IN VITRO ASSEMBLY OF ARTIFICIAL MITOTIC SPINDLES USING DIELECTROPHORESIS
|
|
|
- Valentine Cross
- 6 years ago
- Views:
Transcription
1 The Pennsylvania State University The Graduate School Department of Bioengineering IN VITRO ASSEMBLY OF ARTIFICIAL MITOTIC SPINDLES USING DIELECTROPHORESIS A Thesis in Bioengineering by Vidhya Aravamuthan 2008 Vidhya Aravamuthan Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science August 2008
2 COMMITTEE PAGE The thesis of Vidhya Aravamuthan was reviewed and approved* by the following: William O. Hancock Associate Professor of Bioengineering Thesis Co-Advisor Richard J. Cyr Professor of Biology Thesis Co-Advisor Peter J. Butler Associate Professor of Bioengineering Herbert H. Lipowsky Professor of Bioengineering Head of the Department of Bioengineering * Signatures are on file in the Graduate School.
3 ABSTRACT The separation of duplicated chromosomes during mitosis is achieved by the mitotic spindle which consists of microtubules and associated motor proteins. Many motor proteins are known to interact with microtubules and affect their dynamics and organization. Knockout strategies have been used to probe the spindle in cells, but due to the inherent redundancy built into this complex process, deleting individual motor proteins often has only subtle effects. Single molecule studies have been used to study the properties of isolated motor proteins. However, they do not provide any information on the interaction of motors with complex microtubule assemblies like the spindle. The goal of this project is to develop novel engineering approaches for the study of mitosis that bridges single molecule studies and in vivo studies in cells. The approach is to assemble artificial spindles in vitro to study and characterize interactions of single motors with the microtubules that make up the spindle. To achieve this goal, we have used microelectrodes fabricated on a quartz substrate. AC electric fields were then applied across the electrodes, which results in electroosmotic flows and dielectrophoretic forces. Microtubules assemble at the electrode tip due to the resultant electrokinetic forces and are then immobilized on the neutravidin patterned electrodes. These assembled microtubules were then extended with rhodamine tubulin to obtain overlapping opposed microtubules. The ability to extend microtubules in the flow cell facilitates the study of interesting phenomena like plus tip tracking of motor proteins and the influence of motors on polymerization kinetics. This assembly of microtubules then provides a platform to study the function of single motors and populations of motors and their effect on the spindle. The scope of this research can be further expanded by using beads coated with a single motor type in conjunction with optical traps to impose external forces. This novel experimental tool should provide important clues towards understanding force generation and microtubule rearrangements by molecular motors in the mitotic spindle. iii
4 Table of Contents List of Figures.....v List of Tables...vi Acknowledgements..vii Chapter1 Introduction 1 Background Mitosis and Motor proteins..3 Motor proteins..5 Current approaches used to study mitosis 6 Manipulation of microtubules organization in vitro 8 Dielectrophoresis.9 Electroosmotic flows.10 Electrothermal flow...11 Thesis Overview...12 References..14 Chapter2 Microtubule bundling in vitro by ATK5 Introduction 17 Materials and Methods...20 Results and Discussion ATK5 bundles microtubules in vitro.21 Aster formation..25 Bundling of microtubules..26 Tail of ATK5..27 References..30 Chapter3 Spindle assembly in vitro using Dielectrophoresis Introduction 32 Materials and Methods Preparing microtubules..35 Sample preparation Dielectrophoresis experiment 36 Results and Discussion..38 Spindle assembly...39 Spindle kinetics..41 Age of the electrode...43 Microtubule extension in the flow cell..46 References..48 Conclusion iv
5 List of Figures Figure 1.1: Artificial spindle schematic Figure 1.2: Various stages of mitosis Figure 1.3: Bipolar spindle assembly with Xenopus egg extracts.6 Figure 1.4: Microtubule geometries formed from multiheaded kinesins....7 Figure 1.5: Dielectrophoresis in a castellated electrode. 9 Figure 1.6: Formation of double layer on electrode surface 10 Figure 1.7: Electroosmotic flows..11 Figure 1.8: Simulated electrothermal flow...12 Figure 2.1: Microtubule geometry for different microtubule concentration Figure 2.2: Distribution of the number of microtubules in a bundle Figure 2.3: Interaction of Ncd tail with the microtubule.. 27 Figure 2.4: Possible scenarios of ATK5 binding to the microtubule Figure 3.1: Schematic of artificial spindle Figure 3.2: Accumulation of segmented microtubules at electrode tip Figure 3.3: Spindle assembly on neutravidin coated electrodes Figure 3.4: Intensity distribution of rhodamine extension and cy5 seed.. 40 Figure 3.5: Spindle kinetics Figure 3.6: Microtubules unbinding from the electrode tips...44 Figure 3.7: Age of the electrode Figure 3.9: Extension of biotin seeds in the flow cell with rhodamine tubulin 47 v
6 List of Tables Table 2.1: Distribution of Microtubule structures. 24 Table 3.1: Optimizing binding of biotinylated microtubules to neutravidin surface vi
7 Acknowledgements I would like to thank my thesis advisor Dr.Hancock for his able guidance. The discussions with him were fruitful and enlightening. I thank my thesis co-advisor Dr.Cyr for being helpful and approachable. The many discussions I have had with him and Dr.Hancock were definitive. I would also like to thank Dr.Butler for reading my thesis and for giving valuable comments. My lab colleagues Shankar, Maruti and Gayatri were very helpful all through. This work was built on Maruti s work and hence his guidance on intricate aspects of the project was immense. I thank Jennelle (from Cyr lab) for all her help in molecular biology techniques and all the hearty conversations I have had with her. I would like to thank my husband Narayanan and the rest of my family who stood behind me at all times. vii
8 Chapter 1 Introduction Cell division, a basic process of life, has been a subject of intense research, employing techniques ranging from imaging to deletion studies to biochemical studies. Cell division studies have implications in drug development, cancer treatment and fertility related problems. The key event in cell division is the segregation of sister chromatids into two daughter cells by attaching themselves to microtubules. Among the many motor proteins involved in cell division, kinesin and dynein play key roles in spindle morphogenesis and chromosome segregation. Motor proteins hydrolyze ATP and convert this chemical energy into mechanical work. Motor proteins are involved in many functions like microtubule destabilization (by Mitotic Centromere Associated Kinesin, MCAK) (Maney et al. 1998), in bundling of microtubules (by Arabidopsis thaliana kinesin, ATK5) (Ambrose et al. 2005), and being localized in the kinetochore area (like dynein and CENP-E) (Banks and Heald 2001). Although the function of motor proteins have been studied by deletion studies in cells, there is a possibility that the cell could compensate for that loss of function by error correcting mechanisms, as in the case of ATK1 (Marcus et al. 2003). Mutants of ATK1 show abnormal microtubule accumulation and spindle polarity during early stages of mitosis which is eventually resolved and chromosomes are segregated normally due to the redundancy in the cells (Marcus et al. 2003). Single molecule studies have been used to
9 study the motor kinetics, but this does not give any information on the interaction of motor with a complex microtubule assembly like the spindle. It is our endeavor to bridge between the study of motor proteins in cells and the study of motor proteins at the single molecule level. Our goal is to create an artificial spindle assembly (see Fig 1.1 for schematic) which will enable us to study the function of single motor proteins in the complex geometry of the mitotic spindle. In this thesis, we use engineering approaches like dielectrophoresis and protein patterning to assemble artificial mitotic spindles. This allows an opportunity to introduce single motor proteins and observe their interaction with the spindle and model spindle dynamics. Electrode Electrode Figure 1.1: Artificial spindle schematic - The biotinylated minus ends (blue) of the segmented microtubule binds to the neutravidin patterned electrodes. The purple lines indicate rhodamine extensions in the electrode gap. Microtubules are assembled by dielectrophoresis and further immobilized at the electrode tip due to biotin-neutravidin link. 2
10 Background Mitosis and Motor proteins The mitotic spindle is a dynamic structure brought about by a series of microtubule growth and shortening events. These events are concurrent with the movement of chromosomes towards the equator and to the spindle pole. Before the start of mitosis, both genomic DNA and the centrosome (in animal cells) replicate. The start of mitosis, prophase, sees the movement of centrosomes to form the spindle pole. A tetrameric motor protein from kinesin-5 family, Eg5, is known to affect the separation of centrosome and null mutants of Eg5 have shown monopolar spindles (Zhu et al. 2005). RNA interference mediated depletion of other motor proteins like Klp10A, Klp61 and Klp67A form monopolar spindles as well, while depletion of the kinesin-14 motor, Ncd, causes multipolar spindles (Goshima and Vale 2003). Metaphase sees the alignment of chromosomes at the equator of the cell due to pushing forces. These pushing forces could be due to the polymerization of the microtubules and/or from various motors acting on the microtubule. It is seen that chromosome congression at the metaphase plate is ensured by many kinesins like CENPmeta, Klp3A and Nod (Goshima and Vale 2003). After metaphase, during anaphase, the chromosomes move away from the equator towards the spindle pole. This is achieved by both spindle pole movement and by microtubule depolymerization at both plus and minus ends (Rogers et al. 2004). MCAK, a motor involved in microtubule depolymerization, has been shown to be involved in chromosome separation during Anaphase (Maney et al. 3
11 1998). In telophase, separation of chromosome is complete and the nuclear membrane forms around the separated chromosomes (see Fig 1.2). Figure 1.2: Various stages of mitosis (a) Centrosome movement in prophase (b) Movement of chromosomes to metaphase plate (c) Separation of chromosomes towards spindle pole during anaphase (d) Formation of nuclear membrane around the daughter chromosomes (Figure adapted from Schliwa 2003) Research in microtubules and motors, apart from answering many questions from a pure science perspective, is significant due to its direct consequences in cancer and other diseases. Motors like KIF1A are involved in neurodegenerative disease and KIF5 in Alzheimer s (Seog et al. 2004). Using our proposed artificial spindle, those proteins involved in division and specifically in spindle formation and maintenance like Eg5, MCAK and dynein (Banks and Heald 2001), can be studied individually. 4
12 Motor proteins In this thesis the focus will be on two motors: conventional kinesin and ATK5. Of the 14 kinesin families, conventional kinesin is most well characterized. It is a plus ended motor and for every ATP hydrolyzed it takes one 8 nm step (Coy et al. 1999). It is a processive motor, i.e. it can walk on the microtubule for long distances (~1 µm) (Howard et al. 1989; Block et al. 1990). Every step kinesin takes is due to conformational changes in the head which is in turn tightly coupled to ATP hydrolysis cycle (Hancock and Howard 1999). Conventional kinesin consists of two heavy and two light chains. The heavy chain contains microtubule binding domain and a catalytic domain which in turn is connected to neck linker and coiled coil. The light chain is involved in cargo binding (Hirokawa et al. 1989). While conventional kinesin is a processive plus ended motor with its motor domain at the N terminal, motors in kinesin-14 family protein have their motor domain at the C terminal and move towards the minus ends of the microtubule. Arabidopsis thaliana kinesins (ATK1, ATK2, ATK3, and ATK5) are minus ended mitotic motor proteins belonging to Kinesin 14A sub-family (Mitsui et al. 1993; Mitsui et al. 1994). ATK5 is 83% similar in sequence to ATK1, which is a non processive minus end directed motor (Marcus et al. 2002). ATK5 has been implicated in focusing of spindle pole and spindles and the authors suggest that it is due to TIP action and microtubule bundling that it zippers the microtubules at the pole (Ambrose et al. 2005). 5
13 Current approaches used to study mitosis A study that best mimics spindle assembly in vitro, has been conducted using Xenopus egg extracts (Heald et al. 1997). In this study, the authors assembled chromatin using DNA coated magnetic beads in the presence of Xenopus egg extract. They observed formation of nuclear envelope when DNA beads were incubated with extracts from interphase. Interestingly, in the presence of labeled tubulin, spindle-like structures were observed (see Fig 1.3.a) (Heald et al. 1997). The authors suggest three key step interactions of motors and microtubules lead to bipolar spindle formation (Fig 1.3.b). In the first step, nucleation, chromatin beads randomly interact with microtubules. In second step, dynein groups the minus ends forming a nearly linear bundle and finally the plus ends in the chromatin beads push the microtubules away. b a Figure 1.3: (a) Bipolar spindle assembly in the presence of microtubules and Xenopus egg extracts and DNA coated beads, (b) Possible mechanism of spindle formation through a three step process (Figure reproduced from Heald et al. 1997). 6
14 Another in vitro study that assembles microtubules in various geometries uses oligomeric motors (Nédélec et al. 1997) (see Fig 1.4). In this study, the authors use both stabilized and dynamic microtubules along with oligomeric motors. The relative concentration of microtubules and motors were varied in closed chambers and the various structures formed are observed using dark field microscopy. The authors report formation of spindle-like geometries on incubating the microtubules with both plus and minus end directed motors. Figure 1.4: Microtubule geometries formed by addition of multiheaded kinesins to stabilized microtubules (a) Combination of asters and vortices, (b) Asters, (c) bundles (d), vortices formed for different microtubule to motor ratios. (Figure reproduced from (Nédélec et al. 1997)). Depletion studies in cells have also been undertaken to study interaction of various motors with microtubules. Goshima and Vale (2003) studied the function of different motors by observing the effects on mitosis on depleting motors by RNA interference. They found many redundant or error correcting mechanisms in the cell when a single motor was depleted. For example, four motor proteins - Nod, CENP-meta, Klp3A and Klp67A are involved in alignment of chromosome during metaphase. Hence, it is difficult to study the effect of one motor by depletion studies. 7
15 Manipulation of Microtubule organization in vitro Efforts to immobilize microtubules in certain geometries are being driven by the desire to achieve efficient transport in microfluidic systems. Currently, efforts towards immobilizing microtubules in a particular geometry have been achieved by fabricating open and closed microchannels (Moorjani et al. 2003; Huang et al. 2005). Microtubules have been magnetically functionalized and then aligned by magnetic fields (Platt et al. 2005). An elegant method to control the microtubule alignment is by using dielectrophoresis (Uppalapati et al. 2008). In this technique, the polarized microtubule moves according to the forces generated in the flow cell due to the application of AC electric fields (Uppalapati et al. 2008). The details of this phenomenon will be discussed in the following section. This thesis is built on earlier work done in the Hancock lab using AC electrokinetic mechanisms such as dielectrophoresis, electroosmotic flow to align microtubules. After aligning the microtubules, they will be immobilized by biotinylating the microtubules and binding them to neutravidin coated electrodes. 8
16 Dielectrophoresis Microtubules are protein polymers with a net negative charge and hence applying electric fields to separate or transport or assemble them is possible as has been shown in the Hancock lab (Jia et al. 2004; van den Heuvel et al. 2006; Kim et al. 2007). However, applying DC field causes electrolysis of the electrolyte and bubble generation leading to electrode damage. Applying non-uniform AC electric fields is another option to transport microtubules. Dielectrophoresis is the movement of a polarizable particle in the presence of an electric field. If a particle is more polarizable than its medium, then it moves towards high field regions leading to positive dielectrophoresis. Negative dielectrophoresis occurs when a particle is less polarizable than the medium, leading to movement towards low electric field regions (Fig 1.5) (Morgan et al. 2003). Figure 1.5: Positive (grey particles) and negative (black particles) dielectrophoresis in a castellated electrode (Figure reproduced from Morgan et al. 2003) 9
17 Electroosmotic flows When a charged surface comes in contact with an electrolyte, opposite charges (counter ions) accumulate near the surface of the electrode (see Fig 1.6). The Stern layer is the region of tightly associated counter ions and the diffuse layer is the region adjacent to Stern layer and is part of the bulk solution (Morgan et al. 2003). Figure 1.6: Formation of double layer, ions of opposite polarity on electrode surface (Figure reproduced from Morgan et al. 2003). In the presence of an electric field, these ions move due to the tangential force (see Fig 1.7.a). Fig 1.7.b shows the bulk flow generated by the movement of ions in the double layer. In the other half cycle (refer to Fig 1.7.a), a positive potential is applied to the left electrode and negative ions accumulate on the electrode. Ions move in the same direction as before since direction of tangential component of electric field remains the same. 10
18 Electroosmotic flows are frequency dependent and work well in intermediate frequencies (for the electrodes we used it was ~ 2.5 MHz). At high frequencies, since ions cannot accumulate in half cycle period, there is no electroosmotic flow (Morgan et al. 2003). At very low frequencies, there is no electroosmotic flow due to potential drop across the double layer resulting in zero tangential component acting on the double layer. Figure 1.7: (a) Forces acting on the double layer due to the applied electric field (b) Electroosmotic flow due to the movement of double layer due to tangential component electric field (Figure reproduced from Morgan et al. 2003) Electrothermal flow Although electroosmotic flows are maximal at intermediate frequencies, electrothermal flows are frequency independent (Morgan et al. 2003). Electrothermal flows are generated due to temperature gradients in the flow cell. In our experiments, the main cause of heating is due to irradiation from the fluorescence lamps, which is absorbed by the semi-opaque chrome electrodes. Fig 1.8 shows a simulated electrothermal flow profile. It is apparent from the figure that electrothermal flow is bulk phenomena whereas electroosmotic flow is a surface flow. Also note that the flow is towards the electrode gap, unlike the electroosmotic flow which is away from the electrode gap. 11
19 Hence, for our application of spindle formation, it is necessary to cancel out electrothermal and electroosmotic flow so that microtubules can accumulate on the electrode due to dielectrophoresis (DEP). DEP experiments were performed at 5MHz when electroosmotic and electrothermal flows cancel out each other (Uppalapati et al. 2008) Figure 1.8: Electrothermal flow simulated for one of the two electrode system at electrolyte conductivity of 2.1 mm m -1 and value shown in the box is the maximum velocity (Figure reproduced from Morgan et al. 2003). Thesis Overview Mitosis is a complex phenomenon and many motor proteins are involved in spindle formation and maintenance. We aim to create an in vitro environment with microtubule organization resembling those found in mitotic spindles. So far, Maruti Uppalapati (from the Hancock lab, Pennsylvania State University) and Ying-Ming Huang (from Jackson lab, Pennsylvania State University) have laid the groundwork by aligning microtubules using AC electrokinetic techniques (Uppalapati et al. 2008). They have also patterned 12
20 neutravidin for selective microtubule immobilization (Huang et al. 2008). In addition, new electrode samples for this work have been provided by Raymond Fok (from Jackson lab, Pennsylvania State University). In this thesis, we combine the above techniques to obtain artificial spindles by using segmented microtubules that contain biotinylated segments on their minus ends. We show the formation of artificial spindle and also the possibility of growing the microtubules in the flow cell. We also show that the age of the electrode affects microtubule binding. 13
21 References: Ambrose, J. C.,Li, W.,Marcus, A.,Ma, H. and Cyr, R. (2005). "A minus-end-directed kinesin with plus-end tracking protein activity is involved in spindle morphogenesis." Molecular biology of the cell 16(4): Banks, J. D. and Heald, R. (2001). "Chromosome movement: dynein-out at the kinetochore." Curr Biol 11(4): R Block, S. S. M.,Goldstein, L. L. S. and Schnapp, B. B. J. (1990). "Bead movement by single kinesin molecules studied with optical tweezers." Nature 348(6299): Coy, D. L.,Wagenbach, M. and Howard, J. (1999). "Kinesin takes one 8-nm step for each ATP that it hydrolyzes." J Biol Chem 274(6): Goshima, G. and Vale, R. D. (2003). "The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line." The Journal of cell biology 162(6): Hancock, W. O. and Howard, J. (1999). "Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains." Proc Natl Acad Sci U S A 96(23): Heald, R.,Tournebize, R.,Habermann, A.,Karsenti, E. and Hyman, A. (1997). "Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule selforganization." The Journal of cell biology 138(3): Hirokawa, N.,Pfister, K. K.,Yorifuji, H.,Wagner, M. C.,Brady, S. T. and Bloom, G. S. (1989). "Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration." Cell 56(5): Howard, J.,Hudspeth, A. J. and Vale, R. D. (1989). "Movement of microtubules by single kinesin molecules." Nature 342(6246): Huang, Y. M., Uppalapati M., Hancock W. O., Jackson T. N. (2005). "Microfabricated capped channels for biomolecular motor-based transport." IEEE transactions on advanced packaging 28(4): 564. Huang, Y. M., Uppalapati M., Hancock W. O., Jackson T. N. (2008) "Neutravidin Patterning by Deep UV Irradiation" Lab on a Chip. In Press Jia, L.,Moorjani, S. G.,Jackson, T. N. and Hancock, W. O. (2004). "Microscale transport and sorting by kinesin molecular motors." Biomedical microdevices 6(1): Kim, T.,Kao, M.-T.,Hasselbrink, E. F. and Meyhöfer, E. (2007). "Active alignment of microtubules with electric fields." Nano letters 7(1):
22 Maney, T. T.,Hunter, A. A. W.,Wagenbach, M. M. and Wordeman, L. L. (1998). "Mitotic centromere-associated kinesin is important for anaphase chromosome segregation." The Journal of cell biology 142(3): Marcus, A. I.,Ambrose, J. C.,Blickley, L.,Hancock, W. O. and Cyr, R. J. (2002). "Arabidopsis thaliana protein, ATK1, is a minus-end directed kinesin that exhibits nonprocessive movement." Cell motility and the cytoskeleton 52(3): Marcus, A. I.,Li, W.,Ma, H. and Cyr, R. J. (2003). "A kinesin mutant with an atypical bipolar spindle undergoes normal mitosis." Molecular biology of the cell 14(4): Mitsui, H.,Nakatani, K.,Yamaguchi-Shinozaki, K.,Shinozaki, K.,Nishikawa, K. and Takahashi, H. (1994). "Sequencing and characterization of the kinesin-related genes katb and katc of Arabidopsis thaliana." Plant molecular biology 25(5): Mitsui, H.,Yamaguchi-Shinozaki, K.,Shinozaki, K.,Nishikawa, K. and Takahashi, H. (1993). "Identification of a gene family (kat) encoding kinesin-like proteins in Arabidopsis thaliana and the characterization of secondary structure of KatA." Molecular & general genetics 238(3): Moorjani, S. G., Jia, L., Jackson, T. N. and Hancock, W. O. (2003). "Lithographically patterned channels spatially segregate kinesin motor activity and effectively guide microtubule movements." Nano letters 3(5): 633. Morgan, H. and Green, N. G. AC Electrokinetics: Colloids and Nanoparticles. 1st ed. Vol. 1. Hertfordshire, England: Research Studies P Ltd., 2003 Nédélec, F. J.,Surrey, T.,Maggs, A. C. and Leibler, S. (1997). "Self-organization of microtubules and motors." Nature 389(6648): Platt, M.,Muthukrishnan, G.,Hancock, W. O. and Williams, M. E. (2005). "Millimeter scale alignment of magnetic nanoparticle functionalized microtubules in magnetic fields." Journal of the American Chemical Society 127(45): Rogers, G. C.,Rogers, S. L.,Schwimmer, T. A.,Ems-McClung, S. C.,Walczak, C. E.,Vale, R. D.,Scholey, J. M. and Sharp, D. J. (2004). "Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase." Nature 427(6972): Schliwa, M. ed. Molecular Motors. 1st ed. Vol. 1. Munchen: Wiley-VCH, Seog, D.-H.,Lee, D.-H. and Lee, S.-K. (2004). "Molecular motor proteins of the kinesin superfamily proteins (KIFs): structure, cargo and disease." Journal of Korean medical science 19(1):
23 van den Heuvel, M. G. L.,de Graaff, M. P. and Dekker, C. (2006). "Molecular sorting by electrical steering of microtubules in kinesin-coated channels." Science 312(5775): Uppalapati M., Huang, Y. M., Jackson T. N., Hancock W. O. (2008) "Microtubule Alignment and Manipulation Using AC Electrokinetics" Small. In Press Zhu, C.,Zhao, J.,Bibikova, M.,Leverson, J. D.,Bossy-Wetzel, E.,Fan, J. B.,Abraham, R. T. and Jiang, W. (2005). "Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference." Mol Biol Cell 16(7):
24 Chapter 2 Microtubule bundling in vitro by ATK5 Introduction Kinesins are motor proteins with specific domains for microtubule binding, ATP hydrolysis, dimerization and cargo binding. Although kinesins are structurally diverse, certain key structural domains define their functions. The presence of the motor domain at the N- or C- terminal dictates the movement of the kinesin to plus or minus ends of the microtubule respectively (Vale and Fletterick 1997). Motors like Non claret disjunctional (Ncd) and the Arabidopsis thaliana kinesins ATK1, ATK2, ATK3, and ATK5 are members of the kinesin-14 family and are C-terminal motors that translocate towards the minus end of the microtubule (Mitsui et al. 1993; Mitsui et al. 1994). Some Arabidopsis thaliana kinesins have their motor domain at the C terminal which is then the primary microtubule binding site. The secondary microtubule binding site for ATK2 and ATK3 is at the tail domain which is at the N terminal (Jiang et al. 2007). ATK1 and ATK5 share high sequence similarity with ATK2 and ATK3 (Jiang et al. 2007). ATK5 is a minus ended motor which localizes to the growing plus tip of the microtubule i.e. ATK5 is a plus tip tracker (Ambrose et al. 2005). This plus tip localization is independent of the motor domain i.e. tail and stalk region is sufficient for plus tip activity. It has been seen that EB1, a plus tip tracker, attaches selectively to the growing
25 ends of a microtubule and not to a stabilized microtubule (Mimori-Kiyosue et al. 2000). In vitro studies using purified components of Mal3 (EB1 homologue), kinesin Tea2, Tip1 (Clip170 homologue) showed that Mal3 selectively binds to the growing plus tip and not to the lattice and growing minus ends (Bieling et al. 2007). Mal3 (or EB1) can associate with the growing plus tip either by co-polymerizing (i.e. binds to free tubulin in solution) or due to some structural difference of the microtubule at the plus end. Bieling and colleagues found that EB1 does not localize to the plus tip due to co-polymerization with tubulin. Hence, the plus tip activity is due to the structural difference of the microtubule at the tip either due to the presence of GTP-tubulin or due to presence of open ended structures like the protofilament (Bieling et al. 2007). It is seen that null mutants of ATK5 not only have wide spindle midzone and poles during metaphase and anaphase, but also elongated and bent spindles during prometaphase. This widening of spindles has been attributed to the lack of bundling activity in ATK5 null mutants. It has been proposed by Ambrose and Cyr (2007) that ATK5 uses both its plus-tip tracking ability and minus-ended motility to capture antiparallel microtubules in the spindle and slide them across to decrease the lateral distance. This interaction also creates inward forces preventing elongation of the spindle and further straightens the spindle. The bundling ability of ATK5 implies two microtubule binding sites like another kinesin-14 motor, Ncd, which has an ATP dependent binding site at the motor domain and an ATP independent binding site at the tail (Karabay and Walker 1999). Tail of Ncd is positively charged and it electrostatically interacts with the negatively charged E-hook of the microtubule (Furuta and Toyoshima 18
26 2008). The nature of the ATK5 tail, however, is not very well understood. It could either interact with the microtubule through a plus tip protein complex or directly bind to the microtubule plus end. ATK5 could electrostatically interact with the microtubule like Ncd (Furuta and Toyoshima 2008) and diffuse one dimensionally or bind tightly to the microtubule. 19
27 Materials and Methods The protocol for the following experiments were developed by Christian Ambrose from the Cyr lab (Ambrose and Cyr 2007). Arabidopsis thaliana Kinesin 5 (ATK5) and Drosophila melanogaster kinesin heavy chain (KHC) were expressed in E.Coli BL21(DE3) cells. Cell lysates of ATK5 and KHC were obtained as described previously (Marcus et al. 2002). The transformed bacterial cells were grown overnight and expression induced by adding 0.5 mm isopropyl beta D-thiogalactoside (IPTG). The cells were then centrifuged at 5000 rpm and resuspended in a buffer (containing 1 mm MgATP, 1 mm PMSF, 20% glycerol, 25x protease, 5 mm β-mercaptoethanol (BME)) in PM buffer (50 mm PIPES, 1 mm MgSO 4, 1 mm EGTA, ph 6.9). The cells were sonicated 2 times for 10 secs and centrifuged at 60,000 rpm for 30 mins to remove any insoluble components. The clarified supernatant was then used in these bundling assays. A microtubule mix was prepared from Microtubules (MT). Three concentrations of microtubules were used µm, 0.2 µm and 0.5 µm (1:4 rhodamine to PC tubulin) along with 5 mm MgATP, 0.05 mm Taxol, mg/ml catalase, 0.06 M glucose, 0.24 mg/ml glucose oxidase, 1% BME in PM buffer (50 mm PIPES, 1 mm MgSO 4 and 1 mm EGTA, ph 6.9). 10 µl of cell lysate was mixed with 20 µl of MT mix and flowed into a flow cell. Microtubule-motor interactions were observed using Zeiss Axiovert 8100TV inverted microscope (100x, 1.3 NA oil objective). Microtubules were imaged using Q- Imaging Retiga-SRV camera. The images were recorded using ImagePro Plus
28 Results and Discussion ATK5 is similar in amino acid sequence to ATK2 and ATK3 and is hypothesized to bind to microtubule at the tail domain (Jiang et al. 2007). In order to test the hypothesis that ATK5 bundles microtubules in vitro, ATK5 was expressed in bacterial cells and the bacterial cell lysate was used in bundling assay. A mix of microtubules and ATK5 with ATP was introduced into a flow cell. Fig 2.1 shows fluorescent images of the structures formed for three different microtubule concentrations of ATK5 and KHC. ImageJ analysis of intensities of bundles of microtubules and individual microtubule was done and the number of microtubules involved in a bundle was determined. This analysis was done for all the bundles in a field of view. Finally, a distribution for the number of microtubules in a bundle is plotted in Fig 2.2. ATK5 bundles microtubules in vitro As seen from Fig 2.1, in the presence of ATP as the ratio of microtubule to motor concentration is varied, the microtubule geometry changes from bundles to asters. The number of microtubules incorporated into a bundle also varies with the microtubule to motor ratio. At low microtubule concentration (for fixed ATK5 concentration), almost all the microtubules are involved in small microtubule bundles ( 5 microtubules/bundle). At high microtubule concentrations (0.5 µm) for ATK5, there are small bundles, but almost all the microtubules are involved in asters (see Fig 2.1.a). At low microtubule concentrations (for fixed KHC concentration), there is no definite microtubule structure. 21
29 At high microtubule concentration (0.5 µm) for KHC, almost all the microtubules are involved in bundles (see Fig 2.1.b). Figure 2.1: Microtubule geometry for different microtubule concentration in the presence of ATP a) 0.5 µm MT with ATK5 b) 0.5 µm MT with KHC c) 0.2 µm MT with ATK5 d) 0.2 µm MT with KHC e) 0.13 µm MT with ATK5 f) 0.13 µm MT with KHC Microtubule geometry varies with the change in microtubule concentration as seen above in Fig 2.1. Next, we wanted to determine if the bundle size changes with the microtubule 22
30 concentration. For every condition (i.e. microtubule concentration and motor type), the intensity of the bundle was compared to the intensity of a single microtubule to calculate the number of microtubules in a bundle. This was done for every bundle in the field of view. This analysis gave a distribution for the number of microtubules in a bundle for every motor type and motor concentration as plotted in Fig 2.2. At low microtubule concentrations (0.13 µm and 0.2 µm Microtubules), most of the microtubules are involved in small microtubule bundles ( 5 microtubules in a bundle). As microtubule concentration is increased to 0.5 µm (for both ATK5 and KHC), the number of small bundles reduces and number of larger bundles ( 5 microtubules in a bundle) increases Normalized Frequency No of Microtubules 0.5uM MT ATK5 0.5uM MT KHC 0.2uM ATK5 0.13uM ATK5 Figure 2.2: Distribution of the number of microtubules in a bundle for every motor type and concentration. Fluorescence intensity of every microtubule bundle and single microtubule was measured using ImageJ to obtain the number of microtubules in a bundle. Combining the data from Fig 2.1 and Fig 2.2 we obtain Table 2.1, which gives the distribution of microtubule structures formed by ATK5 and KHC. It can be seen that 23
31 KHC does not form asters for the given microtubule concentrations (0.13 µm, 0.2 µm, 0.5 µm). Whereas at high microtubule concentration (0.5 µm), about 77.3% of the microtubules are involved in bundles. ATK5, on the other hand, forms both bundles and asters. For ATK5, as the microtubule concentration is increased from 0.13µM to 0.2µM and 0.5µM, microtubules form bigger bundles and asters. The density of aster increases with the increase in microtubule concentration i.e. from 0.2 µm to 0.5 µm. The percentage of microtubules involved in bundles falls from 87.5% for 0.13 µm microtubules to 75% for 0.2 µm and 76.3% for 0.5 µm microtubules since microtubules are also involved in the formation of asters. Table 2.1: Distribution of Microtubule structures 0.13µM 0.13 µm 0.2 µm 0.2µM MT 0.5 µm 0.5µM MT ATK5 MT KHC MT ATK5 KHC MT MT KHC ATK5* Percentage of Microtubules in bundles Percentage of Microtubule in Asters Unstructured geometry 87.5% % % 77.3% Yes -- Yes Yes -- Yes
32 Aster Formation In a simulation study done by Surrey and colleagues, a multimeric motor is modeled to be either in free solution or bound to one microtubule or, bound to two microtubules exerting a force on both (Surrey et al. 2001). The simulation study found an inverse correlation between the processivity of a motor and intensity of the aster formed. Also other kinetic parameters such as on and off rates can influence this geometry. We can see from our observations that although KHC is a processive motor, asters are not formed for the given concentrations. However, the above mentioned study simulated oligomeric kinesins, while we used only KHC, which has a microtubule binding domain in the tail (Andrews et al. 1993). Contrastingly, ATK5 which has 83% sequence similarity with ATK1 (Ambrose et al. 2005) formed asters. ATK1 is a minus ended non processive motor (Marcus et al. 2002). Hence, it is reasonable to assume that ATK5 is a non-processive motor. In studies done with non-processive Ncd, it has been found that they act cooperatively (Higuchi and Endow 2002) i.e. when one motor unbinds other Ncd motors bind to the microtubules pushing the microtubule forward (in a gliding assay). Similarly, in this bundling assay, it is possible that the aster formation is due to the cooperativity of ATK5 wherein many ATK5 motors are localized in the aster. Also, the fact that asters are formed in vitro suggests that asters could be formed in vivo too. 25
33 Bundling of Microtubules In our experiments we see that KHC bundles microtubules in vitro at high microtubule concentrations (0.5 µm). Conventional kinesins have been shown to bundle microtubules in vivo. An in vivo live imaging study on fungus Ustilago maydis confirmed the role of conventional kinesin in microtubule bundling (Straube et al. 2006). Their study also indicated that 25% of the times up to 3 microtubules were involved in a bundle which were predominantly straight and only 6% of them were bent (Straube et al. 2006). It has been found that, kinesin in its inactive state folds over to bury the tail domain where the IAK domain in the tail interacts with the head (Hackney and Stock 2000). Kinesin truncated to 960 amino acids (a.a.) folds over due to the presence of IAK domain whereas, truncates till 945 a.a. and 937 a.a. do not have IAK domain leading to unfolding of kinesin molecule and to microtubule binding (Hackney and Stock 2000). The tail of kinesin binds to the microtubule tightly, possibly due to electrostatic interactions with the microtubule. Conventional kinesin is a plus end directed motor, whereas Ncd and ATK5 are minus end directed motors from the Kinesin-14 family. Ncd has two microtubule binding sites and is known to bundle microtubules in vivo through the tail domain (Oladipo et al. 2007). There is a positively charged K-rich region in the tail domain that electrostatically interacts with negatively charged E-hook region of the microtubule (see Fig 2.3) (Furuta and Toyoshima 2008). This electrostatic interaction of Ncd with the microtubule makes Ncd a processive motor under low ionic strength buffers (Furuta and Toyoshima 2008) 26
34 Figure 2.3 Positively charged K-rich region in the tail of Ncd interacts with negatively charged E hook region on the microtubule (Figure reproduced from Furuta and Toyoshima 2008) ATK5 is a mitotic motor which crosslinks microtubules and focuses the spindle pole. It does this by tail/stalk interaction with microtubules (Ambrose et al. 2005). In our experiments we observe that in the presence of ATK5, 2.1% of microtubules are bent and the average angle of the bend is 37.1 o which is more straight compared to microtubules in the presence of KHC (3.3% bent microtubule, 55.3 o angle of bend). Tail of ATK5 ATK5 is known to be a plus end tracker i.e. it remains preferentially attached to plus end of a growing microtubule tip (Ambrose et al. 2005). This preferential attachment to a growing end is through the interaction of the tail/stalk region of ATK5 with the microtubule and not through motor domain. ATK5 has also been implicated in focusing of spindle pole. This is due to the zippering action of ATK5 caused by plus tip 27
35 localization and motor movement towards the minus end. The authors propose that ATK5 can preferentially bind either directly to the microtubule or through a TIP complex (Ambrose et al. 2005). Motor proteins can interact with the growing plus ends by either of three mechanisms active transport to the plus ends by a motor, affinity for the microtubule end structure or by attaching to free tubulin in solution and co-polymerizing to the microtubule end (Cassimeris 2007). From our observations, we see that ATK5 bundles taxol stabilized microtubules. Hence, it is reasonable to conclude that the interaction of the motor to the microtubule is not due to any of the plus tip mechanisms. Rather it is due to direct binding of the motor to the microtubule. Fig 2.4 (a, b, c) below indicates possible scenarios when ATK5 binds tightly and loosely to the plus end. If the tail of the ATK5 is loosely bound to the microtubule (like the electrostatic interaction seen in Ncd (Furuta and Toyoshima 2008)), then we can expect the microtubules to remain straight (see Fig 2.4.b). This is because the motor can translocate and bundle the microtubule. If ATK5 is bound to the plus tip tightly through a protein complex localized at the plus end, then we can expect to see curled microtubules (see Fig 2.4.c.). This is due to anchoring of tail end of the motor on a microtubule while the motor end of the motor moves on another microtubule. What we observe are predominantly straight microtubules and bundling of taxol stabilized microtubules. Hence it is reasonable to conclude that there is weak but direct interaction between the motors and microtubules. 28
36 - Direct binding of tail to the microtubule Plus tip tight binding of ATK5 Figure 2.4.Possible scenarios of ATK5 binding to the microtubule(a) ATK5 starts to walk (bundle) (b) Weak direct binding of motor to the microtubule (c) Tight plus tip binding of ATK5. 29
37 References: Ambrose, J. C. and Cyr, R. (2007). "The kinesin ATK5 functions in early spindle assembly in Arabidopsis." The Plant cell 19(1): Ambrose, J. C.,Li, W.,Marcus, A.,Ma, H. and Cyr, R. (2005). "A minus-end-directed kinesin with plus-end tracking protein activity is involved in spindle morphogenesis." Molecular biology of the cell 16(4): Andrews, S. B.,Gallant, P. E.,Leapman, R. D.,Schnapp, B. J. and Reese, T. S. (1993). "Single kinesin molecules crossbridge microtubules in vitro." Proceedings of the National Academy of Sciences of the United States of America 90(14): Bieling, P.,Laan, L.,Schek, H.,Munteanu, E. L.,Sandblad, L.,Dogterom, M.,Brunner, D. and Surrey, T. (2007). "Reconstitution of a microtubule plus-end tracking system in vitro." Nature 450(7172): Cassimeris, L. (2007). "Tubulin delivery: polymerization chaperones for microtubule assembly?" Developmental cell 13(4): Furuta, K. y. and Toyoshima, Y. Y. (2008). "Minus-end-directed motor Ncd exhibits processive movement that is enhanced by microtubule bundling in vitro." Current biology 18(2): Hackney, D. D. and Stock, M. F. (2000). "Kinesin's IAK tail domain inhibits initial microtubule-stimulated ADP release." Nature cell biology 2(5): Higuchi, H. and Endow, S. A. (2002). "Directionality and processivity of molecular motors." Current opinion in cell biology 14(1): Jiang, S.,Li, M.,Xu, T.,Ren, D. and Liu, G. (2007). "Two kinesins from Arabidopsis, KatB and KatC, have a second microtubule-binding site in the tail domain." Journal of biochemistry and molecular biology 40(1): Karabay, A. and Walker, R. A. (1999). "Identification of microtubule binding sites in the Ncd tail domain." Biochemistry 38(6): Marcus, A. I.,Ambrose, J. C.,Blickley, L.,Hancock, W. O. and Cyr, R. J. (2002). "Arabidopsis thaliana protein, ATK1, is a minus-end directed kinesin that exhibits nonprocessive movement." Cell motility and the cytoskeleton 52(3): Mimori-Kiyosue, Y.,Shiina, N. and Tsukita, S. (2000). "The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules." Current biology 10(14):
38 Mitsui, H.,Nakatani, K.,Yamaguchi-Shinozaki, K.,Shinozaki, K.,Nishikawa, K. and Takahashi, H. (1994). "Sequencing and characterization of the kinesin-related genes katb and katc of Arabidopsis thaliana." Plant molecular biology 25(5): Mitsui, H.,Yamaguchi-Shinozaki, K.,Shinozaki, K.,Nishikawa, K. and Takahashi, H. (1993). "Identification of a gene family (kat) encoding kinesin-like proteins in Arabidopsis thaliana and the characterization of secondary structure of KatA." Molecular & general genetics 238(3): Oladipo, A.,Cowan, A. and Rodionov, V. (2007). "Microtubule motor Ncd induces sliding of microtubules in vivo." Molecular biology of the cell 18(9): Straube, A. A.,Hause, G. G.,Fink, G. G. and Steinberg, G. G. (2006). "Conventional kinesin mediates microtubule-microtubule interactions in vivo." Molecular biology of the cell 17(2): Surrey, T.,Nedelec, F.,Leibler, S. and Karsenti, E. (2001). "Physical properties determining self-organization of motors and microtubules." Science 292(5519): Vale, R. D. and Fletterick, R. J. (1997). "The design plan of kinesin motors." Annu Rev Cell Dev Biol 13:
39 Chapter 3 Spindle assembly in vitro using Dielectrophoresis Introduction In order to exploit the microtubule-motor combination in nanotechnology, there are two main approaches: (i) immobilize motors on the surface and let the microtubules localize themselves, or (ii) immobilize microtubules on the surface and let the motors localize themselves. This selective immobilization is done in order to harness their potential to do directed work. The first approach of selectively immobilizing motors has been achieved by coating a substrate with Polymethylene methacrylate (PMMA) and forming tracks by UV photolithography. Myosin motors were then seen to adsorb only to PMMA tracks and actin motility was observed only on the PMMA tracks (Suzuki et al. 1997). Microtubules similarly have been shown to move along the axis of polytetrafluoroethylene (PTFE) surface functionalized with kinesin (Dennis 1999). Apart from this, lithographically patterned microchannels and rectifiers have been shown to restrict the motion of microtubules (Moorjani 2003; Jia et al. 2004; Huang et al. 2007). The second approach of selectively immobilizing microtubules has been achieved using electric fields, magnetic fields and fluid flow. Microtubules have been functionalized with magnetic particles using biotin-neutravidin interaction and aligned in magnetic fields (Platt et al. 2005). However, use of this sort of alignment for creating artificial spindle is limited due to the reduction of kinesin-microtubule interaction owing to bulkiness of neutravidin. This issue has been addressed using segmented microtubules,
40 allowing greater probability of kinesin to interact with unlabeled microtubule segment (Hutchins et al. 2007). Another approach has been to use fluid flow to align microtubule seeds which then subsequently interact with the surface (Brown 2002). For our goal of creating an artificial spindle, we need to assemble an array of anti-parallel microtubules such that the plus ends of the microtubules face each other. Minus ends of the microtubules needs to be secured and be farthest away (see schematic in Fig 3.1). To achieve this end, we build this work on an earlier work on dielectrophoresis and neutravidin patterning (Huang et al. 2008, Uppalapati et al. 2008). In this work by (Huang et al. 2008) flow cells were formed on top of chrome electrodes and neutravidin was adsorbed in the flow cell area. This neutravidin was patterned by irradiating the backside of the quartz sample with UV. This ensured that neutravidin was present only on the electrode (Huang et al. 2008). They were also successful in assembling microtubules at the tip of two opposing electrodes by dielectrophoresis (DEP) (Uppalapati et al. 2008). Electrode Electrode Figure 3.1: Schematic of artificial spindle formed on neutravidin coated electrodes and segmented microtubueles (Cy5 seeds (blue) and rhodamine extensions (purple)) 33
41 In the present work, we use dielectrophoresis and neutravidin patterning to assemble and bind biotinylated microtubules at the electrode tip. We study the kinetics of microtubule assembly by taking successive images of spindle assembly and analyzing them off-line. We have optimized the patterning process for microtubule assembly. Finally, in order to obtain overlapping microtubules at the electrode gap, we extend the microtubules in the flow cell. Even though microtubules assemble at the electrode tip when the field is on, binding of the microtubules is poor when the field is switched off. This is probably due to the erosion of the electrode with repeated use or due to accumulation of debris. This microtubule binding problem was solved by using new electrodes. With new electrodes we were able to obtain good microtubule binding and extension. Microtubule assembly followed a linear increase with time. 34
42 Materials and Methods Preparing microtubules Purified tubulin was obtained from bovine brains (Williams and Lee 1982). The tubulin was then labeled with rhodamine, biotin and Cy5 as described previously (Hyman 1991). N-ethylmaleimide (NEM) labeled tubulin binds to the minus ends of microtubules, blocking polymerization from the minus ends but does not affect plus end polymerization (Phelps and Walker 2000). To prepare NEM tubulin, 62.9 µm cycled tubulin was incubated with 2.5 mm NEM and 1.25 mm GTP on ice for 10 mins. The reaction was quenched with 14 mm β-mercaptoethanol. Biotin tubulin was mixed with Cy5 tubulin in a 1:4 ratio and polymerized for 20 mins at 37 o C along with 4 mm MgCl 2, 1 mm GTP, 5% DMSO in BRB80 buffer (80 mm PIPES, 1 mm MgCl 2 and 1 mm EGTA). The biotin seeds were then diluted 100 fold along with 10 µm Paclitaxel. 150 µl of biotin seeds were spun for 10 mins in Beckman Airfuge at 300 psi. Spun seeds were then resuspended in a solution containing 1.25 mm GTP, 5% DMSO, 4 mm MgCl 2, 6.29 µm NEM tubulin and RTU (40 µm of 1:4, rhodamine tubulin:cycled tubulin). This solution was incubated at 37 o C for 20 mins and stabilized with 10 µm Paclitaxel. For the extension experiments, biotin seeds as were immobilized on the electrode using DEP (Uppalapati et al. 2008). The seeds were then extended with an extension solution (RTU (10 µm of 1:4, rhodamine tubulin:cycled tubulin), 1.25 mm GTP, 5% DMSO, 4 mm MgCl 2 ) in BRB80 (80 mm PIPES, 1 mm MgCl 2 and 1 mm EGTA). 35
43 Sample Preparation The electrode samples were cleaned in Piranha (1:4 Hydrogen peroxide : Sulfuric acid) for 10 mins. They were then exposed to UV-Ozone for 5 mins. They were then treated using 5% 3-aminoproyltriethoxysilane (APTES) for 30 mins. The hydroxyl groups on the quartz surface form a covalent bond with APTES by displacing the alkoxy groups. The sample was then washed with acetone and DI water and then blow dried. 30 µl of 5% gluteraldehyde was placed on top of the active electrode region and an 18 mm coverslip was placed on top of the gluteraldehyde forming a squash cell (approx µm high) for 15 mins. The aldehyde groups on gluteraldehyde crosslink amine groups on the neutravidin and APTES, thus stably crosslinking neutravidin to the glass surface. After washing the sample with DI water and drying it, neutravidin was similarly incubated for 15 mins under a squash cell. The sample was then washed in a bath containing BRB6 and dried in a bath containing acetone for 30 secs and finally blown dried. Neutravidin was patterned by exposing the backside of the quartz sample with deep UV in the presence of Nitrogen for 110secs using the electrodes as the mask (Huang et al. 2008). Dielectrophoresis experiment A flow cell was created on top of the electrodes and 0.5 mg/ml casein was introduced for surface blocking. The microtubule solution containing 20 mm glucose, 0.02 mg/ml glucose oxidase, mg/ml catalase, 0.5 mg/ml casein, paclitaxel (10µM), 0.35 M β- Mercaptoethanol and segmented microtubule was introduced into the flow cell. A 5MHz 36
44 field was applied across the electrodes (BK Precision, CA) (Uppalapati et al. 2008). Spindle formation was observed using Nikon eclipse E600 microscope (60x, water immersion objective). The images and videos were taken using Photometrics cascade 512B camera and MetaVue Imaging software. 37
45 Results and Discussion In order to construct artificial spindles, we used dielectrophoresis to assemble microtubules and patterned electrodes to selectively bind the microtubules to the electrodes. The electrode surface was selectively coated with neutravidin by backside exposure of the quartz sample to UV (Huang et al. 2008). We made segmented microtubules by growing extensions of rhodamine tubulin from the Cy5-biotinylated seeds which are capped at their minus ends by NEM tubulin. The segmented microtubules were then injected into a flow cell and then the electric field was turned on and the accumulation was visualized under Nikon fluorescence microscope. Fig 3.2 shows the accumulation of segmented microtubules at the tip of the electrode when an AC field (5MHz, 22V) was applied to the electrodes. Electrode Figure 3.2: Accumulation of segmented microtubules at the tip of the electrode when AC field is applied to the electrodes. Cy5 biotin seeds are in green and rhodamine extensions are in red 38
46 Spindle assembly If the microtubules assembled in the right polarities then we would see (green) Cy5 seeds at the electrode tip and (red) rhodamine extensions at the gap. However, we can see from Fig 3.2 that it is difficult to distinguish between the polarities of microtubules when the field is on. The biotin-cy5 seeds are capped at their minus end with NEM tubulin and hence the rhodamine extensions which are seen in Fig 3.3 and Fig 3.4 are from their plus ends only. When the field is turned off, we can be almost sure to obtain only plus ends at the gap. Since the whole flow cell is coated with casein, only those microtubules that have biotin at their tip remain bound to the neutravidin coated electrode. However, we observe that relatively shorter microtubules remain bound at the electrode tip when field is turned off (see Fig 3.3). Figure 3.3: Spindle assembly on neutravidin coated electrodes formed by DEP. Biotin seeds labeled with Cy5 are in red and rhodamine tubulin extensions are in green. 39
47 One way to quantify and confirm the formation of spindle is by measuring the intensity of Cy5 seeds and rhodamine extensions. Ideally, if the segmented microtubules immobilized on the electrodes with minus ends on the electrode and plus ends at the gap, then we expect to see Cy5 intensity peak near the electrode followed by rhodamine intensity peak. Intensity distributions of rhodamine and Cy5 were obtained from ImageJ by drawing a line along the length of the electrode through the gap and measuring intensities along the line (Fig 3.4). Intensities were normalized to the peak intensity value and background noise was subtracted for each filter. Normalized Intensity profile Fluorescence Intensity Electrode _ Along the electrode gap _ Electrode Cy5 seeds Rhodamine extension Figure 3.4: Intensity of rhodamine extension and cy5 seed along the electrode gap. The fluorescence intensity of Cy5 (blue) and Rhodamine (purple) were normalized to the peak intensity value and corrected for background noise. Gap between the electrodes is 20 µm. 40
48 The profile of fluorescence intensity of Cy5 tubulin shows an intensity peak near the electrode indicating the presence of seeds located near the electrode (Fig 3.4). The intensity distribution of rhodamine is wider near the electrodes compared to Cy5 intensity distribution, indicating a longer rhodamine extension compared to the Cy5 seeds. We see overlapping Cy5 and rhodamine intensities indicating non uniform distribution of seed and extension lengths. Overlap is probably due to rhodamine extensions having very small seeds. Thus, we have shown that spindle can be assembled with minus ends at the electrode and plus ends near the gap. Spindle kinetics In order to measure the spindle assembly kinetics, video of microtubule accumulation was recorded as soon as the electric field was applied to the electrodes. The kinetics of spindle assembly was obtained by measuring the intensity of microtubules assembled at the electrode tip. This was done by measuring the fluorescence intensities along the line drawn at the electrode tip using ImageJ. Fig 3.5.a shows a montage of microtubule assembly at various time points. 41
49 a 1500 Fluorescence intensity b Time (sec) Figure 3.5 (a) Snapshot of microtubule accumulation on the electrode at different times (rhodamine filter). Each frame is separated by 19.9 seconds. (b) Accumulation of microtubule at the electrode tip is observed as soon as the field is turned on. The Y axis shows relative fluorescence intensities. Microtubule accumulation at the electrode tips was quantified by measuring the intensity of fluorescence (microtubule accumulation) for series of images using ImageJ. The fluorescence intensity, which is a measure of number of microtubules, is plotted in Fig 3.5.b indicating assembly kinetics of microtubules. Electroosmotic and electrothermal flows as well as DEP forces are generated as soon as the AC electric field is applied (Uppalapati et al. 2008). Hence, it is expected that microtubules will accumulate at the electrode tip linearly with time as soon as the electric field is applied. However, we see a small time lag of 5 secs as soon as the field is turned on. This could be the time the 42
50 microtubules take to accumulate near the electrode surface from the bulk solution. As the microtubules accumulate on the tip of the electrode we expect to see the fluorescence intensity to level due to depletion of microtubules from the solution and/or crowding of microtubules at the electrode. We do observe a moderate plateau after 110 secs indicating saturation of microtubule accumulation on the electrodes. Although, antifade was used along with the microtubules, the effect of photobleaching cannot be ruled out and hence it possible the plateau seen in Fig 3.5 is due to photobleaching of the fluorophores. But frankly, we are do not fully understand the plateau. Age of the electrode Although microtubules are efficiently assembled at the electrode tip by DEP forces, many microtubules diffused away from the electrode once the electric field is turned off (Fig 3.6). This could be due to the presence of non-segmented microtubules and/or nonbiotinylated seeds. Non-biotinylated seeds and non-segmented microtubules are attracted by DEP to the electrode, but they do not bind to the neutravidin coated electrodes. Biotinylated microtubules can also diffuse away due to insufficient neutravidin densities on the electrodes. 43
51 Figure 3.6: Time series of microtubules unbinding from the electrode tips after the electric field is turned off To understand this unbinding issue, we did control experiments on glass slides. Samples were coated with APTES followed by gluteraldehyde and then neutravidin. To check for effect of neutravidin treatment, we compared binding between dried neutravidin surface and un-dried neutravidin surface. Effect of patterning was studied by exposing part of the neutravidin coated glass slide to UV by covering the other half with a quartz coverslip. Binding was studied between the exposed and the unexposed regions of the control sample. We also checked for binding differences between the biotin seeds and the segmented microtubules on dried neutravidin coated glass surfaces. Although the number of bound segmented microtubule (35 microtubules per screen) is slightly less than the number of biotin seeds (50 microtubules per screen), it could be due to non-extension of 44
52 some seeds. Binding of segmented microtubules on acetone dried neutravidin surface also did not vary much from non-dried neutravidin surface. Patterning neutravidin also did not affect the microtubule binding (Table 3.1). However, we did see considerable difference in microtubule binding between new and old quartz sample (see Fig 3.7). Table 3.1: Some control experiments to decide the most important factor in spindle formation (Results are in Number of Microtubules/screen) Sample preparation process Control With the process Drying with acetone Patterning under UV (Unexposed electrode region) This difference in binding between old and new electrodes (Fig 3.7) could be due to insufficient binding of neutravidin to the electrodes. In turn, neutravidin binding could be affected by electrode surface roughness due to pitting. Neutravidin binding could also be affected either due to debris on the electrode or due to electrode thinning (due to electrolysis). Hence, an important factor determining binding is the age of the quartz/electrode sample. a b Figure 3.7: Difference between binding of biotin seeds in (a) the old electrode and (b) the new electrode after electric field is turned off 45
53 Microtubule extension in the flow cell Although we obtained microtubule accumulation with minus ends at the electrode tip and plus ends at the gap, the structure still doesn t resemble a spindle since the microtubules do not overlap. To solve this problem, we set out to bind biotinylated microtubules to the electrodes and polymerize microtubule extensions off of them in the flow cell (Brown 2002). First we immobilized only biotin seeds at the electrode tip using the standard dielectrophoresis procedure. Next, we introduced rhodamine tubulin (1mg/ml in BRB80) in the presence of 4 mm MgCl 2, 1 mm GTP, 5% DMSO. After 10mins, the flow cell is washed with BRB80 to remove the background fluorescence. At room temperature, we see long microtubules that span the 20 µm electrode gap. Hence, the polymerization works to obtain microtubules that are sufficiently long to span the electrode gap. However, one issue with this approach is the skewing of microtubules in the direction of the flow (Fig 3.8). As the solutions (extension solution and wash) are introduced into the flow cell, the electrode-bound microtubules are bent in the direction of the flow (perpendicular to the long axis of the electrode) giving rise to a skewed spindle. Efforts are underway in the Hancock lab to optimize introducing solutions through a syringe pump into the flow cell. This would then facilitate to have straight spindles since the spindles would then experience less force. 46
54 Figure 3.9: Extension of biotin seeds (red) in the flow cell with rhodamine tubulin (green). Biotin seeds are immobilized on the neutravidin coated electrode using DEP. Extension solution is introduced after the field is turned off. Thus, we have shown that assembly and dynamic growth of the mitotic spindle is possible in vitro. This has been achieved by the combination of AC electrokinetic techniques and protein patterning. A further development of this project would involve introducing motors like ATK5 to the artificial spindle setup and study the spindle dynamics and structure. 47
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Self assembly and organization of nanofibers using biological molecular motors
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Self assembly and organization of nanofibers using biological molecular motors Presented by: Jeffrey M. Catchmark Assistant
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
Section 10. Junaid Malek, M.D.
 Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
Chapter 3. DNA Replication & The Cell Cycle
 Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
The Pennsylvania State University. The Graduate School. Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF
 The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering
The Pennsylvania State University The Graduate School Department of Bioengineering CONTROLLED ASSEMBLY OF MICROTUBULES AND MANIPULATION OF KINESIN DRIVEN MICROTUBULE MOTION A Dissertation in Bioengineering
CELL BIOLOGY - CLUTCH CH CYTOSKELETON AND CELL MOVEMENT.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
!! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
Three major types of cytoskeleton
 The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
Cell cycle oscillations
 Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Development Team. Molecular Cell Biology Cytoskeleton ZOOLOGY. Head, Department of Zoology, University of Delhi
 Paper No. : 15 Module : 11 Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Paper Coordinator: Prof. S. Basu Modak Department of Zoology, University
Paper No. : 15 Module : 11 Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Paper Coordinator: Prof. S. Basu Modak Department of Zoology, University
The Pennsylvania State University. The Graduate School. The College of Engineering INTEGRATED ELECTRONICS AND FLUIDIC MEMS FOR BIOENGINEERING
 The Pennsylvania State University The Graduate School The College of Engineering INTEGRATED ELECTRONICS AND FLUIDIC MEMS FOR BIOENGINEERING A Dissertation in Electrical Engineering by Ho Him Raymond Fok
The Pennsylvania State University The Graduate School The College of Engineering INTEGRATED ELECTRONICS AND FLUIDIC MEMS FOR BIOENGINEERING A Dissertation in Electrical Engineering by Ho Him Raymond Fok
CYTOSKELETON, MOTORPROTEINS.
 CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation
 A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
A Polarized Microtubule Array for Kinesin-Powered Nanoscale Assembly and Force Generation NANO LETTERS 2002 Vol. 2, No. 10 1131-1135 Timothy B. Brown and William O. Hancock* Department of Bioengineering,
Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules
 Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Aligning Bacterial Cellulose
 2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
2006 International Conference on Nanotechnology, April 26-28, 2006 Atlanta, GA Aligning Bacterial Cellulose Nicole R. Brown Assistant Professor The School of Forest Resources The Materials Research Institute
Biophysics of Molecules
 Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Practice Exam 3 MCBII
 1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
Nanotechnological Applications of Biomolecular Motor Systems. Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden
 Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Nanotechnological Applications of Biomolecular Motor Systems Stefan Diez Max-Planck-Institute of Molecular Cell Biology and Genetics Dresden Max-Planck-Institute of Molecular Cell Biology and Genetics
Fluorescence Imaging with One Nanometer Accuracy Lab
 I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
I. Introduction. Fluorescence Imaging with One Nanometer Accuracy Lab Traditional light microscope is limited by the diffraction limit of light, typically around 250 nm. However, many biological processes
7.06 Problem Set Four, 2006
 7.06 Problem Set Four, 2006 1. Explain the molecular mechanism behind each of the following events that occur during the cell cycle, making sure to discuss specific proteins that are involved in making
7.06 Problem Set Four, 2006 1. Explain the molecular mechanism behind each of the following events that occur during the cell cycle, making sure to discuss specific proteins that are involved in making
Supplementary Figures 1-6
 1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only)
 Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only) I. Protein Synthesis: creation of new proteins a. Much of the cellular machinery is devoted to synthesizing
Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only) I. Protein Synthesis: creation of new proteins a. Much of the cellular machinery is devoted to synthesizing
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules. Dr. Carissa M. Soto
 Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
Towards Single Molecule Detection of SEB A Mobile Sandwich Immunoassay on Gliding Microtubules Dr. Carissa M. Soto March 8, 2008 Dr. Kim E. Sapsford, Dr. Brett D. Martin, Dr. Amy Szuchmacher Blum, and
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NPLANTS.2015.111 The non-processive rice kinesin-14 OsKCH1 transports actin filaments along microtubules with two distinct velocities Figure S1 The OsKCH1 constructs used in this study Schematic
DOI: 10.1038/NPLANTS.2015.111 The non-processive rice kinesin-14 OsKCH1 transports actin filaments along microtubules with two distinct velocities Figure S1 The OsKCH1 constructs used in this study Schematic
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Biosensors. DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses)
 Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Kinesin motors in the transport along microtubules. Dynein Motor
 Molecular Cell Biology Autumn 2014, Michael Pavlov Based on Chapter 17 and 18.(1-4), MCB book Lecture 9: Cytoskeleton Microtubules: Tubulin Structure of tubulin dimer and organization of tubulin dimers
Molecular Cell Biology Autumn 2014, Michael Pavlov Based on Chapter 17 and 18.(1-4), MCB book Lecture 9: Cytoskeleton Microtubules: Tubulin Structure of tubulin dimer and organization of tubulin dimers
Microtubule Cytoskeleton and Cell Patterning
 Microtubule Cytoskeleton and Cell Patterning 10:00 Anna Akhmanova Microtubule Dynamics 11:00 Lukas Kapitein Motors and Transport 12:00 12:30 Microscopy Facility Tour 13:00-14:30 Lunch and Self-study Jacobson
Microtubule Cytoskeleton and Cell Patterning 10:00 Anna Akhmanova Microtubule Dynamics 11:00 Lukas Kapitein Motors and Transport 12:00 12:30 Microscopy Facility Tour 13:00-14:30 Lunch and Self-study Jacobson
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Nano-Scale Engineering III Bio-Molecular Motors for Engineering
 Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
Nano-Scale Engineering III Bio-Molecular Motors for Engineering Y. C. Lee Department of Mechanical Engineering University of Colorado Boulder, CO 80309-0427 leeyc@colorado.edu March 4, 2014 1 MLD of Hybrid
Thursday 26 May 2016 Afternoon Time allowed: 1 hour 30 minutes
 Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature AS BIOLOGY Paper 1 Thursday 26 May 2016 Afternoon Time allowed: 1 hour 30 minutes Materials
Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature AS BIOLOGY Paper 1 Thursday 26 May 2016 Afternoon Time allowed: 1 hour 30 minutes Materials
EFFECTS OF CYSTEINE MODIFICATION ON MICROTUBULE-MOTOR FUNCTION AND TUBULIN ASSEMBLY. by Kalmia Kniel Phelps
 EFFECTS OF CYSTEINE MODIFICATION ON MICROTUBULE-MOTOR FUNCTION AND TUBULIN ASSEMBLY by Kalmia Kniel Phelps Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in
EFFECTS OF CYSTEINE MODIFICATION ON MICROTUBULE-MOTOR FUNCTION AND TUBULIN ASSEMBLY by Kalmia Kniel Phelps Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in
MATERIAL DATA SHEET. Reagents Provided in Kit
 Lot # XXXXX MATERIAL DATA SHEET HSP70/HSP40 Glow-Fold Protein Refolding Kit Cat. # K-290 Heat shock proteins (HSPs) are a family of highly conserved stress response proteins. Heat shock proteins function
Lot # XXXXX MATERIAL DATA SHEET HSP70/HSP40 Glow-Fold Protein Refolding Kit Cat. # K-290 Heat shock proteins (HSPs) are a family of highly conserved stress response proteins. Heat shock proteins function
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true?
 Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true? A. COPII and COPI vesicles are transported with dynein. B. COPII and COPI vesicles
Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true? A. COPII and COPI vesicles are transported with dynein. B. COPII and COPI vesicles
REVIEW SHEET: Units 9 & 10 Cell Cycle, DNA, & Gene Expression
 REVIEW SHEET: Units 9 & 10 Cell Cycle, DNA, & Gene Expression HONORS BIOLOGY Textbook Reading: Cell Cycle (Ch. 10.1 and 10.2), DNA (Ch. 12), and Gene Expression (Ch. 13) Handouts:! Online Tutorial: Cell
REVIEW SHEET: Units 9 & 10 Cell Cycle, DNA, & Gene Expression HONORS BIOLOGY Textbook Reading: Cell Cycle (Ch. 10.1 and 10.2), DNA (Ch. 12), and Gene Expression (Ch. 13) Handouts:! Online Tutorial: Cell
Chapter 15. Cytoskeletal Systems. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Characterizing the cargo binding and regulatory function of the tail domain in Ncd motor protein
 Characterizing the cargo binding and regulatory function of the tail domain in Ncd motor protein Natalie E. Lonergan Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University
Characterizing the cargo binding and regulatory function of the tail domain in Ncd motor protein Natalie E. Lonergan Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University
Supplemental Data Length Control of the Metaphase Spindle
 Supplemental Data Length Control of the Metaphase Spindle S1 Gohta Goshima, Roy Wollman, Nico Stuurman, Jonathan M. Scholey, and Ronald D. Vale Supplemental Experimental Procedures Cell Culture, RNA Interference,
Supplemental Data Length Control of the Metaphase Spindle S1 Gohta Goshima, Roy Wollman, Nico Stuurman, Jonathan M. Scholey, and Ronald D. Vale Supplemental Experimental Procedures Cell Culture, RNA Interference,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material THE JOURNAL OF CELL BIOLOGY Toso et al., http://www.jcb.org/cgi/content/full/jcb.200809055/dc1 Figure S1. Control experiments for sirna depletions used in Figs. 1 3. The phenotype
Supplemental Material THE JOURNAL OF CELL BIOLOGY Toso et al., http://www.jcb.org/cgi/content/full/jcb.200809055/dc1 Figure S1. Control experiments for sirna depletions used in Figs. 1 3. The phenotype
Plant Molecular and Cellular Biology Lecture 9: Nuclear Genome Organization: Chromosome Structure, Chromatin, DNA Packaging, Mitosis Gary Peter
 Plant Molecular and Cellular Biology Lecture 9: Nuclear Genome Organization: Chromosome Structure, Chromatin, DNA Packaging, Mitosis Gary Peter 9/16/2008 1 Learning Objectives 1. List and explain how DNA
Plant Molecular and Cellular Biology Lecture 9: Nuclear Genome Organization: Chromosome Structure, Chromatin, DNA Packaging, Mitosis Gary Peter 9/16/2008 1 Learning Objectives 1. List and explain how DNA
SUPPLEMENTARY INFORMATION
 Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants NATURE PLANTS www.nature.com/natureplants 1 2 NATURE PLANTS www.nature.com/natureplants NATURE PLANTS
Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants NATURE PLANTS www.nature.com/natureplants 1 2 NATURE PLANTS www.nature.com/natureplants NATURE PLANTS
Docking Studies of the Binding Mode of Dictyostatin and Its Analogues to the Taxoid Binding Site on Tubulin
 Docking Studies of the Binding Mode of Dictyostatin and Its Analogues to the Taxoid Binding Site on Tubulin Christopher B. Hackmeyer Spring Hill College Billy W. Day, Ph.D. Department of Pharmaceutical
Docking Studies of the Binding Mode of Dictyostatin and Its Analogues to the Taxoid Binding Site on Tubulin Christopher B. Hackmeyer Spring Hill College Billy W. Day, Ph.D. Department of Pharmaceutical
Cytoskeleton. B. Balen
 Cytoskeleton B. Balen structural backbone of the cell supports the fragile plasma membrane provides mechanical linkages that let the cell bear stresses without being ripped apart Importance and function
Cytoskeleton B. Balen structural backbone of the cell supports the fragile plasma membrane provides mechanical linkages that let the cell bear stresses without being ripped apart Importance and function
1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called.
 Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
me239 mechanics of the cell homework biopolymers - motivation 3.1 biopolymers - motivation 3. biopolymers biopolymers biopolymers
 3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler
 Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler 23.10. Zelle 30.10. Biologische Makromoleküle I 06.11. Biologische Makromoleküle II 13.11. Nukleinsäuren-Origami (DNA, RNA) 20.11.
Vorlesung Biophysik I - Molekulare Biophysik Kalbitzer/Kremer/Ziegler 23.10. Zelle 30.10. Biologische Makromoleküle I 06.11. Biologische Makromoleküle II 13.11. Nukleinsäuren-Origami (DNA, RNA) 20.11.
C. Incorrect! Second Law: Law of Independent Assortment - Genes for different traits sort independently of one another in the formation of gametes.
 OAT Biology - Problem Drill 20: Chromosomes and Genetic Technology Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick
OAT Biology - Problem Drill 20: Chromosomes and Genetic Technology Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension
 Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Bionanomechanics with Optical Tweezers: Molecular Machines under Tension Erik Schäffer Center for Plant Molecular Biology (ZMBP) University of Tübingen, Germany www.zmbp.uni-tuebingen.de/nano 16 April
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Colchicine. Colchicine. a b c d e
 α1-tub Colchicine Laulimalide RB3 β1-tub Vinblastine α2-tub Taxol Colchicine β2-tub Maytansine a b c d e Supplementary Figure 1 Structures of microtubule and tubulin (a)the cartoon of head to tail arrangement
α1-tub Colchicine Laulimalide RB3 β1-tub Vinblastine α2-tub Taxol Colchicine β2-tub Maytansine a b c d e Supplementary Figure 1 Structures of microtubule and tubulin (a)the cartoon of head to tail arrangement
Prof. R. V. Skibbens. Cell Cycle, Cell Division and Cancer
 Prof. R. V. Skibbens November 18, 2010 BIOS 10: BioScience in the 21 st Century Cell Cycle, Cell Division and Cancer 4 Stages of the cell cycle: Genome segregation Interphase DNA Microtubules Microtubules
Prof. R. V. Skibbens November 18, 2010 BIOS 10: BioScience in the 21 st Century Cell Cycle, Cell Division and Cancer 4 Stages of the cell cycle: Genome segregation Interphase DNA Microtubules Microtubules
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology
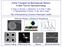 Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
Active Transport by Biomolecular Motors: A New Tool for Nanotechnology H. Hess, C. Brunner, J. Clemmens, K. H. Ernst, T. Nitta, S. Ramachandran, R.Tucker, D. Wu and V. Vogel Dep. of Bioengineering, University
Complications Dawn for Kinetochore Regulation by Aurora
 Complications Dawn for Kinetochore Regulation by Aurora The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Nannas, Natalie
Complications Dawn for Kinetochore Regulation by Aurora The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Nannas, Natalie
Vocab Word 1: Interphase
 Vocab Word 1: Interphase Interphase is the phase of the cell cycle in which a typical cell spends most of its life. During this phase, the cell copies its DNA in preparation for mitosis. Interphase is
Vocab Word 1: Interphase Interphase is the phase of the cell cycle in which a typical cell spends most of its life. During this phase, the cell copies its DNA in preparation for mitosis. Interphase is
The Pennsylvania State University. The Graduate School. College of Engineering MICRO-SCALE HYBRID BIOLOGICAL-ENGINEERED DEVICES
 The Pennsylvania State University The Graduate School College of Engineering MICRO-SCALE HYBRID BIOLOGICAL-ENGINEERED DEVICES POWERED BY BIOMOLECULAR MOTORS A Thesis in Electrical Engineering by Ying-Ming
The Pennsylvania State University The Graduate School College of Engineering MICRO-SCALE HYBRID BIOLOGICAL-ENGINEERED DEVICES POWERED BY BIOMOLECULAR MOTORS A Thesis in Electrical Engineering by Ying-Ming
Mechanisms for focusing mitotic spindle poles by minus end directed motor proteins
 JCB: ARTICLE Mechanisms for focusing mitotic spindle poles by minus end directed motor proteins Gohta Goshima, 1 François Nédélec, 2 and Ronald D. Vale 1 1 The Howard Hughes Medical Institute and the Department
JCB: ARTICLE Mechanisms for focusing mitotic spindle poles by minus end directed motor proteins Gohta Goshima, 1 François Nédélec, 2 and Ronald D. Vale 1 1 The Howard Hughes Medical Institute and the Department
EPIGENTEK. EpiQuik Chromatin Immunoprecipitation Kit. Base Catalog # P-2002 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE
 EpiQuik Chromatin Immunoprecipitation Kit Base Catalog # P-2002 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Chromatin Immunoprecipitation Kit is suitable for combining the specificity of
EpiQuik Chromatin Immunoprecipitation Kit Base Catalog # P-2002 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Chromatin Immunoprecipitation Kit is suitable for combining the specificity of
Rotary DNA Motors INTRODUCTION THE FLASHING FIELD MODEL
 Biophysical Journal Volume 69 December 1995 2256-2267 Rotary DNA Motors Charles Doering,* Bard Ermentrout,* and George 0ster "Center for Nonlinear Studies, Los Alamos National Laboratory, Los Alamos, New
Biophysical Journal Volume 69 December 1995 2256-2267 Rotary DNA Motors Charles Doering,* Bard Ermentrout,* and George 0ster "Center for Nonlinear Studies, Los Alamos National Laboratory, Los Alamos, New
MATERIAL DATA SHEET. Reagents Provided in Kit
 Lot # XXXXX MATERIAL DATA SHEET HSP60/HSP10 Glow-Fold Protein Refolding Kit Cat. # K-300 HSP60 (also known as Chaperonin 60) and HSP10 (also known as Chaperonin 10) are the eukaryotic homologues of prokaryotic
Lot # XXXXX MATERIAL DATA SHEET HSP60/HSP10 Glow-Fold Protein Refolding Kit Cat. # K-300 HSP60 (also known as Chaperonin 60) and HSP10 (also known as Chaperonin 10) are the eukaryotic homologues of prokaryotic
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
doi:10.1038/nature10016 Supplementary discussion on binding site density for protein complexes on the surface: The density of biotin sites on the chip is ~10 3 biotin-peg per µm 2. The biotin sites are
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
3. To understand the cellular function of a protein by means of a reconstitution assay, it is important to also include interacting proteins.
 Propositions 1. Growth-limited sliding is an efficient way to maintain microtubule overlaps. (This thesis) 2. Directional switching by motor proteins can be used to control the length of microtubule overlaps.
Propositions 1. Growth-limited sliding is an efficient way to maintain microtubule overlaps. (This thesis) 2. Directional switching by motor proteins can be used to control the length of microtubule overlaps.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/333/6041/456/dc1 Supporting Online Material for Cilia-Like Beating of Active Microtubule Bundles Timothy Sanchez, David Welch, Daniela Nicastro, Zvonimir Dogic* *To
www.sciencemag.org/cgi/content/full/333/6041/456/dc1 Supporting Online Material for Cilia-Like Beating of Active Microtubule Bundles Timothy Sanchez, David Welch, Daniela Nicastro, Zvonimir Dogic* *To
A smart dust biosensor powered by kinesin motors
 Supplementary Information (SI) to accompany A smart dust biosensor powered by kinesin motors Thorsten Fischer, Ashutosh Agarwal and Henry Hess* *Henry Hess University of Florida Department of Materials
Supplementary Information (SI) to accompany A smart dust biosensor powered by kinesin motors Thorsten Fischer, Ashutosh Agarwal and Henry Hess* *Henry Hess University of Florida Department of Materials
Cells and Tissues. Overview CELLS
 Cells and Tissues WIll The basic unit of structure and function in the human body is the cell. Each of a cell's parts, or organelles, as well as the entire cell, is organized to perform a specific function.
Cells and Tissues WIll The basic unit of structure and function in the human body is the cell. Each of a cell's parts, or organelles, as well as the entire cell, is organized to perform a specific function.
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Localization of the Kinesin-like Protein Xklp2 to Spindle Poles Requires a Leucine Zipper, a Microtubule-associated Protein, and Dynein
 Localization of the Kinesin-like Protein Xklp2 to Spindle Poles Requires a Leucine Zipper, a Microtubule-associated Protein, and Dynein Torsten Wittmann,* Haralabia Boleti, Claude Antony, Eric Karsenti,*
Localization of the Kinesin-like Protein Xklp2 to Spindle Poles Requires a Leucine Zipper, a Microtubule-associated Protein, and Dynein Torsten Wittmann,* Haralabia Boleti, Claude Antony, Eric Karsenti,*
Activity Subject Area(s) Associated Unit Associated Lesson Activity Title Header Image 1 ADA Description: Caption: Image file: Source/Rights:
 Activity Subject Area(s) Biology Associated Unit Associated Lesson Activity Title Breaking News: Molecular Trucks Riding Inside Cells! Header Image 1 ADA Description: An ant carries a 1 millimeter square
Activity Subject Area(s) Biology Associated Unit Associated Lesson Activity Title Breaking News: Molecular Trucks Riding Inside Cells! Header Image 1 ADA Description: An ant carries a 1 millimeter square
CELLULAR PROCESSES; REPRODUCTION. Unit 5
 CELLULAR PROCESSES; REPRODUCTION Unit 5 Cell Cycle Chromosomes and their make up Crossover Cytokines Diploid (haploid diploid and karyotypes) Mitosis Meiosis What is Cancer? Somatic Cells THE CELL CYCLE
CELLULAR PROCESSES; REPRODUCTION Unit 5 Cell Cycle Chromosomes and their make up Crossover Cytokines Diploid (haploid diploid and karyotypes) Mitosis Meiosis What is Cancer? Somatic Cells THE CELL CYCLE
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Ono et al., http://www.jcb.org/cgi/content/full/jcb.201208008/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Chromatin-binding properties of MCM2 and condensin II during
Supplemental material Ono et al., http://www.jcb.org/cgi/content/full/jcb.201208008/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Chromatin-binding properties of MCM2 and condensin II during
Unit 4 Information NUCLEIC ACIDS DNA GENES DOUBLE HELIX REPLICATION MITOSIS
 Unit 4 Information NUCLEIC ACIDS DNA GENES DOUBLE HELIX REPLICATION MITOSIS 1/2/2018 Bell Work What is a Gene? Genes as Medicine As you watch the film, complete the questions on the film guide. When you
Unit 4 Information NUCLEIC ACIDS DNA GENES DOUBLE HELIX REPLICATION MITOSIS 1/2/2018 Bell Work What is a Gene? Genes as Medicine As you watch the film, complete the questions on the film guide. When you
Final exam. Please write your name on the exam and keep an ID card ready.
 Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
GCE. Edexcel GCE Biology / Biology (Human) (6101/01) January Edexcel GCE. Mark Scheme (Results) Biology / Biology (Human) (6101/01)
 GCE Edexcel GCE Biology / Biology (Human) (6101/01) January 2006 Mark Scheme (Results) Edexcel GCE Biology / Biology (Human) (6101/01) Symbols used in the mark scheme Symbol General Principles Meaning
GCE Edexcel GCE Biology / Biology (Human) (6101/01) January 2006 Mark Scheme (Results) Edexcel GCE Biology / Biology (Human) (6101/01) Symbols used in the mark scheme Symbol General Principles Meaning
Microtubule Interactions and Regulation of the Mitotic-Kinesin Like Protein-1 and Kinesin-Like Calmodulin-Binding Protein
 Microtubule Interactions and Regulation of the Mitotic-Kinesin Like Protein-1 and Kinesin-Like Calmodulin-Binding Protein Bettina Edith Deavours Dissertation submitted to the faculty of Virginia Polytechnic
Microtubule Interactions and Regulation of the Mitotic-Kinesin Like Protein-1 and Kinesin-Like Calmodulin-Binding Protein Bettina Edith Deavours Dissertation submitted to the faculty of Virginia Polytechnic
Cell Division. Use Target Reading Skills. This section explains how cells grow and divide.
 Name Date Class Cell Processes Guided Reading and Study Cell Division This section explains how cells grow and divide. Use Target Reading Skills As you read, make a cycle diagram that shows the events
Name Date Class Cell Processes Guided Reading and Study Cell Division This section explains how cells grow and divide. Use Target Reading Skills As you read, make a cycle diagram that shows the events
Chromosome Structural Analysis
 Chromosome Structural Analysis A Practical Approach Edited by WENDY A. BICKMORE Cell Genetics Section, MRC Human Genetics Unit, Western General Hospital, Edinburgh OXJORD UNIVERSITY PRESS 1999 List of
Chromosome Structural Analysis A Practical Approach Edited by WENDY A. BICKMORE Cell Genetics Section, MRC Human Genetics Unit, Western General Hospital, Edinburgh OXJORD UNIVERSITY PRESS 1999 List of
TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS. Kathryn P. Trogden
 TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS Kathryn P. Trogden A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill
TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS Kathryn P. Trogden A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill
Lecture 6. Chromosome Structure and Function in Mitosis
 Lecture 6 Chromosome Structure and Function in Mitosis Outline: Chromosome Organization and Function in Interphase Chromatin effects on replication Effects of nuclear organization on gene expression Chromosomes
Lecture 6 Chromosome Structure and Function in Mitosis Outline: Chromosome Organization and Function in Interphase Chromatin effects on replication Effects of nuclear organization on gene expression Chromosomes
Design Principles of Length Control of Cytoskeletal Structures
 Design Principles of Length Control of Cytoskeletal Structures Lishibanya Mohapatra 1, Bruce L. Goode 2, Predrag Jelenkovic 3, Rob Phillips 4, Jane Kondev 5,* 1 Department of Physics, Brandeis University,
Design Principles of Length Control of Cytoskeletal Structures Lishibanya Mohapatra 1, Bruce L. Goode 2, Predrag Jelenkovic 3, Rob Phillips 4, Jane Kondev 5,* 1 Department of Physics, Brandeis University,
PROTOCOL 1850 Millrace Drive, Suite 3A Eugene, Oregon
 PROTOCOL ATP Synthase Protein Quantity Microplate Assay Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 DESCRIPTION ATP Synthase Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL ATP Synthase Protein Quantity Microplate Assay Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 DESCRIPTION ATP Synthase Protein Quantity Microplate Assay Kit Sufficient materials
Tubulin Polymerization Assay Kit
 The Protein Experts Manual Cytoskeleton, Inc. V 3.0 Tubulin Polymerization Assay Kit Cat. # BK006P cytoskeleton.com Phone: (303) 322.2254 Fax: (303) 322.2257 Customer Service: cserve@cytoskeleton.com Technical
The Protein Experts Manual Cytoskeleton, Inc. V 3.0 Tubulin Polymerization Assay Kit Cat. # BK006P cytoskeleton.com Phone: (303) 322.2254 Fax: (303) 322.2257 Customer Service: cserve@cytoskeleton.com Technical
Comsol Multiphysics Simulations of Microfluidic Systems for Biomedical Applications
 Excerpt from the Proceedings of the COMSOL Conference 008 Hannover Comsol Multiphysics Simulations of Microfluidic Systems for Biomedical Applications Maria Dimaki *,1, Jacob Moresco Lange 1, Patricia
Excerpt from the Proceedings of the COMSOL Conference 008 Hannover Comsol Multiphysics Simulations of Microfluidic Systems for Biomedical Applications Maria Dimaki *,1, Jacob Moresco Lange 1, Patricia
Introduction to Cells
 Introduction to Cells Key terms: Cell Microscopy Nucleus Organelle Micrograph Cytoplasm Define: Cell Microscopy Cell structure Today, all of biology is based on an understanding of what is going on within
Introduction to Cells Key terms: Cell Microscopy Nucleus Organelle Micrograph Cytoplasm Define: Cell Microscopy Cell structure Today, all of biology is based on an understanding of what is going on within
Cell cycle: Cell growth and division (when the replication and segregation of chromosomes occurs). Interphase: Interphase Chromosomes
 Chromosomes exist in different states throughout the life of a cell Cell cycle: Cell growth and division (when the replication and segregation of chromosomes occurs). Interphase: Interphase Chromosomes
Chromosomes exist in different states throughout the life of a cell Cell cycle: Cell growth and division (when the replication and segregation of chromosomes occurs). Interphase: Interphase Chromosomes
Page 1. Name: 1) Which letter indicates a cell structure that directly controls the movement of molecules into and out of the cell?
 Name: 1) Which letter indicates a cell structure that directly controls the movement of molecules into and out of the cell? A) A B) B C) C D) D 2) A single-celled organism is represented in the diagram
Name: 1) Which letter indicates a cell structure that directly controls the movement of molecules into and out of the cell? A) A B) B C) C D) D 2) A single-celled organism is represented in the diagram
Adenine % Guanine % Thymine % Cytosine %
 1. Explain each of the following statements in terms of your knowledge of the structure and function of DNA. (i) In all living organisms the ratio species to another. A C T G is constant but the ratio
1. Explain each of the following statements in terms of your knowledge of the structure and function of DNA. (i) In all living organisms the ratio species to another. A C T G is constant but the ratio
Modulating the microtubule assembly and dynamics by altering the chemical environment
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2016 Modulating the microtubule assembly and dynamics by altering the chemical environment
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2016 Modulating the microtubule assembly and dynamics by altering the chemical environment
Nanobiotechnology. Place: IOP 1 st Meeting Room Time: 9:30-12:00. Reference: Review Papers. Grade: 50% midterm, 50% final.
 Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Nanobiotechnology Place: IOP 1 st Meeting Room Time: 9:30-12:00 Reference: Review Papers Grade: 50% midterm, 50% final Midterm: 5/15 History Atom Earth, Air, Water Fire SEM: 20-40 nm Silver 66.2% Gold
Chapter 8 DNA Recognition in Prokaryotes by Helix-Turn-Helix Motifs
 Chapter 8 DNA Recognition in Prokaryotes by Helix-Turn-Helix Motifs 1. Helix-turn-helix proteins 2. Zinc finger proteins 3. Leucine zipper proteins 4. Beta-scaffold factors 5. Others λ-repressor AND CRO
Chapter 8 DNA Recognition in Prokaryotes by Helix-Turn-Helix Motifs 1. Helix-turn-helix proteins 2. Zinc finger proteins 3. Leucine zipper proteins 4. Beta-scaffold factors 5. Others λ-repressor AND CRO
