Loss of basement membrane, receptor and cytoskeletal lattices in a laminin-deficient muscular dystrophy
|
|
|
- Anne Franklin
- 6 years ago
- Views:
Transcription
1 Research Article 735 Loss of basement membrane, receptor and cytoskeletal lattices in a laminin-deficient muscular dystrophy Peter D. Yurchenco 1, *, Yi-Shan Cheng 1, Kevin Campbell 2 and Shaohua Li 1 1 Department of Pathology & Laboratory Medicine, UMDNJ Robert Wood Johnson Medical School, Piscataway, NJ 08854, USA 2 Howard Hughes Medical Institute, Department of Physiology & Biophysics, University of Iowa College of Medicine, Iowa City, IA 52242, USA *Author for correspondence ( yurchenc@umdnj.edu) Accepted 2 October 2003 Journal of Cell Science 117, Published by The Company of Biologists 2004 doi: /jcs Summary Basement membrane laminins bearing the α2-subunit interact with α-dystroglycan and β1-integrins, cell-surface receptors that are found within the rectilinear costameric lattices of skeletal muscle sarcolemma. Mutations of the α2 subunit are a major cause of congenital muscular dystrophy. To determine whether the costameres are altered as a result of laminin α2-mutations, the skeletal muscle surface of a dystrophic mouse (dy 2J /dy 2J ) lacking the α2-ln domain was examined by confocal and widefield deconvolution immunomicroscopy. Although the dy 2J dystrophic fibers possessed a normal-appearing distribution of α2-laminins and α-dystroglycan within a rectilinear costameric lattice at 6.5 weeks of age, by 11 weeks the surface architecture of these components were found to be disorganized, with frequent effacement of the circumferential and longitudinal lattice striations. The defect in the lattice organization was also noted to be a characteristic of type IV collagen, nidogen, perlecan, β1 D - integrin, dystrophin and vinculin. The development of this pattern change occurring only after birth suggests that although α2-laminins are not essential for the initial assembly of the costameric framework, they play a role in maintaining the stability and organization of the framework. Key words: Dystroglycan, Costamere, Muscular dystrophy, Basement membrane Introduction The sarcolemmal basement membrane is a plasma membraneassociated extracellular matrix that receives the lateral transmission of contractile force generated by the myofibrils and that participates in the linkage between adjacent and aligned myotubes (reviewed by Bloch et al., 2002; Sanes, 2003). Laminin-2 (α2β1γ1) and laminin-4 (α2β2γ1) are major components of these basement membranes, forming surfaceassociated polymers through short-arm LN domain interactions and adhering to the cell surface through their G-domains (reviewed by Colognato and Yurchenco, 2000). Receptor interactions of laminin G-domain in muscle include those of dystroglycan and the α7β1 integrin. α-dystroglycan is bound to its transmembrane moiety β-dystroglycan, and β- dystroglycan binds to dystrophin and other members of the dystrophin-glycoprotein complex (Durbeej et al., 1998; Michele and Campbell, 2003). The laminin-interacting integrin of muscle, α7β1, is also thought to bridge laminin to the underlying cytoskeleton and may provide protective compensation in muscular dystrophies (Burkin et al., 2001; Hodges et al., 1997). An important class of human congenital muscular dystrophy has been found to be caused by point mutations, deletions or complete absence of the laminin α2 subunit (reviewed by Miyagoe-Suzuki et al., 2000). Several mouse strains containing α2 mutations serve as animal models for the disease (Kuang et al., 1998; Miyagoe et al., 1997; Sunada et al., 1995). Among these, the murine dy 2J dystrophy has been shown to result from an in-frame deletion within the N-terminal globular domain of the laminin α2 subunit (Sunada et al., 1995; Xu et al., 1994) that destabilizes the domain and renders it unable to polymerize (Colognato and Yurchenco, 1999; Guo et al., 2003). The dystrophy is characterized by degeneration and regeneration of muscle fibers. Modestly decreased levels of laminin α2 have been detected by immunofluorescence in the sarcolemmal surfaces of these mice, with irregular attenuations of the basement membrane seen at the ultrastructural level (Colognato and Yurchenco, 2000; Sunada et al., 1995; Xu et al., 1994). Similarly, variable loss of the α2 subunit, nearnormal levels of type IV collagen, and compensation by other laminin α-subunits have been appreciated in other laminin-α2 deficient states (Kuang et al., 1998; Patton et al., 1999; Sunada et al., 1994). Mature skeletal muscle fibers possess a rectilinear framework called the costamere, which is thought to be responsible for linking the tightly packed and aligned contractile myofibers to the sarcolemma and basement membrane (Bloch et al., 2002). The costameric framework consists of major repeating circumferential bands of receptors and cytoskeletal components that are aligned with the myofibril Z-line (Z-elements), minor repeating circumferential lines interspersed between the Z-elements (M-elements), and parallel longitudinal striations (L-elements). Vinculin, spectrin, β1-integrin, dystroglycan and laminins have all been found to
2 736 Journal of Cell Science 117 (5) be concentrated at, or largely confined to, the elements of the costameric array, suggesting that these components are all connected to each other and linked to the myofibers Z- and M- discs by desmin and other proteins. The array has been found to be a dynamic structure whose major axis of alignment is sensitive to denervation and muscle agrin (Bezakova and Lomo, 2001). Recently, it was found that α1- and α2-laminins were found to organize dystroglycan, integrin α7β1, vinculin and dystrophin into irregular polygonal arrays in cultured myotubes produced by the fusion of mouse satellite cellderived C2C12 myoblasts, possibly constituting precursor structures to the rectilinear costameres seen in adult muscle (Colognato et al., 1999). The changes produced by the addition of exogenous laminins to the culture medium were found to require both its polymerization and G-domain-mediated cellsurface binding. Because the LN domain of laminin that is involved in polymerization is defective in the dy 2J mouse, because laminin extracted from dy 2J is unable to induce the polygonal array in myotubes (Colognato et al., 1999; Colognato and Yurchenco, 1999), and because the same components organized by laminin into the C2C12 polygonal array are also found in costameric arrays, it seemed that a defect in the costameric array might be found in the skeletal muscle of the dy 2J mouse. We therefore examined the surface distribution of basement membrane, cell surface and several cytoskeletal components in normal and dy 2J mouse muscle. We report that basement membrane, receptor and cortical cytoskeletal lattice patterns appear initially normal in dy 2J muscle but become distorted and effaced by 11 weeks of age. Our findings suggest that although α2-laminins contribute to the stability of the array, they are not uniquely essential for its formation. Materials and Methods Antibody and protein reagents Rabbit polyclonal antibodies specific for type IV collagen, the core protein of perlecan, the G domain of the laminin α2 chain, and placental laminins-2/-4 were prepared and characterized as described previously (Cheng et al., 1997; Handler et al., 1997; Rambukkana et al., 1998; Yurchenco and Ruben, 1987). Polyclonal antibody for nidogen-1 (entactin-1) was generated by immunizing a New Zealand white rabbit with protein purified from the EHS (Engelbreth-Holm- Swarm) laminin-nidogen complex. Purification was accomplished by dissociating nidogen from laminin in 2 M guanidine-hcl in 50 mm Tris-HCl, ph 7.4, 2 mm EDTA on ice and separating the species by two rounds of gel filtration with Sephacryl S-500 (90 cm 2.5 cm column) in the same buffer. The antiserum was affinity purified with nidogen immobilized on Sepharose 4B beads and then cross-absorbed on nidogen-free laminin immobilized on Sepharose beads. Mouse monoclonal IgM antibody IIH6 hybridoma medium specific for α- dystroglycan (Ervasti and Campbell, 1991) was used as conditioned hybridoma medium or purified from medium. Rabbit polyclonal antibody specific for dystrophin was prepared against a dystrophin fusion protein as described previously (Ervasti et al., 1990). Mouse monoclonal IgG antibody 2B1 (van der Flier et al., 1997) specific for the integrin β1 D chain was kindly provided by Arnoud Sonnenberg (Netherlands Cancer Institute). Mouse monoclonal anti-rabbit α- actinin and mouse monoclonal anti-chicken vinculin antibodies were purchased from Sigma (St Louis, MO). FITC-, Cy3- and Cy5- conjugated antibodies specific for mouse IgG, mouse IgM and rabbit IgG, respectively, were purchased from Jackson Immunochemicals (Bar Harbor, ME). Preparation and immunostaining of skeletal muscle Breeding pairs of heterozygous C57BL/6J-Lama 2 dy-2j mice and homozygous C57BL/10ScSn mdx/mdx mice (4-6 weeks of age) were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in the medical school vivarium and treated under an animal protocol approved by the Institutional Animal Care and Use Committee. Homozygous dy 2J /dy 2J offspring and their normal littermates were distinguished from each other by the presence or absence of a distinct gait abnormality that primarily affects the hind limbs. Following anesthetization of mice injected intraperitoneally with 0.05 ml of a 6.5% solution of sodium pentobarbital, the chest cavity was exposed, the left ventricle of the heart cannulated with a 25 gauge butterfly syringe needle, the right atrium incised and the animal perfused for several minutes with phosphate buffered saline (10 mm sodium phosphate, 127 mm NaCl, ph 7.4) followed by a % solution of paraformaldehyde in phosphate buffered saline for 20 minutes. Hind limb muscles were exposed by dissection and prepared for microscopy following teasing, or, for a limited number of samples, cryostat sectioning. Excised portions of muscle were slowly teased with fine forceps into individual fibers and fiber groups and adhered onto glass slides. For cryostat sectioning, a portion of muscle was rapidly plunge-frozen in a propane slush cooled to liquid nitrogen temperatures using a pneumatic-driven plunger. Sections µm thick were cut in a Leica CM1850 cryostat at 18 C. Slides were postfixed in 1.5% paraformaldehyde in phosphate buffered saline for minutes. Following fixation, slides were either used detergent-free or treated with 0.5% Triton-X-100 in phosphate buffered saline for 30 minutes on ice. They were then blocked with 0.5% bovine serum albumin in phosphate buffered saline at 5 C, incubated with primary antibodies (anti-laminin-α2g or anti-laminin-2/-4, 20 µg/ml; antitype IV collagen, perlecan or nidogen, 10 µg/ml, anti-dystroglycan IIH6 conditioned medium, undiluted or 1:1 dilution; anti-integrin β1 D conditioned medium without dilution; anti-vinculin and antidystrophin, 1:20 dilution; anti-α-actinin, 1:400 dilution) diluted in blocking buffer. After washing, the slides were incubated with secondary antibody conjugated to FITC, Cy3 or Cy5 according to the manufacturer s instructions for 1.5 hours at room temperature. Nonimmune mouse IgM, mouse IgG and rabbit IgG were used as controls. The slides were again washed over a 2 hour period. Nuclei were stained with a 10 µg/ml solution of propidium iodide (viewed with a Chroma narrow X HQ TRITC filter set) or stained with 2 µg/ml DAPI. The latter was found to provide better tissue penetration. Coverslips were mounted with 6% 1,4 diazabicyclo(2,2,2)octane (Sigma) in a 9:1 mixture of glycerol:100 mm Tris-HCl, ph 8.6 to inhibit photobleaching. Light microscopy and analysis of digital images Digital confocal images were obtained with either a Zeiss LSM 410 Invert laser scanning microscope fitted with a 63 oil immersion objective (NA=1.4) and capable of discriminating among FITC, TRITC/Cy3 and Cy5 fluorescence, or an Olympus IX81 inverted microscope fitted with a CARV confocal imaging device, PCO SensiCam CCD camera, 60 oil immersion objective (NA=1.4) and DAPI, FITC, TRITC/Cy3 and Cy5 filters and controlled by IPLab (Scanalytics, Fairfax, VA). Slides were also viewed by widefield indirect immunofluorescence using an Olympus IX70 inverted microscope fitted with IX-FLA fluorescence observation attachment, 60 oil immersion objective (NA=1.4), and a MicroMax 5 mhz CCD camera (Princeton Instruments, NJ) controlled by IP Lab. For these images, out-of-focus haze was reduced by digital deconvolution of sets of 16, 32 or 64 serial optical sections recorded at 0.25 µm intervals using an expectation maximization algorithm and a calculated point spread function (Conchello and McNally, 1996; McNally et al., 1999) in the program XCOSM (Institute for Biomedical Computing, Washington University, St Louis, MO). Confocal images were processed in Adobe Photoshop (San Jose, CA)
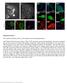
3 Costameres in a laminin-deficient dystrophy 737 Fig. 1. Laminin (Lm) and α- dystroglycan (DG) in normal skeletal muscle. (a-d) Confocal immunofluorescence images of the surface of normal teased muscle fibers immunostained with laminin α2-g domain (a, FITC secondary, green) and α-dystroglycan (b, IIH6, Cy3, red) antibodies (a-c, fixed and detergent treated). Laminin and dystroglycan striated patterns colocalize (arrows in a), with less intense laminin fluorescence between striations. (d) Similar fiber image with laminindystroglycan costained fiber treated with detergent following laminin immunostaining. (e-h) Peripheral nuclei (e, laminin; f, laminin (green) and α-actinin (red); g, oblique optical section showing nucleus protruding beyond plane of sarcolemma (dystroglycan, red; α-actinin, green); h, laminin, green; propidium iodide, red). Peripheral nuclei are sandwiched between α-actinin of the sarcomere (which coincides with the major costameric circumferential striations) and plasma membrane dystroglycan. (i-j) Periodicity of orthogonally oriented longitudinal optical sections (i, confocal image, laminin-2/4 above and α- dystroglycan below; j, deconvoluted widefield image, laminin α2g above and dystroglycan below) of normal fibers. Arrows indicate positions of Z-discs. (k-o) Widefield images reveal repeating pattern of laminin α2g epitope on internal (k,l) and external (m,n) basement membrane surfaces. (k) An oblique view of an internal basement membrane lying between adjacent fibers contained within a computed three-dimensional pixel (voxel) array prepared from 0.25 µm serial deconvoluted optical sections. (l) Location of internal basement membranes within optical cross-section through three adjacent fibers (arrows). (m-o) Images of fixed teased fiber prepared without detergent and stained with laminin α2g-specific antibody following (m) and before (n) deconvolution, and incubated only with secondary antibody (o). Costameric elements (Z, M, L striations) and location of peripheral nucleus (n) indicated in m. and deconvoluted images in IP Labs and, when required, analyzed in three dimensions using Voxblast 3.0 (VayTek, Fairfield, IO). Results Sarcolemmal architecture of normal skeletal muscle The distribution of laminin was examined on teased myotube surfaces with a polyclonal antibody raised against laminin-2/4 and an antibody prepared against the laminin α2 G domain by confocal and widefield deconvolution microscopy (Fig. 1). By either method, and with either reagent, laminin-specific immunofluorescence was present well above background levels over almost the entire surface but was brightest in a regular rectilinear pattern that consisted of alternating broad and narrow circumferential striations intersected by parallel longitudinal lines. The major circumferential striations had a periodicity of 2.2±0.2 µm (mean ± standard deviation, n=26), and the overall pattern was similar to that of the costamere. The prominence of the striations varied somewhat from fiber to fiber, with the intercostameric regions having intensities typically less than a one quarter below that of the brightest regions as measured in IPLab. When laminin immunofluorescence was examined as confocal or deconvoluted longitudinal oriented orthogonal optical sections, the periodic difference in intensity was also noted, but it was less than that for images prepared en face. The laminin pattern array was observed in both detergent-permeabilized and nonpermeabilized fibers, and in both teased fibers and frozen sections of muscle, indicating that the structure was independent of these manipulations. Strikingly, the rectilinear laminin pattern colocalized with a similar but generally more contrasted array of α-dystroglycan in both its major circumferential and longitudinal striations. Many laminin and dystroglycan costained or independently stained images also revealed the existence of finer (minor) circumferential lines lying between the major striations in the laminin-stained images, corresponding to more prominent inter-z striations in the dystroglycan-stained images. The Z-line of the laminin and dystroglycan array was established with antibody to α-actinin. Peripheral nuclei, scattered on the myotube surface, were identified with propidium iodide and DAPI (see below), often projected beyond the sarcolemmal plane. Oblique views and serial sections revealed that the laminin and dystroglycan extended over the outer surface of the nuclei while α-actinin, marking the sarcomere, lay beneath. The laminin and dystroglycan costameric patterns in teased fibers were examined in quadraceps femoris and soleus at 6.5 weeks, and
4 738 Journal of Cell Science 117 (5) Fig. 2. Defects in the surface organization of laminin and dystroglycan in dy 2J muscle. Confocal images of teased dy 2J dystrophic muscle fibers (b-e: b, merged image; c, laminin-α2-g; d, α-dystroglycan). A wild-type control fibril (merged image) is shown in a. (e) A deconvoluted widefield cross-sectional view of dystrophic basement membrane zone (dystroglycan, red; laminin, green; merge, yellow). Note extensive (but not complete) effacement of rectilinear distributions for surface-distributed basement membrane, receptor and cytoskeletal components. Bars, 2 µm in e. quadraceps femoris, adductor magnus, gracilis, soleus and tibialis anterior muscle at 14.5 weeks. The laminin and dystroglycan patterns were found to be continuous or nearcontinuous in all of these muscles, with about the same degree of variability within a muscle as between them. As shown ahead, basement membrane type IV collagen, nidogen and perlecan were also condensed into a rectilinear pattern. It was concluded that laminin-2 and laminin-4 and other major basement membrane components form a continuous sarcolemma coating but are condensed within an rectilinear array that coincides with that of dystroglycan. Sarcolemmal surface architecture of laminin-deficient dystrophic skeletal muscle Skeletal muscle obtained from dy 2J mice aged weeks was examined. The distribution of laminin-α2 and dystroglycan was found to be substantially altered in most fibers (Figs 2-5). Although a faint residual costameric pattern could often still be Fig. 3. Defects in the surface organization of basement membrane, receptor and cytoskeletal components in dy 2J muscle. Confocal images of wild-type (wt) and dystrophic (dy2j) muscle fibers. Muscle fibers immunostained to detect laminin-α2 (Lm) and corresponding α-actinin (αa), dystrophin (Dys) and corresponding dystroglycan (DG), integrin β1d, vinculin (Vn), nidogen (Nd), type IV collagen (Col4) and perlecan (Perl). Nonimmune (wild-type) controls for integrin (C1), vinculin (C2), dystrophin/basement membrane components (C3) and dystroglycan (C4) included. Note the extensive but incomplete effacement of rectilinear distributions for surfacedistributed basement membrane, receptor and cytoskeletal components with preservation of the sarcomeric α-actinin cross-band architecture. Arrows in top row panel indicate locations of peripheral nuclei which overlie the sarcomeres. Bar, 2 µm. appreciated, the laminin and dystroglycan components were now seen to splay beyond the confines of the lattice, obscuring
5 Costameres in a laminin-deficient dystrophy 739 Fig. 5. Age-dependent transitions in dy 2J surface architecture. The orthogonal pattern in 6.5-week and 11-week-old dy 2J /dy 2J mice and their normal littermates was scored. Number of determinations (n, image fields) indicated in panels. The class 2 and 3 changes are characteristics of dystrophic muscle, developing after 6.5 weeks. Fig. 4. Grading of normal and dy 2J surface architectures. Three classes of surface architecture were defined from the dystroglycan immunofluorescence patterns. Class 1, seen in normal muscle, corresponds to a regular rectilinear pattern. Class 2, largely present in dystrophic muscle, consists of a less regular lattice with splaying and with frequent loss of the finer circumferential and longitudinal lattice struts. Class 3 is a dystrophic pattern characterized by substantial loss of the rectilinear pattern with extensive inter-array clearing (shown) or near-complete pattern effacement. Corresponding paired images with 1.5 magnified insets for dystroglycan shown. the normal grid-like pattern and creating a fairly diffuse and sometimes disorganized distribution of epitope (Fig. 2), i.e. the defective fibers possessed varying degrees of effacement of the lattice array pattern both for dystroglycan and laminin. Type IV collagen, nidogen and perlecan were found to possess a surface pattern in normal mouse muscle similar to that for laminin (Fig. 3). This pattern colocalized with that of β1 D integrin receptor, and cytoskeletal dystrophin and vinculin. This pattern was noted to be effaced in dy 2J muscle for all of these components. By contrast, the pattern of sarcomeric α-actinin was found to remain unchanged with preservation of the evenly spaced parallel Z-bands. Temporal progression of surface architecture in dy 2J dystrophic muscle Muscles obtained from younger normal and dy 2J mice (3 weeks, not shown, and 6.5 weeks) were evaluated. The dystroglycan and laminin costameric patterns could not be distinguished from muscle obtained from normal littermates. To quantitate the degree of surface alteration at two ages, muscle fibers were then collected from dystrophic mice aged 6.5 weeks and 11 weeks, and from their normal littermates, and evaluated by confocal microscopy. Gradations of structural abnormality could be appreciated and these were divided into three groups (Fig. 4). Class 1 fibers were defined as fibers with regular lattice patterns, sometimes with a relatively small degree of ligand staining detected beyond the fine rectilinear array. Class 2 fibers were defined as fibers with surfaces characterized by splaying of the pattern accompanied by rounding of the intersections, filament irregularity and/or a piecemeal loss of interlattice segments (particularly the M-line and longitudinal filaments). Class 3 fibers were defined as fibers characterized by extensive loss of the lattice pattern. Some of these surfaces showed preservation of central interlattice clearing, whereas others showed near-complete effacement. As shown (Fig. 5), there was little or no difference seen between normal and dystrophic mice at 6.5 weeks. The normal pattern consisted of ~90% regular orthogonal array. However, profound differences in fiber architecture became evident by 11 weeks of age, i.e. 71% of fiber fields had class 2-3 effacement patterns. The fraction of central nuclei, an indicator of regenerating and regenerated fibers, was estimated from counts of hematoxylinstained nuclei and eosin counterstained cross-sections of
6 740 Journal of Cell Science 117 (5) Fig. 6. Loss of costameric lattice in dy 2J fibers with central and peripheral nuclei. Confocal images of teased muscle fibers from an 11-week-old dy 2J dystrophic mouse stained with DAPI (blue) to detect nuclei and with IIH6 antibody (red) to detect α-dystroglycan. Cross-sectional views (a,b; d,e) reconstructed from serial optical 0.5 µm sections taken approximately at positions indicated by yellow lines in en face views of upper surface (c,f). Red vertical lines indicate lateral borders of fiber in c. Note dystrophic pattern seen in myofiber containing central nuclei (a-c) and peripheral nuclei (d-f). contralateral hindlimb muscle. They were found to be 2% of normal 6.5-week-old muscle (74/3173 nuclei), 6% of dy 2J /dy 2J 6.5-week muscle (250/4254), 1% of normal 11-week-old muscle (108/10767) and 21% of dy 2J /dy 2J 11-week muscle (1957/9542). Increased variation in fiber diameter was noted at 11 weeks. The basement membrane laminin and dystroglycan patterns appeared essentially normal at 6.5 weeks and markedly abnormal at 11 weeks, with the fraction of affected fibers exceeding the fraction containing central nuclei. Both thin and thick 11-week-old fibers were noted to have altered surface architectures. To determine whether the costameric alterations developed in regenerated muscle fibers that contain central nuclei, and primary fibers that contain peripheral nuclei, 11-week-old dystrophic fibers and fiber groups were stained with DAPI to detect nuclei and immunostained with dystroglycan-specific and laminin-2/4- specific antibodies to determine the costameric pattern (Fig. 6). Serial optical sections were used to generate en face views, and orthogonal z-sections were computationally obtained from these. Fibers containing both peripheral and central nuclei were identified. The surface of both fiber types showed effacement of the normal costameric pattern. Collectively, these data suggest that the fibers that present initially at birth, as well as regenerating myofibers, had been affected. Sarcolemmal surface architecture of dystrophin-deficient skeletal muscle The pattern of laminin and α-dystroglycan on fibers teased from 11-week-old homozygous mdx mice was compared with age-matched controls (Fig. 7). The dystrophic fibers exhibited an altered costameric dystroglycan pattern in which the minor Fig. 7. Surface distribution of laminin-2/4 and α-dystroglycan in mdx dystrophic muscle. Confocal images of teased muscle isolated from an 11-week-old mdx/mdx mouse. The pattern of laminin-2/4 (Lm2) revealed prominent Z bands with variable reductions of M- and longitudinal striations, and more prominent selective loss of the α-dystroglycan (DG) inter-z minor circumferential M bands. The furrows are sites of capillaries removed from the sarcolemma. circumferential bands (corresponding to the M-line) and longitudinal striations were reduced in intensity, absent, or irregular, similar to those reported for spectrin in older skeletal muscle of the mdx mouse (Williams and Bloch, 1999). The circumferential M-line and longitudinal striations of the laminin pattern were reduced compared with normal in areas overlying abnormal dystrophic striations. Discussion In the present study we found that basement membrane α2- laminins, type IV collagen, nidogen and perlecan are organized as continuous sarcolemmal sheets containing rectilinear condensations that coincide with the costameric lattice of α- dystroglycan, β1 integrin, dystrophin and vinculin. This lattice becomes disorganized in the dy 2J mouse between 6.5 and 11 weeks of age such that the circumferential and longitudinal condensations are disrupted and/or extend beyond their normal linear borders, obscuring the normal architecture. The pattern is different from the M-line and longitudinal striation defects seen in age-matched mdx mouse muscle (Fig. 8). Previously, we found that laminin-1 and laminin-2 can
7 Costameres in a laminin-deficient dystrophy 741 Fig. 8. Model of normal and dystrophic costameric patterns. Sarcolemmal basement membrane (bm), plasma membrane (pm), cortical cytoskeleton (cc) and underlying sarcomeres (sm) are shown. Normal (wild-type, wt) basement membrane, although continuous, is condensed into circumferential Z (major) and M (minor) bands and longitudinal (L) striations that correspond to the same pattern elements seen with β1-integrin and dystroglycan receptors and cytoskeletal vinculin and dystrophin. The basement membrane, receptor and cytoskeletal dy 2J lattice-like patterns become generally effaced while the receptor (dystroglycan, this study) and cytoskeletal (spectrin) (Williams and Bloch, 1999) M and L elements of mdx muscle are lost, with less-pronounced changes in the laminin pattern. organize dystroglycan, integrin, dystrophin and vinculin into an irregular polygonal array in cultured myotubes (Colognato et al., 1999), the same components found in the rectilinear costameric framework. Although it is not known whether this irregular polygonal array is a precursor to the costamere, the organization of shared components into distinct surface architectures argues for a relationship between the two entities. One possibility is that the irregular polygonal architecture of cultured myotubes is not organized in a rectilinear array simply because the sarcomeres are too sparse and too well separated from the plasma membrane to allow components such as desmin to effectively form a bridge between the sarcomeric Z- disc and sarcolemma, as is thought to be the case in mature muscle (Bloch et al., 2002). It has been proposed that the costameric arrays are the sites of force transmission and that these forces stabilize membrane and cytoskeletal costameric components (Danowski et al., 1992; Sharp et al., 1997). Because a basement membrane forms a coat around each myotube with fusion occurring between adjacent fibers, mechanical forces transmitted between myotubes during contraction must be transmitted across the adjacent basement membranes through the costameric portion. Defects arising in the array appear to be deleterious for muscle function; one such change has been evident in the mdx mouse, a model for muscular dystrophies with defects in dystrophin in which partial compensation is provided by utrophin (Grady et al., 1997). In the mdx mouse, the array defect is characterized by a loss of spectrin and other components over M lines and in the longitudinal strands of the lattice array (Williams and Bloch, 1999), and we found a similar pattern for dystroglycan in these mice as early as 11 weeks of age. In the dy 2J mouse, the α2-laminin subunit is deleted within the LN domain (Sunada et al., 1995). Although defective in self-assembly, the α2-laminins nonetheless remain within basement membranes, probably tethered through nidogen, agrin, integrin, dystroglycan and other cell-surface binding molecules (Colognato and Yurchenco, 1999). The defective laminins, unlike native laminin-1 or laminin-2, were unable to induce a polygonal array of receptors and cytoskeleton in cultured myotubes, which suggests that laminins play an important role in their surface organization (Colognato et al., 1999). 11-week-old dy 2J dystrophic muscle fibers exhibited an effaced pattern of laminin, receptors and cytoskeletal elements reminiscent of the nonpolymerization state seen on cultured myotubes after treatment with dy 2J laminin; however, the normal rectilinear array organization seen in younger mice showed that α2-laminin polymerization cannot be uniquely essential for costameric assembly. Instead, the data argued that α2-laminin polymerization is important for the maintaining the stability of these structures. However, the broader question of whether a laminin is required for costameric assembly remains unresolved. Although the α2 subunit is the major muscle laminin subunit present after birth (Kuang et al., 1999), developing and regenerating skeletal muscle express other laminin α-subunits that associate with the β and γ subunits (Patton et al., 1999). In particular, it has been reported in the severe laminin α2- deficient dy/dy mouse that both the laminin α4 and α5 chains are expressed at increased levels during development and regeneration, with increase of the former seen under a variety of pathological conditions (Patton et al., 1999; Ringelmann et al., 1999). Thus, laminin subunit redundancy and compensation exists and may insure a role of laminin in costameric assembly in either the absence of α2 expression, or the expression of defective α2 subunit. A hypothesis to be considered is that in the presence of partial laminin compensation, a lattice structural defect develops early in the dystrophic muscle, which affects stability, but which is morphologically latent. Progression to the morphological defect might require an initiating event that overstresses the weak lattice such as contraction against load, as has been proposed for non-laminin dystrophies (Campbell, 1995). This work was supported by a grant (NS38469) from the National Institutes of Health (to P.D.Y.). K.P.C. is an Investigator of the Howard Hughes Medical Institute. We thank Andrea Biermann at the Robert W. Johnson Medical School for her technical assistance. References Bezakova, G. and Lomo, T. (2001). Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers. J. Cell Biol. 153, Bloch, R. J., Capetanaki, Y., O Neill, A., Reed, P., Williams, M. W., Resneck, W. G., Porter, N. C. and Ursitti, J. A. (2002). Costameres: repeating structures at the sarcolemma of skeletal muscle. Clin. Orthop. S Burkin, D. J., Wallace, G. Q., Nicol, K. J., Kaufman, D. J. and Kaufman, S. J. (2001). Enhanced expression of the alpha7beta1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J. Cell Biol. 152, Campbell, K. P. (1995). Three muscular dystrophies: loss of cytoskeletonextracellular matrix linkage. Cell 80,
8 742 Journal of Cell Science 117 (5) Cheng, Y. S., Champliaud, M. F., Burgeson, R. E., Marinkovich, M. P. and Yurchenco, P. D. (1997). Self-assembly of laminin isoforms. J. Biol. Chem. 272, Colognato, H. and Yurchenco, P. D. (1999). The laminin alpha2 expressed by dystrophic dy(2j) mice is defective in its ability to form polymers. Curr. Biol. 9, Colognato, H. and Yurchenco, P. D. (2000). Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, Colognato, H., Winkelmann, D. A. and Yurchenco, P. D. (1999). Laminin polymerization induces a receptor-cytoskeleton network. J. Cell Biol. 145, Conchello, J. and McNally, J. (1996). Fast regularization technique for expectation maximization algorithm for computational optical sectioning microscopy. In Three-Dimensional Microscopy: Image Aquisition and Processing III, Vol (ed. C. J. Cogswell, G. S. Kino and T. Wilson), pp Proc. SPIE-The International Society for Optical Engineering. Danowski, B. A., Imanaka-Yoshida, K., Sanger, J. M. and Sanger, J. W. (1992). Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J. Cell Biol. 118, Durbeej, M., Henry, M. D. and Campbell, K. P. (1998). Dystroglycan in development and disease. Curr. Opin. Cell Biol. 10, Ervasti, J. M. and Campbell, K. P. (1991). Membrane organization of the dystrophin-glycoprotein complex. Cell 66, Ervasti, J. M., Ohlendieck, K., Kahl, S. D., Gaver, M. G. and Campbell, K. P. (1990). Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345, Grady, R. M., Teng, H., Nichol, M. C., Cunningham, J. C., Wilkinson, R. S. and Sanes, J. R. (1997). Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90, Guo, L. T., Zhang, X. U., Kuang, W., Xu, H., Liu, L. A., Vilquin, J. T., Miyagoe-Suzuki, Y., Takeda, S., Ruegg, M. A., Wewer, U. M. et al. (2003). Laminin alpha2 deficiency and muscular dystrophy; genotypephenotype correlation in mutant mice. Neuromuscul. Disord. 13, Handler, M., Yurchenco, P. D. and Iozzo, R. V. (1997). Developmental expression of perlecan during murine embryogenesis. Dev. Dyn. 210, Hodges, B. L., Hayashi, Y. K., Nonaka, I., Wang, W., Arahata, K. and Kaufman, S. J. (1997). Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J. Cell Sci. 110, Kuang, W., Xu, H., Vachon, P. H., Liu, L., Loechel, F., Wewer, U. M. and Engvall, E. (1998). Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J. Clin. Invest. 102, Kuang, W., Xu, H., Vilquin, J. T. and Engvall, E. (1999). Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2- deficiency. Lab. Invest. 79, McNally, J. G., Karpova, T., Cooper, J. and Conchello, J. A. (1999). Threedimensional imaging by deconvolution microscopy. Methods 19, Michele, D. E. and Campbell, K. P. (2003). Dystrophin-glycoprotein complex: Post-translational processing and dystroglycan function. J. Biol. Chem. 278, Miyagoe, Y., Hanaoka, K., Nonaka, I., Hayasaka, M., Nabeshima, Y., Arahata, K., Nabeshima, Y. and Takeda, S. (1997). Laminin alpha2 chainnull mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 415, Miyagoe-Suzuki, Y., Nakagawa, M. and Takeda, S. (2000). Merosin and congenital muscular dystrophy. Microsc. Res. Tech. 48, Patton, B. L., Connoll, A. M., Martin, P. T., Cunningham, J. M., Mehta, S., Pestronk, A., Miner, J. H. and Sanes, J. R. (1999). Distribution of ten laminin chains in dystrophic and regenerating muscles. Neuromuscul. Disord. 9, Rambukkana, A., Yamada, H., Zanazzi, G., Mathus, T., Salzer, J. L., Yurchenco, P. D., Campbell, K. P. and Fischetti, V. A. (1998). Role of alpha-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science 282, Ringelmann, B., Roder, C., Hallmann, R., Maley, M., Davies, M., Grounds, M. and Sorokin, L. (1999). Expression of laminin alpha1, alpha2, alpha4, and alpha5 chains, fibronectin, and tenascin-c in skeletal muscle of dystrophic 129ReJ dy/dy mice. Exp. Cell Res. 246, Sanes, J. R. (2003). The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 278, Sharp, W. W., Simpson, D. G., Borg, T. K., Samarel, A. M. and Terracio, L. (1997). Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am. J. Physiol. 273, H Sunada, Y., Bernier, S. M., Kozak, C. A., Yamada, Y. and Campbell, K. P. (1994). Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J. Biol. Chem. 269, Sunada, Y., Bernier, S. M., Utani, A., Yamada, Y. and Campbell, K. P. (1995). Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2j mice. Hum. Mol. Genet. 4, van der Flier, A., Gaspar, A. C., Thorsteinsdottir, S., Baudoin, C., Groeneveld, E., Mummery, C. L. and Sonnenberg, A. (1997). Spatial and temporal expression of the beta1d integrin during mouse development. Dev. Dyn. 210, Williams, M. W. and Bloch, R. J. (1999). Extensive but coordinated reorganization of the membrane skeleton in myofibers of dystrophic (mdx) mice. J. Cell Biol. 144, Xu, H., Wu, X. R., Wewer, U. M. and Engvall, E. (1994). Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat. Genet. 8, Yurchenco, P. D. and Ruben, G. C. (1987). Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J. Cell Biol. 105,
Chimeric protein repair of laminin polymerization ameliorates muscular dystrophy phenotype
 Chimeric protein repair of laminin polymerization ameliorates muscular dystrophy phenotype Karen K. McKee,, Markus A. Rüegg, Peter D. Yurchenco J Clin Invest. 2017;127(3):1075-1089. https://doi.org/10.1172/jci90854.
Chimeric protein repair of laminin polymerization ameliorates muscular dystrophy phenotype Karen K. McKee,, Markus A. Rüegg, Peter D. Yurchenco J Clin Invest. 2017;127(3):1075-1089. https://doi.org/10.1172/jci90854.
64 CuCl 2 in 50 µl 0.1N NaOAc buffer, and 20 µg of each DOTA-antibody conjugate in 40 µl
 Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation
 Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Purification Kits. Fast and Convenient PROSEP -A and PROSEP-G Spin Column Kits for Antibody Purification DATA SHEET
 Â Montage Antibody Purification Kits Fast and Convenient PROSEP -A and PROSEP-G Spin Column Kits for Antibody Purification DATA SHEET Available with immobilized Protein A or Protein G Easy-to-use Antibody
 Montage Antibody Purification Kits Fast and Convenient PROSEP -A and PROSEP-G Spin Column Kits for Antibody Purification DATA SHEET Available with immobilized Protein A or Protein G Easy-to-use Antibody
Strength at the extracellular matrix muscle interface
 Scand J Med Sci Sports 2005: COPYRIGHT & BLACKWELL MUNKSGAARD 2005 Printed in Denmark. All rights reserved DOI: 10.1111/j.1600-0838.2005.00467.x Strength at the extracellular matrix muscle interface M.
Scand J Med Sci Sports 2005: COPYRIGHT & BLACKWELL MUNKSGAARD 2005 Printed in Denmark. All rights reserved DOI: 10.1111/j.1600-0838.2005.00467.x Strength at the extracellular matrix muscle interface M.
Nature Immunology: doi: /ni Supplementary Figure 1
 Supplementary Figure 1 BALB/c LYVE1-deficient mice exhibited reduced lymphatic trafficking of all DC subsets after oxazolone-induced sensitization. (a) Schematic overview of the mouse skin oxazolone contact
Supplementary Figure 1 BALB/c LYVE1-deficient mice exhibited reduced lymphatic trafficking of all DC subsets after oxazolone-induced sensitization. (a) Schematic overview of the mouse skin oxazolone contact
Species predicted to react based on 100% sequence homology: Chicken, Bovine, Dog.
 1 of 5 11/1/2013 10:25 PM Product Pathways - Jak/Stat Pathway Phospho-Stat3 (Tyr705) Antibody #9131 Have you tried your application using our XP monoclonal antibodies? Try products: 9145 PhosphoSitePlus
1 of 5 11/1/2013 10:25 PM Product Pathways - Jak/Stat Pathway Phospho-Stat3 (Tyr705) Antibody #9131 Have you tried your application using our XP monoclonal antibodies? Try products: 9145 PhosphoSitePlus
Materials and Methods Materials Required for Fixing, Embedding and Sectioning. OCT embedding matrix (Thermo Scientific, LAMB/OCT)
 Page 1 Introduction Tissue freezing and sectioning is a rapid method of generating tissue samples (cryosections) for histological analysis, and obviates the need for wax embedding. The method is popular
Page 1 Introduction Tissue freezing and sectioning is a rapid method of generating tissue samples (cryosections) for histological analysis, and obviates the need for wax embedding. The method is popular
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging
 QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
Immunostaining Protocols
 Immunostaining Protocols Lula L. Hilenski, Ph.D. Director Microscopy in Medicine Core Emory University Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on
Immunostaining Protocols Lula L. Hilenski, Ph.D. Director Microscopy in Medicine Core Emory University Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on
LAMININ. For Immunohistochemical Demonstration of Laminin in Paraffin-embedded and Frozen Human Tissue Sections Stock No. IMMH-7
 LAMININ For Immunohistochemical Demonstration of Laminin in Paraffin-embedded and Frozen Human Tissue Sections Stock No. IMMH-7 TABLE OF CONTENTS BACKGROUND AND PRINCIPLE... 4 REAGENTS AND EQUIPMENT PROVIDED...
LAMININ For Immunohistochemical Demonstration of Laminin in Paraffin-embedded and Frozen Human Tissue Sections Stock No. IMMH-7 TABLE OF CONTENTS BACKGROUND AND PRINCIPLE... 4 REAGENTS AND EQUIPMENT PROVIDED...
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Monoclonal antibody to fibronectin which inhibits. Extracellular matrix assembly
 Volume 217, number 1, 124-128 FEB 04787 June 1987 Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly M.A. Chernousov, A.I. Faerman, M.G. Frid, O.Yu. Printseva and V.E. Koteliansky
Volume 217, number 1, 124-128 FEB 04787 June 1987 Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly M.A. Chernousov, A.I. Faerman, M.G. Frid, O.Yu. Printseva and V.E. Koteliansky
Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney
 1 Supplementary text and data for: 2 3 4 5 Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney Authors: David A. D. Munro 1*, Peter Hohenstein 2, and Jamie A.
1 Supplementary text and data for: 2 3 4 5 Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney Authors: David A. D. Munro 1*, Peter Hohenstein 2, and Jamie A.
Confocal immunofluorescence microscopy
 Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
Nature Methods: doi: /nmeth Supplementary Figure 1. Retention of RNA with LabelX.
 Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
*Corresponding author. Tel: ;
 1 SUPPLEMENTARY DATA 2 3 4 5 6 7 8 9 10 11 Integrin 2 1 in nonactivated conformation can induce focal adhesion kinase signaling Maria Salmela 1, Johanna Jokinen 1,2, Silja Tiitta 1, Pekka Rappu 1, Holland
1 SUPPLEMENTARY DATA 2 3 4 5 6 7 8 9 10 11 Integrin 2 1 in nonactivated conformation can induce focal adhesion kinase signaling Maria Salmela 1, Johanna Jokinen 1,2, Silja Tiitta 1, Pekka Rappu 1, Holland
INVESTIGATION OF MSC DIFFERENTIATION ON ELECTROSPUN NANOFIBROUS SCAFFOLDS
 With support of NSF Award no. EEC-0754741 INVESTIGATION OF MSC DIFFERENTIATION ON ELECTROSPUN NANOFIBROUS SCAFFOLDS NSF Summer Undergraduate Fellowship in Sensor Technologies Emily Wible (Bioengineering)
With support of NSF Award no. EEC-0754741 INVESTIGATION OF MSC DIFFERENTIATION ON ELECTROSPUN NANOFIBROUS SCAFFOLDS NSF Summer Undergraduate Fellowship in Sensor Technologies Emily Wible (Bioengineering)
ab Hypoxic Response Human Flow Cytometry Kit
 ab126585 Hypoxic Response Human Flow Cytometry Kit Instructions for Use For measuring protein levels by flow cytometry: hypoxia-inducible factor 1-alpha (HIF1A) and BCL2/adenovirus E1B 19 kda proteininteracting
ab126585 Hypoxic Response Human Flow Cytometry Kit Instructions for Use For measuring protein levels by flow cytometry: hypoxia-inducible factor 1-alpha (HIF1A) and BCL2/adenovirus E1B 19 kda proteininteracting
SensoLyte Anti-alpha-Synuclein Quantitative ELISA Kit (Human/Mouse/Rat) *Colorimetric*
 Catalog # Kit Size SensoLyte Anti-alpha-Synuclein Quantitative ELISA Kit (Human/Mouse/Rat) *Colorimetric* AS-55550 One 96-well strip plate This kit is optimized to detect human/mouse/rat alpha-synuclein
Catalog # Kit Size SensoLyte Anti-alpha-Synuclein Quantitative ELISA Kit (Human/Mouse/Rat) *Colorimetric* AS-55550 One 96-well strip plate This kit is optimized to detect human/mouse/rat alpha-synuclein
Product Information. Before you begin. Component A 1 vial of 30 ul vial of 300 ul each Glycerol. Tris
 Glowing Products for Science Mix-n-Stain Antibody Labeling Kits Size: 1 labeling per kit Storage: -20 o C Stability: Stable for at least 1 year from date of receipt when stored as recommended. Components:
Glowing Products for Science Mix-n-Stain Antibody Labeling Kits Size: 1 labeling per kit Storage: -20 o C Stability: Stable for at least 1 year from date of receipt when stored as recommended. Components:
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell culture. HeLa cells were cultured as monolayers in Dulbecco s Minimal Essential
 Supporting Online Material Materials and methods Cell culture. HeLa cells were cultured as monolayers in Dulbecco s Minimal Essential Medium (Gibco BRL, Invitrogen Corporation, Carlsbad, CA, USA), supplemented
Supporting Online Material Materials and methods Cell culture. HeLa cells were cultured as monolayers in Dulbecco s Minimal Essential Medium (Gibco BRL, Invitrogen Corporation, Carlsbad, CA, USA), supplemented
1. Cross-linking and cell harvesting
 ChIP is a powerful tool that allows the specific matching of proteins or histone modifications to regions of the genome. Chromatin is isolated and antibodies to the antigen of interest are used to determine
ChIP is a powerful tool that allows the specific matching of proteins or histone modifications to regions of the genome. Chromatin is isolated and antibodies to the antigen of interest are used to determine
ANAT Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales. UNSW Copyright Notice
 ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
PROTOCOL TO PREPARE PLANTAR FOOTSKIN FOR MORPHOMETRY. I. Removal and Fixation of Plantar Skin (see video)
 PROTOCOL TO PREPARE PLANTAR FOOTSKIN FOR MORPHOMETRY I. Removal and Fixation of Plantar Skin (see video) 1. Sacrifice the animal a. Anaesthetize the animal by placing in a closed chamber with isoflurane.
PROTOCOL TO PREPARE PLANTAR FOOTSKIN FOR MORPHOMETRY I. Removal and Fixation of Plantar Skin (see video) 1. Sacrifice the animal a. Anaesthetize the animal by placing in a closed chamber with isoflurane.
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Radius 24-Well Cell Migration Assay (Fibronectin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Fibronectin Coated) Catalog Number CBA-125-FN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Fibronectin Coated) Catalog Number CBA-125-FN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Human IL10RB ELISA Pair Set ( CRFB4 )
 Human IL10RB ELISA Pair Set ( CRFB4 ) Catalog Number : SEK10945 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human IL10RB ELISA Pair Set ( CRFB4 ) Catalog Number : SEK10945 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Recombinant Biglycan for the Treatment! of Duchenne Muscular Dystrophy!!! PPMD Annual Conference, Jun 2013! By Joel B. Braunstein, MD!
 Recombinant Biglycan for the Treatment! of Duchenne Muscular Dystrophy!!! PPMD Annual Conference, Jun 2013! By Joel B. Braunstein, MD! Tivorsan Overview Commercial operations began in 2010 around biglycan
Recombinant Biglycan for the Treatment! of Duchenne Muscular Dystrophy!!! PPMD Annual Conference, Jun 2013! By Joel B. Braunstein, MD! Tivorsan Overview Commercial operations began in 2010 around biglycan
Mouse ICAM-1 / CD54 ELISA Pair Set
 Mouse ICAM-1 / CD54 ELISA Pair Set Catalog Number : SEK50440 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the General
Mouse ICAM-1 / CD54 ELISA Pair Set Catalog Number : SEK50440 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the General
-Actinin-2 Is a New Component of the Dystrophin Glycoprotein Complex 1
 Archives of Biochemistry and Biophysics Vol. 365, No. 2, May 15, pp. 216 222, 1999 Article ID abbi.1999.1172, available online at http://www.idealibrary.com on -Actinin-2 Is a New Component of the Dystrophin
Archives of Biochemistry and Biophysics Vol. 365, No. 2, May 15, pp. 216 222, 1999 Article ID abbi.1999.1172, available online at http://www.idealibrary.com on -Actinin-2 Is a New Component of the Dystrophin
Supplementary material to Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease
 Supplementary material to Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease Gyula Batta, Lilla Soltész, Tamás Kovács, Tamás Bozó, Zoltán
Supplementary material to Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease Gyula Batta, Lilla Soltész, Tamás Kovács, Tamás Bozó, Zoltán
How to run Alpha assay: How to setup an Alpha assay Make your own assay!
 How to run Alpha assay: How to setup an Alpha assay Make your own assay! 1 2009 PerkinElmer AlphaLISA kits - recommendations before starting the assay Samples: Phenol red and hemoglobin: choose AlphaLISA
How to run Alpha assay: How to setup an Alpha assay Make your own assay! 1 2009 PerkinElmer AlphaLISA kits - recommendations before starting the assay Samples: Phenol red and hemoglobin: choose AlphaLISA
Contents. The Right Surface for Every Cell Extracellular Matrices and Biologically Coated Surfaces ECM Mimetic and Advanced Surfaces...
 Contents The Right Surface for Every Cell... 1 Extracellular Matrices and Biologically Coated Surfaces... 2 Corning Matrigel Matrix... 2 Corning BioCoat Cultureware... 3 ECM Mimetic and Advanced Surfaces...
Contents The Right Surface for Every Cell... 1 Extracellular Matrices and Biologically Coated Surfaces... 2 Corning Matrigel Matrix... 2 Corning BioCoat Cultureware... 3 ECM Mimetic and Advanced Surfaces...
Mouse Factor XII Total ELISA Kit
 Mouse Factor XII Total ELISA Kit Catalog No: IMFXIIKT-TOT Lot No: SAMPLE INTENDED USE This mouse coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor XII
Mouse Factor XII Total ELISA Kit Catalog No: IMFXIIKT-TOT Lot No: SAMPLE INTENDED USE This mouse coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor XII
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A
 Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Post-expansion antibody delivery, after epitope-preserving homogenization.
 Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
NAG-1 (GDF-15, MIC-1) Prostate Cancer ELISA kit
 NAG-1 (GDF-15, MIC-1) Prostate Cancer ELISA kit Catalog Number: NG1 Store at -0 C. FOR RESEARCH USE ONLY v. 1081 Introduction This sandwich ELISA kit is for determination of NAG-1 (GDF-15, MIC-1) levels
NAG-1 (GDF-15, MIC-1) Prostate Cancer ELISA kit Catalog Number: NG1 Store at -0 C. FOR RESEARCH USE ONLY v. 1081 Introduction This sandwich ELISA kit is for determination of NAG-1 (GDF-15, MIC-1) levels
Generic DELFIA Reagents
 AD0005P-12 (en) 1 Generic DELFIA Reagents For Research Use Only These instructions for use apply to the following reagents: AD0038 DELFIA Eu-N1 PY20 antibody 50 µg vial AD0039 DELFIA Eu-N1 PY20 antibody
AD0005P-12 (en) 1 Generic DELFIA Reagents For Research Use Only These instructions for use apply to the following reagents: AD0038 DELFIA Eu-N1 PY20 antibody 50 µg vial AD0039 DELFIA Eu-N1 PY20 antibody
Self-assembly of Laminin Isoforms*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 50, Issue of December 12, pp. 31525 31532, 1997 Printed in U.S.A. Self-assembly of Laminin Isoforms* (Received for publication, April 11, 1997, and in
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 50, Issue of December 12, pp. 31525 31532, 1997 Printed in U.S.A. Self-assembly of Laminin Isoforms* (Received for publication, April 11, 1997, and in
Supplemental information
 Supplemental information - Control samples (200 subjects) - Immunohistochemistry of rat brain - Immunocytochemistry on neuronal cultures - Immunocompetition assay - Immunoprecipitation - Immunocytochemistry
Supplemental information - Control samples (200 subjects) - Immunohistochemistry of rat brain - Immunocytochemistry on neuronal cultures - Immunocompetition assay - Immunoprecipitation - Immunocytochemistry
PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%)
 1 AFFINITY HIS-TAG PURIFICATION PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%) DESCRIPTION Nickel NTA Magnetic Agarose Beads are products that allow rapid and easy small-scale purification of
1 AFFINITY HIS-TAG PURIFICATION PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%) DESCRIPTION Nickel NTA Magnetic Agarose Beads are products that allow rapid and easy small-scale purification of
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
The World Leader in SPR Technology. Jimmy Page, PhD, Biacore, Inc.
 The World Leader in SPR Technology Jimmy Page, PhD, Biacore, Inc. Objectives of Biacore Experiments Yes/No Data» Is there binding?» Ligand Fishing Concentration Analysis: How MUCH? Active Concentration
The World Leader in SPR Technology Jimmy Page, PhD, Biacore, Inc. Objectives of Biacore Experiments Yes/No Data» Is there binding?» Ligand Fishing Concentration Analysis: How MUCH? Active Concentration
Human IgG Antigen ELISA Kit
 Human IgG Antigen ELISA Kit Catalog No: IHUIGGKT Lot No: SAMPLE INTENDED USE This human immunoglobulin G antigen assay is intended for the quantitative determination of total human IgG antigen in serum,
Human IgG Antigen ELISA Kit Catalog No: IHUIGGKT Lot No: SAMPLE INTENDED USE This human immunoglobulin G antigen assay is intended for the quantitative determination of total human IgG antigen in serum,
Cloning and Characterization of Cytokeratins 8 and 19 in Adult Rat Striated Muscle
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 40, Issue of October 1, pp. 41830 41838, 2004 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Cloning and Characterization
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 40, Issue of October 1, pp. 41830 41838, 2004 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Cloning and Characterization
One-Step Western TM Kit using TMB
 Technical Manual No. 0203 Version 03272008 I Description.. 1 II Kit Contents.. 2 III Applications 3 IV Key Features.. 3 V Storage.. 3 VI One-Step Western TM Protocol. 3 VII Examples. 3 VIII Troubleshooting..
Technical Manual No. 0203 Version 03272008 I Description.. 1 II Kit Contents.. 2 III Applications 3 IV Key Features.. 3 V Storage.. 3 VI One-Step Western TM Protocol. 3 VII Examples. 3 VIII Troubleshooting..
SDS-PAGE and Western Blot. Molecular Basis of Evolution
 1 SDS-PAGE and Western Blot Molecular Basis of Evolution Homology high level of DNA and protein sequence similarity due to common ancestry. Evidence Genomes of related organisms are very similar. Even
1 SDS-PAGE and Western Blot Molecular Basis of Evolution Homology high level of DNA and protein sequence similarity due to common ancestry. Evidence Genomes of related organisms are very similar. Even
Human Pluripotent Stem Cell Functional Identification Kit
 Human Pluripotent Stem Cell Functional Identification Kit Catalog Number SC027B Reagents for the identification of human pluripotent stem cells by in vitro functional differentiation. This package insert
Human Pluripotent Stem Cell Functional Identification Kit Catalog Number SC027B Reagents for the identification of human pluripotent stem cells by in vitro functional differentiation. This package insert
NOTES. The Basal Lamina Is a Physical Barrier to Herpes Simplex Virus-Mediated Gene Delivery to Mature Muscle Fibers
 JOURNAL OF VIROLOGY, Nov. 1996, p. 8117 8123 Vol. 70, No. 11 0022-538X/96/$04.00 0 Copyright 1996, American Society for Microbiology NOTES The Basal Lamina Is a Physical Barrier to Herpes Simplex Virus-Mediated
JOURNAL OF VIROLOGY, Nov. 1996, p. 8117 8123 Vol. 70, No. 11 0022-538X/96/$04.00 0 Copyright 1996, American Society for Microbiology NOTES The Basal Lamina Is a Physical Barrier to Herpes Simplex Virus-Mediated
Multiplex Fluorescent Western Blot Starter Kit for the Bio- Rad ChemiDoc MP
 Page 1 of 7 INSTRUCTIONS: Z-310 Multiplex Fluorescent Western Blot Starter Kit for the Bio- Rad ChemiDoc MP Rockland Immunochemicals and Bio-Rad Laboratories have jointly developed an easy to use multiplex
Page 1 of 7 INSTRUCTIONS: Z-310 Multiplex Fluorescent Western Blot Starter Kit for the Bio- Rad ChemiDoc MP Rockland Immunochemicals and Bio-Rad Laboratories have jointly developed an easy to use multiplex
A Level. A Level Biology. Cells, Microscopes, Cell Cycle and Immunity Questions. AQA, OCR, Edexcel. Name: Total Marks: Page 1
 AQA, OCR, Edexcel A Level A Level Biology Cells, Microscopes, Cell Cycle and Immunity Questions Name: Total Marks: Page 1 Q1.The diagram shows a eukaryotic cell. (a) Complete the table by giving the letter
AQA, OCR, Edexcel A Level A Level Biology Cells, Microscopes, Cell Cycle and Immunity Questions Name: Total Marks: Page 1 Q1.The diagram shows a eukaryotic cell. (a) Complete the table by giving the letter
KPL SignaLOCK ChemiWestern Kits (Film and Imager Analysis)
 KPL SignaLOCK ChemiWestern Kits (Film and Imager Analysis) SignaLOCK HRP ChemiWestern Kit (Film) Catalog No. 54-53-00 SignaLOCK HRP ChemiWestern Kit (Imager) Catalog No. 54-54-00 SignaLOCK AP ChemiWestern
KPL SignaLOCK ChemiWestern Kits (Film and Imager Analysis) SignaLOCK HRP ChemiWestern Kit (Film) Catalog No. 54-53-00 SignaLOCK HRP ChemiWestern Kit (Imager) Catalog No. 54-54-00 SignaLOCK AP ChemiWestern
Stellaris RNA FISH Protocol for Simultaneous IF + FISH in Adherent Cells
 Stellaris RNA FISH Protocol for Simultaneous IF + FISH in Adherent Cells General Protocol & Storage Product Description A set of Stellaris RNA FISH Probes is comprised of up to 48 singly labeled oligonucleotides
Stellaris RNA FISH Protocol for Simultaneous IF + FISH in Adherent Cells General Protocol & Storage Product Description A set of Stellaris RNA FISH Probes is comprised of up to 48 singly labeled oligonucleotides
Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product.
 Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
5 AMP Sepharose 4B. instructions
 instructions 5 AMP Sepharose 4B 5' AMP Sepharose 4B interacts strongly with NAD + -dependent dehydrogenases and ATP-dependent enzymes. Selective elution with gradients of NAD + or NADP + has allowed the
instructions 5 AMP Sepharose 4B 5' AMP Sepharose 4B interacts strongly with NAD + -dependent dehydrogenases and ATP-dependent enzymes. Selective elution with gradients of NAD + or NADP + has allowed the
Antibody Structure. Antibodies
 Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibody Structure supports Function
 Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Combined Digoxigenin-labeled in situ hybridization/ Immunohistochemistry protocol (for fixed frozen cryostat sections)
 Combined Digoxigenin-labeled in situ hybridization/ Immunohistochemistry protocol (for fixed frozen cryostat sections) A. Digoxigenin-UTP labeling of crna antisense probe Refer to laboratory protocol and
Combined Digoxigenin-labeled in situ hybridization/ Immunohistochemistry protocol (for fixed frozen cryostat sections) A. Digoxigenin-UTP labeling of crna antisense probe Refer to laboratory protocol and
Applications of HTRF and Tag-lite Assays for HTP Antibody Screening
 Applications of HTRF and Tag-lite Assays for HTP Antibody Screening Brigitte Devaux, PhD Bristol Myers Squibb, Redwood City CA HTRF Symposium April 25, 2013 1 Introduction Generate human therapeutic antibodies
Applications of HTRF and Tag-lite Assays for HTP Antibody Screening Brigitte Devaux, PhD Bristol Myers Squibb, Redwood City CA HTRF Symposium April 25, 2013 1 Introduction Generate human therapeutic antibodies
rprotein A GraviTrap Protein G GraviTrap rprotein A/Protein G GraviTrap
 GE Healthcare Life Sciences Data file 28-9921-04 AA rprotein A GraviTrap Protein G GraviTrap rprotein A/Protein G GraviTrap Protein sample preparation rprotein A GraviTrap, Protein G GraviTrap, and rprotein
GE Healthcare Life Sciences Data file 28-9921-04 AA rprotein A GraviTrap Protein G GraviTrap rprotein A/Protein G GraviTrap Protein sample preparation rprotein A GraviTrap, Protein G GraviTrap, and rprotein
Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology. Chad Zimprich January 2015
 Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology Chad Zimprich January 2015 Presentation verview HaloTag Fusion Technology Design Functionality
Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology Chad Zimprich January 2015 Presentation verview HaloTag Fusion Technology Design Functionality
Immunofluorescence Staining Protocol for 3 Well Chamber, removable
 Immunofluorescence Staining Protocol for 3 Well Chamber, removable This Application Note presents a simple protocol for the cultivation, fixation, and staining of cells using the 3 Well Chamber, removable.
Immunofluorescence Staining Protocol for 3 Well Chamber, removable This Application Note presents a simple protocol for the cultivation, fixation, and staining of cells using the 3 Well Chamber, removable.
Chapter 17: Immunization & Immune Testing. 1. Immunization 2. Diagnostic Immunology
 Chapter 17: Immunization & Immune Testing 1. Immunization 2. Diagnostic Immunology 1. Immunization Chapter Reading pp. 505-511 What is Immunization? A method of inducing artificial immunity by exposing
Chapter 17: Immunization & Immune Testing 1. Immunization 2. Diagnostic Immunology 1. Immunization Chapter Reading pp. 505-511 What is Immunization? A method of inducing artificial immunity by exposing
Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice
 Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice Holly H. Nguyen*, Vianney Jayasinha*, Bing Xia, Kwame Hoyte, and Paul T. Martin Department
Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice Holly H. Nguyen*, Vianney Jayasinha*, Bing Xia, Kwame Hoyte, and Paul T. Martin Department
The Children s Hospital of Philadelphia Department of Pathology and Laboratory Medicine
 TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
Technical Note Detection of post-immunoprecipitation proteins by Western blot using the Quick Western Kit IRDye 680RD
 Technical Note Detection of post-immunoprecipitation proteins by Western blot using the Quick Western Kit IRDye 680RD Developed for: Aerius, Odyssey Classic, Odyssey CLx and Odyssey Sa Imaging Systems
Technical Note Detection of post-immunoprecipitation proteins by Western blot using the Quick Western Kit IRDye 680RD Developed for: Aerius, Odyssey Classic, Odyssey CLx and Odyssey Sa Imaging Systems
Pinpoint Slide DNA Isolation System Catalog No. D3001
 INSTRUCTIONS Pinpoint Slide DNA Isolation System Catalog No. D3001 Highlights Easily isolates genomic DNA in any targeted microscopic tissue area on a slide. The simple procedure combines Pinpoint tissue
INSTRUCTIONS Pinpoint Slide DNA Isolation System Catalog No. D3001 Highlights Easily isolates genomic DNA in any targeted microscopic tissue area on a slide. The simple procedure combines Pinpoint tissue
AVB Sepharose High Performance
 GE Healthcare Life Sciences Data file 28-927-54 AB Custom designed media AVB Sepharose High Performance AVB Sepharose High Performance is an affinity chromatography medium (resin) designed for the purification
GE Healthcare Life Sciences Data file 28-927-54 AB Custom designed media AVB Sepharose High Performance AVB Sepharose High Performance is an affinity chromatography medium (resin) designed for the purification
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation 5
 Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation Application Note Authors Yoonseok Kam 1, Ned Jastromb 1, Joe Clayton, Paul Held, and Brian P. Dranka 1 1 Agilent
Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation Application Note Authors Yoonseok Kam 1, Ned Jastromb 1, Joe Clayton, Paul Held, and Brian P. Dranka 1 1 Agilent
3D Cell Culture Product Intro. Bio-Byblos Biomedical
 3D Cell Culture Product Intro Bio-Byblos Biomedical Rundown Product Intro Cellusponge Series Go Matrix Applications Upcoming Products Degradable series Cellusponge CB series Cell Alignment plate Vivoalign
3D Cell Culture Product Intro Bio-Byblos Biomedical Rundown Product Intro Cellusponge Series Go Matrix Applications Upcoming Products Degradable series Cellusponge CB series Cell Alignment plate Vivoalign
MATERIAL DATA SHEET. NOTE: Kit contains reagents sufficient for 10 x 30 μl reactions and 5 Western Blots (minigel. Reagents Provided in Kit
 Lot # XXXXX ITCH/AIP4 Ubiquitin Ligase Kit Cat. # K-270 MATERIAL DATA SHEET The mammalian Itchy homolog, or ITCH, (also known as Atrophin-1-interacting protein 4 or AIP4) is a HECT domain class ubiquitin
Lot # XXXXX ITCH/AIP4 Ubiquitin Ligase Kit Cat. # K-270 MATERIAL DATA SHEET The mammalian Itchy homolog, or ITCH, (also known as Atrophin-1-interacting protein 4 or AIP4) is a HECT domain class ubiquitin
ab TripleStain IHC Kit: M&M&R on human tissue (DAB, Red/AP & DAB/Ni)
 ab183287 TripleStain IHC Kit: M&M&R on human tissue (DAB, Red/AP & DAB/Ni) Instructions for Use For the detection of Rabbit and Mouse Primary antibodies on Human tissue or cell samples. This product is
ab183287 TripleStain IHC Kit: M&M&R on human tissue (DAB, Red/AP & DAB/Ni) Instructions for Use For the detection of Rabbit and Mouse Primary antibodies on Human tissue or cell samples. This product is
Electrophoresis and transfer
 Electrophoresis and transfer Electrophoresis Cation = positively charged ion, it moves toward the cathode (-) Anion = negatively charged ion, it moves toward the anode (+) Amphoteric substance = can have
Electrophoresis and transfer Electrophoresis Cation = positively charged ion, it moves toward the cathode (-) Anion = negatively charged ion, it moves toward the anode (+) Amphoteric substance = can have
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION. Transcriptional output transiently spikes upon mitotic exit
 SUPPLEMENTARY INFORMATION Transcriptional output transiently spikes upon mitotic exit Viola Vaňková Hausnerová 1, 2, Christian Lanctôt 1* 1 BIOCEV and Department of Cell Biology, Faculty of Science, Charles
SUPPLEMENTARY INFORMATION Transcriptional output transiently spikes upon mitotic exit Viola Vaňková Hausnerová 1, 2, Christian Lanctôt 1* 1 BIOCEV and Department of Cell Biology, Faculty of Science, Charles
Immunofluorescence of organoids embedded in Basement Membrane Matrix
 Immunofluorescence of organoids embedded in Basement Membrane Matrix Sol Degese 1, Gabe Benton 1 1 Organoid Resource Lab (ORL), Trevigen, Inc., 8405 Helgerman Court, Gaithersburg, MD 20877 Introduction
Immunofluorescence of organoids embedded in Basement Membrane Matrix Sol Degese 1, Gabe Benton 1 1 Organoid Resource Lab (ORL), Trevigen, Inc., 8405 Helgerman Court, Gaithersburg, MD 20877 Introduction
Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced
 Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
The Nuclear Area Factor (NAF): a measure for cell apoptosis using microscopy and image analysis
 The Nuclear Area Factor (NAF): a measure for cell apoptosis using microscopy and image analysis Mark A. DeCoster Department of Biomedical Engineering and Institute for Micromanufacturing, Louisiana Tech
The Nuclear Area Factor (NAF): a measure for cell apoptosis using microscopy and image analysis Mark A. DeCoster Department of Biomedical Engineering and Institute for Micromanufacturing, Louisiana Tech
Protein A Mag Sepharose Xtra Protein G Mag Sepharose Xtra
 GE Healthcare Data file 28-9768-1 AA Protein sample preparation Protein A Mag Sepharose Xtra Xtra products are magnetic beads designed for efficient, high capacity small-scale purification/screening of
GE Healthcare Data file 28-9768-1 AA Protein sample preparation Protein A Mag Sepharose Xtra Xtra products are magnetic beads designed for efficient, high capacity small-scale purification/screening of
Figure S1. Figure S2. Figure S3 HB Anti-FSP27 (COOH-terminal peptide) Ab. Anti-GST-FSP27(45-127) Ab.
 / 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
/ 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands).
 Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Franzens-Universitaet Graz, Humboldtstrasse 50, 8010 Graz. Phone: ++43 (0) Fax: ++43 (0)
 Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation Andrea Seper 1, Vera H. I. Fengler 1, Sandro Roier 1, Heimo Wolinski 1, Sepp D. Kohlwein 1, Anne
Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation Andrea Seper 1, Vera H. I. Fengler 1, Sandro Roier 1, Heimo Wolinski 1, Sepp D. Kohlwein 1, Anne
Supplementary Material. Levels of S100B protein drive the reparative process in acute muscle injury and muscular dystrophy
 Supplementary Material Levels of protein drive the reparative process in acute muscle injury and muscular dystrophy Francesca Riuzzi 1,4 *, Sara Beccafico 1,4 *, Roberta Sagheddu 1,4, Sara Chiappalupi
Supplementary Material Levels of protein drive the reparative process in acute muscle injury and muscular dystrophy Francesca Riuzzi 1,4 *, Sara Beccafico 1,4 *, Roberta Sagheddu 1,4, Sara Chiappalupi
Affinity Chromatography Media. Cellufine Sulfate. Technical Data Sheet
 Affinity Chromatography Media Cellufine Sulfate Technical Data Sheet Cellufine Sulfate For concentration, purification and depyrogenation of virus, viral/microbial antigens and heparin-binding proteins
Affinity Chromatography Media Cellufine Sulfate Technical Data Sheet Cellufine Sulfate For concentration, purification and depyrogenation of virus, viral/microbial antigens and heparin-binding proteins
WesternMAX Alkaline Phosphatase Chemiluminescent Detection Kits
 WesternMAX Alkaline Phosphatase Chemiluminescent Detection Kits Code N221-KIT N220-KIT Description WesternMAX Chemiluminescent AP Kit, Anti-Mouse Includes: Alkaline Phosphatase (AP) Conjugated Anti-Mouse
WesternMAX Alkaline Phosphatase Chemiluminescent Detection Kits Code N221-KIT N220-KIT Description WesternMAX Chemiluminescent AP Kit, Anti-Mouse Includes: Alkaline Phosphatase (AP) Conjugated Anti-Mouse
CHAPTER 3 ANTIBODY STRUCTURE I
 CHAPTER 3 ANTIBODY STRUCTURE I See APPENDIX: (3) OUCHTERLONY ANALYSIS; (6), EQUILIBRIUM DIALYSIS; (7) CROSS-REACTIVITY Electrophoretic separation of serum proteins identifies the GAMMA-GLOBULIN fraction
CHAPTER 3 ANTIBODY STRUCTURE I See APPENDIX: (3) OUCHTERLONY ANALYSIS; (6), EQUILIBRIUM DIALYSIS; (7) CROSS-REACTIVITY Electrophoretic separation of serum proteins identifies the GAMMA-GLOBULIN fraction
Supplemental Materials. Matrix Proteases Contribute to Progression of Pelvic Organ Prolapse in Mice and Humans
 Supplemental Materials Matrix Proteases Contribute to Progression of Pelvic Organ Prolapse in Mice and Humans Madhusudhan Budatha, Shayzreen Roshanravan, Qian Zheng, Cecilia Weislander, Shelby L. Chapman,
Supplemental Materials Matrix Proteases Contribute to Progression of Pelvic Organ Prolapse in Mice and Humans Madhusudhan Budatha, Shayzreen Roshanravan, Qian Zheng, Cecilia Weislander, Shelby L. Chapman,
Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ
 Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ Research at the nanoscale is more effective, when research teams can quickly and easily observe and characterize a wide
Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ Research at the nanoscale is more effective, when research teams can quickly and easily observe and characterize a wide
phab Amine and Thiol Reactive Dyes for Antibody Internalization Studies Nidhi Nath, Ph.D. Group Leader, Protein Analysis Promega Corporation
 phab Amine and Thiol Reactive Dyes for Antibody Internalization Studies Nidhi Nath, Ph.D. Group Leader, Protein Analysis 1 Outline 1. phab Dyes 2. Protocols for conjugating phab Dyes to antibodies 3. Applications:
phab Amine and Thiol Reactive Dyes for Antibody Internalization Studies Nidhi Nath, Ph.D. Group Leader, Protein Analysis 1 Outline 1. phab Dyes 2. Protocols for conjugating phab Dyes to antibodies 3. Applications:
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
ab Mouse and Rabbit AP/Fast-Red (ABC) Detection IHC Kit
 ab128967 - Mouse and Rabbit AP/Fast-Red (ABC) Detection IHC Kit Instructions for Use For the detection of a specific antibody bound to an antigen in tissue sections. This product is for research use only
ab128967 - Mouse and Rabbit AP/Fast-Red (ABC) Detection IHC Kit Instructions for Use For the detection of a specific antibody bound to an antigen in tissue sections. This product is for research use only
Rat IGF-1 ELISA Kit (rigf-1-elisa)
 Rat IGF-1 ELISA Kit (rigf-1-elisa) Cat. No. EK0377 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Rat IGF-1 ELISA Kit (rigf-1-elisa) Cat. No. EK0377 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Materials and methods. by University of Washington Yeast Resource Center) from several promoters, including
 Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
