M. It is expressed in units such a g/cc,
|
|
|
- Lenard Alvin Gallagher
- 6 years ago
- Views:
Transcription
1 Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing an object on a balance. Determination of the volume of a solid can be done several ways. If the object has a regular geometrical shape like a cube, the appropriate dimensions call be measured and the volume calculated by an appropriate formula. If the solid is irregular its volume can be determined by displacement of a liquid by a weighed amount of the solid. Graduated cylinder filled with water. (Note the level of the water is read at The bottom of its curved upper surface, which is called the meniscus.) Rubber stoppers are added to the cylinder. The water level rises to 45 ml. The difference in volume, 45 ml - 30 ml or 15 ml, is the volume of the rubber stoppers. RECORD ALL INFORMATION ON WORKSHEET PROVIDED Part A Obtain either a block or a cylinder of metal, and measure its various dimensions (in cm) and its mass (in g). Then, calculate its volume (in cm 3 ) and finally, its density (in g/cm 3 ). Repeat the measurements for confidence. (How do you calculate the volume?) Part B Use the same piece of metal and determine its density (in g/ml) by the method of volume displacement in water. (This method has some limitations. What are some?) Repeat the determination for precision.
2 Part C. Density of a prepared solution Each student or group prepares a sodium chloride solution with a given (by instructor) concentration. For example if the concentration is 5.00 % by mass, then the student needs to weigh exactly 5.00 g of NaCl and add g of water to it. According to the formula you prepared a 5.00% NaCl solution. masssolute % mass 100% masssolution Procedure 1. You will prepare the solution in a 250ml Erlenmeyer flaks. Based on your concentration calculate how much salt and how much water you need to add to the beaker to prepare a 100 g solution. 2. Use a small beaker to weigh the correct amount of salt. Put the small beaker onto the balance and tare the balance. Add slowly with a spatula the correct amount of salt into the beaker. Once you are close to the desired mass, write the amount of salt measured and add the salt to a 250ml Erlenmeyer. 3. For measuring out the water use a small beaker (100 ml). Tare the balance with the beaker on it and add the exact amount of water to it (make sure you do not spill any water onto the balances!). Add the water to the Erlenmeyer and stir until all the salt is dissolved. 4. Once your salt is completely dissolved we can proceed and measure the volume and mass of the solution. Take a small, empty and dry beaker and measure its mass. Take the beaker back to your place. 5. Use a 10ml volumetric pipet, draw up 10.00ml of solution (make sure to use pipet pumps) and transfer the solution to the dry beaker. 6. Reweigh the mass of the beaker with the 10.00ml solution and record your new mass. 7. Repeat step 4 6 a second time in order to have two trials. 8. Calculate the mass of the solution and calculate the density of the solution for each trial; calculate the average density of the solution. 9. Make sure to rinse your volumetric pipet a few times with RO water. Graphing exercise with class data: After obtaining your average density for you solution, write your concentration and density on the white board. Once all groups have finished their work use the data to prepare a graph. Plot the density of the different solutions as a function of concentration on a graphing paper. Make sure you label the axis properly and draw a best fit line through your data.
3 Part D. Density of floating golf ball Procedure Obtain a golf ball from the instructor. Carefully place it in a 400 ml beaker and add enough water to cover it (about 300 ml). Add solid sugar, a scoop at a time, with stirring to dissolve the solid from each scoop until your golf ball just floats. Make sure the ball floats in the solution and does not break the surface of the solution. In case you added too much sugar you can correct this by adding extra water. Determine the density of the sugar solution (which should be the same as the density of the ball) by transferring 10.00ml of the solution with a volumetric pipet to a pre-weighed small beaker. Measure the mass of the beaker with the solution after the transfer and calculate the net mass of the 10.00ml solution. Finally calculate the density of the sugar solution and therefore the density of the ball. Make sure to rinse all your glassware with plenty of RO water in order to wash out all the sugar.
4 DATA Experiment 2 Densities Name Part A. Description of metal Mass Length Diameter Height Width Volume Density Average density Part B Use the same piece of metal and determine its density (in g/ml) by the method of volume displacement in water. (This method has some limitations. What are some?) Repeat the determination for precision. Initial volume reading Final volume reading V H2O = V metal Mass (from above) Density Average density Some Final Thoughts: Find out from your instructor what the metal is. Which method gave you a more accurate density result? How could you improve the accuracy?
5 Part C Density of prepared solution DATA Assigned concentration of solution: % Prepare solution: Mass of solute (NaCl) Mass of solvent (water) Density: Trial 1 Trial 2 Trial 3 (if needed) Mass of empty beaker Volume of solution Mass of cylinder plus solution Calculations: Net mass of solution Density of solution (for ea. Trial) Average density: Graph: Plot all the class date on a graphing paper. Make sure you label the axis properly. X-axis is concentration and y-axis is density. Answer the following question: Is the data linear? Are all the points close to the trend line? What does it mean if some points are somewhat away from the trend line? Explain.
6 Part D Determine Density of floating golf ball. Mass of empty beaker Volume of transferred solution Mass of beaker plus solution Net mass of solution Density of ball Density Problems: Fill in the following table: Density (g/ml) Mass (grams) Volume (ml) 15.7 g 1.90 ml 3.44 g/ml 148 ml 7.7 g/ml 21.0 g Answer the following questions: 1. Iron (Fe) has a density of 7.87 g/cm 3. If a block of Fe has a length of 15.0 cm and width of 13.0 cm and a height of 10.0 cm what is the mass of the block in grams? grams 2. The following is a table of densities of metals Metal density (g/ml) Nickel 8.91 Titanium 4.50 Lead Zinc 7.14 Tin 7.23 a. If a block of pure metal has a mass of 74.9 and a volume of 6.60 ml what is the identity of the metal? b. If the mass of a piece of metal is g and it is 2.20 cm long, 2.00 cm wide and 1.09 cm thick, what is the identity of the element?
SIGNIFICANT FIGURES WORKSHEET
 SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) 0.02 2.) 0.020 3.) 501 4.) 501.0 5.) 5,000 6.) 5,000. 7.) 6,051.00 8.) 0.0005 9.) 0.1020
SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) 0.02 2.) 0.020 3.) 501 4.) 501.0 5.) 5,000 6.) 5,000. 7.) 6,051.00 8.) 0.0005 9.) 0.1020
Measurement and Density - Experiment 1
 Measurement and Density - Experiment 1 Purpose: The purpose of this experiment is to acquaint you with some common metric units of length, volume, and mass. You will also measure the density of an unknown
Measurement and Density - Experiment 1 Purpose: The purpose of this experiment is to acquaint you with some common metric units of length, volume, and mass. You will also measure the density of an unknown
DETERMINATION OF DENSITY
 DETERMINATION OF DENSITY Dr. Barbara B. Bunn. Adapted from Experiments in General Chemistry ; Wiley 1996J.G. Wardeska, T.T-S. Huang, R.W. Kopp; used by permission. Tested by Ms. Janice Orr s Chemistry
DETERMINATION OF DENSITY Dr. Barbara B. Bunn. Adapted from Experiments in General Chemistry ; Wiley 1996J.G. Wardeska, T.T-S. Huang, R.W. Kopp; used by permission. Tested by Ms. Janice Orr s Chemistry
Introduction to Density
 Introduction to Density INTRODUCTION Which is heavier, a pound of aluminum or a pound of lead? The answer, of course, is neither, but many people confuse the words "heavy" and "dense". "Heavy" refers to
Introduction to Density INTRODUCTION Which is heavier, a pound of aluminum or a pound of lead? The answer, of course, is neither, but many people confuse the words "heavy" and "dense". "Heavy" refers to
Demonstration of osmosis
 Demonstration of osmosis We will try to carry out a classical experiment on demonstration of osmosis. The principle is shown in the figure. Water moves from the solution of lower osmolality, across the
Demonstration of osmosis We will try to carry out a classical experiment on demonstration of osmosis. The principle is shown in the figure. Water moves from the solution of lower osmolality, across the
An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+
 An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+ LAB ADV COMP 8 From Advanced Chemistry with Vernier, Vernier Software & Technology, 2004 INTRODUCTION A titration, as you recall, is a
An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+ LAB ADV COMP 8 From Advanced Chemistry with Vernier, Vernier Software & Technology, 2004 INTRODUCTION A titration, as you recall, is a
Salinity in Seawater
 Salinity in Seawater Objective To familiarize students with the different methods used for measuring salinity of water. Introduction: Salinity exerts profound impacts on the marine environment. It controls
Salinity in Seawater Objective To familiarize students with the different methods used for measuring salinity of water. Introduction: Salinity exerts profound impacts on the marine environment. It controls
Name Honors Chemistry / /
 Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley)
 Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Analysis of Calcium Carbonate Tablets
 Experiment 9 Analysis of Calcium Carbonate Tablets Prepared by Ross S. Nord, Eastern Michigan University PURPOSE To perform a gravimetric exercise to determine weight percent of active ingredient in a
Experiment 9 Analysis of Calcium Carbonate Tablets Prepared by Ross S. Nord, Eastern Michigan University PURPOSE To perform a gravimetric exercise to determine weight percent of active ingredient in a
Experiment #3. Density and Specific Gravity.
 Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
R = kc Eqn 5.1. R = k C Eqn 5.2. R = kc + B Eqn 5.3
 5 Standard addition 5.1 Introduction An interference is anything that causes an analysis to be incorrect, which means in practice, that the measured concentration for the presented sample is wrong. There
5 Standard addition 5.1 Introduction An interference is anything that causes an analysis to be incorrect, which means in practice, that the measured concentration for the presented sample is wrong. There
Experiment 2: The Chromatography of Organic Compounds
 Experiment 2: The Chromatography of Organic Compounds INTRODUCTION When performing an organic reaction, it is very common to observe the formation of other compounds in addition to your desired product;
Experiment 2: The Chromatography of Organic Compounds INTRODUCTION When performing an organic reaction, it is very common to observe the formation of other compounds in addition to your desired product;
Standard Test Procedures Manual
 STP 205-10 Standard Test Procedures Manual Section: 1. SCOPE 1.1. Description of Test This method describes the quantitative determination of the distribution of particle sizes in soils. The distribution
STP 205-10 Standard Test Procedures Manual Section: 1. SCOPE 1.1. Description of Test This method describes the quantitative determination of the distribution of particle sizes in soils. The distribution
Experiment 2: Preparation of the Artificial Sweetener Dulcin
 Experiment 2: Preparation of the Artificial Sweetener Dulcin Organic compounds known as sugars are carbohydrates that occur widely in nature. For example, sucrose (aka table sugar) is found in sugar can,
Experiment 2: Preparation of the Artificial Sweetener Dulcin Organic compounds known as sugars are carbohydrates that occur widely in nature. For example, sucrose (aka table sugar) is found in sugar can,
Experiment 13: Determination of Molecular Weight by Freezing Point Depression
 1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
Experiment 3: The Chromatography of Organic Compounds
 Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
IDENTIFYING UNKNOWN SUBSTANCES
 IDENTIFYING UNKNOWN SUBSTANCES LAB 15 EXPERIMENT STUDENT BOOK Chapter 1, page 25 TOOLBOX Page 4 and 36 Goal Identify unknown substances with the help of different tests. 1. What is the independent variable
IDENTIFYING UNKNOWN SUBSTANCES LAB 15 EXPERIMENT STUDENT BOOK Chapter 1, page 25 TOOLBOX Page 4 and 36 Goal Identify unknown substances with the help of different tests. 1. What is the independent variable
Chapter 8. Gravimetric Analysis
 Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a :
Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a :
Name Lab Section Date. Sediment Lab
 Name Lab Section Date. Investigating Stokes Law Sediment Lab ds = density of solid, g/cm dw = density of water, g/cm g = gravity, 980 cm/second 2 D = particle diameter in centimeters μ = molecular viscosity,
Name Lab Section Date. Investigating Stokes Law Sediment Lab ds = density of solid, g/cm dw = density of water, g/cm g = gravity, 980 cm/second 2 D = particle diameter in centimeters μ = molecular viscosity,
5-4 Chemical changes Trilogy
 5-4 Chemical changes Trilogy.0 A student investigated the reaction of sodium carbonate with dilute hydrochloric acid. The student used the apparatus shown in Figure. Figure Sodium carbonate This is the
5-4 Chemical changes Trilogy.0 A student investigated the reaction of sodium carbonate with dilute hydrochloric acid. The student used the apparatus shown in Figure. Figure Sodium carbonate This is the
Determine whether the metal is magnesium, iron, or zinc based on the value of the calculated molar mass.
 Gases Part A: A student working at METAL Company found an unlabelled bottle of a metal in the lab. The metal could be magnesium, iron, or zinc. Each of these metals react with dilute hydrochloric acid
Gases Part A: A student working at METAL Company found an unlabelled bottle of a metal in the lab. The metal could be magnesium, iron, or zinc. Each of these metals react with dilute hydrochloric acid
v = 5.00cm x 3.00cm x 2.00cm 2. What is the volume of a marble that has a mass of 3.0 g and a density of 2.7 g/ml? Formula (density) Substitution
 Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
EXPERIMENT 3: Identification of a Substance by Physical Properties
 EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
CONSERVATION OF MATTER AND CHEMICAL PROPERTIES
 1 CONSERVATION OF MATTER AND CHEMICAL PROPERTIES I. OBJECTIVES AND BACKGROUND The object of this experiment is to demonstrate the conservation of matter- or more particularly, the conservation of "atoms"
1 CONSERVATION OF MATTER AND CHEMICAL PROPERTIES I. OBJECTIVES AND BACKGROUND The object of this experiment is to demonstrate the conservation of matter- or more particularly, the conservation of "atoms"
DETERMINATION of the EMPIRICAL FORMULA
 DETERMINATION of the EMPIRICAL FORMULA One of the fundamental statements of the atomic theory is that elements combine in simple whole number ratios. This observation gives support to the theory of atoms,
DETERMINATION of the EMPIRICAL FORMULA One of the fundamental statements of the atomic theory is that elements combine in simple whole number ratios. This observation gives support to the theory of atoms,
A TEACHER-DIRECTED, GUIDED DISCOVERY ACTIVITY FOR ADVANCED PHYSICAL SCIENCE STUDENTS
 A TEACHER-DIRECTED, GUIDED DISCOVERY ACTIVITY FOR ADVANCED PHYSICAL SCIENCE STUDENTS 1 2 FEATURES OF THIS ACTIVITY Problem solving Cooperative learning Requires student reasoning Requires student initiative
A TEACHER-DIRECTED, GUIDED DISCOVERY ACTIVITY FOR ADVANCED PHYSICAL SCIENCE STUDENTS 1 2 FEATURES OF THIS ACTIVITY Problem solving Cooperative learning Requires student reasoning Requires student initiative
Density What is density? How do you measure density?
 Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
LABORATORY MODULE UNCONFINED COMPRESSION OF HYDROGEL DISKS
 LABORATORY MODULE UNCONFNED The unconfined compression test is by far the most popular mechanical testing configuration to measure the mechanical properties of cylindrical samples. amples with non-planar
LABORATORY MODULE UNCONFNED The unconfined compression test is by far the most popular mechanical testing configuration to measure the mechanical properties of cylindrical samples. amples with non-planar
(aq) + 5e - Mn 2+ (aq) + 4H 2
 EXPERIMENT 20 Titrimetric Determination of Iron INTRODUCTION Potassium permanganate is widely used as an oxidizing agent in titrimetric analysis. In acidic solution, a permanganate ion undergoes reduction
EXPERIMENT 20 Titrimetric Determination of Iron INTRODUCTION Potassium permanganate is widely used as an oxidizing agent in titrimetric analysis. In acidic solution, a permanganate ion undergoes reduction
LAD B3 (pg! 1 of 6! ) Analysis by Redox Titration Name Per
 LAD B3 (pg! 1 of 6! ) Name Per Introduction As you know, one common type of reaction in chemistry is oxidation-reduction. It involves the transfer of electrons from one species to another. Atoms undergo
LAD B3 (pg! 1 of 6! ) Name Per Introduction As you know, one common type of reaction in chemistry is oxidation-reduction. It involves the transfer of electrons from one species to another. Atoms undergo
The determination of copper in brass
 The determination of copper in brass Objective - To determine the amount of copper in a brass sample Background Brass is an alloy made of copper and zinc. Most brass contains about 60% copper. The proportions
The determination of copper in brass Objective - To determine the amount of copper in a brass sample Background Brass is an alloy made of copper and zinc. Most brass contains about 60% copper. The proportions
A Hydrogen Powered Bottle Rocket
 A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION
 GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
Density Answers to the End of module questions
 IDS 101 Density Answers to the End of module questions 1. You are experimenting with the mysterious substance X. You carefully measure the mass of a uniform 2 ml sample of pure substance X. You slice it
IDS 101 Density Answers to the End of module questions 1. You are experimenting with the mysterious substance X. You carefully measure the mass of a uniform 2 ml sample of pure substance X. You slice it
GEL Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Grade: /25
 GEL 4250 - Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Name: Section: Grade: /25 COMPLETE & TURN IN ONLY PAGES THAT HAVE A FIELD FOR YOUR NAME. ALL OTHER PAGES
GEL 4250 - Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Name: Section: Grade: /25 COMPLETE & TURN IN ONLY PAGES THAT HAVE A FIELD FOR YOUR NAME. ALL OTHER PAGES
D =? D = M = 10 g = 5 g/cm 3 M = 10 g V 2 cm 3 V = 2 cm 3 5 g/cm 3
 Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Rev Experiment 10
 Experiment 10 SPECTROPHOTOMETRIC DETERMINATION OF IRON IN DRINKING WATER 2 lab periods Reading: 1) Chapter 17, pg 393-403, Quantitative Chemical Analysis, 8 h Edition, Daniel C. Harris (7 th Edition: Chapter
Experiment 10 SPECTROPHOTOMETRIC DETERMINATION OF IRON IN DRINKING WATER 2 lab periods Reading: 1) Chapter 17, pg 393-403, Quantitative Chemical Analysis, 8 h Edition, Daniel C. Harris (7 th Edition: Chapter
Zinc 17. Part 2 Practical work
 Zinc 17 Part 2 Practical work 18 Zinc and Zirconia (per group) Teacher s notes Using carbon to extract copper from copper oxide could be used as an introduction to extracting less-reactive metals by displacement.
Zinc 17 Part 2 Practical work 18 Zinc and Zirconia (per group) Teacher s notes Using carbon to extract copper from copper oxide could be used as an introduction to extracting less-reactive metals by displacement.
Partner: Cathy 22 March Separation and Qualitative Determination of Cations and Anions
 Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
Forensics with TI-Nspire Technology
 Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com Science Objectives Identify characteristics of different soils to demonstrate that a suspect has been at a scene.
Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com Science Objectives Identify characteristics of different soils to demonstrate that a suspect has been at a scene.
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter.
 1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
Experimental techniques
 Unit 1 Experimental techniques In this unit you will come to understand the general principles behind various experimental techniques. Experimental investigation is a very important aspect of Physical
Unit 1 Experimental techniques In this unit you will come to understand the general principles behind various experimental techniques. Experimental investigation is a very important aspect of Physical
Gravimetric Analysis: Determination of % Sulfur in Fertilizer
 Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
SurTec 717 Alkaline Zinc/Nickel Electroplating Process (Electrolyte based on Sodium)
 SurTec 717 Alkaline Zinc/Nickel Electroplating Process (Electrolyte based on Sodium) Properties tolerates higher temperatures superior metal distribution produces Zn/Ni alloy deposits containing 12-15
SurTec 717 Alkaline Zinc/Nickel Electroplating Process (Electrolyte based on Sodium) Properties tolerates higher temperatures superior metal distribution produces Zn/Ni alloy deposits containing 12-15
EXPERIMENT 6. Determination of the Ideal Gas Law Constant - R. Magnesium metal reacts with hydrochloric acid according to the following reaction,
 EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
A Hydrogen Powered Bottle Rocket
 A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION
 EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
look down at cross on paper paper cross on paper
 1 The equation for the reaction between sodium thiosulfate and hydrochloric acid is given below. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + S(s) + SO 2 (g) + H 2 O(l) The speed of this reaction was investigated
1 The equation for the reaction between sodium thiosulfate and hydrochloric acid is given below. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + S(s) + SO 2 (g) + H 2 O(l) The speed of this reaction was investigated
Chem 2115 Experiment #9. Consumer Chemistry: Determining the Iron Content in Supplements
 Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
Overview. Learning Objectives: This module provides step-by-step instructions in how to do the Bulk Density Protocol.
 Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example,
 SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
Recrystallization with a Single Solvent
 Experiment: Recrystallization Part II: Purification of Solids In Part I of the recrystallization experiment, you learned about the factors which make a good recrystallization solvent, and you learned how
Experiment: Recrystallization Part II: Purification of Solids In Part I of the recrystallization experiment, you learned about the factors which make a good recrystallization solvent, and you learned how
1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
Skills in Science. Lab equipment. (Always draw 2D) Drawings below are NOT to scale. Beaker - A general purpose container with a pouring lip.
 Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
Experiment 30A ENERGY CONTENT OF FUELS
 Experiment 30A ENERGY CONTENT OF FUELS FV 12/10/2012 MATERIALS: 12-oz. aluminum beverage can with top cut out and holes on side, thermometer, 100 ml graduated cylinder, 800 ml beaker, long-stem lighter,
Experiment 30A ENERGY CONTENT OF FUELS FV 12/10/2012 MATERIALS: 12-oz. aluminum beverage can with top cut out and holes on side, thermometer, 100 ml graduated cylinder, 800 ml beaker, long-stem lighter,
Preparation of copper(ii) sulfate from copper(ii) nitrate
 Student s Name: Date: Background Preparation of copper(ii) sulfate from copper(ii) nitrate The purpose of this laboratory activity is to prepare copper(ii) sulfate from copper(ii) nitrate. This is done
Student s Name: Date: Background Preparation of copper(ii) sulfate from copper(ii) nitrate The purpose of this laboratory activity is to prepare copper(ii) sulfate from copper(ii) nitrate. This is done
Lab Question from Board papers Class-09[2018] 1. How will you separate a mixture of iron filings, iodine and common salt?
![Lab Question from Board papers Class-09[2018] 1. How will you separate a mixture of iron filings, iodine and common salt? Lab Question from Board papers Class-09[2018] 1. How will you separate a mixture of iron filings, iodine and common salt?](/thumbs/77/74991575.jpg) Lab Question from Board papers Class-09[2018] SET-1 Short Answer Type Questions (2 marks) 1. How will you separate a mixture of iron filings, iodine and common salt? Ans. (i) Remove iron filings with the
Lab Question from Board papers Class-09[2018] SET-1 Short Answer Type Questions (2 marks) 1. How will you separate a mixture of iron filings, iodine and common salt? Ans. (i) Remove iron filings with the
Metal Finishing Products and Service META-MATE ZINCATE 40 "A CONCENTRATED LIQUID ZINCATE FORMULATION FOR THE PRETREATMENT OF ALUMINUM AND ITS ALLOYS"
 Metal Chem,inc. Metal Finishing Products and Service 29 Freedom Court Greer, SC 29650 864.877.6175 Fax 864.877.6176 DATA SHEET META-MATE ZINCATE 40 "A CONCENTRATED LIQUID ZINCATE FORMULATION FOR THE PRETREATMENT
Metal Chem,inc. Metal Finishing Products and Service 29 Freedom Court Greer, SC 29650 864.877.6175 Fax 864.877.6176 DATA SHEET META-MATE ZINCATE 40 "A CONCENTRATED LIQUID ZINCATE FORMULATION FOR THE PRETREATMENT
The table below shows some of the properties of the elements cobalt and nickel.
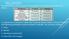 BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS
 EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
To identify and classify various types of chemical reactions.
 Cycle of Copper Reactions Minneapolis Community and Technical College v.11.17 Objectives: To observe and document copper s chemical changes in five different reactions and verify that copper is conserved
Cycle of Copper Reactions Minneapolis Community and Technical College v.11.17 Objectives: To observe and document copper s chemical changes in five different reactions and verify that copper is conserved
Evaluation copy. Total Dissolved Solids. Computer INTRODUCTION
 Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Equation Writing and Predicting Products Chemistry I Acc
 Introduction: Equation Writing and Predicting Products Chemistry I Acc If you examine your bicycle after it has been left out in the rain a number of times you will find that it has begun to rust. Rust
Introduction: Equation Writing and Predicting Products Chemistry I Acc If you examine your bicycle after it has been left out in the rain a number of times you will find that it has begun to rust. Rust
The Crystal Forest Favorite Holiday Demonstrations
 The Crystal Forest Favorite Holiday Demonstrations SCIENTIFIC Introduction Put a new twist on crystal growing. In this class participation demonstration, students cut out and assemble miniature trees and
The Crystal Forest Favorite Holiday Demonstrations SCIENTIFIC Introduction Put a new twist on crystal growing. In this class participation demonstration, students cut out and assemble miniature trees and
EXPERIMENT 5. Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline
 EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
H N 2. Decolorizing carbon O. O Acetanilide
 Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Method to 500 µg/l CH 2 O Powder Pillows
 , 8110 DOC316.53.01042 MBTH Method 1 Method 8110 3 to 500 µg/l CH 2 O Powder Pillows Scope and Application: For water. 1 Adapted from Matthews, T.G. and Howell, T.C., Journal of the Air Pollution Control
, 8110 DOC316.53.01042 MBTH Method 1 Method 8110 3 to 500 µg/l CH 2 O Powder Pillows Scope and Application: For water. 1 Adapted from Matthews, T.G. and Howell, T.C., Journal of the Air Pollution Control
2. Crystallization. A. Background
 2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
VISCOSITY, INHERENT (One Point)
 VISCO.02-1 VISCOSITY, INHERENT (One Point) PRINCIPLE SCOPE A weighed starch sample is dispersed in sodium hydroxide solution using a standard technique. Relative viscosity of the sample dispersion is determined
VISCO.02-1 VISCOSITY, INHERENT (One Point) PRINCIPLE SCOPE A weighed starch sample is dispersed in sodium hydroxide solution using a standard technique. Relative viscosity of the sample dispersion is determined
Activity 6: Test Ability of Mushroom Extracts to Increase Reaction Rate
 Activity 6: Test Ability of Mushroom Extracts to Increase Reaction Rate Cellobiase that breaks down the 1,4 b-glucoside linkages in cellobiose is produced by many organisms. Fungi, such as molds, yeasts
Activity 6: Test Ability of Mushroom Extracts to Increase Reaction Rate Cellobiase that breaks down the 1,4 b-glucoside linkages in cellobiose is produced by many organisms. Fungi, such as molds, yeasts
Module 2, Add on Lesson Turbidity Sensor. Teacher. 90 minutes
 Module 2, Add on Lesson Turbidity Sensor Teacher 90 minutes Purpose Construct a sensor to measure the turbidity of water Graph data and reason about curves and linear relationships Calibrate the turbidity
Module 2, Add on Lesson Turbidity Sensor Teacher 90 minutes Purpose Construct a sensor to measure the turbidity of water Graph data and reason about curves and linear relationships Calibrate the turbidity
Calculating Ballast By Troy Averill
 Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
SPECTROPHOTOMETRIC DETERMINATION OF IRON
 SPECTROPHOTOMETRIC DETERMINATION OF IRON In this experiment you will determine trace amounts of iron using spectrophotometric methods. BACKGROUND In solution ferrous iron combines with 2,2 bipyridyl to
SPECTROPHOTOMETRIC DETERMINATION OF IRON In this experiment you will determine trace amounts of iron using spectrophotometric methods. BACKGROUND In solution ferrous iron combines with 2,2 bipyridyl to
Measuring Manganese Concentration Using Spectrophotometry
 Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
The total amount in grams of solid material dissolved in 1 kg of seawater.
 MS20 Laboratory Objectives To understand the definition of salinity To learn the sources and sinks for salt in the ocean To measure the salinity of a water sample by mass evaporation To measure the salinity
MS20 Laboratory Objectives To understand the definition of salinity To learn the sources and sinks for salt in the ocean To measure the salinity of a water sample by mass evaporation To measure the salinity
TESTING THE WATERS HOW GOOD IS THAT BOTTLED WATER AND HOW EFFECTIVE IS YOUR WATER FILTER
 TESTING THE WATERS HOW GOOD IS THAT BOTTLED WATER AND HOW EFFECTIVE IS YOUR WATER FILTER TEACHER NOTES This experiment is designed for students working singly or in groups of two. One run through the series
TESTING THE WATERS HOW GOOD IS THAT BOTTLED WATER AND HOW EFFECTIVE IS YOUR WATER FILTER TEACHER NOTES This experiment is designed for students working singly or in groups of two. One run through the series
Principles of Gel Filtration Chromatography
 Edvo-Kit #108 Principles of Gel Filtration Chromatography Experiment Objective: The objective of this experiment is to introduce the principles of gel fi ltration chromatography as a method that separates
Edvo-Kit #108 Principles of Gel Filtration Chromatography Experiment Objective: The objective of this experiment is to introduce the principles of gel fi ltration chromatography as a method that separates
Total Dissolved Solids
 Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Spectrophotometry of DNA and RNA
 Spectrophotometry of DNA and RNA Many of the techniques used to study cells are focused on characterization of the molecules that make up cells. Since the molecules are invisible, they are studied using
Spectrophotometry of DNA and RNA Many of the techniques used to study cells are focused on characterization of the molecules that make up cells. Since the molecules are invisible, they are studied using
LABORATORY 3 SOIL ANALYSIS
 VEGETATION DESCRIPTION AND ANALYSIS 2017 LABORATORY 3 SOIL ANALYSIS OBJECTIVE This lab will obtain four key soil parameters from the samples collected from Shawnee Gowan s Grizzly Glacier project relevés.
VEGETATION DESCRIPTION AND ANALYSIS 2017 LABORATORY 3 SOIL ANALYSIS OBJECTIVE This lab will obtain four key soil parameters from the samples collected from Shawnee Gowan s Grizzly Glacier project relevés.
Properties of Water Lab: Water and solutions
 Properties of Water Lab: Water and solutions Background: Water has several unique properties. The hydrogen bonding that occurs between molecules in water results in much higher melting and boiling points
Properties of Water Lab: Water and solutions Background: Water has several unique properties. The hydrogen bonding that occurs between molecules in water results in much higher melting and boiling points
Soil Particle Density Protocol
 Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
ADVANCED PLACEMENT ENVIRONMENTAL SCIENCE
 NAME: DATE: MODS: ADVANCED PLACEMENT ENVIRONMENTAL SCIENCE Carolina: 18-1030 Population Growth in Lemna minor 1 Objectives Upon completion of this exercise, you should be able to Identify the similarities
NAME: DATE: MODS: ADVANCED PLACEMENT ENVIRONMENTAL SCIENCE Carolina: 18-1030 Population Growth in Lemna minor 1 Objectives Upon completion of this exercise, you should be able to Identify the similarities
Accomplishments. 1 Nevada Renewable Energy Consortium Quarterly Progress Report
 Subtask.4.: Chemically promoted mechanical dewatering of wastewater sludge FINAL REPORT for work completed January, 200 December 3, 200. (PI: Charles Cornella, UNR) More than 6,000 wastewater treatment
Subtask.4.: Chemically promoted mechanical dewatering of wastewater sludge FINAL REPORT for work completed January, 200 December 3, 200. (PI: Charles Cornella, UNR) More than 6,000 wastewater treatment
Calcium and Magnesium; Chlorophosphonazo Rapid Liquid Method Method to 1000 µg/l Ca and Mg as CaCO 3 (ULR) Pour-Thru Cell
 Hardness, Total DOC316.53.01045 Calcium and Magnesium; Chlorophosphonazo Rapid Liquid Method Method 8374 4 to 1000 µg/l Ca and Mg as CaCO 3 (ULR) Pour-Thru Cell Scope and application: For boiler and ultrapure
Hardness, Total DOC316.53.01045 Calcium and Magnesium; Chlorophosphonazo Rapid Liquid Method Method 8374 4 to 1000 µg/l Ca and Mg as CaCO 3 (ULR) Pour-Thru Cell Scope and application: For boiler and ultrapure
INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES. Introduction. Electrochemistry Revised 4/28/14
 INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES Introduction Electrochemical Cells In this part of the experiment, four half cells are created by immersing metal strips of zinc, copper,
INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES Introduction Electrochemical Cells In this part of the experiment, four half cells are created by immersing metal strips of zinc, copper,
MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE. CHEMISTRY OCTOBER hour
 MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE CANDIDATE NAME SCHOOL NAME CLASS/SECTION NATIONAL ASSESSMENT AT FORM III CHEMISTRY
MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE CANDIDATE NAME SCHOOL NAME CLASS/SECTION NATIONAL ASSESSMENT AT FORM III CHEMISTRY
COMPOSITION OF SEAWATER
 COMPOSITION OF SEAWATER Ocean water is a combination of freshwater and a variety of dissolved substances. Salinity is a measure of the amount of dissolved salts in seawater, measured in parts per thousand
COMPOSITION OF SEAWATER Ocean water is a combination of freshwater and a variety of dissolved substances. Salinity is a measure of the amount of dissolved salts in seawater, measured in parts per thousand
UGANDA STANDARD. Fruit and vegetable products Determination of soluble solids Refractometric method US ISO First Edition 2009-mm-dd
 UGANDA STANDARD US ISO 2173 First Edition 2009-mm-dd Fruit and vegetable products Determination of soluble solids Refractometric method Reference number US ISO 2173:2003 UNBS 2009 US ISO 2173: 2003 Compliance
UGANDA STANDARD US ISO 2173 First Edition 2009-mm-dd Fruit and vegetable products Determination of soluble solids Refractometric method Reference number US ISO 2173:2003 UNBS 2009 US ISO 2173: 2003 Compliance
USEPA 1,2 Bicinchoninate Method 3 Method 8506 (CuVer 1) and Method 8026 (CuVer 2) 0.04 to 5.00 mg/l Cu Powder Pillows or AccuVac Ampuls
 Copper DOC316.53.01039 USEPA 1,2 Bicinchoninate Method 3 Method 8506 (CuVer 1) and Method 8026 (CuVer 2) 0.04 to 5.00 mg/l Cu Powder Pillows or AccuVac Ampuls Scope and application: For water, wastewater
Copper DOC316.53.01039 USEPA 1,2 Bicinchoninate Method 3 Method 8506 (CuVer 1) and Method 8026 (CuVer 2) 0.04 to 5.00 mg/l Cu Powder Pillows or AccuVac Ampuls Scope and application: For water, wastewater
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard.
 1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
EXPERIMENT. The Reaction of Magnesium with Hydrochloric Acid; The Molar Volume of Hydrogen
 EXPERIMENT The Reaction of Magnesium with Hydrochloric Acid; The Molar Volume of Hydrogen PURPOSE In this experiment you will determine the volume of the hydrogen gas which is produced when a sample of
EXPERIMENT The Reaction of Magnesium with Hydrochloric Acid; The Molar Volume of Hydrogen PURPOSE In this experiment you will determine the volume of the hydrogen gas which is produced when a sample of
) and it s ideal van t Hoff factor is 4. Note that polyatomic ions do not break up into their constituent elements.
 Freezing Point Depression: Determining CaCl2 Van t Hoff Factor Minneapolis Community and Technical College C1152 v.12.15 I. Introduction The physical properties of solutions that depend on the number of
Freezing Point Depression: Determining CaCl2 Van t Hoff Factor Minneapolis Community and Technical College C1152 v.12.15 I. Introduction The physical properties of solutions that depend on the number of
European Pharmacopoeia
 i PHARMACOPOEIAL DISCUSSION GROUP REV 1 - CORR 1 G02 Bulk density and tapped density of powders It is understood that sign-off covers the technical content of the draft and each party will adapt it as
i PHARMACOPOEIAL DISCUSSION GROUP REV 1 - CORR 1 G02 Bulk density and tapped density of powders It is understood that sign-off covers the technical content of the draft and each party will adapt it as
CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017)
 CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017) SEPARATION AND RECOVERY OF ORGANIC COMPOUNDS, THIN LAYER CHROMATOGRAPHY, COLUMN CHROMATOGRAPHY, CRYSTALLIZATION, AND MELTING POINTS Overview
CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017) SEPARATION AND RECOVERY OF ORGANIC COMPOUNDS, THIN LAYER CHROMATOGRAPHY, COLUMN CHROMATOGRAPHY, CRYSTALLIZATION, AND MELTING POINTS Overview
Module 2, Add on Lesson Turbidity Sensor. Student. 90 minutes
 Module 2, Add on Lesson Turbidity Sensor Student 90 minutes Purpose Construct a sensor to measure the turbidity of water Graph data and reason about curves and linear relationships Calibrate the turbidity
Module 2, Add on Lesson Turbidity Sensor Student 90 minutes Purpose Construct a sensor to measure the turbidity of water Graph data and reason about curves and linear relationships Calibrate the turbidity
MAKING SOLID SOLUTIONS WITH ALKALI HALIDES (AND BREAKING THEM)
 MAKING SOLID SOLUTIONS WITH ALKALI HALIDES (AND BREAKING THEM) John B. Brady Department of Geology Smith College Northampton, MA 01063 jbrady@science.smith.edu INTRODUCTION When two cations have the same
MAKING SOLID SOLUTIONS WITH ALKALI HALIDES (AND BREAKING THEM) John B. Brady Department of Geology Smith College Northampton, MA 01063 jbrady@science.smith.edu INTRODUCTION When two cations have the same
