality constant of the fit. Error bars in (a) and (b) are the standard error of 16 signals of eight pulses each Figure 5.7: TEM images of silica coated nanorods with silica shell of (a) 6nm, (b) 18nm, and (c) 38nm, respectively Figure 5.8: Photoacoustic signal amplitude of gold nanorods with varying silica shell thickness as a function of temperature in different environments: (a) water (b) silicon oil (c) toluene. The silica thickness is: 0 nm (circle), 6 nm (triangle), 18 nm (square), and 38 nm (rhombus) xix
20 Figure 6.1: Schematic illustration directional functionalization and Fluorescent labeling of antibody Figure 6.2: Schematic illustration of the bioconjugation processes. (a) Three chemistry schemes show antibodies directional conjugate to silica coated gold nanorod surface. (b) Antibodies are conjugate to silica surface with random direction Figure 6.3: (a) TEM image illustrate the surface morphology of dye doped silica coated gold nanorods. (b) UV-Vis extinction spectra dye doped silica coated gold nanorod solution. An noticeable absorption appear in the wavelength around 550 nm indicate the dye, rhodamine b isothiolcyanate, have been loaded in to silica matrix Figure 6.4: UV-Vis extinction spectra of antibody conjugated silica coated gold nanorod solution. An noticeable absorption appear in the wavelength around 280 nm indicate the conjugation of antibody to the gold nanorods Figure 6.5: The bright field images and fluorescence images show the cell uptake of antibody conjugated gold nanorods in EGFR(+) A431 cell, in EGFR(-) MD435, and the blocking test suggesting the molecular specificity of the nanorods Figure 6.6: The fluorescence images show the cell uptake of antibody conjugated gold nanorods in EGFR(+) A431 cell, with antibody conjugated gold nanorods. The first column shows the fluoresence signal from rhodamine b isothiocyanate in the silica shells, the second column show the signal from Alexa fluor 350 dye label on the antibody, and the third column show the sgnal from live stain, calceiin-am xx
21 Figure 6.7: Cell viability data after 48 hr exposure to anti-egf receptor antibody conjugated silica coated gold nanorods at different concentrations in A431 cells Figure 7.1: Comparison of PA signal generated from a nanoparticle and tissue as a function of time for two different time intervals in the optical pulse train. (a) The time interval is equal to the time duration of the input optical pulse. (b) The time interval is shorter than the time duration of the input pulse, so that overlap of the optical pulse in the pulse train results in the change of pulse shape, which further induces the change only in the PA single from the nanoparticle Figure 7.2: Pressure wave generated by two kinds of laser pulses. (a) long laser pulse, the stress is not confined; (b) short laser pulse, the stress confinement is satisfied Figure 7.3: The optical set up of multipulse interferometer, where the operation wavelength is 1064 nm with around 6.5 nanosecond pulse width..163 Figure 7.4: (a) 4 pulse Michelson interferometer with different delay between pulses with assumption that the original pulse has 7 nanosecond pulse width. (b) The derivative of the pulse intensity as a function of time, which is proportional to the generated PA signal Figure 7.5: The two pulses used for the dynamic photoacoustic imaging, the energy for the two types of pulses are adjusted to be the same; the inset shows the experimental set up for signal calibration using multipulse interferometer based dynamic photoacoustic imaging system xxi
22 Figure 7.6: Measured laser pulses from the output of the Michelson interferometer with different delay between adjacent pulses to achieve three different duration of pulse width Figure 7.7: Optical extinction spectra of the samples are adjusted to the same level at the imaging wavelength of 1064 nm Figure 7.8: Photoacoustic signal calibrated using flat top long pulse (red symbols) and short pulse (blue symbols) for (a) silica coated nanorod solution and (b) dye solution under the same condition Figure 7.9: (a) Optical absorption of water, and (b) photoacoustic response of water under three different laser fluences of both short and flat top long optical pulses Figure 8.1: A xenograft nude mouse model of AsPc-1 (Mpanc96) pancreatic cancer cells xxii
23 Chapter 1: Introduction Molecular imaging is an emerging imaging principle which can visually represent the biological processes both spatially and temporally down to the sub-cellular level in vivo 1-3. The information provided by the imaging technique is expected to have a profound impact on facilitating early diagnosis of diseases, accelerating the development of new drugs, and improving the efficacy of therapy. In general, molecular imaging highly relies on probes to sense the occurrence of molecular biological events, and to generate signals which could then be picked up by diagnostic imaging modalities 4. The advances in the design of molecular probes not only have equipped traditional anatomical medical imaging with new capabilities, but also, in some cases, stimulated developments of new imaging modalities and renaissance of existing medical imaging modalities. Photoacoustic (PA) molecular imaging is one of these imaging technologies which unites the merits from both optical imaging and ultrasound imaging 5-9. It shares with optical imaging, that it uses non-ionizing radiation sources and provides higher contrast and higher sensitivity than ultrasound imaging. Unlike optical imaging, which requires ballistic photons during the imaging procedure, photoacoustic imaging requires only diffusive photons to excite the ultrasound signal from the imaging target; therefore, it is capable of imaging much deeper into the tissue. In combination with molecular probes, photoacoustic molecular imaging has been demonstrated by several research groups using various photoacoustic molecular probes However, the molecular probes used for most of these studies were contrast agents directly adopted without modifications from optical imaging modalities 12. In this research, we show that the mechanism of photoacoustic signal generation from nanometer-sized contrast agents is distinct from that of optically homogeneous 1
24 materials, such as tissue 13. In tissue, the photoacoustic signal generation is mainly dependent on the optical absorption and thermal properties of tissue itself. However, we have discovered that, the amplitude of the photoacoustic signal generated from nanocontrast agents depends not only on the optical absorption of the nanoparticles, but more importantly, on the dynamic processes of the heat conduction from the nanoparticles to the ambient, and the thermal properties of the surrounding materials Based on this finding, we expanded our investigation on the photoacoustic response of the nanoparticles to further improve the performance of photoacoustic contrast agents by optimizing and exploiting the heat conduction process between the nanoparticle and its surrounding materials and the thermal properties of its ambient. In addition, this fundamental difference in the thermal responses results in diverse temporal response and frequency spectrum of the PA signal generated from the nanoparticle and its ambient; in return, it creates opportunities to dynamically manipulate the PA signals by exploiting varying excitations. 1.1 THE DEMAND OF MOLECULAR IMAGING Before the advent of modern medical imaging, physicians relied on external symptoms to diagnose diseases. The symptoms, however, are the late manifestations of the disease and could vary from patient to patient. Moreover, for some diseases the external symptoms could be easily ignored. Without medical imaging, painful exploratory surgeries would, therefore, be performed frequently to just scout out the anatomic symptoms. Such surgery could bring complications to the treatment, may downgrade the quality of the patient's life, and waste resources of medical care system. The less invasive medical imaging, which could help physicians to early diagnose diseases, make evidence-based decisions, and better understand the effect of treatments, 2
25 has great benefits to patients and the society, for it provides a fast, comfortable, and costefficient way to diagnose and treat diseases. Present medical imaging modalities are only capable to provide information of nonspecific anatomic structures or, in some cases, physiological changes of organs But these capabilities are no longer sufficient to the modern biomedical research and clinical applications 1. For instance, anatomic imaging techniques in general can only detect a lesion of centimeter size or larger. However, lesions at centimeter size level usually indicate later stages of the cancer, which may have already passed the best timing for proper treatments 17. The outstanding progress of nanotechnology and fundamental biology in the past decades has allowed researchers to create various nanomaterials which contain molecular specific moieties and can selectively reach the targets Some of them were designed to contain additional imaging moieties which will respond to specific excitation signals 4, Such molecular specific nanomaterials are called molecular probes or biomarkers 1-2,22. Visualizing the bio-distributions of these molecular probes, called molecular imaging, could provide physicians with information on the traces of the cellular or molecular pathway both spatially and temporally at cellular or sub-cellular level noninvasively 1-2,25. Such information permits the in vivo study of the mechanism of the disease such as inflammation, atherosclerosis, cancer or degenerative diseases 1, and is expected to have a major impact on improving early diagnosis, customizing individual treatment, and hastening drug development. 3
26 A number of evolving molecular imaging modalities have been developed from main available medical imaging modalities, and some of them are available on the market. These include single photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI), ultrasound, and optical imaging. Among these imaging modalities, SPECT and PET use radioactive probes; and MRI requires magnetic field and radio frequency field. To be more specifically, SPECT involves using a gamma ray camera, which is used to image gamma radiation emitting radioisotopes and is useful in analyzing the injected medication for further assessment. The isotopes involved in the imaging technique have relative long half life compared to PET which provides a cost benefit. Because of this, one of the major applications for SPECT is to measure the regional cerebral blood flow in brain, which can provide an 88% accuracy to diagnose Alzheimer disease. On the other hand, PET also involves detections of gamma rays to form three dimensional images of dynamic functional processes in the body. This imaging technique has been mostly applied to image cancer metastasis. In comparison with SPECT, the typical probes clinically used in PET have a half life in the range of hours, and often require a cyclotron on site, which dramatically increase the cost of the imaging procedure. The major advantage of PET is the high sensitivity of detecting mol/l to mol/l concentrations of the probes. In contrast to these two radioactive imaging techniques, MRI doesn't involve ionizing radiation. The magnetic field in MRI is used to align the magnetization of some atomic nuclei in the body, and the radio frequency field is used to systematically alter this alignment. The high spatial resolution of MRI provides this imaging technique a good candidate to image brain, muscles, heart, and tumors. Two major categories of mostly used imaging techniques which don't arouse safety issues are ultrasound imaging and optical imaging. In the ultrasound imaging, a transducer sends out high frequency sound 4
27 waves, typically between 2 to 18 MHz, and detects the echoes which are reconstructed into images of inner body organs and blood flow in real time. The non-ionizing nature of ultrasound imaging is widely recognized, so that it has been commonly used during the course of pregnancy to monitor the growth of fetus. In addition, it is a flexible, low cost imaging technique, therefore imaging of tissue, muscles, bone surfaces and interfaces between tissue solid and fluid have been highly applied in clinical diagnosis. Another imaging modality involves non-ionizing probes is the optical imaging. Light sources used in this modality include ultra violate (UV), visible and near infrared (NIR). There are two major categories in optical imaging, one involves the detection of diffusive photons, and the other one detects ballistic photons. Any object that scatters light can create diffusive optical images. The diffusive photons can detect hemodynamics changes, which have been utilized in measuring changes in blood volumes as well as the oxygen saturation level in blood, providing specific information in early cancer detection before tumor formation. Diffusive optical imaging is also highly used in the field of studying the function and time course of the brain, where an IR laser acts as the light source, and fiber bundles as the detector to sense the variation in the absorption and scattering of the light path after passing through the brain tissue. Typical spatial resolution of diffusive optical imaging is in the range of a few millimeters, and temporal resolution of a few milliseconds. On the contrary, ballistic optical imaging relies on detecting photons that travel in a straight line from scattered medium. Although tissue is an optical turbid medium, if the detector is close enough to the medium, a small portion of the coherent light can still be captured. Two widely used optical imaging techniques based on ballistic photons are ballistic scanner and optical coherent tomography (OCT), which can provide a high resolution as far as diffraction limit can approach. 5
28 1.2 THE CHALLENGE OF MOLECULAR IMAGING Molecular imaging, however, is still in its infancy despite many efforts. In order to bring molecular imaging to clinical practice, the main challenge of designing and validating proper molecular probes that could recognize the target molecules of interest and generate sufficient signals which could be detected by existing imaging techniques needs to be overcome 1. The challenges are either from the fundamental limitation of the imaging modalities or from the restrain of the materials. For instance, the molecular probes of PET usually need to be produced with a cyclotron, and most of the biomarkers have very short shelf life 26. The molecular probes of SPECT are radioactive, and the spatial resolution of SPECT is only in the range of 3 to 6 mm, and the temporal resolution is in the millisecond range 27. In addition, both PET and SPECT require ionizing excitation sources during imaging which potentially have harmful side effects to the human body. The molecular probes of MRI require high magnetic susceptibility, which narrows down the substances to very few magnetic materials in nature 28. Even with proper magnetic materials, MRI has a low imaging sensitivity due to the fact that the difference between atoms in the high energy state and the low energy state is very small; the sensitivity of MRI is only in the range between 10 3 mol/l and 10 5 mol/l, which results in the requirement of potentially high magnetic field to drive this procedure 2. In contrast to MRI, the sensitivity of optical imaging is higher, and their molecular probes do not require any radioactive labeling or magnetic properties 2. Most importantly, the constituent materials of optical imaging probes are versatile, which makes optical imaging agents to be one of the most rapid growth areas in molecular probe development However, due to optical absorption and scattering by tissue, most optical imaging modalities are limited by shallow imaging depth and are difficult to be transferred to real clinical applications 2. Several optical imaging techniques have been 6
29 developed to overcome the limitations of imaging depth. Two prevailing examples are diffusive optical tomography (DOT) 34 and PA imaging 5. DOT has limited resolution at the scale of millimeters due to the fact that the image formation is based on scattered photons instead of ballistic photons On the contrary, PA imaging, as a hybrid imaging modality, which inherits the advantages from both optical imaging and ultrasound imaging, has an image resolution in the micro-meter scale which is bound only by the frequency of the transducer selected 5,9. PA imaging circumvents the shallow imaging depth compared to optical imaging by using a light source only for locally heating the imaging target and using ultrasound transducers to detect the sound waves generated by thermal expansion of the materials instead of detecting photons. The generated sound wave is less scattered than light waves in turbid materials such as tissue, which allows the PA imaging technique to image deeper into tissue than regular ballistic imaging techniques. 1.3 PHOTOACOUSTIC IMAGING Alexander Graham Bell in 1880 first discovered that light can produce sound in solid sample if incident light was rapidly interrupted. Bell used spinning slotted wheel as a chopper to "chop" the sunlight and found that the resulting sound depends strongly upon the composition of the materials. Since then the application of using PA effect has seen two major renaissances 37. The first transformation was facilitated by lasers and piezoelectric transducers to create an ultra-sensitive spectroscopy technique to characterize the absorptive properties of gaseous, liquid, and solid materials in the form of bulk, powders and surface films It has also been very helpful in characterizing photo-induced chemical reactions 40, and in chemical, thermal, and structural sensing 41, or in the characterization of two-photon absorption cross sections 42. Theoretical descriptions 7
30 (a) (b) Pulsed Laser Ultrasound (c) CW laser Figure 1.1: This cartoon illustrates the general idea of molecular imaging and its role in cancer therapy. (a) molecular probe specifically targets to cancer cell; (b) pulsed laser induces photoacoustic signal to generate photoacoustic images to image cancer at the cell level; (c) when CW laser irradiation is on, the nanocontrast agents are heated up and locally annihilate cancer cells without damaging the surrounding tissue. 8
31 of various experimental situations were developed by a number of authors 43-46, and summarized for instance in Ref 47. More recently, a second renewed interest has been developed, which is motivated from biomedical imaging and image-guided therapy 5, although photoacoustic techniques have been used in the life sciences earlier 48. The electromagnetic waves used in PA imaging have been limited to three major spectral regions for the reasons of providing non-ionizing excitations and achieving relative good penetration depth. These spectral regions include the visible spectrum from 400 nm to 700 nm, the near-ir range from 700 nm to 1100 nm and the radio frequency (RF) spectrum. The RF spectrum excitation is often referred to as the thermoacoustic imaging, whose mechanism is the same as PA imaging. Because of the high photon energy, UV rays are excluded as the excitation source since it can be detrimental to the integumentary system such as the epidermis and the cornea. Terahertz frequency electromagnetic waves are also excluded, because the major constituent material of tissue is water which absorbs strongly in that spectral range. The optical responses to the visible and near-ir spectra including scattering and absorption from biological tissues are highly related to the molecular constituents and their electronic and vibrational structures of the tissues; and the RF counterpart is related to the electronic properties of the tissues, described by the complex conductivity. In PA imaging, short pulsed lasers (usually in nanosecond) are used to excite the imaging target, the light is partially absorbed and generates phonons. These phonons are converted to a pressure wave due to transient thermal-elastic expansion and propagate to the detector as a sound wave. Since biological tissues are optically turbid medium, photons are highly scattered, and partially diffusive into the medium. PA imaging requires only the diffusive photons, since it is based on optical absorption, and as a result, it provides physiologically specific optical absorption contrast in the images due to the 9
32 light (or RF) absorption. This is in drastic contrast to pure ultrasound imaging, whose contrast relies on the different impedance of the sound waves between interfaces, therefore ultrasound imaging doesn't provide scout images and is unable to reveal either oxygen saturation or hemoglobin concentration. The depth of PA imaging is governed by the ultrasound attenuation, and similarly to ultrasound imaging, there is tradeoff between the spatial resolution and imaging depth, which is dependent upon the frequency of the transducer. As an example, PA signals with 50 MHz bandwidth can provide 3 mm penetration depth with an axial spatial resolution of 15 um, and a lateral spatial resolution of 45 um; in contrast, a PA signal with 5 MHz bandwidth can penetrate as far as 50 mm, by sacrificing the spatial resolution into 700 um. Besides the optical absorption from endogenous molecules in biological tissues, the absorption can be enhanced exogenously by molecular probes in PA imaging. The introduction of molecular specific exogenous imaging agents with proper design brings PA imaging molecular sensitivity, even deeper imaging depth, and better contrast based on a much stronger light-to-sound conversion efficiency. The benefits of deep penetration depth, relatively high resolution and bio-labeling using molecular probes make PA molecular imaging an ideal candidate for early stage cancer detection, diagnosis and surgical procedure guidance during treatment. These overall advantages will make the physical pain and discomfort of invasive imaging a thing of the past, while allowing for diagnostic imaging eventually to be done in a more comfort setting. 1.4 PA CONTRAST AGENTS Introduction of molecular specific PA contrast agents brings molecular imaging capabilities to PA imaging. PA molecular imaging has been demonstrated by several research groups. Mallidi et al. has reported photoacoustic molecular imaging of skin 10
33 cancer using gold nanospheres as molecular probes 11. Gold nanorods reported by Song et al. have also been used as tracers to map lymph drainage to identify sentinel lymph nodes in rat models 49. Kim et al. performed photoacoustic molecular tomography using gold nanocage as molecular probes 50. Silver nanoparticles, such as antibody conjugated silver nanoplates, have also been used as molecular probes to image pancreatic cancer. Other than plasmonic nanoparticles, contrast enhanced indocyanine green dye-enhanced single walled carbon nanotubes have been introduced as the molecular probes in PA imaging of U87MG tumor in live mice 51. Glioblastoma brain tumors have been imaged using a small molecule organic dye conjugated with a cyclopeptide as the molecular probe for PA imaging in vivo 52. However, the PA imaging agents used for these studies, in most cases, are optical absorbing nanostructures adopted from optical imaging contrast agents. Albeit it works in certain degree to directly adopt the optical imaging agents for PA molecular imaging, the fundamental difference between PA imaging and optical imaging suggests that present strategies may not be an optimal route to improve the PA molecular imaging for future clinical applications. In order to develop strategies to increase light-to-acoustic conversion of nanoparticles, it requires a brief review of the fundamental difference between the photoacoustic effect from optical absorbing nanostructure and tissue. The photoacoustic effect is the physical phenomena of an acoustic wave excitation by the interaction of modulated electromagnetic wave, i.e. laser pulses, with matter. There are five important mechanisms responsible for the photoacoustic signal generation including dielectric breakdown, vaporization, thermo-elastic expansion, electrostriction, and radiation pressure 53. The first two mechanisms involve an irreversible change of state in materials and are rarely used for imaging. Electrostriction and radiation pressure, in general, contribute very minor to the photoacoustic generation and can be neglected 53. In 11
34 biomedical imaging applications, consequently, thermo expansion is considered as a main mechanism. In this case, for optically homogeneous materials, such as tissue, radiation is absorbed, the absorption energy is converted to heat through non-radiative relaxation, and the heated zone produces localized compressed stress and attempts to expand. This thermo-expansion results in an outgoing thermo-acoustic wave that can be detected by an ultrasound transducer and used to reconstruct images. In order to effectively generate the photoacoustic signal, several parameters and constrains need to be considered in each step of the process. In the first step, the optical absorption coefficient is the dominating factor. After the absorption of the radiation, two relaxation processes could happen. One is radiative and the other is non-radiative. To effectively convert absorbed energy into heat, it requires the non-radiative process to be faster than the radiative process. Also, to prevent the heat from dissipating, the temporal duration of the excitation laser pulse ( t ) must be shorter than the characteristic time of the thermal relaxation 47. Finally, to effectively transfer the thermal energy into an acoustic wave, the laser pulse width should be shorter than the time that the acoustic wave travels across the heated zone 47. In this condition, the stress is confined and accumulated in the heated area until the stop of the pulse which means the PA generation is independent of the laser pulse duration 47. Therefore, in the condition of thermal and stress confinement, the amplitude of photoacoustic signal is related to laser fluence, optical absorption of materials, and thermal properties of the materials. 1.5 OVERALL RESEARCH GOALS The overall goal of this research is to improve the contrast and sensitivity of PA molecular imaging by optimizing the design of plasmonic nanoparticle based contrast agents and imaging system. 12
35 Specifically, plasmonic nanoparicles, using gold nanorods as an example, will be prepared as a platform to develop PA molecular probes using wet chemistry methods. By adjusting the aspect ratio of the nanorods, the maximum optical absorption will be carefully tuned to the wavelength where tissue has the minimum absorption. The size of the gold nanorods will be controlled during the synthesis process to optimize their intercellular transportation and endocytosis. The optical properties of nanoparticles will be studied by ultraviolet-visible (UV-Vis) spectroscopy. The size and shape of the particles will be confirmed by transmission electron microscopy (TEM). To demonstrate our hypothesis, the optimal heat transfer and thermal expansion will be manipulated by multilayer dielectric shells and adhesive materials between the core and the shell of the nanoparticles. Initially, biocompatible dielectrics such as silica, and lipid layers will be used as the coating materials to build signal-enhanced contrast agents which are named photoacoustic nanoamplifiers. The morphology and the thickness of the shells will be studied using TEM. The photoacoustic signal response of the nanoamplifiers will be characterized by an integrated ultrasound/photoacoustic (US/PA) imaging system. We will functionalize the surface of the synthesized nanoamplifiers with polyethylene glycol to improve nanoparticle stability and to conjugate monoclonal anti-epidermal growth factor receptor (anti-egfr) antibodies to target human epidermal growth factor receptor which is highly expressed in a variety of human tumors including non-small cell lung cancer (NSCLC), breast, head and neck, gastric, colorectal, esophageal, prostate, bladder, renal, pancreatic, and ovarian cancers. Other biomarkers will also be investigated to expand the targeting capabilities of the photoacoustic molecular probes developed in this research. The stability of the bioconjugated nanoparticles will be studied by Zeta potential analyzer and the labeling efficiency will be measured using fluorescent spectroscopy and inductively coupled plasma mass spectrometry (ICP-MS) to evaluate 13
36 the contrast between normal tissue and cancerous cells. Cytotoxicity studies will be performed to ensure biological safety. A human pancreatic cancer line will be used to determine the potential toxicity of the developed molecular probes. These extra steps allow transferring these contrast agents to in vivo study and practical clinical applications to be achieved. A multiple arm Michelson interferometer will be built and integrated with a tunable OPO laser system to allow for the generation of nanosecond pulse trains with administrable time intervals. The manipulation of the time intervals between the excitation pulses is validated through modulating the individual arm length of the interferometer. The manipulation reflects dynamically in the PA response of the nanoparticle apart from the homogenous tissue, which provides unprecedentedly enhanced contrast of the PA imaging in a dynamic fashion. 1.6 REFERENCES 1 Weissleder, R. Molecular imaging in cancer. Science (New York, N.Y.) 312, , (2006). 2 Weissleder, R. & Mahmood, U. Molecular imaging. Radiology 219, , (2002). 3 Grenier, N. & Brader, P. Principles and basic concepts of molecular imaging. Pediatric Radiology 41, , (2011). 4 Debbage, P. & Jaschke, W. Molecular imaging with nanoparticles: giant roles for dwarf actors. Histochemistry and cell biology 130, , (2008). 5 Emelianov, S. Y., Li, P. C. & O'Donnell, M. Photoacoustics for molecular imaging and therapy. Physics Today 62, 34-39, (2009). 14
37 6 Oraevsky, A. A., Jacques, S. L., Esenaliev, R. O. & Tittel, F. K. in OSA Proceedings on Advances in Optical Imaging and Photon Migration Vol. 21 Optical Society of America Proceedings (OSA, Washington, 1994). 7 Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nature Photonics 3, , (2009). 8 Wang, X. D. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature Biotechnology 21, , (2003). 9 Xu, M. H. & Wang, L. H. V. Photoacoustic imaging in biomedicine. Review of Scientific Instruments 77, , (2006). 10 Homan, K. et al. Prospects of molecular photoacoustic imaging at 1064 nm wavelength. Optics Letters 35, , (2010). 11 Mallidi, S. et al. Multiwavelength Photoacoustic Imaging and Plasmon Resonance Coupling of Gold Nanoparticles for Selective Detection of Cancer. Nano Letters 9, , (2009). 12 Yang, X., Stein, E. W., Ashkenazi, S. & Wang, L. V. Nanoparticles for photoacoustic imaging. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 1, , (2009). 13 Chen, Y. S. et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Optics Express 18, , (2010). 14 Chen, Y.-S. et al. Silica-coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Letters 11, , (2011). 15 Prince, J. L. & Links, J. M. Medical imaging signals and system. ( Prentice Hall, 2006). 15
38 16 Bushberg, J. T., Seibert, J. A., Leidholdt, E. M. & Boone, J. M. The Essential Physics of Medical Imaging. 2 edn, (Lippincott Williams & Wilkins, 2001). 17 Robinson, P. Imaging liver metastases: current limitations and future prospects. British journal of radiology 73, (2000). 18 Chauhan, V. P., Stylianopoulos, T., Boucher, Y. & Jain, R. K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annual Review of Chemical and Biomolecular Engineering 2, , (2011). 19 Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature reviews. Drug discovery 7, , (2008). 20 Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nature reviews. Clinical oncology 7, , (2010). 21 Petros, R. a. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nature reviews. Drug discovery 9, , (2010). 22 Cormode, D. P., Skajaa, T., Fayad, Z. a. & Mulder, W. J. M. Nanotechnology in medical imaging: probe design and applications. Arteriosclerosis, thrombosis, and vascular biology 29, , (2009). 23 Hahn, M. a., Singh, A. K., Sharma, P., Brown, S. C. & Moudgil, B. M. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Analytical and bioanalytical chemistry 399, 3-27, (2011). 24 Koo, H. et al. Nanoprobes for biomedical imaging in living systems. Nano Today 6, , (2011). 25 Grenier, N. & Brader, P. Principles and basic concepts of molecular imaging. Pediatric radiology 41, , (2011). 16
39 26 Phelps, M. E. Molecular imaging with positron emission tomography. Annual Review of Nuclear and Particle Science 52, , (2002). 27 Wester, H. J. Nuclear imaging probes: From bench to bedside. Clinical Cancer Research 13, , (2007). 28 Huang, J., Zhong, X. D., Wang, L. Y., Yang, L. L. & Mao, H. Improving the Magnetic Resonance Imaging Contrast and Detection Methods with Engineered Magnetic Nanoparticles. Theranostics 2, , (2012). 29 Cho, E. C., Glaus, C., Chen, J. Y., Welch, M. J. & Xia, Y. N. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends in Molecular Medicine 16, , (2010). 30 Douma, K., Megens, R. T. A. & van Zandvoort, M. Optical molecular imaging of atherosclerosis using nanoparticles: shedding new light on the darkness. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology 3, , (2011). 31 Luo, G. P. et al. Quantum dots in cancer therapy. Expert Opinion on Drug Delivery 9, 47-58, (2012). 32 Swierczewska, M., Lee, S. & Chen, X. Y. Inorganic Nanoparticles for Multimodal Molecular Imaging. Molecular Imaging 10, 3-16, (2011). 33 Zhang, Y., Hong, H., Myklejord, D. V. & Cai, W. B. Molecular Imaging with SERS-Active Nanoparticles. Small 7, , (2011). 34 Gibson, A. P., Hebden, J. C. & Arridge, S. R. Recent advances in diffuse optical imaging. Physics in Medicine and Biology 50, R1-R43, (2005). 35 Habermehl, C., Schmitz, C. H. & Steinbrink, J. Contrast enhanced high-resolution diffuse optical tomography of the human brain using ICG. Optics Express 19, , (2011). 17
40 36 Boas, D. A., Chen, K., Grebert, D. & Franceschini, M. A. Improving the diffuse optical imaging spatial resolution of the cerebral hemodynamic response to brain activation in humans. Optics Letters 29, , (2004). 37 Bell, A. G. On the production and reproduction of sound by light: the photophone. American Journal of Science 20, , (1880). 38 Patel, C. K. N. & Tam, A. C. Pulsed optoacoustic spectroscopy of condensed matter. Reviews of Modern Physics 53, , (1981). 39 Rosencwaig, A. Photoacoustic-spectroscopy. Annual Review Of Biophysics and Bioengineering 9, 31-54, (1980). 40 Braslavsky, S. E. & Heibel, G. E. Time-resolved photothermal and photoacoustic methods applied to photoinduced processes in solution. Chemical Reviews 92, , (1992). 41 Tam, A. C. & Patel, C. K. N. 2-photon absorption-spectra and cross-section measurements in liquids. Nature 280, , (1979). 42 Tam, A. C. Applications of photoacoustic sensing techniques. Reviews of Modern Physics 58, , (1986). 43 Adams, M. & Kirkbright, G. Analytical optoacoustic spectrometry. Part III. The optoacoustic effect and thermal diffusivity. The Analyst 102, 281, (1977). 44 McDonald, F. A. & Wetsel, G. C. Generalized theory of photoacoustic effect. Journal of Applied Physics 49, , (1978). 45 Rosencwaig, A. & Gersho, A. Photoacoustic Effect with Solids: A Theoretical Treatment. Science 190, , (1975). 46 Rosencwaig, A. & Gersho, A. Theory of photoacoustic effect with solids. Journal of Applied Physics 47, 64-69, (1976). 47 Gusev, V. E. & Karabutov, A. A. Laser Optoacoustics (AIP, 1993). 18
41 48 Cahen, D., Bults, G., Garty, H. & Malkin, S. Photoacoustics in life sciences. Journal of Biochemical and Biophysical Methods 3, , (1980). 49 Song, K. H., Kim, C., Maslov, K. & Wang, L. V. Noninvasive in vivo spectroscopic nanorod-contrast photoacoustic mapping of sentinel lymph nodes. European Journal of Radiology 70, , (2009). 50 Kim, C. et al. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. Acs Nano 4, , (2010). 51 Zerda, A. d. l. et al. Ultrahigh Sensitivity Carbon Nanotube Agents for Photoacoustic Molecular Imaging in Living Mice. Nano Letters 10, , (2010). 52 Meng-Lin, L. et al. Simultaneous molecular and hypoxia imaging of brain tumors in vivo using spectroscopic photoacoustic tomography. Proceedings of the IEEE 96, , (2008). 53 Scruby, C. B. & Drain, L. E. Laser Ultrasonics Techniques and Applications. (CRC Press, 1990). 19
42 Chapter 2: Design, Synthesis, and Characterization of Gold Nanorods Metal nanoparticles (MNPs) have become highly versatile contrast agents for optical imaging of cells in-vitro and of biological tissue. While it has been known that the quantum yield from spherical gold or silver nanoparticles is orders of magnitude higher than that of organic dyes 1, these spherical particles have recently also been used to study dynamic interactions in cells using their sensitivity to the proximity of other MNPs 2-3. The formation of MNPs into other shapes, such as rods, shells, cubes, triangles, bipyramids, etc., has allowed a relatively free choice of the wavelength, including the transparent range of wavelengths in the near infra-red (NIR) range where tissues absorb minimally 4-5. The large surface area and simple chemistry of the nanoparticles makes it easy to develop complex recognition and targeting strategies for molecular imaging and site specific therapy 6. In addition, new imaging and therapeutic techniques, such as photoacoustic molecular imaging and photothermal therapy, have been developed where unique properties of these particles are used to achieve enhanced contrast between MNPs and tissue 7-9. Among these new developing techniques, molecular specific photoacoustic and ultrasound image-guided photothermal therapy were proposed to treat various medical conditions, such as cancers The photothermal therapy relies on the resonant absorption of light by the nanoparticle and the conversion of the electromagnetic energy into heat to destroy malignant tissue At the same time, nanoparticle-facilitated absorption of pulsed light leads to the production of sound waves used for photoacoustic imaging. In image-guided photothermal therapy, photoacoustic imaging can be used both to confirm the delivery of MNPs to the desired location in tissue and to visualize temperature maps during photothermal therapy 10. The absolute as well as relative strength 20
43 of the surface plasmon resonant absorption and scattering that contribute to the well defined extinction band of gold or silver MNPs depend on the nanoparticle material composition, shape, and environment 14. While large scattering cross sections are needed for dark-field imaging, large absorption cross sections are required for photoacoustic imaging 15. Therefore nanospheres, nanoshells, nanocages, nanoplates and nanorods have been investigated and are used in facilitating photoacoustic imaging as contrast enhancement agents. Gold nanorods are of particular interests as their sizes and aspect ratios can be tailored and controlled during chemical synthesis, which results in a tunable resonance ranging from the visible to the NIR spectrum, and the resulting nanoparticle can be designed to possess a very high absorption cross section. 2.1 OPTICAL PROPERTIES OF GOLD NANORODS Gold nanorods, as studied in many research groups have the appealing fact that their plasmonic resonance is tunable. Due to the elongated structure, nanorods possess two resonance wavelengths. The shorter wavelength between 500 nm to 600 nm which is also found in nanospheres is the transverse surface plasmon resonance; the peak at longer wavelength, also known as the longitudinal plasmon resonance occurs when incident light is polarized along the long axis of the nanorod, causing surface electron oscillating along that axis. The location of the transverse resonance is inert to the shape of the nanorod, however, the longitudinal plasmon band is sensitive to the aspect ratio which leads to interesting applications of nanorod especially in biosensing as well as in the rapid development of photoacoustic imaging as a contrast agent. With increasing rod length, the peak usually red shifts due to the reduced restoring force because charges are separated at a longer distance. 21
44 In this section, the surface plasmon is first reviewed as a background study, then analytical description of plasmonic nanospheres using Mie theory is introduced, followed by its modified form using the Rayleigh-Ganns theory to describe elongated nanostructures. More specific numerical calculations are conducted with respect to rod shape geometry of the particular nanoparticles used in this study, and two approaches are utilized: one is the discrete dipole approximation (DDA) and the other is full vectoral finite difference time domain (FDTD) method. Both of these numerical methods are briefly introduced and the examples of calculations using these methods are followed in particular dealing with nanorod structures involved in this research Surface Plasmons and Localized Surface Plasmon Resonance In the physics community, a plasmon is a quasi-particle, similarly to photons and phonons; it is a quanta of plasma oscillation. Therefore, the collective valence electron oscillations are referred to as the plasmons. When these oscillations are coupled with light, it is called plasmon polariton. Surface plasmons are collective charge oscillations that occur at the interface between a dielectric and a metal (or a conductor). The history of plasmons can be retrieved back to the 4th century AD, and the most famous example that almost every plasmonics book mentioned is the Lycurgus cup. Experimental efforts started in 1902 by R. W. Wood who discovered anomalous diffraction from metal gratings, which is later referred to as Wood's anomalous 16. These early experimental works were followed by Maxwell Garnett who built the Drude model to describe the permittivities of metal 17 and Lord Rayleigh who gave optical properties of nanospheres, especially scattering in terms of wavelength Until 1908, Gustav Mie developed the Mie theory that can describe light scattering from metal sphere in a rigorous manner 22. The terminology of "plasmon" was coined by David Pine in 1956, 22
45 who named this quanta of electron oscillations in analogy to plasma oscillations in gas, this term now is called "bulk plasmon", which is distinct from surface plasmons 23 ; and the term "polariton" was proposed by J. Hopfield, which is used to describe the coupled oscillation between photons and bound electrons 24. Not until 1968 that R. Ritchie first connected the plasmon resonance to the Wood's anomalous by explaining this abnormal phenomenon in terms of excitation of surface plasmon resonances inside the gratings 25. In the same year, it were A. Otto, et al. that experimentally demonstrated how to excite surface plasmons in metal film which made a big leap in this field 26. In relation with surface plasmons, there is surface plasmon resonance, which is often referred to as the resonant collective charge oscillation excited by the electromagnetic field. In nanoparticles, it is a quasi-zero dimensional surface, so only localized surface plasmon (LSP) modes exist. In this study, we focus on modifying the nanoparticles' shape, size and surface to achieve a molecular specific nano-probe for assisting PA imaging and PA thermal cancer therapy Mie Theory Among all the theoretical studies of the light and small particle interactions, the theory that Gustav Mie developed can provide a lot of details on scattering and absorption of the plane wave by spherical nanoparticles. In the experiment, the far field extinction coefficients can be obtained for a nanoparticle solution; and the PA signal generation is often associated with the absorption of the nanoparticle and the dynamic process of transferring the absorbed energy into heat. In the nanoparticle solution, the incident light is either scattered or absorbed; the total extinction, which can be described using a dimensionless quantity, the extinction coefficients Q ext, is the summation of the 23
46 scattering and absorption coefficients. Therefore, it is relevant to study the scattering coefficient Q scat, which in the far field is 2 R Q lim Ra a 2 * scat s s E E sind d (1) where a is the radius of the spherical particle and R is the distance from the observer to the object. In the quasi-static limit, which happens when the radius of the particle is much smaller than the wavelength, which is also called the Rayleigh scattering range, the scattering coefficient of a dipole is Q scat k 8k R m d R o 3 m 2 d 2 (2) where is the polarizability of the dipole scatterer, m is the permittivity of the metal, and d is the permittivity of the dielectric medium that the metal particle is embedded in. And the extinction coefficient under this approximation is '' '' k 12 kr d m Qabs 2 ' 2 R 0 2d m (3) where the double prime at the superscript represents the imaginary part of the permittivity, and single prime represents the real part. Therefore, for a spherical nanoparticle, when it is on resonance under the quasi-static approximation, the real part of the metal should be around if the surrounding medium is water. For ellipsoidal particles, a depolarization factor A is considered which is related to the geometric factor. The depolarization factor is related to the three axes of the ellipsoid abc,, in the following manner: 24
47 A A x y abc 2 abc 2 abc Az ds s a s b s c ds s a s b s c ds s a s b s c And the polarizability along x, y, z directions are modified accordingly: 4 abc 0 d m d x, y, z 3 d Ax, y, z m d (4) (5) Rayleigh-Gans Calculation When the size of the particle is larger than what is described in the Rayleigh scattering from the last section, but still not too large, it is possible to use the Rayleigh- Gans theory to approximate the scattering of a particles with arbitrary shape. The condition for a valid Rayleigh-Gans approximation is: kd m 1 1 (6) where m is the ratio between the complex refractive index of the metal particle to its surrounding medium Numerical Calculation on Gold nanorods With the advent of successful synthesis of arbitrary shaped nanostructures, in depth understanding, design and control of their optical properties are highly demanded. For an arbitrary shaped nanoparticle, there are no analytical solutions of the scattering cross-section, although at the quasi-static limit, the Mie theory can give a relatively accurate estimation on the resonance frequencies. To meet this demand, numerical approaches including differential equation methods, such as the finite difference time domain (FDTD) method, finite element method (FEM), etc.; volume integral equation 25
48 methods such as the method of moments (MoM), the discrete dipole approximation (DDA); as well as surface integral equation based methods, such as the T-matrix approach, have been extensively studied All of these approaches have advantages and disadvantages. Both of the FDTD method and FEM are flexible in terms of particle shape, however, since the computation has to be repeated for different orientations with respect to the incident field, it will be inefficient in terms of high computation resource requirement when dealing with randomly orientated particles, i.e. nanoparticles suspended in chemical solutions. The volume integral equation method can also be applied to study arbitrary shapes of targets, one advantage of this approach over differential equation approach is that the calculation needs only to be carried out within the scatterer. In DDA, the structure of the particle is discretised by mutually interacting dipoles, so to accurately evaluate a nanosized particle, a high density of dipoles is still desired. The T matrix approach, also known as the null field method (NFM) or extended boundary condition method (EBCM) is more advantageous over the other methods in terms of calculating ensembles of randomly orientated scatterers, however, such method itself doesn t treat imhomogeneous materials, which requires special implementation and will increase the complexity of calculation; moreover, high refractive index (either real or imaginary part) of the target material could cause numerical inaccuracy. To deal with the gold-silica core-shell nanorods discussed in this report, FDTD and DDA are prior to NFM due to the fact that both FDTD and DDA are easier to implement for irregular and inhomogeneous particles, and the large imaginary part in refractive index of the core material gold can lead to numerical problems when using NFM. Compared to DDA, FDTD needs to perform in the spatial domain which is larger than the particle itself, which leads to more unknowns than in DDA. Yurkin et al. did a systematic comparison of DDA and FDTD for different size parameters and refractive 26
49 index, and concluded that DDA is more sensitive to refractive index change. For our purpose, to study the optical properties of gold-silica core-shell nanorods and engineer the thickness of the mantle to control the longitudinal plasmon resonance, the sensitivity of the peak wavelength shift with respect to the change of the refractive index of the surrounding media where the gold nanorod embedded is a good indicator to reveal the success of silica coating. Based on these reasons, DDA is chosen as the numerical method in this report. In this section, we present a detailed study showing dependence of optical properties, especially the shift of plasmon resonances in terms of the aspect ratio of the nanorods in both DDA and FDTD approaches. Discrete Dipole Approximation DDA is used to compute scattering and absorption by nanoparticles of arbitrary geometry and composition with dimensions comparable to or smaller than the incident wavelength. It was first proposed by Purcell and Pennypacker in 1973, who modeled the particle by a cubic array of point dipoles, which interact with each other and the incident wave to produce the complex amplitude at each location. A comprehensive introduction of DDA can be found in Flatau, only key formulism is reviewed in this section. E inc E Consider a particle discretised by N point dipoles (i=1,,n), with incident wave o exp i kr i t at the th i location, the polarization is i i i to describe the relation between the polarization and the field is: 27 P E where the field Ei includes both the incident wave and the contribution from the rest of N-1 dipoles at that location. E E E E AP (7) i inc, i dip, i inc, i ij j i j where AP is the field induced by dipole ij j P at the j th j location. The matrix element A ij
50 ikrij rij rij 3 2 rij rij 3 exp ikr 1 Ι Ι j (8) th j i j dipole, rij r r, and I 3 is the 3 by 3 r ij 2 Aij k, i rij rij where r ij is the distance between the i th and identity matrix. When i j, A is just the inverse of the polarizability ij with the scattering problem reduces to solve 3N linear equations. ij i. So dealing N A P ij j Einc, i (9) j1 Once the polarization at each location is solved, the extinction and absorption cross-section as well as the scattered field can be obtained, for example: N 4 k 1 * * Cabs 2 Im i i P i k P i E i1 P α 3 (10) o Spectral Calculation Using Finite Difference Time Domain Method Numerical analysis was also performed using full vectoral numerical simulations based on finite difference time domain (FDTD) method obtained from commercially available software (Lumerical Inc.). A non-uniform grid with total-field/scattered-field (TF/SF) source and perfect matched layer (PML) absorbing boundaries were applied during the simulation. Gold and dielectric permittivities were based on Johnson and Christy and the silica refractive index used was The ambient environment considered in the simulation was water with a refractive index of CTAB and PEG layers were ignored. The spectral dependence of the absorption and scattering cross sections for both polarizations were collected and added. Nanorods were modeled as cylinders with semi-spherical caps of constant radius. Ellipsoids were calculated to have the same volume as the original gold nanorod, with progressively shortened long axis and circular cross section. 28
51 2.2 OPTICAL PROPERTIES INSIDE HUMAN BODY The contrast of PA imaging is strongly influenced by the optical absorption of materials and the incident light. Therefore, in order to design proper imaging contrast agents for imaging in the biology body, it is important to have a basic understanding on the light propagation inside the tissue and optical absorption of both tissue and blood. This section gives a brief overview of the optical properties of tissue and blood, the two major components of vertebrates Optical Properties of Tissue Because tissue is heterogeneous on length scales between 1 and 1000 m, in the visible (Vis) to near infra-red (NIR) range, when light travels inside the biological tissues, the dominate light-tissue interaction is scattering. Scattering is mainly caused by the mismatch of the refractive index of different tissue components. The main scatters of tissue components are cell nuclei and mitochondria. The scattering process changes the internal light distribution, polarization state, and the reflection. A. Bashkatov et al. studied the optical properties of human skin, subcutaneous adipose and mucous tissues within the spectral range from 400 to 2000 nm, both in terms of absorption and scattering coefficients for the different components 29. The skin has three major layers, which are epidermis, dermis, and the subcutaneous fat. The epidermis is around 100 um thick, which is free from blood; the dermis is a vascularized layer around 1 to 4 mm thick, therefore the optical absorption of this layer is dominated by the absorption of blood, which will be explained in more detail in section 2.2.2; the subcutaneous fat layer is around 1 to 6 mm thick depending upon the subject. The subcutaneous adipose tissue is composed of droplets of lipids with nerves, capillaries and fibrils filling the gaps. Therefore, the absorption of the adipose tissue is dominated by the absorption of lipids, water and hemoglobin. And the droplets of lipids 29
52 act as main scatterers inside this layer. The mucous tissue contains epithelial and proper layers; and the proper layers determine the optical properties of the mucous tissue because it takes much more volume ratio compared to the epithelial layer in the mucous tissue. The absorption coefficients and reduced scattering coefficients of in-vitro biological tissues are shown in Figure 2.1 (a) and (b) respectively. The minimum absorption of tissue happens at wavelength around 1100 nm, and the absorption peaks at around 410 nm, 1430 nm and 1925 nm, respectively. Compared to visible light, the near infra-red (NIR) light in the spectral range of nm can penetrate deeper into the biological tissue; the reason resides at that the light with longer wavelength is less scattered estimated using the Rayleigh scattering coefficients which is in the order of 4 1/. Because of the inhomogenous size distribution of the lipid droplets inside the tissue, the experimental results can be fitted fairly accurately as shown in Figure 2.1 (b) at wavelength longer than 700 nm using a combined contribution from Mie scatterers and Rayleigh scatters, in essence, the scattering decays fast as wavelength increases. 30
53 Figure 2.1: (a) The absorption coefficient of in-vitro measured biological tissue between spectral range of 400 nm to 2000 nm; (b) The reduced scattering coefficients of the averaged experimental data. (Adapted from ref 29) 31
54 2.2.2 Optical Properties of Blood Human blood is composed of blood cells suspended in a liquid called plasma, among these, 55% of the entire blood volume is plasma which contains 90% of water. The blood cells are composed of 99% of erythrocytes (red blood cells), and 1% of leucocytes (white blood cells) and thrombocytes (blood platelets), which in total take up the rest of 45% volume ratio of blood. Inside the red blood cells, 97% of the dry content is the ion-containing protein, which is responsible for transportation of oxygen and other respiratory gases, called hemoglobin. Therefore understanding the optical properties of oxyhemoglobin and deoxygenated hemoglobin can help in better designing the molecular probes. The level of oxygen saturation in the red blood cells can affect its absorption of light especially in the wavelength range from 590 nm to 1150 nm. A. Roggan et al. have investigated the optical absorption of red blood cells with various levels of oxygen saturation as a function of wavelength, which is cited in Figure It is seen at the wavelength around 1100 nm, where the overall absorption from both oxygenated and deoxygenated hemoglobin has a dip. With this information, it indicates a better light delivery to the molecular probes with less disturbance from the blood cells could happen at the vicinity of that wavelength. By keeping this design rule in mind, we designed and synthesized photothermal stable plasmonic nanoparticle resonant at 1064 nm, which also matches the excitation wavelength of the Nd:YAG laser commonly used in the lab and provide easy calibration of the designed molecular probe. 32
55 Figure 2.2: The optical spectrum of oxygenated and deoxygenated diluted blood. (Adapted from Ref 30) 2.3 SYNTHESIS OF GOLD NANORODS CTAB stabilized gold nanorods were synthesized by seed-mediated growth following Jana et al. 31 and Nikoobakht et al. 32 : 5 ml of CTAB (aq) solution (0.20 M) was first mixed with 5 ml of HAuCl 4(aq) solution (0.5 mm). Then, 0.60 ml of ice-cold NaBH 4(aq) solution (0.01 M) was added to the mixture and vigorously stirred for 2 min at 25 C, which resulted in the formation of a brownish yellow seed solution. The growth solution was made by adding ml AgNO 3(aq) (4 mm) and then 5 ml of HAuCl 4(aq) (1 mm) solutions to 5 ml of CTAB (aq) (0.20 M) solution, under gentle mixing, followed by 70 L of ascorbic acid ( M) solution. To grow nanorods, 12 L of the seed solution was added to the growth solution at C under gentle stirring for 30 seconds. The transparency of the solution changed to burgundy red within min. The solution then aged for another 12 hours at C, before being centrifuged at 18,000 g for 45 min, twice. The collected nanorods were re-dispersed in deionized water and ultrafiltrated (18 mωcm). In the second step, the stabilization agent, CTAB, on the 33
56 surface of the gold nanorods was replaced by mpeg-thiol through ligand exchange. Briefly, the CTAB-stabilized gold nanorod dispersion was added to an equal volume of mpeg-thiol (0.2 mm) aqueous solution under vigorous stirring. The mixture was sonicated for 5 minutes and left to react for 2 hours. Excess mpeg-thiol molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3000 g for 10 min and the PEGylated gold nanorods were re-suspended in water. 2.4 CHARACTERIZATION OF GOLD NANORODS Optical properties of gold nanoparticles are characterized by ultraviolet to visible (UV-Vis) extinction spectroscopy. Extinction spectra are collected from a 100 L nanoparticle suspension in a 96-well microliter plate reader (BioTek Synergy HT). The shape and morphology changes of the gold nanorods are monitored by transmission electron microscopy (TEM) imaging. For TEM imaging, a drop of the gold nanorod suspension is placed on copper-formvar grids and blotted dry with a filter paper. The grids are imaged using the TEM mode of a Hitachi S-5500 FESEM equipped with a field emission electron source operated at 30 kv. 2.5 RESULTS AND DISCUSSIONS Since the longitudinal resonance of the gold nanorods is mainly governed by its aspect ratio ratio, resonance wavelength tuning with respect to different aspect ratios are studied and numerical results are listed in Figure 2.3 using the DDA method. In the numerical method involved using discrete dipole approximation (DDA), an open-source software package DDSCAT 7.0 written in Fortran 90, which was first developed by Draine and Flatau is employed. The calculation is carried out with implementation of a new particle description developed by the author. In the DDA calculated spectra, we considered all materials to be at room temperature 25 C, and employed the refractive 34
57 index of gold, water solution and synthesized amorphous silica has been described in literatures. Because all gold nanorods are PEGylated as described in the synthesis section, the gold nanorods considered here are core-shell structures with a gold core and a thin Polyethyleneglycol (PEG) shell. The refractive index of PEG is chosen as an approximated value of 1.42 at all wavelengths, which is an intermediate dielectric coating when further surface modification of bare gold nanorods is needed such as coating the gold nanorods with a silica cell, which will be detailed in the next chapter. Because of the limitation of the computational facility, 2647 dipoles are calculated, for further improvement of the accuracy of this calculation, dipole number in the order of 104 is preferred. Figure 2.3 illustrates the change of the longitudinal resonance of three gold-peg core-shell nanorods with the same effective radius of 17 nm, but different aspect ratios (AR) of , and , respectively. We have set an initial aspect ratio of 3.5 according to the estimation from the experimental SEM images of nanorods synthesized in the lab as an input in DDSCAT. The aspect ratio here is defined as: 2R L AR 2R (11) where L is the length of the nanorod without counting the end caps, and R is the radius of the end caps. In the calculation, after discretising the particles into point dipoles, the actual aspect ratio ( AR ' ) which corresponds to the calculated positions of the resonance wavelengths are given as output. In the program, the aspect ratio is defined as: NLAY AR' 1 (12) NFAC 2 where NLAY denotes the number of slices (layers) in the cylinder portion of the nanorod after slicing the target into discrete pieces, and NFAC represents number of single 35
58 unit cells (point dipoles) in each slice of the inner core material, which is gold in this case. One will notice that the amount of off-set of the actual calculated AR will deviate from the initial set value of AR depending on the density of dipoles evaluated in the calculation. The denser the point dipoles are, the less deviation it will be. Figure 2.3 (b) shows the sensitivity of the peak wavelength shift with respect to slight change of the aspect ratio, which also coincides with the approximate linear relationship between the aspect ratio and the plasmon band shift as observed in non-coated gold nanorods. Figure 2.4 shows the TEM images obtained for the size reduced gold nanorods using the slow growth seed mediated method discussed in the synthesis section. By proper control of the ph value in the growth solution, a successful size reduction was achieved from an average length of 125 nm gradually down to an average length of 65 nm. The achieved shorter rod length provides the possibilities of better circulation of the nanorods as molecular probes inside the biological target. 36
59 (a) (b) (a) (a) (b) Figure 2.3: (a) Longitudinal plasmon spectra with different aspect ratios of the coreshell structured nanorods; (b) Linear relationship of the aspect ratio of the rod versus the peak wavelength. 37
60 ph ph 50 nm 50 nm 50 nm Average length:125nm Average length: 90nm Average length:65nm HAuCl 4 NaBH 4 CTAB Seeds Ascorbic acid AgNO 3 CTAB HCl [ph] Figure 2.4: Slow growth seed mediated method for nanorod synthesis. The TEM images show the nanorod dimension has been successfully reduced from an average length of 125 nm to 65 nm. 38
61 2.6 REFERENCES 1 Yguerabide, J. & Yguerabide, E. E. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications - II. Experimental characterization. Analytical Biochemistry 262, , (1998). 2 Kumar, S., Harrison, N., Richards-Kortum, R. & Sokolov, K. Plasmonic nanosensors for imaging intracellular biomarkers in live cells. Nano Letters 7, , (2007). 3 Aaron, J., Travis, K., Harrison, N. & Sokolov, K. Dynamic Imaging of Molecular Assemblies in Live Cells Based on Nanoparticle Plasmon Resonance Coupling. Nano Letters 9, , (2009). 4 Chang, S. S., Shih, C. W., Chen, C. D., Lai, W. C. & Wang, C. R. C. The shape transition of gold nanorods. Langmuir 15, , (1999). 5 Lee, K. & El-Sayed, M. A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. Journal Of Physical Chemistry B 110, , (2006). 6 Kumar, S., Aaron, J. & Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nature Protocols 3, , (2008). 7 Mallidi, S., Larson, T., Aaron, J., Sokolov, K. & Emelianov, S. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Optics Express 15, , (2007). 8 Li, P. C. et al. In vivo Photoacoustic Molecular Imaging with Simultaneous Multiple Selective Targeting Using Antibody-Conjugated Gold Nanorods. Optics Express 16, , (2008). 39
62 9 Mallidi, S. et al. Multiwavelength Photoacoustic Imaging and Plasmon Resonance Coupling of Gold Nanoparticles for Selective Detection of Cancer. Nano Letters 9, , (2009). 10 Shah, J. et al. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. Journal of Biomedical Optics 13, 9, (2008). 11 Sethuraman, S., Aglyamov, S. R., Smalling, R. W. & Emelianov, S. Y. Remote temperature estimation in intravascular photoacoustic imaging. Ultrasound in Medicine and Biology 34, , (2008). 12 Shah, J., Aglyamov, S. R., Sokolov, K., Milner, T. E. & Emelianov, S. Y. Ultrasound imaging to monitor photothermal therapy - Feasibility study. Optics Express 16, , (2008). 13 West, J. L. & Halas, N. J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annual Review of Biomedical Engineering 5, , (2003). 14 Kelly, K. L., Coronado, E., Zhao, L. L. & Schatz, G. C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. Journal Of Physical Chemistry B 107, , (2003). 15 Jain, P. K., Lee, K. S., El-Sayed, I. H. & El-Sayed, M. A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. Journal Of Physical Chemistry B 110, , (2006). 16 Wood, R. W. XLII. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Philosophical Magazine Series 6 4, , (1902). 40
63 17 Garnett, J. C. M. Colours in Metal Glasses and in Metallic Films. Philosophical Transactions of the Royal Society of London. Series A, Containing Papers of a Mathematical or Physical Character 203, , (1904). 18 Strutt, J. On the light from the sky, its polarization and colour,. Philosophical Magazine 41, , (1871). 19 Strutt, J. On the scattering of light by small particles. Philosophical Magazine, 41, , (1871). 20 Strutt, J. On the electromagnetic theory of light. Philosophical Magazine 12, , (1881). 21 Strutt, J. On the transmission of light through an atmosphere containing small particles in suspension, and on the origin of the blue of the sky. Philosophical Magazine 47, , (1899). 22 Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Annalen der Physik 330, , (1908). 23 Pines, D. Collective Energy Losses in Solids. Reviews of Modern Physics 28, , (1956). 24 Hopfield, J. J. Theory of the Contribution of Excitons to the Complex Dielectric Constant of Crystals. Physical Review 112, , (1958). 25 Ritchie, R. H., Arakawa, E. T., Cowan, J. J. & Hamm, R. N. Surface-Plasmon Resonance Effect in Grating Diffraction. Physical Review Letters 21, , (1968). 26 Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Zeitschrift für Physik A Hadrons and Nuclei 216, , (1968). 41
64 27 Kahnert, F. M. Numerical methods in electromagnetic scattering theory. Journal of Quantitative Spectroscopy and Radiative Transfer 79 80, Zhao, J. et al. Methods for Describing the Electromagnetic Properties of Silver and Gold Nanoparticles. Accounts of Chemical Research 41, , (2008). 29 Bashkatov, A. N., Genina, E. A., Kochubey, V. I. & Tuchin, V. V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. Journal of Physics D-Applied Physics 38, , (2005). 30 Roggan, A., Friebel, M., Dorschel, K., Hahn, A. & Muller, G. Optical properties of circulating human blood in the wavelength range NM. Journal of Biomedical Optics 4, 36-46, (1999). 31 Jana, N. R., Gearheart, L. & Murphy, C. J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Advanced Materials 13, , (2001). 32 Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chemistry of Materials 15, , (2003). 42
65 Chapter 3: Design and Preparation of Photostable Photoacoustic Nanocontrast Agents During photoacoustic imaging, gold nanorods are exposed to high-energy nanosecond laser pulses. The nanorods absorb that light and generate substantial heat that can lead to nanorod reshaping and an associated reduction in absorption cross section. For the purpose of photothermal therapy guided by molecular photoacoustic imaging and ultrasound, it is highly desirable to have plasmonic nanoabsorbers with a more photothermally stable form, which can be a coating that is chemically easy to modify. However, nanoparticle melting has been shown for nanospheres and nanorods to occur at significantly lower temperatures than bulk melting of the metal, in part because surface reorganization processes dominate 1-4 (Figure 3.1). Results of numerical simulations indicate that for thin nanorods, the surface melting can extend into the interior 5. Nanorods held at fixed temperatures can already melt and form spheres below 100 C to 250 C depending on the surface coating and the environment in which they are embedded 3,6. Several studies have shown that embedding the nanorods in a solid environment, such as carbon or PMMA, significantly increases photothermal stability; in the latter case below the glass transition temperature only 6-7 Silica coating of nanorods has been shown based on several strategies 8-12, and silica can easily be used for bioconjugation In this chapter, we provide a detailed study of the stability of silica-coated gold nanorods of different shell thickness under nanosecond laser pulse, and compare the results to the photothermal stability of CTAB, and PEG-coated nanorods. Among these new developing techniques, molecular specific photoacoustic and ultrasound image-guided photothermal therapy was proposed to treat various medical conditions, such as cancer. The photothermal therapy relies on the resonant absorption of light by the nanoparticle and the conversion of the electromagnetic energy into heat to 43
66 destroy malignant tissue. At the same time, nanoparticle-facilitated absorption of pulsed light leads to the production of sound waves used for photoacoustic imaging. In imageguided photothermal therapy, photoacoustic imaging can be used both to confirm the delivery of MNPs to the desired location in tissue and to visualize temperature maps during photothermal therapy. The absolute as well as relative strength of the surface plasmon resonant absorption and scattering that contribute to the well defined extinction band of gold or silver MNPs depend on the nanoparticle material composition, shape, and environment. While large scattering cross sections are needed for dark-field imaging, large absorption cross sections are required for photoacoustic imaging. Therefore nanospheres, nanoshells, nanocages, nanoplates and nanorods are used in photoacoustic imaging. Gold nanorods are particularly interesting as they are small, easy to synthesize, have a tunable resonance in the red and NIR spectrum, and posses a very high absorption cross section. 3.1 SYNTHESIS OF SILICA COATED GOLD NANORODS The stabilization agent, CTAB, on the surface of the gold nanorods was replaced by mpeg-thiol through ligand exchange. Briefly, the CTAB-stabilized gold nanorod dispersion was added to an equal volume of mpeg-thiol (0.2 mm) aqueous solution under vigorous stirring. The mixture was sonicated for 5 minutes and left to react for 2 hours. Excess mpeg-thiol molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the PEGylated gold nanorods were resuspended in water. A modified Stöber method 9,15 was used to grow a silica shell of controlled thickness on the PEGylated gold nanorods or polyelectrolyte-coated gold nanorods. The gold nanorod suspension (1.2 ml) was added under vigorous stirring to 1.8 ml of isopropanol, then an ammonia solution (3.8%) in isopropanol was added 44
67 slowly under vigorous stirring until the solution reached ph = 11. Finally, 0.04 ml 0.40 ml of a solution of TEOS in isopropanol (100 mm) was added under gentle stirring, depending on the desired shell thickness. The reaction mixture was allowed to react for 2 hours. The above procedure produces silica shells with an adjustable thickness from 6 nm to 20 nm. The 75 nm coating was achieved by extending the reaction time to 8 hours with the highest concentration of TEOS (0.4 ml of TEOS). 3.2 CHARACTERIZATION OF SILICA COATED GOLD NANORODS The optical property and morphology of silica coating was characterized using method described in the previous chapter. The nanorod stability under nanosecond pulsed laser exposure was optically characterized by ultraviolet to visible (UV-Vis) extinction spectroscopy. Extinction spectra were collected from a 50L nanorod suspension in a 96-well microliter plate reader (BioTek Synergy HT) at room temperature before and after laser exposure. An 800 nm wavelength laser beam, generated by a tunable OPO laser system (Vibrant, OPOTEK, Inc.), was collimated to fully illuminate each well from the top. The experimental set up is schematically illustrated in Figure 3.2 (a). Three hundred pulses of 7 ns pulse duration at 10 Hz repetition rate were applied. 3.3 RESULTS AND DISCUSSIONS It is known that gold nanorods without any surface modification are highly unstable under high fluence laser irradiation. The experimental set-up for testing the thermal instability of bare gold nanorods is introduced in section 3.2, and schematically shown in Figure 3.2 (a). After 300 laser pulse irradiation, with a laser pulse width at 7ns and fluence at 20mJ/cm 2, it is seen from the extinction spectra that the resonance peak 45
68 Absorbance Absorbance (a) Before 50nm (b) Wavelength (nm) After 50nm Wavelength (nm) 900 Wavelength (nm) Figure 3.1: Measured UV-Vis extinction spectra of (a) gold nanorods before 300 laser pulse irradiation, and (b) same gold nanorods after 300 laser pulse irradiation. The insets display corresponding TEM images. 46
69 (a) Mirror Pulsed Laser Radiation 7 nsec, 10 Hz, 300 Pulses (b) Sample 96 Well Plate PA signal characterization Transducer Thermal Meter 7.5 MHz F=25.4mm F/#=2 Optical Window Pulsed Laser Glass Tube and Sample Figure 3.2: Experimental set up for examining of the thermal stability of uncoated and coated gold nanorods. (a) The 96 well set up; (b) photoacoustic signal calibration set up. 47
70 shifts to a shorter wavelength. TEM measurement confirms the morphology change of the bare gold nanorods under such fluences. The results reaffirm that the surface modification, such as coating the gold nanorods with silicon dioxide is highly desired. It is relevant to study the spectra change, especially the shift of resonance with the silica coating numerically, because it can provide guidance in designing the desired molecular probe. With the power of numerical simulation methods introduced in chapter 2, especially when comparing the time consumption between DDA and FDTD, numerical calculations using DDA are first conducted to investigate the sensitivity of the longitudinal plasmon resonance shift due to different thickness of the silica coating with fixed aspect ratio and effective radius of the core. A series of silica coated gold nanorods with coating thickness varying from 0 to 2, 4, 8, 16 and 24nm are studied using DDA. Calculated extinction efficiency and the resonance wavelength shift with respect to the shell thickness are shown in Figure 3.3 (a) and (b), respectively under these circumstances. The peak locations were estimated by fitting the data with a nonlinear Lorentzian function. Statistic data show the sensitivity of the shift due to the change of the coating thickness decreases with thicker silica, and closes to saturation when the thickness is in the orders of the effective radius of the nanorod which is similar as observed in silica coated gold nanospheres. Besides the shell thickness effect on the shift of resonance, simulation also shows that the change of composite of the shell material can also result in noticeable shift of the resonance wavelength. When the coating material changes from bare water solution with index of refraction around 1.33, to PEG (n~ 1.42) and to silica (n~1.45), significant shifts of the peak positions are observed in the simulated data which agrees well with the spectra measured of lab synthesized core-shell nanorods with the corresponding geometric and composite parameters. Figure 3.4 (a) shows the agreement of the 48
71 simulation data with the experimental measured spectra of both gold-peg and gold-silica core-shell nanorods using DDA. The simulation result of the longitudinal plasmon resonance wavelength for the PEG coated nanorod is nm, which is 1.3% off the experimental data of 782 nm, while for the silica coated gold nanorod, the simulation result of nm well matches the experimental data of 804nm. Another noticeable difference is the much broadened linewidth of the peak measured in the lab compared with the simulation, which is caused by the normal distribution of the particle size and randomness of the particle orientation while suspended in the chemical solution. An SEM image of such silica coated nanorods is shown in the center image of Figure 3.4 (b). A typical UV-Vis extinction spectrum and a corresponding TEM image of gold nanorods with 10 nm of silica shell are shown in Figure 3.5. It is seen from the TEM image that the nanorods are uniformly coated with silica. As expected, the UV-Vis spectrum has two extinction bands at 530 nm (transverse with polarization perpendicular to the long axis) and at ~800 nm (longitudinal with polarization along the long axis). While the transverse peak does not shift significantly from bare PEG-coated to silicacoated nanorods, the longitudinal resonance shifts by ~20 nm (Figure 3.5 (a)). A FDTD simulation of the gold nanorods before and after silica coating is also shown in Figure 3.5 (a). The experimental spectra are significantly broader due to some inhomogeneity of the aspect ratio 16. The peak position coincides for a nanorod length of 35 nm and a diameter of 9 nm, which is very close to the main experimental size and aspect ratio. The peak position of the simulated spectrum for the silica-coated nanorod is further to the red than seen in the experiment, which is due to a more porous nature compared to the fused silica assumed in the simulation. 49
72 (a) (b) (a) ) (b) Figure 3.3: (a) Normalized extinction efficiency spectra reveal the change of resonance wavelength with respect to a change in the thickness of the silica shell. (b) The shift of peaks as a function of silica thickness show increasing wavelength with increasing thickness and saturation of the trend when the thickness is around 3 times of the effective radius of the gold nanorod core. 50
73 Figure 3.4: (a) the comparison of simulation and experimental results of longitudinal surface plasmon spectra of PEG-coated gold nanorod and silica-coated gold nanorod, the experimental data (in solid black and red curves) well matches the experimental data (in red and black dots) for both kinds of nanorods. The nanorod has an aspect ratio of 3.127, with thickness of the PEG layer around 8nm, and silica coating around 24nm. The effective radius of the gold nanorod in the core is 8.145nm. (b) Shows left to right: SEM image of bare gold nanorod with around 40nm length and 10 nanometer diameter end-caps; SEM image of the silica coated gold nanorod which gives spectra in red curve of Figure 3 (a); Element mapping of a silica coated gold nanorod showing different composite of the core-shell nanorod (Hitachi S Scanning Electron Microscope/Scanning Transmission Electron Microscopy) 51
74 Normalized Extinction (a) (b) Wavelength (nm) (a) (b) 50 nm Wavelength (nm) Figure 3.5: (a) Experimental and simulated extinction spectra of the PEG-coated gold nanorods, and silica-coated gold nanorods, where solid blue ( ) and solid red ( ) curves correspond to experimental data of PEG-coated and silicacoated gold nanorods. The dotted blue (----), dotted red (----), and dotted green (----) curves correspond to FDTD calculated data of PEG-coated, porous silica-coated, and fused silica-coated gold nanorods, respectively. (b) TEM image illustrates one example of the silica-coated gold nanorods produced. 52
75 The thermal stability of CTAB and PEG coated gold nanorods, as well as silicacoated gold nanorods with 6 nm and 20 nm thickness was compared using UV-Vis spectroscopy. The longitudinal plasmon peak is a good indicator for shape changes of the nanorods, because the peak position strongly depends on the aspect ratio 17. Indeed, after irradiating the aqueous nanorod solutions with 300 laser pulses at various fluences, the longitudinal peak changes shape in all cases above some threshold fluence (Figure 3.6). Fluences below 4 mj/cm 2 did not induce any spectral changes of the longitudinal peak in all cases. However, fluences above 8 mj/cm 2 induced a 10% amplitude decrease for the CTAB-coated gold nanorods, while no changes were observed for the PEG-coated and silica-coated nanorods. Further increase of the fluence led to a dramatic decrease of the amplitude, a strong shift to the blue of the longitudinal peak, and a strong increase of the absorption in the nm range, which all are consistent with a rounding of the gold nanorods. Bare PEG-coated nanorods showed the same spectral trends as CTAB-coated nanorods, only at slightly elevated fluences starting at 12 mj/cm 2. In contrast, already a 6 nm silica shell induced a stabilizing effect, although above 12 mj/cm 2 the peak also decreases and shifts to the blue at a similar rate as for the PEG-coated nanoparticles. However, no shoulder in the nm range develops here, indicating a different process of change and possible material loss. The 20 nm silica-coated gold nanorods, in contrast, are very robust and show no change in the spectrum under pulsed laser exposure, and only show a small spectral change of ~10 % above 750 nm at 20 mj/cm 2 (Figure 3.6 (d)). It is therefore clear that the uncoated gold nanorods change shapes due to the nanosecond laser pulses, lowering their aspect ratio. Thick silica coating protects the gold nanorods, while thin silica coating shows only a limited protection, and material may be lost when the aspect ratio is reduced. 53
76 Absorbance Extinction Absorbance Extinction Extinction Extinction mJ/cm 2 4mJ/cm 2 8mJ/cm 2 12mJ/cm 2 16mJ/cm 2 20mJ/cm mJ/cm 2 4mJ/cm 2 8mJ/cm 2 12mJ/cm 2 16mJ/cm 2 20mJ/cm Wavelength (nm) (a) Wavelength (nm) (b) mJ/cm 2 2 4mJ/cm 4mJ/cm 2 2 8mJ/cm 8mJ/cm mJ/cm mJ/cm 2 16mJ/cm mJ/cm 2 20mJ/cm mJ/cm mJ/cm 2 4mJ/cm 2 8mJ/cm 2 12mJ/cm 2 16mJ/cm 2 20mJ/cm (nm) Wavelength (nm) (nm) (a) (c) (b) Wavelength (nm) Wavelength (nm) (b) (d) Figure 3.6: Measured UV-Vis extinction spectra of (a) CTAB-coated gold nanorods, (b) PEGylated (PEG-coated) gold nanorods, (c) 6 nm silica-coated gold nanorods, and (d) 20 nm silica-coated gold nanorods before and after laser irradiation with various fluences. 54
77 Comparisons between the morphology change and the corresponding extinction spectra have been conducted. Figure 3.7 shows TEM images corresponding to the UV- Vis spectra in Figure 3.6 for PEG-, 6 nm and 20 nm silica-coated gold nanorods before and after 300 laser pulses with 7ns pulse width at a fluence of 20 mj/cm 2. PEG-coated gold nanorods changed from rod-shaped to either spherical, ellipsoidal, or " " shape an ellipsoid with an equatorial thickening first observed by Chang 1 (Figure 3.7 (a-b)). In contrast, already a silica coating of around 6 nm improved the shape stability, and only few cases of severe shape change were observed (Figure 3.7 (c-d)). Instead, the pulsed laser irradiation caused a decrease in length and therefore aspect ratio of the gold cores from 3.9 ± 0.38 to 3.0 ± 0.25 (n=100). This can explain the blue shift of the plasmon peak in the spectrum (Figure 3.6 (c)). A further increase in the silica thickness to around 20 nm nearly completely stabilized the rod shape, and almost no shape deformation was observed after laser irradiation. Additionally, the heating of the gold core by the nanosecond pulsed laser irradiation did not cause any apparent morphological changes to the silica layer for either the 6 nm or the 20 nm thick shells. FDTD simulations were performed to calculate extinction and absorption cross sections to confirm the interpretation of the experimental results, and the results are summarized in Figure 3.8. Unprotected gold nanorods form ellipsoidal particles upon strong pulsed heating, which strongly shifts the resonance to the blue. Ellipsoidal particles were assumed to have the same volume as the original nanorod, and are labeled here by the fraction of the long axis relative to the original nanorod length. Even pure rounding without changing the length (100%) already causes a strong shift to the blue. Further rounding with shortening of the long axis leads to resonances in the nm range with strong shoulders to the blue (75% - 45%). Therefore already a small 55
78 After pulsed laser irradiation Before pulsed laser irradiation (a) PEG-coated Au NRs 6 nm silica-coated Au NRs 20 nm silica-coated Au NRs (c) (e) 50 nm 50 nm (b) (d) (f) 50 nm 50 nm Figure 3.7: TEM images show the morphology evolutions of various gold nanorods before and after 300 pulses of 20mJ/cm 2 laser irradiation. (a) and (b): PEG coated gold nanorods; (c) and (d): 6 nm silica-coated gold nanorods; (e) and (f): 20 nm silica-coated gold nanorods. Absorbtion Cross Section (x m 2 ) % Fused silica -1.5 nm Porous silica 90% -3 nm Gold -4.5 nm 75% 60% 45% Wavelength (nm) M N O P Q R S T U V W Figure 3.8: FDTD simulated absorption cross section spectra for unpolarized light for various gold nanoparticles. Ellipsoids have the same volume as the original nanorod and are labeled as the fraction of the long axis relative to the original nanorod length. Shortened silica-coated nanorods are labeled for their reduction at each end. For details see the text. 56
79 rounding effect leads to the increased absorption in the wavelength range of nm seen for CTAB and PEG-coated gold nanorods. A closer examination of the TEM images of the 6 nm silica-coated gold nanorods shows a shrinking of the gold nanorod core in length due to the laser-pulse exposure, but the silica shell retained its shape, thus creating a small gap between the gold and silica at the tips of the rod (Figure 3.9). The fractions of nanorods exhibiting this gap formation are outlined in yellow in Figure 3.9 (a). A similar hollow cap region between the gold nanorod and its silica coating is not found with the thicker silica coating. Simulations of such nanorods are shown in Figure 3.8. Different from the simulation of the ellipsoids, only the long axis of the gold nanorods was shortened without changing the shape, while keeping the silica shell unchanged, and filling the void with water. Only very small reductions in nanorod length of 1.5, 3.0 and 4.5 nm at each end were simulated. These relatively small changes agree with the TEM images, and are sufficient to induce a blue shift of the resonance by about 50 nm and 100 nm, respectively. Shifts of this magnitude for such shortened nanorods in a silica shell confirm the spectra seen in Figure 3.4. Most prominently, the resonances remain symmetric except for 4.5 nm reduction at each end, and the amplitudes do not decay as quickly; removing 3 and 5 nm from each end corresponds to 17% and 29% length reduction, respectively, and gives about twice the cross section compared to ellipsoids of similar length (Figure 3.8). Also evidence from the images is that the 6 nm silica-coated nanorods appear to form aggregates with fused silica shells, which is not found for either PEG-coated or 20 nm silica-coated nanorods. We assume this aggregation is induced by centrifugation, but additional investigation is required to completely define the cause. 57
80 (a) 50 nm (b) 15 nm Figure 3.9: (a) The TEM image illustrates the 6 nm silica-coated gold nanorods after irradiated by 300 pulses of 20 mj/cm 2 laser light; the yellow circles indicate the nanorods which have hollow gaps between gold and silica. (b) The zoom-in view of the hollow gaps of a silica-coated gold nanorod. The image shows the gaps were formed in the tips and about 2 nm. 58
81 The capability to produce a stable photoacoustic response is critical to photoacoustic image-guided photothermal therapy, since the photoacoustic contrast and the photoacoustic temperature mapping are based on the assumption that the absorbance of the photoabsorbers remains the same during imaging. The photoacoustic contrast depends on the difference in photoabsorber concentration and, at a constant concentration; the temperature induced photoacoustic signal change is due to the change of the temperature dependent Grüeneisen coefficient. Clearly, a shape change due to the pulsed laser irradiation would cause false temperature measurements due to the change in absorption cross section. The stability of the photoacoustic response was investigated by measuring the photoacoustic amplitude generated by PEG-coated and 20 nm silica-coated gold nanorods during exposure to 300 pulses at fluences of 4 mj/cm 2 and 18 mj/cm 2 (Figure 3.10), and the experimental set up is illustrated in Figure 3.2 (b). For both types of nanorods the photoacoustic amplitude was stable at 4 mj/cm 2 as expected, because this fluence is below the damage threshold as seen in the UV-Vis spectra (Figure 3.6). At 18 mj/cm 2, the signal from PEG-coated nanorods dropped by 40% in the first 100 pulses, while the 20 nm silica-coated gold nanorods produced a stable signal over the full 300 pulses. Therefore, gold-silica core-shell nanorods are a promising candidate for photothermal therapy, photoacoustic imaging, and molecular imaging. Several mechanisms have been suggested for the increased stability seen with the silica-coated nanorods. The covalent binding of the PEG-thiol already leads to an increased stability, indicating its role in the stabilization of surface gold atoms or a change in the interfacial heat resistance; the influence of the chemical nature of the environment close to the nanorod surface has been shown 18. PEG induced stability also agrees with the increased thermal stability of PVA stabilized nanorods 3. Another factor could be the steric barrier created by a rigid encapsulation, as has been shown for carbon 59
82 and PMMA 7. Especially the fact that PMMA only stabilized the rods significantly below the glass transition would speak for such a mechanism, as would our result that shows an undeformed silica shell with a shortened gold core. Silica stabilization of gold nanorods has also been demonstrated under annealing up to 200 C, which also showed a small change in the structure of the silica as judged by the longitudinal plasmon peak position 11. The slight red shift seen in these silica-coated nanorods synthesized using the layer-by-layer technique, is not found in our experiments. This difference may be due to our coupling agent we use PEG thiol that is both chemically bound to the gold and the silica, or to the time-scale of the heating. Finally, a change in the thermal conductivity could lead to an improved cooling due to a more efficient heat transfer. The influence of the heat transfer to the environment on the stability of nanorods has been shown for femtosecond laser pulses 3,19. Silica has a three times higher thermal diffusivity compared to water and the thickness-dependent thermal stability enhancement we have found could be in part be due the faster heat distribution over a larger area. However, the thermal relaxation time which is defined as d 2 / with being the thermal diffusivity, is still shorter than the pulse width of the laser even for a thickness of d 20 nm for both water and silica. In addition, the more porous structure of silica fabricated by the Stöber method is not considered, which has been shown to play an important role 19. The reduction in nanorod length at high laser irradiation may suggest that gold nanorods may shed atoms, an effect seen with silver but not with gold 7. 60
83 Normalized Intensity Normalized Intensity (a) Number of Pulses (b) Number of Pulses Figure 3.10: Photoacoustic signal intensity of PEG-coated gold nanorods (red symbols) and silica-coated gold nanorods (blue symbols) versus number of pulses with fluence (a) 4 mj/cm 2 and (b) 18 mj/cm 2. 61
84 3.4 REFERENCES 1 Chang, S. S., Shih, C. W., Chen, C. D., Lai, W. C. & Wang, C. R. C. The shape transition of gold nanorods. Langmuir 15, , (1999). 2 Mohamed, M. B., Ismail, K. Z., Link, S. & El-Sayed, M. A. Thermal reshaping of gold nanorods in micelles. Journal Of Physical Chemistry B 102, , (1998). 3 Petrova, H. et al. On the temperature stability of gold nanorods: comparison between thermal and ultrafast laser-induced heating. Physical Chemistry Chemical Physics 8, , (2006). 4 Plech, A., Kotaidis, V., Gresillon, S., Dahmen, C. & von Plessen, G. Laserinduced heating and melting of gold nanoparticles studied by time-resolved x-ray scattering. Physical Review B 70, , (2004). 5 Wang, Y. T., Teitel, S. & Dellago, C. Surface-driven bulk reorganization of gold nanorods. Nano Letters 5, , (2005). 6 Liu, Y., Mills, E. & Composto, R. Tuning optical properties of gold nanorods in polymer films through thermal reshaping. Journal of Materials Chemistry 19, , (2009). 7 Khalavka, Y., Ohm, C., Sun, L., Banhart, F. & Soennichsen, C. Enhanced thermal stability of gold and silver nanorods by thin surface layers. Journal Of Physical Chemistry C 111, , (2007). 8 Jana, N. R., Gearheart, L. & Murphy, C. J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Advanced Materials 13, , (2001). 62
85 9 Pastoriza-Santos, I., Perez-Juste, J. & Liz-Marzan, L. M. Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chemistry of Materials 18, , (2006). 10 Heitsch, A., Smith, D. K., Patel, R., Ress, D. & Korgel, B. A. Multifunctional particles: Magnetic nanocrystals and gold nanorods coated with fluorescent dyedoped silica shells. Journal Of Solid State Chemistry 181, , (2008). 11 Omura, N., Uechi, I. & Yamada, S. Comparison of Plasmonic Sensing between Polymer- and Silica-coated Gold Nanorods. Analytical Sciences 25, , (2009). 12 Gorelikov, I. & Matsuura, N. Single-step coating of mesoporous silica on cetyltrimethyl ammonium bromide-capped nanoparticles. Nano Letters 8, , (2008). 13 Kumar, S., Aaron, J. & Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nature Protocols 3, , (2008). 14 Chen, Y.-S. et al. in SPIE Photonics West Chen, Y. S. et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Optics Express 18, , (2010). 16 Qiu, L. et al. Single gold nanorod detection using confocal light absorption and scattering spectroscopy. Ieee Journal of Selected Topics in Quantum Electronics 13, , (2007). 17 Lee, K. & El-Sayed, M. A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. Journal Of Physical Chemistry B 110, , (2006). 63
86 18 Mohamed, M. B., Ahmadi, T. S., Link, S., Braun, M. & El-Sayed, M. A. Hot electron and phonon dynamics of gold nanoparticles embedded in a gel matrix. Chemical Physics Letters 343, 55-63, (2001). 19 Hu, M., Wang, X., Hartland, G. V., Salgueirino-Maceira, V. & Liz-Marzan, L. M. Heat dissipation in gold-silica core-shell nanoparticles. Chemical Physics Letters 372, , (2003). 64
87 Chapter 4: Characterization of Photoacoustic Nanoamplifier The conventional strategy of designing plasmonic photoacoustic contrast agents is usually to maximize the optical absorption cross-section of nanoparticles by tailoring their size and shape, and to tune the peak position and the relative amplitude of the absorption and scattering cross-sections 1-2. Photoacoustic imaging has been successfully demonstrated using plamonic nanomaterials such as gold nanorods, nanocages, and nanoshells with high and tunable optical absorption cross-sections 3-4. In chapter 3, we have presented a route to prepare the photostable photoacoustic nano-contrast agent by embedding gold nanorods in non-absorbing shell materials, such as silica and demonstrated that silica coating indeed can enhance the stability of the gold nanorods, thus maintaining their photoacoustic responses. In this chapter, we investigate how the silica coating affects the photoacoustic amplitude of gold nanorods. The results show that silica shell can boost the photoacoustic signal. We further investigate the origin of this photoacoustic signal amplification. We found the silica shell alters the optical property of nanoparticles very insignificantly and the signal enhancement is mainly attributed from the heat transfer mechanism from the gold nanoparticle to the ambient signal-generating medium. This finding introduces a new approach to significantly increase the contrast-tonoise ratio (CNR) of contrast-enhanced nanoparticle-augmented photoacoustic imaging. 4.1 PREPARATION AND CHARACTERIZATION OF NANOPARTICLES PEGylated and Silica coated gold nanorods were prepared using the method described in chapters 2 and 3. Multilayer electrolyte coated gold nanorods were synthesized by following the procedure reported by Pastoriza-Santos et al 5. A brief summary of the synthesis procedure is described as following. 5 ml of as-prepared CTAB gold nanorod dispersion was added drop-wise to 5 ml of PSS (2 g/l, 6 mm 65
88 NaCl) aqueous solution under vigorous stirring. Stirring was continued for 3 hours, and excess PSS molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min, and the PSS coated gold nanorods were re-suspended in 5 ml of ultra-filtrated deionized water. Thereafter, it was added drop-wise to 5 ml of PAH (2 g/l, 6 mm NaCl) aqueous solution under vigorous stirring followed by continuous stirring for 3 hours. The mixture was filtered by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min to eliminate excess PAH, and redispersed in 5 ml of ultra-filtrated deionized water. Five ml of PSS/PAH coated gold nanorods were mixed with 5 ml of PVP (MW Da, 4g/L) aqueous solution and stirred overnight. Excess PVP molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the precipitate was redispersed in 1 ml of ultrafiltrated deionized water. The optical properties, as well as shape and morphology of the particles were investigated using the methods described in section 2.4 of chapter 2 and section 3.2 of chapter ULTRASOUND AND PHOTOACOUSTIC IMAGING OF PHANTOMS A polyvinyl alcohol (PVA) tissue-mimicking phantom with four inclusions containing gelatin mixed with gold nanorods of different types, as shown in Figure 4.1 (a), was used in the imaging studies. To fabricate this phantom, a 5 mm thick slab was made of 15 wt% of PVA mixed with 0.2 wt% of silica ultrasound scatterers (40 m in diameter). Inside the slab, four plastic spacers were placed to create four molds aligned horizontally 10 mm from the top surface of the phantom. Two 12-hour freeze-and-thaw cycles were applied to crosslink PVA, the plastic spacers were removed, and the molds were filled with 200 µl of a one-to-one (vol:vol) mixture of a 10 wt% aqueous gelatin solution at 60 C and aqueous solution of gold nanorods of a particular type (PEGylated 66
89 nanorods and nanorods coated with 6, 20 and 75 nm silica). The optical density of all solutions was matched to be the same (OD = 3.0 in 1 cm optical path). Prior to imaging, the phantom was stored in the fridge at 4 C. The geometry of the phantom and imaging setup are shown in Figure 4.1 (b). The ultrasound micro-imaging system (Vevo 2100, VisualSonics, Inc.) was used to capture both ultrasound and photoacoustic signals. As shown in Figure 4.1 (c), a 40 MHz array ultrasound transducer (MS550, VisualSonics, Inc.) was mounted on a one-dimensional positioning stage. The position of the transducer was adjusted so that the inclusion was located in the focal region of the ultrasound transducer. The phantom was placed in a water cuvette with an optical window on one side. A laser beam (5 ns pulse duration, 10 Hz repetition rate) generated from a wavelength tunable OPO laser system (Premiscan, GWU, Inc.) pumped by a pulsed Nd- YAG laser (Quanta-Ray, Spectra Physics, Inc.) uniformly irradiated the phantom with inclusions through the optical window. The acquired ultrasound and photoacoustic images were captured in real-time and then further processed off-line. A quantitative analysis of the photoacoustic signal was performed to investigate the enhancement of the contrast-to-noise ratio (CNR) in photoacoustic imaging due to the silica coating of the gold nanorods. As shown in Figure 4.2, relatively large areas of the inclusion and the phantom background, further divided into 166 rectangular regions measuring 160 m 100 m, were selected. The size was chosen to be on the order of, but larger than a speckle. The average intensity of the photoacoustic signal amplitude within each region was calculated and, separately for the inclusion and the background, the average intensities of the 166 rectangular regions were used to calculate the mean value and standard error. The CNR of the photoacoustic image was calculated using the following expression: 67
90 (a) PEG 6nm 20nm 75nm (b) Array Transducer 5mm Pulsed Laser Radiation (c) Phantom with Inclusion Optical Window Nanosecond Pulsed Laser Laser Control Transmit/Receive Electronics Trigger Computer/Data Acquisition 1 D positioning Control Ultrasound Photoacoustic Image Figure 4.1: (a) Photograph of the phantom prepared for ultrasound and photoacoustic imaging. (b) Schematic illustration of the experimental setup. (c) A block diagram of the experimental setup for the combined photoacoustic and ultrasound imaging. 68
91 (a) (b) (c) (c) (d) Figure 4.2: (a) Photograph of the phantom prepared for ultrasound and photoacoustic imaging. (b) Schematic illustration of the experimental setup. (c) A block diagram of the experimental setup for the combined photoacoustic and ultrasound imaging. 69
92 2 2( Ai A ) b CNR 10log i b (4.1) 2 where Ai and i represent the mean value and variance of the photoacoustic signal in 2 the inclusion, and Ab and b represent the mean value and variance of the photoacoustic signal in the background. 4.3 PHOTOACOUSTIC SIGNAL MEASUREMENT OF GOLD NANOPARTICLES The experimental setup for measuring the photoacoustic response from nanoparticles is schematically illustrated in Figure 4.3. A single element focused ultrasound transducer (7.5 MHz center frequency, 50.4 mm focal distance, and 13 mm aperture, Panametrics Inc., V320) was mounted above the sample a thin-walled glass tube containing the particular type of nanoparticles. The sample was uniformly irradiated using 60 laser pulses (5 ns pulse duration, 10 Hz pulse repetition rate) with a fluence of 4 mj/cm 2. For each experiment, the set of 60 photoacoustic signals was captured and stored for off-line processing. The amplitude of the recorded photoacoustic signals was first compensated for the pulse-to-pulse fluctuation of the laser fluence, which was measured simultaneously. Then, the photoacoustic signal for each laser pulse was normalized to the maximum photoacoustic signal recorded within the set, and then the mean and standard deviation were computed. No systematic changes that would indicate a change in the nanorods shape were detected. 70
93 (a) Transducer (b) T Laser Control Pulsed Laser Trigger Transmit/Receive Electronics Signal Ultrasound & Photoacoustic Signal Computer/ Data Acquisition Glass Tub Au NRs Aq Soluti Figure 4.3: A block diagram of the experimental setup for the characterization of the photoacoustic response of gold nanoparticles. 71
94 The photoacoustic responses from gold nanorod suspensions in water were characterized by using the setup shown in Figure 4.4 (a). The extinction of each 100 L gold nanorod solution in a standard 96-well plate was first adjusted to 4.5 (1 cm optical path) using UV-Vis measurements. A 1 mm diameter glass tube was fixed in an acrylate water tank containing an optical window inlay. The glass tube contains an inlet and an outlet for injecting the 20 μl aliquot into the glass tube without moving the tube during the experiment. The position of the transducer was adjusted so that the injected solution within the tube was located in the center of the ultrasound beam, and the distance between the transducer and the glass tube was kept constant during the entire experiment. To exchange the solvent from water to oil, 2 μl of the gold nanorod aqueous solution (OD = 4.5 with 1 cm optical path) was added to a custom-made cone-shaped well created in an optically transparent acrylate plate. The plate was placed in a lowtemperature vacuum oven to evaporate the solvent. This procedure was repeated 10 times until an overall 20 μl aliquot of gold nanorods was step-wise added to the well and the solvent was evaporated. Using this approach, nanoparticles were deposited into 4 coneshaped wells. Thereafter, 20 μl of silicon oil was added to each well. The experimental setup to measure the photoacoustic response from the samples is shown schematically in Figure 4.4 (b). The bottom of the sample plate was immersed in water to ensure good acoustic coupling and signal transmission. The collimated laser beam irradiated the sample from the top and the ultrasound transducer was placed below the plate near the bottom of the water tank. The position of the transducer was adjusted so that the sample was located at the intersection of laser and ultrasound beams and the distance between transducer and sample was kept constant during the entire experiment. 72
95 ducer (b) (a) Transducer (c) (b) Laser Light Sample Holder Optical Transparent Polyacrylate Sample Holder Electronics ter/ isition Glass Tube With Au NRs Aqueous Solution Optical Window Transducer Silicon Oil Au NRs Figure 4.4: Schematic illustration of the experimental setup for measuring the photoacoustic response from (a) aqueous solution of nanorods and (b) nanorods in oil. 73
96 4.4 RESULTS AND DISCUSSIONS PEGylated and silica-coated gold nanorods prepared for this study are illustrated in Figure 4.5. The silica coating procedure followed our previously reported methods 6-7. Briefly, cetyltrimethyl-ammoniumbromide (CTAB) stabilized gold nanorods were first prepared with the seed mediated growth method 8-9. Then CTAB was replaced by mpegthiol through ligand exchange, and then silica was grown onto the PEGylated gold nanorods via the modified Stöber method using tetraethyl orthosilicate (TEOS) As seen in Figure 4.5, there is conformal and isotropic silica coating of the gold nanorods with controllable silica thickness. In order to investigate the effect of the silica coating on the photoacoustic signal response, we performed a photoacoustic phantom imaging study using method described in section 4.2. Figure 4.6 shows a comparison of ultrasound, photoacoustic, and the spatially coregistered combined ultrasonic and photoacoustic images of phantoms with inclusions containing PEGylated gold nanorods, and gold-silica core shell nanorods with silica shells of 6 nm, 20 nm, and 75 nm thickness, respectively. Inclusion with PEGylated gold nanorods produced a much weaker photoacoustic signal than inclusions with silica coated nanorods even the nanorods with 6 nm silica shell had noticeable photoacoustic signal enhancement. As the thickness of the silica shell increases from 6 nm to 20 nm, the photoacoustic signal increases and, consequently, the photoacoustic image appears brighter. The signal enhancement is slightly reduced for the nanorods with 75 nm silica shell. The quantitative analysis of the photoacoustic signal enhancement is presented in Figure 4.7. The amplitude of the signal and background noise were obtained in black and white squares as shown in Figure 4.2, and the details of the signal calibration method was introduced in section
97 Figure 4.5: (a) TEM image of PEGylated gold nanorods; TEM images of gold-silica core-shell nanorods with (b) 6 ± 0.5 nm (N = 100) and (c) 20 ± 3.6 nm (N = 100) thickness of silica coating; (d) SEM image of gold-silica core-shell nanorods with 75 ± 5.0 nm (N = 100) thickness of silica coating (the silica shell is too thick for TEM). 75
98 (c) Combined (b) Photoacousic (a) Ultrasound PEGylated Au NRs 6 nm Silica-Au NRs 20 nm Silica-Au NRs 75 nm Silica-Au NRs Figure 4.6: (a) Ultrasound, (b) photoacoustic, and (c) combined ultrasound and photoacoustic images (top to bottom) of inclusions containing (I) PEGylated gold nanorods and gold-silica core-shell nanorods with (II) 6 nm silica coating, (III) 20 nm silica coating, and (IV) 75 nm silica coating (left to right). Each image covers 6 mm by 6 mm field of view. 76
99 Contrast to noise ratio (db) Photoacoustic Signal (A.U.) (a) Inclusion Signal Background Noise (b) Silica Thickness (nm) Figure 4.7: (a) Amplitude of the photoacoustic signal from the background (green dots) and inclusions (blue dots) containing gold nanorods with varying thickness of the silica coating. The error bars show plus/minus one standard error. (b) Contrast-to-noise ratios (CNRs) of the photoacoustic images of the inclusions containing gold nanorods with varying thickness of the silica coating. 77
100 The average amplitude of the normalized photoacoustic signal measured from the inclusions containing PEGylated gold nanorods, 6 nm, 20 nm, and 75 nm silica coated gold nanorods is ± 5.40, ± 11.00, ± 15.00, and ± 12.00, respectively (Figure 4.7 (a)). The photoacoustic signal from the gold nanorods with 20 nm silica shell is about 3.8 times higher compared to PEGylated nanorods while the amplitude of the photoacoustic signal from the background (i.e., noise) remains relatively the same for each phantom. Correspondingly, the contrast-to-noise ratio (CNR) of the photoacoustic images of inclusions with PEGylated, 6 nm, 20 nm, and 75 nm silica coated gold nanorods were 9.0 db, 10.1 db, 13.5 db, and 10.7 db, respectively (Figure 3b). Again, the 20 nm silica coated gold nanorods exhibit a more than 2.8 times (4.5 db) enhancement of contrast and CNR compared to traditional PEGylated gold nanorods. Since the photoacoustic amplitude is proportional to the optical absorption, one would expect that the strong increase in the photoacoustic signal is a result of a changed absorption cross-section of the gold nanorods due to the change of the immediate environment (i.e. the silica shell acting as a layer between the gold surface of the nanorod and the surrounding material such as water). To show that this is not the case, a series of UV-Vis spectra of gold nanorods before and after silica coating were collected. To ensure that the concentration of gold nanorods was constant, 100 L of the nanorod solution was taken at the beginning and at different time points during the silica shell growth process from the growth solution. Figure 4.8 (a) shows the measured extinction spectra of gold nanorods recorded at the start of the silica coating process and as the silica coating reached 75 nm thickness. The synthesized gold nanorods, as illustrated in Figure 4.5, have an aspect ratio of 3.9±0.38 resulting in a longitudinal plasmon resonance at 786 nm. After coating the nanorods with a silica shell of 6 nm, 20 nm and 75 nm thickness, the longitudinal peak red shifts to 790 nm, 802 nm, and 806 nm correspondingly. The 78
101 amplitude of extinction after 6 nm silica coating drops 3% and then gradually increases back to the same amplitude as for the PEGylated gold nanorods due to the increasing contribution of light scattering. The shape of the peak is preserved suggesting that the silica coating did not alter the shape or cause noticeable aggregation of the nanorods. This was also confirmed by an analysis of the TEM images. Most importantly, the amplitude of the extinction at the resonance is unchanged. Finite difference time domain (FDTD) simulations (FDTD Solutions, Lumerical Inc.) confirmed that the silica coating insignificantly alters the amplitude of the extinction cross-section of gold nanorods (~1.8%), and that the amplitude of the absorption crosssection, which is responsible for the photoacoustic signal, is also practically unchanged (~2%). Figure 4.8 (b) presents the calculated absorption and scattering cross-sections for gold nanorods before and after 10 nm, 20 nm, and 60 nm silica coating. The scattering cross-section, which is nearly 2 orders of magnitude lower than the absorption crosssection and is shown in an enlarged scale in Figure 4.8 (b) for clarity. The peaks are less broad compared to measured spectra this is due to small variations in the aspect ratio of the synthesized nanorods. The insignificant scattering by nanorods with and without silica shell indicates that a small number of spheroid particles in the synthesis solution leads to the increased background seen in the experiment (Figure 4.8 (a)). However, without background signal, the area under the measured peak decreases only slightly with silica thickness, which most likely indicates some decrease in the yield of the synthesis. The peak is shifted further to the red compared to the experiment because, as discussed previously 6, the silica shell is less dense than the fused silica used in the simulation. Because the absorption cross-section changes insignificantly and the laser wavelength in each photoacoustic experiment was adjusted to the peak absorption of each sample, we can exclude a change in the absorption cross-section as the cause of the photoacoustic 79
102 signal amplification. After excluding a change in the absorptive properties of the gold nanorod due to silica coating as the reason for the increase in the photoacoustic response, changes in the thermal transport from the gold nanorod to the signal generating fluid environment were investigated. The amplitude of the photoacoustic signal for a small source at nanosecond time scales is related to the temporal temperature gradient generated in the surrounding medium by the heat diffusing from the nanorod Assuming a diffusive heat transport process, adding a shell of any material with finite heat conductance and capacity will only broaden a heat pulse and deteriorate the photoacoustic signal. However, the same signal deterioration results from a high interfacial thermal resistance between the heat pulse exiting the gold nanorod and entering the ambient medium. This high resistance would lead to a much higher nanoparticle temperature and a slower heat release into the adjacent medium. Conversely, a low interfacial thermal resistance leads to fast heat transfer and a temperature profile that much more closely resembles the profile of the laser pulse and leads to a stronger photoacoustic signal. The interfacial thermal resistance originates from the scattering or reflection of phonons at the gold dielectric interface and causes a discontinuity of the thermal profile at the interface of the form T R Q, where Q is the heat flux through the interface and R is the interfacial resistance
103 Absorption Cross Section Extinction (a) nm 6 nm 20 nm 75 nm (b) Wavelength (nm) X (m 2 ) 2 1 Absorption 0 nm 10 nm 20 nm 60 nm Scattering Wavelengh (nm) Scattering Cross Section X (m 2 ) Figure 4.8: (a) Extinction spectra of a gold nanorod solution before and after 6 nm, 20 nm, and 75 nm silica coating. (b) FDTD simulated absorption (solid curve) and scattering (dashed curve) cross-section spectra of gold nanorods before (black line) and after coating with 10 nm (red line), 20 nm (blue line), and 60 nm (orange line) silica. The simulations were performed using the refractive index of fused silica. Note that scattering is nearly 2 orders of magnitude lower than the absorption. 81
104 In the case of PEGylated gold nanorods, the interfaces of gold to surface layer, and surface layer to water play a role. In the case of silica-coated gold nanorods, the interfaces of gold to surface layer, surface layer to silica, and silica to water exist. Studies of the heat conductance from flat gold surfaces coated with alkyl-thiols of varying length showed that the heat transfer process was linear with chain length but that it was dominated by the Au-S interface resistance 17. The interfacial thermal conductance, which is the inverse of the interfacial thermal resistance, for this interface was found to be MW m -2 K -1, very similar to Au-Pd nanoparticles stabilized with monohydroxy(1- mercaptoundec-11-yl)tetraethylene glycol (EG-4) and CTAB 18, and gold nanorods stabilized with CTAB 19. This value is about two times the interfacial thermal conductance for citrate stabilized gold nanoparticles in water 19-20, while the calculated value for a pure gold-water interface of 170 MW m -2 K -1 lies in between 21. However, these values depend strongly on the solvent; the interfacial conductivity for the same thiol coated Au-Pd nanoparticles in toluene is an order of magnitude lower The thermal conductance for the interface of a thin solution-grown silica layer to gold is not known. Some reports place the conductance relatively low for solid-solid interfaces, with the Audielectric conductance at 40 MW m -2 K -1 independent of the dielectric 24, and Au-glass conductance at 100 MW m -2 K -1 for glass with a silica content of 53% 25, and 60 MW m -2 K -1 for gold on thermally grown thin silica films 26. However, the structure of the silica and details of the interface play an important role 26 and interfacial heat conductance in excess of 220 MW m -2 K -1 have been measured for more grainy gold films 27,[ which is in better agreement with what our results suggest. In contrast, the silica to water interface has a very high thermal conductance in excess of 1000 MW m -2 K -1 as determined by molecular dynamics The large spread of interfacial thermal conductances between different interfaces highlights the importance of the molecular interactions, such as 82
105 hydrogen bonding between the hydroxyl head groups of silica and water. Accordingly, terminal functionality of thiols and silanes has been shown to strongly influence the effective interfacial conductance for gold and silica to water with hydrophobic groups, showing ~50 MW m -2 K -1 for gold 28 and ~200 MW m -2 K -1 for silica 29. While it is clear that introducing a silica-water surface will increase the interfacial conductance, the gold to silica surface may have a relatively high thermal interface resistance. However, coating gold nanoparticles with a shell has been found to increase the heat conductance from the gold core to the ambient medium. Ge and coworkers coated gold spheres with a polymeric shell and found the heat conductance to be faster than expected from bulk materials properties and interfacial conductance if a small amount of a co-solvent was added to the aqueous medium 18. Very much in agreement with our results, Hu et al. reported faster heat dissipation from silica coated gold nanospheres than from bare gold nanoparticles in water 30. We therefore expected that the photoacoustic signal enhancement must come from an increased thermal transfer through the gold interface induced by changing the interface from gold-(peg)-water to gold- (PEG)-silica-water, and that the signal increase should be sensitive to an exchange of the environment from water to oil. To experimentally demonstrate the influence of the interfacial heat conductance on the photoacoustic response, two sets of experiments were conducted to separately investigate the two interfaces of silica coated gold nanorods. The first set of experiments was focused on studying the thermal conductance at the gold/silica interface in aqueous solution. Three types of gold nanorods were prepared: CTAB stabilized gold nanorods, gold nanorods coated with mpeg-thiol (MW 2,000 Da), and CTAB stabilized gold nanorods coated with an electrostatically stabilized multilayer created by sequential layer-by-layer deposition of poly(styrenesulphonate) (PSS, MW 14,900 Da), 83
106 poly(allylamine hydrochloride) (PAH, MW 15,000 Da), and poly(vinylpyrrolidone) (PVP, MW 10,000 Da) following the procedure reported by Pastoriza-Santos et al. 5. These three types of gold nanorods have all been successfully used for subsequent silica coating 5-7,30, but have an increasing thickness of the organic passive layer. The thickness of CTAB, PEG, and multilayer polyelectrolyte are about 4-5 nm 31, 6-7 nm 32-33, and 7-8 nm 32, respectively. The concentrations were adjusted so that the extinctions of each gold nanorod type were the same. The amplitudes of the photoacoustic signal from 60 pulses were recorded, and the average and the standard deviation are reported for each nanorod type as shown in Figure 4.9. Before silica coating, CTAB and PEGylated gold nanorods show similar photoacoustic signal amplitudes, while a slightly higher signal was found for the polyelectrolyte coated gold nanorods. After coating the nanorods with 20 nm of silica, all three types of nanorods show significantly elevated photoacoustic signals, but to different levels. The silica coated CTAB gold nanorods show the largest enhancement and the silica-coated polyelectrolyte-multilayer gold nanorods show the lowest signal enhancement. These different nanorods represent different interfaces of gold with silica and have been shown to modulate the interfacial heat conductance 32. Since the CTAB layer is extremely thin, and the CTAB is almost totally exchanged with silica during the coating process 34 ; these nanorods come closest to the exact interface between gold and silica. However, since both the PEG layer and the polyelectrolyte multilayer have finite thickness, the results show a decreasing enhancement with increasing layer thickness. It is unclear, however, whether this thickness dependence represents a direct correlation, or whether factors such as interpenetration of silica into the organic layer or surface coverage play a role. Such influences are suggested by the CTAB nanorod experiments, 84
107 Photoacoustic Singal (A.U.) Silica Coating CTAB 2K-PEG Polyelectrolytes Types of Au NRs Figure 4.9: Average amplitudes of the photoacoustic signal from three types of gold nanorods before and after silica coating. The error bar represents plus/minus one standard deviation of the photoacoustic signal measured using 60 laser pulses of low (4 mj/cm 2 ) fluence. 85
108 which show a large variation between batches and are known to be the least controlled way to generate the silica coating. In another set of experiments, the interface between silica and the surrounding media was investigated (experimental set up for this experiments is shown in Figure 4.4). Three different solvents, the silica growth solution (a water/ipa mixture which contains 26 volume % of water), water, and silicone oil were utilized to immerse PEGylated gold nanorods and silica-coated gold nanorods with various coating thickness. The absolute value of the photoacoustic signal in oil, IPA, and water is generally different due to the differences in Grüneisen parameter and experimental setups, but this is of no importance to the arguments made here. Figure 4.10 shows an increasing photoacoustic signal with increasing silica thickness from 0 nm to 20 nm in polar solvent, while in oil, the opposite trend, a decrease in photoacoustic signal with increasing silica thickness up to 20 nm is observed. PEGylated gold nanorods have a less hydrophilic surface due to the methyl terminal groups than silica, and, when immersed in oil, a higher interfacial thermal conductance could be expected compared to water. On the other hand, the hydroxyl groups of the silica surface are able to form hydrogen bonds with water, and have been shown to have a high interfacial thermal conductance. The equal photoacoustic signal from PEG and CTAB coated nanorods is in agreement with the thermal behavior seen for Au-Pd nanoshells 18, although our PEG is larger and methyl rather than hydroxyl-terminated. The strongly increased signal due to silica coating in water contradicts a direct coupling of the silica with the gold surface as this should have a decreased interfacial conductance 25. The results shown in Figure 4.6 and Figure 4.10 (b) demonstrate, however, that the parameters that characterize each interface have a strong influence on the thermal conductance, and therefore on the photoacoustic signal. Whether the signal increase by a factor of 3 can be explained fully 86
109 by an improved thermal interfacial conductance or whether increased thermal diffusion along water channels in the silica shell may play a role, and whether the improved conductance leads to non-linear effects in the photoacoustic signal generation due to the temperature dependence of the thermal expansion coefficient of water 12 is currently under investigation. It is interesting that the photoacoustic response change due to the introduction of a silica shell is not a simple step but a biphasic profile in the case of the polar solvent and a continuous decrease in the case of oil. The maximum at around 20 nm silica shell thickness in polar solvents is significantly higher than the signal at thicker shells (p < 10-4, pairwise t-test). We attribute the gradual increase to imperfections in the coating for thin silica layers below ~20 nm. For thicker silica layers we expect that the thermal pulse profile exhibits some broadening due to the finite thermal conduction of silica, even though silica has a good thermal conduction. The signal enhancement effect due to an increase in interfacial conductance is partially canceled by this signal broadening above a thickness of 20 nm in polar solvent. Additionally, the increased scattering of the larger silica-coated nanoparticles could lead to a reduced local fluence, and so also contribute to the reduced PA signal. On the other hand, in silicon oil, the interfacial conductance of the silica/oil interface is lower than that of the PEG/oil interface, and together with the heat pulse broadening and the scattering both contribute to the signal reduction. Figure 4.11 shows a schematic summary of the proposed mechanism for the photoacoustic signal enhancement from silica-coated nanoparticles, emphasizing the importance of the heat transfer to the water. While nanoparticles with no shell have a slower heat transfer resulting in a broader heat peak due to their interfacial properties, adding a silica shell reduces the interfacial resistance and leads to a sharper peak even though its amplitude may be reduced due to the heat capacity of silica. At very large 87
110 thickness the influence of the bulk silica is stronger leading to a further reduced and somewhat broader temperature peak. Because the pressure is roughly proportional to the slope, but includes also a geometric term that grows with the size of the heated liquid, the slightly sharper pulse of a larger particle leads to a higher photoacoustic signal. Additionally, changes in the frequency spectrum of the signals may be possible, which we have not investigated here. In conclusion, this study has shown that silica coated gold nanorods can amplify the photoacoustic response without altering the optical absorption of nanoparticles other than shifting it slightly to the red. The signal enhancement of silica coated gold nanorods in water depends on the silica thickness in a biphasic way. The enhancement could be attributed to changes in the interfacial heat conduction from gold to water due to the silica. The biphasic behavior reflects the balance between changing the interfacial conduction and the profile flattening due to a finite bulk heat conductivity and capacity by adding a dielectric shell. Silica coating of gold nanoparticles appears to be a simple way to increase their usefulness as contrast agents for photoacoustic imaging. 88
111 PA Signal (A.U.) PA Signal (A.U.) PA Signal (A.U.) (a) Silica growth solution (IPA+water) (b) (c) Water Silicon oil Silica Thickness (nm) Figure 4.10: Photoacoustic signal amplitude of gold nanorods with varying thickness of the silica coating in (a) silica growth solution (IPA containing 26 vol% water), (b) water, and (c) silicon oil. In polar environment the signal is amplified, and shows a maximum at ~20 nm shell thickness, while in nonpolar environment the signal monotonically decreases. The vertical error bar represents plus/minus one standard deviation of the photoacoustic signal measured using 60 laser pulses of low (4 mj/cm 2 ) fluence. The horizontal error bars represent plus/minus one standard deviation of the silica thickness measured by TEM. 89
112 %(?Y) %(?Y) %(?Y) %(?Y) %(?Y) %(?Y) Types of Au Nanorods Temperature profile of surrounding liquid A (a) (b) (c) Au SiO T T T Figure 4.11: Schematic summary of the proposed thermal transport processes from the nanoparticle to the environment and the resulting temporal profiles of the temperature (T) near the surface and the amplitude of the photoacoustic signal (P) far from the surface of the nanoparticle. (a) A bare nanoparticle with high interfacial resistance leads to a broadened temperature profile and a smaller amplitude of the photoacoustic pressure signal; (b): introducing a silica shell leads to a minimal interfacial resistance between gold (Au) and SiO2 and SiO2 and water. The resulting sharper temperature profile, and because the temperature profile is at a larger distance, the photoacoustic signal is increased; (c) a thick shell leads to a broadened temperature peak and again a decrease in the photoacoustic signal, although it may still be higher than that for the bare nanoparticle. B B B t A t A t Photoacoustic signal amplitude P P P B A B B A A t t t 90
113 4.5 REFERENCES 1 Jain, P. K., Lee, K. S., El-Sayed, I. H. & El-Sayed, M. A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. Journal Of Physical Chemistry B 110, , (2006). 2 Lee, K. & El-Sayed, M. A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. Journal Of Physical Chemistry B 110, , (2006). 3 Agarwal, A. et al. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. Journal of Applied Physics 102, , (2007). 4 Oraevsky, A. A., Karabutov, A. A. & Savateeva, E. V. in Proceedings of SPIE The International Society for Optical Engineering 4434, (SPIE, Bellingham, 2001). 5 Pastoriza-Santos, I., Perez-Juste, J. & Liz-Marzan, L. M. Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chemistry of Materials 18, , (2006). 6 Chen, Y. S. et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Optics Express 18, , (2010). 7 Chen, Y. S. et al. On Stability of Molecular Therapeutic Agents for Noninvasive Photoacoustic and Ultrasound Image-guided Photothermal Therapy. Proceedings of SPIE The International Society for Optical Engineering 7564, , (2010). 91
114 8 Jana, N. R., Gearheart, L. & Murphy, C. J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Advanced Materials 13, , (2001). 9 Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chemistry of Materials 15, , (2003). 10 Lu, Y., Yin, Y. D., Mayers, B. T. & Xia, Y. N. Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Letters 2, , (2002). 11 Stober, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. Journal of Colloid and Interface Science 26, 62-&, (1968). 12 Calasso, I. G., Craig, W. & Diebold, G. J. Photoacoustic point source. Physical Review Letters 86, , (2001). 13 Cox, B. T. & Beard, P. C. Fast calculation of pulsed photoacoustic fields in fluids using k-space methods. Journal of the Acoustical Society of America 117, , (2005). 14 Diebold, G. J., Beveridge, A. C. & Hamilton, T. J. The photoacoustic effect generated by an incompressible sphere. Journal of the Acoustical Society of America 112, , (2002). 15 Murad, S. & Puri, I. K. Molecular simulation of thermal transport across hydrophilic interfaces. Chemical Physics Letters 467, , (2008). 16 Swartz, E. T. & Pohl, R. O. Thermal-boundary resistance. Reviews of Modern Physics 61, , (1989). 92
115 17 Wang, Z. H. et al. Ultrafast flash thermal conductance of molecular chains. Science 317, , (2007). 18 Ge, Z. B., Cahill, D. G. & Braun, P. V. AuPd metal nanoparticles as probes of nanoscale thermal transport in aqueous solution. Journal of Physical Chemistry B 108, , (2004). 19 Schmidt, A. J. et al. Probing the gold nanorod-ligand-solvent interface by plasmonic absorption and thermal decay. Journal of Physical Chemistry C 112, , (2008). 20 Plech, A., Kotaidis, V., Gresillon, S., Dahmen, C. & von Plessen, G. Laserinduced heating and melting of gold nanoparticles studied by time-resolved x-ray scattering. Phys. Rev. B 70, (2004). 21 Merabia, S., Shenogin, S., Joly, L., Keblinski, P. & Barrat, J. L. Heat transfer from nanoparticles: A corresponding state analysis. Proceedings of the National Academy of Sciences of the United States of America 106, , (2009). 22 Douma, K., Megens, R. T. A. & van Zandvoort, M. Optical molecular imaging of atherosclerosis using nanoparticles: shedding new light on the darkness. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology 3, , (2011). 23 Wilson, O. M., Hu, X. Y., Cahill, D. G. & Braun, P. V. Colloidal metal particles as probes of nanoscale thermal transport in fluids. Physical Review B 66, (2002). 24 Stoner, R. J. & Maris, H. J. Kapitza conductance and heat-flow between solids at temperatures from 50 to 300 k. Physical Review B 48, , (1993). 25 Juve, V. et al. Cooling dynamics and thermal interface resistance of glassembedded metal nanoparticles. Physical Review B 80, (2009). 93
116 26 Burzo, M. G., Komarov, P. L. & Raad, P. E. Thermal transport properties of goldcovered thin-film silicon dioxide. IEEE Transactions on Components and Packaging Technologies 26, 80-88, (2003). 27 Kato, R. & Hatta, I. Thermal Conductivity and Interfacial Thermal Resistance: Measurements of Thermally Oxidized SiO2 Films on a Silicon Wafer Using a Thermo-Reflectance Technique. International Journal of Thermophysics 29, , (2008). 28 Hu, M., Goicochea, J. V., Michel, B. & Poulikakos, D. Thermal rectification at water/functionalized silica interfaces. Applied Physics Letters 95, (2009). 29 Schoen, P. A. E., Michel, B., Curioni, A. & Poulikakos, D. Hydrogen-bond enhanced thermal energy transport at functionalized, hydrophobic and hydrophilic silica-water interfaces. Chemical Physics Letters 476, , (2009). 30 Hu, M., Wang, X., Hartland, G. V., Salgueirino-Maceira, V. & Liz-Marzan, L. M. Heat dissipation in gold-silica core-shell nanoparticles. Chemical Physics Letters 372, , (2003). 31 Murphy, C. J. et al. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. Journal of Physical Chemistry B 109, , (2005). 32 Alpert, J. & Hamad-Schifferli, K. Effect of Ligands on Thermal Dissipation from Gold Nanorods. Langmuir 26, , (2010). 33 Shiotani, A., Mori, T., Niidome, T., Niidome, Y. & Katayama, Y. Stable incorporation of gold nanorods into N-isopropylacrylamide hydrogels and their rapid shrinkage induced by near-infrared laser irradiation. Langmuir 23, , (2007). 94
117 34 Gorelikov, I. & Matsuura, N. Single-step coating of mesoporous silica on cetyltrimethyl ammonium bromide-capped nanoparticles. Nano Letters 8, , (2008). 95
118 Chapter 5: The Mechanism of Photoacoustic Generation in Nanoparticle Solution The introduction of plasmonic nanoparticles as photoacoustic contrast agents and their enhanced optical absorption due to surface plasmon polaritons brought molecular sensitivity to photoacoustic imaging 1-8. In the previous chapter, we have prepared the photostable photoacoustic contrast agents using gold nanorods as the template, and demonstrated the photoacoustic signal amplitude could be further enhanced through silica coating. With the presence of nanoparticles, the medium containing the nano-sized strong optical absorbers in a weakly absorbing tissue is no longer optically homogeneous the medium is heterogeneous on the scale of the thermal diffusion length of the tissue. Unlike in systems that are homogeneous or where the heterogeneities are much larger than the thermal diffusion length and therefore the photoacoustic signal is produced directly by the illuminated absorber The origin of the photoacoustic signal in the case of plasmonic nanoparticles is expected to depend on the heat transfer from the nanoparticle to the environment, and has not been explored in detail so far. In this chapter, we estimate the relative contribution from a nanoparticle and its liquid environment based on continuums mechanics, and show experimentally with gold nanorods and gold nanospheres that the photoacoustic signal has the thermal signature of the colloidal solvent. The finding suggests that silica coated gold nanorods could be a better candidate for temperature sensing and can be applied for photoacoustic molecular image guided photothermal therapy. 5.1 SYNTHESIS OF HYDROPHOBIC SILICA COATED GOLD NANORODS PEGylated and Silica coated gold nanorods were prepared using the method described in chapters 2 and 3. The gold nanospheres were prepared using citric reduction 96
119 method. Briefly, 95 ml of HAuCl 4(aq) solution (0.27 mm) solution was heated to boiling temperature, and 5 ml of trisodium citrate solution (34 mm) was added. The transparency of the solution changed to blue in 30 seconds and burgundy red within 90 seconds. The solution then aged for another 1 hour at refluxing before being centrifuged at 30,000 g for 45 min, twice. The collected citrate gold nanospheres were re-dispersed in ultrafiltrated (18 MΩcm, Thermo Scientific Barnstead Diamond water purification systems), deionized water. The PEGylated gold nanospheres were produced from citrate stabilized gold nanospheres by exchanging citrate with the biocompatible mpeg-thiol, and silica coated gold nanospheres were prepared by coating silica directly on PEGylated gold nanospheres via a modified Stöber method, as described earlier in chapter 3. Hydrophobic nanospheres for non-aqueous solvents were prepared by replacing the citrate of citrate-stabilized nanospheres by octadecane-1-thiol, and the organic soluble silica coated nanospheres was prepared by functionalizing the silica surface with trimethoxy(octadecyl)silane. The detailed procedure of the nanoparticle synthesis has been described earlier Briefly, the citrate-stabilized gold nanosphere dispersion was added to an equal volume of mpeg-thiol (0.1 mm) aqueous solution under vigorous stirring. The mixture was sonicated for 30 seconds and left to react for 1 hour. Excess mpeg-thiol molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the PEGylated gold nanospheres were re-suspended in THF. The PEGylated gold nanospheres in THF were mixed with an equal volume of ethanol solution of dodecanethiol (5 mg/ml). The mixture was sonicated for 30 min at room temperature and then 60 min at 50 ºC and then allowed to react overnight at room temperature. The functionalized gold nanospheres were centrifuged at 8,000 g for 20 min, twice, then re-suspended into toluene or into silicon oil. For the hydrophobation of silica coated nanospheres, 300 μl of OTMS in chloroform solution (2.4%) was added dropwise 97
120 into 3 ml of silica coated gold nanospheres solution (0.78 mm (in ethanol) containing 30 μl of NH4OH (32%) under vigorous stirring. The mixture was allowed to react for 24 hours. Ethanol and excess OTMS was removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the hydrophobic silica coated gold nanospheres were re-suspended in organic solutions, such as toluene and silicon oil. 5.2 PHOTOACOUSTIC MEASUREMENTS AND ANALYSIS A custom-made ultrasound and photoacoustic imaging system was used to test the photoacoustic effect of the nanoparticle solutions. As illustrated in Figure 5.1, a 1 mm diameter glass tube was fixed in an acrylate water tank with an optical window inlay. The glass tube contains an inlet and an outlet for injecting the samples into the glass tube without moving the tube during the experiment. At the fluences used here, the photoacoustic signal generated in the glass wall is negligible. The water tank also has an outlet and an inlet which connects to the temperature controlled water circulator. During the experiment, 50 L of gold nanoparticle solution, each with a peak optical density (O.D.) of 1.5 were injected into the tube through the outlet. The water temperature was set at either 4 C or 15 C and then increased stepwise until 45 C. When the desired temperature was reached, it was stabilized for at least 5 minutes at each temperature to ensure equilibration of the temperature of the colloidal solution. A single element focused ultrasound transducer (Panametrics Inc., V320) with 7.5 MHz center frequency, 50.4 mm focal distance, and 13 mm aperture was mounted on a one dimensional positioning stage. The nanoparticle solution was aligned with the focus of the transducer, and the distance between transducer and glass tube was kept constant during the entire experiment. Seven nanosecond laser pulses produced by a Nd:YAG pumped OPO (Spectra Physics) laser were introduced through the optical window into the water tank and uniformly irradiated 98
121 the sample. A laser fluence of 5 mj/cm 2 was chosen to prevent bubble formation. The wavelength was matched to the peak absorption of each nanoparticle solution. For each temperature, the photoacoustic signal of each pulse was captured and stored for off-line processing. The amplitude of the recorded photoacoustic signal from each pulse was first compensated by the fluence fluctuation factor (calculated from recorded power meter readings per pulse), then normalized to the maximum photoacoustic signal recorded. 128 pulses were recorded and separated evenly to sixteen sets with eight pulses per set. Each set of signals was averaged and the peak amplitude of the averaged signal of each set was recorded. The mean value and the standard error of sixteen averaged peak amplitudes were calculated. Photoacoustic signals from pure solvents were recorded in the same way as those with nanoparticles, but using a wavelength of 920 nm for silicon oil and 1180 nm for water, and fluences of 30 mj/cm 2, respectively. Measurements for pure toluene failed because no absorption bands were available within the wavelength range of the laser system. 99
122 M1 OPO Nd:YAG Trigger M2 ND BS L1 L2 I PM Computer /Digitizer M3 ND Amplifier Transmit /Receive Sample UT Temperature Control Unit Transducer Thermal Meter Optical Window Pulsed Laser Glass Tube and Sample Figure 5.1: Schematic illustration the combined photoacoustic/ultrasound system used in this study, where M1-3 represent mirror 1 to 3; L1-2 are lens 1-2; ND represent neutral density filters; BS represent beam splitter; PM represent power meter, and UT represent ultrasound transducer. In the dashed square, it shows the close-up schematic illustration of the sample irradiated by a pulsed laser beam while photoacoustic transients were measured using the ultrasound transducer. 100
123 5.3 ESTIMATION OF THE PHOTOACOUSTIC SIGNAL FOR A NANOSPHERE IN A FLUID Sphere with no Shell We consider a sphere of radius a surrounded by a homogeneous infinite fluid, as shown in Figure 1 in the main text. Under spherical symmetry the strain tensor for the displacement u(r) = (u r (r),0,0) induced by the increase of the temperature T above equilibrium in each material is: ur 0 0 r ur 0 0 r ur 0 0 r (5.1) and the stress tensor is: ur ur ur 1 ur ur rr KT K r r r 3 r r ur ur ur 1 ur ur KT K r r r 3 r r ur ur ur 1 ur ur KT K r r r 3 r r, r, r, 0 (5.2) Both tensors depend on the compressive modulus K, the shear modulus µ, and the thermal expansion coefficient for each material. The equation of motion is: 2 u r rr 2 rr 2 t r r. Inserting (5.2) gives: u r K T K 4 u r K 4 r 2 r r 2 u K T c u 2 u 2 2 L 2 t r 3 r 3 r r r r r r (5.3) 101
124 It is convenient to introduce a potential for the displacement 13 : u t (5.4) where the component needs to be zero because of the spherical symmetry. For the potential (5.3) converts to the photoacoustic wave equation: 2 2 K T 2 r 2 K T 2 c 2 L c 2 L t t r r r t or using the spherical Laplace operator: 2 1 K T c t c t L L (5.5) (5.6) where we introduced the longitudinal and transverse speed of sound: 4 K 3 cl ; ct ; (5.7) In a fluid µ = 0, and we will also assume that we can neglect any transversal sound in the analysis because we are interested in the signal in the fluid far away from the surface of the nanoparticle. Then (5.6) reduces to: 2 1 T 2 2 c t t L (5.8) At the interface between the metal sphere (s) and the fluid medium (m) the following boundary conditions need to be fulfilled: u u and (5.9) rs ra rm ra rrs ra rrm ra Converting to potentials transforms (5.9) into: s m r ra r ra (5.10) 2 tot 2 Ks Ts 2 s 2 s 2 4 s m 2 Tm m 2 m s c c L 2 T c s L m m 2 s s t r r r r r s t r r r 102
125 In order to solve a one-dimensional problem, the potential =r is introduced. Equations (5.6) and (5.8) become: 2 2 s r, t 2 sr, t Ks T s s t c 2 L r s 2 t r t tot r, t r, t T r, t 2 2 m 2 m 2 m c 2 L rc m 2 L m m t r t s (5.11) We make the following assumptions for the temperature profile: the sphere is at a homogeneous temperature so that there is only a time dependence of T s (t), and the temperature profile in the fluid medium is separable into space and time dependencies, and the spatial component is a simple exponential with characteristic length r 0 so that T tot 0 (r,t) = T m (t) exp(-(r-a)/r 0 ) (see also Figure 5.2). Temporal Fourier transformation of (5.11) leads to: 2 Fs r, 2 2 Kss Fs r, cl ir G s s 2 r s 2 Fm r, Fm r, cl ir c m L m mgm e 2 r ( ra) ro (5.12) where F s and F m are the Fourier transforms of, and G s and G m are the transforms of T s (t) and T m (t), respectively. Equations (5.12) have the general solution: r Ks s r r Fs r, i Gs C3 cos C1 sin s c L c s Ls c r r 2r r r F r ic G e C e C e r a i r i r 2 L m r c 0 L c m Lm m, L m m m cm r0 (5.13) 103
126 where the C i 's are complex integration constants that depend on. C 3 must be zero because the potential = /r must be finite at r = 0. Additionally, no wave can be incoming from infinity, so that C 4 must be zero also. The boundary conditions (5.10) are Fourier transformed to determine C 1 and C 2 : 2 K 2 Fs r, s i r sgs cl 4 c 2 2, s Ts Fs r s r r r r ra ra 2 2 r F, m 0 m r cl i r m mgm e 2 s r ra and 1 F r, F r, 1 F r, F r, a r a a r a s s m m 2 2 ra ra ra ra (5.14) Neglecting the transversal sound term of the sphere, and after significant algebra the solutions are: a ir/ cl ir/ cl s s a 1i 2 e e cl c L s s m Fs r, i Gs r a m a a m c m a 2 i 1 sin i cos cl m s c L c s L m s c s c L s r0 m 3 r 0 a ir/ cl ir/ c s Ls m a 1 1i e e a s a c Lm igm 2 r c 0 a a m a a m L a m 2 i i 1 sin i cos a c L c m L m s c L c s L m s c L c s Ls (5.15) and 104
127 a a a a sin cos 2 cl c s s ir a / c L c L s L c s Ls m Fm r, i Gse m a a a m a 1 i sin cos s c Lm c Ls c L s s c Ls r a r r a mc r e igm ra/ r0 L 1 2 m a c L r a c m Lm r0 a a c Lm r0 a ira / cl m mcl a e m 2 a cl m r0 a m a a a m a i 2 2 a c L m s cl c m L c s L s s cl s igm 1 1 sin cos r0 r0 r0 m r0 r0 a r0 m a sin a a a s a a cl a m s c Ls a r0 m r0 r0 a a 1 2 cos 2 2 cl a s s a a c L c m Ls (5.16) The first term in (5.16) is the contribution from the sphere to the signal measured in the fluid a distance r from the sphere. The second term represents a near-pressure term that decays exponentially and does not contribute to the signal measured at r, if r is large enough. The third term represents the signal from the heating of the fluid around the metal sphere. Equations (5.15) and (5.16) are written in a form to suggest the following dimensionless parameters: a a r (5.17) 0 m s ; m ; rn ; r ; cl c s L a m s The parameters s and m are small (< 0.001) for the range of frequencies used in photoacoustic imaging with ultrasound transducers (f = 2π = 1 50 MHz), the sizes of 105
128 spheres of interest (a < 100 nm), and the typical speeds of sound (c = 1 10 km/s). We therefore expand the sine and cosine up to third order: 2 2 2acL s s ira / c Lm s Fm r, igs e 2 2 2rs s 61 i m r0 2 rn m 1 rn m 2 mclr m r ra/ r0 igm e rn m 2 rn m 2 s rn r rn mrn 2 1 s 6 rn m 2 rn 2 1 mcla m i r a igm e / cl m 2 1 r n m s r i s 6m i (5.18) The signal to first order from the sphere and the fluid measured at a distance r >> a, r 0 is then, respectively 2 2 ac sphere L s s ira / c, L m s Fm r igs e 3 1i 2 2 / c rn m rn rn medium mcla m i r a Lm m, m 1 im F r ig e The relative contributions are then: sphere Fm r, Gs s 1 medium 3 2 F r, G 3 2r 2r r m m m n n n m (5.19) (5.20) (5.21) Conversion to pressure gives: sphere P Gs m s 1 medium P G 3 2r 3 2r 2 r m m m n n n (5.22) If no interfacial resistance exists between the sphere and the fluid, the temperature is continuous at the interface and (5.21) simplified to: 106
129 F r, 1 sphere m s medium 3 2 m m n n n, 32 2 F r r r r (5.23) If interfacial resistance exists, the heat generated in the sphere will be released in a broadened peak in the fluid, and assuming a Gaussian profile with width, such as T(t)=(2) -1/2 exp(-t 2 /2 2 ) can be assumed in both the sphere and the fluid, the ratio of the contributions to the pressure measured some distance away from the source is then sphere Pm 2 m s s 1 e (5.24) medium 3 2 P 3 2r 2r r m m n n n Sphere with a thin shell Introducing a shell of thickness dr around a sphere of radius a, under otherwise the same conditions as in the case with no shell, leads to the system of differential equations in (temporal) Fourier space: Fm r, 2 Fm r, cl i rc 2, m L m mgm r r Fs r, Kss Fs r, cl i r G 2 s s r s Fsh r, Kshsh Fsh r, cl i r G 2, sh sh r r sh (5.25) where the index sh indicates the space a r a+dr of the shell. In order to keep the mathematical effort limited, we assume a temperature profile inside the shell that is linear, which should be fulfilled for thin shells or very good thermal conductors. The shell therefore only leads to a drop in dimensionless temperature T 1 over the whole thickness. 1 sh it r a Gsh r, T r, te dt Gs 1 T (5.26) 2 dr The general solutions for (5.25) with the limiting conditions for r = 0 and as before included are: 107
130 c r 2 r r r F r ic r e G C e Lm 0 0 ra/ r0 m m, Lm m 0 2 m cl r0 m ir / cl s Kshsh r r a sh r sh r Fsh r, i Gsh 1 T C1 cos C2 sin sh dr c L c sh Lsh Kss r i Fs r, i Gs ir / c L ir / s Ls e e c C s 1 2 s (5.27) Applying the boundary conditions analog to (5.13) to the interfaces at r = a and r = a + dr, leads to expressions for F m, F sh, and F s. Within F m the contribution for the solid sphere and shell and the fluid can again be separated by setting s and sh or m = 0, respectively. After expanding sine and cosine to fourth order for F sphere m and first order for F medium m, and collecting lowest order terms, one arrives at the following expressions for the sphere with shell and the fluid, respectively:, 1 F r i G a dr sphere 3 m s n i radr c Lm dr 1 4 T dr 3 2 T 3dr 1 2 T 2 c 2 s dr dr dr 3 i dr dr c s sh n n n s r n n n m n 1 n 1 sh e r (5.28) radr 3 medium 2 r r n Fm r, igm ma e 2a rn mrn r 3 e igm ma 2 cl m 2 c rmdrn md 2 3 rn r n L sh 2 1 i m drn 1 shdrn r 2 2 radr 2 cl 2 c i m Lm c Lm 2 1 2drn drn rn 2 2drn 2 rmdrn 2 r c mdrn r n L c sh Lsh 2 r 1 i m drn 1 shdrn r (5.29) 108
131 Here dr n =dr/a has been introduced. The ratio of the two contributions at large distances r is then: F r, G T dr dr dr dr dr dr sphere m s s sh n n n sh n n n medium m m 3m n 2 n n n 2 n n 2 n n 2 n F r, G r r r dr r dr r dr r (5.30) Equation (5.30) reduces to (5.21) for dr n =0 as expected. Additionally, for r n 1 and dr n < 1, only the highest order term in dr n is considered so that equation (5.30) becomes: F r, G F r, G dr sphere m s s sh 3 medium drn 4 T 1 2 m m 3m n 3m (5.31) This means, that for dr < 1 the contribution from the solid sphere increases linearly with the thickness, considering that T < CHARACTERIZATION OF GOLD NANORODS Optical properties of gold nanoparticles are characterized by ultraviolet to visible (UV-Vis) extinction spectroscopy. Extinction spectra are collected from a 100 L nanoparticle suspension in a 96-well microliter plate reader (BioTek Synergy HT). The shape and morphology changes of the gold nanorods are monitored by transmission electron microscopy (TEM) imaging. For TEM imaging, a drop of the gold nanorod suspension is placed on copper-formvar grids and blotted dry with a filter paper. The grids are imaged using the TEM mode of a Hitachi S-5500 FESEM equipped with a field emission electron source operated at 30 kv. 5.5 RESULTS AND DISCUSSIONS For an optically heterogeneous medium the photoacoustic signal results in general from both the particles and the surrounding medium. Their relative magnitudes depend on the size of the absorber and the size of the heated region in the surrounding, as well as 109
132 the temporal profile of the heating in the absorber and the surrounding. In order to show that the photoacoustic signal for a plasmonic nanoparticle in a liquid environment with good thermal conductivity is generated mostly from the surrounding, we consider a metallic sphere of radius a heated by light absorption to have the temperature (Figure 5.2). We use a continuums approach and make the following assumptions for our estimation: (1) the problem has spherical symmetry, so that transversal wave components can be neglected; (2) the sphere is small enough and the absorption time scales are long so that the sphere can be considered to be heated homogeneously, an assumption made frequently and justified by the very high thermal conductivity of the gold and other noble metals we are interested in Ref 20; (3) the temperature profile in the surrounding at time t and position r from the center of the sphere can be written as T tot m (r, t)=t r m (r)t m (t)=exp[- (r-a)/r 0 ]T m (t), with a characteristic decay length of r 0. We then solve the equation of motion in each material in spherical coordinates (r,, ): 2 u r rr 2 rr 2 t r r (5.32) For the radial displacement u r and the diagonal stress tensor with components ii =-KT+K( rr +2 )+2[ ii -( rr +2 )]/3; here is the volumetric expansion coefficient, K and are the compressive and shear modulus, respectively, and is the strain tensor. With c 2 L =(K+4/3)/ the longitudinal speed of sound and the density, equation (5.32): 2 2 ur K T 2 ur 2 ur c 2 L 2 (5.33) t r r r r Introducing the potential so that u / t and P / t, gives the r photoacoustic wave equation that has to be solved in both media: 2 1 K T c t c t L L (5.34) 110
133 r PA a r 0 Figure 5.2: (a) Schematic representation of the photoacoustic response generated from a sphere and the surrounding liquid. (b) Generalized temperature profile without interfacial resistance as a function of time and distance, summarizing the major assumptions made for the analytical pressure amplitude estimation. 111
134 Boundary conditions at the interface between the sphere and surrounding fluid are that the radial stresses and displacements are continuous 13. Additionally, the displacement at the sphere center needs to be finite (and zero), and no incoming wave (traveling from infinity) exists for the surrounding. With r, the Laplace operator can be reduced to a simple second derivative in equation (5.34) and it can be solved in (temporal) Fourier space with F r, F, G F T t, T t s s m G F, and F denotes the Fourier transform of the potential ( ), temperature in the particle ( T s ), and m temperature in the environment ( T m ), respectively. The solution is given in the supplement. Since the pressure is measured in the medium far from the nanoparticle, the contributions from the solid sphere and the liquid environment can be separated by setting the liquid and the metal volume expansion coefficients, respectively, to zero. The ratio of the two pressure contributions is then approximately: sphere P Gs m s 1 medium P G 3 2r 3 2r 2 r m m m n n n (5.35) Here medium P and P are the pressure contribution at frequency from the sphere m m sphere (s) and the medium (m) measured far away from the nanoparticle in the medium, and r r / 0 a. If no interfacial heat resistance exists between the nanosphere and the n fluid, then spectral temperatures at the interface are equal and is interfacial heat resistance at the interface, then G G. If there earlier in section The ratio of the contributions to the pressure measured some distance away from the source is then reduced to: 112 s m s G G, and the temperature undergoes a jump at the interface. In order to emphasize the importance of the heat transfer from the solid sphere to the fluid environment a Gaussian temperature profile of width for both the sphere and 1/ the medium, such as T t 2 exp t / 2 can be assumed as mentioned m
135 sphere Pm 2 m s s 1 e medium 3 2 P 3 2r 2r r m m n n n (5.36) which is same as equation (5.24). From equation (5.36), it is clear that the spatial decay of the temperature profile, the temporal broadening due to a finite heat transfer through the interface, and the characteristics of the interface influence the balance of the two contributions. It should be pointed out that equation (5.36) assumes a temporal temperature profile in the sphere; for the situation of constant fluence, however, the temperature in the sphere is higher with large interfacial resistance than with lower resistance. This effect is included in the Gaussian profile to the order of approximation used here. For a gold sphere in water and rn 1, little broadening of a nanosecond laser pulse m s, and the range of frequencies used in photoacoustic imaging with ultrasound transducers (f = 2π = 1 50 MHz), so that m s 113 / 2 1 and / 0.2 at 20 C in aqueous solvent, and equation (5.36) predicts the pressure ratio s m between the sphere and the water medium of about one percent. Therefore, the photoacoustic signal is generated mostly in the fluid surrounding the spherical particle. Water is ideally suited to study the origin of the photoacoustic signal from a nanoparticle colloid because the thermal expansion of water at atmospheric pressure vanishes at 3.98 C, and therefore any photoacoustic signal generated by the heating of water at this temperature vanishes, while sound generated by the sphere will still be transported 14. Experiments were performed with polyethylene glycol stabilized gold spheres, and silica coated gold spheres with silica thickness of 6, 18 and 38 nm (Figure 5.3 (a), (b) and (c)) using a flow capillary suspended in a thermostated bath, 5 ns pulses from a Nd:YAG pumped OPO laser, and imaging with a single element focused 7.5 MHz ultrasound transducer (as shown in Figure 5.1). The PEGylated gold nanospheres were produced from citrate stabilized gold nanospheres by exchanging citrate with the
136 biocompatible mpeg-thiol, and silica coated gold nanospheres were prepared by coating silica directly on PEGylated gold nanospheres via a modified Stöber method The peak optical density of each sample was adjusted to 1.5 to achieve equal absorption, and the laser was tuned to the peak wavelength of each sample. We verified with Mie theory that at the extinction peak the scattering contribution to the extinction cross-section due to the addition of a silica shell was well below 10% for silica shells between 0 nm and 40 nm thickness (see supplement for the experimental extinction spectra of gold nanosphere solutions). A laser fluence of 5 mj/cm 2 was chosen to prevent bubble formation in organic solvents, and to avoid damage to the nanoparticles. We have not found that the fluence used here causes any damage to the bare or coated nanospheres, and the PA signal remained stable over at least 128 laser pulses. 114
137 (a) 50nm 50nm (b) 50nm 50nm 50nm (c) 50nm 50nm Figure 5.3: TEM images of silica coated nanospheres with silica shell of (a) 6nm, (b) 18nm, and (c) 38nm, respectively. 115
138 PA Signal (A.U.) PA Signal (A.U.) PA Signal (A.U.) (a) (b) (c) Temperature ( o C) Figure 5.4: Photoacoustic signal amplitude of gold nanospheres with varying silica shell thickness as a function of temperature in different environments: (a) water (b) silicon oil (c) toluene. The silica thickness is: 0 nm (circle), 6 nm (triangle), 18 nm (square), and 38 nm (rhombus). 116
139 Figure 5.4 (a) shows that the photoacoustic signal of PEGylated and silica-coated gold nanospheres in aqueous solution indeed falls below noise level around 4 C. The photoacoustic signal increases nearly linearly with temperature with a slope that depends on the silica thickness. The slope is lowest for the PEGylated gold nanospheres and is maximal for 18 nm silica. An approximately linear dependence of the photoacoustic signal with temperature owing to the raise of the Grüneisen parameter of water has been seen for gold nanospheres in water above 25 C 18, and for homogeneous aqueous absorbers without nanoparticles, where the photoacoustic signal also disappeared at 4 C.[32] The photoacoustic signal therefore originates mostly from the surrounding water, and the signal from the absorber is below the noise level even if the size of the nanoparticle is increased 2-fold by the silica coating. The relative contributions of the nanoparticle, including shell, and the water can be estimated by fitting the measured photoacoustic signal to the equation nanoparticle contribution NP P out NP Ptot P b H2O T, assuming that the, including the PEG-thiol capping and binding layer, is constant, and the Grüneisen parameter s temperature dependence T is dominated by that of the expansion coefficient, as demonstrated in Figure 5.5. The proportionality factor b is a function of shell thickness, and the nanoparticle contribution to the signal NP P, which is below noise level for the fluence used here, also varies linearly with thickness (Figure 5.6). The good fit to the thermal expansion of water, the magnitude of the nanoparticle contribution, and its linear dependence with thickness all confirm the trends of the theoretical estimate for a simple sphere with and without a shell. 117
140 Grüneisen parameter (dimensionless) PA Signal Amplitude (A.U.) Temperature ( o C) Figure 5.5: Temperature dependence of the Gruneisen parameter (left axis) for water (open circle) and toluene (open triangle), and of the measured photoacoustic signal (right axis) of water (solid circle) and silicon oil (solid square). The error bar represents the standard error of 16 signals of eight pulses each. 118
141 PA Signal (A.U.) Measured PA Signal (A.U.) Calculated Grüneisen parameter Contribution particle/water (%) Amplification b (a.u.) (a) (b) Temperature ( o C) (c) Temperature ( o C) Contribution from P NP (%) residual amplification 750 contribution at 25 C Silica thickness (nm) Figure 5.6: (a) Measured photoacoustic signal of water as a function of temperature compared to the calculated Grüneisen parameter (line). (b) Fits of the photoacoustic signal of the nanoparticles in water at varying shell thickness with the thermal expansion coefficient of water. (c) Results of the fits in (b) showing the relative and the absolute nanoparticle contribution pnp, and the proportionality constant of the fit. Error bars in (a) and (b) are the standard error of 16 signals of eight pulses each. 119
142 The increase in slope reflects the increase in amplitude of the photoacoustic signal at 20 C, which we have recently reported for the silica-coated nanorods 15. The amplitude of the photoacoustic signal is determined by the Grüneisen parameter of the solvent, as well as the details of the profile of heat transferred from the nanoparticles to the liquid, which depends on the optical absorption of the nanoparticles, the laser fluence, and the interfacial heat conductivities between the two materials. We had concluded there that the signal increase due to silica coating was mainly due to changes in the interfacial heat resistance between the gold and water, and an enhanced efficiency of the heat transfer between the solid and water due to the coating, since all other parameters were approximately constant. The effect of the heat transfer between nanoparticle and solvent on the photoacoustic signal was further investigated by replacing the aqueous solvent with two non-polar solvents with different thermal properties: polydimethylsiloxane (silicon oil) and toluene. The surface functionalization of the bare and silica-coated nanoparticles was changed to be able to suspend them in non-aqueous solvent. As shown in Figure 5.3 (b), the citrate of the citrate-stabilized nanospheres was replaced by octadecane-1-thiol, and the silica surface was functionalized with trimethoxy(octadecyl)silane by copolymerization 19. Silicon oil (Figure 5.4 (b)) shows three different features of the photoacoustic signal compared to water: a non-zero signal at 4 C, a bi-phasic and mostly opposite slope of the temperature dependence, and a decrease of the signal amplitude with silicon coating thickness. The signal follows the bi-phasic profile of the Grüneisen parameter of pure silicon oil, measured using the intrinsic absorption of the oil without nanoparticles (Figure 5.5), signaling that the oil is the major signal-generating medium despite the differences to water. However, the temperature dependence is more complex, as the 120
143 signal is not proportional to the measured Grüneisen coefficient of oil, and there is a leveling off at high temperatures. One possible reason may be residues of water in the silica shell left from the solvent exchange. The fact that silica coating the nanospheres reduces the photoacoustic signal in the oil over most of the temperature range is in agreement with our recent findings at 20 C 15. Toluene is known to have a very high interfacial resistance with citrate coated gold nanospheres and could therefore be a good test of the role that thermal interfacial transfer has on the balance of the contributions from gold and fluid to the signal. Figure 5.4 (c) shows the photoacoustic signal as a function of temperature in toluene. Between 15 and 45 C the signal follows the trend of the temperature dependent Grüneisen parameter of toluene (Figure 5.5) with a slight negative slope. A small signal amplification due to the addition of a silica shell can be seen, which differs from our finding for nanorods at 25 C 15. This difference may come in part from changes in the absorption cross section of gold spheres when a silica shell is added, which are not present for the longitudinal resonance of nanorods. Same set of experiments were also conducted for silica coated gold nanorods with same silica coating thickness ranging from 6 nm, 18 nm to 30 nm, respectively (Figure 5.7). Similar trends of the photoacoustic signal (Figure 5.8) were also observed for the silica coated gold nanorods in the three different solvent using the same experimental set up and under the same conditions. 121
144 (a) 100 nm (b) 100 nm (c) 100 nm Figure 5.7: TEM images of silica coated nanorods with silica shell of (a) 6nm, (b) 18nm, and (c) 38nm, respectively. 122
145 PA Signal Amplitude (A.U.) PA Signal Amplitude (A.U.) PA Signal Amplitude (A.U.) (a) (b) (c) Temperature ( o C) Figure 5.8: Photoacoustic signal amplitude of gold nanorods with varying silica shell thickness as a function of temperature in different environments: (a) water (b) silicon oil (c) toluene. The silica thickness is: 0 nm (circle), 6 nm (triangle), 18 nm (square), and 38 nm (rhombus). 123
146 In conclusion, the photoacoustic signal of a solution of gold nanospheres (or nanorods) in different solvents shows that the dominant photoacoustic signal is produced by its surrounding medium and hence carries the information of the thermal properties of the surrounding medium. This is despite the fact that the heat is generated by the absorption of light in the nanoparticle and therefore carries also the signature of the absorptivity of the gold nanoparticle and its interface to the solvent by determining the amount and time-dependence of the heat provided to the solvent. Photoacoustics can therefore be a new tool for investigating the thermal properties and transport through nanoparticle interfaces. 5.6 REFERENCES 1 Bayer, C. L. et al. Multiplex photoacoustic molecular imaging using targeted silica-coated gold nanorods. Biomedical Optics Express 2, , (2011). 2 de la Zerda, A., Kim, J. W., Galanzha, E. I., Gambhir, S. S. & Zharov, V. P. Advanced contrast nanoagents for photoacoustic molecular imaging, cytometry, blood test and photothermal theranostics. Contrast Media & Molecular Imaging 6, , (2011). 3 Homan, K. A. et al. Silver Nanoplate Contrast Agents for in Vivo Molecular Photoacoustic Imaging. Acs Nano 6, , (2012). 4 Jokerst, J. V. & Gambhir, S. S. Molecular Imaging with Theranostic Nanoparticles. Accounts of Chemical Research 44, , (2011). 5 Luke, G. P., Yeager, D. & Emelianov, S. Y. Biomedical Applications of Photoacoustic Imaging with Exogenous Contrast Agents. Annals of Biomedical Engineering 40, , (2012). 124
147 6 Mallidi, S., Luke, G. P. & Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends in Biotechnology 29, , (2011). 7 Manohar, S., Ungureanu, C. & Van Leeuwen, T. G. Gold nanorods as molecular contrast agents in photoacoustic imaging: the promises and the caveats. Contrast Media & Molecular Imaging 6, , (2011). 8 Wilson, K., Homan, K. & Emelianov, S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nature Communications 3, (2012). 9 Diebold, G. J., Sun, T. & Khan, M. I. Photoacoustic monopole radiation in 1- dimension, 2-dimension, and 3-dimension. Physical Review Letters 67, , (1991). 10 Khan, M. I. & Diebold, G. J. The photoacoustic effect generated by an isotropic solid sphere. Ultrasonics 33, , (1995). 11 Pastoriza-Santos, I., Perez-Juste, J. & Liz-Marzan, L. M. Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chemistry of Materials 18, , (2006). 12 Thierry, B., Ng, J., Krieg, T. & Griesser, H. J. A robust procedure for the functionalization of gold nanorods and noble metal nanoparticles. Chem. Commun., , (2009). 13 Gusev, V. E. & Karabutov, A. A. Laser Optoacoustics (AIP, 1993). 14 Hunter, S. D. et al. Acoustic-signals of nonthermal origin from high-energy protons in water. Journal of the Acoustical Society of America 69, , (1981). 125
148 15 Chen, Y.-S. et al. Silica-coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Letters 11, , (2011). 16 Frens, G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nature-Physical Science 241, 20-22, (1973). 17 Stober, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. Journal of Colloid and Interface Science 26, 62-&, (1968). 18 Shah, J. et al. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. Journal of Biomedical Optics 13, 9, (2008). 19 Chen, Y.-S. et al. in SPIE Photonics West Egerev, S. et al. Acoustic signals generated by laser-irradiated metal nanoparticles. Applied Optics 48, C38-C45, (2009). 126
149 Chapter 6: Development of Dye Doped Molecular Specific Photoacoustic Nano-amplifier Molecular Probes In previous chapters, we have developed photostable PA nanoamplifiers which are expected to significantly enhance the contrast of PA images. In order to use the developed PA nanoamplifiers for PA molecular imaging, they are required to be conjugated with molecular specific moieties for molecular labeling capability. In this chapter, we focus on developing the chemical strategies to conjugate biomarkers on the silica surface of the PA nanoamplifiers. We first functionalize the surface of the synthesized nanoamplifiers with functional groups through silane chemistry. The particles, then, are conjugated to the monoclonal anti-epidermal growth factor receptor (anti-egfr) antibodies to target human epidermal growth factor receptor which is expressed or highly expressed in a variety of human tumors including non-small cell lung cancer (NSCLC), breast, head and neck, gastric, colorectal, esophageal, prostate, bladder, renal, pancreatic, and ovarian cancers 1. After conjugation, polyethylene glycol polymers are filled into the gaps between antibody and silica to improve particle colloidal stability and pharmokinetic property in biological environment, also prevent the nonspecific cell from uptaking the nanoamplifiers. 6.1 SPECIFIC TARGETING OF NANOPARTICLE Transport nano-agents from circulation system in the body to cancer cells require the following steps 2-4. First, the nano-agents should be able to flow around in the circulation system. Second, the nano-agents need to pass through vessel walls. Finally, the nano-agents should penetrate through interstitial space to reach target cancer cells. When the nanoagents reach tumor, there are several ways for specific accumulation which, in general, could be achieved using passive and active strategies 5-6. The former 127
150 approach uses the unique properties of the tumor microenvironment. In tumors, in order to serve fast growing cancer cells, tissues are significantly angiogenic, the vasculature is tortuous and leaky, and their diameters are irregular and the walls are thin, which also lead to the heterogeneous blood flow 2,7-8. Because of this morphologically abnormal organization of the vasculature, tumors are usually lack of functional lymphatic vessels 2,7-8. The extravasation and the limited lymphalic drain allows the enhanced permeability and retention (EPR) effect 2,7-8. As the result of EPR effect, the accumulation of nanoparticles with specific size and long circulation half-life in the tumor has been found up to 100 times than in normal tissue 9. Therefore, in this case, no active targeting ligands are required, when these angiogenic tissues are of pathological interest. The degree of nanoparticle accumulation depends on the size of the interendothelial gap junctions and tran-endothelial channels. In general, the particles with the size smaller than 200nm are the most effective for extravasating the tumor microvasculature 9. The general strategy of passive form targeting is to use biocompatible polymers such as dextran and polyethylene glycol as the particle coating. The limitation of the passive delivery is the inability to achieve sufficient accumulation and thus cause undesired system side effect In comparison to passive targeting, the other target strategy is active (or pathotropic targeting). Active targeting is to deliver the nanoagents to uniquely identified site without causing undesired system side effect elsewhere, which is typically achieved through the manipulation of pharmakinetic, size, and shape of the nanoagents It is known that the surface of cancer cells express many molecules which could be used as markers for recognizing the cancer cells. Many of these molecules are involved in promoting tumor angeogenesis. Tumor also tends to develop lymphatic vessels. Several markers of lymphatic endothelium have been discovered. Active tumor targeting is typically achieved by both local and systemic administration of nanoagents with targeting 128
151 molecules conjugated on the particle surface that can recognize and bind to the specific ligands that are unique to cancer cells. Several different types of molecules, therefore, can be attached to nanoparticles to yield active targeting. A range of biomoieties can be conjugated to the nanoparticles including low molecular weight ligands (folic acid, thiamine, dimercaptosuccinic acid), peptides (RGD, LHRD, antigenic peptides, internalization peptides), proteins (BSA, transferrin, antibodies, lectins, cytokines, fibrinogen, thrombin), polysaccharides (hyaluronic acid, chitosan, dextran, oligosaccharides, heparin), polyunsaturated fatty acids (palmitic acid, phospholipids), DNA, plasmids, sirna, and so forth. 6.2 ACTIVE TARGETING USING ANTIBODY AS BIOMARKER In addition to all the moieties mentioned in 6.1, monoclonal antibodies represent one of the most interesting targeting molecules used to achieve the active targeted delivery of a nanoagent. Albet most of the mentioned small molecular moieties have shown proven selectivity and clear advantages such as the ability of crossing biological membranes, they still have limitations. For instance, certain regions of the TAT-peptide also known as protein transduction domains are able to pass biological membranes by mechanisms that are independent of transporters and receptor-mediated endocytosis. But the susceptibility of the peptides to be denatured by proteases and their short vascular circulation lifetimes are their main drawbacks. Synthetic molecules of DNA or RNA known as aptamers are also able to recognize specific proteins. The main advantage of using aptamers compared to antibodies is the in vitro selection process without using animals, in addition to their small size, lack of immunogenicity, and ease of isolation. However, the main disadvantages of using aptamers compared to antibodies are the high cost to produce them especially in large quantities and their susceptibility to enzymatic 129
152 degradation. The advantages of antibodies over small molecule markers such as aptamers, therefore, include predictable pharmacokinetic properties, longer circulation half-life, not susceptible to nuclease degradation, and so on. Besides, antibody technologies are widely distributed because the early intellectual property either never existed or has expired. For instance, some of these antibody-based drugs have already undergone clinical development and have been successfully translated into the clinical test. Such examples include rituximab (Rituxan ), trastuzumab (Herceptin ), cetuximab (Erbitux ), and bevacizumab (Avastin ). Rituximab was approved by the FDA for treating B-cell lymphoma in Another successful therapeutic mab is trastuzumab, which binds to HER2 receptors and was approved by the FDA for treating breast cancer in The conjugation of nanoparticles with antibodies combines the properties of the nanoparticles themselves with the specific and selective recognition ability of the antibodies to antigens. 6.3 DIRECTIONAL CONJUGATION OF ANTIBODY TO SILICA SURFACE We developed three chemistry schemes to directionally conjugate EFG-receptors on the silica surface and one random directional conjugation scheme as a reference for comparing the targeting efficacy of the three directional conjugations. For the directional conjugation, we adopted the method reported by Kumar et al. to functionalize the nonbinding region (FC) of antibody 13. Specifically, Anti-EGFR (Ab-3) Mouse mab (225) is used as an example of biomarker to test our conjugation strategies. Oxidizing polysaccharide residues in a glycoprotein of antibodies with sodium periodate (NaIO4) generate aldehyde groups on the heavy chain. Then, we develop chemical strategies to directionally conjugate the functionalized antibodies on both silica and lipid surface. 130
153 Table 6.1 Monoclonal antibodies approved for human therapy by FDA and/or EMEA
154 6.4 MULTI-IMAGING MOLECULAR PROBE BASED ON PA NANOAMPLIFIER Dye doped silica coated gold nanoparticles reveal several advantages over dye coated silica nanoparticles. First, dye molecules that are trapped into silica matrix can show reduced photobleaching and photodamage, since silica matrix could prevent dye molecules from contacting the oxygen. In addition, very broad range of the small molecular dyes are hydrophobic, thus, in order to utilize these dyes in aqueous biological environment, chemical modification of the structure of these dyes are usually required or the dyes are required to be embedded into a hydrophobic carrier such as a lipid core. Using a hydrophobic silica precursor, the hydrophobic dye can be brought from organic phase into hydrophilic silica matrix, which allows these dyes to be used in hydrophilic environment. Finally, in most cases, excitation of a localized surface plasmon polariton leads to high electromagnetic energy concentrated in regions near the particle surface. The dye molecules doped in silica coated gold nanorods are at the proximity of the gold nanoparticle surface and thus, are expected to better enhance several linear and nonlinear optical processes. By taking these advantages, the dye doped silica coated metallic nanoparticles have been utilized for the development of various surface-enhanced spectroscopy and imaging techniques, such as surface-enhanced Raman scattering (SERS), surface-enhanced one and multi-photon fluorescence imaging and surface enhanced second-harmonic generation. In combination with the characteristic enhancement of the photoacoustic signal, dye doped silica coated gold nanorods can provide versatile applications for biomedical imaging. Several methods have been developed to trap dye molecules into the silica shell. When using Stöber method to coat silica onto nanoparticle surfaces, which are composed of hydrolysis of a silica alkoxide precursor (such as tetraethyl orthosilicate, TEOS) in an alcohol (such as isopropyl alcohol or ethanol) and aqueous ammonium hydroxide 132
155 mixture. Silicic acid is produced during hydrolysis and, when its concentration is above its solubility in ethanol, it nucleates homogeneously and forms silica particles of nanometer size. To dope the dyes into silica matrix, the hydrophilic dyes can be dissolved in alcohol solution and capsulated in the silica matrix during silica deposition. The method is comparatively simple and both organic and inorganic dyes can be incorporated using this method. However, the main disadvantage of the approach is that the dye could leak out from the silica matrix, since the dye molecules are not physically bound to the silica matrix. To prevent the leakage of the encapsulated dyes from the silica matrix, a method has been optimized to synthesize dye-doped silica nanoparticles by covalently attaching organic fluorescent dye molecules to the silica matrix. The procedure involves two steps: The dye is chemically bound to an amine-containing Saline agent (such as 3- aminopropyltriethoxysilane, APTS), and then APTS and TEOS are allowed to hydrolyze and co-condense in a mixture of water, ammonia, and ethanol, resulting in dye-doped silica nanoparticles. This approach enables the incorporation of a variety of organic dye molecules into the silica nanoparticles. However, the limitation of this approach is that the co-condensation of APTS-dye complex to silica matrix could induce the morphological change of the silica, and the high concentration of APTS could also lead to instability of silica growth solution. Therefore, the amount of the dye that could be incorporated is limited. In order to overcome the limitations, in this chapter, a new chemical scheme was developed to covalently bind fluorescent dyes into silica shell but prevent the cause the instability of silica growth solution. One of the improvement of the silica coating process, described in chapter 3 is that the PEG-thiol was used as silica-to-gold coupling agent. The charge neutral PEG stabilized the gold nanorods in silica growth solution and thus resulted in a better control of the silica coating process. Instead of PEG-thiol, in the 133
156 protocol to prepare dye doped silica gold nanorods, the PEG-thiol with an amine end group was used instead. The amine reactive dyes first reacted with amine groups of the amine-peg-thiol linker. The dye-peg-thiol complexes then were used to replace the CTAB on the gold nanorod surface through ligand exchange. The silica was then coated on the PEGylated gold nanorods by using the modified Stöber method described in chapter 3. This method provides two advantages over the method described previously. First, since the dye doped process and silica coating process is separated, the stability of silica process is not disturbed and the morphology of the silica coating is also remained. In addition, the distance between dyes and gold can be adjusted by using different molecular weight of heterobifunctional PEG linkers to achieve an optimal plasmonic enhancement. 6.5 METHODS Preparation of Dye Doped Silica Coated Gold Nanorods In this chapter, amine reactive dye, Rhodamine B isothiocyanate (RITC) was used as an example to dope silica coated gold nanorods. The isocyanate group (-N=C=O) is a highly unsaturated and extremely reactive group. It can react with both electron donor and electron acceptor functional groups. The most important groups that react with isocyanates are amino, hydroxyl and carboxyl groups. Among these groups, isocyanates and primary amino group has highest reactivity and the reaction does not require any catalyst. Isocyanate group reacts with amino groups at 0-25 o C to form substituted urea. In particular, 5 mg of RITC and 10 mg of amine-peg-thiol was dissolved in 5mL of water. The mixture was stirred for 24 hours in dark. After RITC-PEG solution was ready, the CTAB-stabilized gold nanorod dispersion was added to an equal volume of RITC-PEGthiol (0.2 mm) aqueous solution under vigorous stirring. The mixture was sonicated for 5 134
157 minutes and left to react for 2 hours. Excess RITC-PEG-thiol molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3000 g for 10 min and the RITC- PEGylated gold nanorods were re-suspended in water Fluorescent labeling of antibody Commercial antibody labeling kid, Alexa Fluor 355, was used to prepare fluorescent antibodies for bioconjugation efficacy study. Specifically, 1mL of sodium bicarbonate (1M) solution was prepared. Antibody was diluted to 1 mg/ml by phosphate buffered saline at ph 7.2, then add one-tenth volume of 1 M sodium bicarbonate buffer. Transfer 100 µl of the antibody solution to mix with dye in a small vial. Incubate the solution for 1 hour at room temperature. Every minutes, gently invert the vial several times in order to mix the two reactants and increase the labeling efficiency. After reaction, the antibody-dye mixture was transferred to a purification spin column which contains purification resin. Place the spin column in to a 15 ml plastic centrifuge tube and spin in centrifuge for 5 minutes at 1100 g using a swinging bucket rotor. After centrifugation, the collection tube will contain labeled protein in approximately 100 µl of PBS, ph 7.2 with 2 mm sodium azide; free dye will remain in the column bed Determining degree of labeling The degree of labeling was determined by ultraviolet to visible (UV-Vis) extinction spectroscopy. Absorbance spectra are collected from a 100 L nanoparticle suspension in a 96-well microlitter plate reader (BioTek Synergy HT). The following equation is used to calculate the concentration of protein in the sample: A 280 (A ) dilution factor Pr otein concentration (M) = (6.1) 135
158 where 203,000 is the molar extinction coefficient (ε) in cm 1 M 1 of a typical IgG at 280 nm, and 0.19 is a correction factor for the Fluorophore s contribution to the absorbance at 280 nm. The degree of labeling can be estimated from calculating the protein concentration and the measured absorbance at 346 nm by the following equation: Dye(mole) A 346 dilution factor [Pr otein(mole)] protein concentration (M) (6.2) Surface Functionalization of Silica Surface Maleimide-PEG 5K Functionalized Silica Coated Gold Nanorods Silica surface of silica coated gold nanorods were functionalized by silane coupling agent, maleimide-peg 5K -silane, to decorate maleimide groups and a layer of PEG on particle surface via acid catalyzed silane hydrolysis. Specifically, 2 ml of acetate buffer (10mM, ph 4.75) was mix with 5 ml of aqueous silica coated gold nanorods solution (10 13 particles/ml). The mixture then was added to a 22mL of separately prepared maleimide-peg 5K -silane solution which contains 10mg of maleimide-peg 5K - silane, 20mL of ethanol, and 2mL of water. The reaction was stirred on a hotplate at room temperature for 48 hours. After which, the excess free maleimide-peg 5k -silane was removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3000 g for 10 min and the maleimide-peg 5k functionalized gold nanorods were re-suspended in water. Thiol Functionalized Silica Coated Gold Nanorods (3-mercaptopropyl) trimethoxysilane was used as coupling agent to graft thiol moieties on the surface of silica coated gold nanorods under base condition. In particular, silica coated gold nanorods was diluted by ethanol to the concentration of particles/ml. Two hundred fifty L of concentrated Ammonium hydroxide reagent (30%) was added to 30 ml of diluted silica coated gold nanorod solution under stirring. 136
159 After 5 minutes stirring, 3mL of (3-mercaptopropyl) trimethoxysilane (2% Vol./Vol. in ethanol) solution was added to the mixture and the reaction was stirred at room temperature for 48 hours. The excess (3-mercaptopropyl) trimethoxysilane molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3000 g for 10 min and the epoxide functionalized gold nanorods were re-suspended in water. Epoxide Functionalized Silica Coated Gold Nanorods The silica surface of the silica coated gold nanorods was activated by expoxysilane chemistry to graft epoxide functional group. Briefly, silica coated gold nanorods were diluted by ethanol to the concentration of particles/ml. Twenty milliliter of glycidyloxypropyl trimethoxysilane (2% Vol./Vol. in ethanol) was added into the diluted nanoparticle solution. After reaction with stirring for 48 hour at 37 C, excess glycidyloxypropyl trimethoxysilane molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3000 g for 10 min and the epoxide functionalized gold nanorods were re-suspended in water Conjugation of Antibodies on Silica Surface Directional Conjugation We adopted the method reported by Kumar et al. to control the binding orientation of antibodies on the surface of silica coated gold nanorods. In this process, the polysaccharide residues in a glycoprotein of antibodies were oxidized with sodium periodate (NaIO 4 ) to generate aldehyde groups on the heavy chain.[47] Then, in order to tag desired functional groups on the heavy chain, an aldehyde reactive heterobifunctional linker was coupled to the antibody through aldehyde-to-hydrazide reaction. Theses functional group then were used to conjugate with surface functionalized silica coated 137
160 Figure 6.1: Schematic illustration directional functionalization and Fluorescent labeling of antibody gold nanorods prepared in section Specifically, Anti-EGFR (Ab-3) Mouse mab (225) will be used as an example of biomarker to test our conjugation strategies. Two heterobifunctional hydrazide linkers, n-k-maleimidoundecanoic acid hydrazide and, hydrazide-polyethylene glycol-thiol, were used to conjugate antibody to different surface functionalized silica coated gold nanorods. To prepare antibody-linker complex, first, dilute or re-suspend antibody solution (1:1 targeting/antibiotin antibody) to 1mg/mL in 1 ml of 100 mm Na 2 HPO 4, ph 7.5. Ten milliliter of 100 mm NaIO 4 was added to and incubate with 100 ml of antibody solution in the dark for 30 minutes at room temperature. After that, the reaction was quenched with 500 ml of 1X PBS. The linker was dissolved in PBS buffer to prepare 40mM linker solution, then added 2 ml of this linker solution to the antibody solution and incubated for 1 hour. One milliliter of (4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid) (HEPES) buffer (40mM) was added to the antibody-linker mixture to stop the reaction. The excess linker was removed by centrifuge filtration (Amicon ultra-15, Millipore) at 2000 g at 4 ºC until 75% of solution was filtered. Finally, resuspend the retained solution in 40 mm HEPES to a final volume of 1 ml and an antibody concentration of 100 g/ ml. To conjugate functionalized antibodies to silica coated gold nanorods. One hundred microliter of malimide functionalized antibody solution was mixed with 1 ml of thiol functionalized silica coated gold nanorod solution. The mixture was shaken gently in the shaker for one hour at room temperature. After 138
161 which, 1mL of PEG-thiol (1mg/mL in HEPES buffer) was added to the antibody solution and the mixture was shaken at room temperature for 30 minutes. Finally, the excess free antibodies and linkers were removed by centrifuge filtration (Sartorius, 300K MWCO). Similar protocol was used to conjugated thiol functionalized antibody to both expoxide and malimide-peg functionalized silica coated gold nanorods. Random Directional Conjugation In comparison with directional conjugation, a random directional conjugation scheme to conjugate Anti-EGFR (Ab-3) Mouse mab (225) to thiol functionalized silica coated gold nanorod was also developed. In particular, the sulfosuccinimidyl-4-(nmaleimidomethyl)cyclohexane-1-carboxylate (sulfo-smcc) linker was used to cross link the antibody on the silica surface. Specifically, sulfo-smcc was dissolved in 0.1 M sodium phosphate buffer (ph 7) to the concentration 40mM. One hundred micro-liter of antibody solution (1mg/mL in 1 ml of 100 mm Na 2 HPO 4, ph 7) was mixed with sulfo- SMCC solution. In this mixture, the NHS ester of sulfo-smcc was reacted first with the amine group of antibody. The excess linker was removed by centrifuge filtration. Finally, the malimide functionalized antibody solution was incubated with thiol functionalized silica coated gold nanorod solution to non-directionally conjugate antibody on the silica surface Cytotoxicity test Cytotoxicity studies were performed to ensure biological safety. Cell lines, such as skin cancer cell line, A431, were used to determine potential toxicity. For cell viability studies, five thousand cells (per 100μL of media) were seeded in each well of a 96-well plate. The cells were allowed to attach and grow to be confluent in the 96-well plate for 24 hr. After which the media was removed and replaced with suspensions of anti-egf 139
162 receptor antibody conjugated silica coated gold nanorods solution at various concentrations representing 10 8 particles/ml, particles/ml, and particles/ml will be incubated with the cells for 48 hours. Next, an 20 μl of [3-(4,5-dimethylthiazol-2- yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2h-tetrazolium (MTS) solution will be added to determine mitochondrial activity. Dehydrogenase enzymes prduced in metabolically active cells educes the MTS to formazan product, which absorbs light at approximately 490 nm. A higher absorbance at 490 nm indicates more cell viability. Cell viability was determined by comparing the absorbance of wells containing no nanoparticles to that of wells containing nanoparticles using a one-way ANOVA F test Optical Microscopy Imaging A431 and MDA-MB-435 cells were grown on 5mm round microscope slide cover slips in a 96 well plate. The antibody conjugated silica coated gold nanorods were dispersed in the media. One hundred microliter of gold nanorod solution with concentration of particles/ml was added into each well to be incubated with 5000 cells (per 100 μl of media) on the cover slips for 24 hr. After 24 hr, the media containing free gold nanoparticle solution was removed and the coverslip was washed 3x with 5 ml of DPBS. Then the cells were fixed with a solution of 1% formalin in DPBS for 20 min on the cover slips. Finally the formalin-dpbs was aspirated away and the cover slips were mounted on slides using Fluoromount and sealed on the edges with nail polish. Images of the cells were captured using a Leica Inverted Microscope with fluorescence and bright field modes. 140
163 Figure 6.2: Schematic illustration of the bioconjugation processes. (a) Three chemistry schemes show antibodies directional conjugate to silica coated gold nanorod surface. (b) Antibodies are conjugate to silica surface with random direction. 141
164 6.6 RESULTS AND DISCUSSIONS PREPARATION OF DYE DOPED SILICA COATED GOLD NANORODS The TEM image, as shown in Figure 6.3 (a), illustrates the surface morphology of RITC doped silica coated gold nanorods. The silica shell is conformally coated on the gold nanorod surface. Based on the TEM image, the RITC molecules have negligible effect on the silica coating. And thus, the silica shell thickness can be controlled using the same method described in chapter 3. A noticeable absorption appears in the wavelength around 550 nm indicating the dye molecules, rhodamine b isothiolcyanate, have been loaded in to the silica matrix. We have discussed in chapter 2 that without dye molecular involved, after PEGylation, the longitudinal absorption peak of the gold nanorods will slightly blue shift for about 3 to 5 nm, then after silica coating, the resonance peak will red shift about 20 nm. However, it is noticed that the PEGlyation process using RITC-PEG-thiol molecules can cause the longitudinal absorption peak wavelength of the gold nanorods to first red shift about 5 nm instead of blue shift. After silica coating the absorption peak wavelength will further red shift about 40 nm. These red shifts are attributed from the high index value of the RITC molecules, which provide an added opportunity of producing nanoparticles resonating at near infrared wavelength but with a smaller aspect ratio. Figure 6.3 (b) shows, the UV- Vis extinction spectra of dye-doped silica coated gold nanorod solution. A small absorption appears on the shoulder of the transverse peak around 550 nm, which confirms the successful loading of the RITC molecules on the surface of gold nanorods. 142
165 Extinction (a) 50 nm (b) Optical absorption of Dye Wavelength (nm) Figure 6.3: (a) TEM image illustrates the surface morphology of dye doped silica coated gold nanorods. (b) UV-Vis extinction spectra dye doped silica coated gold nanorod solution. A noticeable absorption appear in the wavelength around 550 nm indicate the dye, rhodamine b isothiolcyanate, have been loaded in to silica matrix. 143
166 6.6.2 ANTIBODY CONJUGATION OF SILICA COATED GOLD NANORODS Thiol to mailimide reaction is one of the popular standard reaction used for bioconjugation. In the directional bio-conjugation sachems, two chemical strategies based on thiol to mailimide reaction are developed. The differences between these two thiol-tomailimide conjugations are that in the first reaction, the thiol group was first functionalized on the silica surface and the antibody was linked to nanoparticle through a short (19 Å) crosslinker. In contrast to this conjugation, in the second thiol-to-malimide conjugation, a layer of PEG (5K Da) separate the nanoparticles and antibody. The conjugation efficacy and the nanoparticle solution stability in biological environment of these two was compared. Instead of using malimid groups, the third conjugation use epoxide-to-thiol reaction. Epoxysilane agents have been applied widely to a variety of polymer composite materials to enhance the stability. Epoxy functionalized surfaces are also very attractive systems for covalent biomolecule immobilization beacuse epoxy is reactive under mild experimental conditions toward many nucleophiles including amine, hydroxyl and thiol groups which are commonly seen in the biological molecules. The chemical bond thus formed is very stable. Epoxy-functionalized surface is also stable under normal storage conditions. Therefore, epoxysilane modification provides a chemically reactive surface for covalent conjugate antibody on the silica surface. After back fill the PEG-thiol in to the gap between antibody and nanoparticle, the stability test showed that the conjugated nanoparticles were stable in the biological solution, such as saline solution. As shown in Figure, the UV-Vis spectra of the conjugated naoparticles produced from this conjugation scheme reveal a noticeable absorption peak at 280nm which indicates that the conjugation works between epoxy reactive silica surface and the 144
167 Extinction Optical absorption of Antibody at 280nm Optical absorption of RITC Wavelength (nm) Figure 6.4: UV-Vis extinction spectra of antibody conjugated silica coated gold nanorod solution. A noticeable absorption appear in the wavelength around 280 nm indicate the conjugation of antibody to the gold nanorods. 145
168 thiolated antibody. The targeting specification of this antibody conjugated gold nanorods were test with cancer cells, and the result was recorded using fluorescent, and bright field optical microscopy. The result is illustrated in Figure 6.5. It shows a high cell uptake of nanoparticles in the case of nanoparticle and A431 cell incubation. However, based on the fluorescent images when the MDA-MB 435 cells were incubated with nanoparticle, there was no cell uptake occured because MDA-MB435 cells are not EGF-receptor express. To ensure that the cell uptake in the A431 cells is not due to the nonspecific uptake, a blocking test was also performed. The free anti-egfr antibodies which amount are 100 times higher than total EGF recetors of the incubating cells were used to block the receptors on the A431 cells. Since free antibodies block the receptor. The cells showed very low cell uptake of nanoparticles. The result indicates that after conjugated with antibody, we have created the photoacoustic moelcular probes which are molecular specific. The targeting efficacy of the four antibody conjugation schemes (three directional conjugation, one nondirectional conjugation) were also compare using cell uptaking test, then the cell uptake was compared using fluorescent imaging. The images from these four cases indicate the first case, using malimide functionalized antibody to conjugate thiolized silica surface provides the highest conjugation efficacy and the expoxy approach provides the second highest conjugation efficacy. As we expected, the noorientic conjugation has the poorest targeting capability. 146
169 Figure 6.5: The bright field images and fluorescence images show the cell uptake of antibody conjugated gold nanorods in EGFR(+) A431 cell, in EGFR(-) MD435, and the blocking test suggesting the molecular specificity of the nanorods 147
170 6.6.3 CYTOTOXICITY TEST OF ANTIBODY CONJUGATION OF SILICA COATED GOLD NANORODS We applied the method methioned in section to test the cytotoxicity of the silica coated gold nanorods. According to testing on A431 cell, concentrations of silica coated gold nanoparticles up to particles/ml in media were not shown any toxicity effect. As shown in Figure 6.6, cells exposed to no nanoparticles and to concentrations of up to particles/ml showed no statistically significant variation in cell viability. The results suggested the dye doped silica coated nanoparticle which conjugate to antibody throught epoxy-to-thiol reaction did not exhibit no short term cytotoxic effects, even at concentrations as high as particles/ml. 148
171 (Directional) Rhodamine isothiocyanate Alexa Fluor 350-antibody Calcein-AM (I) (II) (III) (Nondirectional) Figure 6.6: The fluorescence images show the cell uptake of antibody conjugated gold nanorods in EGFR(+) A431 cell, with antibody conjugated gold nanorods. The first column shows the fluoresence signal from rhodamine b isothiocyanate in the silica shells, the second column show the signal from Alexa fluor 350 dye label on the antibody, and the third column show the sgnal from live stain, calceiin-am. 149
172 125 nontargeted targeted control Cell viability (%) Gold nanorod concentration (particles/ml) Figure 6.7: Cell viability data after 48 hr exposure to anti-egf receptor antibody conjugated silica coated gold nanorods at different concentrations in A431 cells 150
173 6.7 REFERENCES 1 Shin, D. M. et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clinical Cancer Research 7, , (2001). 2 Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nature reviews. Clinical oncology 7, , (2010). 3 Chauhan, V. P., Stylianopoulos, T., Boucher, Y. & Jain, R. K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annual Review of Chemical and Biomolecular Engineering 2, , (2011). 4 Jain, R. K. Transport of molecules across tumor vasculature. Cancer metastasis reviews 6, , (1987). 5 Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature reviews. Drug discovery 7, , (2008). 6 Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology 2, , (2007). 7 Narang, A. S. & Varia, S. Role of tumor vascular architecture in drug delivery. Advanced drug delivery reviews 63, , (2011). 8 Ruoslahti, E. Specialization of tumour vasculature. Nature reviews. Cancer 2, 83-90, (2002). 9 Gu, F. X. et al. Targeted nanoparticles for cancer therapy. Nanotoday 2, 14-21, (2007). 10 Bae, Y. H. & Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. Journal of Controlled Release 153, , (2011). 151
174 11 Ruoslahti, E., Bhatia, S. N. & Sailor, M. J. Targeting of drugs and nanoparticles to tumors. The Journal of cell biology 188, , (2010). 12 Wang, M. & Thanou, M. Targeting nanoparticles to cancer. Pharmacological Research 62, 90-99, (2010). 13 Kumar, S., Aaron, J. & Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nature Protocols 3, , (2008). 14 Arruebo, M., Valladares, M. & González-Fernández, Á. Antibody-Conjugated Nanoparticles for Biomedical Applications. Journal of Nanomaterials 2009, 1-24, (2009). 152
175 Chapter 7: Design of Multipulse Imaging System In biological tissues, the strong scattering and obscure nature often induce a large background noise and make it difficult to obtain a sharp and high contrast image with decent penetration depth. Two approaches have been investigated in enhancing the contrast of the biomedical images, and both involve the introduction of imaging agents. One approach is to introduce contrast agents, which are commonly plasmonic nanoparticles, resonating at certain wavelength to enhance the strength of the obtained signal at the imaging target. The second approach is to introduce dynamic agents, which through certain mechanism can suppress the background signal, and improve the imaging contrast. The dynamic imaging concept has been widely studied in optical imaging modalities 1. One way to introduce dynamic contrast agents in optical imaging is to design the contrast agents which can response in nonlinear optical effects, such as two photon fluorescence, two photon luminescence, and hyper Rayleigh scattering 2-6. However, these approaches share the similar drawbacks as seen in conventional optical imaging modalities of shallow imaging depth, which will be impractical to in vivo medical imaging when large penetration depth is required. Another way to achieve dynamic medical imaging is via certain signal modulation of the dynamic agents through magnetic, optical, thermal or other types of perturbations, which can create a selective response from solely the imaging agents which have been attached to the imaging targets; when demodulating the obtained signals, the background signal can be suppressed. In PA imaging modality, the dynamic imaging concept has been rarely studied. One major reason might reside in the lack of probes specifically designed for PA imaging. The main goal of the PA dynamic agents is to response specifically to the signal 153
176 and creates modulated acoustic signal instead of optical responses apart from the background. The main goal of this study is to demonstrate a dynamic contrast enhanced photoacoustic molecular imaging using photoacoustic nanoamplifiers. In the previous chapters, design, modification and optimization of the molecular probe tailored specifically for PA imaging have been discussed. We were able to achieve a new type of photoacoustic molecular imaging probe which can produce several folds stronger PA response, provide better thermal and biological environment stability, and molecular specification for a more efficient delivery and accumulation to the imaging targets. In this chapter, we focus on design a matching, customarized imaging system for these molecular probes to achieve the dynamic PA imaging scheme which can take better advantage of the unique photoacoustic property of the new developed molecular probes for an improved contrast. More specifically, a multi-arm interferometer was built in integration with the OPO system in order to create a nanosecond pulse train excitation source and fine tune the amplitude and the frequency spectrum of the PA signal generated from the molecular probes. Using multiple pulses with interval allows us to grasp a dynamic control of the PA signal generated from the target separately from the tissue, and to a certain extent, create matches with the frequency range of the imaging transducer to improve the detection efficiency as well as the image contrast. 7.1 THE DESIGN PRINCIPLES OF MULTIPULSE SYSTEM FOR DYNAMIC PA IMAGING In this paradigm, we aim to demonstrate that dynamic PA imaging schemes can be achieved by using multiple nanosecond optical pulse excitations when the time interval between pulses can be tailored at will. The nomenclature of PA dynamic imaging derives from the controllable "on" and "off" of the integrated PA signal of the imaging 154
177 target, with the background tissue bearing different responses. This difference originates from the breakdown of the stress confinement in the nanoparticle. It is known that the thermal relaxation time th, which characterizes the thermal diffusion, is estimated as proportional to the square of the characteristic dimension 2 th dc / th d c of the heated area r, ; while the stress relaxation time s, which characterizes the pressure propagation, is linearly proportional to the characteristic dimension as d / v s c s, and v s is the ultrasound velocity in the medium. When optically homogeneous medium, such as tissue, is illuminated by nanosecond pulsed laser, the entire illumination area is heated with the temperature gradient only at the heating boundary, thus d c is equal to the dimension of the illumination area r, which leads to th and s in the scale of second and micro-second respectively, provided r is in millimeter scale; therefore both thermal and stress are confined. Differently, when the nanoparticles with absorption peak wavelength tailored to a specific wavelength away from the tissue are illuminated by the same pulsed laser, only the nanoparticles other than the surrounding tissue are heated due to absorption; d c then coincides with the dimension of the nanoparticle a. Since the dimension of the nanoparticle is in the nanometer range, by taking into account the thermal diffusivity th of the material, th is in the scale of few tens of nanoseconds. In contrast, the stress relaxation time s is in few tens of picosecond scale, therefore stress is not confined in the nanoparticle case. In general, when stress confinement is satisfied, the generated PA signal is independent of the shape of the laser pulse, on the contrary, when the stress confinement is broken down, the PA signal is related to both the gradient of the optical pulse and the thermal response function of the nanoparticle. In this sense, the "dynamics" in the imaging is achieved by manipulating the temporal overlap of the optical pulse train, which can be obtained using multi-arm Michelson interferometer. The 155
178 time intervals between the excitation optical pulses are controlled by modulating the individual arm length in the interferometer. Figure 7.1 shows two scenarios of the different intervals of the optical pulse train. In the first case, when the optical pulse interval is same as the individual pulse width, the sum of the pulse train generates a hat-top pulse; in the second case, however, when the time interval is shorter, the overlap of the pulses builds up a triangle shaped input. Since the energy of both pulses are the same, when the stress confinement is satisfied for tissue, its PA response will stay remain unchanged. However, in contrast to tissue, the stress confinement is not satisfied for nanoparticle solution. Therefore, the change of the optical pulse shape leads to the change of photoacoustic profile and creates an "on" and "off" pattern, which will not occur in the surrounding tissue area. This "twinkled" change is a dynamic effect which can be used indirectly to improve the contrast of the PA imaging. Using multiple pulse train to create enhanced PA signal has been reported 7. The enhancement in the PA signal reported was a purely linear effect, where picosecond optical pulses instead of nanosecond pulses were used as the excitation, which creates a major difference. For short laser pulses in the picosecond scale, thermal and stress confinement were simultaneously satisfied in both tissue and nanoparticle ranges; in this way, the enhancement was only attributed to the accumulation of the heat energy deposited in the illuminated area, and signal would increase as pulse number increases. However, this linear effect would also lead to increment in the background signal at the same time, so the contrast of the image was not drastically improved even an increased PA signal can be generated. Another big advantage of using a tunable nanosecond pulse train arises from the fact that the unsatisfaction of the stress confinement condition can cause deformation of the PA signal in the time domain, which shifts the center frequency 156
179 Laser Pulses Pulse shape: retangular Pulse duration: 5nsec Pulse number: 4 x z d c a y NPs x z r=d c y Tissue (I) I PA 0 Off PA 0 On time 4 4 time o time I PA PA (II) On 0 On time time o time Figure 7.1: Comparison of PA signal generated from a nanoparticle and tissue as a function of time for two different time intervals in the optical pulse train. (a) The time interval is equal to the time duration of the input optical pulse. (b) The time interval is shorter than the time duration of the input pulse, so that overlap of the optical pulse in the pulse train results in the change of pulse shape, which further induces the change only in the PA single from the nanoparticle. 157
180 of the PA signal in the frequency domain and could possibly bring the peak of the PA signal into the detection window of the transducer at lower frequency, which will only increase the PA signal in the target area rather than the background, hence increase the contrast of the image. The theoretical model for the dynamic PA imaging is based on spherical absorbers embedded in a homogenous medium. The pressure transient, which is related to the PA signal, can be expressed as 8 : For r For r a: a: Ie vs PL r, t B t t bk r, t c v 2r (7.1) k0 Ie vs PL r, t B t bkr, t (7.2) c 2r v k0 where Ie Ie / 0 is the energy input per unit mass per unit time, which is proportional to the time derivative of the input laser pulse, and 0 is the pulse duration; B is the bulk modulus, is the thermal expansion coefficient, c v is the specific heat, which are all considered as constants. The transient pressure wave is generated as the laser is turned on, when turning the laser off, it is equivalent mathematically to add a negative pulse in the time domain. Therefore,,,, P r t t P r t t P r t (7.3) L L 0 The pressure wave propagates inside the absorber surface to the center then to the surface of the absorber, which takes time 2 a/ v. Two extreme conditions were considered. First, when a long laser pulse is the excitation source, which means the laser pulse duration 0 c, the negative pulse and the initial positive pulse are well separated; second, when an ultra-short laser pulse is the excitation, 0 c. c s
181 For the long pulse scenario, it is similar as the stress confinement is unsatisfied. It has been explained by 8 that during the laser illumination, the pressure inside the absorber grows uniformly and generates a compressive wave which propagates into the medium; the growth of the pressure inside the absorber at location r will stop until the tensile wave that propagates into the center reaches r ; this procedure takes time t a r / vs. This tensile wave will keep propagating and after passing the center of the absorber, it acts as a compressive wave and propagates to the other side of the absorber until gets reflected at the absorber/medium interface. The wave will be partially transmitted and partially reflected back to the absorber and repeat the same process similarly to plane wave propagation in a 2 dimensional Fabry-Perot system. In this case, the generated pressure wave will be two separated pulse (one positive and one negative), related to I e as shown in Figure 7.2 (a). When the laser pulse is short, which is in analogous to the scenario that the stress confinement is satisfied; the pressure wave generated is an N shape as shown in Figure 7.2 (b). This is also the similar situation as underwent in the tissue, no matter what kind of pulse profile is chosen, as long as the pulse energy is the same, the generated PA signal should be inertly dependent upon the shape of the input laser. 159
182 (a) In the unit of 0 (b) Figure 7.2: Pressure wave generated by two kinds of laser pulses. (a) Long laser pulse, the stress is not confined; (b) short laser pulse, the stress confinement is satisfied. (Adapted from Ref 8) 160
183 7.2 OPTIMIZATION OF THE EXCITATION SOURCE TO ENHANCE THE CONTRAST OF PHOTOACOUSTIC IMAGING Generating a photoacoustic signal in a nanoparticle system requires one extra step of heat transfer to dissipate heat from the nanoparticle to its surrounding media. For coreshell nanoparticles, the speed of heat dissipation could be adjusted by carefully choosing the shell material. Therefore, the heat dissipation speed in nanoparticles could be different from that in tissue. This difference could cause different photoacoustic signatures in terms of either intensity or frequency. By carefully designing the optical excitation, it is possible to maximize the difference of photoacoustic response to enhance the contrast or increase the sensitivity of the image. In this study, we used the multibeam interferometer to dynamically tune the pulse shape and duration Multiple Beam Interferometer Our theoretical analysis has indicated that when a pulse train is carefully chosen, the PA response can be tuned accordingly because in the nanoparticle region, stress is not confined. For the homogeneous absorbing medium, this PA signal is less dependent on the shape of the laser pulse because the stress confinement in this region is always satisfied. Therefore, we anticipate that if we could design a system to dynamically tune the pulse shape and duration, we could turn the nanoparticle signal "on" and "off" to differentiate the nanoparticle region from high intensity background signal. The multiple arm Michelson interferometer, as schematically shown in Figure 7.3, was built and integrated with wavelength tunable OPO laser and PA imaging system to provide a multiple pulse train with tunable pulse interval. Four pulses were created through the interferometer. To achieve a good beam splitting quality at the two beam splitters with a ratio of 1:1, the laser pulse was first passed through a polarizing beam splitter. The P polarized portion of the incidence thus was transmitted to the interferometer, with the S 161
184 polarized portion reflected and collected by a beam dump. The two delay lines were built on linear guides, which can provide flexibility and tunability of changing the delay length, thus manipulating the pulse intervals and shape. Due to space limitation, the longest arm was designed to achieve 21 nanosecond delay with respect to the first pulse in the pulse train. The shortest interval was chosen to be 3 nanoseconds, so that the four pulses joined as a single pulse with a Gaussian profile, as shown in the black curve in Figure 7.4 (a); at an intermediate 5 nanosecond pulse intervals, the four pulses form a flat top pulse, close to a rectangular pulse shape in the temporal domain; as the delay lines keep tuning, and when they were tuned to the maximum length, the intervals reach 7 nanosecond, the four pulses form an elongated pulse with rippled top, which is shown as the red curve in Figure 7.4 (a). By following the theoretical analysis, the generated PA signal from the nanoparticle solution, should be proportional to the time derivative of the laser pulses, which is shown correspondingly in Figure 7.4 (b). The larger the difference between the laser pulse slope, the larger the difference should be observed in the PA intensity when imaging the nanoparticle solution. 7.3 SIGNAL CALIBRATION Photoacoustic signal was calibrated using a needle hydrophone with frequency ranging from 1 khz to 30 MHz to measure two sets of samples under varying laser pulse durations and fluences. The experimental set up is shown in the inset of Figure 7.5. The samples were contained in a quartz cuvette, where the optical absorption is negligible at the imaging wavelength. A needle hydrophone was placed in the sample solution without direct illumination by the impinging pulsed laser. The laser pulse duration was modulated using the multipulse interferometer system introduced earlier in this chapter. Three 162
185 tunable tunable Beam Dump M10 M9 M2 PBS1 M1 PBS2 WP M8 M3 M7 M4 BS2 BS Laser M6 M5 M12 M11 Figure 7.3: The optical set up of multipulse interferometer, where the operation wavelength is 1064 nm with around 6.5 nanosecond pulse width. The symbols in the set up are: M, mirror; PBS, polarizing beam splitter; BS, beam splitter; WP, wave plate. The mirrors M5, M6, M11 and M12 are on linear guides, so the length of these two delay lines are tunable. 163
186 Intensity Intensity (a) pulse 1nsec 2nsec 3nsec 4nsec 5nsec 6nesc 7nesc (b) t (nsec) 1nsec 2nsec 3nsec 4nsec 5nsec 6nesc 7nesc t (nsec) Figure 7.4: (a) 4 pulse Michelson interferometer with different delay between pulses with assumption that the original pulse has 7 nanosecond pulse width. (b) The derivative of the pulse intensity as a function of time, which is proportional to the generated PA signal. 164
187 different pulse durations were achieved with distinct separations, one with the pulse width equal to the single pulse duration around 10 nanoseconds with controlled pulse intensity generated from the Nd:YAG laser system; the other two pulse durations were 18 nanoseconds and 39 nanoseconds (flat top pulse) by passing two and four pulses respectively as shown in Figure 7.6. Only results from the shortest and longest pulses were compared due to the most obvious discrepancy generated in the resultant photoacoustic signals. The two utilized laser pulse signals are shown in Figure 7.5, where the single pulse and the flat top long pulse were adjusted to carry the same amount of energy for a fair comparison. The samples used in the calibration were tetraaryldiamine dye solution and the developed silica coated gold nanoparticle solution with both of the optical absorption spectra adjusted to the same value at 1064 nm. The absorption spectra for the dye and nanoparticle solutions are shown in Figure 7.7. Signal calibration of a control sample was also conducted using water as the imaging solution, because water exhibits nonzero optical absorption at 1064 nm and it fulfills both stress and thermal confinement as a homogenous medium. 7.4 RESULTS AND DISCUSSIONS Figure 7.8 shows the calibrated photoacoustic signal for both the silica coated gold nanorod solution and dye solution with short and long pulse excitations under the same condition. The extrapolations of the detected signals all cross zero amplitude at zero fluence, indicating the collected signals were genuine photoacoustic signals, rather than 165
188 Laser Intensity (A.U.) Experimental setup: Pulsed Laser Needle hydrophone Quartz cuvette Sample Nanosecond (nsec) Figure 7.5: The two pulses used for the dynamic photoacoustic imaging, the energy for the two types of pulses are adjusted to be the same; the inset shows the experimental set up for signal calibration using multipulse interferometer based dynamic photoacoustic imaging system. 166
189 Normalized Laser Intensity (A.U.) Normalized Laser Intensity (A.U.) Normalized Laser Intensity (A.U.) nsec Nanosecond (nsec) nsec Nanosecond (nsec) nsec Nanosecond (nsec) Figure 7.6: Measured laser pulses from the output of the Michelson interferometer with different delay between adjacent pulses to achieve three different duration of pulse width. 167
190 Extinction (A.U.) Silica coated Au NRs Dye (Tetraaryldiamine) Wavelength(nm) Figure 7.7: Optical extinction spectra of the samples are adjusted to the same level at the imaging wavelength of 1064 nm. 168
191 speckles. It is confirmed that the short pulse can indeed generate a photoacoustic signal with stronger amplitude than flat top laser pulses under the same fluence as predicted from our theory. This effect has been explained explicitly in section 7.1, and illustrated by Figure 7.1. However, in comparing the nanoparticle and dye solutions, we had assumed that the dye solution is an optical absorbing homogenous medium, consequently, we had expected the nanoparticle solution to exhibit a much more dramatic signal discrepancy while switching from short to optical long pulses, and the signal from the dye solution should have been inert from the change of the pulse shape. On the contrary, it is seen from Figure 7.8 (b) that the slope change of the dye solution is much more dramatic, indicating the dye solution was actually a more extreme "nanoparticle" solution without satisfying the stress confinement condition. We further investigated the dye solution, and the calculated concentration of the dye molecule suggests the separation distance between the dye molecules is longer than 80 nm to ensure inefficient thermal coupling to satisfy its condition as an inhomogenous medium. The dye solution used in the calibration was a "nanoparticle solution" with nanoparticle size of only one nanometer. We confirmed our idea by conducting another series of experiments using pure de-ionized water as a control experiment. Water is an optical homogenous system with non-zero optical absorption at the wavelength of 1064 nm. The measured optical absorption spectra of water is shown in Figure 7.9 (a), its photoacoustic responses for both short and long pulses are shown in Figure 7.9 (b). The observation confirms the prediction that optically homogeneous medium respond equally to optical pulses with different durations, and exhibiting no difference as long as the excitation energy of the pulse is the same. 169
192 PA Intensity (A.U.) PA Intensity (A.U.) PA Intensity (A.U.) PA Intensity (A.U.) (a) SiO 2 Au NRs Short pulse Flat top pulse (b) Dye Short Pulse Flat-top Pulse Fluence (mj/cm 2 ) Fluence (mj/cm 2 ) Fluence (mj/cm 2 ) Fluence (mj/cm 2 ) Figure 7.8: Photoacoustic signal calibrated using flat top long pulse (red symbols) and short pulse (blue symbols) for (a) silica coated nanorod solution and (b) dye solution under the same condition. 170
193 PA Intensity (A.U.) Extinction (A.U.) (a) Water Wavelength(nm) (b) Short Pulse Flat-top Pulse Fluence (mj/cm 2 ) Figure 7.9: (a) Optical absorption of water, and (b) photoacoustic response of water under three different laser fluences of both short and flat top long optical pulses. 171
194 7.5 REFERENCES 1 Wei, Q. S. & Wei, A. Optical Imaging with Dynamic Contrast Agents. Chemistrya European Journal 17, , (2011). 2 Tong, L., Wei, Q. S., Wei, A. & Cheng, J. X. Gold Nanorods as Contrast Agents for Biological Imaging: Optical Properties, Surface Conjugation and Photothermal Effects. Photochemistry and Photobiology 85, 21-32, (2009). 3 Zipfel, W. R., Williams, R. M. & Webb, W. W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nature Biotechnology 21, , (2003). 4 He, G. S., Tan, L.-S., Zheng, Q. & Prasad, P. N. Multiphoton absorbing materials: Molecular designs, characterizations, and applications. Chemical Reviews 108, , (2008). 5 Larson, D. R. et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 300, , (2003). 6 Wang, H. F. et al. In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proceedings of the National Academy of Sciences of the United States of America 102, , (2005). 7 Liu, T., Wang, J., Petrov, G. I., Yakovlev, V. V. & Zhang, H. F. Photoacoustic generation by multiple picosecond pulse excitation. Medical Physics 37, , (2010). 8 Sun, J. M. & Gerstman, B. S. Photoacoustic generation for a spherical absorber with impedance mismatch with the surrounding media. Physical Review E 59, , (1999). 172
195 Chapter 8: Future Directions The objective of this study was to create molecular probes and pairing imaging schemes for contrast enhanced photoacoustic imaging to facilitate early cancer detection and improve efficacy of cancer therapy. The study is to better understand the photoacoustic imaging mechanism, overcoming critical imaging conditions, for example, when the imaging target itself exhibits strong optical absorptions, therefore baring a strong background signal. To achieve this goal, we designed and developed photostable PA nanoamplifiers with molecular specifications, and in addition, developed a dynamic imaging system to suppress background signals for further contrast enhancement. Specifically, chapter 2, 3 and 6 discussed the design and synthesis of the molecular probes; chapter 4 and 5 introduced the characterization of these agents and explained their physical mechanism of the photoacoustic signal amplification; finally, chapter 7 demonstrated enhanced photoacoustic imaging in tissue mimicking phantom using a dynamic imaging system in parallel with the developed molecular probes. In this chapter, I will provide possible future directions of this field. 8.1 FUTURE DIRECTIONS FOR PHOTOACOUSTIC MOLECULAR IMAGING In order to bridge our preclinical studies to practical clinical applications, one crucial step is to demonstrate in vivo contrast enhanced photoacoustic molecular imaging using the molecular probes and image system in small animal model. To validate our concept, we choose to visualize blood-laden liver, which has been one of the most challenging imaging target that often encounters low imaging contrast due to the high background signal attributed from the strong optical absorption of hemoglobin in blood. In addition, it is crucial to detect the formation of metastatic lesions in patients with 173
196 cancer history, and liver is the second most common site of metastasis, only after lymph nodes, for cancers such as colon, breast, pancreas, and stomach 1-2. We will choose a xenograft nude mouse model of hepatic metastasis to demonstrate the feasibility of proposed contrast enhanced PA molecular imaging since the xenograft model of liver metastasis is histologically similar to human tumor. This provides a platform where our PA nano-amplifier augmented molecular imaging modality can validate its capability. In particular, the liver metastasis will be induced via intrasplenic injection of AsPc-1 (Mpanc96) pancreatic cancer cells 3. One million cancer cells in a total volume of 20 μl consisting of equal volumes of PBS and matrigel will be injected to the spleen of an immunodeficient mouse, a nu/nu mouse. All mice will be monitored for tumor development by ultrasound imaging and after four weeks, 0.45mg of PA molecular probes in PBS will be injected to the mouse through tail vein injection. The liver of the mouse will be imaged to validate the performance of proposed imaging technique. After six weeks, mice will be sacrificed and organs will be excised and stored in Bouin s fixative solution. All of the animal procedures will be performed in accordance with a protocol approved by the Institutional Animal Care and Usage Committee. The accuracy of photoacoustic molecular imaging will be assessed by histopathology. 174
197 Figure 8.1: A xenograft nude mouse model of AsPc-1 (Mpanc96) pancreatic cancer cells. 8.2 REFERENCES 1 de Groen, P. C., Gores, G. J., LaRusso, N. F., Gunderson, L. L. & Nagorney, D. M. Medical progress - Biliary tract cancers. New England Journal of Medicine 341, , (1999). 2 Robinson, P. Imaging liver metastases: current limitations and future prospects. British journal of radiology 73, (2000). 3 Partecke, I. L. et al. In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. Bmc Cancer 11, (2011). 175
Translational Multimodality Optical Imaging
 Translational Multimodality Optical Imaging Fred S. Azar Xavier Intes Editors 0 ARTECH H O U S E BOSTON LONDON artechhouse.com Contents Foreword Preface xv xvii CHAPTER1 Introduction to Clinical Optical
Translational Multimodality Optical Imaging Fred S. Azar Xavier Intes Editors 0 ARTECH H O U S E BOSTON LONDON artechhouse.com Contents Foreword Preface xv xvii CHAPTER1 Introduction to Clinical Optical
BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering
 BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering Outline What is Medical Imaging? History of Medical Imaging X-Ray Imaging
BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering Outline What is Medical Imaging? History of Medical Imaging X-Ray Imaging
Photoacoustic Imaging in Biomedicine Critical Review by Saurabh Vyas Group 9: Interventional Photoacoustic Ultrasound CIS II: 600.
 Photoacoustic Imaging in Biomedicine Critical Review by Saurabh Vyas Group 9: Interventional Photoacoustic Ultrasound CIS II: 600.446, Spring 2011 Introduction Photoacoustic imaging (PA Imaging) is the
Photoacoustic Imaging in Biomedicine Critical Review by Saurabh Vyas Group 9: Interventional Photoacoustic Ultrasound CIS II: 600.446, Spring 2011 Introduction Photoacoustic imaging (PA Imaging) is the
Cellular imaging using Nano- Materials. A Case-Study based approach Arun Murali, Srivats V
 Cellular imaging using Nano- Materials A Case-Study based approach Arun Murali, Srivats V Agenda Discuss a few papers Explain a couple of new imaging techniques and their benefits over conventional imaging
Cellular imaging using Nano- Materials A Case-Study based approach Arun Murali, Srivats V Agenda Discuss a few papers Explain a couple of new imaging techniques and their benefits over conventional imaging
Absorption of an electromagnetic wave
 In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
Spectra Chacracterizations of Optical Nanoparticles
 THAI NGUYEN UNIVERSITY OF EDUCATION Spectra Chacracterizations of Optical Nanoparticles Chu Viet Ha Department of Physics 18/2018 1 THAI NGUYEN UNIVERSITY OF EDUCATION Address 20 Luong Ngoc Quyen Street,
THAI NGUYEN UNIVERSITY OF EDUCATION Spectra Chacracterizations of Optical Nanoparticles Chu Viet Ha Department of Physics 18/2018 1 THAI NGUYEN UNIVERSITY OF EDUCATION Address 20 Luong Ngoc Quyen Street,
Biophotonics?? Biophotonics. technology in biomedical engineering. Advantages of the lightwave
 Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Enhanced Light Trapping in Periodic Aluminum Nanorod Arrays as Cavity Resonator
 Enhanced Light Trapping in Periodic Aluminum Nanorod Arrays as Cavity Resonator Rosure B. Abdulrahman, Arif S. Alagoz, Tansel Karabacak Department of Applied Science, University of Arkansas at Little Rock,
Enhanced Light Trapping in Periodic Aluminum Nanorod Arrays as Cavity Resonator Rosure B. Abdulrahman, Arif S. Alagoz, Tansel Karabacak Department of Applied Science, University of Arkansas at Little Rock,
Contents Preface xiii Introduction Fabrication and manufacturing technology for optical MEMS
 Contents Preface xiii 1 Introduction 1 1.1 Optical MEMS and optofluidics 1 1.2 History 1 1.2.1 Processes and materials 1 1.2.2 Early devices and systems 2 1.3 Progress in optical MEMS and optofluidics
Contents Preface xiii 1 Introduction 1 1.1 Optical MEMS and optofluidics 1 1.2 History 1 1.2.1 Processes and materials 1 1.2.2 Early devices and systems 2 1.3 Progress in optical MEMS and optofluidics
TARGETED IMAGING. Maureen Chan and Ruwani Mahathantila
 TARGETED IMAGING Maureen Chan and Ruwani Mahathantila Overview 2 Introduction to fluorescent imaging Fluorescent agents Quantum Dots Physical properties How QDs work In Vivo QD imaging Future Video What
TARGETED IMAGING Maureen Chan and Ruwani Mahathantila Overview 2 Introduction to fluorescent imaging Fluorescent agents Quantum Dots Physical properties How QDs work In Vivo QD imaging Future Video What
Compact Plasmonic Blackbody for Cancer Theranosis in Near-Infrared II Window
 Supporting Information for Compact Plasmonic Blackbody for Cancer Theranosis in Near-Infrared II Window Jiajing Zhou,, Yuyan Jiang,, Shuai Hou, Paul Kumar Upputuri, Di Wu,, Jingchao Li, Peng Wang, Xu Zhen,
Supporting Information for Compact Plasmonic Blackbody for Cancer Theranosis in Near-Infrared II Window Jiajing Zhou,, Yuyan Jiang,, Shuai Hou, Paul Kumar Upputuri, Di Wu,, Jingchao Li, Peng Wang, Xu Zhen,
NANO 243/CENG 207 Course Use Only
 L5: Nanomedicine in Diagnostics and Bioimaging April 17, 2018 Cancer Diagnostics Diagnostics plays an important role throughout cancer treatment Before treatment, accurately locate tumors, stage the disease,
L5: Nanomedicine in Diagnostics and Bioimaging April 17, 2018 Cancer Diagnostics Diagnostics plays an important role throughout cancer treatment Before treatment, accurately locate tumors, stage the disease,
Biomedical Applications of Molecular Spectroscopy
 Biomedical Applications of Molecular Spectroscopy Mike Kayat B&W Tek, Inc 19 Shea Way Newark, DE 19713 United States of America +1 302 368 7824 mikek@bwtek.com 1 Overview Molecular spectroscopy is a large
Biomedical Applications of Molecular Spectroscopy Mike Kayat B&W Tek, Inc 19 Shea Way Newark, DE 19713 United States of America +1 302 368 7824 mikek@bwtek.com 1 Overview Molecular spectroscopy is a large
Photoacoustic imaging of vascular networks in transgenic mice
 Photoacoustic imaging of vascular networks in transgenic mice J.G. Laufer 1, J.O. Cleary 1,2, E.Z. Zhang 1, M.F. Lythgoe 2, P.C. Beard 1 1. Department of Medical Physics and Bioengineering, University
Photoacoustic imaging of vascular networks in transgenic mice J.G. Laufer 1, J.O. Cleary 1,2, E.Z. Zhang 1, M.F. Lythgoe 2, P.C. Beard 1 1. Department of Medical Physics and Bioengineering, University
Supplementary Data. In-Vivo Tumor Targeting and Spectroscopic Detection with Surface- Enhanced Raman Nanoparticle Tags
 Supplementary Data In-Vivo Tumor Targeting and Spectroscopic Detection with Surface- Enhanced Raman Nanoparticle Tags X.-M. Qian, 1 Xiang-Hong Peng, 2 Dominic O. Ansari, 1 Qiqin Yin-Goen, 3 Georgia Z.
Supplementary Data In-Vivo Tumor Targeting and Spectroscopic Detection with Surface- Enhanced Raman Nanoparticle Tags X.-M. Qian, 1 Xiang-Hong Peng, 2 Dominic O. Ansari, 1 Qiqin Yin-Goen, 3 Georgia Z.
Biophotonics. Light Matter Interactions & Lasers. NPTEL Biophotonics 1
 Biophotonics Light Matter Interactions & Lasers NPTEL Biophotonics 1 Overview In this lecture you will learn, Light matter interactions: absorption, emission, stimulated emission Lasers and some laser
Biophotonics Light Matter Interactions & Lasers NPTEL Biophotonics 1 Overview In this lecture you will learn, Light matter interactions: absorption, emission, stimulated emission Lasers and some laser
Basic principles of quantification using optical techniques
 Contents Basic principles of quantification using optical techniques Adrian Taruttis Helmholtz Zentrum München Chair for Biological Imaging Technische Universität München Light/ tissue interactions Planar
Contents Basic principles of quantification using optical techniques Adrian Taruttis Helmholtz Zentrum München Chair for Biological Imaging Technische Universität München Light/ tissue interactions Planar
Supporting Information for. Electrical control of Förster energy transfer.
 1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-07-1-0231 TITLE: Spectroscopic Photoacoustic Tomography of Prostate Cancer PRINCIPAL INVESTIGATOR: Xueding Wang CONTRACTING ORGANIZATION: University Of Michigan Ann Arbor, MI 48109-1274
AD Award Number: W81XWH-07-1-0231 TITLE: Spectroscopic Photoacoustic Tomography of Prostate Cancer PRINCIPAL INVESTIGATOR: Xueding Wang CONTRACTING ORGANIZATION: University Of Michigan Ann Arbor, MI 48109-1274
Plasmonics using Metal Nanoparticles. Tammy K. Lee and Parama Pal ECE 580 Nano-Electro-Opto-Bio
 Plasmonics using Metal Nanoparticles Tammy K. Lee and Parama Pal ECE 580 Nano-Electro-Opto-Bio April 1, 2007 Motivation Why study plasmonics? Miniaturization of optics and photonics to subwavelength scales
Plasmonics using Metal Nanoparticles Tammy K. Lee and Parama Pal ECE 580 Nano-Electro-Opto-Bio April 1, 2007 Motivation Why study plasmonics? Miniaturization of optics and photonics to subwavelength scales
Characterisation of Fe-Ni amorphous thin films for possible magnetostrictive sensor applications
 Characterisation of Fe-Ni amorphous thin films for possible magnetostrictive sensor applications Contents 9.1 Introduction 9.2 Experiment 9.3 Results and Discussions 9.4 Conclusion 9.1 Introduction Magnetostrictive
Characterisation of Fe-Ni amorphous thin films for possible magnetostrictive sensor applications Contents 9.1 Introduction 9.2 Experiment 9.3 Results and Discussions 9.4 Conclusion 9.1 Introduction Magnetostrictive
Design for Manufacturability (DFM) in the Life Sciences
 T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
Principles of translational medicine: imaging, biomarker imaging, theranostics
 Principles of translational medicine: imaging, biomarker imaging, theranostics Compiled by: Endre Mikus PhD, CEO Budapest, 21/9/2015 Imaging and imaging biomarkers An imaging biomarker is an anatomic,
Principles of translational medicine: imaging, biomarker imaging, theranostics Compiled by: Endre Mikus PhD, CEO Budapest, 21/9/2015 Imaging and imaging biomarkers An imaging biomarker is an anatomic,
Bioengineering (BIOE)
 Bioengineering (BIOE) 1 Bioengineering (BIOE) Courses BIOE 5301. Biosignals. 3 Credit Hours. This course offers a deep overview of the signals in the Biomedical fields. Signals are studied in several modalities,
Bioengineering (BIOE) 1 Bioengineering (BIOE) Courses BIOE 5301. Biosignals. 3 Credit Hours. This course offers a deep overview of the signals in the Biomedical fields. Signals are studied in several modalities,
Femtosecond micromachining in polymers
 Femtosecond micromachining in polymers Prof. Dr Cleber R. Mendonca Daniel S. Corrêa Prakriti Tayalia Dr. Tobias Voss Dr. Tommaso Baldacchini Prof. Dr. Eric Mazur fs-micromachining focus laser beam inside
Femtosecond micromachining in polymers Prof. Dr Cleber R. Mendonca Daniel S. Corrêa Prakriti Tayalia Dr. Tobias Voss Dr. Tommaso Baldacchini Prof. Dr. Eric Mazur fs-micromachining focus laser beam inside
Detecting Gene Expression In-Vivo Using Differential Laser. Absorption. Senior Thesis - Physics, May By Hermonta M Godwin
 Detecting Gene Expression In-Vivo Using Differential Laser Absorption Senior Thesis - Physics, May 2002 By Hermonta M Godwin Advisor: Professor William E. Cooke College of William and Mary Abstract: The
Detecting Gene Expression In-Vivo Using Differential Laser Absorption Senior Thesis - Physics, May 2002 By Hermonta M Godwin Advisor: Professor William E. Cooke College of William and Mary Abstract: The
MEDICAL EQUIPMENT (1) TOPIC 1: RECORDING AND PROCESSING OF BIOSIGNALS
 MEDICAL EQUIPMENT (1) TOPIC 1: RECORDING AND PROCESSING OF BIOSIGNALS Term 1 2013/14 Prof. Yasser Mostafa Kadah www.k-space.org Measurement Basics Measuring is the experimental determination of a measured
MEDICAL EQUIPMENT (1) TOPIC 1: RECORDING AND PROCESSING OF BIOSIGNALS Term 1 2013/14 Prof. Yasser Mostafa Kadah www.k-space.org Measurement Basics Measuring is the experimental determination of a measured
Master of Molecular Imaging Course Outline
 Master of Molecular Imaging Course Outline Graduate Outcomes On completion of the course, graduates will have achieved the following skills, knowledge and attributes: chemistry/pharmacy physics/engineering
Master of Molecular Imaging Course Outline Graduate Outcomes On completion of the course, graduates will have achieved the following skills, knowledge and attributes: chemistry/pharmacy physics/engineering
Abstract: Keywords: Gold nanoparticle, Folic acid, Folate, Hela cell, Mcf7 cell, Targeting, 4-aminothiophenol, 6- mercapto-1-hexanol
 Abstract: Cancer Nanotechnology Treatment through Folate Conjugated Gold Nanoparticles ( * ) Ali Shakeri-Zadeh and G.Ali Mansoori ( ** ) University of Illinois at Chicago (*) Invited paper. (**) Corresponding
Abstract: Cancer Nanotechnology Treatment through Folate Conjugated Gold Nanoparticles ( * ) Ali Shakeri-Zadeh and G.Ali Mansoori ( ** ) University of Illinois at Chicago (*) Invited paper. (**) Corresponding
Nanomaterials for Imaging Technology. Nadeem A. Kizilbash, Ph.D. Assistant Professor Department of Chemistry Quaid-i-Azam University Islamabad
 Nanomaterials for Imaging Technology Nadeem A. Kizilbash, Ph.D. Assistant Professor Department of Chemistry Quaid-i-Azam University Islamabad Introduction Nanotechnology, most basically put, is the molecular
Nanomaterials for Imaging Technology Nadeem A. Kizilbash, Ph.D. Assistant Professor Department of Chemistry Quaid-i-Azam University Islamabad Introduction Nanotechnology, most basically put, is the molecular
Building An Ultrafast Photon-Induced Near-field Transmission Electron Microscope
 Building An Ultrafast Photon-Induced Near-field Transmission Electron Microscope Dr. Tom T.A. Lummen École Polytechnique Fédérale de Lausanne -- LUMES Photonic Instruments 2013 September 11, 2013 Zürich,
Building An Ultrafast Photon-Induced Near-field Transmission Electron Microscope Dr. Tom T.A. Lummen École Polytechnique Fédérale de Lausanne -- LUMES Photonic Instruments 2013 September 11, 2013 Zürich,
Molecular Imaging: Definition, Overview and Goals
 This tutorial will define what is currently considered molecular imaging. It will provide history and an overview, discuss the goals and the advantages of molecular imaging. It will clarify what is and
This tutorial will define what is currently considered molecular imaging. It will provide history and an overview, discuss the goals and the advantages of molecular imaging. It will clarify what is and
Advances in Intense Pulsed Light Solutions For Display Manufacturing. XENON Corporation Dr. Saad Ahmed Japan IDW 2016
 Advances in Intense Pulsed Light Solutions For Display Manufacturing XENON Corporation Dr. Saad Ahmed Japan IDW 2016 Talk Outline Introduction to Pulsed Light Applications in Display UV Curing Applications
Advances in Intense Pulsed Light Solutions For Display Manufacturing XENON Corporation Dr. Saad Ahmed Japan IDW 2016 Talk Outline Introduction to Pulsed Light Applications in Display UV Curing Applications
Dynamics of Energy Transfer in Large. Plasmonic Aluminum Nanoparticles
 Supporting Information Dynamics of Energy Transfer in Large Plasmonic Aluminum Nanoparticles Kenneth J. Smith,#, Yan Cheng,#, Ebuka S. Arinze,#, Nicole E. Kim, Arthur E. Bragg, Susanna M. Thon Department
Supporting Information Dynamics of Energy Transfer in Large Plasmonic Aluminum Nanoparticles Kenneth J. Smith,#, Yan Cheng,#, Ebuka S. Arinze,#, Nicole E. Kim, Arthur E. Bragg, Susanna M. Thon Department
Introduction. (b) (a)
 Introduction Whispering Gallery modes (WGMs) in dielectric micro-cavities are resonant electromagnetic modes that are of considerable current interest because of their extremely high Q values leading to
Introduction Whispering Gallery modes (WGMs) in dielectric micro-cavities are resonant electromagnetic modes that are of considerable current interest because of their extremely high Q values leading to
Chapter-IV OPTICAL PROPERTIES
 Chapter-IV OPTICAL PROPERTIES 4.1 Ultraviolet/ Visible Spectroscopy UV- visible spectroscopy of wavelength is shorter than visible light, but longer than X- rays. UV-Violet color is the shortest wavelength
Chapter-IV OPTICAL PROPERTIES 4.1 Ultraviolet/ Visible Spectroscopy UV- visible spectroscopy of wavelength is shorter than visible light, but longer than X- rays. UV-Violet color is the shortest wavelength
We will begin momentarily at 2pm ET. Slides available now! Recordings available as an exclusive ACS member benefit.
 We will begin momentarily at 2pm ET Slides available now! Recordings available as an exclusive ACS member benefit. www.acs.org/acswebinars Contact ACS Webinars at acswebinars@acs.org 1 Have Questions?
We will begin momentarily at 2pm ET Slides available now! Recordings available as an exclusive ACS member benefit. www.acs.org/acswebinars Contact ACS Webinars at acswebinars@acs.org 1 Have Questions?
Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging
 Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging Introduction In this application note, we will discuss the application of quantum dots in fluorescence imaging, both
Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging Introduction In this application note, we will discuss the application of quantum dots in fluorescence imaging, both
WHITE-LIGHT SPECTRAL INTERFEROMETRY FOR SURFACE PLASMON RESONANCE SENSOR APPLICATIONS
 A WHITE-LIGHT SPECTRAL INTERFEROMETRY FOR SURFACE PLASMON RESONANCE SENSOR APPLICATIONS NG SIU PANG DOCTOR OF PHILOSOPHY CITY UNIVERSITY OF HONG KONG SEPTEMBER 2010 A B CITY UNIVERSITY OF HONG KONG 香港城市大學
A WHITE-LIGHT SPECTRAL INTERFEROMETRY FOR SURFACE PLASMON RESONANCE SENSOR APPLICATIONS NG SIU PANG DOCTOR OF PHILOSOPHY CITY UNIVERSITY OF HONG KONG SEPTEMBER 2010 A B CITY UNIVERSITY OF HONG KONG 香港城市大學
Quantifying biological forces involved in the process of cell-mediated cytolysis
 Quantifying biological forces involved in the process of cell-mediated cytolysis Experimentation based on responses of living cells (Cell-based assays) represents an important stratum in biological and
Quantifying biological forces involved in the process of cell-mediated cytolysis Experimentation based on responses of living cells (Cell-based assays) represents an important stratum in biological and
Active delivery of single DNA molecules into a plasmonic nanopore for. label-free optical sensing
 Supporting Information: Active delivery of single DNA molecules into a plasmonic nanopore for label-free optical sensing Xin Shi 1,2, Daniel V Verschueren 1, and Cees Dekker 1* 1. Department of Bionanoscience,
Supporting Information: Active delivery of single DNA molecules into a plasmonic nanopore for label-free optical sensing Xin Shi 1,2, Daniel V Verschueren 1, and Cees Dekker 1* 1. Department of Bionanoscience,
Supplementary Figure S1. Scheme for the fabrication of Au nanohole array pattern and
 Supplementary Figure S1. Scheme for the fabrication of Au nanohole array pattern and the growth of hematite nanorods on the Au nanohole array substrate. (a) Briefly, the 500 nm sized PS monolayer was assembled
Supplementary Figure S1. Scheme for the fabrication of Au nanohole array pattern and the growth of hematite nanorods on the Au nanohole array substrate. (a) Briefly, the 500 nm sized PS monolayer was assembled
Tunable Nanoscale Plasmon Antenna for Localization and Enhancement of Optical Energy. Douglas Howe
 Tunable Nanoscale Plasmon Antenna for Localization and Enhancement of Optical Energy Douglas Howe Applied Optics Spring 2008 Table of Contents Abstract... 3 Introduction... 4 Surface Plasmons... 4 Nano
Tunable Nanoscale Plasmon Antenna for Localization and Enhancement of Optical Energy Douglas Howe Applied Optics Spring 2008 Table of Contents Abstract... 3 Introduction... 4 Surface Plasmons... 4 Nano
Miniature fibre optic probe for minimally invasive photoacoustic sensing
 Miniature fibre optic probe for minimally invasive photoacoustic sensing Sunish J. Mathews*, Edward Z. Zhang, Adrien E. Desjardins and Paul C. Beard Department of Medical Physics and Biomedical Engineering,
Miniature fibre optic probe for minimally invasive photoacoustic sensing Sunish J. Mathews*, Edward Z. Zhang, Adrien E. Desjardins and Paul C. Beard Department of Medical Physics and Biomedical Engineering,
Enhancement of connectivity and flux pinning in MgB2 superconducting bulks and wires
 University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2009 Enhancement of connectivity and flux pinning in MgB2 superconducting
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2009 Enhancement of connectivity and flux pinning in MgB2 superconducting
Spectroscopy and Imaging IV
 PROGRESS IN BIOMEDICAL OPTICS AND IMAGING Vol. 16 No. 55 Clinical and Biomedical Spectroscopy and Imaging IV J. Quincy Brown Volker Decked Edifors 22-24 June 2015 Munich, Germany Sponsored by SPIE (United
PROGRESS IN BIOMEDICAL OPTICS AND IMAGING Vol. 16 No. 55 Clinical and Biomedical Spectroscopy and Imaging IV J. Quincy Brown Volker Decked Edifors 22-24 June 2015 Munich, Germany Sponsored by SPIE (United
Supporting Information: Gold nanorod plasmonic upconversion microlaser
 Supporting Information: Gold nanorod plasmonic upconversion microlaser 1 Materials Synthesis and Properties Ce Shi, Soheil Soltani, Andrea M. Armani 1.1 Nanorod synthesis First the gold nanorods (NRs)
Supporting Information: Gold nanorod plasmonic upconversion microlaser 1 Materials Synthesis and Properties Ce Shi, Soheil Soltani, Andrea M. Armani 1.1 Nanorod synthesis First the gold nanorods (NRs)
Vertical plasmonic nanowires for 3D nanoparticle trapping
 Vertical plasmonic nanowires for 3D nanoparticle trapping Jingzhi Wu, Xiaosong Gan * Centre for Micro-Photonics, Faculty of Engineering and Industrial Sciences, Swinburne University of Technology, PO Box
Vertical plasmonic nanowires for 3D nanoparticle trapping Jingzhi Wu, Xiaosong Gan * Centre for Micro-Photonics, Faculty of Engineering and Industrial Sciences, Swinburne University of Technology, PO Box
Crystallographic Characterization of GaN Nanowires by Raman Spectral Image Mapping
 Crystallographic Characterization of GaN Nanowires by Raman Spectral Image Mapping Heerad Farkhoor, Adam Schwartzberg, Jeffrey Urban August 12, 2009 Abstract Obtaining structural information of nano-structured
Crystallographic Characterization of GaN Nanowires by Raman Spectral Image Mapping Heerad Farkhoor, Adam Schwartzberg, Jeffrey Urban August 12, 2009 Abstract Obtaining structural information of nano-structured
Tapered Optical Fiber Probe Assembled with Plasmonic Nanostructures for Surface-Enhanced Raman Scattering Application
 Supporting Information Tapered Optical Fiber Probe Assembled with Plasmonic Nanostructures for Surface-Enhanced Raman Scattering Application Zhulin Huang, Xing Lei, Ye Liu, Zhiwei Wang, Xiujuan Wang, Zhaoming
Supporting Information Tapered Optical Fiber Probe Assembled with Plasmonic Nanostructures for Surface-Enhanced Raman Scattering Application Zhulin Huang, Xing Lei, Ye Liu, Zhiwei Wang, Xiujuan Wang, Zhaoming
Solar Cells and Photosensors.
 Designing Photonic Crystals in Strongly Absorbing Material for Applications in Solar Cells and Photosensors. Minda Wagenmaker 1, Ebuka S. Arinze 2, Botong Qiu 2, Susanna M. Thon 2 1 Mechanical Engineering
Designing Photonic Crystals in Strongly Absorbing Material for Applications in Solar Cells and Photosensors. Minda Wagenmaker 1, Ebuka S. Arinze 2, Botong Qiu 2, Susanna M. Thon 2 1 Mechanical Engineering
REVOLUTIONIZING EARLY CANCER DETECTION
 cover story Written by candace o connor Photos by geoff story IMAGING WITH LIGHT AND SOUND: REVOLUTIONIZING EARLY CANCER DETECTION For years, the field of optical imaging in biological tissue had languished,
cover story Written by candace o connor Photos by geoff story IMAGING WITH LIGHT AND SOUND: REVOLUTIONIZING EARLY CANCER DETECTION For years, the field of optical imaging in biological tissue had languished,
Folic Acid-Conjugated Chitosan Functionalized Gold Nanoparticles for Targeted Delivery of 5-Fluorouracil in Breast Cancer
 Proceedings of the 3 rd World Congress on Recent Advances in Nanotechnology (RAN'18) Budapest, Hungary April 10-12, 2018 Paper No. NDDTE 103 DOI: 10.11159/nddte18.103 Folic Acid-Conjugated Chitosan Functionalized
Proceedings of the 3 rd World Congress on Recent Advances in Nanotechnology (RAN'18) Budapest, Hungary April 10-12, 2018 Paper No. NDDTE 103 DOI: 10.11159/nddte18.103 Folic Acid-Conjugated Chitosan Functionalized
Title: Localized surface plasmon resonance of metal nanodot and nanowire arrays studied by far-field and near-field optical microscopy
 Contract Number: AOARD-06-4074 Principal Investigator: Heh-Nan Lin Address: Department of Materials Science and Engineering, National Tsing Hua University, 101, Sec. 2, Kuang Fu Rd., Hsinchu 30013, Taiwan
Contract Number: AOARD-06-4074 Principal Investigator: Heh-Nan Lin Address: Department of Materials Science and Engineering, National Tsing Hua University, 101, Sec. 2, Kuang Fu Rd., Hsinchu 30013, Taiwan
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-07-1-0231 TITLE: Spectroscopic Photoacoustic Tomography of Prostate Cancer PRINCIPAL INVESTIGATOR: Xueding Wang CONTRACTING ORGANIZATION: University Of Michigan Ann Arbor, MI 48109-1274
AD Award Number: W81XWH-07-1-0231 TITLE: Spectroscopic Photoacoustic Tomography of Prostate Cancer PRINCIPAL INVESTIGATOR: Xueding Wang CONTRACTING ORGANIZATION: University Of Michigan Ann Arbor, MI 48109-1274
Plasmonic Nanostructures II
 Plasmonic Nanostructures II Dr. Krüger / Prof. M. Zacharias, IMTEK, Propagation of SPPs Propagation distance decreases with decreasing strip width! 2 Dr. Krüger / Prof. M. Zacharias, IMTEK, Bound and leaky
Plasmonic Nanostructures II Dr. Krüger / Prof. M. Zacharias, IMTEK, Propagation of SPPs Propagation distance decreases with decreasing strip width! 2 Dr. Krüger / Prof. M. Zacharias, IMTEK, Bound and leaky
Microstructural Characterization of Materials
 Microstructural Characterization of Materials 2nd Edition DAVID BRANDON AND WAYNE D. KAPLAN Technion, Israel Institute of Technology, Israel John Wiley & Sons, Ltd Contents Preface to the Second Edition
Microstructural Characterization of Materials 2nd Edition DAVID BRANDON AND WAYNE D. KAPLAN Technion, Israel Institute of Technology, Israel John Wiley & Sons, Ltd Contents Preface to the Second Edition
Example SimphoSOFT simulation of Thulium-Ion-Doped Pulsed Amplification
 Rare-earth ions can undergo fluorescence, cross-relaxation (or self-quenching), upconversion, and other non-radiative relaxations. For rare-earth ions, these processes are important for lasers, optical
Rare-earth ions can undergo fluorescence, cross-relaxation (or self-quenching), upconversion, and other non-radiative relaxations. For rare-earth ions, these processes are important for lasers, optical
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Mass spectrometry characterization of Au 25, Au 38, Au 144, Au 333, Au ~520 and Au ~940 nanoclusters. (a) MALDI-mass spectra of Au 144, Au 333, Au ~520 and
Supplementary Figures Supplementary Figure 1. Mass spectrometry characterization of Au 25, Au 38, Au 144, Au 333, Au ~520 and Au ~940 nanoclusters. (a) MALDI-mass spectra of Au 144, Au 333, Au ~520 and
Nanodiamond-Based Therapeutic Delivery Films For the Treatment of Cancer and Inflammation
 Nanodiamond-Based Therapeutic Delivery Films For the Treatment of Cancer and Inflammation Dean Ho, Ph.D. Assistant Professor Departments of Biomedical and Mechanical Engineering Lurie Comprehensive Cancer
Nanodiamond-Based Therapeutic Delivery Films For the Treatment of Cancer and Inflammation Dean Ho, Ph.D. Assistant Professor Departments of Biomedical and Mechanical Engineering Lurie Comprehensive Cancer
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
LOW TEMPERATURE PHOTONIC SINTERING FOR PRINTED ELECTRONICS. Dr. Saad Ahmed XENON Corporation November 19, 2015
 LOW TEMPERATURE PHOTONIC SINTERING FOR PRINTED ELECTRONICS Dr. Saad Ahmed XENON Corporation November 19, 2015 Topics Introduction to Pulsed Light Photonic sintering for Printed Electronics R&D Tools for
LOW TEMPERATURE PHOTONIC SINTERING FOR PRINTED ELECTRONICS Dr. Saad Ahmed XENON Corporation November 19, 2015 Topics Introduction to Pulsed Light Photonic sintering for Printed Electronics R&D Tools for
micromachines ISSN X
 Micromachines 2012, 3, 55-61; doi:10.3390/mi3010055 Article OPEN ACCESS micromachines ISSN 2072-666X www.mdpi.com/journal/micromachines Surface Plasmon Excitation and Localization by Metal-Coated Axicon
Micromachines 2012, 3, 55-61; doi:10.3390/mi3010055 Article OPEN ACCESS micromachines ISSN 2072-666X www.mdpi.com/journal/micromachines Surface Plasmon Excitation and Localization by Metal-Coated Axicon
for Bioanalytical Applications
 Conferinţa Diaspora in Cercetarea Ştiinţifică şi Invăţămantul Superior din Romania Bucuresti, 21-24 Septembrie 2010 Multifunctional Plasmonic Nanosensors for Bioanalytical Applications Simion Astilean
Conferinţa Diaspora in Cercetarea Ştiinţifică şi Invăţămantul Superior din Romania Bucuresti, 21-24 Septembrie 2010 Multifunctional Plasmonic Nanosensors for Bioanalytical Applications Simion Astilean
262 Chapter 16. Figure Fiber-optic-enabled arrays using fluorescence for high-speed screening. 1
 262 Chapter 16 Figure 16.13 Fiber-optic-enabled arrays using fluorescence for high-speed screening. 1 Figure 16.14 Fluorescent array microsphere sensors. 10 to have low background fluorescence to maximize
262 Chapter 16 Figure 16.13 Fiber-optic-enabled arrays using fluorescence for high-speed screening. 1 Figure 16.14 Fluorescent array microsphere sensors. 10 to have low background fluorescence to maximize
3 Pulsed laser ablation and etching of fused silica
 3 Pulsed laser ablation and etching of fused silica 17 3 Pulsed laser ablation and etching of fused silica Material erosion caused by short laser pulses takes place far from equilibrium and may be based
3 Pulsed laser ablation and etching of fused silica 17 3 Pulsed laser ablation and etching of fused silica Material erosion caused by short laser pulses takes place far from equilibrium and may be based
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Nanoscale Materials Inspires Innovation and Drives Economic Development
 Nanoscale Materials Inspires Innovation and Drives Economic Development 5nm Greg Salamo & Alex Biris InAs Quantum Dots What is Nanoscience? The effort to understand and design structures at the nano size
Nanoscale Materials Inspires Innovation and Drives Economic Development 5nm Greg Salamo & Alex Biris InAs Quantum Dots What is Nanoscience? The effort to understand and design structures at the nano size
INTEGRATED OPTICAL ISOLATOR
 INTEGRATED OPTICAL ISOLATOR Presented by Gokhan Ozgur Advisor: Dr. Gary Evans July 02, 2004 Electrical Engineering - SMU INTRODUCTION They are used to eliminate light that is back-reflected, from splices
INTEGRATED OPTICAL ISOLATOR Presented by Gokhan Ozgur Advisor: Dr. Gary Evans July 02, 2004 Electrical Engineering - SMU INTRODUCTION They are used to eliminate light that is back-reflected, from splices
Acoustic Characterization of Materials
 Acoustic Characterization of Materials Bernhard R. Tittmann Group Leader CAV Introduction Graduate Students 4 Ph.D. Candidates 4 U.S. 1 Non-US 3 M.S Candidates 3 U.S. 1 Non U.S. Visiting Scholars Dr.
Acoustic Characterization of Materials Bernhard R. Tittmann Group Leader CAV Introduction Graduate Students 4 Ph.D. Candidates 4 U.S. 1 Non-US 3 M.S Candidates 3 U.S. 1 Non U.S. Visiting Scholars Dr.
A Survey of Laser Types. Gas Lasers
 Mihail Pivtoraiko Andrei Rozhkov Applied Optics Winter 2003 A Survey of Laser Types Laser technology is available to us since 1960 s, and since then has been quite well developed. Currently, there is a
Mihail Pivtoraiko Andrei Rozhkov Applied Optics Winter 2003 A Survey of Laser Types Laser technology is available to us since 1960 s, and since then has been quite well developed. Currently, there is a
Medical instrumentationi 11/19/2010
 Medical instrumentationi BIOEN 302 11/19/2010 Medical instrumentation Definition: instrument for sensing, diagnostics, therapeutics or surgery of human being. 2 Medical instrumentation Definition: instrument
Medical instrumentationi BIOEN 302 11/19/2010 Medical instrumentation Definition: instrument for sensing, diagnostics, therapeutics or surgery of human being. 2 Medical instrumentation Definition: instrument
ECE 541/ME 541 Microelectronic Fabrication Techniques
 ECE 541/ME 541 Microelectronic Fabrication Techniques MW 4:00-5:15 pm Metrology and Characterization Zheng Yang ERF 3017, email: yangzhen@uic.edu ECE541/ME541 Microelectronic Fabrication Techniques Page
ECE 541/ME 541 Microelectronic Fabrication Techniques MW 4:00-5:15 pm Metrology and Characterization Zheng Yang ERF 3017, email: yangzhen@uic.edu ECE541/ME541 Microelectronic Fabrication Techniques Page
Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ
 Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ Research at the nanoscale is more effective, when research teams can quickly and easily observe and characterize a wide
Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ Research at the nanoscale is more effective, when research teams can quickly and easily observe and characterize a wide
1st Faculty of Medicine, Charles University in Prague Center for Advanced Preclinical Imaging (CAPI)
 ADVANTAGES Optical Imaging OI Optical Imaging is based on the detection of weak light by a highly sensitive and high resolution CCD camera DISADVANTAGES High sensitivity Limited penetration depth Easy
ADVANTAGES Optical Imaging OI Optical Imaging is based on the detection of weak light by a highly sensitive and high resolution CCD camera DISADVANTAGES High sensitivity Limited penetration depth Easy
CREOL, The College of Optics & Photonics, University of Central Florida
 Metal Substrate Induced Control of Ag Nanoparticle Plasmon Resonances for Tunable SERS Substrates Pieter G. Kik 1, Amitabh Ghoshal 1, Manuel Marquez 2 and Min Hu 1 1 CREOL, The College of Optics and Photonics,
Metal Substrate Induced Control of Ag Nanoparticle Plasmon Resonances for Tunable SERS Substrates Pieter G. Kik 1, Amitabh Ghoshal 1, Manuel Marquez 2 and Min Hu 1 1 CREOL, The College of Optics and Photonics,
DETECTION OF LASER ULTRASONIC SURFACE DISPLACEMENT BY WIDE APERTURE FIBER OPTIC AMPLIFIER M.L. Rizzi and F. Corbani CESI, Milano, Italy
 DETECTION OF LASER ULTRASONIC SURFACE DISPLACEMENT BY WIDE APERTURE FIBER OPTIC AMPLIFIER M.L. Rizzi and F. Corbani CESI, Milano, Italy Abstract: In the frame of the European Project INCA, CESI is in charge
DETECTION OF LASER ULTRASONIC SURFACE DISPLACEMENT BY WIDE APERTURE FIBER OPTIC AMPLIFIER M.L. Rizzi and F. Corbani CESI, Milano, Italy Abstract: In the frame of the European Project INCA, CESI is in charge
Production and analysis of optical gratings and nanostructures created by laser based methods
 Summary of the Ph.D. thesis Production and analysis of optical gratings and nanostructures created by laser based methods Kiss Bálint Supervisor: Dr. Vass Csaba Research fellow Doctoral School in Physics
Summary of the Ph.D. thesis Production and analysis of optical gratings and nanostructures created by laser based methods Kiss Bálint Supervisor: Dr. Vass Csaba Research fellow Doctoral School in Physics
LIST OF ABBREVIATIONS
 vii TABLE OF CONTENTS CHAPTER TITLE PAGE DECLARATION DEDICATION ACKNOWLEDGEMENTS ABSTRACT ABSTRAK TABLE OF CONTENTS LIST OF TABLES LIST OF FIGURES LIST OF ABBREVIATIONS LIST OF SYMBOLS LIST OF APPENDICES
vii TABLE OF CONTENTS CHAPTER TITLE PAGE DECLARATION DEDICATION ACKNOWLEDGEMENTS ABSTRACT ABSTRAK TABLE OF CONTENTS LIST OF TABLES LIST OF FIGURES LIST OF ABBREVIATIONS LIST OF SYMBOLS LIST OF APPENDICES
Plastic Coated Silica/Silica (Low OH) FIBER CROSS SECTION Polyimide and Acrylate Coated. Nylon and Tefzel Coated
 DESCRIPTION When looking for a high quality fiber with superior transmission and a numerical aperture (N.A.) of 0.22 for efficient light coupling, the is the fiber of choice. The Anhydroguide fiber is
DESCRIPTION When looking for a high quality fiber with superior transmission and a numerical aperture (N.A.) of 0.22 for efficient light coupling, the is the fiber of choice. The Anhydroguide fiber is
Supporting Information for The Influence of the CTAB Surfactant on the Colloidal Seed-Mediated Synthesis of Gold Nanorods
 Supporting Information for The Influence of the CTAB Surfactant on the Colloidal Seed-Mediated Synthesis of Gold Nanorods Danielle K. Smith and Brian A. Korgel* Department of Chemical Engineering, Texas
Supporting Information for The Influence of the CTAB Surfactant on the Colloidal Seed-Mediated Synthesis of Gold Nanorods Danielle K. Smith and Brian A. Korgel* Department of Chemical Engineering, Texas
Compact hybrid plasmonic-si waveguide structures utilizing Albanova E-beam lithography system
 Compact hybrid plasmonic-si waveguide structures utilizing Albanova E-beam lithography system Introduction Xu Sun Laboratory of Photonics and Microwave Engineering, Royal Institute of Technology (KTH),
Compact hybrid plasmonic-si waveguide structures utilizing Albanova E-beam lithography system Introduction Xu Sun Laboratory of Photonics and Microwave Engineering, Royal Institute of Technology (KTH),
SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION
 APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
MEDICAL PHYSICS (MED PHYS)
 Medical Physics (MED PHYS) 1 MEDICAL PHYSICS (MED PHYS) MED PHYS/PHYSICS 265 INTRODUCTION TO MEDICAL PHYSICS Primarily for premeds and other students in the medical and biological sciences. Applications
Medical Physics (MED PHYS) 1 MEDICAL PHYSICS (MED PHYS) MED PHYS/PHYSICS 265 INTRODUCTION TO MEDICAL PHYSICS Primarily for premeds and other students in the medical and biological sciences. Applications
Qswitched lasers are gaining more interest because of their ability for various applications in remote sensing, environmental monitoring, micro
 90 Qswitched lasers are gaining more interest because of their ability for various applications in remote sensing, environmental monitoring, micro machining, nonlinear frequency generation, laserinduced
90 Qswitched lasers are gaining more interest because of their ability for various applications in remote sensing, environmental monitoring, micro machining, nonlinear frequency generation, laserinduced
Diagnostic Medical Image Processing
 Diagnostic Medical Image Processing Introduction WS 2010/11 Joachim Hornegger, Dietrich Paulus, Markus Kowarschik Lehrstuhl für Mustererkennung (Informatik 5) Friedrich-Alexander-Universität Erlangen-Nürnberg
Diagnostic Medical Image Processing Introduction WS 2010/11 Joachim Hornegger, Dietrich Paulus, Markus Kowarschik Lehrstuhl für Mustererkennung (Informatik 5) Friedrich-Alexander-Universität Erlangen-Nürnberg
Contents. Foreword... (vii) Prologue... (ix) Preface... (xi) Acknowledgements... (xiii)
 Contents Foreword... (vii) Prologue... (ix) Preface... (xi) Acknowledgements... (xiii) Chapter 1: Nano Science and Technologies 1.1 Concepts of Nano Science and Nanotechnologies... 1 1.1.1 Introduction...
Contents Foreword... (vii) Prologue... (ix) Preface... (xi) Acknowledgements... (xiii) Chapter 1: Nano Science and Technologies 1.1 Concepts of Nano Science and Nanotechnologies... 1 1.1.1 Introduction...
Supporting Information For
 Supporting Information For Stimuli-Responsive Functionalized Mesoporous Silica Nanoparticles for Drug Release in Response to Various Biological Stimuli Xin Chen a,b, Xiaoyu Cheng a,b, Alexander H. Soeriyadi
Supporting Information For Stimuli-Responsive Functionalized Mesoporous Silica Nanoparticles for Drug Release in Response to Various Biological Stimuli Xin Chen a,b, Xiaoyu Cheng a,b, Alexander H. Soeriyadi
resonant photothermal sensitizers
 Supporting Information White and brightly coloured 3D printing based on resonant photothermal sensitizers Alexander W. Powell, Alexandros Stavrinadis, Ignacio de Miguel, Gerasimos Konstantatos*, Romain
Supporting Information White and brightly coloured 3D printing based on resonant photothermal sensitizers Alexander W. Powell, Alexandros Stavrinadis, Ignacio de Miguel, Gerasimos Konstantatos*, Romain
In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating. melanoma cells
 In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells Yun He 1,, Lidai Wang 1,,, Junhui Shi 1,, Junjie Yao 1, Lei Li 1, Ruiying Zhang 1, Chih-Hsien
In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells Yun He 1,, Lidai Wang 1,,, Junhui Shi 1,, Junjie Yao 1, Lei Li 1, Ruiying Zhang 1, Chih-Hsien
Imaging Quantum Dots using FUJIFILM LAS 4000
 Imaging Quantum Dots using FUJIFILM LAS 4000 Application Note John Pizzonia, Ph.D. 9-28-07 Quantum dots (also known as nanocrystals) are a special class of materials known as semiconductors, which are
Imaging Quantum Dots using FUJIFILM LAS 4000 Application Note John Pizzonia, Ph.D. 9-28-07 Quantum dots (also known as nanocrystals) are a special class of materials known as semiconductors, which are
Research Projects in Nano-Technology
 Research Projects in Nano-Technology Ibrahim Abdulhalim Our research is multidisciplinary combining nanostructures, liquid crystals, devices and methods for biosensing and biomedical optical imaging applications.
Research Projects in Nano-Technology Ibrahim Abdulhalim Our research is multidisciplinary combining nanostructures, liquid crystals, devices and methods for biosensing and biomedical optical imaging applications.
Fiber and Electro-Optics Research Center Virginia Polytechnic Institute and State University Blacksburg, Virginia 24061
 OPTICAL FIBER METHODS FOR AUTOCLAVE AND EPOXY CURE MONITORING B. Zimmermann, C. DiFrancia K. Murphy, A.Vengsarkar,' and R. Claus Fiber and Electro-Optics Research Center Virginia Polytechnic Institute
OPTICAL FIBER METHODS FOR AUTOCLAVE AND EPOXY CURE MONITORING B. Zimmermann, C. DiFrancia K. Murphy, A.Vengsarkar,' and R. Claus Fiber and Electro-Optics Research Center Virginia Polytechnic Institute
Deliverable 2.1: Definition of paradigms representing exemplary breast lesions cases
 Project title: Smart Optical and Ultrasound Diagnostics of Breast Cancer Grant Agreement: 731877 Call identifier: H2020-ICT-2016-1 Topic: ICT-29-2016 Photonics KET 2016 Deliverable 2.1: Definition of paradigms
Project title: Smart Optical and Ultrasound Diagnostics of Breast Cancer Grant Agreement: 731877 Call identifier: H2020-ICT-2016-1 Topic: ICT-29-2016 Photonics KET 2016 Deliverable 2.1: Definition of paradigms
Computer Assisted Surgery Basics of medical imaging
 Computer Assisted Surgery Basics of medical imaging Prof. Leo Joskowicz School of Engineering and Computer Science The Hebrew University of Jerusalem, ISRAEL Medical Image Processing Basics of medical
Computer Assisted Surgery Basics of medical imaging Prof. Leo Joskowicz School of Engineering and Computer Science The Hebrew University of Jerusalem, ISRAEL Medical Image Processing Basics of medical
Cell analysis and bioimaging technology illustrated
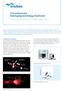 Cell analysis and bioimaging technology illustrated The Cell Analysis Center Scientific Bulletin Part 1 Sysmex has been studying and exploring principles of automated haematology analysers, making full
Cell analysis and bioimaging technology illustrated The Cell Analysis Center Scientific Bulletin Part 1 Sysmex has been studying and exploring principles of automated haematology analysers, making full
Motivation: Biofunctional Composite Nanoparticles
 1 Motivation: Biofunctional Composite Nanoparticles Iron Oxide Core Magnetic core Alternating magnetic field Brownian and Neél relaxation Bi-Material Composites MRI contrast enhancement and RF thermal
1 Motivation: Biofunctional Composite Nanoparticles Iron Oxide Core Magnetic core Alternating magnetic field Brownian and Neél relaxation Bi-Material Composites MRI contrast enhancement and RF thermal
Fundamentals of X-ray diffraction and scattering
 Fundamentals of X-ray diffraction and scattering Don Savage dsavage@wisc.edu 1231 Engineering Research Building (608) 263-0831 X-ray diffraction and X-ray scattering Involves the elastic scattering of
Fundamentals of X-ray diffraction and scattering Don Savage dsavage@wisc.edu 1231 Engineering Research Building (608) 263-0831 X-ray diffraction and X-ray scattering Involves the elastic scattering of
August 15, 2018 $ " % ' & OMe. NEt2 MeO
 Taking a deep look: a near infrared fluorescent dye for long term bioimaging ~ Promising photostable tool for single molecule tracking and multicolor imaging ~ August 15, 2018 A team of researchers at
Taking a deep look: a near infrared fluorescent dye for long term bioimaging ~ Promising photostable tool for single molecule tracking and multicolor imaging ~ August 15, 2018 A team of researchers at
