3 The abbreviations used are: PI, phosphatidylinositol; Pak, p21-activated
|
|
|
- Irene Johnston
- 6 years ago
- Views:
Transcription
1 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 47, pp , November 24, by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Opposing Roles for Akt1 and Akt2 in Rac/Pak Signaling and Cell Migration * Received for publication, January 25, 2006, and in revised form, September 12, 2006 Published, JBC Papers in Press, October 1, 2006, DOI /jbc.M Guo-Lei Zhou 1, David F. Tucker, Sun Sik Bae, Kanav Bhatheja, Morris J. Birnbaum, and Jeffrey Field 2 From the Department of Pharmacology and the Howard Hughes Medical Institute, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania The Akt/PKB isoforms have different roles in animals, with Akt2 primarily regulating metabolic signaling and Akt1 regulating growth and survival. Here we show distinct roles for Akt1 and Akt2 in mouse embryo fibroblast cell migration and regulation of the cytoskeleton. Akt1-deficient cells responded poorly to platelet-derived growth factor while Akt2-deficient cells had a dramatically enhanced response, resulting in a substantial increase in dorsal ruffling. Swapping domains between Akt1 and Akt2 demonstrated that the N-terminal region containing the pleckstrin homology domain and a linker region distinguishes the two isoforms, while the catalytic domains are interchangeable. Akt2 knock-out cells also migrated faster than wild-type cells, especially through extracellular matrix (ECM), while Akt1 knock-out cells migrated more slowly than wild-type cells. Consistently, Akt2 knock-out cells had elevated Pak1 and Rac activities, suggesting that Akt2 inhibits Rac and Pak1. Both Akt2 and Akt1 associated in complexes with Pak1, but only Akt2 inhibited Pak1 in kinase assays, suggesting an underlying molecular basis for the different cellular phenotypes. Together these data provide evidence for an unexpected functional link between Akt2 and Pak1 that opposes the actions of Akt1 on cell migration. Cell movement requires the coordinated rearrangement of the actin cytoskeleton to extend the leading edge of the cell, attach the leading edge to substrates, and then contract the cell body behind the cell. Small hair-like protrusions called filopodia are not well characterized but they are likely to help polarize cells and form a leading edge. Filopodia are followed by much larger extensions called lamellipodia, which often fold back over the peripheral edges to form ruffles. In addition to these peripheral ruffles, ruffles can take the shape of distinct waves over the dorsal surface of cells called dorsal ruffles or circular ruffles. Peripheral ruffles help direct lateral movement while the dorsal ruffles promote movement off of the plane of the cell. All of these structures are rich in filamentous actin (F actin) and the actin cytoskeleton plays a central role in cell motility * This work was supported in part by Grants GM48241 (to J. F.) and DK56886 (to M. J. B.) from the National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 1 Supported by a National Scientist Development grant from the American Heart Association ( N). 2 To whom correspondence should be addressed: Dept. of Pharmacology, University of Pennsylvania School of Medicine, 3620 Hamilton Walk, Philadelphia, PA Tel.: ; Fax: ; field@ pharm.med.upenn.edu. through filopodia and lamellipodia at the leading edge as well as promoting contraction at the trailing edge. Coordinating the actions of the cytoskeleton in response to growth factors and chemo-attractants requires the activation of multiple cell signaling pathways through phosphatidylinositol (PI) 3 3-kinase (PI 3-kinase). PI 3-kinase-generated phospholipids serve as second messengers that activate small GTPases from the Rho family to regulate the actin cytoskeleton. Rho, Rac, Cdc42, and their targets are among the best characterized molecules that direct cell motility. Cdc42 regulates filopodia, Rac regulates lamellipodia and ruffles, while Rho regulates stress fibers and cell contraction (1). In addition, signals from PI 3-kinase promote both cell survival and glucose metabolism through a distinct effector, the Akt/protein kinase B (PKB). The Akt family consists of three isoforms: Akt1 ( ), Akt2 ( ), and Akt3 ( ). All Akt isoforms contain an N-terminal pleckstrin homology (PH) domain for phospholipid binding followed by a short linker domain, a catalytic domain and a C-terminal regulatory tail domain. The isoforms share many common substrates through their preferential phosphorylation of a motif with the sequence of RXRXX(S/T) (2). The cell survival substrates include Bad and Forkhead (3 5), while metabolic signaling targets regulate the insulin-responsive glucose transporter GLUT4 (6). Although the survival and metabolic signals are relatively well characterized, more recent studies suggest that Akt also regulates cell migration (7 11). Akt1 is also rapidly recruited to the leading edge of migrating cells and is required for cell polarization (8, 12, 13). Conversely, while Rac regulates motility, it can protect cells from apoptosis through its effector, the p21-activated protein kinase (Pak), to stimulate phosphorylation of Bad (14 17). Thus, a growing body of literature has emerged showing that the cytoskeletal and cell survival pathways downstream of PI 3-kinase are interconnected by several overlapping signals. Isoform-specific knock-out mice have revealed distinct roles for two of the isoforms, Akt1 and Akt2. Akt1 knock-out mice are small throughout their lifespan, and cells derived from them have high rates of apoptosis suggesting that Akt1 regulates growth and survival (18, 19). Akt2 knockouts have metabolic defects resembling diabetes mellitus including elevated blood glucose (20). In addition, adipocytes from Akt2, but not Akt1 3 The abbreviations used are: PI, phosphatidylinositol; Pak, p21-activated protein kinase; DMEM, Dulbecco s modified Eagle s medium; GTP S, guanosine 5 -O-(thiotriphosphate); PBS, phosphate-buffered saline; PDGF, platelet-derived growth factor; WT, wild type; GST, glutathione S-transferase; ECM, extracellular matrix; 1KO, Akt knock-out; 2KO, Akt2 knock-out; DKO, double knock-out. NOVEMBER 24, 2006 VOLUME 281 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 36443
2 knock-out mice, display significant defects in uptake of hexose and glucose transporter 4 translocation suggesting that Akt2 is the major isoform used for insulin signaling (6). The isoforms may also have differential effects on cell motility depending on the cell type (10, 11, 21, 22). Pak kinases are serine/threonine protein kinases identified through screens for direct effectors of the small GTPases Rac and Cdc42. Paks are divided into two groups, A and B, based on sequence similarities. Group A consists of three closely related family members, Pak1 (Pak ), Pak2 (Pak ), and Pak3 (Pak ). Group B contains Pak4, Pak5, and Pak6 (23 25). Activation of Pak1 stimulates some of the changes in the actin cytoskeleton associated with Rac and Cdc42, including stimulation of cell ruffling, especially dorsal ruffles, and inhibition of stress fibers (26 29). The cell ruffling phenotypes are likely to be regulated by a Pak/LIMK/cofilin pathway (30), while the effects of Pak on stress fibers are caused by phosphorylation and inhibition of a Rho GEF (31). However, the precise role of Pak in regulating these structures is complicated by feedback activation of Rac, which occurs when Pak localizes to the membrane, so in some experimental paradigms even kinase-dead Pak can stimulate ruffling by activating Rac (32). Whereas most of the regulation of Pak is through small GTPases, Pak kinases are also regulated by GTPase-independent mechanisms. Growth factor receptors recruit Pak to the membrane through the adaptor proteins Nck and Grb2 (33 36). The PIX proteins also bind Pak through SH3 domains (37, 38). PIX proteins are guanine exchangers for Rac and Cdc42 but can activate Pak through both GTPase-dependent and GTPaseindependent mechanisms (39). PIX is also responsible for the feedback activation of Rac by membrane-targeted Pak (32). Several protein kinases also regulate Pak. PDK-1 phosphorylates Pak at threonine 423, a site that is also autophosphorylated when Pak is activated by Rac or Cdc42 (40). Cdc2 phosphorylates Pak at threonine 212 (41, 42). Another protein kinase, Akt1, can also stimulate Pak by phosphorylation of serine 21, but other mechanisms contribute to Akt activation of Pak too (9, 14, 43, 44). We report here that Akt2, unlike Akt1, is a negative regulator of Pak1. Cells lacking Akt2 have enhanced dorsal ruffling in response to the growth factor PDGF as well as elevated levels of activated Rac and Pak1. Akt2 knock-out cells also have increased migration, especially invasion through extracellular matrix (ECM). These studies suggest opposing roles for Akt1 and Akt2 in regulation of Rac/Pak signaling, the actin cytoskeleton, and cell migration. EXPERIMENTAL PROCEDURES Materials Dulbecco s modified Eagle s medium (DMEM), fetal bovine serum, trypsin-edta (1 ), recombinant human platelet-derived growth factor-bb (PDGF-BB) were from Invitrogen (Carlsbad, CA). FuGENE 6 transfection reagent, Complete protease inhibitor mixture tablets, histone 4 and fibronectin were from Roche Applied Science. Recombinant Akt1 and Akt2 and the Rac activation assay kit were from Upstate Biotechnology Inc. (Charlottesville, VA). Rabbit polyclonal Pak1 antibodies (N20 and its agarose-conjugated form, for Western blot and immunoprecipitation, respectively) and protein A/G PLUS agarose were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Rabbit polyclonal Pak1 antibody for immunofluorescence was from Cell Signaling Technology (Beverly, MA). Highly cross-absorbed Alexa-Fluor 488 goat anti-rabbit IgG(H L), marina blue goat anti-mouse IgG(H L), and Alexa-Fluor 594 phalloidin were from Molecular Probes (Eugene, OR). Transwell membranes were from Costar (Corning, NY). Matrigel Invasion Chambers (8 micron pore size) were from BD Biosciences (San Diego, CA). [ - 32 P]ATP and ECL Western blotting detection reagents were from Amersham Biosciences (Piscataway, NJ). The peptide Bad S112 (ETRSRHSSYP) was synthesized by Invitrogen. Construction of Wild Type, Kinase-dead, and Chimeric Akt Retroviral Constructs Wild-type Akt1 and Akt2 in the retroviral vector, pmigr1, have been described previously (6). Kinase-dead (KD) Akt2 construct was made based on the pmigr-mycakt2 by introduction of a K181M mutation (21). Two primers: CCGCTATTATGCCATGATGATCCTGCG- CAAGGAGG (F) and GGCGATAATACGGTACTACTAG- GACGCGTTCCTCC (R) were used in PCR with Pfu DNA polymerase. The successful introduction of the point mutation was confirmed by DNA sequencing. Akt chimeras were generated by PCR ligation-pcr mutagenesis (45). Murine Akt1 or Akt2 cdna in puc19 were used as templates for PCR. All Akt constructs bear an N-terminal c-myc epitope tag, and lack a native initiation codon. Primer pairs were designed to amplify cdna sequences corresponding to the following domains of Akt1 and Akt2: PH domain (Akt1 residues 1 113, Akt2 residues 1 112), PH and linker region (Akt1 residues 2 143, Akt2 residues 2 145), PH, linker and catalytic (Akt1 residues 2 349, Akt2 residues 2 350), linker, catalytic, and regulatory tail (Akt1 residues , Akt2 residues ), catalytic (Akt1 residues , Akt2 residues ), catalytic and regulatory tail (Akt1 residues , Akt2 residues ), and regulatory tail (Akt1 residues , Akt2 residues ). The primer sequences were as follows: puc19/ecori/c-myc(f) for the N terminus of both Akt1 and Akt2:CGGCCAAATTCCACCA- TGG; puc19/bamhi/stop(r) for the C terminus of both Akt1 and Akt2: CTGCAGGTCGACTCTAGAGGATCCTC; Akt1P- H(R):CTGCCTCTTGAGTCCATCGGCC; Akt1Linker (F): GAAGAAGAGACGATGGACTTCCGATC; Akt1Linker(R): GTGCTTGGGCTTGGCCAGGGAC; Akt1Catalytic(F): CGT- GTGACCATGAACGAGTTTGAG; Akt1Catalytic(R): GAA- GGGCAGGCGGCGGCCACAC; Akt1Tail(F): TACAACCA- GGACCACGAGAAGCTGTTC; Akt2PH(R): CTGCTTCAG- ACTGTTGGCGACCATC; Akt2Linker(F): CGGGCCCAGG- TGAGGAC; Akt2Linker(R): GGCCCGTGCCTTGTTGAC- AGC; Akt2Catalytic(F): AAAGTGACCATGAATGACTTCG- ATTATC; Akt2Catalytic(R): GAATGGCAGGCGGCCACAC; Akt2Tail(F): TACAACCAGGACCACGAGCGC. The amplified fragments were gel-purified from agarose and subject to blunt-end ligation in the desired combinations. Ligation reactions were used as templates for a second round of PCR to amplify the full-length chimera. PCR products corresponding to full-length chimera were gel-purified from agarose and cloned into puc19 as EcoRI/BamHI fragments. Chimera integrity was verified by automated DNA sequencing. For mamma JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 281 NUMBER 47 NOVEMBER 24, 2006
3 lian expression, wild-type and chimeric Akt were subcloned into the retroviral vector pmigr2, a derivative of pmigr, as EcoRI/BamHI fragments. Retroviral Infection of Akt2KO MEFs Ecotropic BOSC23 packaging cells (a gift from Dr. Warren S. Pear, The University of Pennsylvania) were grown to 70% confluence in 100-mm dishes. BOSC23 cells were transfected with 6 g of each pmigr retroviral construct using FuGENE6. Retroviral supernatants were harvested 48 h post-transfection and passed through a m filter. Cell-free supernatants were used to infect spontaneously immortalized Akt2KO MEFs in the presence of 8 g/ml polybrene. An additional round of infection was performed using cell-free retroviral supernatants harvested 72 h post-transfection. Infection efficiencies were assessed by GFP expression and were routinely greater than 90%, as determined by flow cytometry or fluorescence microscopy. Akt chimera expression and kinase activity was comparable in mouse fibroblasts (not shown). Cell Culture and Transfection Cells were maintained in DMEM supplemented with fetal bovine serum as previously described (9). For transfection of HEK 293T cells, cells were split and grown for a day until 60 80% confluent and transfected using FuGENE 6. For one single well of a 6-well plate, 2 g of plasmids and 6 l of FuGENE 6 were used. The cells were allowed to express the proteins for 24 h after DNA was added. The cells were harvested and cell lysate was prepared as previously described (9). Pak Kinase Assay For kinase assays using endogenous Pak from MEF cells, cell lysates were prepared, then Pak1 was immunoprecipitated using an agarose-conjugated Pak1 antibody. For kinase assays using transfected Pak in 293T cells, Myc-Pak was immunoprecipitated with a Myc monoclonal antibody. Pak kinase assays were conducted as previously described using either histone 4 or a Bad S112 peptide (ETRSRHSSYP) as a substrate (9). Kinase assay results were detected by autoradiography and quantified by densitometry. Rac Activation Assay Rac activation assays were conducted as recommended by the supplier. Cells were plated onto 100-mm plates and grown 24 h to 75 80% confluent, serumstarved for 24 h, and then harvested. Where indicated, the cells were stimulated with 10 ng/ml PDGF for 5 min prior to harvesting. To harvest, the cells were washed twice with icecold PBS before adding 0.8 ml 1 Mg 2 Lysis/Wash Buffer (MLB). The cells were then scraped from the plate and transferred to microtubes on ice. To prepare the cell lysates they were incubated with 10% (v/v) glutathione-agarose for 10 min at 4 C on a rotator. The samples were spun down at 14,000 rpm for 5 s, and the supernatants were transferred to new tubes as cell lysates. The cell lysates were used immediately for a pulldown of active Rac with Pak1-PBD-agarose. 10 l of Pak1 PBDagarose were added to 500 l of cell lysate, and the samples were rotated at 4 C for 60 min. The agarose beads were collected by spinning at 14,000 rpm for 5 s, and the supernatants were removed. The beads were washed three times with MLB and suspended in 2 SDS buffer for Rac detection by Western blot. To serve as positive and negative controls, cell lysates prepared the same way from wild-type MEF cells were preloaded with 100 M (final concentration) of either GTP S or GDP in the presence of 10 M EDTA and incubated at 30 C for 15 min. The reaction was terminated by adding MgCl 2 to 60 mm. Pulldown assays were also conducted with these samples as above, except the incubation time was for 30 min. Phase Imaging and Immunofluorescence MEF cells were observed and pictures were taken using Phase 3 Imaging Systems (Glen Mills, PA). Time lapse images were taken at 30-s intervals. For immunostaining, MEF cells were grown on a Nunc chamber overnight and starved for 24 h, and then stimulated with PDGF for 10 min. The cells were fixed with 3.7% paraformaldehyde for 30 min and then premeabilized with PBS containing 0.5% Triton for 20 min. Following incubation with PBS containing 3% bovine serum albumin and goat serum for 30 min at 37 C, cells were incubated with diluted antibodies (1:50 for Pak; 1:100 for Rac) for 1 2 h at room temperature, washed with PBS containing 0.1% Triton three times and then incubated with Alexa-Fluor 594 phalloidin, 1:500 dilution of Alexa-Fluor 488 goat anti-rabbit IgG (H L), or Marina blue goat anti-mouse IgG (H L) for 45 min at room temperature. After washing with PBS containing 0.1% Triton several times, the samples were mounted with Vectashield Mounting Medium from Vector Laboratories, Inc. (Burlingame, CA). The samples were observed and pictures were taken as previously described (9). Cell Migration and Invasion Assays MEF cells were grown overnight until subconfluent and then serum-starved for 24 h. Cells were detached with Trypsin-EDTA, collected in prewarmed DMEM containing trypsin inhibitor (Boehringer), washed once with the same medium and kept in it until used. For migration in transwells, about cells in a 100- l volume were added to the upper well of 24-well chamber plates (6.5 mm diameter, 8 M pores) coated with fibronectin and DMEM containing 30 ng/ml PDGF was added to the bottom part of the well. After incubation at 37 C with 5% CO 2 for 18 h, the membranes were fixed with a solution with 10% acetic acid and 10% methanol, stained with 0.4% crystal violet in 10% ethanol for 30 min. Then, the non-migrating cells, which stay on the upper side of the membrane, were removed by gently wiping the membrane with a cotton swab. For invasion assays through Matrigel, a chamber with 8 M pore size coated with Matrigel Basement Membrane Matrix was used cells in a 500- l volume were loaded into the top well. The medium in the bottom well, incubation, and staining procedures were the same as described for the transwell assay. After the cells were stained and dried, the migrated cells were counted under a Nikon TMS inverted microscope using a 20 lens. RESULTS Enhanced Dorsal Ruffling in Akt2 Knock-out MEFs Akt1 can stimulate Pak1 through multiple mechanisms (9, 43, 44). However, all of the studies on the interactions between Akt1 and Pak have been based on cell transfection or in vitro studies. To date, there is no genetic evidence for a role of Akt1 in Pak signaling, nor have any studies documented an interaction between Akt2 and Pak. To study the interaction between Akt and Pak we initiated studies with mouse embryo fibroblast (MEF) cell lines derived from Akt knock-out mice as described NOVEMBER 24, 2006 VOLUME 281 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 36445
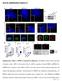
4 FIGURE 1. Morphological and cytoskeletal changes in Akt KO cells. A, phase images of MEF cells. Cells were grown in DMEM with serum for 24 h before pictures were taken using Phase-3 Imaging Systems. Arrowhead, cell with branched structure for Akt 1KO cells; arrow, cell with large lamellipodia for Akt 2KO cells. B, time-lapse phase images of 2KO cells stimulated with PDGF showing dorsal ruffles. Cells were grown overnight, starved for 24 h followed by stimulation with 10 ng/ml of PDGF and photographed at the indicated times (Min). The arrowheads follow a cell with a typical dorsal ruffle, while the arrows follow a cell with an atypical pattern of a dorsal ruffles (See Results ). C, actin cytoskeleton of MEF cells. Cells were grown overnight, serum-starved 24 h followed by stimulation with 10 ng/ml PDGF for 10 min, where indicated. Cells were fixed with 3.7% paraformadehyde, the actin cytoskeleton was stained with phalloidin and photographed. Arrow indicates a typical cell with developed stress fibers in Akt 1KO cells, while arrowheads indicate cells with dorsal ruffles in Akt 2KO and DKO cells. D, confirmation of reexpression of Akt2 and Akt1 in 2KO cells using Akt1 and Akt2 isoform-specific antibodies in Western blots. Cells grown in DMEM were lysed, the lysates were normalized for total protein, resolved on a 10% SDS-PAGE and subjected to Western blots. Samples were reprobed with actin to assure equal loading. WTM and 2KOM represent WT and 2KO cells harboring empty vector. E, effect of loss of Akt on dorsal ruffling formation in MEF cells. Percentage of cells with dorsal ruffles was calculated after starvation and PDGF stimulation. Cells were grown overnight, starved, and stimulated with PDGF for 10 min before fixation and staining. The cytoskeleton was stained with phalloidin and observed under a fluorescence microscope. Four random fields each with cells were counted in each experiment and the experiment was repeated three times with similar results. The data are shown as the mean S.E. F, reexpression of Akt2 (2KOM2), but not Akt1 (2KOM1) in 2KO cells rescued the dorsal ruffles after PDGF stimulation. The experiment was done in same way as in E. previously (6). The cell lines tested were: wild type (WT), Akt1 knock-out (1KO), Akt2 knock-out (2KO), and Akt1/Akt2 double knock-out (DKO). For reexpression of Akt isoforms, stable cell lines were made by introducing pmigr-based Akt1 or Akt2 into WT or 2KO backgrounds. These cell lines include: WT cells with empty vector (WTM); 2KO cells with empty vector (2KOM); 2KO cells with Akt2 (2KOM2) and 2KO cells with Akt1 (2KOM1). We first examined morphological alterations caused by the loss of the Akt isoforms. As shown in Fig. 1A, 1KO cells are larger and more spread than WT and the other two KO lines, 2KO or DKO. Many Akt1 KO cells have a branched structure (Fig. 1A, indicated with an arrowhead). When stained for F-actin with phalloidin, Akt1 KO cells have highly developed stress fibers compared with WT cells (Fig. 1C, arrow). While stress fibers are caused by Rho activation, they are also observed when Pak activity is reduced because Pak phosphorylates and inhibits a Rho guanine nucleotide exchange factor (31). In contrast to the Akt1 KO cells, Akt2 KO cells are smaller, but have more lamellipodia (Fig. 1A, indicated with an arrow), a structure JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 281 NUMBER 47 NOVEMBER 24, 2006
5 associated with high Rac and Pak activity (1). DKO cells are similar to 2KO cells in cell morphology. Most strikingly, when cells were starved and then stimulated with PDGF, 75% of the 2KO cells formed dorsal ruffles (Fig. 1, B, C, and E), compared with less than 10% of the wild-type cells. When stained with phalloidin, the dorsal ruffles were seen as actin rings (Fig. 1C, arrowheads). Most cells had a single ring, although some cells had 2 3 actin rings (Fig. 1C). In contrast, no dorsal ruffles were observed in the 1KO cells (Fig. 1, C and E). About 50% of the DKO cells formed dorsal ruffles (Fig. 1, C and E). Dorsal ruffles are vertical ruffles that appear directly behind the leading edge on the dorsal surface (46); they are formed when the cell membrane behind the leading edge projects dorsally, and the ruffles move along the dorsal surface until they disappear (47). Dorsal ruffles are also observed when cells express active mutants of Pak (29). In time-lapse microscopy under phase contrast conditions, we observed that most dorsal ruffles initiated s after PDGF stimulation. The dorsal ruffles were first observed as phase dark rings close to the cell periphery. They then moved over the dorsal surface, decreasing in diameter until they disappeared. Most dorsal ruffles were no longer visible 15 min after PDGF stimulation (Fig. 1B, arrowheads), although in rare cases ruffles were visible as long as 30 min after PDGF stimulation (not shown). We also observed an atypical dorsal ruffling pattern in some of the cells. In the atypical cells, the ruffle initially expanded toward the cell periphery, and after reaching the edge, it reversed direction to contract toward the center like a typical ruffle (Fig. 1B, arrow). Thus, loss of either Akt1 or Akt2 caused aberrant cell ruffling, although in different ways. We performed two types of controls to address the specificity of these observations. First, we reintroduced Akt2 into 2KO cells expressed from a pmigr vector (2KOM2). Second, we reexpressed Akt1 in the 2KO cells (2KOM1). The expression of Akt1 and Akt2 were verified using isoform-specific Akt antibodies in Western blots (Fig. 1D). As expected, wild-type levels of PDGF-stimulated dorsal ruffling were observed in the cells reexpressing Akt2 (Fig. 1F). However, expressing Akt1 in the Akt2 knock-out cells failed to rescue the dorsal ruffling phenotype (Fig. 1F). N-terminal Domains Distinguish Akt Isoforms Because only Akt2 rescued dorsal ruffling, we defined the region essential for rescue using a domain swap strategy. Based on structural studies, Akt1 and Akt2 can be divided into four regions, the N-terminal PH domain, the linker domain, the catalytic domain and the C-terminal regulatory tail domain (Fig. 2A). A series of chimeras between Akt1 and Akt2 were constructed to swap the domains. Three chimeric constructs rescued the dorsal ruffling (Fig. 2B, upper panel). Interestingly all three of these included the N terminus of Akt2 in the fusion, and all constructs with this domain from Akt2 rescued the cells. The construct with the smallest fragment of Akt2 that rescued cell ruffling, Akt2211, contained the N terminus of Akt2, but the catalytic and regulatory domains of Akt1. We next subdivided the N terminus into the PH and linker domains. There was a partial rescue by the Akt2 PH domain by itself (Akt2111), but rescue was best when the linker domain was also included. The PH domain from Akt1 did not prevent Akt2 from rescuing cells (Akt1222). We conclude that the linker domain distinguishes Akt2 from Akt1, while the other domains are largely interchangeable. We also constructed a kinase-dead mutant of Akt2, K181M and tested if the catalytic activity of Akt2 is required for the rescue. The kinase-dead Akt2 failed to rescue 2KO cells demonstrating that the catalytic activity of Akt2 is required to suppress cell ruffling (Fig. 2B, lower panel). Increased Cell Invasion through ECM by 2KO Cells Cell ruffles are derived from lamellipodia and are required for cell migration. To determine if cell migration was affected by loss of Akt, we performed cell migration studies. First, we measured cell migration in a transwell assay (9). Cells were loaded into the upper chamber of a two chamber well separated from the lower chamber by a membrane. The lower chamber contained PDGF as a chemo-attractant. After a period of migration, the membrane was fixed and stained and the upper surface of the membrane was wiped clean of cells. The remaining cells, which are now on the lower surface, were counted and scored as migrated cells. We found that 2KO cells harboring an empty vector (2KOM) have higher migration rates than WT or WT cells harboring an empty vector, while 1KO cells had a lower migration rate (Fig. 3A). Reexpression of Akt2 (2KOM2), but not expression of Akt1 (2KOM1) rescued the migration phenotypes (Fig. 3, A and C). 2KO cells overexpressing Akt1 migrated somewhat faster than the 2KO cells consistent with the known role of Akt1 to stimulate migration. Because a recent study found that peripheral ruffles are required for migration within the plane of the cell, while dorsal ruffles are associated with cell invasion through a cell matrix (47), we also performed the invasion studies using chambers coated with the matrix Matrigel. This matrix is rich in collagen fibers and approximates tissue invasion more accurately than the standard membranes. In Matrigel as well, the 2KOM cells migrated significantly faster (more than twice) compared with WT cells, while 1KO cells had a small reduction in migration rates (Fig. 3B). As seen in the ruffling and transwell experiments, reexpressing Akt2 in the 2KO cells, but not Akt1, rescued the migration phenotypes in the Matrigel assays (Fig. 3, B and D.) These data suggest that the increased ruffling of 2KO stimulates migration, especially invasion through ECM. Loss of Akt2 Leads to Activation of Rac and Pak1 Because the predominant phenotypes associated with loss of Akt2 were effects on cell ruffling, we measured Rac and Pak activities in these cells, because both are required for cell ruffling. For Rac activation assays, cells were starved and stimulated with PDGF. Rac activity was then measured by a Pak-PBD pull-down assay. In this assay, the Rac binding domain of Pak (PBD) fused to GST and bound to glutathione-agarose beads was used to capture Rac from cell lysates. Following incubation, the beads were spun down and the Rac captured by the PBD was probed on Western blots. As the Pak domain only binds the GTP-bound form of Rac, only the activated Rac is recovered. Cell lysates preloaded with GTP S or GDP were used as positive and negative controls, respectively. As shown in Fig. 4A, the 2KO cells had elevated levels of activated Rac compared with WT cells, while no significant differences were observed in either 1KO or DKO cells. NOVEMBER 24, 2006 VOLUME 281 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 36447
6 FIGURE 2. The N terminus distinguishes Akt1 from Akt2 in rescue experiments. A, Akt domain structure and chimeras between Akt1 and Akt2. Akt isoforms have 4 domains: PH domain, Linker domain, catalytic domain (Catalytic), and regulatory tail domain (Regulatory tail). The PH domain for Akt1 and Akt2 are residues and 1 112, respectively. All constructs have an N-terminal Myc tag and start from residue 2 (see Experimental Procedures ). B, upper panel, rescue of dorsal ruffle formation in 2 KO cells by Akt chimeras. Akt2KO MEFs were infected twice with retrovirus packaged in BOSC23 cells in the presence of 8 g/ml polybrene (see Experimental Procedures for details). The infection efficiency was estimated to be 90 95% by observing GFP under a fluorescence microscope. The cells were grown in Nunc chambers overnight, starved 24 h stimulated with PDGF and then by fixed and stained. The actin cytoskeleton was stained with phalloidin, and the cells were then observed under a fluorescence microscope. Four random fields each with GFP-positive cells were counted in each experiment and the experiment was repeated three times with similar results. The data are shown as the mean S.E. B, lower panel, rescue of dorsal ruffles by kinase-dead Akt2 and PH domain and linker domain chimeras. The experiment was done as in the upper panel JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 281 NUMBER 47 NOVEMBER 24, 2006
7 FIGURE 3. Deficiency of Akt2 results in increased migration, especially invasion through ECM. A, increased migration of Akt 2KO cells in a transwell assay. Cells were grown overnight, starved for 24 h and detached (see Experimental Procedures ) cells were plated in the upper well and DMEM containing 30 ng/ml PDGF was added to the bottom well. After 18 h of incubation, the cells migrating across the membrane were stained and counted. Four random fields were counted and the number of the migrated cells was used as an index for migration. The experiment was repeated at least three times with similar results. The data are presented as the mean S.E. B, increased invasion of Akt 2KO cells through ECM in a Matrigel assay. Cells were starved for 24 h and detached cells were loaded into the upper well, and the experiment carried out as in A. The data are presented as the mean S.E. C, rescue of Akt2 KO cells by Akt2 (2KOM2) but not Akt1 (2KOM1). The experiment was done as in A. D, rescue of Akt2 KO cells in Matrigel invasion by Akt2 (2KOM2) but not Akt1 (2KOM1). The experiment was done as in B. We also measured the activity of Pak in the various cell lines. As we observed maximum levels of dorsal ruffles in 2KO cells 8 10 min after PDGF stimulation (not shown), we treated the cells with PDGF for 10 min before harvesting the cells. Pak was immunoprecipitated from the cell lysates and then used in kinase assays with histone 4 as a substrate. As shown in Fig. 4C, 2KO cells had elevated Pak activity compared with WT cells. Conversely, 1KO cells had slightly lower Pak activity than WT cells. Finally, DKO cells also had elevated Pak activity, but the activation was less than that of the 2KO cells, consistent with a positive role for Akt1 in Pak regulation. These data also demonstrate that, like Rac, Pak is substantially activated in the 2KO cells, while Pak activity is lower in the 1KO cells. The Rac activity (Fig. 4B) and Pak activity (Fig. 4D) both returned to wildtype levels in the 2KO cells reexpressing Akt2 (2KOM2). Taken together, dorsal ruffle formation and Rac/Pak activation are specifically stimulated by loss of Akt2. Akt2 Associates with Pak1 and Inhibits Its Activity The elevated levels of activated Rac and Pak in 2KO cells suggested that Akt2 inhibits their activities. To determine the mechanism of inhibition, we first determined if Akt2 phosphorylates Pak or Rac but were unable to detect any phosphorylation with a phosphoserine 71 specific antibody against Rac (48), or 32 P labeling Akt2 Is a Negative Regulator of Rac/Pak Signaling in kinase assays using recombinant proteins (data not shown). Thus, phosphorylation does not appear to be the mechanism for Akt2 regulation of Pak. As one important mechanism for Pak regulation is through binding of other proteins, we tested if the two Akt isoforms could physically interact with Pak. We first used a transfection system to determine if they co-immunoprecipitate. We transfected 293T cells with HA-Akt1 or HA-Akt2 along with Myc-Pak1 and then performed immunoprecipitations with anti-myc to immunoprecipitate Pak1 and probed with anti-ha to detect Akt. We found that both Akt1 and Akt2 were found in the immunoprecipitates, suggesting that both isoforms could physically associate with Pak1 (Fig. 5A). Interestingly, WT Pak1 associated more readily with both Akt1 and Akt2 compared with kinase-dead (KD or K299R) Pak. The latter can only be detected with extended exposure. We also tested if Akt2 formed a complex with Pak in MEF cells by immunoprecipitating Pak1 and blot with Akt2 antibody. As shown in Fig. 5B, Akt2 was detected in the precipitates demonstrating that the endogenous proteins associate. We performed pull-down assays using recombinant proteins as well, but were unable to detect a complex between Akt and Pak (data not shown) suggesting that the association may be indirect, or the direct binding is too weak to be detected under these experimental conditions. To test the effects of Akt1 and Akt2 on Pak1 activity, we performed Pak kinase assays using either recombinant proteins or Pak from Akt co-transfected cells. As a substrate, we used a peptide derived from the region surrounding amino acid 112 of Bad. This peptide is readily phosphorylated by Pak1, but not by Akt, so it permits measurement of Pak1 activity in the presence of Akt. It also lacks the Akt phosphorylation site (Bad S136) so it is not likely to be affected by Akt. In an in vitro kinase assay, we found that the kinase active form of Akt2 strongly inhibited Pak1 (Fig. 5C, lane 5), while the kinase dead form of Akt2 showed only a modest inhibition (Fig. 5C, lane 4). We speculate that like kinase dead Pak, the kinase dead Akt2 probably does not associate with Pak as well as active Akt2 and thus does not inhibit Pak as well. In a transfection assay, we found that Akt2 cotransfection inhibited the basal activity of Pak (Fig. 5D, lane 3 versus lane 2). We also observed marginal activation of Pak by Akt1 (Fig. 5D, lane 4 versus lane 2), consistent with the results from Akt1 KO cells that support a positive role for Akt1 in Pak NOVEMBER 24, 2006 VOLUME 281 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 36449
8 FIGURE 4. Elevated Rac and Pak activities in Akt2 KO cells. A, elevated Rac activity in 2KO cells. Whole cell lysates (WCL) were prepared after cells were starved for 24 h and stimulated with 10 ng/ml PDGF for 5 min. Active (GTP-bound) Rac was pulled-down from cell lysates by GST-fused Pak PBD domain and detected in a Western blot with a Rac monoclonal antibody. Cell lysates prepared from WT MEF cells and subsequently loaded with GTP S or GDP were included to serve as positive and negative controls, respectively (See Experimental Procedures ). Recombinant His-tagged Rac was used as a marker for molecular weight. B, reexpression of Akt2 in 2KO cells rescued the elevated Rac activity, as detected in a Rac activation assay using PBD pull-down. The experiment was done in a similar way as in A. C, elevated Pak activity in 2KO cells as determined in a Pak kinase assay (KA) using histone 4 as a substrate. Cells were grown overnight, starved for 24 h, and stimulated with PDGF for 10 min before being lysed. Pak was immunoprecipitated with Pak1 antibody and the KA mixture was run on a 10% SDS-PAGE, the phosphate incorporated onto the substrate was detected with autoradiography and quantified. D, reexpression of Akt2 in 2KO cells rescued elevated Pak kinase activity. The experiment was done in a similar way with C. FIGURE 5. Akt association with Pak and effects on Pak activity. A, Akt co-immunoprecipitates with Pak1 in a transfection system. 293T cells were transfected with plasmids expressing a HA-tagged Akt1 or Akt2 and Myc-tagged Pak1, the cells were harvested and Pak was immunoprecipitated from the cell lysates with anti-myc antibody. The co-immunoprecipitated Akt was detected in a Western blot with anti-ha antibody (irrelevant lanes were cropped out). The experiment was repeated three times with similar results. B, Akt co-immunoprecipitates with Pak in MEF cell lysates. MEF cells were harvested and Pak was immunoprecipitated with a conjugated Pak1 antibody, the co-immunoprecipitated Akt2 was detected in a Western blot with an Akt2 antibody. The recombinant His-Akt2 served as a marker for molecular weight. C, Akt2 strongly inhibits Pak activity in an in vitro kinase assay. Kinase assays contained 0.2 g ofakt (recombinant, both active and inactive), 0.1 g of baculovirus Pak and 20 g of Bad S112 peptide as a substrate in a volume of 25 l. Pak phosphorylation of the peptides was detected by autoradiography. D, Akt2 inhibits Pak activity in a transfection system. 293T cells were co-transfected with the indicated Akt and Pak plasmids. After expression for 24 h, cells were harvested and Myc-Pak was immunoprecipitated from the lysates. The Pak kinase assay was done as in C. A total of 2 g of plasmid DNA was used to transfect each well of a 6-well plate, with a ratio of Akt:Pak of 2:1, vector plasmids were used instead of Akt in lane 2. regulation. Together, these data suggest that both Akt1 and Akt2 can associate with Pak; however, while Akt1 activates Pak, Akt2 inhibits Pak. Rac and Pak Are Required for Dorsal Ruffles in MEF Cells Expression of activated Pak in NIH3T3 cells stimulates dorsal ruffles following PDGF treatment (29), while expression of active Rac stimulates peripheral ruffles. We performed experiments in the 2KO cells to test if Pak is required for dorsal ruffling. First, we stained serum-starved 2KO cells for Pak, Rac and the actin cytoskeleton (Fig. 6A). Pak localized diffusely throughout the cell before PDGF treatment. After cells were stimulated for 10 min with PDGF, Pak translocated to the dorsal ruffles along with actin. Rac also co-localized to the dorsal ruffles. These data, together with hyperactivation of Pak and Rac in 2KO cells, suggest that Pak and Rac are involved in dorsal ruffle formation in the 2KO cells. Second, we inhibited the Rac/ Pak pathway to determine if its loss prevented cell ruffling. As the MEF cells were poorly transfected, we used retroviral transduction to express a dominant negative mutant of Pak in the 2KO cells (Fig. 6B). The expression of the mutant Pak was confirmed on Western blots (data not shown). Cells expressing the dominant negative Pak (K299R) had reduced ruffling (Fig. 6B). DISCUSSION Akt1, Akt2, and Akt3 are closely related protein kinases with a shared preferred phosphorylation consensus motif. They are all also activated through similar mechanisms involving PI 3-kinase and PDK-1. Yet the different isoforms have distinct roles in the widely studied metabolic and survival pathways as first revealed in knockout mice. In addition to isoform specific roles in the metabolic and survival functions, the isoforms now appear to have different roles in the relatively newly identified cytoskeleton and cell motility functions (8 11, 21, 22, 49) JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 281 NUMBER 47 NOVEMBER 24, 2006
9 FIGURE 6. Roles for Pak and Rac in dorsal ruffle formation. A, colocalization of Rac and Pak to the actin ring of dorsal ruffles in PDGF treated 2KO cells. Before stimulation with PDGF following cell starvation, Pak was diffusely located in the whole cell (arrow). In contrast, after stimulation with PDGF, most of the Pak protein translocated to the actin ring of dorsal ruffles (arrowhead). Rac also localized to dorsal ruffles. B, retrovirus expression system was used to express kinase-dead Pak, K299R, in the 2KO cells by establishing stable cell lines through puromycin selection (2KO/KDPak). The cells were starved and stimulated with PDGF. Four random fields each with cells were counted, and cells with dorsal ruffling were scored. The data are shown as the mean S.E. Interestingly, where roles for Akt1 and Akt2 in cell motility have been reported, distinct and, in some cases, opposing functions for the two isoforms are often observed. In fibroblasts, Akt1 has repeatedly been found to promote invasion (8 11). Akt1, but not Akt2 is also important for endothelial cell migration through regulation of the nitrous oxide signaling pathway (10). However, three studies found that Akt2, but not Akt1 stimulates motility of breast and ovarian cancer cells, while Akt1 actually inhibits motility in these cells (11, 21, 22). Despite apparently contradictory studies on the roles of the two isoforms, two observations are common to the different studies. The first is that Akt1 promotes motility in fibroblasts and endothelial cells, and secondly, Akt2 promotes motility in epithelial cells. Remarkably, in cells where one isoform stimulates motility, the other isoform usually has a limited or even opposing role. Several models have been proposed to account for the specificities of closely related isoforms of signaling enzymes such as the Akt family including (1) differential expression in target tissues, (2) distinct effectors, despite common preferred substrate motifs (3), differential compartmentalization of isoforms perhaps through association with unique scaffold proteins. It is not likely that differential expression of the Akt1 and Akt2 isoforms in target tissues accounts for the different phenotypes observed in the two knock-out cells. This is because we observed similar levels of both isoforms in cells, and more importantly, expression of Akt2 rescued the phenotypes of Akt2 KO cells, both biochemical and biological, whereas overexpression of Akt1 failed to rescue either dorsal ruffling or migration. A similar pattern was observed in studies of hexose transport in adipocytes, where reexpression of Akt2 but not Akt1 rescued the phenotypes of Akt2 knock-out adipocytes (6). Another model to account for the isoform specificity is subtle differences in the targets of the two Akt isoforms. Since the demonstration of Pak as a downstream target of Akt (14), three mechanisms have been proposed to account for Akt1 activation of Pak: 1) phosphorylation of serine 21 (9), 2) activation of subunits of heterotrimeric G proteins (43), and 3) assembly of a complex by the adaptor ArgBP2 (discussed below) (44). Each study found that Akt1 could activate Pak independently of Rac and Cdc42 but their relative contributions remain to be compared. We have previously reported that Akt1 phosphorylates serine 21 of Pak1 (9). In numerous experiments with Akt2, using either peptide substrates in kinase assays or a serine 21 phosphospecific antibody in Western blots, we have been unable to detect any phosphorylation of Pak at serine 21 by Akt2. While this suggests that the ability to phosphorylate Pak may contribute to the different roles in Pak regulation between the two Akt isoforms, we have also found that there are differences in the ability of different preparations of Akt1 to phosphorylate this site. The difference may, in part, be due to differences in the Akt1 activation procedures. 4 Moreover, because chimeras between Akt1 and Akt2 show that the kinase domains are interchangeable, it is likely that substrate specificity does not distinguish the two isoforms. A third model for isoform specificity is compartmentalization by binding to specific adaptors or scaffolds. Several scaffold proteins bind preferentially to one of the Akt isoforms. One of them POSH, is an adaptor protein that links the Rac target, MLK to JNK signaling. Of relevance to this study, Akt2 is a negative regulator of the signal through POSH (50). Hence, this signal may lead to some of the observed phenotypes. However, POSH activation does not stimulate motility and cell ruffling, but primarily stimulates JNK and NF B leading to high levels of apoptosis (51, 52), whereas Akt2 knockouts have elevated levels of cell ruffling and no significant growth or survival phenotypes. This argues against POSH as the primary target in our studies. Another adaptor protein called ArgBP2 was recently shown to bind to all three Akt isoforms and is specifically phosphorylated by Akt1. Additionally, it binds to Pak and promotes Akt activation of Pak (44). While ArgBP2 remains an attractive candidate for facilitating activation of Pak by Akt1, it probably does not account for the negative regulation of Rac and Pak by Akt2. We also note that neither POSH nor ArgBP2 bind the PH and linker domains of Akt, which are critical in distinguishing them. Thus, the relevant scaffolds remain to be identified. Together, these argue for a distinct mechanism of action by the Akt isoforms on the Rac/Pak pathway based on differences between their PH and linker domains. Interestingly, the linker domain, which is more important in distinguishing the isoforms, is the least homologous domain. In addition, although few biochemical differences are found between the isoforms, Akt2 is predominantly found in the membrane and cytoskeleton, while Akt1 is predominantly found in the cytosol, lending support to the compartmentalization model (6, 21). We speculate that the PH and linker domains of Akt2 direct it to membranous and cytoskeletal compartments, where Akt2 inhibits Pak. Although we do not find that Akt2 phosphorylates Pak1, it may associate better in its kinase active conformation, since the kinase activity of Akt2 is required for Pak inhibition. Rac and its effector Pak have been extensively studied and are well established regulators of cell ruffling and cell motility so 4 G. L. Zhou and J. Field, unpublished observations. NOVEMBER 24, 2006 VOLUME 281 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 36451
10 they are obvious candidates responsible for the phenotypes in the Akt1 and Akt2 knock-out cells. While most studies have identified activators of Pak, there are a growing number of inhibitors of Rac and Pak. In addition to the traditional GTPase-activating proteins (GAPs) and phosphatases, a number of other proteins inhibit Rac and Pak. The integrin binding protein nischarin binds and inhibits Pak (53). Loss of the scaffold protein VASP also activates Pak and Rac causing increases in cell spreading and reduced cell migration and reorientation, although the direct target of VASP has not been established (54, 55). In addition, merlin, the product of the gene responsible for neurofibromatosis type 2, binds and inhibits Pak. Loss of merlin leads to activation of both Rac and Pak, with subsequent increases in cell ruffling (56, 57). In this case, Pak is probably the primary target of merlin and Rac becomes activated through a feedback loop involving PIX. Where tested, each of these inhibitors acts by binding Pak and inhibiting its kinase activity. Thus, while either Rac or Pak could be the primary target of Akt2, our demonstration that Akt2 can associate with Pak and inhibit its kinase activity suggests that direct association of Akt2 and Pak1 may be responsible for the inhibition. In this case then it is likely that the PIX feedback loop causes the Rac activation in the Akt2 knockouts. The phenotypes of the 2KO cells also suggest that Pak is a primary target of Akt2. Loss of Akt2 increased migration, especially in the Matrigel assays. Note that the type of ruffle that predominates in the Akt2 knock-out cells is a dorsal ruffle. A study comparing the effects of the two ruffles on cell motility concluded that dorsal ruffles promote invasion through extracellular matrix, while peripheral ruffles primarily promote lateral migration (47). Thus, the more dramatic increase in cell migration in the 2KO cells through an ECM compared with the uncoated membranes is likely to be caused by the increase in dorsal ruffling. Rac is required for cell ruffling, but the roles of its effectors are not as well established. Activation of Rac stimulates peripheral cell ruffling (lamellipodia formation), but not dorsal ruffling (1, 58, 59). However, expression of a dominant negative Rac in cells inhibits dorsal ruffle formation (59). Thus, Rac activity is necessary, but not sufficient for inducing dorsal ruffling. It is likely that only a subset of its effectors mediate dorsal ruffling. Of relevance, expression of hyperactive Pak mutants stimulates dorsal ruffling (29). Thus, the high rates of dorsal ruffling and increased Matrigel invasion also point to Pak as the primary target of Akt2. In conclusion, we find non-overlapping roles for Akt1 and Akt2 in regulation of Rac/Pak signaling and MEF cell migration. Akt1 is a relatively weak activator of Pak1 while Akt2 is a relatively strong inhibitor of Pak1. Furthermore, the two isoforms play opposing roles in regulating cytoskeleton and cell migration. Akt, especially Akt2, is often over expressed in tumors. Our findings suggest that strategies that inhibit Akt2 alone may increase invasion and metastasis by some tumors. Acknowledgments We thank Sung-Ro Jo, Kristin Roovers, and Benjamin Fryer for helpful discussions and Shenghao Jin for the KD Pak1 retrovirus construct. REFERENCES 1. Bar-Sagi, D., and Hall, A. (2000) Cell 103, Brazil, D. P., and Hemmings, B. A. (2001) Trends Biochem. Sci. 26, Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Blenis, J., and Greenberg, M. E. (1999) Cell 96, Datta, S. R., Dudek, H., Tao, X., Master, S., Fu, H., Gotoh, Y., and Greenberg, M. E. (1997) Cell 91, Downward, J. (1999) Nat. Cell Biol. 1, E33 E35 6. Bae, S. S., Cho, H., Mu, J., and Birnbaum, M. J. (2003) J. Biol. Chem. 278, Morales-Ruiz, M., Fulton, D., Sowa, G., Languino, L. R., Fujio, Y., Walsh, K., and Sessa, W. C. (2000) Circ. Res. 86, Higuchi, M., Masuyama, N., Fukui, Y., Suzuki, A., and Gotoh, Y. (2001) Curr. Biol. 11, Zhou, G. L., Zhuo, Y., King, C. C., Fryer, B. H., Bokoch, G. M., and Field, J. (2003) Mol. Cell. Biol. 23, Ackah, E., Yu, J., Zoellner, S., Iwakiri, Y., Skurk, C., Shibata, R., Ouchi, N., Easton, R. M., Galasso, G., Birnbaum, M. J., Walsh, K., and Sessa, W. C. (2005) J. Clin. Investig. 115, Yoeli-Lerner, M., Yiu, G. K., Rabinovitz, I., Erhardt, P., Jauliac, S., and Toker, A. (2005) Mol. Cell 20, Servant, G., Weiner, A. D., Herzmark, P., Balla, T., Sedat, J. W., and Bourne, H. R. (2000) Science 287, Chung, C. Y., Potikyan, G., and Firtel, R. A. (2001) Mol. Cell 7, Tang, Y., Zhou, H., Chen, A., Pittman, R. N., and Field, J. (2000) J. Biol. Chem. 275, Schurmann, A., Mooney, A. F., Sanders, L. C., Sells, M. A., Wang, H.-G., Reed, J.-C., and Bokoch, G. M. (2000) Mol. Cell. Biol. 20, Jakobi, R., Moertl, E., and Koeppel, M. A. (2001) J. Biol. Chem. 276, Gnesutta, N., Qu, J., and Minden, A. (2001) J. Biol. Chem. 276, Chen, W. S., Xu, P. Z., Gottlob, K., Chen, M. L., Sokol, K., Shiyanova, T., Roninson, I., Weng, W., Suzuki, R., Tobe, K., Kadowaki, T., and Hay, N. (2001) Genes Dev. 15, Cho, H., Thorvaldsen, J. L., Chu, Q., Feng, F., and Birnbaum, M. J. (2001) J. Biol. Chem. 276, Cho, H., Mu, J., Kim, J. K., Thorvaldsen, J. L., Chu, Q., Crenshaw III, E. B., Kaestner, K. H., Bartolomei, M. S., Shulman, G. I., and Birnbaum, M. J. (2001) Science 292, Arboleda, M. J., Lyons, J. F., Kabbinavar, F. F., Bray, M. R., Snow, B. E., Ayala, R., Danino, M., Karlan, B. Y., and Slamon, D. J. (2003) Cancer Res. 63, Irie, H. Y., Pearline, R. V., Grueneberg, D., Hsia, M., Ravichandran, P., Kothari, N., Natesan, S., and Brugge, J. S. (2005) J. Cell Biol. 171, Manser, E., Leung, T., Salihuddin, H., Zhao, Z.-S., and Lim, L. (1994) Nature 367, Abo, A., Qu, J., Cammarano, J., Dan, C., Fritsch, A., B., V., Belisle, B., and Minden, A. (1998) EMBO J. 17, Bokoch, G. M. (2003) Annu. Rev. Biochem. 72, Manser, E., Huang, H.-Y., Loo, T.-H., Chen, X.-Q., Dong, J.-M., Leung, T., and Lim, L. (1997) Mol. Cell. Biol. 17, Sells, M. A., and Chernoff, J. (1997) Trends Cell Biol. 7, Sells, M. A., Knaus, U. G., Bagrodia, S., Ambrose, D. M., Bokoch, G. M., and Chernoff, J. (1997) Curr. Biol. 7, Dharmawardhane, S., Schurmann, A., Sells, M. A., Chernoff, J., Schmid, S. L., and Bokoch, G. M. (2000) Mol. Biol. Cell 11, Edwards, D. C., Sanders, L. C., Bokoch, G. M., and Gill, G. N. (1999) Nat. Cell Biol. 1, Alberts, A. S., Qin, H., Carr, H. S., and Frost, J. A. (2005) J. Biol. Chem. 280, Obermeier, A., Ahmed, S., Manser, E., Yen, S. C., Hall, C., and Lim, L. (1998) EMBO J. 17, Bokoch, G. M., Wang, Y., Bohl, B. P., Sells, M., Quilliam, L. A., and Knaus, JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 281 NUMBER 47 NOVEMBER 24, 2006
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
Flag-Rac Vector V12 V12 N17 C40. Vector C40 pakt (T308) Akt1. Myc-DN-PAK1 (N-SP)
 a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
Cdc42 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
Gα 13 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
ASPP1 Fw GGTTGGGAATCCACGTGTTG ASPP1 Rv GCCATATCTTGGAGCTCTGAGAG
 Supplemental Materials and Methods Plasmids: the following plasmids were used in the supplementary data: pwzl-myc- Lats2 (Aylon et al, 2006), pretrosuper-vector and pretrosuper-shp53 (generous gift of
Supplemental Materials and Methods Plasmids: the following plasmids were used in the supplementary data: pwzl-myc- Lats2 (Aylon et al, 2006), pretrosuper-vector and pretrosuper-shp53 (generous gift of
Supplementary data. sienigma. F-Enigma F-EnigmaSM. a-p53
 Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.
 Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Gα i Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
Polyclonal ARHGAP25 antibody was prepared from rabbit serum after intracutaneous
 Preparation and purification of polyclonal antibodies Polyclonal ARHGAP25 antibody was prepared from rabbit serum after intracutaneous injections of glutathione S-transferase-ARHGAP25-(509-619) (GST-coiled
Preparation and purification of polyclonal antibodies Polyclonal ARHGAP25 antibody was prepared from rabbit serum after intracutaneous injections of glutathione S-transferase-ARHGAP25-(509-619) (GST-coiled
SUPPLEMENTARY INFORMATION
 (Supplementary Methods and Materials) GST pull-down assay GST-fusion proteins Fe65 365-533, and Fe65 538-700 were expressed in BL21 bacterial cells and purified with glutathione-agarose beads (Sigma).
(Supplementary Methods and Materials) GST pull-down assay GST-fusion proteins Fe65 365-533, and Fe65 538-700 were expressed in BL21 bacterial cells and purified with glutathione-agarose beads (Sigma).
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplemental Data Supplementary Figure Legends and Scheme Figure S1.
 Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Supplementary methods
 Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
We performed RT-PCR, cloning, sequencing and qrt-pcr in murine melanoma. cell lines and melanocytic tumors from RET-mice in accordance with the method
 Supplementary Material and Methods Quantitative RT-PCR (qrt-pcr) We performed RT-PCR, cloning, sequencing and qrt-pcr in murine melanoma cell lines and melanocytic tumors from RET-mice in accordance with
Supplementary Material and Methods Quantitative RT-PCR (qrt-pcr) We performed RT-PCR, cloning, sequencing and qrt-pcr in murine melanoma cell lines and melanocytic tumors from RET-mice in accordance with
Nature Structural & Molecular Biology: doi: /nsmb.1583
 Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Supplemental Materials and Methods
 Supplemental Materials and Methods Co-immunoprecipitation (Co-IP) assay Cells were lysed with NETN buffer (20 mm Tris-HCl, ph 8.0, 0 mm NaCl, 1 mm EDT, 0.5% Nonidet P-40) containing 50 mm β-glycerophosphate,
Supplemental Materials and Methods Co-immunoprecipitation (Co-IP) assay Cells were lysed with NETN buffer (20 mm Tris-HCl, ph 8.0, 0 mm NaCl, 1 mm EDT, 0.5% Nonidet P-40) containing 50 mm β-glycerophosphate,
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
Thermo Scientific GTPase Research Tools
 Thermo Scientific Research Tools Active Pull-Down Assays We offer two different tools to study biology, one for active monitoring and one for global profiling. The Thermo Scientific Pierce Active Pull-Down
Thermo Scientific Research Tools Active Pull-Down Assays We offer two different tools to study biology, one for active monitoring and one for global profiling. The Thermo Scientific Pierce Active Pull-Down
System. Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells. Application Note No. 4 / March
 System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay. Josephine MY Ko and Maria Li Lung *
 In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
Supplemental Information. Pacer Mediates the Function of Class III PI3K. and HOPS Complexes in Autophagosome. Maturation by Engaging Stx17
 Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
Supplemental Materials and Methods
 Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Notes on experimental technique: 1. Enzyme activity was measured by noting the increase in absorbance at 341 nm, as NADPH +
 Case 2 Glucose-6-phosphate dehydrogenase activity and cell growth Focus concept The activity of the pentose phosphate pathway enzyme glucose-6-phosphate dehydrogenase has been found to be important in
Case 2 Glucose-6-phosphate dehydrogenase activity and cell growth Focus concept The activity of the pentose phosphate pathway enzyme glucose-6-phosphate dehydrogenase has been found to be important in
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS.
 Supplemental Data: LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS. Scott Jepson, Bryan Vought, Christian H.
Supplemental Data: LINGO-1, A TRANSMEMBRANE SIGNALING PROTEIN, INHIBITS OLIGODENDROCYTE DIFFERENTIATION AND MYELINATION THROUGH INTERCELLULAR SELF- INTERACTIONS. Scott Jepson, Bryan Vought, Christian H.
Supplemental Online Material. The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/-
 #1074683s 1 Supplemental Online Material Materials and Methods Cell lines and tissue culture The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/- knock-out animals
#1074683s 1 Supplemental Online Material Materials and Methods Cell lines and tissue culture The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/- knock-out animals
supplementary information
 DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells
 Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Rab5 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
RheB Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Arf6 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Arf6 Activation Assay Kit Catalog Number: 82401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Arf6 Activation Assay Kit Catalog Number: 82401 20 assays NewEast Biosciences 1 Table of Content Product
Supplementary Materials
 Supplementary Materials Supplementary Figure 1. PKM2 interacts with MLC2 in cytokinesis. a, U87, U87/EGFRvIII, and HeLa cells in cytokinesis were immunostained with DAPI and an anti-pkm2 antibody. Thirty
Supplementary Materials Supplementary Figure 1. PKM2 interacts with MLC2 in cytokinesis. a, U87, U87/EGFRvIII, and HeLa cells in cytokinesis were immunostained with DAPI and an anti-pkm2 antibody. Thirty
Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands).
 Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Xu et al., Supplementary Figures 1-7
 Xu et al., Supplementary Figures 1-7 Supplementary Figure 1. PIPKI is required for ciliogenesis. (a) PIPKI localizes at the basal body of primary cilium. RPE-1 cells treated with two sirnas targeting to
Xu et al., Supplementary Figures 1-7 Supplementary Figure 1. PIPKI is required for ciliogenesis. (a) PIPKI localizes at the basal body of primary cilium. RPE-1 cells treated with two sirnas targeting to
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12119 SUPPLEMENTARY FIGURES AND LEGENDS pre-let-7a- 1 +14U pre-let-7a- 1 Ddx3x Dhx30 Dis3l2 Elavl1 Ggt5 Hnrnph 2 Osbpl5 Puf60 Rnpc3 Rpl7 Sf3b3 Sf3b4 Tia1 Triobp U2af1 U2af2 1 6 2 4 3
doi:10.1038/nature12119 SUPPLEMENTARY FIGURES AND LEGENDS pre-let-7a- 1 +14U pre-let-7a- 1 Ddx3x Dhx30 Dis3l2 Elavl1 Ggt5 Hnrnph 2 Osbpl5 Puf60 Rnpc3 Rpl7 Sf3b3 Sf3b4 Tia1 Triobp U2af1 U2af2 1 6 2 4 3
Fig. S1 TGF RI inhibitor SB effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of
 Fig. S1 TGF RI inhibitor SB525334 effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of different concentrations of SB525334. Cells were lysed and
Fig. S1 TGF RI inhibitor SB525334 effectively blocks phosphorylation of Smad2 induced by TGF. FET cells were treated with TGF in the presence of different concentrations of SB525334. Cells were lysed and
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1
 Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
ab G alpha i Activation Assay Kit
 ab173234 G alpha i Activation Assay Kit Instructions for Use For the simple and fast measurement of G alpha i activation. This product is for research use only and is not intended for diagnostic use. Version
ab173234 G alpha i Activation Assay Kit Instructions for Use For the simple and fast measurement of G alpha i activation. This product is for research use only and is not intended for diagnostic use. Version
Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by
 Supplementary Methods and Figures Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer s disease treatment Wenchao Sun 1, Seongsoo Lee 1,2, Xiaoran
Supplementary Methods and Figures Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer s disease treatment Wenchao Sun 1, Seongsoo Lee 1,2, Xiaoran
No wash 2 Washes 2 Days ** ** IgG-bead phagocytosis (%)
 Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
SUPPLEMENTARY INFORMATION
 The Supplementary Information (SI) Methods Cell culture and transfections H1299, U2OS, 293, HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. H1299 and 293 cells were
The Supplementary Information (SI) Methods Cell culture and transfections H1299, U2OS, 293, HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. H1299 and 293 cells were
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Post-translational modification
 Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Supplementary Fig. 1 Proteomic analysis of ATR-interacting proteins. ATR, ARID1A and
 Supplementary Figure Legend: Supplementary Fig. 1 Proteomic analysis of ATR-interacting proteins. ATR, ARID1A and ATRIP protein peptides identified from our mass spectrum analysis were shown. Supplementary
Supplementary Figure Legend: Supplementary Fig. 1 Proteomic analysis of ATR-interacting proteins. ATR, ARID1A and ATRIP protein peptides identified from our mass spectrum analysis were shown. Supplementary
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary figures Supplementary Figure 1: Suv39h1, but not Suv39h2, promotes HP1α sumoylation in vivo. In vivo HP1α sumoylation assay. Top: experimental scheme. Middle: we
SUPPLEMENTARY INFORMATION Supplementary figures Supplementary Figure 1: Suv39h1, but not Suv39h2, promotes HP1α sumoylation in vivo. In vivo HP1α sumoylation assay. Top: experimental scheme. Middle: we
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Stargazin regulates AMPA receptor trafficking through adaptor protein. complexes during long term depression
 Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity.
 Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity. (A) Amino acid alignment of HDA5, HDA15 and HDA18. The blue line
Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity. (A) Amino acid alignment of HDA5, HDA15 and HDA18. The blue line
Supplemental data. Supplemental Materials and Methods
 Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured
 Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
SUPPLEMENTARY INFORMATION FIGURE LEGENDS
 SUPPLEMENTARY INFORMATION FIGURE LEGENDS Fig. S1. Radiation-induced phosphorylation of Rad50 at a specific site. A. Rad50 is an in vitro substrate for ATM. A series of Rad50-GSTs covering the entire molecule
SUPPLEMENTARY INFORMATION FIGURE LEGENDS Fig. S1. Radiation-induced phosphorylation of Rad50 at a specific site. A. Rad50 is an in vitro substrate for ATM. A series of Rad50-GSTs covering the entire molecule
Online Supporting Material for. The Bisecting GlcNAc on N-Glycans Inhibits Growth. Factor Signaling and Retards Mammary Tumor
 Online Supporting Material for The Bisecting GlcNAc on N-Glycans Inhibits Growth Factor Signaling and Retards Mammary Tumor Progression Yinghui Song 1, Jason A. Aglipay 1, Joshua D. Bernstein 2, Sumanta
Online Supporting Material for The Bisecting GlcNAc on N-Glycans Inhibits Growth Factor Signaling and Retards Mammary Tumor Progression Yinghui Song 1, Jason A. Aglipay 1, Joshua D. Bernstein 2, Sumanta
HPV E6 oncoprotein targets histone methyltransferases for modulating specific. Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu,
 1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
Description of supplementary material file
 Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Antibodies used in this study Figure S1. Akt expression in neutrophils from WT and individual Akt isoform knockout mice
 ntibodies used in this study The anti β-actin monoclonal antibody (Sigma-ldrich, St. Louis, MO) was generated against a slightly modified human β-actin N-terminal peptide, c-sp-sp-sp-ile-la-la-leu-val-ile-
ntibodies used in this study The anti β-actin monoclonal antibody (Sigma-ldrich, St. Louis, MO) was generated against a slightly modified human β-actin N-terminal peptide, c-sp-sp-sp-ile-la-la-leu-val-ile-
supplementary information
 DOI: 10.1038/ncb2116 Figure S1 CDK phosphorylation of EZH2 in cells. (a) Comparison of candidate CDK phosphorylation sites on EZH2 with known CDK substrates by multiple sequence alignments. (b) CDK1 and
DOI: 10.1038/ncb2116 Figure S1 CDK phosphorylation of EZH2 in cells. (a) Comparison of candidate CDK phosphorylation sites on EZH2 with known CDK substrates by multiple sequence alignments. (b) CDK1 and
Anti-HB-EGF (Human) mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Supplementary Fig.1 Luton
 Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supporting Information
 Supporting Information Su et al. 10.1073/pnas.1211604110 SI Materials and Methods Cell Culture and Plasmids. Tera-1 and Tera-2 cells (ATCC: HTB- 105/106) were maintained in McCoy s 5A medium with 15% FBS
Supporting Information Su et al. 10.1073/pnas.1211604110 SI Materials and Methods Cell Culture and Plasmids. Tera-1 and Tera-2 cells (ATCC: HTB- 105/106) were maintained in McCoy s 5A medium with 15% FBS
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Taylor et al., http://www.jcb.org/cgi/content/full/jcb.201403021/dc1 Figure S1. Representative images of Cav 1a -YFP mutants with and without LMB treatment.
Supplemental material THE JOURNAL OF CELL BIOLOGY Taylor et al., http://www.jcb.org/cgi/content/full/jcb.201403021/dc1 Figure S1. Representative images of Cav 1a -YFP mutants with and without LMB treatment.
INVESTIGATION OF THE BINDING SPECIFICITY OF IGF-IR USING MONOCLONAL ANTIBODIES
 INVESTIGATION OF THE BINDING SPECIFICITY OF IGF-IR USING MONOCLONAL ANTIBODIES By Mehrnaz Keyhanfar, Pharm.D. A thesis submitted to the University of Adelaide, South Australia in fulfilment of the requirements
INVESTIGATION OF THE BINDING SPECIFICITY OF IGF-IR USING MONOCLONAL ANTIBODIES By Mehrnaz Keyhanfar, Pharm.D. A thesis submitted to the University of Adelaide, South Australia in fulfilment of the requirements
Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna
 Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected with the wwp-luc reporter, and FLAG-tagged FHL1,
 Supplementary Data Supplementary Figure Legends Supplementary Figure 1 FHL-mediated TGFβ-responsive reporter transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected
Supplementary Data Supplementary Figure Legends Supplementary Figure 1 FHL-mediated TGFβ-responsive reporter transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected
Grb2-Mediated Alteration in the Trafficking of AβPP: Insights from Grb2-AICD Interaction
 Journal of Alzheimer s Disease 20 (2010) 1 9 1 IOS Press Supplementary Material Grb2-Mediated Alteration in the Trafficking of AβPP: Insights from Grb2-AICD Interaction Mithu Raychaudhuri and Debashis
Journal of Alzheimer s Disease 20 (2010) 1 9 1 IOS Press Supplementary Material Grb2-Mediated Alteration in the Trafficking of AβPP: Insights from Grb2-AICD Interaction Mithu Raychaudhuri and Debashis
CDK5 is essential for TGF-β1-induced epithelial-mesenchymal transition and breast cancer progression
 Supplementary information for: CDK5 is essential for TGF-β1-induced epithelial-mesenchymal transition and breast cancer progression Qian Liang, Lili Li, Jianchao Zhang, Yang Lei, Liping Wang, Dong-Xu Liu,
Supplementary information for: CDK5 is essential for TGF-β1-induced epithelial-mesenchymal transition and breast cancer progression Qian Liang, Lili Li, Jianchao Zhang, Yang Lei, Liping Wang, Dong-Xu Liu,
Transfection of CRISPR/Cas9 Nuclease NLS ribonucleoprotein (RNP) into adherent mammalian cells using Lipofectamine RNAiMAX
 Transfection of CRISPR/Cas9 Nuclease NLS ribonucleoprotein (RNP) into adherent mammalian cells using Lipofectamine RNAiMAX INTRODUCTION The CRISPR/Cas genome editing system consists of a single guide RNA
Transfection of CRISPR/Cas9 Nuclease NLS ribonucleoprotein (RNP) into adherent mammalian cells using Lipofectamine RNAiMAX INTRODUCTION The CRISPR/Cas genome editing system consists of a single guide RNA
Regulation of autophagic activity by ζ proteins associated with class III. phosphatidylinositol-3 kinase. Mercedes Pozuelo Rubio
 ONLINE SUPPORTING INFORMATION Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3 kinase Mercedes Pozuelo Rubio entro Andaluz de Biología Molecular y
ONLINE SUPPORTING INFORMATION Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3 kinase Mercedes Pozuelo Rubio entro Andaluz de Biología Molecular y
Analysing protein protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate Hong Wang and Xin Zeng
 Analysing protein protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate Hong Wang and Xin Zeng Department of Molecular Genetics, Biochemistry and Microbiology,
Analysing protein protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate Hong Wang and Xin Zeng Department of Molecular Genetics, Biochemistry and Microbiology,
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in
 Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated
 Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
7.012 Problem Set 5. Question 1
 Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by. 5 AGACACAAACACCAUUGUCACACUCCACAGC; Rand-2 OMe,
 Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
Materials and methods Oligonucleotides and DNA constructs RNA oligonucleotides and 2 -O-methylated oligonucleotides were synthesized by Dharmacon Inc. (Lafayette, CO). The sequences were: 122-2 OMe, 5
McNair Scholars Journal
 McNair Scholars Journal Volume 10 Issue 1 Article 9 2006 Exploring Important Protein Interactions in the Mammalian Diaphanous-related Formins: A Closer Look at the Relationship between the Intracellular
McNair Scholars Journal Volume 10 Issue 1 Article 9 2006 Exploring Important Protein Interactions in the Mammalian Diaphanous-related Formins: A Closer Look at the Relationship between the Intracellular
For quantitative detection of mouse IGF-1 in serum, body fluids, tissue lysates or cell culture supernatants.
 Mouse IGF-1 ELISA Kit (migf-1-elisa) Cat. No. EK0378 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Mouse IGF-1 ELISA Kit (migf-1-elisa) Cat. No. EK0378 96 Tests in 8 x 12 divisible strips Background Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a polypeptide protein hormone similar
Nature Medicine doi: /nm.2558
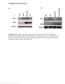 Supplementary. Fig. 1. (a) Sirt1 and mutant HTT (detected by HTT 81-90 antibody) protein levels were detected by Western blotting in cerebral cortex of N171-82Q mice. (b) Sirt1 and mutant HTT (detected
Supplementary. Fig. 1. (a) Sirt1 and mutant HTT (detected by HTT 81-90 antibody) protein levels were detected by Western blotting in cerebral cortex of N171-82Q mice. (b) Sirt1 and mutant HTT (detected
WesternMAX Alkaline Phosphatase Chemiluminescent Detection Kits
 WesternMAX Alkaline Phosphatase Chemiluminescent Detection Kits Code N221-KIT N220-KIT Description WesternMAX Chemiluminescent AP Kit, Anti-Mouse Includes: Alkaline Phosphatase (AP) Conjugated Anti-Mouse
WesternMAX Alkaline Phosphatase Chemiluminescent Detection Kits Code N221-KIT N220-KIT Description WesternMAX Chemiluminescent AP Kit, Anti-Mouse Includes: Alkaline Phosphatase (AP) Conjugated Anti-Mouse
