Gangliogenesis in leech: morphogenetic processes leading to segmentation in the central nervous system
|
|
|
- Dorothy Johnson
- 6 years ago
- Views:
Transcription
1 Dev Genes Evol (1998) 208:28 36 Springer-Verlag 1998 ORIGINAL ARTICLE selor&:daniel H. Shain Felipe-Andrés Ramírez-Weber Joyce Hsu David A. Weisblat Gangliogenesis in leech: morphogenetic processes leading to segmentation in the central nervous system csim&:received: 10 October 1997 / Accepted: 12 December p&:Abstract Using intracellular lineage tracers to study the main neurogenic lineage (N lineage) of the glossiphoniid leech embryo, we have characterized events leading from continuous columns of segmental founder cells (nf and ns primary blast cells) to discrete, segmentally iterated ganglia. The separation between prospective ganglia was first evident as a fissure between the posterior boundary of nf- and the anterior boundary of ns-derived progeny. We also identified the sublineages of nf-derived cells that contribute parallel stripes of cells to each segment. These stripes of cells project ventrolaterally from the dorsolateral margin of each nascent ganglion to the ventral body wall. The position and orientation of the stripes suggests that they play a role in forming the posterior segmental nerve; they are not coincident with the ganglionic boundary, and they form well after the separation of ganglionic primordia. Previous work has shown that cells in the anterior stripe express the leech engrailed-class gene. Thus, in contrast to the role of cells expressing engrailed in Drosophila, the stripes of N-derived cells expressing an engrailed-class gene in leech do not seem to play a direct role in segmentation or segment polarity. dwk&:key words Gangliogenesis Leech Annelid Engrailed Nerve formation&bdy: Introduction Forming the nervous system requires not only the genesis and differentiation of specific neurons and glia, but Edited by J. Campos-Ortega D.H. Shain F.-A. Ramírez-Weber 1 J. Hsu 2 D.A. Weisblat ( ) Department of Molecular and Cell Biology, 385 LSA, University of California, Berkeley, CA , USA Present addresses: 1 Department of Biochemistry and Biophysics, University of California, San Francisco, CA 94143, USA 2 Washington University School of Medicine, St. Louis, MI 63110, USA&/fn-block: also the morphogenetic processes by which these cells become organized spatially. Glossiphoniid leeches, such as Theromyzon rude, are well-suited for studying the role of cell lineage and cell-cell interactions in development because their embryos are large (~800 µm for T. rude) and hardy; in addition, their embryos undergo stereotyped cleavages that give rise to identifiable cells, accessible for experimental manipulation throughout development. Also, the adult leech central nervous system (CNS) is relatively simple in terms of its organization. The bilaterally symmetric CNS comprises a rostral, unsegmented, supraesophageal ganglion and 32 segmentally iterated ventral neuromeres: 4 fused neuromeres make up the anterior subesophageal ganglion; 7 fused neuromeres form a caudal ganglion associated with the posterior sucker; the remaining 21 neuromeres occur as distinct ganglia in the midbody of the animal, separated from adjacent ganglia by interganglionic connective nerves (Stent et al. 1992). Each segmental ganglion contains approximately 400 neurons (Macagno 1980), most of which are bilaterally paired. Many neurons in the adult leech have been assigned individual identities on the basis of morphological, physiological, and/or biochemical criteria (Muller et al. 1981). Previous work has established that the segmented nervous system, nephridia, epidermis, and musculature of the leech arise from rostrocaudally arrayed columns of segmental founder cells (blast cell bandlets), via intermediate structures called the germinal bands and germinal plate (see Fig. 1). The blast cell bandlets arise from five bilaterally paired stem cells (M, N, O/P, O/P and Q teloblasts) and are initially continuous (i.e., unsegmented). Within the germinal bands, they occupy discrete mesodermal and ectodermal layers, with the mesodermal (m) bandlet lying between the prospective endoderm (the yolk-filled macromeres) and the four ectodermal bandlets (n, o, p and q; Fig. 1; Whitman 1887; reviewed by Stent et al. 1992). As in other triploblastic animals, the leech nerve cord is primarily of ectodermal origin, yet lies within the mesodermally-derived coelom. Thus, the nerve cord is separated from the other major
2 ectodermal derivative, the epidermis, by layers of longitudinal, circular and oblique muscle fibers. Most ( ) of the neurons in an adult ganglion are descendants of the bilateral N teloblasts (Kramer and Weisblat 1985). In this study, we investigated the processes by which neural precursors become re-organized from continuous, superficial columns of cells (the ectodermal bandlets) into discrete, segmentally iterated ganglia. For this purpose, we followed the time course of gangliogenesis by differentially labeling the N lineage with fluorescent lineage tracers. By microinjecting individual primary and secondary blast cells, we also determined the contribution of specific blast cell progeny at selected times during development. We were particularly interested in relating events of gangliogenesis to the formation of two segmentally iterated transverse stripes of N-derived cells. As described previously (Wedeen and Weisblat 1991; Lans et al. 1993; Ramirez et al. 1995), these stripes of cells transiently express the leech engrailed-class gene during gangliogenesis. Based on the apparent homology between the stripes of engrailed-class gene expression in leech and arthropods, we previously postulated that the leech engrailed-class gene played a role in segmenting the CNS (Ramirez et al. 1995). We present evidence here that these cells may instead play a role in forming one of the segmental nerves by which ganglia innervate the body wall. Materials and methods Embryos Theromyzon rude embryos were obtained from specimens collected in the lakes of Golden Gate Park, San Francisco, and were cultured as previously described (Torrence and Stuart 1986), except that they were maintained at 23 C. Primary blast cells and their progeny were designated according to the system of Zackson (1984), as extended by Bissen and Weisblat (1989). Lineage tracer injections Fluorescent lineage tracer [either fluorescein-dextran amine (FDA; Molecular Probes) or tetramethylrhodamine-dextran amine (RDA; Molecular Probes)] was injected into the N teloblast after its birth as previously described (Weisblat et. al. 1980b). To follow the development of individual blast cell clones, primary (nf, ns, at 7 12 h clonal age; see next paragraph) or secondary (nf.a, nf.p, ns.a, ns.p, at clonal age ~20 h) blast cells were injected with a second lineage tracer. Both teloblast and blast cell injections were performed under a dissecting microscope. Successful teloblast injections were those in which the embryo survived and the injected cell produced a well-labeled, normal complement of progeny, as judged by a continuous column of primary blast cells in the early embryo and the normal complement of segmentally iterated definitive progeny in the older embryo as described previously (Weisblat et al. 1984; Weisblat and Shankland 1985; Kramer and Weisblat 1985). Successful blast cell injections were those in which the size, shape and distribution of labeled cells in the segment containing the doubly labeled clone were consistent with those of the segments anterior and posterior to it. Unsuccessful injections were those in which the embryo or injected cell died, or gave rise to broken columns of primary blast cells or irregular distributions of 29 blast cell progeny. By these criteria, the rate of successful injections was about 90% for teloblasts and 30% for blast cells. Blast cell injections were carried out without attempting to identify the specific cells being injected, so the estimated success rate is averaged over all types of cells. By this procedure, we obtained roughly ten times as many successful injections of nf.a cells as of nf.p cells and similar ratios were obtained for ns.a versus ns.p injections. This discrepancy probably reflects differences in relative size of the secondary blast cells, and perhaps also differences in their hardiness and/or their positions within the germinal bandlet. Overall, more than 200 T. rude embryos were dissected for the observations reported here. Clonal age determination The age of cell clones derived from primary or secondary n blast cells in T. rude embryos was determined relative to the age of the first labeled clone in the N lineage. The rate of blast cell genesis was determined empirically by injecting the N teloblast with RDA and allowing development to proceed for several hours before fixation. The number of labeled blast cells was then divided by the time interval between teloblast injection and fixation. From this we determined that primary blast cells were produced at a rate of approximately 1.6 cells per hour at 23 C. Thus, assuming that the teloblast cell cycles producing nf and ns blast cells are of equal length in Theromyzon, as in Helobdella (Bissen and Weisblat 1989), consecutive n blast cells differ in age by about 0.6 h. Using this number, the age of any given primary or secondary n blast cell clone was estimated by counting the clones between it and the leading edge of a labeled bandlet. The error of this method was approximately ± 1 h due to the time span in which embryos were injected and the time it takes a cell to recover from the trauma of injection (Bissen and Weisblat 1989). Determination of the number of N lineage progeny at specific time points Primary blast cell clonal progeny were counted for a total of 46 clones at time points ranging from h clonal age, using serial optical sections obtained by confocal microscopy. Outlines of cells labeled with lineage tracer were readily discerned by sectioning through focal planes comprising the progeny of a given clone. Histochemistry Embryos were fixed in 2% formaldehyde [in 0.2 M 4(-2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), ph 7.2] containing Hoechst (1 mg/ml final concentration) overnight at 4 C. The vitelline membrane was removed manually, and the germinal plate was dissected from the yolk with fine pins (Fine Science Tools, Cat. No ). Germinal plates were mounted in 80% glycerol containing 4% n-propylgallate; for viewing, embryos were placed on a slide, drawn out of the glycerol solution with pins and covered with a cover slip. Microscopy A Zeiss Axiophot microscope was used to examine and photograph dissected germinal plates. Slides were taken using Ektachrome 400 film (Kodak) and scanned with a SprintScan 35 Plus (Polaroid) slide scanner. Image enhancement (e.g., adjustment of color levels, merging of images) was performed with Adobe Photoshop (version 4.0). Confocal microscopy was performed with a MRC 600 system (Bio-Rad) attached to a Nikon epifluorescence microscope with a 40 oil immersion lens. A z-series for each N lineage clone was collected in fluorescein and rhodamine channels at intervals of µm. For some color images, a z-series was projected onto a single plane using SOM software (Bio-Rad). In
3 30 some preparations, relative clonal volumes were estimated from confocal z-series by tracing the contour of the clone for each confocal section, then cutting and weighing the contours for each clone. Embryo sectioning To obtain transverse views, germinal plates were prepared from fixed T. rude embryos as described above, then dehydrated through a graded series of alcohols (50 95%) and embedded in glycol methacrylate resin (JB-4; Polysciences) according to the manufacturer s instructions. The embedded embryos were then cut into sections roughly 20 µm thick using glass knives and a Sorvall MT2-B ultramicrotome. Sections were placed onto dry, gelatincoated slides, then expanded by the addition of water drops. The slides were re-dried and coverslips were mounted in either 80% glycerol in 100 mm TRIS, ph 9.0, or the glycol methacrylate embedding medium. Sections were viewed and photographed as described above. Results Overview An overview of the events leading from blast cell formation to discrete ganglia was obtained by injecting N teloblasts with lineage tracer and examining the labeled embryos at various times thereafter. For this purpose approximately 50 dissected germinal plates were examined, from embryos in which both N teloblasts had been injected with lineage tracer (see Materials and methods). In such preparations, the great similarity in midbody segments and the strict anteroposterior progression of development allowed us to infer the temporal sequence of events in a typical midbody segment by examining consecutive segments within the germinal plate. A summary of these results is outlined in Fig. 1, and representative preparations of T. rude embryos fixed at relevant embryonic stages are shown in Fig. 2. Briefly, alternating nf and ns primary blast cells formed a column of cells as they emerged from the bilateral pair of N teloblasts (N L and N R ). At clonal age h, primary nf blast cells divided unequally to generate two secondary blast cells, a larger anterior cell (nf.a) and a smaller posterior cell (nf.p). The ns blast cell underwent a more equal mitosis at clonal age ~20 h, generating anterior (ns.a) and posterior (ns.p) cells. These and subsequent cell divisions are oriented such that the n bandlets remain as single columns of cells as the germinal bands coalesce to form the germinal plate (clonal age ~40 h; Fig. 2 K, L; Bissen and Weisblat 1989). Following coalescence, further divisions of blast cell progeny led to the formation of segmentally iterated bulges within the n bandlets (Fig. 2 I, J). Between clonal ages h, a transverse cleft appeared near the lateral edge of each bulge. These clefts elongated medially and met at the ventral midline, forming a fissure that subdivided the N-derived cells into prospective ganglia. Later (clonal age ~60 h), two laterally directed stripes of cells emerged from the posterior region of each nf clone (Fig. 2E H). As described previously (Ramirez 1995), and in homology with the leech Helobdella triserialis (Wedeen and Weisblat 1991; Tsubokawa and We- Fig. 1 Composite drawing showing the temporal progression of morphogenetic events in the N lineage during formation of midbody ganglia in the CNS of Theromyzon rude. (Contributions from the four other lineages are not shown.) Bilaterally paired N teloblasts (N L and N R ) give rise to coherent columns of cells (n bandlets). Each bandlet comprises two alternating classes of primary blast cells (ns and nf), whose distinct fates are first indicated by the differences in size of their respective progeny (ns.a and ns.p, nf.a and nf.p). Upon entering the germinal plate, contralateral n blast cell clones align along the ventral midline (dashed line) and subsequently give rise to the bulk of the segmental ganglia of the ventral nerve cord, along with some segmentally iterated peripheral neurons (nz1, nz2 and nz3) and a few epidermal cells (not shown). Morphogenetic processes associated with gangliogenesis include: formation of lateral bulges through proliferation of n blast cell clones; formation of transverse fissures that separate ganglionic primordia and divide the lateral bulges into distinct anterior and posterior lobes; outgrowth of two ventrolateral stripes of cells from each posterior lobe (the anterior of which expresses the leech engrailed-class gene); and neural differentiation, including outgrowth of segmental nerves, designated AA (anterior-anterior), MA (medial-anterior), PP (posterior-posterior) and UP (ultra-posterior). In addition, neurons project longitudinally via bilaterally paired connective nerves (not shown) and a median unpaired nerve tract (Faivre s nerve; not shown). Approximate clonal ages for n blast cells and their derivatives are indicated at right. Anterior is up. Not drawn to scale&/f :c.gi
4 31 deen, personal communication), cells in the more anterior stripe (~6 cells) transiently express the leech engrailed-class gene. By clonal age ~75 h, most cells in both stripes had disappeared (Fig. 2C, D), leaving only the previously described peripheral neurons nz1, nz2 and nz3 (Braun and Stent 1989a; Lans et al. 1993). Most features of the mature ganglia were observed by clonal age 100 h (Fig. 2A, B), including the projection of axons along the segmental nerves designated AA (anterior-anterior), MA (medial-anterior), PP (posterior-posterior), and UP (ultra-posterior). Cell proliferation in the nf and ns clones is depicted graphically in Fig. 3. Formation of a linear array of segmental founder cells: clonal ages 0 45 h Previous analyses of the N lineage in clitellate annelids have shown that two primary n blast cells are required to Fig. 3 Proliferation of nf and ns clones during gangliogenesis. The cells in 46 individually labeled primary blast cell clones were counted (see Materials and methods) at approximate clonal ages ranging from 32 to 95 h. The results are grouped within 10 h intervals centered on the times indicated. Each bar represents data from 1 8 independent clones; error bars indicate the standard deviation for bins with multiple data points. For the first two intervals (5 15 h and h), clone sizes (1 cell and 2 cells, respectively for both nf and ns clones) are known by direct observation of the first mitoses and their homology to those in the closely related genus Helobdella (Zackson 1984; Bissen and Weisblat 1989)&/f :c.gi Fig. 2A L Key stages during gangliogenesis in T. rude. Each panel on the left is a double exposure fluorescence photomicrograph showing several segments from the germinal plates of embryos in which the N L teloblasts were injected with tetramethylrhodamine-dextran amine (RDA; red) and the N R teloblasts with fluorescein-dextran amine (FDA; green); the resultant embryos were fixed and dissected at progressively later developmental stages. Panels showing developmentally more advanced segments are at the top of the figure, to match the developmental gradient in the germinal plate. In the double exposure photomicrographs (left panels), yellow areas result from slightly overlapping images caused by imperfect alignment of the two filter sets. The right panel in each pair shows nuclei in the same portion of the germinal plate, revealed by counterstaining with Hoechst (blue); lighter areas correspond to higher nuclear densities. The range of clonal ages within each panel is as follows: A, B h; C, D h; E, F h; G, H h; I, J h; K, L h (Scale bar 20 µm)&/f :c.gi
5 32 Fig. 4 Fissure formation. Photomicrograph showing roughly ten segments of a germinal plate in which both n bandlets were labeled with RDA (white); the ventral midline runs through the center of the long axis (cf. Fig. 2). Arrows mark the two most anterior pairs of lateral bulges in which the fissure has not yet formed. Arrowheads indicate the position of the fissure on either side of the midline. Blast cell clones in the marked segments range from ~42 h (bottom) to ~52 h (top). Anterior is up (Scale bar 20 µm)&/f :c.gi per segment, the length of the prospective segmental ganglion within the bandlet is the length of two primary blast cells (40 50 µm in the fixed specimens of T. rude used in our experiments). As described by Lans et al. (1993), there is a disparity in the age of n and q primary cell clones relative to consegmental m, o and p clones, and this disparity increases as one progresses from anterior to posterior segments. One consequence of this was that anterior n blast cell clones were approximately ~40 h of age upon entering the germinal plate, while posterior clones were only ~25 h when they entered the germinal plate (data not shown). Accordingly, the size of the nf and ns clones at coalescence (Fig. 2K, L) varied from ~2 to ~10 cells between anterior and posterior regions of the germinal plate (Fig. 3). make a single segmental complement of N-derived progeny, and that primary n blast cells adopt distinct nf and ns fates in exact alternation at some point prior to their first mitoses [described for Helobdella triserialis (Hirudinea) by Weisblat et al. 1980a; Zackson 1984; Bissen and Weisblat 1987, and for Eisenia foetida (Oligochaeta) by Storey 1989]. Since there are two n blast cell clones Fig. 5A E Fissures form between nf.p and ns.a secondary blast cell clones. Double exposure photomicrographs, showing segments of embryos in which one (A) or both (B E) N teloblasts were injected with RDA; later, primary (A) or secondary (B E) n blast cells were injected with FDA and their doubly labeled clones therefore appear as yellow. Arrowheads identify the location of the fissures. A Confocal micrograph showing several planes of a preparation in which nf (top) and ns (bottom) primary blast cell fills were fixed at clonal ages ~50 and ~48 h, respectively. Secondary blast cell fills were fixed at clonal age ~55 h and photographed on a compound microscope: B nf.a; C nf.p; D ns.a; E ns.p (Scale bar 25 µm)&/f :c.gi Fissures appearing between the nf.p and ns.a clones separate ganglionic primordia: clonal ages h Within the germinal plate, the n bandlets eventually lost their linear geometry and formed segmentally iterated bulges by clonal age 45 h (Fig. 2I, J). Presumably, this shape change resulted from oriented cell divisions and/or cell rearrangements as the nf and ns clones increased to ~25 30 cells each (Fig. 3). Cell growth may have also contributed to this process, since the combined volume of the nf and ns clones at this clonal age (~50 h) was at least 50% greater than the combined volumes of primary nf and ns blast cells. The overt separation of the n bandlets into discrete ganglionic primordia began between h clonal age with the formation of bilateral transverse clefts, just posterior to the most lateral extent of the segmentally iterated bulges (Fig. 4). The clefts elongated medially and met at the ventral midline, subdividing the bandlets into discrete ganglionic primordia. The fissure reached the midline within three segments (i.e. 6 blast cell clones) anterior to where
6 the cleft was first visible; since primary blast cells were born at the rate of 1.6 per hour, we conclude that the entire process of fissure formation is complete within ~4 h. To investigate the possibility that the interganglionic fissures form between specific cells of the N lineage, we determined the location of each N-derived primary (nf and ns) and secondary (nf.a, nf.p, ns.a and ns.p) blast cell clone as the fissure formed (Fig. 5). For this purpose, we examined about 50 embryos each in which nf, ns, nf.a or ns.a clones were labeled and 5 embryos each in which nf.p or ns.p clones were labeled by direct injection of the primary or secondary blast cell (see Materials and methods). We found that the entire nf clone lay anterior to the fissure, and the ns clone lay posterior (Fig. 5A). There was no intermingling of nf- and ns-derived progeny at this stage (~50 h). The nf.p and ns.a clones contributed progeny that lined the anterior and posterior boundaries of the fissure, respectively; nf.p formed a narrow stripe of 4 6 cells at the posterior margin of one ganglionic primordium (Fig. 5C), while ns.a occupied the anterior lobe of the next posterior hemiganglion (Fig. 5D). The nf.a and ns.p clones contributed progeny internal to the boundaries of the fissure; nf.a-derived cells were contained within the posterior lobe of the hemiganglion (Fig. 5B), and ns.p occupied a medioposterior position that abutted the midline, connecting the ns.a subclone with nf (Fig. 5E). The relative positions of these clones within prospective ganglia indicate that nf.p- and ns.a-derived cells separate from each other, thus subdividing the initially continuous n bandlets into discrete ganglionic primordia. As a result of fissure formation, the segmentally iterated bulges were separated into two distinct populations of cells in each segment (the anterior and posterior lobes, respectively). These lobes were oriented transverse to the anteroposterior axis of the germinal plate and marked the anterior and posterior margins of each prospective ganglion. The region between these projections is occupied by cells from other lineages, as described elsewhere (Weisblat and Shankland 1985; Torrence and Stuart 1986; Lans et al. 1993). As development proceeded, the nf clone expanded laterally further than the ns clone. Thus, on the basis of this criterion, the two clones could be distinguished from each other in the germinal plate (Weisblat et al. 1980a; Zackson 1984; Wedeen and Weisblat 1991; compare Figs. 1, 2 and 5). Ventrolateral stripes of nf-derived cells emerge from the posterior lobe of the ganglion: clonal ages h 33 Fig. 6A C Ventrolateral stripes of cells, arising from nf.a and nf.p clones, connect the ganglion to the ventral body wall. A Digitally merged photomicrographs showing one half of a germinal plate, sectioned (~10 µm thick) transverse to the longitudinal axis of the embryo at the level of an RDA-labeled nf clone (~65 h clonal age); this preparation was counterstained with Hoechst (blue). In this view, a ventrolateral stripe (arrows) extends from the dorsolateral margin of the ganglion (gm) toward the epidermis (ep) of the body wall. Ventral midline (vml) is at the right; dorsal is up. B and C Double exposure photomicrographs showing segmental ganglia from the dissected germinal plate of an embryo in which both N teloblasts were injected with RDA (red) and, later, individual nf or nf.a blast cells were injected with FDA; the doubly labeled clones appear yellow in these preparations. B In an nf clone fixed at ~65 h clonal age, both ventrolateral stripes are yellow, indicating that they arose from the doubly labeled nf clone. C In an nf.a clone fixed at ~65 h, the anterior stripe (arrow) is yellow, indicating that it arose from the nf.a clone, while the posterior stripe (arrowhead) is red, indicating that it arose from the nf.p clone (Scale bar 20 µm)&/f :c.gi During this phase of development, the ns clone expanded along the anteroposterior axis, ultimately spanning the length of the ganglion. Most of this expansion was directed posteriorly along the medial portion of the nascent ganglion and resulted from clonal growth of the ns.p subclone, which increased to ~16 cells during this period. The nf clone, in contrast, expanded laterally, particularly along its ventral aspect, forming two stripes of cells near the ventral surface of the germinal plate. These nfderived stripes were directly adjacent to each other with their cells forming a staggered mediolateral array. Examination of transverse sections at ~65 h clonal age revealed that these lateral stripes of cells transiently con-
7 34 Fig. 7A F Clonal contribution of definitive N-derived progeny. Digitally merged photomicrographs showing segmental ganglia containing the progeny of individual blast cells that were microinjected with FDA (green) and fixed at ~100 h clonal age. Germinal plates were counterstained with Hoechst to visualize the overall distribution of nuclei (blue). The injected blast cells in each panel are: A ns; B ns.a; C ns.p; D nf; E nf.a; F nf.p. Note that the cellular distributions shown in B and C sum to that shown in A, while those shown in E and F sum to that shown in D. In addition, while all ns-derived neurons are confined to within a single ganglion, the nf.a and nf.p clones each give rise to peripheral neurons and to cells in the adjacent posterior ganglion. In addition to the cell bodies, tracer-labeled axons are visible in one or more of the segmental or connective nerves in each panel (see Table 1 for details). (Scale bar 16 µm in D, 20 µm in all other panels) :c.gif/& nected the dorsal margin of the ganglion with the ventral body wall (Fig. 6A). No such projections were observed in the ns clone. Previous work in Helobdella triserialis and T. rude (Ramirez et al. 1995) has shown that the leech engrailedclass gene is expressed in the more anterior of the two nf projections, and that the ns clone shows no detectable levels of the engrailed-class gene at this stage. Direct injection of secondary blast cells revealed that the nf.a clone gave rise to the anterior ventral stripe of cells; by subtraction we deduce that nf.p gives rise to the posterior ventral stripe of cells (Fig. 6B, C; see also Fig. 5B, C, in which the stripes of cells are just starting to form). Thus, cells expressing the leech engrailed-class gene arise from the nf.a subclone, and appear specifically in the anterior ventrolateral stripe (Fig. 6C). By clonal age ~75 h, most cells in both the nf.a and nf.p stripes had disappeared as in Helobdella (Wedeen and Weisblat 1991). Whether these cells die or migrate into the ganglion remains to be established. The result that the transverse stripes of cells arise after the ganglionic primordia have already separated, and anterior to the fissures separating the primordia, suggests that, contrary to our previous hypothesis (Ramirez et al. 1995), these stripes are probably not the nf-derived cells required for separating adjacent ganglia. Contributions of N-derived blast cell progeny to the mature ganglion: clonal age 100 h To determine the relative positions of N-derived primary and secondary blast cell clones and their axonal projections in mature ganglia, blast cells were injected with FDA and fixed at ~100 h clonal age (Fig. 7). By this point, the embryos were at stage 11 of development and exhibited neuronally mediated behaviors including shortening and bending in response to tactile stimuli (Stent et al. 1992). The overall morphology of the nf and ns clones was similar to that observed at ~50 h clonal age (cf. Figs. 5, 7). In addition to an increase in cell number (Fig. 3), these clones also contained morphologically differentiated neurons, whose axons were visible in the segmental nerves and in the connectives of tracer-labeled specimens (Table 1). Progeny derived from the ns clone were confined to a single ganglion (Fig. 7A); the ns.a subclone occupied the anterior lobe of each hemiganglion (Fig. 7B), and the ns.p subclone occupied a medio-lateral position that contacted ns.a at its anterior edge and extended to the posterior boundary of the ganglion (Fig. 7C). The ns.p clone also contributed at least one neuron lying lateral to the rest of the ns.p clone, and just posterior to ns.a (Fig. 7A, C). The nf clone contributed the majority of its progeny to the posterior lobe of the hemiganglion (Fig. 7D). The respective contributions of the nf.a and nf.p subclones are shown in Fig. 7E and F, respectively. The nf.a subclone occupied the anterior region of the posterior lobe, while nf.p remained as a relatively thin stripe of cells (compare with Fig. 5C) that formed the posterior boundary of the ganglion. In contrast with the ns clone, several nf-derived neurons lay outside the ganglion, either peripherally (forming the ventrolateral stripes described above) or in the adjacent, posterior ganglion (Fig. 7D F). Most of the cells in the ventrolateral stripes disappeared, but cells at the distal end of each stripe persisted and became the previously identified peripheral neurons of the N lineage (nz1, nz2 and nz3; Weisblat et al. 1978; Weisblat et al.
8 35 Table 1 Projections of N-derived neurons into connectives and segmental nerves. Embryos in which individual primary or secondary blast cells (top row) had been injected with FDA (clonal ages ~10-25 h) were fixed and dissected ~100 h later (i.e. clonal ages ranging from ~110 h for primary blast cell clones to ~125 h for secondary blast cell clones), then examined for the distribution of labeled axons within the connectives and segmental nerves. Only well labeled clones showing the normal distribution of labeled cell bodies were scored; the number of clones scored for each case is indicated in parentheses. Note that projections into the contralateral posterior connectives and into Faivre s nerve were seen from one or more of the secondary blast cell clones, but not from any of the primary blast cell clones from which they arose. This discrepancy may result from differences in clonal age at the time of fixation. Abbreviations not given in Figure 1 are AC, anterior connective; PC, posterior connective; AF, anterior Faivre s nerve; PF, posterior Faivre s nerve&/tbl.c:&tbl.b: :b.lbt/& 1984; Torrence and Stuart 1986; Braun and Stent 1989a). The nz1 and nz2 neurons arose from the anterior, nf.aderived stripe of cells (Fig. 7E); nz3 arose from the posterior, nf.p-derived stripe (Fig. 7F). This is consistent with the observations that, in Helobdella, the engrailedclass gene is expressed in nz1 and nz2 neurons (in addition to others) but not in nz3, a pattern which apparently persists throughout the life of the leech (Wedeen and Weisblat 1991; Lans et al. 1993). Cells originating from both nf.a and nf.p migrated into the adjacent, posterior ganglion and intermingled with cells derived from the posterior, ns.a clone (Fig. 7E, F). This migration began at ~90 h clonal age, well after the boundary between adjacent ganglia had been established (see Fig. 5). Finally, both ns and nf clones projected axonal processes into the segmental nerve tracts. Table 1 summarizes the main contributions of each primary and secondary blast cell clone to the connectives and segmental nerves. Discussion ns ns.a ns.p nf nf.a nf.p (3) (5) (2) (5) (6) (2) Ipsilateral AA MA PP UP AC PC Contralateral AA 3 5 MA 3 5 PP UP AC PC Midline AF 1 PF 1 Formation of ganglionic primordia in leech We have characterized the morphogenetic processes leading to gangliogenesis in the glossiphoniid leech CNS by following the clonal development of segmental founder cells (the nf and ns blast cells and their progeny) in the main neurogenic (N) lineage of Theromyzon rude. A key event in this process was the formation of a fissure between the nf.p and ns.a secondary blast cell clones over the course of 3 4 h (~50 h clonal age). This fissure subdivided an initially continuous column of cells (the n bandlets) into discrete ganglionic primordia. The mechanism by which this fissure forms remains to be determined. Possible mechanisms include: (1) active cell movements within the n bandlet leading to separation of the nf.p and ns.a clones; (2) cell movements in one or more of the adjacent lineages, such as the underlying mesodermal layer, that pull the nf.p and ns.a clones apart passively; and (3) clone-specific decreases in cellular affinities resulting in delamination of the nf.p and ns.a clones. Previous experiments, in which nf and ns clones were deleted at the 2-cell stage (clonal age ~18 20 h in T. rude) are consistent with the notion of early differences between the nf and ns clones. In those experiments (Ramirez et al. 1995), ablation of an nf clone (which results in two ns clones becoming adjacent to each other in the germinal band) resulted in the fusion of the two hemiganglia on the affected side. By contrast, ablation of an ns clone (which results in two nf clones becoming adjacent to each other in the germinal band) reduced the size of the affected ganglion, but did not affect the separation of ganglia. One interpretation of this result is that, at some point after ablation of the nf clone, the two adjacent ns clones came together and then failed to separate. If so, this scenario may reflect differences between nf and ns clones in cell motility and/or affinity. We speculate that the nf.p clone serves as a spacer clone, of which the cells undergo a decrease in affinity with respect to the adjacent, ns.a-derived cells at ~50 h clonal age, allowing the ganglionic primordia to separate. Significance of early engrailed-class gene expression in the N lineage Previous studies have shown that the initial expression of the leech engrailed-class gene in the N lineage is in segmentally iterated stripes of nf-derived cells, prior to overt segmentation, and near the prospective morphological boundaries of future segments (Wedeen and Weisblat 1991; Lans et al. 1993; Ramirez et al. 1995). On this basis, and by analogy with what is known of the function of engrailed in Drosophila (Kornberg 1981a, b; DiNardo et al. 1985; Kornberg et al. 1985; Poole et al. 1985), it was proposed that one function of the annelid engrailed homologs may be in segmenting the nervous system by separating the n bandlets into discrete ganglionic primordia. In the work reported here, we have extended the previous lineage analysis to show that the stripe of cells expressing the leech-engrailed-class gene arises more specifically from within the nf.a blast cell clone, and that a
9 36 posterior stripe of cells arises from the nf.p blast cell clone. We also determined that these stripes of cells appear at ~60 h clonal age, well after ganglionic primordia have separated. Moreover, the stripes of cells lie anterior to the ganglionic boundary. These data suggest that expression of the leech engrailed-class gene is not associated with the morphogenetic processes of ganglion separation. Two observations suggest that the stripes of nf-derived cells may participate in forming segmental nerves that connect the ganglia to the body wall. First, as described here and previously (Braun and Stent 1989a; Wedeen and Weisblat 1991), identified peripheral neurons arise from the distal ends of both the anterior and posterior stripes of cells (nz1 and nz2 from the anterior, nf.aderived stripe and nz3 from the posterior, nf.p-derived stripe). These neurons lie on the PP and UP branches of the posterior segmental nerve, respectively. Braun and Stent (1989b) also showed that nz3 is required for formation of the UP nerve. Second, we find that the stripes of cells project ventrolaterally from the dorsolateral aspect of the ganglion, forming a cellular bridge to the ventral body wall. These features make the two stripes of cells good candidates for providing a guidance pathway for the posterior nerve tracts. The expression of the leech engrailed-class gene may play a role in regulating the specificity of this process in the anterior stripe of nf.aderived cells. 2.p&:Acknowledgements We thank Duncan Stuart for guidance in collecting and handling T. rude, Françoise Huang for instruction in manipulating digital images, Bob Goldstein, FH, Marc Pilon and Cathy Wedeen for helpful discussions and G Rubin for use of his color printer. This work was supported by NIH NRSA 1 F32 HD to DHS and by NIH Grant HD to DAW. References Bissen ST, Weisblat DA (1987) Early differences between alternate n blast cells in leech embryos. J Neurobiol 18: Bissen ST, Weisblat DA (1989) The durations and compositions of cell cycles in embryos of the leech, Helobdella triserialis. Development 106: Braun J, Stent GS (1989a) Axon outgrowth along segmental nerves in the leech: I. Identification of candidate guidance cells. Dev Biol 132: Braun J, Stent GS (1989b) Axon outgrowth along segmental nerves in the leech: II. Identification of actual guidance cells. Dev Biol 132: DiNardo S, Kuner J, Theis J, O Farrell PH (1985) Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43: Kornberg T (1981a) Compartments in the abdomen of Drosophila and the role of the engrailed locus. Dev Biol 86: Kornberg T (1981b) engrailed: a gene controlling compartment and segment formation in Drosophila. Proc Nat Acad Sci USA 78: Kornberg T, Siden I, O Farrell PH, Simon M (1985) The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell 40: Kramer AP, Weisblat DA (1985) Developmental neural kinship groups in the leech. J Neurosci 5: Lans D, Wedeen CJ, Weisblat DA (1993) Cell lineage analysis of the expression of an engrailed homolog in leech embryos. Development 117: Macagno ER (1980) Number and distribution of neurons in leech segmental ganglia. J Comp Neurol 190: Muller KJ, Nicholls JG, Stent GS (1981) Neurobiology of the leech. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY Poole SJ, Kauvar LM, Drees B, Kornberg T (1985) The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell 40: Ramirez, FA (1995) Morphogenesis and engrailed-class gene expression during development of the glossiphoniid leech central nervous system. Ph.D. thesis, University of California, Berkeley Ramirez FA, Wedeen CJ, Stuart DK, Lans D, Weisblat DA (1995) Identification of a neurogenic sublineage required for CNS segmentation in an annelid. Development 121: Stent GS, Kristan WB, Torrence SA, French KA, Weisblat DA (1992) Development of the leech nervous system. Int Rev Neurobiol 33: Storey KG (1989) Cell lineage and pattern formation in the earthworm embryo. Development 107: Torrence SA, Stuart DK (1986) Gangliogenesis in leech embryos: migration of neural precursor cells. J Neurosci 6: Wedeen CJ, Weisblat DA (1991) Segmental expression of an engrailed-class gene during early development and neurogenesis in an annelid. Development 113: Weisblat DA, Shankland M (1985) Cell lineage and segmentation in the leech. Philos Trans R Soc London 313: Weisblat DA, Sawyer RT, Stent GS (1978) Cell lineage analysis by intracellular injection of a tracer enzyme. Science 202: Weisblat DA, Harper G, Stent GS, Sawyer RT (1980a) Embryonic cell lineage in the nervous system of the glossiphoniid leech Helobdella triserialis. Dev Biol 76: Weisblat DA, Zackson SL, Blair SS, Young JD (1980b) Cell lineage analysis by intracellular injection of fluorescent tracers. Science 209: Weisblat DA, Kim SY, Stent GS (1984) Embryonic origin of cells in the leech Helobdella triserialis. Dev Biol 104: Whitman CO (1887) A contribution to the history of the germ layers in Clepsine. J Morphol 1: Zackson SL (1984) Cell lineage, cell-cell interaction, and segment formation in the ectoderm of a glossiphoniid leech embryo. Dev Biol 104:
Cell Lineage in the Development of the Leech Nervous System
 Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
Cell position and developmental fate in leech embryogenesis
 Proc. Nati. Acad. Sci. USA Vol. 87, pp. 8457-8461, November 1990 Developmental Biology Cell position and developmental fate in leech embryogenesis (cell lineage/transfating/equivalence group/determination)
Proc. Nati. Acad. Sci. USA Vol. 87, pp. 8457-8461, November 1990 Developmental Biology Cell position and developmental fate in leech embryogenesis (cell lineage/transfating/equivalence group/determination)
Developmental origin of segmental identity in the leech mesoderm
 Development 117, 177-189 (1993) Printed in Great Britain The Company of Biologists Limited 1993 177 Developmental origin of segmental identity in the leech mesoderm Lidia Gleizer * and Gunther S. Stent
Development 117, 177-189 (1993) Printed in Great Britain The Company of Biologists Limited 1993 177 Developmental origin of segmental identity in the leech mesoderm Lidia Gleizer * and Gunther S. Stent
Micromere fate maps in leech embryos: lineage-specific differences in rates of cell proliferation
 Development 120, 3427-3438 (1994) Printed in Great Britain The Company of Biologists Limited 1994 3427 Micromere fate maps in leech embryos: lineage-specific differences in rates of cell proliferation
Development 120, 3427-3438 (1994) Printed in Great Britain The Company of Biologists Limited 1994 3427 Micromere fate maps in leech embryos: lineage-specific differences in rates of cell proliferation
Establishment of segment polarity in the ectoderm of the leech Helobdella
 Development 128, 1629-1641 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV3380 1629 Establishment of segment polarity in the ectoderm of the leech Helobdella Elaine C. Seaver*
Development 128, 1629-1641 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV3380 1629 Establishment of segment polarity in the ectoderm of the leech Helobdella Elaine C. Seaver*
Cell lineage analysis of pattern formation in the Tubifex embryo. II. Segmentation in the ectoderm
 Int. J. Dev. Biol. 44: 797-805 (2000) Ectodermal segmentation in Tubifex 797 Original Article Cell lineage analysis of pattern formation in the Tubifex embryo. II. Segmentation in the ectoderm AYAKI NAKAMOTO,
Int. J. Dev. Biol. 44: 797-805 (2000) Ectodermal segmentation in Tubifex 797 Original Article Cell lineage analysis of pattern formation in the Tubifex embryo. II. Segmentation in the ectoderm AYAKI NAKAMOTO,
High Resolution Cell Lineage Tracing Reveals Developmental Variability in Leech
 DEVELOPMENTAL DYNAMICS 238:3139 3151, 2009 RESEARCH ARTICLE High Resolution Cell Lineage Tracing Reveals Developmental Variability in Leech Stephanie E. Gline,* Dian-Han Kuo, Alberto Stolfi, and David
DEVELOPMENTAL DYNAMICS 238:3139 3151, 2009 RESEARCH ARTICLE High Resolution Cell Lineage Tracing Reveals Developmental Variability in Leech Stephanie E. Gline,* Dian-Han Kuo, Alberto Stolfi, and David
Segmentation in leech development
 Development 104 Supplement, 161-168 (1988) Printed in Great Britain @ The Company of Biologists Limited 1988 L6T Segmentation in leech development DAVD A. WESBLAT, DAVD J. PRCE and CATHY J. WEDEEN Department
Development 104 Supplement, 161-168 (1988) Printed in Great Britain @ The Company of Biologists Limited 1988 L6T Segmentation in leech development DAVD A. WESBLAT, DAVD J. PRCE and CATHY J. WEDEEN Department
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
Segmental expression of an engrailed-c\ass gene during early development and neurogenesis in an annelid
 Development 113, 805-814 (1991) Printed in Great Britain The Company of Biologists Limited 1991 805 Segmental expression of an engrailed-c\ass gene during early development and neurogenesis in an annelid
Development 113, 805-814 (1991) Printed in Great Britain The Company of Biologists Limited 1991 805 Segmental expression of an engrailed-c\ass gene during early development and neurogenesis in an annelid
In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary
 Supplementary Methods In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary somatosensory cortex (S1), forepaw
Supplementary Methods In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary somatosensory cortex (S1), forepaw
Introduction to Histology
 Introduction to Histology Histology The term "Histology" is derived from the Greek word for a tissue "Histos", and "-logos" = the study of Histology : Is the study of tissues and how they are arranged
Introduction to Histology Histology The term "Histology" is derived from the Greek word for a tissue "Histos", and "-logos" = the study of Histology : Is the study of tissues and how they are arranged
Cell lineage and pattern formation in the earthworm embryo
 Development 107, 519-531 (1989) Printed in Great Britain The Company of Biologists Limited 1989 519 Cell lineage and pattern formation in the earthworm embryo KATE G. STOREY Department of Zoology, University
Development 107, 519-531 (1989) Printed in Great Britain The Company of Biologists Limited 1989 519 Cell lineage and pattern formation in the earthworm embryo KATE G. STOREY Department of Zoology, University
Movements and stepwise fusion of endodermal precursor cells in leech
 Dev Genes Evol (1998) 208:117 127 Springer-Verlag 1998 ORIGINAL ARTICLE selor&:nai-jia L. Liu Deborah E. Isaksen Constance M. Smith David A. Weisblat Movements and stepwise fusion of endodermal precursor
Dev Genes Evol (1998) 208:117 127 Springer-Verlag 1998 ORIGINAL ARTICLE selor&:nai-jia L. Liu Deborah E. Isaksen Constance M. Smith David A. Weisblat Movements and stepwise fusion of endodermal precursor
Developmental Biology
 Developmental Biology 336 (2009) 112 121 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Evolution of Developmental Control Mechanisms
Developmental Biology 336 (2009) 112 121 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Evolution of Developmental Control Mechanisms
Reading. Lecture III. Nervous System Embryology. Biology. Brain Diseases. September 5, Bio 3411 Lecture III. Nervous System Embryology
 Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Lecture III. Nervous System Embryology
 Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Later Development. Caenorhabditis elegans. Later Processes 10/06/12. Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development
 Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
BMP-2 and BMP-4 signalling in the developing spinal cord of human and rat embryos
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 74, No. 3, pp. 359 364 DOI: 10.5603/FM.2015.0054 Copyright 2015 Via Medica ISSN 0015 5659 www.fm.viamedica.pl BMP-2 and BMP-4 signalling in the developing
O R I G I N A L A R T I C L E Folia Morphol. Vol. 74, No. 3, pp. 359 364 DOI: 10.5603/FM.2015.0054 Copyright 2015 Via Medica ISSN 0015 5659 www.fm.viamedica.pl BMP-2 and BMP-4 signalling in the developing
Sydney Brenner: Present
 Sydney Brenner: 1927 - Present Born in the small town of Germiston, South Africa Won a scholarship to the University of Witwatersrand at the age of 15 Received his PhD from Exeter College, Oxford Made
Sydney Brenner: 1927 - Present Born in the small town of Germiston, South Africa Won a scholarship to the University of Witwatersrand at the age of 15 Received his PhD from Exeter College, Oxford Made
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Nature Neuroscience: doi: /nn Supplementary Figure 1. Recombination within the neurogenic lineage is clonal and specific to NSCs.
 Supplementary Figure 1 Recombination within the neurogenic lineage is clonal and specific to NSCs. (a) Relative abundance of distinct cell types within the recombined population, assessed 1.3 days (i.e.
Supplementary Figure 1 Recombination within the neurogenic lineage is clonal and specific to NSCs. (a) Relative abundance of distinct cell types within the recombined population, assessed 1.3 days (i.e.
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
The fate of the CNS midline progenitors in Drosophila as revealed by a new method for single cell labelling
 Development 120, 1895-1906 (1994) Printed in Great Britain The Company of Biologists Limited 1994 1895 The fate of the CNS midline progenitors in Drosophila as revealed by a new method for single cell
Development 120, 1895-1906 (1994) Printed in Great Britain The Company of Biologists Limited 1994 1895 The fate of the CNS midline progenitors in Drosophila as revealed by a new method for single cell
Chapter 47. Development
 Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Cell lineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates
 Development 129, 5789-5801 2002 The Company of Biologists Ltd doi:10.1242/dev.00155 5789 Cell lineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates
Development 129, 5789-5801 2002 The Company of Biologists Ltd doi:10.1242/dev.00155 5789 Cell lineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
What s the most complex problem in biology?
 Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Slow intermixing of cells during Xenopus embryogenesis contributes to the consistency of the blastomere fate map
 Development 105. 9-15 (1989) < Printed in Great Britain The Company of Biologists Limited 1989 Slow intermixing of cells during Xenopus embryogenesis contributes to the consistency of the blastomere fate
Development 105. 9-15 (1989) < Printed in Great Britain The Company of Biologists Limited 1989 Slow intermixing of cells during Xenopus embryogenesis contributes to the consistency of the blastomere fate
RUTGERS. Executive Director NJ Commission on Spinal Cord Research PO Box 360 Trenton, NJ
 RUTGERS W. M. KECK CENTER FOR COLLABORATIVE NEUROSCIENCE 604 Allison Road, D251, Piscataway, NJ 08854-8082 USA Dept. of Cell Biology and Neuroscience (732) 445-6sn, (732) 445-2061, Fax: (732) 445-2063
RUTGERS W. M. KECK CENTER FOR COLLABORATIVE NEUROSCIENCE 604 Allison Road, D251, Piscataway, NJ 08854-8082 USA Dept. of Cell Biology and Neuroscience (732) 445-6sn, (732) 445-2061, Fax: (732) 445-2063
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
ANAT2341 Embryology Introduction: Steve Palmer
 ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
Piscataway, NJ February 2007
 Final Progress Report for the New Jersey Commission on Spinal Cord Research Submitted by David P. Crockett, PhD for the late Ira B. Black, MD Department of Neuroscience and Cell Biology UMDNJ-Robert Wood
Final Progress Report for the New Jersey Commission on Spinal Cord Research Submitted by David P. Crockett, PhD for the late Ira B. Black, MD Department of Neuroscience and Cell Biology UMDNJ-Robert Wood
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Exam 1 ID#: October 1, 2006
 Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
The durations and compositions of cell cycles in embryos of the leech, Helobdella triserialis
 Development 15, 15118 (1989) Printed in Great Britain The Company of Biologists Limited 1989 15 The durations and compositions of cell cycles in embryos of the leech, Helobdella triserialis SHIRLEY T.
Development 15, 15118 (1989) Printed in Great Britain The Company of Biologists Limited 1989 15 The durations and compositions of cell cycles in embryos of the leech, Helobdella triserialis SHIRLEY T.
Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration?
 Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
The cell lineage of the muscles of the Drosophila head
 J. Embryol. exp. Morph. 85,285-294 (1985) 285 Printed in Great Britain The Company of Biologists Limited 1985 The cell lineage of the muscles of the Drosophila head K. VIJAY RAGHAVAN* A LUDWIN PINTO Molecular
J. Embryol. exp. Morph. 85,285-294 (1985) 285 Printed in Great Britain The Company of Biologists Limited 1985 The cell lineage of the muscles of the Drosophila head K. VIJAY RAGHAVAN* A LUDWIN PINTO Molecular
OF SHEAR ZONES IN POLYCRYSTALLINE
 Tectonophysics, 78 (1981) 677-685 677 Elsevier Scientific Publishing Company, Amsterdam - Printed in The Netherlands THE DEVELOPMENT CAMPHOR OF SHEAR ZONES IN POLYCRYSTALLINE J.L. URAI and F.J. HUMPHREYS
Tectonophysics, 78 (1981) 677-685 677 Elsevier Scientific Publishing Company, Amsterdam - Printed in The Netherlands THE DEVELOPMENT CAMPHOR OF SHEAR ZONES IN POLYCRYSTALLINE J.L. URAI and F.J. HUMPHREYS
Nature Methods: doi: /nmeth.3553
 Supplementary Figure 1 Programming the reconstitution of fully ECM-embedded 3D microtissues by DNA-programmed assembly (DPAC). (a) Timeline for a three-component tissue synthesis by DPAC. The process proceeds
Supplementary Figure 1 Programming the reconstitution of fully ECM-embedded 3D microtissues by DNA-programmed assembly (DPAC). (a) Timeline for a three-component tissue synthesis by DPAC. The process proceeds
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12116 Supplementary Figure 1 Theoretical prediction of temperature profiles. a, sketch of sample, immersed into X-ray beam, its environment (agarose and buffer solution are not distinguished),
doi:10.1038/nature12116 Supplementary Figure 1 Theoretical prediction of temperature profiles. a, sketch of sample, immersed into X-ray beam, its environment (agarose and buffer solution are not distinguished),
Finishing Drosophila grimshawi Fosmid Clone DGA23F17. Kenneth Smith Biology 434W Professor Elgin February 20, 2009
 Finishing Drosophila grimshawi Fosmid Clone DGA23F17 Kenneth Smith Biology 434W Professor Elgin February 20, 2009 Smith 2 Abstract: My fosmid, DGA23F17, did not present too many challenges when I finished
Finishing Drosophila grimshawi Fosmid Clone DGA23F17 Kenneth Smith Biology 434W Professor Elgin February 20, 2009 Smith 2 Abstract: My fosmid, DGA23F17, did not present too many challenges when I finished
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Why Study Developmental Neurobiology? Terrific scientific challenge.
 Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Supplemental Information. Boundary Formation through a Direct. Threshold-Based Readout. of Mobile Small RNA Gradients
 Developmental Cell, Volume 43 Supplemental Information Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients Damianos S. Skopelitis, Anna H. Benkovics, Aman Y. Husbands,
Developmental Cell, Volume 43 Supplemental Information Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients Damianos S. Skopelitis, Anna H. Benkovics, Aman Y. Husbands,
Developmental Biology. Cell Fate, Potency, and Determination
 Developmental Biology Cell Fate, Potency, and Determination Definitions Fate The sum of all structures that the cell or its descendants will form at a later stage of normal development. Cell Fate & Fate
Developmental Biology Cell Fate, Potency, and Determination Definitions Fate The sum of all structures that the cell or its descendants will form at a later stage of normal development. Cell Fate & Fate
Cell Lineage and Fate Map of the Primary Somatoblast of the Polychaete Annelid Capitella teleta
 Integrative and Comparative Biology, volume 50, number 5, pp. 756 767 doi:10.1093/icb/icq120 SYMPOSIUM Cell Lineage and Fate Map of the Primary Somatoblast of the Polychaete Annelid Capitella teleta Néva
Integrative and Comparative Biology, volume 50, number 5, pp. 756 767 doi:10.1093/icb/icq120 SYMPOSIUM Cell Lineage and Fate Map of the Primary Somatoblast of the Polychaete Annelid Capitella teleta Néva
Nature Neuroscience: doi: /nn Supplementary Figure 1. Comprehensive opto-mechanical design of the dual-axis microscope.
 Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Lecture 20: Drosophila melanogaster
 Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
COPYRIGHTED MATERIAL. Tissue Preparation and Microscopy. General Concepts. Chemical Fixation CHAPTER 1
 CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
204 Part 3.3 SUMMARY INTRODUCTION
 204 Part 3.3 Chapter # METHODOLOGY FOR BUILDING OF COMPLEX WORKFLOWS WITH PROSTAK PACKAGE AND ISIMBIOS Matveeva A. *, Kozlov K., Samsonova M. Department of Computational Biology, Center for Advanced Studies,
204 Part 3.3 Chapter # METHODOLOGY FOR BUILDING OF COMPLEX WORKFLOWS WITH PROSTAK PACKAGE AND ISIMBIOS Matveeva A. *, Kozlov K., Samsonova M. Department of Computational Biology, Center for Advanced Studies,
Medicinal Leech Electrophysiology: Retzius Readings
 Introduction Medicinal Leech Electrophysiology: Retzius Readings Hannah Liechty A09522659 May 22, 2014 Phys 173 Spring 2014 Initially when this lab began, the idea was to be able to set up a system that
Introduction Medicinal Leech Electrophysiology: Retzius Readings Hannah Liechty A09522659 May 22, 2014 Phys 173 Spring 2014 Initially when this lab began, the idea was to be able to set up a system that
Supplementary Figure 1
 Supplementary Figure 1 Preaxial dominance during salamander limb development. Panel (a, b) shows RNA in situ hybridisation on whole-mount limbs with Sox9 sense probe as a control. Note the absence of reactivity
Supplementary Figure 1 Preaxial dominance during salamander limb development. Panel (a, b) shows RNA in situ hybridisation on whole-mount limbs with Sox9 sense probe as a control. Note the absence of reactivity
SUPPLEMENTARY INFORMATION. Transcriptional output transiently spikes upon mitotic exit
 SUPPLEMENTARY INFORMATION Transcriptional output transiently spikes upon mitotic exit Viola Vaňková Hausnerová 1, 2, Christian Lanctôt 1* 1 BIOCEV and Department of Cell Biology, Faculty of Science, Charles
SUPPLEMENTARY INFORMATION Transcriptional output transiently spikes upon mitotic exit Viola Vaňková Hausnerová 1, 2, Christian Lanctôt 1* 1 BIOCEV and Department of Cell Biology, Faculty of Science, Charles
Lecture 20: Drosophila embryogenesis
 Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
Mystery microscope images
 Mystery microscope images Equipment: 6X framed microscopy images 6X cards saying what each microscope image shows 6X cards with the different microscopy techniques written on them 6X cards with the different
Mystery microscope images Equipment: 6X framed microscopy images 6X cards saying what each microscope image shows 6X cards with the different microscopy techniques written on them 6X cards with the different
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization.
 Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
Characterization and Functional Analysis of a Newly Identified Human MT5-MMP Transcript Variant Isolated from Multipotent NT2 Cells
 Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2006 Characterization and Functional Analysis of a Newly Identified Human MT5-MMP Transcript Variant Isolated
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2006 Characterization and Functional Analysis of a Newly Identified Human MT5-MMP Transcript Variant Isolated
ANNOUNCEMENTS. HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134)
 ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP019695 TITLE: Fabricating Neural and Cardiomyogenic Stem Cell Structures by a Novel Rapid Prototyping--the Inkjet Printing Method
Differentiation. Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU)
 Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Article. Semaphorin and Eph Receptor Signaling Guide a Series of Cell Movements for Ventral Enclosure in C. elegans
 Current Biology 22, 1 11, January 10, 2012 ª2012 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2011.12.009 Semaphorin and Eph Receptor Signaling Guide a Series of Cell Movements for Ventral Enclosure
Current Biology 22, 1 11, January 10, 2012 ª2012 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2011.12.009 Semaphorin and Eph Receptor Signaling Guide a Series of Cell Movements for Ventral Enclosure
Secondary embryonic axis formation by transplantation of D quadrant micromeres in an oligochaete annelid
 Access Development the most First recent posted version epress online at online on http://dev.biologists.org/lookup/doi/10.1242/dev.055384 9 December publication 2010 as date 10.1242/dev.055384 9 December
Access Development the most First recent posted version epress online at online on http://dev.biologists.org/lookup/doi/10.1242/dev.055384 9 December publication 2010 as date 10.1242/dev.055384 9 December
A leech homolog of twist: evidence for its inheritance as a maternal mrna
 Gene 199 (1997) 31 37 A leech homolog of twist: evidence for its inheritance as a maternal mrna Julio G. Soto *, Brad. H. Nelson 1, David A. Weisblat Department of Molecular and Cell Biology, 385 LSA,
Gene 199 (1997) 31 37 A leech homolog of twist: evidence for its inheritance as a maternal mrna Julio G. Soto *, Brad. H. Nelson 1, David A. Weisblat Department of Molecular and Cell Biology, 385 LSA,
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
(A) Schematic depicting morphology of cholinergic DA and DB motor neurons in an L1
 SUPPLEMENTAL MATERIALS Supplemental Figure 1 Figure S1. Expression of the non-alpha nachr subunit ACR-2. (A) Schematic depicting morphology of cholinergic DA and DB motor neurons in an L1 animal. Wide-field
SUPPLEMENTAL MATERIALS Supplemental Figure 1 Figure S1. Expression of the non-alpha nachr subunit ACR-2. (A) Schematic depicting morphology of cholinergic DA and DB motor neurons in an L1 animal. Wide-field
Developmental Biology 3230 Exam 1 (Feb. 6) NAME
 DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Nature Methods: doi: /nmeth Supplementary Figure 1. Retention of RNA with LabelX.
 Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
CURRICULUM VITAE Anna Martí-Subirana EDUCATION
 CURRICULUM VITAE Anna Martí-Subirana EDUCATION Master of Arts (MA) English Literature, American Modern and Contemporary Poetry. Arizona State University, Tempe, AZ, November 2007. My thesis explored the
CURRICULUM VITAE Anna Martí-Subirana EDUCATION Master of Arts (MA) English Literature, American Modern and Contemporary Poetry. Arizona State University, Tempe, AZ, November 2007. My thesis explored the
In situ study of chorion gene amplification in ovarian follicle cells of Drosophila nasuta
 J. Biosci., Vol. 15, Number 2, June 1990, pp. 99-105. Printed in India. In situ study of chorion gene amplification in ovarian follicle cells of Drosophila nasuta P. K. TIWARI* and S. C. LAKHOTIA Cytogenetics
J. Biosci., Vol. 15, Number 2, June 1990, pp. 99-105. Printed in India. In situ study of chorion gene amplification in ovarian follicle cells of Drosophila nasuta P. K. TIWARI* and S. C. LAKHOTIA Cytogenetics
MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE
 Neurodevelopment MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE UCSD Brain Development Proliferation Differentiation Migration Axon and dendrite development Synaptogenesis Synapse Pruning Apoptosis
Neurodevelopment MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE UCSD Brain Development Proliferation Differentiation Migration Axon and dendrite development Synaptogenesis Synapse Pruning Apoptosis
THE RELATIONS BETWEEN DNA, RNA, AND PROTEIN IN NORMAL EMBRYONIC CELL NUCLEI AND SPONTANEOUS TUMOUR CELL NUCLEI
 THE RELATIONS BETWEEN DNA, RNA, AND PROTEIN IN NORMAL EMBRYONIC CELL NUCLEI AND SPONTANEOUS TUMOUR CELL NUCLEI JOHN SEED, Ph.D From the Department of Radiotherapeutics, University of Cambridge, Cambridge,
THE RELATIONS BETWEEN DNA, RNA, AND PROTEIN IN NORMAL EMBRYONIC CELL NUCLEI AND SPONTANEOUS TUMOUR CELL NUCLEI JOHN SEED, Ph.D From the Department of Radiotherapeutics, University of Cambridge, Cambridge,
SUPPLEMENTARY INFORMATION
 Normalized dorsal trunk length (µm) Normalized dorsal trunk length (TC1-5) (µm) DOI: 1.138/ncb2467 a b c d btl>src64 Src42, btl>src64 e Src64 f g h DT Src64 DT Midgut Src64 Midgut i Src64 Atpα j k l DT
Normalized dorsal trunk length (µm) Normalized dorsal trunk length (TC1-5) (µm) DOI: 1.138/ncb2467 a b c d btl>src64 Src42, btl>src64 e Src64 f g h DT Src64 DT Midgut Src64 Midgut i Src64 Atpα j k l DT
regression t value two sample t values regression line
 Suppl. Table 1: Testing SCRE sequence information without the structural constraint SCRE t test on difference of coefficients SCRE t value sequence only t value Dm1 6.62 6.84 1.29 Dm2 7.06 7.46 1.84 Dm3
Suppl. Table 1: Testing SCRE sequence information without the structural constraint SCRE t test on difference of coefficients SCRE t value sequence only t value Dm1 6.62 6.84 1.29 Dm2 7.06 7.46 1.84 Dm3
Chapter 3. DNA Replication & The Cell Cycle
 Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission.
 Conversion of Ectoderm to Mesoderm by Cytoplasmic Extrusion in Leech Embryos Author(s): Brad H. Nelson and David A. Weisblat Source: Science, New Series, Vol. 253, No. 5018 (Jul. 26, 1991), pp. 435-438
Conversion of Ectoderm to Mesoderm by Cytoplasmic Extrusion in Leech Embryos Author(s): Brad H. Nelson and David A. Weisblat Source: Science, New Series, Vol. 253, No. 5018 (Jul. 26, 1991), pp. 435-438
-RT RT RT -RT XBMPRII
 Base pairs 2000 1650 1000 850 600 500 400 Marker Primer set 1 Primer set 2 -RT RT RT -RT XBMPRII kda 100 75 50 37 25 20 XAC p-xac A 300 B Supplemental figure 1. PCR analysis of BMPRII expression and western
Base pairs 2000 1650 1000 850 600 500 400 Marker Primer set 1 Primer set 2 -RT RT RT -RT XBMPRII kda 100 75 50 37 25 20 XAC p-xac A 300 B Supplemental figure 1. PCR analysis of BMPRII expression and western
Cell commitment and cell interactions in the ectoderm of Drosophila melanogaster
 Development Supplement 2, 1991, 3946 Printed in Great Britain The Company of Biologists Limited 1991 39 Cell commitment and cell interactions in the ectoderm of Drosophila melanogaster ISABELLA STUTTEM
Development Supplement 2, 1991, 3946 Printed in Great Britain The Company of Biologists Limited 1991 39 Cell commitment and cell interactions in the ectoderm of Drosophila melanogaster ISABELLA STUTTEM
Background for Schizophrenia Research Article. Sekar A, et al., Schizophrenia risk from complex variation of complement component 4, Nature, 2016
 Background for Schizophrenia Research Article Sekar A, et al., Schizophrenia risk from complex variation of complement component 4, Nature, 2016 Topics The Major Histocompatibility Complex (MHC) region
Background for Schizophrenia Research Article Sekar A, et al., Schizophrenia risk from complex variation of complement component 4, Nature, 2016 Topics The Major Histocompatibility Complex (MHC) region
Supplementary Figure 1
 Supplementary Figure 1 (A) Schematic of sequential hybridization and barcoding. (B) Schematic of the FISH images of the cell. In each round of hybridization, the same spots are detected, but the dye associated
Supplementary Figure 1 (A) Schematic of sequential hybridization and barcoding. (B) Schematic of the FISH images of the cell. In each round of hybridization, the same spots are detected, but the dye associated
(From the Division of Biological and Medical Research, Argonne National Laboratory, Lemont, Illinois) Methods and Materials
 OBSERVATIONS ON FIBRILLOGENESIS IN THE CONNECTIVE TISSUE OF THE CHICK EMBRYO WITH THE AID OF SILVER IMPREGNATION* BY F. WASSERMANN, M.D., AND L. KUBOTA (From the Division of Biological and Medical Research,
OBSERVATIONS ON FIBRILLOGENESIS IN THE CONNECTIVE TISSUE OF THE CHICK EMBRYO WITH THE AID OF SILVER IMPREGNATION* BY F. WASSERMANN, M.D., AND L. KUBOTA (From the Division of Biological and Medical Research,
SUPPLEMENTARY INFORMATION
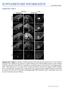 doi:10.1038/nature11094 Supplementary Figures Supplementary Figure 1: Analysis of GFP expression in P0 wild type and mutant ( E1-4) Fezf2-Gfp BAC transgenic mice. Epifluorescent images of sagittal forebrain
doi:10.1038/nature11094 Supplementary Figures Supplementary Figure 1: Analysis of GFP expression in P0 wild type and mutant ( E1-4) Fezf2-Gfp BAC transgenic mice. Epifluorescent images of sagittal forebrain
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
